Abstract
Helicobacter pylori has been shown to induce chronic active gastritis and peptic ulcer and may contribute to the development of duodenal ulcer. Previous studies have shown that H. pylori mediates apoptosis of gastric epithelial cells via a Fas-dependent pathway. However, evidence for the induction of such a mechanism in intestinal epithelial cells (IEC) by H. pylori infection has not been demonstrated yet. This study was performed (i) to ascertain that H. pylori can induce IEC apoptosis; (ii) to delineate the role of the cag pathogenicity island (PAI), cagE, and vacA gene products in this process; and (iii) to verify whether the Fas-dependent pathway is involved in this phenomenon. When T84 cells were exposed to VacA+/cag PAI+ H. pylori strains (CCUG 17874 and 60190), they exhibited apoptosis hallmarks as assessed by morphological studies, as well as annexin V and 3,3′-dihexyloxacarbocyanine iodide staining. In contrast, few or no apoptotic features could be detected after incubation with an isogenic mutant of strain 60190 in which the cagE gene was disrupted (60190:C− strain) or with a VacA−/cag PAI− H. pylori strain (G21). In addition, activation of caspase-3 during infection with VacA+/cag PAI+ H. pylori strains was inhibited by pretreatment of IEC with an antagonistic anti-Fas antibody (ZB4). Taken together, these findings indicate that H. pylori triggers apoptosis in IEC via a Fas-dependent pathway following a process that depends on the expression of the cag PAI.
It is commonly accepted that Helicobacter pylori is the main etiologic agent responsible for gastric and duodenal ulcer diseases (9, 21, 27, 40). Furthermore, this pathogen plays a causative role in the development of gastric adenocarcinoma, which occurs after several steps such as intestinal metaplasia and dysplasia (3, 9, 23, 47). Analysis of biopsy specimens from patients with H. pylori-induced gastritis show gastric cell hyperproliferation associated with infiltration of the mucosa by polymorphonuclear leukocytes (PMN). This increase in cell proliferation is likely to be counterbalanced by cell loss mediated by an apoptotic process (21, 23, 37, 43, 52, 55). Previous studies have suggested that adherence of different bacteria to epithelial cells triggers the production of proinflammatory cytokines such as interleukin-8 (IL-8), IL-6, IL-12, tumor necrosis factor alpha, IL-1β, and gamma interferon (IFN-γ) (2, 11, 13, 22, 36, 42, 45, 50, 58). Release of some of these cytokines induces the recruitment of inflammatory cells and could be involved in the destruction of the functional epithelial barrier (9, 10, 19, 24). We and others have shown that excessive recruitment of PMN by itself or following a process involving enzymatic and radical oxygen intermediate release is able to induce epithelial cell death (12, 28, 35, 62). Previous studies have demonstrated that Fas ligand and Fas antigen expressed by gastric epithelial cells are upregulated when exposed to tumor necrosis factor alpha, IL-1β or IFN-γ. This feature increases epithelial cell susceptibility to apoptosis by homotypic interactions in gastric epithelium or by heterotypic interactions with Fas ligand expressed by infiltrated activated T lymphocytes and PMN (21, 22, 24, 26, 28, 30, 48). Another pathway of the apoptotic process has been proposed showing that the direct bacterium-epithelial cell contact can by itself induce gastric epithelial cell death (6, 41, 52, 53). Along these lines, several putative virulence factors, such as products of the genes in the pathogenicity island (PAI) named cag, surface urease, and the cytotoxin VacA, have been shown by in vitro and in vivo studies to promote the rapid destruction of gastric epithelial cells after interaction with H. pylori (5, 16, 27, 46, 49, 53, 61).
In spite of extensive studies, the physiopathology of duodenal ulcer in patients infected with H. pylori remains to be elucidated. Studies of antral and duodenal biopsy specimens show that H. pylori could adhere to epithelial cell membranes by different forms of adhesion (41), but the consequences of such an interaction on the turnover and the onset of apoptosis of epithelial cells have been poorly investigated. Previous studies have shown a correlation between the development of duodenal ulcer in H. pylori infection and the level of apoptosis in the antral mucosal epithelium (27), but the molecular events mediating enhanced epithelial cell apoptosis associated with duodenal ulcer diseases remain under investigation. To date, there is no gastric epithelial cell model able to grow as a polarized monolayer. In contrast, differentiated T84 monolayers display high transepithelial resistance (31), a well-organized brush border, and the capacity to release IL-8 at the basal cell surface under adhesion with H. pylori (8, 19, 33). The T84 cell line thus appears an interesting model to study the interaction of H. pylori with an epithelial monolayer. In this study we sought to determine whether the direct interaction of H. pylori with intestinal epithelial cells (IEC) could by itself induce their apoptotic cell death. It has been previously shown by Corthesy-Theulaz et al. that this pathogen could adhere to the apical membrane of T84 cells and secondarily induce a reorganization of the brush border and a deep invagination, allowing intimate contact with the bacteria (8). Based on these observations, we used the T84 cell line to study the interaction between H. pylori and IEC. We show in the present study that VacA+/cag PAI+ H. pylori strains but not the VacA−/cag PAI− or VacA+/cagE strains could induce apoptosis of the highly differentiated human IEC (T84) by a Fas-dependent pathway.
MATERIALS AND METHODS
T84 cell culture.
T84 cells (passages 65 to 90) (American Type Culture Collection), a human colonic carcinoma cell line, were grown as confluent monolayers in a 1:1 mixture of Dulbecco-Vogt-modified Eagle medium and Hanks' F-12 medium supplemented with 15 mM HEPES (pH 7.5), 14 mM NaHCO3, 5% newborn calf serum, penicillin-streptomycin, and 8 mg of ampicillin per ml (31). Monolayers were grown on six-well plates or on 5-cm2 collagen-coated polycarbonate filters (Costar, Cambridge, Mass.) and were used 6 to 8 days after plating. For biochemical studies and flow cytometric analysis, 5 × 108 CFU of different strains of H. pylori were added to 5 × 106 T84 cells grown on six-well plates.
Bacterial strains.
We used the wild-type urease+/VacA+/cag PAI+ wild-type cytotoxic H. pylori strain 60190 (ATCC 49503) and its isogenic mutant (60190:C−). As previously described by Tummuru et al., in the 60190:C− strain the cagE gene was disrupted by insertional mutagenesis, leading to a nonpolar mutation (these strains were kindly donated by T. L. Cover and M. J. Blaser, Nashville, Tenn.) (54). In addition, we used the wild-type CCUG 17874 (urease+/VacA+/cag PAI+) cytotoxic H. pylori strain (from the culture collection of the University of Göteborg, Göteborg, Sweden) and the wild-type G21 (urease+/VacA−/ cag PAI−) H. pylori clinical isolate (kindly donated by N. Figura, Siena, Italy).
Preparation of bacterial suspensions.
Bacteria were grown for 3 to 4 days at 37°C under microaerophilic conditions in Columbia agar (Oxoid, Basingstoke, United Kingdom) supplemented with 10% sheep blood (Oxoid) and 1% Vitox (Oxoid). Immediately before the start of the experiments, the bacteria were suspended at a final concentration of 5 × 108 CFU/ml in the culture medium used for T84 cells (19, 45).
Electron microscopy study.
A total of 5 × 108 CFU of different strains of H. pylori was gently distributed on the apical surface of T84 monolayers grown on 5-cm2 filters. After 48 h of coculture, T84 cells were rinsed extensively in Hanks' balanced salt solution and were fixed for 1 h at 4°C with 2% paraformaldehyde in 0.1 M sodium cacodylate (pH 7.4). Monolayers were rinsed in cacodylate buffer, postfixed in 1% OsO4 for 1 h at 4°C, dehydrated through graded ethanol washes, and embedded in epoxy resin. Oriented 1-mm sections were obtained with diamond knives, and multiple areas for thin sections were selected in T84 cells and were then sectioned, mounted on copper mesh grids, and stained with uranyl acetate and lead citrate. Ultrathin sections were examined on a JEOL 1200 EXII electron microscope.
Flow cytometric analysis. (i) CD95 and CD95 ligand immunostaining.
T84 cells grown on six-well plates were dissociated using 0.1% trypsin and 0.03% EDTA, withdrawn, pelleted, and resuspended in phosphate-buffered saline (PBS)–0.1% bovine serum albumin, and 106 cells were stained by a two-step method with anti-Fas antibody (10 μg/ml) (ZB4; Immunotech) and rabbit-anti-mouse linked to fluorescein isothiocyanate (FITC) (dilution, 1:20) (Dako). After being washed the cells were fixed in 0.4% formaldehyde and analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.). Data acquisition and analysis were performed using CellQuest software.
(ii) DiOC6 staining.
After coculture experiments with H. pylori strains, T84 cells monolayers were dissociated as described above and their mitochondrial potential was assessed using 3,3′-dihexyloxacarbocyanine iodide (DiOC6). Briefly, 106 cells were washed in cold PBS, incubated for 30 min with 40 nM DiOC6 at 37°C in the dark, and ultimately analyzed by flow cytometry (excitation wavelength, 488 nm; emission wavelength, 529 nm).
(iii) FITC-labeled annexin V and propidium iodide staining.
FITC-conjugated annexin V binds to phosphatidylserine once it is exposed to the outer layer of the plasma membrane during the apoptotic program. T84 cells (106) dissociated as described above were incubated with FITC-labeled annexin V diluted in HNS buffer (10 mM HEPES-NaOH [pH 7.4], 140 mM NaCl, 5 mM CaCl2) for 30 min at 37°C in the dark, as specified by the manufacturer (Roche, Mannheim, Germany). The staining was analyzed by flow cytometry using 488-nm excitation and a 515-nm bandpass filter for fluorescein detection. Propidium iodide (1 μg/ml) was added to the cell suspension just before analysis by flow cytometry.
Western immunoblot analysis.
Confluent T84 monolayers grown on six-well plates were exposed for different periods to H. pylori strains, washed in Hanks' balanced salt solution, and gently scraped into lysis buffer at 4°C (at a density of 25 × 106 cells/ml) (10 mM HEPES, 3.5 mM MgCl2, 150 mM NaCl, 1% NP-40, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 25 μM leupeptin, 5 mM benzamidine, 1 μM pepstatin, 25 μM aprotinin, 50 mM sodium β-glycerophosphate, 20 mM sodium pyrophosphate, 0.5 mM dithiothreitol). Cell lysates were centrifuged for 15 min at 4°C and denaturated by boiling in reducing sodium dodecyl sulfate (SDS) sample buffer. Protein lysates (50 μg) were analyzed by migration in SDS-polyacrylamide gel electrophoresis (10 to 15% polyacrylamide gels) and subsequently electrophoretically transferred to a nitrocellulose sheet. The nitrocellulose sheet was incubated in blocking buffer and then probed with the first antibody overnight at 4°C. This labeling was visualized by using peroxidase-conjugated secondary anti-rabbit (1:10,000) or anti-mouse (1:5,000) antibodies (Dako, Santa Barbara, Calif.) and enhanced chemiluminescence (ECL kit; Amersham, Little Chalfont, England). The different antibodies used were anti-phospho-ERK1/2 (dilution, 1:1,000) (New England Biolabs, Inc, Beverly, Mass.), anti-poly-(ADP-ribose)-polymerase (PARP) (1 μg/ml) (PharMingen, San Diego, Calif.), anti-caspase-3 (dilution, 1:3,000) (Transduction Laboratories, San Diego, Calif.), anti-caspase-8 (dilution, 1:3,000) (Santa Cruz Biotechnology, Santa Cruz, Calif.), anti-phospho-p38 (dilution, 1:1,000) (New England Biolabs), and anti-phospho-Jun kinase (dilution, 1:1000) (Promega Corp., Madison, Wis.).
DEVD-pNA cleavage assay.
Caspase activity was measured using a continuous colorimetric assay. Briefly, control cells or cells exposed for various periods to H. pylori strains were gently scraped into PBS–2 mM dithiothreitol. After sonification for 2 8-s bursts, lysates were centrifuged at 15,000 × g and 50-μg samples of cell extracts were each incubated with 200 μM acetyl-Asp-Glu-Val-Asp-p-nitroanilide (DEVD-pNA) (Alexis Corp., San Diego, Calif.) preferentially cleaved by members of the CPP32 family of cysteine protease. Release of pNA was monitored at 410 nm at 37°C. Recording was performed over the linear range of the assay, and the specificity of the caspase assay was controlled by adding DEVD-CHO, an apopain/CPP-32 inhibitor (DEVD-CHO) (100 μM) (Alexis Corp.), to the cell extracts. Substrates without lysates served as negative controls.
The SAP kinase inhibitor SB202190 was purchased from Calbiochem (San Diego, Calif.).
Data analysis.
Values are expressed as the mean and standard error of the mean of at least three independent experiments when not otherwise stated.
RESULTS
H. pylori induces intestinal epithelial cell apoptosis in a VacA/cag PAI-dependent manner.
Unlike untreated cells (Fig. 1a), T84 monolayers infected for 48 h with 60190 (Fig. 1b) or with the H. pylori CCUG 17874 strain (VacA+/cag PAI+) (Fig. 1c) exhibited ultrastructural apoptotic changes. These features include loss of brush border and formation of condensed or marginated nuclear chromatin, reduced cytoplasmic size, and vacuolation. Epithelial cells infected for 48 h with the cagE isogenic mutant (60190:C−) H. pylori 60190 strain showed less severe apoptotic features (data not shown), which were even less detectable when T84 cells were incubated with the VacA−/cag PAI− G21 strain (Fig. 1d).
FIG. 1.
Apoptosis of T84 cells after 48 h of coculture with VacA+/cag PAI+ H. pylori strains. Confluent T84 monolayers were incubated for 48 h with three different H. pylori strains, CCUG 17874, G21, and 60190, and morphological hallmarks of apoptosis were analyzed by electron microscopy. (a) Control untreated T84 cells. (b) T84 cells after 48 h of coculture with the VacA+/cag PAI+ 60190 strain, displaying brush border disorganization, cytoplasmic shrinkage, and chromatin condensation. (c) T84 cells infected with the wild-type VacA+/cag PAI+ CCUG 17874 H. pylori strain, showing morphological apoptotic features. (d) Coculture (48 h) with the wild-type VacA−/cag PAI− G21 strain, showing the lack of induction of numerous apoptotic features in T84 cells.
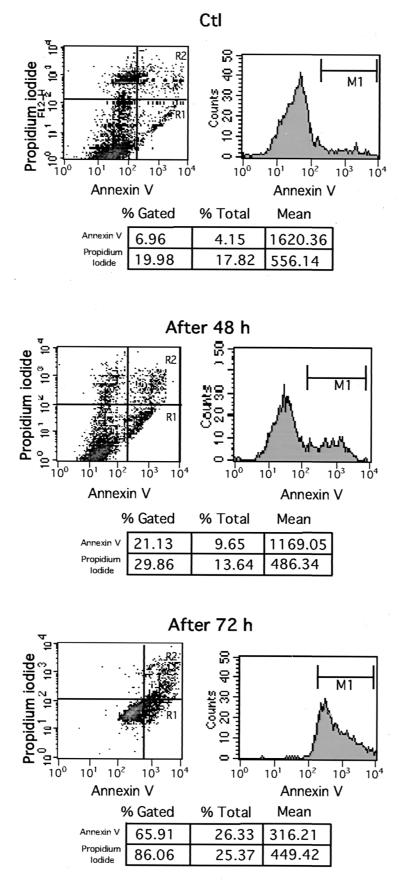
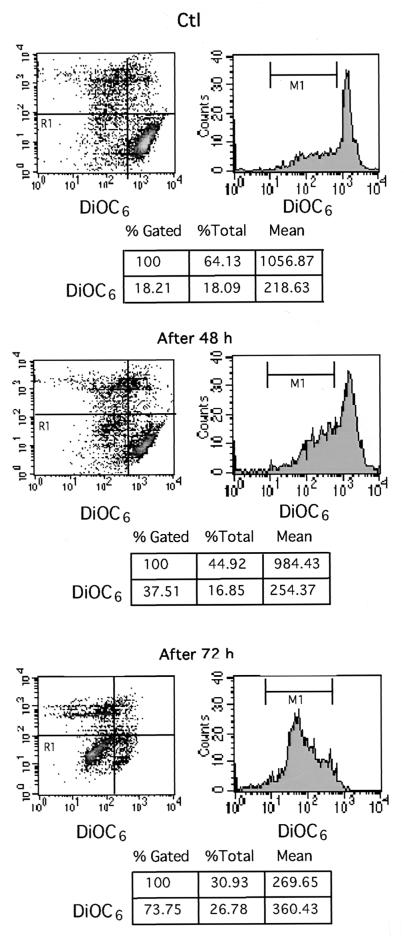
Apoptosis of T84 cells exposed to H. pylori was confirmed by flow cytometric analysis of cells stained with annexin V-FITC and DiOC6. Whereas DiOC6 staining was used to monitor the disruption of mitochondrial transmembrane potential which constitutes a early critical event in several apoptotic processes (4, 59, 60), annexin V-FITC was used to detect exposure of phosphatidylserine on the outer leaflet of the cell membrane, a further step of the apoptotic program (20). To analyze only living cells committed to apoptosis, both necrotic and late apoptotic cells stained with propidium iodide were gated out. As shown in Fig. 2, the percentage of annexin V-labeled T84 apoptotic cells was 21.13% after 48 h of infection and increased to 65.91% after 72 h of infection compared to 6.9% in uninfected T84 cells. Consistently, apoptotic cells exhibiting a collapsed mitochondrial potential as assessed by DiOC6 staining were in the range of 37.51% after 48 h of infection and 73.75% after 72 h compared to 18.21% in uninfected control cells (Fig. 3).
FIG. 2.
Annexin V staining and flow cytometric analysis of T84 cells apoptosis after 48 and 72 h of coculture with H. pylori strains. T84 confluent monolayers were left uninfected as controls (Ctl) or infected with 5 × 108 CFU of (VacA+/cag PAI+) CCUG 17874 strain per filter for 48 h (After 48 h) or 72 h (After 72 h). T84 monolayers were stained with FITC-annexin V and propidium iodide before being subjected to flow cytometeric analysis. The area marked R1 indicates annexin V-labeled early apoptotic cells, opposite to necrotic or late apoptotic cells stained by both annexin V and iodide propidium (area marked R2). The apoptotic cell population contained in the area marked R1 and the cell population contained in area marked R2 are shown in the charts below the diagrams. Data are from a representative experiment (n = 3).
FIG. 3.
DiOC6 staining and flow cytometric analysis of T84 cell apoptosis after 48 and 72 h of coculture with H. pylori strains. The conditions of coculture were the same as for the experiment in Fig. 2. Control (Ctl) cells or T84 cells infected for 48 h (After 48 h) or 72 h (After 72 h) were incubated with DiOC6 (40 nM) before being subjected to analysis by flow cytometry by a method described in Materials and Methods. The area at the low left (R1) indicates apoptotic cells with a drop in mitochondrial potential, and the percentage of apoptotic cells is given in the charts below the diagrams. Data are from a representative experiment (n = 3).
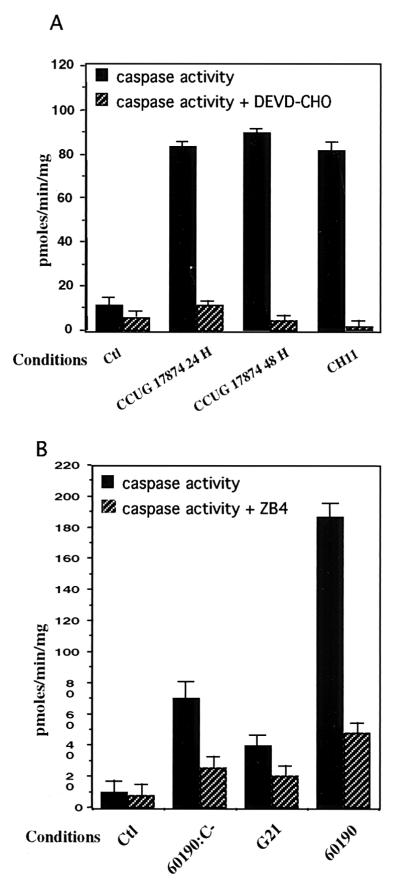
VacA+/cag PAI+ H. pylori strains induce caspase-3 activation in T84 cells by a Fas-dependent pathway.
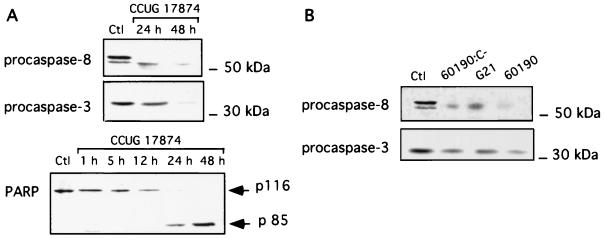
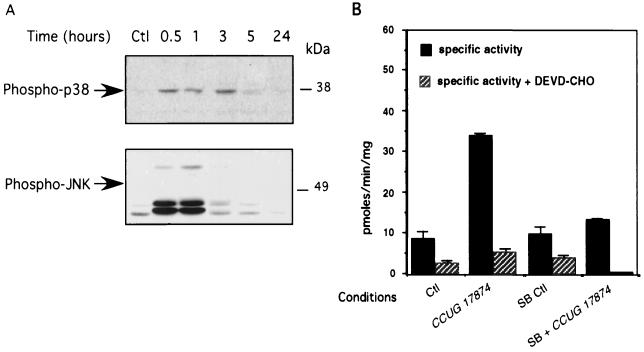
To further assess the apoptotic events of IEC exposed to H. pylori, caspase activity assays were performed after 24 and 48 h of incubation with the wild-type (VacA+/cag PAI+) CCUG 17874 H. pylori strain. Cell extracts were incubated for 24 h with DEVD-pNA, a colorimetric caspase-3 substrate. Specificity of the cleavage was verified by adding the caspase-3 inhibitor (DEVD-CHO) (100 μM). A fourfold increase in caspase activity was observed after 24 and 48 h of infection compared to that in uninfected T84 cells (Fig. 4A). To further delineate the precise function of VacA and cag PAI gene products in the onset of the apoptotic process, we tested the ability of the VacA+/cag PAI+ 60190 strain, its isogenic mutant, 60190:C− (VacA+/cagE), and the G21 (VacA−/cag PAI−) strain to induce caspase activity in T84 cells (Fig. 4B). Whereas a 24-h incubation of T84 cells with the 60190 strain strongly stimulated caspase-3 activity, the cagE mutant strain induced only a weak caspase-3 activation and G21 did not induce any activity. Taken together, these findings suggest that the concomitant expression of the cytotoxin VacA and the gene products of the cag PAI, at least cagE, may play a crucial role in the onset of the apoptotic process. To investigate the mechanism through which H. pylori mediates T84 cell apoptosis, we first tested the possible involvement of the Fas pathway by preincubating T84 cells with an antagonistic anti-Fas antibody (ZB4) before infecting them with the three different strains 60190:C−, G21, and 60190. As shown in Fig. 4B, T84 cell apoptosis was strongly decreased, suggesting that H. pylori mediated its apoptogenic effect via a Fas-dependent pathway. An agonistic anti-Fas antibody (CH11) used as positive control induced caspase-3 activation to the same extent as the CCUG 17874 strain did (Fig. 4A). The amounts of procaspase-8, procaspase-3, and poly-(ADP-ribose)-polymerase (PARP) were evaluated by Western blot analysis in T84 cells exposed for 24 or 48 h to the CCUG 17874 strain and strains 60190:C−, G21, and 60190. As shown in Fig. 5, IEC expressed an abundant level of procaspase-3 (17), which decreased after a 48-h incubation with the wild-type VacA+/cag PAI+ CCUG 17874 strain (Fig. 5A) or after a 24-h incubation with the VacA+/cag PAI+ 60190 H. pylori strain (Fig. 5B), suggesting the activation of caspase-3. Accordingly, PARP, a target of caspase-3, was cleaved under the same conditions. The amount of procaspase-8 was also dramatically decreased after a 24-h infection with strain 60190 (Fig. 5B) and the wild-type VacA+/cag PAI+ CCUG 17874 strain (Fig. 5A), indicating the activation of this upstream caspase. Interestingly, unlike caspase-3, caspase-8 was also activated after 24 h of incubation with the G21 and 60190:C− strains (Fig. 5B).
FIG. 4.
H. pylori induces caspase-3 activity in epithelial cells by a Fas-dependent pathway. (A) T84 cells grown on six-well plates were left uninfected (Ctl) or were infected for 24 h (CCUG 17874 24 h) or 48 h (CCUG 17874 48 h) with 5 × 108 CFU of CCUG 17874. T84 cells were treated for 5 h with CH11 antibody (1 μg/ml) (CH11), used as positive controls for caspase-3 activity. Caspase-3-like activity was inhibited by the addition of DEVD-CHO (100 μM) to extracts before the addition of chromogenic substrate (caspase activity + DEVD-CHO). (B) Involvement of the Fas receptor pathway in H. pylori-induced epithelial cell apoptosis. T84 cells grown on six-well plates were left uninfected (Ctl) or were infected for 48 h with the (VacA+/cag PAI+) 60190 strain (60190), the 60190:C− (VacA+/cagE) isogenic mutant (60190:C)−, or the G21 (VacA−/cag PAI−) wild-type H. pylori strain (G21). To inhibit Fas-dependent apoptosis, T84 cells were preincubated for 2 h with the antagonistic anti-Fas receptor antibody (ZB4) (1 μg/ml) before being subjected to 48 h of incubation with H. pylori strains (caspase activity + ZB4). Cell extracts prepared as described in Materials and Methods were assessed for DEVD-pNA-hydrolyzing activity, and measurements of activity was recorded over 24 h. One representative experiment is shown, with error bars corresponding to triplicates (n = 3).
FIG. 5.
Different requirements for H. pylori strains induce caspase-3, caspase-8, and PARP cleavage in IEC. (A) Western blot analyses of procaspase-8 and procaspase-3 disappearance and PARP cleavage in IEC in response to infection with H. pylori CCUG 17874 (VacA+/cag PAI+). T84 cells grown on 5-cm2 filters were incubated for 48 h with the wild-type CCUG 17874 strain (CCUG 17874), and cell lysates were analyzed using specific anti-procaspase-8, anti-caspase-3, and anti-PARP antibodies. (B) Procaspase-8 and procaspase-3 activation in T84 cells during 24 or 48 h of infection with different H. pylori strains was analyzed by the same technique as above, using anti-procaspase-8 and anti-procaspase-3 antibodies. These strains were the wild-type strain 60190 (VacA+/cag PAI+) (60190), the 60190:C− isogenic mutant (VacA+/cagE) (60190:C−), and the wild-type G21 (VacA−/cag PAI−) strain (G21) and were cocultured with IEC cells for 24 h. Ctl indicates lysates from control T84 cells. These data correspond to the most representative of three independent experiments.
Assessment of Fas receptor expression by flow cytometry.
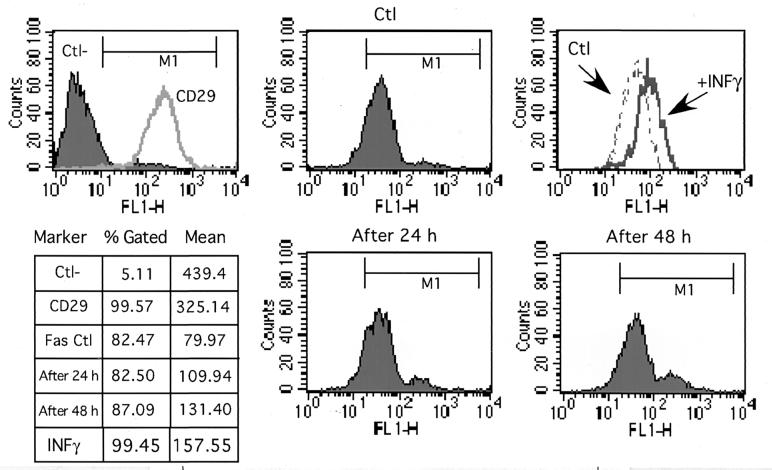
Since Fas receptor has been reported to be upregulated by various proapoptotic stimuli, it was of interest to determine whether H. pylori VacA+/cag PAI+ strains could induce Fas upregulation on IEC. To this aim, T84 cells were infected for 24 h or 48 h with CCUG and the level of cell surface Fas expression was analyzed by flow cytometry (Fig. 6). The effect of H. pylori on Fas receptor expression was compared to that obtained after treatment with IFN-γ (400 UI/ml), which is known to upregulate Fas expression on IEC (1). As shown in Fig. 6, compared to the effect of IFN-γ, only a weak upregulation of the Fas receptor expression was detected after a 48-h incubation with H. pylori CCUG 17874 and no significant effect was observed with the 60190:C− or G21 strain (data not shown).
FIG. 6.
Infection with H. pylori strains failed to affect Fas receptor expression on T84 cells. Cell surface Fas expression was analyzed by flow cytometry using the specific anti-Fas monoclonal antibody (ZB4; Immunotech) and secondary antibodies linked to FITC. T84 control cells (Ctl) or cells after 24 or 48 h of incubation with the H. pylori wild-type CCUG 17874 strain were stained with anti-Fas antibodies. Antibodies to CD29 were used for the positive control (CD29), and irrelevant immunoglobulin G1 was used for the negative control (Ctl−). T84 cells were treated for 24 h with IFN-γ (400 IU/ml) to control Fas upregulation. The percentage of marked cells and mean of fluorescence are reported in the chart.
H. pylori induces early MAP kinase activation in intestinal epithelial T84 cells.
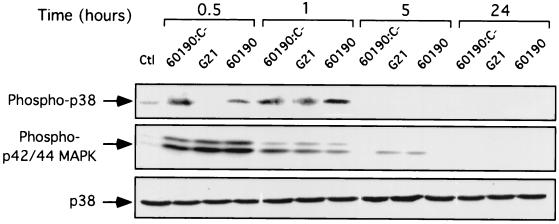
Since the implication of the mitogen-activated protein (MAP) kinase cascade in the regulation of apoptosis has been extensively studied (56), we sought to determine the effects of the different H. pylori strains on the activities of the extracellular signal-regulated kinases p42/p44 (ERK1/2) and p38 MAP kinase in the intestinal epithelial cell line T84. Confluent monolayers were infected for up to 24 h with the three different H. pylori strains, 60190:C−, G21, and 60190, using a ratio of 5 × 108 bacteria to 5 × 106 T84 cells. Time courses of the different MAP kinase activations in T84 cell extracts were assessed by Western blot analysis using phospho-specific antibodies to ERK1/2, p38, and c-Jun kinase (JNK). As shown in Fig. 7, control cells displayed low or undetectable ERK1/2 activation whereas the three H. pylori strains induced a substantial ERK1/2 activation, peaking at 30 min of infection and slowly declining to the basal level by 5 h (Fig. 7). Similarly, phosphorylation of p38 in cell extracts increased as early as 30 min after infection with 60190:C− and 60190 but was more clearly detectable after 1 h of infection with all the three strains used (60190:C−, G21, and 60190) (Fig. 7). As shown in Fig. 8A, similar kinetics for p38 MAP kinase and JNK kinase activation were detected after T84 cell infection with the wild-type H. pylori CCUG 17874 strain. To determine whether the p38 and JNK activation was required for H. pylori-mediated intestinal epithelial cell death, T84 cells were treated with a stress-activated protein (SAP) kinase inhibitor, SB202190 (30 μM), which targets both p38 and JNK, before and for 24 h after infection with wild-type (VacA+/cag PAI+) H. pylori strain CCUG 17874. As shown in Fig. 8B, caspase-3 activation, which characterizes the apoptotic process, was markedly reduced during SAP kinase inhibition in control and infected cells. These results indicate that SAP kinase (p38 and JNK) activation was involved in the regulation of apoptosis induced by infection with the CCUG 17874 H. pylori strain.
FIG. 7.
ERK 1/2 and p38 are activated early in IEC during infections with H. pylori VacA+/cag PAI+ strains. T84 cells grown on six-well plates were incubated for various periods with wild-type 60190 (VacA+/cag PAI+) (60190), isogenic mutant (VacA+/cagE) 60190:C− (60190:C−), or wild-type G21 (VacA−/cag PAI−) (G21) H. pylori strains (108 bacteria/well). Lysates from control cells (Ctl) or cells after various times of infection were analyzed by immunoblotting for the expression of activated MAP kinase using phosphospecific antibodies against ERK1/2 and p38. An immunoblot analysis for p38 (nonphosphorylated) expression was performed on the same membrane to control for equal amounts of proteins. Results of one representative experiment (n = 3) are presented.
FIG. 8.
Apoptosis induced by infection with a VacA+/cag PAI+ H. pylori strain is prevented by inhibition of p38 MAP kinase and JNK activation in T84 cells. (A) Western blot analyses of p38 MAP kinase and JNK activation in T84 cells during incubation with the wild-type (Vac+/cag PAI+) CCUG 17874 H. pylori strain. Activation of p38 MAP kinase and JNK in T84 cells control (Ctl) or during infection with a the CCUG 17874 strain was assessed by immunoblotting using antibodies to phosphorylated p38 and JNK. (B) Caspase-3 activity analysis of p38 MAP kinase inhibition by pretreatment with the SAP kinase inhibitor SB (202190). Activation of caspase-3 was assessed in T84 cell control lysates (Ctl) or after 48 h of incubation with H. pylori CCUG 17874. These results were compared to those obtained after 1 h of pretreatment of T84 cells with the SAP kinase inhibitor SB 202190 (30 μM) (SB Ctl) or pretreatment of T84 cells before 48 h of infection with the CCUG 17874 H. pylori strain (SB + CCUG 17874). Results of one representative experiment (n = 3) are presented.
DISCUSSION
Previous studies have already established that a correlation exists between the development of duodenal ulcer in H. pylori infection and the level of apoptosis in antral mucosal epithelium (37, 40, 41, 52). One hypothesis could be that during H. pylori infection in the gastric antrum, the physiological mechanism of both gastrin and gastric secretion is impaired, leading to the subsequent development of a duodenal ulcer (27). In this study we provide evidence that in vitro infection of T84 intestinal epithelial cells induced apoptosis. Furthermore, we demonstrated that expression of the cytotoxin VacA, associated with the cag PAI gene products, is involved in the induction of H. pylori-mediated intestinal cell apoptosis. This is in agreement with previous data showing that in vivo or in vitro gastric epithelial cells apoptosis could be induced by a cag PAI-positive, VacA-positive (type I) H. pylori strain but not with a cag PAI-negative, VacA-negative (type II) strain (24, 37, 45, 48, 57). Furthermore, Galmiche et al. have recently shown that VacA targets mitochondria, where it potentiates or favors apoptosis (14), and Moss et al. have recently provide evidence that the cag PAI is associated with increased apoptosis of gastric epithelial cells (38). Our findings also indicate that the product of the cagE gene (CagE), which mediates IL-8 secretion and NF-κB activation in gastric epithelial cells, may also be involved in the onset of T84 cell apoptosis (39, 49, 50).
The product of the cagE gene has previously been shown to be necessary for H. pylori to induce the migration of PMN through an epithelial monolayer via the induction of IL-8 secretion by IEC (19). Apart from this effect, our in vitro model allowed us to demonstrate that CagE was also necessary for a direct apoptogenic effect of H. pylori on IEC independently of PMN transmigration. These data are consistent with previous findings demonstrating that CagE contributes to gastric epithelial cell cycle progression and to an apoptotic response (44). Moreover, our results indicate that the apoptogenic effect of H. pylori was markedly inhibited by preincubation with antagonist anti-Fas antibodies, suggesting that the Fas receptor pathway is involved in the induction of the apoptotic process. To support this hypothesis, we found that caspase-8 and caspase-3 were activated in a time-dependent manner during coculture with a VacA+/cag PAI+ H. pylori strain. These results are consistent with recent data showing that caspase-8, caspase-3, and caspase-9 were activated in gastric epithelial cells during infection with H. pylori (51). It is well established that Fas cross-linking with Fas ligand or with an agonist anti-Fas antibody induces the recruitment of the death-inducing signaling complex, leading to the activation of procaspase-8 (18, 56). Activated caspase-8 was released into the cytosol and in turn activated a complex cascade including caspase-3, a postmitochondrial executioner caspase (7). Activation of the caspase cascade in a sequential order leads to downstream biochemical changes such as mitochondrial permeability transition, exposure of phosphatidylserine to the cell surface, and cleavage of proteins such as PARP, involved in the DNA repair system (20, 29, 32, 59, 60). We verified that most of these molecular events did occur during infection of T84 cells with the VacA+/cag PAI+ CCUG 17874 and 60190 H. pylori strains, whereas strains defective in the vacA/cag PAI or cagE genes failed to induce efficient caspase activation. Based on the observation that T84 cells constitutively express Fas ligand on both the basolateral and apical sides (28), one can hypothesize that juxtacrine or paracrine Fas-Fas ligand interaction may occur during infection with H. pylori. This hypothesis was also advanced by Rudi et al., who showed that H. pylori-induced apoptosis of gastric epithelial cells is mediated by activation of the CD95 receptor and ligand system (48). In the present study, we verified that induction of apoptosis was not associated with a significant increase in Fas receptor expression, in contrast to previous data showing that apoptosis of gastric epithelial cells induced by H. pylori was associated with increased Fas receptor expression (24, 48). Since T84 epithelial cells express both Fas and Fas ligand while gastric epithelial cells express only Fas and since they react roughly as gastric epithelial cells in terms of caspase pathway activation and IL-8 secretion, the T84 epithelial cell line may be an interesting model for use to study the apoptotic mechanisms triggered by H. pylori. Considering the crucial role played by MAP kinases in the control of IEC homeostasis (1, 15), we investigated whether ERK1/2 and SAP kinase (p38 and JNK) were activated in response to H. pylori infection. Our results show that ERK1/2 was rapidly activated on contact with H. pylori, regardless of the VacA/cag PAI status of bacteria. It therefore appears, in contrast to the conditions for the onset of the apoptotic process, that expression of VacA and cag PAI gene products was not required for ERK1/2 kinase activation in IEC, ruling out the involvement of these genes in the control of H. pylori-induced apoptosis. These results are in agreement with previous work showing that activation of the ERK1/2 pathway following several stress stimuli, such as Fas ligation, did not have an antiapoptotic function in different epithelial cell lines (15, 56). Similarly, both SAP kinases (JNK and p38) were activated in response to H. pylori as early as 30 min after infection. However, we found that the VacA−/cag PAI− strain activated p38 in a more delayed fashion. We thus hypothesized that SAP kinase activation might play a key role in the control of apoptosis induced by the VacA+/cag PAI+ H. pylori strain. To address this question, we used SB202190 a broad inhibitor of SAP kinase, since it blocks JNK and p38 activities. Indeed, pretreatment with this inhibitor markedly decreased caspase-3 activation induced by VacA+/cag PAI+ strains, thus highlighting the importance of the two SAP kinases in the control of the onset of apoptosis. Consistent with our results, early and transient MAP kinase activation in gastric epithelial cells during infection with H. pylori has been previously reported by Keates et al. (25). Moreover, these studies also show that the cag PAI− strain induced far less phosphorylation of p38 than did the cag PAI+ strains (34). Nevertheless, more studies are needed to further delineate the relationship between the SAP kinase activation pathway and the Fas-dependent apoptotic process occurring during infection with VacA+/cag PAI+ H. pylori strains. It would be of particular interest to elucidate the signaling events occurring upstream of the MAP kinase activation and to identify the bacterial and host factors specifically involved in IEC cell death mediated by H. pylori. Taken together, our in vitro model provides evidence that unlike type II H. pylori strains, type 1 strains are able to trigger apoptosis of IEC via a Fas-dependent pathway.
ACKNOWLEDGMENTS
We thank M. Mari and D. Sadoulet for their excellent technical assistance in the electron microscopy study.
These studies were supported by the Institut National de la Santé et de la Recherche Médicale (INSERM).
REFERENCES
- 1.Abreu-Martin M T, Palladino A A, Faris M, Carramanzana N M, Nel A E, Targan S R. Fas activates the JNK pathway in human colonic epithelial cells: lack of a direct role in apoptosis. Am J Physiol. 1999;276:G599–G605. doi: 10.1152/ajpgi.1999.276.3.G599. [DOI] [PubMed] [Google Scholar]
- 2.Bauditz J, Ortner M, Bierbaum M, Niedobittek G, Lochs H, Schreiber S. Production of IL-12 in gastritis relates to infection with Helicobacter pylori. Clin Exp Immunol. 1999;117:316–323. doi: 10.1046/j.1365-2249.1999.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser M J, Perez-Perez G I, Kleanthous H, Cover T L, Peek R M, Chou P H, Stemmermann G N, Namura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 4.Castedo M, Hirsch T, Susin S A, Zamzami N, Marchetti P, Macho A, Kroemer G. Sequential acquisition of mitochondrial and plasma membrane alterations during early lymphocyte apoptosis. J Immunol. 1996;157:512–521. [PubMed] [Google Scholar]
- 5.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. Cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen G, Sordillo E M, Ramey W G, Reidy J, Helt P R, Krajewski S, Reed J C, Blaser M J, Moss S F. Apoptosis in gastric epithelial cells is induced by Helicobacter pylori and accompanied by increased expression of BAK. Biochem Biophys Res Commun. 1997;239:626–632. doi: 10.1006/bbrc.1997.7485. [DOI] [PubMed] [Google Scholar]
- 7.Cohen G M. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corthesy-Theulaz I, Porta N, Pringault E, Racine L, Bogdanova A, Kraehenbuhl J P, Blum A L, Michetti P. Adhesion of Helicobacter pylori to polarized T84 human intestinal cell monolayers is pH dependent. Infect Immun. 1996;64:3827–3832. doi: 10.1128/iai.64.9.3827-3832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Covacci A, Telford J L, del Guidice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 10.Crabtree J E, Peichl P, Wyatt J I, Stachl U, Lindley I J. Gastric interleukin-8 and IgA IL-8 autoantibodies in Helicobacter pylori infection. Scand J Immunol. 1993;37:65–70. doi: 10.1111/j.1365-3083.1993.tb01666.x. [DOI] [PubMed] [Google Scholar]
- 11.Crabtree J E, Covacci A, Farmery S M, Xiang Z, Tompkins D S, Perry S, Lindley I J D, Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with cagA positive phenotype. J Clin Pathol. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies G R, Simmonds N J, Stevens T R, Sheaff M T, Banatvala N, Laurenson I F, Blake D R, Rampton D S. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35:179–185. doi: 10.1136/gut.35.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan X G, Chua A, Fan X J, Keepling P W. Increased gastric production of interleukin-8 and tumor necrosis factor in patients with Helicobacter pylori infection. J Clin Pathol. 1995;48:133–136. doi: 10.1136/jcp.48.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galmiche A, Rassow J, Doye A, Cagnol S, Chambard J C, Contamin S, Thillot V, Just I, Ricci V, Solcia E, Obberghen E V, Boquet P. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 2000;19:6361–6370. doi: 10.1093/emboj/19.23.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson S, Tu S, Oyer R, Anderson S M, Johnson G L. Epidermal growth factor protects epithelial cells against Fas-induced apoptosis. Requirement for Akt activation. J Biol Chem. 1999;274:17612–17618. doi: 10.1074/jbc.274.25.17612. [DOI] [PubMed] [Google Scholar]
- 16.Guarino A, Bisceglia M, Canani R B, Boccia M C, Mallardo G, Bruzzese E, Massari P, Rappuoli R, Telford J. Enterotoxic effect of the vacuolating toxin produced by Helicobacter pylori in Caco-2 cells. J Infect Dis. 1998;178:1373–1378. doi: 10.1086/314427. [DOI] [PubMed] [Google Scholar]
- 17.Guy-Grand D, Disanto J P, Henchoz P, Malassis-Séris M, Vassalli P. Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-γ, TNF) in the induction of epithelial cell death and renewal. Eur J Immunol. 1998;28:730–744. doi: 10.1002/(SICI)1521-4141(199802)28:02<730::AID-IMMU730>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 18.Hirata H, Takahashi A, Kobayashi S, Yonehara S, Sawai H, Okazaki T, Yamamoto K, Sasada M. Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. J Exp Med. 1998;187:587–600. doi: 10.1084/jem.187.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofman V, Ricci V, Galmiche A, Brest P, Auberger P, Rossi B, Boquet P, Hofman P. Effect of Helicobacter pylori on polymorphonuclear leukocyte migration across polarized T84 epithelial cell monolayers: role of vacuolating toxin vacA and cag pathogenicity island. Infect Immun. 2000;68:5225–5233. doi: 10.1128/iai.68.9.5225-5233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homburg C H E, de Haas M, von dem Borne A E G K, Verhoeven A J, Reutelingsperger C P M, Roos D. Human neutrophils lose their surface FcγRIII and acquire annexin V binding sites during apoptosis in vitro. Blood. 1995;85:532–540. [PubMed] [Google Scholar]
- 21.Houghton J, Korah R M, Condon M R, Kim K H. Apoptosis in Helicobacter pylori-associated gastric and duodenal ulcer disease is mediated via the Fas antigen pathway. Dig Dis Sci. 1999;44:465–478. doi: 10.1023/a:1026628601284. [DOI] [PubMed] [Google Scholar]
- 22.Houghton J, Macrera-Bloch L S, Harrison L, Kim K H, Korah R M. Tumor necrosis factor alpha and interleukin 1 beta up-regulate gastric mucosal Fas antigen expression in Helicobacter pylori infection. Infect Immun. 2000;68:1189–1195. doi: 10.1128/iai.68.3.1189-1195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones N L, Shannon P T, Cutz E, Yeger H, Sherman P M. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol. 1997;151:1695–1703. [PMC free article] [PubMed] [Google Scholar]
- 24.Jones N L, Day A S, Jennings H A, Sherman P M. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect Immun. 1999;67:4237–4242. doi: 10.1128/iai.67.8.4237-4242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keates S, Keates A C, Warny M, Peek R M, Jr, Murray P G, Kelly C P. Differential activation of mitogen-activated protein kinases in AGS gastric epithelial cells by cag+ and cag−Helicobacter pylori. J Immunol. 1999;163:5552–5559. [PubMed] [Google Scholar]
- 26.Kim J M, Eckmann L, Savidge T C, Lowe D C, Witthöft T, Kagnoff M F. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Investig. 1998;102:1815–1823. doi: 10.1172/JCI2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohda K, Tanaka K, Aiba Y, Yasuda M, Miwa T, Koga Y. Role of apoptosis induced by Helicobacter pylori infection in the development of duodenal ulcer. Gut. 1999;44:456–462. doi: 10.1136/gut.44.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le'Negrate G, Selva E, Auberger P, Rossi B, Hofman P. Sustained polymorphonuclear leukocytes (PMNL) transmigration induces apoptosis in T84 intestinal epithelial cells. J Cell Biol. 2000;150:1479–1488. doi: 10.1083/jcb.150.6.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome c and dATP dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 30.Liles W C, Kiener P A, Ledbetter J A, Aruffo A, Klebanoff S J. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996;184:429–440. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madara J L, Colgan S, Nusrat A, Delp C, Parkos C. A simple approach to measurement of electrical parameters of cultured epithelial monolayers: use in assessing PMNL-epithelial monolayers. J Tissue Cult Methods. 1992;14:209–216. [Google Scholar]
- 32.Martin S J, Finucane D M, Amarante-Mendes G P, O'Brien G A, Green D R. Phosphatidylserine externalization during CD95-induced apoptosis of cells and cytoplasts requires ICE/CED-3 protease activity. J Biol Chem. 1996;271:28753–28756. doi: 10.1074/jbc.271.46.28753. [DOI] [PubMed] [Google Scholar]
- 33.McCormick B A, Hofman P M, Kim J, Carnes D, Miller S, Madara J L. Surface binding of Salmonella typhimurium to intestinal epithelia: imprinting of pathways directing PMNL movement on the underlying matrix. J Cell Biol. 1995;131:5785–5791. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer-ter-Vehn T, Covacci A, Kist M, Pahl H L. Helicobacter pylori activates MAP kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J Biol Chem. 2000;275:16064–16072. doi: 10.1074/jbc.M000959200. [DOI] [PubMed] [Google Scholar]
- 35.Mizuki I, Shimoyama T, Fukuda S, Liu Q, Nakaji S, Munakata A. Association of gastric epithelial apoptosis with the ability of Helicobacter pylori to induce a neutrophil oxidative burst. J Med Microbiol. 2000;49:521–524. doi: 10.1099/0022-1317-49-6-521. [DOI] [PubMed] [Google Scholar]
- 36.Moss S F, Legon S, Davies J, Calam J. Cytokine gene expression in Helicobacter pylori associated antral gastritis. Gut. 1994;35:1567–1570. doi: 10.1136/gut.35.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moss S F, Calam J, Agarwal B, Wang S, Holt P R. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moss S F, Sordillo E M, Abdalla A M, Makarov V, Hanzely Z, Perez-Perez G I, Blaser M J, Holt P R. Increased gastric epithelial cell apoptosis associated with colonization with cagA+ Helicobacter pylori strains. Cancer Res. 2001;61:1406–1411. [PubMed] [Google Scholar]
- 39.Munzenmaier A, Lange C, Glocker E, Covacci A, Moran A, Bereswill S, Baeuerle P A, Kist M, Pahl H L. A secreted/shed product of Helicobacter pylori activates transcription factor nuclear factor-kappa B. J Immunol. 1997;159:6140–6147. [PubMed] [Google Scholar]
- 40.NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 41.Noach L A, Rolf T M, Tytgat G N J. Electron microscopic study of association between Helicobacter pylori and gastric and duodenal mucosa. J Clin Pathol. 1994;47:699–704. doi: 10.1136/jcp.47.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oppenheim J J, Zachariae C O C, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene intercrine cytokine family. Annu Rev Immunol. 1991;9:617. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 43.Peek R M, Moss S F, Tham K T, Perez-Perez G I, Wang S, Miller G G, Atherton J C, Holt P R, Blaser M J. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst. 1997;89:863–868. doi: 10.1093/jnci/89.12.863. [DOI] [PubMed] [Google Scholar]
- 44.Peek R M, Blaser M F, Mays D J, Forsyth M H, Cover T L, Song S Y, Krishna U, Pietenpol J A. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 1999;59:6124–6131. [PubMed] [Google Scholar]
- 45.Ricci V, Zarrilli R, Sommi P, Romano M. Mechanisms of Helicobacter pylori-induced damage to gastric mucosa. J Dig Prot. 1999;1:9–20. [Google Scholar]
- 46.Rieder G, Hatz R A, Moran A P, Walz A, Stolte A, Enders G. Role of adherence in interleukin-8 induction in Helicobacter pylori-associated gastritis. Infect Immun. 1997;65:3622–3630. doi: 10.1128/iai.65.9.3622-3630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rokkas T, Ladas S, Liatsos C, Petridou E, Papatheodorou G, Theocharis S, Karameris A, Raptis S. Relationship of Helicobacter pylori cagA status to gastric cell proliferation and apoptosis. Dig Dis Sci. 1999;44:487–493. doi: 10.1023/a:1026636803101. [DOI] [PubMed] [Google Scholar]
- 48.Rudi J, Kuck D, Strand S, von Herbay A, Mariani S M, Krammer P H, Galle P R, Stremmel W. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J Clin Investig. 1998;102:1506–1514. doi: 10.1172/JCI2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Segal E D, Lange C, Covacci A, Tomkins L S, Falkow S. Induction of host signal transduction pathway by Helicobacter pylori. Proc Natl Acad Sci USA. 1997;94:7595–7599. doi: 10.1073/pnas.94.14.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma S A, Tummuru M K, Blaser M J, Kerr L D. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kB in gastric epithelial cells. J Immunol. 1998;160:2401–2407. [PubMed] [Google Scholar]
- 51.Shibayama K, Doi Y, Shibata N, Nada T, Iinuma Y, Arakawa Y. Apoptotic signaling pathway activated by Helicobacter pylori infection and increase of apoptosis-inducing activity under serum-starved conditions. Infect Immun. 2001;69:3181–3189. doi: 10.1128/IAI.69.5.3181-3189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shirin H, Moss S F. Helicobacter pylori induced apoptosis. Gut. 1998;43:592–594. doi: 10.1136/gut.43.5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smoot D T. How does Helicobacter pylori cause mucosal damage? Direct mechanisms. Gastroenterology. 1997;113:531–534. doi: 10.1016/s0016-5085(97)80008-x. [DOI] [PubMed] [Google Scholar]
- 54.Tummuru M K, Sharma S A, Blaser M J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18:867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- 55.Wagner S, Bell W, Westermann J, Logan R P H, Bock C T, Trautwein C, Bleck J S, Manns M P. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology. 1997;113:1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- 56.Wallach D, Varfolomeev E E, Malinin N L, Goltsev Y V, Kovalenko A V, Boldin M P. Tumor necrosis receptor and Fas signaling mechanisms, Annu. Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 57.Xiang Z, Censini S, Bayeli P F, Telford J L, Figura N, Rappuoli R, Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating toxin. Infect Immun. 1995;63:94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut. 1998;42:609–617. doi: 10.1136/gut.42.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere J L, Petit P X, Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zamzami N, Susin S A, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zarrilli R, Ricci V, Romano M. Molecular response of gastric epithelial cells to Helicobacter pylori infection. Cell Microbiol. 1999;1:93–99. doi: 10.1046/j.1462-5822.1999.00018.x. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Q B, Nakshabendi I M, Dawodu J B, Gemmell C G, Russel R I. Association of cytotoxin production and PMNL activation by strains of Helicobacter pylori isolated from patients with peptic ulceration and chronic gastritis. Gut. 1996;38:841–845. doi: 10.1136/gut.38.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]