Abstract
The roles of endogenous cytokines induced by either intact staphylococcal microorganisms or staphylococcal exotoxins were examined using human whole-blood cultures. To accomplish this, interleukin-18 binding protein (IL-18BP) and tumor necrosis factor binding protein (TNFbp) were used to neutralize IL-18 and TNF, respectively, whereas an anti-IL-12 monoclonal antibody was used to neutralize IL-12 and the IL-1 receptor antagonist (IL-1Ra) was used to block IL-1 receptors. Heat-killed Staphylococcus epidermidis and Staphylococcus aureus, as well as the staphylococcal superantigens toxic shock syndrome toxin-1 (TSST-1) and staphylococcus enterotoxin B (SEB) induced gamma interferon (IFN-γ) production. Staphylococcus spp.-induced production of IFN-γ required the presence of endogenous IL-18, IL-12, and TNF. In contrast, TSST-1-induced IFN-γ was not significantly reduced in the presence of IL-18BP, anti-IL-12 antibodies, IL-1Ra, or anti-TNFbp. SEB-induced IFN-γ was significantly inhibited only by anti-IL-12 antibodies, indicating that endogenous IL-18, IL-1, and TNF are not required for SEB-induced IFN-γ. In conclusion, the mechanisms of IFN-γ stimulation by intact staphylococcal microorganisms and by exotoxins differ, and this is likely due to the different receptors which are triggered on the cell membranes. In contrast to its role in the interactions between staphylococci and host cells, IL-18 does not appear to play a major role in superantigen-induced IFN-γ.
Sepsis due to gram-positive bacteria is a life-threatening disease in which the presence of microorganisms in the bloodstream induces an overwhelming production of proinflammatory cytokines, such as tumor necrosis factor (TNF), interleukin-1β (IL-1β), and gamma interferon (IFN-γ), which are thought to play a central role in the pathogenesis of this syndrome (23). In contrast, in staphylococcal toxic shock syndrome (TSS), the bacteria rarely invade the host, and the symptoms are caused by superantigen-induced systemic inflammation. Staphylococcal components such as lipoteichoic acids (LTA) and peptidoglycans (PG) (3, 18), as well as the superantigens TSS toxin-1 (TSST-1) and staphylococcal enterotoxin B (SEB) (16), are believed to be the main inducers of cytokine production during these two severe illnesses.
LTA and PG are major components of Staphylococcus aureus, which induces a strong cytokine response (3). These constituents of the cell wall of most gram-positive bacteria stimulate monocytes through CD14 and Toll-like receptor 2 (TLR-2) (PG) or TLR-4 (LTA) (5, 28, 31). In contrast, the superantigens TSST-1 and SEB lead to the activation of both lymphocytes and monocytes through the major histocompatibility complex class II molecules on antigen-presenting cells and the specific Vβ elements of the T-cell receptor (4, 16). Through these two distinct pathways, macrophages are triggered to produce and release proinflammatory cytokines, including IL-1, IL-18, TNF-α, and IL-12. IFN-γ production likely originates from lymphocytes and natural killer cells upon secondary stimulation with IL-18 and IL-12 (9).
IL-18, originally described as IFN-γ inducing factor (20), has the ability to induce the synthesis of IL-1β, TNF-α, IL-8, and chemokines (25), probably through activation of nuclear factor-κB (17). This places IL-18 in the group of proinflammatory cytokines. IL-18 alone does not induce significant IFN-γ production from T lymphocytes (22), but in the presence of secondary stimulants, particularly IL-12, IL-2, mitogens, or microbial agents, large amounts of IL-18-induced IFN-γ production are observed (9, 22). Although IL-12 alone is able to induce IFN-γ synthesis, the addition of IL-18 increases this production severalfold. IL-1, TNF, and IL-18 potentiate the synthesis of each other, and in turn, they stimulate production of the second-wave cytokines such as IL-8 and IL-6 (2, 8, 9). Recently, IL-18 binding protein (IL-18BP), which binds and prevents IL-18 activity, was described (13, 21).
The aim of the present study was to characterize the cytokine network induced by whole staphylococcal microorganisms on the one hand and by the staphylococcal components LTA, PG, TSST-1, and SEB on the other hand. We have assessed the role of endogenous IL-18, IL-1, TNF, and IL-12 in the induction of IFN-γ, TNF-α, and IL-8 by Staphylococcus epidermidis and S. aureus, TSST-1, SEB, PG, and LTA in human whole-blood cultures by using specific cytokine inhibitors, such as IL-18BP, IL-1 receptor antagonist (IL-1Ra), TNF-binding protein (TNFbp), and anti-IL-12 antibodies (anti-IL-12 Ab).
MATERIALS AND METHODS
Volunteer selection.
The study was approved by the Combined Colorado Investigational Review Board, and each blood donor gave informed consent. For this study we used whole blood from seven healthy, nonsmoking volunteers between 23 and 45 years of age. The volunteers did not report recent infectious or inflammatory disease and had abstained from using cyclooxygenase inhibitors 2 weeks before the study. Blood was collected by venipuncture into heparinized tubes (sodium heparin; final concentration, 20 U/ml; Elkins-Sinn, Cherry Hill, N.J.). We performed separate replicate experiments (sets of three experiments for each stimulus) and pooled the data to perform the statistics.
Reagents.
RPMI culture medium (Cellgro Mediatech, Herndon, Va.) was supplemented with 10 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin (Gibco BRL, Grand Island, N.Y.) per ml. S. epidermidis and S. aureus were grown in brain heart infusion broth for 24 h, washed in pyrogen-free saline, and boiled as described previously (1). The concentration of the heat-killed organisms was adjusted to 10 per white blood cell. Purified TSST-1, SEB, PG, and LTA were obtained from Sigma Chemical Co. (St. Louis, Mo.). Lipopolysaccharide (LPS) contamination of the stimuli used in this study was excluded in control experiments using polymyxin B preincubation (2 μg/ml, 2 h) for the various stimuli. Polymyxin B did not alter the cytokine response induced by the various stimuli, demonstrating the absence of LPS contamination.
IL-18BP was expressed in COS cells as the “a” isoform and purified as a His-tagged protein purified over talon as previously described (13, 21). Recombinant human IL-1Ra was a kind gift of Daniel Tracey (Upjohn, Kalamazoo, Mich.). TNFbp (p55 TNF soluble receptor [22]) was a kind gift of Carl Edwards (Amgen, Boulder, Colo.). Murine anti-human IL-12 Ab (clone 11.5.14) was kindly supplied by Genetics Institute (Cambridge, Mass.).
Whole-blood cultures.
One-half of a milliliter of either RPMI culture medium or RPMI containing S. epidermidis, TSST-1, SEB, or LTA in the presence of the various specific inhibitors (IL-18BP, IL-1Ra, TNFbp, and anti-IL-12 Ab) was added to 12- by 75-mm round-bottom polypropylene tubes (Falcon; Becton Dickinson Labware, Franklin Lakes, N.J.), and 0.5 ml of blood was added. The loosely capped tubes were incubated at 37°C (5% CO2) in an upright position. After 24 h of incubation, the blood was mixed by rapid vortexing and 0.5 ml was transferred into 1.5-ml Eppendorf tubes (Brinkmann Instruments, Westbury, N.Y.). Triton X-100 (Bio-Rad Laboratories, Richmond, Calif.) was then added (1% final concentration), and the blood was mixed until lysed. The remaining 0.5 ml of blood was incubated for an additional 24 h, mixed by rapid vortexing, and lysed with Triton X-100. The lysed blood was stored at −70°C until used for the assay. In these studies, intracellular and secreted cytokine concentrations were determined together and the results represent total cytokine production (26).
Cytokine assays.
Measurements of IFN-γ, IL-8, and TNF-α were performed by electrochemiluminescence as previously described (25, 26). The detection limit of the IFN-γ assay was 62 pg/ml and of the IL-8 and TNF-α assays was 40 pg/ml.
Statistical analysis.
Data were expressed as means ± standard errors of the means (SEM). Differences between groups were analyzed with the Wilcoxon test (double sided). The significance of the dose-response inhibitory effects presented in Fig. 1A was analyzed by the Kruskal-Wallis test.
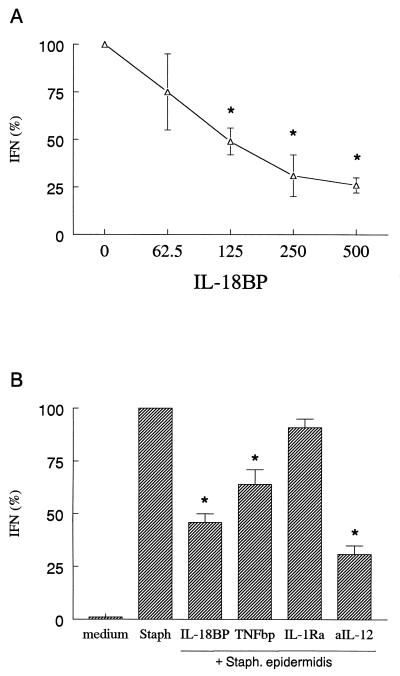
FIG. 1.
S. epidermidis-induced IFN-γ production in whole blood. Whole blood was stimulated with S. epidermidis (10:1 ratio of organisms to leukocytes) in the absence or in the presence of various concentrations of IL-18BP (A) or with fixed amounts of IL-18BP (125 ng/ml), IL-1Ra (10 μg/ml), TNFbp (10 μg/ml), or anti-IL-12 Ab (2.5 μg/ml) (aIL-12) (B). After 48 h the culture was assayed for IFN-γ. The amount of IFN-γ induced by S. epidermidis was set at 100% for each donor and the percent change (mean ± SEM) for each condition is shown. A 100% S. epidermidis stimulation represents 11.1 ± 2.5 ng of IFN-γ/ml. ∗, P < 0.05 versus S. epidermidis alone (n = 7 donors).
RESULTS
Staphylococcus spp.-induced IFN-γ production in whole blood.
Human whole blood stimulated with heat-killed S. epidermidis induced significant amounts of IFN-γ, and this production was inhibited by IL-18BP in a dose-dependent manner (Fig. 1A). In addition to IL-18BP, anti-IL-12 Ab and TNFbp were also able to inhibit S. epidermidis-induced IFN-γ, whereas IL-1Ra did not have a significant effect (Fig. 1B). No effect of IL-18BP, IL-1Ra, TNFbp, or anti-IL-12 Ab on S. epidermidis-induced synthesis of IL-8 was apparent (data not shown). Similarly, IFN-γ production induced by S. aureus was significantly inhibited by IL-18BP (67%; P < 0.05) and TNFbp (44%; P < 0.05) but not by IL-1Ra (9%; P > 0.05).
Induction of IFN-γ, TNF-α, and IL-8 by TSST-1, SEB, PG, and LTA in whole-blood cultures.
The stimulation of whole blood with TSST-1 resulted in a marked stimulation of IFN-γ (47 ± 24 ng/ml), TNF-α (240 ± 53 pg/ml), and IL-8 (25 ± 6 ng/ml), corresponding to a 29-, 10-, and 26-fold increase over unstimulated cultures, respectively. SEB stimulation led to the production of IFN-γ (143 ± 55 ng/ml), TNF-α (459 ± 89 pg/ml), and IL-8 (66 ± 12 ng/ml), with a 48-, 37-, and 43-fold increase over unstimulated cultures, respectively. LTA also induced TNF-α (139 ± 73 pg/ml) and IL-8 (52 ± 27 ng/ml), corresponding to a 12- and 38-fold increase over unstimulated cultures, respectively. However, IFN-γ concentrations after LTA stimulation were below the detection limit (n = seven donors). PG induced significant amounts of IFN-γ (49 ± 19 pg/ml) and IL-8 (417 ± 108 pg/ml), representing a 9- and 23-fold increase over unstimulated cells.
Role of endogenous cytokines in TSST-1-induced cytokine production.
As shown in Fig. 2A, in the presence of IL-18BP or IL-1Ra, there was a nonsignificant reduction in IFN-γ levels. However, the combination of both IL-18BP and IL-1Ra reduced the IFN-γ level by 47% (P < 0.05). Similarly, anti-IL-12 Ab reduced the IFN-γ level by 39% and the combination of anti-IL-12 Ab plus IL-18BP inhibited IFN-γ by 49% (P < 0.05). The addition of TNFbp or the combination of TNFbp and IL-18BP to the whole-blood culture did not affect IFN-γ production (data not shown).
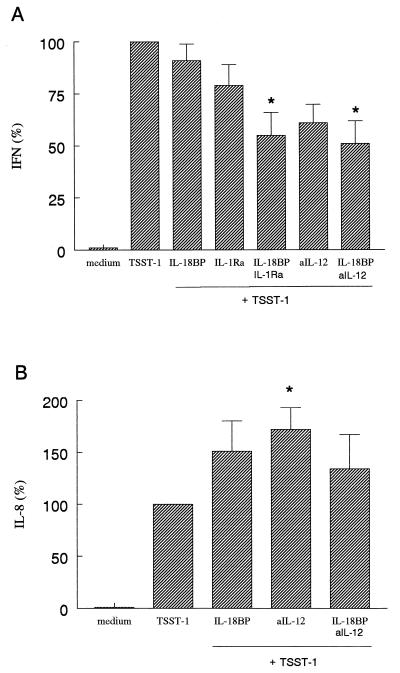
FIG. 2.
TSST-1-induced IFN-γ and IL-8 production in whole blood. Whole blood was stimulated with TSST-1 (1.5 μg/ml) in the absence or in the presence of IL-18BP (125 ng/ml), IL-1Ra (10 μg/ml), or anti-IL-12 Ab (2.5 μg/ml) (aIL-12). After 24 h the culture was assayed for IL-8 (B), and after 48 h it was assayed for IFN-γ (A). The amount of either IL-8 or IFN-γ induced by TSST-1 was set at 100% for each donor and the percent change (mean ± SEM) for each condition is shown. A 100% TSST-1 stimulation represents 47 ± 24 ng/ml (IFN-γ) and 25 ± 6 ng/ml (IL-8). ∗, P < 0.05 versus TSST-1 alone (n = 7 donors).
IL-8 production was not reduced by any of the cytokine inhibitors. In contrast, neutralizing IL-12 resulted in a significant increase in IL-8 production (85% increase of TSST-1 production; P < 0.05) (Fig. 2B). Although this increase was smaller in the presence of both anti-IL-12 Ab and IL-18BP, IL-8 levels were still greater than with TSST-1 alone (31% increase of TSST-1 production; P < 0.05) (Fig. 2B). The addition of IL-1Ra or TNFbp or the combination of either IL-1Ra or TNFbp plus IL-18BP to the whole blood culture did not decrease IL-8 production (data not shown). IL-1Ra suppressed TSST-1-induced TNF-α production by 25% (P > 0.05), and the combination of IL-1Ra and IL-18BP did not have an additional effect on TNF-α production. Neutralizing IL-18 and IL-12 did not influence TSST-1-induced TNF-α production (data not shown).
Role of endogenous cytokines in SEB-induced cytokine production.
As shown in Fig. 3A, only anti-IL-12 Ab significantly inhibited IFN-γ production (40% of SEB-induced IFN-γ production [P < 0.05]). IL-18BP did not decrease IFN-γ and the combination of anti-IL-12 Ab and IL-18BP was less effective than anti-IL-12 Ab. Blocking IL-1 receptors also had no effect on SEB induction of IFN-γ (data not shown). Neutralization of TNF-α by TNFbp either alone or in combination with IL-18BP also had no effect (data not shown).
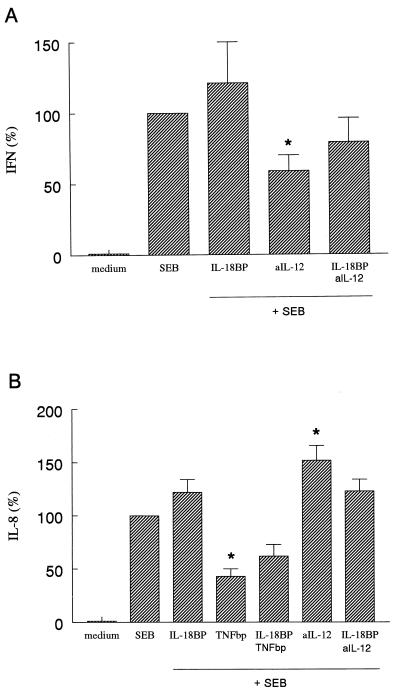
FIG. 3.
SEB-induced IFN-γ and IL-8 production in whole blood. Whole blood was stimulated with SEB (2 μg/ml) in the absence or in the presence of IL-18BP (125 ng/ml), TNFbp (10 μg/ml), or anti-IL-12 Ab (2.5 μg/ml) (aIL-12). After 24 h the culture was assayed for IL-8 (B), and after 48 h it was assayed for IFN-γ (A). The amount of either IL-8 or IFN-γ induced by SEB was set at 100% for each donor and the percent change (mean ± SEM) for each condition is shown. A 100% SEB stimulation represents 143 ± 55 ng/ml (IFN-γ) and 66 ± 12 ng/ml (IL-8). ∗, P < 0.05 versus SEB alone (n = 6 donors).
IL-8 production was strongly decreased by TNFbp (53%; P < 0.05). The combination of IL-18BP and TNFbp did not reduce IL-8 any further. In fact, the neutralization of IL-18 and TNF led to less inhibition of SEB-induced IL-8 (28%; not significant). In contrast, blocking IL-12 had a significant stimulating effect on SEB-induced IL-8 production (52%; P < 0.05) (Fig. 3B). IL-18BP had no effect on SEB-induced IL-8. The addition of IL-1Ra or the combination of IL-1Ra and IL-18BP did not affect IL-8 production (data not shown). TNF-α concentrations in whole blood were not influenced by neutralizing IL-18, IL-1, or IL-12 (data not shown), indicating that TNF-α synthesis by SEB is independent of these cytokines.
Role of endogenous cytokines in LTA- and PG-induced cytokine production.
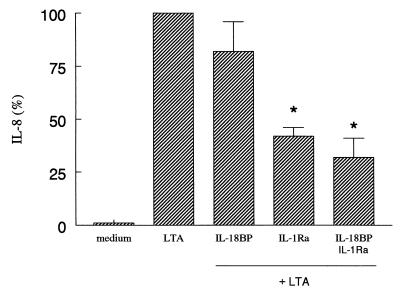
In contrast to the production of TNF-α and IL-8, LTA did not induce significant production of IFN-γ in whole blood. LTA-induced IL-8 was significantly inhibited by IL-1Ra (55%; P < 0.01). The combination of IL-18BP plus IL-1Ra resulted in a 69% decrease of IL-8 (P < 0.05) (Fig. 4). The addition of TNFbp or anti-IL-12 Ab or the combination of either TNFbp or anti-IL-12 Ab and IL-18BP to the whole-blood culture did not decrease IL-8 production (data not shown). TNF-α production was not modulated by neutralizing endogenous IL-18 and IL-1, although a trend towards increased production was measured when anti-IL-12 Ab alone or IL-18BP and anti-IL-12 Ab were added together (59% versus LTA production; P > 0.05) (data not shown).
FIG. 4.
LTA-induced IL-8 production in whole blood. Whole blood was stimulated with LTA (2 μg/ml) in the absence or in the presence of IL-18BP (125 ng/ml) or IL-1Ra (10 μg/ml). After 24 h the culture was assayed for IL-8. The amount of IL-8 induced by LTA was set at 100% for each donor and the percent change (mean ± SEM) for each condition is shown. A 100% LTA stimulation represents 52 ± 27 ng of IL-8/ml. ∗, P < 0.05 versus LTA alone (n = 6 donors).
PG-induced production of IFN-γ was significantly inhibited by IL-18BP (58% ± 19%; P < 0.05), IL-1Ra (61% ± 5%; P < 0.05), and TNFbp (77% ± 13%; P < 0.05). Combination of IL-18BP with both IL-1Ra and TNFbp further reduced the IFN-γ production to less than 5% (P < 0.01). In contrast, IL-18BP and TNFbp had no effects on PG-induced IL-8 synthesis (P > 0.05; n = 5), and only IL-1Ra had a moderate, but significant, inhibitory effect (43% ± 17%; P < 0.05).
DISCUSSION
As previously reported in the literature, both whole staphylococcal microorganisms and the staphylococcal antigens LTA, PG, TSST-1, and SEB are potent stimuli of TNF, IFN-γ, and IL-8 production (3, 16, 32). Only cell-associated IL-18 could be measured after stimulation of cells with the various stimuli (not shown), suggesting that either cell-associated IL-18 is responsible for the biological effects or that small, undetectable amounts of IL-18 act in a paracrine or autocrine manner to exert its effects. In our experiments, whereas S. epidermidis- and S. aureus-induced IFN-γ production was strongly reduced by IL-18BP, anti-IL-12 Ab, and TNFbp, only anti-IL-12 Ab decreased SEB-induced and TSST-1-induced IFN-γ. Even the effect of anti-IL-12 Ab was much less pronounced for the superantigen-stimulated IFN-γ than for S. epidermidis stimulation, and IL-18BP, TNFbp, and IL-1Ra had no effects. It is therefore apparent that the cytokine pathways leading to IFN-γ induction by intact microorganisms and superantigens are different. In contrast to Staphylococcus-induced IFN-γ synthesis, the IFN-γ production induced by SEB and TSST-1 was not reduced by IL-18BP, indicating that endogenous IL-18 does not play a central role in superantigen-induced IFN-γ. We summarized these results in Table 1. Our data are in line with those of Lauw and colleagues showing no effect of anti-IL-18 Ab in an SEB model of TSS in mice (Lauw et al., unpublished data).
TABLE 1.
Differential roles of IL-18, IL-12, IL-1, and TNF for the induction of IFN-γ by staphylococcal cell wall components and superantigens
| Stimulus | IFN-γ production in presence of cytokine inhibitora
|
|||
|---|---|---|---|---|
| IL-18BP | Anti-IL-12 Ab | IL-1Ra | TNFbp | |
| Staphylococcus organisms | ++ | ++ | − | + |
| PG | ++ | ND | ++ | ++ |
| SEB | − | + | − | − |
| TSST-1 | − | + | ± | − |
Inhibitory effect: ++, strong; +, moderate; ±, weak; −, none.
The importance of Staphylococcus-induced cytokine production is emphasized by the severity of the two clinical syndromes caused by staphylococcal infections in which the systemic inflammation caused by overwhelming cytokinemia is thought to play a pivotal role: Staphylococcus sepsis and TSS (7). Whereas in the former the cytokine release is triggered by invasion of the circulation by intact microorganisms, in the latter the staphylococci rarely invade the host and the cytokine production is triggered by exotoxins acting as superantigens. In the present study, we investigated whether the cytokine network induced by either intact S. aureus and S. epidermidis microorganisms or the staphylococcal superantigens is divergent.
Several cytokines are known to be involved in IFN-γ production, either directly or following costimulation with other cytokines or microbial agents. An important role for IFN-γ induction was attributed to endogenous IL-18. Reduction of LPS-induced IFN-γ in IL-1β-converting enzyme-deficient macrophages lacking a functional IL-18 can be greater than 90%, despite normal levels of IL-12 (10, 11, 30), a cytokine which appears to be essential for IFN-γ production (15). IL-12, produced by phagocytic cells in response to bacteria, is able to induce IFN-γ production by natural killer and T cells (27). Two mechanisms may account for the stimulatory effects of IL-12 on IFN-γ production. First, IL-12 upregulates production of IL-18 (14), and second, IL-12 increases the responsiveness of T and B cells to IL-18 by up-regulation of IL-18 receptor expression (34). In addition to IL-12 and IL-18, other cytokines important for the induction of IFN-γ are IL-1 and TNF, which potentiate IFN-γ production together with IL-12 (6, 29, 33). In the present study we show that stimulation of human whole blood with heat-killed Staphylococcus spp. induces the release of IFN-γ in an IL-18-, IL-12-, and TNF-dependent manner. This is probably due to effects exerted at the level of PG stimulation of cytokine synthesis, as similar effects of the cytokine inhibitors were seen when cells were incubated with PG. In contrast, TSST-1- and SEB-induced IFN-γ production is not significantly reduced by IL-18BP, TNFbp, and IL-1Ra, whereas it is slightly inhibited by anti-IL-12 Abs.
Differences were also apparent when production of the chemokine IL-8 was assessed: direct IL-8 stimulation by intact microorganisms and at least partial dependency of IL-8 production on endogenous TNF in the case of SEB were observed. In addition, the TNF production was not inhibited by any of the cytokine inhibitors used, confirming the proximal place of TNF in the cytokine cascade.
The differences between the cytokine patterns induced either by intact staphylococci or by staphylococcal exotoxins are probably mediated by the differential receptors they stimulate on the cell membranes to trigger cytokine synthesis. The gram-positive microorganisms are recognized by pattern recognition receptors such as TLR-2 and TLR-6 on the cell membrane (24, 28, 31), and CD14 can facilitate this process (5). Gram-positive components such as LTA have been suggested to be involved, but LTA did not stimulate IFN-γ synthesis, possibly due to the absence of alanine substituents lost in the purification procedures (19). In contrast, staphylococcal PG strongly stimulated IFN-γ synthesis and play a pivotal role in TLR-2- and CD14-mediated cytokine induction by Staphylococcus spp. (24, 31). Disintegration of the microorganisms and release of bacterial DNA, which would stimulate monocytes through TLR-9, are also likely to be important (12).
In contrast to this pathway, the staphylococcal exotoxins TSST-1 and SEB induce proliferation of T cells through direct binding to the major histocompatibility complex class II molecules on antigen-presenting cells and through stimulation of T-cell receptor-specific Vβ elements. This pathway leads to the induction of proinflammatory cytokines (3–5). Our observation that TSST-1 and SEB induce IFN-γ, TNF-α, and IL-8 is consistent with previous studies (8–10). Several investigators have reported that direct contact is needed between monocytes and T cells to induce TNF-α and IL-1β by superantigens (25, 26). In contrast, other reports show the release of IL-1, TNF-α, and IL-6 in response to TSST-1 and SEB by monocytes in the absence of T cells (9, 10, 27, 28). In whole-blood cultures, contact between monocytes and T cells likely takes place. This pathway of IFN-γ stimulation appears to be independent of endogenous IL-18, TNF, and IL-1 and only marginally dependent on IL-12.
In conclusion, Staphylococcus microorganisms and the staphylococcal exotoxins TSST-1 and SEB are potent stimuli of IFN-γ, TNF-α, and IL-8 production. However, the cytokine pathways leading to production of IFN-γ and IL-8 by these stimuli are different, and this is likely due to differential receptors which are recognized and triggered on the cell membranes. These differences may in part explain the clinical differences between staphylococcal sepsis and staphylococcal TSS.
ACKNOWLEDGMENTS
This study was partly supported by NIH grant AI-15614 (to C.A.D.). M.G.N. was supported by a grant from Stichting “De Drie Lichten,” Leiden, The Netherlands.
REFERENCES
- 1.Aiura K, Gelfand J A, Burke J F, Thompson R C, Dinarello C A. Interleukin-1 (IL-1) receptor antagonist prevents Staphylococcus epidermidis-induced hypotension and reduces circulating levels of tumor necrosis factor and IL-1β in rabbits. Infect Immun. 1993;61:3342–3350. doi: 10.1128/iai.61.8.3342-3350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutler B E. Tumor necrosis factors. The molecules and their emerging roles in medicine. New York, N.Y: Raven Press; 1992. [Google Scholar]
- 3.Bhakdi S, Klonisch T, Nuber P, Fisher W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991;59:4616–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi Y, Kotzin B, Hernon L, Callahan J, Marrack P, Kappler J. Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc Natl Acad Sci USA. 1989;86:8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cleveland M G, Gorham J D, Murphy T L, Tuomanen E, Murphy K M. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–1912. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Andrea A, Aste-Amezaga M, Valiante N M, Xiaojing M, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis J P, Chesney P J, Wand P J, LaVenture M. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors and prevention. N Engl J Med. 1980;303:1429–1435. doi: 10.1056/NEJM198012183032501. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello C A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 9.Dinarello C A. IL-18: a Th1-inducing, proinflammatory cytokine and a new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 10.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 11.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell R A, Sato V, Harding M W, Livingston D J, Su M S-S. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 12.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 13.Kim S-H, Eisenstein M, Reznikov L, Fantuzzi G, Novick D, Rubinstein M, Dinarello C A. Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc Natl Acad Sci USA. 2000;97:1190–1195. doi: 10.1073/pnas.97.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauw F N, Dekkers P E, te Velde A A, Speelman P, Levi M, Kurimoto M, Hack C E, van deventer S J, van der Poll T. Interleukin-12 induces sustained activation of multiple host inflammatory mediator systems in chimpanzees. J Infect Dis. 1999;179:646–652. doi: 10.1086/314636. [DOI] [PubMed] [Google Scholar]
- 15.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C Y, Ferrante J, Stewart C, Sarmiento U, Faherty D A, Gately M K. Interleukin-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 16.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto S, Tsuji-Takayama K, Aizawa Y, Koide K, Takeuchi M, Ohta T, Kurimoto M. Interleukin-18 activates NF-κB in murine T helper type 1 cells. Biochem Biophys Res Commun. 1997;234:545–457. doi: 10.1006/bbrc.1997.6665. [DOI] [PubMed] [Google Scholar]
- 18.Mattsson E, Verhage L, Rollof J, Fleer A, Verhoef J, van Dijk H. Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumour necrosis factor-alpha, interleukin-1 beta and interleukin-6. FEMS Immunol Med Microbiol. 1993;7:281–287. doi: 10.1111/j.1574-695X.1993.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 19.Morath S, Geyer A, Hartung T. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J Exp Med. 2001;193:393–398. doi: 10.1084/jem.193.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura K, Okamura H, Wada M, Nagata K, Tamura T. Endotoxin-induced serum factor that stimulates gamma interferon production. Infect Immun. 1989;57:590–595. doi: 10.1128/iai.57.2.590-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novick D, Kim S-H, Fantuzzi G, Reznikov L L, Dinarello C A, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 22.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces interferon-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 23.Okusawa S, Gelfand J A, Ikejima T, Connolly R J, Dinarello C A. Interleukin-1 induces a shock-like state in rabbits. J Clin Investig. 1988;81:1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozinsky A, Underhill D M, Fontenot J D, Hajjar A M, Smith K D, Wilson C B, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puren A J, Fantuzzi G, Gu Y, Su M S-S, Dinarello C A. Interleukin-18 (IFNγ inducing factor) induces IL-8 and IL-1β via TNFα production from non-CD14+ human mononuclear cells. J Clin Investig. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puren A J, Razeghi P, Fantuzzi G, Dinarello C A. Interleukin-18 enhances lipopolysaccharide-induced interferon-gamma production in human whole blood cultures. J Infect Dis. 1998;178:1830–1834. doi: 10.1086/314481. [DOI] [PubMed] [Google Scholar]
- 27.Schoenhaut D S, Chua A O, Wolitzky A G, Quinn P M, Dwyer C M, McComas W, Familetti P C, Gately M K, Gubler U. Cloning and expression of murine IL-12. J Immunol. 1992;148:3433–3440. [PubMed] [Google Scholar]
- 28.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning C J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 29.Skeen M J, Ziegler H K. Activation of γδ cells for production of IFN-γ is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J Immunol. 1995;154:5832–5841. [PubMed] [Google Scholar]
- 30.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto K, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 32.Wakabayashi G, Gelfand J G, Jung W K, Connolly R J, Burke J F, Dinarello C A. Staphylococcus epidermidis induces complement activation, tumor necrosis factor and interleukin-1, a shock-like state and tissue injury in rabbits without endotoxemia. Comparison to Escherichia coli. J Clin Investig. 1991;87:1925–1935. doi: 10.1172/JCI115218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wherry J C, Schreiber R D, Unanue E R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991;59:1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, Nakanishi K. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]