Abstract
Despite the impacts of neurodegeneration on speech function, little is known about how to comprehensively characterize the resulting speech abnormalities using a set of objective measures. Quantitative phenotyping of speech motor impairments may have important implications for identifying clinical syndromes and their underlying etiologies, monitoring disease progression over time, and improving treatment efficacy. The goal of this research was to investigate the validity and classification accuracy of comprehensive acoustic-based articulatory phenotypes in speakers with distinct neurodegenerative diseases. Articulatory phenotypes were characterized based on acoustic features that were selected to represent five components of motor performance: Coordination, Consistency, Speed, Precision, and Rate. The phenotypes were first used to characterize the articulatory abnormalities across four progressive neurologic diseases known to have divergent speech motor deficits: amyotrophic lateral sclerosis (ALS), progressive ataxia (PA), Parkinson’s disease (PD), and the nonfluent variant of primary progressive aphasia and progressive apraxia of speech (nfPPA+PAOS). We then examined the efficacy of articulatory phenotyping for disease classification. Acoustic analyses were conducted on audio recordings of 217 participants (i.e., 46 ALS, 52 PA, 60 PD, 20 nfPPA+PAOS, and 39 controls) during a sequential speech task. Results revealed evidence of distinct articulatory phenotypes for the four clinical groups and that the phenotypes demonstrated strong classification accuracy for all groups except ALS. Our results highlight the phenotypic variability present across neurodegenerative diseases, which, in turn, may inform (1) the differential diagnosis of neurological diseases and (2) the development of sensitive outcome measures for monitoring disease progression or assessing treatment efficacy.
Keywords: neurodegenerative disease, differential diagnosis, acoustic analyses, articulatory features, domain knowledge
INTRODUCTION
Neurodegenerative diseases affect millions worldwide and often result in debilitating speech deficits (Batista & Pereira, 2016; Hartelius et al., 2008). Over the past two decades, researchers have been characterizing the varied presentations of speech impairment across different neurodegenerative diseases—an effort referred to as speech phenotyping (Kent & Kim, 2003b; Lansford et al., 2014; Poole et al., 2017; Rusz et al., 2011, 2018; Schalling & Hartelius, 2004; Skodda, Visser, et al., 2011; Tsuboi et al., 2015; Utianski et al., 2018; Vogel et al., 2017). The comprehensive phenotyping of behaviors, such as speech, is of broad interest to the clinical neurology community for its potential to inform (1) the differential diagnosis of neurological diseases and (2) the development of sensitive outcome measures for monitoring disease progression or assessing treatment efficacy (Miravitlles et al., 2012; Titova & Chaudhuri, 2017). The purpose of this study was to characterize the articulatory phenotypes of four divergent neurodegenerative conditions and determine the potential utility of these phenotypes for informing disease classification.
The Need for Quantitative Measures of Speech
Speech abnormalities are often one of the earliest signs of disease onset (Duffy, 2013). Yet, while the oral motor exam is a key part of the neurological assessment, specific speech impairments (e.g., uncoordinated articulatory movements, inconsistent syllable productions) are less routinely relied upon in the diagnostic process. The underutilization of speech in the neurology clinic may be, in part, due to the lack of a quantitative, clinically meaningful method for characterizing speech abnormalities. One of the most established paradigms for characterizing speech motor impairments was developed by Darley, Aronson, and Brown (DAB) over five decades ago (Darley et al., 1969a, 1969b). For the articulatory subsystem, speech-language pathologists are instructed to rate speakers on several auditory-perceptual dimensions, such as “imprecise consonants,” “distorted vowels,” or “irregular articulatory breakdowns.” This paradigm has been widely adopted by speech clinicians and has highlighted the heterogeneity of speech impairments across neurologic populations. However, the subjectivity of this approach has raised questions regarding its accuracy and reliability (Borrie et al., 2012; Kent, 1996; Zyski & Weisiger, 1987). Researchers have, therefore, been exploring the utility of objective, instrumental-based measures of speech (Green et al., 2013). Acoustic analyses are one of the most rapidly developing speech analytic techniques because they are widely available, non-invasive, efficient, and affordable.
The Potential Clinical Utility of Measures of Articulatory Function
Articulatory motor function is of particular interest for informing both disease classification and sensitive outcome measures. From a diagnostic perspective, articulatory abnormalities manifest in unique motor symptoms (e.g., irregular articulatory breakdowns) that correspond with underlying neuropathologies of different movement disorders (e.g., cerebellar degeneration) (Ackermann, 2008; Darley et al., 1969b, 1969a; Hirose, 1986; Kent et al., 1979; Poole et al., 2017; Spencer & Slocomb, 2007). Importantly, when examining the speech of speakers with neurodegenerative diseases, identifying features that are spared is arguably as important as identifying those that are impaired. For example, slow rate and imprecision are sensitive markers of both ALS and progressive ataxia (PA) (Duffy, 2020), but irregular articulatory breakdowns are specific to PA (Duffy, 2020), as speakers with ALS have been shown to exhibit less articulatory variability than control speakers (Mefferd et al., 2014). Similarly, although a slow rate of speech is present in most dysarthria subtypes (Duffy, 2020), preserved or even increased rate has been reported in speakers with PD (Blanchet & Snyder, 2009; Kim et al., 2009; Rowe et al., 2020). These findings and others provide the rationale for further investigation into the efficacy of articulatory features as indicators of different movement disorders (Cordella et al., 2017; Green et al., 2013; Orozco-Arroyave et al., 2016; Shellikeri et al., 2016; Takakura et al., 2019). Moreover, articulatory impairments are strongly associated with speech intelligibility (De Bodt et al., 2002; Kent et al., 1989; Lee et al., 2014; Rong et al., 2015; Turner et al., 1995; Weismer et al., 2001) and have been shown to be highly sensitive to changes in disease status (Rong et al., 2015). Indeed, the growing focus on articulatory features has yielded studies demonstrating the potential for acoustic-based articulatory features to improve early disease detection (Allison et al., 2017) and serve as a useful tool for monitoring disease progression or assessing the efficacy of behavioral or pharmaceutical treatments (Green et al., 2018).
A Framework for Comprehensively Characterizing Articulatory Motor Impairments
Although the classification of speech motor disorders is more accurate when based on multiple speech features rather than individual features (Ballard et al., 2016; Basilakos et al., 2017), there is currently no established set of measures that can comprehensively characterize articulatory function (Green et al., 2013). Over the past several decades, articulation has been described using a wide variety of labels (e.g., “distinctiveness,” “imprecision,” “coupling,” “coarticulation”). The vast number of dimensions and lack of conceptual frameworks have led to challenges with interpreting measures and defining the constructs that might be most important for treatment or differential diagnosis (Berisha et al., 2021; Miller, 1992). Our recent work has thus sought to develop and validate a unifying framework for profiling the diversity of articulatory motor abnormalities described in the extant speech motor literature. The framework characterizes articulatory function using five key components of motor control (Rowe et al., 2020, 2021; Rowe & Green, 2019): Coordination, Consistency, Speed, Precision, and Rate. The goal of this framework is to provide a simple, comprehensive, and testable scheme that can allow for comparisons of articulatory function across clinical populations with divergent deficits.
Acoustic Correlates of Articulatory Impairments in Different Neurodegenerative Diseases
Throughout the past several decades, the articulatory patterns of many neurodegenerative diseases have been characterized using acoustic-based articulatory features. While the majority of acoustic studies have focused on disease detection (i.e., distinguishing individuals with a disease from healthy controls) (Mei et al., 2021), a subset of studies investigated articulatory differences across multiple clinical groups (see Table 1, which uses our framework as an organization scheme). To date, most studies have focused on distinguishing speakers with PD from speakers with other diseases. The most consistent findings are that speakers with PD exhibit (1) greater impairments in Precision compared to speakers with multiple sclerosis (MS) (Kuo & Tjaden, 2016), Huntington’s disease (HD) (Ackermann et al., 1995), early stage or mild ALS (Mefferd, 2015; Rowe et al., 2020), and PA (Ackermann et al., 1995); (2) less impaired repetition Rate compared to speakers with PA (Ziegler, 2002), late stage ALS (Rowe et al., 2020), cerebellar variant of multiple systems atrophy (MSA-C) (Rusz et al., 2019), and MS (Tjaden & Watling, 2003); (3) less impaired Coordination compared to speakers with early ALS (Rowe et al., 2020), MSA (Daoudi et al., 2021; Rusz et al., 2019; Tykalova et al., 2017), and progressive supranuclear palsy (PSP) (Tykalova et al., 2017); and (4) less impaired Consistency compared to speakers with MSA (Rusz et al., 2015), PSP (Skodda et al., 2012), HD (Ackermann et al., 1995), and MS (Tjaden & Watling, 2003). Although these findings provide important information regarding how diseases differ on specific articulatory features, this research is limited in the diversity of populations and articulatory characteristics examined in one study. Indeed, Ziegler (2002) investigated three divergent populations (i.e., PA, PD, and AOS) but primarily examined articulatory Rate (Ziegler, 2002). Similarly, Rusz and colleagues examined measures of Coordination, Consistency, Precision, and Rate but only in populations with atypical parkinsonian syndromes (APS) (i.e., MSA, PD, and PSP) (Rusz et al., 2015). No study, to our knowledge, has examined a wide range of both neurological/ pathophysiological deficits and articulatory characteristics, which is crucial for modeling and comparing the diversity of articulatory deficits across disease types.
Table 1.
Findings from current literature on distinguishing neurodegenerative populations using acoustic-based articulatory features (< indicates poorer performance on that measure).
| ALS | AOS | APS | HD | MS | PA | PD | |
|---|---|---|---|---|---|---|---|
| ALS | |||||||
| AOS | NA | ||||||
| APS | NA | NA | |||||
| HD | NA | NA | NA | ||||
| MS | NA | NA | NA | NA | |||
| PA | NA |
Consistency syllable variability: PA = AOS [1] Repetition Rate alternating motion rate: PA < AOS [1] |
NA |
Consistency syllable variability: PA > HD [2] Precision incomplete closure: PA < HD [2] |
NA | ||
| PD |
Coordination voice onset time: ePD > eALS [3] lPD = lALS [3] Consistency voice onset time variability: ePD = eALS [3] lPD = lALS [3] Speed second formant slope: ePD < eALS [3] lPD > lALS [3] Precision second formant slope variability: ePD = eALS [3] lPD > lALS [3] vowel space area: PD = ALS [4] vowel dispersion: PD < ALS [5] Repetition Rate sequential motion rate: ePD = eALS [3] lPD > lALS [3] |
Consistency syllable variability: PD = AOS [1] Repetition Rate alternating motion rate: PD = AOS [1] |
Coordination devoicing of plosives and fricatives: PD > MSA [13] voice onset time: PD = MSA-C [6] PD > MSA-P [6] voice onset time (voiced): PD > MSA [7] PD = PSP [7] voice onset time (voiceless): PD > MSA [7] PD > PSP [7] Consistency syllable variability: PD > MSA [8] PD > MSA-C [8] PD = MSA-P [8] PD > PSP [8,9] Precision resonant frequency attenuation: PD = MSA-C [8] PD = MSA-P [8] vowel area index: PD = MSA [8] PD > PSP [8] PD > PSP [10] Repetition Rate alternating motion rate: PD = MSA [9] PD = PSP [9,10] sequential motion rate: PD > MSA-C [7] PD = MSA-P [7] |
Consistency syllable variability: PD > HD [2] Precision incomplete closure: PD < HD [2] |
Coordination stop gap duration: PD > MS [11] Consistency syllable variability: PD > MS [11] Precision second formant interquartile range: PD < MS [12] Repetition Rate alternating motion rate: PD > MS [11] sequential motion rate: PD > MS [11] |
Consistency syllable variability: PD = PA [1,2] Precision incomplete closure: PD < PA [2] Repetition Rate alternating motion rate: PD > PA [1] |
Note. AOS = apraxia of speech; ALS = amyotrophic lateral sclerosis (e = early, l = late); APS = atypical parkinsonian syndrome; HD = Huntington’s disease; MS = multiple sclerosis; MSA = multiple systems atrophy; MSA-C = cerebellar variant of MSA; MSA-P = parkinsonian variant of MSA; PA = progressive ataxia; PD = Parkinson’s disease (e = early, l = late); PSP = progressive supranuclear palsy;
AOS refers to acquired AOS rather than neurodegenerative, the latter of which is referred to as progressive AOS (PAOS).
Current Study
In the current study, we used our acoustic-based framework to comprehensively characterize articulatory motor abnormalities in four populations with divergent neurodegenerative diseases. We then assessed the efficacy of articulatory phenotyping for disease classification. Importantly, our intention is not to use speech as the sole indicator of disease, but rather to explore the limits of the information speech can provide for informing the complex process of neurologic diagnosis. We examined ALS, which involves upper and lower motor neuron degeneration; PA, which involves cerebellar circuit degeneration; PD, which involves basal ganglia circuit degeneration; and the nonfluent variant of primary progressive aphasia and progressive apraxia of speech (nfPPA+PAOS), which involves left frontal lobe degeneration. The clinical groups were chosen to represent a wide diversity of neurological abnormalities and pathophysiologies that reflect and extend the clinico-anatomic model of Darley and colleagues (Darley et al., 1969a, 1969b; Duffy, 2020; Kent et al., 2000) (see Figure 1). Our research objectives and questions were as follows:
- To characterize the articulatory phenotypes of four divergent neurodegenerative diseases.
- What are the articulatory phenotypes of neurodegenerative diseases known to have divergent speech motor deficits?
- Do the acoustic features measure distinct constructs and, therefore, provide unique information about articulatory function for each neurodegenerative disease?
- To determine the accuracy of neurodegenerative disease classification based on articulatory phenotypes.
- What is the efficacy of articulatory phenotypes for classifying divergent neurodegenerative diseases?
- Is a profile of articulatory features more diagnostically useful than individual features for neurodegenerative diseases?
Figure 1.
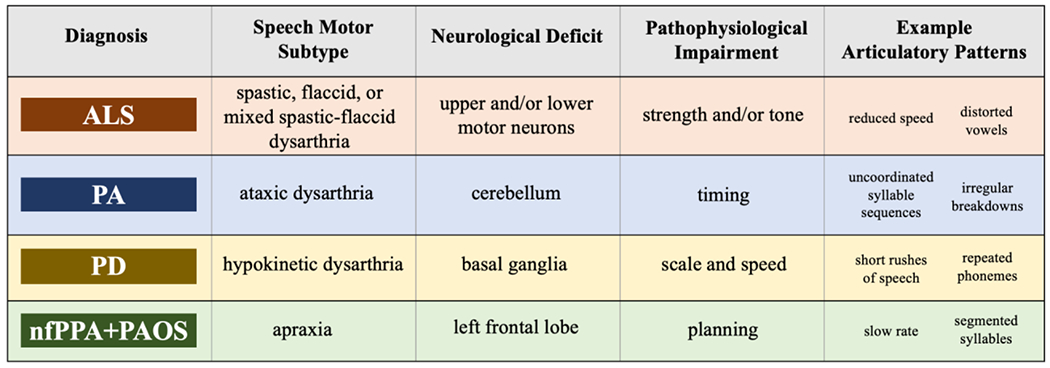
The clinical groups selected for this study along with their associated speech motor subtype, presumed neurological deficit and pathophysiological mechanism, and example articulatory patterns based on the Darley, Aronson, and Brown (DAB) model (ALS = amyotrophic lateral sclerosis; PA = progressive ataxia; PD = Parkinson’s disease; nfPPA+PAOS = the nonfluent variant of primary progressive aphasia and progressive apraxia of speech).
MATERIALS AND METHODS
Participants
Data from the four groups of speakers with neurodegenerative diseases (N = 46 for ALS, 52 for PA, 60 for PD, and 20 for nfPPA+PAOS) and neurotypical controls (N = 39) were obtained from several different extant databases (see Table 2). The participants in the four clinical groups presented with either dysarthria (i.e., ALS, PA, and PD) or AOS (nfPPA+PAOS), as determined by two speech-language pathologists, and demonstrated a mild speech impairment on average. The presence of AOS was based on established criteria as operationalized previously by Cordella and colleagues (Cordella et al., 2019). These criteria were adapted from the Apraxia of Speech Rating Scale (ASRS), which is a validated clinical indicator of AOS among individuals with nfPPA and PAOS (Strand et al., 2014), and were evaluated during AOS-specific tasks (e.g., multisyllable word repetition) and spontaneous speech to make a holistic assessment of AOS.
Table 2.
Demographic data, dataset, and speech characteristics of each speaker group.
| Group | N | Datasets | Mean Age | Percent Female | Categorical Severity 1 (Normal) to 5 (Profound) |
Continuous Severity 0 (Normal) to 100 (Profoundly Impaired) |
|---|---|---|---|---|---|---|
| ALS | 46 | 25 from Beiwe dataset* 18 from MGH ALS Clinic 3 from XRMB dataset |
55.26 (10.01) | 40% | 1.99 (.51) | 19.28 (15.25) |
| PA | 52 | 28 from NYU 15 from U of Washington 9 from U of Strathclyde |
46.12 (17.10) | 48% | 1.87 (.49) | 19.35 (13.54) |
| PD | 60 | 42 from U of Buffalo 18 from XRMB dataset |
64.60 (10.63) | 37% | 1.65 (.40) | 10.08 (8.70) |
| nfPPA+PAOS | 20 | 20 from MGH FTD Unit | 70.50 (7.81) | 59% | 1.98 (.75) | 19.61 (18.67) |
| Controls | 39 | 23 from XRMB dataset 10 from MGH 6 from Beiwe dataset* |
59.97 (9.50) | 49% | 1.53 (.42) | 8.44 (7.34) |
Note. ALS = amyotrophic lateral sclerosis; MGH FTD = Massachusetts General Hospital Frontotemporal Disorders; NYU = New York University; PA = progressive ataxia; PD = Parkinson’s disease; nfPPA+PAOS = the nonfluent variant of primary progressive aphasia and progressive apraxia of speech; XRMB = X-Ray Microbeam Speech Production Database (Westbury, 1994);
= collected remotely through cell phone recordings.
All participants were native English speakers, had normal hearing and vision, and had no cognitive impairments that may have interfered with the ability to follow directions. Control participants also had no history of speech, language, or neurological impairments. Information regarding the specific screening protocols for hearing and cognition was not available for all databases. However, standardized hearing screenings were performed for the XRMB dataset and at the MGH ALS Clinic, and cognitive screenings were conducted with the ALS Cognitive Behavior Screen (Woolley et al., 2010) at the MGH ALS Clinic and with the Standardized Mini-Mental State Examination (Molloy & Clarnette, 1999) at the University of Buffalo.
All neurological diagnoses were based on clinical evaluations by neurologists who specialized in each respective disease. The diagnosis of ALS was based on El Escorial criteria (Brooks et al., 2000); PA was used as an umbrella term to encompass speakers with Friedreich’s ataxia (FA) and cerebellar/spinocerebellar ataxia, whose diagnoses were confirmed with genetic testing (Klockgether et al., 2019; Pandolfo, 2008); the diagnosis of PD was based on criteria developed by the International Parkinson and Movement Disorder Society (MDS) (Postuma et al., 2015); lastly, the diagnosis of PPA was based on Mesulam’s guidelines and a prominent, isolated language deficit (Mesulam, 2001), and the classification of the nonfluent variant was based on criteria outlined by Gorno-Tempini and colleagues (Gorno-Tempini et al., 2011).
Data Collection
Acoustic data were collected from all participants during the sequential motion rate (SMR) task, in which they were instructed to repeat /pataka/ as quickly and accurately as possible on one breath. Data were compiled using several methods, including remote collection, which are denoted in Table 2. All data were collected at a range of sampling frequencies between 16000 and 44100 Hz, which is sufficient for the analyses in this study (Kent et al., 2002). All recordings were screened for audio quality to ensure there was no environmental or signal noise during the speech sample. The in-person data was collected using professional quality microphones (e.g., AKG C410, Shure SM81 Condenser, Olympus VN-702PC digital recorder). The remote data was collected on mobile phones using the Beiwe application, an open-source software designed for Android and iOS devices that records using uncompressed, lossless formats (Berry et al., 2019). Speakers in the Beiwe dataset were instructed to record their speech samples in quiet environments. The feasibility of the Beiwe platform for detecting and tracking speech abnormalities in speakers with ALS has been previously demonstrated (Berry et al., 2019). Other recent work has similarly supported the use of remote data collection for speech telehealth and research given its strong reliability (Pierce et al., 2021) and accessibility (Schneider et al., 2021; Sevitz et al., 2021). Furthermore, a study comparing acoustic measures extracted from cell phone recordings and those extracted from professional recording devices revealed few differences between the two (Zhang et al., 2021). Nonetheless, the literature base examining the potential effects of remote data collection is new and, therefore, still inconclusive. Indeed, another study found that remote data collection may impact certain spectral (e.g., second formant slope, which we used to calculate our measures of Speed and Precision) and durational (e.g., voice onset time, which we used to calculate our measure of Consistency) measures (Ge et al., 2021). Thus, for the current study, we conducted statistical analyses to test for the potential effects of remote data collection on the second formant slope and voice onset time. Still, it should be noted that remote data collection may introduce other spectral and noise distortions that are not captured in this sole analysis.
Task Selection
The diadochokinetic (DDK) task (Fletcher, 1972), composed of the SMR task and alternating motion rate (AMR) task, is one of the few widely implemented, objective assessments for evaluating articulatory function in the clinic (Duffy, 2020). The SMR and AMR tasks involve repeating a syllable sequence (i.e., /pataka/) or single syllables (i.e., /pa/, /ta/, /ka/), respectively, as quickly and accurately as possible on one breath. Although the DDK task does not reflect functional communication (e.g., connected speech), prior work has demonstrated that DDK performance is a leading indicator of impending decreases in speaking rate (Nishio & Niimi, 2006; Rong et al., 2015) and that it is associated with other functional measures of speech, such as intelligibility (Becker et al., 2017; Samlan & Weismer, 1995). Moreover, the task assesses a speaker’s ability to produce rapid muscle contractions of target articulators (e.g., lips, jaw, tongue), which challenges the motor system while imposing a relatively low burden on cognitive and linguistic functions. As a result, the DDK task been found to be an informative tool for distinguishing between speech motor disorders (Kent et al., 2022; Sidtis et al., 2011; Tjaden & Watling, 2003; Ziegler & Wessel, 1996). The SMR task, in particular, has strong diagnostic utility because it requires the ability to rapidly transition between movements, which is characteristically difficult for speakers with motor planning deficits (McNeil et al., 1995). Speakers with AOS often perform adequately on the AMR task but tend to exhibit variable error patterns on multisyllabic sequences in the SMR task (Duffy et al., 2014). Overall, the motoric demands this task imposes on speakers, particularly on those with vulnerable oral motor systems, could expose granular changes in articulatory function that may be diagnostically informative (Rong, 2020; Wang et al., 2009).
Severity Confound
Speech impairment severity is a common confound when attempting to categorize speech motor populations, as significant imbalances in severity could drive group classification (Kim et al., 2011). Including only speakers within a certain level allows us to determine the true discriminatory power of the acoustic features, which increases confidence that severity differences are not the primary driver of phenotype differences. To control for severity, we excluded speakers who had an articulatory severity of “Severe” or “Profound” based on the SMR task. For the severity ratings, two licensed speech-language pathologists with clinical expertise in speech motor disorders rated each speaker’s level of articulatory severity on a categorical scale of “Normal”, “Mild”, “Moderate”, “Severe”, or “Profound” (King et al., 2012). The clinicians were blind to speaker identity and diagnosis and were allowed to listen to the recordings as many times as needed. While the categorical ratings were used to stratify the speakers, the clinicians were also asked to rate each speaker on a 100-point visual analogue scale with endpoints labeled “No Impairment” (0) on the left and “Profound Impairment” (100) on the right. This method, which is consistent with procedures used in prior studies (Awan et al., 2009; Rowe et al., 2021), provides information about potential fine-grained differences in speaker severity. Lastly, in addition to excluding speakers who were identified as “Severe” or “Profound”, we investigated the presence of statistically significant differences in both categorical and continuous severity ratings. A randomly selected 10% of the recordings were repeated for intrarater reliability (which was good to excellent for both severity ratings). Given our large sample size of 217, we felt 10% was an appropriate number of samples to assess for reliability, which also aligns with prior work (Tjaden et al., 2013). Interrater reliability (which was also good to excellent for both severity ratings) was assessed using 100% of the recordings since all recordings were rated by both clinicians.
Acoustic Analysis
The acoustic recordings were analyzed using Praat (Boersma, 2014). For the analysis, formant settings were set to a maximum frequency of 5500 Hz for women and 5000 Hz for men. Of the acoustic recordings from the clinical populations, 16 files (8%) were not able to be analyzed due to formant mistracking (5 [8%] from the ALS group, 4 [7%] from the PA group, 12 [17%] from the PD group, and 4 [15%] from the nfPPA+PAOS group). Each spectrogram from the remaining acoustic recordings was parsed for the first three repetitions of the sequence /pataka/ in which formant tracking was the most valid. For most participants, this was the second or third set of three repetitions. Formant tracking was empirically validated through visual inspection, which ensured robust formant tracking across the entire three repetitions with minimal deviation from the resonant band. Selecting well-defined repetitions from the front end of the speech sample allowed for more experimental control, as later repetitions may be influenced by factors such as fatigue or respiratory capacity. We selected only three repetitions because we sought to develop a brief protocol that could be used across speakers with a range of severities (e.g., speakers who are unable to produce more than three repetitions).
The first author and a trained research assistant segmented all of the SMR samples. To obtain interrater reliability (which was good to excellent for all five acoustic measures), the trained research assistant then re-measured the boundaries of 10% of the samples that the first author measured. To obtain intrarater reliability (which was also good to excellent for all five acoustic measures), the first author re-measured the boundaries of 10% of the samples she originally measured. The data required for each feature was extracted using a custom Praat script (Boersma, 2014), and the features were subsequently calculated using custom MATLAB (MathWorks, 2019) and R scripts (R Core Team, 2014). See Table 3 for the definition of each component and the methods used to calculate each corresponding acoustic feature. Figure 2 illustrates the portions of the spectrogram and waveform from which the five features were extracted.
Table 3.
Description of the components and features for the articulatory phenotypes.
| Definition | Feature | Parsing and Calculation Methods | |
|---|---|---|---|
| Coordination | appropriate temporal alignment of movements to meet task demands | Proportion of Stop Gap to Total Syllable Length | 1. Parse each syllable from consonant release (defined by amplitude burst on spectrogram) to subsequent vowel offset (defined by final glottal pulse on spectrogram) and from subsequent vowel offset to subsequent consonant release 2. Calculate duration between vowel offset in /pa/ and release of /t/, between vowel offset in /ta/ and release of /k/, and between vowel offset in /ka/ and release of subsequent /p/ (i.e., gaps during syllable transitions) 3. Calculate duration between release of /p/ to release of /t/, between release of /t/ to release of /k/, and between release of /k/ and release of subsequent /p/ (i.e., full syllable length) 4. Calculate average gap duration and average full syllable length 5. Calculate proportion of average gap duration to average full syllable length |
|
↑ values = greater gap time during syllable transition and thus
↓ Coordination |
|||
| Consistency | stability of speech sounds across multiple repetitions | Across-Repetition Variability in Voice Onset Time | 1. Parse each consonant from release to subsequent vowel onset (defined by first glottal pulse on spectrogram) 2. Calculate duration between consonant release and subsequent vowel onset for each repetition (i.e., voice onset time [VOT]) 3. Calculate standard deviation in voice onset time between three repetitions and multiply by 100 to obtain coefficient of variation 4. Calculate average standard deviation of VOT across /p/, /t/, and /k/ |
|
↑ values = greater variability in voice onset time and thus
↓ Consistency |
|||
| Speed | quickness of movement during each syllable repetition | Second Formant Slope of /k/ (Hz/ms) | 1. Parse each repetition of /ka/ from vowel onset to vowel offset 2. Run custom Praat script to extract formant time series 3. Divide difference between frequency at vowel midpoint and frequency at vowel onset (i.e., F2-F1) by difference between time at vowel midpoint and time at vowel onset (i.e., T2-T1) for consonant-vowel transition in /ka/ (i.e., F2 slope) 4. Calculate average F2 slope across all three repetitions of /pataka/ |
|
↓ values = reduced rate of vocal tract configuration and thus
↓ Speed |
|||
| Precision | clearness and distinctiveness of consonants | Across-Consonant Variability in Second Formant Slope | 1. Parse each repetition of /pa/, /ta/, and /ka/ from vowel onset to vowel offset 2. Run custom Praat script to extract formant time series 3. Divide F2-F1 by T2-T1 for each consonant-vowel transition in /pa/, /ta/, and /ka/ 4. Calculate standard deviation of F2 slope between consonants within each repetition of /pataka/ 5. Calculate average standard deviation of F2 slope across all three repetitions of /pataka/ |
|
↓ values = reduced variability in place of articulation and thus
↓ Precision |
|||
| Rate | quickness of completion of syllable sequences | Sequential Motion Rate (syll/sec) | 1. Parse first three repetitions from release of first /p/ to vowel offset in third /ka/ 2. Divide duration of three repetitions by nine (i.e., number of syllables produced) |
|
↓ values = reduced syllables produced per second and thus
↓ Rate |
Figure 2.
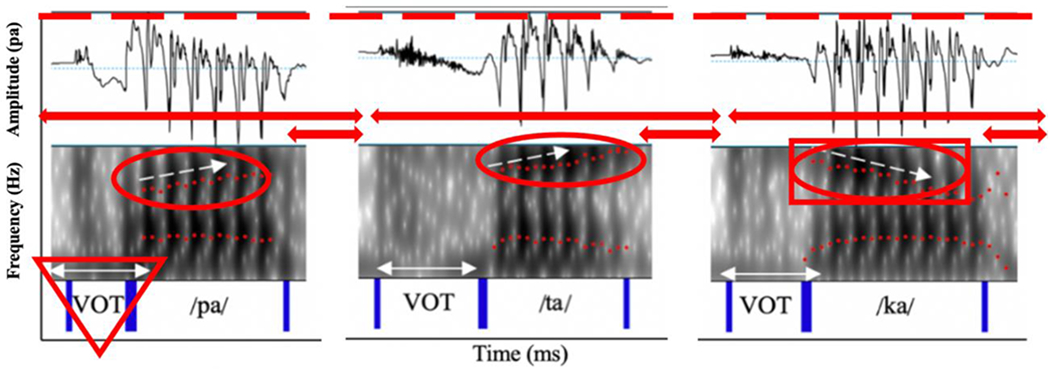
Acoustic spectrogram and waveform of an utterance of /pataka/ produced by a speaker with amyotrophic lateral sclerosis (ALS). Each shape outlines the portions of the signal from which the five components were extracted. 1. Red Arrows = Coordination (proportion of stop gap [i.e., short red arrow] to total syllable length [i.e., long red arrow]); 2. Red Triangle = Consistency (standard deviation of voice onset times [VOT] for each consonant across three repetitions); 3. Red Circles = Precision (standard deviation of three formant slopes for /pa/, /ta/, and /ka/ within each repetition); 4. Red Square = Speed (mean of formant slope for /ka/ across three repetitions); and 5. Red Dashed Line = Rate (syllables produced per second).
Coordination (GapSyllProp):
Coordination is arguably one of the most difficult articulatory constructs to capture acoustically. Based on the DAB paradigm and converging evidence from the speech literature, we expect to see the greatest Coordination deficits in speakers with PA and nfPPA+PAOS (Darley et al., 1969a, 1969b; Ogar et al., 2005; Spencer & France, 2016). Previous work has examined gap duration during DDK tasks as an indicator of coordinative or timing difficulties in populations with ataxia (Ozawa et al., 2001; Schalling et al., 2007; Schalling & Hartelius, 2004). Similarly, recent studies have documented segmented syllable repetitions in speakers with AOS/PAOS (Bouvier et al., 2021; Deger & Ziegler, 2002; Duffy, 2006; Kent & Rosenbek, 1983; Melle & Gallego, 2012; Staiger & Ziegler, 2008; Takakura et al., 2019). While pausing can indicate a linguistic or cognitive deficit in spontaneous speech (Rochester, 1973), intersyllabic pausing during a non-speech syllable repetition task, such as the DDK, can indicate difficulty with motorically planning or executing an utterance (Oytam et al., 2005). In the current study, our measure of Coordination was the relative duration of the silence between two articulatory gestures during each syllable transition (e.g., /pa1/ to /ta1/, /ta1/ to /ka1/, or /ka1/ to /pa2/). In typical speakers, these transitions should be completed with little to no silence, whereas an abnormally prolonged stop gap relative to syllable length may indicate difficulty sequencing the closing and release gestures produced for the different stop consonants. Overall, this measure reflects the ability to transition smoothly between articulatory gestures—a key underlying construct of Coordination (Caruso et al., 1988; Gracco & Abbs, 1986). We used the proportion of gap duration to syllable length to control for potential confounding effects of rate. Furthermore, a proportion provides more information than absolute gap duration because it allows us to determine the relative difficulty of transitioning between sounds relative to the difficulty producing sounds. The measure is intended to be able to distinguish between a speaker who is slow overall (e.g., they produce equally long syllables and gaps) from a speaker who has difficulty with syllable transitions (e.g., they produce a markedly longer gap), the latter of which may be more indicative of a coordinative deficit.
Consistency (RepVarVOT):
For our measure of Consistency, we used the across-repetition variability in voice onset time given its sensitivity to populations that lack stability in repeated sound productions (Hertrich & Ackermann, 1994; Kent et al., 1997; Kent & Rosenbek, 1983; McNeil et al., 1995; Rong, 2020; Rowe & Green, 2019). While variability in syllable length is another measure frequently used to index inconsistency (Ackermann et al., 1995; Rong, 2020; Ziegler, 2002), variability in VOT may be more sensitive than syllable length to smaller across-repetition alterations in the speech signal (Kent et al., 1997).
Speed (F2Slope):
For our measure of Speed, we used the second formant slope in the consonant transition of /k/ because previous research has demonstrated that the second formant slope is strongly associated with articulatory movement speed (Kent & Kim, 2003a; Kim et al., 2009; Yunusova et al., 2012). In light of prior work suggesting that formant slope is best calculated using stimuli that result in large changes in vocal tract configuration (Kim et al., 2009), /k/ was used in this study due to its robust transition.
Precision (ConVarF2Slope):
For our measure of Precision, we used the across-consonant variability in second formant slope in the consonant transitions of /p/, /t/, and /k/ given previous work demonstrating the utility of second formant slope (i.e., frequency range divided by time) as a cue for place and manner of articulation (Chen & Alwan, 2000; Delattre et al., 1955). The frequency range in second formant transitions depends on vocal tract length (e.g., bilabials tend to produce lower frequency ranges than alveolars) (MacKay, 2014), and the transition time depends on the rate of vocal tract constriction (e.g., plosives tend to produce more abrupt transition rates than fricatives) (Delattre et al., 1960). Thus, large differences between the transition slopes of three distinct consonants would indicate greater distinctiveness. This measure is relatively novel measure but was recently validated against kinematic and perceptual measures of Precision, revealing significant correlations of .72 and .68, respectively (Rowe et al., 2021).
Rate (RepRate):
Lastly, for our measure of repetition Rate, we used the number of syllables produced per second during the SMR task because this measure has been shown to be highly sensitive to articulatory decline in speech motor populations (Nishio & Niimi, 2006; Tjaden & Watling, 2003; Ziegler & Wessel, 1996).
Statistical Analysis
All statistical analyses were completed in R (R Core Team, 2014).
Interrater and Intrarater Reliability
Interrater and intrarater reliability for the acoustic and perceptual analyses were calculated using ICC (2,1) intraclass correlation coefficient (ICC) (2,1) (i.e., two-way random single measures used for consistency/absolute agreement).
Controlling for Articulatory Severity across Clinical Groups
Between-group differences in articulatory severity (both continuous and categorical) were assessed using a one-way analysis of variance (ANOVA) with Tukey’s multiple comparison tests to determine which groups were different from one another.
Controlling for Effect of Remote Data Collection on Formant Slope and Voice Onset Time
For the two groups of speakers that included both remote and in-person data (i.e., ALS and controls), we conducted independent t-tests on severity-matched groups to determine the effect of remote data collection on the second formant slope and voice onset time. If remotely collecting data resulted in systematic noise in the signal, we would expect there to be significant differences between the two groups.
Controlling for Effect of Sex and Age on Speech Performance
Because there were unequal ratios of males and females and different age ranges in each group of speakers, we conducted independent t-tests to examine the effect of sex on each of the five acoustic features and Pearson correlations to examine the relationship of age with each of the five acoustic features.
Presence of Articulatory Phenotypes (Research Question 1)
What are the articulatory phenotypes of neurodegenerative diseases known to have divergent speech motor deficits? (Research Question 1a):
To examine whether the overall articulatory profiles of the four clinical groups were different from one another and from controls, we conducted a multivariate analysis of variance (MANOVA) with diagnosis as the independent variable and the five articulatory components as the dependent variables. Post hoc one-way ANOVAs with Tukey’s multiple comparison tests were then conducted to determine which articulatory components were significantly different between groups.
Do the acoustic features measure distinct constructs and, therefore, provide unique information about articulatory function for each neurodegenerative disease? (Research Question 1b):
To assess whether the features measured distinct constructs for each neurodegenerative disease, we conducted correlations for each pair of acoustic features. We conducted weighted Pearson correlations for the clinical groups as a whole (due to the unequal sample sizes across groups) and unweighted Pearson correlations for each clinical group individually.
Classification Accuracy of Articulatory Phenotypes (Research Question 2)
What is the efficacy of articulatory phenotypes for classifying divergent neurodegenerative diseases? (Research Question 2a):
To examine the classification accuracy of articulatory phenotyping, we used a multivariate linear discriminant analysis (LDA). The goal of this analysis was to identify distinguishing characteristics (i.e., phenotypes) of groups through independent variable weightings, known as discriminant vectors, which optimize the ratio of between-group to within-group variance. Because classical LDA identifies discriminant vectors between two groups, the groups were partitioned for the analysis into binary comparisons (e.g., ALS compared to the other three clinical groups). The predictive performance of the LDA was evaluated using k-fold (k = 5) cross-validation. Importantly, all speakers from the clinical groups in this study were symptomatic and already diagnosed with a neurodegenerative disease. Thus, controls were not included in the LDA because this analysis sought to assess whether speech phenotyping informs differential diagnosis (i.e., confirming which disease each speaker had) rather than disease detection.
Is a profile of articulatory features more diagnostically useful than individual features for neurodegenerative diseases? (Research Question 2b):
To compare how the individual features performed compared to the profile of features in classifying the four clinical groups, we conducted univariate LDAs with each feature individually. We assessed the accuracy of the LDAs using receiver operating characteristic (ROC) curves. Area under the curve (AUC), sensitivity, and specificity were calculated for each curve. Independent samples t-tests were then used to examine whether there was a statistical difference between the AUCs from the univariate LDAs in Research Question 2b and the multivariate LDA using all five features in Research Question 2a.
RESULTS
Interrater and Intrarater Reliability
ICC (2,1) demonstrated good to excellent interrater reliability for all five components measured acoustically and for the SMR severity ratings (see Table 4).
Table 4.
Inter- and intrarater reliability with significance levels (* p < .05, ** p < .01, *** p < .001) for acoustic measures of each component and perceptual ratings of articulatory severity.
| Measure | Interrater Reliability | Intrarater Reliability |
|---|---|---|
| Coordination (GapSyllProp) | ICC (2,1) = .91 [.79, .96]*** | ICC (2,1) = .78 [.51, .91]*** |
| Consistency (RepVar.VOT) | ICC (2,1) = .81 [.69, .88]*** | ICC (2,1) = .77 [.63, .86]*** |
| Speed (F2Slope) | ICC (2,1) = .77 [.49, .91]*** | ICC (2,1) = .85 [.65, .94]*** |
| Precision (ConVar.F2Slope) | ICC (2,1) = .83 [.53, .95]*** | ICC (2,1) = .80 [.52, .93]*** |
| Rate (RepRate) | ICC (2,1) = .99 [.99, 1.00]*** | ICC (2,1) = .99 [.99, 1.00]*** |
| Severity | ||
| Articulatory Severity Rating (Continuous) | ICC (2,1) = .74 [.67, .79]*** | ICC (2,1) = .86 [.67, .94]*** |
| Articulatory Severity Rating (Categorical) | ICC (2,1) = .67 [.59, .74]*** | ICC (2,1) = .76 [.49, .90]** |
Controlling for Articulatory Severity across Clinical Groups
A one-way ANOVA revealed a significant effect of categorical severity ratings (F(3, 174) = 4.72, p < .01) and continuous severity ratings (F(3, 174) = 6.44, p < .001) based on diagnosis. Tukey’s multiple comparison tests demonstrated significant differences in categorical severity ratings between speakers with PD and ALS and in continuous severity ratings between speakers with PD and each of the other three clinical groups. Our findings for speakers with PD should thus be considered with the knowledge that this clinical group was significantly less severe than the other three clinical groups. There were no significant differences in severity for any of the other pairs.
Controlling for Effect of Remote Data Collection on Formant Slope and Voice Onset Time
Independent samples t-tests revealed no significant effect of remote data collection on the second formant slope (ALS: p = .13; controls: p = .23) nor on voice onset time (ALS: p = .78; controls: p = .92).
Controlling for Effect of Sex and Age on Speech Performance
Independent samples t-tests revealed no significant effect of sex on any of the five acoustic features for each of the five speaker groups, and Pearson correlations revealed no significant associations between age and any of the five acoustic features for each of the five speaker groups.
Presence of Articulatory Phenotypes
What are the articulatory phenotypes of neurodegenerative diseases known to have divergent speech motor deficits? (Research Question 1a):
A MANOVA was conducted to determine whether the overall articulatory profiles of the four clinical groups were different from one another and from controls. The multivariate result was significant for all articulatory components (Coordination: F(4, 212) = 25.04, p < .001; Consistency: F(4, 212) = 11.84; Speed: F(4, 212) = 8.22; Precision: F(4, 212) = 7.87; Rate: F(4, 212) = 36.70), indicating a difference in articulatory performance depending on speaker diagnosis. One-way ANOVAs with Tukey’s multiple comparison tests were also conducted to determine which groups were significantly different from one another (see Table 5). Figures 3 and 4 illustrate the articulatory performance of the four clinical groups and controls in raw scores and z scores, respectively.
Table 5.
Effect sizes and confidence intervals for between-group comparisons of performance on the articulatory components. The arrow indicates the group that was more impaired on the component (e.g., when comparing ALS and PA on Consistency, the arrow is pointing left because speakers with PA have more impaired Consistency than speakers with ALS) (* = p < .05, ** = p < .01, *** = p < .001). Statistically significant differences are in bold.
| Control | ALS | PA | PD | nfPPA+PAOS | ||
|---|---|---|---|---|---|---|
| COORDINATION | Control | |||||
| ALS | ↑.48 [.04, .92] | |||||
| PA | ↑.25 [−.17, .66] | ←.29 [−.11, .69] | ||||
| PD | ←.13 [−.27, .53] | ←.64* [.23, 1.04] | ←.40 [.02, .78] | |||
| nfPPA+PAOS | ←1.80*** [1.12, 2.46] | ←1.95*** [1.28, 2.61] | ←2.09*** [1.43, 2.74] | ←1.93*** [1.31, 2.54] | ||
|
| ||||||
| CONSISTENCY | Control | |||||
| ALS | ←.32 [−.11, .75] | |||||
| PA | ←.87*** [.41, 1.33] | ←.68** [.26, 1.10] | ||||
| PD | ↑.35 [−.06, .76] | ↑.65 [.24, 1.06] | ↑1.14*** [.71, 1.57] | |||
| nfPPA+PAOS | ←.26 [−.28, .80] | ←.02 [−.51, .54] | ↑.55* [.02, 1.07] | ←.54 [.02, 1.05] | ||
|
| ||||||
| SPEED | Control | |||||
| ALS | ←.95*** [.47, 1.43] | |||||
| PA | ←.73*** [.28, 1.17] | ↑.14 [−.26, .54] | ||||
| PD | ←.86*** [.41, 1.31] | ↑.13 [−.25, .52] | ←.03 [−.34, .40] | |||
| nfPPA+PAOS | ←1.29*** [.67, 1.89] | ←.42 [−.11, .95] | ←.49 [−.04, 1.01] | ←.57 [.05, 1.08] | ||
|
| ||||||
| PRECISION | Control | |||||
| ALS | ←.96*** [.48, 1.44] | |||||
| PA | ←.70** [.25, 1.14] | ↑.21 [−.19, .61] | ||||
| PD | ←.45 [.03, .86] | ↑.49 [.09, .88] | ↑.26 [−.12, .63] | |||
| nfPPA+PAOS | ←1.55*** [.91, 2.19] | ←.41 [−.13, .94] | ←.60 [.07, 1.13] | ←.93** [.39, 1.46] | ||
|
| ||||||
| RATE | Control | |||||
| ALS | ←1.15*** [.64, 1.64] | |||||
| PA | ←1.61*** [1.05, 2.15] | ←.18 [−.22, .57] | ||||
| PD | ←.78* [.33, 1.21] | ↑.60* [.19, 1.00] | ↑.93*** [.52, 1.34] | |||
| nfPPA+PAOS | ←3.57*** [2.60, 4.53] | ←1.50*** [.89, 2.10] | ←1.67*** [1.06, 2.28] | ←2.90*** [2.17, 3.62] | ||
Note. ALS = amyotrophic lateral sclerosis; PA = progressive ataxia; PD = Parkinson’s disease; nfPPA+PAOS = the nonfluent variant of primary progressive aphasia and progressive apraxia of speech.
Figure 3.
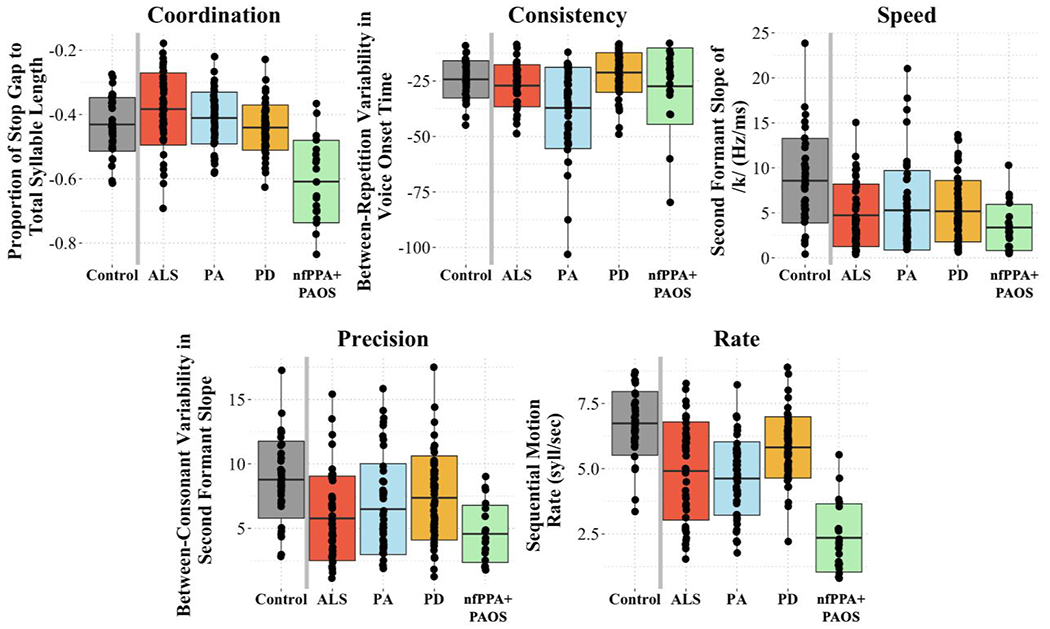
Boxplots demonstrating mean performance on each component of articulatory motor control for the four clinical groups and controls.
Figure 4.
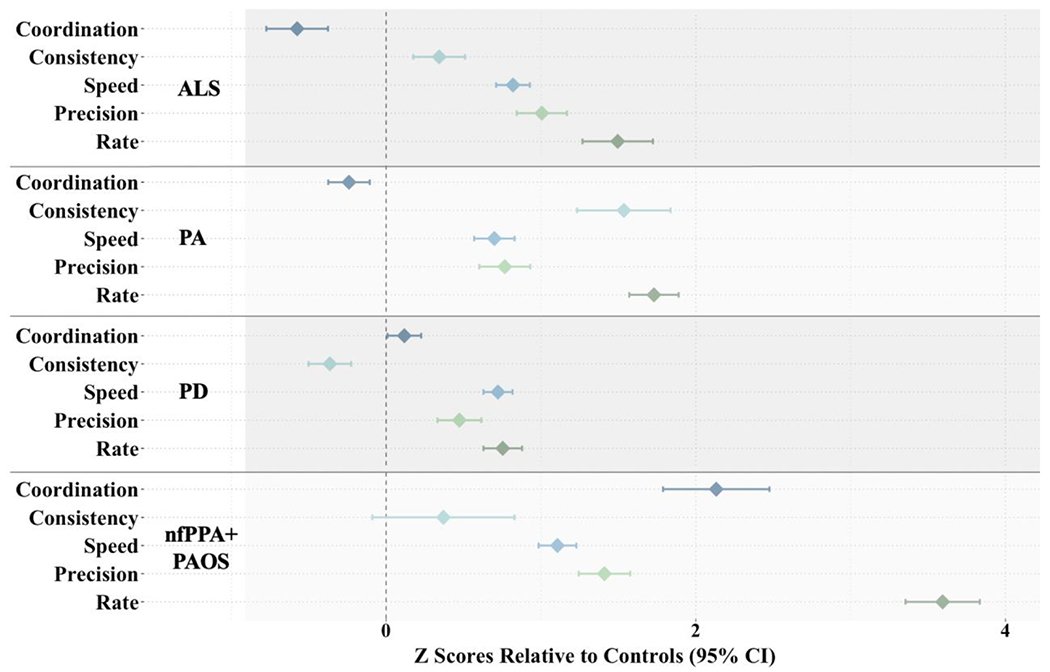
Forest plot demonstrating divergent patterns of articulatory performance across the four clinical groups. Each diamond represents the mean z score (clinical group compared to controls) with the 95% confidence interval (CI) for each articulatory component. Z scores to the right of the vertical dotted line (i.e., positive z scores) indicate that the clinical group is more impaired than controls.
Do the acoustic features measure distinct constructs and, therefore, provide unique information about articulatory function for each neurodegenerative disease? (Research Question 1b):
Weighted and unweighted Pearson correlations were used to assess whether the features measured distinct constructs for each neurodegenerative disease. Our findings revealed weak to moderate correlations for all pairs of acoustic features in the clinical groups as a whole and in each clinical group individually (see Table 6).
Table 6.
Correlation matrices of performance on the acoustic features for the clinical groups as a whole and for each clinical group individually (* = p < .05, ** = p < .01, *** = p < .001).
| Pairwise Correlations | All | ALS | PA | PD | nfPPA+PAOS |
|---|---|---|---|---|---|
| Coordination x Consistency | −.03 | .05 | −.24 | −.15 | .68 |
| Coordination x Speed | −.15* | −.07 | −.20 | −.09 | −.30 |
| Coordination x Precision | −.16* | −.13 | −.19 | −.14 | .36 |
| Coordination x Rate | .09 | −.08 | −.21 | .09 | .57** |
|
| |||||
| Consistency x Speed | .01 | .04 | .03 | .08 | −.08 |
|
| |||||
| Consistency x Precision | −.002 | .003 | −.18 | .19 | .08 |
|
| |||||
| Consistency x Rate | .25*** | .10 | .22 | .09 | .33 |
|
| |||||
| Speed x Precision | .68*** | .73*** | .63*** | .54*** | .17 |
|
| |||||
| Speed x Rate | .43*** | .58*** | .51*** | .29* | −.18 |
|
| |||||
| Precision x Rate | .58*** | .72*** | .70*** | .31** | .53* |
Note. ALS = amyotrophic lateral sclerosis; PA = progressive ataxia; PD = Parkinson’s disease; nfPPA+PAOS = the nonfluent variant of primary progressive aphasia and progressive apraxia of speech.
Classification Accuracy of Articulatory Phenotypes
What is the efficacy of articulatory phenotypes for classifying divergent neurodegenerative diseases? (Research Question 2a):
Multivariate LDAs with five-fold cross validation were used to determine the classification accuracy of the articulatory profiles for the four clinical groups. ROC curve analyses revealed acceptable to good average AUC, sensitivity, and specificity values for each clinical group except ALS (see Figure 5). Interpretation of ROC performance was based on guidelines described in Safari and colleagues (Safari et al., 2016).
Figure 5.
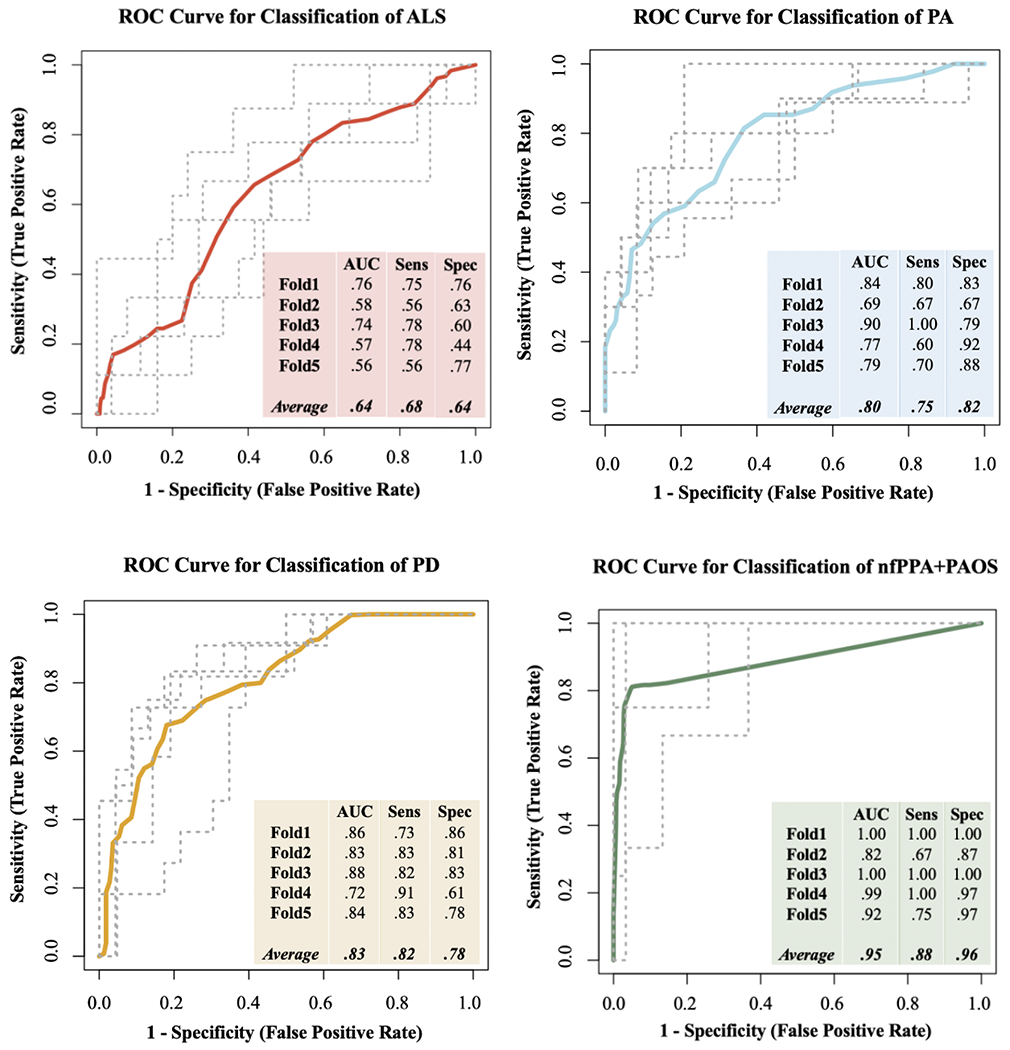
Receiver operating characteristic (ROC) curves based on the linear discriminant analysis (LDA) for each clinical group. Area under the curve (AUC), sensitivity, and specificity values are given for each of the five validation folds, along with the averages of each.
Is a profile of articulatory features more diagnostically useful than individual features for neurodegenerative diseases? (Research Question 2b):
Univariate LDAs with five-fold cross validation were also conducted with each of the five features. Independent samples t-tests demonstrated that the average AUC of the clinical groups using the multivariate LDA was significantly higher than the average AUCs of all clinical groups using the univariate LDAs. This finding indicates that the profile of features performed significantly better than individual features at classifying the four clinical groups (see Table 7).
Table 7.
Average AUC (in bold), sensitivity, and specificity based on the five cross-validation folds of the univariate (i.e., only including individual features) and multivariate (i.e., including all features) LDAs for the clinical groups as a whole and for each group individually.
| ALS | PA | PD | nfPPA+PAOS | Average AUC for All Clinical Groups | Comparison of Average AUCs for All Clinical Groups | ||
|---|---|---|---|---|---|---|---|
| Coord | .69, .62, .79 | .57, .67, .58 | .70, .74, .69 | .88, .88, .85 | .71 | Coord-All Features | p < .05* |
|
|
|||||||
| Con | .55, .66, .55 | .75, .76, .74 | .74, .79, .66 | .39, .53, .52 | .61 | Con-All Features | p < .001*** |
| Speed | .36, .41, .51 | .53, .66, .50 | .54, .60, .63 | .63, .80, .58 | .52 | Speed-All Features | p < .001*** |
| Prec | .56, .73, .51 | .43, .39, .67 | .63, .67, .64 | .69, .73, .69 | .58 | Prec-All Features | p < .001*** |
| Rate | .45, .54, .54 | .67, .69, .66 | .74, .79, .66 | .91, .93, .85 | .69 | Rate-All Features | p < .05* |
| All Features | .64, .64, .66 | .80, .75, .82 | .83, .82, .78 | .95, .88, .96 | .81 | ||
Note. ALS = amyotrophic lateral sclerosis; PA = progressive ataxia; PD = Parkinson’s disease; nfPPA+PAOS = the nonfluent variant of primary progressive aphasia and progressive apraxia of speech.
DISCUSSION
Overall, there were three central findings of our study: (1) The clinical groups exhibited unique articulatory phenotypes, with the primary distinguishing features of reduced Consistency for speakers with PA, preserved repetition Rate for speakers with PD, and reduced Coordination and repetition Rate for speakers with nfPPA+PAOS; (2) The articulatory features provided unique information about speech motor performance for each clinical group, as indicated by weak to moderate pairwise comparisons for most pairs of acoustic features; and (3) Classification accuracy was better for acoustic-based profiles than for individual features, with acceptable to good sensitivity and specificity for all clinical groups except speakers with ALS.
Presence of Articulatory Phenotypes
PD Phenotype: Impaired Speed and Rate Compared to Controls, but Preserved Rate Compared to Other Clinical Groups
For speakers with PD, the finding of decreased Speed relative to controls is consistent with the DAB classification of hypokinetic dysarthria, which posits that hypokinesia leads to reduced movement size and speed (Darley et al., 1969a, 1969b). Moreover, acoustic studies have demonstrated reduced second formant slope in speakers with PD (Chiu et al., 2019; Connor et al., 1989; Kim et al., 2009; Martel-Sauvageau & Tjaden, 2017; Rosen et al., 2006; Tjaden et al., 2013; Tjaden & Wilding, 2004; Walsh & Smith, 2012; Weismer et al., 2001). Our finding of preserved repetition Rate relative to the other clinical groups also aligns with previous acoustic work examining multiple clinical groups, which demonstrated less impaired repetition Rate in speakers with PD compared to those with ALS (Rowe et al., 2020), PA (Ziegler, 2002), MS (Tjaden & Watling, 2003), and MSA-C (Rusz et al., 2019). Similarly, Darley and colleagues identified short rushes of speech or increased rate as a hallmark characteristic of basal ganglia damage (Darley et al., 1969a, 1969b). Researchers have thus proposed that Speed and Rate can dissociate in speakers with PD (Weismer & Berry, 2003). These acoustic findings align with expectations advanced in the literature on the neural mechanisms of hypokinetic dysarthria due to PD (Braak et al., 2003; Guenther, 2016). Indeed, a loss of cortical regulation due to damage to the basal ganglia (Braak et al., 2003) may result in slowed movement Speed. Simultaneously, impaired signaling of when to execute the subsequent speech motor unit may reduce the duration of motor programs, resulting in “bursts of rapid speech” or increased Rate (Guenther, 2016). The neurological abnormalities underlying impaired Rate, in particular, may be more widespread, as recent work found that Rate remained unchanged for speakers with basal ganglia deficits (Rusz, Tykalova, Novotny, Zogala, et al., 2021).
Contrary to prior work, Precision was not significantly reduced compared to controls or the other clinical groups. This finding was surprising given that hypokinesia secondary to PD has been known to downscale articulatory displacements (Ackermann & Ziegler, 1991; Darley et al., 1969a, 1969b; Darling & Huber, 2011; Forrest et al., 1989; Walsh & Smith, 2012; Yunusova et al., 2008, 2011). Numerous studies have found that speakers with PD tend to have reduced vowel dispersion compared to speakers with ALS (Mefferd, 2015), reduced vowel area index compared to speakers with PSP (Skodda et al., 2012), reduced consonant closure compared to speakers with HD and PA (Ackermann et al., 1995), and reduced second formant interquartile range compared to speakers with MS (Kuo & Tjaden, 2016). Given these prior findings, it is possible that second formant slope variability does not adequately capture the imprecision characteristic of hypokinetic dysarthria. However, our negative findings for Precision may also be due to the mild severity of the speakers. Additional work is needed to further investigate the manifestation of imprecision in speakers with PD across the severity continuum.
In sum, the articulatory phenotype of PD in our study tended to be less impaired than those of the other speakers, but two notable articulatory abnormalities were (1) reduced Speed and repetition Rate relative to controls and (2) preserved repetition Rate relative to speakers in the other clinical groups.
nfPPA+PAOS Phenotype: Impaired Coordination, Speed, Precision, and Rate Compared to Controls, but Only Impaired Coordination and Rate Compared to Other Clinical Groups
For speakers with nfPPA+PAOS, our findings align with the primary perceptual speech characteristics associated with PAOS: slow rate, distorted substitutions, syllable segregation, excess/equal stress, and poorly sequenced SMRs (Duffy, 2006; Duffy et al., 2021). First, our finding of impaired Speed and Rate align with the perceptual characteristic of slow rate, which is also consistent with recent acoustic work demonstrating reduced articulatory and repetition Rate and lengthened vowels in PAOS (Cordella et al., 2017, 2019; Duffy et al., 2015, 2017; Laganaro et al., 2012; Takakura et al., 2019; Utianski et al., 2018; van der Merwe, 2009; Wilson et al., 2010). Similarly, our finding of reduced Coordination may capture perceptual characteristics of PAOS such as syllable segregation and poorly sequenced SMRs. Indeed, we found that speakers with nfPPA+PAOS produced abnormally long gaps before each new syllable (i.e., between /pa/ and /ta/, /ta/ and /ka/, and /ka/ and subsequent /pa/). Prior work has demonstrated similar findings of segmented syllables both in speakers with PAOS (Bouvier et al., 2021; Duffy, 2006; Takakura et al., 2019) and non-degenerative AOS (Deger & Ziegler, 2002; Kent & Rosenbek, 1983; Melle & Gallego, 2012; Staiger & Ziegler, 2008). While pausing between syllables could be a compensatory behavior, pausing during a maximum performance task, in which speakers are asked to repeat sounds as quickly as possible, is more likely indicative of the extra time needed to sequence and specify the motor program (Strand et al., 2014). Atypical pausing between syllables may also be associated with articulatory groping (Shriberg et al., 2017a, 2017b, 2017c, 2017d). Neural models of AOS have proposed that groping reflects an inability to access subsequent speech units from the left premotor cortex (Guenther, 2016; Miller & Guenther, 2021). From this perspective, the observed longer gaps in the nfPPA+PAOS group can be interpreted as discoordination at the planning level. Research continues to explore the link between areas of brain atrophy (e.g., gray matter of premotor, supplementary motor, and primary motor cortices, bilaterally; Josephs et al., 2012) and the clinical manifestations of PAOS.
One surprising finding for this clinical group was preserved Consistency using across-repetition variability in VOT. This finding was surprising considering prior work which has shown that changes in VOT are sensitive to incorrect sound productions in speakers with AOS (Wambaugh et al., 1997), and sound substitutions are a common error type in AOS (Strand et al., 2014). However, although VOT tends to change as position of occlusion changes (Baum & Ryan, 1993; Klatt, 1975), the range of VOT values can overlap across different voiceless sounds (Auzou et al., 2000). Therefore, inconsistencies in substitution patterns may not have been evident using our Consistency measure with this specific task.
In sum, the articulatory phenotype of nfPPA+PAOS in our study was characterized by (1) impaired Coordination, Speed, and repetition Rate relative to controls and (2) impaired Coordination and repetition Rate relative to speakers in the other clinical groups.
PA Phenotype: Impaired Consistency, Speed, Precision, and Rate Compared to Controls, but Only Impaired Consistency Compared to Other Clinical Groups
Consistent with our findings, deficits in Speed, Precision, and Rate have been documented in prior acoustic work on speakers with PA (Speed: Folker et al., 2012; Lansford & Liss, 2014; Precision: Folker et al., 2012; Skodda et al., 2013; Rate: Ozawa et al., 2001; Schalling et al., 2007; Singh et al., 2010; Tykalova et al., 2016) and in the DAB classification of ataxic dysarthria (Darley et al., 1969a, 1969b). Our finding of reduced Consistency in speakers with PA relative to controls is also congruent with prior work (Brendel et al., 2015; Darley et al., 1969a, 1969b; Gentil, 1990; Kashyap et al., 2018; Portnoy & Aronson, 1982; Tykalova et al., 2016), which reflects the effects of cerebellar damage on motor function in individuals with PA (Schmahmann, 2004). Indeed, the cerebellum is thought to regulate the timing among movements in a sequence (Guenther, 2016; Spencer & Slocomb, 2007; Tourville & Guenther, 2011), particularly for behaviors that involve explicit temporal goals (Ivry et al., 2002; Schmitz-Hübsch et al., 2012), such as the SMR task. Thus, in contrast to the substitution errors seen in speakers with nfPPA+PAOS, inconsistency secondary to cerebellar deficits can manifest as variability in movement timing (Ziegler et al., 1993; Ziegler & Wessel, 1996), such as timing of laryngeal control, which was reflected in our measure of across-repetition variability in VOT. Beyond appropriate timing of movements, the cerebellum is responsible for fine-tuning or refining movements (Chiu et al., 1996; Guenther, 2016; Kent et al., 1979). Several authors have hypothesized that in ataxic dysarthria, feedforward motor commands are disrupted, which forces the motor control system to rely on sensory feedback commands (Guenther, 2016; Parrell et al., 2017). The reliance on sensory feedback, which requires longer delays, may result in lengthened speech segments (Kent et al., 1979) and, therefore, may contribute to findings of reduced Speed and Rate in speakers with PA.
Our finding of intact Coordination was surprising given that incoordination is often cited as a key deficit associated with cerebellar dysfunction (Ackermann et al., 2007; Ackermann & Hertrich, 1997; Darley et al., 1969a, 1969b; Kent et al., 1979, 1997; Tykalova et al., 2016; Vogel et al., 2018). Furthermore, previous work has identified increased gap durations for speakers with ataxia in the DDK task (Ozawa et al., 2001; Schalling et al., 2007; Schalling & Hartelius, 2004). However, we examined the proportion of gap to syllable duration, which will be smaller if the syllable is longer. Ozawa and colleagues also examined this proportion in speakers with ataxia and, similar to our results, found no significant differences from controls (Ozawa et al., 2001). The results for speakers with PA in conjunction with those of the nfPPA+PAOS group suggest that incoordination may manifest differently depending on the neurological/pathophysiological abnormalities. Indeed, while both AOS and ataxic dysarthria may lead to disruptions in motor planning/programming (Kent et al., 1997, 2000; Spencer & Rogers, 2005), cortical thinning in the left frontal lobe—as seen in AOS—may manifest in difficulty with accessing the motor plan for the subsequent gesture, which we quantified using a measure of syllable transition; on the other hand, cerebellar deficits—as seen in ataxic dysarthria—may be best quantified using measures of movement smoothness to reflect deficits in timing (Chang et al., 2005; Mark & Steve, 1993). The distinction in how Coordination manifests depending on the loci of neurological deficit highlights the need for more than one operational definition of Coordination. Identifying the precise construct of interest will allow researchers to develop quantitative measures that more accurately represent the articulatory deficit specific to a certain clinical group. Importantly, prior work has shown that speakers with ataxic dysarthria tend to perform better on SMRs than on AMRs (Duffy, 2013), which may have also contributed to our null findings for Coordination. In addition to the influence of task, our findings may have been influenced by our assessment of only three repetitions, as it is possible that such a small number of repetitions is insufficient for capturing discoordination in this population. Future work should further investigate measures of Coordination—in a more sensitive task for the population (i.e., AMRs, more repetitions)—that can detect the coordinative deficits characteristic of speakers with PA.
Taken together, the articulatory phenotype for speakers with PA in our study was characterized by (1) deficits across all articulatory components except Coordination (as indexed by intersyllabic pausing) relative to controls and (2) impaired Consistency relative to the other clinical groups.
ALS Phenotype: Impaired Speed, Precision, and Rate Compared to Controls, but No Salient Differentiator from Other Clinical Groups
In contrast to hypokinetic dysarthria, AOS, and ataxic dysarthria, the neural mechanisms underlying mixed spastic-flaccid dysarthria secondary to ALS are less conclusive. Flaccid dysarthria results from damage to the lower motor neurons (LMNs), which are known as the final common pathway for transmitting neural information to the muscles. The nerves that are involved thus determine which articulators are affected. For example, an impaired trigeminal nerve will likely result in jaw weakness and an impaired hypoglossal nerve will likely result in tongue weakness. Although the disease initially manifests in different body locations depending on the person (e.g., some speakers initially present with tongue weakness, whereas others may present with leg weakness), eventually all nerves become affected, which, in the case of speech, results in broad deficits across multiple articulatory domains (Samlan & Weismer, 1995; Weismer & Green, 2015). Darley and colleagues posit that this general reduction in muscle strength may contribute to imprecise consonant production in speakers with flaccid dysarthria (Darley et al., 1969a, 1969b), which aligns with our findings and those in prior acoustic studies (Lee et al., 2017; Lee & Fischer, 2019; Turner et al., 1995; Yunusova et al., 2005, 2012).
The spastic component of dysarthria involves damage to the upper motor neurons (UMNs), which are responsible for carrying information from the primary cortex to the LMNs. Damage to the UMNs is associated with muscle spasticity, which may result in slow and labored speech (Darley et al., 1969a, 1969b; Langmore & Lehman, 1994). Indeed, consistent with previous acoustic literature, we found reduced Speed and repetition Rate in speakers with ALS (Speed: Lansford & Liss, 2014; Mulligan et al., 1994; Weismer et al., 1992, 2001; Yunusova et al., 2012; Rate: Mulligan et al., 1994; Rong, 2020; Shellikeri et al., 2021).
In sum, a divergent profile for ALS emerged when examining differences in the five articulatory components relative to controls. No articulatory components, however, distinguished speakers with ALS from the other three clinical groups. This finding may reflect the heterogeneity in bulbar involvement and neuropathology across individuals with ALS (DePaul et al., 1988; Langmore & Lehman, 1994; Lawyer & Netsky, 1953; Shellikeri et al., 2017; Stipancic et al., 2021). Future work is needed to further investigate the potential within-group phenotypic variability in speakers with ALS.
Lack of Redundancy among Acoustic Features Regardless of Clinical Diagnosis and Different Relationships between Acoustic Features Depending on Clinical Diagnosis
An important consideration in this study was whether the five features were assessing different constructs and, therefore, contributing unique information to the characterization of articulatory function for each neurodegenerative disease. This objective is typically addressed by examining pairwise correlations between the constructs that are hypothesized to be distinct (Streiner & Norman, 2015). Overall, examining the associations between performance on the acoustic measures demonstrated (1) a lack of redundancy among the five acoustic features regardless of the clinical diagnosis and (2) different relationships between features depending on the clinical diagnosis. First, pairwise analyses revealed weak to moderate correlations between most pairs of acoustic features for the clinical groups as a whole and for each clinical group individually, which indicates a lack of redundancy in the measures and that investigating all five constructs may be important for fully characterizing articulatory function for each disease. Although two of the measures (i.e., Coordination and Consistency) have since been updated, the weak to moderate correlations we found in this study are consistent with our prior work assessing the divergent validity of the five features in this framework (Rowe et al., 2021; Rowe & Green, 2019). Importantly, while only a small number of the associations in the individual clinical group results were significant (two to three out of 10 per clinical group), including all participants in the analysis resulted in more significant correlations (five out of 10 for the whole group). This finding suggests that the lack of significant associations in the individual group results may be related to insufficient statistical power.
We also found distinct relationships between articulatory constructs depending on the clinical group, which may provide additional disease-specific knowledge about the manifestation of different neurological deficits. For example, Speed and Precision were strongly correlated in speakers with ALS but exhibited a negligible association in speakers with nfPPA+PAOS. The strong positive correlation in ALS suggested that most speakers who exhibited reduced Speed also exhibited reduced Precision, which may reflect the uniform effects of muscle weakness on these articulatory constructs. While a correlation of .73 does not indicate redundancy or multicollinearity, a strong correlation between these two constructs suggests that, for speakers with ALS, measuring Speed and Precision will provide similar information about articulatory function. The weak Speed-Precision relationship in speakers with nfPPA+PAOS, on the other hand, indicate that performance on the two constructs may diverge in this population. Interestingly, the correlation between Speed and displacement, the latter of which is a measure of Precision, has been used in prior studies to index neuromuscular coordination, with lower correlations indicating a decoupling of motor systems (Missitzi et al., 2004). Thus, the differences in the Speed-Precision relationship in ALS compared to nfPPA+PAOS offer further information about articulatory function for each group.
Classification Accuracy of Articulatory Phenotypes
Use of Individual Features versus Profiles of Features for Classifying Clinical Groups
In addition to validating the use of our acoustic-based framework for characterizing articulatory abnormalities across multiple divergent populations, this study investigated its utility for classifying neurodegenerative diseases. To that end, it was important to consider whether a profile of features was more diagnostically useful than individual features. When considering all five acoustic measures in the classification of the four clinical groups, our ROC analysis based on the multivariate LDA yielded good to excellent average AUCs for distinguishing PA (.80), PD (.83), and nfPPA+PAOS (.95) from the other clinical groups but a poor average AUC for distinguishing ALS (.64). One potential reason for the poor performance in classifying ALS is the lack of significant differences in articulatory performance in the between-clinical group comparisons, which may be related to the subtypes within ALS, such as bulbar- versus spinal-onset or spastic- versus flaccid-dominant dysarthria.
Consistent with prior work (Ballard et al., 2016; Basilakos et al., 2017), we found that a combination of features exhibited stronger performance than individual features with classifying clinical groups, suggesting that a profile was able to capture the diversity in articulatory abnormalities more adequately across groups. Both Ballard and Basilakos and colleagues (2016 and 2017, respectively) demonstrated that combining measures that were significantly impaired in AOS (e.g., pairwise variability index and envelope modulation spectrum) resulted in improved classification accuracy (Ballard et al., 2016; Basilakos et al., 2017). The improvement in performance in our study, however, may provide support for examining features that are both impaired and spared. Indeed, a closer examination of the univariate analysis revealed that even features that were unaffected, such as Coordination in ALS and Consistency in PD, exhibited acceptable specificity and/or sensitivity for classification of the respective clinical groups. Taken together, our findings suggest that the acoustic-based profiles we identified in this study have promising clinical utility for informing the classification of PA, PD, and nfPPA+PAOS.
LIMITATIONS
There are several limitations of this study. While we assigned acoustic features to each articulatory component based on descriptions and definitions used in prior literature, the components can manifest in a variety of ways. Our definition of “appropriate temporal alignment of movements to meet task demands” can subsume coordinative indices such as movement smoothness, motor equivalence, and timing/sequencing (Caruso et al., 1988), all of which can be measured using different features. Thus, a measure that reflects Coordination for one population may not detect this deficit in another population known to have incoordination, as we saw in our findings for speakers with PA.
Second, although our statistical testing suggested that remotely recording speech using the Beiwe app had no measurable effect on the acoustic features we extracted, more work is needed to understand the potential limitations of using smartphone-based speech analytic platforms for recording speech in a home setting.
Third, while we controlled for articulatory severity in the ALS, PA, and nfPPA+PAOS groups by restricting the severity range, there were significant differences in categorical severity ratings between PD and ALS, and in continuous severity ratings between PD and all other clinical groups. Although the severity ratings of all four clinical groups were similar, additional work is needed to examine the articulatory phenotypes of groups that are fully matched on articulatory severity. Similarly, we controlled for sex and age by conducting independent t-tests and Pearson correlations, respectively, which revealed no significant differences or correlations with the acoustic measures. However, it is important to note that lack of significant effects does not indicate that there was no influence of these variables on our findings. Indeed, two recent studies demonstrated a significant effect of sex and age on voice onset time and sequential motion rate in de-novo Parkinsonian speakers with and healthy controls (Rusz et al., 2022; Rusz, Tykalova, Novotny, Ruzicka, et al., 2021). Thus, our findings should be considered within the context of this work.
Furthermore, our study did not consider potential subtypes within each clinical group (e.g., bulbar- versus spinal-onset ALS, phonetic versus prosodic PAOS, tremor-dominant PD versus PD characterized by postural instability and gait disorders). Similarly, speakers in the nfPPA+PAOS group may perform differently on the SMR task depending on their level of concomitant aphasia (Henry et al., 2016), as speakers with severe aphasia may produce more errors that are driven by phonological deficits rather than motoric deficits. While PPA and PAOS often co-occur in neurodegenerative cases (Gorno-Tempini et al., 2011), PAOS can exist in the absence of aphasia (Josephs et al., 2012). Future work should, therefore, investigate primary PAOS in speakers with no/negligible aphasia to further contribute to our understanding of the articulatory characteristics in the potential phonetic and prosodic subtypes of primary PAOS (Utianski et al., 2018). Overall, identifying the articulatory characteristics that distinguish clinical subtypes would provide valuable information that may further inform diagnostic and treatment decision-making.
Given that some of the data were acquired from secondary sources, we did not have access to disease severity, disease duration, and medication status for all participants in the study. While not controlling for these variables may limit the generalizability of our findings, we do not view the absence of this information as a confound for our results. The purpose of this study was to examine disease classification accuracy based solely on speech function, and there are many cases where speech severity is distinct from disease severity, duration, or medication use (Ball et al., 2002; Barnett et al., 2020; Cavallieri et al., 2021; Fabbri et al., 2017; Rusz, Tykalova, Novotny, Zogala, et al., 2021; Skodda, Flasskamp, et al., 2011; Tykalova et al., 2022). Thus, given the study’s focus on speech function, we believe the most critical component to control for was speech severity.
Finally, although weak pairwise correlations suggest a lack of redundancy in the five features, we cannot conclude that the five components are all necessary and sufficient for characterizing articulatory function. In addition, while we found that the multivariate LDA performed better than the univariate LDAs in classifying the clinical groups, we did not examine the performance of different combinations of the five features (e.g., Coordination and Rate), and we do not know the extent to which adding even more features will further increase the classification accuracy. As indicated in the Methods, the features we used to represent each component are considered candidate measures that should be used to generate testable hypotheses and continually revisited and refined in light of new knowledge or evidence. Similarly, we derived our features from the SMR task, which may reveal different articulatory deficits than would a connected speech task. A sentence production task, for example, would allow for a more functional assessment of the speaker’s articulatory abilities in different phonetic contexts. Future work is thus needed to further investigate the optimal task and number of features for both characterizing and classifying neurodegenerative diseases.
CONCLUSIONS AND CLINICAL IMPLICATIONS
This study sought to assess the articulatory phenotypes of four neurodegenerative populations known to have divergent speech motor deficits and determine the efficacy of articulatory phenotyping for classifying different diseases. We aimed to address a primary limitation of the current differential diagnosis literature by expanding the range of neurological/pathophysiological deficits and articulatory characteristics examined in one study. We found evidence of distinct articulatory phenotypes for the four clinical groups (i.e., ALS, PA, PD, and nfPPA+PAOS), which highlights the phenotypic variability present across neurodegenerative diseases. Additionally, the phenotypes demonstrated strong classification accuracy for characterizing neurodegenerative diseases, which emphasizes the potential clinical utility of using a comprehensive profile of articulation. With further research, speech phenotyping could (1) provide diagnostic information about different movement disorders and (2) guide the development of sensitive outcome measures. Future work should continue to investigate the efficacy of this framework for identifying articulatory profiles across and within speech motor populations and for developing new features that best reflect the articulatory characteristics of populations with divergent neurological/pathophysiological deficits.
ACKNOWLEDGEMENTS
This work was supported by the following grants awarded by the National Institute on Deafness and Other Communication Disorders (NIDCD), part of the National Institute of Health (NIH): R01DC013547 (PI: Jordan R. Green), K24DC016312 (PI: Jordan R. Green), R01DC014296 (PI: Bradford C. Dickerson), and F31DC019556 (PI: Hannah P. Rowe). The authors would also like to thank Dr. Kris Tjaden from the University at Buffalo, Dr. John Sidtis from New York University, and Megan Quimby from Massachusetts General Hospital (MGH) for generously sharing their datasets and the University of Wisconsin–Madison for making the X-Ray Microbeam Speech Production (XRMB) Database available.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from MGH, New York University, University of Buffalo, University of Strathclyde, and University of Washington, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the respective institution.
REFERENCES
- Ackermann H (2008). Cerebellar contributions to speech production and speech perception: Psycholinguistic and neurobiological perspectives. Trends in Neurosciences, 31(6), 265–272. [DOI] [PubMed] [Google Scholar]
- Ackermann H, & Hertrich I (1997). Voice onset time in ataxic dysarthria. Brain and Language, 56(3), 321–333. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Hertrich I, & Hehr T (1995). Oral diadochokinesis in neurological dysarthrias. Folia Phoniatrica et Logopaedica, 47(1), 15–23. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Mathiak K, & Riecker A (2007). The contribution of the cerebellum to speech production and speech perception: Clinical and functional imaging data. The Cerebellum, 6(3), 202–213. [DOI] [PubMed] [Google Scholar]
- Ackermann H, & Ziegler W (1991). Articulatory deficits in Parkinsonian dysarthria: An acoustic analysis. Journal of Neurology, Neurosurgery & Psychiatry, 54(12), 1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison KM, Yunusova Y, Campbell TF, Wang J, Berry JD, & Green JR (2017). The diagnostic utility of patient-report and speech-language pathologists’ ratings for detecting the early onset of bulbar symptoms due to ALS. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 18(5–6), 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auzou P, Ozsancak C, Morris RJ, Jan M, Eustache F, & Hannequin D (2000). Voice onset time in aphasia, apraxia of speech and dysarthria: A review. Clinical Linguistics and Phonetics, 14(2), 131–150. [Google Scholar]
- Awan SN, Roy N, & Dromey C (2009). Estimating dysphonia severity in continuous speech: Application of a multi-parameter spectral/cepstral model. Clinical Linguistics and Phonetics, 23(11), 825–841. [DOI] [PubMed] [Google Scholar]
- Ball L, Beukelman D, & Pattee G (2002). Timing of speech deterioration in people with amyotrophic lateral sclerosis. Journal of Medical Speech-Language Pathology, 10(4), 231–235. [Google Scholar]
- Ballard KJ, Azizi L, Duffy JR, McNeil MR, Halaki M, O’Dwyer N, Layfield C, Scholl DI, Vogel AP, & Robin DA (2016). A predictive model for diagnosing stroke-related apraxia of speech. Neuropsychologia, 81, 129–139. [DOI] [PubMed] [Google Scholar]
- Barnett C, Green JR, Marzouqah R, Stipancic KL, Berry JD, Korngut L, Genge A, Shoesmith C, Briemberg H, Abrahao A, Kalra S, Zinman L, & Yunusova Y (2020). Reliability and validity of speech and pause measures during passage reading in ALS. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 21(1–2), 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilakos A, Yourganov G, den Ouden D-B, Fogerty D, Rorden C, Feenaughty L, & Fridriksson J (2017). A multivariate analytic approach to the differential diagnosis of apraxia of speech. Journal of Speech, Language, and Hearing Research, 60(12), 3378–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista P, & Pereira A (2016). Quality of life in patients with neurodegenerative diseases. Journal of Neurology and Neuroscience, 7(1), 1–7. [Google Scholar]
- Baum SR, & Ryan L (1993). Rate of speech effects in aphasia: Voice onset time. Brain and Language, 44, 431–445. [DOI] [PubMed] [Google Scholar]
- Becker J, Barbe MT, Hartinger M, Dembek TA, Pochmann J, Wirths J, Allert N, Mücke D, Hermes A, Meister IG, Visser-Vandewalle V, Grice M, & Timmermann L (2017). The effect of uni- and bilateral thalamic deep brain stimulation on speech in patients with essential tremor: Acoustics and intelligibility. Neuromodulation: Technology at the Neural Interface, 20(3), 223–232. [DOI] [PubMed] [Google Scholar]
- Berisha V, Krantsevich C, Hahn PR, Hahn S, Dasarathy G, Turaga P, & Liss J (2021). Digital medicine and the curse of dimensionality. Npj Digital Medicine, 4(153), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JD, Paganoni S, Carlson K, Burke K, Weber H, Staples P, Salinas J, Chan J, Green JR, Connaghan K, Barback J, & Onnela JP (2019). Design and results of a smartphone-based digital phenotyping study to quantify ALS progression. Annals of Clinical and Translational Neurology, 6(5), 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet PM, & Snyder GJ (2009). Speech rate deficits in individuals with Parkinson’s disease: A review of the literature. Journal of Medical Speech-Language Pathology, 17(1), 1–7. [Google Scholar]
- Boersma P (2014). The use of Praat in corpus research. In Durand J, Gut U, & Kristoffersen G (Eds.), The Oxford handbook of corpus phonology (pp. 342–360). Oxford University Press. [Google Scholar]
- Borrie SA, McAuliffe MJ, & Liss JM (2012). Perceptual learning of dysarthric speech: A review of experimental studies. Journal of Speech, Language, and Hearing Research, 55(1), 290–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier L, Monetta L, Vitali P, Laforce R Jr., & Martel-Sauvageau V (2021). A preliminary look into the clinical evolution of motor speech characteristics in primary progressive apraxia of speech in Québec French. American Journal of Speech-Language Pathology, 30, 1459–1476. [DOI] [PubMed] [Google Scholar]
- Braak H, Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, & Braak E (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging, 24(2), 197–211. [DOI] [PubMed] [Google Scholar]
- Brendel B, Synofzik M, Ackermann H, Lindig T, Schölderle T, Schöls L, & Ziegler W (2015). Comparing speech characteristics in spinocerebellar ataxias type 3 and type 6 with Friedreich ataxia. Journal of Neurology, 262(1), 21–26. [DOI] [PubMed] [Google Scholar]
- Brooks B, Miller R, Swash M, & Munsat T (2000). El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders, 1, 293–299. [DOI] [PubMed] [Google Scholar]
- Caruso AJ, Abbs JH, & Gracco VL (1988). Kinematic analysis of multiple movement coordination during speech in stutterers. Brain, 111(2), 439–455. [DOI] [PubMed] [Google Scholar]
- Cavallieri F, Budriesi C, Gessani A, Contardi S, Fioravanti V, Menozzi E, Pinto S, Moro E, Valzania F, & Antonelli F (2021). Dopaminergic treatment effects on dysarthric speech: Acoustic analysis in a cohort of patients with advanced Parkinson’s disease. Frontiers in Neurology, 11, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J-J, Wu T-I, Wu W-L, & Su F-C (2005). Kinematical measure for spastic reaching in children with cerebral palsy. Clinical Biomechanics, 20(4), 381–388. [DOI] [PubMed] [Google Scholar]
- Chen WS, & Alwan A (2000). Place of articulation cues for voiced and voiceless plosives and fricatives in syllable-initial position. 6th International Conference on Spoken Language Processing, 1–4. [Google Scholar]
- Chiu M-J, Chen R-C, & Tseng C-Y (1996). Clinical correlates of quantitative acoustic analysis in ataxic dysarthria. European Neurology, 36, 310–314. [DOI] [PubMed] [Google Scholar]
- Chiu Y-F, Forrest K, & Loux T (2019). Relationship between F2 slope and intelligibility in Parkinson’s disease: Lexical effects and listening environment. American Journal of Speech-Language Pathology, 28(2S), 887–894. [DOI] [PubMed] [Google Scholar]
- Connor NP, Ludlow CL, & Schulz GM (1989). Stop consonant production in isolated and repeated syllables in Parkinson’s disease. Neuropsychologia, 27(6), 829–838. [DOI] [PubMed] [Google Scholar]
- Cordella C, Dickerson BC, Quimby M, Yunusova Y, & Green JR (2017). Slowed articulation rate is a sensitive diagnostic marker for identifying non-fluent primary progressive aphasia. Aphasiology, 31(2), 241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordella C, Quimby M, Touroutoglou A, Brickhouse M, Dickerson BC, & Green JR (2019). Quantification of motor speech impairment and its anatomic basis in primary progressive aphasia. Neurology, 92(17), e1992–e2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoudi K, Das B, Milhé De Saint Victor S, Foubert-Samier A, Pavy-Le Traon A, Rascol O, Meissner WG, & Woisard V (2021). Distortion of voiced obstruents for differential diagnosis between parkinson’s disease and multiple system atrophy. Interspeech. [Google Scholar]
- Darley FL, Aronson AE, & Brown JR (1969a). Clusters of deviant speech dimensions in the dysarthrias. Journal of Speech and Hearing Research, 12, 462–496. [DOI] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, & Brown JR (1969b). Differential diagnostic patterns of dysarthria. Journal of Speech and Hearing Research, 12, 246–269. [DOI] [PubMed] [Google Scholar]
- Darling M, & Huber JE (2011). Changes to articulatory kinematics in response to loudness cues in individuals with Parkinson’s disease. Journal of Speech, Language, and Hearing Research, 54(5), 1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bodt MS, Hernández-Díaz Huici ME, & Van De Heyning PH (2002). Intelligibility as a linear combination of dimensions in dysarthric speech. Journal of Communication Disorders, 35(3), 283–292. [DOI] [PubMed] [Google Scholar]
- Deger K, & Ziegler W (2002). Speech motor programming in apraxia of speech. Journal of Phonetics, 30(3), 321–335. [Google Scholar]
- Delattre PC, Liberman AM, & Cooper FS (1955). Acoustic loci and transitional cues for consonants. The Journal of the Acoustical Society of America, 27(4), 769–773. [Google Scholar]
- Delattre PC, Liberman AM, & Cooper FS (1960). Second and third formant transitions in English fricatives. The Journal of the Acoustical Society of America, 32(11), 1501–1501. [Google Scholar]
- DePaul R, Abbs JH, Caligiuri M, Gracco VL, & Brooks BR (1988). Hypoglossal, trigeminal, and facial motoneuron involvement in amyotrophic lateral sclerosis. Neurology, 38, 281–283. [DOI] [PubMed] [Google Scholar]
- Duffy JR (2006). Apraxia of speech in degenerative neurologic disease. Aphasiology, 20(6), 511–527. [Google Scholar]
- Duffy JR (2013). Examination of motor speech disorders. In Duffy JR (Ed.), Motor speech disorders: Substrates, differential diagnosis, and management (pp. 69–104). Elsevier Health Sciences. [Google Scholar]
- Duffy JR (2020). Motor speech disorders: Substrates, differential diagnosis, and management. Elsevier Health Sciences. [Google Scholar]
- Duffy JR, Hanley H, Utianski R, Clark H, Strand E, Josephs KA, & Whitwell JL (2017). Temporal acoustic measures distinguish primary progressive apraxia of speech from primary progressive aphasia. Brain and Language, 168, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JR, Strand EA, Clark H, Machulda M, Whitwell JL, & Josephs KA (2015). Primary progressive apraxia of speech: Clinical features and acoustic and neurologic correlates. American Journal of Speech-Language Pathology, 24(2), 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JR, Strand EA, & Josephs KA (2014). Motor speech disorders associated with primary progressive aphasia. Aphasiology, 28(8–9), 1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JR, Utianski RL, & Josephs KA (2021). Primary progressive apraxia of speech: From recognition to diagnosis and care. Aphasiology, 35(4), 560–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M, Guimarães I, Cardoso R, Coelho M, Guedes LC, Rosa MM, Godinho C, Abreu D, Gonçalves N, Antonini A, & Ferreira JJ (2017). Speech and voice response to a levodopa challenge in late-stage Parkinson’s disease. Frontiers in Neurology, 8, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher SG (1972). Time-by-count measurement of diadochokinetic syllable rate. Journal of Speech and Hearing Research, 15(4), 763–770. [DOI] [PubMed] [Google Scholar]
- Folker JE, Murdoch BE, Rosen KM, Cahill LM, Delatycki MB, Corben LA, & Vogel AP (2012). Differentiating profiles of speech impairments in Friedreich’s ataxia: A perceptual and instrumental approach. International Journal of Language and Communication Disorders, 47(1), 65–76. [DOI] [PubMed] [Google Scholar]
- Forrest K, Weismer G, & Turner GS (1989). Kinematic, acoustic, and perceptual analyses of connected speech produced by Parkinsonian and normal geriatric adults. The Journal of the Acoustical Society of America, 85(6), 2608–2622. [DOI] [PubMed] [Google Scholar]
- Ge C, Xiong Y, & Mok P (2021). How reliable are phonetic data collected remotely? Comparison of recording devices and environments on acoustic measurements. Interspeech, 3984–3988. [Google Scholar]
- Gentil M (1990). Acoustic characteristics of speech in Friedreich’s disease. Folia Phoniatrica et Logopaedica, 42(3), 125–134. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, & Grossman M (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracco VL, & Abbs JH (1986). Variant and invariant characteristics of speech movements. Experimental Brain Research, 65(1), 156–166. [DOI] [PubMed] [Google Scholar]
- Green JR, Allison KM, Cordella C, Richburg BD, Pattee GL, Berry JD, Macklin EA, Pioro EP, & Smith RA (2018). Additional evidence for a therapeutic effect of dextromethorphan/quinidine on bulbar motor function in patients with amyotrophic lateral sclerosis: A quantitative speech analysis. British Journal of Clinical Pharmacology, 84(12), 2849–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JR, Yunusova Y, Kuruvilla MS, Wang J, Pattee GL, Synhorst L, Zinman L, & Berry JD (2013). Bulbar and speech motor assessment in ALS: Challenges and future directions. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 14(7–8), 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH (2016). Neural control of speech. The MIT Press. [Google Scholar]
- Hartelius L, Elmberg M, Holm R, Lövberg A-S, & Nikolaidis S (2008). Living with dysarthria: Evaluation of a self-report questionnaire. Folia Phoniatrica et Logopaedica, 60(1), 11–19. [DOI] [PubMed] [Google Scholar]
- Henry ML, Wilson SM, Babiak MC, Mandelli ML, Beeson PM, Miller ZA, & Gorno-Tempini ML (2016). Phonological processing in primary progressive aphasia. Journal of Cognitive Neuroscience, 28(2), 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertrich I, & Ackermann H (1994). Acoustic analysis of speech timing in Huntington’s disease. Brain and Language, 47, 182–196. [DOI] [PubMed] [Google Scholar]
- Hirose H (1986). Pathophysiology of motor speech disorders (dysarthria). Folia Phoniatrica et Logopaedica, 38, 61–88. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM, Zelaznik HN, & Diedrichsen J (2002). The cerebellum and event timing. Annals of the New York Academy of Sciences, 978, 302–317. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, Lowe VJ, Jack CR, & Whitwell JL (2012). Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain, 135(5), 1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap B, Pathirana PN, Horne M, Power L, & Szmulewicz D (2018). Quantitative assessment of syllabic timing deficits in ataxic dysarthria. 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 425–428. [DOI] [PubMed] [Google Scholar]
- Kent RD (1996). Hearing and believing: Some limits to the auditory-perceptual assessment of speech and voice disorders. American Journal of Speech-Language Pathology, 5(3), 7–23. [Google Scholar]
- Kent RD, Kent JF, Rosenbek JC, Vorperian HK, & Weismer G (1997). A speaking task analysis of the dysarthria in cerebellar disease. Folia Phoniatrica et Logopaedica, 49(2), 63–82. [DOI] [PubMed] [Google Scholar]
- Kent RD, Kent JF, Weismer G, & Duffy JR (2000). What dysarthrias can tell us about the neural control of speech. Journal of Phonetics, 28(3), 273–302. [Google Scholar]
- Kent RD, Kent JF, Weismer G, Martin RE, Sufit RL, Brooks BR, & Rosenbek JC (1989). Relationship between speech intelligibility and the slope of the second-formant transitions in dysarthric subjects. Clinical Linguistics and Phonetics, 3(4), 347–358. [Google Scholar]
- Kent RD, Kent RA, & Read C (2002). The acoustic analysis of speech. Singular Publishing Company. [Google Scholar]
- Kent RD, & Kim J-Y (2003a). Toward an acoustic typology of motor speech disorders. 17(6), 427–445. [DOI] [PubMed] [Google Scholar]
- Kent RD, Kim Y, & Chen L (2022). Oral and laryngeal diadochokinesis across the life span: A scoping review of methods, reference data, and clinical applications. Journal of Speech, Language, and Hearing Research, 65(2), 574–623. [DOI] [PubMed] [Google Scholar]
- Kent RD, & Kim Y-J (2003b). Towards an acoustic typology of motor speech disorders. Clinical Linguistics and Phonetics, 17(6), 427–445. [DOI] [PubMed] [Google Scholar]
- Kent RD, Netsell R, & Abbs JH (1979). Acoustic characteristics of dysarthria associated with cerebellar disease. Journal of Speech, Language, and Hearing Research, 22(3), 627–648. [DOI] [PubMed] [Google Scholar]
- Kent RD, & Rosenbek JC (1983). Acoustic patterns of apraxia of speech. Journal of Speech, Language, and Hearing Research, 26(2), 231–249. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kent RD, & Weismer G (2011). An acoustic study of the relationships among neurologic disease, dysarthria type, and severity of dysarthria. Journal of Speech, Language, and Hearing Research, 54(2), 417–429. [DOI] [PubMed] [Google Scholar]
- Kim Y, Weismer G, Kent RD, & Duffy JR (2009). Statistical models of F2 slope in relation to severity of dysarthria. Folia Phoniatrica et Logopaedica, 61(6), 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JM, Watson M, & Loff GL (2012). Practice patterns of speech-language pathologists assessing intelligibility of dysarthric speech. Journal of Medical Speech-Language Pathology, 20(1), 1–17. [Google Scholar]
- Klatt DH (1975). Voice onset time, frication, and aspiration in word-initial consonant clusters. Journal of Speech and Hearing Research, 18(4), 686–706. [DOI] [PubMed] [Google Scholar]
- Klockgether T, Mariotti C, & Paulson HL (2019). Spinocerebellar ataxia. Nature Reviews Disease Primers, 5(24). [DOI] [PubMed] [Google Scholar]
- Kuo C, & Tjaden K (2016). Acoustic variation during passage reading for speakers with dysarthria and healthy controls. Journal of Communication Disorders, 62, 30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganaro M, Croisier M, Bagou O, & Assal F (2012). Progressive apraxia of speech as a window into the study of speech planning processes. Cortex, 48(8), 963–971. [DOI] [PubMed] [Google Scholar]
- Langmore SE, & Lehman ME (1994). Physiologic deficits in the orofacial system underlying dysarthria in amyotrophic lateral sclerosis. Journal of Speech, Language, and Hearing Research, 37(1), 28–37. [DOI] [PubMed] [Google Scholar]
- Lansford KL, & Liss JM (2014). Vowel acoustics in dysarthria: Speech disorder diagnosis and classification. Journal of Speech, Language, and Hearing Research, 57(1), 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansford KL, Liss JM, & Norton RE (2014). Free-classification of perceptually similar speakers with dysarthria. Journal of Speech, Language, and Hearing Research, 57(6), 2051–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawyer T, & Netsky MG (1953). Amyotrophic lateral sclerosis: A clinicoanatomic study of fifty-three cases. Archives of Neurology and Psychiatry, 69(2), 171–192. [DOI] [PubMed] [Google Scholar]
- Lee J, & Fischer JC (2019). Single word–based acoustic vowel space in individuals with dysarthria secondary to amyotrophic lateral sclerosis. Perspectives of the ASHA Special Interest Groups, 4(5), 1171–1188. [Google Scholar]
- Lee J, Hustad KC, & Weismer G (2014). Predicting speech intelligibility with a multiple speech subsystems approach in children with cerebral palsy. Journal of Speech, Language, and Hearing Research, 57(5), 1666–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Littlejohn MA, & Simmons Z (2017). Acoustic and tongue kinematic vowel space in speakers with and without dysarthria. International Journal of Speech-Language Pathology, 19(2), 195–204. [DOI] [PubMed] [Google Scholar]
- MacKay IRA (2014). Consonants. In MacKay IRA (Ed.), Acoustics in hearing, speech, and language sciences: An introduction (pp. 239–254). Pearson. [Google Scholar]
- Mark H, & Steve GM (1993). Physiologic studies of dysmetria in patients with cerebellar deficits. Canadian Journal of Neurological Sciences, 20(S3), S83–S92. [PubMed] [Google Scholar]
- Martel-Sauvageau V, & Tjaden K (2017). Vocalic transitions as markers of speech acoustic changes with STN-DBS in Parkinson’s disease. Journal of Communication Disorders, 70, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MathWorks. (2019). Matlab optimization toolbox (r2019a). The MathWorks. [Google Scholar]
- McNeil MR, Odell KH, Miller SB, & Hunter L (1995). Consistency, variability, and target approximation for successive speech repetitions among apraxic, conduction aphasic, and ataxic dysarthria speakers. Clinical Aphasiology, 23, 39–55. [Google Scholar]
- Mefferd A (2015). Articulatory-to-acoustic relations in talkers with dysarthria: A first analysis. Journal of Speech, Language, and Hearing Research, 58(3), 576–589. [DOI] [PubMed] [Google Scholar]
- Mefferd AS, Pattee GL, & Green JR (2014). Speaking rate effects on articulatory pattern consistency in talkers with mild ALS. Clinical Linguistics and Phonetics, 28(11), 799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei J, Desrosiers C, & Frasnelli J (2021). Machine learning for the diagnosis of Parkinson’s disease: A review of literature. Frontiers in Aging Neuroscience, 13, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melle N, & Gallego C (2012). Differential diagnosis between apraxia and dysarthria based on acoustic analysis. The Spanish Journal of Psychology, 15(2), 495–504. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M (2001). Primary progressive aphasia. Annals of Neurology, 49(4), 425–432. [PubMed] [Google Scholar]
- Miller HE, & Guenther FH (2021). Modelling speech motor programming and apraxia of speech in the DIVA/GODIVA neurocomputational framework. Aphasiology, 35(4), 424–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N (1992). Variability in speech dyspraxia. Clinical Linguistics and Phonetics, 6(1), 77–85. [DOI] [PubMed] [Google Scholar]
- Miravitlles M, Calle M, & Soler-Cataluña JJ (2012). Clinical phenotypes of COPD: Identification, definition and implications for guidelines. Archivos de Bronconeumología (English Edition), 48(3), 86–98. [DOI] [PubMed] [Google Scholar]
- Missitzi J, Geladas N, & Klissouras V (2004). Heritability in neuromuscular coordination: Implications for motor control strategies. Medicine and Science in Sports and Exercise, 36, 233–240. [DOI] [PubMed] [Google Scholar]
- Molloy W, & Clarnette R (1999). Standardized mini-mental state examination (SMMSE): A user’s guide. New Grange Press. [Google Scholar]
- Mulligan M, Carpenter J, Riddel J, Delaney MK, Badger G, Krusinski P, & Tandan R (1994). Intelligibility and the acoustic characteristics of speech in amyotrophic lateral sclerosis (ALS). Journal of Speech, Language, and Hearing Research, 37(3), 496–503. [DOI] [PubMed] [Google Scholar]
- Nishio M, & Niimi S (2006). Comparison of speaking rate, articulation rate and alternating motion rate in dysarthric speakers. Folia Phoniatrica et Logopaedica, 58(2), 114–131. [DOI] [PubMed] [Google Scholar]
- Ogar J, Slama H, Dronkers N, Amici S, & Luisa Gorno-Tempini M (2005). Apraxia of speech: An overview. Neurocase, 11(6), 427–432. [DOI] [PubMed] [Google Scholar]
- Orozco-Arroyave JR, Hönig F, Arias-Londoño JD, Vargas-Bonilla JF, Daqrouq K, Skodda S, Rusz J, & Nöth E (2016). Automatic detection of Parkinson’s disease in running speech spoken in three different languages. The Journal of the Acoustical Society of America, 139(1), 481–500. [DOI] [PubMed] [Google Scholar]
- Oytam Y, Neilson PD, & O’Dwyer NJ (2005). Degrees of freedom and motor planning in purposive movement. Human Movement Science, 24(5–6), 710–730. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Shiromoto O, Ishizaki F, & Watamori T (2001). Symptomatic differences in decreased alternating motion rates between individuals with spastic and with ataxic dysarthria: An acoustic analysis. Folia Phoniatrica et Logopaedica, 53(2), 67–72. [DOI] [PubMed] [Google Scholar]
- Pandolfo M (2008). Friedreich ataxia. Neurological Review, 65(10), 1296–1303. [DOI] [PubMed] [Google Scholar]
- Parrell B, Agnew Z, Nagarajan S, Houde J, & Ivry RB (2017). Impaired feedforward control and enhanced feedback control of speech in patients with cerebellar degeneration. The Journal of Neuroscience, 37(38), 9249–9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JL, Tanner K, Merrill RM, Shnowske L, & Roy N (2021). A field-based approach to establish normative acoustic data for healthy female voices. Journal of Speech, Language & Hearing Research, 64(3), 691–706. [DOI] [PubMed] [Google Scholar]
- Poole ML, Brodtmann A, Darby D, & Vogel AP (2017). Motor speech phenotypes of frontotemporal dementia, primary progressive aphasia, and progressive apraxia of speech. Journal of Speech, Language, and Hearing Research, 60(4), 897–911. [DOI] [PubMed] [Google Scholar]
- Portnoy RA, & Aronson AE (1982). Diadochokinetic syllable rate and regularity in normal and in spastic and ataxic dysarthric subjects. Journal of Speech and Hearing Disorders, 47(3), 324–328. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, & Deuschl G (2015). MDS clinical diagnostic criteria for Parkinson’s disease: MDS-PD Clinical Diagnostic Criteria. Movement Disorders, 30(12), 1591–1601. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Rochester SR (1973). The significance of pauses in spontaneous speech. Journal of Psycholinguistic Research, 2(1), 51–81. [DOI] [PubMed] [Google Scholar]
- Rong P (2020). Automated acoustic analysis of oral diadochokinesis to assess bulbar motor involvement in amyotrophic lateral sclerosis. Journal of Speech, Language, and Hearing Research, 63(1), 59–73. [DOI] [PubMed] [Google Scholar]
- Rong P, Yunusova Y, Wang J, & Green JR (2015). Predicting early bulbar decline in amyotrophic lateral sclerosis: A speech subsystem approach. Behavioural Neurology, 2015, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen KM, Kent RD, Delaney AL, & Duffy JR (2006). Parametric quantitative acoustic analysis of conversation produced by speakers with dysarthria and healthy speakers. Journal of Speech, Language, and Hearing Research, 49(2), 395–411. [DOI] [PubMed] [Google Scholar]
- Rowe HP, & Green JR (2019). Profiling speech motor impairments in persons with amyotrophic lateral sclerosis: An acoustic-based approach. Interspeech, 4509–4513. [Google Scholar]
- Rowe HP, Gutz SE, Maffei MF, & Green JR (2020). Acoustic-based articulatory phenotypes of amyotrophic lateral sclerosis and Parkinson’s disease: Towards an interpretable, hypothesis-driven framework of motor control. Interspeech, 4816–4820. [Google Scholar]
- Rowe HP, Stipancic KL, Lammert AC, & Green JR (2021). Validation of an acoustic-based framework of speech motor control: Assessing criterion and construct validity using kinematic and perceptual measures. Journal of Speech, Language, and Hearing Research, 64(12), 4736–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusz J, Benova B, Ruzickova H, Novotny M, Tykalova T, Hlavnicka J, Uher T, Vaneckova M, Andelova M, Novotna K, Kadrnozkova L, & Horakova D (2018). Characteristics of motor speech phenotypes in multiple sclerosis. Multiple Sclerosis and Related Disorders, 19, 62–69. [DOI] [PubMed] [Google Scholar]
- Rusz J, Bonnet C, Klempíř J, Tykalová T, Baborová E, Novotný M, Rulseh A, & Růžička E (2015). Speech disorders reflect differing pathophysiology in Parkinson’s disease, progressive supranuclear palsy and multiple system atrophy. Journal of Neurology, 262(4), 992–1001. [DOI] [PubMed] [Google Scholar]
- Rusz J, Cmejla R, Ruzickova H, & Ruzicka E (2011). Quantitative acoustic measurements for characterization of speech and voice disorders in early untreated Parkinson’s disease. The Journal of the Acoustical Society of America, 129(1), 350–367. [DOI] [PubMed] [Google Scholar]
- Rusz J, Tykalova T, Novotny M, Ruzicka E, & Dusek P (2021). Distinct patterns of speech disorder in early-onset and late-onset de-novo Parkinson’s disease. Npj Parkinson’s Disease, 7(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusz J, Tykalova T, Novotny M, Zogala D, Ruzicka E, & Dusek P (2022). Automated speech analysis in early untreated Parkinson’s disease: Relation to gender and dopaminergic transporter imaging. European Journal of Neurology, 29(1), 81–90. [DOI] [PubMed] [Google Scholar]
- Rusz J, Tykalova T, Novotny M, Zogala D, Sonka K, Ruzicka E, & Dusek P (2021). Defining speech subtypes in de novo Parkinson disease: Response to long-term levodopa therapy. Neurology, 97(21), e2124–e2135. [DOI] [PubMed] [Google Scholar]
- Rusz J, Tykalová T, Salerno G, Bancone S, Scarpelli J, & Pellecchia MT (2019). Distinctive speech signature in cerebellar and parkinsonian subtypes of multiple system atrophy. Journal of Neurology, 266(6), 1394–1404. [DOI] [PubMed] [Google Scholar]
- Safari S, Baratloo A, Elfil M, & Negida A (2016). Evidence based emergency medicine: Receiver operating curve and area under the curve. Emergency, 4(2), 111–113. [PMC free article] [PubMed] [Google Scholar]
- Samlan RA, & Weismer G (1995). The relationship of selected perceptual measures of diadochokinesis to speech intelligibility in dysarthric speakers with amyotrophic lateral sclerosis. American Journal of Speech-Language Pathology, 4(2), 9–13. [Google Scholar]
- Schalling E, Hammarberg B, & Hartelius L (2007). Perceptual and acoustic analysis of speech in individuals with spinocerebellar ataxia (SCA). Logopedics Phoniatrics Vocology, 32(1), 31–46. [DOI] [PubMed] [Google Scholar]
- Schalling E, & Hartelius L (2004). Acoustic analysis of speech tasks performed by three individuals with spinocerebellar ataxia. Folia Phoniatrica et Logopaedica, 56(6), 367–380. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD (2004). Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. The Journal of Neuropsychiatry and Clinical Neurosciences, 16(3), 367–378. [DOI] [PubMed] [Google Scholar]
- Schmitz-Hübsch T, Eckert O, Schlegel U, Klockgether T, & Skodda S (2012). Instability of syllable repetition in patients with spinocerebellar ataxia and Parkinson’s disease: Syllable repetition capacity in SCA and PD. Movement Disorders, 27(2), 316–319. [DOI] [PubMed] [Google Scholar]
- Schneider SL, Habich L, Weston ZM, & Rosen CA (2021). Observations and considerations for implementing remote acoustic voice recording and analysis in clinical practice. Journal of Voice, 21, 1–8. [DOI] [PubMed] [Google Scholar]
- Sevitz JS, Kiefer BR, Huber JE, & Troche MS (2021). Obtaining objective clinical measures during telehealth evaluations of dysarthria. American Journal of Speech-Language Pathology, 30(2), 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellikeri S, Green JR, Kulkarni M, Rong P, Martino R, Zinman L, & Yunusova Y (2016). Speech movement measures as markers of bulbar disease in amyotrophic lateral sclerosis. Journal of Speech, Language, and Hearing Research, 59(5), 887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellikeri S, Karthikeyan V, Martino R, Black SE, Zinman L, Keith J, & Yunusova Y (2017). The neuropathological signature of bulbar-onset ALS: A systematic review. Neuroscience and Biobehavioral Reviews, 75, 378–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellikeri S, Marzouqah R, Brooks BR, Zinman L, Green JR, & Yunusova Y (2021). Psychometric properties of rapid word-based rate measures in the assessment of bulbar amyotrophic lateral sclerosis: Comparisons with syllable-based rate tasks. Journal of Speech, Language, and Hearing Research, 64(11), 4178–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg LD, Strand EA, Fourakis M, Jakielski KJ, Hall SD, Karlsson HB, Mabie HL, McSweeny JL, Tilkens CM, & Wilson DL (2017a). A diagnostic marker to discriminate childhood apraxia of speech from speech delay: I. Development and description of the pause marker. Journal of Speech, Language, and Hearing Research, 60(4), S1096–S1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg LD, Strand EA, Fourakis M, Jakielski KJ, Hall SD, Karlsson HB, Mabie HL, McSweeny JL, Tilkens CM, & Wilson DL (2017b). A diagnostic marker to discriminate childhood apraxia of speech from speech delay: II. Validity studies of the pause marker. Journal of Speech, Language, and Hearing Research, 60(4), S1118–S1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg LD, Strand EA, Fourakis M, Jakielski KJ, Hall SD, Karlsson HB, Mabie HL, McSweeny JL, Tilkens CM, & Wilson DL (2017c). A diagnostic marker to discriminate childhood apraxia of speech from speech delay: III. Theoretical coherence of the pause marker with speech processing deficits in childhood apraxia of speech. Journal of Speech, Language, and Hearing Research, 60(4), S1135–S1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg LD, Strand EA, Fourakis M, Jakielski KJ, Hall SD, Karlsson HB, Mabie HL, McSweeny JL, Tilkens CM, & Wilson DL (2017d). A diagnostic marker to discriminate childhood apraxia of speech from speech delay: IV. The pause marker index. Journal of Speech, Language, and Hearing Research, 60(4), S1153–S1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidtis JJ, Ahn JS, Gomez C, & Sidtis D (2011). Speech characteristics associated with three genotypes of ataxia. Journal of Communication Disorders, 44(4), 478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Epstein E, Myers LM, Farmer JM, & Lynch DR (2010). Clinical measures of dysarthria in Friedreich ataxia. Movement Disorders, 25(1), 108–111. [DOI] [PubMed] [Google Scholar]
- Skodda S, Flasskamp A, & Schlegel U (2011). Instability of syllable repetition as a marker of disease progression in Parkinson’s disease: A longitudinal study. Movement Disorders, 26(1), 59–64. [DOI] [PubMed] [Google Scholar]
- Skodda S, Grönheit W, & Schlegel U (2012). Instability of syllable repetition in progressive supranuclear palsy. Journal of Neural Transmission, 119(4), 457–462. [DOI] [PubMed] [Google Scholar]
- Skodda S, Schlegel U, Klockgether T, & Schmitz-Hübsch T (2013). Vowel articulation in patients with spinocerebellar ataxia. International Journal of Speech and Language Pathology and Audiology, 1, 63–71. [Google Scholar]
- Skodda S, Visser W, & Schlegel U (2011). Acoustical analysis of speech in progressive supranuclear palsy. Journal of Voice, 25(6), 725–731. [DOI] [PubMed] [Google Scholar]
- Spencer KA, & France AA (2016). Perceptual ratings of subgroups of ataxic dysarthria: Subgroups of ataxic dysarthria. International Journal of Language and Communication Disorders, 51(4), 430–441. [DOI] [PubMed] [Google Scholar]
- Spencer KA, & Rogers MA (2005). Speech motor programming in hypokinetic and ataxic dysarthria. Brain and Language, 94(3), 347–366. [DOI] [PubMed] [Google Scholar]
- Spencer K, & Slocomb D (2007). The neural basis of ataxic dysarthria. The Cerebellum, 6(1), 58–65. [DOI] [PubMed] [Google Scholar]
- Staiger A, & Ziegler W (2008). Syllable frequency and syllable structure in the spontaneous speech production of patients with apraxia of speech. Aphasiology, 22(11), 1201–1215. [Google Scholar]
- Stipancic KL, Yunusova Y, Campbell TF, Wang J, Berry JD, & Green JR (2021). Two distinct clinical phenotypes of bulbar motor impairment in amyotrophic lateral sclerosis. Frontiers in Neurology, 12, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand EA, Duffy JR, Clark HM, & Josephs K (2014). The apraxia of speech rating scale: A tool for diagnosis and description of apraxia of speech. Journal of Communication Disorders, 51, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streiner DL, & Norman GR (2015). Validity. In Cairney J (Ed.), Health measurement scales: A practical guide to their development and use (5th ed., pp. 227–253). Oxford University Press. [Google Scholar]
- Takakura Y, Otsuki M, Sakai S, Tajima Y, Mito Y, Ogata A, Koshimizu S, Yoshino M, Uemori G, Takakura S, & Nakagawa Y (2019). Sub-classification of apraxia of speech in patients with cerebrovascular and neurodegenerative diseases. Brain and Cognition, 130, 1–10. [DOI] [PubMed] [Google Scholar]
- Titova N, & Chaudhuri KR (2017). Personalized medicine in Parkinson’s disease: Time to be precise. Movement Disorders, 32(8), 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden K, Richards E, Kuo C, Wilding G, & Sussman J (2013). Acoustic and perceptual consequences of clear and loud speech. Folia Phoniatrica et Logopaedica, 65(4), 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden K, & Watling E (2003). Characteristics of diadochokinesis in multiple sclerosis and Parkinson’s disease. Folia Phoniatrica et Logopaedica, 55(5), 241–259. [DOI] [PubMed] [Google Scholar]
- Tjaden K, & Wilding GE (2004). Rate and loudness manipulations in dysarthria: Acoustic and perceptual findings. Journal of Speech, Language, and Hearing Research, 47, 766–783. [DOI] [PubMed] [Google Scholar]
- Tourville JA, & Guenther FH (2011). The DIVA model: A neural theory of speech acquisition and production. Language and Cognitive Processes, 26(7), 952–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Watanabe H, Tanaka Y, Ohdake R, Yoneyama N, Hara K, Nakamura R, Watanabe H, Senda J, Atsuta N, Ito M, Hirayama M, Yamamoto M, Fujimoto Y, Kajita Y, Wakabayashi T, & Sobue G (2015). Distinct phenotypes of speech and voice disorders in Parkinson’s disease after subthalamic nucleus deep brain stimulation. Journal of Neurology, Neurosurgery, and Psychiatry, 86(8), 856–864. [DOI] [PubMed] [Google Scholar]
- Turner GS, Tjaden K, & Weismer G (1995). The influence of speaking rate on vowel space and speech intelligibility for individuals with amyotrophic lateral sclerosis. Journal of Speech, Language, and Hearing Research, 38(5), 1001–1013. [DOI] [PubMed] [Google Scholar]
- Tykalova T, Novotny M, Ruzicka E, Dusek P, & Rusz J (2022). Short-term effect of dopaminergic medication on speech in early-stage Parkinson’s disease. Npj Parkinson’s Disease, 8(1), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tykalova T, Pospisilova M, Cmejla R, Jerabek J, Mares P, & Rusz J (2016). Speech changes after coordinative training in patients with cerebellar ataxia: A pilot study. Neurological Sciences, 37(2), 293–296. [DOI] [PubMed] [Google Scholar]
- Tykalova T, Rusz J, Klempir J, Cmejla R, & Ruzicka E (2017). Distinct patterns of imprecise consonant articulation among Parkinson’s disease, progressive supranuclear palsy and multiple system atrophy. Brain and Language, 165, 1–9. [DOI] [PubMed] [Google Scholar]
- Utianski RL, Duffy JR, Clark HM, Strand EA, Botha H, Schwarz CG, Machulda MM, Senjem ML, Spychalla AJ, Jack CR, Petersen RC, Lowe VJ, Whitwell JL, & Josephs KA (2018). Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain and Language, 184, 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe A (2009). A theoretical framework for the characterization of pathological speech sensorimotor control. In McNeil MR (Ed.), Clinical management of sensorimotor speech disorders (2nd ed., pp. 3–18). Thieme Medical Publishers. [Google Scholar]
- Vogel AP, Poole ML, Pemberton H, Caverlé MWJ, Boonstra FMC, Low E, Darby D, & Brodtmann A (2017). Motor speech signature of behavioral variant frontotemporal dementia: Refining the phenotype. Neurology, 89(8), 837–844. [DOI] [PubMed] [Google Scholar]
- Vogel AP, Rommel N, Oettinger A, Stoll LH, Kraus E-M, Gagnon C, Horger M, Krumm P, Timmann D, Storey E, Schöls L, & Synofzik M (2018). Coordination and timing deficits in speech and swallowing in autosomal recessive spastic ataxia of Charlevoix–Saguenay (ARSACS). Journal of Neurology, 265(9), 2060–2070. [DOI] [PubMed] [Google Scholar]
- Walsh B, & Smith A (2012). Basic parameters of articulatory movements and acoustics in individuals with Parkinson’s disease: Articulatory Movement Parameters in PD. Movement Disorders, 27(7), 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh JL, West JE, & Doyle PJ (1997). A VOT analysis of apraxic/aphasic voicing errors. Aphasiology, 11(4/5), 521–532. [Google Scholar]
- Wang Y-T, Kent RD, Duffy JR, & Thomas JE (2009). Analysis of diadochokinesis in ataxic dysarthria using the Motor Speech Profile Program™. Folia Phoniatrica et Logopaedica, 61(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismer G, & Berry J (2003). Effects of speaking rate on second formant trajectories of selected vocalic nuclei. The Journal of the Acoustical Society of America, 113(6), 3362–3378. [DOI] [PubMed] [Google Scholar]
- Weismer G, & Green JR (2015). Speech production in motor speech disorders: Lesions, models, and a research agenda. In Redford MA (Ed.), The Handbook of Speech Production (1st ed., pp. 298–330). John Wiley & Sons, Inc. [Google Scholar]
- Weismer G, Jeng J-Y, Laures JS, Kent RD, & Kent JF (2001). Acoustic and intelligibility characteristics of sentence production in neurogenic speech disorders. Folia Phoniatrica et Logopaedica, 53(1), 1–18. [DOI] [PubMed] [Google Scholar]
- Weismer G, Martin R, Kent RD, & Kent JF (1992). Formant trajectory characteristics of males with amyotrophic lateral sclerosis. The Journal of the Acoustical Society of America, 91(2), 1085–1098. [DOI] [PubMed] [Google Scholar]
- Westbury JR (1994). X-ray microbeam speech production database user’s handbook. University of Wisconsin. [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, Miller BL, & Gorno-Tempini ML (2010). Connected speech production in three variants of primary progressive aphasia. Brain, 133(7), 2069–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SC, Profile S, York MK, Strutt AM, Schulz PE, Woolley SC, York MK, Moore DH, Strutt AM, Murphy J, Schulz PE, & Katz JS (2010). Detecting frontotemporal dysfunction in ALS: Utility of the ALS Cognitive Behavioral Screen (ALS-CBS). Amyotrophic Lateral Sclerosis, 11, 303–311. [DOI] [PubMed] [Google Scholar]
- Yunusova Y, Green JR, Greenwood L, Wang J, Pattee GL, & Zinman L (2012). Tongue movements and their acoustic consequences in amyotrophic lateral sclerosis. Folia Phoniatrica et Logopaedica, 64(2), 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunusova Y, Weismer GG, & Lindstrom MJ (2011). Classifications of vocalic segments from articulatory kinematics: Healthy controls and speakers with dysarthria. Journal of Speech, Language, and Hearing Research, 54(5), 1302–1311. [DOI] [PubMed] [Google Scholar]
- Yunusova Y, Weismer G, Kent R, & Rusche N (2005). Breath-group intelligibility in dysarthria characteristics and underlying correlates. Journal of Speech, Language, and Hearing Research, 48, 1294–1310. [DOI] [PubMed] [Google Scholar]
- Yunusova Y, Weismer G, Westbury J, & Lindstrom M (2008). Articulatory movements during vowels in speakers with dysarthria and healthy controls. Journal of Speech, Language, and Hearing Research, 51, 596–611. [DOI] [PubMed] [Google Scholar]
- Ziegler W (2002). Task-related factors in oral motor control: Speech and oral diadochokinesis in dysarthria and apraxia of speech. Brain and Language, 80(3), 556–575. [DOI] [PubMed] [Google Scholar]
- Ziegler W, Hartmann E, & Hoole P (1993). Syllabic timing in dysarthria. Journal of Speech and Hearing Research, 36, 683–693. [DOI] [PubMed] [Google Scholar]
- Ziegler W, & Wessel K (1996). Speech timing in ataxic disorders: Sentence production and rapid repetitive articulation. Neurology, 47(1), 208–214. [DOI] [PubMed] [Google Scholar]
- Zyski BJ, & Weisiger BE (1987). Identification of dysarthria types based on perceptual analysis. Journal of Communication Disorders, 20(5), 367–378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from MGH, New York University, University of Buffalo, University of Strathclyde, and University of Washington, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the respective institution.


