Abstract
Methods for the rapid detection of Escherichia coli and its related toxins are key to minimizing the risk of exposure to foodborne pathogens. The present study aimed to detect E. coli in food specimens using culture and polymerase chain reaction (PCR) techniques. One hundred and fifty samples from different types of food, comprising beef (n=60), chicken (n=72), and fish (n=18), were analyzed for the identification of E. coli by conventional and PCR methods. The results showed that out of 150 food samples, 44 (29.3%) were positive by culture, and 50 (33.3%) were positive by PCR. Significant differences were detected between sample types with culture (p-value < 0.005). When culture was considered the gold standard, the sensitivity of PCR was 100%, while the specificity was 94.34%. The six-hour pre-enrichment and PCR analysis are reliable in fast detection of E. coli in food samples. Hence, the identification of food pathogens using molecular-based methods would become more useful in routine diagnostic laboratories
Keywords: food poisoning, food pathogens, culture, enrichment, e. coli, pcr, food
Introduction
Food-borne illnesses are now becoming a serious threat to public health. These illnesses are caused by microorganisms (bacteria, viruses, fungi, and other parasites) or by chemical substances that contaminate food or water and cause more than 2500 diseases [1]. Worldwide, it has been reported that 600 million people get sick with food-borne illnesses, and 420,000 die each year from food poisoning [1, 2]. Escherichia coli is a large group of gram-negative bacteria and, in most cases, is harmless. E. coli is also one of the most important microorganisms used for the monitoring of water and food safety. Some strains of E. coli (O157:H7 (STEC)) are commonly associated with food poisoning outbreaks [3]. Over 700 strains or serotypes of E. coli exist in nature, water, and foods. E. coli possesses quite a few virulence factors encoded on mobile genetic elements and/or plasmids or localized in pathogenicity islands. These virulence factors are expressed as endotoxins, exotoxins, adhesion, invasion, or iron acquisition factors.
Health and human concerns are concentrated on the few pathogenic strains, such as strain O157:H7 (the cause of bloody diarrhoea from eating undercooked or raw meat), non-O157:O7 strains (O26, O145, O111, O121, and O45), and Shiga toxin-producing E. coli [4]. Methods for the rapid detection of E. coli and its related toxins are key to minimizing the risk of foodborne pathogens. Consequently, many detection methods have been developed, particularly to detect E. coli in water and foods. Bacterial isolation and identification by culturing are the standard methods for the detection of foodborne pathogens in addition to Gram staining, bacterial count, and biochemical testing [5]. These methods are labour-intensive, slow, and time-consuming (2-10 days for initial results up to confirmation). Therefore, accurate, fast, and low-cost detection methods are needed to assess the microbiological status of food [6].
To minimize the risk of E. coli, many efforts including improvement of diagnostic methods should be spent especially in food establishments. Other techniques, such as adenosine triphosphate (ATP) and optical methods, have also been used for the rapid detection of E. coli. However, such methods involve high costs, sensitive chemicals, and periodic maintenance [7-10]. Some recent rapid detection technologies for pathogenic bacteria include immunoassays, nucleic acid-based assays such as polymerase chain reaction (PCR), DNA microarrays, and antibody-based assays [11-14]. It has been shown that these methods have a short operating time, and their detection time is also low (2-24 hours) [11-14]. However, some of these methods still have low sensitivity and selectivity and, hence, need improvements in accuracy to be of any practical use. The present study aimed to detect E. coli in food samples using culture and PCR techniques.
Materials and methods
One hundred fifty samples from different types of food were purchased from hotels, restaurants, and cafeterias in Mecca city, Saudi Arabia. The selected food samples, consisting of beef (n=60), chicken (n=72), and fish (n=18), were labelled, recorded, and immediately transported in an icebox with freeze packs under sterile conditions to the laboratory of the Department of Environment and Health Research, the Custodian of the Two Holy Mosque Institute for Hajj and Umrah Research, and analysed within two hours hours of collection. If delayed, the samples were refrigerated at 0-4 °C for no more than 24 hours after collection. The isolates were grown at 37 °C and 150 rpm for 10 hours. Two types of growth media were used: (i) Luria-Bertani (LB) broth for general purposes and (ii) Tryptone Bile X-glucuronide (TBX) agar medium, a selective medium for the enumeration and differentiation of E. coli from other coliforms [15]. The two growth media were used for the identification of E. coli by conventional (culture and biochemical testing) and PCR methods.
Standard microbiological methods
Isolation and identification of E. coli were performed by standard microbiological methods. The homogenized samples were transferred into the nutrient broth (5 ml/test tube) and MacConkey agar (Oxoid Limited, Hampshire, United Kingdom). Any grown colony was picked, Gram stained, subjected to different biochemical tests (sugar fermentation, indole production, methyl-red, Voges-Proskauer, and citrate utilization tests), and then subcultured onto Eosin Methylene Blue (EMB) agar.
PCR after pre-enrichment
The pre-enrichment of samples was performed according to the method described by Medici et al. [16] with some modifications. Approximately 25 g samples were homogenized with 225 mL of buffered peptone water (BPW) medium (Oxoid, CM0509) and then divided into two aliquots. While one aliquot was incubated at 37 °C for 24 hours, the other aliquot was subjected to pre-enrichment culture for six hours. The first aliquot was used for DNA extraction by enzyme and freeze/thaw methods, and the second aliquot was used to confirm the presence of E. coli by standard culture methods, followed by biochemical and serological confirmatory tests. The DNA was extracted from the first aliquots by the Boil Lysis Method following Ahmed and Dablool [17]. Briefly, the aliquot was boiled at 100 °C for 10 minutes. Insoluble material was discarded through centrifugation for two minutes, and the supernatant was used as a template in the PCR. A PCR mixture (50 µL) containing one 100 pmol of each primer, 3 µL of DNA template, and a 25 µL solution of Taq PCR Master Mix polymerase (Promega Corporation, Madison, Wisconsin, United States) was used to conduct the PCR. Using Mastercycler® personal PCR equipment (Eppendorf, Hamburg, Germany), DNA was amplified under the following conditions: heat denaturation at 94 °C for three minutes, followed by 30 amplification cycles (one minute at 94 °C, 65 seconds at 62 °C, and 90 seconds at 72 °C), and an elongation step of seven minutes at 72 °C. The primers used were Afa FP (5' GCT GGG CAG CAAACT GAT AAC TCT C 3') and Afa RP (5' CAT CAA GCT GTT TGTTCG TCC GCC G 3'), which amplify a 480 -bp fragment within the conserved Afa gene sequence of pathogenic E. coli [18] using Mastercycler. The products were electrophoresed in a 1.5% agarose gel for one hour at 120 V, stained with ethidium bromide and visualized under ultraviolet light using a gel documentation system via an ultraviolet transilluminator (UVP BioDoc-It® Imaging System; Ultra-Violet Products Ltd, Cambridge, United Kingdom).
Data analysis
Data were analysed, and statistical analyses were made by using the chi-square method using IBM SPSS Statistics for Windows, Version 25.0 (Released 2017; IBM Corp., Armonk, New York, United States). A p < 0.05 was considered statistically significant.
Results
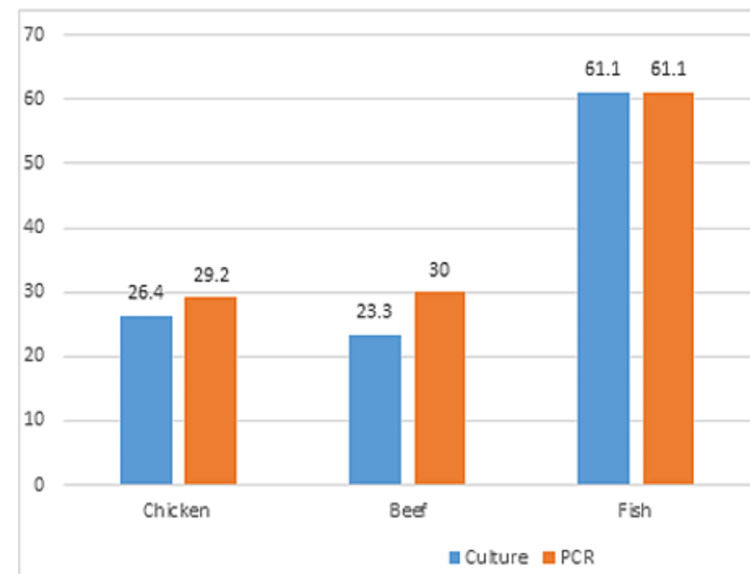
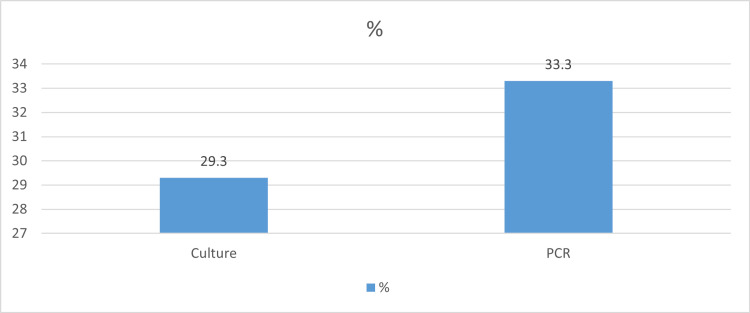
Out of 150 food samples, 44 (29.3%) were positive by culture method and 50 (33.3%) were positive by PCR assay, as shown in Table 1 and Figure 1. Figure 2 shows the results of the food analysis for E. coli. PCR (Figure 3) showed the highest rate of E. coli in fish samples (61.1%), followed by beef (30.0%) and chicken (29.2%). Culture showed the highest rate of E. coli in fish samples (61.1%), followed by chicken (26.4%) and beef (23.3%).
Table 1. Frequencies of positive samples for Escherichia coli in different samples.
PCR: polymerase chain reaction
| Result | Chicken (n=72) | Beef (n=60) | Fish (n=18) | Total | p-value |
| Culture | 19 (26.4%) | 14 (23.3%) | 11 (61.1%) | 44 (29.3%) | 0.006 |
| PCR | 21 (29.2%) | 18 (30.0%) | 11 (61.1%) | 50 (33.3%) | 0.29 |
Figure 1. Distribution of Escherichia coli isolates among food samples.
PCR: polymerase chain reaction
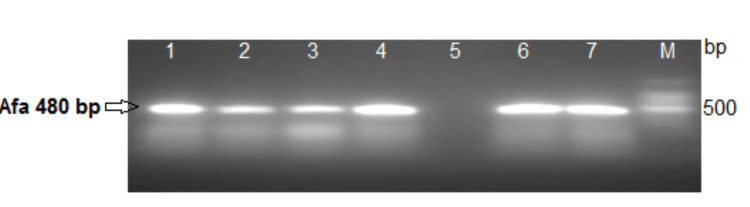
Figure 2. PCR analysis of Escherichia coli isolates among food samples.
Lane 1: Control positive; Lanes 2, 3, 4, 6 and 7: positive Afa gene of Escherichia coli strains (480 bp); Lane 5: Control negative; Lane M: 100-bp DNA ladder.
PCR: polymerase chain reaction
Figure 3. Frequencies of positive samples for Escherichia coli by using culture and polymerase chain reaction.
Significant differences were detected between sample types with culture (p-value < 0.05), as shown in Table 1. When culture was considered the gold standard, the sensitivity of PCR was 100%, while the specificity was 94.34%, with a positive predictive value (PPV) of 89.82% and a negative predictive value (NPV) of 100% (Table 2).
Table 2. Sensitivity and specificity of PCR method when culture is gold standard.
PCR: polymerase chain reaction; PPV: positive predictive value; NPV: negative predictive value
| PCR | |
| Sensitivity | 100% |
| Specificity | 94.34% |
| PPV | 89.82% |
| NPV | 100% |
Discussion
Food hygiene, particularly microbiological safety, is an important concern in the food industry, especially during mass gatherings. Listeria monocytogenes, E. coli, Staphylococcus aureus, Salmonella enterica, and Bacillus cereus are the common food-borne pathogenic bacteria that cause the majority of food-borne disease outbreaks [19]. Methods for the rapid detection of food-borne infections are becoming increasingly crucial in many food analyses, as the traditional approaches are time-consuming and labour-intensive.
E. coli detection in foods is one of the most useful hygienic criteria because of the involvement of the bacterium in causing diseases in addition to its use as an indicator of the presence of other pathogens in foods [20]. The present study aimed to detect E. coli using culture and PCR techniques in different types of food (chicken, beef, and fish) purchased from catering establishments in Mecca city during mass gathering events. The results of the present study showed that 33.3% of the food samples were positive by PCR, while 29.3% of them were positive by culture. Detection of E. coli by culture followed by standard biochemical identification remains the method of choice, especially during outbreaks due to the potential need to compare the results with other typing methods.
Culture results showed the highest rate of E. coli in fish samples (61.1%), followed by chicken (26.4%) and beef (23.3%). However, culture methods may be laborious, have low sensitivity, be time-consuming and require a long time from two days to more than a week for pathogen confirmation. More rapid, sensitive, and specific methods, such as nucleic acid-based methods, have low numbers of false-positive results and no false-negative results and are thus more reliable to detect and identify E. coli in food samples.
Previously, many PCR-based detection methods for the determination of E. coli in food specimens have been developed that target E. coli genes [21]. By using PCR, the present study showed that the highest rate of E. coli was detected more in fish samples, followed by beef and chicken. In a previous study, all E. coli in food specimens were detected after an enrichment period of six hours [22]. In another study from India, 50% of the food samples tested were considered positive for E. coli, where the highest level of incidence of E. coli was found in beef samples (91%) [23]. In Mexico, 44% of the food samples were positive for E. coli [24]. The PCR method was tested by Nguyen et al. on three different types of samples, including chicken meat, and it was shown that after 12 hours of enrichment against foodborne pathogens such as E. coli, the lowest detection level of 10 CFU/mL was achieved [25]. Therefore, the three main food-borne pathogens may be reliably and effectively detected using this multiplex PCR assay in conjunction with a pre-enrichment method.
When culture was considered the gold standard, the sensitivity of PCR was 100%, while the specificity was 94.34%. Comparatively, in a study by Zhang et al., the maximum sensitivity of 100 CFU/mL for four pathogens, including E. coli, was obtained after 24 hours of enrichment from eight experimentally contaminated food samples [26]. The importance of the present study may be seen in the mass gatherings as the presence of a large number of people in overcrowded conditions considerably increases the risk of occurrence of food poisoning and gastrointestinal diseases caused by uncooked food, so that earlier PCR assays also yielded the simultaneous detection of food-borne pathogens, including E. coli, at low concentrations [27].
Conclusions
The PCR achieved was successful in detecting the presence of E. coli in food specimens (33.3%) with 100% sensitivity and 94.34% specificity, while the culture method detected E. coli in 29.3% of the specimens. The six-hour pre-enrichment of samples and PCR analysis using Afa gene-specific primers can reliably and effectively detect E. coli rapidly in food samples. Hence, the identification of food pathogens using molecular-based methods will become more useful in routine diagnostic laboratories, especially during mass gatherings where the risk of occurrence of food poisoning is high.
Acknowledgments
The author thanks the Custodian of the Two Holy Mosques Institute for Hajj and Umrah Research for providing lab facilities in the Department of Health and Environmental Research.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.WHO (World Health Organisation) (2015a. World Health Organization: Food Safety Fact Sheet. 2022. https://www.who.int/news-room/fact-sheets/detail/food-safety https://www.who.int/news-room/fact-sheets/detail/food-safety
- 2.Who Estimates Of The Global Burden Of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007-2015. Geneva, Switzerland: World Health Organization; 2015. WHO estimates of the global burden of foodborne diseases. Foodborne diseases burden epidemiology reference group 2007- 2015http: //www.who.int/foodsafety/publications/foodborne_disease/fergreport/ en/ [Google Scholar]
- 3.Variation in virulence among clades of Escherichia coli O157:H7 associated with disease outbreaks. Manning SD, Motiwala AS, Springman AC, et al. Proc Natl Acad Sci U S A. 2008;105:4868–4873. doi: 10.1073/pnas.0710834105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Predicting the presence of non-O157 Shiga toxin-producing Escherichia coli in ground beef by using molecular tests for Shiga toxins, intimin, and O serogroups. Bosilevac JM, Koohmaraie M. Appl Environ Microbiol. 2012;78:7152–7155. doi: 10.1128/AEM.01508-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strategies for the detection of Escherichia coli O157:H7 in foods. Deisingh AK, Thompson M. J Appl Microbiol. 2004;96:419–429. doi: 10.1111/j.1365-2672.2003.02170.x. [DOI] [PubMed] [Google Scholar]
- 6.Indicator organisms for safety and quality—uses and methods for detection: minireview. Tortorello ML. J AOAC Int. 2003;86:1208–1217. [PubMed] [Google Scholar]
- 7.A novel, optical, on-line bacteria sensor for monitoring drinking water quality. Højris B, Christensen SC, Albrechtsen HJ, Smith C, Dahlqvist M. Sci Rep. 2016;6:23935. doi: 10.1038/srep23935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evaluation of ATP measurements to detect microbial ingress by wastewater and surface water in drinking water. Vang ÓK, Corfitzen CB, Smith C, Albrechtsen HJ. Water Res. 2014;64:309–320. doi: 10.1016/j.watres.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Monitoring microbiological changes in drinking water systems using a fast and reproducible flow cytometric method. Prest EI, Hammes F, Kötzsch S, van Loosdrecht MC, Vrouwenvelder JS. Water Res. 2013;47:7131–7142. doi: 10.1016/j.watres.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 10.Online drinking water quality monitoring: review on available and emerging technologies. Banna MH, Imran S, Francisque A, Najjaran H, Sadiq R, Rodriguez M, Hoorfar M. Crit Rev Environ Sci Technol. 2014;44:1370–1421. [Google Scholar]
- 11.A rapid multiplexed chemiluminescent immunoassay for the detection of Escherichia coli O157:H7, Yersinia enterocolitica, Salmonella typhimurium, and Listeria monocytogenes pathogen bacteria. Magliulo M, Simoni P, Guardigli M, Michelini E, Luciani M, Lelli R, Roda A. J Agric Food Chem. 2007;55:4933–4939. doi: 10.1021/jf063600b. [DOI] [PubMed] [Google Scholar]
- 12.Application of multiplex PCR assay based on uidR and fliCH7 genes for detection of Escherichia coli O157:H7 in milk. Kumar A, Grover S, Kumar Batish V. J Gen Appl Microbiol. 2013;59:11–19. doi: 10.2323/jgam.59.011. [DOI] [PubMed] [Google Scholar]
- 13.Sensitive quantification of Escherichia coli O157:H7, Salmonella enterica , and Campylobacter jejuni by combining stopped polymerase chain reaction with chemiluminescence flow-through DNA microarray analysis. Donhauser SC, Niessner R, Seidel M. Anal Chem. 2011;83:3153–3160. doi: 10.1021/ac2002214. [DOI] [PubMed] [Google Scholar]
- 14.Rapid and simultaneous quantitation of Escherichia coli 0157:H7, Salmonella, and Shigella in ground beef by multiplex real-time PCR and immunomagnetic separation. Wang L, Li Y, Mustaphai A. J Food Prot. 2007;70:1366–1372. doi: 10.4315/0362-028x-70.6.1366. [DOI] [PubMed] [Google Scholar]
- 15.Is TBX agar a suitable medium for monitoring Escherichia coli in bathing water using the membrane filtration method? Jozić S, Vukić Lušić D, Aljinović A, et al. Environ Monit Assess. 2019;191:558. doi: 10.1007/s10661-019-7733-4. [DOI] [PubMed] [Google Scholar]
- 16.Evaluation of DNA extraction methods for use in combination with SYBR green I real-time PCR to detect Salmonella enterica serotype enteritidis in poultry. De Medici D, Croci L, Delibato E, Di Pasquale S, Filetici E, Toti L. Appl Environ Microbiol. 2003;69:3456–3461. doi: 10.1128/AEM.69.6.3456-3461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quality improvement of DNA extracted by boiling method in Gram negative bacteria. Ahmed OB, Dablool AS. Int J Bioassays. 2017;6:5347–5349. [Google Scholar]
- 18.Common virulence factors and genetic relationships between O18:K1:H7 Escherichia coli isolates of human and avian origin. Moulin-Schouleur M, Schouler C, Tailliez P, et al. J Clin Microbiol. 2006;44:3484–3492. doi: 10.1128/JCM.00548-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foodborne pathogens. Bintsis T. AIMS Microbiol. 2017;3:529–563. doi: 10.3934/microbiol.2017.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapid detection of Escherichia coli in fresh foods using a combination of enrichment and PCR analysis. Choi Y, Lee S, Lee H, et al. Korean J Food Sci Anim Resour. 2018;38:829–834. doi: 10.5851/kosfa.2018.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evaluation of culture- and PCR-based detection methods for Escherichia coli O157:H7 in inoculated ground beeft. Arthur TM, Bosilevac JM, Nou X, Koohmaraie M. J Food Prot. 2005;68:1566–1574. doi: 10.4315/0362-028x-68.8.1566. [DOI] [PubMed] [Google Scholar]
- 22.Multiplex real-time PCR assays for detection of eight Shiga toxin-producing Escherichia coli in food samples by melting curve analysis. Singh P, Mustapha A. Int J Food Microbiol. 2015;215:101–108. doi: 10.1016/j.ijfoodmicro.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Species specific PCR based detection of Escherichia coli from Indian foods. Godambe LP, Bandekar J, Shashidhar R. https://doi.org/10.1007/s13205-017-0784-8. 3 Biotech. 2017;7:130. doi: 10.1007/s13205-017-0784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prevalence of Escherichia coli and Salmonella spp. in street-vended food of open markets (tianguis) and general hygienic and trading practices in Mexico City. Estrada-Garcia T, Lopez-Saucedo C, Zamarripa-Ayala B, Thompson MR, Gutierrez-Cogco L, Mancera-Martinez A, Escobar-Gutierrez A. Epidemiol Infect. 2004;132:1181–1184. doi: 10.1017/s0950268804003036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Multiplex PCR for simultaneous identification of E. coli O157:H7, Salmonella spp. and L. monocytogenes in food. Nguyen TT, Van Giau V, Vo TK. 3 Biotech. 2016;6:205. doi: 10.1007/s13205-016-0523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simultaneous detection of Listeria monocytogenes, Staphylococcus aureus, Salmonella enterica and Escherichia coli O157:H7 in food samples using multiplex PCR method. Zhang D, Zhang H, Yang L, Guo J, Li X, Feng Y. J Food Saf. 2009;29:348–363. [Google Scholar]
- 27.A novel multiplex PCR assay for rapid and simultaneous detection of five pathogenic bacteria: Escherichia coli O157:H7, Salmonella, Staphylococcus aureus, Listeria monocytogenes, and Vibrio parahaemolyticus. Kim JS, Lee GG, Park JS, et al. J Food Prot. 2007;70:1656–1662. doi: 10.4315/0362-028x-70.7.1656. [DOI] [PubMed] [Google Scholar]