Abstract
Anaplasma marginale, an intraerythrocytic ehrlichial pathogen of cattle, establishes persistent infections in both vertebrate (cattle) and invertebrate (tick) hosts. The ability of A. marginale to persist in cattle has been shown to be due, in part, to major surface protein 2 (MSP2) variants which are hypothesized to emerge in response to the bovine immune response. MSP2 antigenic variation has not been studied in persistently infected ticks. In this study we analyzed MSP2 in A. marginale populations from the salivary glands of male Dermacentor variabilis persistently infected with A. marginale after feeding successively on one susceptible bovine and three sheep. New MSP2 variants appeared in each A. marginale population, and sequence alignment of the MSP2 variants revealed multiple amino acid substitutions, insertions, and deletions. These results suggest that selection pressure on MSP2 occurred in tick salivary glands independent of the bovine immune response.
Anaplasmosis is a tick-borne disease of cattle caused by the obligate intraerythrocytic ehrlichia Anaplasma marginale. The only known site of development of A. marginale in cattle is within erythrocytes (21). The number of infected erythrocytes increases logarithmically, and removal of these infected cells by phagocytosis results in the development of anemia and icterus without hemoglobinemia and hemoglobinuria (18). Biological transmission of A. marginale is effected by feeding ticks, while mechanical transmission occurs when infected blood is transferred to susceptible animals by biting flies or by blood-contaminated fomites. Cattle that recover from acute infection remain persistently infected and are protected from clinical disease, thus serving as reservoirs for mechanical and biological transmission by ticks. Approximately 20 species of ticks have been incriminated as vectors worldwide (7, 9). The development cycle of A. marginale in ticks is complex and coordinated with the tick feeding cycle (14–16). In the developmental cycle that was described in male ticks transferred from infected to susceptible hosts, the first site of development of A. marginale occurs in gut cells after the ticks have been removed from an infected host. After the ticks feed a second time, many other tick tissues become infected, including the salivary glands from which the ehrlichiae are transmitted to cattle during feeding. Male ticks were found to become persistently infected with A. marginale and were able to transmit A. marginale to multiple hosts (12, 14–16).
Major surface protein 2 (MSP2) is one of the six MSPs that have been identified on A. marginale (1, 17). MSP2 (∼36 kDa) is encoded by a polycistronic mRNA containing msp2 and three other genes (2, 3). Cattle immunized with MSP2 were partially protected against challenge, and MSP2 was strongly recognized by B and T cells from immune cattle (4, 5, 8, 19, 20). MSP2 antigenic variants were found to emerge during persistent infection in cattle, encoded by a single hypervariable region in the central part of the protein (2, 3, 10, 11). MSP2 variants have been posited to arise from templated intragenic recombination between the multiple genomic msp2 copies and the polycistronic expression site which generates complex mosaics of sequences in the expression site (3).
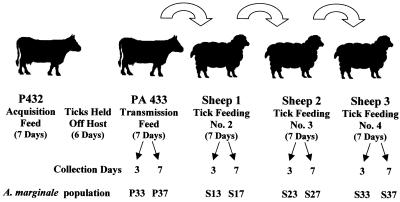
The present study was undertaken to determine whether MSP2 variants arise in the absence of bovine-acquired immune response in male Dermacentor variabilis ticks persistently infected with A. marginale. Two splenectomized calves (PA432 and PA433, 2 to 6 months old), determined to be free of infection by an A. marginale-specific competitive enzyme-linked immunosorbent assay ELISA (25), were used. Calf PA432 was inoculated with 106 ml of blood from PA431 (infected with the tick-transmissible Virginia isolate [12, 14–16]; parasitemia = 0.9%) and served as the donor for infection of D. variabilis males originally collected from Oklahoma and reared at the Oklahoma State University Centralized Tick Rearing Facility. PA433 was used for the first successive feeding in order to confirm tick transmission of A. marginale. Three sheep (S1, S2, and S3) were used for the second to the fourth successive tick feedings. The calves were monitored three times a week by examination of stained blood smears and determination of the packed cell volume. Once infection was detected in blood smears, the calves were monitored daily. The experimental design is depicted in Fig. 1. Calf PA432 was infested with 781 male D. variabilis ticks that were placed in orthopedic stockinettes attached to the calf when the ascending parasitemia was 4.4%. The ticks were allowed to feed for 7 days, after which they were removed and placed in a humidity chamber for 6 days. The ticks were then allowed to feed on calf PA433 for 7 days, after which they were transferred directly and successively to feed for 7 days on sheep 1, 2, and 3. During feeding the ticks were not exposed to A. marginale-specific antibodies because the four hosts (one calf and three sheep) were not infected with the ehrlichiae. Forty ticks were removed from each host (PA433 and sheep 1, 2, and 3) on days 3 and 7 of tick feeding (Fig. 1). The ticks were dissected, and the salivary glands from groups of 20 ticks were pooled in 500 μl of RNALater (Ambion, Austin, Tex.). The samples were placed at 4°C overnight and then frozen at −70°C until used for cloning and sequence analysis.
FIG. 1.
Experimental design. Calf PA432 was inoculated with 106 ml of infected blood (Virginia isolate of A. marginale) from PA431 (parasitemia = 0.9%) and served as the donor for infection of D. variabilis males. Calf PA432 was infested with 781 male D. variabilis ticks that were placed in orthopedic stockinettes attached to the calf when the ascending parasitemia was 4.4%. The ticks were allowed to feed for 7 days, after which they were removed and held in a humidity chamber for 6 days. The ticks were then allowed to feed on calf PA433 for 7 days, and then they were transferred directly and successively to feed for 7 days on sheep 1, 2, and 3. Forty ticks were removed from each host (PA433 and sheep 1, 2, and 3) on days 3 and 7 of tick feeding. The ticks were dissected, and salivary glands from the groups of 20 ticks were pooled and used for msp2 expression site cloning and sequence analysis.
Genomic DNA was isolated from erythrocytic stages of A. marginale from 1 ml of infected blood using Tri-Reagent (Sigma) (6). DNA from A. marginale-infected D. variabilis salivary glands was extracted from 40 salivary glands (from 20 ticks) using 500 μl of Tri-Reagent and homogenized with a 1-ml tuberculin syringe with a 25-gauge needle. The 2.9-kbp genomic expression site for msp2 lacking orf4 and its 5′ flanking region (3) was amplified using the oligonucleotide primers MSP25 (5′-GGATTTTGTGGTCGGGTTTGTAT-3′) and MSP23 (5′-CACCGGTTGATGAAGTTTGC-3′) in a 50-μl volume PCR (0.2 μM concentration of each primer, 1.5 mM MgSO4, 0.2 mM deoxynucleoside triphosphate, 1× avian myeloblastosis virus-Tfl reaction buffer, 5 U of Tfl DNA polymerase) employing the Access RT-PCR system (Promega). Reactions were performed in an automated DNA thermal cycler (Eppendorf Mastercycler) for 35 cycles. After an initial denaturation step of 30 s at 94°C, each cycle consisted of a denaturing step of 30 s at 94°C, an annealing step of 30 s at 58°C, and an extension step of 3 min at 68°C. The program ended by storing the reactions at 4°C. PCR products were electrophoresed on 1% agarose gels to check the size of amplified fragments. Fragments with the correct size (2.9 kbp) were extracted from agarose (Wizard; Promega) and cloned into the pGEM-T vector (Promega). Plasmid DNA was isolated (Wizard SV96 Plasmid DNA Purification System; Promega) and sequenced with primers AB782, AB765, and AB191 (3) at the Core Sequencing Facility, Department of Biochemistry and Molecular Biology, Noble Research Center, Oklahoma State University, using ABI Prism dye-terminator cycle sequencing protocols developed by Applied Biosystems (Perkin-Elmer Corp., Foster City, Calif.). At least five sequences were obtained from each A. marginale population (Fig. 1). Nucleotide sequences were analyzed using the program AlignX (Vector NTI Suite V5.5; InforMax). The sequences reported here have been assigned GenBank accession numbers AF354464 to AF354486. The msp1α gene was amplified and sequenced as reported previously (6).
MSP2 variants appeared in each A. marginale population derived from tick salivary glands (Table 1). A total of 21 different msp2 variants were identified in the 66 clones examined from tick salivary gland-derived A. marginale. Variants P33_B5, P33_B10, P37_C1, P37_C7, P37_D5, S23_A5, S27_B2, and S33_B10 (representing the 38% of all different variants identified in tick salivary glands) were present in two to five of the eight populations analyzed, and variant P37_C10 (5%) was present in seven of the eight tick salivary gland-derived A. marginale populations. Variants P33_A12, P33_A5, P37_B11, P37_C2, S13_E8, S17_F11, S17_G3, S23_A3, S27_A9, S27_B4, S33_C11, and S33_D2 (57%) were each present in only one of the populations. With the exception of population S37, new msp2 variants appeared in each A. marginale population, suggesting conditions of disequilibrium for A. marginale multiplication in tick salivary glands. Selection for and against MSP2 variants occurred during multiplication of A. marginale in tick salivary glands. A shift in the predominant MSP2 variant on each population (master sequence) occurred during persistent infection in tick salivary glands (Table 1). Therefore, analysis of msp2 variants present in A. marginale populations derived from tick salivary glands revealed the most diversity in this expression site in persistently infected salivary glands after successive feeding. This result is similar to the findings reported by Barbet et al. (3) in persistently infected cattle and in salivary glands of ticks fed on these animals.
TABLE 1.
Sequence variants in the MSP2 hypervariable region on each A. marginale population
| Sequence variant (% total)a | No. of sequencesb found in:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PA432 | PA433 at day:
|
S1 at day:
|
S2 at day:
|
S3 at day:
|
|||||
| 3 | 7 | 3 | 7 | 3 | 7 | 3 | 7 | ||
| P33_A12 (1.35) | X | ||||||||
| P33_A5 (1.35) | X | ||||||||
| P33_B5 (13.5) | XXX | XX | X | X | |||||
| XX | X | ||||||||
| P33_B10 (8.1) | X | X | XX | X | X | ||||
| P37_B11 (1.35) | X | ||||||||
| P37_C1 (5.4) | X | X | X | X | |||||
| P37_C2 (1.35) | X | ||||||||
| P37_C7 (6.8) | X | XX | X | X | |||||
| P37_C10 (21.6) | X | XX | XX | X | X | X | X | XX | |
| X | XX | X | |||||||
| X | |||||||||
| P37_D5 (5.4) | X | X | XX | ||||||
| S13_E8 (1.35) | X | ||||||||
| S17_F11 (1.35) | X | ||||||||
| S17_G3 (1.35) | X | ||||||||
| S23_A3 (1.35) | X | ||||||||
| S23_A5 (8.1) | X | X | XX | ||||||
| XX | |||||||||
| S27_A9 (1.35) | X | ||||||||
| S27_B2 (6.8) | X | XX | |||||||
| XX | |||||||||
| S27_B4 (1.35) | X | ||||||||
| S33_B10 (4.05) | XX | X | |||||||
| S33_C11 (2.7) | X | X | |||||||
| S33_D2 (1.35) | X | ||||||||
| 4323 (1.35) | X | ||||||||
| 4327 (1.35) | X | ||||||||
The number of Xs represents the number of sequences for each variant identified in A. marginale populations.
The percent total was calculated as the number of identical sequences/total number of sequences examined (n = 74).
The experimental design used for this study allowed us to monitor the antigenic variation of MSP2 in tick hosts without exposure of the organisms to A. marginale-specific antibodies. The bovine subject used for the first transmission feeding (PA433) was a susceptible calf that was serologically negative for A. marginale, and the use of sheep for the other three successive feedings allowed for tick feeding in the absence of bovine host factors. However, as has been demonstrated for other bacterial species (13), the mammalian innate immune response could have an effect on A. marginale multiplication in ticks. Nevertheless, the main role for MSP2 antigenic variation in persistently infected cattle has been attributed to bovine acquired immune response (3).
Sequence alignment of MSP2 variants derived from tick salivary glands revealed multiple amino acid substitutions, insertions, and deletions (Fig. 2). Conserved amino acid positions were similar to those reported by Barbet et al. (3) in A. marginale populations of the Oklahoma isolate transmitted between cattle and ticks. In order to evaluate possible artifactual changes introduced by PCR, 487 and 202 bp of the region upstream and downstream of the hypervariable region, respectively, were sequenced and analyzed in nine clones derived from independent PCR reactions (one clone per tick-derived A. marginale population). The error rate of the polymerase in the PCR was 0.001 for both regions, equivalent to 1 bp for every two hypervariable regions (423 bp each). Therefore, this error rate was not likely to result in the multiple base substitutions, insertions, and deletions that were observed in the msp2 hypervariable region.
FIG. 2.
Alignment of MSP2 variant sequences identified in the tick salivary gland-derived A. marginale populations. Multiple amino acid substitutions, insertions, and deletions were detected. Alignment of protein sequences was performed using the program AlignX (Vector NTI Suite V 5.5; InforMax, Inc.) with an engine based on the CLUSTAL W algorithm (27). Conserved amino acids are shown in red, amino acids conserved in 11 to 20 or 21 sequences are shown in blue, and amino acid positions present in 1 to 10 or 21 sequences are shown in black.
In contrast to the emergence of variants observed in msp2, msp1α was found to be conserved. The sequence of msp1α that was cloned from A. marginale populations derived from infected erythrocytes of donor cow PA432, which was used for infection of ticks, was the same as the sequence of msp1α from salivary glands of last group of ticks collected after 7 days of feeding from sheep 3 (population S37; Fig. 1). This result agrees with the conservation of MSP1a in the different environments of bovine blood, tick salivary glands, and cell culture as reported by Bowie et al. (M. V. Bowie, J. de la Fuente, K. M. Kocan, E. F. Blouin, and A. F. Barbet, unpublished results).
The msp2 variants in A. marginale derived from infected cow PA432 were studied to evaluate the structure of the original A. marginale population in cattle (Table 1). Our results indicated that for the Virginia isolate, predominant sequence variants do not change on passage of A. marginale between the acute infection in cattle and tick salivary glands sampled on day 3 of tick transmission feeding (Table 1). Of the four msp2 variants identified in the erythrocytic population of A. marginale in PA432, two (P33_B5 and P37_C10; Table 1) were also present in tick salivary gland-derived A. marginale populations, while variants 4323 and 4327 (Table 1) disappeared from A. marginale populations in persistently infected tick salivary glands, suggesting selection against these msp2 variants. As the multiplication progressed in tick salivary glands, more diversity was observed in msp2 sequences (Table 1). While four different variants were identified in A. marginale from infected erythrocytes of PA432 and tick salivary glands after 3 days of first transmission-feeding on PA433, four to eight variants were identified in A. marginale populations after successive tick feeding (Table 1).
Differing results on MSP2 variation in tick salivary glands were reported in previous studies. Rurangirwa et al. (22) reported restriction of MSP2 to two variant types in groups of salivary glands collected on day 3 of tick feeding, while Barbet et al. (3) reported that MSP2 variants in pooled tick salivary glands collected on day 7 of tick feeding were similar to those found in erythrocytic A. marginale during acquisition feeding of the ticks on acutely infected cattle. We were concerned that the differences noted in these two studies may have been related to the presence of different A. marginale developmental stages present at the collection time. On day 3 of tick feeding, the predominant A. marginale form in salivary gland colonies would have been the reticulated or vegetative form, while colonies in salivary glands on day 7 of tick feeding would have contained both reticulated and dense forms (15). Once the salivary glands become persistently infected, both reticulated and dense forms would have been present in the A. marginale salivary gland populations. Therefore, we analyzed A. marginale populations from ticks collected on days 3 and 7 of the first transmission feeding on PA433 (Fig. 1) to determine whether the collection day influenced the msp2 sequence data. In this study, we did not observe a restriction of the MSP2 to two type variants as reported by Rurangirwa et al. (22), and our findings were similar to those of Barbet et al. (3) and Rurangirwa et al. (23) for Oklahoma and Idaho isolates of A. marginale; these authors, respectively, demonstrated the emergence of multiple MSP2 variants in tick salivary glands. Barbet et al. (3) found that the MSP2 variants in D. variabilis salivary glands were similar to the variant types found in erythrocytic A. marginale of the acutely infected bovine used for the acquisition feeding of ticks, while Rurangirwa et al. (23) found that new, different MSP2 variants were expressed by several A. marginale isolates in salivary glands of D. andersoni. Our results using the Virginia isolate of A. marginale in D. variabilis denote similarities with those of Barbet et al. (3), in that we found that the predominant MSP2 variant in tick salivary glands on day 3 of the first tick feeding was similar to the predominant variant type found in erythrocytic A. marginale of the acutely infected bovine used for acquisition feeding of ticks. However, on day 7 of the same tick feeding, the predominant MSP2 variant in tick salivary glands had changed. The differences observed in these experiments could be related to several factors, including the A. marginale geographic isolate and the species of tick. Furthermore, in all the experiments published so far, groups of tick salivary glands have been analyzed, leaving the possibility that individual ticks may express different MSP2 variants. A dendrogram for the comparison of MSP2 variant sequences showed that some variants clustered according to the sampled A. marginale population (Fig. 3). This finding suggests some MSP2 sequence homogeneity in the population at a particular stage of parasite multiplication. However, clustering of variants according to the sampling day (3 or 7) was not demonstrated.
FIG. 3.
Dendogram constructed from the analysis of MSP2 variant sequences found in tick salivary gland-derived A. marginale populations based on a sequence distance method utilizing the neighbor-joining algorithm of Saitou and Nei (24) employing Vector NTI Suite 5.5. Sequence variants were assigned to the first A. marginale population in which they appeared. The clustering of similar sequences in different populations is shown.
The pattern of A. marginale development in ticks is different from that of the protozoan parasites, Theileria and Babesia spp., in which midgut infections clear as the parasites move into the salivary glands from where they are transmitted to the vertebrate host. The mechanism of persistent infection in ticks is not clearly understood. Movement of A. marginale in ticks appears to be correlated with tick feeding. Development of colonies of A. marginale in gut cells does not commence until the ticks have been removed from the parasitemic host, and infection of salivary glands and other tick tissues does not occur until the ticks feed a second time (12, 15, 16). We do not know whether persistent infections of A. marginale in tick salivary glands is due wholly to multiplication of the ehrlichiae within salivary gland cells or else results from the continued movement of organisms from the tick gut cells or other tissues to the salivary glands. At any rate, male ticks maintain fairly constant levels of infection, which is most likely due to the continued multiplication of the A. marginale (16).
The present study demonstrated that selection pressures on A. marginale in persistently infected tick salivary glands resulted in more heterogeneous populations of msp2 sequences and the emergence of new MSP2 variants. It was recently proposed that the emergence of MSP2 variants during persistent infections in cattle was due to selection by the host immune response (3). However, our results have demonstrated that new MSP2 variants emerge in tick salivary glands in the absence of exposure to the bovine immune system. These results are similar to those of Singer and Elmendorf (26), who showed that the emergence of antigenic variants of Giardia lamblia occurred in immunodeficient mice and gerbils. The development of MSP2 variants may be influenced by other host or tick factors. Further studies are needed to define the mechanism of antigenic variation in MSP2, and these studies would be enhanced by the use clonal populations of A. marginale.
Acknowledgments
This research was supported by the project no. 1669 of the Oklahoma Agricultural Experiment Station, the Endowed Chair for Food Animal Research (K. M. Kocan, College of Veterinary Medicine, Oklahoma State University), The NIH Centers for Biomedical Research Excellence through a subcontract to J. de la Fuente from the Oklahoma Medical Research Foundation, and the Oklahoma Center for the Advancement of Science and Technology, Applied Research Grant, AR00(1)-001.
A. F. Barbet (University of Florida) is acknowledged for critical reading of the manuscript and helpful suggestions. Dollie Clawson and Brian McEwen (Department of Veterinary Pathobiology, Oklahoma State University) are acknowledged for technical assistance. Sue Ann Hudiburg and Janet J. Rogers (Core Sequencing Facility, Department of Biochemistry and Molecular Biology, Noble Research Center, Oklahoma State University) are acknowledged for oligonucleotide synthesis and DNA sequencing, respectively.
REFERENCES
- 1.Barbet A F, Blentlinger R, Lundgren A M, Blouin E F, Kocan K M. Comparison of surface proteins of Anaplasma marginale grown in tick cell culture, tick salivary glands and cattle. Infect Immun. 1998;67:102–107. doi: 10.1128/iai.67.1.102-107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbet A F, Lundgren A, Yi J, Rurangirwa F R, Palmer G H. Antigenic variation of Anaplasma marginale by expression of MSP2 mosaics. Infect Immun. 2000;68:6133–6138. doi: 10.1128/iai.68.11.6133-6138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbet A F, Yi J, Lundgren A, McEwen B R, Blouin E F, Kocan K M. Antigenic variation of Anaplasma marginale: MSP2 diversity during cyclic transmission between ticks and cattle. Infect Immun. 2001;69:3057–3066. doi: 10.1128/IAI.69.5.3057-3066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown W C, Zhu D, Shkap B, McGuire T C, Blouin E F, Kocan K M, Palmer G H. The repertoire of Anaplasma marginale antigens recognized by CD4+ lymphocyte clones from protectively immunize cattle is diverse and includes major surface protein 2 (MSP-2) and MSP-3. Infect Immun. 1998;66:5414–5422. doi: 10.1128/iai.66.11.5414-5422.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown W C, McGuire T C, Zhu D, Lewin H A, Sosnow J, Palmer G H. Highly conserved regions of the immunodominant major surface protein 2 of the genogroup II ehrlichial pathogen Anaplasma marginale are rich in naturally derived CD4+ T lymphocyte epitopes that elicit strong recall responses. J Immunol. 2001;166:1114–1124. doi: 10.4049/jimmunol.166.2.1114. [DOI] [PubMed] [Google Scholar]
- 6.de la Fuente J, Van Den Bussche R A, Kocan K M. Molecular phylogeny and biogeography of North American isolates of Anaplasma marginale (Rickettsiaceae: Ehrlichieae) Vet Parasitol. 2001;97:65–76. doi: 10.1016/s0304-4017(01)00378-8. [DOI] [PubMed] [Google Scholar]
- 7.Dikmans G. The transmission of anaplasmosis. Am J Vet Res. 1950;11:5–16. [Google Scholar]
- 8.Eid G, French D M, Lundgren A M, Barbet A F, McElwain T F, Palmer G H. Expression of major surface protein 2 antigenic variants during acute Anaplasma marginale rickettsemia. Infect Immun. 1996;64:836–841. doi: 10.1128/iai.64.3.836-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewing S A. Proceedings of the Seventh National Anaplasmosis Conference. Mississippi State: Mississippi State University; 1981. Transmission of Anaplasma marginale by arthropods; pp. 395–423. [Google Scholar]
- 10.French D M, McElwain T F, McGuire T C, Palmer G H. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect Immun. 1998;66:1200–1207. doi: 10.1128/iai.66.3.1200-1207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French D M, Brown W C, Palmer G H. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect Immun. 1999;67:5834–5840. doi: 10.1128/iai.67.11.5834-5840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge N L, Kocan K M, Blouin E F, Murphy G L. Developmental studies of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in male Dermacentor andersoni (Acari: Ixodidae) infected as adults using nonradioactive in situ hybridization. J Med Ent. 1996;33:911–920. doi: 10.1093/jmedent/33.6.911. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi F, Smith F D, Ozinsky A, Hawn T R, Yi E C, Goodlett D R, Eng J K, Akira S, Underhill D M, Anderem A. The innate immune rsponse to bacterial flagelin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 14.Kocan K M. Development of Anaplasma marginale: coordinated development of a rickettsial organisms and its tick host. In: Sauer J R, Hair J A, editors. Morphology, physiology and behavioral ecology of ticks. Chichester, England: Horwood; 1986. pp. 472–505. [Google Scholar]
- 15.Kocan K M, Stiller D, Goff W L, Claypool P L, Edwards W, Ewing S A, McGuire T C, Hair J A, Barron S J. Development of Anaplasma marginale in male Dermacentor andersoni transferred from infected to susceptible cattle. Am J Vet Res. 1992;53:499–507. [PubMed] [Google Scholar]
- 16.Kocan K M, Goff W L, Stiller D, Claypool P L, Edwards W, Ewing S A, Hair J A, Barron S J. Persistence of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in male Dermacentor andersoni (Acari: Ixodidae) transferred successively from infected to susceptible cattle. J Med Ent. 1992;29:657–668. doi: 10.1093/jmedent/29.4.657. [DOI] [PubMed] [Google Scholar]
- 17.Kocan K M, Blouin E F, Barbet A F. Anaplasmosis control: past, present and future. Ann N Y Acad Sci. 2000;916:501–509. doi: 10.1111/j.1749-6632.2000.tb05329.x. [DOI] [PubMed] [Google Scholar]
- 18.Kuttler K L. Anaplasma infections in wild and domestic ruminants: a review. J Wildl Dis. 1984;20:12–20. doi: 10.7589/0090-3558-20.1.12. [DOI] [PubMed] [Google Scholar]
- 19.Palmer G H, Oberle S M, Barbet A F, Goff W L, Davis W C, McGuire T C. Immunization of cattle with a 36-kilodalton surface protein induces protection against homologous an heterologous Anaplasma marginale challenge. Infect Immun. 1988;56:1526–1531. doi: 10.1128/iai.56.6.1526-1531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer G H, Eid G, Barbet A F, McGuire T C, McElwain T F. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect Immun. 1994;63:3808–3816. doi: 10.1128/iai.62.9.3808-3816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ristic M, Watrach A M. Anaplasmosis. VI. Studies and a hypothesis concerning the cycle of development of the causative agent. Am J Vet Res. 1963;24:67–276. [PubMed] [Google Scholar]
- 22.Rurangirwa F R, Stiller D, French D M, Palmer G H. Restriction of major surface protein 2 (MSP2) variants during tick transmission of the ehrlichia Anaplasma marginale. Proc Natl Acad Sci USA. 1999;96:3171–3176. doi: 10.1073/pnas.96.6.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rurangirwa F R, Stiller D, Palmer G H. Strain diversity in major surface protein 2 expression during tick transmission of Anaplasma marginale. Infect Immun. 2000;68:3023–3027. doi: 10.1128/iai.68.5.3023-3027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saitou N, Nei M. The neighboring-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 25.Saliki J T, Blouin E F, Rodgers S J, Kocan K M. Use of tick cell culture-derived Anaplasma marginale antigen in a competitive ELISA for serodiagnosis of anaplasmosis. Ann N Y Acad Sci. 1998;849:273–281. doi: 10.1111/j.1749-6632.1998.tb11059.x. [DOI] [PubMed] [Google Scholar]
- 26.Singer S M, Elmendorf H G. Biological selection of variant-specific surface proteins in Giardia lamblia. J Infect Dis. 2001;183:119–124. doi: 10.1086/317659. [DOI] [PubMed] [Google Scholar]
- 27.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]