Abstract
Ephedrae Herba (Ephedra), known as “MaHuang” in China, is the dried straw stem that is associated with the lung and urinary bladder meridians. At present, more than 60 species of Ephedra plants have been identified, which contain more than 100 compounds, including alkaloids, flavonoids, tannins, sugars, and organic phenolic acids. This herb has long been used to treat asthma, liver disease, skin disease, and other diseases, and has shown unique efficacy in the treatment of COVID-19 infection. Because alkaloids are the main components causing toxicity, the safety of Ephedra must be considered. However, the nonalkaloid components of Ephedra can be effectively used to replace ephedrine extracts to treat some diseases, and reasonable use can ensure the safety of Ephedra. We reviewed the phytochemistry, pharmacology, clinical application, and alkaloid toxicity of Ephedra, and describe prospects for its future development to facilitate the development of Ephedra.
Keywords: Ephedrae Herba, phytochemistry, pharmacological effect, clinical application, alkaloid toxicity
1. Introduction
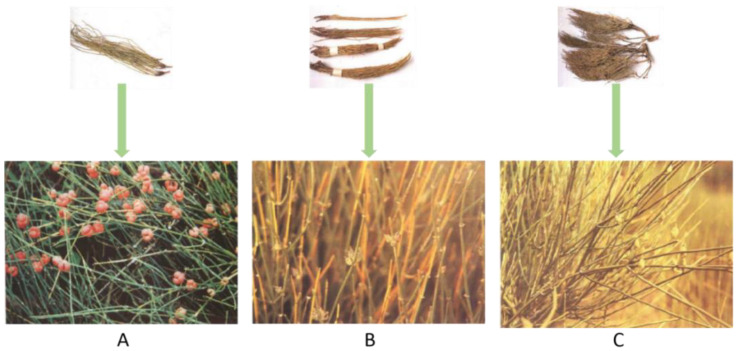
Ephedrae Herba (Ephedra), also known as “MaHuang” in China, grows mostly in dry desert environments and has been used in traditional Chinese medicine for more than 5000 years [1]. In the 2020 edition of the Pharmacopoeia of the People’s Republic of China, Ephedra include the dried straw stems of Ephedra sinica Stapf, Ephedra intermedia Schrenk et C. A. Mey., or Ephedra equisetina Bge (Figure 1A–C). The whole plant is acrid, slightly bitter, warm in nature, and associated with the lung and bladder meridians. It is often used in the clinical treatment of asthma, increasing blood pressure, and analgesia. Moreover, Ephedra can also be used to treat COVID-19 infections to improve the symptoms. Many beneficial components in Ephedra, including alkaloids, flavonoids, polysaccharides, and so on, have unique pharmacological effects. However, the mechanism of its efficacy is not completely explained in the literature, and the corresponding molecular structure has not been fully described and summarized. In addition, the U.S. Food and Drug Administration (FDA) announced in 2004 that additional dietary supplements cannot be used with Ephedra, and Ephedra use in other countries is also restricted [2]. The main reason for this is mainly due to the Ephedra alkaloids having certain toxicity (dose-related), although evidence shows that the nonalkaloid components in Ephedra are still capable of treating diseases. However, its clinical safety is still unknown, and the toxicity of Ephedra has not been thoroughly discussed. In this review, we present an overall overview of the phytochemical properties, pharmacological activities, clinical application, and alkaloid toxicity of Ephedra. By analyzing and integrating the study results, we describe the practical value of Ephedra and propose possible research future directions. We outline the shortcomings of the existing studies, thereby providing a valuable compilation and analysis of the current research on Ephedra plants and laying a foundation for the future Ephedra research.
Figure 1.
Three types of Ephedra listed in the 2020 edition of the Pharmacopoeia of the People’s Republic of China: (A) Ephedra sinica Stapf; (B) Ephedra intermedia Schrenk et C. A. Mey; (C) Ephedra equisetina Bge.
2. Phytochemistry
Ephedra has a complex chemical composition and contains various types of compounds, including alkaloids, flavonoids, tannins, polysaccharides, and organic phenolic acids. Modern medical studies have shown that Ephedra has a wide range of pharmacological effects on the central nervous system, cardiovascular system, and smooth muscle [3,4,5]. Its active constituents mostly include Ephedra alkaloids, and the nonalkaloid components exhibit antioxidant, immunosuppressive, and hypoglycemic properties [6,7].
2.1. Alkaloids
Alkaloids are the main active components of Ephedra, in which a total of 29 have been identified (Table 1). The contents of the following three pairs of stereoisomeric amphetamine alkaloids are the highest: L-ephedrine and D-pseudoephedrine, L-norephedrine and D-norpseudoephedrine, and L-methylephedrine and D-methylpseudoephedrine. These three pairs of stereoisomers are generally considered to be the active ingredients of Ephedra (collectively referred to as ephedrines). Many methods can be used to determine the content of ephedrine alkaloids [8,9,10]. Ibragic et al. established a method for the rapid determination of ephedrine and pseudoephedrine content within 7 min using UPLC-UV, and measured the total content of ephedrine at a dry weight of 20.8 mg/g and pseudoephedrine at a dry weight of 34.7 mg/g. These two compounds accounted for 90% and 99% of the total alkaloid content, respectively [11]. In addition, the alkaloids in Ephedra include imidazole, amphetamine-type, quinoline, and pyrrolidine alkaloids, among others (Table 1, Figure S1).
Table 1.
Alkaloids in Ephedra sinica Stapf (structural formulas are shown in Figure S1).
| No. | Classification | Compound Name | Ref. |
|---|---|---|---|
| 1 | Macrocyclic spermine alkaloids | Ephedradine A | [12] |
| 2 | Ephedradine B | [13] | |
| 3 | Ephedradine C | [13] | |
| 4 | Ephedradine D | [14] | |
| 5 | Imidazole alkaloids | Feruloylhistamine | [15] |
| 6 | Amphetamine-type alkaloids | D(–)-Ephedrine | [16] |
| 7 | L(+)-Pseudoephedrine | [17] [18] |
|
| 8 | D(–)Norephedrine | [17] [18] |
|
| 9 | L(+)-Noreseudoephedrine | [17] [18] |
|
| 10 | D(–)Methylephedrine | [17] [18] |
|
| 11 | L(+)-Methylpseudoephedrine | [17] [18] |
|
| 12 | Ephedroxane | [19] [20] |
|
| 13 | 3, 4-Dimethyl-5-pheyloxazolidine | [20] | |
| 14 | 2, 3, 4-Trimethyl-5-phenyloxazolidine | [20] | |
| 15 | O-benzoyl-L(+)-pseudoephedrine | [20] | |
| 16 | O-benzoyl-D(–)-ephedrine | [20] | |
| 17 | Hordenine | [21] | |
| 18 | (S)-N-((1R, 2S)-1-hydroxy-1-phenylpropan-2-yl)-5-oxopyrrolidine-2-carboxamide | [22] | |
| 19 | Quinoline alkaloids | Transtorine | [23] |
| 20 | 6-Methoxykynurenic acid | [24] | |
| 21 | Kynurenic acid | [24] | |
| 22 | 6-Hydroxykynurenic acids | [24] | |
| 23 | Ephedralone | [25] [26] |
|
| 24 | Pyrrolidine alkaloids Other alkaloids |
cis-3, 4-Methanoproline | [27] |
| 25 | Maokonine | [28] | |
| 26 | (±)-1-Phenyl-2-imido-1-propanol | [29] | |
| 27 | Tetramethylpyrazine | [30] | |
| 28 | Benzylamine | [31] | |
| 29 | N-methybenzlamine | [32] |
2.2. Flavonoids
Flavonoids generally refer to a series of compounds in which two benzene rings (A and B rings) with phenolic hydroxyl groups are connected to each other through the three central carbon atoms. The total flavone content (TFC) extracted from Ephedra by ethanol in a water bath can be analyzed by HPLC/PDA/MS, and is approximately 0.29% [33,34]. At present, more than 40 kinds of flavonoids, such as quercetin, luteolin, and rutin, have been isolated from Ephedra. The results of pharmacological studies have shown that flavonoids in Ephedra can scavenge diphenylpicrohydrazide free radicals, and the strength of its antioxidant effect is related to the number and structure of hydroxyl groups of its active ingredients [35] (Table 2, Figure S2).
Table 2.
Flavonoids in Ephedra sinica Stapf (structural formulas are shown in Figure S2).
| No. | Classification | Compound Name | Ref. |
|---|---|---|---|
| 31 | Flavonols | Herbacetin | [36] |
| 32 | Kaempferol | [37] | |
| 33 | Quercetin | [37] | |
| 34 | Herbacetin 7-methylether | [36] | |
| 35 | Rutin | [38] | |
| 36 | Herbacetin 8-methyl ether3-O-glucoside-7-O-rutinoside | [39] | |
| 37 | Herbacetin 7-O-(6″-quinylglucoside) | [39] | |
| 38 | Herbacetin 3-O-rhamnoside 8-O-glucoside | [26] | |
| 39 | Pollenitin B | [40] | |
| 40 | Herbacetin-8-methyl ether 3-O-glucoside | [36] | |
| 41 | Herbacetin 7-O-glucoside | [40] | |
| 42 | Kaempferol 3-O-rhamnoside 7-O-glucoside | [40] | |
| 43 | Herbacetin 7-O-neohesperidoside | [40] | |
| 44 | Kaempferol-3-O-glucoside-7-O-rhamnoside | [40] | |
| 45 | Kaempferol 3-O-rhamnoside | [39] | |
| 46 | Quercetin 3-O-rhamnoside | [39] | |
| 47 | Quercetin-3-O-glucoside | [38] | |
| 48 | Dihydroflavonol | Dihydroquercetin | [37] |
| 49 | 3-Hydroxynaringenin | [41] | |
| 50 | Flavonone | 3′, 4′, 5, 7-Tetrahydroxy flavanone | [37] |
| 51 | Naringenin | [41] | |
| 52 | Hesperidin | [42] | |
| 53 | Flavanols | (–)-epicatechin | [43] |
| 54 | (–)-epiafzelechin | [37] | |
| 55 | Gallocatechin | [44] | |
| 56 | Epigallocatechin | [37] | |
| 57 | Leucoanthpcyanin | [45] | |
| 58 | Catechin | [40] | |
| 59 | Afzelechin | [37] | |
| 60 | Leucocyanidin | [46] | |
| 61 | Symplocoside | [40] | |
| 62 | Flavones | Tricin | [36] |
| 63 | Luteolin | [47] | |
| 64 | Luteolin-7-glucoside | [33] | |
| 65 | Apigeni | [37] | |
| 66 | 3-Methoxyherbacetin | [36] | |
| 67 | Apigenin-5-rhamnoside | [36] | |
| 68 | 6-C-glycosyl-chrysoeriol | [48] | |
| 69 | Swertisin | [49] | |
| 70 | Isovitexin | [50] | |
| 71 | Isovitexin-2″-O-rhamnoside | [40] | |
| 72 | Apigenin-7-O-glucoside | [38] | |
| 73 | Vitexin | [40] | |
| 74 | Lucenin III | [39] | |
| 75 | 2″, 2′″-Di-O-β-glucopyranosyl-vicenin II | [51] | |
| 76 | 6, 8-di-C-hexosyl apigenin | [44] | |
| 77 | 6/8-C-hexosyl-8/6-C-pentasyl apigenin | [44] | |
| 78 | Anthocyan | Leucodelphinidin | [46] |
| 79 | Leucopelargonin | [46] |
2.3. Tannins
The tannins make up a class of polyphenolic compounds with complex structures that are widely found in plants. They usually exist in condensed form in Ephedra, including dimer, trimer, and tetramer proanthocyanidins, as well as hydrolytic tannins (Table 3, Figure S3). The latest method for detecting tannins in Ephedra is gel permeation chromatography, developed by a team from Japan [52].
Table 3.
Tannins in Ephedra sinica Stapf (structural formulas are shown in Figure S3).
| No. | Classification | Compound Name | Ref. |
|---|---|---|---|
| 80 | Dimer proanthocyanidins | Ephedrannin A | [53] |
| 81 | Ephedrannin B | [53] | |
| 82 | Muhuannin A | [54] | |
| 83 | Muhuannin D | [53] | |
| 84 | Muhuannin B | [53] | |
| 85 | Muhuannin E | [53] | |
| 86 | Muhuannin C | [38] | |
| 87 | Muhuannin F | [55] | |
| 88 | Muhuannin G | [55] | |
| 89 | Muhuannin H | [56] | |
| 90 | Muhuannin I | [55] | |
| 91 | Muhuannin J | [56] | |
| 92 | Muhuannin K | [55] | |
| 93 | Ephedrannin D1 | [44] | |
| 94 | Ephedrannin D2 | [44] | |
| 95 | Ephedrannin D3 | [40] | |
| 96 | Ephedrannin D4 | [44] | |
| 97 | Ephedrannin D5 | [44] | |
| 98 | Ephedrannin D6 | [44] | |
| 99 | Ephedrannin D7 | [44] | |
| 100 | Ephedrannin D8 | [44] | |
| 101 | Ephedrannin D9 | [44] | |
| 102 | Ephedrannin D10 | [44] | |
| 103 | Ephedrannin D11 | [44] | |
| 104 | Ephedrannin D12 | [44] | |
| 105 | Ephedrannin D13 | [44] | |
| 106 | Ephedrannin D14 | [44] | |
| 107 | Trimer proanthocyanidins | Ephedrannin Tr1 | [44] |
| 108 | Ephedrannin Tr2 | [44] | |
| 109 | Ephedrannin Tr3 | [44] | |
| 110 | Ephedrannin Tr4 | [44] | |
| 111 | Ephedrannin Tr5 | [44] | |
| 112 | Ephedrannin Tr6 | [44] | |
| 113 | Ephedrannin Tr7 | [44] | |
| 114 | Ephedrannin Tr8 | [44] | |
| 115 | Ephedrannin Tr9 | [44] | |
| 116 | Ephedrannin Tr10 | [44] | |
| 117 | Ephedrannin Tr11 | [44] | |
| 118 | Ephedrannin Tr12 | [44] | |
| 119 | Ephedrannin Tr13 | [44] | |
| 120 | Ephedrannin Tr14 | [40] | |
| 121 | Ephedrannin Tr15 | [40] | |
| 122 | Tetramer proanthocyanidins | Ephedrannin Te1 | [40] |
| 123 | Ephedrannin Te2 | [40] | |
| 124 | Ephedrannin Te3 | [40] | |
| 125 | Ephedrannin Te4 | [40] | |
| 126 | Ephedrannin Te5 | [40] | |
| 127 | Hydrolytic tannins | Nilocitin | [39] |
2.4. Polysaccharides
Polysaccharides are macromolecular components in Ephedra plants. At present, the main polysaccharides isolated from Ephedra are polysaccharides A, B, C, D, and E, and hyperbranched acidic polysaccharides (ESP-B4). Among them, the relative molecular masses of Ephedra polysaccharides A, B, C, D, and E are 1.2 × 106, 1.5 × 106, 9 × 104, 6.6 × 103, and 3.4 × 104, respectively. In addition, when Ephedra stems were continuously extracted by the water extraction method (liquid–solid ratio 5) in a water bath at 90 °C for 3 h, the monosaccharide composition of the water-soluble polysaccharides in Ephedra was obtained as 43.1% glucose, 36.4% galactose, 14.9% mannose, 3.7% arabinose, and 1.7% gluconic acid [57].
2.5. Organic Acids
Cottiglia et al. separated phenolic acids, such as nebrodenside A, nebrodenside B, and O-coumaric acid glucoside, from Ephedra for the first time in 2005 [43]. Since then, scientists have also successively separated organic acids such as trans-cinnamic acid and syringin from Ephedra (Table 4, Figure S4).
Table 4.
Organic acids in Ephedra sinica Stapf (structural formulas are shown in Figure S4).
| No. | Classification | Compound Name | Ref. |
|---|---|---|---|
| 128 | Organic acids | Nebrodenside A | [22] [43] |
| 129 | Nebrodenside B | [22] [43] |
|
| 130 | O-coumaric acid glucoside | [22] [58] |
|
| 131 | Trans-cinnamic acid | [58] | |
| 132 | Syringin | [45] | |
| 133 | O-Coumaric acid | [25] | |
| 134 | ρ-Hydroxybenzoic acid | [56] | |
| 135 | Protocatechuic acid | [58] | |
| 136 | Quinaldic acid | [59] | |
| 137 | 2-Hydroxyl-5-methoxybenzoic acid | [55] | |
| 138 | Iso-ferulic acid | [55] | |
| 139 | Vanillic acid | [56] | |
| 140 | Caffeic acid | [58] | |
| 141 | Chlorogenic acid | [58] | |
| 142 | (3R)-3-O-β-D-glucopyranosyl-3-phenylpropanoic acid | [22] | |
| 143 | Malic acid | [60] | |
| 144 | Citric acid | [60] | |
| 145 | Oxalic acid | [60] | |
| 146 | Fumaric acid | [60] | |
| 147 | 4-O-β-D-glucoside benzoic acid | [61] | |
| 148 | 5-(hydroxy-isopropyl)-cyclohexenecarboxylic acid | [58] | |
| 149 | Pseudolaroside B | [61] | |
| 150 | n-hexacosane acid | [62] | |
| 151 | Trans-aconitic acid | [50] |
2.6. Organic Volatile Essential Oil
The volatile oil in Ephedra is one of its medicinal material bases [63,64]. The content of volatile oil in Ephedra is low, at only approximately 0.15% [65]. Different planting methods, processing methods, and extraction techniques affect the content of volatile oil in Ephedra [66,67,68]. We provide a summary of the 30 common volatile oils in Ephedra in Table 5 and Figure S5.
Table 5.
Organic volatile essential oil in Ephedra sinica Stapf (structural formulas are shown in Figure S5).
| No. | Classification | Compound Name | Ref. |
|---|---|---|---|
| 152 | Organic volatile essential oil | β-Sitosterol | [63] |
| 153 | 9Z, 12Z-Octadecadienoic acid | [63] | |
| 154 | 9-E-Octadecenoic acid | [63] | |
| 155 | Ergost-5-en-3β-ol | [63] | |
| 156 | Nonacosanol | [63] | |
| 157 | L-α-terpineol | [69] | |
| 158 | Linolenic acid | [63] | |
| 159 | Terpineol acetate | [70] | |
| 160 | 3, 7, 11, 15-Tetramethyl-2-hexadecen-1-ol | [63] | |
| 161 | Stearic acid | [63] | |
| 162 | Globulol | [70] | |
| 163 | γ-eudesmol | [69] | |
| 164 | Linalool | [69] | |
| 165 | Eicosanoic acid | [63] | |
| 166 | Cis-2-ρ-menthen-7-ol | [69] | |
| 167 | Terpinen-4-ol | [69] | |
| 168 | β-terpineol | [71] | |
| 169 | Myrcene | [71] | |
| 170 | Dihydrocarveol | [71] | |
| 171 | 1, 3, 4-Trimethyl-3-cyclohexene-1-carboxaldehyde | [71] | |
| 172 | Trans-phytol | [72] | |
| 173 | Linolenic acid methyl ester | [72] | |
| 174 | γ-Sitosterol | [72] | |
| 175 | 1, 4-Cineole | [69] | |
| 176 | 1, 8-Cineole | [69] | |
| 177 | ρ-Cymene | [69] | |
| 178 | Limonene | [69] | |
| 179 | γ-Terpinene | [69] | |
| 180 | Hexadecanoic acid | [69] | |
| 181 | Dibutyl phthalate | [69] |
2.7. Other Ingredients
In addition to the above compounds isolated and identified from Ephedra, other ingredients such as lignans, naphthalenes, esters, terpenoids, and quinones have been identified (Table 6, Figure S6).
Table 6.
Other components in Ephedra sinica Stapf (structural formulas are shown in Figure S6).
| No. | Classification | Compound Name | Ref. |
|---|---|---|---|
| 182 | Lignans | DL-Syringaresinol | [25] |
| 183 | Sesquipinsapol B | [55] | |
| 184 | Naphthalenes | Methyl-2,3-methylenedioxy-6-naphthalenecarboxylic acid methyl ester | [58] |
| 185 | Esters | Ethyl caprylate | [73] |
| 186 | Terpenoids | (–)-α-Terpineol-8-O-β-D-glucopyranoside | [55] |
| 187 | (+)-α-Terpineol-8-O-β-D-glucopyranoside | [55] | |
| 188 | Geranyl-β-D-glucopyranoside | [55] | |
| 189 | Daucosterol | [55] | |
| 190 | Sitosterol | [55] | |
| 191 | Stigmasterol-3-O-β-D-glucopyranoside | [73] | |
| 192 | Quinones | Physcion | [58] |
| 193 | Rhein | [58] | |
| 194 | Phenols | ρ-Aminophenol | [58] |
| 195 | Rhododendrol-4′-O-β-D-glucopyranoside | [61] | |
| 196 | Vinylguaiacol | [72] | |
| 197 | Di-tert-butylphenol | [72] | |
| 198 | Antiarol | [72] | |
| 199 | Ureas | Allantoin | [44] |
3. Pharmacological Effects
3.1. Antipyretic and Diaphoretic Effects
The ephedrine, volatile oil, and their decoctions in Ephedra all produce different degrees of antipyretic and diaphoretic effects. The diaphoretic effect is especially notable. Wang et al. investigated the heat-inducing mechanism of Ephedra by adopting UPLC-Q/TOF-MS, and discovered that the 2-adrenoceptor (β2-AR) is a signal channel that modulates human perspiration [74]. Jo et al. randomly divided patients into an ephedrine injection group and normal saline injection control group and observed that esophageal temperature and hemodynamic variables remained stable in the patients injected with ephedrine, whereas those in the control group injected with normal saline substantially decreased. Compared with the control group, the index finger temperature of the patients in the ephedrine group was markedly lower. This showed that ephedrine can minimize the core temperature, maintain the overall temperature, and stabilize the maintenance of hemodynamic variables [75]. Carlisle et al. studied the effect of intraperitoneal ephedrine on the metabolic rate of rats in cold (−8 °C) and thermoneutral (22 °C) environments. They found that the diaphoretic effect of ephedrine is closely related to the ambient temperature: the higher the ambient temperature, the better the diaphoretic and heat-generating effect [76]. This also further confirmed that the mechanism of Ephedra diaphoretic may be closely related to the function of the central nervous system [77]. In addition, other researchers have found that Ephedra can reduce the IL-1β level and inhibit HSP70 and NF-κB to promote hypothalamic homeostasis and inhibit heat stress [78]. To summarize, Ephedra plays antipyretic and sweating roles mainly by inhibiting the release of fever factors and controlling the perception by the central nervous system of environmental temperature, but the specific pathway still needs further exploration.
3.2. Inhibiting Asthma
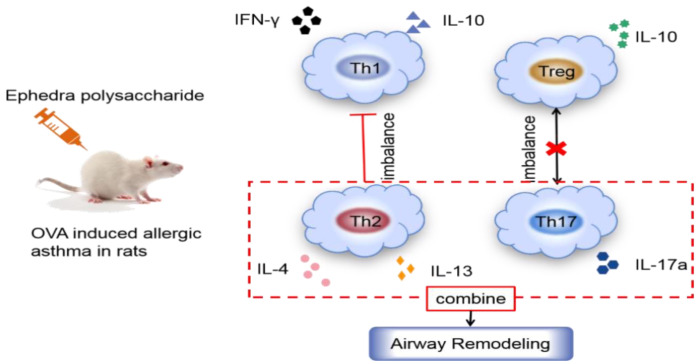
In traditional Chinese medicine, Ephedra is thought to stimulate warmth, relax the qi machine, open the skin, and unblock the qi in the lungs; because of its bitterness, the lungs are internally rejuvenated, which calms coughing. Mei et al. randomly divided Wistar rats into five groups, including a control group and an aspirin group, and the groups received three different doses of ephedrine plaster (6, 12, or 24 g/kg). The antiasthma effect of ephedrine plaster was evaluated with an ovalbumin (OVA)-induced asthma rat model. The fever caused by inflammation in the rats in the ephedrine plaster groups was significantly reduced in a dose-dependent manner; the increase in eosinophils caused by OVA was also reduced, and the lung dry weight ratio in the antiasthma test significantly decreased [79]. These findings indicated that the Ephedra-gypsum extract had antipyretic and antiasthmatic effects. Ma et al. established a mouse asthma model induced by OVA, used an enzyme-linked immunosorbent assay (ELISA) to assess Th1/Th2 and Th17 cytokine levels, and assessed Th17 cell flow cytometry with an ELISA assay to determine the antiasthmatic effect of a Mahuang decoction (consisting of Ephedra, Glycyrrhizae Radix et Rhizoma, Cinnamomi Ramulus, and Armeniacae Semen Amarum), with Ephedra being the key ingredient. The results showed that the Mahuang decoction could inhibit Th17 cells, and effectively inhibited the progression of asthma, which indicated that the Mahuang decoction could be used as a therapeutic drug for patients with allergic asthma [80]. Recently, ELISA, quantitative real-time reverse transcription (qRT)-PCR, and flow cytometry (FCM) were used to measure the levels of inflammatory factors and inflammatory cells in asthmatic model rats, which showed that Ephedra polysaccharides could improve the symptoms of asthmatic rats by regulating the immune imbalance in Th1/Th2 and Th17/Treg cells [81]; the specific pathway is shown in Figure 2. Huang et al. used selective and rapid HPLC/MS-MS to study the ephedrine, pseudoephedrine, methylephedrine, and other ephedrines in an asthma rat model established by the OVA sensitization method after using a Mahuang decoction. The blood concentration of the main components of the decoction showed that it could control or improve asthma, with a lag between the peak drug concentration and the maximum therapeutic effect [82]. Tang et al. found that the Mahuang decoction can be taken together with theophylline to treat cough and asthma [83]. In addition, aerosol administration of Ephedra aqueous extract could inhibit asthma, because it inhibited IL-13 and eotaxin protein expression, reduced airway inflammation, and controlled eosinophil infiltration [84].
Figure 2.
Ephedra polysaccharide regulates OVA-induced inflammatory response in asthma.
3.3. Anti-Inflammatory Effect
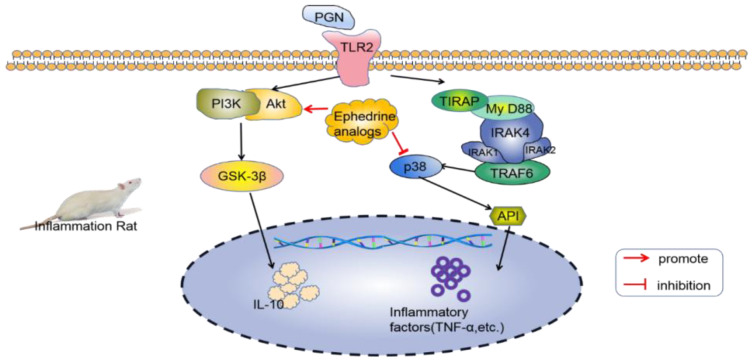
Ephedra contains anti-inflammatory chemicals. In 1985, ephedrine, pseudoephedrine, and ephedrine analogs were found to have strong anti-inflammatory activities in vivo. Its anti-inflammatory effect may be achieved by inhibiting the biosynthesis of prostaglandin E2 [85]. Ephedra protected mice from endotoxin injury by inhibiting the expression of inflammatory factors induced by Gram-positive bacteria (G+), which was due to the Toll-like receptor 2 (TLR2) stimulation by peptidoglycan (PGN) in macrophages. Ephedrine administration can produce an anti-inflammatory effect by stimulating the expression of IL-10 in dendritic cells (DCs) and inhibiting the production of induced inflammatory factors (such as TNF-α) through the PI3K/Akt and PGN pathways. Moreover, ephedrine can act on PI3K, Akt, and the downstream GSK-3β and p38 pathways, leading to the increased expression of IL-10 and the decreased expression of inhibitory inflammatory factors [86,87]; the specific mechanism is summarized in Figure 3.
Figure 3.
Mechanism through which Ephedra inhibits inflammatory factors in mice with peritonitis.
He et al. found that Ephedra had a strong inhibitory effect on the secretion of inflammatory mediators and inflammatory cell infiltration in the lung tissue of asthmatic rats; it inhibited the expressions of IL-21, IL-21R, STAT3, and p-STAT3 in lung tissue [88]. In addition, pseudoephedrine has anti-inflammatory effects. Wu et al. used high-performance liquid chromatography with a photodiode array detector (HPLC-PDA) and utilized the fingerprint screening of anti-inflammatory components to identify the characteristics and active components of Suhuangzhenke capsules (SHs) as quality control markers. The results showed that pseudoephedrine significantly inhibited inflammatory mediator NO production in LPS-stimulated RAW264.7 macrophages, which proved that pseudoephedrine also has anti-inflammatory effects [89]. Zhai et al. found that Ephedra could substantially reduce the degree of alveolitis and pulmonary fibrosis, and had a therapeutic effect on idiopathic pulmonary fibrosis rats induced by bleomycin [90,91]. When Ephedra plays an anti-inflammatory role, many of the active substances or receptors are the same or belong to the same family as those that are active when relieving asthma, fever, and sweating. Therefore, effective substances in Ephedra may simultaneously play different roles through the same pathway, which provides ideas for further understanding the role of some of the pathways.
3.4. Hepatoprotection Effect
In 1984, Japanese researchers successfully isolated feruloyl histamine from Ephedra and found that it has an inhibitory effect on histidine decarboxylase and antihepatotoxicity [15]. Furthermore, Han et al. found that the alkaloids in Ephedra protected the livers of rats in a model of D-galactosamine–lipopolysaccharide (D-GalN/LPS)-induced acute liver failure by inhibiting the high expression of tumor necrosis factor [92]. Ghasemi et al. determined the hepatoprotective effect of an Ephedra extraction on carbon tetrachloride (CCl4)-induced chronic and acute liver failure mouse models, and the antioxidant activity of Ephedra extraction was determined by 2,2′-diphenyl-1-pyridohydrazine (DPPH) and β-carotene bleaching methods. The findings revealed that after treatment with an Ephedra extraction, all parameters of liver inflammation significantly decreased in liver injury mice, which could be attributed to the hepatoprotective effects of Ephedra, which functioned to inhibit oxidative stress and reduce liver inflammation [93]. Ephedra significantly inhibited the apoptosis of hepatocytes and could thus treat acute liver failure by inhibiting the activities of D-galactosamine (GalN) and lipopolysaccharide (LPS). This thereby inhibited the activities of serum alanine aminotransferase (ALT); total bilirubin (T Bil); and caspases 8, 9, and 3, resulting in curbing liver failure. The authors speculated that Ephedra may increase the level of transcription activating factor 3 (STAT3) by increasing the level of IL-6, which may be an effective therapeutic pathway through which Ephedra treats acute liver failure [94]. Song et al. found that an ephedrine alkaloid-free Ephedra extraction regulated lipid metabolism by inhibiting the production of free radicals and helping with the recovery of liver function [95]. The above studies show that the active ingredients in Ephedra can protect the liver (mainly due to its antioxidative and antiapoptotic properties), inhibit the secretion of inflammatory factors, as well as regulate liver lipid metabolism to produce a unique hepatoprotective effect. Ephedra is more distinctive in traditional Chinese medicine. According to ancient books, it is effective in treating jaundice, although the foreign literature claims that it is harmful to the liver [96,97]; therefore, additional investigation is necessary regarding the suitably of the application of Ephedra for the liver.
3.5. Antibacterial and Antifungal Effect
The stems and seeds of Ephedra contain many antibacterial components [98,99], and the inhibitory effects and mechanisms of Ephedra on different types of bacteria are different. Phenolic compounds isolated from Ephedra have remarkable antibacterial activity against Gram-negative and -positive bacteria and fungi [99]. Ali et al. reported that three types of wild Ephedra (Ephedra Strobilacea, Ephedra Pachyclada, and Ephedra Procera) and their respective callus cultures showed strong antibacterial activities, but the original plants exhibited higher antibacterial activity against damaged tissues [100]. Zang et al. tested the antibacterial activity of 12 kinds of A-type proanthocyanidins isolated from Ephedra and found that these compounds inhibited Gram-positive and -negative bacteria and fungi at concentrations ranging from 0.00515 to 1.38 mmol·L−1 [44]. Similarly, the 4-quinolone-2-carboxylic acid isolated from Ephedra showed strong antibacterial activity against common bacteria such as Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus [23]. An Ephedra extraction also produced an excellent inhibitory effect on Aspergillus flavus and Aflatoxin. Researchers evaluated the effect of methanol extracts of the aerial parts and roots of Ephedra on the growth of Aspergillus parasiticus NRRL 2999 and the Aflatoxin B(1) (AFB(1)) produced by itself; they found that the concentration of the methanol extract positively correlated with the inhibition of fungal growth and the production rate of AFB(1). The IC50 and effective concentration values of AFB(1) were 559.74 and 3.98 μg·mL−1, respectively. The methanol extract also inhibited the growth of fungi [101]. All the extracts described above in Ephedra that play an antibacterial role are not Ephedra alkaloids, which means that Ephedra does not rely much on alkaloid components when it plays a specific role, which has reference value for subsequent studies on the safety of Ephedra drug use.
3.6. Anticancer and Analgesia
Although Ephedra is suitable for treating sweating and fever, it is also commonly used for pain relief in clinics. A study reported that a flavonoid aglycone present in herbacetin 7-O-neohsperidin in Ephedra has anticancer cell proliferation effects and analgesic properties [102]. It could also inhibit the abnormal proliferation of cancer cells induced by the hepatocyte growth factor (HGF) by inhibiting C-Met phosphorylation and its tyrosine kinase activity through the PI3K/Akt pathway [103,104]. Moreover, the expressions of tyrosinase genes, as well as B16F10 melanoma cells, were significantly dose-dependently inhibited by ephedrine A and B [105]. In another study, the remaining components in the aqueous phase, after the methanol extract of Ephedra was re-extracted with butanol and ethyl acetate, were able to significantly grow tumor blocks in BDF1 mice (30 mg/kg/d) [106]. Chinese herbal formulae incorporating Ephedra have been investigated primarily for the treatment of lung cancer, breast cancer, thyroid tumor, and other tumors. According to the above findings, Ephedra can be used for the treatment of clinical cancer, but in-depth research on the molecular mechanisms through which Ephedra functions in the treatment of various cancers has not yet been conducted, which may affect the subsequent clinical application of Ephedra.
3.7. Antivirus Effect
According to the Compendium of Materia Medica·Cao Bu, Ephedra ranked first among the drugs used for the treatment of heat toxic skin syndrome and Qi-Fen syndrome. Modern studies have also shown that Ephedra has a strong antiviral effect. Japanese scientists found that an Ephedra extract can induce the nuclear translocation of nuclear factor-κB (NF-κB) to eliminate the latent virus reservoir in people with human immunodeficiency virus type 1 (HIV-1) [107]. Additionally, Chinese scientists found that a Mahuang decoction could increase the levels of IL-2 and interferon-γ in mice by inhibiting the TLR4/MyD88/TRAF6 signaling pathway, and reduce the level of IL-4 and the expression of NF-κB, thus producing antiviral effects on mice infected with influenza virus (IFV) [108]. According to the prediction of network pharmacology, Mahuang decoctions may act on AKT1, TNF, TP53, IL6, JUN, and other targets through the PI3K/Akt, MAPK, and JAKSTAT signaling pathways, so as to prevent and treat influenza A [71].
4. Clinical Application
4.1. Treatment of Coronavirus Disease 2019 (COVID-19)
On 11 February 2020, the World Health Organization officially designated COVID-19 as a pandemic, which has spread around the world [109]. Traditional Chinese medicine showed a strong COVID-19 prevention and treatment effect, and a series of prescriptions showed therapeutic effects, such as Shufeng Jiedu capsules, a Maxing Shigan decoction, Lianhua Qingwen capsules/granules, a Qingfei Paidu decoction (QFPDT), etc. Among them, the QFPDT, which consists of 21 herbs, such as Ephedra, gypsum, and licorice, produced effective results across more than 10 provinces and cities in China. According to the study, the formula may effectively slow disease advancements in mild cases, decrease the duration of common and severe symptoms, and shorten hospital stay durations [110]. Yao et al. divided 42 patients with COVID-19 into a treatment group and a control group to estimate the therapeutic effect of Lianhua Qingwen granules; the control group received only basic treatment (Western medicine treatment). The results showed that compared with the control group, the fever symptoms of 18 patients in the treatment group decreased, accounting for 85.7% of all patients, which was significantly better than the 57.1% achieved in the control group (p < 0.05). Cough symptoms disappeared in seven cases (46.7%), which was significantly better than the 5.6% achieved in the control group (p = 0.012). Furthermore, the therapeutic impact of the treatment group was statistically superior to that of the control group in terms of reducing muscular pain, runny nose, headache, vomiting, and a lack of appetite [111]. In addition, the drug pair containing Ephedra played a key role in the treatment of COVID-19 [112,113]. Ang et al. found that when Ephedra was paired with gypsum fiber, it was effective in the treatment of pediatric COVID-19 [114]. The latest study showed that quinoline-2-carboxylic acid in Ephedra could effectively antagonize the active compounds produced by the interaction between angiotensin-converting enzyme 2 (ACE2) and SARS-CoV-2 spike protein receiver binding domain (SARS-CoV-2 RBD) and inhibit the viral infection, thus showing the potential of using Ephedra to treat COVID-19 infection [115]. This provides strong evidence that Ephedra is an effective medicine used for the treatment of COVID-19 infections. Through network pharmacological prediction, the therapeutic targets of Ephedra are likely TNF-α, IL2, FOS, ALB, and PTGS2 in the pathways related to respiration, nerves, blood circulation, and digestion [112].
Additionally, Ephedra is a pharmaceutical substance frequently used in the therapeutic treatment of COVID-19. Wang et al. reviewed the prescriptions of traditional Chinese medicine for patients with severe COVID-19, and they performed correlation analysis on drugs according to the principles of prescription analysis. They finally identified 1532 effective prescriptions; Ephedra was one of the high-frequency prescriptions. In the prescription analysis by Fan et al., Ephedra was also used up to 142 times. The above findings showed that Ephedra plays an important role in the treatment of COVID-19 infection [116,117]. These data fully demonstrate that Ephedra plays an important role in the treatment of COVID-19 infection, and provides inspiration for the future direction of the traditional Chinese medicine (TCM) treatment of disease.
4.2. Treatment of Asthma
Asthma is the most common chronic respiratory disease in the world and is associated with a persistent inflammatory response caused by a variety of immune cells. Ephedra, which has multiple targets and multiple function pathways, can effectively be used for the treatment of asthma. According to historical ancient books, in ancient times, Ephedra was used in combination with other drugs to treat cough and asthma in east Asia [118]. The ephedrine, pseudoephedrine, and volatile oils contained in Ephedra have antiasthmatic effects, among which ephedrine is the most powerful. Hou et al. found that in Qingfei Xiaoyan pills, the nonselective β-AR agonist ephedrine was the main bronchodilator, and ephedrine could synergize with lignins, such as arctiin, arctigenin, descurainoside, and descurainolide B, to produce a bronchodilation effect, and to treat cough and asthma [119]. Ephedra can be used in the clinic to treat asthma because it can regulate the immune imbalance in Th1/Th2 and Th17/Treg cells.
4.3. Raising Blood Pressure and Treating Muscle Weakness
In many circumstances, the blood pressure of the human body rapidly drops, such as during a major bleeding accident during an operation, in pregnant women, or in those who suddenly change their position, especially middle-aged and elderly people. This situation can sometimes be life-threatening. Muscle weakness is a disease of neuromuscular junction transmission dysfunction, which is clinically characterized by skeletal muscle fatigue. The common challenge in treating the two disorders is how to rapidly and forcefully develop muscular strength in order to raise blood pressure and alleviate muscle weakness. Because of the effect of ephedrine in stimulating the secretion of adrenaline and in increasing muscle strength, ephedrine is commonly used clinically to rescue hypotension and myasthenia gravis [120]. Santana et al. retrospectively evaluated several patients with idiopathic scoliosis whose mean arterial pressure dropped to below 60 mmHg during an operation and found a substantial association between blood pressure elevation and ephedrine consumption [121]. When Kitaura et al. intravenously injected 4 mg of ephedrine into patients with Parkinson’s disease whose blood pressure was significantly reduced, they observed unexpectedly large contractions and increased blood pressure from 78 to 168 mmHg, and the heart rate increased from 52 beats per minute (bpm) to 84 bpm. This phenomenon occurred every time when 4 mg of ephedrine was injected, showing that ephedrine has an effect on blood pressure [122]. Wang compared the efficacy and safety of the drugs (norepinephrine, phenylephrine, and ephedrine) used for hypotension in women with pre-eclampsia under spinal anesthesia during cesarean section. The results showed that the heart rate increase caused by norepinephrine and phenylephrine was significantly lower than that of ephedrine (80.5 ± 12 vs. 84.9 ± 7.1 bpm; p = 0.02), and fewer episodes of ephedrine tachycardia occurred, indicating that ephedrine not only quickly raised blood pressure, but was also safer [123]. The pressure-boosting mechanism of ephedrine may involve increasing the heart rate, and enhancing the myocardial contractility and cardiac output, thus increasing systemic and pulmonary blood pressure levels and the vasoconstriction response, which can then produce a positive inotropic effect, thus increasing the blood pressure [124]. In addition, a Xiaoxuming decoction containing Ephedra was used to effectively treat patients with myasthenia gravis and recurrent myasthenia gravis [125].
4.4. Analgesic Effect
Ephedra can produce an analgesic effect through the pseudoephedrine and polysaccharide contained in Ephedra, which can relax smooth muscle and relieve blood stasis [126]. Schachtel et al. conducted analgesia tests on 640 patients with upper respiratory tract infection through acetylsalicylic acid (ASA) and pseudoephedrine (PSE), and found that the analgesic effect and tolerance were good [127]. Basu found that the administration of oral pseudoephedrine in adults reduced the pain and trauma in the middle ear during aviation flight [128]. Yoshimura et al. reported that Ephedra extract components, other than Ephedra alkaloids, could inhibit cellular mesenchymal-to-epithelial transition factor (c-Met) to relieve pain [52]. The analgesic effect of Ephedra is presently thought to be produced by pseudoephedrine and polysaccharides in Ephedra, but the mechanism through which the effect is produced is not yet clear.
4.5. Treatment of Skin Diseases
Skin allergy is an allergic reaction, manifested as erythema, papules, and itching. Ephedra extract has antibacterial activity, which can prevent the invasion of microorganisms, thereby promoting wound healing. Many ancient Chinese medicine prescriptions for the treatment of skin diseases are related to Ephedra, such as Mahuang Lianqiao Chixiaodou decoctions for the treatment of dermatitis, Maxing Shigan decoctions for the treatment of skin allergies, etc. [129]. In all of these, Ephedra is the main drug. Although Ephedra is frequently applied in skin diseases, studies on its target and molecular mechanism are lacking, which will seriously affect the promotion of the clinical use of Ephedra.
4.6. Treatment of Gynecological Diseases
In traditional Chinese medicine, gynecological diseases are mostly considered syndromes of yang deficiency and cold coagulation. Ephedra can regulate yang, which can not only warm the meridians, but also invigorate qi and dispel cold. Adams et al. found that in California, many herbs including Ephedra are widely used to treat female dysmenorrhea and premenstrual syndrome [130]. Jaradat et al. also found that Ephedra is the most commonly used plant for breast cancer treatment in Palestine [131]. In addition, researchers used XTT analysis (tetrazolium salt reduction) to detect the cytotoxicity of ovarian cancer cell lines (A2780 and A2780CisR) and noncancerous kidney cells (HEK-293) after using Ephedra, and found that Ephedra treatment reduced sensitivity to cisplatin and the cytotoxicity of ovarian cancer cells [132].
5. Alkaloid Toxicity
The misuse and abuse of products containing Ephedra and its extracts have led to many toxic events over the past few decades. As of 2004, the U.S. Food and Drug Administration had received more than 18,000 reports of toxic reactions related to Ephedra [133], and banned the sale of dietary supplements containing Ephedra [2]. Toxic reactions when using Ephedra include excitement, sweating, dysuria, and increased blood pressure, as well as more severe cases including arrhythmia, nephritis, gallstones, and possibly death due to heart or respiratory failure [134,135,136]. Scientists found that a high dose (1000 mg/kg) of Ephedra aqueous extract significantly increased the number of basophils in renal tubules, the weight of salivary glands, and the hypertrophy acinar cells of both female and male F344 rats [137]. When ephedrine and caffeine were used together, F344 rats showed muscle fiber degeneration or even loss [138]. In another group of animal experiments, high concentrations of Ephedra or ephedrine (dosage equivalent to 12.5 to 50 mg/kg of ephedrine) caused cardiac toxicity in rats, which positively correlated with the dosage [139].
The cause of the adverse reactions to Ephedra is its alkaloids; thus, scientists have analyzed the safety of Ephedra extracts without Ephedra alkaloids. Takemoto et al. used ephedra extract (EHE) and ephedra extract without ephedra alkaloids (EFE) to test the activity time, forced swimming time, and pentobarbital-induced sleep time of mice in an open field. They found that both could increase the activity of mice in the open field, reduce the immobility time in forced swimming, and reduce the sleep time in sleep tests. However, compared with mice in the EHE group, the mice in the EFE group did not have arrhythmia, suggesting that EFE may be used as a substitute for EHE without side effects in the future [7]. Kobayashi also found that EHE without Ephedra alkaloids not only suppressed pain, but also did not have the side effects of Ephedra alkaloids such as sleep deprivation and arrhythmia [140]. Both EHE and EFE showed similar analgesic effects after oral administration, whereas EFE did not show the side effects of EHE [126]. In addition, it was reported that two groups of subjects were injected with EHE and EFE respectively for treatment, and the incidence of adverse events in the EHE treatment group was higher than that in the EFE group, but it was not statistically significant [141]. In addition, EFE preserved the anticancer properties of Ephedra, which indicated its developmental potential as a new therapeutic drug [142]. More studies are indicating that the nonalkaloid components in Ephedra can equally replace Ephedra extracts to treat some diseases without producing the side effects related to the latter. This suggests that further clinical studies are needed to determine the safety and efficacy of the nonalkaloid components of Ephedra. Currently available information shows no serious adverse events, and Ephedra is not listed as a poisonous medicinal material in the Chinese Pharmacopoeia.
However, prescription is a commonly used form of administration in Chinese medicine; that is, Ephedra is rarely used alone for treatment, but is instead used in combination with other drugs. Therefore, the safety of the prescription containing Ephedra must be known. When the concentration is 100 times higher than the adult clinical dosage, that is, a body weight of 23,000 mg/kg, a Mahuang Dingchuan decoction (consisting of Ephedra, Ginkgo Semen, Farfarae Flos, Pinelliae Rhizoma, Mori Cortex, Perillae Fructus, Scutellariae Radix, and Glycyrrhizae Radix et Rhizoma) had no obvious acute toxicity to mice and was relatively safe [143]. However, the mice died 1 h after the high-dose administration of a Mahuang decoction (consisting of Ephedra, Cinnamomi Ramulus, Armeniacae Semen Amarum, and Glycyrrhizae Radix et Rhizoma), with an LD50 of 51.07 g/kg [144]. Therefore, attention should be paid to the dosage when using any prescription containing Ephedra in clinical practice.
6. Conclusions and Future Perspective
We reviewed the botany, chemical composition, pharmacological action, clinical application, and toxicity of Ephedra. At present, more than 60 species of Ephedra have been identified, which can be mainly divided into Ephedra sinica Stapf, Ephedra intermediate Schrenk et C. A. Mey, and Ephedra equisetina Bge, which contain more than 100 compounds including alkaloids, flavonoids, tannins, sugars, and organic phenolic acids. The pharmacological effects that have been identified include antipyretic, antiasthmatic, anti-inflammatory, and liver protective effects, among others. Its clinical applications are extensive, as it can be used to treat asthma, liver disease, skin disease, and COVID-19 infection. However, its toxicity cannot be ignored. Ephedra alkaloids represent the main cause of toxic reactions, but evidence shows that Ephedra extracts without alkaloids have similar efficacy in treating some diseases with no adverse reactions.
Although Ephedra has a long been widely applied, some aspects require further study. First, more than 20 medicinal plants of Ephedra have been studied for their pharmacological activities, but the chemical constituents and pharmacological activities of many other Ephedra plants have not been studied. Therefore, to expand upon the applications of Ephedra, these untested species must be examined. Second, the mechanisms through which the many components of Ephedra treat some diseases remain unclear; for example, the effects of Ephedra polysaccharides on hyperlipidemia and its immunosuppression mechanism are still unclear, as are the effective substances and mechanisms of action of Ephedra in producing antibacterial properties. Therefore, further studies of its molecular mechanism, pharmacokinetics, and clinical efficacy are required. Third, the safety of Ephedra must also be examined. More attention should be paid to the acute, subacute, and long-term toxicity of Ephedra alkaloids and to the determination of the target organs suffering from toxicity; in vivo and clinical studies must be conducted to determine the range of safe doses of Ephedra, standardize its safe use, and expand the scope of clinical application. Fourth, as Ephedra is usually used together with other traditional Chinese medicines in the form of a prescription; however, the data on its active ingredients and mechanisms of action after combined use are still unclear. Finally, new research techniques and means are essential to promote the application of Ephedra. To summarize, we think that with increased research on traditional Chinese medicine, including Ephedra, we should first fully collect, accumulate, and study the data, so that our understanding of traditional Chinese medicine is accurate and reliable. On this basis, we should explore and summarize the application and efficacy of Ephedra or extend its application in other directions. Then, with a large amount of experimental data and repeated clinical verification, we can obtain relatively reliable conclusions. Notably, although we can try to find the specific indications of drugs by applying experimental methods of modern medicine, we cannot completely rely on them to guide clinical drug use, because the scope of drug dosage and the effect and mechanism of action for treating diseases are not yet clear enough. Therefore, the clinical use of drugs should not only be guided by practical experience, but should also be based on the main principle of combining traditional Chinese medicine theory with scientific research data. The drug application scope should be determined or expanded on the premise of ensuring safety; otherwise, the effectiveness and safety of drug use cannot be guaranteed.
In this study, through the review of many studies, we systematically summarized the clinical application of Ephedra for the first time. We collated a large number of toxicity studies on EHE and EFE components, and we and summarized the botany, chemical components, and pharmacological effects of Ephedra. We also described the future development direction of Ephedra.
Abbreviations
Ephedra, Ephedra sinica Stapf; COVID-19, novel coronavirus pneumonia 2019; TFC, total flavonoid content; β2-AR, 2-adrenoceptor; OVA, ovalbumin; ELISA, enzyme-linked immunosorbent assay; G+, Gram-positive bacteria; PGN, peptidoglycan; TLR2, Toll-like receptor 2; DCs, dendritic cells; HPLC-PDA, high-performance liquid chromatography with photodiode array detector; SH, Suhuangzhenke capsule; D-GalN/LPS, D-galactosamine–lipopolysaccharide; CCl4, carbon tetrachloride; DPPH, 2, 2′-diphenyl-1-pyridohydrazine; SOD, superoxide dismutase; HFD, high-fat diet; NAFLD, nonalcoholic fatty liver disease; ROS, reactive oxygen species; ALT, alanine aminotransferase; T.Bil, total bilirubin; AFB(1), aflatoxin B(1); HGF, hepatocyte growth factor; NF-κB, nuclear factor-κB; HIV-1, human immunodeficiency virus type 1; IFV, influenza virus; QFPDT, Qingfei Paidu decoction; ASA, acetylsalicylic acid; PSE, pseudoephedrine; c-Met, cellular mesenchymal-to-epithelial transition factor; NOAEL, no observable adverse effect level; EHE, Ephedra extract; EFE, ephedrine alkaloid-free Ephedra Herb extract; TRPV1, transient receptor potential vanilloid 1; TCM, traditional Chinese medicine; bpm, beat per minute.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28020663/s1. Figure S1: Alkaloids isolated from Ephedra sinica Stapf. Figure S2: Flavonoids isolated from Ephedra sinica Stapf. Figure S3: Tannins isolated from Ephedra sinica Stapf. Figure S4: Organic phenolic acids isolated from Ephedra sinica Stapf. Figure S5: Organic volatile essential oils isolated from Ephedra sinica Stapf. Figure S6: Other ingredients isolated from Ephedra sinica Stapf.
Author Contributions
S.T. analyzed the data and wrote the manuscript. J.R., L.K., G.Y., C.L., Y.H., H.S. and X.-J.W. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the National Natural Science Foundation of China (81830110, 81903818) and the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT2020224).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Xie M., Yang Y., Wang B., Wang C. Interdisciplinary investigation on ancient Ephedra twigs from Gumugou Cemetery (3800 B.P.) in Xinjiang region, northwest China. Microsc. Res. Tech. 2013;76:663–672. doi: 10.1002/jemt.22216. [DOI] [PubMed] [Google Scholar]
- 2.Nelson R. FDA issues alert on ephedra supplements in the USA. Lancet. 2004;363:135. doi: 10.1016/S0140-6736(03)15315-9. [DOI] [PubMed] [Google Scholar]
- 3.Kim B.Y., Cao L.H., Kim J.Y. Common responses in gene expression by Ephedra herba in brain and heart of mouse. Phytother. Res. 2011;25:1440–1446. doi: 10.1002/ptr.3434. [DOI] [PubMed] [Google Scholar]
- 4.Andraws R., Chawla P., Brown D.L. Cardiovascular effects of ephedra alkaloids: A comprehensive review. Prog. Cardiovasc. Dis. 2005;47:217–225. doi: 10.1016/j.pcad.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Dillon P.F., Root-Bernstein R.S., Lieder C.M. Antioxidant-independent ascorbate enhancement of catecholamine inducedcontractions of vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2004;286:2353–2360. doi: 10.1152/ajpheart.00968.2003. [DOI] [PubMed] [Google Scholar]
- 6.Wan J.Y., Tian Y.F., Wan H.T., Yu L., Zhou F.H., Li C., He Y. Pharmacokinetics of compatible effective components of Mahuang Decoction in febrile rats. Zhongguo Zhong Yao Za Zhi. 2019;44:2149–2155. doi: 10.19540/j.cnki.cjcmm.20190125.002. [DOI] [PubMed] [Google Scholar]
- 7.Takemoto H., Hyuga S., Takahashi J., Hyuga S., Odaguchi H., Uchiyama N., Maruyama T., Yamashita T., Hyuga M., Oshima N., et al. Ephedrine Alkaloids-Free Ephedra Herb Extract, EFE, Has No Adverse Effects Such as Excitation, Insomnia, and Arrhythmias. Biol. Pharm. Bull. 2018;41:247–253. doi: 10.1248/bpb.b17-00803. [DOI] [PubMed] [Google Scholar]
- 8.Pellati F., Benvenuti S. Determination of ephedrine alkaloids in Ephedra natural products using HPLC on a pentafluorophenylpropyl stationary phase. J. Pharm. Biomed. Anal. 2008;48:254–263. doi: 10.1016/j.jpba.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Roman M.C. Determination of ephedrine alkaloids in botanicals and dietary supplements by HPLC-UV: Collaborative study. J. AOAC Int. 2004;87:1–14. doi: 10.1093/jaoac/87.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gul R., Jan S.U., Faridullah S., Sherani S., Jahan N. Preliminary Phytochemical Screening, Quantitative Analysis of Alkaloids, and Antioxidant Activity of Crude Plant Extracts from Ephedra intermedia Indigenous to Balochistan. Sci. World J. 2017;2017:5873648. doi: 10.1155/2017/5873648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibragic S., Sofić E. Chemical composition of various Ephedra species. Bosn. J. Basic Med. Sci. 2015;15:21–27. doi: 10.17305/bjbms.2015.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurosawa W., Kan T., Fukuyama T. Stereocontrolled total synthesis of (-)-ephedradine A (orantine) J. Am. Chem. Soc. 2003;125:8112–8113. doi: 10.1021/ja036011k. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J., Hesse M. The spermine alkaloids of Chaenorhinum minus. Planta Med. 1988;54:430–433. doi: 10.1055/s-2006-962490. [DOI] [PubMed] [Google Scholar]
- 14.Nezbedová L., Hesse M., Drandarov K., Werner C. Dihydroxyverbacine is the terminal precursor in the biosynthesis of aphelandrine and orantine. Tetrahedron. Lett. 2000;41:7859–7862. doi: 10.1016/S0040-4039(00)01392-7. [DOI] [Google Scholar]
- 15.Hikino H., Kiso Y., Ogata M., Konno C., Aisaka K., Kubota H., Hirose N., Ishihara T. Pharmacological actions of analogues of feruloylhistamine, an imidazole alkaloid of Ephedra roots. Planta Med. 1984;50:478–480. doi: 10.1055/s-2007-969777. [DOI] [PubMed] [Google Scholar]
- 16.Aghdasi M., Bojnoordi M.M., Mianabadi M., Nadaf M. Chemical components of the Ephedra major from Iran. Nat. Prod. Res. 2016;30:369–371. doi: 10.1080/14786419.2015.1058794. [DOI] [PubMed] [Google Scholar]
- 17.Ballero M., Foddis C., Sanna C., Scartezzini P., Poli F., Petitto V., Serafini M., Stanzione A., Bianco A., Serilli A.M., et al. Pharmacological activities on Ephedra nebrodensis Tineo. Nat. Prod. Res. 2010;24:1115–1124. doi: 10.1080/14786410802680902. [DOI] [PubMed] [Google Scholar]
- 18.Groves R.A., Hagel J.M., Zhang Y., Kilpatrick K., Levy A., Marsolais F., Lewinsohn E., Sensen C.W., Facchini P.J. Transcriptome profiling of khat (Catha edulis) and Ephedra sinica reveals gene candidates potentially involved in amphetamine-type alkaloid biosynthesis. PLoS ONE. 2015;10:e0119701. doi: 10.1371/journal.pone.0119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hikino H., Konno C., Takata H., Tamada M. Antiinflammatory principle of Ephedra Herbs. Chem. Pharm. Bull. 1980;28:2900–2904. doi: 10.1248/cpb.28.2900. [DOI] [PubMed] [Google Scholar]
- 20.Krizevski R., Bar E., Shalit O.R., Levy A., Hagel J.M., Kilpatrick K., Marsolais F., Facchini P.J., Ben-Shabat S., Sitrit Y., et al. Benzaldehyde is a precursor of phenylpropylamino alkaloids as revealed by targeted metabolic profiling and comparative biochemical analyses in Ephedra spp. Phytochemistry. 2012;81:71–79. doi: 10.1016/j.phytochem.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Kader M.S., Kassem F.F., Abdallah R.M. Two alkaloids from Ephedra aphylla growing in Egypt. Nat. Prod. Sci. 2003;9:52–55. [Google Scholar]
- 22.Zhang D., Deng A.J., Ma L., Li Z.H., Zhang Z.H., Jiang J.D., Qin H.L. Phenylpropanoids from the stems of Ephedra sinica. J. Asian Nat. Prod. Res. 2016;18:260–267. doi: 10.1080/10286020.2015.1070831. [DOI] [PubMed] [Google Scholar]
- 23.Al-Khalil S., Alkofahi A., el-Eisawi D., al-Shibib A. Transtorine, a new quinoline alkaloid from Ephedra transitoria. J. Nat. Prod. 1998;61:262–263. doi: 10.1021/np9702998. [DOI] [PubMed] [Google Scholar]
- 24.Starratt A.N., Caveney S. Quinoline-2-carboxylic acids from Ephedra species. Phytochemistry. 1996;42:1477–1478. doi: 10.1016/0031-9422(96)00126-4. [DOI] [Google Scholar]
- 25.Nawwar M.A.M., Barakat H.H., Buddrust J., Linscheidt M. Alkaloidal, lignan and phenolic constituents of Ephedra alata. Phytochemistry. 1985;24:878–879. doi: 10.1016/S0031-9422(00)84920-1. [DOI] [Google Scholar]
- 26.Hussein S.A.M., Barakat H.H., Nawar M.A.M., Willuhn G. Flavonoids from Ephedra aphylla. Phytochemistry. 1997;45:1529–1532. doi: 10.1016/S0031-9422(97)00092-7. [DOI] [Google Scholar]
- 27.Starratt A.N., Caveney S. Four cyclopropane amino acids from Ephedra. Phytochemistry. 1995;40:479–481. doi: 10.1016/0031-9422(95)00342-5. [DOI] [Google Scholar]
- 28.Tamada M., Endo K., Hikino H. Maokonine, hypertensive principle of Ephedra roots. Planta Med. 1978;34:291–293. doi: 10.1055/s-0028-1097453. [DOI] [PubMed] [Google Scholar]
- 29.Zhao W., Deng A.J., Du G.H., Zhang J.L., Li Z.H., Qin H.L. Chemical constituents of the stems of Ephedra sinica. J. Asian Nat. Prod. Res. 2009;11:168–171. doi: 10.1080/10286020802573552. [DOI] [PubMed] [Google Scholar]
- 30.Khan I.A., Abourashed E.A. Leung’s Encyclopedia of Common Natural Ingredients: Used in Food, Drugs and Cosmetics. John Wiley & Sons; Hoboken, NJ, USA: 2011. [Google Scholar]
- 31.Fan Y.B. The Research on Activity of Non-Alkaloid in Ephedra. Hubei University of Chinese Medicine; Wuhan, China: 2010. [Google Scholar]
- 32.Chen A.L., Stuart E.H., Chen K.K. The occurrence of methylbenzylamine in the extract of Ma Huang. J. Pharm. Sci. 1931;20:339–345. doi: 10.1002/jps.3080200406. [DOI] [Google Scholar]
- 33.Al-Rimawi F., Abu-Lafi S., Abbadi J., Alamarneh A.A.A., Sawahreh R.A., Odeh I. Analysis Of Phenolic And Flavonoids Of Wild Ephedra Alata Plant Extracts By Lc/Pda And Lc/Ms And Their Antioxidant Activity. Afr. J. Tradit. Complement. Altern. Med. AJTCAM. 2017;14:130–141. doi: 10.21010/ajtcam.v14i2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X., Fu W., Wang Z.B., Kuang H.X., Wang Q.H. Research progress of Ephedra polysaccharides. China J. Tradit. Chin. Med. Pharm. 2019;34:3138–3139. [Google Scholar]
- 35.Okawa M., Kinjo J., Nohara T., Ono M. DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of flavonoids obtained from some medicinal plants. Biol. Pharm. Bull. 2001;24:1202–1205. doi: 10.1248/bpb.24.1202. [DOI] [PubMed] [Google Scholar]
- 36.Purev O., Pospíšil F., Motl O. Flavonoids from Ephedra sinica stapf. Collect. Czech Chem. C. 1988;53:3193–3196. doi: 10.1135/cccc19883193. [DOI] [Google Scholar]
- 37.Tao H.M. Flavonoids from roots of Ephedra sinica. Chin Tradit Herb Drugs. 2011;42:1678–1682. [Google Scholar]
- 38.Kasahara Y., Shimoyama N., Konno C., Hikino H. Structure of mahuannin C, a hypotensive principle of Ephedra roots. Heterocycles. 1983;20:1741–1744. [Google Scholar]
- 39.Nawwar M.A., El-Sissi H.I., Barakat H.H. Flavonoid constituents of Ephedra alata. Phytochemistry. 1984;23:2937–2939. doi: 10.1016/0031-9422(84)83045-9. [DOI] [Google Scholar]
- 40.Amakura Y., Yoshimura M., Yamakami S., Yoshida T., Wakana D., Hyuga M., Hyuga S., Hanawa T., Goda Y. Characterization of phenolic constituents from ephedra herb extract. Molecules. 2013;18:5326–5334. doi: 10.3390/molecules18055326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villalobos A. Compositions and Methods for Enhancing Weight-Loss by Cyclical Administration of Compounds. 7,977,378. U.S. Patent. 2011 July 12;
- 42.Bouchard N.C., Howland M.A., Greller H.A., Hoffman R.S., Nelson L.S. Ischemic stroke associated with use of an ephedra-free dietary supplement containing synephrine. Mayo. Clin. Proc. 2005;80:541–545. doi: 10.4065/80.4.541. [DOI] [PubMed] [Google Scholar]
- 43.Cottiglia F., Bonsignore L., Casu L., Deidda D., Pompei R., Casu M., Floris C. Phenolic constituents from Ephedra nebrodensis. Nat. Prod. Res. 2005;19:117–123. doi: 10.1080/14786410410001704714. [DOI] [PubMed] [Google Scholar]
- 44.Zang X., Shang M., Xu F., Liang J., Wang X., Mikage M., Cai S. A-type proanthocyanidins from the stems of Ephedra sinica (Ephedraceae) and their antimicrobial activities. Molecules. 2013;18:5172–5189. doi: 10.3390/molecules18055172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang B.M., Wang Z.B., Xin P., Wang Q.H., Bu H., Kuang H.X. Phytochemistry and pharmacology of genus Ephedra. Chin. J. Nat. Med. 2018;16:811–828. doi: 10.1016/S1875-5364(18)30123-7. [DOI] [PubMed] [Google Scholar]
- 46.Friedrich H., Wiedemeyer H. Die gerbstoffbildner in Ephedra helvetica. Planta Med. 1976;30:163–173. doi: 10.1055/s-0028-1097713. [DOI] [PubMed] [Google Scholar]
- 47.Porter P.L., Wallace J.W. C-glycosylflavones from species of Ephedra. Biochem. Syst. Ecol. 1988;16:261–262. doi: 10.1016/0305-1978(88)90003-8. [DOI] [Google Scholar]
- 48.Wallace J.W. C-Glycosylflavones In The Gnetopsida: A Preliminary Report. Am. J. Bot. 1979;66:343–346. doi: 10.1002/j.1537-2197.1979.tb06233.x. [DOI] [Google Scholar]
- 49.Ohba S., Yoshida K., Kondo T. Swertisin dihydrate. Acta Cryst. C. 2004;60:893–896. doi: 10.1107/S0108270104028355. [DOI] [PubMed] [Google Scholar]
- 50.Lv M., Chen J., Gao Y., Sun J., Zhang Q., Zhang M., Xu F., Zhang Z. Metabolomics based on liquid chromatography with mass spectrometry reveals the chemical difference in the stems and roots derived from Ephedra sinica. J. Sep. Sci. 2015;38:3331–3336. doi: 10.1002/jssc.201500529. [DOI] [PubMed] [Google Scholar]
- 51.Oshima N., Maruyama T., Yamashita T., Uchiyama N., Amakura Y., Hyuga S., Hyuga M., Nakamori S., Takemoto H., Kobayashi Y., et al. Two flavone C-glycosides as quality control markers for the manufacturing process of ephedrine alkaloids-free Ephedra Herb extract (EFE) as a crude drug preparation. J. Nat. Med. 2018;72:73–79. doi: 10.1007/s11418-017-1111-8. [DOI] [PubMed] [Google Scholar]
- 52.Morio Y., Yoshiaki A., Sumiko H., Masashi H., Shunsuke N., Takuro M., Naohiro O., Nahoko U., Jinwei Y., Hideki O., et al. Quality Evaluation and Characterization of Fractions with Biological Activity from Ephedra Herb Extract and Ephedrine Alkaloids-Free Ephedra Herb Extract. Chem. Pharm. Bull. 2020;68:140–149. doi: 10.1248/cpb.c19-00761. [DOI] [PubMed] [Google Scholar]
- 53.Tao H., Wang L., Cui Z., Zhao D., Liu Y. Dimeric proanthocyanidins from the roots of Ephedra sinica. Planta Med. 2008;74:1823–1825. doi: 10.1055/s-0028-1088321. [DOI] [PubMed] [Google Scholar]
- 54.Hikino H. Structures of mahuannin A and B, hypotensive principles of Ephedra roots. Heterocycles. 1982;19:97–105. doi: 10.3987/R-1982-08-1381. [DOI] [Google Scholar]
- 55.Tao H., Wang L., Cui Z. Study on chemical constituents of root of Ephedra sinica. Chin. Tradit. Herb. Drugs. 2010;41:533–536. [Google Scholar]
- 56.Chumbalov T., Chekmeneva L., Polyakov V. Phenolic acids of Ephedra equisetina. Chem. Nat. Compd. 1977;13:238–239. doi: 10.1007/BF00563959. [DOI] [Google Scholar]
- 57.Soua L., Koubaa M., Barba F.J., Fakhfakh J., Ghamgui H.K., Chaabouni S.E. Water-Soluble Polysaccharides from Ephedra alata Stems: Structural Characterization, Functional Properties, and Antioxidant Activity. Molecules. 2020;25:2210. doi: 10.3390/molecules25092210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao W. Study on the Chemical Constituents of Ephedra Sinica. Chinese Academy of Medical Science & Peking Union Medical College; Beijing, China: 2009. [Google Scholar]
- 59.Lee C.-H., Lee H.-S. Growth inhibiting activity of quinaldic acid isolated from Ephedra pachyclada against intestinal bacteria. J. Korean Soc. Appl. Biol. Chem. 2009;52:331–335. doi: 10.3839/jksabc.2009.059. [DOI] [Google Scholar]
- 60.Zhang L.R., Zou G.L., Yang T.M. Review of development in ephedta’s chemical researching and using. J. South-Cent. Univ Natl. (Nat. Sci.) 2000;19:87–90. [Google Scholar]
- 61.Wang Y.Y. Study on chemical constitutents of Ephedrae herba. Anti Infect. Pharm. 2014;11:416–418. [Google Scholar]
- 62.Ma Q.Y., Li C.S., Zhou J., Huang S.Z., Zhao Y.X. Study on chemical constituents of the ancient Ephedra species found in Yanghai, Xinjiang. J. Anhui Agric. Sci. 2012;40:7089–7090. [Google Scholar]
- 63.Wang L., Zhao D., Liu Y. GC-MS analysis of the supercritical CO2 fluid extraction of Ephedra sinica roots and its antisudorific activity. Chem. Nat. Compd. 2009;45:434–436. doi: 10.1007/s10600-009-9321-2. [DOI] [Google Scholar]
- 64.Tellez M.R., Khan I.A., Schaneberg B.T., Crockett S.L., Rimando A.M., Kobaisy M. Steam distillation–solid-phase microextraction for the detection of Ephedra sinica in herbal preparations. J. Chromatogr. A. 2004;1025:51–56. doi: 10.1016/S0021-9673(03)01035-5. [DOI] [PubMed] [Google Scholar]
- 65.Zhou L., Wu D., Tang Y. Research progress of chemical constituents in Ephedra. J. Nanjing Univ. TCM. 2008;24:71–72. [Google Scholar]
- 66.Yi L.Z., Gao J.M., Liu X.Q., Liang Y.Z. Comparative on the components from different parts between wild growing and cultivated planting of Ephedra sinica. Chin. Tradit. Herb. Drugs. 2007;9:1298–1301. [Google Scholar]
- 67.Lao Y.X., Chen K., Lin W.J., Lin L. GC-MS comparative analysis of the volatile oil of Ephedra sinica with different extraction methods. Res. Pract. Chin. Med. 2005:53–56. [Google Scholar]
- 68.Xue J., Wang L.H., Liu L.J., Chai H.F. Analysis of Volatile Components of Ephedra from Different Places Based on GC-MS. J. Chin. Med. Mater. 2020;43:359–362. [Google Scholar]
- 69.Ji L., Xu Z.L., Pan G.G., Yang J. GC-MS analysis of constituent of essential oils from stems of Ephedra sinica Stapf, E.intermedia Schrenk et C. A. Mey. and E. equisetina Bge. Chin. J. Chin. Mat. Med. 1997;22:489–492. [PubMed] [Google Scholar]
- 70.Wang Y.H., Wang Q.H., Xia Y.G., Kuang H.X. GC-MS analysis of volatile oils from Ephedra sinica Stapf from Datong county, Shanxi province. Acta Chin. Med. Pharm. 2011;39:58–60. [Google Scholar]
- 71.Sun J.Y. Study on the new effective components of Ephedra. Chin. Herb. Med. 1983;14:9–10. [Google Scholar]
- 72.Kallassy H., Fayyad-Kazan M., Makki R., El-Makhour Y., Rammal H., Leger D.Y., Sol V., Fayyad-Kazan H., Liagre B., Badran B. Chemical Composition and Antioxidant, Anti-Inflammatory, and Antiproliferative Activities of Lebanese Ephedra Campylopoda Plant. Med. Sci. Monit. Basic Res. 2017;23:313–325. doi: 10.12659/MSMBR.905056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang Y.F., Lu Y., Wu G.F., Xiao M.Y., Wu H.Z. Chemical constituents of Ephedra root. Chin Tradit Pat. Med. 2010;32:1758–1760. [Google Scholar]
- 74.Wang Z., Cui Y., Ding G., Zhou M., Ma X., Hou Y., Jiang M., Liu D., Bai G. Mahuannin B an adenylate cyclase inhibitor attenuates hyperhidrosis via suppressing β(2)-adrenoceptor/cAMP signaling pathway. Phytomedicine Int. J. Phytother. Phytopharm. 2017;30:18–27. doi: 10.1016/j.phymed.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Jo Y.Y., Kim J.Y., Kim J.S., Kwon Y., Shin C.S. The effect of ephedrine on intraoperative hypothermia. Korean J. Anesthesiol. 2011;60:250–254. doi: 10.4097/kjae.2011.60.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carlisle H.J., Stock M.J. Temperature-dependent effects of ephedrine in the cold. Physiol. Behav. 1996;60:1147–1150. doi: 10.1016/0031-9384(96)00217-X. [DOI] [PubMed] [Google Scholar]
- 77.Stohs S.J., Badmaev V. A Review of Natural Stimulant and Non-stimulant Thermogenic Agents. Phytother. Res. 2016;30:732–740. doi: 10.1002/ptr.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim W., Lee W., Huh E., Choi E., Jang Y.P., Kim Y.K., Lee T.H., Oh M.S. Ephedra sinica Stapf and Gypsum Attenuates Heat-Induced Hypothalamic Inflammation in Mice. Toxins. 2019;12:16. doi: 10.3390/toxins12010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mei F., Xing X.-f., Tang Q.-f., Chen F.-l., Guo Y., Song S., Tan X.-m., Luo J.-B. Antipyretic and anti-asthmatic activities of traditional Chinese herb-pairs, Ephedra and Gypsum. Chin. J. Integr. Med. 2016;22:445–450. doi: 10.1007/s11655-014-1952-x. [DOI] [PubMed] [Google Scholar]
- 80.Ma C.H., Ma Z.Q., Fu Q., Ma S.P. Ma Huang Tang ameliorates asthma though modulation of Th1/Th2 cytokines and inhibition of Th17 cells in ovalbumin-sensitized mice. Chin. J. Nat. Med. 2014;12:361–366. doi: 10.1016/S1875-5364(14)60044-3. [DOI] [PubMed] [Google Scholar]
- 81.Zhang B.B., Zeng M.N., Zhang Q.Q., Wang R., Jia J.F., Cao B., Liu M., Guo P.L., Zhang Y.H., Zheng X.K., et al. Ephedrae Herba polysaccharides inhibit the inflammation of ovalbumin induced asthma by regulating Th1/Th2 and Th17/Treg cell immune imbalance. Mol. Immunol. 2022;152:14–26. doi: 10.1016/j.molimm.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 82.Huang P., Tang Y., Li C., Zhou H., Yu L., Wan H., He Y. Correlation study between the pharmacokinetics of seven main active ingredients of Mahuang decoction and its pharmacodynamics in asthmatic rats. J. Pharm. Biomed. Anal. 2020;183:113144. doi: 10.1016/j.jpba.2020.113144. [DOI] [PubMed] [Google Scholar]
- 83.Tang J., Ji H., Shi J., Wu L. Ephedra water decoction and cough tablets containing ephedra and liquorice induce CYP1A2 but not CYP2E1 hepatic enzymes in rats. Xenobiotica. 2016;46:141–146. doi: 10.3109/00498254.2015.1060371. [DOI] [PubMed] [Google Scholar]
- 84.Wang J., Xiong Y., Xiong B., Wang S.P. Effects of aerosolized aqueous extract of Ephedra on airway inflammation in asth matic mice. Chongqing Med. J. 2013;42:304–307. [Google Scholar]
- 85.Kasahara Y., Hikino H., Tsurufuji S., Watanabe M., Ohuchi K. Antiinflammatory actions of ephedrines in acute inflammations1. Planta Med. 1985;51:325–331. doi: 10.1055/s-2007-969503. [DOI] [PubMed] [Google Scholar]
- 86.Zheng Y., Yang Y., Li Y., Xu L., Wang Y., Guo Z., Song H., Yang M., Luo B., Zheng A., et al. Ephedrine hydrochloride inhibits PGN-induced inflammatory responses by promoting IL-10 production and decreasing proinflammatory cytokine secretion via the PI3K/Akt/GSK3β pathway. Cell. Mol. Immunol. 2013;10:330–337. doi: 10.1038/cmi.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He W., Ma J., Chen Y., Jiang X., Wang Y., Shi T., Zhang Q., Yang Y., Jiang X., Yin S., et al. Ephedrine hydrochloride protects mice from staphylococcus aureus-induced peritonitis. Am. J. Transl. Res. 2018;10:670–683. [PMC free article] [PubMed] [Google Scholar]
- 88.He Y., Lou X., Jin Z., Yu L., Deng L., Wan H. Mahuang decoction mitigates airway inflammation and regulates IL-21/STAT3 signaling pathway in rat asthma model. J. Ethnopharmacol. 2018;224:373–380. doi: 10.1016/j.jep.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 89.Wu X., Liu Q., Chen D., Qin W., Lu B., Bi Q., Wang Z., Jia Y., Tan N. Identification of quality control markers in Suhuang antitussive capsule based on HPLC-PDA fingerprint and anti-inflammatory screening. J. Pharm. Biomed. Anal. 2020;180:113053. doi: 10.1016/j.jpba.2019.113053. [DOI] [PubMed] [Google Scholar]
- 90.Zhai H.Q., Hhang J.R., Gao M.C., Liu Y., Zhang S.F., Wang X.H., Meng F.Y., Wang Y.P. Comparative study between Ephedra sinica Stapf and Fructus Schisandrae Chinensis on ET-1 and 6-keto-prostaglandin F1α in rats with idiopathic pulmonary fibrosis. Genet. Mol. Res. GMR. 2014;13:3761–3771. doi: 10.4238/2014.May.13.3. [DOI] [PubMed] [Google Scholar]
- 91.Zhai H.Q., Zhang S.F., Gao M.C., Liu Y., Ou M., Meng F.Y., Wang Y.Y. Effects of Herba Ephedra Sinicae and Fructus Schisandrae Chinensis on pathology of rats with bleomycin A(5)-induced idiopathic pulmonary fibrosis. Zhong Xi Yi Jie He Xue Bao J. Chin. Integr. Med. 2011;9:553–557. doi: 10.3736/jcim20110514. [DOI] [PubMed] [Google Scholar]
- 92.Han Y., Zhu J., Wu Z. Ephedra protects rats against acute liver failure induced by D-galactosamine and lipopolysaccharide. Zhonghua Gan Zang Bing Za Zhi = Zhonghua Ganzangbing Zazhi = Chin. J. Hepatol. 2016;24:127–129. doi: 10.3760/cma.j.issn.1007-3418.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 93.Ghasemi M., Azarnia M., Jamali M., Mirabolghasemi G., Nazarian S., Naghizadeh M.M., Rajabi M., Tahamtani Y. Protective effects of Ephedra pachyclada extract on mouse models of carbon tetrachloride- induced chronic and acute liver failure. Tissue Cell. 2014;46:78–85. doi: 10.1016/j.tice.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 94.Yamada I., Goto T., Takeuchi S., Ohshima S., Yoneyama K., Shibuya T., Kataoka E., Segawa D., Sato W., Dohmen T., et al. Mao (Ephedra sinica Stapf) protects against D-galactosamine and lipopolysaccharide-induced hepatic failure. Cytokine. 2008;41:293–301. doi: 10.1016/j.cyto.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 95.Song M.K., Um J.Y., Jang H.J., Lee B.C. Beneficial effect of dietary Ephedra sinica on obesity and glucose intolerance in high-fat diet-fed mice. Exp. Ther. Med. 2012;3:707–712. doi: 10.3892/etm.2012.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.LiverTox . LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD, USA: 2012. Chinese and other Asian Herbal Medicines. [PubMed] [Google Scholar]
- 97.Lee A.Y., Jang Y., Hong S.H., Chang S.H., Park S., Kim S., Kang K.S., Kim J.E., Cho M.H. Ephedrine-induced mitophagy via oxidative stress in human hepatic stellate cells. J. Toxicol. Sci. 2017;42:461–473. doi: 10.2131/jts.42.461. [DOI] [PubMed] [Google Scholar]
- 98.Caveney S., Charlet D.A., Freitag H., Maier-Stolte M., Starratt A.N. New observations on the secondary chemistry of worldEphedra(Ephedraceae) Am. J. Bot. 2001;88:1199–1208. doi: 10.2307/3558330. [DOI] [PubMed] [Google Scholar]
- 99.Khan A., Jan G., Khan A., Gul Jan F., Bahadur A., Danish M. In Vitro Antioxidant and Antimicrobial Activities of Ephedra gerardiana (Root and Stem) Crude Extract and Fractions. Evid. Based Complement. Altern. Med. Ecam. 2017;2017:4040254. doi: 10.1155/2017/4040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parsaeimehr A., Sargsyan E., Javidnia K. A comparative study of the antibacterial, antifungal and antioxidant activity and total content of phenolic compounds of cell cultures and wild plants of three endemic species of Ephedra. Molecules. 2010;15:1668–1678. doi: 10.3390/molecules15031668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bagheri-Gavkosh S., Bigdeli M., Shams-Ghahfarokhi M., Razzaghi-Abyaneh M. Inhibitory effects of Ephedra major Host on Aspergillus parasiticus growth and aflatoxin production. Mycopathologia. 2009;168:249–255. doi: 10.1007/s11046-009-9220-x. [DOI] [PubMed] [Google Scholar]
- 102.Oshima N. Efficient Preparation of Ephedrine Alkaloids-free Ephedra Herb Extract and Its Antitumor Effect and Putative Marker Compound. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2017;137:173–177. doi: 10.1248/yakushi.16-00233-3. [DOI] [PubMed] [Google Scholar]
- 103.Hyuga S., Hyuga M., Oshima N., Maruyama T., Kamakura H., Yamashita T., Yoshimura M., Amakura Y., Hakamatsuka T., Odaguchi H., et al. Ephedrine alkaloids-free Ephedra Herb extract: A safer alternative to ephedra with comparable analgesic, anticancer, and anti-influenza activities. J. Nat. Med. 2016;70:571–583. doi: 10.1007/s11418-016-0979-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hyuga S., Hyuga M., Yoshimura M., Amakura Y., Goda Y., Hanawa T. Herbacetin, a constituent of ephedrae herba, suppresses the HGF-induced motility of human breast cancer MDA-MB-231 cells by inhibiting c-Met and Akt phosphorylation. Planta Med. 2013;79:1525–1530. doi: 10.1055/s-0033-1350899. [DOI] [PubMed] [Google Scholar]
- 105.Kim I.S., Yoon S.J., Park Y.J., Lee H.B. Inhibitory effect of ephedrannins A and B from roots of Ephedra sinica STAPF on melanogenesis. Biochim. Et Biophys. Acta. 2015;1850:1389–1396. doi: 10.1016/j.bbagen.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 106.Nam N.H., Lee C.W., Hong D.H., Kim H.M., Bae K.H., Ahn B.Z. Antiinvasive, antiangiogenic and antitumour activity of Ephedra sinica extract. Phytothe Res. 2003;17:70–76. doi: 10.1002/ptr.901. [DOI] [PubMed] [Google Scholar]
- 107.Murakami T., Harada H., Suico M.A., Shuto T., Suzu S., Kai H., Okada S. Ephedrae herba, a component of Japanese herbal medicine Mao-to, efficiently activates the replication of latent human immunodeficiency virus type 1 (HIV-1) in a monocytic cell line. Biol. Pharm. Bull. 2008;31:2334–2337. doi: 10.1248/bpb.31.2334. [DOI] [PubMed] [Google Scholar]
- 108.Peng X.Q., Zhou H.F., Zhang Y.Y., Yang J.H., Wan H.T., He Y. Antiviral effects of Yinhuapinggan granule against influenza virus infection in the ICR mice model. J. Nat. Med. 2016;70:75–88. doi: 10.1007/s11418-015-0939-z. [DOI] [PubMed] [Google Scholar]
- 109.Bajgain K.T., Badal S., Bajgain B.B., Santana M.J. Prevalence of comorbidities among individuals with COVID-19: A rapid review of current literature. Am. J. Infect. Control. 2021;49:238–246. doi: 10.1016/j.ajic.2020.06.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhong L.L.D., Lam W.C., Yang W., Chan K.W., Sze S.C.W., Miao J., Yung K.K.L., Bian Z., Wong V.T. Potential Targets for Treatment of Coronavirus Disease 2019 (COVID-19): A Review of Qing-Fei-Pai-Du-Tang and Its Major Herbs. Am. J. Chin. Med. 2020;48:1051–1071. doi: 10.1142/S0192415X20500512. [DOI] [PubMed] [Google Scholar]
- 111.Yao K.T., Liu M.Y., Li X., Hang J.H., Cai H.B. Retrospective clinical analysis on treatment of coronavirus disease 2019 with traditional Chinese medicine Lianhua Qingwen. Chin J Exp Trad Med. 2020;26:8–12. [Google Scholar]
- 112.Li X., Qiu Q., Li M., Lin H., Cao S., Wang Q., Chen Z., Jiang W., Zhang W., Huang Y., et al. Chemical composition and pharmacological mechanism of ephedra-glycyrrhiza drug pair against coronavirus disease 2019 (COVID-19) Aging. 2021;13:4811–4830. doi: 10.18632/aging.202622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gao K., Song Y.P., Song A. Exploring active ingredients and function mechanisms of Ephedra-bitter almond for prevention and treatment of Corona virus disease 2019 (COVID-19) based on network pharmacology. BioData Min. 2020;13:19. doi: 10.1186/s13040-020-00229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ang L., Lee H.W., Kim A., Lee J.A., Zhang J., Lee M.S. Herbal medicine for treatment of children diagnosed with COVID-19: A review of guidelines. Complement. Ther. Clin. Pract. 2020;39:101174. doi: 10.1016/j.ctcp.2020.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mei J., Zhou Y., Yang X., Zhang F., Liu X., Yu B. Active components in Ephedra sinica stapf disrupt the interaction between ACE2 and SARS-CoV-2 RBD: Potent COVID-19 therapeutic agents. J. Ethnopharmacol. 2021;278:284–296. doi: 10.1016/j.jep.2021.114303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang C., Ming H., Jia W., Su W., Zhan L.R., Luo D., Yang J.Y. Analysis of medication regularity and pharmacodynamic characteristics of traditional Chinese medicine treatment in 444 severe cases of COVID-19. Zhongguo Zhong Yao Za Zhi. 2020;45:3007–3012. doi: 10.19540/j.cnki.cjcmm.20200427.501. [DOI] [PubMed] [Google Scholar]
- 117.Fan T., Chen Y., Bai Y., Ma F., Wang H., Yang Y., Chen J., Lin Y. Analysis of medication characteristics of traditional Chinese medicine in treating COVID-19 based on data mining. Zhejiang Da Xue Xue Bao. Yi Xue Ban = J. Zhejiang University. Med. Sci. 2020;49:260–269. doi: 10.3785/j.issn.1008-9292.2020.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shergis J.L., Wu L., May B.H., Zhang A.L., Guo X., Lu C., Xue C.C. Natural products for chronic cough: Text mining the East Asian historical literature for future therapeutics. Chronic Respir. Dis. 2015;12:204–211. doi: 10.1177/1479972315583043. [DOI] [PubMed] [Google Scholar]
- 119.Hou Y., Cheng B., Zhou M., Fang R., Jiang M., Hou W., Bai G. Searching for synergistic bronchodilators and novel therapeutic regimens for chronic lung diseases from a traditional Chinese medicine, Qingfei Xiaoyan Wan. PLoS ONE. 2014;9:e113104. doi: 10.1371/journal.pone.0113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brun G.C. Mechanism of the Vasoconstrictor Action of Ephedrine: II. Interaction between Ephedrine and Adrenaline. Acta Pharmacol. Et Toxicol. 1947;3:239–251. doi: 10.1111/j.1600-0773.1947.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 121.Santana L., Kiebzak G.M., Toomey N., Maul T.M. Blood pressure measurements during intraoperative pediatric scoliosis surgery. Saudi J. Anaesth. 2020;14:152–156. doi: 10.4103/sja.SJA_570_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kitaura A., Houri K., Nakao S. Ephedrine-Induced Increases in Blood Pressure and Heart Rate Due to Suspected Cardiac Sympathetic Denervation Supersensitivity in a Patient with Parkinson’s Disease Under Spinal Anesthesia. Am. J. Case Rep. 2019;20:1104–1107. doi: 10.12659/AJCR.916188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang X., Mao M., Liu S., Xu S., Yang J. A Comparative Study of Bolus Norepinephrine, Phenylephrine, and Ephedrine for the Treatment of Maternal Hypotension in Parturients with Preeclampsia During Cesarean Delivery Under Spinal Anesthesia. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019;25:1093–1101. doi: 10.12659/MSM.914143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liles J.T., Dabisch P.A., Hude K.E., Pradhan L., Varner K.J., Porter J.R., Hicks A.R., Corll C., Baber S.R., Kadowitz P.J. Pressor responses to ephedrine are mediated by a direct mechanism in the rat. J. Pharm.. Exp. 2006;316:95–105. doi: 10.1124/jpet.105.090035. [DOI] [PubMed] [Google Scholar]
- 125.Xiong X.J. Experience of treating facial neuritis, unexplained limb weakness, cervical spondylosis, acute myelitis, acute radiculitis, Guillain Barre syndrome, multiple sclerosis, myasthenia gravis, motor neuron disease, dermatomyositis with Xiaoxuming Decoction. Zhongguo Zhong Yao Za Zhi. 2020;45:2735–2751. doi: 10.19540/j.cnki.cjcmm.20200328.501. [DOI] [PubMed] [Google Scholar]
- 126.Nakamori S., Takahashi J., Hyuga S., Tanaka-Kagawa T., Jinno H., Hyuga M., Hakamatsuka T., Odaguchi H., Goda Y., Hanawa T. Ephedra Herb extract activates/desensitizes transient receptor potential vanilloid 1 and reduces capsaicin-induced pain. J. Nat. Med. 2017;71:105–113. doi: 10.1007/s11418-016-1034-9. Correction in J. Nat. Med. 2018, 72, 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schachtel B.P., Voelker M., Sanner K.M., Gagney D., Bey M., Schachtel E.J., Becka M. Demonstration of the analgesic efficacy and dose-response of acetylsalicylic acid with pseudoephedrine. J. Clin. Pharmacol. 2010;50:1429–1437. doi: 10.1177/0091270009360978. [DOI] [PubMed] [Google Scholar]
- 128.Basu A. Middle-ear pain and trauma during air travel. Clin. Evidince. 2007;2007:0501. [PMC free article] [PubMed] [Google Scholar]
- 129.Fan Y.P., Xiong X.J. Chinese classical formulas Ephedra associated prescriptions for treatment of skin diseases. Zhongguo Zhong Yao Za Zhi. 2018;43:2431–2434. doi: 10.19540/j.cnki.cjcmm.2018.0074. [DOI] [PubMed] [Google Scholar]
- 130.Adams J.D., Jr., Garcia C. Women’s health among the Chumash. Evid. -Based Complement. Altern. Med. Ecam. 2006;3:125–131. doi: 10.1093/ecam/nek021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jaradat N.A., Shawahna R., Eid A.M., Al-Ramahi R., Asma M.K., Zaid A.N. Herbal remedies use by breast cancer patients in the West Bank of Palestine. J. Ethnopharmacol. 2016;178:1–8. doi: 10.1016/j.jep.2015.11.050. [DOI] [PubMed] [Google Scholar]
- 132.Ben-Arye E., Lavie O., Samuels N., Khamaisie H., Schiff E., Raz O.G., Mahajna J. Safety of herbal medicine use during chemotherapy in patients with ovarian cancer: A "bedside-to-bench" approach. Med. Oncol. 2017;34:54. doi: 10.1007/s12032-017-0910-9. [DOI] [PubMed] [Google Scholar]
- 133.Palamar J. How ephedrine escaped regulation in the United States: A historical review of misuse and associated policy. Health Policy. 2011;99:1–9. doi: 10.1016/j.healthpol.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 134.Wang Z.C., Li F.J., Yang J. Talking about the adverse reactions of Ephedra. Sci. Technol. Inf. 2010;13:407–408. [Google Scholar]
- 135.Pittler M.H., Schmidt K., Ernst E. Adverse events of herbal food supplements for body weight reduction: Systematic review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2005;6:93–111. doi: 10.1111/j.1467-789X.2005.00169.x. [DOI] [PubMed] [Google Scholar]
- 136.Powell T., Hsu F.F., Turk J., Hruska K. Ma-huang strikes again: Ephedrine nephrolithiasis. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1998;32:153–159. doi: 10.1053/ajkd.1998.v32.pm9669437. [DOI] [PubMed] [Google Scholar]
- 137.Han H.Y., Huh J.I., Han S.R., Kang M.G., Yoon S., Han J.S., Lee B.S., Kim J.A., Min B.S. Assessing the safety of an Ephedrae Herba aqueous extract in rats: A repeat dose toxicity study. Regul. Toxicol. Pharmacol. RTP. 2018;94:144–151. doi: 10.1016/j.yrtph.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 138.Nyska A., Murphy E., Foley J.F., Collins B.J., Petranka J., Howden R., Hanlon P., Dunnick J.K. Acute hemorrhagic myocardial necrosis and sudden death of rats exposed to a combination of ephedrine and caffeine. Toxicol. Sci. Off. J. Soc. Toxicol. 2005;83:388–396. doi: 10.1093/toxsci/kfi034. [DOI] [PubMed] [Google Scholar]
- 139.Dunnick J.K., Kissling G., Gerken D.K., Vallant M.A., Nyska A. Cardiotoxicity of Ma Huang/caffeine or ephedrine/caffeine in a rodent model system. Toxicol. Pathol. 2007;35:657–664. doi: 10.1080/01926230701459978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kobayashi Y. Analgesic Effects and Side Effects of Ephedra Herb Extract and Ephedrine Alkaloids-free Ephedra Herb Extract. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2017;137:187–194. doi: 10.1248/yakushi.16-00233-5. [DOI] [PubMed] [Google Scholar]
- 141.Odaguchi H., Hyuga S., Sekine M., Nakamori S., Takemoto H., Huang X., Oshima N., Shimada N., Yang J., Amakura Y., et al. The Adverse Effects of Ephedra Herb and the Safety of Ephedrine Alkaloids-free Ephedra Herb Extract (EFE) Yakugaku Zasshi J. Pharm. Soc. Jpn. 2019;139:1417–1425. doi: 10.1248/yakushi.19-00122. [DOI] [PubMed] [Google Scholar]
- 142.Oshima N., Yamashita T., Hyuga S., Hyuga M., Kamakura H., Yoshimura M., Maruyama T., Hakamatsuka T., Amakura Y., Hanawa T., et al. Efficiently prepared ephedrine alkaloids-free Ephedra Herb extract: A putative marker and antiproliferative effects. J. Nat. Med. 2016;70:554–562. doi: 10.1007/s11418-016-0977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li C., Zheng M.Y., Xu Y.J. Acute Toxicity of Mahuang Dingchuan Decoction and Lurong Dabu Decoction. Acta Chin. Med. Pharmacol. 2018;46:31–35. [Google Scholar]
- 144.Liu Y.G., Luo J.B., Jiang Y.P. Study on the acute toxicity, anti-inflammatory and anti-allergic effects of Mahuang Decoction. Chin. Tradit. Pat. Med. 2005;2005:101–102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.