Abstract
Ruminants are foregut fermenters that have the remarkable ability of converting plant polymers that are indigestible to humans into assimilable comestibles like meat and milk, which are cornerstones of human nutrition. Ruminants establish a symbiotic relationship with their microbiome, and the latter is the workhorse of carbohydrate fermentation. On the other hand, during carbohydrate fermentation, synthesis of propionate sequesters H, thus reducing its availability for the ultimate production of methane (CH4) by methanogenic archaea. Biochemically, methane is the simplest alkane and represents a downturn in energetic efficiency in ruminants; environmentally, it constitutes a potent greenhouse gas that negatively affects climate change. Prevotella is a very versatile microbe capable of processing a wide range of proteins and polysaccharides, and one of its fermentation products is propionate, a trait that appears conspicuous in P. ruminicola strain 23. Since propionate, but not acetate or butyrate, constitutes an H sink, propionate-producing microbes have the potential to reduce methane production. Accordingly, numerous studies suggest that members of the genus Prevotella have the ability to divert the hydrogen flow in glycolysis away from methanogenesis and in favor of propionic acid production. Intended for a broad audience in microbiology, our review summarizes the biochemistry of carbohydrate fermentation and subsequently discusses the evidence supporting the essential role of Prevotella in lignocellulose processing and its association with reduced methane emissions. We hope this article will serve as an introduction to novice Prevotella researchers and as an update to others more conversant with the topic.
Keywords: Prevotella, carbohydrate metabolism, propionate, methane emissions, sustainable agriculture
1. Introduction
The genus Prevotella was named after the French microbiologist André Romain Prévot [1]. It belongs to the Prevotellaceae family, which also includes the kindred genera Paraprevotella, Alloprevotella and Hallella [2]. The genus Prevotella comprises more than 50 anaerobic, non-spore-forming, Gram-negative species which are largely saccharolytic [3], and generate short-chain fatty acids (SCFAs) as fermentation products [4,5]. Prevotella is ubiquitous. For example, it is found in various body environments (oral cavity, urogenital and gastrointestinal tracts) [3,6,7,8,9,10] and across multiple species of animals, including humans, livestock, rodents and insects [1,2,3,4,11,12,13,14,15,16]. In the phylum Bacteroidetes, the phylogenetic and biological distinction between the genera Bacteroides and Prevotella is rather unclear [17,18], and this explains apparent controversies found in the early literature. Consequently, many former Bacteroides spp. were reclassified as Prevotella, Porphyromonas, Parabacteroides and Alistipes [19].
The rumen microbiome includes bacteria, ciliate protozoa, archaea, fungi and viruses, and their abundance follows the same order [20,21,22,23,24]. As in other organisms, the prevalent bacteria phyla in the rumen are Bacteroidetes and Firmicutes (synonyms Bacteroidota and Bacillota, respectively) which include a series of fiber-degrading bacteria [25,26,27]. Prevotella is often reported as a dominant genus of the rumen microbiome [28]. For example, in a comprehensive study, including 742 animals from 35 countries and 32 species of ruminants and camelids, Henderson and collaborators reported that the most abundantly identified taxa corresponded to members of the genera Prevotella, Ruminococcus, and Butyrivibrio, as well as unclassified members of the orders Clostridiales and Bacteroidales and of the families Ruminococcaceae and Lachnospiraceae [29]. Another large study including seven farms and 816 Holstein cows from the UK and Italy and 200 Nordic Red cows from Sweden and Finland also reported Bacteroidales, Spirochaetales, and WCHB1-41 as the most common bacterial taxa, and all operational taxonomic units (OTUs) found to be heritable in the Nordic red cohort were related to the Prevotellaceae family [26]. Moreover, a study including 695 samples from eight sites in the four compartments of the stomach of buffaloes also reported Prevotella among the most abundant taxa [30]. Prevotella was also shown as one of the most frequent taxa in a study that assembled 10,000 ruminal metagenomes [31]. A series of other studies with smaller cohorts also hinted towards the important role of Prevotellaceae as ruminal bacteria in cattle [18,32,33,34], buffalo [35,36,37,38,39], sheep [12,40,41,42,43], goat [11,44,45,46], domestic yak [47,48,49,50] and deer [51,52,53].
Over half a century ago, Robert Hungate and collaborators put forward the notion that the rumen microbiome is highly influenced by ingested feedstuff, a selection process driven by microbial biochemical efficiency, and the incorporation of new microbes into niches created by highly competitive microorganisms displacing less-competitive ones [20]. Thus, the abundance of Prevotella in metagenomic studies spanning several species of ruminants is perhaps a reflection of their biochemical efficiency and more generally of their adaptation to the rumen environment. As shown below, genetics of the host and its interaction with the microbiome are also important in determining the microbiome’s population structure and its metabolic efficiency [54,55].
Prevotella is central to carbohydrate and hydrogen metabolism and high abundance of Prevotella in ruminants is associated with a healthy microbiome [36,56,57,58,59,60,61,62,63]. As found in other Bacteroidetes and more generally in fiber-degrading bacteria, the Prevotella genome is endowed with polysaccharide utilization loci (PUL), which are gene clusters that encode proteins specialized in the processing of complex carbohydrates [3,64,65,66]. Ruminants ingest insufficient amounts of glucose in their diet, and to compensate for this limiting nutrient they engage in gluconeogenesis. Prevotella is able to break down a variety of polysaccharides and has the capacity to synthesize propionate, which in turn is the most important substrate for gluconeogenesis in the liver of ruminants [4,5,12,59,67]. Metabolic profiling of the Prevotella genome revealed enrichment of roles that include amino acid, carbohydrate, lipid, cofactors and vitamins, nucleotide and energy (ATP) metabolism, genetic information processing, membrane transport, replication and repair and translation [1,3,13,36,68,69,70]. This is likely a reflection of adaptations to an ecological niche where carbohydrates and free amino acids are limiting factors.
One of the end products of ruminal fermentation is methane (CH4) which is a contaminant greenhouse gas and represents a loss of energy for animals [71,72,73]. Thus, procuring a rumen microenvironment that minimizes methane production is energetically and environmentally favorable. Central to rumen fermentation is the intra- and extracellular flux of hydrogen, which can be diverted between the production of fatty acids or methane [74]. Hydrogen production is an intrinsic problem of ruminal fermentation. More than half of sequenced ruminal microbes encode hydrogenases in one or more of 26 hydrogenases subgroups. In a meta-study that assembled 10,000 bacterial metagenome-assembled genomes (MAGs) more than 6000 of those MAGs contained genes encoding [NiFe]-, [FeFe]-, and Fe-hydrogenases, with more than 3000 encoding enzymes for production of H2, mostly in Firmicutes, and 95 additional MAGs encoded hydrogenases for H2-uptake and methyl-CoM reductases for methanogenesis [31]. In metatranscriptomic experiments in the sheep rumen, half of electron bifurcating [FeFe]-hydrogenases expressed belonged to the class Clostridia. Interestingly, several H2-uptake pathways, including methanogenesis, fumarate and nitrite reduction, and acetogenesis were found to be differentially expressed in sheep with high and low methane emissions. This opens opportunities for experimental manipulation of the microbiome to favor expression of pathways that compete with methanogenesis [75,76]. Descriptive and comparative metagenomic studies in multiple species of ruminants have revealed that Prevotella is not only beneficial for efficient biosynthesis of nutrients by ruminants but also significantly ameliorates negative effects of ruminal metabolism on the environment. In this article, we summarize the contribution of Prevotella to ruminal metabolism and their association with lower emissions of methane derived from carbohydrate fermentation.
2. Ruminal Carbohydrate Fermentation
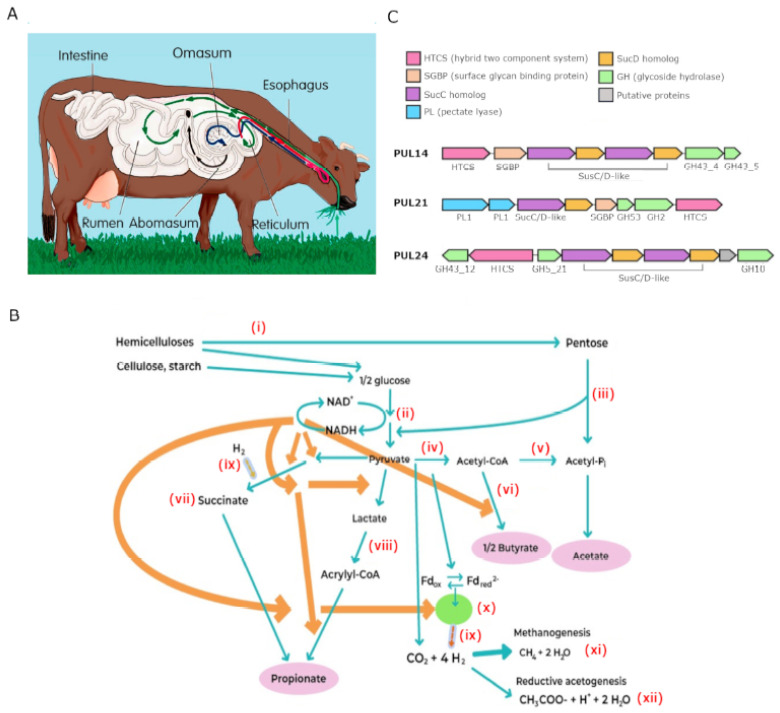
Plant tissues are mostly carbohydrates that constitute the main source of energy for ruminants and their commensal microorganisms [54,77,78,79]. Carbohydrates are structural components of the plant cell wall, but also exist as intracellular pools of carbon [80,81]. The digestive system of ruminants is complex (Figure 1A); in addition to the small and large intestines, the pancreas, and the gallbladder, there is a stomach with four compartments: the rumen, reticulum, omasum and abomasum [82]. The fermentation process starts with chewing of forage or concentrate to reduce the size of particles and to increase the surface area of the feed that will make contact with the aqueous environment of the rumen, where fermentative microbes intercept feedstuff [21,83]. The first two components of the ruminant stomach (rumen and reticulum) constitute the forestomach or fermentation vat [82]. Scratching of the rumen surface by fibers induce vigorous contractions of the rumen, which helps mixing the ingesta, but the reticulum also experiences contractions that further mix feedstuff and help regurgitation of feed boluses that need remastication for more efficient fermentation (Figure 1A) [21,60,61,84]. Indeed, it has been demonstrated that feedstuff surface area is a more important parameter of fermentation efficiency than the crystallinity of plant materials [21].
Figure 1.
Ruminal carbohydrates fermentation. (A) Ruminants lack enzymes for digestion of cellulose contained in the cell wall of plant material. Therefore, digestion of carbohydrates starts with initial mastication and passage of feedstuff to the rumen (green arrow), where cellulolytic bacteria partially degrade plant cell walls. Semi-digested material flows then to the reticulum, which facilitates regurgitation (red arrow) and rechewing of feed particles. Remasticated, finer feed particles are mobilized to the omasum (blue arrow). Finally, feedstuff passes to the abomasum (black arrow), which is considered the true stomach where an acidic pH facilitates digestion of microbial and plant proteins. (B) Simplified schematics of carbohydrate fermentation. (i) Breakdown of polysaccharides to monosaccharides; (ii) A glucose molecule is oxidized into two molecules of pyruvate with concomitant reduction of NAD+ to NADH; (iii) Pentose metabolism through the pentose cycle and transketolase cleavage; (iv) Pyruvate oxidative decarboxylation with production of CO2, reduced ferredoxin and acetyl-CoA. An alternative reaction releases formate instead CO2 and reduced ferredoxin; (v) Acetate production; (vi) Butyrate production; (vii) Propionate production via the succinate pathway; (viii) Propionate production via acrylate pathway. (ix) Interspecies hydrogen transference; (x) Production of molecular hydrogen through electron confurcation; (xi) Hydrogenotrophic methanogenesis; (xii) Reductive acetogenesis; which is a smaller H2 sink than (xi). Not all reactants or products are shown. Figure 1B was modified from [85]. Orange thick arrows indicate points where the redox couple (NAD+ and NADH) promote chemical reactions. (C) Genetic architectures of some PUL loci in P. copri, the central SusC/D proteins, the hybrid two component system (HTCS), glycoside hydrolases (GH), pectate lyases (PL) and surface glycan binding proteins (SGBP) are depicted. Figure 1C was modified from [86].
The anaerobic nature of the rumen provides a suitable niche for microorganisms that act as workhorses for carbohydrate fermentation [20,21,61,83,87]. Bacterial density in the rumen may be as high as 1010 cells per g or ruminal content [21]. Nutrients are extracted by microbes under thermodynamically favorable conditions in an aqueous solution rich in bicarbonate, contributed by the saliva of animals, which buffers acidity in the rumen [21]. It has been reported that saliva production in adult cows may exceed 200 L per day [88]. An anaerobic environment also ensures only partial oxidation of carbohydrates to CO2 and H2O and enables enzymatic extraction of energy by microorganisms in the form of intermediate energetic molecules called volatile fatty acids (VFAs), most commonly propanoic acid, acetic acid, and butyric acid [74,84]. Maintenance of constant and favorable temperature and pH for microbial activity in the rumen is essential to achieve optimal metabolic efficiency [89]. Appropriate temperature is maintained by the exergonic nature of fermentation itself (flux of electrons during fermentation) [75], while VFAs should be continuously mobilized through the ruminal epithelium to the portal vein, and finally to the liver, to be distributed but also to prevent excessive pH reduction in the ruminal chamber [21].
What follows is a simplified description of the biochemical reactions during carbohydrate fermentation (Figure 1B). For a more complete overview, the reader is encouraged to read several excellent articles published by experts in this field [21,60,74,77,83,84,90,91,92]. Lignocellulose is the most abundant carbohydrate-containing compound on Earth, as it constitutes the dry matter in vegetation. Lignocellulose is composed of two types of polymers, cellulose and hemicellulose that are the core feedstuffs of ruminants. Additionally, livestock fed with processed concentrates derived from cereals (wheat, corn, barley, etc.) ingests substantial amounts of starch. All three (cellulose, hemicellulose and starch) contain hexose, a sugar molecule with six carbon atoms (C6H12O6), which through glycolysis (EMP pathway) is converted into pyruvate (CH3COCO2H), ATP, NADH and H2O [61,93]. Since glycolysis comprises two phases—one that consumes two molecules of ATP used for phosphorylation of one molecule of glucose, and a posterior one that produces four molecules of ATP—the net gain of the process is two molecules each of ATP, pyruvate, NADH and H2O. Pyruvate is a central intermediary in fermentation and its fate is determined by reducing equivalent disposal (electron transference in redox reactions). For example, pyruvate is decarboxylated to Acetyl-CoA by a hydrogenase, with the consequent production of hydrogen and CO2, which in turns reduces ferredoxin or CO2 to produce formate [93,94,95]. Oxidation of NADH to NAD transforms pyruvate into lactate in a reversible fashion or gives rise to succinate from reduction of oxaloacetate. Succinate is finally metabolized to propionate and lactate can be metabolized to acetate, butyrate or propionate [21,74,96]. Hemicelluloses also contain pentose, a sugar with five carbon atoms (C5H10O5), through which the pentose cycle gives rise to acetate and ribose 5-phosphate [74,97]. The latter, through anabolic reactions, leads to the production of acetyl-CoA, propionyl-CoA, pyruvate and oxaloacetate. In general, reoxidation of reduced cofactors carried out by hydrogenases also transfers electrons to H+ to form H2 (molecular hydrogen), which is intercepted by methanogens for the production of methane from CO2 and H2O [55,74,76,98]. Since propionate production, but not acetate or butyrate production, implies utilization of H (constitutes a H sink), the profile of VFAs is intimately linked to methane production [5,74,99]. Moreover, hydrogenotrophic acetogenic bacteria (homo-acetogens) use the Wood-Ljungdahl pathway to metabolize CO2 and H2 into acetate [75,100]. In Figure 1B we present a simplified description of the chemical reactions taking place in carbohydrate fermentation.
The aqueous medium in the rumen contains secreted bacterial enzymes and soluble nutrients like sugars, peptides and amino acids, which can be utilized by other bacteria that lack the ability to extract such nutrients [89]. However, microbes in the ruminal aqueous media (planktonic ones) likely do not play a pivotal role in fermentation and represent instead transient states mainly constituted by daughter cells that eventually will attach to a biofilm formed on feed particles [21,101]. A biofilm is a consortia of structurally and functionally organized bacteria formed by the activity of cellulolytic bacteria that produce mono- and disaccharide sugars, which are subsequently used to produce SCFA. One such SCFA (formate) is utilized by some Archaea, i.e., Methanobrevibacter species, for methanogenesis [101]. Methanogens utilize the hydrogen released during reverse oxidation of reduced cofactors and produce methane, thus contributing to reducing acidification of the rumen [55,74,76,96]. The syntrophic relationship established in biofilms favors fermentation efficiency and sustainability [90]. Evolution of the fermentative rumen environment attempts to maximize the production of energy for the host and its microbiome and has, obviously, not taken into consideration the anthropocentric inconvenience of methane emissions. Therefore, extensive livestock production systems represent a predicament wherein production of meat and dairy products, which is directly proportional to carbohydrates fermentation, is expected to be maximized, while emissions of greenhouse gasses like methane should be urgently reduced [54,102,103,104].
3. Role of Prevotella in Lignocellulose Processing
The rumen is a seemingly infinite collection of microbes that establish imbricate interactions both with their host and among themselves. As a whole, they produce SCFAs that are absorbed by the rumen epithelium for growth and the eventual production of meat and milk [99,105,106]. Despite their high level of organization, the microbiome configurations are unstable and influenced by host diet and by interspecies competition [1,23,40,54,105].
Energetic efficiency of animals refers to the percentage of ingested calories that are converted into animal products, e.g., meat and milk [107,108,109]. Genome-wide studies have shown that specific genomic regions are associated with energetic efficiency [110] but the predicted heritability is only moderate [111]. The remaining variability in feed efficiency among ruminants could be explained by the rumen microbiome [106,112,113]. Interestingly, Shabat and collaborators [106] found an inverse correlation between feed efficiency and microbiome richness. Highly efficient animals harbored higher concentrations of the SCFAs propionate, butyrate, valerate, and isovalerate as well as higher propionate-to-acetate ratio. Lower-efficiency animals were enriched in bacterial genes that represent a more diverse array of metabolic functions and also in methanogens archaea. The authors concluded that highly efficient animals process a less diverse array of metabolites and specialize in the production of fatty acids with a concomitant reduction in methane production [106]. Further results published by the same group revealed that proteomics data are more informative to delineate the actual microbiome metabolic profile than metagenomics data, thus fueling a long-standing debate concerning the intrinsic divergence between gene content and actual protein expression in metagenomics studies [114]. Nevertheless, these and a myriad of additional studies led to the proposal of a host genetics-microbiome axis as the master control mechanism of metabolism in ruminants [103,115].
Saccharification refers to the process whereby carbohydrates are hydrolyzed to component sugar by bacterial enzymes. In Bacteroidetes, like Prevotella, such enzymes are organized as PULs (Figure 1C), which are sets of co-regulated genes active in saccharification of complex carbohydrates (glycans) [65]. However, PULs are not exclusive to ruminants, but rather are widespread across species [3,64,65,66]. The central part of PULs corresponds to a starch utilization system (Sus; Figure 1C), best characterized in Bacteroides thetaiotaomicron but ubiquitous in Bacteroidetes [116]. A comprehensive collection of carbohydrate-active enzymes (CAZymes) is maintained at the PULDB site (www.cazy.org; accessed on 12 September 2022) [117] and the dbCAN-PUL site (https://bcb.unl.edu/dbCAN_PUL; accessed on 12 September 2022) [118]. Computational tools for prediction of PULs have also been developed [117,119,120]. At the time of writing, five classes of CAZymes are indexed in the CAZy database. Glycoside hydrolases (GH; EC 3.2.1.-; 173 families) hydrolyze glycosidic bonds between carbohydrates and other moieties. Glycosyl transferases (GT; EC 2.4.x.y; 115 families) catalyze glycosidic bonds between activated donor and specific acceptor molecules. Polysaccharide lyases (PL; EC 4.2.2.-; 42 families) cleave uronic acid-containing polysaccharides to produce unsaturated hexeuronic acid and a new reducing end. Carbohydrate esterases (CE; 20 families) catalyze the acylation of substituted saccharides. Lastly, there are 17 families of redox enzymes that perform functions auxiliary to CAZymes, e.g., lignin degradation [121].
Prevotella genomes contain many PULs that encode CAZymes specialized in the degradation of non-cellulosic plant fibers. So far, for Prevotella alone, the CAZy database contains entries for 9858 CAZymes which represent 191 CAZymes families in 85 bacterial strains. Of those, 22 correspond to carbohydrate binding enzymes; 12 are carbohydrate esterases; 116 are glycoside hydrolases; 26 are glycosyl transferases; and 15 are polysaccharide lyases. The five strains with the larger number of characterized CAZymes are Prevotella sp. E13-3 (267 CAZymes), P. ruminicola 23 (274 CAZymes), P. sp. E15-14 (278 CAZymes), P. ruminicola KHP1 (279 CAZymes) and P. bryantii B14 (310 CAZymes) [117,121]. However, we acknowledge that the number of CAZymes characterized in each strain may be a reflection of the anthropocentric interest they generate more than their actual CAZymes repertoire encoded in their genomes. A genetic toolbox for studying PULs in Prevotella has been developed [86] and it should shed light into the specific role of more Prevotella CAZymes in the years to come. PULs stratified a series of Prevotella species into specialists and generalists and exhibited considerable diversity within the generalists [3]. Accetto and Avguštin [122] characterized the PULs from 39 species and 50 genomes of Prevotella from diverse habitats. Approximately 1200 full-length SusD proteins, arranged in tandem with SusC proteins, were detected but the average number per species was quite variable, with species harboring a reduced number of SusD proteins exhibiting a proportional number of CAZymes. Out of 25 SusD protein groups, only three groups targeted hemicellulose or pectin, suggesting that Prevotella catalytic activity centers mainly on glycans and plant storage polysaccharides. On the basis of their SusD enzymes, the Prevotella species could be clustered into five groups, and the ruminal Prevotella clustered together with oral and gut species. Finally, six SusD protein groups included peptidases, evidencing a role in protein degradation and peptide transport [122]. An independent study reported similar results for Prevotella bryantii, which exhibited high catalytic affinity for plant storage and the cell wall polysaccharide β-glucan [123]. In enrichment cultures inoculated with ruminal content from cattle fed a total mixed ration, Prevotella poorly fermented hemicellulose, but turned dominant in cultures supplemented with xylan [14]. PULs containing Sus-like homologs have been reported in metagenomic studies in cattle [124,125,126,127], reindeer [126,128,129], moose [27] and sheep [126]. A Bacteroidetes phylotype (SRM-1) found in the reindeer metagenome was demonstrated to have glycoside hydrolase activity, apparently from members of the GH5 family of endoglucanases, and was shown to be active on β-glucans, xylans, xyloglucans and mannan substrates [128]. In general, it has been proposed that the efficiency of Prevotella for the digestion of xylans and pectins resides in a multitude of enzymes encoded in its gene clusters, especially the glycoside hydrolases xylanase GH10 and beta xylosidase GH43 [130]. One way of quantifying the relative contribution of specific bacterial taxa to carbohydrate fermentation is through comparisons of relative abundances of taxonomically preclassified sequences encoding CAZymes. In a deep sequenced metagenomics study in goats, Prevotella was the most abundant genus and was found to contribute 30% hemicellulases, 36% enzymes for lignocellulose pretreatment, 98.8% ferulolyl esterases and 71.1% acetylxylan esterases [11] of all sequences encoding such enzymes.
The ability of Prevotella to ferment carbohydrates is also reflected in the productivity of cattle. In a small cohort of lactating Holstein cows, it was reported that animals with high content protein and fat (HPF) in their milk harbored higher content of SCFAs (acetate, butyrate and propionate) in their ruminal fluid. Species in the genus Prevotella occupied 68.8% of all species identified and were more abundant in the HPF group. Among taxa found at higher abundance in the HPF group, functional annotation of bacterial genes using KEGG, eggNOG and CAZy databases, demonstrated an enrichment in functions pertaining to metabolism of carbohydrates, amino acids, pyruvate, insulin and lipids as well as transport [68]. Analogously, Conte and collaborators also reported a positive correlation between the abundance of Prevotella and lipid metabolism in cows [131]. In fattening yak in Tibet, Prevotella was the most abundant genus in the ruminal microbiome, and its abundance was positively and negatively correlated with polyunsaturated and saturated fatty acids, respectively [132].
In vitro, Prevotella bryantii has been reported to be involved in the de novo synthesis of amino acids from ammonia in the presence of amino acids and peptides, and the extent to which it is used for protein synthesis should have implications for carbohydrate fermentation efficiency [133]. The proteolytic ability of Prevotella ruminicola has been reported [70]. Also, in vitro experiments demonstrated that vitamin B12 increased propionate synthesis by Prevotella ruminicola 23, but in the absence of this vitamin the main synthesis product was succinate [67]. Interestingly, a positive correlation between the abundance of Prevotella and vitamin B12 was found in the rumen of Holstein cattle [134]. Together, the capability of Prevotella to synthesize lipids and amino acids, and its proteolytic capability confer great adaptability to the ruminal environment.
4. Prevotella and Its Association with Reduced Methane Emissions
Tropospheric greenhouse gasses, mainly methane, ozone and carbon dioxide regulate temperature and allow life on Earth to flourish, but such amenable temperatures have been fine-tuned over long periods of time. Research has shown that even small fluctuations in temperature, as a result of increasing greenhouse gas emissions, may have dramatic effects on life on Earth, e.g., on coral reefs, forests, biodiversity, etc. [135,136,137]. Following the industrial revolution, temperature has been on the rise, and such a trend has been exacerbated after the 1980s, mainly due to the use of fossil fuels and the expansion of agriculture, including livestock production systems [135]. Ruminants generate massive amounts of methane as a byproduct of carbohydrate fermentation [60,74,76,98,138]. Unlike gasses emitted during the combustion of fossil fuels, carbon emitted by ruminants is part of the carbon cycle since it was initially sequestered as CO2 by photosynthetic organisms. Specifically, the problem lies in the fact that ruminants belch out CH4 as one of the end products of ruminal microbial metabolism, which is a more potent greenhouse gas. CH4 is eventually converted back into CO2 but only after a period of ~20 years. It is generally accepted that ruminants contribute approximately 20% of global anthropogenic methane [139,140]. Methane oxidation in the troposphere contributes to the formation of ozone and carbon dioxide and therefore boosts global warming [72,141]. Although methanogenesis metabolizes only a handful of substrates, it is a very sophisticated mechanism that includes three different pathways and more than 200 genes [142]. In order to reduce the environmental footprint of livestock systems, it is imperative to implement production practices that lower methane emissions. The rumen microbiome stands out as one of the most promising targets for such interventions. Moreover, ruminal methane production also represents a loss of energy [102,143]; therefore, microbiome configurations that reduce methane production have the potential to both reduce the environmental footprint and increase energetic efficiency of production animals.
Although part of ruminal methane is generated via methylotrophic methanogenesis [144,145], the majority arises from hydrogenotrophic methanogenesis using H2 and CO2 as substrates [23,146]. In the latter pathway, the archaea genus Methanobrevibacter appears dominant, and its abundance is favored by a rich forage diet because it promotes synthesis of ATP [147]. Interestingly, the abundance of methanogenic archaea in the rumen holds only a weak correlation with methane emissions [36,102,103]. On the other hand, a series of studies in ruminants using a halogenated methane analog, bromochloromethane (BCM), which inhibits methanogenesis, showed that increases in ruminal H2 and H2 emissions were accompanied by an increase in the synthesis of propionate and isovalerate and by expanded populations of Prevotella spp. and Fibrobacter succinogenes [148,149,150,151]. Prevotella could sequester H by the succinate or the acrylate pathways that ferment sugars or lactate, respectively [67,152]. Although it is unlikely that BCM administration will be used as a practice to mitigate methane emissions—because it is itself considered a greenhouse gas—those experiments revealed that some microorganisms could efficiently divert the H2 pool away from methanogenesis.
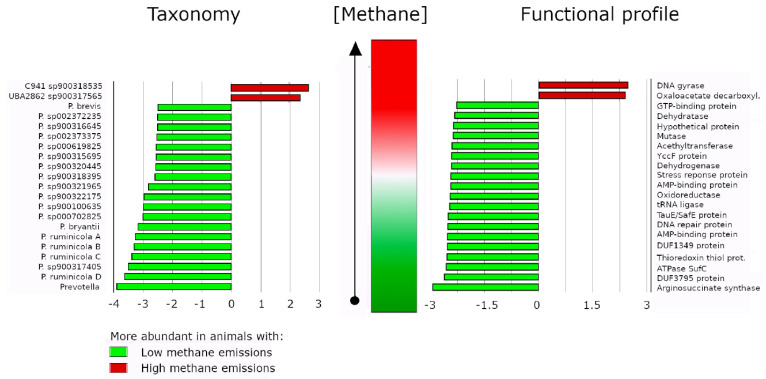
A series of metagenomics studies have outlined a strong correlation between the abundance of Prevotella and lower methane emissions. For instance, in a cohort of Colombian buffalo, it was reported that animals with lower emissions of methane harbored higher densities of P. ruminicola, P. bryantii, P. brevis and 22 other Prevotella species. The authors hypothesized that Prevotella could contribute to reductions in methane emissions by diverting H towards the production of propionic acid and away from methanogenesis. Interestingly, no differences in the abundance of methanogenic archaea were observed between animals with low and high methane emissions. Metabolic profiling revealed that among the top Prevotella enzymes that increased in abundance were methylmalonyl CoA mutase (MCM), NADP-specific glutamate dehydrogenase (NADP-GDH), a sulfite exporter TauE/SafE family protein, and a FeS cluster assembly ATPase SufC protein [36]. Because data in this study capture in great detail what several studies suggest, we summarize in Figure 2 the prevalence of many Prevotella species (green bars in left panel) and Prevotella enzymes (green bars in right panel) in animals exhibiting lower methane emissions. Interestingly, vitamin B12 is a cofactor of MCM [153], and increased abundance of Prevotella was associated with augmented concentrations of vitamin B12 [134], which in turn influenced propionate synthesis in Prevotella ruminicola 23 [67]. In feedlot lambs, liver vitamin B12 concentration correlated with higher content of propionate metabolizing enzymes, but exogenous application of vitamin B2 did not accentuate such correlation [154]. Thus, supplementation of vitamin B12 is unlikely a good strategy to promote propionate biosynthesis as a means to lower methane production, although more investigations are still required to rule out such a possibility. The majority of studies concerning feed processing efficiency and its relationship with methane production have been conducted on dairy cows, buffalo or goats. In a study involving small cohorts of Holstein or Jersey steers under the same diet regime, it was found that methane production and VFA concentrations (mainly acetate and butyrate) were significantly higher in the Jersey cohort, while ruminal pH was lower. A series of Prevotella species, including P. micans, P. copri, P. oris, P. baroniae were found at a significantly higher density in the Holstein cohort, which produced less methane. Only P. shahii was found at reduced abundance [155].
Figure 2.
Inverse correlation between Prevotella abundance and methane emissions. Data from Aguilar-Marin et al., 2020 [36]. Results from linear discriminant analysis with software LEfSe. All presented results had a p-value < 0.05 and showed that many species of Prevotella (left panel) or proteins from Prevotella (right panel) were more abundant in animals that exhibited lower methane emissions (green bars). Red bars depict taxa or proteins that were more abundant in animals with high methane emissions. P: Prevotella. Suffixes A, B, C and D in Prevotella ruminicola species are arbitrary and are used here only to denote that four different strains of such a species were found differentially accumulated. For the sake of space, names of proteins in the right panel were arbitrarily abbreviated. All green bars correspond to Prevotella proteins while the two red bars correspond to proteins from Bacteroides ovatus (DNA gyrase, top bar) and [Ruminoccocacea bacterium AB4001] (Oxoloacetate decarboxylase). Full names of proteins can be found in Figure 6 of Aguilar-Marin et al., 2022 [36].
A variety of studies have reported that the cashew nut shell liquid (CNSL), a phenolic subproduct of cashew nut production, has the potential to reduce ruminal methane emissions through the modification of the microbiome [156,157,158,159,160]. In a small cohort of Holstein cows, animals were subjected to a diet containing concentrate and hay (60:40) for four weeks (control time point) and then to the same diet supplemented with pellets containing 4 g/100 kg of body weight per day of CNLS (CNLS time point). In one of the trials, methane production per unit of dry matter was reduced by 38.3% and energy loss decreased from 9.7% to 6.1%. Bacterial species related to the production of formate (Ruminococcus flavefaciens, Butyrivibrio fibrisolvens, and Treponema bryantii) decreased in abundance while others related to propionate production (Prevotella ruminicola, Selenomonas ruminantium, Anaerovibrio lipolytica, and Succinivibrio dextrinosolvens) increased in abundance [160]. These experiments need validation in larger cohorts but highlight an important point: not only do propionate-producing species like Prevotella ruminicola contribute to a reduction in methane production, but the abundance of such bacteria is boosted by the suppression of methane generation. In other words, a feedback loop that progressively increases the relative abundance of propionate-producing bacteria and at the same time reduces methane emissions could be established by using chemical suppressors of methanogenesis and parallel strategies to foster abundance of such taxa. Conversely, it was reported that supplementation of nitrate led to a reduction of methane emissions in lactating cows and an increase in acetate to propionate ratio with a concomitant decrease in abundance of Prevotella [161].
In a factorial experimental design, buffaloes and bovines were fed two different roughage concentrate dietary mixes (70:30, low concentrate, LC; 40:60, high concentrate, HC) and their microbial diversity, ruminal methane emissions and nutrient utilization were determined. HC led to reduced methane emissions. Bacteroidetes increased their abundance in the HC diet, and Prevotella was the dominant genus in the rumen with higher relative abundance in bovines (52%) than in buffalo (32%); however the HC diet also increased Prevotella abundance in buffalo [162]. Yet, reductions in methane production are not exclusively associated with Prevotella. In studies on Holstein dairy cows whose diet was supplemented with two methane-mitigating agents (grape marc or a mixture of lipids and tannins) a series of contigs resembling Faecalibacterium sequences were found to be differentially accumulated in animals that received methane-mitigating agents. In an independent cohort, it was found that the abundance of such contigs correlated with methane production. No differential abundance of Prevotella was reported in that study [163].
Another major player in ruminal methane production is host genetics. Studies on ruminal content transplantation where modifications of the host microbiome structure and milk production efficiency of animals were only transient [164,165], suggested a role of the host genotype on microbiome composition. Host genotype has also been reported to influence CH4 emissions [166,167]. Thus, interest has arisen in the relative contribution of the microbiome and the host genotype on methanogenesis and the relationships among them. Difford and collaborators [168] assessed the hypothesis that the microbiome composition is heritable and that methane emissions are influenced by both the genome of the host and its microbiome. In a cohort of 750 lactating Holstein dairy cows from farms in Denmark, methane emissions were quite variable. There was a 41% mean difference between the top and bottom 10% emitters (519.28 ± 28.5 g/d vs. 303.8 ± 11.9 g/d, respectively). The vast majority of OTU’s found in >50% of cows corresponded to the phylum Bacteroidetes (72.2%). Using a linear mixed model, it was found that the archaeal genus Methanobrevibacter had a statistically significant heritability (h2) (0.22 ± 0.09). Host genetics and ruminal microbes explained 21% and 13% of the variation observed in methane production, respectively [168]. Similar results were obtained using Bayesian modeling, with even a smaller proportion of the variance in methane emissions explained by the microbiome [169]. The take-home message of these studies is that both host genetics and microbiome composition should be taken into consideration for designing strategies to mitigate ruminal methane emissions. Inside the ruminal microbiome, Prevotella appears a strong candidate to integrate methane mitigation strategies due to its frequent association with lower methane emissions and its high relative abundance in the rumen.
5. Concluding Remarks
Prevotella appears as a major player in ruminal metabolism. Several attributes of such a bacterium vindicate its preeminence. Namely, it is remarkably abundant in many species of ruminants; one of its fermentation products is propionic acid and has, therefore, the potential to compete with methanogenesis and archaea for hydrogen utilization. Consequently, its abundance has been found inversely correlated with methane emissions. Given the urgency to reduce greenhouse gas emissions, Prevotella has the potential to be used as an antimethanogenic agent. Several questions remain unanswered in that respect. For example, what is the appropriate formulation to supplement livestock feedstuff with Prevotella? Would exogenously administered Prevotella persist in the ruminal microbiome of receptor animals? Experiments involving administration of probiotic formulations that include Prevotella or transplantation of ruminal content from animals harboring high density of Prevotella will shed light into those questions.
Acknowledgments
Special thanks to Alyssa Butters and Stefan Gavriliuc (University of Calgary) for their excellent criticism of a previous version of the manuscript. Figure 1A is a modified version of an illustration produced by Addisson Wesley Longman Inc. Publicly available at https://www.mun.ca/biology/scarr/Ruminant_Digestion.html.
Abbreviations
| OTU | Operational taxonomic unit |
| PUL | Polysaccharide utilization loci |
| SCFA | Short-chain fatty acid |
| VFA | Volatile fatty acid |
| H2 | Molecular hydrogen |
| CH4 | Methane |
| NiFe-hydrogenase | A hydrogenase that binds NiFe at its active site |
| FeFe-hydrogenases | A hydrogenase that binds FeFe at its active site |
| Fe-hydrogenases | A hydrogenase that binds Fe at its active site |
| MAG | Metagenome-assembled genome |
| EMP pathway | Embden-Meyerhof-Parnas glycolytic pathway |
| CAZymes | Carbohydrate-active enzymes |
| Methyl-CoM | Methyl coenzyme M |
| MCM | Methylmalonyl CoA mutase |
| CNSL | Cashew nut shell liquid |
| BCM | Bromochloromethane |
| HPF | High content protein and fat |
| KEGG | Kyoto encyclopedia of genes and genomes |
| eggNOG | Clusters of ortholog groups database |
| CAZy | Carbohydrate-active enzymes database |
| NADP-GDH | NADP-specific glutamate dehydrogenase |
| TauE/SafE | Sulfite exporter family protein |
| FeS | Iron-sulfur cluster |
| SufC | ATPase of the ABC superfamily |
| CNSL | Cashew nut shell liquid |
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tett A., Pasolli E., Masetti G., Ercolini D., Segata N. Prevotella Diversity, Niches and Interactions with the Human Host. Nat. Rev. Microbiol. 2021;19:585–599. doi: 10.1038/s41579-021-00559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg E. The Prokaryotes. Springer; Berlin/Heidelberg, Germany: 2014. The Family Prevotellaceae; pp. 825–827. [Google Scholar]
- 3.Accetto T., Avguštin G. The Diverse and Extensive Plant Polysaccharide Degradative Apparatuses of the Rumen and Hindgut Prevotella Species: A Factor in Their Ubiquity? Syst. Appl. Microbiol. 2019;42:107–116. doi: 10.1016/j.syapm.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Chen T., Long W., Zhang C., Liu S., Zhao L., Hamaker B.R. Fiber-Utilizing Capacity Varies in Prevotella- versus Bacteroides-Dominated Gut Microbiota. Sci. Rep. 2017;7:2594. doi: 10.1038/s41598-017-02995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Portincasa P., Bonfrate L., Vacca M., De Angelis M., Farella I., Lanza E., Khalil M., Wang D.Q.-H., Sperandio M., Di Ciaula A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022;23:1105. doi: 10.3390/ijms23031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi H., Shibata K., Sakamoto M., Tomita S., Benno Y. Prevotella copri Sp. Nov. and Prevotella stercorea Sp. Nov., Isolated from Human Faeces. Int. J. Syst. Evol. Microbiol. 2007;57:941–946. doi: 10.1099/ijs.0.64778-0. [DOI] [PubMed] [Google Scholar]
- 7.Könönen E., Gursoy U.K. Oral Prevotella Species and Their Connection to Events of Clinical Relevance in Gastrointestinal and Respiratory Tracts. Front. Microbiol. 2021;12:798763. doi: 10.3389/fmicb.2021.798763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richter H.E., Carnes M.U., Komesu Y.M., Lukacz E.S., Arya L., Bradley M., Rogers R.G., Sung V.W., Siddiqui N.Y., Carper B., et al. Association between the Urogenital Microbiome and Surgical Treatment Response in Women Undergoing Midurethral Sling Operation for Mixed Urinary Incontinence. Am. J. Obstet. Gynecol. 2022;226:93.e1–93.e15. doi: 10.1016/j.ajog.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubourg G., Morand A., Mekhalif F., Godefroy R., Corthier A., Yacouba A., Diakite A., Cornu F., Cresci M., Brahimi S., et al. Deciphering the Urinary Microbiota Repertoire by Culturomics Reveals Mostly Anaerobic Bacteria From the Gut. Front. Microbiol. 2020;11:513305. doi: 10.3389/fmicb.2020.513305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas-White K., Forster S.C., Kumar N., Van Kuiken M., Putonti C., Stares M.D., Hilt E.E., Price T.K., Wolfe A.J., Lawley T.D. Culturing of Female Bladder Bacteria Reveals an Interconnected Urogenital Microbiota. Nat. Commun. 2018;9:1557. doi: 10.1038/s41467-018-03968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dao T.-K., Do T.-H., Le N.-G., Nguyen H.-D., Nguyen T.-Q., Le T.-T.-H., Truong N.-H. Understanding the Role of Prevotella Genus in the Digestion of Lignocellulose and Other Substrates in Vietnamese Native Goats’ Rumen by Metagenomic Deep Sequencing. Animals. 2021;11:3257. doi: 10.3390/ani11113257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietary H.-J., Callaway R., Wu Q.-C., Wang W.-K., Zhang F., Li W.-J., Wang Y.-L., Lv L.-K., Yang H.-J. Dietary Cysteamine Supplementation Remarkably Increased Feed Efficiency and Shifted Rumen Fermentation toward Glucogenic Propionate Production via Enrichment of Prevotella in Feedlot Lambs. Microorganisms. 2022;10:1105. doi: 10.3390/microorganisms10061105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovatcheva-Datchary P., Nilsson A., Akrami R., Lee Y.S., De Vadder F., Arora T., Hallen A., Martens E., Björck I., Bäckhed F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015;22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Emerson E.L., Weimer P.J. Fermentation of Model Hemicelluloses by Prevotella Strains and Butyrivibrio Fibrisolvens in Pure Culture and in Ruminal Enrichment Cultures. Appl. Microbiol. Biotechnol. 2017;101:4269–4278. doi: 10.1007/s00253-017-8150-7. [DOI] [PubMed] [Google Scholar]
- 15.Amat S., Lantz H., Munyaka P.M., Willing B.P. Prevotella in Pigs: The Positive and Negative Associations with Production and Health. Microorganisms. 2020;8:1584. doi: 10.3390/microorganisms8101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagpal R., Wang S., Solberg Woods L.C., Seshie O., Chung S.T., Shively C.A., Register T.C., Craft S., McClain D.A., Yadav H. Comparative Microbiome Signatures and Short-Chain Fatty Acids in Mouse, Rat, Non-Human Primate, and Human Feces. Front. Microbiol. 2018;9:2897. doi: 10.3389/fmicb.2018.02897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah H.N., Collins D.M. Prevotella, a New Genus to Include Bacteroides Melaninogenicus and Related Species Formerly Classified in the Genus Bacteroides. Int. J. Syst. Bacteriol. 1990;40:205–208. doi: 10.1099/00207713-40-2-205. [DOI] [PubMed] [Google Scholar]
- 18.Flint H.J., Duncan S.H. Bacteroides and Prevotella. In: Lou Tortorello M., Batt C.A., editors. Encyclopedia of Food Microbiology—Reference Work. Elsevier; Amsterdam, The Netherlands: 2014. pp. 203–208. [Google Scholar]
- 19.Rajilić-Stojanović M., de Vos W.M. The First 1000 Cultured Species of the Human Gastrointestinal Microbiota. FEMS Microbiol. Rev. 2014;38:996–1047. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hungate R.E., Bryant M.P., Mah R.A. The rumen bacteria and protozoa. Annu. Rev. Microbiol. 1964;18:131–166. doi: 10.1146/annurev.mi.18.100164.001023. [DOI] [PubMed] [Google Scholar]
- 21.Russell J.B. Rumen Microbiology and Its Role in Ruminant Nutrition. Department of Microbiology, Cornell University; Ithaca, NY, USA: 2002. [Google Scholar]
- 22.Mizrahi I., Wallace R.J., Moraïs S. The Rumen Microbiome: Balancing Food Security and Environmental Impacts. Nat. Rev. Microbiol. 2021;19:553–566. doi: 10.1038/s41579-021-00543-6. [DOI] [PubMed] [Google Scholar]
- 23.Morgavi D.P., Kelly W.J., Janssen P.H., Attwood G.T. Rumen Microbial (meta)genomics and Its Application to Ruminant Production. Animal. 2013;7((Suppl. 1)):184–201. doi: 10.1017/S1751731112000419. [DOI] [PubMed] [Google Scholar]
- 24.Hungate R.E. The Rumen and Its Microbes. Elsevier; Amsterdam, The Netherlands: 2013. [Google Scholar]
- 25.Pinnell L.J., Reyes A.A., Wolfe C.A., Weinroth M.D., Metcalf J.L., Delmore R.J., Belk K.E., Morley P.S., Engle T.E. Bacteroidetes and Firmicutes Drive Differing Microbial Diversity and Community Composition Among Micro-Environments in the Bovine Rumen. Front. Vet. Sci. 2022;9:897996. doi: 10.3389/fvets.2022.897996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace R.J., Sasson G., Garnsworthy P.C., Tapio I., Gregson E., Bani P., Huhtanen P., Bayat A.R., Strozzi F., Biscarini F., et al. A Heritable Subset of the Core Rumen Microbiome Dictates Dairy Cow Productivity and Emissions. Sci. Adv. 2019;5:eaav8391. doi: 10.1126/sciadv.aav8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svartström O., Alneberg J., Terrapon N., Lombard V., de Bruijn I., Malmsten J., Dalin A.-M., El Muller E., Shah P., Wilmes P., et al. Ninety-Nine de Novo Assembled Genomes from the Moose (Alces Alces) Rumen Microbiome Provide New Insights into Microbial Plant Biomass Degradation. ISME J. 2017;11:2538–2551. doi: 10.1038/ismej.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Hara E., Neves A.L.A., Song Y., Guan L.L. The Role of the Gut Microbiome in Cattle Production and Health: Driver or Passenger? Annu. Rev. Anim. Biosci. 2020;8:199–220. doi: 10.1146/annurev-animal-021419-083952. [DOI] [PubMed] [Google Scholar]
- 29.Henderson G., Cox F., Ganesh S., Jonker A., Young W., Global Rumen Census Collaborators. Janssen P.H. Rumen Microbial Community Composition Varies with Diet and Host, but a Core Microbiome Is Found across a Wide Geographical Range. Sci. Rep. 2015;5:14567. doi: 10.1038/srep14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong F., Wang T., Gao N.L., Liu Z., Cui K., Duan Y., Wu S., Luo Y., Li Z., Yang C., et al. The Microbiome of the Buffalo Digestive Tract. Nat. Commun. 2022;13:823. doi: 10.1038/s41467-022-28402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie F., Jin W., Si H., Yuan Y., Tao Y., Liu J., Wang X., Yang C., Li Q., Yan X., et al. An Integrated Gene Catalog and over 10,000 Metagenome-Assembled Genomes from the Gastrointestinal Microbiome of Ruminants. Microbiome. 2021;9:137. doi: 10.1186/s40168-021-01078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dowd S.E., Callaway T.R., Wolcott R.D., Sun Y., McKeehan T., Hagevoort R.G., Edrington T.S. Evaluation of the Bacterial Diversity in the Feces of Cattle Using 16S rDNA Bacterial Tag-Encoded FLX Amplicon Pyrosequencing (bTEFAP) BMC Microbiol. 2008;8:125. doi: 10.1186/1471-2180-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holman D.B., Gzyl K.E. A Meta-Analysis of the Bovine Gastrointestinal Tract Microbiota. FEMS Microbiol. Ecol. 2019;95:fiz072. doi: 10.1093/femsec/fiz072. [DOI] [PubMed] [Google Scholar]
- 34.Furman O., Shenhav L., Sasson G., Kokou F., Honig H., Jacoby S., Hertz T., Cordero O.X., Halperin E., Mizrahi I. Stochasticity Constrained by Deterministic Effects of Diet and Age Drive Rumen Microbiome Assembly Dynamics. Nat. Commun. 2020;11:1904. doi: 10.1038/s41467-020-15652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nathani N.M., Kothari R.K., Patel A.K., Joshi C.G. Functional Characterization Reveals Novel Putative Coding Sequences in Prevotella Ruminicola Genome Extracted from Rumen Metagenomic Studies. J. Mol. Microbiol. Biotechnol. 2015;25:292–299. doi: 10.1159/000437265. [DOI] [PubMed] [Google Scholar]
- 36.Aguilar-Marin S.B., Betancur-Murillo C.L., Isaza G.A., Mesa H., Jovel J. Lower Methane Emissions Were Associated with Higher Abundance of Ruminal Prevotella in a Cohort of Colombian Buffalos. BMC Microbiol. 2020;20:364. doi: 10.1186/s12866-020-02037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh K.M., Reddy B., Patel A.K., Panchasara H., Parmar N., Patel A.B., Shah T.M., Bhatt V.D., Joshi C.G. Metagenomic Analysis of Buffalo Rumen Microbiome: Effect of Roughage Diet on Dormancy and Sporulation Genes. Meta Gene. 2014;2:252–268. doi: 10.1016/j.mgene.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kala A., Kamra D.N., Kumar A., Agarwal N., Chaudhary L.C., Joshi C.G. Impact of Levels of Total Digestible Nutrients on Microbiome, Enzyme Profile and Degradation of Feeds in Buffalo Rumen. PLoS ONE. 2017;12:e0172051. doi: 10.1371/journal.pone.0172051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou C., Gu Q., Zhou X., Xia Z., Muhammad W.I., Tang Q., Liang M., Lin B., Qin G. Ruminal Microbiota Composition Associated with Ruminal Fermentation Parameters and Milk Yield in Lactating Buffalo in Guangxi, China-A Preliminary Study. J. Anim. Physiol. Anim. Nutr. 2019;103:1374–1379. doi: 10.1111/jpn.13154. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y., Cao P., Wang L., Zhao Z., Chen Y., Yang Y. Bacterial Community Diversity Associated with Different Levels of Dietary Nutrition in the Rumen of Sheep. Appl. Microbiol. Biotechnol. 2017;101:3717–3728. doi: 10.1007/s00253-017-8144-5. [DOI] [PubMed] [Google Scholar]
- 41.Wang L., Zhang K., Zhang C., Feng Y., Zhang X., Wang X., Wu G. Dynamics and Stabilization of the Rumen Microbiome in Yearling Tibetan Sheep. Sci. Rep. 2019;9:19620. doi: 10.1038/s41598-019-56206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu S., Zhang G., Liu Z., Wu P., Yu Z., Wang J. Repeated Inoculation with Fresh Rumen Fluid before or during Weaning Modulates the Microbiota Composition and Co-Occurrence of the Rumen and Colon of Lambs. BMC Microbiol. 2020;20:29. doi: 10.1186/s12866-020-1716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace R.J., McKain N., Broderick G.A. Breakdown of Different Peptides by Prevotella (Bacteroides) Ruminicola and Mixed Microorganisms from the Sheep Rumen. Curr. Microbiol. 1993;26:333–336. doi: 10.1007/BF01576265. [DOI] [PubMed] [Google Scholar]
- 44.Li B., Zhang K., Li C., Wang X., Chen Y., Yang Y. Characterization and Comparison of Microbiota in the Gastrointestinal Tracts of the Goat (Capra Hircus) During Preweaning Development. Front. Microbiol. 2019;10:2125. doi: 10.3389/fmicb.2019.02125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wetzels S.U., Mann E., Metzler-Zebeli B.U., Wagner M., Klevenhusen F., Zebeli Q., Schmitz-Esser S. Pyrosequencing Reveals Shifts in the Bacterial Epimural Community Relative to Dietary Concentrate Amount in Goats. J. Dairy Sci. 2015;98:5572–5587. doi: 10.3168/jds.2014-9166. [DOI] [PubMed] [Google Scholar]
- 46.Lv X., Chai J., Diao Q., Huang W., Zhuang Y., Zhang N. The Signature Microbiota Drive Rumen Function Shifts in Goat Kids Introduced to Solid Diet Regimes. Microorganisms. 2019;7:516. doi: 10.3390/microorganisms7110516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su Y., Su J., Li F., Tian X., Liu Z., Ding G., Bai J., Li Z., Ma Z., Peppelenbosch M.P. Yak Gut Microbiota: A Systematic Review and Meta-Analysis. Front. Vet. Sci. 2022;9:889594. doi: 10.3389/fvets.2022.889594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo W., Zhou M., Ma T., Bi S., Wang W., Zhang Y., Huang X., Guan L.L., Long R. Survey of Rumen Microbiota of Domestic Grazing Yak during Different Growth Stages Revealed Novel Maturation Patterns of Four Key Microbial Groups and Their Dynamic Interactions. Anim. Microbiome. 2020;2:23. doi: 10.1186/s42523-020-00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xin J., Chai Z., Zhang C., Zhang Q., Zhu Y., Cao H., Zhong J., Ji Q. Comparing the Microbial Community in Four Stomach of Dairy Cattle, Yellow Cattle and Three Yak Herds in Qinghai-Tibetan Plateau. Front. Microbiol. 2019;10:1547. doi: 10.3389/fmicb.2019.01547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao C., Wang L., Ke S., Chen X., Kenéz Á., Xu W., Wang D., Zhang F., Li Y., Cui Z., et al. Yak Rumen Microbiome Elevates Fiber Degradation Ability and Alters Rumen Fermentation Pattern to Increase Feed Efficiency. Anim. Nutr. 2022;11:201–214. doi: 10.1016/j.aninu.2022.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z.P., Liu H.L., Li G.Y., Bao K., Wang K.Y., Xu C., Yang Y.F., Yang F.H., Wright A.-D.G. Molecular Diversity of Rumen Bacterial Communities from Tannin-Rich and Fiber-Rich Forage Fed Domestic Sika Deer (Cervus Nippon) in China. BMC Microbiol. 2013;13:151. doi: 10.1186/1471-2180-13-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y., Sun Y., Shi Z., Liu Z., Zhao C., Lu T., Gao H., Zhu F., Chen R., Zhang J., et al. Gut Microbiota of Wild and Captive Alpine Musk Deer (Moschus Chrysogaster) Front. Microbiol. 2019;10:3156. doi: 10.3389/fmicb.2019.03156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gruninger R.J., Sensen C.W., McAllister T.A., Forster R.J. Diversity of Rumen Bacteria in Canadian Cervids. PLoS ONE. 2014;9:e89682. doi: 10.1371/journal.pone.0089682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belanche A., Patra A.K., Morgavi D.P., Suen G., Newbold C.J., Yanez-Ruiz D.R. Gut Microbiome Modulation in Ruminants: Enhancing Advantages and Minimizing Drawbacks. Frontiers Media SA; Lausanne, Switzerland: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danielsson R., Dicksved J., Sun L., Gonda H., Müller B., Schnürer A., Bertilsson J. Methane Production in Dairy Cows Correlates with Rumen Methanogenic and Bacterial Community Structure. Front. Microbiol. 2017;8:226. doi: 10.3389/fmicb.2017.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J.N., Méndez–García C., Geier R.R., Iakiviak M., Chang J., Cann I., Mackie R.I. Metabolic Networks for Nitrogen Utilization in Prevotella Ruminicola 23. Sci. Rep. 2017;7:7851. doi: 10.1038/s41598-017-08463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsuda T., Sasaki Y., Kawashima R. Physiological Aspects of Digestion and Metabolism in Ruminants: Proceedings of the Seventh International Symposium on Ruminant Physiology. Academic Press; Cambridge, MA, USA: 2012. [Google Scholar]
- 58.Wallace R.J., McKain N., Broderick G.A., Rode L.M., Walker N.D., Newbold C.J., Kopecny J. Peptidases of the Rumen Bacterium, Prevotella Ruminicola. Anaerobe. 1997;3:35–42. doi: 10.1006/anae.1996.0065. [DOI] [PubMed] [Google Scholar]
- 59.Miyazaki K., Martin J.C., Marinsek-Logar R., Flint H.J. Degradation and Utilization of Xylans by the Rumen AnaerobePrevotella bryantii(formerlyP. Ruminicolasubsp.brevis) B14. Anaerobe. 1997;3:373–381. doi: 10.1006/anae.1997.0125. [DOI] [PubMed] [Google Scholar]
- 60.Wolin M.J. The Rumen Fermentation: A Model for Microbial Interactions in Anaerobic Ecosystems. In: Alexander M., editor. Advances in Microbial Ecology: Volume 3. Springer; Boston, MA, USA: 1979. pp. 49–77. [Google Scholar]
- 61.Hobson P.N., Stewart C.S. The Rumen Microbial Ecosystem. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2012. [Google Scholar]
- 62.Dodd D., Moon Y.-H., Swaminathan K., Mackie R.I., Cann I.K.O. Transcriptomic Analyses of Xylan Degradation by Prevotella Bryantii and Insights into Energy Acquisition by Xylanolytic Bacteroidetes. J. Biol. Chem. 2010;285:30261–30273. doi: 10.1074/jbc.M110.141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kabel M.A., Yeoman C.J., Han Y., Dodd D., Abbas C.A., de Bont J.A.M., Morrison M., Cann I.K.O., Mackie R.I. Biochemical Characterization and Relative Expression Levels of Multiple Carbohydrate Esterases of the Xylanolytic Rumen Bacterium Prevotella Ruminicola 23 Grown on an Ester-Enriched Substrate. Appl. Environ. Microbiol. 2011;77:5671–5681. doi: 10.1128/AEM.05321-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fehlner-Peach H., Magnabosco C., Raghavan V., Scher J.U., Tett A., Cox L.M., Gottsegen C., Watters A., Wiltshire-Gordon J.D., Segata N., et al. Distinct Polysaccharide Utilization Profiles of Human Intestinal Prevotella Copri Isolates. Cell Host Microbe. 2019;26:680–690.e5. doi: 10.1016/j.chom.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grondin J.M., Tamura K., Déjean G., Abbott D.W., Brumer H. Polysaccharide Utilization Loci: Fueling Microbial Communities. J. Bacteriol. 2017;199:e00860-16. doi: 10.1128/JB.00860-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gálvez E.J.C., Iljazovic A., Amend L., Lesker T.R., Renault T., Thiemann S., Hao L., Roy U., Gronow A., Charpentier E., et al. Distinct Polysaccharide Utilization Determines Interspecies Competition between Intestinal Prevotella spp. Cell Host Microbe. 2020;28:838–852.e6. doi: 10.1016/j.chom.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 67.Strobel H.J. Vitamin B12-Dependent Propionate Production by the Ruminal Bacterium Prevotella Ruminicola 23. Appl. Environ. Microbiol. 1992;58:2331–2333. doi: 10.1128/aem.58.7.2331-2333.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu X., Huang S., Huang J., Peng P., Liu Y., Han B., Sun D. Identification of the Potential Role of the Rumen Microbiome in Milk Protein and Fat Synthesis in Dairy Cows Using Metagenomic Sequencing. Animals. 2021;11:1247. doi: 10.3390/ani11051247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi N., Yamada T. Pathways for Amino Acid Metabolism by Prevotella Intermedia and Prevotella Nigrescens. Oral Microbiol. Immunol. 2000;15:96–102. doi: 10.1034/j.1399-302x.2000.150205.x. [DOI] [PubMed] [Google Scholar]
- 70.Griswold K.E., Mackie R.I. Degradation of Protein and Utilization of the Hydrolytic Products by a Predominant Ruminal Bacterium, Prevotella Ruminicola B14. J. Dairy Sci. 1997;80:167–175. doi: 10.3168/jds.S0022-0302(97)75924-1. [DOI] [PubMed] [Google Scholar]
- 71.Holmes C.D., Prather M.J., Søvde O.A., Myhre G. Future Methane, Hydroxyl, and Their Uncertainties: Key Climate and Emission Parameters for Future Predictions. Atmos. Chem. Phys. 2013;13:285–302. doi: 10.5194/acp-13-285-2013. [DOI] [Google Scholar]
- 72.Johnson K.A., Johnson D.E. Methane Emissions from Cattle. J. Anim. Sci. 1995;73:2483–2492. doi: 10.2527/1995.7382483x. [DOI] [PubMed] [Google Scholar]
- 73.Yan T., Mayne C.S., Gordon F.G., Porter M.G., Agnew R.E., Patterson D.C., Ferris C.P., Kilpatrick D.J. Mitigation of Enteric Methane Emissions through Improving Efficiency of Energy Utilization and Productivity in Lactating Dairy Cows. J. Dairy Sci. 2010;93:2630–2638. doi: 10.3168/jds.2009-2929. [DOI] [PubMed] [Google Scholar]
- 74.Ungerfeld E.M. Metabolic Hydrogen Flows in Rumen Fermentation: Principles and Possibilities of Interventions. Front. Microbiol. 2020;11:589. doi: 10.3389/fmicb.2020.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greening C., Geier R., Wang C., Woods L.C., Morales S.E., McDonald M.J., Rushton-Green R., Morgan X.C., Koike S., Leahy S.C., et al. Diverse Hydrogen Production and Consumption Pathways Influence Methane Production in Ruminants. ISME J. 2019;13:2617–2632. doi: 10.1038/s41396-019-0464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lan W., Yang C. Ruminal Methane Production: Associated Microorganisms and the Potential of Applying Hydrogen-Utilizing Bacteria for Mitigation. Sci. Total Environ. 2019;654:1270–1283. doi: 10.1016/j.scitotenv.2018.11.180. [DOI] [PubMed] [Google Scholar]
- 77.Baldwin R.L., Allison M.J. Rumen Metabolism. J. Anim. Sci. 1983;57((Suppl. 2)):461–477. [PubMed] [Google Scholar]
- 78.Sutton J.D. Carbohydrate Digestion and Glucose Supply in the Gut of the Ruminant. Proc. Nutr. Soc. 1971;30:243–248. doi: 10.1079/PNS19710047. [DOI] [PubMed] [Google Scholar]
- 79.Andrieu S., Wilde D. Gut Efficiency; the Key Ingredient in Ruminant Production: Elevating Animal Performance and Health. Wageningen Academic Publishers; Wageningen, The Netherlands: 2008. [Google Scholar]
- 80.Fettke J., Fernie A.R. Intracellular and Cell-to-Apoplast Compartmentation of Carbohydrate Metabolism. Trends Plant Sci. 2015;20:490–497. doi: 10.1016/j.tplants.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 81.Leegood R.C., Sharkey T.D., Von Caemmerer S., von Caemmerer S. Photosynthesis: Physiology and Metabolism. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2000. [Google Scholar]
- 82.Umphrey J.E., Staples C.R. General Anatomy of the Ruminant Digestive System. University of Florida, Cooperative Extension Service, Institute of Foods and Agriculture Sciences (EDIS); Gainesville, FL, USA: 1992. [Google Scholar]
- 83.Russell J.B., Hespell R.B. Microbial Rumen Fermentation. J. Dairy Sci. 1981;64:1153–1169. doi: 10.3168/jds.S0022-0302(81)82694-X. [DOI] [PubMed] [Google Scholar]
- 84.Murphy M.R. Nutrition, Digestion and Absorption: Fermentation in the Rumen. In: McSweeney P.L.H., McNamara J.P., editors. Encyclopedia of Dairy Sciences (Third Edition) Elsevier; Amsterdam, The Netherlands: 2021. pp. 93–97. [Google Scholar]
- 85.McSweeney P.L.H., McNamara J.P., editors. Encyclopedia of Dairy Sciences—Reference Work. Elsevier; San Diego, CA, USA: 2022. [Google Scholar]
- 86.Li J., Gálvez E.J.C., Amend L., Almási É., Iljazovic A., Lesker T.R., Bielecka A.A., Schorr E.-M., Strowig T. A Versatile Genetic Toolbox for Prevotella Copri Enables Studying Polysaccharide Utilization Systems. EMBO J. 2021;40:e108287. doi: 10.15252/embj.2021108287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Puniya A.K., Singh R., Kamra D.N. Rumen Microbiology: From Evolution to Revolution. Springer; Berlin/Heidelberg, Germany: 2015. [Google Scholar]
- 88.Maekawa M., Beauchemin K.A., Christensen D.A. Chewing Activity, Saliva Production, and Ruminal pH of Primiparous and Multiparous Lactating Dairy Cows. J. Dairy Sci. 2002;85:1176–1182. doi: 10.3168/jds.S0022-0302(02)74180-5. [DOI] [PubMed] [Google Scholar]
- 89.Moran J. Tropical Dairy Farming: Feeding Management for Small Holder Dairy Farmers in Humid Tropics. Csiro Publishing; Clayton, Australia: 2005. [Google Scholar]
- 90.Leng R.A. The Rumen—A Fermentation Vat or a Series of Organized Structured Microbial Consortia: Implications for the Mitigation of Enteric Methane Production by Feed Additives. Livestock Res. Rural Dev. 2011;23:258. [Google Scholar]
- 91.Nakamura S., Nakashio S., Yamakawa K., Tanabe N., Nishida S. Carbohydrate Fermentation by Clostridium Difficile. Microbiol. Immunol. 1982;26:107–111. doi: 10.1111/j.1348-0421.1982.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 92.Annison E.F., Lewis D. Metabolism in the Rumen. John Wiley & Sons; Hoboken, NJ, USA: 1959. [Google Scholar]
- 93.Hamar D., Borchers R. Glycolytic Pathway in Rumen Microorganisms. J. Anim. Sci. 1967;26:654–657. doi: 10.2527/jas1967.263654x. [DOI] [PubMed] [Google Scholar]
- 94.Romano A.H., Conway T. Evolution of Carbohydrate Metabolic Pathways. Res. Microbiol. 1996;147:448–455. doi: 10.1016/0923-2508(96)83998-2. [DOI] [PubMed] [Google Scholar]
- 95.Ibrahim R., Varin L., De Luca V., Romeo J. Evolution of Metabolic Pathways. Elsevier; Amsterdam, The Netherlands: 2000. [Google Scholar]
- 96.Ungerfeld E.M., James Newbold C. Engineering Rumen Metabolic Pathways: Where We Are, and Where Are We Heading. Frontiers Media SA; Lausanne, Switzerland: 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thurston B., Dawson K.A., Strobel H.J. Pentose Utilization by the Ruminal Bacterium Ruminococcus Albus. Appl. Environ. Microbiol. 1994;60:1087–1092. doi: 10.1128/aem.60.4.1087-1092.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McAllister T.A., Newbold C.J. Redirecting Rumen Fermentation to Reduce Methanogenesis. Aust. J. Exp. Agric. 2008;48:7–13. doi: 10.1071/EA07218. [DOI] [Google Scholar]
- 99.Baldwin R.L., 6th, Connor E.E. Rumen Function and Development. Vet. Clin. N. Am. Food Anim. Pract. 2017;33:427–439. doi: 10.1016/j.cvfa.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 100.Karekar S., Stefanini R., Ahring B. Homo-Acetogens: Their Metabolism and Competitive Relationship with Hydrogenotrophic Methanogens. Microorganisms. 2022;10:397. doi: 10.3390/microorganisms10020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harrison J.J., Turner R.J., Marques L.L.R., Ceri H. Biofilms: A New Understanding of These Microbial Communities Is Driving a Revolution That May Transform the Science of Microbiology. Am. Sci. 2005;93:508–515. doi: 10.1511/2005.56.508. [DOI] [Google Scholar]
- 102.Tapio I., Snelling T.J., Strozzi F., Wallace R.J. The Ruminal Microbiome Associated with Methane Emissions from Ruminant Livestock. J. Anim. Sci. Biotechnol. 2017;8:7. doi: 10.1186/s40104-017-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martínez-Álvaro M., Auffret M.D., Stewart R.D., Dewhurst R.J., Duthie C.-A., Rooke J.A., Wallace R.J., Shih B., Freeman T.C., Watson M., et al. Identification of Complex Rumen Microbiome Interaction Within Diverse Functional Niches as Mechanisms Affecting the Variation of Methane Emissions in Bovine. Front. Microbiol. 2020;11:659. doi: 10.3389/fmicb.2020.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sejian V., Gaughan J., Baumgard L., Prasad C., editors. Climate Change Impact on Livestock: Adaptation and Mitigation. Springer; New Delhi, India: 2015. [Google Scholar]
- 105.Loor J.J., Elolimy A.A., McCann J.C. Dietary Impacts on Rumen Microbiota in Beef and Dairy Production. Anim. Front. 2016;6:22–29. doi: 10.2527/af.2016-0030. [DOI] [Google Scholar]
- 106.Shabat S.K.B., Sasson G., Doron-Faigenboim A., Durman T., Yaacoby S., Berg Miller M.E., White B.A., Shterzer N., Mizrahi I. Specific Microbiome-Dependent Mechanisms Underlie the Energy Harvest Efficiency of Ruminants. ISME J. 2016;10:2958–2972. doi: 10.1038/ismej.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moe P.W. Energy Metabolism of Dairy Cattle. J. Dairy Sci. 1981;64:1120–1139. doi: 10.3168/jds.S0022-0302(81)82692-6. [DOI] [PubMed] [Google Scholar]
- 108.McBride B.W., Kelly J.M. Energy Cost of Absorption and Metabolism in the Ruminant Gastrointestinal Tract and Liver: A Review. J. Anim. Sci. 1990;68:2997–3010. doi: 10.2527/1990.6892997x. [DOI] [PubMed] [Google Scholar]
- 109.Oltjen J.W., Kebreab E., Lapierre H. Energy and Protein Metabolism and Nutrition in Sustainable Animal Production. Springer; Berlin/Heidelberg, Germany: 2013. [Google Scholar]
- 110.Pryce J.E., Arias J., Bowman P.J., Davis S.R., Macdonald K.A., Waghorn G.C., Wales W.J., Williams Y.J., Spelman R.J., Hayes B.J. Accuracy of Genomic Predictions of Residual Feed Intake and 250-Day Body Weight in Growing Heifers Using 625,000 Single Nucleotide Polymorphism Markers. J. Dairy Sci. 2012;95:2108–2119. doi: 10.3168/jds.2011-4628. [DOI] [PubMed] [Google Scholar]
- 111.Moore S.S., Mujibi F.D., Sherman E.L. Molecular Basis for Residual Feed Intake in Beef Cattle. J. Anim. Sci. 2009;87:E41–E47. doi: 10.2527/jas.2008-1418. [DOI] [PubMed] [Google Scholar]
- 112.Rubino F., Carberry C., Waters S.M., Kenny D., McCabe M.S., Creevey C.J. Divergent Functional Isoforms Drive Niche Specialisation for Nutrient Acquisition and Use in Rumen Microbiome. ISME J. 2017;11:932–944. doi: 10.1038/ismej.2016.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sasson G., Kruger Ben-Shabat S., Seroussi E., Doron-Faigenboim A., Shterzer N., Yaacoby S., Berg Miller M.E., White B.A., Halperin E., Mizrahi I. Heritable Bovine Rumen Bacteria Are Phylogenetically Related and Correlated with the Cow’s Capacity to Harvest Energy from Its Feed. MBio. 2017;8:e00703-17. doi: 10.1128/mBio.00703-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sasson G., Moraïs S., Kokou F., Plate K., Trautwein-Schult A., Jami E., Bayer E.A., Becher D., Mizrahi I. Metaproteome Plasticity Sheds Light on the Ecology of the Rumen Microbiome and Its Connection to Host Traits. ISME J. 2022;16:2610–2621. doi: 10.1038/s41396-022-01295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roehe R., Dewhurst R.J., Duthie C.-A., Rooke J.A., McKain N., Ross D.W., Hyslop J.J., Waterhouse A., Freeman T.C., Watson M., et al. Bovine Host Genetic Variation Influences Rumen Microbial Methane Production with Best Selection Criterion for Low Methane Emitting and Efficiently Feed Converting Hosts Based on Metagenomic Gene Abundance. PLoS Genet. 2016;12:e1005846. doi: 10.1371/journal.pgen.1005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Foley M.H., Cockburn D.W., Koropatkin N.M. The Sus Operon: A Model System for Starch Uptake by the Human Gut Bacteroidetes. Cell. Mol. Life Sci. 2016;73:2603–2617. doi: 10.1007/s00018-016-2242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Terrapon N., Lombard V., Drula É., Lapébie P., Al-Masaudi S., Gilbert H.J., Henrissat B. PULDB: The Expanded Database of Polysaccharide Utilization Loci. Nucleic Acids Res. 2018;46:D677–D683. doi: 10.1093/nar/gkx1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ausland C., Zheng J., Yi H., Yang B., Li T., Feng X., Zheng B., Yin Y. dbCAN-PUL: A Database of Experimentally Characterized CAZyme Gene Clusters and Their Substrates. Nucleic Acids Res. 2021;49:D523–D528. doi: 10.1093/nar/gkaa742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Terrapon N., Lombard V., Gilbert H.J., Henrissat B. Automatic Prediction of Polysaccharide Utilization Loci in Bacteroidetes Species. Bioinformatics. 2015;31:647–655. doi: 10.1093/bioinformatics/btu716. [DOI] [PubMed] [Google Scholar]
- 120.Stewart R.D., Auffret M.D., Roehe R., Watson M. Open Prediction of Polysaccharide Utilisation Loci (PUL) in 5414 Public Bacteroidetes Genomes Using PULpy. bioRxiv. 2018:421024. doi: 10.1101/421024. [DOI] [Google Scholar]
- 121.Drula E., Garron M.-L., Dogan S., Lombard V., Henrissat B., Terrapon N. The Carbohydrate-Active Enzyme Database: Functions and Literature. Nucleic Acids Res. 2022;50:D571–D577. doi: 10.1093/nar/gkab1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Accetto T., Avguštin G. Polysaccharide Utilization Locus and CAZYme Genome Repertoires Reveal Diverse Ecological Adaptation of Prevotella Species. Syst. Appl. Microbiol. 2015;38:453–461. doi: 10.1016/j.syapm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 123.McGregor N., Morar M., Fenger T.H., Stogios P., Lenfant N., Yin V., Xu X., Evdokimova E., Cui H., Henrissat B., et al. Structure-Function Analysis of a Mixed-Linkage β-Glucanase/Xyloglucanase from the Key Ruminal Bacteroidetes Prevotella Bryantii B(1)4. J. Biol. Chem. 2016;291:1175–1197. doi: 10.1074/jbc.M115.691659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosewarne C.P., Pope P.B., Cheung J.L., Morrison M. Analysis of the Bovine Rumen Microbiome Reveals a Diversity of Sus-like Polysaccharide Utilization Loci from the Bacterial Phylum Bacteroidetes. J. Ind. Microbiol. Biotechnol. 2014;41:601–606. doi: 10.1007/s10295-013-1395-y. [DOI] [PubMed] [Google Scholar]
- 125.Naas A.E., Mackenzie A.K., Mravec J., Schückel J., Willats W.G.T., Eijsink V.G.H., Pope P.B. Do Rumen Bacteroidetes Utilize an Alternative Mechanism for Cellulose Degradation? MBio. 2014;5:e01401–e01414. doi: 10.1128/mBio.01401-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Glendinning L., Genç B., Wallace R.J., Watson M. Metagenomic Analysis of the Cow, Sheep, Reindeer and Red Deer Rumen. Sci. Rep. 2021;11:1990. doi: 10.1038/s41598-021-81668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ferrer M., Golyshina O.V., Chernikova T.N., Khachane A.N., Reyes-Duarte D., Santos V., Martins Dos A.P., Strompl C., Elborough K., Jarvis G., et al. Novel Hydrolase Diversity Retrieved from a Metagenome Library of Bovine Rumen Microflora. Environ. Microbiol. 2005;7:1996–2010. doi: 10.1111/j.1462-2920.2005.00920.x. [DOI] [PubMed] [Google Scholar]
- 128.Mackenzie A.K., Naas A.E., Kracun S.K., Schückel J., Fangel J.U., Agger J.W., Willats W.G.T., Eijsink V.G.H., Pope P.B. A Polysaccharide Utilization Locus from an Uncultured Bacteroidetes Phylotype Suggests Ecological Adaptation and Substrate Versatility. Appl. Environ. Microbiol. 2015;81:187–195. doi: 10.1128/AEM.02858-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pope P.B., Mackenzie A.K., Gregor I., Smith W., Sundset M.A., McHardy A.C., Morrison M., Eijsink V.G.H. Metagenomics of the Svalbard Reindeer Rumen Microbiome Reveals Abundance of Polysaccharide Utilization Loci. PLoS ONE. 2012;7:e38571. doi: 10.1371/journal.pone.0038571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Flint H.J., Bayer E.A. Plant Cell Wall Breakdown by Anaerobic Microorganisms from the Mammalian Digestive Tract. Ann. N. Y. Acad. Sci. 2008;1125:280–288. doi: 10.1196/annals.1419.022. [DOI] [PubMed] [Google Scholar]
- 131.Conte G., Dimauro C., Daghio M., Serra A., Mannelli F., McAmmond B.M., Van Hamme J.D., Buccioni A., Viti C., Mantino A., et al. Exploring the Relationship between Bacterial Genera and Lipid Metabolism in Bovine Rumen. Animal. 2022;16:100520. doi: 10.1016/j.animal.2022.100520. [DOI] [PubMed] [Google Scholar]
- 132.Hu R., Zou H., Wang H., Wang Z., Wang X., Ma J., Shah A.M., Peng Q., Xue B., Wang L., et al. Dietary Energy Levels Affect Rumen Bacterial Populations That Influence the Intramuscular Fat Fatty Acids of Fattening Yaks (Bos Grunniens) Animals. 2020;10:1474. doi: 10.3390/ani10091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Atasoglu C., Valdés C., Walker N.D., Newbold C.J., Wallace R.J. De Novo Synthesis of Amino Acids by the Ruminal Bacteria Prevotella Bryantii B14, Selenomonas Ruminantium HD4, and Streptococcus Bovis ES1. Appl. Environ. Microbiol. 1998;64:2836–2843. doi: 10.1128/AEM.64.8.2836-2843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Franco-Lopez J., Duplessis M., Bui A., Reymond C., Poisson W., Blais L., Chong J., Gervais R., Rico D.E., Cue R.I., et al. Correlations between the Composition of the Bovine Microbiota and Vitamin B12 Abundance. mSystems. 2020;5:e00107-20. doi: 10.1128/mSystems.00107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hertzberg M., Siddons A., Schreuder H. Role of Greenhouse Gases in Climate Change. Energy Environ. 2017;28:530–539. doi: 10.1177/0958305X17706177. [DOI] [Google Scholar]
- 136.Hoegh-Guldberg O., Poloczanska E.S., Skirving W., Dove S. Coral Reef Ecosystems under Climate Change and Ocean Acidification. Front. Mar. Sci. 2017;4:158. doi: 10.3389/fmars.2017.00158. [DOI] [Google Scholar]
- 137.Cornwall C.E., Comeau S., Kornder N.A., Perry C.T., van Hooidonk R., DeCarlo T.M., Pratchett M.S., Anderson K.D., Browne N., Carpenter R., et al. Global Declines in Coral Reef Calcium Carbonate Production under Ocean Acidification and Warming. Proc. Natl. Acad. Sci. USA. 2021;118:e2015265118. doi: 10.1073/pnas.2015265118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Buccioni A., Cappucci A., Mele M. Methane Emission from Enteric Fermentation: Methanogenesis and Fermentation. In: Sejian V., Gaughan J., Baumgard L., Prasad C., editors. Climate Change Impact on Livestock: Adaptation and Mitigation. Springer; New Delhi, India: 2015. pp. 171–186. [Google Scholar]
- 139.Bhatta R., Enishi O. Measurement of Methane Production from Ruminants. Asian-Australas. J. Anim. Sci. 2007;20:1305–1318. doi: 10.5713/ajas.2007.1305. [DOI] [Google Scholar]
- 140.Moss A.R., Jouany J.-P., Newbold J. Methane Production by Ruminants: Its Contribution to Global Warming. Ann. Zootech. 2000;49:231–253. doi: 10.1051/animres:2000119. [DOI] [Google Scholar]
- 141.Seid A. Review on Methane Emission from Dairy Farms and Its Impact on Global Warming. Austin J. Vet. Sci. Anim. Husb. 2019;6:1052–1056. doi: 10.26420/austinjvetscianimhusb.1052.2019. [DOI] [Google Scholar]
- 142.Lyu Z., Shao N., Akinyemi T., Whitman W.B. Methanogenesis. Curr. Biol. 2018;28:R727–R732. doi: 10.1016/j.cub.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 143.Appuhamy J.A.D.R.N., France J., Kebreab E. Models for Predicting Enteric Methane Emissions from Dairy Cows in North America, Europe, and Australia and New Zealand. Glob. Chang. Biol. 2016;22:3039–3056. doi: 10.1111/gcb.13339. [DOI] [PubMed] [Google Scholar]
- 144.Neill A.R., Grime D.W., Dawson R.M. Conversion of Choline Methyl Groups through Trimethylamine into Methane in the Rumen. Biochem. J. 1978;170:529–535. doi: 10.1042/bj1700529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Poulsen M., Schwab C., Jensen B.B., Engberg R.M., Spang A., Canibe N., Højberg O., Milinovich G., Fragner L., Schleper C., et al. Methylotrophic Methanogenic Thermoplasmata Implicated in Reduced Methane Emissions from Bovine Rumen. Nat. Commun. 2013;4:1428. doi: 10.1038/ncomms2432. [DOI] [PubMed] [Google Scholar]
- 146.Anand A., Sharma A. Survival Strategies in Cold-Adapted Microorganisms. Springer; Singapore: 2022. Use of Proteomics and Transcriptomics to Identify Proteins for Cold Adaptation in Microbes; pp. 285–319. [Google Scholar]
- 147.Liu Y., Whitman W.B. Metabolic, Phylogenetic, and Ecological Diversity of the Methanogenic Archaea. Ann. N. Y. Acad. Sci. 2008;1125:171–189. doi: 10.1196/annals.1419.019. [DOI] [PubMed] [Google Scholar]
- 148.Denman S.E., Tomkins N.W., McSweeney C.S. Quantitation and Diversity Analysis of Ruminal Methanogenic Populations in Response to the Antimethanogenic Compound Bromochloromethane. FEMS Microbiol. Ecol. 2007;62:313–322. doi: 10.1111/j.1574-6941.2007.00394.x. [DOI] [PubMed] [Google Scholar]
- 149.Goel G., Makkar H.P.S., Becker K. Inhibition of Methanogens by Bromochloromethane: Effects on Microbial Communities and Rumen Fermentation Using Batch and Continuous Fermentations. Br. J. Nutr. 2009;101:1484–1492. doi: 10.1017/S0007114508076198. [DOI] [PubMed] [Google Scholar]
- 150.Denman S.E., Martinez Fernandez G., Shinkai T., Mitsumori M., McSweeney C.S. Metagenomic Analysis of the Rumen Microbial Community Following Inhibition of Methane Formation by a Halogenated Methane Analog. Front. Microbiol. 2015;6:1087. doi: 10.3389/fmicb.2015.01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mitsumori M., Shinkai T., Takenaka A., Enishi O., Higuchi K., Kobayashi Y., Nonaka I., Asanuma N., Denman S.E., McSweeney C.S. Responses in Digestion, Rumen Fermentation and Microbial Populations to Inhibition of Methane Formation by a Halogenated Methane Analogue. Br. J. Nutr. 2012;108:482–491. doi: 10.1017/S0007114511005794. [DOI] [PubMed] [Google Scholar]
- 152.Purushe J., Fouts D.E., Morrison M., White B.A., Mackie R.I., Coutinho P.M., Henrissat B., Nelson K.E. Comparative Genome Analysis of Prevotella Ruminicola and Prevotella Bryantii: Insights into Their Environmental Niche. Microb. Ecol. 2010;60:721–729. doi: 10.1007/s00248-010-9692-8. [DOI] [PubMed] [Google Scholar]
- 153.Takahashi-Iñiguez T., García-Hernandez E., Arreguín-Espinosa R., Flores M.E. Role of Vitamin B12 on Methylmalonyl-CoA Mutase Activity. J. Zhejiang Univ. Sci. B. 2012;13:423–437. doi: 10.1631/jzus.B1100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Daugherty M.S., Galyean M.L., Hallford D.M., Hageman J.H. Vitamin B12 and monensin effects on performance, liver and serum vitamin B12 concentrations and activity of propionate metabolizing hepatic enzymes in feedlot lambs. J. Anim. Feed Sci. 1986;62:452–463. doi: 10.2527/jas1986.622452x. [DOI] [PubMed] [Google Scholar]
- 155.Islam M., Kim S.-H., Ramos S.C., Mamuad L.L., Son A.-R., Yu Z., Lee S.-S., Cho Y.-I., Lee S.-S. Holstein and Jersey Steers Differ in Rumen Microbiota and Enteric Methane Emissions Even Fed the Same Total Mixed Ration. Front. Microbiol. 2021;12:601061. doi: 10.3389/fmicb.2021.601061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tamori K., Matsunaga B., Boonsaen P., Khongpradit A., Sawanon S., Nagashima K., Koike S., Kobayashi Y. Feeding Cashew Nut Shell Liquid Decreases Methane Production from Feces by Altering Fecal Bacterial and Archaeal Communities in Thai Local Ruminants. Anim. Sci. J. 2021;92:e13569. doi: 10.1111/asj.13569. [DOI] [PubMed] [Google Scholar]
- 157.Su C., Shinkai T., Miyazawa N., Mitsumori M., Enishi O., Nagashima K., Koike S., Kobayashi Y. Microbial Community Structure of the Bovine Rumen as Affected by Feeding Cashew Nut Shell Liquid, a Methane-inhibiting and Propionate-enhancing Agent. Anim. Sci. J. 2021;92:e13503. doi: 10.1111/asj.13503. [DOI] [PubMed] [Google Scholar]
- 158.Watanabe Y., Suzuki R., Koike S., Nagashima K., Mochizuki M., Forster R.J., Kobayashi Y. In Vitro Evaluation of Cashew Nut Shell Liquid as a Methane-Inhibiting and Propionate-Enhancing Agent for Ruminants. J. Dairy Sci. 2010;93:5258–5267. doi: 10.3168/jds.2009-2754. [DOI] [PubMed] [Google Scholar]
- 159.Narabe C., Kamiyama S., Saito M., Boonsaen P., Khongpradit A., Sawanon S., Suzuki Y., Koike S., Kobayashi Y. Cashew Nut Shell Liquid Potentially Mitigates Methane Emission from the Feces of Thai Native Ruminant Livestock by Modifying Fecal Microbiota. Anim. Sci. J. 2021;92:e13614. doi: 10.1111/asj.13614. [DOI] [PubMed] [Google Scholar]
- 160.Shinkai T., Enishi O., Mitsumori M., Higuchi K., Kobayashi Y., Takenaka A., Nagashima K., Mochizuki M., Kobayashi Y. Mitigation of Methane Production from Cattle by Feeding Cashew Nut Shell Liquid. J. Dairy Sci. 2012;95:5308–5316. doi: 10.3168/jds.2012-5554. [DOI] [PubMed] [Google Scholar]
- 161.Veneman J.B., Muetzel S., Hart K.J., Faulkner C.L., Moorby J.M., Perdok H.B., Newbold C.J. Does Dietary Mitigation of Enteric Methane Production Affect Rumen Function and Animal Productivity in Dairy Cows? PLoS ONE. 2015;10:e0140282. doi: 10.1371/journal.pone.0140282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dixit S., Kumar S., Sharma R., Banakar P.S., Deb R., Tyagi A.K. Rumen Microbial Diversity, Enteric Methane Emission and Nutrient Utilization of Crossbred Karan-Fries Cattle (Bos Taurus) and Murrah Buffalo (Bubalus Bubalis) Consuming Varied Roughage Concentrate Ratio. Anim. Biotechnol. 2022:1–19. doi: 10.1080/10495398.2022.2053696. [DOI] [PubMed] [Google Scholar]
- 163.Ross E.M., Moate P.J., Marett L., Cocks B.G., Hayes B.J. Investigating the Effect of Two Methane-Mitigating Diets on the Rumen Microbiome Using Massively Parallel Sequencing. J. Dairy Sci. 2013;96:6030–6046. doi: 10.3168/jds.2013-6766. [DOI] [PubMed] [Google Scholar]
- 164.Weimer P.J., Cox M.S., de Paula T.V., Lin M., Hall M.B., Suen G. Transient Changes in Milk Production Efficiency and Bacterial Community Composition Resulting from near-Total Exchange of Ruminal Contents between High- and Low-Efficiency Holstein Cows. J. Dairy Sci. 2017;100:7165–7182. doi: 10.3168/jds.2017-12746. [DOI] [PubMed] [Google Scholar]
- 165.Weimer P.J., Stevenson D.M., Mantovani H.C., Man S.L.C. Host Specificity of the Ruminal Bacterial Community in the Dairy Cow Following near-Total Exchange of Ruminal Contents. J. Dairy Sci. 2010;93:5902–5912. doi: 10.3168/jds.2010-3500. [DOI] [PubMed] [Google Scholar]
- 166.Lassen J., Løvendahl P. Heritability Estimates for Enteric Methane Emissions from Holstein Cattle Measured Using Noninvasive Methods. J. Dairy Sci. 2016;99:1959–1967. doi: 10.3168/jds.2015-10012. [DOI] [PubMed] [Google Scholar]
- 167.Donoghue K.A., Bird-Gardiner T., Arthur P.F., Herd R.M., Hegarty R.F. Genetic and Phenotypic Variance and Covariance Components for Methane Emission and Postweaning Traits in Angus Cattle. J. Anim. Sci. 2016;94:1438–1445. doi: 10.2527/jas.2015-0065. [DOI] [PubMed] [Google Scholar]
- 168.Zhu Z., Difford G.F., Noel S.J., Lassen J., Løvendahl P., Højberg O. Stability Assessment of the Rumen Bacterial and Archaeal Communities in Dairy Cows Within a Single Lactation and Its Association With Host Phenotype. Front. Microbiol. 2021;12:636223. doi: 10.3389/fmicb.2021.636223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang Q., Difford G., Sahana G., Løvendahl P., Lassen J., Lund M.S., Guldbrandtsen B., Janss L. Bayesian Modeling Reveals Host Genetics Associated with Rumen Microbiota Jointly Influence Methane Emission in Dairy Cows. ISME J. 2020;14:2019–2033. doi: 10.1038/s41396-020-0663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.