Abstract
Sepsis is a major cause of morbidity and mortality worldwide. Sepsis-associated coagulation disorders are involved in the pathogenesis of multiorgan failure and lead to a subsequently worsening prognosis. Alongside the global impact of the COVID-19 pandemic, a great number of research papers have focused on SARS-CoV-2 pathogenesis and treatment. Significant progress has been made in this regard and coagulation disturbances were once again found to underlie some of the most serious adverse outcomes of SARS-CoV-2 infection, such as acute lung injury and multiorgan dysfunction. In the attempt of untangling the mechanisms behind COVID-19-associated coagulopathy (CAC), a series of similarities with sepsis-induced coagulopathy (SIC) became apparent. Whether they are, in fact, the same disease has not been established yet. The clinical picture of CAC shows the unique feature of an initial phase of intravascular coagulation confined to the respiratory system. Only later on, patients can develop a clinically significant form of systemic coagulopathy, possibly with a consumptive pattern, but, unlike SIC, it is not a key feature. Deepening our understanding of CAC pathogenesis has to remain a major goal for the research community, in order to design and validate accurate definitions and classification criteria.
Keywords: sepsis, COVID-19, SARS-CoV-2 infection, coagulopathy, immunothrombosis, disseminated intravascular coagulopathy, viscoelastic tests
1. Introduction
A global estimate on sepsis for the year 2017 found an incidence of 48.9 million cases and nearly 11 million sepsis-related deaths [1]. Along with the epidemiological burden, there are gaps in the early diagnosis strategies, prognostic markers and individualized therapy of sepsis that continue to be problematic [2]. Therefore, sepsis has remained a topic of great interest of both researchers and clinicians for the last decades. Since its emergence in late 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been diagnosed in over 600 million patients and has caused over 6 million deaths worldwide, according to the World Health Organization [3]. The numerous questions regarding SARS-CoV-2 pathogenesis and therapeutics have been addressed with great participation and responsibility from the scientific community, so that at this point one can attempt to grasp the peculiarities of this systemic viral disease [4]. Dysregulated immune responses, multiorgan failure, vascular involvement and coagulopathy are some of the mutual gross features of coronavirus disease 2019 (COVID-19) and sepsis, but the molecular pathways behind the clinical pictures are not so clearly overlapping [5]. Some authors would rather consider COVID-19 a viral form of sepsis, while others highlight the various distinguishing patterns between the two [5,6]. Since coagulation abnormalities represent an often-fatal complication of both diseases and a number of treatment options are being assessed in this regard, this paper aims to review the current data on the pathogenic sequences responsible for the pathogen invasion (i.e., bacterial pathogen, or SARS-CoV-2) evolving towards an apparent form of coagulopathy. In the first part, the intricate interactions between pathogens, the immune system and hemostasis are outlined. In the last part, these processes are embedded within larger-scale patterns, i.e., the clinically relevant forms of coagulopathy.
2. Materials and Methods
Our narrative review was based on the medical literature indexed in PubMed, including in the search process English language articles, from 2000 till 2022. The following keywords were used in the screening process: “sepsis”, “COVID-19”, “SARS-CoV-2”, “coagulation”, “coagulopathy”, “immunothrombosis” and “thromboinflammation”.
After excluding case reports and studies with participants under the age of 18 years old, we selected articles presenting data on coagulation dysregulations in sepsis and/or COVID-19, the pathogenesis of coagulopathy in sepsis and COVID-19, the effector cells of coagulopathy in sepsis and COVID-19, the clinical aspects of coagulopathy in sepsis and COVID-19 and the pathogenesis of disseminated intravascular coagulation (DIC). These aspects were initially searched for in the titles, then in the abstracts, and, when necessary (title and/or abstract were not explicit enough), a full-text evaluation was performed. In cases of further doubts, the supervisor (D.A.I.) was queried.
After the selection strategy mentioned above was performed, thenumber of papers included in our review was 296.
3. Molecular Mechanisms of Inflammation–Coagulation Crosstalkin Sepsis and COVID-19
3.1. The Triggers—PAMPs/DAMPs/PRRs Interplay
Highly conserved molecular patterns pertaining to pathogens, known as pathogen-associated molecular patterns (PAMPs), and endogenous substances released by cellular injury, called damage-associated molecular patterns (DAMPs), are sought for and recognized by specialized receptors, pattern recognition receptors (PRRs). The interactions between PAMPs, DAMPs and PRRs fire intracellular signaling cascades and/or generate the assembly of inflammasome, which enhances the immune response by producing various cytokines and/or pyroptosis [7,8]. Coagulation abnormalities follow this sequence and contribute to the process called immunothrombosis [9].
3.1.1. Pattern Recognition Receptors
Toll-like receptors (TLRs) and C-type lectin receptors (CLRs) are found on cell membranes and endosomes; conversely, nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs) and absent in melanoma-2 (AIM2)-like receptors (ALRs) bind intracellular ligands [7]. In infectious diseases, the common endpoint is the activation of shared signaling pathways: nuclear factor kappa B (NF-κB)signaling, inflammasome signaling, mitogen-activatedprotein kinase (MAPK) signaling and TANK-binding kinase 1—interferon regulatory factor 3 (TBK1–IRF-3)signaling [10,11].
The NF-κB signaling pathway has been commonly associated with the synthesis of various cytokines and chemokines, but, recently, its role in modulating coagulation has become more apparent. The coagulation factor III or tissue factor (TF), factor VIII (FVIII), urokinase-type plasminogen activator (u-PA), plasminogen activator inhibitor-1 (PAI-1), tissue factor pathway inhibitor (TFPI), antithrombin and thrombomodulin have been identified as endpoints in the NF-κB pathway (Figure 1A) [12,13,14,15,16].
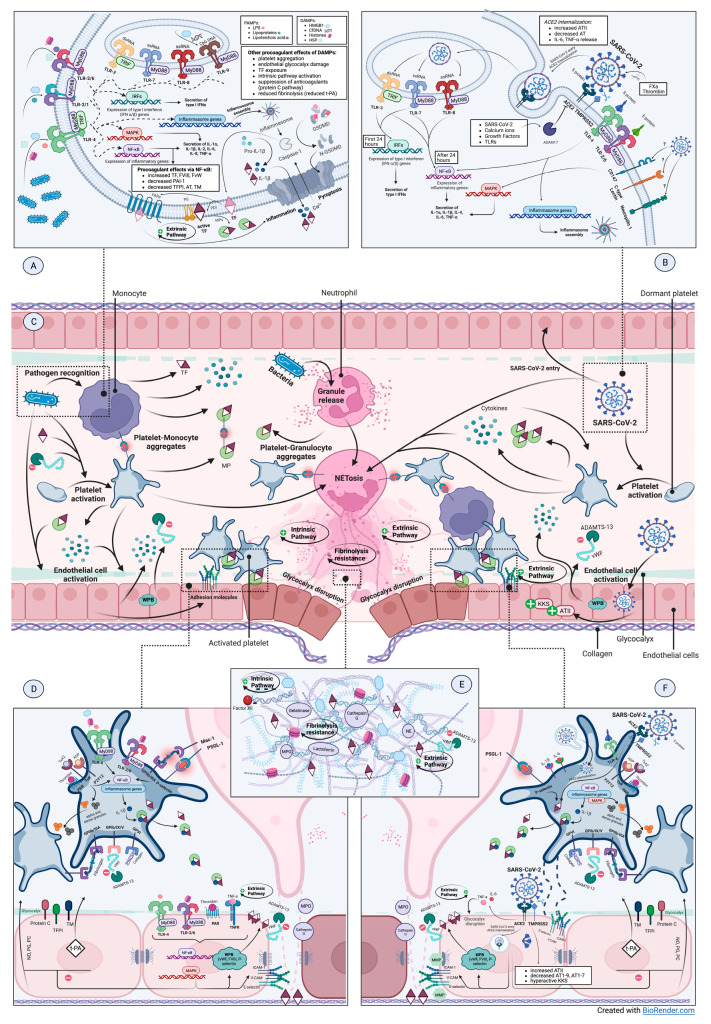
Figure 1.
A comparative overview of pathogenic mechanisms involved in sepsis-induced coagulopathy and COVID-19-associated coagulopathy. (A). Pathogen recognition pathways in bacterial sepsis. (B). Molecular mechanisms of SARS-CoV-2 entry and subsequent cell injury. (C). Intravascular interplay of pathogens (i.e., bacteria and SARS-CoV-2), effector cells and humoral factors in the context of sepsis-induced coagulopathy versus COVID-19-associated coagulopathy. (D). Platelet and endothelium dysfunctions in bacterial sepsis. (E). NETs components and NET-associated prothrombotic status. (F). Platelet and endothelium dysfunctions in COVID-19. Abbreviations: ACE2, angiotensin-converting enzyme 2; ADAM17, a disintegrin and metalloprotease 17; ADAMTS13, a disintegrin and metalloprotease with a thrombospondin type 1 motif, member 13; ADP, adenosine diphosphate; AT, antithrombin; ATII/1-9/1-7, angiotensin II/1-9/1-7; CD147, cluster of differentiation 147; Cf/CpG DNA, cell-free/cytosine-guanine pair desoxyribonucleic acid; CLR, C-type lectin receptor; DAMP, damage-associated molecular pattern; FVIII/X/XII, coagulation factor VIII/X/XII; GP-Iba-IX-V, glycoprotein Iba-IX-V; GPIIb/IIIa, glycoprotein IIb/IIIa; GPVI, glycoprotein VI; GSDMD, Gasdermin D; HMGB1, high mobility group box 1; Hsp, heat-shock proteins; ICAM-1, intercellular adhesion molecule 1, IFN, interferon; IL, interleukin; IRF, interferon-regulatory factor; KKS, kallikrein-kinin system; LPS, lipopolysaccharides; Mac-1, macrophage 1 antigen; MAPK, mitogen-activated protein kinase; MMP, matrix metalloprotease; MP, microparticle; MPO, myeloperoxidase; MyD88, myeloid differentiation primary response 88; NE, neutrophil elastase; NET, neutrophil extracellular trap; NF-κB, nuclear factor kappa B; NO, nitric oxide; PAI-1, plasminogen activator inhibitor-1; PAMP, pathogen-associated molecular pattern; PAR, protease-activated receptor; PC, prostacyclin; PDI, protein disulfide isomerase; PG, prostaglandin; PS, phosphatidyl-serine; PSGL-1, P-selectin glycoprotein ligand-1; ss/dsRNA, single-stranded/double-stranded ribonucleic acid; TF, tissue factor; TFPI, tissue factor pathway inhibitor; TLR, Toll-like receptor; TM, thrombomodulin; TMPRSS2, transmembrane protease serine 2; TNF-α, tumor necrosis factor α TNFR, TNF receptor; t-PA, tissue-type plasminogen activator; TRIF, toll-interleukin 1 receptor-domain-containing adapter-inducing interferon-beta; TxA2, thromboxane A2; TxR, thromboxane receptor; VCAM-1, vascular cell adhesion molecule 1; vWF, von Willebrand Factor; WPB, Weibel Palade Bodies.
Following inflammasome activation, active caspases are generated, promoting the synthesis of proinflammatory cytokines and the cleavage of Gasdermin D (GSDMD), with subsequent pyroptosis (Figure 1A) [8,9]. Caspase 1 and GSDMD-mediated TF activation and release has been reported in murine macrophages following bacterial stimuli and in human monocytic cell lines [17]. Previous studies found that the suppression of inflammasome-mediated pyroptosis in macrophages can diminish TF-induced coagulation disorders [17]. This led to a number of trials investigating the therapeutical value of inflammasome pathway modulators in SARS-CoV-2 infection [18].
3.1.2. Pathogen-Associated Danger Signals
The most-studied PAMPs are constituents of the bacterial wall or membraneand bacterial nucleic acids. Lipopolysaccharides (LPS) are abundant in the outer leaflet of the outer membrane of gram-negative bacteria; their ability to replicate a septic shock pathogenic scenario by binding TLR4 in vitro made them a reliable research tool in the field of sepsis pathophysiology [11]. The SARS-CoV-2 S protein was found to be a TLR4 ligand and NF-κB pathway activator in monocytes and macrophages [19,20,21,22]. SARS-CoV-2 ribonucleic acid (RNA) can activate TLR3 and TLR7 signaling pathways, with interferon α (IFN-α) and IFN-β synthesis in the first 24 h; afterwards, signaling is directed towards proinflammatory cytokine secretion: interleukin 1α (IL-1α), IL-1β, IL-4, IL-6, through the NF-κB pathway (Figure 1B) [23]. Similar to other viral infections, the vigorous cytokine response can often have a systemic impact [24]. The authors suggested that early responses are meant to protect the host, while the subsequent amplification of inflammatory reactions can maladaptively lead to cytokine storm.
3.1.3. Endogenous Danger Signals
The most citedDAMPsare high mobility group box 1 (HMGB1), cell-free desoxyribonucleic acid (cfDNA), histones, heat-shock proteins (Hsp), mitochondria-derived DAMPs and metabolism-associated molecular patterns (MAMPs) [25,26,27].
HMGB1 can bind several PRRs, such as TLR2, TLR4 and TLR9, activating macrophages and endothelial cells (ECs) with the synthesis of proinflammatory cytokines and barrier disruption [28,29,30,31]. HMGB1 modulates fibrinolysis by interacting with plasminogen and tissue-type plasminogen activator (t-PA) and promotes coagulation via TF exposure and anticoagulant protein C pathway inhibition [29,32,33,34]. The role of HMGB1 in sepsis pathogenesis has already been outlined by several studies stating that levels of HMGB1 are elevated in non-survivors, in patients with more severe organ failure and DIC; targeting HMGB1 may improve outcomes in sepsis experimental models [35,36,37,38]. In SARS-CoV-2 infection, HMGB1 is thought to promote acute respiratory distress syndrome (ARDS) development and correlates with disease severity and mortality [39].
Cell-free DNA (CfDNA) activates coagulation via factors XI (FXI) and XII (FXII); accordingly, electron microscopy studies noticed the binding of cfDNA to FXII and high molecular weight kininogen [40]. In this context, the procoagulant activity could be explained by the negative charges and the double-stranded pattern within the DNA structure [41]. The fibrinolytic system is influenced by cfDNA in a concentration-dependent manner: low-physiological levels of cfDNA activate t-PA, enhancing fibrinolysis, while increased cfDNA concentrations determine t-PA inactivation by PAI-1; these processes promote organ damage via microvascular dysfunction in DIC [42,43,44]. Increased PAI-1 levels are thought to be specific of sepsis-associated coagulopathy since they serve as excellent severity markers [45]. Some authors also found increased PAI-1 levels associated withthe cytokine storm of COVID-19, especially IL-6 levels [46]. However, authors comparing the two diseases found significantly higher PAI-1 levels in COVID-19 patients, which could explain the lower D-dimer levels in the COVID-19 group versus the sepsis group in their study [47]. This may limit the plasmin degradation of crosslinked fibrin and the release of D-dimers, explaining the lower levels of D-dimers compared with sepsis found in this study.
Histones exhibit important cytotoxic, proinflammatory and prothrombotic features and have emerged as an important prognostic tool in severe forms of COVID-19 as well as in sepsis; the therapeutic implications for septic patients are currently being investigated [48,49]. Histones promote platelet aggregation and bind prothrombotic molecules such as fibrinogen and adhesion proteins [50,51]. Other interactions concern anticoagulant protein C and tissue factor pathway inhibitor (TFPI) pathways suppression and antithrombin dysfunction through glycocalyx disruption [52,53,54].
3.1.4. How Does SARS-CoV-2 Infect Cells?
SARS-CoV-2 entry in susceptible cells is facilitated by the interaction between spike protein (S) and specific cellular receptors, among which angiotensin-converting enzyme 2 (ACE2) is the most important (Figure 1B). Some other host (co-)receptors, such as CLRs, cluster of differentiation 147 (CD147), phosphatidyl serine (PS) receptors and neuropilin 1 have been cited to contribute to SARS-CoV-2 infection, but none has yet emerged as another essential entry pathway [55,56,57]. However, CD147 was found to initiate proinflammatory signaling via the MAPK pathway, contributing to cytokine storms [58,59]. Host cell proteases—transmembrane protease serine 2 (TMPRSS2) at the cellular surface and cathepsin L within endosomes—are responsible forviral internalization via S protein cleavage [60]. It was shown that activated coagulation FX and thrombin may also cleave S protein and promote viral entry in a potential inflammation–coagulation positive feedback loop (Figure 1B) [61].
Since coagulation disorders have become one of the major systemic effects of SARS-CoV-2 infection, a great amount of research has been focused on viral interactions with the main cellular players of thrombosis—the blood cells. SARS-CoV-2 can infect red blood cells (RBCs) by attaching to Band-3 protein on the erythrocyte membrane; the interaction of S1 spike protein with Band-3 protein leads to alterations in carbon dioxide uptake and oxygen release from hemoglobin, inducing tissue hypoxic changes [62]. Russo et al. found high levels of oxidized glutathione and significant decreases in the enzymes responsible for thereduction inoxidative stress in the RBCs of patients with COVID-19; this led to an increased susceptibility to reactive oxygen species (ROS), resulting in cellular lysis and the inability to carry oxygen [63]. After entering RBCs, SARS-CoV-2 interacts with the 1-betachains of hemoglobin through several viral proteins (ORF1ab, ORF3a, ORF7a, ORF8a and ORF10), causing hemoglobin denaturation and aggravating hypoxia [64]. Hypoxia has been indicated to induce a prothrombotic state and to be associated with venous thromboembolism [65,66]. Shen X.R. et al. reported the presence of viral particles in T lymphocytes and demonstrated that the infection was ACE2/TMPRSS2-independent [67]. Other authors proposed a potential entry pathway of SARS-CoV-2 in T lymphocytes via CD147 [55].
A study by Shen S. et al. revealed a lack of ACE2 expression in both human platelets and megakaryocytes inimmunofluorescence assay (IFA) and Western blot assay testing, suggesting a possible interaction of the viral spike protein with CD147, KREMEN1 and NRP1 [68]. Based on the functions of CD147 in signaling pathways and of NRP1 in cardiovascular, neuronal and immune systems, SARS-CoV-2 interaction with platelets is suspected to regulate the platelet-mediated immune response and promote coagulation dysfunction in COVID-19 [55,56,68,69]. Other authors observed that both ACE2 and TMPRSS2, a serine protease for spike protein priming, are expressed in human platelets, suggesting the possibility that an ACE2-dependent SARS-CoV-2 and platelet interaction is possible, with a consecutive platelet hyperactivation and thrombosis risk in COVID-19 patients [70]. Another study suggests a possible interaction between SARS-CoV-2 viral spike protein and platelet CD42b receptor, thereby promoting interactions with monocytes and cytokine production by monocytes inducing immunothrombosis [71].
Although the interaction mechanisms communicated between platelets and SARS-CoV-2 are not completely understood, may vary among authors and more in-depth studies are necessary, all of the studies performed regarding this point of interest concur to the point that interactions between the virus and platelets are frequent, leading to platelet hyperactivation and thrombosis.
3.2. Effector Cells—Monocytes, Neutrophils, Platelets, Endothelial Cells
3.2.1. Monocytes—The Main Source of TF in Inflammation-Induced Coagulopathy
In addition totheir role in conducting responses against foreign pathogens, monocytes promote the activation of coagulation via the extrinsic pathway during inflammatory states; TF is at least partially responsible for this effect [72]. In COVID-19, two functional subsets of monocytes have been identified. Classical, proinflammatory monocytes are numerous in patients with severe disease, while anti-inflammatory monocytes are reduced in these patients and correlate with lung damage markers [73]. This pattern is unlike mononuclear responses in sepsis.
Increasing TF release occurs upon stimulation by LPS, lipoteichoic acid, C-reactive protein or hypoxia (Figure 1A) [74,75,76]. In SARS-CoV-2 infection, platelet–monocyte interactions are a major stimulus for monocyte activation with robust cytokine and chemokine secretion and TF expression [77]. Subsequent to any of these triggers, TF suffers a thiol-disulfide exchange and is turned into the fully procoagulant form by the enzyme called protein disulfide isomerase (PDI) [78]. If fully procoagulant TF is not formed (i.e., the disulfide bond does not form), a stabilized TF/FVII complex, unable to bind FX, interacts with protease-activated receptors (PARs) and it triggers proinflammatory pathways [78]. Therefore, TF release can equally lead to procoagulant and proinflammatory outcomes (Figure 1A).
3.2.2. Activated Neutrophils—At the Intersection of Hemostatic Pathways
Dysregulated neutrophils’ activity in sepsis and septic shock may generate prothrombotic and hyperinflammatory states of varying intensities [79,80]. COVID-19 pathogenesis is increasingly found to rely on a significant contribution from neutrophils. Histological findings were the first aspects pointing in this direction. Autopsies of COVID-19 patients showed important neutrophil infiltrates in pulmonary capillaries [81,82]. In hospitalized patients, bronchoalveolar lavages showed an excessive neutrophil response relative to the other etiologies of pneumonia [83]. On peripheral blood counts, a neutrophil to lymphocyte ratio at admission, as well as its subsequent dynamic changes, have been proposed by several authors to correlate with disease severity, the need for mechanical ventilation and survival [84,85,86,87].
At least three mechanisms could explain neutrophils’ role in coagulation abnormalities: neutrophil extracellular traps (NETs) formation, neutrophil-derived microparticles (MPs) and neutrophil inflammasome activation.
NETs are a key mediator in the maladaptive inflammation–coagulation interplay, associated with sepsis severity, the development of DIC and mortality [88,89,90,91]. The ability of neutrophils to release NETs is one of the most dangerous consequences of neutrophil activation in COVID-19, causing microvascular thrombosis, endothelial dysfunction, tissue damage and eventually organ failure [92,93]. A certain neutrophil population, called low-density granulocytes, is more active in generating NETs and has been identified in greater proportions in COVID-19 patients, enhancing hypercoagulability-mediated organ damage and a poor prognosis [94,95,96].
The stimuli for NETs release are various: PAMPs (i.e., LPS, viral-derived PAMPs), DAMPs, cytokines and chemokines, infection-associated ROS production, activated platelets and ECs (Figure 1C) [97,98,99,100]. SARS-CoV-2-infected neutrophils are able to generate NETs as long as viral replication is underway and with the participation of the ACE2—TMPRSS2 entry pathway or CLRs [101,102,103]. CLRs, together with TLR2, also mediate a specific pathway for immunothrombosis potentiation in COVID-19 consisting of viral-induced platelet activation with vesicle shedding and NETosis [104]. As far as cytokines are concerned, IL-8 promotes NETs release in sepsis as well as in COVID-19, while IL-1β is rather particular for SARS-CoV-2 infection [99,105,106].
The activation of coagulation is mediated through several mechanisms, since NETs not only contain the right triggers but they also create the right conditions for hemostatic processes to take place by engaging platelets, ECs, coagulation factors and inorganic polyphosphate (Figure 1E) [107,108]. The negatively charged web-like surface of NETs (due to histones and nucleic acids) serves as a catalytic medium for the contact pathway activation of coagulation via factor FXII [109,110]. Conversely, interactions between NETs and platelets lead to the activation of the extrinsic pathway; cited mediators are TLR4 signaling, C5a, histones H3 and H4, von Willebrand factor (vWF), fibronectin, P-selectin, HMGBP1 and TF [50,52,108,109,111,112,113,114,115].
Coagulation pathways are further hastened through the inhibition of activated protein C by histones, TFPI degradation by NE and cathepsin G and antithrombin inactivation by NE and cytokine release [52,116,117,118,119,120]. Fibrinolysis pathways also represent a target of NETs components. NE alters plasminogen, mitigating the clot dissolving effects of activated plasmin; moreover, the binding of NE to extracellular DNA protects it from circulating alpha-1-proteinase inhibitor, thus prolonging its procoagulant activity [121,122]. Fibrinolysis resistance can also be explained by the formation of fibrin–DNA tight complexes, which are insensitive to plasmin [114,123,124]. CfDNA activates PAI-1, hindering fibrinolysis via this additional mechanism [42].
Neutrophil activation exposes the endothelium to lytic enzymes—MPO, NE, cathepsin G, ROS and NETs, which can break the functional and anatomical barriers (Figure 1D,F) [125,126]. NETs exert damaging effects on ECs, activating them and promoting more inflammation and thrombosis by TF and adhesion molecules expression, glycocalyx disruption and matrix metalloproteinases activation; accordingly, several study groups found increased levels of syndecan-1 in DIC septic patients [127,128,129,130].
MPsare another mechanism of neutrophil involvement in coagulopathies. The presence of TF in neutrophil-derived MPs has been a subject of controversy [131]. Even though TF in neutrophils could also be acquired from monocytes, several studies managed to induce in vitro TF production in neutrophils with P-selectin or C5a stimulation [132,133]. Currently, it seems that neutrophil-derived MPs can generate thrombin via the intrinsic pathway; interactions between NETs and PS facilitate the assembly of the latter and increase the effectiveness of thrombin generation by MPs [134]. The MPO presence in neutrophil MPs contributes to endothelial barrier disruption and the associated procoagulant phenotype [135].
3.2.3. Platelets’ Function—Much More Than Primary Hemostasis
The last few decades’ research has uncovered the major role of plateletsin coagulation–inflammation crosstalk; unsurprisingly, the amount of evidence of platelets’ involvement in sepsis continues to grow and to get more attractive from a therapeutical perspective. SARS-CoV-2 infection is no exception, especially since micro- and macrothrombotic events are such a frequent finding [136,137,138].
The activation of platelets in infectious circumstances can be caused either by classical endogenous factors or by pathogen-associated exogenous factors—directly or indirectly. Common endogenous platelet activators are adenosine diphosphate (ADP), thromboxane A2 (TxA2), thrombin (acting on PARs), subendothelial collagen and vWF exposure (via glycoprotein VI (GPVI) and glycoprotein Ibα-IX-V (GP-Ibα-IX-V), respectively), and TF and complement component C1q via the C1q receptor (Figure 1D,F) [139,140,141]. Sepsis can associate an acquired type of ADisintegrinandMetalloprotease with ThromboSpondin type 1 motif, member13 (ADAMTS13) deficiency, which generates large circulating vWF multimers, excessively activating platelets and worsening organ dysfunction [142].
Platelets from COVID-19 patients exhibit a particular hyperreactivity to low-dose common agonists (such as collagen, α-thrombin or ADP) compared to healthy controls and other hospitalized patients, including those with ARDS of other etiology, as was found by a number of authors [143,144,145]. The hyperreactivity of thrombocytes was associated withviral RNA entry, increased RNA blood levels and spike protein [70,145,146]. This reduced activation threshold of platelets was proposed by Zaid et al. to explain the hypercoagulable state of COVID-19 patients persisting despite heparin-based anticoagulation regimens [143]. Accordingly, some authors propose additional antithrombotic measures, i.e., antiplatelet agents, following observations of platelet-neutrophil immunothrombi [147]. Moreover, another work of Zaid et al., which found no correlation between D-dimers and platelet number or degranulation markers, suggested that platelet activation may occur independent of coagulation pathways [144].
The exogenous, bacterial-mediated activation of platelets via TLRs has been described [148,149,150,151]. Indirect bacterial–platelet interaction can be facilitated by fibrinogen, vWF, complement and immunoglobulins; IgG and Fcγ receptor IIa may induce platelet activation in staphylococcal and streptococcal infections [152]. In SARS-CoV-2 infection, antibodies are not a key element early in pathogenesis, but considering the widespread propagation of the virus and the high vaccination rates, some authors cite this mechanism to potentially gain more importance in the future [144]. Direct bacterial-mediated platelet activation has been cited in some Staphylococcus species via integrin αIIbβ3 [153,154]. SARS-CoV-2 S protein also contains an integrin binding domain, accounting for another plausible entry pathway [155]. Staphylococcal α-toxin may directly promote thrombosis: it stimulates the activation of platelets via increased calcium influx and factor V (FV) release from α-granules [156].
In SARS-CoV-2 infection, attempts to identify viral RNA in platelets and megakaryocytes haveled to both positive and negative outcomes in different study groups [145,157,158]. The cause of this discrepancy is still unclear; it could be related to different techniques of platelet isolation [159]. Zhang et al. argue that platelets express both ACE2 and TMPRSS2 and that direct viral and protein S binding is responsible forthe subsequent proinflammatory and procoagulant state, mediated through the MAPK pathway (Figure 1F) [142]. However, other studies failed to identify ACE2 and TMPRSS2 mRNA or proteins [144,145]. If and when viral entry is mediated by ACE2 and TMPRSS2, a local disequilibrium of the RAA system is expected, leading to increased TF expression and thrombosis [160,161]. Moreover, Zhang et al. confirmed that SARS-CoV-2 entry promotes ACE2 internalization in platelets, which could eventually explain the conflicting results of other authors regarding ACE2 expression [70]. Alternatively, viral entry could also be mediated by CD147, KREMEN1, neuropilin 1, heparan sulfate, sialic acid or MPs, the latter of which were found to trigger vigorous prothrombotic granules’ release, possibly followed by cell death [158,162]. Since a definitive answer is still lacking in this regard, the main culprit of platelet hyperreactivity—either viral genome or external factors—cannot be clearly identified.
Upon activation by various ligands in sepsis, platelets not only enhance their prothrombotic functions but also gain a particularly proinflammatory phenotype. Despite being anucleated, platelets can assemble NLR family pyrin domain containing 3 (NLRP3) inflammasome and produce IL-1β through IL-1β pre-mRNA processing following LPS-TLR4 stimulation [163,164,165,166,167]. IL-1β is unique, as it generates an autocrine activation loop, with further platelet activation and subsequent IL-1β synthesis; it is packed in platelet-derived MPs and it promotes megakaryocyte maturation [164,165,168,169]. In a comparable way to IL-1β, TF pre-mRNA is spliced to TF mRNA upon activation and shed as active TF [170,171]. The immune response to SARS-CoV-2 infection is characterized by high innate immunity reactivity with excessive and various cytokine release—IL-6, IL-2, IL-4, IL-13, IL-12, tumor necrosis factor α(TNF-α)and IL-7 [172]. Among them, IL-6 and TNF-α can directly activate platelets; both IL-6 and IL-1β can prime platelets before stimulation by classical agonists (Figure 1F) [160]. Following activation and/or priming through the aforementioned modalities, platelets release their α and dense granules and a variety of stored cytokines as shown by the experimental detection of increased FV and FXIII and platelet factor 4 release, CD40L, P-selectin exposure and IL-1 de novo synthesis (which partially mirrors the positive feedback mechanism in sepsis) [70,144,145].
Platelet–leukocyte interactions are initiated by the corresponding receptors’ pairing following activation: P-selectin and PSGL1 on neutrophils and monocytes, integrin αIIbβ3 or GPIb, through fibrinogen and macrophage 1 antigen (Mac-1) fibrinogen receptor on neutrophils (Figure 1C) [112,173,174,175,176]. Several studies found increased platelet–monocyte, platelet–neutrophil and platelet–T-cell interactions in COVID-19 patients [145,177]. These interactions lead to MPs release, TF exposure and eventually fibrin formation [145,178]. Significant correlations with inflammatory markers and disease severity were found by several authors [179,180].
Microparticle sheddingis another consequence of thrombocyte activation during sepsis. TLR4 signaling and GPIV ligands are some of the main triggers [165,181]. MPs contain IL-1β and can expose TF and binding sites for coagulation factors (particularly vitamin K-dependent coagulation factors) (Figure 1D,F) [165,182,183]. Their role in COVID-19 pathogenesis is becoming more and more apparent as well. Substantiating the prothrombotic potential of MPs in COVID-19, PS and TF have been identified in their composition; a connection with systemic thrombin formation and pulmonary disease severity was subsequently inferred [144,184,185,186]. Accordingly, markers of platelet activation were found in tracheal aspirates from patients with severe COVID-19 [180]. Other effects of increased MPs release are enhanced bacterial clearance andthe carrying of DAMPs, lipid mediators and platelet-associated receptors (i.e., P-selectin and integrin αIIbβ3) to other membranes [166,187,188,189]. Platelet-derived MPs have been associated with altered prognosis in sepsis due to myocardial dysfunction and the apoptosis of vascular smooth muscle cells and endothelium [190,191,192]. Apoptosis and endothelial cell dysfunction are frequent findings in various scenarios of increased blood levels of inflammatory cytokines—TNFα, IL-6 and CRP—due to persistent immune activation [193,194]. In SARS-CoV-2 infection, severe forms were associated with a lower number of active, PS-containing MPs (versus non-severe forms), possibly due to consumption or sequestration in certain organs [144]. Authors suggested that, since inflammation markers correlated better with MPs concentrations than D-dimers, inflammatory processes and not thrombosis arewhat primarily impact MPs dynamics [144].
In the septic proinflammatory environment, a new generation of thrombopoietin-independent thrombocytes is formed within the bone marrow [72,195]. This inflammation-induced megakaryopoiesis, stimulated by IL-6 and IL-3, leads to a different lineage of megakaryocytes and thrombocytes, which contains more TLRs and IL receptors, produces more IL-6 and TNF-α and exposes the major histocompatibility complex class II (MHC class II), facilitating antigen presentation [196,197,198,199,200,201]. Megakaryocytes are increasingly cited contributors to SARS-CoV-2 infection pathogenesis as well. Unlike platelets, ACE2 and TMPRSS2 protein expression in megakaryocytes is stated with more certainty [70]. As a consequence, pulmonary residing megakaryocytes are theoretically susceptible to SARS-CoV-2 infection and may transfer viral particles when circulating platelets are generated [144]. The diverse repertoire of cytokines and chemokines released by platelets upon activation can also be transferred during megakaryopoiesis. Furthermore, SARS-CoV-2 can locally enhance the production of this new generation of thrombocytes; in line with this theory, increased thrombopoietin levels were found in COVID-19 patients [160,202].
3.2.4. Endothelium Coordinates Both Inflammatory Responses and Coagulation
The endothelium represents an autonomous “organ”, whose integrity helps preserve a specific homeostatic state regarding hemostasis and inflammation.The activation of ECs is a proven key element of sepsis pathogenesis. Accordingly, several endothelium-derived markers serve as diagnostic tools or therapeutic targets [203,204,205,206]. In COVID-19, the first studies pointing to the major role of endothelial dysfunction focused on the histological findings of endotheliitis, a disrupted endothelial barrier, thrombosis and the microangiopathy of alveolar capillaries [207].
The activation of ECs in sepsis is mainly due to the binding of TNF-α and thrombin to TNF receptors and PAR-1, respectively; bacterial LPS and HMGB1 can also play a secondary role (Figure 1D) [72,208]. However, the primary insult in COVID-19-associated endotheliopathy is represented by the direct SARS-CoV-2 entry via ACE2 and TMPRSS2 (Figure 1F) [209]. Both viral particles per se (i.e., S protein) and the associated reduction in ACE2 expression contribute to the activation of ECs and to the subsequent shift towards a proinflammatory and prothrombotic phenotype (Figure 1F). Currently under researchers’ scrutiny, the pathway of angiopoietin 1 (Angpt1)—tyrosine kinase with immunoglobulin-like loops and epidermal growth factor homology domains-2 (Tie2) represents a major factor in ECs homeostasis, particularly when facing inflammation-derived prothrombotic tendencies [206]. Tie2 signaling alterations with excessive angiopoietin-2 release could lead to a phenotype switch in both COVID-19 and the early phases of sepsis [206,210].
A unique feature of sepsis endotheliopathy is the impact of mechanical insults. Shear stress has been indicated by some studies to maintain an anti-inflammatory and anticoagulant state via the NF-κB pathway [211,212]. Therefore, authors have hypothesized that, if adequate shear stress and ECs function were to be preserved, sepsis outcomes could benefit from this anticoagulant and anti-inflammatory phenotype and from the subsequent attraction of endothelial cells progenitors [213].
Regardless of the initial trigger, ECs acquire a proinflammatory phenotype mediated through classical signaling pathways—NF-κB and MAPK [208]. The transcription of adhesion molecule genes (such as intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1) and E-selectin), ROS synthesis and matrix metalloproteinases release may result from direct cytokine activity, ACE2 down-regulation or S-protein-related damage (Figure 1F) [178,208,214,215,216,217]. The subsequent increased cytokine production (TNF-a and IL-6 in particular) further promotes endothelial dysfunction: IL-6 contributes to vascular permeability and TNF-a worsens glycocalyx disruption in both diseases [218]. In COVID-19, excessive complement activation via the lectin pathway and a particular hyperactive state of the KKS pathway stand out, increasing vascular permeability [216,219,220].
Following this proinflammatory response, procoagulant mechanisms are subsequently engaged. NF-κB activation determines Weibel–Palade bodies’ exocytosis, with the release of vWF multimers, FVIII and P-selectin [178,217,221,222,223]. The release of vWF multimers in the context of sepsis-associated ADAMTS13 deficiency determines microvascular thrombosis with an impact on organ dysfunction severity (Figure 1D) [142,224]. The protein C anticoagulant system becomes dysfunctional in sepsis due to impaired protein C synthesis and activation, via thrombomodulin and endothelial protein C receptor deficiency [222]. Conversely, activated protein C has been found to mediate inflammation–coagulation crosstalk, similarly to the thrombin-related activation of PARs [225,226]. Other procoagulant modifications found in sepsis, as well as in COVID-19, are reduced TFPI and t-PA synthesis and increased PAI-1 expression [178,208,218,223,227].
Endothelial glycocalyx has become a major link in endothelial dysfunction pathogenesis in multiple diseases. Sepsis-associated glycocalyx injury is initiated through the lytic activity of specific enzymes: glucuronidases (i.e., heparanase), hyaluronidase, plasmin, cathepsin B, proteases and by reactive oxygen species (ROS) (Figure 1D) [226,228]. Hypervolemia (mostly determined by aggressive fluid resuscitation within sepsis management) has been cited to be a glycocalyx-disrupting factor; atrial natriuretic peptide may be incriminated in pathogenesis [229]. Following glycocalyx disruption, various adhesion molecules are expressed, leukocyte transmigration is facilitated and platelets are attracted to the injury site, initiating microthrombus formation [230]. The anticoagulant roles of glycosaminoglycans are therefore compromitted with resulting TFPI and antithrombin dysfunction [231]. In COVID-19, increased syndecan-1, heparan sulfate and hyaluronic acid levels, which correlated with disease severity, point towards significant glycocalyx disruption, but its role in SARS-CoV-2 coagulopathy remains a topic of on-going research [232,233].
A comparative summary of the major commonalities between sepsis and COVID-19 regarding all molecular and cellular mechanisms is found in Table 1.
Table 1.
Mechanisms of sepsis and COVID-19-associated coagulopathies.
| Commonalities between Sepsis and COVID-19-Associated Coagulopathies | Characteristics of Sepsis-Induced Coagulopathy | Characteristics of COVID-19-Associated Coagulopathy |
|---|---|---|
| TLRs and CLRs found on cell membranes and endosomes interact with PAMPs and DAMPs [10,11]. The synthesis of proinflammatory cytokines is activated via MAPK and NF-κB pathways [10,11,209]. Increased PAI-1 levels can be found in both COVID-19 and sepsis. [45,46] TF, FVIII, u-PA, PAI-1, TFPI, antithrombin and thrombomodulin are endpoints of the NF-κB pathway [12,13,14,15,16]. Histones promote platelet aggregation, bind prothrombotic molecules, and damage the antithrombotic properties of the endothelial glycocalyx [50,51]. Activated neutrophils generate prothrombotic and hyperinflammatory states via NETs formation and inflammasome activation, leading to immunothrombosis [88,89,90,91,92,93,94,95,96]. Intercellular interactions between platelets, endothelial cells, neutrophils and monocytes lead to MPs release and TF exposure [145,179]. Monocytes, as a source of soluble TF, promote the activation of coagulation via the extrinsic pathway [72,73]. Active platelets and MPs induce adhesion molecules’ expression and the dysfunction of endothelial cells [127,128,129,130]. The antithrombin pathway and protein C anticoagulant system become dysfunctional due to impaired protein C synthesis and activation via thrombomodulin and endothelial protein C receptor deficiency [52,53,54,226,227]. Tie2, NF-κB and MAPK signaling lead to ECs acquisitionof a proinflammatory and prothrombotic phenotype [207,208,209,210,211,212,213]. IL-6 contributes to vascular permeability and TNF-α worsens the glycocalyx disruption of endothelial cells [219]. |
HMGB1 can modulate fibrinolysis by interacting with plasminogen and t-PA, can promote coagulation via TF exposure on macrophages and inhibits the protein C pathway [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. cfDNA activates coagulation via FXI and FXII [40,41]. High cfDNA concentrations inactivate t-PA by PAI-1 [42,43,44]. Increased TF release from monocytes occurs upon stimulation by LPS [74,75,76]. Fibrinolysis resistance is enhanced via plasminogen altering within NETs, the formation of fibrin–DNA tight complexes insensitive to plasmin and the activation of PAI-1 by cfDNA [42,114,123,124]. Sepsis is associated withADAMTS13 deficiency, which generates large circulating vWF multimers, excessively activating platelets [234,235,236,237]. Platelet activation can arise via direct and indirect bacterial–platelet interactions [153]. A septic proinflammatory status induces (via IL-6 and IL-3 stimulation) the release of thrombopoietin-independent thrombocytes with more numerous TLRs and interleukin receptors, more IL-6 and TNF-α [72,196]. Endothelial cells’ activation can be triggered by TNF-α and thrombin, bacterial LPS and other PAMPs, HMGB1 and other DAMPs, cytokines, such as IFN-γ and IL-1β and shear stress [72,207,208,209,210,211,212,213,214]. Reduced TFPI and t-PA synthesis and increased PAI-1 expression by ECs potentiate the prothrombotic status [179,209,219,224,228]. Glycocalyx injury is initiated by specific enzymes (glucuronidases, hyaluronidases, plasmin, ROS) and hypervolemia (via excessive fluid administration within sepsis management) [227,228,229,230,231]. |
Activated coagulation FX and thrombin may cleave S protein and promote viral entry in a potential inflammation–coagulation positive feedback loop [61]. Platelet–monocyte interactions are themain stimulus for monocyte activation with robust cytokine and chemokine secretion and TF expression [77]. Low-density granulocytes are found in greater proportions in COVID-19 patients; they are more active in generating NETs, further enhancing hypercoagulability-mediated organ damage [94,95,96]. Platelets from COVID-19 patients exhibit a particular hyperreactivity to low-dose common agonists (such as collagen, α-thrombin or ADP) [143,144,145]. IL-6 and TNF-α can directly activate platelets; IL-6 and IL-1β can prime platelets before stimulation by classical agonists [161]. Pulmonary-residing megakaryocytes are theoretically susceptible to SARS-CoV-2 infection and may transfer viral particles and cytokines when new circulating platelets are generated [144]. SARS-CoV-2 can infect endothelial cells via ACE2 and TMPRSS2, with endotheliitis as a result of direct viral entry [208]. S-protein-related damage increases adhesion molecules’ exposure, ROS synthesis and matrix metalloproteinases’ release with the disruption of the endothelial barrier [179,209,215,216,217,218,219]. COVID-19 induces a hyperactive state of the KKS pathway, promoting vascular permeability [217,220,221]. Excessive complement activation via the lectin pathway accentuates endothelial-derived immunothrombosis [217,220,221]. |
ACE2—angiotensin-converting enzyme 2; ADAMTS13—ADisintegrinandMetalloprotease with ThromboSpondin type 1 motif, member13; ADP—adenosinediphosphate; cfDNA—cell-free desoxyribonucleic acid; CLR—C-type lectin receptor; COVID-19—coronavirus disease 2019; DAMP—damage-associated molecular pattern; EC—endothelial cell; FVIII, FX, FXI, FXII—coagulation factor VIII, X, XI, XII; HMGB1—high mobility group box 1; IFN-γ—interferonγ; IL-1β, IL-3, IL-6—interleukin 1β, 3, 6; KKS—kallikrein-kinin system; LPS—lipopolysaccharides; MAPK—mitogen-activated protein kinase; MP—microparticle; NET—neutrophil extracellular trap; NF-κB—nuclear factor kappa B; PAI-1—plasminogen activator inhibitor-1; PAMP—pathogen-associated molecular pattern; ROS—reactive oxygen species; TF—tissue factor; TFPI—tissue factor pathway inhibitor; Tie2—tyrosine kinase with immunoglobulin-like loops and epidermal growth factor homology domains-2; TLR—Toll-like receptor; TMPRSS2—transmembrane protease serine 2; TNF-α—tumor necrosis factor α; tPA—tissue type plasminogen activator; TxA2—thromboxane A2; u-PA—urokinase-type plasminogen activator; vWF—von Willebrand factor.
4. From Theory to Practice—Clinical Aspects of Coagulopathy in Sepsis and COVID-19
4.1. Progression of Coagulation Disorders—The Pathways towards DIC
As was highlighted in the paragraphs above, septic patients have numerous theoretical reasons to develop nearly any type of coagulopathy. Accordingly, some authors state that practically all patients fulfilling sepsis criteria suffer from a certain type and severity of coagulopathy [238]. In COVID-19, describing and defining the associated coagulopathy (CAC) has been a matter of intense research ever since the global spread and the severe course of SARS-CoV-2 infection became apparent.
4.1.1. What Is DIC?
DIC is defined as “an acquired syndrome characterized by intravascular activation of coagulation with loss of localization arising from different causes; it can originate from and cause damage to the microvasculature, which if sufficiently severe, can produce organ dysfunction” [239]. From a pathogenic standpoint, sepsis-associated DIC is a form of suppressed fibrinolysis-type DIC: the very subtle rise in fibrinolytic activityis highly disproportionate compared to the uncontrolled activation of coagulation (due to abnormally high levels of PAI-1, a reduction in t-PA, TFPI and protein C system activity), a process called “fibrinolysis shutdown” [44,240]. A vicious circle, with continuously worsening hemostasis dysfunction and consequent inflammatory maladaptive responses, leadsto the final stages of DIC, substantially worsening the prognosis for septic patients [241,242].
Within the clinical setting, DIC can take the form of a wide spectrum of manifestations, both manifest and subclinical, sometimes progressing towards a common classical form of consumptive coagulopathy. Sensible markers of coagulation activation and fibrinolysis such as thrombin-antithrombin complex (TAT) and plasmin-α2-plasmin inhibitor complex (PIC) can identify the clinically silent forms of coagulopathy; standard coagulation tests showing slight modifications are characteristic of early forms of septic coagulopathy; eventually, completely impaired coagulation panels are found in what is defined as overt DIC [239,243,244]. Overt DIC criteria, despite having high sensitivity and specificity, diagnose advanced stages of coagulopathy, when therapeutic interventions are no longer beneficial. Updated criteria of the International Society on Thrombosis and Haemostasis (ISTH)on DIC diagnosis in sepsis introduced the concept of early-stage DIC in septic patients, namely sepsis-induced coagulopathy (SIC) [245]. The SIC score is only composed of platelet count, prothrombin time (PT) and sequential organ failure assessment (SOFA) score; it remains the most widely employed tool for sepsis coagulopathy assessment [245].
4.1.2. Is Coagulopathy of COVID-19 a Form of DIC?
One of the first important steps towards understanding CAC pathogenesis was launching the hypothesis that coagulopathy in COVID-19 could mirror the natural evolution of the infection, thus displaying an initial respiratory limited phase, possibly followed by a disseminated, systemic phase [5]. This two-phase progression of the disease differs substantially from that of bacterial sepsis. In fact, it appears that severity scores for bacterial sepsis (i.e., SOFA score) do not perform as well in predicting COVID-19 outcomes [246,247,248].
Some authors advanced the term “Pulmonary Intravascular Coagulation” to depict the phenomena behind COVID-19 acute lung injury [249,250,251]. Alveolar fibrin deposits represent one of the main histological markers of COVID-19; therefore, the disruption of the alveolar–endothelial barrier and microvascular thrombosis due to unbalanced activity of coagulation and fibrinolytic systems are factors thought to underly the pulmonary histological features of SARS-CoV-2 infection [252,253,254,255]. An accelerated fibrin turn-over is characteristic of the pulmonary phase of CAC with both endothelial PAI-1 and alveolar (u-PA) excessive release, followed by intense D-dimers formation [256,257]. By contrast, this phase associates a systemic activation of the coagulation cascadewith “fibrinolysis shutdown”—similarly to SIC—as found by viscoelastic testing, despite the overwhelming increase in serum D-dimers [234,251,255,256,258]. In fact, several authors have recently pointed out that D-dimer levels are only associated with fibrin formation dynamics, and high D-dimers do not necessarily imply systemic hyperfibrinolysis; therefore, in COVID-19, evidence points towards an extravascular—i.e., pulmonary—source of D-dimers [259].
A clinically relevant systemic form of coagulopathy can eventually develop in SARS-CoV-2 infection. In advanced stages, it can take the highly fatal form of overtDIC—higher DIC scores (in accordance with ISTH) were found with a high prevalence of up to 70%, in COVID-19 non-survivors [260]. However, the systemic phase of CAC can take different intermediary forms before progressing towards overtDIC. In the earliest stages, probably overlapping the pulmonary phase, a trend towards platelet- and fibrinogen-mediated hypercoagulability is shown by viscoelastic assays [255]. A slight prolongation of PT and activated partial thromboplastin time (aPTT) can occur and this anomaly remains subtle for a longer period of the disease course, possibly due to FVIII and fibrinogen synthesis within the acute phase response [257]. An increased thrombin generation potential can be identified in both sepsis and SARS-CoV-2 infection; this enhanced activation of coagulation is maintained in patients with COVID-19 despite prophylactic anticoagulation, unlike septic patients [147]. Moreover, the pathologic changes in plasma thrombin and plasmin generation found in this study permitted enhanced fibrin formation in both COVID-19 and sepsis, with unexpectedlydelayed times to thrombin, plasmin and fibrin formation in COVID-19, suggesting a loss of coagulation-initiating mechanisms in severe COVID-19 [147]. The platelet count remains only slightly decreased until an overt DIC stage is reached and, unlike SIC, it is not so clearly associated with mortality [257,261].
Other cited forms of coagulopathy that have been described in association with SARS-CoV-2 infection are a form of hemolytic microangiopathy due to ADAMTS13/vWF disequilibrium, vasculitis linked to antiphospholipid and anti-β2-glycoprotein antibodies, and possibly hemophagocytic lymphohistiocytosis [235,236,257,262].
Considering the aspects reviewed in the paragraphs above on SIC and CAC pathogenesis, we depicted numerous shared mechanisms when it comes to coagulation–inflammation interplay. Nevertheless, in a more in-depth assessment, subtle differences become apparent and the resulting clinical picture does not display as many similarities between the two diseases.
Overall, CAC is a very complex form of coagulopathy, displaying two anatomically and pathogenically different phases; even though it could at times fulfill DIC definition and criteria, other factors should be considered before setting the equal sign between the two. Consequently, a collective effort is being made to accurately define and classify CAC; a systematic approach is provided by Ibaet al. in one of their latest reviews [5].
4.2. Searching for the Proof—Coagulation Studies
4.2.1. Standard Coagulation Tests
Typical laboratory findings in SIC and sepsis-associated DIC. ISTH diagnostic criteria for SIC and sepsis-associated overtDIC employ the most important markers in sepsis coagulopathy, with high availability in clinical circumstances: platelet count, D-dimers or fibrinogen/fibrin degradation products (FDP), PT, serum fibrinogen levels and SOFA score [245]. Thrombocytopenia is very frequent in sepsis and serves as a marker of poor prognosis [237]. A very low or rapidly falling platelet count can be a typical sign of DIC and it predicts the length of intensive care unit (ICU) stay, mortality and increased risk of bleeding [263]. Increased D-dimer levels are a frequent finding in sepsis-associated DIC [264]. High levels of D-dimers are associated with 28-day mortality in patients with infection or sepsis [265], a level of 4μg/mL or more inducing a 12.6-fold increase in mortality in patients with severe sepsis [266].However, several authors [264,267,268] have implied that D-dimers do not accurately correlate with mortality. For instance, a sepsis subgroup, with advanced illness and overtDIC criteria but particularly low D-dimer levels showed very high mortality rates [268]. There are also authors that support both situations, demonstrating that both low and extremely elevated D-dimer values are associated with a higher risk of death in patients with sepsis [269]. Both COVID-19 and sepsis are associated with the endogenous activation of coagulation and fibrinolysis, presenting elevated fibrinogen, D-dimers, solublethrombomodulin and plasmin–antiplasmin complexes. [147]. Although COVID-19 and sepsis patients presented significantly high levels of D-dimers and TF-containing MPs’ activity compared to healthy controls, the serum levels of D-dimers were significantly lower in patients with COVID-19 compared to patients with sepsis [47]. Patients with COVID-19 also presented high levels of active PAI-1 (whereas patients with sepsis did not), which may limit the plasmin degradation of crosslinked fibrin and the release of D-dimers, explaining the lower levels of D-dimers compared with sepsis [47].
The “fibrinolysis shutdown” that occurs in sepsis due to increased PAI-1 synthesis has been incriminated in this scenario [267]. This hypothesis is supported by corresponding lower PIC levels in these patients [268]. Standard coagulation tests PT and aPTT cannot accurately measure in vivo hemostatic processes; however, when prolonged due to excessive consumption, they correlate with DIC severity and bleeding risk [263]. Among clotting factors, FVIII and fibrinogen are considered less valuable in assessing DIC: FVIII is frequently increased due to excessive release together with vWF and fibrinogen, being an acute phase protein that maintains a close-to-normal serum level until the very advanced phases of DIC [270,271].
What stands out in CAC? There are at least two aspects that distinguish SIC and CAC clinical pictures: D-dimers and platelet count. As was highlighted before, in COVID-19, D-dimers are thought to originate from a process of intense fibrin formation and subsequent degradation within alveoli, in the context of pulmonary intravascular coagulation [249,272]. D-dimers in COVID-19 correlate with poor prognosis [258,273]. They have particularly high levels even in early phases, much higher than in classical DIC settings, and are not initially associated with consumptive coagulopathy markers such as prolonged standard coagulation tests or hypofibrinogenemia [274,275,276,277]. Regarding platelet count, a definitive conclusion is yet to be proposed. Some authors found worsening thrombocytopenia with increased COVID-19 severity, but plenty of studies showed that, even though mild thrombocytopenia (100–150 × 109/L) is frequent, platelet counts lower than that are identified in a very small group of patients [261,278,279]. This could be explained by a compensatory response of platelet production [280]. Overall, further investigations are needed to determine whether low platelet count is a prognostic marker in COVID-19 and whether it arises mainly due to the viral infection itself or to other factors as well (antiphospholipid syndrome, immune thrombocytopenic purpura, hemophagocytic syndrome, heparin-induced thrombocytopenia, drug-induced myelosuppression, etc.) [251].
Another distinctive feature has been outlined by the major work of Tanget al.,who followed the dynamics of coagulation markers in COVID-19 patients [276]. It was shown that COVID-19 coagulopathy, as it can be described by standard coagulation tests, is a continuously evolving process. Specifically, after approximately 7–10 days from admission, non-survivors underwent a massive shift in their biological picture—from slightly increased PT and aPTT, high D-dimers and near-normal fibrinogen (the characteristics of a suppressed-fibrinolytic-type DIC) towards extremely prolonged PT and aPTT, extremely high D-dimer levels and very low fhbrinogen levels (the characteristics of an enhanced-fibrinolytic-type DIC) [276,281]. The Histological findings of alveolar hemorrhage along thrombosis in the autopsies of COVID-19 patients could be a manifestation of this profile switch of CAC, as was suggested by Asakuraet al. [281,282].
4.2.2. Advanced Coagulation Studies—Viscoelastic Tests in SIC and CAC
Viscoelastic tests (VETs), i.e., thrombelastography and thromboelastometry, are advanced techniques, using whole blood to evaluate in real time the dynamics of clot formation and the distinct input of platelets, fibrinogen, coagulation factors or fibrinolysis to thrombus development. Unlike standard coagulation tests, viscoelastic assays offer a more comprehensive insight on how global coagulation unfolds in vivo.
In the last few decades, VETs have been used to evaluate SIC and sepsis-associated overt DIC, considering the persistent need to predict and prevent poor outcomes and complications in sepsis more efficiently. Up until now, a few conclusions couldbe drawn from research work using VETs in sepsis. Three viscoelastic patterns were found in septic patients—hypocoagulability, normocoagulability and hypercoagulability; the abnormal patterns were identified with a prevalence ranging from 43% to 100% [283]. Hypocoagulable patterns identified in multiple studies, using various protocols, correlated with mortality rates [284,285,286,287]. Certain VET parameters can predict and diagnose DIC in septic patients, particularly in the phase of consumptive coagulopathy [288,289,290,291]. In accordance with the pathogenic sequence of sepsis progression, an early tendency towards hypercoagulation and early “fibrinolysis shutdown” hasbeen identified through VETs in some study groups; these modifications increase morbidity and mortality [240]. Eventually, abnormal VET patterns turned out to be particularly sensible in detecting coagulopathy as they seem to impact prognosis despite near-normal standard coagulation panels [292].
In COVID-19 patients, a hypercoagulable state with accentuated clot kinetics, clot strength and “fibrinolysis shutdown” wasfound through VETs [256,293,294]. The hypofibrinolytic pattern together with high D-dimer levels was associated with an increased risk of thromboembolic events [295]. Unlike sepsis patients, COVID-19 patients rarely display hypocoagulable VET patterns. Further studies are required to determine the prognostic value of VETs for micro- and macrothrombosis in COVID-19 and their possible role in the therapeutic approach of CAC.
5. Conclusions
The molecular mechanisms behind the inflammatory and immunological responses of bacterial sepsis, on the one hand, and COVID-19, on the other hand, together with the associated coagulation disorders, reveal multiple similarities between SIC and CAC pathogenesis.
Humoral and cellular actors mediate the common pathways of inflammation–coagulation crosstalk. Both bacterial- and viral-derived PAMPs initiate immune responses following binding to PRRs. Subsequent inflammasome and NF-κB pathway activations are responsible for the initial coagulation activation, mainly via the extrinsic pathway. Cellular effectors are further involved in the unfolding of widespread immunothrombosis. Monocytes represent the main source of circulating TF and subsequent extrinsic pathway enhancement. NETs formation and platelet activation establish excellent structural and functional circumstances for inflammation and thrombosis to potentiateeach other. In addition, ECs dysfunction equally worsens hemostatic and immune disorders in SIC, as well as in CAC.
The resulting clinical picture of SIC and CAC shows, however, some distinctive features. In fact, these two coagulopathies seem to share the same “ingredients” (i.e., molecular mechanisms), but they may not share the same “recipe” (i.e., dynamics). CAC is marked by a pulmonary-localized initial phase of intravascular coagulation due to the important respiratory tropism of SARS-CoV-2. This phase may be followed by a disseminated form of coagulopathy, potentially evolving towards a consumptive pattern.
CAC does not smoothly superimpose over classical SIC and/or DIC definition and criteria. Therefore, proposals of new definitions and/or classifications for CAC should be supported and an effort to clinically validate them encouraged.
Author Contributions
Conceptualization, G.T., E.C.B., M.L., C.E.C.-T., S.I.N., E.M. and D.A.I.; Data curation, G.T., E.C.B., M.L., C.E.C.-T., S.I.N., E.M. and D.A.I.; Formal analysis, G.T., E.C.B., M.L., C.E.C.-T., S.I.N., E.M. and D.A.I.; Investigation, G.T., E.C.B., M.L., C.E.C.-T., S.I.N., E.M. and D.A.I.; Methodology, G.T., E.C.B., M.L., C.E.C.-T., S.I.N., E.M. and D.A.I.; Resources, G.T., E.C.B., M.L., C.E.C.-T., S.I.N., E.M. and D.A.I.; Software, G.T., E.C.B., M.L., C.E.C.-T., S.I.N., E.M. and D.A.I.; Supervision, D.A.I.; Validation, G.T., E.C.B., M.L., C.E.C.-T., S.I.N., E.M. and D.A.I.; Visualization, G.T., E.C.B., M.L., C.E.C.-T., S.I.N., E.M. and D.A.I.; Writing—original draft, G.T.; Writing—review and editing, G.T., E.C.B., M.L., C.E.C.-T., S.I.N., E.M. and D.A.I. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Opal S. The Current Understanding of Sepsis and Research Priorities for the Future. Virulence. 2014;5:1. doi: 10.4161/viru.26803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. [(accessed on 24 October 2022)]. Available online: https://covid19.who.int/
- 4.Gusev E., Sarapultsev A., Solomatina L., Chereshnev V. SARS-CoV-2-Specific Immune Response and the Pathogenesis of COVID-19. Int. J. Mol. Sci. 2022;23:1716. doi: 10.3390/ijms23031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iba T., Warkentin T.E., Thachil J., Levi M., Levy J.H. Proposal of the Definition for COVID-19-Associated Coagulopathy. J. Clin. Med. 2021;10:191. doi: 10.3390/jcm10020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koçak Tufan Z., Kayaaslan B., Mer M. COVID-19 and Sepsis. Turk. J. Med. Sci. 2021;51:3301–3311. doi: 10.3906/sag-2108-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamkanfi M., Dixit V.M. Mechanisms and Functions of Inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Yu P., Zhang X., Liu N., Tang L., Peng C., Chen X. Pyroptosis: Mechanisms and Diseases. Signal Transduct. Target. Ther. 2021;6:128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu R., Wang N., Comish P.B., Tang D., Kang R. Inflammasome-Dependent Coagulation Activation in Sepsis. Front. Immunol. 2021;12:641750. doi: 10.3389/fimmu.2021.641750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li D., Wu M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct Target. 2021;6:291. doi: 10.1038/s41392-021-00687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raymond S.L., Holden D.C., Mira J.C., Stortz J.A., Loftus T.J., Mohr A.M., Moldawer L.L., Moore F.A., Larson S.D., Efron P.A. Microbial Recognition and Danger Signals in Sepsis and Trauma. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:2564–2573. doi: 10.1016/j.bbadis.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swiatkowska M., Szemraj J., Cierniewski C.S. Induction of PAI-1 Expression by Tumor Necrosis Factor Alpha in Endothelial Cells Is Mediated by Its Responsive Element Located in the 4G/5G Site. FEBS J. 2005;272:5821–5831. doi: 10.1111/j.1742-4658.2005.04979.x. [DOI] [PubMed] [Google Scholar]
- 13.Angus D.C., van der Poll T. Severe Sepsis and Septic Shock. N. Engl. J. Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 14.Begbie M., Notley C., Tinlin S., Sawyer L., Lillicrap D. The Factor VIII Acute Phase Response Requires the Participation of NFkappaB and C/EBP. Thromb. Haemost. 2000;84:216–222. doi: 10.1055/s-0037-1613999. [DOI] [PubMed] [Google Scholar]
- 15.Blanch-Ruiz M.A., Ortega-Luna R., Martínez-Cuesta M.Á., Álvarez Á. The Neutrophil Secretome as a Crucial Link between Inflammation and Thrombosis. Int. J. Mol. Sci. 2021;22:4170. doi: 10.3390/ijms22084170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S.F., Malik A.B. NF-ΚB Activation as a Pathological Mechanism of Septic Shock and Inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:622–645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 17.Wu C., Lu W., Zhang Y., Zhang G., Shi X., Hisada Y., Grover S.P., Zhang X., Li L., Xiang B., et al. Inflammasome Activation Triggers Blood Clotting and Host Death through Pyroptosis. Immunity. 2019;50:1401–1411.e4. doi: 10.1016/j.immuni.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yap J.K.Y., Moriyama M., Iwasaki A. Inflammasomes and Pyroptosis as Therapeutic Targets for COVID-19. J. Immunol. 2020;205:307. doi: 10.4049/jimmunol.2000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y., Kuang M., Li J., Zhu L., Jia Z., Guo X., Hu Y., Kong J., Yin H., Wang X., et al. SARS-CoV-2 Spike Protein Interacts with and Activates TLR41. Cell Res. 2021;31:818. doi: 10.1038/s41422-021-00495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirato K., Kizaki T. SARS-CoV-2 Spike Protein S1 Subunit Induces pro-Inflammatory Responses via Toll-like Receptor 4 Signaling in Murine and Human Macrophages. Heliyon. 2021;7:E06187. doi: 10.1016/j.heliyon.2021.e06187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khadke S., Ahmed N., Ahmed N., Ratts R., Raju S., Gallogly M., de Lima M., Sohail M.R. Harnessing the Immune System to Overcome Cytokine Storm and Reduce Viral Load in COVID-19: A Review of the Phases of Illness and Therapeutic Agents. Virol. J. 2020;17:154. doi: 10.1186/s12985-020-01415-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohn K.M., Lee S.G., Kim H.J., Cheon S., Jeong H., Lee J., Kim I.S., Silwal P., Kim Y.J., Paik S., et al. COVID-19 Patients Upregulate Toll-like Receptor 4-Mediated Inflammatory Signaling That Mimics Bacterial Sepsis. J. Korean Med. Sci. 2020;35:e343. doi: 10.3346/jkms.2020.35.e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bortolotti D., Gentili V., Rizzo S., Schiuma G., Beltrami S., Strazzabosco G., Fernandez M., Caccuri F., Caruso A., Rizzo R. Tlr3 and Tlr7 Rna Sensor Activation during SARS-CoV-2 Infection. Microorganisms. 2021;9:1820. doi: 10.3390/microorganisms9091820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbu E.C., Chiţu-Tişu C.E., Lazăr M., Olariu C., Bojincă M., Ionescu R.A., Ion D.A., Bădărău I.A. Hepatic Osteodystrophy: A Global (Re)View of the Problem. Acta Clin. Croat. 2017;56:512–525. doi: 10.20471/acc.2017.56.03.19. [DOI] [PubMed] [Google Scholar]
- 25.Zhu X., Zhang W., Wu C., Wang S., Smith F.G., Jin S., Zhang P. The Novel Role of Metabolism-Associated Molecular Patterns in Sepsis. Front. Cell Infect. Microbiol. 2022;12:915099. doi: 10.3389/fcimb.2022.915099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin S.J. Cell Death and Inflammation: The Case for IL-1 Family Cytokines as the Canonical DAMPs of the Immune System. FEBS J. 2016;283:2599–2615. doi: 10.1111/febs.13775. [DOI] [PubMed] [Google Scholar]
- 27.Rajaee A., Barnett R., Cheadle W.G. Pathogen- A Nd Danger-Associated Molecular Patterns and the Cytokine Response in Sepsis. Surg. Infect. 2018;19:107–116. doi: 10.1089/sur.2017.264. [DOI] [PubMed] [Google Scholar]
- 28.Lu B., Nakamura T., Inouye K., Li J., Tang Y., Lundbäck P., Valdes-Ferrer S.I., Olofsson P.S., Kalb T., Roth J., et al. Novel Role of PKR in Inflammasome Activation and HMGB1 Release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scaffidi P., Misteli T., Bianchi M.E. Release of Chromatin Protein HMGB1 by Necrotic Cells Triggers Inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 30.Sha Y., Zmijewski J., Xu Z., Abraham E. HMGB1 Develops Enhanced Proinflammatory Activity by Binding to Cytokines. J. Immunol. 2008;180:2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- 31.Wang H., Ward M.F., Sama A.E. Targeting HMGB1 in the Treatment of Sepsis. Expert Opin. Ther. Targets. 2014;18:257–268. doi: 10.1517/14728222.2014.863876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelmann B., Massberg S. Thrombosis as an Intravascular Effector of Innate Immunity. Nat. Rev. Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 33.Maugeri N., Campana L., Gavina M., Covino C., de Metrio M., Panciroli C., Maiuri L., Maseri A., D’Angelo A., Bianchi M.E., et al. Activated Platelets Present High Mobility Group Box 1 to Neutrophils, Inducing Autophagy and Promoting the Extrusion of Neutrophil Extracellular Traps. J. Thromb. Haemost. 2014;12:2074–2088. doi: 10.1111/jth.12710. [DOI] [PubMed] [Google Scholar]
- 34.Ito T., Kawahara K., Nakamura T., Yamada S., Nakamura T., Abeyama K., Hashiguchi T., Maruyama I. High-Mobility Group Box 1 Protein Promotes Development of Microvascular Thrombosis in Rats. J. Thromb. Haemost. 2007;5:109–116. doi: 10.1111/j.1538-7836.2006.02255.x. [DOI] [PubMed] [Google Scholar]
- 35.Musumeci D., Roviello G.N., Montesarchio D. An Overview on HMGB1 Inhibitors as Potential Therapeutic Agents in HMGB1-Related Pathologies. Pharmacol. Ther. 2014;141:347–357. doi: 10.1016/j.pharmthera.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Lee W., Yuseok O., Yang S., Lee B.S., Lee J.H., Park E.K., Baek M.C., Song G.Y., Bae J.S. JH-4 Reduces HMGB1-Mediated Septic Responses and Improves Survival Rate in Septic Mice. J. Cell Biochem. 2019;120:6277–6289. doi: 10.1002/jcb.27914. [DOI] [PubMed] [Google Scholar]
- 37.Lee W., Ku S.K., Bae J.S. Zingerone Reduces HMGB1-Mediated Septic Responses and Improves Survival in Septic Mice. Toxicol. Appl. Pharmacol. 2017;329:202–211. doi: 10.1016/j.taap.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Stevens N.E., Chapman M.J., Fraser C.K., Kuchel T.R., Hayball J.D., Diener K.R. Therapeutic Targeting of HMGB1 during Experimental Sepsis Modulates the Inflammatory Cytokine Profile to One Associated with Improved Clinical Outcomes. Sci. Rep. 2017;7:5850. doi: 10.1038/s41598-017-06205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-kuraishy H.M., Al-Gareeb A.I., Alkazmi L., Habotta O.A., Batiha G.E.S. High-Mobility Group Box 1 (HMGB1) in COVID-19: Extrapolation of Dangerous Liaisons. Inflammopharmacology. 2022;30:811. doi: 10.1007/s10787-022-00988-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oehmcke S., Mörgelin M., Herwald H. Activation of the Human Contact System on Neutrophil Extracellular Traps. J. Innate Immun. 2009;1:225–230. doi: 10.1159/000203700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gansler J., Jaax M., Leiting S., Appel B., Greinacher A., Fischer S., Preissner K.T. Structural Requirements for the Procoagulant Activity of Nucleic Acids. PLoS ONE. 2012;7:e50399. doi: 10.1371/journal.pone.0050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komissarov A.A., Florova G., Idell S. Effects of Extracellular DNA on Plasminogen Activation and Fibrinolysis. J. Biol. Chem. 2011;286:41949–41962. doi: 10.1074/jbc.M111.301218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D., Wrobleski S.K., Wakefield T.W., Hartwig J.H., Wagner D.D. Extracellular DNA Traps Promote Thrombosis. Proc. Natl. Acad. Sci. USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fourrier F. Severe Sepsis, Coagulation, and Fibrinolysis: Dead End or One Way? Crit. Care Med. 2012;40:2704–2708. doi: 10.1097/CCM.0b013e318258ff30. [DOI] [PubMed] [Google Scholar]
- 45.Levy J.H., Iba T. Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. Anesthesiology. 2020;132:1238–1283. doi: 10.1097/ALN.0000000000003122. [DOI] [PubMed] [Google Scholar]
- 46.Kang S., Tanaka T., Inoue H., Ono C., Hashimoto S., Kioi Y., Matsumoto H., Matsuura H., Matsubara T., Shimizu K., et al. IL-6 Trans-Signaling Induces Plasminogen Activator Inhibitor-1 from Vascular Endothelial Cells in Cytokine Release Syndrome. Proc. Natl. Acad. Sci. USA. 2020;117:22351–22356. doi: 10.1073/pnas.2010229117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell R.A., Hisada Y., Denorme F., Grover S.P., Bouck E.G., Middleton E.A., Wolberg A.S., Rondina M.T., Mackman N. Comparison of the Coagulopathies Associated with COVID-19 and Sepsis. Res. Pract. Thromb. Haemost. 2021;5:e12525. doi: 10.1002/rth2.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saffarzadeh M., Juenemann C., Queisser M.A., Lochnit G., Barreto G., Galuska S.P., Lohmeyer J., Preissner K.T. Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PLoS ONE. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y., Wan D., Luo X., Song T., Wang Y., Yu Q., Jiang L., Liao R., Zhao W., Su B. Circulating Histones in Sepsis: Potential Outcome Predictors and Therapeutic Targets. Front. Immunol. 2021;12:650184. doi: 10.3389/fimmu.2021.650184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuchs T.A., Bhandari A.A., Wagner D.D. Histones Induce Rapid and Profound Thrombocytopenia in Mice. Blood. 2011;118:3708–3714. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakahara M., Ito T., Kawahara K.-I., Yamamoto M., Nagasato T., Shrestha B., Yamada S., Miyauchi T., Higuchi K., Takenaka T., et al. Recombinant Thrombomodulin Protects Mice against Histone-Induced Lethal Thromboembolism. PLoS ONE. 2013;8:e75961. doi: 10.1371/journal.pone.0075961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Massberg S., Grahl L., von Bruehl M.L., Manukyan D., Pfeiler S., Goosmann C., Brinkmann V., Lorenz M., Bidzhekov K., Khandagale A.B., et al. Reciprocal Coupling of Coagulation and Innate Immunity via Neutrophil Serine Proteases. Nat. Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 53.Ammollo C.T., Semeraro F., Xu J., Esmon N.L., Esmon C.T. Extracellular Histones Increase Plasma Thrombin Generation by Impairing Thrombomodulin-Dependent Protein C Activation. J. Thromb. Haemost. 2011;9:1795–1803. doi: 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 54.Biswas I., Panicker S.R., Cai X.S., Giri H., Rezaie A.R. Extracellular Histones Bind Vascular Glycosaminoglycans and Inhibit the Anti-Inflammatory Function of Antithrombin. Cell Physiol. Biochem. 2021;55:605–617. doi: 10.33594/000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang K., Chen W., Zhang Z., Deng Y., Lian J.Q., Du P., Wei D., Zhang Y., Sun X.X., Gong L., et al. CD147-Spike Protein Is a Novel Route for SARS-CoV-2 Infection to Host Cells. Signal Transduct. Target. Ther. 2020;5:283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., et al. Neuropilin-1 Facilitates SARS-CoV-2 Cell Entry and Infectivity. Science. 2020;370:856. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thépaut M., Luczkowiak J., Vivès C., Labiod N., Bally I., Lasala F., Grimoire Y., Fenel D., Sattin S., Thielens N., et al. DC/L-SIGN Recognition of Spike Glycoprotein Promotes SARS-CoV-2 Trans-Infection and Can Be Inhibited by a Glycomimetic Antagonist. PLoS Pathog. 2021;17:e1009576. doi: 10.1371/journal.ppat.1009576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirato K., Takanari J., Kizaki T. Standardized Extract of Asparagus Officinalis Stem Attenuates SARS-CoV-2 Spike Protein-Induced IL-6 and IL-1β Production by Suppressing P44/42 MAPK and Akt Phosphorylation in Murine Primary Macrophages. Molecules. 2021;26:6189. doi: 10.3390/molecules26206189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geng J., Chen L., Yuan Y., Wang K., Wang Y., Qin C., Wu G., Chen R., Zhang Z., Wei D., et al. CD147 Antibody Specifically and Effectively Inhibits Infection and Cytokine Storm of SARS-CoV-2 and Its Variants Delta, Alpha, Beta, and Gamma. Signal Transduct. Target. Ther. 2021;6:347. doi: 10.1038/s41392-021-00760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022;23:3. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kastenhuber E.R., Mercadante M., Nilsson-Payant B., Johnson J.L., Jaimes J.A., Muecksch F., Weisblum Y., Bram Y., Chandar V., Whittaker G.R., et al. Coagulation Factors Directly Cleave SARS-CoV-2 Spike and Enhance Viral Entry. Elife. 2022;11:77444. doi: 10.7554/eLife.77444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khawaja U.A., Shamsoddin E., Desideri L.F., Tovani-Palone M.R. Infection of Red Blood Cells by SARS-CoV-2: New Evidence. Einstein. 2021;19:eCE6285. doi: 10.31744/einstein_journal/2021CE6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russo A., Tellone E., Barreca D., Ficarra S., Laganà G. Implication of COVID-19 on Erythrocytes Functionality: Red Blood Cell Biochemical Implications and Morpho-Functional Aspects. Int. J. Mol. Sci. 2022;23:2171. doi: 10.3390/ijms23042171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu W., Li H. COVID-19: Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism. Biol. Med. Chem. 2020 doi: 10.26434/CHEMRXIV.11938173.V9. preprint . [DOI] [Google Scholar]
- 65.Ninivaggi M., de Laat M., Lancé M.M.D., Kicken C.H., Pelkmans L., Bloemen S., Dirks M.L., van Loon L.J.C., Govers-Riemslag J.W.P., Lindhout T., et al. Hypoxia Induces a Prothrombotic State Independently of the Physical Activity. PLoS ONE. 2015;10:e0141797. doi: 10.1371/journal.pone.0141797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoeper M.M., Granton J. Intensive Care Unit Management of Patients with Severe Pulmonary Hypertension and Right Heart Failure. Am. J. Respir. Crit. Care Med. 2011;184:1114–1124. doi: 10.1164/rccm.201104-0662CI. [DOI] [PubMed] [Google Scholar]
- 67.Shen X.R., Geng R., Li Q., Chen Y., Li S.F., Wang Q., Min J., Yang Y., Li B., Jiang R.-D., et al. ACE2-Independent Infection of T Lymphocytes by SARS-CoV-2. Signal Transduct. Target. Ther. 2022;7:83. doi: 10.1038/s41392-022-00919-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen S., Zhang J., Fang Y., Lu S., Wu J., Zheng X., Deng F. SARS-CoV-2 Interacts with Platelets and Megakaryocytes via ACE2-Independent Mechanism. J. Hematol. Oncol. 2021;14:72. doi: 10.1186/s13045-021-01082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Savla S.R., Prabhavalkar K.S., Bhatt L.K. Cytokine Storm Associated Coagulation Complications in COVID-19 Patients: Pathogenesis and Management. Expert Rev. Anti Infect. Ther. 2021;19:1397–1413. doi: 10.1080/14787210.2021.1915129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., Liu M., Zhao X., Xie Y., Yang Y., et al. SARS-CoV-2 Binds Platelet ACE2 to Enhance Thrombosis in COVID-19. J. Hematol. Oncol. 2020;13:120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li T., Yang Y., Li Y., Wang Z., Ma F., Luo R., Xu X., Zhou G., Wang J., Niu J., et al. Platelets Mediate Inflammatory Monocyte Activation by SARS-CoV-2 Spike Protein. J. Clin. Invest. 2022;132:e150101. doi: 10.1172/JCI150101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mussbacher M., Salzmann M., Brostjan C., Hoesel B., Schoergenhofer C., Datler H., Hohensinner P., Basílio J., Petzelbauer P., Assinger A., et al. Cell Type-Specific Roles of NF-ΚB Linking Inflammation and Thrombosis. Front. Immunol. 2019;10:85. doi: 10.3389/fimmu.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christensen E.E., Jørgensen M.J., Nore K.G., Dahl T.B., Yang K., Ranheim T., Huse C., Lind A., Nur S., Stiksrud B., et al. Critical COVID-19 Is Associated with Distinct Leukocyte Phenotypes and Transcriptome Patterns. J. Intern. Med. 2021;290:677. doi: 10.1111/joim.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paffen E., Vos H.L., Bertina R.M. C-Reactive Protein Does Not Directly Induce Tissue Factor in Human Monocytes. Arterioscler. Thromb. Vasc. Biol. 2004;24:975–981. doi: 10.1161/01.ATV.0000126681.16619.69. [DOI] [PubMed] [Google Scholar]
- 75.Åberg M., Siegbahn A. Tissue Factor Non-Coagulant Signaling—Molecular Mechanisms and Biological Consequences with a Focus on Cell Migration and Apoptosis. J. Thromb. Haemost. 2013;11:817–825. doi: 10.1111/jth.12156. [DOI] [PubMed] [Google Scholar]
- 76.Mattsson E., Hartung T., Morath S., Egesten A. Highly Purified Lipoteichoic Acid from Staphylococcus Aureus Induces Procoagulant Activity and Tissue Factor Expression in Human Monocytes but Is a Weak Inducer in Whole Blood: Comparison with Peptidoglycan. Infect. Immun. 2004;72:4322–4326. doi: 10.1128/IAI.72.7.4322-4326.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hottz E.D., Martins-Gonçalves R., Palhinha L., Azevedo-Quintanilha I.G., de Campos M.M., Sacramento C.Q., Temerozo J.R., Soares V.C., Gomes Dias S.S., Teixeira L., et al. Platelet-Monocyte Interaction Amplifies Thromboinflammation through Tissue Factor Signaling in COVID-19. Blood Adv. 2022;6:5085. doi: 10.1182/bloodadvances.2021006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Egorina E.M., Sovershaev M.A., Hansen J.B. The Role of Tissue Factor in Systemic Inflammatory Response Syndrome. Blood Coagul. Fibrinolysis. 2011;22:451–456. doi: 10.1097/MBC.0b013e328346ef3f. [DOI] [PubMed] [Google Scholar]
- 79.Stiel L., Meziani F., Helms J. Neutrophil Activation during Septic Shock. Shock. 2018;49:371–384. doi: 10.1097/SHK.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 80.Brown K., Brain S., Pearson J., Edgeworth J., Lewis S., Treacher D. Neutrophils in Development of Multiple Organ Failure in Sepsis. Lancet. 2006;368:157–169. doi: 10.1016/S0140-6736(06)69005-3. [DOI] [PubMed] [Google Scholar]
- 81.Bian X.W., Yao X.H., Ping Y.F., Yu S., Shi Y., Luo T., He Z.C., Tang R., Chen C., Fu W.J., et al. Autopsy of COVID-19 Patients in China. Natl. Sci. Rev. 2020;7:1414–1418. doi: 10.1093/nsr/nwaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lazar M., Barbu E.C., Chitu C.E., Anghel A.M.J., Niculae C.M., Manea E.D., Damalan A.C., Bel A.A., Patrascu R.E., Hristea A., et al. Pericardial Involvement in Severe COVID-19 Patients. Medicina. 2022;58:1093. doi: 10.3390/medicina58081093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z., Guo L., Yang J., Wang C., Jiang S., et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe. 2020;27:883–890.e2. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang H., Zhang Y., Mo P., Liu J., Wang H., Wang F., Zhao Q. Neutrophil to CD4+ Lymphocyte Ratio as a Potential Biomarker in Predicting Virus Negative Conversion Time in COVID-19. Int. Immunopharmacol. 2020;85:106683. doi: 10.1016/j.intimp.2020.106683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cai J., Li H., Zhang C., Chen Z., Liu H., Lei F., Qin J.J., Liu Y.M., Zhou F., Song X., et al. The Neutrophil-to-Lymphocyte Ratio Determines Clinical Efficacy of Corticosteroid Therapy in Patients with COVID-19. Cell Metab. 2021;33:258–269.e3. doi: 10.1016/j.cmet.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moisa E., Corneci D., Negoita S., Filimon C.R., Serbu A., Negutu M.I., Grintescu I.M. Dynamic Changes of the Neutrophil-to-Lymphocyte Ratio, Systemic Inflammation Index, and Derived Neutrophil-to-Lymphocyte Ratio Independently Predict Invasive Mechanical Ventilation Need and Death in Critically Ill COVID-19 Patients. Biomedicines. 2021;9:1656. doi: 10.3390/biomedicines9111656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anghel A.M.J., Niculae C.M., Manea E.D., Lazar M., Popescu M., Damalan A.C., Bel A.A., Nedelcu I.M., Patrascu R.E., Hristea A. The Impact of Tocilizumab on Radiological Changes Assessed by Quantitative Chest CT in Severe COVID-19 Patients. J. Clin. Med. 2022;11:1247. doi: 10.3390/jcm11051247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abrams S.T., Morton B., Alhamdi Y., Alsabani M., Lane S., Welters I.D., Wang G., Toh C.H. A Novel Assay for Neutrophil Extracellular Trap Formation Independently Predicts Disseminated Intravascular Coagulation and Mortality in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2019;200:869–880. doi: 10.1164/rccm.201811-2111OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar S., Gupta E., Kaushik S., Srivastava V.K., Saxena J., Mehta S., Jyoti A. Quantification of NETs Formation in Neutrophil and Its Correlation with the Severity of Sepsis and Organ Dysfunction. Clin. Chim. Acta. 2019;495:606–610. doi: 10.1016/j.cca.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 90.Luo L., Zhang S., Wang Y., Rahman M., Syk I., Zhang E., Thorlacius H. Proinflammatory Role of Neutrophil Extracellular Traps in Abdominal Sepsis. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307:L586–L596. doi: 10.1152/ajplung.00365.2013. [DOI] [PubMed] [Google Scholar]
- 91.Czaikoski P.G., Mota J.M.S.C., Nascimento D.C., Sônego F., Castanheira F.V.E.S., Melo P.H., Scortegagna G.T., Silva R.L., Barroso-Sousa R., Souto F.O., et al. Neutrophil Extracellular Traps Induce Organ Damage during Experimental and Clinical Sepsis. PLoS ONE. 2016;11:e0148142. doi: 10.1371/journal.pone.0148142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Becker K., Beythien G., de Buhr N., Stanelle-Bertram S., Tuku B., Kouassi N.M., Beck S., Zickler M., Allnoch L., Gabriel G., et al. Vasculitis and Neutrophil Extracellular Traps in Lungs of Golden Syrian Hamsters With SARS-CoV-2. Front. Immunol. 2021;12:640842. doi: 10.3389/fimmu.2021.640842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ackermann M., Anders H.J., Bilyy R., Bowlin G.L., Daniel C., de Lorenzo R., Egeblad M., Henneck T., Hidalgo A., Hoffmann M., et al. Patients with COVID-19: In the Dark-NETs of Neutrophils. Cell Death Differ. 2021;28:3125–3139. doi: 10.1038/s41418-021-00805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Obermayer A., Jakob L.M., Haslbauer J.D., Matter M.S., Tzankov A., Stoiber W. Neutrophil Extracellular Traps in Fatal COVID-19-Associated Lung Injury. Dis. Markers. 2021;2021:5566826. doi: 10.1155/2021/5566826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cabrera L.E., Pekkarinen P.T., Alander M., Nowlan K.H.A., Nguyen N.A., Jokiranta S., Kuivanen S., Patjas A., Mero S., Pakkanen S.H., et al. Characterization of Low-Density Granulocytes in COVID-19. PLoS Pathog. 2021;17:e1009721. doi: 10.1371/journal.ppat.1009721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morrissey S.M., Geller A.E., Hu X., Tieri D., Ding C., Klaes C.K., Cooke E.A., Woeste M.R., Martin Z.C., Chen O., et al. A Specific Low-Density Neutrophil Population Correlates with Hypercoagulation and Disease Severity in Hospitalized COVID-19 Patients. JCI Insight. 2021;6:e148435. doi: 10.1172/jci.insight.148435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clark S.R., Ma A.C., Tavener S.A., McDonald B., Goodarzi Z., Kelly M.M., Patel K.D., Chakrabarti S., McAvoy E., Sinclair G.D., et al. Platelet TLR4 Activates Neutrophil Extracellular Traps to Ensnare Bacteria in Septic Blood. Nat. Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 98.Gupta A.K., Joshi M.B., Philippova M., Erne P., Hasler P., Hahn S., Resink T.J. Activated Endothelial Cells Induce Neutrophil Extracellular Traps and Are Susceptible to NETosis-Mediated Cell Death. FEBS Lett. 2010;584:3193–3197. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 99.Yipp B.G., Kubes P. NETosis: How Vital Is It? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 100.Pilsczek F.H., Salina D., Poon K.K.H., Fahey C., Yipp B.G., Sibley C.D., Robbins S.M., Green F.H.Y., Surette M.G., Sugai M., et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus Aureus. J. Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 101.Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., Schneider A.H., Caetité D., Tavares L.A., Paiva I.M., et al. SARS-CoV-2-Triggered Neutrophil Extracellular Traps Mediate COVID-19 Pathology. J. Exp. Med. 2020;217:e20201129. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sung P.S., Hsieh S.L. C-Type Lectins and Extracellular Vesicles in Virus-Induced NETosis. J. Biomed. Sci. 2021;28:46. doi: 10.1186/s12929-021-00741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arcanjo A., Logullo J., Menezes C.C.B., de Souza Carvalho Giangiarulo T.C., dos Reis M.C., de Castro G.M.M., da Silva Fontes Y., Todeschini A.R., Freire-de-Lima L., Decoté-Ricardo D., et al. The Emerging Role of Neutrophil Extracellular Traps in Severe Acute Respiratory Syndrome Coronavirus 2 (COVID-19) Sci. Rep. 2020;10:19630. doi: 10.1038/s41598-020-76781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sung P.-S., Yang S.-P., Peng Y.-C., Sun C.-P., Tao M.-H., Hsieh S.-L. CLEC5A and TLR2 Are Critical in SARS-CoV-2-Induced NET Formation and Lung Inflammation. J. Biomed. Sci. 2022;29:52. doi: 10.1186/s12929-022-00832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yaqinuddin A., Kashir J. Novel Therapeutic Targets for SARS-CoV-2-Induced Acute Lung Injury: Targeting a Potential IL-1β/Neutrophil Extracellular Traps Feedback Loop. Med. Hypotheses. 2020;143:109906. doi: 10.1016/j.mehy.2020.109906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaiser R., Leunig A., Pekayvaz K., Popp O., Joppich M., Polewka V., Escaig R., Anjum A., Hoffknecht M.L., Gold C., et al. Self-Sustaining IL-8 Loops Drive a Prothrombotic Neutrophil Phenotype in Severe COVID-19. JCI Insight. 2021;6:e150862. doi: 10.1172/jci.insight.150862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McDonald B., Davis R.P., Kim S.J., Tse M., Esmon C.T., Kolaczkowska E., Jenne C.N. Platelets and Neutrophil Extracellular Traps Collaborate to Promote Intravascular Coagulation during Sepsis in Mice. Blood. 2017;129:1357–1367. doi: 10.1182/blood-2016-09-741298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Skendros P., Mitsios A., Chrysanthopoulou A., Mastellos D.C., Metallidis S., Rafailidis P., Ntinopoulou M., Sertaridou E., Tsironidou V., Tsigalou C., et al. Complement and Tissue Factor-Enriched Neutrophil Extracellular Traps Are Key Drivers in COVID-19 Immunothrombosis. J. Clin. Invest. 2020;130:6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.von Brühl M.L., Stark K., Steinhart A., Chandraratne S., Konrad I., Lorenz M., Khandoga A., Tirniceriu A., Coletti R., Köllnberger M., et al. Monocytes, Neutrophils, and Platelets Cooperate to Initiate and Propagate Venous Thrombosis in Mice in Vivo. J. Exp. Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gould T.J., Vu T.T., Swystun L.L., Dwivedi D.J., Mai S.H.C., Weitz J.I., Liaw P.C. Neutrophil Extracellular Traps Promote Thrombin Generation through Platelet-Dependent and Platelet-Independent Mechanisms. Arterioscler. Thromb. Vasc. Biol. 2014;34:1977–1984. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 111.Kim S.W., Lee J.K. Role of HMGB1 in the Interplay between NETosis and Thrombosis in Ischemic Stroke: A Review. Cells. 2020;9:1794. doi: 10.3390/cells9081794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Etulain J., Martinod K., Wong S.L., Cifuni S.M., Schattner M., Wagner D.D. P-Selectin Promotes Neutrophil Extracellular Trap Formation in Mice. Blood. 2015;126:242–246. doi: 10.1182/blood-2015-01-624023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martinod K., Wagner D.D. Thrombosis: Tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gould T.J., Vu T.T., Stafford A.R., Dwivedi D.J., Kim P.Y., Fox-Robichaud A.E., Weitz J.I., Liaw P.C. Cell-Free DNA Modulates Clot Structure and Impairs Fibrinolysis in Sepsis. Arterioscler. Thromb. Vasc. Biol. 2015;35:2544–2553. doi: 10.1161/ATVBAHA.115.306035. [DOI] [PubMed] [Google Scholar]
- 115.Iba T., Levy J.H. Inflammation and Thrombosis: Roles of Neutrophils, Platelets and Endothelial Cells and Their Interactions in Thrombus Formation during Sepsis. J. Thromb. Haemost. 2018;16:231–241. doi: 10.1111/jth.13911. [DOI] [PubMed] [Google Scholar]
- 116.Yang S., Qi H., Kan K., Chen J., Xie H., Guo X., Zhang L. Neutrophil Extracellular Traps Promote Hypercoagulability in Patients with Sepsis. Shock. 2017;47:132–139. doi: 10.1097/SHK.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 117.Osada K., Minami T., Arioka T., Sakai T., Tawara S., Kawasaki K., Fareed J., Matsuzaki O. Thrombomodulin Alfa Attenuates the Procoagulant Effect and Cytotoxicity of Extracellular Histones through the Promotion of Protein C Activation. Thromb. Res. 2017;160:51–57. doi: 10.1016/j.thromres.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 118.Noubouossie D.F., Whelihan M.F., Yu Y.-B., Sparkenbaugh E., Pawlinski R., Monroe D.M., Key N.S. In Vitro Activation of Coagulation by Human Neutrophil DNA and Histone Proteins but Not Neutrophil Extracellular Traps. Blood. 2017;129:1021–1029. doi: 10.1182/blood-2016-06-722298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maroney S.A., Mast A.E. Tissue Factor Pathway Inhibitor and Bacterial Infection. J. Thromb. Haemost. 2011;9:119. doi: 10.1111/j.1538-7836.2010.04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Simmons J., Pittet J.F. The Coagulopathy of Acute Sepsis. Curr. Opin. Anaesthesiol. 2015;28:227. doi: 10.1097/ACO.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scapini P., Nesi L., Morini M., Tanghetti E., Belleri M., Noonan D., Presta M., Albini A., Cassatella M.A. Generation of Biologically Active Angiostatin Kringle 1-3 by Activated Human Neutrophils. J. Immunol. 2002;168:5798–5804. doi: 10.4049/jimmunol.168.11.5798. [DOI] [PubMed] [Google Scholar]
- 122.Da Cruz D.B., Helms J., Aquino L.R., Stiel L., Cougourdan L., Broussard C., Chafey P., Riès-Kautt M., Meziani F., Toti F., et al. DNA-Bound Elastase of Neutrophil Extracellular Traps Degrades Plasminogen, Reduces Plasmin Formation, and Decreases Fibrinolysis: Proof of Concept in Septic Shock Plasma. FASEB J. 2019;33:14270–14280. doi: 10.1096/fj.201901363RRR. [DOI] [PubMed] [Google Scholar]
- 123.Varjú I., Longstaff C., Szabó L., Farkas Á.Z., Varga-Szabó V.J., Tanka-Salamon A., Machovich R., Kolev K. DNA, Histones and Neutrophil Extracellular Traps Exert Anti-Fibrinolytic Effects in a Plasma Environment. Thromb. Haemost. 2015;113:1289–1299. doi: 10.1160/TH14-08-0669. [DOI] [PubMed] [Google Scholar]
- 124.Longstaff C., Varjú I., Sótonyi P., Szabó L., Krumrey M., Hoell A., Bóta A., Varga Z., Komorowicz E., Kolev K. Mechanical Stability and Fibrinolytic Resistance of Clots Containing Fibrin, DNA, and Histones. J. Biol. Chem. 2013;288:6946–6956. doi: 10.1074/jbc.M112.404301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Radermecker C., Detrembleur N., Guiot J., Cavalier E., Henket M., d’Emal C., Vanwinge C., Cataldo D., Oury C., Delvenne P., et al. Neutrophil Extracellular Traps Infiltrate the Lung Airway, Interstitial, and Vascular Compartments in Severe COVID-19. J. Exp. Med. 2020;217:e20201012. doi: 10.1084/jem.20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Caillon A., Trimaille A., Favre J., Jesel L., Morel O., Kauffenstein G. Role of Neutrophils, Platelets, and Extracellular Vesicles and Their Interactions in COVID-19-Associated Thrombopathy. J. Thromb. Haemost. 2022;20:17–31. doi: 10.1111/jth.15566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rabinovitch M. NETs Activate Pulmonary Arterial Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2016;36:2035–2037. doi: 10.1161/ATVBAHA.116.308206. [DOI] [PubMed] [Google Scholar]
- 128.Carmona-Rivera C., Zhao W., Yalavarthi S., Kaplan M.J. Neutrophil Extracellular Traps Induce Endothelial Dysfunction in Systemic Lupus Erythematosus through the Activation of Matrix Metalloproteinase-2. Ann. Rheum. Dis. 2015;74:1417–1424. doi: 10.1136/annrheumdis-2013-204837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Folco E.J., Mawson T.L., Vromman A., Bernardes-Souza B., Franck G., Persson O., Nakamura M., Newton G., Luscinskas F.W., Libby P. Neutrophil Extracellular Traps Induce Endothelial Cell Activation and Tissue Factor Production Through Interleukin-1α and Cathepsin, G. Arterioscler. Thromb. Vasc. Biol. 2018;38:1901–1912. doi: 10.1161/ATVBAHA.118.311150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mao J.Y., Zhang J.H., Cheng W., Chen J.W., Cui N. Effects of Neutrophil Extracellular Traps in Patients with Septic Coagulopathy and Their Interaction with Autophagy. Front. Immunol. 2021;12:757041. doi: 10.3389/fimmu.2021.757041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Osterud B., Rao L., Olsen J. Induction of Tissue Factor Expression in Whole Blood: Lack of Evidence for the Presence of Tissue Factor Expression in Granulocytes—PubMed. Thromb. Haemost. 2000;83:861–867. doi: 10.1055/s-0037-1613934. [DOI] [PubMed] [Google Scholar]
- 132.Ritis K., Doumas M., Mastellos D., Micheli A., Giaglis S., Magotti P., Rafail S., Kartalis G., Sideras P., Lambris J.D. A Novel C5a Receptor-Tissue Factor Cross-Talk in Neutrophils Links Innate Immunity to Coagulation Pathways. J. Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 133.Maugeri N., Brambilla M., Camera M., Carbone A., Tremoli E., Donati M.B., de Gaetano G., Cerletti C. Human Polymorphonuclear Leukocytes Produce and Express Functional Tissue Factor upon Stimulation. J. Thromb. Haemost. 2006;4:1323–1330. doi: 10.1111/j.1538-7836.2006.01968.x. [DOI] [PubMed] [Google Scholar]
- 134.Wang Y., Luo L., Braun O., Westman J., Madhi R., Herwald H., Mörgelin M., Thorlacius H. Neutrophil Extracellular Trap-Microparticle Complexes Enhance Thrombin Generation via the Intrinsic Pathway of Coagulation in Mice. Sci. Rep. 2018;8:4020. doi: 10.1038/s41598-018-22156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pitanga T.N., de Aragão França L., Rocha V.C.J., Meirelles T., Matos Borges V., Gonçalves M.S., Pontes-de-Carvalho L.C., Noronha-Dutra A.A., Dos-Santos W.L.C. Neutrophil-Derived Microparticles Induce Myeloperoxidase-Mediated Damage of Vascular Endothelial Cells. BMC Cell Biol. 2014;15:21. doi: 10.1186/1471-2121-15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Belani P., Schefflein J., Kihira S., Rigney B., Delman B.N., Mahmoudi K., Mocco J., Majidi S., Yeckley J., Aggarwal A., et al. COVID-19 Is an Independent Risk Factor for Acute Ischemic Stroke. AJNR Am. J. Neuroradiol. 2020;41:1361–1364. doi: 10.3174/ajnr.A6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang C., Shen L., Le K.-J., Pan M.-M., Kong L.-C., Gu Z.-C., Xu H., Zhang Z., Ge W.-H., Lin H.-W. Incidence of Venous Thromboembolism in Hospitalized Coronavirus Disease 2019 Patients: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2020;7:151. doi: 10.3389/fcvm.2020.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., Wang X., Hu C., Ping R., Hu P., et al. Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan. A Retrospective Observational Study. Am. J. Respir. Crit. Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Linden M.D. Platelet Physiology. Methods Mol. Biol. 2013;992:13–30. doi: 10.1007/978-1-62703-339-8_2. [DOI] [PubMed] [Google Scholar]
- 140.Coughlin S.R. Thrombin Signalling and Protease-Activated Receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 141.Kral J.B., Schrottmaier W.C., Salzmann M., Assinger A. Platelet Interaction with Innate Immune Cells. Transfus. Med. Hemother. 2016;43:78–88. doi: 10.1159/000444807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bockmeyer C.L., Claus R.A., Budde U., Kentouche K., Schneppenheim R., Lösche W., Reinhart K., Brunkhorst F.M. Inflammation-Associated ADAMTS13 Deficiency Promotes Formation of Ultra-Large von Willebrand Factor. Haematologica. 2008;93:137–140. doi: 10.3324/haematol.11677. [DOI] [PubMed] [Google Scholar]
- 143.Zaid Y., Guessous F., Puhm F., Elhamdani W., Chentoufi L., Morris A.C., Cheikh A., Jalali F., Boilard E., Flamand L. Platelet Reactivity to Thrombin Differs between Patients with COVID-19 and Those with ARDS Unrelated to COVID-19. Blood Adv. 2021;5:635. doi: 10.1182/bloodadvances.2020003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zaid Y., Puhm F., Allaeys I., Naya A., Oudghiri M., Khalki L., Limami Y., Zaid N., Sadki K., ben El Haj R., et al. Platelets Can Associate with SARS-CoV-2 RNA and Are Hyperactivated in COVID-19. Circ. Res. 2020;127:1404–1418. doi: 10.1161/CIRCRESAHA.120.317703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kanth Manne B., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C., Petrey A.C., Tolley N.D., Guo L., Cody M., et al. Platelet Gene Expression and Function in Patients with COVID-19. Blood. 2020;136:1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grobbelaar L.M., Venter C., Vlok M., Ngoepe M., Laubscher G.J., Lourens P.J., Steenkamp J., Kell D.B., Pretorius E. SARS-CoV-2 Spike Protein S1 Induces Fibrin(Ogen) Resistant to Fibrinolysis: Implications for Microclot Formation in COVID-19. Biosci. Rep. 2021;41:20210611. doi: 10.1042/BSR20210611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bouck E.G., Denorme F., Holle L.A., Middelton E.A., Blair A.M., de Laat B., Schiffman J.D., Yost C.C., Rondina M.T., Wolberg A.S., et al. COVID-19 and Sepsis Are Associated with Different Abnormalities in Plasma Procoagulant and Fibrinolytic Activity. Arterioscler. Thromb. Vasc. Biol. 2021;41:401. doi: 10.1161/ATVBAHA.120.315338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vogel S., Bodenstein R., Chen Q., Feil S., Feil R., Rheinlaender J., Schäffer T.E., Bohn E., Frick J.S., Borst O., et al. Platelet-Derived HMGB1 Is a Critical Mediator of Thrombosis. J. Clin. Invest. 2015;125:4638–4654. doi: 10.1172/JCI81660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Berthet J., Damien P., Hamzeh-Cognasse H., Arthaud C.A., Eyraud M.A., Zéni F., Pozzetto B., McNicol A., Garraud O., Cognasse F. Human Platelets Can Discriminate between Various Bacterial LPS Isoforms via TLR4 Signaling and Differential Cytokine Secretion. Clin. Immunol. 2012;145:189–200. doi: 10.1016/j.clim.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 150.Zhang G., Han J., Welch E.J., Ye R.D., Voyno-Yasenetskaya T.A., Malik A.B., Du X., Li Z. Lipopolysaccharide Stimulates Platelet Secretion and Potentiates Platelet Aggregation via TLR4/MyD88 and the CGMP-Dependent Protein Kinase Pathway. J. Immunol. 2009;182:7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Semeraro F., Ammollo C.T., Morrissey J.H., Dale G.L., Friese P., Esmon N.L., Esmon C.T. Extracellular Histones Promote Thrombin Generation through Platelet-Dependent Mechanisms: Involvement of Platelet TLR2 and TLR4. Blood. 2011;118:1952–1961. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Arman M., Krauel K., Tilley D.O., Weber C., Cox D., Greinacher A., Kerrigan S.W., Watson S.P. Amplification of Bacteria-Induced Platelet Activation Is Triggered by FcγRIIA, Integrin AIIbβ3, and Platelet Factor 4. Blood. 2014;123:3166–3174. doi: 10.1182/blood-2013-11-540526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Miajlovic H., Zapotoczna M., Geoghegan J.A., Kerrigan S.W., Speziale P., Foster T.J. Direct Interaction of Iron-Regulated Surface Determinant IsdB of Staphylococcus Aureus with the GPIIb/IIIa Receptor on Platelets. Microbiology. 2010;156:920–928. doi: 10.1099/mic.0.036673-0. [DOI] [PubMed] [Google Scholar]
- 154.Brennan M.P., Loughman A., Devocelle M., Arasu S., Chubb A.J., Foster T.J., Cox D. Elucidating the Role of Staphylococcus Epidermidis Serine-Aspartate Repeat Protein G in Platelet Activation. J. Thromb. Haemost. 2009;7:1364–1372. doi: 10.1111/j.1538-7836.2009.03495.x. [DOI] [PubMed] [Google Scholar]
- 155.Sigrist C.J., Bridge A., le Mercier P. A Potential Role for Integrins in Host Cell Entry by SARS-CoV-2. Antivir. Res. 2020;177:104759. doi: 10.1016/j.antiviral.2020.104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Garciarena C.D., McHale T.M., Watkin R.L., Kerrigan S.W. Coordinated Molecular Cross-Talk between Staphylococcus Aureus, Endothelial Cells and Platelets in Bloodstream Infection. Pathogens. 2015;4:869. doi: 10.3390/pathogens4040869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bury L., Camilloni B., Castronari R., Piselli E., Malvestiti M., Borghi M., KuchiBotla H., Falcinelli E., Petito E., Amato F., et al. Search for SARS-CoV-2 RNA in Platelets from COVID-19 Patients. Platelets. 2021;32:284–287. doi: 10.1080/09537104.2020.1859104. [DOI] [PubMed] [Google Scholar]
- 158.Koupenova M., Corkrey H.A., Vitseva O., Tanriverdi K., Somasundaran M., Liu P., Soofi S., Bhandari R., Godwin M., Parsi K.M., et al. SARS-CoV-2 Initiates Programmed Cell Death in Platelets. Circ. Res. 2021;129:631. doi: 10.1161/CIRCRESAHA.121.319117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jevtic S.D., Nazy I. The COVID Complex: A Review of Platelet Activation and Immune Complexes in COVID-19. Front. Immunol. 2022;13:594. doi: 10.3389/fimmu.2022.807934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brambilla M., Canzano P., Becchetti A., Tremoli E., Camera M. Alterations in Platelets during SARS-CoV-2 Infection. Platelets. 2021;33:1. doi: 10.1080/09537104.2021.1962519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu Z., Hu R., Zhang C., Ren W., Yu A., Zhou X. Elevation of Plasma Angiotensin II Level Is a Potential Pathogenesis for the Critically Ill COVID-19 Patients. Crit. Care. 2020;24:290. doi: 10.1186/s13054-020-03015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Maugeri N., de Lorenzo R., Clementi N., Antonia Diotti R., Criscuolo E., Godino C., Tresoldi C., Bio Angels For Covid-BioB Study Group. Bonini C., Clementi M., et al. Unconventional CD147-dependent Platelet Activation Elicited by SARS-CoV-2 in COVID-19. J. Thromb. Haemost. 2022;20:434. doi: 10.1111/jth.15575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schwertz H., Tolley N.D., Foulks J.M., Denis M.M., Risenmay B.W., Buerke M., Tilley R.E., Rondina M.T., Harris E.M., Kraiss L.W., et al. Signal-Dependent Splicing of Tissue Factor Pre-MRNA Modulates the Thrombogenicity of Human Platelets. J. Exp. Med. 2006;203:2433–2440. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brown G.T., Narayanan P., Li W., Silverstein R.L., McIntyre T.M. Lipopolysaccharide Stimulates Platelets through an IL-1β Autocrine Loop. J. Immunol. 2013;191:5196–5203. doi: 10.4049/jimmunol.1300354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brown G.T., McIntyre T.M. Lipopolysaccharide Signaling without a Nucleus: Kinase Cascades Stimulate Platelet Shedding of Proinflammatory IL-1β-Rich Microparticles. J. Immunol. 2011;186:5489–5496. doi: 10.4049/jimmunol.1001623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lindemann S., Tolley N.D., Dixon D.A., McIntyre T.M., Prescott S.M., Zimmerman G.A., Weyrich A.S. Activated Platelets Mediate Inflammatory Signaling by Regulated Interleukin 1beta Synthesis. J. Cell Biol. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Denis M.M., Tolley N.D., Bunting M., Schwertz H., Jiang H., Lindemann S., Yost C.C., Rubner F.J., Albertine K.H., Swoboda K.J., et al. Escaping the Nuclear Confines: Signal-Dependent Pre-MRNA Splicing in Anucleate Platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Beaulieu L.M., Lin E., Mick E., Koupenova M., Weinberg E.O., Kramer C.D., Genco C.A., Tanriverdi K., Larson M.G., Benjamin E.J., et al. Interleukin 1 Receptor 1 and Interleukin 1β Regulate Megakaryocyte Maturation, Platelet Activation, and Transcript Profile during Inflammation in Mice and Humans. Arterioscler. Thromb. Vasc. Biol. 2014;34:552–564. doi: 10.1161/ATVBAHA.113.302700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Qiao J., Wu X., Luo Q., Wei G., Xu M., Wu Y., Liu Y., Li X., Zi J., Ju W., et al. NLRP3 Regulates Platelet Integrin AIIbβ3 Outside-in Signaling, Hemostasis and Arterial Thrombosis. Haematologica. 2018;103:1568–1576. doi: 10.3324/haematol.2018.191700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ivanov I.I., Apta B.H.R., Bonna A.M., Harper M.T. Platelet P-Selectin Triggers Rapid Surface Exposure of Tissue Factor in Monocytes. Sci. Rep. 2019;9:13397. doi: 10.1038/s41598-019-49635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mackman N., Tilley R.E., Key N.S. Role of the Extrinsic Pathway of Blood Coagulation in Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2007;27:1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 172.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 Infection: The Role of Cytokines in COVID-19 Disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Patko Z., Csaszar A., Acsady G., Peter K., Schwarz M. Roles of Mac-1 and Glycoprotein IIb/IIIa Integrins in Leukocyte-Platelet Aggregate Formation: Stabilization by Mac-1 and Inhibition by GpIIb/IIIa Blockers. Platelets. 2012;23:368–375. doi: 10.3109/09537104.2011.625098. [DOI] [PubMed] [Google Scholar]
- 174.Ehlers R., Ustinov V., Chen Z., Zhang X., Rao R., Luscinskas F.W., Lopez J., Plow E., Simon D.I. Targeting Platelet-Leukocyte Interactions: Identification of the Integrin Mac-1 Binding Site for the Platelet Counter Receptor Glycoprotein Ibalpha. J. Exp. Med. 2003;198:1077–1088. doi: 10.1084/jem.20022181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Simon D.I., Chen Z., Xu H., Li C.Q., Dong J.F., McIntire L.V., Ballantyne C.M., Zhang L., Furman M.I., Berndt M.C., et al. Platelet Glycoprotein Ibalpha Is a Counterreceptor for the Leukocyte Integrin Mac-1 (CD11b/CD18) J. Exp. Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lipińska-Gediga M. Platelets in Sepsis—Are There Any New Aspects? Anaesthesiol. Intensive Ther. 2017;49:167–172. doi: 10.5603/AIT.a2017.0025. [DOI] [PubMed] [Google Scholar]
- 177.Taus F., Salvagno G., Canè S., Fava C., Mazzaferri F., Carrara E., Petrova V., Barouni R.M., Dima F., Dalbeni A., et al. Platelets Promote Thromboinflammation in SARS-CoV-2 Pneumonia. Arterioscler. Thromb. Vasc. Biol. 2020;40:2975. doi: 10.1161/ATVBAHA.120.315175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang J., Tecson K.M., McCullough P.A. Endothelial Dysfunction Contributes to COVID-19-Associated Vascular Inflammation and Coagulopathy. Rev. Cardiovasc. Med. 2020;21:315–319. doi: 10.31083/j.rcm.2020.03.126. [DOI] [PubMed] [Google Scholar]
- 179.le Joncour A., Biard L., Vautier M., Bugaut H., Mekinian A., Maalouf G., Vieira M., Marcelin A.G., Rosenzwajg M., Klatzmann D., et al. Neutrophil–Platelet and Monocyte–Platelet Aggregates in COVID-19 Patients. Thromb. Haemost. 2020;120:1733. doi: 10.1055/s-0040-1718732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pão C.R.R., Righy C., Franco S., Souza T.M.L., Kurtz P., et al. Platelet Activation and Platelet-Monocyte Aggregate Formation Trigger Tissue Factor Expression in Patients with Severe COVID-19. Blood. 2020;136:1330. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Semple J.W., Italiano J.E., Freedman J. Platelets and the Immune Continuum. Nat. Rev. Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 182.Reid V.L., Webster N.R. Role of Microparticles in Sepsis. Br. J. Anaesth. 2012;109:503–513. doi: 10.1093/bja/aes321. [DOI] [PubMed] [Google Scholar]
- 183.de Stoppelaar S.F., van ’t Veer C., van der Poll T. The Role of Platelets in Sepsis. Thromb. Haemost. 2014;112:666–677. doi: 10.1160/TH14-02-0126. [DOI] [PubMed] [Google Scholar]
- 184.Canzano P., Brambilla M., Porro B., Cosentino N., Tortorici E., Vicini S., Poggio P., Cascella A., Pengo M.F., Veglia F., et al. Platelet and Endothelial Activation as Potential Mechanisms Behind the Thrombotic Complications of COVID-19 Patients. JACC Basic Transl. Sci. 2021;6:202–218. doi: 10.1016/j.jacbts.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rosell A., Havervall S., von Meijenfeldt F., Hisada Y., Aguilera K., Grover S.P., Lisman T., Mackman N., Thålin C. Patients With COVID-19 Have Elevated Levels of Circulating Extracellular Vesicle Tissue Factor Activity That Is Associated With Severity and Mortality-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2021;41:878–882. doi: 10.1161/ATVBAHA.120.315547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zahran A.M., El-Badawy O., Ali W.A., Mahran Z.G., Mahran E.E.M.O., Rayan A. Circulating Microparticles and Activated Platelets as Novel Prognostic Biomarkers in COVID-19; Relation to Cancer. PLoS ONE. 2021;16:e0246806. doi: 10.1371/journal.pone.0246806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Record M., Carayon K., Poirot M., Silvente-Poirot S. Exosomes as New Vesicular Lipid Transporters Involved in Cell-Cell Communication and Various Pathophysiologies. Biochim. Biophys. Acta. 2014;1841:108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 188.Köffel R., Wolfmeier H., Larpin Y., Besançon H., Schoenauer R., Babiychuk V.S., Drücker P., Pabst T., Mitchell T.J., Babiychuk E.B., et al. Host-Derived Microvesicles Carrying Bacterial Pore-Forming Toxins Deliver Signals to Macrophages: A Novel Mechanism of Shaping Immune Responses. Front. Immunol. 2018;9:1688. doi: 10.3389/fimmu.2018.01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Varon D., Shai E. Platelets and Their Microparticles as Key Players in Pathophysiological Responses. J. Thromb. Haemost. 2015;13((Suppl. 1)):S40–S46. doi: 10.1111/jth.12976. [DOI] [PubMed] [Google Scholar]
- 190.Kuckleburg C.J., Tiwari R., Czuprynski C.J. Endothelial Cell Apoptosis Induced by Bacteria-Activated Platelets Requires Caspase-8 and -9 and Generation of Reactive Oxygen Species. Thromb. Haemost. 2008;99:363–372. doi: 10.1160/TH07-07-0474. [DOI] [PubMed] [Google Scholar]
- 191.Azevedo L.C.P., Janiszewski M., Pontieri V., de Almeida Pedro M., Bassi E., Tucci P.J.F., Laurindo F.R.M. Platelet-Derived Exosomes from Septic Shock Patients Induce Myocardial Dysfunction. Crit. Care. 2007;11:R120. doi: 10.1186/cc6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Janiszewski M., do Carmo A.O., Pedro M.A., Silva E., Knobel E., Laurindo F.R.M. Platelet-Derived Exosomes of Septic Individuals Possess Proapoptotic NAD(P)H Oxidase Activity: A Novel Vascular Redox Pathway. Crit. Care Med. 2004;32:818–825. doi: 10.1097/01.CCM.0000114829.17746.19. [DOI] [PubMed] [Google Scholar]
- 193.Munteanu D., Negru A., Mihailescu R., Tiliscan C., Tudor A.-M., Lazăr M., Aramă Ș.S., Ion D., Popescu C., Aramă V. Evaluation of Bone Mineral Density and Correlations with Inflammation Markers in Romanian HIV-Positive Patients Undergoing Combined Antiretroviral Therapy. Farmacia. 2017;65:114–119. [Google Scholar]
- 194.Barbu E.C., Chiţu-Tișu C.E., Lazăr M., Olariu C.M., Olteanu D., Bojincă M., Abagiu A.O., Aramă V., Ion D.A., Bădărău I.A. Body Composition Changes in Patients with Chronic Hepatitis C. J. Gastrointest. Liver Dis. 2016;25:323–329. doi: 10.15403/jgld.2014.1121.253.hpc. [DOI] [PubMed] [Google Scholar]
- 195.Garcia C., Compagnon B., Poëtte M., Gratacap M.P., Lapébie F.X., Voisin S., Minville V., Payrastre B., Vardon-Bounes F., Ribes A. Platelet Versus Megakaryocyte: Who Is the Real Bandleader of Thromboinflammation in Sepsis? Cells. 2022;11:1507. doi: 10.3390/cells11091507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pariser D.N., Hilt Z.T., Ture S.K., Blick-Nitko S.K., Looney M.R., Cleary S.J., Roman-Pagan E., Saunders J., Georas S.N., Veazey J., et al. Lung Megakaryocytes Are Immune Modulatory Cells. J. Clin. Invest. 2021;131:e137377. doi: 10.1172/JCI137377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kaser A., Brandacher G., Steurer W., Kaser S., Offner F.A., Zoller H., Theurl I., Widder W., Molnar C., Ludwiczek O., et al. Interleukin-6 Stimulates Thrombopoiesis through Thrombopoietin: Role in Inflammatory Thrombocytosis. Blood. 2001;98:2720–2725. doi: 10.1182/blood.V98.9.2720. [DOI] [PubMed] [Google Scholar]
- 198.Valet C., Magnen M., Qiu L., Cleary S.J., Wang K.M., Ranucci S., Grockowiak E., Boudra R., Conrad C., Seo Y., et al. Sepsis Promotes Splenic Production of a Protective Platelet Pool with High CD40 Ligand Expression. J. Clin. Invest. 2022;132:e153920. doi: 10.1172/JCI153920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zufferey A., Speck E.R., Machlus K.R., Aslam R., Guo L., McVey M.J., Kim M., Kapur R., Boilard E., Italiano J.E., et al. Mature Murine Megakaryocytes Present Antigen-MHC Class I Molecules to T Cells and Transfer Them to Platelets. Blood Adv. 2017;1:1773–1785. doi: 10.1182/bloodadvances.2017007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang J., Xie J., Wang D., Han X., Chen M., Shi G., Jiang L., Zhao M. CXCR4high Megakaryocytes Regulate Host-Defense Immunity against Bacterial Pathogens. Elife. 2022;11:e78662. doi: 10.7554/eLife.78662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chiţu-Tişu C.-E., Barbu E.-C., Lazăr M., Ionescu R., Bojinca M., Ion D., Bădărău I.A. An Overview of Bone Disease in HIV-Infected Patients. Acta Med. Mediterr. 2015;31:1139–1151. [Google Scholar]
- 202.Comer S.P., Cullivan S., Szklanna P.B., Weiss L., Cullen S., Kelliher S., Smolenski A., Murphy C., Altaie H., Curran J., et al. COVID-19 Induces a Hyperactive Phenotype in Circulating Platelets. PLoS Biol. 2021;19:e3001109. doi: 10.1371/journal.pbio.3001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ushiyama A., Kataoka H., Iijima T. Glycocalyx and Its Involvement in Clinical Pathophysiologies. J. Intensive Care. 2016;4:59. doi: 10.1186/s40560-016-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Endo S., Shimazaki R., Gando S., Oda S., Ootomo Y., Miura M., Ogura S., Eguchi Y., Kotani J., Yamashita N., et al. An Open-Label, Randomized, Phase 3 Study of the Efficacy and Safety of Antithrombin Gamma in Patients with Sepsis-Induced Disseminated Intravascular Coagulation Syndrome. J. Intensive Care. 2018;6:75. doi: 10.1186/s40560-018-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ito T., Maruyama I., Shimazaki S., Yamamoto Y., Aikawa N., Hirayama A., Honda G., Saito H. Effects of Thrombomodulin Alfa on Hemostatic Parameters in Disseminated Intravascular Coagulation: Post Hoc Analysis of a Phase 3 Randomized Controlled Trial. Res. Pract. Thromb. Haemost. 2020;4:1141–1149. doi: 10.1002/rth2.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Higgins S.J., de Ceunynck K., Kellum J.A., Chen X., Gu X., Chaudhry S.A., Schulman S., Libermann T.A., Lu S., Shapiro N.I., et al. Tie2 Protects the Vasculature against Thrombus Formation in Systemic Inflammation. J. Clin. Invest. 2018;128:1471–1484. doi: 10.1172/JCI97488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020;383:120. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Khakpour S., Wilhelmsen K., Hellman J. Vascular Endothelial Cell Toll-like Receptor Pathways in Sepsis. Innate Immun. 2015;21:827–846. doi: 10.1177/1753425915606525. [DOI] [PubMed] [Google Scholar]
- 209.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado del Pozo C., Prosper F., et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell. 2020;181:905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Smadja D.M., Guerin C.L., Chocron R., Yatim N., Boussier J., Gendron N., Khider L., Hadjadj J., Goudot G., Debuc B., et al. Angiopoietin-2 as a Marker of Endothelial Activation Is a Good Predictor Factor for Intensive Care Unit Admission of COVID-19 Patients. Angiogenesis. 2020;23:611–620. doi: 10.1007/s10456-020-09730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhou J., Li Y.S., Chien S. Shear Stress-Initiated Signaling and Its Regulation of Endothelial Function. Arterioscler. Thromb. Vasc. Biol. 2014;34:2191–2198. doi: 10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Orr A.W., Sanders J.M., Bevard M., Coleman E., Sarembock I.J., Schwartz M.A. The Subendothelial Extracellular Matrix Modulates NF-KappaB Activation by Flow: A Potential Role in Atherosclerosis. J. Cell Biol. 2005;169:191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kutikhin A.G., Sinitsky M.Y., Yuzhalin A.E., Velikanova E.A. Shear Stress: An Essential Driver of Endothelial Progenitor Cells. J. Mol. Cell Cardiol. 2018;118:46–69. doi: 10.1016/j.yjmcc.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 214.O’Sullivan J.M., Gonagle D.M., Ward S.E., Preston R.J.S., O’Donnell J.S. Endothelial Cells Orchestrate COVID-19 Coagulopathy. Lancet Haematol. 2020;7:e553–e555. doi: 10.1016/S2352-3026(20)30215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Buzhdygan T.P., DeOre B.J., Baldwin-Leclair A., Bullock T.A., McGary H.M., Khan J.A., Razmpour R., Hale J.F., Galie P.A., Potula R., et al. The SARS-CoV-2 Spike Protein Alters Barrier Function in 2D Static and 3D Microfluidic in-Vitro Models of the Human Blood-Brain Barrier. Neurobiol. Dis. 2020;146:105131. doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Thomas M.C., Pickering R.J., Tsorotes D., Koitka A., Sheehy K., Bernardi S., Toffoli B., Nguyen-Huu T.P., Head G.A., Fu Y., et al. Genetic Ace2 Deficiency Accentuates Vascular Inflammation and Atherosclerosis in the ApoE Knockout Mouse. Circ. Res. 2010;107:888–897. doi: 10.1161/CIRCRESAHA.110.219279. [DOI] [PubMed] [Google Scholar]
- 217.Xue J., Thippegowda P.B., Hu G., Bachmaier K., Christman J.W., Malik A.B., Tiruppathi C. NF-KappaB Regulates Thrombin-Induced ICAM-1 Gene Expression in Cooperation with NFAT by Binding to the Intronic NF-KappaB Site in the ICAM-1 Gene. Physiol.Genom. 2009;38:42–53. doi: 10.1152/physiolgenomics.00012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: The Vasculature Unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.van de Veerdonk F.L., Kouijzer I.J.E., de Nooijer A.H., van der Hoeven H.G., Maas C., Netea M.G., Brüggemann R.J.M., van de Veerdonk F.L. Outcomes Associated with Use of a Kinin B2 Receptor Antagonist Among Patients With COVID-19. JAMA Netw. Open. 2020;3:e2017708. doi: 10.1001/jamanetworkopen.2020.17708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement Associated Microvascular Injury and Thrombosis in the Pathogenesis of Severe COVID-19 Infection: A Report of Five Cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Michels A., Albánez S., Mewburn J., Nesbitt K., Gould T.J., Liaw P.C., James P.D., Swystun L.L., Lillicrap D. Histones Link Inflammation and Thrombosis through the Induction of Weibel-Palade Body Exocytosis. J. Thromb. Haemost. 2016;14:2274–2286. doi: 10.1111/jth.13493. [DOI] [PubMed] [Google Scholar]
- 222.Nightingale T., Cutler D. The Secretion of von Willebrand Factor from Endothelial Cells; an Increasingly Complicated Story. J. Thromb. Haemost. 2013;11((Suppl. 1)):192–201. doi: 10.1111/jth.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Escher R., Breakey N., Lämmle B. ADAMTS13 Activity, von Willebrand Factor, Factor VIII and D-Dimers in COVID-19 Inpatients. Thromb. Res. 2020;192:174–175. doi: 10.1016/j.thromres.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Booth K.K., Terrell D.R., Vesely S.K., George J.N. Systemic Infections Mimicking Thrombotic Thrombocytopenic Purpura. Am. J. Hematol. 2011;86:743–751. doi: 10.1002/ajh.22091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Levi M., van der Poll T. Inflammation and Coagulation. Crit. Care Med. 2010;38:26–34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 226.van der Poll T., Parker R.I. Platelet Activation and Endothelial Cell Dysfunction. Crit. Care Clin. 2020;36:233–253. doi: 10.1016/j.ccc.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 227.Keith P., Day M., Perkins L., Moyer L., Hewitt K., Wells A. A Novel Treatment Approach to the Novel Coronavirus: An Argument for the Use of Therapeutic Plasma Exchange for Fulminant COVID-19. Crit. Care. 2020;24:128. doi: 10.1186/s13054-020-2836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Iba T., Levy J.H. Derangement of the Endothelial Glycocalyx in Sepsis. J. Thromb. Haemost. 2019;17:283–294. doi: 10.1111/jth.14371. [DOI] [PubMed] [Google Scholar]
- 229.Bruegger D., Schwartz L., Chappell D., Jacob M., Rehm M., Vogeser M., Christ F., Reichart B., Becker B.F. Release of Atrial Natriuretic Peptide Precedes Shedding of the Endothelial Glycocalyx Equally in Patients Undergoing On- and off-Pump Coronary Artery Bypass Surgery. Basic Res. Cardiol. 2011;106:1111–1121. doi: 10.1007/s00395-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 230.Joffre J., Hellman J., Ince C., Ait-Oufella H. Endothelial Responses in Sepsis. Am. J. Respir. Crit. Care Med. 2020;202:361–370. doi: 10.1164/rccm.201910-1911TR. [DOI] [PubMed] [Google Scholar]
- 231.Ito T., Kakuuchi M., Maruyama I. Endotheliopathy in Septic Conditions: Mechanistic Insight into Intravascular Coagulation. Crit. Care. 2021;25:95. doi: 10.1186/s13054-021-03524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Vollenberg R., Tepasse P.R., Ochs K., Floer M., Strauss M., Rennebaum F., Kabar I., Rovas A., Nowacki T. Indications of Persistent Glycocalyx Damage in Convalescent COVID-19 Patients: A Prospective Multicenter Study and Hypothesis. Viruses. 2021;13:2324. doi: 10.3390/v13112324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang D., Li L., Chen Y., Ma J., Yang Y., Aodeng S., Cui Q., Wen K., Xiao M., Xie J., et al. Syndecan-1, an Indicator of Endothelial Glycocalyx Degradation, Predicts Outcome of Patients Admitted to an ICU with COVID-19. Mol. Med. 2021;27:151. doi: 10.1186/s10020-021-00412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Eslamifar Z., Behzadifard M., Soleimani M., Behzadifard S. Coagulation Abnormalities in SARS-CoV-2 Infection: Overexpression Tissue Factor. Thromb. J. 2020;18:38. doi: 10.1186/s12959-020-00250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rodríguez Y., Novelli L., Rojas M., de Santis M., Acosta-Ampudia Y., Monsalve D.M., Ramírez-Santana C., Costanzo A., Ridgway W.M., Ansari A.A., et al. Autoinflammatory and Autoimmune Conditions at the Crossroad of COVID-19. J. Autoimmun. 2020;114:102506. doi: 10.1016/j.jaut.2020.102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.McGonagle D., O’Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune Mechanisms of Pulmonary Intravascular Coagulopathy in COVID-19 Pneumonia. Lancet Rheumatol. 2020;2:e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Venkata C., Kashyap R., Christopher Farmer J., Afessa B. Thrombocytopenia in Adult Patients with Sepsis: Incidence, Risk Factors, and Its Association with Clinical Outcome. J. Intensive Care. 2013;1:9. doi: 10.1186/2052-0492-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Levi M., Schultz M., van der Poll T. Sepsis and Thrombosis. Semin. Thromb. Hemost. 2013;39:559–566. doi: 10.1055/S-0033-1343894. [DOI] [PubMed] [Google Scholar]
- 239.Taylor F.B., Jr., Toh C.-H., Hoots W.K., Wada H., Levi M. Towards Definition, Clinical and Laboratory Criteria, and a Scoring System for Disseminated Intravascular Coagulation. Thromb. Haemost. 2001;86:1327–1330. doi: 10.1055/s-0037-1616068. [DOI] [PubMed] [Google Scholar]
- 240.Schmitt F.C.F., Manolov V., Morgenstern J., Fleming T., Heitmeier S., Uhle F., Al-Saeedi M., Hackert T., Bruckner T., Schöchl H., et al. Acute Fibrinolysis Shutdown Occurs Early in Septic Shock and Is Associated with Increased Morbidity and Mortality: Results of an Observational Pilot Study. Ann. Intensive Care. 2019;9:19. doi: 10.1186/s13613-019-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Iba T., Umemura Y., Wada H., Levy J.H. Roles of Coagulation Abnormalities and Microthrombosis in Sepsis: Pathophysiology, Diagnosis, and Treatment. Arch. Med. Res. 2021;52:788–797. doi: 10.1016/j.arcmed.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 242.Toh C.H., Dennis M. Disseminated Intravascular Coagulation: Old Disease, New Hope. BMJ. 2003;327:974–977. doi: 10.1136/bmj.327.7421.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Levi M. The Coagulant Response in Sepsis. Clin. Chest Med. 2008;29:627–642. doi: 10.1016/j.ccm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 244.Asakura H., Takahashi H., Uchiyama T., Eguchi Y., Okamoto K., Kawasugi K., Madoiwa S., Wada H. Proposal for New Diagnostic Criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb. J. 2016;14:42. doi: 10.1186/s12959-016-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Iba T., di Nisio M., Levy J.H., Kitamura N., Thachil J. New Criteria for Sepsis-Induced Coagulopathy (SIC) Following the Revised Sepsis Definition: A Retrospective Analysis of a Nationwide Survey. BMJ Open. 2017;7:e017046. doi: 10.1136/bmjopen-2017-017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Moisa E., Corneci D., Negutu M.I., Filimon C.R., Serbu A., Popescu M., Negoita S., Grintescu I.M. Development and Internal Validation of a New Prognostic Model Powered to Predict 28-Day All-Cause Mortality in ICU COVID-19 Patients—The COVID-SOFA Score. J. Clin. Med. 2022;11:4160. doi: 10.3390/jcm11144160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Raschke R.A., Agarwal S., Rangan P., Heise C.W., Curry S.C. Discriminant Accuracy of the SOFA Score for Determining the Probable Mortality of Patients With COVID-19 Pneumonia Requiring Mechanical Ventilation. JAMA. 2021;325:1469. doi: 10.1001/jama.2021.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lazar M., Barbu E.C., Chitu C.E., Anghel A.M.J., Niculae C.M., Manea E.D., Damalan A.C., Bel A.A., Patrascu R.E., Hristea A., et al. Mortality Predictors in Severe SARS-CoV-2 Infection. Medicina. 2022;58:945. doi: 10.3390/medicina58070945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Belen-Apak F.B., Sarıalioğlu F. Pulmonary Intravascular Coagulation in COVID-19: Possible Pathogenesis and Recommendations on Anticoagulant/Thrombolytic Therapy. J. Thromb. Thrombolysis. 2020;50:278. doi: 10.1007/s11239-020-02129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The Role of Cytokines Including Interleukin-6 in COVID-19 Induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Asakura H., Ogawa H. COVID-19-Associated Coagulopathy and Disseminated Intravascular Coagulation. Int. J. Hematol. 2021;113:45. doi: 10.1007/s12185-020-03029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., Pesenti A., Peyvandi F., Tripodi A. Hypercoagulability of COVID-19 Patients in Intensive Care Unit: A Report of Thromboelastography Findings and Other Parameters of Hemostasis. J. Thromb. Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., Bikdeli B., Ahluwalia N., Ausiello J.C., Wan E.Y., et al. Extrapulmonary Manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 254.Kohansal Vajari M., Shirin M., Pourbagheri-Sigaroodi A., Akbari M.E., Abolghasemi H., Bashash D. COVID-19-Related Coagulopathy: A Review of Pathophysiology and Pharmaceutical Management. Cell Biol. Int. 2021;45:1832–1850. doi: 10.1002/cbin.11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Iba T., Connors J.M., Nagaoka I., Levy J.H. Recent Advances in the Research and Management of Sepsis-Associated DIC. Int. J. Hematol. 2021;113:24. doi: 10.1007/s12185-020-03053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ibañez C., Perdomo J., Calvo A., Ferrando C., Reverter J.C., Tassies D., Blasi A. High D Dimers and Low Global Fibrinolysis Coexist in COVID19 Patients: What Is Going on in There? J. Thromb. Thrombolysis. 2021;51:308–312. doi: 10.1007/s11239-020-02226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Levi M., Iba T. COVID-19 Coagulopathy: Is It Disseminated Intravascular Coagulation? Intern. Emerg. Med. 2021;16:309. doi: 10.1007/s11739-020-02601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hardy M., Bareille M., Lecompte T., Mullier F. Don’t Let D-Dimer Fool You: Elevated D-Dimer Plasma Levels Should Not Imply ‘Hyperfibrinolysis’. Thromb. Res. 2022;214:63. doi: 10.1016/j.thromres.2022.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hosseini S., Behnam-Roudsari S., Alavinia G., Emami A., Toghyani A., Moradi S., Zadeh M., Mohseni S., Shafiee M. Diagnostic and Prognostic Value of Sepsis-Induced Coagulopathy and International Society on Thrombosis and Hemostasis Scoring Systems in COVID-19-Associated Disseminated Intravascular Coagulopathy. J. Res. Med. Sci. 2021;26:102. doi: 10.4103/JRMS.JRMS_1295_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lippi G., Plebani M., Henry B.M. Thrombocytopenia Is Associated with Severe Coronavirus Disease 2019 (COVID-19) Infections: A Meta-Analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Bazzan M., Montaruli B., Sciascia S., Cosseddu D., Norbiato C., Roccatello D. Low ADAMTS 13 Plasma Levels Are Predictors of Mortality in COVID-19 Patients. Intern. Emerg. Med. 2020;15:861–863. doi: 10.1007/s11739-020-02394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Levi M., Meijers J.C. DIC: Which Laboratory Tests Are Most Useful. Blood Rev. 2011;25:33–37. doi: 10.1016/j.blre.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 264.Semeraro F., Colucci M., Caironi P., Masson S., Ammollo C.T., Teli R., Semeraro N., Magnoli M., Salati G., Isetta M., et al. Platelet Drop and Fibrinolytic Shutdown in Patients with Sepsis. Crit. Care Med. 2018;46:E221–E228. doi: 10.1097/CCM.0000000000002919. [DOI] [PubMed] [Google Scholar]
- 265.Rodelo J.R., de La Rosa G., Valencia M.L., Ospina S., Arango C.M., Gómez C.I., García A., Nuñez E., Jaimes F.A. D-Dimer Is a Significant Prognostic Factor in Patients with Suspected Infection and Sepsis. Am. J. Emerg. Med. 2012;30:1991–1999. doi: 10.1016/j.ajem.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 266.Schwameis M., Steiner M.M., Schoergenhofer C., Lagler H., Buchtele N., Jilma-Stohlawetz P., Boehm T., Jilma B. D-Dimer and Histamine in Early Stage Bacteremia: A Prospective Controlled Cohort Study. Eur. J. Intern. Med. 2015;26:782–786. doi: 10.1016/j.ejim.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 267.Levi M., Vincent J.L., Tanaka K., Radford A.H., Kayanoki T., Fineberg D.A., Hoppensteadt D., Fareed J. Effect of a Recombinant Human Soluble Thrombomodulin on Baseline Coagulation Biomarker Levels and Mortality Outcome in Patients with Sepsis-Associated Coagulopathy. Crit. Care Med. 2020;48:1140–1147. doi: 10.1097/CCM.0000000000004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Semeraro F., Ammollo C.T., Caironi P., Masson S., Latini R., Panigada M., Semeraro N., Gattinoni L., Colucci M. Low D-Dimer Levels in Sepsis: Good or Bad? Thromb. Res. 2019;174:13–15. doi: 10.1016/j.thromres.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 269.Han Y.Q., Yan L., Zhang L., Ouyang P.H., Li P., Lippi G., Hu Z. de Performance of D-Dimer for Predicting Sepsis Mortality in the Intensive Care Unit. Biochem. Med. 2021;31:020709. doi: 10.11613/BM.2021.020709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Bakhtiari K., Meijers J.C.M., de Jonge E., Levi M. Prospective Validation of the International Society of Thrombosis and Haemostasis Scoring System for Disseminated Intravascular Coagulation. Crit. Care Med. 2004;32:2416–2421. doi: 10.1097/01.CCM.0000147769.07699.E3. [DOI] [PubMed] [Google Scholar]
- 271.Levi M., de Jonge E., Meijers J. The Diagnosis of Disseminated Intravascular Coagulation. Blood Rev. 2002;16:217–223. doi: 10.1016/S0268-960X(02)00032-2. [DOI] [PubMed] [Google Scholar]
- 272.Hunt B.J., Levi M. Re The Source of Elevated Plasma D-dimer Levels in COVID-19 Infection. Br. J. Haematol. 2020;190:e133. doi: 10.1111/bjh.16907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Levi M., Hunt B.J. Thrombosis and Coagulopathy in COVID-19: An Illustrated Review. Res. Pract. Thromb. Haemost. 2020;4:744–751. doi: 10.1002/rth2.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Levi M., Thachil J., Iba T., Levy J.H. Coagulation Abnormalities and Thrombosis in Patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tang N., Li D., Wang X., Sun Z. Abnormal Coagulation Parameters Are Associated with Poor Prognosis in Patients with Novel Coronavirus Pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Han H., Yang L., Liu R., Liu F., Liu F., Wu K.L., Li J., Liu X.H., Zhu C.L. Prominent Changes in Blood Coagulation of Patients with SARS-CoV-2 Infection. Clin. Chem. Lab. Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 278.Amgalan A., Othman M. Hemostatic Laboratory Derangements in COVID-19 with a Focus on Platelet Count. Platelets. 2020;31:740–745. doi: 10.1080/09537104.2020.1768523. [DOI] [PubMed] [Google Scholar]
- 279.Yang X., Yang Q., Wang Y., Wu Y., Xu J., Yu Y., Shang Y. Thrombocytopenia and Its Association with Mortality in Patients with COVID-19. J. Thromb. Haemost. 2020;18:1469–1472. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wool G.D., Miller J.L. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology. 2021;88:15–27. doi: 10.1159/000512007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Asakura H. Classifying Types of Disseminated Intravascular Coagulation: Clinical and Animal Models. J. Intensive Care. 2014;2:20. doi: 10.1186/2052-0492-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M., et al. Pulmonary Post-Mortem Findings in a Series of COVID-19 Cases from Northern Italy: A Two-Centre Descriptive Study. Lancet Infect. Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Müller M.C., Meijers J.C.M., Vroom M.B., Juffermans N.P. Utility of Thromboelastography and/or Thromboelastometry in Adults with Sepsis: A Systematic Review. Crit. Care. 2014;18:R30. doi: 10.1186/cc13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Haase N., Ostrowski S.R., Wetterslev J., Lange T., Møller M.H., Tousi H., Steensen M., Pott F., Søe-Jensen P., Nielsen J., et al. Thromboelastography in Patients with Severe Sepsis: A Prospective Cohort Study. Intensive Care Med. 2015;41:77–85. doi: 10.1007/s00134-014-3552-9. [DOI] [PubMed] [Google Scholar]
- 285.Ostrowski S.R., Windeløv N.A., Ibsen M., Haase N., Perner A., Johansson P.I. Consecutive Thrombelastography Clot Strength Profiles in Patients with Severe Sepsis and Their Association with 28-Day Mortality: A Prospective Study. J. Crit. Care. 2013;28:317.e1–317.e11. doi: 10.1016/j.jcrc.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 286.Johansson P.I., Stensballe J., Vindeløv N., Perner A., Espersen K. Hypocoagulability, as Evaluated by Thrombelastography, at Admission to the ICU Is Associated with Increased 30-Day Mortality. Blood Coagul. Fibrinolysis. 2010;21:168–174. doi: 10.1097/MBC.0b013e3283367882. [DOI] [PubMed] [Google Scholar]
- 287.Boscolo A., Spiezia L., Campello E., Bertini D., Lucchetta V., Piasentini E., de Cassai A., Simioni P. Whole-Blood Hypocoagulable Profile Correlates with a Greater Risk of Death within 28 Days in Patients with Severe Sepsis. Korean J. Anesthesiol. 2020;73:224–231. doi: 10.4097/kja.19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Adamzik M., Langemeier T., Frey U.H., Görlinger K., Saner F., Eggebrecht H., Peters J., Hartmann M. Comparison of Thrombelastometry with Simplified Acute Physiology Score Ii and Sequential Organ Failure Assessment Scores for the Prediction of 30-Day Survival: A Cohort Study. Shock. 2011;35:339–342. doi: 10.1097/SHK.0b013e318204bff6. [DOI] [PubMed] [Google Scholar]
- 289.Muzaffar S.N., Azim A., Siddiqui S.S. Thromboelastography for Predicting Disseminated Intravascular Coagulation (DIC) in Sepsis. Shock. 2022;57:759. doi: 10.1097/SHK.0000000000001929. [DOI] [PubMed] [Google Scholar]
- 290.Brenner T., Schmidt K., Delang M., Mehrabi A., Bruckner T., Lichtenstern C., Martin E., Weigand M.A., Hofer S. Viscoelastic and Aggregometric Point-of-Care Testing in Patients with Septic Shock—Cross-Links between Inflammation and Haemostasis. Acta Anaesthesiol. Scand. 2012;56:1277–1290. doi: 10.1111/j.1399-6576.2012.02750.x. [DOI] [PubMed] [Google Scholar]
- 291.Sivula M., Pettilä V., Niemi T.T., Varpula M., Kuitunen A.H. Thromboelastometry in Patients with Severe Sepsis and Disseminated Intravascular Coagulation. Blood Coagul. Fibrinolysis. 2009;20:419–426. doi: 10.1097/MBC.0b013e32832a76e1. [DOI] [PubMed] [Google Scholar]
- 292.Kim S.M., Kim S.-I., Yu G., Kim J.S., Hong S.I., Chae B., Shin Y.S., Kim Y.J., Jang S., Kim W.Y. Role of Thromboelastography in the Evaluation of Septic Shock Patients with Normal Prothrombin Time and Activated Partial Thromboplastin Time. Sci. Rep. 2021;11:11833. doi: 10.1038/s41598-021-91221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Pavoni V., Gianesello L., Pazzi M., Stera C., Meconi T., Frigieri F.C. Evaluation of Coagulation Function by Rotation Thromboelastometry in Critically Ill Patients with Severe COVID-19 Pneumonia. J. Thromb. Thrombolysis. 2020;50:281–286. doi: 10.1007/s11239-020-02130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Gergi M., Goodwin A., Freeman K., Colovos C., Volod O. Viscoelastic Hemostasis Assays (VHA) in Septic, Critically Ill COVID-19 Patients: A Practical Guide for Clinicians. Blood Coagul. Fibrinolysis. 2021;32:225. doi: 10.1097/MBC.0000000000000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wright F.L., Vogler T.O., Moore E.E., Moore H.B., Wohlauer M.V., Urban S., Nydam T.L., Moore P.K., McIntyre R.C. Fibrinolysis Shutdown Correlation with Thromboembolic Events in Severe COVID-19 Infection. J. Am. Coll. Surg. 2020;231:193–203.e1. doi: 10.1016/j.jamcollsurg.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.