Abstract
Table olives and olive oils are the main dietary sources of hydroxytyrosol (HT), a natural antioxidant compound that has emerged as a potential aid in protection against cardiovascular risk. Bioavailability studies with olive oils showed that HT is bioavailable from its free form and from conjugated forms such as oleuropein and its aglycone. Still, its low dietary intake, poor bioavailability, and high inter-individual variability after absorption through the gastrointestinal tract hamper its full benefits. In a randomized, controlled, blinded, cross-over study, we investigated the impact of HT metabolism and bioavailability by comparing two olive-derived watery supplements containing different doses of HT (30.58 and 61.48 mg of HT/dosage). Additionally, HT-fortified olive oil was used in the control group. To this aim, plasma and urine samples were evaluated in 12 healthy volunteers following the intake of a single dose of the supplements or fortified olive oil. Blood and urine samples were collected at baseline and at 0.5, 1, 1.5, 2, 4, and 12 h after intake. HT and its metabolites were analyzed using UHPLC-DAD-MS/MS. Pharmacokinetic results showed that dietary HT administered through the food supplements is bioavailable and bioavailability increases with the administered dose. After intake, homovanillic acid, HT-3-O-sulphate, and 3,4-dihydroxyphenylacetic acid are the main metabolites found both in plasma and urine. The maximum concentrations in plasma peaked 30 min after intake. As bioavailability of a compound is a fundamental prerequisite for its effect, these results promise a good potential of both food supplements for protection against oxidative stress and the consequent cardiovascular risk.
Keywords: olive phenolics; hydroxytyrosol; bioavailability; 3,4-dihydroxyphenyl-ethanol; oleuropein; homovanillic acid; hydroxytyrosol sulphate; 3,4-dihydroxyphenylacetic acid; hydroxytyrosol glucuronide
1. Introduction
Olive fruits (Olea europaea L.) contain several types of phenols, mainly tyrosol (Tyr) derivates, phenolic acids, flavonols, and flavones, the contents of which vary with the olive cultivar [1,2], fruit maturity, and time of harvest [3,4]. Hydroxytyrosol (HT; 3,4-dihydroxyphenyl-ethanol) is the main phenolic phytochemical occurring in olives [5]. It occurs either as a simple phenol or esterified with elenolic acid to form oleuropein (Ole) aglycone. It shows potential antioxidant effects, anti-inflammatory effects, and health benefits mainly related to cardiovascular diseases [6,7,8,9]. A health claim for olive oil phenolics has been authorized in the EU. This authorization states that a minimum daily intake of 5 mg of hydroxytyrosol and its derivatives protects low-density lipoprotein (LDL) cholesterol from oxidative damage [10,11]. In fact, it has been shown that olive oil phenolics in general, and HT and its derivatives in particular, bind to LDL cholesterol in humans [12], reducing its oxidation [13,14,15,16].
However, the olive phenolics relevant to health are not only contained in the oil but also in the water-soluble part of the olives. Indeed, HT is a polar molecule only slightly soluble in fats, a feature that hinders its passage through the lipid bilayer membrane in the small intestines.
Clinical evidence related to HT metabolism is based primarily on human studies conducted with olive oils containing different concentrations of naturally present or added olive phenolic compounds [17,18,19,20,21,22,23,24,25,26,27,28]. Overall, these studies showed that HT can be rapidly absorbed from the intestine [19], metabolized in the gut and liver, distributed, and rapidly eliminated via the kidneys. Only a small fraction of free (unchanged) HT is detected in plasma or urine, as HT undergoes several changes through phase I/II metabolism. The main metabolites of the HT transformation result from the direct intestinal phase II conjugation (glucuronidation and sulphation), and from the enzymatic methylation-oxidation that gives rise to homovanillic acid (HVA). Furthermore, enzymatic oxidation of HT via alcohol dehydrogenases and aldehyde dehydrogenases gives rise to 3,4-dihydroxyphenylacetic acid (DOPAC), which can be further methylated via catecholmethyltransferase to form HVA (reviewed in [9,29]). Additionally, and despite their lower concentration in olives, the absorbed Ole and Tyr can be metabolizsed into free HT for absorption in the human body, thus increasing the concentration of HT in circulation [9,29]. Pharmacokinetic data obtained with animal models also point out an extensive and fast uptake of HT, which is distributed in the body and detected dose-dependently in blood and urine as well as in different organs including kidneys, liver, heart, and brain [25,30,31,32,33].
Vegetation water is a by-product of olive oil processing, i.e., the aqueous part of the olive that is separated from the oil. Such liquid by-product is rich in polar phenols, and typically contains 98% of the total phenols from the olive fruit [34]. Olive phenolics are promising functional ingredients for the prevention of cardiovascular disease and inflammatory events. However, in the field of food supplements, the absorption and bioavailability of the active ingredients in humans are important issues that are often underestimated. Moreover, given that the bioavailability of HT and its derivates can be modulated by the matrix with which they are administered [35,36], it is important to clarify whether its absorption, metabolism, and bioavailability occur with a food matrix other than oil before claiming any beneficial implications for human health.
Our study investigates the bioavailability, disposition, and dose response of HT in humans after intake of the proprietary food supplements, Oliphenolia and Oliphenolia bitter. They are produced using the vegetation water generated during olive oil production and contain mainly natural HT as simple phenol or esterified with elenolic acid to form Ole aglycone.
To this end, a randomized, controlled human trial was conducted with 12 healthy volunteers. Kinetics and bioavailability were determined analyzing HT and Ole, as well as their metabolized oxidated-methylated, sulphated, and glucuronidated forms, in both plasma and urine at 0.5 h, 1 h, 1.5 h, 2 h, 4 h, and 12 h after taking 25 mL(one flask) of the liquid supplement, and evaluated as a change from baseline. HT and its metabolites were analyzed with UHPLC-DAD-MS/MS using pure reference standards.
The results indicate that HT is dose-dependently absorbed after intake of the aqueous food supplements; it is metabolised mainly to HVA, HT-3-O-sulphate (HT-3-S), and DOPAC; and it is highly excreted in the urine.
These results promise a good potential of both food supplements for oxidative stress control in vivo. We hypothesized that the food supplements rich in natural HT may provide benefits on risk factors for cardiovascular disease similar to those provided by high-phenolic extra virgin olive oils (EVOOs). Thus, further secondary outcomes of the present study [37] evaluated the potential antioxidant-health benefits of both food supplements, considering the postprandial kinetics of F2α isoprostanes in urine and oxidized LDL in blood, as the HT from these supplements is expected to show positive effects on these markers of lipid peroxidation.
2. Materials and Methods
2.1. Standards and Reagents
HT, Ole, HVA, and DOPAC (all purity ≥ 98%) were from Cayman Chemical (Ann Arbor, MI, USA). Pure HT-3-S (98%) was supplied by ChemCruz (Huissen, The Netherlands); Tyr (purity ≥ 99.5%) was from Sigma-Aldrich (Taufkirchen, Germany). HT-3-G (purity ≥ 97%) was from Biozol (Eching, Germany); citric acid, phosphoric acid, and L(+)-ascorbic acid were from Roth (Karlsruhe, Germany). Water, acetonitrile, methanol, acetic acid, and formic acid (all LC-MS-grade) were purchased from VWR Chemicals (Darmstadt, Germany).
The liquid food supplements Oliphenolia bitter and Oliphenolia, and the EVOO were provided by Fattoria La Vialla S.A.S (Castiglion Fibocchi, Arezzo, Italy). HTEssence Hydroxytyrosol Liquid was kindly provided by Wacker Chemie AG (Munich, Germany).
2.2. Investigational Products (IPs)
The food supplements are derived from olive fruit (Olea europaea L.) vegetation water subjected to filtration and concentration. The general compositions of Oliphenolia bitter (hereafter referred to as IP-1) and Oliphenolia (hereafter IP-2) are shown in Table 1. IP-1 consists of a 94% concentrated vegetation water and 6% lemon juice (Citrus limon L. fructus). IP-2 is composed of 30% further concentrated vegetation water and 70% grape juice (Vitis vinifera L. fructus).
Table 1.
Typical nutritional value of the food supplements, and content of hydroxytyrosol (HT) and derivatives (as free forms) in the food supplements used for the trial. Ole: oleuropein, HVA: homovanillic acid, DOPAC: 3,4-dihydroxyphenylacetic acid. n.d.: not detectable; *: according to producer.
| Nutrional Value * (g/100 mL) | IP-1 | IP-2 |
|---|---|---|
| Energy (kJ) | 75.00 | 555.00 |
| Fat | 0.00 | 0.05 |
| of which saturates | 0.00 | 0.00 |
| Carbohydrate | 4.17 | 32.03 |
| of which sugars | 1.05 | 27.52 |
| Protein | 0.20 | 0.34 |
| Salt | 0.028 | 0.019 |
| Potassium | 0.804 | 0.642 |
| HT and Derivatives (mg/L) | ||
| HT | 1223 | 2459 |
| Ole | 1.65 | 2.99 |
| HVA | n.d | n.d |
| DOPAC | n.d | n.d |
| Total polyphenols * | 10,980 | 10,100 |
Quantification of HT and derivatives in the food supplements was conducted as previously described [38]. The administered dose (25 mL—1 flask) of the food supplements contained 30.6 mg HT: 0.04 mg Ole for IP-1 and 61.5 mg HT: 0.07 mg Ole for IP-2. Additionally, EVOO was spiked with HT to reach a final concentration of 5.77 mg/20 g, concentration recognized to promote human health according to EFSA [11], and used in the control group. The sum of HT and Tyr in the fortified EVOO was further measured after acid hydrolysis by ADSI GmbH (Innsbruck, Austria) via HPLC-UV and resulted equivalent to 12.19 mg (sum of HT + Tyr) per 20 g of fortified EVOO.
Free Tyr, DOPAC and HVA were not detectable in any of the IPs.
2.3. Participants and Study Design
The present study has been registered at ClinicalTrials.gov (identifier: NCT04876261). The protocol was approved by the Ethics Commission of State Medical Association of Rheinland-Pfalz (Mainz, Germany). The clinical study was conducted as set out in the Declaration of Helsinki by daacro GmbH & CO at the Science Park Trier (Trier, Germany). Written informed consent was obtained from all participants.
Volunteers meeting the inclusion and exclusion criteria (Supplementary Table S1) were recruited. Specific diet indications included the requirement to avoid consuming any olive-derived products as well as alcohol and food supplements with hydroxytyrosol, vitamins, minerals, and antioxidants, at least 2–4 days before the first intake and during the whole study. Three days prior to and at each intervention, volunteers avoided moderate or intense physical activity. Volunteers underwent a wash-out period of 6 days between the interventions to avoid interference between the IPs.
The study design was randomized, single-blind, single-dose, three-way cross-over. Twelve healthy male volunteers ingested, after an overnight fast of at least 10 hr, different concentrations of olive phenolics through the respective IP administered with 200 mL of water. Water intake was allowed ad libitum, and a controlled basal diet was administered 2 h after intake.
One volunteer dropped out of the study after the completion of the first intervention period (IP-2) and was subsequently replaced. Considering the data from this subject the sample size for the IP-2 group is 13.
2.4. Sampling
At each intervention visit, a baseline blood sample (9 mL) was collected immediately before administration of the IP. Further blood samples (9 mL each) were collected 0.5, 1, 1.5, 2, 4 and 12 h after the intake. EDTA-plasma samples were obtained and stabilized with 10% V/V of 2 M aqueous citric acid.
At each intervention visit, a baseline urine sample was collected from –240 to 0 min before administration of the IP. A further 6 urine samples were collected after the intervention from 0 to 30 min, 30 to 60 min, 60 to 90 min, 90 to 120 min, 120 to 240 min, and 4 h to 12 h. Urine samples were stabilized with 1.88 g/L of ascorbic acid and their volume was measured in order to be able to convert the proportion of excreted metabolites at each measurement point to the excreted volume in a comparable manner.
Stabilized plasma and urine samples were stored at −80 °C until analysis.
2.5. Sample Processing
The method developed to purify the plasma and concentrate the HT metabolites was based on a previous research study [39]. Prior to analysis, plasma samples were thawed and mixed 1:1 with aqueous phosphoric acid (4%) to reduce phenolic protein interactions. After centrifugation, the supernatant was plated on a 96 well Oasis HLB µElution SPE Plate from Waters (Eschborn, Germany) without conditioning. As washing solutions 200 µL of water and 200 µL of 0.2% acetic acid were added. The phenolic metabolites were eluted with 100 µL of acetonitrile + water (1 + 1) applied in two portions of 50 µL each.
Urine samples were thawed, filtered through 0.20 µm regenerated cellulose filters (Macherey-Nagel, Düren, Germany), and immediately analyzed using UHPLC without further preparation.
Plasma standards were prepared in a control plasma which did not contain the tested compounds; urine standards were prepared in water. These reference standards (5.6–0.02 mg/L) were prepared fresh for each measurement and processed in the same way as the samples.
Peaks of HT metabolites were identified by comparison with reference compounds regarding retention time and MS2 spectra. For quantitation, a linear calibration equation was calculated from the peak area (MS2) vs. concentration plot.
2.6. Analysis of HT and Its Metabolites
All samples were run in duplicate. Measurement was conducted using UHPLC-DAD-MS/MS, with an Acquity UPLC I-Class system coupled to a XEVO-TQS micro mass spectrometer (Waters, Milford, MA, USA). The instrument consisted of a sample manager cooled at 10 °C, a binary pump, a column oven, and a diode array detector (DAD) measuring at 280 nm for confirmation of the peaks. The column oven temperature was set at 40 °C. Eluent B was water with 0.1% formic acid, eluent A was acetonitrile with 0.1% formic acid, and the flow was 0.4 mL/min on an Acquity BEH C18 RP column (50 mm × 2.1 mm, 1.7 µm particle size) combined with an Acquity BEH C18 precolumn (2.1 mm × 5 mm, 1.7 µm), both from Waters (Milford, MA, USA). The gradient started with 1% A for 3 min and rose linearly to 20% A within 1 min, then to 80% A within 1.3 min, then to 100% A in 0.3 min and holding for 1.5 min as a washing step; then back to 1% A within 0.2 min and equilibrating for 1 min. The injection volume was 5 µL.
The peaks were identified using MS/MS in negative mode. The source voltage was kept at 3.4kV, and the cone voltage was 77 V. The source temperature was set at 150 °C and the desolvation temperature at 400 °C with a desolvation gas flow of 800 L/h and a cone gas flow of 50 L/h. Data were acquired and processed using MassLynx 4.1 (Waters, Milford, MA, USA).
2.7. Data Analysis
Each metabolite measurement was conducted in duplicate. The average values of concentration for each of the samples were calculated in Microsoft Excel version 16.0. For the raw statistics, classical statistical methods using median, mean, standard deviation, and confidence intervals were used. Average values were further processed using GraphPad software (San Diego, CA, USA) version 5.00 to represent data in graphs and tables. Pharmacokinetic parameters (maximum plasma concentration Cmax (nmol/L), time to reach Cmax (tmax, h), and mean area under the concentration time curve (AUC0–12h)) were calculated from plasma concentrations, while cumulative concentrations were calculated for the urinary excretion. The data are expressed as mean ± standard error of the mean (SEM) unless otherwise indicated. Student’s unpaired t-test was performed to compare the results before and after ingestion for each test product.
In order to identify potentially hidden data correlations, artificial intelligence algorithms were applied using MATLAB 2019 (Natick, MA, USA) software, including the machine learning and deep learning toolbox version 11.5. This includes the classification of the data using machine learning techniques (such as k-nearest neighbors, decision tree, and support vector machine), deep learning using deep neural networks as well as the analysis of the data using correlation matrix calculations.
3. Results
Table 2 shows the mass transitions used for the quantification and identification of HT and its metabolites as determined by fragmenting standard substances in the chromatograms. These data are further compared to the literature [40]. For HT-S and HT-G only the 3-O-standards were available; as these have the same mass transitions as their 4-O-isomeres, it was concluded that the second peak with the same mass transition detected in a similar retention time window is the 4-O-derivative.
Table 2.
Mass transitions of hydroxytyrosol and its metabolites. HT: hydroxytyrosol, Ole: oleuropein, DOPAC: 3,4-dihydroxyphenylacetic acid, HVA: homovanillic acid, HT-S: hydroxytyrosol sulphates, HT-G: hydroxytyrosol glucuronides.
| Hydroxytyrosol (Metabolites) | [M − H]− (m/z) | Most Abundant Fragment (m/z) (Used for Quantification) |
|---|---|---|
| HT | 153 | 123 |
| Ole | 539 | 275 |
| DOPAC | 167 | 123 |
| HVA | 181 | 137 |
| HT-S | 233 | 153 |
| HT-G | 329 | 153 |
The HT metabolites were separated in a single UHPLC run. The respective limits of quantification (LOQ) were calculated using diluted reference solutions and a signal:noise ratio of 10 (Table 3).
Table 3.
Limit of quantification of HT and its metabolites in plasma and urine. Results are expressed as mean (in mg/L) ± SEM.
| Plasma | Urine | |
|---|---|---|
| HT | 0.02 ± 0.01 | 0.01 ± 0.00 |
| Ole | 0.01 ± 0.00 | 0.01 ± 0.00 |
| HT-3-S | 0.05 ± 0.01 | 0.05 ± 0.00 |
| HVA | 0.08 ± 0.02 | 0.14 ± 0.08 |
| DOPAC | 0.25 ± 0.04 | 0.09 ± 0.03 |
| HT-3-G | 0.64 ± 0.22 | 0.05 ± 0.00 |
Detection of Tyr was interfered due to the co-elution of matrix components; consequently, the method was found to be unsuitable for quantification of Tyr in both plasma and urine.
3.1. HT Metabolites in Plasma
The non-metabolized forms of HT and Ole were almost undetectable inplasma after ingestion of the IPs.
For the fortified EVOO group, the HT metabolites were almost undetectable in all subjects before and after the intake, with the exception of HVA, which was detected (<LOQ) in all but quantifiable only in two subjects before intake and 30 min after.
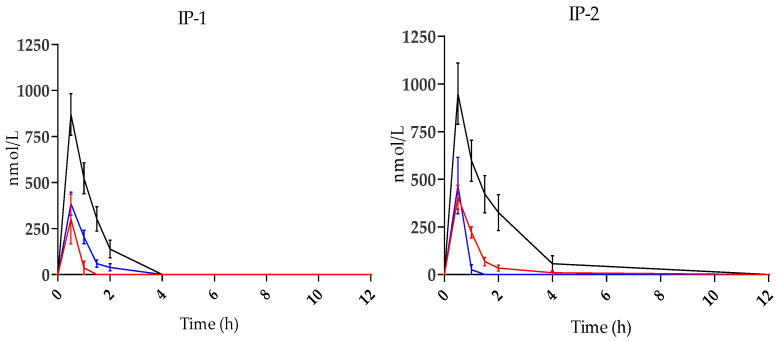
Table 4 illustrates the pharmacokinetic parameters from plasma concentrations of the main metabolites detected after the intake of IP-1 and IP-2. HT was mainly detected as HVA in both the food supplement groups, followed by smaller amounts of HT-3-S and DOPAC.
Table 4.
Pharmacokinetic parameters from plasma concentration. Cmax: peak plasma concentrations expressed in nmol/L ± SEM; tmax: time (in minutes) to reach Cmax; AUC: mean area under the plasma concentration time curve from 0 h to 12 h. Plasma concentrations at baseline (t0) were below the LOQ. n.s: no significant difference between groups.
| HT-3-S | HVA | DOPAC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IP | Cmax | tmax | AUC | Cmax | tmax | AUC | Cmax | tmax | AUC |
| IP-1 | 384.89 n.s ± 62.63 | 30 | 372 n.s | 868.87 n.s ± 111.86 | 30 | 1019 n.s | 301.07 n.s ± 134.43 | 30 | 169 n.s |
| IP-2 | 406.28 n.s ± 62.68 | 30 | 443 n.s | 948.76 n.s ± 160.19 | 30 | 1672 n.s | 466.96 n.s ± 147.68 | 30 | 247 n.s |
As expected, the mean areas under the concentration time curves were higher for IP-2 than for IP-1; however, these differences are not significant (p > 0.05) due to the large intra- and inter-individual variation observed, which is assumed to be mainly due to biological variability.
Comparing the kinetics of plasma concentration of the major metabolites (Figure 1) HVA peaks at 30 min and progressively decreases over the next 3.5 h; HT-3-S reached the maximum concentration 30 min after intake and strongly decreased within 2 h after administration. Of note, HVA and HT-3-S were measurable from 0.5 to 2 h at the lower HT dose and from 0.5 to 4 h at the higher dose. DOPAC showed the maximum concentration at 30 min after the intake of HT through the food supplements followed by a marked decrease until reaching values close to the LOQ one hour after ingestion.
Figure 1.
Mean plasma concentration profile of main HT metabolites before (0 h) and after intake of IP-1 (n = 12) and IP-2 (n = 13). HVA: black lines; HT-3-S: blue lines; DOPAC: red lines. Bars: SEM.
Among the other HT metabolites studied, HT-4-S was undetectable in plasma from all groups, while the glucuronide conjugates were poorly present: HT-3-G was mainly detected in trace amounts (<LOQ) after all the interventions, but measured in one subject at 0.5 and 1 h after intake of IP-2, peaking at 1 h (Cmax: 8.73 nmol/L). HT-4-G was detected (<LOQ) only in few samples after intake of the food supplements but not after intake ofthe fortified EVOO.
3.2. Urinary Excretion of HT Metabolites
The unmetabolized form of Ole was almost undetectable in the urine before and after ingestion of the respective IPs. Free (unchanged) HT was excreted within an hour and in small amounts after the ingestion of IP-1 and IP-2 (0.0004 µmole ± 0.0010 and 0.0024 µmole ± 0.0009, respectively; no significant difference between groups), but not after EVOO intake (p < 0.05 vs. IP-2).
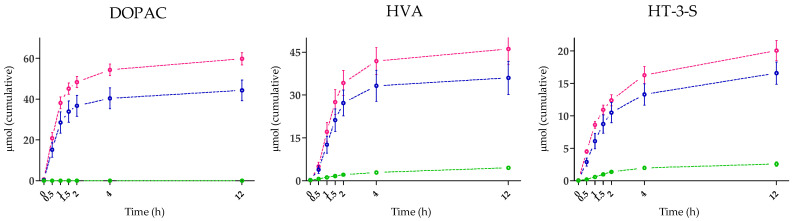
A dose-dependent concentration of all the HT metabolites analyzed was recorded following the IP administration (Table 5). Moreover, the three main metabolites quantified in plasma were extensively excreted in urine after intake in all the interventional groups (Figure 2). For instance, after the ingestion of HT through the aqueous food supplements, the main metabolite excreted was DOPAC, followed by HVA (p < 0.05 vs. DOPAC), and HT-3-S (p < 0.001 vs. HVA), while after EVOO intake the HVA ranked first but was excreted in a similar extent to DOPAC and HT-3-S (not significantly different at the p < 0.05 level).
Table 5.
Mean cumulative (0–12 h) excretion of HT metabolites (micromoles) ± SEM in urine. Significance vs. EVOO was as follows: *: significant at p < 0.001 level, n.s: not significantly different.
| IP | n | HVA | DOPAC | HT-3-S | HT-4-S | HT-3-G | HT-4-G |
|---|---|---|---|---|---|---|---|
| IP-1 | 12 | 36.01 * ± 20.08 | 44.31 * ± 17.52 | 16.58 * ± 6.0 | 5.32 n.s ± 16.23 | 0.46 * ± 0.35 | 0.15 n.s ± 0.10 |
| IP-2 | 13 | 46.16 * ± 5.37 | 59.74 * ± 3.00 | 20.05 * ± 1.55 | 0.16 n.s ± 0.05 | 0.87 * ± 0.13 | 0.41 * ± 0.10 |
| EVOO | 12 | 4.84 ± 3.93 | 4.53 ± 1.76 | 2.57 ± 1.17 | 0.05 ± 0.19 | 0.06 ± 0.09 | 0.01 ± 0.02 |
Figure 2.
Mean urinary excretion of main HT metabolites quantified before (0 h) and after intake of the investigational products. Blue lines: IP-1 (n = 12), pink lines: IP-2 (n = 13), green lines: EVOO (n = 12). Bars: SEM.
The HT-4-S and the glucuronide conjugates were measured after intake in all the interventional groups, but in smaller concentrations than HVA, DOPAC, and HT-3-S. Unlike IP-1, the EVOO and IP-2 groups, containing the lowest and highest dose of HT, respectively, showed a predominant detection of HT-glucuronides over HT-4-S (not significant for EVOO, and p < 0.01 for IP-2).
The mean HT excretion calculated from the accumulated amounts was estimated at 59.6% and 35.8% of the total intake for IP-1 and IP-2, respectively, and 27.6% when administered with HT-enriched EVOO.
4. Discussion
The two food supplements studied here are based on ingredients of vegetable origin, mainly olive concentrate resulting from the aqueous by-product generated during olive oil production, and thus contain HT as the main bioactive component. In addition, they contain lemon juice (IP-1) or grape juice (IP-2) concentrates. Despite olive phenolics being a promising functional ingredient, the bioavailability of HT and its derivates could be modulated by the matrix with which they are administered [35,36]. Therefore, before claiming beneficial properties for human health it is of key importance to verify the absorption, metabolism, and bioavailability of the active ingredient in the context of the food matrix that contains it.
In line with other studies conducted with olive oil and pure HT [12,24,25,41,42,43], our results show that HT administered together with both aqueous food supplements is absorbed from the intestinal tract in varying amounts and rapidly metabolized to phase I and phase II metabolites.
HT metabolites, which were undetected in plasma in the fasting state, were rapidly cleared from plasma in the postprandial phase (Cmax 30 min, complete clearance 2–4 h) and excreted in the urine. The free (unchanged) forms of HT and Ole were almost undetectable in most plasma and urine samples, both before and after the intake of the products by the oral route and regardless of the IP. This result is in line with data in the literature [12,20].
Similar to previous studies reporting a dose-dependent absorption of HT contained in administered oil [17,25,41,42,43], the mean of HT metabolites detected in plasma (as the sum of all quantifiable metabolites) correlated with the ingested dose of HT. This calculation was higher for IP-2 than IP-1 (although this difference did not reach statistical significance), and significantly higher than for fortified EVOO (p < 0.05 vs. IP-1 and IP-2). A large inter-individual variability in the absorption of HT was observed, which has also been reported previously [12,16,19,22,36,44].
The absolute amount of HT in urine (as the sum of all quantifiable metabolites in 12 h) correlated with the dose administered. This degree of correlation for the three different intervention products was fortified EVOO < IP-1 < IP-2. However, the excreted percentage of the total ingested HT was as follows: fortified EVOO < IP-2 < IP-1. Overall the excretion ranged from 28 to 60%, which is consistent with previously published data [25]. Contrary to what is reported in the literature [12,35,36], excretion of the HT administered as a natural component of an aqueous matrix can exceed that of the oily matrix. These results underline the importance of dietary factors influencing bioavailability, such as the possible presence of effectors (positive or negative) in the food matrix and differences in phenol, water, or fat contents, all factors that can influence the absorption of HT itself. Similarly, synergistic interactions with other phenolic components of food matrices cannot be excluded [45].
The highest average concentrations of HT metabolites in plasma (as the sum of all the metabolites) were found 30 min after intake of the food supplements, being significantly different to the intake of EVOO, which only rarely showed levels above the quantification limit. HT is highly metabolized mainly to HVA, HT-3-S, and DOPAC; these metabolites (free forms) could often be detected in plasma samples from the food supplement groups and especially immediately after ingestion, but only very rarely in the fortified-EVOO group. Moreover, after ingestion both DOPAC and HVA, reach the maximum plasma concentration at 30 min for both supplements. DOPAC concentration was lower and its elimination faster than that of HVA; this is likely due to DOPAC’s enzymatic transformation into HVA via catecholmethyltransferase [17,30].
Our findings indicate that regardless of the food matrix (i.e., watery or oily), the free forms of HVA and DOPAC, which result from the oxidation and/or methylation of dietary HT, contribute to a greater extent to the metabolization of HT in the human body. To date, few human studies have looked for the free forms of these metabolites after HT ingestion. Earlier studies conducted with olive oil observed an increase in total HVA (after hydrolysis) in 24 h pooled urine [17] despite the high basal urinary concentration of HVA registered in all volunteers. Another human study conducted after the intake of ~175 mg of pure HT (99.5%) in aqueous solution reported the excretion of DOPAC-glucuronide and HVA in 24 h pooled urine, but not in plasma samples [12]. Overall our results are in line with these previous findings, and in particular with Kontouri and collaborators, who addressed the conversion of HT into DOPAC, HVA, and homovanillic alcohol (both free and total) in humans after the ingestion of olives (fruit). These researchers reported that HT is excreted in urine and plasma mainly as HVA and DOPAC [24].
Among the phase II conjugates, sulphated metabolites (particularly HT-3-S) are preferred over glucuronidated ones. These results are in line with previous studies showing that sulphation at position 3 is preferred to position 4 [31,46], and that sulphates are mainly detected after intake of high HT doses while glucuronates are detected at low dosages [27,31].
Overall, our results show that after ingestion of both aqueous dietary supplements, HT is indeed absorbed and highly metabolized into phase I and phase II metabolites. Moreover, the absence of free HT in the biological samples suggests that its metabolites, especially HVA, DOPAC, and HT-3-S, are more likely to define the positive effects of olive phenolics reported in vivo. These results promise a good potential of both food supplements for the protection against oxidative stress in vivo. Further, the outcomes of this trial let us expect that potential health benefits of both food supplements can be shown in the postprandial phase with the help of lipoxidation markers, as bioavailable HT is expected to counteract lipoxidation.
Acknowledgments
The authors would like to acknowledge the University Hospital Bonn (Germany) for kindly providing plasma samples for the preparation of standard curves in plasma, and Eva Rogel (Institut Kurz GmbH) for her technical support. This research was supported by Fattoria La Vialla, S.A.S. (Castiglion Fibocchi-Arezzo, Italy).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15020325/s1, Table S1: Selection criteria for trial participants.
Author Contributions
C.B.: Conceptualization, investigation, data analysis, original draft writing; S.S.: formal analysis, methodology; C.G.: data review with AI; C.B. and S.S.: review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, approved by the Ethics Committee of State Medical Association of Rheinland-Pfalz (Mainz, Germany), and registered at ClinicalTrials.gov (identifier: NCT04876261).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The sponsor had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rothwell J.A., Pérez-Jiménez J., Neveu V., Medina-Ramon A., M’Hiri N., Garcia Lobato P., Manach C., Knox K., Eisner R., Wishart D., et al. Phenol-Explorer 3.0: A Major Update of the Phenol-Explorer Database to Incorporate Data on the Effects of Food Processing on Polyphenol Content. Database 2013. https://doi.org/10.1093/database/bat070. [(accessed on 3 February 2022)]. Available online: http://www.phenol-explorer.eu. [DOI] [PMC free article] [PubMed]
- 2.Amiot M.J., Fleuriet A., Macheix J.J. Importance and evolution of phenolic compounds in olive during growth and maturation. J. Agric. Food Chem. 1986;34:823–826. doi: 10.1021/jf00071a014. [DOI] [Google Scholar]
- 3.Ryan D., Robards K., Lavee S. Changes in phenolic content of olive during maturation. Int. J. FoodSci. Technol. 1999;34:265–274. doi: 10.1046/j.1365-2621.1999.00261.x. [DOI] [Google Scholar]
- 4.Geropoulos N.K., Kaliora A.C. Olive and Olive Oil Bioactive Constituents. AOCS Press; Urbana, Il, USA: 2015. Effect of Fruit Maturity on Olive Oil Phenolic Composition and Antioxidant Capacity; pp. 123–145. [DOI] [Google Scholar]
- 5.Blekas G., Vassilakis C., Harizanis C., Tsimidou M., Boskou D.G. Biophenolsin table olives. J. Agric. Food Chem. 2002;50:3688–3692. doi: 10.1021/jf0115138. [DOI] [PubMed] [Google Scholar]
- 6.Hohmann C.D., Cramer H., Michalsen A., Kessler C., Steckhan N., Choi K., Dobos G. Effects of high phenolic olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Phytomedicine. 2015;22:631–640. doi: 10.1016/j.phymed.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Covas M.I., de la Torre R., Fitó M. Virgin olive oil: A key food for cardiovascular risk protection. Br. J. Nutr. 2015;113:S19–S28. doi: 10.1017/S0007114515000136. [DOI] [PubMed] [Google Scholar]
- 8.Tejada S., Pinya S., Del Mar Bibiloni M., Tur J.A., Pons A., Sureda A. Cardioprotective Effects of the Polyphenol Hydroxytyrosol from Olive Oil. Curr. Drug Targets. 2017;18:1477–1486. doi: 10.2174/1389450117666161005150650. [DOI] [PubMed] [Google Scholar]
- 9.Karković-Marković A., Torić J., Barbarić M., Jakobušić-Brala C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules. 2019;24:2001. doi: 10.3390/molecules24102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Commission Regulation EC No. 432/2012 Establishing a List of Permitted Health Claims Made on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. [(accessed on 3 February 2022)];Off. J. Eur. Union. 2012 L136:1. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32012R0432. [Google Scholar]
- 11.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties”(ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract”(3779), and “contributes to body defences. EFSA J. 2011;9:2033. [Google Scholar]
- 12.González-Santiago M., Fonollá J., Lopez-Huertas E. Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharmacol. Res. 2010;61:364–370. doi: 10.1016/j.phrs.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Covas M.I., Nyyssönen K., Poulsen H.E., Kaikkonen J., Zunft H.J., Kiesewetter H., Gaddi A., de la Torre R., Mursu J., Bäumler H., et al. EUROLIVE Study Group. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006;145:333–341. doi: 10.7326/0003-4819-145-5-200609050-00006. [DOI] [PubMed] [Google Scholar]
- 14.Fitó M., Guxens M., Corella D., Sáez G., Estruch R., de la Torre R., Francés F., Cabezas C., López-Sabater M.D.C., Marrugat J., et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: A randomized controlled trial. Arch Intern. Med. 2007;167:1195–1203. doi: 10.1001/archinte.167.11.1195. [DOI] [PubMed] [Google Scholar]
- 15.de la Torre-Carbot K., Chávez-Servín J.L., Jaúregui O., Castellote A.I., Lamuela-Raventós R.M., Nurmi T., Poulsen H.E., Gaddi A.V., Kaikkonen J., Zunft H.F., et al. Elevated circulating LDL phenol levels in men who consumed virgin rather than refined olive oil are associated with less oxidation of plasma LDL. J. Nutr. 2010;140:501–508. doi: 10.3945/jn.109.112912. [DOI] [PubMed] [Google Scholar]
- 16.Mateos R., Martínez-López S., BaezaArévalo G., Amigo-Benavent M., Sarriá B., Bravo-Clemente L. Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem. 2016;205:248–256. doi: 10.1016/j.foodchem.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Caruso D., Visioli F., Patelli R., Galli C., Galli G. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism. 2001;50:1426–1428. doi: 10.1053/meta.2001.28073. [DOI] [PubMed] [Google Scholar]
- 18.Miro-Casas E., Covas M.I., Farre M., Fito M., Ortuño J., Weinbrenner T., Roset P., de la Torre R. Hydroxytyrosol disposition in humans. Clin. Chem. 2003;49:945–952. doi: 10.1373/49.6.945. [DOI] [PubMed] [Google Scholar]
- 19.Bonanome A., Pagnan A., Caruso D., Toia A., Xamin A., Fedeli E., Berra B., Zamburlini A., Ursini F., Galli G. Evidence of postprandial absorption of olive oil phenols in humans. Nutr. Metab. Cardiovasc. Dis. 2000;10:111–120. [PubMed] [Google Scholar]
- 20.Miró-Casas E., Covas M.I., Fitó M., Farré-Albadalejo M., Marrugat J., de la Torre R. Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans. Eur. J. Clin. Nutr. 2003;57:186–190. doi: 10.1038/sj.ejcn.1601532. [DOI] [PubMed] [Google Scholar]
- 21.Tuck K.L., Hayball P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002;13:636–644. doi: 10.1016/S0955-2863(02)00229-2. [DOI] [PubMed] [Google Scholar]
- 22.Suárez M., Valls R.M., Romero M.P., Macià A., Fernández S., Giralt M., Solà R., Motilva M.J. Bioavailability of phenols from a phenol-enriched olive oil. Br. J. Nutr. 2011;106:1691–1701. doi: 10.1017/S0007114511002200. [DOI] [PubMed] [Google Scholar]
- 23.Vissers M.N., Zock P.L., Roodenburg A.J., Leenen R., Katan M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002;132:409–417. doi: 10.1093/jn/132.3.409. [DOI] [PubMed] [Google Scholar]
- 24.Kountouri A.M., Mylona A., Kaliora A.C., Andrikopoulos N.K. Bioavailability of the phenolic compounds of the fruits (drupes) of Olea europaea (olives): Impact on plasma antioxidant status in humans. Phytomedicine. 2007;14:659–667. doi: 10.1016/j.phymed.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Visioli F., Galli C., Bornet F., Mattei A., Patelli R., Galli G., Caruso D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000;468:159–160. doi: 10.1016/S0014-5793(00)01216-3. [DOI] [PubMed] [Google Scholar]
- 26.de la Torre R. Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology. 2008;16:245–247. doi: 10.1007/s10787-008-8029-4. [DOI] [PubMed] [Google Scholar]
- 27.Rubió L., Valls R.M., Macià A., Pedret A., Giralt M., Romero M.P., de la Torre R., Covas M.I., Solà R., Motilva M.J. Impact of olive oil phenolic concentration on human plasmatic phenolic metabolites. Food Chem. 2012;135:2922–2929. doi: 10.1016/j.foodchem.2012.07.085. [DOI] [PubMed] [Google Scholar]
- 28.Khymenets O., Farre M., Pujadas M., Ortiz E., Joglar J., Covas M.I., de la Torre R. Direct analysis of glucuronidated metabolites of main olive oil phenols in human urine after dietary consumption of virgin olive oil. Food Chem. 2011;126:306–314. doi: 10.1016/j.foodchem.2010.10.044. [DOI] [Google Scholar]
- 29.Rodríguez-Morató J., Boronat A., Kotronoulas A., Pujadas M., Pastor A., Olesti E., Pérez-Mañá C., Khymenets O., Fitó M., Farré M., et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016;48:218–236. doi: 10.1080/03602532.2016.1179754. [DOI] [PubMed] [Google Scholar]
- 30.D’Angelo S., Manna C., Migliardi V., Mazzoni O., Morrica P., Capasso G., Pontoni G., Galletti P., Zappia V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001;29:1492–1498. [PubMed] [Google Scholar]
- 31.Kotronoulas A., Pizarro N., Serra A., Robledo P., Joglar J., Rubió L., Hernaéz A., Tormos C., Motilva M.J., Fitó M., et al. Dose-dependent metabolic disposition of hydroxytyrosol and formation of mercapturates in rats. Pharmacol. Res. 2013;77:47–56. doi: 10.1016/j.phrs.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 32.López de las Hazas M.C., Rubió L., Kotronoulas A., de la Torre R., Solà R., Motilva M.J. Dose effect on the uptake and accumulation of hydroxytyrosol and its metabolites in target tissues in rats. Mol. Nutr. Food Res. 2015;59:1395–1399. doi: 10.1002/mnfr.201500048. [DOI] [PubMed] [Google Scholar]
- 33.López de las Hazas M.C., Piñol C., Macià A., Romero M.P., Pedret A., Solà R., Rubió L., Motilva M.J. Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. J. Funct. Foods. 2016;22:52–63. doi: 10.1016/j.jff.2016.01.030. [DOI] [Google Scholar]
- 34.Obied H.K., Allen M.S., Bedgood D.R., Prenzler P.D., Robards K., Stockmann R. Bioactivity and analysis of biophenols recovered from olive mill waste. J. Agric. Food Chem. 2005;53:823–837. doi: 10.1021/jf048569x. [DOI] [PubMed] [Google Scholar]
- 35.Visioli F., Galli C., Grande S., Colonnelli K., Patelli C., Galli G., Caruso D. Hydroxytyrosol excretion differs between rats and humans and depends on the vehicle of administration. J. Nutr. 2003;133:2612–2615. doi: 10.1093/jn/133.8.2612. [DOI] [PubMed] [Google Scholar]
- 36.Alemán-Jiménez C., Domínguez-Perles R., Medina S., Prgomet I., López-González I., Simonelli-Muñoz A., Campillo-Cano M., Auñón D., Ferreres F., Gil-Izquierdo Á. Pharmacokinetics and bioavailability of hydroxytyrosol are dependent on the food matrix in humans. Eur. J. Nutr. 2021;60:905–915. doi: 10.1007/s00394-020-02295-0. [DOI] [PubMed] [Google Scholar]
- 37.Bender C., Candi I., Rogel E. Bioefficacy of hydroxytyrosol-rich food supplements on preventing lipid peroxidation in healthy men. bioRxiv. 2022:508834. doi: 10.1101/2022.09.21.508834. [DOI] [Google Scholar]
- 38.Bender C., Straßmann S., Heidrich P. Cellular Antioxidant Effects and Bioavailability of Food Supplements Rich in Hydroxytyrosol. Appl. Sci. 2021;11:4763. doi: 10.3390/app11114763. [DOI] [Google Scholar]
- 39.Feliciano R.P., Mecha E., Bronze M.R., Rodriguez-Mateos A. Development and validation of a high-throughput micro solid-phase extraction method coupled with ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry for rapid identification and quantification of phenolic metabolites in human plasma and urine. J. Chromatogr. A. 2016;1464:21–31. doi: 10.1016/j.chroma.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 40.Suárez M., Romero M.P., Macià A., Valls R.M., Fernández S., Solà R., Motilva M.J. Improved method for identifying and quantifying olive oil phenolic compounds and their metabolites in human plasma by microelution solid-phase extraction plate and liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009;877:4097–4106. doi: 10.1016/j.jchromb.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 41.Marrugat J., Covas M.I., Fitó M., Schröder H., Miró-Casas E., Gimeno E., López-Sabater M.C., de la Torre R., Farré M. Effects of differing phenolic content in dietary olive oils on lipids and LDL oxidation—A randomized controlled trial. Eur. J. Nutr. 2004;43:140–147. doi: 10.1007/s00394-004-0452-8. [DOI] [PubMed] [Google Scholar]
- 42.Weinbrenner T., Fitó M., de la Torre R., Saez G.T., Rijken P., Tormos C., Coolen S., Albaladejo M.F., Abanades S., Schroder H., et al. Olive oils high in phenolic compounds modulate oxidative/antioxidative status in men. J. Nutr. 2004;134:2314–2321. doi: 10.1093/jn/134.9.2314. [DOI] [PubMed] [Google Scholar]
- 43.Covas M.I., de la Torre K., Farré-Albaladejo M., Kaikkonen J., Fitó M., López-Sabater C., Pujadas-Bastardes M.A., Joglar J., Weinbrenner T., Lamuela-Raventós R.M., et al. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free RadicBiol. Med. 2006;40:608–616. doi: 10.1016/j.freeradbiomed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 44.de Bock M., Thorstensen E.B., Derraik J.G., Henderson H.V., Hofman P.L., Cutfield W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013;57:2079–2085. doi: 10.1002/mnfr.201200795. [DOI] [PubMed] [Google Scholar]
- 45.Bohn T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014;72:429–452. doi: 10.1111/nure.12114. [DOI] [PubMed] [Google Scholar]
- 46.Khymenets O., Crespo M., Dangles O., Njara R., Andres-Lacueva C., Visioli F. Human hydroxytyrosol’s absorption and excretion from a nutraceutical. J. Funct. Foods. 2016;23:278–282. doi: 10.1016/j.jff.2016.02.046. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.