Abstract
Here, a QuEChERS (quick, easy, cheap, effective, rugged, and safe) pretreatment method was combined with UPLC-MS/MS to facilitate the rapid and reliable simultaneous detection of five calcium channel blockers (CCBs) in human plasma. For this approach, samples were treated with 1 mL of acetonitrile, 350 mg of magnesium sulfate, and 70 mg of PSA adsorbent prior to centrifugation. Supernatants then underwent gradient elution for 8 min with an Agilent C18 column using an acetonitrile-water solution supplemented with 5 mmol⋅L−1 of ammonium acetate. This technique exhibited a good linear response in the 1–800 ng⋅mL−1 range for the analyzed drugs, with an R2≥ 0.9921, an accuracy of 87.54–113.05%, a matrix effect (ME) of 91.21–116.39%, a precision of 0.19–11.64%, and stability of no more than 10.05%. This time-saving QuEChERS reagent-based pretreatment technique thus allowed for the simultaneous and accurate detection of five CCBs in human plasma samples, providing a promising new basis for therapeutic drug monitoring in patients with hypertension.
Keywords: calcium channel blockers, QuEChERS, UPLC-MS/MS, human plasma, hypertension
1. Introduction
Hypertension is a condition in which patients experienced persistently elevated systemic blood pressure levels outside the normal physiological range, elevating the risk of a range of renal cardiac, cerebral, and peripheral vascular diseases. Achieving sustained blood pressure control in hypertensive patients often necessitates pharmacologic intervention using a range of first-line treatments including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, calcium channel blockers (CCBs), and diuretics.
CCBs are prescribed primarily for the treatment of peripheral vasospasm, migraine headaches, angina pectoris, and arterial hypertension [1]. CCB use rates have risen in recent decades such that they now account for over 30% of hypotensive drug use in patients affected by a range of cardiovascular conditions [2]. However, even when care is taken, these calcium antagonists can cause toxic effects resulting in sudden illness or death [3]. Some reports suggest that CCB use is among the most significant factors contributing to therapeutic cardiovascular drug poisoning and mortality [4].
Based on their affinity and effects on the heart and arterial vessels, these drugs are classified as dihydropyridine CCBs, which primarily affect the vascular smooth muscle, and non-dihydropyridine CCBs, which exhibit specific targeting of myocardial L-type channels [5]. At therapeutic doses, dihydropyridines can thus promote vasodilation [6,7]. Pharmacological selectivity may be lost when toxic levels of these drugs are consumed [8], resulting in potentially severe adverse reactions including low blood pressure, nausea, vomiting, rhythm depression, and cardiac arrest in severe cases [9]. Maintaining an appropriate plasma concentration of these drugs is thus vital to ensure that optimal patient blood levels are maintained and that a proper dose is utilized.
Liquid chromatography-mass spectrometry (LC-MS) approaches are among the most widely applied strategies for the detection of a diverse range of compounds, and they are the primary strategy employed to analyze calcium antagonists in bio-samples including serum, plasma, and milk owing to their excellent sensitivity and acquisition speed [10,11,12,13,14]. The combination of high-performance liquid chromatography and ultraviolet detection (HPLC-UV) [11], gas chromatography-tandem mass spectrometry (GC-MS) [15], and GC with electron-capture detection (GC-ECD) have also been used in various research settings. However, LC-based strategies alone cannot provide sufficient sensitivity when detecting many compounds, given that samples containing complex substrates inevitably contain many spurious peaks for which effective separation cannot be achieved. The GC-MS-based detection of pharmaceutical compounds also necessitates derivatization and time-consuming sample processing.
Appropriate analytical sample preparation is essential to mitigate interference caused by endogenous compounds or drug metabolites when biological samples consist of a complex matrix likely to disturb analyses. To date, many extraction techniques have been designed including protein precipitation (PP) [12], liquid-liquid extraction (LLE) [14], solid-phase extraction (SPE) [11,13], solid-phase microextraction (SPME), and others have been developed and used to measure drug concentrations in blood samples. However, these techniques are subject to certain limitations including high costs and complex workflow techniques. LLE can result in emulsification and consequent drug loss, while SPE requires expensive equipment and complex pre-column derivatization procedures that can impact the subsequent accuracy of quantitative analyses. In this study, a modified QuEChERS approach was used for sample fabrication. This methodological approach was initially published by Anastassiades et al. [16] and was based on SPE and matrix solid-phase dispersion (MSPD) techniques [17,18,19]. This improved strategy consists of three primary steps: (1) Homogenous sample extraction using organic solvents, (2) The separation of the organic layer by adding extracted samples to appropriate inorganic salts, and (3) The purification of specific analytes with an adsorbent [20]. Relative to traditional sample extraction techniques, optimized QuEChERS methods are more efficient, easier to complete, and less expensive.
The present study was developed with the goal of combining the performance of LC-MS/MS detection strategies and the utility of QuEChERS techniques in an effort to enable the rapid and simultaneous quantitative detection of amlodipine, nifedipine, nimodipine, nifedipine, and felodipine in human plasma samples. The resultant protocol only requires tiny samples and easy-to-implement pretreatment procedures, offering a reliable approach to therapeutic drug monitoring in individuals using calcium antagonists.
2. Results
2.1. Methodological Validation
2.1.1. Selectivity
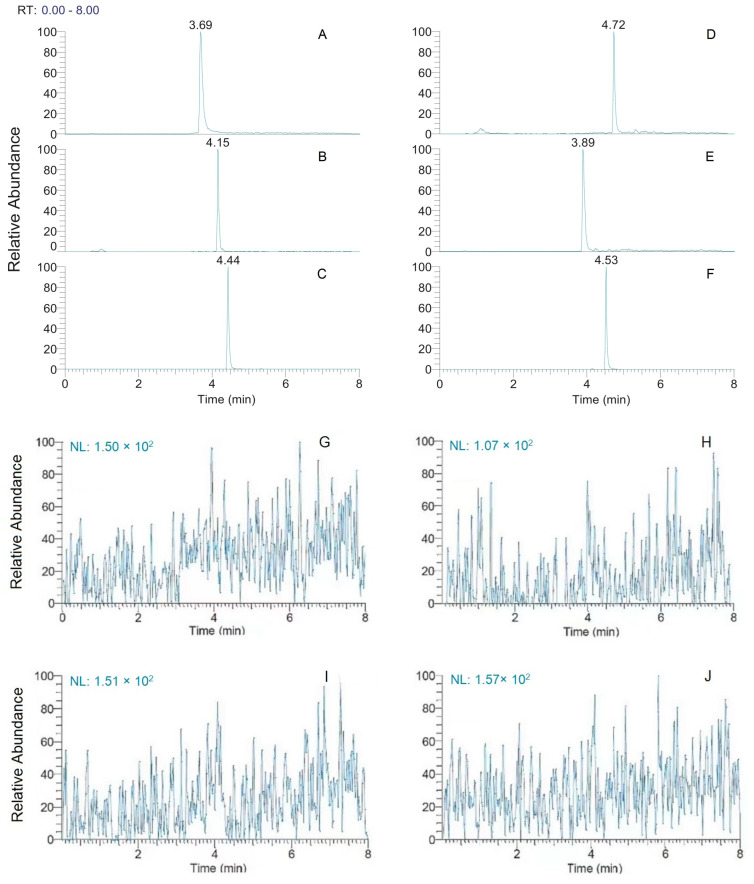
No interference was detected when assessing blank plasma samples. Chromatograms for samples spiked with internal standard (IS), when extracted separately from CCBs and IS, did not exhibit any interfering retention time peaks and presented with good quantitative peak shape. IS concentrations were as follows:20 ng⋅mL−1 for amlodipine (AML) and nimodipine (NIM); 10 ng⋅mL−1 for nifedipine (NIF); 60 ng⋅mL−1 for nitrendipine (NIT); 200 ng⋅mL−1 for felodipine (FEL); 10 ng⋅mL−1 for IS (Figure 1). Carryover in the chromatogram of the blank plasma sample after high-concentration samples was barely visible, ensuring that the accuracy and precision of the method were not compromised. Chromatograms of blank plasma samples evaluating selectivity and carryover are shown in Figure 1G–R.
Figure 1.
Typical chromatograms of IS-propranolol (A), nifedipine (B), nitrendipine (C), felodipine (D), amlodipine (E), and nimodipine (F) in blank plasma samples, the chromatogram of sixblank plasma samples (G–N) and chromatogram of six blank samples after HQC sample analysis (M–R).
2.1.2. Linearity
Peak area ratios for these five CCBs were linear within the 2.0–800 ng⋅mL−1 range, with each exhibiting a linear correlation coefficient (R2) > 0.992. Peak concentrations up to three times the baseline noise level were defined as LODs, while peak concentrations up to 10 times the baseline noise level were defined as LOQs. For details regarding these specific parameters, see Table 1.
Table 1.
Regression equations, LODs, and LOQs of compounds.
| Compounds | Linear Equation | Linear Range (ng·mL−1) | R2 | LODs (ng·mL−1) | LOQs (ng·mL−1) |
|---|---|---|---|---|---|
| AML | Y = −0.171406 + 0.0869037x | 2.0–80 | 0.9951 | 0.014 | 0.048 |
| FEL | Y = −0.0524462 + 0.00288808x | 20–800 | 0.9933 | 0.220 | 0.732 |
| NIF | Y = −0.429218 + 0.421058x | 1.0–40 | 0.9921 | 0.002 | 0.006 |
| NIM | Y = −0.107548 + 0.0537931x | 2.0–80 | 0.9914 | 0.007 | 0.025 |
| NIR | Y = −0.0401095 + 0.0169601x | 6.0–240 | 0.9947 | 0.041 | 0.137 |
2.1.3. Accuracy and Precision
The accuracy of this method ranged from 87.54–113.05%, with inter-day precision ranging from 0.19–11.04% and intra-day precision ranging from 0.28–11.64%. For further detail regarding these parameters, see Table 2.
Table 2.
ME, extraction recovery, accuracy, and precision of five compounds (n = 3).
| Compounds | Spiked (ng·mL−1) | ME (%) | ER (%) | Accuracy (%) | Precision (RSD %) | |
|---|---|---|---|---|---|---|
| Inter-Day | Intra-Day | |||||
| AML | 5 | 102.06 | 95.23 | 101.43 | 0.69 | 2.00 |
| 20 | 99.64 | 102.67 | 105.83 | 6.89 | 1.81 | |
| 80 | 93.59 | 94.06 | 87.54 | 2.24 | 2.86 | |
| FEL | 50 | 97.18 | 106.17 | 103.95 | 3.35 | 0.64 |
| 200 | 94.52 | 94.86 | 106.15 | 11.04 | 2.87 | |
| 800 | 91.21 | 96.41 | 90.15 | 6.36 | 2.61 | |
| NIF | 2.5 | 91.23 | 100.80 | 111.23 | 0.82 | 0.28 |
| 10 | 98.19 | 95.98 | 96.27 | 3.79 | 0.71 | |
| 40 | 92.43 | 91.74 | 91.60 | 4.45 | 2.10 | |
| NIM | 5 | 93.66 | 91.94 | 104.18 | 0.21 | 1.45 |
| 20 | 110.48 | 95.18 | 103.35 | 8.51 | 1.85 | |
| 80 | 95.09 | 97.45 | 92.91 | 5.71 | 11.64 | |
| NIT | 15 | 99.11 | 97.55 | 113.05 | 4.43 | 2.57 |
| 60 | 116.39 | 98.45 | 105.08 | 0.19 | 1.66 | |
| 240 | 108.84 | 94.78 | 96.63 | 3.22 | 2.75 | |
2.1.4. Matrix Effect
The internal standard normalized MF for these five CCBs ranged from 91.21–116.39%, suggesting that these matrix effects are not likely to impact the determinations made using this method. It is generally stipulated that the extraction recovery rate should be greater than 50%, and the extraction recovery rate of this method is between 91.74–106.17%, which meets the requirements. For further details regarding specific values, see Table 2.
2.1.5. Stability
The RSD value corresponding to short-term stability was less than or equal to 10.05%, and sample tray stability was less than or equal to 7.00%. Long-term storage stability was less than or equal to 9.41%. For further details see Table 3.
Table 3.
The stability of the QC samples under four different conditions (n = 3).
| Compounds | Spiked (ng·mL−1) | Stability (RSD%) | |||
|---|---|---|---|---|---|
| 25 °C/24 h | 4 °C/24 h | 4 °C/48 h | −20 °C/7d | ||
| AML | 5 | 1.52 | 1.44 | 2.69 | 6.33 |
| 20 | 2.30 | 1.61 | 0.95 | 0.69 | |
| 80 | 1.50 | 0.48 | 1.62 | 3.36 | |
| FEL | 50 | 6.11 | 2.13 | 4.77 | 5.38 |
| 200 | 1.96 | 3.19 | 7.00 | 4.72 | |
| 800 | 5.47 | 0.85 | 2.45 | 9.41 | |
| NIF | 2.5 | 2.16 | 1.43 | 1.64 | 2.34 |
| 10 | 7.42 | 0.57 | 0.92 | 1.23 | |
| 40 | 0.91 | 1.26 | 0.37 | 4.53 | |
| NIM | 5 | 4.95 | 1.70 | 5.58 | 2.93 |
| 20 | 3.02 | 1.20 | 2.40 | 4.50 | |
| 80 | 0.50 | 5.41 | 3.27 | 7.60 | |
| NIT | 15 | 2.16 | 1.70 | 1.55 | 8.08 |
| 60 | 10.05 | 1.06 | 6.42 | 2.02 | |
| 240 | 2.50 | 3.64 | 2.75 | 9.10 | |
3. Discussion
3.1. Extraction Procedure Optimization
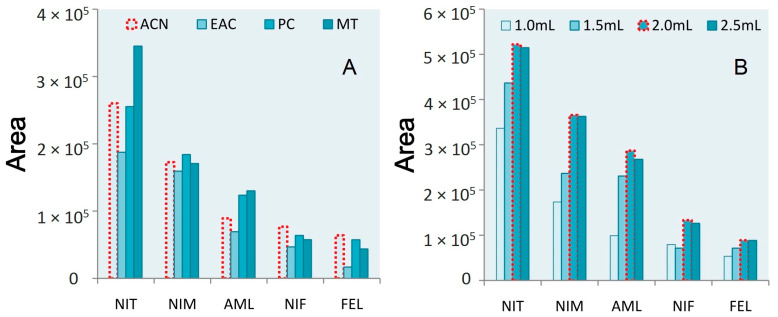
While structurally related, these different dihydropyridine drugs are all distinct from one another such that their relative solubility differs in particular solvents. The extraction effects for methanol (MT), ethyl acetate (EAC), acetonitrile (ACN), and acetone (CP) were therefore explored based on the area index values. Of these four tested extraction reagents, ethyl acetate yielded the smallest area value for each of these CCBs, while methanol exhibited the best extraction effects for nitrendipine, nimodipine, and amlodipine, although its efficacy was relatively poor for the other two drugs. Acetone and acetonitrile exhibited good performance for most of these CCBs, but slightly poorer for amlodipine. Of these two extraction reagents, the area values for drugs purified using acetone were lower than when using acetonitrile. Accordingly, subsequent experiments were performed using acetonitrile (Figure 2A).
Figure 2.
(A) The area results of five CCBs treated with different extraction solvents. (B) The area results of five CCBs treated with different volumes of extractants.
Different acetonitrile volumes were next combined with samples, given the optimized extraction reagent usage. As shown in Figure 2B, the use of 2 mL of acetonitrile yielded larger peak area values.
3.2. Purification Reagent Selection
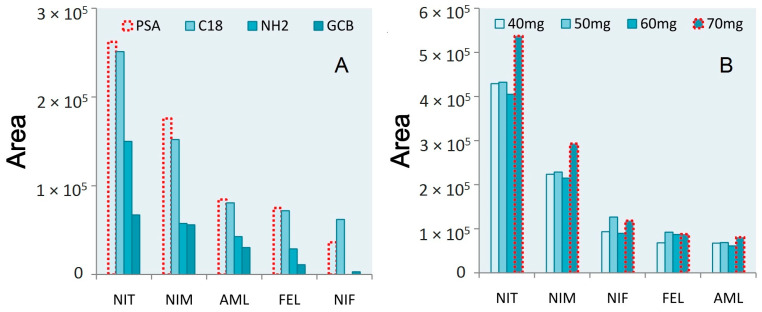
The four purifying agents include g primary–secondary amine (PSA), C18, graphitized carbon black (GCB), and NH2. Area values were used to assess the relative performance of these different agents. PSA exhibited excellent purification efficacy for most tested CCBs in the sample matrix, although C18 exhibited better performance for nifedipine. However, peak areas for the four other analyzed CCBs were lower when using C18 than PSA. The overall rank order purification efficacy was as follows: PSA > C18 > NH2 > GCB (Figure 3A).
Figure 3.
(A) The area results of five CCBs treated with different purifying agents. (B) The area results of five CCBs treated with different amounts of purifying agents.
To better optimize absorbant dosing, four different amounts of PSA (40, 50, 60, and 70 mg) were tested. For these five CCBs, the most prominent peak areas were observed for nitrendipine, nimodipine, and amlodipine when using 70 mg of PSA. In contrast, better responses were observed for the other drugs when using 50 mg of PSA. When pretreatment was performed using 70 mg of PSA, nifedipine and felodipine exhibited smaller peak area values than when 50 mg of PSA was added. As such, 70 mg of PSA was used as an adsorbent in subsequent analyses of these five CCBs (Figure 3B).
3.3. Salting Reagent Selection
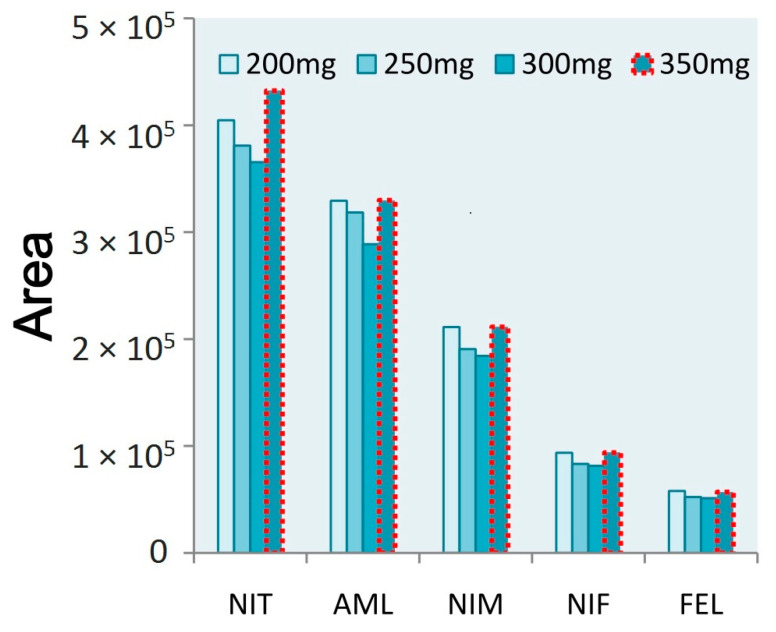
Based on the results of this study, anhydrous MgSO4 was selected as the salting agent. Different concentrations were selected for extraction (250, 300, 350, and 400 mg), with optimal results established based on area values. The highest CCB peak responses were evident when using 350 mg of anhydrous magnesium sulfate. A slightly less pronounced peak response was observed at a 200 mg dose. As the extraction dose was the most critical factor, a salt dose of 350 mg was selected (Figure 4).
Figure 4.
The area results of five CCBs treated with different amounts of MgSO4.
3.4. Comparisons
Most prior studies have primarily focused on a single calcium antagonist, whereas few have described the simultaneous detection of multiple CCBs. In this study, five calcium antagonists were determined simultaneously by QuEChERS-UPLC-MS/MS method for the first time. While these dipine-drugs are derived from the same family, the structural differences in their specific moieties give them each unique physicochemical characteristics. The methodological approach to their detection is also subject to bio-matrix complexity and the low expected concentrations of these compounds in some instances. Our work is challenging.
Few methods for the simultaneous determination of five calcium antagonists have been established in the last five years of research. Most of the methods in Table 4 measured one-three drugs and included only one calcium antagonist. The method we established allows simultaneous determination of five CCBs, which indicates that our method covers an extensive range of drugs and is more applicable. The developed method has LOD and LOQ of 0.014, 0.048 for AML, and 0.007,0.025 for NIM. Existing studies have shown LOD and LOQ greater than 0.2 and 0.5 for AML [11,12] and greater than 0.05 and 0.12 for NIM [13,14]. The new method demonstrated lower LOD and LOQ and, therefore, has higher sensitivity than published methods. The extraction recoveries of each drug in this method were more than 94%, which was higher than those of the corresponding drugs in other studies in Table 4.
Table 4.
Comparison of the proposed method with other published methods for the quantitative detection of CCBs.
| Method | Analyte | LOD(ng/mL) | LOQ(ng/mL) | ER(%) | Ref. |
|---|---|---|---|---|---|
| QuEChERS/UPLC-MS/MS | AML | 0.014 | 0.04 | 97.32 | This work |
| FEL | 0.220 | 80.732 | 96.15 | ||
| NIF | 0.002 | 0.006 | 96.17 | ||
| NIM | 0.007 | 0.025 | 94.85 | ||
| NIT | 0.041 | 0.137 | 96.93 | ||
| PP/first derivative SFS | AML | 1.160 | 3.516 | 95.20 | [21] |
| HPLC-MS/MS | AML | 0.2 | 0.5 | 50.90 | [11] |
| MSPE/HPLC-UV | NIM | 0.28 | 0.84 | 59.87 | [12] |
| PP/SFC-MS/MS | NIM | 0.05 | 0.12 | 91 | [13] |
| SPE/LC-MS/MS | FEL | - | 0.59 | 91.9 | [14] |
| LLE/LC-MS/MS | NIT | - | - | 89.51 | [15] |
Overall, this improved QuEChERS-UPLC-MS/MS method achieved good recovery, precision, and accuracy while maintaining good efficacy and cost-effectiveness, thereby supporting greater environmental conservation. For further comparisons of these different parameters, see Table 4.
In previous work conducted by our group, we applied the QuEChERS pre-treatment technique to assess the concentrations of multiple drug classes. Analyses of multiple tyrosine kinase inhibitors (TKI) concentrations in human plasma [22] and multiple immunosuppressant concentrations in whole human blood [23] have performed well in terms of LOQ and recovery as compared to other assays for similar drugs. In addition, other members ofour group have attempted to use the QuEChERS technique in combination with LC-MS to detect 16illicitly added drugs in capsule dietary supplements containing lipid-lowering drugs, diuretics, and appetite suppressants [24]. They successfully verified the utility of this method as a means of detecting and quantifying the target analytes in dietary supplement samples. Therefore, we propose that this QuEChERS technique applies not only to the field of calcium antagonists but also to a broad rangeof therapeutic drug monitoring applications.
4. Materials and Methods
4.1. Chemicals and Reagents
All CCBs and propranolol hydrochloride were acquired from National Institutes for Food and Drug Control (Beijing, China) and were ≥99.2% pure. Methanol (MT), ethyl acetate (EAC), acetonitrile (ACN), and acetone (CP) were obtained from Fisher Chemical (Shanghai, China). Ammonium acetate was obtained from the Tianjin BODI Chemicals Co., Ltd. (Tianjin, China). Analytical grade magnesium sulfate anhydrous (MgSO4) was from Kemiou Chemical Reagent Co., Ltd. (Tianjin, China). QuEChERS adsorbents were provided by Agilent(Tianjin, China). Ultrapure water was from Watsons.
4.2. Instrument and Analytical Conditions
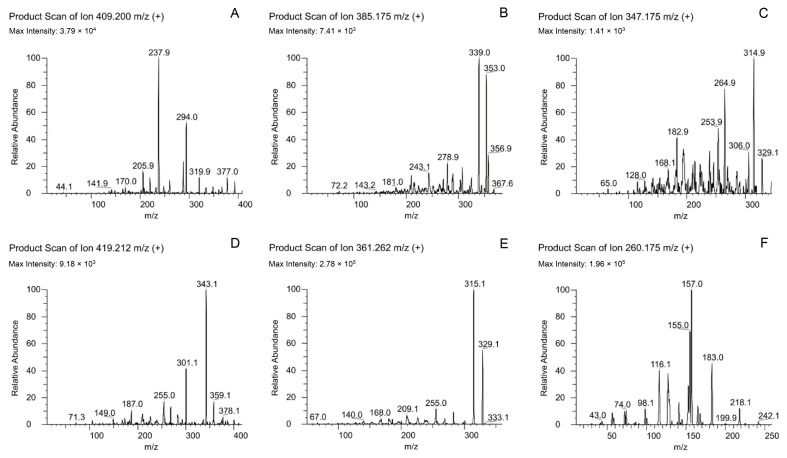
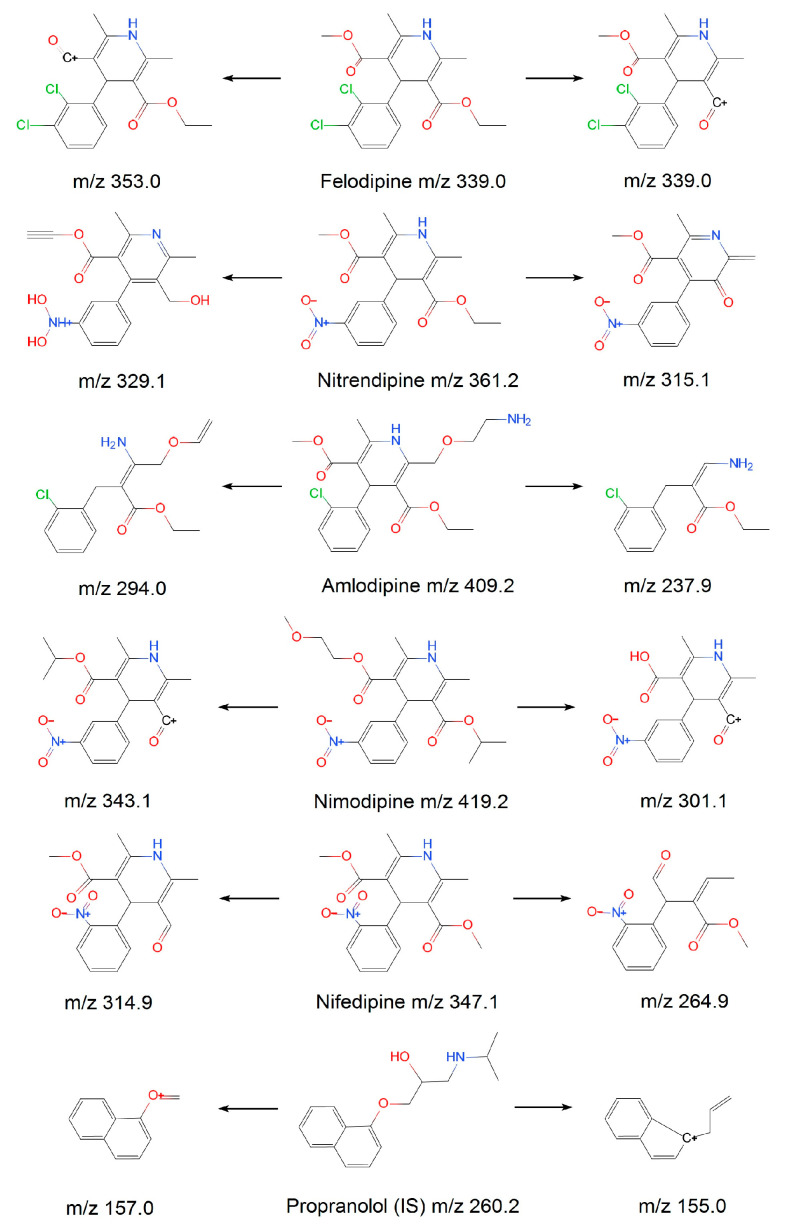
A VanquishTM Flex ultra-HPLC System coupled with a TSQ AltisTM Triple Quadrupole MS Instrument (Thermo Fisher Scientific, Waltham, Massachusetts USA) was used for all analyses. Briefly, samples (5 μL) were spiked into a Poroshell 120 EC-C18 (column temperature 45 °C). Elution was performed using 5 mmol/L ammonium acetate–water (A) and acetonitrile (B) at a 0.3 mL⋅min−1 flow rate using the following settings: 0–1.0 min 20% B; 1.0–3.0 min, linear gradient from 20–90% B; 3.0–6.0 min, 90% B; 6.0–6.1 min, 90–20% B; 6.1–8.0 min, 20% B. The relevant MS parameters were positive mode electrospray ionization source (ESI+); selection reaction monitoring (SRM). Mass transitions of the RF Lens, CE, and RT, are presented in Table 5. Product spectra for each analyte are presented in Figure 5. The chemical structure and possible fragmentation pathways of each compound are shown in Figure 6.
Table 5.
The MS/MS fragment ions, fragmentor voltage, collision voltage, and retention time of the five calcium blockers.
| Compounds | Precursor (m/z) | Product (m/z) | Collision Energy(V) | RFLens(V) | Retention Time (min) |
|---|---|---|---|---|---|
| AML | 409.200 | 237.9 | 12.03 | 53 | 3.89 |
| 294.0 | 12.20 | ||||
| FEL | 385.175 | 339.0 | 13.13 | 53 | 4.72 |
| 353.0 | 13.55 | ||||
| NIF | 347.175 | 264.9 | 12.75 | 46 | 4.15 |
| 314.9 | 8.79 | ||||
| NIM | 419.212 | 301.1 | 20.60 | 55 | 4.53 |
| 343.1 | 10.64 | ||||
| NIR | 361.262 | 315.1 | 13.17 | 64 | 4.44 |
| 329.1 | 13.72 | ||||
| PRO | 260.175 | 155.0 | 25.30 | 47 | 3.69 |
| 157.0 | 20.12 |
Figure 5.
Product ion mass spectra of amlodipine (A), felodipine (B), nifedipine (C), nimodipine (D), nitrendipine (E) and IS-propranolol (F) in positive mode.
Figure 6.
The chemical structure and possible fragmentation pathways of five CCBs.
4.3. Solution Preparation
Each drug and the internal standard (IS) were prepared as a 100 μg⋅mL−1stock solution in methanol. Methanol was then used to dilute these drugs to appropriate working concentrations including NIF:1, 2.5, 5, 10, 25, 40 ng⋅mL−1; AML and NIM: 2, 5, 10, 20, 50, 80 ng⋅mL−1; NIT: 6, 15, 30, 60, 150, 240 ng⋅mL−1; FEL: 20, 50, 100, 200, 500, 800 ng⋅mL−1; Propranolol (IS):10 ng⋅mL−1.
4.4. Plasma Sample Preparation
Plasma samples were collected from healthy human donors and prepared via centrifugation prior to storage at −110 °C. To prepare samples, 50 μL of human plasma was added to a 2 mL polypropylene tube, after which 100 μL each of the IS methanol solution and the mixed standard preparation were added to the same tube and mixed thoroughly. Then, 1 mL of acetonitrile, 350 mg of MgSO4, and 70 mg of PSA were added to the tube in sequence followed by vigorous shaking for 1 min. The entire mixture was taken by centrifugation for 5 min at 1500× g at 4 °C, after which the residue was re-dissolved in 500μLof methanol after being dried under a nitrogen stream. The solution was then passed through a filter membrane using a syringe. The Ethics Committee of Hebei Medical University approved this study.
4.5. Methodological Validation
The readouts assessed to ensure the suitability and efficacy of the developed methods included selectivity, calibration curves, accuracy, precision, analyte stability in biological matrices, and matrix effects, which were analyzed as detailed below.
4.5.1. Selectivity and Carryover
Six blank plasma samples of healthy people and sixplasma samples containing internal standard and standard solution were analyzed to assess methodological selectivity. Responses of interfering components below 20% of the LLOQ for the analyte and 5% of the internal standard are acceptable.
Validation of carryover was assessed by injecting a blank sample after the high-concentration sample. The carryover of the high-concentration standard sample in the blank sample should not exceed 20% of the LLOQ and 5% of the internal standard.
4.5.2. Linearity
Following pretreatment using the developed analytical protocol, a series of calibration samples were analyzed. Linear regression analyses were performed with the Xcalibur system based on the ratio of the detection response for the five CCBs to the response of the IS (dependent variable, y) graphed against the drug concentration in prepared calibration samples (independent variable, x).
4.5.3. Precision and Accuracy
To assess accuracy, QC samples prepared at low, medium, and high concentrations were analyzed, with these analyses being repeated three times for each sample concentration. Intra-day precision was measured by replicating this analysis three times per day at all sample concentrations, while inter-day precision was calculated by repeating three analyses of QC samples on six successive days.
4.5.4. Stability
Stability was validated using QC samples prepared at different concentrations. Short-term stability was assessed after storage for 24 h at room temperature (25 °C), while long-term stability was assessed following storage in the sample tray (4 °C) for 24 or 48 h, or after storage in refrigerator-frozen storage (−20 °C) for 7 days.
4.5.5. Matrix Effect and Extraction Recovery
QC samples, standard analyte solutions, and IS standard solutions were evaluated to assess the matrix effect (ME). The matrix factor (MF) was determined based on the sample peak area’s ratio to the standard solution’s peak area. The normalized matrix factor (MFi) was determined by dividing the MF for a given analyte by the MF for the IS.
The extraction recovery (ER) was calculated by the ratio of the area of the QC sample to the area of the blank sample added with the corresponding concentration standard solution after pretreatment.
5. Conclusions
Here, an LC-MS/MS strategy was successfully combined with an optimized QuEChERS pretreatment technique in order to simultaneously measure levels of amlodipine, nimodipine, nifedipine, nifedipine, and felodipine in samples of human plasma. Plasma pretreatment was straightforward, and the use of pre-weighed QuEChERS reagents can reduce labor and time requirements while also being amenable to use as a pretreatment for analyses of other compounds of interest. This approach achieved an accuracy of 87.54–113.05%, precision from 0.19–11.64%, extraction recovery of 94.85–97.32%,and a stability RSD value ≤ 10.05%, with negligible matrix interference. This method is thus an effective and straightforward approach to therapeutic drug monitoring focused on calcium antagonists.
Author Contributions
Conceptualization, T.Z. and H.L.; methodology, T.Z.; validation, T.Z. and W.J.; investigation, C.J. and Y.Z.; resources, X.Z.; writing—original draft preparation, T.Z.; writing—review and editing, W.J.; supervision, H.L.; funding acquisition, H.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declarationof Helsinki, and approved by the Medical Ethics Committee of Hebei Medical University (P2022090).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds (AML, NIF, NIT, NIM, FEL, Propranolol) are available from the authors.
Funding Statement
This research was funded by the Natural Science Foundation of Hebei Province, grant number H2022329002.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gummin D.D., Mowry J.B., Spyker D.A., Brooks D.E., Beuhler M.C., Rivers L.J., Hashem H.A., Ryan M.L. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin. Toxicol. 2019;57:1220–1413. doi: 10.1080/15563650.2019.1677022. [DOI] [PubMed] [Google Scholar]
- 2.Sørensen H.T., Olsen J.H., Mellemkjaer L., Marie A., Steffensen F.H., McLaughlin J.K., Baron J.A. Cancer risk and mortality in users of calcium channel blockers. A cohort study. Cancer. 2000;89:165–170. doi: 10.1002/1097-0142(20000701)89:1<165::AID-CNCR21>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 3.Wong A., Hoffman R.S., Walsh S.J., Roberts D.M., Gosselin S., Bunchman T.E., Kebede S., Lavergne V., Ghannoum M. EXTRIP workgroup. Extracorporeal treatment for calcium channel blocker poisoning: Systematic review and recommendations from the EXTRIP workgroup. Clin. Toxicol. 2021;59:361–375. doi: 10.1080/15563650.2020.1870123. [DOI] [PubMed] [Google Scholar]
- 4.Mladěnka P., Applová L., Patočka J., Costa V.M., Remiao F., Pourová J., Mladěnka A., Karlíčková J., Jahodář L., Vopršalová M., et al. TOX-OER and CARDIOTOX Hradec Králové Researchers and Collaborators. Comprehensive review of cardiovascular toxicity of drugs and related agents. Med. Res. Rev. 2018;38:1332–1403. doi: 10.1002/med.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nawrot D.A., Ozer L.Y., Al Haj Zen A. A Novel High Content Angiogenesis Assay Reveals That Lacidipine, L-Type Calcium Channel Blocker, Induces In Vitro Vascular Lumen Expansion. Int. J. Mol. Sci. 2022;28:4891. doi: 10.3390/ijms23094891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St-Onge M. Cardiovascular Drug Toxicity. Crit. Care Clin. 2021;37:563–576. doi: 10.1016/j.ccc.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Batalis N.I., Harley R.A., Schandl C.A. Verapamil toxicity: An unusual case report and review of the literature. Am. J. Forensic. Med. Pathol. 2007;28:137–140. doi: 10.1097/01.paf.0000257399.58935.28. [DOI] [PubMed] [Google Scholar]
- 8.Palatnick W., Jelic T. Calcium channel blocker and beta blocker overdose, and digoxin toxicity management. Emerg. Med. Pract. 2020;22((Suppl. S9)):1–42. [PubMed] [Google Scholar]
- 9.Levine M., Curry S.C., Padilla-Jones A., Ruha A.M. Critical care management of verapamil and diltiazem overdose with a focus on vasopressors: A 25-year experience at a single center. Ann. Emerg. Med. 2013;62:252–258. doi: 10.1016/j.annemergmed.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Chen G., Jirjees Sr F., Al Bawab A., McElnay J.C. Quantification of amlodipine in dried blood spot samples by high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2018;1072:252–258. doi: 10.1016/j.jchromb.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Wang W., Li P., Wu F., Wang Z., Zhang T. Development and optimization of a supercritical fluid chromatography tandem mass spectrometry method for the high-throughput determination of nimodipine in beagle plasma. J. Sep. Sci. 2019;42:2179–2186. doi: 10.1002/jssc.201900134. [DOI] [PubMed] [Google Scholar]
- 12.Chen M., Zhou J., Mei L., Yu F., Xie X., Liu Y., Yang Y., Li Y., Mei X. Simultaneous Determination of Felodipine and Metoprolol in Beagle Dog Plasma by Online SPE-LC-MS/MS and Its Application in a Pharmacokinetic Study. Anal. Sci. 2017;33:755–759. doi: 10.2116/analsci.33.755. [DOI] [PubMed] [Google Scholar]
- 13.Mohammad M.A., Mahrouse M.A., Amer E.A.H., Elharati N.S. Validated LC-MS/MS method for the simultaneous determination of enalapril maleate, nitrendipine, hydrochlorothiazide, and their major metabolites in human plasma. Biomed Chromatogr. 2020;34:e4955. doi: 10.1002/bmc.4955. [DOI] [PubMed] [Google Scholar]
- 14.Heidari H., Limouei-Khosrowshahi B. Magnetic solid phase extraction with carbon-coated Fe3O4 nanoparticles coupled to HPLC-UV for the simultaneous determination of losartan, carvedilol, and amlodipine besylate in plasma samples. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2019;1114–1115:24–30. doi: 10.1016/j.jchromb.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Jain B., Jain R., Kabir A., Sharma S. Rapid Determination of Non-Steroidal Anti-Inflammatory Drugs in Urine Samples after In-Matrix Derivatization and Fabric Phase Sorptive Extraction-Gas Chromatography-Mass Spectrometry Analysis. Molecules. 2022;27:7188. doi: 10.3390/molecules27217188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anastassiades M., Lehotay S.J., Stajnbaher D., Schenck F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003;86:412–431. doi: 10.1093/jaoac/86.2.412. [DOI] [PubMed] [Google Scholar]
- 17.Liu W., Su Y., Liu J., Zhang K., Wang X., Chen Y., Duan L., Shi F. Determination of cyflufenamid residues in 12 foodstuffs by QuEChERS-HPLC-MS/MS. Food Chem. 2021;362:130148. doi: 10.1016/j.foodchem.2021.130148. [DOI] [PubMed] [Google Scholar]
- 18.Abdelghani J.I., Al-Degs Y.S. Spectroscopic quantifiication of preservatives in different food matrices using QuEChERS extraction and multivariate calibration with comparison against liquid chromatography. Arab J. Chem. 2022;15:103462. doi: 10.1016/j.arabjc.2021.103462. [DOI] [Google Scholar]
- 19.Hernández-Mesa M., García-Campaña A.M. Determination of sulfonylurea pesticide residues in edible seeds used as nutraceuticals by QuEChERS in combination with ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2020;1617:460831. doi: 10.1016/j.chroma.2019.460831. [DOI] [PubMed] [Google Scholar]
- 20.Rejczak T., Tuzimski T. A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chem. 2015;13:980–1010. doi: 10.1515/chem-2015-0109. [DOI] [Google Scholar]
- 21.Attala K., Eissa M.S., Hasan M.A., El-Henawee M.M., Abd El-Hay S.S. An enhanced first derivative synchronous spectrofluorimetric method for determination of the newly co-formulated drugs, amlodipine and celecoxib in pharmaceutical preparation and human plasma. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020;240:118533. doi: 10.1016/j.saa.2020.118533. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W., Zhao T., Zhen X., Jin C., Li H., Ha J. Rapid Determination of 9 Tyrosine Kinase Inhibitors for the Treatment of Hepatocellular Carcinoma in Human Plasma by QuEChERS-UPLC-MS/MS. Front. Pharmacol. 2022;13:920436. doi: 10.3389/fphar.2022.920436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng M., Song J., Xue H., Li H., Lian K. Simultaneous determination of six immunosuppressants in human whole blood by HPLC-MS/MS using a modified QuEChERS method. Molecules. 2022;27:4087. doi: 10.3390/molecules27134087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao J., Ma C., Duan Q., Gao W., Li H. Simultaneous determination of 16illegally added drugs in capsule dietary supplements using a QuEChERS method and HPLC–MS/MS. Chromatographia. 2021;84:1009–1023. doi: 10.1007/s10337-021-04087-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.