Abstract
A nonhuman primate model for malaria vaccine development allowing reliable, stringent sporozoite challenge and evaluation of both cellular and antibody responses is needed. We therefore constructed a multicomponent, multistage DNA vaccine for the simian malaria species Plasmodium knowlesi including two preerythrocytic-stage antigens, the circumsporozoite protein (PkCSP) and sporozoite surface protein 2 (PkSSP2), and two blood stage antigens, apical merozoite antigen 1 (PkAMA1) and merozoite surface protein 1 (PkMSP1p42), as well as recombinant canarypox viruses encoding the four antigens (ALVAC-4). The DNA vaccine plasmids expressed the corresponding antigens in vitro and induced antiparasite antibodies in mice. Groups of four rhesus monkeys received three doses of a mixture of the four DNA vaccine plasmids and a plasmid encoding rhesus granulocyte-monocyte colony-stimulating factor, followed by boosting with a single dose of ALVAC-4. Three groups received the priming DNA doses by different routes, either by intramuscular needle injection, by intramuscular injection with a needleless injection device, the Biojector, or by a combination of intramuscular and intradermal routes by Biojector. Animals immunized by any route developed antibody responses against sporozoites and infected erythrocytes and against a recombinant PkCSP protein, as well as gamma interferon-secreting T-cell responses against peptides from PkCSP. Following challenge with 100 P. knowlesi sporozoites, 1 of 12 experimental monkeys was completely protected and the mean parasitemia in the remaining monkeys was significantly lower than that in 4 control monkeys. This model will be important in preclinical vaccine development.
Malaria is a major cause of morbidity and mortality throughout tropical and subtropical regions of the world, accounting for an estimated 300 to 500 million infections and 1.5 to 3.0 million deaths annually (35). In the face of the spread of drug-resistant malaria, efforts to develop an effective vaccine have become increasingly critical. Two observations suggest that a malaria vaccine may be achievable. First, immunization with radiation-attenuated sporozoites induces sterile protection in mice and humans (5, 17), mediated predominantly by CD8+ T cells and gamma interferon (IFN-γ) and directed against the intrahepatocytic stage of the parasite. Second, adults in areas endemic for malaria develop partial clinical immunity, which is largely mediated by antibodies directed against blood stage antigens (23). A vaccine may need to induce both types of responses to provide optimal protection. DNA vaccines represent a flexible vaccine delivery system, capable of inducing both antibodies and cell-mediated immune responses to a wide variety of antigens. The flexibility of DNA vaccine technology permits the combination of multiple antigens from both the preerythrocytic and erythrocytic stages of the parasite. Previous studies from our laboratory have shown that DNA vaccines directed against either preerythrocytic-stage antigens (7, 26) or erythrocytic-stage antigens (1) can provide partial protection in the Plasmodium yoelii murine-malaria model. A mixture of DNA vaccines encoding four preerythrocytic-stage Plasmodium falciparum antigens induced both antibodies and T-cell responses to all four components in rhesus monkeys (32). In human volunteers a DNA vaccine encoding the P. falciparum circumsporozoite protein was safe and well tolerated and induced antigen-specific cytotoxic-T-lymphocyte responses in the majority of immunized volunteers (31). However, these first-generation DNA vaccines are not optimally immunogenic or protective; the PfCSP vaccine did not induce antibodies in volunteers, and the protection induced by immunization with P. yoelii DNA vaccines in mice is incomplete. Recent studies have shown that the effectiveness of DNA vaccination against malaria in mice can be increased by use of a prime-boost strategy in which priming doses of DNA vaccine plasmids are followed by a boost with recombinant poxvirus (25, 27). In addition, inclusion of a plasmid encoding murine granulocyte-monocyte colony-stimulating factor (GM-CSF) improves the protection seen with the DNA vaccine alone (34). Finally, combination of the two approaches further improves both protection and immunogenicity (28). We therefore constructed a set of DNA vaccines and recombinant canarypox virus to allow us to test the prime-boost approach in the Plasmodium knowlesi/rhesus monkey model, a system in which both reliable challenge with sporozoites and partial protection after immunization with irradiated sporozoites have been demonstrated (14). Because previous work involving Aotus monkeys with malaria and hepatitis B DNA vaccines had suggested that the route and method of administration can affect both the quality and magnitude of the induced immune response (10, 11), we studied three different methods of administering the priming DNA, intramuscular (i.m.) injection with needle and syringe, i.m. injection with the Biojector, a CO2-driven needleless injection system (11, 12), and a combination of i.m. and intradermal (i.d.) injection with the Biojector.
MATERIALS AND METHODS
Parasites and DNA.
DNA from the H strain (4) of P. knowlesi was the kind gift from Tom Templeton (National Institutes of Health, Bethesda, Md.). H strain sporozoites and infected erythrocytes for immunofluorescence assay (IFA) were provided by William Collins (Centers for Disease Control and Prevention, Atlanta, Ga.). Infectious sporozoites for challenge were obtained by infection of adult female Anopheles dirus mosquitoes (the kind gift from William Collins).
Construction of P. knowlesi DNA vaccine plasmids.
The genes encoding the circumsporozoite protein (PkCSP), sporozoite surface protein 2 (PkSSP2), and apical merozoite antigen 1 (PkAMA1) and the gene fragment encoding the 42-kDa carboxy-terminal fragment of merozoite surface protein 1 (PkMSP1p42) were amplified from P. knowlesi H strain genomic DNA by PCR using the following primer pairs: PkAMAfp, CGGATCCATGAATAAAATATACTACATACT, and PkAMArp, GGGATCCTCAGTAGTAAGGCTTCTCCATCAG; PkMSPfp, GGGATCCAAGAAGCAACTGGAGAATCACGTG, and PkMSPrp, TGGATCCTTAGCTGGAAGAACTACAGAAAACTC; PkCSPfp, CGGATCCATGAAGAACTTCATTCTCTTGGCCGTC, and PkCSPrp, GGGATCCTTAATTGAATAATGCTAGGACTAACAA; PvSSP2-10, CCTGGATCCATGAAGCTACTTCAGAACAAAAGC, and PkSSP2rp2, TGGATCCTTATAACTTGAACTGATCTGCCTCTCCAGCGTC. Primer pairs for PkCSP, PkAMA1, and PkMSP1p42 were based on published sequences (2, 18, 33). The published partial sequence of the H strain PkSSP2/TRAP lacks the 39 most N-terminal amino acids (30). Therefore, for PkSSP2, the 5′ PCR primer was based on the 5′ sequence of the corresponding Plasmodium vivax gene, PvSSP2 (22). PCR products were purified from preparative agarose gels and cloned into the pCR-Script plasmid using the pCR-Script Amp SK(+) cloning kit (Stratagene) according to the manufacturer's instructions. The sequence of the cloned PCR product was determined on an ABI Prism model 377 automated sequencer using the Dye Terminator Cycle Sequencing kit (ABI, Warrington, United Kingdom). From each pCR-Script clone, a BamHI fragment containing the desired P. knowlesi antigen gene was excised and cloned into the BamHI site of DNA vaccine vector VR1020 (16) to produce vaccine plasmids encoding PkCSP (VR2560), PkSSP2 (VR2561), PkMSP1p42 (VR2562), and PkAMA1 (VR2563). Vector VR1020 contains an expression cassette under the control of the promoter from the cytomegalovirus intermediate/early 5′ untranslated region including intron A and flanked at the 3′ end by the bovine growth hormone transcription terminator element. In addition, VR1020 encodes the human tissue plasminogen activator as a carboxy-terminal fusion with the inserted antigen gene. A plasmid encoding rhesus GM-CSF (VR1722) was a kind gift from Richard Hedstrom (Naval Medical Research Center, Silver Spring, Md.).
In vitro expression analysis.
Expression of plasmid-encoded antigens was qualitatively determined by immunoblot analysis of transiently transfected UM449 human melanoma cell cultures as previously described (9, 16). Antigens were detected by reaction with polyclonal murine antisera raised by immunization of female CD-1 mice with plasmids encoding PkCSP, PkSSP2, PkAMA1, and PkMSP1p42 as described below.
Construction of recombinant ALVAC-expressing P. knowlesi antigens.
To optimize expression of foreign genes in recombinant canarypox virus ALVAC (19, 29), occurrences of the sequence TTTTTNT, which serves as an early transcriptional terminator in vaccinia virus, must be modified. A single occurrence of this sequence in PkAMA1 was mutagenized to alter the nucleotide sequence without changing the encoded amino acid using the QuickChange mutagenesis kit (Stratagene, Inc., La Jolla, Calif.) and primers Pkmut1, GTCTCATTAATGACAAAAATTTCTTTGCAACAACAGCGTTATCTC, and Pkmut2, GAGATAACGCTGTTGTTGCAAAGAAATTTTTGTCATTAATGAGAC, according to the manufacturer's instructions. The remaining three P. knowlesi genes contain no instances of the sequence TTTTTNT. A donor vector for recombination of genes under the control of the modified H6 vaccinia virus promoter (13, 20), pC6L/H6, was constructed. The complete H6 promoter was amplified from plasmid pBSH6-1 (Virogenetics, Troy, N.Y.) with primers H6-1, CCGAATTCGATCCCCCAACAAAAACTAATCAG, and H6-2, CGCTGCAGATATCGCGACCATGGGCCCATTACGATACAAACTTAACGG. The PCR product was cloned into pCR-Script, the sequence was verified, and the EcoRI/PstI fragment containing the H6 promoter was subcloned into the EcoRI/PstI site of pC6L (Virogenetics), an ALVAC donor plasmid containing a multiple cloning site flanked by 1.1- and 0.4-kb sequences from the C6 region of the ALVAC genome. The four P. knowlesi genes were amplified by PCR using primer pairs that included bases −28 through −1 of the H6 promoter and cloned into NruI- or EcoRV-digested pC6L/H6 to produce four donor plasmids, each containing a single P. knowlesi gene under the control of the H6 promoter flanked by sequences homologous to the C6 region of ALVAC. Each donor plasmid was used to generate a recombinant ALVAC, ALVAC-PkCSP, ALVAC-PkSSP2, ALVAC-PkAMA1, and ALVAC-PkMSP1p42 by in vitro recombination as described previously (29). The mixture of the four P. knowlesi antigen-encoding ALVAC viruses is designated ALVAC-4.
Animals and immunizations.
Animals were used under protocols approved by Institutional Use and Care of Animals Committees at facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. The experiments were conducted according to the principles set forth by the Institute of Laboratory Animals Resources (12a). Female CD-1 mice (6 to 8 weeks old; Charles River Laboratories, Wilmington, Mass.) were injected i.d. with 50 μg of vaccine plasmid (1 mg/ml) in sterile saline in two sites at the base of the tail at weeks 0, 4, and 8. Serum was taken for analysis prior to immunization and 2 weeks after the second and third doses. Thirteen male and 3 female rhesus macaques (Macaca mulatta; Three Springs Scientific, Inc., Perkasie, Pa.; 18 to 33 months old, 3 to 4 kg) were divided into four groups of four animals and immunized with either a mixture of the four P. knowlesi antigen plasmids and the rhesus macacque GM-CSF plasmid, VR1722, at 500 μg/plasmid (groups 1 to 3) or with 2 mg of VR1020 and 500 μg of VR1722 (group 4). Immunization was either i.m. in the tibialis anterior muscle by needle and syringe (group 1), i.m. by Biojector injection (group 2), or a combination of one i.m. injection by Biojector containing 70% of the total dose and three separate i.d. Biojector injections along the anterolateral aspect of the thigh, each containing 10% of the total dose (groups 3 and 4). DNA immunizations were given at weeks 0, 5, and 9. At week 35 the animals were boosted by i.m. injection (needle and syringe) of either 2.5 × 108 PFU of each of four recombinant ALVAC expressing the four P. knowlesi antigens (groups 1 to 3) or 109 PFU of parental ALVAC (group 4).
Parasites and challenge.
At week 40 sporozoites of P. knowlesi H strain were dissected from the salivary glands of infected A. dirus mosquitoes in E199 medium supplemented with 10% heat-inactivated, random-source, normal rhesus macaque serum. For challenge, 100 sporozoites were injected into the saphenous vein. Beginning on day 6 after challenge peripheral thick and thin blood films were examined to determine parasitemia. Parasitemia in thick films was determined by the method of Earle and Perez (8) and in thin films was determined by separate enumeration of infected and total erythrocytes. Animals were treated with drugs when parasitemias reached 2% or higher.
IFA.
Sera from immunized mice and monkeys were analyzed in IFAs against air-dried sporozoites and infected erythrocytes as previously described (22).
PkCSP EIA.
Ninety-six well enzymatic immunoassay (EIA) plates were coated with recombinant PkCSP protein at 0.3 μg/ml in phosphate-buffered saline (PBS), pH 7.0, for 6 h at room temperature, washed three times with 0.05% Tween 20 (Sigma, St. Louis, Mo.) in PBS, and blocked overnight in 5% nonfat dry milk in PBS at 4°C. Appropriate dilutions of test sera in 3% nonfat dried milk in PBS were added to quadruplicate wells, and the plates were incubated at room temperature for 2 h and washed three times with washing buffer. Plates were incubated with horseradish peroxidase-conjugated goat anti-human immunoglobulin G (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) for 1 h at room temperature, washed three times, and incubated for 20 min with ABTS (2,2′-azinobis[3-ethylbenzthiazolinesulfonic acid]) substrate solution (Kirkegaard and Perry Laboratories), and the optical densities at 405 nm (OD405) were read. The background OD405 in wells treated with preimmune serum were subtracted, and the means and standard errors of the means from the quadruplicate wells are reported.
IFN-γ ELISPOT assay.
IFN-γ enzyme-linked immunospot (ELISPOT) assays were carried out as previously described (15). The following peptide pools were used: pool 1, PkCSP amino acids (aa) 11 to 30, SILLVDLLPTHFEHNVDLSR, aa 21 to 40, HFEHNVDLSRAINVNGVSFN, aa 41 to 60, NVDTSSLGAQQVRQSASRGR, and aa 51 to 70, QVRQSASRGRGLGEKPKEGA; pool 2, PkCSP aa 71 to 90, DKEKKKEKGKEKEEEPKKPN, aa 91 to 110, ENKLKQPNEGQPQAQGDGAN, aa 201 to 220, QGDGANAGQPQAQGDGANAG, aa 251 to 270, GGAPAGGNEGNKQAGKGQGQ, and aa 281 to 300, KVVNDYLHKIRSSVTTEWTP; pool 3, PkCSP aa 306 to 325, GNGVRIRRKAHAGNKKAEDL, aa 311 to 330, IRRKAHAGNKKAEDLTMDDL, aa 315 to 334, AHAGNKKAEDLTMDDLEVEA, and aa 320 to 339, KKAEDLTMDDLEVEACVMDK. Means and standard deviations for quadruplicate wells are reported.
Statistical analyses.
Differences between multiple groups were assessed by one-way analysis of variance followed by Tukey's honestly significant difference test to identify which specific groups differed. Comparisons between two groups were made using a two-tailed Student t test. Correlations between parasitemia and measures of immune response were evaluated using the Pearson product moment correlation. All statistical calculations were performed with SigmaStat, version 2.03, for Windows (SPSS, Inc., Chicago, Ill.).
Nucleotide sequence accession numbers.
The sequences of the PkMSP1 and PkAMA1 H strain genes amplified in the vaccine plasmids were determined and deposited in GenBank with accession no. AF298219 and AF298218, respectively. The nucleotide sequence of the product of PCR amplification of the coding sequence of PkSSP2 was deposited in GenBank with accession no. AF298217.
RESULTS
Construction of vaccine plasmids.
Vaccine plasmids encoding PkCSP, PkSSP2, PkMSP1p42, and PkAMA1 in the VR1020 backbone were constructed as described in Materials and Methods. For PkMSP1 and PkAMA1 PCR amplification was performed with primers based on sequences from the Nuri strain. The published sequence encoding H strain PkSSP2 lacked the sequence encoding 39 N-terminal amino acids (30). The full-length sequence of PkSSP2 was amplified from H strain DNA using a 5′ PCR primer based on the P. vivax SSP2 sequence; the product was sequenced and was found to differ from the published sequence at 3 nucleotides (2 synonymous changes and 1 nonsynonymous change).
In vitro expression.
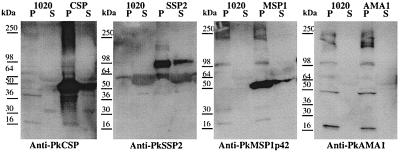
We assessed the ability of the vaccine plasmids to express the desired P. knowlesi gene products in mammalian cells by in vitro transfection of melanoma cell line UM449 and detection of products of the expected molecular weights in denatured sodium dodecyl sulfate-polyacrylamide gel electrophoresis Western blots with antisera raised against DNA vaccine plasmids encoding the P. knowlesi antigens. Figure 1 shows that products of the expected sizes were detected in UM449 cells transfected with plasmids encoding PkCSP (48 kDa), PkSSP2 (95 kDa), and PkMSP1p42 (42 kDa). As a check against the circularity involved in using antisera derived from immunization with a given plasmid to detect the in vitro expression products of the same plasmid, the antisera were tested in IFAs against P. knowlesi sporozoites or infected erythrocytes and were found to have the staining pattern expected for each antigen (data not shown). As for a similar P. vivax AMA1 DNA vaccine (22), no PkAMA1 product was detected in transient transfection assays; however, as described below, immunization with the PkAMA1 plasmid induced antibodies in mice, which showed the expected apical fluorescence pattern in infected erythrocytes. The ability of the four recombinant ALVAC to express PkCSP, PkSSP2, PkAMA1, and PkMSP1p42 was assessed by IFA and Western blotting of infected Vero cells using murine antisera raised by immunization with DNA vaccine plasmids described above. All recombinant ALVAC expressed the corresponding P. knowlesi antigen; for ALVAC PkAMA1, expression could be detected by IFA but not by Western blotting (data not shown).
FIG. 1.
In vitro expression of P. knowlesi candidate vaccine antigens. UM449 cells were transfected either with a control plasmid (1020) or with the indicated antigen plasmid (encoding PkCSP, PkSSP2, PkAMA1, or PkMSP1p42). Protein extracts were prepared from cell pellets (P) or culture supernatants (S) and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described in Materials and Methods. Antigen expression was detected with the antisera indicated beneath each panel.
Murine immunogenicity.
To confirm the in vivo expression and immunogenicity of the P. knowlesi vaccine plasmids, we immunized groups of five CD-1 mice with either a control plasmid (VR1020), each of the individual plasmids encoding PkCSP, PkSSP2, PkAMA1, and PkMSP1p42, or the combination of all four vaccine plasmids, designated tetravalent vaccine, Knowlesi (TVK). Mice were immunized intradermally at the base of the tail with 50 μg of each plasmid three times at 4-week intervals. Sera were obtained 2 weeks after each immunization. Table 1 shows the results of IFA on sporozoites and infected erythrocytes. Mice immunized with either the PkCSP or the PkSSP2 plasmids alone or with TVK produced moderate antisporozoite antibody titers (Table 1); the staining patterns were as expected, diffuse surface staining for PkCSP and patchy staining for PkSSP2. Surprisingly, antisera raised against PkAMA1 also gave moderate antisporozoite antibody titers with a distinctive apical staining pattern. Mice immunized with either the PkMSP1p42 or PkAMA1 plasmids alone or with TVK produced moderate anti-infected erythrocyte antibody titers (Table 1) with the expected staining patterns, an alveolar pattern for PkMSP1p42 and a spotty, apical pattern for PkAMA1. Interestingly, antibodies raised against PkSSP2 did not react in blood stage IFA, consistent with the lack of expression of PfSSP2 (24) and PySSP2 (3) in infected erythrocytes, in spite of the original description of PfSSP2 as a blood stage antigen (21). Immunization with TVK induced comparable or higher titers in response to both sporozoites and infected erythrocytes than did immunization with the individual stage-specific antigen plasmids (Table 1). Neither preimmune sera nor sera from mice immunized with the control plasmid, VR1020, contained antibodies to sporozoites or infected erythrocytes (Table 1).
TABLE 1.
Pooled IFA titers after three doses of P. knowlesi vaccine plasmids in mice
| Vaccine | Sporozoite IFA titera | Blood stage IFA titera |
|---|---|---|
| Control plasmid | <10 | <10 |
| PkCSP | 2,560 | NDc |
| PkSSP2 | 640 | 20 |
| PkAMA1 | 320 | 640 |
| PkMSP1p42 | ND | 320 |
| TVKb | 2,560 | 1,280 |
Titer of pooled sera from five CD-1 mice.
TVK, mixture of PkCSP, PkSSP2, PkAMA1, and PkMSP1p42.
N.D., not done.
Nonhuman primate immunogenicity.
To assess the immunogenicity and protective efficacy of this tetravalent vaccine in nonhuman primates we conducted an immunization-and-challenge study with rhesus monkeys. Based on the results of experiments with the P. yoelii murine model system, which showed enhanced immunogenicity and protective efficacy when a DNA vaccine encoding PyCSP was coinjected with a plasmid encoding murine GM-CSF and boosted with a recombinant poxvirus expressing PyCSP (28), we used a similar immunization regimen with the TVK in rhesus monkeys. In the trial described here, we varied only the route and method of administration of the DNA; three groups of four monkeys were immunized with the TVK and GM-CSF plasmids and boosted with recombinant ALVAC as described in Materials and Methods. One group received the DNA by i.m. injection with needle and syringe, one group received it by i.m. injection with the needleless Biojector device, and one group received it by a combination of the i.m. and i.d. (i.m/i.d.) routes using the Biojector. The group receiving the control vaccine received it via the i.m./i.d. route.
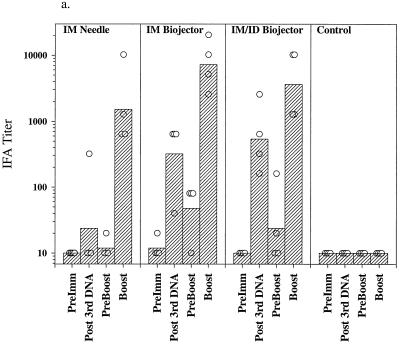
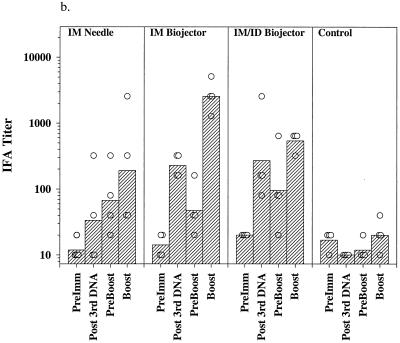
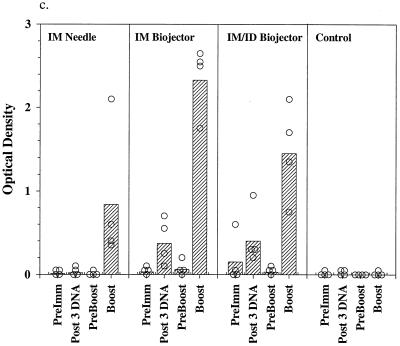
Figure 2 shows the results of IFA and EIA analysis of sera from the immunized monkeys. Preimmune sera and sera from control immunized monkeys contained no detectable antibodies to sporozoites (Fig. 2a) and minimal background titers against infected erythrocytes (Fig. 2b). Following the third dose of DNA the geometric mean antisporozoite and anti-infected red blood (irbc) cell titers were highest in the group immunized by the i.m./i.d. route (1:538 for sporozoites; 1:269 for irbc) although the difference between the two Biojector groups, i.m. and i.m./i.d., was not statistically significant. Titers in the i.m. needle group were barely detectable. Titers declined toward baseline during the 6-month interval between the third DNA dose and the poxvirus boost. Three weeks following the ALVAC boost, geometric mean antisporozoite and anti-irbc IFA titers were highest in the group which had received the DNA prime i.m. by Biojector (1:7,241 for sporozoites, 1:2,560 for irbc; P = 0.097 and 0.046, respectively, compared with needle group by two-tailed t test); the difference between the two Biojector groups was not statistically significant. The response to PkCSP was analyzed by EIA using a recombinant PkCSP as the target antigen. As shown in Fig. 2c, following the third dose of DNA only the monkeys immunized with the Biojector had significant antibodies to PkCSP. Following the canarypox virus boost the highest ODs at a 1:100 dilution of serum were observed in the i.m. Biojector group and were statistically higher than those seen in the i.m./i.d. Biojector group (P = 0.049; two-tailed t test) and the i.m. needle group (P = 0.019; two-tailed t test).
FIG. 2.
Antibody responses in immunized rhesus monkeys. Shown are IFA titers against P. knowlesi sporozoites (a) and P. knowlesi-infected rhesus erythrocytes (b) and OD405 values obtained from a 1:100 dilution of serum in an EIA against recombinant PkCSP. Hatched bars, geometric mean titers (a and b) or mean OD405 values (c) for each group (i.m. needle, i.m. Biojector, i.m./i.d. Biojector, control); circles, individual values from each animal. Serum samples were obtained before immunization (PreImm), 2 weeks following the third dose of DNA (Post 3rd DNA), immediately prior to the viral boost, 26 weeks after the last DNA dose (PreBoost), and 2 weeks following the viral boost (Boost).
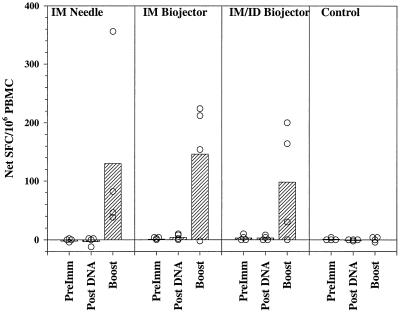
Figure 3 and Table 2 show the results of IFN-γ ELISPOT assays on peripheral blood mononuclear cells (PBMC) from the immunized monkeys. Neither preimmune PBMC nor PBMC obtained 2 weeks after administration of the third DNA dose secreted IFN-γ in response to the three peptide pools (Fig. 3 and data not shown); following the recombinant ALVAC boost, PBMC from 10 of 12 immunized monkeys produced IFN-γ-secreting cells at net frequencies ranging from 29 to 355 spot-forming cells (SFC)/106 PBMC. Table 2 summarizes the results of ELISPOT assays carried out with the three pools of peptides on PBMC obtained after the poxvirus boosting of the animals. Three of 12 experimental monkeys responded to peptide pool 1, 10 of 12 responded to pool 2, and 5 of 12 responded to pool 3 at net frequencies greater than the mean plus 2 standard deviations of the net frequency in control animals. There were no statistically significant differences in the mean IFN-γ responses between the three experimental groups immunized by different routes.
FIG. 3.
IFN-γ ELISPOT responses to pool 2 peptides. Shown are the net numbers of IFN-γ SFC per million PBMC (SFC in cells incubated with peptides in pool 2 minus SFC in cells incubated with medium alone). PBMC were obtained prior to immunization (PreImm), 2 weeks after the third DNA dose (Post DNA), and 2 weeks after the viral boost (Boost). Hatched bars, mean values for each group (i.m. needle, i.m. Biojector, i.m./i.d. Biojector, control); circles, individual values from each animal. Standard deviations of quadruplicate values for individual animals are shown in Table 2.
TABLE 2.
IFN-γ ELISPOT responses after boost of rhesus monkeys with recombinant canarypox virus (ALVAC-4)
| Route | Monkey | Net IFN-γ SFC/106 PBMC (SD)a for pool:
|
||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Needle i.m. | TCD | 5 (1) | 38 (5) | 89 (7) |
| PEH | 1 (3) | 355 (15) | 4 (2) | |
| JGF | 5 (4) | 81 (6) | 1 (3) | |
| PTT | −1 (2) | 45 (9) | 48 (8) | |
| Biojector i.m. | PWA | 3 (1) | 153 (2) | −1 (1) |
| TJE | 10 (2) | 224 (5) | 5 (1) | |
| PPG | −1 (3) | −4 (2) | −8 (2) | |
| PWH | 26 (4) | 211 (7) | 43 (3) | |
| Biojector i.m./i.d. | TJX | −1 (1) | −1 (1) | −3 (1) |
| TXA | 125 (13) | 164 (18) | 44 (12) | |
| PVG | 28 (7) | 199 (12) | 15 (6) | |
| PJE | 1 (1) | 29 (2) | 1 (1) | |
| Control | GKK | 3 (1) | 3 (1) | 1 (1) |
| TXP | 5 (0) | 3 (0) | 8 (1) | |
| KCV | 6 (2) | −5 (2) | −5 (2) | |
| KJT | 3 (1) | −1 (1) | 0 (1) | |
Shown are the means and standard deviations (SD) of the net (peptide-stimulated − medium control) values from quadruplicate wells. Values in boldface exceed the mean + 2 SD of the control animals' responses.
Protective efficacy.
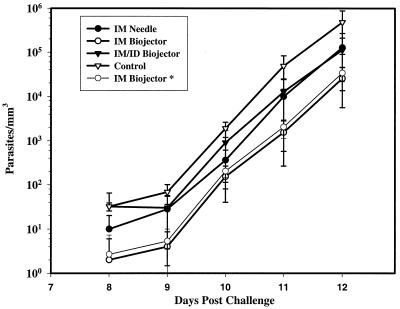
Five weeks after the ALVAC boost the monkeys were challenged by saphenous vein injection of 100 H strain P. knowlesi sporozoites on day 0 of the challenge. Beginning on day 6 daily peripheral blood smears, thick and thin, were examined for P. knowlesi parasites. Animals were treated when the parasitemia reached 2% or on day 14. The individual parasitemias of the monkeys from days 7 to 12 are shown in Table 3, and the mean parasitemias of each group are plotted in Fig. 4. All of the control monkeys (4 of 4) and all but one (PPG) of the TVK-immunized monkeys (11 of 12) became parasitemic, and all but 2 of the parasitemic monkeys (1 control, KJT, and 1 from the i.m./i.d. Biojector group, TJX) required treatment for parasitemia greater than 2% on day 12 or 13 (range of parasitemia at time of treatment, 2 to 29%). On each day of the parasitemic phase of the study, the mean parasitemias in the experimental groups were lower than those in the control group. Analysis of variance on the mean parasitemias on each of days 9 to 12, before any monkeys received treatment, detected statistically significant differences between groups (P = 0.024, day 9; P = 0.004, day 10; P = 0.023, day 11; P = 0.048, day 12). The differences remained statistically significant even if the monkey which did not develop parasitemia, PPG, was excluded from the analysis. Application of Tukey's test identified statistically significant differences between the control group and the i.m. Biojector group on days 9 to 12 (P = 0.016, 0.004, 0.032, and 0.047, respectively) and between the control group and the i.m. needle group on day 10 (P = 0.010). Comparison of control monkeys versus all 12 immunized monkeys by two-tailed t test also identified statistically significantly lower parasitemias in the immunized monkeys on days 9 to 12 (P = 0.010, 0.002, 0.003, and 0.007, respectively). In the group with the lowest mean parasitemia, the i.m. Biojector group, parasitemias were more than 10-fold lower than those in the control group on days 9 to 12. In all groups, parasitemia increased approximately 10-fold every 24 h, consistent with release of 10 merozoites from each infected erythrocyte in a 24-h cycle in P. knowlesi.
TABLE 3.
Parasitemias following challenge with P. knowlesi sporozoitesa
| Monkey | Route | Parasitemia (parasites/mm−3) on day:
|
|||||
|---|---|---|---|---|---|---|---|
| 7 | 8 | 9 | 10 | 11 | 12 | ||
| JGF | Needle i.m. | 0 | 8 | 24 | 464 | 5,600 | 136,000 |
| PEH | 0 | 24 | 24 | 112 | 2,680 | 43,000 | |
| TCD | 0 | 8 | 64 | 656 | 30,800 | 325,200 | |
| PTT | 0 | 0 | 0 | 208 | 528 | 7,300 | |
| PPG | Biojector i.m. | 0 | 0 | 0 | 0 | 0 | 0 |
| PWH | 0 | 0 | 0 | 248 | 1,440 | 32,400 | |
| TJE | 0 | 0 | 8 | 224 | 1,600 | 22,100 | |
| PWA | 0 | 8 | 8 | 136 | 3,120 | 47,000 | |
| PVG | Biojector i.m./i.d. | 0 | 56 | 56 | 1,968 | 4,800 | 245,200 |
| PJE | 0 | 64 | 48 | 488 | 17,680 | 121,000 | |
| TJX | 0 | 8 | 16 | 1,080 | 27,200 | 68,200 | |
| TXA | 0 | 0 | 0 | 72 | 880 | 14,500 | |
| GKK | Control i.m./i.d. | 0 | 24 | 96 | 2,184 | 95,600 | 1,000,000 |
| KCV | 0 | 32 | 88 | 1,528 | 52,800 | 538,400 | |
| KJT | 0 | 40 | 64 | 2,776 | 21,360 | 68,600 | |
| TXP | 0 | 32 | 24 | 1,136 | 25,100 | 329,200 | |
Shown are parasitemias determined from thick-film examination. Monkeys were challenged on day 0 with 100 H strain P. knowlesi sporozoites.
FIG. 4.
Parasitemia in monkeys following challenge with 100 P. knowlesi sporozoites. Shown are means and standard deviations of the parasitemias in each group of challenged monkeys, determined from thick-film examination as described in Materials and Methods. IM Biojector*, means and standard deviations of that group when the single nonparasitemic monkey is excluded.
Association between immune responses and parasitemia.
We attempted to identify immunological markers as correlates of protection by correlating the mean parasitemia for each monkey group on each of days 9 to 12 with each of six immunological variables, geometric mean IFA titer for sporozoites or infected erythrocytes, mean OD value at 1:100 dilution in the PkCSP EIA, and mean frequency of IFN-γ SFC in response to each of three peptide pools. On each of days 9 to 12 there was a significant negative correlation between the IFN-γ response to peptide pool 2 and parasitemia (correlation coefficients were −0.965 [P = 0.035], −0.995 [P = 0.0047], −0.989 [P = 0.011], and −0.973 [P = 0.027] on days 9 to 12, respectively, by Pearson product moment correlation). No other immunological variable was significantly correlated with parasitemia.
DISCUSSION
We have demonstrated the immunogenicity and modest protective efficacy of a four-gene vaccination regimen consisting of a DNA prime with canarypox virus boost in the P. knowlesi/rhesus macaque malaria model system. This study was designed to address the efficacy of this prime boost regimen and to compare the levels of effectiveness of different methods of administration of the DNA prime. We did not here separate the effects of the several components of this regimen. In a separate, recent study we addressed the effect of the addition of the rhesus macaque GM-CSF plasmid to the prime and the use of the recombinant canarypox virus in the boost phase of the regimen. Preliminary results suggest that inclusion of the rhesus macaque GM-CSF plasmid does not improve antibody responses. Induction of optimal antibody responses requires boosting with recombinant canarypox virus; significantly, however, even two doses of the recombinant canarypox virus given without prior boosting with the DNA vaccine plasmids failed to induce detectable antibodies to sporozoites or blood stage parasites (unpublished results). Thus, the effect of the poxvirus boost here was to amplify responses primed by DNA, rather than to induce de novo immune responses.
Immunization of rhesus monkeys with the prime boost regimen tested here induced antibodies against preerythrocytic- and erythrocytic-stage P. knowlesi parasites and against a recombinant PkCSP protein and T cells which secreted IFN-γ in response to incubation with peptides from PkCSP. T cells secreting IFN-γ in response to pooled peptides from PkCSP were detected in 11 of 12 monkeys following the recombinant poxvirus boost at frequencies greater than the mean plus 2 standard deviations of the background frequencies found in control monkeys. No detectable IFN-γ responses were found prior to the poxvirus boost; in this experiment, at least, DNA vaccination alone failed to induce T-cell responses in rhesus monkeys. The maximum frequencies of IFN-γ SFC found in this study were in the range of 200 to 350 SFC/106 PBMC. These frequencies are approximately 10-fold lower than those observed when a similar regimen involving DNA prime with poxvirus boost was used to immunize mice against the P. yoelii CSP in a murine malaria vaccine model (28). The study by Sedegah et al. differs in several important respects from the present study; in the murine study, a single antigen, PyCSP, was used and the poxvirus used in the boost phase was an attenuated vaccinia virus rather than the recombinant canarypox virus used here. It is not clear whether the lower responses observed in the present study are the result of the different levels of responsiveness to this approach in mice and monkeys, to the use of recombinant canarypox virus rather than vaccinia virus, or to possible antigenic competition between the four antigens used in this study.
The vaccination regimen used here provided modest evidence of protection. A single immunized monkey in the i.m. Biojector group was completely protected against sporozoite challenge. Among the remaining immunized monkeys there was a lower mean parasitemia on each of days 9 to 12 of the challenge phase. In the group which had the lowest parasitemias on each day, the i.m. Biojector group, parasitemias were slightly more than 10-fold lower than those observed in controls. Both the absence of parasitemia and the reduced parasitemias in the vaccinated monkeys could, in principle, be due to immune responses directed at either the preerythrocytic or erythrocytic stages of the life cycle. However, the slopes of the parasitemia curves (Fig. 4) do not appear to differ significantly between the immunized and control groups, implying that there was no effective growth-inhibitory response directed against blood stage parasites. It therefore appears more likely that the modest protection seen here was the result of a response directed against the preerythrocytic stages, leading to a reduced number of liver stage schizonts being released into the circulation at the beginning of the blood stage infection. Given an observed multiplication rate of approximately 10-fold in 24 h, 10-fold-lower parasitemias on any given day or a 1-day delay in reaching equivalent parasitemias in the vaccinated monkeys imply an approximately 90% reduction in the liver stage parasite burden. It is interesting that the reduction in parasitemia on each of days 9 to 12 correlated with the magnitude of the IFN-γ response to the peptide pool to which the majority of the immunized monkeys responded. This correlation is not absolute at the level of the individual monkey, however, as the sterilely protected monkey, PPG, made no detectable IFN-γ response to any of the peptide pools tested. This correlation is consistent with experiments with the murine P. yoelii system, which suggest that DNA vaccine-induced protection is IFN-γ dependent (6, 28). There are limitations to the analysis of T-cell immune responses presented here. First, we examined only a subset of possible peptides from PkCSP; we did not assess T-cell responses to PkSSP2 or to the two blood stage antigens. It is possible that a better correlation between T-cell responses and protection at the level of individual monkeys would have been evident if we had been able to evaluate responses to all possible targets. Second, in the present experiments we did not have sufficient numbers of prechallenge PBMC to test the T-cell subset dependence of the IFN-γ response; in the several murine P. yoelii vaccine models, preerythrocytic-stage protection is usually dependent on CD8+ T cells but can be dependent on CD4+ T cells (6). In experiments carried out to develop the rhesus IFN-γ ELISPOT assay, we found that the T-cell responses observed in monkeys immunized by this DNA prime plus canarypox virus boost were CD4+ but not CD8+ T cell dependent (15). As the IFN-γ ELISPOT assay used here is based on pools of 20-mer peptides, it is possible that it is not optimal for detection of CD8+ T-cell responses. Thus, although it appears that the partial protection seen here was the result of an IFN-γ-mediated response directed against preerythrocytic-stage parasites in the liver, the T-cell subset dependence and detailed mechanism of protection remain to be elucidated.
A secondary goal of these experiments was to examine the effect of delivering the priming DNA dose by three different routes, i.m. injection by needle, i.m. injection by the needleless Biojector device, and a combination of the i.m. (70% of dose) and i.d. (30% of dose) routes by the Biojector. The route of administration of the viral boost was i.m. by needle injection in all groups. For all of the immune responses measured following the viral boost the responses consistently followed the rank order Biojector i.m. > Biojector i.m./i.d. > needle i.m. In addition, on each of days 9 to 12 the lowest parasitemias were seen in the Biojector i.m. group. The differences between the i.m. Biojector and i.m./i.d. Biojector groups did not reach statistical significance for any immune system parameter measured. The difference between the i.m. Biojector and the i.m. needle group was statistically significant for the IFA titer in response to blood stage parasites (P = 0.046; two-tailed t test on log-transformed titers) and the PkCSP EIA (P = 0.019; two-tailed t test). In brief, there was a consistent trend for delivery of the DNA priming dose by the i.m. Biojector route to be more effective than the other routes by all tested measures of immune response, including protection. The trend, however, reached statistical significance only in isolated comparisons between the i.m. Biojector and i.m. needle groups.
There are several possible reasons why neither the level of immune response nor protection in this experiment was comparable to those seen in recent studies in the P. yoelii mouse malaria model (27, 28). First, there may be differences in the response of mice and monkeys to the DNA prime-plus-poxvirus boost regimen. Second, the challenge with 100 P. knowlesi H strain sporozoites may be more stringent than the challenge with 100 P. yoelii nonlethal strain sporozoites used in the mouse model. Finally, the recombinant canarypox virus used in the boost phase here may be less immunogenic than the attenuated vaccinia virus used in the murine studies. We have therefore undertaken a study of a similar four-gene P. knowlesi DNA prime-plus-virus boost vaccine regimen using recombinant vaccinia virus rather than canarypox virus in the prime.
In brief, we have demonstrated the ability of a four-gene DNA prime-plus-canarypox virus boost malaria vaccine regimen to induce both antibody and T-cell responses and partial protection against sporozoite challenge. We thus have established a nonhuman primate model for a multicomponent, multistage malaria vaccine in which it is possible to measure both antibody and T-cell responses and in which, following sporozoite challenge, it is possible to assess protective effects at both the preerythrocytic and erythrocytic stages of the parasite life cycle. In this experiment 1 of 4 controls did not require treatment; pooling results from all challenges in this system, 17 of 18 controls required treatment (W. O. Rogers, unpublished data). Given this occasional failure of controls to require treatment, detection of very low levels of erythrocytic-stage protection may be difficult. P. knowlesi in rhesus macaques is not a perfect model for P. falciparum in humans; at a genetic level it is more closely related to P. vivax than to P. falciparum; it has a quotidian rather than a tertian cycle in the blood; it is not a reliable model for cerebral malaria; finally, it may be more lethal in rhesus macaques than is P. falciparum in humans. Nonetheless, we believe that this model will be an important preclinical system with which to test enhanced vaccines and vaccine delivery systems prior to selecting candidates for testing in humans.
ACKNOWLEDGMENTS
This work was supported by Naval Medical Research Center Work Unit STOF 6.2.622787A.0101.870.EFX and by a Cooperative Research and Development Agreement between the Naval Medical Research Center and Aventis Pasteur, Inc.
We thank Daniel Carucci for assistance with the automated counting of the ELISPOTs, William Collins for providing A. dirus mosquitoes, John Sacci for assistance in dissection of mosquitoes and purification of sporozoites, Richard Stout (Bioject, Inc., Portland, Oreg.) for the gift of Biojector syringes, Brett Saladino, Angela King, Donald Randolph, and Bryan Cosling for assistance in care of the rhesus monkeys, Pradeep Rathore for help with production of recombinant PkCSP, and Heidi Lee for technical assistance.
REFERENCES
- 1.Becker S I, Wang R, Hedstrom R C, Aguiar J C, Jones T R, Hoffman S L, Gardner M J. Protection of mice against Plasmodium yoelii sporozoite challenge with P. yoelii merozoite protein 1 DNA vaccines. Infect Immun. 1998;66:3457–3461. doi: 10.1128/iai.66.7.3457-3461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackman M J, Dennis E D, Hirst E M A, Kocken C H, Scott-Finnigan T J, Thomas A W. Plasmodium knowlesi: secondary processing of the malaria merozoite surface protein-1. Exp Parasitol. 1996;83:229–239. doi: 10.1006/expr.1996.0069. [DOI] [PubMed] [Google Scholar]
- 3.Charoenvit Y, Leef M L, Yuan L F, Sedegah M, Beaudoin R L. Characterization of Plasmodium yoelii monoclonal antibodies directed against stage-specific sporozoite antigens. Infect Immun. 1987;55:604–608. doi: 10.1128/iai.55.3.604-608.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin W, Contacos P G, Coatney G R, Kimball H R. A naturally acquired quotidian-type malaria in man transferrable to monkeys. Science. 1965;149:865. doi: 10.1126/science.149.3686.865. [DOI] [PubMed] [Google Scholar]
- 5.Clyde D F, Most H, McCarthy V C, Vanderberg J P. Immunization of man against sporozoite-induced falciparum malaria. Am J Med Sci. 1973;266:169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Doolan D L, Hoffman S L. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165:1453–1462. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 7.Doolan D L, Sedegah M, Hedstrom R C, Hobart P, Charoenvit Y, Hoffman S L. Circumventing genetic restriction of protection against malaria with multi-gene DNA immunization: CD8+ T cell, interferon-gamma, and nitric oxide dependent immunity. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Earle W S, Perez M. Enumeration of parasites in the blood of malarial patients. J Lab Clin Med. 1932;17:1124. [Google Scholar]
- 9.Felgner P L, Gadek T R, Holm M, Roman R, Chan H W, Wenz M, Northrop J P, Rongold G M, Danielsen M. Lipofectin: a highly efficient, lipid-mediated DNA transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gramzinski R A, Maris D C, Doolan D, Charoenvit Y, Obaldia N, Rossan R, Sedegah M, Wang R, Hobart P, Margalith M, Hoffman S. Malaria DNA vaccines in Aotus monkeys. Vaccine. 1997;15:913–915. doi: 10.1016/s0264-410x(96)00270-8. [DOI] [PubMed] [Google Scholar]
- 11.Gramzinski R A, Millan C L B, Obaldia N, Hoffman S L, Davis H L. Immune response to a hepatitis B DNA vaccine in Aotus monkeys: a comparison of vaccine formulation, route, and method of administration. Mol Med. 1998;4:109–118. [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg R S, Maxwell L G, Zahurak M, Yaster M. Preanesthetic medication of children with midazolam using the Biojector jet injector. Anesthesiology. 1995;83:264–269. doi: 10.1097/00000542-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 12a.Grossblatt N, editor. Guide for the care and use of laboratory animals. Washington, D.C.: Institute for Laboratory Animal Resources, National Academy Press; 1996. [Google Scholar]
- 13.Guo P X, Goebel S, Davis S, Perkus M E, Languet B, Desmettre P, Allen G, Paoletti E. Expression in recombinant vaccinia virus of the equine herpesvirus 1 gene encoding glycoprotein gp13 and protection of immunized animals. J Virol. 1989;63:4189–4198. doi: 10.1128/jvi.63.10.4189-4198.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gwadz R W, Cochrane A H, Nussenzweig V, Nussenzweig R S. Preliminary studies on vaccination of rhesus monkeys with irradiated sporozoites of Plasmodium knowlesi and characterization of surface antigens of these parasites. Bull W H O. 1979;57(Suppl. 1):165–173. [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A, Weiss W, Tine J A, Hoffman S L, Rogers W O. ELISPOT assay for detection of peptide specific interferon-secreting cells in Rhesus macaques. J Immunol Methods. 2001;247:49–60. doi: 10.1016/s0022-1759(00)00310-0. [DOI] [PubMed] [Google Scholar]
- 16.Luke C J, Carner K, Liang X, Barbour A G. An OspA-based DNA vaccine protects mice against infection with Borrelia burgdorferi. J Infect Dis. 1997;175:91–97. doi: 10.1093/infdis/175.1.91. [DOI] [PubMed] [Google Scholar]
- 17.Nussenzweig R S, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 18.Ozaki L S, Svec P, Nussenzweig R S, Nussenzweig V, Godson G N. Structure of the Plasmodium knowlesi gene coding for the circumsporozoite protein. Cell. 1983;34:815–822. doi: 10.1016/0092-8674(83)90538-x. [DOI] [PubMed] [Google Scholar]
- 19.Paoletti E, Taylor J, Meignier B, Meric C, Tartaglia J. Highly attenuated poxvirus vectors: NYVAC, ALVAC and TROVAC. Dev Biol Stand. 1995;84:159–163. [PubMed] [Google Scholar]
- 20.Perkus M E, Limbach K, Paoletti E. Cloning and expression of foreign genes in vaccinia virus, using a host range selection system. J Virol. 1989;63:3829–3836. doi: 10.1128/jvi.63.9.3829-3836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robson K J, Hall J R, Jennings M W, Harris T J, Marsh K, Newbold C I, Tate V E, Weatherall D J. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature. 1988;335:79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- 22.Rogers W O, Gowda K, Hoffman S L. Construction and immunogenicity of DNA vaccine plasmids encoding four Plasmodium vivax candidate vaccine antigens. Vaccine. 1999;17:3136–3144. doi: 10.1016/s0264-410x(99)00146-2. [DOI] [PubMed] [Google Scholar]
- 23.Rogers W O, Hoffman S L. Malaria vaccines. In: Wahlgren M, Perlmann P, editors. Malaria: molecular and clinical aspects. London, United Kingdom: Harwood Academic Publishers; 1999. pp. 439–493. [Google Scholar]
- 24.Rogers W O, Malik A, Mellouk S, Nakamura K, Rogers M D, Szarfman A, Gordon D M, Nussler A K, Aikawa M, Hoffman S L. Characterization of Plasmodium falciparum sporozoite surface protein 2. Proc Natl Acad Sci USA. 1992;89:9176–9180. doi: 10.1073/pnas.89.19.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider J, Gilbert S C, Blanchard T J, Hanke T, Robson K J, Hannan C M, Becker M, Sinden R, Smith G L, Hill A V. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 26.Sedegah M, Hedstrom R, Hobart P, Hoffman S L. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sedegah M, Jones T R, Kaur M, Hedstrom R, Hobart P, Tine J A, Hoffman S L. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of a malaria DNA vaccine. Proc Natl Acad Sci USA. 1998;95:7648–7653. doi: 10.1073/pnas.95.13.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sedegah M, Weiss W, Sacci J B, Jr, Charoenvit Y, Hedstrom R, Gowda K, Majam V F, Tine J, Kumar S, Hobart P, Hoffman S L. Improving protective immunity induced by DNA-based immunization: priming with antigen and GM-CSF-encoding plasmid DNA and boosting with antigen-expressing recombinant poxvirus. J Immunol. 2000;164:5905–5912. doi: 10.4049/jimmunol.164.11.5905. [DOI] [PubMed] [Google Scholar]
- 29.Taylor J, Meignier B, Tartaglia J, Languet B, VanderHoeven J, Franchini G, Trimarchi C, Paoletti E. Biological and immunogenic properties of a canarypox-rabies recombinant, ALVAC-RG (vCP65) in non-avian species. Vaccine. 1995;13:539–549. doi: 10.1016/0264-410x(94)00028-l. [DOI] [PubMed] [Google Scholar]
- 30.Templeton T J, Kaslow D C. Cloning and cross-species comparison of the thrombospondin-related anonymous protein (TRAP) gene from Plasmodium knowlesi, Plasmodium vivax, and Plasmodium gallinaceum. Mol Biochem Parasitol. 1997;84:13–25. doi: 10.1016/s0166-6851(96)02775-2. [DOI] [PubMed] [Google Scholar]
- 31.Wang B, Doolan D L, Le T P, Hedstrom R C, Coonan K M, Charoenvit Y, Jones T R, Hobart P, Margalith M, Ng J, Weiss W R, Sedegah M, de Taisne C, Norman J A, Hoffman S L. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 32.Wang R, Doolan D L, Charoenvit Y, Hedstrom R C, Gardner M J, Hobart P, Tine J, Sedegah M, Fallarme V, Sacci J B, Jr, Kaur M, Klinman D M, Hoffman S L, Weiss W R. Simultaneous induction of multiple antigen-specific cytotoxic T lymphocytes in nonhuman primates by immunization with a mixture of four Plasmodium falciparum DNA plasmids. Infect Immun. 1998;66:4193–4202. doi: 10.1128/iai.66.9.4193-4202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waters A P, Thomas A W, Mitchell G H, McCutchan T F. Intra-generic conservation and limited inter-strain variation in a protective minor surface antigen of Plasmodium knowlesi merozoites. Mol Biochem Parasitol. 1991;44:141–144. doi: 10.1016/0166-6851(91)90230-4. [DOI] [PubMed] [Google Scholar]
- 34.Weiss W R, Ishii K J, Hedstrom R C, Sedegah M, Ichino M, Barnhart K, Klinman D M, Hoffman S L. A plasmid encoding murine granulocyte-macrophage colony-stimulating factor increases protection conferred by a malaria DNA vaccine. J Immunol. 1998;161:2325–2332. [PubMed] [Google Scholar]
- 35.World Health Organization. World malaria situation in 1992. Wkly Epidemiol Rec. 1994;69:309–314. [PubMed] [Google Scholar]