Abstract
There is a growing interest in the use of alternative medical systems (AMS), such as traditional Chinese medicine (TCM), ayurveda, homeopathy, and naturopathy, among chronic kidney disease patients. This review summarizes the efficacy and safety of AMS interventions in chronic kidney disease (CKD) patients. A systematic review was conducted in MEDLINE, Embase, Scopus, CINAHL, CENTRAL, and PsycINFO in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Synthesis without meta-analysis (SWiM) guidelines. Randomized controlled trials (RCTs) which evaluated the use of AMS among adult CKD patients were included. The efficacy of each AMS was assessed based on improvement in biochemical markers or reduction in symptom severity scores. All adverse reactions were recorded. Of the 14,583 articles retrieved, 33 RCTs were included. TCM (n=20) and ayurveda (n=6) were the most well-studied. Majority of studies (66.7%) had a sample size <100. Common indications evaluated included improvement in renal function (n=12), proteinuria (n=5), and uremic pruritus (n=5). Among TCM, acupuncture and syndromes-based TCM granules formulation were shown to improve estimated glomerular filtration rate (eGFR) by 5.1-15.5% and 7.07-8.12% respectively. Acupuncture reduced uremic pruritus symptoms by 54.7-60.2% while Huangkui, Shenqi granules, and Tripterygium wilfordii Hook F reduced proteinuria by 18.6-50.7%, 61.8%, and 32.1% respectively. For Ayurveda, camel milk and Nigella sativa oil improved eGFR by 16.9% and 86.8%, respectively, while capsaicin reduced pruritus scores by 84.3%. Homeopathic verum medication reduced pruritus scores by 29.2-41.5%. Nausea was the most common adverse effect reported with alpha-keto amino acids (0.07%), Nigella sativa oil (7.04%), and silymarin (10%). TCM and ayurveda were more well-studied AMS therapies that demonstrated efficacy in CKD patients. RCTs with larger sample sizes are needed to ascertain the efficacy and safety of promising AMS.
Keywords: renal insufficiency, chronic kidney diseases, complementary therapies, therapeutics, alternative medical systems, systematic reviews
Introduction and background
Chronic kidney disease (CKD) is one of the leading causes of death globally, which affects 13.4% of the world’s population [1]. With deterioration in renal function, this leads to the onset of CKD-related complications, such as uremia, anemia, and electrolyte disorders [2]. These complications often manifest as symptoms ranging from pruritus, pain, and insomnia to muscle cramps. This in turn has negative implications on patients’ quality of life [3,4]. Importantly as CKD patients approach end-stage renal disease (ESRD), the prevalence and severity of such symptoms increase [5].
Despite medical breakthroughs and the advent of new therapies in the past decades, optimal treatments for some of the symptoms resulting from CKD-related complications remained unclear, possibly due to their complex pathophysiology. A case in point is uremic pruritus, which is found in around 20% of pre-dialysis CKD patients and 40% of ESRD patients [6]. Although prevalent treatments include the use of emollients, gabapentin, and antihistamines, data related to their efficacy were often derived from small studies and their use is limited by adverse effects [7].
The use of alternative medical systems (AMS) which forms a key pillar of complementary and alternative medicine (CAM) has increased in the past 20 years [8]. AMS is defined as “entire systems of health theory and practice that developed separately from conventional medicine” [9]. Notably, around 18% of dialysis patients have utilized some form of AMS [10,11]. In addition, prescription of AMS therapies such as traditional Chinese medicine (TCM) by professional practitioners often aids in minimizing the risk of side effects, hence increasing their appeal as potential therapeutic alternatives [11].
Prior studies have shown that AMS is effective in reducing symptoms such as pain, nausea, and fatigue in non-CKD patient populations. For instance the use of TCM formulas, such as Liu Junzi Tang and Xiao Banxia Plus Fuling have demonstrated efficacy in treating cancer-related pain and chemotherapy-related nausea and vomiting [12]. In addition, Chinese herbs such as Curcuma longa and Panax ginseng among patients with malignancies have shown efficacy in promoting apoptosis of cancer cells and inhibiting tumor metastasis [13]. Another study showed that a multi-modal Ayurvedic treatment approach was effective in reducing knee osteoarthritis symptoms, such as pain and stiffness, and improving function [14]. With increasing research supporting the use of AMS, this has led to a rise in healthcare institutions adopting and providing such integrated services which are supported by insurance coverage [15].
Among CKD patients, multiple studies have also been conducted to assess the efficacy of AMS in the treatment of CKD-related conditions and symptoms such as uremic pruritis and anemia. For instance, a study that assessed the efficacy of homeopathy verum among CKD patients showed a reduction in pruritus symptoms by 49% after 30 days of treatment [16]. Another study that evaluated the use of TCM patients with glomerulonephritis showed improvement in hemoglobin after 24 weeks of therapy [16,17].
Existing reviews which have assessed the role of AMS are currently limited to specific indications, such as uremic pruritus [18], use of subtypes of AMS in specific CKD subgroups, such as consumption of Chinese herbal medicine in diabetic kidney disease [19], and specific AMS therapies, such as use of Astragalus [20,21]. This review aimed to summarize and evaluate the broad roles and efficacy of AMS as potential alternative therapeutic options for CKD patients. Findings from the review will aid physicians in gaining a better understanding of the efficacy of AMS for CKD patients, which can aid in facilitating purposeful discussions with patients who are using or considering these therapies.
Review
Methods
Protocol and Registration
The protocol for this study was registered on Open Science Framework (https://osf.io/ymks8/) and was composed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and Synthesis without Meta-Analysis (SWiM) reporting guidelines [22,23].
Information Sources and Search
A literature search was conducted in MEDLINE, Embase, Scopus, CENTRAL, CINAHL, and PsycINFO. There was no start date restriction, and studies up to April 2022 were included. Key terms related to CKD, randomized controlled trials (RCT), and AMS were included in the searches. The search terms were adapted from other systematic reviews and the full search strategy is available in Appendix 1 [18,24-26].
Eligibility Criteria
With regards to inclusion criteria, full-text articles in English language which involved RCTs evaluating the use of AMS in adult CKD patients (>18 years old) were included. As defined by the National Center for Complementary and Integrative Health (NCCIH), AMS is a broad category encompassing a variety of medical modalities and refers to an entire system of theory and practice which developed separately from conventional medicine [15]. In this review, we included TCM, naturopathy, homeopathy, and ayurvedic medicine. Non-RCTs, case series, other systematic reviews, and meta-analyses were excluded.
Description of Main Types of AMS
TCM: TCM is a system of medical practice which originated in China and adopts a holistic approach to the medical treatment of a patient based on “syndrome differentiation.” It focuses on the integrity of the human body by emphasizing the intimate relationship between the body and its social and natural environment, as well as dynamic balance of movement [27]. The basic tenet of TCM is based on the flow and balance of vital energy, Qi, which flows through channels in the body called meridians that connect various organs and tissues [28]. Diseases are believed to be brought about by the imbalance of Qi. Hence, by restoring balance via acupoints or intake of herbs, TCM seeks to promote individual wellness and prevent diseases [29].
Naturopathy: Naturopathy is a form of medical practice which is rooted in vitalism and folk medicine and, promotes natural and self-healing ideologies [30]. The unique attribute of naturopathic medicine lies in the reprioritization of the order of therapeutics with increased emphasis on preventive behaviors, lifestyle modifications, nutrition, and exercise, over medical or surgical interventions [31].
Homeopathy: Homeopathy entails the therapeutic administration of substances derived from plants, minerals, or animals that produce effects that correspond to the clinical manifestation of diseases [32]. Its practice is centered on two following theories: “like cures like” and “law of minimum dose.” “Like cures like” refers to the belief that diseases can be treated with substances that produce similar symptoms in healthy individuals, and “law of minimum dose” refers to the belief that the lower the dose the greater its therapeutic efficacy [32].
Ayurvedic medicine: Ayurvedic medicine is one of the oldest alternative medical systems which involves the use of therapeutics derived predominantly from plants, animals, minerals, diet, exercise, and lifestyle changes. Its therapies are centered on the principle of “Panchakarma,” which comprises five karmas (actions) to rejuvenate and remove toxins from one’s body [33].
Study Selection and Data Collection Process
Citations retrieved from the six databases were extracted into Endnote X9 software (Philadelphia, PA: Clarivate) and duplicated citations were removed. During the initial article screening, two independent reviewers (WY and SW) reviewed the titles and abstracts of articles to select relevant articles. Thereafter, the full texts of the identified articles were evaluated. All discrepancies during the article screening process were resolved by discussion with a third reviewer (JJ). Hand-searching of references within identified articles was also performed to enhance the comprehensiveness of the search. A standardized Microsoft Excel data collection form was used for data extraction, and details related to the study characteristics, studied indications of intervention, efficacy, and safety were collected.
Management of Missing Data
For studies with missing data, authors were contacted for clarification to enhance the comprehensiveness of this review. Missing information that could not be retrieved after two email reminders were labeled as unavailable.
Risk of Bias in Individual Studies
The Cochrane Risk of Bias tool version 2.0 (Oxford, England: Cochrane) was utilized in the assessment of the included RCTs [34]. Two independent reviewers (Teo and Chu) performed the risk of bias assessment (Appendix 2). The instrument comprises of the following five domains: risk of bias arising from the randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of reported results. Using the responses derived from the five domains, the overall risk of bias for the individual studies was rated as "low," "some concerns," or "high" risk of bias.
Assessment of Heterogeneity
Clinical and methodological heterogeneity of included studies were analyzed to evaluate if meta-analyses could be performed for specific interventions in this study. Clinical heterogeneity describes variation in characteristics of study participants, intervention, or outcomes while methodological heterogeneity describes variation in study design and risk of bias. This was performed by two independent reviewers (Yeam and Seng). In view of the clinical and methodological heterogeneity across the included studies, a narrative review of the RCTs was conducted.
Synthesis of Data
With regard to the efficacy of AMS interventions, the response rates and any changes in patients’ quality of life were recorded. Additionally, the safety profile of each intervention was evaluated and the reported prevalence, severity, and outcomes of adverse effects were tabulated. The adverse events reported in included studies were categorized using the Common Terminology Criteria for Adverse Events (CTCAE) [35].
Summary Measures
Descriptive statistics were utilized to summarize the characteristics of all included studies. The principal summary measures evaluated in this study were the studied indications, efficacy of each AMS, and safety profile of each AMS.
Results
Study Selection
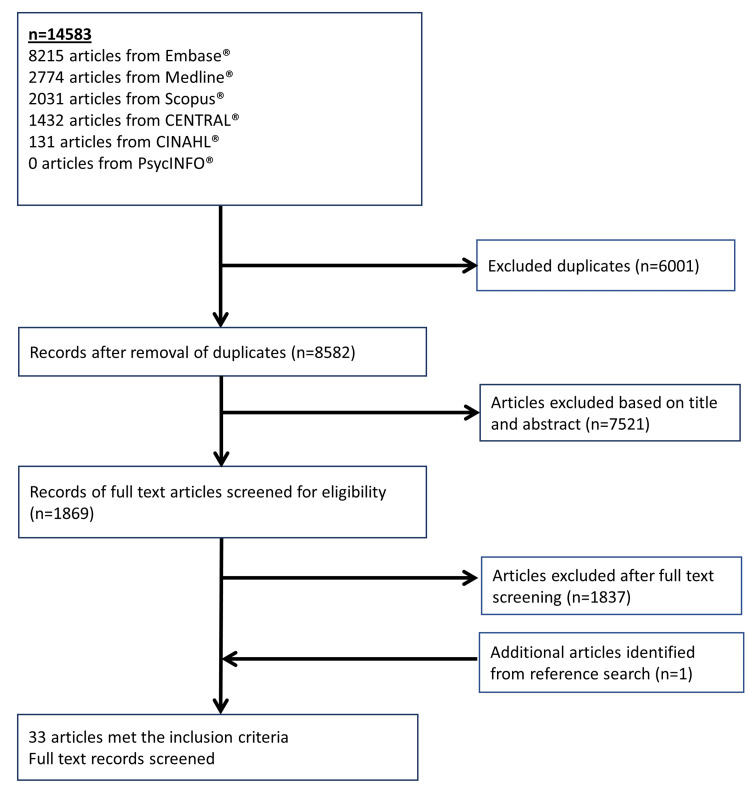
Out of 14,583 retrieved citations, 33 full-text articles were included in this review. The inclusion and exclusion criteria for the studies are shown in Figure 1. The percentage of agreement of articles between the reviewers was 94.0% and all disagreements were resolved after discussion.
Figure 1. Flow chart for the inclusion of articles in this systematic review.
Study Characteristics
Types of AMS studied: Table 1 shows the characteristics of included studies. Among the four main types of AMS, TCM was the most studied (n=20, 60.6%) [3,7,17,36-52], followed by Ayurveda (n=6, 18.2%) [53-58], naturopathy (n=5, 15.2%) [59-63], and homeopathy (n=2, 6.1%) [16,64].
Table 1. Characteristics of included AMS intervention studies.
aQDDHG is composed of Huang Qi, Danshen, Dihuang, Shanyao, and Gan Cao.
bDosage and frequency vary from patient to patient.
ARB: angiotensin receptor blockers; DN: diabetic neuropathy; ESRD: end-stage renal disease; TBN: tianbaoning; TSF: tangshen formula; TWHF: Tripterygium wilfordii Hook F; ZSTL: zishentongluo; QoL: quality of life; QD: once a day; TIW: thrice a week; TID: thrice a day; CKD: chronic kidney disease; QIW: four times a week; HD: hemodialysis; T: test group; C: control group; T1: test group 1; T2: test group 2; C1: control group 1; C2: control group 2
| Study (year of publication) | Study design | Number of patients in treatment and control arm | Indication for use of intervention | Treatment | Comparator | Country | Patient population | Mean age of patients (SD) | Gender (male) (%) |
| Traditional Chinese medicine (n=20) | |||||||||
| Li et al. (2015) [41] | Double-blinded parallel arm | T1:66, C1:32, T2:56, C2:26 | Renal function | 8 g TSF granules and ARB BID x 24 weeks | 8 g placebo TID and ARB BID x 24 weeks | China | Non-dialysis CKD patients | T1:59.5 (10.1), C1:56.7 (9.3), T2:58.9 (9.0), C2:60.8 (10.0) | T1:54.5%, C1:53.1%, T2:58.9%, C2:53.8% |
| Ma et al. (2013) [43] | Parallel arm | T:25, C:20 | Renal function | 150 mL ZSTL solution BID x 3 months | 10 mg benazepril QD x 3 months | China | Early DN patients | T:57, C:57 | T:40%, C:40% |
| Wang et al. (2012) [17] | Double-blinded parallel arm | T1:192, T2:191, C:189 | Renal function | T1: TCM granules BID x 24 weeks, T2: TCM granules BID and 10mg benazepril QD x 24 weeks | 10 mg benazepril QD and TCM placebo TID x 24 weeks | China | CKD stage 3 | T1:47.3 (10.9), T2:49.3 (11.4), C:49.0 (10.5) | T1:54.7%, T2:47.1%, C:47.6% |
| Yu et al. (2017) [50] | Single-blinded parallel arm | T:28, C:25 | Renal function | Acupuncture at Li4, ST36 and K13 acupoint QD x 3 months | Sham acupuncture QD x 3 months | China | CKD stage 2-4 | T:58.5, C:61.0 | T:89.3%, C:88.0% |
| Zhao et al. (2020) [52] | Double-blinded parallel arm | T:171, C:172 | Renal function | Herbal granules TID x 6 months | Placebo granules x TID 6 months | China | CKD stage 3 | T:51.89 (13.12), C:52.03 (12.62) | T:62.0%, C:70.4% |
| Xiang et al. (2016) [47] | Parallel arm | T:51, C:51 | Renal function | QDDHGa tablets BID x 12 weeks | ARB tablets (minimum dosage) | China | DN patients | T:57.21 (13.20), C:58.16 (11.59) | T:24%, C:22% |
| Xu et al. (2016) [49] | Double-blinded parallel arm | T:91, C:86 | Renal function | 500 mg GS-Rb1 (ginseng extract) QD x 6 months | Placebo tablets QD x 6 months | China | CKD stage 2-3 | T:59.2 (8.5), C:58.4 (7.5) | T:72.5%, C:70.9% |
| Chen et al. (2013) [37] | Parallel arm | T:95, C:95 | Proteinuria | 9.6 g of Shenqi particle TID x 48 weeks | Routine care | China | Non-dialysis CKD patients | T:49 (14), C:53 (12) | T:36.2%, C:68.42% |
| Ge et al. (2013) [39] | Parallel arm | T:34, C:31 | Proteinuria | 40 mg TWHF TID x 3 months followed by 20 mg TWHF TID x 3 months | 80 mg valsartan BID x 6 months | China | DN patients | T:51.9 (9.8), C:51.0 (8.9) | T:58.8%, C:54.8% |
| Li et al. (2020) [42] | Double-blinded parallel arm | T:735, C:735 | Proteinuria | Huangkui capsule TID x 12 months | Losartan potassium tablet QD and placebo capsules TIW x 12 months | China | CKD stage 1-3a | T:37.7 (10.9), C:37.1 (10.4) | T:48.0%, C:46.3% |
| Zhang et al. (2014) [51] | Parallel arm | T1:133, T2:136, C:135 | Proteinuria | T1: Huangkui capsule TID x 6 months, T2: Huangkui capsule TID and Losartan potassium tablet QD x 6 months | Losartan tablet potassium QD x 6 months | China | Non-dialysis CKD patients | T1:37.3 (12.5), T2:37.1 (11.1), C:38.1 (12.7) | T1:50.4%, T2:47.1%, C:53.3% |
| Xiong et al. (2020) [48] | Parallel arm | T:62, C:62 | Proteinuria | 60 mg TWHF and 160 mg valsartan QD x 24 weeks | 160 valsartan QD x 24 weeks | China | Non-dialysis CKD patients | T:50.3 (11.8), C:49.6 (12.3) | T:69.4%, C:72.6% |
| Che-Yi et al. (2005) [36] | Double-blinded parallel arm | T:20, C:20 | Uremic pruritus | Acupuncture at Quchi (L11) acupoint TIW x 1 month | Sham acupuncture TIW x 1 month | China | ESRD | T:62.4 (9.1), C:63.2 (7.5) | T:45.0%, C:50.0% |
| Gao et al. (2002) [38] | Double-blinded parallel arm | T:34, C:34 | Uremic pruritus | Acupuncture at Quchi (L11) and Zusanli (ST 36) acupoint BIW x 1 month | Sham acupuncture BIW x 1 month | China | ESRD | 43.6 | 59.0% |
| Nahidi et al. (2018) [7] | Single-blinded parallel arm | T:15, C:11 | Uremic pruritus | Acupuncture at various acupoints TIW x 6 weeks | Sham acupuncture TIW x 6 weeks | Iran | HD | T:54.7 (11.4), C:41.4 (16.2) | T:60%, C:73% |
| Ono et al. (2015) [3] | Parallel arm | T:23, C:17 | Fatigue, insomnia, itchiness, and pain | Acupuncture QIW x 2 months | Routine care | Japan | HD | T:70.0 (9.6), C:67.3 (13.0) | NI |
| Su et al. (2009) [44] | Parallel arm | T:31, C:30 | QoL | Infrared stimulation of Qihai (RN6), Kuamyuan (RN4) and Chungchi (RN3) TIW x 3 months | Heat pad therapy to acupoints TIW x 3 months | China | ESRD | T:61.07 (13.9), C:58.6 (12.6) | T:51.6%, C:56.7% |
| Wang et al. (2019) [45] | Single-blinded parallel arm | T:40, C:40 | Wnt/β-catenin signaling pathway | Qingshen granules TID x 3 months | Placebo granules TID x 3 months | China | CKD stage 3-5 | T:52.1 (10.4), C:54.9 (9.2) | T:55%, C:48% |
| Wang et al. (2020) [46] | Parallel arm | T:136, C:146 | Immune function | Qingshen granules TID x 3 months | Routine care | China | CKD stage 3-5 | T:54.0 (10.5), C:51.8 (12.0) | T:55.1%, C:57.5% |
| Li et al. (2009) [40] | Parallel arm | T:32, C:32 | Vascular endothelial function | TBN tablets (gingko extract) TI x 8 weeks | Routine care | China | Early DN patients | T:66.5 (71.1), C:67.2 (7.2) | T:53.1%, C:50% |
| Ayurveda (n=6) | |||||||||
| Alam et al. (2020) [53] | Parallel arm | T:70, C:66 | Renal function | Sativa oil QD and alpha-keto amino acid tablets TID x 3 months | Alpha-keto amino acid tablets TID x 3 months | India | CKD stage 3-4 | T:49.2, C:48.8 | T:58.6%, C:51.5% |
| Fallahzadeh et al. (2012) [54] | Double-blinded parallel arm | T:30, C:30 | Renal function | 140 mg silymarin tablet QD x 3 months | Placebo tablet QD x 3 months | Iran | Non-dialysis CKD patients | T:55.9 (8.3), C:57.6 (7.5) | T:50%, C:43.3% |
| Hoseini et al. (2019) [55] | Parallel arm | T:22, C:22 | Renal function | Camel milk BID x 3 months | Routine care | Iran | CKD stage 3-4 | 56.7 (11.8) | 52.3% |
| Khajehdehi et al. (2011) [56] | Double-blinded parallel arm | T:28, C:28 | Renal function | 140 mg silymarin TID x 3 months | Placebo tablet TID x 3 months | Iran | Non-dialysis CKD patients | T:55.9 (8.3), C:57.6 (7.5) | T:50%, C:43.3% |
| Makhlough et al. (2010) [57] | Double-blinded parallel arm | T:17, C:17 | Uremic pruritus | 0.03% capsaicin ointment QID x 4 weeks | Placebo ointment QID x 4 weeks | Iran | ESRD | 57 (18.6) | 41.2% |
| Pingali et al. (2020) [58] | Double-blinded parallel arm | T1:18, T2:18, C:19 | Hyperuricemia | T1:500 mg of beleric capsule taken QD T2: 1000 mg of beleric capsule taken QD | C:40 mg of febuxostat taken QD | India | CKD stage 2-3 | T1:53.2 (8.9), T2:50.8 (8.8), C:51.0 (9.8) | T1:72.2%, T2:77.8%, C:73.7% |
| Naturopathy (n=5) | |||||||||
| Khan et al. (2014) [60] | Double-blinded parallel arm | T:80, C:80 | Malnutrition | Alpha-keto amino acid tablets TID x 3 months | Placebo tablets TID x 3 months | India | Non-dialysis CKD patients | T:45.0, C:45.0 | T:59.5%, C:57.8% |
| Prakash et al. (2004) [61] | Double-blinded parallel arm | T:21, C:19 | Malnutrition | Keto amino acid tablets QD x 9 months | Placebo tablets QD x 9 months | India | CKD stage 3-4 | T:52.8 (14.1), C:55.9 (17.6) | T:55.6%, C:43.8% |
| Sedaghattalab et al. (2021) [62] | Double-blinded parallel arm | T:22, C:23 | Inflammation | Watercress extract QD x 1 month | Placebo extract QD x 1 month | Iran | HD | T:58.9 (16), C:63.1 (13) | NI |
| Zare et al. (2019) [63] | Double-blinded parallel arm | T:19, C:21 | Inflammation | Garlic extract tablets TIW x 2 months | Placebo tablets TIW x 2 months | Iran | PD | T:56.0 (16.1), C:52.8 (18.8) | T:42.1%, C:42.8% |
| Boldaji et al. (2019) [59] | Crossover trial | T:22, C:19 | Hypertension, stress, and inflammation | Pomegranate juice TIW x 2 months | Routine care | Iran | ESRD | 47.8 (13.3) | 61% |
| Homeopathy (n=2) | |||||||||
| Cavalcanti et al. (2003) [16] | Double-blinded parallel arm | T:11, C:9 | Uremic pruritus | Homeopathic verum medicationb administered | Placebo medication administered | Brazil | HD | T:47, C:57 | T:64%, C:56% |
| Silveira et al. (2019) [64] | Double-blinded parallel arm | T:18, C:14 | Renal function | Brazilian green propolis pills BID x 3 months | Placebo pills BID x 3 months | Brazil | CKD stage 1-5 | T:52.8 (14.1), C:55.9 (17.6) | T:55.6%, C:43.8% |
Overview of study design and patient characteristics in included studies are as follows: the majority of the studies were conducted in Asia (n=31, 93.9%), whereas most of the studies were performed in China (n=18, 54.5%). Out of all reviewed studies, 20 (60.6%) were blinded to randomized controlled trials. The sample size was greater than 50 patients in 21 (63.6%) trials (Table 1). Non-dialysis patients were recruited in seven studies (21.2%), whereas ESRD patients were recruited in five studies (15.2%). The average duration of follow-up for all studies was 4.9 months (Table 2).
Table 2. Assessment of outcomes and efficacy of different AMS interventions.
aPercentage reduction in symptoms is computed using (mean score at baseline - mean score post-treatment at end of study)/(mean score at baseline) for the treatment group.
bTCM granules are formulated based on four TCM syndrome patterns (Qi Yin/Xue deficiency, blood stasis in the kidney, wind-dampness interfering in the kidney, and endoretention of damp heat) for the treatment of CKD.
cHerbal granules consist of ten herbs: Huangqi, Danggui, Huzhang, Liuyuexue, Tufuling, Niuxi, Shiwei, Dahuang, Jixuecao, and Huangjing.
dDosage and frequency vary from patient to patient.
99mTc-DTPA: 99 m technetium diethylenetri-aminepenta-aceticacid; ARB: angiotensin II receptor blockers; BAID: brachial arterial inner diameter; BIW: twice a week; BUN: blood urea nitrogen; CCR: creatinine clearance rate; CKD-EPI: chronic kidney disease-epidemiology collaboration; DQOL: diabetes quality of life; eGFR: estimated glomerular filtration rate; ESR: erythrocyte sedimentation rate; ELISA: enzyme-linked immunosorbent assay; EQ-5D: EuroQol-5 dimension; FBG: fasting blood glucose; GS-RB1: Ginsenoside Rb1; HbA1c: haemoglobin A1c; ITT: intention to treat; LF: low frequency; MDA: malondialdehyde; MDRD: modification of diet in renal disease; NI: no information; PP: per protocol; QD: once a day; QDDHG: Qidan Dihuang Grain; SCr: serum creatinine; TBN: Tianbaoning; TCM: traditional Chinese medicine; TIW: thrice a week; TSF: Tangshen formula; TwHF: Tripterygium Wilfordii Hook F; TUP: total urine protein; TUV: total urine volume; UAER: urinary albumin excretion rate; VAS: visual analog scale; WHOQOL-BREF: World Health Organization Quality of Life-100 Questionnaire; ZSTL: Zishentongluo; Hs-CRP: high-sensitivity c-reactive protein; ITT: intention to treat; QIW: four times a week; HIF-1α: hypoxia-inducible factor 1-alpha; Wnt1: Wnt family member 1; α-SMA: alpha smooth muscle actin; TNF-α: tumor necrosis factor alpha; TRAF6: tumor necrosis factor receptor associated factor 6; FN: Fibronectin
| Study (year of publication) | Indication for use of intervention | Treatment (dose and duration if available) | Comparator (dose and duration if available) | Tool(s) used to assess outcomes | Outcome | Improvement symptoms (yes / no) | Percentage reductiona/improvement in symptoms (if available) | Follow-up duration |
| Traditional Chinese medicine (n=20) | ||||||||
| Li et al. (2015) [41] | Renal function | 8 g TSF granules TID and ARB BID x 24 weeks | 8 g placebo TID and ARB BID x 24 weeks | WHOQOL-BREF, DQOL | UAER (μg/min) (pre vs post): 105.39±77.29 vs 88.37±108.46, p=0.021 | Yes | -16.1% (UAER) | 6 months |
| 24 h urinary protein (g/24 h) (pre vs post): 1.12±0.75 vs 0.91±0.90, p=0.017 | -18.8% (24 h urinary protein) | |||||||
| Ma et al. (2013) [43] | Renal function | 150 mL ZSTL solution BID x 3 months | 10 mg benazepril QD x 3 months | Radioimmunoassay, ELISA | HbA1c (%) (baseline vs mean change from baseline): 10.68 (8.48, 13.96) vs -4.29 (-5.85, -2.79), p<0.05. | Yes | -40.2% (HbA1c) | 9 months |
| UAER (μg/min) (baseline vs mean change from baseline): 211.52 (164.58, 243.89) vs -106.99 (-121.29, -85.55), p<0.05 | -50.6% (UAER) | |||||||
| SCr (μmol/L) (baseline vs mean change from baseline): 87.17 (70.59, 110.25) vs -3.33 (-11.02, 2.15), p<0.05 | -3.82% (SCr) | |||||||
| CCR (mL/min) (baseline vs mean change from baseline): 139.86 (129.58, 149.52) vs -9.22 (-13.42, -5.82), p <0.05 | -6.59% (CCR) | |||||||
| Wang et al. (2012) [17] | Renal function | T1: TCM granuleb BID x 24 weeks. T2: TCM granuleb BID and 10 mg benazepril QD x 24 weeks. | 10 mg benazepril QD and TCM placebo TID x 24 weeks | MDRD study equation, TCM assessing sheets | eGFR (mL/min/1.73 m2) (pre vs post): T1: 45.26±10.12 vs 48.46±15.90, p<0.05. T2: 44.68±9.82 vs 48.31±17.50, p<0.05. | Yes | 7.07% (eGFR; T1), 8.12% (eGFR; T2) | 6 months |
| 24 h proteinuria (mg/24 h) (pre vs post): T1: 725.98 vs 990.00, p<0.05. T2: 590.00 vs 453.50, p<0.05 | 36.4% (proteinuria; T1), -21.1% (proteinuria; T2) | |||||||
| Urinary albumin/creatinine (mg/gCr) (pre vs post): T2: 0.30 vs 0.22, p<0.05 | -26.7% (urinary albumin/creatinine; T2) | |||||||
| Hb (g/L) (pre vs post): T1: 127.31±18.47 vs 129.57±21.82, p<0.05 | 17.8% (Hb; T1) | |||||||
| Yu et al. (2017) [50] | Renal function | Acupuncture at Li4, ST36 and K13 acupoint QD x 3 months | Sham acupuncture QD x 3 months | NI | SCr levels (mg/dL) (T vs C): baseline: 1.45 vs 1.67, p=0.1298. Post-intervention: 1.41 vs 1.65, p=0.0489. 3-month follow-up: 1.32 vs 1.81, p=0.0467 | Yes | -2.76% (SCr; pre vs post), -9.00% (SCr; pre- vs 3 months follow-up) | 6 months |
| eGFR (mL/min/1.73m2) (T vs C): Baseline: 51.85 vs 42.50, p=0.0855. Post-intervention: 54.50 vs 43.60, p=0.0470. 3-month follow-up: 59.90 vs 40.80, p=0.0191 | 5.11% (eGFR; pre vs post), 15.5% (eGFR; pre-intervention vs 3 months follow-up) | |||||||
| hs-CRP (mg/dL) (T vs C): Baseline: 1.10 vs 0.79, p=0.4361. Post-intervention: 0.80 vs 0.90, p=0.8773 | -27.3% (hs-CRP pre vs post) | |||||||
| Zhao et al. (2020) [52] | Renal function | Herbal granulec TID x 6 months | Placebo granules x TID 6 months | Dye-binding method, Cerebrospinal fluid protein test kit, Determiner L CRE kit | SCr (μmol/L) (pre vs weeks 16, 20 and 24): 148.42±35.90 vs 130.19±29.79, 130.08±30.57, 130.78±32.55, p<0.05 | Yes | -12.3% (SCr; pre vs 16 weeks), -12.4% (SCr; pre vs 20 weeks), -11.9% (SCr; pre vs 24 weeks) | 6 months |
| Xiang et al. (2016) [47] | Renal function | QDDHG tablets BID and ARB (minimum dosage) x 12 weeks | ARB tablets (minimum dosage) | Guidelines for clinical research of Chinese medicine | Albumin (mg/24h) (within treatment group, baseline vs 4 vs 8 vs 12 week): 85.30 (66.00, 176.30) vs 61.50 (49.00, 110.20), p<0.05 vs 51.00 (37.00, 90.00), p<0.05 vs 41.40 (29.00, 68.00), p<0.05 | Yes | -27.9% (Albumin; 4 weeks), -40.2% (Albumin; 8 weeks), -43.9% (Albumin; 12 weeks) | 3 months |
| Proteinuria (g/24h) (within treatment group, baseline vs 4 vs 8 vs 12 week): 0.20 (0.10, 0.30) vs 0.10 (0.10, 0.20), p<0.05 vs 0.10 (0.10, 0.20), p<0.05 vs 0.10 (0.10, 0.20), p<0.05 | -50% (Proteinuria; 4, 8, 12 weeks) | |||||||
| Albumin/creatinine (mg/mol) (within treatment group, baseline vs 4 vs 8 vs 12 week): 20.70 (11.00, 30.50) vs 16.30 (8.10, 25.00), p<0.05 vs 15.00 (7.20, 20.60), p<0.05 vs 10.10 (5.60, 17.00), p<0.05 | -21.3% (albumin/creatinine; 4 weeks), -27.5% (albumin/creatinine; 8 weeks), -51.2% (albumin/creatinine; 12 weeks) | |||||||
| Xu et al. (2016) [49] | Renal function | 500 mg GS-Rb1 (ginseng extract) QD x 6 months | Placebo tablets QD x 6 months | ELISA | Creatinine and urea level (T vs C): 6 months, p<0.01. 12 months, p<0.01 | Yes | - | 12 months |
| Oxidative stress markers (T vs C): 6 months, p<0.01. 12 months, p<0.05 | ||||||||
| TNF-a level (T vs C): 6 months, p<0.05 | ||||||||
| Chen et al. (2013) [37] | Proteinuria | 9.6 g of Shenqi particle TID x 48 weeks | Routine care | MDRD study equation | Proteinuria (g/d) (pre vs post): 5.34±2.74 vs 2.04±2.15, p<0.001 | Yes | -61.8% (proteinuria) | 12 months |
| eGFR (mL/min/1.73 m2) (pre vs post): 84.6±27.0 vs 100.7±37.5, p=0.001 | 19.0% (eGFR) | |||||||
| Ge et al. (2013) [39] | Proteinuria | 40 mg TwHF TID x 3 months, 20 mg TwHF TID x 3 months. | 160 mg valsartan capsules QD x 6 months | Trichloroacetic acid method, Jaffe reaction, MDRD study equation, high-performance liquid chromatography | Urinary protein (g/24 h) (pre vs 1 month, pre vs 3 months, pre vs 6 months): 4.99±2.25 vs 3.23±2.57, p<0.01. 4.99±2.25 vs 2.83±1.57, p<0.01. 4.99±2.25 vs 2.99±1.81, p<0.01 | Yes | -35.3% (urinary protein; 1 months), -43.3% (urinary protein; 3 months), -40.1% (urinary protein; 6 months) | 6 months |
| eGFR (mL/min/1.73 m2) (pre vs 6 months): 43.07±21.65 vs 38.71±23.66, p<0.05 | -10.1% (eGFR; 6 months) | |||||||
| Li et al. (2020) [42] | Proteinuria | Huangkui capsule TID x 12 months | Losartan potassium tablet QD and placebo capsules TIW x 12 months | NI | Proteinuria (mg/24 h) (pre vs post): 1238.9±667.4 vs 1008.8±1104.7, p<0.001 | Yes | -18.6% (proteinuria) | 12 months |
| Zhang et al. (2014) [51] | Proteinuria | T1: Huangkui capsule TID x 6 months. T2: Huangkui capsule TID and Losartan potassium tablet QD x 6 months. | Losartan tablet potassium QD x 6 months | Biuret method, sarcosine oxidase assay | Proteinuria within T1 (pre vs 12 vs 24 weeks): 1045±420 vs 762±533, p<0.001 vs 537±409, p<0.001 | Yes | T1: -27.1% (pre vs 12 weeks), -48.6% (pre vs 24 weeks) | 6 months |
| Proteinuria within T2 (pre vs 12 vs 24 weeks):1073±439 vs 783±658, p<0.001 vs 529±509, p<0.001. | T2: -27.0% (pre vs 12 weeks), -50.7% (pre vs 24 weeks). | |||||||
| Xiong et al. (2020) [48] | Proteinuria | 60 mg TWHF and 160 mg valsartan QD x 24 weeks | 160 valsartan QD x 24 weeks | CKD-EPI equation | Proteinuria (g/24 h) (T vs C, PP analysis): 3.16±0.62 vs 4.28±0.85, p<0.001 | Yes | PP: -26.2% (proteinuria) | 6 months |
| Serum albumin (g/L) (T vs C, PP analysis): 37.65±4.31 vs 33.59±4.56, p<0.001 | PP: 12.1% (serum albumin) | |||||||
| Proteinuria (g/24 h) (T vs C, ITT analysis): 3.36±0.83 vs 4.52±1.06; p<0.001 | ITT: -25.7% (proteinuria) | |||||||
| Serum albumin (g/L) (T vs C, ITT analysis): 36.91±4.42 vs 34.67±4.75, p=0.008 | ITT: 6.46% (serum albumin) | |||||||
| Che-yi et al. (2005) [36] | Uremic pruritus | Acupuncture at Quchi (L11) acupoint TIW x 1 month | Sham acupuncture TIW x 1 month | Validated questionnaire | Pruritus scores (pre vs post vs 3 months follow-up): 38.2±4.8 vs 17.3±5.5 vs 16.5±4.9, p<0.001 | Yes | -54.7% (pruritus scores; pre- vs post-intervention), -56.8% (pruritus scores; pre-intervention vs 3 months follow-up) | 3 months |
| Gao et al. (2002) [38] | Uremic pruritus | Acupuncture at Quchi (L11) and Zusanli (ST 36) acupoint BIW x 1 month | Sham acupuncture BIW x 1 month | NI | Number of patients (complete alleviation vs improvement vs no effect): 24 (70.6%) vs 9 (26.5%) vs 1 (2.9%) | Yes | - | 3 months |
| Nahidi et al. (2018) [7] | Uremic pruritus | 30 minutes of acupuncture, for six weeks, at the following acupoints: Sp6, Sp10, Lv3, Li4, Li11. | 30 minutes of sham acupuncture, for 6 weeks. | VAS | Pruritus scores (pre vs post): 9.87±0.35 vs 3.93±2.85, p<0.001 | Yes | -60.2% (pruritus scores) | 6 weeks |
| Ono et al. (2015) [3] | Fatigue, insomnia, itchiness, and pain | Acupuncture QIW x 2 months | Routine care | VAS, EQ-5D | Headache score (pre vs post): 17.1±26.1 vs 6.2±13.5, p<0.05. | Yes | -63.7% (headache score) | 3 months |
| Blurred vision score (pre vs post): 33.4±32.7 vs 17.0±22.2, p<0.05. | -49.1% (blurred vision score) | |||||||
| Dizziness score (pre vs post): 13.0±21.4 vs 1.4±6.3, p<0.05. | -89.2% (dizziness score) | |||||||
| Ear buzzing (pre vs post): 17.9±27.2 vs 8.0±14.7, p<0.05 | -55.3% (ear buzzing) | |||||||
| Cervical pain (pre vs post): 37.7±39.1 vs 25.3±29.7, p<0.05 | -32.9% (cervical pain) | |||||||
| Stiff shoulders (pre vs post): 29.9±28.6 vs 12.5±21.6, p<0.05 | -58.2% (stiff shoulders) | |||||||
| Back pain (pre vs post): 38.5±33.7 vs 9.3±18.1, p<0.05 | -58.2% (back pain) | |||||||
| Lower limb pain (pre vs post): 29.4±36.4 vs 17.1±23.3, p<0.05 | -41.8% (lower limb pain) | |||||||
| Numbness in upper limb (pre vs post): 18.9±30.4 vs 4.0±29.5, p<0.05 | -78.8% (numbness in upper limb) | |||||||
| Numbness in lower limb (pre vs post): 21.9±34.9 vs 11.0±26.2, p<0.05 | -49.8% (numbness in lower limb) | |||||||
| Itchiness (pre vs post): 38.7±40.7 vs 29.3±31.5, p<0.05 | -24.3% (itchiness) | |||||||
| Difficulty in sleeping (pre vs post): 34.8±36.9 vs 12.8±22.5, p<0.05 | -63.2% (difficulty in sleeping) | |||||||
| Utility in treatment group (pre vs post): 0.66±0.15 vs 0.76±0.17, p<0.05 | 15.2% (utility) | |||||||
| Su et al. (2009) [44] | QoL | Infrared stimulation of Qihai (RN6), Kuamyuan (RN4) and Chungchi (RN3) TIW x 3 months | Heat pad therapy to acupoints TIW x 3 months | Heart rate variability analyser, WHOQOL-BREF questionnaire | LF activity (pre vs post): 49.99±79.08 vs 131.71±214.36, p=0.01 | Yes | 163% (LF activity) | 3 months |
| Fatigue index (pre vs post): 133.90±20.43 vs 121.71±32.68, p=0.02 | -9.10% (fatigue index) | |||||||
| Psychological domain (pre vs post): 18.16±4.30 vs 19.39±0.72, p=0.02 | 6.77% (psychological domain) | |||||||
| Environmental domain (pre vs post): 29.87±4.04 vs 32.00±4.85, p=0.00. | 7.13% (environmental) | |||||||
| Wang et al. (2019) [45] | Wnt/β-catenin signaling pathway | Qingshen granules TID x 3 months | Placebo granules TID x 3 months | ELISA | Effective rates of TCM symptom (T vs C): 80% vs 60%, p=0.024 | Yes | - | 3 months |
| eGFR (mL/min) (T vs C): 15.9±3.2 vs 14.0±4.0, p=0.019 | 17.8% (eGFR) | |||||||
| HIF-1𝛼 (ng/mL) (T vs C): 0.66±0.16 vs 1.39±0.17, p≤0.001 | -61.4% (HIF-1𝛼) | |||||||
| Wnt1 (pg/mL) (T vs C): 314.2±85.8 vs 382.8±85.3, p=0.001 | -16.9% (Wnt1) | |||||||
| 𝛽-catenin (pg/mL) (T vs C): 416.5±13.6 vs 462.1±15.1, p ≤0.001 | -10.0% (𝛽-catenin) | |||||||
| 𝛼-SMA (KU/L) (T vs C): 20.5±3.1 vs 23.5±4.1, p≤0.001 | -20.8% (𝛼-SMA) | |||||||
| E-cadherin (ng/mL) (T vs C): 2166.9±398.6 vs 2370.7±468.0, p=0.039 | -15.1% (E-cadherin) | |||||||
| Wang et al. (2020) [46] | Immune function | Qingshen granules TID x 3 months | Routine care | Flow cytometry, ELISA | CD4+/CD8+ T cell (pre vs post): 1.98±0.86 vs 1.58±0.72, p<0.05. | Yes | -20.2% (CD4+/CD8+ T cell) | 3 months |
| Th17 cell (pre vs post): 2.51±1.05 vs 1.70±0.83, p<0.01. | -32.3% (Th17) | |||||||
| NF-κB p65 (pre vs post): 36.84±12.96 vs 24.86±1.97, p<0.05 | -32.5% (NF-κB p65) | |||||||
| IL-17 (pre vs post): 28.62±13.53 vs 19.78±12.25, p<0.05 | -30.9% (IL-17) | |||||||
| IL-6 (pre vs post): 77.13±20.54 vs 58.42±18.25, p<0.05 | -24.3% (IL-6) | |||||||
| TNF-α (pre vs post): 110.34±23.76 vs 75.49±22.80, p<0.01 | -31.6% (TNF-α) | |||||||
| TRAF6 (pre vs post): 4.94±1.82 vs 2.85±1.53, p<0.01 | -42.3% (TRAF6) | |||||||
| FN (pre vs post): 93.42±20.36 vs 62.86±19.35, p<0.01 | -32.7% (FN) | |||||||
| Col-IV (pre vs post): 36.85±14.58 vs 24.36±13.36, p<0.01 | -33.9% (Col-IV) | |||||||
| Total effective rate (T vs C): 79.41% vs 67.12%, p<0.05. | - | |||||||
| Li et al. (2009) [40] | Vascular endothelial function | TBN tablets (gingko extract) TID x 8 weeks | Routine care | Chemical colorimeter, Radioimmunoassay, ELISA, Siemens Sequoia 512 color Doppler ultrasonography | UAER (μg/min) (pre vs post): 153.30±63.28 vs 85.15±36.82, p<0.01 | Yes | -44.5% (UAER) | 3 months |
| SCr (μmol/L) (pre vs post): 120.76±17.83 vs 105.67±18.13, p<0.01 | -12.5% (SCr) | |||||||
| NO (μmol/L) (pre vs post): 50.16±24.64 vs 70.65±28.71, p<0.01 | 40.8% (NO) | |||||||
| vWF (%) (pre vs post): 182.05±64.13 vs 128.56±48.98, p<0.01 | -29.4% (vWF) | |||||||
| BAID responsive change (%) (pre vs post): 4.91±2.31 vs 6.78±3.89, p<0.01 | 38.1% (BAID responsive change) | |||||||
| Ayurveda (n=6) | ||||||||
| Alam et al. (2020) [53] | Renal function | Sativa oil QD and alpha-keto amino acid tablets TID x 3 months | Alpha-keto amino acid tablets TID x 3 months | Hemogram, renal function test, serum electrolyte test | Hb% (g/dL) (pre vs post): 8.84±1.31 vs 10.24±1.10, p<0.001 | Yes | 15.8% (Hb%) | 3 months |
| 24-h TUV (mL/day) (pre vs post): 1250.69±303.74 vs 1660.14±258.78, p<0.001 | 32.7% (TUV) | |||||||
| eGFR (mL/min) (pre vs post): 22.71±7.28 vs 42.42±17.38, p<0.001 | 86.8% (eGFR) | |||||||
| Fallahzadeh et al. (2012) [54] | Renal function | 140 mg silymarin tablet QD x 3 months | Placebo tablet QD x 3 months | Jaffé method, ELISA MDA assay, MDRD study equation, nephelometry, high-performance liquid chromatography, mercury sphygmomanometer | Urinary TNF-α (pg/mg) (change from baseline): -3.45 (-5.44 to -1.46), p<0.05 | Yes | - | 2 months |
| Urinary MDA (nmol/mg) (change from baseline): -1.5 (-2.87 to -0.13, p<0.05 | ||||||||
| Serum MDA (μmol/L) (change from baseline): -3.43 (-6.02 to -0.83), p<0.05 | ||||||||
| Hoseini et al. (2019) [55] | Renal function | Camel milk BID x 3 months | Routine care | MDRD | eGFR (pre vs post): 26.9±7.39 vs 31.45±8.99, p=0.001 | Yes | 16.9% (eGFR) | 3 months |
| SCr levels (pre vs post): 2.58±0.71 vs 2.2±0.48, p=0.01 | -14.7% (SCr) | |||||||
| BUN (pre vs post): 60.31±22.61 vs 44.38±14.29, p=0.0001 | -26.4% (BUN) | |||||||
| Khajehdehi et al. (2011) [56] | Renal function | 140 mg silymarin TID x 3 months | Placebo tablet TID x 3 months | ELISA | Proteinuria (mg/24h) (pre vs post, patients with type 2 diabetic nephropathy): 4328.7±3038.2 vs 2354.7±1800.1, p=0.001 | Yes | -45.6% (proteinuria) | 2 months |
| IL-8 (pg/mL) (pre vs post, patients with type 2 diabetic nephropathy): 99.1±97.9 vs 43.6±55.0, p=0.002 | -56.0% (IL-8) | |||||||
| TGF-β (pg/mL) (pre vs post, patients with overt type 2 diabetic nephropathy): 522.3±189.2 vs 397.3±55.2, p=0.006 | -23.9% (TGB-β) | |||||||
| IL-8 (pg/mL) (pre vs post, patients with overt type 2 diabetic nephropathy): 41.4±50.3 vs 30.6±75.2, p=0.02 | -26.1% (IL-8) | |||||||
| Makhlough et al. (2010) [57] | Uremic pruritus | 0.03% capsaicin ointment QID x 4 weeks | Placebo ointment QID x 4 weeks | Uremic pruritus scoring questionnaire by Duo | Pruritus score (T vs C): 2.5±2.5 vs 7.2±5.5, p<0.05 | Yes | -84.3% (pruritus score) | |
| Pingali et al. (2020) [58] | Hyperuricemia | T1:500 mg of beleric capsule taken QD. T2: 1000 mg of beleric capsule taken QD | 40 mg of Febuxostat taken QD | Jaffe method, MDRD Study equation, Salbutamol challenge test, Ellman’s method, Chrono-log light transmittance aggregometry, Spectrometry, Colorimetric detection with Griess reagents | SCr (pre vs post): group B: 1.86±0.32 vs 1.64±0.29, p≤0.005. Group C: 2.06±0.26 vs 1.56±0.24, p≤0.0001 | Yes | -11.70%±9.00 (SCr, group B), -24.42%±8.14 (SCr, group C). | 6 months |
| eGFR (pre vs post): group B: 39.13±6.57 vs 45.96±11.14, p≤0.005. Group C: 34.78±5.34 vs 48.93±11.46, p≤0.0001 | 16.96%±14.87 (eGFR, group B), 40.39%±20.98 (eGFR, group C) | |||||||
| Serum uric acid (pre vs post): Group B:8.10±0.67 vs 6.46±0.34, p≤0.0001. Group C: 8.54±0.64 vs 5.63±0.37, p≤0.0001 | 19.84%±6.43 (serum uric acid, group B), 33.88%±4.95 (serum uric acid, group C) | |||||||
| Naturopathy (n=5) | ||||||||
| Khan et al. (2014) [60] | Malnutrition | Alpha-keto amino acid tablets TID x 3 months | Placebo tablets TID x 3 months | Blood tests | Hb% (g/dL) (T vs C): 9.39±0.87 vs 8.91±1.48, p<0.05 | Yes | 19.8% (Hb%) | 3 months |
| FBG (mg/dL) (T vs C): 104.00±8.46 vs 113.78±14.31, p<0.001 | -20.8% (FBG) | |||||||
| Blood urea (mg/dL) (T vs C): 66.07±19.29 vs 79.78±24.79, p<0.001 | -38.1% (blood urea) | |||||||
| SCr (mg/dL) (T vs C): 2.83±1.10 vs 3.33±1.37, p<0.05 | -39.5% (SCr) | |||||||
| 24 h TUP (g/day) (T vs C): 2.06±0.61 vs 2.43±0.97, p<0.01 | -38.3% (TUP) | |||||||
| 24 Hour TUV (mL/day) (T vs C): 1943.23±204.1 vs 1736.76±176.04, p<0.001 | 33.3% (TUV) | |||||||
| GFR (mL/min) (T vs C): 29.4±3.68 vs 23.3±1.63, p<0.001 | 49.2% (GFR) | |||||||
| Prakash et al. (2004) [61] | Malnutrition | Keto amino acid tablets QD x 9 months | Placebo tablets QD x 9 months | 99mTc-DTPA plasma sample method | GFR (mL/min/ 1.73 m2) (pre vs post within C): 28.6±17.6 vs 22.5±15.9, p=0.015. | Progress of renal failure prevented. | - | 9 months |
| Serum total proteins (g%) (pre vs post within C): 7.04±0.66 vs 6.56±0.83, p=0.038 | ||||||||
| Mid-arm circumference (cm) (pre vs post within C): 28.0±4.4 vs 27.3±4.8, p=0.048 | ||||||||
| Sedaghattalab et al. (2021) [62] | Inflammation | Watercress extract QD x 1 month | Placebo extract QD x 1 month | Blood tests, TBA reaction assay, Colorimetric kits, Spectrophotometer | BUN (mg/dL) (pre vs post): 40.6±11.2 vs 34.6±15.1, p<0.04. | Yes | -14.8% (BUN) | 1 month |
| Calcium (mg/dL) (pre vs post): 8.8±1.32 vs 10.4±2, p<0.001 | 18.1% (calcium) | |||||||
| Total oxidant status (μM) (pre vs post): 11.3±3.3 vs 6.9±2.4, p<0.001 | -38.9% (total oxidant status) | |||||||
| Sulfhydryl protein (mmol/L) (pre vs post): 13.1±5.3 vs 7.4±4.3, p<0.001 | -43.5% (sulfhydryl protein) | |||||||
| MDA (mmol/L) (pre vs post): 1.6±0.13 vs 0.42±0.27, p<0.001 | -73.8% (MDA) | |||||||
| Superoxide dismutase (U/mL) (pre vs post): 29.3±6.3 vs 37.1±8.4, p<0.001 | 26.6% (superoxide dismutase) | |||||||
| Zare et al. (2019) [63] | Inflammation | Garlic extract tablets TIW x 2 months | Placebo tablets TIW x 2 months | Human homocysteine kits, ELISA | IL-6 (pg/mL) (pre vs post): 2.2 (0.8, 6.4) vs 0.7 (0.6, 1.2), p<0.001 | Yes | -68.2% (IL-6) | 2 months |
| CRP (mg/L) (pre vs post): 13.0 (5.0, 14.0) vs 2.0 (1.0, 9.0), p<0.001 | -84.6% (CRP) | |||||||
| ESR (mm) (pre vs post): 50.7±28.5 vs 35.4±21.7, p=0.021. | -30.2% (ESR) | |||||||
| Boldaji et al. (2019) [59] | Hypertension, stress, and inflammation | Pomegranate juice TIW x 2 months | Routine care | Mini nutritional assessment | MDA (μmol L-1) (pre vs post): 0.88±0.01vs 0.77±0.01, p<0.001 | Yes | -12.5% (MDA) | 2 months |
| Total antioxidant capacity (mmol L-1) (pre vs post): 0.40±0.08vs 0.49±0.11, p<0.001 | 22.5% (total antioxidant capacity) | |||||||
| IL-6 (ng L-1) (pre vs post): 3.00±1.48 vs 2.09±1.25, p<0.0001 | -30.3% (IL-6) | |||||||
| Homeopathy (n=2) | ||||||||
| Cavalcanti et al. (2003) [16] | Uremic pruritus | Homeopathic verum medicationd administered | Placebo medication administered | Validated scale | Pruritus score (pre vs 15 vs 30 vs 45 vs 60 days): 65±25 vs 46±29, p=0.002 vs 41±30, p=0.002 vs 42±29, p=0.002 vs 38±33, p=0.004 | Yes | -29.2% (pruritus score, pre vs 15 days), -36.9% (pruritus score, pre vs 30 days), -35.4% (pruritus score, pre vs 45 days), -41.5% (pruritus score, pre vs 60 days) | 60 days |
| Silveira et al. (2019) [64] | Renal function | Brazilian green propolis pills BID x 3 months | Placebo pills BID x 3 months | Immunoturbidimetry, ELISA | Proteinuria (mg/24 h) (T vs C, baseline vs 12 months): 695 (95% CI, 483 to 999) vs. 1403 (95% CI, 1031 to 1909); p=0.004 | Yes | -27.6% (proteinuria) | 12 months |
Tools used for outcomes assessments of symptoms during interventions are as follows: the most frequently utilized tools were the abbreviated version of the World Health Organization Quality of Life-100 Questionnaire (n=2) and visual analog scale (n=2).
Risk of bias within studies: Out of all included studies, 14 (42.4%) studies were assessed to be of "low" risk of bias, nine (27.3%) and 10 (30.3%) studies were scored as "some concerns" and "high" risk of bias, respectively.
Results of Individual Studies
Traditional Chinese medicine: The most commonly utilized interventions in the studies were herbal treatments (n=14, 70%) [17, 37-43, 45-49, 51, 52] followed by acupuncture (n=6, 30%) [3,7,36,38,44,50]. For acupuncture treatment, five studies used conventional acupuncture [3,7,36,38,50] while one study used infrared stimulation of acupoints [44]. The common acupoints administered during conventional acupuncture treatment were Li11 (n=3, 60%), ST36 (n=2, 20%), and Li4 (n=2, 20%). The frequency of acupuncture ranged from once a week to once a day, whereas the duration of studies lasted between six weeks to six months. Uremic pruritus (n=3, 60%) was the most commonly studied indication, with reductions in pruritus score observed between 54.7% and 60.2% [7,36,38]. Infrared stimulation was used on RN6, RN4, and RN3 thrice a week for three months [44]. The indication studied was quality of life (QoL). According to QoL scores that were evaluated using the EQ-5D questionnaire, utility increased by 15.2%.
Herbal treatments include six single-herb (n=6, 42.9%) [39-42,48,49,51] and eight multi-herbs formula granules (n=8, 57.1%) [17,37,41,43,45-47,52]. Common single-herb treatments used are Huangkui (n=2, 33.3%) and TWHF (n=2, 33.3%). The frequency of treatment was once to three times a day, for two to 12 months. Proteinuria was the most studied indication (n=4, 66.7%), where the various treatments reduced proteinuria between 27.0% and 61.8% [39,42,48,51]. Common herbs used in the multi-herb formula granules studies included Huang Qi (n=6, 75%), Danggui (n=4, 50%), Salvia miltiorrhiza (n=3, 37.5%), and Poria (n=3, 37.5%). The most commonly studied indication was improvement in renal function (n=5, 62.5%) [17,41,43,47,52]. Serum creatinine decreased (2.76-12.4%) and eGFR increased (7.07-15.5%) across the various treatments.
Ayurveda: The included studies evaluated both plant-based (n=3, 50%) [53,54,56] and animal-based (n=3, 50%) treatments [55,57,58]. The most common treatment studied was plant-based silymarin tablets (n=2, 33.3%) while that for clinical indication was improvement in renal function (n=4, 66.6%) [53-56]. The eGFR increased between 16.9% and 86.8% across the various treatments [53-56].
Naturopathy: Keto amino acids were the most studied naturopathic treatment (n=2, 40%). Common indications studied included anemia and glucose control, where hemoglobin and fasting blood glucose levels in subjects improved by 19.8% and 20.8%, respectively [60,61]. The use of watercress and garlic extract was also studied for inflammation, where the total oxidant status and IL-6 levels were shown to improve by 38.9% and 68.2%, respectively.
Homeopathy: Homeopathic verum was studied for relief of uremic pruritus with improvement in pruritus score between 29.2% and 41.5% [16]. For Brazilian green propolis pills, it was studied for proteinuria where a 27.6% reduction in proteinuria was noted [64].
Safety Profile of AMS Interventions
The adverse events reported by all included studies are shown in Table 3. Adverse events reported were of grade 1 (n=13, 39.4%), grade 2 (n=8, 24.4%), and grade 3 (n=6, 18.2%) severity. There were no life-threatening consequences or death related to adverse effects (grades 4 and 5) reported.
Table 3. Adverse effects reported with AMS usage.
aThe adverse effects were graded based on Common Terminology Criteria for Adverse Effects (CTCAE).
bQDDHG is composed of Huang Qi, Danshen, Dihuang, Shanyao, and Gan Cao.
cDosage and frequency vary from patient to patient.
Grade 1: mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated. Grade 2: moderate; minimal, local, or non-invasive intervention indicated; limiting age-appropriate instrumental activities of daily living. Grade 3: severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living. Grade 4: life-threatening consequences; urgent intervention indicated. Grade 5: death related to adverse effects; AE: adverse events; BID: twice a day; C: control arm; NA: not applicable; QD: once a day; T: treatment arm; TID: thrice a day; TIW: thrice a week
| Study (year) | N (T) | Treatment | Reported adverse effects based on Common Terminology Criteria for Adverse Effects (CTCAE) v5.0a,b,c | Onset of adverse effects (if available) | Management and outcomes of patients | ||
| Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | |||||
| Traditional Chinese medicine (n=19) | |||||||
| Chenet al. (2013) [37] | 95 | 9.6 g of Shenqi particle TID x 48 weeks | NI | Interstitial pneumonia (n=1, 1.1%) | Lung infection (n=5, 5.26%); liver injury (n=3, 3.15%) | NI | NI |
| Geet al. (2013) [39] | 34 | 40 mg Tripterygium Wilfordii Hook F TID x 3 months | Vomiting (n=13, 38.2%) | Hyperkalaemia (n=8, 23.5%); Leukopenia (n=1, 2.9%); Photosensitive dermatitis (n=3, 8.8%) | NA | NI | Patient with decreased white blood cell withdrawn from the study |
| Liet al. (2020) [42] | 735 | Huangkui capsule TID x 12 months | NA | NA | Upper respiratory tract infections (n=21, 2.9%) | NI | NI |
| Zhanget al. (2014) [51] | T1:133 T2:136 | T1: Huangkui capsule TID x 6 months T2: Huangkui capsule TID and Losartan potassium tablet QD x 6 months | Elevated cholesterol (T1: n=5, 3.76%; T2: n=4, 2.94%) | Upper respiratory tract infections (T1: n=4, 3.0%; T2: n=4, 2.94%) | Liver injury (T1: n=3, 2.26%) | NI | NI |
| Xionget al. (2020) [48] | 62 | 60 mg Tripterygium Wilfordii Hook F and 160 mg valsartan QD x 24 weeks | Itchy skin (n=4, 6.45%); nausea (n=3.22%); rash (n=1, 1.61%) | NA | Liver dysfunction (n=12, 19.4%); leukopenia (n=1, 1.61%) | NI | NI |
| Liet al. (2015) [41] | 122 | 8 g Tangshen Formula granules and angiotensin receptor blockers BID x 24 weeks | NA | Anaemia (n=2) | Acute myocardial infarction (n=5) | NI | NI |
| Maet al. (2013) [43] | 25 | 150 mL zishentongluo solution BID x 3 months | NA | NA | NA | NA | NA |
| Wanget al. (2012) [17] | T1:192 T2:191 | T1: TCM granules BID x 24 weeks T2: TCM granules BID and 10 mg benazepril QD x 24 weeks | Dry cough (T2: n=2, 1.04%); Gastrointestinal symptoms (T1: n=7, 3.64%; T2: n=3, 1.57%) | Anaemia (T1: n=7, 3.64%; T2: n=6, 3.14%) | Liver injury (T1: n=2, 1.04%; T2: n=5, 2.61%); hyperkalaemia (T1 n=7, 3.64%; T2 n=18, 9.42%) | NI | NI |
| Yuet al. (2017) [50] | 28 | Acupuncture at Li4, ST36 and K13 acupoint QD x 3 months | Mild pain, bleeding and bruising in some patients | NA | NA | NI | Symptoms resolved spontaneously without any treatment |
| Zhaoet al. (2020) [52] | 171 | Herbal granules TID x 6 months | Mild abnormal liver function test (n=5, 2.92%); Mild discomfort (n=2, 1.17%) | NA | NI | NI | |
| Xianget al. (2016) [47] | 51 | QDDHGb tablets BID x 12 weeks | Insomnia (n=1, 1.96%) | NA | NA | NI | NI |
| Xuet al. (2016) [49] | 91 | 500 mg GS-Rb1 (ginseng extract) QD x 6 months | NA | NA | NA | NI | NI |
| Che-yiet al. (2005) [36] | 20 | Acupuncture at Quchi (L11) acupoint TIW x 1 month | Elbow soreness (n=2; 10.0%) | NA | NA | NI | Symptoms resolved spontaneously after 1 day. |
| Gaoet al. (2002) [38] | 34 | Acupuncture at Quchi (L11) and Zusanli (ST 36) acupoint BIW x 1 month | NA | NA | NA | NA | NA |
| Nahidiet al. (2018) [7] | 15 | Acupuncture at various acupoints TIW x 6 weeks | NA | NA | NA | NA | NA |
| Onoet al. (2015) [3] | 23 | Acupuncture QIW x 2 months | NA | NA | NA | NA | NA |
| Suet al. (2009) [44] | 31 | Infrared stimulation of Qihai (RN6), Kuamyuan (RN4) and Chungchi (RN3) TIW x 3 months | NA | NA | NA | NA | NA |
| Wanget al. (2019) [45] | 41 | Qingshen granules TID x 3 months | NA | NA | NA | NA | NA |
| Wanget al. (2020) [46] | 136 | Qingshen granules TID x 3 months | NA | NA | NA | NA | NA |
| Liet al. (2009) [40] | 32 | Tianbaoning tablets (gingko extract) TID x 8 weeks | NA | NA | NA | NA | NA |
| Ayurveda (n=6) | |||||||
| Alamet al. (2020) [53] | 70 | Sativa oil QD and alpha-keto amino acid tablets TID x 3 months | NA | NA | NA | NA | NA |
| Fallahzadehet al. (2012) [54] | 30 | 140 mg silymarin tablet QD x 3 months | Nausea and vomiting (n=3, 10%); headache (n=2,6.67%) | NA | NA | NI | NI |
| Hoseiniet al. (2019) [55] | 22 | Camel milk BID x 3 months | NA | Abdominal pain (n=1, 4.5%) | NA | NI | NI |
| Khajehdehiet al. (2011) [56] | 28 | 140 mg silymarin TID x 3 months | NA | NA | NA | NA | NA |
| Makhlough et al. (2010) [57] | 17 | 0.03% capsaicin ointment QID x 4 weeks | NA | Severe skin burning (n=1, 2.8%) | NA | NI | NI |
| Pingaliet al. (2020) [58] | 18 | 1000 mg of beleric capsule taken QD | Mild gastrointestinal intolerance (n=2, 11.1%) | NA | NA | NA | NA |
| Naturopathy (n=5) | |||||||
| Khanet al. (2014) [60] | 80 | Alpha-keto amino acid tablets TID x 3 months | Nausea (n=5, 6.25%); diarrhoea (n=5, 6.25%) | NA | NA | NA | NA |
| Prakashet al. (2004) [61] | 21 | Keto amino acid tablets QD x 9 months | NA | NA | NA | NI | NI |
| Sedaghattalabet al. (2021) [62] | 22 | Watercress extract QD x 1 month | NA | NA | NA | NA | NA |
| Zareet al. (2019) [63] | 19 | Garlic extract tablets TIW x 2 months | NA | NA | NA | NI | NI |
| Boldajiet al. (2019) [59] | 22 | Pomegranate juice TIW x 2 months | Stomach discomfort (n=1, 4.54%) | NA | NA | NI | NI |
| Homeopathy (n=2) | |||||||
| Cavalcantiet al. (2003) [16] | 11 | Homeopathic verum medicationc administered | NA | NA | NA | NA | NA |
| Silveiraet al. (2019) [64] | 18 | Brazilian green propolis pills BID x 3 months | NA | NA | NA | NA | NA |
Adverse effects associated with traditional Chinese medicine: the use of acupuncture is associated with mild pain, bleeding, bruising, and elbow soreness (grade 1) [36,40]. These symptoms resolved spontaneously without additional treatment. For single-herb treatments, TWHF treatment was associated with vomiting (38.2%, grade 1), itchy skin (6.45%, grade 1), nausea (3.22%, grade 1), and rash (1.61%, grade 1) [39,48]. Grade 2 adverse events associated with its use included hyperkalemia (23.5%), leukopenia (2.9%), and photosensitive dermatitis (8.8%), while grade 3 adverse effects included liver dysfunction (19.4%) and severe leukopenia (1.61%). Subjects who developed leukopenia were withdrawn from the study.
For Huangkui treatment, adverse effects observed included elevated cholesterol (2.94-3.76%) (grade 1), upper respiratory tract infection (2.94-3%) (grade 2 and grade 3), and liver injury (2.26%) (grade 3) [42,51]. The use of Shenqi particles was associated with interstitial pneumonia (1.1%, grade 2), lung infection (5.26%, grade 3), and liver injury (3.15%, grade 3) [37]. Anemia (grade 2) and acute myocardial infarction (grade 3) were observed for subjects using Tangshen formula granules [41]. The use of QDDHG tablets was associated with insomnia (1.96%, grade 1) [47].
Adverse effects associated with ayurveda: Silymarin treatment was associated with nausea and vomiting (10%), as well as headache (6.67%) [54,56]. Beleric capsule treatment was associated with mild gastrointestinal intolerance (11.1%) [58]. Adverse effects from both studies were of grade 1 severity. Abdominal pain (4.5%, grade 2) was observed with the treatment of camel milk [55].
Adverse effects associated with naturopathy: All adverse effects reported for naturopathy treatments are of grade 1 severity (Table 3). They include nausea (6.25%) and diarrhea (6.25%) with the use of alpha-keto amino acid [60], as well as stomach discomfort (4.54%) with the use of pomegranate juice [59].
Adverse effects associated with homeopathy: No adverse effects were reported with the use of homeopathy (Table 3).
Summary of Efficacy and Safety Profile of AMS Interventions
Table 4 shows a summary related to the efficacy and safety profiles of AMS interventions.
Table 4. Summary of efficacy and safety profile of AMS indicated for CKD symptoms.
aQDDHG is composed of Huang Qi, Danshen, Dihuang, Shanyao, and Gan Cao.
bDosage and frequency vary from patient to patient.
Percentage reduction in symptoms is computed using (mean score at baseline - mean score post-treatment at end of study)/(mean score at baseline) for the treatment group.
99mTc-DTPA: 99 m technetium diethylenetri-aminepenta-aceticacid, BAID: brachial arterial inner diameter, BID: twice a day, ELISA: enzyme-linked immunosorbent assay, GS-Rb1: Ginseng extract, MDRD: Modification of Diet in Renal Disease, QoL: Quality of life, TBN: Tianbaoning, TID: thrice a day, TIW: thrice a week, TwHF: Tripterygium Wilfordii Hook F, ZSTL: zishentongluo
| Type of AMS | Common doses and treatment regimens and duration of therapy | Indications | Percentage reduction in CKD symptoms (if available) | Adverse effects reported (%) |
| TCM (n=20) [3,7,17,36-52] | Herbal | Renal function | 2.76% to 51.2% | Elbow soreness (10%); liver injury and dysfunction (1.04-19.40%); dry cough, pneumonia, and upper respiratory infections (1.04-5.26%); hyperkalemia (3.64-23.50%); vomiting (38.2%); leukopenia and anemia (1.61-2.90%); photosensitive dermatitis, itchy skin, and rash (1.65-8.8%); gastrointestinal symptoms, nausea, and vomiting (1.57-38.2%); insomnia (1.96%) |
| Herbal granules TID x 6 months; QDDHG tabletsa BID x 12 weeks; 500 mg GS-Rb1 QD x 6 months; 8 g TSF granules and ARB BID x 24 weeks; 150 mL ZSTL solution BID x 3 months; TCM granules BID x 24 weeks | ||||
| Acupuncture | ||||
| Acupuncture at Li4, ST36 and K13 acupoint QD x 3 months | ||||
| Herbal | Proteinuria | -61.8% to -18.6% | ||
| 9.6 g of Shenqi particle TID x 48 weeks; 40 mg TWHF TID x 3 months followed by 20 mg TWHF TID x 3 months; Huangkui capsule TID x 12 months; 60 mg TWHF and 160 mg valsartan QD x 24 weeks | ||||
| Acupuncture | Uremic pruritus | -60.2% to -54.7% | ||
| Acupuncture at Quchi (L11) acupoint TIW x 1 month; acupuncture at Quchi (L11) and Zusanli (ST 36) acupoint BIW x 1 month; acupuncture at various acupoints TIW x 6 weeks | ||||
| Acupuncture QIW x 2 months | Fatigue, insomnia, itchiness, and pain | -89.2% to 15.2% | ||
| Infrared stimulation of Qihai (RN6), Kuamyuan (RN4) and Chungchi (RN3) TIW x 3 months | QoL | -9.10% to 163% | ||
| Qingshen granules TID x 3 months | Wnt/β-catenin signaling pathway | -61.4% to 17.8% | ||
| Qingshen granules TID x 3 months | Immune function | 42.3% to -20.2% | ||
| TBN tablets (gingko extract) TI x 8 weeks | Vascular endothelial function | 44.5% to 40.8% | ||
| Ayurveda (n=6) [53-58] | Plant-based | Renal function | -56.0% to 86.8% | Nausea, vomiting, and headache (6.67-10%); abdominal pain (4.5%) severe skin burning (2.8%) |
| Sativa oil QD and alpha-keto amino acid tablets TID; 140 mg silymarin TID x 3 months; 140 mg silymarin tablet QD x 3 months | ||||
| Animal-based | ||||
| Camel milk BID x 3 months | ||||
| 0.03% capsaicin ointment QID x 4 weeks | Uremic pruritus | -84.3% | ||
| 1000/500 mg of beleric capsule taken QD | Hyperuricemia | -24.4% to 40.4% | ||
| Naturopathy (n=5) [59-63] | Alpha-keto amino acid tablets TID x 3 months; Keto amino acid tablets QD x 9 months | Malnutrition | 39.5% to 49.2% | Nausea (6.25%); diarrhea (6.25%); stomach discomfort (4.45%) |
| Watercress extract QD x 1 month; garlic extract tablets TIW x 2 months | Inflammation | -73.8% to 26.6% | ||
| Pomegranate juice TIW x 2 months | Hypertension, stress, and inflammation | -30.3% to 22.5% | ||
| Homeopathy (n=2) [16,64] | Homeopathic verum medicationb administered x 60 days; Brazilian green propolis pills BD x 3 months | Uremic pruritus | -41.5% to -29.2% | - |
| Renal function | -27.6% |
Discussion
To the best of our knowledge, this is the first review that has summarized findings related to the therapeutic uses of AMS for CKD patients in RCTs. Among the four classes of AMS, TCM was the most studied class which has demonstrated efficacy in improving CKD-related symptoms and outcomes [3,7,36-52]. Among the TCM interventions evaluated, Huangkui, TWHF, and acupuncture have shown efficacy in reducing proteinuria and relieving uremic pruritus symptoms. The therapeutic basis of TCM for CKD is rooted in the restoration of vital energy and nourishment of blood, dispelling of heat and reduction of dampness, and regulation of Yin and Yang in the body [65]. In Western medicine, this is seen in a reduction in inflammation and oxidative stress, as well as boosting micro-circulation and enhancement of metabolism [52]. For example, Huangkui, also known as Abelmoschus manihot, reduces proteinuria by removing oxygen radicals, improving the circulation, and clearance of immune complexes as well as reducing inflammation and renal tubular epithelial injury [66]. It is also noted that triptolide, the key constituent of TWHF, suppresses the nuclear factor kappa b (NF-κB) signaling pathway and prevents the trigger of T lymphocytes and some inflammatory cytokines (TNF-α, IL-1β, IL-6, and IFN-γ), in addition to its podocyte-protective capabilities [67-70]. Notably, two of the included studies demonstrated that a combination of TCM and Western medicine, such as the intake of Huangkui capsule and losartan tablet to alleviate proteinuria, is more efficacious than taking TCM or Western medicine alone. This adds to existing evidence on potential applications of TCM alongside conventional medical therapy. Among patients on TWHF, regular checks of potassium and liver enzymes should be performed due to the risk of hyperkalemia and raised liver enzymes.
With regards to the use of acupuncture, it results in the release of endogenous opiate-like substances that have been proposed to dull the peripheral and central perception of itching [71]. Stimulation of acupoints via far infrared (FIR) treatments has also been revealed to boost skin microcirculation, lessen emotional anxiety and promote excretion of waste products by improving the autonomic nervous system [44,72-76]. Enhanced circulation via a stronger autonomic nervous system is postulated to relieve CKD-related symptoms as the development of renal failure is attributed to poor circulation in the field of TCM [73,77]. Currently, renowned hospitals in the United States such as the Mayo Clinic and Duke University Medical Center have started providing acupuncture, along with other treatments. With growing evidence related to the efficacy and safety of TCM, there has been greater receptivity from medical doctors related to applications and use of TCM in clinical practice [78]. It is however important to note that TCM is not without any side effects. For example, the use of Huangkui should be cautioned in patients with hyperlipidemia or liver disease as its use has been associated with elevated lipid levels and liver injury. On the other hand, acupuncture appears to be relatively safe with mild side effects, such as elbow soreness. More studies related to TCM are required to further assess their long-term safety profile, and they should be prescribed with careful consideration of each patient’s health condition.
For Ayurveda, silymarin was one of the most studied interventions which demonstrated efficacy in improving renal function. Ayurvedic therapies are derived predominantly from plants, animals, minerals, exercise, and lifestyle changes. They are believed to rejuvenate and remove toxins from one’s body. In conventional medicine, the therapeutic effects of ayurveda for CKD are ascribed to their anti-inflammatory and anti-oxidant properties. For instance, silymarin has shown efficacy in in vitro studies in attenuating inflammatory stress in renal tissue by suppressing the NF-κB signaling pathway and hence TNF production [79-85]. Other Ayurvedic treatments, such as the application of capsaicin ointment, were also found to alleviate uremic pruritus. Topical capsaicin, a natural alkaloid derived from red chili pepper, has been discovered to relieve uremic pruritus by binding specifically to type C sensory neurons and resulting in the release of substance P, as well as suppressing its synthesis, transport, and storage thereafter [57]. Relatively few side effects were observed for the Ayurvedic therapies discussed above. Despite the promising benefits associated with Ayurvedic treatments, it is currently less globally recognized as compared to TCM [86]. Further research is necessary to evaluate their efficacy and safety profile to improve their acceptance in clinical practice as adjunctive treatments, in particular for CKD patients.
For naturopathy, its unique attribute lies in the reprioritization of the order of therapeutics, with increased emphasis on non-invasive treatments, such as lifestyle modifications and nutrition, over medical or surgical interventions. In this review, one of the more studied interventions is the use of ketoanalogues of essential amino acids (KAs). The addition of KAs to a low-protein diet has been shown to improve renal function and uremia. Notably, while lowering protein intake may improve renal function in CKD patients by altering immunologic events and reducing hypertrophy and hyperfiltration in the remaining nephrons, it may result in malnutrition [87-89]. However, the supplementation of KAs not only averts malnutrition by ensuring adequate consumption of amino acids but also alleviates uremia [61]. The absence of amino nitrogen in KAs allows them to become transaminated by taking nitrogen from non-essential amino acids and hence, reducing the production of urea via re-using the amino group [90,91]. Relatively few and mild adverse effects were observed for the included naturopathic therapies, rendering them attractive treatment options. Additionally, as naturopathic treatments are usually non-invasive, they can be easily combined with conventional medications. Of note, 28 health systems, hospitals, and cancer treatment centers in the United States currently have at least one licensed naturopathic physician at their premises [92]. With increasing research evaluating the efficacy and safety profile of naturopathic treatments, its role as potential adjunctive treatment for CKD patients is also likely to expand in the future.
Lastly, homeopathy has also shown efficacy in improving CKD-related symptoms and outcomes. Homeopathy entails the therapeutic administration of substances derived from plants, minerals, or animals which produce effects that correspond to the clinical manifestation of diseases. In this review, the use of Brazilian green propolis pills and homeopathic verum medication were found to improve renal function and alleviate uremic pruritus, respectively. Brazilian green propolis was reported to improve renal function via a few mechanisms. Firstly, it decreases proteinuria via its ability to reduce urinary oxidative stress and macrophage infiltration into the kidneys [93]. Secondly, chrysin, a flavonoid in propolis has been shown to decrease podocyte apoptosis in patients with diabetic nephropathy and lessen glomerular injury [94]. Lastly, propolis has also been shown to decrease blood pressure via acetylcholine-induced vasodilation and from its antioxidant properties [93,95-97]. With regard to the safety of homeopathic treatment, no adverse effects were reported across included studies. However, the practice of homeopathy is relatively restricted, with 36% of states in the United States requiring homeopathic practitioners to either be licensed Western medicine or Naturopathic practitioners [32]. Consequently, more research is necessitated to validate the efficacy and safety of homeopathic treatment as adjunctive therapy for CKD patients.
Limitations
The following limitations should be considered in conjunction with this review. Firstly, due to the clinical and methodological heterogeneity of the studies, meta-analyses were not performed. As the pool of evidence for AMS trials for CKD patients grows, subsequent reviews should consider conducting meta-analyses for the efficacy of AMS treatments for CKD patients. In addition, there could have been exclusion of potentially applicable studies even though an extensive search strategy was used. To prevent this, the references of included studies were also hand-searched as part of our search strategy. Another limitation of the study relates to the inclusion of only articles in English language. Researchers should consider the inclusion of studies in other languages such as Chinese and Tamil in future reviews. Finally, although results of the included studies were reported normalized Z scores, care should be taken when interpreting these values and comparing the efficacy of various AMS classes. This is due to considerable diversity in types of outcomes evaluated and comparator arms and tools adopted for evaluation of outcomes. Overall, it is hoped that with greater standardization of study outcomes for AMS therapies in future studies, these normalized Z scores can enable more purposeful comparisons of the efficacy of the different AMS classes.
Conclusions
In this review, TCM and naturopathy were the most studied AMS which have shown efficacy for indications, such as improvement in renal function, proteinuria, and uremic pruritus in CKD patients. Most studies recruited small number of patients and larger RCTs are necessary to continue assessing and validating these potential AMS therapies. Medical professionals who plan to incorporate the use of AMS in daily practice should tailor treatment based on each patient’s health condition and be cognizant of their associated adverse effects, if any.
Acknowledgments
Wei Yi Teo, Jun Jie Benjamin Seng, and Shu Wen Felicia Chu contributed equally to the work and should be considered joint-first authors.
Appendices
Appendix 1: details of search strategy used
Table 5. Search strategy for MEDLINE.
| S/N | Key terms | Search terms employed |
| 1 | Randomized controlled trials | ({"Randomized controlled trial" (Publication Type) OR "randomized controlled trials as topic" (MeSH Terms) OR "randomized controlled trial" (All Fields) OR "randomized controlled trial" (All Fields)}) OR ({"random allocation" (MeSH Terms) OR ("random" {All Fields} AND "allocation" {All Fields}) OR "random allocation" (All Fields) OR "randomized" {All Fields}) AND controlled (All Fields) AND study (All Fields)} OR {"randomized controlled trial" (Publication Type) OR "randomized controlled trials as topic" (MeSH Terms) OR "randomized controlled trial" (All Fields) OR "randomized controlled trial" (All Fields)} OR {randomized (All Fields) AND controlled (All Fields) AND study (All Fields)}) |
| 2 | Chronic kidney disease | ({"Renal insufficiency, chronic"(MeSH Terms) OR ("renal"{All Fields} AND "insufficiency"{All Fields} AND "chronic"{All Fields}) OR "chronic renal insufficiency"(All Fields) OR ("chronic"{All Fields} AND "kidney"{All Fields} AND "disease"{All Fields} OR "chronic kidney disease"{All Fields}) OR ("renal insufficiency, chronic"{MeSH Terms} OR ("renal"{All Fields} AND "insufficiency"{All Fields} AND "chronic"{All Fields}) OR ("chronic renal insufficiency"{All Fields} OR ("chronic"{All Fields} AND "kidney"{All Fields} AND "insufficiency"{All Fields}) OR "chronic kidney insufficiency"{All Fields}) OR ("renal insufficiency, chronic"{MeSH Terms} OR "renal"{All Fields} AND "insufficiency"{All Fields} AND "chronic"{All Fields}) OR "chronic renal insufficiency"(All Fields) OR ("chronic"{All Fields} AND "renal"{All Fields} AND "disease"{All Fields}) OR "chronic renal disease"{All Fields} OR "kidney failure, chronic"(MeSH Terms) OR ("kidney"{All Fields} AND "failure"{All Fields} AND "chronic"{All Fields}) OR "chronic kidney failure"{All Fields} OR ("chronic"{All Fields} AND "renal"{All Fields} AND "disease"{All Fields}) OR ("renal insufficiency, chronic"{MeSH Terms} OR "renal"{All Fields} AND "insufficiency"{All Fields} AND "chronic"{All Fields}) OR "chronic renal insufficiency"{All Fields} OR ("chronic"{All Fields} AND "renal"{All Fields} AND "insufficiency"{All Fields}) OR ("renal insufficiency"(MeSH Terms) OR ("renal"{All Fields} AND "insufficiency"{All Fields}) OR "renal insufficiency"{All Fields} OR ("kidney"{All Fields} AND "failure"{All Fields}) OR "kidney failure"{All Fields}) OR ("renal insufficiency"(MeSH Terms) OR ("renal"{All Fields} AND "insufficiency"{All Fields}) OR "renal insufficiency"{All Fields} OR ("renal"{All Fields} AND "failure"{All Fields}) OR "renal failure"{All Fields}) OR ("kidney failure, chronic"(MeSH Terms) OR ("kidney"{All Fields} AND "failure"{All Fields} AND "chronic"{All Fields}) OR "chronic kidney failure"{All Fields} OR ("end"{All Fields} AND "stage"{All Fields} AND "renal"{All Fields} AND "failure"{All Fields}) OR "end stage renal failure"{All Fields}) OR ("kidney failure, chronic"(MeSH Terms) OR ("kidney"{All Fields} AND "failure"{All Fields} AND "chronic"{All Fields}) OR "chronic kidney failure"{All Fields} OR ("end"{All Fields} AND "stage"{All Fields} AND "renal"{All Fields} AND "disease"{All Fields}) OR "end stage renal disease"{All Fields}) OR predialysis{All Fields} OR pre-dialysis{All Fields} OR non-dialysis{All Fields} OR "renal dialysis"(MeSH Terms) OR ("renal"{All Fields} AND "dialysis"{All Fields}) OR "renal dialysis"{All Fields}) OR ("kidney diseases"{MeSH Terms} OR "kidney"{All Fields} AND "diseases"{All Fields}) OR "kidney diseases"{All Fields} OR ("kidney"{All Fields} AND "disease"{All Fields}) OR "kidney disease"{All Fields}) OR ("haemodialysis"{All Fields} OR "renal dialysis"{MeSH Terms}) OR ("renal"{All Fields} AND "dialysis"{All Fields}) OR "renal dialysis"{All Fields} OR "hemodialysis"{All Fields}) OR ("haemodialysis"{All Fields} OR "renal dialysis"{MeSH Terms}) OR ("renal"{All Fields} AND "dialysis"{All Fields}) OR "renal dialysis"({All Fields} OR "hemodialysis"{All Fields}) OR ("peritoneal dialysis"{MeSH Terms} OR ("peritoneal"{All Fields} AND "dialysis"{All Fields}) OR "peritoneal dialysis"{All Fields}) |
| 3 | Manipulative and body-based methods | ({Randomized controlled trial} OR {randomized controlled study} OR {randomized controlled trial} OR {randomized controlled study}) AND ({chronic kidney disease} OR {chronic kidney insufficiency} OR {chronic renal disease} OR {chronic renal insufficiency} OR {kidney failure} OR {renal failure} OR {end-stage renal failure} OR {end-stage renal disease} OR {predialysis} OR {pre-dialysis} OR {non-dialysis} OR {renal dialysis} OR {Kidney disease} OR {hemodialysis} OR {hemodialysis} OR {peritoneal dialysis}) AND ({Traditional Chinese Medicine} OR {Traditional Medicine} OR {Chinese Herbal Drugs} OR {Alternative Medicine} OR {Complementary Medicine} OR {Complementary therap*} OR {Alternative therap*} OR {Natural Medicine} OR {Integrative Medicine} OR TCM OR Ayurved* OR ayuverda OR Naturopath* OR naturopathy OR Homeopath* OR homeopathy OR {medicinal plant} OR {plant extract} OR {Plant Preparation} OR {Chinese medicinal herb} OR herb OR {natural remedies} OR {oriental medicine} OR {Materia Medica} OR {Diet-based therap*} OR {ethnopharmacology OR ethnomedicine OR kampo OR kanpo OR herbology}) |
Table 6. Search strategy for Embase.
| S/N | Key terms | Search terms employed |
| 1 | Randomized controlled trials | (“Randomized controlled trial”/exp OR “randomized controlled trial” OR {randomized AND controlled AND (“trial”/exp OR trial)} OR “randomized controlled study”/exp OR “randomized controlled study” OR {randomized AND controlled AND (“study”/exp OR study)} OR “randomized controlled trial”/exp OR “randomized controlled trial” OR {randomized AND controlled AND (“trial”/exp OR trial)} OR “randomized controlled study”/exp OR “randomized controlled study” OR {randomized AND controlled AND (“study”/exp OR study)}) |
| 2 | Chronic kidney disease | (“Chronic kidney disease”/exp OR “chronic kidney disease” OR {chronic AND (“kidney”/exp OR kidney) AND (“disease”/exp OR disease)} OR “chronic kidney insufficiency”/exp OR “chronic kidney insufficiency” OR {chronic AND (“kidney”/exp OR kidney) AND insufficiency} OR “chronic renal disease”/exp OR “chronic renal disease” OR {chronic AND (“renal”/exp OR renal) AND (“disease”/exp OR disease)} OR “chronic renal insufficiency”/exp OR “chronic renal insufficiency” OR {chronic AND (“renal”/exp OR renal) AND insufficiency} OR “kidney failure”/exp OR “kidney failure” OR {(“kidney”/exp OR kidney) AND (“failure”/exp OR failure)} OR “renal failure”/exp OR “renal failure” OR {(“renal”/exp OR renal) AND (“failure”/exp OR failure)} OR “end-stage renal failure”/exp OR “end-stage renal failure” OR {“end stage” AND (“renal”/exp OR renal) AND (“failure”/exp OR failure)} OR “end-stage renal disease”/exp OR “end-stage renal disease” OR {“end stage” AND (“renal”/exp OR renal) AND (“disease”/exp OR disease)} OR “predialysis”/exp OR predialysis OR “pre dialysis” OR “non dialysis” OR “renal dialysis”/exp OR “renal dialysis” OR {(“renal”/exp OR renal) AND (“dialysis”/exp OR dialysis)} OR “kidney disease”/exp OR “kidney disease” OR {(“kidney”/exp OR kidney) AND (“disease”/exp OR disease)} OR “hemodialysis”/exp OR hemodialysis OR “hemodialysis”/exp OR hemodialysis OR “peritoneal dialysis”/exp OR “peritoneal dialysis” OR {peritoneal AND (“dialysis”/exp OR dialysis)}) |
| 3 | Manipulative and body-based methods | (“Alternative medicine”/exp OR “alternative medicine” OR {alternative AND (“medicine”/exp OR medicine)} OR “alternative therapy”/exp OR “alternative therapy” OR {alternative AND (“therapy”/exp OR therapy)} OR “complementary medicine”/exp OR “complementary medicine” OR {complementary AND (“medicine”/exp OR medicine)} OR “complementary therapy” OR {complementary AND (“therapy”/exp OR therapy)} OR “natural medicine” OR {(“natural”/exp OR natural) AND (“medicine”/exp OR medicine)} OR “integrative medicine”/exp OR “integrative medicine” OR {integrative AND (“medicine”/exp OR medicine)} OR “massage”/exp OR massage OR chiropractice OR “yoga”/exp OR yoga OR “tai chi”/exp OR “tai chi” OR {tai AND chi} OR “moxibustion”/exp OR moxibustion OR “cupping”/exp OR cupping OR “qi gong”/exp OR “qi gong” OR {(“qi”/exp OR qi) AND gong} OR “acupuncture”/exp OR acupuncture OR osteopath* OR naprapath* OR reflexo* OR “shiatsu”/exp OR shiatsu OR “tui na”/exp OR “tui na” OR {tui AND (“na”/exp OR na)}) AND (“spinal manipulation”/exp OR “spinal manipulation” OR {spinal AND (“manipulation”/exp OR manipulation)}) |
Table 7. Search strategy for CINAHL.
CINAHL: Cumulative Index to Nursing and Allied Health Literature
| S/N | Key terms | Search terms employed |
| 1 | Randomized controlled trials | ({Randomized controlled trial} OR {randomized controlled study} OR {randomized controlled trial} OR {randomized controlled study}) |
| 2 | Chronic kidney disease | ({Chronic kidney disease} OR {chronic kidney insufficiency} OR {chronic renal disease} OR {chronic renal insufficiency} OR {kidney failure} OR {renal failure} OR {end-stage renal failure} OR {end-stage renal disease} OR {predialysis} OR {pre-dialysis} OR {non-dialysis} OR {renal dialysis} OR {Kidney disease} OR {hemodialysis} OR {hemodialysis} OR {peritoneal dialysis}) |
| 3 | Manipulative and body-based methods | ({Alternative medicine} OR {Alternative therapy} OR {Complementary Medicine} OR {Complementary therapy} OR {Natural Medicine} OR {Integrative Medicine} OR {Massage} OR {Chiropractice} OR yoga OR {Tai Chi} OR moxibustion OR cupping OR {Qi Gong} OR Acupuncture OR Osteopath* OR Naprapath* OR Reflexo* OR Shiatsu OR {Tui Na} {Spinal manipulation}) |
Table 8. Search strategy for PsycINFO.
| S/N | Key terms | Search terms employed |
| 1 | Randomized controlled trials | ({Randomized controlled trial} OR {randomized controlled study} OR {randomized controlled trial} OR {randomized controlled study}) |
| 2 | Chronic kidney disease | ({Chronic kidney disease} OR {chronic kidney insufficiency} OR {chronic renal disease} OR {chronic renal insufficiency} OR {kidney failure} OR {renal failure} OR {end-stage renal failure} OR {end-stage renal disease} OR {predialysis} OR {pre-dialysis} OR {non-dialysis} OR {renal dialysis} OR {Kidney disease} OR {hemodialysis} OR {hemodialysis} OR {peritoneal dialysis}) |
| 3 | Manipulative and body-based methods | ({Alternative medicine} OR {Alternative therapy} OR {Complementary Medicine} OR {Complementary therapy} OR {Natural Medicine} OR {Integrative Medicine} OR {Massage} OR {Chiropractice} OR yoga OR {Tai Chi} OR moxibustion OR cupping OR {Qi Gong} OR Acupuncture OR Osteopath* OR Naprapath* OR Reflexo* OR Shiatsu OR {Tui Na} {Spinal manipulation}) |
Table 9. Search strategy for Scopus.
| S/N | Key terms | Search terms employed |
| 1 | Randomized controlled trials | (TITLE-ABS-KEY {({randomized AND controlled AND trial} OR {randomized AND controlled AND study} OR {randomized AND controlled AND trial} OR {randomized AND controlled AND study})}) |
| 2 | Chronic kidney disease | (TITLE-ABS-KEY {({randomized AND controlled AND trial} OR {randomized AND controlled AND study} OR {randomized AND controlled AND trial} OR {randomized AND controlled AND study })}) AND (TITLE-ABS-KEY {({chronic AND kidney AND disease } OR {chronic AND kidney AND insufficiency} OR {chronic AND renal AND disease} OR {chronic AND renal AND insufficiency} OR {kidney AND failure} OR {renal AND failure} OR {end-stage AND renal AND failure} OR {end-stage AND renal AND disease} OR {predialysis} OR {pre-dialysis} OR {non-dialysis} OR {renal AND dialysis} OR {kidney AND disease} OR {hemodialysis} OR {hemodialysis} OR {peritoneal AND dialysis})}) |
| 3 | Manipulative and body-based methods | (TITLE-ABS-KEY {(alternative AND medicine) OR (complementary AND medicine) OR (complementary AND therapy) OR (alternative AND therapy) OR (natural AND medicine) OR (integrative AND medicine) OR (massage) OR (chiropractice) OR yoga OR (tai AND chi) OR (moxibustion) OR (cupping) OR (qi AND gong) OR (acupuncture) OR (Osteopath*) OR (Naprapath*) OR (Reflexo*) OR (Shiatsu) OR (Tui Na) OR (Spinal manipulation)}) |
Table 10. Search strategy for Cochrane Central Register of Controlled Trial (CENTRAL).
| S/N | Key terms | Search terms employed |
| 1 | Randomized controlled trials | ({Randomized controlled trial} OR {randomized controlled study} OR {randomized controlled trial} OR {randomized controlled study}) |
| 2 | Chronic kidney disease | ({Chronic kidney disease} OR {chronic kidney insufficiency} OR {chronic renal disease} OR {chronic renal insufficiency} OR {kidney failure} OR {renal failure} OR {end-stage renal failure} OR {end-stage renal disease} OR {predialysis} OR {pre-dialysis} OR {non-dialysis} OR {renal dialysis} OR {Kidney disease} OR {hemodialysis} OR {hemodialysis} OR {peritoneal dialysis}) |
| 3 | Manipulative and body-based methods | ({Alternative medicine} OR {Alternative therapy} OR {Complementary Medicine} OR {Complementary therapy} OR {Natural Medicine} OR {Integrative Medicine} OR {Massage} OR {Chiropractice} OR yoga OR {Tai Chi} OR moxibustion OR cupping OR {Qi Gong} OR Acupuncture OR Osteopath* OR Naprapath* OR Reflexo* OR Shiatsu OR {Tui Na} {Spinal manipulation}) |
Modeled after: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0223564
Appendix 2: risk of bias assessment
Table 11. Risk of bias assessment for AMS studies.
| Study (year of publication) | Domain 1: risk of bias from the randomization process | Domain 2: risk of bias due to deviations from the intended interventions | Domain 3: missing outcome data | Domain 4: risk of bias in measurement of the outcome | Domain 5: risk of bias in selection of the reported result | Overall Risk of bias judgment |
| Traditional Chinese medicine (n=20) | ||||||
| Li et al. (2015) [41] | Low | Low | Low | Low | Low | Low |
| Ma et al. (2013) [43] | Some concerns | Low | Low | Low | Low | Low |
| Wang et al. (2012) [17] | Low | Low | High | Low | Low | Some concerns |
| Yu et al. (2017) [50] | Low | High | Low | Low | Low | High |
| Zhao et al. (2020) [52] | Low | Low | Some concerns | Low | Low | Some concerns |
| Xiang et al. (2016) [47] | Low | Some concerns | Low | Low | Low | Low |
| Xu et al. (2016) [49] | Low | Low | Low | Low | Low | Low |
| Chen et al. (2013) [37] | Low | Low | Low | Low | Low | Low |
| Ge et al. (2013) [39] | Some concerns | Some concerns | Low | Low | Low | Low |
| Li et al. (2020) [42] | Some concerns | Low | Some concerns | High | Low | Some concerns |
| Zhang et al. (2014) [51] | Some concerns | Low | Low | Low | Low | Some concerns |
| Xiong et al. (2020) [48] | Low | Some concerns | Low | Low | Low | Low |
| Che-Yi et al. (2005) [36] | Low | Low | Low | Low | Low | Low |
| Gao et al. (2002) [38] | Some concerns | Some concerns | Low | Low | Low | Low |
| Nahidi et al. (2018) [7] | Low | High | High | Low | Low | High |
| Ono et al. (2015) [3] | Low | High | High | High | Low | High |
| Su et al. (2009) [44] | Some concerns | Some concerns | Some concerns | Some concerns | Low | High |
| Wang et al. (2019) [45] | Low | Low | Low | Low | Low | Low |
| Wang et al. (2020) [46] | Low | High | High | Low | Low | High |
| Li et al. (2009) [40] | Low | Some concerns | Low | Low | Low | Low |
| Ayurveda (n=6) | ||||||
| Alam et al. (2020) [53] | Low | Some concerns | Low | Low | Some concerns | Some concerns |
| Fallahzadeh et al. (2012) [54] | Low | Low | Low | Low | Some concerns | Some concerns |
| Hoseini et al. (2019) [55] | Low | Some concerns | Some concerns | Low | Some concerns | Some concerns |
| Khajehdehi et al. (2011) [56] | Low | Low | Low | Low | Low | Low |
| Makhlough et al. (2010) [57] | Low | Low | Low | Low | Low | Low |
| Pingali et al. (2020) [58] | Low | Low | Low | Low | Low | Low |
| Naturopathy (n=5) | ||||||
| Khan et al. (2014) [60] | Low | Low | Low | Low | Low | Low |
| Prakash et al. (2004) [61] | Low | Low | Low | Low | Low | Low |
| Sedaghattalab et al. (2021) [62] | Low | Low | Low | Low | Low | Low |
| Zare et al. (2019) [63] | Low | Low | Low | Low | Low | Low |
| Boldaji et al. (2019) [59] | Low | Low | Low | Low | Low | Low |
| Homeopathy (n=2) | ||||||
| Cavalcanti et al. (2003) [16] | Low | Low | High | Low | Low | High |
| Silveira et al. (2019) [64] | Low | Low | Low | Low | Low | Low |
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Prevalence and disease burden of chronic kidney disease. Lv JC, Zhang LX. Adv Exp Med Biol. 2019;1165:3–15. doi: 10.1007/978-981-13-8871-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Prevalence and outcomes associated with hypocalcaemia and hypercalcaemia among pre-dialysis chronic kidney disease patients with mineral and bone disorder. Amanda Yong MH, Benjamin Seng JJ, Cheryl Tan YL, Wong J, How P. Singapore Med J. 2022 doi: 10.4103/singaporemedj.SMJ-2021-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Efficacy and cost effectiveness of the acupuncture treatment using a new skin stimulus tool called M-test which is a measure based on symptoms accompanied with body movements: a pragmatic RCT targeting hemodialysis patients. Ono S, Mukaino Y. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/802846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Association of anemia and mineral and bone disorder with health-related quality of life in Asian pre-dialysis patients. Wee HL, Seng BJ, Lee JJ, Chong KJ, Tyagi P, Vathsala A, How P. Health Qual Life Outcomes. 2016;14:94. doi: 10.1186/s12955-016-0477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The prevalence of symptoms in end-stage renal disease: a systematic review. Murtagh FE, Addington-Hall J, Higginson IJ. Adv Chronic Kidney Dis. 2007;14:82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 6.CKD-associated pruritus: new insights into diagnosis, pathogenesis, and management. Verduzco HA, Shirazian S. Kidney Int Rep. 2020;5:1387–1402. doi: 10.1016/j.ekir.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acupuncture effect on pruritus in hemodialysis patients: a randomized clinical trial. Nahidi Y, Badiee S, Torabi S, Abbasi Z, Nazemian F, Saki A. Iranian Red Crescent Medical Journal. 2018 [Google Scholar]
- 8.Potential factors that influence usage of complementary and alternative medicine worldwide: a systematic review. Tangkiatkumjai M, Boardman H, Walker DM. BMC Complement Med Ther. 2020;20 doi: 10.1186/s12906-020-03157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey WB, Crocker AC, Coleman WL, Elias E, Feldman H. Developmental-Behavioral Pediatrics (Fourth Edition) Philadelphia, PA: Elsevier Saunders; 2009. Developmental-Behavioral Pediatrics (Fourth Edition) [Google Scholar]
- 10.Usage of complementary and alternative medicine among patients with chronic kidney disease on maintenance hemodialysis. Arjuna Rao AS, Phaneendra D, Pavani ChD, Soundararajan P, Rani NV, Thennarasu P, Kannan G. J Pharm Bioallied Sci. 2016;8:52–57. doi: 10.4103/0975-7406.171692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Understanding traditional Chinese medicine - a doctor’s viewpoint. Ho NK. https://pubmed.ncbi.nlm.nih.gov/11874155/ Singapore Med J. 2001;42:487–492. [PubMed] [Google Scholar]
- 12.Traditional Chinese medicine in the treatment of symptoms in patients with advanced cancer. Ling Y. Ann Palliat Med. 2013;2:141–152. doi: 10.3978/j.issn.2224-5820.2013.04.05. [DOI] [PubMed] [Google Scholar]
- 13.Therapeutic effects of ten commonly used Chinese herbs and their bioactive compounds on cancers. Liu W, Yang B, Yang L, et al. Evid Based Complement Alternat Med. 2019;2019 doi: 10.1155/2019/6057837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Effectiveness of an Ayurveda treatment approach in knee osteoarthritis - a randomized controlled trial. Kessler CS, Dhiman KS, Kumar A, et al. Osteoarthritis Cartilage. 2018;26:620–630. doi: 10.1016/j.joca.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Washington, DC: National Academies Press; 2005. Complementary and Alternative Medicine in the United States. [PubMed] [Google Scholar]
- 16.Effects of homeopathic treatment on pruritus of haemodialysis patients: a randomised placebo-controlled double-blind trial. Cavalcanti AM, Rocha LM, Carillo R Jr, Lima LU, Lugon JR. Homeopathy. 2003;92:177–181. doi: 10.1016/j.homp.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Optimized project of traditional Chinese medicine in treating chronic kidney disease stage 3: a multicenter double-blinded randomized controlled trial. Wang YJ, He LQ, Sun W, et al. J Ethnopharmacol. 2012;139:757–764. doi: 10.1016/j.jep.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Complementary and alternative medicine therapies for uremic pruritus - a systematic review of randomized controlled trials. Yeam CT, Yo TE, Tan YL, Liew A, Seng JJ. Complement Ther Med. 2021;56 doi: 10.1016/j.ctim.2020.102609. [DOI] [PubMed] [Google Scholar]
- 19.Chinese herbal medicine for diabetic kidney disease: a systematic review and meta-analysis of randomised placebo-controlled trials. Zhang L, Yang L, Shergis J, et al. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-025653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Astragalus (a traditional Chinese medicine) for treating chronic kidney disease. Zhang HW, Lin ZX, Xu C, Leung C, Chan LS. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD008353.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Efficacy and safety of the Fu-Zheng-Qu-Zhuo method on retarding the progress of chronic kidney disease (stage 3-4): a systematic review and meta-analysis. Liu SY, Huang P, Zhang N. Ann Transl Med. 2019;7 doi: 10.21037/atm.2018.12.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. Campbell M, McKenzie JE, Sowden A, et al. BMJ. 2020;368 doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Moher D, Liberati A, Tetzlaff J, Altman DG. BMJ. 2009;339:0. [PMC free article] [PubMed] [Google Scholar]
- 24.The role of mind-body interventions in pre-dialysis chronic kidney disease and dialysis patients - a systematic review of literature. Chu SW, Yeam CT, Low LL, Tay WY, Foo WY, Seng JJ. Complement Ther Med. 2021;57 doi: 10.1016/j.ctim.2020.102652. [DOI] [PubMed] [Google Scholar]
- 25.Manipulative and body-based methods in chronic kidney disease patients: a systematic review of randomized controlled trials. Chu SW, Ng WJ, Yeam CT, et al. Complement Ther Clin Pract. 2022;48 doi: 10.1016/j.ctcp.2022.101593. [DOI] [PubMed] [Google Scholar]
- 26.Prevalence and risk factors for elevated anxiety symptoms and anxiety disorders in chronic kidney disease: a systematic review and meta-analysis. Huang CW, Wee PH, Low LL, et al. Gen Hosp Psychiatry. 2021;69:27–40. doi: 10.1016/j.genhosppsych.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Theory of traditional Chinese medicine and therapeutic method of diseases. Lu AP, Jia HW, Xiao C, Lu QP. World J Gastroenterol. 2004;10:1854–1856. doi: 10.3748/wjg.v10.i13.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evidence-based Chinese medicine: theory and practice. [Article in Chinese] Zhang JH, Li YP, Zhang BL. Zhongguo Zhong Yao Za Zhi. 2018;43:1–7. doi: 10.19540/j.cnki.cjcmm.20171127.001. [DOI] [PubMed] [Google Scholar]
- 29.Systems biology of meridians, acupoints, and Chinese herbs in disease. Lin LL, Wang YH, Lai CY, et al. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/372670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evidence-based medicine and naturopathy. Jagtenberg T, Evans S, Grant A, Howden I, Lewis M, Singer J. J Altern Complement Med. 2006;12:323–328. doi: 10.1089/acm.2006.12.323. [DOI] [PubMed] [Google Scholar]
- 31.About naturopathic medicine. [ Oct; 2022 ];https://naturopathic.org/page/aboutnaturopathicmedicine 24 [Google Scholar]
- 32.Homeopathy. [ Oct; 2022 ];https://www.nccih.nih.gov/health/homeopathy 24 [Google Scholar]
- 33.A glimpse of Ayurveda - the forgotten history and principles of Indian traditional medicine. Jaiswal YS, Williams LL. J Tradit Complement Med. 2017;7:50–53. doi: 10.1016/j.jtcme.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.RoB 2: a revised tool for assessing risk of bias in randomised trials. Sterne JA, Savović J, Page MJ, et al. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 35.Common Terminology Criteria for Adverse Events (CTCAE) [ Oct; 2022 ];https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm 24 [Google Scholar]
- 36.Acupuncture in haemodialysis patients at the Quchi (LI11) acupoint for refractory uraemic pruritus. Che-Yi C, Wen CY, Min-Tsung K, Chiu-Ching H. Nephrol Dial Transplant. 2005;20:1912–1915. doi: 10.1093/ndt/gfh955. [DOI] [PubMed] [Google Scholar]
- 37.Efficacy and safety of traditional Chinese medicine (Shenqi particle) for patients with idiopathic membranous nephropathy: a multicenter randomized controlled clinical trial. Chen Y, Deng Y, Ni Z, et al. Am J Kidney Dis. 2013;62:1068–1076. doi: 10.1053/j.ajkd.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Acupuncture treatment for 34 cases of uremic cutaneous pruritus. Gao H, Zhang W, Wang Y. https://pubmed.ncbi.nlm.nih.gov/11977515/ J Tradit Chin Med. 2002;22:29–30. [PubMed] [Google Scholar]
- 39.Treatment of diabetic nephropathy with Tripterygium wilfordii Hook F extract: a prospective, randomized, controlled clinical trial. Ge Y, Xie H, Li S, et al. J Transl Med. 2013;11 doi: 10.1186/1479-5876-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Effect of Ginkgo leaf extract on vascular endothelial function in patients with early stage diabetic nephropathy. Li XS, Zheng WY, Lou SX, Lu XW, Ye SH. Chin J Integr Med. 2009;15:26–29. doi: 10.1007/s11655-009-0026-8. [DOI] [PubMed] [Google Scholar]
- 41.Efficacy and safety of tangshen formula on patients with type 2 diabetic kidney disease: a multicenter double-blinded randomized placebo-controlled trial. Li P, Chen Y, Liu J, et al. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Efficacy and safety of Abelmoschus manihot for IgA nephropathy: a multicenter randomized clinical trial. Li P, Lin H, Ni Z, et al. Phytomedicine. 2020;76 doi: 10.1016/j.phymed.2020.153231. [DOI] [PubMed] [Google Scholar]
- 43.Effects of zishentongluo in patients with early-stage diabetic nephropathy. Ma J, Xu L, Dong J, Wei H, Zhi Y, Ma X, Zhang W. Am J Chin Med. 2013;41:333–340. doi: 10.1142/S0192415X13500249. [DOI] [PubMed] [Google Scholar]
- 44.Effects of far infrared acupoint stimulation on autonomic activity and quality of life in hemodialysis patients. Su LH, Wu KD, Lee LS, Wang H, Liu CF. Am J Chin Med. 2009;37:215–226. doi: 10.1142/S0192415X09006783. [DOI] [PubMed] [Google Scholar]
- 45.Based on HIF-1α/Wnt/β-Catenin pathway to explore the effect of Qingshen granules on chronic renal failure patients: a randomized controlled trial. Wang Y, Zhang L, Jin H, Wang D. Evid Based Complement Alternat Med. 2019;2019 doi: 10.1155/2019/7656105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Effects of Qingshen granules on immune function in patients with comorbid chronic renal failure and damp-heat syndrome: a multicenter, randomized, controlled trial. Wang D, Wang Y, Li C, Liu S, Zhang L, Jin H. Evid Based Complement Alternat Med. 2020;2020 doi: 10.1155/2020/5057894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Additive effect of Qidan Dihuang grain, a traditional Chinese medicine, and angiotensin receptor blockers on albuminuria levels in patients with diabetic nephropathy: a randomized, parallel-controlled trial. Xiang L, Jiang P, Zhou L, et al. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/1064924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evaluation of the efficacy and safety of TWHF in diabetic nephropathy patients with overt proteinuria and normal eGFR. Xiong C, Li L, Bo W, Chen H, XiaoWei L, Hongbao L, Peng Z. J Formos Med Assoc. 2020;119:685–692. doi: 10.1016/j.jfma.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Impact of extended ginsenoside Rb1 on early chronic kidney disease: a randomized, placebo-controlled study. Xu X, Lu Q, Wu J, Li Y, Sun J. Inflammopharmacology. 2017;25:33–40. doi: 10.1007/s10787-016-0296-x. [DOI] [PubMed] [Google Scholar]
- 50.Acupuncture on renal function in patients with chronic kidney disease: a single-blinded, randomized, preliminary controlled study. Yu JS, Ho CH, Wang HY, Chen YH, Hsieh CL. J Altern Complement Med. 2017;23:624–631. doi: 10.1089/acm.2016.0119. [DOI] [PubMed] [Google Scholar]
- 51.Efficacy and safety of Abelmoschus manihot for primary glomerular disease: a prospective, multicenter randomized controlled clinical trial. Zhang L, Li P, Xing CY, et al. Am J Kidney Dis. 2014;64:57–65. doi: 10.1053/j.ajkd.2014.01.431. [DOI] [PubMed] [Google Scholar]
- 52.Efficacy and safety of Chinese herbal formula granules in treating chronic kidney disease stage 3: a multicenter, randomized, placebo-controlled, double-blind clinical trial. Zhao J, Sun W, Chen J, et al. Evid Based Complement Alternat Med. 2020;2020 doi: 10.1155/2020/4073901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evaluation of safety and efficacy profile of Nigella sativa oil as an add-on therapy, in addition to alpha-keto analogue of essential amino acids in patients with chronic kidney disease. Alam MA, Nasiruddin M, Haque SF, Khan RA. Saudi J Kidney Dis Transpl. 2020;31:21–31. doi: 10.4103/1319-2442.279943. [DOI] [PubMed] [Google Scholar]
- 54.Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: a randomized, double-blind, placebo-controlled trial. Fallahzadeh MK, Dormanesh B, Sagheb MM, et al. Am J Kidney Dis. 2012;60:896–903. doi: 10.1053/j.ajkd.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 55.The efficacy of camel milk and Tarangabin (manna of Alhagi maurorum (combination therapy on glomerular filtration rate in patients with chronic kidney disease: a randomized controlled trial. Hoseini SM, Anushiravani M, Mojahedi MJ, et al. https://pubmed.ncbi.nlm.nih.gov/32257889/ Avicenna J Phytomed. 2020;10:170–180. [PMC free article] [PubMed] [Google Scholar]
- 56.Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Khajehdehi P, Pakfetrat M, Javidnia K, Azad F, Malekmakan L, Nasab MH, Dehghanzadeh G. Scand J Urol Nephrol. 2011;45:365–370. doi: 10.3109/00365599.2011.585622. [DOI] [PubMed] [Google Scholar]
- 57.Topical capsaicin therapy for uremic pruritus in patients on hemodialysis. Makhlough A, Ala S, Haj-Heydari Z, Kashi Z, Bari A. https://pubmed.ncbi.nlm.nih.gov/20404425/ Iran J Kidney Dis. 2010;4:137–140. [PubMed] [Google Scholar]
- 58.A randomized, double-blind, positive-controlled, prospective, dose-response clinical study to evaluate the efficacy and tolerability of an aqueous extract of Terminalia bellerica in lowering uric acid and creatinine levels in chronic kidney disease subjects with hyperuricemia. Pingali U, Nutalapati C, Koilagundla N, Taduri G. BMC Complement Med Ther. 2020;20 doi: 10.1186/s12906-020-03071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pomegranate juice improves cardiometabolic risk factors, biomarkers of oxidative stress and inflammation in hemodialysis patients: a randomized crossover trial. Barati Boldaji R, Akhlaghi M, Sagheb MM, Esmaeilinezhad Z. J Sci Food Agric. 2020;100:846–854. doi: 10.1002/jsfa.10096. [DOI] [PubMed] [Google Scholar]
- 60.A randomized clinical trial to evaluate the efficacy and safety of α-Keto amino acids in stage 3 and 4 of chronic kidney disease. Khan I, Nasiruddin M, Haque SF, Khan RA. https://www.researchgate.net/publication/285116719_A_randomized_clinical_trial_to_evaluate_the_efficacy_and_safety_of_a-Keto_amino_acids_in_stage_3_and_4_of_chronic_kidney_disease Asian J Pharm Clin Res. 2014;7:21–24. [Google Scholar]
- 61.Randomized, double-blind, placebo-controlled trial to evaluate efficacy of ketodiet in predialytic chronic renal failure. Prakash S, Pande DP, Sharma S, Sharma D, Bal CS, Kulkarni H. J Ren Nutr. 2004;14:89–96. doi: 10.1053/j.jrn.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 62.Effects of Nasturtium officinale extract on antioxidant and biochemical parameters in hemodialysis patients: a randomized double-blind clinical trial. Sedaghattalab M, Razazan M, Sadeghi H, et al. Evid Based Complement Alternat Med. 2021;2021 doi: 10.1155/2021/1632957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evaluating the effect of garlic extract on serum inflammatory markers of peritoneal dialysis patients: a randomized double-blind clinical trial study. Zare E, Alirezaei A, Bakhtiyari M, Mansouri A. BMC Nephrol. 2019;20 doi: 10.1186/s12882-019-1204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: a randomized, double-blind, placebo-controlled trial. Silveira MA, Teles F, Berretta AA, Sanches TR, Rodrigues CE, Seguro AC, Andrade L. BMC Nephrol. 2019;20 doi: 10.1186/s12882-019-1337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.New theoretical study on the biological origins of Chinese medicinal herbs. Xie Z, Liang A. https://pubmed.ncbi.nlm.nih.gov/7492355/ Zhongguo Zhong Yao Za Zhi. 1995;20:259–261. [PubMed] [Google Scholar]
- 66.Treatment of chronic kidney disease using a traditional Chinese medicine, Flos Abelmoschus manihot (Linnaeus) Medicus (Malvaceae) Chen Y, Cai G, Sun X, Chen X. Clin Exp Pharmacol Physiol. 2016;43:145–148. doi: 10.1111/1440-1681.12528. [DOI] [PubMed] [Google Scholar]
- 67.Triptolide: a potent inhibitor of NF-kappa B in T-lymphocytes. Liu H, Liu ZH, Chen ZH, Yang JW, Li LS. https://pubmed.ncbi.nlm.nih.gov/11501157/ Acta Pharmacol Sin. 2000;21:782–786. [PubMed] [Google Scholar]
- 68.Triptolide attenuates oxidative stress, NF-κB activation and multiple cytokine gene expression in murine peritoneal macrophage. Wu Y, Cui J, Bao X, Chan S, Young DO, Shen DL. Int J Mol Med. 2006;17:141–150. [PubMed] [Google Scholar]
- 69.Triptolide protects podocytes from puromycin aminonucleoside induced injury in vivo and in vitro. Zheng CX, Chen ZH, Zeng CH, Qin WS, Li LS, Liu ZH. Kidney Int. 2008;74:596–612. doi: 10.1038/ki.2008.203. [DOI] [PubMed] [Google Scholar]
- 70.Triptolide reduces proteinuria in experimental membranous nephropathy and protects against C5b-9-induced podocyte injury in vitro. Chen ZH, Qin WS, Zeng CH, et al. Kidney Int. 2010;77:974–988. doi: 10.1038/ki.2010.41. [DOI] [PubMed] [Google Scholar]
- 71.Brain opiates at work in acupuncture? Pomeranz BH. New Scientist. 1977;6:12–13. [Google Scholar]
- 72.Far-infrared therapy: a novel treatment to improve access blood flow and unassisted patency of arteriovenous fistula in hemodialysis patients. Lin CC, Chang CF, Lai MY, Chen TW, Lee PC, Yang WC. J Am Soc Nephrol. 2007;18:985–992. doi: 10.1681/ASN.2006050534. [DOI] [PubMed] [Google Scholar]
- 73.Biological effect of far-infrared therapy on increasing skin microcirculation in rats. Yu SY, Chiu JH, Yang SD, Hsu YC, Lui WY, Wu CW. Photodermatol Photoimmunol Photomed. 2006;22:78–86. doi: 10.1111/j.1600-0781.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 74.The effects of repeated thermal therapy for two patients with chronic fatigue syndrome. Masuda A, Kihara T, Fukudome T, Shinsato T, Minagoe S, Tei C. J Psychosom Res. 2005;58:383–387. doi: 10.1016/j.jpsychores.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 75.Sleep-enhancing effects of far-infrared radiation in rats. Honda K, Inoué S. Int J Biometeorol. 1988;32:92–94. doi: 10.1007/BF01044900. [DOI] [PubMed] [Google Scholar]
- 76.The study of far infrared therapeutic apparatus on animal model. Wu BJ. China Med Devices Infor. 2001;7:36–38. [Google Scholar]
- 77.The clinical experience of wide spectrum far infrared therapy. Hung YH. Taiwan Neph Nurses Ass. 2002;1:70–71. [Google Scholar]
- 78.Top U.S. hospitals embrace Chinese herbal medicine. 2014. https://www.unifiedpractice.com/top-u-s-hospitals-embrace-chinese-herbal-medicine/ https://www.unifiedpractice.com/top-u-s-hospitals-embrace-chinese-herbal-medicine/
- 79.Dietary supplementation of silymarin protects against chemically induced nephrotoxicity, inflammation and renal tumor promotion response. Kaur G, Athar M, Alam MS. Invest New Drugs. 2010;28:703–713. doi: 10.1007/s10637-009-9289-6. [DOI] [PubMed] [Google Scholar]
- 80.Silibinin protects mice from T cell-dependent liver injury. Schümann J, Prockl J, Kiemer AK, Vollmar AM, Bang R, Tiegs G. J Hepatol. 2003;39:333–340. doi: 10.1016/s0168-8278(03)00239-3. [DOI] [PubMed] [Google Scholar]
- 81.Effects of plant-derived polyphenols on TNF-alpha and nitric oxide production induced by advanced glycation endproducts. Chandler D, Woldu A, Rahmadi A, et al. Mol Nutr Food Res. 2010;54:141–150. doi: 10.1002/mnfr.200900504. [DOI] [PubMed] [Google Scholar]
- 82.Inhibitory effect of silibinin on tumour necrosis factor-alpha and hydrogen peroxide production by human monocytes. Bannwart CF, Peraçoli JC, Nakaira-Takahagi E, Peraçoli MT. Nat Prod Res. 2010;24:1747–1757. doi: 10.1080/14786410903314492. [DOI] [PubMed] [Google Scholar]
- 83.Silymarin inhibits in vitro T-cell proliferation and cytokine production in hepatitis C virus infection. Morishima C, Shuhart MC, Wang CC, et al. Gastroenterology. 2010;138:671-81, 681.e1-2. doi: 10.1053/j.gastro.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Effect of silymarin administration on TNF-α serum concentration in peritoneal dialysis patients. Nazemian F, Karimi G, Moatamedi M, Charkazi S, Shamsara J, Mohammadpour AH. Phytother Res. 2010;24:1654–1657. doi: 10.1002/ptr.3175. [DOI] [PubMed] [Google Scholar]
- 85.Silymarin: a novel antioxidant with antiglycation and antiinflammatory properties in vitro and in vivo. Wu CH, Huang SM, Yen GC. Antioxid Redox Signal. 2011;14:353–366. doi: 10.1089/ars.2010.3134. [DOI] [PubMed] [Google Scholar]
- 86.Ayurveda. [ Oct; 2022 ]. https://www.mountsinai.org/health-library/treatment/ayurveda https://www.mountsinai.org/health-library/treatment/ayurveda
- 87.Initiation of dialysis. Hakim RM, Lazarus JM. J Am Soc Nephrol. 1995;6:1319–1328. doi: 10.1681/ASN.V651319. [DOI] [PubMed] [Google Scholar]
- 88.Nutritional markers and survival in maintenance dialysis patients. Pollock CA, Ibels LS, Allen BJ. Nephron. 1996;74:625–641. doi: 10.1159/000189468. [DOI] [PubMed] [Google Scholar]
- 89.Role of dietary factors in the progression of chronic renal disease. Klahr S, Buerkert J, Purkerson ML. Kidney Int. 1983;24:579–587. doi: 10.1038/ki.1983.197. [DOI] [PubMed] [Google Scholar]
- 90.Utilisation of ammonia nitrogen for protein synthesis in man, and the effect of protein restriction and uraemia. Richards P, Metcalfe-Gibson A, Ward EE, Wrong O, Houghton BJ. Lancet. 1967;2:845–849. doi: 10.1016/s0140-6736(67)92588-3. [DOI] [PubMed] [Google Scholar]
- 91.Supplements of keto acids in patients with chronic renal failure--more than modulators of nitrogen economy. Druml W. https://pubmed.ncbi.nlm.nih.gov/11603097/ Wien Klin Wochenschr. 2001;113:17–18. [PubMed] [Google Scholar]
- 92.Naturopathic medicine is growing in U.S. Medical Centers of Excellence. [ Oct; 2022 ];https://www.prnewswire.com/news-releases/naturopathic-medicine-is-growing-in-us-medical-centers-of-excellence-300601605.html 2018 3006016 [Google Scholar]
- 93.Brazilian red propolis attenuates hypertension and renal damage in 5/6 renal ablation model. Teles F, da Silva TM, da Cruz Júnior FP, et al. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chrysin ameliorates podocyte injury and slit diaphragm protein loss via inhibition of the PERK-eIF2α-ATF-CHOP pathway in diabetic mice. Kang MK, Park SH, Kim YH, et al. Acta Pharmacol Sin. 2017;38:1129–1140. doi: 10.1038/aps.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anti-hypertensive effects of Brazilian propolis in spontaneously hypertensive rats. Kubota Y, Umegaki K, Kobayashi K, et al. Clin Exp Pharmacol Physiol. 2004;31:29–30. doi: 10.1111/j.1440-1681.2004.04113.x. [DOI] [PubMed] [Google Scholar]
- 96.Antihypertensive effects of Brazilian propolis: identification of caffeoylquinic acids as constituents involved in the hypotension in spontaneously hypertensive rats. Mishima S, Yoshida C, Akino S, Sakamoto T. Biol Pharm Bull. 2005;28:1909–1914. doi: 10.1248/bpb.28.1909. [DOI] [PubMed] [Google Scholar]
- 97.Antihypertensive effects of flavonoids isolated from brazilian green propolis in spontaneously hypertensive rats. Maruyama H, Sumitou Y, Sakamoto T, Araki Y, Hara H. Biol Pharm Bull. 2009;32:1244–1250. doi: 10.1248/bpb.32.1244. [DOI] [PubMed] [Google Scholar]