Abstract
Cholera toxin B subunit (CTB) is an efficient mucosal carrier molecule for the generation of mucosal antibody responses and/or induction of systemic T-cell tolerance to linked antigens. CTB binds with high affinity to GM1 ganglioside cell surface receptors. In this study, we evaluated how conjugation of a peptide or protein antigen to CTB by chemical coupling or genetic fusion influences the T-cell-activating capacity of different antigen-presenting cell (APC) subsets. Using an in vitro system in which antigen-pulsed APCs were incubated with antigen-specific, T-cell receptor-transgenic T cells, we found that the dose of antigen required for T-cell activation could be decreased >10,000-fold using CTB-conjugated compared to free antigen. In contrast, no beneficial effects were observed when CTB was simply admixed with antigen. CTB conjugation enhanced the antigen-presenting capacity not only of dendritic cells and B cells but also of macrophages, which expressed low levels of cell surface major histocompatibility complex (MHC) class II and were normally poor activators of naive T cells. Enhanced antigen-presenting activity by CTB-linked antigen resulted in both increased T-cell proliferation and increased interleukin-12 and gamma interferon secretion and was associated with up-regulation of CD40 and CD86 on the APC surface. These results imply that conjugation to CTB dramatically lowers the threshold concentration of antigen required for immune cell activation and also permits low-MHC II-expressing APCs to prime for a specific immune response.
Cholera toxin B subunit (CTB) and the closely related Escherichia coli heat-labile enterotoxin B subunit (LTB) are highly efficient carrier molecules for the induction of mucosal antibody responses (10, 17, 27, 31) and oral tolerance (2, 17, 42). The therapeutic applications of CTB-mediated oral tolerance, as demonstrated in animal models, include the prevention and treatment of T-cell-mediated autoimmune diseases (7, 44, 49), immunoglobulin E (IgE)-mediated allergic reactions (37, 47, 55), and infection-induced pathological inflammatory conditions (32, 43). The mechanism behind CTB's or LTB's efficacy as a mucosal carrier molecule has not been fully defined but is believed to be associated with the strong binding of CTB or LTB to the GM1 receptor present on most cells in the body, including epithelial cells and leukocytes. Efficient binding to GM1 could potentially increase the uptake of antigen across the mucosa and lead to an enhanced presentation of the conjugated molecule to the immune system (17, 34). Another possibility is that CTB per se has immunomodulating properties. Indeed, it has been shown that CTB induces major histocompatibility complex (MHC) class II expression on B cells (14), enhances antigen presentation by macrophages (Mφ) in the absence of enhanced MHC II expression (29), and blocks the development of diabetes in NOD mice through the development of regulatory cells (40).
Mucosal antigen-specific antibody formation and tolerance induction share the requirement for an initial immune activation (13). The first step in antigen-specific T-cell activation is controlled by antigen-presenting cells (APC) that adsorb, process, and present antigens in a complex with MHC class II on the cell surface together with costimulatory signals. APC utilize multiple mechanisms for antigen uptake, which vary according to cell type. B cells have membrane-bound antibody receptors that recognize and bind one specific antigen, whereas other APC such as dendritic cells (DC) and Mφ have a broader range of binding specificities through Fc receptors and C-type multilectin receptors and can also absorb antigens by macropinocytosis and phagocytosis (5). The nature of the APC involved and the nature of the cytokines present and/or induced are important determinants for the outcome of the subsequent T-cell response. Thus, the level of T-cell activation depends on the densities of specific peptide-loaded MHC class II and of costimulatory molecules such as CD40, CD80, and CD86 present on the APC surface (30, 50), as well as on the levels of cytokines produced, such as interleukin-1 (IL-1), IL-12, and IL-18 (21, 35, 53).
In the present study we have evaluated whether exposure of different APC to an antigen coupled to CTB by chemical or genetic means can modulate their cognate T-cell-activating capacity. To this end defined APC populations were pulsed with either free peptide or protein antigens, or with CTB-linked derivatives thereof. The pulsed APC were then incubated together with purified T cells from antigen-specific T-cell-receptor (TCR)-transgenic mice, and the proliferative responses and cytokine profiles were measured in these cultures. It was found that DC and B cells were efficient APC that could present free peptide and protein antigens to naive transgenic T cells, whereas Mφ could not. Exposing the different APC to CTB-linked rather than free antigen greatly enhanced their antigen-presenting capacity, decreasing >104-fold the amount of antigen required to stimulate a proliferative response by cognate T cells. Furthermore, Mφ also functioned as efficient APC when exposed to the CTB-conjugated antigens. The enhanced T-cell-proliferative responses obtained were associated with increased levels of secreted IL-12 and gamma interferon (IFN-γ) and with increased expression of CD40 and CD86 on the APC surface.
MATERIALS AND METHODS
Chemical conjugation of influenza virus HA peptide or OVA to CTB.
A synthetic peptide corresponding to amino acid residues 108 to 119 of influenza virus hemagglutinin H1 subtype (HA peptide) was purchased from Neosystem (Strasbourg, France). Whole ovalbumin (OVA) was purchased from Sigma (St. Louis, Mo.). Recombinant CTB (rCTB) was produced and purified from Vibrio cholerae strain 358 as described elsewhere (23).
The HA peptide and OVA protein were chemically coupled to rCTB using N-succinimidyl (3-[2-pyridyl]-dithio)propionate (SPDP; Pharmacia Biotech, Uppsala, Sweden) as a bifunctional coupling reagent as described previously (37). The conjugates were analyzed in a GM1 enzyme-linked immunosorbent assay (ELISA) using biotinylated anti-CTB monoclonal antibodies (46) and were shown to have retained GM1-binding activity.
Construction and purification of CTB fusion proteins.
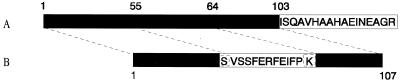
Two recombinant CTB fusion molecules were made, one in which peptide 323-339 of OVA was fused to the C terminus of CTB (CTB::OVAp [Fig. 1A]) and one in which a peptide corresponding to the influenza virus HA residues 108 to 119 replaced residues 56 to 63 in the CTB structure (CTB56-63HAp [Fig. 1B]).
FIG. 1.
Construction of the genetic CTB fusion proteins. (A) Addition of the 17-amino-acid OVA peptide to the C terminus of CTB. (B) Insertion of the 13-amino-acid HA peptide into the CTB molecule, replacing residues 56 to 64 of the mature protein and resulting in a protein of 107 instead of 103 amino acids. The first residue of the peptide and residue 55 of CTB are both serine. Additionally, the last residue of the peptide and residue 63 of CTB are both lysine. Open rectangles, HA or OVA peptide; solid rectangles, mature CTB protein. The serine residue at position 55 and the lysine residue at position 63 of CTB that are shared with the peptide sequence in CTB55-64HA are boxed.
(i) CTB::OVAp.
Synthetic oligonucleotides encoding the 17-amino-acid OVA peptide were synthesized (Innovagen AB, Lund, Sweden). The oligonucleotides were annealed and then ligated onto the 3′ end of a CTB gene. DNA sequencing of the final plasmid, pML-CTB::OVA, confirmed the final sequence of the gene fusion.
(ii)CTB56-63HAp.
Synthetic oligonucleotides encoding the 12-amino-acid HA peptide were synthesized (Innovagen AB). The oligonucleotides were inserted into plasmid pCB56-63gp12 (3) between positions 55 and 64 of mature CTB. DNA sequencing was used to confirm the sequence of the insert in pCB56-64HA.
(iii) Expression and purification of CTB fusion proteins.
Recombinant proteins carrying peptides inserted into CTB are more stable than those carrying peptides linked to the N or C terminus (4). Thus, whereas CTB56-63HAp could be expressed in V. cholerae, CTB::OVAp was expressed in E. coli to avoid cleavage by extracellular V. cholerae proteases known to readily destroy C-terminal peptide extensions on CTB (38).
pCB56-63HAp was transferred into V. cholerae strain JS1569 by electroporation (23). The protein was precipitated from the growth medium using hexametaphosphate (23) and was then redissolved in a minimal volume of 0.2 M Tris-HCl (pH 8.0) and dialyzed against phosphate-buffered saline (PBS), pH 7.2.
pML-CTB::OVA was transferred into E. coli BL21 (16). The CTB gene used lacked the signal peptide directing transport of the synthesized protein into the periplasmic space. This resulted in the cytoplasmic accumulation of the product (CTB::OVAp) as monomers, which formed insoluble inclusion bodies. These were dissolved in 6.5 M urea and reassembled by dialysis (26).
The CTB::OVAp and CTB56-64HAp fusion proteins were further purified by ion exchange (Resource Q column; Pharmacia Biotech) and fast protein liquid chromatography (FPLC) gel filtration (Superdex 200 16/60 column; Pharmacia Biotech) using the Biologic Workstation FPLC system (Bio-Rad, Richmond, Calif.).
By means of GM1 ELISA using biotinylated anti-CTB monoclonal antibodies, the CTB fusion proteins were shown to have retained GM1-binding activity.
Generation of APC.
For the generation of bone marrow-derived DC and Mφ male BALB/c mice (B&K Universal AB, Stockholm, Sweden) were killed and bone marrow was flushed from the femur and tibia and depleted of erythrocytes with ammonium chloride.
DC were generated from bone marrow precursors as described previously (19). On day 6, nonadherent cells were collected and further purified on metrizamide (Sigma) by density centrifugation at 800 × g.
To generate Mφ (52), plastic adherent cells were removed by incubation in a 20-ml flask overnight at 37°C, and the remaining cells were cultured at 5 × 105/ml in 75-cm2 flasks (20 ml/flask) in complete medium containing 20 ng of colony-stimulating factor 1 (Sigma)/ml. Adherent Mφ were retrieved on day 7 by a 5-min incubation at 37°C with 5 ml of PBS containing 2.5 U of dispase I (Boehringer Mannheim). B cells were purified from adherent-cell-depleted spleen cell suspensions by use of B220-specific magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) generating >90% pure B220-positive cells.
Purification of HA-specific TCR-transgenic T cells.
T cells were purified from peripheral lymph nodes or spleens of naive BALB/c mice expressing a transgenic α/β TCR specific for peptide 111–119 of influenza virus HA in the context of I-Ed (20) (a kind gift from H. von Boehmer, Harvard, Cambridge, Mass.) and of BALB/c mice expressing a transgenic α/β TCR specific for peptide 323–339 of OVA (33) (a kind gift from Nils Lycke, University of Göteborg) using T-cell purification columns (R & D) followed by panning on petri dishes coated with anti-IAb,d monoclonal antibodies (5 μg/ml; PharMingen, San Diego, Calif.). More than 98% of the resulting T-cell population was CD3+, of which approximately 10% expressed the transgenic TCR in HA-TCR animals and 50 to 70% expressed the transgenic TCR in OVA-TCR animals.
FACS analysis.
APC were analyzed either (i) immediately following isolation or in vitro generation or (ii) 24 h after 106 antigen-pulsed APC had been incubated either alone or together with 106 antigen-specific TCR-transgenic T cells in flat-bottom 24-well plates (Nunc). Cells were analyzed by fluorescence-activated cell sorter (FACS) using the following antibodies from PharMingen: fluorescein isothiocyanate (FITC)-conjugated anti-IAd/IEd clone 39-10-8, FITC–anti-mouse CD40 clone HM40-3, phycoerythrin (PE)-conjugated anti-mouse CD80 clone 16-10A1, PE–anti-mouse CD86 clone GL1, PE–anti-mouse B220 clone RA3-6B2, and peridinin chlorophyll protein–anti-mouse CD3ε clone 145-2C11. TCR-transgenic T cells were analyzed using PE–anti-mouse CD3ε clone 145-2C11 from PharMingen and an FITC-labeled rat clonotypic monoclonal antibody, 6.5, recognizing the HA-specific transgenic TCR (54) or an FITC-labeled KJ1-26 monoclonal antibody recognizing OVA-specific transgenic TCR (28).
Proliferation tests.
APC were irradiated at 900 rads and then incubated with graded amounts of antigen for 90 min at 37°C, extensively washed, and plated in triplicate at 104 or 105 cells/well in flat-bottom 96-well plates (Nunc) together with 105 HA-specific or OVA-specific transgenic T cells in complete medium. Plates were incubated for 2 to 3 days at 37°C. Culture supernatants were collected at 48 h and frozen at −70°C until assayed for cytokine content. One microcurie of [6-3H]thymidine (Amersham, Little Chalfont, Buckinghamshire, United Kingdom) was added to each well 8 h before harvesting, and incorporated radioactivity was measured. Results are expressed either as antigen-specific [3H]thymidine incorporation (in counts per minute) or as stimulation indexes (SI), defined as the ratio between the amounts of [3H]thymidine incorporated into T cells incubated with antigen-treated APC and [3H]thymidine incorporated into T cells incubated with mock-treated APC.
Cytokine measurements.
Culture supernatants were analyzed for IL-1β and IFN-γ content using Duoset ELISAs for mouse IL-1β and IFN-γ from R & D according to instructions. IL-12 and IL-18 were similarly measured using OptEIA mouse IL-12 (p40), IL-12 (p70), and IL-18 sets from PharMingen. IL-4 and IL-10 were measured either by ELISA or by a more sensitive modified cell ELISA method using specific antibody pairs from PharMingen (6). The sensitivity of both assays was 30 pg/ml.
RESULTS
Coupling of antigen to CTB enhances antigen presentation in vitro.
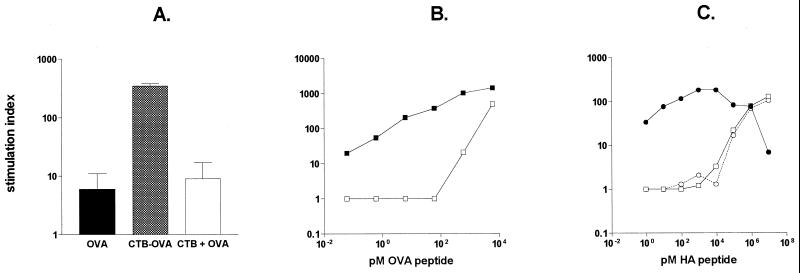
To examine the effect of CTB as a carrier protein on antigen presentation by different APC, the antigens were coupled to CTB, either chemically or genetically, and incubated with APC. Using whole spleen cell suspensions as APC, we found that conjugation of proteins or peptides to CTB greatly reduced the antigen concentration required for effective presentation to cognate TCR-transgenic T cells (Fig. 2). When APC were preincubated with a fixed concentration of OVA or CTB-OVA, corresponding to 10−8 M OVA, prior to presentation to OVA-specific TCR-transgenic T cells, the CTB-OVA conjugate gave rise to a proliferative response 80-fold higher than that obtained with free OVA (Fig. 2A). Similarly, the CTB fusion protein carrying OVA peptide (CTB::OVAp) was effective at a concentration >104-fold lower than the concentration of free OVA peptide required (Fig. 2B). Similar results were obtained when an HA peptide genetically fused to CTB was compared to a free HA peptide, using HA-specific TCR-transgenic T cells as the readout system (Fig. 2C). The proliferative responses obtained were antigen specific, as T cells from wild-type BALB/c animals did not proliferate in response to either HA or OVA peptide or in response to the corresponding CTB derivatives (data not shown).
FIG. 2.
Conjugation of antigen to CTB enhances antigen presentation in vitro. Spleen cells were incubated with antigen in free form or conjugated to CTB together with purified antigen-specific TCR-transgenic T cells. Data are expressed as the proliferative responses obtained in response to free or CTB-conjugated whole OVA (A), OVA peptide (B), or HA peptide (C). (A) APC and OVA-specific TCR-transgenic T cells were incubated with 10−8 M OVA or OVA-CTB or 10−8 M OVA plus 6 × 10−9 M CTB. (B) APC and OVA-specific TCR-transgenic T cells were incubated with graded amounts of OVA peptide (□) or with CTB-conjugated OVA peptide (■). (C) APC and HA-specific TCR-transgenic T cells were incubated with graded amounts of free HA peptide (□), CTB-conjugated HA peptide (●), or the equivalent amounts of unconjugated HA peptide and CTB (○).
A physical interaction between the antigen and the CTB molecule was required in order for the beneficial effect on antigen presentation to occur, since coadministration of free CTB and free antigen did not result in any enhancement of the T-cell-proliferative responses compared to those obtained with antigen alone (Fig. 2A and C).
Conjugation of antigen to CTB enhances antigen presentation by DC, B cells, and Mφ.
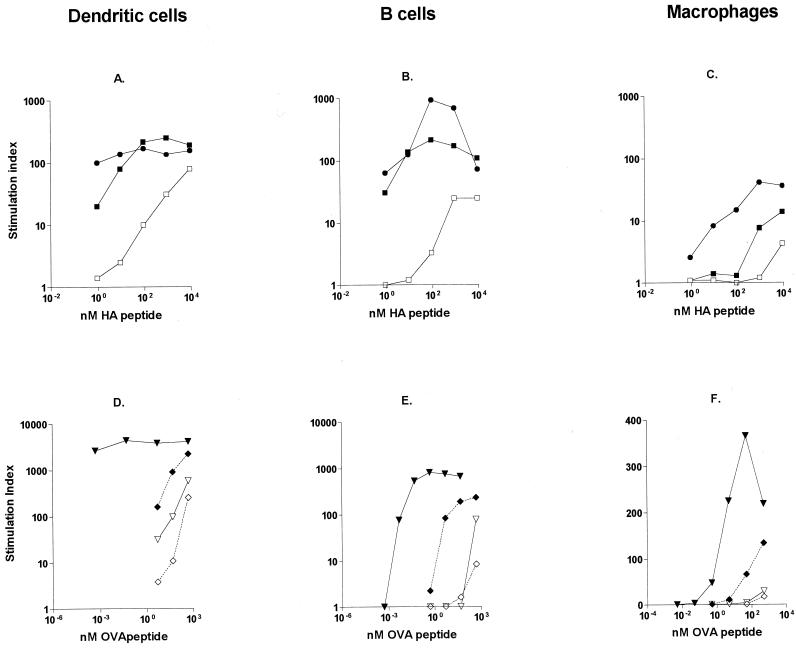
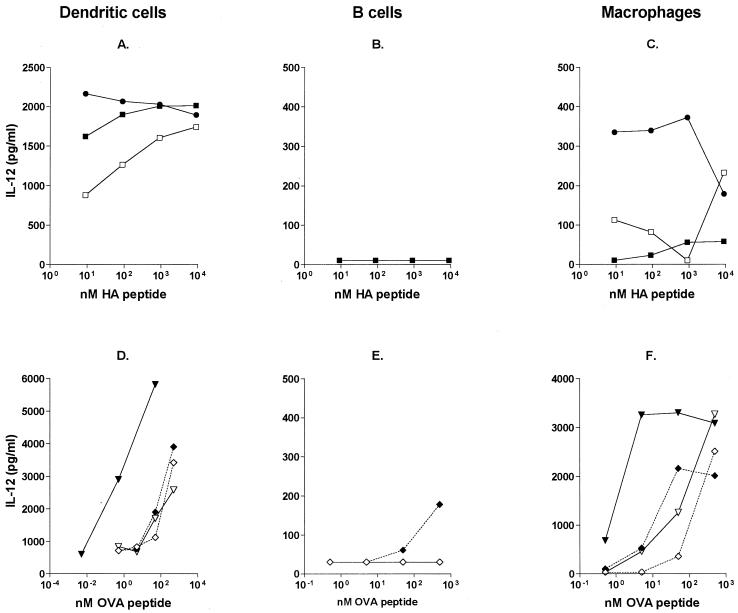
In this study, we used three different types of APC: those generated from bone marrow precursors grown in the presence of specific cytokines to differentiate into either DC or Mφ and B cells purified from the spleens of naive BALB/c animals. The different types of APC were first incubated for 90 min with the HA or OVA peptide, OVA, or their CTB fusion protein derivatives and then extensively washed and added at a 1:10 cell ratio to cultures of purified T cells from HA- or OVA-specific TCR-transgenic animals. Under these conditions, DC and B cells, which constitutively express MHC II, were efficient APC for free peptide or protein antigens, requiring approximately 10 and 100 nM free antigen, respectively, to induce a detectable in vitro T-cell-proliferative response (Fig. 3A, B, D, and E). I contrast, Mφ treated with free peptide or protein antigens were not able to induce any significant proliferation by the HA- or OVA-specific TCR-transgenic T cells (Fig. 3C and F). These results are in accordance with FACS data showing significant MHC II expression on DC and B cells but not on Mφ (data not shown).
FIG. 3.
Conjugation of antigen to CTB enables the use of Mφ as APC. DC, B cells, and Mφ were pulsed with free antigen or with CTB-coupled antigen and incubated with antigen-specific TCR-transgenic T cells. (A through C) Data are expressed as the SI obtained with different concentrations of HA peptide (□), chemically conjugated CTB-HA peptide (■), and genetically coupled CTH-HA peptide (●) using either DC (A), B cells (B), or Mφ (C) as APC. (D through F) Data are expressed as the SI obtained with different concentrations of OVA (◊), CTB-OVA (⧫), OVA peptide (▿), and CTB-OVA peptide (▾) using either DC (D), B cells (E), or Mφ (F) as APC. Data represent one of two experiments using 105 purified T cells with 104 APC.
Conjugation of HA or OVA peptide to CTB enhanced the proliferative response of the HA- or OVA-specific TCR-transgenic T cells 20- to 100-fold, depending on the dose of the antigen and the type of APC. The proliferative responses to CTB-linked antigens were strongest when DC or B cells were used as APC (Fig. 3A, B, D, and E). However, conjugation of antigen to CTB also induced strong T-cell proliferation when Mφ were used as APC (Fig. 3C and F). Both genetic and chemical CTB-peptide conjugates could be utilized for this purpose (Fig. 3A to C), and CTB conjugation was shown to be an efficient means of enhancing the subsequent proliferative response to both peptide (OVA peptide) and protein (OVA) antigens (Fig. 3D to F).
The enhanced antigen presentation obtained with CTB-conjugated antigen could be blocked if anti-CTB immune serum from mice (used at 1/100) or GM1 (10 μM) was included during the preincubation step (data not shown), showing that the enhanced antigen presentation was dependent on CTB-mediated binding to GM1 receptors on APC.
CTB-conjugated antigens induce enhanced IFN-γ and IL-12 secretion in vitro.
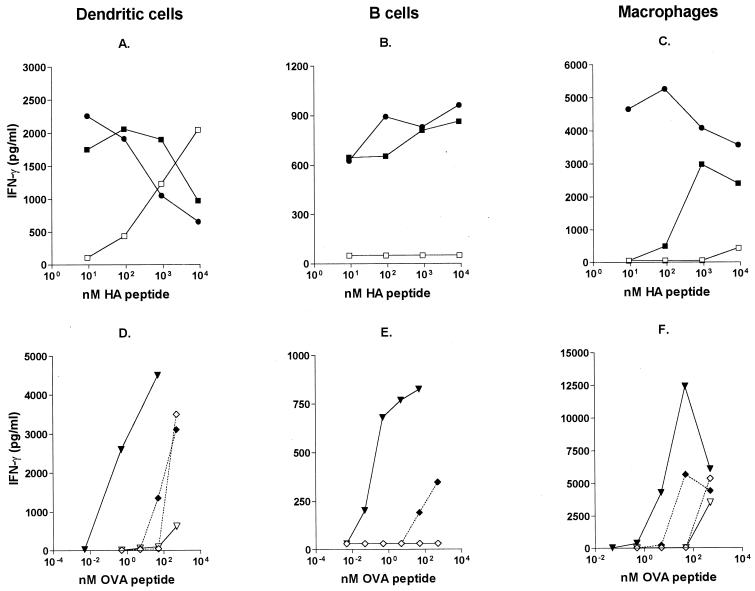
We compared the ability of different APC to induce IFN-γ production when pulsed with free or CTB-coupled antigens. IFN-γ secretion was measured in 48-h supernatants from cultures of OVA-specific or HA-specific TCR-transgenic T cells incubated with APC that had been pretreated with different concentrations of OVA, OVA peptide, or HA peptide or with CTB derivatives thereof.
Firstly, we compared the effect of HA peptide with that of CTB-coupled HA peptide on IFN-γ production when DC, B cells, or Mφ were used as APC. HA peptide alone induced low levels of IFN-γ unless given at very high doses, and it never induced IFN-γ secretion when B cells were used as APC (Fig. 4A to C). When HA peptide had been chemically or genetically coupled to CTB, the levels of secreted IFN-γ were considerably higher, and secretion occurred at >1,000-fold lower concentrations. Furthermore, CTB-coupled HA peptide also induced measurable IFN-γ responses when B cells were utilized as the source of APC (Fig. 4B).
FIG. 4.
CTB-conjugated antigens induce enhanced IFN-γ secretion in vitro. (A through C) IFN-γ production by HA-specific TCR-transgenic T cells following incubation with HA peptide or HA-CTB-treated APC. Data are expressed as the concentrations of IFN-γ in 48-h culture supernatants obtained with different concentrations of HA peptide (□), chemical CTB-HA peptide (■), and genetic CTB-HA peptide (●) using DC (A), B cells (B), or Mφ (C) as APC. (D through F) IFN-γ production by OVA-specific TCR-transgenic T cells following incubation with antigen-pulsed DC (D), B cells (E), or Mφ (F). Data are expressed as the concentrations of IFN-γ in 48-h culture supernatants using different concentrations of OVA (◊), CTB-OVA (⧫), OVA peptide (▿), and CTB-OVA peptide (▾). Data represent one of two experiments using 105 purified T cells with 105 APC.
Secondly, we compared genetic and chemical CTB-HA constructs. In general, the difference in ability to induce IFN-γ responses between the genetic and chemical CTB-HA constructs was small (Fig. 4A to C). However, when Mφ were used as APC, the genetic construct was superior to the chemical conjugate (Fig. 4C). When a lower dose of APC was used (104 APC/well), the level of IFN-γ produced was generally lower, although the pattern of secretion was the same as that with the higher APC dose.
Thirdly, we compared the effect of CTB conjugation of a peptide (OVA peptide) and a protein (OVA) antigen. We found that similar concentrations of free OVA and OVA peptide were required to induce IFN-γ responses by DC but that OVA consistently induced higher levels of IFN-γ than did the peptide (Fig. 4D). Neither OVA nor the OVA peptide could induce any measurable IFN-γ responses when B cells were used as APC (Fig. 4E). Similarly to the CTB56-63HA fusion protein, CTB::OVA hybrid protein was far superior to free OVA peptide at inducing IFN-γ responses, with respect to both the dose of antigen required and the magnitude of the IFN-γ response obtained. When CTB was chemically conjugated to native OVA, IFN-γ responses did occur at a lower OVA concentration, but the differences were not as prominent as those seen with peptide antigens (Fig. 4D to F).
We also analyzed these cultures for the presence of Th2 cytokines, e.g., IL-4 and IL-10, but there were no measurable levels of any of these cytokines irrespective of the source of APC or the nature of the antigen (data not shown).
IL-12 is an important factor in inducing IFN-γ responses (35). We therefore measured the levels of IL-12 in the culture supernatants analyzed above for IFN-γ.
Nonconjugated antigen induced substantial levels of IL-12p40 when DC were used as APC. Thus, HA peptide, OVA peptide, and OVA at ≥10 nM gave rise to strong IL-12p40 responses, which could be induced at even lower antigen doses when the antigen was coupled to CTB (Fig. 5A and D). IL-12p70 could also be detected in these cultures. The pattern of IL-12p70 secretion was similar to that of IL-12p40, but the levels detected were considerably lower (data not shown). Antigen-pulsed Mφ secreted low but measurable levels of IL-12p40, and these levels increased when CTB-conjugated antigens were used (Fig. 5C and F). Irrespective of the antigen formulation or the levels of IFN-γ detected, there were only negligible levels of IL-12 in cultures containing B cells as APC (Fig. 5B and E). Furthermore, both Mφ and DC secreted low levels of IL-12 after exposure to CTB-conjugated antigens in the absence of specific T cells (data not shown).
FIG. 5.
CTB-conjugated antigens induce enhanced IL-12p40 secretion in vitro. Shown is IL-12p40 production by antigen-specific TCR-transgenic T cells following incubation with antigen-pulsed APC. (A through C) Data are expressed as concentrations of IL-12p40 in 48-h culture supernatants obtained with different concentrations of HA peptide (□), chemical CTB-HA peptide (■), and genetic CTB55-63HAp (●) using DC (A), B cells (B), or Mφ (C) as APC. (D through F) IL-12p40 production by OVA-specific TCR-transgenic T cells following incubation with antigen-pulsed DC (D), B cells (E), or Mφ (F). Data are expressed as concentrations of IL-12p40 in 48-h culture supernatants obtained with different concentrations of OVA (◊), CTB-OVA (⧫), OVA peptide (▿), and CTB::OVAp (▾). Data represent one of two experiments using 105 purified T cells with 105 APC.
The CTB56-63HA fusion protein and the chemical conjugate between CTB and HA peptide induced similar levels of IL-12p40 from DC, whereas the genetic constructs were superior at inducing IL-12p40 production in Mφ (Fig. 5A and C).
As with IFN-γ production, lower doses of APC (104/well) gave a similar pattern of IL-12p40 secretion, but 4 to 10 times lower than that obtained with 105 APC (data not shown). Furthermore, all IL-12p40 responses were dependent on the presence of T cells, as no IL-12p40 could be detected in pure APC cultures (data not shown).
IL-1β and IL-18 are also implicated in the induction of IFN-γ responses (9, 21). We therefore analyzed the cultures for the presence of IL-1β and IL-18, but there were no measurable levels of either of these cytokines irrespective of the source of APC or the nature of the antigen (data not shown).
CTB as a carrier molecule promotes APC maturation in vitro.
The levels of MHC and costimulatory molecules on the surfaces of the APC influence not only the induction of a T-cell response but also the magnitude and the cytokine pattern of that response (30, 50). To determine whether CTB-conjugated antigen influenced the phenotype of different APC populations, antigen-pulsed or untreated DC or Mφ were incubated for 24 h in the presence or absence of antigen-specific TCR-transgenic T cells and then analyzed for levels of MHC II, CD40, CD80, and CD86 by FACS. Contaminating T cells were gated away using a PerCP-labeled anti-CD3 antibody. When DC had been incubated in the absence of any antigen or any specific T cells, they expressed high levels of MHC II, CD40, CD80, and CD86, whereas Mφ incubated in the same way had low expression of MHC II and CD40 and high levels of CD80 and CD86 (data not shown).
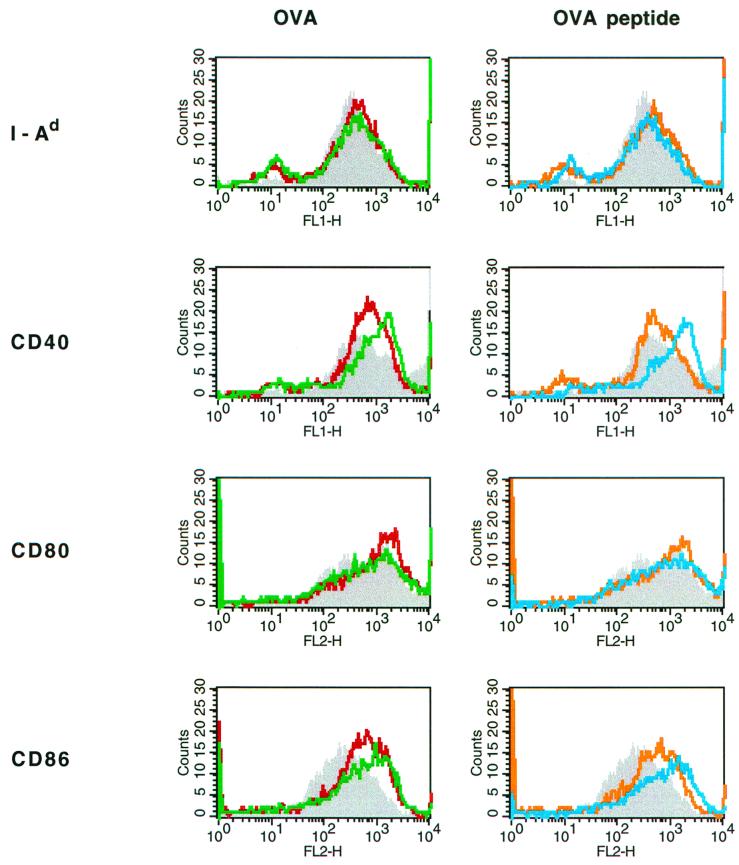
Incubation of antigen-pulsed APC together with OVA-specific TCR-transgenic T cells induced a phenotypic change in the APC, as shown for Mφ in Fig. 6. Mφ pulsed with free OVA (Fig. 6, left) or OVA peptide (Fig. 6, right) had substantially enhanced surface expression of both CD40 and CD86 compared to unpulsed Mφ (Fig. 6). The levels of MHC II remained unaltered, while a weak up-regulation of CD80 was observed. When APC had been pretreated with CTB-coupled OVA or OVA peptide, the cell surface densities of both CD40 and CD86 were further enhanced (Fig. 6A and B). Interestingly, APC that had been pretreated with CTB-OVA or CTB-OVA peptide up-regulated CD40 and CD86 on their cell surfaces in the absence of specific T cells as well (data not shown), although to a much lesser extent than when T cells were present. Thus, CTB-coupled antigen per se can induce maturation of APC.
FIG. 6.
Mφ incubated with CTB-coupled antigens up-regulate their cell surface expression of CD40 and CD86 in vitro. Mφ were pulsed with OVA, CTB-OVA, OVA peptide, or CTB-coupled OVA peptide, or were left untreated, and then incubated for 24 h with OVA-specific TCR-transgenic T cells. The Mφ cell surface levels of MHC II, CD40, CD80, and CD86 were then evaluated by FACS analysis. Data are presented as fluorescent intensities and were recorded for unpulsed (shaded), OVA-pulsed (red line), and CTB-OVA-pulsed (green line) Mφ (A) and for unpulsed (shaded), OVA peptide-pulsed (orange line), and CTB-OVA peptide-pulsed (blue line) Mφ.
DISCUSSION
In this study we show that conjugation of an antigen to CTB greatly enhances the T-cell-activating capacity of different types of pulsed APC incubated together with naive antigen-specific TCR-transgenic T cells. The enhanced antigen presentation of CTB-conjugated antigens was shown to be dependent on retained binding activity of the CTB-antigen complex to GM1 receptors on the APC, and the resulting T-cell response was shown to be antigen specific. In addition to enhancing antigen presentation by DC and B cells, which express high levels of MHC class II, the conjugation of antigen to CTB also allowed Mφ, which express low levels of MHC class II and are normally unable to activate naive T cells, to present antigen efficiently. Enhanced antigen presentation was associated with increased expression of IL-12 and IFN-γ, as well as with increased expression of CD40 and CD86 on the APC.
That coupling of antigen to CTB promotes antigen presentation by increased uptake of the coupled antigen through binding to the GM1 receptor on the APC was deduced from experiments showing that both anti-CTB antiserum and free GM1 ganglioside could block the CTB-mediated antigen presentation. CTB has a high affinity for its receptor, the GM1 ganglioside (KA, ∼109 mol−1) (18). The binding to GM1 leads to cellular internalization of CTB into vesicles (25, 36). Binding to GM1 has previously been shown to be essential for the immunogenicity of CTB (34), and it has been suggested that GM1 binding represents a danger signal per se (36). We could document a phenotypic activation of the APC following incubation with CTB-conjugated antigen, as evidenced by an up-regulated expression of both CD40 and CD86, as well as a pronounced secretion of IL-12. Thus, we propose that GM1 binding greatly improves the uptake of the CTB-conjugated antigen by APC, leading to a more abundant presentation of the corresponding peptides on MHC II, which, together with the enhanced levels of costimulatory molecules such as CD40 and CD86 induced on the APC surface in association with an enhanced IL-12 secretion by the APC, augments the T-cell-activating potential of the APC. It has previously been shown that mannosylation of peptides promotes mannose-receptor-mediated antigen absorption by DC, thus reducing 200- to 1,000-fold the threshold concentration of peptide required (48). We show here that CTB conjugation of antigen represents another potent mechanism for receptor-mediated uptake of antigens by APC and that this effect is universal, as all APC express GM1.
The increased proliferation of transgenic cells observed with CTB-coupled antigens is compatible with the notion that feeding of CTB-conjugated antigens can induce either a mucosal antibody response or systemic tolerance in vivo, or both. The inductive phase of oral tolerance as well as that of the mucosal immune response is preceded by antigen-specific T-cell activation in vivo, proliferation in the regional draining lymph nodes, and differentiation into a memory-like state (41). This indicates that antigen-directed differentiation occurs as part of T-cell tolerance.
DC are important inducers of both T-cell immunity and T-cell tolerance (5, 51). The enhanced presentation of CTB-conjugated antigens by DC in vitro is therefore in line with both the enhanced antibody responses and the induction of peripheral T-cell tolerance in vivo, which have been observed when different CTB-coupled antigens are administered mucosally. To what extent B cells contribute to T-cell priming, leading to antibody responses and/or to peripheral tolerance, is not clear. B cells are not required for immune priming in vivo (12) and turn off rather than activate naive T cells (15). At the same time, some mucosal adjuvants can promote strong antibody responses by simultaneously activating and targeting antigen to B cells (1).
Interestingly, the use of CTB-conjugated antigens stimulated Mφ to become potent activators of naive T cells despite their documented low expression of MHC molecules. The cytokine responses obtained with Mφ were indeed comparable to those obtained with DC and B cells, even though the proliferative responses induced were considerably lower. The enhanced antigen-presenting capacity was associated with a strong up-regulation of CD40 and CD86 on the Mφ surface, together with enhanced levels of secreted IL-12. We can only speculate about the in vivo relevance of this finding. CTB has been used extensively as a carrier molecule for the induction of antibody responses and for the generation of systemic T-cell tolerance by administration of CTB-antigen conjugates at mucosal surfaces (7, 8, 11, 31, 42, 44, 49) where Mφ are abundant. Mφ can be found in both organized mucosal lymphoid tissues such as lymph nodes and in diffuse mucosal lymphoid tissues such as lamina propria. It is therefore plausible that Mφ are involved in CTB-mediated activation of T cells present in the mucosal tissues. Whether antigens presented by Mφ in vivo would prime for mucosal antibody responses and/or for systemic T-cell tolerance remains an open question.
The beneficial effect of CTB conjugation was more pronounced when peptides rather than whole proteins were used as antigens. One should bear in mind that the readout system was monoclonal, as the TCR-transgenic T cells are restricted in their specificity to one single epitope (20, 33), whereas several different epitopes are available with whole OVA. Furthermore, the size of the CTB-antigen complex might impact on the uptake, transport, and processing of the antigen. Thus, on a weight basis, CTB-coupled whole OVA protein is approximately 2.5 times larger than the CTB-OVA peptide conjugate. We further observed that the genetically engineered CTB56–63HA fusion protein was superior to the chemical conjugate between CTB and HA peptide in promoting HA-specific T-cell activation. This might be due to the homogeneity and better stability of the genetic construct, structural considerations such as the positioning of the peptide antigen in the CTB molecule, and the numbers of HA peptides present on each CTB molecule.
The in vitro responses to CTB-coupled HA or OVA were strongly IFN-γ dominated despite the documented Th2/Th3 profile that is observed in vivo following mucosal delivery of CTB-conjugated antigens (45). However, IFN-γ is induced locally in the gut following oral antigen feeding (22, 24) and has been shown to be an important component in the development of oral tolerance (22, 24). Thus, the enhanced IFN-γ responses observed with CTB-conjugated antigens are in line with in vivo data. However, the development of systemic T-cell tolerance and of mucosal IgA responses depends on factors present in the local mucosal milieu (56), e.g., transforming growth factor β, and these factors are most likely not present in our in vitro system. We also found that the IFN-γ responses could develop independently of IL-12p40, which was particularly evident when large numbers of B cells were used as APC. This observation is in line with in vitro data showing that anti-IL-12 antibodies fail to block the priming for IFN-γ if IL-2 is present (39). The induction of IL-12 was dependent on T cells, most likely through CD40 binding, as evidenced by the fact that little IL-12 was induced in 48-h DC cultures in the absence of T cells, irrespective of the APC type and antigen formulation.
It is well established that CTB is a highly efficient carrier molecule for the induction of mucosal antibody responses (8, 11, 31) as well as for the induction of mucosally induced systemic T-cell (42) and systemic B-cell (37, 47, 55) tolerance. The latter observations have led to the development of antigen-specific tolerogenic strategies to prevent and/or to treat T-cell-mediated autoimmune (7, 44, 49), IgE-mediated allergic (37, 47, 55), and infection-induced pathological inflammatory conditions (43) by administration of CTB-conjugated antigens through a mucosal surface. In this study, we show that CTB promotes presentation of coupled antigens not only by DC and B cells but also by Mφ, which are normally poor activators of naive T cells due to their low levels of surface MHC class II. We suggest that this enhanced antigen presentation represents an important mechanism contributing to the efficacy of CTB as a carrier molecule in vivo.
ACKNOWLEDGMENTS
A. George-Chandy and K. Eriksson contributed equally to this work.
We thank Marianne Lindblad and Gun Wallerström for help with CTB preparation, conjugation, purification, and characterization and Margareta Fredriksson for excellent technical assistance.
These studies were supported by the Swedish Medical Research Council, SIDA/SAREC's Special Program for AIDS and related diseases, The Swedish Strategic Foundation program in Infection and Vaccinology, the Swedish Society for Medical Research, and the Swedish Technical Research Council.
REFERENCES
- 1.Ågren L C, Ekman L, Löwenadler B, Lycke N Y. A genetically engineered nontoxic vaccine adjuvant that combines B cell targeting with immunomodulation by cholera toxin A1 subunit. J Immunol. 1997;158:3936–3946. [PubMed] [Google Scholar]
- 2.Arakawa T, Yu J, Chong D K, Hough J, Engen P C, Langridge W H. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat Biotechnol. 1998;16:934–938. doi: 10.1038/nbt1098-934. [DOI] [PubMed] [Google Scholar]
- 3.Bäckström M, Holmgren J, Schödel F, Lebens M. Characterization of an internal permissive site in the cholera toxin B-subunit and insertion of epitopes from human immunodeficiency virus-1, hepatitis B virus and enterotoxigenic Escherichia coli. Gene. 1995;165:163–171. doi: 10.1016/0378-1119(95)00444-b. [DOI] [PubMed] [Google Scholar]
- 4.Bäckström M, Lebens M, Schödel F, Holmgren J. Insertion of a HIV-1-neutralizing epitope in surface-exposed internal region of the cholera toxin B-subunit. Gene. 1994;149:211–217. doi: 10.1016/0378-1119(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Beech J T, Bainbridge T, Thompson S J. Incorporation of cells into an ELISA system enhances antigen-driven lymphokine detection. J Immunol Methods. 1997;205:163–168. doi: 10.1016/s0022-1759(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 7.Bergerot I, Ploix C, Petersen J, Moulin V, Rask C, Fabien N, Lindblad M, Mayer A, Czerkinsky C, Holmgren J, Thivolet C. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc Natl Acad Sci USA. 1997;94:4610–4614. doi: 10.1073/pnas.94.9.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergquist C, Lagergård T, Lindblad M, Holmgren J. Local and systemic antibody responses to dextran-cholera toxin B subunit conjugates. Infect Immun. 1995;63:2021–2025. doi: 10.1128/iai.63.5.2021-2025.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromander A K, Holmgren J, Lycke N. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J Immunol. 1991;146:2908–2914. [PubMed] [Google Scholar]
- 10.Czerkinsky C, Russell M W, Lycke N, Lindblad M, Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989;57:1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czerkinsky C, Svennerholm A M, Quiding M, Jonsson R, Holmgren J. Antibody-producing cells in peripheral blood and salivary glands after oral cholera vaccination of humans. Infect Immun. 1991;59:996–1001. doi: 10.1128/iai.59.3.996-1001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein B M M, Di Rosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Förster I, Lieberam I. Peripheral tolerance of CD4 T-cells following local activation in adolescent mice. Eur J Immunol. 1996;26:3194–3202. doi: 10.1002/eji.1830261253. [DOI] [PubMed] [Google Scholar]
- 14.Francis M L, Ryan J, Jobling M G, Holmes R K, Moss J, Mond J J. Cyclic AMP-independent effects of cholera toxin on B cell activation. II. Binding of ganglioside GM1 induces B cell activation. J Immunol. 1992;148:1999–2005. [PubMed] [Google Scholar]
- 15.Fuchs E J, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 16.Grodberg J, Dunn J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988;170:1245–1253. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmgren J, Czerkinsky C, Sun J-B, Svennerholm A-M. Oral vaccination, mucosal immunity and oral tolerance with special reference to cholera toxin. In: Kaufmann S H E, editor. Concepts in vaccine development. Berlin, Germany: Walter de Gruyter; 1996. pp. 437–458. [Google Scholar]
- 18.Holmgren J, Lindholm L, Lönnroth I. Interaction of cholera toxin and toxin derivatives with lymphocytes. J Exp Med. 1974;139:801–819. doi: 10.1084/jem.139.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinmann R M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okomoto I, Usui M, Ikeda M, Kurimoto M. IFN-γ-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- 22.Kweon M-N, Fujihashi K, VanCott J L, Higuchi K, Yamamoto M, McGhee J R, Kiyono H. Lack of orally induced systemic unresponsiveness in IFN-γ knockout mice. J Immunol. 1998;160:1687–1693. [PubMed] [Google Scholar]
- 23.Lebens M, Johansson S, Osek J, Lindblad M, Holmgren J. Large-scale production of Vibrio cholerae toxin B subunit for use in oral vaccines. Bio/Technology. 1993;11:1574–1578. doi: 10.1038/nbt1293-1574. [DOI] [PubMed] [Google Scholar]
- 24.Lee L O, Miller S D, Hurst S D, Tan L J, Cooper C J, Barrett T A. Interferon gamma induction during oral tolerance reduces T-cell migration to sites of inflammation. Gastroenterology. 2000;119:129–138. doi: 10.1053/gast.2000.8542. [DOI] [PubMed] [Google Scholar]
- 25.Lencer W I, Hirst T R, Holmes R K. Membrane traffic and the cellular uptake of cholera toxin. Biochim Biophys Acta. 1999;1450:177–190. doi: 10.1016/s0167-4889(99)00070-1. [DOI] [PubMed] [Google Scholar]
- 26.L'Hoir C, Renard A, Martial J A. Expression in Escherichia coli of two mutated genes encoding the cholera toxin B subunit. Gene. 1990;89:47–52. doi: 10.1016/0378-1119(90)90204-5. [DOI] [PubMed] [Google Scholar]
- 27.Lipscombe M, Charles I G, Roberts M, Dougan G, Tite J, Fairweather N F. Intranasal immunization using the B subunit of the Escherichia coli heat-labile toxin fused to an epitope of the Bordetella pertussis P.69 antigen. Mol Microbiol. 1991;5:1385–1392. doi: 10.1111/j.1365-2958.1991.tb00785.x. [DOI] [PubMed] [Google Scholar]
- 28.Marrack P, Shimonkevitz R, Hannum C, Haskins K, Kappler J. The major histocompatibility complex-restricted antigen receptor on T cells. IV. An antiidiotypic antibody predicts both antigen and I-specificity. J Exp Med. 1983;158:1635–1646. doi: 10.1084/jem.158.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matousek M P, Nedrud J G, Cieplak W J, Harding C V. Inhibition of class II major histocompatibility complex antigen processing by Escherichia coli heat-labile enterotoxin requires an enzymatically active A subunit. Infect Immun. 1998;66:3480–3484. doi: 10.1128/iai.66.7.3480-3484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAdam A J, Schweitzer A N, Sharpe A H. The role of B7 costimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol Rev. 1998;165:231–247. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 31.McKenzie S J, Halsey J F. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J Immunol. 1984;133:1818–1824. [PubMed] [Google Scholar]
- 32.McSorley S J, Rask C, Pichot R, Julia V, Czerkinsky C, Glaichenhaus N. Selective tolerization of Th1-like cells after nasal administration of a cholera toxoid-LACK conjugate. Eur J Immunol. 1998;28:424–432. doi: 10.1002/(SICI)1521-4141(199802)28:02<424::AID-IMMU424>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 33.Murphy K M, Heimberger A B, Loh D Y. Induction by antigen of intrathymic apoptosis of CD4+ CD8+ TCR1o thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 34.Nashar T O, Webb H M, Eaglestone S, Williams N A, Hirst T R. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc Natl Acad Sci USA. 1996;93:226–230. doi: 10.1073/pnas.93.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 36.Rappuoli R, Pizza M, Douce G, Dougan G. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol Today. 1999;20:493–500. doi: 10.1016/s0167-5699(99)01523-6. [DOI] [PubMed] [Google Scholar]
- 37.Rask C, Holmgren J, Fredriksson M, Lindblad M, Nordström I, Sun J-B, Czerkinsky C. Prolonged oral treatment with low doses of allergen conjugated to cholera toxin B subunit suppresses immunoglobulin E antibody responses in sensitized mice. Clin Exp Allergy. 2000;30:1024–1032. doi: 10.1046/j.1365-2222.2000.00849.x. [DOI] [PubMed] [Google Scholar]
- 38.Schödel F, Will H, Johansson S, Sanchez J, Holmgren J. Synthesis in Vibrio cholerae and secretion of hepatitis B virus antigen fused to Escherichia coli heat-labile enterotoxin subunit B. Gene. 1991;99:255–259. doi: 10.1016/0378-1119(91)90135-x. [DOI] [PubMed] [Google Scholar]
- 39.Sedar R A, Gazzinelli R, Sher A, Paul W E. Interleukin l2 acts directly on CD4+ T cells to enhance priming for interferon γ production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobel D O, Yankelevich B, Goyal D, Nelson D, Mazumder A. The B-subunit of cholera toxin induces immunoregulatory cells and prevents diabetes in the NOD mouse. Diabetes. 1998;47:186–191. doi: 10.2337/diab.47.2.186. [DOI] [PubMed] [Google Scholar]
- 41.Sun J, Dirden-Kramer B, Ito K, Ernst P B, van Houten N. Antigen-specific T cell activation and proliferation during oral tolerance induction. J Immunol. 1999;162:5868–5875. [PubMed] [Google Scholar]
- 42.Sun J B, Holmgren J, Czerkinsky C. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc Natl Acad Sci USA. 1994;91:10795–10799. doi: 10.1073/pnas.91.23.10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun J B, Mielcarek N, Lakew M, Grzych J M, Capron A, Holmgren J, Czerkinsky C. Intranasal administration of a Schistosoma mansoni glutathione S-transferase–cholera toxoid conjugate vaccine evokes antiparasitic and antipathological immunity in mice. J Immunol. 1999;163:1045–1052. [PubMed] [Google Scholar]
- 44.Sun J B, Rask C, Olsson T, Holmgren J, Czerkinsky C. Treatment of experimental autoimmune encephalomyelitis by feeding myelin basic protein conjugated to cholera toxin B subunit. Proc Natl Acad Sci USA. 1996;93:7196–7201. doi: 10.1073/pnas.93.14.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun J B, Xiao B G, Lindblad M, Li B L, Link H, Czerkinsky C, Holmgren J. Oral administration of cholera toxin B subunit conjugated to myelin basic protein protects against experimental autoimmune encephalomyelitis by inducing transforming growth factor-β-secreting cells and suppressing chemokine expression. Int Immunol. 2000;19:1449–1457. doi: 10.1093/intimm/12.10.1449. [DOI] [PubMed] [Google Scholar]
- 46.Svennerholm A-M, Holmgren J. Identification of Escherichia coli heat-labile enterotoxin by means of a ganglioside immunosorbent assay (GM1-ELISA) procedure. Curr Microbiol. 1978;1:19–23. [Google Scholar]
- 47.Tamura S, Hatori E, Tsuruhara T, Aizawa C, Kurata T. Suppression of delayed-type hypersensitivity and IgE antibody responses to ovalbumin by intranasal administration of Escherichia coli heat-labile enterotoxin B subunit-conjugated ovalbumin. Vaccine. 1997;15:225–229. doi: 10.1016/s0264-410x(96)00135-1. [DOI] [PubMed] [Google Scholar]
- 48.Tan M C A A, Mommaas A M, Drijfhout J W, Jordens R, Onderwater J J M, Verwoerd D, Mulder A A, van der Heiden A N, Scheidegger D, Oomen L C J M, Ottenhoff T H M, Tulp A, Neefjes J J, Koning F. Mannose receptor-mediated uptake of antigens strongly enhances HLA class II-restricted antigen presentation by cultured dendritic cells. Eur J Immunol. 1997;27:2426–2435. doi: 10.1002/eji.1830270942. [DOI] [PubMed] [Google Scholar]
- 49.Tarkowski A, Sun J-B, Holmdahl R, Holmgren J, Czerkinsky C. Treatment of experimental autoimmune arthritis by nasal administration of a type II collagen-cholera toxoid conjugate vaccine. Arthritis Rheumatism. 1999;42:1628–1634. doi: 10.1002/1529-0131(199908)42:8<1628::AID-ANR10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 50.van Gool S W, Vandenberghe P, de Boer M, Ceuppens J L. CD80, CD86 and CD40 provide accessory signals in a multiple-step T-cell activation model. Immunol Rev. 1996;153:47–83. doi: 10.1111/j.1600-065x.1996.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 51.Viney J L, Mowat A M, O'Malley J M, Williamson E, Fanger N A. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol. 1998;160:5815–5825. [PubMed] [Google Scholar]
- 52.Warren M K, Vogel S N. Bone-marrow derived macrophages: development and regulation of differentiation markers by colony-stimulating factor and interferons. J Immunol. 1985;134:982–989. [PubMed] [Google Scholar]
- 53.Weaver C T, Unanue E R. The costimulatory function of antigen-presenting cells. Immunol Today. 1990;11:49–55. doi: 10.1016/0167-5699(90)90018-5. [DOI] [PubMed] [Google Scholar]
- 54.Weber S, Traunecker A, Oliveri F, Gerhard W, Karjalainen K. Specific low-affinity recognition of major histocompatibility complex plus peptide by soluble T-cell receptor. Nature. 1992;356:793–796. doi: 10.1038/356793a0. [DOI] [PubMed] [Google Scholar]
- 55.Wiedermann U, Jahn-Schmid B, Lindblad M, Rask C, Holmgren J, Kraft D, Ebner C. Suppressive versus stimulatory effects of allergen/cholera toxoid (CTB) conjugates depending on the nature of the antigen in a murine model of type I allergy. Int Immunol. 1999;11:1717–1724. doi: 10.1093/intimm/11.10.1717. [DOI] [PubMed] [Google Scholar]
- 56.Wolvers D A W, Coenen-de Roo C J J, Mebius R E, van der Cammen M J F, Tirion F, Miltenburg A M M, Kraal G. Intranasally induced immunological tolerance is determined by characteristics of the draining lymph nodes: studies with OVA and human cartilage gp-39. J Immunol. 1999;162:1994–1998. [PubMed] [Google Scholar]