Abstract
Introduction:
Regenerative endodontics is a developing field of dentistry and aims to recover the physiological and anatomical functions of the tooth for cases of severe dental caries, pulpal pathologies and dental trauma.
Materials and Methods:
This umbrella review seeks to discover the scientific evidence on the effectiveness and the factors result in successful regenerative endodontic therapies in teeth with necrotic pulps and with incomplete root development. The study was conducted following the PRISMA Guidelines. There were no restrictions regarding search period. A comprehensive literature search was carried out in EMBASE, LILACS, PubMed, Cochrane, Scopus, and Google Scholar. A quality evaluation was conducted by using AMSTAR-2. A descriptive analysis of the included systematic reviews and meta-analysis were conducted.
Results:
Thirteen descriptive systematic reviews and 7 meta-analyses were included. Three articles evidenced low methodological quality according to AMSTAR-2 tool. Overall success rates for the endodontic regeneration procedures ranged from 50% to 98% and the survival rates were between 94% and 100%. Pulp regeneration had a high success rate, evidenced by factors such as the resolution of symptoms, healing, increased root length, dentin thickening and recovery of sensitivity. Follow-up varied from 1 to 48 months for the original studies included in the systematic reviews and meta-analyses.
Conclusions:
Endodontic practice offers the clinician a good treatment option in case of necrotic pulp with immature roots such as the endodontic regeneration, that is supported by high and moderate quality scientific literature.
Key Words: Apexification, Regenerative Endodontics, Root Canal Therapy, Systematic Review, Umbrella Review
Introduction
Regenerative endodontics is a developing field of dentistry whose main objective is to give continuity to the development of the tooth and recovery of its functions [1, 2]. Currently, techniques have been implemented that allow the treatment of permanent teeth with immature roots that have suffered pulp necrosis due to etiological factors, such as dental caries and dentoalveolar trauma [1, 3]. This implies a transformation in the way in which these cases have traditionally been approached that can also result in the recovery of dental health in all its anatomical and physiological components, such as complete root formation, recovery of sensitivity, revascularization, and re-innervation [1, 4]. Until a few years ago, immature teeth with pulpal necrosis were treated with apexification of calcium hydroxide and apical barrier with biomaterials [5]. However, the literature reports limited results regarding the thickening of the dentin and increasing the length of the root, which incurs a high risk of fracture and tooth loss [6-8].
Currently, there are various pulp regeneration techniques, such as the use of platelet derivatives, scaffolds, stem cells, and growth factors, [9-11]. These have been described in clinical trials and systematic reviews that attempt to standardize their use in decision-making based on good quality scientific evidence [12]. In general, regenerative endodontics comprises procedures with molecular and biological bases designed to replace dental structures affected by early pulp pathology, such as cement, periodontal ligament, and bone [13-15]. When regeneration of the pulp and periapical tissues is implemented, procedures are carried out that facilitate fundamental biological and structural processes, such as cell differentiation and enzyme secretion. However, there are controversial issues regarding the type of cells in regenerative processes [12-14]. For example, odontoblasts at the coronal level present different phenotypes compared to those found at the apical level and require different regenerative procedures [12-14]. Another example is scaffolding; tissues are three-dimensional structures and need an appropriate matrix to promote cell growth and differentiation. The nature of this structure can be a stimulated blood clot or a material of natural or synthetic origin [13, 14]. A third component is signaling, which is formed by growth factors and other enzymes that stimulate cell proliferation and differentiation. In this sense, the thickening of the dentin walls can be caused by the production of cement or dentin thanks to molecular signals and cells [1, 16, 17]. Several regeneration mechanisms have been mentioned in the literature: First, vital pulp cells that remain in the apical region can induce Hertwig epithelial cells so that they proliferate and differentiate into odontoblast-like cells [13, 14, 18]. Second, stem cells from the dental pulp present in permanent teeth may differentiate into odontoblast-like cells [11, 13, 14]. Third, regeneration may occur due to the presence of stem cells in the periodontal ligament that proliferate from the apical region [14, 18]. The fourth mechanism is attributed to the presence of stem cells found in the apical papilla or medullary bone; in this case, the induction of bleeding transports stem cells of mesenchymal origin from the bone to the lumen of the duct, a stimulated blood clot is rich in growth factors, generating a stimulus for the differentiation and maturation of fibroblasts, odontoblasts, and cementoblasts [19-22].
Knowledge of the biological activities that modulate cellular events during the pulp regeneration process allows us to identify how and in which cases regenerative treatment should be carried out and to devise improved regeneration protocols that improve the prognosis [19-21]. Some considerations for the success of endodontic regeneration are related in scientific texts and articles, for example, good disinfection of the canals, the proportion of a scaffold a blood clot or a scaffold of synthetic or natural origin that facilitates the activity of cells [10], an adequate coronal seal to prevent bacterial invasion, and occasionally the administration of growth factors to facilitate the process of cellular and molecular interaction [10, 22, 23]. The expected results of these procedures are total development of the root in length and thickening of the walls, health of the ligament and bone, revascularization, and recovery of sensitive functions [24].
Many studies comparing the techniques traditionally used and current pulp regeneration techniques have found that these new procedures are associated with higher increases in root length and thickness as compared to apexification [6, 7], as well as increased survival rates. However, knowledge on this topic is still very diverse and abundant, making it necessary to standardize the techniques of pulp regeneration according to the different methods [10]. This will facilitate clinical decision-making based on quality scientific studies [25].
Accordingly, the objective of this umbrella review is to discover the existing scientific evidence in systematic reviews and/or meta-analyses on the effectiveness of and the factors demonstrating the success of different types of regenerative endodontic therapy in teeth with necrotic pulps and with incomplete root development.
Materials and Methods
Study protocol and registration
This paper was written in accordance with the PRISMA statement for systematic reviews and meta-analysis [26]. The study protocol was registered at the International Prospective Register of Systematic Reviews-PROSPERO-(Protocol code: CRD42019124402; Available from https://www.crd.york.ac.uk/ prospero/display_record.php?RecordID=124402). In addition, the study was approved by the Ethics Committee of the Faculty of Dentistry of the University of Antioquia (Act 11/2019).
Design, search strategy, and period
For study purposes, an umbrella review was conducted following the methodology recommended by the Joanna Briggs Institute for development of a protocol applied to systematic reviews and meta-analyses [27]. The following PICO question was used: What are the effectiveness of and the factors evidencing the success (O) of different types of regenerative endodontic therapy (I, C) in teeth with a diagnosis of pulpal necrosis and incomplete root development (P)? According to this question, the eligibility criteria were:
Study design: We included systematic reviews and meta-analyses. As far as possible, they should accomplish the main criteria established for the Cochrane Collaboration [28] and/or the Centre for Review and Dissemination [29].
Participants: Patients in all age ranges presenting necrotic pulps and immature open apex teeth.
Interventions/control: Different types of regenerative endodontic therapy.
Outcome: The effectiveness of and factors evidencing the success of different types of regenerative endodontic therapy. For that purpose, the search focused on effect measures such as improvements in signs and symptoms, the lengthening of the tooth root, changes in the thickness of the dentin walls, apical foramen closure, sensitivity tests, and other clinical and socio-demographic variables.
Exclusion criteria: Other formats such as theoretical reviews, intervention, observational, or analytical studies, critical and theoretical essays, and clinical guides.
Table 1 lists the main characteristics related to database sources, the search equations, and definitions employed for the search strategy according to the MeSH terms/thesaurus. To identify the largest amount of information possible, we did not use time filters, and we included papers from all countries, selecting those in English, Portuguese, or Spanish.
Table 1.
Characteristics of the search strategy used for the umbrella review
| 1. |
Type of literature: Published material |
Source:
|
| Grey literature |
|
|
| 2. | Search terms |
|
Two reviewers (W.J.R.G. & A.A.A.S.) independently searched for titles and abstracts of potentially eligible articles. If the information met the eligibility criteria, the article was selected for full reading. The reviewers checked the reference list of the articles selected to find further studies not identified in the initial searches. All articles selected for inclusion were processed for data extraction. Disagreements were resolved by discussion and consultation with the other member of the research team (E.P.V).
Critical appraisal and studies’ analysis
The three authors (W.J.R.G., E.P.V., and A.A.A.S.) reviewed the quality of the selected studies. To guarantee the process’ quality, a pilot test with five articles was carried out, and we calculated a simple concordance index with a score of 90%. First, all reports were evaluated using the PRISMA checklist for systematic reviews (SR) [26]. Second, the AMSTAR-2 tool was used which is a checklist of 16 items [30]. Each item is answered with yes, no, cannot answer, or not applicable. Of the possible answers, only yes counts as a point in the total score for assessing the review. AMSTAR-2 characterizes quality at four levels: high, moderate, low, or critically low, according to the guidance document provided by the creators of the instrument. Toward that end, the checklist and final score can be calculated by introducing the data at https://amstar.ca/ Amstar Checklist php.
For each study included in the umbrella, we carried out an assessment of possible risk of bias (low, high, or unclear) using the information available for each systematic review/meta-analysis regarding the quality of the original studies, the methods employed for critical appraisal, and the accuracy of the tools used for combining the findings of the studies. In addition, we identified the level of heterogeneity of the included studies for each systematic review/meta-analysis, if available. No study was excluded from the umbrella review on the basis of quality.
We carried out a descriptive analysis of the main characteristics of the included reviews: the first author and year of publication, objective(s) of study, number, and type of original articles included in the review, type of study (systematic review or meta-analysis), main results, limitations, and gaps according to the reported findings.
Results
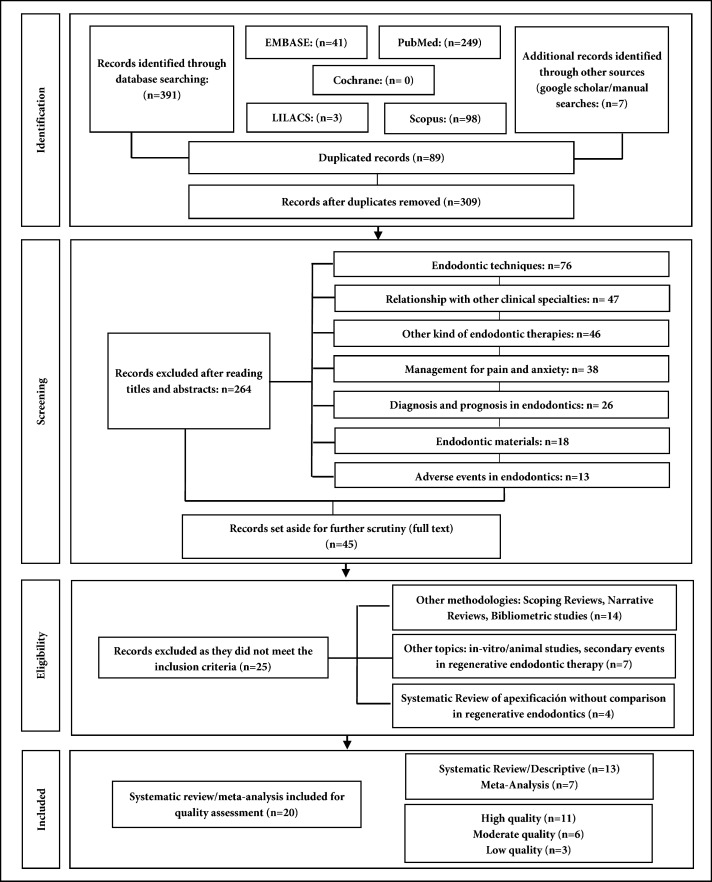
The initial search resulted in 398 records. After eliminating duplicates, 309 records were selected for revision of title and abstract, 264 were excluded and 45 articles remained for full reading; ultimately, 20 publications were included [31-50]. Reasons for exclusion are shown in Figure 1.
Figure 1.
Selection process of studies for the umbrella review
Table 2 shows the quality appraisal of the 20 studies included in the umbrella review. These systematic reviews/meta-analyses were conducted in the period 2012–2020.[31-50] According to the AMSTAR-2 tool, 11 studies (55%) were categorized as high quality [35, 36, 39, 41-45, 47, 48, 50], six (30%) of moderate quality [32, 33, 37, 38, 40, 49] and three (15%) were considered low quality [31, 34, 46]. Ten systematic reviews (50%) did not specify heterogeneity among their analyzed original studies [31-35, 37, 39, 40, 46, 49], three SRs (15%) reported low heterogeneity [41, 48, 50], four SRs (20%) reported high heterogeneity [36, 38, 44, 47], and the remaining three SRs (15%) were considered as having variable heterogeneity depending on the specific characteristics of the included original studies [42, 43, 45]. The risk of bias was unclear in nine studies (45%) [31-34, 37, 38, 40, 46, 47], moderate in ten studies (50%) [35, 36, 39, 41-43, 45, 48-50], and high in one study (5.3%) [44]. Seven studies (35%) did not clarify quality assessment procedures in terms of the instrument used [31, 33-35, 38, 40, 46]. Ten studies (50%) used the Cochrane Collaboration Tool [28] in different versions [36, 39, 41-45, 47, 49, 50]. The Newcastle Ottawa Scale (NOS) [51] was used in three systematic reviews (15%) [32, 42, 48], in one of which [42] as a complementary tool. Finally, one systematic review (5%) [37] used quality assessment procedures from the Oxford Centre for Evidence Medicine and the Center for Evidence-Based Management (CEBMa) [52].
Table 2.
Quality appraisal and summary of the systematic reviews/meta-analyses (n=20)
| Author | Appraisal rating (PRISMA) | AMSTAR Score (%)/Quality | Heterogeneity | Risk of Bias | Instrument (s) of Quality Assessment | Comments |
|---|---|---|---|---|---|---|
| Pramila R [ 31 ] | 11/27 | 56/Low | Not specified | Unclear (the authors did not specify quality appraisal for the included studies) | Not specified | This review is lacking for some essential elements of a systematic review (the quality appraisal of the studies selected) |
| Kontakiotis EG [ 32 ] | 18/27 | 75/Moderate | Not specified | Unclear | Newcastle-Ottawa scale (NOS) | This systematic review included case reports |
| Antunes LS [ 33 ] | 22/27 | 75/Moderate | Not specified | Unclear (the authors did not specify quality appraisal for the included studies) | Not specified | This review is lacking for some essential elements of a systematic review (the quality appraisal of the studies selected) |
| Conde MCM [ 34 ] | 7/27 | 56/Low | Not specified | Unclear (the authors did not specify quality appraisal for the included studies) | Not specified | This review is lacking for some essential elements of a systematic review (the quality appraisal of the studies selected) |
| Lolato A [ 35 ] | 24/27 | 88/High | Not specified | Moderate | Not specified | This systematic review did not specify the heterogeneity in the studies, and did not justify the conduction of a meta-analysis, although present some statistics. The existing literature lacks high-level clinical studies |
| Meschi M [ 36 ] | 23/27 | 94/High | High | Moderate | Cochrane Collaboration’s tool | There was considerable heterogeneity between the RCTs with regard to the type of therapy, type of APCs, assessment method, and study quality, and therefore the data could not be analyzed quantitatively |
| Ragab RA [ 37 ] | 20/27 | 75/Moderate | Not specified | Unclear | Critical appraisal approach used by the Oxford Centre for Evidence Medicine | This systematic review included case reports |
| Bucchi C [ 38 ] | 16/27 | 75/Moderate | High (exact value was not specified) | Unclear (the authors did not specify quality appraisal for the included studies) | Not specified | This review is lacking for some essential elements of a systematic review (the quality appraisal of the studies selected). The review also included animal studies |
| Duggal M [ 39 ] | 19/27 | 88/High | Not specified | Moderate | Cochrane Collaboration’s tool | The existing literature lacks high-level clinical studies |
| Kahler B [ 40 ] | 15/27 | 75/Moderate | Not specified | Unclear (the authors did not specify quality appraisal for the included studies) | Not specified | This review is lacking for some essential elements of a systematic review (the quality appraisal of the studies selected) |
| Nicoloso GF [ 41 ] | 25/27 | 88/High | Low (I < 50%) | Moderate | Cochrane Collaboration’s tool | The results must be carefully interpreted, considering the quality assessment of the included studies, and the risk of bias for some of them |
| Tong HJ [ 42 ] | 25/27 | 94/High | Variable (depending on the subgroup analysis) | Moderate | For observational studies (cohort and case-control studies) the Newcastle-Ottawa scale was used. For studies with randomized controlled trials and uncontrolled prospective trial designs the authors used the Cochrane Collaboration´s tool and for uncontrolled longitudinal studies, a modification including the judgment of not applicable was introduced for domains such as randomization and allocation concealment | The existing literature evidenced many knowledge gaps according to the studies´ findings |
| Torabinejad M [ 43 ] | 25/27 | 88/High | Variable (depending on the subgroup analysis (Low for survival rates (I2<50%, P>0.10) and High for success rates) | Moderate | A quality appraisal instrument developed for the authors in a previous study and the Cochrane Collaboration’s tool | The existing literature lacks high-level clinical studies |
| Chisini LA [ 44 ] | 23/27 | 88/High | High (exact value was not specified) | High | Cochrane Collaboration’s tool | The results must be carefully interpreted, considering the quality assessment of the included studies, and the risk of bias for some of them |
| Metlerska J [ 45 ] | 20/27 | 81/High | Variable (depending on the subgroup analysis) | Moderate | Cochrane Collaboration’s tool | The methodological quality of the studies was generally poor (in case of RCT) |
| Murray PE [ 46 ] | 12/27 | 56/Low | Not specified | Unclear (the authors did not specify quality appraisal for the included studies) | Not specified | This review is lacking for some essential elements of a systematic review (the quality appraisal of the studies selected) |
| do Couto AM [ 47 ] | 26/27 | 81/High | High (exact value was not specified) | Unclear | Cochrane Collaboration’s tool | The existing literature evidenced many knowledge gaps according to the studies’ findings |
| Nicoloso GF [ 48 ] | 26/27 | 88/High | Low (I<50%) | Moderate | Newcastle-Ottawa scale (NOS) | The existing literature evidenced many knowledge gaps according to the studies’ findings |
| Rossi-Fedele G [ 49 ] | 21/27 | 75/Moderate | Not specified | Moderate | Cochrane Collaboration’s tool and Joanna Briggs Institute Critical Appraisal Checklist for Case Reports | This systematic review included case reports and animal studies |
| Koç S [ 50 ] | 27/27 | 100/High | Low (I=0%) | Moderate | Cochrane Collaboration’s tool | The existing literature evidenced many knowledge gaps according to the studies’ findings |
General characteristics of the studies included are summarized in Table 3. Seven (35%) included quantitative synthesis (meta-analysis) [41-43, 45, 46, 48, 50], seven (35%) were from European countries [32, 35, 36, 38, 44, 45, 50], five (25%) were carried out in Brazil [33, 34, 41, 47, 48], three (15%) in Asian countries [31, 37, 39], three (15%) in the United States [42, 43, 46], and the other two (10%) were from Australia [40, 49]. Variability was observed taking into account the type and number of original studies included in the 20 qualitative and quantitative syntheses carried out. The minimum number of analyzed studies was three in the case of the meta-analysis conducted by Nicoloso et al. [48] and the maximum was 144 in the case of the descriptive systematic review carried out by Torabinejad et al.[43].
Table 3.
Main characteristics of the included systematic reviews and meta-analyses (n=20)
| First author, Country | Type of review | Number of included studies | Type of studies included | Type of intervention | Irrigation | Scaffold | Intraconduct medication |
|---|---|---|---|---|---|---|---|
| Pramila R, India [ 31 ] | Descriptive | 31 | Randomized clinical trials | Pulp regeneration, platelet derivatives, revascularization | 1.25% NaOCl to 5.25%, saline and 2% chlorhexidine | Blood clot, platelet-rich plasma and platelet-rich fibrin | Triantibiotic paste |
| Kontakiotis EG, Greece [ 32 ] | Descriptive | 51 | High-level cohort studies, case series, and case reports | Pulp regeneration | NaOCl and EDTA | Not specified | Triantibiotic paste, biantibiotic paste and calcium hydroxide |
| Antunes LS, Brazil [ 33 ] | Descriptive | 11 | Clinical research studies and serial case reports | Pulp revascularization | 17% EDTA and 2% to 6% NaOCl | Blood clot, platelet-rich plasma and platelet-rich fibrin | Triantibiotic paste, biantibiotic paste and calcium hydroxide |
| Conde MCM, Brazil [ 34 ] | Descriptive | 3 | Randomized clinical trials | Regenerative stem cell therapy | NaOCl and EDTA | Scaffolding of natural and synthetic origin | Not specified |
| Lolato A, Italy [ 35 ] | Descriptive | 4 | Prospective studies with a comparative design | Pulp regeneration with platelet derivatives | 17% EDTA and 5.25 NaOCl | Blood clot, platelet-rich plasma and platelet-rich fibrin | Triantibiotic paste, biantibiotic paste and calcium hydroxide |
| Meschi M, Belgium [ 36 ] | Descriptive | 48 | Clinical trials and case reports | Pulp regeneration with platelet derivatives | NaOCl and EDTA | Blood clot, platelet rich plasma and platelet rich fibrin combined with natural membranes | Calcium hydroxide and triantibiotic paste |
| Ragab RA, India [ 37 ] | Descriptive | 32 | Case reports | Pulp regeneration with blood-derived scaffolding or clot formation | NaOCl 1.25% to 5.25%, saline and 2% chlorhexidine, 17% EDTA | Platelet-rich plasma, induction of blood clot, and collagen scaffold | Triantibiotic paste, biantibiotic paste, formocresol and calcium hydroxide |
| Bucchi C, Spain [ 38 ] | Descriptive | 33 | Clinical trials in humans and animals | Pulp regeneration | NaOCl from 1% to 6%, chlorhexidine, saline, sodium thiosulfate and H2O2 | Not specified | Triantibiotic paste, biantibiotic paste and calcium hydroxide |
| Duggal M, Singapore [ 39 ] | Descriptive | 22 | Controlled clinical trials | Apexification and revascularization | 1 to 6% NaOCl alone or in combination with 17% EDTA and saline or distilled water | Blood clot, platelet-rich plasma and platelet-rich fibrin | Calcium hydroxide and triantibiotic paste |
| Kahler B, Australia [ 40 ] | Descriptive | 6 | Randomized clinical trials | Apexification and revascularization | NaOCl between 1% and 2.5%, EDTA at 17%, some studies used chlorhexidine and saline solution | Blood clot and collagplug | Calcium hydroxide, formocresol and triantibiotic paste |
| Nicoloso GF, Brazil [ 41 ] | Meta-analysis | 14 for systematic reviews (7 for meta-analyses) | Randomized clinical trials | Apexification and revascularization | 1 to 5.25% NaOCl in combination with 17% EDTA and saline | Blood clot | Not specified |
| Tong HJ, USA [ 42 ] | Meta-analysis | 14 for systematic reviews (5 for meta-analyses) | Randomized clinical trials | Apexification and revascularization | 1 to 2.5% NaOCl in combination with 17% EDTA and saline | Platelet-rich plasma and blood clot | Calcium hydroxide and triantibiotic paste |
| Torabinejad M , USA [ 43 ] | Meta-analysis | 144 for systematic reviews (5 for meta-analyses) | Clinical trials | Apexification and revascularization | NaOCl between 0.5% and 6%, saline and EDTA at 17% | Platelet-rich plasma and blood clot | Calcium hydroxide and triantibiotic paste |
| Chisini LA, Italy [ 44 ] | Descriptive | 5 | Prospective or retrospective clinical studies | Apexification with MTA and revascularization | NaOCl at 0.5% and 5.25%, saline and EDTA at 17% | Blood clot | Triantibiotic paste, biantibiotic paste and calcium hydroxide |
| Metlerska J, Poland [ 45 ] | Meta-analysis | 26 for systematic reviews (3 for meta-analyses) | Clinical trials 5 and case reports 21 | Pulp regeneration with platelet derivatives | NaOCl between 0.5% and 5.25%, saline serum and 17% EDTA were also used | Platelet-rich plasma membrane and platelet-rich fibrin | Calcium hydroxide and triantibiotic paste |
| Murray PE, USA [ 46 ] | Meta-analysis | 20 for systematic reviews and meta-analyses | Not specified | Pulp regeneration with platelet rich plasma and PRF | NaOCl 1.25% to 5.25%, saline and chlorhexidine at 2%, EDTA at 17% | Blood clot and platelet-rich plasma | Not specified |
| Do Couto AM, Brazil [ 47 ] | Descriptive | 8 | Randomized clinical trials | Pulp revascularization with PTA | NaOCl and EDTA | Blood clot, platelet-rich plasma, platelet-rich fibrin, hydrogel and collagen | Triantibiotic paste and calcium hydroxide |
| Nicoloso GF, Brazil [ 48 ] | Meta-analysis | 3 for systematic reviews and meta-analyies | Randomized clinical trials | Pulp revascularization and apexification | Various concentrations of NaOCl, chlorhexidine and/or EDTA CH | Blood clot | Triantibiotic paste, biantibiotic paste and calcium hydroxide |
| Rossi-Fedele G, Australia [ 49 ] | Descriptive | 7 | Case reports, clinical trials in humans and animals | Pulp revascularization | NaOCl at 1.25% and 5.25%, saline solution and chlorhexidine at 2%, 2% chlorhexidine, H2O2, 17% EDTA | Blood clot, platelet-rich plasma and platelet-rich fibrin | Not specified |
| Koç S, Turkey [ 50 ] | Meta-analysis | 18 for systematic review (7 for meta-analysis) | Clinical trials and Prospective or retrospective clinical studies | Pulp regeneration with blood-derived scaffolding or clot formation and collagen barrier | NaOCl at 1.25% and 5.25%, saline solution and chlorhexidine at 2%, 2% chlorhexidine, H2O2, 17% EDTA | Blood clot, platelet-rich plasma and platelet-rich fibrin and collagen | Triantibiotic paste, biantibiotic paste and calcium hydroxide, combination of calcium hydroxide and 2% chlorhexidine gel |
PRP: Platelet-Rich Plasma, PRF: Platelet-Rich Fibrin, BC: Blood Clot, EDTA: Ethylenediaminetetraacetic acid, MTA: Mineral trioxide aggregate, CH: Chlorhexidine, NaOCl: sodium hypochlorite, H 2 O 2 : hydrogen peroxide
All systematic reviews/meta-analyses included original studies in which endodontic regeneration procedures were performed (Table 3). However, there is variability among the kind of scaffolds used. For instance, two SRs (10%) did not specify the type of scaffold [32, 38] while in the rest, the use of blood clots and derivatives of blood plasma (platelet-rich plasma and platelet-rich fibrin), stem cells, and synthetic and natural scaffolds were examined [31, 33-37, 39-50]. Irrigation protocols varied, but all studies included at least 0.5% to 6% sodium hypochlorite and 17% Ethylenediamine Tetraacetic Acid (EDTA) together or alternating. Eight studies (40%) mentioned the use of other irrigants, such as hydrogen peroxide and 2% chlorhexidine [31, 37-40, 48-50]. Four SRs (20%) did not specify the type of intra-conduct medication used in the procedures [34, 41, 46, 49]. The remaining 15 (75%) [31-33, 35-40, 42-45, 47, 48, 50] reported implementing this kind of medication in the appointment just prior to the revascularization process, by using tri-antibiotic paste [53], bi-antibiotic paste, or calcium hydroxide; only one study (5%) reported the use of Formocresol [37].
Table 4 shows the summary of different outcomes related to the effectiveness and factors evidencing the success of endodontic regeneration. The global period for the process varied from one to 48 months (considering the initial–final of each research). Eleven systematic reviews/meta-analyses (55%) included original studies with follow-up times of four months or less [31, 34, 35, 38-40, 42, 46, 47, 49]. The systematic review carried out by Kontakiotis et al. in 2014 included original studies with a follow-up of 48 months [32].
Table 4.
Summary of outcomes related to the effectiveness and factors evidencing the success rates (%) of regenerative endodontics as provided for the systematic reviews/meta-analyses included (n=20)
| Author | Characteristics related to effectiveness | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follow up (months) | General success rates | General survival rates | Specific factors evidencing success of endodontic regeneration therapy | |||||||
| Min | Max | Resolution of symptoms | Apical lesion resolution | Apical closure (tooth root) | Dentin thickening | Increased tooth root length | Response to pulp sensibility tests | |||
| Pramila R [ 31 ] | 3 | 25 | -- | -- | 100.0 | 100.0 | 100.0 | 100.0 | 67.0 | -- |
| Kontakiotis EG [ 32 ] | 6 | 48 | -- | -- | 100.0 | -- | -- | -- | -- | -- |
| Antunes LS [ 33 ] | 12 | 18 | 76.0 | -- | -- | -- | -- | -- | -- | -- |
| Conde MCM [ 34 ] | 3 | 36 | 76.0 | -- | 100.0 | -- | -- | -- | -- | -- |
| Lolato A [ 35 ] | 3 | 18 | -- | -- | 100.0 | 100.0 | -- | 5.0- 15.0 | -- | Cold-Electrical: 50.0 |
| Meschi M [ 36 ] | 5 | 36 | 50.0 | -- | 76.0- 92.0 | 87.0- 95.0 | -- | -- | -- | -- |
| Ragab RA [ 37 ] | 6 | 36 | -- | -- | 100.0 | -- | -- | -- | -- | -- |
| Bucchi C [ 38 ] | 3 | 36 | -- | -- | 100.0 | 88.0 | 84.3 | 88.0 | 84.1 | -- |
| Duggal M [ 39 ] | 1 | 36 | 98.0 | -- | 40.0- 100.0 | 89.7 | -- | 20.0- 80.0 | 0.0- 100.0 | -- |
| Kahler B [ 40 ] | 3 | 36 | 89.7 | 98.6 | 100.0 | 98.0 | -- | -- | -- | -- |
| Nicoloso GF [ 41 ] | 11 | 18 | -- | 94.0 | 100.0 | 80.0- 90.0 | 100.0 | 100.0 | 100.0 | -- |
| Tong HJ [ 42 ] | 1 | 12 | 89.7 | 100.0 | 100.0 | 94.0 | BC: 76.0 PRP: 82.0 | 48.0- 70.0 | BC: 80.0 PRP: 94.0 | -- |
| Torabinejad M [ 43 ] | 6 | 36 | 91.3 | 97.1- 97.8 | 100.0 | -- | -- | -- | 79.0 | -- |
| Chisini LA [ 44 ] | 1 | 23 | 87.9 | -- | 100.0 | -- | -- | 13.0 | 8.5 | -- |
| Metlerska J [ 45 ] | 6 | 18 | -- | -- | 100.0 | 10.0- 98.0 | 20.0- 93.0 | 20.0- 93.0 | 10.0- 99.0 | Positive sensitivity: 13.3- 80.0 |
| Murray PE [ 46 ] | 3 | 25 | 76.0 | -- | -- | 89.0- 100.0 | BC: 58.8 PRF: 85.2 PRP: 85.1 |
PRP: 100.0 PRF: 100.0 BC: 100.0 |
PRP: 64.2 PRF: 74.1 BC: 64.1 |
-- |
| do Couto AM [ 47 ] | 4 | 19 | -- | -- | -- | -- | -- | -- | -- | -- |
| Nicoloso GF [ 48 ] | 8 | 35 | 89.0 | -- | 100.0 | -- | -- | -- | -- | -- |
| Rossi-Fedele G [ 49 ] | 1 | 19 | 50.0 | -- | 100.0 | 50.0 | -- | -- | -- | -- |
| Koç S [ 50 ] | 8 | 46 | 94.8 | -- | -- | -- | -- | -- | -- | -- |
PRP: Platelet-Rich Plasma, PRF: Platelet-Rich Fibrin, BC: Blood Clot
12 systematic reviews/meta-analyses (60%) reported general success rates [31, 34, 36, 39, 40, 42-44, 46, 48-50], and four (20%) reported survival rates [40-43] for endodontic regeneration procedures. In the first case, lower success rates (50%) were reported by Meschi et al. [36] and by Rossi-Fedele et al.[49]. The remaining studies reported general success rates equal to or greater than 76%. In the second case, the studies reported survival rates equal to or greater than 94% (Table 4).
Table 4 shows to the success percentages for endodontic regeneration according to different factors, as reported by the analyzed systematic reviews/meta-analyses. Fifteen articles (75%) reported a resolution of symptoms in 100% of cases [31, 32, 34, 35, 37-45, 48, 49]. Eleven studies (55%) reported reduction between 50% and 100% in the size of the lesion [31, 35, 36, 38-42, 45, 49]. Six studies (30%) reported an apical closure (tooth root) in ranges higher than 80% [31, 38, 41, 42, 45, 46]. A thickening of the dentinal walls was reported in six articles included in this review (30%), with ranges between 80% and 100% of cases [31, 38, 39, 41, 45, 46]. Considering the root length, a variable percentage of increase in root length ranging from 0% to 100% was seen.
This situation was reported in nine (45%) analyzed articles [31, 38, 39, 41-46]. The recovery of pulp sensitivity was seldom analyzed (in only two articles, 10%); the authors emphasized that it can be achieved in between 13% and 80% of cases [35, 45].
The limitations of the studies are summarized in Table 5. The more frequent limitations reported for the systematic reviews/meta-analyses were: a lack of clarity between survival and success (n=20, 100%), the success of endodontic regeneration was defined with different parameters (n=16, 80%), small sample sizes included in some systematic reviews (n=15, 75%), and some had not followed a suitable methodology protocol for this type of study and do not present a record for this purpose (n=13, 65%).
Table 5.
Summary of the limitations reported in the systematic reviews/meta-analyses included (n =20)
| Limitations | n (%) |
|---|---|
| Some studies included in the systematic reviews do not clarify the difference between survival and success. | 20 (100.0) |
| In the studies included in the systematic reviews, the success of endodontic regeneration was defined with different parameters, so there is a difference in the concept of success for the different authors. | 16 (80.0) |
| Studies with small sample sizes were included in some of the systematic reviews | 15 (75.0) |
| Some of the systematic reviews do not follow a methodology protocol for this type of study and do not present a record for this purpose. | 13 (65.0) |
| Amongst the different studies included in the systematic reviews there are different regeneration protocols, some include natural and systematic scaffolds, use of stem cells, or blood clot only. Also, they use different materials in cervical barriers such as Biodentine, MTA, ect. | 12 (60.0) |
| The systematic reviews expose a lack of scientific evidence with methodological rigor and propose to carry out more and better clinical trials. | 8 (40.0) |
| In the evaluation of the risk of bias of the studies, different methodologies were presented in the systematic reviews, which does not allow a standardization of quality. | 8 (40.0) |
| Only publications in English language were included in our review and no grey literature was searched, which may be a limitation in the study selection process. | 4 (20.0) |
| Many studies included in these reviews were uncontrolled longitudinal studies and randomized controlled clinical trials with high levels of bias. There were also systematic reviews of clinical case studies. | 2 (10.0) |
| Some of the clinical trials included in these studies did not report sample size calculations. | 2 (10.0) |
| Systematic reviews where meta-analyzes were carried out argued high methodological heterogeneity in the included studies. | 2 (10.0) |
| Some of these systematic reviews compared animal studies to human studies. | 1 (5.0) |
Discussion
Main findings
Pulp regeneration is a procedure performed on teeth that suffer pulp necrosis due to dental trauma or bacterial infections [16]. The primary goals of dentistry and regenerative endodontics is to maintain dentition, its functionality, and aesthetics [54]. The effectiveness and success of endodontic regeneration procedures in systematic reviews and meta-analyses discussed in this general review include factors such as survival, symptom relief, healing of apical injury, thickening of dentin walls, and increasing root length. This is the long-term expectation [16, 55].
Possible explanations from the scientific literature
It should be noted that there are conceptual differences between success and survival of endodontically treated teeth. Survival refers to keeping the tooth in the mouth for an indeterminate time and under conditions not necessarily ideal, while success, in addition to tooth survival, refers to returning its optimal health and functionality [55].
For teeth that have suffered pulp necrosis from trauma or caries, the treatment traditionally implemented is apexification either with calcium hydroxide or with biomaterials such as Biodentine (Septodont Ltd., Saint Maur des Fraussés, Francia) and mineral trioxide aggregate (MTA) [56, 57]. Variability among survival rates has been reported, and this relates to the technique and type of material used. For instance, in calcium hydroxide apexification, the average survival rate is 88% [7, 56]. However, a disadvantage of this procedure is the high number of appointments to achieve the stimulus for the formation of an apical barrier. In the case of apexification with biomaterials such as mineral trioxide aggregate (MTA), the literature reports a lower number of appointments and higher survival rates ranging from 97% to 100% in follow-up periods up to 36 months [7, 56]. High survival rates have also been reported in teeth treated with regenerative endodontics, ranging from 94% to 98% at 48-month follow-up [40-43].
Another parameter for analysis of the effectiveness and success of apexification and pulp regeneration procedures is the resolution of symptomatology. The objective in this case is to control spontaneous or percussion pain and the presence of the sinuous tract [58]. For apexification procedures with calcium hydroxide or a biomaterial, symptom control occurs in between 98% and 100% of patients [39, 59, 60]. For pulp regeneration procedures, 85% (n=17) of the articles included in this umbrella review reported 100% effectiveness in the resolution of symptoms [31-35, 37, 38, 40-49], and the two remaining articles (10%) reported elimination of symptoms in 40% to 100% of study cases [36, 39]. In general terms, variability in the percentages can be attributed to factors related to the technique and the systemic condition of the patient [61].
Currently, diagnostic aids such as X-ray and tomographic images are an important complement to the evolution of endodontic treatments, ideally a tomographic follow-up being indicated to show changes at bone level resulting from pulp regeneration treatment [62, 63]. Regardless of the imagery technique used, one factor that shows success is reduction in the size of the lesion caused by pulp necrosis that evolves into symptomatic or asymptomatic apical periodontitis. The radiographic assessment of evolution can be performed by means of standardized indices, such as the periapical index, which allows comparison of the size of the lesion in tracking intervals [64]. This umbrella review found that 11 studies (55%) reported a reduction between 50% and 100% in the size of the lesion for pulp regeneration [31, 35, 36, 38-42, 45, 49]. The other studies did not specify this result, only mentioning that periapical lesions had disappeared when the follow-up was conducted. Regarding biomaterials for apexification, an injury size reduction of 94% was reported at follow-up periods of 6 to 18 months; for calcium hydroxide apexification, there was a decrease in injury by 87% [6, 56, 59, 65].
In addition to seeking survival and resolution of injury and symptoms of teeth with pulp necrosis and immature roots, regenerative endodontic therapy also seeks to maintain or reactivate the biological process of tooth development, generating thickening of the dentin walls and root length gain. These are factors that demonstrate the success of such therapies and that represent an advantage over apexification procedures [16].
Thickening of the dentin root walls can be achieved with regenerative endodontic therapy in a tooth with immature root suffering necrosis, usually in stages of early development when even the position and mineralization of dentin have not reached their peak due to the age of the patient, and has a great potential for healing and neoformation of soft and mineralized tissues [14]. The treatment is complex and variable and may depend on modifications made to the basic regeneration protocol [2]. For regenerative endodontic procedures, a thickening of the dentinal walls in 80% to 100% of cases was reported in six articles in this umbrella review [31, 38, 39, 41, 45, 46]. Ten (50%) of these systematic reviews/meta-analyses did not report whether there was thickening of the dentin walls [32-34, 36, 37, 40, 43, 47-49]. It should be noted that measuring the thickening of dentin in teeth with pulp necrosis and immature roots is a difficult factor to evaluate, usually done with radiographic and tomographic images, which makes a significant difference in the reported results [48, 59]. Despite the variability with regard to this factor, it is known to be an exclusive manifestation of pulp regeneration therapy [66].
Similarly, an increase in root length is another factor that verifies the success of endodontic regeneration. Inducing continuation of the tooth’s root development is necessary to improve its long-term prognosis and increase the chances of survival [66]. Monitoring this is best accomplished by X-ray and tomographic images, which allow calculation of the differences observed in the control visits [63]. Only three systematic reviews reported success percentages equal to or below 10% in their original studies regarding increase of tooth root length [39, 44, 45]. That is why this success factor occurs mostly in regenerative endodontic procedures, having an obvious advantage over apexification [31, 37, 44], for which success rates less than 10% have been reported for both MTA and calcium hydroxide [65, 67, 68].
One of the most interesting aspects that has become a challenge in regenerative endodontics is restoring the tooth’s important functions, such as recovery of blood flow and the ability to perceive sensations [4]. This is a very active biological process that involves the genesis of tissues that begin with the formation of the clot inside the root canal to enable, together with bioactive materials, the neoformation of vascular and nervous tissues, specific cell differentiation and migration, gene expression, and the secretion of enzymes such as growth factors and signaling molecules/enzymes [21, 57]. In the articles included in this umbrella review, the recovery of pulp sensibility was under-analyzed; however, some authors stated that it can be achieved in 13% to 80% of cases [33, 35, 45]. Clinical trials have shown significant pulp responses in up to 18-month follow-ups [69, 70].
Inducing tissue produced during the regenerative process to recover physiological functions must be understood from a model of imitation of embryonic tissue development through repair or regeneration [18]. Tissue repair is the replacement of lost tissue with other cell lines and results in deprivation of the main biological function, while tissue regeneration is the reconstruction of damaged tissue from the same cells, which restores lost biological function [21]. Wound healing is a histological process in which the structure formed within the duct has its own identity, as described by some original histological studies that observed fibrous connective tissue with fibroblasts, mesenchymal cells, and blood vessels. These may indicate an extension of periapical tissue and periodontal ligament [21, 71]. Neurons and nerve packets were also observed in some cases [21].
The success of regenerative endodontics can be sensitive to different factors, such as the technique used and the patient's response [38, 72]. The basic protocol of regeneration is defined by international associations as consisting of the formation of a blood clot as the basis of the process; however, these protocols are usually modified in different clinical trials with the use of natural or synthetic scaffolding as blood derivatives, for example, platelet-rich plasma and platelet-rich fibrin that provide growth factors [1, 2, 4]. These act as a guide for cell migration and differentiation.[46] The use of these scaffoldings has been linked to greater success in some aspects of regenerative endodontics; however, the evidence is not entirely conclusive [34, 45, 46]. Differences in the irrigation protocol may also intervene [73, 74].
Another difference is that the biomaterial that constitutes the cervical barrier must offer physical and chemical characteristics that render it biocompatible and resistant to the conditions of the medium, such as its ability to seal, its elastic module, and its resistance to different forces. Currently, bioactive cements are used, MTA being the material most studied over time [75]. A systematic review of studies analyzing the biocompatibility of materials shows that there are no statistically significant differences between Biodentine (Septodont Ltd., Saint Maur des Fraussés, France) and MTA (Loma Linda University, CA, USA); both have good interaction with dental and periapical tissue [76], but Biodentine has advantages over MTA in its shorter setting time and lower risk of changes in pigmentation [75]. When comparing MTA (Loma Linda University, CA, USA) with Biodentine or EndoSequence BC Sealer (Brasseler, Savannah, GA, USA), the literature reports almost similar success rates in their use as repair materials or apical filling materials to achieve seal in ranges of 92% to 99% [57, 75-78].
Other modifications to regenerative endodontic protocols have been reported as experimental techniques, including stem cell implantation and stimulation of growth factors present in dentin through irrigants such as EDTA [13, 79]. Original studies describe the role of growth factors in regeneration in general and how it applies when it comes from external sources [13, 79]. These factors can be extracted from cultured cells that release their secretome into the environment, which is made up of growth factors, cytokines, free proteins, and macro/micro vesicles [13, 79]. For regenerative endodontics, a secretome rich in growth factors from differentiated cells in cultures could be obtained and applied to the root canal where the clot is located [19].
A very important aspect of pulp regeneration is the patient’s response to the therapy. This may be conditioned by several situations, such as tobacco use, chronic diseases, or even biological differences between healthy patients that can intervene in long-term success [5, 69, 80]. The response in healing may also be affected by the presence of local infections, such as apical periodontitis and abscesses. Some original studies have highlighted factors such as age, genetic profile, and phenotype as conditions influencing the healing process [81]. Research in the specific field of regenerative endodontics and scarring from genotype or phenotype injury is scarce, but this may be a key factor in long-term forecasting and should be investigated by clinical trials with substantial follow-up periods [1, 82, 83].
Strengths and limitations of this umbrella review
The results of this umbrella review are based on the results of the systematic reviews included but not on the actual original studies. For this reason, the findings are dependent on the methodological quality of the systematic reviews as well as the individual studies chosen. Nevertheless, this umbrella review followed the protocol for systematic reviews and was recorded in PROSPERO. Also, the quality of each article was evaluated in order to select the most relevant and useful information for clinical decision-making. The systematic reviews included proceeded from a few countries in Europe, Asia, and North America; only Brazil represents Latin America. This could have an iceberg effect because the results of the original studies are according to the populations studied, but regenerative treatments are applied globally. Another limitation of this review is that much literature may have been omitted because it was in languages other than English or because it was not published in a recognized journal.
Conclusions and recommendations
Pulp regeneration and apexification are highly successful procedures; this is evidenced by factors such as survival, resolution of symptoms, and scarring of the lesion. We can see that these factors do not present significant differences in the success rates reported in the original articles of the systematic reviews we have included. Tooth vitality, gain in root length, functioning odontoblasts, and thickening of the dentin walls and in some cases recovery of nerve/vascular physiological function are to name a few excellent advantages that pulp regeneration offers. This has been reported to improve the long-term prognosis of the teeth and offers the clinician several treatment options supported by high-quality scientific literature in cases of pulp necrosis occurring with immature roots.
More clinical trials are needed with rigorous methodologies to evaluate regenerative endodontic techniques and their results in basic and modified protocols. Even more innovative studies, such as the use of growth factors and stem cells will be essential for the future. Studies should consolidate the wide variety of regenerative protocols and outcomes to obtain high-quality scientific evidence to support decision-making in clinical practice. Finally, it is recommended that clinical studies that analyze healing behaviors be carried out in patients with different genotypic and phenotypic profiles as this could be a determining factor in prognosis. All socio-demographic variables of the populations studied should be taken into account for future studies, proposing analyses in different countries to compare how this may influence the results of regenerative treatments.
Acknowledgements
This systematic review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest:
‘None declared’.
References
- 1.Jung C, Kim S, Sun T, Cho Y-B, Song M. Pulp-dentin regeneration: current approaches and challenges. J Tissue Eng. 2019;10:204173141881926. doi: 10.1177/2041731418819263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trope M. Regenerative potential of dental pulp. J Endod. 2008;34(7 Suppl):S13–7. doi: 10.1016/j.joen.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Burczynska A, Struzycka I, Dziewit L, Wroblewska M. Periapical abscess - etiology, pathogenesis and epidemiology. Przegl Epidemiol. 2017;71(3):417–28. [PubMed] [Google Scholar]
- 4.Cymerman JJ, Nosrat A. Regenerative Endodontic Treatment as a Biologically Based Approach for Non-Surgical Retreatment of Immature Teeth. J Endod. 2020;46(1):44–50. doi: 10.1016/j.joen.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Shah N, Logani A, Bhaskar U, Aggarwal V. Efficacy of revascularization to induce apexification/apexogensis in infected, nonvital, immature teeth: a pilot clinical study. J Endod. 2008;34(8):919–25; Discussion 1157. doi: 10.1016/j.joen.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Vidal K, Martin G, Lozano O, Salas M, Trigueros J, Aguilar G. Apical Closure in Apexification: A Review and Case Report of Apexification Treatment of an Immature Permanent Tooth with Biodentine. J Endod. 2016;42(5):730–4. doi: 10.1016/j.joen.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Lin JC, Lu JX, Zeng Q, Zhao W, Li WQ, Ling JQ. Comparison of mineral trioxide aggregate and calcium hydroxide for apexification of immature permanent teeth: A systematic review and meta-analysis. J Formos Med Assoc. 2016;115(7):523–30. doi: 10.1016/j.jfma.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Morales Martínez D, Covo Morales E, Díaz Caballero A. Efectos adversos en el periápice relacionados con el uso del mineral trióxido agregado en apexificación: revisión sistemática [Relationship between adverse effects on the peri apex with the use of mineral trioxide aggregate on apexification, systematic review] Av Odontoestomatol. 2014;30(2):95–8. [Google Scholar]
- 9.Araújo PRDS, Silva LB, Neto APDS, Almeida De Arruda JA, Álvares PR, Sobral APV, Júnior SA, Leão JC, Braz Da Silva R, Sampaio GC. Pulp Revascularization: A Literature Review. Open Dent J. 2017;10(1):48–56. doi: 10.2174/1874210601711010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahler B, Lin LM. A review of regenerative endodontics: current protocols and future directions. J Istanb Univ Fac Dent. 2017;51(3 Suppl 1):S41–S51. doi: 10.17096/jiufd.53911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saberi EA, Karkehabadi H, Mollashahi NF. Cytotoxicity of Various Endodontic Materials on Stem Cells of Human Apical Papilla. Iran Endod J. 2016;11(1):17–22. doi: 10.7508/iej.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shamszadeh S, Asgary S, Nosrat A. Regenerative Endodontics: A Scientometric and Bibliometric Analysis. J Endod. 2019;45(3):272–80. doi: 10.1016/j.joen.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Duncan HF, Kobayashi Y, Shimizu E. Growth Factors and Cell Homing in Dental Tissue Regeneration. Curr Oral Health Rep. 2018;5(4):276–85. doi: 10.1007/s40496-018-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashemi-Beni B, Khoroushi M, Foroughi MR, Karbasi S, Khademi AA. Tissue engineering: Dentin - pulp complex regeneration approaches (A review) Tissue Cell. 2017;49(5):552–64. doi: 10.1016/j.tice.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Babaki D, Matin MM. Odontoblast-like cytodifferentiation of dental stem cells: a review. Iran Endod J. 2020;15(2):79–89. doi: 10.22037/iej.v15i2.27569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SG, Malek M, Sigurdsson A, Lin LM, Kahler B. Regenerative endodontics: a comprehensive review. Int Endod J. 2018;51(12):1367–88. doi: 10.1111/iej.12954. [DOI] [PubMed] [Google Scholar]
- 17.Kawashima N, Okiji T. Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit Anom (Kyoto) 2016;56(4):144–53. doi: 10.1111/cga.12169. [DOI] [PubMed] [Google Scholar]
- 18.Digka A, Sakka D, Lyroudia K. Histological assessment of human regenerative endodontic procedures (REP) of immature permanent teeth with necrotic pulp/apical periodontitis: A systematic review. Aust Endod J. 2020;46(1):140–53. doi: 10.1111/aej.12371. [DOI] [PubMed] [Google Scholar]
- 19.Martins LF, Costa RO, Pedro JR, Aguiar P, Serra SC, Teixeira FG, Sousa N, Salgado AJ, Almeida RD. Mesenchymal stem cells secretome-induced axonal outgrowth is mediated by BDNF. Sci Rep. 2017;7:1. doi: 10.1038/s41598-017-03592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abou Neel EA, Chrzanowski W, Salih VM, Kim H-W, Knowles JC. Tissue engineering in dentistry. J Dent. 2014;42(8):915–28. doi: 10.1016/j.jdent.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Shi X, Mao J, Liu Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl Med. 2020;9(4):445–64. doi: 10.1002/sctm.19-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nosrat A, Ryul Kim J, Verma P, P SC. Tissue engineering considerations in dental pulp regeneration. Iran Endod J. 2014;9(1):30–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Orti V, Collart-Dutilleul PY, Piglionico S, Pall O, Cuisinier F, Panayotov I. Pulp Regeneration Concepts for Nonvital Teeth: From Tissue Engineering to Clinical Approaches. Tissue Eng Part B Rev. 2018;24(6):419–42. doi: 10.1089/ten.TEB.2018.0073. [DOI] [PubMed] [Google Scholar]
- 24.Diogenes A, Ruparel NB, Shiloah Y, Hargreaves KM. Regenerative endodontics: A way forward. J Am Dent Assoc. 2016;147(5):372–80. doi: 10.1016/j.adaj.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad P, Dummer PMH, Noorani TY, Asif JA. The top 50 most‐cited articles published in the International Endodontic Journal. Int Endod J. 2019;52(6):803–18. doi: 10.1111/iej.13083. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–40. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thomas J. Cochrane handbook for systematic reviews of interventions. 6a ed. Wiley Online Library; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centre for Reviews and Dissemination (CRD)-University of York. CDR Database. [[cited 2018 July 30]]. Available from: https://www.york.ac.uk/crd /
- 30.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017:358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pramila R, Muthu M. Regeneration potential of pulp-dentin complex: Systematic review. J Conserv Dent. 2012;15(2):97–103. doi: 10.4103/0972-0707.94571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kontakiotis EG, Filippatos CG, Agrafioti A. Levels of evidence for the outcome of regenerative endodontic therapy. J Endod. 2014;40(8):1045–53. doi: 10.1016/j.joen.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Antunes LS, Salles AG, Gomes CC, Andrade TB, Delmindo MP, Antunes LA. The effectiveness of pulp revascularization in root formation of necrotic immature permanent teeth: A systematic review. Acta Odontol Scand. 2016;74(3):161–9. doi: 10.3109/00016357.2015.1069394. [DOI] [PubMed] [Google Scholar]
- 34.Conde MC, Chisini LA, Demarco FF, Nor JE, Casagrande L, Tarquinio SB. Stem cell-based pulp tissue engineering: variables enrolled in translation from the bench to the bedside, a systematic review of literature. Int Endod J. 2016;49(6):543–50. doi: 10.1111/iej.12489. [DOI] [PubMed] [Google Scholar]
- 35.Lolato A, Bucchi C, Taschieri S, Kabbaney AE, Fabbro MD. Platelet concentrates for revitalization of immature necrotic teeth: a systematic review of the clinical studies. Platelets. 2016;27(5):383–92. doi: 10.3109/09537104.2015.1131255. [DOI] [PubMed] [Google Scholar]
- 36.Meschi N, Castro AB, Vandamme K, Quirynen M, Lambrechts P. The impact of autologous platelet concentrates on endodontic healing: a systematic review. Platelets. 2016;27(7):613–33. doi: 10.1080/09537104.2016.1226497. [DOI] [PubMed] [Google Scholar]
- 37.Ragab RA, Abellatif AE, Eldokky NA. Regeneration of Necrotic ImmatureTeeth: Systematic Review. Indian Journal of Science and Technology. 2016;9(40):1–15. [Google Scholar]
- 38.Bucchi C, Valdivia-Gandur I, Sánchez-Bizjak R, Tallón-Walton V, Manzanares-Céspedes C. Regenerative endodontic therapy: a systematic review of clinical protocols. Int J Clin Exp Med. 2017;10(3):2006–15. [Google Scholar]
- 39.Duggal M, Tong HJ, Al-Ansary M, Twati W, Day PF, Nazzal H. Interventions for the endodontic management of non-vital traumatised immature permanent anterior teeth in children and adolescents: a systematic review of the evidence and guidelines of the European Academy of Paediatric Dentistry. Eur Arch Paediatr Dent. 2017;18(3):139–51. doi: 10.1007/s40368-017-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahler B, Rossi-Fedele G, Chugal N, Lin LM. An Evidence-based Review of the Efficacy of Treatment Approaches for Immature Permanent Teeth with Pulp Necrosis. J Endod. 2017;43(7):1052–7. doi: 10.1016/j.joen.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Nicoloso GF, Potter IG, Rocha RO, Montagner F, Casagrande L. A comparative evaluation of endodontic treatments for immature necrotic permanent teeth based on clinical and radiographic outcomes: a systematic review and meta-analysis. Int J Paediatr Dent. 2017;27(3):217–27. doi: 10.1111/ipd.12261. [DOI] [PubMed] [Google Scholar]
- 42.Tong HJ, Rajan S, Bhujel N, Kang J, Duggal M, Nazzal H. Regenerative Endodontic Therapy in the Management of Nonvital Immature Permanent Teeth: A Systematic Review-Outcome Evaluation and Meta-analysis. J Endod. 2017;43(9):1453–64. doi: 10.1016/j.joen.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Torabinejad M, Nosrat A, Verma P, Udochukwu O. Regenerative Endodontic Treatment or Mineral Trioxide Aggregate Apical Plug in Teeth with Necrotic Pulps and Open Apices: A Systematic Review and Meta-analysis. J Endod. 2017;43(11):1806–20. doi: 10.1016/j.joen.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 44.Chisini LA, Grazioli G, Francia A, San Martin AS, Demarco FF, Conde MCM. Revascularization versus apical barrier technique with mineral trioxide aggregate plug: A systematic review. Giornale italiano di endodonzia. 2018;32(1):9–16. [Google Scholar]
- 45.Metlerska J, Fagogeni I, Nowicka A. Efficacy of Autologous Platelet Concentrates in Regenerative Endodontic Treatment: A Systematic Review of Human Studies. J Endod. 2019;45(1):20–30 e1. doi: 10.1016/j.joen.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Murray PE. Platelet-Rich Plasma and Platelet-Rich Fibrin Can Induce Apical Closure More Frequently Than Blood-Clot Revascularization for the Regeneration of Immature Permanent Teeth: A Meta-Analysis of Clinical Efficacy. Front Bioeng Biotechnol. 2018;6:139. doi: 10.3389/fbioe.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.do Couto AM, Espaladori MC, Leite APP, Martins CC, de Aguiar MCF, Abreu LG. A Systematic Review of Pulp Revascularization Using a Triple Antibiotic Paste. Pediatr Dent. 2019;41(5):341–53. [PubMed] [Google Scholar]
- 48.Nicoloso GF, Goldenfum GM, Pizzol T, Scarparo RK, Montagner F, de Almeida Rodrigues J, Casagrande L. Pulp Revascularization or Apexification for the Treatment of Immature Necrotic Permanent Teeth: Systematic Review and Meta-Analysis. J Clin Pediatr Dent. 2019;43(5):305–13. doi: 10.17796/1053-4625-43.5.1. [DOI] [PubMed] [Google Scholar]
- 49.Rossi-Fedele G, Kahler B, Venkateshbabu N. Limited Evidence Suggests Benefits of Single Visit Revascularization Endodontic Procedures - A Systematic Review. Braz Dent J. 2019;30(6):527–35. doi: 10.1590/0103-6440201902670. [DOI] [PubMed] [Google Scholar]
- 50.Koc S, Del Fabbro M. Does the Etiology of Pulp Necrosis Affect Regenerative Endodontic Treatment Outcomes? A Systematic Review and Meta-analyses. J Evid Based Dent Pract. 2020;20(1):101400. doi: 10.1016/j.jebdp.2020.101400. [DOI] [PubMed] [Google Scholar]
- 51.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 52.Barends E, Rousseau DM B BR. CEBMa Guideline for Critically Appraised Topics in Management and Organizations. Amsterdam: The Center for Evidence-Based Management; 2017. [Google Scholar]
- 53.Mohammadi Z, Jafarzadeh H, Shalavi S, Yaripour S, Sharifi F, Kinoshita JI. A Review on Triple Antibiotic Paste as a Suitable Material Used in Regenerative Endodontics. Iran Endod J. 2018;13(1):1–6. doi: 10.22037/iej.v13i1.17941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Setzer FC, Kim S. Comparison of Long-term Survival of Implants and Endodontically Treated Teeth. J Dent Res. 2014;93(1):19–26. doi: 10.1177/0022034513504782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feigin K, Shope B. Regenerative Endodontics. J Vet Dent. 2017;34(3):161–78. doi: 10.1177/0898756417722022. [DOI] [PubMed] [Google Scholar]
- 56.Guerrero F, Mendoza A, Ribas D, Aspiazu K. Apexification: A systematic review. J Conserv Dent. 2018;21(5):462–5. doi: 10.4103/JCD.JCD_96_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knorr A, Boldrin Mestieri L, Pinheiro LS, Mendes RA, Gonzalez Hernandez PA, Barletta FB, Grecca FS. Cytotoxicity and Bioactivity of Calcium Silicate-based Cements in a Culture of Stem Cells from the Apical Papilla. Iran Endod J. 16(4):225–31. doi: 10.22037/iej.v16i4.30747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen SJ, Chen LP. Radiographic outcome of necrotic immature teeth treated with two endodontic techniques: A retrospective analysis. Biomed J. 2016;39(5):366–71. doi: 10.1016/j.bj.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rafter M. Apexification: a review. Dent Traumatol. 2005;21(1):1–8. doi: 10.1111/j.1600-9657.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 60.Torabinejad M, Parirokh M, Dummer PMH. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part II: other clinical applications and complications. Int Endod J. 2018;51(3):284–317. doi: 10.1111/iej.12843. [DOI] [PubMed] [Google Scholar]
- 61.Yamada Y, Nakamura-Yamada S, Kusano K, Baba S. Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. Int J Mol Sci. 2019;20:5. doi: 10.3390/ijms20051132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saidi A, Naaman A, Zogheib C. Accuracy of Cone-beam Computed Tomography and Periapical Radiography in Endodontically Treated Teeth Evaluation: A Five-Year Retrospective Study. J Int Oral Health. 2015;7(3):15–9. [PMC free article] [PubMed] [Google Scholar]
- 63.Campello AF, Gonçalves LS, Guedes FR, Marques FV. Cone-beam computed tomography versus digital periapical radiography in the detection of artificially created periapical lesions: A pilot study of the diagnostic accuracy of endodontists using both techniques. Imaging Sci Dent. 2017;47(1):25–31. doi: 10.5624/isd.2017.47.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maia Filho EM, Calisto AM, De Jesus Tavarez RR, de Castro Rizzi C, Bezerra Segato RA, Bezerra da Silva LA. Correlation between the Periapical Index and Lesion Volume in Cone-beam Computed Tomography Images. Iran Endod J. 2018;13(2):155–8. doi: 10.22037/iej.v13i2.15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alobaid AS, Cortes LM, Lo J, Nguyen TT, Albert J, Abu-Melha AS, Lin LM, Gibbs JL. Radiographic and Clinical Outcomes of the Treatment of Immature Permanent Teeth by Revascularization or Apexification: A Pilot Retrospective Cohort Study. J Endod. 2014;40(8):1063–70. doi: 10.1016/j.joen.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fuks AB, Nuni E. Pulp therapy for the young permanent dentition. Pediatric Dentistry: Elsevier; 2019: 482–96. [Google Scholar]
- 67.Lee L-W, Hsiao S-H, Chang C-C, Chen L-K. Duration for Apical Barrier Formation in Necrotic Immature Permanent Incisors Treated With Calcium Hydroxide Apexification Using Ultrasonic or Hand Filing. J Formos Med Assoc. 2010;109(8):596–602. doi: 10.1016/S0929-6646(10)60097-6. [DOI] [PubMed] [Google Scholar]
- 68.Silujjai J, Linsuwanont P. Treatment Outcomes of Apexification or Revascularization in Nonvital Immature Permanent Teeth: A Retrospective Study. J Endod. 2017;43(2):238–45. doi: 10.1016/j.joen.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 69.Shah D, Lynd T, Ho D, Chen J, Vines J, Jung H-D, Kim J-H, Zhang P, Wu H, Jun H-W, Cheon K. Pulp–Dentin Tissue Healing Response: A Discussion of Current Biomedical Approaches. J Clin Med. 2020;9(2):434. doi: 10.3390/jcm9020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nageh M, Ahmed GM, El-Baz AA. Assessment of Regaining Pulp Sensibility in Mature Necrotic Teeth Using a Modified Revascularization Technique with Platelet-rich Fibrin: A Clinical Study. J Endod. 2018;44(10):1526–33. doi: 10.1016/j.joen.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 71.Meschi N, Hilkens P, Lambrichts I, Van den Eynde K, Mavridou A, Strijbos O, De Ketelaere M, Van Gorp G, Lambrechts P. Regenerative endodontic procedure of an infected immature permanent human tooth: an immunohistological study. Clin Oral Investig. 2016;20(4):807–14. doi: 10.1007/s00784-015-1555-8. [DOI] [PubMed] [Google Scholar]
- 72.Roshene R. Regeneration of dental pulp-A review. J Pharm Sci. 2015;7(10):858–60. [Google Scholar]
- 73.Kahler B, Hu JY, Marriot-Smith C, Heithersay G. Splinting of teeth following trauma: a review and a new splinting recommendation. Aust Dent J. 2016;61(Suppl 1):59–73. doi: 10.1111/adj.12398. [DOI] [PubMed] [Google Scholar]
- 74.Farhad Mollashahi N, Saberi E, Karkehabadi H. Evaluation of Cytotoxic Effects of Various Endodontic Irrigation Solutions on the Survival of Stem Cell of Human Apical Papilla. Iran Endod J. 2016;11(4):293–7. doi: 10.22037/iej.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaur M. MTA versus Biodentine: Review of Literature with a Comparative Analysis. J Clin Diagn Res. 2017;11(8):ZG01–ZG5. doi: 10.7860/JCDR/2017/25840.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solanki NP, Venkappa KK, Shah NC. Biocompatibility and sealing ability of mineral trioxide aggregate and biodentine as root-end filling material: A systematic review. J Conserv Dent. 2018;21(1):10–5. doi: 10.4103/JCD.JCD_45_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aly MM, Taha SEE, El Sayed MA, Youssef R, Omar HM. Clinical and radiographic evaluation of Biodentine and Mineral Trioxide Aggregate in revascularization of non-vital immature permanent anterior teeth (randomized clinical study) Int J Paediatr Dent. 2019;29(4):464–73. doi: 10.1111/ipd.12474. [DOI] [PubMed] [Google Scholar]
- 78.Kahler B, Chugal N, Lin L. Alkaline Materials and Regenerative Endodontics: A Review. Materials (Basel) 2017;10(12):1389. doi: 10.3390/ma10121389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bakhtiar H, Mazidi S A, Mohammadi Asl S, Ellini MR, Moshiri A, Nekoofar MH, Dummer PMH. The role of stem cell therapy in regeneration of dentine-pulp complex: a systematic review. Prog Biomater. 2018;7(4):249–68. doi: 10.1007/s40204-018-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saoud T, Ricucci D, Lin L, Gaengler P. Regeneration and Repair in Endodontics A Special Issue of the Regenerative Endodontics A New Era in Clinical Endodontics. Dent J (Basel) 2016;4(1) doi: 10.3390/dj4010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fouad AF, Verma P. Healing after regenerative procedures with and without pulpal infection. J Endod. 2014;40(4 Suppl):S58–64. doi: 10.1016/j.joen.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 82.Chand V, Joseph S, George J, John M, Anand S, Nair M. Regenerative Endodontics-Treatment options and challenges to success. Int J Oral Care Res. 2015;3(1):89–95. [Google Scholar]
- 83.Mukherjee P, Patel A, Chandak M, Kashikar R. Minimally Invasive Endodontics a Promising Future Concept: A Review Article. Int J Sci Stud. 2017;5(1):245–51. [Google Scholar]