Abstract
Excessive daytime sleepiness is a recognized non-motor symptom that adversely impacts the quality of life of people with Parkinson’s disease (PD), yet effective treatment options remain limited. Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is an effective treatment for PD motor signs. Reliable daytime sleep-wake classification using local field potentials (LFPs) recorded from DBS leads implanted in STN can inform the development of closed-loop DBS approaches for prompt detection and disruption of sleep-related neural oscillations. We performed STN DBS lead recordings in three nonhuman primates rendered parkinsonian by administrating neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Reference sleep-wake states were determined on a second-by-second basis by video monitoring of eyes (eyes-open, wake and eyes-closed, sleep). The spectral power in delta (1–4 Hz), theta (4–8 Hz), low-beta (8–20 Hz), high-beta (20–35 Hz), gamma (35–90), and high-frequency (200–400 Hz) bands were extracted from each wake and sleep epochs for training (70% data) and testing (30% data) a support vector machines classifier for each subject independently. The spectral features yielded reasonable daytime sleep-wake classification (sensitivity: 90.68±1.28; specificity: 88.16±1.08; accuracy: 89.42±0.68; positive predictive value; 88.70±0.89, n=3). Our findings support the plausibility of monitoring daytime sleep-wake states using DBS lead recordings. These results could have future clinical implications in informing the development of closed-loop DBS approaches for automatic detection and disruption of sleep-related neural oscillations in people with PD to promote wakefulness.
Keywords: Parkinson’s Disease, Sleep-Wake Disturbances, Daytime Sleepiness, Subthalamic Nucleus, Nonhuman Primates, Support Vector Machines, Deep Brain Stimulation, MPTP
Introduction
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is an effective treatment option for Parkinson’s disease (PD) motor signs i.e., rigidity, tremor, bradykinesia, and akinesia (Anderson et al., 2017; Mao et al., 2019; Zhang et al., 2021). Current DBS approaches are typically not optimized to treat non-motor symptoms of PD, however. Sleep-wake disturbances, which include insomnia, sleep fragmentation, REM sleep behavior disorder, and excessive daytime sleepiness, are debilitating non-motor symptoms of PD (Baumann, 2019; Bruin et al., 2012; Suzuki et al., 2015, 2011). The propensity to fall asleep unintentionally within a matter of seconds while conducting daily routines is a major factor impacting the quality of life of some people with PD (Frucht et al., 1999; Hobson et al., 2002; Salawu and Olokoba, 2015; Yeung and Cavanna, 2014), yet an effective treatment option remains elusive for excessive daytime sleepiness (Rodrigues et al., 2016). Automatic detection of sleep-related neural oscillations from DBS lead sensing can inform DBS optimization for prompt detection and disruption of sleep-related neural oscillations which can potentially aid management of daytime sleepiness in people with PD. In this study, as a critical first step, we aim to test whether daytime sleep-wake states can be reliably differentiated using short-duration (1-second long epochs) STN local field potentials (LFPs) recorded from DBS leads.
The STN is a key input nucleus of the basal ganglia which is involved in the regulation of sleep-wake behavior through its distinct anatomical connection with the brainstem, thalamic, and cortical structures (Castillo and Benarroch, 2020; Hasegawa et al., 2020; Lazarus et al., 2013; Vetrivelan et al., 2010). For this reason, DBS of the STN has been explored as a treatment for sleep-wake disturbances (Amara et al., 2017, 2011; Baumann-Vogel et al., 2017; Deli et al., 2015; Sharma et al., 2018; Zuzuarregui and Ostrem, 2020), though the efficacy of traditional continuous high-frequency STN DBS on objective outcomes of sleep-wake states has been inconsistent (Amara et al., 2017; Baumann-Vogel et al., 2017; Iranzo, 2002; Yin et al., 2021). To overcome this limitation, there is an increasing interest in identifying putative neurophysiological biomarkers of sleep-wake states that can support DBS optimization to deliver sleep-wake dependent stimulation (closed-loop DBS) and potentially improve the therapeutic efficacy of STN DBS (Baumgartner et al., 2021). To this end, several studies have examined the potential of DBS lead recordings of STN LFPs for sleep stage classification (Baumgartner et al., 2021; Chen et al., 2019; Christensen et al., 2019; Thompson et al., 2018). To what extent short-duration STN LFP recorded from DBS lead can facilitate daytime sleep-wake classification has not been explored, despite data suggesting daytime sleep-wake states can modulate subthalamic LFPs (Devergnas et al., 2014; Escobar Sanabria et al., 2017).
Given people with PD may fall asleep suddenly (sleep attack) during the daytime (Hirayama et al., 2008; Hobson et al., 2002; Körner et al., 2004; Yeung and Cavanna, 2014), it is imperative to conceptualize closed-loop DBS strategies engineered to sense patients’ daytime sleep-wake states continuously to facilitate prompt detection and disruption of sleep-related neural oscillations. A critical first step towards the realization of effective closed-loop DBS strategies would be to test the feasibility of daytime sleep-wake state classification using short duration (e.g.,1-sec long) STN LFP recorded from DBS lead (see Figure 1). We extracted delta (1–4 Hz), theta (4–8 Hz), low-beta (8–20 Hz), high-beta (20–35 Hz), gamma (35–90 Hz), and high-frequency (HF, 200–400 Hz) LFP power of each sleep-wake epochs as features. These features were used to train and test a support vector machines (SVM) classifier for differentiating daytime sleep-wake states. This approach is based on other studies demonstrating the promise of machine learning-based personalized closed-loop DBS strategies (Bore et al., 2020; Connolly et al., 2015; Houston et al., 2019; Neumann and Rodriguez-Oroz, 2021). In this study, we sought to determine whether short-duration STN LFP can differentiate daytime sleep-wake states reliably. Moreover, we aimed to characterize whether subthalamic spectral features modulate similarly or differentially across subjects with sleep-wake states and whether the features that facilitate sleep-wake classification are consistent across subjects. The study findings will inform the development of on-demand, subject-specific STN DBS approaches to mitigate excessive daytime sleepiness while also treating typical motor signs in people with PD.
Figure 1.
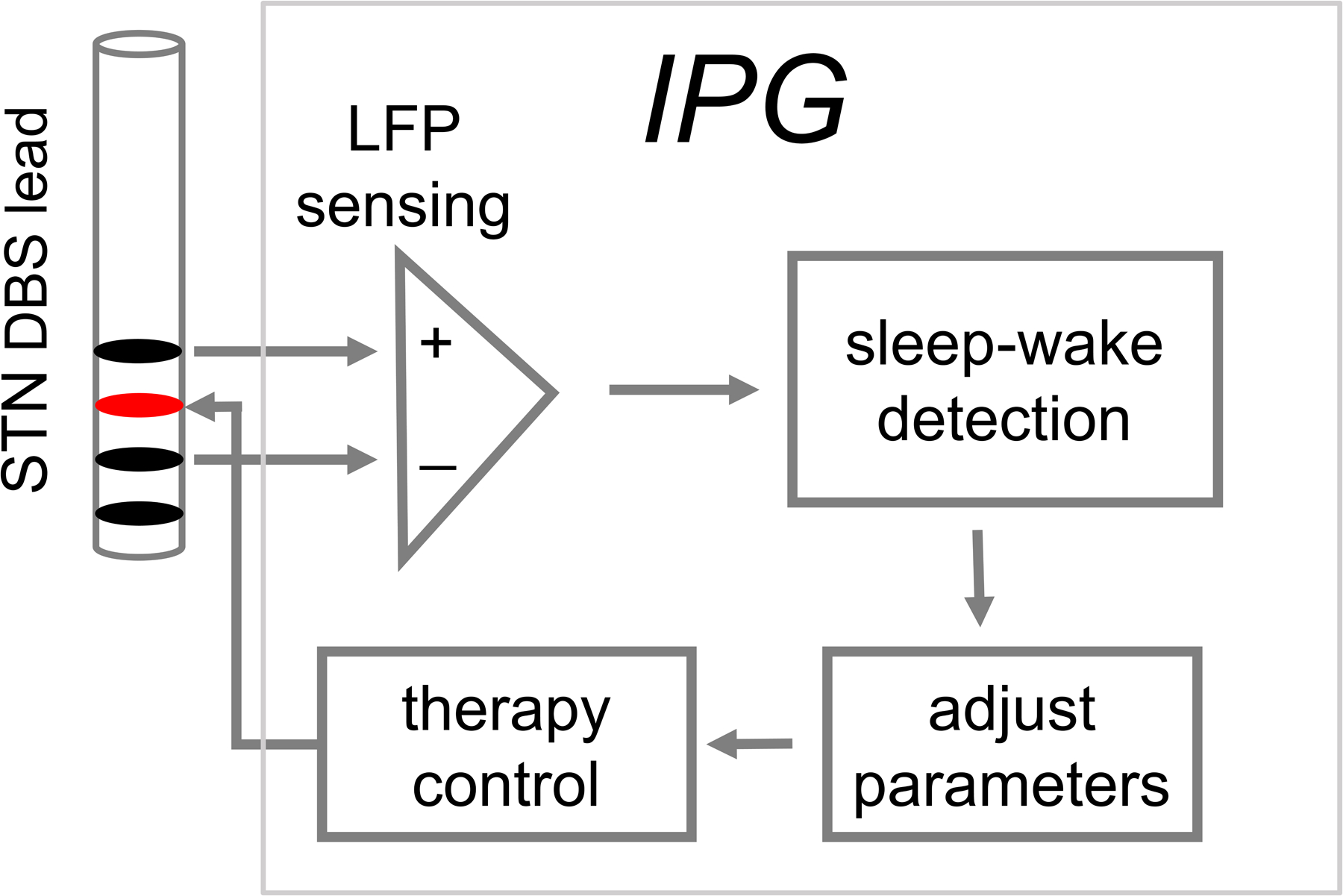
An example of a closed-loop DBS paradigm for automatic detection and disruption of sleep-related neural oscillations. A major first step towards the realization of this paradigm will be a reliable identification of daytime sleep-wake states using DBS lead sensing. This manuscript examines the plausibility of classifying daytime sleep-wake states using DBS lead recorded STN local field potentials.
Methods
Experimental Protocol and Data Recording
All procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee and complied with the US Public Health Service policy on the humane care and use of laboratory animals Three adult female rhesus macaque NHPs (Subject P, 18 years; Subject J, 16 years; and Subject B, 23 years) were used in this study. All subjects were implanted with two 8-channels scaled-down versions of a human DBS leads (0.5 mm contact height, 0.5 mm inter contact spacing, 0.625 mm diameter, NuMed) targeting the STN and globus pallidus internus (GPi). Details of surgical procedures are described in previous publications (Escobar Sanabria et al., 2017; Yu et al., 2021). In addition to DBS leads, Subjects J and P were instrumented with a 96-channel Utah array (Blackrock) to record neural activity from the primary motor cortex (MC), while Subject B was instrumented with 96 channel Microdrive (Gray Matter Research) with microelectrodes targeting basal ganglia, motor thalamus, and motor cortices.
Neural recordings were performed while subjects were seated on a primate chair with their head fixed. A Tucker Davis Technology (TDT) workstation was used for continuously recording the neural data sampled at ~24,000 Hz. Once the normal state data collection was completed, the subject was rendered moderately parkinsonian by administrating the neurotoxin compound 1-methyl-4-phenyl-1,2,3,6, tetrahydropyridine (MPTP). The subject’s parkinsonian state was determined by the modified Unified Parkinson’s Disease Rating Scale (mUPDRS), which involved the rating of axial motor symptoms (gait, posture, balance, turning, and defense reaction) and rigidity, bradykinesia, akinesia, and tremor (for arms and legs) using a 0–3 scale (0=normal and 3=severe) with a maximum total score=42 (Yu et al., 2021). The severity of the parkinsonian state was determined as mild, mUPDRS<18; moderate, mUPDRS 18–31; and severe, mUPDRS≥32 (Yu et al., 2021). Subject J received three intramuscular (IM) injections on consecutive days to achieve a mild parkinsonian state and one intracarotid injection to advance the subject’s parkinsonian state to moderate. Subject P and Subject B were rendered parkinsonian progressively by administering IM injections weekly or biweekly (0.2–0.8 mg/Kg). Subject P received 27 IM injections while Subject B received 42 IM injections to attain moderate parkinsonian state. The results presented in the manuscript are from 23 sessions for subject P (9 normal, 14 post-MPTP), 27 sessions from subject J (10 normal and 17 post-MPTP), and 18 sessions from subject B (10 normal, 8 post-MPTP).
Data Analysis
The reference daytime sleep or wake state of the subject was determined via a video camera tracking the opening and closing of the eyes. A video camera (sampling at 30 frames/second for Subject J and P, and 20 frames/second for Subject B) was focused on the eyes of the subject and synchronized to the neural recording system. A Custom MATLAB image segmentation algorithm was used for analyzing the video of one eye and calculating the eye-opening area for each video frame. The processing of the algorithm is detailed in our previous work (Escobar Sanabria et al., 2017). The video frame with an eye area greater than 0.5 was considered an indicator of an awake state while the video frame with an eye area less than 0.2 was considered an indicator of a sleep state. Using this approach, the wake (eyes-open) and sleep (eyes-closed) epochs were determined on a second-by-second basis. This approach is similar to literature where eye-opening and closing have been used for the identification of daytime sleep episodes (Kratzel et al., 2021) as well as used as reference vigilance states to guide algorithm development for automatic detection of sleep-wake states from electroencephalogram (EEG) (Nguyen et al., 2017). In addition to video monitoring of the eyes, the power in the delta (1–4 Hz) band of MC was also calculated during eyes-open and eyes-closed epochs to verify that there was a significant increase in low-frequency MC power during the eyes-closed condition compared to eyes-open. For Subject P and J, the resultant neural activity in MC was obtained by averaging all channels (after discarding non-functional noisy channels) of the Utah array and for Subject B a channel in MC was used for analysis. In all subjects’ delta power was higher (p<0.001) during eyes-closed condition compared to eyes-open.
The DBS lead location was estimated by fusing MRI and CT scans and the contact pairs spanning STN were identified. The neural oscillations from the selected contacts were bandpass filtered from 1–900 Hz using an IIR Butterworth filter of order 3 and then resampled to ~2000 Hz. The local field potentials (LFPs) were obtained by subtracting the neural recordings from adjacent contacts of the DBS lead. The DBS lead contact pair demonstrating the highest therapeutic benefit to PD motor signs for each subject was selected for further analysis.
The power spectral density (PSD) of LFP was calculated using the Welch method with a window size of 1024 samples, 50% overlap, and 4098 FFTs resulting in a frequency resolution of 0.49 Hz. In Subject P LFP normalization was required to compare pre-MPTP and post-MPTP PSD (due to change in the ground) hence the LFP was scaled to have unit standard deviation before PSD computation. For each recording session, trapezoidal integration of the PSD was performed to obtain total power in delta (1–4 Hz), theta (4–8 Hz), low-beta (8–20 Hz), high-beta (20–35 Hz), gamma (35–90 Hz), and HF (200–400 Hz) bands of STN LFP on a second-by-second basis. The resultant time series (power in the respective band) was smoothed by an order 4 filter before parsing out the epochs which belonged to wake (eyes-open) and sleep (eyes-closed) periods as determined by the video assessment of subject’s eye. We observed that each recording session (typically five minutes in duration) usually had more wake epochs than sleep. To limit session-to-session variability the number of wake epochs was matched to sleep epochs for each session i.e., selecting only the first 20 wake epochs for comparison if there were 20 sleep epochs detected for a respective session and vice-versa. All available wake state data were used for comparing normal and post-MPTP wake spectral power in respective frequency bands.
The spectral features derived from 1-sec long sleep/wake epochs from Subject P (n=674), Subject J (n=518), and Subject B (n=668) were then used for training and testing a support vector machines (SVM) classifier (linear kernel) to determine the feasibility of daytime sleep-wake classification using short-duration LFPs recorded using the DBS lead. A hold-out cross-validation technique was employed to train (70% of the data) and test (30% of the data) an SVM classifier for each subject independently. This process was repeated 15 times so that different subsets of data accumulated across sessions can be part of training and test sets. For each iteration true positive (TP), true negative (TN), false positive (FP), and false negative (FN) observations were noted from which the performance of the SVM classifier for differentiating sleep (+ve class) and wake (−ve class) state was determined by calculating , , , and . Moreover, for each subject 10-fold cross-validation (SVM, linear kernel) using each spectral feature was performed and the area under the receiver operating characteristic (ROC) curve was noted. Based on this metric, the contribution of specific spectral features in relation to all features for sleep-wake classification was determined for each subject. All data analysis reported in this manuscript was performed using MATLAB (Mathworks Inc., Natick, MA) inbuilt functions (version 2020b).
Statistical Analysis
Normality was not assumed, and the Wilcoxon rank-sum (WRS) test was performed to report statistical results. Kruskal-Wallis test was employed when more than two variables were compared followed by post-hoc analysis using the Tukey-HSD method. Results in the manuscript are presented as mean±2.58×SEM (i.e., mean±99% confidence interval) unless stated otherwise. Reported p-values are an outcome of the WRS or Kruskal-Wallis test unless stated otherwise. Statistical tests were performed using the statistical toolbox of MATLAB (Mathworks Inc., Natick, MA). The test results were considered significant at p<0.05.
Results
Effect of MPTP (parkinsonism) on STN power spectrum and daytime sleep-wake behavior:
The data reported in this study were obtained from normal and moderately parkinsonian (mUPDRS>18) NHP subjects. The effect of parkinsonism on the STN power spectrum for each subject is summarized in Figure 2. The wake state low-beta (8–20 Hz) power increased in all subjects following MPTP administration. Furthermore, delta (1–4 Hz) and HF (200–400 Hz) power was lower during wake in parkinsonian state compared to normal in all subjects (Figure 2). In the parkinsonian state compared to normal, daytime sleep increased in Subject P (p<0.001) and Subject B (p<0.001), but not in Subject J (p=0.46), Figure 3.
Figure 2.
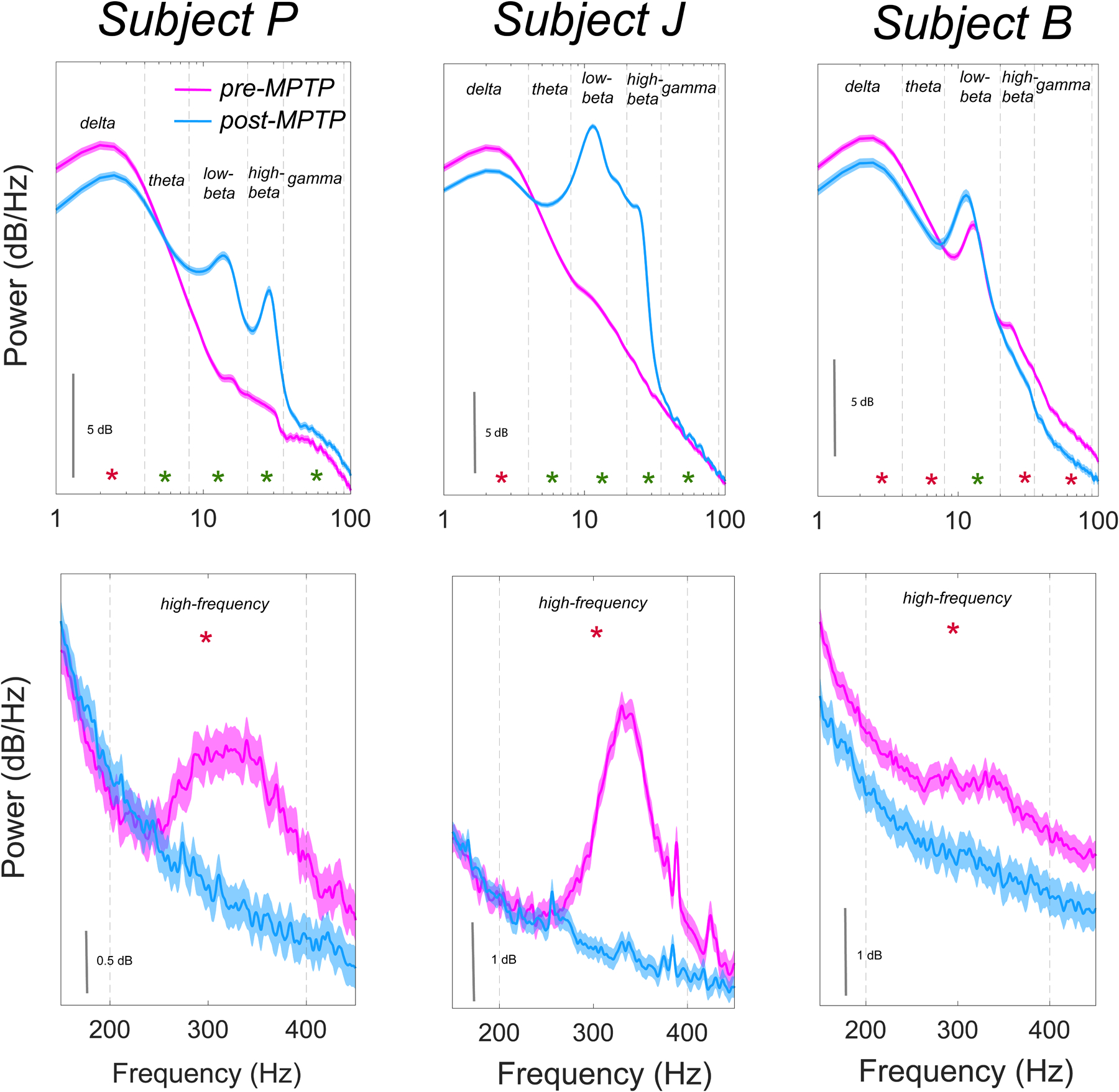
Effect of induction of parkinsonism with MPTP on the STN PSD (1–100 Hz; top and 150–450 Hz; bottom) for respective subjects during wake. A significant difference between pre-MPTP and post-MPTP STN spectral power in respective frequency bands is highlighted with * (green; increase in power and red; decrease in power compared to pre-MPTP state; p<0.001). MPTP administration led to spectral power decrease in delta (1–4 Hz) and high-frequency (200–400 Hz) bands in all subjects and an increase in low-beta (8–20 Hz) band in all subjects.
Figure 3.
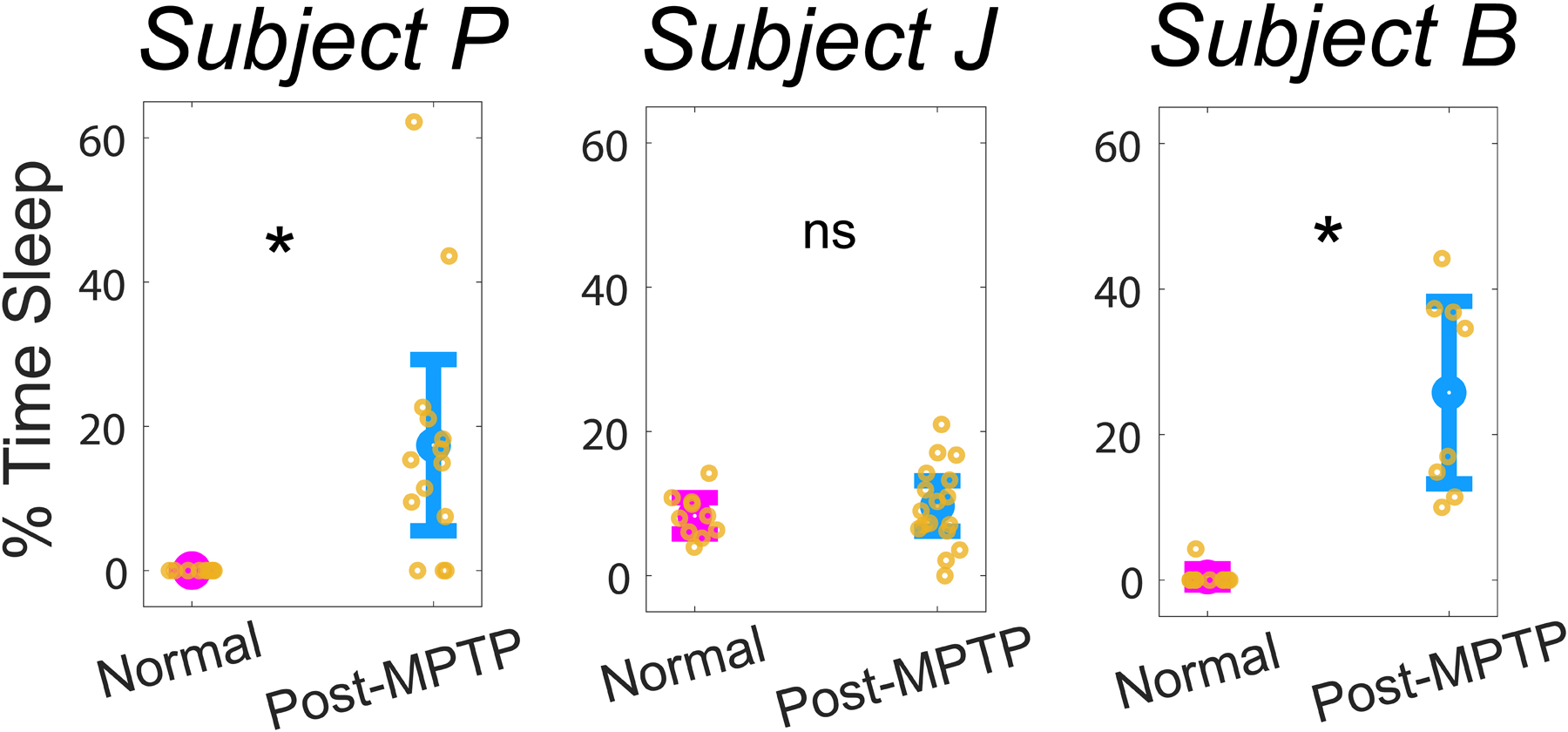
Behavioral changes in daytime sleep after MPTP administration. * Represents a significant increase (p<0.001) in daytime sleepiness which was observed in Subject P and Subject B, but not in Subject J (p=0.46). The percentage of time subject was asleep during each recording session is highlighted with a yellow circular marker.
Effect of parkinsonian daytime sleep on STN power spectrums:
The trial average PSDs for sleep and wake states for each subject is shown in Figure 4 A and the means for respective spectral features are summarized in Figure 4 B. All subjects showed an increase (p<0.001) in delta and theta power during sleep compared to wake. Also, high-beta, gamma and HF power was observed to decrease (p<0.001) in all subjects during sleep compared to wake. No change (p=0.24) in low-beta power was observed during sleep compared to wake in Subject P. Low-beta power decreased (p<0.001) in Subject J but increased (p<0.001) in Subject B during sleep compared to wake.
Figure 4.
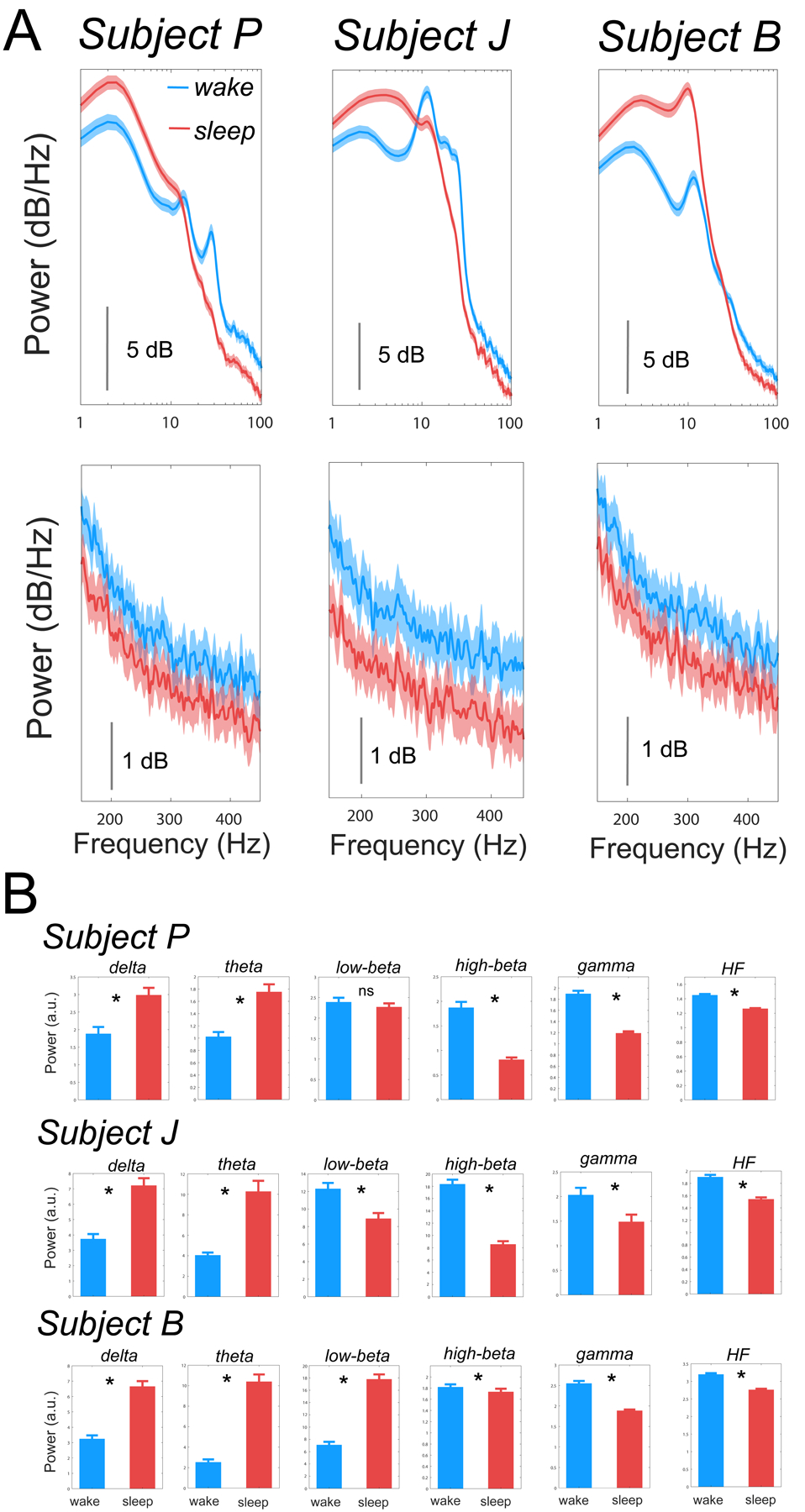
The effect of sleep-wake state on the STN PSD (1–100 Hz; top and 150–450 Hz; bottom) is shown in A. Means of respective spectral features for all subjects during wake and sleep states are summarized in B. Bouts of daytime sleep resulted in subject-specific changes in power in the low-beta (8–20 Hz) band but consistent increase in delta (1–4 Hz) and theta (4–8 Hz) bands and decrease in high-beta (20–35 Hz), gamma (35–90 Hz), and HF (200–400 Hz) bands. * Represents significant difference and ns represents non-significant difference between wake and sleep power in respective frequency bands.
Daytime sleep-wake classification using STN LFPs:
The power of STN LFPs in delta, theta, low-beta, high-beta, gamma and HF bands for respective subjects were used as a feature for sleep-wake classification. We obtained reasonable sensitivity (SE, Subject P: 90.87±1.60, Subject J: 94.50±1.14, Subject B: 86.66±1.10), specificity (SP, Subject P: 80.56±1.13, Subject J: 95.52±1.12, Subject B: 88.40±1.00), accuracy (ACC, Subject P: 85.70±0.61, Subject J: 95.02±0.78, Subject B: 87.56±0.66), and positive predictive value (PPV, Subject P: 82.38±0.70, Subject J: 95.51±1.08, Subject B: 88.22±0.88). The distribution of SE, SP, ACC, and PPV for each subject is summarized in Figure 5 A. The contribution of respective STN spectral features for sleep-wake classification in relation to all features is shown in Figure 5 B. The potential of respective spectral features for sleep-wake discrimination was variable across subjects. For example, In Subject P, gamma power was the most informative single feature, in Subject J, power in the HF band was most informative, while in Subject B, theta power was the most informative feature for sleep-wake discrimination.
Figure 5.
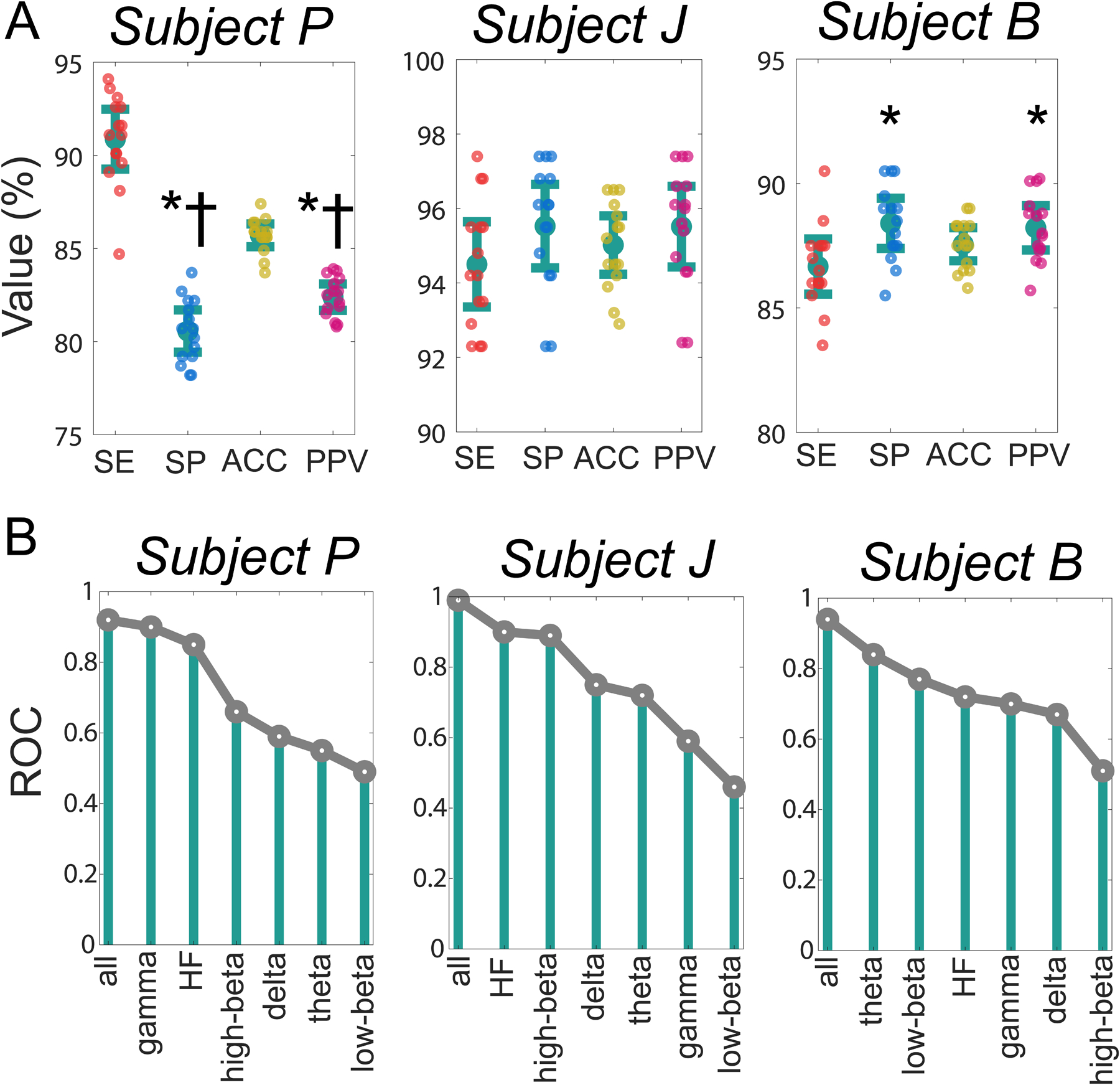
Performance of SVM classifier for differentiating daytime sleep-wake states on 70% training and 30% testing data for each iteration. Classifier sensitivity (SE), specificity (SP), accuracy (ACC), and positive predictive value (PPV) are reported as a measure of SVM classifier performance in A. * and † represent a significant difference from SE and ACC, respectively. The utility of each feature for sleep-wake classification in relation to all features using 10-fold cross-validation ROC is presented in B. Compared to all features, the feature contributing most to daytime sleep-wake classification was subject-specific.
Discussion
Classification of daytime sleep-wake states using STN LFPs
The neural underpinnings of daytime sleep-wake states have been largely informed by the cortical neural oscillations recorded using surface EEG (Balandong et al., 2018; Breitenbach et al., 2020; Qian et al., 2017; Skorucak et al., 2020). Despite recognizing that daytime sleepiness is common in people with PD, the neurophysiology of daytime sleep in parkinsonism is not properly understood. Only limited studies have attempted to understand brain oscillations during daytime sleep in parkinsonism (Devergnas et al., 2014; Escobar Sanabria et al., 2017; Urrestarazu et al., 2009). Moreover, to what extent the signatures of daytime sleep-wake states can be determined reliably from STN LFPs recorded using DBS lead remains unclear. An improved understanding of neurophysiological biomarkers of sleep-wake states derived from DBS lead sensing can inform DBS approaches for automatic detection and disruption of sleep-related neural oscillations. The primary finding of this study was a reasonable (accuracy>0.85 in all subjects) daytime sleep-wake discrimination capability of features extracted from short-duration (1-sec long) LFPs recorded from the DBS lead. Moreover, we observed across subject variability in the effectiveness of STN spectral features towards daytime sleep-wake classification. The findings of this study support the idea of optimizing DBS strategies in the future to sense subjects’ sleep-wake states (via subject-specific features) continuously and deliver sleep-wake dependent stimulation to disrupt sleep-related neural oscillations and promote daytime wakefulness in people with PD.
Our observation of considerable across-subject variability in wake power spectrum in parkinsonian state was similar to prior studies (Aman et al., 2020; Connolly et al., 2015; Yu et al., 2021) and guided our decision to train and test the SVM classifier independently for each subject (Figure 4 A). This approach was further motivated by previous studies showing superior performance of a classifier trained with subject-specific spectral features as opposed to features unified across subjects (Chen et al., 2019). For each subject, the wake and daytime sleep detection rate using a 1-second-long epoch was reasonable (>85%), which supported our hypothesis of the plausibility of daytime sleep-wake state classification using DBS lead recorded short-duration STN LFP. However, in Subject P while the sleep detection rate (SE) was high, the positive predictive value (PPV) was lower than SE suggesting the misclassification of wake epochs as sleep. Furthermore, in Subject B, we observed that the wake detection rate was slightly better than the sleep detection rate (Figure 5 A). In this study for simplicity, the SVM classifier was trained with a linear kernel. Given the variability in the distribution of features across subject future studies could compare the performance of other SVM kernels (e.g., quadratic, cubic, and Gaussian) to determine if kernel selection can yield more balanced sleep and wake detection rates. Although reasonable daytime sleep-wake classification was obtained for each subject (Figure 5 A), the contribution of respective spectral features towards sleep-wake discrimination was variable across subjects (Figure 5 B). This suggests the potential need for the incorporation of subject-specific trends to achieve robust sleep-wake classification.
STN beta band oscillations – relevance to motor signs and sleep
Beta oscillations are excessively synchronized in the parkinsonian state and are believed to be associated with daytime motor complications since the suppression of beta oscillations generally leads to improvement in motor behavior (Feldmann et al., 2022; Giannicola et al., 2010; Kuhn et al., 2008; Neumann et al., 2016; Oswal et al., 2013). These findings have led to increased interest in the development of beta-based closed-loop DBS approaches for managing parkinsonian symptoms (Bocci et al., 2021; Johnson et al., 2016; Little et al., 2013). Aligned with this thought, previous sleep studies performed in PD patients also emphasized the STN beta oscillations to understand its pattern of modulation across wake and sleep states (Thompson et al., 2018; Urrestarazu et al., 2009; van Rheede et al., 2022). These studies demonstrated a reduction in STN beta power during sleep (especially during non-REM sleep). Our data showed a reduction in STN high-beta power during daytime sleep compared to wake in all subjects, while low-beta power was reduced during sleep compared to wake in Subject J, did not change in Subject P, and increased in Subject B (Figure 4). To further understand the relative importance of respective STN spectral features in daytime sleep-wake classification, we studied the utility of each spectral feature in relation to all features for sleep-wake classification to understand if the features facilitating sleep-wake classification are unique to each subject or similar across subjects. High-beta power (Subjects P and J) and low-beta power (Subject B) was observed to be important discriminator of daytime sleep-wake states (Figure 5 B).
An explanation for the reduction in beta power during sleep may partly come from the hypothesis that sleep itself may alleviate PD motor signs (Askenasy and Yahr, 1990; Stefani et al., 2006) and therefore lead to a reduction in beta power. In Subject B, however, bouts of daytime sleep were associated with a marked increase in the low-beta power. Another study in the MPTP-treated NHPs (n=2) reported no change in STN beta power (10–17 Hz) during non-REM sleep compared to wake (Mizrahi-Kliger et al., 2020). Taken together, these observations point towards complex dynamics of beta oscillations relative to sleep and potential subject-specific variability in the polarity of sleep-related modulation. Future studies with a higher sample size are warranted to obtain a more generalized behavior regarding the patterns of beta oscillations in the STN across sleep-wake states and how it correlates with the underlying change in sleep-wake behavior.
Relevance of STN oscillatory activity in higher frequencies (gamma and HF)
STN gamma power decreased in all subjects during the episodes of daytime sleep compared to wake. Unlike beta, the pathophysiology of gamma oscillations in parkinsonism is less clear. We did not observe a consistent pattern of change in STN gamma oscillations following MPTP administration (power increased in Subject J and P but decreased in Subject B, Figure 2). Cortical gamma oscillations are associated with attention (Bauer, 2006; Jensen et al., 2007). Subthalamic gamma power is also shown to increase during conditions that require heightened attention for example initiating movement in response to a cue (Jenkinson et al., 2013; Joundi et al., 2012). A condition of reduced attention like daytime sleep may explain why STN gamma power was reduced in all subjects. Our findings were comparable to other sleep studies that have also reported reduced STN gamma power during NREM sleep in people with PD (Chen et al., 2019; Thompson et al., 2018).
The parkinsonian state was associated with a significant decrease in HF (200–400 Hz) STN power compared to normal. This change was most pronounced in Subject J and Subject P (Figure 2). Other studies in human patients have reported synchronization of HF STN oscillations during ON-medication and ON-DBS conditions suggesting the pathophysiological relevance of STN HF power (Foffani, 2003; Ozturk et al., 2021; Petersson et al., 2019) and providing a rationale for exploring the polarity of modulation of STN HF power with sleep-wake states. Only limited studies have explored the dynamics of subthalamic HF power during sleep; one study has reported a reduction in HF subthalamic power during NREM sleep in people with PD (Thompson et al., 2018), and a previous study from our group in NHPs also observed a reduction in HF power during 15 s epochs of daytime sleep (Escobar Sanabria et al., 2017). Our analysis found a reduction in HF power during daytime sleep in all subjects compared to wake. Furthermore, in relation to all features, the ranking of respective spectral features based on their relative importance for differentiating sleep-wake states showed HF STN power to be an important discriminator of sleep-wake states across subjects (Figure 5 B). This suggests HF power may have an association with vigilance and can be a reliable feature across subjects for sleep detection.
Although further studies are warranted to fully understand the functional role of HF oscillations in the basal ganglia, our results and others confirm that HF oscillations are responsive to vigilance and can have utility in the continuous monitoring of daytime sleep-wake states via DBS lead sensing. There is currently one commercial DBS device (Medtronic Percept) with DBS lead sensing capabilities to detect neural oscillations below 100 Hz (Cagle et al., 2021; Feldmann et al., 2022, 2021; Medtronic, 2020; Thenaisie et al., 2021; van Rheede et al., 2022). The findings of this study along with other studies in the literature showing the pathophysiological relevance and distinct sleep-wake modulation of basal ganglia HF oscillations (Aman et al., 2020; Foffani, 2003; Johnson et al., 2021; Tsiokos et al., 2013; Verma et al., 2022) provide a rationale for the development of DBS systems with sensing capability at higher frequencies (e.g. 100–400 Hz) to utilize HF oscillations as a potential biomarker in closed-loop DBS systems.
Excessive daytime sleepiness in the MPTP model of PD
In the parkinsonian state, daytime sleep was observed in all subjects. The increase in daytime sleep compared to the normal state was significant in Subjects P and B but surprisingly it was not significant in Subject J (Figure 3). Given previous reports of presence of daytime sleep in the prodromal stage of PD (Abbott et al., 2005; Barraud et al., 2009; Davin et al., 2022; Zhou et al., 2017), we expected that all subjects will exhibit a significant increase in daytime sleep in the parkinsonian state compared to normal. One explanation for this unexpected observation could be higher bouts of daytime sleep in Subject J in a normal state compared to Subject P and B (Figure 3). Secondly, the mode of induction of parkinsonism via MPTP administration was also different in Subject J (IC+IM) compared to Subjects P and B (low dose repeated IM injections). The slow low-dose administration of MPTP is shown to produce brain pathology beyond the dopaminergic system (Masilamoni and Smith, 2018) and hence could lead to sleep-wake disturbances in NHPs like PD patients as shown by other studies (Barraud et al., 2009; Belaid et al., 2014; Davin et al., 2022), which could explain why daytime sleep was more pronounced in Subjects P and B in the parkinsonian state compared to normal.
Limitations and Future Directions
Excessive daytime sleepiness that is observed in people with PD is multifactorial (Knie et al., 2011; Videnovic, 2018) and likely not fully replicated by the NHP MPTP model of PD. Future studies should perform basal ganglia recordings using DBS leads in PD patients during episodes of daytime sleep to better understands whether the features discussed in this manuscript can differentiate sleep-wake states and aid optimization of DBS to mitigate excessive daytime sleepiness in people with PD. Another limitation, as is common with NHP preclinical studies, was the limited number of study subjects. Nevertheless, within-subject comparison, as it was performed in this study, of behavioral changes in daytime sleepiness and STN power spectrum due to induction of parkinsonism is not feasible to perform in people with PD. Although we showed the plausibility of differentiating daytime sleep-wake states reliably using DBS lead recordings (accuracy>85% in all subjects), future work is needed for testing the real-time monitoring of daytime sleep-wake states using DBS lead sensing and the features discussed in this study. Additionally, a longitudinal study regarding classification stability and the extent to which the specific features (for example beta and HF power) contribute to the pathophysiology of excessive daytime sleepiness is required. This can be performed by selectively suppressing and facilitating beta and HF oscillations (Foffani, 2003; Holt et al., 2019; Ozturk et al., 2021; Sanabria et al., 2022, 2020), respectively via DBS or dopaminergic treatment and measure the effect on excessive daytime sleepiness.
Conclusion
The main finding of this study was the demonstration of daytime sleep-wake classification using LFPs recorded from DBS leads. The study also provides support for utilizing HF (200–400 Hz) STN power for monitoring daytime sleep-wake states in addition to power at lower frequencies (<100 Hz) that are more traditionally explored. Moreover, the findings support the overarching goal of developing closed-loop DBS strategies for continuously monitoring the sleep-wake states of the subject and delivering therapy accordingly to minimize the frequency of bouts of daytime sleepiness in people with PD, while also treating PD motor signs. Finally, our findings reinforce the idea that physiology-based therapies will need to adopt a subject-specific personalized approach, in which activity across sleep-wake states are characterized and effective subject-specific neurophysiological biomarkers are identified to guide DBS.
Highlights.
Daytime sleepiness occurs in Parkinson’s disease (PD), but treatment options remain lacking.
DBS is an effective treatment option for PD motor signs.
Daytime sleep-wake states can be classified based on DBS lead recordings with >85% accuracy.
Results can guide closed-loop DBS systems for mitigating daytime sleepiness in PD.
Acknowledgment
This work was supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (NINDS) R01 NS110613, R01 NS058945, R01 NS037019, R37 NS077657, P50-NS123109, P50-NS098573, MnDRIVE (Minnesota’s Discovery, Research and Innovation Economy) Brain Conditions Program, MN-REACH, and the Engdahl Family Foundation.
Declaration of Competing Interest
JLV serves as a consultant for Medtronic, Boston Scientific, and Abbott. He also serves on the Executive Advisory Board for Abbott and is a member of the scientific advisory board for Surgical Information Sciences. He has research support through the National Institutes of Health. He has no competing non-financial interest to disclose. All other authors have no competing interests to disclose.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Abbott RD, Ross GW, White LR, Tanner CM, Masaki KH, Nelson JS, Curb JD, Petrovitch H, 2005. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 65, 1442–1446. [DOI] [PubMed] [Google Scholar]
- Aman JE, Johnson LA, Sanabria DE, Wang J, Patriat R, Hill M, Marshall E, MacKinnon CD, Cooper SE, Schrock LE, Park MC, Harel N, Vitek JL, 2020. Directional deep brain stimulation leads reveal spatially distinct oscillatory activity in the globus pallidus internus of Parkinson’s disease patients. Neurobiology of disease 139, 104819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara AW, Walker HC, Joop A, Cutter G, DeWolfe JL, Harding SM, Standaert DG, 2017. Effects of Subthalamic Nucleus Deep Brain Stimulation on Objective Sleep Outcomes in Parkinson’s Disease. Mov Disord Clin Pract 4, 183–190. 10.1002/mdc3.12375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara AW, Watts RL, Walker HC, 2011. The effects of deep brain stimulation on sleep in Parkinson’s disease. Ther Adv Neurol Disord 4, 15–24. 10.1177/1756285610392446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D, Beecher G, Ba F, 2017. Deep Brain Stimulation in Parkinson’s Disease: New and Emerging Targets for Refractory Motor and Nonmotor Symptoms. Parkinson’s Disease 2017, 1–13. 10.1155/2017/5124328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askenasy JJ, Yahr MD, 1990. Parkinsonian tremor loses its alternating aspect during non-REM sleep and is inhibited by REM sleep. Journal of Neurology, Neurosurgery & Psychiatry 53, 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balandong RP, Ahmad RF, Mohamad Saad MN, Malik AS, 2018. A Review on EEG-Based Automatic Sleepiness Detection Systems for Driver. IEEE Access 6, 22908–22919. 10.1109/ACCESS.2018.2811723 [DOI] [Google Scholar]
- Barraud Q, Lambrecq V, Forni C, McGuire S, Hill M, Bioulac B, Balzamo E, Bezard E, Tison F, Ghorayeb I, 2009. Sleep disorders in Parkinson’s disease: the contribution of the MPTP non-human primate model. Experimental Neurology 219, 574–582. [DOI] [PubMed] [Google Scholar]
- Bauer M, 2006. Tactile Spatial Attention Enhances Gamma-Band Activity in Somatosensory Cortex and Reduces Low-Frequency Activity in Parieto-Occipital Areas. Journal of Neuroscience 26, 490–501. 10.1523/JNEUROSCI.5228-04.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CR, 2019. Sleep–wake and circadian disturbances in Parkinson disease: a short clinical guide. J Neural Transm 126, 863–869. 10.1007/s00702-019-02039-3 [DOI] [PubMed] [Google Scholar]
- Baumann-Vogel H, Imbach LL, Sürücü O, Stieglitz L, Waldvogel D, Baumann CR, Werth E, 2017. The Impact of Subthalamic Deep Brain Stimulation on Sleep–Wake Behavior: A Prospective Electrophysiological Study in 50 Parkinson Patients. Sleep. 10.1093/sleep/zsx033 [DOI] [PubMed] [Google Scholar]
- Baumgartner AJ, Kushida CA, Summers MO, Kern DS, Abosch A, Thompson JA, 2021. Basal Ganglia Local Field Potentials as a Potential Biomarker for Sleep Disturbance in Parkinson’s Disease. Front. Neurol 12, 765203. 10.3389/fneur.2021.765203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaid H, Adrien J, Laffrat E, Tande D, Karachi C, Grabli D, Arnulf I, Clark SD, Drouot X, Hirsch EC, Francois C, 2014. Sleep Disorders in Parkinsonian Macaques: Effects of L-Dopa Treatment and Pedunculopontine Nucleus Lesion. Journal of Neuroscience 34, 9124–9133. 10.1523/JNEUROSCI.0181-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci T, Prenassi M, Arlotti M, Cogiamanian FM, Borellini L, Moro E, Lozano AM, Volkmann J, Barbieri S, Priori A, Marceglia S, 2021. Eight-hours conventional versus adaptive deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. npj Parkinsons Dis. 7, 88. 10.1038/s41531-021-00229-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bore JC, Campbell BA, Cho H, Gopalakrishnan R, Machado AG, Baker KB, 2020. Prediction of mild parkinsonism revealed by neural oscillatory changes and machine learning. Journal of Neurophysiology 124, 1698–1705. 10.1152/jn.00534.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenbach J, Baumgartl H, Buettner R, 2020. Detection of Excessive Daytime Sleepiness in Resting-State EEG Recordings: A Novel Machine Learning Approach Using Specific EEG Sub-Bands and Channels. AMCIS 2020 Proc. 10. [Google Scholar]
- Bruin VMS, Bittencourt LRA, Tufik S, 2012. Sleep-Wake Disturbances in Parkinson’s Disease: Current Evidence regarding Diagnostic and Therapeutic Decisions. Eur Neurol 67, 257–267. [DOI] [PubMed] [Google Scholar]
- Cagle JN, Wong JK, Johnson KA, Foote KD, Okun MS, De Hemptinne C, 2021. Suppression and Rebound of Pallidal Beta Power: Observation Using a Chronic Sensing DBS Device. Frontiers in Human Neuroscience 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PR, Benarroch EE, 2020. How could the basal ganglia control sleep? Neurology 95, 302–304. 10.1212/WNL.0000000000010008 [DOI] [PubMed] [Google Scholar]
- Chen Y, Gong C, Hao H, Guo Y, Xu S, Zhang Y, Yin G, Cao X, Yang A, Meng F, Ye J, Liu H, Zhang J, Sui Y, Li L, 2019. Automatic Sleep Stage Classification Based on Subthalamic Local Field Potentials. IEEE Trans. Neural Syst. Rehabil. Eng 27, 118–128. 10.1109/TNSRE.2018.2890272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen E, Abosch A, Thompson JA, Zylberberg J, 2019. Inferring sleep stage from local field potentials recorded in the subthalamic nucleus of Parkinson’s patients. J Sleep Res 28, e12806. 10.1111/jsr.12806 [DOI] [PubMed] [Google Scholar]
- Connolly AT, Jensen AL, Baker KB, Vitek JL, Johnson MD, 2015. Classification of pallidal oscillations with increasing parkinsonian severity. Journal of Neurophysiology 114, 209–218. 10.1152/jn.00840.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davin A, Chabardès S, Belaid H, Fagret D, Djaileb L, Dauvilliers Y, David O, Torres-Martinez N, Piallat B, 2022. Early onset of sleep/wake disturbances in a progressive macaque model of Parkinson’s disease. Sci Rep 12, 17499. 10.1038/s41598-022-22381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli G, Aschermann Z, Ács P, Bosnyák E, Janszky J, Faludi B, Makkos A, Kovács M, Komoly S, Balás I, Dóczi T, Kovács N, 2015. Bilateral Subthalamic Stimulation can Improve Sleep Quality in Parkinson’s Disease. JPD 5, 361–368. 10.3233/JPD-150540 [DOI] [PubMed] [Google Scholar]
- Devergnas A, Pittard D, Bliwise D, Wichmann T, 2014. Relationship between oscillatory activity in the cortico-basal ganglia network and parkinsonism in MPTP-treated monkeys. Neurobiology of Disease 68, 156–166. 10.1016/j.nbd.2014.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar Sanabria D, Johnson LA, Nebeck SD, Zhang J, Johnson MD, Baker KB, Molnar GF, Vitek JL, 2017. Parkinsonism and vigilance: alteration in neural oscillatory activity and phase-amplitude coupling in the basal ganglia and motor cortex. Journal of Neurophysiology 118, 2654–2669. 10.1152/jn.00388.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann LK, Lofredi R, Neumann W-J, Al-Fatly B, Roediger J, Bahners BH, Nikolov P, Denison T, Saryyeva A, Krauss JK, Faust K, Florin E, Schnitzler A, Schneider G-H, Kühn AA, 2022. Toward therapeutic electrophysiology: beta-band suppression as a biomarker in chronic local field potential recordings. npj Parkinsons Dis. 8, 44. 10.1038/s41531-022-00301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann LK, Neumann W, Krause P, Lofredi R, Schneider G, Kühn AA, 2021. Subthalamic beta band suppression reflects effective neuromodulation in chronic recordings. Euro J of Neurology 28, 2372–2377. 10.1111/ene.14801 [DOI] [PubMed] [Google Scholar]
- Foffani G, 2003. 300-Hz subthalamic oscillations in Parkinson’s disease. Brain 126, 2153–2163. 10.1093/brain/awg229 [DOI] [PubMed] [Google Scholar]
- Frucht S, Rogers JD, Greene PE, Gordon MF, Fahn S, 1999. Falling asleep at the wheel: motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology 52, 1908–1908. [DOI] [PubMed] [Google Scholar]
- Giannicola G, Marceglia S, Rossi L, Mrakic-Sposta S, Rampini P, Tamma F, Cogiamanian F, Barbieri S, Priori A, 2010. The effects of levodopa and ongoing deep brain stimulation on subthalamic beta oscillations in Parkinson’s disease. Experimental Neurology 226, 120–127. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Selway R, Gnoni V, Beniczky S, Williams SC, Kryger M, Ferini-Strambi L, Goadsby P, Leschziner GD, Ashkan K, Rosenzweig I, 2020. The subcortical belly of sleep: New possibilities in neuromodulation of basal ganglia? Sleep medicine reviews 52, 101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama M, Nakamura T, Hori N, Koike Y, Sobue G, 2008. The video images of sleep attacks in Parkinson’s disease: Video of Sleep Attacks in PD. Mov. Disord 23, 288–290. 10.1002/mds.21830 [DOI] [PubMed] [Google Scholar]
- Hobson DE, Lang AE, Martin WRW, Razmy A, Rivest J, Fleming J, 2002. Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. Jama 287, 455–463. [DOI] [PubMed] [Google Scholar]
- Holt AB, Kormann E, Gulberti A, Pötter-Nerger M, McNamara CG, Cagnan H, Baaske MK, Little S, Köppen JA, Buhmann C, Westphal M, Gerloff C, Engel AK, Brown P, Hamel W, Moll CKE, Sharott A, 2019. Phase-Dependent Suppression of Beta Oscillations in Parkinson’s Disease Patients. J. Neurosci 39, 1119–1134. 10.1523/JNEUROSCI.1913-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston B, Thompson M, Ko A, Chizeck H, 2019. A machine-learning approach to volitional control of a closed-loop deep brain stimulation system. J. Neural Eng 16, 016004. 10.1088/1741-2552/aae67f [DOI] [PubMed] [Google Scholar]
- Iranzo A, 2002. Sleep symptoms and polysomnographic architecture in advanced Parkinson’s disease after chronic bilateral subthalamic stimulation. Journal of Neurology, Neurosurgery & Psychiatry 72, 661–664. 10.1136/jnnp.72.5.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson N, Kühn AA, Brown P, 2013. Gamma oscillations in the human basal ganglia. Experimental neurology 245, 72–76. [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP, 2007. Human gamma-frequency oscillations associated with attention and memory. Trends in neurosciences 30, 317–324. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Aman JE, Yu Y, Sanabria DE, Wang J, Hill M, Dharnipragada R, Patriat R, Fiecas M, Li L, Schrock LE, Cooper SE, Johnson MD, Park MC, Harel N, Vitek JL, 2021. High‐Frequency Oscillations in the Pallidum: A Pathophysiological Biomarker in Parkinson’s Disease? Movement Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Nebeck SD, Muralidharan A, Johnson MD, Baker KB, Vitek JL, 2016. Closed-Loop Deep Brain Stimulation Effects on Parkinsonian Motor Symptoms in a Non-Human Primate – Is Beta Enough? Brain Stimulation 9, 892–896. 10.1016/j.brs.2016.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joundi RA, Brittain JS, Green AL, Aziz TZ, Brown P, 2012. Oscillatory activity in the subthalamic nucleus during arm reaching in Parkinson’s disease. Experimental neurology 236, 319–326. [DOI] [PubMed] [Google Scholar]
- Knie B, Mitra MT, Logishetty K, Chaudhuri K, 2011. Excessive daytime sleepiness in patients with Parkinson’s disease. CNS drugs 25, 203–212. [DOI] [PubMed] [Google Scholar]
- Körner Y, Meindorfner C, Möller JC, Stiasny-Kolster K, Haja D, Cassel W, Oertel WH, Krüger H-P, 2004. Predictors of sudden onset of sleep in Parkinson’s disease. Mov Disord. 19, 1298–1305. 10.1002/mds.20163 [DOI] [PubMed] [Google Scholar]
- Kratzel L, Glos M, Veauthier C, Rekow S, François C, Fietze I, Penzel T, 2021. Video-based sleep detection using ocular signals under the standard conditions of the maintenance of wakefulness test in patients with sleep disorders. Physiol. Meas 42, 014004. 10.1088/1361-6579/abdb7e [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Kempf F, Brucke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider G-H, Hariz MI, Vandenberghe W, Nuttin B, Brown P, 2008. High-Frequency Stimulation of the Subthalamic Nucleus Suppresses Oscillatory Activity in Patients with Parkinson’s Disease in Parallel with Improvement in Motor Performance. Journal of Neuroscience 28, 6165–6173. 10.1523/JNEUROSCI.0282-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus M, Chen JF, Urade Y, Huang ZL, 2013. Role of the basal ganglia in the control of sleep and wakefulness. Current opinion in neurobiology 23, 780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, Foltynie T, Limousin P, Ashkan K, FitzGerald J, Green AL, 2013. Adaptive deep brain stimulation in advanced Parkinson disease. Annals of neurology 74, 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Ling Z, Pan L, Xu X, Cui Z, Liang S, Yu X, 2019. Comparison of Efficacy of Deep Brain Stimulation of Different Targets in Parkinson’s Disease: A Network Meta-Analysis. Front. Aging Neurosci 11, 23. 10.3389/fnagi.2019.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masilamoni GJ, Smith Y, 2018. Chronic MPTP administration regimen in monkeys: a model of dopaminergic and non-dopaminergic cell loss in Parkinson’s disease. J Neural Transm 125, 337–363. 10.1007/s00702-017-1774-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medtronic PI, 2020. Green light for deep brain stimulator incorporating neurofeedback. Nat. Biotechnol 38, 1014–1015. [DOI] [PubMed] [Google Scholar]
- Mizrahi-Kliger AD, Kaplan A, Israel Z, Deffains M, Bergman H, 2020. Basal ganglia beta oscillations during sleep underlie Parkinsonian insomnia. Proc Natl Acad Sci USA 117, 17359–17368. 10.1073/pnas.2001560117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann W, Rodriguez-Oroz MC, 2021. Machine Learning Will Extend the Clinical Utility of Adaptive Deep Brain Stimulation. Mov Disord 36, 796–799. 10.1002/mds.28567 [DOI] [PubMed] [Google Scholar]
- Neumann W-J, Degen K, Schneider G-H, Brücke C, Huebl J, Brown P, Kühn AA, 2016. Subthalamic synchronized oscillatory activity correlates with motor impairment in patients with Parkinson’s disease: Correlation of Subthalamic B Oscillations and PD Symptoms. Mov Disord. 31, 1748–1751. 10.1002/mds.26759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Ahn S, Jang H, Jun SC, Kim JG, 2017. Utilization of a combined EEG/NIRS system to predict driver drowsiness. Sci Rep 7, 43933. 10.1038/srep43933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswal A, Brown P, Litvak V, 2013. Synchronized neural oscillations and the pathophysiology of Parkinsonʼs disease: Current Opinion in Neurology 26, 662–670. 10.1097/WCO.0000000000000034 [DOI] [PubMed] [Google Scholar]
- Ozturk M, Viswanathan A, Sheth SA, Ince NF, 2021. Electroceutically induced subthalamic high-frequency oscillations and evoked compound activity may explain the mechanism of therapeutic stimulation in Parkinson’s disease. Commun Biol 4, 393. 10.1038/s42003-021-01915-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson P, Halje P, Cenci MA, 2019. Significance and Translational Value of High-Frequency Cortico-Basal Ganglia Oscillations in Parkinson’s Disease. JPD 9, 183–196. 10.3233/JPD-181480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D, Wang B, Qing X, Zhang T, Zhang Y, Wang X, Nakamura M, 2017. Drowsiness Detection by Bayesian-Copula Discriminant Classifier Based on EEG Signals During Daytime Short Nap. IEEE Trans. Biomed. Eng 64, 743–754. 10.1109/TBME.2016.2574812 [DOI] [PubMed] [Google Scholar]
- Rodrigues TM, Castro Caldas A, Ferreira JJ, 2016. Pharmacological interventions for daytime sleepiness and sleep disorders in Parkinson’s disease: Systematic review and meta-analysis. Parkinsonism & Related Disorders 27, 25–34. 10.1016/j.parkreldis.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Salawu F, Olokoba A, 2015. Excessive Daytime Sleepiness and Unintended Sleep Episodes Associated with Parkinson’s Disease. Oman Med J 30, 3–10. 10.5001/omj.2015.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria DE, Aman JE, Amaya V, Johnson LA, Farooqi H, Wang J, Hill M, Patriat R, Sovell-Brown K, Molnar GF, Darrow D, McGovern R, Cooper SE, Harel N, MacKinnon CD, Park MC, Vitek JL, 2022. Controlling pallidal oscillations in real-time in Parkinson’s disease using evoked interference deep brain stimulation (eiDBS): Proof of concept in the human. Brain Stimulation 15, 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria DE, Johnson LA, Yu Y, Busby Z, Nebeck S, Zhang J, Harel N, Johnson MD, Molnar GF, Vitek JL, 2020. Real-time suppression and amplification of frequency-specific neural activity using stimulation evoked oscillations. Brain Stimulation 13, 1732–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VD, Sengupta S, Chitnis S, Amara AW, 2018. Deep Brain Stimulation and Sleep-Wake Disturbances in Parkinson Disease: A Review. Front. Neurol 9, 697. 10.3389/fneur.2018.00697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorucak J, Hertig-Godeschalk A, Schreier DR, Malafeev A, Mathis J, Achermann P, 2020. Automatic detection of microsleep episodes with feature-based machine learning. Sleep 43, zsz225. 10.1093/sleep/zsz225 [DOI] [PubMed] [Google Scholar]
- Stefani A, Galati S, Peppe A, Bassi A, Pierantozzi M, Hainsworth AH, Bernardi G, Orlacchio A, Stanzione P, Mazzone P, 2006. Spontaneous sleep modulates the firing pattern of Parkinsonian subthalamic nucleus. Exp Brain Res 168, 277–280. 10.1007/s00221-005-0175-y [DOI] [PubMed] [Google Scholar]
- Suzuki K, Miyamoto M, Miyamoto T, Hirata K, 2015. Parkinson’s Disease and Sleep/Wake Disturbances. Curr Neurol Neurosci Rep 15, 8. 10.1007/s11910-015-0525-5 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Miyamoto M, Miyamoto T, Iwanami M, Hirata K, 2011. Sleep Disturbances Associated with Parkinson’s Disease. Parkinson’s Disease 2011, 1–10. 10.4061/2011/219056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thenaisie Y, Palmisano C, Canessa A, Keulen BJ, Capetian P, Jiménez MC, Bally JF, Manferlotti E, Beccaria L, Zutt R, Courtine G, Bloch J, van der Gaag NA, Hoffmann CF, Moraud EM, Isaias IU, Contarino MF, 2021. Towards adaptive deep brain stimulation: clinical and technical notes on a novel commercial device for chronic brain sensing. J. Neural Eng 18, 042002. 10.1088/1741-2552/ac1d5b [DOI] [PubMed] [Google Scholar]
- Thompson JA, Tekriwal A, Felsen G, Ozturk M, Telkes I, Wu J, Ince NF, Abosch A, 2018. Sleep patterns in Parkinson’s disease: direct recordings from the subthalamic nucleus. J Neurol Neurosurg Psychiatry 89, 95–104. 10.1136/jnnp-2017-316115 [DOI] [PubMed] [Google Scholar]
- Tsiokos C, Hu X, Pouratian N, 2013. 200–300 Hz movement modulated oscillations in the internal globus pallidus of patients with Parkinson’s Disease. Neurobiology of disease 54, 464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrestarazu E, Iriarte J, Alegre M, Clavero P, Rodríguez-Oroz MC, Guridi J, Obeso JA, Artieda J, 2009. Beta activity in the subthalamic nucleus during sleep in patients with Parkinson’s disease. Mov Disord. 24, 254–260. 10.1002/mds.22351 [DOI] [PubMed] [Google Scholar]
- van Rheede JJ, Feldmann LK, Busch JL, Fleming JE, Mathiopoulou V, Denison T, Sharott A, Kühn AA, 2022. Diurnal modulation of subthalamic beta oscillatory power in Parkinson’s disease patients during deep brain stimulation. npj Parkinsons Dis. 8, 88. 10.1038/s41531-022-00350-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma AK, Acosta Lenis SF, Aman JE, Sanabria DE, Wang J, Pearson A, Hill M, Patriat R, Schrock LE, Cooper SE, Park MC, Harel N, Howell MJ, MacKinnon CD, Vitek JL, Johnson LA, 2022. Basal ganglia engagement during REM sleep movements in Parkinson’s disease. npj Parkinsons Dis. 8, 116. 10.1038/s41531-022-00382-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivelan R, Qiu M-H, Chang C, Lu J, 2010. Role of Basal Ganglia in Sleep–Wake Regulation: Neural Circuitry and Clinical Significance. Front. Neuroanat 4. 10.3389/fnana.2010.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnovic A, 2018. Disturbances of Sleep and Alertness in Parkinson’s Disease. Curr Neurol Neurosci Rep 18, 29. 10.1007/s11910-018-0838-2 [DOI] [PubMed] [Google Scholar]
- Yeung EYH, Cavanna AE, 2014. Sleep Attacks in Patients With Parkinson’s Disease on Dopaminergic Medications: A Systematic Review. Mov Disord Clin Pract 1, 307–316. 10.1002/mdc3.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Bai Y, Guan B, Jiang Y, Wang Z, Meng F, Yang A, Zhang J, 2021. A quantitative analysis of the effect of bilateral subthalamic nucleus-deep brain stimulation on subjective and objective sleep parameters in Parkinson’s disease. Sleep Medicine 79, 195–204. 10.1016/j.sleep.2020.10.021 [DOI] [PubMed] [Google Scholar]
- Yu Y, Escobar Sanabria D, Wang J, Hendrix CM, Zhang J, Nebeck SD, Amundson AM, Busby ZB, Bauer DL, Johnson MD, Johnson LA, Vitek JL, 2021. Parkinsonism Alters Beta Burst Dynamics across the Basal Ganglia–Motor Cortical Network. J. Neurosci 41, 2274–2286. 10.1523/JNEUROSCI.1591-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang F, Li W, Wang N, Han C, Fan S, Li P, Xu L, Zhang J, Meng F, 2021. Relationship between electrode position of deep brain stimulation and motor symptoms of Parkinson’s disease. BMC Neurol 21, 122. 10.1186/s12883-021-02148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhang J, Lam SP, Chan JW, Mok V, Chan A, Li SX, Liu Y, Tang X, Yung WH, Wing YK, 2017. Excessive Daytime Sleepiness Predicts Neurodegeneration in Idiopathic REM Sleep Behavior Disorder. Sleep 40. 10.1093/sleep/zsx041 [DOI] [PubMed] [Google Scholar]
- Zuzuarregui JRP, Ostrem JL, 2020. The Impact of Deep Brain Stimulation on Sleep in Parkinson’s Disease: An update. Journal of Parkinson’s Disease 10, 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


