Abstract
Background
A medication review can be defined as a structured evaluation of a patient's medication conducted by healthcare professionals with the aim of optimising medication use and improving health outcomes. Optimising medication therapy though medication reviews may benefit hospitalised patients.
Objectives
We examined the effects of medication review interventions in hospitalised adult patients compared to standard care or to other types of medication reviews on all‐cause mortality, hospital readmissions, emergency department contacts and health‐related quality of life.
Search methods
In this Cochrane Review update, we searched for new published and unpublished trials using the following electronic databases from 1 January 2014 to 17 January 2022 without language restrictions: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP). To identify additional trials, we searched the reference lists of included trials and other publications by lead trial authors, and contacted experts.
Selection criteria
We included randomised trials of medication reviews delivered by healthcare professionals for hospitalised adult patients. We excluded trials including outpatients and paediatric patients.
Data collection and analysis
Two review authors independently selected trials, extracted data and assessed risk of bias. We contacted trial authors for data clarification and relevant unpublished data. We calculated risk ratios (RRs) for dichotomous data and mean differences (MDs) or standardised mean differences (SMDs) for continuous data (with 95% confidence intervals (CIs)). We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach to assess the overall certainty of the evidence.
Main results
In this updated review, we included a total of 25 trials (15,076 participants), of which 15 were new trials (11,501 participants). Follow‐up ranged from 1 to 20 months. We found that medication reviews in hospitalised adults may have little to no effect on mortality (RR 0.96, 95% CI 0.87 to 1.05; 18 trials, 10,108 participants; low‐certainty evidence); likely reduce hospital readmissions (RR 0.93, 95% CI 0.89 to 0.98; 17 trials, 9561 participants; moderate‐certainty evidence); may reduce emergency department contacts (RR 0.84, 95% CI 0.68 to 1.03; 8 trials, 3527 participants; low‐certainty evidence) and have very uncertain effects on health‐related quality of life (SMD 0.10, 95% CI ‐0.10 to 0.30; 4 trials, 392 participants; very low‐certainty evidence).
Authors' conclusions
Medication reviews in hospitalised adult patients likely reduce hospital readmissions and may reduce emergency department contacts. The evidence suggests that mediation reviews may have little to no effect on mortality, while the effect on health‐related quality of life is very uncertain. Almost all trials included elderly polypharmacy patients, which limits the generalisability of the results beyond this population.
Plain language summary
What are the benefits and risks of medication reviews for hospitalised adults?
Key messages
Medication reviews in hospitalised adults likely reduce hospital readmissions but may have little to no effect on mortality.
What is a medication review?
A medication review is a structured intervention conducted by healthcare professionals in order to optimise an individual patient’s medication and improve health outcomes.
What did we want to find out?
Whether medication reviews improve the health of hospitalised adult patients.
What did we do?
We searched for trials that examined medication reviews compared with usual care or trials that examined two or more types of medication reviews in hospitalised adults. We compared and summarised the results of the trials and rated our confidence in the evidence.
What did we find?
We found that medication reviews in hospitalised adult patients likely reduce hospital readmissions and may reduce emergency department contacts. However, medication reviews may have little to no effect on mortality, and it is unclear if medication reviews have an effect on health‐related quality of life.
What are the limitations of the evidence?
Almost all trials included elderly patients taking a high number of medications, so we may not be able to generalise the results to other types of patients.
How up to date is this evidence?
We searched electronic databases and other sources for trials that had been published up to January 2022.
Summary of findings
Summary of findings 1. Medication review compared with standard care for hospitalised adult patients.
| Medication review compared with standard care for hospitalised adult patients | |||||
|
Patient or population: hospitalised adult patients Intervention: medication review Comparison: standard care | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Assumed risk with standard care | Corresponding risk with medication review | ||||
|
Mortality (all‐cause) Median follow‐up 6 months (range 1 to 20 months) |
High‐risk population | RR 0.96 (0.87 to 1.05) | 10,108 (18 trials) | ⊕⊕⊝⊝ Lowb,c |
|
| 200 per 1000a | 194 per 1000 (174 to 216) | ||||
| Very high‐risk population | |||||
| 400 per 1000a | 388 per 1000 (332 to 432) | ||||
|
Hospital readmission (all‐cause) Median follow‐up 6 months (range 1 to 12 months) |
High‐risk population | RR 0.93 (0.89 to 0.98) | 9561 (17 trials) |
⊕⊕⊕⊝ Moderated |
|
| 500 per 1000a | 465 per 1000 (445 to 490) | ||||
| Very high‐risk population | |||||
| 650 per 1000a | 605 per 1000 (579 to 637) | ||||
|
Hospital emergency department contacts (all‐cause) Median follow‐up 3 months (range 1 to 12 months) |
High‐risk population | RR 0.84 (0.68 to 1.03) | 3527 (8 trials) | ⊕⊕⊝⊝ Lowe,f | |
| 300 per 1000a | 249 per 1000 (204 to 309) | ||||
| Very high‐risk population | |||||
| 400 per 1000a | 332 per 1000 (272 to 412) | ||||
|
Health‐related quality of lifeg Median follow‐up 3 months (range 3 to 6 months) |
— | — | SMD 0.10** (‐0.10 to 0.30) | 392 (4 trials) |
⊕⊝⊝⊝ Very lowg,h |
| * The basis for the assumed riskwith standard care is provided in footnotes. The corresponding riskwith medication review (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** > 0 favours medication reviews. 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect (Cohen 1988) CI: confidence interval; NA: not applicable; RR: risk ratio. SMD: standardised mean difference | |||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low certainty: We are very uncertain about the estimate | |||||
aThe assumed risk with standard care is based on published trial data. The ‘very high‐risk’ estimates are based on the included trials with the highest risk in the control group at 12 months follow‐up for mortality (Gillespie 2009), hospital readmissions (Lea 2020) and emergency department contacts (Kempen 2021). The ‘high‐risk’ estimates are based on the included trials with the lowest risk (albeit still a high‐risk, hospitalised population) in the control group at 12 months follow‐up for mortality and hospital readmissions (Scullin 2007) and emergency department contacts (Gillespie 2009).
bDowngrade for indirectness. Follow‐up ranged from 3 to 20 months for mortality. In 13 of 18 trials follow‐up was less than 12 months. Short follow‐up may be inadequate as changes to preventive medications may take years before having an effect on mortality (downgraded 1 category for indirectness).
cThe 95% CI ranges from 0.87 to 1.05 and includes both important benefit and important harm (i.e. more than 5% change in mortality) (downgraded 1 category for imprecision).
dAnalysis restricted to ‘low’ risk of bias trials showed that the confidence interval overlapped 1 (downgraded 1 category for study limitations).
eThe 95% CI ranges from 0.68 to 1.03 and includes important benefit (i.e. more than 20% reduction in emergency department contacts) (downgraded 1 category for imprecision).
fSubgroup analysis comparing trials with ‘high’ and ‘low’ risk of bias showed that the effect of medication reviews was smaller in trials with low risk of bias (interaction test: P value = 0.07) (downgraded 1 category for study limitations).
gScales used to assess health‐related quality of life: EuroQol‐visual analogue scale (EQ‐VAS) (3 trials) and QUALIDEM (1 trial).
hThe 95% CI ranges from ‐0.10 to 0.30 and includes important benefit, i.e. Cohen’s d of 0.2 (downgraded 1 category for imprecision).
iThe included trials all reported missing outcome data for 31% to 53% of participants, resulting in a high risk of attrition bias (downgraded 2 categories for study limitations).
Background
Evidence links polypharmacy (most often defined as the use of five or more medications (Masnoon 2017)) to an increased risk of adverse events (e.g. falls) (Bourgeois 2010; Hallas 1996; Obreli‐Neto 2012; Rothschild 2000; Ziere 2006), poorer medication adherence (Pasina 2014), greater economic burden (Classen 1997), emergency department contacts and hospital admissions (Kongkaew 2008; Schneeweiss 2002; Zed 2008), drug‐related deaths and overall mortality (Ebbesen 2001; Gnjidic 2012). Therefore, it is important to distinguish between appropriate and inappropriate polypharmacy (Masnoon 2017). This is particularly relevant among elderly patients, for whom the benefit‐harm balance of each medication might change with age‐related physiological changes, frailty and multiple coexisting conditions (El Desoky 2007; Mangoni 2004). Generally, an increasing number of medications is associated with an increasing number of inappropriate medications (Steinman 2006). The challenge of inappropriate polypharmacy is expected to grow in the future, as individuals in most parts of the world live longer with multiple chronic conditions and new treatment options emerge (CDC 2011; Christensen 2009; European Communities 2006; Pefoyo 2015; WHO 2019).
Several interventions have been developed to ensure the appropriateness of prescribing and thereby improve clinical outcomes (Cooper 2015; Rankin 2018; Spinewine 2007b), and medication reviews constitute such an intervention. Medication reviews vary from simple point‐of‐care medication list revisions to comprehensive interventions necessitating access to all clinical data and involvement of other healthcare professionals before shared decision‐making with the patient. To aid the process of reviewing patients' medications, several criteria have been formulated to identify potentially inappropriate medications, especially for older adults (American Geriatrics Society 2019; Hanlon 1992; Holt 2010; Laroche 2007a; McLeod 1997; Naugler 2000; O'Mahony 2015; Samsa 1994). However, the applicability and effectiveness of applying these various criteria in clinical practice remains uncertain (Gallagher 2008b; Hill‐Taylor 2016; Laroche 2007b; Lozano‐Montoya 2015; Lund 2010; O'Mahony 2020; Spinewine 2007b).
Based on previous systematic reviews and meta‐analyses of randomised trials of medication review interventions (Christensen 2016; Dautzenberg 2021; Hohl 2015; Huiskes 2017; Renaudin 2016), the effect on clinical outcomes is uncertain and the best method for conducting medication reviews is unknown. By updating one of these reviews (Christensen 2016), we aim to clarify whether medication reviews can reduce mortality, hospital readmissions, emergency department contacts, adverse drug events and/or increase health‐related quality of life among hospitalised adult patients. In several predefined subgroup analyses, we will also examine whether some methods of medication review are more effective than others.
Description of the condition
Inappropriate medication use is a significant cause of patient morbidity and mortality. Inappropriate medication use could be the use of medications or combinations thereof with an unfavourable benefit‐harm balance, but may also include under‐use of medications. An unfavourable benefit‐harm balance entails that the harms (or risk thereof) of a given medication exceed the beneficial effects for an individual patient. This could include the use of medications without correct indication or dosage, with unfavourable interactions with certain conditions or other medications, with unacceptable adverse effects or risks, without necessary biochemical monitoring, or with inadequate patient adherence to therapy. In this review we focus on hospitalised adult patients as this is a population with a high risk of inappropriate medication use.
Description of the intervention
Any medication review delivered by healthcare professionals with the aim of optimising medication use and improving health outcomes, i.e. optimising the effectiveness and minimising the harms (without impairing the benefit) of the prescribed medication.
How the intervention might work
More appropriate prescribing and medication use (i.e. ensuring that treatment is correctly indicated and monitored and that the individual patient receives the right medication and dosage) could reduce harms and improve the effectiveness of medication therapy, possibly leading to reduced morbidity and mortality.
Why it is important to do this review
Medication reviews are performed in many parts of the world in different settings. However, despite the widespread use of medication reviews, it is still uncertain whether medication reviews for hospitalised adult patients reduce patient morbidity and mortality. In addition, the best method for medication review is presently unknown.
Objectives
We examined the effect of medication review interventions in hospitalised adult patients compared with standard care or other types of medication reviews on all‐cause mortality, hospital readmissions, emergency department contacts and health‐related quality of life.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials (RCTs) in any language, published or unpublished, with randomisation on an individual level or an aggregated level (i.e. cluster‐randomised trials).
Types of participants
We included trials of hospitalised adult patients (i.e. adult patients admitted to hospital).
We excluded trials of outpatients, patients solely seen in the emergency department (i.e. not admitted to a hospital) and paediatric patients.
Types of interventions
We included any medication review of a patient's pharmacotherapy delivered by a healthcare professional with the aim of optimising medication use and improving health outcomes. The intervention entails an evaluation of each medication's relevance, benefit and harms in relation to the patient, and results in a recommendation or a direct change in the medication. We included trials comparing medication review with usual care or comparing two or more types of medication reviews.
We excluded:
trials aimed solely at increasing a patient's knowledge about current medication, improving adherence or reducing costs;
trials in which the results of medication review were to be primarily implemented after discharge from hospital (e.g. intervention consisting of a letter to the patient's general practitioner);
trials reviewing only portions of a patient's medication related to a specific condition or to a single class of medications (e.g. only diabetes medications or antidepressants were reviewed).
Types of outcome measures
We assessed the outcomes at the longest follow‐up available in line with the previous versions of this review.
Primary outcomes
Mortality (all‐cause)
Secondary outcomes
Mortality (due to adverse drug events)
Hospital readmission (all‐cause)
Hospital readmission (due to adverse drug events)
Hospital emergency department contacts (all‐cause)
Hospital emergency department contacts (due to adverse drug events)
Adverse drug events (defined as when someone is harmed by a medication)
Health‐related quality of life
We included any trial that reported follow‐up data on either primary or secondary outcomes. When outcome data were reported at more than one time point, we used the outcome data with the longest follow‐up.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases on 30 October 2019 and updated the search on 17 January 2022:
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (1 January 2014 to 17 January 2022);
MEDLINE (Ovid) (1 January 2014 to 17 January 2022);
Embase (Ovid) (1 January 2014 to 17 January 2022);
CINAHL (EBSCO) (1 January 2014 to 17 January 2022).
In addition, we searched the following trial registries on 30 October 2019 and updated the search on 17 January 2022:
ClinicalTrials.gov;
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP).
The search strategies were developed for Ovid MEDLINE and were adapted for the other databases (Appendix 1). We used the Cochrane RCT Sensitivity/Precision‐Maximizing Filter to limit our search to RCTs (Lefebvre 2011). In this update, we limited our search from January 2014 as publications prior to 2014 would have been identified in previous versions of the Cochrane Review. Search strategies from the previous version of the review can be seen in Appendix 1.
Searching other resources
We searched the reference lists of all included trials and relevant reviews for additional trials. We searched MEDLINE (PubMed, January 2022) for relevant publications by the lead authors (first and last) of the included trials. We contacted content experts in the field and corresponding with authors of the included trials to identify additional trials.
Data collection and analysis
Selection of studies
Two review authors (CB, SSC) independently assessed trials for inclusion in two rounds using Covidence systematic review software (Covidence). First, we screened titles and abstracts for potentially includable publications. Then we screened the full text of all potential publications for inclusion. Disagreements were resolved by discussion and if consensus could not be reached we involved an additional review author (MC, AL).
Data extraction and management
Two review authors (CB, SSC) independently extracted data from all included trials into a standardised data sheet. Disagreements were resolved by discussion and if consensus could not be reached we involved an additional review author (MC, AL).
Data included:
Trial characteristics: author name, publication year, journal name, methods of randomisation.
Participants: number of participants, country, age, gender, type of department, morbidities, medication history, inclusion and exclusion criteria.
Intervention: description of medication review, the profession of the reviewer (pharmacist, physician, other), explanation of how medication could be changed (recommendation by letter to patient's general practitioner, meeting between pharmacist and responsible physician, reviewing physicians responsible for direct change of prescription) and implementation rate of the suggested medication changes, co‐interventions that could influence the change in prescription.
Outcome: outcome assessor, timing of outcomes.
Results for each group and for each outcome at each time point; number of participants randomly assigned and included in the analysis; and number of participants who withdrew, were lost to follow‐up or were excluded.
Other characteristics: funding source.
Assessment of risk of bias in included studies
Two review authors (CB, SSC) independently assessed each trial and outcome for risk of bias using Cochrane’s tool for assessing risk of bias in randomised trials (Higgins 2011a). We assessed trials as having low, unclear or high risk of bias for the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other biases. In addition, we assessed contamination bias (EPOC 2017), as specific recommendations in medication review could also be applied to similar participants in the control group (e.g. advice to stop treatment with a specific medication). For cluster‐randomised and cross‐over trials we assessed additional domains specific to these designs using the items recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Disagreements were resolved by discussion and if consensus could not be reached we involved an additional review author (MC, AL).
Measures of treatment effect
We used the risk ratio (RR) with 95% confidence intervals (CIs) for dichotomous data, and for continuous data we used the mean difference (MD) with 95% CIs or the standardised mean difference (SMD) with 95% CIs if outcomes were measured using different scales. We analysed the mean scores of final assessments. When interpreting results presented as SMDs we used the assumptions of Cohen with 0.2 representing a small effect, 0.5 a moderate effect and 0.8 a large effect (Cohen 1988). If trials reported quality of life data on more than one rating scale, we used the rating scale we deemed most appropriate (e.g. in a trial recruiting mostly patients with dementia, we included data from the dementia‐specific quality of life rating scale; Curtin 2020).
Unit of analysis issues
We included parallel‐group, cluster‐randomised trials and cluster‐randomised cross‐over trials. To avoid unit of analysis error, when possible we used data adjusted for clustering in cluster‐randomised trials (see Data synthesis below) and adjusted for clustering and time effect in the cluster‐randomised cross‐over trial (we received reanalysed data from the trial authors). For the subgroup analysis comparing extended versus basic medication reviews, we included some trials in the analysis with three intervention arms (e.g. extended medication review, basic medication review and standard of care). For such trials we split the number of participants in the standard of care group evenly amongst the two medication review groups. When the standard of care group included an uneven number of participants or events, we used random.org to randomly allocate the last participant or event to either arm.
Dealing with missing data
We contacted the authors of all included trials by email requesting missing data. In our primary analysis we used available case analysis and in a sensitivity analysis we assumed that data were available for all randomised participants (see Data synthesis and Sensitivity analysis). Our sensitivity analysis can be viewed as a form of imputation with zero events.
Assessment of heterogeneity
We assessed statistical heterogeneity using the I² statistic across trials in each analysis. We interpreted I² values in line with the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). Additionally, we visually inspected the forest plots for signs of heterogeneity (e.g. non‐overlapping CIs).
Assessment of reporting biases
We judged trials as having a low risk of selective outcome reporting if outcomes in trial reports were reported in accordance with the trial protocol or registry information and there was no other evidence of selective outcome reporting (e.g. reported outcomes or time points did not differ from what was planned in the protocol). If only some relevant outcomes (i.e. mortality and hospital contacts) were reported and there was no information on pre‐specification of outcomes in the protocol or registry information, we judged the trial as having low risk of selective outcome reporting if all reported outcomes were negative (i.e. not statistically significant or unfavourable and statistically significant) and having unclear risk of bias if one or more reported outcomes were positive (favourable and statistically significant). If some of the relevant pre‐specified outcomes were not reported, we judged the trial as having a high risk of selective outcome reporting if one or more of the reported outcomes were positive. We assessed the overall publication bias using a funnel plot for our primary outcome (all‐cause mortality).
Data synthesis
We analysed trials comparing medication review with standard care and trials comparing different types of medication review separately. However, in multi‐arm trials we pooled two or more medication review intervention groups into a single group before comparing to standard care. In our primary analysis, we included trials randomised on an individual level and cluster‐randomised trials adjusted for clustering (i.e. excluding cluster‐randomised cross‐over trials) and only assessed outcomes measured post‐discharge. The primary analysis was based on the available case intention‐to‐treat principle. Patients who died in hospital were only retained in our primary mortality analysis, and excluded from the secondary analyses (e.g. hospital readmissions). Results from trials not included in meta‐analysis are reported descriptively in the Effects of interventions section. We analysed the data using Review Manager 5.4.1 (RevMan 2020). We calculated pooled RRs and estimated 95% CIs using the Mantel‐Haenszel method for dichotomous data when the meta‐analysis only included individually randomised trials, and we used inverse variance for analyses including cluster‐randomised trials adjusted for clustering. Due to the anticipated large heterogeneity in clinical setting, patient population and methodology of medication reviews between trials, we used a random‐effects model. Based on the estimates of absolute risk reduction derived from Table 1 we calculated the number needed to treat for the main outcomes for ‘high‐risk’ and ‘very high‐risk’ populations when the results suggested an intervention effect of medication reviews. For continuous data, we calculated pooled MDs for outcomes measured on the same scale or SMDs for outcomes measured on multiple scales, and estimated 95% CIs using the random‐effects model with the inverse variance method.
Subgroup analysis and investigation of heterogeneity
We planned to explore our findings by performing the following prespecified subgroup analyses.
Trials of participants taking a mean of ≥ 10 different medications (often defined as excessive polypharmacy; Masnoon 2017) versus trials of participants taking a mean of < 10 different medications.
Trials in which the medication review was performed by a person or team with the capability of directly changing the participant’s medication versus trials where the medication review was carried out by healthcare professionals who were not allowed to change the participants’s medications, but could only recommend changes to a responsible physician.
Trials in which the medication review intervention explicitly used published criteria (e.g. Beers’ criteria (Beers 1997), START/STOPP criteria (Gallagher 2008a), or an electronic decision support system based on these (O'Mahony 2020)) versus trials in which the medication review intervention was non‐criteria‐based.
Trials with a high implementation rate (≥ 50%) of identified drug‐related problems versus trials with a low implementation rate (< 50%). The implementation rate of identified drug‐related problems is used to describe the proportion of implemented medication changes out of all suggested changes.
Trials with an overall low risk of bias versus trials with an overall high risk of bias. We defined overall low risk of bias trials as trials with low risk of selection bias, detection bias and selective outcome reporting concerning the relevant outcome. In addition, cluster‐randomised trials and cross‐over trials needed all design‐specific domains to be low risk for the overall risk of bias to be low. We judged all other trials as having an overall high risk of bias.
Trials with extended medication review interventions versus basic medication review interventions. We defined extended medication reviews as reviews that included intervention components performed after the medication review, e.g. post‐discharge follow‐up phone calls, motivational interviewing at discharge, additional contact with general practitioners or other co‐interventions that can influence outcomes after the initial medication review.
To minimise multiplicity issues, we restricted these subgroup analyses to the dichotomous outcomes: mortality (all‐cause), hospital readmissions (all‐cause) and hospital emergency department contacts (all‐cause).
Sensitivity analysis
We performed the following sensitivity analyses to test the robustness of our findings:
A full intention‐to‐treat sensitivity analysis assuming that data were available for all randomised participants for the primary outcome (i.e. in contrast to the primary analysis using available case intention‐to‐treat analysis). This analysis assumes that randomised participants with missing outcome data had no events. Further, in the full intention‐to‐treat analysis of the secondary outcomes that occurred after hospital discharge, we excluded participants who died in hospital.
An analysis using a fixed‐effect model.
An analysis including cluster‐randomised cross‐over trials adjusted for clustering and time.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the overall certainty of the evidence (Guyatt 2008). We constructed a Table 1 for mortality (all‐cause), hospital readmissions (all‐cause), hospital emergency department contacts (all‐cause) and health‐related quality of life, as these were the most reliable and patient‐relevant outcomes. The conclusion is phrased in line with the GRADE recommendations (Santesso 2020).
Results
Description of studies
Results of the search
In this update, we identified 4614 new records in our database search (Figure 1). By reading titles and abstracts, we excluded 4339 references. We obtained full‐text publications for 275 references and excluded 196 of these. The remaining 79 publications reported on 15 new finished and published trials (Blum 2021; Bonetti 2018; Cossette 2017; Curtin 2020; Graabaek 2019; Gustafsson 2017; Juanes 2018; Kempen 2021; Lea 2020; Lenssen 2018; Nielsen 2017; O'Mahony 2020; Ravn‐Nielsen 2018; Song 2021; SUREPILL 2015), and 22 ongoing trials, which were included in our review.
1.
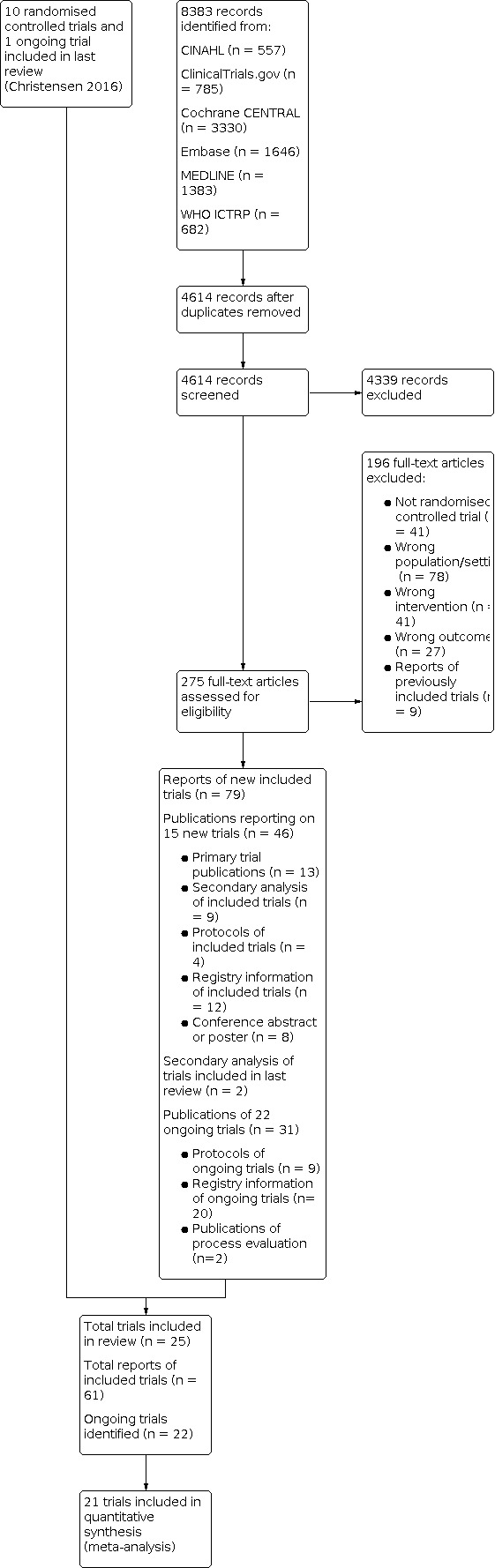
We contacted the authors of seven of the 22 ongoing trials to inquire about the status of the trials. We received a reply from all trial authors. Three trials were still ongoing (ACTRN12618000979257 2018; Loffler 2014; NCT04028583 2019), and four were finished with manuscripts under preparation (NCT03156348 2017; NCT03393299 2018; NCT03666793 2018; Ranaudin 2017).
In previous versions of the review, we obtained additional data from three trials (Gillespie 2009; Lisby 2010; Lisby 2015). During this update of the review, we received additional data from seven trials (Blum 2021; Gustafsson 2017; Kempen 2021; Lea 2020; Lenssen 2018; O'Mahony 2020; Ravn‐Nielsen 2018). In addition, we received additional descriptions of the methods for 12 trials (Bladh 2011; Blum 2021; Bonnerup 2014; Cossette 2017; Gustafsson 2017; Juanes 2018; Kempen 2021; Lea 2020; Lenssen 2018; Lisby 2010; O'Mahony 2020; Ravn‐Nielsen 2018).
In summary, with 10 trials included in the previous version and 15 newly included trials, this review now includes 25 trials (see Characteristics of included studies).
Included studies
Setting
The 25 trials included a total of 15,076 participants and reported follow‐up from 1 to 20 months. Trial reports were published between 2006 and 2021. Two trials were conducted in the US (Farris 2014; Schnipper 2006), one in Canada (Cossette 2017), one in Brazil (Bonetti 2018), one in South Korea (Song 2021), 18 in Europe (one in Belgium (Dalleur 2014); six in Denmark (Bonnerup 2014; Graabaek 2019; Lisby 2010; Lisby 2015; Nielsen 2017; Ravn‐Nielsen 2018); one in Germany (Lenssen 2018); two in Ireland (Curtin 2020; Gallagher 2011); one in Northern Ireland (Scullin 2007); one in Norway (Lea 2020); one in Spain (Juanes 2018); four in Sweden (Bladh 2011; Gillespie 2009; Gustafsson 2017; Kempen 2021); and one in the Netherlands (SUREPILL 2015)). A multinational trial was conducted in Switzerland, the Netherlands, Belgium and Ireland (Blum 2021) and another in Ireland, Scotland, Spain, Italy, Belgium and Iceland (O'Mahony 2020).
Nine trials included participants admitted to departments of internal medicine (Bladh 2011; Bonnerup 2014; Dalleur 2014; Gillespie 2009; Kempen 2021; Lea 2020; Lenssen 2018; Lisby 2010; Scullin 2007), one was from a cardiology department (Bonetti 2018), one from a nephrology department (Song 2021), two from surgical departments (Lisby 2015; SUREPILL 2015), three from acute admission departments (Graabaek 2019; Nielsen 2017; Ravn‐Nielsen 2018), one from a general medicines service (Schnipper 2006), four from both internal medicine and surgical departments (Blum 2021; Farris 2014; Gustafsson 2017; O'Mahony 2020), two from a tertiary medical referral hospital (Gallagher 2011; Juanes 2018), and two did not specify which departments participants were included from (Cossette 2017; Curtin 2020).
Participants
Fourteen trials used age as an inclusion criterion (nine trials included participants of 65 years or older (Cossette 2017; Gallagher 2011; Graabaek 2019; Gustafsson 2017; Juanes 2018; Kempen 2021; Lenssen 2018; Lisby 2015; O'Mahony 2020), two included 70 years or older (Blum 2021; Lisby 2010), two included 75 years or older (Curtin 2020; Dalleur 2014) and one included 80 years or older (Gillespie 2009)). In general, the mean trial participant age was around 75 years (range of means: 53 to 87 years), the mean proportion of women was 55% (range of means: 40% to 71%), and the mean number of medications per participant was 9 (range of means: 7 to 16).
Design
Twenty‐two trials were randomised at an individual level. Three trials were cluster‐randomised, of which one trial was at ward‐level at three hospitals (SUREPILL 2015), one trial at physician‐level at four hospitals (Blum 2021), and one trial at ward‐level at four hospitals in a cross‐over design, where each ward acted as its own control (Kempen 2021). Twenty trials compared medication review with standard care (Bladh 2011; Blum 2021; Bonetti 2018; Bonnerup 2014; Cossette 2017; Curtin 2020; Dalleur 2014; Gallagher 2011; Gillespie 2009; Gustafsson 2017; Lea 2020; Lenssen 2018; Lisby 2010; Lisby 2015; Nielsen 2017; O'Mahony 2020; Schnipper 2006; Scullin 2007; Song 2021; SUREPILL 2015), four trials had three intervention groups and compared two different types of medication reviews and standard care (Farris 2014; Graabaek 2019; Kempen 2021; Ravn‐Nielsen 2018), and one trial compared two different types of medication reviews (Juanes 2018).
Types of interventions
Who performed the medication reviews
The medication review was performed by a pharmacist in 13 trials (Bladh 2011; Cossette 2017; Farris 2014; Gillespie 2009; Graabaek 2019; Gustafsson 2017; Juanes 2018; Lea 2020; Lenssen 2018; Nielsen 2017; Ravn‐Nielsen 2018; Schnipper 2006; Song 2021), by a team of pharmacists and pharmacy technicians in two trials (Scullin 2007; SUREPILL 2015), by a physician in four trials (Curtin 2020; Dalleur 2014; Gallagher 2011; O'Mahony 2020), by a pharmacist and/or a physician specialised in clinical pharmacology in three trials (Bonnerup 2014; Lisby 2010; Lisby 2015), by a team of cardiovascular pharmacy residents and cardiologists in one trial (Bonetti 2018), by a trained research physician and pharmacist in one trial (Blum 2021) and by a pharmacist, who collaborated with a physician and sometimes a nurse, in one trial (Kempen 2021).
The content of the medication reviews
Medication reviews were non‐criteria‐based in 19 trials. In six trials, the medication review was done using published criteria: the validated Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP) criteria was used in two trials (Dalleur 2014; Gallagher 2011); the latter trial also used the Screening Tool to Alert to Right Treatment (START) criteria. In two trials, the medication review was based on a computerised decision support system encompassing the STOPP/START criteria, i.e. SENATOR software (O'Mahony 2020), or the systematic tool to reduce inappropriate prescribing (STRIP) software (Blum 2021). In one trial, the medication review was done using the STOPPFrail Screening Tool of Older Persons Prescriptions in Frail adults with limited life expectancy criteria (Curtin 2020), and in one trial the medication review was based on a web‐based clinical decision support system (MiniQ) (Bladh 2011).
In 19 trials, the intervention group received other co‐interventions (e.g. discharge counselling or written information to a primary care physician) in addition to a basic medication review (see Appendix 2 for overview of co‐interventions). In six trials there were no co‐interventions, i.e. interventions were basic medication reviews.
The implementation of the medication reviews
In six trials, the medication reviews resulted in a written recommendation to the prescribing physicians (Farris 2014; Juanes 2018; Lisby 2010; Lisby 2015; Nielsen 2017; O'Mahony 2020), in eight trials, the medication reviews were discussed with the prescribing physicians (Bladh 2011; Blum 2021; Cossette 2017; Gustafsson 2017; Lea 2020; Lenssen 2018; Schnipper 2006; SUREPILL 2015), in six trials the recommendations were both discussed and written down (Bonnerup 2014; Curtin 2020; Dalleur 2014; Gallagher 2011; Graabaek 2019; Ravn‐Nielsen 2018), and five trials did not specify how the medication review was delivered (Bonetti 2018; Gillespie 2009; Kempen 2021; Scullin 2007; Song 2021).
The proportion of medication reviews that resulted in a recommendation for medication changes in the medication review group was reported in five trials and ranged from 58% to 91% in the included trials (58% (Gallagher 2011), 60% (Schnipper 2006), 66% (Gustafsson 2017), 86% (Blum 2021), and 91% (Curtin 2020)). The proportion of medication review recommendations that were subsequently implemented by the prescribing physicians was reported in 16 trials and ranged from 15% to 93% in the included trials (15% (O'Mahony 2020), 18% (Lisby 2015), 36% (Bladh 2011), 39% (Lisby 2010), 40% (Dalleur 2014), 43% (Blum 2021), 55% (Lea 2020), 57% (Graabaek 2019), 66% (Ravn‐Nielsen 2018), 65% (Bonnerup 2014), 72% (Lenssen 2018), 73% (Kempen 2021), 75% (Gillespie 2009), 82% (Gustafsson 2017; Song 2021), 88% (Curtin 2020), and 93% (Gallagher 2011)).
Data not included in the meta‐analysis
Four trials were not included in any of our meta‐analyses due to incomplete data or methodological issues; instead the results are reported descriptively below. Two of these trials reported hospital readmissions and hospital emergency department contacts as a composite outcome and separate data on readmissions and emergency department contacts could not be obtained from the authors (Schnipper 2006; Song 2021). In one trial, a subgroup of patients were randomised more than once and to both the control and intervention groups for separate hospitalisations, and we were unable to get separate data for the participants being randomised only once (Cossette 2017). One trial had substantial methodological shortcomings and we deemed the risk of bias too high to include the trial in the meta‐analysis and reported the results descriptively instead (see Risk of bias in included studies) (SUREPILL 2015). We did not include the cluster‐randomised cross‐over trial Kempen 2021 in our primary meta‐analyses due to the high to risk of bias inherent to the cross‐over design (Higgins 2022), but we included it in the sensitivity analyses (see Analysis 4.7; Analysis 4.8; Analysis 4.9).
4.7. Analysis.

Comparison 4: Sensitivity analysis ‐ Trials comparing medication reviews with standard care, Outcome 7: Mortality (all‐cause) ‐ including adjusted data from cluster‐randomised cross‐over trials
4.8. Analysis.

Comparison 4: Sensitivity analysis ‐ Trials comparing medication reviews with standard care, Outcome 8: Hospital readmissions (all‐cause) ‐ including adjusted data from cluster‐randomised cross‐over trials
4.9. Analysis.

Comparison 4: Sensitivity analysis ‐ Trials comparing medication reviews with standard care, Outcome 9: Hospital emergency department contacts (all‐cause) ‐ including adjusted data from cluster‐randomised cross‐over trials
Excluded studies
See Characteristics of excluded studies for the complete list of excluded studies with reasons for exclusion.
Risk of bias in included studies
The risk of bias in the included trials is described in the Characteristics of included studies section (see Figure 2 and Figure 3 for a graphical display).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. White spaces in this figure represent instances where it was not possible to make a judgement regarding objective or non‐objective outcomes.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial. White spaces in this figure represent instances where it was not possible to make a judgement regarding outcomes (e.g. outcome not included in relevant trial).
Allocation
We judged that 17 trials had a low risk of selection bias as they reported adequate allocation sequence generation and allocation concealment (Bladh 2011; Blum 2021; Bonnerup 2014; Cossette 2017; Curtin 2020; Farris 2014; Gallagher 2011; Gillespie 2009; Graabaek 2019; Gustafsson 2017; Kempen 2021; Lea 2020; Nielsen 2017; O'Mahony 2020; Ravn‐Nielsen 2018; Schnipper 2006; Scullin 2007). Four trials had an unclear risk of selection bias (Bonetti 2018; Lisby 2010; Lisby 2015; Song 2021) (for details see Appendix 3). Four trials had a high risk of selection bias (Dalleur 2014; Juanes 2018; Lenssen 2018; SUREPILL 2015). In two of these, the randomisation list was accessible to the person including participants (Juanes 2018; Lenssen 2018). In one trial, it was not described how the randomisation sequence was generated, but a study nurse both generated the sequence and included participants (Dalleur 2014). In the last trial, there was no description of the method for cluster‐randomisation, and both intervention groups (each including participants from three surgical wards) had the exact same number of participants, which we judged as unlikely to have happened by chance (SUREPILL 2015). For details see Appendix 3.
Blinding
We judged the risk of performance bias to be high in all trials. Twenty‐four trials described directly or indirectly that participants or personnel were not blinded, whereas the remaining trial was described as double‐blinded (Dalleur 2014). However, we deemed it unlikely that it is possible to blind the ward physicians responsible for implementation of the medication review intervention.
Nine trials reported blinded outcome assessment (Curtin 2020; Farris 2014; Gillespie 2009; Gustafsson 2017; Kempen 2021; Lea 2020; Lenssen 2018; Schnipper 2006; Song 2021), and the remaining 16 trials did not report blinded outcome assessment. For the outcomes mortality, readmissions and/or emergency department contacts, we judged it unlikely that awareness of group assignments would lead to a risk of detection bias and we judged that these outcomes had a low risk of detection bias for all trials.
All eight trials assessing hospital readmissions due to adverse drug events used blinded outcome assessment (i.e. low risk of detection bias) (Blum 2021; Gillespie 2009; Graabaek 2019; Gustafsson 2017; Kempen 2021; Lenssen 2018; Ravn‐Nielsen 2018; Schnipper 2006). Two trials assessed hospital emergency department contacts due to adverse drug events, of which one had blinded outcome assessment (Schnipper 2006). We assessed the other trial as having an unclear risk of detection bias as blinding of the causality assessment was not described (Gillespie 2009). Six trials assessed adverse drug events, of which five had blinded outcome assessment (Blum 2021; Curtin 2020; Farris 2014; Lenssen 2018; Schnipper 2006). We assessed the last trial as having a high risk of detection bias as the assessment was performed by an unblinded physician (Gallagher 2011).
Seven trials assessed health‐related quality of life after a follow‐up period and we judged five of these trials as having low risk of detection bias, as they either reported blinded outcome assessment (Blum 2021; Bonnerup 2014), or sent questionnaires to participants by postal mail (Lisby 2010; Lisby 2015; SUREPILL 2015). We assessed the remaining two trials as having a high risk of detection bias (Bladh 2011; Curtin 2020). One trial because the outcome assessors was not blinded (Bladh 2011), and the other trial because participants were not blinded (Curtin 2020). In the latter trial many participants had dementia, and the outcome assessors (nurses) assessed health‐related quality of life (secondary outcome).
Incomplete outcome data
For the outcomes mortality, readmissions and/or emergency department contacts, we judged that 18 trials had a low risk of attrition bias due to almost complete follow‐up in many cases through national registers (Bladh 2011; Blum 2021; Bonnerup 2014; Curtin 2020; Gallagher 2011; Gillespie 2009; Graabaek 2019; Gustafsson 2017; Juanes 2018; Kempen 2021; Lea 2020; Lenssen 2018; Lisby 2010; Lisby 2015; Nielsen 2017; O'Mahony 2020; Ravn‐Nielsen 2018; Song 2021). Five trials had a high risk of attrition bias because of a relatively high loss to follow‐up (15% to 58%) or unbalanced loss to follow‐up between groups (Bonetti 2018; Cossette 2017; Dalleur 2014; Schnipper 2006; SUREPILL 2015). In these trials participants may have been lost to follow‐up because of having an event (e.g. participant did not reply to telephone concerning readmissions as participant was hospitalised). Two trials had an unclear risk of attrition bias due to discrepancies between participants reported lost to follow‐up and participants excluded from analysis (Farris 2014; Scullin 2007).
Of the seven trials reporting on health‐related quality of life, all had a high risk of attrition bias, primarily because of relatively high loss to follow‐up for this outcome (23% to 54%) (Bladh 2011; Blum 2021; Bonnerup 2014; Curtin 2020; Lisby 2010; Lisby 2015; SUREPILL 2015). For details, see Appendix 3.
Selective reporting
Eighteen trials had low risk of reporting bias (Bladh 2011; Blum 2021; Bonnerup 2014; Cossette 2017; Curtin 2020; Farris 2014; Gallagher 2011; Gillespie 2009; Gustafsson 2017; Juanes 2018; Kempen 2021; Lenssen 2018; Lisby 2010; Lisby 2015; Nielsen 2017; O'Mahony 2020; Scullin 2007; SUREPILL 2015). We judged one trial as having a high risk of selective outcome reporting (Lea 2020). In the protocol, follow‐up was planned to be up to 12 months, and in the publication there was no statistically significant effect of medication reviews at 12 months, however the authors also reported a statistically significant effect of medication reviews on mortality at 20 months, a time point that was not pre‐specified in the protocol. They did not report any other outcomes at 20 months (Lea 2020). We judged six trials as having unclear risk of selective outcome reporting: five trials had no trial registrations to compare with published results (Bonetti 2018; Dalleur 2014; Graabaek 2019; Schnipper 2006; Song 2021), and for one trial the protocol was dated after trial initiation and trial registration was after trial completion (trial start September 2013, date of protocol March 2014, trial completion April 2015 and trial registration March 2017 (Ravn‐Nielsen 2018)).
The funnel plot for all‐cause mortality showed no sign of publication bias (Figure 4).
4.

Other potential sources of bias
Two trials were cluster‐randomised and we judged that these had low risk of contamination bias as all participants at a specific ward received the same intervention (Blum 2021; SUREPILL 2015). We judged the remaining 23 trials as having a high risk of contamination bias as intervention components may have been delivered unintentionally to other patients on the wards.
Twenty trials had a low risk of other bias. Four trials had a high risk of other bias (Dalleur 2014; Kempen 2021; Scullin 2007; SUREPILL 2015), and one trial had an unclear risk of other bias (Bonnerup 2014). For details see Appendix 3.
Effects of interventions
See: Table 1
Medication review compared with standard care for hospitalised adult patients
See Table 1 for the main comparison.
Mortality (all‐cause)
Nineteen trials reported all‐cause mortality and of these we included 18 trials with data from 10,108 participants and a median follow‐up of six months (range: 1 to 20 months) in a meta‐analysis (Analysis 1.1). We found that medication reviews in hospitalised adults may have little to no effect on mortality (risk ratio (RR)) 0.96, 95% confidence interval (CI) 0.87 to 1.05; I2 = 0%; low‐certainty evidence). The last trial is a cluster‐randomised, cross‐over trial (Kempen 2021), and we included it in the sensitivity analysis (Analysis 4.7).
1.1. Analysis.

Comparison 1: Primary outcome ‐ Trials comparing medication reviews with standard care, Outcome 1: Mortality (all‐cause)
Mortality (due to adverse drug events)
One trial reported in‐hospital mortality due to adverse drug events at six months follow‐up (Ravn‐Nielsen 2018). Mortality outside the hospital was not assessed for causality (i.e. drug‐related or not) because of sparse information from the primary care sector regarding the causes of death. In this trial the effect of medication reviews on in‐hospital mortality due to adverse drug events was uncertain (RR 0.69, 95% CI 0.24 to 1.96).
Hospital readmissions (all‐cause)
Twenty‐three trials reported all‐cause hospital readmissions. Seventeen trials with data from 9561 participants and a median follow‐up of six months (range: 1 to 12 months) reported all‐cause hospital readmissions as a dichotomous outcome and we included them in a meta‐analysis (Analysis 2.1). We found that medication reviews in hospitalised adults likely reduce hospital readmissions (RR 0.93, 95% CI 0.89 to 0.98; I2 = 0%; moderate‐certainty evidence). This corresponds to a relative risk reduction of 7%, equal to a number needed to treat of 22 (95% CI 14 to 77) for a very high‐risk population and 29 (95% CI 18 to 100) for a high‐risk population after a median follow‐up of six months (Table 1).
2.1. Analysis.

Comparison 2: Secondary outcomes ‐ Trials comparing medication reviews with standard care, Outcome 1: Hospital readmissions (all‐cause)
Four trials reported continuous data for 448 participants with three months of follow‐up and we included them in a meta‐analysis (Analysis 2.2). The effect of medication reviews on the number of readmissions per participant was uncertain (mean difference (MD) 0.01, 95% CI ‐0.14 to 0.17; I2= 26%). Three trials reported continuous data for 1449 participants with 12 months of follow‐up and we included them in a meta‐analysis (Analysis 2.3). The effect of medication reviews on the number of readmissions per participant was uncertain (MD ‐0.13, 95% CI ‐0.28 to 0.03; I2 = 0%).
2.2. Analysis.

Comparison 2: Secondary outcomes ‐ Trials comparing medication reviews with standard care, Outcome 2: Hospital readmissions (all‐cause) ‐ 3 months
2.3. Analysis.

Comparison 2: Secondary outcomes ‐ Trials comparing medication reviews with standard care, Outcome 3: Hospital readmissions (all‐cause) ‐ 12 months
Six trials were not included in the meta‐analysis. Two trials reported the effect of medication reviews on hospital readmissions and hospital emergency department contacts as a composite outcome (RR 1.02, 95% CI 0.65 to 1.61) (Schnipper 2006) and RR 1.31, 95% CI 0.69 to 2.45 (Song 2021)). One trial reported the effect on 30‐day post‐discharge readmissions (i.e. not follow‐up at time of randomisation): the RR was 0.73 (95% CI 0.43 to 1.22) (Cossette 2017). Another trial reported the mean number of hospital readmissions per participant at three months follow‐up as: 0.73 (95% CI 0.37 to 1.09) in the medication review group, and 1.07 (95% CI 0.67 to 1.47) in the control group (P = 0.09) (Bonnerup 2014). One trial reported hospital readmissions at three months: the RR was 1.31 (95% CI 0.98 to 1.76) (unadjusted for clustering) (SUREPILL 2015). The last trial is a cluster‐randomised cross‐over trial and we included it in the sensitivity analysis (Analysis 4.8) (Kempen 2021).
Hospital readmissions (due to adverse drug events)
Eight trials reported hospital readmissions due to adverse drug events. Six trials with data from 4836 participants and a median follow‐up of six months (range: 6 to 12 months) reported dichotomous data on hospital readmissions due to adverse drug events and we included them in a meta‐analysis (Analysis 2.4). Medication reviews may reduce hospital readmissions due to adverse drug events (RR 0.75, 95% CI 0.58 to 0.98; I2 = 63%).
2.4. Analysis.

Comparison 2: Secondary outcomes ‐ Trials comparing medication reviews with standard care, Outcome 4: Hospital readmissions (due to drug‐related adverse events)
Two trials also reported continuous data for hospital readmissions due to adverse drug events for 428 participants with 12 months follow‐up and we included them in a meta‐analysis (Analysis 2.5). Medication reviews may reduce the number of hospital readmissions due to adverse drug events per participant (MD ‐0.18, 95% CI ‐0.26 to ‐0.10; I2 = 0%). One trial reported continuous data for 329 participants with six months follow‐up, and the effect of medication reviews on the number of readmissions due to adverse drug events per participant was uncertain (MD 0.00, 95% CI ‐0.20 to 0.20) (Gustafsson 2017).
2.5. Analysis.

Comparison 2: Secondary outcomes ‐ Trials comparing medication reviews with standard care, Outcome 5: Hospital readmissions (due to drug‐related adverse events) ‐ 12 months
Two trials were not included in the meta‐analysis. One trial reported hospital readmissions and hospital emergency department contacts due to adverse drug events as a composite outcome (RR 0.52, 95% CI 0.16 to 1.72) (Schnipper 2006). The second trial reported results as a hazard ratio (HR): HR 0.89, 95% CI 0.69 to 1.16 for basic medication reviews versus usual care, and HR 1.12, 95% CI 0.87 to 1.45 for extended medication review versus usual care (Kempen 2021). The estimates were adjusted for clustering, study period effect and unplanned hospital visits 12 months before inclusion.
Hospital emergency department contacts (all‐cause)
Fourteen trials reported all‐cause hospital emergency department contacts. Eight trials with data from 3527 participants with a median follow‐up of six months (range: 1 to 12 months) reported dichotomous data on hospital emergency department contacts and we included them in meta‐analysis (Analysis 2.6). We found that medication reviews in hospitalised adults may reduce emergency department contacts (RR 0.84, 95% CI 0.68 to 1.03; I2 = 31%; low‐certainty evidence).
2.6. Analysis.

Comparison 2: Secondary outcomes ‐ Trials comparing medication reviews with standard care, Outcome 6: Hospital emergency department contacts (all‐cause)
Five trials reported continuous data on hospital emergency department contacts. Of these, four trials reported continuous data for 448 participants with three months of follow‐up and we included them in a meta‐analysis (Analysis 2.7). The effect of medication reviews on the number of emergency department contacts per participant was uncertain (MD ‐0.05, 95% CI ‐0.14 to 0.04; I2 = 0%). One trial reported continuous data for 368 participants with 12 months of follow‐up and the medication reviews may have reduced the number of emergency department contacts per participant (MD ‐0.23, 95% CI ‐0.43 to ‐0.03) (Gillespie 2009).
2.7. Analysis.

Comparison 2: Secondary outcomes ‐ Trials comparing medication reviews with standard care, Outcome 7: Hospital emergency department contacts (all‐cause) ‐ 3 months
Six trials were not included in the meta‐analysis. Two trials reported the effect of medication reviews on hospital readmissions and hospital emergency department contacts as a composite outcome (Schnipper 2006; Song 2021) (see results above). One trial reported the effect of medication reviews on 30‐day post‐discharge emergency department contacts (i.e. not follow‐up at time of randomisation): RR 1.02, 95% CI 0.63 to 1.63 (Cossette 2017). One trial reported the effect of medication reviews on the median number of emergency department contacts per participant at 180 days post‐discharge (median: 0 in all intervention groups, P = 0.87) (Graabaek 2019). Another trial reported the effect on the mean number of emergency department contacts per participant at three months (medication review group: 0.19, 95% CI 0.07 to 0.30, control group: 0.25, 95% CI 0.06 to 0.43, P = 0.83) (Bonnerup 2014). The last trial is a cluster‐randomised cross‐over trial and we included it in the sensitivity analysis (Analysis 4.9) (Kempen 2021).
Hospital emergency department contacts (due to adverse drug events)
One trial with data from 368 participants with 12 months of follow‐up reported the effect of mediation reviews on emergency department contacts due to adverse drug events as a dichotomous outcome (RR 0.45, 95% CI 0.14 to 1.45) (Gillespie 2009). This trial also reported continuous data on the effect of medication reviews on the number of emergency department contacts per participant, which was uncertain (MD ‐0.03, 95% CI ‐0.07 to 0.01).
Adverse drug events
Five trials reported adverse drug events. One trial reported the effect of medication reviews on adverse drug events (RR 1.08, 95% CI 0.53 to 2.18) (Schnipper 2006). Another trial reported the effect of medication reviews on falls as an adverse drug event (RR 0.69, 95% CI 0.33 to 1.46) (Gallagher 2011). Another trial reported the effect of medication reviews on falls and non‐vertebral fractures as adverse drug events (RR 0.90, 95% CI 0.48 to 1.69 for falls and RR 0.23, 95% CI 0.03 to 1.95 for non‐vertebral fractures) (Curtin 2020). One trial reported adverse events and adverse drug events as a composite outcome (Farris 2014). We were unable to get separate data on adverse drug events from the author and therefore we did not include data for this outcome. The last trial reported the effect of medication reviews on falls (HR 0.96, 95% CI 0.79 to 1.15 (not adjusted for clustering)) (Blum 2021).
Health‐related quality of life
Seven trials reported health‐related quality of life using a variety of scales. Two trials used the EuroQol‐visual analogue scale (EQ‐VAS) (Lisby 2010; Lisby 2015), where patients provide an overall assessment of their health. Three trials reported results measured using both the EQ‐VAS and EQ‐5D (Blum 2021; Bonnerup 2014; SUREPILL 2015). The EQ‐5D is a five‐dimensional health state classification. One trial reported EQ‐5D, EQ‐VAS and self‐rated global health (Bladh 2011). One trial reported both ICECAP‐O, which targeted older people, and the QUALIDEM questionnaire, a dementia‐specific instrument (Curtin 2020). Four trials reported no baseline assessment (Blum 2021; Lisby 2010; Lisby 2015; SUREPILL 2015).
Four trials with data from 569 participants and follow‐up from three to six months reported continuous data using either the EQ‐VAS or QUALIDEM and we included them in the meta‐analysis (Analysis 2.8) (Bladh 2011; Curtin 2020; Lisby 2010; Lisby 2015). We found that the effect of medication reviews on health‐related quality of life is very uncertain (standardised mean difference (SMD) 0.10, 95% CI ‐0.10 to 0.30; I2 = 0%; very low‐certainty evidence).
2.8. Analysis.

Comparison 2: Secondary outcomes ‐ Trials comparing medication reviews with standard care, Outcome 8: Health‐related quality of life
Three trials were not included in the primary meta‐analysis. One trial reported the effect of medication reviews on the EQ‐VAS score after a median follow‐up of three months (medication review group 70, interquartile range (IQR) 60 to 80, and control group 70, IQR 60 to 80, P = 0.10) (SUREPILL 2015). One trial reported the effect of medication reviews on the EQ‐VAS score after 12 months (adjusted MD 2.26, 95% CI 0.18 to 4.34) (Blum 2021). The last trial reported the effect of medication review as the mean difference between baseline and three months follow‐up for EQ‐5D (intervention group 0.03, 95% CI ‐0.02 to 0.08, control group 0.01, 95% CI ‐0.05 to 0.07, P = 0.65) and EQ‐VAS (intervention group 8.47, 95% CI 0.98 to 12.78, control group 6.89, 95% CI 2.32 to 14.62, P = 0.72) (Bonnerup 2014). We did not include data from Bonnerup 2014 in the meta‐analysis because data are reported for all participants in the intervention group and not separately for the subgroup of participants that received a medication review.
Subgroup analysis and investigation of heterogeneity
For the subgroup analysis we only report full results when the interaction test had a P value of 0.1 or lower.
Comparison of trials with participants taking a ‘mean number of ≥ 10 different medications’ versus trials with participants taking a ‘mean of < 10 different medications’ uncovered little to no difference in the effect of medication reviews on mortality (Analysis 3.1), or hospital readmissions (Analysis 3.2), but found a seemingly stronger effect of medication reviews on hospital emergency department contacts in trials with a ‘mean number of < 10 different medications’ (RR 0.59, 95% CI 0.38 to 0.94) compared with trials with participants with a ‘mean number of ≥ 10 different medications’ (RR 0.97, 95% CI 0.86 to 1.10) (test for interaction: P = 0.04) (Analysis 3.3).
3.1. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 1: Mortality (all‐cause): trials of participants taking a mean of ≥ 10 different medications versus trials of participants taking a mean of < 10 different medications
3.2. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 2: Hospital readmissions (all‐cause): trials of participants taking a mean of ≥ 10 different medications versus trials of participants taking a mean of < 10 different medications
3.3. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 3: Hospital emergency department contacts (all‐cause): trials of participants taking a mean of ≥ 10 different medications versus trials of participants taking a mean of < 10 different medications
Comparison of trials with and without medication review based on explicit criteria found little to no difference in the effect on mortality (Analysis 3.4), hospital readmissions (Analysis 3.5), and hospital emergency department contacts (Analysis 3.6).
3.4. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 4: Mortality (all‐cause): trials with criteria‐based medication review versus trials with non‐criteria‐based medication review
3.5. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 5: Hospital readmissions (all‐cause): trials with criteria‐based medication review versus trials with non‐criteria‐based medication review
3.6. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 6: Hospital emergency department contacts (all‐cause): trials with criteria‐based medication review versus trials with non‐criteria‐based medication review
Comparison of trials with high and low implementation rates found little to no difference in the effect on mortality (Analysis 3.7), hospital readmissions (Analysis 3.8), and hospital emergency department contacts (Analysis 3.9).
3.7. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 7: Mortality (all‐cause): trials with low implementation rate versus trials with high implementation rate
3.8. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 8: Hospital readmissions (all‐cause): trials with low implementation rate versus trials with high implementation rate
3.9. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 9: Hospital emergency department contacts (all‐cause): trials with low implementation rate versus trials with high implementation rate
Comparison of trials with low overall risk of bias and high overall risk of bias found no difference in the effect on hospital readmissions (Analysis 3.11), but found a seemingly stronger effect of medication reviews on mortality in trials with high overall risk of bias (RR 0.82, 95% CI 0.68 to 0.99) compared with trials with low overall risk of bias (RR 1.01, 95% CI 0.90 to 1.12) (test for interaction: P = 0.06) (Analysis 3.10) and emergency department contacts in trials with high overall risk of bias (RR 0.49, 95% CI 0.25 to 0.96) compared with trials with low overall risk of bias (RR 0.93, 95% CI 0.83 to 1.06) (test for interaction: P = 0.07) (Analysis 3.12).
3.11. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 11: Hospital readmissions (all‐cause): trials with low risk of bias versus trials with high risk of bias
3.10. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 10: Mortality (all‐cause): trials with low risk of bias versus trials with high risk of bias
3.12. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 12: Hospital emergency department contacts (all‐cause): trials with low risk of bias versus trials with high risk of bias
Comparison of trials of extended versus basic medication review interventions found little to no difference in the effect on mortality (Analysis 3.13), hospital readmissions (Analysis 3.14), and hospital emergency department contacts (Analysis 3.15).
3.13. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 13: Mortality (all‐cause): trials with extended medication reviews versus trials with basic medication reviews
3.14. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 14: Hospital readmissions (all‐cause): trials with extended medication reviews versus trials with basic medication reviews
3.15. Analysis.

Comparison 3: Subgroup analysis ‐ Trials comparing medication reviews with standard care, Outcome 15: Hospital emergency department contacts (all‐cause): trials with extended medication reviews versus trials with basic medication reviews
Sensitivity analysis
Our sensitivity analysis using a full intention‐to‐treat analysis yielded results fairly similar to our primary analysis for mortality (Analysis 4.1), readmissions (Analysis 4.2), and emergency department contacts (Analysis 4.3).
4.1. Analysis.

Comparison 4: Sensitivity analysis ‐ Trials comparing medication reviews with standard care, Outcome 1: Mortality (all‐cause) ‐ alternative ITT analysis
4.2. Analysis.

Comparison 4: Sensitivity analysis ‐ Trials comparing medication reviews with standard care, Outcome 2: Hospital readmissions (all‐cause) ‐ alternative ITT analysis
4.3. Analysis.

Comparison 4: Sensitivity analysis ‐ Trials comparing medication reviews with standard care, Outcome 3: Hospital emergency department contacts (all‐cause) ‐ alternative ITT analysis
Our sensitivity analysis using a fixed‐effect model did not change our results for mortality or hospital readmissions (Analysis 4.4; Analysis 4.5), but the statistical precision for hospital emergency department contacts increased somewhat using a fixed‐effect model (RR 0.87, 95% CI 0.76 to 0.98) (Analysis 4.6) compared to a random‐effects model (RR 0.84, 95% CI 0.68 to 1.03) (Analysis 2.6).
4.4. Analysis.

Comparison 4: Sensitivity analysis ‐ Trials comparing medication reviews with standard care, Outcome 4: Mortality (all‐cause) ‐ fixed‐effect
4.5. Analysis.

Comparison 4: Sensitivity analysis ‐ Trials comparing medication reviews with standard care, Outcome 5: Hospital readmissions (all‐cause) ‐ fixed‐effect
4.6. Analysis.

Comparison 4: Sensitivity analysis ‐ Trials comparing medication reviews with standard care, Outcome 6: Hospital emergency department contacts (all‐cause) ‐ fixed‐effect
Our sensitivity analyses for mortality, hospital readmissions and emergency department contacts including adjusted results from the cluster‐randomised cross‐over trial Kempen 2021 had results fairly similar to our primary analyses (Analysis 4.7; Analysis 4.8; Analysis 4.9).
Trials comparing two or more types of medication reviews
See Appendix 4: 'Summary of findings table 2' for the main comparisons between basic medication review interventions and extended medication review interventions for hospitalised adult patients. Only five of the included trials in this review compared two types of medication reviews head‐to‐head within the same trial, therefore we did not perform any subgroup analyses for this comparison.
Mortality (all‐cause)
Four trials with data from 2087 participants and follow‐up ranging from 3 to 12 months reported all‐cause mortality and we included them in meta‐analysis (Analysis 5.1) (Farris 2014; Graabaek 2019; Juanes 2018; Ravn‐Nielsen 2018). We found that it is very uncertain whether there is a difference in the effect on mortality between extended medication reviews and basic medication reviews (RR 1.27, 95% CI 0.95 to 1.71; I2 = 0%; very low‐certainty evidence).
5.1. Analysis.

Comparison 5: Trials comparing extended medication reviews with basic medication reviews, Outcome 1: Mortality (all‐cause)
Hospital readmissions (all‐cause)
Four trials reported all‐cause hospital readmissions and, of these, we included three trials with data from 1918 participants and follow‐up ranging from 3 to 12 months in a meta‐analysis (Analysis 5.2) (Farris 2014; Graabaek 2019; Ravn‐Nielsen 2018). We found that extended medication reviews may have little to no effect on hospital readmissions compared with basic medication reviews (RR 0.99, 95% CI 0.73 to 1.26; I2 = 58%; low‐certainty evidence).
5.2. Analysis.

Comparison 5: Trials comparing extended medication reviews with basic medication reviews, Outcome 2: Hospital readmissions (all‐cause)
One trial not included in the meta‐analysis compared the effect of medication reviews on hospital readmissions and hospital emergency department contacts as a composite outcome and found that the effect of basic medication reviews compared with extended medication review interventions had an effect of RR 1.05 (95% CI 0.66 to 1.66) (Juanes 2018).
Hospital emergency department contacts (all‐cause)
Three trials reported all‐cause hospital emergency department contacts. Of these, two trials with data from 1522 participants and follow‐up ranging from three to six months were included in the meta‐analysis (Analysis 5.3) (Farris 2014; Ravn‐Nielsen 2018). We found that extended medication reviews likely have little to no effect on hospital readmissions compared with basic medication reviews (RR 1.00, 95% CI 0.71 to 1.41; I2 = 0%; moderate‐certainty evidence). One trial not included in the meta‐analysis reported hospital readmissions and hospital emergency department contacts as a composite outcome (see results above) (Juanes 2018).
5.3. Analysis.

Comparison 5: Trials comparing extended medication reviews with basic medication reviews, Outcome 3: Hospital emergency department contacts (all‐cause)
Sensitivity analysis
Our sensitivity analysis using a full intention‐to‐treat analysis yielded results fairly similar to our primary analysis for mortality, hospital readmissions and hospital emergency department contacts (Analysis 6.1; Analysis 6.2; Analysis 6.3).
6.1. Analysis.

Comparison 6: Sensitivity analysis ‐ Trials comparing extended medication reviews with basic medication reviews, Outcome 1: Mortality (all‐cause) ‐ alternative ITT analysis
6.2. Analysis.

Comparison 6: Sensitivity analysis ‐ Trials comparing extended medication reviews with basic medication reviews, Outcome 2: Hospital readmissions (all‐cause) ‐ alternative ITT analysis
6.3. Analysis.

Comparison 6: Sensitivity analysis ‐ Trials comparing extended medication reviews with basic medication reviews, Outcome 3: Hospital emergency department contacts (all‐cause) ‐ alternative ITT analysis
Our sensitivity analysis using a fixed‐effect model did not change our results for mortality (Analysis 6.4) or hospital emergency department contacts (Analysis 6.6), but the statistical precision increased for hospital readmissions (RR 0.94, 95% CI 0.83 to 1.06) (Analysis 6.5) compared to a random‐effects model (RR 0.99, 95% CI 0.78 to 1.26).
6.4. Analysis.

Comparison 6: Sensitivity analysis ‐ Trials comparing extended medication reviews with basic medication reviews, Outcome 4: Mortality (all‐cause) ‐ fixed‐effect
6.6. Analysis.

Comparison 6: Sensitivity analysis ‐ Trials comparing extended medication reviews with basic medication reviews, Outcome 6: Hospital emergency department contacts (all‐cause) ‐ fixed‐effect
6.5. Analysis.

Comparison 6: Sensitivity analysis ‐ Trials comparing extended medication reviews with basic medication reviews, Outcome 5: Hospital readmissions (all‐cause) ‐ fixed‐effect
Discussion
Summary of main results
In this Cochrane Review, we included 25 trials enrolling 15,076 hospitalised adults and comparing a medication review intervention to standard care or to a different type of medication review intervention. The participants were primarily elderly patients receiving polypharmacy and trial follow‐up was variable, ranging from 1 to 20 months. We found that medication reviews may have little to no effect on mortality, likely reduce hospital readmissions, and may reduce emergency department contacts. The evidence is very uncertain about the effect of medication reviews on health‐related quality of life and also whether different types of medication reviews are more effective than others. Sensitivity analyses did not significantly alter the results.
Overall completeness and applicability of evidence
We included trials with hospitalised adult patients as this population is at high risk of medication harms and, at the same time, has a high risk of mortality, hospital contacts and a further decline in health‐related quality of life. The mean age of trial participants was around 75 years, and the median number of medications taken was around eight. As almost all trials included elderly patients receiving polypharmacy, the generalisability of results is limited beyond this population, e.g. to younger and perhaps less frail patients receiving fewer medications and with a lower risk of readmissions. The number needed to treat was 22 for a very high‐risk population and 29 for a high‐risk population to prevent one hospital readmission for a median follow‐up of six months. Nonetheless, follow‐up differed greatly between the trials. Only 30% (n = 7) of the trials had follow‐up at 12 months for one or more outcomes and a large proportion (43%, n = 10) had a follow‐up of only one to three months. The short follow‐up in the trials should be a caveat when interpreting the results of this review, bearing in mind that many medications are used for chronic diseases, where drug harms may occur after long‐term treatment (e.g. bleeding ulcers from non‐steroidal anti‐inflammatory drugs (NSAIDs)) or for risk conditions with long‐term prevention in mind (e.g. treatment of dyslipidaemia or diabetes for prevention of cardiovascular disease).
Mortality was only a primary outcome in one trial (Bonetti 2018), but it was reported as an outcome in all but five trials (Cossette 2017; Lenssen 2018; Schnipper 2006; Song 2021; SUREPILL 2015). Only a single trial found an effect on mortality, with a 30% relative risk reduction in mortality at 20 months (Lea 2020). However, the large effect should be interpreted with caution, as the time point was not prespecified in the protocol and the prespecified 12‐month results showed a relative risk reduction of 21%, which was not statistically significant. We did not identify any specific trial characteristics that could explain the marked effect on mortality not seen in the other included trials and therefore the effect may likely be spurious.
Five trials reported hospital readmissions due to drug‐related adverse events (Gillespie 2009; Graabaek 2019; Gustafsson 2017; Lenssen 2018; Ravn‐Nielsen 2018). We found that medication reviews resulted in a relative risk reduction of 25% in hospital readmissions due to drug‐related adverse events. However, it is possible that a medication review resulting in the discontinuation of medications might minimise adverse drug events, but lead to the undertreatment of other conditions. For example, less use of antihypertensives could lead to fewer readmissions due to falls, but more readmissions due to stroke. Unfortunately, evaluations of both overtreatment and particularly undertreatment are subjective and may be inconsistently captured as an adverse drug event due to heterogeneity in definitions and methods for obtaining the outcome across trials. Consequently, the effect of medication reviews on hospital readmissions and emergency department contacts due to adverse drug events should be considered with caution.
We excluded trials of interventions solely aimed at increasing knowledge of or adherence to medication as well as interventions that were only related to a specific part of the medication or had to be implemented after discharge. Nonetheless, the interventions in the 25 included trials differed regarding the content of the medication review, and many trials included additional co‐interventions (see Appendix 2). Based on this review, it is difficult to tease out the effects of the individual components of the medication review interventions. Medication review interventions generally consist of a medication reconciliation and a critical review of the prescriptions. The co‐interventions most often involved communication across health care sectors with written information to primary care physicians (n = 10) and/or discharge counselling (n = 11).
Our subgroup analysis found a stronger effect of medication reviews on hospital emergency department contacts in trials where participants used a mean of fewer than 10 medications as opposed to trials where participants used a mean of 10 or more medications. However, as this finding goes against the anticipated intervention effect and was not consistent across outcomes, it is likely a spurious finding. Our subgroup analysis comparing trials in which the medication review intervention explicitly used published criteria with trials in which the medication review intervention was non‐criteria‐based found no difference in effect. Criteria‐based medication reviews may be more uniform and should guide the physician to parts of the patient’s medication that might need to be adjusted. Nevertheless, it cannot be ruled out that some trials might have used the criteria in the intervention without stating it. Our subgroup analysis revealed no difference in effect between trials with co‐interventions (i.e. extended medication reviews) and trials with medication reviews alone (basic medication reviews), nor did we find any differences in effect between head‐to‐head trials comparing different types of medication reviews. However, the content of the medication reviews varied within the trials (e.g. use of patient interviews focusing on patient knowledge and preferences, use of laboratory results, and contact with relatives or home nurses), and this might influence the effect of the trials, as much as any co‐interventions. The subgroup analysis of trials with high risk of bias versus trials with low risk of bias showed a difference in effect on mortality and on hospital emergency department contacts in favour of a stronger effect in trials with high risk of bias. Because of this, our results for these outcomes should be interpreted with caution since bias could lead to an overestimation of the true intervention effect and this is also reflected in the lower certainty of evidence. Some of the included trials had a relatively low level of implementation, but surprisingly our subgroup analysis comparing trials with a high implementation rate with trials with a low implementation rate did not reveal any differences in effect – again highlighting the heterogenous nature of the medication review interventions.
Medication review interventions are time‐consuming and hence costly. Therefore, it is relevant to assess whether the interventions are cost‐effective. Four of the included trials estimated the cost of a medication review per participant to be between 24 to 170 USD (Gillespie 2009; Gustafsson 2017; Kempen 2021; Ravn‐Nielsen 2018; Rasmussen 2019 (secondary analysis of Ravn‐Nielsen 2018); Sjölander 2019 (secondary analysis of Gustafsson 2017)). A formal health economic analysis has been published based on one of these trials (Ravn‐Nielsen 2018), and it reported no difference in overall societal cost between the groups and concluded that the costs of the additional time used on medication reviews, patient interviews and follow‐ups outweighed the decrease in costs of readmissions (Rasmussen 2019 secondary reference of Ravn‐Nielsen 2018). A future cost‐effectiveness analysis based on data from our Cochrane Review could contribute to a general cost‐benefit analysis of medication review interventions.
Quality of the evidence
All studies included in this review were randomised trials, and we included data from 25 trials with 15,076 participants, contributing to the high statistical precision for many of our effect estimates. Overall, we rated the certainty of evidence from very low to moderate. Most of the included trials had some issues related to risk of bias, some had problems due to imprecision or indirectness, and some had issues with inadequate reporting. The nature of the intervention precludes blinding of participants, which may introduce performance bias. We judged outcomes to have a low risk of bias when outcome assessors were unaware of group assignment and when outcomes were fairly objective (e.g. readmissions), but whether this was sufficient to prevent detection bias is debatable. Further, most included trials had low risk of attrition bias due to almost complete follow‐up through national registers for the important outcomes mortality and readmissions. As only a minor proportion of trials (24% of all participants) had high or unclear risk of attrition bias is unlikely to substantially impact the overall results and our interpretation.
Another important source of bias is contamination bias. Although it seems unlikely that participants in the control groups should have received a similar intervention, some contamination bias might have occurred, e.g. increasing physicians' and nurses' focus on appropriate pharmacotherapy, thereby introducing bias towards the null. Surprisingly we only identified two cluster‐randomised trials with a design more able to minimise contamination bias (Blum 2021; SUREPILL 2015), though one of the trials had considerable methodological shortcomings (SUREPILL 2015). Another cluster‐randomised trial also had a cross‐over design, where around 15% of participants in the standard of care group also received an unintended medication review component (Kempen 2021). Furthermore, since included wards acted as both intervention and control arms in the trial the risk of contamination bias was marked. Nevertheless, the inclusion of the cluster‐randomised cross‐over trial in the sensitivity analysis did not alter the main findings significantly.
Potential biases in the review process
Our review was done using standard Cochrane methodology, based on a comprehensive literature search and is further strengthened by the inclusion of unpublished data. Our original version was based on a peer‐reviewed Cochrane protocol and the changes for this update were decided before data extraction, except for the inclusion of the sensitivity analyses with adjusted data from the cluster‐randomised cross‐over trial (see Differences between protocol and review).
Agreements and disagreements with other studies or reviews
Many reviews have attempted to examine the effects of medication review on hospitalised adult patients. A systematic review assessed the impact of in‐hospital, pharmacist‐led medication reviews and reported no effect on mortality, all‐cause readmissions or all‐cause emergency department contacts, though a beneficial effect of medication reviews on drug‐related readmissions was found (Renaudin 2016). A more recent systematic review included randomised and quasi‐randomised trials and assessed the effect of medication review as an isolated intervention and with several co‐interventions for preventing hospital readmissions in older adults (Dautzenberg 2021). The review reported that medication reviews in combination with medication reconciliation, patient education, professional education and transitional care reduced hospital readmissions compared to usual care. However, without co‐interventions there was no effect of medication reviews. In our review, we did not find a difference in effect for trials with or without co‐interventions (i.e. extended medication reviews). In future trials, the focus should be on examining which combination of medication review components and co‐interventions may yield the strongest effects.
In this review, we report a beneficial effect on readmissions (moderate‐certainty evidence), which was not found in the previous version of the review (Christensen 2016), though the point estimates were quite similar and the findings could be due to increased statistical power of the analysis in the current review.
Authors' conclusions
Implications for practice.
This systematic review provides evidence that medication reviews for hospitalised elderly polypharmacy patients likely reduce hospital readmissions and may reduce emergency department contacts. However, the beneficial effect of reviewing patients' medication does not seem to expand to increased survival, and the effect on quality of life is very uncertain. Based on our data, it seems reasonable to implement medication reviews in some form for hospitalised patients to prevent readmission. However, it is uncertain which form of medication review is most effective.
Implications for research.
Future trials of medication reviews should ensure a high implementation rate, long follow‐up and assess the impact of different types of co‐interventions on intervention effects. For example, by using a factorial design. The evidence for an effect on health‐related quality of life is limited and future trials should include this important outcome, preferably using a generalisable measure (e.g. EQ‐5D) and try to minimise risk of attrition bias from loss to follow‐up. Furthermore, risk of contamination bias is an important issue when investigating the effect of medication reviews and use of a cluster‐randomised design may minimise such bias. However, such trials should appropriately adjust for clustering and transparently report their methodology so that data may be included in future meta‐analyses.
What's new
| Date | Event | Description |
|---|---|---|
| 20 January 2023 | New citation required and conclusions have changed | We have updated the review (search performed January 2022) and included 15 new trials (i.e. a total of 25 trials with 15,076 participants are now included). The update led to changes to the conclusions. |
| 20 January 2023 | New search has been performed | New searches were performed on 30 October 2019 and 17 January 2022. We identified 15 new trials. |
History
Protocol first published: Issue 2, 2011 Review first published: Issue 2, 2013
| Date | Event | Description |
|---|---|---|
| 20 February 2016 | New search has been performed | Review updated (Christensen 2016). |
| 4 March 2014 | Feedback has been incorporated | Minor amendments were made. |
Acknowledgements
Cochrane Effective Practice and Organisation of Care (EPOC) supported the review authors in the development of this review. We thank Poul Miller (Information Specialist) at the EPOC group for updating the search strategy and identifying records for our review update. We thank Ana Juanes, Benoit Cossette, Denis O’Mahony, Dorthe Krogsgaard Bonnerup, Lene Ravn‐Nielsen, Maria Gustafsson, Marianne Lea, Marianne Lisby, Rebekka Lenssen, Susanna M. Wallerstedt, Thomas Kempen, Ulrika Gillespie and their co‐authors for supplying us with additional trial data or descriptions of methods used.
The following people conducted the editorial process for this review:
Sign‐off Editor (final editorial decision): Mary Ann O’Brien, Department of Family and Community Medicine, University of Toronto, Toronto, Ontario, Canada
Managing Editor (selected peer reviewers, provided comments, collated peer reviewer comments, provided editorial guidance to authors, edited the article): Lara Kahale and Sam Hinsley, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Jenny Bellorini, Cochrane Central Production Service
Peer reviewers (provided comments and recommended an editorial decision): Meagan Hayashi, Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, AB, Canada (clinical/content review), Thomas Kempen, Uppsala University, Uppsala, Sweden; Primary Care and Health, Uppsala, Sweden; Netherlands Institute for Health Services Research, Utrecht, the Netherlands (clinical/content review), Pratibha Thomas, Rajiv Gandhi University of Health Sciences, Bengaluru, India (consumer review), Rachel Richardson, Associate Editor, Cochrane Evidence Production and Methods Directorate (methods review), Joanne Abbott, Cochrane Pain, Palliative and Supportive Care (search review).
Appendices
Appendix 1. Search strategies
MEDLINE
Ovid MEDLINE <update 17 January 2022>
1 (medication adj2 (review? or reconcil* or counsel*)).ti,ab,kf.
2 ((inappropriate or appropriate or improve* or optim* or quality) adj5 (pharmacotherapy or medication* or medicine* or prescri*)).ti,ab,kf.
3 (deprescrib* or deprescript* or de‐prescrib* or de‐prescript*).ti,ab,kf.
4 ((beer* or shan? or mcleod?) adj3 criter*).ti,ab,kf.
5 integrated medicine? management.ti,ab,kf.
6 ("fit for the aged" adj3 (criter* or list? or instrument or classif*)).ti,ab,kf.
7 ((forta or rasp or priscus) adj3 (criter* or list? or instrument)).ti,ab,kf.
8 (stopp criter* or stopp list?).ti,ab,kf.
9 inappropriate prescribing/
10 potentially inappropriate medication list/
11 medication reconciliation/
12 pharmacy service, hospital/og
13 hospital*.ti,ab,kf,hw.
14 or/1‐11
15 13 and 14
16 12 or 15
17 exp randomized controlled trial/
18 controlled clinical trial.pt.
19 randomi#ed.ti,ab.
20 placebo.ab.
21 randomly.ti,ab.
22 Clinical Trials as topic.sh.
23 trial.ti.
24 or/17‐23
25 exp animals/ not humans/
26 24 not 25
27 16 and 26
28 (2014* or 2015* or 2016* or 2017* or 2018* or 2019*).dt,dp,ed,ep,yr.
29 27 and 28
EMBASE
Ovid EMBASE <update 17 January 2022>
1 (medication adj2 (review? or reconcil* or counsel*)).ti,ab,kw.
2 ((inappropriate or appropriate or improve* or optim* or quality) adj5 (pharmacotherapy or medication* or medicine* or prescri*)).ti,ab,kw.
3 (deprescrib* or deprescript* or de‐prescrib* or de‐prescript*).ti,ab,kw.
4 ((beer* or shan? or mcleod?) adj3 criter*).ti,ab,kw.
5 integrated medicine? management.ti,ab,kw.
6 ("fit for the aged" adj3 (criter* or list? or instrument or classif*)).ti,ab,kw.
7 ((forta or rasp or priscus) adj3 (criter* or list? or instrument)).ti,ab,kw.
8 (stopp criter* or stopp list?).ti,ab,kw.
9 inappropriate prescribing/
10 potentially inappropriate medication/
11 medication therapy management/
12 hospital*.ti,ab,kw,hw.
13 or/1‐11
14 12 and 13
15 hospital pharmacy/
16 "organization and management"/
17 15 and 16
18 14 or 17
19 random*.ti,ab.
20 factorial*.ti,ab.
21 (crossover* or cross over*).ti,ab.
22 ((doubl* or singl*) adj blind*).ti,ab.
23 (assign* or allocat* or volunteer* or placebo*).ti,ab.
24 crossover procedure/
25 single blind procedure/
26 randomized controlled trial/
27 double blind procedure/
28 or/19‐27
29 exp animal/ not human/
30 28 not 29
31 18 and 30
32 limit 31 to embase
33 (2014* or 2015* or 2016* or 2017* or 2018* or 2019*).yr.
34 32 and 33
The Cochrane Library
The Cochrane Library <update 17 January 2022>, Wiley
#1 (medication near/2 (review? or reconcil* or counsel*)):ti,ab
#2 ((inappropriate or appropriate or improve* or optim* or quality) near/5 (pharmacotherapy or medication* or medicine* or prescri*)):ti,ab
#3 (deprescrib* or deprescript* or de‐prescrib* or de‐prescript*):ti,ab
#4 ((beer* or shan? or mcleod?) near/3 criter*):ti,ab
#5 (integrated next medicine? next management):ti,ab
#6 ("fit for the aged" near/3 (criter* or list? or instrument or classif*)):ti,ab
#7 ((forta or rasp or priscus) near/3 (criter* or list? or instrument)):ti,ab
#8 ((stopp next criter*) or (stopp next list?)):ti,ab
#9 [mh "inappropriate prescribing"]
#10 [mh "potentially inappropriate medication list"]
#11 [mh "medication reconciliation"]
#12 [mh "pharmacy service, hospital"/OG]
#13 hospital*:ti,ab,kw
#14 {or #1‐#11}
#15 #13 and #14
#16 #12 or #15
(with Cochrane Library publication date Between Jan 2014 and Dec 2019)
CINAHL
EbscoHost CINAHL <update 17 January 2022>
S1 TI (medication N2 (review? or reconcil* or counsel*)) OR AB (medication N2 (review? or reconcil* or counsel*))
S2 TI ((inappropriate or appropriate or improve* or optim* or quality) N5 (pharmacotherapy or medication* or medicine* or prescri*)) OR AB ((inappropriate or appropriate or improve* or optim* or quality) N5 (pharmacotherapy or medication* or medicine* or prescri*))
S3 TI (deprescrib* or deprescript* or de‐prescrib* or de‐prescript*) OR AB (deprescrib* or deprescript* or de‐prescrib* or de‐prescript*)
S4 TI ((beer* or shan? or mcleod?) N3 criter*) OR AB ((beer* or shan? or mcleod?) N3 criter*)
S5 TI (integrated medicine? management) OR AB (integrated medicine? management)
S6 TI ("fit for the aged" N3 (criter* or list? or instrument or classif*)) OR AB ("fit for the aged" N3 (criter* or list? or instrument or classif*))
S7 TI ((forta or rasp or priscus) N3 (criter* or list? or instrument)) OR AB ((forta or rasp or priscus) N3 (criter* or list? or instrument))
S8 TI (stopp criter* or stopp list?) OR AB (stopp criter* or stopp list?)
S9 (MH "Inappropriate Prescribing")
S10 (MH "Medication Reconciliation")
S11 (MH "Medication Management")
S12 (MH "Pharmacy Service")
S13 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12
S14 TI hospital* OR AB hospital* OR MW hospital*
S15 S13 AND S14
S16 MH randomized controlled trials
S17 MH double‐blind studies
S18 MH single‐blind studies
S19 MH random assignment
S20 MH pretest‐posttest design
S21 MH cluster sample
S22 TI (randomised OR randomized)
S23 AB (random*)
S24 TI (trial)
S25 MH (sample size) AND AB (assigned OR allocated OR control)
S26 MH (placebos)
S27 PT (randomized controlled trial)
S28 AB (control W5 group)
S29 MH (crossover design) OR MH (comparative studies)
S30 AB (cluster W3 RCT)
S31 MH animals+
S32 MH (animal studies)
S33 TI (animal model*)
S34 S31 OR S32 OR S33
S35 MH (human)
S36 S34 NOT S35
S37 S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30
S38 S37 NOT S36
S39 S15 AND S38
S40 S39 Limiters ‐ Published Date: 20140101‐20191231; Exclude MEDLINE records
WHO International Clinical Trials Registry Platform (ICTRP)
ICTRP <update 17 January 2022>
medication reconciliation OR medication review
ClinicalTrials.gov
ClinicalTrials.gov <update 17 January 2022>
Intervention/treatment: medication reconciliation OR medication review
Other terms: hospital OR hospitalization
Interventional Studies
Search strategies from previous version of the review
MEDLINE
Ovid MEDLINE <update 18 November 2014> 1 Pharmacy service, hospital/ [ML] 2 ((PHARMACEUTICAL CARE or PHARMACY or PHARMACIES or PHARMACIST? or PRESCRIBING) and (inpatient? or hospital$ or WARD? or UNIT or UNITS)).ti. 3 ((PHARMACEUTICAL CARE or PHARMACY or PHARMACIES or PHARMACIST? or PRESCRIBING) adj2 (inpatient? or hospital$ or WARD? or UNIT or UNITS)).ab. 4 Medication Systems, Hospital/ [ML] 5 ((medication? or prescribing or prescription? or dispensing) adj2 system?).ti,ab. and (hospital$ orWARD orWARDS or (CARE adj2 UNIT?) or INPATIENT?).ti,hw. 6 (stopp or beer’s criteria).ti,ab. [Term added Aug 2011] 7 or/1‐6 [Hosp Pharm/Med Systems] 8 exp Hospitals/ or exp Hospital Units/ [ML] 9 (hospital$ or WARD or WARDS).ti. 10 Hospitalization/ [ML] 11 hospital$.ab. Medication review in hospitalised patients to reduce morbidity and mortality (Review) 70 Copyright © 2016 The Cochrane Collaboration. Published by JohnWiley & Sons, Ltd. 12 “length of stay”/ or Patient admission/ or Patient discharge/ or Patient readmission/ or Patient transfer/ [ML] 13 ((patient? or hospital$).ti,hw. and (discharg$ or admission? or admitting or readmission? or readmit$ or transfer?).ti.) or “length of stay”.ti. 14 (((patient? or hospital?) adj2 (discharg$ or admission? or admitting or readmission? or transfer?)) or “length of stay”).ab. 15 Inpatients/ [ML] 16 (inpatient? or in‐patient?).ti. 17 exp HOSPITAL DEPARTMENTS/ or HOSPITAL SHARED SERVICES/ [ML] 18 MEDICAL STAFF, HOSPITAL/ or HOSPITALISTS/ [ML] 19 or/8‐18 [Hospitals/Hospitalization/Inpatients] 20 (pharmacy or pharmacies or pharmacist? or prescription? or prescribing).ti. 21 (pharmacist‐led or pharma$ initiated or ((driven or lead or led) adj2 pharmacist?)).ab. 22 (PRESCRIBING adj2 PATTERN?).ab. 23 (“physician‐pharmacist?” or “doctor‐pharmacist?”).ti,ab. 24 ((IMPROV$ or OPTIMI?ING or OPTIMI?E? or OPTIMAL$) and (DOSING or DOSAGE or PHARMAC$ or PRESCRIB$ or PRESCRIPT$)).ti. or ((IMPROV$ or OPTIMI?ING or OPTIMI?E? or OPTIMAL$) adj2 (PHARMACEUTICAL CARE or PHARMACY or PRESCRIB$ or PRESCRIPT$)).ab. 25 ((pharmaceutical adj (care or consult$)) or (pharmacist? adj2 (care or consult$ or intervention? or managed))).ab. 26 (((prescription? or prescribing or medication?) adj4 review$) or (pharmacist? adj2 review$)).ti,ab. 27 ((drug therapy or drug regime? or medication? or medicineS or pharmacy or pharmacist? or pharmaceutical or PRESCRIB$ or prescription?) adj2 (audit$ or monitor$ or RECONCIL$ or review?)).ti,ab. 28 ((medication? or prescrib$ or pharmac$) adj2 (manage? or management or service? or system?)).ti,ab. 29 ((“drug therapy” or dosage? or dose? or medication? or PRESCRIPTION? or PRESCRIB$ or PHARMACIST? or PHARMACEUTICAL CARE) adj2 (managing or management or monitor$)).ti,ab. 30 (drug? review? or drug? assess$ or drug? audit? or drug? reconcil$).ti,ab. 31 (“drug utili?ation” adj2 (review? or reconcil$ or audit?)).ab. or (“drug utili?ation” and (review? or reconcil$ or audit?)).ti. 32 Medication adherence/ [ML] 33 Pharmacists/ or Pharmacists’ Aides/ [ML] 34 Pharmaceutical Services/ or Drug Information Services/ [ML] 35 Clinical Pharmacy Information Systems/ [ML] 36 Prescriptions/ or Drug Prescriptions/ or Pharmaceutical Preparations/ or Drug Therapy/ or DrugDosage Calculations/ or Electronic Prescribing/ or Medication Systems/ [ML] 37 Drug Monitoring/ or Medication Therapy Management/ [ML] 38 Drug Therapy/ or Drug Therapy, Computer‐Assisted/ [ML] 39 POLYPHARMACY/ or POLYPHARM$.ti. [ML] 40 MEDICATION ERRORS/ [ML] 41 Drug utilization review/ [ML] 42 Drug Utilization/ [ML] 43 inappropriate prescribing/ [Term added Aug 2011] 44 ((Medication? or prescrib$ or prescription? or drug therap$) adj2 assessment?).ti,ab. [Term added Aug 2011] 45 (inappropriate$ adj2 (medicine? or medication? or prescrib$ or drug?)).ti,ab. [Term added Aug 2011] 46 or/20‐45 [PHARMA/DRUG CONCEPTS ‐‐combine with hospital concepts] 47 (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly. ab. or trial.ti. 48 exp animals/ not humans.sh. 49 47 not 48 [Cochrane RCT Filter 6.4.d Sens/Precision Maximizing] 50 7 and 49 [Hosp Pharma & RCT] 51 19 and 46 and 49 [Hospitals & Pharma/Drug sets & RCT] 52 50 or 51 53 limit 52 to yr=“1980 ‐Current” 54 (2012$ or 2013$ or 2014$).ed,ep,dp. [Entry date, E‐pub date, Pub Date] 55 (198$ or 199$ or 2$).ep. [Electronic publication date 1980 to present] 56 (201108$ or 201109$ or 20111$).ed,dp. [August 2011‐Dec2011] 57 52 and 54 58 (52 and 55) not 57 59 (52 and 56) not (or/57‐58) 60 52 and 2011$.dp,ep,yr,ed. [2011 all date search] 61 60 not (or/57‐59) 62 57 or 58 or 59 or 61 [Results to export Jan 7 2013 update search] 63 remove duplicates from 62
EMBASE
Ovid EMBASE <update 18 November 2014> 1 *hospital pharmacy/ not outpatient?.ti. [EM] 2 hospital? pharmacy.ti. 3 ((pharmaceutical care or pharmacist? or prescribing) adj4 (inpatient? or hospital$ or ward? or ICU or intensive care or (emergency adj2 (room? or department? or unit or units)))).ti. 4 ((pharmaceutical care or pharmacist? or prescribing) adj3 (inpatient? or hospital$ or ward? or ICU or intensive care or (emergency adj2 (room? or department? or unit or units)))).ab. 5 ((medication? or prescribing or prescription? or dispensing) adj2 system?).ti,ab. and (hospital$ or ward or wards or (care adj2 unit?) or inpatient?).ti,hw. 6 (medication? adj4 (review$ or audit$)).ti. and (hospital$ or ward or wards or (care adj2 unit?) or inpatient?).ti,hw. 7 (stopp or beer’s criteria).ti,ab. [Term added Aug 2011] 8 or/1‐7 [Hosp Medication Rev or Hosp Pharm‐‐combine with Filters] 9 ((medication? or medicine?) adj4 (review or audit)).ti. 10 ((medication? or medicine?) adj2 (review or audit)).ab. 11 (((prescription? or prescribing) adj4 review$) or (pharmacist? adj2 review$)).ti,ab. 12 ((drug formulary or drug therapy or drug regime? or medication? or medicines or pharmacy or pharmacist? or pharmaceutical or prescrib$ or prescription?) adj3 (audit$ or monitor$ or reconcil$)).ti,ab. 13 (drug? review? or drug? assess$ or drug? audit? or drug? reconcil$).ti,ab. 14 (“drug utili?ation” adj2 (reconcil$ or audit?)).ab. or (“drug utili?ation” adj4 (reconcil$ or audit?)).ti. [line moved] 15 inappropriate prescribing/ [Term added Aug 2011] 16 ((Medication? or prescrib$ or prescription? or drug therap$) adj2 assessment?).ti,ab. [Term added Aug 2011] 17 (inappropriate$ adj2 (medicine? or medication? or prescrib$ or drug?)).ti,ab. [Term added Aug 2011] 18 or/9‐17 [Medication Review/Audit] 19 exp *Hospital/ [EM] 20 exp *Ward/ [EM] 21 (hospital$ or WARD or WARDS).ti. 22 *Hospitalization/ [EM] 23 *Hospital care/ or *Intensive care/ [EM] 24 *“length of stay”/ or *hospital admission/ or *Hospital discharge/ or *Hospital readmission/ or *Patient transport/ [EM] 25 (((patient? or hospital$) and (discharg$ or admission? or admitting or readmission? or readmit$ or transfer?)) or “length of stay”).ti. 26 (((patient? or hospital?) adj2 (discharg$ or admission? or admitting or readmission? or transfer?)) or “length of stay”).ab. 27 *hospital patient/ [EM] 28 (inpatient? or in‐patient?).ti. 29 *Hospital service/ [EM] 30 *Hospital personnel/ or *Hospital physician/ or *Medical staff/ or *Resident/ [EM] 31 or/19‐30 [Hospitals/Hospitalization/Inpatients] 32 (pharmacy or pharmacies or pharmacist? or prescription? or prescribing).ti. 33 (pharmacist‐led or pharma$ initiated or ((driven or lead or led) adj2 pharmacist?)).ab. 34 (prescribing adj2 pattern?).ab. 35 (“physician‐pharmacist?” or “doctor‐pharmacist?”).ti,ab. 36 ((improv$ or optimi?ing or optimi?e? or optimal$) and (dosing or dosage or pharmac$ or prescrib$ or prescript$)).ti. or ((improv$ or optimi?ing or optimi?e? or optimal$) adj2 (pharmaceutical care or pharmacy or prescrib$ or prescript$)).ab. 37 ((pharmaceutical adj (care or consult$)) or (pharmacist? adj2 (care or consult$ or intervention? or managed))).ab. 38 ((medication? or prescrib$ or pharmac$) adj2 (manage? or management or service? or system?)).ti,ab. 39 ((“drug therapy” or dosage? or dose? or medication? or PRESCRIPTION? or PRESCRIB$ or PHARMACIST? or PHARMACEUTICAL CARE) adj2 (managing or management or monitor$)).ti,ab. (11654) 40 *Patient compliance/ and (medication? or pharmac$ or drug? or prescrib$ or prescription?).ti. 41 *Pharmacist/ or *Pharmacy technician/ [EM] 42 *Pharmaceutical care/ [EM] 43 *medical information system/ and (medication? or pharmac$ or drug? or prescrib$ or prescription?).ti,hw. [EM] 44 *Prescription/ [EM] 45 *Medication therapy management/ or *Recommended drug dose/ or *Optimal drug dose/ [EM] 46 *Polypharmacy/ or POLYPHARM$.ti. [EM] 47 *Medication error/ [EM] 48 *“drug use”/ [EM] 49 *Drug utilization/ [EM] 50 *DRUG FORMULARY/ 51 or/32‐50 [Pharmacy/Prescribing/Med Use] 52 medical audit/ 53 *medical audit/ or *monitoring/ [EM] 54 monitoring/ 55 (audit? or monitoring or reconcil$).ti. 56 or/52,54‐55 [Monitoring/Audit broad] 57 randomized controlled trial/ or controlled study/ or controlled clinical trial/ [EM] 58 pretest posttest control group design/ 59 clinical study/ or major clinical study/ or clinical trial/ 60 multicenter study/ 61 random$.ti. or (randomi?ed or randomly).ab. or controlled.ti. 62 (clinical study/ or major clinical study/ or clinical trial/) and random$.ti. 63 crossover‐procedure/ or double‐blind procedure/ or single‐blind procedure/ [EM] 64 or/57‐63 [Trials Filter EM] 65 (animal model? or animal experiment? or animal study? or animal trial? or canine or feline or bovine or cow or cows or mice or dog? or cat or cats or rabbit? or rat or rats or veterinar$).ti. or (animal or veterinary).hw. [EM] 66 (editorial or letter or note or “review” or trade or survey).pt. [EM] 67 systematic review/ or meta‐analysis/ or (systematic adj3 review).ti. or (meta‐analy$ or metaanaly$).ti. or (literature adj2 review).ti. 68 64 not (or/65‐67) [EPOC RCT Filter EM] 69 18 and 31 [Drug Review/Audit & Hosp] 70 31 and 51 and 56 [Hosp & Pharma & Monitoring‐‐Broad search] 71 (or/69‐70) and 68 [RCT Results 2] 72 8 and 68 [Med Rev Hosp & RCT Results 1] 73 72 or 71 [RCT Results] 74 (20113$ or 20114$ or 20115$ or 2012$ or 2013$ or 2014$).em. [Entry week Aug 2011 to Nov 2014] 75 (“2011” or “2012” or “2013” or “2014”).yr. 76 73 and (74 or 75) [Results Nov 18, 2014] 77 remove duplicates from 76
The Cochrane Library
The Cochrane Library <update 18 November 2014>, Wiley #1 (“PHARMACEUTICAL CARE” near/2 inpatient* or PHARMACY near/2 inpatient* or PHARMACIES near/2 inpatient* or PHARMACIST* near/2 inpatient* or PRESCRIBING near/2 inpatient*):ab or (stopp or (Beer N2 criteria)):ti,ab #2 (“PHARMACEUTICAL CARE” near/2 hospital*or PHARMACY near/2 hospital* or PHARMACIES near/2 hospital* or PHARMACIST* near/2 hospital* or PRESCRIBING near/2 hospital*):ab #3 (“PHARMACEUTICAL CARE” near/2WARD* or PHARMACY near/2WARD* or PHARMACIES near/2WARD* or PHARMACIST* near/2 WARD* or PRESCRIBING near/2 WARD*):ab #4 (“PHARMACEUTICAL CARE” near/2 UNIT or PHARMACY near/2 UNIT or PHARMACIES near/2 UNIT or PHARMACIST* near/2 UNIT or PRESCRIBING near/2 UNIT):ab #5 (“PHARMACEUTICAL CARE” near/2 UNITS or PHARMACY near/2 UNITS or PHARMACIES near/2 UNITS or PHARMACIST* near/2 UNITS or PRESCRIBING near/2 UNITS):ab #6 (medication* near/2 system* or prescribing near/2 system* or prescription* near/2 system* or dispensing near/2 system*):ti,kw and (hospital* or WARD or WARDS or INPATIENT* or CARE near/2 UNIT*):ti,kw #7 MeSH descriptor: [Pharmacy Service, Hospital] this term only #8 MeSH descriptor: [Medication Systems, Hospital] this term only #9 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8) #10 MeSH descriptor: [Hospitalization] explode all trees #11 MeSH descriptor: [Inpatients] this term only #12 MeSH descriptor: [Hospital Departments] explode all trees #13 MeSH descriptor: [Hospital Shared Services] this term only #14 MeSH descriptor: [Hospital Units] explode all trees #15 MeSH descriptor: [Medical Staff, Hospital] explode all trees #16 (hospital* or WARD or WARDS):ti #17 hospital*:ab #18 (patient* or hospital*):ti,kw and (discharge* or admission* or admitting or readmission* or readmit* or transfer*):ti or “length of stay”:ti #19 (Patient* near/2 discharg* or Patient* near/2 admission* or Patient* near/2 admitting or Patient* near/2 readmission* or Patient* near/2 transfer*) or “length of stay”:ab #20 (hospital* near/2 discharg* or hospital* near/2 admission* or hospital near/2 admitting or hospital near/2 readmission* or hospital near/2 transfer*) or “length of stay”:ab #21 (inpatient* or in‐patient*):ti #22 (#10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21) #23 (pharmacy or pharmacies or pharmacist* or prescription* or prescribing):ti #24 (“pharmacist‐led” or “pharma* initiated” or pharmacist* near/2 driven or pharmacist* near/2 lead or pharmacist* near/2 led):ab #25 Prescribing near/2 Pattern*:ab #26 (“physician‐pharmacist*” or “doctor‐pharmacist*”):ti,ab #27 (IMPROV* or OPTIMI*ING or OPTIMI*E* or OPTIMAL*):ti and (DOSING or DOSAGE or PHARMAC* or PRESCRIB* or PRESCRIPT*):ti #28 (IMPROV* near/2 “PHARMACEUTICAL CARE” or OPTIMI*ING near/2 “PHARMACEUTICAL CARE” or OPTIMI*E* near/2 “PHARMACEUTICAL CARE” or OPTIMAL* near/2 “PHARMACEUTICAL CARE”):ab #29 (IMPROV* near/2 PHARMACY or OPTIMI*ING near/2 PHARMACY or OPTIMI*E* near/2 PHARMACY or OPTIMAL* near/2 PHARMACY):ab #30 (IMPROV* near/2 PRESCRIB* or OPTIMI*ING near/2 PRESCRIB* or OPTIMI*E* near/2 PRESCRIB* or OPTIMAL* near/ 2 PRESCRIB*):ab #31 (IMPROV* near/2 PRESCRIPT* or OPTIMI*ING near/2 PRESCRIPT* or OPTIMI*E* near/2 PRESCRIPT*or OPTIMAL* near/2 PRESCRIPT*):ab #32 “pharmaceutical care” or “pharmaceutical consult*” or (pharmacist* near/2 care or pharmacist* near/2 consult* or pharmacist* near/2 intervention* or pharmacist* near/2 managed):ab #33 (prescription* near/4 review* or prescribing near/4 review* or medication* near/4 review*OR pharmacist* near/2 review*):ti,ab #34 (“drug therapy” near/2 audit* or “drug regime*” near/2 audit* ormedication* near/2 audit* ormedicine* near/2 audit* or pharmacy near/2 audit* or pharmacist* near/2 audit* or pharmaceutical near/2 audit* or PRESCRIB* near/2 audit* or prescription* near/2 audit*):ti,ab #35 (“drug therapy” near/2monitor* or “drug regime*” near/2monitor* ormedication* near/2monitor* ormedicine* near/2monitor* or pharmacy near/2 monitor* or pharmacist* near/2 monitor* or pharmaceutical near/2 monitor* or PRESCRIB* near/2 monitor* or prescription* near/2 monitor*):ti,ab #36 (“drug therapy” near/2 RECONCIL* or “drug regime*” near/2 RECONCIL* or medication* near/2 RECONCIL* or medicine* near/2 RECONCIL* or pharmacy near/2 RECONCIL* or pharmacist* near/2 RECONCIL* or pharmaceutical near/2 RECONCIL* or PRESCRIB* near/2 RECONCIL* or prescription* near/2 RECONCIL*):ti,ab #37 (“drug therapy” near/2 review* or “drug regime*” near/2 review* or medication* near/2 review* or medicine* near/2 review* or pharmacy near/2 review* or pharmacist* near/2 review* or pharmaceutical near/2 review* or PRESCRIB* near/2 review* or prescription* near/2 review*):ti,ab #38 (medication* near/2 manage* or prescrib* near/2 manage* or phamac* near/2 manage*):ti,ab #39 (medication* near/2 management or prescrib* near/2 management or pharmac* near/2 management):ti,ab #40 (medication* near/2 service* or prescrib* near/2 service* or pharmac* near/2 service*):ti,ab #41 (medication* near/2 system* or prescrib* near/2 system* or pharmac* near/2 system*):ti,ab #42 (“drug therapy” near/2 managing or dosage* near/2 managing or dose* near/2 managing or medication* near/2 managing or PRESCRIPTION* near/2 managing or PRESCRIB* near/2 managing or PHARMACIST* near/2 managing or “PHARMACEUTICAL CARE” near/2 managing):ti,ab #43 (“drug therapy” near/2 management or dosage* near/2 management or dose* near/2 management or medication* near/2 management or PRESCRIPTION* near/2 management or PRESCRIB* near/2 management or PHARMACIST* near/2 management or “PHARMACEUTICAL CARE” near/2 management):ti,ab #44 (“drug therapy” near/2 monitor* or dosage* near/2 monitor* or dose* near/2 monitor* or medication* near/2 monitor* or PRESCRIPTION* near/2monitor* or PRESCRIB* near/2monitor* or PHARMACIST* near/2monitor* or “PHARMACEUTICAL CARE” near/2 monitor*):ti,ab #45 (“drug* review*” or “drug* assess*” or “drug* audit*” or “drug* reconcil*”):ti,ab #46 (“drug utili*ation” near/2 review* or “drug utili*ation” near/2 reconcil* or “drug utili*ation” near/2 audit*):ab #47 (review* or reconcil* or audit*):ti and “drug utili*ation”:ti #48 MeSH descriptor: [Medication Adherence] this term only #49 MeSH descriptor: [Pharmacists] this term only #50 MeSH descriptor: [Pharmacists’ Aides] explode all trees #51 MeSH descriptor: [Pharmaceutical Services] this term only #52 MeSH descriptor: [Drug Information Services] this term only #53 MeSH descriptor: [Clinical Pharmacy Information Systems] this term only #54 MeSH descriptor: [Prescriptions] this term only #55 MeSH descriptor: [Drug Prescriptions] this term only #56 MeSH descriptor: [Drug Dosage Calculations] this term only #57 MeSH descriptor: [Pharmaceutical Preparations] this term only #58 MeSH descriptor: [Electronic Prescribing] this term only #59 MeSH descriptor: [Medication Systems] this term only #60 MeSH descriptor: [Drug Monitoring] this term only #61 MeSH descriptor: [Medication Therapy Management] this term only #62 MeSH descriptor: [Drug Therapy] this term only #63 MeSH descriptor: [Drug Therapy, Computer‐Assisted] this term only #64 MeSH descriptor: [Medication Errors] this term only #65 MeSH descriptor: [Drug Utilization Review] this term only #66 MeSH descriptor: [Drug Utilization] this term only #67 MeSH descriptor: [Polypharmacy] this term only #68 Polypharm*:ti #69 Polypharmacy or polypharm*:ti #70 MeSH descriptor: [Inappropriate Prescribing] this term only #71 ((Medication or medications or prescrib* or prescription or prescriptions or drug therap*) near/2 assessment):ti,ab #72 (inappropriate* near/2 (medicine or medicines or medication or medications or prescrib* or drug or drugs)):ti,ab #73 (#23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72) #74 (#9 or (#22 and #73)) #75 (medication near/2 review) (Word variations have been searched) #76 hospital* or inpatient*:ti,ab,kw #77 #75 and #76 in Cochrane Reviews (Reviews and Protocols)
CINAHL
EbscoHost CINAHL <update 18 November 2014> S1 (MH “Pharmacy Service”) S2 TI ( pharmaceutical care or pharmacy or pharmacies or pharmacist* or prescribing ) S3 (MH “Medication Systems”) OR TI (medication* n2 system) or (prescribing n2 system) or (prescription* n2 system) or (dispensing n2 system) OR TI (medication* n2 systems) or (prescribing n2 systems) or (prescription* n2 systems) or (dispensing n2 systems) OR TI ((medication N2 assessment) or (prescrib* N2 assessment) or (prescription N2 assessment) or (drug therap* N2 assessment)) OR AB ((medication N2 assessment) or (prescrib* N2 assessment) or (prescription N2 assessment) or (drug therap* N2 ass ... S4 TI ( hospital* OR inpatient ward or wards or intensive care or ICU or emergency department* or unit ) OR MW ( hospital* OR inpatient ward or wards or intensive care or ICU or emergency department* ) S5(MH“Adolescent,Hospitalized”)OR(MH“Aged,Hospitalized”)OR(MH“Child,Hospitalized”)OR(MH“Emergency Patients”) OR (MH “Infant, Hospitalized”) OR (MH “Inpatients”) S6 (MH “Hospitals+”) OR (MH “Hospital Units+”) OR TI ( inpatient* or hospital$ or WARD* or UNIT or UNITS ) S7 (MH “Hospitalization”) OR (MH “Length of Stay”) OR (MH “Patient Admission”) OR (MH “Patient Discharge”) OR (MH “Discharge Planning+”)OR(MH“PatientDischarge Education”)OR (MH“Early PatientDischarge”)OR (MH“Transfer,Discharge”) OR (MH “Patient Dumping”) OR (MH “Readmission”) OR (MH “Transfer, Intrahospital”) S7 S8 (MH “Medication Reconciliation”) S9 TI ( (drug therapy N2 reconcil*) or (drug therapy N2 audit*) or (drug therapy N2 review*) ) or AB ( (drug therapy N2 reconcil*) or (drug therapy N2 audit*) or (drug therapy N2 review*) ) OR TI ( (medicine* N2 reconcil*) or (medicine* N2 audit*) or (medicine* N2 review*) ) or AB ( (medicine* N2 reconcil*) or (medicine* N2 audit*) or (medicine* N2 review*) ) S10 (MH “Nursing Audit”) OR (MH “Audit”) S11 TI ( medication* or medicine* or drug therap* or prescrib* or prescript* or medication* ) or MW ( medication* or medicine* or drug therap* or prescrib* or prescript* or medication* ) S12 S10 and S11 S13 S1 or S2 or S3 S14 S4 or S5 or S6 or S7 S15 S8 or S9 or S12 S16 S13 and S14 S17 S14 and S15 S18 TI ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) ) or AB ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) ) S19 (MM “Clinical Trials+”) S20 TI ( “clinical study” or “clinical studies” ) or AB ( “clinical study” or “clinical studies” ) S21 TI random* or AB random* S22 TI controlled or AB controlled S23 TI ( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” ) or AB ( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” ) S24 S18 or S19 or S20 or S21 or S22 or S23 S25 TI ( (stopp or “beer’s criteria”) ) OR AB ( (stopp or “beer’s criteria”) ) S26 S16 or S17 or S25 S27 S24 and S26 S28 TI medication review* S29 S27 or S28 S30 (MH “Pharmacy Service”) S31 TI ( pharmaceutical care or pharmacy or pharmacies or pharmacist* or prescribing ) S32 (MH“Medication Systems”)OR TI (medication* n2 system) or (prescribing n2 system) or (prescription* n2 system) or (dispensing n2 system) OR TI (medication* n2 systems) or (prescribing n2 systems) or (prescription* n2 systems) or (dispensing n2 systems) OR TI ((medication N2 assessment) or (prescrib* N2 assessment) or (prescription N2 assessment) or (drug therap* N2 assessment)) OR AB ((medication N2 assessment) or (prescrib* N2 assessment) or (prescription N2 assessment) or (drug therap* N2 ass ... S33 TI ( hospital* OR inpatient ward or wards or intensive care or ICU or emergency department* or unit ) OR MW ( hospital* OR inpatient ward or wards or intensive care or ICU or emergency department* ) S34 (MH “Adolescent, Hospitalized”) OR (MH “Aged, Hospitalized”) OR (MH “Child, Hospitalized”) OR (MH “Emergency Patients”) OR (MH “Infant, Hospitalized”) OR (MH “Inpatients”) S35 (MH “Hospitals+”) OR (MH “Hospital Units+”) OR TI ( inpatient* or hospital$ or WARD* or UNIT or UNITS ) Medication review in hospitalised patients to reduce morbidity and mortality (Review) 76 Copyright © 2016 The Cochrane Collaboration. Published by JohnWiley & Sons, Ltd. S36 (MH “Hospitalization”) OR (MH “Length of Stay”) OR (MH “Patient Admission”) OR (MH “Patient Discharge”) OR (MH “Discharge Planning+”)OR(MH“PatientDischarge Education”)OR (MH“Early PatientDischarge”)OR (MH“Transfer,Discharge”) OR (MH “Patient Dumping”) OR (MH “Readmission”) OR (MH “Transfer, Intrahospital”) S37 (MH “Medication Reconciliation”) S38 TI ( (drug therapy N2 reconcil*) or (drug therapy N2 audit*) or (drug therapy N2 review*) ) or AB ( (drug therapy N2 reconcil*) or (drug therapy N2 audit*) or (drug therapy N2 review*) ) OR TI ( (medicine* N2 reconcil*) or (medicine* N2 audit*) or (medicine* N2 review*) ) or AB ( (medicine* N2 reconcil*) or (medicine* N2 audit*) or (medicine* N2 review*) ) S39 (MH “Nursing Audit”) OR (MH “Audit”) S40 TI ( medication* or medicine* or drug therap* or prescrib* or prescript* or medication* ) or MW ( medication* or medicine* or drug therap* or prescrib* or prescript* or medication* ) S41 S39 and S40 S42 S30 or S31 or S32 S43 S33 or S34 or S35 or S36 S44 S37 or S38 or S41 S45 S42 and S43 S46 S43 and S44 S47 TI ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) ) or AB ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) ) S48 (MM “Clinical Trials+” S49 TI ( “clinical study” or “clinical studies” ) or AB ( “clinical study” or “clinical studies” ) S50 TI random* or AB random* S51 TI controlled or AB controlled S52 TI ( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” ) or AB ( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” ) S53 S47 or S48 or S49 or S50 or S51 or S52 S54 TI ( (stopp or “beer’s criteria”) ) OR AB ( (stopp or “beer’s criteria”) S55 S45 or S46 or S54 S56 S53 and S55 S57 TI medication review* S58 S56 or S57
EPOC Specialised Register
Reference Manager, EPOC Specialised Register <update 18 November 2014>
TI: {Medication} AND {review} OR TI: {prescription} AND {review} OR TI: {prescription} AND {audit} OR TI: {medication} AND {audit} OR TI: {medication} AND {reconcil} OR TI: {prescription} AND {reconcil} OR TI: {prescrib} AND {reconcil} OR TI: {prescrib} AND {audit} OR TI: {prescrib} AND {review} OR TI: {pharmacist} AND {audit} OR TI: {pharmacist} AND {review} OR TI: {hospital pharmacist} OR TI: {hospital AND prescribe} OR AB: hospital prescribe OR Keyword: (Pharmacy Service,Hospital*) OR TI: (inappropriate OR assessment) AND TI: (medication OR medicine OR drug OR prescrib OR prescrip) NOTE: Due to the limited searching capabilities of RefMan, this strategy was searched in separate parts.
International Pharmaceutical Abstracts
Ovid International Pharmaceutical Abstracts <17 August 2011 to 12 May 2015> 1 Pharmacy service, hospital.mp. 2 ((PHARMACEUTICAL CARE or PHARMACY or PHARMACIES or PHARMACIST? or PRESCRIBING) and (inpatient? or hospital$ or WARD? or UNIT or UNITS)).ti. 3 ((PHARMACEUTICAL CARE or PHARMACY or PHARMACIES or PHARMACIST? or PRESCRIBING) adj2 (inpatient? or hospital$ or WARD? or UNIT or UNITS)).ab. 4 Medication Systems, Hospital.mp. 5 ((medication? or prescribing or prescription? or dispensing) adj2 system?).ti,ab. and (hospital$ orWARD orWARDS or (CARE adj2 UNIT?) or INPATIENT?).ti,hw. 6 (stopp or beer’s criteria).ti,ab. 7 1 or 2 or 3 or 4 or 5 or 6 8 (hospital$ or WARD or WARDS).ti. 9 Hospitalization.mp. 10 hospital$.ab. 11 (“length of stay” or Patient admission or Patient discharge or Patient readmission or Patient transfer).mp. 12 ((patient? or hospital$).ti,hw. and (discharg$ or admission? or admitting or readmission? or readmit$ or transfer?).ti.) or “length of stay”.ti. 13 Inpatients.mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name] 14 (inpatient? or in‐patient?).ti. 15 HOSPITAL SHARED SERVICES.mp. 16 (MEDICAL STAFF, HOSPITAL or HOSPITALISTS).mp. 17 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18 (pharmacy or pharmacies or pharmacist? or prescription? or prescribing).ti. 19 (pharmacist‐led or pharma$ initiated or ((driven or lead or led) adj2 pharmacist?)).ab. 20 (PRESCRIBING adj2 PATTERN?).ab. 21 (“physician‐pharmacist?” or “doctor‐pharmacist?”).ti,ab. 22 ((IMPROV$ or OPTIMI?ING or OPTIMI?E? or OPTIMAL$) and (DOSING or DOSAGE or PHARMAC$ or PRESCRIB$ or PRESCRIPT$)).ti. or ((IMPROV$ or OPTIMI?ING or OPTIMI?E? or OPTIMAL$) adj2 (PHARMACEUTICAL CARE or PHARMACY or PRESCRIB$ or PRESCRIPT$)).ab. 23 ((pharmaceutical adj (care or consult$)) or (pharmacist? adj2 (care or consult$ or intervention? or managed))).ab. 24 (((prescription? or prescribing or medication?) adj4 review$) or (pharmacist? adj2 review$)).ti,ab. 25 ((drug therapy or drug regime? or medication? or medicineS or pharmacy or pharmacist? or pharmaceutical or PRESCRIB$ or prescription?) adj2 (audit$ or monitor$ or RECONCIL$ or review?)).ti,ab. 26 ((medication? or prescrib$ or pharmac$) adj2 (manage? or management or service? or system?)).ti,ab. 27 ((“drug therapy” or dosage? or dose? or medication? or PRESCRIPTION? or PRESCRIB$ or PHARMACIST? or PHARMACEUTICAL CARE) adj2 (managing or management or monitor$)).ti,ab. 28 (drug? review? or drug? assess$ or drug? audit? or drug?reconcil$).ti,ab. 29 (“drug utili?ation” adj2 (review? or reconcil$ or audit?)).ab. or (“drug utili?ation” and (review? or reconcil$ or audit?)).ti. 30 Medication adherence.mp. 31 (Pharmacists or Pharmacists’ Aides).mp. 32 (Pharmaceutical Services or Drug Information Services).mp. 33 Clinical Pharmacy Information Systems.mp. 34 (Prescriptions orDrug Prescriptions or Pharmaceutical Preparations or Drug Therapy orDrugDosage Calculations or Electronic Prescribing or Medication Systems).mp. 35 (Drug Monitoring or Medication Therapy Management).mp. 36 (Drug Therapy or Drug Therapy, Computer‐Assisted).mp. 37 POLYPHARMACY.mp. or POLYPHARM$.ti. 38 MEDICATION ERRORS.mp. 39 Drug utilization review.mp. 40 Drug Utilization.mp. 41 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 42 (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti. 43 animals/ not humans.sh. 44 42 not 43 45 (((patient? or hospital?) adj2 (discharg$ or admission? or admitting or readmission? or transfer?)) or “length of stay”).ab. 46 17 or 45 47 7 and 44 48 41 and 44 and 45 49 47 or 48
Appendix 2. Co‐interventions
| Medication reconciliation at discharge | Discharge counselling | Written information to the patient at discharge/discharge letter | Post‐discharge telephone contact with the patient | Post‐discharge telephone contact with primary care physician | Written information to primary care physician | Post‐discharge telephone contact to community pharmacy | Written information to community pharmacy | |
| Bladh 2011 | X | X | X | |||||
| Blum 2021 | X | |||||||
| Bonetti 2018 | X | X | X | |||||
| Bonnerup 2014 | ||||||||
| Cossette 2017 | ||||||||
| Curtin 2020 | ||||||||
| Dalleur 2014 | X | |||||||
| Farris 2014 | X | X | X | X | X | |||
| Gallagher 2011 | ||||||||
| Gillespie 2009 | X | X | X | |||||
| Graabaek 2019 | X | |||||||
| Gustafsson 2017 | ||||||||
| Juanes 2018 | X | |||||||
| Kempen 2021 | X | X | X | |||||
| Lea 2020 | X | X | ||||||
| Lenssen 2018 | X | X | ||||||
| Lisby 2010 | X | |||||||
| Lisby 2015 | ||||||||
| Nielsen 2017 | ||||||||
| O'Mahony 2020 | ||||||||
| Ravn‐Nielsen 2018 | X | X | X | X | X | X | ||
| Schnipper 2006 | X | X | ||||||
| Scullin 2007 | X | X | X | X | ||||
| Song 2021 | X | X | ||||||
| SUREPILL 2015 | X | X | X | X |
Appendix 3. Risk of bias in included studies
Allocation (selection bias)
Of the four trials with unclear risk of selection bias, three trials did not report who included the participants and whether they had knowledge of the allocation group before trial inclusion (Lisby 2010; Lisby 2015; Song 2021), and in the last trial, the same persons were responsible for both participant enrolment and performing the medication review and it was unclear how the randomisation was done (Bonetti 2018).
Incomplete outcome data (attrition bias)
Eleven trials reported one or more of the following outcomes: mortality (due to drug‐related adverse events), hospital re‐admission (due to drug‐related adverse events), hospital emergency department contact (due to drug‐related adverse events) or drug‐related adverse events. Of these, we assessed seven trials as having low risk of attrition bias for all outcomes (Blum 2021; Curtin 2020; Gillespie 2009; Graabaek 2019; Gustafsson 2017; Kempen 2021; Ravn‐Nielsen 2018). We judged two trials as having a high risk of attrition for several outcomes: one trial had high risk of attrition bias for adverse drug events and hospital readmissions (due to adverse drug events) due to a high loss to follow‐up (33%) (Lenssen 2018). The other trial had high risk of attrition bias for hospital readmissions (due to adverse drug events), hospital emergency department contacts (due to adverse drug events) and adverse drug events due to large dropout with uneven distribution (Schnipper 2006). We judged two trials as having unclear risk of attrition bias for drug‐related adverse events: one trial showed discrepancies between reported participants lost to follow‐up and participants excluded from analysis (Farris 2014), and one trial measured outcomes using registry data and contact with general practitioners and participants, but did not report how often data were unavailable (Gallagher 2011). As adverse events, such as falls, could lead to loss to follow‐up, we judged this outcome as unclear.
Other potential sources of bias
Of the four trials judged as having high risk of other biases, one trial stated that "In order to avoid contamination bias, two of the four geriatricians involved in the inpatient geriatric consultation team during the study period were allocated to the intervention group because they used the STOPP criteria in their current practice, while the other two, who had never worked with the STOPP criteria, were allocated to the control group" (Dalleur 2014). This entails a risk of unevenly distributed physician competencies leading to high risk of bias. We judged another trial as having high risk of other biases in their data analysis (Scullin 2007). Firstly, there was a difference of 20 participants between the two intervention groups, which should not have been possible because the trial randomised in blocks of 10 in each group. Secondly, data from a surgical ward were excluded from the analysis without an explanation. We judged one trial as high risk of other biases for several reasons (SUREPILL 2015). Firstly, groups were unbalanced at baseline, probably because of cluster‐randomisation using only six clusters. Secondly, there were exactly 547 participants in each intervention group and exactly 362 participants from each group returned the questionnaires about readmissions and quality of life, which we judged as very unlikely when randomising at cluster level. The last trial was a cluster‐randomised cross‐over trial, and we judged it as having high risk of other bias due to the cross‐over design where all wards were allocated to all three interventions during trial, giving a risk of contamination and herd effect (Kempen 2021). Medication reviews may result in physicians gaining knowledge about which drugs should be discontinued or prescribed, thereby introducing a carry‐over effect of the intervention.
In one trial, baseline data were reported for the entire population and not for the subgroup of participants (35% of all participants) who received a medication review (Bonnerup 2014). Thus, we judged the trial as unclear risk of other biases as it was not possible to evaluate baseline differences.
Appendix 4. Summary of findings table: trials comparing two or more types of medication reviews for hospitalised adult patients
| Extended medication review compared with basic medication review for hospitalised adult patients | |||||
|
Patient or population: hospitalised adult patients Intervention: extended medication review Comparison: basic medication review | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk with basic medication review | Corresponding risk with extended medication review | ||||
|
Mortality (all‐cause) Median follow‐up 6 months (range 3 to 6 months) |
High‐risk population |
RR 1.27 (0.95 to 1.71) |
2087 (4 trials) | ⊕⊝⊝⊝ very lowb,c | |
| 200 per 1000a | 254 per 1000 (190 to 342) | ||||
| Very high‐risk population | |||||
| 400 per 1000a | 508 per 1000 (380 to 684) | ||||
|
Hospital readmissions (all‐cause) Median follow‐up 3 months (range 1 to 6 months) |
High‐risk population |
RR 0.99 (0.78 to 1.26) |
1918 (3 trials) | ⊕⊕⊝⊝ lowd,e | |
| 500 per 1000a | 495 per 1000 (390 to 630) | ||||
| Very high‐risk population | |||||
| 650 per 1000a | 644 per 1000 (507 to 819) | ||||
|
Hospital emergency department contacts (all‐cause) 6 months follow‐up |
High‐risk population |
RR 1.00 (0.71 to 1.41) |
1522 (2 trials) | ⊕⊕⊕⊝ moderatef | |
| 300 per 1000a | 300 per 1000 (213 to 423) | ||||
| Very high‐risk population | |||||
| 400 per 1000a | 400 per 1000 (284 to 564) | ||||
| * The basis for the assumed riskwith basic medication review is provided in footnotes. The corresponding riskwith extended medication review (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** > 0 favours medication reviews CI: confidence interval; NA: not applicable; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aThe assumed risk with standard care is based on published trial data. The ‘very high‐risk’ estimates are based on the included trials with the highest risk in the control group at 12 months follow‐up for mortality (Gillespie 2009), hospital readmissions (Lea 2020) and emergency department contacts (Kempen 2021). The ‘high‐risk’ estimates are based on the included trials with the lowest risk (albeit still a high risk, hospitalised population) in the control group at 12 months follow‐up for mortality and hospital readmissions (Scullin 2007) and emergency department contacts (Gillespie 2009).
bDowngrade for indirectness (follow‐up ranged from 3 to 6 months for mortality. Short follow‐up may be inadequate as changes to preventive medications may take years before showing an effect on outcomes (downgraded 1 category for indirectness).
cThe 95% CI ranges from 0.95 to 1.71 and includes important benefit, i.e. 5% reduction in mortality, and very important harm, i.e. 20% increase in mortality (downgraded 2 categories for imprecision).
dInconsistency: downgraded by one level: I2 = 58%.
eThe 95% CI ranges from 0.78 to 1.26 and includes both important benefit, i.e. 15% reduction in hospital readmissions, and important harm (downgraded 1 category for imprecision).
fThe 95% CI ranges from 0.71 to 1.41 and includes both important benefit, i.e. 20% reduction in emergency department contacts, and important harm (downgraded 1 category for imprecision).
Data and analyses
Comparison 1. Primary outcome ‐ Trials comparing medication reviews with standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mortality (all‐cause) | 18 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.87, 1.05] |
Comparison 2. Secondary outcomes ‐ Trials comparing medication reviews with standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Hospital readmissions (all‐cause) | 17 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.89, 0.98] | |
| 2.2 Hospital readmissions (all‐cause) ‐ 3 months | 4 | 448 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.14, 0.17] |
| 2.3 Hospital readmissions (all‐cause) ‐ 12 months | 3 | 1449 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.28, 0.03] |
| 2.4 Hospital readmissions (due to drug‐related adverse events) | 6 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.58, 0.98] | |
| 2.5 Hospital readmissions (due to drug‐related adverse events) ‐ 12 months | 2 | 428 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.26, ‐0.10] |
| 2.6 Hospital emergency department contacts (all‐cause) | 8 | 3527 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.68, 1.03] |
| 2.7 Hospital emergency department contacts (all‐cause) ‐ 3 months | 4 | 448 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.14, 0.04] |
| 2.8 Health‐related quality of life | 4 | 392 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.10, 0.30] |
Comparison 3. Subgroup analysis ‐ Trials comparing medication reviews with standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mortality (all‐cause): trials of participants taking a mean of ≥ 10 different medications versus trials of participants taking a mean of < 10 different medications | 15 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.83, 1.03] | |
| 3.1.1 Study population taking a mean of ≥ 10 different medications | 6 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.82, 1.11] | |
| 3.1.2 Study population taking a mean of < 10 different medications | 9 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.77, 1.08] | |
| 3.2 Hospital readmissions (all‐cause): trials of participants taking a mean of ≥ 10 different medications versus trials of participants taking a mean of < 10 different medications | 14 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.90, 0.99] | |
| 3.2.1 Study population taking a mean of ≥ 10 different medications | 6 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.89, 1.01] | |
| 3.2.2 Study population taking a mean of < 10 different medications | 8 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.87, 1.01] | |
| 3.3 Hospital emergency department contacts (all‐cause): trials of participants taking a mean of ≥ 10 different medications versus trials of participants taking a mean of < 10 different medications | 8 | 3527 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.68, 1.03] |
| 3.3.1 Study population taking a mean of ≥ 10 different medications | 5 | 2948 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.10] |
| 3.3.2 Study population taking a mean of < 10 different medications | 3 | 579 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.38, 0.94] |
| 3.4 Mortality (all‐cause): trials with criteria‐based medication review versus trials with non‐criteria‐based medication review | 18 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.87, 1.05] | |
| 3.4.1 Criteria‐based medication review | 6 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.82, 1.15] | |
| 3.4.2 Non‐criteria‐based medication review | 12 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.84, 1.07] | |
| 3.5 Hospital readmissions (all‐cause): trials with criteria‐based medication review versus trials with non‐criteria‐based medication review | 16 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.89, 0.98] | |
| 3.5.1 Criteria‐based medication review | 5 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.91, 1.05] | |
| 3.5.2 Non‐criteria‐based medication review | 11 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.85, 0.96] | |
| 3.6 Hospital emergency department contacts (all‐cause): trials with criteria‐based medication review versus trials with non‐criteria‐based medication review | 8 | 3527 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.68, 1.03] |
| 3.6.1 Criteria‐based medication review | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.15, 2.40] |
| 3.6.2 Non‐criteria‐based medication review | 7 | 3409 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.68, 1.04] |
| 3.7 Mortality (all‐cause): trials with low implementation rate versus trials with high implementation rate | 18 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.87, 1.05] | |
| 3.7.1 Low implementation rate | 5 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.85, 1.27] | |
| 3.7.2 High implementation rate | 13 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.83, 1.04] | |
| 3.8 Hospital readmissions (all‐cause): trials with low implementation rate versus trials with high implementation rate | 17 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.89, 0.98] | |
| 3.8.1 Low implementation rate | 5 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.90, 1.05] | |
| 3.8.2 High implementation rate | 12 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.86, 0.96] | |
| 3.9 Hospital emergency department contacts (all‐cause): trials with low implementation rate versus trials with high implementation rate | 8 | 3527 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.68, 1.03] |
| 3.9.1 Low implementation rate | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.17, 1.49] |
| 3.9.2 High implementation rate | 6 | 3321 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.83, 1.05] |
| 3.10 Mortality (all‐cause): trials with low risk of bias versus trials with high risk of bias | 18 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.87, 1.05] | |
| 3.10.1 Low risk of bias | 12 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.90, 1.12] | |
| 3.10.2 High risk of bias | 6 | Risk Ratio (IV, Random, 95% CI) | 0.82 [0.68, 0.99] | |
| 3.11 Hospital readmissions (all‐cause): trials with low risk of bias versus trials with high risk of bias | 16 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.89, 0.98] | |
| 3.11.1 Low risk of bias | 11 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.90, 1.00] | |
| 3.11.2 High risk of bias | 5 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.79, 0.97] | |
| 3.12 Hospital emergency department contacts (all‐cause): trials with low risk of bias versus trials with high risk of bias | 8 | 3527 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.68, 1.03] |
| 3.12.1 Low risk of bias | 5 | 3217 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.83, 1.06] |
| 3.12.2 High risk of bias | 3 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.25, 0.96] |
| 3.13 Mortality (all‐cause): trials with extended medication reviews versus trials with basic medication reviews | 18 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.87, 1.05] | |
| 3.13.1 Extended medication review | 11 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.82, 1.06] | |
| 3.13.2 Basic medication review | 10 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.87, 1.21] | |
| 3.14 Hospital readmissions (all‐cause): trials with extended medication reviews versus trials with basic medication reviews | 17 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.92, 1.01] | |
| 3.14.1 Extended medication review | 11 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.87, 1.02] | |
| 3.14.2 Basic medication review | 9 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.92, 1.07] | |
| 3.15 Hospital emergency department contacts (all‐cause): trials with extended medication reviews versus trials with basic medication reviews | 8 | 3527 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.74, 1.02] |
| 3.15.1 Extended medication review | 5 | 1703 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.60, 1.01] |
| 3.15.2 Basic medication review | 5 | 1824 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.61, 1.15] |
Comparison 4. Sensitivity analysis ‐ Trials comparing medication reviews with standard care.
Comparison 5. Trials comparing extended medication reviews with basic medication reviews.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Mortality (all‐cause) | 4 | 2087 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.95, 1.71] |
| 5.2 Hospital readmissions (all‐cause) | 3 | 1918 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.78, 1.26] |
| 5.3 Hospital emergency department contacts (all‐cause) | 2 | 1522 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.71, 1.41] |
Comparison 6. Sensitivity analysis ‐ Trials comparing extended medication reviews with basic medication reviews.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Mortality (all‐cause) ‐ alternative ITT analysis | 4 | 2142 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.93, 1.68] |
| 6.2 Hospital readmissions (all‐cause) ‐ alternative ITT analysis | 3 | 1996 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.31] |
| 6.3 Hospital emergency department contacts (all‐cause) ‐ alternative ITT analysis | 2 | 1602 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.68, 1.36] |
| 6.4 Mortality (all‐cause) ‐ fixed‐effect | 4 | 2087 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.96, 1.74] |
| 6.5 Hospital readmissions (all‐cause) ‐ fixed‐effect | 3 | 1918 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.06] |
| 6.6 Hospital emergency department contacts (all‐cause) ‐ fixed‐effect | 2 | 1522 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.70, 1.41] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bladh 2011.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 400 participants randomised (199 to medication review and 201 to control) Patients admitted to 2 internal medicine wards at a university hospital in Sweden Median (IQR) age: medication review group 81 (72 to 87) years, control group 82 (75 to 86) years 39% male Median number of drugs: 7 (+1 drug on demand) |
|
| Interventions | Medication reviews were performed with a computer support system (MiniQ) that identified potentially inappropriate prescribing (PIP) according to 3 drug‐specific quality indicators, and included oral feedback to prescribing physicians. PIPs were (1) drugs that should be avoided in the elderly, for example long‐acting benzodiazepines and drugs with anticholinergic action, (2) 3 or more psychotropic drugs (i.e. antipsychotics, anxiolytics, hypnotic‐sedatives and antidepressants) and (3) potentially serious drug‐drug interactions: Category D interactions according to the Pharmaceutical Specialties in Sweden (FASS) specifying drug combinations that should be avoided. Participants in the control group received normal care. | |
| Outcomes |
Primary outcome: EQ‐5D index
Secondary outcomes: 'self‐rated global health' (registered as an integer from 1 (very poor) to 5 (very good)) and 'attitudes towards the medication report' (evaluated by questionnaires sent to participants' GPs after discharge from hospital) PIP items: (1) drugs that should be avoided in the elderly, (2) 3 or more psychotropics and (3) potentially serious interactions Potential drug‐related problems (DRPs) identified only in the intervention group (mortality not reported as an outcome per se) All outcomes had 6 months of follow‐up |
|
| Notes | Funding: Swedish National Board of Health and Welfare | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised (1:1) to intervention or control group. Two persons without knowledge about the study protocol performed the randomisation using sequentially numbered envelopes. Authors confirm that the allocation sequence was generated using random generator software. |
| Allocation concealment (selection bias) | Low risk | A ward physician or nurse judged whether the medical condition of the patient allowed inclusion in the study. Sequentially numbered, sealed envelopes were opened after participant details were written and transferred to the assignment card via a carbon paper inside the envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but probably not blinded. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Mortality data were likely gathered from a non‐biased national register (through unique patient‐specific social security number). |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Readmission data should be unbiased, as they were gathered from a non‐biased register. |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | High risk | It was not stated whether the person who called the participants and asked about EQ‐5D was blinded to group allocation. The trial authors confirmed that the person who called the participants was not blinded. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | No missing data were described; all data should be available from a non‐biased national register. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | No missing data were described; all data should be available from a non‐biased national register. |
| Incomplete outcome data (attrition bias) Health‐related quality of life | High risk | The HRQL follow‐up was completed for 204 participants (59%). Lost to follow‐up: intervention group 69 (20 died, 7 declined, 42 not reached despite repeated attempts), control group 72 (15 died, 6 declined, 51 not reached despite repeated attempts). |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were collected and were similar to information provided on www.clinicaltrials.gov. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Blum 2021.
| Study characteristics | ||
| Methods | European multicentre, cluster‐randomised, controlled trial | |
| Participants | 2008 participants randomised (963 to 54 intervention clusters and 1045 to 56 control clusters) Participants admitted to 4 study centres in Bern (Switzerland), Utrecht (the Netherlands), Brussels (Belgium) and Cork (Ireland); 85 medical clusters and 25 surgical clusters Median (IQR) age: 79 (74 to 84) years 56% male Median (IQR) number of drugs: medication review group 10 (7 to 13), control group 9 (7 to 12) |
|
| Interventions |
Intervention group: Pharmacotherapy optimisation based on the Systematic Tool to Reduce Inappropriate Prescribing through (1) systematic medication review by a physician and a pharmacist, with support of the STRIP Assistant, a software‐based tool taking into account the predictable adverse medication effects, advising safe and appropriate therapy using established STOPP/START criteria, monitoring clinically relevant interactions and dosing appropriately in accordance with renal function, (2) drug discussion and adaptation with the prescribing physician, (3) shared decision‐making with the patient and (4) generation of a report with specific recommendations for the patient’s general practitioner. Control group: Usual practice and a sham intervention using a questionnaire (Medication Adherence Measure Questionnaire, ©MMAS30–32*) by a team member (the physician or the pharmacist) to mimic the intervention and improve blinding of the patient and other blinded team members. |
|
| Outcomes |
Primary outcome: drug‐related hospital admission within 1 year after enrolment Secondary outcomes: number of any hospitalisations, mortality, number of falls, quality of life, degree of polypharmacy, activities of daily living, patient’s drug compliance, as well as the number of significant drug–drug interactions, drug overuse and underuse and potentially inappropriate medication |
|
| Notes | Funding: This work is part of the project OPERAM: OPtimising thERapy to prevent Avoidable hospital admissions in the Multimorbid elderly supported by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 634238, and by the Swiss State Secretariat for Education, Research, and Innovation (SERI) under contract number 15.0137. This project was also partially funded by the Swiss National Scientific Foundation (SNSF 320030_188549). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | In this study design, each prescribing hospital physician defines a cluster. Physicians were allocated 1:1 to either the intervention arm or the control arm, using a probabilistic minimisation method implemented by a web‐based clinical trial management system (WebSpirit hosted by the Clinical Trials Unit (CTU) Bern). Minimisation was done according to country to ensure a balanced distribution of hospitals. The minimisation algorithm was implemented using randomisation lists generated by an independent statistician in Stata (StataCorp., Stata Statistical Software Version 14). |
| Allocation concealment (selection bias) | Low risk | Only system administrators who were otherwise not involved in the conduct of the trial had access to the randomisation lists, to ensure concealment of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The intervention team consisted of a doctor and a pharmacist; neither was blinded to enable direct interactions with both the attending hospital doctors and the participants. The participants, hospital doctors and general practitioners were partially blinded and received only general information on the trial without specific details about the intervention. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Outcome assessors were fully blinded. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Outcome assessors were fully blinded. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (due to adverse drug events) | Low risk | An independent blinded adjudication committee at each trial site, consisting of doctors and pharmacists, consecutively adjudicated all hospital admissions (both medical and surgical) for drug relatedness according to a previously published standardised adjudication guideline. When a hospital admission (at the index hospital or any other hospital) was identified, a second unblinded team gathered data on hospital admission and concealed all information identifying the intervention allocation before sending it to the adjudication team outcome assessors who were fully blinded. |
| Blinding of outcome assessment (detection bias) Adverse drug events | Low risk | Outcome assessors were fully blinded. |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | Low risk | The teams conducting follow‐up telephone calls were fully blinded. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | During follow‐up, 10 (0.5%) participants were lost to follow‐up (7 in the intervention group and 3 in the control group), and 118 (5.9%) withdrew from the trial (50 in the intervention group and 62 in the control group). |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | During follow‐up, 10 (0.5%) participants were lost to follow‐up (7 in the intervention group and 3 in the control group), 118 (5.9%) withdrew from the trial (50 in the intervention group and 62 in the control group), and 385 (19.2%) died (179 in the intervention group and 206 in the control group). |
| Incomplete outcome data (attrition bias) Hospital readmissions (due to adverse drug events) | Low risk | During follow‐up, 10 (0.5%) participants were lost to follow‐up (7 in the intervention group and 3 in the control group), 118 (5.9%) withdrew from the trial (50 in the intervention group and 62 in the control group), and 385 (19.2%) died (179 in the intervention group and 206 in the control group). |
| Incomplete outcome data (attrition bias) Adverse drug events | Low risk | During follow‐up, 10 (0.5%) participants were lost to follow‐up (7 in the intervention group and 3 in the control group), 118 (5.9%) withdrew from the trial (50 in the intervention group and 62 in the control group), and 385 (19.2%) died (179 in the intervention group and 206 in the control group). |
| Incomplete outcome data (attrition bias) Health‐related quality of life | High risk | Loss to follow‐up was 41% for the medication review group and 38% for the control group. |
| Selective reporting (reporting bias) | Low risk | No unpublished outcomes when paper is compared to published protocol or trial registration at clinicaltrials.gov |
| Contamination bias | Low risk | Cluster‐randomised. Cluster‐randomisation was at the doctor and not hospital level. Physicians may have worked in the same department, but the wards were in distinct locations, and each physician was responsible for his/her own non‐overlapping ward. |
| Other bias | Low risk | No evidence of other types of bias. Recruitment bias: low risk of bias (support for judgement: to limit selection bias, the recruitment team were fully blinded). Baseline imbalance: low risk of bias (support for judgement: Table 1 suggests no major baseline imbalance). Loss of clusters: low risk of bias (support for judgement: no loss of clusters). Incorrect analysis: low risk of bias (support for judgement: adjusted for clustering). Comparability with individually randomised trials: low risk of bias (support for judgement: allocated by attending physician resulting in patients being admitted to different wards, so no risk of herd effect). |
Bonetti 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 133 participants were randomised (66 to medication review and 67 to control) Participants were admitted to the cardiology ward at a Brazilian tertiary hospital Mean age: 65 years Mean number of medications at discharge: intervention 7 (± 2), control: 8 (± 3) |
|
| Interventions | Patients who were allocated to the intervention group or their caregivers received individual counselling sessions regarding the discharge prescriptions. These sessions included a thorough assessment of the pharmacotherapy and interventions from cardiologists in order to correct any medication issues, as well as an explanation about the indications, benefits, therapeutic targets, dose, dosing schedule, routes, storage, length of therapy, refill pharmacy and possible adverse drug events for each prescribed drug. A leaflet containing the information provided in the verbal counselling was delivered by the pharmacists. Subsequently, patients were contacted by telephone 3 and 15 days post‐discharge to reinforce the previous counselling session. All pharmacist interventions were performed and described according to the Descriptive Elements of Pharmacist Interventions Characterization Tool (DEPICT). The control group received usual care from pharmacists and other healthcare providers. |
|
| Outcomes |
Primary outcome: mortality rate, hospital readmissions (related and unrelated to heart disease) and emergency department visits (related and unrelated to heart disease) within 30 days Secondary outcomes: medication adherence based on the results of the MedTake, Beliefs about Medicines Questionnaire (BMQ), and Adherence to Refills and Medications Scale (ARMS) instruments, all completed 30 days post‐discharge |
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were allocated to either the intervention group or control group in a 1:1 ratio using a random number list generated by a third person using Microsoft Office Excel 2010. |
| Allocation concealment (selection bias) | Unclear risk | Two cardiovascular pharmacy residents were responsible for patient enrolment according to the eligibility criteria and for performing the intervention. It is unclear whether including staff knew allocation group. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but probably not blinded. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Not everyone in the ambulatory setting was blinded to the group allocation, but mortality is objective and will likely not be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Outcomes were assessed by asking patients and not by records. "We asked the patients whether they visited emergency departments during this period and whether they were admitted to another hospital" (page 2 methods section). Not everyone in the ambulatory setting was blinded to the group allocation, but the outcome of readmissions is objective and will likely not be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Outcomes were assessed by asking patients and not by records. "We asked the patients whether they visited emergency departments during this period and whether they were admitted to another hospital" (page 2 method section). Not everyone in the ambulatory setting was blinded to the group allocation, but the outcome of readmissions is objective and will likely not be influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | High risk | Loss to follow‐up: intervention: 15% (9/60), control: 16% (10/63). Authors were contacted but did not reply. The trial had considerable loss to follow‐up. Mortality was likely not assessed through registries and reason for dropout was stated as “patients did not attend the ambulatory”. Thus, despite loss to follow‐up being balanced there is a substantial risk that these high‐risk participants may have been lost to follow‐up because of death. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | High risk | Loss to follow‐up: intervention: 15% (9/60), control: 16% (10/63). |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | High risk | Loss to follow‐up: intervention: 15% (9/60), control: 16% (10/63). |
| Selective reporting (reporting bias) | Unclear risk | No clinical trial to compare protocol and publication of results. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Bonnerup 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 375 participants randomised (124 to high‐risk subgroup: 64 to medication review and 60 to control group) Patients admitted to 1 acute medical department at a university hospital in Denmark Mean age: medication review group (not available), control group 78.2 years; mean number of drugs: 11.0 |
|
| Interventions | Patients presenting with risk of prescribing errors identified by a risk score called MERIS (ranging from 0 to 37). A MERIS score between 14 and 26 warranted a medication review by a clinical pharmacist, whereas a risk score ≥ 26 led to medication review by a clinical pharmacologist. Medication reviews consisted of (1) collecting information concerning the participant's drug treatment and the clinical status of the participant, (2) conducting a participant interview and (3) performing a critical examination of a participant's overall drug treatment. Recommendations or information arising from the medication reviews were delivered to hospital physicians as a note in the electronic medical record. If fast response was needed (e.g. if the participant was about to be discharged, if urgent action was required), the note was accompanied by direct contact with a physician. Participants in the control group received usual care. |
|
| Outcomes |
Primary outcome: number of prescribing errors during participants' hospitalisation Secondary outcomes: health care utilisation (divided into all‐cause readmissions, contacts with general practitioners and visits to emergency departments), health‐related quality of life, mortality All outcomes had 90 days of follow‐up (after hospital discharge) |
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was generated by a computer program in the hospital pharmacy in random blocks of a maximum of 20. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque and sealed envelopes containing randomisation codes were delivered to study pharmacists who allocated participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but probably not blinded. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Mortality data were taken from a non‐biased national register (participants identifiable through unique patient‐specific social security numbers). |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Readmission data were taken from a non‐biased national register. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Emergency department contacts were taken from a non‐biased national register. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | No missing data were described; all data should be available from a non‐biased national register. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | No missing data were described; all data should be available from a non‐biased national register. |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | No missing data were described; all data should be available from a non‐biased national register. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were collected and were similar to information provided on www.clinicaltrials.gov. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Unclear risk | Only participants with high risk of prescription errors (35%) received a medication review out of all participants allocated to the medication review group. Baseline data for this subgroup were not reported, and we therefore judged the trial as having unclear risk of other biases. |
Cossette 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 321 participants were randomised (162 to medication review and 159 to control) Participants were admitted at the Centre Hospitalier Universitaire de Sherbrooke, Québec, Canada Mean age: 81 years 40% male |
|
| Interventions | Medication review group: CAS‐based pharmacist‐physician intervention. For the intervention group, the study pharmacist analysed the CAS alerts daily and determined their clinical relevance based on their clinical experience. For clinically relevant alerts, the pharmacist then developed a geriatric pharmaco‐therapeutic plan to be discussed with the treating physician to reduce PIM use. The clinical relevance of the CAS alerts in the control group was only determined by the study pharmacists after the control patient was discharged from the hospital. Participants in the control group received usual care. |
|
| Outcomes |
Primary outcome: change in medication, defined as the number of discontinued drugs or drugs with a dosage decrease out of the total number of drugs for which the pharmacist suggested a drug cessation or dosage decrease. The change in medication was evaluated within 48 hours after the CAS. Secondary outcomes: length of stay, emergency room visits and readmissions within 30 days of hospital discharge, and in‐hospital death |
|
| Notes | Funding: grant from the Merck funds of the Faculty of Medicine and Health Sciences of the University of Sherbrooke | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were randomly assigned to the control and intervention groups with a 1:1 ratio using block sizes of 2, 4 and 6 and stratification by hospital site. Individuals with no clinical involvement in the trial generated the randomisation sequence using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and programmed its implementation using a computer that produced daily the lists of intervention and control patients. |
| Allocation concealment (selection bias) | Low risk | No description of who enrolled participants. The trial authors were contacted to explain the allocation concealment further. Their reply was as follows: “Every evening at midnight, an automated extraction of ALL hospitalised patients was made and put in a file (inaccessible to us). An automated algorithm was started 15 min later to see if there is a flag related to our inclusion criteria or if the patient had already been randomised for that visit and a second list was then generated only with patients who were flagged and were not randomised during their active visit. The system then randomised by assigning flagged patients to either the control group or the intervention group. The pharmacist then accessed a web page showing ONLY the patients in the intervention group.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the pharmacists and physicians conducting the interventions was not feasible. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Not described but will not likely influence readmission. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Not described but will not likely influence ED contacts. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | High risk | Loss to follow‐up: 20.9% (61/ 292). Loss‐to follow‐up was unevenly distributed between groups (of the 61 participants lost to follow‐up 26% were in the control group and 74% were in the intervention group). High risk of bias due to the large dropout with uneven distribution. |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | High risk | Loss to follow‐up: 20.9% (61/ 292). Loss‐to follow‐up was unevenly distributed between groups (of the 61 participants lost to follow‐up 26% was in the control group and 74% were in the intervention group). High risk of bias due to the large dropout with uneven distribution. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Number of falls from randomisation to the end of the hospitalisation was a prespecified secondary outcome according to clinicaltrials.gov record, but not reported in the publication. Other reported outcomes were negative. |
| Contamination bias | High risk | No cluster‐randomisation. Of 98 physicians caring for study participants, 44% took care of patients in both the intervention and control groups leading to high risk of contamination bias. |
| Other bias | Low risk | 231 patients were included in the analysis: 209 had 1 hospitalisation, 21 had 2 hospitalisations and 1 had 3 hospitalisations. Ten patients were included in both the control and intervention groups for separate hospitalisations. We assessed that the relatively small cross‐over effect is not likely to influence the results. |
Curtin 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 130 participants were randomised (65 to medication review and 65 to control) Participants were admitted to 2 acute hospitals (Cork University Hospital and Mercy University Hospital, Ireland) Mean age: 85 years 38% male Mean number of regular prescribed medications: 11 |
|
| Interventions | After baseline data collection was completed, the research physician used the STOPPFrail criteria to identify de‐prescribing targets. For participants randomised to the intervention arm, a medication withdrawal plan was devised by the research physician. The recommended medication withdrawal plan was communicated directly to one of the participant’s attending physicians and also documented in the patient’s medical record. The attending physician assessed whether to accept the drug withdrawal plan and implement the recommended changes. Participants in the control group received usual pharmaceutical care (i.e. hospital physician and pharmacist care). |
|
| Outcomes |
Primary outcome: mean change in the number of long‐term prescribed medicines consumed by participants at 3 months after randomisation Secondary outcomes: measured at 3 months and included the following:
|
|
| Notes | Funding: Denis Curtin, Emma Jennings and Denis O’Mahony are supported by the European Union’s Horizon 2020 research and innovation programme (grant number 634238). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to study arms in a 1:1 ratio, using block randomisation. Block sizes of 4 and 6 were generated using the website randomization.com by an administrator external to the study. Randomisation was not stratified by hospital site. |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was concealed in sequentially numbered, opaque envelopes until the research physician had enrolled participants, completed baseline data collection and identified deprescribing targets using the STOPPFrail criteria. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The attending physicians and participants were not blinded to intervention or control group allocation. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Outcome data were collected by 3 research physicians who were blinded to the group allocation of participants. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Outcome data were collected by 3 research physicians who were blinded to the group allocation of participants. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Outcome data were collected by 3 research physicians who were blinded to the group allocation of participants. |
| Blinding of outcome assessment (detection bias) Adverse drug events | Low risk | Outcome data were collected by 3 research physicians who were blinded to the group allocation of participants. |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | High risk | Outcome data were collected by 3 research physicians who were blinded to the group allocation of participants. They contacted directors of nursing homes by telephone and requested that a nurse or care assistant, familiar with the participant, complete the QUALIDEM instrument. Where possible, the ICECAP‐O was to be completed by the same person who completed the questionnaire at baseline. In some instances, the research physicians contacted the relevant person by telephone to complete the ICECAP‐O. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | One participant withdrew from the intervention group during follow‐up. 1 of 65 in medication review group and 0 of 65 in control group were lost to follow‐up. Judged as low risk. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | One participant withdrew from the intervention group during follow‐up. Judged as low risk. |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | One participant withdrew from the intervention group during follow‐up. Judged as low risk. |
| Incomplete outcome data (attrition bias) Adverse drug events | Low risk | One participant withdrew from the intervention group during follow‐up. Judged as low risk. |
| Incomplete outcome data (attrition bias) Health‐related quality of life | High risk | High loss to follow‐up: ICECAP‐O: intervention group 64% (38/59), control group 51% (30/59) QUALIDEM: intervention group 37% (22/59), control group 36% (21/59) |
| Selective reporting (reporting bias) | Low risk | No unpublished outcomes when paper is compared to trial registration at clinicaltrials.gov. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Dalleur 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 158 participants randomised (77 to medication review and 81 to control) Patients admitted to non‐geriatric medical wards at a teaching hospital in Belgium Median (IQR) age: medication review 84 years (81 to 87), control 86 (81 to 89) years 37% male Median number of drugs: 7 |
|
| Interventions | Each participant's medications were routinely reviewed by the inpatient consultation team geriatrician, who used an implicit approach (i.e. no explicit tool was used). In the intervention group, in addition to the usual inpatient geriatric consultation team care, geriatricians performed the following 2 steps: (1) they applied 64 STOPP criteria to systematically screen the list of medications being taken by the patient on admission for potentially inappropriate medications (PIMs) (‘duplicate drug classes’ was not considered because the concept of duplication is perceived differently by clinicians), and (2) they made oral and written recommendations to the ward physician during hospitalisation for discontinuation of PIMs. Participants in the control group received standard care from the inpatient geriatric consultation team. |
|
| Outcomes | Primary outcome: proportion of PIMs discontinued (or corrected in case of dosage‐related or duration‐related PIMs) between hospital admission and discharge (according to the discharge letter) Secondary outcomes: (1) characteristics associated with discontinuation of PIMs at discharge, (2) proportion of PIMs that were still discontinued 1 year after discharge and (3) clinical significance of STOPP‐related recommendations. Mortality after 1 year was also reported. | |
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Eligible patients were allocated by the study nurse to control or intervention group by simple randomisation using drawing of lots (without matching for age or geriatric profile). No description of how drawing of lots was performed. |
| Allocation concealment (selection bias) | High risk | Study nurse assigned participants and performed randomisation using an apparently open design, which could lead to lack of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Attending ward physician (responsible for prescriptions during hospitalisation and at discharge), outcome evaluator and participants were blinded to group assignment, but it is unlikely that it is possible to blind ward physicians responsible for patient care and implementation of the intervention. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Study nurse provided the outcome evaluator with a list of participants included in the trial, which did not specify allocation group. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | High risk | Follow‐up data on mortality were not available for 58% of randomised participants. |
| Selective reporting (reporting bias) | Unclear risk | The trial had no trial registrations to compare with published results and we therefore judged it as having unclear risk of selective outcome reporting. |
| Contamination bias | High risk | Participants in the control group were treated at the same wards (as intervention group participants) and there is therefore a risk of contamination bias. |
| Other bias | High risk | The paper states: "In order to avoid contamination bias, two of the four geriatricians involved in the inpatient geriatric consultation team during the study period were allocated to the intervention group because they used the STOPP criteria in their current practice, while the other two, who had never worked with the STOPP criteria, were allocated to the control group". This entails a risk of unevenly distributed physician competencies. |
Farris 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 936 participants randomised (314 to enhanced medication review, 315 to minimal medication review, 313 to control) Participants admitted to departments of internal medicine, family medicine, cardiology and orthopaedics at a Midwestern Academic Health Centre in the USA Mean age: 61.0 years Mean number of drugs: 11.0 |
|
| Interventions | Participants in the minimal and enhanced intervention groups received medication reconciliation at admission and pharmacist visits every 2 to 3 days for patient education during inpatient stay, discharge counselling and discharge medication list. Counselling was tailored for each participant and focused on goals of therapy, medication administration and barriers to adherence including cost and patient concerns. Participants in the enhanced intervention group also received a telephone call 3 to 5 days post‐discharge, and primary care physician and community pharmacist received a discharge care plan focused on medication changes and recommendations. The care plan was faxed to the primary care physician and to the community pharmacist within 24 hours of discharge but usually within 6 hours. The care plan included the discharge medication list, plans for dosage adjustments and monitoring and recommendations for preventing adverse drug events, with participant‐specific concerns such as adherence or cost issues highlighted. The control group received usual care. Usual care was medication reconciliation at admission according to hospital policy, nurse discharge counselling and a discharge medication list for patients. The usual care discharge summary was transcribed and received in the mail by the primary care physician several days or weeks after discharge. |
|
| Outcomes | Primary outcome: Medication Appropriateness Index (MAI) Secondary outcomes: adverse events, preventable adverse events as a composite variable of combined hospital readmission, emergency department visit or unscheduled general practitioner office visit during 30‐day and 90‐day follow‐up periods | |
| Notes | Funding: the National Heart, Lung and Blood Institute (1RO1 HL082711). Drs Carter, Kaboli and Christensen were also supported by the Comprehensive Access and Delivery Research and Evaluation (CADRE), Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (HFP 04–149). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised according to a statistician‐generated randomisation scheme. |
| Allocation concealment (selection bias) | Low risk | Allocated to groups by sequentially numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and staff were aware of interventions, but not whether allocation was to minimal or enhanced group medication review. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Records from the primary care provider and the pharmacy were obtained for all participants. Hospitalisation records were obtained from the university hospital and from community hospitals, when such an event occurred. Research staff were blinded. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Trained research assistants contacted all participants by telephone to gather self‐reported adverse events and symptoms and self‐reported healthcare utilisation. Primary care provider and pharmacy records were obtained for all participants. Hospitalisation records were obtained from the university hospital and from community hospitals when such an event occurred. Research staff were blinded when assessing readmission data |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Trained research assistants contacted all participants by telephone to gather self‐reported adverse events and symptoms and self‐reported healthcare utilisation. Primary care provider and pharmacy records were obtained for all participants. Hospitalisation records were obtained from the university hospital and from community hospitals, when such an event occurred. Research staff were blinded when assessing emergency department contact assessment. |
| Blinding of outcome assessment (detection bias) Adverse drug events | Low risk | Trained research assistants contacted all participants by telephone to gather self‐reported adverse events and symptoms and self‐reported healthcare utilisation Primary care provider and pharmacy records were obtained for all participants. Research staff were blinded. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Unclear risk | Loss to follow‐up: medication review 3% (16/623) participants (3% (8/312) minimal intervention group and 3% (8/311) in the enhanced intervention group) and control 2% (5/313 participants). Unclear whether participants lost to follow‐up had mortality data available (i.e. loss to follow‐up due to death). |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Unclear risk | Discrepancies in the trial publication between the number of participants reported as study completers and the number of participants included in the analysis of hospital readmissions. |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Unclear risk | Discrepancies in the trial publication between the number of participants reported as study completers and the number of participants included in the analysis of hospital emergency department contacts. |
| Incomplete outcome data (attrition bias) Adverse drug events | Unclear risk | Discrepancies in the trial publication between the number of participants reported as study completers and the number of participants included in the analysis of adverse events. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were included in trial and were similar to information provided on www.clinicaltrials.gov. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Gallagher 2011.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 400 participants randomised (200 to medication review and 200 to control) Participants were admitted via the emergency department under the care of attending physicians at a tertiary medical centre at the University Hospital in Ireland Median (IQR) age: medication review 75 (71 to 80) years, control 77 (71 to 82) years 47% male Mean number of drugs: 7.7 |
|
| Interventions | A primary research physician evaluated the pharmacotherapy of participants in the intervention group using specific criteria for potentially inappropriate prescribing and prescribing omissions (STOPP/START criteria). The research physician discussed with attending medical team and provided written communication of interventions within 24 hours after hospitalisation. Team members were not obliged to follow up. No reporting of co‐interventions. Participants in the control group received usual hospital physician and pharmacist care. |
|
| Outcomes |
Primary outcome: Medication Appropriateness Index (MAI) and Assessment of Underutilisation Index (AOU)
Secondary outcomes: mortality, frequency of general practitioner visits, hospital readmissions, falls. All outcomes had 6 months of follow‐up. |
|
| Notes | Funding: The Health Research Board of Ireland, Clinical Research Training Fellowship number CRT/2006/029 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was determined by an independently generated random numbers table using StatsDirect software. |
| Allocation concealment (selection bias) | Low risk | The random numbers table was retained, independent of investigators, by a physician external to the study, who also assigned participants to groups using a sealed envelope system. Group allocation was concealed from the research physician and from participants until baseline data had been collected and inclusion criteria verified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study described as not blinded. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Data collected by research physician aware of assignments, but this will likely not influence assessment of mortality. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Data collected by research physician aware of assignments, but this will likely not influence assessment of hospital readmissions. |
| Blinding of outcome assessment (detection bias) Adverse drug events | High risk | Data collected by research physician, who was aware of assignments. An interrater reliability analysis of outcome measurements was conducted to ensure no bias towards more favourable ratings in the intervention group as compared with the control group (n = 40). Nevertheless, the causality assessment of falls is highly subjective and may lead to bias. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | No loss to follow‐up was described. Primary researcher obtained data from general practitioner, community pharmacist and hospital records. No description or evidence in publication of loss to follow‐up. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | Primary researcher obtained data through contact with participants, their general practitioners or community pharmacists, and from hospital records. No description (or evidence in publication) of loss to follow‐up. |
| Incomplete outcome data (attrition bias) Adverse drug events | Unclear risk | Assessment of falls (adverse events) was obtained by telephone contact with participants or their general practitioners. No description of how many times participants could not be contacted. Lack of contact could be related to falls and might not reveal whether participants had experienced a fall not requiring medical assistance. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were included in trial and were similar to information provided on www.clinicaltrials.gov. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Gillespie 2009.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 400 participants randomised (199 to medication review and 201 to control) Participants were admitted to 2 acute internal medicine wards at a university hospital in Sweden Mean age: 87 years 41% male Mean number of drugs: 8.0 |
|
| Interventions |
Medication review group: the list of current medications was reconciled to ensure that the medication list was correct. Thereafter, a drug review was performed, and advice was given to participant's physician on drug selection, dosages and monitoring needs, with the final decision made by the physician in charge. Participants were educated and monitored throughout the admission process, and received discharge counselling. Co‐interventions: information about discharge medications (e.g. rationale for changes, therapeutic goals, monitoring needs for newly commenced drugs) was provided to primary care physicians (general practitioners) by study pharmacists. A follow‐up telephone call was made to participants 2 months after discharge. Control group: patients in the control group received standard care without pharmacist involvement in the healthcare team at the ward level. Standard care usually included the same elements as those of the enhanced service but was less extensive, focusing mainly on the cause of admission, and was performed by physicians and nurses. |
|
| Outcomes |
Primary outcome: frequency of hospital visits (emergency department and readmissions (total and drug‐related))
Secondary outcome: cost of hospital care
Mortality not stated as an outcome, but measured All outcomes had 12 months of follow‐up |
|
| Notes | Funding: Uppsala County Council, University Hospital of Uppsala, Uppsala University, Apoteket AB and Swedish Society of Pharmaceutical Sciences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated to groups using closed‐envelope technique. Authors confirm that the allocation sequence was generated using a random generator software. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed in blocks of 20 (each block contained 10 intervention and 10 control allocations). Investigators included participants and once included the Clinical Trial Unit would reveal the allocation. In principle, due to the fixed blocks and unblinded design, the allocation of the last participants in a block could be predicted. However, this would likely have little impact on the overall risk of bias and we judged the trial as having low risk of selection bias. The authors confirmed the allocation procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial described as not blinded. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Mortality data were likely taken from a non‐biased national register (participants identifiable through unique patient‐specific social security number). |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | The 2 researchers responsible for analysing readmission data were blinded regarding the group to which participants had been randomised. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (due to adverse drug events) | Low risk | The physician in charge of the participant was required to document in the medical record whether readmissions were drug‐related. Physicians making this decision were blinded as to whether patients were study participants. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Emergency department contact data were likely taken from a non‐biased national register. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (due to adverse drug events) | Unclear risk | Blinding of outcome assessment was not described for emergency department contacts. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | States that no participants were lost to follow‐up. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | States that no participants were lost to follow‐up. |
| Incomplete outcome data (attrition bias) Hospital readmissions (due to adverse drug events) | Low risk | States that no participants were lost to follow‐up. |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | States that no participants were lost to follow‐up. |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (due to adverse drug events) | Low risk | States that no participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were included in trial and were similar to information provided on www.clinicaltrials.gov. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Graabaek 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 600 participants randomised (200 to the extended medication review, 200 to basic medication review and 200 to the control group) Patients were included from the medical acute admission unit at Hospital South West Jutland Mean age: 74 years 51% male Median number of drugs: 6 |
|
| Interventions | The 3 groups consisted of a control group (usual care) and 2 intervention groups named ED (basic intervention) and STAY (extended intervention). All patients received usual care including medication history, medication reconciliation and medication review by a physician without any structured instrument as part of the normal procedure. Both the ED group and the STAY group received a pharmacist‐led medication review (including patient interview and medication reconciliation) on admission. Furthermore, patients in the STAY group transferred to a specialised ward received a medication review during inpatient stay together with patient counselling and a medication report at discharge. | |
| Outcomes |
Primary outcome measure: number of patients with a medication‐related readmission within 30 days from discharge Secondary outcome measures: mortality (overall, during index admission, within 30 days after discharge or 31 to 180 days after discharge), patients with readmissions (acute and planned, both including medication‐related readmissions) within 30 days after discharge and number of visits to the emergency department, the hospital or a general practitioner within 180 days after discharge |
|
| Notes | Funding: Hospital South West Jutland, University of Southern Denmark, Region of Southern Denmark, Sygehusapotekernes og Amgros' forsknings ‐ og udviklingspulje, Actavis Legat, Karola Jørgensens Forskningsfond, and Edith & Vagn Hedegaard Jensens Fond | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomised using a 1:1:1 allocation ratio to 1 of 3 groups in blocks of 15 (each block contained 5 patients from each group) using the opaque closed‐envelope technique. |
| Allocation concealment (selection bias) | Low risk | The patients were randomised using the opaque closed‐envelope technique. The pharmacist opened the envelope at the bedside after patient consent was obtained. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The group allocation was not blinded to the patient, the pharmacist or other healthcare professionals present at the ward. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Data were collected from the nationwide registers from the Danish Health Authorities: the Civil Registration System, the National Health Insurance Service Registry and the National Patient Registry. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Data were collected from the nationwide registers from the Danish Health Authorities: the Civil Registration System, the National Health Insurance Service Registry and the National Patient Registry. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (due to adverse drug events) | Low risk | Two researchers, with expertise in clinical pharmacology and geriatrics, individually conducted the analysis of the outcome. Information about group allocation was blinded to these researchers. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Data were collected from the nationwide registers from the Danish Health Authorities: the Civil Registration System, the National Health Insurance Service Registry, and the National Patient Registry. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | No description of missing data for this outcome. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | No description of missing data for this outcome. |
| Incomplete outcome data (attrition bias) Hospital readmissions (due to adverse drug events) | Low risk | A total of 9 patients were excluded from the analysis of medication‐related readmission within 30 days (7 patients died during inpatient stay and 2 patients died during 30‐day follow‐up). Four patients had an acute readmission before they died within 30 days and they were hence included in the analysis. |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | No description of missing data for this outcome. |
| Selective reporting (reporting bias) | Unclear risk | No clinical trial registration to compare protocol and publication of results. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Gustafsson 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 460 participants randomised (230 to medication reviews and 230 to control) Patients aged ≥ 65 years with dementia or cognitive impairment admitted to 3 wards at 2 hospitals located in Northern Sweden were included Mean age: 83.1 years 36% male Mean number of drugs: control group 8.3, intervention group 8.4 |
|
| Interventions | Three clinical pharmacists with postgraduate degrees in clinical pharmacy and long experience in performing medication reviews in primary care and hospital wards conducted the interventions. The pharmacists were already part of the different ward teams at the time when the study started. The additional service provided by the clinical pharmacists consisted of medication reconciliation, medication review and participation in ward rounds. Participants in the control group received usual care. |
|
| Outcomes |
Primary outcome: risk of drug‐related readmissions at 180 days Secondary outcomes: all‐cause readmission at 30 days and 180 days, mortality, cost analysis (not yet analysed), time to institutionalisation (not yet analysed) and adherence to quality indicators (not yet analysed) |
|
| Notes | Funding: grants from the Swedish Dementia Association, the County Council of Västerbotten, the Janne Elgqvists foundation, the Swedish Society of Medicine, and the foundation for Medical Research in Skellefteå | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was prepared before study start using a throwing dice method by an independent person who was not engaged in the trial in any other way. The sequence was performed in blocks of 6 to 36 (each block contained between 3 and 18 intervention allocations and the same number of control allocations). Randomisation was stratified at ward level. To accomplish this, each ward used their own randomisation blocks, consecutively starting a new block after completion of the preceding, meaning that there were an equal number of control and intervention participants in each ward. |
| Allocation concealment (selection bias) | Low risk | When a patient formally entered the trial, an employee of the Department of Pharmacology and Clinical Neuroscience, who was not involved in the interventions, provided the treatment allocation according to the randomisation scheme. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants and pharmacists were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | An independent, blinded external expert group consisting of one specialist in geriatrics, one specialist in internal medicine and one clinical pharmacist working in another county assessed the outcomes. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | An independent, blinded external expert group consisting of one specialist in geriatrics, one specialist in internal medicine and one clinical pharmacist working in another county assessed the outcomes. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (due to adverse drug events) | Low risk | An independent, blinded external expert group consisting of one specialist in geriatrics, one specialist in internal medicine and one clinical pharmacist working in another county assessed the outcomes. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Not described but will not likely influence ED contacts. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | One participant withdrew from the control group before discharge; none lost to follow‐up in both groups (why assessed as low risk of bias). |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | One participant withdrew from the control group before discharge; none lost to follow‐up in both groups (why assessed as low risk of bias). |
| Incomplete outcome data (attrition bias) Hospital readmissions (due to adverse drug events) | Low risk | One participant withdrew from the control group before discharge; none lost to follow‐up in both groups (why assessed as low risk of bias). |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | One participant withdrew from the control group before discharge; none lost to follow‐up in both groups (why assessed as low risk of bias). |
| Selective reporting (reporting bias) | Low risk | Mortality was not prespecified as outcome on clinicaltrials.gov, but direction of effect does not suggest selective reporting. We therefore judged the outcome of mortality as having low risk of bias for selective reporting. The 30‐day readmission analysis was not pre‐specified in the trial registration at clinicaltrials.gov. The trial authors were contacted and replied “The 30‐days readmission analysis was not pre‐specified in the study protocol However, because of the increased use of 30‐ day readmission as an indicator of quality of care, the outcome was added as a post‐hoc analysis after the study was started”. Frequency of hospital visits (readmissions and emergency department) during the 6‐month follow‐up is a secondary outcome on clinicaltrials.gov, but emergency department contacts not reported in the publication. The trial authors sent data on readmissions and ED visits and the direction of effect does not suggest selective reporting. We therefore judged both outcomes as low risk of bias for selective reporting. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Juanes 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 118 participants randomised (59 to medication review and 59 to control) Patients 65 years or older, with a length of stay in ED longer than 12 hours, decompensation of HF and/or COPD and polypharmacy (4 or more drugs) were included from a tertiary referral hospital from Catalonia, Spain Mean age: 80 years 50% male Mean number of medications taken regularly at home: intervention 10.5 (3.5), control 10.0 (3.3) |
|
| Interventions | The intervention consisted of a pharmaceutical care programme focusing on the resolution of potential drug‐related problems, from admission to ED until discharge. The pharmaceutical care programme comprised the following steps:
Patients in the control group received standard pharmaceutical care, initiated at admission to the ward and consisting of medication review and prescriptions' validation, analogous to step 3 in the intervention group. The study compares the effects of an extended medication review and a basic medication review. |
|
| Outcomes |
Primary outcome: drug‐related negative outcomes (DNO) defined as health problems that patients experience owing to drug use or non‐use Secondary outcomes: patients readmitted within 180 days to the same ED and/or to the hospital ward: patients readmitted owing to decompensation of HF and/or exacerbation of COPD within 180 days after inclusion in the study |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by the hospital’s pharmacology department using SPSS V.18 (SPSS, Chicago, Illinois, USA) to create a dedicated application to randomise patients to one of the of 2 study groups (distribution 1:1). The application used a seed obtained by rolling two dice to select the row and column from a random‐number table; therefore, while replicable but unpredictable, the series was perfectly balanced between groups in 10‐case blocks. |
| Allocation concealment (selection bias) | High risk | Trial authors were contacted for clarification. They replied that the pharmacology department created the randomisation scheme. The pharmacist had access to the entire randomisation. After assessing the suitability of the patient through compliance of the inclusion criteria, the pharmacist assigned each individual a group according to the randomisation scheme, chronologically (in function of the onset of the episode in the ED) and correlative according to with the randomisation scheme. We assessed the risk of bias as high, because the pharmacist had access to the randomisation form (which was made in advance by rolling dice) and thus the allocation was not hidden (e.g. if 2 patients arrived at the same time). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither patients nor healthcare professionals were blinded to the treatment group. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | A single pharmacist was responsible for the implementation of the programme and the assessment of results, which might have led to observer bias. However, it is unlikely that it would affect mortality. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | A single pharmacist was responsible for the implementation of the programme and the assessment of results, which might have led to observer bias. However, it is unlikely that it would affect hospital readmissions. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | A single pharmacist was responsible for the implementation of the programme and the assessment of results, which might have led to observer bias. However, it is unlikely that it would affect hospital emergency department contacts. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | States none lost to follow‐up. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | States none lost to follow‐up. |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | States none lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Average cost of hospital stay is an outcome according to the clinical trial registration, but not reported or mentioned in the publication. However, as reported results are negative and cost‐effectiveness analyses are often published as secondary publication we therefore assessed the risk of selective reporting bias as low. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Kempen 2021.
| Study characteristics | ||
| Methods | Multicentre, cluster‐randomised, cross‐over trial | |
| Participants | 2637 participants randomised (922 to comprehensive medication review, 823 to comprehensive medication review plus follow‐up and 892 to usual care) Participants were admitted to 8 wards within 4 hospitals in 3 Swedish counties Median age: 81 years 48.5% male Median number of drugs: 9 |
|
| Interventions |
Intervention group 1: comprehensive medication review, including a thorough medication reconciliation, a comprehensive medication review in collaboration with the ward physician and patient, and another medication reconciliation before discharge Intervention group 2: comprehensive medication review with active follow‐up. This includes the same as intervention group 1 but with the following additions: when relevant an electronic medication review referral is sent to the patient's primary care physician upon discharge. A first phone call to the patient or carer is made 2 to 7 days after the patient is discharged and a second phone call is made 30 days after hospital discharge with the aim to find out how the patient is managing the medication and if any problems, concerns or questions have arisen, and to provide the patient with a motivational "boost". Control group: the control group will receive usual hospital care. Usual care includes medication reconciliation upon admission, and a medication report addressing the patient's medication treatment to be given to the patient or carer upon hospital discharge and to be attached to the electronic discharge letter. This report contains a motivation and explanation to the changes in medication treatment that have been made during hospital stay, as well as the patient's updated medication list. No clinical pharmacist will be involved. |
|
| Outcomes |
Primary outcome: incidence of unplanned hospital visits Secondary outcomes: all‐cause mortality rates, unplanned hospital admissions, emergency department visits, unplanned medication‐related hospital admissions and unplanned primary care visits at 30 days, 3 months, 6 months and 12 months, unplanned hospital visits at 30 days, 3 months and 6 months, time from hospital discharge to first unplanned hospital visit, and costs of hospital‐based care at 6 months and 12 months |
|
| Notes |
Design: the cross‐over design of the Kempen trial is complex. The clusters comprised 2 wards per hospital from 4 hospitals (8 clusters in total). Cross‐over and randomisation took place at a cluster (i.e. ward) level within each hospital. Each ward participated in the trial for 6 consecutive 8‐week study periods, which were divided into 2 separate blocks of 3 study periods each. During each period, 1 of 3 treatments (intervention 1 or 2 or control) was provided at the ward, with permuted block randomisation ensuring that each treatment was performed within each block. Participants did not cross over into other groups as individual patients could only be included in the trial once. Funding: The Medication Reviews Bridging Healthcare (MedBridge) trial has received governmental research grants RFR‐555601, RFR‐641791, and RFR‐735911 from the Uppsala‐Örebro Regional Research Council, grants LUL‐527721, LUL‐614061, LUL‐716201, and LUL‐821261 from Region Uppsala, grants CFUG‐658451 and CFUG‐698771 from Region Gävleborg, and grants LTV‐675921, LTV‐712341, LTV‐736641, and LTV‐840112 from Region Västmanland; funding from the Swedish Pharmacists Association (Sveriges Farmaceuter), the Thuréus Fund for Geriatric Research (Thuréus stiftelse för främjande av geriatrisk forskning), and the Geriatric Fund (Geriatriska fonden); and grants FA 2017:38 and FA 2018:43 from the Swedish Heart and Lung Association (Riksförbundet HjärtLung) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomised sequence was generated at Uppsala Clinical Research Center using SAS software (SAS Institute Inc). |
| Allocation concealment (selection bias) | Low risk | The computer‐generated codes were held by the statistician to assure allocation concealment until the moment of randomisation. The randomised cluster design did not allow for patient recruitment before randomisation, resulting in a risk of selection bias. However, we believe that risk of bias is low. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blinded study (outcomes assessor). |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | All primary and secondary outcome data collection and assessments were blinded to treatment allocation. Data on mortality were extracted from the national death registry. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | All primary and secondary outcome data collection and assessments were blinded to treatment allocation. Data on hospital admissions were extracted from the patients' electronic medical record. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (due to adverse drug events) | Low risk | All primary and secondary outcome data collection and assessments were blinded to treatment allocation. Unplanned hospital admissions were assessed by 2 final‐year undergraduate pharmacy students with a validated method to identify unlikely or possibly medication‐related admissions (AT‐HARM10). In case of doubt, an experienced clinical pharmacist was available to have the deciding vote. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | All primary and secondary outcome data collection and assessments were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | Registry data. No description of loss to follow‐up. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | Registry data. No description of loss to follow‐up. |
| Incomplete outcome data (attrition bias) Hospital readmissions (due to adverse drug events) | Low risk | Registry data. No description of loss to follow‐up. |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | Registry data. No description of loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No difference in outcomes when publication is compared to information on clinicaltrials.gov. |
| Contamination bias | High risk | Cluster‐randomised, cross‐over design. There is a risk of contamination bias, despite cluster‐randomisation due to the cross‐over design. Unintended intervention components were received by 132 of the 892 control patients (14.7%) during index admission (14 control patients (1.6%) received a comprehensive medication review during the index admission). |
| Other bias | High risk | Recruitment bias: low risk of bias (support for judgement: included admitted internal medicine patients. Inclusion unlikely to be associated with group allocation.) Baseline imbalance: low risk of bias (support for judgement: few clusters (n = 8) but due to cross‐over design all clusters are allocated to all 3 interventions). Loss of clusters: low risk of bias (support for judgement: no loss of clusters). Incorrect analysis: low risk of bias (support for judgement: adjusted for clustering and time). Comparability with individually randomised trials: high risk of bias (support for judgement: all wards are allocated to all 3 interventions during trial so risk of contamination and herd effect). Suitable design: high risk of bias (support for judgement: types of patients admitted are influenced by seasonal period effects and also over time quality improvement initiatives and other factors may be introduced influencing outcomes). Carry‐over effect: high risk of bias (support for judgement: medication reviews may result in physicians gaining knowledge about which drugs should be discontinued or prescribed, thereby introducing carry‐over effect of intervention). First period data: high risk of bias (support for judgement: first period data not available). Analysis: low risk of bias (support for judgement: analysed at ward level so no within‐person design. Analysis adjusted for study period.) |
Lea 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 399 participants randomised (200 to medication review and 199 to control) Patients acutely admitted to the internal medicine ward, Oslo University hospital 111 (Ullevaal), Norway Median age: 79 years 45% male Median number of regular drugs: 8 (range 4 to 19) |
|
| Interventions | Intervention participants received pharmacist‐led medicines management comprising medicines reconciliation at admission, repeated medicines reviews throughout the stay and medicines reconciliation and tailored information at discharge, according to the integrated medicines management model. Control participants received standard care. |
|
| Outcomes |
Primary outcome: time to first hospital readmission or death within 12 months after discharge Secondary outcomes:
|
|
| Notes | Supported by South‐Eastern Norway Regional Health Authority (Ph.D. grant number 12/00718 to author ML). Additional support was provided by the Hospital Pharmacies Enterprise and Oslo University Hospital and Diakonhjemmet Hospital. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number generator program and a permuted block design were used to generate the randomisation sequence, which was delivered to the study pharmacists in sequentially numbered, opaque, sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | The investigators were blinded to block size, which was randomly varied. Randomisation took place following patient inclusion and baseline assessments. A study pharmacist assigned the envelope with the lowest number to the individual participant and signed the allocation before the envelope was opened. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was neither feasible to blind participants nor study pharmacists to the allocation. It was also known by ward staff which of the patients belonged to the intervention group. Ward staff were, however, unable to distinguish between patients randomised to the control group and patients not participating in the trial. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Data on mortality were gathered from a non‐biased national register, the Norwegian Cause of Death Registry (through unique patient‐specific social security number). |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Data on readmissions were gathered from a non‐biased national register, the Norwegian Cause of Death Registry (through unique patient‐specific social security number). |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | Registry data. No description of loss to follow‐up. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | Registry data. No description of loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | In the protocol follow‐up was planned to be assessed at 12 months for both readmission and death. In the publication, the outcomes are reported at 30 days, 6 months and 12 months for both outcomes, and at 20 months for the outcome mortality. There was a statistically significant effect on mortality at 20 months, but not at 12 months. In the publication the changes are described: "We had originally planned a follow‐up of 12 months. However, as both the inclusion period and the retrieval of outcome data took longer than planned, we decided to extend the follow‐up of all patients to December 31, 2017, to increase statistical power. This amendment was described in the statistical analysis plan, which was finalized and signed before any outcome data files were available." Because the changes to follow‐up time were planned before data were analysed, we believe that the risk of bias for mortality is low. However, because data were available at 20 months, but not reported for the hospital readmissions, we judged the trial as having a high risk of selective reporting bias. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Lenssen 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 61 participants were randomised (31 to medication review and 30 to control) Patients admitted to 4 departments of non‐intensive care units at the German University Hospital Aachen, Germany: the Departments of Urology, Neurology, Internal Medicine III (Gastroenterology and Metabolic Disorders) and Internal Medicine I (Cardiology, Pneumology and Angiology) Mean age: 77.6 years 40% male Mean number of medications 16.8 |
|
| Interventions | The comprehensive pharmaceutical care service included a detailed medication history, medication reconciliation and a medication safety check directly after inclusion in the study and during the entire stay on the co‐operating wards. Medication was checked for new prescriptions, and a medication review was repeated with each newly prescribed drug. Medication safety checks included the plausibility of medication, check for drug allergies, renal/liver dysfunction and dosage adjustment, relevant laboratory data, contraindications, dosage, drug‐drug interactions, adverse drug reactions, medications before surgery or other interventions, adequate duration of drug therapy, need for patient information and therapeutic drug monitoring. Transitional care at discharge included medication reconciliation at discharge and providing recommendations for the discharge letter. After discharge, the comprehensive pharmaceutical care service ended, and all study patients received their ‘standard care’. Standard care did not include clinical pharmacists in the healthcare team on the ward. |
|
| Outcomes |
Primary outcome: the occurrence of drug‐related readmissions (DRRs), measured over 1 year at 4 pre‐defined contact times after discharge. DRR was defined as re‐hospitalisation of a discharged patient due to an adverse drug reaction (ADR) in any hospital. Secondary outcomes: adverse drug reactions, potentially inappropriate medication (PIM) using the PRISCUS list and the number of changes in medication after discharge were documented. |
|
| Notes | Funding: Supported by the Foerderinitiative Pharmazeutische Betreuung e.V. and the Apothekerstiftung Nordrhein. RL received a research grant from the “Foerderinitiative Pharmazeutische Betreuung e.V.”. RL, UJ and AE received a research grant from the Apotheker‐Stiftung Nordrhein. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was generated at www.randomization.com. |
| Allocation concealment (selection bias) | High risk | Patients were randomised successively after being included in the study. The trial authors were contacted and asked to clarify their process for randomisation. They replied: "The randomisation list was accessible by the person, who included the patients, but was first accessed, when the enrollment of a patient was finalised." Therefore judged as high risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but probably not blinded. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Three pharmacists assessed all cases of ADR‐suspicious symptoms and hospital readmissions. They were blinded regarding the allocation of the patients to the control or intervention group. Readmissions was not an outcome in the clinical trial, but the data were reported descriptively in the publication. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (due to adverse drug events) | Low risk | Three pharmacists assessed all cases of ADR‐suspicious symptoms and hospital readmissions. They were blinded regarding the allocation of the patients to the control or intervention group. |
| Blinding of outcome assessment (detection bias) Adverse drug events | Low risk | Three pharmacists assessed all cases of ADR‐suspicious symptoms and hospital readmissions. They were blinded regarding the allocation of the patients to the control or intervention group. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | No description of missing data. Readmissions was not an outcome in the clinical trial registration, but the outcome is reported in the publication. Authors report data from all patients randomised to the study. |
| Incomplete outcome data (attrition bias) Hospital readmissions (due to adverse drug events) | High risk | Loss to follow‐up: 33% (20/60). The inability to reach a patient (e.g. the patient moved to new address or died) caused censoring. |
| Incomplete outcome data (attrition bias) Adverse drug events | High risk | Loss to follow‐up: 33% (20/60). The inability to reach a patient (e.g. the patient moved to new address or died) caused censoring. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported in trial publication and similar to information on clinicaltrials.gov. Readmissions was not an outcome in the clinical trial, but the data were reported descriptively in the publication. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Lisby 2010.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 100 participants randomised (50 to medication review and 50 to control) Participants were admitted to an acute ward of internal medicine at a regional hospital in Denmark Mean age: 79.2 years 39% male Mean number of drugs: 10.2 |
|
| Interventions | The intervention included clinical pharmacists and clinical pharmacologists (physicians) and was accomplished in 2 steps. First, a clinical pharmacist systematically collected information about participants' medication; second, collected medical histories were discussed with a clinical pharmacologist according to participants' entire medical records, including medical histories and laboratory test results. Discrepancies, inappropriate drugs, doses, routes, dosing schedules and inappropriate interactions between drugs were described in a note with recommendations for change. Ward physicians were not obliged to follow these recommendations. No co‐interventions were reported. Patients assigned the control arm received the usual routine for medication prescription. |
|
| Outcomes |
Primary outcome: length of hospital stay (hours) Secondary outcomes: time to first admission, readmissions, emergency department visits, visits to outpatient care clinic, general practitioner visits, specialist visits, after‐hours care, quality of life assessment, mortality. All outcomes had 3 months of follow‐up. |
|
| Notes | Funding: ALIS, Amgros I/S, which is a publicly owned pharmaceutical procurement service for the 5 regional authorities in Denmark | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were randomly assigned to intervention or control by a computer‐generated code. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial described as not blinded. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Mortality data were likely gathered from a non‐biased national register (through unique patient‐specific social security number). |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Readmission data were likely gathered from a non‐biased national register. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Hospital emergency department contact data were likely gathered from a non‐biased national register. |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | Low risk | Questionnaires including pre‐paid envelopes were sent by postal mail. Because of the design of the intervention, it was not possible to blind the participants toward group assignment. Participant knowledge about the assignment was assessed as unlikely to influence the outcome. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | No loss to follow‐up was described; all data should be readily available from a non‐biased national register. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | No loss to follow‐up was described; all data should be readily available from a non‐biased national register. |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | No loss to follow‐up was described; all data should be readily available from a non‐biased national register. |
| Incomplete outcome data (attrition bias) Health‐related quality of life | High risk | Loss to follow‐up: intervention: 34% (17/50), control: 29% (14/49). In addition, on average 4 to 6 responses were missing for each question in the questionnaire, though missingness was equally distributed between the intervention and control arm. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were included in trial and were similar to information on www.clinicaltrials.gov. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Lisby 2015.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 108 participants randomised (53 to medication review and 55 to control) Participants were admitted to an orthopaedic ward at a regional hospital in Denmark Mean age: 80.5 years 29% male Mean number of drugs: 6.7 |
|
| Interventions | Systematic medication review by a clinical pharmacist and a clinical pharmacologist. A clinical pharmacist obtained medication history through medical records, electronic prescribing system, registry of drug purchase and interview after a ward physician had prescribed in‐hospital medication. Subsequently, the case was discussed with a clinical pharmacologist, and a note with comments and recommendations for medication changes was prepared and handed directly to the physician responsible for the ward round. Ward physicians were not obliged to follow these recommendations. No co‐interventions were reported. The participants assigned controls were treated routinely, that is, a ward physician obtaining medication history, performing a review and prescribing the in‐hospital medication. |
|
| Outcomes |
Primary outcome: time to first unscheduled physician contact (general practitioner, emergency department, ambulatory care or hospital) after discharge from the orthopaedic department
Secondary outcomes: admission time, time to first readmission, number of readmissions, emergency department visits, visits to outpatient care clinic, general practitioner contacts if first contact with general practitioner included medication issues, contacts with physicians outside working hours, medical specialist contacts, quality of life assessment, mortality All outcomes had 3 months of follow‐up |
|
| Notes | Funding: The Health Insurance Foundation in Denmark | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were randomly assigned to intervention or control by a computer‐generated code. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial described as not blinded. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Mortality data were taken from a non‐biased national register (participants identifiable through unique patient‐specific social security number). |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Readmission data were likely gathered from a non‐biased national register. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Emergency department contacts were likely gathered from a non‐biased national register. |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | Low risk | Questionnaires including pre‐paid envelopes were sent by postal mail. Because of the design of the intervention, it was not possible to blind the participants toward group assignment. We assessed participant knowledge about the assignment as unlikely to influence the outcome. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | No loss to follow‐up was described; all data should be readily available from a non‐biased national register. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | No loss to follow‐up was described; all data should be readily available from a non‐biased national register. |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | No loss to follow‐up was described; all data should be readily available from a non‐biased national register. |
| Incomplete outcome data (attrition bias) Health‐related quality of life | High risk | Fifty‐five (54%) of 102 participants returned the questionnaire. Loss to follow‐up: intervention: 50% (25/50), control: 57% (30/52). |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were included in trial and were similar to information provided on www.clinicaltrials.gov. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Nielsen 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial. | |
| Participants | 404 participants randomised (202 to medication review and 202 to control). Retrospective period: 1142 randomised (189 to control and 953 randomised to no enrolment) Participants were included from 3 Danish acute medicine wards Mean age: 73.1 years 48% male Median number of drugs: 8 For the first 8 months, the clinical pharmacists were alternately absent for 2 months (retrospective periods) and present for 2 months (prospective periods) at the wards, giving two 2‐month periods at each centre with no clinical pharmacists present. After these 8 months, the clinical pharmacists were present at all 3 wards for the remaining time. In the retrospective periods, patients were included for a second, retrospective control group, which would allow the assessment of a potential educational bias from the intervention to the prospective control group. In the prospective periods, the patients were stratified by centre and randomised to the intervention or the prospective control. |
|
| Interventions | The intervention comprised secondary medication history, medication reconciliation, medication review based on the pre‐admission medication, and entry of proposed prescriptions in the electronic medication module (EMM). The intervention was made before a physician attended to the patient and entered admission medication orders into the EMM. The control group patients received standard hospital care with no clinical pharmacist involvement. |
|
| Outcomes |
Primary outcome: the proportion of patients with harm as indicated by one or more medication‐related triggers (in‐hospital ADEs detected with the Adverse Drug Event Trigger Tool) Secondary outcomes: harms per patient, median length of stay, re‐admission within 1st year, days to 1st readmission, deceased with 1st year, time to death |
|
| Notes | Funding: supported by grants from Hospital Pharmacies and Amgros’ Research and Development Foundation, The Health Foundation (Helsefonden) and Region Zealand Health Scientific Research Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation lists were generated using a computer‐based randomisation tool and kept at the hospital pharmacy by the pharmacist responsible for clinical trials. |
| Allocation concealment (selection bias) | Low risk | Allocation was revealed to the clinical pharmacist by telephone whenever the clinical pharmacist had enrolled a patient. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither the clinical pharmacists nor the healthcare personnel or patients were blinded to the participant allocation. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Based on hospital records; not sure if blinded but will likely not influence assessment. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Based on hospital records; not sure if blinded but will likely not influence assessment. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | Deaths were collected from the electronic patient records (national registry data are integrated in the Danish electronic patient records). |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | Readmissions were collected from the electronic patient records. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were included in trial and were similar to information provided on www.isrctn.com. Direct cost for the hospital is listed as an secondary outcome in the trial registration, but not reported in the trial publication. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
O'Mahony 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 1537 participants randomised (772 to medication review and 752 to control) Patients were admitted to 6 European medical centres (Ireland, Scotland, Spain, Italy, Belgium and Iceland), (13 medical and eight surgical clusters) Median (IQR) age: 78 (72 o 84) years 53% male Median (IQR) number of medications: 10 (8 to 13) |
|
| Interventions | Customised SENATOR software input information included patients’ diagnoses (ICD‐10 codes), prescription drugs (ATC codes) and doses, renal function (MDRD formula), liver function (liver transaminases, INR), cardiac rhythm (electrocardiogram) and complete blood count. By applying STOPP/START criteria (version 2) alongside potentially relevant drug‐drug and drug‐disease interaction information from local databases, SENATOR software produced an individualised medication advice report. Primary researchers subsequently notified senior attending physicians of intervention arm patients of the SENATOR reports and inserted copies into patients’ medical records, i.e. printed reports into paper‐based records or electronic reports into electronic records. Participants in the control group received standard pharmaceutical care as provided at each site at time of randomisation. |
|
| Outcomes |
Primary outcome: the occurrence of probable or certain ADRs within 14 days of randomisation Secondary outcomes: primary endpoint derivatives Tertiary outcomes: all‐cause mortality, re‐hospitalisation, composite healthcare utilisation and health‐related quality of life |
|
| Notes | Funding: supported by the European Commission’s Seventh Framework Programme (FP7/2007–2013) (grant number 305930) as part of the SENATOR project | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent statistician used random block sizes to generate stratum‐specific randomisation lists. |
| Allocation concealment (selection bias) | Low risk | The randomisation lists were integrated into the eCRF so that researchers could not access them, and any given allocations were only revealed once patients were unambiguously enrolled into the trial and their screening information was irreversibly entered onto the eCRF. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Primary researchers who recruited the patients were not blinded and attending hospital physicians were not blinded. However, the patients were blinded and those assessing the primary outcome (i.e. ADR) were also blinded. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Mortality data were assessed from patients’ medical records and national registries in all but one site, where death was established by telephone follow‐up. Since the outcome of mortality is assessed from records in most countries, lack of blinding will likely not influence mortality. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Readmissions were assessed by post‐hospital discharge follow‐up call by the local site research staff who were not blinded. However, the outcome of readmissions is objective and will likely not be influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | Loss to follow‐up: 6.8% (52/766) in the medication review group, 7.4% (56/752) in the control group. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | Loss to follow‐up: 5.4% (41/766) in the medication review group, 5.3% (40/752) in the control group. |
| Selective reporting (reporting bias) | Low risk | Health‐related quality of life was prespecified in a trial registry, but according to the corresponding author it was decided not to measure the outcome due to lack of resources. Therefore we assessed as low risk of selective reporting. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Ravn‐Nielsen 2018.
| Study characteristics | ||
| Methods | Randomised, clinical, multicentre trial | |
| Participants | 1498 participants randomised (498 to basic medication review, 497 to extended medication review and 503 to control) Participants were admitted to 4 different acute admission wards in Denmark Median (IQR) age: basic group 72 (63 to 87) years, extended group 71 (63 to 79) years and control group 83 (65 to 80) years 54% male Median (IQR) number of drugs: control 9 (7 to 12), basic intervention 10 (7 to 13), extended intervention 10 (7 to 12) |
|
| Interventions | Patients were randomised 1:1:1 to usual care, a basic intervention or an extended intervention group. In the basic intervention group, a structured, patient‐centred medication review was conducted by a clinical pharmacist once shortly after the patient was admitted, when laboratory data were available and the primary medical admission note was written. In the extended intervention group, a similar medication review was conducted. In addition, on discharge of the patient, a medication reconciliation was conducted. Follow‐up calls with the PCP and nursing home or caregiver were conducted when any change in medication was made during the index hospitalisation. The primary pharmacy was called when the clinical pharmacist from the hospital found it necessary, for example, to delete old prescriptions or address problems concerning dose‐dispensed medication. The interview in the follow‐up telephone call was also based on principles of motivational interview and was routinely performed twice. The first interview was conducted 1 week after discharge, whereas a second interview was performed 6 months after discharge. If required, additional follow‐ups could be arranged. |
|
| Outcomes |
Primary outcome: the occurrence of readmission within 30 and 180 days and the occurrence of a pre‐specified composite endpoint of readmissions and emergency department (ED) visits within 180 days Secondary outcomes: drug‐related readmissions within 30 and 180 days after inclusion and all‐cause and drug‐related mortality |
|
| Notes | Funding: unrestricted grants from The Hospitals Pharmacies’ and Amgros’ Research Development Foundation, public regional research foundations for Southern Denmark and Zealand, and the Actavis Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were randomly assigned to the usual care, basic intervention or extended intervention group in a 1:1:1 ratio using block randomisation (blocks of 6 and 4) with the sequentially numbered, opaque, sealed envelope technique. |
| Allocation concealment (selection bias) | Low risk | The patients were randomly assigned to the usual care, basic intervention or extended intervention group in a 1:1:1 ratio using block randomisation (blocks of 6 and 4) with the sequentially numbered, opaque, sealed envelope technique. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial could not be entirely blinded because, for example, the intervening pharmacist and the patient would know the result of the allocation. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Information about readmissions, ED visits and deaths was gathered from the non‐biased National Patient Register (through unique patient‐specific social security number). |
| Blinding of outcome assessment (detection bias) Mortality (due to drug‐related adverse events) | Low risk | Information about deaths was drawn from the National Patient Register. Evaluation of whether a death should be classified as drug‐related was done by clinical pharmacists and a clinical pharmacologist, who were blinded to study allocation. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Information about readmissions, ED visits and deaths was gathered from the non‐biased National Patient Register (through unique patient‐specific social security number). |
| Blinding of outcome assessment (detection bias) Hospital readmissions (due to adverse drug events) | Low risk | Information about readmissions, ED visits and deaths was collected from the non‐biased National Patient Register (through unique patient‐specific social security number). The clinical pharmacists and clinical pharmacologist who evaluated whether a readmission or death should be classified as drug‐related were blinded to study allocation. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Information about readmissions, ED visits and deaths was gathered from the non‐biased National Patient Register (through unique patient‐specific social security number). |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Low risk | Loss to follow‐up 2% (32/1499) (13 excluded after randomisation, 19 withdrew consent). Information about readmissions, ED visits and deaths was collected from the National Patient Register. |
| Incomplete outcome data (attrition bias) Mortality (due to drug‐related adverse events) | High risk | Information about deaths was drawn from the National Patient Register. For drug‐related mortality, only in‐hospital deaths were assessed with respect to causality (i.e. drug‐related or not). Deaths outside the hospital did not undergo causality assessment because information from the primary care sector regarding the circumstances of death was usually too sparse. The study does not report how many participants died at home or at the hospital, therefore we assessed the risk of bias as high. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | Loss to follow‐up 2% (32/1499) (13 excluded after randomisation, 19 withdrew consent). Information about readmissions, ED visits and deaths was collected from the National Patient Register. |
| Incomplete outcome data (attrition bias) Hospital readmissions (due to adverse drug events) | Unclear risk | There were more dropouts in the extensive intervention group than in the usual care or the basic intervention group. Authors argue that this is because some patients in the extensive intervention group were frustrated by the additional healthcare contacts and therefore withdrew their informed consent. Information about readmissions, ED visits and deaths was drawn from the National Patient Register. |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | Loss to follow‐up 2% (32/1499) (13 excluded after randomisation, 19 withdrew consent). Information about readmissions, ED visits and deaths was collected from the National Patient Register. |
| Selective reporting (reporting bias) | Unclear risk | We assessed bias as unclear as data were registered in clinical trials.gov after study completion. The date on the protocol is after the trial start (protocol from March 2014, inclusion of participants began September 2013). There are no unpublished outcomes when trial publication is compared to information on clinicaltrials.gov. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Schnipper 2006.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 178 participants randomised (92 to medication review and 86 to control) Participants were admitted to the general medicine service at a university hospital in the USA Mean age: 59.3 years 34% male Median number of drugs: 8.0 |
|
| Interventions | The pharmacist intervention on the day of discharge consisted of several parts. First, discharge medication regimens were compared with preadmission regimens, and all discrepancies were reconciled with the assistance of the medical team. Participants were screened for previous drug‐related problems, including non‐adherence, lack of efficacy and side effects. The pharmacist reviewed the indications, directions for use and potential adverse effects of each discharge medication with the participant, and discussed significant findings with the medical team. Co‐interventions: follow‐up telephone call, during which the pharmacist compared the participant's self‐reported medication list with the discharge list, exploring any discrepancies The pharmacist also asked about medication adherence, possible adverse drug events and adherence with scheduled follow‐up and laboratory appointments. Significant findings were communicated to the participant's primary care physician. Patients assigned to usual care received a routine review of medication orders by a ward‐based pharmacist and medication counselling by a nurse at the time of discharge |
|
| Outcomes |
Primary outcome: preventable adverse drug events
Secondary outcomes: all adverse drug events (preventable or not), participant satisfaction, health care utilisation (readmission + emergency department contact), medication adherence, medication discrepancies All outcomes had 30 days of follow‐up |
|
| Notes | Funding: supported by the Division of General Medicine at Brigham and Women’s Hospital (BWH), Boston, MA, the Fish and Anderson Fundsat BWH and an unrestricted grant from the Merck Co Foundation, West Point, PA. Dr Schnipper is supported by Mentored Clinical Scientist Development Award HL072806 from the National Heart, Lung and Blood Institute, Bethesda, MD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a computer‐generated algorithm. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes were opened only after patient consent was obtained. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial was described as not blinded. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Outcomes were assessed by research assistants and manuscript authors blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (due to adverse drug events) | Low risk | Outcomes were assessed by research assistants and manuscript authors blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | Outcomes were assessed by research assistants and manuscript authors blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (due to adverse drug events) | Low risk | Outcomes were assessed by research assistants and manuscript authors blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) Adverse drug events | Low risk | Outcomes were assessed by research assistants and manuscript authors blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | High risk | Uneven loss to follow‐up: medication review 36% (33/92) participants; control group 21% (18/84 participants). Assessed as high‐risk due to large dropout with uneven distribution. |
| Incomplete outcome data (attrition bias) Hospital readmissions (due to adverse drug events) | High risk | Uneven loss to follow‐up: medication review 36% (33/92) participants; control group 21% (18/84 participants). Assessed as high‐risk due to large dropout with uneven distribution. |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | High risk | Uneven loss to follow‐up: medication review 36% (33/92) participants; control group 21% (18/84 participants). Assessed as high‐risk due to large dropout with uneven distribution. |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (due to adverse drug events) | High risk | Uneven loss to follow‐up: medication review 33/92 participants; control group 18/84 participants. Assessed as high‐risk due to large dropout with uneven distribution. |
| Incomplete outcome data (attrition bias) Adverse drug events | High risk | Uneven loss to follow‐up: medication review 36% (33/92) participants; control group 21% (18/84 participants). Assessed as high‐risk due to large dropout with uneven distribution. |
| Selective reporting (reporting bias) | Unclear risk | No clinical trial registration or protocol to compare publication of results. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
Scullin 2007.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 762 participants randomised (371 to medication review and 391 to control) Participants were admitted to medical wards at 3 general hospitals in Northern Ireland Mean age: 70.1 years 47% male |
|
| Interventions | A clinical pharmacist (aided by a pharmacy technician) constructed an accurate medication history by using a variety of sources and reviewed drug treatment daily, taking into account therapeutic goals, relevant clinical chemistry and haematology results and, when appropriate, therapeutic drug monitoring. The intervention also included medication counselling tailored to suit the needs of each individual participant. The control group received traditional clinical pharmacy services, which were in place across the participating hospitals. |
|
| Outcomes |
Primary outcome: difference in length of hospital stay
Secondary outcomes: time to hospital readmission, number of readmissions, healthcare practitioner satisfaction All outcomes had 30 days of follow‐up |
|
| Notes | Funding: Department of Health, Social Services and Public Safety (Northern Ireland), under its Executive Programme Fund scheme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated to groups using closed‐envelope technique, therefore indirectly regarded as random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Allocated to groups using closed‐envelope technique, but no description of whether they were sequentially numbered or opaque. Randomisation was performed in blocks of 20 (each block contained 10 intervention and 10 control allocations), which could reveal allocation. No description of who included participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described as blinded. |
| Blinding of outcome assessment (detection bias) Mortality (all‐cause) | Low risk | Data collected by researchers aware of assignments, but this will likely not influence assessment of mortality. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | Data collected by researchers aware of assignments, but this will likely not influence assessment of readmissions. Readmission data were collected from the hospital computer system. |
| Incomplete outcome data (attrition bias) Mortality (all‐cause) | Unclear risk | Discrepancies in the publication concerning reporting of mortality data, as 7 participants seem to be missing from the medication review group and 1 from the control group. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | High risk | Discrepancies in the publication concerning reporting of hospital readmission data. The publication states that 141 participants (40.8%) were readmitted in the medication review group versus 171 participants (49.3%) in the control group. However, when percentages were used to calculate the total number of participants, 25 participants seemed to be missing from the medication review group (346 versus 371), but only 1 was described as lost to follow‐up and 42 as missing from the control group (349 versus 391); only 7 were described as lost to follow‐up. This could have influenced the outcome of hospital readmissions. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes seem to have been reported. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | High risk | Unequal randomisation with 371 participants assigned to the medication review group and 391 to the control group, which should not have been possible when block sizes were 20. This may have been possible if each block was per hospital, but this was not described. Very unclear reporting of data. Data not reported for surgical wards; no reason stated for excluding data. No protocol available. |
Song 2021.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 100 participants randomised (50 to medication review and 50 to control) Participants were admitted to the nephrology ward at Seoul National University Hospital (SNUH) in South Korea Mean age: 52.5 years 60% male Median number of drugs: 9 |
|
| Interventions | Pharmacists conducted a structured, patient‐centred medication review, communicated with healthcare professionals and patients, and documented inpatients diagnosed with chronic kidney disease daily. The service included a (1) medication reconciliation service to reduce discrepancies in medicines prescribed within 24 hours after admission compared with those in medicines prescribed before admission, (2) medication evaluation and management service to promote the appropriateness of the pharmacotherapy, and (3) discharge pharmaceutical care transition service to reduce the medication discrepancies before and after discharge. Participants in the control group received usual care from pharmacists and physicians. |
|
| Outcomes |
Primary outcome: the average number of DRPs per patient at discharge.
Secondary outcomes:
|
|
| Notes | Funding: The Korea Health Technology R&D Project of the Ministry of Health & Welfare, grant number HI13C0731, and the National Research Foundation of Korea of the Ministry of Science and ICT (MSIT), grant number NRF‐2019R1G1A1100325, South Korea | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study participants were randomised in a 1:1 ratio to the clinical pharmacist intervention or control group using a centralised, secure, computer‐generated program. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients and pharmacist conducting the medication reviews were not blinded. The pharmacists who performed the assessment at admission, discharge and post‐discharge were blinded (blinded outcome assessment). |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | The pharmacists who performed the assessment at admission, discharge and post‐discharge were blinded (blinded outcome assessment). |
| Blinding of outcome assessment (detection bias) Hospital emergency department contacts (all‐cause) | Low risk | The pharmacists who performed the assessment at admission, discharge and post‐discharge were blinded (blinded outcome assessment). |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | Low risk | 50 participants were allocated to the intervention group; outcome presented for 48 participants (1 lost to follow‐up (to other hospital), and 1 participant died). 50 participants were allocated to the control group; outcome presented for 47 participants (1 lost to follow‐up (to other study), and 2 participants withdrew their consent). |
| Incomplete outcome data (attrition bias) Hospital emergency department contacts (all‐cause) | Low risk | 50 participants were allocated to the intervention group; outcome presented for 48 participants (1 lost to follow‐up (to other hospital), and 1 participant died). 50 participants were allocated to the control group; outcome presented for 47 participants (1 lost to follow‐up (to other study), and 2 participants withdrew their consent). |
| Selective reporting (reporting bias) | Unclear risk | No clinical trial registration to compare protocol and publication of results. |
| Contamination bias | High risk | No cluster‐randomisation. |
| Other bias | Low risk | No evidence of other types of bias. |
SUREPILL 2015.
| Study characteristics | ||
| Methods | Cluster‐randomised controlled trial. | |
| Participants | 1094 participants randomised (547 to medication review and 547 to control) Participants were included from at least 2 surgical wards from 3 different types of hospital: an academic hospital, a tertiary teaching hospital and a community teaching hospital in The Netherlands 57% male |
|
| Interventions | Patients admitted at an intervention ward received bedside care from the ward‐based pharmacy team. These interventions were tailored to cover critical steps in the medication process during the surgical pathway. On admission the pharmacy practitioner performed medication reconciliation in consultation with the patient. This included verification of the current use of community pharmacy medication. During hospitalisation, the hospital pharmacist reviewed the medication charts daily and optimised drug therapy when needed. The pharmacist combined information from CPOE‐alerts, laboratory results and medical record information, in liaison with the ward doctor (face‐to‐face communication). At discharge the pharmacy practitioner reviewed the medication prescriptions by comparing them with the medication at admission. Unintended discrepancies were discussed with the ward doctor. In addition, the pharmacy practitioner performed patient counselling and sent a complete list of discharge medication to the community pharmacy and general practitioner. Participants in the control group received standard care. |
|
| Outcomes |
Primary outcome: the number of preventable ADEs on control and intervention wards during the study. Secondary outcomes: postoperative complications were registered prospectively by doctors in the Dutch National Surgical Adverse Event Registration database. Furthermore, the duration of hospital stay and health‐related quality of life were assessed approximately 3 months after admission using the validated questionnaires EuroQol–5D and EQ‐VAS. In addition, the number and duration of any readmissions within 3 months were recorded, measured from the patients’ questionnaires. |
|
| Notes | Funding: ZonMw, the Dutch Organization for Health Research and Development (project number 170882706) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described. |
| Allocation concealment (selection bias) | High risk | Described as randomised at ward level and that "consecutive patients admitted for elective surgery with expected hospital stay longer than 48 h were included." There were 3 intervention wards and 3 control wards and in total 547 patients were included in the intervention group and 547 in the control group. There is no description of how informed consent was obtained from patients in either group and the equal number of patients in each group is unlikely to have happened by chance. This suggests possible selection bias and is why we judged the trial as high‐risk. The trial authors did not respond regarding our queries to clarify methods of randomisation and allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described, but probably not blinded. |
| Blinding of outcome assessment (detection bias) Hospital readmissions (all‐cause) | Low risk | The number and duration of any readmissions within 3 months were recorded, measured from the patients’ questionnaires. If patients reported readmission to the original hospital, this was confirmed by checking the hospital information system. It is not stated whether the study was blinded, but the outcome of readmissions is objective and will likely not be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | Low risk | Health‐related quality of life was assessed approximately 3 months after admission using the validated questionnaire EuroQol–5D. The questionnaire was sent to the patients who filled out and returned it. Participant knowledge about the assignment was assessed as unlikely to influence the outcome. |
| Incomplete outcome data (attrition bias) Hospital readmissions (all‐cause) | High risk | High loss to follow‐up: 34% (185/547) in both the medication review group and the control group. Data were assessed from patients’ questionnaires. If patients reported readmission to the original hospital, this was confirmed by checking the hospital information system. 547 participants were randomised to both control and intervention group. Unclear why they only reported data from 362 participants in both groups. |
| Incomplete outcome data (attrition bias) Health‐related quality of life | High risk | High loss to follow‐up: 32% (338/1062). Approximately 3 months after the hospital admission, questionnaires were sent to 1062 of 1094 participants; the remaining 32 patients had died or had no recorded address. Some 755 patients (71.1%) returned the questionnaire, and 724 (68.2%) completed the quality of life assessment and data concerning readmission. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were included in the trial and were similar to the information provided in the protocol. Direct (non‐)medical costs and indirect non‐medical costs, and extra costs per prevented ADE, were secondary outcomes in the protocol, but not reported in the trial publication. |
| Contamination bias | Low risk | Cluster‐randomised. |
| Other bias | High risk | Major methodological issues were identified leading to high risk of bias. First, it is uncanny that there are exactly the same number of participants who returned questionnaires about quality of life and readmission in each group. In total, there are data from 724 participants, which are evenly distributed between the control group and the intervention group with 362 in each (see Table 4). Recruitment bias: low risk (support for judgement: included elective surgical patients. Inclusion unlikely to be associated with group allocation.) Baseline imbalance: high risk of bias (support for judgement: 3 surgical wards from 3 hospitals were included in the medication review group and 3 surgical wards in the control group. Since only one type of surgical ward exists in each hospital and due to only 3 clusters this creates a high risk of baseline imbalance on important prognostic factors (i.e. co‐morbidities and types of drug treatment). This is also suggested in Table 1 were there are statistically significant differences in important characteristics (e.g. patients undergoing vascular surgery and patients with co‐morbidities were over‐represented in the intervention wards)). Loss of clusters: low risk of bias (support for judgement: no loss of clusters Incorrect analysis: high risk of bias (support for judgement: no adjustment for clusters) Comparability with individually randomised trials: high risk of bias (support for judgement: familiarity with medication reviews among surgeons at medication review wards may improve adherence to recommendations by pharmacists compared with trials randomised at individual level (i.e. herd effect)). |
Abbreviations:
ADE: adverse drug event ADR: adverse drug reaction AOU: Assessment Of Underutilization index ATC: Anatomical Therapeutic Chemical Classification System CAS: computerised alert systems COPD: chronic obstructive pulmonary disease CPOE: computerised physician order entry DRP: drug‐related problem DRR: drug‐related readmission ED: emergency department EQ‐5D: standardised instrument of the EuroQol Group used to measure health outcomes FASS: Pharmaceutical Specialities in Sweden GP: general practitioner HF: heart failure HRQL: health‐related quality of life ICECAP‐O: ICEpop CAPability measure for Older people INR: international normalised ratio IQR: interquartile range MAI: Medication Appropriateness Index MDRD: modification of diet in renal disease PIM: potentially inappropriate medication PIP: potentially inappropriate prescribing START: Screening Tool to Alert to Right Treatment STOPP: Screening Tool of Older Persons’ potentially inappropriate Prescriptions
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| [no author] 2001 | Not a randomised controlled trial |
| AbuRuz 2021 | Outcome did not meet inclusion criteria (prevalence of DRPs and no follow‐up data after discharge) |
| ACTRN12605000264684 2005 | Population did not meet inclusion criteria (medication review done in general practice) |
| ACTRN12605000376640 2005 | Population did not meet inclusion criteria (the intervention was a pharmacist transition co‐ordinator for older adults undergoing first‐time transfer from a hospital to a long‐term care facility. The medication review was performed by the community pharmacist within 10 to 14 days of the transfer to long‐term care facility). |
| ACTRN12611000370909 2011 | Population did not meet inclusion criteria (residential aged care facilities) |
| ACTRN12611000995976 2011 | Intervention did not meet inclusion criteria (all medication review recommendations were sent to the general practitioners (to be implemented after discharge)) |
| ACTRN12611001262998 2011 | Population did not meet inclusion criteria (included outpatients) |
| ACTRN12616001034426 2016 | Intervention did not meet inclusion criteria (medication management plan in the medical discharge summary) |
| ACTRN12616001474448 2016 | Intervention did not meet inclusion criteria (discharge service) |
| ACTRN12617001293358 2017 | Population did not meet inclusion criteria (intervention done after discharge) |
| ACTRN12618000250235 2018 | Population did not meet inclusion criteria (included outpatients) |
| ACTRN12618000794202 2018 | Population did not meet inclusion criteria (included outpatients) |
| ACTRN12619000729123 2019 | Intervention did not meet inclusion criteria (ADR risk assessment and medication management plan) |
| Ahmad 2012 | Intervention did not meet inclusion criteria (to be implemented after discharge) |
| Al‐Hashar 2018 | Intervention did not meet inclusion criteria (medication reconciliation) |
| Alicic 2016 | Population did not meet inclusion criteria (intervention to be done after discharge) |
| Allen 1986 | Intervention did not meet inclusion criteria (geriatric team) |
| Al Mazroui 2009 | Population did not meet inclusion criteria (included outpatients) |
| Al‐Rashed 2002 | Intervention did not meet inclusion criteria (only drug information) |
| Awdishu 2016 | Intervention did not meet inclusion criteria (physicians randomised to receive clinical decision support in the electronic health record) |
| Balaban 2015 | Intervention did not meet inclusion criteria (hospital‐based Community Health Workers provided coaching and assistance in navigating the transition from hospital to home through hospital visits and weekly telephone outreach) |
| Basoor 2011 | Intervention did not meet inclusion criteria (Heart Failure Discharge Checklist) |
| Bell 2016 | Intervention did not meet inclusion criteria (medication reconciliation, inpatient pharmacist counselling, adherence aids and individualised telephone follow‐up after discharge) |
| Bhagwan 2018 | Intervention did not meet inclusion criteria (medication reconciliation) |
| Boland 2016 | Not a randomised controlled trial |
| Bolas 2004 | Intervention did not meet inclusion criteria (only medication reconciliation) |
| Bondesson 2013 | Not a randomised controlled trial |
| Bonnet‐Zamponi 2010 | Not a randomised controlled trial |
| Bonnet‐Zamponi 2013 | Not a randomised controlled trial (used a Zelen design by which consent is obtained after randomisation, leading to selection bias) |
| Brecher 2015 | Population did not meet inclusion criteria (chronic care geriatric facility) |
| Briggs 2015 | Population did not meet inclusion criteria (medication review done in the Emergency Department) |
| Bruhwiler 2018 | Intervention did not meet inclusion criteria (standardised prescription check)) |
| Bruhwiler 2019 | Intervention did not meet inclusion criteria (medication reconciliation at discharge) |
| Bulow 2018 | Not a randomised controlled trial |
| Burleson 2003 | Intervention did not meet inclusion criteria (medication history) |
| Burnett 2009 | Outcome did not meet inclusion criteria (medication appropriateness) |
| Capobussi 2016 | Intervention did not meet inclusion criteria (Computerised Decision Support Systems) |
| Cardinale 1993 | Not a randomised controlled trial |
| Chen 2014 | Full text in Chinese. The study is excluded based on the abstract. Intervention did not meet inclusion criteria (continuity of care model). |
| Chiu 2018 | Not a randomised controlled trial |
| Ciechanover 1987 | Not a randomised controlled trial |
| Connor 2012 | Outcome did not meet inclusion criteria (no follow‐up data after discharge according to study authors) |
| Crotty 2004 | Intervention did not meet inclusion criteria (medication reconciliation) |
| CTRI/2014/08/004900 2014 | Population did not meet inclusion criteria (included outpatients) |
| Dalton 2018 | Not a randomised controlled trial (publication comparing the interventions of O'Connor 2016 and O'Sulivan 2016. Both trials are excluded in this review because outcome did not meet inclusion criteria (no follow‐up data after discharge according to study authors)). |
| Dalton 2019 | Not a randomised controlled trial |
| Dalton 2019a | Not a randomised controlled trial (publication comparing the interventions of O'Connor 2016 and O'Sulivan 2016. Both trials are excluded in this review because outcome did not meet inclusion criteria (no follow‐up data after discharge according to study authors)). |
| Dauphinot 2017 | Population did not meet inclusion criteria (included outpatients) |
| DRKS00007696 2015 | Not a randomised controlled trial |
| DRKS00013321 2017 | Population did not meet inclusion criteria (patients received medication review in community pharmacies) |
| DRKS00013538 2017 | Not a randomised controlled trial |
| Dudley‐Brown 2016 | Intervention did not meet inclusion criteria (Medication Therapy Management for patients with inflammatory bowel disease) |
| Ee 2019 | Population did not meet inclusion criteria (included outpatients at rehabilitation hospital) |
| Erku 2017 | Population did not meet inclusion criteria (outpatient visits at the diabetes illness follow‐up care clinic) |
| EUCTR‐004236‐38‐GB 2016 | Population did not meet inclusion criteria (intervention done in primary care) |
| Franchi 2016 | Intervention did not meet inclusion criteria (e‐learning educational programme) |
| Frankenthal 2014 | Population did not meet inclusion criteria (chronic geriatric facility) |
| Frankenthal 2017 | Population did not meet inclusion criteria (chronic geriatric facility (long‐term follow‐up of Frankenthal 2014)) |
| Gallagher 2015 | Outcome did not meet inclusion criteria (a cost‐effectiveness analysis) |
| Gallagher 2015a | Outcome did not meet inclusion criteria (a cost‐effectiveness analysis) |
| Gallagher 2016 | Outcome did not meet inclusion criteria (a cost‐effectiveness analysis) |
| Gallagher 2017 | Outcome did not meet inclusion criteria (a cost‐effectiveness analysis) |
| Garcia 2015 | Population did not meet inclusion criteria (received pharmacist follow‐up programme at discharge, 3 months and 12 months after discharge; recommendations were communicated to the patients’ general practitioners) |
| Gattis 1999 | Population did not meet inclusion criteria (included outpatients) |
| Geneletti 2019 | Intervention did not meet inclusion criteria (discharge letter send to the general practitioner) |
| George 2011 | Population did not meet inclusion criteria (included outpatients (patients seen at surgical preadmission clinic)) |
| Gines 2016 | Population did not meet inclusion criteria (medication review in the Emergency Department. Recommendations to modify treatment are send to General Practitioner). |
| Goldberg 2019 | Not a randomised controlled trial |
| Granados 2020 | Outcome did not meet inclusion criteria (medication errors) |
| Hedegaard 2014 | Intervention did not meet inclusion criteria (the medication review focused on thrombo‐preventive agents and potential adherence problems related to these) |
| Hedegaard 2015 | Intervention did not meet inclusion criteria (the medication review focused on thrombo‐preventive agents and potential adherence problems related to these) |
| Hellstrom 2011 | Not a randomised controlled trial |
| Heselmans 2015 | Outcome did not meet inclusion criteria (no follow‐up data after discharge according to study authors) |
| Ho 2013 | Intervention did not meet inclusion criteria (medication reconciliation, patient education and educational and medication refill reminder calls) |
| Hohl 2017 | Not a randomised controlled trial |
| Ilting‐Reuke 2018 | Intervention did not meet inclusion criteria (only focused on treatment of delirium) |
| Iltingreuke 2019 | Intervention did not meet inclusion criteria (telecare service) |
| ISRCTN01624723 2007 | Not a randomised controlled trial |
| ISRCTN11674947 2012 | Population did not meet inclusion criteria (included outpatients) |
| ISRCTN11751440 2017 | Not a randomised controlled trial. The study was a prospective, single‐centre, unblinded, dual arm interventional study. Allocation was through cluster‐randomisation by admission date; allocation to the intervention or control arm was based on which CTU team the patient was admitted under, which alternated between the 2 teams on a daily basis. |
| ISRCTN17219647 2017 | Population did not meet inclusion criteria (patients not admitted to hospital) |
| ISRCTN32281812 2017 | Population did not meet inclusion criteria (patients to be seen at outpatient clinic (Medication Therapy Services clinic)) |
| ISRCTN38449870 2013 | Population did not meet inclusion criteria (intervention done by general practitioners) |
| JPRN‐UMIN000024250 2016 | Population did not meet inclusion criteria (included patients at rehabilitation hospital) |
| JPRN‐UMIN000033814 2018 | Not a randomised controlled trial |
| Kang 2018 | Not a randomised controlled trial |
| Karapinar‐Çarkıt 2017 | Outcome did not meet inclusion criteria (cost‐effectiveness) |
| KCT0005994 | Outcome did not meet inclusion criteria (difference in the number of ADEs and PIMs) |
| Kelly 2011 | Not a randomised controlled trial |
| Kindstedt 2020 | Population did not meet inclusion criteria (included outpatients) |
| Koehler 2009 | Intervention did not meet inclusion criteria (no medication review) |
| Kripalani 2011 | Intervention did not meet inclusion criteria (medication reconciliation, inpatient pharmacist counselling, low‐literacy adherence aids and telephone follow‐up) |
| Lea 2018 | Intervention did not meet inclusion criteria (conference abstract describes the baseline characteristics of multi‐morbid patients included in a randomised controlled trial, but does not describe the intervention) |
| Leguelinel‐Blache 2018 | Not a randomised controlled trial |
| Lind 2016 | Outcome did not meet inclusion criteria (length of stay) |
| Lind 2017 | Outcome did not meet inclusion criteria (the primary outcome measure was a comparison of changes in the Electronic Medication Module (EMM) and changes proposed by CPs) |
| Lipton 1992 | Outcome did not meet inclusion criteria (medication appropriateness) |
| Lonnbro 2017 | Population did not meet inclusion criteria (included outpatients) and not randomised |
| Mao 2015 | Intervention did not meet inclusion criteria (multidisciplinary disease management programme) |
| Marinovic 2021 | Outcome did not meet inclusion criteria (post‐discharge unintentional medication discrepancies) |
| Martinez 2019 | Population did not meet inclusion criteria (included patients in the emergency department) |
| Marusic 2013 | Intervention did not meet inclusion criteria (counselling) |
| Mateti 2018 | Population did not meet inclusion criteria (included outpatients) |
| McCoy 2012 | Intervention did not meet inclusion criteria (pharmacy surveillance and clinical decision support) |
| McDonald 2018 | Outcome did not meet inclusion criteria (medication appropriateness) |
| McMullin 1999 | Not a randomised controlled trial |
| Mendes 2021 | Intervention did not meet inclusion criteria (article discussing the findings of Blum 2021) |
| Michalek 2014 | Outcome did not meet inclusion criteria (no follow‐up data after discharge) |
| Mishra 2017 | Population did not meet inclusion criteria (included outpatients) |
| Nachtigall 2019 | Not a randomised controlled trial (quasi‐randomised) |
| Naughton 1994 | Intervention did not meet inclusion criteria (geriatric team) |
| NCT00205140 2005 | Not a randomised controlled trial |
| NCT00279656 2006 | Intervention did not meet inclusion criteria (pharmaceutical care) |
| NCT00351676 2006 | Population did not meet inclusion criteria (primary care) and not a randomised controlled trial |
| NCT00416026 2006 | Intervention did not meet inclusion criteria (telephone counselling intervention) |
| NCT00541606 | Population did not meet inclusion criteria (ambulatory care setting) |
| NCT00773942 2010 | Population did not meet inclusion criteria (intervention done in community pharmacies and family medicine clinics) |
| NCT00844025 2010 | Outcome did not meet inclusion criteria (number of unsolved drug‐related problems) |
| NCT01034761 2009 | Outcome did not meet inclusion criteria (the percentage of elderly patients who receive a specified high‐risk medication from the Beer's list) |
| NCT01134900 2010 | Intervention did not meet inclusion criteria (pharmacy surveillance and clinical decision support) |
| NCT01164137 2013 | Population did not meet inclusion criteria (medication reconciliation in the patients' home after hospital discharge) |
| NCT01212211 2011 | Population did not meet inclusion criteria (included outpatients) |
| NCT01356563 2011 | Population did not meet inclusion criteria (included outpatients) |
| NCT01467050 2012 | Outcome did not meet inclusion criteria (number of patients with probable and definite adverse drug events in hospital) |
| NCT01467128 2012 | Outcome did not meet inclusion criteria (number of patients with definite and possible adverse drug events during their hospital admission. No follow‐up data after discharge according to study authors.) |
| NCT01503554 2011 | Not a randomised controlled trial |
| NCT01513265 2012 | Not a randomised controlled trial |
| NCT01602744 2012 | Population did not meet inclusion criteria (participants live in the geriatric hospital permanently) |
| NCT01627483 2010 | Intervention did not meet inclusion criteria (medication review was performed once during the hospital stay and twice after discharge from the hospital) |
| NCT01739816 2014 | Population did not meet inclusion criteria (pharmacies) |
| NCT01814280 2013 | Not a randomised controlled trial |
| NCT01906710 2013 | Not a randomised controlled trial |
| NCT01969526 2016 | Population did not meet inclusion criteria (intervention conducted in primary care teams) |
| NCT02047448 2017 | Population did not meet inclusion criteria (intervention is done both in hospital and after discharge) |
| NCT02052505 2015 | Not a randomised controlled trial (controlled before‐and‐after study) |
| NCT02085837 2014 | Population did not meet inclusion criteria (included outpatients) |
| NCT02102503 2018 | Population did not meet inclusion criteria (included outpatients) |
| NCT02122965 2013 | Not a randomised controlled trial |
| NCT02149940 2013 | Not a randomised controlled trial |
| NCT02165618 2014 | Not a randomised controlled trial |
| NCT02202096 2016 | Intervention did not meet inclusion criteria (medication reconciliation, patient education and discharge counselling) |
| NCT02232126 2014 | Population did not meet inclusion criteria (social worker preformed in‐home assessment and telephone follow‐up after discharge from hospital) |
| NCT02275572 2014 | Population did not meet inclusion criteria (primary care) |
| NCT02317666 2015 | Population did not meet inclusion criteria (medication review by the community pharmacist in collaboration with the patient’s general practitioner) |
| NCT02379455 2017 | Population did not meet inclusion criteria (included outpatients) |
| NCT02598115 2016 | Not a randomised controlled trial (a multicentric, stepped wedge, cluster‐randomised trial. This involves sequential roll‐out of an intervention to participants over a number of time periods. By the end of the study, all participants will have received the intervention, although the order in which participants receive the intervention is determined at random.) |
| NCT02664948 2015 | Not a randomised controlled trial (quasi‐randomised) |
| NCT02712268 2013 | Not a randomised controlled trial |
| NCT02740764 2016 | Population did not meet inclusion criteria (included outpatients) |
| NCT02871115 2019 | Population did not meet inclusion criteria (included outpatients) |
| NCT02942927 2019 | Population did not meet inclusion criteria (medication review to be done by community pharmacist and family doctor) |
| NCT03123640 2017 | Duplicate (identical reference to ongoing study NCT03123640) |
| NCT03272607 2019 | Stepped wedge design with only 3 clusters. Not considered to be a randomised trial. |
| NCT03354845 2017 | Outcome did not meet inclusion criteria (cost savings). No relevant outcomes in publication of results: Charissa Ann Jia Ming Ee, Kheng Hock Lee, Hee Lim Tan and Lian Leng Low. Effectiveness and feasibility of de‐prescribing of symptomatic medications in a Singapore rehabilitation hospital. Proceedings of Singapore Healthcare, 2018. |
| NCT03360305 2019 | Population did not meet inclusion criteria (patients seen in the Emergency Department, and discharged from there) |
| NCT03369652 2017 | Not a randomised controlled trial |
| NCT03369652 2018 | Not a randomised controlled trial. Author sent protocol. |
| NCT03445767 2018 | Population did not meet inclusion criteria (included outpatients) |
| NCT03557944 2019 | Population did not meet inclusion criteria (included outpatients) |
| NCT03671629 2021 | Population did not meet inclusion criteria (included outpatients) |
| NCT03695081 2019 | Not a randomised controlled trial |
| NCT03713112 2019 | Population did not meet inclusion criteria (included outpatients) |
| NCT03722017 2021 | Population did not meet inclusion criteria (included outpatients) |
| NCT03750500 2018 | Population did not meet inclusion criteria (included outpatients ‐ home‐based falls prevention programme) |
| NCT03824106 2023 | Population did not meet inclusion criteria (included outpatients) |
| NCT03902028 2021 | Intervention did not meet inclusion criteria (reinforced multidisciplinary follow‐up) |
| NCT03909035 2021 | Population did not meet inclusion criteria (included outpatients) |
| NCT03922529 2019 | Intervention did not meet inclusion criteria (to be implemented after discharge from hospital) |
| NCT04077281 2019 | Intervention did not meet inclusion criteria (telemedicine) |
| NCT04082871 2019 | Population did not meet inclusion criteria (included outpatients) |
| NCT04087109 2020 | Population did not meet inclusion criteria (residents of long‐term care facilities) |
| NCT04151797 2019 | Not a randomised controlled trial |
| NCT04188470 2019 | Population did not meet inclusion criteria (primary care) |
| NCT04228900 2020 | Population did not meet inclusion criteria (patients not admitted to hospital) and not a randomised controlled trial |
| NCT04251520 2020 | Population did not meet inclusion criteria (included outpatients) |
| NCT04278794 2020 | Population did not meet inclusion criteria (included outpatients) |
| NCT04360746 2020 | Population did not meet inclusion criteria (geriatric rehabilitation department) and not a randomised controlled trial |
| NCT04391218 2020 | Population did not meet inclusion criteria (patients hospitalised at home) |
| NCT04417400 2020 | Population did not meet inclusion criteria (included patients in pharmacy practice) |
| NCT04553107 2020 | Population did not meet inclusion criteria (primary care) |
| NCT04556786 2020 | Population did not meet inclusion criteria (included outpatients) |
| NCT04722588 2021 | Intervention did not meet inclusion criteria (introducing clinical pharmacists to the interdisciplinary emergency department team) and not a randomised controlled trial |
| NCT04796701 2021 | Population did not meet inclusion criteria (included outpatients) and not a randomised controlled trial |
| Nymoen 2019 | Not a randomised controlled trial |
| O'Brien 2018 | Outcome did not meet inclusion criteria (cost‑effectiveness analysis) |
| O'Connor 2016 | Outcome did not meet inclusion criteria (no follow‐up data after discharge according to study authors) |
| O'Sullivan 2016 | Outcome did not meet inclusion criteria (no follow‐up data after discharge according to study authors) |
| Okere 2015 | Not a randomised controlled trial |
| Pazan 2017 | Outcome did not meet inclusion criteria (conference abstract for Pazan 2018 (excluded ‐ no follow‐up data after discharge) |
| Pazan 2018 | Outcome did not meet inclusion criteria (secondary analysis of Wehling 2016 (excluded reference) |
| Pazan 2019 | Outcome did not meet inclusion criteria (secondary analysis of Wehling 2016 (excluded reference) |
| Pfister 2017 | Not a randomised controlled trial |
| Phatak 2016 | Intervention did not meet inclusion criteria (medication reconciliation, discharge counselling and post‐discharge phone calls) |
| Pope 2011 | Intervention did not meet inclusion criteria (medication review implemented after discharge) |
| Rainville 1999 | Intervention did not meet inclusion criteria (only heart failure medication reviewed) |
| Sakthong 2018 | Population did not meet inclusion criteria (included outpatients) |
| Saltvedt 2005 | Outcome did not meet inclusion criteria (medication use) |
| Santolaya‐Perrin 2016 | Population did not meet inclusion criteria (medication review conducted in the Emergency Department. Recommendation to modify the treatment is sent to the Primary Care Physician) |
| Santolaya‐Perrin 2019 | Population did not meet inclusion criteria (medication review conducted in the Emergency Department. Recommendation to modify the treatment is sent to the Primary Care Physician) |
| Schmader 1997 | Not a randomised controlled trial |
| Schmader 2004 | Intervention did not meet inclusion criteria (geriatric team) |
| Sjoberg 2013 | Intervention did not meet inclusion criteria (medication review restricted to fall risk‐increasing drugs) |
| SLCTR//011 2018 | Population did not meet inclusion criteria (included outpatients) |
| SLCTR//029 2013 | Not a randomised controlled trial |
| Smith 1996 | Not a randomised controlled trial |
| Spinewine 2007 | Not a randomised controlled trial (quasi‐randomised trial, used alternate randomisation) |
| Stowasser 2002 | Intervention did not meet inclusion criteria (medication reconciliation) |
| Tallon 2016 | Not a randomised controlled trial |
| Tan 2018 | Population did not meet inclusion criteria (included outpatients) |
| TCTR20181104004 2018 | Population did not meet inclusion criteria (included outpatients) |
| Van Der Linden 2013 | Not a randomised controlled trial (planned RCT in clinical trial registration, but not RCT in final publication from 2017) |
| Van Der Linden 2014 | Not a randomised controlled trial (planned RCT in clinical trial registration, but not RCT in final publication from 2017) |
| Van der Linden 2017 | Not a randomised controlled trial (planned RCT in clinical trial registration, but not RCT in final publication from 2017) |
| Verbeek 2016 | Population did not meet inclusion criteria (included outpatients) |
| Walker 2009 | Not a randomised controlled trial |
| Wehling 2015 | Outcome did not meet inclusion criteria (no follow‐up data after discharge, the primary endpoint was the FORTA score) |
| Wehling 2016 | Outcome did not meet inclusion criteria (no follow‐up data after discharge, the primary endpoint was the FORTA score) |
| Williams 2012 | Intervention did not meet inclusion criteria (medication reconciliation and discharge counselling) |
| Zhao 2015 | Intervention did not meet inclusion criteria (only cardiovascular drugs) |
| Zhao 2015a | Intervention did not meet inclusion criteria (only cardiovascular drugs) |
ADR: adverse drug reaction CTU: clinical teaching unit CP: clinical pharmacist DRP: drug‐related problem FORTA: Fit fOR The Aged PIM: potentially inappropriate medication RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12618000979257 2018.
| Study name | The Australian Team Approach to Polypharmacy Evaluation and Reduction (AusTAPER) study for older hospital inpatients |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention group: The AusTAPER is a web‐based software application that can be used as a generic tool for a collaborative medication review between the participant, hospital team, study pharmacist and GP. At an initial consultation with the pharmacist, data will be entered on the participant’s medications, dosages and indications; any reported side effects; the patient’s priorities and preferences for treatment; and medication‐related data such as blood pressure and creatinine (if known). The TAPER App tool performs a ‘machine screen’ comprising (1) interaction checker; and (2) listing of potentially inappropriate medicines (including the Screening Tool of Older Person's potentially inappropriate prescriptions, the Beers List, anticholinergic and serotonergic burden, and QT‐prolonging drugs). This screen is also supported by existing evidence‐based resources providing numbers needed to treat/harm, and decision aids for de‐prescribing and tapering guidelines were available. The research staff and study pharmacist record notes in the web‐based AusTAPER App. "We will liaise with GPs for follow‐up post‐discharge, and the GP will also record notes in the AusTAPER App, which is intuitive to use for GPs familiar with practice software." The key steps for TAPER are: (1) Study pharmacist consultation (approximately 30 mins): the medication data and this information will be entered into the TAPER App. Through the application of automated filters within the TAPER App, potentially inappropriate medications, medication interactions and warnings will be identified and flag medications that are candidates for discontinuation or dose reduction. Based on these data, the study pharmacist will generate preliminary recommendations for medicines optimisation. (2) Hospital and usual doctor consultation (same day when feasible): the study pharmacist will liaise with both the hospital multi‐disciplinary team and the participant’s usual GP. A prioritised plan for appropriate discontinuations and a template for monitoring frequency, duration and criteria for medicine recommencement will then be confirmed with the agreement of the hospital multi‐disciplinary team and GP (if possible). The study pharmacist will then carry out a comprehensive medication review focused on medications suitable for discontinuation or dose reduction informed by this list, report medication‐related adverse effects from the patient, and review the patient’s goals for treatment. The pharmacist will make recommendations based on this review and add these to the TAPER clinical pathway. (3) Review and then commencement of pause‐and‐monitor discontinuation. This is an opportunity for the hospital doctor and participant to agree and refine the AusTAPER plan, including monitoring. The pause‐and‐monitor approach addresses key barriers to de‐prescribing, which patients report by making clear a shared understanding of the withdrawal plan that includes monitoring and agreed criteria for restarting medicines if necessary. Monitoring during the implementation of the intervention will be individualised as needed/agreed upon. At each monitoring visit, patients will have a brief consultation (with the study pharmacist pre‐discharge, and with the GP post‐discharge) to review progress with the AusTAPER plan and address any concerns (such as perceived adverse drug withdrawal events). Control group: those in the control group will receive standard hospital care |
| Outcomes |
Primary outcome: total number of current regular medicines including prescribed medicines, over‐the‐counter and complementary and alternative medicines, and herbal and mineral supplements Secondary outcomes:
|
| Starting date | July 2018 |
| Contact information | Principal investigator: Prof Christopher Etherton‐Beer, WA Centre for Health & Ageing (WACHA), WA Institute for Medical Research, Royal Perth Hospital, Australia, Phone: +61 8 9224 2746. Email: christopher.etherton‐beer@uwa.edu.au |
| Notes | www.anzctr.org.au/ (accessed February 2020); trial ID: ACTRN12618000979257 |
Andersen 2021.
| Study name | Optimization of Nutrition And Medication (OptiNAM) for acutely admitted older patients: protocol for a randomized single blinded controlled trial |
| Methods | Randomised controlled trial |
| Participants | Setting: Hvidovre Hospital, Denmark Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention group: optimisation of nutrition and medication (1) Inter‐professional optimisation of medication prescribing: Study participants in the intervention group receive optimisation of medication prescribing at admission day (baseline) regardless of nutritional state. The intervention is performed in co‐operation between a clinical pharmacist and a medical physician. (2) Nutritional intervention: If positive screening for malnutrition or risk of malnutrition a dietetic intervention is initiated and if positive screening below interventions is initiated: dysphagia: occupational therapy intervention; oral cavity problems: odontological intervention; depression: geriatric intervention; low ADL: occupational therapy intervention; if positive screening for poor muscle strength: physiotherapeutic intervention Control group: the control group receives standard care |
| Outcomes |
Primary outcomes:
Secondary outcomes: (All secondary outcomes are assessed at baseline (admission day), week 8 and week 16):
Other outcome measures:
|
| Starting date | 15 October 2018 |
| Contact information | Ove Andersen, Department of Clinical Research, Copenhagen University Hospital Amager and Hvidovre, Denmark. Email: Ove.Andersen@regionh.dk |
| Notes |
Funding: The Capital Region’s strategic funds (2,000,000 DKK); Capital Region’s fund for transitional research (2,011,807 DKK); Danish Regions (1,000,000 DKK); The Danish Research Unit for Hospital Pharmacy, Amgros I/S, Copenhagen (1,000,000 DKK)), Denmark; and the Læge Sofus Carl Emil Friis og Hustru Olga Doris Friis' Legat (190,000 DKK) Trial ID: ClinicalTrials.gov NTC03741283 |
ChiCTR1800017706 2018.
| Study name | Elderly patients multi‐drug management: a multicenter randomized controlled trial |
| Methods | Cluster‐randomisation; in this study, a third‐party randomisation method was used to allocate 5 centres: 2 centres as control group and 3 centres as intervention group |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | Pharmacists intervention with aid of intelligentialised medication review system |
| Outcomes |
|
| Starting date | January 2019 |
| Contact information | Study leader: Yan Suying; email: yansuying10@xwhosp.org; phone: +86 13708905057 |
| Notes | Chinese Clinical Trial Registry (accessed February 2020) Trial ID: ChiCTR1800017706 |
Diplock 2017.
| Study name | The Alice Springs Hospital Readmission Prevention Project (ASHRAPP): a randomised control trial |
| Methods | Randomised control trial |
| Participants | Setting: Alice Springs Hospital, the regional referral centre for remote Central Australia Inclusion criteria:
Exclusion criteria:
|
| Interventions | Participants allocated to the intervention group will be provided with a multi‐dimensional and case based transitional care package led by a team consisting of a medical officer, nurse, Aboriginal Health Practitioner and pharmacist. At each admission, the participants will have the following provided during their inpatient stay.
The intervention team will facilitate the following activities following discharge through liaison with the participant, family and/or primary health care provider:
Participants allocated to the control group will receive usual care |
| Outcomes | All outcomes will be assessed at 12 months Primary outcome: number of all‐cause hospital admissions Secondary outcomes:
|
| Starting date | Anticipated date of first participant enrolment 29 June 2015 |
| Contact information | Prof Graeme Maguire, Monash University and Baker IDI Heart & Diabetes Institute, Melbourne, Australia. Email: gabrielle.diplock@bakeridi.edu.au |
| Notes | The trial was stopped early after inclusion of 113 participants (210 were planned to be included), due to lack of funding/staff/facilities and participant recruitment difficulties. Data collected is being analysed. |
Grischott 2018.
| Study name | Improving inappropriate medication and information transfer at hospital discharge: a cluster‐RCT |
| Methods | Double‐centre, double‐blind, cluster‐randomised, parallel‐controlled clinical trial |
| Participants |
Setting: Institute of Primary Care of the University of Zurich, Switzerland Inclusion criteria:
Exclusion criteria:
|
| Interventions | In the intervention group, the senior hospital physicians take part in a 2‐hour teaching session on how to integrate a structured medication review and specific elements of communication into the daily discharge routine. The senior physicians are responsible for instructing their assistant physicians in patient recruitment and carrying out the correct discharge procedure. The assistant physicians critically review their patients’ medication lists, discuss the results of these reviews and their suggestions with the patients and compile revised medication lists which they then communicate to the patients’ general practitioners with an invitation for discussion. The senior hospital physicians in the control group undergo a 2‐hour instruction addressing multi‐morbidity, patient in‐ and exclusion, and the handling of the different data collection forms. Their assistant physicians will follow the 'usual' discharge routine of their clinics. |
| Outcomes |
Primary outcome: time (in days) without readmission to hospital within 6 months after discharge Secondary outcomes:
|
| Starting date | January 2017 |
| Contact information | Dr. med. Stefan Neuner‐Jehle MPH, University Hospital Zurich, Switzerland. Phone: +41 44 255 98 55. Email: stefan.neuner‐jehle@usz.ch |
| Notes | Trial ID: ISRCTN18427377 |
Ie 2020.
| Study name | Protocol of a randomised controlled trial on the efficacy of medication optimisation in elderly inpatients: medication optimisation protocol efficacy for geriatric inpatients (MPEG) trial |
| Methods | Randomised controlled trial |
| Participants |
Setting: the medical wards of a university‐affiliated community hospital in Japan Inclusion criteria:
Exclusion criteria:
|
| Interventions | All participants will be subjected to medication reconciliation by ward‐based pharmacists. For those assigned to the intervention group, the multidisciplinary de‐prescribing team will conduct the medication optimisation intervention within 48 hours of allocation. Overall, the intervention will consist of a medication review, followed by the development of a medication optimisation proposal based on the STOPP/START criteria and a medication optimisation protocol. |
| Outcomes |
Primary outcome: a composite of all‐cause death, unscheduled hospital visits and rehospitalisation until 48 weeks after randomisation Secondary outcomes (assessed at 24 weeks and 48 weeks post‐randomisation):
In addition to the above‐listed outcomes, the ones listed below, occurring within 48 weeks after randomisation, will be assessed including the event dates:
|
| Starting date | Date of first enrolment: 1 March 2019 |
| Contact information | Dr Kenya Ie. Email: kenya.ie@marianna‐u.ac.jp |
| Notes |
Funding: The Ministry of Education, Science, Sports and Culture, Grant‐in‐Aid for Young Scientists, 2018–2021 (grant number 18K15434, Kenya Ie) Trial ID: UMIN000035265 |
Johansen 2018.
| Study name | Interdisciplinary collaboration across secondary and primary care to improve medication safety in the elderly (IMMENSE study): study protocol for a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants |
Setting: the study is carried out at 2 acute internal medicine wards at the University Hospital of North Norway (UNN); a geriatric internal medicine ward at UNN Tromsø and a general acute internal medicine ward at UNN Harstad Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention group: patients randomised to the intervention group receive the IMM‐based intervention including: (1) MedRec at admission, (2) medication review and monitoring during the hospital stay, (3) patient counselling designed to meet the needs of each individual patient, (4) MedRec at discharge together with an updated and structured medication list given to patients and submitted to primary care at discharge and (5) a follow‐up phone call to the patient’s GP and nurses in home care service/nursing home to inform about and discuss current medication therapy and recommendations. The study pharmacist is performing all steps in close collaboration with the hospital physician who has the medical responsibility for the patients. Control group: the control group receives standard care. Patients assigned to standard care receive treatment from a team consisting of physicians, nurses, nurse assistants. Standard care may include elements as MedRec, medication review and patient counselling performed by physicians or nurses during the hospital stay. However, it is not standardised, structured or involving pharmacists. |
| Outcomes |
Primary outcome: the rate of "acute readmissions and ED visits" 12 months after discharge from the index hospital stay in the intervention group compared with the control group Secondary outcomes:
|
| Starting date | 21 September 2016. Estimated study completion date: September 2021 |
| Contact information | Jeanette Schultz Johansen; email: jeajoh@uit.no |
| Notes | Trial ID: NCT02816086 |
JPRN‐UMIN000035265 2018.
| Study name | Efficacy of medication optimization protocol for older inpatients: a randomized controlled trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria: Patients aged 65 years and older Exclusion criteria: Patients whose attending physicians do not agree to participate in the study |
| Interventions |
Intervention group: a multidisciplinary team‐based medication review and de‐prescribing proposal based on STOPP/START criteria and a medication optimisation protocol Control group: usual care |
| Outcomes | Primary outcomes: composite of death, unscheduled visits, and re‐hospitalisation until 12 months after randomisation |
| Starting date | March 2019 |
| Contact information | Scientific contact: Ie Kenya, Kawasaki city, Kanagawa, Japan. Email: iekenya0321@gmail.com |
| Notes | Trial ID: UMIN000035265 |
Kogamine 2018.
| Study name | Study protocol for a single‐centre, prospective, non‐blinded, randomised, 12‐month, parallel‐group superiority study to compare the efficacy of pharmacist intervention versus usual care for elderly patients hospitalised in orthopaedic wards |
| Methods | A single‐centre, prospective, non‐blinded, randomised, 12‐month, parallel‐group, superiority study |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention group: pharmacist interventions include medication reconciliation, advice to patients' physicians in stopping unnecessary or inappropriate medications and starting necessary medications, patient education, monitoring and providing written summary information about discharge medications to patients, primary care physicians and community pharmacists from admission to discharge Control group: usual care includes medication reconciliation, patient education, monitoring and providing information about discharge medications |
| Outcomes |
Primary outcome: the readmission rate within 1 year after randomisation Secondary outcomes: the proportion of patients who undergo Emergency Department (ED) visits, all‐cause death, a new fracture, acute myocardial infarction and ischaemic stroke Other outcomes include the number of medications, potentially inappropriate medications and potential prescribing omissions |
| Starting date | November 2017 |
| Contact information | Kenichi Sugawara, National Hospital Organization Tochigi Medical Center, Department of Pharmacy, Nakatomatsuri, Utsunomiya, Tochigi, Japan. Email: ksuga1@tochigi‐mc.jp |
| Notes | Trial ID: UMIN000029404 |
Loffler 2014.
| Study name | Optimizing polypharmacy among elderly hospital patients with chronic diseases ‐ study protocol of the cluster randomized controlled POLITE‐RCT trial |
| Methods | Cluster‐randomised controlled trial |
| Participants |
Clusters:
Inclusion criteria:
Exclusion criteria:
Participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | During inpatient treatment of participants affected by polypharmacy, a pharmacist specially trained in communication skills performs a narrative‐based medication review. Thus, 2 approaches are combined here: the face‐to‐face clinical “brown bag” medication review, and the patient‐centred approach of narrative medicine. Apart from detecting potentially inadequate medication, a major aim is to identify patient preferences and to include them ‐ when possible ‐ into a hierarchically structured list of evidence‐based medication recommendations. Thus, priorities for medication modification can be based on both 'objective' pharmaceutical considerations and 'subjective' participant preferences. |
| Outcomes |
Primary outcomes: (1) health‐related quality of life (EQ‐5D), and (2) the difference in the number of prescribed long‐term pharmaceutical agents between intervention and control groups at T3 Secondary outcomes: the appropriateness of prescribed medication (PRISCUS list, Beers criteria, MAI), patient satisfaction (TSQM), patient empowerment (PEF‐FB‐9), patient autonomy (IADL), falls (frequency and severity), rehospitalisation and death. For all participants ensured with the largest public German health insurance provider (AOK), cost‐effectiveness will be analysed by the Scientific Institute of the AOK (WIdO). |
| Starting date | November 2013 |
| Contact information | Principal Investigator: Christin Löffler, Institute of General Practice, Rostock University Medical Center, Rostock, Germany |
| Notes | www.controlled‐trials.com (accessed May 2015) Trial ID: ISRCTN42003273 |
Logan 2020.
| Study name | Comprehensive geriatric assessment for frail older people with chronic kidney disease to increase attainment of patient‐identified goals ‐ a cluster randomised controlled trial ‐ The GOAL Trial |
| Methods | Cluster‐randomised trial |
| Participants |
Setting: Australia Inclusion criteria:
Exclusion criteria:
|
| Interventions | Sites will be randomly allocated to either provide a Comprehensive Geriatric Assessment to participants or usual care. A skilled multi‐disciplinary team will provide specialist co‐ordinated care (known as comprehensive geriatric assessment) and will address medical, social, mental health and physical needs. The control sites will receive usual care. |
| Outcomes |
Primary outcome: Goal Attainment Scaling at 3 months
Secondary outcomes:
Other outcome measures: Process evaluation (time frame: 12 months) Qualitative analysis of structured interviews |
| Starting date | 15 March 2021 |
| Contact information | Laura Robison +61427911414. Email: goal@uq.edu.au |
| Notes | Trial ID: NCT04538157 |
NCT03123640 2018.
| Study name | Improving drug safety in emergency patients ‐ a randomized controlled trial (EPIMERR) |
| Methods | Randomised controlled trial |
| Participants |
Setting: emergency department, Diakonhjemmet Hospital Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention group: the pharmacist conducts medication reconciliation and medication review while the patient is admitted to the emergency department. The pharmacist presents results from medication reconciliation to physicians at the emergency department before the medical history is obtained. Further, the pharmacist will discuss drug‐related problems obtained during the medication review with the physicians to customise and optimise the medication treatment for each patient. Control group: standard treatment without pharmacist intervention in the emergency department |
| Outcomes |
Primary outcome: proportion of patients readmitted (time frame: 12 months from inclusion) Secondary outcomes:
|
| Starting date | 24 April 2017 Actual primary completion date: 16 May 2018 Estimated study completion date: December 2021 |
| Contact information | Lisbeth Damlien Nymoen, Ph.D. candidate and project pharmacist, Diakonhjemmet Hospital |
| Notes | www.clinicaltrials.gov (accessed April 2020) Trial ID: NCT03123640 |
NCT03156348 2017.
| Study name | Impact of clinical pharmacist on adverse drug events in older adults |
| Methods | Randomised controlled trial |
| Participants |
Setting: The Clinical Hospital of the University of Chile Inclusion criteria:
Exclusion criteria:
|
| Interventions | The intervention group, in addition to the usual care, will receive the Clinical Pharmacist Care during hospitalisation, discharge and during 2 months post‐discharge, through a home visit at 30 ± 5 days post‐discharge and a telephone call at 60 ± 5 days. During hospitalisation and at discharge a clinical pharmacist (CP) will monitor daily pharmacological safety and efficacy of the medication to asses and make appropriate recommendations. CP will explain the use reasons of each of the drugs. At 30 days post‐discharge, the CP will review the updated clinical record of patient and conduct a home visit to enhance and ask about adherence, self‐medication, medication use at that time and possible results of laboratory tests performed and clarify doubts regarding the use of current medications. The same activities will be made at 60 days by telephone, to reinforce the recommendations. |
| Outcomes |
Primary outcome: incidence of adverse drug events at 90 days post‐discharge (time frame: 90 days post‐discharge) Secondary outcomes:
|
| Starting date | May 2015 |
| Contact information | Principal Investigator: Marcela Jirón, PhD University of Chile |
| Notes | Trial ID: NCT03156348 |
NCT03393299 2018.
| Study name | Impact of the systematic use of the criteria STOPP/START in short stay geriatric (REVOR) |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: use of STOPP/START criteria during medication reconciliation Control: medication reconciliation done as usual, without the consideration of the STOPP/START criteria |
| Outcomes |
Primary outcome: SF‐12 quality of life scale at inclusion and at 2 months Secondary outcomes:
|
| Starting date | January 2018 |
| Contact information | Anne Frey Geoffret. Email: ageoffret@hospitalporteverte.com |
| Notes | www.clinicaltrials.gov (accessed February 2020) Trial ID: NCT03393299 |
NCT03666793 2018.
| Study name | Comprehensive management of drug prescriptions throughout the elderly person's hospital care (OPTISORT) |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria: patients hospitalised in the department of short geriatric stay Exclusion criteria: patients already included in the study and readmitted in the same service |
| Interventions |
Intervention group: received a medication reconciliation at hospital admission and discharge, a medication review during hospitalisation, and transmission of therapeutic modifications to the general practitioner and community pharmacist at discharge Control group: received the geriatric unit’s usual management |
| Outcomes |
Primary outcome: Rate of readmission at 30 days (only direct re‐admissions to emergency and geriatric short‐stay services in participating centres will be counted) Secondary outcomes:
|
| Starting date | September 2018 |
| Contact information | Principal Investigator: Fabien Visade, MD |
| Notes | Trial ID: NCT03666793 |
NCT03827031.
| Study name | Impact of multidisciplinary medication assessment review in surgery departments (CHIROPMEV) |
| Methods | Randomised controlled trial |
| Participants |
Setting: surgery departments Inclusion criteria:
Exclusion criteria:
|
| Interventions | There are 3 study arms: intervention 1 (multidisciplinary medication review), intervention 2 (multidisciplinary medication review with community pharmacist follow‐up) and control The multidisciplinary medication review entails medication reconciliation and pharmaceutical analysis by a clinical pharmacist, and a physician performs a clinical examination and analysis of the medical record. Both participate in a collaborative interview. The hospital physician calls the community pharmacist to discuss proposed changes on the order and to establish a new prescription. At the end of the stay, the clinical pharmacist will conduct an exit interview with the patient. For participants in intervention group 2, a summary of the follow‐up report stating the therapeutic modifications will be sent to the community pharmacist and physician. |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | Estimated study start date: June 2022 |
| Contact information | Jean‐Marie Kinowski. Email: jean.marie.kinowski@chu-nimes.fr |
| Notes | Trial ID: NCT03827031 |
NCT04028583 2019.
| Study name | Tool for Inappropriate Prescription Evaluation: The TaIPE Study (TaIPE) |
| Methods | Randomised controlled trial |
| Participants |
Setting: Centre Hospitalier Universitaire Vaudois Inclusion criteria: all patients meeting the admission criteria of the acute care for elders (ACE) unit will be eligible Exclusion criteria: none |
| Interventions |
Intervention group: PIM‐Check In the PIM‐Check group, a medication review will be conducted using PIM‐Check within 72 hours of the patient's admittance to the unit. The physician will decide whether to accept these recommendations or not and implement prescribing changes if agreed. Active comparator group: STOPP/START group In the STOPP/START group, medication lists will be analysed within 72 hours of patient's admittance and optimised according to STOPP/START criteria. The second physician will decide whether to accept these recommendations or not and implement prescribing changes if agreed. |
| Outcomes |
Primary outcome: Rate of potentially inappropriate prescriptions (PIPs) reduction in the PIM‐Check group compared to STOPP/START (time frame: 18 months) Secondary outcomes:
|
| Starting date | February 2018 |
| Contact information | Akram Farhat, PharmD, MPH, PhD, akram.farhat@hotmail.com Chantal Csajka, PharmD, PhD +41 21 314 42 63 chantal.csajka@chuv.ch |
| Notes | Trial ID: NCT04028583 |
NCT04617340.
| Study name | Effect of a trAnSitional Pharmacist Intervention in geRiatric Inpatients on Hospitals Visits After dischargE (ASPIRE) |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | The interventional group receives a multifaceted clinical pharmacy intervention: (1) assessing patient and caregiver preferences, (2) medication reconciliation on admission, (3) comprehensive medication review before discharge, (4a) compiling a patient friendly medication list, (4b) optimising communication with healthcare providers in primary care, (4c) providing a copy of the medication list for the community pharmacist, (4d) contacting the general practitioner by phone, (4e) contacting, if applicable the home care nurse or the nurse from the nursing home by phone, (5) motivation interview before discharge with patients and caregivers, (6) post‐discharge follow‐up |
| Outcomes |
Primary outcome: time to all‐cause unplanned hospital visit after discharge (time frame: up to 6 months after discharge)
Secondary outcomes:
|
| Starting date | February 2021 |
| Contact information | Julie Hias +3216343080. Email: julie.1.hias@uzleuven.be |
| Notes | Trial ID: NCT04617340 |
Pevnick 2021.
| Study name | The Pharmacist Discharge Care (PHARM‐DC) study: a multicentre RCT of pharmacist‐directed transitional care to reduce post‐hospitalisation utilisation |
| Methods | Randomised controlled trial |
| Participants |
Setting: an academic medical centre (Brigham and Women’s Hospital in Boston, Massachusetts) and a university‐affiliated hospital (Cedars‐Sinai Medical Center in Los Angeles, California) Inclusion criteria:
Exclusion criteria:
|
| Interventions | The PHARMacist Discharge Care intervention includes medication reconciliation at admission and discharge, medication review, increased communication with caregivers, providers and retail pharmacies, and patient education and counselling during and after discharge Control patients will receive usual care, which may at times include pharmacist consultation and/or post‐discharge phone call(s) if deemed clinically necessary and requested or performed by physicians, nurses or pharmacists in the course of usual clinical care |
| Outcomes |
Primary outcome: 30‐day post‐discharge utilisation (readmissions, observation stays or emergency department visits) Secondary outcomes: the rates of 30‐day post‐discharge utilisation stratified by: (1) receipt of different intervention components, (2) diagnosis of congestive heart failure at admission, (3) having 3 or more high‐risk medications prior to admission, (4) having 10 or more medications prior to admission, (5) study site, (6) patient medication adherence and literacy, (7) quintiles of patient socioeconomic status, (8) discharge on weekends (compared to weekdays) |
| Starting date | December 2019 |
| Contact information | Joshua M Pevnick, Division of General Internal Medicine, Department of Medicine, Cedars‐Sinai Medical Center, Los Angeles, CA, United States of America. Email: joshua.pevnick@cshs.org. |
| Notes |
Funding: The National Institute on Aging of the National Institutes of Health, United States under award R01AG058911 and NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881 Trial ID: NCT04071951 |
Ranaudin 2017.
| Study name | Impact of a pharmacist‐led medication review on hospital readmission in a pediatric and elderly population: study protocol for a randomized open‐label controlled trial |
| Methods | Randomised controlled trial |
| Participants |
Setting: recruitment will be performed in a paediatric department and a geriatric and internal medicine department Inclusion criteria:
Exclusion criteria:
|
| Interventions | A total of 1400 hospitalised patients will be randomised into 2 groups: (1) the experimental group (group receiving a pharmacist‐led medication review) and (2) the control group (group receiving usual care). Participants in the intervention group receive a pharmacist‐led medication review including the following: (1) a medical and pharmaceutical admission medication reconciliation and treatment review, (2) a medical and pharmaceutical medication reconciliation at discharge and treatment review and (3) medication liaison service. |
| Outcomes |
Primary outcome: The rate of all‐cause hospital readmission and/or all‐cause death and/or emergency department visits occurring within 30 days after the patient discharge from initial hospitalisation Secondary outcomes:
|
| Starting date | May 2016 |
| Contact information | Correspondence: renaudin.pierre@ap‐hm.fr |
| Notes | Trial ID: NCT02734017 |
RBR‐42nd7q.
| Study name | Medical record model oriented to problems with the use of medications in patients with heart failure admitted to the intensive care unit |
| Methods | Randomised controlled trial |
| Participants |
Setting: Brazil Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention group: the Drug Therapy Intervention Group will receive care in which the problems related to medications will be diagnosed through the application of the diagnostics chart: a) adverse clinical findings and medications (DAM), followed by the establishment of conducts to solve drug‐related problems; (b) adjustment of drug treatment according to the previous step, which will be carried out by prescribers and nurses; (c) monitoring drug treatment on the evolution of drug‐related problems, achieving therapeutic goals and evaluating new drug‐related problems. A pharmaceutical conduct and therapeutic goals (care plan) will be recorded in the patient's medical record according to Classification for Drug related problems PCNE, V.8.01. Control group: the control group will receive the identification of drug‐related problems, however, instead of the subsequent intervention, the patients in this group will be subjected to the usual health care at the study sites. Care consists of evaluation of prescriptions by pharmacists, bedside visits and medication reconciliation. |
| Outcomes |
Primary outcome: length of stay in the ICU (in days) Secondary outcomes: the Sequential Organ Failure Assessment (SOFA) and death scores |
| Starting date | Date first enrollment: 16 March 2018 |
| Contact information | Tâmara Natasha Gonzaga de Andrade Santos Andrade Santos Phone: + 55‐79 99905‐1756, email: tamara_farmacia@hotmail.com |
| Notes | Trial ID: RBR‐42nd7q |
SLCTR/2019/039.
| Study name | A randomised control trial to evaluate the impact of a clinical pharmacist on optimizing the quality use of medicines according to the Acute Coronary Syndrome (ACS) secondary prevention guidelines and medication adherence following discharge in patients with ACS |
| Methods | Randomised controlled trial |
| Participants |
Setting: the medical wards of a teaching hospital, Peradeniya Inclusion criteria: male and female patients above 18 years of age who are diagnosed and managed as ACS by the medical team Exclusion criteria:
|
| Interventions | The intervention will be delivered by the principal investigator, and a qualified pharmacist will assist the process. Interventions planned: medication history will be recorded by the clinical pharmacist at the time of recruitment and it will be reconciled against the inward medication plan; patient's medication adherence will be assessed at the time of recruitment by using a validated adherence assessment tool (Brief Medication Questionnaire); continuous medication review by the clinical pharmacist daily during the patient's stay at the ward and discharge medication review at the time of discharge(BNF, AMH and ACS therapeutic guidelines will be used for the review); identification and resolution of medication‐related problems (wrong drug choice, wrong dose, drug interactions etc.); discharge medication counselling with written information and labelled medicine by the clinical pharmacist; follow‐up after discharge at 1‐month, 3‐month and 6‐month intervals by the clinical pharmacist at the medical clinic; hospital readmissions, changes to medications, adverse drug reactions will be checked during the respective period at each follow‐up and medication‐related issues will be resolved at the follow‐ups; at the end of the study period (6 months after follow‐up) patient's medication adherence will be reassessed using the same tool to check the improvement |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | Date of first enrollment 15 November 2019 |
| Contact information | Dr ACM Fahim, Department of Pharmacy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya.Email: fahim.cader@gmail.com |
| Notes | Trial ID: SLCTR/2019/039 |
Vasilevskis 2019.
| Study name | A patient‐centered deprescribing intervention for hospitalized older patients with polypharmacy: rationale and design of the Shed‐MEDS randomized controlled trial |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention group: Shed‐Meds: a patient‐centred de‐prescribing intervention. Participants assigned to the intervention group will receive a clinical review of their prescribed medications by a research clinician (pharmacist, physician and/or nurse practitioner) followed by a patient interview to assess their willingness to discontinue or reduce some of their medicines based on the clinical recommendations of the team. Hospital and outpatient providers also will be part of the de‐prescribing decision process. De‐prescribing actions will be initiated in the hospital prior to discharge and continue through the skilled nursing facility stay. Control group: participants assigned to the control group will receive usual care as it is normally provided by the hospital and skilled nursing facility treatment teams. Research staff will monitor their prescribed medications in both care settings but not make any recommendations or changes unless a safety issue is identified. |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | Patient enrollment for the Shed‐MEDS trial began March 2017 and is scheduled to end October 2020 |
| Contact information | Principal Investigator: Sandra F Simmons, PhD Vanderbilt University Medical Center |
| Notes | — |
Abbreviations:
ACS: acute coronary syndrome ADL: activities of daily living ALT: alanine transaminase CRP: C‐reactive protein ED: emergency department EQ‐5D: standardised instrument of the EuroQol Group used to measure health outcomes GFR/eGFR: glomerular filtration rate/estimated glomerular filtration rate GP: general practitioner HDU: high‐dependency unit HRQoL: health‐related quality of life IADL: instrumental activities of daily living ICU: intensive care unit IMM: integrated medicine management INR: international normalised ratio MAI: Medication Appropriateness Index MCH: mean corpuscular haemoglobin MCV: mean corpuscular volume NGAL: neutrophil gelatinase‐associated lipocalin PEF‐FB‐9: "Fragebogen zur Partizipativen Ent‐scheidungsfindung (revidierte 9‐Item‐Fassung)" ‐ tool used to measure patient empowerment PIM: potentially inappropriate medication RCT: randomised controlled trial TSQM: Treatment Satisfaction Questionnaire for Medication
Differences between protocol and review
We hereby describe the differences between the current and last versions of this review (Christensen 2016). All changes were made prior to the review update unless specifically stated.
In this update, we included health‐related quality of life as a secondary outcome because of the direct patient relevance and potential for using the data in further cost‐utility analysis.
In this update, we also compared extended versus basic medication reviews.
Subgroup analysis
We added a subgroup analysis comparing trials with extended medication reviews versus trials with basic medication reviews.
We added a subgroup analysis comparing trials with a high implementation rate (over 50%) of identified drug‐related problems versus trials with low implementation rate (50% or below).
In the last version of the review the subgroup analysis comparing trials with participants at high risk of medication errors and adverse drug events versus low‐risk participants was based on the trial inclusion and exclusion criteria (i.e. high‐risk if trial eligibility criteria restricted trial population to participants at high risk, e.g. elderly patients or patients with multiple co‐medications). However, this strategy resulted in the majority of trials being coded as high‐risk. We therefore removed the analysis and instead added a subgroup based on a simpler and more objective stratification using the average number of medications (i.e. above or below 10 medications).
Sensitivity analysis
We added an analysis including cluster‐randomised cross‐over trials adjusted for clustering and period effect. We added the sensitivity analysis post hoc.
Contributions of authors
CB: adjusted the protocol, selected trials, extracted data, assessed risk of bias, was responsible for data management and data analysis, was involved in the interpretation of the results, drafted the manuscript, contributed to additional manuscript writing and approved the final version of the manuscript.
SSC: adjusted the protocol, selected trials, extracted data, assessed risk of bias, contributed to writing the manuscript and approved the final version of the manuscript.
AL: designed, conducted, analysed and reported the previous two versions of this review. For this update AL adjusted the protocol, assessed risk of bias, supervised data extraction and analysis, was involved in the interpretation of the results, contributed to writing the manuscript and approved the final version of the manuscript.
MC: designed, conducted, analysed and reported the previous two versions of this review. For this update MC adjusted the protocol, assessed risk of bias, supervised data extraction and analysis, was involved in the interpretation of the results, contributed to writing the manuscript and approved the final version of the manuscript.
Sources of support
Internal sources
-
The Nordic Cochrane Centre, Denmark
The centre provided research facilities for the initial review.
-
Department of Clinical Pharmacology, Bispebjerg Hospital, Denmark
The department provided research facilities for the update.
External sources
-
TrygFonden, Denmark
Both review authors were salaried by a grant from TrygFonden, a non‐profit foundation, for the initial review. Review authors did not receive funding for the update.
Declarations of interest
AL, CB, MC and SSC all declare no conflicts of interest relevant to this review.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bladh 2011 {published and unpublished data}
- Bladh L, Ottosson E, Karlsson J, Klintberg L, Wallerstedt SM. Effects of a clinical pharmacist service on health-related quality of life and prescribing of drugs: a randomised controlled trial. BMJ Quality & Safety 2011;20(9):738-46. [DOI] [PubMed] [Google Scholar]
- NCT01016301. Effects of a clinical pharmacist service on health-related quality of life. https://clinicaltrials.gov/ct2/show/NCT01016301 (first received 19 November 2009).
Blum 2021 {published data only}
- Adam L, Moutzouri E, Baumgartner C, Loewe AL, Feller M, M’Rabet-Bensalah K, et al. Rationale and design of OPtimising thERapy to prevent Avoidable hospital admissions in Multimorbid older people (OPERAM): a cluster randomised controlled trial. BMJ Open 2019;9(6):e026769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum MR, Sallevelt BTGM, Spinewine A, O'Mahony D, Moutzouri E, Feller M, et al. Optimizing Therapy to Prevent Avoidable Hospital Admissions in Multimorbid Older Adults (OPERAM): cluster randomised controlled trial. BMJ 2021;13(374):n1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley EK, Sallevelt BTGM, Huibers CJA, Murphy KD, Spruit M, Shen Z, et al. Intervention protocol: OPtimising thERapy to prevent avoidable hospital Admission in the Multi-morbid elderly (OPERAM): a structured medication review with support of a computerised decision support system. BMC Health Services Research 2020;20(1):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02986425. OPtimising thERapy to Prevent Avoidable Hospital Admissions in the Multimorbid Older People. https://clinicaltrials.gov/ct2/show/NCT02986425 (first received 8 December 2016).
- NTR6012. OPtimising thERapy to prevent Avoidable hospital admissions in the Multimorbid elderly. https://www.trialregister.nl/trial/5435 (first received 28 July 2016).
- Sallevelt BTGM, Huibers CJA, Heij JMJO, Egberts TCG, Puijenbroek EP, Shen Z, et al. Frequency and acceptance of clinical decision support system-generated STOPP/START signals for hospitalised older patients with polypharmacy and multimorbidity. Drugs & Aging 2022;39(1):59-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerah L, Henrard S, Wilting I, O'Mahony D, Rodondi N, Dalleur O, et al. Prevalence of drug-drug interactions in older people before and after hospital admission: analysis from the OPERAM trial. BMC Geriatrics 2021;21(1):571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonetti 2018 {published data only}
- Bonetti AF, Bagatim BQ, Mendes AM, Rotta I, Reis RC, Fávero ML, et al. Impact of discharge medication counseling in the cardiology unit of a tertiaryhospital in Brazil: a randomized controlled trial. Clinics 2008;73:e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonnerup 2014 {published and unpublished data}
- Bonnerup DK, Lisby M, Sædder EA, Brock B, Truelshøj T, Sørensen CA, et al. Effects of stratified medication review in high-risk patients at admission to hospital: a randomised controlled trial. Therapeutic Advances in Drug Safety 2020;20(11):1-10. [DOI: 10.1177/2042098620957142] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnerup DK, Lisby M, Sædder EA, Brock B, Truelshøj T, Sørensen CA, et al. Effects of stratified medication reviews in high-risk patients at admission to hospital: a randomised controlled trial. In: Risk Stratification of Medication Reviews: Physicians’ Perspectives, Test of Algorithm and Interventions [PhD Thesis by Dorthe Bonnerup]. Aarhus, Denmark: Faculty of Health Sciences, Aarhus University, 2014:82-100. [Google Scholar]
- NCT01819974. Effect of medication reviews performed in high risk patients. https://clinicaltrials.gov/ct2/show/NCT01819974 (first received 28 March 2013).
Cossette 2017 {published and unpublished data}
- Cossette B, Éthier JF, Joly-Mischlich T, Bergeron J, Ricard G, Brazeau S, et al. Reduction in targeted potentially inappropriate medication use in elderly inpatients: a pragmatic randomized controlled trial. European Journal of Clinical Pharmacology 2017;73(10):1237-45. [DOI] [PubMed] [Google Scholar]
- Cossette B, Ethier J, Joly-Mischlich T, Bergeron J, Ricard G, Brazeau S, et al. Reduction in potentially inappropriate medication use in elderly inpatients: a pragmatic randomized controlled trial. Journal of the American Geriatrics Society 2017;65:S109-10. [DOI] [PubMed] [Google Scholar]
- NCT02570945. Trial of a pharmacist-physician intervention model to reduce high-risk drug use by hospitalised elderly patients. https://clinicaltrials.gov/show/NCT02570945 (first received 7 October 2015).
Curtin 2020 {published data only}
- Curtin D, Jennings E, Daunt R, Curtin S, Randles M, Gallagher P, et al. Deprescribing in older people approaching end of life: a randomized controlled trial using STOPPFrail criteria. Journal of the American Geriatrics Society 2020;68(4):762-9. [DOI] [PubMed] [Google Scholar]
- Curtin D, Jennings E, Daunt R, Randles M, Gallagher P, O'Mahony D. 206 Deprescribing in frail older people transitioning to long-term care: a randomized controlled trial using STOPPFrail criteria 67th Annual & Scientific Meeting of the Irish Gerontological Society, Innovation, Advances and Excellence in Ageing, 26-28 September 2019, Cork, Ireland. Age & Ageing 2019;48:iii17-65. [Google Scholar]
- NCT03501108. Discontinuation of long-term medications in older people entering nursing home care (STOPPFrail) [Medication rationalization for older people awaiting long-term nursing home care: a randomized controlled trial using the STOPPFrail criteria]. https://clinicaltrials.gov/ct2/show/NCT03501108;(first received 18 April 2018).
Dalleur 2014 {published data only}
- Dalleur O, Boland B, Losseau C, Henrard S, Wouters D, Speybroeck N, et al. Reduction of potentially inappropriate medications using the STOPP criteria in frail older inpatients: a randomised controlled study. Drugs & Aging 2014;31(4):291-8. [DOI] [PubMed] [Google Scholar]
Farris 2014 {published data only}
- Farris KB, Carter BL, Xu Y, Dawson JD, Shelsky C, Weetman DB, et al. Effect of a care transition intervention by pharmacists: an RCT. BMC Health Services Research 2014;14:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallagher 2011 {published data only (unpublished sought but not used)}
- Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clinical Pharmacology and Therapeutics 2011;89(6):845-54. [DOI] [PubMed] [Google Scholar]
Gillespie 2009 {published and unpublished data}
- Alassaad A, Bertilsson M, Gillespie U, Sundström J, Hammarlund-Udenaes M, Melhus H. The effects of pharmacist intervention on emergency department visits in patients 80 years and older: subgroup analyses by number of prescribed drugs and appropriate prescribing. PLOS One 2014;9(11):e111797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alassaad A, Melhus H, Hammarlund-Udenaes M, Bertilsson M, Gillespie U, Sundström J. A tool for prediction of risk of rehospitalisation and mortality in the hospitalised elderly: secondary analysis of clinical trial data. BMJ Open 2015;5(2):e007259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund-Udenaes M, Toss H, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Archives of Internal Medicine 2009;169(9):894-900. [DOI] [PubMed] [Google Scholar]
Graabaek 2019 {published data only}
- Graabaek T, Hedegaard U, Christensen MB, Clemmensen MH, Knudsen T, Aagaard L. Effect of a medicines management model on medication-related readmissions in older patients admitted to a medical acute admission unit-A randomized controlled trial. Journal of Evaluation in Clinical Practice 2019;25(1):88-96. [DOI] [PubMed] [Google Scholar]
- Graabaek T, Knudsen T, Clemmensen MH, Aagaard L. Comparison of pharmacist-led medication review at different stages during the inpatient stay. In: European Journal of Hospital Pharmacy. Vol. 21. 2014:A210.
Gustafsson 2017 {published and unpublished data}
- Gustafsson M, Sjölander M, Pfister B, Jonsson J, Schneede J, Lövheim H. Pharmacist participation in hospital ward teams and hospital readmission rates among people with dementia: a randomized controlled trial. European Journal of Clinical Pharmacology 2017;73(7):827-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson M, Sjolander M, Pfister B, Schneede J, Lovheim H. Effects of pharmacists' interventions on inappropriate drug use and drug-related readmissions in people with dementia-a secondary analysis of a randomized controlled trial. Pharmacy (Basel) 2018;6(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01504672. A randomized controlled pharmacist intervention study to reduce drug-related problems and readmissions among old people with dementia. https://clinicaltrials.gov/ct2/show/NCT01504672 (first received 5 January 2012).
- Sjolander M, Lindholm L, Pfister B, Jonsson J, Schneede J, Lovheim H, et al. Impact of clinical pharmacist engagement in ward teams on the number of drug-related readmissions among older patients with dementia or cognitive impairment: an economic evaluation. Research in Social & Administrative Pharmacy: RSAP 2019;15(3):287-91. [DOI] [PubMed] [Google Scholar]
Juanes 2018 {published data only}
- Juanes A, Garin N, Mangues MA, Herrera S, Puig M, Faus MJ, et al. Impact of a pharmaceutical care programme for patients with chronic disease initiated at the emergency department on drug-related negative outcomes: a randomised controlled trial. European Journal of Hospital Pharmacy 2018;25(5):274-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02368548. Randomized clinical trial of a pharmaceutical care program in chronic patients users of an emergency department. https://clinicaltrials.gov/ct2/show/NCT02368548 (first received 23 February 2015).
Kempen 2021 {published and unpublished data}
- Kempen T, Bertilsson M, Hadziosmanovic N, Lindner KJ, Melhus H, Nielsen E, et al. Effectiveness of hospital-based comprehensive medication reviews including post-discharge follow-up on older patients' healthcare utilisation (the MedBridge trial): pragmatic clusterrandomized crossover trial. JACCP Journal of the American College of Clinical Pharmacy 2020;3(8):1583. [Google Scholar]
- Kempen T, Gillespie U. Identified drug-related problems and actions taken to solve them: intervention delivery within a clinical trial on comprehensive medication reviews in older hospitalised patients. International Journal of Clinical Pharmacy 2020;42(1):224. [DOI: 10.1007/s11096-019-00945-w] [DOI] [Google Scholar]
- Kempen TGH, Bertilsson M, Hadziosmanovic N, Lindner KJ, Melhus H, Nielsen EI, et al. Effects of hospital-based comprehensive medication reviews including postdischarge follow-up on older patients’ use of health care. JAMA Network Open 2021;4(4):e216303. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempen TGH, Bertilsson M, Lindner KJ, Sulku J, Nielsen EI, Högberg A, et al. Medication Reviews Bridging Healthcare (MedBridge): study protocol for a pragmatic cluster-randomised crossover trial. Contemporary Clinical Trials 2017;61:126-32. [DOI: 10.1016/j.cct.2017.07.019] [DOI] [PubMed] [Google Scholar]
- Kempen TGH, Cam H, Kälvemark A, Lindner KJ, Melhus H, Nielsen EI, et al. Intervention fidelity and process outcomes of medication reviews including post-discharge follow-up in older hospitalized patients: process evaluation of the MedBridge trial. Journal of Clinical Pharmacy and Therapeutics 2020;45(5):1021-9. [DOI] [PubMed] [Google Scholar]
- NCT02999412. Medication reviews bridging healthcare: a cluster-randomised crossover trial (MedBridge). https://clinicaltrials.gov/ct2/show/NCT02999412 (first received 21 December 2016).
Lea 2020 {published and unpublished data}
- Lea M, Mowé M, Molden E, Kvernrød K, Skovlund E, Mathiesen L. Effect of medicines management versus standard care on readmissions in multimorbid patients: a randomised controlled trial. BMJ Open 2020;10(12):e041558. [DOI: doi: 10.1136/bmjopen-2020-041558.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02336113. Oslo pharmacist intervention study - effect on readmissions. https://clinicaltrials.gov/ct2/show/NCT02336113 (first received 12 January 2015).
Lenssen 2018 {published data only}
- Lenssen R, Schmitz K, Griesel C, Heidenreich A, Schulz JB, Trautwein C, et al. Comprehensive pharmaceutical care to prevent drug-related readmissions of dependent-living elderly patients: a randomized controlled trial. BMC Geriatrics 2018;18(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02413957. Medication reconciliation in comparison to an extensive medication safety check. https://clinicaltrials.gov/ct2/show/NCT02413957 (first received 10 April 2015).
Lisby 2010 {published and unpublished data}
- Lisby M, Thomsen A, Nielsen LP, Lyhne NM, Breum-Leer C, Fredberg U, et al. The effect of systematic medication review in elderly patients admitted to an acute ward of internal medicine. Basic and Clinical Pharmacology and Toxicology 2010;106(5):422-7. [DOI] [PubMed] [Google Scholar]
Lisby 2015 {published data only}
- Lisby M, Bonnerup DK, Brock B, Gregersen PA, Jensen J, Larsen ML, et al. Medication review and patient outcomes in an orthopedic department: a randomized controlled study. Journal of Patient Safety 2018;14(2):74-81. [DOI] [PubMed] [Google Scholar]
Nielsen 2017 {published and unpublished data}
- ISRCTN08043800. Pharmacists' review of medicine during admission to hospital. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN08043800 2010.
- Nielsen TRH, Honoré PH, Rasmussen M, Andersen SE. Clinical effects of a pharmacist intervention in acute wards - a randomized controlled trial. Basic & Clinical Pharmacology & Toxicology 2017;121(4):325-33. [DOI] [PubMed] [Google Scholar]
O'Mahony 2020 {published data only}
- Dalton K, Curtin D, O'Mahony D, Byrne S. Computer-generated STOPP/START recommendations for hospitalised older adults: evaluation of the relationship between clinical relevance and rate of implementation in the SENATOR trial. Age & Ageing 2020;49(4):615-21. [DOI] [PubMed] [Google Scholar]
- Dalton K, O'Mahony D, Cullinan S, Byrne S. Factors affecting prescriber implementation of computer-generated medication recommendations in the SENATOR trial: a qualitative study. Drugs & Aging 2020;37(9):703-13. [DOI] [PubMed] [Google Scholar]
- Lavan AH, O'Mahony D, Gallagher P, Fordham R, Flanagan E, Dahly D, et al. The effect of SENATOR (Software ENgine for the Assessment and optimisation of drug and non-drug Therapy in Older peRsons) on incident adverse drug reactions (ADRs) in an older hospital cohort - Trial Protocol. BMC Geriatrics 2019;19(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony D, Gudmundsson A, Soiza RL, Petrovic M, Jose Cruz-Jentoft A, Cherubini A, et al. Prevention of adverse drug reactions in hospitalized older patients with multi-morbidity and polypharmacy: the SENATOR* randomized controlled clinical trial. Age & Ageing 2020;49(4):605-614. Erratum in: Age & Ageing. 2021 Nov 10;50(6):e10-1. [DOI: ] [DOI] [PubMed] [Google Scholar]
Ravn‐Nielsen 2018 {published and unpublished data}
- NCT03079375. The pharmacist follows you and your medication from hospital to your daily life and investigate what this means to you. https://clinicaltrials.gov/ct2/show/NCT03079375 (first received 2 December 2019).
- Rasmussen MK, Ravn-Nielsen LV, Duckert ML, Lund ML, Henriksen JP, Nielsen ML, et al. Cost-consequence analysis evaluating multifaceted clinical pharmacist intervention targeting patient transitions of care from hospital to primary care. JACCP Journal of the American College of Clinical Pharmacy 2019;2(2):123-30. [Google Scholar]
- Ravn-Nielsen LV, Duckert ML, Lund ML, Henriksen JP, Nielsen ML, Eriksen CS, et al. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. JAMA Internal Medicine 2018;178:375-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravn-Nielsen LV, Duckert M-L, Lund ML, Henriksen JP, Nielsen ML, Eriksen CS, et al. The impact of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. In: Pharmacoepidemiology and Drug Safety. Vol. 27. 2018:377. [DOI] [PMC free article] [PubMed]
Schnipper 2006 {published data only (unpublished sought but not used)}
- Schnipper JL, Kirwin JL, Cotugno MC, Wahlstrom SA, Brown BA, Tarvin E, et al. Role of pharmacist counselling in preventing adverse drug events after hospitalization. Archives of Internal Medicine 2006;166(5):565-71. [DOI] [PubMed] [Google Scholar]
Scullin 2007 {published data only}
- Scullin C, Scott MG, Hogg A, McElnay JC. An innovative approach to integrated medicines management. Journal of Evaluation in Clinical Practice 2007;13(5):781-8. [DOI] [PubMed] [Google Scholar]
Song 2021 {published data only}
- Song YK, Jeong S, Han N, Na H, Jang HY, Sohn M, et al. Effectiveness of clinical pharmacist service on drug-related problems and patient outcomes for hospitalized patients with chronic kidney disease: a randomized controlled trial. Journal of Clinical Medicine 2021;10(8):1788. [DOI: 10.3390/jcm10081788] [DOI] [PMC free article] [PubMed] [Google Scholar]
SUREPILL 2015 {published and unpublished data}
- Boer M, Ramrattan MA, Kiewiet JJ, Boeker EB, Gombert-Handoko KB, Lent-Evers NA, et al. Cost-effectiveness of ward-based pharmacy care in surgical patients: protocol of the SUREPILL (Surgery & Pharmacy In Liaison) study. BMC Health Services Research 2011;11:55. [DOI: 10.1186/1472-6963-11-55] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTR2258. The economic and clinical impact of a clinical pharmacy team on surgical patients: the SUREPILL study. https://www.trialregister.nl/trial/2134 2010.
- Surgery and Pharmacy in Liaison (SUREPILL) Study Group. Effect of a ward-based pharmacy team on preventable adverse drug events in surgical patients (SUREPILL study). British Journal of Surgery 2015;102(10):1204-12. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
[no author] 2001 {published data only}
- [no author]. Hospital pharmacists and the NSF for CHD. Pharmaceutical Journal 2001;267(7169):518. [Google Scholar]
AbuRuz 2021 {published data only}
- AbuRuz S, Jaber D, Basheti I, Sadeq A, Arafat M, AlAhmad M, et al. Impact of pharmacist interventions on drug-related problems in general surgery patients: a randomised controlled trial. European Journal of Hospital Pharmacy 2021;28(Suppl 2):e72-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
ACTRN12605000264684 2005 {unpublished data only}
- ACTRN12605000264684. Intervention to improve quality use of medicines in elderly people: effect on health outcomes. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=338 (first received 2 September 2005).
ACTRN12605000376640 2005 {unpublished data only}
- ACTRN12605000376640. Improving evidence based prescribing in older adults moving from hospital to long-term facilities with a pharmacist transition coordinator: a randomised controlled trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12605000376640 2005.
ACTRN12611000370909 2011 {unpublished data only}
- ACTRN12611000370909. Deprescribing in frail older people: a randomised controlled trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336757 (first received 11 April 2011). [DOI] [PMC free article] [PubMed]
ACTRN12611000995976 2011 {unpublished data only}
- ACTRN12611000995976. Application of a prescribing indicators tool to assist in identifying and resolving drug-related problems in older Australians - a randomized controlled trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=347487 (first received 19 September 2011).
ACTRN12611001262998 2011 {unpublished data only}
- ACTRN12611001262998. A pilot study to assess the feasibility of a prospective randomised controlled trial of a patient-centred medicines management approach to reduce the burden of iatrogenic symptoms in palliative care. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12611001262998 2011.
ACTRN12616001034426 2016 {unpublished data only}
- ACTRN12616001034426. Evaluation of partnered pharmacist charting at admission and discharge. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371206 (first received 4 August 2016).
ACTRN12616001474448 2016 {unpublished data only}
- ACTRN12616001474448. Pharmacist involvement in helping patients at discharge from hospital and the effect on their wellbeing and readmission to hospital. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12616001474448 2016.
ACTRN12617001293358 2017 {unpublished data only}
- ACTRN12617001293358. Impact of a chronic disease management model in patients with decompensated liver disease: The Australian Liver Failure Trial (ALFIE). https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372421 (first received 7 September 2017).
ACTRN12618000250235 2018 {unpublished data only}
- ACTRN12618000250235. (M)ultifactorial (I)ntervention in Patients with (P)eripheral (A)rterial (D)isease - a randomised controlled trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12618000250235 2018.
ACTRN12618000794202 2018 {unpublished data only}
- ACTRN12618000794202. Comprehensive geriatric assessment and interventions in older lung cancer patients. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372397 (first received 10 May 2018).
ACTRN12619000729123 2019 {unpublished data only}
- ACTRN12619000729123. Preventing adverse drug reactions in older Australians. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12619000729123 2019.
Ahmad 2012 {published data only}
- Ahmad A, Nijpels G, Dekker JM, Kostense PJ, Hugtenburg JG. Effect of a pharmacist medication review in elderly patients discharged from the hospital. Archives of Internal Medicine 2012;172(17):1346-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Al‐Hashar 2018 {published data only}
- Al-Hashar A, Al-Zakwani I, Eriksson T, Sarakbi A, Al-Zadjali B, Al Mubaihsi S, et al. Impact of medication reconciliation and review and counselling, on adverse drug events and healthcare resource use. International Journal of Clinical Pharmacy 2018;40(5):1154-64. [DOI] [PubMed] [Google Scholar]
Alicic 2016 {published data only}
- Alicic RZ, Short RA, Corbett CL, Neumiller JJ, Gates BJ, Daratha KB, et al. Medication intervention for chronic kidney disease patients transitioning from hospital to home: study design and baseline characteristics. American Journal of Nephrology 2016;44(2):122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 1986 {published data only}
- Allen CM, Becker PM, McVey LJ, Saltz C, Feussner JR, Cohen HJ. A randomized, controlled clinical trial of a geriatric consultation team. Compliance with recommendations. JAMA 1986;255(19):2617-21. [PMID: ] [PubMed] [Google Scholar]
Al Mazroui 2009 {published data only}
- Al Mazroui NR, Kamal MM, Ghabash NM, Yacout TA, Kole PL, McElnay JC. Influence of pharmaceutical care on health outcomes in patients with Type 2 diabetes mellitus. British Journal of Clinical Pharmacology 2009;67(5):547-57. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Rashed 2002 {published data only}
- Al-Rashed SA, Wright DJ, Roebuck N, Sunter W, Chrystyn H. The value of inpatient pharmaceutical counselling to elderly patients prior to discharge. British Journal of Clinical Pharmacology 2002;54(6):657-64. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Awdishu 2016 {published data only}
- Awdishu L, Coates CR, Lyddane A, Tran K, Daniels CE, Lee J, et al. The impact of real-time alerting on appropriate prescribing in kidney disease: a cluster randomized controlled trial. Journal of the American Medical Informatics Association: JAMIA 2016;23(3):609-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balaban 2015 {published data only}
- Balaban RB, Galbraith AA, Burns ME, Vialle-Valentin CE, Larochelle MR, Ross-Degnan D. A patient navigator intervention to reduce hospital readmissions among high-risk safety-net patients: a randomized controlled trial. Journal of General Internal Medicine 2015;30(7):907-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basoor 2011 {published data only}
- Basoor A, Doshi N, Cotant J, Todorov M, Saleh T, Patel K, et al. Result of quality improvement discharge tool in congestive heart failure-randomized controlled trial. Chest 2011;140(4). [DOI: 10.1378/chest.1113754] [DOI]
Bell 2016 {published data only}
- Bell SP, Schnipper JL, Goggins K, Bian A, Shintani A, Roumie CL, et al. Effect of pharmacist counseling intervention on health care utilization following hospital discharge: a randomized control trial. Journal of General Internal Medicine 2016;31(5):470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhagwan 2018 {published data only}
- Bhagwan V, Kirke C, Meehan F, Hickey M, Kinlan E, Grimes T. A quality improvement project addressing peri-operative medication reconciliation and administration in elective surgical patients using five or more medications pre-admission. International Journal of Pharmacy Practice 2018;26:35-6. [Google Scholar]
Boland 2016 {published data only}
- Boland B, Guignard B, Dalleur O, Lang PO. Application of STOPP/START and Beers criteria: compared analysis on identification and relevance of potentially inappropriate prescriptions. European Geriatric Medicine 2016;7(5):416-23. [Google Scholar]
Bolas 2004 {published data only}
- Bolas H, Brookes K, Scott M, McElnay J. Evaluation of a hospital-based community liaison pharmacy service in Northern Ireland. Pharmacy World and Science 2004;26(2):114-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bondesson 2013 {published data only}
- Bondesson A, Eriksson T, Kragh A, Holmdahl L, Midlov P, Hoglund P. In-hospital medication reviews reduce unidentified drug-related problems. European Journal of Clinical Pharmacology 2013;69(3):647-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bonnet‐Zamponi 2010 {published data only}
- Bonnet-Zamponi D, Lacaille S, Tubach F, Paillaud E, Aquino J, Verny M, et al. A new intervention to decrease mortality and readmissions in very elderly inpatients: the OMAGE trial. Journal of the American Geriatrics Society 2010;58:S3-4. [Google Scholar]
Bonnet‐Zamponi 2013 {published data only}
- Bonnet-Zamponi D, d'Arailh L, Konrat C, Delpierre S, Lieberherr D, Lemaire A, et al. Drug-related readmissions to medical units of older adults discharged from acute geriatric units: results of the Optimization of Medication in AGEd multicenter randomized controlled trial. Journal of the American Geriatrics Society 2013;61(1):113-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brecher 2015 {published data only}
- Brecher DB. Intervention with the STOPP/START criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. Journal of Pain and Symptom Management 2015;49(5):967-8. [Google Scholar]
Briggs 2015 {published data only}
- Briggs S, Pearce R, Dilworth S, Higgins I, Hullick C, Attia J. Clinical pharmacist review: a randomised controlled trial. Emergency Medicine Australasia 2015;27(5):419-26. [DOI] [PubMed] [Google Scholar]
Bruhwiler 2018 {published data only}
- Bruhwiler LD, Beeler PE, Boni F, Wiedemeier PG, Hersberger KE, Lutters M. A pragmatic in-hospital service to reduce workload of community pharmacists after discharge: a randomised controlled trial. International Journal of Clinical Pharmacy 2018;40(1):207. [Google Scholar]
Bruhwiler 2019 {published data only}
- Bruhwiler LD, Beeler PE, Boni F, Giger R, Wiedemeier PG, Hersberger KE, et al. A RCT evaluating a pragmatic in-hospital service to increase the quality of discharge prescriptions. International Journal for Quality in Health Care 2019;31(8):G74-80. [DOI: 10.1093/intqhc/mzz043] [DOI] [PubMed] [Google Scholar]
Bulow 2018 {published data only}
- Bulow C, Faerch KU, Armandi H, Jensen BN, Sonne J, Christensen HR, et al. Important aspects of pharmacist-led medication reviews in an acute medical ward. Basic & Clinical Pharmacology & Toxicology 2018;122(2):253-61. [DOI] [PubMed] [Google Scholar]
Burleson 2003 {published data only}
- Burleson BS. Impact of pharmacist-conducted medication histories on number of pharmacist's interventions, drug cost, medication errors, re-admission rates, and mortality during hospitalization of medicine patients. In: ASHP Midyear Clinical Meeting. Vol. 38. 2003:P-610.
Burnett 2009 {published data only}
- Burnett KM, Scott MG, Fleming GF, Clark CM, McElnay JC. Effects of an integrated medicines management program on medication appropriateness in hospitalized patients. American Journal of Health-System Pharmacy 2009;66(9):854-9. [DOI] [PubMed] [Google Scholar]
Capobussi 2016 {published data only}
- Capobussi M, Banzi R, Moja L, Bonovas S, Gonzalez-Lorenzo M, Liberati EG, et al. Computerized decision support systems: EBM at the bedside. Recenti Progressi in Medicina 2016;107(11):589-91. [DOI] [PubMed] [Google Scholar]
Cardinale 1993 {published data only}
- Cardinale V. Clinical R.Ph.s in a team prove to be cost-effective. Drug Topics 1993;Jan Suppl:1. [Google Scholar]
Chen 2014 {published data only}
- Chen F, Chen J, Zheng P. Application of continuity of care mode for senile patients with chronic disease. Chinese Nursing Research 2014;28(11C):4119-21. [Google Scholar]
Chiu 2018 {published data only}
- Chiu KC, Lee WK, See YW, Chan HW. Outcomes of a pharmacist-led medication review programme for hospitalised elderly patients. Hong Kong Medical Journal 2018;24(2):98-106. [DOI: 10.12809/hkmj176871] [DOI] [PubMed] [Google Scholar]
Ciechanover 1987 {published data only}
- Ciechanover M, Kohn D. A drug-reduction trial in elderly patients. Harefuah 1987;112(11):543-5. [PubMed] [Google Scholar]
Connor 2012 {unpublished data only}
- Connor MO, O'Sullivan D, Gallagher P, Byrne S, Eustace J, O'Mahony D. Prevention of adverse drug events in hospitalised older patients: a randomised controlled trial. Irish Journal of Medical Science 2012;181:S181-223. [Google Scholar]
Crotty 2004 {published data only}
- Crotty M, Rowett D, Spurling L, Giles LC, Phillips PA. Does the addition of a pharmacist transition coordinator improve evidence-based medication management and health outcomes in older adults moving from the hospital to a long-term care facility? Results of a randomized, controlled trial. American Journal of Geriatric Pharmacotherapy 2004;2(4):257-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
CTRI/2014/08/004900 2014 {unpublished data only}
- CTRI/2014/08/004900. Impact of pharmaceutical care on quality of life among chronic kidney disease patients on hemodialysis. http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=8802&EncHid=&modid=&compid=%27,%278802det%27 2014.
Dalton 2018 {published data only}
- Dalton K, O'Mahony D, O'Sullivan D, O'Connor NM, Byrne S. Prescriber acceptance of STOPP/START recommendations for hospitalised older adults: pharmacist versus physician-secondary analysis of data from two randomised controlled trials. In: International Journal of Pharmacy Practice. Vol. 26. 2018:21-2.
Dalton 2019 {published data only}
- Dalton K, Curtin D, O'Mahony D, Byrne S. Evaluation of the clinical relevance of computer-generated STOPP/START recommendations for hospitalised older adults. International Journal of Pharmacy Practice 2019;27:6. [Google Scholar]
Dalton 2019a {published data only}
- Dalton K, O'Mahony D, O'Sullivan D, O'Connor MN, Byrne S. Prescriber implementation of STOPP/START recommendations for hospitalised older adults: a comparison of a pharmacist approach and a physician approach. Drugs & Aging 2019;36(3):279-88. [DOI] [PubMed] [Google Scholar]
Dauphinot 2017 {published data only}
- Dauphinot V, Jean-Bart E, Krolak-Salmon P, Mouchoux C. A multi-center, randomized, controlled trial to assess the efficacy of optimization of drug prescribing in an elderly population, at 18 months of follow-up, in the evolution of functional autonomy: the OPTIM study protocol. BMC Geriatrics 2017;17(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
DRKS00007696 2015 {unpublished data only}
- DRKS00007696. Impact of a a pharmaceutical consultation service on palliative care pharmacotherapy. http://www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00007696 2015.
DRKS00013321 2017 {unpublished data only}
- DRKS00013321. GLICEMIA 2.0 – a randomized, controlled trial of secondary and tertiary prevention in a type 2 diabetic population. GLICEMIA 2.0 – a randomized, controlled trial of secondary and tertiary prevention in a type 2 diabetic population - GLICEMIA 2.0 2017.
DRKS00013538 2017 {unpublished data only}
- DRKS00013538. Perioperative drug therapy management in very old patients by a geriatric-pharmaceutical team. http://www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00013538 2017.
Dudley‐Brown 2016 {published data only}
- Dudley-Brown S, Andros V, Hinkle J, Caines J. Adherence and long-term IBD value-added effectiveness (alive): effectiveness of a patient fulfillment model in a large academic center IBD clinic. Inflammatory Bowel Diseases 2016;22:S30. [Google Scholar]
Ee 2019 {published data only}
- Ee CAJM, Lee KH, Tan HL, Low LL. Effectiveness and feasibility of deprescribing of symptomatic medications in a Singapore rehabilitation hospital. Proceedings of Singapore Healthcare 2019;28(1):31-8. [Google Scholar]
Erku 2017 {published data only}
- Erku DA, Belachew SA, Tegegn HG, Ayele AA. The impact of pharmacist-led medication therapy management on medication adherence in patients with type 2 diabetes mellitus: a randomized controlled study. Value in Health 2017;20(9):A402. [DOI] [PMC free article] [PubMed] [Google Scholar]
EUCTR‐004236‐38‐GB 2016 {unpublished data only}
- EUCTR-004236-38-GB. Optimising treatment for mild systolic hypertension in the elderly. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016-004236-38-GB 2016.
Franchi 2016 {published data only}
- Franchi C, Tettamanti M, Djade CD, Pasina L, Mannucci PM, Onder G, et al. E-learning in order to improve drug prescription for hospitalized older patients: a cluster-randomized controlled study. British Journal of Clinical Pharmacology 2016;82(1):53-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frankenthal 2014 {published data only}
- Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. Journal of the American Geriatrics Society 2014;62(9):1658-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Frankenthal 2017 {published data only}
- Frankenthal D, Israeli A, Caraco Y, Lerman Y, Kalendaryev E, Zandman-Goddard G. Long-term outcomes of medication intervention using the screening tool of older persons potentially inappropriate prescriptions screening tool to alert doctors to right treatment criteria. Journal of the American Geriatrics Society 2017;65(2):e33-8. [DOI] [PubMed] [Google Scholar]
Gallagher 2015 {published data only}
- Gallagher J, O'Sullivan D, McCarthy S, Gillespie P, Woods N, O'Mahony D, et al. A cost-effectiveness analysis of hospital pharmacist review in older patients. Value in health 2015;18(7):A732-3. [Google Scholar]
Gallagher 2015a {published data only}
- Gallagher J, McCarthy S, O'Sullivan D, O'Mahony D, Gillespie P, Woods N, et al. Economic evaluation of a software-supported structured pharmacist medication review in hospitalised older patients. International Journal of Pharmacy Practice 2015;23:13-4. [Google Scholar]
Gallagher 2016 {published data only}
- Gallagher J, O'Sullivan D, McCarthy S, Gillespie P, Woods N, O'Mahony D, et al. Structured pharmacist review of medication in older hospitalised patients: a cost-effectiveness analysis. Drugs & Aging 2016;33(4):285-94. [DOI] [PubMed] [Google Scholar]
Gallagher 2017 {published data only}
- Gallagher J, O'Sullivan D, McCarthy S, Gillespie P, Woods N, O'Mahony D, et al. Structured pharmacist review of medication in older hospitalised patients: a cost effectiveness analysis. European Journal of Hospital Pharmacy 2017;24:A6. [DOI] [PubMed] [Google Scholar]
Garcia 2015 {published data only}
- Garcia BH, Giverhaug T, Hogli JU, Skjold F, Smabrekke L. A pharmacist-led follow-up program for patients with established coronary heart disease in North Norway - a randomized controlled trial. Pharmacy Practice 2015;13(2):575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gattis 1999 {published data only}
- Gattis WA, Hasselblad V, Whellan DJ, O'Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) Study. Archives of Internal Medicine 1999;159(16):1939-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Geneletti 2019 {published data only}
- Geneletti L, Chanoine S, Lugosi M, Allenet B, Bouilet L, Bedouch P, et al. Impact of hospital-city communication based on the multiprofessional and collaborative development of the discharge letter on the continuity of patients' medication management-city study. European Journal of Hospital Pharmacy 2019;26:A172. [Google Scholar]
George 2011 {published data only}
- George LJW, Senturk-Raif R, Hodgkinson MR, Emmerton M, Larmour I. Impact of a surgical preadmission clinic pharmacist on the quality of medication management from preadmission to discharge: a randomised controlled study. Journal of Pharmacy Practice and Research 2011;41(3):212-6. [Google Scholar]
Gines 2016 {published data only}
- Gines A, Sanchez Navarro I, Santolaya Perrin R, Galan N, Sierra J, Moreno MT, et al. Inappropriate prescribing in elderly patients attending the emergency room. European Journal of Hospital Pharmacy 2016;23:A56. [Google Scholar]
Goldberg 2019 {published data only}
- Goldberg EM, Resnik L, Mor V, Marks SJ, Merchant RC, Kennedy M. Feasibility and initial efficacy of gapcare: the geriatric acute and post-acute care coordination fall prevention program. Academic Emergency Medicine 2019;26:S125. [Google Scholar]
Granados 2020 {published data only}
- Granados J, Salazar-Ospina A, Botero-Aguirre JP, Valencia-Quintero AF, Ortiz N, Amariles P. Effect and associated factors of a clinical pharmacy model in the incidence of medication errors (EACPharModel) in the Hospital Pablo Tobon Uribe: study protocol for a stepped wedge randomized controlled trial (NCT03338725). Trials 2020;21(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedegaard 2014 {published data only}
- Hedegaard U, Kjeldsen L J, Pottegard A, Bak S, Hallas J. Multifaceted intervention including motivational interviewing to support medication adherence after stroke/transient ischemic attack: a randomized trial. Cerebrovascular Diseases Extra 2014;4(3):221-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedegaard 2015 {published data only}
- Hedegaard U, Kjeldsen LJ, Pottegård A, Hallas J. A multifaceted pharmacist intervention to support medication adherence after stroke and transient ischemic attack. International Journal of Clinical Pharmacy 2015;37(1):187. [Google Scholar]
Hellstrom 2011 {published data only}
- Hellstrom LM, Bondesson A, Hoglund P, Midlov P, Holmdahl L, Rickhag E, et al. Impact of the Lund Integrated Medicines Management (LIMM) model on medication appropriateness and drug-related hospital revisits. European Journal of Clinical Pharmacology 2011;67(7):741-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Heselmans 2015 {published data only}
- Heselmans A, Krieken J, Cootjans S, Nagels K, Filliers D, Dillen K, et al. Medication review by a clinical pharmacist at the transfer point from ICU to ward: a randomized controlled trial. Journal of Clinical Pharmacy and Therapeutics 2015;40(5):578-83. [DOI] [PubMed] [Google Scholar]
Ho 2013 {published data only}
- Ho M, Lambert-Kerzner A, Carey E, Fahdi I, Bryson C, Melnyk DS, et al. Multifaceted intervention to improve medication adherence and secondary prevention measures (Medication study) after acute coronary syndrome hospital discharge. Circulation 2013;128(24):2706-7. [DOI] [PubMed] [Google Scholar]
Hohl 2017 {published data only}
- Hohl CM, Partovi N, Ghement I, Wickham ME, McGrail K, Reddekopp LN, et al. Impact of early in-hospital medication review by clinical pharmacists on health services utilization. PLOS One 2017;12(2):e0170495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ilting‐Reuke 2018 {published data only}
- Ilting-Reuke K, Duning T, Roos A, Ohrmann P, Hempel G. Management of delirium in an acute care hospital. European Journal of Hospital Pharmacy 2018;25:A124-5. [Google Scholar]
Iltingreuke 2019 {published data only}
- Iltingreuke K, Roos A, Ohrmann P, Duning T, Hempel G. Management of delirium in elderly people: a randomized controlled trial. International Journal of Clinical Pharmacy 2019;41(1):295. [Google Scholar]
ISRCTN01624723 2007 {unpublished data only}
- ISRCTN01624723. Medication error and adverse event detection and resolution by a pharmacist in the Emergency Department at Southampton General Hospital. Sub-study on patient views about medication. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN01624723 2007.
ISRCTN11674947 2012 {published data only}
- ISRCTN11674947. The Malaysian Falls Assessment and Intervention Trial. lhttps://www.isrctn.com/ISRCTN11674947 (first received 27 November 2012).
ISRCTN11751440 2017 {published data only}
- ISRCTN11751440. Hospital pharmacists successfully work with the acute care team to stop home medications deemed no longer necessary. Impact of Deprescribing Rounds on Outpatient Prescriptions: An Interventional Trial 2017.
ISRCTN17219647 2017 {unpublished data only}
- ISRCTN17219647. GeriMedRisk®, a telemedicine geriatric pharmacology consultation service to address adverse drug events in long-term care. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN17219647 2017. [DOI] [PMC free article] [PubMed]
ISRCTN32281812 2017 {unpublished data only}
- ISRCTN32281812. Effectiveness of a pharmacist intervention in patients with lung disease. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN32281812 2017.
ISRCTN38449870 2013 {unpublished data only}
- ISRCTN38449870. Reduction of inappropriate medication and adverse drug events in older patients. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN38449870 2013.
JPRN‐UMIN000024250 2016 {unpublished data only}
- JPRN-UMIN000024250. SADA-5 (Strategy of Assessment for Drug Appropriateness) criteria as a powerful tool for detecting potentially inappropriate medications with using another explicit criteria; a randomized control trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000024250 2016.
JPRN‐UMIN000033814 2018 {unpublished data only}
- JPRN-UMIN000033814. The Intervention study for Medication assist provided by pharmacist for the elderly with polypharmacy. https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000038567 (first received 20 August 2018).
Kang 2018 {published data only}
- Kang JE, Yu JM, Choi JH, Chung IM, Pyun WB, Kim SA, et al. Development and clinical application of an evidence-based pharmaceutical care service algorithm in acute coronary syndrome. Journal of Clinical Pharmacy and Therapeutics 2018;43(3):366-76. [DOI] [PubMed] [Google Scholar]
Karapinar‐Çarkıt 2017 {published data only}
- Karapinar-Çarkıt F, Knaap R, Bouhannouch F, Borgsteede SD, Janssen MJA, Siegert CEH, et al. Cost-effectiveness of a transitional pharmaceutical care program for patients discharged from the hospital. PLOS One 2017;12(4):e0174513. [DOI] [PMC free article] [PubMed] [Google Scholar]
KCT0005994 {published and unpublished data}
- Lee S, Yu YM, Han E, Park MS, Lee J-H, Chang MJ. Effect of pharmacist intervention on 30-adverse drug event after discharge in elderly patients through a comprehensive medication reconciliation: a randomized clinical trial. https://assets.researchsquare.com/files/rs-1092054/v1/41fe85c2-dbf0-4942-9766-b16624807138.pdf?c=1648497962 9 December 2021:(preprint). [DOI: 10.21203/rs.3.rs-1092054/v1] [DOI] [PMC free article] [PubMed]
Kelly 2011 {published data only}
- Kelly DM, Frick EM, Hale LS. How the medication review can help to reduce risk of falls in older patients. JAAPA 2011;24(4):30-4, 55. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kindstedt 2020 {published data only}
- Kindstedt J, Svahn S, Sjölander M, Glader EL, Lövheim H, Gustafsson M. Investigating the effect of clinical pharmacist intervention in transitions of care on drug-related hospital readmissions among the elderly: study protocol for a randomised controlled trial. BMJ Open 2020;10(4):e036650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koehler 2009 {published data only}
- Koehler BE, Richter KM, Youngblood L, Cohen BA, Prengler ID, Cheng D, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. Journal of Hospital Medicine 2009;4(4):211-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kripalani 2011 {published data only}
- Kripalani S, Roumie CL, Dalal AK, Cawthon C, Businger A, Eden S, et al. Pharmacist intervention for low literacy in cardiovascular disease (PILL-CVD): a randomized controlled trial. Journal of General Internal Medicine 2011;26:S121-2. [Google Scholar]
Lea 2018 {published data only}
- Lea M, Mathiesen L, Mowe M, Molden E. Baseline characteristics of multimorbid patients included in a RCT. International Journal of Clinical Pharmacy 2018;40(1):269. [Google Scholar]
Leguelinel‐Blache 2018 {published data only}
- Leguelinel-Blache G, Castelli C, Roux-Marson C, Bouvet S, Andrieu S, Cestac P, et al. Impact of collaborative pharmaceutical care on in-patients' medication safety: study protocol for a stepped wedge cluster randomized trial (MEDREV study). Trials 2018;19(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lind 2016 {published data only}
- Lind KB, Soerensen CA, Salamon SA, Jensen TM, Kirkegaard H, Lisby M. Impact of clinical pharmacist intervention on length of stay in an acute admission unit: a cluster randomised study. European Journal of Hospital Pharmacy: Science & Practice 2016;23(3):171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lind 2017 {published data only}
- Lind KB, Soerensen CA, Salamon SA, Kirkegaard H, Lisby M. Consequence of delegating medication-related tasks from physician to clinical pharmacist in an acute admission unit: an analytical study. European Journal of Hospital Pharmacy: Science and Practice 2017;24(5):272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipton 1992 {published data only}
- Lipton HL, Bero LA, Bird JA, McPhee SJ. The impact of clinical pharmacists' consultations on physicians' geriatric drug prescribing. A randomized controlled trial. Medical Care 1992;30(7):646-58. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lonnbro 2017 {published data only}
- Lonnbro J, Wallerstedt SM. Clinical relevance of the STOPP/START criteria in hip fracture patients. European Journal of Clinical Pharmacology 2017;73(4):499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mao 2015 {published data only}
- Mao CT, Liu MH, Hsu KH, Fu TC, Wang JS, Huang YY, et al. Effect of multidisciplinary disease management for hospitalized heart failure under a national health insurance programme. Journal of Cardiovascular Medicine 2015;16(9):616-24. [DOI] [PubMed] [Google Scholar]
Marinovic 2021 {published data only}
- Marinović I, Bačić Vrca V, Samardžić I, Marušić S, Grgurević I, Papić I, et al. Impact of an integrated medication reconciliation model led by a hospital clinical pharmacist on the reduction of post-discharge unintentional discrepancies. Journal of Clinical Pharmacy and Therapeutics 2021;46(5):1326-33. [DOI] [PubMed] [Google Scholar]
Martinez 2019 {published data only}
- Martinez MF, Herrada L, Munoz A, Chavez C, Sandoval T, Jiron M. Effect of a clinical pharmacist intervention on the emergency department revisits: a randomized controlled trial. In: Pharmacoepidemiology and Drug Safety. Vol. 28. 2019:199‐200.
Marusic 2013 {published data only}
- Marusic S, Gojo-Tomic N, Erdeljic V, Bacic-Vrca V, Franic M, Kirin M, et al. The effect of pharmacotherapeutic counselling on readmissions and emergency department visits. International Journal of Clinical Pharmacy 2013;35(1):37-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mateti 2018 {published data only}
- Mateti UV, Nagappa AN, Attur RP, Nagaraju SP, Rangaswamy D. Impact of pharmaceutical care on clinical outcomes among hemodialysis patients: a multicenter randomized controlled study. Saudi Journal of Kidney Diseases and Transplantation 2018;29(4):801-8. [DOI] [PubMed] [Google Scholar]
McCoy 2012 {published data only}
- McCoy AB, Cox ZL, Neal EB, Waitman LR, Peterson NB, Bhave G, et al. Real-time pharmacy surveillance and clinical decision support to reduce adverse drug events in acute kidney injury: a randomized, controlled trial. Applied clinical informatics 2012;3(2):221-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2018 {published data only}
- McDonald E, Lee T, Wu P, Downar J, Goodine C. The medsafer pilot results: electronic deprescribing for hospitalized older adults with polypharmacy. BMJ Evidence-based Medicine 2018;23:A25-6. [Google Scholar]
McMullin 1999 {published data only}
- McMullin ST, Hennenfent JA, Ritchie DJ, Huey WY, Lonergan TP, Schaiff RA, et al. A prospective, randomized trial to assess the cost impact of pharmacist-initiated interventions. Archives of Internal Medicine 1999;159(19):2306-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mendes 2021 {published data only}
- Mendes A. Multimorbidity, optimising treatment and preventing hospital admissions in older people. Journal of Prescribing Practice 2021;3(9).
Michalek 2014 {published data only}
- Michalek C, Wehling M, Schlitzer J, Frohnhofen H. Effects of "Fit fOR The Aged" (FORTA) on pharmacotherapy and clinical endpoints--a pilot randomized controlled study. European Journal of Clinical Pharmacology 2014;70(10):1261-7. [DOI] [PubMed] [Google Scholar]
Mishra 2017 {published data only}
- Mishra A, Sai Krishna G, Sravani A, Kurian TD, Kurian J, Ramesh M, et al. Impact of pharmacist-led collaborative patient education on medication adherence and quality of life of schizophrenia patients in a tertiary care setting. Bulletin of Faculty of Pharmacy, Cairo University 2017;55(2):345-9. [Google Scholar]
Nachtigall 2019 {published data only}
- Nachtigall A, Heppner HJ, Thurmann PA. Influence of pharmacist intervention on drug safety of geriatric inpatients: a prospective, controlled trial. Therapeutic Advances in Drug Safety 2019;10:2042098619843365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naughton 1994 {published data only}
- Naughton BJ, Moran MB, Feinglass J, Falconer J, Williams ME. Reducing hospital costs for the geriatric patient admitted from the emergency department: a randomized trial. Journal of the American Geriatrics Society 1994;42(10):1045-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT00205140 2005 {unpublished data only}
- NCT00205140. Effect of pharmacist initiated recommendations on prescribing for participants in the senior meds personalized medication review. https://clinicaltrials.gov/ct2/show/NCT00205140 (first received 20 September 2005).
NCT00279656 2006 {unpublished data only}
- NCT00279656. Benefit of a collaborative approach to improve the quality of medicines use in elderly inpatients. https://clinicaltrials.gov/show/NCT00279656 (first received 19 January 2006).
NCT00351676 2006 {unpublished data only}
- NCT00351676. Capturing outcomes of clinical activities performed by a rounding pharmacist practising in a team environment (COLLABORATE). https://www.clinicaltrials.gov/ct2/show/NCT00351676 (first received 13 July 2006). [DOI] [PubMed]
NCT00416026 2006 {unpublished data only}
- NCT00416026. Translating research: patient decision support/coaching. https://clinicaltrials.gov/show/NCT00416026 (first received 27 December 2006).
NCT00541606 {unpublished data only}
- NCT00541606. Pharmacist assisted medication program enhancing the regulation of diabetes. https://clinicaltrials.gov/ct2/show/NCT00541606 (first received 10 October 2007).
NCT00773942 2010 {unpublished data only}
- NCT00773942. Design & evaluation of a medication therapy management program to improve patient safety in medicare beneficiaries. https://clinicaltrials.gov/ct2/show/NCT00773942 (first received 16 October 2008).
NCT00844025 2010 {unpublished data only}
- NCT00844025. Pharmaceutical care and clinical outcomes for the elderly taking potentially inappropriate medication. https://clinicaltrials.gov/ct2/show/NCT00844025 (first received 13 February 2009).
NCT01034761 2009 {unpublished data only}
- NCT01034761. Using clinical alerts to decrease inappropriate medication prescribing. https://clinicaltrials.gov/show/NCT01034761 (first received 17 December 2009).
NCT01134900 2010 {unpublished data only}
- NCT01134900. Achieving medication safety during acute kidney injury. https://clinicaltrials.gov/ct2/show/NCT01134900 (first received 2 June 2010).
NCT01164137 2013 {unpublished data only}
- NCT01164137. Eliminating risk of preventable adverse drug events at the hospital-community interface of care. https://clinicaltrials.gov/ct2/show/NCT01164137 (first received 16 July 2010).
NCT01212211 2011 {unpublished data only}
- NCT01212211. Hyponatremia in the elderly: benefit from a change in drug therapy? https://clinicaltrials.gov/ct2/show/NCT01212211 (first received 30 September 2010).
NCT01356563 2011 {unpublished data only}
- NCT01356563. Efficacy study of pharmacist intervention on medication-related problems in hemodialysis patients. https://clinicaltrials.gov/ct2/show/NCT01356563 (first received 19 May 2011).
NCT01467050 2012 {unpublished data only}
- NCT01467050. Prevention of adverse drug events (ADEs) in hospitalised older patients. https://clinicaltrials.gov/ct2/show/NCT01467050 (first received 8 November 2011).
NCT01467128 2012 {unpublished data only}
- NCT01467128. Adverse drug event prevention using structured pharmacist review. https://clinicaltrials.gov/ct2/show/NCT01467128 (first received 8 November 2011).
NCT01503554 2011 {unpublished data only}
- NCT01503554. Combined social worker and pharmacist transitional care program. https://clinicaltrials.gov/show/NCT01503554 (first received 4 January 2012).
NCT01513265 2012 {unpublished data only}
- NCT01513265. Rationalisation of polypharmacy in the elderly by the RASP instrument. https://clinicaltrials.gov/ct2/show/NCT01513265 (first received 20 January 2012).
NCT01602744 2012 {unpublished data only}
- NCT01602744. The use of STOPP/START criteria for medication intervention among elderly population living in a geriatric hospital. https://clinicaltrials.gov/show/NCT01602744 (first received 21 May 2012).
NCT01627483 2010 {unpublished data only}
- NCT01627483. Randomized controlled trial of effects of physician's medication reviews on prescribing in older hip fracture patients. https://www.clinicaltrials.gov/ct2/show/NCT01627483 (first received 25 June 2012).
NCT01739816 2014 {unpublished data only}
- NCT01739816. Polymedication check - a randomised controlled trial. https://clinicaltrials.gov/ct2/show/NCT01739816 (first received 4 December 2012).
NCT01814280 2013 {unpublished data only}
- NCT01814280. Reducing medication errors on basis of an individual risk assessment. https://clinicaltrials.gov/ct2/show/NCT01814280 (first received19 March 2013).
NCT01906710 2013 {unpublished data only}
- NCT01906710. The Pharmacy Intervention Team Hospital-based (PITH) for People Study: effect on clinical and economic outcomes. https://clinicaltrials.gov/ct2/show/NCT01906710 (first received 24 July 2013).
NCT01969526 2016 {published data only}
- NCT01969526. Effectiveness of a multifactorial intervention on frailty. https://clinicaltrials.gov/ct2/show/NCT01969526 (first received 25 October 2013).
NCT02047448 2017 {unpublished data only}
- NCT02047448. Improving medication adherence through a transitional care pharmacy practice model. https://clinicaltrials.gov/ct2/show/NCT02047448 (first received 28 January 2014).
NCT02052505 2015 {unpublished data only}
- NCT02052505. Reducing inappropriate prescribing for psychiatric patients using nurse-led medication reviews. https://clinicaltrials.gov/ct2/show/NCT02052505 (first received 3 February 2014).
NCT02085837 2014 {unpublished data only}
- NCT02085837. Healthy transitions in late stage kidney disease. https://clinicaltrials.gov/ct2/show/NCT02085837 (first received 13 March 2014).
NCT02102503 2018 {unpublished data only}
- NCT02102503. Motivational interviewing and medication review in coronary heart disease. https://clinicaltrials.gov/ct2/show/NCT02102503 (first received 3 April 2014).
NCT02122965 2013 {unpublished data only}
- NCT02122965. The effect of medication review in high-risk emergency department patients. https://clinicaltrials.gov/ct2/show/NCT02122965 (first received 25 April 2014).
NCT02149940 2013 {published data only}
- NCT02149940. Feasibility of pharmaceutical interventions in elderly heart failure patients. https://clinicaltrials.gov/ct2/show/NCT02149940 (first received 29 May 2014).
NCT02165618 2014 {published data only}
- NCT02165618. Rationalisation of polypharmacy by the geriatric consultation team. https://www.clinicaltrials.gov/ct2/show/NCT02165618 (first received 17 June 2014).
NCT02202096 2016 {unpublished data only}
- NCT02202096. A pilot randomized trial of a comprehensive transitional care program for colorectal cancer patients. https://clinicaltrials.gov/ct2/show/NCT02202096 (first received 28 July 2014).
NCT02232126 2014 {unpublished data only}
- NCT02232126. Social work intervention focused on transitions (SWIFT). https://clinicaltrials.gov/show/NCT02232126 (first received 5 September 2014).
NCT02275572 2014 {unpublished data only}
- NCT02275572. Clinical and economical assessment of an intervention to reduce potentially inappropriate medication in polymedicated elderly patients (REMEI). https://clinicaltrials.gov/ct2/show/NCT02275572 (first received 27 October 2014).
NCT02317666 2015 {unpublished data only}
- NCT02317666. DBI - tool for medication reviews in older people. https://clinicaltrials.gov/ct2/show/NCT02317666 (first received 16 December 2014).
NCT02379455 2017 {unpublished data only}
- NCT02379455. Cooperation for improved pharmacotherapy in home-dwelling elderly people receiving polypharmacy- The COOP Study. https://clinicaltrials.gov/ct2/show/NCT02379455 (first received 5 March 2015).
NCT02598115 2016 {unpublished data only}
- NCT02598115. Impact of collaborative pharmaceutical care on hospital admission drug prescriptions for patients 65 years of age and older. https://clinicaltrials.gov/ct2/show/NCT02598115 (first received 5 November 2015).
NCT02664948 2015 {unpublished data only}
- NCT02664948. Early geriatric follow-up in older acute medical patients. https://clinicaltrials.gov/show/NCT02664948 (first received 27 January 2016).
NCT02712268 2013 {unpublished data only}
- NCT02712268. Intervention to improve prescribing in elderly patients. https://clinicaltrials.gov/ct2/show/NCT02712268 (first received 18 March 2016).
NCT02740764 2016 {unpublished data only}
- NCT02740764. Optimization of drug prescribing in an elderly population of geriatric consultations (OPTIM) [Optimization of drug prescribing in an elderly population of geriatric consultations and living at home]. https://clinicaltrials.gov/ct2/show/NCT02740764 (first received 15 April 2016).
NCT02871115 2019 {unpublished data only}
- NCT02871115. Pilot study of a pharmacy intervention for older adults with cancer. https://clinicaltrials.gov/ct2/show/NCT02871115 (first received 18 August 2016).
NCT02942927 2019 {unpublished data only}
- NCT02942927. Team approach to polypharmacy evaluation and reduction. https://clinicaltrials.gov/ct2/show/NCT02942927 (first received 24 October 2016).
NCT03123640 2017 {unpublished data only}
- NCT03123640. Drug safety in emergency patients - RTC on the effect of medication reconciliation and review on readmission rate. https://clinicaltrials.gov/show/NCT03123640 (first received 21 April 2017).
NCT03272607 2019 {unpublished data only}
- NCT03272607. Reducing post-discharge adverse drug events amongst the elderly: a multi-centre electronic deprescribing intervention. https://clinicaltrials.gov/ct2/show/NCT03272607 (first received 5 September 2017).
NCT03354845 2017 {unpublished data only}
- NCT03354845. Deprescribing of symptomatic medications in rehabilitative or subacute care patients. https://clinicaltrials.gov/show/NCT03354845 (first received 28 November 2017).
NCT03360305 2019 {unpublished data only}
- NCT03360305. GAPcare: the geriatric acute & post-acute care coordination program for fall prevention in the emergency department. https://clinicaltrials.gov/ct2/show/NCT03360305 (first received 4 December 2017).
NCT03369652 2017 {unpublished data only}
- NCT03369652. Telephone contact between hospital and general practitioner about medication review for older patients [Can telephone contact after discharge between geriatrician, clinical pharmacist and general practitioner about medication review in hospital improve the medication in older patients? A feasibility study]. https://clinicaltrials.gov/ct2/show/NCT03369652 (first received 12 December 2017).
NCT03369652 2018 {unpublished data only}
- NCT03369652. Telephone contact between hospital and general practitioner about medication review for older patients. https://clinicaltrials.gov/ct2/show/NCT03369652 (first received 12 December 2017).
NCT03445767 2018 {unpublished data only}
- NCT03445767. Structured polypharmacy management before elective non-cardiac surgery in frail and elderly people. https://clinicaltrials.gov/show/NCT03445767 (first received 26 February 2018).
NCT03557944 2019 {unpublished data only}
- NCT03557944. Team approach to polypharmacy evaluation and reduction (pharmacy). https://clinicaltrials.gov/ct2/show/NCT03557944 (first received 15 June 2018).
NCT03671629 2021 {unpublished data only}
- NCT03671629. Pharmacist intervention to reduce drug-related readmissions among the elderly. https://clinicaltrials.gov/ct2/show/NCT03671629 (first received 14 September 2018).
NCT03695081 2019 {unpublished data only}
- NCT03695081. Patient pathway pharmacist - optimal drug-related care. https://clinicaltrials.gov/ct2/show/NCT03695081 (first received 3 October 2018).
NCT03713112 2019 {unpublished data only}
- NCT03713112. Efficacy and feasibility of de-prescribing rounds in a Singapore rehabilitative hospital- a pilot randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT03713112 (first received 19 October 2018).
NCT03722017 2021 {unpublished data only}
- NCT03722017. Drug Reduction in Older Patients: the DROP trial. https://clinicaltrials.gov/ct2/show/NCT03722017 (first received 26 October 2018).
NCT03750500 2018 {unpublished data only}
- NCT03750500. Clinical impact of a digital home-based falls prevention program on elderly people. https://clinicaltrials.gov/ct2/show/NCT03750500 (first received 23 November 2018).
NCT03824106 2023 {published data only}
- NCT03824106. Frailty rehabilitation. https://clinicaltrials.gov/ct2/show/NCT03824106 (first received 31 January 2019).
NCT03902028 2021 {unpublished data only}
- NCT03902028. Optimized medico-pharmaceutical collaboration in the drug management of patients with heart failure. https://clinicaltrials.gov/ct2/show/NCT03902028 (first received 3 April 2019).
NCT03909035 2021 {unpublished data only}
- NCT03909035. A collaborative approach to medication reviews for older patients with polypharmacy. https://clinicaltrials.gov/ct2/show/NCT03909035 (first received 9 April 2019).
NCT03922529 2019 {unpublished data only}
- NCT03922529. Modified application of cardiac rehabilitation for older adults. https://clinicaltrials.gov/ct2/show/NCT03922529 (first received 22 April 2019).
NCT04077281 2019 {unpublished data only}
- NCT04077281. Improving medication prescribing-related outcomes for vulnerable elderly in transitions. https://clinicaltrials.gov/show/NCT04077281 (first received 4 September 2019).
NCT04082871 2019 {unpublished data only}
- NCT04082871. Medication review in women with depression and anxiety. https://clinicaltrials.gov/ct2/show/NCT04082871 (first received 9 September 2019).
NCT04087109 2020 {unpublished data only}
- NCT04087109. MedSafer e-care: an automated deprescribing solution (E-CARE Study). https://clinicaltrials.gov/ct2/show/NCT04087109 (first received 12 September 2019).
NCT04151797 2019 {unpublished data only}
- NCT04151797. Impact of a mobile geriatric team with a pharmacist on the optimisation of prescriptions in elderly inpatients (PHARMOG). https://clinicaltrials.gov/ct2/show/NCT04151797 (first received 5 November 2029).
NCT04188470 2019 {unpublished data only}
- NCT04188470. Optimising drug therapy in polymedicated patients according to the person-centered care model [Open randomised multicentric controlled trial to evaluate the impact of an intervention to improve drug appropriateness in polymedicated patients according to the person-centered care model by a multidisciplinary team at primary care]. https://clinicaltrials.gov/ct2/show/NCT04188470 (first received 6 December 2019).
NCT04228900 2020 {unpublished data only}
- NCT04228900. Malnutrition in older adults: an intervention based on medication review and individual nutritional plan [Nutrition and medication management in home-dwelling older adults. Study II: malnutrition in older adults: an intervention based on medication review and individual nutritional plan]. https://clinicaltrials.gov/ct2/show/NCT04228900 (first received 14 January 2020).
NCT04251520 2020 {unpublished data only}
- NCT04251520. The role of the pharmacist and pharmacogenomics in the care of seriously ill patients. https://clinicaltrials.gov/ct2/show/record/NCT04251520 (first received 5 February 2020).
NCT04278794 2020 {unpublished data only}
- NCT04278794. Optimizing hospital-to-home transitions for older adults with stroke and multimorbidity [Optimizing hospital-to-home transitions for older adults with stroke and multimorbidity: a pragmatic trial of an outpatient-based virtual transitional care intervention]. https://clinicaltrials.gov/ct2/show/record/NCT04278794 (first received 20 February 2020).
NCT04360746 2020 {unpublished data only}
- NCT04360746. The influence of clinical pharmacist on the quality of drug prescribing and rehabilitation outcomes in post-acute hip fractured patients. https://clinicaltrials.gov/ct2/show/NCT04360746 (first received 24 April 2020).
NCT04391218 2020 {unpublished data only}
- NCT04391218. A multidisciplinary intervention including a clinical decision support system and an app for drug therapy management in older patients (FARMA-CANP). https://clinicaltrials.gov/ct2/show/NCT04391218 (first received 18 May 2020).
NCT04417400 2020 {unpublished data only}
- NCT04417400. Evaluation of medicines use review in Slovenia (SLOPUZ) [Benefits of medicines use review service in Slovenia: a randomized controlled trial]. https://clinicaltrials.gov/ct2/show/NCT04417400 (first received 4 June 2020).
NCT04553107 2020 {unpublished data only}
- NCT04553107. Reducing costs by deprescribing medications [Reducing healthcare costs in older adults by deprescribing unnecessary, harmful, and costly medications]. https://clinicaltrials.gov/ct2/show/NCT04553107 (first received 17 September 2020).
NCT04556786 2020 {unpublished data only}
- NCT04556786. A pharmacist-led transitions of care program [The impact of a pharmacist-led transitional care program on health outcomes of uninsured populations]. https://clinicaltrials.gov/ct2/show/NCT04556786 (first received 21 September 2020).
NCT04722588 2021 {unpublished data only}
- NCT04722588. Integrating the clinical pharmacists into emergency department teams (ED-PHARM) [Integrating the clinical pharmacist into the emergency department interdisciplinary team: a study protocol for a multicentre trial applying a non-randomized stepped wedge study design]. https://clinicaltrials.gov/ct2/show/NCT04722588 (first received 25 January 2021). [DOI] [PMC free article] [PubMed]
NCT04796701 2021 {unpublished data only}
- NCT04796701. Effect of a transitional care intervention [Effect of a transitional care intervention from hospital to home on readmissions among older medical patients: a quasi-experimental study]. https://clinicaltrials.gov/ct2/show/record/NCT04796701 (first received 15 March 2021).
Nymoen 2019 {published data only}
- Nymoen LD, Solsvik IK, Flatebo TE, Nilsen M, Godo A, Oie E, et al. Drug related hospital admissions: identifying highrisk patients. International Journal of Clinical Pharmacy 2019;41(1):304-5. [Google Scholar]
O'Brien 2018 {published data only}
- O'Brien G L, O'Mahony D, Gillespie P, Mulcahy M, Walshe V, O'Connor M N, et al. Cost- effectiveness analysis of a physician-implemented medication screening tool in older hospitalised patients in Ireland. Drugs & Aging 2018;35(8):751-62. [DOI] [PubMed] [Google Scholar]
O'Connor 2016 {published data only}
- NCT01467128. Adverse drug event (ADE) incidence in older patients following hospital admission and pharmacist review to older persons' prescriptions and its' effect on ADE reduction in hospital: a randomised controlled trial. www.clinicaltrials.gov/show/NCT01467128 (first received 8 November 2011).
- O'Connor MN, O'Sullivan D, Gallagher PF, Eustace J, Byrne S, O'Mahony D. Prevention of hospital-acquired adverse drug reactions in older people using screening tool of older persons' prescriptions and screening tool to alert to right treatment criteria: a cluster randomized controlled trial. Journal of the American Geriatrics Society 2016;64(8):1558-66. [DOI] [PubMed] [Google Scholar]
O'Sullivan 2016 {published data only}
- O'Sullivan D, O'Mahony D, O'Connor MN, Gallagher P, Gallagher J, Cullinan S, et al. Prevention of adverse drug reactions in hospitalised older patients using a software-supported structured pharmacist intervention: a cluster randomised controlled trial. Drugs & Aging 2016;33(1):63-73. [DOI] [PubMed] [Google Scholar]
Okere 2015 {published data only}
- Okere AN, Renier CM, Tomsche JJ. Evaluation of the influence of a pharmacist-led patient-centered medication therapy management and reconciliation service in collaboration with emergency department physicians. Journal of Managed Care & Specialty Pharmacy 2015;21(4):298-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pazan 2017 {published data only}
- Pazan F, Burkhardt H, Frohnhofen H, Weiss C, Throm C, Kuhn-Thiel A, et al. Optimization of drug treatment by using FORTA: results from the FORTAMED study. European Geriatric Medicine 2017;8:S221. [Google Scholar]
Pazan 2018 {published data only}
- Pazan F, Burkhardt H, Frohnhofen H, Weiss C, Throm C, Kuhn-Thiel A, et al. Changes in prescription patterns in older hospitalized patients: the impact of FORTA on disease-related over- and under-treatments. European Journal of Clinical Pharmacology 2018;74(3):339-47. [DOI] [PubMed] [Google Scholar]
Pazan 2019 {published data only}
- Pazan F, Burkhardt H, Frohnhofen H, Weiss C, Throm C, Kuhn-Thiel A, et al. Higher Fit-fOR-The-Aged (FORTA) scores comprising medication errors are associated with impaired cognitive and physical function tests in the VALFORTA trial. Drugs & Aging 2019;36(3):269-77. [DOI] [PubMed] [Google Scholar]
Pfister 2017 {published data only}
- Pfister B, Jonsson J, Gustafsson M. Drug-related problems and medication reviews among old people with dementia. BMC Pharmacology & Toxicology 2017;18(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phatak 2016 {published data only}
- Phatak A, Prusi R, Ward B, Hansen LO, Williams MV, Vetter E, et al. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and postdischarge call-backs (IPITCH Study). Journal of Hospital Medicine 2016;11(1):39-44. [DOI] [PubMed] [Google Scholar]
Pope 2011 {published data only}
- Pope G, Wall N, Peters CM, O'Connor M, Saunders J, O'Sullivan C, et al. Specialist medication review does not benefit short-term outcomes and net costs in continuing-care patients. Age & Ageing 2011;40(3):307–12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rainville 1999 {published data only}
- Rainville EC. Impact of pharmacist interventions on hospital readmissions for heart failure. American Journal of Health-System Pharmacy 1999;56(13):1339-42. [PMID: ] [PubMed] [Google Scholar]
Sakthong 2018 {published data only}
- Sakthong P, Sangthonganotai T. A randomized controlled trial of the impact of pharmacist-led patient-centered pharmaceutical care on patients' medicine therapy-related quality of life. Research in Social & Administrative Pharmacy 2018;14(4):332-9. [DOI] [PubMed] [Google Scholar]
Saltvedt 2005 {published data only}
- Saltvedt I, Spigset O, Ruths S, Fayers P, Kaasa S, Sletvold O. Patterns of drug prescription in a geriatric evaluation and management unit as compared with the general medical wards: a randomised study. European Journal of Clinical Pharmacology 2005;61(12):921-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Santolaya‐Perrin 2016 {published data only}
- Santolaya-Perrin R, Jimenez-Diaz G, Galan-Ramos N, Moreno Carvajal MT, Rodriguez-Camacho JM, Sierra-Sanchez JF, et al. A randomised controlled trial on the efficacy of a multidisciplinary health care team on morbidity and mortality of elderly patients attending the Emergency Department. Study design and preliminary results. Farmacia Hospitalaria 2016;40(5):371-84. [DOI] [PubMed] [Google Scholar]
Santolaya‐Perrin 2019 {published data only}
- Santolaya-Perrin R, Calderon-Hernanz B, Jimenez-Diaz G, Galan-Ramos N, Moreno-Carvajal MT, Rodriguez-Camacho JM, et al. The efficacy of a medication review programme conducted in an emergency department. International Journal of Clinical Pharmacy 2019;41(3):757-66. [DOI] [PubMed] [Google Scholar]
Schmader 1997 {published data only}
- Schmader KE, Hanlon JT, Landsman PB, Samsa GP, Lewis IK, Weinberger M. Inappropriate prescribing and health outcomes in elderly veteran outpatients. Annals of Pharmacotherapy 1997;31(5):529-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schmader 2004 {published data only}
- Schmader KE, Hanlon JT, Pieper CF, Sloane R, Ruby CM, Twersky J, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. American Journal of Medicine 2004;116(6):394-401. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sjoberg 2013 {published data only}
- Sjoberg C, Wallerstedt SM. Effects of medication reviews performed by a physician on treatment with fracture-preventing and fall-risk-increasing drugs in older adults with hip fracture - a randomized controlled study. Journal of the American Geriatrics Society 2013;61(9):1464-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
SLCTR//011 2018 {unpublished data only}
- SLCTR//011. Clinical pharmacy services to improve management outcomes of chronic kidney disease of uncertain etiology patients undergoing hemodialysis. http://www.who.int/trialsearch/Trial2.aspx?TrialID=SLCTR/2018/011 2018.
SLCTR//029 2013 {unpublished data only}
- SLCTR//029. Impact of a ward based clinical pharmacist on improving the quality use of medicines in patients with chronic non communicable diseases, compared to standard practice in a Sri Lankan teaching hospital. http://www.who.int/trialsearch/Trial2.aspx?TrialID=SLCTR/2013/029 2013.
Smith 1996 {published data only}
- Smith DM, Cox MR, Brizendine EJ, Hui SL, Freedman JA, Martin DK, et al. An intervention on discharge polypharmacy. Journal of the American Geriatrics Society 1996;44(4):416-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Spinewine 2007 {published data only (unpublished sought but not used)}
- Spinewine A, Swine C, Dhillon S, Lambert P, Nachega JB, Wilmotte L, et al. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized, controlled trial. Journal of the American Geriatrics Society 2007;55(5):658-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stowasser 2002 {published data only}
- Stowasser DA, Collins DM, Stowasser M. A randomised controlled trial of medication liaison services - patient outcomes. Journal of Pharmacy Practice and Research 2002;32:133-40. [Google Scholar]
Tallon 2016 {published data only}
- Tallon M, Barragry J, Allen A, Breslin N, Deasy E, Moloney E, et al. Impact of the collaborative Pharmaceutical Care at Tallaght Hospital (PACT) model on medication appropriateness of older patients. European Journal of Hospital Pharmacy: Science & Practice 2016;23(1):16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2018 {published data only}
- Tan PJ, Khoo EM, Chinna K, Saedon NI, Zakaria MI, Ahmad Zahedi AZ, et al. Individually-tailored multifactorial intervention to reduce falls in the Malaysian Falls Assessment and Intervention Trial (MyFAIT): a randomized controlled trial. PLOS One 2018;13(8):e0199219. [DOI] [PMC free article] [PubMed] [Google Scholar]
TCTR20181104004 2018 {unpublished data only}
- TCTR20181104004. Effects of pharmaceutical care on medication appropriateness in elderly patients treated at Uttaradit Hospital. http://www.thaiclinicaltrials.org/show/TCTR20181104004 (first received 4 November 2018).
Van Der Linden 2013 {published data only}
- Van Der Linden L, Decoutere L, Flamaing J, Spriet I, Willems L, Milisen K, et al. Reduction of polypharmacy in geriatric inpatients using the RASP list: a cluster-randomized controlled trial. European Geriatric Medicine 2013;4:S183. [Google Scholar]
Van Der Linden 2014 {published data only}
- Van Der Linden L. Reduction of inappropriate prescribing in older persons using the RASP list: a cluster-randomised controlled trial. European Journal of Hospital Pharmacy 2014;21:A212. [Google Scholar]
Van der Linden 2017 {published data only}
- Van der Linden L, Decoutere L, Walgraeve K, Milisen K, Flamaing J, Spriet I, et al. Combined use of the rationalization of home medication by an adjusted STOPP in older patients (RASP) list and a pharmacist-led medication review in very old inpatients: impact on quality of prescribing and clinical outcome. Drugs & Aging 2017;34(2):123-33. [DOI] [PubMed] [Google Scholar]
Verbeek 2016 {published data only}
- Verbeek A, Oonk N, Munster E, Movig K, Ter Huurne K, Nijmeijer H, et al. The effect of a structured medication review on quality of life in patients with Parkinson's disease. An interim analysis. Movement Disorders 2016;31:S170-1. [Google Scholar]
Walker 2009 {published data only}
- Walker PC, Bernstein SJ, Tucker Jones JN, Piersma J, Kim HW, Regal RE, et al. Impact of a pharmacist-facilitated hospital discharge program: a quasi-experimental study. Archives of Internal Medicine 2009;169(21):2003-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wehling 2015 {published data only}
- Wehling M, Kuhn-Thiel A, Throm C, Burkhardt H, Frohnhofen H, Pazan F, et al. Clinical validation of the FORTA (Fit fOR The Aged) List in a prospective randomized controlled clinical study. European Geriatric Medicine 2015;6:S146. [Google Scholar]
Wehling 2016 {published data only}
- Wehling M, Burkhardt H, Kuhn-Thiel A, Pazan F, Throm C, Weiss C, et al. VALFORTA: a randomised trial to validate the FORTA (Fit fOR The Aged) classification. Age & Ageing 2016;45(2):262-7. [DOI] [PubMed] [Google Scholar]
Williams 2012 {published data only}
- Williams LM, Hauser D, De Luca A. The medication REACH program. Pharmacotherapy 2012;32(10):e295. [Google Scholar]
Zhao 2015 {published data only}
- Zhao S, Zhao H, Du S, Qin Y. The impact of clinical pharmacist support on patients receiving multi-drug therapy for coronary heart disease in China: a long-term follow-up study. European Journal of Hospital Pharmacy 2015;22(6):323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2015a {published data only}
- Zhao S J, Zhao HW, Du S, Qin Y H. The impact of clinical pharmacist support on patients receiving multi-drug therapy for coronary heart disease in China. Indian Journal of Pharmaceutical Sciences 2015;77(3):306-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12618000979257 2018 {unpublished data only}
- ACTRN12618000979257. The Australian Team Approach to Polypharmacy Evaluation and Reduction (AusTAPER) study for older hospital inpatients. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12618000979257 (first received 12 June 2018).
Andersen 2021 {published data only}
- Andersen AL, Houlind MB, Nielsen RL, Jørgensen LM, Treldal C, Damgaard M, et al. Optimization of Nutrition And Medication (OptiNAM) for acutely admitted older patients: protocol for a randomized single-blinded controlled trial. Trials 2021;22(2):616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03741283. Optimisation of nutrition and medication for acutely admitted older medical patients. https://clinicaltrials.gov/show/NCT03741283 (first received 14 November 2018).
ChiCTR1800017706 2018 {unpublished data only}
- ChiCTR1800017706. Elderly patients multi-drug management: a multicenter randomized controlled tiral. https://www.chictr.org.cn/showproj.aspx?proj=28852 (first received 10 August 2018).
Diplock 2017 {published data only}
- ACTRN12615000808549. Alice Springs Hospital Readmission Prevention Project: analysis of a multi-dimensional transitional care program in patients frequently admitted to hospital. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365384&isReview=true (first received 4 August 2015).
- Diplock G, Ward J, Stewart S, Scuffham P, Stewart P, Reeve C, et al. The Alice Springs Hospital Readmission Prevention Project (ASHRAPP): a randomised control trial. BMC Health Services Research 2017;17(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grischott 2018 {published and unpublished data}
- Grischott T, Zechmann S, Rachamin Y, Markun S, Chmiel C, Senn O, et al. Improving inappropriate medication and information transfer at hospital discharge: study protocol for a cluster RCT. Implementation Science 2018;13(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN18427377. Hospital discharge study. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN18427377 2018.
- Rachamin Y, Grischott T, Neuner-Jehle S. Implementation of a complex intervention to improve hospital discharge: process evaluation of a cluster randomised controlled trial. BMJ Open 2021;11(5):e049872. [DOI: 10.1136/bmjopen-2021-049872] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ie 2020 {published data only}
- Ie K, Hirose M, Sakai T, Motohashi I, Aihara M, Otsuki T, et al. Protocol of a randomised controlled trial on the efficacy of medication optimisation in elderly inpatients: medication optimisation protocol efficacy for geriatric inpatients (MPEG) trial. BMJ Open 2020;10(10):e041125. [DOI: 10.1136/bmjopen-2020-041125] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johansen 2018 {published data only}
- Johansen JS, Halvorsen KH, Havnes K, Wetting HL, Svendsen K, Garcia BH. Intervention fidelity and process outcomes of the IMMENSE study, a pharmacist-led interdisciplinary intervention to improve medication safety in older hospitalized patients. Journal of Clinical Pharmacy and Therapeutics 2021 Dec 21 [Epub ahead of print]. [DOI: 10.1111/jcpt.13581] [DOI] [PubMed]
- Johansen JS, Havnes K, Halvorsen KH, Haustreis S, Skaue LW, Kamycheva E, et al. Interdisciplinary collaboration across secondary and primary care to improve medication safety in the elderly (IMMENSE study): study protocol for a randomised controlled trial. BMJ Open 2018;8(1):e020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
JPRN‐UMIN000035265 2018 {unpublished data only}
- JPRN-UMIN000035265. Efficacy of medication optimization protocol for older inpatients: a randomized controlled trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000035265 2018.
Kogamine 2018 {published and unpublished data}
- JPRN-UMIN000029404. A single-center, prospective, non-blinded, randomized, 12-month, parallel-group, superiority study to compare the efficacy of pharmacist intervention versus usual care for older patients hospitalized in the orthopedic ward. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000029404 2017. [DOI] [PMC free article] [PubMed]
- Komagamine J, Sugawara K, Kaminaga M, Tatsumi S. Study protocol for a single-centre, prospective, non-blinded, randomised, 12-month, parallel-group superiority study to compare the efficacy of pharmacist intervention versus usual care for elderly patients hospitalised in orthopaedic wards. BMJ Open 2018;8(7):e021924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loffler 2014 {published and unpublished data}
- ISRCTN42003273. Polypharmacy reduction in patients treated for chronic diseases. https://www.isrctn.com/ISRCTN42003273 (first received 30 January 2014).
- Loffler C, Drewelow E, Paschka SD, Frankenstein M, Eger J, Jatsch L, et al. Optimizing polypharmacy among elderly hospital patients with chronic diseases - study protocol of the cluster randomized controlled POLITE-RCT trial. Implementation Science 2014;9:151. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Logan 2020 {published data only}
- Logan B, Viecelli AK, Johnson DW, Tong A, Comans T, Janda M, et al. The goal trial protocol: comprehensive geriatric assessment for frail older people with chronic kidney disease to increase attainment of patient-identified goals-a cluster randomised controlled trial. Nephrology 2020;25(Suppl 3):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04538157. Comprehensive geriatric assessment for frail older people with chronic kidney disease - the GOAL trial [Comprehensive geriatric assessment for frail older people with chronic kidney disease to increase attainment of patient-identified goals - a cluster randomised controlled trial- the GOAL trial]. https://clinicaltrials.gov/ct2/show/NCT04538157 (first received 3 September 2020). [DOI] [PMC free article] [PubMed]
NCT03123640 2018 {unpublished data only}
- NCT03123640. Improving drug safety in emergency patients -a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT03123640 (first received 21 April 2017).
NCT03156348 2017 {unpublished data only}
- NCT03156348. Impact of clinical pharmacist on adverse drug events in older adults. https://clinicaltrials.gov/show/NCT03156348 (first received 17 May 2017).
NCT03393299 2018 {unpublished data only}
- NCT03393299. Impact of the systematic use of the criteria STOPP/START in short stay geriatric. https://clinicaltrials.gov/ct2/show/NCT03393299 (first received 8 January 2018).
NCT03666793 2018 {unpublished data only}
- NCT03666793. Comprehensive management of drug prescriptions throughout the elderly person's hospital care. https://clinicaltrials.gov/show/NCT03666793 (first received 12 September 2018).
NCT03827031 {unpublished data only}
- NCT03827031. Impact of multidisciplinary medication assessment review in surgery departments [Implementation and impact of multidisciplinary medication review in surgery departments on medication management of elderly patients]. https://clinicaltrials.gov/ct2/show/record/NCT03827031 (first received 1 February 2019).
NCT04028583 2019 {unpublished data only}
- NCT04028583. Tool for inappropriate prescription evaluation: the TaIPE study. https://clinicaltrials.gov/show/NCT04028583 (first received 22 July 2019).
NCT04617340 {published data only}
- NCT04617340. Effect of a trAnSitional Pharmacist Intervention in geRiatric Inpatients on Hospitals Visits After dischargE [The Effect of a trAnSitional Pharmacist Intervention in geRiatric Inpatients on Hospitals Visits After dischargE (ASPIRE): a randomized controlled trial]. https://clinicaltrials.gov/ct2/show/record/NCT04617340 (first received 5 November 2020). [DOI] [PubMed]
Pevnick 2021 {published data only}
- NCT04071951. Pharmacist intervention to reduce hospital readmissions. https://clinicaltrials.gov/ct2/show/NCT04071951 (first received 28 August 2019).
- Pevnick JM, Keller MS, Kennelty KA, Nuckols TK, Ko EM, Amer K, et al. The Pharmacist Discharge Care (PHARM-DC) study: a multicenter RCT of pharmacist-directed transitional care to reduce post-hospitalization utilization. Contemporary Clinical Trials 2021;106:106419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranaudin 2017 {published and unpublished data}
- NCT02734017. Impact of a medication review on hospital readmission: (ConcReHosp). https://clinicaltrials.gov/ct2/show/NCT02734017 (first received 12 April 2016).
- Renaudin P, Baumstarck K, Daumas A, Esteve MA, Gayet S, Auquier P, et al. Impact of a pharmacist-led medication review on hospital readmission in a pediatric and elderly population: study protocol for a randomized open-label controlled trial. Trials 2017;18(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
RBR‐42nd7q {published data only}
- RBR-42nd7q. Medical record model oriented to problems with the use of medications in patients with heart failure admitted to the intensive care unit. https://ensaiosclinicos.gov.br/rg/RBR-42nd7q (first received 13 March 2020).
SLCTR/2019/039 {published data only}
- SLCTR/2019/039. Assessment of the impact of a clinical pharmacist compared to standard care in improving quality use of medicines, patient adherence and reducing hospital readmissions in patients diagnosed with acute coronary syndrome [A randomised control trial to evaluate the impact of a clinical pharmacist on optimizing the quality use of medicines according to the Acute Coronary Syndrome (ACS) secondary prevention guidelines and medication adherence following discharge in patients with ACS]. https://slctr.lk/trials/slctr-2019-039 (first received 10 November 2019).
Vasilevskis 2019 {published and unpublished data}
- NCT02979353. A randomized controlled trial to deprescribe for older patients with polypharmacy. https://clinicaltrials.gov/ct2/show/NCT02979353 (first received 1 December 2016).
- Vasilevskis EE, Shah AS, Hollingsworth EK, Shotwell MS, Mixon AS, Bell SP, et al. A patient-centered deprescribing intervention for hospitalized older patients with polypharmacy: rationale and design of the Shed-MEDS randomized controlled trial. BMC Health Services Research 2019;19(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
American Geriatrics Society 2019
- By the 2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers criteria for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society 2019;67(4):674-94. [DOI: 10.1111/jgs.15767] [DOI] [PubMed] [Google Scholar]
Beers 1997
- Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Archives of Internal Medicine 1997;157(14):1531-6. [PubMed] [Google Scholar]
Bourgeois 2010
- Bourgeois FT, Shannon MW, Valim C, Mandl KD. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiology and Drug Safety 2010;19(9):901-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2011
- Centers for Disease Control and Prevention. Health, United States, 2011. Atlanta: Centers for Disease Control and Prevention, 2012. [Google Scholar]
Christensen 2009
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet 2009;374(9696):1196-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Classen 1997
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997;277(4):301-6. [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988. [Google Scholar]
Cooper 2015
- Cooper JA, Cadogan CA, Patterson SM, Kerse N, Bradley MC, Ryan C, et al. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open 2015;5(12):e009235. [DOI: 10.1136/bmjopen-2015-009235] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 2022. Available at covidence.org.
Dautzenberg 2021
- Dautzenberg L, Bretagne L, Koek HL, Tsokani S, Zevgiti S, Rodondi N, et al. Medication review interventions to reduce hospital readmissions in older people. Journal of the American Geriatrics Society 2021;69(6):1646-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Ebbesen 2001
- Ebbesen J, Buajordet I, Erikssen J, Brors O, Hilberg T, Svaar H, et al. Drug-related deaths in a department of internal medicine. Archives of Internal Medicine 2001;161(19):2317-23. [DOI] [PubMed] [Google Scholar]
El Desoky 2007
- El Desoky ES. Pharmacokinetic-pharmacodynamic crisis in the elderly. American Journal of Therapeutics 2007;14(5):488-98. [DOI] [PubMed] [Google Scholar]
EPOC 2017
- Cochrane Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. https://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/suggested_risk_of_bias_criteria_for_epoc_reviews.pdf (accessed January 2020).
European Communities 2006
- European Communities. Population statistics 2006. www.epp.eurostat.ec.europa.eu/cache/ITY_OFFPUB/KS-EH-06-001/EN/KS-EH-06-001-EN.PDF (accessed 1 April 2012).
Gallagher 2008a
- Gallagher P, Ryan C, Byrne S, Kennedy J, O'Mahony D. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. International Journal of Clinical Pharmacology and Therapeutics 2008;46(2):72-83. [DOI] [PubMed] [Google Scholar]
Gallagher 2008b
- Gallagher P, O'Mahony D. STOPP (Screening Tool of Older Persons' potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers' criteria. Age & Ageing 2008;37(6):673-9. [DOI] [PubMed] [Google Scholar]
Gnjidic 2012
- Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. Journal of Clinical Epidemiology 2012;65(9):989-95. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al, for the GRADE Working Group. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallas 1996
- Hallas J. Drug related hospital admissions in subspecialities of internal medicine. Danish Medical Bulletin 1996;43(2):141-55. [PubMed] [Google Scholar]
Hanlon 1992
- Hanlon JT, Schmader KE, Samsa GP, Weinberger M, Uttech KM, Lewis IK, et al. A method for assessing drug therapy appropriateness. Journal of Clinical Epidemiology 1992;45(10):1045-51. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2022
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Hill‐Taylor 2016
- Hill-Taylor B, Walsh KA, Stewart S, Hayden J, Byrne S, Sketris IS. Effectiveness of the STOPP/START (Screening Tool of Older Persons' potentially inappropriate Prescriptions/Screening Tool to Alert doctors to the Right Treatment) criteria: systematic review and meta-analysis of randomized controlled studies. Journal of Clinical Pharmacy and Therapeutics 2016;41(2):158-69. [DOI: 10.1111/jcpt.12372] [DOI] [PubMed] [Google Scholar]
Hohl 2015
- Hohl CM, Wickham ME, Sobolev B, Perry JJ, Sivilotti ML, Garrison S, et al. The effect of early in-hospital medication review on health outcomes: a systematic review. British Journal of Clinical Pharmacology 2015;80(1):51-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holt 2010
- Holt S, Schmiedl S, Thurmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Deutsches Arzteblatt international 2010;107(31-2):543-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huiskes 2017
- Huiskes VJ, Burger DM, den Ende CH, den Bemt BJ. Effectiveness of medication review: a systematic review and meta-analysis of randomized controlled trials. BMC Family Practice 2017;18(1):5. [DOI: 10.1186/s12875-016-0577-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kongkaew 2008
- Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Annals of Pharmacotherapy 2008;42(7):1017-25. [DOI] [PubMed] [Google Scholar]
Laroche 2007a
- Laroche ML, Charmes JP, Merle L. Potentially inappropriate medications in the elderly: a French consensus panel list. European Journal of Clinical Pharmacology 2007;63(8):725-31. [DOI] [PubMed] [Google Scholar]
Laroche 2007b
- Laroche ML, Charmes JP, Nouaille Y, Picard N, Merle L. Is inappropriate medication use a major cause of adverse drug reactions in the elderly? British Journal of Clinical Pharmacology 2007;63(2):177-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Lozano‐Montoya 2015
- Lozano-Montoya I, Velez-Diaz-Pallares M, Delgado-Silveira E, Montero-Errasquin B, Cruz Jentoft AJ. Potentially inappropriate prescribing detected by STOPP-START criteria: are they really inappropriate? Age & Ageing 2015;44(5):861-6. [DOI] [PubMed] [Google Scholar]
Lund 2010
- Lund BC, Carnahan RM, Egge JA, Chrischilles EA, Kaboli PJ. Inappropriate prescribing predicts adverse drug events in older adults. Annals of Pharmacotherapy 2010;44(6):957-63. [DOI] [PubMed] [Google Scholar]
Mangoni 2004
- Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. British Journal of Clinical Pharmacology 2004;57(1):6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Masnoon 2017
- Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatrics 2017;17(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
McLeod 1997
- McLeod PJ, Huang AR, Tamblyn RM, Gayton DC. Defining inappropriate practices in prescribing for elderly people: a national consensus panel. CMAJ 1997;156(3):385-91. [PMC free article] [PubMed] [Google Scholar]
Naugler 2000
- Naugler CT, Brymer C, Stolee P, Arcese ZA. Development and validation of an improving prescribing in the elderly tool. Canadian Journal of Clinical Pharmacology 2000;7(2):103-7. [PubMed] [Google Scholar]
O'Mahony 2015
- O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age & Ageing 2015;44(2):213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Obreli‐Neto 2012
- Obreli-Neto PR, Nobili A, Oliveira Baldoni A, Guidoni CM, Lyra Junior DP, Pilger D, et al. Adverse drug reactions caused by drug-drug interactions in elderly outpatients: a prospective cohort study. European Journal of Clinical Pharmacology 2012;68(12):1667-76. [DOI] [PubMed] [Google Scholar]
Pasina 2014
- Pasina L, Brucato AL, Falcone C, Cucchi E, Bresciani A, Sottocorno M, et al. Medication non-adherence among elderly patients newly discharged and receiving polypharmacy. Drugs & Aging 2014;31(4):283-9. [DOI] [PubMed] [Google Scholar]
Pefoyo 2015
- Pefoyo AJ, Bronskill SE, Gruneir A, Calzavara A, Thavorn K, Petrosyan Y, et al. The increasing burden and complexity of multimorbidity. BMC Public Health 2015;15:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rankin 2018
- Rankin A, Cadogan CA, Patterson SM, Kerse N, Cardwell CR, Bradley MC, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD008165. [DOI: 10.1002/14651858.CD008165.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Renaudin 2016
- Renaudin P, Boyer L, Esteve MA, Bertault-Peres P, Auquier P, Honore S. Do pharmacist-led medication reviews in hospitals help reduce hospital readmissions? A systematic review and meta-analysis. British Journal of Clinical Pharmacology 2016;82(6):1660-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Review Manager (RevMan). Version 5.4.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, September 2020.
Rothschild 2000
- Rothschild JM, Bates DW, Leape LL. Preventable medical injuries in older patients. Archives of Internal Medicine 2000;160(18):2717-28. [DOI] [PubMed] [Google Scholar]
Samsa 1994
- Samsa GP, Hanlon JT, Schmader KE, Weinberger M, Clipp EC, Uttech KM, et al. A summated score for the medication appropriateness index: development and assessment of clinimetric properties including content validity. Journal of Clinical Epidemiology 1994;47(8):891-6. [DOI] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE Working Group. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI: doi: 10.1016/j.jclinepi.2019.10.014.] [DOI] [PubMed] [Google Scholar]
Schneeweiss 2002
- Schneeweiss S, Hasford J, Gottler M, Hoffmann A, Riethling AK, Avorn J. Admissions caused by adverse drug events to internal medicine and emergency departments in hospitals: a longitudinal population-based study. European Journal of Clinical Pharmacology 2002;58(4):285-91. [DOI] [PubMed] [Google Scholar]
Spinewine 2007b
- Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet 2007;370(9582):173-84. [DOI] [PubMed] [Google Scholar]
Steinman 2006
- Steinman MA, Landefeld CS, Rosenthal GE, Berthenthal D, Sen S, Kaboli PJ. Polypharmacy and prescribing quality in older people. Journal of the American Geriatrics Society 2006;54(10):1516-23. [DOI] [PubMed] [Google Scholar]
WHO 2019
- World Health Organization. Medication Safety in Polypharmacy: technical report. Geneva. World Health Organization 20 June 2019:https://apps.who.int/iris/handle/10665/325454. License: CC BY-NC-SA 3.0 IGO.
Zed 2008
- Zed PJ, Abu-Laban RB, Balen RM, Loewen PS, Hohl CM, Brubacher JR, et al. Incidence, severity and preventability of medication-related visits to the emergency department: a prospective study. Canadian Medical Association Journal 2008;178(12):1563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziere 2006
- Ziere G, Dieleman JP, Hofman A, Pols HA, Cammen TJ, Stricker BH. Polypharmacy and falls in the middle age and elderly population. British Journal of Clinical Pharmacology 2006;61(2):218-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Christensen 2011
- Christensen M, Lundh A. Medication review of hospitalized patients to prevent morbidity and mortality. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No: CD008986. [DOI: 10.1002/14651858.CD008986] [DOI] [Google Scholar]
Christensen 2013
- Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database of Systematic Reviews 2013, Issue 2. Art. No: CD008986. [DOI: 10.1002/14651858.CD008986.pub2] [DOI] [PubMed] [Google Scholar]
Christensen 2016
- Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD008986. [DOI: 10.1002/14651858.CD008986.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


