Abstract
Background:
Infancy perfluoroalkyl substances (PFAS) exposure from breastfeeding is partially determined by the transfer efficiencies (TEs) of PFAS from maternal serum into breast milk. However, to our knowledge there are no studies of such TEs in highly exposed populations.
Objectives:
We estimated the TEs of PFAS from maternal serum into colostrum and breast milk in a cohort of women with a wide range of PFAS exposures.
Methods:
The Ronneby Mother–Child Cohort was established in 2015 after PFAS contamination was discovered in the public drinking water of Ronneby, Sweden. We measured seven PFAS in matched samples of maternal serum at delivery and colostrum and breast milk. We calculated the TE (in percentage) as the ratio of PFAS in colostrum or breast milk to serum multiplied by 100 and evaluated whether TEs varied by PFAS, lactation stage, or exposure level using a series of linear mixed-effects models with a random intercept for each woman.
Results:
This study included 126 mothers. PFAS associated with firefighting foams [i.e., perfluorohexane sulfonic acid (PFHxS) and perfluorooctane sulfonic acid (PFOS)] were substantially elevated in the serum, colostrum, and breast milk samples of highly exposed women in the cohort and showed strong correlation. PFHxS and PFOS also contributed the largest fraction of total PFAS on average in colostrum and breast milk. Median TEs varied from 0.9% to 4.3% and were higher for perfluoroalkyl carboxylic acids, including perfluorooctanoic acid, than perfluoroalkane sulfonic acids, including PFHxS and PFOS. TEs varied by exposure level, but there was not a consistent pattern in this variation.
Discussion:
PFAS concentrations in the colostrum and breast milk of highly exposed women were higher than the concentrations in low-exposed women, and TEs were of a similar magnitude across exposure categories. This implies that breastfeeding may be an important route of PFAS exposure for breastfeeding infants with highly exposed mothers, although the relative contribution of breastfeeding vs. prenatal transplacental transfer remains to be clarified. https://doi.org/10.1289/EHP11292
Introduction
Perfluoroalkyl substances (PFAS) are a diverse class of synthetic chemicals containing an aliphatic fluorinated carbon chain.1 PFAS have unique chemical properties, including chemical and thermal stability and water and oil repellency.2 They have been widely used since the 1950s in various industrial and commercial applications, such as personal care products and cosmetics, firefighting foams, textile treatments, food contact materials, medical devices, and membranes used by the chemical and energy sectors.3 However, the same properties that make PFAS desirable in so many applications also make them extremely persistent in the environment and bioaccumulative. Widespread human exposure through diet and other pathways, combined with the long biological half-lives of some PFAS, has led to detectable levels in most human blood samples.2,4,5
Although governmental regulation on the manufacture and use of certain PFAS is increasing,6,7 the extreme environmental persistence of PFAS means that existing contamination will remain a concern. This is particularly relevant for populations living near contaminated sites, where environmental PFAS concentrations and human exposures are typically highest.4 These sites include fluorochemical manufacturing facilities, manufacturing facilities where PFAS are used, airports and military bases that use aqueous film forming firefighting foams (AFFFs), and landfills.4,8 Highly exposed communities continue to be identified globally.2,9
Infants and children are particularly vulnerable to environmental toxicants, including PFAS. Children often have higher body burdens than adults owing to differences in exposure sources, body size, and body surface area.10 PFAS developmental toxicity has been demonstrated in animal models,11,12 and epidemiological studies have linked prenatal and childhood PFAS exposures to health outcomes, including dyslipidemia, changes in fetal and postnatal growth, impaired immunity, and delayed onset of puberty.10,13 Furthermore, it is increasingly understood that changes during sensitive periods of development may have potentially adverse consequences for health later in life.14
A critical step in the breastfeeding exposure pathway is the transfer of PFAS from maternal serum into colostrum and breast milk. Cumulative infancy exposure can be estimated by multiplying the transfer efficiency (TE; the fraction of PFAS transferred from maternal serum into breast milk), by the maternal PFAS concentration and then by the cumulative milk volume consumed. Although breastfeeding has beneficial effects for mother and child,15 it is also an important source of PFAS exposure for infants and young children. At low exposure levels, the duration of exclusive breastfeeding has been associated with an increase in infancy serum PFAS concentrations up to as high as 30% per month.16,17 One study also found that postnatal exposure from exclusive breastfeeding the month after delivery was higher than prenatal exposure.18 However, there is an overall lack of information on potential PFAS exposures from breastfeeding.19
Although a limited number of studies have estimated TEs using paired samples of maternal serum and breast milk, these studies had several major limitations.18,20–23 First, they were all conducted in populations exposed to low levels of PFAS. Therefore, PFAS concentrations in milk samples were often below the limit of detection (LOD) or limit of quantification (LOQ). To our knowledge, no studies have estimated PFAS TEs in highly exposed women although this is the population where infant exposures via breast milk are most concerning. In addition, because breast milk composition changes throughout lactation, it is possible that PFAS TEs may also change. However, no study has evaluated this possibility. Finally, all but one existing study had a sample size of women.
To address these limitations, we measured the transfer of PFAS from maternal serum into colostrum and breast milk in a cohort of women from Ronneby, Sweden, where many residents were exposed to PFAS from drinking water that was highly contaminated by AFFFs.24 We hypothesized that PFAS would be measurable in colostrum and breast milk and that TEs would vary by PFAS compound and lactation stage. We also explored whether TEs varied by the level of maternal exposure (high vs. background exposure).
Methods
Ronneby Mother–Child Cohort
In 2013, it was discovered that one of the two municipal water supplies in Ronneby, Blekinge county, Sweden, had been delivering water that was highly contaminated by PFAS from AFFF runoff at a nearby military airport (Figure 1).24 Military records documented that AFFF usage at the airport began in the mid-1980s, although the start of widespread water contamination is unknown.24 The total PFAS level in outgoing drinking water in December 2013 in this municipal water supply was (the Swedish action level at the time was ) and the waterworks was immediately closed.24 In the other Ronneby waterworks, the total PFAS was .24 In contrast, the total PFAS concentration in the water supply of the nearby municipality of Karlshamn was .24
Figure 1.
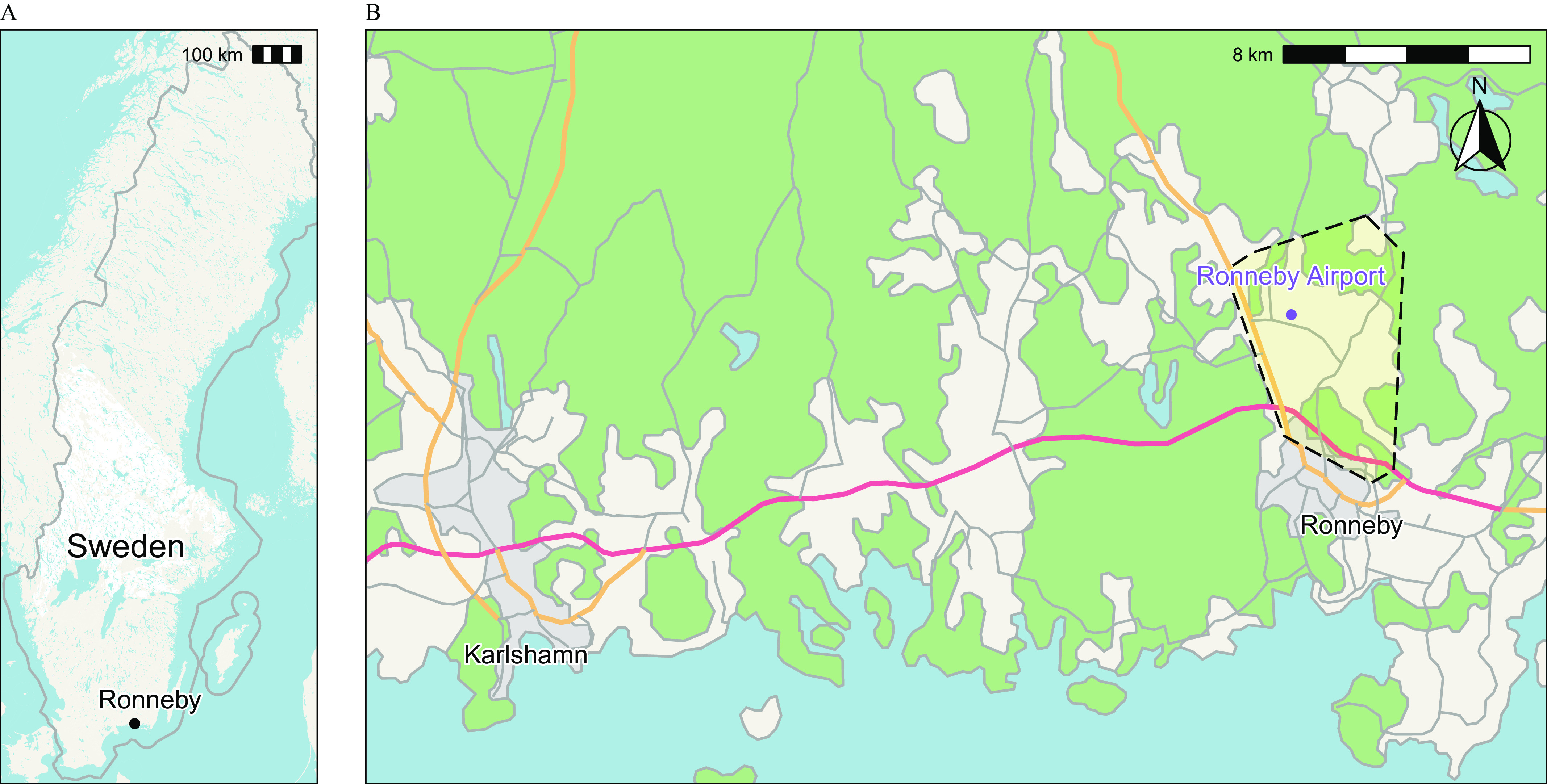
(A) Map of Sweden. (B) Location of the Ronneby Mother–Child Cohort. The dashed yellow polygon outlines the area receiving highly contaminated drinking water in 2013. Background maps: ©Lantmäteriet.25
We established the prospective Ronneby Mother–Child Cohort after the contamination was discovered and drinking water from the contaminated waterworks was turned off, with the purpose of investigating the transfer of PFAS from mother to child. All pregnant women in the Ronneby municipality between 2015 and 2020 were invited to participate at their maternal health care center. Women from Karlshamn were also invited to participate starting in 2018 after population biomonitoring found that most individuals in Ronneby, regardless of drinking water source, had higher serum PFAS concentrations compared with residents from Karlshamn.24 The final cohort included 263 women, with 225 from Ronneby, 35 from Karlshamn, and 3 who were missing location data.
A maternal blood sample was collected at the time of delivery from all participants. The blood was centrifuged and the serum sample was collected in a micro tube (Sarstedt). It was then shipped from the Karlskrona hospital to the Laboratory of Occupational and Environmental Medicine in Lund on dry ice and stored at until analysis. Mothers collected a colostrum sample 3–4 d postpartum and a breast milk sample 4–12 wk postpartum in screw cap polypropylene tubes (Sarstedt). Both samples were collected at home using either hand expression or a pump and were stored in the mothers’ home freezers at until they were returned to the maternal health care center during a maternal follow-up visit ( postpartum). They were stored at until they were retrieved and transferred to the Laboratory of Occupational and Environmental Medicine in Lund on ice and stored at . They were shipped on dry ice to the Norwegian Institute of Public Health, arrived frozen, and were stored at until analysis. Study participants also completed questionnaires with information on breastfeeding history, smoking habits, medical drug use, education, occupation, and residential history.
The study was approved by the regional ethical review board in Lund, Sweden (no. 2017/437, with amendments). Written informed consent was provided by all participants.
PFAS Analysis
Seven PFAS were measured in maternal serum, colostrum, and breast milk samples [perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), perfluorohexane sulfonic acid (PFHxS), perfluoroheptane sulfonic acid (PFHpS), and perfluorooctane sulfonic acid (PFOS)]. Serum samples were analyzed at the Division of Occupational and Environmental Medicine at Lund University using the method described by Norén et al.26 In brief, an aliquot of serum was added with an internal PFAS standard. Serum proteins were then precipitated with acetonitrile, and the mixture was vigorously shaken for 30 min. The samples were analyzed using liquid chromatography/tandem mass spectrometry (LC-MS/MS) (QTRAP 5500; AB Sciex). Total PFOS was measured as the total non–isomer-specific compounds. Four reference samples, created by pooling several serum samples, were used as a quality control to estimate between-run precision, and 164 serum samples were analyzed in duplicate to estimate between-batch precision (Table S1). The LOQ for PFAS measurements in serum was . The laboratory participates in the interlaboratory comparison investigations and external quality assurance schemes for analyses of PFAS and is approved by the European Human Biomonitoring Initiative (HBM4EU) project and the University of Erlangen-Nuremberg.
Colostrum and breast milk samples were analyzed for PFAS concentrations at the Department of Food Safety, Norwegian Institute of Public Health. The samples were prepared and analyzed using LC-MS/MS, following the method detailed by Thomsen et al.27 In brief, the samples were thawed and homogenized in an incubator at 37°C. An aliquot of was transferred to an Eppendorf tube, to which internal standards and acetonitrile were added. The sample was then mixed on a whirl mixer and further centrifuged. The supernatant was transferred to an autosampler vial and 0.1 M formic acid was added. The sample extracts were injected on a column-switching LC system coupled to a triple quadrupole mass spectrometer. High quality of measurements was assured by analyzing two in-house quality control samples ( each). The mean concentrations of PFAS in the two in-house control samples were in the range of . The relative standard deviations for the two sets of replications were 28% and 11.1% for PFOA, 28.0 and 33.8% for PFNA, 48.2% and 28.8% for PFDA, 64.2% and 15.9% for PFHxS, and 20.2% and 8.8% for PFOS. The LOQ for all seven PFAS in colostrum and breast milk was . For the quantification of PFOS, the total area of the linear and branched isomers was integrated.
Statistical Analysis
We categorized participants into three exposure groups based on their maternal serum PFHxS concentrations at delivery given that PFHxS is a strong indicator of exposure to AFFF-contaminated water in Ronneby.24 Participants were included in the background exposure group if their PFHxS concentration was percentile of PFHxS concentration in women receiving maternal health care in Karlshamn. This cutoff was selected to represent background levels of exposure while accounting for the fact that some women in Karlshamn may have spent time working or visiting in Ronneby. Participants who were not included in the background group were then categorized as in either the high exposure group ( percentile in PFHxS concentration in the non-background population) or the intermediate exposure group ( percentile in PFHxS concentration in the non-background population). Of the 126 women with a serum sample at delivery and a paired colostrum or breast milk sample, 25 women were categorized as being in the background group, 76 women in the intermediate group, and 25 women in the highly exposed group (Table 1, Tables S5 and S6, and Figure S1).
Table 1.
Baseline characteristics of the 126 mothers from the Ronneby Mother–Child Cohort who were included in this study, displayed as (%; excludes missing) or .
| Characteristic | Overall | PFAS exposure categorya | ||
|---|---|---|---|---|
| Background | Intermediate | High | ||
| 126 | 25 | 76 | 25 | |
| Maternal age at delivery | ||||
| Year of delivery | ||||
| 2015 | 6 (5) | 0 (0) | 2 (3) | 4 (16) |
| 2016 | 27 (21) | 0 (0) | 20 (26) | 7 (28) |
| 2017 | 32 (25) | 3 (12) | 21 (28) | 8 (32) |
| 2018 | 31 (25) | 10 (40) | 19 (25) | 2 (8) |
| 2019 | 27 (21) | 11 (44) | 12 (16) | 4 (16) |
| 2020 | 3 (2) | 1 (4) | 2 (3) | 0 (0) |
| Parity | ||||
| Primiparous | 48 (38) | 10 (40) | 27 (36) | 11 (44) |
| Multiparous | 77 (62) | 15 (60) | 48 (64) | 14 (56) |
| Smoking status | ||||
| Never smoker | 80 (67) | 18 (78) | 45 (62) | 17 (71) |
| Current smoker | 7 (6) | 0 (0) | 6 (8) | 1 (4) |
| Past smoker | 32 (27) | 5 (22) | 21 (29) | 6 (25) |
| Missing | 7 | 2 | 4 | 1 |
| Education status | ||||
| Less than high school | 3 (2) | 0 (0) | 3 (4) | 0 (0) |
| High school | 51 (42) | 8 (35) | 29 (40) | 14 (58) |
| University () | 63 (52) | 15 (65) | 40 (55) | 8 (33) |
| Other | 3 (2) | 0 (0) | 1 (1) | 2 (8) |
| Missing | 6 | 2 | 3 | 1 |
| Location of maternal care | ||||
| Karlshamn | 23 (18) | 20 (80) | 3 (4) | 0 (0) |
| Ronneby | 103 (82) | 5 (20) | 73 (96) | 25 (100) |
Note: PFAS, perfluoroalkyl substances; PFHxS, perfluorohexane sulfonic acid; SD, standard deviation.
Exposure levels were categorized based on maternal serum PFHxS concentrations (, ng/mL) at delivery. Background: . Intermediate: . High: .
We calculated the average relative contribution of each PFAS to the sum of the seven measured PFAS (PFOA, PFNA, PFDA, PFUnDA, PFHxS, PFHpS, and PFOS) for each exposure category and sample matrix (serum, colostrum, and breast milk). We estimated bivariate correlations between each PFAS in each matrix for the full cohort, as well as stratified by exposure group. Correlations were estimated using Spearman rank correlations given that the PFAS concentrations were not normally distributed. In these descriptive analyses we used the measured PFAS concentration when available and otherwise used the LOQ divided by the square root of 2.
We then calculated the TE (in percentage) of each PFAS from maternal serum (S) into colostrum (C) or breast milk (B) for all PFAS measured in the study, limiting our analyses to samples that measured to increase the accuracy of the estimates and reduce noise from imputation-related error. TE was calculated as follows:
| (1) |
where i was either colostrum or breast milk; was the PFAS concentration (in nanograms per milliliter) in the serum sample; and was the PFAS concentration in either colostrum or breast milk. We summarized TEs using the range, median, and interquartile range (IQR). Sample quantiles were calculated using sample quantile definition 7 from Hyndman and Fan,28 which is the default quantile algorithm in R.
For PFAS measured with a high quantification frequency ( in at least 50% of maternal serum samples and 50% of combined colostrum and breast milk samples), we further investigated potential variation in TE by PFAS compound, lactation stage, and exposure level. In these secondary analyses we used measured PFAS values when available but otherwise used the LOQ divided by the square root of 2. First, we estimated TE stratified by exposure category. Then, to evaluate whether TE varied significantly by PFAS compound, lactation stage, or exposure level, we modeled TE using linear mixed-effects models with a random intercept for each woman to account for within-subject correlation. TE was log-transformed to improve the normality of the residuals. In our first model, we evaluated the significance of PFAS and lactation stage by modeling TE as a function of these two indicator variables, including an interaction between the two predictors. This can be written as follows:
| (2) |
where is the natural logarithm of the TE for woman i and PFAS p (either PFOA, PFNA, PFHxS, or PFOS); is the vector of coefficients for an indicator variable of PFAS; is the main effect of lactation stage (colostrum or breast milk); is the vector of coefficients for the interaction term between PFAS and lactation stage; is a subject-specific intercept; and is the within-subject error. We used Wald -tests to evaluate the overall significance of PFAS and lactation stage29 considering as statistically significant. In our second model, we similarly estimated TE as a function of PFAS and exposure level, allowing for an interaction between the two terms and stratifying the model by lactation stage. We again used Wald -tests to evaluate the overall significance of exposure level.
All statistical analyses were conducted in R [version 4.2.0; R Development Core Team (2022-04-22)] using the Tidyverse.30 Mixed effects models were run using the package nlme (version 3.1.157),31 and the map of Ronneby in Figure 1 was constructed using the package sf (version 1.0.7).32
Results
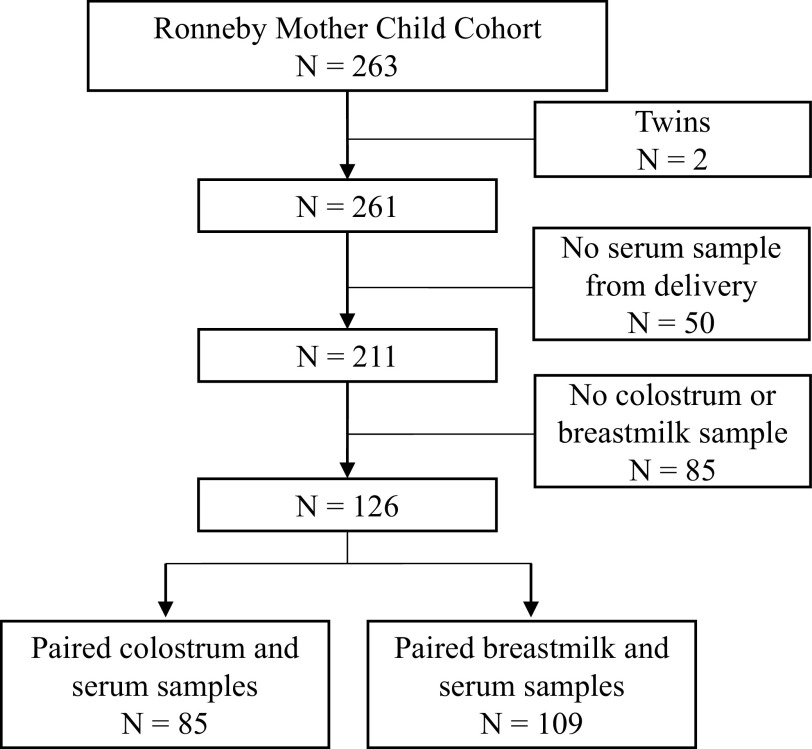
Of the 263 mother–child pairs participating in the Ronneby Mother–Child Cohort, 211 had PFAS concentrations measured from maternal serum collected at the time of delivery. Of these, 126 also had PFAS concentrations measured in colostrum () or breast milk (). A study selection flow chart is included as Figure 2. Summary characteristics of the study population are included in Table 1. Four mothers were enrolled twice in the study because they each had two children in the cohort and provided serum and breast milk samples in both instances.
Figure 2.
Study selection flowchart. refers to the number of mother–child pairs included or excluded. In the case of twins, each mother–twin pair is considered .
The population characteristics of women with intermediate and high PFAS exposures were generally similar to those with background exposures (Table 1). However, women in the high exposure group had lower educational attainment than the other two groups: Only 32% of women in the high exposure group attended university, compared with 60% of women in the background exposure group. Women in the background group also generally gave birth later with respect to calendar year than women in the intermediate and high exposure groups.
Women who provided at least one colostrum or breast milk sample differed from women who did not by several important characteristics. They were slightly older, more likely to be a never-smoker, and more likely to have attended university. In addition, women with a colostrum or breast milk sample were more likely to have received maternal care in Karlshamn than those who did not (18.3% compared with 8.2%) and, as a result, had lower median concentrations of PFOA, PFHxS, and PFOS (Tables S2 and S3).
PFAS Concentrations
We measured seven PFAS in all three matrices (serum, colostrum, and breast milk). All seven PFAS were quantifiable in at least 76% of serum samples (Table 2). PFAS concentrations in colostrum and breast milk were considerably lower than in maternal serum. The PFAS with the highest quantification frequency in colostrum and breast milk samples was PFOS (97% ), whereas PFDA had the lowest quantification frequency (2% ). PFHxS and PFOS had the highest median concentrations in all matrices (Table 2).
Table 2.
PFAS concentrations (ng/mL) in maternal serum at delivery and in colostrum and breast milk samples.
| PFAS | Serum () |
Colostrum () |
Breast milk () |
|---|---|---|---|
| PFOA | |||
| % >LOQ | 100 | 98 | 94 |
| 5th–95th perc. | 0.43–5.93 | 0.01–0.2 | 0.01–0.15 |
| Median (IQR) | 1.31 (0.84–2.6) | 0.03 (0.02–0.08) | 0.03 (0.02–0.06) |
| PFNA | |||
| % >LOQ | 99 | 45 | 75 |
| 5th–95th perc. | 0.17–0.73 | 0.01–0.02 | 0.01–0.01 |
| Median (IQR) | 0.38 (0.26–0.52) | (–0.01) | 0.01 (0.01–0.01) |
| PFDA | |||
| % >LOQ | 94 | 2 | 2 |
| 5th–95th perc. | –0.45 | –0.01 | –0.01 |
| Median (IQR) | 0.22 (0.15–0.32) | (–) | (–) |
| PFUnDA | |||
| % >LOQ | 90 | 9 | 3 |
| 5th–95th perc. | –0.44 | –0.01 | –0.01 |
| Median (IQR) | 0.2 (0.15–0.32) | (–) | (–) |
| PFHxS | |||
| % >LOQ | 100 | 71 | 71 |
| 5th–95th perc. | 0.29–67.47 | 0.01–1.73 | 0.01–0.98 |
| Median (IQR) | 6.02 (0.88–26.12) | 0.1 (–0.48) | 0.07 (–0.32) |
| PFHpS | |||
| % >LOQ | 76 | 34 | 36 |
| 5th–95th perc. | –3.77 | 0.01–0.11 | 0.01–0.05 |
| Median (IQR) | 0.42 (0.1–1.74) | (–0.02) | (–0.02) |
| PFOS | |||
| % >LOQ | 100 | 95 | 99 |
| 5th–95th perc. | 1.89–92.55 | 0.01–0.96 | 0.01–1.08 |
| Median (IQR) | 11.29 (3.81–36.58) | 0.12 (0.03–0.32) | 0.13 (0.04–0.39) |
Note: IQR, interquartile range; LOQ, limit of quantification; perc., percentile; PFAS, perfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHpS, perfluoroheptane sulfonic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUnDA, perfluoroundecanoic acid.
PFAS concentrations were generally higher in women attending maternal health care in Ronneby () than in women receiving care in Karlshamn () (Table S4 and Figure S2). When we used serum PFHxS concentrations to group women by their exposure levels, 25 women were categorized as being in the background group (serum PFHxs ), 76 women in the intermediate group (0.78 PFHxs ), and 25 women in the highly exposed group (serum PFHxS ) (Table 1, Tables S5 and S6, and Figure S1). Concentrations of AFFF-associated PFAS were much higher among the intermediate and high exposed groups compared with the background group. For example, the median serum concentration of PFHxS in the high exposure group was compared with in the intermediate group and in the background group (Table S6). PFAS concentrations in colostrum and breast milk were similarly higher in the intermediate and highly exposed groups compared with the background group (Tables S7 and S8).
The relative contribution of each PFAS to the sum of seven PFAS (PFOA, PFNA, PFDA, PFUnDA, PFHxS, PFHpS, and PFOS) varied by both exposure group and sample matrix (serum, colostrum, and breast milk) (Figure 3 and Table S9). The average relative contribution of PFHxS to the sum of PFAS was higher in the intermediate and high exposure groups than the background group for all matrices. On average, PFOA contributed a higher percentage of total PFAS in colostrum and breast milk than in serum, whereas PFOS had a higher relative contribution in serum than in colostrum and breast milk.
Figure 3.
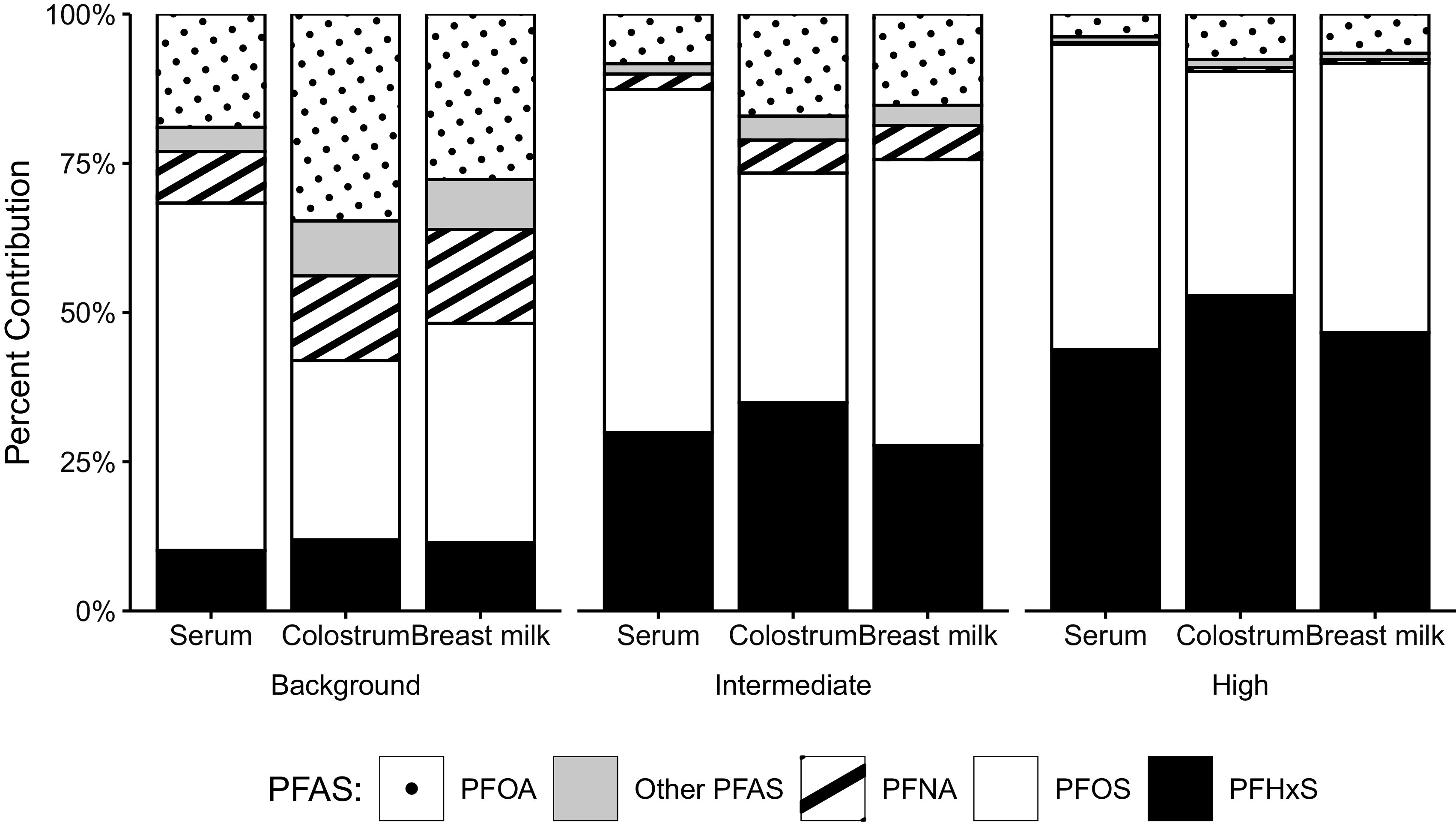
The average relative contribution of each PFAS to the sum of seven PFAS (PFOA, PFNA, PFDA, PFUnDA, PFHxS, PFHpS, and PFOS), by matrix and exposure category. The underlying numeric data for this figure are presented in Table S9. Note: PFAS, perfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHpS, perfluoroheptane sulfonic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUnDA, perfluoroundecanoic acid.
Concentrations of PFOA, PFHxS, PFHpS, and PFOS were highly correlated with one another within each matrix. The strongest correlations were found for PFOS and PFHxS (Figure S3). For example, PFOS and PFHxS were strongly correlated with one another in serum (), colostrum (), and breast milk (). Each of these PFAS were also highly correlated across the three matrices. For example, PFHxS concentrations in maternal serum were strongly correlated with PFHxS concentrations in colostrum () and breast milk (). Concentrations of other PFAS correlated somewhat across matrices (e.g., PFNA correlation between serum and ) but less with other PFAS. When we evaluated correlations separately in the background exposure group and in a combined intermediate and high exposure group, correlations across AFFF-related PFAS were much stronger in the combined intermediate- and high-exposure group (Figures S4 and S5).
Transfer Efficiency
TEs for each PFAS and lactation stage are shown in Table 3 and Figure 4. Median TEs varied from 0.9% ( for PFOS, ) to 4.3% ( for PFUnDA, ) and were higher for perfluoroalkyl carboxylic acids (PFCAs: PFOA, PFNA, PFDA, and PFUnDA) compared with perfluoroalkane sulfonic acids (PFSAs: PFHxS, PFHpS, and PFOS). For example, the median for PFOA and PFNA was 2.2% (IQR: 1.7–3.1) and 2.5% (IQR: 1.8–3.4), whereas for PFHxS and PFOS it was 1.2% (IQR: 1.0–1.5) and 1.0% (IQR: 0.8–1.2), respectively. For all PFAS except PFOS, the median was higher than the median .
Table 3.
Transfer efficiencies (%) of PFAS from maternal serum at delivery into colostrum () and breast milk (), limited to samples .
| PFAS | Median (IQR) | Range | |
|---|---|---|---|
| PFOA | 83 | 2.8 (2.31–3.81) | 0.79–8.2 |
| PFNA | 38 | 3.18 (2.25–3.85) | 1.2–12.5 |
| PFDA | 2 | 3.27 (2.83–3.72) | 2.38–4.17 |
| PFUnDA | 8 | 4.26 (3.71–5.54) | 2.33–9.09 |
| PFHxS | 60 | 1.65 (1.17–2.21) | 0.68–4.13 |
| PFHpS | 29 | 2.19 (1.68–3.16) | 0.68–5.31 |
| PFOS | 81 | 0.86 (0.65–1.19) | 0.33–3.06 |
| PFOA | 103 | 2.16 (1.73–3.08) | 0.79–6 |
| PFNA | 82 | 2.5 (1.79–3.42) | 0.98–7.69 |
| PFDA | 2 | 2.72 (2–3.45) | 1.28–4.17 |
| PFUnDA | 3 | 2.33 (2.06–2.95) | 1.79–3.57 |
| PFHxS | 77 | 1.24 (1–1.5) | 0.36–2.94 |
| PFHpS | 36 | 1.33 (0.93–1.87) | 0.38–12.5 |
| PFOS | 108 | 1.02 (0.85–1.2) | 0.37–3.26 |
Note: B, breast milk; C, colostrum; IQR, interquartile range; LOQ, limit of quantification; PFAS, perfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHpS, perfluoroheptane sulfonic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUnDA, perfluoroundecanoic acid; S, serum; TE, transfer efficiency.
Figure 4.
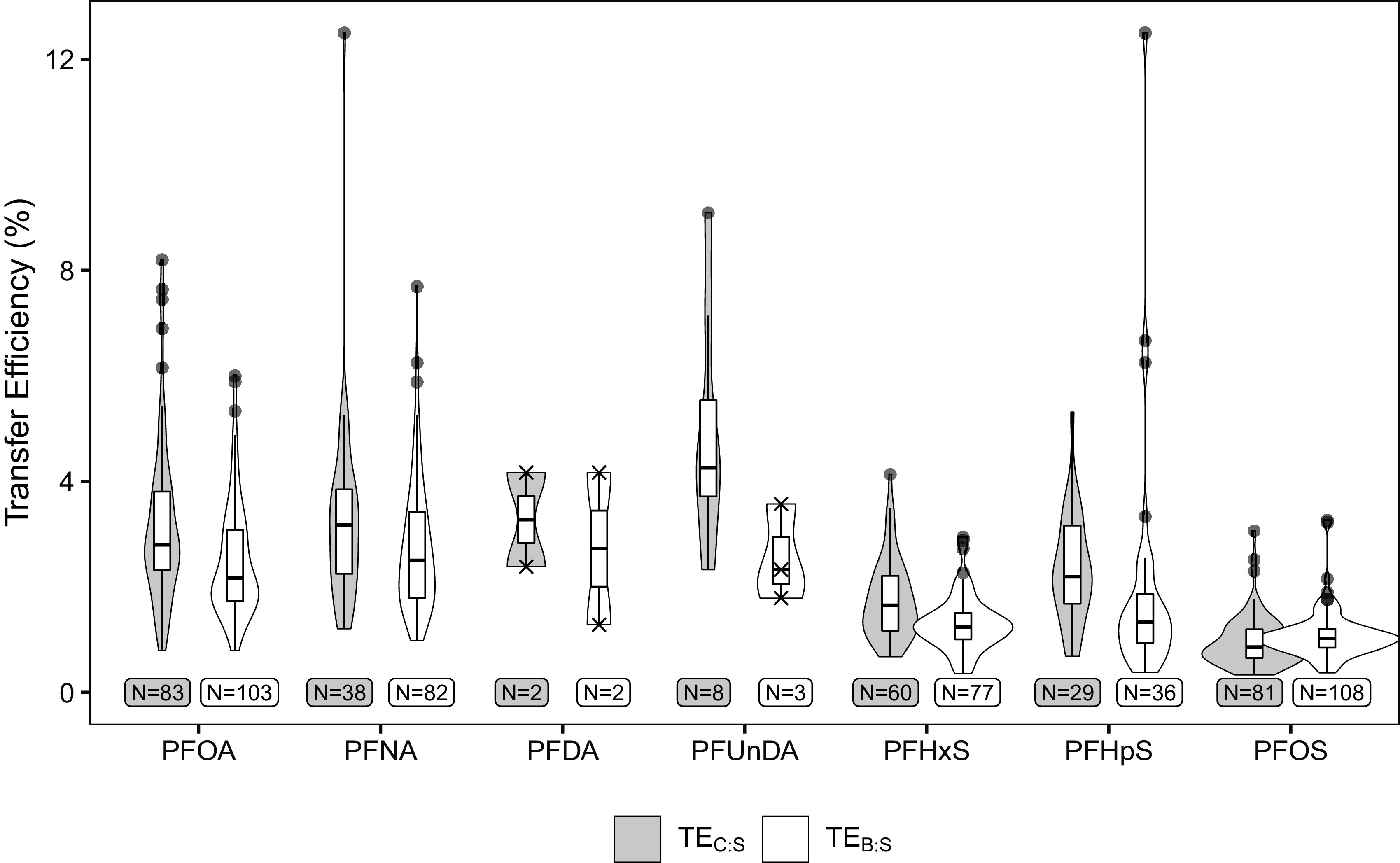
Distribution of PFAS TEs (%) from maternal serum at delivery into colostrum () and breast milk (), limited to samples . Tukey outliers are marked as points, whereas individual TEs when are marked as Xs. The underlying numeric data for this figure are presented in Table 3. Note: B, breast milk; C, colostrum; LOQ, limit of quantification; PFDA, perfluorodecanoic acid; PFHpS, perfluoroheptane sulfonic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFUnDA, perfluoroundecanoic acid; S, serum; TE, transfer efficiency.
Four PFAS (PFOA, PFNA, PFHxS, and PFOS) were in of combined colostrum and breast milk samples and were included in further analyses. When we stratified TEs by exposure group, there was no clear pattern in TE by exposure (Table 4). In our first mixed-effects model, both PFAS and lactation stage were significant predictors of TE ( for PFAS; for lactation stage) although the directional effect of lactation stage was not consistent across PFAS (Table S10). In our models of TE by PFAS and exposure level (stratified by sample type), exposure level was also overall a significant predictor of TE in both colostrum () and breast milk (). However, the direction of the exposure-level effect was not consistent across PFAS, agreeing with our stratified analysis (Tables S11 and S12).
Table 4.
Transfer efficiencies (%) of PFAS from maternal serum at delivery into colostrum () and breast milk (), stratified by exposure group.
| Exposure categorya | Transfer efficiency [median (IQR)] | ||||
|---|---|---|---|---|---|
| PFOA | PFNA | PFHxS | PFOS | ||
| Background | 13 | 2.94 (2–3.77) | 2.58 (1.78–3.1) | 1.86 (1.31–2.36) | 0.66 (0.4–0.92) |
| Intermediate | 56 | 2.74 (1.99–3.82) | 2.60 (1.81–3.74) | 1.33 (0.85–1.87) | 0.76 (0.61–1.09) |
| High | 16 | 2.83 (2.57–3.65) | 2.63 (2.03–3.64) | 1.81 (1.23–2.27) | 1.13 (0.92–1.26) |
| Background | 21 | 2.25 (1.82–3.45) | 3.03 (2.27–3.57) | 1.86 (1.39–2.36) | 0.99 (0.78–1.2) |
| Intermediate | 68 | 2.18 (1.72–2.96) | 2.63 (2.09–3.98) | 1.12 (0.79–1.36) | 1.00 (0.83–1.2) |
| High | 20 | 1.98 (1.7–2.51) | 1.94 (1.57–2.89) | 1.41 (1.14–1.54) | 1.09 (1–1.19) |
Note: Samples were included and substituted with the LOQ divided by the square root of 2 when a measured concentration was not available. B, breast milk; C, colostrum; IQR, interquartile range; LOQ, limit of quantification; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; S, serum; TE, transfer efficiency.
Exposure levels were categorized based on maternal serum PFHxS concentrations (, ng/mL) at delivery. Background: . Intermediate: . High: .
Discussion
In this study, we examined the transfer of PFAS from maternal serum into colostrum and breast milk in a cohort of women with a wide range of PFAS exposures. PFAS concentrations were correlated across both sample matrices and PFAS compound. In the intermediate and high exposure groups, correlations were extremely strong for AFFF-associated PFAS, including PFHxS and PFOS. This confirmed that AFFF runoff was indeed the dominant source of PFAS exposure in this population. Median TEs varied from 0.9% ( for PFOS) to 4.3% ( for PFUnDA), and were higher for PFCAs than PFSAs. TEs in colostrum generally had a wider spread than those in breast milk, possibly reflecting the larger interindividual variation of colostrum composition.33 In our mixed-effects models, we found significant differences in TE by PFAS and lactation stage. When we stratified women by exposure categories (background, intermediate, and high), there were also some differences in TE by exposure level. However, there was not a consistent pattern for these differences, and the magnitude was small compared with differences in TE by PFAS compound.
Comparison with Previous Studies
We conducted an informal literature review on PubMed in November 2021 with the search terms (“PFAS” OR perfluoro*) AND (breastmilk OR “breast milk” OR “milk, human” [MeSH Terms]) AND (“exposure” OR “transfer”) and identified five existing studies that estimated TEs in breast milk (Table 5). Compared with these previous studies, our study had a wider range of maternal AFFF-associated PFAS exposures and included more highly exposed women. For example, the median maternal serum PFHxS concentration in our study was (range: 0.15–189), compared with the median maternal concentration of (range: 0.8–31) in a cohort of 100 women in France,20 (range: 0.012–0.36) in a cohort of 50 women in China,18 and (range: 0.16–4.1) in a cohort of 41 women in Norway.21 Similarly, the median maternal serum PFOS concentration in our study was (range: 0.92–310), compared with a median maternal concentration of (range: 0.32–24.5) in Cariou et al.,20 (range: 0.83–13.2) in Liu et al.,18 and (range: 2.3–15) in Haug et al.21 This wide range in exposures allowed us to investigate potential variation in TEs by exposure levels, as well as estimate TEs for several PFAS compounds (PFDA, PFUnDA, and PFHpS) that were unquantifiable in previous studies.
Table 5.
Literature comparison.
| Country | Reference | Total paired samples () |
Sampling period | Transfer efficiency ( of paired samples in breast milk) [% ()] | ||||
|---|---|---|---|---|---|---|---|---|
| Maternal serum | Breast milk | PFOA | PFNA | PFHxS | PFOS | |||
| Sweden | Kärrman et al.,22a | 12 | 3 wk postpartum | 3 wk postpartum | 12 (1) | 1 (2) | 2 (12) | 1 (12) |
| Norway | Haug et al.,21a | 19 | After delivery | After delivery | 3.8 (10) | — | — | 1.4 (19) |
| Korea | Kim et al.,23a | 17 | 1 d prior to delivery | 3–10 d postpartum | 2.5 (8) | — | 0.8 (15) | 1.1 (17) |
| China | Liu et al.,18b | 50 | 1 wk postpartum | 1 wk postpartum | 9 (50) | 4 (50) | — | 2 (50) |
| France | Cariou et al.,20a,c | 19 | At delivery | 4–5 d postpartum | 3.8 (10) | — | 1.2 (9) | 1.4 (19) |
| Sweden | Present Studyb | 85 | At delivery | 3–4 d postpartum | 2.8 (83) | 3.2 (38) | 1.7 (60) | 0.9 (81) |
| Sweden | Present Studyb | 109 | At delivery | 4–12 wk postpartum | 2.2 (103) | 2.5 (82) | 1.2 (77) | 1.0 (108) |
Note: —, not applicable; LOQ, limit of quantification; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; TE, transfer efficiency.
TE summarized as mean.
TE summarized as median.
TEs from the 2015 paper by Cariou et al.20 were received by direct correspondence with the authors and reflect a correction to their original publication.
Our results generally agree with previous studies on the transfer of PFOA and PFOS into breast milk (Table 5). In the five previous studies that estimated TEs for both PFOA and PFOS (all conducted at low levels of exposure), all five found that PFOA had a higher mean or median TE than PFOS.18,20–23 The estimated TEs for PFOS were between 1% and 2%, similar to our estimated median of 0.9% and of 1.0%. Estimated TEs for PFOA were more variable across previous studies. This also makes sense in the context of our results given that we found that the TEs of PFOA had a greater spread than those of PFOS.
Existing literature on the transfer of PFHxS and PFNA into human milk is limited and inconsistent. Two previous studies estimated TEs for PFNA in paired maternal serum and breast milk samples, although one study only included two samples.22 In the study by Liu et al. (), the estimated median TE of PFNA was 4%,18 which is higher than the median and for PFNA found in the present study. We found that the median and were higher for PFNA than the corresponding median TEs for PFOS, a result which agrees with Liu et al.18 Notably, this contradicts the 2020 European Food Safety Authority (EFSA) report, “Risk to Human Health Related to the Presence of Perfluoroalkyl Substances in Food,” which stated “[t]he [transfer] ratio… for PFNA… occurs at similar levels for PFOS but at a lesser extent than for PFOA.”11
Three previous studies estimated TE for PFHxS, with estimated mean TEs varying from 0.8% to 2%. However, these three studies were limited by their small sample size and by the low concentrations of PFHxS in maternal serum, which ranged from 0.89 to .20,22,23 In contrast, we estimated TEs in a large population with a wide range of PFHxS exposures (mean maternal serum concentration), and found TE values that fell in the middle of these estimates (median ; median ). Because AFFF-contaminated groundwater often has a high level of PFHxS34–36 and high concentrations of PFHxS have been found in AFFF-exposed populations,37–39 this TE is of particular concern for contaminated populations.
This study is one of the first to quantify PFHpS in breast milk40 and provides the first estimates of TEs for this PFAS. PFHpS concentrations were elevated in the serum of exposed mothers in this cohort, as well in exposed individuals from a larger biomonitoring cohort in Ronneby.24 PFHpS has also been identified as a component PFAS in several AFFF formulations.41 This indicates that, as with PFHxS, PFHpS contamination may be of particular concern in AFFF-exposed communities, such as Ronneby. We found that the median and were the highest of the three PFSAs included in the study, although they were lower than the median TEs for PFCAs. The health effects of early life PFHpS exposures remain relatively unstudied.
Potential Biological Mechanisms
The specific biological processes regulating the transfer of PFAS from maternal serum into breast milk are not known. The mechanism(s) likely differ from those of other persistent organic pollutants given that PFAS do not accumulate in lipids and fatty tissue.42 Instead, PFAS in human blood are found primarily bound to albumin, the most abundant protein in the blood.43,44 Serum albumin is transported directly from plasma into human milk via transcytosis.45 Some nutrients, including zinc and copper, have been shown to cotransport with albumin,46 and it is possible that PFAS are also cotransferred this way. If so, PFAS-specific TEs may partially reflect differences in PFAS-albumin binding affinity. The concentration of albumin in colostrum and breast milk is relatively consistent,47 which could explain why we did not see large differences in TEs by lactation stage.
Another potential biological mechanism that could determine the transfer of PFAS into breast milk is active transport. PFAS have similar structures to those of natural fatty acids and may be transported in a similar manner. Long-chain fatty acids are transported across the basal layer of the mammary epithelium by specific transport proteins, such as CD36 [fatty acid translocase (FAT)] and members of the fatty acid transport proteins/solute carrier 27 (FATP/SLC2.7A) and acyl-CoA synthetase (ACSL) families, although the precise mechanisms regulating this active transfer are still unknown.48 Other transporters, especially those in the ATP-binding cassette () and Solute Carrier () superfamilies, are known to be highly active during lactation and act as mediators for the active transport of other toxic chemicals, including specific drugs and environmental pollutants,49 and may play a role. A better understanding of the biological processes governing the transfer of PFAS would help scientists anticipate the risk of transfer for PFAS that have not been directly measured in populations. In addition, it may help elucidate why some women have higher transfer ratios than others and identify infants at risk of high exposure.
Strengths and Limitations
The Ronneby Mother–Child Cohort is a unique cohort in which many of the women were exposed to highly contaminated drinking water for several decades. As a result, exposures in our study population spanned a wide range, with maternal serum concentrations varying from background to extremely high levels of AFFF-associated PFAS. This allowed us to explicitly test whether maternal exposure levels impacted PFAS TE. One limitation of the study was that, as in previous studies, concentrations of PFNA and PFHxS in colostrum and breast milk from low-exposed mothers were often . This may have limited our power to detect differences in TEs across exposure groups. Other PFAS in our study (PFDA and PFUnDA) also had a low rate of quantification across all colostrum and breast milk samples, so the estimated TEs for these PFAS compounds may not be as accurate. A second limitation was that women from background-exposed Karlshamn were more likely to provide colostrum and breast milk samples than highly exposed women from Ronneby, again possibly limiting our power to detect differences by exposure level. Despite this, we were still able to include many women at high levels of exposure. The method of milk collection (hand expression vs. a pump) and time of sample was not standardized, which may have induced some sample heterogeneity. However, given that all women received the same collection instructions, this should not induce any systematic bias in our results. Finally, both and were estimated using the same maternal serum concentrations collected at delivery. Maternal serum PFAS concentrations have been shown to decrease over lactation,17 and therefore our estimates of may also reflect a decreasing maternal body burden. However, obtaining paired serum and breast milk samples at two occasions would have required additional health care visits and would likely have come at the cost of declining participation rate and reduced study power.
This study adds much needed information to the existing literature. To the best of our knowledge, it is the largest study of breast milk TE to date and the first large study of PFHxS and PFHpS, two PFAS that are often elevated in AFFF-exposed communities.24,50 In addition, this study was one of the first to compare the transfer of multiple PFAS, and we found a strong pattern suggesting that functional group may play an important role in TE. Finally, this is the first study that we are aware of to measure TEs in both colostrum and breast milk.
Conclusions
In this large study of women with a wide range of PFAS exposures, we found that PFAS concentrations in colostrum and breast milk were much lower than in maternal serum at delivery. Median TEs of seven PFAS varied from 0.9% to 4.3%. Given that cumulative infant exposure is a function of maternal serum concentrations multiplied by the TE and by the cumulative milk consumption, our finding that TE is similar across exposure levels suggests that infants of highly exposed mothers are at risk of high exposures from breast milk. We also found a wide spread of TEs across individuals, especially for PFOA and PFNA, indicating that some infants may be exposed to higher levels of PFAS than others. The use of one summary TE value in risk assessments may mask this large variation and understate the risk for some children. Future research should evaluate the contribution of breastfeeding to cumulative prenatal and infancy PFAS exposures, with the ultimate goal of developing evidence-based breastfeeding recommendations for highly exposed populations.
Supplementary Material
Acknowledgments
We acknowledge the participating mothers and the staff at the Karlskrona hospital, who donated time and collected the samples. Thanks also to M. Lewandowski for her administrative support.
This work was funded by the Swedish Research Council for the Environment, Agricultural Sciences and Spatial Planning, Formas [grants 216-2014-1709 (to K.J.), 2017-00896 (to C.N.), and 2019-02344 (to C.N.)]. The funding source was not involved in the design or execution of the research. In-kind funding from the University of Gothenburg is also acknowledged.
References
- 1.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541, PMID: , 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131–147, PMID: , 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cousins IT, Goldenman G, Herzke D, Lohmann R, Miller M, Ng CA, et al. 2019. The concept of essential use for determining when uses of PFASs can be phased out. Environ Sci Process Impacts 21(11):1803–1815, PMID: , 10.1039/c9em00163h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Silva AO, Armitage JM, Bruton TA, Dassuncao C, Heiger-Bernays W, Hu XC, et al. 2021. PFAS exposure pathways for humans and wildlife: a synthesis of current knowledge and key gaps in understanding. Environ Toxicol Chem 40(3):631–657, PMID: , 10.1002/etc.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindstrom AB, Strynar MJ, Libelo EL. 2011. Polyfluorinated compounds: past, present, and future. Environ Sci Technol 45(19):7954–7961, PMID: , 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- 6.European Commission. 2020. Commission staff working document: poly- and perfluoroalkyl substances. https://ec.europa.eu/environment/pdf/chemicals/2020/10/SWD_PFAS.pdf [accessed 9 August 2021].
- 7.U.S. Environmental Protection Agency. 2021. PFAS Strategic Roadmap: EPA’s Commitments to Action 2021–2024. https://www.epa.gov/system/files/documents/2021-10/pfas-roadmap_final-508.pdf [accessed 11 November 2022].
- 8.Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. 2016. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett 3(10):344–350, PMID: , 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldenman G, Fernandes M, Holland M, Tugran T, Nordin A, Schoumacher C, et al. 2019. The Cost of Inaction: a Socioeconomic Analysis of Environmental and Health Impacts Linked to Exposure to PFAS. http://norden.diva-portal.org/smash/get/diva2:1295959/FULLTEXT01.pdf [accessed 13 July 2021].
- 10.Rappazzo KM, Coffman E, Hines EP. 2017. Exposure to perfluorinated alkyl substances and health outcomes in children: a systematic review of the epidemiologic literature. Int J Environ Res Public Health 14(7):691, PMID: , 10.3390/ijerph14070691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.EFSA Panel on Contaminants in the Food Chain, Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J.. 2020. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J 18(9):e06223, PMID: , 10.2903/j.efsa.2020.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau C, Butenhoff JL, Rogers JM. 2004. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol Appl Pharmacol 198(2):231–241, PMID: , 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 13.Liew Z, Goudarzi H, Oulhote Y. 2018. Developmental exposures to perfluoroalkyl substances (PFASs): an update of associated health outcomes. Curr Environ Health Rep 5(1):1–19, PMID: , 10.1007/s40572-018-0173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. 2012. Developmental origins of non-communicable disease: implications for research and public health. Environ Health 11(1):42, PMID: , 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Section on Breastfeeding: Eidelman AI, Schanler RJ, Johnston M, Landers S, Noble L.. 2012. Breastfeeding and the use of human milk. Pediatrics 129(3):e827–e841, PMID: , 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 16.Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jørgensen E. 2015. Breastfeeding as an exposure pathway for perfluorinated alkylates. Environ Sci Technol 49(17):10466–10473, PMID: , 10.1021/acs.est.5b02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mondal D, Weldon RH, Armstrong BG, Gibson LJ, Lopez-Espinosa MJ, Shin HM, et al. 2014. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ Health Perspect 122(2):187–192, PMID: , 10.1289/ehp.1306613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Li J, Liu Y, Chan HM, Zhao Y, Cai Z, et al. 2011. Comparison on gestation and lactation exposure of perfluorinated compounds for newborns. Environ Int 37(7):1206–1212, PMID: , 10.1016/j.envint.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 19.LaKind JS, Verner MA, Rogers RD, Goeden H, Naiman DQ, Marchitti SA, et al. 2022. Current breast milk PFAS levels in the United States and Canada: after all this time, why don’t we know more? Environ Health Perspect 130(2):25002, PMID: , 10.1289/EHP10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cariou R, Veyrand B, Yamada A, Berrebi A, Zalko D, Durand S, et al. 2015. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environ Int 84:71–81, PMID: , 10.1016/j.envint.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Haug LS, Huber S, Becher G, Thomsen C. 2011. Characterisation of human exposure pathways to perfluorinated compounds—comparing exposure estimates with biomarkers of exposure. Environ Int 37(4):687–693, PMID: , 10.1016/j.envint.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Kärrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, et al. 2007. Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ Health Perspect 115(2):226–230, PMID: , 10.1289/ehp.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SK, Lee KT, Kang CS, Tao L, Kannan K, Kim KR, et al. 2011. Distribution of perfluorochemicals between sera and milk from the same mothers and implications for prenatal and postnatal exposures. Environ Pollut 159(1):169–174, PMID: , 10.1016/j.envpol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, Nielsen C, Li Y, Hammarstrand S, Andersson EM, Li H, et al. 2021. Serum perfluoroalkyl substances in residents following long-term drinking water contamination from firefighting foam in Ronneby, Sweden. Environ Int 147:106333, PMID: , 10.1016/j.envint.2020.106333. [DOI] [PubMed] [Google Scholar]
- 25.Lantmäteriet (Swedish mapping and land registration authority). 2021. GSD-Sverigekartan 1:1 miljon [in Swedish]. https://www.lantmateriet.se/sv/Kartor-och-geografisk-information/geodataprodukter/produktlista/sverigekartor/ [accessed 19 January 2022].
- 26.Norén E, Lindh C, Glynn A, Rylander L, Pineda D, Nielsen C. 2021. Temporal trends, 2000–2017, of perfluoroalkyl acid (PFAA) concentrations in serum of Swedish adolescents. Environ Int 155:106716, PMID: , 10.1016/j.envint.2021.106716. [DOI] [PubMed] [Google Scholar]
- 27.Thomsen C, Haug LS, Stigum H, Frøshaug M, Broadwell SL, Becher G. 2010. Changes in concentrations of perfluorinated compounds, polybrominated diphenyl ethers, and polychlorinated biphenyls in Norwegian breast-milk during twelve months of lactation. Environ Sci Technol 44(24):9550–9556, PMID: , 10.1021/es1021922. [DOI] [PubMed] [Google Scholar]
- 28.Hyndman RJ, Fan Y. 1996. Sample quantiles in statistical packages. Am Stat 50(4):361–365, 10.1080/00031305.1996.10473566. [DOI] [Google Scholar]
- 29.Pinheiro JC, Bates DM. 2006. Mixed-Effects Models in S and S-PLUS. Dordrecht, Netherlands: Springer Science & Business Media. [Google Scholar]
- 30.Wickham H, Averick M, Bryan J, Chang W, D’Agostino McGowan L, François R, et al. 2019. Welcome to the Tidyverse. J Open Source Softw 4(43):1686, 10.21105/joss.01686. [DOI] [Google Scholar]
- 31.Pinheiro J, Bates D, DebRoy S, Sarkar D, Heisterkamp S, Van Willigen B, et al. 2021. nlme: linear and nonlinear mixed effects models. https://CRAN.R-project.org/package=nlme [accessed 24 January 2022].
- 32.Pebesma E. 2018. Simple features for R: standardized support for spatial vector data. R J 10(1):439–446, 10.32614/RJ-2018-009. [DOI] [Google Scholar]
- 33.Gidrewicz DA, Fenton TR. 2014. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr 14(1):216, PMID: , 10.1186/1471-2431-14-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Backe WJ, Day TC, Field JA. 2013. Zwitterionic, cationic, and anionic fluorinated chemicals in aqueous film forming foam formulations and groundwater from U.S. military bases by nonaqueous large-volume injection HPLC-MS/MS. Environ Sci Technol 47(10):5226–5234, PMID: , 10.1021/es3034999. [DOI] [PubMed] [Google Scholar]
- 35.Dauchy X, Boiteux V, Colin A, Hémard J, Bach C, Rosin C, et al. 2019. Deep seepage of per- and polyfluoroalkyl substances through the soil of a firefighter training site and subsequent groundwater contamination. Chemosphere 214:729–737, PMID: , 10.1016/j.chemosphere.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Hatton J, Holton C, DiGuiseppi B. 2018. Occurrence and behavior of per-and polyfluoroalkyl substances from aqueous film-forming foam in groundwater systems. Remediation (NY) 28(2):89–99, 10.1002/rem.21552. [DOI] [Google Scholar]
- 37.Barton KE, Starling AP, Higgins CP, McDonough CA, Calafat AM, Adgate JL. 2020. Sociodemographic and behavioral determinants of serum concentrations of per- and polyfluoroalkyl substances in a community highly exposed to aqueous film-forming foam contaminants in drinking water. Int J Hyg Environ Health 223(1):256–266, PMID: , 10.1016/j.ijheh.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rotander A, Toms LML, Aylward L, Kay M, Mueller JF. 2015. Elevated levels of PFOS and PFHxS in firefighters exposed to aqueous film forming foam (AFFF). Environ Int 82:28–34, PMID: , 10.1016/j.envint.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Trowbridge J, Gerona RR, Lin T, Rudel RA, Bessonneau V, Buren H, et al. 2020. Exposure to perfluoroalkyl substances in a cohort of women firefighters and office workers in San Francisco. Environ Sci Technol 54(6):3363–3374, PMID: , 10.1021/acs.est.9b05490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng G, Schreder E, Dempsey JC, Uding N, Chu V, Andres G, et al. 2021. Per- and polyfluoroalkyl substances (PFAS) in breast milk: concerning trends for current-use PFAS. Environ Sci Technol 55(11):7510–7520, PMID: , 10.1021/acs.est.0c06978. [DOI] [PubMed] [Google Scholar]
- 41.Nicol L, Kreißig J, Corden C, Keyte I, Whiting R, Matulina A, et al. 2020. The Use of PFAS and Fluorine-Free Alternatives in Fire-Fighting Foams. https://echa.europa.eu/documents/10162/28801697/pfas_flourine-free_alternatives_fire_fighting_en.pdf/d5b24e2a-d027-0168-cdd8-f723c675fa98 [accessed 7 July 2022].
- 42.MacManus-Spencer LA, Tse ML, Hebert PC, Bischel HN, Luthy RG. 2010. Binding of perfluorocarboxylates to serum albumin: a comparison of analytical methods. Anal Chem 82(3):974–981, PMID: , 10.1021/ac902238u. [DOI] [PubMed] [Google Scholar]
- 43.Alesio JL, Slitt A, Bothun GD. 2022. Critical new insights into the binding of poly- and perfluoroalkyl substances (PFAS) to albumin protein. Chemosphere 287(pt 1):131979, PMID: , 10.1016/j.chemosphere.2021.131979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forsthuber M, Kaiser AM, Granitzer S, Hassl I, Hengstschläger M, Stangl H, et al. 2020. Albumin is the major carrier protein for PFOS, PFOA, PFHxS, PFNA and PFDA in human plasma. Environ Int 137:105324, PMID: , 10.1016/j.envint.2019.105324. [DOI] [PubMed] [Google Scholar]
- 45.Monks J, Neville MC. 2004. Albumin transcytosis across the epithelium of the lactating mouse mammary gland. J Physiol 560(pt 1):267–280, PMID: , 10.1113/jphysiol.2004.068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S, Kelleher SL. 2016. Molecular regulation of lactation: the complex and requisite roles for zinc. Arch Biochem Biophys 611:86–92, PMID: , 10.1016/j.abb.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Lönnerdal B, Erdmann P, Thakkar SK, Sauser J, Destaillats F. 2017. Longitudinal evolution of true protein, amino acids and bioactive proteins in breast milk: a developmental perspective. J Nutr Biochem 41:1–11, PMID: , 10.1016/j.jnutbio.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 48.McManaman JL. 2020. Lipid Transport Across the Mammary Gland. In: Ion Transport Across Epithelial Tissues and Disease. Hamilton KL, Devor DC, eds. Cham, Switzerland: Springer, 241–277. [Google Scholar]
- 49.García-Lino AM, Álvarez-Fernández I, Blanco-Paniagua E, Merino G, Álvarez AI. 2019. Transporters in the mammary gland—contribution to presence of nutrients and drugs into milk. Nutrients 11(10):2372, PMID: , 10.3390/nu11102372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nilsson S, Smurthwaite K, Aylward LL, Kay M, Toms LM, King L, et al. 2022. Serum concentration trends and apparent half-lives of per- and polyfluoroalkyl substances (PFAS) in Australian firefighters. Int J Hyg Environ Health 246:114040, PMID: , 10.1016/j.ijheh.2022.114040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.