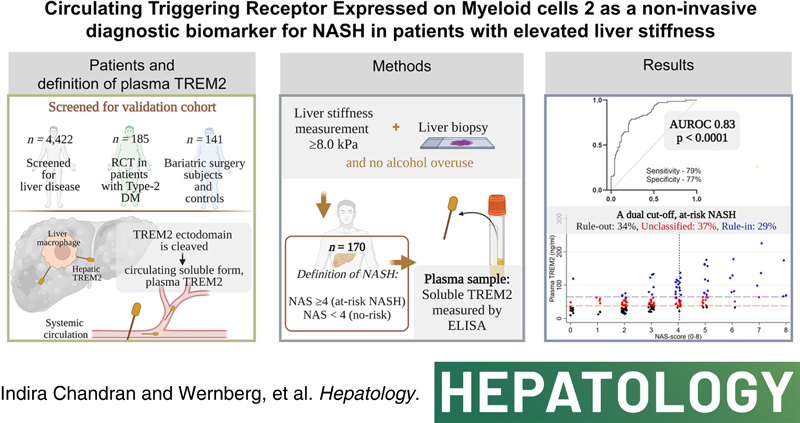
Background and Aims:
Reliable noninvasive biomarkers are an unmet clinical need for the diagnosis of NASH. This study investigates the diagnostic accuracy of the circulating triggering receptor expressed on myeloid cells 2 (plasma TREM2) as a biomarker for NASH in patients with NAFLD and elevated liver stiffness.
Approach and Results:
We collected cross‐sectional, clinical data including liver biopsies from a derivation (n = 48) and a validation cohort (n = 170) of patients with elevated liver stiffness measurement (LSM ≥ 8.0 kPa). Patients with NAFLD activity scores (NAS) ≥4 were defined as having NASH. Plasma TREM2 levels were significantly elevated in patients with NASH of the derivation cohort, with an area under the receiver operating characteristics curve (AUROC) of 0.92 (95% confidence interval [CI], 0.84–0.99). In the validation cohort, plasma TREM2 level increased approximately two‐fold in patients with NASH, and a strong diagnostic accuracy was confirmed (AUROC, 0.83; 95% CI, 0.77–0.89; p < 0.0001). Plasma TREM2 levels were associated with the individual histologic features of NAS: steatosis, lobular inflammation, and ballooning (p < 0.0001), but only weakly with fibrosis stages. Dual cutoffs for rule‐in and rule‐out were explored: a plasma TREM2 level of ≤38 ng/ml was found to be an optimal NASH rule‐out cutoff (sensitivity 90%; specificity 52%), whereas a plasma TREM2 level of ≥65 ng/ml was an optimal NASH rule‐in cutoff (specificity 89%; sensitivity 54%).
Conclusions:
Plasma TREM2 is a plausible individual biomarker that can rule‐in or rule‐out the presence of NASH with high accuracy and thus has the potential to reduce the need for liver biopsies and to identify patients who are eligible for clinical trials in NASH.
INTRODUCTION
NAFLD affects approximately 24% of the adult population worldwide.1 The progression of NAFLD to the more severe form of the disease, NASH, is accompanied by hepatocyte ballooning, hepatic necroinflammation, and fibrosis, and for some it eventually advances to end‐stage liver disease.2 Currently, liver biopsy is the gold standard to diagnose NASH.3 However, the use of liver biopsy is limited by its inherently invasive nature and risk of bleeding.4 A multitude of noninvasive blood biomarkers have been assessed for their diagnostic accuracy in NASH, but none have qualified for routine clinical use partly because of their modest accuracy in independent validation.4 Many validated and commonly used markers in NAFLD and NASH diagnostics reflect fibrosis, whereas only a few diagnose steatosis, ballooning, or inflammation.4,5 Consequently, there is still an unmet clinical need for noninvasive biomarkers that can diagnose NASH as well as identify patients who are eligible for treatment and clinical trials.
Liver macrophages encompassing Kupffer cells and monocyte‐derived macrophages play central roles in the etiology of hepatic inflammation in NASH including a proinflammatory role. Moreover, the ability to detect hepatic inflammation is widely recognized to facilitate the diagnosis of NASH,6 making macrophage‐derived proteins potential markers of NASH. Triggering receptor expressed on myeloid cells are a class of cell surface receptor proteins of the immunoglobulin superfamily that mediates multiple pathophysiological processes in various diseases.7 Recent studies have demonstrated that Trem2 is involved in inflammation‐associated pathologies in the liver.8–13 For example, Trem2 expression is upregulated in nonparenchymal hepatic cells, including macrophages, in both mouse and human livers during injury.8 Increased hepatic macrophage expression of Trem2 has also been associated with higher NAFLD activity scores (NAS) in histopathological assessment.9 Further, three independent studies identified distinct Trem2‐positive hepatic macrophage subpopulations to be associated with advanced stages of NASH.10–12 Recently, an integrated analysis of single‐cell RNA‐sequencing data from cirrhotic and healthy liver samples showed an enrichment of Trem2 during NAFLD progression that was particularly associated with advanced NASH.13 Taken together, an accumulating amount of data links the expression of triggering receptor expressed on myeloid cells (TREM2) on liver macrophages with the severity of NASH. Intriguingly, other studies have shown that a cleaved and soluble form of TREM2 can be found in blood. This soluble form is generated by shedding of the TREM2 ectodomain from the macrophage surface after cleavage by disintegrin and metalloproteases14–16 (Figure S1). Therefore, we hypothesized that increased hepatic expression of cellular TREM2 in patients with NASH would result in increased levels of plasma TREM2 that could potentially serve as a biomarker to identify suspected patients with NASH with evidence of fibrosis.
In this study, we explored the potential of plasma TREM2 as a noninvasive biomarker to distinguish patients with NASH. Accordingly, the main outcome was diagnosis of NASH, defined as NAS ≥4,17 in subjects with an elevated liver stiffness measurement (LSM) (≥8 kPa). The secondary endpoint was to explore optimal rule‐in/rule‐out cut‐offs for plasma TREM2.
PATIENTS AND METHODS
Study design and participants
For this combined derivation and validation study, we selected patients who were at a moderate to high risk of NAFLD and NASH (obesity and/or type 2 diabetes mellitus [T2DM]) and controls who were lean and healthy (Table 1, Figure 1). The participants were included via three clinical studies that were independently approved by the regional committees on health research ethics and registered at ClinicalTrials.gov (see details, Table 1). Participants were recruited from May 2016 until August 2021 from the University Hospital of South Denmark, Esbjerg, and Odense University Hospital, Odense. Study 1 comprised patients with severe obesity; Study 2, patients with T2DM; and Study 3, patients screened for liver disease. We also included 10 healthy participants. Inclusion and exclusion criteria for each study are provided in Table 1. All studies were conducted in accordance with the guidelines of the Declaration of Helsinki and the principles of good clinical practice. All subjects gave written informed consent for study participation and a separate biobank consent.
TABLE 1. Details of participants and methods used in this study.
| Lean healthy controls | Study 1: patients with severe obesity | Study 2: patients with T2DM | Study 3: patients screened for liver disease | |
|---|---|---|---|---|
| Derivation | n = 10 | n = 38 | – | – |
| Validation | – | n = 59 | n = 36 | n = 75 |
| Inclusion criteria |
|
|
|
|
| Exclusion criteria | (1) Use of prescription drugs, (2) Any chronic disease, (3) Use of antibiotics within 6 months, (4) Alcohol use >8 g for women and 16 g for men per day, (5) Abnormal US and/or elevated TE, (6) Hospital contacts within 6 months. | (1) Chronic liver disease other than NAFLDa, (2) Decompensated cirrhosisb, (3) Pregnancy, (4) Severe/deadly illness, (5) Hepatotoxic medicationc, (6) Alcohol use >12 g for women and >24 g for men per dayd, (7) Following low carbohydrate diete, (8) >10 kg weight loss within the last 3 monthse, (9) Use of antibiotics within the last 2 monthse. | ||
| Inclusion period | May 2016–Mar. 2018 | Aug. 2018–ongoing | Nov. 2016–Jun. 2021 | Oct. 2017–ongoing |
| Study name and ethics approvalf | Gala‐HP S‐20160006G | PROMETHEUS S‐20160006G | REDUCTION S‐20150217 | SIPHON S‐20170087 |
| Registration | OPEN.rsyd.dk, OP‐239 | ClinicalTrials.gov: NCT03535142 | ClinicalTrials.gov: NCT03068078 | ClinicalTrials.gov: NCT03308916 |
Note: Study 3 included patients with alcohol overuse, however, patients with alcohol overuse were excluded in Studies 1 and 2 (see Figure 2, consort diagram).
Abbreviations: BMI, Body Mass Index; OPEN, Odense Patient data Explorative Network; T2DM, type 2 diabetes mellitus; TE, transient elastography; US, ultrasound.
Liver biopsy and biochemical results showing evidence of viral hepatitis, autoimmune liver disease, cholestatic liver disease, and/or primary hemochromatosis.
Defined as manifestation of ascites, overt hepatic encephalopathy, jaundice, hepatorenal syndrome, or variceal hemorrhage.
Tamoxifen, amiodarone, systemic glucocorticoids, and methotrexate.
Only Study 1 and 2.
Only Study 2.
Identifying number from Ethics committee in Region of Southern of Denmark.
FIGURE 1.
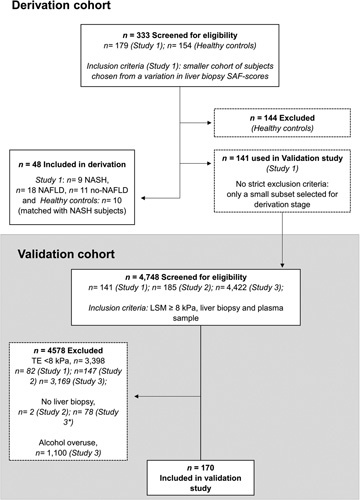
Consort diagram. Study 1, patients with severe obesity and matched controls; Study 2, patients with type 2 diabetes; and Study 3, patients screened for liver disease. LSM, liver stiffness measurement; SAF, steatosis, activity, and fibrosis; TE, transient elastography. *Reasons for not undergoing liver biopsy: TE scan on biopsy day <8 kPa (50%), patient did not consent to liver biopsy (29.5%), other reasons (20.5%).
Derivation cohort (n = 48)
We included candidates with severe obesity (n = 38) and controls who were lean and healthy (n = 10) in the derivation cohort for Study 1 (Table 1 and Figure 1). Study 1 is an ongoing single‐center, prospective case‐control study on future bariatric surgery patients and matched controls for body mass index (BMI), sex, and age. The study with healthy controls is a completed study, where all participants were extensively investigated.18 Patients with severe obesity from Study 1 were also included in the discovery analysis of Trem2 expression in hepatic tissues (n = 27).
Validation cohort (n = 170)
We included only patients with an LSM ≥8.0 kPa, a well‐established cutoff to rule in significant fibrosis.19,20 Patients were selected from three different studies (see Table 1 and Figure 1): Study 1 (described previously, n = 59); Study 2, a liver biopsy‐controlled single‐center randomized controlled trial in patients with T2DM (n = 36); and Study 3, a prospective single‐center study where subjects were screened for liver disease (transient elastography [TE] scan and blood samples), and participants with an LSM ≥8.0 kPa were offered a liver biopsy (n = 75).
Investigations
All samples and data were cross‐sectionally obtained after a minimum of 10 h of fasting and were collected on the same day. TE scans were performed by experienced staff using FibroScan 502 touch (Echosens, Paris, France) to obtain an LSM.21 The LSM was performed on the same day as the liver biopsy, except for in Study 3, where the biopsy date could vary (median 16 days, interquartile range [IQR] 28). The controls who were lean and healthy did not receive a liver biopsy, but all participants had an LSM <5.5 kPa (Table 1). Blood was drawn by trained staff and immediately processed and stored at −80°C by a single technician for biobanking. All routine biochemical analyses were performed by a local and central laboratory using commercially available kits.
Histopathological assessment
Liver biopsies were obtained percutaneously using the Menghini method, with a 16‐18G suction needle. Histologic assessments were performed according to the NASH Clinical Research Network classification system22: steatosis (0–3), lobular inflammation (0–3), and ballooning (0–2). NAS 0–8 is the sum score of these three features. We also applied the steatosis, activity, and fibrosis (SAF) scoring system.23 Hepatic fibrosis was semiquantitatively assessed using Kleiner fibrosis stages22: no fibrosis (F0), perisinusoidal or periportal (F1), perisinusoidal fibrosis in combination with portal and/or periportal fibrosis (F2), bridging fibrosis (F3), and cirrhosis (F4). Two pathologists highly experienced in the evaluation of NAFLD biopsies performed scorings and the clinical data were anonymized for them. In Study 1, pathologist T.C. performed all readings and in Study 2 and 3, pathologist S.D. performed all readings. We defined patients with NASH as liver biopsies with NAS ≥4.
Protein analysis
TREM2 (ab224881, Abcam. Cambridge, UK), matrix metalloproteinase 2 (MMP2) (ab267813, Abcam), tissue inhibitor metalloproteinase 1 (TIMP1) (ab187394, Abcam), or tissue inhibitor metalloproteinase 2 (TIMP2) (ab270213, Abcam) levels in plasma samples were analyzed using the respective human enzyme‐linked immunosorbent assay kits according to manufacturer's instructions. Briefly, 50 μl of each standard and sample was added to appropriate wells followed by the addition of 50 μl of antibody cocktail (detector and capture antibody mixture) and was incubated at room temperature (RT) for 1 hr with gentle shaking (400 rpm). After washing, 100 μl of 3,3′,5,5′‐Tetramethylbenzidine development solution was added to all wells and incubated for 10 min in the dark at RT with gentle shaking (400 rpm). Approximately 100 μl of stop solution was added to all wells followed by the measurement of absorbance at 450 nm in a UV/Vis microplate spectrophotometer (Thermo Scientific, Multiskan GO). Plasma levels of adiponectin and leptin were determined using multiplex kits (R‐PLEX human adiponectin, K151YTR‐2 and RV‐PLEX human leptin, K151V5D‐1, respectively; both Meso Scale Discovery, Maryland, USA) according to the manufacturer's instructions.
Data management
Study data were entered into and managed with a secured Research Electronic Data Capture database hosted by Open Patient data Explorative Network (https://www.sdu.dk/en/forskning/open). All writers had access to the study data and reviewed the final manuscript. The methods and results of this study were reported in accordance with the Liver‐FibroSTARD standards24,25 (Table S1). Murine gene expression data set submitted to GEO, sumission ID GSE207309.
Murine NASH model
NASH was introduced in mice and gene expression profilling performed as described in the supplementary methods. All animal experiments were approved by the Danish Animal Experiments Inspectorate (approval #2020‐15‐0201‐00603) and adhered to the ARRIVE guidelines.
Statistical analyses
All continuous data are presented as medians (± IQR), and categorical variables are expressed as absolute frequencies and percentages unless otherwise noted. We used unpaired two‐tailed Student t‐test (parametric data) or Mann‐Whitney U‐test (nonparametric data) for between‐group comparisons. Correlations between two groups were computed using Pearson correlation coefficient (two‐tailed p‐value). In the case of Trem2, Cd68, Colony stimulating factor 2 receptor alpha (Csfr2a), Integrin alpha X (Itgax), and Integrin alpha M (Itgam) gene expression, p‐values were adjusted for multiple testing using the Benjamini‐Hochberg procedure. The diagnostic accuracy of plasma TREM2 and other comparative noninvasive tests in the derivation and validation cohort were evaluated using area under receiver operating curves (AUROCs). We determined optimal singular cut‐off values for plasma TREM2 by maximizing the Youden index and determined rule‐in and rule‐out cutoffs by optimizing to 90% specificity and 90% sensitivity, respectively. To test for robustness and relative performance, we did subpopulation analyses on each independent cohort. We used a multivariable, ordered logistic regression to evaluate the correlation between plasma TREM2 concentration and NAS as a semiquantitative variable, to go beyond the dichotomization of NAS done in diagnostic testing. Before performing the ordered logistical regression analysis, we verified the proportional odds assumption by testing whether the coefficients were equal across categories using a maximum likelihood ratio test. We also investigated possible confounding variables of plasma TREM2 by linear and ordered logistic regression adjusting for age, sex, BMI, and hemoglobin A1c (HbA1c). A p‐value of 0.05 was considered statistically significant. STATA 17.0 and GraphPad Prism version 9.1.0.221 were used for the statistical analysis and generation of figures. The syntax is available on request.
All other information regarding materials and methods is provided in the Supporting Information.
RESULTS
Patient characteristics
In total, 4748 participants with risk factors were screened for eligibility to participate in the three studies from which a derivation cohort (n = 48) and a validation cohort (n = 170) of patients with elevated liver stiffness were recruited (Figure 1). Patient characteristics are shown in Table 1 and the participant selection processes in Figure 1.
Determination of Trem2 in hepatic tissue – discovery phase and proof of concept
First, we investigated expression levels of Trem2 in livers from a 52‐week Western diet‐fed, murine NASH model. Whole liver messenger RNA (mRNA)‐sequencing study revealed a 7‐fold increase in Trem2 expression in livers from Western diet‐fed mice as compared with chow diet‐fed mice (Figure 2A). Trem2 expression followed the expression of other mononuclear phagocyte surface markers Cd68, Csf2ra, Itgax, and Itgam (Figure 2A), and single‐cell RNA‐sequencing confirmed enrichment of Trem2 mRNA in macrophages (Bendixen et al., unpublished data). We then analyzed TREM2 expression in hepatic RNA samples from 27 patients with obesity from the derivation cohort (Table 1) (n = 6, NASH, NAS ≥4; n = 21, NAS <4). TREM2 expression was 3.3‐fold (p = 0.004) higher in patients with NASH than in subjects with NAS <4 (Figure 2B), with an AUROC of 0.83 (Figure 2C, Table S2).
FIGURE 2.
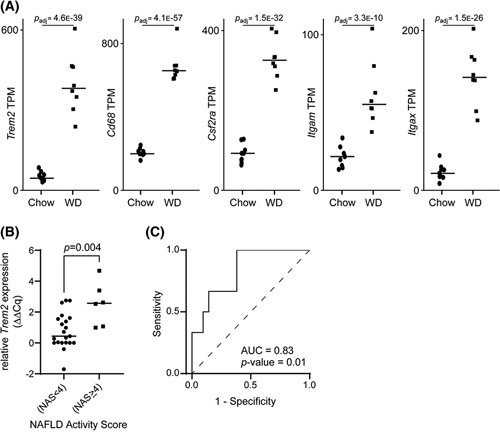
Exploratory analysis of triggering receptor expressed on myeloid cells 2 (Trem2) association with NASH. (A) Whole liver messenger RNA‐sequencing analysis showing increased expression of Trem2, Cd68, Colony stimulating factor 2 receptor alpha (Csfr2a), Integrin alpha X (Itgax), and Integrin alpha M (Itgam) in livers from Western diet (WD)‐fed mice as compared with chow diet‐fed mice. (B) TREM2 expression in liver tissue obtained from patients with NAFLD activity score (NAS) ≥4 and subjects with NAS <4 from 27 liver biopsies and categorized according to NAS. (C) Diagnostic accuracy of TREM2 expression between patients with NAS ≥4 and subjects with NAS <4.
Diagnostic performance of plasma TREM2 in the derivation cohort (n = 48)
Plasma TREM2 levels were significantly higher in patients with NASH than in subjects with NAS <4 (Figure 3A). All samples had TREM2 levels above the lower limit of detection (78.1 pg/ml). Plasma TREM2 diagnosed NASH with excellent accuracy (AUROC, 0.92; 95% confidence interval [CI], 0.84–0.99; p < 0.0001) and had a sensitivity of 100% (95% CI, 0.76–1.0) and specificity of 86% (95% CI, 0.71–0.94) (Figure 3B, Table S2). We also compared the performance of plasma TREM2 with other explorative NASH biomarkers in a subgroup of 28 subjects with obesity (n = 7 NASH and n = 21 NAS <4).4 Plasma TREM2 (AUROC, 0.95) showed higher diagnostic accuracy to discriminate patients with NASH as compared with TIMP1 (AUROC, 0.87), TIMP2 (AUROC, 0.53), and MMP2 (AUROC, 0.54) (Figure 3C, Table S2). From the same subgroup of patients as mentioned above, we examined liver biopsies from 27 available patients (n = 6, NASH; n = 21, NAS <4) to determine the correlation between hepatic TREM2 mRNA and plasma TREM2. A significant association was observed between hepatic mRNA and plasma levels (r = 0.72; p < 0.0001), suggesting that changes in the hepatic expression of TREM2 were reflected peripherally (Figure 3D, Table 2).
FIGURE 3.
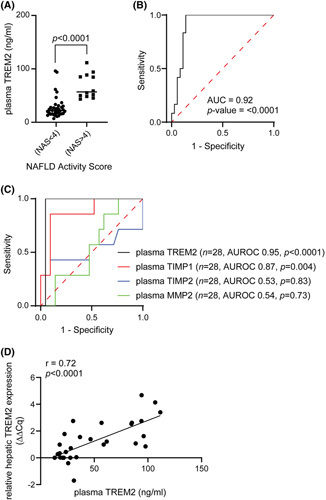
Plasma triggering receptor expressed on myeloid cells 2 (TREM2) levels can accurately discriminate patients with NAFLD activity score (NAS) ≥4 and subjects with NAS <4 in the derivation cohort. (A) Quantitative enzyme‐linked immunosorbent assay analysis of TREM2 levels in plasma extracted from patients with NAS ≥4 and subjects with NAS <4 categorized according to NAS (n = 48). (B) Diagnostic accuracy of plasma TREM2 levels. (C) Diagnostic accuracy of plasma TREM2, tissue inhibitor metalloproteinase 1 (TIMP1), tissue inhibitor metalloproteinase 2 (TIMP2), and matrix metalloproteinase 2 (MMP2) between patients with NAS ≥4 and subjects with NAS <4 (n = 28). (D) Correlation between hepatic TREM2 messenger RNA levels and plasma TREM2 levels in patients with biopsy‐proven NAFLD (n = 27).
TABLE 2. Patient characteristics.
| Derivation cohort (n = 48) | Validation cohort (n = 170) | ||||
|---|---|---|---|---|---|
| Lean healthy controls | Study 1: patients with severe obesity | Study 1: patients with severe obesity | Study 2: patients with T2DM | Study 3: patients screened for liver disease | |
| n = 10 | n = 38 | n = 59 | n = 36 | n = 75 | |
| Age (mean, SD) | 45 (12) | 41 (13) | 45 (13) | 56 (10) | 57 (10) |
| Sex (Male, n %) | 3 (30.0) | 10 (26.3) | 27 (45.8) | 16 (44.4) | 46 (61.3) |
| Metabolic phenotype | |||||
| BMI (kg/m2) | 24.1 (4.3) | 43.1 (8.4) | 42.7 (8.6) | 38.0 (12.1) | 35.0 (10.3) |
| Obesea | 0 (0.0) | 38 (100.0) | 59 (100.0) | 29 (80.6) | 60 (80.0) |
| Prediabetesb | 1 (10.0) | 14 (36.8) | 25 (71.4) | 0 (0.0) | 30 (85.7) |
| T2DM | 0 (0.0) | 7 (18.4) | 20 (33.9) | 36 (100.0) | 32 (42.7) |
| Dyslipidemiac | 0 (0.0) | 8 (21.1) | 19 (32.2) | 17 (47.2) | 31 (41.3) |
| Hypertensiond | 0 (0.0) | 15 (39.5) | 34 (57.6) | 22 (61.1) | 44 (58.7) |
| Biochemistry | |||||
| ALT (U/L) | 17.5 (11.3) | 30.0 (31.8) | 53.0 (42.0) | 37.0 (26.8) | 44.0 (27.0) |
| AST (U/L) | 25.0 (10.0) | 22.5 (11.3) | 36.5 (28.3) | 27.0 (16.8) | 33.0 (24.5) |
| GGT (U/L) | 18.5 (7.5) | 26.0 (32.0) | 44.0 (61.0) | 40.0 (48.8) | 64.0 (108.0) |
| Albumin (g/L) | 45.5 (4.8) | 42.0 (2.0) | 43.0 (4.0) | 44.0 (2.0) | 45.0 (4.0) |
| Triglycerides | 0.8 (0.6) | 1.4 (0.8) | 1.7 (1.3) | 1.7 (1.2) | 1.6 (1.3) |
| Total cholesterol | 4.8 (0.9) | 4.4 (1.3) | 4.4 (1.1) | 4.1 (0.9) | 4.2 (1.4) |
| Plasma TREM2 (ng/ml) | 16.0 (11.0) | 32.2 (36.8) | 55.7 (62.2) | 47.0 (39.0) | 41.6 (40.5) |
| TREM2 in NAS <4 | NA | 24.2 (15.6) | 33.0 (27.2) | 46.5 (20.2) | 30.4 (17.4) |
| TREM2 in NAS ≥4 | NA | 57.0 (37.5) | 86.5 (81.9) | 74.4 (51.7) | 63.6 (39.8) |
| Adiponectin (μg/ml) | NA | 172.7 (129) | 142.0 (86.4) | 131.9 (172.2) | 142.6 (201.2) |
| Leptin (ng/ml) | NA | 990.1 (704.4) | 806.9 (703.9) | 593.3 (528.4) | 359.7 (631.4) |
| Liver assessment | |||||
| TE (kPa) | 3.9 (1.4) | 6.8 (3.3) | 11.8 (10.6) | 11.0 (3.9) | 11.9 (8.0) |
| NASe group (%) 0–1/2–3/4–5/≥6 | NA | 34/34/26/5 | 14/39/37/10 | 8/56/33/3 | 13/47/31/9 |
| SAF score (%) no NAFLD/ NAFLD/ NASH | NA | 29/47/24 | 10/56/34 | 8/67/25 | 12/55/33 |
| Fibrosis stagef (%) 0/1/2/3/4 | NA | 29/50/16/5/0 | 9/52/25/9/5 | 3/33/55/6/3 | 3/41/29/20/7 |
Note: All descriptive data are medians (± interquartile range), or counts (%), or else noted in table.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, γ‐glutamyl transpeptidase; NA, not applicable; NAS, NAFLD activity score; SAF, steatosis, activity, and fibrosis; T2DM, type 2 diabetes mellitus; TE, transient elastography.
BMI ≥30 kg/m2.
Fasting glucose between 5.6–7.0 mM, but not T2DM.
Taking prescriptions on statins or fibrates.
Taking prescriptions on antihypertensive drugs and stating they have arterial hypertension.
NASH Clinical Research Network classification system groups.
Kleiner fibrosis stages.
Diagnostic performance of plasma TREM2 in the validation cohort (n = 170)
Plasma TREM2 was 2.1‐fold elevated in NASH patients compared to NAS <4 subjects (Figure 4A). The plasma TREM2 showed high diagnostic accuracy with an AUROC of 0.83 (95% CI, 0.77–0.89; p < 0.0001) to differentiate patients with NASH from subjects with NAS <4 (Figure 4B, Table S2).
FIGURE 4.
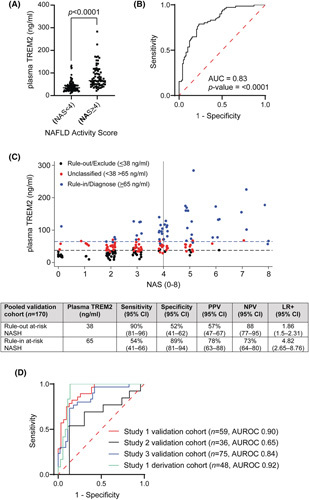
Plasma triggering receptor expressed on myeloid cell 2 (TREM2) levels can identify patients with NASH from subjects with NAFLD activity score (NAS) <4 in a large validation study (n = 170). Study 1, patients with severe obesity; Study 2, patients with type 2 diabetes; and Study 3, patients screened for liver disease. (A) Quantitative enzyme‐linked immunosorbent assay analysis of TREM2 levels in plasma extracted from patients with NAS ≥4 and subjects with NAS <4 categorized according to NAS. (B) Diagnostic accuracy of plasma TREM2 levels in patients with NAS ≥4 and subjects with NAS <4. (C) Plasma TREM2 levels according to NAFLD activity score (0–8) with dual cutoff rule‐in NAS ≥4 (≥65 ng/ml) and rule‐out NAS <4 (≤38 ng/ml) patients and diagnostic accuracy test probabilities for plasma TREM2 with 95% confidence intervals for NAS ≥4 (at‐risk NASH), with 2 × 2 tables. LR+, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value. (D) Diagnostic accuracy of plasma TREM2 across different validation cohorts.
We evaluated a dual cut‐off strategy for plasma TREM2 concentration to rule out and rule in NASH. A cutoff at 38 ng/ml was optimal to rule‐out (exclude) patients with NASH, with a sensitivity of 90% (95% CI, 81%–96%) and specificity 52%; whereas a plasma TREM2 level of 65 ng/ml was the optimal rule‐in (diagnose) cutoff, with a specificity of 89% (95% CI, 81%–94%) and sensitivity 54%. This approach divided the validation cohort into three groups: NAS <4 (n = 58), NASH (n = 49), and an intermediate group of unclassified patients (n = 63, 37%) (Figure 4C).
Next, we examined the ability of plasma TREM2 to discriminate patients with NASH from subjects with NAS <4 in the individual patient cohorts from Studies 1, 2, and 3. Plasma TREM2 levels consistently showed high diagnostic ability to rule‐in patients with NASH and rule‐out subjects with NAS <4 in Study 1 (AUROC derivation cohort, 0.92; validation cohort, 0.9) and Study 3 (AUROC, 0.84) cohorts. However, we observed a low diagnostic accuracy for plasma TREM2 in Study 2, a study of patients with T2DM (AUROC, 0.65) (Figure 4E, Table S2). Consequently, we performed a post hoc diagnostic accuracy analysis on only the patients with T2DM in the Study 1 and Study 3 cohorts that showed an AUROC of 0.90 and 0.83 respectively (Table S2).
To further validate the ability of plasma TREM2 levels to discriminate patients with NASH, we performed subgroup analyses and applied two additional classifications: at‐risk NASH, defined as NAS ≥4 and Fibrosis ≥2;21 and strict NASH according to the SAF‐definition,23 defined as steatosis ≥1, lobular inflammation ≥1, and ballooning ≥1. Analysis of the three NASH definitions in the 170 participants of the validation cohort revealed that 42% had NAS ≥4, 30% had at‐risk NASH, and 32% had strict NASH. The prevalence of NASH according to the different classifications in each cohort can be found in Table S3. Plasma TREM2 levels differentiated at‐risk NASH and strict NASH with AUROCs of 0.76 and 0.77, respectively (Figure S2A,B).
We then compared the diagnostic accuracy of different classifications of NASH (NAS >4, at‐risk NASH, and strict NASH) to other noninvasive test markers involved in NAFLD diagnosis such as FibroScan‐aspartate aminotransferase (FAST) score,26 Fibrosis‐4 (FIB‐4),27 Forns index,28 aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio,29 and NAFLD fibrosis score (NFS)30 and proposed a dual cutoff for all the above‐mentioned tests. In comparative receiver operating characteristic analyses, NAS ≥4 plasma TREM2 (AUROC, 0.84), at‐risk NASH (AUROC, 0.77), and strict NASH (AUROC, 0.78) individually performed on par with FAST‐score (AUROC, 0.78), but out‐performed FIB‐4 (AUROC, 0.58), Forns (AUROC, 0.55), AST/ALT (AUROC, 0.56), and NFS (AUROC, 0.54) (Table S4) (Figure S2C–E).
Association of plasma TREM2 with NASH progression and Fibrosis
To further understand the association of plasma TREM2 with the different histological components of NASH, we examined the correlation of plasma TREM2 with individual features of steatosis, inflammation, and ballooning. We observed significantly elevated levels of plasma TREM2 in patients with moderate to severe steatosis (grade 1–3), marked ballooning (grade 1 and 2), or moderate to severe inflammation (grade 1–3) compared with patients with low scores (Figure 5A–C).
FIGURE 5.
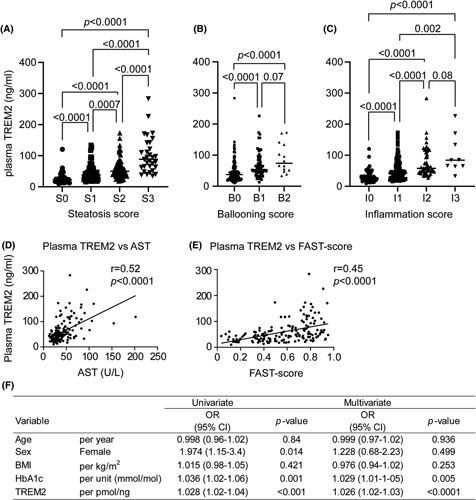
Plasma triggering receptor expressed on myeloid cells 2 (TREM2) levels are strongly associated with NAFLD progression. Distribution of subjects according to NASH Clinical Research Network histologic scoring system for (A) steatosis, (B) hepatocellular ballooning, and (C) lobular inflammation. Correlation between plasma TREM2 levels and plasma aspartate aminotransferase (AST) levels (D), and FibroScan‐AST score (E) in patients with biopsy‐proven NAFLD. (F) Logistic regression analysis for the prediction of NASH in the validation cohort (n = 170) patients with biopsy‐proven NAFLD. ALT, alanine aminotransferase; BMI, body mass index; HbA1c, hemoglobin A1c; OR, odds ratio.
Then, we tested the association of plasma TREM2 with well‐recognized NASH progression markers including AST and FAST score. The plasma TREM2 level exhibited a moderate correlation (Pearson) with the plasma AST level (r = 0.52, p < 0.0001) and FAST‐score (r = 0.45, p < 0.0001) (Figure 5D,E). Meanwhile, plasma TREM2 levels also exhibited a weak correlation with BMI (r = 0.16; p = 0.02) (Figure S3A). Multivariable logistic regression analysis revealed that plasma TREM2 level and HbA1c were independent predictors of NASH in the validation cohort, but not BMI (Figure 5F). We also analyzed the association of plasma TREM2 with markers of adipose tissue inflammation such as adiponectin and leptin. Although plasma TREM2 showed no significant correlation with adiponectin (r = 0.04; p = 0.52) (Figure S3B), leptin exhibited a weak correlation with plasma TREM2 levels (r = 0.24; p = 0.0006) (Figure S3C).
Finally, we investigated the association between plasma TREM2 and fibrosis. We observed a proportional increase in plasma TREM2 levels according to the fibrosis stages F0–F2, however, there was no stepwise increased association of plasma TREM2 levels with fibrosis stages F3–F4 (Figure 6A). The diagnostic accuracy of plasma TREM2 levels to differentiate advanced fibrosis (F3–F4) as compared with patients with F0–F2 fibrosis was low (AUROC, 0.61) (Figure 6B). Then, we tested the association of plasma TREM2 with well‐recognized fibrosis markers including NFS and FIB‐4. The plasma TREM2 level exhibited a weak correlation (Pearson) with the FIB‐4 (r = 0.05; p = 0.56) and NFS (r = 0.08; p = 0.34) (Figure 6C,D).
FIGURE 6.
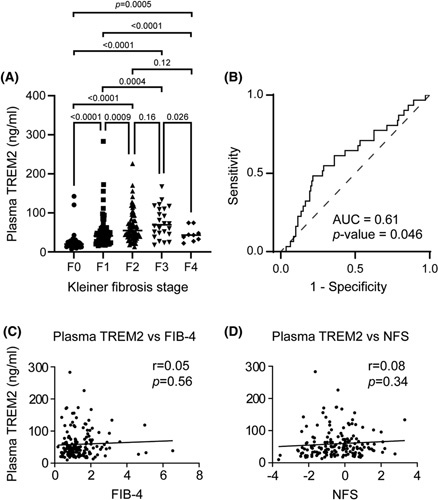
Association of plasma triggering receptor expressed on myeloid cells 2 (TREM2) with fibrosis. (A) Distribution of subjects according to Kleiner fibrosis stages (F0 [no fibrosis], F1 [perisinusoidal or periportal], F2 [perisinusoidal and portal/periportal], F3 [bridging fibrosis], and F4 [cirrhosis]). (B) Diagnostic accuracy of plasma TREM2 between minimal (F0‐2 stages) and advanced fibrosis (F3‐4 stages) patients. Correlation between plasma TREM2 levels and Fibrosis‐4 (C), and NAFLD fibrosis score (NFS) (D) in patients with biopsy‐proven NAFLD.
DISCUSSION
This study evaluated the diagnostic potential of plasma TREM2 as a noninvasive biomarker of NASH in three well‐characterized¸ biopsy‐controlled, clinical cohorts, totaling over 200 patients with elevated liver stiffness. Although previous studies have reported a positive association between TREM2 expression and NASH in liver tissues from patients with NAFLD,8–13 no studies have yet evaluated the diagnostic potential of elevated plasma TREM2 levels to identify NASH. We found that plasma TREM2 identified NASH with high diagnostic accuracy (AUROC, 0.92). Plasma TREM2 also performed better than other explorative individual biomarkers of NASH such as TIMP1, TIMP2, and MMP2.4 The plasma TREM2 level was strongly associated with the NAS and individual histologic features (steatosis, ballooning, and lobular inflammation). Further, the proposed rule‐in and rule‐out cutoff for NASH allowed us to exclude NASH in 34% of patients, and diagnose NASH in 29%, leaving only 37% of patients for a biopsy. Importantly, the performance of plasma TREM2 in the diagnosis of NASH across different patient cohorts with varied metabolic risk factors makes plasma TREM2 a reliable hepatic biomarker with the potential for generalizability. Moreover, the participants included in our study cohorts were recruited from primary and secondary care settings and were not highly selected patients recruited at tertiary hepatology settings. This means that plasma TREM2 was tested in a rather low‐prevalence population and even in such a setting, it still showed clinical potential.
We observed a low diagnostic accuracy for plasma TREM2 to diagnose patients at‐risk for NASH in the Study 2 cohort with 100% patients with T2DM as compared with Study 1 (34%) and Study 3 (43%) cohorts. However, the patients with T2DM in the Study 1 and Study 3 cohorts separately showed an AUROC of 0.91 and 0.83 respectively for plasma TREM2 to diagnose patients with NASH that suggests the negligible role of T2DM in the low diagnostic accuracy in the Study 2 cohort. Additionally, we found no key attributable demographic or clinical variables that could be suspected of this discrepancy. HbA1c was an independent predictor for belonging to a higher NAS score group, but a similar usage of antidiabetic medications by patients with T2DM across the three study cohorts substantiated the inconsistency in the role of T2DM in the plasma TREM2 concentration in patients with NASH (Figure S4). However, given the bidirectional role of T2DM in NASH,31,32 more investigations are required to further evaluate the relevance of circulating TREM2 in patients with T2DM and in patients who receive glucose‐lowering agents. In this direction, when we analyzed the role of adipose tissue inflammation markers such as leptin and adiponectin, the proinflammatory leptin showed a weak correlation with plasma TREM2, suggesting a possible interference of adipose tissue inflammation in NASH plasma TREM2 levels. However, detailed investigations are required to understand the contribution of TREM2 from adipose tissue in NASH‐associated plasma TREM2 concentration.
The ability of plasma TREM2 to identify patients with NASH and its strong association with NAFLD severity makes it a plausible individual biomarker for NASH. When we investigated TREM2 further in NASH progression, we found a strong positive association between plasma TREM2 and hepatocyte ballooning and inflammation. However, the diagnostic accuracy of plasma TREM2 to differentiate advanced fibrosis from lower fibrosis stages was low. One of the limiting factors that may have contributed to this observation is the low number of included patients with fibrosis grade F3 and F4 in the validation pool. In our view, the weak association of plasma TREM2 to advanced fibrosis is not unexpected, but biologically plausible because a single marker will likely not reflect a disease spectrum with widely different and heterogeneous histopathological features. Instead, single promising markers like plasma TREM2 should be further tested and externally validated in combination with specific markers of fibrosis such as TE or ProC3.33 Composite biomarker panels to increase the diagnostic utility of individual markers is a well‐recognized strategy and has previously been reported in NASH. For example, Cytokeratin 18 (CK18) is the most extensively evaluated single test for NASH diagnosis, but its overall moderate accuracy has provoked inclusion of CK18 in multiple biomarker panels including NASHTest (AUROC, 0.69–0.79) and NASH Diagnostic panel (AUROC, 0.73–0.91) with improved diagnostic accuracy.4 Likewise, the sensitivity and specificity of TIMP1 as a NASH biomarker were increased by combining it with age, hyaluronic acid, and amino‐terminal propeptide of type III collagen.34 Recently, NIS4 (combination of miR‐34a‐5p, alpha‐2 macroglobulin, YKL‐40, and HbA1c) was found to be effective to rule‐in or rule‐out at‐risk noncirrhotic NASH (NAS ≥4 and F ≥2) in patients with metabolic risk factors and suspected disease.17
LSM may be perceived as a limitation for routine analysis as the TE device is not yet widely available in primary care centers. However, LSM allows for the exclusion of patients with low risk of significant fibrosis and hence at‐risk NASH, and thus enabling preselection of patients from a low‐prevalence population. In addition, LSM was used to derive the FAST score in suspected NAFLD cases.26 Further, American Association for the Study of Liver Diseases and European Association for the Study of Liver guidelines35,36 propose that LSM by vibration‐controlled transient elastography may be used to identify patients who are at risk for steatohepatitis and/or advanced fibrosis.
Finally, although our study was performed with a robust design with well‐described prospective cohorts, larger validation studies should be conducted with patients recruited from multiple regions to improve generalizability for NASH diagnosis and to validate the proposed rule‐in and rule‐out cut‐off values.
In conclusion, we have identified and validated plasma TREM2 as a reliable noninvasive biomarker to diagnose NASH with elevated liver stiffness in a large cohort of patients with histologically characterized NAFLD.
Supplementary Material
Acknowledgments
AUTHOR CONTRIBUTIONS
Vineesh Indira Chandran contributed to conceptualization, methodology, investigation, visualization, formal analysis and writing of the original draft. Final approval for the version to be published. Charlotte Wilhelmina Wernberg contributed to methodology, resources, investigation, formal analysis, and writing of the original draft. Final approval for the version to be published. Mette Munk Lauridsen contributed to methodology, resources, investigation and review and editing. Maria Kløjgaard Skytthe contributed to investigation and writing ‐ review and editing. Sofie Marchsteiner Bendixen contributed to investigation and writing ‐ review and editing. Frederik Tibert Larsen contributed to investigation and writing ‐ review and editing. Camilla Dalby Hansen contributed to resources, investigation and writing ‐ review and editing. Lea Gadegaard Grønkjær contributed to resources, investigation and writing ‐ review and editing. Majken Storm Siersbæk contributed to investigation. Tina Di Caterino contributed to investigation and formal analysis. Sönke Detlefsen contributed to investigation and formal analysis. Holger Jon Møller contributed to investigation, validation and writing ‐ review and editing. Kim Ravnskjaer contributed to funding acquisition, supervision writing ‐ review and editing. Lars Grøntved contributed to conceptualization, funding acquisition, supervision and writing ‐ review and editing. Maja Sofie Thiele contributed to resources, formal analysis and writing ‐ review and editing. Søren Kragh Moestrup contributed to funding acquisition, conceptualization, supervision and writing ‐ review and editing. Aleksander Krag contributed to funding acquisition, conceptualization, methodology, resources, formal analysis and writing of the original draft. Final approval for the version to be published. Jonas Heilskov Graversen contributed to funding acquisition, conceptualization, methodology, formal analysis, supervision and writing of the original draft. Final approval for the version to be published. [Correction added August 1, 2022 after first online publication: Sönke Detlefsen's author contributions were included for this article].
CONFLICT OF INTEREST
Jonas Heilskov Graversen owns stock in Deliver Pharma. Maje Sofie Thiele advises GE Healthcare. She is on the speakers’ bureau for Echosens, Norgine, and Siemens Healthcare.
Footnotes
Funding information
Danmarks Grundforskningsfond, Grant/ Award Number: DNRF141; H2020 Health, Grant/Award Number: 668031; Innovationsfonden, Grant/Award Number: 1048-00010B
Abbreviations: AST, aspartate aminotransferase; AUROC, area under receiver operating characteristic curve; BMI, body mass index; CI, confidence interval; Csf2ra, Colony stimulating factor 2 receptor alpha; FAST, FibroScan‐aspartate aminotransferase; HbA1c, hemoglobin A1c; Itgam, Integrin alpha M; Itgax, Integrin alpha X; LSM, liver stiffness measurement; MMP2, matrix metalloproteinase 2; mRNA, messenger RNA; NAS, NAFLD activity score; NFS, NAFLD fibrosis score; SAF, steatosis, activity, and fibrosis; T2DM, Type 2 diabetes mellitus; TE, transient elastography; TIMP1, tissue inhibitor metalloproteinase 1; TIMP2, tissue inhibitor metalloproteinase 2; TREM2, triggering receptor expressed on myeloid cells 2.
Vineesh Indira Chandran and Charlotte Wilhelmina Wernberg contributed equally.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.hepjournal.com.
Contributor Information
Aleksander Krag, Email: akrag@health.sdu.dk.
Jonas Heilskov Graversen, Email: jgraversen@health.sdu.dk.
REFERENCES
- 1.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 2.Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411–28. [DOI] [PubMed] [Google Scholar]
- 3.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–44. [DOI] [PubMed] [Google Scholar]
- 4.Wong VWS, Adams LA, de Lédinghen V, Wong GLH, Sookoian S. Noninvasive biomarkers in NAFLD and NASH — current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15:461–78. [DOI] [PubMed] [Google Scholar]
- 5.Castera L, Friedrich‐Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1264–81.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145–59. [DOI] [PubMed] [Google Scholar]
- 7.Deczkowska A, Weiner A, Amit I. The physiology, pathology, and potential therapeutic applications of the TREM2 signaling pathway. Cell. 2020;181:1207–117. [DOI] [PubMed] [Google Scholar]
- 8.Perugorria MJ, Esparza‐Baquer A, Oakley F, Labiano I, Korosec A, Jais A, et al. Non‐parenchymal TREM‐2 protects the liver from immune‐mediated hepatocellular damage. Gut. 2019;68:533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong X, Kuang H, Ansari S, Liu T, Gong J, Wang S, et al. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single‐cell secretome gene analysis. Mol Cell. 2019;75:644–60.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daemen S, Gainullina A, Kalugotla G, He L, Chan MM, Beals JW, et al. Dynamic shifts in the composition of resident and recruited macrophages influence tissue remodeling in NASH. Cell Rep. 2021;34:108626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seidman JS, Troutman TD, Sakai M, Gola A, Spann NJ, Bennett H, et al. Niche‐specific reprogramming of epigenetic landscapes drives myeloid cell diversity in nonalcoholic steatohepatitis. Immunity. 2020;52:1057–74.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramachandran P, Dobie R, Wilson‐Kanamori JR, Dora EF, Henderson BEP, Luu NT, et al. Resolving the fibrotic niche of human liver cirrhosis at single‐cell level. Nature. 2019;575:512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govaere O, Cockell S, Tiniakos D, Queen R, Younes R, Vacca M, et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci Transl Med. 2020;12:eaba4448. [DOI] [PubMed] [Google Scholar]
- 14.Schlepckow K, Kleinberger G, Fukumori A, Feederle R, Lichtenthaler SF, Steiner H, et al. An Alzheimer‐associated TREM2 variant occurs at the ADAM cleavage site and affects shedding and phagocytic function. EMBO Mol Med. 2017;9:1356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feuerbach D, Schindler P, Barske C, Joller S, Beng‐Louka E, Worringer KA, et al. ADAM17 is the main sheddase for the generation of human triggering receptor expressed in myeloid cells (hTREM2) ectodomain and cleaves TREM2 after histidine 157. Neurosci Lett. 2017;660:109–114. [DOI] [PubMed] [Google Scholar]
- 16.Wunderlich P, Glebov K, Kemmerling N, Tien NT, Neumann H, Walter J. Sequential proteolytic processing of the triggering receptor expressed on myeloid cells‐2 (TREM2) protein by ectodomain shedding and γ‐secretase‐dependent intramembranous cleavage. J Biol Chem. 2013;288:33027–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison SA, Ratziu V, Boursier J, Francque S, Bedossa P, Majd Z, et al. A blood‐based biomarker panel (NIS4) for non‐invasive diagnosis of non‐alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:970–85. [DOI] [PubMed] [Google Scholar]
- 18.Trošt K, Ahonen L, Suvitaival T, Christiansen N, Nielsen T, Thiele M, et al. Describing the fecal metabolome in cryogenically collected samples from healthy participants. Sci Rep. 2020;10:885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–30. [DOI] [PubMed] [Google Scholar]
- 20.Papatheodoridi M, Hiriart JB, Lupsor‐Platon M, Bronte F, Boursier J, Elshaarawy O, et al. Refining the Baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J Hepatol. 2021;74:1109–1116. [DOI] [PubMed] [Google Scholar]
- 21.Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182–91. [DOI] [PubMed] [Google Scholar]
- 22.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 23.Nascimbeni F, Bedossa P, Fedchuk L, Pais R, Charlotte F, Lebray P, et al. Clinical validation of the FLIP algorithm and the SAF score in patients with non‐alcoholic fatty liver disease. J Hepatol. 2020;72:828–38. [DOI] [PubMed] [Google Scholar]
- 24.Guéchot J, Boursier J, de Ledinghen V, Poynard T, Carrat F, Leroy V, et al. Liver‐FibroSTARD checklist and glossary: tools for standardized design and reporting of diagnostic accuracy studies of liver fibrosis tests. Clin Chem Lab Med. 2015;53:1135–7. [DOI] [PubMed] [Google Scholar]
- 25.Boursier J, de Ledinghen V, Poynard T, Guéchot J, Carrat F, Leroy V, et al. An extension of STARD statements for reporting diagnostic accuracy studies on liver fibrosis tests: the Liver‐FibroSTARD standards. J Hepatol. 2015;62:807–15. [DOI] [PubMed] [Google Scholar]
- 26.Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan W‐K, et al. FibroScan‐AST (FAST) score for the non‐invasive identification of patients with non‐alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 28.Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez‐Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. [DOI] [PubMed] [Google Scholar]
- 29.Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol. 1999;94:1018–22. [DOI] [PubMed] [Google Scholar]
- 30.Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–97.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019;1:312–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomah S, Alkhouri N, Hamdy O. Nonalcoholic fatty liver disease and type 2 diabetes: where do diabetologists stand? Clin Diabetes Endocrinol. 2020;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniels SJ, Leeming DJ, Eslam M, Hashem AM, Nielsen MJ, Krag A, et al. ADAPT: an algorithm incorporating PRO‐C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatology. 2019;69:1075–86. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–113. [DOI] [PubMed] [Google Scholar]
- 35.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- 36.European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL‐EASD‐EASO clinical practice guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. [DOI] [PubMed] [Google Scholar]


