Abstract
N-acetyl-β-D-hexosaminidases (EC 3.2.1.52) are exo-acting glycosyl hydrolases that remove N-acetyl-β-D-glucosamine (Glc-NAc) or N-acetyl-β-D-galactosamine (Gal-NAc) from the non-reducing ends of various biomolecules including oligosaccharides, glycoproteins, and glycolipids. The same enzymes are sometimes called N-acetyl-β-D-glucosaminidases and this review article employs the shorthand descriptor HEX(NAG) to indicate that the terms HEX or NAG are used interchangeably in the literature. The wide distribution of HEX(NAG) throughout the biosphere and its intracellular location in lysosomes combine to make it an important enzyme in food science, agriculture, cell biology, medical diagnostics, and chemotherapy. For more than 50 years, researchers have employed chromogenic derivatives of N-acetyl-β-D-glucosaminide in basic assays for biomedical research and clinical chemistry. Recent conceptual and synthetic innovations in molecular fluorescence sensors, along with concurrent technical improvements in instrumentation have produced a growing number of new fluorescent imaging and diagnostics methods. A systematic summary of the recent advances in optical sensors for HEX(NAG) is provided under the following headings: assessing kidney health, detection and treatment of infectious disease, fluorescence imaging of cancer, treatment of lysosomal disorders, and reactive probes for chemical biology. The article concludes with some comments on likely future directions.
Keywords: enzyme, fluorescence, molecular probe, kidney disease, cancer imaging
Graphical Abstract
INTRODUCTION
The field of enzymology has employed colorimetric and fluorometric enzyme substrates for many decades with an early emphasis on enzyme assays that quantified the kinetic parameters or identified enzyme inhibitors. Over time, the technology has expanded in various directions and colorimetric/fluorometric enzyme substrates are now incorporated into a variety of different methods for imaging and diagnostics. Several recent review articles have recently summarized the broad strategies used to create colorimetric/fluorometric enzyme substrates,1 2 3 and the more specific topic of reactive probes for glycosidase enzymes.4 However, to the best of our knowledge this review article is the first to collate the different chromogenic and fluorogenic substrates for N-acetyl-β-D-hexosaminidases, a major sub-group of glycosyl hydrolase enzymes with increasing biomedical importance. As described below these activity-based molecular probes are the basis for a wide range of biomedical sensing applications in clinical diagnostics, high throughput drug screening, in-vivo imaging/fluorescence-guided surgery, gene and enzyme replacement therapies, and fundamental chemical biology.
A detailed discussion of the taxonomy for glycosyl hydrolase enzymes is beyond the scope of this article and only a brief summary is provided here. In short, N-acetyl-β-D-hexosaminidases (EC 3.2.1.52) are exo acting glycosyl hydrolases that remove N-acetyl-β-D-glucosamine (Glc-NAc) or N-acetyl-β-D-galactosamine (Gal-NAc) from the non-reducing ends of various biomolecules including oligosaccharides, glycoproteins, and glycolipids.5 According to the carbohydrate-active enzyme (CAZy) classification system, these enzymes are assigned to one of three glycosyl hydrolase families; GH3, GH20 or GH84.6 7 Members of the GH20 family are usually called β-N-acetyl-D-hexosaminidases (HEX) because they can recognize both GlcNAc and GalNAc sugar units, whereas members of the GH3 and GH84 families are usually called N-acetyl-β-D-glucosaminidases (NAG) because they only recognize GlcNAc. This taxonomy is not applied rigorously and some research subfields continue to use the name N-acetyl-β-D-glucosaminidases (NAG) for members of the GH20 family, in part because these enzymes were historically assigned to an older designation of β-N-acetyl-D-glucosaminidases (EC 3.2.1.30) that was subsequently merged into EC 3.2.1.52 in 1992.8 To avoid any ambiguity, this article consistently refers to these enzymes as N-acetyl-β-D-hexosaminidases but employs the shorthand descriptor HEX(NAG) to make clear that the literature terms HEX or NAG refer interchangeably to the same enzymes.
In humans, the two most important N-acetyl-β-D-hexosaminidases from the GH20 family are HEX(NAG) A (acidic) and HEX(NAG) B (basic) with isoelectric points of 5.4 and 7.9, respectively.7 HEXA is a heterodimeric structure comprised of an α and β subunit, and its primary physiological substrate is GM2 ganglioside.8 HEXB is a homodimer comprised of two β subunits and does not cleave GM2 ganglioside, but it will hydrolyze simple colorimetric/fluorometric HEX(NAG) substrates. The optimal pH for HEX(NAG) activity is ~5 which is consistent with its acidic location in lysosomes. The classical “hexosaminidase” mechanism is substrate-assisted catalysis with the oxygen of the substrate’s C-2 acetamido group forming a reactive oxazoline intermediate.7 The active site of HEX(NAG) can selectively accommodate N-acetyl glucose or N-acetyl galactose isomers and will accept an aglycone unit that is relatively hydrophobic.9 A functionally related enzyme is human O-GlcNAcase (OGA), which catalyzes the removal of O-GlcNAc from protein serine or threonine residues and is implicated in Alzheimer’s disease and osteoarthritis.10 The search for chemical inhibitors of OGA employs simple enzyme inhibition assays and uses fluorescent GlcNAc substrates;11 12 however, there has been limited development of optical sensors for specific imaging of OGA activity in biomedical samples. Therefore, OGA will not be discussed any further here.13 14
The aims of this review article are to summarize the potential of HEX(NAG) as a useful biomarker in several, quite different diseases, and to describe the colorimetric/fluorometric enzyme substrates that are used as optical sensors in biomedical research and clinical chemistry. The narrative focuses on the optical sensors listed in Scheme 1 which are derivatives of N-acetyl-β-D-glucosamine with different dyes as the aglycones. Upon substrate cleavage, the released dyes (optical reporters) undergo changes in absorption or fluorescence signal which enables spectroscopic detection or fluorescence imaging. The sections below describe how these HEX(NAG)-selective substrates have been used for the following biomedical applications; assessing kidney health, detection and treatment of infectious disease, fluorescence imaging of cancer, treatment of lysosomal disorders, and reactive probes for chemical biology.
Scheme 1.
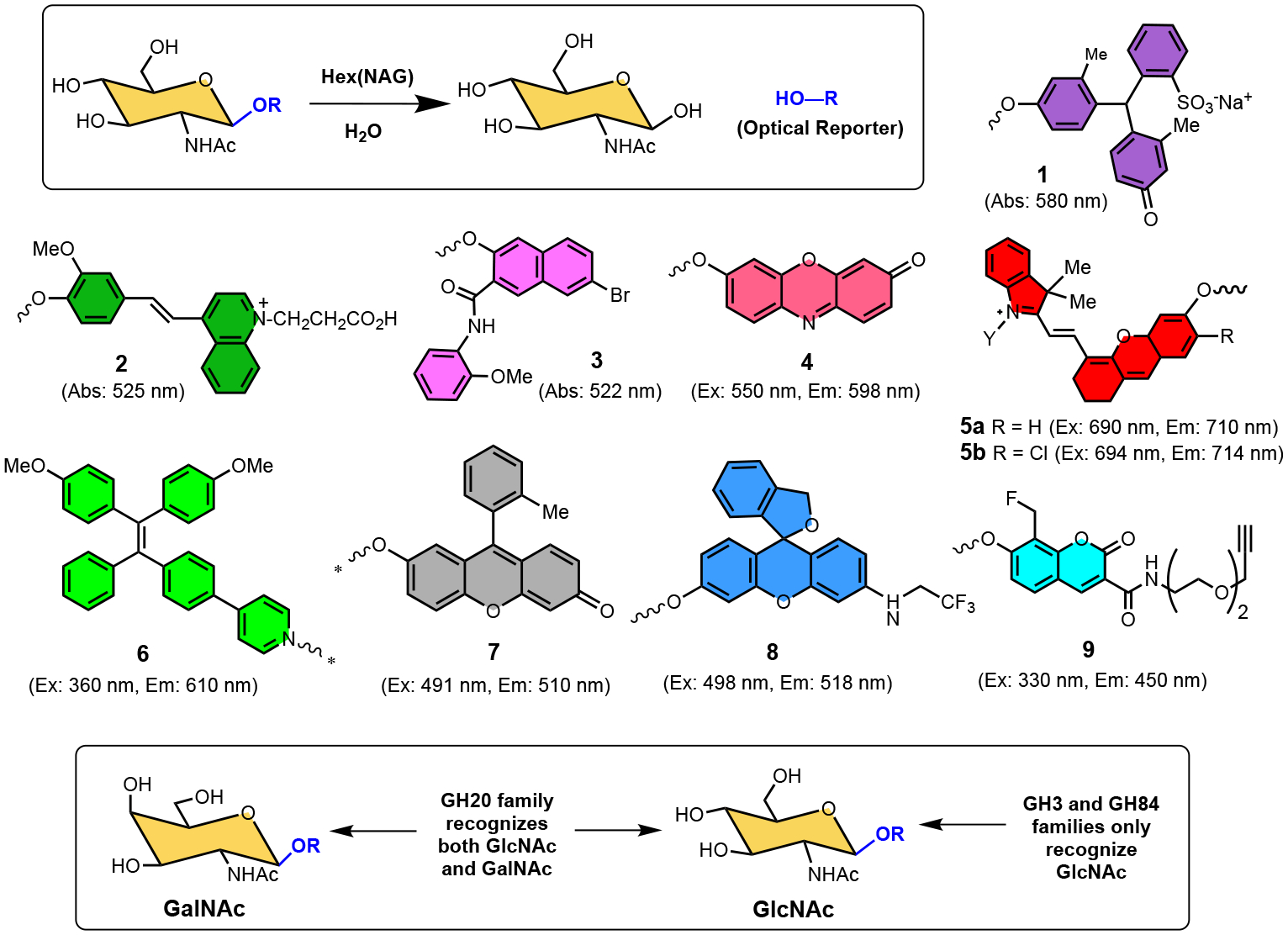
Enzymatic cleavage of the chromogenic or fluorogenic N-acetyl-β-D-glucosaminide substrates, 1 – 9, by HEX(NAG) releases an optical reporter that produces a detectable change in signal. Substrates 1 - 3 are chromogenic and substrates 4 - 9 are fluorogenic. The * shown on optical reporters 6 and 7 are reminders that there is a self-immolative linker between the N-acetyl-β-D-glucosaminide and the optical reporter. The lower box depicts the structural difference between GlcNAc and GalNAc, and the substrate selectivities of GH3, GH20 and GH84 glycosyl hydrolase families.
ASSESSING KIDNEY HEALTH
Within the kidneys, HEX(NAG) is predominantly found in the proximal and distal convoluted tubular cells and its concentration in the urine of healthy patients is low due to its large molecular size and inability to cross the glomerular basal membrane (Figure 1a). An increased concentration of urinary HEX(NAG) indicates renal tubular cell breakdown or more precisely loss of lysosomal integrity and for several decades, urinary HEX(NAG) has been measured and treated as a urinary biomarker for kidney disease (Figure 1b).15 The underlying disorder could be acute kidney Injury (AKI) which is defined as a sudden and serious loss of kidney function. Early detection of AKI is imperative, so the condition can be reversed before it deteriorates to renal replacement therapy or possibly death.16 It is worth emphasizing the global scale of AKI-related mortality which exceeds that of breast cancer, heart failure or diabetes.17 A separate but related kidney disorder is chronic kidney disease (CKD) which is a slow irreversible loss of kidney cells and nephrons over months or years.18 Globally, more than a million people die of CKD each year.19 Early stage detection of CKD is challenging since most patients are asymptomatic and the diagnosis is often made incidentally during an encounter for an unrelated health concern. Many urinary biomarkers have been investigated over several decades as potential indicators of AKI or CKD, and HEX(NAG) is one of the few urinary protein biomarkers that is an enzyme.20 21 22 There are many risk factors for elevated urinary HEX(NAG) including the following: diabetes, opportunistic infection including COVID-19, sepsis, malaria, pregnancy, severe trauma, hypovolemia, old age, acute organ failure, major surgery, nephrotoxic antibiotics or cancer chemotherapy, kidney transplantation, cirrhosis, dosing with imaging contrast-agent, and autoimmune disorders.23 24 25 26 27 28 29 30 31 32 33 34 35 Provided in Table S1 is a list of typical changes in urinary HEX(NAG) levels that have been reported in a wide range of clinical circumstances. In most cases, HEX(NAG) levels are elevated several fold, but in some cases the increases are less than 2-fold, and there is often considerable dispersion in the measured values for a specific patient cohort. Thus, there are many clinical variables that can affect HEX(NAG) levels and ongoing research seeks to identify meaningful correlations with other urinary biomarkers and patient risk factors. There is no doubt that future work to improve the prognostic value of urinary HEX(NAG) levels will be facilitated if the assays could be: (a) expanded in throughput to ensure larger sample numbers and thus improve the statistical significance of observed trends and analytical conclusions, (b) conducted more easily in low-resource settings that facilitate early detection and longitudinal tracking of kidney disease especially in underserved communities, and (c) conducted directly within the living subject using weakly invasive imaging methods that eliminate the potential artifacts that arise when urine samples are stored or transported.15
Figure 1.
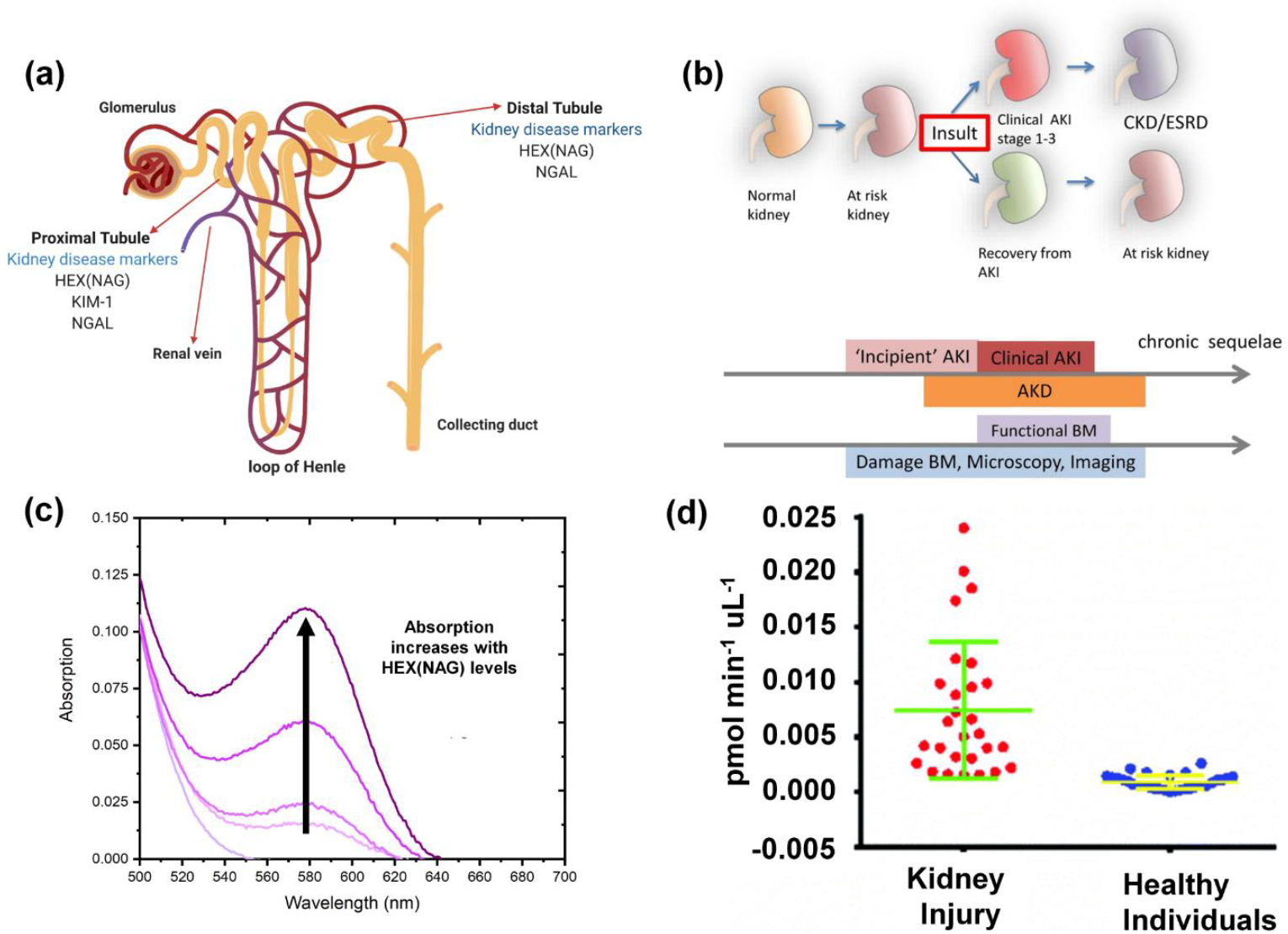
(a) Location of urinary biomarkers in the nephron. (b) Summary of the different stages of kidney disease and the use of urinary markers as indicators. (Reprinted (adapted) with permission from Springer Nature copyright 2021, https://creativecommons.org/licenses/by/4.0/),15 (c) Typical absorption profile for colorimetric substrate 1. (d) Plot of urinary HEX(NAG) levels measured using fluorometric substrate 4. (Reproduced from ref43 with permission from the Royal Society of Chemistry)
For the last few decades, clinical studies have measured HEX(NAG) in patient-provided urine samples using a small number of standard colorimetric or fluorometric assays.36 The basis of the more cost-effective colorimetric assay is enzyme cleavage of a suitably designed chromogenic substrate which releases a dye with a distinctive absorbance profile. The assay can be conducted as a single endpoint reading after multiple assay manipulations, or it can be monitored continuously over time as a kinetic assay. The Km value for most colorimetric HEX(NAG) substrates is typically in the high micromolar range, so relatively high substrate concentrations are needed to ensure enzyme saturation and a short assay incubation time.37 38 This means the released dye must exhibit a distinct red-shifted absorbance band for selective detection and quantification without interference from the high level of unreacted substrate as a background signal. The simplest colorimetric substrate for HEX(NAG) detection is 4-nitrophenyl N-acetyl-β-D-glucosaminide (pNP-NAG) which releases 4-nitrophenol for absorbance detection at 400 nm.39 Although cheap and convenient, the pNP-NAG assay is suspectable to interference by light absorbing components in the urine sample. This drawback is obviated by using chromogenic HEX(NAG) substrates that release dyes with longer wavelength absorption maxima bands. A current commercial colorimetric assay for HEX(NAG) detection uses sodium-3-cresolsulfonphthaleinyl-N-acetyl-β-D-glucosaminide (substrate 1 in Scheme 1) and releases the intensely colored dye, meta-cresol purple, which is detected at 580 nm after the assay is stopped by increasing the assay pH > 9 (Figure 1c).40 41 42
Fluorometric assays have inherently higher sensitivity than colorimetric assays which means the incubation time can be shorter and relatively dilute samples can be successfully analyzed. In addition, fluorometric assays can avoid interference problems due to background absorption that are encountered in colorimetric assays. Fluorescence “turn on” assays are less susceptible to artifacts than “turn off” assays and a range of fluorogenic HEX(NAG) probes have been prepared over the years. A common commercial fluorogenic substrate for HEX(NAG) detection is 4-methylumbelliferyl-N-acetyl-β-d-glucosaminide (4-MU-NAG). Upon cleavage by HEX(NAG) enzyme, 4-MU-NAG releases 4-methylumbelliferone, a fluorescent dye which emits at 460 nm.44 45 46 A recent successful effort to extend the emission band to a longer wavelength led to substrate 4 which was found to be rapidly cleaved by human HEX(NAG) to produce highly fluorescent resorufin dye for fluorescent detection at 598 nm.43 47 Substrate 4 was first used to quantify urinary HEX(NAG) activity in a mouse model of kidney disease and subsequently validated for use in urine samples from human patients. The graph in Figure 1d compares the HEX(NAG) activity in 58 urine samples from renal injury patients (n= 28) and healthy individuals (n=30). Overall, the mean urinary HEX(NAG) value for the patients with different degrees of kidney injury was 8.2 times the mean urinary value for the healthy patients.
An alluring prospect with fluorescence assays48, is the possibility of multiplex detection methods that can simultaneously report the outcome of multiple sensing assays conducted in the same sample. A good example is the multiplex assay in Figure 2a which treats a urine sample with three fluorescent probes that sense the presence of three different urinary enzymes as known biomarkers of kidney disease. One of the three fluorescent probes is HEX(NAG) substrate 5a which releases a near-infrared fluorescent dye that emits at 710 nm, and the other two probes in the assay are a substrate for urinary γ-glutamyl transferase (GGT) that releases a dye for detection at 440 nm, and a substrate for alanine aminopeptidase (AAP) that releases a dye for detection at 590 nm.49 Shown in Figure 2b is a typical data set from a study that measured the change in the three fluorescent probes (plus two other biomarkers) over time after treatment with the nephrotoxin cisplatin.
Figure 2.
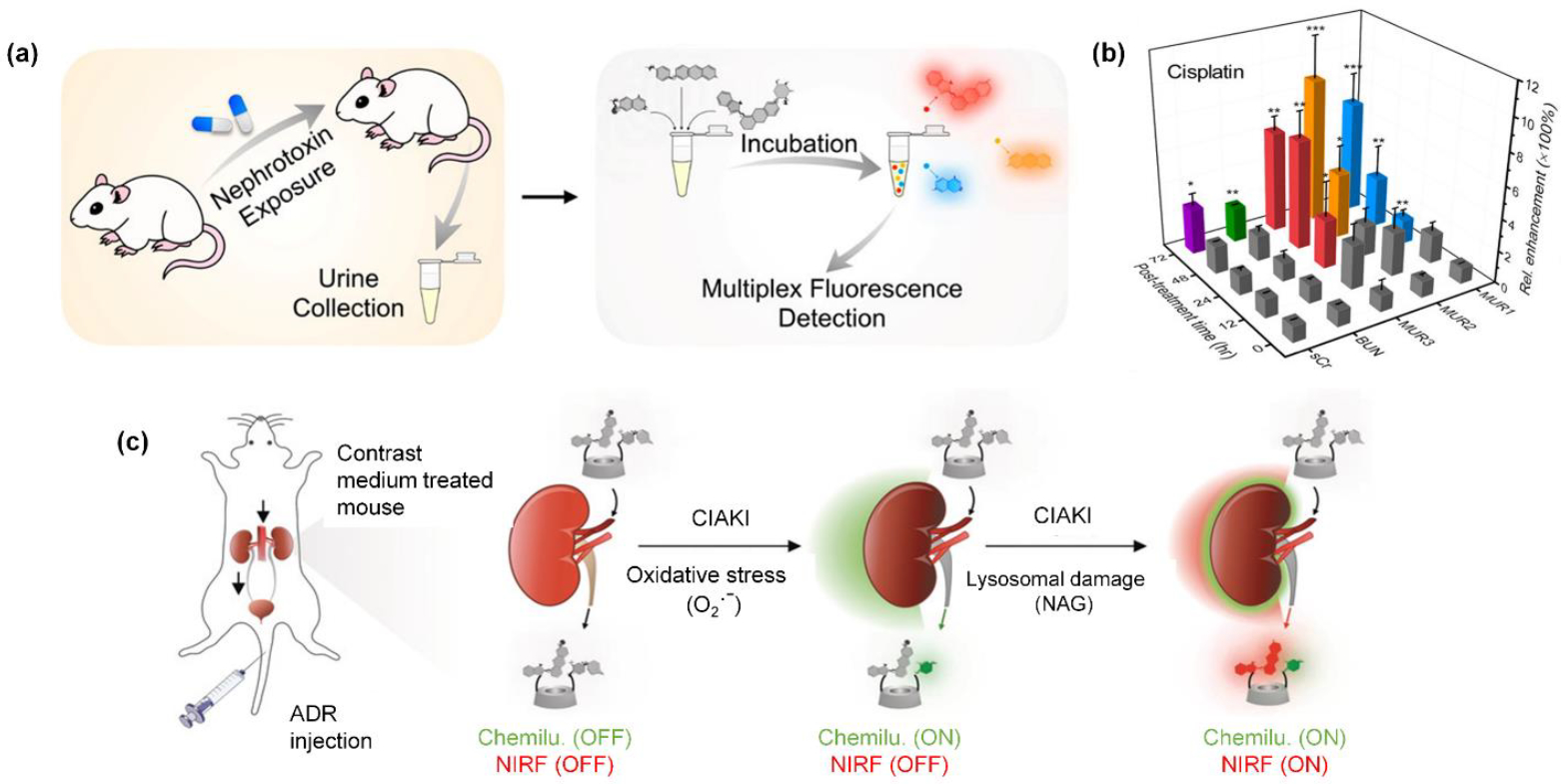
(a) Multiplex detection of multiple urinary biomarkers using a mixture of three fluorescent probes including substrate 5a for HEX(NAG). (b) Plot of signals from five urinary biomarkers (three are enzyme biomarkers, including HEX(NAG), as a function of time after treatment with the nephrotoxin cisplatin. (Reprinted (adapted) with permission.49 copyright (2020) American Chemical Society) (c) Activatable duplex reporter (ADR) for real time in vivo imaging of contrast agent induced AKI (CIAKI). The ADR has high renal clearance efficiency and it emits chemiluminescence and near-infrared fluorescence output signals that can be activated by oxidative stress or released HEX(NAG), respectively (Reprinted (adapted) with permission,50 copyright (2019).
Recently, a more direct approach to measuring the presence of kidney injury biomarkers has been proposed.50 The underlying concept is to dose the subject with an activatable reporter that is enzymatically cleaved to produce an optical signal for real-time in vivo imaging.51 A more ambitious version of the idea is an activatable duplex reporter (ADR) that can report two separate enzyme activities that are known to correlate with kidney disease. To date, the technology has been evaluated in a preclinical mouse model of kidney damage caused by dosage of excess contrast agent. The process described in Figure 2c shows an ADR that releases a near-infrared fluorescent reporter (derivative of substrate 5a) in the presence of the HEX(NAG) enzyme and a chemiluminescent reporter in the presence of oxidative stress (O2.−).50 The ADR was found to exhibited high renal clearance and permitted duplex optical imaging of the correlated biomarkers in the damaged kidneys of living mice.
DETECTION AND TREATMENT OF INFECTIOUS DISEASE
The field of diagnostic microbiology employs chromogenic enzyme substrates for detecting the presence of specific microorganisms within environmental and clinical samples.52 53 The synthetic substrates are incorporated into agar growth media and the color and morphology of colonies on the agar can often be correlated with microorganism identity.54 This simple technology is especially attractive in low and mid-income countries because it requires fewer lab resources than more sophisticated molecular tests based on PCR techniques.55 56 HEX(NAG) enzymes play an essential role in fungal nutrient utilization and morphogenesis making them essential enzymes, but HEX(NAG) expression levels depend on the fungal species and microenvironment.57 58 Therefore, commercial or customized chromogenic agar containing a chromogenic HEX(NAG) substrate is often quite useful for detecting and differentiating clinical strains of yeast and other fungal species.53 For example, a chromogenic HEX(NAG) substrate usually can discriminate Candida albicans or Candida dubliniensis from other yeasts. Shown in Figure 3a is the result of an assay that treated agar containing substrate 2 with Candida albicans.59 The presence of Candida dubliniensis yielded very similar colonies, whereas colonies of Candida kefyr or Candida tropicalis were uniformly pink, and colonies of other Candida species were white. Very recent work by several independent research groups has established that commercial chromogenic agar assays can be used to identify the presence of Candida auris, a multidrug-resistant species that is frequently encountered in hospital settings.60 61 62 A current drawback with the technique is the variation in interpretation when the assay is assessed by the naked eye. Looking to the future, it should be possible to mitigate this problem by using smart-phone technology to capture a digital photographic image of the assay and assess it using appropriate image recognition software.63
Figure 3.
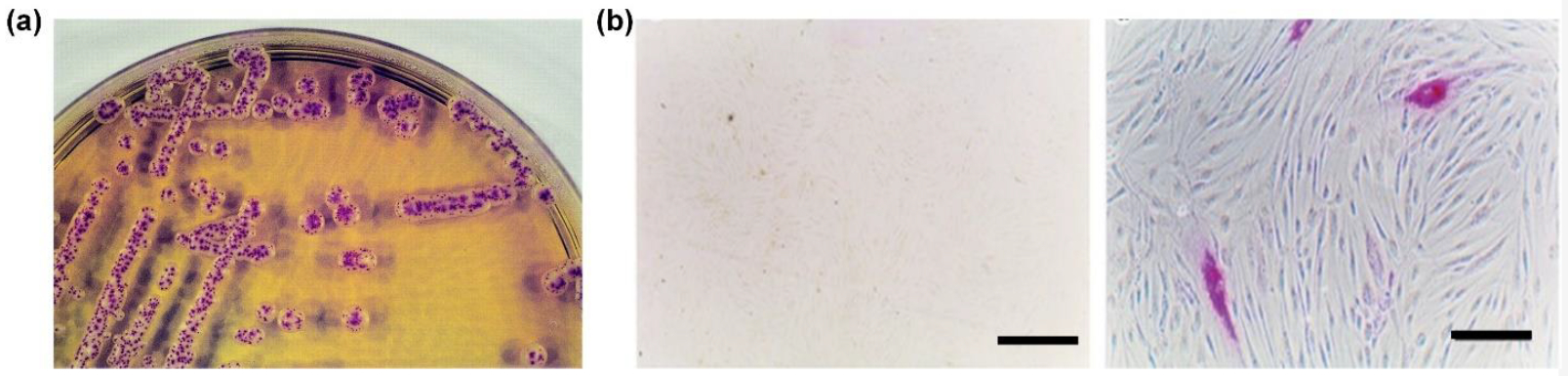
(a) Colonies of Candida albicans showing deep-red spots due to cleavage of chromogenic substrate 2 within the agar by the HEX(NAG) enzyme produced by the fungus. (Reprinted (adapted) with permission,59 copyright (2002) (b) Cultured cells showing purple color due to cleavage of added chromogenic substrate 3 by HEX(NAG); (left) Sandhoff fibroblasts lacking HEX(NAG) activity (scale bar = 160 mm) and (right) transfected Sandhoff fibroblasts with elevated HEX(NAG) activity (scale bar = 40 mm), reprinted with permission from Springer Nature.64
HEX(NAG) is a central enzyme in the life cycle of insects and microbial pathogens, because it controls the degradation of chitin, a linear polysaccharide within insect exoskeletons, crustacean shells and fungal cell walls. Thus, HEX(NAG) inhibitors are under active investigation as pest control agents or as potential drugs for treating opportunistic infections.7 65 66 The research often involves computer docking studies of enzyme/inhibitor complexes,67 and standard enzyme inhibition measurements using a commercial chromogenic substrate such as pNP-NAG or fluorogenic substrate such as 4-MU-NAG.
FLUORESCENCE IMAGING OF CANCER
Cancer cells are highly dependent on lysosomal recycling programs for survival and lysosomal enzymes are key players in tumor growth or suppression.68 Moreover, lysosomal proteases and hydrolases are often upregulated and mislocalized in cancer. Clinical analysis of urine, blood serum and tissue from colon cancer patients suggests that HEX(NAG) levels are elevated in colorectal tumors.69 70 This raises the possibility of HEX(NAG) acting as an enzyme target for cancer imaging and fluorescence guided surgery of colorectal cancer using endoscopic techniques. Fluorescence microscopy studies of cells treated with fluorogenic HEX(NAG) substrates produces localized fluorescence in the cell lysosomes.9 71 72 73 One example is substrate 6, which is cleaved by lysosomal HEX(NAG) to release an unstable phenolate intermediate which undergoes a self-immolative fragmentation to produce an insoluble tetraphenylethylene dye. Rapid self-aggregation of the dye leads to increased fluorescent signal due to the Aggregation Induced Emission (AIE) effect.71 As displayed in Figure 4a, cells treated with substrate 6 and the molecular probe Lysotracker Green DND-26 produced a very high level of fluorescence colocalization in cell lysosomes. An independent study developed the fluorogenic HEX(NAG) substrate 7 which also undergoes a self-immolative fragmentation after enzymatic cleavage by HEX(NAG).9 Fluorescence microscopy colocalization experiments confirmed selective targeting of the signal to cell lysosomes, and additional studies imaged lysosome dynamics. The micrographs in Figure 4b show a representative example of lysosome enlargement that occurs after cell treatment with chloroquine, a well-known lysosomotropic agent. To date, the most compelling evidence that fluorogenic HEX(NAG) substrates have promise for cancer imaging in human patients was produced by a study that evaluated the HEX(NAG) substrate 8. After validation experiments in living cancer cells the study demonstrated effective ex vivo imaging of small metastatic nodules (<1 mm) in a mouse model of disseminated colorectal cancer. Next, substrate 8 was applied to a small number of surgical samples from colorectal cancer patients and, as indicated by the example in Figure 4c, the tumor lesion was clearly visualized within a few minutes after application.74 The promising results support further development of fluorogenic HEX(NAG) substrates for fluorescence guided surgery and rapid pathology of colorectal cancer. A drawback with substrate 8 is the visible emission at 519 nm which is not optimal for imaging of cancer in thick biological samples; undoubtedly, imaging performance would be improved by switching to a fluorogenic HEX(NAG) substrate that absorbs and emits near-infrared light which can penetrate more deeply through skin and tissue.
Figure 4.
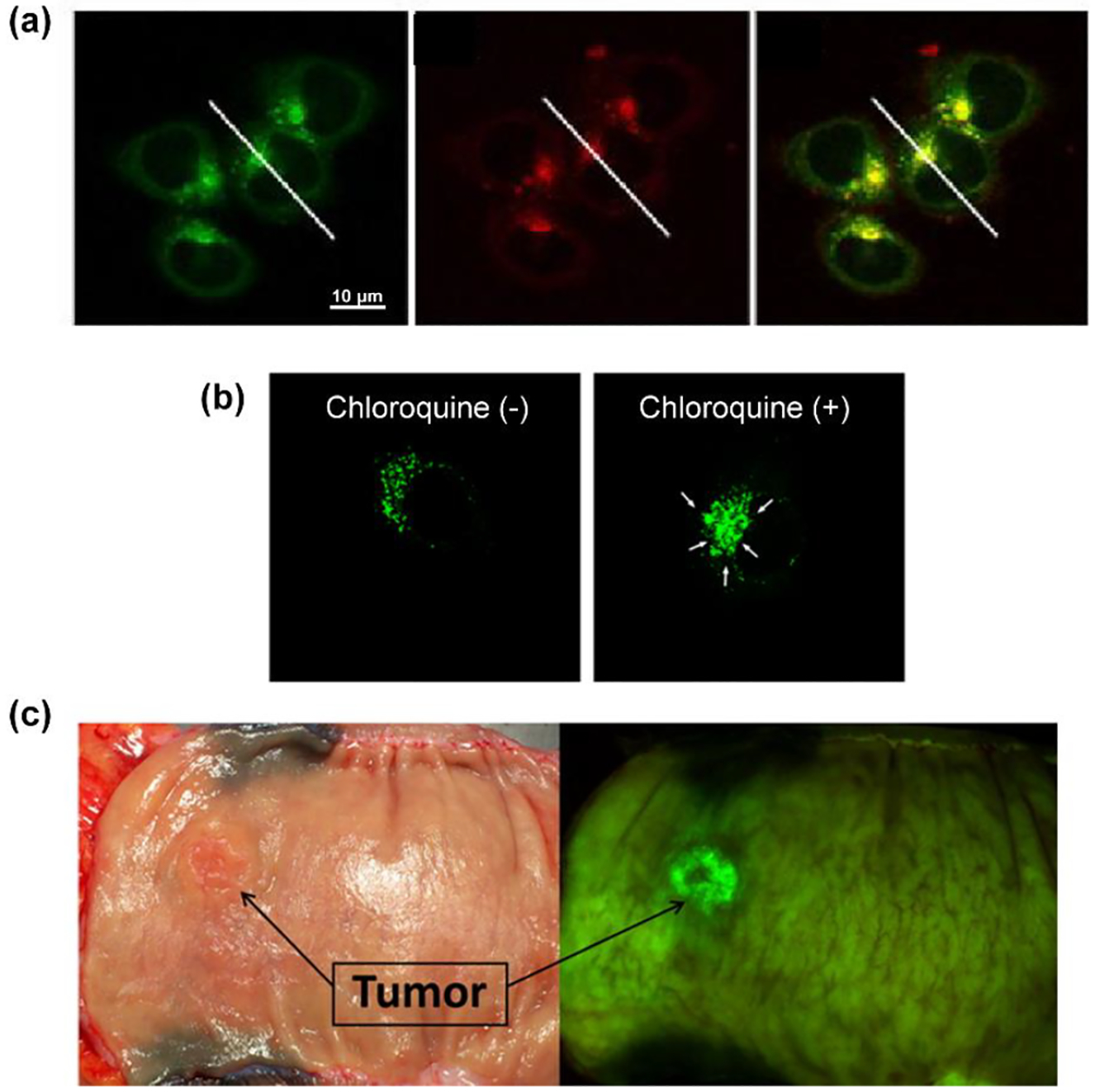
(a) Fluorescence micrographs of HCT116 colorectal cancer cells showing high colocalization of LysoTracker Green DND-26 (left) with HEX(NAG) substrate 6 (middle) and the merged images (right). (Reprinted (adapted) with permission.71 Copyright (2019) American Chemical Society). (b) Dynamic change in fluorescence images of chloroquine-treated HeLa cervical cancer cells stained with fluorescent HEX(NAG) substrate 7. Reproduced with permission from Chem. Pharm. Bull. 68. 526–533. Copyright 2021,The Pharmaceutical Society of Japan.9 (c) White light and fluorescence images of a surgical specimen from a colorectal cancer patient at 20 minutes after application of fluorescent HEX(NAG) substrate 8. (Reprinted (adapted) with permission.74 Copyright (2016) American Chemical Society)
TREATMENT OF LYSOSOMAL DISORDERS
The HEXA isoform of human N-acetyl-β-D-hexosaminidase is a heterodimeric structure comprised of an α and β subunit.8 Hereditary mutations in the gene which encodes for the α subunit are thought to cause Tay-Sachs disease which is a decreased capability to hydrolyze GM2 ganglioside and related glycolipids. This leads to accumulation of GM2 in the lysosomes of nerve cells which promotes cell toxicity and neurodegenerative disease. A related disorder is Sandhoff’s disease which is caused by mutations in the gene for the β subunit.75
An interesting potential strategy to treat these lysosomal disorders is the concept of Pharmacological Chaperones (Figure 5), which employs inhibitor molecules as stabilizing agents to increase the amount of folded and functional HEX(NAG) that is trafficked from the endoplasmic reticulum to the lysosomes.76 One study evaluated a library of 50,000 compounds as potential inhibitors of purified HEX(NAG) enzyme in a real-time high throughput screen using 4-MU-NAG as a fluorogenic substrate. Three compounds were identified that specifically produced a three-fold increase in the levels of a mutant HEXA α subunit in the lysates of fibroblasts derived from patients with adult Tay-Sachs disease.77 A separate study worked to improve the high throughput screening process by developing a one-step, cell-based screening assay that used fluorogenic 4-MU-NAG to assess HEX(NAG) activity within a 96-well format.78
Figure 5:
Treatment options for lysosomal disorders. Created with BioRender.com.
Another innovative potential strategy to treat lysosomal disorders is Enzyme Replacement Therapy (Figure 5).76 In the context of diseases caused by a deficiency of HEX(NAG), the basic idea is to deliver exogeneous HEX(NAG) to cell lysosomes. Effective implementation requires engineered HEX(NAG) with appended targeting elements to ensure lysosome accumulation. A recent study used genetic expansion combined with bioorthogonal ligation techniques to create synthetic HEX(NAG) with multiple copies of attached mannose-6-phosphate (M6P) units which were expected to bind M6P receptors in the Golgi complex and induce transport of the engineered HEX(NAG) to cell lysosomes.79 The study also prepared and evaluated several fluorogenic HEX(NAG) substrates for cell imaging performance, including a substrate called GalNAc-NIR-MP, an N-acetyl-β-D-galactoside derivative that is recognized and cleaved by HEX(NAG). The aglycone unit in this GalNAc-NIR-MP substrate was the fluorogenic near-infrared dye, 5b, with an appended morpholine unit to promote substrate accumulation in the cell lysosomes. A series of fluorescence microscopy experiments validated GalNAc-NIR-MP as a useful near-infrared fluorogenic substrate for cell imaging of HEX(NAG) activity. The data included: (a) colocalization of the near-infrared signal from 5b with Lysotracker Green dye, and (b) decreased near-infrared signal when the cells were treated with a HEX(NAG) inhibitor. Using patient-derived primary cells that lacked endogenous HEX(NAG), the study observed enhanced cell delivery of the engineered HEX(NAG) with attached multiple MP6 units compared to unmodified HEX(NAG). Histochemical experiments using GM2-ganglioside antibody showed a time-dependent decrease in lysosomal GM2-ganglioside levels in cells supplemented with the engineered HEX(NAG). The study concluded that engineered lysosomal enzymes, such as HEX(NAG), with appended M6P units as lysosome targeting agents is an attractive approach to Enzyme Replacement Therapy. The favorable fluorescence imaging properties of GalNAc-NIR-MP and its near-infrared reporter unit 5b make it an appealing candidate for other in-vivo HEX(NAG) imaging applications, such as the cancer imaging describe in the section above.
Gene Therapy is an alternative way to increase the levels of lysosomal enzymes in patients with inherited lysosomal storage disorders (Figure 5).80 Efforts using adeno-associated virus (AAV) gene therapy to treat Tay-Sachs and Sandhoff diseases have progressed from promising results in cell and animal models of the disease to the first clinical trials in human patients.80 81 64 The preclinical stages of these gene therapy studies employed chromogenic HEX(NAG) substrates such as commercially available 3 to determine if transfected cells and tissues express enhanced levels of functional HEX(NAG). The insolubility of the released dye makes substrate 3 quite suitable for bright field cell microscopy and histochemical staining.82 83 The images in Figure 3b illustrate how transfected Sandhoff fibroblasts with elevated HEX(NAG) activity exhibit an intense purple color due to high levels of selective enzymatic cleavage of 3.64
REACTIVE PROBES FOR CHEMICAL BIOLOGY
Increasing numbers of carbohydrate-based molecular probes are being developed as helpful tools for experimental glycobiology.84 85 The immobilization of carbohydrate molecules within spatially defined microarrays enables rapid interrogation of complex biological samples and simultaneous identification of proteins such as lectins or antibodies with specific carbohydrate affinity.86 Carbohydrate microarrays based on reactive probe molecules can be used to detect the presence of specific glycosidase activities. A recent study constructed a microarray containing fluorescent glycoside substrates whose fluorescence signals increase upon glycosidase-catalyzed cleavage of the glycosidic bonds (Figure 6a).87 An amine modified glass slide was covalently coated with bovine serum albumin (BSA), and then five different fluorogenic monosaccharide or disaccharide substrates with terminal hydrazide groups were attached to spatially distinct regions on the BSA-coated surface using a robotic high-precision pin-type microarrayer. One of the five glycosyl substrates was 5b an N-acetyl-β-D-glucosaminide derivative with a near-infrared dye as the aglycone. Cleavage of the glycosidic bond by HEX(NAG) revealed the dye whose enhanced near-infrared fluorescence was imaged by a suitable camera (Figure 6a). The microarray was used to compare the reactivity for different glycosidase/substrate pairs, and carbohydrate selectivity profiles were generated. For example, HEX(NAG) was found to catalyze the cleavage of immobilized N-acetyl-β-D-glucosaminide substrate 5b faster than the corresponding N-acetyl-β-D-galactosaminide substrate, a selectivity preference that matched the solution-state Michaelis-Menten kinetic parameters. Additional, proof-of-concept experiments used the microarrays to quantify enzyme inhibition. The data in the right side of Figure 6b shows determination of the IC50 value for HEX(NAG) inhibition by the known inhibitor compound PUGNAc. In the future, it seems this microarray technology could be utilized for HEX(NAG) biomarker discovery,88 89 90 or as a rapid screening platform within several of the drug discovery projects described above that evaluate large libraries of small molecules for capacity to act as a HEX(NAG) inhibitor.
Figure 6.
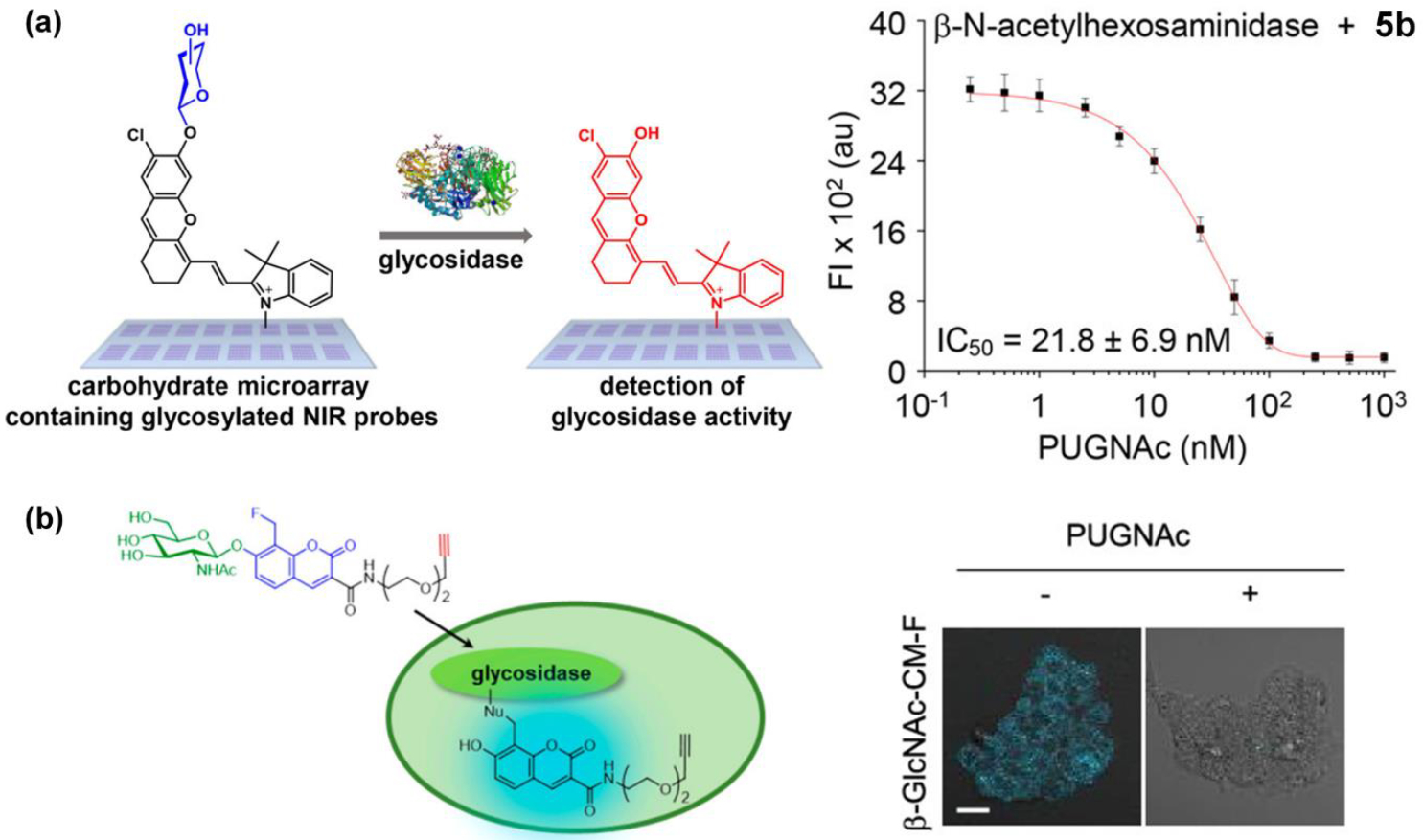
(a) (left) Carbohydrate microarrays containing different glycosyl substrates including the HEX(NAG) substrate 5b, (right) Quantification of IC50 value for HEX(NAG) inhibition by the inhibitor PUGNAc. (Reprinted (adapted) with permission.87 Copyright (2018) American Chemical Society). (b) (left) Cleavage of fluorinated fluorescent probe 9 leads to covalent labeling of HEX(NAG). (right) fluorescent labeling of intracellular HEX(NAG) by probe 9 is blocked by cell pretreatment with inhibitor PUGNAc. (Reprinted (adapted) with permission.92 Copyright (2019) American Chemical Society).
Chemical biologists are also using suitably designed reactive probe molecules to label and identify unknown enzymes within complicated biological media such as cell culture. An increasingly popular method is based on a substrate molecule that has been carefully designed to be cleaved by a specific enzyme class and generate an unstable intermediate that rapidly reacts with the enzyme surface and labels it for subsequent separation and identification steps. One elegant and generalizable molecular design aims to generate a reactive quinone methide intermediate which can be trapped by nucleophiles near the enzyme active site.91 An example of this paradigm is the trifunctional fluorogenic probe 9 shown in Figure 6b.92 Selective cleavage of this probe by HEX(NAG) enzyme produces an unstable phenoxy intermediate which spontaneously undergoes an elimination process to release fluoride anion and generate a quinone methide that reacts with a nucleophilic residue on the HEX(NAG) enzyme. The two cell micrographs in Figure 6b show that probe 9 can fluorescently label intracellular HEX(NAG), and that the process can be prevented by selective inhibition of the enzyme by pretreating the cells with PUGNAc a known HEX(NAG) inhibitor compound. Not only does this chemical biology method place a covalent fluorescent label on the HEX(NAG) surface but the trifunctional probe is also equipped with an alkyne group for subsequent attachment of a biotin unit and separation of the labeled HEX(NAG) by affinity chromatography. In principle, probe 9 can be used to identify new classes of HEX(NAG) enzymes in a wide range of complex biological specimens.
CONCLUSIONS
The wide distribution of HEX(NAG) throughout the biosphere and its intracellular location in lysosomes combine to make it an important enzyme in food science, agriculture, cell biology, medical diagnostics, and chemotherapy. For more than 50 years, researchers have employed chromogenic derivatives of N-acetyl-β-D-glucosaminide in basic assays for biomedical research and clinical chemistry. Conceptual and synthetic advances in molecular fluorescence sensors, along with concurrent technical improvements in instrumentation have produced a growing number of new fluorescent imaging and diagnostics methods. As described in this article, new methods for HEX(NAG) detection have been applied in clinical diagnostics, high throughput drug screening, in vivo imaging and fluorescence guided surgery, gene therapy, enzyme replacement therapy, and fundamental chemical biology. In some cases, the method can be readily improved with some simple optimization of the fluorescent sensor. For example, the use of fluorescent HEX(NAG) substrates for in vivo imaging of cancer (Figure 4c) will be enhanced by switching the fluorescent reporter from green emitting substrate 8 to a near-infrared substrate such as 5. Indeed, further enhancement of in vivo image contrast will be gained by extending the emission wavelength of the fluorescent reporter to 808 nm (which minimizes the variation in background signal due to changes in hemoglobin oxygenation93) or into the near-infrared II window >1000 nm.94 From a molecular design perspective, the task is more complicated than a simple exchange of the optical reporter component within the substrate chemical structure. As summarized in recent reviews, optimization of colorimetric/fluorometric enzyme substrates is a multiparameter problem that has to produce a molecule with a proper combination of chemical, physical, spectral, and enzyme recognition properties.1 2 3 In some cases, the probe optimization process can be achieved by an iterative cycle of rational design, but in other cases it may be more efficient to screen libraries of substrate candidates.
Looking beyond colorimetric and fluorometric reporters, it is surprising that the development of chemiluminescent HEX(NAG) substrates has not been reported beyond two early papers in 1991 and some peripheral mention in the patent literature.95 96 97 98 Most recently, HEX(NAG) sensing platforms have been described based on surface-enhanced Raman spectroscopy and electrochemical technologies.99 89 100 From the perspective of bioconjugate chemistry there is an opportunity to develop next-generation substrates with more sophisticated chemical structures that can distinguish between the different isoforms of HEX(NAG), and between members of the different GH3, GH20 and GH84 families of β-N-acetyl-D-hexosaminidases. A selective fluorescent sensor for O-GlcNAcase (OGA, catalyzes the removal of O-GlcNAc from protein serine or threonine residues) would be a very helpful contribution to research on the biology of protein O-GlcNAcylation and its role in Alzheimer’s disease and osteoarthritis.10
Supplementary Material
ACKNOWLEDGEMENT
We are grateful for funding support provided by NIH grants R35GM136212 and T32GM075762 and an ADT grant from the University of Notre Dame.
Footnotes
Supporting Information.
The following file is available free of charge at …
Table S1, a list of typical changes in urinary HEX(NAG) levels in different clinical circumstances.
(PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Pala L; Sirec T; Spitz U Modified Enzyme Substrates for the Detection of Bacteria: A Review. Molecules 2020, 25 (16), 1–30. 10.3390/molecules25163690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Rajapaksha AA; Fu YX; Guo WY; Liu SY; Li ZW; Xiong CQ; Yang WC; Yang GF Review on the Recent Progress in the Development of Fluorescent Probes Targeting Enzymes. Methods Appl. Fluoresc 2021, 9 (3), 1–27. 10.1088/2050-6120/abf988. [DOI] [PubMed] [Google Scholar]
- (3).Zeng Z; Liew SS; Wei X; Pu K Hemicyanine-Based Near-Infrared Activatable Probes for Imaging and Diagnosis of Diseases. Angew. Chem. Int. Ed 2021, 60, 26454–26475. 10.1002/anie.202107877. [DOI] [PubMed] [Google Scholar]
- (4).Wei X; Feng Y; Chen M; Zhang S; Chen M; Zhang J; Zhang Y; Ye Q; Ding Y; Xue L; Wu Q Recent Advances in Glycosidase Probes Used in Escherichia Coli Detection. Curr. Med. Chem 2020, 28 (26), 5386–5410. 10.2174/0929867328999201224111615. [DOI] [PubMed] [Google Scholar]
- (5).Slámová K; Bojarová P; Petrásková L; Křen V β-N-Acetylhexosaminidase: What’s in a Name…? Biotechnol. Adv 2010, 28 (6), 682–693. 10.1016/j.biotechadv.2010.04.004. [DOI] [PubMed] [Google Scholar]
- (6).Cantarel BI; Coutinho PM; Rancurel C; Bernard T; Lombard V; Henrissat B The Carbohydrate-Active EnZymes Database (CAZy): An Expert Resource for Glycogenomics. Nucleic Acids Res 2009, 37, 233–238. 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Liu T; Yan J; Yang Q Comparative Biochemistry of GH3, GH20 and GH84 β-N-Acetyl-Dhexosaminidases and Recent Progress in Selective Inhibitor Discovery. Curr. Drug Targets 2012, 13 (4), 512–525. 10.2174/138945012799499730. [DOI] [PubMed] [Google Scholar]
- (8).Liu T; Duan Y; Yang Q Revisiting Glycoside Hydrolase Family 20 β-N-Acetyl-D-Hexosaminidases: Crystal Structures, Physiological Substrates and Specific Inhibitors. Biotechnol. Adv 2018, 36 (4), 1127–1138. 10.1016/j.biotechadv.2018.03.013. [DOI] [PubMed] [Google Scholar]
- (9).Miura K; Aoyama Y; Natsu Y; Koyama R; Hirano T; Nishio T; Hakamata W Development of Specific Fluorogenic Substrates for Human β-N-Acetyl-D-Hexosaminidase a for Cell-Based Assays. Chem. Pharm. Bull 2020, 68 (6), 526–533. 10.1248/CPB.C20-00069. [DOI] [PubMed] [Google Scholar]
- (10).Mueller T; Ouyang X; Johnson MS; Qian W-J; Chatham JC; Darley-Usmar V; Zhang J New Insights Into the Biology of Protein O-GlcNAcylation: Approaches and Observations. Front. Aging 2021, 1, 1–28. 10.3389/fragi.2020.620382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Elbatrawy AA; Kim EJ; Nam G O-GlcNAcase: Emerging Mechanism, Substrate Recognition and Small-Molecule Inhibitors. ChemMedChem 2020, 15 (14), 1244–1257. 10.1002/cmdc.202000077. [DOI] [PubMed] [Google Scholar]
- (12).Wang X; Li W; Marcus J; Pearson M; Song L; Smith K; Terracina G; Lee J; Hong KLK; Lu SX et al. MK-8719, a Novel and Selective O-GlcNAcase Inhibitor That Reduces the Formation of Pathological Tau and Ameliorates Neurodegeneration in a Mouse Model of Tauopathy. J. Pharmacol. Exp. Ther 2020, 374 (2), 252–263. 10.1124/jpet.120.266122. [DOI] [PubMed] [Google Scholar]
- (13).Kim EJ In Vitro Biochemical Assays for O-GlcNAc-Processing Enzymes. ChemBioChem 2017, 18 (15), 1462–1472. 10.1002/cbic.201700138. [DOI] [PubMed] [Google Scholar]
- (14).Worth M; Li H; Jiang J Deciphering the Functions of Protein O-GlcNAcylation with Chemistry. ACS Chem. Biol 2017, 12 (2), 326–335. 10.1021/acschembio.6b01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Katagiri D; Wang F; Gore JC; Harris RC; Takahashi T Clinical and Experimental Approaches for Imaging of Acute Kidney Injury. Clin. Exp. Nephrol 2021, 25, 685–699. 10.1007/s10157-021-02055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Kellum JA; Romagnani P; Ashuntantang G; Ronco C; Zarbock A; Anders HJ Acute Kidney Injury. Nat. Rev. Dis. Prim 2021, 7 (52), 1–17. 10.1038/s41572-021-00284-z. [DOI] [PubMed] [Google Scholar]
- (17).Lewington Andrew JP., Cerda Jorge., Metha R Raising Awareness of Acute Kidney Injury: A Global Perspective of a Silent Killer. Kidney Int 2013, 84 (3), 457–467. 10.1117/12.2549369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chawla LS; Kimmel PL Acute Kidney Injury and Chronic Kidney Disease: An Integrated Clinical Syndrome. Kidney Int 2012, 82 (5), 516–524. 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- (19).Bikbov B; Purcell CA; Levey AS; Smith M; Abdoli A; Abebe M; Adebayo OM; Afarideh M; Agarwal Sanjay Kumar, et al. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395 (10225), 709–733. 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Price RG Urinary Enzymes, Nephrotoxicity and Renal Disease. Toxicology 1982, 23 (2–3), 99–134. 10.1016/0300-483x(82)90092-0. [DOI] [PubMed] [Google Scholar]
- (21).Bujnowska A; Bȩdzichowska A; Jobs K; Kalicki B Novel Early Markers of Chronic Kidney Disease. Pediatr Med Rodz 2019, 15 (3), 234–239. 10.15557/PiMR.2019.0039. [DOI] [Google Scholar]
- (22).Beker BM; Corleto MG; Fieiras C; Musso CG Novel Acute Kidney Injury Biomarkers: Their Characteristics, Utility and Concerns. Int. Urol. Nephrol 2018, 50, 705–713. 10.1007/s11255-017-1781-x. [DOI] [PubMed] [Google Scholar]
- (23).Ronco C; Reis T; Husain-Syed F Management of Acute Kidney Injury in Patients with COVID-19. Lancet Respir. Med 2020, 8 (7), 738–742. 10.1016/s2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).So Ra Kim, Yong-ho Lee, Sang-Guk Lee, Eun Seok Kang B; Cha Bong-Soo, Jeong-Ho Kim, B.-W. L Urinary N-Acetyl-β-D-Glucosaminidase, an Early Marker of Diabetic Kidney Disease, Might Reflect Glucose Excursion in Patients with Type 2 Diabetes. Medicine (Baltimore) 2016, 95 (27), 4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Hong N; Lee M; Park S; Lee Y; Jin S; Kim JH; Lee B Elevated Urinary N-Acetyl- β -D- Glucosaminidase Is Associated with A1c Ratio in Type 1 Diabetes Patients with Early Diabetic Kidney Disease. Sci. Rep 2018, 8 (1), 1–8. 10.1038/s41598-018-25023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Xiong WFS; Shu YF; Deng MCR; Zheng L; Wang LPQ Urinary Tubular Biomarkers in Short-Term Type 2 Diabetes Mellitus Patients : A Cross-Sectional Study 2012, 82–88. 10.1007/s12020-011-9509-7. [DOI] [PubMed] [Google Scholar]
- (27).Bagshaw SM; George C; Bellomo R Early Acute Kidney Injury and Sepsis: A Multicentre Evaluation. Crit. Care 2008, 12 (2), 1–9. 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kersting S; Koomans HA; Hené RJ; Verdonck LF Acute Renal Failure after Allogeneic Myeloablative Stem Cell Transplantation: Retrospective Analysis of Incidence, Risk Factors and Survival. Bone Marrow Transplant 2007, 39 (6), 359–365. 10.1038/sj.bmt.1705599. [DOI] [PubMed] [Google Scholar]
- (29).Benoit DD; Hoste EA; Depuydt PO; Offner FC; Lameire NH; Vandewoude KH; Dhondt AW; Noens LA; Decruyenaere JM Outcome in Critically Ill Medical Patients Treated with Renal Replacement Therapy for Acute Renal Failure: Comparison between Patients with and Those without Haematological Malignancies. Nephrol. Dial. Transplant 2005, 20 (3), 552–558. 10.1093/ndt/gfh637. [DOI] [PubMed] [Google Scholar]
- (30).Kaufmann M; Schlossbauer M; Hubauer U; Stadler S; Fischer M; Wallner S; Hupf J; Zimmermann M; Orso E; Zeman Florian, et al. N-Acety-b-D-Glucosaminidase: A Potential Biomarker for Early Detection of Acute Kidney Injury in Acute Chest Pain. Nephrology 2020, 25 (2), 135–143. 10.1111/nep.13664. [DOI] [PubMed] [Google Scholar]
- (31).Guillon B; Ecarnot F; Marcucci C; Ducloux D; Chatot M; Badoz M; Bonnet B; Chopard R; Frey P; Meneveau Nicolas, et al. Incidence, Predictors, and Impact on Six-Month Mortality of Three Different Definitions of Contrast-Induced Acute Kidney Injury After Coronary Angiography. Am. J. Cardiol 2018, 121 (7), 818–824. 10.1016/j.amjcard.2017.12.029. [DOI] [PubMed] [Google Scholar]
- (32).Susantitaphong P; Cruz DN; Cerda J; Abulfaraj M; Alqahtani F; Koulouridis I; Jaber BL World Incidence of AKI: A Meta-Analysis. Clin. J. Am. Soc. Nephrol 2013, 8 (9), 1482–1493. 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Wu H; Huang J Drug-Induced Nephrotoxicity: Pathogenic Mechanisms, Biomarkers and Prevention Strategies. Curr. Drug Metab 2018, 19 (7), 559–567. 10.2174/1389200218666171108154419. [DOI] [PubMed] [Google Scholar]
- (34).Lei L; Li L; Zhang H Advances in the Diagnosis and Treatment of Acute Kidney Injury in Cirrhosis Patients. Biomed Res. Int 2017, 2017, 1–7. 10.1155/2017/8523649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Driza AR; Kapoula GV; Bagos PG Urinary N-Acetyl-β-D-Glucosaminidase (UNAG) as an Indicative Biomarker of Early Diabetic Nephropathy in Patients with Diabetes Mellitus (T1DM, T2DM): A Systematic Review and Meta-Analysis. Diabetology 2021, 2 (4), 272–285. 10.3390/diabetology2040025. [DOI] [Google Scholar]
- (36).Price RG Measurement of N-Acetyl-Beta-Glucosaminidase and Its Isoenzymes in Urine Methods and Clinical Applications. Eur. J. Clin. Chem. Clin. Biochem 1992, 30 (10), 693–705. [PubMed] [Google Scholar]
- (37).Macauley MS; Whitworth GE; Debowski AW; Chin D; Vocadlo DJO -GlcNAcase Uses Substrate-Assisted Catalysis. J. Biol. Chem 2005, 280 (27), 25313–25322. 10.1074/jbc.M413819200. [DOI] [PubMed] [Google Scholar]
- (38).Kim EJ; Kang DO; Love DC; Hanover JA Enzymatic Characterization of O-GlcNAcase Isoforms Using a Fluorogenic GlcNAc Substrate. Carbohydr. Res 2006, 341 (8), 971–982. 10.1016/j.carres.2006.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Shibata H; Yagi T Rate Assay of N-Acetyl-β-D-Hexosaminidase with 4-Nitrophenyl N-Acetyl-β-D-Glucosaminidase as an Artificial Substrate. Clin. Chim. Acta 1996, 251 (1), 53–64. 10.1016/0009-8981(96)06292-4. [DOI] [PubMed] [Google Scholar]
- (40).Spasovski D Estimate and Evaluation of Drug Nephrotoxicity Caused with Most Used Medicals in Patients with Rheumathoid Arthritis. Arch. Clin. Nephrol 2016, 2 (1), 32–37. [Google Scholar]
- (41).Percinkova S; Slaninka-micevska M; Balkanov T; Dejanova B; Alabakovska S Symmetric Dimethyl Arginine and N-Acetyl-β-D-Glucosaminidase Lysozimuria of Proximal Renal Tubules as a Target for Nephrotoxicity in Patients with Rheumatoid Arthritis Treated with Disease Modifying Antirheumatic Drugs. J. Nephropathol 2013, 2 (1), 36–52. 10.5812/nephropathol.8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Tantawy AAG; El Bablawy N; Adly AAM; Ebeid FSE Early Predictors of Renal Dysfunction in Egyptian Patients with β-Thalassemia Major and Intermedia. Mediterr. J. Hematol. Infect. Dis 2014, 6 (1), 1–8. 10.4084/MJHID.2014.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Yan F; Tian X; Luan Z; Feng L; Ma X; James TD NAG-Targeting Fluorescence Based Probe for Precision Diagnosis of Kidney Injury. Chem. Commun 2019, 55 (13), 1955–1958. 10.1039/C8CC10311A. [DOI] [PubMed] [Google Scholar]
- (44).Chen SM; Lin CE; Chen HH; Cheng YF; Cheng HW; Imai K Effect of Prednisolone on Glyoxalase 1 in an Inbred Mouse Model of Aristolochic Acid Nephropathy Using a Proteomics Method with Fluorogenic Derivatization-Liquid Chromatography-Tandem Mass Spectrometry. PLoS One 2020, 15 (1), 1–19. 10.1371/journal.pone.0227838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Linko-lopponen S, Makinen M A Microtiter Plate Assay for N-Acetyl-β-D-Glucosaminidase Using a Fluorogenic Substrate. Anal. Biochem 1985, 148 (1), 50–53. [DOI] [PubMed] [Google Scholar]
- (46).Kristz W; Samaan GJ; Leberre C; Demelier J; Biou D Semi-Automated Fluorometric Assay for Urinary Total and B N-Acetyl-β-D-Glucosaminidase : Analytical Investigation. J. Clin. Lab. Anal 1991, 5 (1), 1–2. [DOI] [PubMed] [Google Scholar]
- (47).Morsby JJ; Dharmarwardana M; McGarraugh H; Smith BD Supramolecular Optimization of the Visual Contrast for Colorimetric Indicator Assays That Release Resorufin Dye. Chem. Commun 2020, 56 (65), 9296–9299. 10.1039/d0cc03551c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Huang J; Li J; Lyu Y; Miao Q; Pu K Molecular Optical Imaging Probes for Early Diagnosis of Drug-Induced Acute Kidney Injury. Nat. Mater 2019, 18 (10), 1133–1143. 10.1038/s41563-019-0378-4. [DOI] [PubMed] [Google Scholar]
- (49).Cheng P; Miao Q; Huang J; Li J; Pu K Multiplex Optical Urinalysis for Early Detection of Drug-Induced Kidney Injury. Anal. Chem 2020, 92 (8), 6166–6172. 10.1021/acs.analchem.0c00989. [DOI] [PubMed] [Google Scholar]
- (50).Huang J; Lyu Y; Li J; Cheng P; Jiang Y; Pu K A Renal-Clearable Duplex Optical Reporter for Real-Time Imaging of Contrast-Induced Acute Kidney Injury. Angew. Chem. Int. Ed 2019, 58 (49), 17796–17804. 10.1002/anie.201910137. [DOI] [PubMed] [Google Scholar]
- (51).Soleimany AP; Bhatia SN Activity-Based Diagnostics: An Emerging Paradigm for Disease Detection and Monitoring. Trends Mol. Med 2020, 26 (5), 450–468. 10.1016/j.molmed.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Perry JD A Decade of Development of Chromogenic Culture Media for Clinical Microbiology in an Era of Molecular Diagnostics. Clin. Microbiol. Rev 2017, 30 (2), 449–479. 10.1128/CMR.00097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Orenga S; James AL; Manafi M; Perry JD; Pincus DH Enzymatic Substrates in Microbiology. J. Microbiol. Methods 2009, 79 (2), 139–155. 10.1016/j.mimet.2009.08.001. [DOI] [PubMed] [Google Scholar]
- (54).Burton M; Perry JD; Stanforth SP; Turner HJ The Synthesis of Novel Chromogenic Enzyme Substrates for Detection of Bacterial Glycosidases and Their Applications in Diagnostic Microbiology. Bioorganic Med. Chem 2018, 26 (17), 4841–4849. 10.1016/j.bmc.2018.08.023. [DOI] [PubMed] [Google Scholar]
- (55).Orekan J; Barbe B; Oeng S; Ronat J-B; Letchford J; Jacobs J; Affolabi D; Hardy L Culture Media for Clinical Bacteriology in Low- and Middle-Income Countries: Challenges, Best Practices for Preparation and Recommendations for Improved Access. Clin. Microbiol. Infect 2021, 27 (10), 1400–1408. 10.1016/j.cmi.2021.05.016. [DOI] [PubMed] [Google Scholar]
- (56).Dennis EK; Chaturvedi S; Chaturvedi V So Many Diagnostic Tests, So Little Time: Review and Preview of Candida Auris Testing in Clinical and Public Health Laboratories. Front. Microbiol 2021, 12, 1–13. 10.3389/fmicb.2021.757835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Pusztahelyi T; Pocsi I Chitinase but N-Acetyl-β-D-Glucosaminidase Production Correlates to the Biomass Decline in Penicillium and Aspergillus Species. Acta Microbiol. Immunol. Hung 2014, 61 (2), 131–143. [DOI] [PubMed] [Google Scholar]
- (58).Zhang A; Mo X; Zhou N; Wang Y; Wei G; Chen J; Chen K; Ouyang P A Novel Bacterial β-N-Acetyl Glucosaminidase from Chitinolyticbacter Meiyuanensis Possessing Transglycosylation and Reverse Hydrolysis Activities. Biotechnol. Biofuels 2020, 13 (1), 1–14. 10.1186/s13068-020-01754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Cooke VM; Miles RJ; Price RG; Midgley G; Khamri W; Richardson AC New Chromogenic Agar Medium for the Identification of Candida Spp. Appl. Environ. Microbiol 2002, 68 (7), 3622–3627. 10.1128/AEM.68.7.3622-3627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).De Jong AW; Dieleman C; Carbia M; Tap RM; Hagen F Performance of Two Novel Chromogenic Media for the Identification of Multidrug-Resistant Candida Auris Compared with Other Commercially Available Formulations. J. Clin. Microbiol 2021, 59 (4), 1–9. 10.1128/JCM.03220-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Mulet Bayona JV; Salvador García C; Tormo Palop N; Gimeno Cardona C Evaluation of a Novel Chromogenic Medium for Candida Spp. Identification and Comparison with CHROMagar™ Candida for the Detection of Candida Auris in Surveillance Samples. Diagn. Microbiol. Infect. Dis 2020, 98 (4), 1–5. 10.1016/j.diagmicrobio.2020.115168. [DOI] [PubMed] [Google Scholar]
- (62).Borman AM; Fraser M; Johnson EM Chromagartmcandida plus: A Novel Chromogenic Agar That Permits the Rapid Identification of Candida Auris. Med. Mycol 2021, 59 (3), 253–258. 10.1093/mmy/myaa049. [DOI] [PubMed] [Google Scholar]
- (63).Ding X; Mauk MG; Yin K; Kadimisetty K; Liu C Interfacing Pathogen Detection with Smartphones for Point-of-Care Applications. Anal. Chem 2019, 91 (1), 655–672. 10.1021/acs.analchem.8b04973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Bourgoin C; Emiliani C; Kremer EJ; Gelot A; Tancini B; Gravel RA; Drugan C; Orlacchio A; Poenaru L; Caillaud C Widespread Distribution of β-Hexosaminidase Activity in the Brain of a Sandhoff Mouse Model after Coinjection of Adenoviral Vector and Mannitol. Gene Ther 2003, 10 (21), 1841–1849. 10.1038/sj.gt.3302081. [DOI] [PubMed] [Google Scholar]
- (65).Dong Y; Hu S; Zhao X; He Q; Yang Q; Zhang L Virtual Screening, Synthesis, and Bioactivity Evaluation for the Discovery of β-N-Acetyl-D-Hexosaminidase Inhibitors. Pest Manag. Sci 2020, 76 (9), 3030–3037. 10.1002/ps.5852. [DOI] [PubMed] [Google Scholar]
- (66).Santana AG; Vadlamani G; Mark BL; Withers SGN -Acetyl Glycals Are Tight-Binding and Environmentally Insensitive Inhibitors of Hexosaminidases. Chem. Commun 2016, 52 (51), 7943–7946. 10.1039/c6cc02520j. [DOI] [PubMed] [Google Scholar]
- (67).Dong L; Shen S; Xu Y; Wang L; Feng R; Zhang J; Lu H Computational Studies on the Potency and Selectivity of PUGNAc Derivatives against GH3, GH20, and GH84 β-N-Acetyl-D-Hexosaminidases. Front. Chem 2019, 7, 1–11. 10.3389/fchem.2019.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Fennelly C; Amaravadi RK Lysosomal Biology in Cancer. Methods Mol. Biol 2017, 1594, 293–308. 10.1007/978-1-4939-6934-0_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Iwasaki H; Shimura T; Kataoka H Current Status of Urinary Diagnostic Biomarkers for Colorectal Cancer. Clin. Chim. Acta 2019, 498, 76–83. 10.1016/j.cca.2019.08.011. [DOI] [PubMed] [Google Scholar]
- (70).Szajda SD; Borzym-Kluczyk M; Snarska J; Puchalski Z; Zwierz K N-Acetyl-β-D-Hexosaminidase and Its Isoenzymes A and B in Blood Serum and Urine, as a Potential Colon Cancer Markers. Hepatogastroenterology 2009, 56 (94–95), 1287–1298. [PubMed] [Google Scholar]
- (71).Wang Q; Li C; Chen Q; Zhang P; Wang D; Kang M; Jiang G; Wang J Lysosome-Targeting Red-Emitting Aggregation-Induced Emission Probe with Large Stokes Shift for Light-Up in Situ Visualization of β- N-Acetylhexosaminidase. Anal. Chem 2019, 91 (20), 12611–12614. 10.1021/acs.analchem.9b03832. [DOI] [PubMed] [Google Scholar]
- (72).Dong L; Shen S; Lu H; Jin S; Zhang J Novel Glycosylated Naphthalimide-Based Activatable Fluorescent Probe: A Tool for the Assessment of Hexosaminidase Activity and Intracellular Hexosaminidase Imaging. ACS Sensors 2019, 4 (5), 1222–1229. 10.1021/acssensors.8b01617. [DOI] [PubMed] [Google Scholar]
- (73).Wang Y; Mu S; Li S; Fu G; Liu X; Gao H; Zhang H A Fluorescent Probe for Bioimaging of Hexosaminidases Activity and Exploration of Drug-Induced Kidney Injury in Living Cell. Talanta 2021, 228, 1–9. 10.1016/j.talanta.2021.122189. [DOI] [PubMed] [Google Scholar]
- (74).Matsuzaki H; Kamiya M; Iwatate RJ; Asanuma D; Watanabe T; Urano Y Novel Hexosaminidase-Targeting Fluorescence Probe for Visualizing Human Colorectal Cancer. Bioconjug. Chem 2016, 27 (4), 973–981. 10.1021/acs.bioconjchem.6b00037. [DOI] [PubMed] [Google Scholar]
- (75).Bley AE; Giannikopoulos OA; Hayden D; Kubilus K; Tifft CJ; Eichler FS Natural History of Infantile G M2 Gangliosidosis. Pediatrics 2011, 128 (5), 1233–1241. 10.1542/peds.2011-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Thomas R; Kermode AR Enzyme Enhancement Therapeutics for Lysosomal Storage Diseases: Current Status and Perspective. Mol. Genet. Metab 2019, 126 (2), 83–97. 10.1016/j.ymgme.2018.11.011. [DOI] [PubMed] [Google Scholar]
- (77).Tropak MB; Blanchard JE; Withers SG; Brown ED; Mahuran D High-Throughput Screening for Human Lysosomal β-N-Acetyl Hexosaminidase Inhibitors Acting as Pharmacological Chaperones. Chem. Biol 2007, 14 (2), 153–164. 10.1016/j.chembiol.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Mauri V; Lotfi P; Segatori L; Sardiello M A Rapid and Sensitive Method for Measuring N-Acetylglucosaminidase Activity in Cultured Cells. PLoS One 2013, 8 (6), 1–9. 10.1371/journal.pone.0068060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Hyun JY; Kim S; Lee HS; Shin I A Glycoengineered Enzyme with Multiple Mannose-6-Phosphates Is Internalized into Diseased Cells to Restore Its Activity in Lysosomes. Cell Chem. Biol 2018, 25 (10), 1255–1267. 10.1016/j.chembiol.2018.07.011. [DOI] [PubMed] [Google Scholar]
- (80).Nagree MS; Scalia S; McKillop WM; Medin JA An Update on Gene Therapy for Lysosomal Storage Disorders. Expert Opin. Biol. Ther 2019, 19 (7), 655–670. 10.1080/14712598.2019.1607837. [DOI] [PubMed] [Google Scholar]
- (81).McCurdy VJ; Johnson AK; Gray-Edwards HL; Randle AN; Bradbury AM; Morrison NE; Hwang M; Baker HJ; Cox NR; Sena-Esteves Miguel, et al. Therapeutic Benefit after Intracranial Gene Therapy Delivered during the Symptomatic Stage in a Feline Model of Sandhoff Disease. Gene Ther 2021, 28 (3–4), 142–154. 10.1038/s41434-020-00190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Hayashi M Histochemical Demonstration of N-Acetyl-β-Glucosaminidase Employing Napthol AS-BI N-Acetyl-β-Glucosaminide as Substrate. J. Histochem. Cytochem 1965, 13 (5), 355–360. 10.1177/13.5.355. [DOI] [PubMed] [Google Scholar]
- (83).Brekk OR; Korecka JA; Crapart CC; Huebecker M; MacBain ZK; Rosenthal SA; Sena-Esteves M; Priestman DA; Platt FM; Isacson Ole, et al. Upregulating β-Hexosaminidase Activity in Rodents Prevents α-Synuclein Lipid Associations and Protects Dopaminergic Neurons from α-Synuclein-Mediated Neurotoxicity. Acta Neuropathol. Commun 2020, 8 (127), 1–14. 10.1186/s40478-020-01004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Burke HM; Gunnlaugsson T; Scanlan EM Recent Advances in the Development of Synthetic Chemical Probes for Glycosidase Enzymes. Chem. Commun 2015, 51 (53), 10576–10588. 10.1039/c5cc02793d. [DOI] [PubMed] [Google Scholar]
- (85).Singh M; Watkinson M; Scanlan EM; Miller GJ Illuminating Glycoscience: Synthetic Strategies for FRET-Enabled Carbohydrate Active Enzyme Probes. RSC Chem. Biol 2020, 1 (5), 352–368. 10.1039/d0cb00134a. [DOI] [PubMed] [Google Scholar]
- (86).Haab BB; Klamer Z Advances in Tools to Determine the Glycan-Binding Specificities of Lectins and Antibodies. Mol. Cell. Proteomics 2020, 19 (2), 224–232. 10.1074/mcp.R119.001836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Hyun JY; Kang NR; Shin I Carbohydrate Microarrays Containing Glycosylated Fluorescent Probes for Assessment of Glycosidase Activities. Org. Lett 2018, 20 (4), 1240–1243. 10.1021/acs.orglett.8b00180. [DOI] [PubMed] [Google Scholar]
- (88).Hogenkamp DG; Arakane Y; Kramer KJ; Muthukrishnan S; Beeman RW Characterization and Expression of the β-N-Acetylhexosaminidase Gene Family of Tribolium Castaneum. Insect Biochem. Mol. Biol 2008, 38 (4), 478–489. 10.1016/j.ibmb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- (89).Kumar DN; Pinker N; Shtenberg G Porous Silicon Fabry−Perot Interferometer for N-Acetyl-B-D-Glucosaminidase Biomarker Monitoring. ACS Sensors 2020, 5, 1969–1976. [DOI] [PubMed] [Google Scholar]
- (90).Pásztói M; Sódar B; Misják P; Pálóczi K; Kittel Á; Tóth K; Wellinger K; Géher P; Nagy G; Lakatos Tamás, et al. The Recently Identified Hexosaminidase D Enzyme Substantially Contributes to the Elevated Hexosaminidase Activity in Rheumatoid Arthritis. Immunol. Lett 2013, 149 (1–2), 71–76. 10.1016/j.imlet.2012.10.012. [DOI] [PubMed] [Google Scholar]
- (91).Abe A; Kamiya M A Versatile Toolbox for Investigating Biological Processes Based on Quinone Methide Chemistry: From Self-Immolative Linkers to Self-Immobilizing Agents. Bioorganic Med. Chem 2021, 44, 1–21. 10.1016/j.bmc.2021.116281. [DOI] [PubMed] [Google Scholar]
- (92).Hyun JY; Park SH; Park CW; Kim HB; Cho JW; Shin I Trifunctional Fluorogenic Probes for Fluorescence Imaging and Isolation of Glycosidases in Cells. Org. Lett 2019, 21 (12), 4439–4442. 10.1021/acs.orglett.9b01147. [DOI] [PubMed] [Google Scholar]
- (93).Nassif IA; Zhou X; Yücel YH; Toronov V Wavelength Optimization in the Multispectral Photoacoustic Tomography of the Lymphatic Drainage in Mice. Photoacoustics 2018, 12, 75–81. 10.1016/j.pacs.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Chen C; Tian R; Zeng Y; Chu C; Liu G Activatable Fluorescence Probes for “Turn-On” and Ratiometric Biosensing and Bioimaging: From NIR-I to NIR-II. Bioconjug. Chem 2020, 31 (2), 276–292. 10.1021/acs.bioconjchem.9b00734. [DOI] [PubMed] [Google Scholar]
- (95).Kricka LJ Clinical Applications of Chemiluminescence. Anal. Chim. Acta 2003, 500 (1–2), 279–286. 10.1016/S0003-2670(03)00809-2. [DOI] [Google Scholar]
- (96).Wang T; Li X United States Patent US 9,399,657 B2, 2016.
- (97).Sasamoto K; Zenko R; Ueno K; Ohkura Y Chemiluminiscence Assay of N-Acetyl-β-D-Glucosaminidase in Urine o-Aminopthalyhydrazido-N-Acetyl-β-D-Glucosaminide. Chem. Pharm. Bull 1991, 39 (5), 1317–1319. [DOI] [PubMed] [Google Scholar]
- (98).Sasamoto K; Ohkura Y A New Chemiluminogenic Substrate for N-Acetyl-β-D-Glucosaminidase, 4’-(6’-Diethylaminobenzofuranyl)Phtalyhydrazido-N-Acetyl-β-D-Glucosaminidase. Chem. Pharm. Bull 1991, 39 (2), 411–416. [Google Scholar]
- (99).Nirala NR; Asiku J; Dvir H; Shtenberg G N-Acetyl-β-D-Glucosaminidase Activity Assay for Monitoring Insulin-Dependent Diabetes Using Ag-Porous Si SERS Platform. Talanta 2022, 239, 1–9. 10.1016/j.talanta.2021.123087. [DOI] [PubMed] [Google Scholar]
- (100).Vibulcharoenkitja P; Suginta W; Schulte A Electrochemical N-Acetyl-β-D-Glucosaminidase Urinalysis: Toward Sensor Chip-Based Diagnostics of Kidney Malfunction. Biomolecules 2021, 11 (10), 1433. 10.3390/biom11101433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


