Abstract
Peptidoglycan polysaccharide (PG-PS) is a primary structural component of bacterial cell walls and causes rheumatoid-like arthritis in rats. Recently, glycine has been shown to be a potential immunomodulator; therefore, the purpose of this study was to determine if glycine would be protective in a PG-PS model of arthritis in vivo. In rats injected with PG-PS intra-articularly, ankle swelling increased 21% in 24 to 48 h and recovered in about 2 weeks. Three days prior to reactivation with PG-PS given intravenously (i.v.), rats were divided into two groups and fed a glycine-containing or nitrogen-balanced control diet. After i.v. PG-PS treatment joint swelling increased 2.1 ± 0.3 mm in controls but only 1.0 ± 0.2 mm in rats fed glycine. Infiltration of inflammatory cells, edema, and synovial hyperplasia in the joint were significantly attenuated by dietary glycine. Tumor necrosis factor alpha (TNF-α) mRNA was detected in ankle homogenates from rats fed the control diet but not in ankles from rats fed glycine. Moreover, intracellular calcium was increased significantly in splenic macrophages treated with PG-PS; however, glycine blunted the increase about 50%. The inhibitory effect of glycine was reversed by low concentrations of strychnine or chloride-free buffer, and it increased radiolabeled chloride influx nearly fourfold, an effect also inhibited by strychnine. In isolated splenic macrophages, glycine blunted translocation of the p65 subunit of NF-κB into the nucleus, superoxide generation, and TNF-α production caused by PG-PS. Further, mRNA for the beta subunit of the glycine receptor was detected in splenic macrophages. This work supports the hypothesis that glycine prevents reactive arthritis by blunting cytokine release from macrophages by increasing chloride influx via a glycine-gated chloride channel.
Peptidoglycan polysaccharide (PG-PS) is a primary structural component of bacterial cell walls, and injection of PG-PS induces arthritis in the rat that resembles human rheumatoid arthritis (29). The mechanism of the pathogenic action of PG-PS remains largely unclear, but it is thought that inflammation is due, in part, to stimulation of secretion of tumor necrosis factor alpha (TNF-α), interleukin-1, and other inflammatory cytokines as well as oxygen radicals from a variety of inflammatory cells including macrophages (28, 29). Indeed, PG-PS is concentrated in macrophages in the spleen, liver, and mesenteric lymph nodes after systemic injection in rats (28). Furthermore, PG-PS was detected in the joints of patients with septic arthritis and rheumatoid arthritis, those PG-PS-containing cells were mostly macrophages surrounded by T lymphocytes. Recently, macrophages were reported to be required in bacterial and adjuvant-induced arthritis (15). The depletion of macrophages attenuated the severity of the arthritic lesions in joints.
NF-κB activation and TNF-α production from macrophages have been shown to be important in the etiology of rheumatoid arthritis and bacterial cell wall-induced arthritis (8, 23). Treatment with anti-TNF-α antibody (7) or gene therapy with IκB superrepressor inhibits the severity of recurrent PG-PS-induced arthritis (23). Anti-TNF-α therapy is the most efficient new strategy in arthritis treatment from clinical trials, and new drugs such as soluble TNF receptors represent exciting new therapies for inflammatory arthritis, especially for patients who do not respond to methotrexate (39). However, widespread clinical use of these agents has limitations, not the least of which is expense. Simpler and more economical treatment is needed.
Glycine is a nonessential amino acid and an inhibitory neurotransmitter in the central nervous system. It can stimulate glycine-gated chloride channels, leading to increased chloride influx that hyperpolarizes neuronal membranes and inhibits excitatory signal transduction (4, 26). Recently, glycine has been shown to be immunosuppressive in several studies (35, 40). Glycine ameliorates kidney and liver injury during endotoxin shock (12), an effect that was due to the blocking of intracellular calcium signaling and the production of TNF-α in hepatic Kupffer cells via a glycine-gated chloride channel (13, 41). Therefore, the purpose of this study was to investigate whether glycine reduces PG-PS-induced arthritis in vivo and whether a glycine-gated chloride channel is involved.
(A preliminary account of this work has appeared elsewhere [18].)
MATERIALS AND METHODS
Arthritis model.
Male Lewis rats (175 to 200 g) were injected intra-articularly with PG-PS (5 μg; Lee Labs, Garrison, Ga.) in one ankle and sterile saline in the other ankle as a control (30). After 2 weeks, rats were divided randomly into control and glycine treatment groups; one group was given nitrogen-balanced AIN-76 diet (20% casein), while the other group received a glycine-containing diet (15% casein + 5% glycine; Harlan Teklad, Madison, Wis.). Three days later, all rats were injected intravenously (i.v.) with 200 μg of PG-PS to reactivate arthritis.
Joint swelling was evaluated by measurement of ankle diameter, using a digital caliper by an individual who was blinded to the treatment groups. Each joint was also evaluated using a limp score based on a scale from 0 to 4: 0, normal gait; 1, slight alteration of motion; 2, occasional limp; 3, frequent limp but occasional joint use; 4, withdrawal of paw and no use of joint. One day after the peak of swelling during the reactivation phase, blood samples were collected for serum glycine measurements. During the experimental period, body weight and food consumption were monitored.
Histology analysis.
After i.v. injection of PG-PS, ankles were harvested 1 day after the peak of inflammation. Rats were anesthetized with pentobarbital, and ankles were amputated, skinned, and fixed in 10% formalin for histological evaluation. Tissues were stained with hematoxylin and eosin. Histology was scored from 0 (no damage) to 5 (severe damage) by evaluating panus formation, inflammatory cell infiltration, synovial lining distension, edema, and bone erosion by a observer blinded to treatment groups (2).
Glycine measurement.
Rat serum was collected at the peak of inflammation, and the glycine concentration was determined. In brief, glycine was extracted from serum and benzoylated, and the resulting hippuric acid was extracted and dried by nitrogen (24, 25). The concentration of a colored conjugate of hippuric acid with dimethylaminobenzaldehyde was determined spectrophotometrically at 458 nm.
Splenic macrophage isolation and culture.
Rats were anesthetized, and the spleen was isolated using aseptic techniques. The spleen was teased apart, rinsed through mesh with minimal essential medium, (MEM), and centrifuged; then cells were resuspended in RPMI 1640 containing 10% fetal bovine serum (FBS) and antibiotics (100 U of penicillin G per ml and 100 μg of streptomycin sulfate 1 per ml). Ten milliliters of 0.15 mM NH4Cl buffer was added to the cell suspension for 1 min to lyse red cells. Splenocytes were centrifuged (500 × g), the pellet was resuspended in medium, cell number was determined using a hemocytometer, and viability was assessed from trypan blue exclusion (>90%). To purify macrophages, cells were seeded on coverslips or 24-well plates and cultured in RPMI 1640 for 30 min at 37°C in a 5% CO2 atmosphere. Nonadherent cells were gently removed by replacing media three to five times, and the number of adherent cells was calculated by difference. Purity of macrophages was above 95% as determined by Wright-Giemsa staining. For all experiments, cells were incubated in RPMI 1640 24 h before use.
Measurement of intracellular calcium.
The intracellular calcium concentration ([Ca2+]i) in splenic macrophages was measured using the fluorescent [Ca2+]i indicator dye fura-2 and a microspectrofluorometer (InCyt Im2 imaging, Cincinnati, Ohio) interfaced with an inverted microscope (TMS-F; Nikon, Tokyo, Japan). Splenic macrophages cultured on coverslips were incubated in modified Hanks' buffer (115 mM NaCl, 5 mM KCl, 0.3 mM Na2HPO4, 0.4 mM KH2PO4, 5.6 mM glucose, 0.8 mM MgSO4, 1.26 mM CaCl2, 15 mM HEPES [pH 7.4]) containing 5 μM fura-2-acetoxymethyl ester (Molecular Probes, Eugene, Oreg.) at room temperature for 30 min. Coverslips with macrophages loaded with fura-2 were rinsed and placed in chambers with buffer at room temperature. Changes in fluorescence intensity of fura-2 at excitation wavelengths of 340 and 380 nm and emission at 510 nm were monitored. Each value was corrected by subtracting the system dark noise and autofluorescence. [Ca2+]i was determined from the equation [Ca2+]I = Kd[(R − Rmin)/(Rmax − R)] × (F0/Fs). Cells were selected at random and analyzed using InCyt Im2 image acquisition and analysis software.
Measurement of chloride uptake.
Assays for uptake of chloride were conducted as described previously (32). Briefly, about 5 × 105 cells were incubated with buffer (20 mM HEPES, 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 2.5 mM CaCl2, 10 mM glucose) for 30 min at room temperature. Coverslips were gently blotted dry and incubated for 5 s in a petri dish with 2 ml of buffer containing 2 μCi of 36Cl− per ml. The coverslips were washed with ice-cold buffer, which terminated chloride uptake. Radioactivity was detected by liquid scintillation spectroscopy using a Beckman LC6000SC scintillation counter (Beckman Instruments Inc., Fullerton, Calif.). Protein was determined by a Lowry assay (19).
Detection of NF-κB in splenic macrophages.
The p65 subunit of NF-κB was detected in splenic macrophages by a method described previously (14). Isolated splenic macrophages were incubated for 24 h before the addition of PG-PS (20 μg/ml) in the presence or absence of 1 mM glycine. After 30 min, cells were fixed with 100% ice-cold methanol for 10 min and blocked with 10% nonimmune goat serum (NGS; Sigma) for 30 min. Rabbit anti-p65 (Rockland, Gilbertsville, Pa.) antibody in 10% NGS was added for 30 min, followed by the addition of rhodamine isothiocyanate-conjugated goat anti-rabbit immunoglobulin G antibody in 10% NGS. Nuclear DNA was stained using Hoechst 33258, and p65 and nuclear DNA were visualized with a fluorescence microscope.
Superoxide release assay.
Superoxide production was determined from the superoxide dismutase-inhibitable reduction of cytochrome c as described elsewhere (21). Splenic macrophages (∼106 /ml) were seeded onto 24-well plates with MEM plus 15% FBS, and cytochrome c was added to each well at a final concentration of 0.8 mg/ml. Cells were incubated with glycine (1 mM) for 3 min at room temperature before addition of PG-PS. Cells were incubated for 15 min at 37°C, supernatants were collected, and the reduction of cytochrome c was measured by spectrophotometry at 550 nm. The differences in absorption between samples incubated in the presence and absence of superoxide dismutase (85 U/ml) were compared, and superoxide concentration was calculated using a millimolar extinction coefficient of 18.5.
RT-PCR.
Ankles or splenic macrophages were homogenized, and RNA was extracted with phenol-chloroform and ethanol precipitation (1). For the synthesis of cDNA, reverse transcription (RT) was performed using murine leukemia virus reverse transcriptase. Primers for glycine receptor were designed based on the cloned sequence of highly conserved regions in the beta subunit of the glycine receptor from rat spinal cord (41). The forward primer 5′-AGGTCATCTTCACCCTGAGGAGA-3′ and the reverse primer 5′-CCAAGTCCAGTGTTGACTTCAATG-3′ generated a 575-bp DNA fragment. Primers for TNF-α were 5′-TACTGAACTTCGGGGTGATTGGTCC-3′ and 5′-CAGCCTTGTCCCTTGAAGAGAACC-3′, generating a 294-bp DNA fragment. Primers for glyceraldehyde-3-phosphate dehydrogenase (G3PDH) were 5′-TGAAGGTCGGTGTCAACGGATTTG-3′ and 5′-GTACATCCGTACTCCAGGTGGTG-3′ generating a 982-bp DNA fragment. Primers were prepared at the nucleotide synthesis facility at the University of North Carolina. Briefly, cDNA was added to a mixture containing 20 μM primer, 2 mM deoxynucleoside triphosphates, polymerase buffer, and Taq polymerase (1.25 U). The positive controls for G3PDH and TNF-α were plasmids of PCR fragments from G3PDH and TNF-α cloned into the subcloning vector pUC19. Amplification products were separated on a 1.5% agarose gel and visualized by UV illumination.
DNA sequencing.
The PCR product generated using glycine receptor primers from splenic macrophages was subcloned into Escherichia coli, using a TOPO TA clone kit (Invitrogen, Carlsbad, Calif.). Positive clones with the DNA fragment were selected and amplified, and DNA was isolated from plasmid (Miniprep DNA purification system; Promega). Plasmid DNA was sequenced in the facility of University of North Carolina.
Statistical analysis.
All results were expressed as means ± standard error of the means (SEM). Statistical differences between groups were determined by using analysis of variance (ANOVA) with either Tukey's post-hoc test or Scheffe's post-hoc test where appropriate. Scores were compared by the Mann-Whitney rank sum test. P < 0.05 was selected as the criterion for significance before initiation of the study.
RESULTS
Body weight and food consumption.
During the course of this study, body weight and food consumption were monitored. On average, rats grew at the same rate over the course of the study, with a maximal increase of 40 ± 0.5 g in 25 days. Food consumption increased steadily over the first 18 days of the experiment. Rats were then randomly divided into two groups; one group received a glycine-containing diet, and the other control received a diet for 3 days prior to i.v. injection of PG-PS. Rats in the two groups studied did not have significantly different body weights throughout the experiment. Food consumption was also not different before reactive arthritis. After the reinjection of PG-PS, only on day 18, rats given the control diet consumed 15.6 ± 0.5 g of food/day, while rats fed the glycine diet consumed about 19.4 ± 1.2 g/day (P < 0.05).
Effect of glycine on PG-PS-induced arthritis.
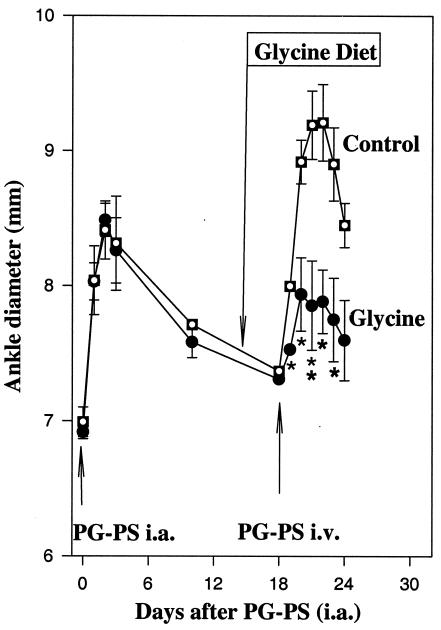
In this model, acute arthritis was induced by intra-articlular injection of PG-PS, while recurrent arthritis was reactivated i.v. After intra-articular injection of PG-PS, the ankles of PG-PS-treated rats swelled about 21% in 2 to 3 days (Fig. 1 [representative experiment repeated twice]). The effect subsided substantially 4 days after PG-PS and was nearly back to baseline in 2 weeks. At that time, rats were divided into two groups and fed the control or glycine-containing diet. Three days later, arthritis was reactivated by an i.v. injection of PG-PS. The diameters of ankles of rats fed the control diet reached a maximal value of 9.2 ± 0.2 mm, an increase of 2.1 ± 0.3 mm over baseline; however, ankles of rats fed the glycine diet had a maximal increase of only 1.0 ± 0.2 mm above baseline (Fig. 1). Thus, feeding dietary glycine prior to PG-PS reactivation prevented joint swelling significantly.
FIG. 1.
Effect of dietary glycine on ankle diameter in PG-PS induced arthritis. Arthritis was induced in rats fed control diet by intra-articular (i.a.) injection of PG-PS (5 μg) into the ankle joint as described in Materials and Methods, followed by recovery for 2 weeks. Three days prior to i.v. injection of PG-PS (200 μg), half of the rats were switched to 5% glycine diet and control rats received a diet balanced for nitrogen. Ankle diameter was measured as described in Materials and Methods. Data are expressed as mean ± SEM (n = 5) (∗, P < 0.05; ∗∗, P < 0.01 [one-way ANOVA with Tukey's post-hoc test]).
Ankle function was also impaired after injection of PG-PS. After the intra-articular injection of PG-PS, the average limp score was nearly maximal at 3.5 ± 0.4 and decreased to zero by day 5. After i.v. PG-PS, rats fed the glycine diet had an average limp score of 1.2 ± 0.7, significantly lower than that of the control group (3.6 ± 0.4).
Serum was collected immediately after the peak of ankle swelling on day 22. In the control group, the serum glycine concentration was 0.18 ± 0.15 mM. In rats fed glycine, the glycine concentration was 0.95 ± 0.10 mM, similar to values shown to be protective in a model of endotoxin shock (12).
Inflammation due to PG-PS.
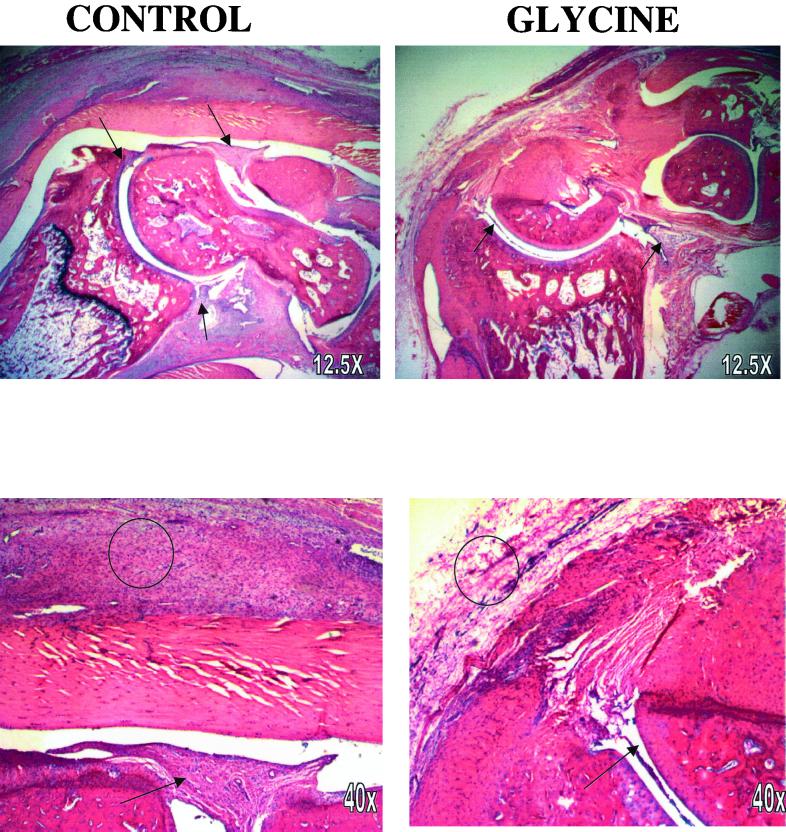
One day after the peak of joint swelling, severe infiltration of inflammatory cells and swelling both in the synovium and surrounding tissue were observed in rats fed the control diet (Fig. 2). Neovascularization with occasional areas of hemorrhage and a few lymphocytic foci, chiefly in the synovium, were observed in PG-PS-treated control rats. Synovial cell hyperplasia was significant, with an increase in the number and size of synovial cells in the tibiotarsal joint and in the bursa of the Achilles tendon. In rats fed the glycine-containing diet, only a slight increase in the number and size of synovial cells was observed. Several parameters of histological changes are summarized in Table 1. Synovial hypertrophy, increases in inflammatory cells, and edema were the main pathological changes detected in the joints from control rats. Dietary glycine significantly attenuated the synovial extension, edema, and inflammatory infiltration to different extents. There was moderate cartilage destruction in control ankles, while bone destruction was less extensive in joints of rats in the glycine group (Table 1). Thus, consistent with the joint swelling (Fig. 1), glycine blunted pathological changes in the joint caused by PG-PS by about 50%.
FIG. 2.
Histological analysis of ankle joints. Ankles were collected 1 day after the peak of inflammation after PG-PS reactivation as described in Materials and Methods and stained with hematoxylin and eosin. Typical low-power (12.5×; top row) and higher-power (40×; bottom row) photomicrographs are presented. Synovial tissue is marked by arrows, and areas of inflammation are indicated by circles.
TABLE 1.
Effect of glycine on the histological parameters of PG-PS-induced arthritis
| Parameter | Score (mean ± SEM) (n = 5/group)
|
|
|---|---|---|
| Control diet | 5% Glycine diet | |
| Panus formation | 2.3 ± 0.4 | 1.2 ± 0.4 |
| Inflammatory cell infiltration | 3.2 ± 0.5 | 1.4 ± 0.4b |
| Synovial hyperplasia | 3.5 ± 0.4 | 2.0 ± 0.3b |
| Edema | 3.3 ± 0.5 | 1.5 ± 0.4b |
| Bone erosion | 2.8 ± 0.5 | 1.0 ± 0.6b |
| Total score | 14.5 ± 1.3 | 7.1 ± 1.5b |
Histology was scored in a blinded fashion as described in Materials and Methods.
P < 0.05 compared to control joints (Mann-Whitney rank sum test).
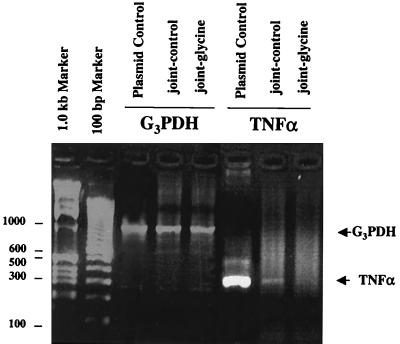
One day after the peak of reactivation of arthritis by i.v. injection of PG-PS, ankles were harvested and homogenized and RNA was isolated. mRNA for TNF-α was detected in ankles from rats fed the control diet (Fig. 3) but not in ankles from rats fed the glycine-containing diet.
FIG. 3.
TNF-α mRNA in ankles. Ankle mRNA transcripts were amplified by RT-PCR from rats fed either control (joint-control) or glycine (joint-glycine) diet 1 day after reactivation of arthritis using PG-PS. Ankles were harvested, and RNA was isolated as described in Materials and Methods. Markers (1.0 kb and 100 bp) and positive controls specifically for TNF mRNA and G3PDH, the housekeeping gene, were assayed simultaneously. The data are representative of two independent experiments.
Additionally, isolated splenic macrophages (0.8 × 106) were incubated with or without glycine (1 mM) for 1 h. The addition of glycine lowered the increase in TNF-α caused by PG-PS (50 μg/ml) from 1,014 ± 60 pg/ml to 717 ± 86 pg/ml (P < 0.05). In the absence of PG-PS, TNF-α levels were below detection limits.
Effect of PG-PS on intracellular calcium in splenic macrophages.
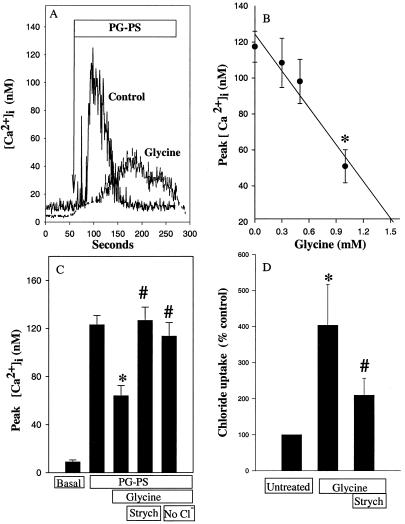
Since rat joints are small, technical difficulties were encountered in obtaining an adequate number of synovial macrophages. Therefore, we used splenic macrophages as a model to study the effect of glycine in intracellular signaling. [Ca2+]i was monitored after the addition of PG-PS (10 μg/ml) plus 5% rat serum to splenic macrophages. Without addition of rat serum, PG-PS did not increase calcium; however, with serum, PG-PS quickly increased [Ca2+]i about 17-fold over basal values in about 1 min; the level returned to baseline in 4 to 6 min (Fig. 4A). In contrast, after 3 min of preincubation with 1 mM glycine, the increase in [Ca2+]i was slow and peaked at a level much lower than in control cells. On average, PG-PS increased [Ca2+]i in splenic macrophages to 122 ± 8 nM. The maximal increase was blunted about 50% by 1 mM glycine (Fig. 4C). Furthermore, the inhibitory effect of glycine was dose dependent (Fig. 4B; half-maximal effect = 0.55 mM glycine).
FIG. 4.
Effects of PG-PS and glycine on [Ca2+]i and radiolabeled chloride uptake in cultured splenic macrophages. (A) [Ca2+]i was measured with the fluorescent indicator fura-2 as described in Materials and Methods in isolated splenic macrophages. Representative traces from cultured splenic macrophages preincubated in modified Hanks' balanced salt solution buffer in the presence or absence of glycine (1 mM) for 3 min and challenged with PG-PS (10 μg/ml). are shown. (B) Conditions as in panel A except that the concentration of glycine was varied. Data are expressed as means ± SEM (n = 6 to 10 cells) from three individual experiments. ∗, P < 0.05 versus no glycine by one-way ANOVA with Scheffe's post-hoc test. (C) Glycine (1 mM) or glycine plus strychnine (Strych; 1 μM) was added for 3 min. in the presence or absence of chloride-containing buffer (no Cl−) prior to calcium measurements. Data are mean ± SEM from five independent experiments (13 to 28 cells per group). ∗, P < 0.05 compared to basal; #, P < 0.05 compared to glycine group by one-way ANOVA. (D) Non treated splenic macrophages were incubated with 36Cl− (2 μCi/ml) in the presence or absence of glycine or glycine plus strychnine (1 μM). Data are presented as percentage of 36Cl− uptake in untreated controls from five independent experiments (mean ± SEM). ∗, P < 0.05 compared to untreated group; #, P < 0.05 compared to glycine group by one-way ANOVA on ranks with Tukey's post-hoc test.
To determine if the effect of glycine occurred through a glycine-gated chloride channel, the specific glycine receptor antagonist strychnine was studied. Preincubation of strychnine (1 μM) with glycine reversed the inhibitory effect of glycine completely (Fig. 4C). Chloride-free buffer also reversed the inhibitory effect of glycine significantly.
Glycine increases chloride influx.
Hyperpolarization of the plasma membrane due to influx of chloride is the mechanism of the inhibitory effect of glycine in the central nervous system and in a variety of white blood cells (40). To investigate whether glycine affects chloride influx in splenic macrophages, radiolabeled chloride influx was measured. Indeed, glycine increased chloride uptake by splenic macrophages about fourfold (Fig. 4D). This increase of chloride influx by glycine was reduced significantly by strychnine.
Molecular evidence for a glycine receptor in splenic macrophages.
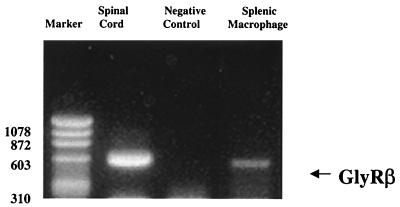
Figure 5 demonstrates the detection of mRNA of the beta subunit of the glycine receptor in splenic macrophages. The 575-bp fragment from splenic macrophages was identical to the positive control from the spinal cord, consistent with the finding that splenic macrophages contain a beta subunit similar to the 58-kDa subunit in the spinal cord. Furthermore, the PCR product from splenic macrophages was sequenced and found to have 98% homology with a cDNA segment of the beta subunit of the glycine receptor (GenBank accession no. X81202). This is the first study to provide molecular evidence demonstrating the presence of a glycine-gated chloride channel in splenic macrophages.
FIG. 5.
Glycine receptor mRNA in splenic macrophages. Total RNA was isolated from splenic macrophages as described in Materials and Methods. The glycine receptor was identified by RT-PCR using primers specific for conserved regions in the beta subunit, (GlyRβ). The negative control was total RNA from splenic macrophages without RT. The PCR product was confirmed by sequencing as described in Materials and Methods. The data are representative of three independent experiments. Sizes are indicated in base pairs.
Detection of NF-κB in splenic macrophages.
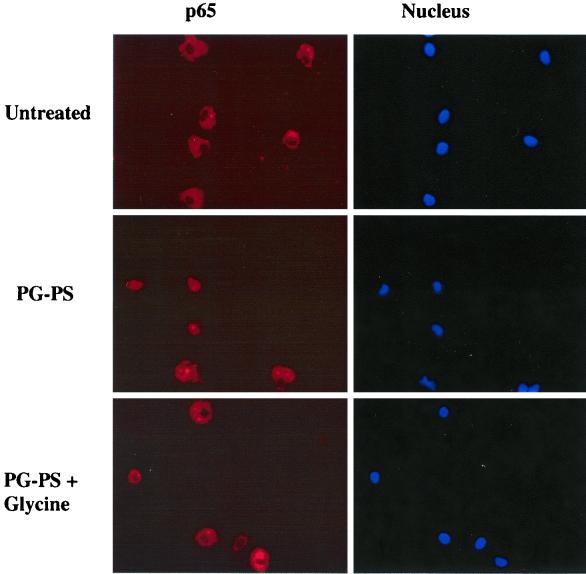
The pivotal transcription factor NF-κB is important for the production of TNF-α. In untreated splenic macrophages, the p65 subunit of NF-κB was detected in cytoplasm, indicating that it was in an inactive form (Fig. 6, top row). PG-PS stimulated the translocation of p65 into the nucleus in about 60% of the cells (Fig. 6, middle row). In contrast, glycine (1 mM) blocked the nuclear translocation of p65 by PG-PS nearly completely (Fig. 6, bottom row).
FIG. 6.
Effects of PG-PS and glycine on nuclear translocation of the p65 subunit of NF-κB. Splenic macrophages were stimulated with PG-PS (20 μg/ml) in the presence or absence of glycine (1 mM). The p65 subunit in cells was detected with an anti-p65 antibody as described in Materials and Methods. Nuclear DNA was stained using Hoechst 33342, and the p65 subunit and nuclear DNA were visualized with a fluorescence microscope. The data are representative of three independent experiments. Top row, untreated cells; middle row, cells treated with PG-PS (20 μg/ml) plus 5% rat serum; bottom row, glycine-pretreated cells treated with PG-PS.
Superoxide generation by splenic macrophages is inhibited by glycine.
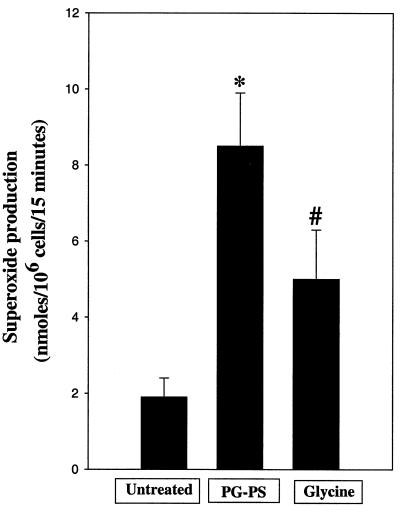
Phagocytes express NADPH oxidase in the cell membrane; therefore, the effect of PG-PS on superoxide production by splenic macrophages was evaluated (Fig. 7). In the absence of PG-PS, cells generated superoxide at rates of 1.9 ± 0.5 nmol 106 cells/15 min. PG-PS (10 μg/ml) increased values to 8.5 ± 1.4 nmol/106 cells/15 min. Interestingly, the increase in superoxide release due to PG-PS was reduced significantly (about 45%) by glycine.
FIG. 7.
Effect of glycine on superoxide production. Splenic macrophages were incubated in MEM with 15% FBS, HEPES, and antibiotics, and superoxide production was measured as described in Materials and Methods. Briefly, glycine (1 mM) was added to cells 3 min before the addition of PG-PS (10 μg/ml). Data are the means ± SEM from four independent experiments. ∗, P < 0.05 by one-way ANOVA with Tukey's post-hoc test compared with control group; #, P < 0.05 by one-way ANOVA with Tukey's post-hoc test compared with PG-PS alone.
DISCUSSION
Glycine inhibits PG-PS-induced arthritis.
The PG-PS-induced arthritis model has been used widely in studies of the etiology of rheumatoid arthritis because it mimics the clinical patterns observed in arthritic patients (3). One intra-articular dose of PG-PS causes a synovitis that reverts in a few days. Reactivation after a small i.v. dose of PG-PS causes a more severe, chronic, cyclic inflammation which is produced in the injured joint and eventually causes permanent destruction (31). This cell wall complex is resistant to breakdown in the body and remains in the tissue for long periods of time (3). PG-PS is retained in the joints and synovial fluid of some rheumatoid arthritis patients (16, 22, 37). Interestingly, overgrowth of small bowel bacteria induces reactivation of arthritis possibly due to PG-PS; however, the mechanism of the pathogenic actions of PG-PS is still unclear (11, 29, 34). PG-PS might induce a variety of persistent inflammatory diseases (i.e., carditis, granulomatus hepatitis, and enterocolitis) by production of cytokines and oxygen radicals (29). Here, PG-PS-induced both acute and reactive inflammation in PG-PS-injected ankles, as expected (Fig. 1), and elevated TNF-α mRNA in swollen ankles (Fig. 3).
Dietary glycine increases blood glycine levels four- to fivefold to levels which are protective in endotoxin shock and which prevent experimental liver tumors (12, 27). In vivo, glycine significantly blunted joint swelling (Fig. 1) and reduced the limp score of rats during reactive arthritis. Additionally, TNF-α mRNA (Fig. 3) could not be detected in ankles from rats fed glycine, and glycine reduced TNF-α production by macrophages about 30% in vitro (Results). Therefore, it is hypothesized that dietary glycine inhibits ankle inflammation by blunting TNF-α production.
Anti-inflammatory effect of glycine and role of the glycine receptor.
The mechanism of PG-PS activation of macrophages is not completely understood. It is suggested that PG-PS shares a similar signaling pathway with lipopolysaccharide (LPS) by activating CD14 (6, 10). Recent work has demonstrated that knockout mice which lack the Toll-like receptor 4 (TLR4) do not respond to LPS but do respond to PG-PS; however, mice without TLR2 respond similarly to wild-type mice after LPS but not PG-PS (36). Moreover, TLR2 receptors are responsive to gram-positive bacteria (i.e., to PG-PS) and activity of TLR2 is potentiated by CD14 (42). These studies support the hypothesis that TLR2 and CD14 are involved in signaling due to PG-PS.
It is well known that intracellular calcium signals are important for activation and release of cytokines from inflammatory cells (5, 38). Voltage-dependent calcium channels are opened based on the membrane potential of the cell membrane (17). Signal transduction leads to depolarization of the plasma membrane, causing calcium influx. Like LPS, PG-PS increases intracellular calcium in splenic macrophages, an effect dependent on serum binding proteins (Fig. 4).
In phagocytes, one mechanism for superoxide generation is via NADPH oxidase, which is regulated, directly or indirectly, by calcium signaling. For example, increases in intracellular calcium activates protein kinase C, resulting in an increase in NADPH oxidase which leads to the production of oxidants, activation of NF-κB, and stimulation of TNF-α production (33). PG-PS was reported to activate NF-κB and increase the production of TNF-α (9, 10). In this study, translocation of the p65 subunit of NF-κB into the nucleus and production of TNF-α both in vitro and in vivo were also observed (Fig. 3 and Results). Interestingly, glycine blunts the elevation of intracellular calcium after PG-PS in a dose-dependent manner (Fig. 4B), inhibits superoxide production (Fig. 7), and blunts both nuclear translocation of NF-κB (Fig. 6) and TNF-α production (Results). Differences in amplitude and duration of intracellular calcium signals activate a variety of transcription factors. For example, the amplitude of the calcium signal has been reported to be critical for NF-κB activation (5). Changes in the intracellular calcium signal patterns in macrophages by glycine (Fig. 4) may explain the beneficial effect of glycine in PG-PS-induced arthritis. Moreover, the results suggest that intracellular calcium plays an important role in the intracellular signaling of PG-PS.
Glycine has long been known to be an inhibitory neurotransmitter in the spinal cord (4) and acts by binding to receptors which are localized largely in postsynaptic neuronal membranes. Glycine receptors in the neuron are comprised of three distinct protein subunits: a 48-kDa alpha subunit, a 58-kDa beta subunit, and a cytoplasmic anchoring protein, gephyrin. Glycine triggers the opening of this chloride channel, leading to influx of chloride that hyperpolarizes the cell membrane in opposition to the depolarizing action of excitatory signals. In splenic macrophages, the inhibitory effect of glycine is blocked by the high-affinity specific glycine receptor against strychnine and is dependent on extracellular chloride (Fig. 4C and D). Recently, evidence for a glycine-gated chloride channel has also been obtained for other macrophages, such as Kupffer cells (40). Here, the beta subunit of the glycine-gated chloride channel, which was 98% identical to the cDNA segment of the mouse glycine receptor from the GenBank database, was detected in splenic macrophages (Fig. 5). Similarly, glycine increased the influx of radiolabeled chloride into cells (Fig. 4D). Thus, the glycine receptor of splenic macrophages is nearly identical to glycine receptors described elsewhere (40). This finding supports the hypothesis that glycine has direct effects on inflammatory cells.
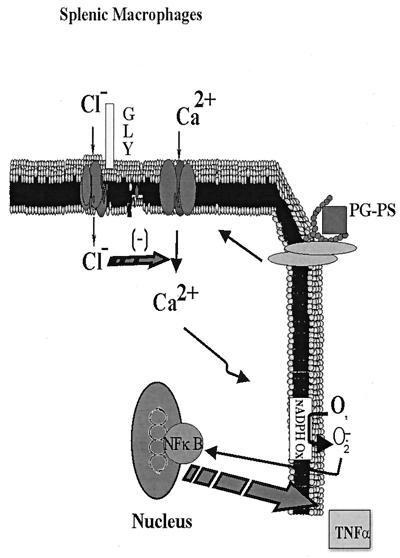
Based on the data presented in this study, the following mechanism is proposed to explain these findings (Fig. 8). PG-PS activates CD14 and TLR2, which stimulates phospholipase C and increases intracellular calcium, a critical signal in the inflammatory response. This causes assembly of NADPH oxidase, most likely via protein kinase C, leading to superoxide production, which causes translocation of NF-κB, resulting in TNFα production. Glycine blunts PG-PS-induced increases in [Ca2+]i, an effect reversed by low concentrations of strychnine and depletion of extracellular chloride (Fig. 4C and D). Inhibition of superoxide production and TNF-α generation by glycine is most likely due to the blunting of calcium signals. These data are consistent with the hypothesis that glycine activates a glycine receptor (Fig. 5), leading to the influx of chloride which hyperpolarizes the macrophage membrane and decreases the opening time of voltage-dependent calcium channels. In vivo treatment with glycine blocks PG-PS-induced arthritis likely via this mechanism.
FIG. 8.
Working hypothesis for the mechanism of action of glycine in PG-PS-induced arthritis in the rat. PG-PS from gram-positive bacteria plays an important role in the etiology of arthritis. It is proposed that PG-PS stimulates CD14 and TLR2, resulting in increases in intracellular calcium. This stimulates NADPH oxidase and generates superoxide, which activates NF-κB, leading to TNF-α production. Glycine (GLY) blunts PG-PS-induced increases in [Ca2+]i, an effect reversed by low concentrations of strychnine or depletion of chloride. The inhibition of superoxide production and TNF-α generation by glycine is most likely due to blunting of the intracellular calcium signaling pathway. These data are consistent with the hypothesis that glycine activates a glycine receptor, leading to influx of chloride which hyperpolarizes the macrophage membrane and decreases the opening time of voltage-dependent calcium channels. In vivo treatment with glycine blocks PG-PS-induced arthritis most likely via a similar mechanism.
Clinical significance.
In clinical trials, therapy against TNF-α with soluble anti-TNF-α receptor or anti-TNF-α antibody reduced the severity of rheumatoid arthritis (20). Soluble TNF receptors which neutralize TNF-α before joints are damaged has become a new exciting strategy for therapy (39). Combination therapy of these drugs with methotrexate appears particularly effective in patients whose disease persists despite traditional drug therapy. However, these therapies are limited due to expense or adverse side effects. Glycine, a dietary nutrient, is anti-inflammatory and has protective effects in experimental arthritis by reducing TNF-α production (Fig. 3). Since dietary glycine is easily administered, inexpensive, and nontoxic, it could supplement current therapies for arthritis.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical expertise of Julie Vorobiov, Center for Gastrointestinal Biology and Disease (P30 KD34987).
This work was supported by grants from NIH.
REFERENCES
- 1.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guadinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 2.Conway J G, Wakefield J A, Brown R H, Marron B E, Sekut L, Stimpson S A, McElroy A, Menius J A, Jeffreys J J, Clark R L, McGeehan G M, Connolly K M. Inhibition of cartilage and bone destruction in adjuvant arthritis in the rat by a matrix metalloproteinase inhibitor. J Exp Med. 1995;182:449–457. doi: 10.1084/jem.182.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cromartie W J, Craddock J G, Schwab J H, Anderle S K, Yang C-H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977;146:1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis D R, Hosli L, Johnston G A R. Inhibition of spinal neurons by glycine. Nature. 1967;215:1502–1503. doi: 10.1038/2151502a0. [DOI] [PubMed] [Google Scholar]
- 5.Dolmetsch R E, Lewis R S, Goodnow C C, Healy J I. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 6.Dziarski R, Tapping R I, Tobias P S. Binding of bacterial peptidoglycan to CD14. J Biol Chem. 1998;273:8680–8690. doi: 10.1074/jbc.273.15.8680. [DOI] [PubMed] [Google Scholar]
- 7.Feldmann M, Brennan F M, Maini R N. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 8.Foxwell B, Browne K, Bondeson J, Clarke C, de Martin R, Brennan F, Feldmann M. Efficient adenoviral infection with IkappaB alpha reveals that macrophage tumor necrosis factor alpha production in rheumatoid arthritis is NF-kappaB dependent. Proc Natl Acad Sci USA. 1998;95:8211–8215. doi: 10.1073/pnas.95.14.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta D, Jin Y, Dziarski R. Peptidoglycan induces transcription and secretion of TNF-alpha and activation of lyn, extracellular signal-regulated kinase, and rsk signal transduction proteins in mouse macrophages. J Immunol. 1995;155:2620–2630. [PubMed] [Google Scholar]
- 10.Gupta D, Kirkland T N, Viriyakosol S, Dziarski R. CD14 is a cell-activating receptor for bacterial peptidoglycan. J Biol Chem. 1996;271:23310–23316. doi: 10.1074/jbc.271.38.23310. [DOI] [PubMed] [Google Scholar]
- 11.Hazenberg M P, Klasen I S, Kool J, Ruseler-van Embden J G, Severijnen A J. Are intestinal bacteria involved in the etiology of rheumatoid arthritis? APMIS. 1992;100:1–9. doi: 10.1111/j.1699-0463.1992.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 12.Ikejima K, Iimuro Y, Forman D T, Thurman R G. A diet containing glycine improves survival in endotoxin shock in the rat. Am J Physiol. 1996;271:G97–G103. doi: 10.1152/ajpgi.1996.271.1.G97. [DOI] [PubMed] [Google Scholar]
- 13.Ikejima K, Qu W, Stachlewitz R F, Thurman R G. Kupffer cells contain a glycine-gated chloride channel. Am J Physiol. 1997;272:G1581–G1586. doi: 10.1152/ajpgi.1997.272.6.G1581. [DOI] [PubMed] [Google Scholar]
- 14.Jobin C, Hellerbrand C, Licato L L, Brenner D A, Sartor R B. Mediation by NF-kappa B of cytokine induced expression of intercellular adhesion molecule 1 (ICAM-1) in an intestinal epithelial cell line, a process blocked by proteasome inhibitors. Gut. 1998;42:779–787. doi: 10.1136/gut.42.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinne R W, Schmidt-Weber C B, Hoppe R, Buchner E, Palombo-Kinne E, Nuruberg E, Emmrich F. Long-term amelioration of rat adjuvant arthritis following systemic elimination of macrophages by clodronate-containing liposomes. Arthritis Rheum. 1995;38:1777–1790. doi: 10.1002/art.1780381211. [DOI] [PubMed] [Google Scholar]
- 16.Klasen I S, Melief M J, Swaak T J, Severijnen A J, Hazenberg M P. Responses of synovial fluid and peripheral blood mononuclear cells to bacterial antigens and autologous antigen presenting cells. Ann Rheum Dis. 1993;52:127–132. doi: 10.1136/ard.52.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause K H, Welsh M J. Voltage-dependent and Ca2+-activated ion channels in human neutrophils. J Clin Investig. 1990;85:491–498. doi: 10.1172/JCI114464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Bradford B U, Stimpson S A, Schwab J H, Thurman R G. Splenic macrophages contain a glycine-gated chloride channel: studies with peptidoglycan-polysaccharide. Arthritis Rheum. 1999;42:S337. [Google Scholar]
- 19.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Maini R N, Elliott M, Brennan F M, Williams R O, Feldmann M. TNF blockade in rheumatoid arthritis: implications for therapy and pathogenesis. APMIS. 1997;105:257–263. doi: 10.1111/j.1699-0463.1997.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 21.McCord J M, Fridovich I. Superoxide dismutase: an enzymatic function of erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 22.Melief M J, Hoijer M A, Van Paassen H C, Hazenberg M P. Presence of bacterial flora-derived antigen in synovial tissue macrophages and dendritic cells. Br J Rheumatol. 1995;34:1112–1116. doi: 10.1093/rheumatology/34.12.1112. [DOI] [PubMed] [Google Scholar]
- 23.Miagkov A V, Kovalenko D V, Brown C E, Didsbury J R, Cogswell J P, Stimpson S A, Baldwin A S, Makarov S S. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci USA. 1998;95:13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohmori S, Ikeda M, Kira S, Ogata M. Colorimetric determination of hippuric acid in urine and liver homogenate. Anal Chem. 1977;49:1494–1496. doi: 10.1021/ac50019a009. [DOI] [PubMed] [Google Scholar]
- 25.Ohmori S, Ikeda M, Watanabe Y, Hirota K. A simple and specific determination of glycine in biological samples. Anal Biochem. 1978;90:662–670. doi: 10.1016/0003-2697(78)90159-8. [DOI] [PubMed] [Google Scholar]
- 26.Rajendra S, Lynch J W, Schofield P R. The glycine receptor. Pharmacol Ther. 1997;73:121–146. doi: 10.1016/s0163-7258(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 27.Rose M L, Madren J, Bunzendahl H, Thurman R G. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis. 1999;20:793–798. doi: 10.1093/carcin/20.5.793. [DOI] [PubMed] [Google Scholar]
- 28.Sartor R B, Bond T M, Schwab J H. Systemic uptake and intestinal inflammatory effects of luminal bacterial cell wall polymers in rats with acute colonic injury. Infect Immun. 1988;56:2101–2108. doi: 10.1128/iai.56.8.2101-2108.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwab J H. Phlogistic properties of peptidoglycan-polysaccharide polymers from cell walls of pathogenic and normal-flora bacteria which colonize humans. Infect Immun. 1993;61:4535–4539. doi: 10.1128/iai.61.11.4535-4539.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwab J H. Bacterial cell-wall induced arthritis: models of chronic recurrent polyarthritis and reactivation of monoarticular arthritis. In: Henderson B, Edwards J C W, Pettipher E R, editors. Mechanisms and models in rheumatoid arthritis. London, England: Academic Press; 1995. pp. 431–446. [Google Scholar]
- 31.Schwab J H, Anderle S K, Brown R R, Dalldorf F G, Thompson R C. Pro- and anti-inflammatory roles of interleukin-1 in recurrence of bacterial cell wall-induced arthritis is rats. Infect Immun. 1991;59:4436–4442. doi: 10.1128/iai.59.12.4436-4442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz R D, Suzdak P D, Paul S M. Gamma-aminobutyric acid (GABA) and barbiturate receptor mediated 36Cl− uptake in rat brain synaptoneurosomes: evidence for rapid desensitization of the GABA receptor-coupled chloride ion channel. Mol Pharmacol. 1986;30:419–426. [PubMed] [Google Scholar]
- 33.Sen C K, Roy S, Packer L. Involvement of intracellular Ca2+ in oxidant-induced NF-κB activation. FEBS Lett. 1996;385:58–62. doi: 10.1016/0014-5793(96)00346-8. [DOI] [PubMed] [Google Scholar]
- 34.Severijnen A J, Van Kleef R, Hazenberg M P, van de Merwe J P. Cell wall fragment from major residents of the human interstinal flora induce chronic arthritis in rats. J Rheumatol. 1989;16:1061–1068. [PubMed] [Google Scholar]
- 35.Stachlewitz R F, Li X, Smith S, Bunzendahl H, Graves L M, Thurman R G. Glycine inhibits growth of T lymphocytes by an IL-2-independent mechanism. J Immunol. 2000;164:176–182. doi: 10.4049/jimmunol.164.1.176. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 37.van der Heijden I M, Wilbrink B, Tchetverikov I, Schouls L M, Hazenberg M P, Breedveld F C, Tak P P. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatioid arthritis and other arthritides. Arthritis Rheum. 2000;43:593–598. doi: 10.1002/1529-0131(200003)43:3<593::AID-ANR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe N, Suzuki J, Kobayashi Y. Role of calcium in tumor necrosis factor-α produced by activated macrophages. J Biochem. 1996;120:1190–1195. doi: 10.1093/oxfordjournals.jbchem.a021540. [DOI] [PubMed] [Google Scholar]
- 39.Weinblatt M E, Kremer J M, Bankhurst A D, Bulpitt K J, Fleischmann R M, Fox R I, Jackson C G, Lange M, Burge D J. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;28:253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- 40.Wheeler M D, Ikejima K, Enomoto N, Stachlewitz R F, Seabra V, Zhong Z, Yin M, Schemmer P, Rose M L, Rusyn I, Bradford B U, Thurman R G. Glycine: a new anti-inflammatory immunonutrient. Cell Mol Life Sci. 1999;56:843–856. doi: 10.1007/s000180050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wheeler M D, Seabra V, Thurman R G. Molecular evidence for glycine-gated chloride channel in Kupffer cells. In: Wisse E, Knook D L, Wake K, editors. Cells of the hepatic sinusoid. Leiden, The Netherlands: The Kupffer Cell Foundation; 1999. pp. 153–155. [Google Scholar]
- 42.Yoshimura A, Lien E, Ingalls R R, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]