This systematic review and meta-analysis evaluates the efficacy and safety of mRNA COVID-19 vaccines in children aged 5 to 11 years.
Key Points
Question
Are messenger RNA (mRNA) COVID-19 vaccines safe and effective in children aged 5 to 11 years?
Findings
In this systematic review and meta-analysis including 17 studies with 10 935 541 vaccinated and 2 635 251 unvaccinated children aged 5 to 11 years, COVID-19 vaccination was associated with lower risks of SARS-CoV-2 infection, symptomatic COVID-19, hospitalization, and multisystem inflammatory syndrome in children. While vaccination, compared with placebo, was associated with higher incidences of adverse events, the overall frequency of severe adverse events, including myocarditis, was low.
Meaning
These data support the safety and efficacy of mRNA COVID-19 vaccines among children aged 5 to 11 years and endorse the universal age-based recommendations.
Abstract
Importance
Evidence of the efficacy and safety of messenger RNA (mRNA) COVID-19 vaccines in children aged 5 to 11 years has been emerging. Collecting these data will inform clinicians, families, and policy makers.
Objective
To evaluate the efficacy and safety of mRNA COVID-19 vaccines in children aged 5 to 11 years in a systematic review and meta-analysis.
Data Sources
PubMed and Embase databases were searched on September 29, 2022, without language restrictions.
Study Selection
Randomized clinical trials and observational studies comparing vaccinated vs unvaccinated children aged 5 to 11 years and reporting efficacy or safety outcomes were included. Studies reporting safety outcomes in vaccinated children only (ie, no control group) were also included.
Data Extraction and Synthesis
Two investigators independently extracted relevant data from each study. Odds ratios (ORs) for efficacy and safety outcomes and incidences of adverse events (AEs) following vaccination were synthesized using a random-effects model. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses and Meta-analysis of Observational Studies in Epidemiology reporting guidelines.
Main Outcomes and Measures
The primary outcome was SARS-CoV-2 infections with or without symptoms. The secondary outcomes included symptomatic SARS-CoV-2 infections, hospitalizations, and multisystem inflammatory syndrome in children. The incidences of each AE following vaccination were also evaluated.
Results
Two randomized clinical trials and 15 observational studies involving 10 935 541 vaccinated children (median or mean age range, 8.0-9.5 years) and 2 635 251 unvaccinated children (median or mean age range, 7.0-9.5 years) were included. Two-dose mRNA COVID-19 vaccination compared with no vaccination was associated with lower risks of SARS-CoV-2 infections with or without symptoms (OR, 0.47; 95% CI, 0.35-0.64), symptomatic SARS-CoV-2 infections (OR, 0.53; 95% CI, 0.41-0.70), hospitalizations (OR, 0.32; 95% CI, 0.15-0.68), and multisystem inflammatory syndrome in children (OR, 0.05; 95% CI, 0.02-0.10). Two randomized clinical trials and 5 observational studies investigated AEs among vaccinated children. Most vaccinated children experienced at least 1 local AE following the first injection (32 494 of 55 959 [86.3%]) and second injection (28 135 of 46 447 [86.3%]). Vaccination was associated with a higher risk of any AEs compared with placebo (OR, 1.92; 95% CI, 1.26-2.91). The incidence of AEs that prevented normal daily activities was 8.8% (95% CI, 5.4%-14.2%) and that of myocarditis was estimated to be 1.8 per million (95% CI, 0.000%-0.001%) following the second injection.
Conclusions and Relevance
In this systematic review and meta-analysis, COVID-19 mRNA vaccines among children aged 5 to 11 years were associated with measures of efficacy in preventing SARS-CoV-2 infection and severe COVID-19–related illnesses. While most children developed local AEs, severe AEs were rare, and most of AEs resolved within several days. These data provide evidence for future recommendations.
Introduction
SARS-CoV-2 infection and COVID-19–related illnesses have affected all age groups and caused over 6.5 million deaths across the globe as of September 2022.1 During the early phase of the COVID-19 pandemic, studies showed that children with COVID-19 tended to present with milder symptoms than adults.2 However, as the pandemic progressed, emerging evidence suggested that children were still at risk of developing severe illnesses and complications from COVID-19, including respiratory failure, myocarditis, and multisystem inflammatory syndrome in children (MIS-C).3,4,5
COVID-19 messenger RNA (mRNA) vaccines have been extensively tested in adults and shown to be safe and effective measures to prevent SARS-CoV-2 infection, severe progression, and persistent sequelae known as post-COVID conditions.6,7,8,9,10 As these vaccines are now widely available for adolescents and children, the evidence in these populations is accumulating.11,12,13,14,15,16 A randomized clinical trial (RCT) showed a high vaccine efficacy (100%; 95% CI, 75.3%-100% for 1 month) in preventing symptomatic COVID-19 in adolescents aged 12 to 15 years.16 In addition, mRNA vaccines were also shown to be effective in reducing hospitalization and MIS-C in adolescents aged 12 to 18 years.14,17 Moreover, the risk of vaccine-related serious adverse events (AEs), including myocarditis, in this age group was reported to be low.16,18
However, the benefit of mRNA vaccines in children aged 5 to 11 years is not firmly established. While RCTs showed vaccine efficacy of 85% to 95% in preventing symptomatic SARS-CoV-2 infection in this age group,15,19 not all studies have confirmed their effectiveness in preventing acute COVID-19 and hospitalizations due to COVID-19–related illnesses.13,20 Furthermore, concerns for the safety of mRNA vaccines have been raised.21,22 Therefore, pooled evidence regarding the efficacy and safety of mRNA vaccines among children aged 5 to 11 years will inform physicians, families, and policy makers.
We conducted a systematic review and meta-analysis to synthesize the current literature and demonstrate the pooled evidence regarding the efficacy and safety of the mRNA COVID-19 vaccines in children aged 5 to 11 years.
Methods
This research was conducted under the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline.23,24 The protocol was registered in PROSPERO (CRD42022363439). An institutional review board exemption was granted.
Data Sources and Search
We used a 2-level strategy to search for all RCTs and observational studies evaluating the safety or efficacy of COVID-19 vaccines in children. First, a comprehensive literature search was conducted using PubMed and Embase on September 29, 2022. The search terms included (COVID-19 or SARS-CoV-2) and (vaccination or mRNA vaccines or COVID-19 vaccines or vaccine) and (children or child or 5-11 or 5 to 11) and (2019-nCoV vaccine mRNA-1273 or mRNA 1273 or M 1273 or TAK 919 or BNT162 vaccine or BNT162b2). Second, we performed an additional manual search of secondary sources, including references from initially identified articles, to comprehensively collect relevant articles. No restrictions on language and publication date were applied.
Eligibility Criteria
We included studies meeting the following criteria: (1) studies published in a peer-reviewed journal; (2) human subject studies involving children aged 5 to 11 years; (3) studies investigating mRNA vaccines; and (4A) studies comparing vaccinated vs unvaccinated children and reporting the efficacy or safety outcomes or (4B) studies reporting the safety outcomes in vaccinated children. We excluded articles without original patients’ data (eg, guidelines, correspondence, and reviews). To avoid patient duplication, we only included the largest study when 2 or more reports from the same cohort and period were identified. The risk of bias in RCTs and comparative observational studies were evaluated using the Cochrane Collaboration risk of bias 2.0 tool and a tool for assessing risk of bias in nonrandomized studies (ROBINS), respectively.25,26
Data Extraction
Two investigators (R.K. and A.W.) screened the search results independently to identify the studies based on the inclusion and exclusion criteria and assessed the eligibility. After screening the title and abstract, we retrieved the full text of potentially eligible reports for further review. Disagreements were resolved through consensus.
Data Items
Baseline characteristics, such as age, sex, ethnicity, preexisting comorbidities (obesity, cardiovascular disease, pulmonary disease, diabetes, and chronic kidney disease), observational period, and follow-up duration were extracted. Regarding the COVID-19 vaccine, we collected the type of vaccine (eg, BNT162b2 by Pfizer-BioNTech and mRNA-1273 by Moderna) and doses.
Outcomes
The primary outcome was SARS-CoV-2 infections with or without symptoms. The secondary outcomes included symptomatic SARS-CoV-2 infections, hospitalizations due to COVID-19–related illnesses or conditions, MIS-C, any reported AEs, and AEs that prevented normal daily activities. Among vaccinated children only, further details of the incidence of each AE, such as any local AEs, injection site pain, injection site redness, injection site swelling, any systemic AEs, fatigue, fever, headache, chills, myalgia, and myocarditis (defined by Medical Dictionary for Regulatory Activities or International Statistical Classification of Diseases, Tenth Revision, Clinical Modification), were assessed. Data from the Vaccine Adverse Event Reporting System were not used to estimate the incidence of each AE because the total number of injections was unavailable. The overall certainty of evidence in each outcome was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation approach.27
Data Synthesis and Analysis
For studies comparing vaccinated and unvaccinated children, we extracted unadjusted or adjusted (whenever available) odds ratios (ORs) of the safety and efficacy outcomes and synthesized the ORs with 95% CIs for each outcome using the Review Manager (RevMan) version 5.4 (Nordic Cochrane Center, the Cochrane Collaboration) with a random-effects model. Furthermore, we performed subgroup analyses according to the predominant subvariants (delta vs omicron) and the given dose (2 doses vs 1 dose) when 2 or more studies reported the outcomes of interest. We classified studies specifying that they investigated the omicron subvariant or those initiated after November 2021 into the omicron subgroup and those completed before November 2021 into the delta subgroup, while studies that continued over November 2021 were assumed to overlap. For studies involving vaccinated children, we pooled the logarithm of the incidence of each AE among vaccinated children and performed a 1-group meta-analysis by the Wald method with a random-effects model using OpenMetaAnalyst version 21.11.14.28 The synthesized logarithm was back-transformed into the original scale. Heterogeneity was assessed using I2, with more than 50% indicating substantial heterogeneity. Publication bias was assessed by Egger tests and funnel plots using the Comprehensive Meta-Analysis version 2 (Biostat).29 As a sensitivity analysis, we performed the Duval and Tweedy trim-and-fill analysis when a publication bias was suggested.30
Results
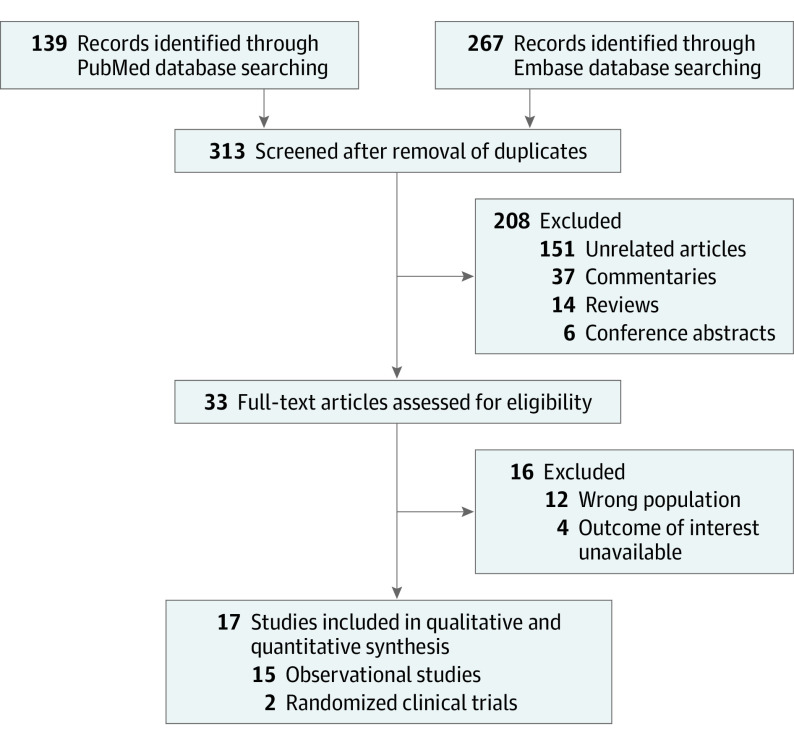
We identified 313 reports through the initial database and subsequent manual search. After removing 280 items based on the title and abstract, we retrieved the full text of 33 articles. We excluded studies investigating different age ranges, studies investigating non-mRNA vaccines, and studies not reporting outcomes of interest. Finally, we included 2 RCTs and 15 observational studies, including 12 cohort studies and 3 case-control studies (Figure 1).12,13,14,15,19,20,31,32,33,34,35,36,37,38,39,40,41,42 Baseline characteristics are summarized in Table 1 and eTable 1 in Supplement 1. There were 10 935 541 vaccinated children (mean or median age range, 8.0-9.5 years; 46.0%-55.9% female individuals) and 2 635 251 unvaccinated children (mean or median age range, 7.0-9.5 years; 44.3%-51.7% female individuals). The median follow-up duration ranged from 7 to 90 days. The risk of bias assessment is summarized in eTables 2 and 3 in Supplement 1.
Figure 1. Flowchart of Study Selection.
Of 313 identified articles, 2 randomized clinical trials and 15 observational studies were included, comparing efficacy or safety outcomes among vaccinated vs unvaccinated children or reporting adverse events reporting in vaccinated children.
Table 1. Baseline Characteristics.
| Source | Design | Country | Observational period | Predominant variant | Vaccine type | Vaccination status | Cohort size, No. | Age, y | No. (%) | Follow-up duration | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | At least 1 comorbidity | ||||||||||
| Cohen-Stavi et al,32 2022 | Retrospective cohort | Israel | November 23, 2021, to January 7, 2022 | Omicron | BNT162b2 | Vaccinated | 94 728 | Median (IQR), 8 (7-10) | 46 082 (49) | 31 087 (33) | 7-21 d After second dose |
| 14-27 d After first dose | |||||||||||
| Unvaccinated | 94728 | Median (IQR), 8 (7-10) | 46 083 (49) | 31 087 (33) | NA | ||||||
| Fleming-Dutra et al,31 2022 | Retrospective case-control | US | December 26, 2021, to February 21, 2022 | Omicron | BNT162b2 | Vaccinated | 15 778 | Median (IQR), 9 (7-10) | 7646 (48.5) | NA | 14-30/30-90 d After second dose |
| Unvaccinated | 58 430 | Median (IQR), 8 (6-10) | 28 958 (49.6) | NA | NA | ||||||
| Price et al,14 2022 | Retrospective case-control | US | December 19, 2021, to February 17, 2022 | Omicron | BNT162b2 | Vaccinated | 70 | NA | NA | NA | ≥14 d After second dose |
| Unvaccinated | 467 | NA | NA | NA | NA | ||||||
| Fowlkes et al,13 2022 | Prospective cohort | US | December 14, 2021, to February 12, 2022 | Omicron | BNT162b2 | 2 Doses | 682 | NA | NA | NA | 14-82 d After second dose |
| 1 Dose | 69 | NA | NA | NA | NA | ||||||
| Unvaccinated | 301 | NA | NA | NA | NA | ||||||
| Tan et al,34 2022 | Prospective cohort | Singapore | January 21, 2022, to April 8, 2022 | Omicron | BNT162b2 | 2 Doses | 173 237 | NA | NA | NA | ≥7-60 d After second dose |
| 1 Dose | 30 656 | NA | NA | NA | ≥1-30 d After first dose | ||||||
| Unvaccinated | 52 043 | NA | NA | NA | NA | ||||||
| Sacco et al,35 2022 | Retrospective cohort | Italy | January 17, 2022, to April 10, 2022 | Omicron | BNT162b2 | 2 Doses | 1 063 035 | NA | 519 315 (48.9) | NA | 0-84 d After second dose |
| 1 Dose | 134 386 | NA | 64 556 (48.0) | NA | NA | ||||||
| Unvaccinated | 1 768 497 | NA | 857 295 (48.5) | NA | NA | ||||||
| Klein et al,20 2022 | Retrospective cohort | US | December 3, 2021, to January 29, 2022 | Omicron | BNT162b2 | Vaccinated | 582 | NA | NA | NA | 14-67 d After second dose |
| Unvaccinated | 8599 | NA | NA | NA | NA | ||||||
| Creech et al,19 2022a | RCT | US and Canada | August 2021 to November 2021 | Delta | BNT162b2 | Vaccinated | 3007 | Mean (SD), 8.5 (1.7) | 1453 (48.3) | NA | ≥14 d After first/second dose |
| Unvaccinated | 995 | Mean (SD), 8.5 (1.6) | 514 (51.7) | NA | NA | ||||||
| Walter et al,15 2022 | RCT | US, Finland, Spain, and Poland | June 7, 2021, to October 8, 2021 | Delta | mRNA-1273 | Vaccinated | 1518 | Mean (SD), 8.2 (1.93) | 719 (47.4) | 312 (20.6) | ≥7 d After second dose, median follow-up time: 2.3 (0-2.5) mo |
| Unvaccinated | 750 | Mean (SD), 8.1 (1.97) | 367 (48.9) | 152 (20.3) | NA | ||||||
| Zambrano et al,12 2022 | Retrospective case-control | US | December 20, 2021, to April 7, 2022 | Omicron | BNT162b2 | Vaccinated | 53 | NA | NA | NA | 28-120 d After second dose |
| Unvaccinated | 321 | NA | NA | NA | NA | ||||||
| Amir et al,36 2023b | Retrospective cohort | Israel | December 26, 2021, to January 8, 2022 | Omicron | BNT162b2 | Vaccinated | 56 819 | NA | 48.3% | NA | 14-35 d After second dose |
| Unvaccinated | 572 859 | NA | 44.3% | NA | NA | ||||||
| Shi et al,37 2022 | Retrospective case-control | US | December 19, 2021, to February 28, 2022 | Omicron | BNT162b2 | Vaccinated | 48 | Median (IQR), 9 (8-11) | 23 (46.0) | NA | NA |
| Unvaccinated | 301 | Median (IQR), 7 (8-9) | 136 (44.4) | NA | NA | ||||||
| Block et al,38 2022 | Retrospective cohort | US | January 1, 2021, to January 31, 2022 | Delta-omicron | BNT162b2 and mRNA-1273 | 2 Doses | 41 742 | NA | NA | NA | NA |
| 1 Dose | 48 986 | NA | NA | NA | NA | ||||||
| Dose unknown | 30 199 | NA | NA | NA | NA | ||||||
| Unvaccinated | 76 960 | NA | NA | NA | NA | ||||||
| Hause et al,40 2022 | Retrospective single-arm | US | May 17, 2022, to July 31, 2022 | Omicron | BNT162b2 | 3 Doses | 3249 | NA | NA | NA | NA |
| 2 Doses | 3249 | NA | NA | NA | NA | ||||||
| 1 Dose | 3249 | NA | NA | NA | NA | ||||||
| Hause et al,39 2022 | Retrospective single-arm | US | November 3, 2021, to February 27, 2022 | Delta-omicron | BNT162b2 | 2 Doses | 7 266 633 | NA | NA | NA | 0-7 d After second dose |
| 1 Dose | 9 235 783 | NA | NA | NA | 0-7 d After first dose | ||||||
| Capponi et al,42 2022 | Retrospective single-arm | Italy | February 1, 2022, to February 28, 2022 | Omicron | BNT162b2 | 2 Doses | 345 | NA | NA | NA | 1/2/3 d After second dose |
| 1 Dose | 332 | NA | NA | NA | 1/2/3 d After first dose | ||||||
| Bloise et al,41 2022 | Prospective single-arm | Italy | December 15, 2021, to January 31, 2022 | Omicron | BNT162b2 | 2 Doses | 449 | Mean (SD), 9.5 (0.35) | NA | NA | 24-48 h/7 d/20 d after second dose |
| 1 Dose | 569 | Mean (SD), 9.5 (0.35) | 318 (55.9) | 459 (80.7) | 24-48 h/7 d/20 d after first dose | ||||||
Abbreviations: NA, not available; RCT, randomized clinical trial.
Age group, 6-11 years.
Age group, 5-10 years; the baseline is only shown for percentage because the absolute numbers were unavailable.
Comparison Between Vaccinated and Unvaccinated Children
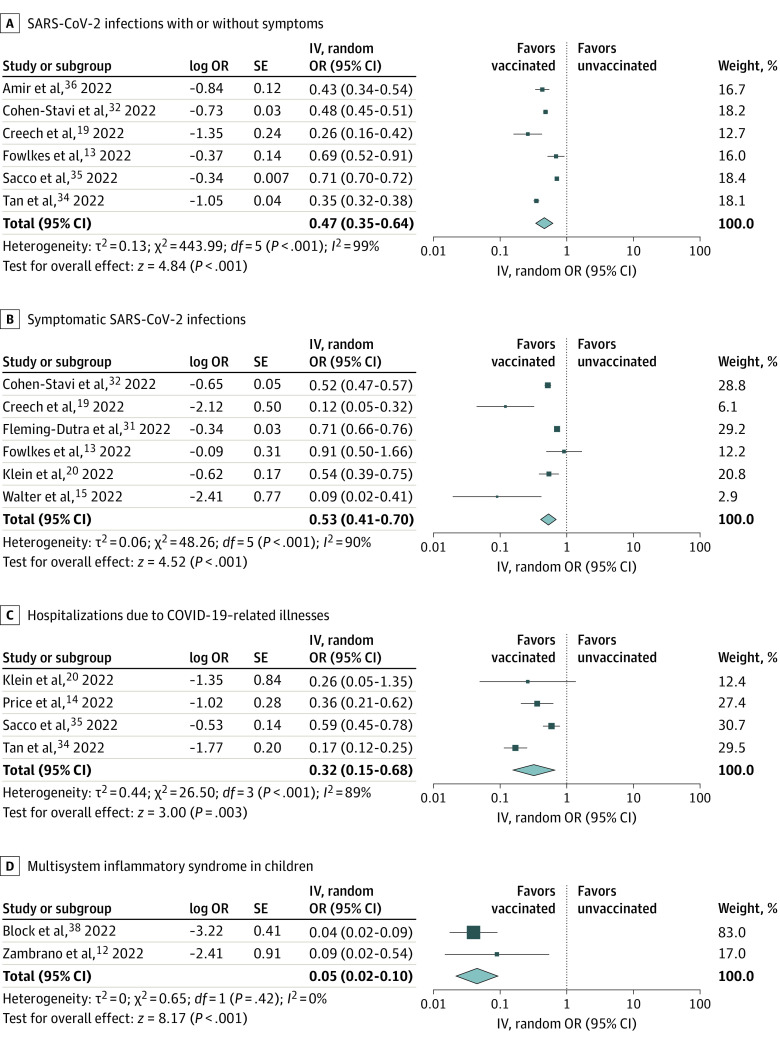
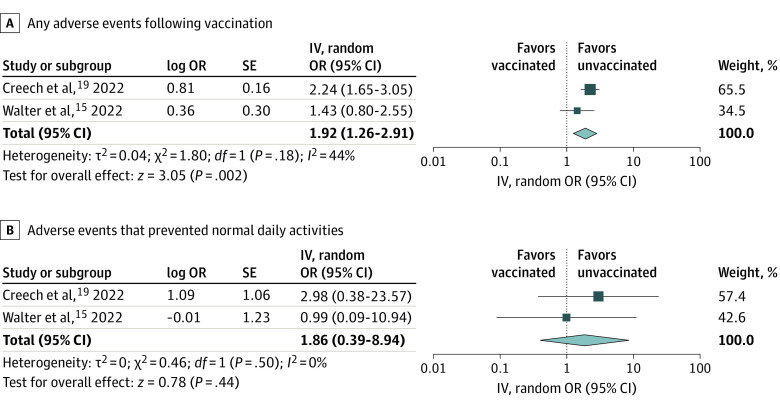
Two RCTs and 11 observational studies compared vaccinated vs unvaccinated children.12,13,14,15,19,20,31,32,34,35,36,37,38 The overall certainty of evidence (Grading of Recommendations Assessment, Development, and Evaluation) for each outcome is summarized in eTable 4 in Supplement 1. Two-dose mRNA COVID-19 vaccination compared with no vaccination among children aged 5 to 11 years was associated with lower risks of SARS-CoV-2 infections with or without symptoms (OR, 0.47; 95% CI, 0.35-0.64; I2 = 99%; high certainty) (Figure 2A), symptomatic SARS-CoV-2 infections (OR, 0.53; 95% CI, 0.41-0.70; I2 = 90%; high certainty) (Figure 2B), hospitalizations due to COVID-19–related illnesses (OR, 0.32; 95% CI, 0.15-0.68; I2 = 89%; moderate certainty) (Figure 2C), and MIS-C (OR, 0.05; 95% CI, 0.02-0.10; I2 = 0%; moderate certainty) (Figure 2D). Vaccination compared with placebo was significantly associated with higher risks of any AEs (OR, 1.92; 95% CI, 1.26-2.91; I2 = 44%; moderate certainty) (Figure 3A) and nonsignificantly associated with higher risks of AEs that prevented normal daily activities (OR, 1.86; 95% CI, 0.39-8.94; I2 = 0%; moderate certainty) (Figure 3B). Publication bias was not suggested (eFigure 1A-C in Supplement 1). Myocarditis or AEs that led to discontinuation from the trials were not observed.15,19
Figure 2. Forest Plots Showing the Odds Ratio (OR) of Efficacy Outcomes Following the Second Dose of Messenger RNA (mRNA) Vaccine.
Two-dose mRNA COVID-19 vaccination was associated with lower risks of SARS-CoV-2 infections with or without symptoms, symptomatic SARS-CoV-2 infections, hospitalizations due to COVID-19–related illnesses, and multisystem inflammatory syndrome in children. ORs were calculated using random-effects model. IV indicates inverse variance.
Figure 3. Forest Plots Showing the Odds Ratio (OR) for Adverse Events.
Vaccination was associated with higher risks for adverse events, although it was not statistically significant for adverse events that prevented normal daily activities. ORs were calculated using random-effects model. IV indicates inverse variance.
Among the 6 studies that reported SARS-CoV-2 infections with or without symptoms, 5 were conducted in the omicron-predominant period,13,32,34,35,36 which showed a similar finding to the primary analysis (eFigure 2 in Supplement 1). Among the 6 studies that reported symptomatic SARS-CoV-2 infections, 2 were conducted in the delta-predominant period,15,19 and 4 were conducted in the omicron-predominant period.13,20,31,32 Whereas the OR was lower in the delta-predominant period (eFigure 3A in Supplement 1), COVID-19 vaccination was still associated with lower risks of symptomatic SARS-CoV-2 infections in the omicron-predominant period (eFigure 3B in Supplement 1).
While 1-dose vaccination was associated with lower risks of SARS-CoV-2 infections with or without symptoms and hospitalizations due to COVID-19–related illnesses (eFigure 4 in Supplement 1), the effect size was smaller than 2-dose vaccination.
Vaccinated Children and AEs
Seven studies (2 RCTs and 5 single-arm observational studies) investigated the AEs among vaccinated children.15,19,38,39,40,41,42 Most vaccinated children experienced at least 1 local AE(s) following the first injection (32 494 of 55 959 [86.3%]; 95% CI, 74.1%-93.3%; I2 = 99%) (eFigure 5A in Supplement 1) and second injection (28 135 of 46 447 [86.3%]; 95% CI, 73.8%-93.4%; I2 = 99%) (eFigure 6A in Supplement 1). Less than half of the vaccinated children developed at least 1 systemic AE(s) following the first injection (20 369 of 55 380 [45.1%]; 95% CI, 34.3%-56.5%; I2 = 99%) (eFigure 5B in Supplement 1) and second injection (20 182 of 45 998 [56.4%]; 95% CI, 38.8%-72.5%; I2 = 99%) (eFigure 6B in Supplement 1). AEs that prevented normal daily activities were observed in 4.9% (2883 of 55 380; 95% CI, 3.1%-7.7%; I2 = 97%) (eFigure 5C in Supplement 1) and 8.8% (3805 of 47 516; 95% CI, 5.4%-14.2%; I2 = 99%) (eFigure 6C in Supplement 1) following the first and second injections, respectively. Myocarditis was observed in 1.3 per million (12 of 9 291 923; 95% CI, 0.000%-0.001%; I2 = 93%) (eFigure 5D in Supplement 1) and 1.8 per million (13 of 7 316 924; 95% CI, 0.000%-0.001%; I2 = 91%) (eFigure 6D in Supplement 1) following the first and second injections, respectively. The frequency of each AE following the first and second injections is summarized in Table 2. During the follow-up period, 4 deaths of 16 608 847 injections were observed after vaccination. Two had complex medical histories, such as autonomic instability and frequent intensive care unit admissions, while 1 had evidence of influenza infection on autopsy, and the other one’s autopsy report is under review. No evidence of causal associations between vaccination and deaths or explanations about other possible etiologies was found in these reports. Funnel plots and Egger tests for the AEs (eFigures 7A-D in Supplement 1) suggested overreporting publication bias of myocarditis. The sensitivity analysis using the trim-and-fill technique showed a similar result (eFigure 8 in Supplement 1).
Table 2. Adverse Events (AEs) Following Each Dose of Messenger RNA COVID-19 Vaccination.
| Factor | Dose 1 | Dose 2 | ||||
|---|---|---|---|---|---|---|
| Event | Total | Pooled proportion, % (95% CI) | Event | Total | Pooled proportion, % (95% CI) | |
| Any local AEs | 32 494 | 55 949 | 86.3 (74.1-93.3) | 28 135 | 46 447 | 86.3 (73.8-93.4) |
| Pain | 31 287 | 55 949 | 81.1 (66.1-90.4) | 27 311 | 46 447 | 79.6 (64.6-89.3) |
| Redness | 2121 | 52 945 | 3.9 (3.3-4.6) | 2070 | 43 459 | 5.1 (4.1-6.3) |
| Swelling | 2621 | 55 617 | 5.7 (3.1-10.1) | 2786 | 46 102 | 7.8 (3.8-15.1) |
| Any systemic AEs | 20 369 | 55 380 | 45.1 (34.3-56.5) | 20 182 | 45 998 | 56.4 (38.8-72.5) |
| Fatigue | 12 170 | 55 949 | 24.6 (16.3-35.3) | 13 224 | 46 447 | 33.1 (19.5-50.4) |
| Fever | 4543 | 55 949 | 5.7 (4.0-8.1) | 6788 | 46 447 | 15.2 (11.5-19.9) |
| Headache | 8566 | 55 949 | 16.4 (10.8-24.1) | 10 209 | 46 447 | 25.3 (14.0-41.3) |
| Chills | 2492 | 55 378 | 5.0 (2.8-8.5) | 3881 | 45 996 | 8.8 (3.2-21.9) |
| Myalgia | 4441 | 55 615 | 7.3 (4.9-10.9) | 5365 | 46 100 | 10.5 (5.4-19.3) |
| Myocarditis | 12a | 9 291 923 | 1.3/Million (0.000-0.001) | 13a | 7 316 924 | 1.8/Million (0.000-0.001) |
| AEs | ||||||
| That prevented normal daily activities | 2883 | 55 380 | 4.9 (3.1-7.7) | 3805 | 47 516 | 8.8 (5.4-14.2) |
| That required hospitalization | 11 | 52 376 | 0.21‰ (0.000-0.001) | 9 | 47 516 | 0.19‰ (0.000-0.001) |
When reported as <5 cases per million doses for confidentiality, we added 5 to avoid underestimation.
Regarding the duration of AEs, the RCTs showed that the median duration of each symptom ranged from 1 to 2 days, except for local pain and redness, which lasted for 3 days or more.15,19 Another prospective cohort study showed that 4 of 449 children (0.8%) had symptoms 7 days after the second injection, including headache and lymphadenopathy.41
Discussion
In this meta-analysis of 17 studies, we demonstrated 2 important findings: (1) mRNA COVID-19 vaccination in children aged 5 to 11 years was associated with lower risks of SARS-CoV-2 infections, severe COVID-19–related illnesses, and hospitalizations due to COVID-19 and (2) while mRNA COVID-19 vaccination compared with placebo was associated with any AEs, most of them were nonsevere and transient.
Since its emergence, SARS-CoV-2 has rapidly spread across the world and caused substantial mortality and long-term sequelae.1,43 Furthermore, as the COVID-19 pandemic progressed, the virus has transformed and negatively impacted children’s school life, mental health, and social development.44,45,46 This meta-analysis collected published data investigating the mRNA vaccines and indicated the associations between vaccination and lower risks of SARS-CoV-2 infections and COVID-19–related illnesses. Although children tend to develop milder symptoms than adults due to COVID-19,2 severe cases have still been identified.4,5,47,48 Our finding that mRNA vaccination in children aged 5 to 11 years was not only associated with lower risks of acute infection but also associated with lower risks of hospitalization and MIS-C was important since it will aid in addressing the misunderstandings that vaccination does not benefit children.
Studies have shown that the protective effect of COVID-19 vaccines wanes over time.49 In addition, the different effects according to subvariants have been suggested.50 Our meta-analysis classified studies into the 2 subgroups and evaluated the reported effectiveness of the vaccines against the different subvariants (delta vs omicron). While our findings showed that the association between vaccination and lower risks of symptomatic COVID-19 was more pronounced against the delta subvariant than omicron, it should be noted that vaccination was still associated with lower risks of symptomatic COVID-19 in the omicron-predominant time than no vaccination. Since our study could not include enough studies to evaluate hospitalizations and MIS-C according to the subvariants, further investigations regarding severe COVID-19–related illnesses are needed.
Whereas the efficacy of mRNA vaccines has been shown worldwide, their safety should be monitored closely and continuously. In the present study, while most children developed local AEs, the severe AEs were rare, and most of the AEs resolved within several days. As a concerning AE, postvaccination myocarditis has been reported.51,52,53 Although the clear mechanism has not been fully understood, several possible explanations have been suggested.54,55 When the immune system detects the vaccines’ mRNA as an antigen, it may activate proinflammatory cascades, potentially resulting in the development of myocarditis.54,55 Antibodies against SARS-CoV-2 spike protein have also been hypothesized to cross-react with human peptides.54 Reassuringly, the frequency of postvaccination myocarditis appears to be very low in adolescents aged 12 to 17 years, with most cases presenting favorable prognoses.18,51,52 A multicenter study in Italy reported that the incidence of myocarditis following mRNA COVID-19 vaccination was 0.04 to 3.2 of 100 000 individuals.53 According to the Vaccine Adverse Event Reporting System, myocarditis consisted of 4.3% (397 of 9246) of the total reported AEs in adolescents.56 In children aged 5 to 11 years, several myocarditis cases have also been identified.38,39 We indicated that the incidence of postvaccination myocarditis in this age group was also very low, even with the potential overreporting, although the design of our 1-group meta-analysis cannot suggest a causal relationship due to the absence of a comparator.
In the present study, mRNA vaccination was found to be associated with a lower risk of MIS-C. Importantly, the risk of developing myocarditis after SARS-CoV-2 infection is higher than after COVID-19 mRNA vaccination.5,57,58 A previous nationwide study in Israel showed that the risk of developing myocarditis following SARS-CoV-2 infection was 18 times higher than the control group, while it was 3 times higher following vaccination than the control.58 Likewise, the frequency of postvaccination myocarditis may be comparable with or slightly higher than the annual incidence rate of myocarditis before the COVID-19 pandemic in children.59 Furthermore, the prognoses of MIS-C provoked by SARS-CoV-2 infection are likely to be much poorer than mRNA postvaccination myocarditis.5,18,48,51 Given the incidence rate of MIS-C following SARS-CoV-2 infection among children aged 5 to 11 years, the protective effect of mRNA vaccines against MIS-C should be considered when discussing the risk-benefit balance of vaccination.
Despite the accumulating evidence, vaccine hesitancy still remains a global issue.21,60,61 The low uptake of vaccines among children and unwillingness of parents to vaccinate their children have been reported.21,22,62,63 According to surveys in the US, 25% to 46% of parents were unsure or reluctant to vaccinate their children.22,63,64 While 83% of parents acknowledged that “it would be bad if children got COVID-19,”63 26% did not believe that vaccines would protect their children from COVID-19. Notably, even when parents themselves were vaccinated, numerous parents considered that vaccination is unnecessary for their children because they are not seriously concerned about COVID-19 risks and its complications in children.21 Misunderstandings about vaccines’ effectiveness and concerns about their safety have repeatedly been suggested as major drivers of vaccine hesitancy among parents.21,22,61,65,66 More than 80% of parents have reported being concerned about possible serious or rare AEs of COVID-19 vaccines.64 In this context, the safety and effectiveness of mRNA COVID-19 vaccines in children aged 5 to 11 years drawn by previous reports and determined by our meta-analysis were informative. Furthermore, it should be noted that unvaccinated children may be causing continuous household viral transmission, perhaps leading to the prolongation of the pandemic worldwide.67,68,69 Although practical modalities to solve this multifactorial problem are not fully defined,70 our findings can help address the hesitancy related to mRNA COVID-19 vaccines.
Limitations
This study had several limitations. First, although we planned to evaluate all mRNA vaccines, most studies included only investigated BNT162b2 by Pfizer-BioNTech. Therefore, the external validity of our findings is unclear. However, as previous studies have shown similar efficacy and safety profiles between mRNA vaccines,9,10 our findings may translate into other mRNA vaccines. Second, the incidence of AEs in our study was estimated by 1-group meta-analyses. In principle, AEs should be evaluated by comparisons with background rates. However, since only 2 RCTs actively monitored AEs and compared vaccinated vs unvaccinated children, which did not detect any cases of myocarditis or AEs that led to discontinuation from the study,15,19 we had to adopt a 1-group meta-analysis design to estimate the frequency of AEs. In addition, as no RCTs reported postvaccination myocarditis, myocarditis cases in observational studies were used to estimate the incidence. Due to the nature of passive reporting, the incidence might have been underestimated. Nevertheless, we performed trim-and-fill analyses to address the potential reporting bias and demonstrated a similar result.30 Therefore, although our estimation cannot suggest causal relationships between some AEs and vaccination, the safety of the vaccines can still be highlighted. Third, the detailed history and patient characteristics of serious AEs following vaccination were unavailable. Future studies focusing on the risk factors and prognoses of serious AEs among children are warranted. Fourth, since this study only evaluated the vaccine effectiveness for acute infection, vaccine efficacy against post-COVID conditions and long-term safety remains unknown. Fifth, only 1 study in our analysis investigated the third dose.40 Since booster doses are expected to provide additional protection against infection and severe progression,71,72 further investigations on additional doses and omicron-bivalent vaccines are awaited.
Conclusions
In this systematic review and meta-analysis, COVID-19 vaccination in children aged 5 to 11 years was associated with lower risks of SARS-CoV-2 infection, COVID-19–related illnesses, and hospitalizations due to COVID-19–related illnesses. While most children developed local AEs, severe AEs were rare, and most of AEs resolved within several days. Our findings can help inform physicians and other clinicians, parents, and policy makers about the effectiveness and safety of COVID-19 vaccination for children aged 5 to 11 years. Future studies with longer follow-up are warranted to monitor the long-term safety of mRNA vaccines.
eTable 1. Baseline characteristics
eTable 2. Risk of bias assessment for randomized trials
eTable 3. Risk of bias assessment for observational studies
eTable 4. The overall certainty of each outcome
eFigure 1. Funnel plots for outcomes comparing vaccinated and unvaccinated children
eFigure 2. Forest plots for SARS-CoV-2 infections with or without symptoms in the omicron-predominant period
eFigure 3. Forest plots for symptomatic SARS-CoV-2 infections according to the predominant subvariants
eFigure 4. Forest plots comparing 1-dose vaccination versus no vaccination
eFigure 5. Forest plots showing the proportion of adverse events following the 1st dose of mRNA vaccination
eFigure 6. Forest plots showing the proportion of adverse events following the 2nd dose of mRNA vaccine
eFigure 7. Funnel plots for the proportion of each adverse events following the 2nd dose of mRNA vaccination
eFigure 8. Sensitivity analysis using the trim-and-fill technique for myocarditis
Data sharing statement
References
- 1.World Health Organization . WHO coronavirus (COVID-19) dashboard. Published March 9, 2022. Accessed March 10, 2022. https://covid19.who.int/
- 2.Yasuhara J, Kuno T, Takagi H, Sumitomo N. Clinical characteristics of COVID-19 in children: a systematic review. Pediatr Pulmonol. 2020;55(10):2565-2575. doi: 10.1002/ppul.24991 [DOI] [PubMed] [Google Scholar]
- 3.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. ; International COVID-19 PICU Collaborative . Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174(9):868-873. doi: 10.1001/jamapediatrics.2020.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandes DM, Oliveira CR, Guerguis S, et al. ; Tri-State Pediatric COVID-19 Research Consortium . Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J Pediatr. 2021;230:23-31.e10. doi: 10.1016/j.jpeds.2020.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team . Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334-346. doi: 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azzolini E, Levi R, Sarti R, et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA. 2022;328(7):676-678. doi: 10.1001/jama.2022.11691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385(26):2413-2420. doi: 10.1056/NEJMoa2115624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385(7):585-594. doi: 10.1056/NEJMoa2108891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kildegaard H, Lund LC, Højlund M, Stensballe LG, Pottegård A. Risk of adverse events after covid-19 in Danish children and adolescents and effectiveness of BNT162b2 in adolescents: cohort study. BMJ. 2022;377:e068898. doi: 10.1136/bmj-2021-068898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zambrano LD, Newhams MM, Olson SM, et al. ; Overcoming COVID-19 Investigators . BNT162b2 mRNA vaccination against COVID-19 is associated with decreased likelihood of multisystem inflammatory syndrome in U.S. children ages 5-18 years. Clin Infect Dis. 2022;ciac637. doi: 10.1093/cid/ciac637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowlkes AL, Yoon SK, Lutrick K, et al. Effectiveness of 2-dose BNT162b2 (Pfizer BioNTech) mRNA vaccine in preventing SARS-CoV-2 infection among children aged 5-11 years and adolescents aged 12-15 years: PROTECT cohort, July 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(11):422-428. doi: 10.15585/mmwr.mm7111e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price AM, Olson SM, Newhams MM, et al. ; Overcoming Covid-19 Investigators . BNT162b2 protection against the omicron variant in children and adolescents. N Engl J Med. 2022;386(20):1899-1909. doi: 10.1056/NEJMoa2202826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter EB, Talaat KR, Sabharwal C, et al. ; C4591007 Clinical Trial Group . Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386(1):35-46. doi: 10.1056/NEJMoa2116298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frenck RW Jr, Klein NP, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239-250. doi: 10.1056/NEJMoa2107456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zambrano LD, Newhams MM, Olson SM, et al. ; Overcoming COVID-19 Investigators . Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12-18 years: United States, July-December 2021. MMWR Morb Mortal Wkly Rep. 2022;71(2):52-58. doi: 10.15585/mmwr.mm7102e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuhara J, Masuda K, Aikawa T, et al. Myopericarditis after COVID-19 mRNA vaccination among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. Published online December 5, 2022. doi: 10.1001/jamapediatrics.2022.4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creech CB, Anderson E, Berthaud V, et al. ; KidCOVE Study Group . Evaluation of mRNA-1273 Covid-19 vaccine in children 6 to 11 years of age. N Engl J Med. 2022;386(21):2011-2023. doi: 10.1056/NEJMoa2203315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein NP, Stockwell MS, Demarco M, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19-associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5-17 years: VISION network, 10 states, April 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(9):352-358. doi: 10.15585/mmwr.mm7109e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubé E, Gagnon D, Pelletier C. COVID-19 vaccination in 5-11 years old children: drivers of vaccine hesitancy among parents in Quebec. Hum Vaccin Immunother. 2022;18(1):2028516. doi: 10.1080/21645515.2022.2028516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teasdale CA, Ratzan S, Rauh L, Lathan HS, Kimball S, El-Mohandes A. COVID-19 Vaccine coverage and hesitancy among New York City parents of children aged 5-11 years. Am J Public Health. 2022;112(6):931-936. doi: 10.2105/AJPH.2022.306784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372(160):n160. doi: 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 25.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 26.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.OpenMeta[Analyst]. Accessed January 4, 2023. http://www.cebm.brown.edu/openmeta/
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 31.Fleming-Dutra KE, Britton A, Shang N, et al. Association of Prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during omicron predominance. JAMA. 2022;327(22):2210-2219. doi: 10.1001/jama.2022.7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen-Stavi CJ, Magen O, Barda N, et al. BNT162b2 vaccine effectiveness against omicron in children 5 to 11 years of Age. N Engl J Med. 2022;387(3):227-236. doi: 10.1056/NEJMoa2205011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.González S, Olszevicki S, Gaiano A, et al. Effectiveness of BBIBP-CorV, BNT162b2 and mRNA-1273 vaccines against hospitalisations among children and adolescents during the omicron outbreak in Argentina: a retrospective cohort study. Lancet Reg Health Am. 2022;13:100316. doi: 10.2139/ssrn.4087375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan SHX, Cook AR, Heng D, Ong B, Lye DC, Tan KB. Effectiveness of BNT162b2 vaccine against omicron in children 5 to 11 years of age. N Engl J Med. 2022;387(6):525-532. doi: 10.1056/NEJMoa2203209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sacco C, Del Manso M, Mateo-Urdiales A, et al. ; Italian National COVID-19 Integrated Surveillance System and the Italian COVID-19 vaccines registry . Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection and severe COVID-19 in children aged 5-11 years in Italy: a retrospective analysis of January-April, 2022. Lancet. 2022;400(10346):97-103. doi: 10.1016/S0140-6736(22)01185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amir O, Goldberg Y, Mandel M, et al. Initial protection against SARS-CoV-2 omicron lineage infection in children and adolescents by BNT162b2 in Israel: an observational study. Lancet Infect Dis. 2023;23(1):67-73. doi: 10.1016/S1473-3099(22)00527-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi DS, Whitaker M, Marks KJ, et al. ; COVID-NET Surveillance Team . Hospitalizations of children aged 5-11 years with laboratory-confirmed COVID-19: COVID-NET, 14 states, March 2020-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(16):574-581. doi: 10.15585/mmwr.mm7116e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination: PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. doi: 10.15585/mmwr.mm7114e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hause AM, Shay DK, Klein NP, et al. Safety of COVID-19 vaccination in United States children ages 5 to 11 years. Pediatrics. 2022;150(2):e2022057313. doi: 10.1542/peds.2022-057313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hause AM, Baggs J, Marquez P, et al. Safety monitoring of Pfizer-BioNTech COVID-19 vaccine booster doses among children aged 5-11 years: United States, May 17-July 31, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(33):1047-1051. doi: 10.15585/mmwr.mm7133a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bloise S, Marcellino A, Frasacco B, et al. Cross-sectional survey on BNT162b2 mRNA COVID-19 vaccine serious adverse events in children 5 to 11 years of age: a monocentric experience. Vaccines (Basel). 2022;10(8):1224. doi: 10.3390/vaccines10081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capponi M, Pulvirenti F, Cinicola BL, et al. Short-term side effects and SARS-CoV-2 infection after COVID-19 Pfizer-BioNTech vaccine in children aged 5-11 years: an Italian real-world study. Vaccines (Basel). 2022;10(7):1056. doi: 10.3390/vaccines10071056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe A, So M, Iwagami M, et al. One-year follow-up CT findings in COVID-19 patients: a systematic review and meta-analysis. Respirology. 2022;27(8):605-616. doi: 10.1111/resp.14311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerber JS, Offit PA. COVID-19 vaccines for children. Science. 2021;374(6570):913. doi: 10.1126/science.abn2566 [DOI] [PubMed] [Google Scholar]
- 45.Letourneau N, Luis MA, Kurbatfinski S, et al. COVID-19 and family violence: a rapid review of literature published up to 1 year after the pandemic declaration. EClinicalMedicine. 2022;53:101634. doi: 10.1016/j.eclinm.2022.101634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fainardi V, Meoli A, Chiopris G, et al. Long COVID in children and adolescents. Life (Basel). 2022;12(2):285. doi: 10.3390/life12020285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yasuhara J, Watanabe K, Takagi H, Sumitomo N, Kuno T. COVID-19 and multisystem inflammatory syndrome in children: a systematic review and meta-analysis. Pediatr Pulmonol. 2021;56(5):837-848. doi: 10.1002/ppul.25245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe A, Yasuhara J, Karube T, et al. Extracorporeal membrane oxygenation in children with COVID-19: a systematic review and meta-analysis. Pediatr Crit Care Med. Published online November 17, 2022. doi: 10.1097/PCC.0000000000003113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924-944. doi: 10.1016/S0140-6736(22)00152-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327(7):639-651. doi: 10.1001/jama.2022.0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murase H, Zhu Y, Sakaida K, et al. Case report: five patients with myocarditis after mRNA COVID-19 vaccination. Front Pediatr. 2022;10:977476. doi: 10.3389/fped.2022.977476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hadley SM, Prakash A, Baker AL, et al. Follow-up cardiac magnetic resonance in children with vaccine-associated myocarditis. Eur J Pediatr. 2022;181(7):2879-2883. doi: 10.1007/s00431-022-04482-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Massari M, Spila Alegiani S, Morciano C, et al. ; TheShinISS-Vax|COVID Surveillance Group . Postmarketing active surveillance of myocarditis and pericarditis following vaccination with COVID-19 mRNA vaccines in persons aged 12 to 39 years in Italy: a multi-database, self-controlled case series study. PLoS Med. 2022;19(7):e1004056. doi: 10.1371/journal.pmed.1004056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471-484. doi: 10.1161/CIRCULATIONAHA.121.056135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schauer J, Buddhe S, Colyer J, et al. Myopericarditis after the Pfizer messenger ribonucleic acid coronavirus disease vaccine in adolescents. J Pediatr. 2021;238:317-320. doi: 10.1016/j.jpeds.2021.06.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feldstein LR, Tenforde MW, Friedman KG, et al. ; Overcoming COVID-19 Investigators . Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074-1087. doi: 10.1001/jama.2021.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078-1090. doi: 10.1056/NEJMoa2110475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arola A, Pikkarainen E, Sipilä JO, Pykäri J, Rautava P, Kytö V. Occurrence and features of childhood myocarditis: a nationwide study in Finland. J Am Heart Assoc. 2017;6(11):e005306. doi: 10.1161/JAHA.116.005306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Machingaidze S, Wiysonge CS. Understanding COVID-19 vaccine hesitancy. Nat Med. 2021;27(8):1338-1339. doi: 10.1038/s41591-021-01459-7 [DOI] [PubMed] [Google Scholar]
- 60.Carcelen AC, Prosperi C, Mutembo S, et al. COVID-19 vaccine hesitancy in Zambia: a glimpse at the possible challenges ahead for COVID-19 vaccination rollout in sub-Saharan Africa. Hum Vaccin Immunother. 2022;18(1):1-6. doi: 10.1080/21645515.2021.1948784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reilev M, Olesen M, Kildegaard H, et al. Changing characteristics over time of individuals receiving COVID-19 vaccines in Denmark: a population-based descriptive study of vaccine uptake. Scand J Public Health. 2022;50(6):686-692. doi: 10.1177/14034948221108246 [DOI] [PubMed] [Google Scholar]
- 62.Nguyen KH, Nguyen K, Geddes M, Allen JD, Corlin L. Trends in adolescent COVID-19 vaccination receipt and parental intent to vaccinate their adolescent children, United States, July to October, 2021. Ann Med. 2022;54(1):733-742. doi: 10.1080/07853890.2022.2045034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hammershaimb EA, Cole LD, Liang Y, et al. COVID-19 vaccine acceptance among US parents: a nationally representative survey. J Pediatric Infect Dis Soc. 2022;11(8):361-370. doi: 10.1093/jpids/piac049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gray A, Fisher CB. Determinants of COVID-19 vaccine uptake in adolescents 12-17 years old: examining pediatric vaccine hesitancy among racially diverse parents in the United States. Front Public Health. 2022;10:844310. doi: 10.3389/fpubh.2022.844310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fisher CB, Gray A, Sheck I. COVID-19 pediatric vaccine hesitancy among racially diverse parents in the United States. Vaccines (Basel). 2021;10(1):31. doi: 10.3390/vaccines10010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lyngse FP, Mortensen LH, Denwood MJ, et al. Household transmission of the SARS-CoV-2 Omicron variant in Denmark. Nat Commun. 2022;13(1):5573. doi: 10.1038/s41467-022-33328-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sumner KM, Karron RA, Stockwell MS, et al. ; CDC Genomic Sequencing Laboratory, SEARCh and C-HEaRT Study Teams . Impact of age and symptom development on SARS-CoV-2 transmission in households with children: Maryland, New York, and Utah, August 2020-October 2021. Open Forum Infect Dis. 2022;9(8):ofac390. doi: 10.1093/ofid/ofac390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Esposito S, Giordano R, Paini G, Puntoni M, Principi N, Caminiti C. Can we get out of the COVID pandemic without adequate vaccination coverage in the pediatric population? Ital J Pediatr. 2022;48(1):150. doi: 10.1186/s13052-022-01339-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reddinger JL, Levine D, Charness G. Can targeted messages reduce COVID-19 vaccination hesitancy? a randomized trial. Prev Med Rep. 2022;29:101903. doi: 10.1016/j.pmedr.2022.101903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Magen O, Waxman JG, Makov-Assif M, et al. Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2022;386(17):1603-1614. doi: 10.1056/NEJMoa2201688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Munro APS, Feng S, Janani L, et al. ; COV-BOOST study group . Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis. 2022;22(8):1131-1141. doi: 10.1016/S1473-3099(22)00271-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Callaway E. New Omicron-specific vaccines offer similar protection to existing boosters. Nature. 2022;609(7926):232-233. Published online September 1, 2022. doi: 10.1038/d41586-022-02806-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline characteristics
eTable 2. Risk of bias assessment for randomized trials
eTable 3. Risk of bias assessment for observational studies
eTable 4. The overall certainty of each outcome
eFigure 1. Funnel plots for outcomes comparing vaccinated and unvaccinated children
eFigure 2. Forest plots for SARS-CoV-2 infections with or without symptoms in the omicron-predominant period
eFigure 3. Forest plots for symptomatic SARS-CoV-2 infections according to the predominant subvariants
eFigure 4. Forest plots comparing 1-dose vaccination versus no vaccination
eFigure 5. Forest plots showing the proportion of adverse events following the 1st dose of mRNA vaccination
eFigure 6. Forest plots showing the proportion of adverse events following the 2nd dose of mRNA vaccine
eFigure 7. Funnel plots for the proportion of each adverse events following the 2nd dose of mRNA vaccination
eFigure 8. Sensitivity analysis using the trim-and-fill technique for myocarditis
Data sharing statement