Abstract
Iron uptake systems which are critical for bacterial survival and which may play important roles in bacterial virulence are often carried on mobile elements, such as plasmids and pathogenicity islands (PAIs). In the present study, we identified and characterized a ferric dicitrate uptake system (Fec) in Shigella flexneri serotype 2a that is encoded by a novel PAI termed the Shigella resistance locus (SRL) PAI. The fec genes are transcribed in S. flexneri, and complementation of a fec deletion in Escherichia coli demonstrated that they are functional. However, insertional inactivation of fecI, leading to a loss in fec gene expression, did not impair the growth of the parent strain of S. flexneri in iron-limited culture media, suggesting that S. flexneri carries additional iron uptake systems capable of compensating for the loss of Fec-mediated iron uptake. DNA sequence analysis showed that the fec genes are linked to a cluster of multiple antibiotic resistance determinants, designated the SRL, on the chromosome of S. flexneri 2a. Both the SRL and fec loci are carried on the 66,257-bp SRL PAI, which has integrated into the serX tRNA gene and which carries at least 22 prophage-related open reading frames, including one for a P4-like integrase. This is the first example of a PAI that carries genes encoding antibiotic resistance and the first report of a ferric dicitrate uptake system in Shigella.
Pathogenicity islands (PAIs) are increasingly recognized as playing a vital role in bacterial virulence. PAIs are distinct virulence cassettes that often integrate into tRNA genes and encode bacteriophage-like integrases. Such islands may occupy large regions of the chromosome and often carry mobile elements, such as insertion sequences and transposons (25). PAIs have been found in many bacterial species, including Yersinia spp. (9, 13), enteropathogenic, enterohemorrhagic, and uropathogenic Escherichia coli (24, 38, 51), Salmonella enterica serovar Typhimurium (61), Vibrio cholerae (32), Helicobacter pylori (14), and Shigella flexneri (2, 42, 57, 71). Some strains of uropathogenic E. coli and S. enterica serovar Typhimurium may harbor at least five PAIs (19, 74). A variety of virulence determinants may be carried on PAIs, including genes encoding fimbriae, hemolysins (31, 64), type III secretion systems (15, 27), and iron uptake systems (13, 42, 71, 75). Various Shigella spp. produce the siderophores enterobactin and/or aerobactin, which are involved in iron uptake (34, 50). The aerobactin locus in S. flexneri was recently shown to be carried on the SHI-2 PAI (42, 71). This was the first report of an iron transport system being carried on a PAI in Shigella.
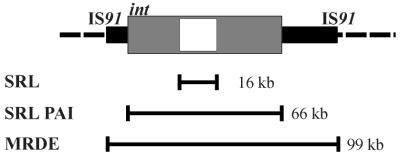
A number of PAI-like elements in Shigella spp. have been described. These include the SHI-2 PAI and a family of structurally related elements (42, 71) and the she PAI, which also belongs to a larger family of structurally related elements (2, 57). One of the characteristics of PAIs is their tendency to excise spontaneously from their sites of integration in the chromosome (26). In the S. flexneri 2a strain YSH6000, the spontaneous loss of multiple antibiotic resistance is accompanied by the deletion of an approximately 99-kb chromosomal region (56). The deletion of this region also coincides with a 50% decrease in contact hemolysis, a trait that correlates closely with virulence in Shigella spp. (56). These findings suggested that the 99-kb region is a deletable genomic element that carries multiple antibiotic resistance determinants. Preliminary sequence analysis of the 99-kb deletable element, which we have termed the multiple resistance deletable element (MRDE), demonstrated that the four antibiotic resistance determinants associated with the element are clustered within a 16-kb region (54) which we have termed the Shigella resistance locus (SRL). We recently found that the loss of multiple antibiotic resistance also occurs via a second type of spontaneous deletion event involving a distinct 66-kb element contained within the 99-kb MRDE (66). In the present study, we demonstrate that the 66-kb element is a PAI, termed the SRL PAI, that encodes a functional ferric dicitrate uptake system.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Bacterial strains and plasmids used in this study are listed in Table 1. Strains were grown routinely with aeration at 37°C in either 2YT broth (40) or Luria-Bertani broth (LB) (5) with the addition of ampicillin (100 μg/ml), kanamycin (50 μg/ml), or tetracycline (12.5 μg/ml) when necessary.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| S. flexneri | ||
| YSH6000 | Serotype 2a, wild-type Japanese isolate; Strr Apr Cmr Tetr | 58 |
| YSH6000T | Derivative of YSH6000; MRDE deletant; Strs Aps Cms Tets | 44 |
| SBA1366 | Derivative of YSH6000; SRL deletant; Strs Aps Cms Tets | 66 |
| SBA1415 | fecl::kan allelic exchange into SBA1366 | This work |
| E. coli | ||
| DH5α | F− φ80d lacZΔ M15Δ(lacZYA-argF) U169 endA1 recA1 hsdR17 deor thi-1 supE441 gyrA96 relA1 | Bethesda Research Laboratories |
| AA93 | Z1418 (F−araD139 ΔlacU169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR thi aroB) Δfec | 49 |
| SBA844 | SBA845 with pSBA491 | This work |
| SBA845 | Z1418 Δfec with pWSK129 | This work |
| Plasmids | ||
| pWSK29 | pSC101-based low-copy-number vector; Apr ΔlacZ | 73 |
| pWSK129 | pSC101-based low-copy-number vector; Knr ΔlacZ | 73 |
| pCACTUS | pSC101-based low-copy-number suicide vector with sacB, Cmr | 67 |
| pUC4-KIXX | Source of Kmr from Tn5, Apr, Kmr | 6 |
| pSBA361 | 27.6-kb BamHI fragment generated by marker rescue from YSH6000 cloned into pWSK29; Tetr Apr | This work |
| pSBA491 | 8.9-kb HindIII fragment containing fecIRABCDE subcloned from pSBA361 into pWSK 129; Knr | This work |
| pSBA509 | 24-kb BamHI fragment generated by marker rescue from YSH6000 cloned into pWSK129; Apr, Knr | This work |
Molecular techniques.
Plasmid DNA was isolated using a modified alkaline lysis method (35), while genomic DNA was isolated as described previously (5). Restriction digests were carried out using enzymes supplied by Roche Molecular Biochemicals or New England Biolabs. Transformation of E. coli and S. flexneri strains was performed following electroporation (63) with a Bio-Rad gene pulser at 1.8 kV, 25 μF, and 200 Ω in 0.1-cm electroporation cuvettes.
RNA was extracted from E. coli and S. flexneri strains for expression analysis of the fec locus. Inocula were prepared by growing bacteria overnight in LB supplemented with antibiotics where necessary. Following centrifugation at 10,000 × g for 1 min, cells were washed in 1 ml of Fec medium (49) modified by the addition of 2′,2-dipyridyl (0.4 mM) and citrate (1 mM). Fifty milliliters of modified Fec medium was inoculated with 100 μl of each bacterial suspension and incubated with aeration until early exponential phase (4 h). Cells were centrifuged at 1,300 × g for 10 min, and the supernatant was discarded. RNA was extracted as described previously (62) and treated with DNase (Roche) to remove DNA contamination. RNA dot blots were probed with either a fecA DNA fragment or a recA fragment that served as a control for sample loading. Probes were derived by PCR amplification (fecA, 5′-GTTGTCGTCATAAGAGCGG-3′ and 5′-GCTCCCATTTCGCTCGGC-3′; recA, 5′-CTACGCACGTAAACTGGGCG-3′ and 5′-ACCGGTAGTGGTTTCCGGG-3′) and labeled with digoxigenin (Roche) as recommended by the manufacturer. The RNA concentration of matched strain pairs SBA844-AA93, YSH6000-YSH6000T, and SBA1366-SBA1415 was standardized by absorbance at 260 nm. The equal loading of RNA on membranes was confirmed by dot blot analysis with the recA-derived probe.
PCR and sequencing.
Standard PCR, single strand-specific PCR (sspPCR), and inverse PCR were performed as described previously (47; PCR Applications Manual, 2nd ed., Roche Molecular Biochemicals; J. Novak and L. Novak, Promega Notes Magazine 61:26–29, 1997). Long-range PCR was carried out with the Expand long-range PCR kit (Roche).
Nucleotide sequencing of the PAI in S. flexneri strain YSH6000 was carried out by sequencing genomic clones, inverse PCR products, sspPCR products, and a long-range PCR product. Sequence reactions were conducted using the BigDye system (PE Biosystems Inc.). Reaction products were analyzed on an Applied Biosystems model 373A DNA sequencing system. Sequence editing was carried out using Sequencher 3.0 for Macintosh. Sequence analysis and database comparisons were performed using BlastN and BlastX (3). Analysis of proteins was carried out using previously described web-based analysis tools (28, 29, 43, 46, 60).
Assays of growth under iron-limited conditions.
Modified Fec medium (49) for growth under iron-limited conditions consisted of LB containing 0.4 mM 2′,2-dipyridyl, 1 mM citrate, and kanamycin when required. Inocula were prepared by growing strains overnight in 2.5 ml of LB or LB containing kanamycin for the maintenance of plasmids. To remove exogenous iron, bacteria were centrifuged at 10,000 × g for 1 min and resuspended in modified Fec medium. Following a second wash in modified Fec medium, bacterial suspensions were standardized by absorbance at 600 nm. Fifty milliliters of the modified Fec medium was inoculated with 0.1 ml of the standardized bacterial suspension. Aerated cultures were incubated at 37°C. Two-milliliter samples were taken over a 24-h period (2, 4, 6, 8, 12, and 24 h), and the absorbance at 600 nm was measured. Four cultures of each strain were grown simultaneously. Viable counts were also performed at 0 and 24 h to compare with absorbance readings.
Nucleotide sequence accession number.
Nucleotide sequences have been deposited in GenBank under the accession number AF326777.
RESULTS
Identification and functional analysis of the fec iron transport locus in S. flexneri 2a.
Sequencing of the regions surrounding the SRL using marker-rescued clones (described below) revealed a locus that was homologous to the ferric dicitrate transport (fec) genes located at min 97.3 of the E. coli K-12 genome (68). Like the E. coli fec locus, the Shigella locus consists of two operons carrying the regulatory genes, fecI and fecR, and the downstream structural genes, fecABCDE. The S. flexneri fec genes showed more than 99% nucleotide identity with the E. coli K-12 genes, but there were differences in the regions flanking the locus. The E. coli K-12 locus is flanked upstream by IS1 and downstream by an IS911 element that is insertionally disrupted by an IS30 and a truncated IS2. In contrast, the S. flexneri fec locus was flanked downstream by an intact IS911. The sequence directly downstream of IS911 is identical in E. coli and S. flexneri. Upstream of the S. flexneri fec locus were the first 61 bp of IS1 followed by remnants of IS3, IS629, and IS903-like elements. Prior to this report, the fec locus had been identified only in E. coli strains (36, 53, 65, 72), although fecA, fecD, and fecE homologs had been observed in H. pylori (69). This is the first report of an intact ferric dicitrate transport system in a Shigella sp.
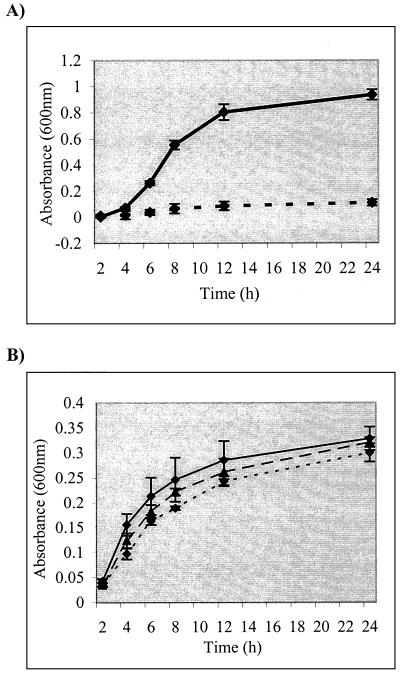
The ferric dicitrate iron transport system has been well characterized in E. coli K-12 (17). It is capable of maintaining the growth of E. coli under iron-limited conditions in the absence of other iron uptake systems. Because of the high similarity between the S. flexneri and E. coli fec loci, the function of the SRL PAI fec locus was tested in an E. coli Δfec strain, AA93 (49). In LB alone, all strains grew equally well (data not shown). However, the growth rate of AA93 under iron-limited conditions (LB supplemented with 0.4 mM 2,2′-dipyridyl and 1 mM citrate) was greatly reduced (Fig. 1A). Complementation of AA93 with the cloned S. flexneri fec locus (pSBA491), but not the cloning vector alone, restored its ability to grow under iron-limited conditions (Fig. 1A), demonstrating that the S. flexneri fec locus is functional.
FIG. 1.
Growth of E. coli (A) and S. flexneri (B) under iron-limiting conditions in medium supplemented with citrate. (A) SBA844 (solid line) is a Δfec strain complemented with the S. flexneri fec locus, and SBA845 (broken line) is the Δfec strain carrying an empty vector. (B) S. flexneri strains used were the wild-type SBA1366 (solid line), the fecI::kan strain SBA1415 (short dashes), and the MRDE deletant strain YSH6000T (long dashes). Error bars represent 2 standard deviations.
Although the fec genes are functional in E. coli, it was necessary to establish their ability to function in S. flexneri. FecI is a member of the sigma 70 factor subclass that responds to extracytoplasmic stimuli and regulates extracytoplasmic functions (37). In E. coli, the fecI gene is essential for the transcription of the fecABCDE operon (48). Therefore, to study the function of the fec locus in S. flexneri, we inactivated the fecI gene by inserting the kan cassette from pUC4-KIXX into the unique XhoI site within fecI. To introduce the fecI mutation into the S. flexneri chromosome, a 5.1-kb PCR product containing fecI::kan, fecR, and the 5′ end of fecA was cloned into the suicide vector pCACTUS and subsequently introduced by electroporation into SBA1366 (YSH6000, SRL−). A double-crossover mutant, SBA1415, was selected by growth at 42°C in the presence of sucrose. The mutation was confirmed by PCR using primers within, and external to, the pCACTUS construct (data not shown).
To test whether the Fec iron transport system had a role in the growth of S. flexneri 2a YSH6000 under iron-limited conditions, the fecI::kan mutant was compared to its parent (SBA1366) and strain YSH6000T, which has undergone a spontaneous excision of the 99-kb element. When strains were cultured under iron-limited conditions in medium supplemented with citrate, there was no significant difference in growth rate between any of the strains (Fig. 1B); neither mutation nor loss of the fec locus had any effect on the growth of S. flexneri 2a strain YSH6000.
There were several possible explanations for the lack of phenotypic difference between the wild-type strain and the fecI mutant, including the possibility that the fec locus is not transcribed in S. flexneri or that fecI may not be essential for transcription of the fec structural genes in S. flexneri. Both of these hypotheses were tested by RNA dot blot analysis of the expression of fec in YSH6000, YSH6000T, AA93, SBA844, SBA1366, and SBA1415 cells grown under iron-limited conditions (see Materials and Methods).
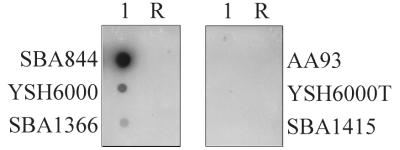
Although analysis with a recA probe confirmed that equal amounts of RNA were loaded for each pair on the dot blot, fecA mRNA was undetectable in the S. flexneri fecI mutant, SBA1415, but readily detectable in the isogenic parent strain, SBA1366, and in the wild-type strain YSH6000 (Fig. 2). As expected, fecA transcript was undetectable in the negative control strains YSH6000T and AA93, which do not carry the fec locus, but was detected in an AA93 strain complemented with the S. flexneri fec locus (SBA844). These results demonstrated that the fec locus is expressed in YSH6000 in a fecI-dependent manner and therefore suggest that this strain carries additional iron uptake systems that compensate for the fec mutation when grown in laboratory culture media.
FIG. 2.
Transcriptional analysis of fecA in S. flexneri and E. coli. An RNA dot blot compares matched strain pairs (E. coli SBA844-AA93 and S. flexneri YSH6000-YSH6000T and SBA1366-SBA1415) for transcription of fecA under iron-limited conditions. Lanes: 1, undiluted sample; R, samples treated with RNase to ensure that there was no DNase contamination. SBA844, YSH6000, and SBA1366 carry the S. flexneri fec locus, while AA93 and YSH6000T do not. SBA1415 carries the fec locus with a kan cassette inserted in fecI.
Southern hybridization showed that the fec locus is present in a single copy in YSH6000 and absent in YSH6000T (data not shown). Therefore, if a second iron uptake system exists in YSH6000, it must belong to another class. Recently, aerobactin-mediated iron uptake systems were found on proposed PAIs on the chromosome of several S. flexneri serotypes (42, 71). To test whether such a system existed in YSH6000, we examined this strain for the presence of iucA, the first gene in the aerobactin biosynthesis operon. A PCR product was amplified from strains YSH6000 and YSH6000T using primers designed from the SHI-2 PAI iucA region. The sequence of the PCR product from YSH6000T confirmed that an iucA gene identical to that from the SHI-2 PAI was present in YSH6000T. Therefore, it seems possible that an aerobactin locus and/or other types of iron uptake systems may have compensated for the loss of fec function in SBA1415.
The fec locus is present on a PAI.
Iron transport systems have been identified on several PAIs, including the high-pathogenicity island (HPI) of Yersinia spp. and E. coli (10, 33, 59), Salmonella pathogenicity island 1 of S. enterica serovar Typhimurium (30), and PAI-VICFT073 in uropathogenic E. coli, which carries a putative iron transport system (23). Several of these systems, as well as the aerobactin iron transport system on the SHI-2 PAI of S. flexneri (42, 71), have roles in virulence. Based on the findings presented below, we demonstrated that the S. flexneri fec locus also is carried on a PAI that in addition carries the multiple antibiotic resistance genes of the SRL.
Southern hybridization analysis demonstrated that the fec locus is present in S. flexneri 2a YSH6000 but is absent in the spontaneous, antibiotic-sensitive derivative YSH6000T, suggesting that the fec locus and the SRL are physically linked. This was confirmed by DNA sequencing, which showed that the tetracycline and chloramphenicol resistance determinants characterized by Rajakumar et al. (57) are on the same BamHI fragment as the fec locus (Fig. 3). We have found that the fec locus is present in a variety of Shigella strains. Thirty-five of 55 Shigella strains examined by high-stringency Southern analysis carried the fecA gene. Long-range PCR analysis of a single sample strain from each Shigella species with the same antibiotic resistance profile as strain YSH6000 confirmed that in each case the fec locus is linked to the tetracycline resistance gene, as it is in strain YSH6000 (data not shown). This suggests that the SRL PAI is present in all species of Shigella.
FIG. 3.
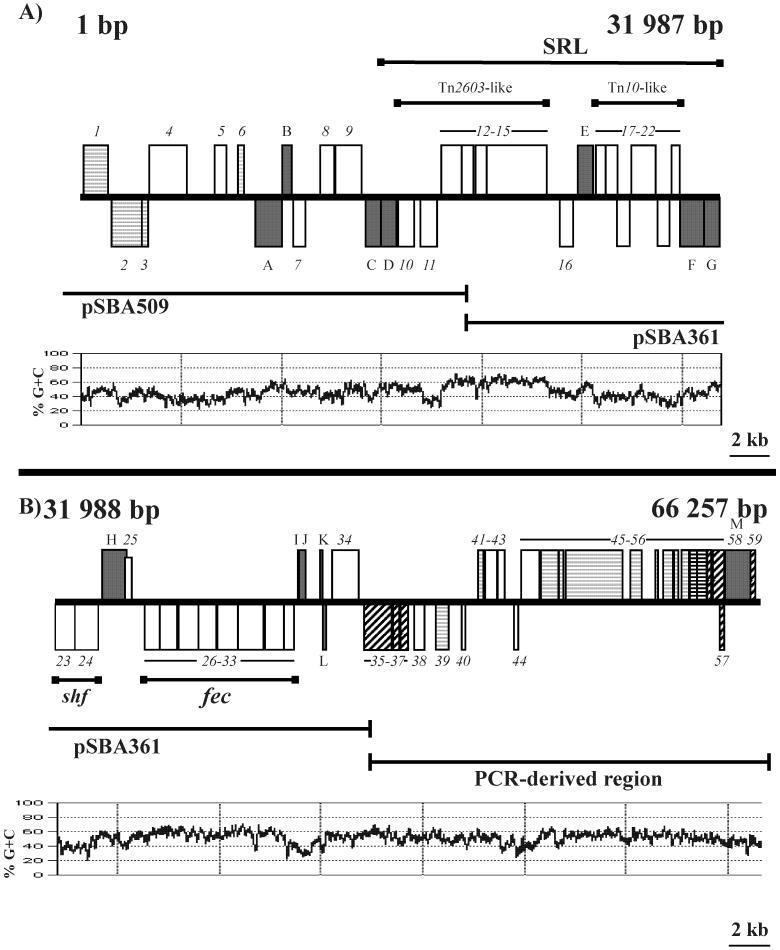
Genetic organization of the SRL PAI. A map of the SRL PAI sequence from bp 1 to 31,987 (A) and 31,988 to 66,257 (B) is shown. The ORFs are represented by boxes above the line (forward orientation) or below the line (reverse orientation). The unlabeled open boxes represent ORFs of unknown function. IS elements (grey boxes) have been designated A through M (Table 3). Boxes with light grey horizontal lines represent the CP4 prophage-related ORFs, while boxes with diagonal black lines represent 933L prophage-related ORFs. Boxes with black horizontal lines represent ORFs that have homologs on both the 933L and CP4 prophages. Sequence data were derived from the genomic subclones pSBA509 and pSBA361 and PCR-derived products indicated below each map. The G+C content of the SRL PAI was plotted using a window size of 100 bp.
The antibiotic resistance determinants of strain YSH6000 delete spontaneously from the chromosome at a frequency of 10−5 to 10−6 (66), suggesting that the SRL, and therefore the closely linked fec locus, may be carried on a mobile element. To gain a better understanding of the element, the regions flanking the SRL were sequenced using plasmid clones obtained by marker rescue of the resistance determinants encoded by the SRL. BamHI fragments of 27.6 kb, carried on pSBA361, and 24 kb, carried on pSBA509 (Fig. 3), were cloned by selection for tetracycline and ampicillin resistance, respectively. The remainder of the element was sequenced from DNA fragments derived by inverse PCR, sspPCR, and long-range PCR (Fig. 3).
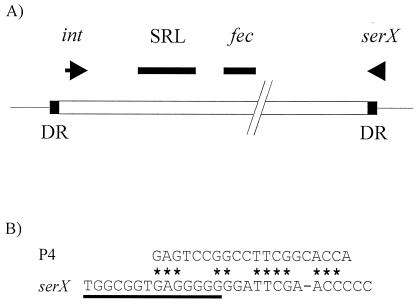
Analysis of the DNA sequence showed that a large genetic element has inserted into the 3′ terminal region of the serX tRNA gene in YSH6000 (Fig. 4A). The element is bounded on the serX-distal side by a 14-bp direct repeat (DR) of the 3′-terminal 14-bp sequence of serX (Fig. 4A). The DNA sequences upstream of serX and downstream of the serX-distal DR are almost identical to sequences that are contiguous with the serX gene in E. coli, a species that is closely related to S. flexneri. Notably, the 3′ termini of tRNA genes commonly serve as integration sites for PAIs and prophages (25). In addition, the 3′ terminus of serX has sequence similarity to a P4 att site (Fig. 4B), implying that it may act as an integration site for prophage-like or PAI-like elements. The sequence of the genetic element revealed the presence of a P4 bacteriophage-like integrase gene 161 bp upstream of the 14-bp serX-distal DR. The integration of the element into the 3′ end of a tRNA gene, the presence of an integrase gene near one boundary of the element, and the recent finding that the element undergoes spontaneous, integrase-mediated, precise excision to restore the serX locus as it is organized in E. coli (66) led us to conclude that the element is a PAI, which we have termed the SRL PAI.
FIG. 4.
Structure of the SRL PAI and the DR. (A) The SRL PAI is represented by the open box. The shaded boxes represent the 14-bp DR. The right-hand DR is the last 14 bp of serX. (B) The 14-bp DR and the potential att site. The lower sequence shows the 3′ end of the S. flexneri YSH6000 serX gene. The region representing the 14-bp DR is underlined. The upper sequence represents the P4 core att site. ∗, conserved nucleotide.
Interestingly, there was a significant discrepancy in the lengths of the 66.2-kb SRL PAI and the previously described 99-kb deletable element carrying multiple antibiotic resistance in YSH6000 (56). This discrepancy has been explained by the recent finding that the SRL PAI is entirely contained within a yet-larger distinct genetic element, termed the MRDE, which is flanked by IS91-like elements and is also capable of precise excision from the chromosome (66) (Fig. 5). This is similar to the Y. pestis 6/69 HPI, which is contained within a larger, deletable 102-kb chromosomal region and which also carries genes for hemin utilization. The boundaries of the 102-kb region are defined by two IS100 elements which mediate the spontaneous deletion of the HPI and flanking chromosomal DNA in this strain (9).
FIG. 5.
Overview of the genetic organization of the S. flexneri 2a YSH6000 chromosome surrounding the SRL PAI. The three deletable elements in the region corresponding to min 23 of the E. coli chromosome are shown. The dashed line represents the chromosomal region identical to E. coli, while the solid line represents the 99-kb MRDE. The grey box denotes the SRL PAI, while the white box is the SRL. The MRDE is flanked by IS91-like sequences.
Genetic organization of the SRL PAI.
The SRL PAI is 66,257 bp in length (Fig. 3), beginning 161 bp upstream of the int gene and ending at the 3′ terminus of the intact serX gene. The SRL PAI contains 59 open reading frames (ORFs) (Table 2; Fig. 3), excluding the ORFs associated with insertion sequences, and has an average G+C content of 49.8 mol%. Although the overall G+C content is not significantly different from that of the S. flexneri chromosome (51 mol%), significant deviations occur in the regions homologous to Tn2603, Tn10, and the fec locus, which have G+C contents of 57, 39, and 58 mol%, respectively. This is consistent with the fact that Tn2603 and Tn10 are laterally acquired elements and suggests that the fec locus may also have been laterally acquired by the PAI relatively recently.
TABLE 2.
ORFs contained within the SRL PAI
| ORF | Gene | Position (bp) | Related proteina | % Similarityb | Protein accession no. |
|---|---|---|---|---|---|
| 1 | int | 619–1836 | Integrase CP4-57 (SlpA) | 49 | P32053 (U36840) |
| 2 | orf2 | 3535–2006 | Yfjl | 45 | P52124 |
| 3 | orf3 | 3812–3507 | AlpA (CP4-57) | 66 | P33997 |
| 4 | orf4 | 3868–5730 | No similarity | ||
| 5 | orf5 | 7104–7697 | No similarity | ||
| 6 | orf6 | 8449–8745 | Yfjl (frameshift) | 73 | P52125 |
| 7 | orf7 | 11582–10980 | LysR-like transcriptional regulator | 79 | P39376 |
| 8 | orf8 | 12300–12989 | Hypothetical protein in LysR-AraE | 63 | P03813 |
| 9 | orf9 | 13055–14353 | DcuA (anaerobic decarboxylate transporter) | 70 | P04539 |
| 10 | aadA1 | 16963–16172 | AadA1 (streptomycin resistance) | 95 | P04826 |
| 11 | oxa-1 | 17906–17076 | Oxa-1 (ampicillin resistance) | 99 | P13661 |
| 12 | intI1 | 18116–19129 | IntI1 | 99 | P09999 |
| 13 | tnpM | 19098–19682 | TnpM | 100 | P04162 |
| 14 | tnpR | 19808–20368 | TnpR | 98 | P04130 |
| 15 | tnpA | 20371–23337 | TnpA | 98 | BAA78805.1 |
| 16 | cat | 24641–23982 | Cat (chloramphenicol Resistance) | 98 | P00483 |
| 17 | orf17 | 25759–26256 | YdjB | 79 | BAA78832.1 |
| 18 | orf18 | 26365–26949 | JemC | 79 | AF162223 |
| 19 | tetR | 27553–26927 | TetR | 98 | P04483 |
| 20 | tetA(B) | 27632–28837 | TetA(B) (tetracycline resistance) | 99 | P02980 |
| 21 | tetC | 29543–28950 | TetC | 99 | BAA78836 |
| 22 | tetD | 29631–30047 | TetD | 100 | BAA78837 |
| 23 | orf23 | 32594–31983 | CapU (hexosyltransferase homolog) | 99, 73 | AAD34405, AB011549 |
| 24 | orf24 | 33389–32622 | Shf | 93, 92.3 | AF134403, U61977 |
| 25 | orf25 | 34749–35096 | Hypothetical ORF o137 | 98 | S56511 |
| 26 | fecE | 36488–35721 | FecE | 100 | P15031 |
| 27 | fecD | 37445–36492 | FecD | 100 | P15029 |
| 28 | fecC | 38419–37442 | FecC | 100 | P15030 |
| 29 | fecB | 39339–38437 | FecB | 98 | P15028 |
| 30 | orf30 | 40424–39384 | FecA precursor | 100 | P13036 |
| 31 | fecA | 41707–40421 | FecA | 100 | M63115 |
| 32 | fecR | 42747–41794 | FecR | 100 | P23485 |
| 33 | fecI | 43265–42744 | FecI | 100 | P23484 |
| 34 | orf34 | 45173–46531 | Putative periplasmic protein (Campylobacter jejuni) | 52 | AL139076 |
| 35 | orf35 | 48155–46770 | L0015 | 98 | AAC31494 |
| 36 | orf36 | 48552–48205 | L0014 | 98 | AAC31493 |
| 37 | orf37 | 48950–48549 | L0013 | 99 | AAC31492 |
| 38 | orf38 | 49805–49284 | No similarity | ||
| 39 | orf39 | 50940–50404 | YfjJ | 55 | P52125 |
| 40 | orf40 | 51855–51679 | Hha/YmoA (Yersinia enterocolitica) | 75, 75 | P23870, P27720 |
| 41 | orf41 | 52491–52769 | Vis (P4) | 53 | —c |
| 42 | orf42 | 52858–53466 | No similarity | ||
| 43 | orf43 | 53637–53888 | No similarity | ||
| 44 | orf44 | 54534–54319 | No similarity | ||
| 45 | orf45 | 54667–55584 | No similarity | ||
| 46 | orf46 | 55669–56541 | YfjP, YeeP | 65, 97 | P52131, P76359 |
| 47 | orf47 | 56924–59761 | Ag43 (Flu) | 78 | P39180 |
| 48 | orf48 | 60145–60705 | YfjQ | 88 | P52132 |
| 49 | orf49 | 61390–61530 | YfjX, KlcA | 81, 51 | P52139, P52603 |
| 50 | orf50 | 61782–62273 | YfjY, YeeS | 80, 99 | p52140, P76362 |
| 51 | orf51 | 62336–62557 | YeeT | 98 | P76363 |
| 52 | orf52 | 62720–63094 | YeeU, YfjZ | 91, 75 | P76364, P52141 |
| 53 | orf53 | 63141–63515 | L0007, YeeV, YpjJ | 93, 87, 74 | AAC31485.1, P76365, O46953 |
| 54 | orf54 | 63512–64003 | L0008, YeeW | 96, 77 | AAC31487.1, P76366 |
| 55 | orf55 | 64015–64212 | L0009 | 88 | AAC31488.1 |
| 56 | orf56 | 64297–64860 | L0010 | 85 | AAC31489.1 |
| 57 | orf57 | 64878–64651 | L0011 | 80 | AAC31490.1 |
| 58 | orf58 | 64929–65933 | IS/328 transposase (Y. enterocolitica) | 83 | O56897 |
| 59 | orf59 | 66204–66452 | L0012 | 90 | AAC31491.1 |
Related proteins are from E. coli unless otherwise stated.
At the amino acid level.
—, data from reference 52.
The deduced product of the int gene, located near the left boundary of the SRL PAI, has significant sequence similarity at the amino acid level to several integrase proteins from the P4 prophage Int family, including those from the CP4-57 cryptic prophage of E. coli (49% similar) and the V. cholerae PAI (52% similar). Integrases from the other S. flexneri PAIs, she PAI and SHI-2, showed 47 and 45% similarity to the SRL PAI Int, respectively. The putative Int protein encoded on the SRL PAI possesses a conserved motif (R, HXXR, Y) necessary for the function of P4-like integrases (1, 4), suggesting that the integrase may be functional.
Twelve ORFs on the SRL PAI have significant sequence similarity, ranging from 45 to 88%, to ORFs carried on the CP4-57 prophage (Fig. 3). Although the physical spacing of the SRL PAI ORFs differed from that of their homologues in CP4-57, their order and orientation are conserved, with the exception of orf2 and orf3 on the SRL PAI, which are inverted compared to their CP4-57 homologues, yfjI and alpA, respectively. A number of these SRL PAI ORFs, including orf16, orf39, and orf48, are truncated in comparison to their CP4-57 homologues. Of the 12 ORFs homologous to CP4-57 ORFs, seven have homologues in CP4-44, another cryptic prophage in E. coli K-12 (8). Similarity between the SRL ORFs and ORFs on CP4-44 ranged from 87 to 99%. The SRL PAI also carries homologues of ORFs L0007 to L0015 from a third prophage, 933L, situated on the enterohemorrhagic E. coli (EHEC) locus of enterocyte effacement (LEE) PAI. Two of these ORFs, ORFs 53 and 54, are also common to CP4-44. Thus, a total of 22 SRL PAI ORFs, comprising just under 20% of the PAI sequence, appear to have a prophage origin. ORF 47 of the SRL PAI is homologous to autotransporter protein Antigen 43 (87% similarity), encoded by the cryptic prophage CP4-44, YpjA (37% similarity), encoded by a CP4-57 ORF of unknown function, and Sap (72% similarity), encoded by an ORF of unknown function on the she PAI of S. flexneri 2a.
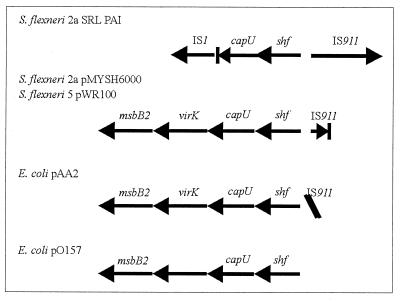
The SRL PAI carries six intact insertion sequences and seven IS remnants that have undergone deletions (Table 3). IS elements and their remnants, including IS1, IS200, IS600, IS629, and IS1328, which are present on the SRL PAI, are commonly found in other PAIs (7, 21, 39, 71). The SRL PAI also carries an ORF, designated shf, that is almost identical to the previously described shf ORF carried on the virulence plasmids of S. flexneri serotype 2a strain YSH6000 (55) and serotype 5 (11). The shf ORFs have sequence characteristics that are common to some mobile elements, such as retroviral integrases and IS transposases. Close homologues of shf (>83% similarity at the protein level) are also found on the plasmids pAA2 of enteroaggregative and diffusely adhering E. coli (18) and pO157 of EHEC (12), as well as a chromosomal locus bearing two overlapping genes, sat1 and sat2, in S. enterica serovar Typhimurium. In Shigella and E. coli, shf is part of a larger locus that includes a hexosyltransferase homologue, capU, an msbB homologue, and an IS911 remnant (Fig. 6).
TABLE 3.
Insertion sequences on the SRL PAI
| Fig. 3 label | Position(s) | IS element | Comment(s) (% nucleotide identity) |
|---|---|---|---|
| A | 10421–9112 | IS629 | Intact (99) |
| B | 10422–10905 | IS200 | First 118 bp truncated; transposase gene frameshift (98) |
| C | 15323–14554 | IS600 | Deletion of bp 746–801; no intact ORFs (97) |
| D | 16091–15324 | IS1 | Intact (99) |
| E | 24865–25632 | IS1 | Intact (99) |
| F | 31219–30060 | IS10 | Transposase truncated by 16 bp (99) |
| G | 31987–31220 | IS1 | Intact (97) |
| H | 33590–34827 | IS911 | Intact (92) |
| I | 43448–43508 | IS1 | Truncated at bp 61 |
| J | 43507–43741 and 43749–43827 | IS3 | Internal deletion and truncation (87) |
| K | 44562–44609 and 44621–44666 | IS903-like | Remnant only; no intact ORFs (86) |
| L | 44884–44718 | IS629 | Remnant only; last 163 bp (97) |
| M | 64929–66203 | IS1328 | Truncated; transposase intact (83) |
FIG. 6.
Genetic organization of the shf locus in S. flexneri and E. coli, showing the extent of the conserved shf locus in several bacterial strains. pMYSH6000 is carried by YSH6000, the strain that contains the SRL PAI. pWR100 is borne by S. flexneri 5 strain M90T (70). pAA2 is from enteroaggregative E. coli strain O42 (18), and pO157 is carried by EHEC O157:H7 (12). shf and capU are conserved in all loci, although they are truncated in the S. flexneri chromosome due to an IS1 insertion. An IS911 is found upstream of three of the loci, although it is intact only on the S. flexneri SRL PAI chromosomal locus and is oriented in the reverse direction on pAA2 in enteroaggregative E. coli. In addition, the plasmid-borne loci exhibit a conserved organization with regard to shf, capU, virK, and msbB2, with pO157 having an alternative gene in place of virK.
DISCUSSION
This study confirms our hypothesis that multiple antibiotic resistance in S. flexneri strain YSH6000 is encoded by a PAI. While our previous work suggested that such a PAI might be approximately 99 kb in length (56), we have shown that the SRL PAI is only 66.2 kb in length and is located on a distinct 99-kb element which, like the PAI, is capable of excision from the chromosome (66). Our conclusion that the antibiotic resistance determinants of the SRL are carried on a PAI is based on several characteristic sequence features and genetic properties common to other PAIs. These include a chromosomal insertion site in the 3′ terminus of a tRNA gene, the presence of short DRs at the boundaries of the element, the presence of a P4-like integrase gene near one end of the element, and the recent demonstration that the element undergoes precise, integrase-mediated excision from the chromosome (66). In addition, the SRL PAI typically contains a large number of IS elements and transposons. A striking feature of the PAI is the large number of ORFs that are related to the CP4 group of prophages and the 933L prophage, which is associated with the EHEC LEE PAI. Of particular interest is the almost complete conservation in the organization and orientation of homologous ORFs in the SRL PAI and CP4, suggesting very strongly that the SRL PAI shares a common ancestry with these prophages.
The SRL PAI represents the first example of a PAI that contains multiple antibiotic resistance genes. One other mobile genetic element that encodes antibiotic resistance, the SXT element of V. cholerae, has some features in common with the SRL PAI. The SXT element is capable of integrase-mediated, site-specific integration into and excision from the chromosome and carries all of the genes required for conjugative self-transfer to new hosts. However, although the SRL PAI undergoes site-specific, integrase-mediated excision from the chromosome, it does not appear to carry any of the genes required for conjugative transfer. Therefore, the SRL PAI appears to be quite a distinct type of genetic element. Whether the SRL PAI is capable of being transferred laterally to new hosts is unknown. However, the finding that the fec and SRL loci are linked in several tested strains from each of the four Shigella spp. suggests that the SRL PAI has disseminated throughout the genus Shigella.
In addition to antibiotic resistance, the SRL PAI encodes an iron uptake system. Iron is an essential nutrient in bacteria, where it is a component of the electron transport system (45) and an essential cofactor for a variety of enzymes. While iron is readily available in the environment, in the human host it is stored in tissues, such as the liver, or chelated by extracellular proteins, such as transferrin and lactoferrin (22, 41). Intracellular pathogens, such as Shigella, can scavenge iron from within the cells they invade, but they must also obtain iron from the extracellular environment of the host. To achieve this they produce extracellular high-affinity, low-molecular-weight iron chelators, called siderophores (16, 45). E. coli and some S. flexneri and Shigella boydii strains produce the catechol siderophore enterobactin (50), while some S. flexneri, S. boydii, and Shigella sonnei strains also produce the dihydroxamate siderophore aerobactin (34). In the present study we have identified a third type of siderophore system, a ferric dicitrate system, in S. flexneri 2a. The fec system in YSH6000 is only the second example of iron uptake genes carried on a PAI in Shigella. However, iron uptake genes are also carried on PAIs in S. enterica serovar Typhimurium, Yersinia spp., and some pathogenic strains of E. coli (13, 33, 75).
Our work also describes the first example of a ferric dicitrate uptake system in the genus Shigella. Until now, this type of iron uptake system has been found only in the commensal strains E. coli B and E. coli K12, E. coli strains causing bovine mastitis (36), and EHEC O157:H7 strain EDL933 (65). Although it was demonstrated that the fec locus is functional and is expressed in S. flexneri strain YSH6000, we could not demonstrate any alteration in the growth rate of a fecI mutant strain grown in iron-limited culture media. This finding suggests that YSH6000 expresses additional iron uptake systems that are capable of compensating for the loss of Fec function. This is consistent with previous reports of siderophore production in S. flexneri and the presence of iucA, one of the genes involved in aerobactin synthesis, in strain YSH6000. Indeed the presence of multiple iron uptake systems in a single strain is not unusual. For example, E. coli strains may have up to five iron(III) transport systems (20). The possession of more than one iron uptake system may confer on bacteria a greater ability to survive in different niches outside or inside the host. Since the Fec system is expressed in nonpathogenic E. coli strains, which unlike Shigella spp. do not invade intestinal cells, its primary role in S. flexneri may be in the uptake of iron from the intestinal lumen, where exogenous citrate is available for the chelation of iron.
In conclusion, our discovery of the SRL PAI in S. flexneri has revealed yet another type of genetic element that may be involved in the lateral transfer of antibiotic resistance. In addition, this element also encodes the first ferric dicitrate uptake system known to exist in Shigella spp. Future studies will address whether the SRL PAI is naturally mobilized to new bacterial hosts.
ACKNOWLEDGMENTS
This work was supported by a project grant from the National Health and Medical Research Council, Canberra, Australia.
We acknowledge the excellent technical assistance of Ian McPherson and Vicki Vallance.
REFERENCES
- 1.Abremski K E, Hoess R H. Evidence for a second conserved arginine residue in the integrase family of recombination proteins. Protein Eng. 1992;5:87–91. doi: 10.1093/protein/5.1.87. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hasani K, Rajakumar K, Dieter B, Robins-Browne R, Adler B, Sakellaris H. Genetic organization of the she pathogenicity island in Shigella flexneri2a. Microb Pathog. 2001;30:1–8. doi: 10.1006/mpat.2000.0404. [DOI] [PubMed] [Google Scholar]
- 3.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argos P, Landy A, Abremski K, Egan J B, Haggard-Ljungquist E, Hoess R H, Kahn M L, Kalionis B, Narayana S V, Pierson III L S, et al. The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J. 1986;5:433–440. doi: 10.1002/j.1460-2075.1986.tb04229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons Inc.; 1995. [Google Scholar]
- 6.Barany F. Single-stranded hexameric linkers: a system for in-phase insertion mutagenesis and protein engineering. Gene. 1985;37:111–123. doi: 10.1016/0378-1119(85)90263-x. [DOI] [PubMed] [Google Scholar]
- 7.Blanc-Potard A B, Solomon F, Kayser J, Groisman E A. The SPI-3 pathogenicity island of Salmonella enterica. J Bacteriol. 1999;181:998–1004. doi: 10.1128/jb.181.3.998-1004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goedon M A, Rose D J, Mau B, Shao Y. The complete sequence of Escherichia coliK-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 9.Buchrieser C, Prentice M, Carniel E. The 102-kilobase unstable region of Yersinia pestiscomprises a high-pathogenicity island linked to a pigmentation segment which undergoes internal rearrangement. J Bacteriol. 1998;180:2321–2329. doi: 10.1128/jb.180.9.2321-2329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchrieser C, Brosch R, Bach S, Guiyoule A, Carniel E. The high-pathogenicity island of Yersinia pseudotuberculosis can be inserted into any of the three chromosomal asntRNA genes. Mol Microbiol. 1998b;30:965–978. doi: 10.1046/j.1365-2958.1998.01124.x. [DOI] [PubMed] [Google Scholar]
- 11.Buchrieser C, Glaser P, Rusniok C, D'Hauteville H, Kunst F, Sansonetti P, Parsot C. The virulence plasmid pWR100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri. Mol Microbiol. 2000;38:760–771. doi: 10.1046/j.1365-2958.2000.02179.x. [DOI] [PubMed] [Google Scholar]
- 12.Burland V, Shao Y, Perna N T, Plunkett G, Sofia H J, Blattner F R. The complete DNA sequence and analysis of the large virulence plasmid of Escherichia coli O157:H7. Nucleic Acids Res. 1998;26:4196–4204. doi: 10.1093/nar/26.18.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carniel E, Guilvout I, Prentice M. Characterization of a large chromosomal “high-pathogenicity island” in biotype 1B Yersinia enterocolitica. J Bacteriol. 1996;178:6743–6751. doi: 10.1128/jb.178.23.6743-6751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cirillo D M, Valdivia R H, Monack D M, Falkow S. Macrophage-dependent induction of the Salmonellapathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- 16.Crosa J H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989;53:517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crosa J H. Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol Mol Biol Rev. 1997;61:319–336. doi: 10.1128/mmbr.61.3.319-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czeczulin J R, Whittam T S, Henderson I R, Navarro-Garcia F, Nataro J P. Phylogenetic analysis of enteroaggregative and diffusely adherent Escherichia coli. Infect Immun. 1999;67:2692–2699. doi: 10.1128/iai.67.6.2692-2699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dozois C M, Curtiss R., III Pathogenic diversity of Escherichia coliand the emergence of ‘exotic’ islands in the gene stream. Vet Res. 1999;30:157–179. [PubMed] [Google Scholar]
- 20.Earhart C F. Uptake and metabolism of iron and molybdenum. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1075–1090. [Google Scholar]
- 21.Elliot S J, Wainwright L A, McDaniel T K, Jarvis K G, Deng Y, Lai L-C, McNamara B, Donnenberg M S, Kaper J B. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coliE2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 22.Gray-Owen S, Schryvers A. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–191. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 23.Guyer D M, Kao J-S, Mobley H L T. Genomic analysis of a pathogenicity island in uropathogenic Escherichia coliCFT073: distribution of homologous sequence among isolates from patients with pyelonephritis, cystitis, and catheter-associated bacteriuria and from faecal samples. Infect Immun. 1998;66:4411–4417. doi: 10.1128/iai.66.9.4411-4417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hacker J, Bender L, Ott M, Wingender J, Lund B, Marre R, Goebel W. Deletions of chromosomal regions coding for fimbriae and hemolysins occur in vitro and in vivo in various extraintestinal Escherichia coliisolates. Microb Pathog. 1990;8:213–225. doi: 10.1016/0882-4010(90)90048-u. [DOI] [PubMed] [Google Scholar]
- 25.Hacker J, Blum-Oehler G, Muhldorfer I, Tschape H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 26.Hacker J, Kaper J B. The concept of pathogenicity islands. In: Kaper J B, Hacker J, editors. Pathogenicity islands and other mobile virulence elements. Washington, D.C.: American Society for Microbiology; 1999. pp. 1–11. [Google Scholar]
- 27.Hensel M, Shea J E, Waterman S R, Mundy R, Nikolaus T, Banks G, Vazquez-Torres A, Gleeson C, Fang F C, Holden D W. Genes encoding putative effector proteins of the type III secretion system of Salmonellapathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann K, Bucher P, Falquet L, Bairoch A. The PROSITE database, its status in 1999. Nucleic Acids Res. 1999;27:215–219. doi: 10.1093/nar/27.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofmann K, Stoffel W. TMbase—a database of membrane spanning proteins segments. Biol Chem Hoppe-Seyler. 1993;347:166. [Google Scholar]
- 30.Janakiraman A, Slauch J M. The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium. Mol Microbiol. 2000;35:1146–1155. doi: 10.1046/j.1365-2958.2000.01783.x. [DOI] [PubMed] [Google Scholar]
- 31.Kao J S, Stucker D M, Warren J W, Mobley H L. Pathogenicity island sequences of pyelonephritogenic Escherichia coliCFT073 are associated with virulent uropathogenic strains. Infect Immun. 1997;65:2812–2820. doi: 10.1128/iai.65.7.2812-2820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karaolis D K, Johnson J A, Bailey C C, Boedeker E C, Kaper J B, Reeves P R. A Vibrio choleraepathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci USA. 1998;95:3134–3139. doi: 10.1073/pnas.95.6.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karch H, Schubert S, Zhang D, Zhang W, Schmidt H, Olschlager T, Hacker J. A genomic island, termed high-pathogenicity island, is present in certain non-O157 Shiga toxin-producing Escherichia coliclonal lineages. Infect Immun. 1999;67:5994–6001. doi: 10.1128/iai.67.11.5994-6001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawlor K M, Payne S M. Aerobactin genes in Shigellaspp. J Bacteriol. 1984;160:266–272. doi: 10.1128/jb.160.1.266-272.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LeGouill C, Parent J-L, Rola-Pleszczynsk M, Stankova J. Analysis of recombinant plasmids by a modified alkaline lysis method. Anal Biochem. 1994;219:164. doi: 10.1006/abio.1994.1250. [DOI] [PubMed] [Google Scholar]
- 36.Lin J, Hogan J S, Smith K L. Antigenic homology of the inducible ferric citrate receptor (FecA) of coliform bacteria isolated from herds with naturally occurring bovine intramammary infections. Clin Diagn Lab Immunol. 1999;6:966–969. doi: 10.1128/cdli.6.6.966-969.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lonetto M A, Brown K L, Rudd K E, Buttner M J. Analysis of the Streptomyces coelicolor sigEgene reveals the existence of a subfamily of eubacterial RNA polymerase sigma factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDaniel T K, Kaper J B. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coliK-12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 39.Mellies J L, Navarro-Garcia F, Okeke I, Frederickson J, Nataro J P, Kaper J B. espC pathogenicity island of enteropathogenic Escherichia coliencodes an enterotoxin. Infect Immun. 2001;69:315–324. doi: 10.1128/IAI.69.1.315-324.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 41.Moffett D, Moffett S, Schauf C. Human physiology: foundations and frontiers. 2nd ed. St. Louis, Mo: Mosby-Year Book Inc.; 1993. [Google Scholar]
- 42.Moss J E, Cardozo T J Z A, Groisman E A. The selC-associated SHI-2 pathogenicity island of Shigella flexneri. Mol Microbiol. 1999;33:74–83. doi: 10.1046/j.1365-2958.1999.01449.x. [DOI] [PubMed] [Google Scholar]
- 43.Nakai K, Horton P. PSORT: a program for detecting the sorting signals of proteins and predicting their subcellular localization. Trends Biochem Sci. 1999;24:34–35. doi: 10.1016/s0968-0004(98)01336-x. [DOI] [PubMed] [Google Scholar]
- 44.Nakata N, Sasakawa C, Okada N, Tobe T, Fukuda I, Suzuki T, Komatsu K, Yoshikawa M. Identification and characterization of virK, a virulence-associated large plasmid gene essential for intercellular spreading of Shigella flexneri. Mol Microbiol. 1992;6:2387–2395. doi: 10.1111/j.1365-2958.1992.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 45.Neilands J B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Ochman H, Gerber A S, Hartl D L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ochs M, Angerer A, Enz S, Braun V. Surface signaling in transcriptional regulation of the ferric citrate transport system of Escherichia coli: mutational analysis of the alternative sigma factor FecI supports its essential role in fectransport gene transcription. Mol Gen Genet. 1996;250:455–465. doi: 10.1007/BF02174034. [DOI] [PubMed] [Google Scholar]
- 49.Ochs M, Veitinger S, Kim I, Welz D, Angerer A, Braun V. Regulation of citrate-dependent iron transport of Escherichia coli: fecRis required for transcription activation by FecI. Mol Microbiol. 1995;15:119–132. doi: 10.1111/j.1365-2958.1995.tb02226.x. [DOI] [PubMed] [Google Scholar]
- 50.Payne S M, Niesel D W, Pixotto S S, Lawlor K M. Expression of hydroxymate and phenolate siderophores by Shigella flexneri. J Bacteriol. 1983;155:949–955. doi: 10.1128/jb.155.3.949-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perna N T, Mayhew G F, Posfai G, Elliot S, Donnenberg M S, Kaper J B, Blattner F R. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coliO157:H7. Infect Immun. 1998;66:3810–3817. doi: 10.1128/iai.66.8.3810-3817.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polo S, Sturniolo T, Deho G, Ghisotti D. Identification of a phage-coded DNA-binding protein that regulates transcription from late promoters in bacteriophage P4. J Mol Biol. 1996;257:745–755. doi: 10.1006/jmbi.1996.0199. [DOI] [PubMed] [Google Scholar]
- 53.Pressler U, Staudenmaier H, Zimmermann L, Braun V. Genetics of the iron dicitrate transport system of Escherichia coli. J Bacteriol. 1988;170:2716–2724. doi: 10.1128/jb.170.6.2716-2724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajakumar K, Bulach D, Davies J, Ambrose L, Sasakawa C, Adler B. Identification of a chromosomal Shigella flexnerimulti-antibiotic resistance locus which shares sequence and organizational similarity with the resistance region of the plasmid NR1. Plasmid. 1997;37:159–168. doi: 10.1006/plas.1997.1280. [DOI] [PubMed] [Google Scholar]
- 55.Rajakumar K, Luo F, Sasakawa C, Adler B. Evolutionary perspective on a composite Shigella flexneri2a virulence plasmid-borne locus comprising three distinct genetic elements. FEMS Microbiol Lett. 1996;144:13–20. doi: 10.1111/j.1574-6968.1996.tb08502.x. [DOI] [PubMed] [Google Scholar]
- 56.Rajakumar K, Sasakawa C, Adler B. A spontaneous 99-kb chromosomal deletion results in multi-antibiotic susceptibility and an attenuation of contact haemolysis in Shigella flexneri2a. J Med Microbiol. 1996;45:64–75. doi: 10.1099/00222615-45-1-64. [DOI] [PubMed] [Google Scholar]
- 57.Rajakumar K, Sasakawa C, Adler B. Use of a novel approach, termed island probing, identifies the Shigella flexneri shepathogenicity island which encodes a homolog of the immunoglobulin A protease-like family of proteins. Infect Immun. 1997;65:4606–4614. doi: 10.1128/iai.65.11.4606-4614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sasakawa C, Kamata K, Sakai T, Murayama S Y, Makino S, Yoshikawa M. Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infect Immun. 1986;51:470–475. doi: 10.1128/iai.51.2.470-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schubert S, Rakin A, Karch H, Carniel E, Heesemann J. Prevalence of the “high-pathogenicity island” of Yersinia pestis species among Escherichia colistrains that are pathogenic to humans. Infect Immun. 1998;66:480–485. doi: 10.1128/iai.66.2.480-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schultz J, Milpetz F, Bork P, Ponting C P. SMART, a simple modular architecture research tool: identification of signalling domains. Proc Natl Acad Sci USA. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shea J E, Hensel M, Gleeson C, Holden D W. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simms D, Cizdziel P E, Chomczynski P. TRIzol: a new reagent for optimal single-step isolation of RNA. Focus (Gaithersburg) 1993;15:99–102. [Google Scholar]
- 63.Smith M, Jesse J. High efficiency bacterial electroporation: 1 × 1010E. colitransformants/microgram. Focus. 1990;12:38–40. [Google Scholar]
- 64.Swenson D L, Bukanov N O, Berg D E, Welch R A. Two pathogenicity islands in uropathogenic Escherichia coliJ96: cosmid cloning and sample sequencing. Infect Immun. 1996;64:3736–3743. doi: 10.1128/iai.64.9.3736-3743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torres A G, Payne S M. Haem iron-transport system in enterohaemorrhagic Escherichia coliO157:H7. Mol Microbiol. 1997;23:825–833. doi: 10.1046/j.1365-2958.1997.2641628.x. [DOI] [PubMed] [Google Scholar]
- 66.Turner, S. A., S. N. Luck, H. Sakellaris, K. Rajakumar, and B. Adler. Nested deletion events of the SRL pathogenicity island of Shigella flexneri 2a. J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 67.Van den Bosch L, Manning P, Morona R. Regulation of O-antigen chain length is required for Shigella flexnerivirulence. Mol Microbiol. 1997;23:765–775. doi: 10.1046/j.1365-2958.1997.2541625.x. [DOI] [PubMed] [Google Scholar]
- 68.Veitinger S, Braun V. Localization of the entire fec region at 97.3 minutes on the Escherichia coliK-12 chromosome. J Bacteriol. 1992;174:3838–3839. doi: 10.1128/jb.174.11.3838-3839.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Velayudhan J, Hughes N J, McColm A A, Bagshaw J, Clayton C L, Andrews S C, Kelly D J. Iron acquisition and virulence in Helicobacter pylori: a major role for FeoB, a high-affinity ferrous iron transporter. Mol Microbiol. 2000;37:274–286. doi: 10.1046/j.1365-2958.2000.01987.x. [DOI] [PubMed] [Google Scholar]
- 70.Venkatesan M M, Goldberg M B, Rose D J, Grotbeck E J, Burland V, Blattner F R. Complete DNA sequence and analysis of the large virulence plasmid of Shigella flexneri. Infect Immun. 2001;69:3271–3285. doi: 10.1128/IAI.69.5.3271-3285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vokes S A, Reeves S A, Torres A G, Payne S M. The aerobactin iron transport system genes in Shigella flexneriare present within a pathogenicity island. Mol Microbiol. 1999;33:63–73. doi: 10.1046/j.1365-2958.1999.01448.x. [DOI] [PubMed] [Google Scholar]
- 72.Wagegg W, Braun V. Ferric citrate transport in Escherichia colirequires outer membrane receptor protein FecA. J Bacteriol. 1981;145:156–163. doi: 10.1128/jb.145.1.156-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- 74.Wood M W, Jones M A, Watson P R, Hedges S, Wallis T S, Galyov E E. Identification of a pathogenicity island required for Salmonellaenteropathogenicity. Mol Microbiol. 1998;29:883–891. doi: 10.1046/j.1365-2958.1998.00984.x. [DOI] [PubMed] [Google Scholar]
- 75.Zhou D, Hardt W D, Galan J E. Salmonella typhimuriumencodes a putative iron transport system within the centisome 63 pathogenicity island. Infect Immun. 1999;67:1974–1981. doi: 10.1128/iai.67.4.1974-1981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]