Abstract
Chronic alcohol use increases risk of alcohol withdrawal symptoms (AW) and disrupts stress biology and resilient coping, thereby promoting excessive alcohol intake. Chronic alcohol intake and multiple alcohol detoxifications are known to impair brain medial prefrontal cortex (mPFC) and striatal functioning, regions involved in regulating stress, craving and alcohol intake. In two related studies, we examined whether AW predicts this functional brain pathology and whether Prazosin versus Placebo treatment may reverse these effects. In Study 1, patients with AUD (N=45) with varying AW levels at treatment entry were assessed to examine the AW effects on corticostriatal responses to stress, alcohol cue and neutral images with fMRI. In Study 2, 23 AUD patients entering a 12-week randomized controlled trial (RCT) of Prazosin, an alpha1 adrenergic antagonist that decreased withdrawal-related alcohol intake in laboratory animals, participated in two fMRI sessions at pre-treatment and also at week 9–10 of chronic treatment (Placebo: N=13; Prazosin: N=10) to assess Prazosin treatment effects on alcohol-related cortico-striatal dysfunction. Study 1 results indicated that higher AW predicted greater disruption in brain mPFC and striatal response to stress and alcohol cues (p<.001, family-wise error corrected-FWE), and also subsequently greater heavy drinking days (HDD) in early treatment (p<.01). In study 2, Prazosin versus Placebo treatment reversed mPFC-striatal dysfunction (p<.001, FWE), which in turn, predicted fewer drinking days (p<.01) during the 12-week treatment period. These results indicate AW is a significant predictor of alcohol-related prefrontal-striatal dysfunction and Prazosin treatment reversed these effects, that in turn, contributed to improved alcohol treatment outcomes.
INTRODUCTION
Alcohol misuse is a leading cause of global disease burden and Alcohol Use Disorder (AUD) is a chronic relapsing illness associated with significant medical morbidity and mortality worldwide. 1 Currently approved medications for the treatment of AUD show modest therapeutic efficacy, partly due to no clearly identified alcohol use-related pathophysiology that links to specific clinical moderators of treatment response. 2,3 Current clinical protocols for the treatment of severe alcohol withdrawal (alcohol withdrawal syndrome) target acute delirium tremens, seizures and mortality risk, 4 but these do not specifically treat risk of relapse and heavy drinking that often ensues post- acute withdrawal treatment. 5,6
Recent basic and clinical neuroscience research has shown that severe AUD and multiple alcohol detoxifications damages the brain medial prefrontal cortex and striatal pathway and is associated with excessive alcohol intake. 7–10 Chronic heavy alcohol intake also disrupts functioning of this medial prefrontal cortex (mPFC) and striatal pathway and such disruptions leads to greater alcohol seeking, higher stress and cue reactivity and higher risk of alcohol relapse outcomes. 7,10–15 Interestingly, Prazosin, an alpha1-adrenergic antagonist, reduces adrenergic hyperactivity and AW-related excessive drinking in laboratory animals, 16,17 reduces stress-related alcohol seeking and relapse risk in AUD patients, 8,18 and rescues mPFC working memory function during uncontrollable stress challenge in laboratory animals. 19
Although prior clinical studies report mixed findings with Prazosin for drinking outcomes in AUD patients, 20, 21,22 our recent proof-of-concept (POC) clinical trial indicated that AW moderated Prazosin treatment effects with its efficacy observed only in those with significant AW at treatment entry. 23 These results suggest the need to further examine such moderators and related pathophysiology in order to improve alcohol use treatment outcomes. 3,24 As AW are not observed in all patients with AUD and severity of the illness plays a significant role in its pathophysiology and chronic relapsing nature, 2 understanding the neurobiological mechanisms that drive AW-related pathophysiology can help in targeting treatments to reverse such pathophysiology to improve treatment outcomes. 3,5 Thus, we conducted 2 related studies in treatment seeking individuals with AUD with varying AW levels at treatment entry, to assess the effects of withdrawal symptoms on cortico-striatal responses to stress, alcohol cue and neutral images with fMRI in Study 1, and to assess whether Prazosin vs. Placebo treatment may reverse the stress and alcohol cue-related cortico-striatal dysfunction and contribute to improved treatment outcomes in Study 2. Based on previous work, we hypothesized that more severe AW will predict greater corticostriatal dysfunction to stress, alcohol and neutral cues in Study 1, and that chronic Prazosin treatment will reverse this corticostriatal dysfunction, that in turn, will contribute to improved alcohol use outcomes.
METHODS
Participants
Treatment seeking AUD patients who exhibited varying levels of AW upon treatment entry (assessed by the Clinical Institute of Withdrawal Assessment for Alcohol-Revised: CIWA-Ar) 25 were studied. Study 1 included 45 AUD patients (31 men, 14 women), while Study 2 included 23 AUD patients (16 men, 8 women) who also participated in Study 1. Male and female participants were included in the studies if they were: 18–65 years of age, met current DSM-IV-TR criteria for alcohol dependence, read and write English to complete study evaluations, were able to sign informed consent, and were excluded if they met the following criteria: DSM-IV-TR criteria for dependence of any other psychoactive substance except cannabis, nicotine, and caffeine; current use of psychoactive drugs, including anxiolytics, antidepressants (except SSRIs), naltrexone, or antabuse; any acute psychotic disorder or current Axis I requiring specific psychiatric attention; significant underlying medical conditions such as cerebral, renal, thyroid, hepatic, or cardiac pathology that would interfere or be of potential harm during the study; or were hypotensive as indicated by a sitting blood pressure below 90/60 mmHG. All participants signed a written informed consent and study procedures were approved by the Human Investigation Committee of the Yale University School of Medicine.
Once eligible participants signed informed consent and completed baseline assessments, they were admitted to either the outpatient Clinical Research Program at the Yale Stress Center or the Clinical Neuroscience Research Unit (CNRU), an inpatient treatment and research facility of the Connecticut Mental Health Center (CMHC) for treatment initiation and study participation. All participants completed an assessment of alcohol withdrawal symptoms (AW) during the intake using the Clinical Institute of Withdrawal Assessment-Alcohol revised (CIWA-Ar), 25 conducted by a clinical research staff member. Three participants in Study 1 chose to initiate treatment via admission to the CNRU, but did not receive medical detoxification on the basis of further medical evaluation. No participants in Study 2 were admitted to the CNRU prior to study initiation. Additional demographic and physical health examination, including electrocardiogram (EKG), CBC/laboratory and liver function tests were assessed in the intake period. Urine toxicology testing for ethyl glucuronide (EtG) and other illicit drugs and a breathalyzer was conducted to confirm recent alcohol use and objective assessment of drug use at baseline.
Study Design
Patients in Study 1 and Study 2 were presented with challenging (stress, alcohol cues) and non-challenging, control (neutral-relaxing) visual images in a block design as per our previous work 26,27 to assess the influence of AW symptoms on integrity of corticostriatal responses during functional magnetic resonance imaging (fMRI) scanning. Study 2 included patients who were enrolled in a larger 12-week randomized controlled trial (RCT) of Prazosin (16 mg/day) versus Placebo, titrated over the course of 2 weeks as described in a recently published study 23 and who volunteered for two fMRI scans, the first at baseline prior to study medication initiation and also completed a second fMRI scan during weeks 9–10 of the RCT. (Design Figure 1a). All Study 2 patients titrated up to the full 16 mg/day study dose. Study medication, dosing, titration and additional details are provided in Supplemental Methods and in the previously RCT. 23
Figure 1.
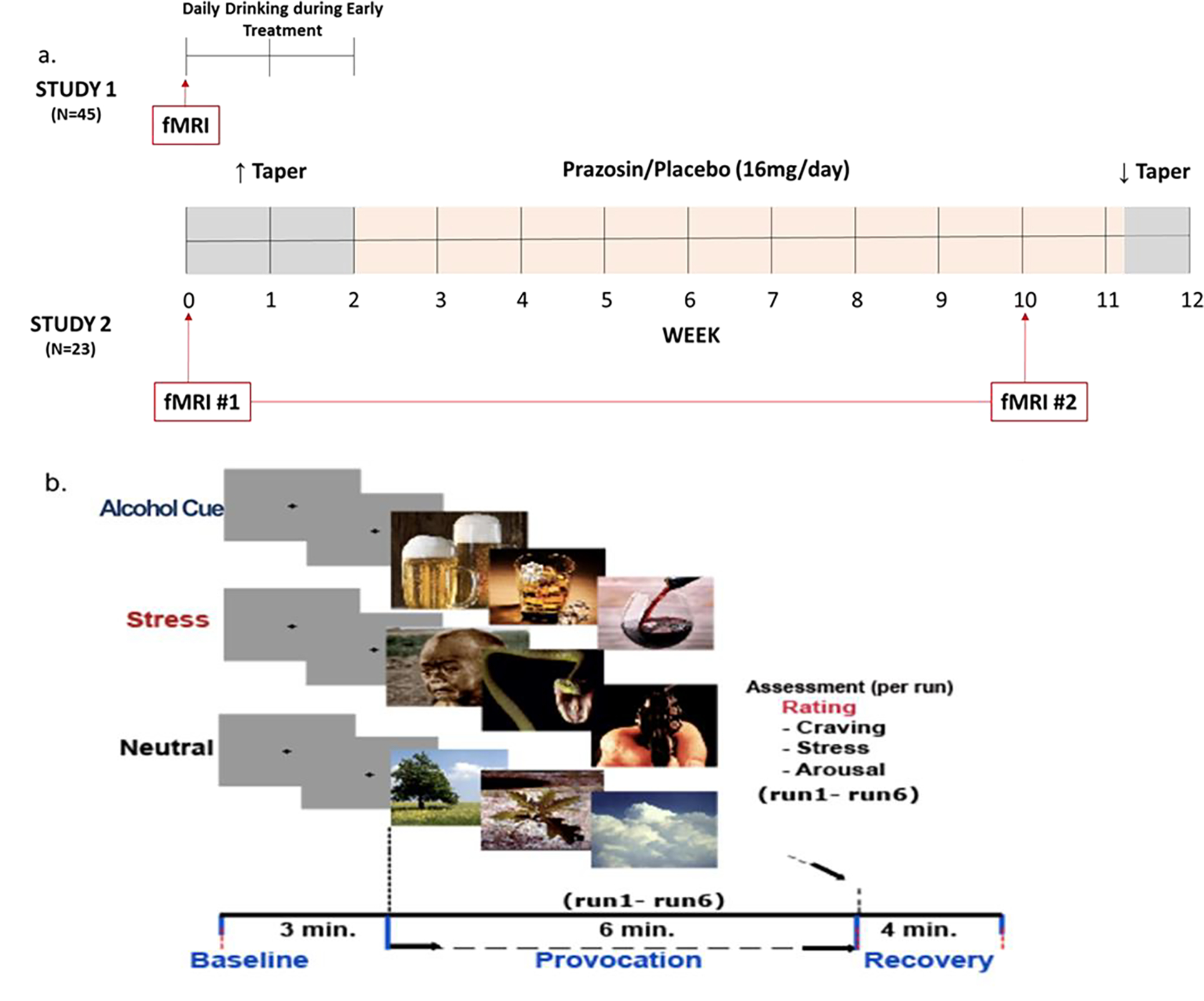
a: Study Designs: Study 1 assessed whether AW predicts brain functional pathology in medial prefrontal cortex (mPFC) and striatal pathway involved in regulating brain stress and reward responses using functional magnetic resonance imaging (fMRI) exposure to challenge (stress, alcohol cues) and non-challenge control (neutral relaxing) conditions (shown in Figure 1b), and prospective assessment of daily drinking during early treatment in 45 AUD patients. Study 2 assessed if chronic Prazosin relative to Placebo treatment reversed the pre-treatment mPFC and striatal functional pathology assessed in fMRI#1 with a second fMRI scan (fMRI#2) in 23 AUD participants who participated in both study 1 and were also randomized to Prazosin vs. Placebo in a 12-week randomized controlled study of Prazosin and underwent brain imaging at pre-treatment and at week 10. Daily drinking outcomes were assessed in each study for time period specified. Figure 1b: fMRI Scan Procedure: Sustained Emotion/Reward Provocation (SEP) blocks during the fMRI experiment showing a sample stimuli presentation block with 3 baseline gray fixation 1-min runs, followed by 6 challenge stimuli 1-min runs (e.g. stress/alcohol cue/neutral images) and a 4-minute recovery period, based on previous work. 26,27 All participants were exposed to 3 stimulus conditions (Stress, Alcohol Cue or Neutral) of the above presentation to assess functional mPFC-striatal brain responses. Separate sets of images per condition that were matched on emotional intensity were used for the second scan in Study 2.
Study Procedures
Patients in Study 1 (N=45) participated in an fMRI scan after completion of the intake procedures prior to initiating treatment. After the fMRI scan all 45 patients in Study 1 participated in a 2-week treatment engagement phase during which time they reported daily alcohol intake via interactive voice recording (IVR) on landline phone or using a Smartphone App, as well as reporting alcohol use in up to twice weekly face-to-face research appointments. The Study 2 sample (N=23) included patients were those who participated in Study 1 with a baseline/pretreatment scan and also volunteered to enroll in a Prazosin vs Placebo RCT and participated in the second fMRI scan during chronic Prazosin/Placebo treatment in week 9–10 (see Supplemental Methods for participant details and medication dosing and titration). All Study 1 participants were offered the chance to participate in Study 2, and 23 agreed and completed the MRI scans for Study 2. For study 2, treatment outcome data for the 10 weeks between fMRI scan 1 and Scan 2 was utilized to assess prediction of treatment outcome.
fMRI Acquisition and Task
fMRI Acquisition.
MRI images were obtained using a 3-T Siemens Prisma MRI system equipped with a standard quadrature head coil, using T2*-sensitive gradient-recalled single shot echo planar pulse sequence. On each scanning day, AUD patients were alcohol free assessed via breathalyzer and were tested for scanning under fasting between 8–10 AM after which they were provided a snack. The scanning procedure included obtaining anatomical images, high-resolution 3D Magnetization Prepared Rapid Gradient Echo sequences, diffusion tensor imaging sequence, resting state functional MRI sequences and functional images obtained sequentially. Functional, blood oxygen level dependent (BOLD) signals were acquired with a 64-channel head coil using a multi-band accelerated, echo planar imaging sequence, which is the focus of the current studies. Seventy-five axial slices parallel to the AC-PC line covering the whole brain were acquired with TR = 1000 msec, TE = 30 msec, bandwidth = 1894 Hz/pixel, flip angle = 55 degrees, field of view = 220 × 220 mm, slice thickness = 2 mm and no gap.
fMRI Challenge Task Procedures:
All fMRI brain scans were conducted starting at 8:00 AM using a 3-T Siemens Trio MRI system. During a 2 hour scan, patients were exposed to a previously validated sustained emotion/reward provocation (SEP) task presented as a block design involving repeated successful presentation of, 66 highly stressful and aversive (images of terror, violence, disgust, threat, victimization, mutilation, etc.), 66 alcohol cue (images of alcoholic beverages or their intake) and 66 neutral relaxing (images of natural landscapes) pictures. 26,28At the beginning of each condition (Stress, alcohol cue, neutral), three 60-second runs of gray fixation blocks preceded each picture block to serve as a baseline period within each condition. 26 Following this baseline period, pictures were presented for 5 seconds per image with a 1-s inter-stimulus interval (ISI), over six successive runs of 66 s each (11 images per run) to induce a sustained functional challenge (alcohol cue, stress) versus a control (neutral-relaxing) brain state to assess functional integrity of the target brain pathways. Condition order was randomly generated and counterbalanced across subjects. Participants rated their levels of craving, stress, and arousal after each run on a scale of 1–9 (1= not at all, 9=very much so) (Figure 1b for fMRI scan design) (also see Supplemental information).
Alcohol Use Treatment Outcomes:
Patients completed 4–5 minute surveys daily to report each day’s alcohol intake (amount and alcohol type consumed) using either an interactive voice response (IVR) phone call or Smartphone App for the two week treatment engagement period in Study 1 and throughout the 10 week period between scans in Study 2, as part of the Prazosin RCT. 29 Compliance was monitored, and daily survey completion was reinforced at each study visit. Alcohol intake was also assessed weekly using the 7-day Substance Use Calendar (SUC), based on the Time-Line Follow Back (TLFB) assessment, 30 and the SUC was used if daily phone data were missing on a particular day as in previous research. 31
Statistical Analysis
Study 1 and 2:
fMRI Data Preprocessing and Analysis:
General Linear Models (GLM) were used for individual level analysis on each voxel in the entire brain volume with a regressor (time viewing each image) for each run per condition using BioImageSuite. 32 Temporal filtering was carried out by including drift correction in the GLM. Each trial was spatially smoothed using a 6 mm Gaussian kernel and individually normalized to generate beta-maps (3.44mm x 3.44 mm x 4mm). To consider individual variation in brain anatomy, we performed three sequential registrations which were then applied to the individually normalized beta maps. This advanced multistep registration approach has been validated as a robust method to accommodate individual differences in brain anatomy in numerous fMRI studies, including our previous work.15,26–27 This sophisticated sequential three-stage registration method includes a linear individual registration of raw functional image into a 2D anatomical image, followed by 2D to 3D (1×1×1mm) linear registration and a nonlinear registration to reference 3D image space in the Montreal Neurological Institute (MNI) space.
For Study 1 group analysis, AW X Condition effects were examined using voxel-based whole brain linear mixed-effects(LME) modeling using AFNI 3dLME (http://afni.nimh.nih.gov), firstly, using linear mixed regression of AW as the independent predictor of brain activity during each condition (alcohol cue-A, stress-S, neutral-N), and secondly, post-hoc secondary follow up analysis to identify source of AW prediction of fMRI brain response by dividing the sample into High and Low AW scores (median cut-off) implemented as a 2 (group: High/Low AW) × 3 (condition: alcohol cue, stress, neutral) design. Finally, we assessed whether specific AW-related functional pathology in the mPFC and dorsal and ventral striatal regions (ROI: region of interest) during stress (S) and alcohol cue (A) relative to neutral (N) exposure predicted drinking during the early 2-week treatment period using random effects regression analyses.
Similarly for Study 2, whole brain group level voxel-based LME analysis was conducted with Treatment Period (Pre-treatment, Chronic treatment) X Group (Prazosin, Placebo) X Condition (Alcohol cue, Stress, Neutral) as Fixed Effects and Subjects as the Random effect factor. Secondary analysis to assess specific contrasts were assessed in specific ROIs of the mPFC and dorsal and ventral striatal regions. Furthermore, regression analyses also assessed whether medication-related change in the mPFC region of interest (ROI) brain function predicted alcohol intake during treatment. For all voxel-based whole brain analysis, family-wise error (FWE) correction was applied using Monte Carlo simulation in AFNI 3dClustSim program (version 16.3.05, October 2016), with a p< 0.001 significance threshold, at a cluster correction of α =0.05.
Study 1 and 2 Specific Treatment Outcomes:
The primary outcome of interest was percent of heavy drinking days (HDD%), percent of any drinking days (DD%) and average drinks/day (AvgD) during treatment engagement in weeks 1–2 for Study 1, and in Study 2 for the period between fMRI scan1 and fMRI scan 2 from weeks 1–10. Alcohol intake was coded daily for heavy drinking day (1) or not (0), any drinking day (1) or not (0) and drinks per day (continuous variable). Heavy drinking days were defined using the 4+/5+ cutoff for women and men. Due to a positive skew, number of drinks per day was log-transformed. Control variables that were modeled in all analyses were gender (0=male), inpatient (0=outpatient), and dropout (0=completer, 1=dropout prior to 4 weeks, 2=dropout after 4 weeks).
RESULTS
Demographic, mean AW symptoms and clinical characteristics of patients in both studies are presented in Table 1.
Table 1.
Demographic and Clinical Characteristics.
| Study 1 b | Study 2 b | |||||
|
|
|
|||||
| Placebo | Prazosin | |||||
|
|
|
|
||||
| n=45 | n=13 | n=10 | ||||
| N | % | N | % | N | % | |
|
|
|
|
||||
| Gender – no. of males | 31 | 68.9% | 10 | 76.9% | 6 | 60.0% |
| Race | ||||||
| Caucasian | 25 | 55.6% | 8 | 61.5% | 7 | 70.0% |
| African American | 16 | 35.6% | 5 | 38.5% | 3 | 30.0% |
| Other | 4 | 8.9% | 0 | 0% | 0 | 0.0% |
| No. of regular smokers | 24 | 53.3% | 7 | 53.8% | 4 | 40.0% |
| Stabilized on antidepressants | 2 | 4.4% | 0 | 0.0% | 0 | 0.0% |
| Lifetime depression - % | 11 | 24.4% | 3 | 23.1% | 3 | 30.0% |
| Lifetime anxiety (incl. PTSD) - % | 12 | 26.7% | 7 | 53.8% | 5 | 50.0% |
| Lifetime anxiety (without PTSD) - % | 6 | 13.3% | 3 | 23.1% | 2 | 20.0% |
| M | SD | M | SD | M | SD | |
|
|
|
|
||||
| Age a | 38.33 | 11.15 | 41.92 | 9.84 | 39.10 | 10.65 |
| Years of Education a | 13.73 | 2.22 | 13.62 | 1.56 | 13.00 | 2.40 |
| Years of alcohol use a | 14.69 | 10.63 | 16.54 | 10.29 | 13.90 | 7.68 |
| Past 30 days alcohol use a | 18.73 | 8.27 | 17.69 | 7.09 | 21.80 | 8.78 |
| Ave Drinks/Day | 3.69 | 2.75 | 4.13 | 3.22 | 5.53 | 2.88 |
| % Drinking Days | 61.74 | 31.09 | 54.05 | 30.45 | 74.60 | 21.16 |
| % Heavy Drinking Days | 36.16 | 31.91 | 42.62 | 31.23 | 49.21 | 28.67 |
| CIWA Total Score a | 4.41 | 4.69 | 3.46 | 4.70 | 5.2 | 5.96 |
Data indicate means and standard deviations. All variables: p>.05.
All participants met criteria for current Alcohol Use Disorders (AUD) as defined by the Diagnostic and Statistical Manual (DSM-IV-TR; DSM-5) as determined via the Standard Clinical Interview for DSM-IV (SCID). Alcohol withdrawal (AW) symptoms were assessed by the Clinical Institute of Withdrawal Assessment – Alcohol Revised (CIWA-Ar).
Frequency of specific AW reported by Study 1 patients divided into high and low AW groups based on a median split is presented in Figure 2.
Figure 2.
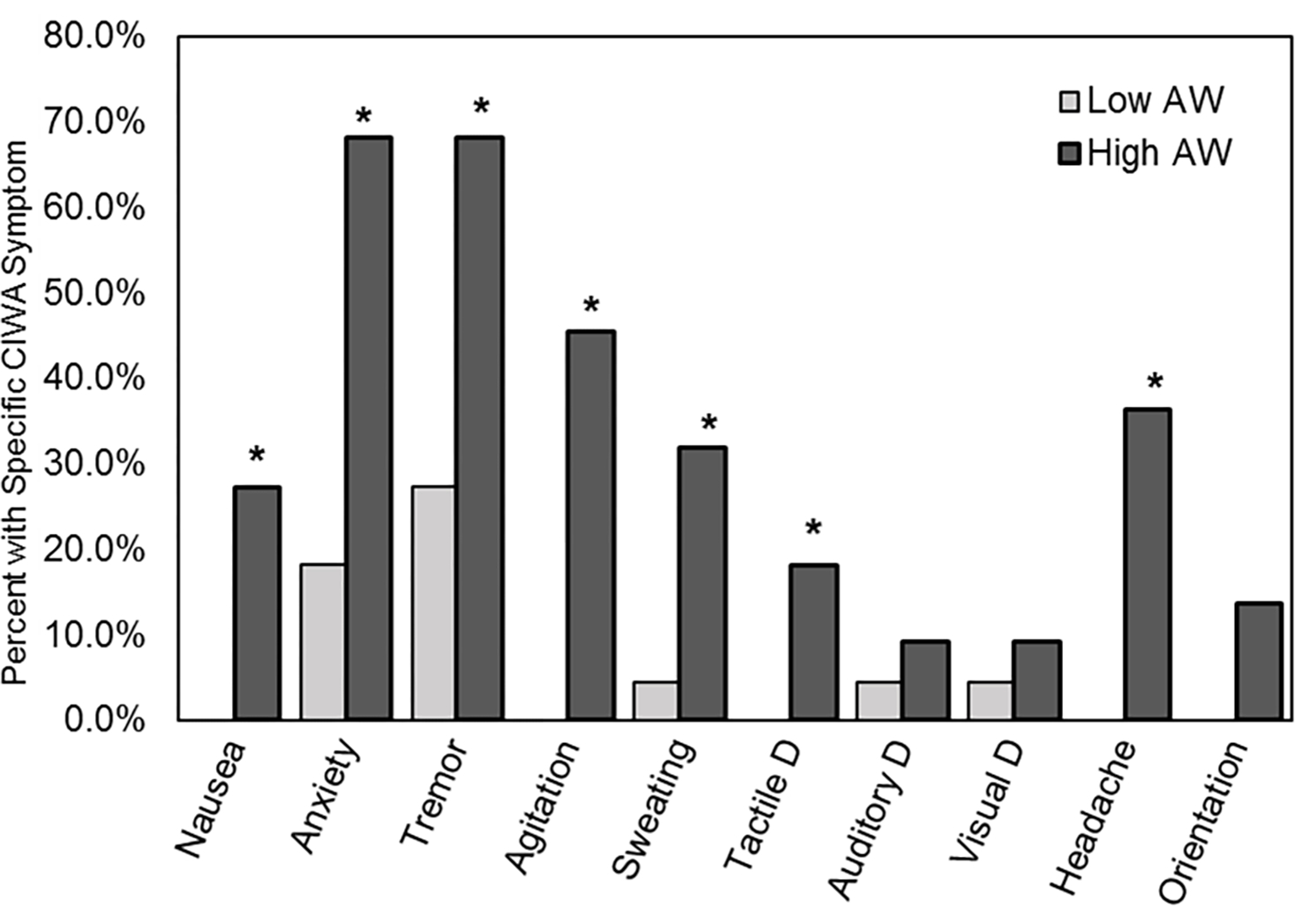
Study 1 percent of patients reporting specific AW from the CIWA-Ar divided into high and low AW subgroups based on the median of 4 and above for the HIGH and below 4 for the LOW AW groups. CIWA-Ar is a 13-item interviewer guided measure to evaluate current alcohol withdrawal (AW) signs and symptoms. The assessment includes objective measures (e.g., pulse), participants’ responses to questions, such as orientation to time and space (“What day is this? Where are you? Who am I?”), and observations by the interviewer (evidence of tremor or paroxysmal sweats). Item responses range from 0 indicating no evidence of the symptom to 4 indicating highest severity of symptoms. Possible total scores could range from 0 to 67. A symptom was considered positive if a participant had a score of 1 or more for that item. * indicates significant χ2 p<.05.
Study 1:
fMRI Rating Results for Alcohol Craving, Stress and Arousal.
Linear mixed effects models were conducted with Condition (S, A, N) as within-subjects factor and CIWA scores as a between-subjects factor as a moderator. A significant condition main effect was found in craving, arousal, and stress ratings during the provocation period during the viewing of pictures (p’s<.01). There were no significant AW main effect or AW x condition interactions. Within each provocation period, there was a significant difference between conditions in craving (F(2, 90.25) = 17.49, p <0.001), arousal (F(2, 91.17) = 24.31, p <0.001), and stress ratings (F(2, 90.25) = 17.49, p <0.001). Specifically, there was a significant increase in the alcohol cue and stress conditions compared to neutral condition in craving (Alcohol cue: t=5.31, p<0.001, Stress: t=4.92, p<0.001), subjective stress ratings (Alcohol cue: t=2.37, p<0.05, Stress: t=8.84, p<0.001) and arousal (Alcohol cue: t=3.07, p<0.001, Stress: t=6.96, p<0.001) (see Figure S1).
fMRI Results:
Significant AW X Condition interaction effects in the whole brain LME analyses indicated higher AW were significantly associated with fMRI brain response in mPFC, striatal and related limbic-cortical networks (p<.001, cluster corrected at .05, shown in red/yellow; Figure 3a). Secondary simple effects contrasts between high vs low AW groups indicated specific that high AW group showed blunted ventro-medial PFC (VmPFC), lateral PFC, amygdala, insula, hypothalamus and ventral striatum responses to stress (S-N) and to alcohol cues (A-N), and greater dorsal striatal responses to alcohol cues, relative to neutral cues relative to the low AW group (p<.001, Figure 3b, Table ST1).
Figure 3.
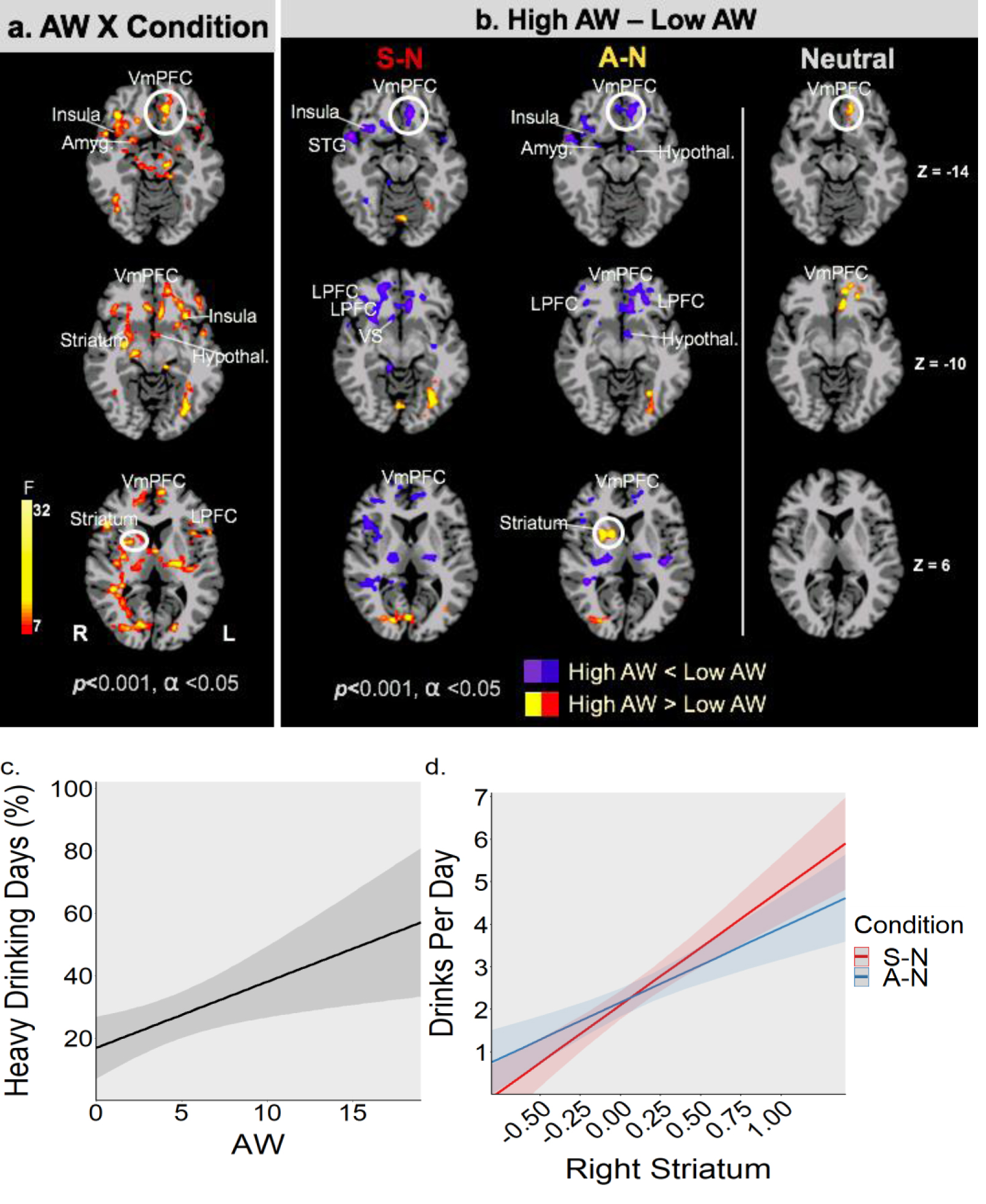
a: Significant whole brain voxel-wise LME analysis indicates AW (continuous scores) X Condition (Stress-S, alcohol cue-A, neutral-N) interaction effects (shown in red/yellow) in functional brain responses in the VmPFC, dorsolateral PFC, insula, amygdala, striatal, hypothalamic regions, consistent with brain regions involved in functional regulation of stress and reward cue responses (FWE corrected at p<0.001, cluster corrected at α<.05). Figure 3b: Post-hoc secondary analyses to understand the AW Symptom X Condition significant brain effects shown in 3a was conducted by dividing AUD patients into high and low AW scores (median split). Figure 3b shows disrupted VmPFC and related limbic and striatal responses to functional challenge of stress (S) and alcohol cue (A) relative to neutral (N) and under neutral-relaxed (N) state responses (FWE p<.001, α<.05). As hypothesized, VmPFC and lateral PFC regions as well as limbic (Amyg: amygdala, insula, Hypothal: hypothalamus) and striatal (VS: ventral striatum) show reduced/blunted responses during stress and alcohol cue challenge in high AW versus low AW group (blue/purple shows hypoactivation), while Right Striatum (dorsal) shows hyperactive responses during alcohol cue exposure (red/yellow shows hyperactivation). Furthermore, hyperactive and disrupted VmPFC functioning during exposure to neutral-relaxing images is shown in high AW relative to the low AW group. (see Supplemental Table ST1). Figure 3c: Higher AW (CIWA-Ar continuous scores) at intake prospectively predicted higher 2-week drinking during early treatment (Percent heavy drinking days, p <0.01). Error bands are ± SEM. Figure 3d: Higher R dorsal striatum response during challenge (A: alcohol cue; S: stress) relative to neutral (A-N or S-N) predicted higher number of drinks consumed on a drinking day (S-N: Drinks Per Day; p < 0.01; A-N: Drinks Per Day; p <0.035), in the early treatment phase. Error bands are ± SEM.
Prediction of Treatment Outcome:
Higher AW (CIWA-Ar continuous scores) at intake prospectively predicted higher 2-week drinking during early treatment (Percent heavy drinking days – HDD%: (b=2.11, 95%, CI[.57, 3.66], p <0.01; Drinks Per Day: b= 0.16, CI[0.019, 0.3], p<0.028), where each unit increase in alcohol withdrawal (AW) score predicted a 2.11% increased risk of heavy drinking per day assessed (HDD%, shown in Figure 3c) during the early treatment phase. Furthermore, higher the R dorsal striatum response during challenge (A: alcohol cue; S: stress) relative to neutral (A-N or S-N), greater the number of drinks consumed on a drinking day (S-N: Drinks Per Day; b=2.71, 95%CI[1.17, 4.25], p < 0.01; A-N: Drinks Per Day; b=1.75, 95%CI[.14, 3.37], p <0.035), in the early 2 week treatment period (see Figure 3d). For each unit increase in right striatum activation in A-N or the S-N contrast, there was an increased risk of drinking 1.75 more drinks (A-N) or 2.71 more drinks (S-N) per drinking day, respectively, over the 14-day early treatment period.
Study 2:
Effects of Prazosin Treatment on Task Ratings:
To understand the effect of treatment on changes in ratings during the fMRI task, a 2 X 2 X 3 linear mixed model was conducted with group (Prazosin, Placebo) as a between-subjects factor, and treatment (Chronic, Pre) and condition (Alcohol, Stress, Neutral) as within-subjects factors. Significant treatment effects were found for alcohol craving and arousal ratings. For alcohol craving, there were significant effects of Treatment time (F=70.6, p<0.001) and Treatment time X Condition (F = 3.0, p < 0.05), where Prazosin relative to Placebo group showed reductions in craving across all conditions, but specifically significantly lower craving in the alcohol cue condition (p<.05) (see Supplemental Figure S2).
For arousal ratings, there were significant effect of Medication Group X Treatment Time (F=9.8, p<0.01) and Medication Group X Treatment X Condition effect (F = 3.64, p < 0.05), where the Prazosin group showed a greater reduction in arousal ratings compared to the Placebo group across all conditions (p<.01), and in particular, the Prazosin group showed significantly lower emotional arousal relative to the Placebo group in the alcohol cue condition (p<.05) (Supplemental Figure S2).
Effects of Treatment on fMRI Response:
In voxel-based whole brain analysis, a significant interaction of Chronic Prazosin treatment on VmPFC and striatal response to challenge was observed (Treatment Group X Scan Period X Condition, p<.001, FWE). Chronic Prazosin relative to pre-treatment increased VmPFC activation to stress, while the Placebo treated group showed no such VmPFC improvement during stress or alcohol cue challenge, and instead showed increased R striatum activation with alcohol cue challenge (p<.01, FWE) (Figure 4). Furthermore, using the VmPFC ROI response to stress and to alcohol cues with chronic Prazosin relative to Placebo treatment, we found higher VmPFC response was associated with significantly fewer drinking days during treatment (Stress: b= −40.33, 95% CI[−78.55, −2.11], t=−2.23, p<.039; Alcohol Cues: b= −39.65, 95%CI[−55.8, −2.7], t=−2.21, p<.04).
Figure 4.
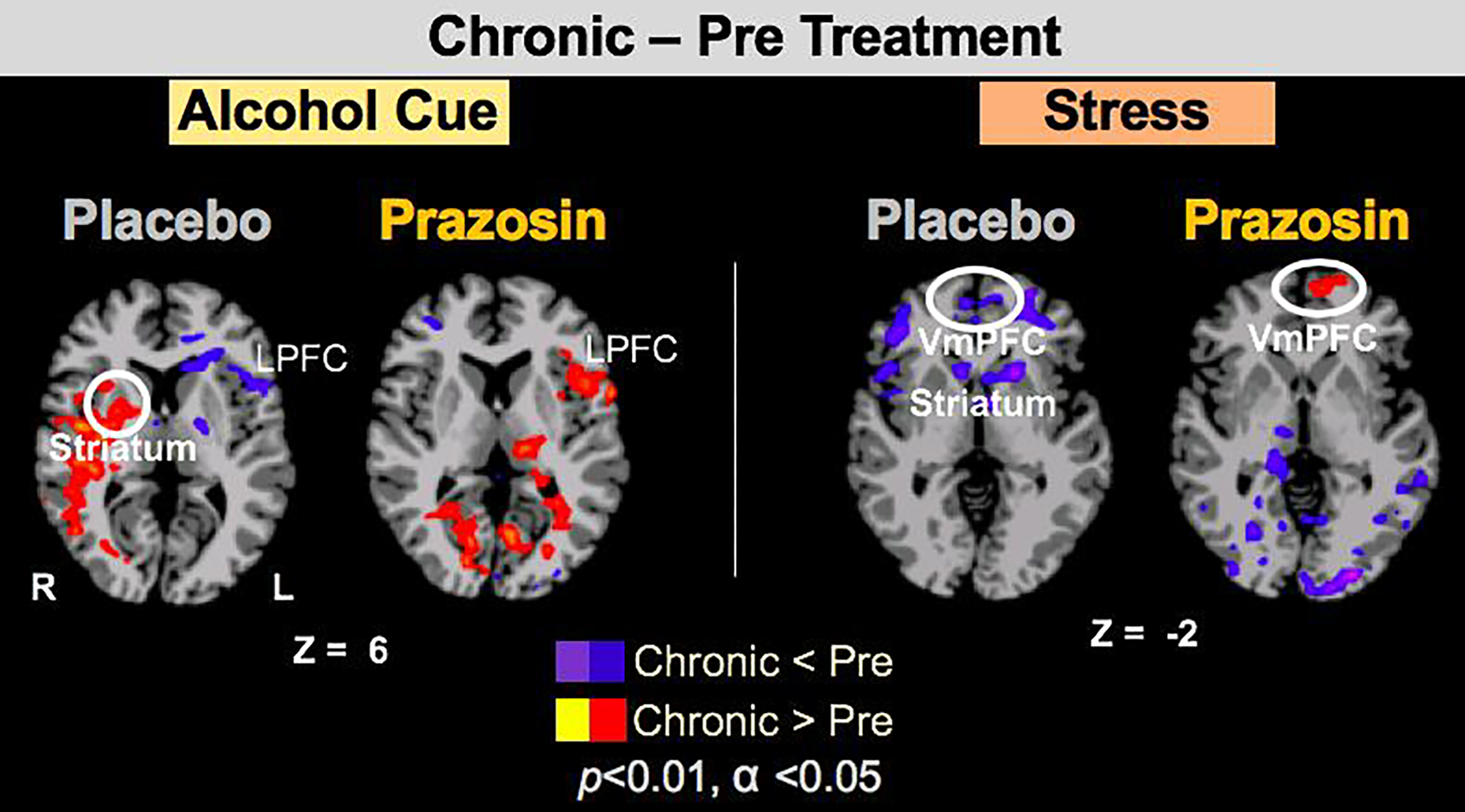
In Study 2, whole brain voxel-wise analysis of Medication Group X Treatment Period X Condition resulted in VmPFC and Right striatum effects (p<.001, α<.05, FWE). Here we show change in fMRI Brain Responses to Stress and Alcohol Cues with Prazosin versus Placebo Chronic Treatment relative to Pre-treatment in targeted VmPFC and striatal regions of interest at the p<.01 threshold. The Prazosin group showed improved VmPFC function with chronic treatment relative to pre-treatment during stress cue exposure (shown in red) not seen in the Placebo group; while the Placebo group showed increased R Striatum response (shown in Red) to alcohol cues and blunted VmPFC (shown in blue) response to stress challenge not seen in the Prazosin group.
DISCUSSION
Findings from two related studies integrating data from experimental neuroimaging and clinical outcomes show that AW at treatment entry predicts dysfunctional responses to stress and alcohol cue challenge in the key mPFC-striatal pathway in patients entering AUD treatment. Greater severity of AW was associated with greater disruption of mPFC-striatal functioning during stress and alcohol cue challenge, and it also predicted heavy drinking days during early treatment. These findings support the well-known clinical observation that AUD patients with greater AW severity are at greater risk of relapse and treatment failure, 33–35 supporting the need to utilize AW as a prognostic indicator of AUD treatment response as shown with our recent clinical trial of Prazosin. 23 Expanding on the clinical data that alcohol use outcomes are significantly improved by Prazosin relative to Placebo treatment in those with significant AW, 29 current findings from Study 2 show that chronic Prazosin treatment improves mPFC-striatal functional responses to stress and to alcohol cue exposure, and that such improvements predict improved Prazosin clinical treatment response.
Higher alcohol withdrawal scores are associated with heavier drinking and poses a significant challenge in AUD treatment. 33,35 Despite this well known clinical observation, there are no FDA approved medications to target greater AUD severity marked by positive AW, alcohol-related brain pathophysiology and related poor alcohol use outcomes. 5 Anticonvulsants are successfully used to treat acute alcohol withdrawal, and may decrease early alcohol relapse risk; 34 however, their sustained use to target post-withdrawal excessive drinking presents a significant side effect burden, and there is no evidence that they may reverse disrupted brain mPFC-striatal pathophysiology associated with chronic alcohol intake and alcohol withdrawal. 11 Similarly, recent evidence shows high AW severity significantly impacts AUD treatment efficacy of Gabapentin, 36 but it is unclear whether Gabapentin improves mPFC-striatal functional pathology shown in current findings to be associated with AW, and representing one of the disrupted brain pathways that may be targeted to improve drinking outcomes.
The medial PFC encompassing the orbitofrontal cortex and the rostral anterior cingulate exercises inhibitory control over the striatum and is involved in regulating stress and drug cue challenge states in order to facilitate adaptive goal-directed behaviors. 37 Thus, disruption of mPFC brain function associated with alcohol withdrawal can render the brain striatal regions involved in habitual behaviors hyperactive to alcohol-related stimuli, thereby driving excessive drinking as predicted by addiction models 38 and supported by Study 1 findings. Furthermore, basic science studies show noradrenergic hyperactivity in the prefrontal network is a central feature of AW, 11,16,17 and clinical studies show a history of multiple withdrawal episodes and poor functional recovery are associated with alcohol-related mPFC-striatal pathophysiology. 7,9 Consistent with this previous work, study 1 findings showed not only greater mPFC blunted response to stress and higher striatal response to alcohol cues, but that hyperactive striatal responses to cues predicted greater drinks per day in the early phase of treatment.
Following up on Study 1 findings, chronic Prazosin versus Placebo treatment improved mPFC and striatal functioning during fMRI, and such Prazosin-related improved mPFC function predicted lower drinking days during treatment in Study 2. While previous research has shown mixed efficacy of Prazosin treatment in reducing drinking outcomes, 20–22 recent findings from a clinical trial of Prazosin showed a significant moderating effect of AW on Prazosin’s efficacy with respect to drinking outcomes. 23 Notably, Prazosin/Placebo treatment did not affect sleep quality or blood pressure significantly either in the previous reported larger RCT nor in the current brain imaging sample,, suggesting that its effects were not due to a direct effect on AW per se, but rather on the cortico-striatal motivational dysfunction associated with AW. Also, as AUD patients were randomly assigned to Prazosin and Placebo treatment groups after balancing on demographic and clinical AUD symptoms, any potential individual variation in gray matter volume or white matter integrity were less likely to influence the fMRI responses. Thus, current findings suggest AW may be a significant moderator of chronic alcohol-related mPFC and striatal dysfunction, that in turn, predicts Prazosin treatment response in a subgroup of individuals who participated in the above mentioned RCT. Remarkably, the effects of AW severity on mPFC-striatal function indicated not only that higher AW severity predicted greater disruption of mPFC and striatal function, but also that chronic Prazosin treatment improved functional disruption of the mPFC-striatal pathway, that in turn significantly improved heavy drinking outcomes.
Current findings have significant clinical implications. AW is a key diagnostic and clinical feature of AUD which is influenced not only by drinking severity, but also genetic and environmental susceptibility factors. 39,40 Repeated alcohol withdrawal and concomitant AW symptoms results in disruption of the PFC-striatal pathway, that in turn, facilitates stress-related and alcohol cue-related excessive drinking, to influence AUD course and risk of treatment failure. 12,41 Significant heterogeneity in AUD clinical features has resulted in only modest efficacy of currently approved medications of Naltrexone, Acamprosate and Antabuse, with no established moderator to guide targeted treatment to improve efficacy. 2,5 Addressing this clinical dilemma, current findings integrated a novel neuroimaging design combined with specific treatment to identify AW severity as a key moderator of mPFC-striatal pathophysiology in AUD. In addition, because Prazosin treatment for AUD involved t.i.d. dosing which could affect medication compliance, the effects of alpha1-adrenergic antagonists with longer half-life, such as doxazosin, may also be studied for their effects on cortico-striatal dysfunction in AUD. Furthermore, both Prazosin and Doxazosin are widely used currently in clinical practice and available in generic formulations rather inexpensively and may be utilized off label for AUD, given current and previous findings.
The current findings are limited by small sample size and future studies could benefit from further assessment of sex differences, and of Prazosin effects on sleep and blood pressure and their concomitant impact on brain functioning. Despite these caveats, the results represent a proof-of-concept novel approach to addressing inherent heterogeneity in AUD with key disease-related clinical and neurobehavioral markers that may be specifically targeted with treatment to improve clinical outcomes.
Supplementary Material
ACKNOWLEDGEMENTS:
We thank the staff at the Yale Stress Center, the Clinical Neuroscience Research Unit of the Connecticut Mental Health Center (CMHC), the Yale New Haven Health Investigational Drug Service (Yale IDS), and staff at the Magnetic Resonance Research Center (Yale MRRC), and also Dr. Todd Constable, Ms. Cheryl Lacadie, Ms. Rachel Hart, Zubaida Dabre, Mary Kurjanowicz and Ryan Douglas. Disclosure forms are provided by the authors.
FUNDING AND DISCLOSURES:
Supported by National Institute of Alcohol Abuse and Alcoholism grants R01-AA013892 and R01-AA020504, the National Center for Advanced Translational Sciences (NCATS) for the Yale Clinical and Translational Science Award UL1-TR00125(Yale CTSA) in support of the Yale Center of Clinical Investigation (YCCI), and by the Connecticut State Department of Mental Health and Addiction Services. The authors have nothing to disclose relating to the current study.
BIBLIOGRAPHY AND REFERENCES CITED
- 1.Collaboration G Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2018;6736:1–21. [Google Scholar]
- 2.Garbutt JC. The state of pharmacotherapy for the treatment of alcohol dependence. J Subst Abuse Treat. 2009;36(1):S15–23; quiz S24–15. [PubMed] [Google Scholar]
- 3.Sinha R Prazosin for the Treatment of Alcohol Use Disorders. Am J Psychiatry. 2018;175(12):1159–1160. [DOI] [PubMed] [Google Scholar]
- 4.Kosten TR, O’Connor PG. Management of drug and alcohol withdrawal. N Engl J Med. 2003;348(18):1786–1795. [DOI] [PubMed] [Google Scholar]
- 5.Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res. 2015;39(4):579–584. [DOI] [PubMed] [Google Scholar]
- 6.Schuckit MA. Recognition and management of withdrawal delirium (delirium tremens). N Engl J Med. 2014;371(22):2109–2113. [DOI] [PubMed] [Google Scholar]
- 7.Duka T, Trick L, Nikolaou K, Gray MA, Kempton MJ, Williams H, Williams SC, Critchley HD, Stephens DN. Unique brain areas associated with abstinence control are damaged in multiply detoxified alcoholics. Biol Psychiatry. 2011;70(6):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, Sinha R. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcoholism, clinical and experimental research. 2012;36:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Overall JE, Burr G, Wolf AP. Recovery of brain glucose metabolism in detoxified alcoholics. American Journal of Psychiatry. 1994;151:178–183. [DOI] [PubMed] [Google Scholar]
- 10.Heinz A, Deserno L, Zimmermann US, Smolka MN, Beck A, Schlagenhauf F. Targeted intervention: Computational approaches to elucidate and predict relapse in alcoholism. Neuroimage. 2017;151:33–44. [DOI] [PubMed] [Google Scholar]
- 11.George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A. 2012;109(44):18156–18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaine SK, Sinha R. Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology. 2017;122:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Williams SS, Stephens DN, Duka T. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology. 2012;37(10):2267–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schonig K, Bartsch D, Hope BT, Spanagel R, Sommer WH. Losing Control: Excessive Alcohol Seeking after Selective Inactivation of Cue-Responsive Neurons in the Infralimbic Cortex. J Neurosci. 2015;35(30):10750–10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seo D, Lacadie CM, Tuit K, Hong K-I, Constable RT, Sinha R. Disrupted Ventromedial Prefrontal Function, Alcohol Craving, and Subsequent Relapse Risk. JAMA Psychiatry. 2013;70:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The α 1 -Adrenergic Receptor Antagonist, Prazosin, Reduces Alcohol Drinking in Alcohol-Preferring (P) Rats. Alcoholism: Clinical and Experimental Research. 2009;33:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker BM, Rasmussen DD, Raskind MA, Koob GF. α1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lê AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2011;218:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnsten AFT. Stress weakens prefrontal networks: molecular insults to higher cognition. Nature Neuroscience. 2015;18:1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson TL, Saxon AJ, Stappenbeck C, Malte CA, Lyons R, Tell D, Millard SP, Raskind M. Double-Blind Randomized Clinical Trial of Prazosin for Alcohol Use Disorder. American Journal of Psychiatry. 2018:appi.ajp.2018.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrakis IL, Desai N, Gueorguieva R, Arias A, O’Brien E, Jane JS, Sevarino K, Southwick S, Ralevski E. Prazosin for Veterans with Posttraumatic Stress Disorder and Comorbid Alcohol Dependence: A Clinical Trial. Alcoholism: Clinical and Experimental Research. 2016;40:178–186. [DOI] [PubMed] [Google Scholar]
- 22.Wilcox CE, Tonigan JS, Bogenschutz MP, Clifford J, Bigelow R, Simpson T. A Randomized, Placebo-controlled, Clinical Trial of Prazosin for the Treatment of Alcohol Use Disorder. Journal of Addiction Medicine. 2018;12:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinha R, Wemm S, Fogelman N, Milivojevic V, Morgan PM, Angarita GA, Hermes G, Fox HC. Moderation of prazosin’s efficacy by alcohol withdrawal symptoms. American Journal of Psychiatry. 2021;178(5):447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinman RA, Ostacher MJ. Prazosin and Alcohol Use Disorder. Am J Psychiatry. 2019;176(2):165. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of Alcohol Withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). British Journal of Addiction. 1989;84:1353–1357. [DOI] [PubMed] [Google Scholar]
- 26.Sinha R, Lacadie CM, Constable RT, Seo D. Dynamic neural activity during stress signals resilient coping. Proceedings of the National Academy of Sciences. 2016;113(31):8837–8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaine SK, Wemm S, Fogelman N, Lacadie C, Seo D, Scheinost D, Sinha R. Association of prefrontal-striatal functional pathology with alcohol abstinence days at treatment initiation and heavy drinking after treatment initiation. American Journal of Psychiatry. 2020;177(11):1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang PJ, Bradley MM, Cuthbert Greenwald M, Dhman A, Vaid D, Hamm A, Cook E, Bertron A, Petry M, Bruner R, Mcmanis M, Zabaldo D, Martinet S, Cuthbert S, Ray D, Koller K, Kolchakian M, Hayden S. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. International Affective Picture System (IAPS. 1997. [Google Scholar]
- 29.Sinha R, Wemm S, Fogelman N, Milivojevic V, Morgan PM, Angarita GA, Hermes G, Fox HC. Moderation of Prazosin’s Efficacy by Alcohol Withdrawal Symptoms. American Journal of Psychiatry. 2021;178(5), 447–458. The American journal of psychiatry, 10.1176/appi.ajp.2020.20050609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobell LC, Sobell MB.Timeline Follow-Back, in: Litten RZ, Allen JP (eds) Measuring Alcohol Consumption. New Jersey: Humana Press;1992, 41–72. [Google Scholar]
- 31.Kranzler HR, Armeli S, Tennen H, Covault J, Feinn R, Arias AJ, Pettinati H, Oncken C. A double-blind, randomized trial of sertraline for alcohol dependence: moderation by age of onset [corrected] and 5-hydroxytryptamine transporter-linked promoter region genotype. Journal of clinical psychopharmacology. 2011;31:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duncan JS, Papademetris X, Yang J, Jackowski M, Zeng X, Staib LH. Geometric strategies for neuroanatomic analysis from MRI. NeuroImage. 2004;23:S34–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuckit MA, Danko GP, Smith TL, Hesselbrock V, Kramer J, Bucholz K. A 5-Year Prospective Evaluation of DSM-IV Alcohol Dependence With and Without a Physiological Component. Alcoholism: Clinical & Experimental Research. 2003;27:818–825. [DOI] [PubMed] [Google Scholar]
- 34.Myrick H, Anton RF. Clinical management of alcohol withdrawal. CNS spectrums. 2000;5(2):22–32. [DOI] [PubMed] [Google Scholar]
- 35.Malcolm R, Roberts JS, Wang W, Myrick H, Anton RF. Multiple previous detoxifications are associated with less responsive treatment and heavier drinking during an index outpatient detoxification. Alcohol. 2000;22:159–164. [DOI] [PubMed] [Google Scholar]
- 36.Anton RF, Latham P, Voronin K, Book S, Hoffman M, Prisciandaro J, Bristol E. Efficacy of Gabapentin for the Treatment of Alcohol Use Disorder in Patients With Alcohol Withdrawal Symptoms: A Randomized Clinical Trial. JAMA Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences. 2009;106:912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 2016;3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kendler KS, Sundquist K, Ohlsson H, Palmér K, Maes H, Winkleby MA, Sundquist J. Genetic and Familial Environmental Influences on the Risk for Drug Abuse. Archives of General Psychiatry. 2012;69:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinha R Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becker HC. Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology. 2017;122:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


