Abstract
To examine the hypothesis that the ancestral role of the toxR gene in the family Vibrionaceae is control of the expression of outer membrane protein (OMP)-encoding genes for adaptation to environmental change, we investigated the role of the toxR gene in Vibrio anguillarum, an important fish pathogen. The toxR gene of V. angullarum (Va-toxR) was cloned from strain PT-87050 isolated from diseased ayu (Plecoglossus altivelis), and the sequence was analyzed. The toxR sequence was 63 to 51% identical to those reported for other species of the family Vibrionaceae. Distribution of the Va-toxR gene sequence in V. anguillarum strains of various serotypes was confirmed by using DNA probe and PCR methods. An isogenic toxR mutant of V. anguillarum PT-24, isolated from diseased ayu, was constructed by using an allelic exchange method. The wild-type strain and the toxR mutant did not differ in the ability to produce a protease(s) and a hemolysin(s) or in pathogenicity for ayu when examined by the intramuscular injection and immersion methods. A 35-kDa major OMP was not produced by the toxR mutant. However, a 46-kDa OMP was hardly detected in the wild-type strain but was produced as the major OMP by the toxR mutant. For the toxR mutant, the MICs of two β-lactam antibiotics were higher and the minimum bactericidal concentration of sodium dodecyl sulfate was lower than for the wild-type strain. Analysis of the N-terminal amino acid sequences of the 35- and 46-kDa OMPs indicated that these proteins are the porin-like OMPs and are related to the toxR-regulated major OMPs of the family Vibrionaceae. The results indicate that the toxR gene is not involved in virulence expression in V. anguillarum PT-24 and that toxR regulation of major OMPs is universal in the family Vibrionaceae. These results support the hypothesis that the ancestral role of the toxR gene is regulation of OMP gene expression and that only in some Vibrio species has ToxR been appropriated for the regulation of a virulence gene(s).
The toxR gene was first discovered as a positive transcriptional regulator of the ctx gene encoding the cholera toxin of Vibrio cholerae (33). The toxR gene was subsequently shown to encode a transmembrane protein that plays a pivotal role in the coordinate regulation of ctx and many other genes, including the tcp gene encoding toxin-coregulated pili and the ompU and ompT genes encoding major outer membrane proteins (OMPs), in this microorganism (6, 12, 34, 35). The toxR gene was also detected in Vibrio parahaemolyticus and was shown to stimulate expression of the tdh gene encoding thermostable direct hemolysin, a major virulence factor of this microorganism (29). In addition, the toxR gene is involved in the stimulation of hemolysin gene expression and regulation of OMP expression in Vibrio vulnificus (27). Although the nucleotide sequence identities among the three toxR genes were 52 to 63%, the amino acid sequences of the proposed activity domain and the presumed transmembrane domain were well conserved in the amino acid sequences deduced from the three toxR sequences (27, 29). These findings suggest that the toxR gene is a regulatory gene controlling the expression of the genes encoding important extracellular virulence factors and other virulence-associated genes in vibrios. ToxR regulation of the genes in V. cholerae is mediated by transcriptional activation of the toxT gene, the second regulatory gene in the cascade, and the toxT gene is present in the tcp operon (12). However, ToxR regulation of the ompU gene is not mediated by ToxT and ToxR activation of the ompU gene is stronger than that of the toxT gene (5). The ctx operon and the tcp operon of V. cholerae are parts of phage genomes (23, 24, 64). It was therefore hypothesized that the ompU branch of the ToxR cascade may be the primary role of ToxR and that the toxT-mediated branch may have been introduced later (5). Furthermore, the product of the tcpP gene was shown to positively regulate toxT expression and overexpression of TcpP can activate toxT transcription in the absence of ToxR (18).
In addition, the toxR gene seems to be a regulatory gene widely distributed in the family Vibrionaceae; the toxR genes of Vibrio hollisae, Vibrio fischeri, a nonpathogenic species, and a strain of deep-sea bacterium belonging to the genus Photobacterium have been cloned and analyzed (50, 63, 65). The nucleotide sequences homologous to the internal portion of the toxR gene were detected in at least three other species of the genus Vibrio (Vibrio fluvialis, Vibrio alginolyticus, and Vibrio mimicus) and two subspecies of Photobacerium damselae (44). The toxR gene was shown to mediate control of the expression of the pressure-responsive porin-like OMPs in the Photobacterium species (65). This indicates that toxR regulation of OMP expression is not directly related to virulence expression. However, a recent report indicated that ToxR-dependent modulation of OmpU and OmpT in V. cholerae is critical for bile resistance, virulence factor expression, and colonization in the infant-mouse model (48, 49). It is ideal to study the virulence expression of a pathogen in its natural host, but it is difficult to perform such experiments with humans. Examination in the natural host of an animal pathogen that is related to the human pathogens may provide valuable information on the pathogenic mechanism. A goldfish model for Mycobacterium marinum is a successful example for the study of Mycobacterium tuberculosis pathogenicity (56, 61).
Vibrio anguillarum is classified in the genus Listonella based on analysis of the 5S rRNA sequence (30), but it is still referred to as V. anguillarum in many publications. This microorganism is the etiologic agent of vibriosis, a terminal hemorrhagic septicemia, in various marine and freshwater fish (58). Vibriosis is one of the most important infectious diseases of salmonid fish throughout the world, and in Japan, vibriosis of cultured ayu (Plecoglossus altivelis), a salmonid-related fish, is an especially serious problem (39). Various virulence factors of V. anguillarum have been proposed. Extracellular products such as proteases (11, 19, 20, 22, 43) and hemolysins (37, 59) and cell-associated hemagglutinating activity (60) are considered to be possible virulence factors. Chemotactic motility is required for invasion of the host (45). The iron-sequestering system encoded on the 65-kb virulence plasmid and regulated by the AngR protein and other regulators is an important mechanism for the organism's proliferation in the host (7, 8, 66). OMPs may serve as auxiliary virulence factors. The OMPs involved in iron-sequestering systems have been well characterized (1, 2, 9). A 40-kDa porin and a 35-kDa porin have been studied (51, 55), but these porins are structurally and functionally similar to well-characterized porins of Escherichia coli and other organisms (10, 51, 54).
In this study, we cloned and analyzed the toxR gene of V. anguillarum (Va-toxR) and demonstrated universal distribution of the Va-toxR sequence in the representative strains of V. anguillarum. We then constructed a toxR mutant of the V. anguillarum strain isolated from diseased ayu by an allelic exchange method. The effect of the toxR mutation on the expression of extracellular virulence factors, lethality to the natural host, and OMPs were analyzed to examine the hypothesis that the ancestral role of the toxR gene may be a global regulator rather than a virulence regulator.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains used for the DNA colony hybridization test and the PCR test are listed in Table 1. These strains are our laboratory stock strains. V. anguillarum strains PT-87050 and PT-24 were used for cloning and mutant construction and are included in Table 1. V. parahaemolyticus AQ3815ΔToxR was as reported previously (29). E. coli strains SY327λpir, SM10λpir, and MC1061 were described previously (4, 34). The plasmids employed or constructed in this study are described in Table 2.
TABLE 1.
Results of tests for the detection of the Va-toxR gene in various species of the genus Vibrio, E. coli, and other organisms isolated from diseased fish
| Species | Strainb | Detection of the Va-toxR genea by:
|
|
|---|---|---|---|
| DNA probe | PCR | ||
| V. anguillarum | PT-24 (A, ayu, 1973, Japan) | + | + |
| V. anguillarum | pSH-801 (A, ayu, 1980, Japan) | + | + |
| V. anguillarum | PT-87050 (A, ayu, 1987, Japan) | + | + |
| V. anguillarum | ET-78063 (A, Japanese eel, 1978, Japan) | + | + |
| V. anguillarum | ET-506 (A, European eel, 1975, Japan) | + | + |
| V. anguillarum | ATCC19264 (A, cod, 1956, Norway) | + | + |
| V. anguillarum | PT-493 (B, ayu, 1975, Japan) | + | + |
| V. anguillarum | pB-82006 (B, ayu, 1982, Japan) | + | + |
| V. anguillarum | ET-208 (B, European eel, 1974, Japan) | + | + |
| V. anguillarum | PT-81016 (C, ayu, 1981, Japan) | + | + |
| V. anguillarum | PT-82009 (C, ayu, 1982, Japan) | + | + |
| V. anguillarum | ET-79052 (C, Japanese eel, 1979, Japan) | + | + |
| V. anguillarum | RH-8101 (C, red seabream, 1981, Japan) | + | + |
| V. anguillarum | MT-8201 (C, red seabream, 1982, Japan) | + | + |
| V. anguillarum | HT-77003 (C, yellowtail, 1977, Japan) | + | + |
| V. anguillarum | FY-8701 (C, flounder, 1987, Japan) | + | + |
| V. anguillarum | LS-174 (C, coho salmon, 1974, USA) | + | + |
| V. anguillarum | 775 (C, coho salmon, 1973, USA) | + | + |
| V. anguillarum | PB-15 (D, ayu, 1966, Japan) | + | + |
| V. anguillarum | PB-28 (E, ayu, 1967, Japan) | + | + |
| V. anguillarum | ET-1 (F, Japanese eel, 1971, Japan) | + | + |
| V. anguillarum | pT-8001 (G, ayu, 1980, Japan) | + | + |
| V. anguillarum | pT-8005 (H, ayu, 1980, Japan) | + | + |
| V. anguillarum | pT-8002 (I, ayu, 1980, Japan) | + | + |
| V. aestuarianus | ATCC 35048 | − | − |
| V. alginolyticus | NCMB1903 | − | − |
| V. alginolyticus | 219 | − | − |
| V. alginolyticus | 220 | − | − |
| V. campbellii | ATCC 25920 | − | − |
| V. carchariae | ATCC 35084 | − | − |
| V. cholerae O1 | NIH41 | − | − |
| V. cholerae O1 | NIH35A3 | − | − |
| V. cholerae O139 | MO45 | − | − |
| V. cholerae non-O1 | AM2 | − | − |
| V. cincinnatiensis | ATCC 35912 | − | − |
| V. damsela | ATCC 33539 | − | − |
| V. damsela | RIMD2222001 | − | − |
| V. diazotrophicus | ATCC 33466 | − | − |
| V. fischeri | ATCC 7744 | − | − |
| V. fluvialis | NCTC11327 | − | − |
| V. fluvialis | RIMD2220002 | − | − |
| V. furnisii | RIMD2223001 | − | − |
| V. gazogenes | ATCC 29988 | − | − |
| V. harveyi | ATCC 14126 | − | − |
| V. hollisae | 525-82 | − | − |
| V. ichthyoenteri | IFO15847 | − | − |
| V. iliopiscarius | ATCC 51760 | − | − |
| V. logei | ATCC 15382 | − | − |
| V. mediterranei | ATCC 43341 | − | − |
| V. metschnikovii | IAM1039 | − | − |
| V. metschnikovii | RIMD2208006 | − | − |
| V. mimicus | RIMD2218002 | − | − |
| V. mytili | ATCC 51288 | − | − |
| V. navarrensis | ATCC 51183 | − | − |
| V. nereis | ATCC 25917 | − | − |
| V. nigripulchritudo | ATCC 27043 | − | − |
| V. ordalii | ATCC 33509 | − | − |
| V. orientalis | ATCC 33934 | − | − |
| V. parahaemolyticus | AQ3815 | − | − |
| V. parahaemolyticus | WP1 | − | − |
| V. parahaemolyticus | AQ4037 | − | − |
| V. parahaemolyticus | AT4 | − | − |
| V. pelagius | ATCC 25916 | − | − |
| V. penaeicida | IFO15640 | − | − |
| V. proteolyticus | NCMB1326 | − | − |
| V. splendidus | ATCC 33125 | − | − |
| V. tubiashii | ATCC 19109 | − | − |
| V. vulnificus | RIMD2219022 | − | − |
| Escherichia coli | HB101 | − | − |
| Aeromonas hydrophila | ET-79069 | NT | − |
| Aeromonas hydrophila | ET-2 | NT | − |
| Aeromonas salmonicida | NCMB1102 | NT | − |
| Aeromonas salmonicida | NCMB2020 | NT | − |
| Edwardsiella tarda | NUF251 | NT | − |
| Edwardsiella tarda | ET-82021 | NT | − |
| Enterococcus seriolicida | ATCC 49156 | NT | − |
| Enterococcus seriolicida | No.4 | NT | − |
| Pasteurella piscicida | SJ-9107 | NT | − |
| Pasterurella piscicida | OT-8447 | NT | − |
| Pseudomonas anguilliseptica | NCMB1950 | NT | − |
| Pseudomonas anguilliseptica | SH-83454 | NT | − |
| Streptococcus iniae | HS95-06 | NT | − |
| Streptococcus iniae | YT-9509 | NT | − |
+, detected; −, not detected; NT, not tested.
Detailed information for each V. anguillarum strain is given in parentheses in the following order: serotype, origin (name of fish) of isolation, year of isolation, and country of isolation.
TABLE 2.
Plasmids used in this study
| Plasmid | Description | Source or reference |
|---|---|---|
| pUC118 | Cloning vector | 62 |
| pUC119 | Cloning vector | 62 |
| pUC1318 | Vector for addition of restriction sites | 25 |
| pRE112 | Suicide vector carrying chloramphenicol resistance gene and sacB gene | 13 |
| pKTN170 | 2.3-kb SspI-BamHI fragment carrying Vp-htpG gene cloned into SmaI site of pUC118 | (M. Nishibuchi, J. Okuda, and K. Kumagai, unpublished data) |
| pKON21 | 9.6-kb SacI fragment carrying Va-htpG and Va-toxR genes cloned from cellular DNA of V. anguillarum PT-87050 into SacI site of pUC118 | This study |
| pKON23 | 2.8-kb PstI-SalI fragment of pKON21 carrying Va-toxR gene cloned into PstI-SalI site of pUC119 | This study |
| pKON24 | 2.5-kb BamHI fragment carrying mutated Va-toxR gene with 330-bp sequence deletion cloned into BamHI site of pUC1318 | This study |
| pKON25 | 2.5-kb SacI fragment of pKON24 carrying deletion-containing Va-toxR gene cloned into SacI site of pRE112 | This study |
Cloning of the Va-toxR gene.
The SacI digest of the cellular DNA of V. anguillarum PT-87050 was size fractionated by agarose gel electrophoresis, and DNA fragments of approximately 9.6 kb were cloned into the SacI site of pUC118 in E. coli MC1061. The transformants were grown on Luria-Bertani (LB) agar containing ampicillin and were screened by the DNA colony blot hybridization method with the V. parahaemolyticus htpG (Vp-htpG) gene probe under slightly stringent conditions (in a solution containing 40% formamide and washing at 65°C) as described previously (42). The probe-positive recombinant plasmid thus selected was named pKON21. The pKON21 DNA digested with appropriate restriction enzymes was examined by Southern blot hybridization with the V. parahaemolyticus toxR (Vp-toxR) gene probe under moderately stringent conditions (in a solution containing 35% formamide and washing at 61.5°C) as described previously (42). A 2.8-kb probe-positive PstI-SalI fragment of pKON21 was subcloned into pUC119 that had been cleaved with PstI and SalI, resulting in pKON23.
Nucleotide sequence analysis.
The nucleotide sequence of the 2.8-kb PstI-SalI insert of pKON23 was determined with the ABI-Prism Big Dye Terminator Cycle Sequencing Ready Reaction Kit and an ABI-Prism 377 sequencer (Perkin-Elmer, Applied Biosystems Division, Foster City, Calif.) in accordance with the manufacturer's instructions.
DNA probes.
To prepare the Vp-htpG probe DNA, a 1.5-kb HindIII fragment internal to the Vp-htpG gene (M. Nishibuchi, J. Okuda, and K. Kumagai, unpublished data) was isolated from pKTN170 and used as the probe DNA. The Vp-toxR probe DNA that is internal to the Vp-toxR coding region was prepared as described previously (29). The Va-toxR probe DNA was prepared by PCR using pKON23 as the template and VA-U1 and VA-D1 as the primers (Table 3). The PCR conditions used to amplify the Va-toxR probe sequence were as follows. The PCR mixture consisted of 2 μl of pKON23 solution in distilled water (10 ng/μl), 5 μl of 10× buffer (ExTaq buffer; Takara, Tokyo, Japan), 4 μl of 2.5 mM deoxynucleoside triphosphates (dNTPs), 1 μl of each primer (20 pmol/μl), 0.5 μl of Taq polymerase (ExTaq; Takara), and 36.5 μl of distilled water. The amplification condition were 30 cycles of 95°C for 1 min, 67°C for 1.5 min, and 72°C for 1.5 min and then an extra extension step of 72°C for 5 min. The 342-bp amplicon was purified by gel electrophoresis and used as the Va-toxR probe DNA. The probe DNAs were labeled by the random priming method with 32P-labeled dCTP (29).
TABLE 3.
Sequences of oligonucleotides used as PCR primers
| Primer | Orientation | Positions | Base sequencea |
|---|---|---|---|
| VA-U1 | Sense | 397–419b | GCCAGTGAGTCTATCCAAGACAT |
| VA-U2 | Sense | 432–453b | CACTTCGCAACCCGAAGAGACA |
| VA-D1 | Antisense | 717–738b | CTGCTTAGGTGCCAGTTCTCCA |
| VA-KS1 | Sense | 180–208c | GAGAGGATCCATTGTGAGCGGATAACAATTTCACACAGG |
| VA-KS2 | Sense | 557–583b | GAGAGGTACCGTGACTCGAGCAACCCCCTACTCACAA |
| VA-KS3 | Sense | 27–51b | TGATTTGCTAGTAAGATGAGGCTGG |
| VA-KS4 | Sense | 1459–1484b | GTTCTAGTCAAAGTAAGGACTTTACC |
| VA-KR1 | Antisense | 195–226b | AGAGGGTACCGATAATCTTCTTATTGTTGGCATGTTTAACCG |
| VA-KR2 | Antisense | 311–334c | AGAGGGATCCCGCCAGGGTTTTCCCAGTCACGAC |
| VA-KR3 | Antisense | 1459–1484b | GGTAAAGTCCTTACTTTGACTAGAAC |
| VA-KR4 | Antisense | 27–51b | CCAGCCTCATCTTACTAGCAAATCA |
The underlined sequences are extra bases added for cloning purposes, and these do not appear in the sequences deposited in the GenBank database.
The positions of primers correspond to the base positions of the V. anguillarum toxR gene deposited in the GenBank database (accession number AB042547).
The positions of primers correspond to the base positions of the pUC119 nucleotide sequence deposited in the GenBank database (accession number U07650).
DNA colony blot hybridization test for detection of the Va-toxR gene.
DNA colony blots were prepared, and DNA colony hybridization was performed under stringent conditions (in a solution containing 50% formamide and washing at 65°C) as described previously (42).
PCR for detection of the Va-toxR gene.
A 1-ml portion of the test strain grown in LB broth with shaking (160 rpm) at 37°C overnight was boiled for 5 min. The supernatant was obtained by centrifugation (15,000 × g) on a tabletop centrifuge and diluted 1:10 with distilled water. A 10-μl portion of this preparation was used as the template for PCR amplification. Primers VA-U2 and VA-D1 (listed in Table 3) were employed, and the PCR procedure was as follows. The PCR mixture consisted of 10 μl of the diluted boiled culture supernatant, 5 μl of 10× buffer (ExTaq buffer; Takara), 4 μl of 2.5 mM dNTPs, 1 μl of each primer (20 pmol/μl), 0.25 μl of Taq polymerase (ExTaq; Takara), and 28.75 μl of distilled water. The amplification condition were 20 cycles of 95°C for 1 min, 62°C for 1.5 min, and 72°C for 1.5 min and then an extra extension step of 72°C for 5 min. A 10-μl portion of the reaction mixture was resolved by electrophoresis in a 2% agarose gel to detect 307-bp amplicons.
Construction of a Va-toxR mutant of V. anguillarum.
The Va-toxR gene of V. anguillarum PT-24 was replaced with the mutated Va-toxR gene by using an allelic exchange method with suicide vector plasmid pRE112. A 330-bp fragment was deleted from the Va-toxR coding region to construct the mutated Va-toxR gene by using the PCR method that follows. A 1.5-kb upstream sequence and a 1.0-kb downstream sequence of the deletion region were amplified by using pKON23 as the template. The 1.5-kb upstream sequence was amplified with primers VA-KS1 and VA-KR1 (Table 3). These primers were designed so that the amplicon has a BamHI site at the 5′ end and a KpnI site at the 3′ end. The 1.0-kb downstream sequence was amplified by using primers VA-KS2 and VA-KR2 (Table 3). These primers were designed so that the amplicon has a KpnI site at the 5′ end and a BamHI site at the 3′ end. The PCR amplification was carried out by using KOD DNA polymerase (TOYOBO, Osaka, Japan) in accordance with the manufacturer's specifications with a change in the amplification conditions to 30 cycles of denaturation at 95°C for 30 s, annealing at 73°C for the 1.5-kb fragment and at 80°C for the 1.0-kb fragment for 30 s, and an extension step of 72°C for 30 s. The 1.5- and 1.0-kb amplicons were purified by gel electrophoresis and digested with KpnI and BamHI. The two digested fragments were cloned simultaneously into BamHI-cleaved pUC1318, resulting in pKON24. The 2.5-kb insert flanked by the SacI recognition sequences was excised from pKON24 by digestion with SacI and cloned into SacI-cleaved pRE112. The resulting plasmid, pKON25, was first constructed in the E. coli SY327λpir background and transformed into E. coli SM10λpir. pKON25 was then mobilized from this strain into V. anguillarum PT-24 by conjugation. Transconjugants were selected on thiosulfate-citrate-bile-sucrose agar (Difco Laboratories, Detroit, Mich.) supplemented with chloramphenicol (5 μg/ml). The chromosomal integration of pKON25 in the transconjugant was confirmed by two PCR methods. First, the wild-type toxR gene of PT-24 and the mutated toxR gene were detected as 1,458- and 1,128-bp amplicons, respectively, using amplification with primers VA-KS3 and VA-KR3 (Table 3). The PCR mixture consisted of 1 μl of boiled culture supernatant, 5 μl of 10× buffer (ExTaq buffer, Takara), 4 μl of 2.5 mM dNTPs, 1 μl of each primer (20 pmol/μl), 0.25 μl of Taq polymerase (ExTaq; Takara), and 37.75 μl of distilled water. The amplification condition were 30 cycles of 95°C for 1 min, 63°C for 1.5 min, and 72°C for 1.5 min and then an extra extension step of 72°C for 5 min. Additionally, the distance (7.4 kb) between the wild-type and mutated toxR genes in the chromosome was confirmed by PCR amplification with primers VA-KS4 and VA-KR4 (Table 3), followed by estimation of the amplicon size by gel electrophoresis. The PCR mixture consisted of 5 μl of cellular DNA (24 ng/μl), 5 μl of 10× buffer (ExTaq buffer; Takara), 6 μl of 2.5 mM dNTPs, 1 μl of each primer (20 pmol/μl), 0.5 μl of Taq polymerase (ExTaq; Takara), and 31.5 μl of distilled water. The amplification condition were 34 cycles of 95°C for 1 min, 67°C for 1.5 min, and 72°C for 2.5 min and then an extra extension step of 72°C for 5 min. PT-24/pKON25 grown overnight in LB broth containing 5% sucrose with shaking at 37°C was inoculated onto LB agar containing 5% sucrose. The colonies were analyzed by the PCR method with primers VA-KS3 and VA-KR3 as described above. One strain that retained the mutated Va-toxR gene and lost the wild-type Va-toxR gene was selected. Finally, the nucleotide sequence of the amplicon corresponding in size to the mutated Va-toxR gene was determined. This confirmed that the desired mutation had been introduced into this V. anguillarum strain.
Protease assay.
Proteolytic activity was examined by the casein plate method as described by Gardel and Mekalanos (14), except that the casein plate inoculated with the test strain was incubated at 25°C for 18 h. In another proteolytic assay, the extracellular products (ECP) of the test strain were prepared by the cellophane plate technique and the proteolytic activity of the ECP was measured by the method described by Inamura et al. (19). Briefly, a sterile sheet of cellophane was placed on the surface of a nutrient agar plate and 0.2 ml of the seed culture of the test strain in LB broth was spread on the cellophane sheet. After 24 h of incubation at 25°C, bacterial growth was washed off the cellophane sheet with 4 ml of 0.01 M phosphate-buffered saline (pH 7). The supernatant of the suspension was obtained by centrifugation (10,000 × g, 5 min), and the supernatant was used as the ECP. A reaction mixture containing 0.5 ml of azocasein at 5 mg/ml, 0.4 ml of distilled water, and 0.1 ml of an ECP sample solution was incubated at 25°C for 20 min. The reaction was stopped by addition of 3.5 ml of 5% trichloroacetic acid, the precipitate was removed by centrifugation at 5,600 × g for 5 min, and 4.5 ml of 0.5 M NaOH was added to the supernatant. The proteolytic activity of each sample was measured by determining the A440.
Extracellular hemolysin assay.
The test strain was streaked onto sheep blood agar purchased from Eiken Chemical Co., Ltd., Tokyo, Japan (product number E-MR93). The result was recorded after incubation at 25°C for 40 h.
Cell-associated hemolysin and hemagglutination assays.
The assays for the cell-associated hemolysin and hemagglutination were carried out as described by Gardel and Mekalanos (14), except that sheep erythrocytes were used in place of chicken and human erythrocytes. The cell-associated hemolysin titer and the hemagglutination titer were expressed as the reciprocal of the highest dilution of the sample that gave a positive reaction.
Pathogenicity tests.
Healthy ayu weighing 3 g, on average, were challenged with the test strain grown on triptic soy agar (Difco) at 25°C for 24 h. Twenty fish in each test group were injected intramuscularly with 0.05 ml of 0.85% NaCl solution containing a known number of bacterial cells (the intramuscular injection method) or immersed for 10 min in 0.5% NaCl solution containing a known number of bacterial cells (the immersion method). Control fish received the same treatment, except that bacterial cells were not added to the NaCl solutions. After these treatments, fish were kept in flowthrough fresh water maintained at 22°C in 40-liter tanks. Mortality was recorded daily for 7 days. Kidneys of dead fish were subjected to bacterial isolation, and the isolated bacterium was confirmed to be the test strain by the agglutination test with anti-V. anguillarum J-O-1 (serotype A) rabbit antiserum.
Extraction and analysis of OMPs.
The test strain was grown with shaking (125 rpm) for 16 h in modified LB broth where the pH or NaCl concentration was varied at 25°C or in LB broth at pH 7.0, 1.0% NaCl and various temperatures. The outer membrane of the test strain was extracted by a modification of the method described by Simon et al. (51). Briefly, bacterial cells were harvested by centrifugation at 10,000 × g for 10 min at 4°C, washed in 10 mM HEPES, and disrupted by sonication on ice with a W-225R sonicator (Heat Systems-Ultrasonics, Inc., Farmingdale, N.Y.) for 3 min at an output control setting of 6. The suspension was centrifuged at 6,000 × g for 5 min to remove intact cells. The cell fragments were collected by sedimentation at 20,000 × g for 1 h at 4°C. The cytoplasmic membrane in this preparation was solubilized with 0.7% sarcosyl, and the remaining outer membrane was collected by centrifugation at 20,000 × g for 1 h at 4°C. An outer membrane preparation containing 24 μg of protein was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis using a 5% stacking gel and a 10% separating gel. The gels were stained with Coomassie brilliant blue R-250. The molecular weights of the proteins were estimated with standard low-molecular-weight markers purchased from Bio-Rad (Hercules, Calif.).
N-terminal amino acid sequencing.
The 35-kDa major OMP detected in the OMP preparation of PT-24 and the 46-kDa OMP detected in PT-24ΔtoxR (explained below) were separated by SDS-polyacrylamide gel electrophoresis as described above and transferred to an Immobilon-P membrane filter (Millipore, Bedford, Mass.). The membrane was stained with Coomassie brilliant blue R-250. The portions of the filter containing the 35- and 46-kDa major OMP bands were cut out, and the N-terminal amino acid sequence of each protein was determined by the standard technique (32).
MIC.
MICs of antibiotics were determined by the twofold agar dilution method recommended by the Japan Society of Chemotherapy. The test strain was grown in LB broth to mid-logarithmic phase at 25°C. The concentration of the organism was then adjusted to 106 CFU/ml, and 5 μl of the culture was inoculated onto LB agar containing the test antibiotic. The MIC was determined after 18 h of incubation at 25°C.
Determination of resistance to anionic detergents.
A minimum bactericidal concentration (MBC) assay was performed essentially as described by Provenzano et al. (49) with some modifications. The test strain was grown in LB broth (containing 1% NaCl) with shaking (160 rpm) overnight. Ten microliters of the seed culture was inoculated into 5 ml of fresh LB broth and incubated with shaking for 12 h (V. anguillarum), 6 h (V. parahaemolyticus AQ3815), or 7.5 h (V. parahaemolyticus AQ3815ΔToxR). This broth culture was diluted 1:500 in fresh LB broth. Twenty microliters of the diluted culture containing 1.2 × 105 to 2 × 105 CFU of each test strain was inoculated into 2 ml of LB broth containing the test anionic detergent and incubated with shaking for 24 h. One hundred microliters of the culture was then inoculated onto LB agar medium and incubated for up to 36 h. The incubation temperatures for V. anguillarum and V. parahaemolyticus were 25 and 37°C, respectively, throughout the experiment. The MBC was defined as that at which no viable bacteria were recovered (49). The assay starting with the same seed culture was performed in triplicate. Values in Table 6 were obtained from three independent experiments performed as described by Provenzano et al. (49). The anionic detergents examined included bile (cholic acid sodium salt; Nacalai Tesque, Inc. Kyoto, Japan), deoxycholate (DC; deoxycholic acid sodium salt; Sigma), and SDS (sodium lauryl sulfate; Nacalai Tesque, Inc.). A log2 dilution of the test detergent was employed (discussed below).
TABLE 6.
MBCs of anionic detergents for V. anguillarum PT-24 and PT-24ΔtoxR and V. parahaemolyticus AQ3815 and AQ3815ΔToxR
| Species and strain | MBC (%) of:
|
||
|---|---|---|---|
| Bile | DC | SDS | |
| V. anguillarum | |||
| PT-24 | 2.0 | 0.2 | 0.4 |
| PT-24ΔtoxR | 2.0 | 0.2 | 0.1 |
| V. parahaemolyticus | |||
| AQ3815 | 2.0 | 0.2 | 0.8 |
| AQ3815ΔToxR | 1.0 | 0.2 | 0.1 |
Nucleotide sequence accession number.
The nucleotide sequence of the Va-toxR gene will appear in the GenBank nucleotide sequence database under accession number AB042547.
RESULTS
Analysis of the Va-toxR gene sequence.
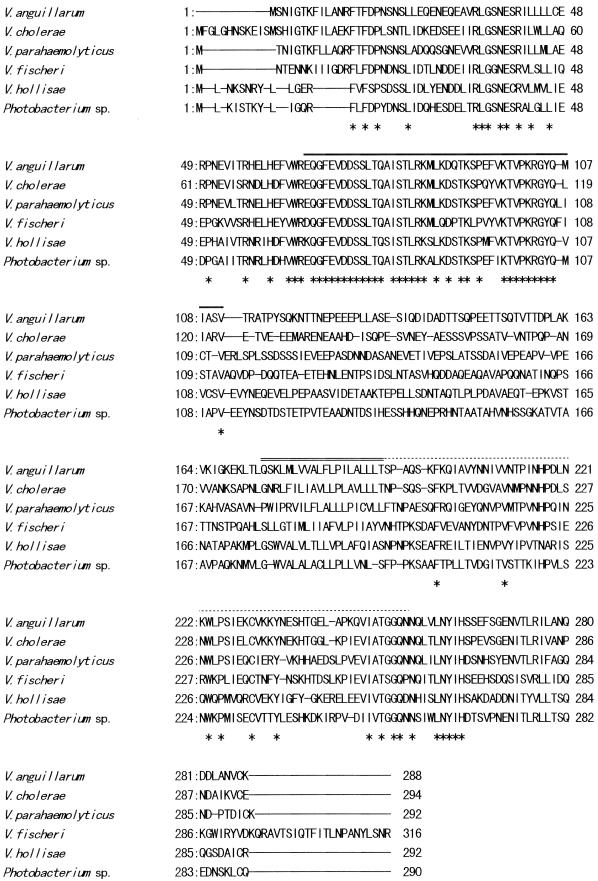
We hypothesized that the toxR-htpG gene arrangement is universal in vibrios and utilized the Vp-htpG gene probe to clone the DNA fragment of V. anguillarum containing both the htpG and toxR genes (discussed later). A Vp-htpG gene probe-positive 9.6-kb SacI fragment was first cloned from cellular DNA of V. anguillarum PT-87050. A 2.8-kb PstI-SalI fragment that hybridized with the Vp-toxR gene probe was then subcloned from the 9.6-kb SacI fragment. Nucleotide sequence analysis of the 2.8-kb PstI-SalI fragment revealed an open reading frame of 864 bp encoding 288 amino acid residues. The sequence was 63, 62, 57, 57, and 51% identical to those of the toxR genes of V. parahaemolyticus, V. cholerae, V. fisheri, Photobacterium sp., and V. hollisae, respectively. The amino acid sequence encoded by the V. anguillarum toxR homologue was 59, 60, 45, 44, and 38% identical to those of the ToxRs, respectively. An alignment of the amino acid sequences is shown in Fig. 1. The sequence of V. anguillarum contained the sequences of the conserved regions (discussed below). We therefore judged that the open reading frame is the coding region of the Va-toxR gene.
FIG. 1.
Comparison by alignment of V. anguillarum, V. cholerae, V. parahaemolyticus, V. fischeri, V. hollisae, and Photobacterium sp. ToxR amino acid sequences predicted from the nucleotide sequences. A line in the sequence indicates absence of the residue. An asterisk indicates that the residue is conserved in all of the sequences. An overline, a double overline, and a dotted overline indicate the presumed transcription activation domain, the transmembrane domain, and the periplasmic domain, respectively (44). The sequences were taken from those reported in the literature (29, 35, 49, 63, 65).
Distribution of the Va-toxR sequence.
The presence or absence of the Va-toxR-specific sequence in various strains of V. anguillarum and non-V. anguillarum species was examined by the DNA probe and PCR methods (Table 1). A 341-bp sequence internal to the Va-toxR gene was used as the probe DNA in the DNA colony blot hybridization test performed under stringent conditions. All strains of V. anguillarum belonging to various serotypes and isolated from various sources over 31 years gave positive results, whereas the strains belonging to 34 other species of the genus Vibrio yielded negative results. PCR primer sequences were selected from the regions not conserved among the toxR sequences of various Vibrio species, and the PCR condition were optimized to achieve specific annealing. As a result, examination by the PCR method gave the same results as those obtained by the DNA probe method. Selected fish-pathogenic bacteria not belonging to the genus Vibrio were also examined by the PCR method and exhibited negative results (discussed later).
Effect of toxR mutation on proteolytic activity, hemolytic activity, and hemagglutination.
A 330-bp sequence spanning the region from the ATG start codon to a point near the end of the presumed activity domain was deleted from the cloned Va-toxR gene. The Va-toxR gene in V. anguillarum PT-24 rather than PT-87050 (discussed below) was replaced with the mutated Va-toxR gene by an allelic exchange method, and the mutant strain was designated PT-24ΔtoxR.
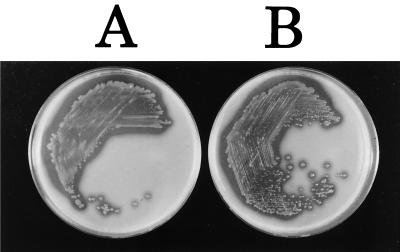
The degrees of proteolytic activity of PT-24 and PT-24ΔtoxR were visualized as the zones of clearing around bacterial growth on casein plates, where they were very similar (Fig. 2). The A440 values of extracellular proteases of PT-24 and PT-24ΔtoxR measured by the cellophane plate technique were 0.0734 ± 0.00175 and 0.0870 ± 0.0174, respectively (results of three independent experiments). The difference between the two strains was not statistically significant (P > 0.2).
FIG. 2.
Extracellular protease production on casein plates. A, PT-24. B, PT-24ΔtoxR.
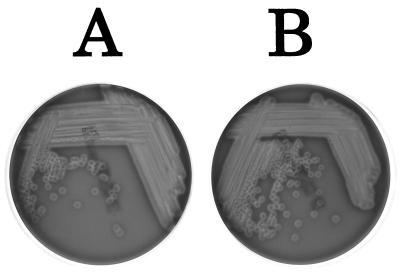
The hemolytic activities of the two strains were visualized as the zone of clearing around bacterial growth on sheep blood agar plates and were similar in level (Fig. 3). The hemagglutination assay of Gardel and Mekalanos (14) was performed since this method also allows semiquantitative measurement of a cell-associated hemolysin(s). PT-24 and PT-24ΔtoxR showed the same hemolytic titer of 4,096 against sheep erythrocytes. Neither PT-24 nor PT-24ΔtoxR manifested hemagglutination activity in this assay.
FIG. 3.
Hemolysin production on sheep blood agar plates. A, PT-24. B, PT-24ΔtoxR.
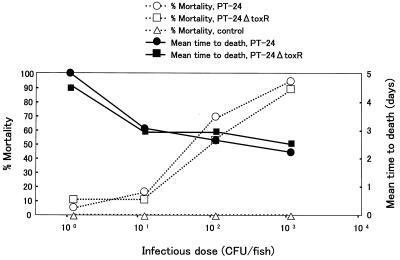
Effect of toxR mutation on pathogenicity for ayu.
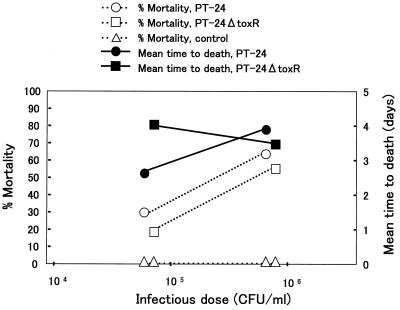
Healthy ayu were challenged with PT-24 and PT-24ΔtoxR. The results of the challenge by the intramuscular injection and immersion methods are summarized in Fig. 4 and 5, respectively. The percent mortality and the average time to death for the two strains did not markedly differ in either of the challenge tests. The calculated 50% lethal doses of PT-24 and PT-24ΔtoxR by the intramuscular injection method were 101.7 and 101.9 CFU/fish, respectively, and those by the immersion method were 105.4 and 105.6 CFU/ml, respectively.
FIG. 4.
Results of pathogenicity tests for ayu done by the intramuscular injection method.
FIG. 5.
Results of pathogenicity tests for ayu done by the immersion method.
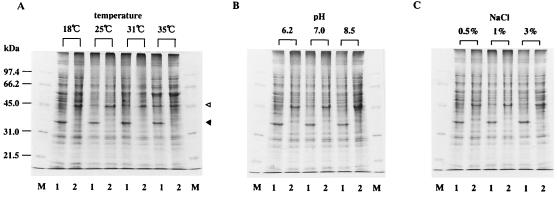
Effect of toxR mutation on the expression of OMPs.
The OMP profiles of PT-24 and PT-24ΔtoxR grown under varied cultural conditions were compared. The cultural conditions varied included the incubation temperature and the pH and NaCl concentration of the culture medium (Fig. 6). PT-24 (Fig. 6, lane 1) invariably produced a 35-kDa major OMP (indicated by the closed triangle), whereas PT-24ΔtoxR (Fig. 6, lane 2) did not produce this protein under any of the cultural conditions tested. On the other hand, PT-24ΔtoxR produced a 46-kDa major OMP (indicated by the open triangle) and PT-24 produced this protein at negligible levels except when it was grown at 35°C.
FIG. 6.
OMP profiles of V. anguillarum PT-24 and PT-24ΔtoxR. The standard cultural condition was growth in LB broth with 1% NaCl pH 7.0 and at 25°C for 16 h with shaking (125 rpm). The incubation temperature (A), pH (B), or NaCl concentration (C) was varied as indicated, and OMP preparations were analyzed by SDS-polyacrylamide gel electrophoresis. Lanes: M, molecular mass standards (molecular masses are indicated on the left); 1, PT-24; 2, PT-24ΔtoxR. The positions of the 35- and 46-kDa major OMPs (explained in the text) are indicated by the closed and open triangles, respectively.
The sequence of the first 16 amino acid residues at the N-terminal end of the 35-kDa major OMP was GELYNQDGTSLEMGGR. The N-terminal amino acid sequence of the first 17 residues of the 46-kDa major OMP was AEIYANDTTAVKLKGEV. The sequences of the 35- and 46-kDa OMPs showed 44 to 100% and 29 to 47% sequence identities with the N-terminal amino acid sequences of the OMPs of Vibrio species and other gram-negative bacteria (Table 4).
TABLE 4.
Proteins that show amino acid sequence homology with the 35- and 46-kDa major OMPs of V. anguillarum PT-24 at the N-terminal end
| Organism | Related protein (reference[s]), % identity with V. anguillarum PT-24
|
|
|---|---|---|
| 35-kDa major OMPa | 46-kDa major OMPb | |
| V. anguillarum | Omp35La (55), 100 40-kDa major OMP (10), 97 | Omp35La (55), 29 |
| V. cholerae | OmpU (53), 56 | OmpT (28), 35 |
| V. vulnificus | 38-kDa OMP (27), 87 | 38-kDa OMP (27), 35 |
| Photobacterium sp. | OmpL (65), 50 | OmpH (65), 29 |
| E. coli | OmpC (36), 44 | OmpF (21, 40), 47 OmpC (36), 41 PhoE (46), 41 |
| Haemophilus influenzae type b | OMP P2 (17, 38), 45 | OMP P2 (17, 38), 41 |
| Salmonella enterica serovar Typhimurium | OmpF (GenBank accession no. Z31594), 41 | |
Sixteen N-terminal amino acid residues of the 35-kDa major OMP were compared.
Seventeen N-terminal amino acid residues of the 46-kDa major OMP were compared.
Effect of toxR mutation on sensitivity to β-lactam antibiotics.
MICs of selected β-lactam antibiotics against V. anguillarum PT-24 and PT-24ΔtoxR were determined (Table 5). There was no significant difference between PT-24 and PT-24ΔtoxR in susceptibility to imipenem, ceftazidime, and moxalactam (MIC difference, <2-fold). However, PT-24ΔtoxR was less sensitive to cefotaxime and aztreonam than was PT-24 (16- and 4-fold MIC differences, respectively).
TABLE 5.
Susceptibilities of V. anguillarum PT-24 and PT-24ΔtoxR to β-lactam antibiotics
| Strain | MIC (μg/ml) of antibiotica
|
||||
|---|---|---|---|---|---|
| AZT | CAZ | CTX | LMOX | IPM | |
| PT-24 | 3.13 | 12.5 | 0.025 | 3.13 | 3.13 |
| PT-24ΔtoxR | 12.5 | 12.5 | 0.39 | 3.13 | 1.56 |
AZT, aztreonam; CAZ, ceftazidime; CTX, cefotaxime; LMOX, moxalactam; IPM, imipenem.
Effect of toxR mutation on resistance to anionic detergents.
The MBCs of bile, DC, and SDS against PT-24 and PT-24ΔtoxR were determined (Table 6). Those against V. parahaemolyticus AQ3815, a wild-type strain isolated from a patient with diarrhea, and its toxR null mutant, AQ3815ΔToxR, were also determined under the same conditions as a control set (Table 6; discussed below). Mutation in the toxR gene reduced the resistance of V. anguillarum PT-24 to SDS and that of V. parahaemolyticus AQ3815 to bile and SDS.
DISCUSSION
In this study, we demonstrated that the toxR gene is present in V. anguillarum. This provides some evidence to support the hypothesis that the toxR genes are universally distributed in the family Vibrionaceae. Osorio and Klose aligned the amino acid sequences deduced from the partial toxR genes of Vibrio species and Photobacterium species and showed conservation of the homologous sequences corresponding to the presumed transcriptional activation, transmembrane, and periplasmic domains (44). The homologous sequences corresponding to these presumed domains were also detected in the deduced amino acid sequence of V. anguillarum ToxR (indicated by three kinds of overlines in Fig. 1). The results suggest that V. anguillarum ToxR is likely to encode a function(s) shared with the ToxRs of other bacterial species.
The htpG genes of V. cholerae, V. parahaemolyticus, V. vulnificus, and V. hollisae were located immediately upstream of the toxR genes, and the homology between the htpG genes of V. cholerae and V. parahaemolyticus was considerably higher than that between the toxR genes of the two species (29, 47, 63; M. Nishibuchi, J. Okuda, and K. Kumagai, unpublished data). As reported in our previous study on the V. hollisae toxR gene (63), cloning of the Va-toxR gene was achieved by utilizing the Vp-htpG gene probe because the assumption that the htpG and toxR loci are physically linked was valid. It appears very likely that the htpG-toxR gene arrangement is conserved in the family Vibrionaceae and that this cloning strategy will be applicable to cloning of the toxR gene from other members of this family.
There is a growing interest in the methods used to isolate V. anguillarum from the marine environment and to identify isolated strains (3, 31). We showed in our previous studies that the toxR gene is a useful target for the development of genetic identification methods because interspecies homology values of the toxR gene are much lower than those of rRNA genes (26, 63). Other workers have also agreed with this idea (44). Both the DNA probe and PCR methods established in this study specifically detected the toxR gene sequence in V. anguillarum but not in other species of the genus Vibrio (Table 1). The PCR method is more practical than the DNA probe method. The Va-toxR-specific PCR method was also shown to be useful in distinguishing V. anguillarum from other fish-pathogenic bacteria. This PCR method can therefore be useful in identifying V. anguillarum strains isolated from marine environments and diseased fish. Direct application of the V. hollisae toxR-specific PCR method to environmental samples was shown to be helpful in the isolation of V. hollisae (63). Likewise, the Va-toxR-specific PCR method may prove useful for the isolation of V. anguillarum from environmental samples.
We first tried to replace the toxR gene with the mutated Va-toxR gene by using a suicide vector-mediated allelic exchange method with V. anguillarum PT-87050, from which the Va-toxR gene was cloned. However, no transconjugant was obtained after extensive efforts to conjugally transfer pKON25 into PT-87050. As reported by Singer et al. (52), some strains of V. anguillarum have an apparent defect in homologous recombination that limits the use of marker exchange techniques for the construction of specific chromosomal gene mutations. We considered that V. anguillarum PT-87050 is probably defective in homologous recombination. We therefore changed the recipient strain of the conjugation to PT-24 and succeeded in constructing the mutant.
The involvement of the Va-toxR gene in the regulation of virulence and OMP expression was examined by comparing PT-24 and PT-24ΔtoxR. Unlike the findings on V. cholerae, V. parahaemolyticus, and V. vulnificus (27, 29, 33), the toxR gene was not implicated in control of the expression of extracellular virulence factors in V. angullarum PT-24. Furthermore, direct evidence that the toxR gene is not associated with virulence expression was obtained in the pathogenicity tests, in which the natural host, ayu fish, was challenged with the test strains. The immersion method simulates a natural course of infection, and the intramuscular injection method examines the ability of the bacterium to proliferate in vivo and cause septicemia. No remarkable difference was seen between the two test strains in these pathogenicity tests (Fig. 4 and 5). These results are direct evidence that ToxR is not involved in virulence regulation and support the hypothesis that the ancestral role of the toxR gene is not the control of virulence. Rather, the toxR regulation of virulence genes in the three human-pathogenic vibrios may be fortuitous events. The ctx and tcp genes of V. cholerae are carried by bacteriophages and are present almost exclusively in serovars O1 and O139 (23, 24, 64), suggesting that these virulence genes were acquired by these serovars through phage conversion. There is enough evidence suggesting that the tdh gene encoding thermostable direct hemolysin was acquired by V. parahaemolyticus through horizontal transfer (41, 57). In addition, the levels of stimulation by the toxR gene of the V. parahaemolyticus tdh gene and the V. vulnificus hemolysin gene were much lower than that observed for the V. cholerae ctx gene (27, 29, 33). The acquired genes were probably placed under toxR control by different mechanisms in each genetic background after gene transfer. Control of the expression of acquired virulence genes by a preexisting regulator is also known in Salmonella. The PhoP and PhoQ proteins form a two-component global regulatory system in both pathogenic and nonpathogenic organisms. Many horizontally acquired virulence genes seem to have been appropriated so that the expression of the acquired genes is controlled by the PhoP-PhoQ system in Salmonella enterica serovar Typhimurium (15, 16).
OMPs have been examined in four of the reported toxR-bearing species. The toxR gene was shown to be involved in the control of the expression of major OMPs in all cases. Miller and Mekalanos (34) reported that OmpU required the toxR gene for expression and OmpT was expressed maximally in the absence of a functional toxR gene in V. cholerae. Similar observations were made on V. vulnificus (38- and 36-kDa OMPs, respectively; 27) and Photobacterium sp. (OmpL and OmpH, respectively; 65). The 35- and 46-kDa major OMPs of V. anguillarum, respectively, appear to correspond to these toxR-regulated major OMPs. Similarities of the N-terminal sequence among these proteins support their relatedness (Table 4). The 35-kDa major OMP of V. anguillarum PT-24 is homologous to the OMPs of V. cholerae, V. vulnificus, and Photobacterium sp. under positive ToxR regulation, with the V. vulnificus 38-kDa OMP showing the highest homology. The 46-kDa major OMP of V. anguillarum PT-24 was homologous, to some extent, to the OMPs of V. cholerae and Photobacterium sp. under negative ToxR regulation, although higher homologies with the porin family OMPs of other gram-negative bacteria were detected. Furthermore, the abundance of the ToxR-regulated OMPs is influenced by cultural conditions and the toxR gene seems to mediate transfer of the environmental signal to expression of the OMPs (34, 65). The Va-toxR-mediated expression of the V. anguillarum 46-kDa major OMP was influenced by the incubation temperature. toxR-regulated OmpL and OmpH of Photobacterium sp. have been suggested to be porin proteins that might be involved in pressure-mediated alterations in membrane structure in this deep-sea bacterium (65). The 35- and 46-kDa major OMPs of V. anguillarum were postulated to be porin proteins because of the similarity of their N-terminal amino acid sequences to those of well-characterized porin proteins of gram-negative bacteria (Table 4). In particular, the 35-kDa major OMP probably corresponds to the porin-like protein termed Omp35La (55), which is highly related to the 40-kDa major OMP (10). Hydrophilic molecules, such as β-lactam antibiotics, pass the outer membrane through the porin channels. We demonstrated that toxR mutation induced resistance to β-lactam antibiotics such as cefotaxine and aztreonam (Table 5). It is likely that the 35-kDa major OMP forms a porin channel through which these β-lactam antibiotics can penetrate, although the possibility that other ToxR-regulated proteins are involved cannot be ruled out.
Provenzano et al. reported that toxR mutation in human intestinal pathogenic Vibrio species, including V. cholerae, V. mimicus, V. fluvialis, and V. parahaemolyticus, lowered the MBCs of anionic detergents (bile, DC, and SDS) against these species and altered their OMP profiles (49). The authors proposed from these results that the ToxR-dependent OMPs may mediate enhanced resistance to these detergents. The enhanced bile resistance would help human enteric pathogens to persist in the intestinal environment. The toxR mutation reduced the resistance of V. anguillarum PT-24 to SDS but not to bile and DC (Table 6). As a control experiment, we included the wild type and a toxR mutant strain of V. parahaemolyticus AQ3815 that we used in our previous study on ToxR (29). Our results indicated that toxR expression contributes to increased resistance of AQ3815 to bile and SDS but not to DC. The result that the toxR mutant of V. parahaemolyticus did not exhibit lowered resistance to DC in our test could be due to the sensitivity of the MBC determination method. We employed a log2 dilution of the test detergent because the assay using concentrations of the test detergent closer than the log2 difference did not allow us to obtain reproducible results (data not shown). It seems reasonable that toxR expression increased the resistance of V. anguillarum PT-24 to neither bile nor DC because this organism is not an enteric pathogen. Expression of the toxR gene clearly increased the resistance of V. anguillarum and V. parahemolyticus to SDS in our experiment. If ToxR-dependent OMP expressions are associated with the protection of these pathogens against anionic detergents in general, the protection by the OMPs may not always be related to intraintestinal events. Therefore, control of the expression of Omp35La porin and the 46-kDa major OMP may play an important role in the adaptation of V. anguillarum to varying environmental conditions, such as fresh versus marine water, aquatic versus in vivo environments, and the aquatic environment contaminated with antibiotics or detergents.
In conclusion, the results obtained in this study indicate that ToxR regulation of OMP expression is not associated with virulence expression in V. anguillarum PT-24. This supports the hypothesis that the ancestral role of the toxR gene is control of the expression of OMP-encoding genes, so that the members of the family Vibrionaceae can adapt themselves to varying environmental conditions. We suspect that the ToxR regulation of a virulence gene(s) in only several pathogenic Vibrio species reflects the appropriation of ToxR for virulence gene expression.
ACKNOWLEDGMENTS
This research was supported in part by the COE program “Making Regions: Proto-Areas, Transformations and New Formations in Asia and Africa”; by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan; by the “Research for the Future” Program of The Japan Society for the Promotion of Science (JSPS-RFTF97L00706); and by the US-Japan Cooperative Medical Science Program, Japanese Panel.
We are grateful to Yohko Takeda for technical assistance and to Dieter M. Schifferi and Robert Kay for supplying plasmids. We also thank Joon Haeng Rhee for preliminary communication of data.
REFERENCES
- 1.Actis L A, Potter S A, Crosa J H. Iron-regulated outer membrane protein OM2 of Vibrio anguillarum is encoded by virulence plasmid pJM1. J Bacteriol. 1985;161:736–742. doi: 10.1128/jb.161.2.736-742.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Actis L A, Tolmasky M E, Crosa L M, Crosa J H. Characterization and regulation of the expression of FatB, an iron transport protein encoded by the pJM1 virulence plasmid. Mol Microbiol. 1995;17:197–204. doi: 10.1111/j.1365-2958.1995.mmi_17010197.x. [DOI] [PubMed] [Google Scholar]
- 3.Austin B, Alsina M, Austin D A, Blanch A R, Grimont F, Grimont P A D, Jofre J, Koblavi S, Larsen J L, Pedersen K, Tiainen T, Verdonck L, Swings J. Identification and typing of Vibrio anguillarum: a comparison of different methods. Syst Appl Microbiol. 1995;18:285–302. [Google Scholar]
- 4.Casadaban M J, Cohen S N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138:179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- 5.Champion G A, Neely M N, Brennan M A, DiRita V J. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol Microbiol. 1997;23:323–331. doi: 10.1046/j.1365-2958.1997.2191585.x. [DOI] [PubMed] [Google Scholar]
- 6.Crawford J A, Kaper J B, DiRita V J. Analysis of ToxR-dependent transcription activation of ompU, the gene encoding a major envelope protein in Vibrio cholerae. Mol Microbiol. 1998;29:235–246. doi: 10.1046/j.1365-2958.1998.00925.x. [DOI] [PubMed] [Google Scholar]
- 7.Crosa J H. A plasmid associated with virulence in the marine fish pathogen Vibrio anguillarum specifies an iron-sequestering system. Nature. 1980;284:566–568. doi: 10.1038/284566a0. [DOI] [PubMed] [Google Scholar]
- 8.Crosa J H. Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol Mol Biol Rev. 1997;61:319–336. doi: 10.1128/mmbr.61.3.319-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosa J H, Hodges L L. Outer membrane proteins induced under conditions of iron limitation in the marine fish pathogen Vibrio anguillarum 775. Infect Immun. 1981;31:223–227. doi: 10.1128/iai.31.1.223-227.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davey M, Hancock R E W, Mutharia L M. Influence of culture conditions on expression of the 40-kilodalton porin protein of Vibrio anguillarum serotype O2. Appl Environ Microbiol. 1998;64:138–146. doi: 10.1128/aem.64.1.138-146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denkin S M, Nelson D R. Induction of protease activity in Vibrio anguillarum by gastrointestinal mucus. Appl Environ Microbiol. 1999;65:3555–3560. doi: 10.1128/aem.65.8.3555-3560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiRita V J. Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae. Mol Microbiol. 1992;6:451–458. doi: 10.1111/j.1365-2958.1992.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 13.Edwards R A, Keller L H, Schifferli D M. Improved alleic exchange vectors and their use to analyze 987P fimbria gene expression. Gene. 1998;207:149–157. doi: 10.1016/s0378-1119(97)00619-7. [DOI] [PubMed] [Google Scholar]
- 14.Gardel C L, Mekalanos J J. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect Immun. 1996;64:2246–2255. doi: 10.1128/iai.64.6.2246-2255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groisman E A. The ins and outs of virulence gene expression: Mg2+ as a regulatory signal. Bioessays. 1998;20:96–101. doi: 10.1002/(SICI)1521-1878(199801)20:1<96::AID-BIES13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Groisman E A, Ochman H. How Salmonella became a pathogen. Trends Microbiol. 1997;5:343–349. doi: 10.1016/S0966-842X(97)01099-8. [DOI] [PubMed] [Google Scholar]
- 17.Hansen E J, Hasemann C, Clausell A, Capra J D, Orth K, Moomaw C R, Slaughter C A, Latimer J L, Miller E E. Primary structure of the porin protein of Haemophilus influenzae type b determined by nucleotide sequence analysis. Infect Immun. 1989;57:1100–1107. doi: 10.1128/iai.57.4.1100-1107.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hase C, Mekalanos J J. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA. 1998;95:730–734. doi: 10.1073/pnas.95.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inamura H, Muroga K, Nakai T. Toxicity of extracellular products of Vibrio anguillarum. Fish Pathol. 1984;19:89–96. [Google Scholar]
- 20.Inamura H, Nakai T, Muroga K. An extracellular protease produced by Vibrio anguillarum. Bull Jpn Soc Sci Fish. 1985;51:1915–1920. [Google Scholar]
- 21.Inokuchi K, Mutoh N, Matsuyama S, Mizushima S. Primary structure of the ompF gene that codes for a major protein of Escherichia coli K-12. Nucleic Acids Res. 1982;10:6957–6968. doi: 10.1093/nar/10.21.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanemori Y, Nakai T, Muroga K. The role of extracellular protease produced by Vibrio anguillarum. Fish Pathol. 1987;22:153–158. [Google Scholar]
- 23.Karaolis D K R, Johnson J A, Bailey C C, Boedeker E C, Kaper J B, Reeves P R. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci USA. 1998;95:3134–3139. doi: 10.1073/pnas.95.6.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karaolis D K R, Somara S, Maneval Jr D R, Johnson J A, Kaper J B. A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature. 1999;399:375–379. doi: 10.1038/20715. [DOI] [PubMed] [Google Scholar]
- 25.Kay R, McPherson J. Hybrid pUC vectors for addition of new restriction enzyme sites to the ends of DNA fragments. Nucleic Acids Res. 1987;15:2778. doi: 10.1093/nar/15.6.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y B, Okuda J, Matsumoto C, Takahashi N, Hashimoto S, Nishibuchi M. Identification of Vibrio parahaemolyticus strains at the species level by PCR targeted to the toxR gene. J Clin Microbiol. 1999;37:1173–1177. doi: 10.1128/jcm.37.4.1173-1177.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S E, Shin S H, Kim S Y, Kim Y R, Shin D H, Chung S S, Lee Z H, Lee J Y, Jeong K C, Choi S H, Rhee J H. Vibrio vulnificus has the transmembrane transcription activator ToxRS stimulating the expression of the hemolysin gene vvhA. J Bacteriol. 2000;182:3405–3415. doi: 10.1128/jb.182.12.3405-3415.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C C, Crawford J A, DiRita V J, Kaper J B. Molecular cloning and transcriptional regulation of ompT, a ToxR-repressed gene in Vibrio cholerae. Mol Microbiol. 2000;35:189–203. doi: 10.1046/j.1365-2958.2000.01699.x. [DOI] [PubMed] [Google Scholar]
- 29.Lin Z, Kumagai K, Baba K, Mekalanos J J, Nishibuchi M. Vibrio parahaemolyticus has a homolog of the Vibrio cholerae toxRS operon that mediates environmentally induced regulation of the thermostable direct hemolysin gene. J Bacteriol. 1993;175:3844–3855. doi: 10.1128/jb.175.12.3844-3855.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonell M T, Colwell R R. Phylogeny of the Vibrionaceae, and recommendation for two new genera, Listonella and Shewanella. Syst Appl Microbiol. 1985;6:171–182. [Google Scholar]
- 31.Martinez-Picado J, Alsina M, Blanch A R, Cerda M, Jofre J. Species-specific detection of Vibrio anguillarum in marine aquaculture environments by selective culture and DNA hybridization. Appl Environ Microbiol. 1996;62:443–449. doi: 10.1128/aem.62.2.443-449.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 33.Miller V L, Mekalanos J J. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc Natl Acad Sci USA. 1984;81:3471–3475. doi: 10.1073/pnas.81.11.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller V L, Taylor R K, Mekalanos J J. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell. 1987;48:271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- 36.Mizuno T, Chou M Y, Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane: DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983;258:6932–6940. [PubMed] [Google Scholar]
- 37.Munn T W. Haemolysin production by Vibrio anguillarum. FEMS Microbiol Lett. 1978;3:265–268. [Google Scholar]
- 38.Munson R S, Tolan R W. Molecular cloning, expression, and primary sequence of outer membrane protein P2 of Haemophilus influenzae type b. Infect Immun. 1989;57:88–94. doi: 10.1128/iai.57.1.88-94.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muroga K, Egusa S. Vibriosis of ayu: a review. J Fac Appl Biol Sci. 1988;27:1–17. [Google Scholar]
- 40.Mutoh N, Inokuchi K, Mizushima S. Amino acid sequence of the signal peptide of OmpF, a major outer membrane protein of Escherichia coli. FEBS Lett. 1982;137:171–174. doi: 10.1016/0014-5793(82)80341-4. [DOI] [PubMed] [Google Scholar]
- 41.Nishibuchi M, Kaper J B. Thermostable direct hemolysin gene of Vibrio parahaemolyticus: a virulence gene acquired by a marine bacterium. Infect Immun. 1995;63:2093–2099. doi: 10.1128/iai.63.6.2093-2099.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishibuchi M, Taniguchi T, Misawa T, Khaeomanee-iam V, Honda T, Miwatani T. Cloning and nucleotide sequence of the gene (trh) encoding the hemolysin related to the thermostable direct hemolysin of Vibrio parahaemolyticus. Infect Immun. 1989;57:2691–2697. doi: 10.1128/iai.57.9.2691-2697.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norqvist A, Norrman B, Wolf-Watz H. Identification and characterization of a zinc metalloprotease associated with invasion by the fish pathogen Vibrio anguillarum. Infect Immun. 1990;58:3731–3736. doi: 10.1128/iai.58.11.3731-3736.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osorio C R, Klose K E. A region of the transmembrane regulatory protein ToxR that tethers the transcriptional activation domain to the cytoplasmic membrane displays wide divergence among Vibrio species. J Bacteriol. 2000;182:526–528. doi: 10.1128/jb.182.2.526-528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Toole D, Milton L, Wolf-Watz H. Chemotactic motility is required for invasion of the host by the fish pathogen Vibrio anguillarum. Mol Microbiol. 1996;19:625–637. doi: 10.1046/j.1365-2958.1996.412927.x. [DOI] [PubMed] [Google Scholar]
- 46.Overbeeke N, Bergmans H, Van Mansfeld F, Lugtenberg B. Complete nucleotide sequence of phoE, the structural gene for the phosphate limitation inducible outer membrane pore protein of Escherichia coli K12. J Mol Biol. 1983;163:513–532. doi: 10.1016/0022-2836(83)90110-9. [DOI] [PubMed] [Google Scholar]
- 47.Parsot C, Mekalanos J J. Expression of ToxR, the transcriptional activator of the virulence factors in Vibrio cholerae, is modulated by the heat shock response. Proc Natl Acad Sci USA. 1990;87:9898–9902. doi: 10.1073/pnas.87.24.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Provenzano D, Klose K E. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc Natl Acad Sci USA. 2000;97:10220–10224. doi: 10.1073/pnas.170219997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Provenzano D, Schuhmacher D A, Barker J L, Klose K E. The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species. Infect Immun. 2000;68:1491–1497. doi: 10.1128/iai.68.3.1491-1497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reich K A, Schoolnik G K. The light organ symbiont Vibrio fischeri possesses a homolog of the Vibrio cholerae transmembrane transcriptional activator ToxR. J Bacteriol. 1994;176:3085–3088. doi: 10.1128/jb.176.10.3085-3088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon M, Mathes A, Blanch A, Engelhardt H. Characterization of a porin from the outer membrane of Vibrio anguillarum. J Bacteriol. 1996;178:4182–4188. doi: 10.1128/jb.178.14.4182-4188.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singer J T, Ma C, Boettcher K J. Overcoming a defect in generalized recombination in the marine fish pathogen Vibrio anguillarum 775: construction of a recA mutant by marker exchange. Appl Environ Microbiol. 1996;62:3727–3731. doi: 10.1128/aem.62.10.3727-3731.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sperandio V, Bailey C, Giron J A, DiRita V J, Silveira W D, Vettore A L, Kaper J B. Cloning and characterization of the gene encoding the OmpU outer membrane protein of Vibrio cholerae. Infect Immun. 1996;64:5406–5409. doi: 10.1128/iai.64.12.5406-5409.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki S, Kurose K, Kusuda R. Characterization of porin-like major outer membrane proteins of Listonella anguillara serotypes J-O-1, -2 and -3. Biochem Mol Biol Int. 1994;32:605–613. [PubMed] [Google Scholar]
- 55.Suzuki S, Kurose K, Yasue K, Kusuda R. Antigenicity and N-terminal amino acid sequence of a 35-kDa porin-like protein of Listonella (Vibrio) anguillarum: comparison among different serotypes and other bacterial species. Lett Appl Microbiol. 1996;23:303–306. doi: 10.1111/j.1472-765x.1996.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 56.Talaat A M, Reimschuessel R, Wasserman S S, Trucksis M. Goldfish, Carassius auratus, a novel animal model for the study of Mycobacterium marinum pathogenesis. Infect Immun. 1998;66:2938–2942. doi: 10.1128/iai.66.6.2938-2942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terai A, Baba K, Shirai H, Yoshida O, Takeda Y, Nishibuchi M. Evidence for insertion sequence-mediated spread of the thermostable direct hemolysin gene among Vibrio species. J Bacteriol. 1991;173:5036–5046. doi: 10.1128/jb.173.16.5036-5046.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thune R L, Stanley L A, Cooper K. Pathogenesis of Gram-negative bacterial infections in warm water fish. Annu Rev Fish Dis. 1993;3:37–68. [Google Scholar]
- 59.Toranzo A E, Barja J L. Virulence factors of bacteria pathogenic for coldwater fish. Annu Rev Fish Dis. 1993;3:5–36. [Google Scholar]
- 60.Toranzo A E, Barja J L, Colwell R R, Hetrick F M, Crosa J H. Haemagglutinating, haemolytic and cytotoxic activities of Vibrio anguillarum and related vibrios isolated from striped bass on the Atlantic Coast. FEMS Microbiol Lett. 1983;18:257–262. [Google Scholar]
- 61.Trucksis M. Fishing for mycobacterial virulence genes: a promising animal model. ASM News. 2000;66:668–674. [Google Scholar]
- 62.Vieira J, Messing J. The pUC plasmids, and M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 63.Vuddhakul V, Nakai T, Matsumoto C, Oh T, Nishino T, Chen C H, Nishibuchi M, Okuda J. Analysis of the gyrB and toxR gene sequences of Vibrio hollisae and the development of the gyrB- and toxR-targeted PCR methods for the isolation and identification of V. hollisae from the environment. Appl Environ Microbiol. 2000;66:3506–3514. doi: 10.1128/aem.66.8.3506-3514.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 65.Welch T J, Bartlett D H. Identification of a regulatory protein required for pressure-responsive gene expression in the deep-sea bacterium Photobacterium species strain SS9. Mol Microbiol. 1998;27:977–985. doi: 10.1046/j.1365-2958.1998.00742.x. [DOI] [PubMed] [Google Scholar]
- 66.Wertheimer A M, Verweij W, Chen Q, Crosa L M, Nagasawa M, Tolmasky M E, Actis L A, Crosa J H. Characterization of the angR gene of Vibrio anguillarum: essential role in virulence. Infect Immun. 1999;67:6496–6509. doi: 10.1128/iai.67.12.6496-6509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]