Summary
Increasing numbers of transgender individuals are presenting for gender-affirming medical care. For trans women, gender-affirming hormone therapy (GAHT) promotes feminization but also inhibits spermatogenesis. There is a common untested assumption that this inhibition is permanent, resulting in infertility. In this longitudinal study, we report the recovery of viable spermatozoa in nine trans women who stopped GAHT for reproductive purposes. Our preliminary findings suggest that the negative impact of GAHT on spermatogenesis can be reversed, casting doubt on previous claims that GAHT in trans women inevitably leads to permanent infertility. Larger studies are needed to confirm our findings, which have implications not only for fertility counseling and the reproductive options of transgender individuals but also efforts to restrict access to GAHT based on fertility grounds.
Keywords: spermatogenesis, gender-affirming hormones, estrogen, anti-androgens, transgender, fertility, azoospermia
Graphical abstract
Highlights
-
•
This study follows trans women after discontinuation of hormone therapy
-
•
The authors examine the subsequent ability of these women to produce sperm
-
•
Trans women exhibit spermatogenesis after GAHT cessation
-
•
Hormone treatment for trans women does not lead to permanent infertility
Trans women often commence gender-affirming hormonal therapy (GAHT) to promote feminization. But GAHT can also impair fertility. de Nie et al.’s report of successful restoration of spermatogenesis in trans women after discontinuation of GAHT suggests that GAHT’s impact on fertility can be reversed, which has important clinical implications.
Introduction
Increasing numbers of transgender, gender diverse, and non-binary (henceforth, trans) people are seeking hormonal intervention as part of gender-affirming medical care. For trans women, gender-affirming hormone therapy (GAHT) typically consists of estrogen combined with an anti-androgen. Such treatment promotes feminization but is also known to impair spermatogenesis.1 Specifically, multiple studies have observed that most trans women receiving GAHT are not producing mature sperm at the time of gender-affirming surgery.2,3,4,5,6,7,8 Some of these studies reported relatively normal spermatogenesis in a variable minority of trans women (e.g., 11%–40%), but Vereecke et al. found that none of the 97 individuals they studied had histological evidence of complete spermatogenesis at gonadectomy.2,7,8
Current international clinical guidelines therefore recommend that trans women “should be informed about sperm preservation options and encouraged to consider banking their sperm prior to hormone therapy.”9 Such advice seems sensible, especially in light of prominent claims from leaders in the transgender health field that GAHT “eventually results in irreversible infertility.”1 However, proof that GAHT causes “irreversible infertility” requires longitudinal follow up and, importantly, an assessment of whether or not loss of spermatogenesis can be reversed after GAHT is ceased. With that in mind, we identified a small cohort of trans women, each of whom had elected to stop GAHT for reproductive purposes. We assessed their subsequent ability to produce sperm and observed the recovery of spermatogenesis following azoospermia in several individuals.
Results
In total, nine trans women met our study criteria (Table 1). Four of the nine had stopped GAHT to conceive with current partners; the remaining five stopped because they were hoping to bank sperm for potential future reproductive purposes. Their mean age was 26.1 years (range: 18–32 years), and the median duration of GAHT was 36 months (range: 6–216 months). All used estrogen in combination with an anti-androgen (spironolactone or cyproterone acetate). Seven individuals had been on oral estradiol (median dose: 4 mg daily; range: 2–4 mg daily), while the other two had been on topical estradiol.
Table 1.
Clinical characteristics of nine trans women who stopped GAHT and their subsequent semen analyses
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial presentation to gender clinic | Location | The Netherlands | The Netherlands | The Netherlands | The Netherlands | The Netherlands | The Netherlands | Australia | The Netherlands | The Netherlands |
| age at first presentation to gender clinic | 26 | 18 | 25 | 28 | 26 | 32 | 28 | 26 | 27 | |
| age at diagnosis of gender dysphoria | 28 | 21 | 25 | 28 | 27 | 33 | 28 | 27 | 29 | |
| relevant medical history | left testicular torsion | androgenetic alopecia | N/A | commenced hormone treatment on own initiative after purchasing online | started hormone treatment in Lebanon; depression | N/A | N/A | N/A | used oral contraceptives for 2 months in 2012 | |
| concurrent medication | N/A | finasteride 1mg/day (for alopecia) | N/A | N/A | paroxetine 20 mg/day | N/A | N/A | N/A | N/A | |
| baseline serum hormone levels | ||||||||||
| estradiol (pmol/L) | 123 | 72 | 82 | 705 | ND | 95 | 122 | 127 | 124 | |
| testosterone (nmol/L) | 27 | 19 | 15 | <0.5 | ND | 21 | 14.8 | 29 | 33 | |
| LH (IU/L) | 2.8 | 3.6 | 2.4 | <0.1 | ND | 5.2 | 4.2 | 5.1 | 9 | |
| FSH (IU/L) | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Fertility history and previous counseling | previous children | nil | nil | nil | nil | nil | nil | nil | nil | nil |
| reproductive desires prior to commencing GAHT | wish for fertility preservation, wants children in the future. | used illicitly obtained hormones, no prior fertility counseling | not discussed | used illicitly obtained hormones, no prior fertility counseling | used illicitly obtained hormones, no prior fertility counseling | no prior fertility counseling | fertility counseling was provided; not wanting children, planning to adopt; fertility preservation declined | fertility counseling was provided; declined fertility preservation | fertility counseling was provided; declined fertility preservation | |
| prior fertility preservation | semen cryopreservation: 43 vials suitable for ICSI | none | none | none | none | none | none | none | none | |
| Estrogen therapy | age started | 28 | 18 | 23 | 24 | 24 | 33 | 28 | 27 | 30 |
| agent | estradiol patches | estradiol valerate | estradiol valerate | estradiol valerate | estradiol valerate | estradiol valerate | estrodial valerate | estradiol valerate | estradiol valerate | |
| starting dose | 100 mcg/24 h twice a week | 4 mg/day | 2 mg/twice a day | 2 mg/twice a day | 2 mg/twice a day | 2 mg/twice a day | 4 mg/day | 2 mg/twice a day | 2 mg/twice a day | |
| age stopped | 31 | 18 | 41 | 32 | 27 | 36 | 29 | 33 | 33 | |
| dose at cessation | 100 mcg/24 h twice a week | 2 mg/day | estrogel 0.06% 1.25 mg twice a day | estradiol valerate 2 mg /twice a day | estradiol valerate 2 mg/day | estradiol valerate 2 mg/twice a day | 4 mg/day | estradiol valerate 2 mg/twice a day | estradiol valerate 2 mg/twice a day | |
| duration of estrogen therapy (months) | 22 | 6 | 216 | 99 | 36 | 36 | 14 | 56 (period of cessation in 2014) | 36 | |
| Anti-androgen therapy | age started | 28 | 18 | 23 | 24 | 24 | 33 | 28 | 27 | 29 |
| agent | cyproterone acetate | spironolactone | cyproterone acetate | cyproterone acetate | spironolactone | cyproterone acetate | cyproterone acetate | cyproterone acetate | cyproterone acetate | |
| starting dose | 25 mg/day | 100 mg/day | 100 mg/day | 50 mg/day | 100 mg/day | 50 mg/day | 25 mg/day | 50 mg/twice a day | 50 mg/day | |
| age stopped | 31 | 18 | 41 | 32 | 27 | 36 | 29 | 33 | 33 | |
| dose at cessation | 10 mg/day | 100 mg/day | 50 mg/day | 50 mg/day | 100 mg/day | 12.5 mg/day | 25 mg/alternate days | 20 mg/day | 50 mg/day | |
| duration on anti-androgen (months) | 17 | 6 | 216 | 93 | 36 | 36 | 14 | 56 (period of cessation in 2014) | 40 | |
| Gender affirming surgery | None | None | Breast augmentation | Breast augmentation | None | None | None | None | None | |
| Physical response to hormone treatment | physical changes | breast development, decreased muscle strength | unknown | unknown | unknown | unknown | breast development, decreased facial hair growth | Tanner stage 3-4 breast development | breast development | decreased muscle strength, decreased body hair growth, softer skin, breast development |
| subsequent serum hormone levels | ||||||||||
| timing | 20 months after commencement | ND | 204 months after commencement | 93 months after commencement | ND | 15 months after commencement | 13 months after commencement | 24 months after re-initiation of hormones | 36 months after commencement of estradiol | |
| estradiol (pmol/L) | 641 | ND | 120 | 286 | ND | 246 | 556 | 320 | 154 | |
| testosterone (nmol/L) | 0.4 | ND | 0.9 | 0.2 | ND | 4.4 | 0.9 | 0.7 | 0.4 | |
| LH (IU/L) | 0.1 | ND | 0.3 | <0.1 | ND | 1.1 | 0.2 | 1.2 | <0.1 | |
| FSH (IU/L) | n/d | ND | ND | ND | ND | ND | ND | ND | ND | |
| Cessation of anti-androgens/estrogen | rationale | wishes natural conception with new partner | wants to cryopreserve semen to keep all options open | wants to conceive with partner | wants to cryopreserve semen to keep all options open, partner has active child wish | prefers genetically related offspring over adoption | wants to conceive with partner | wants to conceive with partner | wants to cryopreserve semen to keep all options open | strong parental desire |
| physical changes after cessation | increased hair growth and higher volume of ejaculate | unknown | none, no hot flushes, no increased hair growth | acne | increased hair loss/baldness | increased facial hair growth | breast atrophy, testicular volume increase | higher volume of ejaculation, increased sexual function, increased muscle strength | increased hair growth (body and face) | |
| subsequent serum hormone levels | ||||||||||
| timing | 3 months after cessation | ND | ND | ND | 3 months after cessation | 22 months after cessation | 6 months after cessation | 7 months after cessation | 13 months after cessation | |
| estradiol (pmol/L) | 82 | ND | ND | ND | ND | ND | 164 | ND | ND | |
| testosterone (nmol/L) | 17.0 | ND | ND | ND | 18 | 11 | 18.8 | 21 | 16 | |
| LH (IU/L) | 3.1 | ND | ND | ND | ND | ND | 5.2 | 9.2 | 11 | |
| FSH (IU/L) | 9.0 | ND | ND | ND | 3.3 | 6.2 | 17.2 | 19 | 23 | |
| Semen specimen #1 after stopping hormones | time after stopping GAHT (months) | 3 | 3 | 27∗∗ | 4 | 3 | 7 | 1 | 4 | 7 |
| volume (mL) | 1.2 | 4.5∗ | 2.1∗ | 3.5∗ | 1.3 | 3.4∗ | 0.2 | unknown | 1 | |
| sperm concentration (million/mL) | 3.3 | 48∗ | 77.7∗ | 0.2 | 1.2 | 31.3∗ | 0 | 0 | 0 | |
| sperm motility (%) | 20 | 69∗ | 51∗ | 19 | 17 | 40∗ | nil | 0 | 0 | |
| Semen specimen #2 after stopping hormones | time after stopping GAHT (months) | 7 | 3 | N/A | N/A | 3 | 22 | 8 | 7 | 7 |
| volume (mL) | 1.3 | 5∗ | N/A | N/A | 1.7∗ | 1.6∗ | 1 | unknown | 2.5∗ | |
| sperm concentration (million/mL) | 3.8 | 55∗ | N/A | N/A | 0.8 | 22.3∗ | <2 | 0 | 0 | |
| sperm motility (%) | 41∗ | 55∗ | N/A | N/A | 49∗ | 63∗ | 6 | 0 | 0 | |
| Semen specimen #3 after stopping hormones | time after stopping GAHT (months) | 8 | 3 | N/A | N/A | 3 | 22 | 9 | 10 | 13 |
| volume (mL) | 4.8∗ | 6∗ | N/A | N/A | 0.9 | 2.5∗ | 2∗ | 3.2∗ | 3.2∗ | |
| sperm concentration (million/mL) | 20.2∗ | 53∗ | N/A | N/A | 0.4 | 7.1 | <2 | 9 | 0 | |
| sperm motility (%) | 48∗ | 75∗ | N/A | N/A | 56∗ | 13 | 50∗ | 44∗ | 0 | |
| Semen specimen #4 after stopping hormones | time after stopping GAHT (months) | N/A | N/A | N/A | N/A | N/A | 22 | N/A | 11 | N/A |
| volume (mL) | N/A | N/A | N/A | N/A | N/A | 2.2∗ | N/A | 2.7∗ | N/A | |
| sperm concentration (million/mL) | N/A | N/A | N/A | N/A | N/A | 21∗ | N/A | 11.8 | N/A | |
| sperm motility (%) | N/A | N/A | N/A | N/A | N/A | 43∗ | N/A | 36∗ | N/A | |
| Additional follow-up | Patient 1: Partner became pregnant 4 months after cessation of hormone treatment through natural conception Patient 3: Partner became pregnant 40 months after cessation of hormone treatment through natural conception Patient 6: Partner became pregnant 20 months after cessation of hormone treatment through natural conception Patient 9: After failing to identify any mature sperm on repeated semen specimens, further physical examination and investigations were performed. Examination revealed a right testicle volume of 2 ml, left testicle volume of 3 ml and normal consistency on both sides without any abnormalities of the epididymis or vas deferens. Scrotal ultrasound revealed: right testicle volume of 2.0 ml, left testicle volume 3.7 ml, normal testicular structure and vascularisation, normal epididymis. Karyotype was 46XY with no Y chromosome abnormalities seen. Testicular sperm extraction was subsequently performed. Complete spermatogenesis was sporadically encountered in both testicles, and mature spermatozoa were harvested 17 months after stopping hormones. |
|||||||||
The single asterisk (∗) indicates that semen parameters were above the normal WHO reference range for semen volume (≥1.5 mL), sperm concentration (≥15 million/mL), or progressive motility (≥32%).
The double asterisk (∗∗) indicates that Patient 3’s first semen analysis was significantly delayed due to a move across continents, during which time she was unable to be with her partner. They were eventually reunited after which the semen analysis was performed.
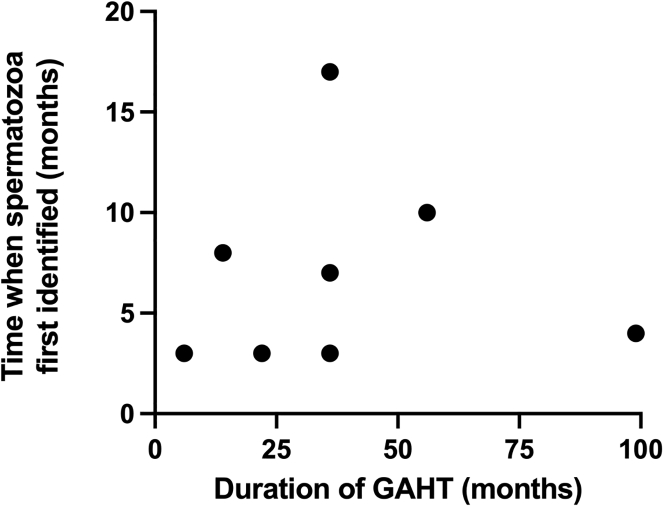
Following cessation of GAHT, viable spermatozoa were eventually documented in all nine cases (Table 1). Initial semen collection and analysis occurred 3–27 months after GAHT was ceased. Six had sperm present on their initial semen analyses, but the other three had documented azoospermia on their initial analyses at 1, 4, and 7 months. Spermatozoa were later recovered from the semen of two of these individuals at 8 and 10 months. For the remaining individual, azoospermia was still present at 13 months, but eventually, testicular sperm extraction at 17 months recovered mature spermatozoa from both testes. Four individuals yielded at least one normal semen analysis; the other four had either low volume, low sperm concentration, and/or low sperm motility (see Table 1 for full description of semen volume, sperm concentration, and motility at each semen analysis). There was no obvious relationship between the duration of GAHT and the timing of when spermatozoa were first identified (Figure 1), although our small sample size and the heterogeneity in the timing of semen collection within the cohort limit any firm conclusions in this regard.
Figure 1.
Relationship between GAHT duration and the time when spermatozoa were first identified after stopping GAHT
Note: patient 3 was excluded from this analysis given that their first semen analysis was significantly delayed (see Table 1).
Three of the four trans women who stopped GAHT to naturally conceive with their partners successfully did so after 4, 20, and 40 months (for the latter individual, it should be noted that she was separated from her partner for a substantial duration after GAHT cessation, which is likely to have contributed to the extended time it took to conceive). For the remaining trans woman, there had not been any report of a natural conception at the most recent follow up 28 months after stopping GAHT.
Discussion
The prevailing dogma in transgender health is that GAHT in trans women “eventually results in irreversible infertility.”1 This view has fueled controversy over the provision of GAHT, especially to adolescents, and is reflected in arguments to support legal efforts to restrict young people’s access to gender-affirming care.10
While there are numerous reports that GAHT impairs spermatogenesis, there is also indirect evidence to suggest that the impact may be transient. For example, Alford et al. recently described a trans woman who—prior to gender-affirming genital surgery and following a course of follicle-stimulating hormone (FSH) and clomiphene—successfully cryopreserved sperm from semen specimens collected 6–10 weeks after GAHT cessation.11 Similarly, Adeleye et al. reported the presence of mature sperm among three trans women for whom GAHT had been withdrawn 3–6 months earlier.12 However, for all four cases, the extent to which spermatogenesis had been previously impaired by GAHT was uncertain. As noted earlier, up to 40% of trans women have evidence of normal spermatogenesis at the time of gonadectomy despite GAHT.8 Thus, it is conceivable that spermatogenesis in each of these four cases had not been impaired. Consequently, these previous reports do not resolve the question of whether spermatogenesis can recover after GAHT cessation.
Our observation that six trans women had sperm identified from their initial semen analyses after GAHT cessation provides further indirect evidence that GAHT-induced impairment of spermatogenesis can be reversible. A priori, the probability that at least one of these six patients had ceased producing mature sperm due to GAHT is very high (p(azoospermia in at least one of the 6 patients) = 1 – p(finding mature sperm in all six patients) = 1 – p(finding mature sperm in one patient)6 = 1 − 0.46 = 99.6%), indicating that spermatogenesis is likely to have recovered in some of these individuals. More noteworthy, though, is our observation that three trans women with documented azoospermia subsequently produced mature sperm afterward. This provides evidence that spermatogenesis can recover following GAHT cessation.
In summary, our data strongly suggest that the impact of GAHT on spermatogenesis can be reversed and cast doubt on claims that GAHT inevitably leads to permanent infertility. Our findings also have important implications for fertility counseling in transgender health. For example, many trans women (or non-binary individuals) receiving feminizing GAHT and who have their gonads intact believe that they are permanently infertile, as do their clinicians. Our observations will help such women make better informed reproductive choices moving forward. Similarly, for trans women (or non-binary individuals) wanting to commence GAHT in the future, our findings may influence their decision-making regarding fertility preservation (e.g., some may be less inclined to freeze their sperm knowing that they may be able to produce sperm should they stop GAHT later on). Nonetheless, we would still recommend sperm cryopreservation prior to GAHT for anyone who might want to be a genetic parent in the future. After all, recovery of spermatogenesis took many months in some cases, during which time testosterone levels increased and are likely to have had negative physical and psychological consequences. Moreover, previous studies found that semen quality is decreased in individuals with a history of GAHT compared with individuals without GAHT.13 This is in line with our observation that invasive testicular sperm extraction was required in one case, and >55% (5/9) of individuals had impaired semen quality after stopping GAHT.
Limitations of the study
There are several important limitations that should be noted. Firstly, there was a lack of data on semen quality before and during GAHT. In this way, it is possible that some of our participants may have had abnormal semen parameters even before treatment, in which case their abnormal semen analysis results after GAHT should be interpreted with caution. Secondly, assessment of hormone levels, such as testosterone, luteinizing hormone (LH), and FSH, following GAHT cessation in our cohort would have been useful as a guide to help indicate the resumption of normal reproductive endocrine function—including at the time of semen collection—but monitoring of these levels was haphazard among our patients. Finally, our sample size was obviously limited and ultimately only included three patients in whom reversible azoospermia could be confirmed. Looking ahead, future studies that include a larger number of such patients will therefore be important to validate and expand upon our findings.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Transgender women’s semen samples | Sexual health clinic in Coffs Harbor, Australia, and Amsterdam UMC, Netherlands | N/A |
Resource availability
Lead contact
Further information and requests should be directed to Ken Pang (ken.pang@mcri.edu.au).
Materials availability
This study did not generate new unique reagents or materials.
Experimental model and human participant details
Human participant details
Trans women were identified based on: i) their attendance at either a sexual health clinic in Coffs Harbor, Australia, or the Center of Expertise on Gender Dysphoria at the Amsterdam UMC, Netherlands; ii) their desire to temporarily stop GAHT for reproductive purposes; and iii) a minimum of at least six months of GAHT use. Informed consent to participate in this study was subsequently obtained from nine eligible participants. As this study adhered to a case series design, no sample size calculation was performed. This study was conducted in accordance with the Medical Ethics Review Committee of VU University Medical Center (IRB00002991).
Method details
Semen samples were collected in accordance with standard sperm banking procedures. This included instructions to patients to ensure a suitable abstinence period (e.g., avoiding intercourse or masturbation for several days before collection), to avoid use of any lubricants, and to deliver samples for analysis within an hour of collection (in any cases where the specimen was produced off-site). Semen characteristics (semen volume, sperm concentration and sperm motility) were manually assessed by qualified clinical laboratory technicians. Volume was determined using a wide-bore volumetric pipette, and sperm concentration and motility were assessed using a Makler counting chamber (Sefi-Medical Instruments LTD, Haifa, Israel) and a phase contrast microscope (200–400x). Consistent with expert andrology advice, patients generally waited several months before re-attempting semen collection if semen analysis revealed azoospermia, since the timeline for sperm production is typically at least two months. Relevant clinical data were extracted from the medical records and included: confirmed gender incongruence, age at commencement of GAHT; type of estrogen and anti-androgen therapy, duration and dose; reasons for stopping GAHT; subsequent semen analysis results; and reproductive outcomes.
Quantification and statistical analysis
Baseline characteristics are presented as mean with SD when normally distributed, and as median with interquartile range (IQR) when non-normally distributed. In Table 1 the characteristics for all participants of this study are displayed and semen parameters that were above the reference values for human semen as determined by the World Health Organization (semen volume (≥1.5mL), sperm concentration (≥15 million/mL) or progressive motility (≥32%)) were indicated with an asterisk. Patient 3’s first semen analysis was significantly delayed due to a move across continents, during which she was unable to be with her partner. They were eventually reunited after which the semen analysis was performed. Therefore, patient 3 was excluded from Figure 1 where the relationship was plotted between GAHT duration and time when spermatozoa were first identified after stopping GAHT.
Acknowledgments
The authors would like to thank Professor Deb Gook for her expert andrology advice and Dr. Anja Ravine for her helpful feedback on the manuscript. K.C.P. also wishes to acknowledge fellowship support from the Hugh Williamson Foundation Trust and the Royal Children's Hospital Foundation.
Author contributions
Conceptualization, K.C.P., N.M.v.M., and I.d.N.; methodology, K.C.P., N.M.v.M., and I.d.N.; validation, K.C.P. and I.d.N.; formal analysis, K.C.P. and I.d.N.; investigation, E.V., I.d.N., A.P., and C.C.; writing – original draft, K.C.P.; writing – review & editing, I.d.N., N.M.v.M., C.C., A.P., M.d.H., A.M., J.H., E.V., and K.C.P.; visualization, C.C., A.P., M.d.H., A.M., J.H., and E.V.; supervision, M.d.H., A.M., and J.H.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: December 20, 2022
Data and code availability
Data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Tangpricha V., den Heijer M. Oestrogen and anti-androgen therapy for transgender women. Lancet Diabetes Endocrinol. 2017;5:291–300. doi: 10.1016/S2213-8587(16)30319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vereecke G., Defreyne J., Van Saen D., Collet S., Van Dorpe J., T'Sjoen G., Goossens E. Characterisation of testicular function and spermatogenesis in transgender women. Hum. Reprod. 2021;36:5–15. doi: 10.1093/humrep/deaa254. [DOI] [PubMed] [Google Scholar]
- 3.Schneider F., Neuhaus N., Wistuba J., Zitzmann M., Heß J., Mahler D., van Ahlen H., Schlatt S., Kliesch S. Testicular functions and clinical characterization of patients with gender Dysphoria (GD) undergoing sex reassignment surgery (SRS) J. Sex. Med. 2015;12:2190–2200. doi: 10.1111/jsm.13022. [DOI] [PubMed] [Google Scholar]
- 4.Schulze C. Response of the human testis to long-term estrogen treatment: morphology of Sertoli cells, Leydig cells and spermatogonial stem cells. Cell Tissue Res. 1988;251:31–43. doi: 10.1007/BF00215444. [DOI] [PubMed] [Google Scholar]
- 5.Matoso A., Khandakar B., Yuan S., Wu T., Wang L.J., Lombardo K.A., Mangray S., Mannan A.A.S.R., Yakirevich E. Spectrum of findings in orchiectomy specimens of persons undergoing gender confirmation surgery. Hum. Pathol. 2018;76:91–99. doi: 10.1016/j.humpath.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Leavy M., Trottmann M., Liedl B., Reese S., Stief C., Freitag B., Baugh J., Spagnoli G., Kölle S. Effects of elevated beta-estradiol levels on the functional morphology of the testis - new insights. Sci. Rep. 2017;7 doi: 10.1038/srep39931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jindarak S., Nilprapha K., Atikankul T., Angspatt A., Pungrasmi P., Iamphongsai S., Promniyom P., Suwajo P., Selvaggi G., Tiewtranon P. Spermatogenesis abnormalities following hormonal therapy in transwomen. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/7919481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang D.D., Swenson E., Mason M., Turner K.R., Dugi D.D., Hedges J.C., Hecht S.L. Effects of estrogen on spermatogenesis in transgender women. Urology. 2019;132:117–122. doi: 10.1016/j.urology.2019.06.034. [DOI] [PubMed] [Google Scholar]
- 9.Coleman E., Bockting W., Botzer M., Cohen-Kettenis P., DeCuypere G., Feldman J., Fraser L., Green J., Knudson G., Meyer W.J., et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int. J. Transgenderism. 2012;13:165–232. doi: 10.1080/15532739.2011.700873. [DOI] [Google Scholar]
- 10.A new push to ban medical treatments for transgender children . 2020. The Economist.https://www.economist.com/united-states/2020/01/30/a-new-push-to-ban-medical-treatments-for-transgender-children [Google Scholar]
- 11.Alford A.V., Theisen K.M., Kim N., Bodie J.A., Pariser J.J. Successful ejaculatory sperm cryopreservation after cessation of long-term estrogen therapy in a transgender female. Urology. 2020;136:e48–e50. doi: 10.1016/j.urology.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Adeleye A.J., Reid G., Kao C.N., Mok-Lin E., Smith J.F. Semen parameters among transgender women with a history of hormonal treatment. Urology. 2019;124:136–141. doi: 10.1016/j.urology.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Wallberg K.A., Häljestig J., Arver S., Johansson A.L.V., Lundberg F.E. Sperm quality in transgender women before or after gender affirming hormone therapy - a prospective cohort study. Andrology. 2021;9:1773–1780. doi: 10.1111/andr.12999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data reported in this paper will be shared by the lead contact upon request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.