Abstract
Streptococcus intermedius is a member of the normal flora of the mouth but is also an opportunistic pathogen associated with purulent infections at oral and nonoral sites. Intermedilysin (ILY) has been shown to be a cytolysin capable of generating pores in the cell membrane of erythrocytes demonstrable by electron microscopy. This effect has been shown to be specific for human cells. Since polymorphonuclear cells (PMNs) are the main cell involved in innate immunity we investigated the effect of purified intermedilysin from Streptococcus intermedius on PMN function. Active ILY at a concentration of 40 ng/μl caused a significant decrease in the number of intact PMNs after 60 min. The active cytolysin, when compared with heat-inactivated ILY, did not appear to be chemotactic for the PMNs but did cause an increase in intracellular calcium, with increased cell surface CD11b expression, metabolic burst, and phagocytosis of Staphylococcus aureus. These findings may have implications for the role of ILY in deep-seated abscesses.
The oral commensal bacterium Streptococcus intermedius is a member of the Anginosus group of streptococci (10, 22) and is associated with endogenous infections leading to abscess formation in the oral cavity and at deep-seated sites, notably in the brain and other head and neck sites (1, 4, 7, 23). Despite the clinical significance of this bacterial species, the determinants for its virulence remain unknown, and no clear correlation between a specific cell product and infection has yet been shown (20). However, recent studies have demonstrated that Streptococcus intermedius secretes a human-specific cytolysin, named intermedilysin (ILY), that directly damages host cells, including human cell lines derived from the organs associated with infections by this streptococcus (18). In the same study, purified ILY was analyzed and shown to be a 54-kDa protein in the mature form, exhibiting between 42 and 71% primary sequence homology against the cholesterol-binding cytolysin (thiol-activated cytotoxin) pneumolysin from Streptococcus pneumoniae, as determined by amino acid sequence homology to five internal ILY peptide fragments.
The ily gene seems to be derived from the same ancestor as thiol-activated cytolysins and membrane pore formation (30 to 50 nm in diameter) in erythrocytes, as observed by electron microscopy (19). However, ILY showed several differences from other thiol-activated cytotoxins, notably a lack of activation by dithiothreitol, resistance to treatments with established inhibitors of this group of toxins, and a greatly reduced level of inhibition in the presence of cholesterol and anti-streptolysin O antibody (18).
Subsequent determination of the distribution of the ily gene within the Anginosus species group has demonstrated that the ily gene exists only in S. intermedius and is present in all strains and that no closely related homologue to the toxin gene is distributed within the other species of the group, namely Streptococcus anginosus and Streptococcus constellatus. Furthermore, assays of ILY hemolytic activity and expression levels revealed by Western blotting using ILY-specific monoclonal antibodies have clearly demonstrated that strains of S. intermedius from deep-seated abscesses express approximately 6- to 10-fold more ILY than do strains from normal habitats, such as dental plaque, or strains obtained from peripheral infection sites. No such pattern of relative expression was observed for the other potential virulence determinants examined, hyaluronidase, chondroitin sulfate depolymerase, and sialidase (neuraminidase) (20). The monospecific distribution of ILY and its specificity for human cells have recently been confirmed in another study (8).
At sublethal concentrations, the related cytotoxin pneumolysin has been shown to have a range of effects on the immune system (16), including the inhibition of respiratory burst in neutrophils and stimulation of interleukin-1 and tumor necrosis factor production from human monocytes (5, 21). Indeed, the effects on host cells of pneumolysin and other related cytolysins, by direct cytotoxic activity or by interference with the defense and immune cell functions, including chemotaxis and migration, respiratory burst, phagocytosis, and other modulations of the immune response, have been catalogued extensively (2). On the basis of the evidence described above, we were interested to determine the effects of sublethal concentrations of ILY on human neutrophils, as these form the major component of the phagocytes present within abscesses (17) and therefore any deleterious effects on human neutrophils by ILY would presumably be instrumental in rendering a substantial part of the local defenses ineffective, a situation which is taken to be the case with abscess formation following infection. The studies reported here were carried out using purified ILY from culture supernatants as part of an ongoing program of research on ILY as a significant virulence factor in the pathogenesis of the serious infections caused by S. intermedius.
Using multiparameter flow cytometric analysis of polymorphonuclear cells (PMNs) in whole blood from normal, healthy subjects, we examined PMN viability, calcium flux, shape change, cell surface CD11b expression, respiratory burst, membrane permeability, chemotaxis, and phagocytosis in the presence of active and heat-inactivated ILY. Whole blood was used, and assays were performed rapidly after venesection to ensure that the PMNs were minimally activated prior to challenge with the ILY (12, 14). S. intermedius is usually found in deep-seated abscesses, and the analysis of PMNs from such sites is not possible. However, ILY may exert its effects on PMNs in the capillaries surrounding the abscess, and the use of whole blood therefore does have a physiological rational. With established methods (13), we have shown that in the presence of active ILY, viable PMN cell numbers are decreased, PMNs are activated with increased expression of CD11b and Staphylococcus aureus phagocytosis, and cell membrane is rendered permeable, resulting in a sustained influx of calcium.
MATERIALS AND METHODS
Samples.
All blood samples were taken into anticoagulant sodium citrate (Vacutainers; BD, Oxford, England) from the anticubital vein of normal volunteers who gave informed consent.
Reagents and equipment.
2,7-Dichlorofluorescein diacetate (DCFDA) and LDS-751 were obtained from Molecular Probes Europe (Leiden, The Netherlands). Tyrodes buffer, Hanks' balanced salt solution, glucose, ethanol, methanol, dimethyl sulfoxide (DMSO), phorbol myristate acetate (PMA), N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP), and Fluo-3 acetoxymethyl ester (Fluo-3 AM) were from Sigma (Poole, England). Phycoerythrin (PE)-conjugated CD11b (PE CD11b) and mouse immunoglobulin G2a isotype control were from BD. Fluorescein isothiocyanate (FITC)-conjugated CD45 (FITC-CD45) and propidium iodide were from Beckman Coulter (High Wycombe, England). Phosphate-buffered saline (PBS), horse blood agar, and digest broth were from Oxoid (Basingstoke, England). RPMI culture medium, Polymorph Prep, and HEPES buffer were from Life Technologies (Paisley, Scotland). Plastic petri dishes, test tube caps, glass microscope slides, coverslips, propan-2-ol, Harris hematoxylin, and cedarwood oil were from Merck. Cellulose nitrate filters (3 μm) were from Sartorius (Epsom, England). Fluorospheres were from Dako (Ely, England). Filter paper circles (8.5 cm) were from Whatman (Maidstone, England). Purified ILY had a protein content of 40 μg/ml and an activity of 5 U/μl, where 1 U is defined as the amount of ILY giving 50% hemolysis of 10 μl of washed erythrocytes (18). Inactivated ILY was prepared by heating at 100°C for 2 min, as ILY has been shown to be inactivated at temperatures above 50°C (18).
Determining hemolytic activity of purified ILY and establishing the appropriate concentration for use in assays with unwashed blood.
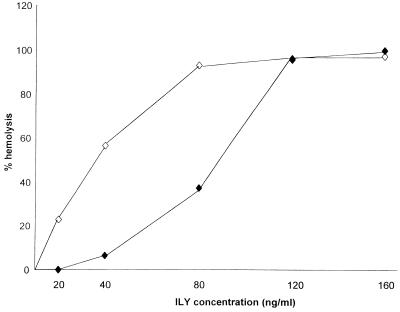
In order to approximate physiological conditions more closely, all experiments were carried out with unwashed but diluted blood. Preliminary observations showed that using 3.5 versus 2.5% whole unwashed blood reduced the hemolytic activity of ILY (see Fig. 1), and subsequently 2.5% unwashed blood was used in all experiments.
FIG. 1.
Percent hemolysis obtained in diluted whole 2.5% (◊) and 3.5% (⧫) unwashed blood when incubated for 1 h with increasing amounts of active ILY. The graph is representative of three similar experiments.
To optimize the concentration of ILY, 25 μl of whole, fresh, unwashed human blood was added to a range of volumes of purified ILY, with the final assay volumes adjusted to 1 ml with PBS. Control tubes were set up in parallel with 25 μl of lysed blood (100% control) and no blood (0% control). Following 1 h of incubation at 37°C, the tubes were centrifuged at 3,000 rpm for 5 min, and the optical density of the resulting supernatants was measured at 540 nm. The percent hemolysis was obtained relative to the 100% control after correction with the 0% control value, and the volume of purified ILY giving approximately 50% hemolysis was determined.
Bacterial cultures.
Staphylococcus aureus Wood 46 was inoculated from a 24-h horse blood agar plate into digest broth supplemented with 0.1% glucose and incubated aerobically (5% CO2) at 37°C for 24 h. The culture was washed six times with Tyrodes buffer. To ensure standard bacterial challenge, the bacteria were fixed in 70% cold ethanol, washed two times with Tyrodes buffer, labeled with propidium iodide (50 μg/ml in saline), and then stored aliquoted at −20°C until required. The aliquoted bacteria were then washed two times with Tyrodes buffer and resuspended to an optical density at 600 nm of 0.6 in Tyrodes buffer. Previous studies in which fixed and unfixed bacteria were used to stimulate PMNs were found to give similar results in terms of shape change, CD62L shedding, and metabolic burst activity (13).
Measurement of neutrophil count.
Aliquots of blood (25 μl) were diluted in 1 ml of PBS, and either active or heat-inactivated ILY 80 (ng) was added. The diluted blood samples were then analyzed at timed intervals (0, 30, and 60 min) on the ADVIA 120 hematology system (Bayer, N.Y.), which generated a differential white cell count.
Measurement of intracellular calcium in neutrophils.
Aliquots of blood (20 μl) were incubated at 37°C for 5 min with 10 μl of 5 mM Fluo-3 AM made in Tyrodes buffer (from a stock 1 M solution in DMSO) and 2 μl of LDS-751 (from a stock saturated solution in methanol) and diluted with 1,967 μl of Tyrodes buffer. A baseline level of fluorescence for the Fluo-3 AM in gated PMNs was established on the flow cytometer, and then 1 μl of either Tyrodes buffer, fMLP (from a 1 mM stock solution), or active or heat-inactivated ILY (40 ng) was added to the reaction mix, and the change in fluorescence intensity of the Fluo-3 AM was measured over time.
Measurement of CD11b expression, shape change, and metabolic burst in neutrophils.
CD11b antigen expression, shape change, and metabolic burst activity were measured in stimulated PMNs as previously described (13). Aliquots of blood (25 μl) were incubated at 37°C with PE-CD11b (10 μl) for 5 min prior to the addition of 965 μl of warm PBS at 37°C containing 0.01% LDS-751 (from a saturated stock solution in methanol) and 1 μM DCFDA (from a stock 10 mM solution in ethanol). The PMNs were then stimulated by the addition of 1 μl of either PBS, PMA (from a 3 mM stock solution in DMSO), or active or heat-inactivated ILY (40 ng). Aliquots (40 μl) of the reaction mix were then taken at timed intervals (0, 10, and 20 min), diluted in 960 μl of ice-cold PBS, and analyzed immediately by flow cytometry. The same batch of anti-CD11b antibodies was used throughout the study.
Measurement of membrane permeability in neutrophils.
Aliquots of blood (25 μl) were incubated at 37°C with FITC-CD45 (10 μl) for 5 min prior to the addition of 965 μl of warm PBS at 37°C. The PMNs were then stimulated by the addition of 1 μl of either PBS, PMA (from a 3 mM stock solution in DMSO), or active or heat-inactivated ILY (40 ng). Aliquots (40 μl) of the reaction mix were then taken at timed intervals (0, 30, and 60 min), diluted in 960 μl of a solution of propidium iodide (50 μg/ml in saline), incubated for 20 min at room temperature, and analyzed within 30 min by flow cytometry. The same batch of CD45 was used throughout the study. In one experiment, changes in forward scatter, CD11b and metabolic burst were measured in a single series of tubes with subsequent addition of propidium iodide and FITC-CD45 to show that the propidium iodide uptake occurred after cell activation.
Measurement of phagocytosis of S. aureus by neutrophils.
Aliquots of blood (150 μl) were incubated at 37°C for 5 min with FITC-CD45 (30 μl). Aliquots of labeled blood (50 μl) were diluted in 950 μl of warm (37°C) Tyrodes buffer before addition of either 3 μl of PBS or active or heat-inactivated ILY (120 ng) for 3 min. Then 500 μl of the diluted blood was added to 500 μl of propidium iodide-labeled S. aureus and incubated in a shaking waterbath at 37°C. At timed intervals (0, 10, and 20 min), aliquots (40 μl) were removed and diluted in 500 μl of ice-cold Hanks' balanced salt solution.
Flow cytometry.
Blood cells were analyzed on a FACScan (BD) equipped with CellQuest software as previously described (14). The instrument had a standard optical filter configuration with band pass 530/30-nm and 585/44-nm filters for FL1 and FL2, respectively and a long-pass 650-nm filter for FL3. For the analysis of forward scatter, metabolic burst, CD11b expression, and calcium mobilization, data were acquired in real time with a primary gate set on a dual-parameter histogram of log red fluorescence intensity of LDS-751 and linear side scatter. This facilitated identification of leukocytes within the blood. A second gate was set around those cells with nuclear fluorescence and side scatter characteristics of PMN cells. Changes in the log green fluorescence and log orange fluorescence together with those of forward light scatter and side light scatter were then recorded on the gated PMNs. For analysis of phagocytosis and cell membrane permeability, PMNs were identified in a plot of FITC-CD45 and linear side scatter. Changes in the log green-red fluorescence were then recorded on the gated PMNs when appropriate spectral compensation was made for PE emissions entering the FL3 (LDS-751) channel and LDS-751 emissions entering the FL2 (PE) channel and similarly for the FITC (FL1) and PE (FL2) spectral overlap. This was facilitated by single-stained preparations of PMNs. The changes in PMN size (forward scatter), metabolic burst activity (FL1), and CD11b (FL2) expression were recorded at 0, 10, and 20 min, with the change in membrane permeability (FL3) recorded at 0, 30, and 60 min. Median channel values were used to determine changes in these parameters.
For the measurement of phagocytosis, the increase above background levels at time zero of the percentage of red fluorescent (FL3) neutrophils was recorded at 10 and 20 min. The change in calcium mobilization (FL1) was recorded continuously over a period of 512 s. The flow cytometer was calibrated before use with Fluorosphere beads labeled with green, orange, and red fluorochromes. Although diluted whole blood was used, each sample for analysis contained at least 10,000 PMNs. We have previously investigated the reproducibility of the live whole blood procedure by multiple analysis (five times) of a normal blood sample. These studies showed that the values obtained for the percentage of positively stained cells, their mean-median fluorescence intensity, and the coefficient of variation varied minimally (<0.05%) when between 1,000 and 7,000 gated cells were counted.
Chemotaxis assay.
Blood (10 ml) was taken into a plastic tube containing preservative-free heparin (100 μl). Blood (3.5 to 5.0 ml) was layered over 3.5 ml of Polymorph Prep in a 15-ml conical tube at room temperature. The tube was centifuged at 500 × g for 20 min at 20°C. The PMN cells were then collected and washed once by centifugation at 400 × g for 20 min in RPMI medium containing 20 mM HEPES buffer. The cells were resuspended in the same medium to give a cell count of 106/ml. Filter paper circles were soaked in either RPMI with 20 mM HEPES for random locomotion, 4% casein for a positive control, diluted active ILY, diluted heat-inactivated ILY (equivalent to 40 ng/ml), 4% casein with active ILY, or 4% casein with heat-inactivated ILY and placed in sterile petri dishes. Strips of cellulose nitrate paper were then placed over the soaked filter papers, and 10-mm-diameter test tube caps were filled with cell suspension and then inverted over the nitrocellulose strips. The petri dishes were incubated in a damp chamber at 37°C for 1 h. The cellulose nitrate strips were then removed, washed with PBS, fixed in propan-2-ol for 10 min, hydrated in water, and stained with Harris hematoxylin for 30 min. They were then washed in water, fixed twice in propan-2-ol for 10 min each, and immersed in cedar oil until clear. The filters were mounted on slides with cedar oil under large coverslips. The distance traveled by the neutrophils was measured using the leading-front method, in which the starting surface is identified by microscopy. The field of focus was then moved down through the cell-filled area in the filter until the furthest two to five cells were in focus. The distance traveled by the neutrophils was measured on a micrometer. A minimum of three fields in each filter were examined. Movement of PMNs by random locomotion through the filters was very small, and the leading front of normal cells in the positive control filter was usually 50 to 100 μm from the starting surface.
Statistical analysis.
PMN size, CD11b expression, metabolic burst activity, propidium iodide uptake, and phagocytic ability in the presence of buffer or active or heat-inactivated ILY were compared using parametric statistics and Student's paired t test.
RESULTS
Determining hemolytic activity of purified ILY and optimizing the flow cytometry experimental conditions.
A plot of the percent hemolysis for 25 and 35 μl of blood in PBS at a total volume of 1 ml and volume of ILY added is shown in Fig. 1. For convenience, a volume of 1 μl (40 ng) of ILY with 25 μl of blood was chosen for use in experiments. This reaction mix gave suboptimal hemolysis and represented the equivalent of 5 U of ILY.
Measurement of neutrophil count.
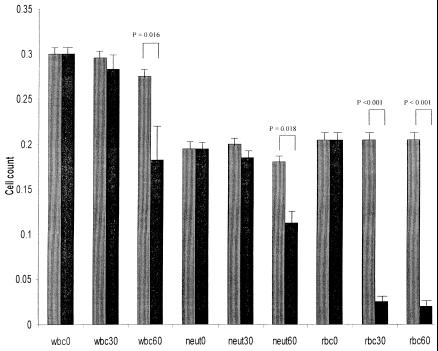
In blood incubated with active ILY, the number of erythrocytes decreased significantly (P < 0.01) within the first 30 min. There was also a decrease in the number of white cells at 60 min (P = 0.016) compared to time zero, and this was predominantly due to a decrease in the number of neutrophils (P = 0.018) (Fig. 2), although small decreases in the number of monocytes and lymphocytes were also observed (data not shown).
FIG. 2.
Decrease in white blood cell (wbc), neutrophil (neut), and red blood cell (rbc) counts (109/liter) in diluted whole blood at 0, 30, and 60 min after the addition of 1 μl (40 ng, 5 U) of active (black bars) or heat-inactivated (gray bars) ILY.
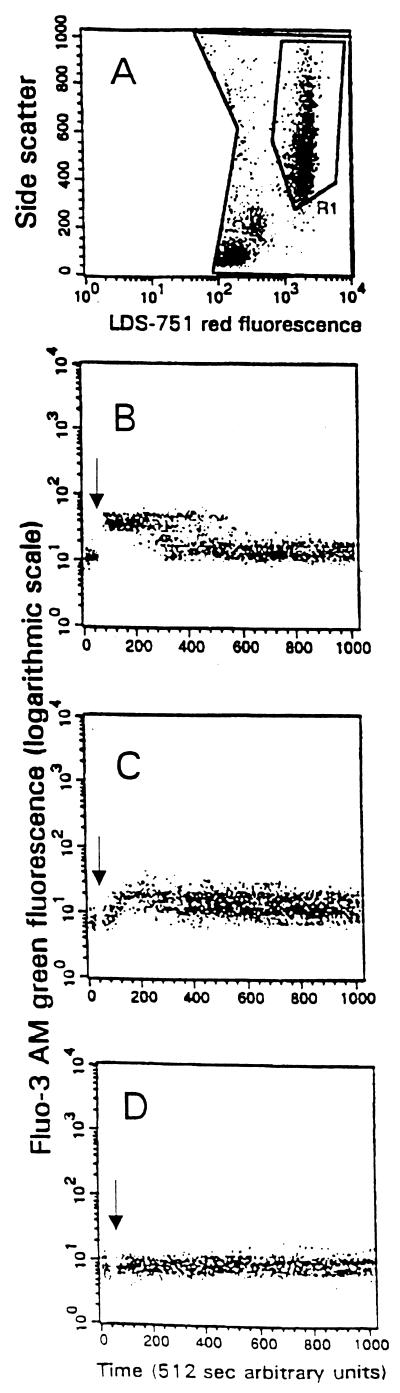
Measurement of intracellular calcium in neutrophils.
PMNs were identified by their side scatter and nuclear red fluorescence characteristics in region R1 of histogram A (Fig. 3). The green fluorescence associated with Fluo-3 AM within the PMNs was recorded over time in histograms B, C, and D. A baseline level of fluorescence was established, and then the agonist, fMLP, active ILY, or heat-inactivated ILY, in histograms B, C, and D, respectively, was added (denoted by the arrow). Histogram B shows a rapid increase in fluorescence intensity of Fluo 3 with a rapid decrease back to the baseline level, whereas in histogram C there is a slower increase in fluorescence intensity which is maintained over the whole time period. In histogram D there is no change in the fluorescence intensity, showing that there had been no increase in intracellular calcium concentration. Addition of PBS gave results similar to those in histogram D (data not shown).
FIG. 3.
(A) Identification of PMNs in region R1 of a dot plot of LDS-751 red fluorescence (x axis, logarithmic scale) plotted against Fluo-3 AM green fluorescence (y axis, logarthmic scale). (B, C, and D) Change in fluorescence of Fluo-3 AM resulting from increased intracellular calcium when fMLP or active or heat-inactivated ILY (40 ng) was added, respectively.
Measurement of CD11b expression, shape change, and metabolic burst in neutrophils.
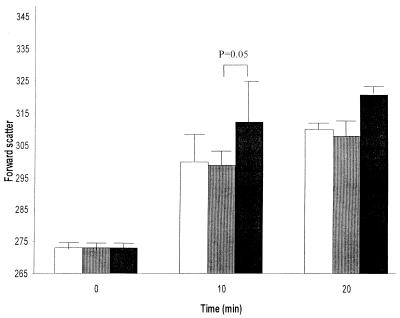
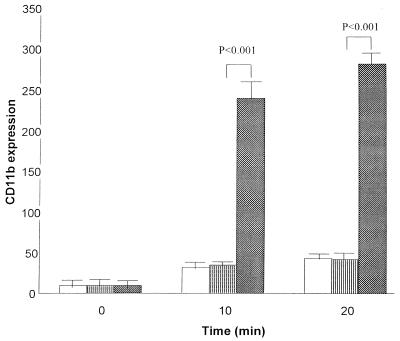
PMNs in diluted whole blood, when mixed with PBS or active or heat-activated ILY, exhibited an increase in size, as measured by forward scatter over the 20-min incubation period (Fig. 4). This was greater in PMNs incubated with active ILY than in PMNs incubated with heat-inactivated ILY or PBS (P = 0.05). Similarly, a significant (P < 0.01) increase in the expression of CD11b, as determined by fluorescently labeled antibody binding, occurred in PMN cells incubated with active ILY compared to those incubated with heat-inactivated ILY or PBS at 10 and 20 min (Fig. 5).
FIG. 4.
Change in forward scatter (median values) of PMNs in diluted whole blood at 0, 10, and 20 min after the addition of 1 μl of PBS (open bars) or active (black bars) or heat-inactivated (gray bars) ILY (40 ng). The mean and standard error of four experiments are shown.
FIG. 5.
Change in CD11b antigen expression (median fluorescence values) of PMNs in diluted whole blood at 0, 10, and 20 min after the addition of 1 μl of PBS (white bars) or active (stippled bars) or heat-inactivated (striped bars) ILY (40 ng). The mean and standard error of four experiments are shown.
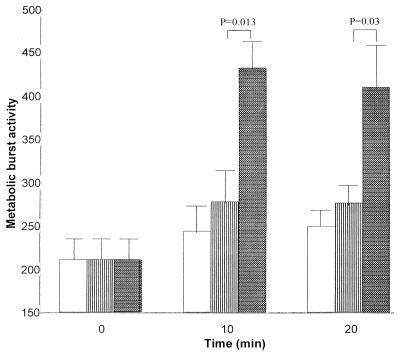
The metabolic burst activity was measured as an increase in fluorescence associated with the dye DCFDA. This dye has low fluorescence in its native state and is readily taken up by PMNs. However, when it is oxidized by H2O2 and other reactive oxygen species produced during a metabolic burst, it becomes more highly fluorescent. The increase in fluorescence may then be used as a measure of the metabolic burst. A significant (P < 0.01) increase in the metabolic burst activity occurred in PMNs incubated with active ILY compared with those incubated with heat-inactivated ILY or PBS at 10 min (Fig. 6). In PMNs incubated with active ILY, the metabolic burst was reduced at 20 min compared to that at 10 min.
FIG. 6.
Change in metabolic burst activity (median fluorescence values) of PMNs in diluted whole blood at 0, 10, and 20 min after the addition of 1 μl of either PBS (white bars) or active (stippled bars) or heat-inactivated (striped bars) ILY (40 ng). The mean and standard error of four experiments are shown.
Measurement of membrane permeability.
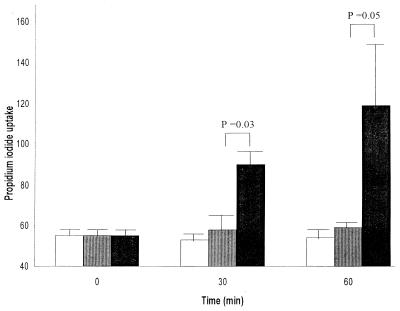
Propidium iodide is a fluorescent dye that is excluded from cells with intact membranes. However, alteration in permeability of the cell membrane would allow the dye to enter the cell, which would then become fluorescent. PMNs in diluted whole blood incubated with active ILY for 60 min showed a significant (P = 0.05) increase in propidium iodide uptake compared to cells in diluted blood incubated with heat-inactivated ILY or buffer (Fig. 7), suggesting that the ILY had induced an alteration in cell membrane permeability.
FIG. 7.
Change in propidium iodide uptake (median fluorescence values) of PMNs in diluted whole blood at 0, 30, and 60 min after the addition of 1 μl of either PBS (white bars) or active (black bars) or heat-inactivated (grey bars) ILY (40 ng). The means and standard errors of four experiments are shown.
Measurement of phagocytosis of S. aureus by neutrophils.
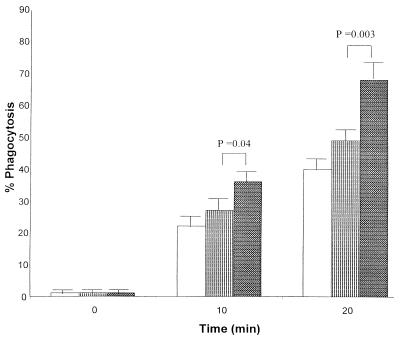
When incubated with either PBS or active or heat-inactivated ILY, PMNs were able to phagocytose propidium iodide-labeled S. aureus. However, this phagocytosis was considerably greater in PMNs incubated with active ILY than in those incubated with heat-inactivated ILY or buffer at 10 min (P = 0.04) and at 20 min (P < 0.01) (Fig. 8).
FIG. 8.
Change in phagocytic activity as a percentage of the positive control values of PMNs in diluted whole blood at 0, 10, and 20 min after the addition of 1 μl of either PBS (white bars) or active (dark grey bars) or heat-inactivated (light grey bars) ILY (40 ng). The means and standard errors of four experiments are shown.
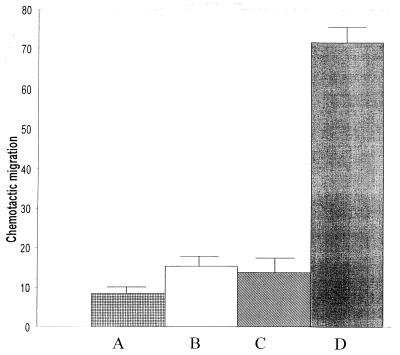
Measurement of chemotaxis.
Active ILY acted as a weak chemoattractant for PMNs compared to RPMI (P = 0.01), but this effect was not found to be significantly different from that of the heat-inactivated ILY (Fig. 9). Neither the active nor heat-inactivated form of ILY had an inhibitory effect on the chemotactic effect of casein (data not shown).
FIG. 9.
Chemotactic activity (micrometers of migration) of PMNs towards (A) RPMI, (B) active ILY, (C) heat-inactivated ILY (40 ng), and (D) casein.
DISCUSSION
In virtually all vertebrates, the first blood-borne cells to arrive at sites of tissue injury or inflammation are the PMNs. Together with monocytes and tissue macrophages, they carry out many of the major functional responses of the innate immune system (15). Among the most important functional responses of activated PMNs is the ability to rearrange their actin cytoskeleton and move toward inflammatory sites (6). This process, referred to as diapedesis, is required for PMNs to be able to exit the microvasculature and enter the inflammatory site. Coincident with rearrangement of the actin cytoskeleton, activated PMNs are able to assemble the subunits of NADPH oxidase at the plasma membrane and undergo a respiratory burst, resulting in release of superoxide plus a range of other reactive oxygen species. In addition, activated PMNs release their granule constituents, including hydrolytic enzymes, metal-binding proteins, and peroxidases (3). The degranulation process also results in the fusion of granule membranes with the cytoplasmic membrane, providing the activated neutrophil with a rich source of new cell surface receptors that can interact with environmental stimuli (24).
The process of adhesion to cell surfaces also augments the ability of PMNs to phagocytose pathogens and foreign particles. In the present study, we have investigated the effect of ILY on many of these cellular changes that occur when PMNs are activated. It has been documented that ILY has a lytic effect on human erythrocytes and cell lines (18). Here we show that it is also capable of decreasing the number of neutrophils in diluted whole blood over a 60-min time period. Although the exact mechanism of this cell loss requires further investigation, the finding that PMNs become more permeable to propidium iodide in the presence of ILY suggests that changes in the integrity of the cell membrane may play a role in the cell loss. Similarly, a related toxin, pneumolysin, has also been shown to cause a drop in PMN number and viability in cultures incubated at 37°C for 30 min (9).
It was also shown that the membrane changes were calcium permissive, since in the presence of active ILY there was an increase in intracellular calcium binding to Fluo 3, resulting in increased fluorescence associated with this dye. The increase in fluorescence was sustained, indicating a continual influx of calcium through the cell membrane or mobilization from intracellular stores. In contrast, in PMNs stimulated with fMLP, there was a rapid increase in the amount of free intracellular calcium, which then returned to baseline levels. No calcium flux was observed in PMNs incubated with heat-inactivated ILY. In a previous study of the effect of aerolysin, a pore-forming toxin produced by Aeromonas hydrophilia, a similar calcium response was observed in HL-60 cells (11). In that study, the calcium influx occurred in two kinetically distinct phases, an initial rapid and transient phase and a second, more sustained phase. These changes in intracellular calcium were found to be the result of pore formation and not due to signal transduction. The first phase was found to be due to pore formation in the cell membrane, and the second phase was due to release from intracellular stores.
Although ILY did not cause much change in the size of the PMNs, as measured by the forward light scatter properties, it did stimulate rapid expression of CD11b at the membrane surface and a metabolic burst. CD11b is a membrane constituent of specific granules, gelatinase granules, and secretory vesicles within the PMN. The mobilization of the secretory vesicles transforms the PMN from a passive cell, well suited for circulation, to a highly responsive, β2-integrin-presenting cell primed for migration into tissues. Elevation of intracellular calcium levels is known to elicit exocytosis of storage granules, but the molecular mechanism by which this occurs is unknown (3). The influx of calcium resulting from the effects of active ILY may therefore have been the main impetus for the observed degranulation.
The generation of reactive oxygen metabolites is also dependent on the components of granules and their degranulation. Both peroxidase-negative granules, which contain flavocytochrome b558 (an essential component of NADPH oxidase), and azurophil granules, which contain myeloperoxidase (which converts the product of NADPH oxidase, H2O2, to hypochlorous acid), are involved in the metabolic burst. The metabolic burst in response to active ILY may be a result of elevated intracellular calcium but may also be due to other stimulatory effects. The observed decrease in metabolic burst between 10 and 20 min of stimulation may be due to an inhibitory effect or to loss of the DCFDA dye from the cell cytoplasm as a result of loss in cell membrane integrity. The latter is supported by the cell membrane's becoming permeable to propidium iodide, indicating that dyes were able to pass into and therefore out of the PMNs. DCFDA is a nonpolar molecule that diffuses through the cell membrane, and once inside, acetyl groups attached to the molecule are cleaved by nonspecific esterases to generate a polar molecule which becomes trapped within the cells. With a change in membrane permeability, the DCFDA may be able to pass out of the cell. Paton and Ferrante (21) have shown that pneumolysin, a toxin produced by S. pneumoniae, had an inhibitory effect on PMN metabolic burst, and Johnson et al. (9) found that the same toxin caused increased lysosomal enzyme secretion and presumably an increased metabolic burst, since the two events occur concomitantly. These contradictory findings may in part reflect the different techniques used for both the isolation and functional analysis.
Interestingly, the rate of phagocytosis of S. aureus by PMNs in the presence of active ILY was significantly greater than in PMNs incubated with heat-inactivated ILY. This may in part have been due to the higher expression of CD11b on PMNs incubated with active ILY. CD11b is a receptor for the complement component C3bi and enhances phagocytosis by binding to complement-immunoglobulin complexes on the bacterial cell surface membrane (24).
The apparent limited chemotaxis of PMNs in the presence of ILY was consistent with the small changes in the size of the cells determined by flow cytometry. As mentioned above, cytoskeletal actin rearrangement occurs prior to diapedesis after stimulation by a chemoattractant. A previous study has suggested that the toxins aerolysin, α-toxin, and streptolysin O were highly chemotactic for PMNs and that the response was as great as for fMLP (11). However, our study and that study are not directly comparable because different positive control chemotractants were used, casein and fMLP. In another study (9), pneumolysin was found to stimulate chemotaxis' at low concentrations but was inhibitory at high concentrations. Cytolytic toxins therefore appear to have variable effects on the chemotaxis of PMNs.
In conclusion, ILY appears not to act as a chemotactic agent but does enhance CD11b expression, metabolic burst activity, and phagocytosis. The changes in CD11b and metabolic burst activity may be the result of the PMNs' becoming permeant, with a continuous influx of Ca2+, the rate of phagocytosis then being enhanced by the increased expression of CD11b on the PMNs. We do not know the concentration of ILY that is present during infections with S. intermedius, but it may be postulated that during abscess formation, ILY could contribute to bacterial survival and persistence by reducing the number of fully functional PMNs. The observation that the rate of phagocytosis of S. aureus was enhanced in the presence of ILY may indicate that this cytotoxin better serves S. intermedius from within the PMN once phagocytosis has taken place, as a mechanism for avoiding subsequent intracellular killing. Clearly, further studies are required to determine whether the observed changes in the cell membrane of PMNs are due to pore formation, which has been observed in erythrocytes, or some other mechanism. All the studies described here were performed on purified ILY, but different responses may have been elicited by the cytolysin when bound to the bacterial cell. Experiments are currently being performed to compare the effect of the wild type compared to an ily gene knockout mutant of S. intermedius on PMN function.
ACKNOWLEDGMENT
We acknowledge the Royal London Hospital Special Trustees Grant.
REFERENCES
- 1.Bantar C, Canigia L F, Relloso S, Lanza A, Bianchini H, Smayevsky J. Species belonging to the “Streptococcus milleri” group: antimicrobial susceptibility and comparative prevalence in significant clinical specimens. J Clin Microbiol. 1996;34:2020–2022. doi: 10.1128/jcm.34.8.2020-2022.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billington S J, Jost B H, Songer J G. Thiol-activated cytolysins: structure, function and role in pathogenesis. FEMS Microbiol Lett. 2000;182:197–205. doi: 10.1016/s0378-1097(99)00536-4. [DOI] [PubMed] [Google Scholar]
- 3.Borregaard N, Cowland J B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 4.Gomez-Garces J-L, Alosand J-I, Cogollos R. Biologic characteristics and antimicrobial susceptibility of 70 clinically significant isolates of Streptococcus milleri group. Diagn Microbiol Infect Dis. 1994;19:69–73. doi: 10.1016/0732-8893(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 5.Houldsworth S, Andrew P W, Mitchell T J. Pneumolysin stimulates production of tumour necrosis factor alpha and interleukin-1β by human mononuclear phagocytes. Infect Immun. 1994;62:1501–1503. doi: 10.1128/iai.62.4.1501-1503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imhof B A, Dunon D. Basic mechanism of leucocyte migration. Horm Metab Res. 1997;29:614–621. doi: 10.1055/s-2007-979112. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs J A, Pietersen H G, Stobberinghand E E, Soeters P B. Streptococcus anginosus, Streptococcus constellatus and Streptococcus intermedius clinical relevance, hemolytic and serologic characteristics. Am J Clin Pathol. 1995;104:547–553. doi: 10.1093/ajcp/104.5.547. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs J A, Scot C S, Scouls L M. Haemolytic activity of the ‘Streptococcus milleri group’ and relationship between haemolysis restricted to human red blood cells and pathogenicity in S. intermedius. J Med Microbiol. 2000;49:55–62. doi: 10.1099/0022-1317-49-1-55. [DOI] [PubMed] [Google Scholar]
- 9.Johnson M K, Boese-Marrazzo D, Pierce W A. Effects of pneumolysin on human polymorphonuclear leukocytes and platelets. Infect Immun. 1981;54:171–176. doi: 10.1128/iai.34.1.171-176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawamura Y, Hou X-G, Sultana F, Miura H, Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships amongst members of the genus Streptococcus. Int J Syst Bacteriol. 1995;45:406–408. doi: 10.1099/00207713-45-2-406. [DOI] [PubMed] [Google Scholar]
- 11.Krause K, Fivaz M, Monod A, van der Goot F G. Aerolysin induces g-protein activation and Ca2+ release from intracellular stores in human granulocytes. J Biol Chem. 1998;273:18122–18129. doi: 10.1074/jbc.273.29.18122. [DOI] [PubMed] [Google Scholar]
- 12.Macey M G, McCarthy D A, Newland A C. The ex vivo function and expression of function associated antigens of peripheral blood neutrophils and monocytes. Exp Hematol. 1994;22:967–972. [PubMed] [Google Scholar]
- 13.Macey M G, McCarthy D A, Howells G L, King G, Newland A C. Multiparameter flow cytometric analysis of polymorphonuclear leucocytes in whole blood from patients with adult rapidly progressive periodontitis reveal low expression of the adhesion molecule L-selectin CD62L. Cytometry. 1998;34:152–158. doi: 10.1002/(sici)1097-0320(19980615)34:3<152::aid-cyto5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy D A, Macey M G. A simple flow cytometric procedure for the determination of surface antigens on unfixed leucocytes in whole blood. J Immunol Methods. 1993;163:155–160. doi: 10.1016/0022-1759(93)90117-p. [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R, Janeway C A., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell T J. Toxins. Journal of Applied Microbiology Symposium Supplement 1998. Vol. 84. London, England: The Society for Applied Microbiology, Blackwell Science; 1998. The role of streptococcal toxins in disease; pp. 119S–124S. [DOI] [PubMed] [Google Scholar]
- 17.Muir R. Chapter 4. In: Anderson J R, editor. Textbook of pathology, 12th ed. London, England: Edward Arnold; 1989. pp. 4–29. [Google Scholar]
- 18.Nagamune H, Ohnishi C, Katsuura A, Fushitani K, Whiley R A, Tsuji A, Matsuda Y. ILY, a novel cytotoxin secreted by Streptococcus intermedius UNS46 isolated from a human liver abscess. Infect Immun. 1996;64:3093–3100. doi: 10.1128/iai.64.8.3093-3100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagamune H, Ohnishi C, Katsuura A, Taoka Y, Fushitani K, Whiley R A, Yamashita K, Tsuji A, Matsuda Y, Maeda T, Korai H, Kitamura S. A cytolytic toxin specific for human cells of a Streptococcus intermedius isolated from human liver abscess. In: Horaud T, Bouvet A, Leclercq R, de Montclos H, Sicard M, editors. Streptococci and the host. Advances in experimental medicine and biology. 418. Proceedings of the XIIIth Lancefield International Symposium on Streptococci and Streptococcal Diseases. New York, N. Y: Plenum Press; 1997. pp. 773–775. [PubMed] [Google Scholar]
- 20.Nagamune H, Whiley R A, Goto T, Inai Y, Maeda T, Hardie J M, Kourai H. Distribution of the intermedilysin gene among the anginosus group streptococci and correlation between intermedilysin production and deep-seated infection with Streptococcus intermedius. J Clin Microbiol. 2000;38:200–226. doi: 10.1128/jcm.38.1.220-226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paton J C, Ferrante A. Inhibition of human polymorphonuclear leukocyte respiratory burst, bacteriocidal activity, and migration by pneumolysin. Infect Immun. 1983;41:1212–1216. doi: 10.1128/iai.41.3.1212-1216.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whiley R A, Beighton D. Emended descriptions and recognition of Streptococcus constellatus, Streptococcus intermedius, and Streptococcus anginosus as distinct species. Int J Syst Bacteriol. 1991;41:1–5. doi: 10.1099/00207713-41-1-1. [DOI] [PubMed] [Google Scholar]
- 23.Whiley R A, Beighton D, Winstanley T G, Fraser H Y, Hardie J M. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus, the Streptococcus milleri group: association with different body sites and clinical infections. J Clin Microbiol. 1992;30:243–244. doi: 10.1128/jcm.30.1.243-244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams M A, Solomkin J S. Integrin-mediated signalling in human neutrophils. J Leukoc Biol. 1999;65:725–736. doi: 10.1002/jlb.65.6.725. [DOI] [PubMed] [Google Scholar]