Abstract
Background:
We aimed to determine the association between maternal infections during pregnancy with risk of Attention-Deficit Hyperactivity Disorder (ADHD) in children.
Methods:
A systematic literature search was performed utilizing the online databases PubMed, Scopus, and Web of Sciences up to July 2020. Random-effects meta-analyses were applied to estimate pooled relative risk (RR). Heterogeneity, study quality and publication bias were assessed through I2 value, Newcastle–Ottawa scale (NOS) and Egger’s test, respectively.
Results:
Thirteen articles involving 1401904 mother-child pairs were included. The result of meta-analysis showed that the risk of ADHD increased by 30% among children whose mothers took any infections during pregnancy (pooled RR=1.30, 95% CI: 1.14–1.49; I2=85.5, P<0.001). Overall, the included studies were good in quality and no publication bias was found (P=0.23, Egger’s test).
Conclusion:
Maternal infections during pregnancy might be associated with an increased risk of ADHD in children.
Keywords: Maternal infection, Pregnancy, Disorder, Hyperactivity
Introduction
Attention-deficit hyperactivity disorder (ADHD) is one of the most common psychiatric diagnosis in both children and adults. It affects approximately 5–10% of school-age children (1) with a worldwide prevalence of 7.2% (95% confidence interval [CI]: 6.7 to 7.8) (2). However, it argued that the statistics for the morbidity of ADHD is along with the degree of bias because there is the degree of underestimation and overestimation in these statistics (3, 4). ADHD may be a risk factor for several adverse outcomes in children, youth and adults such as comorbid mood disorders, substance abuse and dependency (5–7), suicidal behavior (8), social and functional impairments (9), academic performance (10), unintentional injury and mortality (11).
Risk factors of ADHD are the constellation of genetic and environmental factors. Regarding genetic background, several studies aimed to estimate heritability (12) and risk gene identification (13). There are several well-known environmental risk factors for ADHD, e.g. socioeconomic status (14), heavy metals exposures (15), brain injury (16), low birth weight (17) and prenatal smoking (18, 19) and alcohol (20).
One of the prenatal risk factors of ADHD that attracts notable attention is maternal infection during pregnancy (21–23). Several studies have demonstrated that neurodevelopmental disorders may be due to exposure to maternal infection during pregnancy (22, 24, 25). In other words, in utero exposure to infections may influence fetal brain development and following short and long-term effects on function (26). Studies on whether maternal infection during pregnancy may influence the risk of ADHD in children have increased recently and they are still controversial. For example, the findings suggest a null, positive and negative association between maternal infection during pregnancy and ADHD (21–23).
Therefore, we aimed to evaluate the association between maternal infection during pregnancy and the risk of ADHD among children using a systematic review and meta-analysis.
Methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) (27).
This research was approved by the Research Ethics Committee of Hamadan University of Medical Sciences (Ethical code: IR.UMSHA.REC.1401.662).
Search strategy and Study Selection
Potential articles were retrieved from three electronic databases including PubMed, Scopus and Web of Sciences up to July 2020. The search strategy was created using the following MeSH terms and keywords: (“infection” [Mesh Terms] OR “infection” [All Fields] OR “maternal infection”) AND (“Attention Deficit Disorder with Hyperactivity” [Mesh Terms] OR “Attention Deficit Disorder with Hyperactivity” [All Fields] OR “Attention deficit hyperactivity disorder” [All Fields] OR “ADHD” [All Fields] “attention deficit disorder” [All Fields] OR “hyperactivity disorder” [All Fields]) AND (“Pregnancy” [Mesh Terms] OR “Pregnant” [All Fields] OR “Gestational” [All Fields]) AND (“Child” [Mesh Terms] OR “Pediatrics” [Mesh Terms] OR “child” [All Fields] OR “children” [All Fields] OR “children” [All Fields]). Then, this search strategy was modified for search in other electronic databases. The search strategy was restricted to articles in English. The results of initial searches were screened by one author (EA) based on titles and abstract, and then potential eligible articles for inclusion in the systematic review and Meta-analyses were assessed by two authors (EA and KM). Any disagreement was resolved with discussion.
Study eligibility
Observational studies investigating the effect of maternal infection during pregnancy on the ADHD were considered. To be eligible, studies had to report the relative risks (RRs) including odds ratio (OR), risk ratio (RR) and hazard ratio (HR) and their corresponding confidence intervals (CIs) or at least to report data for manually calculating the RRs and CIs. Case reports, small case series, animal and lab studies, reviews, irrelevant original studies, letters and correspondences, editorials, and conference proceedings were excluded.
Data extraction and quality assessment
Data were extracted independently by two authors (EA and KM), with any discrepancies discussed; the extracted data were as follow; first author, year of publication, country, study design, sample size, male % among ADHD cases, age of ADHD cases, type of maternal infection during pregnancy, time of infection ascertainment in the study, the method to confirm maternal infection during pregnancy, the method to ADHD assessment, crude and adjusted RRs and list of confounders in multivariable analyses.
The methodological quality of included cohort and case-control studies was criticized using the Newcastle-Ottawa Scale (NOS) (28) and a modified version of the NOS was used for cross-sectional studies (29). The NOS criticize the quality of each study according to a) selection of study groups (4 items), b) comparability of study groups (2 items) and c) exposure and outcome measurement (3 items). The highest quality study is awarded up to 9 scores. The modified form of NOS assesses the “selection of study groups” in 5 items and the maximum score is 10 for a study. The two authors EA and KM evaluated the quality of included studies separately. Any discrepancies were resolved after discussion and consensus.
Statistical analysis
Heterogeneity across studies was evaluated using I2 value where I2 value over 50% was considered as substantial between-study heterogeneity. In case of significant substantial heterogeneity, Pooled RRs with the corresponding 95% CIs were estimated using a random-effects model, otherwise fixed-effect model was used to estimate pooled association. A forest plot was generated to present the results of pooled associations. Since the study outcome was being expected to be rare among both exposed and unexposed, we approximated pooled RRs by ORs and HRs from included studies (30). Publication bias was checked by visual inspection of the funnel plot and Egger’s test. All analysis were conducted using Stata SE version 11. P <0.05 was considered as a significant level.
Results
Study selection
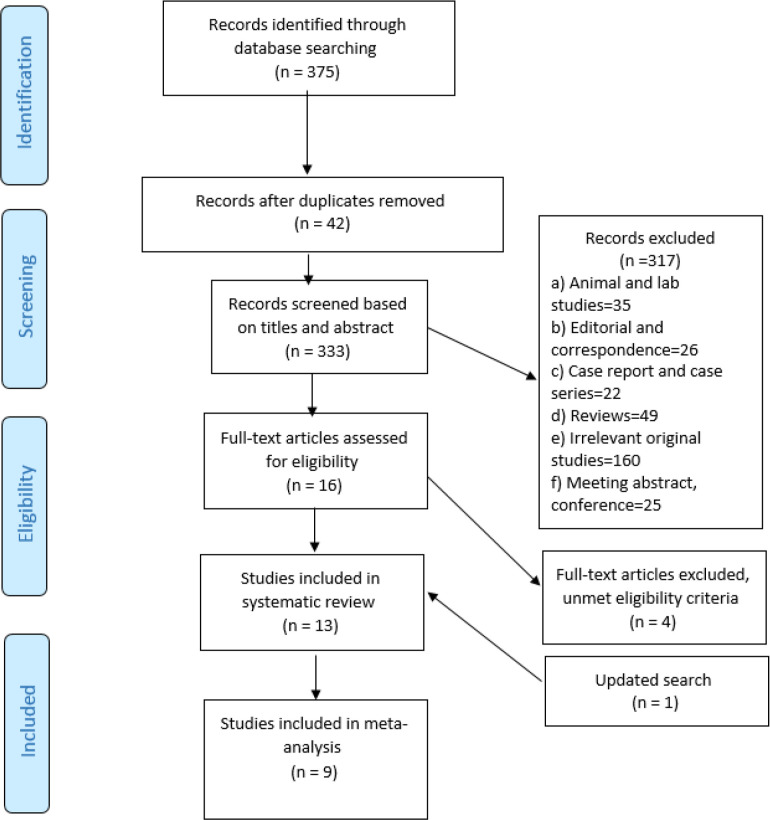
Fig. 1 is the PRISMA flow chart of the study selection process. Initial searches yielded 375 potential articles. After removing 42 duplicate records, 333 articles were screened by titles and abstracts, of which 16 articles met the eligibility criteria. Of them, four articles (23, 31–33) were discarded because did not meet eligibility criteria. After an update search, one other article was retrieved and finally, 13 articles (16, 21, 22, 34–43) were considered for the systematic review and of these, 9 articles (16, 21, 22, 36–39, 42, 43) provides adjusted RRs on association between maternal infection during pregnancy and ADHD and they met the criteria for the meta-analysis.
Fig. 1:
PRISMA flow diagram showing study selection process
Characteristics of included studies in systematic review
Table 1 present characteristics of included studies in systematic review and meta-analysis. This systematic review included five case control (16, 35, 36, 41, 42), five cohort (21, 22, 37–39) and three cross sectional studies (34, 40, 43) that published in English between 2005 and 2019. The total sample size of included studies in the systematic review was 1401904 with a wide range from 92 (34) to 1,066,956 (38). All of the included studies examined ADHD as the study outcome while in one study (37) considered attention problems as an additional outcome. Five studies (16, 21, 22, 36, 38) ascertained maternal infection in pregnancy period, 6 studies (34, 35, 40–43) at time of ADHD diagnosis, one study (37) at time of delivery, and another in both during pregnancy and postpartum period (39). Maternal infection during pregnancy was assessed with different type of methods e.g. in 9 studies (22, 34–36, 39–43) based on mothers’ recall through questionnaires or interviews, in 2 (16, 38) using medical records, in one study (21) with International Classification of Diseases, Ninth Revision (ICD-9) and in another study (36) with examining gestational maternal C-reactive protein (CRP) as a biological marker of maternal infection.
Table 1:
Characteristics of included studies in systematic review and meta analyses
| First author Year of publication | Study design | Sample size | Male % among ADHD cases | Age of ADHD cases | Infection during pregnancy | Method to confirm infection | Method to ADHD assessment | uRR (95% CI) | aRR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Afsharpaiman 2016 (35) | Cross sectional | 92 | 66.7% | Mean (SD): 8.12 (3.25) years | Unknown infections | questionnaire | DSM-IV-TR | 1.00 * (0.06, 16.48) | |
| Arpino 2005 (36) | Case control | 189 | 77.5% | range=6–11 years | Exanthematic infection (i.e. measles, varicella, or rubella) | questionnaire | DSM-IV-TR | 5.26 * (0.81, 34.23) †† | |
| Chudal 2019 (37) | Nested case control | 2158 | 85.4% | Mean (SD): 7.3 (1.9), range: 2–14 | Unknown infection | C-reactive protein (CRP) assayed in stored maternal sera | ICD-10 codes F90.0, F90.1, F90.8, F90.9 | 1.03 * (0.95, 1.11) | 1.05 * (0.96, 1.11) |
| Downey 2015 (38) | Cohort | 826 | 58% | range: 0–2 | Mycoplasma recovered from placenta | interview | DSM-IV-TR | 2.3 *** (1.1, 4.8) | |
| Fever | 14.83 * (4.4, 50.00) | ||||||||
| Urinary tract infection | 16.85 * (8.09, 35.07) | ||||||||
| Vaginal/cervical infection | 26.01 * (12.48, 54.22) | ||||||||
| Periodontal disease | 24.03 * (4.31, 133.76) | ||||||||
| One organism recovered from placenta | Interview | Child Behavior Checklist (CBCL)-defined attention problem † | 2.2 *** (1.3, 3.8) | ||||||
| 2+ organisms recovered from placenta | Child Behavior Checklist (CBCL)-defined attention problem † | 0.9 *** (0.5, 1.7) | |||||||
| Fever | Child Behavior Checklist (CBCL)-defined attention problem † | 14.66 * (4.68, 45.88) | |||||||
| Urinary tract infection | Child Behavior Checklist (CBCL)-defined attention problem † | 17.05 * (8.48, 34.28) | |||||||
| Vaginal/cervical infection | Child Behavior Checklist (CBCL)-defined attention problem † | 12.38 * (5.85, 26.16) | |||||||
| Periodontal disease | Child Behavior Checklist (CBCL)-defined attention problem † | 12.98 * (2.14, 156.52) | |||||||
| Ginsberg 2019 (39) | Cohort | 1066956 | 51.3% | Unknown infections | Medical records | ICD-9 code 314.00, ICD-10 code F90 | 1.03 ** (0.76, 1.41) | ||
| Gustavson 2019 (40) | Cohort | 99947 | Mean:11 range (7–17 | Fever | questionnaire | ICD-10 code F90.0 | 1.45 (1.29, 1.62 ) | 1.30 (1.15–147) | |
| Mann 2011 (21) | Cohort | 84721 | Mean: 6.49 | 71.9% | Genitourinary infections | ICD-9 codes | ICD-9 codes 314.00 314.01 | 1.12 * (1.07, 1.17) | 1.29 * (1.23, 1.35) |
| Candidiasis | 1.01 * (0.9, 1.13) | 1.22 (1.08, 1.37) | |||||||
| Chlamydia/NGU | 1.1 * (0.85, 1.43) | 1.47 (1.12, 1.92) | |||||||
| Gonorrhea | 0.83 * (0.63, 1.08) | 0.99 (0.75, 1.31) | |||||||
| Trichomoniasis | 0.97 * (0.85, 1.1) | 1.26 (1.10, 1.45) | |||||||
| Urinary tract infection | 1.17 * (1.11, 1.24) | 1.25 (1.18, 1.33) | |||||||
| genitourinary infections and preeclampsia | 1.53 * (1.32, 1.77) | ||||||||
| Mellins 2009 (47) | Cross sectional | 340 | perinatally HIV-infected | questionnaire | DSM-IV-TR | 2.45 * (1.2, 4.99) | |||
| Oerlemans 2016 (45) | Case control | 493 | Mean (SD): 11.8 (2.4), range:4–20 | 87.1% | Unknown infections | questionnaire | standardized questionnaires and diagnostic interviews | 1.41 * (0.31, 6.37) | |
| Pineda 2007 (43) | Case control | 486 | Mean (SD): 7.9 (1.5), range:6–11 | 74.5% | urinary infections along other physical illness | questionnaire | Diagnostic Interview for Children and Adolescents (DICA) and the Behavior Assessment System for Children (BASC | 4.20 * (1.70, 10.0 ) | 5.0 * (2.0, 12.9) |
| Severe maternal respiratory infection (flu) | 3.3 * (1.7, 6.4) | 3.1 * (1.5, 6.3) | |||||||
| Syphilis | 1.43 * (0.14, 13.90) | ||||||||
| Measles, Varicella, Rubella | 2.40 * (0.31, 18.35) | ||||||||
| toxoplasmosis | 1.43 * (0.14, 13.9) | ||||||||
| Schmitt 2012 (48) | Cross sectional | 13488 | Mean (SD): 9.8 (4.3), range:3–17 | 78.8% | Unknown infections along other perinatal health problems | Interview | ICD-10 hyperkinetic disorder | 1.88 * (1.6, 2.22) | 1.69 * (1.40, 2.03) |
| Silva 2014 (16) | Case control | 43062 | 77.1% | Urinary tract infection | Medical records | ICD-10 and DSM-IV-TR | 1.37 * (1.21, 1.54) for male | 1.26 * (1.11, 1.44) for male | |
| 1.51 * (1.21, 1.90) for female | 1.33 * (1.04, 1.70) for female | ||||||||
| Werenberg Dreier 2016 (22) | Cohort | 89146 | Fever | Interview | ICD-10 codes DF90.0 DF90.9 | 1.09 (0.99, 1.19) | 1.03 (0.93, 1.13) | ||
| Any infection | 1.09 ** (0.99, 1.19) | 1.01 ** (0.92, 1.11) | |||||||
| Genitourinary infections (cystitis, pyelonephritis, vaginal symptoms) | 1.22 ** (1.11, 1.34) | 1.14 ** (1.03, 1.25) | |||||||
| Persistent viral infections (orofacial herpes infection, genital herpes, condylomas) | 1.03 ** (0.91, 1.16) | 1.01 ** (0.89, 1.14) | |||||||
| Prolong Cough | 1.15 (1.03, 1.29) | 1.05 ** (0.94, 1.19) | |||||||
| Diarrhea | 1.09 (0.99, 1.20) | 0.98 ** (0.89, 1.09) | |||||||
| Other infection | 0.99 ** (0.87, 1.13) | 0.97 ** (0.84, 1.11) |
odds ratio
hazard ratio
risk ratio
outcome studied is attention problem
odds ratio was estimated using adding 0.5 to each cell of 2×2 contingency table
In the all included studies, ADHD was assessed using ICD or Diagnostic and Statistical Manual of Mental Disorders (DSM) while in 2 studies (41, 42) ADHD was confirmed using standardized questionnaires and diagnostic interviews. The results of the quality assessment have demonstrated that overall, the quality of the included study was good e.g., the NOS score of studied included in the meta-analyses was equal to or above 7. The detail of quality assessment is presented in Table 2.
Table 2:
Results of the quality assessment
| Author (yr) | Items | Total NOS stars | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Selection | Comparability | Outcome/exposure | |||||||
| Cohort | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Downey (2015) | * | * | * | * | * | * | * | ******* | |
| Ginsberg (2019) | * | * | * | * | ** | * | * | * | ********* |
| Gustavson (2019) | * | * | * | ** | * | * | * | ******** | |
| Mann (2011) | * | * | * | * | ** | * | * | ******** | |
| Werenberg Dreier (2016) | * | * | * | ** | * | * | ******* | ||
| Case controls | |||||||||
| Oerlemans (2016) | * | * | * | * | ** | * | * | ******** | |
| Pineda (2007) | * | * | * | * | ** | * | ******* | ||
| Silva (2014) | * | * | * | * | ** | * | * | * | ********* |
| Arpino (2005) | * | * | * | * | * | * | ****** | ||
| Chudal (2019) | * | * | * | * | ** | * | * | ******** | |
| Cross sectional studies | |||||||||
| Afsharpaiman (2016) | ** | * | * | **** | |||||
| Mellins (2009) | * | * | ** | * | * | ****** | |||
| Schmitt (2012) | * | * | * | ** | ** | * | ******** | ||
Cohort studies: 1. Representativeness of the exposed cohort, 2. Selection of the non-exposed cohort, 3. Ascertainment of exposure, 4. Demonstration that outcome of interest was not present at start of study, 5. Comparability of cohorts on the basis of the design or analysis controlled for confounders, 6. Assessment of outcome, 7. Was follow-up long enough for outcomes to occur, 8. Adequacy of follow up of cohorts
Case control studies: 1.Is the case definition adequate?, 2. Representativeness of the cases, 3. Selection of Controls, 4. Definition of Controls, 5. Comparability of cases and controls on the basis of the design or analysis, 6. Ascertainment of exposure, 7. Same method of ascertainment for cases and controls, 8. Non-Response rate
Cross sectional studies: 1. Representativeness of the sample, 2. Sample size, 3. Non-respondents, 4. Ascertainment of the exposure (risk factor), 5. The subjects in different outcome groups are comparable, based on the study design or analysis, 6. Assessment of the outcome, 7. Statistical test
Association between maternal infection during pregnancy and ADHD
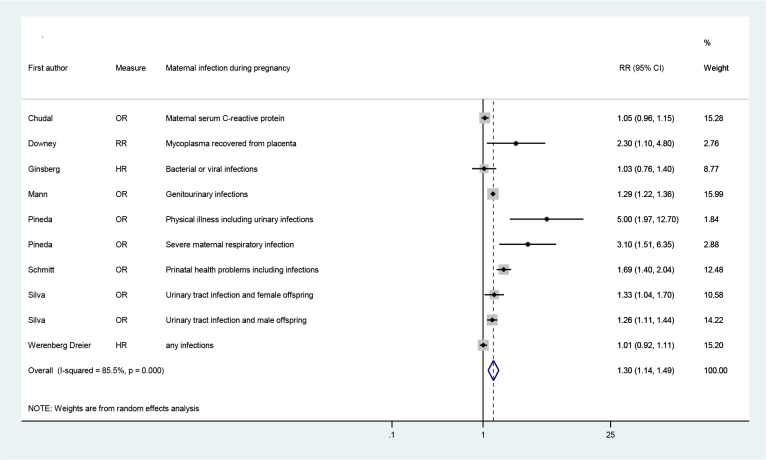
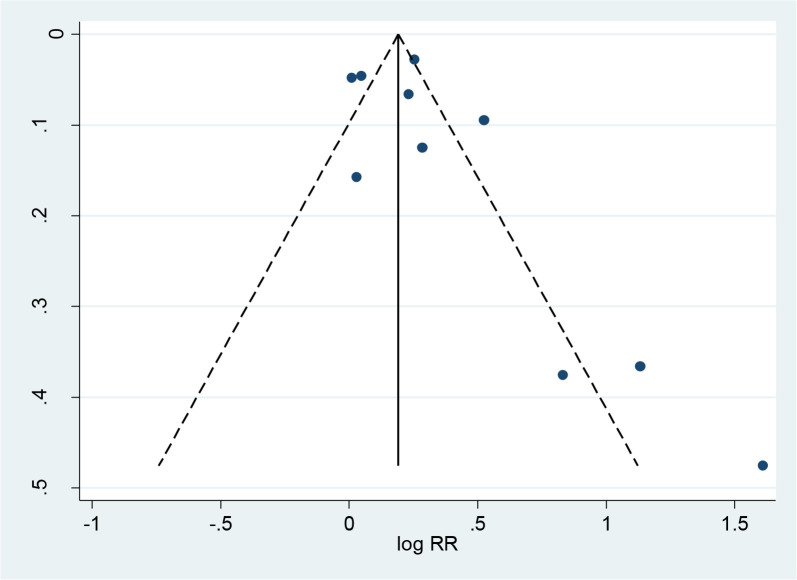
Using a random-effects model with adjusted RRs from 8 eligible studies (16, 21, 22, 36–38, 42, 43) that aimed to evaluate association between maternal infection during pregnancy and ADHD (not including fever), a pooled RR (95% CI of 1.30 (1.14, 1.49) have been yielded (I2=85.5%, p<0.0001) (Fig. 2) and evidence of symmetric funnel plot and no publication bias (p=0.23, Egger’s test, P-value=0.23 and Begg’s test, P-value=0.32) (Fig. 3). The pooled effect estimate did not change when adding adjusted RR for fever during pregnancy from Gustavson et al. (39) to the meta-analysis (overall RR=1.30; 95% CI=1.15, 1.46; I2=84.1%, P-value<0.001) (Forest plot not shown).
Fig. 2:
The overall effect of maternal infection during pregnancy on the risk of ADHD in children
Fig. 3:
Funnel plot assessment of publication bias
Discussion
The present study aimed to review systematically and meta-analysis published studies evaluating the effect of maternal infection during pregnancy on the risk of ADHD in children. Our pooled analysis from observational studies results suggests that maternal infection during pregnancy was significantly associated with a 30% increased risk of ADHD in the children.
Although the biological mechanisms underlying the association between maternal infection during pregnancy and ADHD still not completely defined, however, the finding from both animal studies (26) and observational studies on the association between maternal infection during pregnancy and neurodevelopmental disorders (24, 25) suggested that maternal infection during pregnancy might disrupt some aspects of fetal brain development and the children’s function. The pooled effect measures from meta-analysis of observational studies should be interpreted with caution due to ignoring the many possible sources of biases. Here, we acknowledge that the estimated pooled RR for the association between maternal infection during pregnancy and ADHD may be different from that in reality due to several reasons. First, among the included studies especially in case-control studies, the information about maternal infection during pregnancy was measured by mothers’ recall. This increase the risk of a special type of misclassification bias, known as recall bias. In other words, mothers of children with ADHD are likely to remember infections they took during pregnancy differently than mothers of normal children. Second, the multivariable model across the included studies for evaluating the association between maternal infection during pregnancy and ADHD were heterogonous and most of them did not consider all potential covariates in maternal infection during pregnancy-ADHD pathway.
It argued that potential covariates in maternal infection during pregnancy-ADHD pathway can be as follow; maternal education, calendar year of birth, pre-pregnancy body mass index (BMI), children in household, maternal history of psychiatric disease, maternal age, maternal stress, maternal smoking, birth weight, gestational age at birth, antipyretics (22). Some of them are potential confounders; covariates affecting both maternal infections during pregnancy and ADHD e.g. maternal age and pre-pregnancy BMI, and some others are mediators; covariates that are more likely affected by the maternal infection during pregnancy e.g. birth weight. Of included studies, some studies e.g. Ginsberg et al. (38) and Werenberg Dreier et al. (22) have attempted to adjust possible potential covariates. Finally, in some cases, maternal infection included in meta-analyses as a composite variable. For example in Pineda et al. study (42) maternal infection was evaluated with other exposures e.g. with other physical illness. Here, it is not known how large a proportion of the exposed is truly exposed to infections.
We considered fever during pregnancy from Gustavson et al. study (39) because they have mentioned that infection is the most common cause of fever and they have no attempted to identify microbial causes of fever. Hence, we still believe that the association between fever and other maternal infection indicators during pregnancy and ADHD in children should be evaluated in an independent and comprehensive systematic review with considering issues such as dose response association, episodes in different trimester etc.
Among the included studies, most infection during pregnancy was genitourinary infection. In a study (44), maternal genitourinary infections during pregnancy were important risk factors for autism spectrum disorders in children. Genitourinary infection is a composite variable; however, the risk of ADHD may be different across various genitourinary infections because various pathogens may have specific effects on the risk of ADHD. Since most genitourinary infections are bacterial, general immune activation measures including CRP and cytokines (interleukins) may be more trigged and those resulted in changes in brain morphology (45). On the other hands, genitourinary infections may mediate several factors such as low birth weight (LBW) (46) which is well-established risk factor for ADHD (47).
We found the association between maternal infection during pregnancy and ADHD to be similar to other meta-analysis that evaluated the association of maternal infection during pregnancy with adverse outcomes in children such as with autism spectrum disorders (pooled OR=1.13, 95% CI: 1.03–1.23) (44) type 1 diabetes mellitus (pooled OR=1.31, 95% CI: 1.07, 1.62) (48), fetal congenital heart diseases (pooled OR, 2.28; 95% CI: 1.54, 3.36) (49), stillbirth (pooled RR=2.36, 95% CI: 1.05–5.31) and low birth weight (RR=1.71, 95% CI: 1.03–2.84) (50).
Our study had several strengths: The systematic review and meta-analysis include over 1,400,000 mother-child pairs that resulted in pooled measures with high precision. However, our study has several limitations that should be considered. The degree of selection bias may exist in this systematic review because the initial search may not be comprehensive coverage of all potential literature. For example, of included studies, there were few studies from low and middle-income countries or we included only literature in English. Moreover, it is needed the results to be updated with including published articles during 2020 and 2021. Another limitation is that no information was available on several important variables such as type of infection, maternal infection during the different trimester etc. Finally, pooled estimates from Meta-analysis of observational studies do not necessarily reflect causal associations.
Conclusion
There was a significant increase in the risk of ADHD among children whose mothers took infections during pregnancy. Future larger and unbiased researches are needed to explore the association between various infectious agents and risk of ADHD separately. Moreover, the association between maternal infection during pregnancy and other neurodevelopmental disorders should be studied.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This research was financially supported by the Hamadan University of Medical Sciences, Hamadan, Iran (with No.140109017199).
Footnotes
Conflict of interests
The authors declare that they have no competing interests.
References
- 1.Faraone SV, Sergeant J, Gillberg C, et al. (2003). The worldwide prevalence of ADHD: is it an American condition? World Psychiatry, 2(2):104–13. [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas R, Sanders S, Doust J, et al. (2015). Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics, 135(4): e994–e1001. [DOI] [PubMed] [Google Scholar]
- 3.Bruchmuller K, Margraf J, Schneider S. (2012). Is ADHD diagnosed in accord with diagnostic criteria? Overdiagnosis and influence of client gender on diagnosis. J Consult Clin Psychol, 80(1):128–38. [DOI] [PubMed] [Google Scholar]
- 4.Cuffe SP, Moore CG, McKeown RE. (2005). Prevalence and correlates of ADHD symptoms in the national health interview survey. J Atten Disord, 9(2):392–401. [DOI] [PubMed] [Google Scholar]
- 5.Biederman J, Petty CR, Monuteaux MC, et al. (2010). Adult psychiatric outcomes of girls with attention deficit hyperactivity disorder: 11-year follow-up in a longitudinal case-control study. Am J Psychiatry, 167(4):409–17. [DOI] [PubMed] [Google Scholar]
- 6.Grazioli VS, Gmel G, Rougemont-Bucking A, et al. (2019). Attention deficit hyperactivity disorder and future alcohol outcomes: Examining the roles of coping and enhancement drinking motives among young men. PloS One, 14(6): e0218469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGough JJ, Smalley SL, McCracken JT, et al. (2005). Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry, 162(9):1621–7. [DOI] [PubMed] [Google Scholar]
- 8.Baldessarini RJ, Tondo L, Pinna M, et al. (2019). Suicidal risk factors in major affective disorders. Br J Psychiatry, 1–6. [DOI] [PubMed] [Google Scholar]
- 9.Das D, Cherbuin N, Butterworth P, et al. (2012). A population-based study of attention deficit/hyperactivity disorder symptoms and associated impairment in middle-aged adults. PloS One, 7(2):e31500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mpango RS, Kinyanda E, Rukundo GZ, et al. (2017). Prevalence and correlates for ADHD and relation with social and academic functioning among children and adolescents with HIV/AIDS in Uganda. BMC Psychiatry, 17(1):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen VC, Chan HL, Wu SI, et al. (2019). Attention-Deficit/Hyperactivity Disorder and Mortality Risk in Taiwan. JAMA Netw Open, 2(8):e198714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faraone SV, Perlis RH, Doyle AE, et al. (2005). Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry, 57(11):1313–23. [DOI] [PubMed] [Google Scholar]
- 13.Hayman V, Fernandez TV. (2018). Genetic Insights Into ADHD Biology. Front Psychiatry, 9:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell AE, Ford T, Williams R, et al. (2016). The Association Between Socioeconomic Disadvantage and Attention Deficit/Hyperactivity Disorder (ADHD): A Systematic Review. Child Psychiatry Hum Dev, 47(3):440–58. [DOI] [PubMed] [Google Scholar]
- 15.Lee MJ, Chou MC, Chou WJ, et al. (2018). Heavy Metals’ Effect on Susceptibility to Attention-Deficit/Hyperactivity Disorder: Implication of Lead, Cadmium, and Antimony. Int J Environ Res Public Health, 15(6):1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva D, Colvin L, Hagemann E, et al. (2014). Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics, 133(1): e14–e22. [DOI] [PubMed] [Google Scholar]
- 17.Mick E, Biederman J, Prince J, et al. (2002). Impact of low birth weight on attention-deficit hyperactivity disorder. J Dev Behav Pediatr, 23(1):16–22. [DOI] [PubMed] [Google Scholar]
- 18.Dong T, Hu W, Zhou X, et al. (2018). Prenatal exposure to maternal smoking during pregnancy and attention-deficit/hyperactivity disorder in offspring: A meta-analysis. Reprod Toxicol, 76:63–70. [DOI] [PubMed] [Google Scholar]
- 19.Huang L, Wang Y, Zhang L, et al. (2018). Maternal Smoking and Attention-Deficit/Hyperactivity Disorder in Offspring: A Meta-analysis. Pediatrics, 141(1): e20172465. [DOI] [PubMed] [Google Scholar]
- 20.Pagnin D, Zamboni Grecco ML, Furtado EF. (2019). Prenatal alcohol use as a risk for attention-deficit/hyperactivity disorder. Eur Arch Psychiatry Clin Neurosci, 269(6):681–7. [DOI] [PubMed] [Google Scholar]
- 21.Mann JR, McDermott S. (2011). Are maternal genitourinary infection and pre-eclampsia associated with ADHD in school-aged children? J Atten Disord, 15(8):667–73. [DOI] [PubMed] [Google Scholar]
- 22.Werenberg Dreier J, Nybo Andersen AM, Hvolby A, et al. (2016). Fever and infections in pregnancy and risk of attention deficit/hyperactivity disorder in the offspring. J Child Psychol Psychiatry, 57(4):540–8. [DOI] [PubMed] [Google Scholar]
- 23.Ystrom E, Gustavson K, Brandlistuen RE, et al. (2017). Prenatal Exposure to Acetaminophen and Risk of ADHD. Pediatrics, 140(5):e20163840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atladottir HO, Thorsen P, Ostergaard L, et al. (2010). Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord, 40(12):1423–30. [DOI] [PubMed] [Google Scholar]
- 25.Blomstrom A, Karlsson H, Gardner R, et al. (2016). Associations Between Maternal Infection During Pregnancy, Childhood Infections, and the Risk of Subsequent Psychotic Disorder--A Swedish Cohort Study of Nearly 2 Million Individuals. Schizophr Bull, 42(1):125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khandaker GM, Cousins L, Deakin J, et al. (2015). Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. The lancet Psychiatry, 2015;2(3):258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Shamseer L, Clarke M, et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev, 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells GA, Shea B, O’Connell D, et al. (2019). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed September [Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 29.Modesti PA, Reboldi G, Cappuccio FP, et al. (2016). Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PloS One, 11(1):e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings P. (2009). The relative merits of risk ratios and odds ratios. Arch Pediatr Adolesc Med, 163(5):438–45. [DOI] [PubMed] [Google Scholar]
- 31.Bilenberg N, Hougaard D, Norgaard-Pedersen B, et al. (2011). Twin study on transplacental-acquired antibodies and attention deficit/hyperactivity disorder--A pilot study. J Neuroimmunol, 236(1–2):72–5. [DOI] [PubMed] [Google Scholar]
- 32.Liew Z, Ritz B, Rebordosa C, et al. (2014). Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatrics, 168(4):313–20. [DOI] [PubMed] [Google Scholar]
- 33.Wiggs KK, Rickert ME, Hernandez-Diaz S, et al. (2017). A Family-Based Study of the Association Between Labor Induction and Offspring Attention-Deficit Hyperactivity Disorder and Low Academic Achievement. Behav Genet, 47(4):383–93. [DOI] [PubMed] [Google Scholar]
- 34.Afsharpaiman S, Khosravi MH, Faridchehr M, et al. (2016). Assessment of Toxoplasma Seropositivity in Children Suffering from Attention Deficit Hyperactivity Disorder. Galen Med J, 5(4):188–93. [Google Scholar]
- 35.Arpino C, Marzio M, D’Argenzio L, et al. (2005). Exanthematic diseases during pregnancy and attention-deficit/hyperactivity disorder (ADHD). Eur J Paediatr Neurol, 9(5):363–5. [DOI] [PubMed] [Google Scholar]
- 36.Chudal R, Brown AS, Gyllenberg D, et al. (2020). Maternal serum C-reactive protein (CRP) and offspring attention deficit hyperactivity disorder (ADHD). Eur Child Adolesc Psychiatry, 29(2):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Downey LC, O’Shea TM, Allred EN, et al. (2015). Antenatal and early postnatal antecedents of parent-reported attention problems at 2 years of age. J Pediatr, 166(1):20–5.e1. [DOI] [PubMed] [Google Scholar]
- 38.Ginsberg Y, D’Onofrio BM, Rickert ME, et al. (2019). Maternal infection requiring hospitalization during pregnancy and attention-deficit hyperactivity disorder in offspring: a quasi-experimental family-based study. J Child Psychol Psychiatry, 60(2):160–8. [DOI] [PubMed] [Google Scholar]
- 39.Gustavson K, Ask H, Ystrom E, et al. (2019). Maternal fever during pregnancy and offspring attention deficit hyperactivity disorder. Sci Rep, 9(1):9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mellins CA, Brackis-Cott E, Leu CS, et al. (2009). Rates and types of psychiatric disorders in perinatally human immunodeficiency virus-infected youth and seroreverters. J Child Psychol Psychiatry, 50(9):1131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oerlemans AM, Burmanje MJ, Franke B, et al. (2016). Identifying Unique Versus Shared Pre- and Perinatal Risk Factors for ASD and ADHD Using a Simplex-Multiplex Stratification. J Abnorm Child Psychol, 44(5):923–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pineda DA, Palacio LG, Puerta IC, et al. (2007). Environmental influences that affect attention deficit/hyperactivity disorder: study of a genetic isolate. Eur Child Adolesc Psychiatry, 16(5):337–46. [DOI] [PubMed] [Google Scholar]
- 43.Schmitt J, Romanos M. (2012). Prenatal and perinatal risk factors for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med, 166(11):1074–5. [DOI] [PubMed] [Google Scholar]
- 44.Jiang HY, Xu LL, Shao L, et al. (2016). Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain Behav Immun, 58:165–72. [DOI] [PubMed] [Google Scholar]
- 45.Scola G, Duong A. (2017). Prenatal maternal immune activation and brain development with relevance to psychiatric disorders. Neuroscience, 346:403–8. [DOI] [PubMed] [Google Scholar]
- 46.Schultz R, Read AW, Straton JA, et al. (1991). Genitourinary tract infections in pregnancy and low birth weight: case-control study in Australian aboriginal women. BMJ, 303(6814):1369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franz AP, Bolat GU, Bolat H, et al. (2018). Attention-Deficit/Hyperactivity Disorder and Very Preterm/Very Low Birth Weight: A Meta-analysis. Pediatrics, 141(1): e20171645. [DOI] [PubMed] [Google Scholar]
- 48.Yue Y, Tang Y, Tang J, et al. (2018). Maternal infection during pregnancy and type 1 diabetes mellitus in offspring: a systematic review and meta-analysis. Epidemiol Infect, 146(16):2131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye Z, Wang L, Yang T, et al. (2019). Maternal Viral Infection and Risk of Fetal Congenital Heart Diseases: A Meta-Analysis of Observational Studies. J Am Heart Assoc, 8(9):e011264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He J, Liu ZW, Lu YP, et al. (2017). A Systematic Review and Meta-Analysis of Influenza A Virus Infection During Pregnancy Associated with an Increased Risk for Stillbirth and Low Birth Weight. Kidney Blood Press Res, 42(2):232–43. [DOI] [PubMed] [Google Scholar]