Abstract
We investigated how exogenous variation in exposure to the COVID‐19 pandemic during the first year of life is related to infant development, maternal mental health, and perceived stress. Ninety‐three socioeconomically diverse pregnant women were recruited before the pandemic to participate in a longitudinal study. Infants ranged in age at the beginning of lockdown (0–9.5 months old), thus experiencing different durations of pandemic exposure across the first year of life. The duration of pandemic exposure was not associated with family demographic characteristics, suggesting it captured exogenous variability. We tested associations between this exogenous variation in pandemic exposure and child and family outcomes. We also examined whether mother‐reported disruptive life events were correlated with child and family outcomes. We found no association between duration of pandemic exposure in the first year of life and infant socioemotional problems, infant language development, or maternal mental health and perceived stress symptoms, at 12 or 24 months. However, we found that self‐reported exposure to pandemic‐related disruptive life events predicted greater maternal depression, anxiety, and perceived stress at 12 months, and greater depression and anxiety at 24 months. Socioeconomic status did not moderate these associations. These findings suggest cautious optimism for infants raised during this period.
1. INTRODUCTION
The COVID‐19 pandemic has wreaked devastation on a global scale. In addition to causing millions of deaths, the pandemic disrupted daily lives in countless ways. New York City (NYC), an early American epicenter, was particularly affected with high infection rates, deaths, lockdowns, and job loss. While the pandemic substantially impacted all New Yorkers' lives, mothers and infants may have been particularly affected. Indeed, access to childcare, healthcare, economic resources, and peer socialization were disrupted due to lockdowns. Masking requirements may have interfered with infants' abilities to learn and communicate (Kokkinaki & Hatzidaki, 2022), and correlational work has suggested that mothers reported a decline in mental health during the pandemic during both perinatal and postpartum periods (Morris & Saxbe, 2021; Perzow et al., 2021). These changes and others have raised concern about the impact of the pandemic on infant development and maternal mental health (e.g., Kokkinaki & Hatzidaki, 2022; Venta et al., 2021).
Masking, social isolation, fear of infection, and other environmental changes could affect infants and mothers in numerous ways. Pandemic‐related disruptions may have led to changes in parent‐child interactions in ways that could plausibly support or hinder language and socioemotional skill development. Furthermore, accumulating evidence has indicated declines in maternal mental health due to pandemic‐related stressors (e.g., Grumi et al., 2021). The effect of the pandemic on maternal mental health is an important public health concern in its own right and also has potential implications for infant development (Ierardi et al., 2019; Lam‐Cassettari & Kohlhoff, 2020; Scheiber et al., 2022).
In order to assess the impact of the pandemic on infant development and maternal mental health, the present study leverages exogenous variations in months of exposure to the pandemic during infants' first years of life. We hypothesized that greater duration of pandemic exposure over the first year of life would be associated with lower infant language skills, more infant socioemotional problems, and greater maternal anxiety, depressive, and perceived stress symptoms when infants were 12‐ and 24‐months old.
1.1. Maternal mental health
Pandemic‐related stressors are likely to exacerbate maternal mental health and perceived stress symptoms (Provenzi & Grumi, 2021), potentially leading to declines in mental health (Grumi et al., 2021). In the United Kingdom, Fallon et al. (2021) found clinically relevant cases of anxiety (61%) and depression (43%) in new mothers to be far higher than pre‐pandemic rates (14.6% and 16%, respectively). Similar findings have been reported across the globe (Davenport et al., 2020; Gustafsson et al., 2021; Wu et al., 2020; Zanardo et al., 2020).
Myriad pandemic‐related experiences may contribute to these patterns, including social isolation, fear of infection, financial strain, and caregiver fatigue. These experiences could impact one or more domains of mental health, including depression, anxiety, and perceived stress. McLaughlin et al. (2022) theorized that cumulative exposure to adverse experiences (e.g., an accumulation of stressful pandemic experiences) may be one mechanism driving pandemic‐related upticks in psychopathology. They also propose that specific stressor types (e.g., economic stressors and lifestyle related disruptions) and stress sensitization (e.g., history of adverse early experiences) may put individuals at a greater risk (McLaughlin et al., 2022).
Some empirical work has found support for the cumulative exposure theory. One cross‐sectional survey suggested that experiencing a single stressful life event due to the pandemic was a risk factor for poorer mental health (Rossi et al., 2020). Another study of low‐income families found that caregivers who experienced both pandemic‐related job and income loss reported significantly higher depressive symptoms and life stress (Kalil et al., 2020a). This aligns with pre‐pandemic research reporting that negative life events predict higher levels of maternal depression and anxiety (Mitchell & Ronzio, 2011; Phillips et al., 2015).
While declines in maternal mental health are concerning in their own right, an abundance of research has also linked maternal mental health with infant language and behavior. Specifically, maternal depression, anxiety, and perceived stress have each been associated with poorer socioemotional and language outcomes in the first years of life (Barnett et al., 1991; D'Souza, Crawford, et al., 2019; D'Souza, Waldie, et al., 2019; Harris & Santos, 2020; Pierce et al., 2021; Quevedo et al., 2011; Reck et al., 2018).
Maternal mental health and perceived stress symptoms may shape infant development through impacts on the home language environment and parent‐child interaction, critical factors for scaffolding infant language, and socioemotional development. For example, depressed mothers tend to use less infant‐directed speech and engage in fewer conversational turns with infants (Brookman et al., 2020; Lam‐Cassettari & Kohlhoff, 2020), which in turn are associated with a smaller vocabulary size at 18 months (Brookman et al., 2020). Furthermore, depressed mothers tend to demonstrate more withdrawn and intrusive parenting behaviors (Field et al., 2006), which are associated with greater behavior problems, lower social competence, and lower language skills in offspring (Justice et al., 2019; Wang & Dix, 2013). Interventions that improve parent‐child interactions for mothers experiencing psychological distress have been shown to improve children's behavior problems (Timmer et al., 2011), highlighting the importance of parent‐child interactions as a mechanism to explain the association between maternal mental health and socioemotional development.
Due to the unexpected nature of the pandemic's onset, pandemic‐related studies have largely relied on cross‐sectional, correlational designs to draw conclusions, which limit the ability to draw causal inferences. Two longitudinal studies have investigated these associations in the postnatal period. Overall, these studies detected increased levels of maternal anxiety and depressive symptoms since the onset of the pandemic (Perzow et al., 2021; Racine et al., 2021). Importantly, both studies found that experiencing more pandemic‐related disruptions, such as job loss or loss of childcare, was a risk factor for poorer mental health (Perzow et al., 2021; Racine et al., 2021). To our knowledge, no studies have explored the impact of the pandemic on new mothers or infant development over time.
1.2. Infant development
1.2.1. Language development
Research to date has been mixed regarding the effects of the pandemic on early language development. Indeed, one can imagine various plausible mechanisms by which the pandemic may have facilitated or limited language development. Children's engagement in back‐and‐forth conversational interaction with adults (components of the “home language environment”) has been theorized (Ford et al., 2020; Pace et al., 2017) and empirically backed (Weisleder & Fernald, 2013) as critical processes that scaffold children's emerging language abilities. Social isolation, mask wearing, job loss, and other ecological shifts that occurred during the pandemic may have changed the home language environment in important ways with potential implications for infant language development.
On one hand, it is possible that the pandemic may have led to declines in language development. A recent study observed slightly lower language scores among 6‐ and 12‐month‐olds born during the pandemic, compared with a pre‐pandemic cohort (Imboden et al., 2021). Mask‐wearing and social isolation may have hampered the extent to which children could pick up on language inputs in their environment, and pandemic‐related stress may have reduced the quantity or quality of parent‐child interactions. Indeed, increased perceived stress has been linked with the provision of less sensitive parent‐child interaction (Ursache et al., 2017), though evidence for links between perceived stress and the measures of the home language environment has been mixed (Hart et al., 2022; Pierce et al., 2021). Nonetheless, greater parenting stress (Justice et al., 2019; Magill‐Evans & Harrison, 2001), perceived stress (Pierce et al., 2021), and psychological appraisals of stress (Troller‐Renfree et al., 2022) have each been associated with lower scores on measures of early language development. Some other studies, however, have found no relation between perceived stress and language development (Lehr et al., 2016; Lin et al., 2017).
Alternately, and perhaps counterintuitively, pandemic‐related shifts may have provided increased opportunities for infant language development. For example, parents working remotely or spending more time at home due to job loss may have engaged in more frequent and responsive verbal interactions with their infants. One study found evidence along these lines: Parents who reported both job and income loss during the pandemic were more likely to regularly read with their preschool‐aged child, though this relation was not observed for the experience of either job or income loss alone (Kalil et al., 2020a). Similarly, a separate study found that young children (8‐ to 36‐months‐old) gained more words during the first COVID‐19 lockdown when compared to pre‐pandemic norms (Kartushina et al., 2021). Infant language development may be particularly sensitive to pandemic‐related changes, making this an important area to research.
1.2.2. Socioemotional development
Healthy infant socioemotional development depends on interactions with adults and peers (Brownell & Brown, 1992). Indeed, one way in which adult interaction influences socioemotional development is through pro‐social behavior modeling (Vandell & Wilson, 1987). Additionally, peer interaction during toddlerhood is considered an important aspect of healthy development: social coordination, communication, and engagement in peer play expand rapidly during the second year of life (Brownell & Brown, 1992). The extent to which reduction in peer interactions during this sensitive period may affect infant socioemotional development is unknown.
In many cases, lockdowns restricted social interaction outside of the immediate family unit, limiting opportunities for children to practice these emerging social skills outside of the home. In one qualitative study, parents described the closure of early education programs as negatively impacting their children's socioemotional well‐being and behavior (Egan et al., 2021). Alternatively, it is possible that more time spent at home with family members may have led to more opportunities to learn and practice pro‐social behaviors, thus leading to improved socioemotional development. In the same study, parents also reported increased quality family time as a positive effect of lockdown (Egan et al., 2021).
Importantly, coordinated face‐to‐face interactions are considered essential for socioemotional development (Feldman, 2007), and mask use may reduce opportunities for infants to read faces for emotion. One experimental study found that masks inhibited preschool‐aged children's ability to infer emotional cues via facial expressions (Gori et al., 2021), but no such effects were seen in school‐aged children (Ruba & Pollak, 2020). However, further evidence on how mask use affects infant socioemotional development is limited.
Beyond social isolation and mask wearing, heightened stress and uncertainty during lockdown may have negatively impacted parent‐child interaction quality. It is well‐documented that exposure to early stress is associated with poorer socioemotional development. Indeed, several studies have found that increased postnatal perceived stress correlates with greater socioemotional problems at 12 months (Olafsen et al., 2008; Pesonen et al., 2008; Troller‐Renfree et al., 2022). Adverse childhood experiences (ACEs), such as the death of a family member, are one example of a stress exposure associated with later behavior problems (Clarkson Freeman, 2014). An estimated 140,000 children under age 18 experienced the death of a caregiver due to COVID‐19 (Hillis et al., 2021), raising concern about children's socioemotional trajectories during the pandemic.
One recent study found that infants born during the pandemic scored lower on a parent‐reported measure of personal‐social development at 6 months (Shuffrey et al., 2021), while another study with a similar design did not detect such effects (Imboden et al., 2021). Nonetheless, evidence has suggested that stress‐related psychopathologies have increased in both children and adults since the onset of the pandemic (see McLaughlin et al., 2022 for a review). Though data available on infants to date is limited, it is important to study pandemic‐related socioemotional differences, as these may be precursors to later psychopathology.
1.3. COVID‐19 & socioeconomic status (SES)
Though the pandemic affected all New Yorkers, not all were equally impacted. Many families had the social and financial resources to move out of NYC (Marte, 2020), providing some respite from the stress of living in close quarters with the threat of high infection risk. Some could comfortably work from home and maintain sufficient income. Others experienced devastating losses, financial insecurity, social isolation, and many other disruptive life events.
Importantly, low‐income families were far more likely to experience job and/or income loss during lockdown (Parker et al., 2020). Thus, socioeconomic status (SES) may moderate the impact of the pandemic on infant development and maternal mental health. An analysis from Kalil et al. (2020a) found job and income loss during the pandemic to be associated with less positive parenting interactions among a sample of low‐income caregivers, indicating potential downstream effects of the pandemic for infants experiencing poverty. Similarly, postpartum women with income less than 200% of the federal poverty line tended to report higher depressive and anxiety symptoms during the pandemic compared to high‐income women (Perzow et al., 2021).
Although it is well‐documented that job loss, infections, and deaths were significantly higher in socioeconomically disadvantaged communities (Finch & Hernández Finch, 2020; Hawkins et al., 2020; Karmakar et al., 2021; Parker et al., 2020), some mothers living in disadvantaged circumstances may have fared more favorably during the pandemic. For example, stay‐at‐home mandates and economic relief payments may have provided opportunities for greater quality time in the home. Therefore, it is also possible that mental health symptoms may decrease for low‐income mothers in the context of COVID‐19. Indeed, a recent study found that depressive and anxiety symptoms decreased during the pandemic in a sample of low‐income mothers of 12‐month old's compared with those assessed prior to the pandemic (Premo et al., 2022).
1.4. Current study
The current study tested the effect of pandemic exposure on infant development and maternal mental health. The onset of the pandemic occurred in the midst of an ongoing longitudinal study of child development from pregnancy through age three. Data collection occurred such that all participants had completed a baseline prenatal assessment prior to the pandemic's onset, and all infants in the study reached their first birthday after the pandemic's onset. Thus, the enrolled infants ranged in age when the pandemic began in March 2020.
We leveraged this variation in the infant's age to create an arguably exogenous indicator of pandemic exposure. First, we tested whether the infant's age at pandemic onset was associated with potential confounding demographic characteristics. Theoretically, if the infant's age within our sample varied randomly, we would be well‐positioned to estimate the causal effect of the duration of pandemic exposure within our sample. We hypothesized that a greater duration of pandemic exposure over the first year of life would be associated with poorer infant language skills and greater socioemotional problems at infant ages of 12 and 24 months, as well as greater maternal self‐reports of anxiety, depression, and perceived stress. Our study design did not allow us to identify mechanisms responsible for effects; rather, we sought to test if more time spent living under pandemic conditions was associated with developmental differences in the first 2 years of life.
After the pandemic's onset, when infants were 12‐months old, we collected maternal reports of exposure to pandemic‐related disruptive life events. We hypothesized that a greater number of pandemic‐related disruptive life events would predict poorer infant language, more infant socioemotional problems, and worse maternal mental health. Of note, the maternal report of disruptive life events was not an exogenous measure, and as such, was susceptible to bias due to unmeasured confounds. Finally, we also examined whether SES moderated the relation between both pandemic predictors (duration and disruption) and each of the maternal and infant outcomes. We did not have hypotheses about the directionality of these interactions, as there has been scant literature to date on pandemic‐related SES moderation effects.
2. METHODS
2.1. Study design
Between June 2019 and March 2020, mothers were recruited from local prenatal clinics, parenting/birthing classes, community events, and social media to participate in a longitudinal study examining associations among early experiences and child development. The study aimed to recruit and follow 200 mother‐infant dyads from the prenatal period through age three. However, only 93 mothers were enrolled in the study before recruitment efforts were paused due to the pandemic. Data collection procedures shifted to be remote in March 2020 for those already enrolled in the study for the 12‐ and 24‐month visits.
2.2. Participants
Mothers were recruited from the NYC metropolitan area before the onset of the pandemic. Participants were recruited to be intentionally socioeconomically diverse based on maternal education. Inclusion criteria for the expectant mothers included: being 18 years of age or older, at least 35 weeks pregnant, carrying a singleton fetus with no known neurological or developmental issues, and being able to speak either English or Spanish. All visits were conducted in either English or Spanish according to maternal preference. This study was conducted in alignment with the ethical standards set by the Declaration of Helsinki, and informed consent was obtained from all parents before beginning study procedures. All procedures were approved by the Institutional Review Board of Teachers College, Columbia University.
2.3. Measures
2.3.1. Maternal mental health
Depression
Maternal depressive symptoms were evaluated using the Patient Health Questionnaire (PHQ‐8; Kroenke et al., 2009) at the prenatal, 12‐month, and 24‐month visits. The PHQ‐8 is a valid and reliable 8‐question self‐report scale that assesses the frequency of depressive symptoms over the prior 2 weeks. Items are scored as 0 points for “Not at all,” 1 point for “Several days,” 2 points for “More than half the days,” or 3 points for “Nearly every day.” Scores were considered valid if mothers completed at least 80% of items with higher scores indicating more frequent depressive symptoms = .77).
Anxiety
Maternal anxiety symptoms were evaluated using the Beck Anxiety Inventory (BAI; Beck et al., 1988) at the prenatal, 12‐month, and 24‐month visits. The BAI is a valid and reliable 21‐question self‐report scale that assesses the severity of anxiety symptoms over the prior month. For each item, participants report how much they were bothered by anxiety symptoms on a scale from 0 (not at all) to 3 (severely, I could barely stand it). Scores were considered valid if mothers completed at least 80% of items with higher scores indicating more severe anxiety symptoms = .88).
Perceived stress
Perceived stress was assessed using the Perceived Stress Scale (PSS‐10; Cohen & Williamson, 1988), which was administered at the prenatal, 12‐month, and 24‐month visits. The 10‐item scale assesses the degree to which the respondent has perceived situations as stressful within the last month. Participants responded to each item using a 5‐point Likert scale (1 = never, 5 = very often). Four items were positively stated and thus were reverse scored before summing the items. Higher scores indicated greater perceived stress. Mothers needed to provide valid answers on at least 80% of the items for their score to be considered valid = .80).
2.3.2. Infant outcomes
Socioemotional problems
The Brief Infant‐Toddler Social Emotional Assessment (BITSEA; Briggs‐Gowan, 2004) is a clinical screener for socioemotional issues, behavioral problems, and delays in competency in young children. The BITSEA assesses the occurrence of problem behaviors, overall social competence, and parents' concerns about their child's development. The scale was previously validated in a clinical sample and is used to screen children of 12–36 months old (Briggs‐Gowan, 2004). Mothers completed the BITSEA Problems subscale (31 items) at the 12‐ and 24‐month visit, during which they rated the frequency of 23 behaviors (0 = not true/rarely, 1 = sometimes true/often, 2 = very true/often; e.g., “My child seems nervous, tense, or fearful”). All items were summed to form a composite with higher scores indicating greater problem behaviors. At least 80% completion of the Problems subscale was required to calculate a valid score ( = .82).
Language development
The LENA Developmental Snapshot estimates children's expressive and receptive language ability (LDS; Gilkerson et al., 2017). The LDS is a 52‐item questionnaire that measures language development through the parent's report. Items on the questionnaire progress in difficulty (e.g., “When you talk to your child, does he/she look in the direction of your voice?,” “Does your child produce two or more vowel sounds, such as/ah/or/ooh/?”). For each item, parents are instructed to indicate “yes” if their child consistently demonstrates each skill or behavior either currently or at an earlier developmental stage and “no” if the child has not consistently demonstrated the skills. A developmental age estimate is then calculated based on the parent's report of child language ability. The LDS has been shown to be highly correlated with other performance‐based language assessments and has been validated for children from ages 2–36 months (Gilkerson et al., 2017). Mothers completed the LDS at the 12‐ and 24‐month visits.
2.3.3. Pandemic impact measures
Duration of pandemic exposure
To evaluate the impact of pandemic exposure, it was necessary to select a cut‐off date that established the pandemic's onset in NYC. March 22, 2020, was selected, as this was the date NYC enacted physical distancing requirements and required all nonessential workers to stay home (Thompson et al., 2020). The duration of pandemic exposure was calculated as the number of days that occurred between the pandemic onset date (March 22, 2020) and the infant's first birthday. This created a continuous variable that reflected the duration of pandemic exposure in the first year of life. As such, a lower value indicated that the infant had less pandemic exposure during their first year of life. For example, an infant born on February 22, 2020, would have been 1 month old at the start of the pandemic and experienced 11 months of pandemic exposure in their first year. In contrast, an infant born on June 22, 2019 would have been 9 months old at the start of the pandemic and experienced only 3 months of pandemic exposure in the first year of life. This variable was then transformed into months using the average number of days in a month (i.e., 30.43). For confidentiality purposes, we represent months of pandemic exposure rounded to the second decimal in this manuscript. In analyses, we utilized a continuous variable based on the infant's exact age.
As seen in Figure 1, the onset of the pandemic occurred at various developmental points in our sample. Five women in our sample gave birth shortly after March 22, 2020, so their infants were assigned a value of 12 months of pandemic exposure in the first year. Two of the prenatal visits occurred on or after March 22, 2020, but we retained these cases as their inclusion did not affect the findings (and we assigned these infants a value of 12 months of exposure as well).
FIGURE 1.
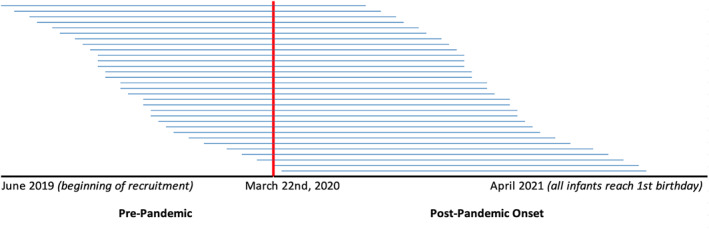
Distribution of pandemic exposure across the sample at recruitment. N = 93. To protect identifiable information, each line represents the average pandemic exposure of three participants, sequential in age. The red line indicates the onset of the pandemic in NYC (March 22, 2020). The left half of the figure represents time each child spent alive prior to the pandemic, and the right half represents the amount of pandemic exposure in the first year of life.
Disruptive life events
One method of assessing stress during a natural disaster is by measuring exposure to stressful life events (King et al., 2012). For example, Laplante et al. (2004) found that the number of stressful events experienced by pregnant mothers during the 1998 Quebec Ice Storm predicted infant language and intellectual abilities at age two (e.g., “Were you injured?” and “How many days were you without power?”).
Here, we used a similar count of the number of disruptive life events experienced by families during the pandemic, using the Epidemic‐Pandemic Impact Inventory (EPII; Grasso et al., 2020), a recently developed scale designed to measure the impact of the COVID‐19 pandemic on personal and family life. The checklist consists of 92 items spread across 10 domains. Due to time constraints, only seven domains (71 items total) were administered at the 12‐month visit: Work and Employment (e.g., “Laid off from job or had to close own business”), Education and Training (e.g., “Had a child in home who could not go to school”), Home Life (e.g., “Childcare and babysitting unavailable when needed”), Social Activities (e.g., “Separated from family or close friends”), Physical Health Problems (e.g., “Increase in health problems not related to this disease”), Infection History (e.g., “Hospital stay due to this disease”), and Positive Change (e.g., “More quality time with partner or spouse”). Participants responded to each item as to whether the event impacted them or a person in their home at any point during the pandemic. We created a cumulative risk index by summing endorsed items from the negative domains of the scale; the Positive Change subscale was excluded from the cumulative risk index. Affirmatively endorsed items (“Yes, impacted me” and/or “Yes, impacted a person in my home”) were collapsed and summed to create a cumulative COVID‐19 Disruptive Life Events Score.
2.3.4. Socioeconomic status
Maternal educational attainment, annual income, and household size data were collected at the prenatal visit. An income‐to‐needs (ITN) ratio was calculated by dividing total household income by the poverty threshold for the respective family size for the year of data collection. As expected, ITN values were positively skewed. Therefore, we winsorized the highest values to 3 standard deviations above the mean and subsequently log‐transformed ITN values for use in all analyses. Some participants reported an income of zero dollars. To enable log transformation, one dollar was added to all income values prior to calculating ITN. Maternal‐reported years of formal education was used as a secondary measure of SES.
2.4. Procedure
Participants were invited to complete surveys during their third trimester of pregnancy, at 12‐ and 24‐month postpartum. We modified procedures to allow for remote data collection during the pandemic; thus, all postpartum surveys were collected virtually.
2.5. Missing data
Of the initial sample (n = 93 mother‐infant dyads; 49 female infants), one infant was excluded from analyses after receiving a diagnosis of autism. Nineteen mothers declined to participate at the 12‐month follow‐up, and another 18 participants declined to participate at the 24‐month visit. Reasons for attrition included relocation, loss of interest, or time constraints. Mothers who completed the 12‐month assessment were more likely to be White ( = .16, p = .07), less likely to be Hispanic ( = −.22, p = .01), more highly educated ( = .09, p = .03), and older ( = .14, p < .001) compared to the prenatal sample. However, no demographic differences were observed between the prenatal sample and the 24‐month sample. We used multiple imputation with 25 iterations for all regression analyses. Our final analytic sample was 92.
2.6. Covariates
Covariates for linear models included infant exact age at the 12‐ and 24‐month assessments; prenatal levels of maternal depression, anxiety, and perceived stress; level of maternal education (continuous); maternal race (two dummy variables for whether the mother was Black or some other race; White was used as the reference group) and ethnicity (dummy variable for whether the mother was Hispanic; not Hispanic was used as the reference group); maternal age at delivery; and maternal age‐adjusted scores from the NIH Toolbox Picture Vocabulary Test (NIH‐TB PVT; Weintraub et al., 2013), a nationally standardized measure of language functioning that was collected at the prenatal visit.
2.7. Analytic Plan
All analyses were conducted in Stata v.16 (StataCorp, 2019). First, we regressed maternal baseline characteristics onto the duration of pandemic exposure to examine whether the duration correlated with pre‐pandemic maternal characteristics. Next, we conducted a linear regression analysis to test whether the duration of pandemic exposure and pandemic‐related disruptive life events predicted infant language skills, socioemotional problems, and maternal mental health and perceived stress symptoms at both 12‐ and 24‐months postpartum. All outcome variables (i.e., maternal mental health, perceived stress, and infant development) were standardized for linear analyses, while the pandemic predictor variables (i.e., duration of exposure and disruptive life events) were unstandardized. According to a post‐hoc power analysis, we were sufficiently powered to detect effect sizes of 0.28 or greater in our regression models with full covariates.
In all models, we controlled for infant exact age at the 12‐ and 24‐month assessments to adjust for variations in the timing of the visit (i.e., to control for the fact that some children were, e.g., slightly older or younger than 12 months at the 12‐month visit). We also tested whether the results changed after the inclusion of additional controls for baseline maternal characteristics measured during the prenatal visit that could also influence our key outcomes. Finally, in our exploratory interaction models, we tested interactions between ITN and both the duration of exposure and disruptive life events in predicting maternal and infant functioning.
To reduce the influence of outliers, linear analyses were conducted using the “robust regression” command in Stata 16.0. One participant obtained the highest possible value on the disruptive life events scale as they selected many extreme and conflicting answer choices, reflective of an extreme and noncontingent response style (Van Vaerenbergh & Thomas, 2013). This response style has the potential to skew data and affect linear analyses (Van Vaerenbergh & Thomas, 2013). Consequently, we set this score to missing, though this participant was included in our analyses that relied on multiple imputation (i.e., their score was imputed as if they had a missing survey response).
3. RESULTS
3.1. Examining baseline correlations with pandemic exposure
Baseline maternal characteristics for the sample at the time of recruitment are presented in Table 1. Here, we also present baseline characteristics regressed onto the duration of pandemic exposure variable. None of the maternal characteristics were significantly associated with the duration of pandemic exposure in the first year of life, indicating that the duration of pandemic exposure was not systematically related to observed participant qualities. This increases our confidence that the duration of pandemic exposure is truly an exogenous variable that was outside of participants' control and is not conflated with other participant characteristics.
TABLE 1.
Descriptive statistics of sample at recruitment and maternal characteristics at baseline regressed on duration of exposure
| N | Mean | SD | Min | Max | b | p‐Value | |
|---|---|---|---|---|---|---|---|
| Prenatal depression | 93 | 4.27 | 3.41 | 0.00 | 13.00 | 0.41 | .09 |
| Prenatal anxiety | 93 | 8.74 | 7.59 | 0.00 | 31.00 | 0.06 | .82 |
| Prenatal perceived stress | 92 | 11.60 | 6.36 | 0.00 | 30.00 | 0.24 | .34 |
| Maternal age at delivery | 93 | 31.11 | 5.65 | 19.00 | 43.00 | −0.26 | .30 |
| Maternal education (years) | 93 | 14.70 | 3.67 | 8.00 | 22.00 | 0.01 | .96 |
| Income‐to‐needs ratio (ITN) | 87 | 5.52 | 8.41 | 0.00 | 43.74 | −0.01 | .97 |
| Age‐adjusted vocabulary | 88 | 95.08 | 18.35 | 65.00 | 146.00 | 0.26 | .26 |
| Black | 93 | 0.20 | 0.00 | 1.00 | 0.42 | .50 | |
| Other race | 93 | 0.46 | 0.00 | 1.00 | −0.65 | .19 | |
| White | 93 | 0.33 | 0.00 | 1.00 | 0.42 | .43 | |
| Hispanic | 93 | 0.58 | 0.00 | 1.00 | 0.08 | .87 |
Note: N = 93. Maternal characteristics at the prenatal time point were regressed onto the duration of pandemic exposure to extract a beta coefficient and p‐value. Continuous variables were standardized for regression analyses, while dichotomous variables remained unstandardized. ITN was log‐transformed for regression analysis. Maternal racial groups (i.e., Black, White, or Other Race) are mutually exclusive.
Although participant characteristics were not significantly associated with the duration of pandemic exposure, some of the magnitudes (e.g., whether Black, prenatal depression) were notable. It is possible we were not powered to detect small differences in baseline characteristics between mothers who gave birth earlier in the sample versus those who gave birth later. To account for this, we ran models both with and without baseline controls to adjust for any possible differences in participants that were associated with infant ages and recruitment practices in the study.
3.2. Descriptive statistics
Table 2 details the descriptive statistics for the COVID‐19 pandemic variables, measures of infant language and socioemotional outcomes, and measures of maternal mental health and perceived stress. Average maternal depression and anxiety scores fell within normal clinical ranges. Notably, mental health symptoms were higher at the prenatal visit—before the pandemic began—than they were at 1‐year (depression: t (72) = 2.19, p = .03; anxiety: t (73) = 4.31, p < .001) and 2‐years postpartum (depression: t (67) = 3.27, p = .002; anxiety: t (66) = 4.06, p < .001). Average levels of perceived stress marginally increased from the prenatal to the 12‐month assessment (t (72) = −1.97, p = .05), but no significant difference in perceived stress was detected between the prenatal and 24‐month visit.
TABLE 2.
Descriptives of key predictor and outcome variables of analytic sample
| Domain | Variable | Time point | N | M | SD | Min | Max |
|---|---|---|---|---|---|---|---|
| Maternal mental health symptoms | Depression | Prenatal | 93 | 4.27 | 3.41 | 0.00 | 13.00 |
| 12 month | 73 | 3.22 | 3.78 | 0.00 | 19.00 | ||
| 24 month | 68 | 2.71 | 2.96 | 0.00 | 17.00 | ||
| Anxiety | Prenatal | 93 | 8.74 | 7.59 | 0.00 | 31.00 | |
| 12 month | 74 | 4.97 | 6.13 | 0.00 | 38.00 | ||
| 24 month | 67 | 4.93 | 6.35 | 0.00 | 32.00 | ||
| Perceived stress | Prenatal | 92 | 11.60 | 6.36 | 0.00 | 30.00 | |
| 12 month | 73 | 13.14 | 6.84 | 0.00 | 32.00 | ||
| 24 month | 69 | 12.23 | 6.31 | 1.00 | 32.00 | ||
| Pandemic experience | Duration of exposure (months) | – | 93 | 7.40 | 2.37 | 2.53 | 12.00 |
| Disruptive life events (count) | 12 month | 71 | 10.70 | 6.18 | 0.00 | 25.00 | |
| Infant outcomes | Socioemotional problems | 12 month | 74 | 7.54 | 6.00 | 1.00 | 32.00 |
| 24 month | 66 | 8.14 | 7.07 | 0.00 | 45.00 | ||
| Language development | 12 month | 73 | 106.68 | 14.79 | 78.00 | 136.00 | |
| 24 month | 59 | 101.23 | 17.29 | 64.00 | 136.00 |
Note: N = 93. All prenatal visits occurred before the pandemic exposure (i.e., March 22, 2020), and all postnatal measures were collected online after the pandemic began.
Average scores on maternally reported language development (M = 106.68 and 101.23) and socioemotional problems (M = 7.54 and 8.14) reflect national norms at 12 and 24 months (see Briggs‐Gowan et al., 2004; Gilkerson et al., 2017). Table 3 presents the correlations between these variables. Notably, mothers with higher education tended to report greater pandemic‐related disruptive life events (r = .40, p < .05), and mothers with greater duration of child pandemic exposure reported higher anxiety at 12 months (r = .23, p < .05) and 24 months, r = .28, p < .05.
TABLE 3.
Pairwise correlations between key variables
| Variables | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | (13) | (14) | (15) | (16) | (17) | (18) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) Duration of exposure | 1.00 | |||||||||||||||||
| (2) Disruptive life events | 0.13 | 1.00 | ||||||||||||||||
| (3) Prenatal depression | 0.17 | 0.28* | 1.00 | |||||||||||||||
| (4) Prenatal anxiety | 0.02 | 0.32* | 0.69* | 1.00 | ||||||||||||||
| (5) Prenatal stress | 0.10 | 0.06 | 0.50* | 0.46* | 1.00 | |||||||||||||
| (6) 12‐month depression | 0.15 | 0.39* | 0.44* | 0.42* | 0.36* | 1.00 | ||||||||||||
| (7) 12‐month anxiety | 0.23* | 0.39* | 0.48* | 0.49* | 0.41* | 0.55* | 1.00 | |||||||||||
| (8) 12‐month stress | 0.10 | 0.26* | 0.15 | 0.18 | 0.34* | 0.64* | 0.44* | 1.00 | ||||||||||
| (9) 24‐month depression | 0.12 | 0.42* | 0.46* | 0.39* | 0.38* | 0.60* | 0.63* | 0.50* | 1.00 | |||||||||
| (10) 24‐month anxiety | 0.28* | 0.37* | 0.41* | 0.42* | 0.24 | 0.33* | 0.73* | 0.32* | 0.62* | 1.00 | ||||||||
| (11) 24‐month stress | 0.04 | 0.21 | 0.24* | 0.23 | 0.46* | 0.60* | 0.52* | 0.66* | 0.62* | 0.46* | 1.00 | |||||||
| (12) 12‐month language | −0.16 | 0.13 | −0.06 | 0.03 | 0.03 | −0.03 | 0.02 | 0.05 | −0.17 | −0.07 | −0.01 | 1.00 | ||||||
| (13) 24‐month language | 0.02 | 0.11 | −0.24 | −0.13 | 0.03 | −0.16 | 0.06 | 0.04 | −0.04 | 0.18 | 0.07 | 0.38* | 1.00 | |||||
| (14) 12‐month infant behavior | −0.13 | 0.07 | 0.10 | 0.21 | 0.09 | 0.05 | 0.34* | 0.21 | 0.15 | 0.40* | 0.17 | −0.10 | 0.01 | 1.00 | ||||
| (15) 24‐month infant behavior | −0.09 | −0.06 | −0.05 | 0.06 | 0.01 | 0.07 | 0.13 | 0.24 | 0.14 | 0.29* | 0.24 | −0.05 | −0.11 | 0.70* | 1.00 | |||
| (16) Maternal age | −0.11 | 0.19 | −0.20 | −0.21* | −0.20 | −0.10 | −0.01 | −0.06 | 0.05 | 0.11 | −0.07 | −0.07 | 0.14 | −0.16 | −0.14 | 1.00 | ||
| (17) Maternal education | 0.01 | 0.40* | −0.10 | −0.03 | −0.19 | 0.01 | −0.05 | −0.04 | −0.09 | −0.04 | −0.05 | 0.01 | 0.28* | −0.15 | −0.27* | 0.23* | 1.00 | |
| (18) NIH‐TB PVT | 0.13 | 0.28* | −0.10 | 0.02 | −0.17 | −0.07 | 0.07 | −0.07 | −0.05 | 0.14 | −0.13 | −0.04 | 0.25 | 0.02 | 0.03 | 0.29* | 0.40* | 1.00 |
Note: N = 93. Infant Behavior refers to socioemotional problems on the BITSEA. NIH‐TB PVT refers to the NIH Toolbox Picture Vocabulary test.
*p < .05.
Of the pandemic‐related disruptive life events reported at the 12‐month visit, commonly endorsed items that impacted either the mothers themselves or someone in their household included: being more sedentary (n = 38), exercising less (n = 38), being unable to do enjoyable hobbies or activities (n = 42), and needing to cancel travel (n = 44) and family celebrations (n = 45). Some mothers were impacted by layoffs or needing to close a business (n = 18), and some were impacted by furloughs (n = 22). Ten mothers reported testing positive for COVID‐19, and two additional mothers reported that someone in their household tested positive. There were no associations between self or household COVID‐19 infection and duration of pandemic exposure, maternal mental health, or infant outcomes.
3.3. Effects of pandemic exposure on maternal mental health
The top row of Table 4 presents results from models that regressed pandemic exposure on our measures of maternal depression, anxiety, and perceived stress at the 12‐ and 24‐month visits, while the second row presents results from separate regressions that examined associations between disruptive life events and the same measures. For each outcome, we present results from models that only controlled for infant age at the time of the assessment, followed by models that controlled for baseline maternal characteristics (see Table 4 note).
TABLE 4.
Associations between pandemic exposure variables and maternal mental health symptoms at 12‐ and 24‐month postpartum
| 12‐month postpartum | 24‐month postpartum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depression | Anxiety | Perceived stress | Depression | Anxiety | Perceived stress | |||||||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | |
| Duration of pandemic exposure | 0.05 | 0.01 | 0.08 | 0.05 | 0.02 | 0.02 | 0.02 | 0.03 | 0.06 | 0.08 | 0.01 | 0.01 |
| (0.05) | (0.05) | (0.05) | (0.05) | (0.06) | (0.06) | (0.06) | (0.06) | (0.05) | (0.05) | (0.06) | (0.06) | |
| Disruptive life events at 12 months | 0.06** | 0.04* | 0.06** | 0.04+ | 0.04+ | 0.04+ | 0.05* | 0.06* | 0.04* | 0.04+ | 0.03 | 0.03 |
| (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.03) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.03) | |
| Controls for maternal characteristics included? | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes |
Note: N = 92. All models control for infant age at the time of the assessment. Maternal mental health symptoms (i.e., outcomes) were standardized, while the duration of pandemic exposure and disruptive life events were not standardized. The duration variable was scaled in months, and the disruptive life events' measure was a count of the number of pandemic‐related disruptive events (see Table 2). Standard errors are in parentheses. Analyses were performed using multiple imputation. Maternal covariates include: prenatal depression, anxiety, and perceived stress; years of education (continuous); race and ethnicity (dummy variables for whether Black, other race, or Hispanic); age at delivery; and age‐adjusted NIH‐TB PVT scores.
+p < .10, *p < .05, **p < .01.
3.3.1. Duration of pandemic exposure
We observed no statistically significant associations between the duration of pandemic exposure and maternal depression, anxiety, or perceived stress at 12‐ or 24‐months postpartum. Effect sizes ranged from 0.01 to 0.08 with full controls added with the largest effects for maternal anxiety at 12 months (0.05, p = .32) and 24 months (0.08, p = .15).
It should be noted that, as reported above, we observed a statistically significant bivariate correlation between the duration of exposure variation and maternal anxiety at 12 months (r = .23, p < .05) and 24 months (r = .28, p < .05), a finding seemingly at odds with the small and nonsignificant associations observed in the regression models. The significance of this correlation is driven by a single extremely high outlier in maternal anxiety at both time points by the same participant. When this outlier is excluded, the correlation is no longer significant at 12 months (r = .15, p = .20) and marginally significant at 24 months (r = .24, p = .06). Robust regression down‐weights the influence of outliers on the regression line (see UCLA Statistical Consulting Group, 2022), explaining the discrepancy between the original pairwise correlations and linear regression findings.
3.3.2. Disruptive life events
For the survey‐based measure of disruptive life events, we observed some notable associations. At the 12‐month visit, each additional pandemic‐related disruptive life event predicted an increase in maternal depression of approximately 0.04 SDs (p = .02) with the inclusion of controls. Similar effects were observed for anxiety ( = 0.04, p = .05) and perceived stress ( = 0.04, p = .10). For each domain of mental health and perceived stress, the endorsement of five additional disruptive life events predicted an increase of approximately 0.20 SDs in symptoms at 12 months.
At the 24‐month visit, we once again observed significant associations with maternal mental health. Disruptive life events significantly predicted maternal depression ( = 0.06, p = .02) and marginally predicted anxiety ( = 0.04, p = .10) with the inclusion of controls. However, disruptive life events did not predict perceived stress ( = 0.03, p = .33) at 24 months with the inclusion of controls.
3.4. Effects of pandemic exposure on infant outcomes
3.4.1. Duration of pandemic exposure
Table 5 presents our key results from robust regressions that were used to predict infant outcomes. The duration of pandemic exposure did not predict infant socioemotional problems at 12 months or 24 months. Though not statistically significant, we observed a notable coefficient for infant language at 12 months, such that each additional month of pandemic exposure predicted a 0.09 SD decrease in language development when full covariates were added (p = .23). However, there was no indication of an effect of pandemic exposure on the infant language at 24 months, with the coefficients falling between −0.01 and 0.01 in the two models.
TABLE 5.
Associations between pandemic experience variables and infant outcomes at 12 and 24 months
| 12 months | 24 months | |||||||
|---|---|---|---|---|---|---|---|---|
| Socioemotional problems | Language development | Socioemotional problems | Language development | |||||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
| Duration of pandemic exposure | 0.01 | −0.03 | −0.11+ | −0.09 | 0.02 | 0.01 | −0.01 | 0.01 |
| (0.05) | (0.06) | (0.06) | (0.07) | (0.05) | (0.06) | (0.07) | (0.07) | |
| Disruptive life events | 0.02 | 0.02 | 0.02 | 0.03 | 0.01 | 0.01 | 0.02 | 0.01 |
| (0.02) | (0.02) | (0.02) | (0.03) | (0.03) | (0.03) | (0.02) | (0.02) | |
| Controls for maternal characteristics included? | No | Yes | No | Yes | No | Yes | No | Yes |
Note: N = 92. All models control for infant age at the time of the assessment. Maternal mental health symptoms (i.e., outcomes) were standardized, while the duration of pandemic exposure and disruptive life events were not standardized. The duration variable was scaled in months, and the disruptive life event measure was a count of the number of pandemic‐related disruptive events (see Table 2). Standard errors are in parentheses. Analyses were performed using multiple imputation. Maternal covariates include: prenatal depression, anxiety, and perceived stress; years of education (continuous); race and ethnicity (dummy variables for whether Black, other race, or Hispanic); age at delivery; and age‐adjusted NIH‐TB PVT scores. Socioemotional problems and language development are standardized, while the duration of pandemic exposure and disruptive life events are unstandardized. Standard errors are in parentheses.
+p < .10, *p < .05, **p < .01.
3.4.2. Disruptive life events
As Table 5 reflects, we observed no associations between the disruptive life events' survey and socioemotional problems or language development at either 12 or 24 months. Coefficients ranged between 0.01 and 0.03 when full controls were added to the model.
3.5. Interactions with SES
Tables 6 and 7 detail the results from additional interaction models, testing whether SES (defined as ITN as measured at the prenatal visit) moderated the relation between each pandemic predictor variable and maternal and infant outcomes. With the inclusion of controls for maternal baseline characteristics, the duration‐of‐pandemic‐by‐SES interaction did not predict maternal (Table 6) or infant outcomes (Table 7) at either 12 months or 24 months. Similarly, the disruptive‐life‐events‐by‐SES interaction did not predict any maternal or infant outcomes. As a robustness check, we tested these interactions with maternal education at both time points and found null results for those models as well (see Supporting Information S1).
TABLE 6.
Associations between pandemic experience measures and maternal mental health symptoms—Interactions with income‐to‐needs
| 12‐month | 24‐month | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depression | Anxiety | Perceived stress | Depression | Anxiety | Perceived stress | |||||||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | |
| Duration of exposure | 0.03 | 0.01 | 0.06 | 0.04 | 0.02 | 0.02 | 0.01 | 0.02 | 0.06 | 0.07 | 0.01 | 0.01 |
| (0.05) | (0.05) | (0.05) | (0.05) | (0.06) | (0.06) | (0.06) | (0.06) | (0.05) | (0.06) | (0.06) | (0.06) | |
| ITN | −0.01 | −0.02 | 0.01 | −0.01 | −0.03 | −0.05 | 0.07 | 0.07 | 0.01 | −0.05 | 0.07 | 0.06 |
| (0.13) | (0.12) | (0.14) | (0.14) | (0.13) | (0.14) | (0.13) | (0.13) | (0.12) | (0.12) | (0.15) | (0.14) | |
| Duration of exposure × ITN | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | −0.01 | −0.01 | −0.01 | 0.01 | −0.01 | −0.01 |
| (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | |
| Disruptive life events | 0.07** | 0.05* | 0.06** | 0.05* | 0.05* | 0.05+ | 0.05* | 0.06* | 0.05* | 0.05+ | 0.04 | 0.04 |
| (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.03) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.03) | |
| ITN | −0.01 | −0.01 | −0.04 | −0.03 | −0.04 | −0.04 | −0.01 | 0.01 | −0.05 | −0.05 | −0.05 | −0.05 |
| (0.05) | (0.05) | (0.05) | (0.05) | (0.06) | (0.06) | (0.05) | (0.05) | (0.06) | (0.06) | (0.06) | (0.06) | |
| Disruptive life events × ITN | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | −0.01 | −0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | |
| Controls included for maternal baseline characteristics? | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes |
Note: N = 92. ITN was winsorized for values +3 SDs above the mean, then log‐transformed. All models control for infant age at the time of the assessment. Maternal mental health symptoms (i.e., outcomes) were standardized, while the duration of pandemic exposure and disruptive life events were not standardized. The duration variable was scaled in months, and the disruptive life event measure was a count of the number of pandemic‐related disruptive events (see Table 2). Standard errors are in parentheses. Analyses were performed using multiple imputation. Maternal covariates include: prenatal depression, anxiety, and perceived stress; race and ethnicity (dummy variables for whether Black, other race, or Hispanic); age at delivery; and age‐adjusted NIH‐TB PVT scores. Standard errors are in parentheses.
Abbreviation: ITN, income‐to‐needs.
+p < .10, *p < .05, **p < .01.
TABLE 7.
Associations between pandemic experience measures and infant outcomes—Interactions with income‐to‐needs
| 12 months | 24 months | |||||||
|---|---|---|---|---|---|---|---|---|
| Socioemotional problems | Language development | Socioemotional problems | Language development | |||||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
| Duration of exposure | 0.02 | −0.02 | −0.06 | −0.05 | 0.02 | 0.01 | −0.01 | 0.01 |
| (0.05) | (0.06) | (0.06) | (0.07) | (0.05) | (0.06) | (0.07) | (0.07) | |
| ITN | 0.02 | 0.03 | 0.10 | 0.10 | −0.08 | −0.06 | 0.06 | 0.03 |
| (0.12) | (0.14) | (0.17) | (0.17) | (0.14) | (0.14) | (0.15) | (0.15) | |
| Duration of exposure × ITN | −0.01 | −0.01 | −0.02 | −0.02 | 0.01 | 0.01 | −0.01 | −0.01 |
| (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | (0.02) | |
| Disruptive life events | 0.02 | 0.02 | 0.03 | 0.04 | 0.02 | 0.02 | 0.02 | 0.01 |
| (0.02) | (0.02) | (0.02) | (0.03) | (0.03) | (0.03) | (0.02) | (0.03) | |
| ITN | 0.01 | −0.01 | −0.05 | −0.07 | −0.07 | −0.09 | −0.04 | −0.04 |
| (0.05) | (0.06) | (0.06) | (0.07) | (0.07) | (0.07) | (0.06) | (0.07) | |
| Disruptive life events × ITN | −0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | (0.01) | |
| Controls included for maternal baseline characteristics? | No | Yes | No | Yes | No | Yes | No | Yes |
Note: N = 92. ITN was winsorized for values +3 SDs above the mean, then log‐transformed. All models control for infant age at the time of the assessment. Maternal mental health symptoms (i.e., outcomes) were standardized, while the duration of pandemic exposure and disruptive life events were not standardized. The duration variable was scaled in months, and the disruptive life event measure was a count of the number of pandemic‐related disruptive events (see Table 2). Standard errors are in parentheses. Analyses were performed using multiple imputation. Maternal covariates include: prenatal depression, anxiety, and perceived stress; race and ethnicity (dummy variables for whether Black, other race, or Hispanic); age at delivery; and age‐adjusted NIH‐TB PVT scores. Standard errors are in parentheses.
Abbreviation: ITN, income‐to‐needs.
+p < .10, *p < .05, **p < .01.
4. DISCUSSION
This study presents evidence of the effects of the pandemic on maternal and infant functioning in a socioeconomically diverse sample of NYC mothers and infants. We leveraged variations in the infant's age from an ongoing longitudinal study of child development to assess the effect of pandemic exposure within the first year of life. The plausible exogeneity of our pandemic exposure measure is a notable strength of our design. Because the children in our study varied in age at the time of the start of the pandemic, we were able to leverage this variation to examine how the amount of exposure to the pandemic during the first year of life affected infant and maternal outcomes. Overall, we did not detect significant associations between the duration of pandemic exposure in the first year of life and either infant functioning or maternal mental health or perceived stress symptoms at 12 or 24 months.
We also investigated associations between pandemic‐related disruptive life events experienced at 12 months and mother‐infant functioning at concurrent and future time points. We did not detect any associations between disruptive life events and infant outcomes at 12 or 24 months. However, we found that greater disruptive life events were associated with greater maternal anxiety, depression, and perceived stress at the 12‐month visit and greater anxiety and depression at the 24‐month visit. Finally, we found that SES did not moderate the relations between and either pandemic duration or disruptive life events and maternal or infant functioning.
4.1. Maternal mental health
We did not detect associations between the duration of pandemic exposure and mental health or perceived stress symptoms at either 12‐ or 24‐months postpartum. This finding suggests that mothers who spent most of their infant's first year during the pandemic did not report statistically different mental health symptoms than those who spent most of that year prior to the pandemic. We can alternatively frame this finding in terms of timing: there was no effect of an infant's age at the start of the pandemic on maternal mental health and perceived stress.
This is particularly interesting, as one might expect poorer mental health for mothers with young infants at the pandemic's onset. Given that declines in mental health are quite common in the postpartum period (Britton, 2008; Wang et al., 2011), it is encouraging that these mothers do not report higher symptomology at 1‐ or 2‐years postpartum. This finding adds a new perspective to the available literature on maternal mental health and COVID‐19, as past research compares pre‐ and post‐pandemic cohorts of new and expectant mothers with no regard to the age of the infant (e.g., Davenport et al., 2020; Fallon et al., 2021; Gustafsson et al., 2021; Wu et al., 2020; Zanardo et al., 2020).
It is important to note that some notable effect sizes were observed for maternal mental health at both time points. Specifically, the standardized coefficient for anxiety was 0.11 and 0.18 at 12 and 24 months, respectively. Though statistically not significant, the consistency of this effect size across time suggests there may be an effect of pandemic exposure on maternal anxiety that we are not powered to detect. However, our small sample also makes our estimates prone to error. The lack of significance combined with a small sample temper our confidence in these estimates. Regardless, we interpret these findings with optimism: exposure to the pandemic does not appear to have a large effect on maternal anxiety in the postpartum period, if any at all.
It is notable that, overall, we witnessed a decline in mental health symptoms between the pre‐pandemic and post‐pandemic onset measure. This is likely due to the timing of the baseline assessment during pregnancy. Previous research indicates that anxiety and depressive symptoms peak before birth and decrease over the course of the postpartum year for many women (Ahmed et al., 2019; Britton, 2008; Liou et al., 2014; Wang et al., 2011), perhaps explaining the sharp decline in depression and anxiety seen in our sample.
We also found that reports of greater disruptive life events were associated with higher maternal anxiety, depression, and perceived stress at 12 months, but only anxiety and depression at 24 months. This aligns with previous literature that an accumulation of negative life events, including those related to the COVID‐19 pandemic, is associated with worsening mental health and perceived stress symptoms (McLaughlin et al., 2022; Mitchell & Ronzio, 2011; Rossi et al., 2020). It is critical to address mental health and perceived stress in mothers, both for their own well‐being and that of their children (West & Newman, 2003).
Of note, unlike the duration of pandemic exposure variable, the disruptive life event scale is not an exogenous indicator; this measure is therefore subject to reporter bias and the effects of unobserved confounding variables. Indeed, pandemic‐related disruptive life event scores were significantly correlated with prenatal levels of anxiety and depression (though not perceived stress)—measures obtained before the pandemic began. This suggests a possible confound: mothers with poorer mental health may have been more likely to report more pandemic‐related disruptive life events. We therefore urge caution in interpreting the link between disruptive life events and maternal mental health.
Additionally, all maternal and infant outcomes in this study were assessed via maternal report due to remote data collection procedures; therefore, the findings reported here should be interpreted with caution. For example, we detected a sizable correlation between maternal education and disruptive life events, such that greater years of education were associated with more disruptive life events, r = .40. Associations such as these may bias interpretations of self‐reported measures. Further research with more objective measurement techniques will be necessary to clarify these associations.
4.2. Language development
By and large, we did not detect associations between the duration of pandemic exposure during the first year of life and measures of infant development at 12‐ or 24‐months of age. As above, we can also interpret this finding in terms of timing: There was no effect of an infant's age at the onset of the pandemic on language or socioemotional development at these time points. In conjunction with the emerging research on the pandemic, we interpret these results with some reassurance. Given the use of masks, social isolation, and other disruptions to infants' typical environments, we hypothesized that infant language and socioemotional development would be impacted. However, the present data suggest little evidence of that.
Increased time spent in the home may have buffered infants from the potential negative effects of the pandemic on their language development. Indeed, one study found that working class parents who were laid‐off during lockdown engaged in more book reading with their children (Kalil et al., 2020b). It is possible that high‐quality parent‐child interactions and opportunities for increased back‐and‐forth conversational turns buffered infants from negative effects of social isolation on language development. A recent experiment offers further reassurance for infant language: Singh et al. (2021) found no effect of opaque mask wearing on word recognition in a sample of infants. While further experimental and longitudinal research is needed, it is possible that pandemic‐related effects on early childhood development will not be as dire as some have feared.
It is also possible that there was a true effect of the pandemic on infant language at 12 months that we were underpowered to detect, but that this effect further diminished as the infants aged and life resumed a bit more normalcy. As described in the Analytic Plan, we were sufficiently powered to detect effects of 0.28 or greater in linear models with full covariates. For context, the standardized coefficient for the duration of exposure predicting language at 12 months was −0.20 with controls, which could indicate a substantively meaningful effect of pandemic exposure on the infant language. Alternatively, this effect at 12 months may just be noise within the data: one‐year‐old infants have very few words and may use other methods to communicate beyond language, rendering this early estimate prone to measurement errors. Overall, the 24‐month estimate is a better representation of infants' current and future language skills, and there is no evidence of an effect of pandemic exposure on 24‐month language development in our sample (standardized coefficient of 0.02).
4.3. Socioemotional development
The lack of association between pandemic exposure and socioemotional problems was also a promising finding. We were concerned that the greater duration of pandemic exposure (and, presumably, less opportunity to engage in un‐masked social interaction with adults and peers) could have negative impacts on socioemotional development by limiting infants' opportunities to gather emotional information from faces (Kokkinaki & Hatzidaki, 2022). Indeed, a recent experiment found that mask‐wearing inhibits preschool‐aged children's ability to infer emotions from pictures of facial expressions (Gori et al., 2021). However, there was no hint of an effect of pandemic exposure on infant socioemotional development.
Though concerns around masking and socioemotional development are valid, caregivers likely did not mask in their homes during lockdown, providing ample opportunity for unmasked interactions. Furthermore, infants pick up on a myriad of inputs from the social environment to infer emotions and develop social competencies. The generalizability of findings obtained in a controlled lab setting without the input of other social features, such as gestures, intonation, etc., likely do not translate into actual experiences.
Furthermore, an abundance of research has found that maternal depression, anxiety, and perceived stress are associated with poorer infant emotion regulation (D'Souza, Waldie, et al., 2019; Feldman et al., 2009; Troller‐Renfree et al., 2022). However, the present sample of mothers did not report greater mental health symptoms due to the duration of pandemic exposure in the postpartum year. This may partially explain the lack of effect seen of the pandemic on infant socioemotional problems.
Nonetheless, there may be downstream effects of the pandemic on infant behavior that are too early to detect in this sample. For example, it is possible that effects of reduced interaction with those outside of the immediate family may not appear until later in development when children start interacting socially with peers. Future studies should follow infants raised during the pandemic as they enter early education settings to evaluate their social adjustment.
We did not find significant associations between disruptive life events and infant outcomes. This was somewhat surprising, given that early life stress exposure often interacts with developing systems to influence behavior (Gee & Casey, 2015). One possible explanation for the lack of detectable associations may be due to the timing of stressful events. For example, some work has reported declines in children's vocabulary when a natural disaster occurred in utero, but not for children who were in the first year of life at the time of the event (Rosales‐Rueda, 2018). Though greater disruptive life events were associated with worse maternal mental health and perceived stress symptoms, it may be that maternal interactions with their children buffered the effects of these events, or that the effects of these events are not yet detectable in infants.
Stress exposure for infants may be particularly deleterious when it occurs prenatally. Indeed, an abundance of research finds significant associations between prenatal stress and both infant language and socioemotional problems (D'Souza, Crawford, et al., 2019; Keim et al., 2011; King et al., 2012; Lin et al., 2017; Madigan et al., 2018). Emerging research on the pandemic supports this hypothesis. One study detected links between pandemic‐related stress in the prenatal period with dysregulated infant temperament at 3‐months postpartum (Provenzi et al., 2021). Similarly, a recent study found that infants born to mothers who were in their first trimester of pregnancy during the peak of the pandemic demonstrated lower scores on a socioemotional assessment at 6 months (Shuffrey et al., 2021). It is possible that prenatal exposure to pandemic‐related stress and/or COVID‐19 infection may operate through an inflammatory cascade to negatively impact infant neurodevelopment (e.g., Martins‐Filho et al., 2020; Shuffrey et al., 2021).
4.4. Moderation by socioeconomic status
We did not observe any significant interactions for either the duration of exposure or disruptive life events and SES. As noted earlier, our small sample prevents us from detecting small associations—a problem that is further compounded by moderation analyses. It is possible that true interactions do indeed exist, but that we were not sufficiently powered to detect them. Future research should continue to test interactive effects by SES to paint a more complete picture of the pandemic's effects on mothers and infants, given very limited research on the potential differential impacts of the pandemic by SES.
4.5. Limitations and conclusion
Overall, the conclusions of this study warrant cautious optimism: the greater duration of the first year of life spent in the pandemic does not appear to be associated with reductions in language development or increases in socioemotional problems at 12 or 24 months of age in our sample. Similarly, mothers who experienced most of the first‐year postpartum during the COVID‐19 pandemic did not report significantly higher mental health symptoms than those who experienced most of that first year prior to the pandemic.
This study is limited by its small sample, relatively high attrition, and reliance on maternal self‐report. Additionally, it is possible there are unobserved differences in mothers who gave birth earlier in the study compared to those who gave birth later, which would threaten the exogeneity of our duration of pandemic exposure variable. Nonetheless, the nonsignificant associations between baseline controls and the duration of exposure measure lend confidence that this variable is truly exogenous. Finally, we cannot disentangle the effects of duration from the effects of timing of exposure, given that the pandemic was ongoing for all participants at the time of postpartum data collection.
Importantly, this study offers one of the first investigations with multiple assessment points and an exogenous indicator of pandemic exposure. This is one of the first studies to investigate the effect of the pandemic on mothers and infants in NYC, an early epicenter of the pandemic in the United States. More research is needed to clarify the effect of the pandemic for mothers and infants using larger samples, objective measurement techniques, and transdiagnostic approaches to aid in the early identification of developmental disorders (Finlay‐Jones et al., 2019). We aim to continue to follow these families to evaluate the impact of early pandemic exposure on subsequent childhood development and physiology.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
This study was made possible by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD093707‐01 to KGN and K99HD104923 to STR). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health. We would like to thank Olivia Colon, Melina Amarante, and Isabel Kovacs for their help with data collection. The authors declare no conflicts of interest with regard to the funding source for this study.
Sperber, J. F. , Hart, E. R. , Troller‐Renfree, S. V. , Watts, T. W. , & Noble, K. G. (2023). The effect of the COVID‐19 pandemic on infant development and maternal mental health in the first 2 years of life. Infancy, 28(1), 107–135. 10.1111/infa.12511
REFERENCES
- Ahmed, A. , Bowen, A. , Feng, C. X. , & Muhajarine, N. (2019). Trajectories of maternal depressive and anxiety symptoms from pregnancy to five years postpartum and their prenatal predictors. BMC Pregnancy and Childbirth, 19(1), 26. 10.1186/s12884-019-2177-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett, B. , Schaafsma, M. F. , Guzman, A.‐M. , & Parker, G. B. (1991). Maternal anxiety: A 5‐year review of an intervention study. Journal of Child Psychology and Psychiatry, 32(3), 423–438. 10.1111/j.1469-7610.1991.tb00321.x [DOI] [PubMed] [Google Scholar]
- Beck, A. T. , Epstein, N. , Brown, G. , & Steer, R. A. (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. 10.1037/0022-006X.56.6.893 [DOI] [PubMed] [Google Scholar]
- Briggs‐Gowan, M. J. (2004). The brief infant‐toddler social and emotional assessment: Screening for social‐emotional problems and delays in competence. Journal of Pediatric Psychology, 29(2), 143–155. 10.1093/jpepsy/jsh017 [DOI] [PubMed] [Google Scholar]
- Briggs‐Gowan, M. J. , Carter, A. S. , Irwin, J. R. , Wachtel, K. , & Cicchetti, D. V. (2004). The Brief infant‐toddler social and emotional assessment: Screening for social‐emotional problems and delays in competence. Journal of Pediatric Psychology, 29(2), 143–155. 10.1093/jpepsy/jsh017 [DOI] [PubMed] [Google Scholar]
- Britton, J. R. (2008). Maternal anxiety: Course and antecedents during the early postpartum period. Depression and Anxiety, 25(9), 793–800. 10.1002/da.20325 [DOI] [PubMed] [Google Scholar]
- Brookman, R. , Kalashnikova, M. , Conti, J. , Xu Rattanasone, N. , Grant, K.‐A. , Demuth, K. , & Burnham, D. (2020). Depression and anxiety in the postnatal period: An examination of infants' home language environment, vocalizations, and expressive language abilities. Child Development, 91(6), e1211–e1230. 10.1111/cdev.13421 [DOI] [PubMed] [Google Scholar]
- Brownell, C. A. , & Brown, E. (1992). Peers and play in infants and toddlers. In Van Hasselt V. B. & Hersen M. (Eds.), Handbook of social development: A lifespan perspective (pp. 183–200). Springer. 10.1007/978-1-4899-0694-6_8 [DOI] [Google Scholar]
- Clarkson Freeman, P. A. (2014). Prevalence and relationship between adverse childhood experiences and child behavior among young children. Infant Mental Health Journal, 35(6), 544–554. 10.1002/imhj.21460 [DOI] [PubMed] [Google Scholar]
- Cohen, S. , & Williamson, G. (1988). Perceived stress in a probability sample of the United States. In The social psychology of health (pp. 31–67). Sage Publications, Inc. [Google Scholar]
- Davenport, M. H. , Meyer, S. , Meah, V. L. , Strynadka, M. C. , & Khurana, R. (2020). Moms are not OK: COVID‐19 and maternal mental health. Frontiers in Global Women's Health, 1. 10.3389/fgwh.2020.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza, S. , Crawford, C. N. , Buckley, J. , Underwood, L. , Peterson, E. R. , Bird, A. , Morton, S. M. B. , & Waldie, K. E. (2019). Antenatal determinants of early childhood talking delay and behavioural difficulties. Infant Behavior and Development, 57, 101388. 10.1016/j.infbeh.2019.101388 [DOI] [PubMed] [Google Scholar]
- D'Souza, S. , Waldie, K. E. , Peterson, E. R. , Underwood, L. , & Morton, S. M. B. (2019). Antenatal and postnatal determinants of behavioural difficulties in early childhood: Evidence from growing up in New Zealand. Child Psychiatry and Human Development, 50(1), 45–60. 10.1007/s10578-018-0816-6 [DOI] [PubMed] [Google Scholar]
- Egan, S. M. , Pope, J. , Moloney, M. , Hoyne, C. , & Beatty, C. (2021). Missing early education and care during the pandemic: The socio‐emotional impact of the COVID‐19 crisis on young children. Early Childhood Education Journal, 49(5), 925–934. 10.1007/s10643-021-01193-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon, V. , Davies, S. M. , Silverio, S. A. , Jackson, L. , De Pascalis, L. , & Harrold, J. A. (2021). Psychosocial experiences of postnatal women during the COVID‐19 pandemic. A UK‐wide study of prevalence rates and risk factors for clinically relevant depression and anxiety. Journal of Psychiatric Research, 136, 157–166. 10.1016/j.jpsychires.2021.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, R. (2007). Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry, 48(3–4), 329–354. 10.1111/j.1469-7610.2006.01701.x [DOI] [PubMed] [Google Scholar]
- Feldman, R. , Granat, A. , Pariente, C. , Kanety, H. , Kuint, J. , & Gilboa‐Schechtman, E. (2009). Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child & Adolescent Psychiatry, 48(9), 919–927. 10.1097/CHI.0b013e3181b21651 [DOI] [PubMed] [Google Scholar]
- Field, T. , Hernandez‐Reif, M. , & Diego, M. (2006). Intrusive and withdrawn depressed mothers and their infants. Developmental Review, 26(1), 15–30. 10.1016/j.dr.2005.04.001 [DOI] [Google Scholar]
- Finch, W. H. , & Hernández Finch, M. E. (2020). Poverty and Covid‐19: Rates of incidence and deaths in the United States during the first 10 weeks of the pandemic. Frontiers in Sociology, 5, 47. 10.3389/fsoc.2020.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay‐Jones, A. , Varcin, K. , Leonard, H. , Bosco, A. , Alvares, G. , & Downs, J. (2019). Very early identification and intervention for infants at risk of neurodevelopmental disorders: A transdiagnostic approach. Child Development Perspectives, 13(2), 97–103. 10.1111/cdep.12319 [DOI] [Google Scholar]
- Ford, A. L. B. , Elmquist, M. , Merbler, A. M. , Kriese, A. , Will, K. K. , & McConnell, S. R. (2020). Toward an ecobehavioral model of early language development. Early Childhood Research Quarterly, 50, 246–258. 10.1016/j.ecresq.2018.11.004 [DOI] [Google Scholar]
- Gee, D. G. , & Casey, B. J. (2015). The impact of developmental timing for stress and recovery. Neurobiology of Stress, 1, 184–194. 10.1016/j.ynstr.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkerson, J. , Richards, J. A. , Greenwood, C. R. , & Montgomery, J. K. (2017). Language assessment in a snap: Monitoring progress up to 36 months. Child Language Teaching and Therapy, 33(2), 99–115. 10.1177/0265659016660599 [DOI] [Google Scholar]
- Gori, M. , Schiatti, L. , & Amadeo, M. B. (2021). Masking emotions: Face masks impair how we read emotions. Frontiers in Psychology, 12, 669432. 10.3389/fpsyg.2021.669432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso, D. J. , Briggs‐Gowan, M. J. , Ford, J. D. , & Carter, A. S. (2020). Epidemic – Pandemic impacts inventory (EPII), (vol. 6). University of Connecticut School of Medicine. [Google Scholar]
- Grumi, S. , Provenzi, L. , Accorsi, P. , Biasucci, G. , Cavallini, A. , Decembrino, L. , Falcone, R. , Fazzi, E. M. , Gardella, B. , Giacchero, R. , Guerini, P. , Grossi, E. , Magnani, M. L. , Mariani, E. M. , Nacinovich, R. , Pantaleo, D. , Pisoni, C. , Prefumo, F. , Sabatini, C. , … Borgatti, R. (2021). Depression and anxiety in mothers who were pregnant during the COVID‐19 outbreak in Northern Italy: The role of pandemic‐related emotional stress and perceived social support. Frontiers in Psychiatry, 12, 716488. 10.3389/fpsyt.2021.716488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson, H. C. , Young, A. S. , Doyle, O. , Nagel, B. J. , Mackiewicz Seghete, K. , Nigg, J. T. , Sullivan, E. L. , & Graham, A. M. (2021). Trajectories of perinatal depressive symptoms in the context of the COVID‐19 pandemic. Child Development, 92(5), e749–e763. 10.1111/cdev.13656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, R. A. , & Santos, H. P. J. (2020). Maternal depression in Latinas and child socioemotional development: A systematic review. PLoS One, 15(3), e0230256. 10.1371/journal.pone.0230256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart, E. R. , Troller‐Renfree, S. V. , Sperber, J. F. , & Noble, K. G. (2022). Relations among socioeconomic status, perceived stress, and the home language environment. International Mind, Brain, and Education Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins, R. B. , Charles, E. J. , & Mehaffey, J. H. (2020). Socio‐economic status and COVID‐19–Related cases and fatalities. Public Health, 189, 129–134. 10.1016/j.puhe.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis, S. D. , Blenkinsop, A. , Villaveces, A. , Annor, F. B. , Liburd, L. , Massetti, G. M. , Demissie, Z. , Mercy, J. A. , Nelson, C. A., III , Cluver, L. , Flaxman, S. , Sherr, L. , Donnelly, C. A. , Ratmann, O. , & Unwin, H. J. T. (2021). COVID‐19–Associated orphanhood and caregiver death in the United States. Pediatrics, 148(6), e2021053760. 10.1542/peds.2021-053760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ierardi, E. , Ferro, V. , Trovato, A. , Tambelli, R. , & Riva Crugnola, C. (2019). Maternal and paternal depression and anxiety: Their relationship with mother‐infant interactions at 3 months. Archives of Women's Mental Health, 22(4), 527–533. 10.1007/s00737-018-0919-x [DOI] [PubMed] [Google Scholar]
- Imboden, A. , Sobczak, B. K. , & Griffin, V. (2021). The impact of the COVID‐19 pandemic on infant and toddler development. Journal of the American Association of Nurse Practitioners, 34(3), 509–519. 10.1097/JXX.0000000000000653 [DOI] [PubMed] [Google Scholar]
- Justice, L. M. , Jiang, H. , Purtell, K. M. , Schmeer, K. , Boone, K. , Bates, R. , & Salsberry, P. J. (2019). Conditions of poverty, parent–child interactions, and toddlers' early language skills in low‐income families. Maternal and Child Health Journal, 23(7), 971–978. 10.1007/s10995-018-02726-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil, A. , Mayer, S. , & Shah, R. (2020a). Impact of the COVID‐19 crisis on family dynamics in economically vulnerable households (SSRN Scholarly Paper ID 3705592). Social Science Research Network. 10.2139/ssrn.3705592 [DOI] [Google Scholar]
- Kalil, A. , Mayer, S. , & Shah, R. (2020b). Impact of the COVID‐19 crisis on family dynamics in economically vulnerable households. In Working papers (No. 2020–143; working papers). Becker Friedman Institute for Research in Economics. https://ideas.repec.org/p/bfi/wpaper/2020‐143.html [Google Scholar]
- Karmakar, M. , Lantz, P. M. , & Tipirneni, R. (2021). Association of social and demographic factors with COVID‐19 incidence and death rates in the US. JAMA Network Open, 4(1), e2036462. 10.1001/jamanetworkopen.2020.36462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartushina, N. , Mani, N. , Aktan‐Erciyes, A. , Alaslani, K. , Aldrich, N. J. , Almohammadi, A. , Alroqi, H. , Anderson, L. , Andonova, E. , Aussems, S. , Babineau, M. , Barokova, M. , Bergmann, C. , Cashon, C. H. , Custode, S. , de Carvalho, A. , Dimitrova, N. , Dynak, A. , Farah, R. , … Mayor, J. (2021). COVID‐19 first lockdown as a window into language acquisition: Associations between caregiver‐child activities and vocabulary gains. PsyArXiv. 10.31234/osf.io/5ejwu [DOI] [Google Scholar]
- Keim, S. A. , Daniels, J. L. , Dole, N. , Herring, A. H. , Siega‐Riz, A. M. , & Scheidt, P. C. (2011). A prospective study of maternal anxiety, perceived stress, and depressive symptoms in relation to infant cognitive development. Early Human Development, 87(5), 373–380. 10.1016/j.earlhumdev.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S. , Dancause, K. , Turcotte‐Tremblay, A.‐M. , Veru, F. , & Laplante, D. P. (2012). Using natural disasters to study the effects of prenatal maternal stress on child health and development. Birth Defects Research Part C: Embryo Today – Reviews, 96(4), 273–288. 10.1002/bdrc.21026 [DOI] [PubMed] [Google Scholar]
- Kokkinaki, T. , & Hatzidaki, E. (2022). COVID‐19 pandemic‐related restrictions: Factors that may affect perinatal maternal mental health and implications for infant development. Frontiers in Pediatrics, 10. 10.3389/fped.2022.846627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke, K. , Strine, T. W. , Spitzer, R. L. , Williams, J. B. W. , Berry, J. T. , & Mokdad, A. H. (2009). The PHQ‐8 as a measure of current depression in the general population. Journal of Affective Disorders, 114(1), 163–173. 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- Lam‐Cassettari, C. , & Kohlhoff, J. (2020). Effect of maternal depression on infant‐directed speech to prelinguistic infants: Implications for language development. PLoS One, 15(7), e0236787. 10.1371/journal.pone.0236787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante, D. P. , Barr, R. G. , Brunet, A. , Du Fort, G. G. , Meaney, M. L. , Saucier, J.‐F. , Zelazo, P. R. , & King, S. (2004). Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatric Research, 56(3), 400–410. 10.1203/01.PDR.0000136281.34035.44 [DOI] [PubMed] [Google Scholar]
- Lehr, M. , Wecksell, B. , Nahum, L. , Neuhaus, D. , Teel, K. S. , Linares, L. O. , & Diaz, A. (2016). Parenting stress, child characteristics, and developmental delay from birth to age five in teen mother–child dyads. Journal of Child and Family Studies, 25(3), 1035–1043. 10.1007/s10826-015-0282-8 [DOI] [Google Scholar]
- Lin, Y. , Xu, J. , Huang, J. , Jia, Y. , Zhang, J. , Yan, C. , & Zhang, J. (2017). Effects of prenatal and postnatal maternal emotional stress on toddlers' cognitive and temperamental development. Journal of Affective Disorders, 207, 9–17. 10.1016/j.jad.2016.09.010 [DOI] [PubMed] [Google Scholar]
- Liou, S.‐R. , Wang, P. , & Cheng, C.‐Y. (2014). Longitudinal study of perinatal maternal stress, depressive symptoms and anxiety. Midwifery, 30(6), 795–801. 10.1016/j.midw.2013.11.007 [DOI] [PubMed] [Google Scholar]
- Madigan, S. , Oatley, H. , Racine, N. , Fearon, R. M. P. , Schumacher, L. , Akbari, E. , Cooke, J. E. , & Tarabulsy, G. M. (2018). A meta‐analysis of maternal prenatal depression and anxiety on child socioemotional development. Journal of the American Academy of Child & Adolescent Psychiatry, 57(9), 645–657.e8. 10.1016/j.jaac.2018.06.012 [DOI] [PubMed] [Google Scholar]
- Magill‐Evans, J. , & Harrison, M. J. (2001). Parent‐child interactions, parenting stress, and developmental outcomes at 4 years. Children's Health Care, 30(2), 135–150. 10.1207/S15326888CHC3002_4 [DOI] [Google Scholar]
- Marte, J. (2020, December 15). Fleeing New Yorkers resulted in an estimated $34 billion in lost income‐study. Reuters. https://www.reuters.com/article/usa‐economy‐nyc‐idINKBN28P1Q8 [Google Scholar]
- Martins‐Filho, P. R. , Tanajura, D. M. , Santos, H. P. , & Santos, V. S. (2020). COVID‐19 during pregnancy: Potential risk for neurodevelopmental disorders in neonates? European Journal of Obstetrics & Gynecology and Reproductive Biology, 250, 255–256. 10.1016/j.ejogrb.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, K. A. , Rosen, M. L. , Kasparek, S. W. , & Rodman, A. M. (2022). Stress‐related psychopathology during the COVID‐19 pandemic. Behaviour Research and Therapy, 154, 104121. 10.1016/j.brat.2022.104121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, S. J. , & Ronzio, C. R. (2011). Violence and other stressful life events as triggers of depression and anxiety: What psychosocial resources protect African American mothers? Maternal and Child Health Journal, 15(8), 1272–1281. 10.1007/s10995-010-0668-6 [DOI] [PubMed] [Google Scholar]
- Morris, A. R. , & Saxbe, D. E. (2021). Mental health and prenatal bonding in pregnant women during the COVID‐19 pandemic: Evidence for heightened risk compared with a prepandemic sample. Clinical Psychological Science, 10(5), 846–855. 10.1177/21677026211049430 [DOI] [Google Scholar]
- Olafsen, K. S. , Kaaresen, P. I. , Handegård, B. H. , Ulvund, S. E. , Dahl, L. B. , & Rønning, J. A. (2008). Maternal ratings of infant regulatory competence from 6 to 12 months: Influence of perceived stress, birth‐weight, and intervention: A randomized controlled trial. Infant Behavior and Development, 31(3), 408–421. 10.1016/j.infbeh.2007.12.005 [DOI] [PubMed] [Google Scholar]
- Pace, A. , Luo, R. , Hirsh‐Pasek, K. , & Golinkoff, R. M. (2017). Identifying pathways between socioeconomic status and language development. Annual Review of Linguistics, 3(1), 285–308. 10.1146/annurev-linguistics-011516-034226 [DOI] [Google Scholar]
- Parker, K. , Minkin, R. , & Bennett, J. (2020, September 24). Economic fallout from COVID‐19 continues to hit lower‐income Americans the hardest. Pew Research Center’s Social & Demographic Trends Project. https://www.pewresearch.org/social‐trends/2020/09/24/economic‐fallout‐from‐covid‐19‐continues‐to‐hit‐lower‐income‐americans‐the‐hardest/ [Google Scholar]
- Perzow, S. E. D. , Hennessey, E.‐M. P. , Hoffman, M. C. , Grote, N. K. , Davis, E. P. , & Hankin, B. L. (2021). Mental health of pregnant and postpartum women in response to the COVID‐19 pandemic. Journal of Affective Disorders Reports, 4, 100123. 10.1016/j.jadr.2021.100123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen, A.‐K. , Räikkönen, K. , Heinonen, K. , Komsi, N. , Järvenpää, A.‐L. , & Strandberg, T. (2008). A transactional model of temperamental development: Evidence of a relationship between child temperament and maternal stress over five years. Social Development, 17(2), 326–340. 10.1111/j.1467-9507.2007.00427.x [DOI] [Google Scholar]
- Phillips, A. C. , Carroll, D. , & Der, G. (2015). Negative life events and symptoms of depression and anxiety: Stress causation and/or stress generation. Anxiety, Stress & Coping, 28(4), 357–371. 10.1080/10615806.2015.1005078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, L. J. , Reilly, E. , & Nelson, C. A. (2021). Associations between maternal stress, early language behaviors, and infant electroencephalography during the first year of life. Journal of Child Language, 48(4), 737–764. 10.1017/S0305000920000501 [DOI] [PubMed] [Google Scholar]
- Premo, E. , Magnuson, K. , Lorenzo, N. , Fox, N. , & Noble, K. (2022). Psychological well‐being of low‐income mothers of one‐year‐olds during the COVID‐19 pandemic. Society for Social Work and Research. https://sswr.confex.com/sswr/2022/webprogram/Paper47233.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzi, L. , & Grumi, S. (2021). The need to study developmental outcomes of children born during the COVID‐19 pandemic. JAMA Pediatrics, 176(1), 103. 10.1001/jamapediatrics.2021.4342 [DOI] [PubMed] [Google Scholar]
- Provenzi, L. , Grumi, S. , Altieri, L. , Bensi, G. , Bertazzoli, E. , Biasucci, G. , Cavallini, A. , Decembrino, L. , Falcone, R. , Freddi, A. , Gardella, B. , Giacchero, R. , Giorda, R. , Grossi, E. , Guerini, P. , Magnani, M. L. , Martelli, P. , Motta, M. , Nacinovich, R. , … Group, M.‐C. S. (2021). Prenatal maternal stress during the COVID‐19 pandemic and infant regulatory capacity at 3 months: A longitudinal study. Development and Psychopathology, 1–9. 10.1017/S0954579421000766 [DOI] [PubMed] [Google Scholar]
- Quevedo, L. A. , Silva, R. A. , Godoy, R. , Jansen, K. , Matos, M. B. , Pinheiro, K. A. T. , & Pinheiro, R. T. (2011). The impact of maternal post‐partum depression on the language development of children at 12 months. Child: Care, Health and Development, 38(3), 420–424. 10.1111/j.1365-2214.2011.01251.x [DOI] [PubMed] [Google Scholar]
- Racine, N. , Hetherington, E. , McArthur, B. A. , McDonald, S. , Edwards, S. , Tough, S. , & Madigan, S. (2021). Maternal depressive and anxiety symptoms before and during the COVID‐19 pandemic in Canada: A longitudinal analysis. Lancet Psychiatry, 8(5), 405–415. 10.1016/S2215-0366(21)00074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck, C. , Van Den Bergh, B. , Tietz, A. , Müller, M. , Ropeter, A. , Zipser, B. , & Pauen, S. (2018). Maternal avoidance, anxiety cognitions and interactive behaviour predicts infant development at 12 months in the context of anxiety disorders in the postpartum period. Infant Behavior and Development, 50, 116–131. 10.1016/j.infbeh.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Robust regression. (n.d.). UCLA Statistical Consulting Group. Retrieved June 10, 2022, from https://stats.oarc.ucla.edu/stata/dae/robust‐regression/ [Google Scholar]
- Rosales‐Rueda, M. (2018). The impact of early life shocks on human capital formation: Evidence from El Niño floods in Ecuador. Journal of Health Economics, 62, 13–44. 10.1016/j.jhealeco.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Rossi, R. , Socci, V. , Talevi, D. , Mensi, S. , Niolu, C. , Pacitti, F. , Di Marco, A. , Rossi, A. , Siracusano, A. , & Di Lorenzo, G. (2020). COVID‐19 pandemic and lockdown measures impact on mental health among the general population in Italy. Frontiers in Psychiatry, 11. 10.3389/fpsyt.2020.00790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruba, A. L. , & Pollak, S. D. (2020). Children's emotion inferences from masked faces: Implications for social interactions during COVID‐19. PLoS One, 15(12), e0243708. 10.1371/journal.pone.0243708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiber, F. A. , Ryckman, K. K. , & Demir‐Lira, Ö. E. (2022). Maternal depressive symptoms and maternal child‐directed speech: A systematic review. Journal of Affective Disorders, 297, 194–207. 10.1016/j.jad.2021.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuffrey, L. C. , Firestein, M. R. , Kyle, M. , Fields, A. , Alcántara, C. , Amso, D. , Austin, J. , Bain, J. M. , Barbosa, J. , Bence, M. , Bianco, C. , Fernández, C. , Goldman, S. , Gyamfi‐Bannerman, C. , Hott, V. , Hu, Y. , Hussain, M. , Factor‐Litvak, P. , Lucchini, M. , … Dumitriu, D. (2021). Birth during the COVID‐19 pandemic, but not maternal SARS‐CoV‐2 infection during pregnancy, is associated with lower neurodevelopmental scores at 6‐months. 10.1101/2021.07.12.21260365 [DOI]
- Singh, L. , Tan, A. , & Quinn, P. C. (2021). Infants recognize words spoken through opaque masks but not through clear masks. Developmental Science, 24(6). 10.1111/desc.13117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp . (2019). Stata statistical software: Release 16 (Version 16) [Computer software]. StataCorp, LLC. [Google Scholar]
- Thompson, C. N. , Baumgartner, J. , Pichardo, C. , Toro, B. , Li, L. , Arciuolo, R. , Chan, P. Y. , Chen, J. , Culp, G. , Davidson, A. , Devinney, K. , Dorsinville, A. , Eddy, M. , English, M. , Fireteanu, A. M. , Graf, L. , Geevarughese, A. , Greene, S. K. , Guerra, K. , … Fine, A. (2020). COVID‐19 outbreak—New York city, February 29–June 1, 2020. MMWR. Morbidity and Mortality Weekly Report, 69(46), 1725–1729. 10.15585/mmwr.mm6946a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer, S. G. , Ho, L. K. L. , Urquiza, A. J. , Zebell, N. M. , Fernandez y Garcia, E. , & Boys, D. (2011). The effectiveness of parent–child interaction therapy with depressive mothers: The changing relationship as the agent of individual change. Child Psychiatry and Human Development, 42(4), 406–423. 10.1007/s10578-011-0226-5 [DOI] [PubMed] [Google Scholar]
- Troller‐Renfree, S. V. , Hart, E. R. , Sperber, J. F. , Fox, N. A. , & Noble, K. G. (2022). Associations among stress and language and socioemotional development in a low‐income sample. Development and Psychopathology, 34(2), 1–9. 10.1017/S0954579421001759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache, A. , Merz, E. C. , Melvin, S. , Meyer, J. , & Noble, K. G. (2017). Socioeconomic status, hair cortisol and internalizing symptoms in parents and children. Psychoneuroendocrinology, 78, 142–150. 10.1016/j.psyneuen.2017.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandell, D. L. , & Wilson, K. S. (1987). Infants' interactions with mother, sibling, and peer: Contrasts and relations between interaction systems. Child Development, 58(1), 176–186. 10.2307/1130299 [DOI] [PubMed] [Google Scholar]
- Van Vaerenbergh, Y. , & Thomas, T. D. (2013). Response styles in survey research: A literature review of antecedents, consequences, and remedies. International Journal of Public Opinion Research, 25(2), 195–217. 10.1093/ijpor/eds021 [DOI] [Google Scholar]
- Venta, A. , Bick, J. , & Bechelli, J. (2021). COVID‐19 threatens maternal mental health and infant development: Possible paths from stress and isolation to adverse outcomes and a call for research and practice. Child Psychiatry and Human Development, 52(2), 200–204. 10.1007/s10578-021-01140-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Wu, T. , Anderson, J. L. , & Florence, J. E. (2011). Prevalence and risk factors of maternal depression during the first three years of child rearing. Journal of Women's Health, 20(5), 711–718. 10.1089/jwh.2010.2232 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , & Dix, T. (2013). Patterns of depressive parenting: Why they occur and their role in early developmental risk. Journal of Family Psychology, 27(6), 884–895. 10.1037/a0034829 [DOI] [PubMed] [Google Scholar]
- Weintraub, S. , Dikmen, S. S. , Heaton, R. K. , Tulsky, D. S. , Zelazo, P. D. , Bauer, P. J. , Carlozzi, N. E. , Slotkin, J. , Blitz, D. , Wallner‐Allen, K. , Fox, N. A. , Beaumont, J. L. , Mungas, D. , Nowinski, C. J. , Richler, J. , Deocampo, J. A. , Anderson, J. E. , Manly, J. J. , Borosh, B. , … Gershon, R. C. (2013). Cognition assessment using the NIH Toolbox. Neurology, 80(11 Supplement 3), S54–S64. 10.1212/WNL.0b013e3182872ded [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisleder, A. , & Fernald, A. (2013). Talking to children matters: Early language experience strengthens processing and builds vocabulary. Psychological Science, 24(11), 2143–2152. 10.1177/0956797613488145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, A. E. , & Newman, D. L. (2003). Worried and blue: Mild parental anxiety and depression in relation to the development of young children's temperament and behavior problems. Parenting: Science and Practice, 3(2), 133–154. 10.1207/S15327922PAR0302_02 [DOI] [Google Scholar]
- Wu, Y. , Zhang, C. , Liu, H. , Duan, C. , Li, C. , Fan, J. , Li, H. , Chen, L. , Xu, H. , Li, X. , Guo, Y. , Wang, Y. , Li, X. , Li, J. , Zhang, T. , You, Y. , Li, H. , Yang, S. , Tao, X. , … Huang, H. (2020). Perinatal depressive and anxiety symptoms of pregnant women during the coronavirus disease 2019 outbreak in China. American Journal of Obstetrics and Gynecology, 223(2), 240.e1–240.e9. 10.1016/j.ajog.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardo, V. , Manghina, V. , Giliberti, L. , Vettore, M. , Severino, L. , & Straface, G. (2020). Psychological impact of COVID‐19 quarantine measures in northeastern Italy on mothers in the immediate postpartum period. International Journal of Gynecology & Obstetrics, 150(2), 184–188. 10.1002/ijgo.13249 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1


