Abstract
Waning antibody levels against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and the emergence of variants of concern highlight the need for booster vaccinations. This is particularly important for the elderly population, who are at a higher risk of developing severe coronavirus disease 2019 (COVID‐19) disease. While studies have shown increased antibody responses following booster vaccination, understanding the changes in T and B cell compartments induced by a third vaccine dose remains limited. We analyzed the humoral and cellular responses in subjects who received either a homologous messenger RNA(mRNA) booster vaccine (BNT162b2 + BNT162b2 + BNT162b2; ‘‘BBB”) or a heterologous mRNA booster vaccine (BNT162b2 + BNT162b2 + mRNA‐1273; ‘‘BBM”) at Day 0 (prebooster), Day 7, and Day 28 (postbooster). Compared with BBB, elderly individuals (≥60 years old) who received the BBM vaccination regimen display higher levels of neutralizing antibodies against the Wuhan and Delta strains along with a higher boost in immunoglobulin G memory B cells, particularly against the Omicron variant. Circulating T helper type 1(Th1), Th2, Th17, and T follicular helper responses were also increased in elderly individuals given the BBM regimen. While mRNA vaccines increase antibody, T cell, and B cell responses against SARS‐CoV‐2 1 month after receiving the third dose booster, the efficacy of the booster vaccine strategies may vary depending on age group and regimen combination.
Keywords: B cell, humoral immunity, immunity/immunization, SARS coronavirus, T cell
1. INTRODUCTION
As of June 2022, over 11 billion coronavirus disease 2019 (COVID‐19) vaccines have been administered around the world. 1 Real‐world data have shown that humoral responses against severe acute syndrome coronavirus 2 (SARS‐CoV‐2) wane as early as 6 months postinfection. Combined with the emergence of variants of interests, the need for booster vaccinations to increase vaccine effectiveness has become evident. 2 , 3
Since the first approved SARS‐CoV‐2 vaccine was in November 2020, there are now over 11 vaccines authorized for emergency use or approved for full use. 4 Of these, two messenger RNA (mRNA) vaccines, BNT162b2 and mRNA‐1273, are the most administered. 1 In November 2021, the Food and Drug Administration approved the use of heterologous vaccine booster combinations. 5 While both homologous and heterologous booster vaccinations are immunogenic in adults, 6 evidence suggests that a heterologous regimen may be better at eliciting neutralizing antibodies and offer better protection against breakthrough infections. 6 , 7 , 8 , 9 , 10 However, the immune mechanisms leading to a more robust humoral response upon heterologous booster strategies, remain obscure. 11 Additionally, knowledge of the efficacy of these various combinations in different age groups is limited.
To investigate this, a cohort of 79 individuals who received two doses of BNT162b2 were recruited as part of the PRIBIVAC study. 7 The individuals received a booster dose (median of 228 days after the second dose) of either BNT162b2 or mRNA‐1273 vaccines. We compared their immune responses with SARS‐CoV‐2 before receiving their third vaccine dose with follow‐ups at 7‐ and 28‐days post boosting (dpb). By 28 dpb, the heterologous mRNA booster vaccine elicited higher neutralizing antibody titers against the Wuhan and Delta strains, stronger receptor‐binding domain (RBD)‐specific memory B cell (MBC) responses, particularly against the Omicron variant (BA.1), and higher levels of antigen‐specific T follicular helper (Tfh) cells in participants over the age of 60.
2. METHODS
2.1. Ethics statement and study population
A total of 79 participants were recruited as part of the PRIBIVAC study, a randomized, subject‐blinded study to compare the immunogenicity and safety of a heterologous (BNT162b2 + BNT162b2 + mRNA‐1273; “BBM”) COVID‐19 booster regimen versus a homologous (BNT162b2 + BNT162b2 + BNT162b2; “BBB”) COVID‐19 booster regimen. 7 Participants were recruited and enrolled at the National Centre for Infectious Diseases in Singapore (ClinicalTrials.gov identifier: NCT05142319). Participants who had received two doses of the BNT162b2 vaccine 174–327 days (median of 228 days) before enrollment were selected. Participants were excluded if they had already been infected with SARS‐CoV‐1 or SARS‐CoV‐2 (as assessed by the absence of antibodies to SARS‐CoV‐2 N protein) or had a history of being immunocompromised (e.g., requiring immunosuppressant medication, undergoing chemotherapy, or diagnosed with leukemia). Participants were randomly given an intramuscular dose of either BNT162b2 or mRNA1273 as a booster. Blood samples were taken before receiving the booster (Day 0) and at 7 days and at 28 days postbooster for immunological analysis (Table 1 and Supporting Information: Table 1).
Table 1.
Demographics of the cohort
| BNT162b2 | mRNA1273 | All | ||||
|---|---|---|---|---|---|---|
| <60 | ≥60 | <60 | ≥60 | |||
| n | n = 21 | n = 21 | n = 17 | n = 20 | n = 79 | |
| n = 42 | n = 37 | |||||
| Age and sex | ||||||
| Age, median (IQR) | 31 (24–58) | 69 (60–78) | 33 (25–59) | 66 (60–84) | ||
| 59 (24–78) | 61 (25–84) | 60 (24–84) | ||||
| Female | n | 15 | 8 | 6 | 13 | 42 |
| % | 71% | 38% | 35% | 65% | 53% | |
| n (%) | 23 (55%) | 19 (51%) | ||||
| Male | n | 6 | 13 | 11 | 37 | |
| % | 29% | 62% | 65% | 35% | 47% | |
| n (%) | 19 (45%) | 18 (49%) | ||||
| Ethnicity | ||||||
| Chinese | n | 17 | 20 | 15 | 20 | 72 |
| % | 81% | 95% | 88% | 100% | 91% | |
| n (%) | 37 (88.1%) | 35 (94.6%) | ||||
| Malay | n | 3 | 0 | 0 | 0 | 3 |
| % | 14% | 0% | 0% | 0% | 4% | |
| n (%) | 3 (7.1%) | 0 (0%) | ||||
| Indian | n | 1 | 0 | 1 | 0 | 2 |
| % | 5% | 0% | 6% | 0% | 2.5% | |
| n (%) | 1 (2.4%) | 1 (2.7%) | ||||
| Other | n | 0 | 1 | 1 | 0 | 2 |
| % | 0% | 5% | 6% | 0% | 2.5% | |
| n (%) | 1 (2.4%) | 1 (2.7%) | ||||
Note: Values in bold are for BNT162b2 and mRNA1273 groups regardless of age.
Abbreviations: IQR, interquartile range; mRNA, messenger RNA.
2.2. Peripheral blood mononuclear cells and plasma collection
Whole blood was collected in BD Vacutainer Cell Preparation Tubes (Becton‐Dickinson). Plasma and peripheral blood mononuclear cell (PBMC) fractions were harvested separately after 20 min centrifugation at 1700g and frozen for downstream analysis. Plasma samples were inactivated by solvent/detergent treatment with a final concentration of 1% Triton X‐100 (Thermo Fisher Scientific) at room temperature for 2 h before use.
2.3. Spike protein flow cytometry‐based assay for antibody detection
The spike protein flow cytometry‐based (SFB) assay was performed as previously described. 11 , 12 HEK293T cells expressing the spike protein of either the Wuhan wild‐type (WT), Delta, or Omicron variants, were seeded at 1.5 × 105 cells per well in 96‐well V‐bottom plates (Thermo Fisher Scientific). Cells were incubated with human plasma samples (diluted 1:100 in 10% fetal bovine serum [FBS]; HyClone) or FBS as negative control (Supporting Information: Figure 11) for 30 min at 4°C, followed by a secondary 30 min incubation at 4°C with a double stain, comprising Alexa Fluor 647‐conjugated antihuman immunoglobulin G (IgG) (1:500 dilution; Thermo Fisher Scientific) and propidium iodide (1:2500 dilution; Sigma‐Aldrich). Cells were acquired using a BD Biosciences LSR4 laser cytometer (Becton‐Dickinson) and analyzed using FlowJo (Tree Star; BD Biosciences). The assay was performed as two independent experiments, each with a technical duplicate.
2.4. Pseudovirus neutralization assay
The pseudotyped lentivirus neutralization assay was performed as previously described with slight modifications. 13 Briefly, a stable cell line expressing human angiotensin‐converting enzyme 2 (ACE2), Chinese hamster ovary (CHO)‐ACE2 (a kind gift from Professor Yee‐Joo Tan, Department of Microbiology, NUS & IMCB, A*STAR), 14 was used for the assay. CHO‐ACE2 cells were seeded at 1.8 × 104 cells per well in a 96‐well black microplate (Corning) in Dulbecco's modified Eagle's medium (DMEM) without Geneticin overnight. Serially diluted heat‐inactivated plasma samples (1:20–1:5120 dilutions) were incubated with an equal volume of pseudovirus expressing S proteins of the respective SARS‐CoV‐2 strain (5 ng of p24 per well) at 37°C for 1 h, before being added to preseeded CHO‐ACE2 cells in duplicate. Wells were topped up with DMEM after 1 h incubation. After 48 h, cells were washed with phosphate‐buffered saline (PBS) and lysed with 1X Passive Lysis Buffer (Promega) with gentle shaking at 125 rpm for 30 min at 37°C. Luciferase activity was subsequently quantified with Luciferase Assay System (Promega) on a GloMax Luminometer (Promega).
2.5. MBC ELISpot
SARS‐CoV‐2 RBD‐specific MBC numbers were counted using ELISpot. MultiScreen HTS IP Filter Plate, 0·45 µm plates (Merck Millipore) were coated overnight at 4°C with purified antihuman‐IgG (MT91/145, Mabtech) or purified anti‐human‐IgA prepared at 15 μg/ml in PBS 1X. Plates were washed four times with PBS 1X and blocked for 30 min at room temperature with Roswell Park Memorial Institute (RPMI) + 10% FBS. A 1 × 106 PBMCs were resuspended in 1 ml RPMI + 10% FBS + 1 μg/ml R848 + 10 ng/ml interleukin (IL‐2), and incubated at 37°C, 5% CO2 for 5 days to differentiate MBCs into antibody‐secreting cells. After incubation, cells were counted, and 100 000 or 400 000 live cells were taken for ELISpot plating to determine RBD‐specific MBC numbers. Total IgG or IgA‐secreting cells were determined by plating 1500 or 3000 live cells. Cells were incubated for 18–22 h at 37°C, with 5% CO2 in the ELISpot plate before detection. A combination of RBD‐WASP/anti‐WASP‐alkaline phosphatase(ALP) or anti‐IgG‐biotinylated/streptavidin‐ALP or anti‐IgA‐biotinylated/streptavidin‐ALP (Mabtech) was used to detect RBD‐specific or total IgG‐ or total IgA‐secreting cells, respectively.
For the detection of MBCs against RBD SARS‐CoV‐2 variant strains (Delta and Omicron), the same procedure was used except the protein (RBD‐WT, RBD‐Delta, or RBD‐Omicron) was coated onto the plate at 10 μg/ml in PBS 1X and after cell incubation, the plates were detected with a combination of anti‐IgG‐biotinylated/streptavidin‐ALP or anti‐IgA‐biotinylated/streptavidin‐ALP (Mabtech). Plates were then read on an IRIS ELISpot reader (Mabtech).
2.6. SARS‐CoV‐2‐specific T cell responses by intracellular cytokine staining
Profiling of SARS‐CoV‐2 T cell responses was performed as previously described. 15 Frozen PBMCs isolated from donor blood were thawed and rested overnight in RPMI (Hyclone) with 5% human serum (Innovative Research). PBMCs were incubated overnight at 37°C in 5% CO2. The next day, PBMCs were stimulated with either pooled PepTivator SARS‐CoV‐2 S and S1 peptides from the Wuhan (WT) strain (0.6 nmol/ml each; Miltenyi Biotec), phorbol myristate acetate (100 ng/ml; Sigma‐Aldrich), and ionomycin (1 μg/ml; Sigma‐Aldrich) (positive control), or left unstimulated (baseline). PBMCs were stimulated for 2 h before being blocked with 1X Brefeldin A and 1X Monensin (Thermo Fisher Scientific) for 4 h. PBMCs were then stained for surface markers (Supporting Information: Table 3) in the dark for 30 min before being fixed and permeabilized for another 30 min using the Foxp3/Transcription Factor Staining Buffer Set (Thermo Fisher Scientific). Cells were then stained for intracellular cytokines (Supporting Information: Table 4) in the dark for 30 min before being transferred to a polystyrene fluorescence‐activated cell sorting tube containing counting beads (Invitrogen). Cells were acquired with a Cytek® Aurora cytometer running SpectroFlo® Version 2.2.0.3 with automated unmixing and analyzed using FlowJo v10.8.0.
2.7. Statistics
Statistical analysis was performed using GraphPad (Prism) 9 software. Unmatched pairwise comparisons were performed using the Mann–Whitney U test while matched pairwise comparisons were performed using the Wilcoxon matched‐pairs signed rank test. Correlation statistical analysis used the Spearman test. Bonferroni corrections for multiple comparisons were applied for Figures 1B,C and 2C (Supporting Information: Table 6).
Figure 1.
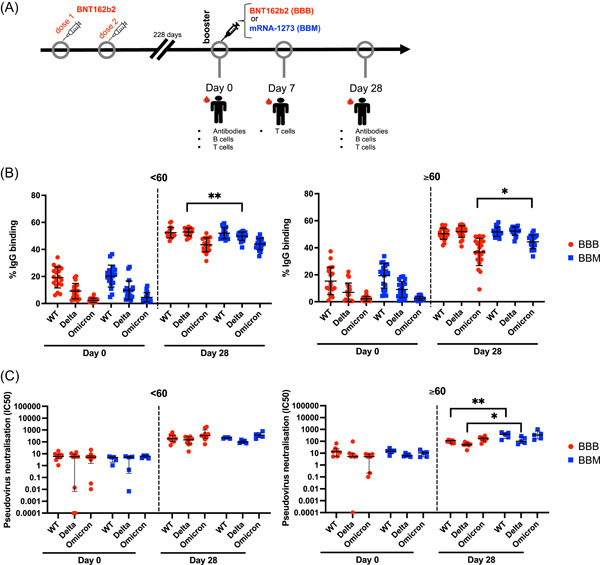
Heterologous vaccination elicits higher levels of severe acute respiratory syndrome coronavirus 2 neutralizing antibodies in participants over the age of 60. (A) Study design: Individuals who had been primed with two doses of BNT162b2 were recruited. When booster shots were due (mean of 228 days after the second vaccination), participants were given either another BNT162b2 or mRNA‐1273. Blood samples were taken on: Day 0 (before the booster) and 28 days after the booster. (B) IgG antispike responses were measured by spike protein flow cytometry based at Day 0 and Day 28 in individuals below 60 years old (left) or above 60 years old (right) from BBB (red circle) or BBM (blue square) groups. Data are presented as % of binding in flow cytometry (fetal bovine serum negative control <1% of binding). (C) Pseudovirus neutralization of plasma antibodies at Day 0 and Day 28 in individuals below 60 years old (left) or above 60 years old (right) from BBB (red circle) or BBM (blue square) groups. Data are presented as the dilution of plasma needed to neutralize 50% of the pseudovirus (IC50) in the log axis. Data are presented with mean and standard deviation (B) and median and interquartile range (C). Mann–Whitney test between BBB versus BBM groups and <60 versus ≥60 groups. Wilcoxon matched‐pairs test for paired samples. *p < 0.05; **p < 0.01. IC50, half maximal inhibitory concentration; IgG, immunoglobulin G; mRNA, messenger RNA; WT, wild‐type.
Figure 2.
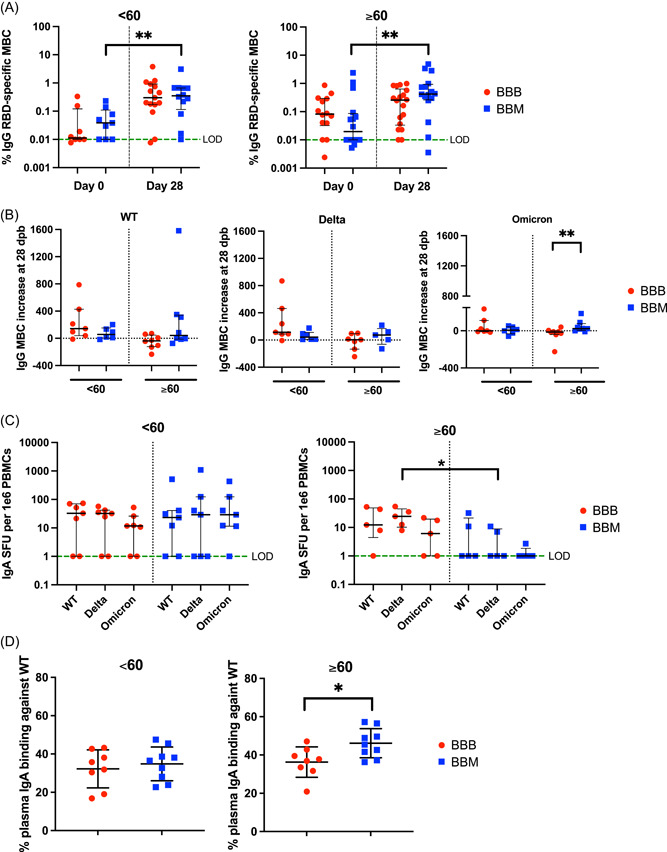
Increased IgG memory B cells against WT and Omicron RBD in elderly individuals under the BBM regimen. (A) Frequency of IgG RBD‐specific memory B cells among total IgG antibody‐secreting cells at Day 0 and Day 28 in individuals below 60 years old (left) or above 60 years old (right) from BBB (red circle) or BBM (blue square) groups. The green dotted line represents the limit of detection (LOD = 0.01). Data are presented in a log axis with median and interquartile range. (B) IgG memory B cell response against RBD from WT, Delta, and Omicron strains in individuals below 60 years old (<60) or above 60 years old (≥60) from BBB (red circle) or BBM (blue square) groups. Data are presented in spot forming unit (SFU) per 1E6 cells in the log axis. The green dotted line represents the (LOD = 1). (C) IgA memory B cell response against RBD from WT, Delta, and Omicron strains in individuals below 60 years old (left) or above 60 years old (right) from BBB (red circle) or BBM (blue square) groups. Data are presented in SFU per 1E6 cells in the log axis. The green dotted line represents (LOD = 1). (D) IgA was measured in the plasma by SFB at Day 28 in individuals below 60 years old (left) or above 60 years old (right) from BBB (red circle) or BBM (blue square) groups. Data are presented as % of binding in flow cytometry. Data are presented with median and interquartile ranges. Mann–Whitney test between BBB versus BBM groups and <60 versus ≥60 groups. Wilcoxon matched‐pairs test for paired samples. IgA, Immunoglobulin A; RBD, receptor‐binding domain; WT, wild‐type. *p < 0.05; **p < 0.01.
3. RESULTS
3.1. Heterologous vaccination elicits higher levels of SARS‐CoV‐2 neutralizing antibodies in participants over 60 years old
Blood samples were obtained before booster vaccination (Day 0) and at 28 days posthomologous or heterologous boosting (Figure 1A). None of them was infected by SARS‐CoV‐2 during the course of the study since they remain seronegative for the N protein. IgG plasma antibodies directed to the full‐length membrane‐bound spike protein from the original Wuhan (WT), Delta, and Omicron (BA.1) strains were quantified by SFB assay. 12 Both booster regimens (BBB and BBM) increased the levels of circulating spike‐specific antibodies in all individuals (Figure 1B). By 28 dpb, BBB and BBM participants in both age groups (<60 and ≥60) presented similar antibody responses against the WT spike protein (Figure 1B). At 28 dpb, all groups showed a significantly lower level of IgG antibodies that bind to the Omicron spike protein relative to WT spike protein binding (Figure 1B and Supporting Information: Table 2). Notably, participants ≥60 in the BBM regimen displayed higher levels of IgG antibodies against Omicron (BA.1) compared with those who received BBB series. (Figure 1B, right panel and Supporting Information: Figure 1). We observed higher levels of IgG antibodies that bind to the Delta spike protein in the BBB group than in the BBM group (Figure 1B, left panel) in participants under the age of 60, but this was not reflected when we looked at the increase between Day 0 and Day 28 (Supporting Information: Figure 1). We found no significant difference in plasma IgG at Day 0 (before booster) against WT, Delta, or Omicron between young and elderly individuals (Supporting Information: Figure 2).
We then assessed the capacity of plasma antibodies to neutralize a pseudovirus carrying the spike protein of the WT, Delta, or Omicron (BA.2) variants (Figure 1C). Both boosters significantly increased the neutralization capacity of serums against the WT pseudovirus in both younger and older individuals. However, elderly individuals in the BBM group had significantly higher neutralizing antibodies at 28 dpb compared with elderly individuals in the BBB group against the WT and Delta strains but not the Omicron strain (Figure 1C, right panel).
3.2. Increased IgG MBCs against WT and Omicron RBD in elderly individuals under the BBM regimen
MBCs play a major role in long‐term protection against pathogens. 16 Therefore, we analyzed the recall responses of RBD‐specific MBC in 63 individuals against the WT, Delta, and Omicron strains (Supporting Information: Table 1). Levels of IgG MBCs against the WT RBD significantly increased over time in both BBM and BBB regimens (Figure 2A). Of note, elderly BBM participants displayed a steep increase in the abundance of IgG MBCs at 28 dpb (Supporting Information: Figure 3A) and the increase was significantly higher than in the BBB group (Supporting Information: Figure 3B). We observed that specific IgG MBC responses against the Omicron RBDs were significantly lower compared with the WT RBD in all groups (< or ≥60 and BBB or BBM, except for the BBB < 60 groups) at Day 28 (Supporting Information:Figure 3C). In elderly individuals receiving the BBM regimen, we observed increased IgG MBC responses against Omicron between Day 0 and Day 28, but not in the BBB group (Figure 2B). We found no significant difference in IgG MBC levels at Day 0 (before booster) against WT, Delta, or Omicron between young and elderly individuals (Supporting Information: Figure 4A–C).
Since IgA antibodies are also important in the protection against SARS‐CoV‐2, 17 we evaluated the profiles of IgA MBCs levels specific for WT, Delta, and Omicron (BA.1) RDBs in BBB and BBM vaccines (Figure 2C). While no differences in IgA MBC levels at Day 0 (before booster) against WT, Delta, or Omicron between the young and elderly were observed (Supporting Information: Figure 4D–F), young individuals of the BBM regimen showed higher IgA MBC levels against Omicron compared to elderly BBM individuals (Supporting Information: Figure 5). In individuals above 60 years old, there were lower levels of MBCs specific for Delta in BBM compared to BBB, while the levels remain similar in individuals below 60 years old. (Figure 2C). Taken together, our data suggest that in the elderly, BBB may be more effective at inducing IgA MBCs against RBD from the variants in the elderly. In contrast, the levels of IgA specific to the WT spike protein in the plasma at Day 28 were higher in the elderly individuals from the BBB group compared with BBM (Figure 2D).
3.3. Circulating T follicular helper cell responses increase over time in both homologous and heterologous booster vaccine regimens
SARS‐CoV‐2‐specific T‐cell recall responses were analyzed by multicolored flow cytometry upon stimulation with peptide cocktails spanning the S protein. Circulating (Tfh) cell responses involved in B cell maturation and differentiation, 18 were identified by the surface expression of CXCR5+CD4+ and the surface expression of CD154 after Spike protein pool peptide stimulation. This strategy allowed for the identification of antigen‐specific populations. 19 The abundance of circulating Tfh cell responses increased over time in young and elderly participants in both BBB and BBM regimens. At 7 and 28 dpb, homologous and heterologous booster vaccination resulted in similar levels of CD154+ Tfh cells (Figure 3A). On the basis of increased levels of Tfh in the elderly after BBM booster vaccination, and that Tfh cells assist B cell maturation, we correlated the difference from Day 0 to Day 28 between CD154+ Tfh and RBD‐specific MBCs and found a positive correlation only for the elderly BBM group (Supporting Information: Figure 6). This suggests that the higher MBC response observed in the elderly after receiving a heterologous mRNA booster may be due to better activation of antigen‐specific Tfh cells.
Figure 3.
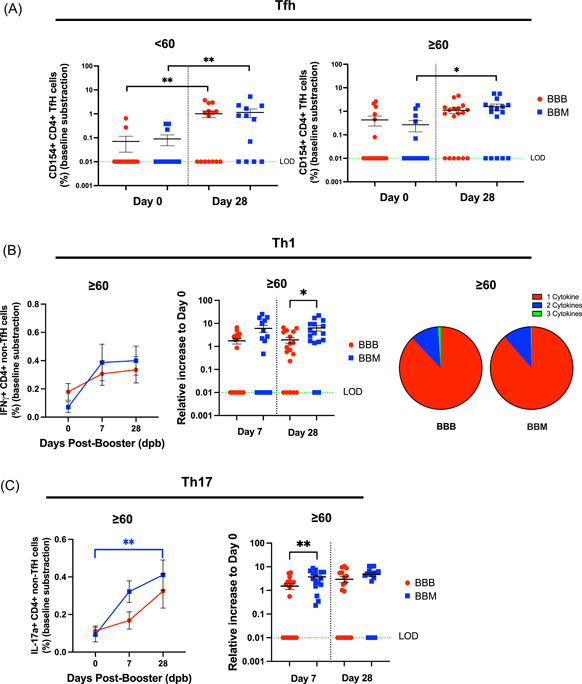
BBM induces more robust severe acute respiratory syndrome coronavirus 2‐specific CD4+ T cell responses in elderly individuals. (A) Frequency of activated Tfh (CD4 + CXCR5 + CD154+) at Day 0 and Day 28 in individuals below 60 years old (left) and above 60 years old (right) from BBB (red circle) or BBM (blue square) groups. (B) Increase in IFNγ in elderly participants from BBB (red circle) or BBM (blue square groups) (left) differences in a relative increase in IFNγ between elderly participants from BBB (red circle) or BBM (blue square) groups between 7 and 28 dpb (middle) polyfunctionality of Th1 cells in BBB and BBM groups (right). The pie charts represent the average proportions of CD4 T cells producing every combination (1, 2, or 3 cytokines) of Th1 cytokines (IFNγ, TNF‐α, and IL‐2). (C) Increase in IL‐17A in elderly participants from BBB (red circle) or BBM (blue square groups) (left) differences in a relative increase in IL‐17A between elderly participants from BBB (red circle) or BBM (blue square) groups between 7 and 28 dpb (Right). Data are presented after baseline subtraction in the log axis. The green dotted line represents the limit of detection (LOD = 0.01). Data are presented with mean and SEM. Mann–Whitney test between BBB versus BBM groups. Wilcoxon matched‐pairs test for paired samples between time points. dpb, days post boosting; IFN‐γ, interferon‐γ; IL‐17A, Interleukin 17A; Th1, T helper type 1; TNF‐α, tumor necrosis factor α. *p < 0.05; **p < 0.01; ***p < 0.001.
3.4. BBM induces more robust SARS‐CoV‐2‐specific CD4+ T cell responses in elderly individuals
CD4+ T cell recall responses after spike protein pool peptide stimulation were profiled by the intracellular expression of IL‐2, interferon γ (IFNγ), and TNF‐α (Th1); IL‐4, IL‐6, and IL‐10 (Th2) and IL‐17A (Th17), respectively. Th1, Th2, and Th17 responses in young participants increased upon BBB and BBM regimens. By 28 dpb, Th1, Th2, and Th17 responses were induced to a similar degree at this age (Supporting Information: Figure 7). On the other hand, while Th1 responses increased in elderly individuals over time, differences between BBB and BBM participants were observed (Figure 3B, left panel and Supporting Information: Figure 8). Particularly, IFNγ and TNF‐α‐producing Th1 responses were significantly higher in elderly BBM participants compared with BBB at 28 dpb (Figure 3B and Supporting Information: Figure 8). We then examined the polyfunctionality of the CD4+ Th1 cells by looking at the coexpression of 1, 2, or 3 cytokines (IL‐2, IFNγ, and TNF‐α). Both BBB and BBM vaccination regimes triggered comparable levels of polyfunctional CD4+ Th1 cells in the elderly (Figure 3B, right panel). Individuals over the age of 60 saw an increase in peptide‐specific CD4 Th2 responses on Day 28 following the booster; however, there was no significant difference between the BBB and BBM regimens (Supporting Information: Figure 9). Intriguingly, Th17 response increased after booster in this age group (Figure 3C, left panel) and the elderly had a greater abundance of IL‐17A‐producing Th17 cells upon heterologous vaccination regime at Day 7. By 28 dpb, both elderly BBM and BBB participants displayed similar Th17 responses (Figure 3C, right panel).
Finally, we examined the CD8+ T cell compartment post‐booster vaccination (Supporting Information: Figure 10). By 28 dpb, IFNγ‐producing CD8+ T cells were induced at a similar degree in young and elderly participants in the BBB and BBM groups (Supporting Information: Figure 10). Similarly, comparable levels of Granzyme B‐producing CD8+ T cells in either age group or vaccine group were observed (Supporting Information: Figure 10).
4. DISCUSSION
In this study, we show that a heterologous mRNA vaccination regimen (BBM) is better at boosting the memory of immune T and B cells against SARS‐CoV‐2 compared with homologous mRNA boosters. A recent study showed that a third dose of mRNA vaccine is beneficial to boost Omicron‐reactive MBC but without looking into heterologous versus homologous boosters and mostly in the young population. 20 Our study provides mechanistic evidence that supports previously published observations on the superiority of heterologous over homologous booster strategies at inducing SARS‐CoV‐2 neutralizing antibody responses. 7 Importantly, our data confirm that this effect is more pronounced in elderly individuals, providing support for age‐differentiated vaccine recommendations.
To elucidate the mechanisms underlying the induction of more potent antibody responses upon heterologous booster regimen, we characterized SARS‐CoV‐2 specific MBC levels in BBM and BBB participants. MBC generation is crucial to ensure long‐term protection against pathogens. 21 Similarly, we have recently reported that vaccine breakthrough cases with the Delta variant were associated with lower MBC levels. 22 In this study, heterologous and homologous booster regimens did not significantly induce different MBC responses against the RBD in younger individuals (below 60 years old). However, in elderly individuals, the heterologous boosting induced a higher level of RBD‐specific MBCs, particularly against the Omicron RBD. This is concordant with previous observations that the third dose of mRNA vaccine elicits Omicron‐reactive MBC. 20 Our data highlighted that this is particularly the case in the elderly population receiving the heterologous booster. A recent study highlighted the existence of a repertoire of cross‐reactive MBCs after two doses of BNT162b2and estimated that 15% of MBCs can cross‐react to variants, including Omicron. 23 We suggest here that heterologous mRNA vaccination in the elderly may favor the selection of this repertoire. It is possible that the elderly had more cumulative exposure to common cold coronaviruses and thus have developed a larger cross‐reactive repertoire to the different coronaviruses and their variants. The mechanisms by which a heterologous mRNA booster may be more effective than a homologous in restimulating this repertoire remain to be explored.
The elderly in the BBM group showed a decrease in IgA MBCs specific to Delta RBD compared with BBB. The levels of IgA MBCs against Delta were maintained in younger individuals from BBB and BBM groups. It might be attributed to a difference in kinetic induction or mobilization of IgA class‐switch cross‐reactive MBCs in heterologous versus homologous boosters at various ages. For example, this might be related to the cell signaling from T cells, 24 and the cytokine environment, 25 which were not examined here and deserve to be investigated further. A larger group would also be needed to confirm this observation against Omicron RBD.
Interestingly, although circulating IgA MBC responses against WT RBD were comparable in elderly individuals from the BBM and BBB groups, we observed higher IgA plasma levels in elderly BBM participants. This could be explained by the different IgA‐secreting potentials of MBCs. Thus, despite similar numbers of IgA MBCs between BBB and BBM, these cells may be more functional to secrete IgA antibodies in the BBM context and could explain the higher level of IgA detected in the plasma. In line with this hypothesis, it was previously shown that following two doses of BNT162b2, MBCs can migrate to mucosal sites and secrete IgA. 26
Besides MBC levels, the quantity and quality of antibody responses also depend on CD4+ T cell help, particularly Tfh cells. 27 Robust Tfh responses have been reported upon mRNA vaccination against SARS‐CoV‐2. 28 Here, we show that the third dose of mRNA vaccine increases the frequency of circulating antigen‐specific Tfh cells. Importantly, elderly individuals who received the BBM regimen displayed higher Tfh levels than those who received the BBB regimen. It has been previously demonstrated that MBC responses against the spike protein are T cell‐dependent during natural SARS‐CoV‐2 infection. 29 In support of this, the strong correlation between Tfh and MBC numbers in elderly BBM participants suggests a plausible interplay between these populations, which may contribute to the significant increase in MBCs. In addition, Tfh levels correlate with neutralizing antibodies and may better aid in SARS‐CoV‐2 clearance. 30 Along with the higher levels of antigen‐specific activated Tfh cells, we also observed a higher induction of Th17, Th1, and Th2 CD4+ T cells in elderly individuals upon a heterologous booster regimen. This marked increase in T cell responses is especially important for the more vulnerable, elderly population, given the vital role T cells play against SARS‐CoV‐2 infection. Th2 CD4+ T cells play an important role in promoting the development of B cells to antibody‐producing plasma via IL‐4 secretion, 31 and may be an additional factor in favor of the higher MBC levels observed in our study. Furthermore, it has been previously reported that asymptomatic patients display a more robust Th17 response compared with symptomatic patients. 32 , 33 An increase in Th17 cells may provide a protective role against SARS‐CoV‐2 infection during a vaccine breakthrough infection. Th1 cells have been well documented as antiviral and play an essential role in virus clearance and consequently limiting the damage caused by the virus, with reports showing a robust Th1 response is a key factor for a favorable SARS‐COV‐2 prognosis. 32 , 33
Our study focused on BBB versus BBM booster regimens, but the efficacy of other combinations of prime and booster vaccines needs to be explored to corroborate that the better performance of heterologous boosting relies on higher T and B cell responses, particularly in elderly individuals. Of note, a study comparing different homologous and heterologous combinations using two doses of mRNA‐1273 as primary vaccination series suggests that MMM is better than MMB in eliciting plasma antibodies. 6 Thus, the mRNA‐1273 could be superior at engaging T and B cell immunity on its own.
In conclusion, heterologous booster vaccination induced a more robust humoral and cellular immune response in elderly individuals than homologous booster vaccination. This study provides important data that can guide policymaking and help healthcare workers decide how best to immunize vulnerable populations.
AUTHOR CONTRIBUTIONS
Angeline Rouers and Nathan Wong conceptualized, processed, acquired, analyzed, and interpreted the data and wrote the manuscript. Yun Shan Goh, Matthew Zirui Tay, Zi Wei Chang, Siew‐Wai Fong, and Anthony Torres‐Ruesta added inputs to the first draft of the manuscript. Yun Shan Goh, Chiew Yee Loh, and Pei Xiang Hor performed the spike protein flow cytometry‐based assays, analyzed, and interpreted the data. Zi Wei Chang, Nathan Wong, Yong Jie Tan, Anthony Torres‐Ruesta, and Siew‐Wai Fong performed the flow cytometry assays, analyzed, and interpreted the data. Vanessa Neo, Isaac Kai Jie Kam, Nicholas Kim‐Wah Yeo, Yuling Huang, and Angeline Rouers performed the B‐cell ELIspot assays, analyzed, and interpreted the data. Yuling Huang, Joel Xu En Wong, and Matthew Zirui Tay performed the pseudovirus neutralization assays, analyzed, and interpreted the data. Xuan Ying Poh, Suma Rao, Po Ying Chia, Sean W. X. Ong , Tau Hong Lee, Ray J. H. Lin, Clarissa Lim, Jefanie Teo, David Chien Lye, and Barnaby E. Young designed and supervised samples collection. The COVID‐19 Study Group (see Supporting Information: Table 5) processed the blood samples. Paul A. Macary and Xinlei Qian provided receptor‐binding domain proteins used in B cell ELISpot assays. Wang Bei, Eve Zi Xian Ngoh, Siti Nazihah Mohd Salleh, Cheng‐I Wang provided the resources and scientific advice for the pseudovirus neutralization assay. Ee Chee Ren, Lisa F. P. Ng, and Laurent Renia conceptualized, designed, and wrote the manuscript. All authors revised and approved the final version of the manuscript.
CONFLICTS OF INTEREST
A patent application for the spike protein flow cytometry‐based assay has been filed (Singapore patent #10202009679P: A method of detecting antibodies and related products) by Yun Shan Goh, Laurent Renia, and Lisa F. P. Ng. The rest of the authors declare no conflict of interest.
Supporting information
Supplementary information.
Supplementary information.
ACKNOWLEDGMENTS
The authors would like to thank all study participants who donated blood and the healthcare workers. They would also like to thank the team at the National Centre for Infectious Diseases and Tan Tock Send Hospital for their help in subject recruitment. The authors are grateful to Professor Yee‐Joo Tan, Department of Microbiology, NUS & IMCB, A*STAR, Singapore, for her kind gift of CHO‐ACE2 cells for the pseudovirus neutralization work. This study was supported by Biomedical Research Council, A*CRUSE (Vaccine monitoring project), A*ccelerate GAP‐funded project (ACCL/19‐GAP064‐R20H‐H) from Agency of Science, Technology and Research (A*STAR), Singapore National Medical Research Council COVID‐19 Research Fund (COVID19RF‐001; COVID19RF‐007; COVID19RF‐0008; COVID19RF‐060; and OFLCG19May‐0034), U.S. Food and Drug Administration (#75F40120C00085), and A*STAR COVID‐19 Research funding (H/20/04/g1/006).
Rouers A, Wong N, Goh YS, et al. Efficient recall of SARS‐CoV‐2 variant‐reactive B cells and T responses in the elderly upon heterologous mRNA vaccines as boosters. J Med Virol. 2022;95:e28258. 10.1002/jmv.28258
Angeline Rouers and Nathan Wong contributed equally as 1st author. Yun Shan Goh, Anthony Torres‐Ruesta, Matthew Zirui Tay, Zi Wei Chang and Siew‐Wai Fong contributed eqully as 2nd author. Lisa F. P. Ng and Laurent Renia contributed equally as last author.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ritchie H, Mathieu E, Rodés‐Guirao L, et al. Coronavirus (COVID‐19) vaccinations [Internet]. Accessed July 1, 2020. https://ourworldindata.org/covid-vaccinations
- 2. Renia L, Goh YS, Rouers A, et al. Lower vaccine-acquired immunity in the elderly population following two-dose BNT162b2 vaccination is alleviated by a third vaccine dose. Nat Commun. 2022; 13(1):4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid‐19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. 11 vaccines granted emergency use listing (EUL) by WHO [Internet] . Accessed July 1, 2022. https://covid19.trackvaccines.org/agency/who/
- 5. USFDA . Coronavirus (COVID‐19) update: FDA expands eligibility for COVID‐19 vaccine boosters. US Food and Drug Administration. 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibility-covid-19-vaccine-boosters
- 6. Atmar RL, Lyke KE, Deming ME, et al. Homologous and heterologous Covid‐19 booster vaccinations. N Engl J Med. 2022;386(11):1046‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poh XY, Tan CW, Lee IR, et al. Antibody response of heterologous vs homologous messenger RNA vaccine boosters against the severe acute respiratory syndrome coronavirus 2 omicron variant: interim results from the PRIBIVAC study, a randomized clinical trial (SARS‐CoV‐2). Clin Infect Dis. 2022. 10.1093/cid/ciac345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Costa Clemens SA, Weckx L, Clemens R, et al. Heterologous versus homologous COVID‐19 booster vaccination in previous recipients of two doses of CoronaVac COVID‐19 vaccine in Brazil (RHH‐001): a phase 4, non‐inferiority, single blind, randomised study. Lancet. 2022;399(10324):521‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mayr FB, Talisa VB, Shaikh O, Yende S, Butt AA. Effectiveness of homologous or heterologous Covid‐19 boosters in veterans. N Engl J Med. 2022;386(14):1375‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tan SHX, Pung R, Wang LF, et al. Association of homologous and heterologous vaccine boosters with COVID‐19 incidence and severity in Singapore. JAMA. 2022;327(12):1181‐1182. 10.1001/jama.2022.1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goh YS, Chavatte JM, Lim Jieling A, et al. Sensitive detection of total anti‐Spike antibodies and isotype switching in asymptomatic and symptomatic individuals with COVID‐19. Cell Reports Medicine. 2021;2(2):100193. 10.1016/j.xcrm.2021.100193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goh YS, Ng LFP, Renia L. A flow cytometry‐based assay for serological detection of anti‐spike antibodies in COVID‐19 patients. STAR Protocols. 2021;2(3):100671. 10.1016/j.xpro.2021.100671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poh CM, Carissimo G, Wang B, et al. Two linear epitopes on the SARS‐CoV‐2 spike protein that elicit neutralising antibodies in COVID‐19 patients. Nat Commun. 2020;11(1):2806. 10.1038/s41467-020-16638-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lip KM, Shen S, Yang X, et al. Monoclonal antibodies targeting the HR2 domain and the region immediately upstream of the HR2 of the S protein neutralize in vitro infection of severe acute respiratory syndrome coronavirus. J Virol. 2006;80(2):941‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fong SW, Yeo NKW, Chan YH, et al. Robust virus‐specific adaptive immunity in COVID‐19 patients with SARS‐CoV‐2 Δ382 variant infection. J Clin Immunol. 2022;42(2):214‐229. 10.1007/s10875-021-01142-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palm AKE, Henry C. Remembrance of things past: long‐term B cell memory after infection and vaccination. Front Immunol. 2019;10:1787. 10.3389/fimmu.2019.01787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chao YX, Rötzschke O, Tan EK. The role of IgA in COVID‐19. Brain Behav Immun. 2020;87:182‐183. https://pubmed.ncbi.nlm.nih.gov/32454136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621‐663. [DOI] [PubMed] [Google Scholar]
- 19. Chattopadhyay PK, Yu J, Roederer M. Live‐cell assay to detect antigen‐specific CD4+ T‐cell responses by CD154 expression. Nat Protoc. 2006;1(1):1‐6. 10.1038/nprot.2006.1 [DOI] [PubMed] [Google Scholar]
- 20. Goel RR, Painter MM, Lundgreen KA, et al. Efficient recall of Omicron‐reactive B cell memory after a third dose of SARS‐CoV‐2 mRNA vaccine. Cell. 2022;185:1875‐1887. https://linkinghub.elsevier.com/retrieve/pii/S0092867422004561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akkaya M, Kwak K, Pierce SK. B cell memory: building two walls of protection against pathogens. Nat Rev Immunol. 2020;20(4):229‐238. 10.1038/s41577-019-0244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tay MZ, Rouers A, Fong SW, et al. Decreased memory B cell frequencies in COVID‐19 delta variant vaccine breakthrough infection. EMBO Mol Med. 2022;14(3):e15227. 10.15252/emmm.202115227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang K, Jia Z, Bao L, et al. Memory B cell repertoire from triple vaccinees against diverse SARS‐CoV‐2 variants. Nature. 2022;603(7903):919‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dullaers M, Li D, Xue Y, et al. A T cell‐dependent mechanism for the induction of human mucosal homing immunoglobulin A‐secreting plasmablasts. Immunity. 2009;30(1):120‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cerutti A, Puga I, Cols M. Innate control of B cell responses. Trends Immunol. 2011;32:202‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mortari EP, Russo C, Vinci MR, et al. Highly specific memory b cells generation after the 2nd dose of BNT162b2 vaccine compensate for the decline of serum antibodies and absence of mucosal IgA. Cells. 2021;10(10):2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crotty S. A brief history of T cell help to B cells. Nat Rev Immunol. 2015;15(3):185‐189. http://www.nature.com/doifinder/10.1038/nri3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mudd PA, Minervina AA, Pogorelyy MV, et al. SARS‐CoV‐2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell. 2022;185(4):603‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pušnik J, Richter E, Schulte B, et al. Memory B cells targeting SARS‐CoV‐2 spike protein and their dependence on CD4+ T cell help. Cell Rep. 2021;35(13):109320. https://www.sciencedirect.com/science/article/pii/S2211124721006963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boppana S, Qin K, Files JK, et al. SARS‐CoV‐2‐specific circulating T follicular helper cells correlate with neutralizing antibodies and increase during early convalescence. PLoS Pathog. 2021;17(7):e1009761. 10.1371/journal.ppat.1009761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maddur MS, Bayry J. B cells drive Th2 responses by instructing human dendritic cell maturation. Oncoimmunology. 2015;4(5):e1005508. https://pubmed.ncbi.nlm.nih.gov/26155405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gil‐Etayo FJ, Suàrez‐Fernández P, Cabrera‐Marante O, et al. T‐Helper cell subset response is a determining factor in COVID‐19 progression. Front Cell Infect Microbiol. 2021;11:624483. https://pubmed.ncbi.nlm.nih.gov/33718270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gutiérrez‐Bautista JF, Rodriguez‐Nicolas A, Rosales‐Castillo A, et al. Negative clinical evolution in COVID‐19 patients is frequently accompanied with an increased proportion of undifferentiated th cells and a strong underrepresentation of the Th1 subset. Front Immunol. 2020;11:596553. https://pubmed.ncbi.nlm.nih.gov/33324414 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Supplementary information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


