Abstract
Objective
To compare the estimates of preterm birth (PTB; 22–36 weeks' gestational age, GA) and stillbirth rates during COVID‐19 pandemic in Italy with those recorded in the three previous years.
Design
A population‐based cohort study of live‐ and stillborn infants was conducted using data from Regional Health Systems and comparing the pandemic period (1 March 2020–31 March 2021, n = 362 129) to an historical period (January 2017–February 2020, n = 1 117 172). The cohort covered 84.3% of the births in Italy.
Methods
Poisson regressions were run in each Region and meta‐analyses were performed centrally. We used an interrupted time series regression analysis to study the trend of preterm births from 2017 to 2021.
Main outcome measures
The primary outcomes were PTB and stillbirths. Secondary outcomes were late PTB (32–36 weeks' GA), very PTB (<32 weeks' GA), and extremely PTB (<28 weeks' GA), overall and stratified into singleton and multiples.
Results
The pandemic period compared with the historical one was associated with a reduced risk for PTB (risk ratio [RR] 0.91, 95% confidence interval [CI] 0.88–0.93), late PTB (RR 0.91, 95% CI 0.88–0.94), very PTB (RR 0.88, 95% CI 0.84–0.91) and extremely PTB (RR 0.88, 95% CI 0.82–0.95). In multiples, point estimates were not very different, but had wider CIs. No association was found for stillbirths (RR 1.01, 95% CI 0.90–1.13). A linear decreasing trend in PTB rate was present in the historical period, with a further reduction after the lockdown.
Conclusions
We demonstrated a decrease in PTB rate after the introduction of COVID‐19 restriction measures, without an increase in stillbirths.
Keywords: epidemiological surveys, neonatal, paediatrics, perinatal, premature birth, stillbirth
Short abstract
Linked article: This article is commented on by Giovanni Sisti and Julie T. Joseph, pp. 285 in this issue. To view this mini commentary visit https://doi.org/10.1111/1471‐0528.17312
1. INTRODUCTION
Soon after the onset of the COVID‐19 pandemic, several reports showed an increased risk of severe illness in pregnant women with SARS‐CoV‐2 compared with those not pregnant, and adverse pregnancy outcomes including preterm birth (PTB). 1 On the other hand, a more recent Italian study showed that most infected pregnant women and newborns had good outcomes. 2 Recently, the Euro‐Peristat Research Network 3 raised concerns about the fact that one major gap in assessing the real effects of the pandemic on maternal and child health was the limited availability of comprehensive population‐based routine data.
Research has also accumulated on the effects of the pandemic on the general population of pregnant women and their infants, possibly due to mitigation strategies and changes in women everyday life. During the early months of the pandemic, a reduced PTB rate, in comparison with that in the previous years, was recorded in Denmark, 4 in one hospital in Ireland 5 and in one Italian Region, 6 where an increase in stillbirths was also observed. Another study, performed in a single hospital in London, reported an increase in stillbirths but not in PTB rates. 7 These reports were based on relatively small samples and were limited, especially for stillbirths, by a possible change in referral patterns of pregnant women.
A first systematic review and meta‐analysis of 31 studies published until 8 January 2021 addressing the indirect effects of the pandemic on perinatal outcomes confirmed a slight reduction in PTB (<37 weeks' gestational age, GA) in high‐income but not in low‐income countries and, vice versa, an increase in stillbirths in low‐income countries only. 8
A more recent systematic review and meta‐analysis of 44 studies 9 found that the odds of PTB during the pandemic period were significantly reduced in single‐centre/single‐health‐authority studies, whereas there was no difference in larger studies based on regional/national data. No difference was documented in the rate of stillbirths in the pandemic period compared with the non‐pandemic one, though these conclusions might be hampered, according to the authors, by more limited data. The review once again concludes that there is still a need for studies in bigger countries largely affected by the COVID‐19 pandemic, such as India, Brazil, UK and Italy, based on national registries to investigate the impact of the pandemic on perinatal health at a population level.
The aim of the present study was to provide national population‐based estimates of the PTB and stillbirth rates during the pandemic period compared with a historical period. To account for the natural variation in PTB over time, and the abrupt implementation of public health measures and disruption of routines of care, we also analysed the temporal trend in monthly incidence of PTB before and after the implementation of mitigation measures.
2. METHODS
We analysed data from the birth certificate (CeDAP), which is filled in at birth for each delivery.
Ten Italian Regions and one Autonomous Province (Piedmont, Lombardy, Veneto, Emilia‐Romagna, Friuli‐Venezia Giulia and the Province of Trento in Northern Italy; Tuscany and Lazio in Central Italy; Apulia, Campania and Sicily in Southern Italy) agreed to participate. These Regions cover 84.3% of all the births in Italy. 10
We defined 1 March 2020–31 March 2021 as the pandemic period: this covered the first two waves of COVID‐19 in Italy, corresponding to several restrictions measures. The historical period included the three previous years, from January 2017 to February 2020. For the Campania Region the comparison period started from January 2018 because 2017 data were not available.
The primary outcomes were PTB (live births between 22 and 36 weeks' GA) and stillbirths, both in singleton and multiple pregnancies. Secondary outcomes were late PTB (32–36 weeks' GA), very PTB (<32 weeks' GA) and extremely PTB (<28 weeks' GA). GA at birth was calculated in completed weeks and was determined on the basis of the last menstrual period or of early ultrasound scans if the last menstrual period was unknown or there was inconsistency between the two. We used unadjusted and adjusted Poisson regression models with robust standard errors to examine the association between birth period (pandemic versus historical) and percentage of preterm births estimating risk ratios (RRs) and 95% confidence intervals (CI) for each outcome. Adjusted analysis included the following variables: maternal country of birth (foreigner versus Italian), maternal age at index birth (continuous), parity (yes/no), maternal education (none or elementary school or primary school diploma; secondary school diploma; University degree), maternal employment (yes/no), pregnancy conceived with assisted reproductive technology (ART, yes/no), sex of the child (female/male). Most of these variables could in fact be considered as mediators or effect modifiers rather than true confounders:11 i.e. mitigation strategies due to COVID‐19 could have influenced maternal lifestyle in pregnancy in different ways depending on mother's origin, age, education, employment and parity. Pregnancy conceived with ART, on the other hand, is an intermediate variable. We therefore chose as the main analysis the unadjusted one. In the adjusted analysis the Lazio Region was not considered because the information on ART was not available for the whole study period.
We further analysed singletons and multiples separately, which could be differently affected by the pandemic restrictions.
In this study there was no patient or public involvement.
Due to privacy regulations, individual data were not shared, and Poisson regression analyses were run in each Region. A meta‐analysis was performed centrally at the Regional Health Agency of Tuscany.
To estimate the heterogeneity of effects in different Regions, the I 2 index was calculated. 12 When there was no evidence of heterogeneity, the pooled estimate of the effect (RR) was calculated using the inverse variance method (fixed effect model); otherwise, the DerSimonian–Laird weights (random effects model) were used. 13 Forest plots were provided to provide graphic illustrations of the effect size estimates for each study as well as the pooled estimate.
We also studied the monthly trend of PTB rates from 2017 to the end of March 2021 using an interrupted time series regression analysis, 14 with 1 March 2020 as the date of interruption. In this quasi‐experimental technique, one looks for a sharp change in outcomes following public health interventions (the interruption corresponding to the implementation date of COVID‐19 mitigation measures). After a visual check of data points, we carried out a log‐linear regression analysis of the (log of the) monthly prevalence of PTB over calendar months and introduced a term estimating the level of discontinuity (gap) on 1 March 2020 (at month 38/39), i.e. at the ‘interruption’. As the prevalence of PTB showed a seasonal trend, we modelled it using Fourier terms (two pairs of sine and cosine functions). 14 In addition, we used the robust Newey–West standard errors for effect estimates to account for residual autocorrelation in the data (‘newey’ command in STATA).
In all models, we also tested whether the slope had been altered by mitigation measures by running a model including a statistical interaction between slope and period (historical versus pandemic period). As there was no evidence of interaction and the models without interaction had a better fit to the data (lower residual MSE), we always used models without interaction.
As a further check of the overall effect of mitigation measures, we carried out a regression of the log of the monthly frequency of PTB over calendar months until February 2020 (just before the pandemic), correcting for seasonal trend and autocorrelation as above. We then computed the expected frequencies for the months following the lockdown (i.e. under the counterfactual scenario of no intervention), and compared them with actual frequencies.
All the analyses were performed using STATA version 15.1 (StataCorp LP).
3. RESULTS
A total of 362 129 live births (351 139 singletons and 10 990 multiples) occurred during the pandemic period, and 1 117 172 live births (1 079 259 singletons and 37 913 multiples) during the historical period. The number and percentage of PTB in different categories of GA and of stillbirths in the two periods are presented in Table 1, together with unadjusted and adjusted overall RR of adverse perinatal outcomes based on meta‐analysis. The pandemic period compared with the historical period was associated with a reduced risk for PTB (<37 weeks' GA), late PTB (32–36 weeks' GA), very PTB (<32 weeks' GA) and extremely PTB (<28 weeks' GA), with very similar estimates in unadjusted and adjusted analyses. No association was found for stillbirths. Forest plots for unadjusted estimates are reported in Figures 1A–D (for liveborn PTB) and Figure S1 (for stillbirths), and those for adjusted estimates are reported in Figures S2–S6.
TABLE 1.
Prevalence of preterm live births and stillbirths in the pandemic period and in the historical period and the overall relative risks of adverse perinatal outcomes during the pandemic versus the historical period, based on meta‐analysis
| Outcome | Births, n (%) | RR (95% CI) | ||
|---|---|---|---|---|
| Pandemic period | Historical period | Unadjusted | Adjusted a | |
| PTB | 25 550 (7.06) | 85 947 (7.69) | 0.91 (0.88–0.93) | 0.92 (0.89–0.96) |
| Late PTB | 22 463 (6.2) | 75 047 (6.72) | 0.91 (0.88–0.94) | 0.93 (0.89–0.96) |
| Very PTB | 3087 (0.85) | 10 900 (0.98) | 0.88 (0.84–0.91) | 0.88 (0.84–0.92) |
| Extremely PTB | 999 (0.28) | 3504 (0.31) | 0.88 (0.82–0.95) | 0.89 (0.82–0.96) |
| Stillbirths | 960 (0.26) | 3004 (0.27) | 1.01 (0.90–1.13) | 0.97 (0.89–1.05) |
Note: PTB = preterm birth (<37 weeks' gestational age, GA); late PTB (32–36 weeks' GA); very PTB (<32 weeks' GA); extremely PTB (<28 weeks' GA).
Adjusted analysis included the following variables: maternal country of birth, maternal age at index birth, parity, maternal educational degree, maternal employment, pregnancy conceived with assisted reproductive technology, sex of the child. Observations with missing variables were excluded from the model. The Lazio Region is not included in adjusted analyses.
FIGURE 1.
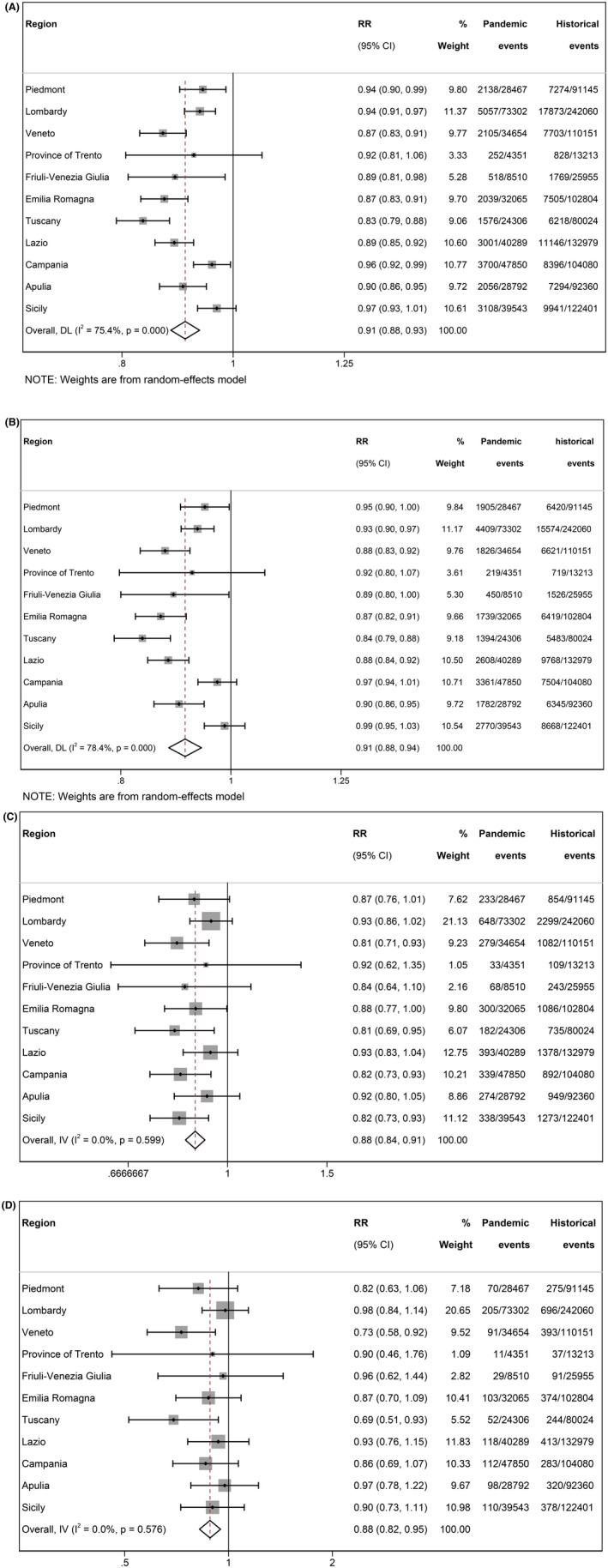
Forest plot for risks of liveborn preterm birth in the pandemic versus the historical period in the studied regions. Unadjusted analysis. (A) Preterm birth: PTB (<37 weeks' GA); (B) late PTB (32–36 weeks' GA); (C) very PTB (<32 weeks' GA); (D) extremely PTB (<28 weeks' GA). Cohort‐specific and overall RR and 95% CI are shown; I 2 = percentage of between‐studies heterogeneity and relative P‐value. % Weight = set of weights attributed to each cohort. Pandemic and historical events = number of preterm births over total live births in the two periods.
Singletons contributed 72.9% of all PTB, and in these the associations remained very similar to those in the whole population of neonates; the unadjusted RR for PTB was 0.92 (95% CI 0.89–0.95), for late PTB 0.93 (95% CI 0.89– 0.96), for very PTB 0.88 (95% CI 0.84–0.93) and for extremely PTB 0.92 (95% CI 0.85–1.00) (Figures S7–S14).
Multiples represented 3.3% of all births and contributed 27.1% of all PTB; in all classes of PTB, point estimates were not very different from those of singletons, but with wider Cis, which encompassed the null value. The unadjusted RR for PTB was 0.97 (95% CI 0.93–1.01), for late PTB 0.97 (95% CI 0.92–1.01), for very PTB 0.91 (95% CI 0.81–1.03) and for extremely PTB 0.78 (95% CI 0.60– 1.01) (Figures S15–S22).
The interrupted time series regression analysed 38 months before lockdown and 13 months after it, showed a decreasing trend in the overall percentage of PTB in the 3 years before the pandemic superimposed to a biannual seasonal oscillation. The de‐seasonalised trend (estimated relative change of PTB percentage) was −0.17% per month (95% CI −0.26 to −0.09%). A further reduction of PTB prevalence (estimated relative change −4.2% compared with the previous period; 95% CI −8.4 to 0.0%) after lockdown and other mitigation strategies was demonstrated (Figure 2), in addition to the continuing decreasing trend.
FIGURE 2.
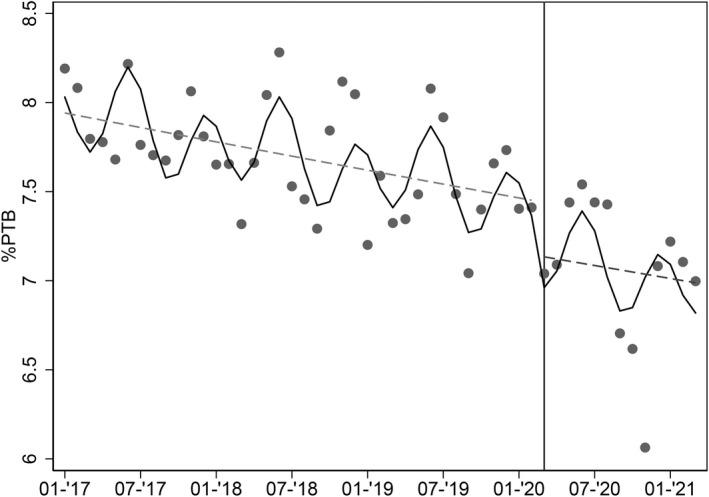
Interrupted time series regression. Each dot represents the average monthly frequency of liveborn preterm births (<37 weeks' GA) over total births. Period starts on 1 January 2017. Solid line: Predicted trend based on the seasonally adjusted regression model. Dashed line: de‐seasonalised trend. The date of implementation of mitigation measures (1 March 2020) is shown as a vertical line.
The comparison between the counterfactual scenario and actual trend after lockdown confirmed the drop in PTB prevalence (estimated mean decrease = −3.8%; 95% CI −7.5 to −0.1%), which was particularly marked in the last months of 2020 (Figure S23).
Similar results were found for the subclass of late PTB (trend in frequency before the pandemic: −0.14% per month; 95% CI −0.22 to −0.06%; further change after lockdown: –4.3%; 95% CI −8.4 to −2.9%) but not for very or extremely PTB, which had much lower frequencies and more scattered data (Figures S24–S26).
Interrupted time series analysis in singletons mirrored the results of the whole population (Figures S27–S30); no interruption was detected for multiple births (data not shown).
4. DISCUSSION
4.1. Main findings
In this study covering 84.3% of the live births in Italy, we found that being born during COVID‐19 pandemics was associated with a reduction of the risk of PTB, as a whole and in all subgroups, compared with the years before. The stillbirth rate was not affected. Even though in Italy a decreasing trend in the overall prevalence of PTB was already present in the historical period (from 2017 onwards), we were able to show a further reduction once the lockdown and mitigation strategies were enforced. The reduction was particularly evident for all the Regions and all GA classes in the last months of 2020.
4.2. Strengths and limitations
Our population‐based study, together with the recent one of Gurol‐Urganci et al. 15 in UK, is the largest ever done as regards both the number of PTB and stillbirths during the COVID‐19 pandemic. This is because both Italy and UK have a larger number of births in comparison with those in northern European countries which have previously published nationwide results, such as Denmark, 4 The Netherlands 16 and Sweden, 17 and also because of the wider time span considered as the pandemic period.
The longer pandemic period considered, besides increasing the sample size, made it possible to study women who were exposed to mitigation strategies during their whole pregnancy.
The large sample size allowed us to study the different categories of PTB and to analyse singletons and multiples separately, though the relative low number of multiple pregnancies precludes definitive answers in this subgroup.
As further limitations: the dataset used does not contain information on lifestyle and social behaviours of pregnant women, which precludes an analysis of possible important and widespread causes for the observed decrease of PTB among the general population.
Information on preterm birth clinical subtypes was not fully available for three Regions and therefore we were not able to analyse whether the decrease in preterm births observed following the lockdown period differed when considering spontaneous versus medically indicated preterm births.
In estimating the total effect of the COVID‐19 pandemic on pregnancy outcomes we did not consider the effect of the SARS‐CoV2 infection on pregnant women – for which data were not available. The COVID‐19 infection is, however, known to increase PTB 1 , 2 so that excluding COVID‐19‐positive women would probably yield further reduced PTB rates.
Finally, our study was a retrospective one using routinely collected data, which are prone to registration errors, although data are filled in by midwifes and doctors soon after birth and are annually checked for the CeDAP report from the Ministry of Health.
4.3. Interpretation
Our data on a reduction in PTB concomitant with the COVID‐19 pandemic period are concordant with those of two recent national‐based studies published after the systematic review and meta‐analysis of Yang et al., 9 which concluded that a reduction in the odds of PTB was observed only in single‐centre studies. The first of these studies was conducted in Israel, 18 on birth data from the Israel national newborn screening programme and showed a 10% decline in all preterm deliveries during the COVID‐19 pandemic national lockdown period. The second one, in UK, 15 used administrative hospital records and found a slightly lower frequency of preterm birth rates (from 6.1 to 6.0%) during the entire pandemic period compared with pre‐lockdown.
In our study, we considered events (PTB and stillbirths) up to the end of March 2021, when most mitigation strategies stopped in Italy. Most of the previous nationwide studies in Europe 4 , 16 , 17 and large regional studies in other countries, 19 , 20 , 21 were instead restricted to 2020 – mostly to the first months of the year. The larger time span considered allowed us in addition to study women who experienced changes in care and social activities for most of or all of their pregnancy. This is not trivial, as multiple factors at different times during pregnancy might have had an impact on the rate of preterm deliveries. Though there are not, so far, studies available on this interesting topic, we can speculate, in accordance with others15, 18 that lifestyle and behaviour (more rest, working from home, reduced exposure to other respiratory pathogens) might have contributed to PTB reduction. Other possible pandemic‐related changes, which are known to impact on PTB, might have been the adoption of a different and healthier diet, 22 and diminished exposure to air pollution. 23
The ancillary but relevant finding of a decreasing trend in the overall percentage of PTB in the 3 years before the pandemic also would deserve further investigation in terms of a possible improvement of healthcare during pregnancy. Finally, the large reduction in activity of Medical Assisted Reproduction services at the beginning of the pandemic period (end of February–April 2020) 24 could be responsible of the marked drop in PTB prevalence we observed in the last months of 2020.
In unadjusted and adjusted Poisson analyses we found an inverse association between being born in the pandemic period and being late PTB, but also very PTB and extremely PTB. With the interrupted time series regression analysis we were able to demonstrate a reduction in the frequency of PTB after the lockdown only for the subgroup of late PTB, not for the other subgroups, which had much lower frequencies and thus more scattered data. Similar results were reported with this type of analysis by Been et al. 16 in the Netherlands and Bian et al. 25 in China, whereas Shah et al. 20 in Canada did not find any significant change either in the rates of all PTB or in the subgroups.
We also analysed separately singletons and multiples; as expected for singletons, who constituted 72.9% of all PTB, we found results very similar to those of the total population, whereas for multiples we probably had a lower power to reach conclusive results. In detail, for multiples, all point estimates were very similar to those of singletons and below 1, indicating a reduction in PTB during the pandemic, but the CIs in the Poisson analyses were wide, and encompassed the null value, especially for very preterm and extremely preterm infants. No data are available in the literature on the pattern of preterm multiples during the COVID‐19 pandemic in comparison with previous years. These data would have been interesting, considering that PTB is largely represented in multiple pregnancies (in our data 61.7% of all multiples are born before 37 weeks' GA); these, in turn, are associated with assisted conception, which possibly decreased during the COVID‐19 period. A sub‐analysis on multiple pregnancies would probably require an international collaboration to obtain larger sample sizes.
Finally, though we elected as the main analysis the unadjusted one because we wanted to avoid correcting for variables which could not be considered ‘true’ confounders, our results were unchanged after adjustment for many covariates considered in previous studies such as maternal ethnicity/country of birth, socio‐economic background/income/education, maternal age, parity and pregnancy conceived with ART. 15 , 17 , 19
5. CONCLUSIONS
We have demonstrated a decrease in the rate of PTB after implementation of measures for COVID‐19 mitigation in Italy, without an increase in stillbirths. Our results are in line with those obtained in other developed countries and, above all, in many European countries, where both COVID 19 restrictions and women's lifestyles are more similar to those in Italy.
Finally, a lesson to be learned from the decrease in PTB rate seen in many countries during the pandemic is the possible importance of lifestyle and environmental aspects related to the occurrence of pregnancies ending preterm. The pandemic period and its restriction measures could therefore represent a large ‘natural experiment’ to explore the prevention of preterm birth, one of the most important goals encouraged by WHO. 26
AUTHOR CONTRIBUTIONS
FR conceived the study, provided overall guidance, drafted the article and reviewed the final version. MP and MP conducted the centralised statistical analysis, assisted with data collection, collaborated in drafting the article and reviewed the final version. SB, LG and AN collaborated in the statistical analysis, collaborated in drafting the article and reviewed the final version. FZ supervised, collaborated in drafting the article and reviewed the final version. PB, SC, SF, MG, EP, EP, RP, EET and LVDP supervised, conducted Regional statistical analyses, collaborated in drafting the article and reviewed the final version. EC, DF, OL, MM, AP, RR and AS supervised, assisted with Regional statistical analysis, collaborated in drafting the article and reviewed the final version. All authors have read and agreed to the published version of the article.
FUNDING INFORMATION
None.
CONFLICT OF INTERESTS
None declared. Completed disclosure of interest forms are available to view online as supporting information.
ETHICS APPROVAL
This study used routinely collected administrative hospital data collected in the Certificato di assistenza al parto (CeDAP). CeDAP is mandatory by law and is run by the Regional Health Systems, which can use personal data without individual consent to evaluate service provision and performance (Italian Ministry of Health). Decree 16 July 2001 (https://www.gazzettaufficiale.it/eli/id/2001/09/19/001G0405/sg).
Supporting information
Appendix S1
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
APPENDIX A.
The AIE (Associazione Italiana di Epidemiologia) Perinatal Health Working Group
Edoardo Corsi (Department of Biomedicine and Prevention, University of Rome Tor Vergata, Rome, Italy; National Centre for Disease Prevention and Health Promotion, Istituto Superiore di Sanità, Rome); Debora Formisano (Unit of Clinical Governance, Azienda USL ‐ IRCCS di Reggio Emilia, Italy); Olivia Leoni (Welfare General Directorate, Lombardy Region, Milan, Italy); Monica Mazzucato (Birth Registry, Coordinating Centre for Rare Diseases, Veneto Region, Padua, Italy); Arianna Polo (Area Rete Ospedaliera, Lazio Regional Authority, Rome, Italy); Raffaella Rusciani (Department of Epidemiology, ASL TO3 Piedmont Region, Turin, Italy); Alessandro Scoppa (Department of Maternal and Child Health, General Directorate for Health, Campania Region, Naples, Italy).
Rusconi F, Puglia M, Pacifici M, Brescianini S, Gagliardi L, Nannavecchia AM, et al. the AIE Perinatal Health Working Group . Pregnancy outcomes in Italy during COVID‐19 pandemic: A population‐based cohort study. BJOG. 2023;130(3):276–284. 10.1111/1471-0528.17315
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. BMJ. 2020;370:m3320. 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donati S, Corsi E, Maraschini A, Salvatore MA, ItOSS‐COVID‐19 Working Group . SARS‐CoV‐2 infection among hospitalised pregnant women and impact of different viral strains on COVID‐19 severity in Italy: a national prospective population‐based cohort study. BJOG. 2022;129(2):221–31. 10.1111/1471-0528.16980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Euro‐Peristat Research Network . Population birth data and pandemic readiness in Europe. BJOG. 2021; 129: 179–84. 10.1111/1471-0528.16946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hedermann G, Hedley PL, Bækvad‐Hansen M, Hjalgrim H, Rostgaard K, Poorisrisak P, et al. Danish premature birth rates during the COVID‐19 lockdown. Arch Dis Child Fetal Neonatal Ed. 2021. Jan;106(1):93–5. 10.1136/archdischild-2020-319990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McDonnell S, McNamee E, Lindow SW, O’Connell MP. The impact of the COVID‐19 pandemic on maternity services: a review of maternal and neonatal outcomes before, during and after the pandemic. Eur J Obstet Gynecol Reprod Biol. 2020;255:172–6. 10.1016/j.ejogrb.2020.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Curtis M, Villani L, Polo A. Increase of stillbirth and decrease of late preterm infants during the COVID‐19 pandemic lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106(4):456. 10.1136/archdischild-2020-320682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O’Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID‐19 pandemic. JAMA. 2020;324(7):705–6. 10.1001/jama.2020.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol‐Urganci I, et al. Effects of the COVID‐19 pandemic on maternal and perinatal outcomes: a systematic review and meta‐analysis. Lancet Glob Health. 2021;9(6):e759–72. 10.1016/S2214-109X(21)00079-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang J, D’Souza R, Kharrat A, Fell DB, Snelgrove JW, Murphy KE, et al. Coronavirus disease 2019 pandemic and pregnancy and neonatal outcomes in general population: a living systematic review and meta‐analysis (updated Aug 14, 2021). Acta Obstet Gynecol Scand. 2022;101(1):7–24. 10.1111/aogs.14277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Directorate‐General of Digitalization, of Health Informative System and of Statistics, Italian Ministry of Health . Certificato di assistenza al parto (CeDAP). Analisi dell’evento nascita–2020[Birth Registry – Year 2020]. [Italian] [https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=3149]. Accessed 10 March 2022.
- 11. Williams TC, Bach CC, Matthiesen NB, Henriksen TB, Gagliardi L. Directed acyclic graphs: a tool for causal studies in paediatrics. Pediatr Res. 2018;84(4):487–93. 10.1038/s41390-018-0071-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester, UK: John Wiley & Sons; 2019. [Google Scholar]
- 13. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 14. Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348–55. Erratum in: Int J Epidemiol. 2020;49(4):1414. 10.1093/ije/dyw098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gurol‐Urganci I, Waite L, Webster K, Jardine J, Carroll F, Dunn G, et al. Obstetric interventions and pregnancy outcomes during the COVID‐19 pandemic in England: a nationwide cohort study. PLoS Med. 2022;19(1):e1003884. 10.1371/journal.pmed.1003884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Been JV, Burgos Ochoa L, Bertens LCM, Schoenmakers S, Steegers EAP, Reiss IKM. Impact of COVID‐19 mitigation measures on the incidence of preterm birth: a national quasi‐experimental study. Lancet Public Health. 2020;5(11):e604–11. 10.1016/S2468-2667(20)30223-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pasternak B, Neovius M, Söderling J, Ahlberg M, Norman M, Ludvigsson JF, et al. Preterm birth and stillbirth during the COVID‐19 pandemic in Sweden: a Nationwide cohort study. Ann Intern Med. 2021;174(6):873–5. 10.7326/M20-6367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leibovitch L, Reichman B, Mimouni F, Zaslavsky‐Paltiel I, Lerner‐Geva L, Wasserteil N, et al. Preterm singleton birth rate during the COVID‐19 lockdown: a population‐based study. Am J Perinatol. 2021;39:1020–6. 10.1055/s-0041-1740012 [DOI] [PubMed] [Google Scholar]
- 19. Simpson AN, Snelgrove JW, Sutradhar R, Everett K, Liu N, Baxter NN. Perinatal outcomes during the COVID‐19 pandemic in Ontario, Canada. JAMA Netw Open. 2021;4(5):e2110104. 10.1001/jamanetworkopen.2021.10104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah PS, Ye XY, Yang J, Campitelli MA. Preterm birth and stillbirth rates during the COVID‐19 pandemic: a population‐based cohort study. CMAJ. 2021;193(30):E1164–72. 10.1503/cmaj.210081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Main EK, Chang SC, Carpenter AM, Wise PH, Stevenson DK, Shaw GM, et al. Singleton preterm birth rates for racial and ethnic groups during the coronavirus disease 2019 pandemic in California. Am J Obstet Gynecol. 2021. Feb;224(2):239–41. 10.1016/j.ajog.2020.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stampini V, Monzani A, Caristia S, Ferrante G, Gerbino M, De Pedrini A, et al. The perception of Italian pregnant women and new mothers about their psychological wellbeing, lifestyle, delivery, and neonatal management experience during the COVID‐19 pandemic lockdown: a web‐based survey. BMC Pregnancy Childbirth. 2021;21(1):473. 10.1186/s12884-021-03904-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. European Environment Agency . Air quality in Europe — 2020 report. Luxembourg: Publications Office of the European Union; 2020 Nov. 164 p. Report No.: 09/2020. 10.2800/786656 [DOI]
- 24. Vermeulen N, Ata B, Gianaroli L, Lundin K, Mocanu E, Rautakallio‐Hokkanen S, et al. A picture of medically assisted reproduction activities during the COVID‐19 pandemic in Europe. Human Reproduction Open. 2020;2020(3):hoaa035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bian Z, Qu X, Ying H, Liu X. Are COVID‐19 mitigation measures reducing preterm birth rate in China? BMJ Glob Health. 2021;6(8):e006359. 10.1136/bmjgh-2021-006359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization . Survive and thrive: transforming care for every small and sick newborn. Geneva; 2019. Licence: CC BY‐NC‐SA 3.0 IGO.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
ICMJE
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


