Abstract
Objective
The aim of this study was to determine the risk of post‐acute sequelae of COVID‐19 associated with the continuous spectrum of BMI.
Methods
Epidemiology of Long COVID (EPILOC) is a population‐based study conducted in Baden‐Württemberg (Germany), including subjects aged 18 to 65 years who tested positive for SARS‐CoV‐2 between October 2020 and April 2021. Eligible subjects answered a standardized questionnaire, including sociodemographic characteristics, lifestyle factors, and the presence of specific symptoms. Participants assessed their current general health recovery and working capacity compared with the pre‐infection situation and provided their body height and weight. Generalized additive models were used to assess the association of BMI with general health recovered, working capacity recovered, and prevalence of fatigue, cognitive impairment, and chest symptoms.
Results
The analyses included 11,296 individuals (41% male), with a mean age of 44.0 (SD 13.7) years. Best general health recovery was observed at BMI of 22.1 (95% CI: 21.0‐27.0) kg/m2 in men and BMI of 21.6 (95% CI: 20.3‐23.1) kg/m2 in women. In addition, we found that increasing BMI was consistently associated with post‐COVID fatigue, neurocognitive impairment, and chest symptoms.
Conclusions
High BMI contributes to impaired recovery after SARS‐CoV‐2 infection; however, a low BMI is associated with impaired recovery as well.
Study Importance.
What is already known?
BMI ≥ 30 kg/m2 is an established risk factor for severe acute COVID‐19.
Some studies found BMI ≥ 30 kg/m2 also to be associated with post‐acute sequelae of COVID‐19.
What does this study add?
This study describes health recovery over the continuous spectrum of BMI.
There is an optimal BMI range in which general health recovery is best; recovery is worse with a higher or lower BMI.
How might these results change the direction of research or the focus of clinical practice?
Future studies should shed light on the biological pathways underlying this association.
Prevention policies for obesity should be considered as a component of future pandemic preparedness strategies.
INTRODUCTION
Post‐acute health problems and other sequelae affecting a significant proportion of COVID‐19 survivors and lasting for up to 12 months have been reported [1, 2, 3]. Body mass index (BMI) ≥ 30 kg/m2 is an established risk factor for severe acute COVID‐19 [4, 5, 6], and it also has been associated with post‐acute sequelae of COVID‐19 [7, 8, 9].
Post‐acute COVID‐19 sequelae are manifold [10]. However, most of the burden, in terms of impaired general health recovery and lost working capacity, is attributable to symptoms of fatigue, cognitive impairment, and chest symptoms (shortness of breath, chest pain, wheezing) [1].
U‐ or J‐shaped associations of BMI with acute complications of COVID‐19 have been observed [11, 12]. Therefore, BMI ≥ 30 kg/m2 but also a low BMI might be associated with post‐acute sequelae of COVID‐19. However, the risk of post‐acute sequelae of COVID‐19 associated with the continuous spectrum of BMI has not yet been determined.
Therefore, we investigated the associations of BMI with self‐reported general health recovered, working capacity recovered, and common symptom clusters such as fatigue, cognitive impairment, and chest symptoms in COVID‐19 survivors. In addition, we considered possible effect modification by important covariates.
METHODS
Study design
Epidemiology of Long COVID (EPILOC) is a population‐based study conducted in the Federal State of Baden‐Württemberg in southwestern Germany [1]. The study included subjects aged 18 to 65 years who tested positive for SARS‐CoV‐2 by polymerase chain reaction (PCR) between October 1, 2020, and April 1, 2021, and whose infection was notified to the local public health authorities.
Eligible subjects were invited to return a standardized questionnaire including sociodemographic characteristics, and lifestyle factors. It evaluated the presence of 30 specific symptoms before and during the acute infection phase and when filling out the questionnaire (i.e., 6 to 12 months after acute infection). For symptoms present at the time of the survey, we also asked whether and to which grade each symptom impaired daily life activities (none, light, moderate, or strong). Clusters of strongly correlated current symptoms were previously identified [1]. The present work focuses on the three most common symptom clusters fatigue (including rapid physical exhaustion or chronic fatigue), cognitive impairment (including concentration difficulties, memory disturbance, and confusion), and chest symptoms (including shortness of breath, chest pain, and wheezing). A cluster was counted as present if at least one included symptom was reported at the time of the survey but was not prevalent before the SARS‐CoV‐2 infection.
In addition, participants assessed their current general health recovery and working capacity compared with the situation before the acute SARS‐CoV‐2 infection (in 10% steps from 0% to 100%). The question was, “What percentage of your original work capacity (before your positive corona test) have you regained today?” The use of this single question has shown similar relations to sick leave and health‐related quality of life in occupational studies [13]. Similarly, the current general health condition compared with the situation before the acute SARS‐CoV‐2 infection was assessed. In addition, participants were asked to provide their current body height (in cm) and weight (in kg, without shoes and light clothing only).
Ethical approval was obtained from the respective ethical review boards of the study centers in Freiburg (21/1484) and Ulm (337/21).
Statistical analysis
We used generalized additive models (GAM) to assess the association of BMI with general health recovered, working capacity recovered, and post‐COVID prevalence of fatigue, cognitive impairment, and chest symptoms. BMI was entered as a smooth term. An identity link and Gaussian error term were used for general health and working capacity recovered as outcomes and a logit link and binomial error term for prevalence outcomes.
All models were fitted for men and women separately and were adjusted for age (continuous), education (university entrance qualification yes/no), smoking status (current or past vs. regular cigarette smoker), and severity of the initial COVID‐19 illness (medically treated yes/no).
Fitted curves for average covariate values with corresponding 95% pointwise confidence intervals (CIs) were plotted by BMI from 15 to 45 kg/m2. For prevalence outcomes, the linear predictor was backtransformed to probabilities using the logistic function. Finally, optima for general health recovered and working capacity recovered were derived numerically, and model‐based 95% CIs using posterior resampling.
We used spline‐factor interaction terms (for discrete factors) and tensor product interactions (for age) to investigate possible effect modification. All analyses have been performed using R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Of 50,457 subjects invited to participate, 12,053 (24%) responded, and 11,296 with available data on BMI and covariates (41% male, mean age 44.0 years) were included in the analysis. Men were more frequently “ever smokers” than women (39.5 vs. 31.4%) and had a higher BMI (27.0 vs. 25.4 kg/m2) on average (Table 1). The average general health recovery was 90% in men and 88% in women. The average working capacity recovery was also 90% in men and 89% in women. Persistent new symptom clusters (not already present before infection) of fatigue, neurocognitive impairment, and chest symptoms were less prevalent in men than in women.
TABLE 1.
Characteristics of the study population
| Men | Women | |||
|---|---|---|---|---|
| N | Mean proportion | N | Mean or proportion | |
| Age (y), mean (SD) | 4674 | 45.0 (13.7) | 6622 | 43.3 (13.6) |
| Age class (y), N (%) | 4674 | 6622 | ||
| <30 | 893 (19.1) | 1516 (22.9) | ||
| 30–<40 | 861 (18.4) | 1233 (18.6) | ||
| 40–<50 | 819 (17.5) | 1183 (17.9) | ||
| 50–<60 | 1359 (29.1) | 1939 (29.3) | ||
| ≥60 | 742 (15.9) | 751 (11.3) | ||
| University entrance qualification, N (%) | 4674 | 2510 (53.7) | 6622 | 3439 (51.9) |
| Ever smoker, N (%) | 4674 | 1846 (39.5) | 6622 | 2078 (31.4) |
| BMI (kg/m2), mean (SD) | 4674 | 27.0 (4.8) | 6622 | 25.4 (5.6) |
| BMI class, N (%) | 4674 | 6622 | ||
| <18.5 kg/m2 | 30 (0.6) | 188 (2.8) | ||
| ≥18.5–<25 kg/m2 | 1706 (36.5) | 3620 (54.7) | ||
| ≥25–<30 kg/m2 | 1989 (42.6) | 1658 (25.0) | ||
| ≥30 kg/m2 | 949 (20.3) | 1156 (17.5) | ||
| Medical treatment of acute SARS‐CoV‐2 infection, N (%) | 4674 | 984 (21.1) | 6622 | 1567 (23.7) |
| Percent general health recovered, mean (SD) | 4604 | 90 (14) | 6497 | 88 (15) |
| Percent general health recovered, N (%) | 4604 | 6497 | ||
| 100% recovery | 2262 (49.1) | 2692 (41.4) | ||
| 90% recovery | 1091 (23.7) | 1688 (26.0) | ||
| ≤80% recovery | 1251 (27.2) | 2117 (32.6) | ||
| Percent working capacity, mean (SD) | 4630 | 90 (16) | 6535 | 89 (17) |
| Percent working capacity, N (%) | 4630 | 6535 | ||
| 100% recovery | 2616 (56.5) | 3294 (50.4) | ||
| 90% recovery | 899 (19.4) | 1400 (21.4) | ||
| ≤80% recovery | 1115 (24.1) | 1841 (28.2) | ||
| Newly developed symptom clusters, a N (%) | ||||
| Any symptom (any grade) | 4674 | 2655 (56.8) | 6622 | 4544 (68.6) |
| Any symptom (moderate to strong) | 1591 (34.0) | 3090 (46.7) | ||
| Fatigue (any grade) | 4586 | 1464 (31.9) | 6489 | 2666 (41.1) |
| Fatigue (moderate to strong) | 828 (18.1) | 1720 (26.5) | ||
| Neurocognitive impairment (any grade) | 4600 | 1187 (25.8) | 6523 | 2291 (35.1) |
| Neurocognitive impairment (moderate to strong) | 526 (11.4) | 1175 (18.0) | ||
| Chest symptoms (any grade) | 4617 | 1204 (26.1) | 6548 | 2168 (33.1) |
| Chest symptoms (moderate to strong) | 553 (12.0) | 1029 (15.7) | ||
The symptom clusters fatigue (including rapid physical exhaustion or chronic fatigue), cognitive impairment (including concentration difficulties, memory disturbance, and confusion), and chest symptoms (including shortness of breath, chest pain, and wheezing) were counted as present if at least one included symptom was reported at the time of the survey but was not present before the SARS‐CoV‐2 infection.
Low and high BMI were associated with both impaired general health and working recovery (Figure 1) in men and women. Best general health recovery was observed in men at BMI of 22.1 (95% CI: 21.0‐27.0) kg/m2, and best working capacity recovered at 24.1 (95% CI: 21.4‐27.1) kg/m2. In women, the best general health recovery was observed at BMI of 21.6 (95% CI: 20.3‐23.1) kg/m2, and the best working capacity recovered at 21.6 (95% CI: NA‐23.3) kg/m2.
FIGURE 1.
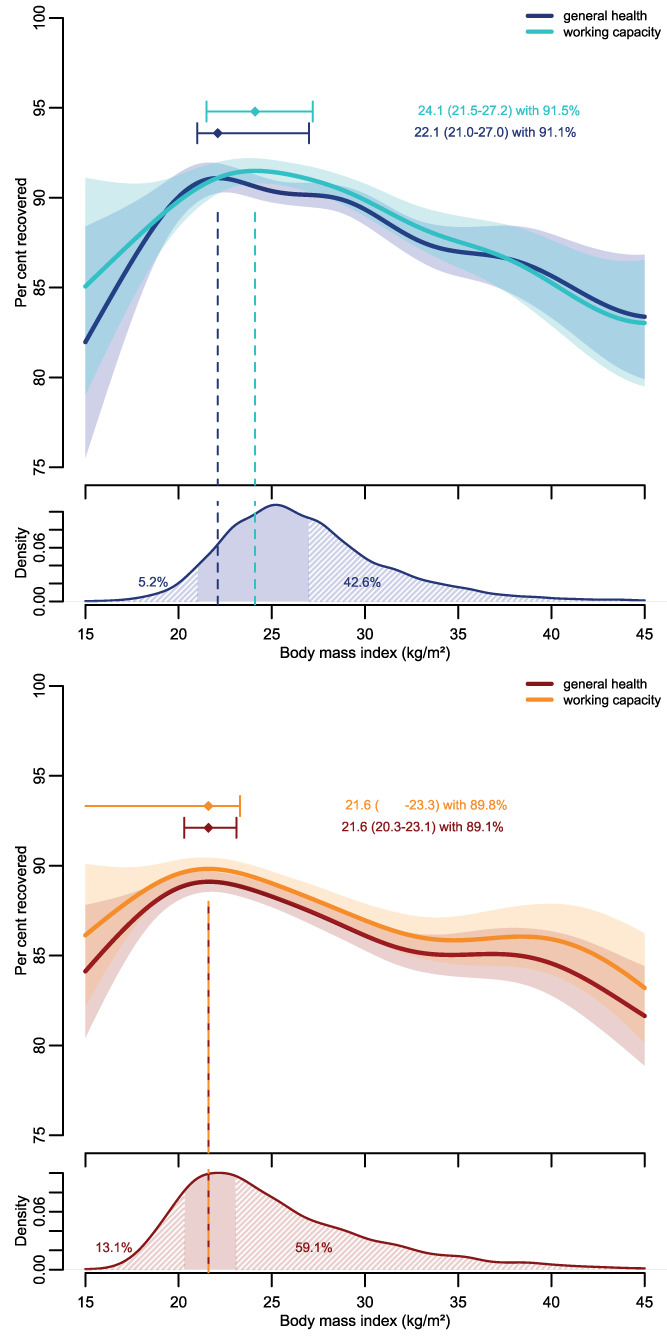
Percent general health and working capacity recovered by body mass index (BMI) in men (top panel, blue colors) and women (bottom panel, red colors), along with the BMI distribution in men and women. The dots with whiskers represent the BMI associated with maximum general health (working capacity) recovered with its corresponding 95% CI. The associations are adjusted for age, education, smoking status, and treatment of acute SARS‐CoV‐2 infection. Shaded areas indicate parts of the BMI distribution where general health recovery was statistically significantly impaired either because of lower or higher than optimal BMI values
General health and working capacity recovered were lower in participants requiring medical treatment during acute infection (i.e., higher severity of the acute COVID disease) (Supporting Information Figure S1). However, the association of BMI with general health and working capacity recovery looked similar in medical treated and not treated subjects. Regarding smoking status, curves also looked similarly in never and ever smokers (Supporting Information Figure S2).
In contrast, age modified the associations of BMI with general health and working capacity recovered (p values < 0.01; Supporting Information Figure S3). A high BMI was less strongly associated with impaired recovery in younger than older men and women.
Regarding symptom clusters, we found that increasing BMI was consistently associated with fatigue, neurocognitive impairment, and chest symptoms in men and women (Figure 2), irrespective of the grade of impairment (any grade, or moderate to strong). There was also a tendency for increased risk at low BMI (< 20 kg/m2) for some symptoms (fatigue in men, neurocognitive impairment of any grade in women, and chest symptoms of grade moderate to strong in women). However, statistical power was rather low at the lower end of the BMI distribution.
FIGURE 2.
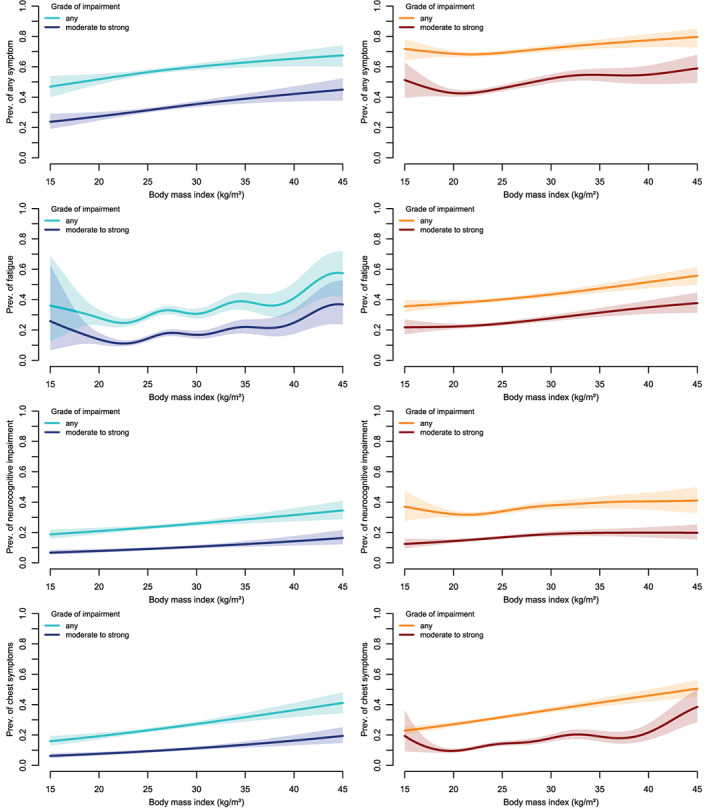
Prevalence of different symptom clusters (not present before infection) by body mass index (BMI) in men (left panels, blue colors) and women (right panels, red colors), by grade of impairment. The associations are adjusted for age, education, smoking status, and treatment of acute SARS‐CoV‐2 infection
DISCUSSION
We found a clear association between increasing BMI and impaired general health and working capacity recovery. In men, general health recovery was statistically significantly compromised above 27.0 kg/m2 and in women above BMI of 23.1 kg/m2. In addition, a low BMI was associated with statistically significantly impaired recovery in men below BMI of 21.0 kg/m2 and in women below BMI of 20.3 kg/m2.
BMI ≥ 30 kg/m2 being a risk factor for post‐acute sequelae of COVID‐19 is in line with other studies [7, 8]. However, a negative association between a low BMI and health outcomes after COVID‐19 has not yet been reported. From a public health perspective, a high BMI is the more relevant issue, as only 5% of men and 13% of women in our cohort had BMI below 21.0 and 20.3 kg/m2, respectively. In contrast, 43% of men and 59% of women had a BMI above 27.0 and 23.1 kg/m2, respectively, and thereby a statistically significant impaired general health recovery.
Interestingly, age had little effect on general health recovery at an optimal or lower BMI: the average general health recovery at BMI of 22.1 kg/m2 was only 0.8% lower in 55‐year‐old compared with 25‐year‐old men, whereas at BMI of 35.0 kg/m2, average general health recovery was 4.1% lower in 55‐year‐old than 25‐year‐old men.
Loosen et al. found hypertension and lipid metabolism disorder, in addition to obesity, to be associated with a Long COVID syndrome diagnosis by a general practitioner [8]. These obesity‐associated metabolic comorbidities accompanied by subclinical inflammation might explain the stronger association in older than younger participants, as these comorbidities develop over time.
The strengths of the present work are the large sample size and the population‐based approach, including many participants who had a mild (or even asymptomatic) infection. In addition, we used a flexible modeling approach instead of relying on predefined BMI categories.
The main limitation of this work is its reliance on self‐reported weight and height and a limited response rate of 24%. In addition, we used BMI at the time of the survey, and body weight might have already been affected by the acute COVID‐19 disease or its sequelae and pandemic‐related influences. Furthermore, a low BMI might result from (known or unknown) health problems already present before infection, and impaired recovery may not necessarily result from a low BMI per se.
Despite these limitations, we show evidence that a high BMI contributes to impaired general health, working capacity recovery, and the presence of post‐COVID sequelae after SARS‐CoV‐2 infection.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
Supporting information
Appendix S1 Supplementary Information
ACKNOWLEDGMENTS
The authors thank all participants who took part in the survey. In addition, the authors acknowledge the participating local health authorities for their administrative and technical support. The authors thank key collaborators on this work: Nelli Edel, Bettina Deibert, Stefanie Döbele, Sabine Gerbersdorf, Katja Hirth, Achim Jerg, Moritz Munk, Sylvia Parthé, Stephan Rusch, Cynthia Stapornwongkul, Michaela Schmid, Patrick Roling, Jennifer Müller, Annika Noghero, and Hanna Tschischka.
Additional members of the EPILOC Phase 1 Study Group: Dietrich August, Christoph Bauer, Benedict Blankenhorn, Ulrike Bopp‐Haas, Stefanie Bunk, Peter Deibert, Armin Dietz, Birgit Friedmann‐Bette, Roland Giesen, Veronika Götz, Sylvia Grote, Beate Grüner, Alexandra Junginger, Oliver Kappert, Johannes Kirsten, Hans‐Georg Kräusslich, Anne Kühn, Nisar P. Malek, Barbara Müller, Andreas Niess, Stefanie Pfau, Isolde Piechotowski, Siegbert Rieg, Sibylle Röttele, Jana Schellenberg, Claudia Schilling, Chantal Schröder, Rainer Schwertz, Monika Spannenkrebs, Gabriele Wagner, Birgit Walter‐Frank, Kersten Wolfers. Open Access funding enabled and organized by Projekt DEAL.
Peter RS, Nieters A, Brockmann SO, et al. Association of BMI with general health, working capacity recovered, and post‐acute sequelae of COVID‐19. Obesity (Silver Spring). 2023;31(1):43‐48. doi: 10.1002/oby.23611
Funding information Baden‐Württemberg Federal State Ministry of Science and Art, Grant/Award Number: MR/S028188/1; German pension fund (“Deutsche Rentenversicherung”) Baden‐Württemberg
REFERENCES
- 1. Peter RS, Nieters A, Kräusslich H‐G, et al; EPILOC Phase 1 Study Group. Post‐acute sequelae of covid‐19 six to 12 months after infection: population based study. BMJ. 2022;379:e071050. doi: 10.1136/bmj-2022-071050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sørensen AIV, Spiliopoulos L, Bager P, et al. A nationwide questionnaire study of post‐acute symptoms and health problems after SARS‐CoV‐2 infection in Denmark. Nat Commun 2022;13:4213. doi:10.1038/s41467‐022‐31897‐x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM; Lifelines Corona Research Initiative. Persistence of somatic symptoms after COVID‐19 in The Netherlands: an observational cohort study. Lancet 2022;400:452‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID‐19 hospital admission. Clin Infect Dis 2020;71:896‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hergens M‐P, Bell M, Haglund P, et al. Risk factors for COVID‐19‐related death, hospitalization and intensive care: a population‐wide study of all inhabitants in Stockholm. Eur J Epidemiol 2022;37:157‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yates T, Zaccardi F, Islam N, et al.; ISARIC4C investigators . Obesity, ethnicity, and risk of critical care, mechanical ventilation, and mortality in patients admitted to hospital with COVID‐19: analysis of the ISARIC CCP‐UK cohort. Obesity (Silver Spring) 2021;29:1223‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whitaker M, Elliott J, Chadeau‐Hyam M, et al. Persistent COVID‐19 symptoms in a community study of 606, 434 people in England. Nat Commun. 2022;13:1957. doi: 10.1038/s41467-022-29521-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loosen SH, Jensen B‐EO, Tanislav C, Luedde T, Roderburg C, Kostev K. Obesity and lipid metabolism disorders determine the risk for development of long COVID syndrome: a cross‐sectional study from 50,402 COVID‐19 patients. Infection. 2022;50:1165‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Desgranges F, Tadini E, Munting A, Regina J, et al. Post‐COVID‐19 syndrome in outpatients: a cohort study. J Gen Intern Med. 2022;37:1943‐1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lopez‐Leon S, Wegman‐Ostrosky T, Perelman C, et al. More than 50 long‐term effects of COVID‐19: a systematic review and meta‐analysis. Sci Rep 2021;11:16144. doi:10.1038/s41598‐021‐95565‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kompaniyets L, Goodman AB, Belay B, et al. Body mass index and risk for COVID‐19‐related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death—United States, March–December 2020. MMWR Morb Mortal Wkly Rep 2021;70:355‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kang IS, Kong KA. Body mass index and severity/fatality from coronavirus disease 2019: a nationwide epidemiological study in Korea. PloS One 2021;16:e0253640. doi:10.1371/journal.pone.0253640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ebener M, Hasselhorn HM. Validation of short measures of work ability for research and employee surveys. Int J Environ Res Public Health 2019;16:3386. doi:10.3390/ijerph16183386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplementary Information


