Abstract
Synucleinopathies, including Parkinson's disease (PD), dementia with Lewy Bodies (DLB), and multiple system atrophy (MSA), are characterized by the misfolding and subsequent aggregation of alpha-synuclein (α-syn) that accumulates in cytoplasmic inclusions bodies in the cells of affected brain regions. Since the seminal report of likely-aggregated α-syn presence within the Lewy bodies by Spillantini et al. in 1997, the keyword “synuclein aggregation” has appeared in over 6000 papers (Source: PubMed October 2022). Studying, observing, describing, and quantifying α-syn aggregation is therefore of paramount importance, whether it happens in tubo, in vitro, in post-mortem samples, or in vivo. The past few years have witnessed tremendous progress in understanding aggregation mechanisms and identifying various polymorphs. In this context of growing complexity, it is of utmost importance to understand what tools we possess, what exact information they provide, and in what context they may be applied. Nonetheless, it is also crucial to rationalize the relevance of the information and the limitations of these methods for gauging the final result. In this review, we present the main techniques that have shaped the current views about α-syn structure and dynamics, with particular emphasis on the recent breakthroughs that may change our understanding of synucleinopathies.
Keywords: Synucleinopathy, Parkinson's disease, α-Synuclein, Oligomerization, Fibril, Amyloid, Fluorescence, Polymorphism, Structural techniques
Abbreviations: Ab, Antibody; AFM, Atomic Force Microscopy; α-syn, alpha-synuclein; AS-PLA, Alpha-synuclein Proximity Ligation Assay; BiFC, Bimolecular Fluorescence Complementation; CD, Circular Dichroism; CFP, Cyan Fluorescent Protein; CR, Congo Red; CSF, Cerebro-spinal fluid; cryo-EM, Cryogenic Electron Microscopy; Cys-Mal, cysteine residue labelled via fluorophore-attached maleimides; DLB, Dementia with Lewy Bodies; ED, Electron diffraction; ELISA, Enzyme-Linked Immunosorbent Assay; EM, Electron Microscopy; ESIPT, Excited-State Intramolecular Proton-Transfer; FlAsH, Fluorescein Arsenical Hairpin; FRET, Förster Resonance Energy Transfer; FTIR, Fourier-Transformed Infra-Red; GCI, Glial Cytoplasmic Inclusions; GFP, Green Fluorescent Protein; LB, Lewy body; LCPs, Luminescent Conjugated Polymers; LRP, Lewy-related pathology; MicroED, Microcrystal Electron Diffraction; mAb, Monoclonal Antibody; MSA, Multiple System Atrophy; NDGA, nordihydroguaiaretic acid; NMR, Nuclear Magnetic Resonance; PCA, Protein Complementation Assay; PD, Parkinson's Disease; PMCA, protein misfolding cyclic amplification; PFF, Pre-Formed Fibril; PK, Proteinase K; ReAsH, Red Arsenical Hairpin; RT-QuIC, real-time quaking-induced conversion; smFRET, single-molecule Förster Resonance Energy Transfer; ssNMR, Solid-State Nuclear Magnetic Resonance; ThS, Thioflavine S; ThT, Thioflavine T; UAA, Unnatural Amino Acid; VCD, Vibrational Circular Dichroism
1. Introduction
1.1. The spectrum of synucleinopathies
Synucleinopathies, such as Parkinson's disease (PD), dementia with Lewy Bodies (DLB), and multiple system atrophy (MSA), are a group of neurodegenerative disorders of unknown aetiology with very diverse clinical and pathological presentations. However, these diseases share certain standard features, the most significant being the aggregation of alpha-synuclein (α-syn), present in cytoplasmic inclusions bodies in the cells of affected brain regions (Papp and Lantos, 1992; Spillantini et al., 1997). These cytoplasmic inclusion bodies are diverse in nature and cellular localization across these synucleinopathies. Lewy bodies (LBs) are found in neurons of PD and DLB patients' brains, while glial cytoplasmic inclusions (GCIs) are detected in oligodendrocytes of MSA patients' brains. LBs are eosinophilic inclusions comprising filamentous structures in a dense core surrounded by a peripheral halo (Wakabayashi et al., 2007) containing numerous membranous components and dysmorphic organelles (Shahmoradian et al., 2019). GCIs are packed triangle or sickle-shaped, filamentous structures (Wakabayashi and Takahashi, 2006).
Neuronal and oligodendroglial pathologies are far more diverse than such a simple description suggests both in their nature or form and the number of anatomical stigmas they present. Behind the established terms, LBs and GCIs, there is, in fact, a myriad of inclusions, both intracellular and extracellular, more or less mature and more or less toxic. Still, all of them are characterized by misfolded, aggregated α-syn.
The neuronal pathology, known as Lewy pathology, is the most studied in PD. Although this pathology has various origins, most research focuses on the characterization of LBs in the lower brainstem nuclei, where they are well structured, with a classic core surrounded by a halo. Cortical LBs cannot be considered “classical” and are less structured (Sakamoto et al., 2002), and, overall, much less studied. This morphological heterogeneity of the two types of LBs is explained by their enrichment in α-syn. Although aggregated filamentous α-syn is a central (not principal) constituent of LBs and GCIs (Laferrière et al., 2022; Mccormack et al., 2016; Spillantini et al., 1997; Wakabayashi and Takahashi, 2006), only around two-thirds of LBs in the Substantia Nigra are composed of α-syn; and they represent no more than one-third of cortical LBs. Finally, the glial inclusions seen in MSA are little studied (except, of course, by pathologists); hence, the status of α-syn aggregates is less evident in these aggregate subtypes.
1.2. Synuclein aggregation leads to synucleinopathies
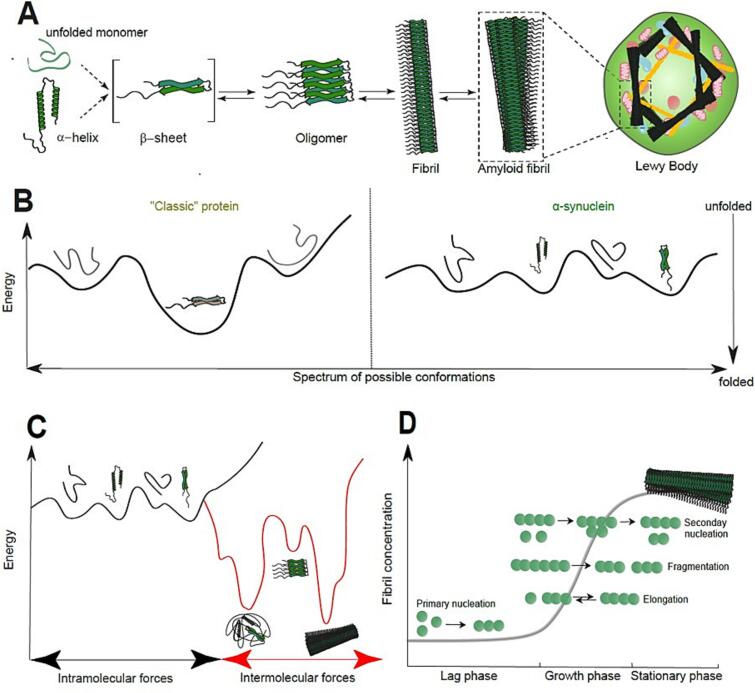
The cornerstone of aggregate formation is the ability of α-syn, a so-called amyloid protein, to aggregate (Bourdenx et al., 2017). Fig. 1A shows a schematic summary of the aggregation pathway of this protein. α-syn exists in various conformational forms. Indeed, it is present in at least two structural isoforms: a soluble, natively unfolded monomer and a membrane-bound, helix-rich form. Some authors have suggested the existence of an alternative, tetrameric, aggregation-resistant organization of the protein (Bartels et al., 2011). Isoforms can undergo dramatic structural changes, resulting in rich sheet-like assemblies that are the precursors of more complex amyloid forms of the protein. From a myriad of in vitro studies, we can deduce that the behaviour of α-syn follows a dynamic equilibrium in which the monomer aggregates. First into several types of small oligomeric species that sheet interactions can stabilize, and then into higher molecular weight, insoluble protofibrils that can polymerize into amyloidogenic fibrils by reassembling those contained in LBs. However, the exact mechanism governing the fundamental conformational change from the standard α-syn monomer to the pathological, disease-associated form remains elusive.
Fig. 1.
α-syn aggregation (adapted from (Bezard and Dehay, 2022). A) Schematic summary of the aggregation pathway of α-syn. Different monomeric species exist in a continuous equilibrium. Aggregation of α-syn usually involves the formation of rich b-sheet-like assemblies, which precede the formation of more complex fibrillated forms of the protein found in Lewy Bodies. B) Energy diagram illustrating protein structural transition. Unlike classic proteins, where different conformational forms are associated with relatively deep energy pits (left), α-syn structure oscillates continuously between various conformations with similar energy levels (right). C) Under special conditions, α-syn can adopt extremely stable forms, termed amyloid structure, which is then very hard to reverse. D) α-syn aggregation process is a highly complex process. Nonetheless, it is generally accepted that amyloid fibril formation is a process that relies on several stages: primary nucleation, growth, and proliferation by elongation and secondary nucleation. These phases are commonly subdivided into three depending on fibril mass: elongation, growth, and stationary.
When applied to α-syn, the notion of folding, or so-called “pathological” conformation, appears restrictive. α-syn is one of the most intrinsically disordered proteins. Its structure oscillates continuously between various conformations, perhaps on a continuum, ranging from a fully unfolded to a fully folded protein (Robustelli et al., 2018). This shape transition can be illustrated by an energy diagram, representing the shape's stability as a function of the energy required to maintain it (Fig. 1B). Imagine that a ball is pushed through this diagram. It will eventually end up ‘locked’ in a pit, the most bottomless pit, for which the free energy will be the lowest, a state of ‘rest.’ This happens to a standard ‘structured’ protein; it takes on the form in which the energy to hold it is lowest, the ‘deep pit’ (Schor et al., 2016). However, under physiological conditions, the conformational energy landscape that characterizes α-syn has no natural “deep pit”; all the energy pits are more or less equal in depth. The protein thus continuously ‘vacillates’ between the different energy states (Fig. 1B).
Other external factors can create ‘energy pits’. These correspond to a β sheet-like organization of the protein (Raskatov and Teplow, 2017) (Fig. 1C): they can be caused by pH changes, post-translational modifications, cleavages, or amino acid substitutions in the protein due, for example, to point mutations (over eight are known to date). The effect of these point mutations has been extensively studied in tubo experiments. Their exact role, however, is not yet well understood. Most of them are located in the N-terminal domain, and, by themselves, do not induce important changes into the conformation of the monomeric form, although they affect α-syn interaction with the membrane (Fares et al., 2014). It is tempting to posit that these mutations increase the self-assembly propensity of α-syn. While some of them indeed do (E46K, H50Q, or A53T), some others (G51D or A53R) seem to produce the opposite effect, thereby preventing aggregation (Villar-Piqué et al., 2016). Interestingly, it has been suggested that the fibres formed by each mutant variant may present structural and biophysical differences that would support the pathological role of the mutant. (Boyer et al., 2020; Ruggeri et al., 2020).
In its β sheet form, α-syn can self-aggregate into large amyloid stacks. These are incredibly stable and correspond to a bottomless energy pit, making the protein pathological. Each folding state has a corresponding energy pit (Fig. 1C): the deeper the pit is, the more difficult it is to reverse the protein's shape. While partially folded states can be changed with minimal energy expenditure, amyloid fibrils produce a bottomless pit, which will require considerable energy expenditure to reverse (and thus return to the unfolded state of the protein).
The formation of the amyloid fibril is a process that relies on several stages: primary nucleation, growth, and proliferation by elongation and secondary nucleation (Perrett et al., 2014). Primary nucleation is critical. It creates the first fibrils. Secondary nucleation can be more problematic. It can lead to an exponential increase in protofibrils and then fibrils. Once the assembly of monomers has started, the amyloid structure acts like a “black hole.” It attracts more and more monomers into the initiated superstructure, making the system increasingly stable in terms of energy, which can concomitantly catalyze the formation of new oligomers (Fig. 1D). In this state, the cell subject to this process is exploited. It will produce more and more α-syn to feed this “monster”.
The final fine structure of amyloid fibres likely depends on several factors. First, the several possible conformations of the monomer β sheets, are the starting point of the process. Then, multiple factors such as pH or particular cellular or extra-cellular environment will affect the aggregation process per se, giving rise to different species of α-syn aggregates (polymorphs) with variable toxicity and manifestations (De Giorgi et al., 2020). These different α-syn fibrils, will spread differently and produce different pathophysiological responses, depending on their particular properties (Lau et al., 2020). This fiber heterogeneity could be at the origin of the different spectrum of synucleinopathies we observe, such as PD, DLB, or MSA, each reflecting the growth and spread of the different amyloid species that would be specific to a given disease manifestation (Peelaerts et al., 2015).
While these insoluble aggregates represent the most stable and thus “final” forms of α-syn aggregates, increasing experimental evidence points to smaller soluble species, usually called oligomers, as the main spreading toxic species. These smaller aggregates, precisely due to their instability and smaller size, would be responsible for cytotoxicity and disease expansion (Emin et al., 2022; Kulenkampff et al., 2021). Be as it may, understanding and monitoring the aggregation process at every level is critical in synucleinopathy research.
1.3. Monitoring aggregation is central to synucleinopathy research
Since the seminal report by Spillantini et al. in 1997 (Spillantini et al., 1997), the number of scientific reports featuring “synucleinopathy” as a keyword has reached 84,804 on PubMed. Among those, the keyword “synuclein aggregation” accounts for 6433 papers (October 2022). Given the above, one understands that studying, observing, describing, and quantifying α-syn aggregation is of paramount importance, whether it happens in tubo (Table 1), in vitro (Table 2), in post-mortem samples (Table 3), or in vivo (Table 4).
Table 1.
In tubo methods.
| Technique | Provided Info | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Circular Dichroism | Secondary structure (and tertiary to some extent) | Simple technique. Compatibility with vesicular dispersions, real-time structural information (kinetics) | Very sensible to light scattering. Limited to studies of one protein. Limited information | Weinreb et al., 1996; Kelly et al., 2005 |
| FTIR | Secondary and tertiary structure | Easy to use. Very sensitive to b-sheets conformations | In tubo studies of one protein. Limited information | Calero and Gasset, 2005 |
| soluble NMR | Strong structural information on soluble α-syn species | Very high structural information. Key for naturally disordered proteins such as α-syn | Need a soluble pure and often isotope-labelled sample. Complex and expensive technique | Eliezer et al., 2001 |
| Solid state NMR | Strong structural resolution on insoluble α-syn fibrils | No need for solubility. Great 3D resolution. Detailed short-range inter-atomic distance | Difficult sample preparation, spectra collection, and analysis. Expensive technique | Heise et al., 2005; Tuttle et al., 2016 |
| “Classic” Diffraction techniques | Diffraction pattern. The tertiary structure of fibrils | Unambiguous structural information about tertiary structures | Limited resolution and utility with non-crystallo-genic samples. | Sunde et al., 1997; Serpell et al., 2000 |
| Microcrystal Electron Diffraction | Diffraction patterns without the need for macroscopic crystallization | Overcome the inability of fibrils to form macro-crystals. Great resolution | Complex equipment is needed. | Shi et al., 2013; Rodriguez et al., 2015 |
| “Classic” EM | Morphology of the fibrils and their size distribution | Relatively accessible. Versatile technique. Possibility to combine with immunochemistry | Limited resolution to resolve relevant structural polymorphisms | Spillantini et al., 1998; Lashuel, 2020 |
| Cryo-EM | Very high resolution on α-syn fibrils | Impressive resolution capabilities. Resolve different fine structural properties of the fibres | Expensive and relatively complex technique. Not readily available | Guerrero-Ferreira et al., 2019; Schweighauser et al., 2020 |
| AFM | Morphology, size distribution. and mechanical properties of fibres | Information about heights, periodicities, mechanical strength and. Kinetic information | Need for synthetic surfaces. May influence the native properties of fibres | Goldberg and Lansbury Jr, 2000; Watanabe-Nakayama et al., 2020 |
| ThT fluorescence | Formation of b-sheet structures (amyloids). Fibril formation kinetics through fluorescence. | Great enhancement of fluorescence upon binding to b-sheets. Real-time monitoring of aggregate formation. | May affect the aggregation process Fluorescence intensity depends on the polymorphs. Not always sensible | Levine III, 1993; De Giorgi et al., 2020 |
| Site-specific labeling | Aggregation monitoring through fluorescence imaging. | Small fluorophores attached to specific and non-perturbing regions. Region-specific information upon aggregation. Through FRET or ESIPT. | Needs a relatively complex chemical label of α-syn before its use. | Shimogawa and Petersson, 2021 |
Table 2.
Live cells methods.
| Technique | Provided Info | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| cell-NMR | α-syn conformation inside cells | Structural information in more relevant cellular environments | Need a soluble pure and isotope-labelled sample. Complex and expensive technique | Selenko and Wagner, 2007; Theillet et al., 2016 |
| ThS | Fluorescence increases upon binding to b-sheet structures | Direct binding to amyloid fibres. Useful as a confirmatory technique for amyloid presence | High backgrounds. It may present specificity problems | Luk et al., 2009; Gaur et al., 2021 |
| Site-specific labelling | Fluorescence imaging of pre-labelled seeds | Cell-permeable, and non-perturbing probes. Information on uptake and interaction with cells | Restricted to labelled pre-formed seeds | Haney et al., 2016; Jun et al., 2019 |
| Protein fusion fluorescence | Direct fluorescence imaging of proteins fused to α-syn | The direct expression on living cells. Quantitative aggregation information through FRET | The bulky probe can alter the native conformation and aggregation process | McLean et al., 2001; Yamasaki et al., 2019 |
| BiFC | Emission of fluorescence by two protein fragments when they are in close proximity | Spatial and temporal resolution for aggregation. Suitable for early oligomers | Bulky probes. Fluorescence potentially not arising from just dimerization | Outeiro et al., 2008, Bae et al., 2014 |
Table 3.
Post-mortem techniques.
| Technique | Provided Info | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| FTIR | Secondary and tertiary structure by IR absorption. Presence of amyloid fibrils | Presence of α-syn inclusion and information about its environment | Basic information. Mainly as an orthogonal technique |
Araki et al., 2015; Shahmoradian et al., 2019 |
| EM | Visualization of α-syn in its context with immuno-electron-dense tags | Visualization of α-syn in its context. Identity of intracellular inclusions | Complex sample preparation. Limited fine fibril resolution | Baba et al., 1998, Bourdenx et al., 2020 |
| Immunohistochemistry | Visual localization of the desired antigen thanks to immunologic specificity. | Direct visualization of the antigen in its context. | Limited by Abs specificity to discern different aggregates | Altay et al., 2022 |
| Western Blot | Separation by mass using electrophoresis combined with immunodetection. | Common method. Separation by mass of species present in a sample. Immunodetection by different Abs | Non-quantitative. Limited by antibody specificity. Mass by itself does not tell much about an aggregate | Peng et al., 2018 |
| AS-PLA | Informs on aggregated protein through an amplification signal of two close-distance antibodies | Good sensitivity towards early aggregates. Recognition of early aggregates in previously not recognized regions | Cannot fundamentally between oligomers and other types of higher-order aggregates | Roberts et al., 2015; Bengoa-Vergniory et al., 2020 |
| ELISA | Quantification of immobilized species by double antibody binding + enzymatic reaction | Fast and relatively easy technique. Quantitative. Powerful combined with previous separation methods | Used Abs are critical. Limited sensibility. Previous sample preparation is critical | Lassen et al., 2018 |
| Purification methods | Obtention of protein fractions from tissue homogenates depending on the shape, size or density of the species. | Straightforward methods. Powerful information when combined with posterior characterization of fractions. | Need of posterior characterization of the fractions | Laferrière et al., 2019 |
| Digestion methods | Informs about aggregate nature through protein solubility/insolubility and/or proteolytic profile | Evaluation of fibrilization nature on digestion-resistance terms. | Limited to “solvent resistance” information | Kushnirov et al., 2020 |
Table 4.
Animal models of α-syn aggregation.
| Technique | Provided Info | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Protein construct fluorescence | In vivo or post-mortem fluorescence. Information about the aggregate formation and its localization | Generation of transgenic animals expressing the fused protein. Possibility of in vivo and post-mortem analysis | Bulky probe. Can alter the native conformation, its behaviour, and the aggregation process | Kaminski Schierle et al., 2011; Osterberg et al., 2015 |
| BiFC | In vivo or post-mortem fluorescence about oligomer formation and propagation | Expression specific at different zones. Oligomerization-specific fluorescence | Bulky probes. Fluorescence potentially not arising from just pure dimerization | Dimant et al., 2013; Kiechle et al., 2019 |
In animal models.
The past few years have witnessed tremendous progress in understanding aggregation mechanisms and identifying various polymorphs. In this context of growing complexity, it is of utmost importance to understand what tools we possess, what information they provide, and what context they may be applied to. Nonetheless, it is also crucial to rationalize the relevance of the information and the limitations of these methods for gauging the final result. In this review, we present the main techniques that have shaped the current views about α-syn structure and dynamics, with particular emphasis on the recent breakthroughs that may change our understanding of synucleinopathies.
2. Semantic-driven review organization
Synucleinopathies research involves various testbeds, i.e. experimental modalities, and the aggregation monitoring methods may apply only to some but not all modalities. We, therefore, propose a review organized in ascending order of model complexity, from in tubo to in vivo approaches. Of course, some techniques have applications in several models and will appear repeatedly. Thus, the first part is dedicated to the various in tubo structural and kinetic techniques that have shaped our understanding of α-syn aggregation and the relations between fine protein structure and pathogenicity. Then, we move towards more complex models, i.e., in vitro cell cultures and, ultimately in vivo (in animals) or post-mortem tissues. Each of these modalities provides specific information and proposes specific challenges to track and monitor α-syn aggregation. Before diving into the presentation of the various techniques and due to the often-confusing terms found in the literature (Bezard, 2022), we would like to explicit the meaning of some terms in the present review:
In tubo suggests an experiment performed in a liquid environment with neither a living cell nor necessarily a physiological buffer.
In vitro reports experiments executed in the presence of living cells which could be cell lines, primary cultures, or differentiated pluripotent stem cells.
In vivo describes an experiment performed on a living organism. Such an organism could be a model organism - such as flies (Drosophila melanogaster), worms (Caenorhabditis elegans), fishes (zebrafish) - or mammals - such as mice, rats, and non-human primates. In most instances, in vivo will mean ‘in living mice”. When performed in another species, it will be indicated.
Post-mortem suggests an experiment performed on biological tissues (here, brain tissue in most cases). The tissue originates from human subjects, patients, or animals previously processed through an in vivo experiment. The nature of the tissue may vary from fresh-frozen (i.e., frozen after death – humans or euthanasia - animals) to fixed cryopreserved frozen (animals) or paraffin-embedded formalin-fixed tissues (most often typical appearance of human post-mortem tissues used in anatomo-pathology).
Recombinant means that the protein in question, here α-syn, has been produced in a bacteria genetically engineered to this end.
Oligomer: The scientific literature describes various early prefibrillar α-syn oligomeric species, coined “oligomers”. They differ from each other in structure, molecular weight, and morphology. Oligomers come in a wide range of molecular weights, with variable levels of secondary structure (ranging from mainly α-helical to β-sheet with different amounts of disordered regions) and exposed hydrophobic areas. Operationally, these oligomers are sometimes termed soluble as opposed to insoluble fibrils. This review uses this operational definition, although we reckon that a more accurate description would provide the sedimentation coefficient since all proteins can be pelleted down if centrifuged rapidly enough for a sufficiently long time.
Fibril: Apart from α-syn oligomers, other (late) conformers associated with synucleinopathies include protofibrils and amyloid fibrils. The fibrillar form of α-syn is mainly located within intracytoplasmic inclusion bodies, i.e., LBs and GCIs are not found free in cytosol and often exhibit a weight in megadaltons.
Polymorphs: During amyloid stacking, α-syn can adopt several distinct structural folds, resulting in fibril polymorphs. Together with their specific fold, their supramolecular properties are inherited and propagated as the polymorphs self-replicate by templated growth.
3. Structural and aggregation-monitoring techniques
3.1. Basic spectroscopic techniques
The process of α-syn aggregation has primarily been studied in cell-free systems. Although non-directly extrapolable, they have contributed much to our basic kinetic and aggregation-mechanisms-related knowledge. Spectroscopical biophysical techniques, such as Circular Dichroism and Fourier-Transformed Infra-Red, are relatively simple techniques that provide helpful essential structural pieces of information. They have been mainly used as complementary and orthogonal techniques.
3.1.1. Circular dichroism
Circular dichroism (CD) is a popular technique for characterizing and following α-syn fibrillization in cell-free systems. It informs about the secondary structure by measuring the difference in absorbance of right and left-circularly polarized light, usually reporting the ellipticity (θ) at different wavelengths. The most common variant of CD in protein study works in the far-UV (180–240 nm), where the signal depends on the environment of the peptide bond, directly related to the secondary structure of α-syn. By knowing the base spectra of the different α-syn forms, it is possible to quantify the relative concentration of each of the species at each time-point in the same sample based on the contribution of each of the species on the spectra (Calero and Gasset, 2005; Kelly et al., 2005). First structural studies on human α-syn used CD to infer the protein structural conformation, suggesting that the protein acquires a random coil structure in solution (Weinreb et al., 1996). Subsequent studies used different forms of α-syn to characterize its structural diversity under various conditions: Davidson et al. used recombinant α-syn to study the effects of lipid binding on the secondary structure of α-syn, observing a shift from unstructured to a highly enriched α -helical conformation (Davidson et al., 1998). Serpell et al. used a set of α-syn strains to study secondary structure upon fibril formation using diffraction techniques and CD. Authors showed a transition from disordered monomer to β-sheet structure upon aggregation in all α-syn species (Serpell et al., 2000). Since then, the CD has become a handy tool for studying and monitoring α-syn structure in in tubo studies (Burré et al., 2013; Rovere, 2019). In recent years, vibrational circular dichroism (VCD) has emerged as an alternative that provides more specific and extensive information about the structure of the protein. In contrast to classic CD, in VCD, the signal arises from vibrational transitions of chemical bonds in response to infrared radiation (Polyanichko et al., 2011). Applied to the study of α-syn, it offers a better sensitivity towards fibrils, providing insights into the size and the higher-order morphology of the fibrils (Van de Vondel et al., 2018) or the possibility of distinguishing fibrillar structures belonging to different levels of fibrillar maturation (Martial et al., 2019).
3.1.2. Fourier-transform infrared
Fourier-transform infrared (FTIR) is an absorption spectroscopy technique based on different vibration modes of heteroatomic bonds in response to infrared radiation. FTIR spectra are conventionally represented in terms of absorbance intensity as a function of wave numbers in units of cm1 (Calero and Gasset, 2005). The FTIR-amyloid study usually focuses on the 1700 to 1600 cm−1 range, a region known as the amide band. In this region, most of the signal arises from stretching the amide carbonyl group, whose vibration frequency strongly depends on its particular electronic context. This gives a particular sensitivity towards β-sheet structures and their relative conformation, allowing to distinguish different β-sheet-containing species (Froula et al., 2019; Sarroukh et al., 2013; Zandomeneghi et al., 2004). FTIR has been routinely used as a complementary technique in saline solution for the study of the structural characteristics of different α-syn species (Biere et al., 2000), oligomeric α-syn species (Hong et al., 2008), and fibrillar α-syn polymorphs (Bourdenx et al., 2020; Bousset et al., 2013)).
More recently, FTIR has been applied to post-mortem human tissue samples. In 2015, Araki et al. used the more radiative-powerful synchrotron radiation FTIR to characterize the β-sheet content in LB of a PD patient. They observed a high content of β-sheet structures in the halo, similar to the β-sheet conformation of in tubo recombinant α-syn fibrils, while the core presented a substantial amount of proteins but with different secondary structures (Araki et al., 2015). In another relevant human-derived tissue study, Shahmoradian et al. used high-definition FTIR imaging with correlative immunofluorescence staining in LB of PD patients. Authors exploited that the presence of different biomolecules, such as lipids, modifies the absorption of infrared radiation to confirm the presence of lipids within the α-syn inclusions (Shahmoradian et al., 2019). Finally, our team leveraged FTIR differential β-sheet sensitivity while analysing patient-derived α-syn. We characterized the α-syn secondary structures in fractionated α-syn extracts from PD patients' mesencephalon, focusing on fractions containing the LBs and fractions supposedly containing soluble α-syn (Bourdenx et al., 2020). While the former exhibited secondary structures typical of large aggregates, the latter contained a minute proportion of smaller aggregates characterized by differential secondary β-sheet conformations unravelled by FTIR. Unexpectedly, we showed that, in nonhuman primates, a small amount of these singular α-syn aggregates was as toxic as larger amyloid fibrils present in the fractions containing the LBs (Bourdenx et al., 2020), thus reinforcing the need for (i) preclinical research in this species and (ii) in-depth characterization of secondary β-sheets structures, notably of patient-derived materials.
3.2. Advanced structural techniques
The insolubility, heterogeneity, and non-crystalline nature of amyloid fibrils have hampered a more detailed structural study with “classic” structural techniques such as X-ray diffraction or soluble nuclear magnetic resonance (NMR). However, more complex structural techniques are vital to understanding the role of species involved in synucleinopathies. Recent years have seen a revolution in structural biology techniques (Iadanza et al., 2018), mainly in cryogenic electron microscopy (cryo-EM) and solid-state NMR (ssNMR) fields, providing near-atomic-resolution structures of amyloid fibrils. They have contributed to the emerging theory of fibril polymorphism as a basis for clinical and pathological heterogeneities (Lau et al., 2020; Shahnawaz et al., 2020; Shrivastava et al., 2020; Tanaka and Komi, 2015). These techniques pave the way for a refined understanding of the different polymorph species, their involvement in diseases, and their interactions with other proteins and small molecules as part of the aggregation process.
3.2.1. Nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a powerful spectroscopic technique that has shed much information on α-syn atomic structure. It provides structural information based on the oscillatory response of nuclei with non-zero spins. When exposed to a magnetic field, spin transitions in these nuclei can be induced by resonant excitation through radiofrequency irradiation. The NMR transition frequencies are sensitive to several orientation-dependent anisotropic interactions, potentially providing vibrant three-dimensional information about covalent bonding, internuclear distances, and electric field gradients (Reif et al., 2021). This makes NMR one of the few techniques well-suited to structurally resolve intrinsically disordered proteins, such as α-syn. Besides, it provides information about specific local conformational structures that encode biological functions in health and disease (Salmon et al., 2010). In the early 2000s, soluble NMR significantly contributed to our comprehension of the intimate structural identity of α-syn in its different conformations. In 2001, Eliezer et al. used NMR to characterize the structure of a recombinant α-syn in a free solution and after binding to membranes (Eliezer et al., 2001). They showed that α-syn protein is mainly unfolded in solution but with the propensity to adopt an α-helical structure in the N-terminal while transitioning towards an α-helical state in the presence of lipidic membranes (Eliezer et al., 2001). Then, further studies shed more light on the structural dynamics of α-syn: Chandra and colleagues showed that the α -helical state upon membrane binding comprises, in fact, only the first 98 N-terminal residues, which are structured in two α -helical regions interrupted by a short break, while the 42C-terminal residues retain a random coil configuration (Chandra et al., 2003). Since then, soluble NMR has remained a powerful method for studying the α-syn monomeric conformation and monitoring its conformational changes (Bodner et al., 2009; Fernandez et al., 2004).
Soluble NMR is not limited to saline solution; the so-called in-cell-NMR is used to study α-syn in more relevant cellular environments. The fact that NMR proteins are radiolabelled can be exploited to create “NMR-visible” proteins in “NMR-invisible” intracellular environments (Selenko and Wagner, 2007). In a remarkable work, Theillet et al. used electroporation to introduce 15 N isotope-enriched α-syn into non-neuronal and neuronal cells. They showed that the dynamic and disordered nature of monomeric α-syn is fundamentally similar to that in tubo but presents amino-terminal acetylation and the overall adoption of more compact structures (Theillet et al., 2016). Unfortunately, NMR presents some limitations. Besides not being a readily available technique, it has a relatively low sensitivity. It requires the use of high-purity samples, usually isotopically enriched proteins, a characteristic that prevents, for instance, from studying native or amplified patient-derived extracts, primarily because of the reported (and concerning) amplification inability to recapitulate the seed structure (Lövestam et al., 2021).
The use of classic NMR is limited by the insolubility of many relevant α-syn aggregated forms. Solid-state NMR (ssNMR) represents a powerful approach to overcoming this limitation. This variant uses highly concentrated protein assemblies forming a solid to semi-solid sample. This sample is then studied under a so-called magic-angle spinning to average the spatial anisotropic contributions and take advantage of local order in fibrils (Habenstein and Loquet, 2016). This variant has been extensively used during the last two decades in the study of aggregated protein structures to overcome the solubility of these relevant species (Heise et al., 2005; Lv et al., 2012; Vilar et al., 2008).
Nonetheless, ssNMR is a complex technique, and characterization at the atomic scale of aggregated species has remained elusive until recently (Meier et al., 2017). Progress in sample preparation and more sophisticated ssNMR methods and analysis have enabled the determination of high-resolution 3D structures of amyloid fibrils. In 2016, Tuttle et al. published the first high-resolution 3D structure of a human α-syn pathogenic fibril showing a Greek-key topology and paving for a better understanding of these fibrils' behaviour (Tuttle et al., 2016). Despite its impressive resolution, ssNMR remains a complex and not always accessible technique. For example, to achieve this impressive resolution, authors had to use samples highly enriched with “NMR-visible isotopes”, collect 68 multidimensional spectra and perform complex computational analysis.
Despite cryo-EM increasing popularity for super-resolve fibril polymorphs structure in an arguably more straightforward manner than ssNMR, this technique is far from having said its last word in the field. For example, cryo-EM can poorly resolve sidechain contacts and their possible interactions between fibres, which likely play a role in cytotoxicity and transmission. Contrary to cryo-EM, the accumulation of short-range inter-atomic distance information inherent to ssNMR allows for a much better resolution and understanding of the role of these elements (Takamuku et al., 2022). Both techniques thus have a complementary role in the refined understanding of polymorph pathology and delicate structure influence.
3.2.2. Diffraction techniques
Diffraction techniques are based on irradiating a high fibrillar content protein sample with a beam of X-rays or electrons, followed by recording several diffraction patterns resulting from the scattering of incoming radiation. The fact that amyloid fibrils do not form homogeneous 3D crystals limits the resolution of diffraction techniques, but they still have been profusely used for studying the amyloid fibril structure. They provided strong evidence for the so-called cross-β structure (Serpell et al., 2000; Sunde et al., 1997). Since then, both X-ray diffraction and electron diffraction have been used as characterization or orthogonal techniques. The latter possesses the advantage that the region where diffraction has been collected can also be imaged, allowing the geometrical relationship between the sample and diffraction pattern (Morris and Serpell, 2012).
In 2013, Shi et al. introduced Microcrystal Electron Diffraction (MicroED) as a remarkable alternative to classic diffraction techniques. It uses tiny three-dimensional crystals in a transmission electron cryo-microscope, irradiating with a reduced electron beam. It provides very high resolutions while overcoming the lack of crystal formation needed for classic diffraction techniques (Shi et al., 2013). In 2015, Rodriguez et al. used MicroED to obtain a fully refined structure with 1.4 Å resolution of the “preNAC” and “NACore” of α-synuclein, a fragment known to be critical in protein fibrillization (Rodriguez et al., 2015).
3.2.3. Imaging techniques
Imaging techniques such as electron microscopy (EM) or atomic force microscopy (AFM) permit visualizing higher molecular weight oligomers, fibrils or aggregates for deciphering their differential morphologies. Even though they are not quantitative, they provide critical structural and morphological information.
3.2.3.1. Electron microscopy (EM) and cryo-electron microscopy (cryo-EM)
EM has been a game-changing technique that grounds our understanding of synucleinopathies (Baba et al., 1998; Goldsbury et al., 2011; Lashuel, 2020; Spillantini et al., 1998). The possibility of immuno-labelling α-syn with electron-dense tags provides information about protein identity, combined with super-resolution lengths and morphologies. Interestingly, EM provided the first hints on the fibrillar composition of LBs in the 60s (Duffy and Tennyson, 1965) while immunolabelled EM shed light on the α-syn composition of the fibrils (Baba et al., 1998; Spillantini et al., 1998). Since then, although it lacks the resolution for a more refined analysis, EM has become a standard tool to study structural properties or simply the presence of α-syn aggregates in in tubo (Shrivastava et al., 2020), in vitro (Mahul-Mellier et al., 2020; Volpicelli-Daley et al., 2011) and in post-mortem samples (Fig. 2A) (Baba et al., 1998; Recasens et al., 2014; Shahmoradian et al., 2019).
Fig. 2.
Relevant examples of α-syn structural imaging techniques. A) Illustrative image of LB from the human brainstem using EM. Here, the LBs present the characteristic dense core and radiating filaments immunolabelled against human α-syn and visualized by the immunoperoxidase method (Baba et al., 1998). B) Cryo-EM maps of filaments purified from the putamen from 4 different MSA cases. Cryo-EM allows almost atomic resolution of fibrils, revealing relevant polymorphisms (Schweighauser et al., 2020). C) Cryo-ET imaging of α-syn aggregates seeded by recombinant PFFs in primary mouse neurons in a 1.4 nm thick tomographic slice. Red arrowheads mark fibrils. Right top magnified view of fibrils (red arrowheads). Right top magnified view of neurofilaments (blue arrowheads) (Trinkaus et al., 2021). D) AFM image of an in tubo-formed helical α-syn fibril. The AFM analysis provided the height profile and periodicity of the fibril (Kumar et al., 2017). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Remarkably, these last years have seen an impressive increase in the resolution capabilities of cryo-EM and image processing techniques, achieving resolutions close to the atomic scale (Kühlbrandt, 2014). Several cryo-EM studies have focused on studying the distinct structures of tubo-produced recombinant α-syn polymorphs, achieving resolutions ranging from 3 to 3.7 Å. These studies have used either full-length α-syn (Guerrero-Ferreira et al., 2019; Li et al., 2018a; Li et al., 2018b) or modified or mutant α-syn (Boyer et al., 2019; Guerrero-Ferreira et al., 2018; Sun et al., 2020; Zhao et al., 2020) to shed light on the relation between the different fine atomic structure of the polymorphs and its fibril-forming mechanisms, seeding capacity and cytotoxicity.
Although of outstanding scientific, technical, and ultimately clinical interests, the question of to what extent these in tubo-formed fibrils resemble those formed in animal, or human brains has yet to be solved. A similar question about Tau filaments suggested that fibrils purified from human post-mortem brains differed from those generated in tubo (Zhang et al., 2019). Burger et al. adopted a different approach. They used homogenates from PD and MSA brains to seed recombinant monomeric α-syn using a protein misfolding cyclic amplification (PMCA) assay (see Section 6.1). The authors resolved the resulting structures using cryo-EM, reporting different polymorphic structures for PD and MSA. (Burger et al., 2021). Although they showed that the fine structure of PMCA-generated fibrils is different from those generated purely from recombinant seeds, the question of to what extent the structure of these fibrils totally reproduces the characteristics and fine structure of in vivo-formed fibrils remains open. Fan et al. used a similar experimental framework to cryo-resolve fibrils amplified using PMCA from the cerebrospinal fluid (CSF) of prodromal and late symptomatic stage post-mortem PD patients. The prodromal sample was obtained from an initially healthy patient who converted to PD 7 years later. Cryo-EM studies showed a modest yet present structural polymorphism between early (prodromal) and late-stage (clinical) disease fibrils. Authors next used these polymorphs to seed the formation of recombinant α-syn fibrils, observing that the resulting recombinant fibrils seeded by the PD late-stage fibrils presented a greater capacity to seed endogenous α-syn aggregation, measured by quantification of phosphorylated α-syn-immunopositive aggregates in primary rat cortical neurons (Fan et al., 2021).
Interestingly, a 2020 study managed to atomically resolve fibrils extracted directly from fresh-frozen brain regions of several individuals with MSA and DLB using sarkosyl solubilization (Schweighauser et al., 2020) (Fig. 2B). Authors found two different types of fibrils in MSA brains, together with non-proteinaceous molecules. Furthermore, they observed that these fibrils were distinct from those found in the brains of DLB patients and those formed in tubo using pathological seeds and recombinant α-syn. Recently, the same group managed to extract and cryo-resolve fibrils from the brain of PD (one individual), PDD, and DLB patients' brains. Authors found all these Lewy pathology fibrils are made of a single proto-filament, which they termed Lewy fold, overall different from the previously reported MSA folds (Yang et al., 2022). These findings are significant in two ways. On the one hand, they once again highlighted the hypothesized relationship between specific fibril polymorphism and specific fibril toxicity (i.e., one polymorph would give rise to one “disease” or pathology phenotype). On the other hand, the authors showed how the fine structure from fibrils purified from the brain presents different structures than those grown in tubo using recombinant α-syn seeded with previously extracted fibrils (Lövestam et al., 2021).
Trinkaus et al. adopted a slightly different strategy in a competitive field of research. They used cryo-EM to image neuronal α-syn inclusions directly in primary mouse cortical neurons preserved by vitrification. Although this study does not achieve the structural richness of fibril-purified studies, it offers a unique high-resolution view of aggregates inside cells. Authors provoked aggregation by culturing the cells either with α-syn preformed fibrils (PFFs) or aggregates purified from MSA patients. Although with some differences, they obtained α-syn fibrils interspersed by numerous cellular organelles (Fig. 2C). Next, they used gold-labelled PFFs to study aggregate seeded growth in a neuroblastoma cell line (SH-SY5Y cells), observing a unidirectional fibril growth seeded mainly by the small fibrils (Trinkaus et al., 2021).
3.2.3.2. Atomic force microscopy (AFM)
AFM is based on scanning the sample with a tip probe, creating a topography image of the proteins, with information about the growth, directionality, and morphology of individual fibrils (Fig. 2D) (Goldsbury et al., 1999). In the field, it has been used to image and study the structural properties and the heterogeneity of α-syn individual oligomers and fibrils formed under different conditions providing both qualitative and quantitative data (e.g., heights and periodicities) (Ding et al., 2002; Goldberg and Lansbury Jr, 2000; Kumar et al., 2017; Li et al., 2018b; Sidhu et al., 2018). It has also been used to image the continuous formation of fibrillar species (in situ AFM), although it is likely that the electrical or chemical properties of the selected substrate might bias the assembly process (Jeong et al., 2013; Moores et al., 2011). Last years have seen the use of High Speed-AFM, allowing the visualization of structural dynamics of individual α-syn species with a much-improved time resolution. It permits imaging of the conformation dynamics of a monomeric and dimeric α-syn (Zhang et al., 2018b) and the fibril growth (Watanabe-Nakayama et al., 2020). The effect of fluorophore upon aggregation pathway is a problem that is usually ignored in fluorescence microscopy. In an exciting approach, Jadavi et al. used AFM-STED correlative microscopy to study fluorophores' effect on in vitro α-syn fibrilization. (Jadavi et al., 2022).
4. Fluorescence-based methods
Fluorescent labelling methods are valuable tools to track α-syn aggregation due to their hypothesized sensitivity towards different α-syn species and their potential ability to provide aggregation information in various models. Choosing a correct fluorescent probe for tracking aggregation is paramount to obtaining meaningful results. Beyond the classic requirements when working with biological samples, such as wavelength, fluorescence intensity, photo-stability, and bio-stability, an ideal aggregation-tracking fluorescent probe should undergo a significant fluorescence change upon binding to a specific structure or upon a change in its microenvironment (Gao et al., 2021; Haney et al., 2016; Shimogawa and Petersson, 2021). Moreover, it is crucial to understand and consider whether (and if yes, how) probe binding alters the functionality of α-syn or modifies its aggregation kinetics or final structure. While it is always a good policy to validate labelling in structure-sensitive experiments like CD, EM, or cell-based fibril seeding assays (Mučibabić et al., 2016), these techniques may not be accurate enough to detect more subtle structural changes.
4.1. Amyloid dyes
The so-called amyloid dyes include, notably, the symmetrical sulfonated azo dye Congo Red (CR) and the benzothiazole dyes thioflavin S (ThS) and thioflavin T (ThT). This family of organic molecules is classically used for studying amyloids due to their affinity and optical changes upon binding to the β-sheet structures present in amyloid structures (Reinke and Gestwicki, 2011). The binding sites of ThT or CR upon the β-sheet structure are reportedly different (Zhang et al., 2018a), explaining why specific samples would produce signals upon CR presence but not with ThT (De Giorgi et al., 2020). The practical outcome is that one can theoretically develop novel dye analogues that can be used to spectroscopically quantify the amount of amyloid richness and variety in a sample.
When bound to β-sheet structures, the optical changes of these compounds depend on different mechanisms. In the case of Congo Red binding, changes can be explained as aromatic rings become torsionally restricted upon binding. Although herein included in the fluorescence section, and indeed fluorescent (Yakupova et al., 2019), Congo-Red stained amyloids have been usually detected upon examination with polarized light in what has been traditionally called apple-green birefringence. (Housmans, 2021). CR has been central in early amyloidogenic research to study aggregation in vitro (Conway et al., 2000) and stain post-mortem tissue (Thiruchelvam et al., 2004). However, its use has been limited more recently, as studies show a problematic nonspecific binding or a likely interference with processes of protein misfolding and aggregation (Frid et al., 2007).
Regarding the thioflavin family, ThS can be used in cellular inclusions (Lázaro et al., 2016; Luk et al., 2009) and post-mortem fractions (Recasens et al., 2014) to confirm the presence of β-sheet structures in an easy way. However, the fact that it does not undergo a shift in the excitation or emission spectra upon binding results in high background fluorescence, making it suboptimal to be used in quantitative measurements (LeVine III, 1999).
ThT deserves special attention due to its widespread use and general reliance as a probe for α-syn fibrillation to the present day. In this case, fluorescence increase upon binding is thought to be due to the loss of rotational freedom between the benzothiazole and aniline rings, hence the nickname of rotor dyes for this family. Briefly, in the unbound state, the ultrafast twisting dynamics around such C—C bond are thought to cause rapid self-quenching of the excited state, resulting in low emission. However, the rotational freedom is restricted upon binding to fibrils, and the excited state is readily populated (Biancalana and Koide, 2010; Kuznetsova et al., 2016). As it binds to β-sheet structures, it theoretically enhances its fluorescence linearly with amyloid fibril mass. Although its use in brain sections is highly limited due to its weak fluorescence emission and potential background interference from other cellular components (Gao et al., 2021; Gaur et al., 2021), ThT fluorescence has become the most common proxy for aggregate formation or presence (Conway et al., 2000; Levine III, 1993).
This reliance on the increase in ThT signal as the aggregation probe cornerstone presents many limitations, even leading to false assumptions. The question of ThT impact upon aggregation itself is seldom considered. Some studies pointed out that ThT interactions with α-syn disordered monomer may promote amyloid aggregation (Coelho-Cerqueira et al., 2014), while others claimed that the effect of ThT on aggregation at moderate concentrations is negligible (Xue et al., 2017). Kumar et al., however, found that the sole presence of ThT during fibrilization modulated the fibrillar conformation, balancing the final fibril population towards different structures. Besides, these ThT-formed structures presented higher in vitro toxicities, reducing the viability of mammalian HEK-293 T cells (Kumar et al., 2017).
Far more concerning is the dogmatic reliance on the ThT signal for the selection/production of recombinant α-syn PFFs. However, polymorph diversity may reflect the pathological diversity (and hence possibly the clinical spectrum) (Lau et al., 2020; Shahnawaz et al., 2020). Sidhu et al. showed that in vitro formed β-sheet structural polymorphs present different ThT binding sites, directly affecting fluorescence intensities (Sidhu et al., 2018). Furthermore, Strohäker et al. observed that PMCA-amplified fibrils seeded by PD or MSA brain extracts present different morphological features supporting their variable ThT fluorescence (Strohäker et al., 2019). Using a similar approach, Shahnawaz et al. observed that MSA-seeded fibrils exhibit significantly lower ThT fluorescence than those amplified from PD samples (Shahnawaz et al., 2020).
Further exploring this puzzling question, De Giorgi and colleagues demonstrated how, while launching PFF formation from monomeric α-syn, a variety of α-syn fibrils are stochastically produced. Some newly generated PFFs are ThT-positive while others are ThT-negative despite exhibiting a clear β-sheet structure demonstrated using notably ssNMR (De Giorgi et al., 2020). The emergence of such ThT-negative fibril polymorphs has been largely experimentally ignored so far or mistaken for fibrillization inhibitions/failures. They present a specific atomic organization and show an exacerbated propensity towards self-replication in cortical neuron primary culture and living mice. Indeed their injection into the substantia nigra pars compacta of 8-week-old wild-type mice induces the formation of phosphorylated S129-positive α-syn aggregates that spreads towards the dorsal striatum, the nucleus accumbens, and the insular cortex (De Giorgi et al., 2020).
Exploring the richness of rotor dyes, De Giorgi et al. showed that fluorescent compounds with a slightly different chemical structure but sharing with ThT its trimethine cyanine skeleton could detect these ThT-negative polymorphs. Notably, SYBR Green, the well-known DNA intercalating dye, could sense the amyloid structure of these ThT-negative polymorphs but appeared to be much less capable of doing so for ThT-positive ones, giving an almost mirror image of the ThT readings (De Giorgi et al., 2020). Thus, as with prion strains and luminescent conjugated polymers (LCPs) (Sigurdson et al., 2007), multiple external probing using these different molecules, now known under the term “fibrilloscope”, could discriminate the α-syn polymorph identities revealed by ssNMR. These data critically highlight the risk of overemphasizing one readout (here ThT positivity) over others at the expense of the description of the richness of the pathology and, hence, of the narrowness of the “therapeutic” tools designed for a single category of α-syn polymorphs. ThT fluorescence alone should not be taken anymore as a proxy of fibrillization.
Research towards new amyloid rotor dyes continues and produces new entities. Needham et al. synthesized a ThT-derived dye, termed ThX, with improved optical properties and higher binding affinity to amylogenic structures. This new probe presents much better sensitivities to earlier pathological amyloid species (Needham et al., 2020). In the same way, Gaur and colleagues developed an amyloid probe, termed RB1, with the ability to selectively stain α-syn aggregates in the cytoplasm of living cells (Gaur et al., 2021). Both, however, would require validation of previously identified ThT-negative strains to demonstrate a clear added value compared to ThT itself.
4.2. Fusion proteins
Beyond amyloid dyes, fluorescence tagging of α-syn monomers, oligomers or fibrils represents a powerful approach for the dynamic structural study of α-syn aggregation.
The aggregate formation is highly affected by the complex cellular environment, but it has primarily been studied in cell-free systems. Cellular models of protein aggregation and neurodegeneration represent the next complexity step to understanding the processes that govern pathological aggregation and propagation. The simplest and most used way to fluorescently label α-syn for live cell study is to express it fused to a fluorescent protein. Integrating the construct into the genome makes it possible to express the fluorescent protein-α-syn construct directly in the live cells model (Falkenburger et al., 2016; McLean et al., 2001; Outeiro and Lindquist, 2003). In the most straightforward approach, the direct visualization of aggregate formation is possible after the expression of the construct, such as α-syn-GFP or α-syn-mCherry. These free constructs are visualized as an even distribution of fluorescence through the cell or around the membrane, while aggregated inclusions appear as bright cytoplasmic spots (Opazo et al., 2008; Osterberg et al., 2015; Perrino et al., 2019). While using fused fluorescent proteins such as GFP or mCherry represents a straightforward method, it presents several problems. The most obvious is the size of these reporters, which are often larger than α-syn. They can potentially disrupt or affect the α-syn folding/misfolding properties, dynamics or toxicity (Mučibabić et al., 2016). Moreover, the expression of fused fluorescent proteins is generally restricted to the C- or N-terminus, which limits and may interfere with the study of aggregation or protein dynamics (Haney et al., 2016; Jun et al., 2019).
In addition to direct visualization, biophysical techniques such as Förster Resonance Energy Transfer (FRET) allow extracting quantitative information upon aggregation, permitting converting a change in fluorescence into an intramolecular distance. Briefly, FRET occurs between two sufficiently close fluorescent molecules when the energy of one fluorophore, the donor, flows to the other, the acceptor, by non-radiative transfer (Fig. 3A). The subsequent FRET signal strongly depends on the distance and the angle between the two fluorophores (Piston and Kremers, 2007). CFP and YFP are probably the most common FRET pairs used in the study of α-syn dynamics, either inter- or intra-molecularly. In the first case, singly- (such as αS-CFP and αS-YFP) or double-tagged (YFP-αS-CFP) α-syn fusion proteins are used as aggregation biosensors and to reveal specific α-syn conformations and aggregations states (Miraglia et al., 2020; Nakamura et al., 2008; Svanbergsson et al., 2021; Yamasaki et al., 2019). In the second, intramolecular FRET (Single-Molecule FRET) uses double-labelled α-syn (such as YFP-αS-CFP) to obtain information about early minor conformations that may initiate protein pathologic aggregation (Moosa et al., 2019). Alternative approaches use polarized light and measure fluorescence anisotropy due to energy migration FRET to distinguish between different aggregated states of α-syn (van Ham et al., 2010).
Fig. 3.
Schematic depiction of FRET and BiFC methods. A) Single-tagged (αS-CFP and αS-YFP) and double-tagged (YFP-αS-CFP, depicted in the image) α-syn can be used as FRET probes. FRET occurs between two sufficiently close fluorescent molecules by non-radiative transfer of energy from one fluorophore, the donor (CFP), flows to the other, the acceptor (YFP). Dimerization can be quantified by the amount of FRET fluorescence emitted by the acceptor protein. B) BiFC is based on the expression of α-syn fused to a fluorescent protein fragment. Fluorescence is only emitted when the two halves are brought into close proximity by protein dimerization, enabling the formation of the full-length fluorescent protein.
4.3. Bimolecular fluorescence complementation (BiFC)
Protein complementation assays (PCA) are valuable methods to study α-syn aggregation in living systems, especially when approaching the formation of early oligomers. These methods are based on the expression of non-bioluminescent/non-fluorescent protein fragments fused on the N- or C-terminal of α-syn. These fragments only emit fluorescence when the two halves are brought into close proximity, i.e. protein dimerization, to enable the formation of the full-length fluorescent protein (Fig. 3B). An early variant of this method uses bioluminescent protein, such as Gaussia Luciferase, to obtain a read-out indicating the formation of α-syn oligomeric species in different cell fractions (Danzer et al., 2011). Bimolecular fluorescence complementation (BiFC) represents a more exciting approach as it is based on the fusion of a fragment of a fluorescent reporter protein (usually GFP or YFP/Venus) to the protein terminus and thus allows also to direct image and monitor the dynamics of α-syn in in vitro cultures or animal models with sub-cellular accuracy (Goncalves et al., 2010). Since Outeiro and colleagues first implemented the BiFC assay to α-syn, the α-syn-BiFC system has been extensively used to study different aspects of α-syn behaviour in living cells, especially regarding early oligomerization events. In this seminal study (Outeiro et al., 2008), authors co-expressed both fragments of GFP-α-syn in H4 neuroglioma cells. In their first paper, the authors found that fluorescence arises only from both α-syn-fused fragments. Besides, the presence of the BiFC system stabilized the oligomers increasing their in vitro toxicity studied by the measure of adenylate kinase released from damaged cells. Studying the release and uptake of α-syn by cells is an interesting application of BiFC in cellular models. By co-culturing cells expressing each of the fragments individually, combined with blotting and immunocytochemistry, several studies have investigated how α-syn propagates between cells (Bae et al., 2018; Bae et al., 2014; Lee et al., 2021). BiFC readout can also be used for the search for different molecular and genetic regulators of α-syn oligomerization (Gonçalves et al., 2016; Lázaro et al., 2016) and pharmacological modifiers of α-syn release and uptake (Dominguez-Meijide et al., 2020).
However, as Frey et al. noted in a recent review (Frey et al., 2021), despite the widespread use of BiFC in recent years, these studies systematically lacked validation and proper characterization regarding several vital aspects. The authors found a marked differential expression of the two fragments, potentially leading to non-specific interactions. Furthermore, they found that Vn-α-syn shows a higher tendency to form oligomeric and higher-order aggregates. They suggest this may lead to fluorescence arising from α-syn dimerization but also from the association or entrapment of α-syn-Vc in the Vn-α-syn aggregates.
In our opinion, these results do not invalidate BiFC but rather define the limits of use. We agree and support the general idea of the importance of well-characterizing and validating our techniques before jumping to conclusions. As discussed, the α-syn aggregation process highly depends on particular conditions, including adding any bulky reporter. Considering how these reporters may modify α-syn aggregation natural dynamics is of utmost importance.
4.4. Fluorogenic biarsenical compounds
Fluorogenic biarsenical compounds (like FlAsH or ReAsH) represent a relatively small tag alternative compared with fluorescent proteins. It is based on the expression of a recombinant α-syn, bearing a tetra-cysteine tagged to the C-terminal region. This tag acts as a selective target for a fluorogenic biarsenical compound, which is later conjugated, either by making react the tag with the previously purified protein or by treating the transfected cells with the biarsenical compound (Roberti et al., 2007; Roberti et al., 2011). The addition of protein tagged with a second biarsenical compound acting as an acceptor (such as ReAsH) can be used to report intermolecular distances in associated and aggregated forms of the protein through FRET interaction. This method has been applied to study the aggregation of α-syn both in in tubo experiments (Dhavale et al., 2017) and living cells (Roberti et al., 2011). Although arsenal compounds represent less bulky alternatives than fused proteins to monitor the formation of different α-syn species, the labelling process for working with live cells is not simple, and it still constitutes a modification of the protein sequence.
4.5. Site-specific fluorescent probes
The selective introduction of one or multiple site-specific small synthetic fluorescent probes in α-syn represents an exciting and relatively new approach with promising application to study structural dynamics in tubo, and to a lesser extent, at least for the moment, in living cells. Besides potentially overcoming the problems of more bulky probes, they can provide rich region-specific information upon aggregation in real-time through fluorescence emission (Shimogawa and Petersson, 2021). A relatively common approach for α-syn-specific labelling is the introduction of a cysteine residue by site-directed mutagenesis, further labelled via fluorophore-attached maleimides (Cys-Mal). Although this method is limited to single-label sequences, it can be combined with native chemical ligation to obtain multi-label sequences (Haney et al., 2016). Alternatively, unnatural amino acid (UAA) mutagenesis represents a versatile and elegant approach to be used alone or in combination with other labelling methods. It is based on the genetic incorporation of UAAs at desired positions of the protein, which act as handles for a fluorophore, later incorporated via click chemistry (Haney et al., 2016; Saal et al., 2018) (for a review of labelling strategies see Shimogawa and Petersson, 2021).
Site-specific labelling with a fluorophore can be exploited to study detailed structural changes using distance-dependent fluorescent interactions such as FRET. Daniels et al. used a set of α-syn proteins with FRET pairs introduced via UAAs to measure distance changes between specific protein regions in response to cyclized nordihydroguaiaretic acid (NDGA) (Daniels et al., 2019). Depending on the case and needs, several of these methods can be combined to obtain site-specific, multiply labelled protein. Such an approach can be capitalized to create single-molecule FRET (smFRET) probes, which can detect conformational subpopulations and dynamics upon aggregation. In an interesting example, Pan et al. used native ligation to join the αS1–55 fragment containing Cys-Mal labelling with the αS56–140 fragment, labelled by UUA. Then, they used smFRET to evaluate the conformational changes in pY39 α-syn that contributed to the aggregation of α-syn (Pan et al., 2020). Remarkably, Lee et al. succeed in the entirely site-specific labelling of α-syn with three different colour fluorophores, combining native chemical ligation with region-specific cysteine protection of protein fragments. This methodology should expand the utility of smFRET, enabling simultaneous measurement of multiple distances and the study of more complex dynamic interactions (Lee et al., 2018).
Another promising alternative is using environment-sensitive probes relying upon excited-state intramolecular proton-transfer (ESIPT). These ESIPT fluorophores owe their interest to their environment-sensitive characteristics and substantial Stokes shifts. Variation between different α-syn states will cause different charge distributions, hydrogen-bond formations and dipole modifications, leading to different fluorometric responses of the fluorophore (Gao et al., 2021; Sedgwick et al., 2018). Fauerbach et al. used a mutant 18Cys α-syn conjugated with an ESIPT-based probe to monitor aggregation processes occurring during the early lag time inaccessible by ThT (Fauerbach and Jovin, 2018). Kucherak et al. used a newly synthesized ESIPT probe to label a cysteine mutant of α-syn, discriminating unstructured, membrane-bound, and fibrillar states of α-syn (Kucherak et al., 2018).
The necessity of exogenously labelling the α-syn protein sequence is the main limitation of these approaches and the reason why site-specific labelling literature refers mainly to cell-free assays, i.e., in tubo experiments. The very few examples of such α-syn labelling applied to living cells have been limited to tracking the fate of α-syn PFFs formed by mixtures of non-labelled and labelled α-syn. Haney et al. incubated hippocampal neurons with PFFs containing a 25% Cys-Mal-labelled α-syn, demonstrating that labelled-α-syn in position 114 presents a similar ability to seed aggregation and toxicity in living cells as wild-type α-syn (Haney et al., 2016). Jun et al. used a small photo-convertible molecule as fluorophore introduced by UAA to study the internalization and subsequent endo-lysosomal trafficking of α-syn (Jun et al., 2019). Zhang et al. used cysteine mutant-tagged α-syn at position 90 to generate fibrils of different lengths that were then applied to SH-SY5Y cells. The authors monitored cell-surface interactions and uptake, reporting that uptake and toxicity are inversely dependent on the PFF average size (Zhang et al., 2020).
5. Immuno-labelling reporting of α-syn aggregation
5.1. Antibodies against α-syn
The present section does not aim to in-depth review the pros and cons of each antibody but to introduce the reader to the variety of antibodies against α-syn for later discussing the techniques for which immunolabelling is required. Indeed, immunolabelling methods, combined with previous purification steps, are critical in understanding the role of α-syn aggregation in synucleinopathies (Spillantini et al., 1998). Shortly after the discovery of the pivotal role of α-syn, several antibodies were raised against different regions of native α-syn -including the NAC region, the N-terminal region, and the C-terminal region (Culvenor et al., 1999; Dickson et al., 1999). The first α-syn antibodies were used in clinical post-mortem studies. Even before the pathological role of α-syn was unravelled in PD, Ueda et al. discovered a 140 amino acids-long protein in amyloid preparation purified from the cortex of AD patients (Ueda et al., 1993). They developed, without knowing, the first α-syn antibodies against the 61–95 amino acids fragment (now known as the NAC fragment). Shortly after the role of α-syn became apparent in PD and DLB, Baba et al. raised a monoclonal antibody (mAB), LB509, against highly purified LBs from DLB cortices. Using this antibody, they confirmed previous work showing the presence of α-syn in LB and highlighted the presence of high-molecular species of putatively aggregated α-syn in LB from human samples using an immunoblotting experiment. LB509 is now a routine Ab in anatomo-pathology. The parallel development of post-translational modification-specific antibodies was of tremendous importance to better understand the influence of those modifications in pathogenesis and identify them as disease signatures. Among the historically first targeted modifications, we can find nitration (the mAb nSyn 8, 14 and 24) (Giasson et al., 2000) or oxidisation (mAb Syn 303, Syn 505, and Syn 514) (Duda et al., 2002). α-syn phosphorylation at serine S129 (PS129) (Fujiwara et al., 2002) deserves a specific highlight as it has developed almost into a synonym of α-syn pathology for most investigators. Posterior research has witnessed the development of many antibodies targeting different epitopes of α-syn or specific disease-associated α-syn post-translational modifications, including phosphorylation, ubiquitination, nitration and C- and N-terminal truncations (Vaikath et al., 2019).
Immunolabelling is undoubtedly an enormously powerful tool in synucleinopathy research, but the results' interpretation should be cautiously conducted. Some of these antibodies, especially those targeting phosphorylated α-syn at serine 129 (pS129-α-syn) or ubiquitinated α-syn, have been developed into recognized and widely used markers of α-syn pathology. However, assuming that phosphorylation equals pathological, i.e., aggregated, α-syn, despite not all pathological α-syn being phosphorylated and not all phosphorylated α-syn being pathological (Bezard, 2022), may have dramatic scientific and (ultimately) therapeutic consequences. In the same way, plenty of known (plus the yet unknown) modifications found in anatomopathological landmarks may interfere with the immunoreactivity of these α-syn antibodies, making interpreting the immunolabelling even more difficult. Likely, the currently used antibodies do not capture the full spectrum of aggregated and not aggregated α-syn pathological populations. Besides, there is a fundamental problem of aiming to the primary structure of α-syn when studying aggregated protein, especially considering the now better-understood heterogeneity underlying α-syn pathology. Indeed, different stages and modalities of disease likely possess different biochemical and structural antibody epitopes. Whether the currently-used antibodies can distinguish the entire proteome spectra underlying different pathology remains fundamentally relevant (Altay et al., 2022).
Accumulated evidence points towards a possible disease-relevant role for α-syn oligomers (Alam et al., 2019; Winner et al., 2011), calling for new tools going beyond primary structure and targeting particular conformations. Unfortunately, intermediate oligomers are unstable, variable and highly heterogeneous (Kulenkampff et al., 2021; Luth et al., 2015). Insight into the biochemical and structural properties of native oligomeric α-syn is therefore challenging to obtain. These factors make generating high-quality and high-specificity conformational antibodies challenging, which is nonetheless critical for the robust interpretation of results (Desai et al., 2021).
Despite this, the last years have seen the development of different antibodies raised against particular protein structural motifs, termed conformation-specific antibodies (Brännström et al., 2014; Covell et al., 2017; Vaikath et al., 2015; van Diggelen et al., 2019). These tools represent an exciting development for studying disease-relevant native oligomers in fixed brain tissue of animals (Duffy et al., 2018) and lysates from human LB (Vaikath et al., 2019). Two recent studies have used conformational antibodies and super-resolution microscopy to study the uptake by neuronal cells of pathologically relevant α-syn oligomers and fibrils (Bigi et al., 2021) and the uptake and secretion of seeds by SH-SY5Y cells (Sang et al., 2021). In an exciting approach, Gibbs et al. used computational modelling to predict conformational epitopes likely to be exposed only on oligomers but not on monomers or insoluble aggregates (Gibbs et al., 2022). They generated monoclonal Ab (mAb) against these epitopes. These mAb present low reactivities with monomers and insoluble fibrils, including post-mortem LBs. Besides, adding the mAb to cultures of primary rat dopaminergic neurons exposed to α-syn oligomers resulted in a higher survival rate, measured by posterior quantitation of TH-positive neurons. Also, they reduced the uptake of PFFs in a cellular seeding assay in rat hippocampal neurons, in which neurons with aggregates of human α-syn were quantified after 14 days of culturing (Gibbs et al., 2022).
The lack of head-to-head comparison through a series of validating steps makes the comparison between various antibodies elusive. Kumar et al. accepted the challenge and performed a systematic antibody characterization through a validation pipeline which allowed a systematic investigation of the specificity of α-syn antibodies using well-defined and well-characterized preparations of various α-syn species, including monomers, fibrils, and different oligomer preparations that were characterized by distinct morphological, chemical and secondary structure properties (Kumar et al., 2020). Testing 18 antibodies, of which 16 were previously reported as conformation- or oligomer-specific antibodies, Kumar et al. convincingly showed that i) none of the antibodies tested were specific for one particular type of α-syn species, including monomers, oligomers or fibrils; ii) all antibodies which were reported to be oligomer-specific also recognized fibrillar α-syn; and iii) a few antibodies showed high specificity for oligomers and fibrils but did not bind to monomers (Kumar et al., 2020). The authors concluded that the great majority of supposedly α-syn aggregate-specific antibodies did not differentiate between oligomers and fibrils, thus highlighting the importance of exercising caution when interpreting results obtained using these antibodies and the need for complete characterization and validation of antibodies before their use in mechanistic studies (Kumar et al., 2020).
5.2. Biochemical methods
The combination of biochemical methods and immunoassays provides precious information about the identity of α-syn species, notably regarding the purification and characterization of those extracted from fresh-tissue samples (Fig. 4). Techniques such as western blotting and dot blotting will not be explicitly explained here, as they are performed routinely in most laboratories. They represent techniques of enormous importance and are usually the final step of the myriad of techniques presented in this section. It is nonetheless worth mentioning filter retardation assay as a potentially powerful technique, as it could allow the capture of aggregated over specific sizes due to specific membrane properties such as acetate membrane (i.e., independently of the supposed conformer specificity of an Ab). The selection of the membrane material and properties is critical in dot blot experiments. Subsequent Dot Blotting with the desired Abs allows for the relative quantification of these species (Bourdenx et al., 2020; Recasens et al., 2014).
Fig. 4.
Biochemical analysis of biological tissues. The combination of purification methods, and characterization techniques, notable immunoassays, provide precious information about the identity of the different α-syn species present in samples extracted from fresh-tissue samples. Characterization of the different fractions may involve, for example, EM (Laferrière et al., 2019), filter retardation assay (Bourdenx et al., 2020), or digestion methods + western blotting (Peng et al., 2018).
One of the most popular methods to study α-syn fibrils derived from ex vivo samples is to evaluate the resistance of aggregates against different protein-digesting chemicals. While relative protein solubility is usually used to purify samples, it also holds potential as an analytical tool. Amyloid fibrils are characterized by their cross-beta fold structure, an incredibly stable fold that confers resistance against solvent agents. α-syn aggregates are diverse and complex, presenting different dynamic regions, with usually a single part of the protomer having the β-fold structure. At the same time, smaller oligomers are usually entirely soluble. Consequently, different polymorphs are expected to fragment differently towards specific solving agents. The evaluation of its resistance and the so-called proteolytic profile towards these agents permits us to infer useful analytical information about the type of aggregates we may be dealing with.
The most well-known example of these agents is Proteinase K (PK). The digestion resistance to this endo-protease is usually cited as one of the main properties of amyloid fibrils. Contrary to other endo-proteases, PK presents a low amino acid specificity, which leads to almost-complete hydrolysis of “non-PK-protected” regions (Kushnirov et al., 2020). Besides PK, further fractionation characterization can be done using different mixtures of protein dissolvent such as urea, SDS, Triton, or trypsin. The subsequent analysis of digestion patterns with Blotting assays, such as western blot or dot blot, permits to evaluate of the differences between different types of samples in digestion-resistance terms (Bourdenx et al., 2020; De Giorgi et al., 2020; Laferrière et al., 2019; Peng et al., 2018). In an elegant approach, Landureu et al. used PK and thermolysin to achieve limited proteolysis. Then, immunoblotting with several Abs directed to different known epitopes was performed to investigate the exposition of different regions. Authors applied it to three previously known α-syn fibril polymorphs displaying different binding properties, confirming a different exposure of α-syn N-terminal domain (Landureau et al., 2021).
Also, of particular relevance in this section, some physicochemical methods permit the separation of different protein fractions found in ex vivo homogenates. For example, sucrose gradient purification allows post-mortem homogenates content based on mass separation. For that, samples are placed on the top of a sucrose step gradient of different concentrations, thus different densities. Ultra-centrifugation of this sucrose gradient at controlled times and speed permits the obtention of various fractions, which can then be studied separately (Emin et al., 2022). In an influential work, Recasens et al. used a sucrose gradient to separate a PD patient mesencephalon homogenate into 20 fractions. Posterior analysis of the fractions with confocal and electronic microscopy, combined with filter retardation assay, permitted the identification of LB-enriched fractions containing pathological α-syn. Most interestingly, they demonstrated the capacity of these purified fractions to induce nigrostriatal neurodegeneration in monkeys and rodents (Recasens et al., 2014). Building on this technique, Bourdenx et al. showed that amyloids with a specific cross-beta structure actually “hide” into the fraction containing supposedly mostly soluble α-syn. They demonstrated that these peculiar conformers could also induce degeneration and pathological changes throughout the brains of primates but not of rodents, thereby proving the need for involving different species to assess the toxicity of α-syn-β-amyloids (Bourdenx et al., 2020). Using only rodents would artificially focus on large aggregates while involving primates widening the spectrum of toxic species (Bourdenx et al., 2020).
Similarly, density floatation gradient and sedimentation assays are practical separation methods after solubilization in solvents such as sarkosyl. In the first case, separation is achieved by exploiting the fact that bigger aggregates are less dense than smaller ones and monomers. This density-based separation is achieved by loading the samples in gradients of iodixanol. Upon centrifugation, the different species move until they reach an equilibrium where their density is the same as the surrounding gradient medium. Again, subsequent blotting with particular Abs allows for relative quantification of the different fragments, such as total α-syn, pS129 α-syn or specific conformation (Bourdenx et al., 2020). Complementary sedimentation assays take advantage of that different species, notably depending on the shape, size and density, tend to form sediments faster (Laferrière et al., 2019). After the first solubilization step, the different entities can be fractionated depending on their sedimentation velocity upon ultracentrifugation, allowing them to isolate particular species or generate sedimentation profiles. Laferriere et al. showed the differential content in aggregates between PD and MSA brains using such sedimentation assay. Applying this to a transgenic mouse model of MSA, they showed that the heavy S129-phospho-α-syn load in these mice was made of soluble α-syn while PD and MSA brains exhibited aggregated and phosphorylated α-syn (Laferrière et al., 2020).
5.3. Enzyme-linked immunosorbent assay (ELISA)
ELISA represents a widely used qualitative method for studying α-syn aggregation as it possesses broad applicability ranging from in tubo to ex vivo tissue or patients' samples. There exist several modalities of ELISA, but, simply put, it is based on the immobilization of the antigen to the surface, either directly or using the first Ab, and the addition of an enzyme-conjugated detection Ab that provides the signal for the quantification of the antigen. ELISA is a relatively simple procedure. It is usually applied to test large pools of samples or purified fractions. The technique's success depends directly on the specificity of the Ab for the desired species. Earlier modalities in the aggregated α-syn study used two monoclonal Ab with identical epitope specificity, so the signal only arises if there is more than one binding site in the structure (El-Agnaf et al., 2006; Kulenkampff et al., 2021). Later studies have used ELISA to quantify the soluble aggregates, i.e., oligomers, using conformational Abs. These studies used Abs raised against epitopes missing on natively unfolded α-syn. Paleologou et al. used a polyclonal rabbit Ab, termed
FILA-1, detecting elevated levels of these soluble aggregates in DLB brain lysates (Paleologou et al., 2009) (but see review by (Kumar et al., 2020)). In this line, Lassen et al. developed a sandwich-type α-syn monoclonal rabbit Ab, MJF14–6–4-2, and demonstrated its ability to detect oligomers in detergent extracts of the whole brain from Thy-1 promoter overexpressing α-syn mice (Lassen et al., 2018). Although promising as a fast and easy diagnosis tool for human-derived samples, these ELISA methods are limited because conformational antibodies are imperfect tools (Kumar et al., 2020). While ELISA usually represents a “routine method”, we believe it becomes precious if it is preceded by fractionation and purification steps earlier mentioned. A careful selection of Abs might then lead to, for instance, quantifying the total α-syn present in patients' brain-derived extracts carefully fractionated using either of the above-described techniques.
We should also highlight the single molecule array (SIMOA), an emergent ELISA variant, which uses femtoliter-sized chambers with Abs immobilized on a paramagnetic bed. In the end, each bed captures either one immunocomplex or none. Fluorescence is then electronically measured, giving this technique a much higher sensibility than ELISA (Kulenkampff et al., 2021). SIMOA has recently been applied to α-syn research by Kumar et al. to assess the immunoreactivity of conformation-specific antibodies (Kumar et al., 2020) and Ren et al. to search for potential biomarkers in PD patients' plasma (Ren et al., 2022).
5.4. Single protein immuno-fluorescent imaging methods
These methods represent unique approaches to image and potentially characterize individual protein aggregates in different systems, notably from human-derived samples. The ability to image individual aggregates provides potentially higher sensibilities than “bulk methods” such as ELISA (Kulenkampff et al., 2021).
sFIDA technology (surface-based fluorescence intensity distribution analysis) represents a promising approach towards the sensitive detection of aggregates, which is especially interesting for the design of biosensors for diagnostics. It is based on the immobilization of Ab directed against linear epitopes of α-syn, such as Syn211, on the glass surface of a microtiter plate and the subsequent treatment with the same Ab functionalized with a fluorophore. Insensitivity towards monomers is achieved as the first immobilized Ab masks the protein epitope. The second Ab, functionalized with a fluorophore, thus permits the detection and counting of single particles of (in theory) all aggregated α-syn subtypes. As a drawback, the technique cannot yet discriminate the size or subtype of aggregate, as aggregates are typically smaller than the resolution limit. Blömeke et al. recently demonstrated the applied sFIDA technology to CSF samples of PD and DLB patients, finding significantly higher levels of α-syn aggregates compared with controls (Blömeke et al., 2022). In a similar approach as sFIDA, the single-molecule pull-down (SiMPull) assay uses a classic immuno-precipitation to immobilize aggregates and a pair of primary-secondary Abs to detect at single protein resolution. The method was applied by Je et al. to the study of α-syn oligomers. Although this method does not directly discriminate between monomers and aggregates, the different intensity distributions can be used to identify the species (Je et al., 2017).
5.5. Alpha-synuclein proximity ligation assay (AS-PLA)
α-syn proximity ligation assay (AS-PLA) represents a suitable method to detect and study the smaller α-syn aggregates, usually oligomers in literature, that other techniques may fail to detect, thanks to its potential higher sensibility. It has particular relevance when it comes to exploring post-mortem tissue surfaces. In some cases, the liability and heterogenicity of early aggregates limit the use of traditional immunohistochemistry with conformational antibodies (Behere et al., 2021). Others might claim that antigen preservation is not the issue but rather the limited knowledge of the conformers in the tissue.
Simply put, PLA methods detect protein-protein multimerization through an amplification reaction mediated by an oligomer chain. This oligomer chain will only bind the two protein–binding antibodies if they are sufficiently close to each other (<40 nm), i.e. possibly aggregated (Sekiya et al., 2022; Söderberg et al., 2006). Although this technique has been profusely used in the study of so-called early oligomers, it is crucial not to mistake its ability to detect smaller species with selectivity. PLA will generate, in principle, signal against all kinds of aggregated or non-aggregated species provided (i) two monomeric proteins are close enough and (ii) the epitope for the Ab is exposed. While some individual fibres may present lower signals with PLA, as epitopes are not close enough (Roberts et al., 2015), this does not mean PLA can universally differentiate between oligomers and other higher aggregated forms of a-syn.
PLA was first applied to an α-syn aggregation study by Roberts et al. by using an α-syn Ab (syn211) displaying blocking activity on the epitope (Roberts et al., 2015). This blocking prevents the liaison of more than one Ab per monomer, making the essay only sensible towards multimeric α-syn species. The authors first demonstrated the technique's sensitivity to detect in tubo recombinant α-syn-formed oligomers preferentially. Then, they applied the method in fixed post-mortem PD brain tissue, observing previously undetected diffuse deposits of α-syn, often in mildly affected regions (Roberts et al., 2015). Over the past few years, AS-PLA became a popular tool to study what authors usually termed early α-syn oligomers. It has been applied to study oligomer distribution in the midbrain of young mice (Behere et al., 2021) or the brain samples of MSA and PD patients (Sekiya et al., 2019). Bengoa-Vergniory and colleagues used AS-PLA in several models and situations to evaluate the presence of oligomers upon treatment with the molecular tweezer CLR01: PD patients' brain extracts, human dopaminergic cultures over-expressing α-syn, and human α-syn-overexpressing mouse model tissue (Bengoa-Vergniory et al., 2020). Sekiya et al. used PLA and classic histochemistry against pS129-α-syn to compare the distribution of early aggregates, i.e. oligomers, with Lewy-related pathology (LRP) distribution. The authors used diverse formalin-fixed paraffin-embedded post-mortem brain samples from neuropathologically-confirmed cases of PD and control patients. First, the authors found the distribution of both oligomers and LRP variables between patients, confirming the heterogenicity of what we collectively call PD. Second, the authors also found a broad distribution of oligomers, which do not necessarily correlate with the presence of LRP. Nonetheless, oligomer presence, for example, in the hippocampus correlated with observed symptoms of dementia, providing another hypothesis for why not all patients fit the Braak staging scheme (Sekiya et al., 2022).
Outside the central nervous system, the potential higher sensibility of AS-PLA has also been used to search for early α-syn oligomeric biomarkers such as in gastro-intestinal biopsy tissue (Ruffmann et al., 2018) or skin biopsies of PD and MSA patients (Mazzetti et al., 2020; Vacchi et al., 2021). Besides using PLA as a marker for α-syn aggregation, it can also be applied to study the colocalization and interaction of α-syn with other proteins in mechanisms possibly relevant to α-syn aggregation, such as the SNARE proteins (Almandoz-Gil et al., 2018), α-tubulin in the murine and human brain (Amadeo et al., 2021) and α-syn with retrograde and anterograde transport proteins in dopaminergic cultures (Bengoa-Vergniory et al., 2020). Interestingly, PLA can also detect modified proteins, such as p-α-syn, by using one Ab targeted to the protein and one targeted to the modification (Arlinghaus et al., 2021).
5.6. Quantification of immunohistochemical reaction
Although immunocytochemical and immunohistochemical studies are the cornerstone of the many post-mortem studies performed on tissue originating from animal models or deceased patients, there is some vagueness in the definition of the measured endpoints. The above discussion gives a sense of the hot debates about the specificity and reliability of the various antibodies. We would like to add to this debate the issue of quantification. To illustrate the lack of generalization in the assessment procedures, allow us to take the example of α-syn phosphorylation at serine 129 (pS129-α-syn) (Fujiwara et al., 2002). This original elegant demonstration suggested excessive phosphorylation of α-syn in synucleinopathy brains (including those in transgenic animals), as well as the underlying alterations in the activities of various kinases and phosphatases and the conformation of α-syn, may contribute to abnormalities of α-syn. Since this publication, in the overwhelming majority of post-mortem and experimental studies, the assessment of α-syn phosphorylation was taken as an indirect but supposedly reliable marker of aggregation without stating the endpoint's proxy nature. However, many papers have been satisfied with even very diffuse pSer129 positivity to qualify experimental histological images as synucleinopathy without considering the presence of actual subcellular inclusions in the measured/quantified signal. In other words, the immunohistochemical signal is often measured through optical density (without defining the experimental process for avoiding background variations) and measured as such or as an assessment of surface staining (Teil et al., 2022). A punctated signal could also be measured either quantitatively (very rarely) (Peng et al., 2018) or semi-quantitatively using a pathology-like rating scale- the actual meaning being more or less defined) (Henrich et al., 2020; Luk et al., 2012). Another option is to bypass the issue of α-syn quantification and use p-syn staining to identify cells that would then be characterized for the expression of other markers (Chu et al., 2019; Henrich et al., 2020). Surprisingly, very few papers report the counting of carefully defined aggregates (here, aggregate means an intracytoplasmic object more extensive than a simple punctum) (De Giorgi et al., 2020; Schmidhuber et al., 2022). We recently elaborated on the need to disclose the analysis of experimental synucleinopathies better, highlighting that the semantic and conceptual efforts should be made by scientists working with human tissues and experimental/animal material (Bezard, 2022).
6. Amplification techniques
At this point, we would like to briefly introduce the amplification techniques. Protein misfolding cyclic amplification (PMCA) and real-time quaking-induced conversion (RT-QuIC) are not stricto sensu approaches for monitoring aggregation but amplifying certain polymorphs of interest. Initially developed to detect different prion strains with high sensitivity, they hold great potential in synucleinopathy diagnosis and research (Fairfoul et al., 2016)(Poggiolini et al., 2022). They are based on the ability of trace amounts of misfolded protein to “trap” monomeric protein into its potential energy pit and grow. A combination of aggregate-growing/aggregate-breaking cycles permits to increase in the template units that can induce further aggregation while, in principle, preserving its ultra-structure. Then, classical laboratory techniques such as western blot or real-time fluorescence should allow signal quantification, while cryo-EM can provide ultra-structural information. (Brandel et al., 2019; Burger et al., 2021).
6.1. Protein misfolding cyclic amplification (PMCA)
PMCA has proved helpful in replicating fibrils from patients' tissue extracts keeping their conformation (Jung et al., 2017). Several studies have recently investigated its potential as a diagnostic tool. Among those, we chose to highlight Shahnawaz et al., who investigated its ability to detect oligomers in the CSF of PD and MSA patients (Shahnawaz et al., 2020), and Fenyi et al., who obtained positive results for detecting and amplifying aggregates in gastrointestinal biopsies using PMCA (Fenyi et al., 2019). Shahnawaz et al. showed very convincingly that the α-syn-PMCA assay can discriminate between CSF samples of PD and MSA patients, with an overall sensitivity of 95.4%. The obtained aggregates were further distinguished using a plethora of techniques, supporting PMCA data. Further, Shahnawaz et al. found that the properties of CSF-originating aggregates were like those of brain-amplified aggregates (Shahnawaz et al., 2020). This result validates the hypothesis of a continuum of pathology between the brain and the body fluids that can be accessed in living patients. It also strengthens the hypothesis of differential conformational strains of PD and MSA α-syn aggregates, paving the way for developing a biochemical assay capable of discriminating between PD and MSA at an early disease stage.
6.2. Real-time quaking-induced conversion (RT-QuIC)
RT-QuIC was born as a variant of PMCA by introducing some modifications in the protocol. Importantly, the real-time aggregation is now followed by ThT fluorescence (hence the name “real-time”) (Atarashi et al., 2011) (of note the need for using complementary dyes because of the ThT fluorescence “bias” and the existence of ThT-negative aggregates possibly as toxic as the positive ones). Several studies have confirmed the α-syn RT-QuIC assay to have high sensitivity and specificity for PD. In 2017, Fairfoul et al. developed an RT-QuIC-based assay to detect aggregated α-syn in DLB and PD cerebrospinal fluid with sensitivities over 90% (Fairfoul et al., 2016). Manne et al., focused on the submandibular glands as more accessible peripheral tissue, finding also positive results (Manne et al., 2020). More recently, Vascellari et al. reliably found misfolded α-syn in the CSF-isolated samples from rapid-eye-movement sleep behaviour disorder patients (Vascellari et al., 2022). Poggiolini et al. contributed to the most comprehensive study to date. Several important features were shown, thanks to the convincing power of their study. Despite a superior sensitivity towards PD (89%), no quantitative parameters correlated with PD severity. Conversely, although sensitivity was lesser for MSA (75%), the quantitative parameters correlated with MSA disease progression. Conversion of RBD patients into PD was somewhat predictable from early RT-QuIC. However, the percentage of converters (31%) was probably too low for concluding the predictivity (9 out of 14 converters showed baseline RT-QuIC positivity) (Poggiolini et al., 2022). Limitations were acknowledged by the authors and the next steps were clearly defined notably regarding the putative usefulness of α-syn RT-QuIC as an early biomarker detecting synucleinopathy in idiopathic RBD patients prior to conversion. This is a result awaited by the entire research community to better understand the relationship between a-syn RT-QuIC signature and the progression from prodromal to different synucleinopathies.
7. Facing the challenge of in vivo reporting of aggregation
Despite the complexity of α-syn aggregation dynamics, the current knowledge comes primarily from cell-free studies and, to a lesser extent, from cellular models. Given the importance of the molecular environment in which α-syn aggregation occurs (Lövestam et al., 2021), animal models designed to monitor aggregation or aggregated α-syn represent a crucial step in our understanding. On the one hand, they allow the validation of in vitro results and the detection of the differences between the processes occurring in the models mentioned above and the in vivo ones. Moreover, they provide a platform for reliably testing protein aggregation upon pharmacological challenges or genetic modifications.
The expression of recombinant α-syn as a fluorescent fusion protein in live organisms represents a straightforward method of studying aggregation in vivo. In a first approach, Kaminski Schierle et al. used an α-syn–YFP transgenic C.elegans worms. The authors used FRET between YFP (donor) and the forming aggregate (acceptor) to study aggregation. To do so, they measured the excited state lifetime of YFP using time-correlated single-photon counting (TCSPC) fluorescence lifetime microscopy. Interestingly, they found that α-syn–YFP forms different species with different associated toxicities in vivo than in vitro, highlighting the importance of the model system over α-syn aggregates structure (Kaminski Schierle et al., 2011).
In an exciting work, Osterberg et al. used previously developed α-syn-GFP transgenic mice for in vivo multiphoton imaging (Unni et al., 2010) to study the seeded aggregation of α-syn in cortical neurons of living mice (Osterberg et al., 2015). The authors showed that the injection of recombinant PFFs in the primary sensory cortex seeded aggregation of transgenically expressed- and endogenous α-syn in cortical neurons of living mice. The in vivo system allowed the imaging of the same neuron over months, observing stage-like maturation of the inclusions and, finally, selective cell death of inclusion-bearing neurons. Besides, the in vivo use of FRAP allowed them to obtain α-syn mobility measured in different conditions and maturation states, detecting slower diffusion kinetics within inclusions. In a recent paper, Schaser et al. developed a transgenic mouse model expressing the human A53T α-syn-GFP fused protein (Schaser et al., 2020). Using a similar experimental approach to Osterberg et al. (Osterberg et al., 2015), Schaser et al. observed the initial formation of aggregates in the axons and a time-dependent retrograde spread of aggregated α-syn in neurons, spreading later to astrocytes (Schaser et al., 2020). Besides mice models, the same group developed a zebrafish model expressing human GFP-α-syn in neurons. FRAP mobility experiments showed that human α-syn in zebrafish behaves similarly to that in mammals. The authors also performed pharmacological and genetic manipulations in the model, concluding that presynaptic aggregation of α-syn cannot be modulated by phosphorylation at serine-129 on its own (Weston et al., 2021).
The development of animal models based on previously described α-syn BiFC has become another valuable tool to monitor α-syn aggregation in living models as it permits detecting the fluorescence directly in vivo or fixed tissue. C. elegans models have been developed to study α-syn transmission in the context of ageing. By expressing each of the α-syn BiFC fragments in different but connected anatomical parts (Kim et al., 2016) or specific neuronal sub-populations (Tyson et al., 2017), the authors monitored the BiFC fluorescence in worms at different ages. They confirmed that α-syn could propagate and aggregate in this system. Prasad et al. recently developed a transgenic α-syn-BiFC Drosophila melanogaster as a more straightforward in vivo test bench amenable to genetic and pharmacologic manipulations. The analysis of different soluble fractions of brain homogenates permitted the assessment of the influence of a set of suspected risk factors or mutations (Prasad et al., 2019).
In 2013, Dimant et al. first developed a BiFC rat model. By co-injecting AAVs encoding human α-syn fused to the N- or C-terminal half of VenusYFP, the authors were able to detect fluorescence and to image α-syn aggregates in fixed sections of the nigrostriatal pathway and in vivo in the cortex, using confocal and two-photon microscopy, respectively (Dimant et al., 2013). More recently, diverse α-syn-BiFC mice models have appeared based on transgenic or AAV approaches. Similarly to Dimant et al., Cai et al. developed a BiFC-based mouse model based on the AAVs co-injection and characterized it at several levels. At four weeks post nigral injection of both AVVs, authors observed venus-YFP colocalizing with TH immunofluorescence in the SN. SN cells were also positive for human α-syn immunohistochemistry. This AAV-mediated human α-syn overexpression was related to ipsilateral nigral neurodegeneration, measured by TH-positive cell counting. YFP was observed in the striatum, supporting an anterograde spread of aggregated ⍺-syn. To explore the nature of aggregates, brain sections were treated with PK showing a significant reduction in fluorescence. The authors concluded that most of this fluorescence was thus caused by non-fibrillar oligomers. Finally, the analysis of Triton-X100- and SDS-treated midbrain homogenates through immunoblotting using an anti-human α-syn Ab shows myriad aggregated α-syn species in the ventral midbrain and the striatum eight weeks post-injection. (Cai et al., 2018). Kiechle et al., on the other hand, have generated two transgenic mouse models, expressing either split Gaussia luciferase or split Venus Yellow Fluorescent Protein (YFP) for aggregate detection within the brain and with subcellular resolution. The authors were able to quantify α-synuclein oligomerization in vivo in the pre-synapse. They confirmed the age-dependency on aggregation and the cell-to-cell transfer of de novo generated α-syn aggregates (Kiechle et al., 2019).
8. Concluding remarks
Researchers have access to an extensive panel of tools for monitoring α-syn aggregation. Although biophysical, non-Ab-based techniques are sound and give rise to unquestionable results in tubo or in vitro experiments, the challenge we collectively face is with the in vivo monitoring of such aggregation. A subsidiary question deals with our ability to produce and use extremely well-characterized antibodies for which we should not claim too rapidly about specificity towards particular conformations. The past few years have witnessed a structural biology revolution demonstrating the possible relationship between exquisite structural details, motifs, and differential pathologies. These results highlight how understanding the particular fibrillization conditions may lead to specific α-syn aggregated species (so-called polymorphs) and thus different pathological manifestations. Such basic science knowledge should now be transferred to in vivo experiments comparing the pathological outcome of exposure to in-depth characterized purely recombinant polymorphs, recombinant-made PMCA- or RT-QuiC-amplified patient-derived seeds, or crude patient-derived extracts. Combining single particle tracking with refined Abs and spatial omics approaches is likely to be the key to unlocking the disease pathology complexity (within a disease) and the diseases' specific polymorphs (if they exist). The in vivo understanding of these conditions leading to specific conformers, and thus specific diseases, is of paramount importance both for advancing the knowledge and for, at last, developing therapeutic approaches for the unrelenting synucleinopathies.
Funding
This review has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (Grant agreement No. #951294 to EB). JEP is a Ph.D. fellow in that ERC program. This study received financial support from the French government in the framework of the University of Bordeaux's IdEx “Investments for the Future” program/GPR BRAIN_2030. The opinions expressed and arguments employed herein do not necessarily reflect the official views of these funding bodies.
Declaration of Competing Interest
EB is a director and shareholder of Motac Neuroscience Ltd. The other authors declare no conflict of interest.
Acknowledgments
The University of Bordeaux and the Centre National de la Recherche Scientifique provided infrastructural support. The authors thank the many colleagues with whom we shared fruitful discussions.
Data availability
No data was used for the research described in the article.
References
- Alam P., et al. α-Synuclein oligomers and fibrils: a spectrum of species, a spectrum of toxicities. J. Neurochem. 2019;150:522–534. doi: 10.1111/jnc.14808. [DOI] [PubMed] [Google Scholar]
- Almandoz-Gil L., et al. In situ proximity ligation assay reveals co-localization of alpha-synuclein and SNARE proteins in murine primary neurons. Front. Neurol. 2018;9:180. doi: 10.3389/fneur.2018.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altay M.F., et al. 2022. Development and validation of an expanded antibody toolset that captures alpha-synuclein pathological diversity in Lewy body diseases. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadeo A., et al. The association between α-synuclein and α-tubulin in brain synapses. Int. J. Mol. Sci. 2021;22:9153. doi: 10.3390/ijms22179153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K., et al. Synchrotron FTIR micro-spectroscopy for structural analysis of Lewy bodies in the brain of Parkinson’s disease patients. Sci. Rep. 2015;5:1–8. doi: 10.1038/srep17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlinghaus R., et al. 2021. Specific Detection of Endogenous S129 Phosphorylated α-synuclein in Tissue Using Proximity Ligation Assay. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi R., et al. Real-time quaking-induced conversion: a highly sensitive assay for prion detection. Prion. 2011;5:150–153. doi: 10.4161/pri.5.3.16893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M., et al. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 1998;152:879. [PMC free article] [PubMed] [Google Scholar]
- Bae E.-J., et al. Glucocerebrosidase depletion enhances cell-to-cell transmission of α-synuclein. Nat. Commun. 2014;5:1–11. doi: 10.1038/ncomms5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae E.-J., et al. LRRK2 kinase regulates α-synuclein propagation via RAB35 phosphorylation. Nat. Commun. 2018;9:1–16. doi: 10.1038/s41467-018-05958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels T., et al. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behere A., et al. Visualization of early oligomeric α-synuclein pathology and its impact on the dopaminergic system in the (Thy-1)-h [A30P] α-syn transgenic mouse model. J. Neurosci. Res. 2021;99:2525–2539. doi: 10.1002/jnr.24927. [DOI] [PubMed] [Google Scholar]
- Bengoa-Vergniory N., et al. CLR01 protects dopaminergic neurons in vitro and in mouse models of Parkinson’s disease. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-020-18689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E. How lazy reading and semantic sloppiness may harm progress in synucleinopathy research. Biomolecules. 2022;12:228. doi: 10.3390/biom12020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E., Dehay B. Maladie de Parkinson-Le rôle de la synucléine. médecine/sciences. 2022;38:45–51. doi: 10.1051/medsci/2021241. [DOI] [PubMed] [Google Scholar]
- Biancalana M., Koide S. Molecular mechanism of thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2010;1804:1405–1412. doi: 10.1016/j.bbapap.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biere A.L., et al. Parkinson’s disease-associated α-synuclein is more fibrillogenic than β-and γ-synuclein and cannot cross-seed its homologs. J. Biol. Chem. 2000;275:34574–34579. doi: 10.1074/jbc.M005514200. [DOI] [PubMed] [Google Scholar]
- Bigi A., et al. Exploring the release of toxic oligomers from α-synuclein fibrils with antibodies and STED microscopy. Life. 2021;11:431. doi: 10.3390/life11050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blömeke L., et al. Quantitative detection of α-Synuclein and Tau oligomers and other aggregates by digital single particle counting. npj Parkinson's Dis. 2022;8:1–13. doi: 10.1038/s41531-022-00330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodner C.R., et al. Multiple tight phospholipid-binding modes of α-synuclein revealed by solution NMR spectroscopy. J. Mol. Biol. 2009;390:775–790. doi: 10.1016/j.jmb.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdenx M., et al. Protein aggregation and neurodegeneration in prototypical neurodegenerative diseases: examples of amyloidopathies, tauopathies and synucleinopathies. Prog. Neurobiol. 2017;155:171–193. doi: 10.1016/j.pneurobio.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Bourdenx M., et al. Identification of distinct pathological signatures induced by patient-derived α-synuclein structures in nonhuman primates. Sci. Adv. 2020;6:eaaz9165. doi: 10.1126/sciadv.aaz9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousset L., et al. Structural and functional characterization of two alpha-synuclein strains. Nat. Commun. 2013;4:1–13. doi: 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer D.R., et al. Structures of fibrils formed by α-synuclein hereditary disease mutant H50Q reveal new polymorphs. Nat. Struct. Mol. Biol. 2019;26:1044–1052. doi: 10.1038/s41594-019-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer D.R., et al. The α-synuclein hereditary mutation E46K unlocks a more stable, pathogenic fibril structure. Proc. Natl. Acad. Sci. 2020;117:3592–3602. doi: 10.1073/pnas.1917914117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandel J.-P., et al. Amplification techniques and diagnosis of prion diseases. Rev. Neurol. 2019;175:458–463. doi: 10.1016/j.neurol.2019.06.002. [DOI] [PubMed] [Google Scholar]
- Brännström K., et al. A generic method for design of oligomer-specific antibodies. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger D., et al. 2021. Cryo-EM structure of alpha-synuclein fibrils amplified by PMCA from PD and MSA patient brains. bioRxiv. [Google Scholar]
- Burré J., et al. Properties of native brain α-synuclein. Nature. 2013;498:E4–E6. doi: 10.1038/nature12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W., et al. Bimolecular fluorescence complementation of alpha-synuclein demonstrates its oligomerization with dopaminergic phenotype in mice. EBioMedicine. 2018;29:13–22. doi: 10.1016/j.ebiom.2018.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calero M., Gasset M. Fourier transform infrared and circular dichroism spectroscopies for amyloid studies. Amyloid Proteins. Springer. 2005:129–151. doi: 10.1385/1-59259-874-9:129. [DOI] [PubMed] [Google Scholar]
- Chandra S., et al. A broken α-helix in folded α-synuclein. J. Biol. Chem. 2003;278:15313–15318. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- Chu Y., et al. Intrastriatal alpha-synuclein fibrils in monkeys: spreading, imaging and neuropathological changes. Brain. 2019;142:3565–3579. doi: 10.1093/brain/awz296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho-Cerqueira E., et al. Pitfalls associated with the use of Thioflavin-T to monitor anti-fibrillogenic activity. Bioorg. Med. Chem. Lett. 2014;24:3194–3198. doi: 10.1016/j.bmcl.2014.04.072. [DOI] [PubMed] [Google Scholar]
- Conway K.A., et al. Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covell D., et al. Novel conformation-selective alpha-synuclein antibodies raised against different in vitro fibril forms show distinct patterns of Lewy pathology in Parkinson’s disease. Neuropathol. Appl. Neurobiol. 2017;43:604–620. doi: 10.1111/nan.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culvenor J.G., et al. Non-Aβ component of Alzheimer’s disease amyloid (NAC) revisited: NAC and α-synuclein are not associated with Aβ amyloid. Am. J. Pathol. 1999;155:1173–1181. doi: 10.1016/s0002-9440(10)65220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M.J., et al. Cyclized NDGA modifies dynamic α-synuclein monomers preventing aggregation and toxicity. Sci. Rep. 2019;9:1–17. doi: 10.1038/s41598-019-39480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer K.M., et al. Heat-shock protein 70 modulates toxic extracellular α-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J. 2011;25:326–336. doi: 10.1096/fj.10-164624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson W.S., et al. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- De Giorgi F., et al. Novel self-replicating α-synuclein polymorphs that escape ThT monitoring can spontaneously emerge and acutely spread in neurons. Sci. Adv. 2020;6:eabc4364. doi: 10.1126/sciadv.abc4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A.A., et al. Rational affinity maturation of anti-amyloid antibodies with high conformational and sequence specificity. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhavale D.D., et al. A sensitive assay reveals structural requirements for α-synuclein fibril growth. J. Biol. Chem. 2017;292:9034–9050. doi: 10.1074/jbc.M116.767053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson D., et al. Widespread alterations of α-synuclein in multiple system atrophy. Am. J. Pathol. 1999;155:1241–1251. doi: 10.1016/s0002-9440(10)65226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimant H., et al. Direct detection of alpha synuclein oligomers in vivo. Acta Neuropathol. Commun. 2013;1:1–10. doi: 10.1186/2051-5960-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding T.T., et al. Annular α-synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain-derived membranes. Biochemistry. 2002;41:10209–10217. doi: 10.1021/bi020139h. [DOI] [PubMed] [Google Scholar]
- Dominguez-Meijide A., et al. Effects of pharmacological modulators of α-synuclein and tau aggregation and internalization. Sci. Rep. 2020;10:1–18. doi: 10.1038/s41598-020-69744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda J.E., et al. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann. Neurol. 2002;52:205–210. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- Duffy P.E., Tennyson V.M. Phase and electron microscopic observations of Lewy bodies and melanin granules in the substantia nigra and locus caeruleus in Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1965;24:398–414. [Google Scholar]
- Duffy M.F., et al. Lewy body-like alpha-synuclein inclusions trigger reactive microgliosis prior to nigral degeneration. J. Neuroinflammation. 2018;15:1–18. doi: 10.1186/s12974-018-1171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf O.M., et al. Detection of oligomeric forms of α-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J. 2006;20:419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- Eliezer D., et al. Conformational properties of α-synuclein in its free and lipid-associated states. J. Mol. Biol. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- Emin D., et al. Small soluble α-synuclein aggregates are the toxic species in Parkinson’s disease. Nat. Commun. 2022;13:1–15. doi: 10.1038/s41467-022-33252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfoul G., et al. Alpha-synuclein RT-Qu IC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Trans. Neurol. 2016;3:812–818. doi: 10.1002/acn3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburger B.H., et al. Cellular models for Parkinson’s disease. J. Neurochem. 2016;139:121–130. doi: 10.1111/jnc.13618. [DOI] [PubMed] [Google Scholar]
- Fan Y., et al. 2021. Different structures and pathologies of α-synuclein fibrils derived from preclinical and postmortem patients of Parkinson’s disease. bioRxiv. [Google Scholar]
- Fares M.-B., et al. The novel Parkinson’s disease linked mutation G51D attenuates in vitro aggregation and membrane binding of α-synuclein, and enhances its secretion and nuclear localization in cells. Hum. Mol. Genet. 2014;23:4491–4509. doi: 10.1093/hmg/ddu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauerbach J.A., Jovin T.M. Pre-aggregation kinetics and intermediates of α-synuclein monitored by the ESIPT probe 7MFE. Eur. Biophys. J. 2018;47:345–362. doi: 10.1007/s00249-017-1272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenyi A., et al. Detection of alpha-synuclein aggregates in gastrointestinal biopsies by protein misfolding cyclic amplification. Neurobiol. Dis. 2019;129:38–43. doi: 10.1016/j.nbd.2019.05.002. [DOI] [PubMed] [Google Scholar]
- Fernandez C.O., et al. NMR of α-synuclein–polyamine complexes elucidates the mechanism and kinetics of induced aggregation. EMBO J. 2004;23:2039–2046. doi: 10.1038/sj.emboj.7600211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey B., et al. Monitoring alpha-synuclein oligomerization and aggregation using bimolecular fluorescence complementation assays: what you see is not always what you get. J. Neurochem. 2021;157:872–888. doi: 10.1111/jnc.15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frid P., et al. Congo red and protein aggregation in neurodegenerative diseases. Brain Res. Rev. 2007;53:135–160. doi: 10.1016/j.brainresrev.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Froula J.M., et al. Defining α-synuclein species responsible for Parkinson’s disease phenotypes in mice. J. Biol. Chem. 2019;294:10392–10406. doi: 10.1074/jbc.RA119.007743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H., et al. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Gao L., et al. Fluorescent probes for bioimaging of potential biomarkers in Parkinson’s disease. Chem. Soc. Rev. 2021;50:1219–1250. doi: 10.1039/d0cs00115e. [DOI] [PubMed] [Google Scholar]
- Gaur P., et al. Fluorescent probe for selective imaging of α-synuclein fibrils in living cells. ACS Chem. Neurosci. 2021;12:1293–1298. doi: 10.1021/acschemneuro.1c00090. [DOI] [PubMed] [Google Scholar]
- Giasson B.I., et al. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Gibbs E., et al. Rational generation of monoclonal antibodies selective for pathogenic forms of alpha-synuclein. Biomedicines. 2022;10:2168. doi: 10.3390/biomedicines10092168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M.S., Lansbury P.T., Jr. Is there a cause-and-effect relationship between α-synuclein fibrillization and Parkinson’s disease? Nat. Cell Biol. 2000;2:E115–E119. doi: 10.1038/35017124. [DOI] [PubMed] [Google Scholar]
- Goldsbury C., et al. Watching amyloid fibrils grow by time-lapse atomic force microscopy. J. Mol. Biol. 1999;285:33–39. doi: 10.1006/jmbi.1998.2299. [DOI] [PubMed] [Google Scholar]
- Goldsbury C., et al. Amyloid structure and assembly: insights from scanning transmission electron microscopy. J. Struct. Biol. 2011;173:1–13. doi: 10.1016/j.jsb.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves S.A., et al. Zooming into protein oligomerization in neurodegeneration using BiFC. Trends Biochem. Sci. 2010;35:643–651. doi: 10.1016/j.tibs.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Gonçalves S.A., et al. shRNA-based screen identifies endocytic recycling pathway components that act as genetic modifiers of alpha-synuclein aggregation, secretion and toxicity. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1005995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Ferreira R., et al. Cryo-EM structure of alpha-synuclein fibrils. elife. 2018;7 doi: 10.7554/eLife.36402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Ferreira R., et al. Two new polymorphic structures of human full-length alpha-synuclein fibrils solved by cryo-electron microscopy. Elife. 2019;8 doi: 10.7554/eLife.48907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenstein B., Loquet A. Solid-state NMR: an emerging technique in structural biology of self-assemblies. Biophys. Chem. 2016;210:14–26. doi: 10.1016/j.bpc.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Haney C.M., et al. Comparison of strategies for non-perturbing labeling of α-synuclein to study amyloidogenesis. Org. Biomol. Chem. 2016;14:1584–1592. doi: 10.1039/c5ob02329g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise H., et al. Molecular-level secondary structure, polymorphism, and dynamics of full-length α-synuclein fibrils studied by solid-state NMR. Proc. Natl. Acad. Sci. 2005;102:15871–15876. doi: 10.1073/pnas.0506109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich M.T., et al. Determinants of seeding and spreading of α-synuclein pathology in the brain. Sci. Adv. 2020;6:eabc2487. doi: 10.1126/sciadv.abc2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong D.-P., et al. Structural characteristics of α-synuclein oligomers stabilized by the flavonoid baicalein. J. Mol. Biol. 2008;383:214–223. doi: 10.1016/j.jmb.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housmans J.A., et al. A guide to studying protein aggregation. FEBS J. 2021 doi: 10.1111/febs.16312. [DOI] [PubMed] [Google Scholar]
- Iadanza M.G., et al. A new era for understanding amyloid structures and disease. Nat. Rev. Mol. Cell Biol. 2018;19:755–773. doi: 10.1038/s41580-018-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadavi S., et al. AFM-STED correlative nanoscopy applied to the study of in-vitro misfolded protein aggregation. Biophys. J. 2022;121:279a. [Google Scholar]
- Je G., et al. Endogenous alpha-synuclein protein analysis from human brain tissues using single-molecule pull-down assay. Anal. Chem. 2017;89:13044–13048. doi: 10.1021/acs.analchem.7b04335. [DOI] [PubMed] [Google Scholar]
- Jeong J.S., et al. Novel mechanistic insight into the molecular basis of amyloid polymorphism and secondary nucleation during amyloid formation. J. Mol. Biol. 2013;425:1765–1781. doi: 10.1016/j.jmb.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Jun J.V., et al. A “clickable” photoconvertible small fluorescent molecule as a minimalist probe for tracking individual biomolecule complexes. J. Am. Chem. Soc. 2019;141:1893–1897. doi: 10.1021/jacs.8b13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung B.C., et al. Amplification of distinct α-synuclein fibril conformers through protein misfolding cyclic amplification. Exp. Mol. Med. 2017;49(4):e314. doi: 10.1038/emm.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski Schierle G.S., et al. A FRET sensor for non-invasive imaging of amyloid formation in vivo. ChemPhysChem. 2011;12:673–680. doi: 10.1002/cphc.201000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S.M., et al. How to study proteins by circular dichroism. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2005;1751:119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Kiechle M., et al. In vivo protein complementation demonstrates presynaptic α-synuclein oligomerization and age-dependent accumulation of 8–16-mer oligomer species. Cell Rep. 2019;29(2862–2874) doi: 10.1016/j.celrep.2019.10.089. [DOI] [PubMed] [Google Scholar]
- Kim D.-K., et al. Anti-aging treatments slow propagation of synucleinopathy by restoring lysosomal function. Autophagy. 2016;12:1849–1863. doi: 10.1080/15548627.2016.1207014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherak O.A., et al. Synthesis of a fluorescent probe for sensing multiple protein states. Eur. J. Org. Chem. 2018;2018:5155–5162. [Google Scholar]
- Kühlbrandt W. The resolution revolution. Science. 2014;343:1443–1444. doi: 10.1126/science.1251652. [DOI] [PubMed] [Google Scholar]
- Kulenkampff K., et al. Quantifying misfolded protein oligomers as drug targets and biomarkers in Alzheimer and Parkinson diseases. Nat. Rev. Chem. 2021;5:277–294. doi: 10.1038/s41570-021-00254-9. [DOI] [PubMed] [Google Scholar]
- Kumar H., et al. Modulation of the extent of structural heterogeneity in α-synuclein fibrils by the small molecule thioflavin T. J. Biol. Chem. 2017;292:16891–16903. doi: 10.1074/jbc.M117.795617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.T., et al. How specific are the conformation-specific α-synuclein antibodies? Characterization and validation of 16 α-synuclein conformation-specific antibodies using well-characterized preparations of α-synuclein monomers, fibrils and oligomers with distinct structures and morphology. Neurobiol. Dis. 2020;146 doi: 10.1016/j.nbd.2020.105086. [DOI] [PubMed] [Google Scholar]
- Kushnirov V.V., et al. Proteinase K resistant cores of prions and amyloids. Prion. 2020;14:11–19. doi: 10.1080/19336896.2019.1704612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova I.M., et al. High fluorescence anisotropy of thioflavin T in aqueous solution resulting from its molecular rotor nature. Anal. Chem. 2016;88:718–724. doi: 10.1021/acs.analchem.5b02747. [DOI] [PubMed] [Google Scholar]
- Laferrière F., et al. TDP-43 extracted from frontotemporal lobar degeneration subject brains displays distinct aggregate assemblies and neurotoxic effects reflecting disease progression rates. Nat. Neurosci. 2019;22:65–77. doi: 10.1038/s41593-018-0294-y. [DOI] [PubMed] [Google Scholar]
- Laferrière F., et al. Overexpression of α-synuclein by oligodendrocytes in transgenic mice does not recapitulate the fibrillar aggregation seen in multiple system atrophy. Cells. 2020;9:2371. doi: 10.3390/cells9112371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferrière F., et al. Similar neuronal imprint and no cross-seeded fibrils in α-synuclein aggregates from MSA and Parkinson’s disease. NPJ Parkinson’s Dis. 2022;8:1–12. doi: 10.1038/s41531-021-00264-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landureau M., et al. The differential solvent exposure of N-terminal residues provides “fingerprints” of alpha-synuclein fibrillar polymorphs. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashuel H.A. Do Lewy bodies contain alpha-synuclein fibrils? And does it matter? A brief history and critical analysis of recent reports. Neurobiol. Dis. 2020;141 doi: 10.1016/j.nbd.2020.104876. [DOI] [PubMed] [Google Scholar]
- Lassen L.B., et al. ELISA method to detect α-synuclein oligomers in cell and animal models. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A., et al. α-Synuclein strains target distinct brain regions and cell types. Nat. Neurosci. 2020;23:21–31. doi: 10.1038/s41593-019-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázaro D.F., et al. The effects of the novel A53E alpha-synuclein mutation on its oligomerization and aggregation. Acta Neuropathol. Commun. 2016;4:1–15. doi: 10.1186/s40478-016-0402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.C., et al. Site-specific three-color labeling of α-synuclein via conjugation to uniquely reactive cysteines during assembly by native chemical ligation. Cell Chem. Biol. 2018;25(797–801) doi: 10.1016/j.chembiol.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.E., et al. Memantine exerts neuroprotective effects by modulating α-synuclein transmission in a parkinsonian model. Exp. Neurol. 2021;344 doi: 10.1016/j.expneurol.2021.113810. [DOI] [PubMed] [Google Scholar]
- Levine H., III Thioflavine T interaction with synthetic Alzheimer’s disease β-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine H., III . Methods in Enzymology. Elsevier; 1999. [18] Quantification of β-sheet amyloid fibril structures with thioflavin T; pp. 274–284. [DOI] [PubMed] [Google Scholar]
- Li B., et al. Cryo-EM of full-length α-synuclein reveals fibril polymorphs with a common structural kernel. Nat. Commun. 2018;9:1–10. doi: 10.1038/s41467-018-05971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., et al. Amyloid fibril structure of α-synuclein determined by cryo-electron microscopy. Cell Res. 2018;28:897–903. doi: 10.1038/s41422-018-0075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövestam S., et al. Seeded assembly in vitro does not replicate the structures of α-synuclein filaments from multiple system atrophy. FEBS Open Bio. 2021;11:999–1013. doi: 10.1002/2211-5463.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk K.C., et al. Exogenous α-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. 2009;106:20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk K.C., et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luth E.S., et al. Purification of α-synuclein from human brain reveals an instability of endogenous multimers as the protein approaches purity. Biochemistry. 2015;54:279–292. doi: 10.1021/bi501188a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv G., et al. Structural comparison of mouse and human α-synuclein amyloid fibrils by solid-state NMR. J. Mol. Biol. 2012;420:99–111. doi: 10.1016/j.jmb.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Mahul-Mellier A.-L., et al. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. 2020;117:4971–4982. doi: 10.1073/pnas.1913904117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne S., et al. α-Synuclein real-time quaking-induced conversion in the submandibular glands of Parkinson’s disease patients. Mov. Disord. 2020;35:268–278. doi: 10.1002/mds.27907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martial B., et al. Vibrational circular dichroism reveals supramolecular chirality inversion of α-synuclein peptide assemblies upon interactions with anionic membranes. ACS Nano. 2019;13:3232–3242. doi: 10.1021/acsnano.8b08932. [DOI] [PubMed] [Google Scholar]
- Mazzetti S., et al. α-Synuclein oligomers in skin biopsy of idiopathic and monozygotic twin patients with Parkinson’s disease. Brain. 2020;143:920–931. doi: 10.1093/brain/awaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccormack A., et al. Purification of α-synuclein containing inclusions from human post mortem brain tissue. J. Neurosci. Methods. 2016;266:141–150. doi: 10.1016/j.jneumeth.2016.03.016. [DOI] [PubMed] [Google Scholar]
- McLean P., et al. α-Synuclein–enhanced green fluorescent protein fusion proteins form proteasome sensitive inclusions in primary neurons. Neuroscience. 2001;104:901–912. doi: 10.1016/s0306-4522(01)00113-0. [DOI] [PubMed] [Google Scholar]
- Meier B.H., et al. Emerging structural understanding of amyloid fibrils by solid-state NMR. Trends Biochem. Sci. 2017;42:777–787. doi: 10.1016/j.tibs.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Miraglia F., et al. Alpha-synuclein FRET biosensors reveal early alpha-synuclein aggregation in the endoplasmic reticulum. Life. 2020;10:147. doi: 10.3390/life10080147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores B., et al. Effect of surfaces on amyloid fibril formation. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosa M.M., et al. Alpha-Synuclein. Springer; 2019. Single-molecule fret detection of early-stage conformations in α-synuclein aggregation; pp. 221–233. [DOI] [PubMed] [Google Scholar]
- Morris K.L., Serpell L.C. Amyloid Proteins. Springer; 2012. X-ray fibre diffraction studies of amyloid fibrils; pp. 121–135. [DOI] [PubMed] [Google Scholar]
- Mučibabić M., et al. The effect of fluorescent labeling on α-synuclein fibril morphology. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2016;1864:1419–1427. doi: 10.1016/j.bbapap.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Nakamura K., et al. Optical reporters for the conformation of α-synuclein reveal a specific interaction with mitochondria. J. Neurosci. 2008;28:12305–12317. doi: 10.1523/JNEUROSCI.3088-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham L.-M., et al. ThX–a next-generation probe for the early detection of amyloid aggregates. Chem. Sci. 2020;11:4578–4583. doi: 10.1039/c9sc04730a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo F., et al. Accumulation and clearance of α-synuclein aggregates demonstrated by time-lapse imaging. J. Neurochem. 2008;106:529–540. doi: 10.1111/j.1471-4159.2008.05407.x. [DOI] [PubMed] [Google Scholar]
- Osterberg V.R., et al. Progressive aggregation of alpha-synuclein and selective degeneration of lewy inclusion-bearing neurons in a mouse model of parkinsonism. Cell Rep. 2015;10:1252–1260. doi: 10.1016/j.celrep.2015.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro T.F., Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003;302:1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro T.F., et al. Formation of toxic oligomeric α-synuclein species in living cells. PLoS One. 2008;3 doi: 10.1371/journal.pone.0001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleologou K.E., et al. Detection of elevated levels of soluble α-synuclein oligomers in post-mortem brain extracts from patients with dementia with Lewy bodies. Brain. 2009;132:1093–1101. doi: 10.1093/brain/awn349. [DOI] [PubMed] [Google Scholar]
- Pan B., et al. Chemoenzymatic semisynthesis of phosphorylated α-synuclein enables identification of a bidirectional effect on fibril formation. ACS Chem. Biol. 2020;15:640–645. doi: 10.1021/acschembio.9b01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M.I., Lantos P.L. Accumulation of tubular structures in oligodendroglial and neuronal cells as the basic alteration in multiple system atrophy. J. Neurol. Sci. 1992;107:172–182. doi: 10.1016/0022-510x(92)90286-t. [DOI] [PubMed] [Google Scholar]
- Peelaerts W., et al. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- Peng C., et al. Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature. 2018;557:558–563. doi: 10.1038/s41586-018-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett S., et al. The physical chemistry of the amyloid phenomenon: thermodynamics and kinetics of filamentous protein aggregation. Essays Biochem. 2014;56:11–39. doi: 10.1042/bse0560011. [DOI] [PubMed] [Google Scholar]
- Perrino G., et al. Quantitative characterization of α-synuclein aggregation in living cells through automated microfluidics feedback control. Cell Rep. 2019;27(916–927) doi: 10.1016/j.celrep.2019.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piston D.W., Kremers G.-J. Fluorescent protein FRET: the good, the bad and the ugly. Trends Biochem. Sci. 2007;32:407–414. doi: 10.1016/j.tibs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Poggiolini I., et al. Diagnostic value of cerebrospinal fluid alpha-synuclein seed quantification in synucleinopathies. Brain. 2022;145:584–595. doi: 10.1093/brain/awab431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyanichko A.M., et al. Vibrational circular dichroism studies of biological macromolecules and their complexes. Circ. Dichroism: Theory Spectroscopy. 2011:67–126. [Google Scholar]
- Prasad V., et al. Monitoring α-synuclein multimerization in vivo. FASEB J. 2019;33:2116–2131. doi: 10.1096/fj.201800148RRR. [DOI] [PubMed] [Google Scholar]
- Raskatov J.A., Teplow D.B. Using chirality to probe the conformational dynamics and assembly of intrinsically disordered amyloid proteins. Sci. Rep. 2017;7:1–7. doi: 10.1038/s41598-017-10525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recasens A., et al. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann. Neurol. 2014;75:351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- Reif B., et al. Solid-state NMR spectroscopy. Nat. Rev. Methods Primers. 2021;1:1–23. doi: 10.1038/s43586-020-00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke A.A., Gestwicki J.E. Insight into amyloid structure using chemical probes. Chem. Biol. Drug Des. 2011;77:399–411. doi: 10.1111/j.1747-0285.2011.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J., et al. Plasma α-synuclein and phosphorylated tau 181 as a diagnostic biomarker panel for de novo Parkinson’s disease. J. Neurochem161: 506-515. 2022 doi: 10.1111/jnc.15601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberti M.J., et al. Fluorescence imaging of amyloid formation in living cells by a functional, tetracysteine-tagged α-synuclein. Nat. Methods. 2007;4:345–351. doi: 10.1038/nmeth1026. [DOI] [PubMed] [Google Scholar]
- Roberti M.J., et al. Confocal fluorescence anisotropy and FRAP imaging of α-synuclein amyloid aggregates in living cells. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R.F., et al. Direct visualization of alpha-synuclein oligomers reveals previously undetected pathology in Parkinson’s disease brain. Brain. 2015;138:1642–1657. doi: 10.1093/brain/awv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robustelli P., et al. Developing a molecular dynamics force field for both folded and disordered protein states. Proc. Natl. Acad. Sci. 2018;115:E4758–E4766. doi: 10.1073/pnas.1800690115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J.A., et al. Structure of the toxic core of α-synuclein from invisible crystals. Nature. 2015;525:486–490. doi: 10.1038/nature15368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovere M. Alpha-Synuclein. Springer; 2019. Circular dichroism and isothermal titration calorimetry to study the interaction of α-synuclein with membranes; pp. 123–143. [DOI] [PubMed] [Google Scholar]
- Ruffmann C., et al. Detection of alpha-synuclein conformational variants from gastro-intestinal biopsy tissue as a potential biomarker for Parkinson’s disease. Neuropathol. Appl. Neurobiol. 2018;44:722–736. doi: 10.1111/nan.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri F.S., et al. The influence of pathogenic mutations in α-synuclein on biophysical and structural characteristics of amyloid fibrils. ACS Nano. 2020;14:5213–5222. doi: 10.1021/acsnano.9b09676. [DOI] [PubMed] [Google Scholar]
- Saal K.-A., et al. Combined use of unnatural amino acids enables dual-color super-resolution imaging of proteins via click chemistry. ACS Nano. 2018;12:12247–12254. doi: 10.1021/acsnano.8b06047. [DOI] [PubMed] [Google Scholar]
- Sakamoto M., et al. Heterogeneity of nigral and cortical Lewy bodies differentiated by amplified triple-labeling for alpha-synuclein, ubiquitin, and thiazin red. Exp. Neurol. 2002;177:88–94. doi: 10.1006/exnr.2002.7961. [DOI] [PubMed] [Google Scholar]
- Salmon L.C., et al. NMR characterization of long-range order in intrinsically disordered proteins. J. Am. Chem. Soc. 2010;132:8407–8418. doi: 10.1021/ja101645g. [DOI] [PubMed] [Google Scholar]
- Sang J.C., et al. Super-resolution imaging reveals α-synuclein seeded aggregation in SH-SY5Y cells. Commun. Biol. 2021;4:1–11. doi: 10.1038/s42003-021-02126-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarroukh R., et al. ATR-FTIR: a “rejuvenated” tool to investigate amyloid proteins. Biochim. Biophys. Acta (BBA)-Biomembranes. 2013;1828 doi: 10.1016/j.bbamem.2013.04.012. 2328-2338. [DOI] [PubMed] [Google Scholar]
- Schaser A.J., et al. Trans-synaptic and retrograde axonal spread of Lewy pathology following pre-formed fibril injection in an in vivo A53T alpha-synuclein mouse model of synucleinopathy. Acta Neuropathol. Commun. 2020;8:1–23. doi: 10.1186/s40478-020-01026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhuber S., et al. A novel C-type lectin receptor-targeted α-synuclein-based Parkinson vaccine induces potent immune responses and therapeutic efficacy in mice. Vaccines. 2022;10:1432. doi: 10.3390/vaccines10091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor M., et al. Analytical methods for structural ensembles and dynamics of intrinsically disordered proteins. Biophys. Rev. 2016;8:429–439. doi: 10.1007/s12551-016-0234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighauser M., et al. Structures of α-synuclein filaments from multiple system atrophy. Nature. 2020;585:464–469. doi: 10.1038/s41586-020-2317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick A.C., et al. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018;47:8842–8880. doi: 10.1039/c8cs00185e. [DOI] [PubMed] [Google Scholar]
- Sekiya H., et al. Wide distribution of alpha-synuclein oligomers in multiple system atrophy brain detected by proximity ligation. Acta Neuropathol. 2019;137:455–466. doi: 10.1007/s00401-019-01961-w. [DOI] [PubMed] [Google Scholar]
- Sekiya H., et al. Discrepancy between distribution of alpha-synuclein oligomers and Lewy-related pathology in Parkinson’s disease. Acta Neuropathol. Commun. 2022;10:1–13. doi: 10.1186/s40478-022-01440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selenko P., Wagner G. Looking into live cells with in-cell NMR spectroscopy. J. Struct. Biol. 2007;158:244–253. doi: 10.1016/j.jsb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Serpell L.C., et al. Fiber diffraction of synthetic α-synuclein filaments shows amyloid-like cross-β conformation. Proc. Natl. Acad. Sci. 2000;97:4897–4902. doi: 10.1073/pnas.97.9.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahmoradian S.H., et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 2019;22:1099–1109. doi: 10.1038/s41593-019-0423-2. [DOI] [PubMed] [Google Scholar]
- Shahnawaz M., et al. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature. 2020;578:273–277. doi: 10.1038/s41586-020-1984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D., et al. Three-dimensional electron crystallography of protein microcrystals. Elife. 2013;2 doi: 10.7554/eLife.01345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogawa M., Petersson E.J. New strategies for fluorescently labeling proteins in the study of amyloids. Curr. Opin. Chem. Biol. 2021;64:57–66. doi: 10.1016/j.cbpa.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A.N., et al. Differential membrane binding and seeding of distinct α-synuclein fibrillar polymorphs. Biophys. J. 2020;118:1301–1320. doi: 10.1016/j.bpj.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu A., et al. Polymorph-specific distribution of binding sites determines thioflavin-T fluorescence intensity in α-synuclein fibrils. Amyloid. 2018;25:189–196. doi: 10.1080/13506129.2018.1517736. [DOI] [PubMed] [Google Scholar]
- Sigurdson C.J., et al. Prion strain discrimination using luminescent conjugated polymers. Nat. Methods. 2007;4:1023–1030. doi: 10.1038/nmeth1131. [DOI] [PubMed] [Google Scholar]
- Söderberg O., et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- Spillantini M.G., et al. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Spillantini M.G., et al. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohäker T., et al. Structural heterogeneity of α-synuclein fibrils amplified from patient brain extracts. Nat. Commun. 2019;10:1–12. doi: 10.1038/s41467-019-13564-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., et al. Cryo-EM structure of full-length α-synuclein amyloid fibril with Parkinson’s disease familial A53T mutation. Cell Res. 2020;30:360–362. doi: 10.1038/s41422-020-0299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunde M., et al. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol. 1997;273:729–739. doi: 10.1006/jmbi.1997.1348. [DOI] [PubMed] [Google Scholar]
- Svanbergsson A., et al. FRET-based screening identifies p38 MAPK and PKC inhibition as targets for prevention of seeded α-Synuclein aggregation. Neurotherapeutics. 2021;18:1692–1709. doi: 10.1007/s13311-021-01070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamuku M., et al. Evolution of α-synuclein conformation ensemble toward amyloid fibril via liquid-liquid phase separation (LLPS) as investigated by dynamic nuclear polarization-enhanced solid-state MAS NMR. Neurochem. Int. 2022;157 doi: 10.1016/j.neuint.2022.105345. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Komi Y. Layers of structure and function in protein aggregation. Nat. Chem. Biol. 2015;11:373–377. doi: 10.1038/nchembio.1818. [DOI] [PubMed] [Google Scholar]
- Teil M., et al. Brain injections of glial cytoplasmic inclusions induce a multiple system atrophy-like pathology. Brain. 2022;145:1001–1017. doi: 10.1093/brain/awab374. [DOI] [PubMed] [Google Scholar]
- Theillet F.-X., et al. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature. 2016;530:45–50. doi: 10.1038/nature16531. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M., et al. Risk factors for dopaminergic neuron loss in human α-synuclein transgenic mice. Eur. J. Neurosci. 2004;19:845–854. doi: 10.1111/j.0953-816x.2004.03139.x. [DOI] [PubMed] [Google Scholar]
- Trinkaus V.A., et al. In situ architecture of neuronal α-Synuclein inclusions. Nat. Commun. 2021;12:1–10. doi: 10.1038/s41467-021-22108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle M.D., et al. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat. Struct. Mol. Biol. 2016;23:409–415. doi: 10.1038/nsmb.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson T., et al. Novel animal model defines genetic contributions for neuron-to-neuron transfer of α-synuclein. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-07383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., et al. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unni V.K., et al. In vivo imaging of α-synuclein in mouse cortex demonstrates stable expression and differential subcellular compartment mobility. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchi E., et al. Alpha-synuclein oligomers and small nerve fiber pathology in skin are potential biomarkers of Parkinson’s disease. NPJ Parkinson’s Dis. 2021;7:1–11. doi: 10.1038/s41531-021-00262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaikath N.N., et al. Generation and characterization of novel conformation-specific monoclonal antibodies for α-synuclein pathology. Neurobiol. Dis. 2015;79:81–99. doi: 10.1016/j.nbd.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Vaikath N.N., et al. Antibodies against alpha-synuclein: tools and therapies. J. Neurochem. 2019;150:612–625. doi: 10.1111/jnc.14713. [DOI] [PubMed] [Google Scholar]
- Van de Vondel E., et al. Vibrational circular dichroism sheds new light on the competitive effects of crowding and β-Synuclein on the fibrillation process of α-synuclein. Biochemistry. 2018;57:5989–5995. doi: 10.1021/acs.biochem.8b00780. [DOI] [PubMed] [Google Scholar]
- van Diggelen F., et al. Two conformationally distinct α-synuclein oligomers share common epitopes and the ability to impair long-term potentiation. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ham T.J., et al. Towards multiparametric fluorescent imaging of amyloid formation: studies of a YFP model of α-synuclein aggregation. J. Mol. Biol. 2010;395:627–642. doi: 10.1016/j.jmb.2009.10.066. [DOI] [PubMed] [Google Scholar]
- Vascellari S., et al. Real-time quaking-induced conversion assays for prion diseases, synucleinopathies, and tauopathies. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.853050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar M., et al. The fold of α-synuclein fibrils. Proc. Natl. Acad. Sci. 2008;105:8637–8642. doi: 10.1073/pnas.0712179105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar-Piqué A., et al. Structure, function and toxicity of alpha-synuclein: the Bermuda triangle in synucleinopathies. J. Neurochem. 2016;139:240–255. doi: 10.1111/jnc.13249. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley L.A., et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Takahashi H. Cellular pathology in multiple system atrophy. Neuropathology. 2006;26:338–345. doi: 10.1111/j.1440-1789.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., et al. The Lewy body in Parkinson's disease: molecules implicated in the formation and degradation of α-synuclein aggregates. Neuropathology. 2007;27:494–506. doi: 10.1111/j.1440-1789.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- Watanabe-Nakayama T., et al. Self-and cross-seeding on α-synuclein fibril growth kinetics and structure observed by high-speed atomic force microscopy. ACS Nano. 2020;14:9979–9989. doi: 10.1021/acsnano.0c03074. [DOI] [PubMed] [Google Scholar]
- Weinreb P.H., et al. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- Weston L.J., et al. In vivo aggregation of presynaptic alpha-synuclein is not influenced by its phosphorylation at serine-129. Neurobiol. Dis. 2021;152 doi: 10.1016/j.nbd.2021.105291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B., et al. In vivo demonstration that α-synuclein oligomers are toxic. Proc. Natl. Acad. Sci. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue C., et al. Thioflavin T as an amyloid dye: fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 2017;4 doi: 10.1098/rsos.160696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakupova E.I., et al. Congo red and amyloids: history and relationship. Biosci. Rep. 2019;39 doi: 10.1042/BSR20181415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T.R., et al. Parkinson’s disease and multiple system atrophy have distinct α-synuclein seed characteristics. J. Biol. Chem. 2019;294:1045–1058. doi: 10.1074/jbc.RA118.004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., et al. Structures of α-synuclein filaments from human brains with Lewy pathology. Nature. 2022;610:791–795. doi: 10.1038/s41586-022-05319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandomeneghi G., et al. FTIR reveals structural differences between native β-sheet proteins and amyloid fibrils. Protein Sci. 2004;13:3314–3321. doi: 10.1110/ps.041024904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., et al. Detection and imaging of Aβ1-42 and tau fibrils by redesigned fluorescent X-34 analogues. Chem Eur J. 2018;24:7210–7216. doi: 10.1002/chem.201800501. [DOI] [PubMed] [Google Scholar]
- Zhang Y., et al. High-speed atomic force microscopy reveals structural dynamics of α-synuclein monomers and dimers. J. Chem. Phys. 2018;148 doi: 10.1063/1.5008874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., et al. Heparin-induced tau filaments are polymorphic and differ from those in Alzheimer’s and Pick’s diseases. Elife. 2019;8 doi: 10.7554/eLife.43584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., et al. Correlation between cellular uptake and cytotoxicity of fragmented α-synuclein amyloid fibrils suggests intracellular basis for toxicity. ACS Chem. Neurosci. 2020;11:233–241. doi: 10.1021/acschemneuro.9b00562. [DOI] [PubMed] [Google Scholar]
- Zhao K., et al. Parkinson’s disease associated mutation E46K of α-synuclein triggers the formation of a distinct fibril structure. Nat. Commun. 2020;11:1–9. doi: 10.1038/s41467-020-16386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.