Background:
Non–vitamin K oral anticoagulants have become the standard therapy for preventing stroke and ischemic thromboembolism in most patients with atrial fibrillation (AF). The effectiveness and safety of non–vitamin K oral anticoagulants in patients on hemodialysis is not well known.
Methods:
From June 2017 through May 2022, AXADIA–AFNET 8 (Compare Apixaban and Vitamin K Antagonists in Patients With Atrial Fibrillation and End-Stage Kidney Disease), an investigator-initiated PROBE (prospective randomized open blinded end point) outcome assessment trial, randomized patients with AF on chronic hemodialysis to either apixaban (2.5 mg BID) or the vitamin K antagonist (VKA) phenprocoumon (international normalized ratio, 2.0 to 3.0). The composite primary safety outcome was defined by a first event of major bleeding, clinically relevant nonmajor bleeding, or all-cause death. The primary efficacy outcome was a composite of ischemic stroke, all-cause death, myocardial infarction, and deep vein thrombosis or pulmonary embolism. Our hypothesis was that apixaban is noninferior to VKA.
Results:
Thirty-nine sites randomized 97 patients (30% women; mean age 75 years; mean CHA2DS2-VASc [congestive heart failure, hypertension, age ≥75 years, diabetes, stroke or transient ischemic attack, vascular disease, age 65 to 74 years, female sex] score, 4.5; baseline characteristics balanced between groups): 48 to apixaban and 49 to VKA. The median follow-up time was 429 days (range, 37 to 1370) versus 506 days (range, 101 to 1379), respectively. Adherence to apixaban was >80% in 44 of 48 patients; the median time in therapeutic range on VKA was 50.7%. Composite primary safety outcome events occurred in 22 patients (45.8%) on apixaban and in 25 patients (51.0%) on VKA (hazard ratio, 0.93 [95% CI, 0.53–1.65]; Pnoninferiority=0.157). Composite primary efficacy outcome events occurred in 10 patients (20.8%) on apixaban and in 15 patients (30.6%) on VKA (P=0.51; log rank). There were no significant differences regarding individual outcomes (all-cause mortality, 18.8% versus 24.5%; major bleeding, 10.4% versus 12.2%; and myocardial infarction, 4.2% versus 6.1%, respectively).
Conclusions:
In this randomized trial comparing apixaban and VKA in patients with AF on hemodialysis with long follow-up, no differences were observed in safety or efficacy outcomes. Even on oral anticoagulation, patients with AF on hemodialysis remain at high risk of cardiovascular events. Larger randomized trials are needed to determine the optimal anticoagulation regimen for patients with AF on hemodialysis.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02933697.
Keywords: atrial fibrillation, randomized controlled trial, renal dialysis, stroke
Clinical Perspective.
What Is New?
In this randomized trial, no differences in safety or efficacy were observed between apixaban 2.5 mg BID and vitamin K antagonists in patients with atrial fibrillation on chronic hemodialysis.
Event rates in patients with atrial fibrillation on hemodialysis remain high on oral anticoagulation, including death or a clinically relevant bleeding event (48.5%) and stroke, myocardial infarction, systemic or pulmonary embolism, or death (26.8%).
What Are the Clinical Implications?
Our data support consideration of apixaban for prevention of cardiovascular complications in patients with atrial fibrillation on chronic hemodialysis, but larger studies are needed.
Additional interventions need to be developed to reduce the very high risk of thromboembolic and bleeding events in this population.
Oral anticoagulation (OAC) by non–vitamin K antagonist oral anticoagulants (NOACs) is the first-line therapy for most patients with atrial fibrillation (AF) to prevent stroke and systemic embolism on the basis of robust data demonstrating equal efficacy with less severe bleeding compared with vitamin K antagonists (VKA).1,2 Exceptions for therapy with NOACs are patients with mechanical heart valves1–3 or rheumatic heart disease.1,2,4 The available trials provide good evidence for the safety and efficacy of NOACs in patients with mild to severe renal failure (chronic kidney disease [CKD] stage II through IV) but excluded individuals with end-stage kidney disease (ESKD) on chronic hemodialysis. Therefore, whether NOACs are safe and effective in patients with AF on hemodialysis is uncertain.5,6 This is of relevance, as patients on hemodialysis who are initiated on VKA show a high risk of bleeding.6–9 Moreover, use of VKA accelerates vascular calcifications in patients with CKD and especially those on hemodialysis, thus further contributing to advanced arteriosclerosis.10 In rare cases, use of VKAs in hemodialysis may lead to the life-threating complication of calciphylaxis.11 Therefore, alternatives for VKA are of growing interest because with the increasing prevalence of older age, AF, and cardiovascular comorbidities, the group of patients with terminal renal failure is also growing rapidly.12,13
Some retrospective and small, prospective, nonrandomized studies in the past suggested that NOACs might be safe and effective in patients on hemodialysis.14–16 The NOAC apixaban is mainly eliminated by means of metabolization and excretion in the liver, rendering apixaban a suitable NOAC in patients with AF on hemodialysis. The investigator-initiated, multicenter, PROBE (prospective randomized open blinded end point) outcome assessment trial AXADIA–AFNET 8 (Compare Apixaban and Vitamin K Antagonists in Patients With Atrial Fibrillation and End-Stage Kidney Disease) compared the NOAC apixaban (2.5 mg BID) with VKA therapy in patients with AF on hemodialysis.
Methods
The rationale and design of AXADIA–AFNET 8 was described previously in detail.17 The primary aim was to compare the safety of the factor Xa inhibitor apixaban with the VKA phenprocoumon in patients with AF on chronic hemodialysis. Phenprocoumon is the most common VKA in Austria, Germany, and Switzerland (>99% of all patients with VKA).
In brief, the trial was conducted in hemodialysis centers in Germany as an investigator-initiated, prospective, parallel-group, single country/national, multicenter phase 3b trial. Because VKA therapy requires repetitive international normalized ratio (INR) measurements to adjust dosage, the study was performed with open-label administration of study drugs. All outcome measures were centrally adjudicated blind to randomization group and anticoagulation therapy.
The trial was conceived, designed, and led by the steering committee (a list of members is provided in the Supplemental Material). The responsible sponsor of the trial was Kompetenznetz Vorhofflimmern eV (AFNET [Atrial Fibrillation Network]; www.kompetenznetz-vorhofflimmern.de), Muenster, Germany. A contract research organization (Proinnovera; Muenster, Germany) and a data management service (GCP-Service; Bremen, Germany) delivered the trial. AXADIA–AFNET8 was financed by Bristol Myers Squibb (Munich, Germany) and Pfizer (Berlin, Germany). The funders did not influence the design, conduct, or interpretation of the trial. All data will be made available upon request after confirmation of regulatory requirements from the sponsor (info@af-net.eu).
Registration, Ethical Approval, and Boards
The trial is registered at EudraCT (Unique identifier: 2015-005503-84) and clinicaltrials.gov (URL: https://www.clinicaltrials.gov; Unique identifier: NCT02933697).
The study protocol was approved by the ethical committee of the Landesaerztekammer (Medical Association) Westfalen-Lippe and the Medical Faculty of the University of Muenster, Germany (reference No. 2016-598-f-A) and by the local ethical boards of all participating sites. Written informed consent was obtained from all patients before study participation, including their consent for long-term follow-up. During the study, all participants were covered by patient insurance contracted with CNA Insurance Company Limited, Cologne, Germany.
A data and safety monitoring board supervised this study (a list of members is provided in the Supplemental Material).
All events that could potentially constitute safety and efficacy outcomes were centrally reviewed and adjudicated by a blinded end point assessment committee (a list of members is provided in the Supplemental Material).
The statistical analysis plan was developed and signed before database lock and unblinding. The full study protocol and statistical analysis plan are available in the Supplemental Material.
Research Hypothesis
The primary goal of this study was to compare the safety of apixaban (2.5 mg BID) with VKA therapy (target range, INR of 2.0 to 3.0). AXADIA was planned in 2013. With regard to the dosage of apixaban within the trial, at that time, no published data were available, nor was the later US Food and Drug Administration label recommending apixaban (5 mg BID) also in patients on hemodialysis. An unpublished pharmacokinetic simulation projected that taking 5 mg BID would lead to higher apixaban exposure, and the lower dose of 2.5 mg BID would lead to a slightly lower apixaban exposure compared with individuals without kidney disease. These considerations, in conjunction with the general high rate of ischemic and bleeding events in hemodialysis, led the steering committee in December 2013 to decide to use apixaban (2.5 mg BID) in the study. The initial hypothesis of the study was that OAC with apixaban would be at least noninferior to VKA therapy in patients with AF on hemodialysis with respect to the safety outcome and effectiveness in stroke prevention would be not inferior.
Patients, Study Design, and Randomization
Adult patients with AF on chronic hemodialysis were centrally and electronically randomized to OAC with apixaban or phenprocoumon in a 1:1 ratio. Key inclusion and exclusion criteria were reported previously17 and included AF documented on at least 2 ECGs at different days, an increased stroke risk estimated by CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 years, diabetes, stroke or transient ischemic attack, vascular disease, age 65 to 74 years, female sex) ≥2, and age ≥18 years. Key exclusion criteria included stroke within 3 months of enrollment, hemodialysis for <3 months, moderate or severe aortic or mitral stenosis, conditions other than AF requiring anticoagulation, active endocarditis, planned AF or flutter ablation, active bleeding, serious bleeding <6 months before enrollment, uncontrolled diabetes, history of cancer, and need for chronic aspirin therapy.
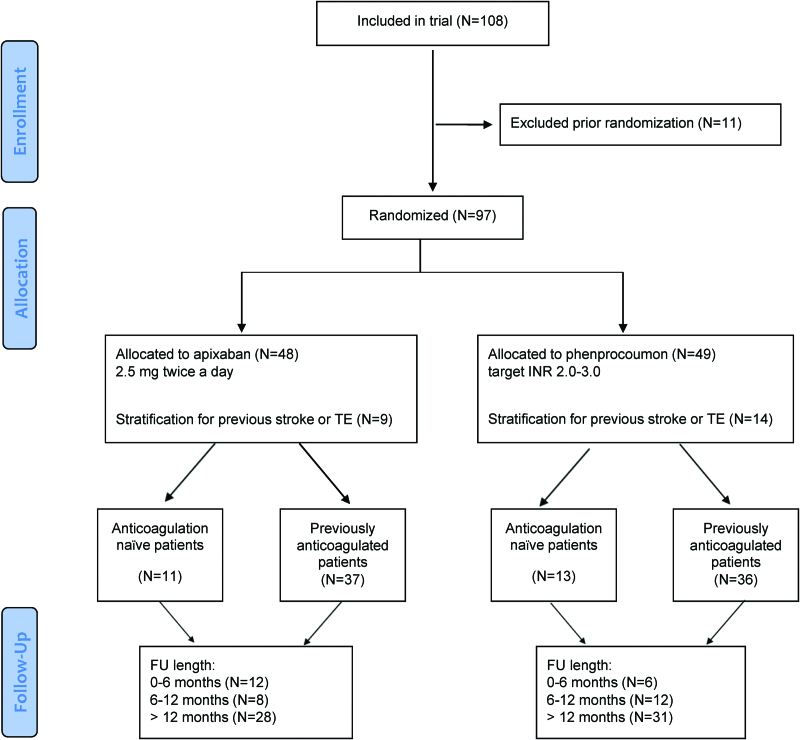
The randomization was stratified by 2 conditions: first, patients with a previous ischemic stroke who met the above criteria were included after >3 months if they were not severely handicapped, as indicated by a value of 0 or 1 on the modified Rankin Scale,18 and were then stratified to assure equal distribution within the groups. Second, randomization was stratified for previous OAC therapy at baseline (anticoagulation-naïve). Patient distribution over these strata is presented in Table S1. The study design and patient allocation were reported in accordance to the CONSORT (Consolidated Standards of Reporting Trials) guideline (patient flow is shown in detail in Figure 1).
Figure 1.
Outline of the AXADIA–AFNET 8 study according to the CONSORT guideline. The included patients and their allocation to the 2 treatment arms are shown in accordance with the CONSORT (Consolidated Standards of Reporting Trials) criteria. AXADIA–AFNET 8 indicates Compare Apixaban and Vitamin K Antagonists in Patients With Atrial Fibrillation and End-Stage Kidney Disease; FU, follow-up; INR, international normalized ratio; and TE, thromboembolism.
Outcomes
The primary outcome measure was a composite of all-cause death, major bleeding events, and clinically relevant, nonmajor bleeding in accordance with the International Society of Thrombosis and Hemostasis consensus.17,19
Secondary outcomes were the efficacy of apixaban compared with phenprocoumon regarding prevention of thromboembolic events, assessed as a composite of myocardial infarction, ischemic stroke, all-cause death, and deep vein thrombosis or pulmonary embolism.17
The primary analysis set consisted of all patients who were randomized and received at least one dose of the study drug. A sensitivity analysis compared events on-treatment (ie, while taking study medication or at maximum 2 days after last intake of the study medication). We report descriptive statistics and analysis for all events and for the events on treatment separately.
Statistics
Continuous variables are presented groupwise by mean±SD as well as median and interquartile range (Q1 and Q3). Skewed data were tested by nonparametric Mann-Whitney U tests; otherwise, t tests were used. Categorical variables are presented by counts and percentages and compared by χ2 or Fisher exact tests, as appropriate. The primary efficacy outcome as well as the secondary outcomes (events of special interest and thromboembolic events as well as all-cause and cardiovascular mortality) were analyzed in the full analysis set using the intention-to-treat (ITT) principle. The primary analysis population consisted of all patients who were randomized and received at least one dose of the study drug. The full statistical analysis plan is included in the Supplemental Material. Event rates were expressed descriptively and by inferential statistics. No adjustment for multiplicity was provided for these analyses.
Two primary statistical null hypotheses were tested in a hierarchical multiple comparison procedure, which controls the familywise error rate, with respect to the primary safety outcome:
H0noninferiority: hazard ration (HR) ≥1.25 versus H1noninferiority: HR <1.25 (proof of noninferiority of apixaban)
H0superiority: HR ≥1 versus H1superiority: HR <1 (proof of superiority of apixaban)
HR denotes the corresponding hazard ratio of the apixaban treatment versus phenprocoumon treatment. The multiple 1-sided significance level was set to α=2.5%. The null hypothesis H0noninferiority was first tested at the local significance level α(H0noninferiority)=2.5%. If the initial hypothesis H0noninferiority could be rejected, subsequently, the hypothesis H0superiority would be tested on a local significance level of 2.5%. The primary statistical analysis provides confirmatory statistical evidence.
H0superiority was analyzed in the full analysis set that consists of all randomized patients, including patients with any kind of protocol violations, applying the ITT principle. H0noninferiority was further analyzed in the per protocol (PP) population, excluding patients with relevant protocol violations. Additional analyses were conducted in the on-treatment population, excluding events that occurred while not receiving study medication. Statistically significant noninferiority was defined as significant differences in both the ITT analysis and the PP analysis. P values for the 2 primary null hypotheses were computed using the z score, z=([ln(HR)-lnM)]/SE (with M as either the noninferiority margin [M=1.25] or the superiority margin [M=1]). Confirmatory evidence was provided by the sequentially rejective testing procedure described previously. All other (2-sided) P values ≤0.05 were considered statistically noticeable. For all time-to-event models, HRs and 2-sided 95% confidence limits were reported. A statistical analysis plan was signed before unblinding of the data and is included in the Supplemental Material.
Sample Size and Power Calculation
AXADIA–AFNET 8 was planned to show noninferiority of the primary safety outcome with 80% power with 64 events. Given the expected combined HR of the composite outcome (HR=0.618), 75 patients would have been sufficient to reach the number of events (on the basis of the log-rank test and the Schoenfeld formula20). To adjust for an approximate loss-to-follow-up rate of 30%, 108 patients were planned to be recruited. At the study start in June 2017, the sample size calculation originally included 222 patients to reach a sufficient power for the superiority null hypothesis. After a blind review of recruitment and event rates in 2020, and in view of newly published data,21 the sample size was changed by an amendment to supply sufficient power for testing the noninferiority null hypothesis. The primary testing strategy and all other aspects of the study design remained unchanged. The data analysis for this article was performed using SAS software (version 9.4, SAS/STAT 14.3; SAS Institute Inc).
Results
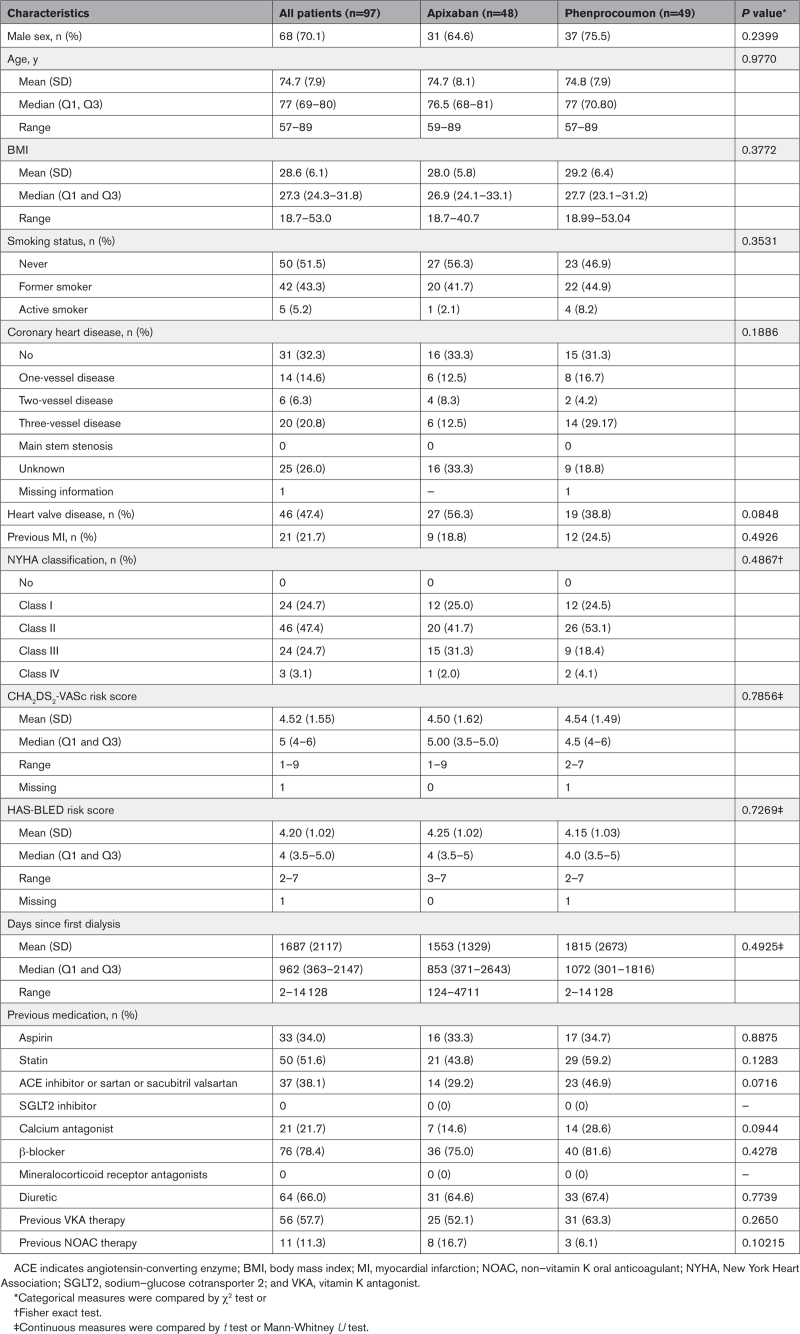
Between June 15, 2017, and May 31, 2022, a total of 108 patients were screened in 39 sites throughout Germany. Of these, 97 patients were randomized to treatment with either apixaban (n=48) or VKA (n=49; Figure 1). A total of 70% of the patients were male, and the average age was 74.7 years (SD 7.9). Mean CHA2DS2-VASc score was 4.5. All randomized patients were treated. Baseline characteristics were well balanced between randomized groups, including the criteria used for stratification (Table 1 and Table S1). The trial was terminated at the planned maximum duration on July 31, 2022.
Table 1.
Baseline Characteristics
Follow-Up
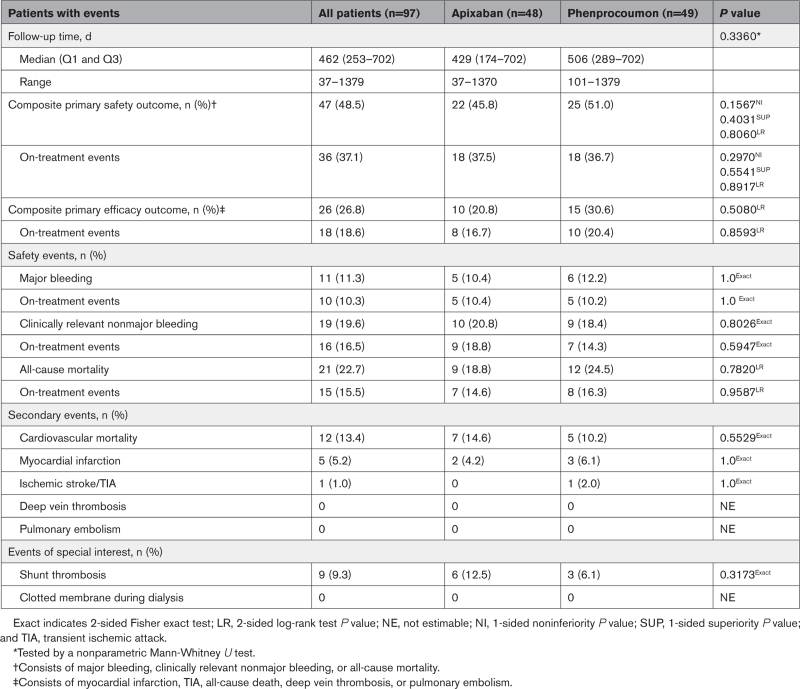
The median follow-up time was 429 days (interquartile range, 174; 702 days) on apixaban therapy and 506 days (interquartile range, 289; 702) on phenprocoumon (Table 2). A total of 60.8% of patients (59/97) were followed up for >12 months after randomization; the maximal follow-up time was 1370 days on apixaban and 1379 days on VKA.
Table 2.
Follow-Up and Outcomes
Primary Safety Outcome
Regarding the primary safety outcome, 47 patients (48.5%) had a least one safety event (major bleeding, clinically relevant nonmajor bleeding, or all-cause death). Of these, 22 events occurred in patients randomized to apixaban and 25 events occurred in patients randomized to VKA (Table 2). With respect to on-treatment events, we observed 18 events on apixaban treatment and 18 safety events on VKA treatment (Table 2). The event rate for the composite primary safety outcome in all patients was 36.4 (apixaban 36.1 versus VKA 36.6) per 100 patient-years. The event rate for all-cause mortality in all patients was 16.2 (apixaban 14.8 versus VKA 17.6) per 100 patient-years. There were no differences in all-cause mortality between the 2 groups (apixaban 18.8% versus VKA 24.5%; Table 2).
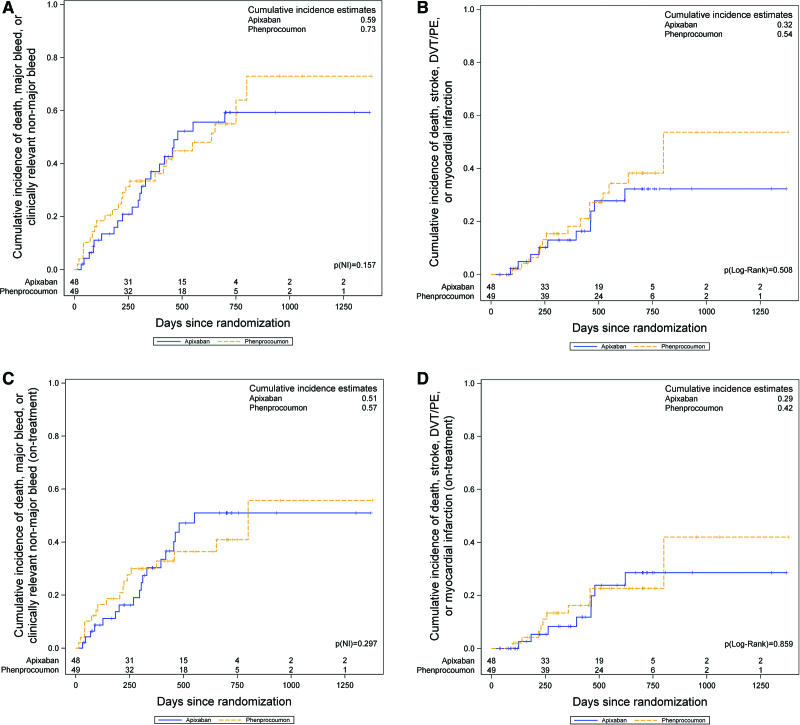
Outcomes were analyzed by Kaplan-Meier estimates comparing the composite primary safety outcome (Figure 2A) and the composite primary efficacy outcome (Figure 2B); the on-treatment results for both outcomes are shown in Figure 2C and 2D. Cumulative incidences of the composite primary safety outcome in the full analysis set was 59% on apixaban and 73% on VKA (Figure 2A); the on-treatment results were 51% and 57%, respectively (Figure 2C).
Figure 2.
Cumulative incidence of the composite primary safety and efficacy outcome. A, Cumulative incidence (inverse Kaplan-Meier) for the primary composite safety outcome (major bleeding, clinically relevant nonmajor bleeding, or all-cause death) in the intention-to-treat population. According to the null hypothesis, the 1-sided noninferiority P value Pnoninferiority is given. B, Cumulative incidence for the primary composite efficacy outcome (myocardial infarction, ischemic stroke, all-cause death, cardiovascular death, and deep vein thrombosis [DVT] or pulmonary embolism [PE]) is shown and compared with a 2-sided log-rank test. C and D, The same results for the on-treatment analysis.
The Cox proportional hazard model for the primary safety outcome incorporating all events estimated an HR of 0.931 (95% CI, 0.525–1.651). An assessment of the proportionality assumption of the Cox regression model is presented in Figure S1. This translates into a noninferiority P value of Pnoninferiority=0.157. Thus, noninferiority could not be shown, and the hierarchical testing procedure was stopped. The predefined PP analysis (1 patient excluded because of low CHA2DS2-VASc risk [<2] at inclusion) showed a consistent result (HR, 0.895 [95% CI, 0.501–1.599]; Pnoninferiority= 0.130).
We performed another sensitivity PP analysis by excluding 7 patients with protocol violations with respect to treatment switches and one patient with respect to the violated inclusion criterion. This analysis is considered an exploratory sensitivity analysis. Details on the additionally excluded patients can be found in Table S2. The exploratory sensitivity PP analysis showed a consistent result (HR, 0.941 [95% CI, 0.518–1.710]; Pnoninferiority= 0.176; Table S1) with the ITT and the preplanned PP analysis. The Cox proportional hazard model for the on-treatment analysis of the primary safety outcome results in an HR of 1.046 (95% CI, 0.544–2.012; Pnoninferiority= 0.297).
The predefined stratified analyses with regard to previous thromboembolism and anticoagulation-naïve patients showed that without previous thromboembolism in the patient’s medical history, apixaban had a similar profile as in the main analysis. Interpretation of the strata with previous thromboembolism was restricted by low power but showed a slight shift of the HR toward favoring VKA (all P>0.05; see Table S1).
Primary Efficacy Outcome
Overall, 26 patients (26.8%) exhibited at least 1 efficacy event (Table 2). Of these, 10 events occurred on apixaban treatment and 15 events occurred on VKA treatment. Most events were cardiovascular deaths (Table 2). The event rate for the composite primary efficacy outcome in all patients was 20.1 (apixaban 16.4 versus VKA 22.0) per 100 patient-years. There was no difference in time to primary efficacy outcome between patient groups (Figure 2B); the on-treatment results are shown in Figure 2D.
Cumulative incidences of the composite primary efficacy outcome in the full analysis set was 32% on apixaban and 54% on VKA (Figure 2B); the on-treatment results were 29% and 42%, respectively (Figure 2D).
The Cox proportional hazard model of all events estimated an HR of 0.764 (95% CI, 0.343–1.700; P=0.5080). The on-treatment analysis of the efficacy events showed an HR of 0.920 (95% CI, 0.363–2.331; P=0.8593) and was thus also consistent with the analysis of all events.
Quality of Anticoagulation
We assessed the quality of anticoagulation by drug accountability for apixaban and the time in therapeutic range (TTR) on the basis of the regular INR controls for phenprocoumon. The TTR was calculated by the Rosendaal method.22
In both study arms, all randomized patients started the assigned treatment (ITT). The median therapy duration (first to last intake) was 355.5 days (range, 3 to 1337 days). Of 48 patients randomized to apixaban, 44 adhered to the intake of medication according to protocol; 4 patients missed >20% of the apixaban doses during the therapy. A detailed presentation of the apixaban intake is given in Figure S2. The median time in target range (TTR) in patients randomized to VKA was 50.7% (range, 0 to 100%; Table S3). Three of the 49 patients randomized to VKA never reached the INR target range (INR, 2 to 3) during the study; 14 of 49 patients achieved a TTR >66% during the study. A detailed presentation of the INR values and TTRs is given in Figure S3.
Discussion
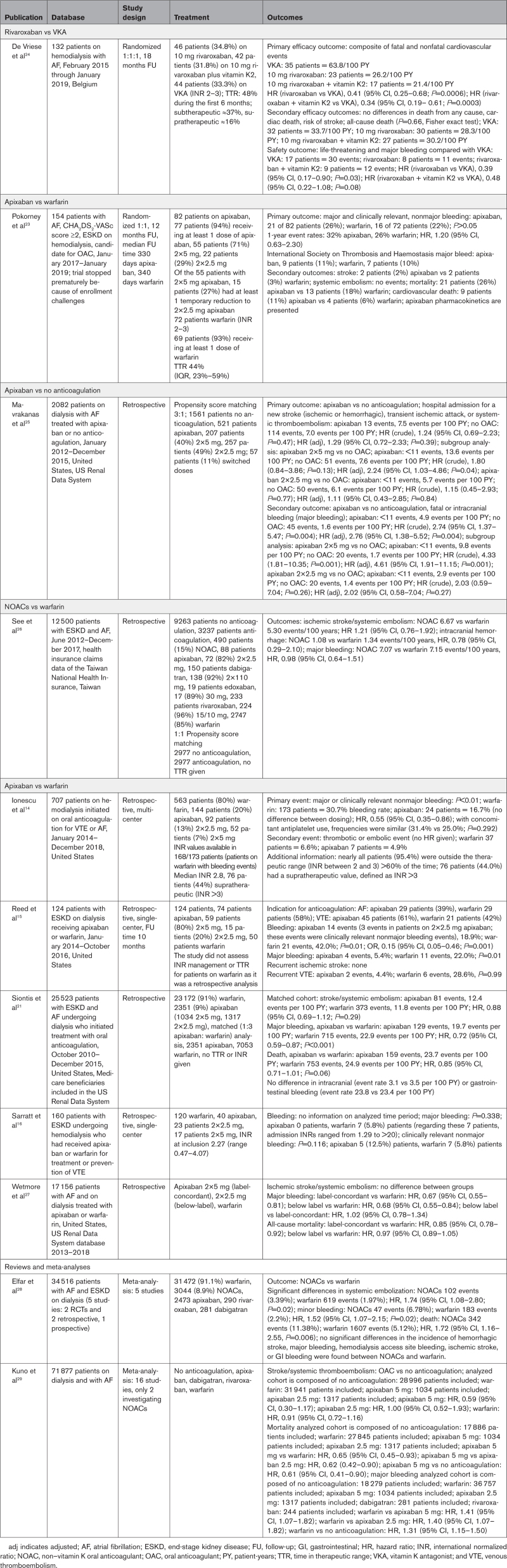
In our AXADIA–AFNET 8 trial, treatment with apixaban (2.5 mg BID) showed no apparent differences in safety and efficacy compared with VKA therapy in patients with AF on chronic hemodialysis (Table 2 and Figure 2A through 2D), although the prespecified noninferiority test requirements were not met because of slow enrollment. Our results cannot exclude a clinically relevant difference with the desirable precision because of a lower number of events than planned. The similarity of the observed event rates supports our conclusion. In view of the paucity of randomized trials in this field (Table 3), our results have clinical implications by providing clinicians with randomized trial data that appear to justify the use of either apixaban or VKA in patients with AF on hemodialysis.
Table 3.
Overview of Studies Analyzing Oral Anticoagulation in Patients on Chronic Hemodialysis
Our results illustrate that patients on chronic hemodialysis with AF remain at high risk of thromboembolic and bleeding events on OAC, calling for the development of additional interventions to reduce these high event rates in this high-risk population.
Comparison With Other Trials and Studies
When AXADIA–AFNET 8 was initiated, there were neither observational studies nor randomized trials comparing NOACs with VKA in patients with AF on hemodialysis. While our trial was running, 2 other randomized trials comparing NOAC with VKA therapy were started.
RENAL-AF (Renal Hemodialysis Patients Allocated Apixaban Versus Warfarin in Atrial Fibrillation), a US study of apixaban, was stopped early in 2019 after randomizing 154 patients because of severe recruitment problems; the results are published in this issue of Circulation.23 In brief, the patients in the US apixaban trial were an average of 10 years younger and had a slightly lower mean CHA2DS2-VASc score of 4.0 compared with 4.5 in our trial. RENAL-AF found no significant differences between apixaban and VKA regarding safety and efficacy, with 1-year Kaplan-Meier estimates for bleeding of 32% for apixaban and 26% for VKA. RENAL-AF used dosages of 5 mg and 2.5 mg apixaban BID, whereas AXADIA–AFNET 8 only used 2.5 mg of apixaban BID. The number of events is too small to draw conclusions, but rates of major bleeding were high in both AXADIA–AFNET 8 and RENAL-AF. Small differences in bleeding events are most likely attributable to chance in addition to potential differences between treatment with 2.5 mg and 5 mg apixaban BID in the US trial.23 The pharmacodynamics data reported in RENAL-AF suggest that the 2.5-mg BID dose tested in AXADIA–AFNET 8 results in plasma concentrations that are comparable to those observed in patients without kidney disease, whereas the 5-mg BID dose results in plasma concentrations comparable to those observed in patients with CKD.23
Another published trial with 132 patients compared VKA with 10 mg of rivaroxaban and 10 mg of rivaroxaban combined with vitamin K2 in 1:1:1 fashion at 3 Belgian centers.24 Although in the Belgian trial, the treatment groups were only slightly smaller than in our trial, the investigators found a significantly lower event rate for both bleeding and thromboembolism on rivaroxaban, resulting in HRs of 0.39 and 0.41, respectively: their event rates per 100 patient-years for the primary efficacy outcome were 63.8 in the VKA group, 26.2 in the 10-mg rivaroxaban group, and 21.4 in the 10-mg rivaroxaban plus vitamin K2 group, whereas we observed only 22.0 in the VKA group and 16.4 in the apixaban group. The event rates for all-cause death in the Belgian trial were 33.7 in the VKA group and 28.3 and 30.2 in the 2 rivaroxaban groups, respectively, whereas we observed 17.6 in the VKA group and 14.8 in the apixaban group. Therefore, our event rates in the VKA group were markedly lower than in the Belgian trial, which may be one reason they found a significant benefit for rivaroxaban.24
Of note, in AXADIA–AFNET 8 and in the other 2 randomized controlled trials,23,24 the mean TTR in the VKA arm was comparable but low (50.7%, 44%, and 48%, respectively), which is lower than the TTR achieved in patients with remaining excretion by the kidneys, although patients on hemodialysis see their doctors 3 times per week. The low TTR also lends support to the use of NOACs in this population.
The primary safety outcome in AXADIA–AFNET 8 comprised relevant safety events, similar to other trials comparing different anticoagulants. The efficacy outcome in the phase III NOAC studies only counted stroke and systemic embolism. The main efficacy outcome of AXADIA–AFNET 8 combined cardiovascular death, stroke, myocardial infarction, pulmonary embolism, and deep vein thrombosis. This combination of events was chosen by the steering committee because each of these outcomes seems relevant for patients and for health care systems and each can be influenced by anticoagulants.
An overview of the key data of these 2 randomized trials,23,24 the few retrospective comparisons of NOACs compared with VKA,14–16,21,25–27 and 2 meta-analyses of the available observational data in patients on hemodialysis28,29 are summarized in Table 3. Taken together, the 3 randomized controlled trials comparing NOAC therapy with VKA, including our trial, found no evidence that NOAC therapy would be unsafe or less effective than VKA. The majority of the observational studies that included patients not receiving OAC also support the use of OAC in patients with AF and stroke risk factors on hemodialysis (Table 3). For considerations about choosing the reduced apixaban dose in our trial, see the Research Hypothesis in the Methods. RENAL-AF provided pharmacokinetic data from the patients on hemodialysis receiving 2.5 mg BID and 5 mg BID, which shows that the dosage of 2.5 mg BID, as chosen in AXADIA–AFNET 8, was associated with identical plasma levels (steady-state area under the curve, 0 to 12) compared with individuals with normal kidney function receiving 5 mg BID.23 These results of RENAL-AF viewed in context of the other published data do not provide a substantial signal supporting one or the other NOAC dose.
Strengths and Weaknesses
Strengths of AXADIA–AFNET 8 are the prospective recruitment in 39 hemodialysis centers in Germany, randomized comparison of a standardized NOAC therapy with apixaban (2.5 mg BID) with intensively controlled VKA therapy, and the high event rate. Careful capture of events and central, blind adjudication of events in a rigorous PROBE design is another strength of the data. The adherence to the assigned drug therapy was high in both arms: all patients received the assigned therapy after randomization (only 4 of 48 patients in the apixaban arm showed some deviations). The TTR in the VKA arm was lower (50.7%) than the TTR in large NOAC trials testing anticoagulation in patients not on hemodialysis. This low TTR is a limitation of the trial but also seems to reflect the usual quality of VKA therapy in patients with AF on hemodialysis because it is comparable to the TTR in RENAL-AF (44%),23 the Belgian trial (48%),24 and other reports of VKA (Table 3). Thus, VKA therapy seems to be difficult to deliver in patients on hemodialysis despite regular visits with coagulation checks during hemodialysis sessions, as also observed in the 2 other randomized trials.
AXADIA–AFNET 8 was not able to recruit the planned number of patients, requiring an adjustment of the planned number of patients on the basis of a blind review of recruitment and events. The high event rate partially compensated for the slow recruitment, but the study was stopped after reaching the maximal defined duration, observing 48 of the planned 64 events. Because of failure to reach the calculated number of patients and events, our study missed the predefined noninferiority margin for apixaban to VKA regarding safety in the full analysis set (1-sided Pnoninferiority=0.1567) and the on-treatment analysis (1-sided Pnoninferiority=0.297; Figure 2A and 2C). Given the observed treatment effect and using the Schoenfeld formula,20 a total of 177 patients (instead of 64) with a relevant safety event would have been necessary to achieve a CI that would have been able to prove noninferiority. With regard to thromboembolic events, as indicated by the composite primary efficacy outcome, there was no signal for differences between the 2 treatment groups (Table 2 and Figure 2B and 2D).
The trial lacked a third arm with no anticoagulation. Thus, the results cannot clarify whether OAC is indicated in patients with AF on hemodialysis. In view of the high thromboembolic event rate observed in AXADIA–AFNET 8, and in context with observational data (Table 3), the initial design without a third arm remains justifiable.
Article Information
Acknowledgments
The authors thank Bristol Myers Squibb, Pfizer, Drs Michael Krekler and Martin Sommer, Proinnovera GmbH, GCP-Service, ZKS (Zentrum für Klinische Studien Muenster), and Drs Eva Freisinger and Katrin Gebauer for their support; as well as the site teams who contributed to recruitment and follow-up and the patients who participated in the trial.
Sources of Funding
AXADIA–AFNET 8 (Compare Apixaban and Vitamin K Antagonists in Patients With Atrial Fibrillation and End-Stage Kidney Disease) was funded by Bristol Myers Squibb and Pfizer.
Paulus Kirchhof was partially supported by European Union BigData@Heart (grant agreement EU IMI 116074), AFFECT-AF (grant agreement 847770), MAESTRIA (grant agreement 965286), British Heart Foundation (grants PG/17/30/32961, PG/20/22/35093, AA/18/2/34218), German Centre for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK), Deutsche Forschungsgemeinschaft (grant Ki 731/4-1), and Leducq Foundation.
Disclosures
Dr Bauersachs reports consulting/lecture fees from Bayer, Bristol Myers Squibb (BMS), Daiichi-Sankyo, Pfizer, and VIATRIS, all outside the submitted work; and research support by the Bavarian State Ministry of Health. Until 2019, Dr Breithardt received speaker honoraria from BMS and Pfizer; he has been a member of scientific advisory boards for BMS, Pfizer, and Bayer Health Care; and during his chairmanship of the AFNET, this institution has received funding for investigator-initiated trials from various companies (for details, please consult http://www.kompetenznetz-vorhofflimmern.de/en/research). Dr Engelbertz has received travel support from Bayer Vital and Abbott outside the submitted work. Dr Haeusler reports speaker honoraria, consulting fees, lecture honoraria, or study grants from Abbott, Alexion, Amarin, AstraZeneca, Bayer Healthcare, Sanofi, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, Bristol Myers Squibb, Biotronik, Medtronic, Portola, Getemed AG, Premier Research, WL Gore and Associates, SUN Pharma, and Edwards Lifesciences. Dr Hewing has received speaker honoraria from BMS, Pfizer, Novartis, Sanofi, Amgen, Daiichi-Sankyo, Berlin Chemie, Bayer, and Astra Zeneca. Dr Reinecke has received speaker honoraria from NeoVasc, Corvia, BMS, MedUpdate, StremedUp, NephroUpdate, and Pfizer; has acted as a consultant for BMS, Pfizer, and Pluristem, receiving in part also financial compensation for this work; has received research grants from the German Federal Ministry for Education and Research (BMBF); and his division within the University Hospital of Muenster has taken or is still taking in multicenter trials of BARD, Bayer, BIOTRONIK, Novartis, and Pluristem, receiving patient fees and financial compensation for these efforts. Dr Gerß has received honoraria from TESARO, QUIRIS Healthcare, Ecker+Ecker, Dr August Wolff, Roche, University Hospital Schleswig-Holstein, and RWTH Aachen University. Dr Rump has received speaker honoraria from Astellas, Baxter, Bayer, Boehringer, Fresenius, StreamedUp, NephroUpdate, and Novartis; has acted as a consultant for Bayer, Medtronic, Novartis, and ReCor, receiving financial compensation for this work; and his department within the University Hospital of Duesseldorf has taken or is still taking in multicenter trials of Bayer, Oxford University, Medtronic, and ReCor, receiving patient fees and financial compensation for these efforts. Dr Kirchhof has received research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), German Centre for Cardiovascular Research, and several drug and device companies active in atrial fibrillation and has received honoraria from several such companies in the past, but not in the past 3 years, and is listed as inventor on 2 patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). Dr Wanner does not report conflicts of interest in respect to the current work; outside this area of research, he has received speaker honoraria from Amgen, Boehringer-Ingelheim, Genzyme-Sanofi, and Shire. Dr Goerlich has received research funding from the European Union and the German Jose Carreras Leukemia Foundation for research unrelated to this trial. The other authors do not report any conflicts of interest.
Supplemental Material
Expanded Methods
Tables S1–S3
Figures S1–S3
Participating sites and local principal investigators
Members of the safety monitoring board
Members of the end point assessment committee
Members of the steering committee
Study protocol
Statistical analysis plan
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- AFNET
- Atrial Fibrillation Network
- AXADIA–AFNET 8
- Compare Apixaban and Vitamin K Antagonists in Patients With Atrial Fibrillation and End-Stage Kidney Disease
- CHA2DS2-VASc
- congestive heart failure, hypertension, age ≥75 years, diabetes, stroke or transient ischemic attack, vascular disease, age 65 to 74 years, female sex
- CKD
- chronic kidney disease
- CONSORT
- Consolidated Standards of Reporting Trials
- ESKD
- end-stage kidney disease
- HR
- hazard ratio
- INR
- international normalized ratio
- ITT
- intention-to-treat
- NOAC
- non–vitamin K oral anticoagulant
- OAC
- oral anticoagulation
- PP
- per protocol
- PROBE
- prospective randomized open blinded end point
- RENAL-AF
- Renal Hemodialysis Patients Allocated Apixaban Versus Warfarin in Atrial Fibrillation
- TTR
- time in therapeutic range
- VKA
- vitamin K antagonist
P. Kirchhof and D. Goerlich contributed equally.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.122.062779.
For Sources of Funding and Disclosures, see page 308.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
Holger Reinecke, Email: holger.reinecke@ukmuenster.de.
Christiane Engelbertz, Email: christianemaria.engelbertz@ukmuenster.de.
Rupert Bauersachs, Email: bauersachs@hotmail.de.
Günter Breithardt, Email: g.breithardt@uni-muenster.de.
Hans-Herbert Echterhoff, Email: nephron@t-online.de.
Joachim Gerß, Email: hoyer@med.uni-marburg.de.
Karl Georg Haeusler, Email: Haeusler_K@ukw.de.
Bernd Hewing, Email: bernd.hewing@gmail.com.
Joachim Hoyer, Email: hoyer@med.uni-marburg.de.
Sabine Juergensmeyer, Email: sabine.juergensmeyer@af-net.eu.
Thomas Klingenheben, Email: klingenheben@aol.com.
Guido Knapp, Email: guido.knapp@tu-dortmund.de.
Lars Christian Rump, Email: christian.rump@med.uni-duesseldorf.de.
Hans Schmidt-Guertler, Email: schmidt-guertler@online.de.
Christoph Wanner, Email: wanner_c@ukw.de.
Paulus Kirchhof, Email: p.kirchhof@uke.de.
Dennis Goerlich, Email: Dennis.Goerlich@ukmuenster.de.
REFERENCES
- 1.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. ; ESC Scientific Document Group. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC): developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 2.Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, et al. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23:1612–1676. doi: 10.1093/europace/euab065 [DOI] [PubMed] [Google Scholar]
- 3.Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K, et al. ; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. doi: 10.1056/NEJMoa1300615 [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Karthikeyan G, Ntsekhe M, Haileamlak A, El Sayed A, El Ghamrawy A, Damasceno A, Avezum A, Dans AML, Gitura B, et al. ; INVICTUS Investigators. Rivaroxaban in rheumatic heart disease-associated atrial fibrillation. N Engl J Med. 2022;387:978–988. doi: 10.1056/NEJMoa2209051 [DOI] [PubMed] [Google Scholar]
- 5.Reinecke H, Brand E, Mesters R, Schaebitz WR, Fisher M, Pavenstaedt H, Breithardt G. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol. 2009;20:705–711. doi: 10.1681/ASN.2007111207 [DOI] [PubMed] [Google Scholar]
- 6.Olesen JB, Lip GY, Kamper AL, Hommel K, Køber L, Lane DA, Lindhardsen J, Gislason GH, Torp-Pedersen C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367:625–635. doi: 10.1056/NEJMoa1105594 [DOI] [PubMed] [Google Scholar]
- 7.Herzog CA, Ma JZ, Collins AJ. Long-term survival of dialysis patients in the United States with prosthetic heart valves: should ACC/AHA practice guidelines on valve selection be modified? Circulation. 2002;105:1336–1341. doi: 10.1161/hc1102.100075 [DOI] [PubMed] [Google Scholar]
- 8.Sood MM, Komenda P, Sood AR, Rigatto C, Bueti J. The intersection of risk and benefit: is warfarin anticoagulation suitable for atrial fibrillation in patients on hemodialysis? Chest. 2009;136:1128–1133. doi: 10.1378/chest.09-0730 [DOI] [PubMed] [Google Scholar]
- 9.Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662–2668. doi: 10.2215/CJN.04550511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy DS, Grewal R, Le TH. Vitamin K deficiency: an emerging player in the pathogenesis of vascular calcification and an iatrogenic consequence of therapies in advanced renal disease. Am J Physiol Renal Physiol. 2020;319:F618–F623. doi: 10.1152/ajprenal.00278.2020 [DOI] [PubMed] [Google Scholar]
- 11.Nigwekar SU, Thadhani R, Brandenburg V. Calciphylaxis. N Engl J Med. 2018;378:1704–1714. doi: 10.1056/NEJMra1505292 [DOI] [PubMed] [Google Scholar]
- 12.Goldstein BA, Arce CM, Hlatky MA, Turakhia M, Setoguchi S, Winkelmayer WC. Trends in the incidence of atrial fibrillation in older patients initiating dialysis in the United States. Circulation. 2012;126:2293–2301. doi: 10.1161/circulationaha.112.099606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bansal N, Fan D, Hsu CY, Ordonez JD, Marcus GM, Go AS. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation. 2013;127:569–574. doi: 10.1161/CIRCULATIONAHA.112.123992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ionescu F, Cooper C, Petrescu I, George J, Mansuri S. Safety of apixaban compared to warfarin in hemodialysis patients: do antiplatelets make a difference? Eur J Haematol. 2021;106:689–696. doi: 10.1111/ejh.13599 [DOI] [PubMed] [Google Scholar]
- 15.Reed D, Palkimas S, Hockman R, Abraham S, Le T, Maitland H. Safety and effectiveness of apixaban compared to warfarin in dialysis patients. Res Pract Thromb Haemost. 2018;2:291–298. doi: 10.1002/rth2.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarratt SC, Nesbit R, Moye R. Safety outcomes of apixaban compared with warfarin in patients with end-stage renal disease. Ann Pharmacother. 2017;51:445–450. doi: 10.1177/1060028017694654 [DOI] [PubMed] [Google Scholar]
- 17.Reinecke H, Juergensmeyer S, Engelbertz C, Gerss J, Kirchhof P, Breithardt G, Bauersachs R, Wanner C. Design and rationale of a randomised controlled trial comparing apixaban to phenprocoumon in patients with atrial fibrillation on chronic haemodialysis: the AXADIA-AFNET 8 study. BMJ Open. 2018;8:e022690. doi: 10.1136/bmjopen-2018-022690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry. 1991;54:1044–1054. doi: 10.1136/jnnp.54.12.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 20.Schoenfeld D. The asymptotic properties of nonparametric tests for comparing survival distributions. Biometrika. 1981;68:316–319. doi: 10.1093/biomet/68.1.316 [Google Scholar]
- 21.Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, Tilea A, Stack AG, Balkrishnan R, Yao X, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138:1519–1529. doi: 10.1161/CIRCULATIONAHA.118.035418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 23.Pokorney SD, Chertow G, Al-Khalidi H, Gallup D, Dignaco P, Mussina K, Bansal N, Gadegbeku C, Garcia D, Merali S, et al. Apixaban for patients with atrial fibrillation on hemodialysis: a multicenter randomized controlled trial. Circulation. 2022;146:1735–1745. doi: 10.1161/CIRCULATIONAHA.121.054990 [DOI] [PubMed] [Google Scholar]
- 24.De Vriese AS, Caluwé R, Van Der Meersch H, De Boeck K, De Bacquer D. Safety and efficacy of vitamin K antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: a multicenter randomized controlled trial. J Am Soc Nephrol. 2021;32:1474–1483. doi: 10.1681/ASN.2020111566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mavrakanas TA, Garlo K, Charytan DM. Apixaban versus no anticoagulation in patients undergoing long-term dialysis with incident atrial fibrillation. Clin J Am Soc Nephrol. 2020;15:1146–1154. doi: 10.2215/cjn.11650919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.See LC, Lee HF, Chao TF, Li PR, Liu JR, Wu LS, Chang SH, Yeh YH, Kuo CT, Chan YH, et al. Effectiveness and safety of direct oral anticoagulants in an Asian population with atrial fibrillation undergoing dialysis: a population-based cohort study and meta-analysis. Cardiovasc Drugs Ther. 2021;35:975–986. doi: 10.1007/s10557-020-07108-4 [DOI] [PubMed] [Google Scholar]
- 27.Wetmore JB, Weinhandl ED, Yan H, Reyes JL, Herzog CA, Roetker NS. Apixaban dosing patterns versus warfarin in patients with nonvalvular atrial fibrillation receiving dialysis: a retrospective cohort study. Am J Kidney Dis. 2022;S0272–6386(22)00621-7. doi: 10.1053/j.ajkd.2022.03.007 [DOI] [PubMed] [Google Scholar]
- 28.Elfar S, Elzeiny SM, Ismail H, Makkeyah Y, Ibrahim M. Direct oral anticoagulants versus warfarin in hemodialysis patients with atrial fibrillation: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:847286. doi: 10.3389/fcvm.2022.847286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuno T, Takagi H, Ando T, Sugiyama T, Miyashita S, Valentin N, Shimada YJ, Kodaira M, Numasawa Y, Briasoulis A, et al. Oral anticoagulation for patients with atrial fibrillation on long-term hemodialysis. J Am Coll Cardiol. 2020;75:273–285. doi: 10.1016/j.jacc.2019.10.059 [DOI] [PubMed] [Google Scholar]