Abstract
Aims
To explore the effect of glucagon-like peptide-1 receptor agonist (GLP-1 RAs) on glycemic control and weight reduction in adults.
Methods
Databases were searched from August 2021 to March 2022. Data were analyzed using mean difference (MD) values with 95% confidence intervals (CIs). Both random-and fixed-effect models were employed. Heterogeneity was explored using pre-specified subgroup analyses and meta-regression. Structural equation modeling fitting was used for the multivariate meta-analysis.
Results
A total of 31 double-blind randomized controlled trials with 22,948 participants were included in the meta-analysis. The MD and 95% CI of the pooled GLP1-RA-induced change in the glycated hemoglobin level was -0.78% (-0.97%, -0.60%) in the random-effects model and -0.45% (-0.47%, -0.44%) in the fixed-effect model, with a high heterogeneity (I2 = 97%). The pooled body weight reduction was -4.05 kg (-5.02 kg, -3.09 kg) in the random-effects model and -2.04 kg (-2.16 kg, -1.92 kg) in the fixed-effect model (I2 = 98%). The standardized pooled correlation coefficient between HbA1c levels and body weight was -0.42. A negative correlation between glycemic control and weight reduction was obtained.
Conclusion
Long-acting GLP-1 RAs significantly reduced the glycated hemoglobin level and body weight in adults.
Introduction
Glucagon-like peptide 1, an incretin secreted from the gut, exerts metabolic effects through glucose-dependent stimulation of insulin secretion, delayed gastric emptying, inhibition of appetite, and increased natriuresis [1]. Glucagon-like peptide receptor agonists (GLP-1 RAs) have been used to treat patients with diabetes since 2007 [2]. and have been approved as anti-obesity drugs since 2014 [3]. However, long-acting GLP-1 RAs have attracted increasing interest due to their better efficacy in diabetes and obesity treatment [4]. Long-acting GLP-1 RA treatment was shown to be associated with a pooled glycated hemoglobin (HbA1c) reduction of 0.99% and a pooled body weight reduction of 2.69 kg (heterogeneity, approximately 90%) [5]. The high heterogeneity can be partially explained by differences in the underlying conditions of participants [6] and the GLP-1 RA interventions [7]. Moreover, participant age and the baseline glycemic level may interact with the results in children [6], indicating the existence of potential effect modifiers. However, further analysis to explore the high heterogeneity and potential effect modifiers in adults is lacking [8, 9].
Glycemic control is intertwined with the weight reduction caused by long-acting GLP-1 RAs through insulin resistance and metabolic changes [10]. Thus, these two outcomes of interest should not be independently estimated. However, previous randomized controlled trials (RCTs) rarely reported the correlation coefficients at the within-study level [11], and to the best of our knowledge, no correlation coefficient was reported in between-study-level meta-analysis [12, 13].
Thus, to explore the high heterogeneity and possible effect modifiers associated with these findings, we performed further univariate meta-analyses of the glycemic control and weight reduction caused by long-acting GLP-1 RAs in adults. Considering the correlation between these outcomes, our study used the structural equation modeling approach for multivariate meta-analysis to jointly estimate the effect sizes for glycemic control and weight reduction in one model and to investigate the associations between these two outcomes of long-acting GLP-1 RA treatment.
Materials & methods
Search strategy and selection criteria
We searched the Medline, Ovid EMBASE, Cochrane Library and ClinicalTrials.gov databases for relevant studies from August 2021 to March 2022 by using the following keywords: “Glucagon-Like Peptide 1” OR “GLP-1” OR “Placebo” OR “Body Weights” OR “Glucose” OR “Glycosylated Hemoglobin A” OR “Trials, Randomized Clinical.” The PRISMA checklist and detailed search strategies are shown in Supplement and S1 Table. To enable a comprehensive search, we did not include limiting parameters for language, article type, year of publication, animal or human subjects, and age of participants.
We included all eligible publications that met the following inclusion criteria: (1) adult participants older than 18 years, either from the general population or including patients with a specific disease; (2) intervention with U.S. Food and Drug Administration approved long-acting GLP-1 RAs, including liraglutide, once-weekly exenatide, dulaglutide, albiglutide, and semaglutide, which were administered orally or subcutaneously, either in same or different doses; (3) comparison with a placebo; (4) glycemic or anthropometric changes as either primary or secondary outcome measures; (5) phase 3 or phase 4 randomized, double-blind, placebo-controlled trials without cross-over or open-label in any study period. We excluded articles that met the following criteria: (1) were duplicated publications or used duplicated populations, such as a post-hoc analysis of an included trial; (2) included participants with other conditions that interfered with outcome assessment, such as pregnancy or weight reduction surgery; (3) assessed other active components in addition to GLP-1 RAs in the treatment arm; (4) performed active comparisons rather than comparisons with placebo; (4) used outcome measures that were not of our interest; (6) reported conference abstracts, review articles, or phase 1 or 2 RCTs. All included trials were assessed for bias using the Cochrane risk-of-bias tool 2.0 [14]. The details of the data extraction in our study were described in supplement (S1 File).
Data were analyzed using the mean difference (MD) with 95% confidence intervals (CIs) for continuous outcomes. For the univariate meta-analysis, we used the statistical software R, version 4.0.3, and the meta package. Both random- and fixed-effect models were employed using DerSimonian and Laird’s method [15]. The results of the meta-analysis are presented in forest plots. Heterogeneity was quantified using the Cochran Q test and I2 statistics [16]. Heterogeneity was explored in pre-specified subgroup analyses by participants’ disease and intervention drugs. Potential effect modifiers were determined in meta-regression analysis. Publication bias was inspected using the symmetry of the funnel plot and Egger’s test [17]. Contour-enhanced funnel plots to enhance the recognition of the causes of asymmetry and trim-and-fill analysis to estimate the effect size were performed if a bias existed. To ensure robustness, a further meta-analysis restricted to articles with a low risk of bias was performed. Since the correlation was not reported in each original study, we set the correlation coefficient between HbA1c level and body weight changes as 0.2, based on a reasonable assumption and previous literature [11]. We used the metaSEM package to fit the structural equation modeling using the maximum likelihood estimation in one step. Effect sizes and effect size variances were the essential arguments to be specified. The results of the multivariate meta-analysis model were visualized by plotting. To explore the direction of the pooled correlation coefficient, we further restricted the multivariate meta-analysis according to participant characteristics. Sensitivity analyses were performed by setting other correlation coefficients and restricting to studies with a low risk of bias.
Results
Description of studies and quality assessment
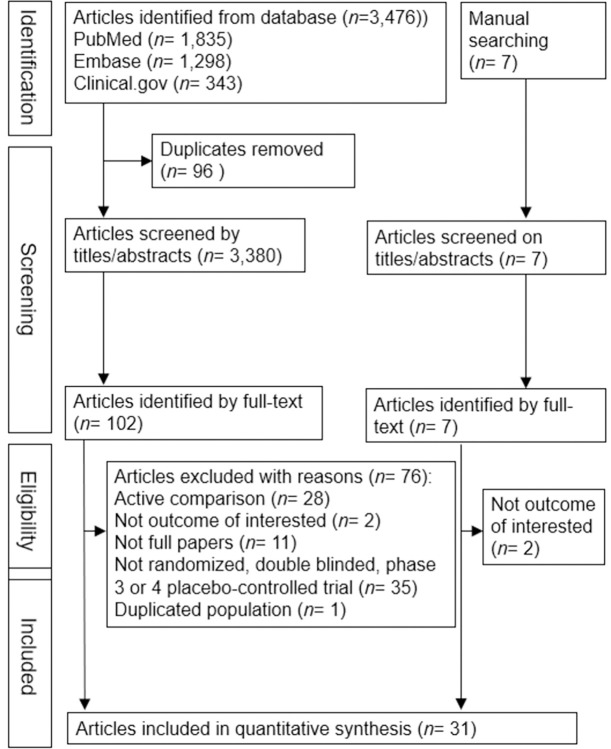
Thirty-one double-blind RCTs [18–47] were included in our meta-analysis (Fig 1). Seven of these were phase 4 trials [24, 25, 29, 35, 38, 42, 43]. The eligible participants ranged from non-diabetic overweight/obese general individuals to patients with schizophrenia [31], obstructive sleep apnea [20], or polycystic ovary syndrome [25]; patients with type 1 diabetes mellitus (DM) [24, 28, 33]; various groups of type 2 DM patients, including drug-naïve patients [19, 41], those treated with insulin [18, 26, 40, 46], those with chronic kidney disease [22, 36], and those with cardiovascular disease [27, 30, 34]. Liraglutide was the most commonly used GLP-1 RA, followed by subcutaneous semaglutide [21, 34, 40, 41, 44, 47], oral semaglutide [19, 30, 36, 46] and dulaglutide [27], once-weekly exenatide [31], and albiglutide [37]. A total of 23,061 participants (mean age, 54.1 years; 54.1% women; baseline body mass index (BMI), 33.7 kg/m2; baseline HbA1c, 7.8%; mean disease duration, 8.2 years; mean study period, 38.1 weeks) were included in the univariate meta-analysis. The baseline characteristics of the included studies are presented in S2 Table. In the GLP-1 RA arm, a total of 12,319 participants (mean age, 54.1 years; 54.4% women, mean baseline BMI, 33.9 kg/m2; mean baseline HbA1c level, 7.7%; mean follow-up duration, 38.1 weeks; mean duration of diabetes, 8.0 years) were included in the multivariate meta-analysis. Most of the included RCTs were assessed as showing high quality with a low risk of bias; only five trials [28, 31, 32, 35, 38] did not use the intention-to-treat analysis and were thus assessed as showing some concerns (S3 Table).
Fig 1. Flowchart of the trial selection process.
Results of the univariate meta-analysis
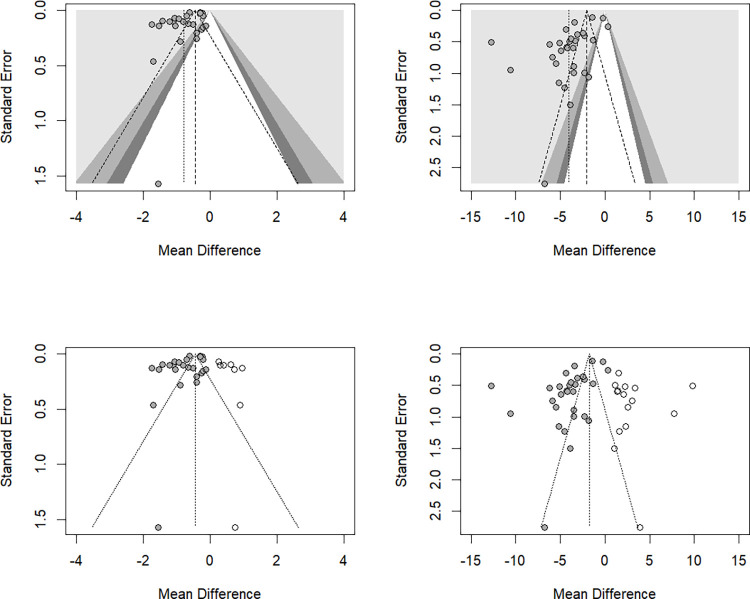
The MD (95% CI) for the pooled HbA1c change caused by GLP-1 RAs was -0.78% (-0.97%, -0.60%) in the random-effect model and -0.45% (-0.47%, -0.44%) in the fixed-effect model, with a high heterogeneity (I2 = 97%). Forest plots are shown in S1 Fig. Subgroup analysis based on participant characteristics showed that the MD (95% CI) for the pooled HbA1c change was -0.99% ([-1.17%, -0.82%], I2 = 94%) in type 2 DM patients, -0.27% ([-0.31%, -0.24%], I2 = 39%) in participants with overweight/obesity, and -0.18% ([-0.35%, -0.01%], I2 = 0%) in type 1 DM patients (S2 Fig). The MD (95% CI) for the pooled HbA1c change was -1.06% ([-1.50%, -0.62%], I2 = 99%) with subcutaneous semaglutide, -0.54% ([-0.76%, -0.33%], I2 = 93%) with liraglutide, -0.94% ([-1.18%, -0.70%], I2 = 89%) with oral semaglutide, and -0.82% ([-1.23%, -0.41%], I2 = 80%) with dulaglutide/exenatide/albiglutide (S3 Fig). Meta-regression (Table 1) showed that the pooled HbA1c reduction significantly interacted with participants’ baseline age (p = 0.032), proportion of female participants (p = 0.017), the baseline HbA1c level (p = 0.018), and gastrointestinal side effect (p = 0.002) but did not interact with baseline body weight or BMI level, duration of diabetes, follow-up period or insulin use. A one-year increase in the participants’ age significantly decreased the pooled HbA1c change by 0.026%; a 1% increase in the proportion of female participants significantly increased the pooled HbA1c change by 0.015%; a 1% increase in the baseline HbA1c level significantly reduced the pooled HbA1c level by 0.262%; and 1% increase in gastrointestinal side effect significantly increase the pooled HbA1c level by 0.017%. The funnel plot (S4 Fig) showed asymmetry, and publication bias was confirmed by Egger’s test (p = 0.004). To ensure that the bias did not contribute to the underlying differences between studies, we further omitted outliers [31], participants without diabetes [31, 44], and participants without type 2 diabetes [24, 28, 33], and the Egger’s test still showed significant bias (S5 Fig). In the assessment of the reasons for this asymmetry, the contour-enhanced funnel plot indicated that studies with a positive MD with any p value were not found (Fig 2). Therefore, we conducted a trim-and-fill analysis to examine the influence of publication bias. The trim-and-fill analysis showed 10 unpublished studies. Considering these unpublished studies, the MD (95% CI) for the pooled HbA1c change was -0.45% (-0.58%, -0.31%), which was also similar to the results of the fixed-effect model. Further analysis by restricting articles with low bias showed robust results (-0.78% [-0.97%, -0.59%], I2 = 98%) (S6 Fig).
Table 1. Univariate meta-regression of the effects of glucagon-like peptide-1 receptor agonists on glycated hemoglobin levels and weight reduction.
| Glycated hemoglobin | Body weight | |||||
|---|---|---|---|---|---|---|
| MD (95% CI) | p | R2(%) | MD (95% CI) | p | R2(%) | |
| Age (years) | -0.026 (-0.049, -0.002) | 0.032 | 17.2 | 0.093 (-0.016, 0.202) | 0.091 | 6.0 |
| Proportion of women (%) | 0.015 (0.003, 0.027) | 0.017 | 17.3 | -0.065 (-0.117, -0.013) | 0.016 | 18.0 |
| Baseline glycated hemoglobin (%) | -0.262 (-0.473, -0.050) | 0.018 | 25.1 | 1.130 (-0.132, 2.393) | 0.077 | 9.6 |
| Baseline body mass index (kg/m2) | 0.021 (-0.045, 0.087) | 0.514 | 0 | -0.217 (-0.553, 0.120) | 0.197 | 3.5 |
| Baseline body weight (kg) | 0.010 (-0.013, 0.033) | 0.374 | 1.8 | -0.045 (-0.157, 0.068) | 0.424 | 0 |
| Duration of diabetes (years) | -0.004 (-0.035, 0.027) | 0.788 | 0 | 0.105 (-0.060, 0.269) | 0.203 | 3.7 |
| Study duration (weeks) | 0.002 (-0.007, 0.011) | 0.660 | 0 | -0.052 (-0.094, -0.010) | 0.018 | 16.2 |
| Gastrointestinal side effect (%) | 0.017 (0.007, 0.027) | 0.002 | 35.1 | -0.059 (-0.110, -0.008) | 0.025 | 15.1 |
| Insulin use (%) | 0.000 (-0.005, 0.005) | 0.863 | 0 | 0.015 (-0.011, 0.040) | 0.249 | 2.2 |
CI, confidence interval; MD, mean difference; R2 (%), percentage of heterogeneity explained
Fig 2. Contour-enhanced and filled funnel plots of glycated hemoglobin level and body weight.
(upper left) Contour-enhanced funnel plot of the pooled glycated hemoglobin level after treatment with a glucagon-like peptide-1 receptor agonist. (lower left) Filled funnel plot of the pooled glycated hemoglobin level after treatment with a glucagon-like peptide-1 receptor agonist. (upper right) Contour-enhanced funnel plot of the pooled body weight after treatment with a glucagon-like peptide-1 receptor agonist. (lower right) Filled funnel plot of the pooled body weight after treatment with a glucagon-like peptide-1 receptor agonist.
The pooled body weight reduction caused by GLP1-RA was -4.05 kg (-5.02 kg, -3.09 kg) in the random-effects model and -2.04 kg (-2.16 kg, -1.92 kg) in the fixed-effect model (I2 = 98%). Forest plots are shown in S1 Fig. Subgroup analysis by participants’ characteristics (S2 Fig) showed that the MD (95% CI) for the pooled body weight change was -3.14% ([-3.84%, -2.44%], I2 = 97%) in type 2 DM patients, -5.77% ([-8.35%, -3.20%], I2 = 99%) in participants with overweight/obesity, and -4.15% ([-5.04%, -3.25%], I2 = 0%) in type 1 DM patients. The MD (95% CI) for the pooled body weight change was -6.58% ([-9.24%, -3.92%], I2 = 98%) with subcutaneous semaglutide, -3.87% ([-4.57%, -3.17%], I2 = 73%) with liraglutide, -2.91% ([-3.48%, -2.34%], I2 = 67%) with oral semaglutide, and -0.47 ([-1.50%, 0.56%], I2 = 98%) with dulaglutide/exenatide/albiglutide (S3 Fig). Meta-regression (Table 1) showed that the pooled body weight reduction significantly interacted with the proportion of female participants (p = 0.016), the follow-up period (p = 0.018) and gastrointestinal side effect (p = 0.025), and had a borderline interaction with participants’ baseline age (p = 0.091) and the participants’ baseline HbA1c level (p = 0.077), but did not interact with baseline BMI level or body weight, insulin use, or the duration of diabetes. A 1% increase in the proportion of female participants significantly decreased the pooled body weight reduction by 0.065 kg; a one-week increase in the treatment duration significantly decreased the pooled body weight reduction by 0.052 kg; a 1% increase in gastrointestinal side effect significantly decreased the pooled body weight reduction by 0.059 kg. Publication bias was present, and the funnel plot and Egger’s test results (p value < 0.001) are shown in S4 Fig. After omitting outliers [45] and restricting the study to participants with diabetes or type 2 diabetes, Egger’s test still showed significant bias (S5 Fig). The contour-enhanced funnel plot also showed the absence of studies with a positive MD, and publication bias existed (Fig 2). The trim-and-fill analysis revealed 15 unpublished studies (Fig 2). Considering these unpublished studies, the MD (95% CI) of the pooled body weight reduction was -1.76 kg (-2.63 kg, -0.88 kg), which was almost half of the current results and similar to the results obtained with the fixed-effect mode. Further analysis by restricting articles with low bias showed similar results: -4.29% ([-5.37%, -3.22%], I2 = 98%) (S6 Fig).
Results of the structural equation modeling multivariate meta-analysis
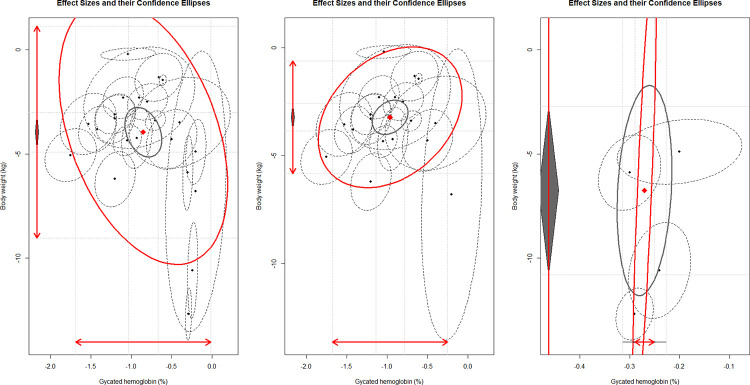
Maximum likelihood estimation worked well in the analysis. Table 2 shows that the pooled HbA1c change induced by GLP1-RAs was -0.85% (95% CI [-1.03%, -0.66%], I2 = 99%), and the pooled body weight change was -4.03 kg (95% CI [-5.11 kg, -2.95 kg], I2 = 99%), which were similar to the results of the univariate meta-analysis. However, overall, the pooled between-study level correlation coefficient between HbA1c and body weight changes from baseline was -0.42, which was the opposite of the within-study level. To explore the negative correlation, we further restricted the multivariate analysis to participants with or without diabetes. The pooled HbA1c change by GLP1-RA was -0.96% (95% CI [-1.14%, -0.79%], I2 = 96%), the pooled body weight change was -3.23 kg (95% CI [-3.86 kg, -2.59 kg], I2 = 95%); the amount of between-study heterogeneity of body weight decreased from 7.36 to 1.77 and the 95% CI became narrower. The pooled correlation coefficient turned to a positive estimate of 0.32. There were only five studies focused on participants without diabetes. The pooled HbA1c change by GLP1-RA was -0.27% (95% CI [-0.31%, -0.23%], I2 = 13%), the pooled body weight change was -6.76 kg (95% CI [-10.81 kg, -2.72 kg], I2 = 99%); the amount of between-study heterogeneity of body weight was much increased to 20.84 with a wide 95% CI due to the limited included articles. However, the pooled correlation coefficient was positive of 0.81. The pooled results for all participants and the results restricted to patients with diabetes are shown in Fig 3. Sensitivity analyses of all participants and patients with diabetes by setting the correlation coefficient to 0.1 and 0.3 and restricting the study selection to studies with a low risk of bias yielded robust results (S4 Table). Further sensitivity analyses of participants without diabetes were not performed due to the limited included articles.
Table 2. The pooled results for glycated hemoglobin level and weight reduction on comparison of glucagon-like peptide-1 receptor agonist and placebo by using the structural equation modeling multivariate meta-analysis according to participant characteristics.
| All participants | Patients with diabetes | Patients without diabetes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Setting r = 0.2 | Estimates (95% CI) | p | I 2 | Estimates (95% CI) | p | I 2 | Estimates (95% CI) | p | I 2 |
| Glycated hemoglobin (%) | -0.85 (-1.03, -0.66) | <0.001 | 99% | -0.96 (-1.14, -0.79) | <0.001 | 96% | -0.27 (-0.31, -0.23) | <0.001 | 13% |
| Body weight (kg) | -4.03 (-5.11, -2.95) | <0.001 | 99% | -3.23 (-3.86, -2.59) | <0.001 | 95% | -6.76 (-10.81, -2.72) | 0.001 | 99% |
| tao of glycated hemoglobin | 0.18 (0.07, 0.30) | <0.001 | 0.13 (0.03, 0.23) | 0.008 | 0 | 0.909 | |||
| tao of body weight | 7.36 (3.10, 11.63) | <0.001 | 1.77 (0.56, 2.99) | 0.004 | 20.84 (-5.32, 47.00) | 0.118 | |||
| Covariance | -0.48 (-1.02, 0.05) | <0.001 | 0.15 (-0.12, 0.43) | 0.281 | 0.04 (-0.14, 0.21) | 0.682 | |||
| Standardized correlation coefficient | -0.42 | 0.32 | 0.81 | ||||||
CI, confidence interval; r, correlation coefficient between glycated hemoglobin and body weight changes within the study level; tao, the variance of effect measure
Fig 3. The pooled effect sizes and their confidence ellipse for changes in glycated hemoglobin level and body weight in patients treated with a glucagon-like peptide-1 receptor agonist and placebo, in participants not restricted and restricted to diabetes.
(left) The pooled effect sizes and their confidence ellipse of the changes in the glycated hemoglobin level and body weight, in a comparison of glucagon-like peptide-1 receptor agonists and placebo in all participants. (middle) The pooled effect sizes and their confidence ellipse for changes in the glycated hemoglobin level and body weight, in a comparison of glucagon-like peptide-1 receptor agonists and placebo in patients with diabetes. (right) The pooled effect sizes and their confidence ellipse for changes in the glycated hemoglobin level and body weight, in a comparison of glucagon-like peptide-1 receptor agonists and placebo in patients without diabetes. x-axis: Effects of glucagon-like peptide-1 receptor agonists on glycated hemoglobin level; y-axis: effects of glucagon-like peptide-1 receptor agonists on body weight changes; black dots: individual studies; ellipses with dashed lines: 95% confidence interval; red diamond: pooled effect and 95% confidence interval; smaller gray ellipse: 95% confidence interval; larger red ellipse: 95% prediction interval.
Discussion
Our meta-analysis demonstrated that long-acting GLP-1 RAs significantly reduced HbA1c levels and body weight in adults. The high heterogeneity in our study might be attributed to the different GLP-1 RAs and the diverse populations ranging from non-diabetic overweight/obese participants to patients with diabetes complicated with end organ damage. The effects of GLP-1 RA were highly correlated with age, sex, baseline condition, treatment duration, and gastrointestinal side effect. For the correlation between glycemic control and weight reduction, the pooled effects were similar, since both effects were estimated independently. However, although the long-acting GLP-1 RA lowered the HbA1c more, it did not cause much decrease in the body weight in our included population. A positive association was found only in a specific condition.
Long-acting GLP-1 RAs showed better efficacy in weight reduction and glycemic control than short-acting GLP-1 RAs [5]. After the first wave of approvals for long-acting GLP-1 RAs from 2009 to 2014 [48–50], semaglutide was approved in 2017 [51], and the oral form of semaglutide was recently approved in 2020 [52]. Thus, meta-analyses published before 2015 [7, 12, 13, 53, 54] did not discuss all the currently available long-acting GLP-1 RAs, while more recent meta-analyses usually targeted semaglutide [8, 9] or focused on emerging outcomes such as cardiovascular or kidney disease [55, 56]. In contrast, our study aimed to investigate the glycemic control and weight reduction caused by long-acting GLP-1 RAs. Previous studies showed high heterogeneity (I2 = 80%-90%) even for findings related to the same GLP-1 RAs [8, 9, 57]. All the potential effect modifiers in our study showed an opposite direction of interaction between glycemic control and weight reduction. Previous meta-analyses have rarely reported this topic and yielded inconsistent results [6, 57]; further studies are warranted to explore a potential effect modifier in the complex combinations between different interventions and target populations. Although unpublished studies (NCT01753362, NCT03480022, NCT02417142, NCT02473809, NCT04325581, NCT03048578, NCT01455441, NCT03466021, NCT04109547, NCT03811574, NCT03693430), withdrawn studies (NCT04057261, NCT02229240), terminated studies (NCT03279731, NCT01628445), studies with an unknown status (NCT01722240, NCT04046822, NCT01722240, NCT02016846, NCT04126603) or those on albiglutide, which was withdraw from the market, all possibly explained the publication bias, the pooled results for the significant HbA1c- and body weight-lowering effects remained robust.
According to a previous within-study-level study [11] and between-study-level network meta-analysis [58], GLP1 RAs showed a higher efficacy for glycemic control, and a compatible higher efficacy for weight reduction is expected. The ecological fallacy in our pooled negative associations between glycemic control and weight reduction may be partially explained by a publication bias, but was better explained by a higher coefficient of variation of GLP-1 RAs for reducing body weight than HbA1c levels and the underlying glucose level [4]. After removing these influential points, studies [20, 31, 44, 45] with prominent weight-reduction effects and modest effects on glycemic changes in non-diabetic participants yielded positive pooled results, supporting our explanation. The mechanism underlying the variable effects of GLP-1 RAs on body weight is not well understood. GLP-1 RAs decreased appetite through direct effects on the hypothalamus, neuronal activation in brain areas, reduced caloric intake, and interference of effective compensatory mechanisms counteracting weight loss [59–61].
Our study provided evidence that the actual effects of GLP-1 RAs on glycemic control and weight reduction were not as high as those reported in previous studies. Thus, a more conservative view of the current published results on GLP-1 RAs is recommended. Clinicians could expect positive associations between weight reduction and glycemic control in diabetes patients treated with GLP-1 RAs. However, marked weight loss in a non-diabetic patient in response to GLP-1 RA treatment did not indicate that clinicians could expect a corresponding glycemic improvement due to the results were interacted with the underlying glucose level.
To the best of our knowledge, our study is the first to consider the correlation between two dependent variables and estimate the relationship between the glycemic control and weight-reducing effects of GLP-1 RAs jointly with an unbiased methodology, a structural equation modeling approach for a multivariate meta-analysis. We comprehensively investigated heterogeneity, effect modifiers, and the reasons for and impact of publication bias. However, the study also had some limitations. First, confirmatory factor and mediation analyses were not performed. We contacted the original authors, but the lack of correlations among the observed variables in individual studies hindered further analysis. Second, our study focused on long-acting GLP1-RAs in comparison with placebo, and future studies should expand the scope to include short-acting GLP1-RAs and comparisons with active components or to sodium-glucose cotransporter-2 inhibitors. Third, the model could not estimate correlations between cardiovascular outcomes and glycemic control and/or weight reduction; the within-study correlation coefficients were not available for categorical variables.
In conclusion, long-acting GLP-1 RAs significantly lowered HbA1c levels and body weight in adults. However, the positive association between glycemic control and weight reduction was only observed in diabetic patients and in non-diabetic participants, but not in all participants with high heterogeneity treated with long-acting GLP-1 RAs.
Supporting information
(PDF)
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
CI, confidence interval; MD, mean difference; SE, standard error; TE, treatment effect.
(JPG)
CI, confidence interval; DM, diabetes mellitus; MD, mean difference; SE, standard error; TE, treatment effect.
(TIF)
CI, confidence interval; GLP1 RA, Glucagon-like peptide-1 receptor agonist; MD, mean difference; sc, subcutaneous; SE, standard error; TE, treatment effect. GLP1 = Others referred to Dulaglutide, once-weekly Exenatide and Albiglutide.
(TIF)
(TIF)
DM, diabetes mellitus; HbA1c, glycated hemoglobin.
(TIF)
CI, confidence interval; MD, mean difference; SE, standard error; TE, treatment effect.
(JPG)
Acknowledgments
We would like to thank Editage for editing and proofreading this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, et al. Glucagon-like peptide 1 (GLP-1). Molecular metabolism. 2019;30:72–130. Epub 2019/11/27. doi: 10.1016/j.molmet.2019.09.010 ; PubMed Central PMCID: PMC6812410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nauck MA, Meier JJ. MANAGEMENT OF ENDOCRINE DISEASE: Are all GLP-1 agonists equal in the treatment of type 2 diabetes? European journal of endocrinology. 2019;181(6):R211–r34. Epub 2019/10/11. doi: 10.1530/EJE-19-0566 . [DOI] [PubMed] [Google Scholar]
- 3.Bessesen DH, Van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. The lancet Diabetes & endocrinology. 2018;6(3):237–48. Epub 2017/09/19. doi: 10.1016/S2213-8587(17)30236-X . [DOI] [PubMed] [Google Scholar]
- 4.Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes—state-of-the-art. Molecular metabolism. 2021;46:101102. Epub 2020/10/18. doi: 10.1016/j.molmet.2020.101102 ; PubMed Central PMCID: PMC8085572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huthmacher JA, Meier JJ, Nauck MA. Efficacy and Safety of Short- and Long-Acting Glucagon-Like Peptide 1 Receptor Agonists on a Background of Basal Insulin in Type 2 Diabetes: A Meta-analysis. Diabetes care. 2020;43(9):2303–12. Epub 2020/09/11. doi: 10.2337/dc20-0498 . [DOI] [PubMed] [Google Scholar]
- 6.Chadda KR, Cheng TS, Ong KK. GLP-1 agonists for obesity and type 2 diabetes in children: Systematic review and meta-analysis. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2021;22(6):e13177. Epub 2020/12/24. doi: 10.1111/obr.13177 . [DOI] [PubMed] [Google Scholar]
- 7.Karagiannis T, Liakos A, Bekiari E, Athanasiadou E, Paschos P, Vasilakou D, et al. Efficacy and safety of once-weekly glucagon-like peptide 1 receptor agonists for the management of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes, obesity & metabolism. 2015;17(11):1065–74. Epub 2015/09/24. doi: 10.1111/dom.12541 . [DOI] [PubMed] [Google Scholar]
- 8.Andreadis P, Karagiannis T, Malandris K, Avgerinos I, Liakos A, Manolopoulos A, et al. Semaglutide for type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes, obesity & metabolism. 2018;20(9):2255–63. Epub 2018/05/15. doi: 10.1111/dom.13361 . [DOI] [PubMed] [Google Scholar]
- 9.Avgerinos I, Michailidis T, Liakos A, Karagiannis T, Matthews DR, Tsapas A, et al. Oral semaglutide for type 2 diabetes: A systematic review and meta-analysis. Diabetes, obesity & metabolism. 2020;22(3):335–45. Epub 2019/10/23. doi: 10.1111/dom.13899 . [DOI] [PubMed] [Google Scholar]
- 10.Schmidt AM. Diabetes Mellitus and Cardiovascular Disease. Arteriosclerosis, thrombosis, and vascular biology. 2019;39(4):558–68. Epub 2019/02/23. doi: 10.1161/ATVBAHA.119.310961 ; PubMed Central PMCID: PMC6532416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umpierrez GE, Pantalone KM, Kwan AY, Zimmermann AG, Zhang N, Fernández Landó L. Relationship between weight change and glycaemic control in patients with type 2 diabetes receiving once-weekly dulaglutide treatment. Diabetes, obesity & metabolism. 2016;18(6):615–22. Epub 2016/03/13. doi: 10.1111/dom.12660 ; PubMed Central PMCID: PMC4934019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet (London, England). 2014;384(9961):2228–34. Epub 2014/09/16. doi: 10.1016/S0140-6736(14)61335-0 . [DOI] [PubMed] [Google Scholar]
- 13.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ (Clinical research ed). 2012;344:d7771. Epub 2012/01/13. doi: 10.1136/bmj.d7771 ; PubMed Central PMCID: PMC3256253 at www.icmje.org/coi_disclosure.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ. Cochrane Handbook for Systematic Reviews of Interventions version 6.2: Cochrane; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. Epub 1986/09/01. doi: 10.1016/0197-2456(86)90046-2 . [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. Epub 2002/07/12. doi: 10.1002/sim.1186 . [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. Epub 1997/10/06. doi: 10.1136/bmj.315.7109.629 ; PubMed Central PMCID: PMC2127453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmann A, Rodbard HW, Rosenstock J, Lahtela JT, de Loredo L, Tornøe K, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes, obesity & metabolism. 2015;17(11):1056–64. Epub 2015/07/17. doi: 10.1111/dom.12539 ; PubMed Central PMCID: PMC5054929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, et al. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison With Placebo in Patients With Type 2 Diabetes. Diabetes care. 2019;42(9):1724–32. Epub 2019/06/13. doi: 10.2337/dc19-0749 . [DOI] [PubMed] [Google Scholar]
- 20.Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. International journal of obesity (2005). 2016;40(8):1310–9. Epub 2016/03/24. doi: 10.1038/ijo.2016.52 ; PubMed Central PMCID: PMC4973216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. The Lancet. 2021;397(10278):971–84. doi: 10.1016/S0140-6736(21)00213-0 [DOI] [PubMed] [Google Scholar]
- 22.Davies MJ, Bain SC, Atkin SL, Rossing P, Scott D, Shamkhalova MS, et al. Efficacy and Safety of Liraglutide Versus Placebo as Add-on to Glucose-Lowering Therapy in Patients With Type 2 Diabetes and Moderate Renal Impairment (LIRA-RENAL): A Randomized Clinical Trial. Diabetes care. 2016;39(2):222–30. Epub 2015/12/19. doi: 10.2337/dc14-2883 . [DOI] [PubMed] [Google Scholar]
- 23.Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, et al. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. Jama. 2015;314(7):687–99. Epub 2015/08/19. doi: 10.1001/jama.2015.9676 . [DOI] [PubMed] [Google Scholar]
- 24.Dejgaard TF, Frandsen CS, Hansen TS, Almdal T, Urhammer S, Pedersen-Bjergaard U, et al. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): A randomised, double-blind, placebo-controlled trial. The Lancet Diabetes and Endocrinology. 2016;4(3):221–32. doi: 10.1016/S2213-8587(15)00436-2 PubMed Central PMCID: PMCNovo Nordisk(Denmark). [DOI] [PubMed] [Google Scholar]
- 25.Frøssing S, Nylander M, Chabanova E, Frystyk J, Holst JJ, Kistorp C, et al. Effect of liraglutide on ectopic fat in polycystic ovary syndrome: A randomized clinical trial. Diabetes, obesity & metabolism. 2018;20(1):215–8. Epub 2017/07/07. doi: 10.1111/dom.13053 . [DOI] [PubMed] [Google Scholar]
- 26.Garvey WT, Birkenfeld AL, Dicker D, Mingrone G, Pedersen SD, Satylganova A, et al. Efficacy and Safety of Liraglutide 3.0 mg in Individuals With Overweight or Obesity and Type 2 Diabetes Treated With Basal Insulin: The SCALE Insulin Randomized Controlled Trial. Diabetes care. 2020;43(5):1085–93. Epub 2020/03/07. doi: 10.2337/dc19-1745 ; PubMed Central PMCID: PMC7171937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet (London, England). 2019;394(10193):121–30. Epub 2019/06/14. doi: 10.1016/S0140-6736(19)31149-3 . [DOI] [PubMed] [Google Scholar]
- 28.Ghanim H, Batra M, Green K, Abuaysheh S, Hejna J, Makdissi A, et al. Liraglutide treatment in overweight and obese patients with type 1 diabetes: A 26-week randomized controlled trial; mechanisms of weight loss. Diabetes, obesity & metabolism. 2020;22(10):1742–52. Epub 2020/05/20. doi: 10.1111/dom.14090 . [DOI] [PubMed] [Google Scholar]
- 29.Gudbergsen H, Overgaard A, Henriksen M, Wæhrens EE, Bliddal H, Christensen R, et al. Liraglutide after diet-induced weight loss for pain and weight control in knee osteoarthritis: a randomized controlled trial. The American journal of clinical nutrition. 2021;113(2):314–23. Epub 2021/01/21. doi: 10.1093/ajcn/nqaa328 . [DOI] [PubMed] [Google Scholar]
- 30.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. The New England journal of medicine. 2019;381(9):841–51. Epub 2019/06/12. doi: 10.1056/NEJMoa1901118 . [DOI] [PubMed] [Google Scholar]
- 31.Ishøy PL, Knop FK, Broberg BV, Bak N, Andersen UB, Jørgensen NR, et al. Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: a randomized, placebo-controlled trial. Diabetes, Obesity and Metabolism. 2017;19(2):162–71. doi: 10.1111/dom.12795 PubMed Central PMCID: PMCAstra Zeneca(Sweden). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SH, Abbasi F, Lamendola C, Liu A, Ariel D, Schaaf P, et al. Benefits of liraglutide treatment in overweight and obese older individuals with prediabetes. Diabetes care. 2013;36(10):3276–82. Epub 2013/07/10. doi: 10.2337/dc13-0354 ; PubMed Central PMCID: PMC3781545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhadiya ND, Dhindsa S, Ghanim H, Mehta A, Makdissi A, Batra M, et al. Addition of Liraglutide to Insulin in Patients With Type 1 Diabetes: A Randomized Placebo-Controlled Clinical Trial of 12 Weeks. Diabetes care. 2016;39(6):1027–35. Epub 2016/05/22. doi: 10.2337/dc15-1136 ; PubMed Central PMCID: PMC5864130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. 2016;375(19):1834–44. doi: 10.1056/NEJMoa1607141 . [DOI] [PubMed] [Google Scholar]
- 35.Mensberg P, Nyby S, Jørgensen PG, Storgaard H, Jensen MT, Sivertsen J, et al. Near-normalization of glycaemic control with glucagon-like peptide-1 receptor agonist treatment combined with exercise in patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2017;19(2):172–80. doi: 10.1111/dom.12797 PubMed Central PMCID: PMCNovo Nordisk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosenzon O, Blicher TM, Rosenlund S, Eriksson JW, Heller S, Hels OH, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. The lancet Diabetes & endocrinology. 2019;7(7):515–27. Epub 2019/06/14. doi: 10.1016/S2213-8587(19)30192-5 . [DOI] [PubMed] [Google Scholar]
- 37.Nauck MA, Stewart MW, Perkins C, Jones-Leone A, Yang F, Perry C, et al. Efficacy and safety of once-weekly GLP-1 receptor agonist albiglutide (HARMONY 2): 52 week primary endpoint results from a randomised, placebo-controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetologia. 2016;59(2):266–74. Epub 2015/11/19. doi: 10.1007/s00125-015-3795-1 ; PubMed Central PMCID: PMC4705137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neeland IJ, Marso SP, Ayers CR, Lewis B, Oslica R, Francis W, et al. Effects of liraglutide on visceral and ectopic fat in adults with overweight and obesity at high cardiovascular risk: a randomised, double-blind, placebo-controlled, clinical trial. The lancet Diabetes & endocrinology. 2021;9(9):595–605. Epub 2021/08/07. doi: 10.1016/S2213-8587(21)00179-0 . [DOI] [PubMed] [Google Scholar]
- 39.Retnakaran R, Kramer CK, Choi H, Swaminathan B, Zinman B. Liraglutide and the preservation of pancreatic β-cell function in early type 2 diabetes: the LIBRA trial. Diabetes care. 2014;37(12):3270–8. Epub 2014/09/25. doi: 10.2337/dc14-0893 . [DOI] [PubMed] [Google Scholar]
- 40.Rodbard HW, Lingvay I, Reed J, de la Rosa R, Rose L, Sugimoto D, et al. Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized, Controlled Trial. The Journal of clinical endocrinology and metabolism. 2018;103(6):2291–301. Epub 2018/04/25. doi: 10.1210/jc.2018-00070 ; PubMed Central PMCID: PMC5991220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorli C, Harashima SI, Tsoukas GM, Unger J, Karsbøl JD, Hansen T, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. The lancet Diabetes & endocrinology. 2017;5(4):251–60. Epub 2017/01/24. doi: 10.1016/S2213-8587(17)30013-X . [DOI] [PubMed] [Google Scholar]
- 42.van Eyk HJ, Paiman EHM, Bizino MB, de Heer P, Geelhoed-Duijvestijn PH, Kharagjitsingh AV, et al. A double-blind, placebo-controlled, randomised trial to assess the effect of liraglutide on ectopic fat accumulation in South Asian type 2 diabetes patients. Cardiovascular diabetology. 2019;18(1):87. Epub 2019/07/11. doi: 10.1186/s12933-019-0890-5 ; PubMed Central PMCID: PMC6615254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanderheiden A, Harrison L, Warshauer J, Li X, Adams-Huet B, Lingvay I. Effect of Adding Liraglutide vs Placebo to a High-Dose lnsulin Regimen in Patients With Type 2 Diabetes: A Randomized Clinical Trial. JAMA Internal Medicine. 2016;176(7):939–47. doi: 10.1001/jamainternmed.2016.1540%J JAMA Internal Medicine. [DOI] [PubMed] [Google Scholar]
- 44.Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183 . [DOI] [PubMed] [Google Scholar]
- 45.Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. International journal of obesity (2005). 2013;37(11):1443–51. Epub 2013/07/03. doi: 10.1038/ijo.2013.120 . [DOI] [PubMed] [Google Scholar]
- 46.Zinman B, Aroda VR, Buse JB, Cariou B, Harris SB, Hoff ST, et al. Efficacy, Safety, and Tolerability of Oral Semaglutide Versus Placebo Added to Insulin With or Without Metformin in Patients With Type 2 Diabetes: The PIONEER 8 Trial. Diabetes care. 2019;42(12):2262–71. Epub 2019/09/19. doi: 10.2337/dc19-0898 ; PubMed Central PMCID: PMC7364672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zinman B, Bhosekar V, Busch R, Holst I, Ludvik B, Thielke D, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. The lancet Diabetes & endocrinology. 2019;7(5):356–67. Epub 2019/03/06. doi: 10.1016/S2213-8587(19)30066-X . [DOI] [PubMed] [Google Scholar]
- 48.Geiser JS, Heathman MA, Cui X, Martin J, Loghin C, Chien JY, et al. Clinical Pharmacokinetics of Dulaglutide in Patients with Type 2 Diabetes: Analyses of Data from Clinical Trials. Clinical Pharmacokinetics. 2016;55(5):625–34. doi: 10.1007/s40262-015-0338-3 [DOI] [PubMed] [Google Scholar]
- 49.Matthews JE, Stewart MW, De Boever EH, Dobbins RL, Hodge RJ, Walker SE, et al. Pharmacodynamics, Pharmacokinetics, Safety, and Tolerability of Albiglutide, a Long-Acting Glucagon-Like Peptide-1 Mimetic, in Patients with Type 2 Diabetes. The Journal of Clinical Endocrinology & Metabolism. 2008;93(12):4810–7. doi: 10.1210/jc.2008-1518%J The Journal of Clinical Endocrinology & Metabolism. [DOI] [PubMed] [Google Scholar]
- 50.Damholt B, Golor G, Wierich W, Pedersen P, Ekblom M, Zdravkovic M. An Open-Label, Parallel Group Study Investigating the Effects of Age and Gender on the Pharmacokinetics of the Once-Daily Glucagon-Like Peptide-1 Analogue Liraglutide. 2006;46(6):635–41. doi: 10.1177/0091270006288215 [DOI] [PubMed] [Google Scholar]
- 51.Marbury TC, Flint A, Jacobsen JB, Derving Karsbøl J, Lasseter K. Pharmacokinetics and Tolerability of a Single Dose of Semaglutide, a Human Glucagon-Like Peptide-1 Analog, in Subjects With and Without Renal Impairment. Clinical Pharmacokinetics. 2017;56(11):1381–90. doi: 10.1007/s40262-017-0528-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Granhall C, Donsmark M, Blicher TM, Golor G, Søndergaard FL, Thomsen M, et al. Safety and Pharmacokinetics of Single and Multiple Ascending Doses of the Novel Oral Human GLP-1 Analogue, Oral Semaglutide, in Healthy Subjects and Subjects with Type 2 Diabetes. Clinical Pharmacokinetics. 2019;58(6):781–91. doi: 10.1007/s40262-018-0728-4 [DOI] [PubMed] [Google Scholar]
- 53.Armstrong MJ, Houlihan DD, Rowe IA, Clausen WH, Elbrønd B, Gough SC, et al. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: individual patient data meta-analysis of the LEAD program. Alimentary pharmacology & therapeutics. 2013;37(2):234–42. Epub 2012/11/21. doi: 10.1111/apt.12149 . [DOI] [PubMed] [Google Scholar]
- 54.Monami M, Dicembrini I, Marchionni N, Rotella CM, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on body weight: a meta-analysis. Experimental diabetes research. 2012;2012:672658. Epub 2012/06/08. doi: 10.1155/2012/672658 ; PubMed Central PMCID: PMC3362858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. The lancet Diabetes & endocrinology. 2019;7(10):776–85. Epub 2019/08/20. doi: 10.1016/S2213-8587(19)30249-9 . [DOI] [PubMed] [Google Scholar]
- 56.Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. The lancet Diabetes & endocrinology. 2018;6(2):105–13. Epub 2017/12/10. doi: 10.1016/S2213-8587(17)30412-6 . [DOI] [PubMed] [Google Scholar]
- 57.Li J, He K, Ge J, Li C, Jing Z. Efficacy and safety of the glucagon-like peptide-1 receptor agonist oral semaglutide in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes research and clinical practice. 2021;172:108656. Epub 2021/01/13. doi: 10.1016/j.diabres.2021.108656 . [DOI] [PubMed] [Google Scholar]
- 58.Xia L, Shen T, Dong W, Su F, Wang J, Wang Q, et al. Comparative efficacy and safety of 8 GLP-1RAs in patients with type 2 diabetes: A network meta-analysis. Diabetes research and clinical practice. 2021;177:108904. Epub 2021/06/09. doi: 10.1016/j.diabres.2021.108904 . [DOI] [PubMed] [Google Scholar]
- 59.Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Rønne J, Alanentalo T, Baquero AF, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6):e133429. doi: 10.1172/jci.insight.133429 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. The Journal of clinical investigation. 2014;124(10):4473–88. Epub 2014/09/10. doi: 10.1172/JCI75276 ; PubMed Central PMCID: PMC4215190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schlögl H, Kabisch S, Horstmann A, Lohmann G, Müller K, Lepsien J, et al. Exenatide-Induced Reduction in Energy Intake Is Associated With Increase in Hypothalamic Connectivity. 2013;36(7):1933–40. doi: 10.2337/dc12-1925%J Diabetes Care. [DOI] [PMC free article] [PubMed] [Google Scholar]