Abstract
The COVID-19 pandemic brought new emphasis on indoor air quality. However, few studies have investigated the impact of air filtration, a COVID-mitigation approach, on indoor air concentrations of semivolatile organic compounds (SVOCs). Using a quasi-experimental design, we quantified the impact of a relatively low-cost “do-it-yourself” air filter (Corsi–Rosenthal Box; CR Box) on indoor air concentrations of 42 PFAS and 24 other SVOCs. We sampled air before (October–November 2021) and during (February–March 2022) deployment of CR Boxes in 17 rooms located in an occupied Providence, Rhode Island office building. We measured sound levels in rooms with CR Boxes operating and not operating. While CR Boxes were deployed, concentrations of seven PFAS (N-EtFOSE, N-EtFOSA, FBSA, PFBS, PFHxS, PFOS, PFNA) were 28–61% lower and concentrations of five phthalates (DMP, DEP, DiBP, BBzP, DCHP) were 29–62% lower. Concentrations of five PFAS and one phthalate increased 23–44% during the intervention period, but the 95% CI of most of these estimates included the null. Daytime sound levels increased 5.0 dB when CR Boxes were operating. These results indicate that CR Boxes reduced exposure to several lower-volatility phthalates and sulfonated PFAS previously reported to be found in office building materials and products, with potentially distracting increases in sound levels.
Keywords: intervention, perfluoroalkyl substances, phthalates, semivolatile organic compounds, indoor air
Short abstract
Few studies have investigated the impact of air filtration on indoor air SVOC concentrations. This study reports that a “do-it-yourself” air filter reduced indoor air concentrations of seven perfluoroalkyl substances and five phthalates.
Introduction
Indoor air filtration is a critical tool to reduce the risk of COVID-19 transmission.1 Affordable and effective air filtration is needed to mitigate the airborne transmission of COVID-19 in schools, offices, personal residences, health care and congregate care facilities, workplaces, etc. In response to this need, a “do-it-yourself” (DIY) air filter consisting of four consumer-grade MERV-13 (or equivalent) filters and a box fan was developed (i.e., Corsi–Rosenthal Box; CR Box). These filters provide comparable or better air filtration performance as commercially available HEPA air filters at a fraction of the cost and thus may be a scalable and sustainable solution to reduce the risk of COVID-19 transmission.2,3
A potential co-benefit of using indoor air filtration to mitigate COVID-19 transmission is reducing air concentrations of indoor pollutants such as semivolatile organic compounds (SVOCs). Previous studies have detected several classes of SVOCs, including phthalates, organophosphate and brominated flame retardants, organochlorines, per- and polyfluoroalkyl substances (PFAS), and synthetic musks in the indoor air of homes and offices.4−7 Moreover, biomonitoring studies indicate widespread exposure to a mixture of these compounds and indoor air may be an important route of exposure to some of these SVOCs.8,9 SVOCs, including phthalates, brominated flame retardants, and organophosphates, have been found on heating, ventilation, and air conditioning (HVAC) filters in buildings with mechanical ventilation.10 Air filtration has been shown to effectively reduce particle concentrations indoors, and a simulation study suggests a similar impact on indoor SVOC air concentrations.11,12 An experimental crossover study in China reported that indoor HEPA air filtration with activated carbon filters in college dormitories reduced gas-phase air concentrations of several phthalates by at least 50%.13 However, this study did not quantify the concentrations of other SVOCs, including PFAS, and it used an air filtration system with both activated carbon and a HEPA filter rather the MERV-13 (or equivalent) filter typically used in CR Boxes.
Lowering indoor air concentrations of SVOCs may improve occupant health. Phthalates, PFAS, and flame retardants have known or presumed health hazards, particularly in susceptible subpopulations like infants and children, who spend the majority of their time indoors.14 Exposure to some phthalates has been associated with an increased risk of childhood asthma and allergy.15 Some PFAS exposures have been associated with reduced vaccine response in children, and these same PFAS may increase the severity of and susceptibility to COVID-19 in adults.16
In Fall 2021, the Brown University School of Public Health installed CR Boxes in classrooms and conference rooms as part of a multilayered approach to mitigate COVID-19 transmission. This provided an opportunity to conduct a quasi-experimental study to determine if CR Boxes reduced concentrations of common indoor air pollutants (i.e., natural experiment). We measured indoor air concentrations of 66 PFAS and other SVOCs in 17 rooms inside an occupied office building prior to and during deployment of the CR Boxes. We also measured sound levels associated with CR Box operation. These results may inform strategies to reduce airborne disease transmission as well as improve indoor air quality, thereby enhancing occupant health.
Materials and Methods
Setting and Study Design
This study took place between October 2021 and March 2022 in an 11-story office building that is the home of the Brown University School of Public Health (Providence, Rhode Island). The 142,000 ft2 building holds offices, conference rooms, classrooms, computer labs, and biological sample storage facilities. The building was built in 1984, and mechanical air handling units were installed in 1999. Each floor in the entire building receives outside air at a constant ventilation rate from side louvers that is sent to an air handling unit with a common ceiling return plenum. The air handling units include MERV-13 filtration. Air heating and cooling is provided with hot and chilled water systems with hot and chilled water coils on each floor, respectively. No additional humidity control is provided beyond that of the air handling, heating, and cooling systems. In rooms with windows, the windows are inoperable.
We conducted the study in 18 classrooms, conference rooms, and computer labs spread across seven floors in the building. All of the sampled rooms were carpeted. We were unable to use data from one room due to a problem with sample collection during the study. Thus, our sample size was 17 rooms (two measurements per room). Of the 17 rooms, 10 were conference rooms (Table 1) and seven rooms were interior rooms without windows. The median room area was 300 ft2 and ranged from 157 to 1084 ft2. Average outdoor concentrations of particulate matter <10 μm at an EPA monitoring site 2.3 km away were 11.2 and 12.0 μg/m3 before and during the intervention, respectively.
Table 1. Characteristics of the 17 Rooms Included in the Intervention.
| characteristic | median [25th, 75th]/N (%) |
|---|---|
| room type | |
| classroom | 5 (29) |
| conference room | 10 (59) |
| computer lab | 2 (12) |
| windows | |
| none | 7 (41) |
| any | 10(59) |
| number of computers and other electronics | 3 [2, 9] |
| room area (ft2) | 300 [276, 787] |
| number of occupants (n)a | 19 [14, 33] |
Estimated from the number of chairs in the room.
We used a quasi-experimental study design to quantify changes in PFAS and other SVOC air concentrations and sound before and during the deployment of CR Boxes. At baseline, between October 25, 2021, and November 18, 2021, we measured air concentrations and ambient sound (assessment methods detailed below). After baseline sampling, CR Boxes were deployed in each of the 18 rooms in a staggered fashion approximately one week after baseline sampling was conducted and operated from 0700 to 1900 on weekdays until December 20, 2021. The CR Boxes were off during the winter break (December 21, 2021, through January 24, 2022), except in some conference rooms used by staff. The CR Boxes were turned back on for approximately 2 weeks before we began sampling during the intervention period. The same air and sound measurements were collected from February 7, 2022, through March 4, 2022, during which time the CR Boxes operated from 0700 to 1900 on weekdays. The building was occupied and classes were in session during both sampling periods.
We recorded the room area, presence and number of windows, type and number of furnishings, maximum number of occupants, and number of electronics in each room using standardized forms.
CR Box Construction and Operation
Each CR Box was assembled using four 50 × 50 × 5 cm3 pleated MERV-13 filters (Tex-Air), a Lasko Power Plus fan, and black electrical tape (Supplemental Figure 1). We affixed a cardboard shroud in front of the fan to improve its efficiency (50 cm diameter circle). The MERV-13 filter media consisted of polyolefin and titanium dioxide (Jim Rosenthal, personal communication). To determine the number of CR Boxes to deploy in each room, we assumed that the CR Boxes had a clean air delivery rate (CADR) of 600 fpm and 85% efficiency (for COVID-19 filtration with the fan set to low speed).2,17 Thus, we placed one to three CR Boxes in each room depending on the room area so that the fans provided approximately six additional effective air changes per hour (Supplemental Table 1). The CR Boxes were placed near walls and in some cases corners to reduce interference with foot traffic.
The CR Boxes operated from 0700 to 1900 h on weekdays using a mechanical outlet timer with the fan set to low speed. During the intervention period, we noted that the CR Boxes were occasionally turned off or unplugged because of perceived sound levels during meetings and classes. We made efforts to keep them on by checking each one throughout the day and asking building occupants to leave them on.
SVOC Air Sampling
We conducted active air sampling in each room, once before installation of the CR Boxes and again when they were operational using previously described methods (Supplemental Figures 2 and 3).6 Briefly, we collected gas and particle-bound phases of air using 160 mm URG personal pesticide samplers (Universal Research Glassware (URG); Chapel Hill, NC) at a target flow rate of 3 L/min over approximately 96 h. We measured the air flow rate at the beginning and end of each sampling period using a TSI Model 4199 air flowmeter. Each sampler contained a 10 μm at 4 L/min impactor-equipped inlet followed by a 25 mm quartz fiber filter and 3 g of XAD sandwiched between two 1 13/16 in. polyurethane foam (PUF) plugs. Prior to packing, the PUF plugs and XAD powder were washed with UHLPC-grade acetonitrile and dried in a 60 °C oven for 3 days. We purchased PUF plugs and Supelco Amberlite XAD-2 from Fisher Scientific (Waltham, MA). In this study, we only analyzed gas-phase PFAS and other SVOC concentrations.
Both before and during the CR Box deployment, we conducted air sampling from Monday morning through Friday morning, except for four rooms where sampling ceased on Thursday night in anticipation of a snowstorm the following day. We positioned samplers near walls or corners, and attempted to place them in the same location of each room before and during the installation of the CR Boxes but had to move them in some cases when the room was relatively small and the CR Box was placed in the original sampling location.
We collected and analyzed several blanks and replicates to evaluate potential contamination and precision of our SVOC sampling. First, using the methods described below, we quantified SVOC concentrations in two solvent blanks (i.e., field naïve samplers) and two field blanks (samplers opened in a room and then processed, one before and one during CR Box deployment). We also collected three technical field replicates by simultaneously sampling in a single residence using the above-described procedures.
PFAS and Other SVOC Air Concentration Quantification
We quantified gas-phase concentrations of 42 PFAS and 24 SVOCs (three brominated flame retardants, two phenols, 12 phthalates, one synthetic musk, three polychlorinated biphenyls, and four organophosphate esters) collected in our air samples (see Supplemental Table 2). After sample collection, the samplers were stored at −20 °C until analysis. Details regarding sample extraction and analysis are provided in Supplemental Methods. In brief, for each sample, we extracted the XAD and PUF with acetonitrile and split the extract for analysis of PFAS and other SVOCs. We spiked aliquots with isotopically labeled PFAS or phthalate internal standards (Supplemental Tables 3 and 4). PFAS were measured using the chromatography scheme, internal standards, and analyte list described in EPA Draft Method 1633 on a high-resolution Thermo QExactive HF-X Orbitrap MS equipped with a Vanquish ultra-high-performance liquid chromatograph (UHPLC-Orbitrap-HRMS). SVOC concentrations were quantified using a high-resolution Thermo QExactive Orbitrap MS equipped with a Thermo Trace 1300 Gas Chromatograph. Two isomeric SVOCs, Galoxolide and Tonalid, could not be separated using this method and are reported as a sum. Sample extracts for either PFAS or SVOCs were analyzed in a single batch.
We used several quality control measurements, including blanks, replicates, and surrogates, to evaluate the quality of our data. PFAS and other SVOC levels were generally nondetectable or low in solvent and field blank samples (Supplemental Tables 5 and 6, Supplemental Figures 4 and 5). Among the PFAS, the maximum field blank levels of PFOSA and FBSA exceeded the minimum measured field sample concentrations, although the field blank concentrations were below the LOD. For the SVOCs, field blank levels of 8 compounds (triphenyl phosphate, benzophenone-3, BDE 100, dihexyl phthalate, di-n-pentyl phthalate, dibutyl phthalate, benzyl butyl phthalate, and dicycloheyl phthalate) exceeded the minimum reported field sample concentrations. Concentrations of most PFAS in the three field replicates were reproducible (coefficient of variation < 30%); however, there were some exceptions that included perfluoro(2-ethoxyethane) sulfonic acid, perfluoro-3-methoxypropanoic acid, perfluoroheptanoic acid, and perfluoroheptanesulfonic acid (Supplemental Table 5). Coefficients of variation were <30% for phthalates, with the exception of dihexyl phthalate (Supplemental Table 6). Recovery of each analyte was assessed by spiking a PUF and XAD sample with all target analytes and extracting. PFAS and other SVOC recoveries ranged from 71 to 110% (Supplemental Tables 7 and 8).
Air Filter PFAS and Other SVOC Concentrations
To better understand how the MERV-13 filters used in CR Boxes may be a source or sink of PFAS or SVOCs, we quantified PFAS and other SVOC concentrations on three filters that had been used for approximately 4 months in one of the intervention rooms and one unused filter of the same brand and size used for the intervention. For the unused filter, we took three 2.5 × 2.5 cm2 samples from the top-center, middle-center, and bottom-center of the filter. For the used filters, we took a single 2.5 × 2.5 cm2 sample from the middle-center of each filter. We quantified the mass of each PFAS and other SVOCs per g of filter (ng/g) using procedures described above with the additional step of sonicating during each extraction for 20 min.
Sound Sampling
We assessed sound levels in each room both with the CR Boxes operating (daytime, 0700–1900 h) and with the CR Boxes not operating (nighttime, 1900–0700 h). Nighttime measurements were made when we were not conducting air sampling in the rooms. We measured ambient continuous sound using a Cirrus Optimus Octave Band Analyzer CR-171B (class 1) (North Yorkshire, U.K.) (dynamic range: 20–140 dB) in 1 s intervals. Calibrations were performed at 90 dB(A) at a frequency of 1 kHz immediately before and after each monitoring session. The microphone of the CR-171B was mounted on a tripod at a height of 1.5 m. Each sound monitor was placed in the exact position of the air samplers. The A-weighted sound level (dB(A)) was used to assess the measured sound as it is the most commonly reported sound weighting system. It emphasizes sounds processed through the auditory system and penalizes both low- and high-frequency sounds.18
The 1-s levels were aggregated by the hour and over the daytime and nighttime 12-h period. Equivalent continuous sound levels were calculated using the following formula
where T is the time period (1-h, day/nighttime), n is the number of 1 s time samples in the time period, and Li is the 1s level determined for each frequency region or for the A-weighted sound level.19
Statistical Analysis
For each PFAS and other SVOC, we calculated the indoor air concentration in ng/m3 over the sampling period using the analyte mass, sampling duration, and average of the start and end of sampling air flow rate (L/min). Next, we examined univariate characteristics of the PFAS and other SVOC air concentrations before and during the intervention by calculating means, medians, percentiles, ranges, and proportions of detected values. For values below the limit of detection (LOD), we used machine-read values unless a concentration of 0 was reported (i.e., no analyte detected). In those cases, we imputed these values with the minimum observed value of that analyte multiplied by its frequency of detection.20 We limited subsequent analyses to those analytes detected in at least 80% of samples before or during the intervention.
We compared PFAS and other SVOC concentrations before and during the CR Box deployment both visually and statistically. Among analytes detected in 80% of samples before or during the intervention, we graphed concentrations before and during the intervention using violin, box-and-whisker, and line plots. We log10-transformed air concentrations prior to modeling to approximate normality assumptions. For each PFAS and other SVOC concentration, we estimated the percent change (and 95% confidence interval [CI]) and geometric mean concentrations before and during the intervention using linear mixed effect models with a random intercept for room and an unstructured covariance matrix. Among the PFAS detected in >80% of samples, we also examined whether sums of PFAS classes changed during CR Box deployment. We limited this analysis to perfluorooctane sulfonamides, perfluoroalkane sulfonamides, perfluorosulfonates, and perfluorocarboxylates, as these had at least two individual PFAS in the respective class.21
Next, we calculated room-specific changes in chemical concentrations by dividing the during-intervention concentration of each chemical by the before-intervention concentration of the same chemical. We plotted these using Plotly (see Supplemental File for interactive graphics that includes features of each room and instructions to interact with the plots).22 We also examined if the log-Koa and molecular weight of respective phthalates and PFAS were related to changes in room air concentrations using graphical methods and linear regression.22
For sound data, we used a linear mixed effects model with a random intercept for room and an unstructured covariance matrix to estimate the change in sound levels before and during the intervention during the daytime (0700–1900) and nighttime (1900–0700) hours.
Results
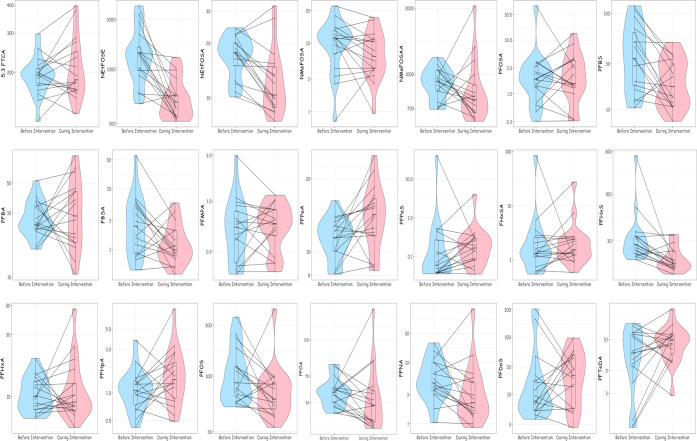
Among the 66 SVOCs measured, 37 were detected in over 80% of samples before or during the intervention (Table 2), including 21 PFAS and 15 other SVOCs. PFAS with the highest median air concentrations before the intervention included 5:3 fluorotelomer carboxylic acid (5:3 FTCA, median = 194 pg/m3), N-ethyl perfluorooctane sulfonamidothanol (NEtFOSE, median = 1430 pg/m3), and N-methyl-perfluorooctane sulfonamido acetic acid (NMeFOSAA, median = 925 pg/m3). Among the other SVOCs, we observed the highest median air concentrations before the intervention for diethyl phthalate (DEP, median = 97.9 ng/m3), di-isobutyl phthalate (DiBP, median = 37.9 ng/m3), and di-n-pentyl phthalate (DnPP, median = 24.5 ng/m3).
Table 2. Univariate Statistics and Frequency of Detection in PFAS (pg/m3) and SVOC (ng/m3) Air Concentrations before and during Intervention (n = 17 Rooms)a.
| pre-intervention |
during intervention |
|||||
|---|---|---|---|---|---|---|
| chemical | % > LOD | median (25th, 75th) | min, max | % > LOD | median (25th, 75th) | min, max |
| 11Cl-PF3OUdS | 35 | 0.57 (0.541, 1.50) | (0.508, 89) | 47 | 1.27 (0.575, 2.12) | (0.503, 10.6) |
| 3:3 FTCA | 6 | 2.84 (2.70, 2.93) | (2.6, 94.5) | 0 | 2.83 (2.75, 3.10) | (2.58, 6.55) |
| 4:2-FTS | 41 | 0.072 (0.070, 0.288) | (0.063, 0.63) | 29 | 0.075 (0.068, 0.178) | (0.062, 0.33) |
| 5:3 FTCA | 100 | 194 (175, 208) | (121, 297) | 100 | 185 (166, 247) | (131, 397) |
| 6:2-FTS | 47 | 0.206 (0.166, 0.515) | (0.156, 24.6) | 59 | 0.480 (0.167, 0.903) | (0.153, 3.81) |
| 7:3 FTCA | 0 | 1.121 (1.069, 1.158) | (1.03, 1.37) | 6 | 1.13 (1.09, 1.37) | (1.02, 35.0) |
| 8:2-FTS | 24 | 0.095 (0.089, 0.114) | (0.086, 2.24) | 18 | 0.094 (0.091, 0.122) | (0.085, 3.22) |
| 9Cl-PF3ONS | 18 | 0.209 (0.196, 0.215) | (0.188, 93.3) | 18 | 0.207 (0.199, 0.268) | (0.187, 6.18) |
| ADONA | 65 | 0.667 (0.393, 1.097) | (0.355, 10.3) | 76 | 0.919 (0.505, 1.822) | (0.355, 40.8) |
| FBSA | 100 | 2.43 (0.884, 5.23) | (0.464, 34.9) | 100 | 0.992 (0.788, 2.08) | (0.397, 5.88) |
| FHxSA | 88 | 1.32 (1.14, 2.62) | (0.548, 83.8) | 94 | 1.54 (1.22, 2.42) | (0.589, 27.4) |
| HFPODA-GenX | 0 | 5.64 (5.38, 5.83) | (5.17, 6.9) | 6 | 5.63 (5.48, 6.91) | (5.14, 201) |
| NEtFOSA | 100 | 17.8 (15, 20.1) | (10.1, 24.3) | 100 | 10.4 (8.84, 14.8) | (7.44, 32.1) |
| NEtFOSAA | 29 | 0.039 (0.037, 0.291) | (0.034, 18.6) | 29 | 0.046 (0.038, 0.111) | (0.036, 4.24) |
| NEtFOSE | 100 | 1430 (973, 1630) | (466, 4060) | 53 | 483 (347, 567) | (317, 1300) |
| NFDHA | 12 | 0.624 (0.594, 0.653) | (0.571, 4.38) | 29 | 0.681 (0.619, 3.924) | (0.567, 4.83) |
| NMeFOSA | 94 | 11.9 (6.99, 15.3) | (0.721, 35.3) | 100 | 6.42 (4.26, 11.2) | (0.934, 23.7) |
| NMeFOSAA | 100 | 925 (840, 1040) | (693, 1190) | 100 | 766 (669, 893) | (611, 2050) |
| NMeFOSE | 65 | 1.76 (0.232, 3.13) | (0.208, 9.26) | 18 | 0.235 (0.218, 0.314) | (0.204, 5.60) |
| PFBA | 100 | 24.5 (22.6, 33.6) | (16.1, 52) | 100 | 28.5 (18, 43.3) | (10.5, 79.9) |
| PFBS | 100 | 34 (17.9, 63.2) | (10.4, 120) | 100 | 17.3 (10.9, 29.8) | (7.56, 49.8) |
| PFDA | 47 | 0.411 (0.336, 1.848) | (0.308, 10.7) | 41 | 0.412 (0.336, 1.24) | (0.308, 95.6) |
| PFDoA | 12 | 0.152 (0.147, 0.157) | (0.139, 9.76) | 29 | 0.157 (0.146, 0.744) | (0.136, 4.62) |
| PFDoS | 100 | 7.61 (5.31, 17.5) | (3.69, 311) | 100 | 21.5 (5.67, 49) | (2.65, 96.5) |
| PFDS | 24 | 0.064 (0.062, 0.076) | (0.058, 33.5) | 59 | 0.388 (0.068, 0.859) | (0.058, 4.45) |
| PFEESA | 35 | 0.006 (0.006, 0.021) | (0.006, 0.07) | 47 | 0.014 (0.007, 0.042) | (0.006, 0.605) |
| PFHpA | 100 | 1.061 (0.737, 1.349) | (0.419, 3.75) | 100 | 1.40 (0.870, 2.14) | (0.489, 8.28) |
| PFHpS | 71 | 0.460 (0.190, 2.788) | (0.149, 30.9) | 76 | 0.511 (0.212, 0.959) | (0.147, 4.22) |
| PFHxA | 100 | 10.0 (7.99, 13.0) | (6.83, 19.6) | 100 | 9.11 (7.89, 12.6) | (5.81, 47.0) |
| PFHxS | 100 | 27.2 (21.6, 33.4) | (18.5, 268) | 100 | 16.7 (15.1, 22.8) | (12.7, 35.5) |
| PFMBA | 24 | 0.063 (0.061, 0.071) | (0.057, 0.46) | 47 | 0.144 (0.061, 0.457) | (0.057, 1.86) |
| PFMPA | 71 | 0.537 (0.228, 0.791) | (0.175, 2.97) | 88 | 0.583 (0.437, 0.817) | (0.186, 1.16) |
| PFNA | 100 | 4.64 (3.46, 9.92) | (1.04, 21) | 82 | 2.20 (1.47, 4.75) | (0.867, 75.4) |
| PFNS | 53 | 0.146 (0.090, 0.489) | (0.081, 36) | 59 | 0.352 (0.092, 0.989) | (0.080, 5.05) |
| PFOA | 100 | 36.6 (30.1, 39.9) | (24.5, 62.6) | 59 | 28.4 (20.6, 37.6) | (18.3, 181) |
| PFOS | 100 | 86.5 (64.7, 124) | (51.4, 357) | 100 | 60.8 (49.0, 86.8) | (33.0, 426) |
| PFOSA | 94 | 1.85 (1.32, 3.26) | (0.308, 43.4) | 88 | 1.55 (1.29, 4.17) | (0.313, 13.3) |
| PFPeA | 100 | 11.4 (10.1, 12.8) | (8.08, 16.3) | 100 | 14.3 (11.7, 16.6) | (8.37, 24.9) |
| PFPeS | 59 | 0.059 (0.039, 0.266) | (0.036, 39.8) | 94 | 0.165 (0.083, 0.295) | (0.035, 3.96) |
| PFTeDA | 88 | 7.39 (5.47, 11.3) | (1.4, 14.3) | 100 | 9.84 (7.29, 11.2) | (2.87, 19.8) |
| PFTrDA | 12 | 0.107 (0.101, 0.111) | (0.097, 12.6) | 18 | 0.111 (0.103, 0.148) | (0.096, 3.22) |
| PFUnA | 24 | 0.051 (0.048, 0.061) | (0.046, 4.73) | 24 | 0.050 (0.048, 0.115) | (0.045, 5.00) |
| BDE 47 | 18 | 0.001 (0.001, 0.001) | (0.001, 0.082) | 6 | 0.001 (0.001, 0.001) | (0.001, 0.064) |
| BDE 99 | 18 | 0.002 (0.002, 0.002) | (0.001, 0.018) | 18 | 0.002 (0.002, 0.002) | (0.001, 0.013) |
| BDE 100 | 41 | 0.002 (0.002, 0.053) | (0.002, 0.888) | 6 | 0.002 (0.002, 0.002) | (0.002, 0.012) |
| BP | 100 | 1.13 (0.82, 1.33) | (0.597, 2.24) | 100 | 1.09 (0.897, 1.30) | (0.302, 2.29) |
| BP3 | 18 | 0.940 (0.915, 1.06) | (0.849, 23.3) | 6 | 0.932 (0.902, 1.13) | (0.843, 7.22) |
| BBzP | 82 | 0.201 (0.165, 0.305) | (0.04, 0.324) | 41 | 0.056 (0.043, 0.168) | (0.040, 0.241) |
| DEHA | 100 | 1.57 (1.22, 1.93) | (0.845, 3.21) | 100 | 1.34 (1.26, 1.76) | (1.01, 3.35) |
| DEHP | 76 | 0.403 (0.347, 0.547) | (0.218, 1.83) | 100 | 0.361 (0.310, 0.562) | (0.280, 1.42) |
| DnBP | 100 | 19.1 (15.9, 39.0) | (6.87, 58.4) | 100 | 11.8 (5.03, 45.3) | (2.38, 74.2) |
| DCHP | 100 | 23.2 (17.4, 44.5) | (12.5, 54.6) | 100 | 14.3 (12.3, 19.8) | (9.96, 43.1) |
| DEP | 100 | 97.9 (81.2, 113) | (40.7, 159) | 100 | 61.4 (50.4, 76.4) | (44.2, 238) |
| DHP | 71 | 0.040 (0.006, 0.071) | (0.006, 0.151) | 35 | 0.006 (0.006, 0.037) | (0.005, 0.119) |
| DiBP | 100 | 37.9 (19.9, 68) | (7.74, 154) | 100 | 11.3 (8.28, 20.3) | (4.89, 193) |
| DMP | 100 | 2.38 (2.04, 3.06) | (1.71, 5.56) | 100 | 1.29 (1.15, 1.72) | (0.827, 3.59) |
| DnOP | 53 | 5.2 (3.41, 7.24) | (3.16, 10.6) | 82 | 7.05 (6.13, 8.28) | (3.18, 17.2) |
| DnPP | 100 | 24.5 (20.4, 50.0) | (8.82, 74.9) | 100 | 15.1 (6.45, 58.1) | (3.05, 95.2) |
| Gal/Ton | 100 | 0.353 (0.316, 0.416) | (0.143, 0.582) | 82 | 0.233 (0.152, 0.422) | (0.110, 0.486) |
| PCB 52 | 88 | 0.003 (0.002, 0.008) | (0.001, 0.027) | 82 | 0.002 (0.002, 0.005) | (0.001, 0.007) |
| PCB 105 | 12 | 0 (0, 0) | (0, 0.011) | 6 | 0 (0, 0) | (0, 0.003) |
| PCB 153 | 24 | 0.001 (0.001, 0.001) | (0.001, 0.023) | 12 | 0.001 (0.001, 0.001) | (0.001, 0.011) |
| TCEP | 100 | 0.164 (0.097, 0.217) | (0.051, 0.518) | 100 | 0.119 (0.107, 0.220) | (0.058, 0.595) |
| TPP | 65 | 0.033 (0.006 0.062) | (0.005, 0.435) | 47 | 0.008 (0.006, 0.026) | (0.005, 0.122) |
| TDCPP | 100 | 0.784 (0.718, 0.919) | (0.39, 3.76) | 100 | 0.875 (0.614, 1.21) | (0.400, 2.05) |
| TCPP | 100 | 0.508 (0.413, 0.691) | (0.285, 1.09) | 100 | 0.580 (0.484, 1.10) | (0.261, 2.34) |
For full names of chemicals, see Supplemental Table 2.
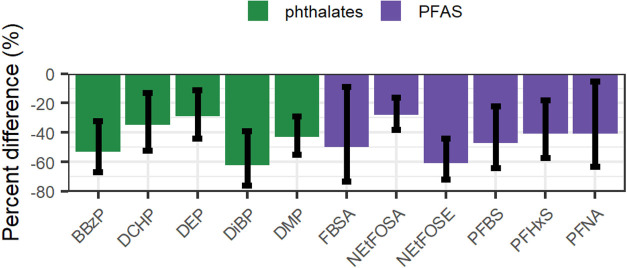
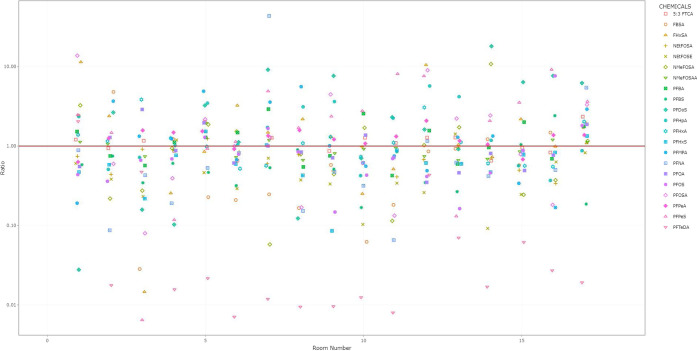
Generally, air concentrations of most PFAS decreased during the intervention period, and in some cases, these decreases were ≥50% (Figure 1 and Table 3). Notably, concentrations of NEtFOSE, N-Ethyl-perfluorooctanesulfonamide (N-EtFOSA), perfluorobutane sulfonic acid (PFBS), perfluorobutane sulfonamide (FBSA), perfluorohexane sulfonic acid (PFHxS), perfluorosulfonic acid (PFOS), and perfluorononanoic acid (PFNA) decreased by 61% (95% CI: −72, −44), 28 (95% CI: −38, −16), 47% (95% CI: −64, −22), 50% (95% CI: −73, −9), 41% (95% CI: −57, −18), 30% (95% CI: −53, 0), and 41% (95% CI: −63, −5), respectively. Concentrations of perfluoro-n-pentanoic acid (PFPeA) significantly increased by 23% (95% CI: 4, 45), and concentrations of perfluorododecanesulfonic acid (PFDoS), perfluoroheptanoic acid (PFHpA), perfluoropentanesulfonic (PFPeS), and perfluorotetradecanoic acid (PFTeDA) increased, but the 95% CI contained zero. The sums of perfluorooctane sulfonamides (−29%; 95% CI: −42, −12) and perfluorosulfonates (−34%; 95% CI: −54, −6) declined during the intervention period. The sums of perfluoroalkane sulfonamides also declined (−27%; 95% CI: −57, 24), while there was no change in the sum of perfluorocarboxylates (7%; 95% CI: −12, 31). All rooms had declines in air concentrations of at least two of the seven PFAS with the largest average decrease during the intervention (NEtFOSE, NEtFOSA, FBSA, PFBS, PFHxS, PFOS, and PFNA) (Figure 3; Supplemental Files 1, 2, and 3). Concentrations of FBSA increased in the two largest (>92 m2 or 1000 ft2) and two smallest rooms (<17 m2 or 180 ft2).
Figure 1.
Box-and-whisker, violin, and line plots of PFAS air concentrations (pg/m3) before and during the CR Box intervention (n = 17 rooms).aFor full names of chemicals, see Supplemental Table 2. bEach dot represents the room-specific PFAS concentration before and during the intervention, with a line connecting the same room. The shaded area represents the density function of PFAS concentrations before and during the intervention.
Table 3. Geometric Mean Air PFAS (pg/m3) and Other SVOC (ng/m3) Concentrations and Percent Differences in Air Concentrations before and during Intervention: Results from Linear Mixed Models (n = 17 rooms)a,b.
| chemical | pre-intervention GM | during-intervention GM | % difference (95% CI) | p-values |
|---|---|---|---|---|
| 5:3 FTCA | 192 | 208 | 8 (−4, 22) | 0.2291 |
| N-EtFOSE | 1330 | 519 | –61 (−72, −44) | <0.0001 |
| N-EtFOSA | 16.9 | 12.2 | –28 (−38, −16) | 0.0007 |
| N-MeFOSA | 9.06 | 6.55 | –28 (−53, 11) | 0.1603 |
| N-MeFOSAA | 0.120 | 0.113 | –6 (−75, 263) | 0.9338 |
| PFOSA | 1.88 | 1.88 | 0 (−42, 73) | 0.9979 |
| PFBS | 35.0 | 18.6 | –47 (−64, −22) | 0.0049 |
| PFBA | 26.9 | 29.2 | 8 (−22, 50) | 0.6327 |
| FBSA | 2.42 | 1.21 | –50 (−73, −9) | 0.0362 |
| PFMPA | 0.49 | 0.539 | 9 (−21, 51) | 0.6125 |
| PFPeA | 11.4 | 13.9 | 23 (4, 45) | 0.0307 |
| PFPeS | 0.12 | 0.173 | 40 (−37, 210) | 0.4183 |
| FHxSA | 1.83 | 1.94 | 6 (−44, 101) | 0.8566 |
| PFHxA | 10.4 | 11.2 | 7 (−20, 44) | 0.6477 |
| PFHxS | 31.5 | 18.6 | –41 (−57, −18) | 0.0057 |
| PFHpA | 1.06 | 1.48 | 39 (−6, 108) | 0.1208 |
| PFOS | 102 | 70.3 | –31 (−53, 0) | 0.0668 |
| PFOA | 36.3 | 32.0 | –12 (−33, 17) | 0.3889 |
| PFNA | 5.19 | 3.05 | –41 (−63, −5) | 0.0443 |
| PFDoS | 13.1 | 18.8 | 44 (−32, 205) | 0.3604 |
| PFTeDA | 6.29 | 8.97 | 42 (−2, 108) | 0.0841 |
| DMP | 2.55 | 1.45 | –43 (−55, −29) | 0.0002 |
| DEP | 96.2 | 67.9 | –29 (−44, −11) | 0.0089 |
| DnBP | 22.4 | 14.1 | –37 (−64, 11) | 0.1281 |
| DiBP | 39.2 | 14.9 | –62 (−76, −39) | 0.001 |
| DnPP | 28.8 | 18.1 | –37 (−64, 11) | 0.1281 |
| BBzP | 0.170 | 0.081 | –53 (−67, −32) | 0.0009 |
| DCHP | 25.8 | 16.7 | –35 (−52, −13) | 0.0121 |
| DEHP | 0.425 | 0.443 | 4 (−26, 46) | 0.8128 |
| DEHA | 1.51 | 1.51 | 0 (−22, 29) | 0.9947 |
| DnOP | 4.99 | 6.85 | 37 (9, 72) | 0.0152 |
| BP | 1.06 | 1.02 | –4 (−30, 32) | 0.8195 |
| galoxolide/tonalid | 0.334 | 0.247 | –26 (−46, 1) | 0.0764 |
| TCEP | 0.146 | 0.143 | –2 (−40, 58) | 0.9309 |
| TDCPP | 0.876 | 0.890 | 2 (−33, 55) | 0.9436 |
| TCPP | 0.538 | 0.717 | 33 (−6, 90) | 0.1294 |
| PCB-52 | 0.004 | 0.003 | –29 (−60, 24) | 0.2443 |
For full names of chemicals, see Supplemental Table 2.
Figure 3.
Room-specific changes in PFAS air concentrations before and during the intervention. aFor full names of chemicals, see Supplemental Table 2. bRoom-specific change presented as a ratio on the y-axis with the during-intervention concentration divided by the before-intervention concentration. Ratios <1 indicate that the chemical concentration declined during the intervention. The red line indicates a ratio of 1 (i.e., no change). cAn interactive version of this figure is available as Supplemental File 1. Rooms are sorted from smallest to largest area (left to right).
The five PFAS whose air concentrations increased during the intervention were infrequently detected on unused filter pieces and concentrations were similar on used filters compared to unused ones (Supplemental Table 9). Average masses of 3:3 fluorotelomer carboxylic acid (3:3 FTCA), NMeFOSAA, PFBS, perfluorohexanoic acid (PFHxA), perfluorohexane sulfonic acid (PFHxS), perfluorooctanoate (PFOA), and perfluorosulfonic acid (PFOS) were higher on used filter pieces compared to unused ones. FBSA, NEtFOSE, and N-methyl-perfluorooctane sulfonamidoethanol (NMeFOSE) were higher on unused filters compared to used ones.
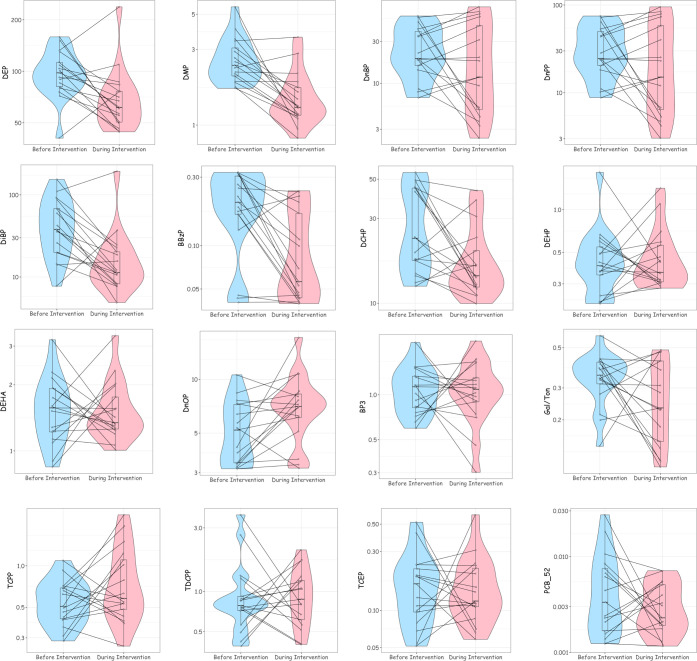
Air concentrations of all phthalates, except di-2-ethylhexyl phthalate (DEHP) and di-n-octyl phthalate (DnOP), decreased during the intervention period (Figure 2 and Table 3). Air concentrations of di-methyl phthalate (DMP) (−43%; 95% CI: −55, −29), DEP (−29%; 95% CI: −44, −11), DiBP (−62%; 95% CI: −76, −39), butyl benzyl phthalate (BBzP) (−53%; 95% CI: −67, −32), and di-cyclohexanoic phthalate (DCHP; −35%; 95% CI: −52, −13) all decreased by at least 25%. Interestingly, DnOP concentrations were 37% (95% CI: 9, 72) higher during the intervention period. Air concentrations of Galoxolide/Tonalid, synthetic fragrances, decreased by 26% (95% CI: −46, 1). Notably, air concentrations of tris(1-chloro-2-propyl) phosphate (TCPP) increased by 33% (95% CI: −6, 90), but this effect estimate included the null value of 0%.
Figure 2.
Box-and-whisker, violin, and line plots of air SVOC concentrations (ng/m3) before and during the CR Box intervention (n = 17 rooms). aFor full names of chemicals, see Supplemental Table 2. bEach dot represents the room-specific SVOC concentration before and during the intervention, with a line connecting the same room. The shaded area represents the density function of SVOC concentrations before and during the intervention.
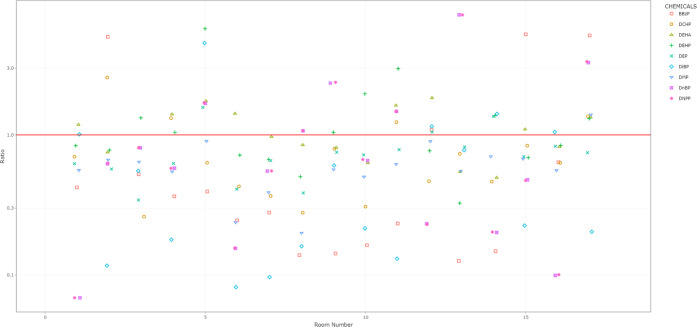
In all rooms, we observed declines in at least two of the five phthalates with >25% decreases in concentrations during the intervention period (Figure 4; Supplemental Files 2, 3, and 4). There was no discernible pattern of increases or decreases in air phthalate concentrations by room size.
Figure 4.
Room-specific changes in phthalate air concentrations before and during the intervention. aFor full names of chemicals, see Supplemental Table 2. bRoom-specific change presented as a ratio on the y-axis with the during-intervention concentration divided by the before-intervention concentration. Ratios <1 indicate that the chemical concentration declined during the intervention. The red line indicates a ratio of 1 (i.e., no change). cAn interactive version of this figure is available as Supplemental File 4. Rooms are sorted from smallest to largest area (left to right).
Brominated diphenyl ethers, polychlorinated biphenyls, and organophosphate ester concentrations were low or below the LOD on unused filter pieces, whereas most phthalates were detected on unused filters (Supplemental Table 10). However, concentrations of nearly all phthalates were higher on used filter pieces compared to unused ones, including DnOP, the one phthalate whose concentration increased during the intervention. In addition, concentrations of organophosphate esters and Galoxolide/Tonalid were higher on used filters than unused ones.
For phthalates, lower log Koa values (β per log-unit decrease in Koa: −0.05; 95% CI: −0.01, −0.09; p-value = 0.03) and molecular weight (β per 100 g decrease in molecular weight: −0.16; 95% CI: −0.01, −0.32; p-value = 0.04) were associated with greater decreases in air concentrations during the intervention (Supplemental Figure 6). Among PFAS, there were no clear associations of log Koa and molecular weight with air concentration change (all p-values > 0.26). However, five of the seven PFAS whose concentrations declined had sulfonyl groups and lower log Koa values (<5.5).
During daytime when the CR Boxes were operating, sound levels increased by 5.0 dB(A) on average (95% CI: 3.1, 7.0; p-value < 0.0001) from 47.7 to 52.7 dB(A); levels increased in all rooms (range: 1.2 to 11.6 dB(A)). During nighttime when CR Boxes were operational, average sound levels increased by 10.1 dB(A) (95% CI: 7.8, 12.4) from 39.3 dB to 49.4 dB(A). Sound increased in all rooms at nighttime when boxes were operational (range: 1.9 to 18.4 dB(A)).
Discussion
In this study, we demonstrated that CR Boxes reduced gas-phase indoor air concentrations of seven PFAS and five phthalates. Of the PFAS whose concentrations declined, five were lower-volatility PFAS with sulfonyl groups (log Koa < 5.5). Phthalates whose concentrations declined were more volatile (log Koa < 11.5). We observed increased concentrations of five PFAS and one phthalate. Three of the five PFAS whose concentrations increased had carboxylic acid groups. Sound levels increased modestly during the intervention. Collectively these results suggest that CR Boxes may be a cost-effective way to reduce indoor air concentrations of some PFAS and other SVOCs, in addition to mitigating COVID-19 transmission.
Most studies evaluating the impact of air filtration on indoor air quality focused on particulate matter with few examining SVOCs.23 SVOCs readily partition between indoor surfaces and are found in both the gas and particle-bound phase in indoor air.7 A simulation study modeled reductions in indoor air concentrations of PAHs and DEHP with air filtration and predicted reduced gas-phase and particle-phase air concentrations.11 An intervention study evaluated the impact of HEPA air filtration on indoor air phthalate concentrations using a randomized crossover design in 18 dormitories in China.13 Similar to the magnitude of our results, this prior study found that filtration reduced air concentrations of DMP, DEP, DnBP, DiBP, and BBzP by 50–90%. The authors reported greater decreases in gas-phase phthalate concentrations compared to particle-phase concentrations, likely due to the presence of activated carbon on the air filters. We note that the MERV-13 filters used in this study contained polyolefin and titanium dioxide and it is possible that the SVOCs interacted with the filter material itself rather than simply trapped particle-bound SVOCs. It is also possible that the gas-phase SVOCs adsorbed onto trapped particles on the MERV-13 filters. Given that many SVOCs are routinely detected in homes and offices and the dynamic nature of SVOCs that readily partition to various surfaces and materials indoors, it is not surprising that exposure could be modified through air filtration.4,6,24,25 A future study with simultaneous quantification of SVOCs in the gas- and particulate-phase of air and on the MERV filter could help elucidate SVOC dynamics in the presence of air filtration.
PFAS and phthalates found or used in indoor environments were among those reduced during the intervention period. Air concentrations of N-EtFOSE, perfluorooctane sulfonamides (individual and sum), and perfluorosulfonates (individual and sum) were reduced during the intervention period. Perfluorooctane sulfonamidoethanols (like N-EtFOSE), perfluorooctane sulfonamides, and perfluorosulfonates (also degradation products of former categories) have been detected in electronics, carpets, carpet protection products, textiles, and upholstery.26 Phthalates with lower log Koa values had greater declines during the intervention. Phthalates like DEP are used in personal care and cleaning products,27 while BBzP is used in some furnishings.28 Reassuringly, pre-intervention air concentrations of PFAS and phthalates were generally on the same order of magnitude as previous studies of gas-phase indoor air concentrations.24,29
We note that the concentrations of one PFAS (PFPeA) and one phthalate (DnOP) significantly increased during the intervention period. Both of these SVOCs have similar physio-chemical properties (e.g., log Koa values) and uses in the indoor environment to other SVOCs whose concentrations did not increase during the intervention. Therefore, the reason for the increase in concentrations of these SVOCs is unclear. However, it may be that there are sources (e.g., carpet treatments and materials) that may be releasing these compounds as a result of the altered partitioning equilibrium induced by the CR boxes. Material testing would be needed to confirm this speculation.
While we detected several SVOCs on unused MERV-13 filters, our results do not suggest that the filters would be a substantial source of the SVOCs that increased during the intervention period. Given the high volume of air passing over the filters and long period of air sampling, it is unlikely that any contamination on the filters was a significant source of SVOCs in the rooms. Indeed, we found higher concentrations of many PFAS, phthalates, organophosphate flame retardants, and a synthetic fragrance chemical on used filters compared to unused ones, suggesting that the air filters acted as a sink. In fact, previous studies have used air filters within HVAC systems to assess indoor SVOC exposures.10 However, future studies should be cautious with the materials used since prior interventions have observed unexpected increases in exposure due to contamination.30 Moreover, future studies should evaluate if the CR Boxes would become a source of PFAS or other SVOCs after long-term use and chemical accumulation on filters. Finally, future studies should determine if filter concentrations are related to measured air concentrations, as this could provide a novel method to simultaneously conduct intervention and exposure assessment studies.
Inhalation of contaminated air can meaningfully contribute to biomarker concentrations of fluorotelomers, perfluoroalkane sulfonamido substances, and low-volatility phthalates, especially in young children.8,31 Given the reductions in air concentrations of several PFAS and phthalates associated with use of CR Boxes in this study, future studies could quantify the impact of air filtration on biomarker concentrations measured in occupants to better estimate potential health impacts of air filter interventions. Indeed, prior intervention studies using dietary modifications and changes in personal care products have observed reduced urinary phthalate concentrations over a few days of intervention.32 These studies may be more appropriate for SVOCs such as phthalates given their shorter biological half-lives relative to biologically persistent SVOCs like PFAS.33
A potential drawback to using CR Boxes is the additional sound they produce. We observed an increase in daytime sound (5 dB(A)) during their operation that translates to a 3-fold rise in the sound intensity and a 41% increase in the sound loudness. Indeed, prior studies suggest that a 5 dB(A) sound level increase would be noticeable to occupants, increasing their annoyance and inducing fatigue.34 In this study, some occupants complained that the CR Boxes interfered with classroom instruction and meetings, particularly when CR Boxes were located near the speaker or listener. Future implementation studies could be conducted to increase the acceptability of CR Boxes and identify low-cost modifications (e.g., placement in room, baffling, etc.) to reduce their sound levels.
Characteristics of the room, as well as behaviors of occupants may influence the degree to which CR Boxes reduce SVOC air concentrations. Given our small sample size, we were not able to systematically examine factors impacting CR Box effectiveness, but we present results for qualitative evaluation in Supplemental Files 1–4. While the concentrations of some PFAS were higher in smaller and larger rooms, we cannot discount the possibility that these increases were due to the behaviors of room occupants (e.g., person applying PFAS-containing cosmetics next to sampler).35 Additionally, the CR Boxes were operated for 12 h per day, allowing for a rebound of air chemical concentrations during the nighttime hours. Despite this, we still observed statistically significant reductions in the concentrations of several PFAS and phthalates. Future studies could investigate factors that influence the effectiveness of CR Boxes to reduce SVOC indoor air concentrations (e.g., materials in room, placement in room, initial concentrations, duration of operation, etc.).
Our study has several notable strengths. First, we used a quasi-experimental design to quantify the potential causal effect of CR Boxes on PFAS and other SVOC indoor air concentrations. An alternative design could have sampled rooms that did not receive the intervention to ensure that unmeasured time-varying factors (e.g., temperature or relative humidity) did not influence our results; however, we did not have the resources to do so. An additional strength was the comprehensive assessment of multiple PFAS and other SVOCs, allowing us to quantify the impact of this air filter intervention on exposure to a mixture of indoor air pollutants. Another strength is that we conducted this study in a single building over a relatively short period to reduce the impact of between-building factors and time-varying confounding factors on our results.
Our study has some limitations. First, our small sample size prevented us from exploring how room-level factors impacted the effectiveness of the CR Boxes on indoor air PFAS and other SVOC concentrations. Second, time-varying confounding is possible and changes in temperature, relative humidity, or room occupancy may have influenced air concentrations of PFAS and SVOCs during the intervention.36 Future studies could evaluate these as potential sources of bias. Third, it is possible that PFAS and other SVOC concentrations decreased during the winter break when the building was unoccupied. Finally, one downside of the CR Boxes was increased sound they produced. In our study, this reduced fidelity to the intervention, as we noted instances when the fans were turned off during the intervention period. However, we expect that this would result in underestimation of the true effect of the CR Boxes on air PFAS and other SVOC concentrations.
In conclusion, we found that CR Boxes reduced indoor air concentrations of seven PFAS and five phthalates by at least 25%. Thus, reducing exposure to a mixture of indoor air pollutants linked to adverse health effects in humans is a co-benefit of conducting air filtration to reduce COVID-19 transmission. Future studies should determine if air filtration is effective at reducing the body burden of these exposures and preventing exposure-related health effects.
Acknowledgments
The authors thank Emilia G. Braun and Elissia Franklin for their assistance with the field work. They appreciate Kevin Travossos providing them with detailed information about the building’s air handling system. They also thank Fiona Dunn and Lucas Scheidl for their assistance in preparing the air samplers. They are grateful for funding from the Brown School of Public Health Dean’s Office and Health Equity Scholars Program. The authors appreciate Jim Rosenthal providing them with information about the composition of the Tex-Air filters.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.2c05169.
Analytic chemistry methods; calculated equivalent air exchanges per hour provided by Corsi–Rosenthal boxes (Table S1); full names, abbreviations, and classes of environmental chemicals included in the present study (Table S2); retention time, quantification ion, and confirming ions used in targeted PFAS and SVOC analyses (Tables S3 and S4); PFAS analytes, their method limit of detection (LOD), blank concentrations, and technical replicate concentrations (Table S5); SVOC analytes, their method limit of detection (LOD), blank concentrations, and technical replicate concentrations (Table S6); recovery of SVOC and PFAS analytes from standards spiked into an XAD and PUF sample (Tables S7 and S8); PFAS and SVOC concentrations on unused and used box air filter pieces (Tables S9 and S10); representative image of an assembled Corsi–Rosenthal box used in the intervention study (Figure S1); representative image of disassembled and assembled SVOC sampling devices (Figure S2); representative image of air sampling device (Figure S3); PFAS and SVOC concentrations in solvent extracts, field blanks, and field sample extracts (Figures S4 and S5); and associations of log-Koa and molecular weight with change in room phthalate and PFAS air concentrations before vs during the intervention (Figure S6) (PDF)
Interactive graphic that includes features of each room and instructions to interact with the plots (HTM)
(HTM)
(HTM)
(HTM)
Funding for this work came from the Brown University School of Public Health. J.M.B. and K.M. were supported by NIEHS R01 ES032386. The Thermo LC-Orbitrap MS was partially funded by NSF Major Research Instrumentation (MRI) award CBET-1919870
The authors declare the following competing financial interest(s): Joseph Braun was financially compensated for his services as an expert witness for plaintiffs in litigation related to PFAS-contaminated drinking water.
Notes
J.M.B. was financially compensated for his services as an expert witness for plaintiffs in litigation related to PFAS-contaminated drinking water.
Supplementary Material
References
- Curtius J.; Granzin M.; Schrod J. Testing mobile air purifiers in a school classroom: Reducing the airborne transmission risk for SARS-CoV-2. Aerosol Sci. Technol. 2021, 55, 586–599. 10.1080/02786826.2021.1877257. [DOI] [Google Scholar]
- Dal Porto R.; Kunz M. N.; Pistochini T.; Corsi R. L.; Cappa C. D. Characterizing the performance of a do-it-yourself (DIY) box fan air filter. Aerosol Sci. Technol. 2022, 56, 564–572. 10.1080/02786826.2022.2054674. [DOI] [Google Scholar]
- Rosenthal J.A Variation on the “Box Fan with MERV 13 Filter” Air Cleaner, https://www.texairfilters.com/a-variation-on-the-box-fan-with-merv-13-filter-air-cleaner/ (accessed on 2022).
- a Mitro S. D.; Dodson R. E.; Singla V.; Adamkiewicz G.; Elmi A. F.; Tilly M. K.; Zota A. R. Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-analysis of U.S. Studies. Environ. Sci. Technol. 2016, 50, 10661–10672. 10.1021/acs.est.6b02023. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rudel R. A.; Camann D. E.; Spengler J. D.; Korn L. R.; Brody J. G. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ. Sci. Technol. 2003, 37, 4543–4553. 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]; c Dodson R. E.; Bessonneau V.; Udesky J. O.; Nishioka M.; McCauley M.; Rudel R. A. Passive indoor air sampling for consumer product chemicals: a field evaluation study. J. Exposure Sci. Environ. Epidemiol. 2019, 29, 95–108. 10.1038/s41370-018-0070-9. [DOI] [PubMed] [Google Scholar]; d Shoeib M.; Harner T.; G M. W.; Lee S. C. Indoor sources of poly- and perfluorinated compounds (PFCS) in Vancouver, Canada: implications for human exposure. Environ. Sci. Technol. 2011, 45, 7999–8005. 10.1021/es103562v. [DOI] [PubMed] [Google Scholar]
- a Dodson R. E.; Rodgers K. M.; Carey G.; Cedeno Laurent J. G.; Covaci A.; Poma G.; Malarvannan G.; Spengler J. D.; Rudel R. A.; Allen J. G. Flame Retardant Chemicals in College Dormitories: Flammability Standards Influence Dust Concentrations. Environ. Sci. Technol. 2017, 51, 4860. 10.1021/acs.est.7b00429. [DOI] [PubMed] [Google Scholar]; b Kim H.; Rebholz C. M.; Wong E.; Buckley J. P. Urinary organophosphate ester concentrations in relation to ultra-processed food consumption in the general US population. Environ Res 2020, 182, 109070 10.1016/j.envres.2019.109070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel R. A.; Dodson R. E.; Perovich L. J.; Morello-Frosch R.; Camann D. E.; Zuniga M. M.; Yau A. Y.; Just A. C.; Brody J. G. Semivolatile endocrine-disrupting compounds in paired indoor and outdoor air in two northern California communities. Environ. Sci. Technol. 2010, 44, 6583–6590. 10.1021/es100159c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler C. J.; Nazaroff W. W. Semivolatile organic compounds in indoor environments. Atmos. Environ. 2008, 42, 9018–9040. 10.1016/j.atmosenv.2008.09.052. [DOI] [Google Scholar]
- a Egeghy P. P.; Lorber M. An assessment of the exposure of Americans to perfluorooctane sulfonate: a comparison of estimated intake with values inferred from NHANES data. J. Exposure Sci. Environ. Epidemiol. 2011, 21, 150–168. 10.1038/jes.2009.73. [DOI] [PubMed] [Google Scholar]; b Bekö G.; Weschler C. J.; Langer S.; Callesen M.; Toftum J.; Clausen G. Children’s phthalate intakes and resultant cumulative exposures estimated from urine compared with estimates from dust ingestion, inhalation and dermal absorption in their homes and daycare centers. PLoS One 2013, 8, e62442 10.1371/journal.pone.0062442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kalloo G.; Wellenius G. A.; McCandless L.; Calafat A. M.; Sjodin A.; Karagas M.; Chen A.; Yolton K.; Lanphear B. P.; Braun J. M. Profiles and Predictors of Environmental Chemical Mixture Exposure among Pregnant Women: The Health Outcomes and Measures of the Environment Study. Environ. Sci. Technol. 2018, 52, 10104–10113. 10.1021/acs.est.8b02946. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Pacyga D. C.; Haggerty D. K.; Nicol M.; Henning M.; Calafat A. M.; Braun J. M.; Schantz S. L.; Strakovsky R. S. Identification of profiles and determinants of maternal pregnancy urinary biomarkers of phthalates and replacements in the Illinois Kids Development Study. Environ. Int. 2022, 162, 107150 10.1016/j.envint.2022.107150. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Prevention C. f. D. C. a.National Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Service, 2022, https://www.cdc.gov/exposurereport/.; d Woodruff T. J.; Zota A. R.; Schwartz J. M. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ. Health Perspect. 2011, 119, 878–885. 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bi C.; Maestre J. P.; Li H.; Zhang G.; Givehchi R.; Mahdavi A.; Kinney K. A.; Siegel J.; Horner S. D.; Xu Y. Phthalates and organophosphates in settled dust and HVAC filter dust of U.S. low-income homes: Association with season, building characteristics, and childhood asthma. Environ. Int. 2018, 121, 916–930. 10.1016/j.envint.2018.09.013. [DOI] [PubMed] [Google Scholar]; b Xu Y.; Liang Y.; Urquidi J. R.; Siegel J. A. Semi-volatile organic compounds in heating, ventilation, and air-conditioning filter dust in retail stores. Indoor Air 2015, 25, 79–92. 10.1111/ina.12123. [DOI] [PubMed] [Google Scholar]
- Shi S.; Zhao B.; Zhang J. J. Effect of residential air cleaning interventions on risk of cancer associated with indoor semi-volatile organic compounds: a comprehensive simulation study. Lancet Planet Health 2018, 2, e532–e539. 10.1016/S2542-5196(18)30236-5. [DOI] [PubMed] [Google Scholar]
- Maestas M. M.; Brook R. D.; Ziemba R. A.; Li F.; Crane R. C.; Klaver Z. M.; Bard R. L.; Spino C. A.; Adar S. D.; Morishita M. Reduction of personal PM2.5 exposure via indoor air filtration systems in Detroit: an intervention study. J. Exposure Sci. Environ. Epidemiol. 2019, 29, 484–490. 10.1038/s41370-018-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Shi J.; Zhao Y.; Xue L.; Li G.; Wang B.; Huang J.; Wu S.; Guo X. Cardiorespiratory responses in healthy young adults with exposure to indoor airborne PAEs: A randomized, crossover trial of air purification. Environ. Int. 2021, 156, 106761 10.1016/j.envint.2021.106761. [DOI] [PubMed] [Google Scholar]
- a Vrijheid M.; Casas M.; Gascon M.; Valvi D.; Nieuwenhuijsen M. Environmental pollutants and child health-A review of recent concerns. Int. J. Hyg. Environ. Health 2016, 219, 331. 10.1016/j.ijheh.2016.05.001. [DOI] [PubMed] [Google Scholar]; b Ghassabian A.; Vandenberg L.; Kannan K.; Trasande L. Endocrine-Disrupting Chemicals and Child Health. Annu Rev Pharmacol Toxicol 2022, 62, 573–594. 10.1146/annurev-pharmtox-021921-093352. [DOI] [PubMed] [Google Scholar]; c Vuong A. M.; Yolton K.; Cecil K. M.; Braun J. M.; Lanphear B. P.; Chen A. Flame retardants and neurodevelopment: An updated review of epidemiological literature. Curr. Epidemiol. Rep. 2020, 7, 220–236. 10.1007/s40471-020-00256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Braun J. M. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Li M. C.; Chen C. H.; Guo Y. L. Phthalate esters and childhood asthma: A systematic review and congener-specific meta-analysis. Environ. Pollut. 2017, 229, 655–660. 10.1016/j.envpol.2017.06.083. [DOI] [PubMed] [Google Scholar]; b Wu W.; Wu C.; Ji C.; Diao F.; Peng J.; Luo D.; Mu X.; Ruan X. Association between phthalate exposure and asthma risk: A meta-analysis of observational studies. Int. J. Hyg. Environ. Health 2020, 228, 113539 10.1016/j.ijheh.2020.113539. [DOI] [PubMed] [Google Scholar]
- a Nielsen C.; Joud A. Susceptibility to COVID-19 after High Exposure to Perfluoroalkyl Substances from Contaminated Drinking Water: An Ecological Study from Ronneby, Sweden. Int. J. Environ. Res. Public Health 2021, 18, 10702. 10.3390/ijerph182010702. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Grandjean P.; Timmermann C. A. G.; Kruse M.; Nielsen F.; Vinholt P. J.; Boding L.; Heilmann C.; Molbak K. Severity of COVID-19 at elevated exposure to perfluorinated alkylates. PLoS One 2020, 15, e0244815 10.1371/journal.pone.0244815. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Program N. T.Systematic Review of Immunotoxicity Associated with Exposure to Perfluorooctanoic Acid (PFOA) Or Perfluorooctane Sulfonate (PFOS). 2016, https://ntp.niehs.nih.gov/ntp/about_ntp/monopeerrvw/2016/july/draftsystematicreviewimmunotoxicityassociatedpfoa_pfos_508.pdf.
- Rosenthal J.IAQ Research-Practice in Action: The Corsi/Rosenthal Box Air Cleaner, 2021. (accessed 2022 October 1).
- ISO. Acoustics: Description, Measurement and Assessment of Environmental Noise; Geneva, Switzerland, 2016.
- Bell L. H.Industrial Noise Control: Fundamentals and Applications; Marcel Dekker, Inc., 1982. [Google Scholar]
- Schildroth S.; Rodgers K. M.; Strynar M.; McCord J.; Poma G.; Covaci A.; Dodson R. E. Per-and polyfluoroalkyl substances (PFAS) and persistent chemical mixtures in dust from U.S. colleges. Environ. Res. 2022, 206, 112530 10.1016/j.envres.2021.112530. [DOI] [PubMed] [Google Scholar]
- Mueller R.; Yingling V.. Naming Conventions and Physical and Chemical Properties of Per- and Polyfluoroalkyl Substances (PFAS); ITRC: Washington, D.C., 2020. https://pfas-1.itrcweb.org/fact_sheets_page/PFAS_Fact_Sheet_Naming_Conventions_April2020.pdf. [Google Scholar]
- Sievert C.Interactive Web-Based Data Visualization with R, Plotly, and Shiny; CRC Press, 2020.
- a Bennett D. H.; Moran R. E.; Krakowiak P.; Tancredi D. J.; Kenyon N. J.; Williams J.; Fisk W. J. Reductions in particulate matter concentrations resulting from air filtration: A randomized sham-controlled crossover study. Indoor Air 2022, 32, e12982 10.1111/ina.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Barn P.; Gombojav E.; Ochir C.; Laagan B.; Beejin B.; Naidan G.; Boldbaatar B.; Galsuren J.; Byambaa T.; Janes C.; et al. The effect of portable HEPA filter air cleaners on indoor PM2.5 concentrations and second hand tobacco smoke exposure among pregnant women in Ulaanbaatar, Mongolia: The UGAAR randomized controlled trial. Sci. Total Environ. 2018, 615, 1379–1389. 10.1016/j.scitotenv.2017.09.291. [DOI] [PubMed] [Google Scholar]
- Morales-McDevitt M. E.; Becanova J.; Blum A.; Bruton T. A.; Vojta S.; Woodward M.; Lohmann R. The Air that we Breathe: Neutral and volatile PFAS in Indoor Air. Environ. Sci. Technol. Lett. 2021, 8, 897–902. 10.1021/acs.estlett.1c00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shoeib M.; Harner T.; Wilford B. H.; Jones K. C.; Zhu J. Perfluorinated sulfonamides in indoor and outdoor air and indoor dust: occurrence, partitioning, and human exposure. Environ. Sci. Technol. 2005, 39, 6599–6606. 10.1021/es048340y. [DOI] [PubMed] [Google Scholar]; b Nilsson H.; Karrman A.; Rotander A.; van Bavel B.; Lindstrom G.; Westberg H. Professional ski waxers’ exposure to PFAS and aerosol concentrations in gas phase and different particle size fractions. Environ Sci Process Impacts 2013, 15, 814–822. 10.1039/c3em30739e. [DOI] [PubMed] [Google Scholar]; c Dodson R. E.; Udesky J. O.; Colton M. D.; McCauley M.; Camann D. E.; Yau A. Y.; Adamkiewicz G.; Rudel R. A. Chemical exposures in recently renovated low-income housing: Influence of building materials and occupant activities. Environ. Int. 2017, 109, 114–127. 10.1016/j.envint.2017.07.007. [DOI] [PubMed] [Google Scholar]; d Kim U. J.; Wang Y.; Li W.; Kannan K. Occurrence of and human exposure to organophosphate flame retardants/plasticizers in indoor air and dust from various microenvironments in the United States. Environ. Int. 2019, 125, 342–349. 10.1016/j.envint.2019.01.065. [DOI] [PubMed] [Google Scholar]
- Glüge J.; Scheringer M.; Cousins I. T.; DeWitt J. C.; Goldenman G.; Herzke D.; Lohmann R.; Ng C. A.; Trier X.; Wang Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process Impacts 2020, 22, 2345–2373. 10.1039/d0em00291g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hubinger J. C. A survey of phthalate esters in consumer cosmetic products. J. Cosmet. Sci. 2010, 61, 457–465. [PubMed] [Google Scholar]; b Koniecki D.; Wang R.; Moody R. P.; Zhu J. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ. Res. 2011, 111, 329–336. 10.1016/j.envres.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Kavlock R.; Boekelheide K.; Chapin R.; Cunningham M.; Faustman E.; Foster P.; Golub M.; Henderson R.; Hinberg I.; Little R.; et al. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of butyl benzyl phthalate. Reprod. Toxicol. 2002, 16, 453–487. 10.1016/S0890-6238(02)00029-1. [DOI] [PubMed] [Google Scholar]
- Tran T. M.; Kannan K. Occurrence of phthalate diesters in particulate and vapor phases in indoor air and implications for human exposure in Albany, New York, USA. Arch. Environ. Contam. Toxicol. 2015, 68, 489–499. 10.1007/s00244-015-0140-0. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S.; Alcedo G.; Saelens B. E.; Zhou C.; Dills R. L.; Yu J.; Lanphear B. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J. Exposure Sci. Environ. Epidemiol. 2013, 23, 378–384. 10.1038/jes.2013.9. [DOI] [PubMed] [Google Scholar]
- Fromme H.; Tittlemier S. A.; Volkel W.; Wilhelm M.; Twardella D. Perfluorinated compounds--exposure assessment for the general population in Western countries. Int. J. Hyg. Environ. Health 2009, 212, 239–270. 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- a Rudel R. A.; Gray J. M.; Engel C. L.; Rawsthorne T. W.; Dodson R. E.; Ackerman J. M.; Rizzo J.; Nudelman J. L.; Brody J. G. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ. Health Perspect. 2011, 119, 914–920. 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Harley K. G.; Kogut K.; Madrigal D. S.; Cardenas M.; Vera I. A.; Meza-Alfaro G.; She J.; Gavin Q.; Zahedi R.; Bradman A.; et al. Reducing Phthalate, Paraben, and Phenol Exposure from Personal Care Products in Adolescent Girls: Findings from the HERMOSA Intervention Study. Environ. Health Perspect. 2016, 124, 1600–1607. 10.1289/ehp.1510514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen G. W.; Burris J. M.; Ehresman D. J.; Froehlich J. W.; Seacat A. M.; Butenhoff J. L.; Zobel L. R. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Persinger M. A.; Tiller S. G.; Koren S. A. Background sound pressure fluctuations (5 DB) from overhead ventilation systems increase subjective fatigue of university students during three-hour lectures. Percept Mot Skills 1999, 88, 451–456. 10.2466/pms.1999.88.2.451. [DOI] [PubMed] [Google Scholar]; b ANSI. Quantities and Procedures for Description and Measurement of Environmental Sound – Part 4: Noise Assessment and Prediction of Long Term Community Response; IHS Markit: London, UK, 2003. [Google Scholar]
- Whitehead H. D.; Venier M.; Wu Y.; Eastman E.; Urbanik S.; Diamond M. L.; Shalin A.; Schwartz-Narbonne H.; Bruton T. A.; Blum A.; et al. Fluorinated Compounds in North American Cosmetics. Environ. Sci. Technol. Lett. 2021, 8, 538–544. 10.1021/acs.estlett.1c00240. [DOI] [Google Scholar]
- a Xu Y.; Little J. C. Predicting emissions of SVOCs from polymeric materials and their interaction with airborne particles. Environ. Sci. Technol. 2006, 40, 456–461. 10.1021/es051517j. [DOI] [PubMed] [Google Scholar]; b Zhou X.; Lian J.; Cheng Y.; Wang X. The gas/particle partitioning behavior of phthalate esters in indoor environment: Effects of temperature and humidity. Environ. Res. 2021, 194, 110681 10.1016/j.envres.2020.110681. [DOI] [PubMed] [Google Scholar]; c Liu C.; Morrison G. C.; Zhang Y. Role of aerosols in enhancing SVOC flux between air and indoor surfaces and its influence on exposure. Atmos. Environ. 2012, 55, 347–356. 10.1016/j.atmosenv.2012.03.030. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.