Abstract
Objectives
The cognitive-protective effects related to the consumption of a variety of fruits are supported by several intervention studies. This systematic review and meta-analysis compared the magnitude of effects following chronic (≥1 week) consumption of frozen, freeze-dried powder including extracts and juices of fruits, covering berries, cherries and citrus, on cognition and mood in adults.
Methods
PubMed, Web of Science, Scopus, and psycARTICLES were searched from inception until February, 2021. Inclusion criteria were randomised controlled trials assessing memory, executive function, psychomotor speed, mood and mini mental state examination in adult participants ≥18 years of age. Cognition was tested by global or domain specific tasks.
Results
Out of 13,861 articles identified, 16 papers were included; 11 studies provided suitable data for meta-analysis. Fourteen studies reported improvement or trend for improvement in cognition, five studies assessed mood and one study supplementing grape juice found trend for mood improvement. From the meta-analysis, cherry juice supplementation was suggested to improve psychomotor speed by −0.37 of standardised mean difference (95% CI [−0.74, 0.01]) in reaction time (P = 0.05).
Conclusions
The meta-analysis did not sufficiently support a role for fruits or fruit forms to improve cognition and mood.
Subject terms: Risk factors, Cognitive control
Introduction
Age-induced cognitive decline is a common and important phenotype that is likely to be associated with increased disease risks such as dementia [1]. Ageing is associated with an increased susceptibility to chronic organ disease and decline of metabolic and immune functions which impact on the brain [1]. Aside from cognitive decline with ageing, an increased age-associated risk of neurodegenerative disorders such as dementia and Alzheimer’s disease is prevalent [2]. Therefore, the measurement and treatment of cognitive impairment is important due to the rising prevalence of dementia [2] and the benefits of early detection and prevention [3].
The rate of age-related cognitive impairment is mainly influenced by lifestyle behaviours, including diet [4, 5]. Fruits and vegetables represent a rich source of antioxidants including vitamins (vitamin C, B complex and E etc.), carotenoids and polyphenols, which have been shown to improve cognition [6–8]. A number of potential underlying mechanisms have been identified including the interaction of fruit polyphenols, carotenoids and vitamins with intracellular neuronal and glial signalling pathways, regulation of cerebral blood flow, and protection against neurotoxins and neuroinflammation [8–10]. A dose-response meta-analysis including nine studies (five cohort studies and four cross-sectional studies) with a total of 31,104 participants suggested approximately a 13% (OR = 0.87, 95% CI 0.77–0.99) reduction in cognitive impairment and dementia risk by an increment of 100 g per day of fruit and vegetable consumption [11]. A number of intervention studies also showed positive results indicating that intake of a range of flavonoid-rich fruit (e.g. blueberry, orange juice) improves both immediate and chronic cognitive performance or mood in older adults [12]. From a nutritional perspective, employing single whole foods instead of single components such as a supplement in interventions is more appropriate since synthetic single nutrient supplements are likely to be metabolised through different pathways to natural bioactive compounds [13].
Fruit can be consumed in a variety of forms following different processing methods, for example as fruit juices, smoothies, frozen fruit, and freeze-dried fruit powders and a recent paper emphasized the need for additional research assessing the effect of processed fruit on health, as fruit in processed forms (e.g. processed powder) provide consumer options whilst also reducing costs and food waste [14]. These different fruit forms could be an effective method to increase overall fruit consumption and it is important to determine the impact of different fruit groups and delivery forms on cognition in order to better inform the public. Therefore, the protective effects on cognition of whole fruit intervention or fruit intervention in different forms (e.g. powder, juice) instead of single molecules, such as polyphenols, are worth exploring.
Inconsistent findings have been reported on fruit interventions for cognitive effects [15, 16]. Whilst small sample size is indicated as a factor in null findings, the length of intervention as well as type and form of fruit may also be important. There could be metabolic difference between acute and chronic polyphenol-rich fruit interventions. The circulating metabolites, including polyphenol metabolites only remained in the cerebral blood flow (CBF) circulation for an approximately 1–2 h peak Cmax in plasma [17–19]. Emerging evidence from acute interventions employing a range of fruit juices and dried powders also indicates that polyphenol-rich fruit-based interventions e.g. citrus, grape, and blackcurrant juice and powder may benefit brain function [20–22].
In terms of nutritional value, fruit juices and dried fruit powders retain polyphenols, vitamins and minerals that are bio-accessible despite fibre and other nutrient losses that occur during processing [23, 24]. Fruit processing, such as smashing or thermal treatment, can damage the cell structure of whole fruit releasing cytoplasmic content that can make bioactive compounds more accessible for absorption [25]. Moreover, the presence of other constituents formed from the technological process or added from food matrix such as sugar could modify the bioavailability of the bioactives due to their ability to bind, solubilize, or stabilize [26]. Research has explored the difference in nutritional value and bioavailability of bioactives between whole fruit and processed fruit. One in vitro gastrointestinal digestion model study showed that mulberry juice still contains 60 - 70% of the anthocyanin content compared to unprocessed raw mulberry [27]. Furthermore, Kuntz et al. compared the bioavailability of selected anthocyanins from grape and blueberry juice with a smoothie and found no difference in plasma pharmacokinetics and recovery of the major anthocyanin species. However, significantly higher concentrations of the phenolic acid 3,4-dihydrobenzoic acid were shown after ingestion of the juice [28].
It is also worth noting that the cognitive domains categorised in intervention studies often vary depending on the cognitive ability that researchers intend to assess with different tasks applied [26]. Therefore, the impact of fruit interventions on specific cognitive domains cannot be quantitatively compared because of the large variability in the assessment tools and scoring interpretations. This concern has also been highlighted in other dietary trials assessing cognition [29]. Here, we have systematically reviewed and meta-analysed available fruit interventions to evaluate the chronic effects (≥1 week) of whole, powdered, and juiced fruit, specifically anthocyanin-rich berries and cherries, and flavonoid-rich citrus fruit on cognition in randomised controlled trials (RCTs). We also categorised specific tasks, depending on the assessed cognitive ability/domain for each RCT, in order to achieve quantitative comparison across RCTs. Due to the impact of mood on cognition, we also included assessment of mood outcomes here [30].
Methods
Study eligibility
We searched for studies investigating the effect of berry, cherry and citrus fruit supplementations on cognition and mood. Berries so defined includes grapes, blueberries, strawberries etc; citrus includes oranges etc; cherry is categorised as stone fruit [31]. The following specific inclusion criteria were applied: (1) study design: RCTs; (2) subjects: adult subjects ≥18 years of age; (3) interventions: only chronic intervention studies for at least one week providing or promoting citrus, cherry, or berries including blueberry, grape, blackberry, raspberry and cranberry in various forms (e.g. their juices) to be consumed without acute supplementation prior to testing; (4) control: control groups without components of citrus, cherry, or berries, likely isoenergetic placebo group; (5) outcomes: cognitive function and mood (described below); (6) Only English-language and peer-reviewed articles were included. No restriction of publication year was applied.
Data sources
This review is in line with the PICOS (population, intervention, comparator, outcome, study design) framework (Supplemental Table 1). The systematic review was conducted with a prospective protocol in accordance with Cochrane Handbook for Systematic Reviews of Interventions version 5.1 [32] and Centre for Reviews and Dissemination Guidelines [33] and was reported according to PRISMA guidelines [34] (Supplemental Table 2). The protocol was registered with PROSPERO, the International Prospective Register of Systematic Reviews (Registration number CRD42018091896). This protocol includes the investigation of the impact of these fruits on the risk factors of cardiovascular diseases, however the analysis has been reported elsewhere [35]. The search started from inception until February 2021 using PubMed, Web of Science, Scopus and psycARTICLES. The search strategy was as following: (Fruit OR citrus OR orange OR berry OR berries OR grape OR blueberry OR blueberries OR blackberry OR strawberry OR strawberries OR blackcurrant OR blackberries OR raspberry OR raspberries OR cranberry OR cranberries OR cherry OR cherries) AND (cogniti* OR memory OR “executive function” OR “reaction time” OR “psychomotor speed” OR attention OR mood) AND (trial OR intervention). Full electronic search strategy for PubMed was added in Supplemental Table 3.
Study selection
Two researchers (YW and JLG) assessed articles independently for inclusion eligibility. All records were exported to EndNote X8 reference management software. Articles were moved to the next screening phase or discarded when full disagreement was reached. Any disagreements that were not resolved were handled by CHR and JKL serving as arbitrators. No disagreements occurred during the selection phase. The selection of eligible studies was based on 2 steps. Firstly, the title and abstract of each study was screened for relevance; full texts were then reviewed for those with potential for inclusion. Reference lists of included papers and relevant systematic reviews were also supplemented by hand-searching for additional articles.
Data abstraction
Data were extracted by YW and JLG independent of each other, their selections for accuracy were reviewed in meeting. Corresponding authors were contacted via e-mail for requested information if there was missing data or for clarification. A pre-defined data extraction form was used to input study data, which includes information on (1) author and published year; (2) study design; (3) population characteristics (ethnicity, mean age, sex, mean BMI, health status and sample size at baseline); (4) treatment details (intervention type, length, dosage and frequency); (5) control group settings; (6) retention rate; (7) measured cognitive testing scores for both experimental group and placebo group at baseline and the longest post-intervention time point to avoid the bias of selectively choosing data (if applicable); (8) recording any data adjustments made for physical activity level among the included studies. Primary outcomes of the analyses were cognitive and mood scores after intervention and placebo treatment. The cognitive function measured in each study was categorized into memory, executive function and psychomotor speed domains for meta-analysis. The domain categorization in this review was based on a commonly used approach to understand cognitive domains [36].
Risk of bias and quality assessment
Study quality was assessed by Cochrane Risk of Bias (RoB2) tool with assessment of five components, D1 of randomisation process, D2 of deviations from intended interventions, D3 of missing outcome data, D4 of measurement of the outcome and D5 of the selection of the reported results [29]. The overall risk of study bias was rated by low risk, some concerns or high risk. Grading of Recommendations Assessment, Development, and Evaluation (GRADE) evidence profile [37] was also applied to evaluate the risk of bias, inconsistency, indirectness, and imprecision of evidence in the aspects of assessed executive function, memory, psychomotor speed and mood in the RCTs included in the present review. Publication bias was also assessed by Funnel plot and Egger’s test where available [38].
Data synthesis
R studio version 3.5.2 [39] and the package “meta” [40] were used to pool and meta-analyse data from collected studies. Means and SDs from endpoint and baseline data for intervention and control group were obtained from long-term (≥1 week) studies. All pooled results were presented as weighted mean difference or standardised mean difference with 2-sided P values and 95% of confidence intervals (CIs) in forest plots. Sensitivity analysis was performed to investigate the impact of studies adjusting for participants’ physical level and also the impact of juice quality on the meta-analysis results [41, 42]. However, insufficient study data was obtained to implement subgroup analysis to further explore the impact of the variations induced by study design and participant characteristics.
As shown in Table 1, based on the categorised cognitive tasks, meta-analyses investigating the effects of berry interventions on memory, executive function and psychomotor speed [43–49] and 2 cherry juice studies assessing executive function and psychomotor speed were carried out [41, 50]. Two grape powder studies assessing MMSE (Mini Mental State Examination), which measures cognitive impairment, were also included [51, 52]. Memory and executive function both encompass the essential cognitive processes in a person’s life [53]. Psychomotor speed assesses the individual’s ability to detect and respond to a stimulus and therefore reflects the relationship between cognition and physical movements [54].
Table 1.
Cognitive domain classification under each intervention type.
| Cognitive domain | Intervention type | Study | Cognitive task entered into meta-analysis | Unit of test scores | Results of the chosen taska |
|---|---|---|---|---|---|
| Memory | grape juice | Lamport, et al., [43] (UK) | Visual verbal learning test-Immediate recall | accuracy/% | No changes |
| cranberry juice | Crews et al., [44] (US) | Delayed free recall | correct numbers | No changes | |
| blueberry powder | Miller, et al., [48] (US) | CVLT list A 1 free recall | correct numbers | No changes | |
| Executive function | grape juice | Lamport, et al., [43] (UK) | RVIP correct | correct responses | No changes |
| cranberry juice | Crews et al., [44] (US) | Digit symbol | raw score | No changes | |
| blueberry powder | Boespflug et al., [46] (US) | 2-Back task | arc | No changes | |
| blueberry juice | Bowtell et al., [45] (UK) | 2-Back task | arc | No changes | |
| cherry juice | Chai, S. C., et al., [41] (US) | Digit span | score | No changes | |
| Kent, et al., [50] (Australia) | Digit span backwards task (short-term memory) | score | Final score between groups (P = 0.02) | ||
| Aronia melanocarpa extract powder | Ahles et al., [49] (Netherlands) | The number cross-out task | Correct responses | No changes | |
| Psychomotor speed | grape juice | Lamport, et al., [43] (UK) | RVIP reaction time | ms | No changes |
| cranberry juice | Crews et al., [44] (US) | Trial making task reaction time | total time | No changes | |
| frozen blueberry | Schrager, et al., [47] (US) | Simple reaction time | ms | No changes | |
| blueberry powder | Boespflug et al., [46] (US) | 2-Back task reaction time ms | ms | No changes | |
| cherry juice | Kent, et al., [50] (Australia) | Trail making task reaction time | ms | No changes | |
| Chai. S. C., et al., [41] (US) | RTI reaction time | ms | No changes | ||
| MMSE | grape powder | Calapai, et al., [51] (Italy) | MMSE | score | Change score between groups (P < 0.01) |
| Lee, et al., [52] (US) | MMSE | score | No changes |
arc Arcsine transformation of the square root of the proportion of correct answers, CVLT California Verbal Learning Test, RVIP Rapid Visual Information Processing, RTI Reaction Time Test, MMSE Mini Mental State Examination.
aAll summarised results were compared to the control group.
Statistical heterogeneity was estimated by Cochrane Q statistics and the consistency of study results was assessed by I2 statistics as an extension of Cochrane Q statistics, which depicts the proportion of the variability in treatment effect rather than sampling error (chance) that accounts for the real study differences (heterogeneity) and an I2 > 50% was considered for high heterogeneity level [55].
Standard deviation of mean difference (SMD) was used for studies assessing MMSE, memory, executive function, and psychomotor speed, where studies used different assessments or different measurement units. Variance of treatment effects across studies due to real treatment differences and/or sampling variability (chance) was assumed by a Hartung-Knapp-Sidik-Jonkman random-effects meta-analysis model [56].
Results
Literature search
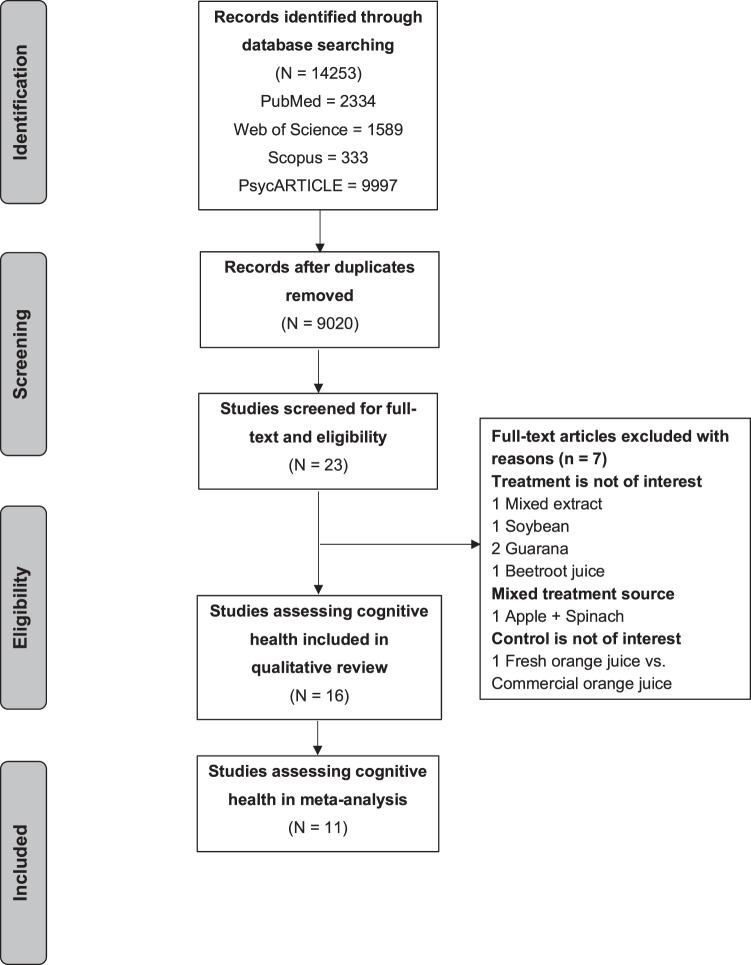
In accordance with PRISMA guidelines [34], the selection process for included studies is shown in Fig. 1. The initial search produced 14,253 articles from the four databases, no additional article was added from manual search through reference lists of articles previously identified. This record was reduced to 9020 articles after duplicates were removed. After screening of titles and abstracts for eligibility, 8997 articles were excluded either due to not being human intervention studies or abstract only. The final selection identified 23 trials assessing cognition, seven articles were further excluded after checking full-text eligibility. Sixteen trials were included in this review, 11 trials from these were included in the meta-analysis.
Fig. 1.
Flow diagram of study selection.
Study characteristics
Among 16 included studies in this review (Table 2), there were 3 crossover RCTs [43, 57, 58] and 13 parallel RCTs [41, 44–52, 59–61]. The average age of the participants in the interventional and control group for included studies were 65.49 ± 15.78 and 65.26 ± 15.61 years old respectively. Thirteen of these studies recruited older participants (aged 60 years or older) [41, 44–48, 50–52, 57, 59–61]. Baseline characteristics of participants varied across interventions, 10 studies included healthy participants at baseline [41, 43–45, 47, 49, 51, 57, 58, 61], five studies included older people manifesting cognitive decline [46, 48, 52, 59, 60] and the remaining one study recruited diagnosed mild to moderate dementia [50].
Table 2.
GRADE evidence profile.
| Certainty assessment | No of patients | Certainty | Importance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Berries, cherries and oranges supplemented randomised controlled trials | Placebo controls | Relative (95% CI) | Absolute (95% CI) | ||
| Executive function (follow up: range 8 weeks to 16 weeks) | ||||||||||||
| 6 | RCT | seriousa | not serious | not serious | not serious | 134 | 135 | 3 | mean 0.08 SMD higher (1.05 lower to 1.08 higher) | 3 | IMPORTANT | |
| Memory (follow up: range 5 weeks to 24 weeks) | ||||||||||||
| 5 | RCT | seriousb | not serious | not serious | not serious | 156 | 151 | 3 | mean 0.02 SMD higher (1.04 lower to 0.92 higher) | 3 | IMPORTANT | |
| Psychomotor speed (follow up: range 5 weeks to 16 weeks) | ||||||||||||
| 7 | RCT | seriousc | not serious | not serious | not serious | 203 | 189 | 3 | mean 0.27 SMD higher (1.08 lower to 2.88 higher) | 3 | IMPORTANT | |
| Mood (follow up: range 1 week to 6 months) | ||||||||||||
| 10 | RCT | not serious | not serious | not serious | not serious | 232 | 225 | 3 | mean 24.9 % lower (0 to 0) | 3 | IMPORTANT | |
Author(s):
Question: Berries, cherries and oranges supplemented randomised controlled trials (RCT) compared to placebo controls for improving cognitive health
CI Confidence interval.
Explanations:
a(Boespflug et al., [46]) study did not specify randomisation and blinding process.
b(Chai et al., [41]) study was not blinded.
c(Boespflug et al., [46]) and (Chai et al., [41]) studies presented high risk of bias due to aforementioned reasons.
There were 9 studies supplementing fruit juice and concentrate [41, 43–45, 50, 57–60], six studies supplemented fruit powder [46, 48, 49, 51, 52, 61] and only one study used whole frozen fruit [47]. Among 16 studies, the mean intervention duration was 13 weeks, ranging from 1 week to 6 months. Two studies supplementing Aronia melanocarpa berry and grape powder used placebo powder composed of maltodextrin as control [49, 62]. One blueberry concentrate intervention used isoenergetic synthetic blackcurrant and apple cordial as control [45]; the orange juice intervention used equicaloric low-flavanone (37 mg) orange-flavoured cordial as control [63]; one cherry juice intervention used flavonoid-devoid apple juice as control [50]; the frozen blueberry intervention used carrot juice with low anthocyanins as control [47]. The rest of the studies used placebo beverage or powder matched for energy, carbohydrate, flavour but devoid of polyphenol content as a control [41, 43, 44, 46, 58–60, 64, 48].
Among the juice supplementations, three studies were accumulated under the groupings of grape juice with mean dosage of 408 ml/d containing173.4 mg, 173.4 mg and 167 mg anthocyanins respectively [43, 59, 60]; three were cherry juice including concentrate with mean dosage of 247 ml/d containing 138 mg anthocyanins, 320 mg anthocyanins, and 450 mg total polyphenols respectively [41, 50, 58], one was cranberry juice with 32 ounces/d (around 942 ml) containing 435 mg total polyphenols [44]. One was blueberry juice concentrate with 30 ml/d [45] containing 387 mg anthocyanidins and one was orange juice study with 500 ml/d containing 305 mg flavanones [57]. Three studies supplemented blueberry powder with mean dosage of 16.3 g/d containing 363 mg anthocyanins, 460.8 mg anthocyanins, and 70 mg total polyphenols respectively [46, 48, 64] and two supplemented grape powder with mean dosage of 48.5 g/d containing 22.3 mg and 12.5 mg anthocyanins respectively [52, 62]; portion conversion of powder to whole fruits were provided in three studies [46, 48, 52]. For example, Lee et al. supplemented grape powder that was comparable to three servings of fresh grapes daily (approx. 504 g/d fresh grapes) [52]. Two studies supplementing blueberry powder were equivalent to providing one cup and 1.5 cups of fresh blueberries as measured in FDA recommended fruit portions respectively [46, 48]. Ahles et al. supplemented 90 mg and 150 mg Aronia melanocarpa berry extract powder providing 16 mg and 27 mg anthocyanins respectively without indicating portions of equivalent whole berry [49]. Schrager et al. supplemented 200 g/d whole frozen blueberries without measuring total polyphenol or anthocyanins levels [47].
Study quality
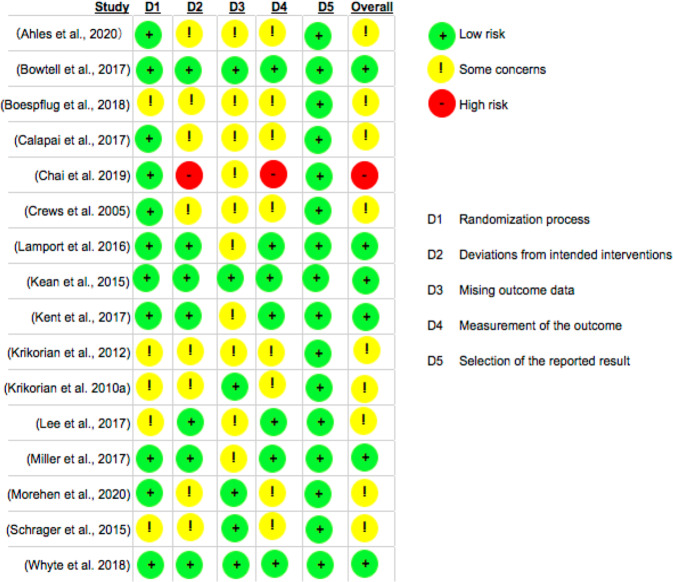
This review assessed RCTs’ quality via RoB2. Figure 2 presents the RoB2 assessment for each study. Eleven studies presented low risk in the evaluation of randomisation process and five studies presented some concerns. Seven studies presented low risk in the evaluation of deviations from intended interventions, eight studies presented some concerns, and one study presented high risk. Six studies presented low risk in the evaluation of missing outcome data, 10 studies presented some concerns. Seven studies presented low risk in the measurement of the outcome, eight studies presented some concerns and one study presented high risk. All 16 studies presented low risk in the selection of the reported result. Six studies presented low risk for overall study risk of bias, nine studies presented some concerns for overall study risk of bias and one study presented high risk for overall study risk of bias.
Fig. 2. RoB2 assessment of study quality.
RoB2: A revised Cochrane risk-of-bias tool for randomized trials.
As summarised in Table 2 of GRADE evidence, there may be serious risk of bias in the assessment of executive function, memory and psychomotor speed in the RCTs included in this review. The risk of bias in the assessment of mood and the risk of inconsistency, indirectness, or imprecision of the assessment of executive function, memory, psychomotor speed and mood was not serious in the RCTs included in this review.
Findings from the studies included in the systematic review
As shown in Table 3, all 16 included studies have assessed cognition with 14 studies reporting improvement or trend for improvement in cognition; 10 studies also assessed mood and one study supplementing grape powder found trend for mood improvement. The largest portion of studies supplemented frozen, juiced or powdered berries (12 out of 16 studies) with blueberries (n = 5) and grapes (n = 5) being the most intensively studied interventions.
Table 3.
Summary of interventions assessing the effect of fruit supplementation on cognition and mood.
| Reference | Study design | Sample (sample at baseline/N, age (years), male (%), mean BMI (kg/m2), health status) | Intervention type, dose and duration | Cognitive tasks | Effects of intervention | Overall risk of bias |
|---|---|---|---|---|---|---|
| Ahles et al. [49] | Double-blind, placebo-controlled, parallel RCT | N = 35; 53 ± 1 years old; male 32%; BMI 29.5 ± 0.4; healthy | 90 mg Aronia melanocarpa, a berry extract (AME) capsule consisted of 16 mg anthocyanins; 150 mg AME consisted of 27 mg anthocyanins; Maltodextrin containing capsules (150 mg) were used as placebo; 24 weeks | Pegboard dominant hand score; Pegboard non-dominant hand score; Total correct—total incorrect; Total edited—total incorrect and missed; Stroop Interference; Mood (T-Scores); BDNF | AME improved psychomotor speed compared to placebo (90 mg AME: change = −3.37; P = 0.009). Attention, cognitive flexibility, BDNF, and mood were not affected. | Moderate |
| Bowtell et al. [45] | Double-blind, parallel-group RCT | N = 12; 67.5 ± 3.0 years old, male 58.33%; BMI 25.9 ± 3.3; | 30 ml blueberry (BB) concentrate (blueberry active) providing 387 mg anthocyanidins, 12 weeks, isoenergetic synthetic blackcurrant and apple cordial as control | Detection Task, Groton maze timed chase test and learning test, sequential letter 1-back and 2-back tasks, fMRI, serum sample | Improved executive function (Groton maze learning task accuracy). Also improved task-related brain activation (Brodman areas, presumes, anterior cingulate, insula and thalamus regions) | Low |
| Boespflug et al. [46] | Double-blind, parallel-group RCT | N = 8; 75.5 ± 4.8 years; male 37.5%; BMI 26.2 ± 3.6; age-related memory decline. | Blueberry powder: 12.5*2 g equivalent to 148 g whole blueberry, 16 weeks, placebo powder: 12*2 g | Sequential letter n-back tasks, fMRI | Trend for improving working memory at larger sample size (effect size d = 1.02). Also increased BOLD activation in the left pre-central gyrus, left middle frontal gyrus, and left inferior parietal lobe during tasks after BB treatment. | Moderate |
| Calapai et al. [51] | Parallel RCT | N = 57; 56–75 years; male 48.2%;BMI 23.2 ± 1.0; healthy. | 250 mg/d Cognigrape-V. vinifera fruit extracted powder and maltodextrin (30–40%), 12 weeks, placebo was composed of maltodextrin | MMSE score, BDI, HARS, RBANS | Improved attention, language, immediate and delayed memory. Supplementation also produced a significant reduction in BDI ( − 15.8%) and HARS ( − 24.9%) scores with respect to baseline levels (p < 0.0001) and placebo (p < 0.0001 for BDI and p < 0.05 for HARS. | Moderate |
| Chai et al. [41] | Parallel RCT | N = 17; 70.0 ± 3.7 years; male 40%; BMI 28.5 ± 3.7; older adults with normal cognitive function | 480 ml tart cherry juice (68 ml Montmorency tart cherry juice concentrate was diluted with 412 ml water) per day for 12 weeks; placebo consisted of mixing unsweetened black cherry flavoured Kool-Aid (Kraft Foods, United States) with water. Dextrose and fructose were added to match the carbohydrate content found in tart cherry | Memory ability, Memory contentment, Memory strategy, digit span, PAL first trial memory, PAL total errors adjusted, RTI movement time, RTI reaction time, RVIP A, RVIP mean latency, SWM strategy, SWM total error | Increased subjective memory in the domain of contentment with memory by 5% and reduced movement time by 4% in comparison with the control drink. Also reduced errors in episodic visual memory by 23% compared to control drink as assessed by PAL task. | High |
| Crews et al. [44] | Double-blind, parallel-group RCT | N = 24: 69.28 ± 6.45 years; male 42%; BMI N/A; healthy. | 909 ml/d 27% cranberry juice for five weeks, placebo drink | Immediate free recall, long term storage, short-term recall, long-term retrieval, consistent long-term retrieval, random long-term retrieval, cued recall, delayed free recall, delayed recognition, Faces I, Faces II, Digit symbol, Part A, Part B, Word page, colour page, colour-word page | A nonsignificant trend (P = 0.123) observed for twice as many subjects of subjective, self-report improved memory in cranberry group compared to placebo controls. | Moderate |
| Lamport et al. [43] | Double-blind, randomized crossover design | N = 25; 43.2 ± 0.6 years; male 0%; BMI 24.6 ± 0.5; healthy. | 355 ml/d concord grape juice, 6 weeks and 12 weeks, energy-, taste-, and appearance-matched placebo | VVLT and VSLT IR & DR, RVIP, Grooved Pegboard, Tower of Hanoi, SBP, DBP, Subjective Mood, Driving performance | Better immediate spatial memory and aspects of driving performance after GJ intake. No difference in mood between groups | Low |
| Kean et al. [57] | Double-blind, randomized, crossover | N = 37; 66.7 ± 5.3 years; male 35.13%; BMI 26.1 ± 1.1; healthy. | High-flavanone (305 mg) 100% orange juice, 500 ml/d for 8 weeks, equicaloric low-flavanone (37 mg) orange-flavoured cordial (500 mL) as control | SBP, DBP, DSST, DSST dual, Go-NoGo RT, LF, LM, Serial Sevens, CERAD Immediate and Delayed, SWM, Immediate and Delayed VPA, PANAS Positive and Negative Affect Scale | Improved cognition (significant drink x visit interaction for global cognitive function and executive function). No effect on mood and BP. | Low |
| Kent et al. [50] | Parallel RCT | N = 24; 78.9 ± 5.2 years; male 51%; BMI 25.7 ± 3.4; mild to moderate dementia. | 200 ml/d cherry juice, 12 weeks, flavonoid-devoid apple juice as control | RAVLT, SPOT, Boston naming test, TMT, Digit Span Backwards Task, Category and Letter Verbal Fluency, SBP, DBP, serum sample | Improved cognition in memory and executive function and reduced SBP of 7.7 mmHg after juice treatment. No effect on Vitamin C and inflammatory markers. | Low |
| Krikorian et al. [59] | Parallel double-blind RCT | N = 11; 76.9 ± 6.1 years; male 52.38%; BMI N/A; mild cognitive impairment | 100% Concord grape juice 6.3–7.8 mL/ kg/d, 3 portions daily, 16 weeks, placebo beverage. | CVLT, GDS, fMRI, SBP, DBP | Attenuated cognitive error (5.03 vs 7.16 interference errors on recognition memory task) and great activation in right superior parietal cortex and right middle frontal cortex regions after juice treatment. No effect on mood and BP. | Moderate |
| Krikorian et al. [60] | Parallel double-blind RCT | N = 5; 78.2 ± 5.0 years; male 66.67%; BMI N/A; older adults with early memory decline but not dementia. | 100% Concord grape juice, 6 and 9 ml/kg/d, 3 portions daily, 12 weeks, placebo beverage. | CVLT, SPALT, GDS, glucose and insulin | Improved memory and insulin level after juice treatment. No effect on mood. | Moderate |
| Lee et al. [52] | Parallel double-blind placebo controlled RCT | N = 5; 72.2 ± 4.7 years; male 50%; BMI N/A mild cognitive decline. | Grape formulation ---freeze-dried grape powder in 8 oz. water, 72 g/d (3 standard servings daily), 6 months, placebo formulation matched in appearance, flavour, smell, volume and content of fructose and glucose but free of polyphenols | ADAS-Cog, MMSE, Hopkins Verbal Learning Test-Revised, Benton Visual Retention Test, Rey-Osterreith CFT, Boston Naming Test, LF, Category Fluency, Stroop, TMT Parts A and B, Wisconsin Card Sorting Test-64, WAIS-III Tasks, Wechsler Test of Adult Reading, Memory Functioning Questionnaire, Hamilton Mood Scales, Neuroimaging sVOI | Attenuated decline in brain metabolites at regions of right posterior cingulate cortex and left superior posterolateral temporal cortex and improved correlated attention after grape treatment. No effect on mood. | Moderate |
| Miller et al. [48] | Parallel double blind placebo-controlled RCT | N = 18; 67.8 ± 4.6 years; male 28%; BMI 24.1 ± 3.7; age-related motor and cognitive decline. | 24 g/d freeze-dried blueberry powder. 90 days, placebo powder | CVLT, ANT, DS, TMT, TST, wMWM, GDS, POMS | Attenuated cognitive error and improved executive function after blueberry treatment. No effects on mood. | Low |
| Morehen et al. [58] | Cross over single blind RCT | N = 11; 18 ± 1 years; male 100%; BMI 27.83 ± 2.51; professional rugby league players | 60 mL cherry concentrate (30 mL*2) in 100 mL water, placebo drink commercially available fruit cordial, mixed with water and maltodextrin, matched for energy and carbohydrate content daily intake for 1 week. | Self-reported subjective wellness including rating of perceived sleep quality, fatigue, muscle soreness, mood and stress using a 1–5 Likert scale. | No significant changes in sleep, fatigue or mood (P > 0.05) were observed pre to post-match or between groups | Moderate |
| Schrager et al. [47] | Parallel RCT | N = 13; 69.5 ± 9.3 years; male 45%; BMI 26.4 ± 3.9; healthy | 6-cup (48 ounce (1.4 kg))/week frozen blueberries, 6 weeks, placebo/carrot juice with low anthocyanins contents | Grip strength, SRT, adaptive gait tests, TMT-B | Reduced errors (76.9% vs 57.1% of participants in BB vs Control had reduced errors) and improved mobility after BB treatment. | Moderate |
| Whyte et al. [61] | Parallel double blind placebo-controlled RCT | N = 29; 70.8 ± 3.88 years; male 38.50%; BMI 27 ± 4; healthy. | 500 mg, 1000 mg blueberry powder or 111 mg purified blueberry extract for 24 weeks, colour matched placebo | Rey’s Auditory Verbal Learning Task (RAVLT), Picture Recognition Task, Corsi Block Task, Stroop Task, and Modified Attention Network Task (MANT), the Serial 3 s, Serial 7 s, and Sternberg task, the PANAS-NOW | No effect on cognition and mood after the blueberry powder intervention. Improved episodic memory performance in delayed word recognition and marginally significant improved visuo-spatial Corsi Block performance at 3, but not 6, months following blueberry extract intervention. | Low |
ADAS The Alzheimer’s Disease Assessment Scale, ADCS-ADL The Alzheimer’s Disease Cooperative Study-Activities of Daily Living, AG Affect Grid, ANT Attention Network Task, BDI Beck Depression Inventory, CBFV Cerebral Blood Flow Velocity, CERAD Consortium to Establish a Registry for Alzheimer’s Disease, CFT Complex Finger Tapping, CPT Continuous Performance Task, CRT Choice Reaction Time, CS Contrast Sensitivity, CVLT California Verbal Learning Test, DBP Diastolic Blood Pressure, DPR Delayed Picture Recognition, DRS The Dementia Rating Scale, DSST Digit Symbol Substitution Test, DV Digit Vigilance, DVR Delayed Verbal Recall, DWR Delayed Word Recognition, EEG Electroencephalogram, fMRI Functional Magnetic Resonance Imaging, FVT Freiburg Vision Test, GDS Geriatric Depression Scale, HARS Hamilton Anxiety Rating Scale, IWR Immediate Word Recall, IVR Immediate Verbal Recall, LF Letter Fluency, LM Letter Memory, MMSE Mini Mental State Examination, NWM Numeric Working Memory, NIRS Near-IR spectroscopy, NPI Neuropsychiatric Inventory, PANAS Positive and Negative Affect Scale, PP Picture Presentation, POMS Profile of Mood States questionnaire, RAVLT Rey Auditory Verbal Learning Test, RBANS Repeatable Battery for the Assessment of Neuropsychological Status, RT Reaction Time, RVIP Rapid Visual Information Processing, SBP Systolic Blood Pressure, SFT Simple Finger Tapping, SPOT Self-ordered Pointing Task, SPALT Spatial Paired Associate Learning Test, SRT Simple Reaction Time, sVOI Standardised Volume of Interest, SWM Spatial Working Memory, TMT Trail Making Task, TST Task Switching Task, VAS Visual Analogue Scales, vMWM Virtual Morris Water Maze, VPA Verbal Paired Association, VSLT Visual Spatial Learning Test, VVLT Visual Verbal Learning Test, WAIS Wechsler Adult Intelligence Scale, WFC Word Fragment Completion, WMS Wechsler Memory Scale, WP Word Presentation.
As shown in Table 3, one frozen blueberry study reported improved motility with a large effect size (Cohen’s d = 1.03) and reduced step errors (76.9% vs 57.1% of participants in intervention vs control group) with a large effect size (Cohen’s d = 1.16) [47]. Ahles et al. found improved psychomotor speed after supplementing 90 mg of Aronia melanocarpa berry extract powder for 24 weeks (P = 0.009) without providing an effect size [49]. For blueberry powder supplementations, Boespflug et al. reported marginally improved accuracy for the blueberry group in the 1-back condition (P = 0.08) for executive function assessment with a large effect size (Cohen’s d = 1.02) [46]; Miller et al. reported significantly fewer repetition errors in the California Verbal Learning test (P = 0.031) with a medium effect size (Cohen’s d = 0.50) and reduced switch cost on a task-switching test (P = 0.033) for executive function assessment across visits, relative to controls, whereas no effect on mood assessment using Geriatric Depression Scale (GDS) and the Profile of Mood States (POMS) was found [48]; Whyte et al. reported no effect on cognition and mood as assessed by the Positive and Negative Affect Schedule-NOW [61]. One blueberry concentrate study also reported improved executive function assessed by working memory (two back test) relative to the control (P = 0.05) [45].
For grape powder supplementation, Calapai et al. reported better attention (P < 0.001); language (P < 0.05); immediate memory (P < 0.0001); delayed memory (P < 0.0001) and MMSE score (P < 0.001) compared to the control without providing effect sizes [51]; Lee et al. also reported better attention/working memory under the domain of executive function, as measured with WAIS-III Digit Span within the intervention group (P = 0.04) without providing effect sizes, no effect on mood as assessed by Hamilton Rating Scale (HRS) was shown [52]. For grape juice supplementations, Lamport et al. reported better immediate spatial memory with a small effect size (Cohen’s d = 0.2) and driving performance (P < 0.05) with a small effect size (Cohen’s d = 0.4) compared to the control [43]; Kirkorian et al. reported attenuated cognitive error (5.03 vs 7.16 interference errors on recognition memory task) with a large effect size (Cohen’s d = 1.0) and no effect on mood as assessed by GDS [59]; Krikorian et al. reported improved Paired Associate Learning (PAL) (P = 0.009), Word List Recall (P = 0.04) with a medium effect size (Cohen’s d = 0.56) and trend for improving mood measured as reduced depressive symptoms (P = 0.08) [60].
For cherry juice supplementations, Chai et al. reported increased subjective memory in the domain of contentment with memory by 5%, reduced movement time by 4% and also reduced errors in episodic visual memory by 23% compared to control drink as assessed by PAL task without providing the effect size [41]. Kent et al. reported better memory and executive as assessed by verbal fluency (P = 0.014) with a large effect size (Cohen’s d = 1.04), short-term memory (P = 0.014) with a medium effect size (Cohen’s d = 0.79) and long-term memory (P ≤ 0.001) with a large effect size (Cohen’s d = 0.94) [50]. Morehen et al. found no significant changes in assessed sleep, fatigue or mood between pre- and post-intervention and between groups [58].
For cranberry juice supplementation, there is no effect (P = 0.123) on self-report memory [44]. For orange juice supplementation, there was improved global cognitive function and executive function (significant drink x visit interaction, P < 0.05) with no effect on Positive and Negative Affect Scale without providing an effect size [57].
Meta-analyses
As shown in Table 1, for studies included in the meta-analysis, memory was assessed as either the number of correct responses or accuracy (%) in Immediate Word Recall, Delayed Word Recall or CVLT List Free Recall (California Verbal Learning Test); executive function was assessed as the total score, or the number of correct responses or arcsine transformation of the square root of the proportion of correct answers in Digit Symbol Substitution Test, Digit Span, 2-Back Task, Rapid Visual Information Processing (RVIP) or Number Cross-out Test; psychomotor speed was assessed as reaction time (RT, ms or s) of Trail Making Task, Reaction Time Test (RTI), Simple Reaction Time, 2-Back Task or RVIP. Studies incorporating grape juice, grape powder, blueberry juice, blueberry powder, cranberry juice and frozen blueberry constituting a berry group along with studies supplementing cherry juice were able to provide sufficient data to run meta-analysis. There was insufficient data for mood to be entered into meta-analysis, but the mood assessment results for individual studies are reported in Table 3.
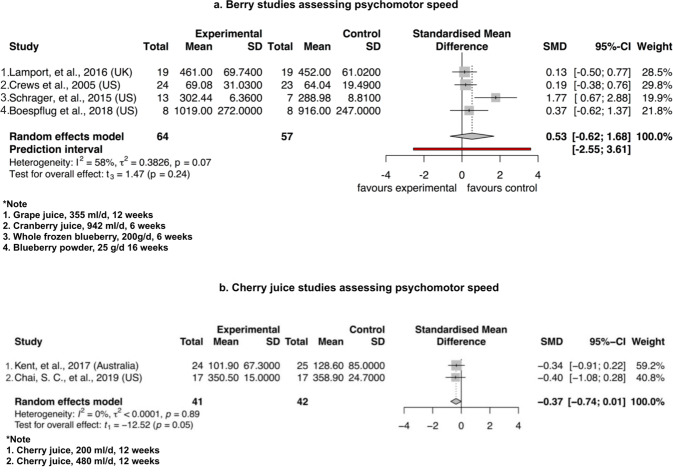
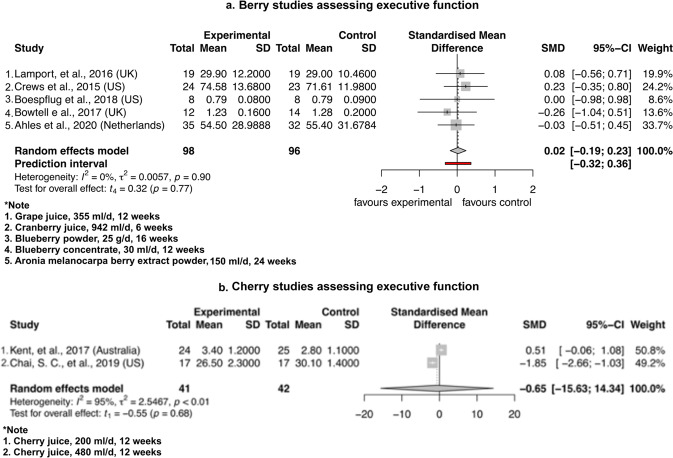
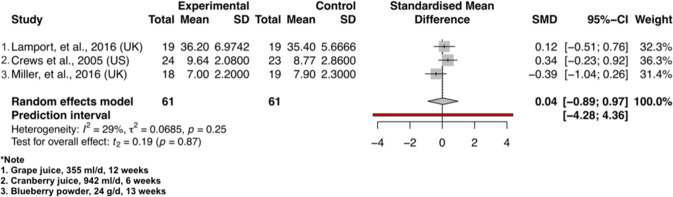
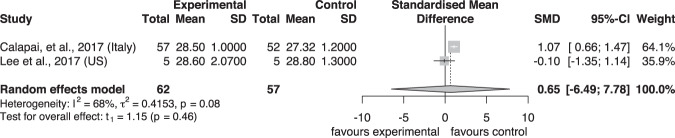
Notably, cherry juice induced a borderline significance in improvement of psychomotor speed after the intervention compared to control (SMD = −0.37, 95% CI – 0.74 to 0.01, P = 0.05, t1 = −12.52) in Fig. 3. The berry group induced no significant difference for psychomotor speed between intervention and control group (SMD = 0.61, 95% CI −1.58 to 2.80, P = 0.36, t4 = 1.19) (Fig. 3). As shown in Fig. 4 for the assessment of executive function, the berry group induced no significant difference between intervention and control group (SMD = 0.02, 95% CI −0.19 to 0.23, P = 0.77, t4 = 0.32) and cherry juice induced no significant difference between the two groups (SMD = −0.65, 95% CI – 15.63 to 14.34, P = 0.68, t1 = −0.55). As shown in Fig. 5 for memory assessment, the berry group induced no significant difference between intervention and control groups (SMD = 0.04, 95% CI −0.89 to 0.97, P = 0.87, t2 = 0.19). Apart from the analysis assessing cognitive domains, two grape powder studies were able to provide MMSE (Mini Mental State Examination) data, but no significant difference was shown between intervention and control groups (SMD = 0.65, 95% CI – 6.49 to 7.78, P = 0.46, t1 = 1.15) (Fig. 6).
Fig. 3. Forest plot of fruit studies assessing psychomotor speed.
Forest plot of a berry studies and b cherry juice studies assessing psychomotor speed.
Fig. 4. Forest plot of fruit studies assessing executive function.
Forest plot of a berry studies and b cherry juice studies assessing executive function.
Fig. 5.
Forest plot of berry studies assessing memory.
Fig. 6. Forest plot of grape powder studies assessing MMSE.
MMSE: Mini Mental State Examination.
Significant heterogeneity among studies was observed in the cherry juice studies assessing executive function (I2 = 95%, P < 0.01) and berry group assessing psychomotor speed (I2 = 72%, P = 0.03). There was no change of heterogeneity in executive function and psychomotor speed assessment after the sensitivity analysis excluding studies applying physical activity adjustments and supplementing concentrate, the sensitivity analysis also suggested no effect of physical activity level and juice quality on the interventional effect (Table 4). Funnel plots and the egger’s test for the berry group showed an overall symmetric distribution of the berry interventions around the standard error for the investigated outcomes of executive function (Egger’s tests P = 0.24) and memory (Egger’s tests P = 0.28); asymmetric distributions were shown for the berry interventions investigating the effect on psychomotor speed (Egger’s tests P = 0.28); cherry interventions investigating the effect on executive function (Egger’s tests P = 0.35) and grape powder interventions investigating the effect on MMSE (Egger’s tests P = 0.24) (Supplemental Fig. 1). Trim and fill method was further implemented to adjust for publication bias (Supplemental Fig. 1).
Table 4.
Sensitivity analysis.
| Berry group assessing memory | Cherry juice study assessing executive function | Cherry juice study assessing psychomotor speed | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | standardised mean difference (95% CI) | P for heterogeneity | P for overall effect | N | standardised mean difference (95% CI) | P for heterogeneity | P for overall effect | N | standardised mean difference (95% CI) | P for heterogeneity | P for overall effect | |
| All studies | 3 | 0.04 [−0.89; 0.97] | 0.25 | 0.87 | 2 | −0.65 [−15.63; 14.34] | <0.01 | 0.68 | 2 | −0.37 [−0.74; 0.01] | 0.89 | 0.05 |
| Without adjusted studiesa | 2 | 0.24 [−1.15; 1.64] | 0.62 | 0.27 | 1 | 0.51 [−0.06; 1.08] | NA | NA | 1 | −0.34 [−0.91; 0.22] | NA | NA |
| berry group assessing executive function | cherry juice study assessing executive function | cherry juice study assessing psychomotor speed | ||||||||||
| N | standardised mean difference (95% CI) | P for heterogeneity | P for overall effect | N | standardised mean difference (95% CI) | P for heterogeneity | P for overall effect | N | standardised mean difference (95% CI) | P for heterogeneity | P for overall effect | |
| All studies | 5 | 0.02 [−0.19; 0.23] | 0.9 | 0.77 | 2 | −0.65 [−15.63; 14.34] | <0.01 | 0.68 | 2 | −0.37 [−0.74; 0.01] | 0.89 | 0.05 |
| Without juice concentrate studies | 4 | 0.07 [−0.13; 0.45] | 0.93 | 0.34 | 1 | 0.51 [−0.06; 1.08] | NA | NA | 1 | −0.34 [−0.91; 0.22] | NA | NA |
aStudy’s statistical analysis has adjusted for participants’ physical activity.
Discussion
Principal findings
Our systematic review collected a range of frozen, freeze-dried, powdered and juiced fruit interventions, specifically berries (n = 12), reporting positive findings on cognition or mood. The largest portion of studies involved fruit juices (nine out of 16 studies) with grape juice (n = 3) and cherry juice (n = 3) being the most intensively studied supplementations. As demonstrated by the meta-analysis, two cherry juice studies including 41 participants receiving cherry juice with mean dosage of 340 ml/d for 12 weeks induced a borderline significant improvement in psychomotor speed. However, it is important to note that only two studies were included and one of those had a high risk of bias [41], which limits the impact of this finding.
Our meta-analyses suggested no differences between the intervention and control groups in any cognitive domains following berry or other fruit-based supplementations. No improvement was observed on any outcomes including executive function and memory, which does not support the notion that the consumption of specific fruit powders or other fruit juices will confer a cognitive-protective benefit. Overall, the individual interventions showing improvements in our systematic review still require further substantiation given that the meta-analysis only suggests that cherry juice may have cognition-protective potential.
Scientific analysis of findings
The systematic review suggested the potential for whole blueberries, blueberry juice concentrate, blueberry powder, grape powder, grape juice, cherry juice, orange juice and cranberry juice supplements to improve cognitive health. Supplementing grape juice also showed potential to improve mood. However, from the meta-analysis, we only found a borderline significant improvement in psychomotor speed following chronic consumption of cherry juice. The participants of the cherry juices studies included here were older, healthy (> 60 years old) or have dementia. It’s worth noting that slower psychomotor speed has been found to be associated with increased risk of all-type dementia (hazard ratio [HR] 3.41, P < 0.0001), Alzheimer’s disease-type dementia (HR 3.18, P < 0.0001), Parkinson’s disease (HR 2.98, P = 0.04) and depressive symptoms (HR 1.53, P = 0.03) [65], and is therefore related to chronic mobility disorders and important for health wellbeing. Cherry juice has high content of flavonoids catechin, epicatechin, procyanidins and anthocyanins [66, 67] and the most recent evidence points towards potential benefits of supplementing flavonoids, ranging from 60 to 768 mg daily on attention, working memory, and psychomotor speed, but the study findings were not conclusive [68].
In our review, grape powder and juice interventions have shown improvement to cognition or mood for at least one of the cognitive aspects [43, 52, 59, 60, 62]. Studies supplementing blueberry powder, cherry juice and cranberry juice also have reported effect on cognitive or mood benefit [46, 48, 50, 64, 69, 70]. Polyphenol-rich fruit juice interventions that reported positive effect on cognitive health have provided anthocyanin levels ranging from 138 to 387 mg or 435 to 450 mg total polyphenol levels daily; fruit powder interventions with positive findings on cognition have provided either anthocyanin levels ranging from 12.5 to 460.8 mg or 70 mg total polyphenol levels daily. Small molecules, especially anthocyanins are the major class of polyphenols in berry and cherry fruit (approximately 92 mg/100 g) [41]. However, the anthocyanin profile in polyphenol-rich fruit is a factor explaining variability in the biological responses observed in dietary interventions with this fruit. For instance, blueberries contain primarily delphinidin, malvidin, and petunidin whereas raspberries and blackberries contain primarily cyanidin, pelargonidin and malvidin [71]. Although consumption of anthocyanins can be in the range of 200 mg/d [72], the bioavailability appears low as they are believed to be poorly absorbed and rapidly excreted [73]. The rate of polyphenol absorption from blueberries could be influenced by dose administered [74], and the matrix of the food source [75], and several studies have suggested that the rate of anthocyanin absorption is influenced by their chemical structure. The bioavailability of polyphenol metabolites will vary between individuals and is dependent on complex absorption, distribution, metabolism and excretion (ADME) mechanisms involving phase I and II metabolism of phenolic molecules [76]. Also, a systematic review has shown that the intake of fruit juice offered similar protection against cognitive decline to the intake of whole fruit [77] and thus a similar proportion of bioactive phytochemicals must remain in the processed products. Unfortunately, the current meta-analyses did not sufficiently support a role for fruits or other fruit forms to improve cognition and mood.
Although our meta-analysis lacks evidence to support improvements in mood by specific fruit interventions, another meta-analysis with 10 observational studies involving 227,852 participants suggested an inverse association of fruit (RR 0.83, 95% CI [0.77, 0.91]; P = 0.006) intake with risk of depression [78]. A previous systematic review has also assessed the association between cognitive benefits and fruit consumption but only included limited evidence from juice interventions, where improvements to memory in mildly cognitive-impaired adults after 12–16 weeks of consumption were illustrated [12]. The high levels of flavonoid metabolites (e.g. anthocyanidin) from flavonoid-rich fruit can transport through blood brain barrier into regions such as the hippocampus to impact on memory and learning [79]. In the pathogenesis of neurological conditions such as Parkinson’s disease, the mechanisms of action of flavonoids have been shown to counteract the damage induced by reactive oxygen species (ROS) and neuroinflammation, modulate synaptic signalling and increase cerebrovascular blood flow [77]. The (poly)phenol metabolites attenuated neuro-inflammatory processes via regulation of nuclear factor (NF)-κB translocation into the nucleus and modulation of IκBα levels by crossing the blood brain barrier endothelium and exerting beneficial effects in different neuronal systems (e.g. cell lines, primary cultures and in vitro three-dimensional human cell model) [80]. Therefore, cerebral metabolism of polyphenol and/or flavonoid molecules plays an important role in the preservation of cognitive function [81].
Implications for health and future research
Currently, the majority of evidence in this area has included the association between intake of fruit combined with vegetable intake and cognitive function. However, the evidence on the association between specific fruit groups and/or forms of fruit with cognition is limited. Although insufficient data were entered for meta-analysis for each fruit subgroup, blueberry and grape powders providing 12.5–460.8 mg/d anthocyanin content were supplemented the most apart from fruit juice and could also be an effective method to increase overall fruit consumption and benefit cognition with moderate to large effect sizes reported [41, 44–48, 50–52, 57, 59–61]. Long-term investigations assessing the impact of whole blueberry intervention on cognition are also scarce and worth exploring, given that a large effect size was reported [47]. Thirteen out of 16 total RCTs in this review recruited older participants and only one study recruited young adults (18 years old athletes) without any improvements to cognition or mood shown. It could be due to that participants with relatively higher cognitive level at the baseline were unlikely to achieve higher cognitive response further following a dietary intervention due to ‘ceiling effects’ [82].
Only one intervention examined the effect of long-term supplementation with whole frozen fruit on cognition in this review, no fresh fruit was identified in the previous interventions, which could be due to the difficulty of storage and allocation during the intervention. Fresh whole fruit is generally how the fruit is consumed, which highlights a novel and necessary intervention in future studies. So far, research has mainly focused on fruit juice interventions, nevertheless, we should take free sugar reduction into account and the daily consumption of fruit juice should not exceed 150 ml per day as set out by Public Health England guidelines [83]. The sugar found in fruit juice is mainly classified as ‘free’ sugars, such as sucrose, whereas in whole fruit the sugars are classified as intrinsic. Increased dietary fructose following sucrose intake is reported to increase de novo lipogenesis and very-low-density lipoprotein levels, which has been shown to increase the risk of developing non-alcoholic fatty liver disease [84]. Therefore, any health promotion effects of increasing fruit intake by juice consumption should be made with caution. Validation of metabolites and biomarkers for cognitive impairment should be incorporated into future trials to help identify the potential mechanisms underlying any influence between fruit-based intake and cognitive health. Due to the impact of fruit processing and food matrix, cognitive research implementing fruit interventions should also consider controlling for factors likely to influence bioavailability in a chronic intervention (>1 week) [26].
Strengths and limitations
To our knowledge, this is the first systematic review and meta-analysis to compare the impact of various forms of specific fruits in isolation from other food supplementation on cognition. Firstly, in addition to the comprehensive search of the literature in the topic, we also applied the newly developed Hartung-Knapp-Sidik-Jonkman method for modelling random effects in meta-analysis. Secondly, the interventions included in this review have assessed either general cognitive performance or specific cognitive domains using well-established cognitive tasks.
There are limitations to our review. Although our systematic review showed positive results in interventions supplementing with blueberry or grape, the small sample size and moderate to high risks of bias for studies quality that used the same cognitive task in the searched literature may partially explain the lack of support from our meta-analysis. It also should be noted that generally the cognitive tasks chosen in each intervention study are not uniform because of the polyfactorial nature of neurocognitive measures [85]. In the current review, not all the domain-specific tasks data could be entered into meta-analyses assessing the effect of the same type of fruit interventions on the specific cognitive domain, thus the effect size derived from the meta-analyses was inevitably under-estimated.
Study quality of 13 studies as assessed by risk of bias in this review presented with moderate to high risks in either randomisation process, intervention deviations, missing outcome data, and/or the measurement of the outcomes (Fig. 2). A crossover design is the most appropriate design when comparing a nutritional intervention against placebo since all participants serve as their own control and only three studies applied a crossover design [43, 57, 58]. Only one citrus intervention RCT supplementing orange juice was included, which led to limited exploration of the effect of citrus fruit intervention on cognition [43]. Due to the small number of studies in each pooled analysis, we were also unable to evaluate whether the effects can be influenced by participant characteristics (e.g. physical activity, sex) or to explore the high heterogeneity by subgroup analysis.
Conclusion
This systematic review has identified berries with the most potential to benefit cognition, however, the meta-analyses only supported a borderline significant improvement to psychomotor speed by two small studies supplementing cherry juice with mean dosage of 340 ml/d for 12 weeks. To our knowledge, this is the first review of the impact on cognitive health following consumption of different varieties of fruit and different processed forms such as freeze-dried powdered fruit or fruit juice. Apart from fruit juice, promising results were also demonstrated among limited studies supplementing whole frozen or powdered forms of fruit (grape powder, frozen and powdered blueberries).
Supplementary information
Author contributions
All authors developed the study concept and design and contributed to the critical revision of the manuscript for important intellectual content; YW wrote the draft of manuscript, conducted data extraction and statistical analysis; all authors contributed to discussion and reviewed/edited the manuscript. All authors have read and approved the final version submitted for publication.
Data availability
The data that support the findings of this study are available from the corresponding author, JL, upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41430-022-01138-x.
References
- 1.Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, et al. Age-associated cognitive decline. Br Med Bull. 2009;92:135–52. doi: 10.1093/bmb/ldp033. [DOI] [PubMed] [Google Scholar]
- 2.Clionsky M, Clionsky E. Dementia screening: saying no to the USPSTF and yes to brief cognitive evaluation. J Alzheimers Dis Parkinsonism. 2014;4:e132. doi: 10.4172/2161-0460.1000e132. [DOI] [Google Scholar]
- 3.Workman B, Dickson F, Green S. Early dementia: optimal management in general practice. Aust Fam Physician. 2010;39:722–6. [PubMed] [Google Scholar]
- 4.Craik FI, Salthouse TA. The handbook of aging and cognition: Psychology press; 2011.
- 5.Greenwood PM, Parasuraman R. Normal genetic variation, cognition, and aging. Behav Cogn Neurosci Rev. 2003;2:278–306. doi: 10.1177/1534582303260641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy DO, Haskell CF. Vitamins and cognition: what is the evidence? Drugs. 2011;71:1957–71. doi: 10.2165/11594130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Vauzour D. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev. 2012;2012:914273. doi: 10.1155/2012/914273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho KS, Shin M, Kim S, Lee SB. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxid Med Cell Longev. 2018;2018:4120458. doi: 10.1155/2018/4120458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraga CG, Galleano M, Verstraeten SV, Oteiza PI. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol Asp Med. 2010;31:435–45. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Spencer JP. The impact of flavonoids on memory: physiological and molecular considerations. Chem Soc Rev. 2009;38:1152–61. doi: 10.1039/b800422f. [DOI] [PubMed] [Google Scholar]
- 11.Jiang X, Huang J, Song D, Deng R, Wei J, Zhang Z. Increased consumption of fruit and vegetables is related to a reduced risk of cognitive impairment and dementia: meta-analysis. Front in aging neurosci. 2017;9:18. [DOI] [PMC free article] [PubMed]
- 12.Lamport DJ, Saunders C, Butler LT, Spencer JP. Fruits, vegetables, 100% juices, and cognitive function. Nutr Rev. 2014;72:774–89. doi: 10.1111/nure.12149. [DOI] [PubMed] [Google Scholar]
- 13.Wright AJA, Dainty JR, Finglas PM. Folic acid metabolism in human subjects revisited: potential implications for proposed mandatory folic acid fortification in the UK. Br J Nutr. 2007;98:667–75. doi: 10.1017/S0007114507777140. [DOI] [PubMed] [Google Scholar]
- 14.Wallace TC, Bailey RL, Blumberg JB, Burton-Freeman B, Chen CYO, Crowe-White KM, et al. Fruits, vegetables, and health: a comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Critical rev in food sci and nutr. 2020;60:2174–2211. [DOI] [PubMed]
- 15.Bell L, Lamport DJ, Butler LT, Williams CM. A review of the cognitive effects observed in humans following acute supplementation with flavonoids, and their associated mechanisms of action. Nutrients. 2015;7:10290–306. doi: 10.3390/nu7125538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Travica N, D’Cunha NM, Naumovski N, Kent K, Mellor DD, Firth J, et al. The effect of blueberry interventions on cognitive performance and mood: a systematic review of randomized controlled trials. Brain Behav Immun. 2020;85:96–105. doi: 10.1016/j.bbi.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Borges G, Mullen W, Mullan A, Lean ME, Roberts SA, Crozier A. Bioavailability of multiple components following acute ingestion of a polyphenol-rich juice drink. Mol Nutr Food Res. 2010;54:S268–77. doi: 10.1002/mnfr.200900611. [DOI] [PubMed] [Google Scholar]
- 18.Mertens-Talcott SU, Rios J, Jilma-Stohlawetz P, Pacheco-Palencia LA, Meibohm B, Talcott ST, et al. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J Agric Food Chem. 2008;56:7796–802. doi: 10.1021/jf8007037. [DOI] [PubMed] [Google Scholar]
- 19.Kay CD, Mazza G, Holub BJ, Wang J. Anthocyanin metabolites in human urine and serum. Br J Nutr. 2004;91:933–42. doi: 10.1079/BJN20041126. [DOI] [PubMed] [Google Scholar]
- 20.Haskell-Ramsay CF, Stuart RC, Okello EJ, Watson AW. Cognitive and mood improvements following acute supplementation with purple grape juice in healthy young adults. Eur J Nutr. 2017;56:2621–31.. doi: 10.1007/s00394-017-1454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alharbi MH, Lamport DJ, Dodd GF, Saunders C, Harkness L, Butler LT, et al. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur J Nutr. 2016;55:2021–9. doi: 10.1007/s00394-015-1016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson AW, Haskell-Ramsay CF, Kennedy DO, Cooney JM, Trower T, Scheepens A. Acute supplementation with blackcurrant extracts modulates cognitive functioning and inhibits monoamine oxidase-B in healthy young adults. J Funct Foods. 2015;17:524–39. doi: 10.1016/j.jff.2015.06.005. [DOI] [Google Scholar]
- 23.Ruxton CHS. Smoothies: one portion or two? Nutr Bull. 2008;33:129–32. doi: 10.1111/j.1467-3010.2008.00696.x. [DOI] [Google Scholar]
- 24.Porrini M, Riso P, Testolin G. Absorption of lycopene from single or daily portions of raw and processed tomato. Br J Nutr. 1998;80:353–61.. doi: 10.1017/S000711459800141X. [DOI] [PubMed] [Google Scholar]
- 25.Celli GB, Brooks MS-L. Impact of extraction and processing conditions on betalains and comparison of properties with anthocyanins––A current review. Food Res Int. 2017;100:501–9. doi: 10.1016/j.foodres.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 26.Eker ME, Aaby K, Budic-Leto I, Brnčić SR, El SN, Karakaya S, et al. A review of factors affecting anthocyanin bioavailability: possible implications for the inter-individual variability. Foods. 2019;9:2. [DOI] [PMC free article] [PubMed]
- 27.Tomas M, Toydemir G, Boyacioglu D, Hall R, Beekwilder J, Capanoglu E. The effects of juice processing on black mulberry antioxidants. Food Chem. 2015;186:277–84. doi: 10.1016/j.foodchem.2014.11.151. [DOI] [PubMed] [Google Scholar]
- 28.Kuntz S, Rudloff S, Asseburg H, Borsch C, Frohling B, Unger F, et al. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br J Nutr. 2015;113:1044–55. doi: 10.1017/S0007114515000161. [DOI] [PubMed] [Google Scholar]
- 29.Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. bmj. 2019;366. [DOI] [PubMed]
- 30.Chepenik LG, Cornew LA, Farah MJ. The influence of sad mood on cognition. Emotion. 2007;7:802–11. doi: 10.1037/1528-3542.7.4.802. [DOI] [PubMed] [Google Scholar]
- 31.Beentje H Plant glossary: Kew Publishing, Royal Botanical Gardens, Kew; 2010.
- 32.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions: John Wiley & Sons; 2011.
- 33.Centre for Reviews and Dissemination UoY. Systematic reviews: CRD’s guidance for undertaking reviews in health care. York, UK: CRD, University of York; 2009.
- 34.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Gallegos JL, Haskell-Ramsay C, Lodge JK. Effects of chronic consumption of specific fruit (berries, citrus and cherries) on CVD risk factors: a systematic review and meta-analysis of randomised controlled trials. Eur J of Nutr. 2021;60:615–639. [DOI] [PMC free article] [PubMed]
- 36.Lezak MD, Howieson DB, Loring DW, Fischer JS Neuropsychological assessment: Oxford University Press, USA; 2004.
- 37.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res ed) 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Team RC. R: A language and environment for statistical computing. 2013.
- 40.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid-Based Ment Health. 2019;22:153–60. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chai SC, Jerusik J, Davis K, Wright RS, Zhang Z. Effect of montmorency tart cherry juice on cognitive performance in older adults: a randomized controlled trial. Food Funct. 2019;10:4423–31. doi: 10.1039/C9FO00913B. [DOI] [PubMed] [Google Scholar]
- 42.Millar CL, Duclos Q, Garcia C, Norris GH, Lemos BS, DiMarco DM, et al. Effects of freeze-dried grape powder on high-density lipoprotein function in adults with metabolic syndrome: a randomized controlled pilot study. Metab Syndr Relat Disord. 2018;16:464–9. doi: 10.1089/met.2018.0052. [DOI] [PubMed] [Google Scholar]
- 43.Lamport DJ, Lawton CL, Merat N, Jamson H, Myrissa K, Hofinan D, et al. Concord grape juice, cognitive function, and driving performance: a 12-wk, placebo-controlled, randomized crossover trial in mothers of preteen children. Am J Clin Nutr. 2016;103:775–83. doi: 10.3945/ajcn.115.114553. [DOI] [PubMed] [Google Scholar]
- 44.Crews WD, Jr, Harrison DW, Griffin ML, Addison K, Yount AM, Giovenco MA, et al. A double-blinded, placebo-controlled, randomized trial of the neuropsychologic efficacy of cranberry juice in a sample of cognitively intact older adults: pilot study findings. J alternative complementary Med (N. Y, NY) 2005;11:305–9. doi: 10.1089/acm.2005.11.305. [DOI] [PubMed] [Google Scholar]
- 45.Bowtell JL, Aboo-Bakkar Z, Conway ME, Adlam AR, Fulford J. Enhanced task-related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl Physiol Nutr Metab. 2017;42:773–9. doi: 10.1139/apnm-2016-0550. [DOI] [PubMed] [Google Scholar]
- 46.Boespflug EL, Eliassen JC, Dudley JA, Shidler MD, Kalt W, Summer SS, et al. Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutritional Neurosci. 2018;21:297–305. doi: 10.1080/1028415X.2017.1287833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schrager MA, Hilton J, Gould R, Kelly VE. Effects of blueberry supplementation on measures of functional mobility in older adults. Appl Physiol Nutr Metab. 2015;40:543–9. doi: 10.1139/apnm-2014-0247. [DOI] [PubMed] [Google Scholar]
- 48.Miller MG, Hamilton DA, Joseph JA, Shukitt-Hale B. Dietary blueberry improves cognition among older adults in a randomized, double-blind, placebo-controlled trial. European journal of nutrition. 2018;57:1169-1180. [DOI] [PubMed]
- 49.Ahles S, Stevens YR, Joris PJ, Vauzour D, Adam J, de Groot E, et al. The effect of long-termaroniamelanocarpaextract supplementation on cognitive performance, mood, and vascular function: a randomized controlled trial in healthy, middle-aged individuals. Nutrients. 2020;12. [DOI] [PMC free article] [PubMed]
- 50.Kent K, Charlton K, Roodenrys S, Batterham M, Potter J, Traynor V, et al. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur J Nutr. 2017;56:333–41.. doi: 10.1007/s00394-015-1083-y. [DOI] [PubMed] [Google Scholar]
- 51.Calapai G, Bonina F, Bonina A, Rizza L, Mannucci C, Arcoraci V, et al. A randomized, double-blinded, clinical trial on effects of a Vitis vinifera extract on cognitive function in healthy older adults. Frontiers in pharmacology. 2017;8(OCT). [DOI] [PMC free article] [PubMed]
- 52.Lee J, Torosyan N, Silverman DH. Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: a double-blinded placebo controlled pilot study. Exp Gerontol. 2017;87:121–8. doi: 10.1016/j.exger.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Best JR, Miller PH. A developmental perspective on executive function. Child Dev. 2010;81:1641–60. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harvey PD, Mohs RC. 5 - Memory Changes with Aging and Dementia. In: Hof PR, Mobbs CV, editors. Functional Neurobiology of Aging. San Diego: Academic Press; 2001. p. 53-63.
- 55.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ: Br Med J. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kean RJ, Lamport DJ, Dodd GF, Freeman JE, Williams CM, Ellis JA, et al. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: an 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am J Clin Nutr. 2015;101:506–14. doi: 10.3945/ajcn.114.088518. [DOI] [PubMed] [Google Scholar]
- 58.Morehen JC, Clarke J, Batsford J, Barrow S, Brown AD, Stewart CE, et al. Montmorency tart cherry juice does not reduce markers of muscle soreness, function and inflammation following professional male rugby League match-play. European Journal of Sport Science. 2020. [DOI] [PubMed]
- 59.Krikorian R, Boespflug EL, Fleck DE, Stein AL, Wightman JD, Shidler MD, et al. Concord grape juice supplementation and neurocognitive function in human aging. J Agric food Chem. 2012;60:5736–42. doi: 10.1021/jf300277g. [DOI] [PubMed] [Google Scholar]
- 60.Krikorian R, Nash TA, Shidler MD, Shukitt-Hale B, Joseph JA. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br J Nutr. 2010;103:730–4. doi: 10.1017/S0007114509992364. [DOI] [PubMed] [Google Scholar]
- 61.Whyte AR, Cheng N, Fromentin E, Williams CM. A randomized, double-blinded, placebo-controlled study to compare the safety and efficacy of low dose enhanced wild blueberry powder and wild blueberry extract (ThinkBlue) in maintenance of episodic and working memory in older adults. Nutrients. 2018;10:660. [DOI] [PMC free article] [PubMed]
- 62.Calapai G, Bonina F, Bonina A, Rizza L, Mannucci C, Arcoraci V, et al. A randomized, double-blinded, clinical trial on effects of a Vitis vinifera extract on cognitive function in healthy older adults. Front Pharm. 2017;8:776. doi: 10.3389/fphar.2017.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keane KM, Haskell-Ramsay CF, Veasey RC, Howatson G. Montmorency Tart cherries (Prunus cerasus L.) modulate vascular function acutely, in the absence of improvement in cognitive performance. Br J Nutr. 2016;116:1935–44.. doi: 10.1017/S0007114516004177. [DOI] [PubMed] [Google Scholar]
- 64.Whyte AR, Lamport DJ, Schafer G, Williams CM. The cognitive effects of an acute wild blueberry intervention on 7-to 10-year-olds using extended memory and executive function task batteries. Food Funct. 2020;11:4793–801. doi: 10.1039/C9FO02284H. [DOI] [PubMed] [Google Scholar]
- 65.Amieva H, Meillon C, Proust-Lima C, Dartigues JF. Is low psychomotor speed a marker of brain vulnerability in late life? digit symbol substitution test in the prediction of Alzheimer, Parkinson, stroke, disability, and depression. Dement Geriatr Cogn Disord. 2019;47:297–305. doi: 10.1159/000500597. [DOI] [PubMed] [Google Scholar]
- 66.Kim DO, Heo HJ, Kim YJ, Yang HS, Lee CY. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J Agric food Chem. 2005;53:9921–7. doi: 10.1021/jf0518599. [DOI] [PubMed] [Google Scholar]
- 67.Bell PG, McHugh MP, Stevenson E, Howatson G. The role of cherries in exercise and health. Scand J Med Sci Sports. 2014;24:477–90. doi: 10.1111/sms.12085. [DOI] [PubMed] [Google Scholar]
- 68.Yang W, Cui K, Li X, Zhao J, Zeng Z, Song R, et al. Effect of polyphenols on cognitive function: evidence from population-based studies and clinical trials. The journal of nutrition, health & aging. 2021;25:1190–1204. [DOI] [PubMed]
- 69.Chai SC, Davis K, Zhang Z, Zha L, Kirschner KF. Effects of tart cherry juice on biomarkers of inflammation and oxidative stress in older adults. Nutrients. 2019;11:228. doi: 10.3390/nu11020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dohadwala MM, Holbrook M, Hamburg NM, Shenouda SM, Chung WB, Titas M, et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr. 2011;93:934–40. doi: 10.3945/ajcn.110.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corona G, Tang F, Vauzour D, Rodriguez-Mateos A, Spencer JPE. Assessment of the anthocyanidin content of common fruits and development of a test diet rich in a range of anthocyanins. J Berry Res. 2011;1:209–16. doi: 10.3233/JBR-2011-022. [DOI] [Google Scholar]
- 72.Zamora-Ros R, Knaze V, Luján-Barroso L, Slimani N, Romieu I, Touillaud M, et al. Estimation of the intake of anthocyanidins and their food sources in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr. 2011;106:1090–9. doi: 10.1017/S0007114511001437. [DOI] [PubMed] [Google Scholar]
- 73.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–42S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 74.Kay CD, Mazza G, Holub BJ. Anthocyanins exist in the circulation primarily as metabolites in adult men. The. J Nutr. 2005;135:2582–8. doi: 10.1093/jn/135.11.2582. [DOI] [PubMed] [Google Scholar]
- 75.Yang M, Koo SI, Song WO, Chun OK. Food matrix affecting anthocyanin bioavailability: review. Curr Med Chem. 2011;18:291–300. doi: 10.2174/092986711794088380. [DOI] [PubMed] [Google Scholar]
- 76.Di Lorenzo C, Colombo F, Biella S, Stockley C, Restani P. Polyphenols and human health: the role of bioavailability. Nutrients. 2021;13:273. doi: 10.3390/nu13010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spencer JPE. The impact of fruit flavonoids on memory and cognition. Br J Nutr. 2010;104:S40–S7.. doi: 10.1017/S0007114510003934. [DOI] [PubMed] [Google Scholar]
- 78.Liu X, Yan Y, Li F, Zhang D. Fruit and vegetable consumption and the risk of depression: a meta-analysis. Nutr (Burbank, Los Angeles Cty, Calif) 2016;32:296–302. doi: 10.1016/j.nut.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 79.Keservani RK, Sharma AK, Kesharwani RK. Medicinal effect of nutraceutical fruits for the cognition and brain health. Sci (Cairo) 2016;2016:3109254. doi: 10.1155/2016/3109254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Figueira I, Garcia G, Pimpão RC, Terrasso AP, Costa I, Almeida AF, et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci Rep. 2017;7:11456. doi: 10.1038/s41598-017-11512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ogoh S. Relationship between cognitive function and regulation of cerebral blood flow. J Physiol Sci. 2017;67:345–351. [DOI] [PMC free article] [PubMed]
- 82.Gaitán A, Garolera M, Cerulla N, Chico G, Rodriguez‐Querol M, Canela‐Soler J. Efficacy of an adjunctive computer‐based cognitive training program in amnestic mild cognitive impairment and Alzheimer’s disease: a single‐blind, randomized clinical trial. Int J Geriatr psychiatry. 2013;28:91–9. doi: 10.1002/gps.3794. [DOI] [PubMed] [Google Scholar]
- 83.Tedstone A, Owtram G, Montel S, O'Kennedy E, Coulton V, Targett V, Perkins C, Sweeney K, Dowd L, Clegg E. Sugar reduction: juice and milk based drinks. A technical report outlining guidelines for industry, 2017 baseline levels for drinks in scope and next steps. London: Public Health England; 2018.
- 84.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Investig. 2009;119:1322–34. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loef M, Walach H. Fruit, vegetables and prevention of cognitive decline or dementia: a systematic review of cohort studies. J Nutr, health aging. 2012;16:626–30. doi: 10.1007/s12603-012-0097-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, JL, upon reasonable request.