Abstract
Over the course of mammalian evolution, the ability to store energy likely conferred a survival advantage when food became scarce. A long-term increase in energy storage results from an imbalance between energy intake and energy expenditure, two tightly regulated parameters that generally balance out to maintain a fairly stable body weight. Understanding the molecular determinants of this feat likely holds the key to new therapeutic development to manage obesity and associated metabolic dysfunctions. Time-restricted feeding (TRF), a dietary intervention that limits feeding to the active phase, can prevent and treat obesity and metabolic dysfunction in rodents fed a high-fat diet - likely by exerting effects on energetic balance. Even when body weight is lower in mice on active-phase TRF, food intake is generally isocaloric as compared to ad libitum fed controls. This discrepancy between body weight and energy intake led to the hypothesis that energy expenditure is increased during TRF. However, at present, there is no consensus in the literature as to how TRF affects energy expenditure and energy balance as a whole, and the mechanisms behind metabolic adaptation under TRF are unknown. This review examines our current understanding of energy balance on TRF in rodents and provides a framework for future studies to evaluate the energetics of TRF and its molecular determinants.
Keywords: Energy balance, Food intake, Energy expenditure, Metabolic switching, Time-restricted eating
Graphical Abstract:
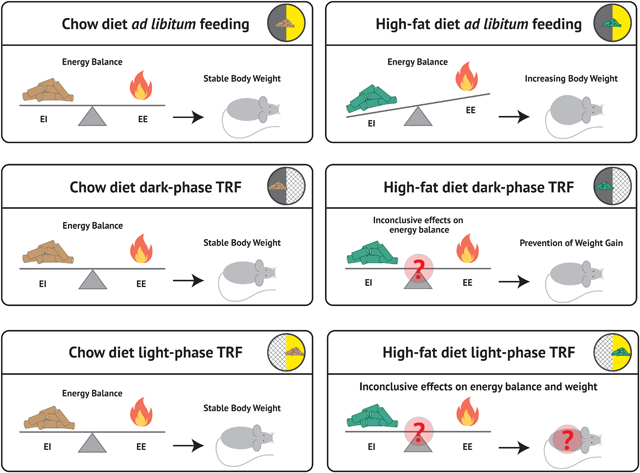
Whole-body energy balance based on daily phase of eating and diet composition.
This figure summarizes the current literature on energy balance under different feeding regimens with each tile of the figure independently representing a unique feeding intervention. The “teeter-totter” In each tile, represents the whole body energy balance; Energy Intake (EI) is represented on the left as food pellets, and Energy Expenditure (EE) is represented on the right side of each scale as a flame. Higher levels of EI or EE are represented as being heavier and tilting the scale in their respective direction. The ensuing effects on body weight are shown on the right side of each tile.
1. Introduction
Body weight is determined by the long-term energetic balance between energy intake and expenditure. Whole-body energy balance is tightly controlled by a host of neuroendocrine signals [1–3]. While highly regulated, energy intake and expenditure are influenced by both internal and external factors including size of adipose tissue depots, recent changes in weight or activity, and dietary or environmental cues regarding the safety and palatability of the food available [1, 2, 4]. The hypothalamus is the main regulator of feeding behavior and energy balance and contains both the suprachiasmatic nucleus (SCN), the central pacemaker of the circadian timing system, and multiple centers of feeding regulation [5]. The close proximity of the SCN to neural circuits regulating behavior, coupled with the roughly 24-hour rhythmic patterns observed in feeding, activity patterns, and body temperature, point to circadian regulation of energy balance [6].
Evidence from randomized controlled trials in humans suggests that eating out-of-sync with endogenous circadian rhythms is associated with poor cardiometabolic health [7–9]. In addition, studies in free-living humans show that caloric intake is often spread across the day, extending eating time to fall within the rest phase [10, 11]. In the gold standard rodent model of diet-induced obesity, mice are provided with ad libitum access to high-fat diet (HFD) and have been found to consume a higher percentage of calories during the light phase than mice fed a standard chow [12–14]. This increased proportion of caloric consumption during the rest phase plays a role in the pathogenesis of weight gain and metabolic disease as numerous studies in rodents show that restricting HFD feeding to the dark/active phase - a paradigm called time-restricted feeding (TRF) - leads to reduced body weight and better metabolic health when compared to animals with ad libitum access to the same HFD (Table 1) [13, 15–22]. In addition to preventing weight gain, switching from ad libitum HFD to dark-phase HFD TRF led to an initial weight loss and prevented further weight gain on a HFD [16], suggesting overall that aligning timing of feeding to circadian rhythms may protect against poor metabolic outcomes. However, the energetics and molecular mechanisms underlying the effects of TRF are incompletely understood. In this review, we aim to discuss the current literature on energy balance under TRF. Our discussion is limited to rodent studies with a TRF duration of at least 4 hours. Human literature on TRF/TRE (time-restricted eating) has been thoroughly reviewed elsewhere [23, 24]. The molecular connection between clock and metabolic regulators is also out of the scope of this review and is discussed in other comprehensive reviews on the subject [25–28].
Table 1.
Summary of relevant TRF studies in rodents. Shading: dark gray indicates study on active-phase TRF, light gray indicates both active and rest-phase TRF included in the studies, no shading indicates all TRF was during the rest phase.
| Study | Title | Species/Sex/Age at beginning | Feeding times of TRF intervention | Diet | Length of intervention | Effects on body weight and energy balance |
|---|---|---|---|---|---|---|
| Hatori et al., 2012 | Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet | C57BL/6J/Males/12 weeks | 1) ZT13–21, 2) ALF | 1) 61% fat (Lab diet, 58Y1), 2) NC ALF control | 18 weeks |
BW: TRF groups lower than ALF groups; Highest in HFD ALF EI: No differences EE: Higher in TRF groups compared to ALF; Higher in HFD compared to chow Activity: Higher in TRF compared to ALF during the late night |
| Chaix et al., 2014 | Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges | C57BL/6J/Males/8–12 weeks | 1) ZT13–22, 2) ZT12–24, 3) ZT10–1 4) ALF | 1) 60% fat (Lab diet, 58Y1), 2) 60% fructose diet (Harlan-TD89247), 3) FS diet (Research Diets-D12266B), 4) NC | 9, 12 or 15 weeks |
BW: Lower in TRF compared to ALF on all diets (1,2, and 3) excluding NC EI: No differences |
| Zarrinpar et al., 2014 | Diet and feeding pattern affect the diurnal dynamics of the gut microbiome | C57BL/6J/Males/10 weeks | 1) ZT13–21, 2) ALF | 1) 60% fat (Lab diet, 58Y1), 2) NC ALF control | 12–38 weeks | BW: Lower in HFD TRF compared to HFD ALF; HFD TRF similar to NC ALF |
| Chung et al., 2016 | Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity | C57BL/6NCrl/females with ovarectomy/Ovx at 7–8 weeks, 10 weeks HFD treatment start, TRF start at 19 weeks | 1) ZT16–24, 2) ALF | 1) 60% fat (Research diets, D12492), 2) NC ALF control | 8 weeks |
BW: HFD TRF lower than HFD ALF but higher than NC ALF EI: Highest in NC ALF; HFD TRF similar to HFD ALF measured in final week EE: NC highest; time of day differences between HFD TRF and HFD ALF Activity: Time of day differences; no overall differences between groups |
| Duncan et al., 2016 | Restricting feeding to the active phase in middle-aged mice attenuates adverse metabolic effects of a high-fat diet | C57BL/6J/Males/12 months | 1) ZT13–21, 2) ALF | 1) 60% fat (Research diets, D12492), 2) LFD ALF control | 21 weeks |
BW: Lower in HFD TRF compared to HFD ALF; Lower in LFD ALF compared to HFD TRF EI: No differences EE: No differences |
| Sundaram et al. 2016 | Time-restricted feeding reduces adiposity in mice fed a high-fat diet | C57BL/6/Males/3 weeks | 1) ZT13–21, 2) ZT12–24, 3) ALF | 1) 45% fat (modified AIN93G diet), 2) AIN93 ALF control | 11 weeks total, 9 weeks TRF |
BW: HFD TRF groups lower compared to HFD ALF EI: No difference between 12-hour HFD TRF and HFD ALF; Lowest in 8-hour HFD TRF EE: Oxygen consumption - higher in 12 hour HFD TRF compared to HFD ALF, but not different between the 8hr TRF and ALF or 12hr TRF groups; EE calculated from the Weir equations was lower in both TRF groups compared to ALF Activity: No differences |
| Olsen et al., 2017 | Time-restricted feeding on weekdays restricts weight gain: A study using rat models of high-fat diet-induced obesity | Sprague-Dawley rats/Males/5,13, & 18 weeks | 1) ZT13–2; 5 day TRF and 2 day ALF 2) ALF | 60% fat (Research diets, D12492) | 12/4/9 weeks |
BW: HFD TRF lower than HFD ALF EI: No differences EE: No differences |
| Woodie et al., 2018 | Restricted feeding for 9h in the active period partially abrogates the detrimental metabolic effects of a Western diet with liquid sugar consumption in mice | C57BL/6NHsd/Males/6–7 weeks | 1) ZT13–22, 2) ALF | 1) 45% fat, with 4.2% fructose/sucrose in water (Test diet, 5TJN), 2) NC | 1) 8 weeks HFSD, next 4 weeks of HFD+TRF. 2) 6 weeks HFSD, next 10 weeks of HFSD+TRF; metabolic phenotyping at 4 and 10 weeks |
BW: No difference in percent weight gain between ALF and TRF EI: Lower in HFD TRF compared to HFD ALF EE: Resting EE was lower in HFD TRF than HFD ALF |
| Cote, 2018 | Limiting feeding to the active phase reduces blood pressure without the necessity of caloric reduction or fat mass loss | Fisher 344 × Brown Norway (F344BN)/Males/6 months | 1) ZT12–24, 2) ALF | 1) HFD 60% (Research Diets, D12492), 2) NC | ~41 days | BW: No differences |
| Chaix et al., 2019 | Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock | Bmal1 Liver-KO, RevErba, b Liver-dKO, CDKO/Males/8–12 weeks | 1) ZT13–21/22, 2) ALF | 60% fat (Lab diet, 58Y1) | 12 weeks |
BW: HFD TRF lower compared to HFD ALF EI: No differences EE: Higher in HFD TRF when compared to HFD ALF Activity: No difference |
| Mitchell et al., 2019 | Daily Fasting Improves Health and Survival in Males Mice Independent of Diet Composition and Calories | C57BL/6J/Males/4 months | 1) ZT9–20, 2) ZT9–23 | NIA diet (7.6% energy from sucrose, 17.7%) WIS purified diet (46% energy from sucrose, 24.4% from fat) | >150 weeks |
BW: NIA TRF lower compared to NIA ALF; No differences between WIS ALF and WIS TRF EI: No difference between TRF and ALF diets EE: N/A |
| Aouichat, 2020 | Time-Restricted Feeding Improves Body Weight Gain, Lipid Profiles, and Atherogenic Indices in Cafeteria-Diet-Fed Rats: Role of Browning of Inguinal White Adipose Tissue | Wistar rats/Males/120–130g | 1) ZT13–21, 2) ALF | Cafeteria (CAF): highly palatable, energy-dense human foods consisting of cookies, cereals, chocolate, crackers, chips, cheese, processed meat, etc.) | 4 weeks |
BW: TRF lower than ALF on same diets EI: No difference between TRF and ALF on same diets |
| Sorrell, 2020 | The central melanocortin system mediates the benefits of time-restricted feeding on energy balance | MC4RKO mice/Males/8 weeks, DIO | 1) ZT12–24, 2) {HFD ZT12–24; LFD ZT0–12}, 3) ALF | 1) 58% HFD (Research Diets, D12331), 2) NC | variable |
BW: DIO mice on intervention 1 or 2 weighed less compared to ALF: MC4RKO intervention 1 mice had lower body weight than MC4RKO ALF EI: interventions 1 and 2 lower EI than ALF EE: TRF lower compared to ALF in DIO and MC4R KO mice |
| Kelly, 2021 | Time-optimized feeding is beneficial without enforced fasting | C57BL/6J/Males/11 weeks | 1) ALF, 2) HFD ZT 12–18, 3) HFD ZT18–24, 4) ZT12–18 and NC at all other times, 5) HFD ZT 18–24 and NC at all other times | 1) HFD 60% (Research Diets, D12492), 2) NC | 8 weeks |
BW: TRF lower than HFD ALF in interventions 2, 3, 4, & 5 (intervention 4 lower than intervention 5) EI: No differences EE: No differences |
| Chaix at al., 2021 | Sex- and age-dependent outcomes of 9-hour time-restricted feeding of a Western high-fat high-sucrose diet in C57BL/6J mice | C57BL/6J /Males & Females /3mo & 1 y | 1) ZT13–22, 2) ALF | 1) Western diet 45% fat, 17% sucrose (Research Diet, D12451) | 12–13 weeks |
BW: HFHS TRF lower than HFHS ALF in males; No differences in females EI: No differences between ALF and TRF EE: No differences between TRF and ALF Activity: 1 year-old males TRF had higher activity than ALF counterparts; No differences in ALF vs. TRF at other ages |
| Das et al., 2021 | Time-restricted feeding normalizes hyperinsulinemia to inhibit breast cancer in obese postmenopausal mouse models | post-menopausal females: 7-week C57BL/6J + ovarectomy or follicle depletion | 1) ZT16–24, 2) ALF | 1) 60% HFD (Research Diets, 12492), 2) NC ALF control | 9 weeks |
BW: TRF lower when compared to ALF EI: No differences between TRF and ALF |
| Davis et al., 2021 | Time-restricted feeding rescues high-fat-diet-induced hippocampal impairment | C57BL/6J/Males/8 weeks | 1) ZT16–24, 2) ALF | 1) HFD 45% (Research Diets, D12451), 2) NC | 2 weeks |
BW: No differences between TRF and ALF on NC or HFD EI: No differences between ALF and TRF groups EE: N/A |
| Wang et al., 2021 | Time-Restricted Feeding Restored Insulin-Growth Hormone Balance and Improved Substrate and Energy Metabolism in MC4RKO Obese Mice | MC4RKO/Males mice on C57BL/6J background/males | 1) ZT13–22/24, 2) ALF | NC | 9 weeks |
BW: TRF lower than ALF EI: No differences EE: No differences |
| Regmi et al., 2021 | Early or delayed time-restricted feeding prevents metabolic impact of obesity in mice | C57BL/6J/Males/8 weeks | 1)early TRF: ZT12–22, 2) delayed TRF: ZT16–2, 3) ALF | 1) HFD 43% (Specialty Feeds, SF16–001) 2) NC | 12 weeks |
BW: Early TRF lower than Delayed TRF; Delayed TRF lower than ALF EI: No difference between groups on same diet EE: No differences Activity: Higher in TRF compared to ALF on same diets |
| Satoh et al.,, 2006 | Time-restricted feeding entrains daily rhythms of energy metabolism in mice | ICR/Males/6 weeks | 1) ZT2–11, 2) ZT11–2, 3) pre-and post TRF was ALF feeding | NC in relevant experiments | 5 days |
EI: no differences reported EE: lower in intervention 1 than during ALF; no differences between ALF and intervention 2 Activity: “slightly less” activity in intervention 1 versus ALF; not different between intervention 2 and ALF |
| Arble et al., 2009 | Circadian timing of food intake contributes to weight gain | C57BL/6/Males/9 weeks | 1) ZT0–12, 2) ZT12–24 | 60% fat (Research diets, D12492) | 6 weeks |
BW: Light fed animals weigh more than dark fed animals EI: No difference between light fed and dark fed Activity: No difference between light fed and dark fed |
| Bray et al., 2010 | Time-of-day-dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice | FVB/N/males/unspecified | 1) (LF ZT0–12, HF ZT12–24), 2) (HF ZT0–12, LF ZT12–24), 3) (LF ZT 12–24), 4) (HFD ZT 12–24), 5) {HFD ZT12–16, LF ZT16–20}, 6) (LF ZT12–20, HF ZT20–24), 7) (HF ZT12–16, LF ZT20–24), 8) (LF ZT12–16, HF ZT20–24) | 1)HFD 45% fat (Research diets, D12451), 2) LFD 10% fat (Research Diets D12450B) | 12 weeks |
Interventions 1 vs. 2
BW: No difference EI: No difference EE: Intervention 1 higher than intervention 2 Activity: no overall difference Interventions 3 vs. 4 BW: No difference EI: No difference EE & Activity: No difference Interventions 5 vs. 6BW: Higher for intervention 6 versus 5 EI: Higher for 6 vs 5 EE: Higher for 6 vs. 5 Activity: No difference Interventions 7 vs. 8 BW: Higher for intervention 8 vs. 7 EI: No difference EE & Activity: No differences |
| Fonken et al., 2010 | Light at night increases body mass by shifting the time of food intake | Swiss–Webster/Males/8 weeks | 1) ZT 15–1, 2) ZT1–15, 3) ALF (in 16:8 L:D or 16:8 L:Dim) | NC | 8 weeks |
BW: In L:D conditions, BW highest in intervention 2; in L:Dim conditions, BW lowest in intervention 1 EI: No differences |
| Salgado-Delgado et al., 2010 | Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work | Wistar rats/Males/11–12 weeks | 1) ZT0–12, 2) ZT12–24, 3) AL; Half of mice were on a shift-work model 5 out of 7 days where mice were in a slow rotating wheel ZT2–10 | NC | 5 weeks | No Forced Work Condition: BW: Highest in intervention 2 EI: no difference Forced Work: BW: Intervention 1 and 3 higher than intervention 2 EI: No difference *Total activity was not reported but forced work with ALF or light feeding altered activity rhythms to have more activity during the day and less at night |
| Tsai et al., 2013 | Influence of dark phase restricted high fat feeding on myocardial adaptation in mice | FVB/N/Males/12 weeks | 1) ZT12-ZT24, 2) ALF | 1) HFD 45% fat (Research diets, D12451), 2) NC | 12–16 weeks |
BW: TRF on HFD reduced body weight gain compared to ALF EI: No difference EE: TRF chow had lower energy expenditure than ALF HFD; No other group differences |
| Yasumoto, 2016 | Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice | C57BL/6J/Males/6 weeks | 1) ZT2–10, 2) ZT 14–22 | HFHS 54.5% kcals fat + 20% w/w sucrose (Oriental Yeast, F2HFHSD) | 1 week |
BW: Higher BW and body fat in light phase fed mice compared to dark phase fed mice EI: Higher in light phase fed mice Activity: Total daily voluntary wheel running in light phase fed mice was lower than dark phase fed mice |
| Acosta-Rodriguez, 2017 | Mice under Caloric Restriction Self-Impose a Temporal Restriction of Food Intake as Revealed by an Automated Feeder System | C57BL/6J/Males/8 weeks + 2 weeks acclimation | 1) ZT0–12, 2) ZT12–24, 3) ALF | NC | 6 weeks |
BW: No differences Food intake: In the first 2.5 hours of feeding, ZT0–12 mice consume more than ALF mice; ZT12–24 mice show no differences Activity: Rhythms differ by feeding condition; Total activity comparisons not reported |
| Kentish et al., 2018 | Time-Restricted Feeding Prevents Ablation of Diurnal Rhythms in Gastric Vagal Afferent Mechanosensitivity Observed in High-Fat Diet-Induced Obese Mice | C57BL/6/Males/12 weeks | 1) ZT0–12, 2) ZT 12–24, 3) ALF | 1) 60% HFD or 2) NC | 8 weeks |
BW: No difference in body weight for any groups on chow; Both TRF groups weighed less than the ALF group EI: Lower in the light phase chow group versus the dark phase chow group; HFD TRF groups displayed no differences; Both HFD TRF groups lower than the HFD ALF group |
| Oishi et al., 2018 | Short-term time-restricted feeding during the resting phase is sufficient to induce leptin resistance that contributes to development of obesity and metabolic disorders in mice | C57BL/6, db/db, ob/ob/Males/8 weeks | 1) ZT2–10, 2) ZT14–22 | HFHS 54.5% kcals fat + 20% w/w sucrose (Oriental Yeast, F2HFHSD) | 2 weeks |
BW: Higher in light phase feeding than dark phase feeding EI: Lower at the start if intervention for light phase fed animals but no differences overall Activity: Lower in light phase fed mice |
| Abe et al., 2019 | Food deprivation during active phase induces skeletal muscle atrophy via IGF-1 reduction in mice | C57BL/6/Males/8 weeks | 1) ZT2–10, 2) ZT14–22 | HFHS 54.5% kcals fat + 20% w/w sucrose (Oriental Yeast, F2HFHSD) | 1 week |
BW: Lower in dark phase fed than light phase fed EI: No differences Activity: Lower in light phase fed than dark phase fed |
| Boucsein et al., 2019 | Hypothalamic leptin sensitivity and health benefits of time-restricted feeding are dependent on the time of day in males mice | C57BL/6/Males/16 weeks | 1) ZT3–9, 2) ZT9–15, 3) ZT15–21, 4) ZT 21–3, 5) ALF | 1) 10% LFD (Research Diets), 2) 60% HFD (Research Diets 12492) | 3 weeks |
BW: Higher in HFD ALF than all HFD TRF groups EI: Higher in HFD ALF than all HFD TRF groups; No differences among TRF groups EE: Lower in HFD ZT3–9 than HFD ALF; No differences between other HFD TRF and HFD ALF groups Activity: No differences between HFD ALF and HFD TRF groups |
| De Goede, 2020 | After-Effects of Time-Restricted Feeding on Whole-Body Metabolism and Gene Expression in Four Different Peripheral Tissues | Wistar/Males/8 weeks | 1) ZT1–11, 2) ZT13–23 | NC | 4 weeks |
BW: Light phase TRF weigh less than dark phase TRF and ALF; No difference between ALF and dark phase TRF EI: Dark phase TRF higher than light phase TRF but lower than ALF Activity: Not different between groups |
| Acosta-Rodriguez, 2022 | Circadian alignment of early onset caloric restriction promotes longevity in males C57BL/6J mice | C57BL/6J/Males/6 weeks | 1) ALF, 2) caloric restriction with: a) continuous feeding, b) bolus feeding @ ZT 0, c) bolus feeding ZT @12, d) continuous feeding ZT12–24, or e) continuous feeding ZT 0–12 | NC | aging survival study |
BW and EI: All CR groups less than ALF regardless of TRF Activity: Daytime feeding misaligns activity; ALF group had lower activity than CR groups at age 6–18 months |
| Szentermai, 2010 | Restricted feeding-induced sleep, activity, and body temperature changes in normal and preproghrelin-deficient mice | C57BL/6J and preproghrelin KO/Males/4 months | 1) ALF baseline, 2) ZT4–8 | NC | 12 days |
BW: KO and WT mice decreased body weight from baseline to post-TRF intervention Activity: Increased under TRF in WT mice but was reduced under TRF in the KO mice |
| Sherman et al., 2011 | Long-term restricted feeding alters circadian expression and reduces the level of inflammatory and disease markers | C57BL/6/Males/11 weeks | 1) ZT3–6, 2) ALF | NC | 16 weeks |
BW: Lower in TRF EI: Lower in TRF Activity: No difference |
| Sherman et al., 2012 | Timed high-fat diet resets circadian metabolism and prevents obesity | C57BL/6/Males/6 week | 1) ZT4–8, 2) ALF | 1) 42% HFD (made in house), 2) NC | 18 weeks |
BW: TRF HFD lower than ALF HFD* EI: ALF HFD higher than TRF HFD; NC ALF higher than NC TRF* Activity: Total activity was higher in TRF versus ALF groups; TRF NC was more active than TRF HFD *statistical comparisons not noted for BW or EI |
Abbreviations: BW- body weight, EI= energy intake, EE= energy expenditure, HF/HFD= High fat diet, LF/ LFD- low fat diet, NC= normal chow, HFHS= high fat high sucrose, RER= respiratory exchange ratio
1.1. Weight defense and common weight loss treatments:
Obesity affects roughly one in three Americans and raises the risks for many chronic diseases including multiple types of cancers, cardiovascular disease, and metabolic syndrome, in turn, contributing greatly to the public health burden [2, 4, 29]. Despite its prevalence, successful treatments for obesity and the often-associated metabolic dysfunction are lacking. Thus, investigations into the mechanisms allowing weight gain to occur and the development of new therapeutic strategies to prevent or reverse obesity are needed and stand to benefit a large proportion of the population.
Dietary and behavioral interventions, such as calorie restriction and exercise programs, are often the first line of treatment for obesity and metabolic syndrome. Yet, in addition to weight regain following the period of caloric restriction, development of disordered eating symptoms has been reported following unmonitored calorie restriction [30–32]. Pharmacotherapy has, in the past, relied on centrally acting appetite suppressants, that induce modest weight loss of roughly 5% that is often reversed following cessation of treatment [30, 33]. Newer anti-diabetic therapies, including glucagon like peptide 1 (GLP-1) receptor agonists and sodium-glucose cotransporter-2 (SGLT2) inhibitors induce significant weight loss [34–36], but gastrointestinal side effects and weight regain after stopping treatment are major concerns with these therapies [34, 36–39]. Roux-en-Y gastric bypass (RYGB) is considered the gold standard for inducing sustained weight loss [40–45], but this surgery is not without risk of mortality, surgical complications, and lifelong nutritional deficiencies [45–48]. Strikingly, whether weight loss is achieved through behavioral, pharmacologic, or even some surgical interventions, weight regain is the norm due in large part to systemic physiologic mechanisms that oppose changes in weight [3, 49, 50].
This opposition to changes in body weight is sometimes referred to as “defense of body weight” or “body weight set-point” and has been observed in human and animal models. Body weight is a manifestation of a multitude of complex processes, and its “set-point,” or “settling-point,” is influenced by a host of internal and external factors including age, genetics, and diet, so that the absolute numerical value of the set-point may change throughout a lifetime [51–53]. Adjustments in food intake and appetite, energy expenditure, or a combination of both that resist weight loss have long been observed in response to changes in a previously stable body weight. These responses underlie the difficulty often faced when trying to lose weight or maintain a reduced body weight. Over the past century, seminal studies have expanded our understanding of energy balance and defense of body weight. A key 1945 study conducted at the University of Minnesota investigated the response to 50% caloric restriction in WWII conscientious objectors. The subjects reported feeling extremely cold and fatigued, mood swings, low libido, and preoccupation with food suggesting reduced energy expenditure and increased drive to find food factors that which encourage a return to the pre-starvation weight [54]. A subsequent phase of this study allowed the men ad libitum food intake where they ate up to 11,000 kilocalories per day and experienced extreme hyperphagia which persisted until the original body fat percentage was reached [55]. Since then, a number of studies have shown that humans, regardless of obesity status, resist weight loss through adaptations in energy intake and energy expenditure and that alterations in energy expenditure persist long after initial weight loss efforts [56–59].
TRF has been shown to prevent weight gain, treat obesity, and curtail metabolic dysfunction in rodent models [13, 14, 16, 60]. These beneficial effects have been seen even without reductions in caloric intake, making it a promising intervention to overcome body weight defense mechanisms [13, 60]. Yet, there is no consensus on how TRF affects energy balance and expenditure- due in part to technical constraints as well as limitations of experimental designs. This review aims to address the current literature on energy balance during TRF, and to provide a framework for future studies to better quantify and evaluate energy balance on TRF. Understanding the effects of TRF on energy balance and the mechanisms that underlie its ability to attenuate metabolic dysfunction on a HFD will move us closer to discovering new treatments and preventive therapies for obesity and metabolic dysfunction.
1.2. Circadian Rhythms
The earth’s rotation creates a roughly 24-hour light-dark cycle that influences most if not all lifeforms on the surface of the planet. We humans and other diurnal animals, align our work and school schedules (active times) to the time when the sun’s natural light is present. Conversely, nocturnal animals, such as mice, are active at night and rest during the light phase. In addition to influencing our behavior and sleep wake cycles, the light-dark rhythms have been internalized to anticipate and coordinate the timing of a host of biological processes.
Many biologic processes and pathways have rhythmic oscillations in their activity that occur over a roughly 24 hour long period; these oscillations are known as circadian rhythms [25, 61]. The term circadian refers to a time period of roughly 24 hours- coming from the Latin word “circa” meaning about, and “diem” meaning day [24, 62]. Circadian rhythmicity has been observed in numerous physiologic functions including sleep-wake cycles, metabolism, and hormonal release. This rhythmicity is governed by a core set of genes, known as “clock genes” that act as transcriptional and translational activators and repressors [24, 25].
In mammals, clock genes are engaged in a circadian rhythms generating feedback loop whereby Bmal1 and Clock are the activating arm and Per, Cry are part of the inhibitory arm [25, 61, 62], Clock genes activity in the suprachiasmatic nucleus (SCN), a small, hypothalamic region, acts as a pacemaker and master regulator of whole body circadian rhythms. The SCN is entrained to light and dark cycles via retinal inputs thus aligning the clock genes’ expression with the light-dark cycles. Interestingly, a clock mechanism is present in most cells of an organisms allowing for circadian rhythms in the activity of most biological processes [25].
Synchronizing and coordinating the activity of different processes has likely conferred a benefit to the organism by preventing the overlap of antagonistic processes [25]. For example, humans feed during the day and fast during their rest period suggesting that the metabolic pathways that are active during the dark and light phases should be different– with the day favoring storage of energy and the night favoring breakdown and use of energy supplies. As the rhythms of feeding and fasting are likely predictable and occur following a circadian rhythm the metabolic pathways allowing for storage versus fuel usage can be upregulated and downregulated based on when the predicted feeding and fasting periods are likely to occur and prevent activation of opposing pathways [25, 61].
1.3. What is time-restricted feeding?
Time-restricted feeding is an intervention that restricts food intake to certain periods of the day. In animal/rodent models, TRF was initially used as a tool to study the underlying circuitry of food anticipatory behavior– a bout of activity that precedes the scheduled provision of food when animals are maintained on calorie restriction and fed during the inactive phase (in most cases). The ability of food to function as an external cue for the regulation of daily activity rhythms, referred to as a Zeitgeber (‘time giver’ in German), sparked further investigations into food timing as a universal Zeitgeber for the biological circadian clock. Thus, early studies of TRF in rodent models tested whether the diurnal oscillatory pattern of expression of “clock genes” that regulate circadian rhythms, could be altered by off-cycle feeding. Because rodents are nocturnal, out of phase feeding occurs when mice are fed during the light-phase. In the early 2000s, key work showed that food could, in fact, be used as a Zeitgeber to entrain peripheral expression of clock genes especially in the liver [63–65] but not in the SCN, the hypothalamic pacemaker and master regulator of whole body circadian rhythms [61, 63–65].
A molecular link between circadian regulation and metabolic function was uncovered through untargeted transcriptomics studies in the liver that showed that the expression of many genes involved in metabolism and energy utilization naturally oscillate throughout the day [66]. This relationship between clock and metabolic homeostasis was further confirmed by the metabolic disturbances observed in mouse models lacking master clock genes. Specifically, Clock and Bmal1 KO and Cry1 and Cry2 double KO mice (cry1−/−; cry2−/−) were reported to have impaired gluconeogenesis [67] or glucose intolerance [68, 69]. In terms of energy balance, cry1−/−; cry2−/− mice do not exhibit the normal bimodal rhythms in activity and respiratory exchange ratio (RER) that are typically observed in wild-type mice [70]. However, these rhythms can be restored under TRF [70], suggesting that timed feeding can be used to introduce a cyclic rhythm of whole body metabolic activity even in the absence of a functional circadian oscillator [14, 70].
Evidence that oscillations of clock and metabolic genes in the periphery were affected by TRF launched the investigations into the metabolic effects of TRF. Early studies in rodents revealed that the timing of food intake affects weight gain trajectories. Restricting high-fat diet access to the dark phase (active phase TRF), the time when mice normally eat, prevented weight gain and accrual of fat mass as compared to ad libitum high-fat fed controls (Table 1) [15, 71–73]. Disruption of normal circadian rhythms, either through forced work or constant light, has also been observed to lead to weight gain and increased fat mass in the absence of increased food intake, but can be prevented by active-phase TRF diet [71, 73].
2. Energy balance under active phase TRF on HFD
2.1. Body weight, and food intake:
In rodents, dark phase TRF often leads to reduced body weight or attenuated weight gain on a HFD in addition to a number of other metabolic benefits including reduced glucose intolerance as well as lowered serum and liver lipid levels [23, 74]. The mechanisms mediating resistance to weight gain on TRF are currently unclear. While caloric intake is a major regulator of body weight, the majority of TRF studies in the dark phase do not report reduced caloric intake as a concurrent finding to reduced body weight (Table 1) [13–15, 17, 19, 60, 75–80].
It is important to note that while the majority of studies report weight loss or prevention of weight gain on HFD, Chaix et al., revealed that body weight effects may be sex specific [60]. While both young and aged males weigh less on dark phase TRF than their ad lib fed counterparts, body weight divergence is not present in female mice. However, sex specific effects on body weight, body composition, and metabolism between C57BL/6 male and female mice on HFD are not unique to TRF interventions. Female versus male mice have been shown to be more resistant to weight gain and insulin resistance development under short-term HFD feeding likely due to protective effects of estrogen [81]. Future studies will clarify whether BW differences and other metabolic benefits can be observed under longer TRF interventions and their interplay with females’ reproductive age, estrogens, and insulin levels. Additionally, a number of other studies do not find reduced body weight on dark phase TRF [76, 82–84]. However, short length of intervention rats versus mice, and diet composition could account for the differing results (Table 1) as diets of differing fat content have been shown to affect food intake and body weight [85, 86].
Overall, male mice on HFD TRF interventions weigh less than their ad lib fed counterparts (Table 1). Although caloric intake is influenced by a host of factors including animal behavior (grinding or playing with the food), seasonal variations in intake, investigator techniques and biases, noises, odors, and social isolation, age, and diet ingredients (e.g. type of fat used for diet composition) [75, 87–91], the agreement of multiple studies outlined above suggests that reduced caloric intake does not explain the reduced body weight seen in dark phase TRF animals.
Another factor that could contribute to differences in body weight between TRF and ad libitum fed animals in the absence of differences in food intake is a change in the balance between nutrient absorption and excretion in the gut. Although at present there is not enough evidence to draw definitive conclusions on how TRF affects those parameters, the idea that these could be affected is strongly supported by several studies showing an effect of TRF on the gut microbiome composition and dynamics and on fecal nutrient composition [22, 92, 93]. Untargeted metabolomics analysis of the feces from TRF versus ad libitum fed mice on HFD showed relatively more byproducts of hemicellulose breakdown in feces from TRF mice, suggesting that TRF mice were likely absorbing less calories from those [22]. TRF also alters the composition of the bile acid pool which could affect lipid absorption and nutrient reabsorption itself [22, 93]. However, since these results were not normalized to total fecal output and caloric load, whether this reflects a difference in caloric extraction from specific nutrient source and/or translates to an overall difference in caloric absorption or excretion remains to be determined. Studies of fecal energy and lipid content will be crucial to understanding how TRF affects nutrient absorption and excretion.
2.2. Active phase HFD TRF and energy expenditure:
Since body weight is determined by both energy intake and expenditure, for body weight to be reduced during active phase TRF, an increase in energy expenditure must be present in the absence of deficit in caloric intake. As follows, a number of studies show increased energy expenditure for animals on dark-phase TRF when compared to ad libitum fed animals [13, 94] which could possibly explain the differences in body weight and improvements in metabolic health. However, findings on energy expenditure are not consistent across studies and many results show no differences in energy expenditure and activity (Table 1) [16, 17, 21, 79].
Overall, the different findings on energy expenditure in TRF could be the result of multiple factors including: the use of different TRF protocols (timing and length), and differences in how or whether energy expenditure data are normalized - for example, to total body weight or to lean mass. Moreover, measurement of energy expenditure in rodents is extremely difficult due to their small size, inaccuracy of measurement systems, and a lack of standardization in the way data are normalized and analyzed [95–97]. The small body size of rodents makes measurements of heat production difficult to assess, so indirect calorimetry systems, which measure the amount of oxygen consumed to carbon dioxide produced are standard metabolic measures [95, 96]. This method is a proxy for direct calorimetry and relies on mathematical and chemical equations of glucose metabolism to calculate metabolic rate. Most studies use the Weir formula [98] (or a derivation) which relies on measures of the amount of oxygen consumed to carbon dioxide produced. However, this formula does not account for differences in substrate utilization.
Substrate utilization could be taken into account by using the respiratory exchange ratio (RER), a measure of the ratio of carbon dioxide produced and oxygen consumed that informs whether carbohydrate or fat is being primarily utilized. Additional consideration of nitrogen excretion in the urine to determine protein usage aids in further calculation of substrate utilization [95]. However, this approach would require very accurate measures of food intake over time as well as accurate determination of dietary macronutrient content. In theory, the differences in metabolism based on substrate utilization are small, and the ability to measure energy expenditure from gas exchange should be very accurate. However, respiratory gas measurements of metabolic rate are extremely variable suggesting that indirect calorimetry may not always be an extremely accurate method of small animal energy expenditure measures and may not capture the, likely very small, differences in energy expenditure on TRF [95, 99]. Moreover, many metabolic cage systems require single housing of the animals away from their home cages- a change that is likely to induce stress in addition to removing socialization and a source of warmth for the animals, potentially confounding measurements of energy expenditure.
Beyond technical difficulties, data processing and presentation is also a point of inconsistency between studies. Some studies normalize energy expenditure to body weight or body weight raised to the power of 0.75, others to lean mass, and some not at all [95, 99, 100]. Because lean mass and fat mass have different metabolic capacities, normalization to body weight does not account for these tissue differences [95, 96, 100]. Moreover, lean mass is a heterogeneous tissue. Thus, its metabolic rate is also not consistent [95, 100]. Further complicating matters, fat mass affects metabolism through secreted adipokines, making normalization by lean mass inadequate for assessing tissue differences [95, 96, 100]. Finally, the regression of energy expenditure on total body mass or lean body mass has a non-zero intercept making it an inaccurate method of normalization that comes up with measures that are physiologically impossible [95, 96, 100]. In the past ten years, consensus guidelines recommend that energy expenditure data be analyzed using analysis of covariance (ANCOVA) with measures of body composition as cofactors [95, 99, 100]. As such, energy expenditure from all papers discussed above would need to be re-analyzed in the same manner to draw proper comparisons between the different studies.
Besides resting metabolic rate, activity also contributes to the overall energy expenditure. While increased activity on TRF has been reported [13], a majority of studies do not report that dark phase TRF alters total activity levels. Rather it has been shown that dark phase TRF modulates circadian rhythms of activity as opposed to total levels [14, 17, 73, 77, 101, 102]. However, similar to aforementioned caveat to accurate measurements of energy expenditure, measurements of activity may be confounded by the single housing and a new environment in many studies. Furthermore, the use of beam breaks and passive infrared sensors to measure activity does not allow activity during REM sleep versus awake behaving activity to be distinguished. As the field moves forward, home cages assessment of both sleep and activity will further our understanding as to how TRF affects total activity as well as circadian patterns of activity.
2.3. Metabolic switching and active phase TRF
In addition to the effects on energy balance, TRF interventions consistently affect metabolic switching as defined by the ability to vary oxidative substrate selection from glucose to fatty acids within the course of a day according to periods of feeding and fasting. Metabolic switching may play a key role in overall metabolic health and longevity and is distinct from metabolic flexibility which describes long-term metabolic shifts [103]. The Respiratory Exchange Ratio (RER) can be used as a proxy to assess metabolic switching across a 24-hour period. The RER is the ratio of the volume of carbon dioxide expelled by the animal to the volume of oxygen consumed as measured by respiratory gas exchange [95]. Although the RER is largely affected by the composition of the diet, its value is a reflection of the metabolic substrate being oxidized [104]. The RER generally falls between 0.7 and 1; with 0.7 and 1 reflecting oxidation of pure fatty acids and carbohydrate respectively, and an RER between 0.7 and 1 reflecting mixed macronutrient substrate usage [104].
While ad libitum HFD fed animals maintain a RER around 0.8– suggesting a use of mixed macronutrient substrates at all times of the day- HFD-fed animals on TRF display a small yet noticeable fluctuation of their RER across the day. During fasting, mice on active phase HFD TRF have increased relative fatty acid oxidation with a lower RER of approximately 0.7–0.8 while above 0.8 during times of food availability [13, 18, 21, 70, 72]. This increase in relative fatty acid oxidation on TRF occurs regardless of diet, but is less pronounced in rodents fed a HFD because the RER is already lower during fed periods [13, 21, 102]. During times of negative energy balance an RER of closer to 0.7 is observed reflecting breakdown of endogenous lipid stores [14, 105]. However, after a meal is consumed, RER increases due to replenished glucose supplies, and RER switches to reflect usage of a larger proportion of glucose and amino acids in addition to the dietary fat [14, 77, 105]. The oscillations in the RER are independent of body weight and also occur in mice lacking a circadian clock machinery [14, 70, 76].
Although the mechanism is incompletely understood, metabolic switching may increase mitochondrial biogenesis and neural plasticity as well as improve glycemic control and may be at least partially responsible for the beneficial effects of dark-phase TRF on metabolic health [105, 106]. While it has been suggested in the literature, no studies that we are aware of draw conclusions on whether metabolic switching is the key to the benefits observed on HFD TRF and future research is needed on this subject to come closer to understanding the mechanisms of TRF.
Interestingly, a recent study aimed at testing the contribution of forced fasting to TRF benefits may support a role for metabolic switching. In this study, mice had access to HFD for six hours during the dark-phase with ad libitum access to chow diet during the rest of the day [77]. The RER of these mice switched between roughly 0.9 (when HFD was available) and 0.75 (no HFD available) with the higher RER suggestive of burning a diet-derived mixed macronutrient substrate while HFD was available and the lower RER suggestive of burning internal fat stores due to low food intake when HFD was not available [77]. Under these conditions, mice were still protected against weight gain compared to mice fed an isocaloric ad libitum HFD suggesting that TRF benefits can be seen even when forced fasting is eliminated under conditions that allow metabolic switching. In studies where fasting was enforced for various durations, it appeared that the longer the fasting period, the greater the metabolic benefits [16]. Yet, whether this is due to the fasting per se or metabolic switching will be a topic for future research. Studies that allow modulation of RER with and without periods of fasting or diet changes will be crucial to disentangling the tightly coupled roles of fasting and metabolic switching in TRF. Answering those questions is particularly relevant from a translational standpoint as an uninterrupted fast is relatively easy to enforce in laboratory animals but likely less feasible in free-living humans. Whether fasting is the key factor in attenuation of weight gain has been the matter of recent research endeavors [107, 108], and future studies examining the role of metabolic switching may uncover a mechanism as to the benefits of fasting and TRF.
3. Dark phase TRF on a chow diet
Time-restricted feeding studies on a chow diet are less common as mice on ad libitum chow are generally considered healthy controls in most metabolism studies, so there is not a large metabolic or circadian phenotype to “treat.” Nevertheless, a number of studies have examined the effects of dark phase chow feeding on energy balance and metabolic health. Studies have generally reported no differences in body weight between ad lib and dark-phase chow fed animals (Table 1) [20, 71, 73, 84, 108, 109]. However, TRF on chow may still have beneficial effects even if weight effects are not present. A long-term TRF study on chow interestingly showed that lean mass was increased over fat mass as the mice aged under TRF [16]. There is also evidence that TRF improves healthspan and lifespan [76, 108] and corrects circadian dysfunction when the normal circadian clock is disrupted [73, 110].
4. Energy balance under rest phase TRF
While there are few studies investigating the consequences of feeding during the natural rest phase in rodents, results are relatively congruent and suggest that feeding during the typical rest period results in adverse metabolic consequences. Specifically, light phase feeding appears to result in increased weight gain [15, 101, 111] and increased fat mass [73] even when food intake is matched between TRF and ad lib fed rodents [15, 73]. However, multiple studies have found reduced body weight in mice fed during the light phase when compared to the ad libitum feeding (Table 1). Yet, these studies report the reduced weight to be accompanied by a reduction in food intake– likely due to the short duration of the feeding intervals (3–4 hours) making the results difficult to interpret [112, 113].
On the energy expenditure side, there are too few studies measuring energy expenditure during rest-phase TRF to draw broad conclusions. Furthermore, results from these studies are mixed. While most assessments of total daily activity find no differences based on daily phase of eating [15, 21, 72, 73, 101, 108], others report higher or lower activity in day fed mice versus ad lib or night-fed animals [101, 113, 114]. Two studies that we are aware of found reduced energy expenditure in light-phase fed animals as compared to ad lib animals using indirect calorimetry [80, 115]. Nonetheless, even when total activity remains the same, changes in patterns of activity that could ultimately lead to shifts in energy balance have been observed under daytime feeding [73, 109, 116]. These shifts include ratio of activity during the light-dark phases [73, 114], sleep-wake architecture [117], and food anticipatory behavior [108, 117]. Thus, more studies of energy expenditure in rest-phase TRF along with measures of circadian patterns sleep and activity are needed.
Collectively, although some evidence suggests that eating during the typical rest phase might have adverse consequences when compared to isocaloric intake during the active phase, the underlying mechanisms and whether it involves changes in whole-body energetic balance still needs to be fully determined. In addition, whether diet composition can further exacerbate metabolic imbalance under rest-phase eating remains to be tested. New study designs involving paired feeding between light and dark phase, various diet compositions, as well as close monitoring of food intake and various parameters of energy expenditure are needed to expand our understanding of the impacts of daily phase of eating. The molecular understanding gained from these studies will help with the development of new strategies to mitigate the effect of out-of-sync feeding that exists in a large fraction of the population and is inherent to the rotating shift workforce in our industrialized world.
5. The effects of TRF on neuronal and hormonal regulators of food intake.
Not much is known about how TRF affects the central control and peripheral regulators of caloric intake. Studies in humans have suggested that TRF may reduce feelings of hunger [118] pointing to potential effects of TRF on the control of feeding. A recent study in mice shows increased plasma concentrations of the appetite suppressing hormone glucagon like peptide-1 (GLP-1) during the dark cycle in HFD fed animals on TRF versus their ad libitum fed counterparts [93], which could potentially explain reductions in appetite or food intake during TRF. Nevertheless, more studies are needed to fully elucidate the effects of TRF on peripheral and central regulators of appetite and feeding and will be important for understanding TRF effect on energy intake.
Ghrelin is currently the only known orexigenic hormone and is mainly secreted by enteroendocrine cells in the stomach. As circulating ghrelin levels are highest just before feeding and increase in response to fasting [119], any measurements of ghrelin will be affected by the timing of the measurements and the fasted or fed status of the mouse making it difficult to interpret findings when mice are entrained to consuming food at certain times. Nevertheless, it has been shown that dark-phase fed animals have higher ghrelin levels than ad lib fed ones [18, 120], but that rest-phase fed animals have lower ghrelin levels than ad lib controls [113]. Finally, in a 12 day light-phase TRF study, pre-proghrelin KO mice were observed to respond to TRF with weight loss mirroring the WT control mice [116] suggesting that the effects of TRF may be independent of ghrelin levels. Overall, there is inconclusive evidence on how TRF affects ghrelin signaling since differences in food intake and body weight make it difficult to interpret the comparisons between feeding interventions.
In addition to ghrelin, leptin levels have also been explored as a proxy to assess TRF effects on feeding. However, the role of leptin in maintaining body weight is not entirely clear. For a long time, it was believed that leptin, which is secreted in proportion to fat mass, serves as a long-term signal to prevent weight gain and reduce food intake [121]. However, evidence of the ability of leptin treatment to reduce body weight is lacking causing some to hypothesize that leptin is actually a signal to prevent weight loss [122, 123]. Nevertheless, a number of studies have investigated leptin levels in TRF animals to gain a better idea of how food intake regulation is altered in TRF interventions. These studies have found that hyperleptinemia was prevented in HFD dark-phase TRF groups as compared to their ad libitum fed counterparts [13, 16, 113]. In HFD TRF fed mice, circulating leptin levels matched that of chow fed controls but were significantly lower than HFD ALF fed animals [18]. Other work in clock mutant mice found that leptin levels were lower in TRF fed WT and clock mutant mice (liver Bmal1 KO, liver Reverb alpha/beta, and Cry double knockout) versus WT ad lib animals [60]. As leptin levels correlate closely with the size of adipose tissue stores, alterations in leptin concentration may be secondary to the reduced fat mass often seen in mice on dark phase TRF rather than a result of TRF itself.
A couple of studies have tried to understand how TRF affects central control of energy balance. Multiple studies have found that melanocortin-4-receptor knockout (MC4R KO) mice still respond to dark phase TRF with a reduced body weight but have conflicting findings on food intake [79, 120]. In sum, there is no clear understanding of how TRF affects central regulation of food intake. It is also difficult to distinguish whether alterations in hormone levels are a cause or result of metabolic adaptations to TRF. Further, body weight differences between ad libitum fed and TRF fed animals make it difficult to compare the effects on food intake and appetite between groups. Tools to turn on or off feeding neurons, such as designer receptors exclusively activated by designer drugs (DREADDs), optogenetics, and inducible models will be helpful in determining the role of the brain in mediating the effects TRF. However, in the absence of methods to control food intake and body weight, it will be difficult to ascertain whether alterations in hormones are causative or secondary to changes in food intake and body weight.
6. Conclusion:
In rodents, active phase TRF ameliorates many of the negative metabolic consequences of HFD intake (Table 1). This does not seem to be the case in rest phase TRF as results vary across studies but point towards less favorable metabolic outcomes (Table 1). The conflicting results in the rest phase TRF studies may be explained by reduced caloric intake. Conversely, reduced intake does not appear to be driving the reduced body weight in animals on active phase TRF, thereby suggesting that increase in energy expenditure may be occurring when feeding is aligned with circadian sleep-wake rhythms. At present, there is not a consensus on how energy expenditure and activity are affected by TRF. Future studies with large samples sizes and using ANCOVA to normalize energy expenditure to body composition will be important to answer this question. Furthermore, investigations into nutrient absorption and fecal energy excretion will also be crucial to our understanding of how TRF affects whole-body energy balance.
In mammals, a compensatory increase in caloric intake and a reduction in energy expenditure are often present following periods of caloric restriction. This makes treating obesity and metabolic dysfunction with caloric restriction in humans likely to fail due to the physiologic propensity towards weight regain. On its own, TRF is a promising intervention for preventing and treating obesity and metabolic dysfunction. However, understanding the molecular mechanisms behind the energetics of active phase TRF may be a fruitful avenue for revealing how to induce weight loss without metabolic compensation or a rebound effect.
Because mice on active phase TRF have a reduced body weight in the absence of a reduction in caloric intake, this is suggestive of an alteration in total body energy balance allowing the mice to “burn” more fuel than their ad lib counterparts. Of exceeding importance is the fact that mice have ad libitum access to food, albeit during specified feeding windows, meaning that the degree to which the mice consume food is of autonomous volition rather than a researcher imposed caloric restriction. HFD hyperphagia has been suggested to be due to increased intake during the light phase rather than increases in consumption during the dark phase [12, 84]. Even when mice have access to chow or a low-fat diet during the light phase and HFD access is restricted to the dark phase, increased caloric intake does not occur [12, 72, 77]. It follows then that body weight set point on a TRF is lower than the body weight set point of mice on ad lib HFD as evidenced by 1) a lack of increased intake in response to a HFD and 2) a lower, stable body weight. What is the mechanism controlling this set point, and how does TRF create a lower body weight set-point than ad libitum feeding when all other factors are equal? Investigations into the intricacies of energy balance during TRF will position us to determine how diet and circadian timing of feeding affect whole-body energy expenditure and body weight set-point. As TRF seems to override HFD driven increases in body weight set-point, examination of the molecular underpinnings of its effects on energy intake and expenditure serve to move us towards findings therapies to prevent and treat obesity and metabolic dysfunction.
Study Importance.
In rodents restricting high fat diet intake to the active phase prevents or reverses weight gain and metabolic dysfunction
Reductions in body weight on active phase TRF do not appear to be driven by reduced caloric intake but may instead be resulting from increased energy expenditure
This review supports and details a new line of investigation and investigational strategies of energy balance on TRF which may lead to new treatments for obesity and metabolic dysfunction in humans
Funding:
MRG is supported by T32DK110966.
SYT is supported by NCATS TL1TR002540.
AC is supported by AHA grant 18CDA34110292 and NIA grant 5R01AG065993.
Footnotes
Disclosure: nothing to declare
Works Cited
- 1.Leibel RL, Molecular physiology of weight regulation in mice and humans. Int J Obes (Lond), 2008. 32 Suppl 7: p. S98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenway FL, Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond), 2015. 39(8): p. 1188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravussin Y, Leibel RL, and Ferrante AW Jr., A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell Metab, 2014. 20(4): p. 565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz MW, et al. , Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocr Rev, 2017. 38(4): p. 267–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saper CB and Lowell BB, The hypothalamus. Current Biology, 2014. 24(23): p. R1111–R1116. [DOI] [PubMed] [Google Scholar]
- 6.Challet E, The circadian regulation of food intake. Nat Rev Endocrinol, 2019. 15(7): p. 393–405. [DOI] [PubMed] [Google Scholar]
- 7.Chellappa SL, et al. , Daytime eating prevents internal circadian misalignment and glucose intolerance in night work. Science advances, 2021. 7(49): p. eabg9910–eabg9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Minguez J, et al. , Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: A randomized, cross-over study. Clin Nutr, 2018. 37(4): p. 1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant CL, et al. , Timing of food intake during simulated night shift impacts glucose metabolism: A controlled study. Chronobiol Int, 2017. 34(8): p. 1003–1013. [DOI] [PubMed] [Google Scholar]
- 10.Gill S, et al. , Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science, 2015. 347(6227): p. 1265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta NJ, Kumar V, and Panda S, A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLOS ONE, 2017. 12(3): p. e0172852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Licholai JA, et al. , Why Do Mice Overeat High-Fat Diets? How High-Fat Diet Alters the Regulation of Daily Caloric Intake in Mice. Obesity (Silver Spring), 2018. 26(6): p. 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatori M, et al. , Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab, 2012. 15(6): p. 848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaix A, et al. , Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab, 2019. 29(2): p. 303–319 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arble DM, et al. , Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring), 2009. 17(11): p. 2100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaix A, et al. , Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab, 2014. 20(6): p. 991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan MJ, et al. , Restricting feeding to the active phase in middle-aged mice attenuates adverse metabolic effects of a high-fat diet. Physiol Behav, 2016. 167: p. 1–9. [DOI] [PubMed] [Google Scholar]
- 18.Sundaram S and Yan L, Time-restricted feeding reduces adiposity in mice fed a high-fat diet. Nutr Res, 2016. 36(6): p. 603–11. [DOI] [PubMed] [Google Scholar]
- 19.Aouichat S, et al. , Time-Restricted Feeding Improves Body Weight Gain, Lipid Profiles, and Atherogenic Indices in Cafeteria-Diet-Fed Rats: Role of Browning of Inguinal White Adipose Tissue. Nutrients, 2020. 12(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kentish SJ, et al. , Time-Restricted Feeding Prevents Ablation of Diurnal Rhythms in Gastric Vagal Afferent Mechanosensitivity Observed in High-Fat Diet-Induced Obese Mice. J Neurosci, 2018. 38(22): p. 5088–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai JY, et al. , Influence of dark phase restricted high fat feeding on myocardial adaptation in mice. J Mol Cell Cardiol, 2013. 55: p. 147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zarrinpar A, et al. , Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab, 2014. 20(6): p. 1006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manoogian ENC, et al. , Time-restricted Eating for the Prevention and Management of Metabolic Diseases. Endocrine Reviews, 2021. 43(2): p. 405–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen MC, et al. , Complex Physiology and Clinical Implications of Time-restricted Eating. Physiological Reviews, 2022. 0(0): p. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaix A, Zarrinpar A, and Panda S, The circadian coordination of cell biology. Journal of Cell Biology, 2016. 215(1): p. 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaix A, et al. , Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annual Review of Nutrition, 2019. 39(1): p. 291–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dibner C, Schibler U, and Albrecht U, The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol, 2010. 72: p. 517–49. [DOI] [PubMed] [Google Scholar]
- 28.Panda S, Circadian physiology of metabolism. Science, 2016. 354(6315): p. 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiegelman BM and Flier JS, Obesity and the Regulation of Energy Balance. Cell, 2001. 104(4): p. 531–543. [DOI] [PubMed] [Google Scholar]
- 30.Longo DL, Heymsfield SB, and Wadden TA, Mechanisms, Pathophysiology, and Management of Obesity. The New England Journal of Medicine, 2017. 376(3): p. 254–266. [DOI] [PubMed] [Google Scholar]
- 31.Provencher V, et al. , Health-At-Every-Size and Eating Behaviors: 1-Year Follow-Up Results of a Size Acceptance Intervention. Journal of the American Dietetic Association, 2009. 109(11): p. 1854–1861. [DOI] [PubMed] [Google Scholar]
- 32.Vartanian LR and Smyth JM, Primum Non Nocere: Obesity Stigma and Public Health. Journal of Bioethical Inquiry, 2013. 10(1): p. 49–57. [DOI] [PubMed] [Google Scholar]
- 33.Han W, et al. , Striatal Dopamine Links Gastrointestinal Rerouting to Altered Sweet Appetite. Cell Metab, 2016. 23(1): p. 103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holst JJ and Madsbad S, Semaglutide seems to be more effective the other GLP-1Ras. Annals of translational medicine, 2017. 5(24): p. 505–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee PC, Ganguly S, and Goh S-Y, Weight loss associated with sodium-glucose cotransporter-2 inhibition: a review of evidence and underlying mechanisms. Obesity Reviews, 2018. 19(12): p. 1630–1641. [DOI] [PubMed] [Google Scholar]
- 36.Wilding JPH, et al. , Once-Weekly Semaglutide in Adults with Overweight or Obesity. New England Journal of Medicine, 2021. 384(11): p. 989–1002. [DOI] [PubMed] [Google Scholar]
- 37.Lean MEJ, et al. , Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. International Journal of Obesity, 2014. 38(5): p. 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies MJ, et al. , Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. Jama, 2015. 314(7): p. 687–99. [DOI] [PubMed] [Google Scholar]
- 39.Rubino D, et al. , Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. Jama, 2021. 325(14): p. 1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biertho L, et al. , Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding. Journal of the American College of Surgeons, 2003. 197(4): p. 536–545. [DOI] [PubMed] [Google Scholar]
- 41.le Roux CW and Bueter M, The physiology of altered eating behaviour after Roux-en-Y gastric bypass. Exp Physiol, 2014. 99(9): p. 1128–32. [DOI] [PubMed] [Google Scholar]
- 42.Hankir MK, et al. , Brain Feeding Circuits after Roux-en-Y Gastric Bypass. Trends Endocrinol Metab, 2018. 29(4): p. 218–237. [DOI] [PubMed] [Google Scholar]
- 43.Lager CJ, et al. , Roux-En-Y Gastric Bypass Vs. Sleeve Gastrectomy: Balancing the Risks of Surgery with the Benefits of Weight Loss. Obes Surg, 2017. 27(1): p. 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olbers T, et al. , Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg, 2006. 244(5): p. 715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffith PS, et al. , Managing complications associated with laparoscopic Roux-en-Y gastric bypass for morbid obesity. Can J Surg, 2012. 55(5): p. 329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim CH, et al. , The future of the Roux-en-Y gastric bypass. Expert Rev Gastroenterol Hepatol, 2016. 10(7): p. 777–84. [DOI] [PubMed] [Google Scholar]
- 47.Meek CL, et al. , The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides, 2016. 77: p. 28–37. [DOI] [PubMed] [Google Scholar]
- 48.Rausa E, et al. , Rate of Death and Complications in Laparoscopic and Open Roux-en-Y Gastric Bypass. A Meta-analysis and Meta-regression Analysis on 69,494 Patients. Obes Surg, 2016. 26(8): p. 1956–63. [DOI] [PubMed] [Google Scholar]
- 49.Berthoud HR and Morrison C, The brain, appetite, and obesity. Annu Rev Psychol, 2008. 59: p. 55–92. [DOI] [PubMed] [Google Scholar]
- 50.Rosenbaum M and Leibel RL, Models of energy homeostasis in response to maintenance of reduced body weight. Obesity (Silver Spring), 2016. 24(8): p. 1620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris RBS, Role of set-point theory in regulation of body weight. The FASEB Journal, 1990. 4(15): p. 3310–3318. [DOI] [PubMed] [Google Scholar]
- 52.Keesey RE and Corbett SW, Metabolic defense of the body weight set-point. Research publications - Association for Research in Nervous and Mental Disease, 1984. 62: p. 87–96. [PubMed] [Google Scholar]
- 53.Muller MJ, Bosy-Westphal A, and Heymsfield SB, Is there evidence for a set point that regulates human body weight? F1000 Med Rep, 2010. 2: p. 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keys A, Brozek J, Henschel A, Mickelsen O & Taylor HL, Experimental Starvation in Man. 1945. [Google Scholar]
- 55.Dulloo AG, Jacquet J, and Girardier L, Poststarvation hyperphagia and body fat overshooting in humans: a role for feedback signals from lean and fat tissues. Am J Clin Nutr, 1997. 65(3): p. 717–23. [DOI] [PubMed] [Google Scholar]
- 56.Fothergill E, et al. , Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity, 2016. 24(8): p. 1612–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leibel RL and Hirsch J, Diminished energy requirements in reduced-obese patients. Metabolism, 1984. 33(2): p. 164–170. [DOI] [PubMed] [Google Scholar]
- 58.Leibel RL, Rosenbaum M, and Hirsch J, Changes in Energy Expenditure Resulting from Altered Body Weight. New England Journal of Medicine, 1995. 332(10): p. 621–628. [DOI] [PubMed] [Google Scholar]
- 59.Rosenbaum M, et al. , Energy intake in weight-reduced humans. 2010. 1350: p. 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaix A, et al. , Sex- and age-dependent outcomes of 9-hour time-restricted feeding of a Western high-fat high-sucrose diet in C57BL/6J mice. Cell Reports, 2021. 36(7): p. 109543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Albrecht U, The circadian clock, metabolism and obesity. Obesity Reviews, 2017. 18(S1): p. 25–33. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi JS, Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet, 2017. 18(3): p. 164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Damiola F, et al. , Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev, 2000. 14(23): p. 2950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hara R, et al. , Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells, 2001. 6(3): p. 269–78. [DOI] [PubMed] [Google Scholar]
- 65.Stokkan KA, et al. , Entrainment of the circadian clock in the liver by feeding. Science, 2001. 291(5503): p. 490–3. [DOI] [PubMed] [Google Scholar]
- 66.Panda S, et al. , Coordinated transcription of key pathways in the mouse by the circadian clock. Cell, 2002. 109(3): p. 307–20. [DOI] [PubMed] [Google Scholar]
- 67.Rudic RD, et al. , BMAL1 and CLOCK, Two Essential Components of the Circadian Clock, Are Involved in Glucose Homeostasis. PLOS Biology, 2004. 2(11): p. e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ikeda H, et al. , Clock gene defect disrupts light-dependency of autonomic nerve activity. Biochemical and Biophysical Research Communications, 2007. 364(3): p. 457–463. [DOI] [PubMed] [Google Scholar]
- 69.Lamia KA, et al. , Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature, 2011. 480(7378): p. 552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vollmers C, et al. , Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A, 2009. 106(50): p. 21453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fonken LK, et al. , Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A, 2010. 107(43): p. 18664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bray MS, et al. , Time-of-day-dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice. Int J Obes (Lond), 2010. 34(11): p. 1589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salgado-Delgado R, et al. , Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology, 2010. 151(3): p. 1019–29. [DOI] [PubMed] [Google Scholar]
- 74.Chaix A, et al. , Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annual review of nutrition, 2019. 39: p. 291–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olsen MK, et al. , Time-restricted feeding on weekdays restricts weight gain: A study using rat models of high-fat diet-induced obesity. Physiol Behav, 2017. 173: p. 298–304. [DOI] [PubMed] [Google Scholar]
- 76.Mitchell SJ, et al. , Daily Fasting Improves Health and Survival in Male Mice Independent of Diet Composition and Calories. Cell Metabolism, 2019. 29(1): p. 221–228.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelly KP, et al. , Time-optimized feeding is beneficial without enforced fasting. Open Biol, 2021. 11(10): p. 210183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Das M, et al. , Time-restricted feeding normalizes hyperinsulinemia to inhibit breast cancer in obese postmenopausal mouse models. Nat Commun, 2021. 12(1): p. 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang W, et al. , Time-Restricted Feeding Restored Insulin-Growth Hormone Balance and Improved Substrate and Energy Metabolism in MC4RKO Obese Mice. Neuroendocrinology, 2021. [DOI] [PubMed] [Google Scholar]
- 80.Boucsein A, Rizwan MZ, and Tups A, Hypothalamic leptin sensitivity and health benefits of time-restricted feeding are dependent on the time of day in male mice. FASEB J, 2019. 33(11): p. 12175–12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maric I, et al. , Sex and Species Differences in the Development of Diet-Induced Obesity and Metabolic Disturbances in Rodents. Front Nutr, 2022. 9: p. 828522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woodie LN, et al. , Restricted feeding for 9h in the active period partially abrogates the detrimental metabolic effects of a Western diet with liquid sugar consumption in mice. Metabolism, 2018. 82: p. 1–13. [DOI] [PubMed] [Google Scholar]
- 83.Cote I, et al. , Limiting feeding to the active phase reduces blood pressure without the necessity of caloric reduction or fat mass loss. Am J Physiol Regul Integr Comp Physiol, 2018. 315(4): p. R751–R758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davis JA, et al. , Time-restricted feeding rescues high-fat-diet-induced hippocampal impairment. iScience, 2021. 24(6): p. 102532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu S, et al. , Dietary Fat, but Not Protein or Carbohydrate, Regulates Energy Intake and Causes Adiposity in Mice. Cell Metab, 2018. 28(3): p. 415–431.e4. [DOI] [PubMed] [Google Scholar]
- 86.Gallop MR, Wilson VC, and Ferrante AW Jr., Post-oral sensing of fat increases food intake and attenuates body weight defense. Cell Rep, 2021. 37(3): p. 109845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ali MA and Kravitz AV, Challenges in quantifying food intake in rodents. Brain research, 2018. 1693(Pt B): p. 188–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bachmanov AA, Tordoff MG, and Beauchamp GK, Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chemical senses, 2001. 26(7): p. 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matikainen-Ankney BA, et al. , An open-source device for measuring food intake and operant behavior in rodent home-cages. eLife, 2021. 10: p. e66173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cameron KM and Speakman JR, The extent and function of ‘food grinding’ in the laboratory mouse (Mus musculus). Lab Anim, 2010. 44(4): p. 298–304. [DOI] [PubMed] [Google Scholar]
- 91.Ellacott KLJ, et al. , Assessment of feeding behavior in laboratory mice. Cell metabolism, 2010. 12(1): p. 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ren J, et al. , Alteration in gut microbiota caused by time-restricted feeding alleviate hepatic ischaemia reperfusion injury in mice. J Cell Mol Med, 2019. 23(3): p. 1714–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dantas Machado AC, et al. , Diet and feeding pattern modulate diurnal dynamics of the ileal microbiome and transcriptome. Cell Reports, 2022. 40(1): p. 111008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Regmi P, et al. , Early or delayed time-restricted feeding prevents metabolic impact of obesity in mice. Journal of Endocrinology, 2021. 248(1): p. 75–86. [DOI] [PubMed] [Google Scholar]
- 95.Speakman J, Measuring Energy Metabolism in the Mouse – Theoretical, Practical, and Analytical Considerations. Frontiers in Physiology, 2013. 4(34). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tschöp MH, et al. , A guide to analysis of mouse energy metabolism. Nat Methods, 2011. 9(1): p. 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fernández-Verdejo R, Aguirre C, and Galgani JE, Issues in Measuring and Interpreting Energy Balance and Its Contribution to Obesity. Curr Obes Rep, 2019. 8(2): p. 88–97. [DOI] [PubMed] [Google Scholar]
- 98.Weir JBDB, New methods for calculating metabolic rate with special reference to protein metabolism. The Journal of physiology, 1949. 109(1–2): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Walsberg GE and Hoffman TCM, Direct calorimetry reveals large errors in respirometric estimates of energy expenditure. Journal of Experimental Biology, 2005. 208(6): p. 1035–1043. [DOI] [PubMed] [Google Scholar]
- 100.Kaiyala KJ and Schwartz MW, Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes, 2011. 60(1): p. 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abe T, et al. , Food deprivation during active phase induces skeletal muscle atrophy via IGF-1 reduction in mice. Arch Biochem Biophys, 2019. 677: p. 108160. [DOI] [PubMed] [Google Scholar]
- 102.Chung H, et al. , Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism, 2016. 65(12): p. 1743–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Storlien L, Oakes ND, and Kelley DE, Metabolic flexibility. Proc Nutr Soc, 2004. 63(2): p. 363–8. [DOI] [PubMed] [Google Scholar]
- 104.Even PC and Nadkarni NA, Indirect calorimetry in laboratory mice and rats: principles, practical considerations, interpretation and perspectives. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2012. 303(5): p. R459–R476. [DOI] [PubMed] [Google Scholar]
- 105.Mattson MP, et al. , Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci, 2018. 19(2): p. 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rajpal A and Ismail-Beigi F, Intermittent fasting and ‘metabolic switch’: Effects on metabolic syndrome, prediabetes and type 2 diabetes. Diabetes, Obesity and Metabolism, 2020. 22(9): p. 1496–1510. [DOI] [PubMed] [Google Scholar]
- 107.Acosta-Rodriguez VA, et al. , Mice under Caloric Restriction Self-Impose a Temporal Restriction of Food Intake as Revealed by an Automated Feeder System. Cell Metab, 2017. 26(1): p. 267–277 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Acosta-Rodríguez V, et al. , Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science, 2022. 376(6598): p. 1192–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Goede P, et al. , After-Effects of Time-Restricted Feeding on Whole-Body Metabolism and Gene Expression in Four Different Peripheral Tissues. Obesity, 2020. 28(S1): p. S68–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hou T, et al. , Active Time-Restricted Feeding Improved Sleep-Wake Cycle in db/db Mice. Front Neurosci, 2019. 13: p. 969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oishi K and Hashimoto C, Short-term time-restricted feeding during the resting phase is sufficient to induce leptin resistance that contributes to development of obesity and metabolic disorders in mice. Chronobiol Int, 2018. 35(11): p. 1576–1594. [DOI] [PubMed] [Google Scholar]
- 112.Sherman H, et al. , Long-term restricted feeding alters circadian expression and reduces the level of inflammatory and disease markers. J Cell Mol Med, 2011. 15(12): p. 2745–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sherman H, et al. , Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J, 2012. 26(8): p. 3493–502. [DOI] [PubMed] [Google Scholar]
- 114.Yasumoto Y, et al. , Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism, 2016. 65(5): p. 714–27. [DOI] [PubMed] [Google Scholar]
- 115.Satoh Y, et al. , Time-restricted feeding entrains daily rhythms of energy metabolism in mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2006. 290(5): p. R1276–R1283. [DOI] [PubMed] [Google Scholar]
- 116.Szentirmai É, et al. , Restricted feeding-induced sleep, activity, and body temperature changes in normal and preproghrelin-deficient mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2010. 298(2): p. R467–R477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Northeast RC, et al. , Sleep homeostasis during daytime food entrainment in mice. Sleep, 2019. 42(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ravussin E, et al. , Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation But Does Not Affect Energy Expenditure in Humans. Obesity, 2019. 27(8): p. 1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nakazato M, et al. , A role for ghrelin in the central regulation of feeding. Nature, 2001. 409(6817): p. 194–198. [DOI] [PubMed] [Google Scholar]
- 120.Sorrell J, et al. , The central melanocortin system mediates the benefits of time-restricted feeding on energy balance. Physiology & Behavior, 2020. 227: p. 113132. [DOI] [PubMed] [Google Scholar]
- 121.Ahima RS and Flier JS, Leptin. Annual Review of Physiology, 2000. 62(1): p. 413–437. [DOI] [PubMed] [Google Scholar]
- 122.Flier JS and Maratos-Flier E, Leptin’s Physiologic Role: Does the Emperor of Energy Balance Have No Clothes? Cell Metab, 2017. 26(1): p. 24–26. [DOI] [PubMed] [Google Scholar]
- 123.Rosenbaum M and Leibel RL, 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol, 2014. 223(1): p. T83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]


