Abstract
Pulmonary arterial hypertension (PAH) is a progressive and fatal disease. Sustained pulmonary vasoconstriction and concentric pulmonary vascular remodeling contribute to the elevated pulmonary vascular resistance and pulmonary artery pressure in PAH. Endothelial cells regulate vascular tension by producing endothelium-derived relaxing factors (EDRFs) and endothelium-derived contracting factors (EDCFs). Homeostasis of EDRF and EDCF production has been identified as a marker of the endothelium integrity. Impaired synthesis or release of EDRFs induces persistent vascular contraction and pulmonary artery remodeling, which subsequently leads to the development and progression of PAH. In this review, the authors summarize how EDRFs and EDCFs affect pulmonary vascular homeostasis, with special attention to the recently published novel mechanisms related to endothelial dysfunction in PAH and drugs associated with EDRFs and EDCFs.
Key Words: endothelial dysfunction, endothelium-derived relaxing factor, pulmonary arterial hypertension, vascular homeostasis
Abbreviations and Acronyms: 5-HT, 5-hydroxytryptamine; ACE, angiotensin-converting enzyme; cGMP, cyclic guanosine monophosphate; EC, endothelial cell; EDCF, endothelium-derived contracting factor; EDRF, endothelium-derived relaxing factor; ET, endothelin; PAH, pulmonary arterial hypertension; PASMC, pulmonary artery smooth muscle cell; PG, prostaglandin; TPH, tryptophan hydroxylase; TXA2, thromboxane A2
Central Illustration
Highlights
-
•
Disrupted EDRF/EDCF homeostasis contributes to the development and progression of PAH/PH.
-
•
Understanding EDRF/ECRF interaction helps in developing therapeutic interventions for PAH/PH.
-
•
Multiple trials are undergoing for drugs based on EDRF/ECRF mechanisms for PAH/PH therapy.
Endothelial dysfunction, characterized by structural changes and functional impairment of the pulmonary artery, plays an important role in the development and progression of pulmonary arterial hypertension (PAH). Normal synthesis and release of endothelium-derived relaxing factors (EDRFs) is considered a sign of endothelial integrity. EDRFs mainly include nitric oxide (NO), prostacyclin, and endothelium-derived hyperpolarizing factors.1 Under the pathologic conditions of PAH, decreased EDRFs, increased endothelium-derived contracting factors (EDCFs) and increased sensitivity of EDCF receptors in endothelial cells (ECs) are the direct cause of endothelial dysfunction.2 Increased EDCFs, including endothelin (ET)-1, EC-derived adenosine diphosphate, angiotensin II, 5-hydroxytryptamine (5-HT), 8-iso-prostaglandin F2α (PGF2α), and thromboxane A2 (TXA2), which can also antagonize the protective and vasodilative effects of EDRFs, ultimately lead to endothelial dysfunction. The chemical properties and classification of these factors are described in detail (Table 1).
Table 1.
Physicochemical Properties and Classification of Endothelium-Derived Factors
| Factor | Property | Classification |
|---|---|---|
| NO | Inorganic gases | EDRF |
| PGI2 | Arachidonic acid metabolites | EDRF |
| H2S | Inorganic gases | EDRF, EDHF |
| K+ | Ion | EDRF, EDHF |
| EETs | Arachidonic acid metabolites | EDRF, EDHF |
| Ang-(1-7) | Bioactive peptide | EDRF |
| Adenosine | Nucleoside | EDRF |
| EC-derived ATP | Nucleotide | EDRF |
| ROS | Oxygen radical | EDCF |
| EC-derived ADP | Nucleotide | EDCF |
| EC-derived Up4A | Nucleotide | EDCF |
| ET-1 | Bioactive peptide | EDCF |
| 5-HT | Small organic molecule | EDCF |
| TXA2 | Arachidonic acid metabolites | EDCF |
| 8-iso-PGF2α | Arachidonic acid metabolites | EDCF |
| Ang II | Bioactive peptide | EDCF |
5-HT = 5-hydroxytryptamine; Ang = angiotensin; EC = endothelial cell; EDCF = endothelium-derived contracting factor; EDHF = endothelium-derived hyperpolarizing factor; EDRF = endothelium-derived relaxing factor; EET = epoxyeicosatrienoic acid; ET-1 = endothelin 1; PG = prostaglandin; ROS = reactive oxygen species; TXA2 = thromboxane A2; Up4A = uridine adenosine tetraphosphate.
It is widely accepted that endothelial dysfunction and apoptosis is critical in the initiation of PAH. EC apoptosis during disease initiation activates a highly proliferating population of pathogenic ECs, which drive PAH progression.3 Broadly, endothelial dysfunction is considered to be a complex set of biological processes involving EDRF/EDCF imbalance, inflammatory cell adhesion, platelet aggregation, increased oxidative stress and glycolysis, endothelial-to-mesenchymal transition, and others.4
Pulmonary artery smooth muscle cells (PASMCs) and pulmonary artery ECs, as direct participants in pulmonary vascular remodeling, have been the main focus of study in the field of PAH.5 For PASMCs, proliferation, migration, and media hyperplasia are thought to contribute significantly to pulmonary artery remodeling.6 For pulmonary artery ECs, endothelial dysfunction is one of the typical vascular alterations in the development of PAH.7 In this review, we discuss the role of EDRFs and EDCFs in endothelial function during PAH progression. On the basis of recent findings, we summarize new mechanisms of endothelial dysfunction and novel related targeted drugs in PAH clinical therapy.
Role of Edcfs and Edrfs in PAH Pathology
Nitric oxide
NO, as a vasodilator, is produced by 2 pathways: the classical L-arginine-to-NO pathway and the nonclassical nitrate-nitrite-to-NO pathway.8 In the classic pathway, the pulmonary vasculature primarily uses NO produced by converting L-arginine through NO synthase. The endothelial NO synthase-mediated biosynthesis of NO in ECs is considered to be the main source of bioavailable NO in the pulmonary circulation.9 Endogenous NO inhibits apoptosis and promotes cell proliferation by promoting the expression of vascular endothelial growth factor, which is essential for angiogenesis in pulmonary vascular development.10 Moreover, NO also can be released into adjacent PASMCs to convert guanosine triphosphate to cyclic guanosine monophosphate (cGMP) by interacting with soluble guanylate cyclase. cGMP achieves its function by activating the downstream cGMP-dependent protein kinase G, cGMP-gated cation channels, and phosphodiesterases. Protein kinase G regulates intracellular calcium [Ca2+]i concentrations by affecting several cytosolic Ca2+ flux regulators to relax vascular tension.
NO synthesis via endothelial NO synthase depends on the availability of substrates and cofactors. Sufficient tetrahydrobiopurine and L-arginine are essential for the maintenance of NO synthesis by endothelial NO synthase. Considerable evidence indicates that L-arginine and tetrahydrobiopurine bioavailability is significantly reduced in pulmonary vascular diseases with endothelial dysfunction.11,12 The L-arginine antagonists asymmetrical dimethylarginine and symmetrical dimethylarginine were remarkably increased in the plasma and tissues of both rats with pulmonary hypertension and patients with idiopathic PAH.13 Asymmetrical dimethylarginine inhibits endothelial NO synthase activity through direct binding, which leads to endothelial NO synthase uncoupling and superoxide accumulation.14 Low tetrahydrobiopurine levels or excess oxidized biopterin (dihydrobiopterin) cause endothelial NO synthase uncoupling and the reduction of oxygen to a superoxide anion. This then scavenges NO and generates other reactive oxygen species, resulting in constrictive and proliferative vascular pathology.15,16
The progression of PAH is significantly associated with a reduction in endothelial NO synthase expression, which is what may contribute to pulmonary vasoconstriction and media hypertrophy.17 In PAH, low endothelial NO synthase levels in pulmonary vascular ECs impair NO production, which may lead to increased vascular tone and other cellular activity in the vascular wall.18 Interestingly, recent studies have shown that the protein expression of endothelial NO synthase did not change in experimental pulmonary hypertension. However, endothelial NO synthase uncoupling leads to its functional loss and an increase in reactive oxygen species production.19 Furthermore, NO is further inactivated by interactions with reactive oxygen species, which results in a reduction in available NO for vasodilation and antiproliferation.
Prostaglandins and their receptors
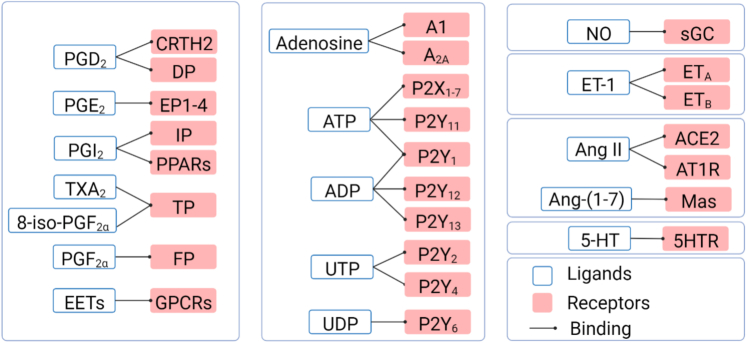
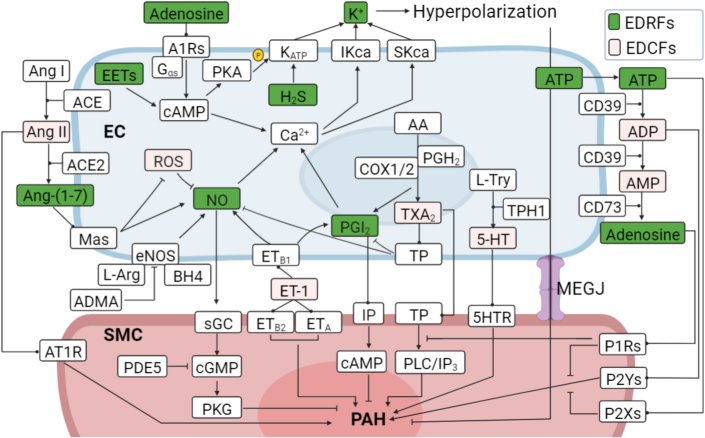
Arachidonic acid is catalyzed by cyclooxygenases and PGI2 synthetases to produce a series of prostaglandins (PGs). The binding relationship between these ligands and receptors20 and their functions are shown in Figure 1. PGD2 effectively increased pulmonary blood flow and reduced pulmonary vascular resistance and pulmonary artery pressure in newborn lambs with pulmonary hypertension.21 However, intravenous PGD2 injection failed to improve hemodynamic parameters and oxygenation in newborn human infants with persistent pulmonary hypertension of the newborn.22 Two subsequent critical clinical studies suggested the importance of the PGD2 signaling pathway in pulmonary hypertension. It was found that the concentration of eicosanoid (including PGD2) increased in the bronchoalveolar lavage fluid of patients with persistent pulmonary hypertension of the newborn.23 In contrast, concentrations of the TXA2 and PGD2 metabolites TX-M and PGD-M simultaneously increased in the urine of patients with primary pulmonary hypertension.24 The PGD2 receptor CRTH2 is up-regulated in circulating T helper type 2 cells in patients with idiopathic PAH and in rodent pulmonary hypertension models, while T helper type 2 cell–specific CRTH2 knockout alleviated pulmonary hypertension in rodents.25 Similarly, macrophage-derived PGD2 dilated blood vessels via PGD2 receptor 1 on PASMCs.26 PGD2 recruits T helper type 2 cells to form a local inflammatory microenvironment through the CRTH2 receptor. In contrast, it can dilate blood vessels through PGD2 receptor 1.
Figure 1.
Endothelium-Derived Factors and Their Receptors
The regulatory effect of endothelium-derived relaxing factors (EDRFs) and endothelium-derived contracting factors (EDCFs) on vascular tension depends on their specific receptor pathways. 5-HT = 5-hydroxytryptamine; 5HTR = 5-hydroxytryptamine receptor; 8-iso-PGF2α = 8-iso-prostaglandin F2α; A1, A2A = type 1 purinergic receptors; ACE2 = angiotensin-converting enzyme 2; ADP = adenosine diphosphate; Ang = angiotensin; AT1R = angiotensin type 1 receptor; ATP = adenosine triphosphate; CRTH2 = prostaglandin D2 receptor 2; DP = prostaglandin D2 receptor; EET = epoxyeicosatrienoic acid; EP = prostaglandin E receptor; ET = endothelin; ETA = endothelin receptor A; ETB = endothelin receptor B; FP = prostaglandin F2α receptor; GPCR = G protein–coupled receptor; IP = prostaglandin I2 receptor; Mas = Mas receptor; P2X = type 2X purinergic receptor; P2Y = type 2Y purinergic receptor; PG = prostaglandin; sGC = soluble guanylate cyclase; TP = thromboxane A2 receptor; TXA2 = thromboxane A2; UDP = uridine diphosphate; UTP = uridine triphosphate.
PGE2 is a widely expressed lipid signaling molecule involved in pain, vascular tension regulation, tissue damage repair, and inflammatory response.27 PGE2 receptors 1, 2, 3, and 4 generally exist on smooth muscle cells. PGE2 receptor 1 activation stimulates intracellular calcium and promotes vasoconstriction. The activation of PGE2 receptors 2 and 4 stimulates the cyclic adenosine monophosphate–protein kinase A signaling pathway to promote vasodilation, while PGE2 receptor 3 inhibits vasodilation in the opposite way.28 PGE2 signaling usually causes airway smooth muscle relaxation. Studies have shown that activation of airway PGE2 receptor 4 had additional benefits for group III pulmonary hypertension treatment,29 while endothelial-specific knockout of PGE2 receptor 4 impaired NO synthesis.30
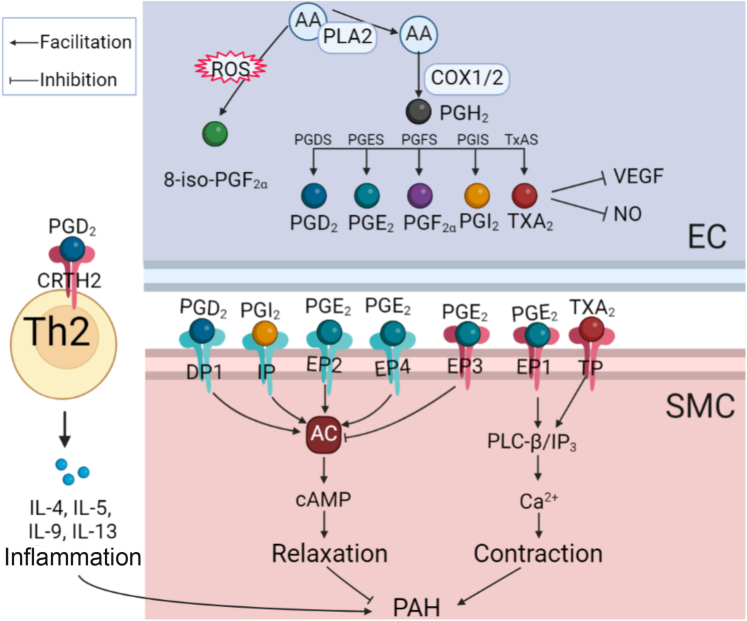
PGF2α appears to contribute to the development of pulmonary hypertension. Earlier case reports suggested that PGF2α metabolism was impaired in the context of extensive pulmonary vascular injury.31 PGF2α and its receptors usually play a role in the reproductive system and renal function, and there is evidence that PGF2α promotes cardiomyocyte hypertrophy in vivo.32 In vitro experiments in the pulmonary arteries of rats demonstrated that PGF2α enhanced pulmonary vasoconstriction under euhydric hypercapnic conditions.33 Hypoxia caused by pulmonary diseases can alter angiogenesis, metabolism, and apoptosis by activating the expression of hypoxia-inducible factors to impair EC function.34 The signaling pathways of PGs in regulating vascular tone are shown in Figure 2.
Figure 2.
Prostaglandin Signaling Regulates Vascular Tone in PAH Progression
PGI2, PGD2, and PGE2 relax smooth muscle cells (SMCs) through IP, PGD2 receptor 1, and EP2 and EP4 receptors, respectively. PGE2 constricts blood vessels through EP1 and EP3. PGD2 promotes inflammation and PAH through CRTH2 of T helper type 2 (Th2) cells. AA = arachidonic acid; AC = adenylate cyclase; cAMP = adenosine cyclic phosphate; COX1/2 = cyclooxygenase 1/2; EC = endothelial cell; IL = interleukin; IP3 = inositol triphosphate; PGDS = prostaglandin D synthase; PGES = prostaglandin E synthase; PGFS = prostaglandin F synthase; PGIS = prostaglandin I synthase; PL = phospholipase; ROS = reactive oxygen species; TxAS = thromboxane synthase; VEGF = vascular endothelial growth factor; other abbreviations as in Figure 1.
In addition to the effects on pulmonary artery ECs and PASMCs, PGD2, PGE2, and PGI2 all increased intracellular cyclic adenosine monophosphate levels to promote apoptosis of fibroblasts and inhibit cell proliferation and transformation. Antifibrotic effects of PGI2 have been reported in dog cardiac hypertrophy models since the 1980s.35 In gamma delta T cells, PGD2 can activate CRTH2 receptors and promote the release of interleukin-10 to inhibit fibrosis.36 PGE2 also plays an antifibrotic role, primarily through binding to PGE2 receptors 2 and 4.37 PGF2α is a potent fibrosis factor, which is abundant in the lung bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis.38 The fibrotic pathway activated by PGF2α is thought to be independent of the transforming growth factor–β pathway in promoting fibrosis. Similar to PGF2α, TXA2 also promotes fibrosis, and various TXA2 receptor antagonists have been used in antifibrosis studies. The TXA2 receptor antagonist NTP42 effectively inhibits inflammatory mast cell infiltration and pulmonary fibrosis and can alleviate experimental pulmonary hypertension.39 In patients with pulmonary fibrosis complicated with PAH, PAH promotes the progression of pulmonary fibrosis by exposing capillary ECs to higher mechanical stress.40
Endothelium-derived hyperpolarizing factor
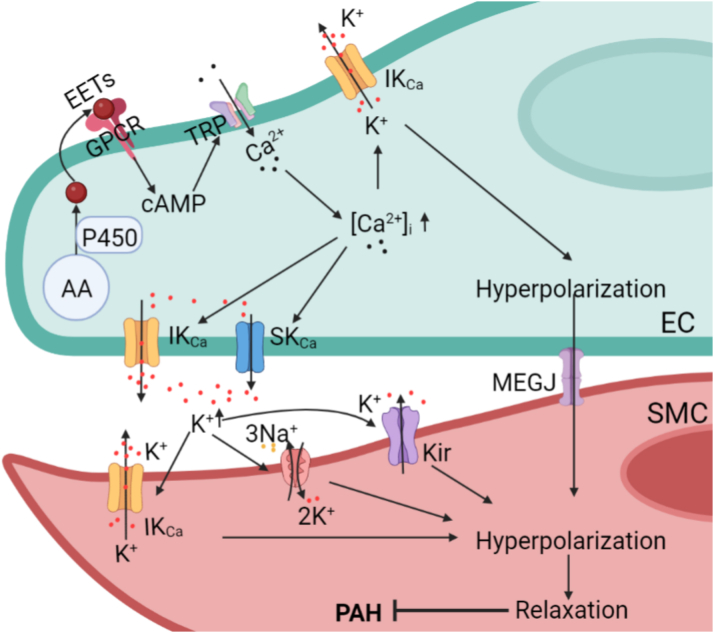
Endothelium-derived hyperpolarizing factor is the third vasodilator, distinct from NO and PGI2, produced by ECs. Only arteries with intact ECs exhibit transient hyperpolarization and sustained relaxation in response to acetylcholine stimulation. Endothelium-derived hyperpolarization–mediated vasodilation involves a complex set of electrochemical signaling processes. This mechanism begins with the activation of small (SKCa) or intermediate (IKCa) conductance through Ca2+-activated K+ channels in ECs by an increased calcium concentration. Then, electric signals are transmitted from hyperpolarized ECs to smooth muscle cells via myoendothelial gap junctions.1 Ultimately, this results in smooth muscle cell hyperpolarization and vasodilation. Endothelium-derived hyperpolarizing factors can be divided into epoxyeicosatrienoic acids,41 potassium ions,42 electric coupling through myoendothelial gap junctions, and others. A recent study suggest that hydrogen sulfide may play an important role in relieving PAH by activating adenosine triphosphate–sensitive potassium channels for vasodilation.43
Epoxyeicosatrienoic acids are generated by arachidonic acid catalyzed by cytochrome P450, and Cyp2c9 is a major subtype of cytochrome P450 involved mainly in epoxyeicosatrienoic acid synthesis in ECs.44 A recent study showed that 14,15-epoxyeicosatrienoic acid dilates blood vessels by binding to G protein–coupled receptor–39 of vascular smooth muscle cells.45 In the proximal pulmonary vessels, ECs rely on releasing PGI2 and NO to dilate the vessels. In the distal arterioles, however, ECs rely primarily on endothelium-derived hyperpolarization to dilate the vessels because more myoendothelial gap junctions exist in arterioles than that in proximal arteries.46 Myoendothelial gap junctions are composed of connexins, which are responsible for the transport of small molecules between cells and the propagation of electric signals. Connexin 40 is decreased in the lung tissues of experimental pulmonary hypertension models and patients with pulmonary hypertension, and the hypoxia-induced decrease of connexin 40 impairs pulmonary artery relaxation by blocking endothelium-derived hyperpolarization to promote PAH development.47
It is speculated that there are 2 ways to achieve electric signal transmission from ECs to smooth muscle cells. One is to achieve rapid polarization of smooth muscle cells through myoendothelial gap junctions. The other is through the K+ released from ECs into the EC–smooth muscle cell intercellular gap, causing inwardly rectifying K+- and Na+/K+-adenosine triphosphatase–induced hyperpolarization of the smooth muscle cells.42 Increased [Ca2+]i in ECs is key to endothelium-derived hyperpolarization: studies have shown that activation of endothelial TRPV4 channels triggers uptake of calcium and activates IKCa and SKCa, promoting vasodilation.48 Additionally, PGs, NO, cyclic adenosine monophosphate, and others can affect the concentration of [Ca2+]i in ECs. Figure 3 shows the mechanism of endothelium-derived hyperpolarizing factors and endothelium-derived hyperpolarization.
Figure 3.
Endothelium-Derived Hyperpolarization Regulates Smooth Muscle Cell Relaxation
EDHFs promotes outflow of K+ by activating SKCa/IKCa to form membrane hyperpolarization. High extracellular concentration of K+ and myoendothelial gap junctions work together to hyperpolarize SMCs for vasodilation. IKCa = intermediate conductance through Ca2+-activated K+ channels; Kir = adenosine triphosphate–sensitive potassium channels; MEGJ = myoendothelial gap junction; P450 = cytochrome P450; SKCa = small conductance through Ca2+-activated K+ channels; TRP = transient receptor potential; other abbreviations as in Figures 1 and 2.
In the progression of PAH, severe remodeling and muscularization of distal pulmonary arterioles causes elevated pulmonary vascular resistance and pulmonary artery pressure. Further study of endothelium-derived hyperpolarization mechanisms may provide a new perspective to elucidate the pathogenesis of PAH, including mechanisms of calcium regulation, the role of conductance Ca2+-activated K+ channel, functional studies of myoendothelial gap junction components, and development of endothelium-derived hyperpolarization agonists.
Endothelin-1
ET is a strong vasoconstrictor produced mainly by ECs but is also produced in small quantities by other types of cells, including PASMCs and lung fibroblasts.49,50 Endothelial dysfunction in PAH progression leads to abnormal ET-1 synthesis. There are 3 paralogs for ETs, EDN1, EDN2, and EDN3, encoding ET-1, ET-2, and ET-3, respectively. ET-1 is the most active isoform, with high expression in vascular ECs and vascular smooth muscle cells, airway epithelium, and airway smooth muscle cells.51 PreproET-1 is sequentially cleaved by endopeptidase and ET-1-converting enzyme to produce proET-1 and bioactive ET-1.52 ET-1 plays an important role in cardiovascular disease because of its biological activity in lung tissue.53
ET-1 works primarily through 2 G protein–coupled receptors, ETA and ETB, which were first identified in the lung.54 Both ETA receptors and ETB receptors mediate vascular smooth muscle cell proliferation, and ETA receptors also mediate vascular contraction. In contrast, ETB receptors on ECs antagonize the contractile effects of ETA by mediating the release of vasodilators and antiproliferative factors and circulating ET-1 clearing.55 Although ETA and ETB receptors’ effects are different, the clinical use of ET receptor antagonists is not specifically differentiated. Interestingly, 2 splicing variants of the ETB receptor, ETB1 and ETB2, perform very different functions,56 which are caused by differences in the distribution of the receptor in tissues. ETA and ETB2 are present mainly in vascular smooth muscle cells and bind to ET-1 to contract blood vessels,57 while ETB1 exists mainly in ECs. After binding to ET-1, ETB1 promotes the synthesis of NO and PGI2 in ECs to antagonize the influence of ETA and ETB2 pathways.58 A recent study showed that the blood vessels of ETB−/− mice were infiltrated by lymphocytes, which contribute to the development of pulmonary hypertension.59 However, there remains controversy whether ETA inhibition alone is superior to ETA/ETB dual inhibition as therapy for PAH.
There have been many studies on the ET signaling pathway’s role in PAH pathogenesis, and its importance has been supported by many laboratory and clinical studies. There is clear evidence showing that the ET system is activated in almost all preclinical PAH models and in all categories of human pulmonary hypertension.60 ET-1 activity is significantly increased in different rat pulmonary hypertension models, including hypoxic pulmonary hypertension rats, monocrotaline-induced pulmonary hypertension rats, and genetically modified pulmonary hypertension rats.61, 62, 63, 64 Moreover, the ability to clear ET-1 from circulation was impaired in experimental pulmonary hypertension models and the lungs of patients with pulmonary hypertension.65 Plasma and lung ET-1 expression is shown to be positively correlated with the severity of disease in patients with PAH.66,67
TXA2 and 8-iso-PGF2α
TXA2 is produced by ECs, neutrophils, platelets, and macrophages and is shown to mediate platelet shape change and aggregation, as well as promote smooth muscle contraction and hypertrophy.68 TXA2 requires TXA2 receptor β rather than TXA2 receptor α to inhibit vascular endothelial growth factor–induced EC migration, NO production, and angiogenesis.69 Activation of phospholipase C-β and inositol triphosphate/diacylglycerol signaling caused by TXA2 receptor–Gq coupling completes intracellular calcium mobilization.70 TXA2-mediated TXA2 receptor–G12/13 coupling phosphorylates myosin light chain via Rho kinase to affect platelet shape.71
It is worth mentioning that 8-iso-PGF2α, a PG derivative transformed from esterified arachidonic acid on the cell membrane by free radical attack, contributes significantly to endothelial dysfunction as a TXA2 receptor activator.72 Through binding and activating the TXA2 receptor, 8-iso-PGF2α participates in hypoxia-induced pulmonary hypertension progression.73 Additionally, 8-iso-PGF2α caused ET-1 accumulation by activating the TXA2 receptor and Rho kinase.74 There is in vitro evidence that H2O2 stimulation can lead to an accumulation of 8-iso-PGF2α in ECs, and the increase of 8-iso-PGF2α in the urine of patients with pulmonary hypertension reflects a lipid peroxidation status as a marker of oxidative stress and inflammation.75
Early work in PAH has shown disruption of the balance between TXA2 and PGI2 in patients,76 which reflects endothelial dysfunction as a major cause of platelet activation and persistent pulmonary vasoconstriction.77 PGI2 achieves its vasodilator and anticoagulation function through PGI2 receptor–Gs subunit coupling and cyclic adenosine monophosphate pathway activation. The increase of cyclic adenosine monophosphate in smooth muscle cells rapidly reduces [Ca2+]i to achieve vasodilation. Studies have shown that PGI2 is similar to PGE2 in its association with pain and inflammation.78 Peroxisome proliferator–activated receptor α and peroxisome proliferator–activated receptor β/δ activation by PGI2 or its analogs can also maintain endothelial function and vasodilation, likely through endothelial NO synthase activation.79 PGI2 and its analogs have been used in the treatment of PAH because of their powerful effects of reducing platelet aggregation and promoting vasodilation, augmenting cardiac output and pulmonary vascular resistance reduction.80
Renin-angiotensin system
The renin-angiotensin system dominates the homeostatic balance of the cardiovascular system and body fluids. There have been many studies demonstrating that the renin-angiotensin system was involved in PAH development through regulating pulmonary vascular remodeling and pulmonary artery pressure.81 The angiotensin-converting enzyme (ACE)–angiotensin II–angiotensin type 1 receptor axis and the ACE2–angiotensin-(1-7)–Mas receptor axis are 2 antagonistic signaling pathways.82
Angiotensin II is a linear polypeptide composed of 8 amino acids and is the most important effector in the renin-angiotensin system. After ACE–angiotensin II–angiotensin type 1 receptor axis activation, angiotensin I is converted to angiotensin II by ACE. Angiotensin II binds to 2 receptors: angiotensin type 1 receptor and angiotensin type 2 receptor. Angiotensin II binds to angiotensin type 1 receptor, which promotes vasoconstriction, inflammation, and oxidative stress, while binding to angiotensin type 2 receptor leads to vasodilation.83 In recent years, Fried et al84 found in studies of nicotine inhalation in mice that angiotensin type 1 receptor–mediated angiotensin II acts on pulmonary blood vessels, leading to increased pulmonary artery pressure and right ventricular hypertrophy. A high-salt diet and high concentrations of angiotensin II can cause pulmonary hypertension with cardiac-renal syndrome.85 In contrast, inhibition of angiotensin II expression (using inhibitors and oxygen enrichment) can effectively improve cardiopulmonary function in rodents and relieve pulmonary hypertension symptoms.86,87
ACE2–angiotensin-(1-7)–Mas axis activation plays the opposite role from the ACE–angiotensin II–angiotensin type 1 receptor axis. ACE2 will competitively inhibit the ACE–angiotensin II–angiotensin type 1 receptor axis, converting angiotensin II into angiotensin-(1-7), causing angiotensin-(1-7) to further interact with Mas and counteracting the proliferation, contraction, inflammation, and other phenotypes of pulmonary blood vessels caused by the ACE–angiotensin II–angiotensin type 1 receptor axis.88 The overexpression of ACE2 in mice shows a greater resistance to hypoxia and attenuates the development of pulmonary hypertension.89
5-Hydroxytryptamine
5-HT is both a neurotransmitter in the central nervous system and a vasoconstrictor in the periphery. 5-HT mediates PAH by promoting pulmonary vascular contraction and remodeling. Moreover, 5-HT can induce the proliferation of pulmonary fibroblasts and smooth muscle cells, which contributes to pulmonary vascular remodeling and narrowing of the vessel lumen.90,91 The International Union of Pharmacology classification divides 5-HT receptors into 8 categories: 5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5A/5B, 5-HT6, 5-HT7, and “orphan” receptors.92 In experimental pulmonary hypertension, antagonism of 5-HT2B receptors has been therapeutic, and activation of 5-HT2B receptors in bone marrow progenitor cells promotes the development of experimental pulmonary hypertension.93,94 Moreover, 5-HT can affect the balance of oxidative stress in PASMCs by enhancing reactive oxygen species production through Src-related kinase-regulated nicotinamide adenine dinucleotide phosphate oxidase 1 and dysregulated nuclear factor [erythroid-derived 2]–like 2 (Nrf-2) antioxidant mechanisms. In this case, the 5-HT1B receptor is involved in experimental pulmonary hypertension by inducing reactive oxygen species production in the lungs.95 In the lamb model of persistent pulmonary hypertension of the newborn, injection of 5-HT increases pulmonary vascular resistance, while injection of the 5-HT2A receptor antagonist ketanserin reduces pulmonary vascular resistance in this experimental model.96
Purinergic signaling in regulation of PAH
Since 1972, numerous studies have demonstrated that adenosine triphosphate acts as an extracellular signaling molecule that controls blood pressure.97 In fact, cells including erythrocytes, ECs, and immune cells can produce nucleotides (adenosine triphosphate, adenosine diphosphate, uridine triphosphate, uridine diphosphate) or nucleosides (adenosine) that bind to purinergic receptors for their biological functions. These purinergic receptors contain 2 subfamilies, namely, P1R and P2R. Among them, P1R contains 4 subtypes, and P2R can be further subdivided into 2 branches: P2XRs and P2YRs.98 The specific classification of these receptors and their corresponding relationships with ligands are reviewed in detail (Figure 1). After being released into extracellular matrix, nucleosides or nucleotides are regulated by a variety of ectonucleotidases. These are classified into 4 groups of enzymes, including ecto-ATPDase 1, 5′-nucleotidase, nucleotide pyrophosphatase/phosphodiesterase and adenosine deaminase. Adenosine triphosphate, in turn, is metabolized by ecto-ATPDase 1, 5′-nucleotidase, and adenosine deaminase into adenosine monophosphate, adenosine, and inosine. Uridine adenosine tetraphosphate is an EDCF synthesized by vascular endothelial growth factor receptor 2 in vascular ECs, while adenosine is a putative EDRF, and EC-derived adenosine diphosphate can be a putative ECRF because of its ability to activate platelet aggregation. This is due mainly to the different types of receptors they activate. These purinergic receptors mediated signaling events are disrupted during PAH progression. Clinical evidence has shown that plasma adenosine concentrations in patients with PAH are lower than in healthy subjects and that intravenous adenosine can effectively reduce pulmonary artery pressure and right ventricular pressure in patients with pulmonary hypertension.99 Experiments in lambs also demonstrate that low doses of adenosine can reduce pulmonary artery pressure by decreasing pulmonary vascular resistance.100 In contrast, the contractile effect of uridine adenosine tetraphosphate on the pulmonary artery is accomplished by activating P2YR, which may involve extracellular calcium influx in vascular smooth muscle cell.101
The complex interaction of EDRFs and EDCFs
The specific function of EDRFs or EDCFs depends on their receptor pathways. There are complex interactions among these factors (Figure 4). ET-1 mediates vasoconstriction by promoting TXA2 release and activation of TXA2 receptors, which depends on the protein kinase Cα pathway.102 The abundance of ET-1 is influenced by several factors, including hypoxia, hyperoxia, reactive oxygen species, growth factors, cytokines, shear stress, thrombin, angiotensin II, and others.103,104 In addition, ET-1 biosynthesis is inhibited by NO and PGI2.105 The vasodilation function of adenosine can be partially achieved by the activation of the adenosine triphosphate–sensitive potassium channel mediated by the A2ARs–Gαs–protein kinase A pathway.106 This process relies on cyclic adenosine monophosphate accumulation and activation of protein kinase A, which phosphorylates the adenosine triphosphate–sensitive potassium channel complex and promotes channel opening. K+ efflux induces hyperpolarization of the cell membrane and eventually vascular expansion.107 The complex interactions of these opposing networks and the imbalance identified in the pathogenesis of PAH highlight the importance of these pathways and the need for further study.
Figure 4.
Interactions Among EDRFs and EDCFs
Neither EDRFs nor EDCFs function independently, and there are complex interactions between them. ACE = angiotensin-converting enzyme; ADMA = asymmetrical dimethylarginine; BH4 = tetrahydrobiopurine; CD39 = ecto-ATPDase 1; CD73 = 5′-nucleotidase; eNOS = endothelial nitric oxide synthase; L-Arg = L-arginine; PKA = protein kinase A; other abbreviations as in Figures 1 and 2.
Drugs Related to Edrfs and Edcfs for PAH Treatment
Over the past 20 years, the treatment and management of patients with pulmonary hypertension have made numerous advancements in drug development and molecular targeting. Currently, 5 different targeted drugs are available for treatment: ET receptor antagonists, phosphodiesterase-5 inhibitors, soluble guanylate cyclase stimulators, PGI2 derivatives, and PGI2 agonists. These different classes of drugs are used in various combinations for pulmonary hypertension treatment, and Table 2 highlights the pulmonary hypertension drugs related to EDRFs and EDCFs.
Table 2.
Drugs Associated With Endothelium-Derived Relaxing Factors and Endothelium-Derived Contracting Factors for PH Treatment
| Drug | Target and Function | Indications |
|---|---|---|
| Macitentan | ETAR/ETBR antagonist | PAH, CTEPH, IPF |
| Bosentan | ETAR/ETBR antagonist | PAH, CTEPH, IPF |
| Ambrisentan | ETAR antagonist | PAH, SSc-PH, IPF |
| Sitaxsentana | ETAR antagonist | PAH |
| ETRQβ-002b | Vaccine for ETAR | PAH |
| Beraprost | IP, EP3 receptor agonist | ASO, PAH |
| Epoprostenol | IP, EP1, EP3 receptor agonist | PAH |
| Selexipag | IP receptor agonist | PAH |
| Iloprost | IP, EP1, EP2 receptor agonist | SSc, PAH |
| Treprostinil | IP, DP1, EP2 receptor agonist | PAH |
| MRE-269b | Selective IP receptor agonist | PAH |
| Riociguat | Oral stimulator of sGC | PAH, CTEPH |
| Cinaciguatb | sGC activator | PPHN |
| Tadalafil | PDE-5 inhibitor | PAH |
| Sildenafil | PDE-5 inhibitor | PAH |
| Vardenafilb | PDE-5 inhibitor | PAH |
| Inhaled NOc | Vasodilator | PAH, PPHN |
| Inhaled nitriteb | Vasodilator | PAH |
| Oral L-Citb | Intermediate for NO synthesis | PAH |
| Oral L-Argb | Substrates for NO synthesis | PAH |
| 6R-BH4b | Cofactor for eNOS | PAH |
| Rodatristat ethylb | TPH1 inhibitor | PAH |
| GSK2586881b | Recombinant human ACE2 | PAH |
ACE2 = angiotensin-converting enzyme 2; ASO = arteriosclerosis obliterans; BH4 = tetrahydrobiopurine; CTEPH = chronic thromboembolic pulmonary hypertension; DP = prostaglandin D2 receptor; eNOS = endothelial nitric oxide synthase; EP = prostaglandin E2 receptor; ETAR = ETA receptor; ETBR = ETB receptor; IP = prostaglandin I2 receptor; IPF = idiopathic pulmonary fibrosis; L-Arg = L-arginine; L-Cit = L-citrulline; PAH = pulmonary arterial hypertension; PDE-5 = phosphodiesterase-5; PH = pulmonary hypertension; PPHN = persistent pulmonary hypertension of the newborn; sGC = soluble guanylate cyclase; SSc = systemic sclerosis; TPH1 = tryptophan hydroxylase 1.
Sitaxsentan was removed from the market because of liver toxicity.
Experimental use only. cInhaled NO is for short-term use or experimental use in patients with PAH.
The therapeutic value of the NO pathway
NO pathway restoration has positive impact on endothelial integrity and is a major target of clinical PAH therapy. The U.S. Food and Drug Administration has approved inhaled NO to treat persistent pulmonary hypertension of the newborn,108 which has prompted further development of portable delivery devices and NO inhalation clinical trials for pulmonary hypertension.16 Oral L-arginine supplementation effectively increases NO production in patients with pulmonary hypertension and improves hemodynamic status and exercise capacity.109 L-citrulline is an intermediate in NO synthesis, and oral L-citrulline supplementation can prevent PAH development.110 Endothelial NO synthase gene-enhanced progenitor cells used to treat PAH significantly improved patients’ 6-minute walk distance, but there was no sustained hemodynamic improvement.111 Sapropterin dihydrochloride is a tetrahydrobiopurine analog involved NO synthesis that is currently under investigation for treatment of PAH (NCT00435331). Moreover, NO can also be formed from nitrite. A clinical trial demonstrated that nitrite inhalation can relieve pulmonary hypertension symptoms via improvements in left and right ventricular filling pressure and pulmonary artery compliance.112 However, the dangers of nitrite overuse have been fully demonstrated. Some recreational or sexual enhancement drugs contain amyl nitrite, and misuse of these drugs can cause serious health damage.
Targeted drugs based on the NO–soluble guanylate cyclase–cGMP–protein kinase G axis
Many experiments have demonstrated that soluble guanylate cyclase activity is impaired during the development and progression of PAH. The oxidation of the heme group in soluble guanylate cyclase attenuates its response to NO and possibly results in heme’s dissociation from soluble guanylate cyclase.113,114 Significant soluble guanylate cyclase up-regulation was found in pulmonary arteries in patients with idiopathic PAH compared with healthy donors. Additionally, soluble guanylate cyclase was also up-regulated in lungs from hypoxic pulmonary hypertension mice and monocrotaline-induced pulmonary hypertension rats, similar to patients with idiopathic PAH.115 On the basis of soluble guanylate cyclase research, 2 new classes of agents have been developed: 1) riociguat, a soluble guanylate cyclase stimulator that activates the native Fe2+–soluble guanylate cyclase and synergizes with NO, significantly improving exercise capacity and pulmonary hemodynamic status in patients with pulmonary hypertension, has been approved for treatment PAH116; and 2) cinaciguat, a soluble guanylate cyclase activator that activates the Fe3+ form, or heme-free form of the enzyme, has been shown to cause pulmonary vasodilation in experimental persistent pulmonary hypertension of the newborn and improve cardiopulmonary hemodynamic parameters in patients with acute decompensated heart failure.117,118
cGMP is metabolized by cGMP-specific 3′,5′-cyclic phosphodiesterase-5 in lung tissue. Phosphodiesterase-5 hydrolyzes the cGMP cyclic phosphate bond to form 5′-guanosine monophosphate, which in turn stimulates protein kinase G. It was reported that phosphodiesterase-5 was elevated both in PASMCs of patients with PAH and cardiomyocytes of patients with right ventricular hypertrophy.119,120 Moreover, phosphodiesterase-5 levels were significantly increased in the pulmonary arteries of hypoxic pulmonary hypertension rats.121 Two phosphodiesterase-5 inhibitors, sildenafil and tadalafil, effectively improved 6-minute walk distance and pulmonary hemodynamic parameters in placebo-controlled trials and have been approved by the Food and Drug Administration for clinical treatment of PAH in adults.108,122 In addition, vardenafil, another phosphodiesterase-5 inhibitor, has shown positive effects in PAH but is not approved for use in this population.123 Phosphodiesterase-5 inhibitors impair cGMP metabolism, resulting in increased levels of intracellular cGMP, which activates protein kinase G by feedback regulation. Protein kinase G activation causes a series of downstream effects, including vasodilation and inhibition of vascular smooth muscle cell proliferation.124 A recent study showed that a novel class of compound, pyrazolo [3,4-b] pyridine derivatives, can not only activate soluble guanylate cyclase to play a vasodilator role but can also regulate vascular remodeling by inhibiting adenosine monophosphate–activated protein kinase.125 These compounds appear to show promise but have not been validated clinically.
PGI2 analogues, PGI2 receptor agonists, and TXA2 inhibitors
PGI2 is a very potent vasodilator but is unstable with a short half-life and therefore has limitations to its clinical use and utility. Therapies in this class started as a synthetic analog to PGI2, epoprostenol, and have since been modified to retain the potent vasodilatory properties while optimizing pharmacokinetics and pharmacodynamics. These agents include beraprost, iloprost, MRE-269, treprostinil, and selexipag. Treprostinil, a long-half-life PGI2 analog, continues to be tested in clinical trials for PAH treatment by intravenous, oral, and inhaled administration.126, 127, 128 Most agonists among them are not specific for PGI2 receptor and are involved in inflammatory and immune processes by activating other PG receptors, such as PGE2 and PGD2 receptor, which may counteract the benefits of PGI2 receptor signaling in endothelial maintenance, vasodilation, and anticoagulation.129 Selexipag, an oral PGI2 receptor agonist, is shown to be highly selective and designed to avoid the effects of PGE2 and PGD2 receptors.130
TXA2 antagonists, TXA2 receptor antagonists, and thromboxane synthase inhibitors can block TXA2-induced platelet aggregation and vasoconstriction to relieve experimental pulmonary hypertension. The thromboxane synthase inhibitor OKY-046 mitigated monocrotaline-induced pulmonary hypertension development by reducing TXA2 production in rats,131 and similar results were also seen in pulmonary hypertension induced by heparin-protamine complexes in goats.132 Additional thromboxane synthase inhibitors include CGS 15435, picotamide, furegrelate sodium, ONO-1301, and others. It is worth mentioning that ONO-1301 is a PGI2 analogue that not only activates PGI2 receptor in the long term but also inhibits thromboxane synthase activity.133 TXA2 antagonists include ramatroban and ramatroban-D4, while TXA2 receptor antagonists include NTP42, YM158 free base, daltroban, picotamide, ICI 192605, LCB-2853, and others. However, none of these drugs is approved for PAH therapy, or they are undergoing clinical trials.
Predictably, interference in the synthesis of PGs and their receptor pathways may ultimately lead to endothelial dysfunction. Specifically, these include arachidonic acid depletion through lipid peroxidation, dysregulated expression profiles of various PG synthases, and PG receptor activation disorders. Hence, multiple considerations are needed to restore endothelial function. Therefore, we can get a glimpse of future research directions that should focus on the development of highly effective free radical scavengers and specific activators and inhibitors for both PG synthases and PG receptors.
Clinical therapeutic drugs for pulmonary hypertension based on the ET-1 signaling pathway
Two ET receptor antagonists are currently in clinical use: selective (for ETA) and nonselective (both ETA and ETB) receptor blockers. Both ET receptor antagonists have been clearly verified to be effective in many preclinical pulmonary hypertension models to improve pulmonary artery EC function, hemodynamic derangements, and right ventricular hypertrophy.134, 135, 136 Currently, some ET receptor antagonists have been approved for PAH clinical therapy: bosentan was the first ET receptor antagonist to have been approved by the Food and Drug Administration in 2001, ambrisentan was approved in 2007, and macitentan was approved in 2013. In addition, sitaxsentan was approved in the European Union, Canada, and Australia, but not in the United States.51,137 It has subsequently been removed from the market because of concerns over liver toxicity.
In general, ET receptor antagonist classification is achieved by differences in pharmacokinetics, basic structure, and receptor affinity, such as bosentan and macitentan being nonselective, while ambrisentan is selective for ETA.137 In terms of immunotherapy, the first experimental vaccine (ETRQβ-002) against ETA for PAH was recently found. ETRQβ-002 can alleviate remodeling of pulmonary arterioles and the right ventricle in monocrotaline-induced and SU5416/hypoxia-induced pulmonary hypertension models by reducing the pressure response, inhibiting ET-1-initiated signal transduction, and effectively reducing right ventricular systolic pressure.138 Whether such immunotherapy is safe and reliable in patients remains to be determined, but vaccination opens new ways to treat PAH.
Tryptophan hydroxylase 1 is a potential target for PAH therapy
Tryptophan hydroxylase (TPH) catalyzes tryptophan to form serotonin, the rate-limiting step in serotonin synthesis.139 There are 2 subtypes of TPHs: TPH1 and TPH2. Previous studies have demonstrated that a portion of 5-HT is produced in pulmonary artery ECs. Endothelium-derived 5-HT promotes PASMC proliferation and PAH development through TPH1. Meanwhile, with PAH developing, 5-HT crosses the intima and contacts PASMCs, causing vasoconstriction, so it is also considered an EDRF. TPH1 is present primarily in the gut and mediates peripheral serotonin production, whereas TPH2 is present exclusively in the central nervous system.139 TPH1 expression is increased in the pulmonary artery ECs of patients with PAH and contributes to PASMC hyperplasia,140 and endothelial TPH1 expression was also increased in experimental pulmonary hypertension models.141 Increasing evidence indicated that TPH1 gene knockout or drug inhibition shows therapeutic effects in experimental pulmonary hypertension models, including the hypoxia-, monocrotaline-, and SU5416/hypoxia-induced rodent pulmonary hypertension models.142,143 Currently, the TPH1 inhibitor rodatristat ethyl (KAR5585) is in the recruitment phase of a clinical trial, and selective inhibitors of TPH1 are expected as new targeted PAH therapies.
The ACE2-angiotensin-(1-7)–Mas axis antagonizes the effect of angiotensin II in PAH
In recent years, a newly developed oral drug using plant cell encapsulation ACE2/angiotensin-(1-7) reduced the experimental pulmonary hypertension phenotype.144 Similarly, a study demonstrated that symptoms in patients with pulmonary hypertension were alleviated when they were given recombinant human ACE2 intravenously.145 Most recently, this recombinant protein was developed into a soluble intravenous injection called GSK2586881, which was evaluated for safety and pharmacokinetics in a PAH phase 2 clinic trial.146 Moreover, the discovery of the micro–ribonucleic acid let-7b, which targets inhibition of ACE2, augmented the development of experimental pulmonary hypertension and revalidated the cardiovascular protective effect of ACE2.147
Conclusions and Perspectives
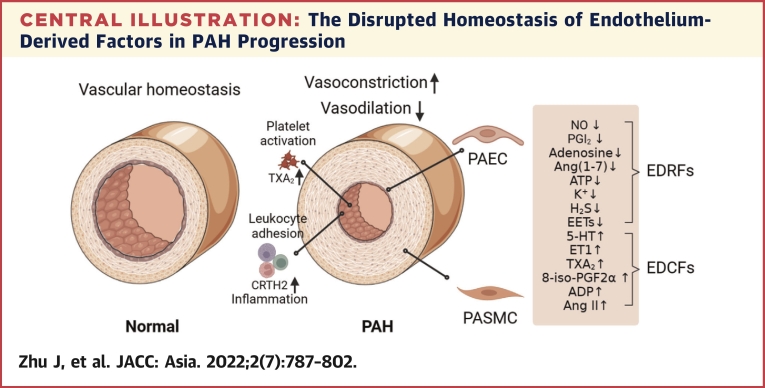
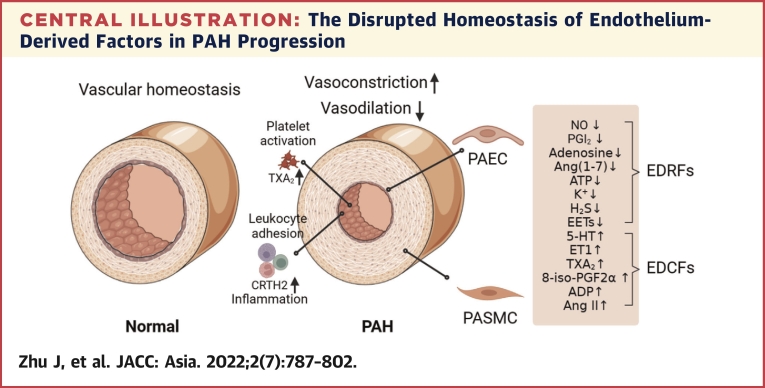
The balance of production between EDCFs and EDRFs is a prerequisite for normal vascular function. Healthy pulmonary artery ECs relax blood vessels by releasing NO, PGI2, and endothelium-derived hyperpolarizing factors, subsequently reducing pulmonary vascular resistance and pulmonary artery pressure. In pathologic conditions of PAH, or under hypoxia, EDRF production and release are reduced, while EDCFs are increased. This results in leukocyte adhesion, platelet aggregation, PASMC proliferation, and ultimately pulmonary artery contraction and remodeling (Central Illustration). As EDRFs and EDCFs dominate vascular contraction and relaxation, blocking EDCF and activating EDRF signals are good solutions for PAH clinical therapies.
Central Illustration.
The Disrupted Homeostasis of Endothelium-Derived Factors in PAH Progression
During pulmonary arterial hypertension (PAH) development, endothelium-derived relaxing factor (EDRF)/endothelium-derived contracting factor (EDCF) imbalance, platelet aggregation, and leukocyte adhesion all lead to endothelial dysfunction and vasoconstriction. 5-HT = 5-hydroxytryptamine; ADP = adenosine diphosphate; Ang = angiotensin; ATP = adenosine triphosphate; CRTH2 = prostaglandin D2 receptor 2; EET = epoxyeicosatrienoic acid; ET = endothelin; PAEC = pulmonary artery endothelial cell; PASMC = pulmonary artery smooth muscle cell; PG = prostaglandin; TXA2 = thromboxane A2.
Clinical treatment for PAH is still a serious challenge. Often a single drug falls short of controlling severe PAH, and combination therapy with targeted drugs has become an attractive option and standard of care.148 Combination therapy with 2 oral pulmonary vasodilators, tadalafil and ambrisentan, was the first to prove the benefit of early treatment targeting multiple pathogenic molecular pathways.149 Since that time multiple studies have shown the benefit of combination therapy and the positive effects of adding additional agents to background therapy for patients with PAH.150 In addition to dual therapy, the therapeutic effects of upfront triple therapy have received attention. A randomized controlled trial study in 2021 included patients with different subtypes of pulmonary hypertension (123 receiving initial triple therapy vs 124 receiving initial dual therapy).151 It is undeniable that the combination of targeted drugs is generally superior to a single drug in PAH therapy. However, there is an urgent need to explore more therapeutic targets and targeted drugs. Optimization on the basis of the combination of multiple targeted drugs may eventually provide an effective solution.
Funding Support and Author Disclosures
This work was funded in part by the National Key Research and Development Program of China (grant 2019YFE0119400), the Natural Science Foundation of China (grants 81970052 and 82170057), and the National Lung, Heart, and Blood Institute of the National Institutes of Health (grant R35 HL135807). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Contributor Information
Haiyang Tang, Email: tanghy2008@yahoo.com.
Jason X-J Yuan, Email: jxyuan@health.ucsd.edu.
References
- 1.Schmidt K., de Wit C. Endothelium-derived hyperpolarizing factor and myoendothelial coupling: the in vivo perspective. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luscher T.F., Boulanger C.M., Dohi Y., et al. Endothelium-derived contracting factors. Hypertension. 1992;19:117–130. doi: 10.1161/01.hyp.19.2.117. [DOI] [PubMed] [Google Scholar]
- 3.Sakao S., Taraseviciene-Stewart L., Lee J.D., et al. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 2005;19:1178–1180. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- 4.Konukoglu D., Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. 2017;956:511–540. doi: 10.1007/5584_2016_90. [DOI] [PubMed] [Google Scholar]
- 5.Thenappan T., Ormiston M.L., Ryan J.J., et al. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360:j5492. doi: 10.1136/bmj.j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang H., Wu K., Wang J., et al. Pathogenic role of mTORC1 and mTORC2 in pulmonary hypertension. J Am Coll Cardiol Basic Trans Science. 2018;3:744–762. doi: 10.1016/j.jacbts.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang H., Babicheva A., McDermott K.M., et al. Endothelial HIF-2alpha contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol. 2018;314:L256–L275. doi: 10.1152/ajplung.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundberg J.O., Weitzberg E., Gladwin M. The T nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Disc. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 9.Chen K., Pittman R.N., Popel A.S. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal. 2008;10:1185–1198. doi: 10.1089/ars.2007.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto H., Yun E.J., Gerber H.P., et al. Epithelial-vascular cross talk mediated by VEGF-A and HGF signaling directs primary septae formation during distal lung morphogenesis. Dev Biol. 2007;308:44–53. doi: 10.1016/j.ydbio.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 11.Block E.R., Herrera H., Couch M. Hypoxia inhibits L-arginine uptake by pulmonary artery endothelial cells. Am J Physiol. 1995;269:L574–L580. doi: 10.1152/ajplung.1995.269.5.L574. [DOI] [PubMed] [Google Scholar]
- 12.Morris C.R., Kato G.J., Poljakovic M., et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pullamsetti S., Kiss L., Ghofrani H.A., et al. Increased levels and reduced catabolism of asymmetric and symmetric dimethylarginines in pulmonary hypertension. FASEB J. 2005;19:1175–1177. doi: 10.1096/fj.04-3223fje. [DOI] [PubMed] [Google Scholar]
- 14.Antoniades C., Shirodaria C., Leeson P., et al. Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: implications for endothelial function in human atherosclerosis. Eur Heart J. 2009;30:1142–1150. doi: 10.1093/eurheartj/ehp061. [DOI] [PubMed] [Google Scholar]
- 15.Davydova M.P., Postnikov A.B., D’Iakonov K.B., et al. [Involvement of tetrahydrobiopterin in local change of endothelium-dependent vasorelaxation in pulmonary hypertension] Ross Fiziol Zh Im I M Sechenova. 2003;89:1516–1522. [PubMed] [Google Scholar]
- 16.Klinger J.R., Abman S.H., Gladwin M.T. Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188:639–646. doi: 10.1164/rccm.201304-0686PP. [DOI] [PubMed] [Google Scholar]
- 17.Giaid A., Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 18.Swisher J.W., Elliott D. Combination therapy with riociguat and inhaled treprostinil in inoperable and progressive chronic thromboembolic pulmonary hypertension. Respir Med Case Rep. 2017;20:45–47. doi: 10.1016/j.rmcr.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y.Y., Zhao Y.D., Mirza M.K., et al. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119:2009–2018. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirata T., Narumiya S. Prostanoid receptors. Chem Rev. 2011;111:6209–6230. doi: 10.1021/cr200010h. [DOI] [PubMed] [Google Scholar]
- 21.Soifer S.J., Morin F.C., III, Heymann M.A. Prostaglandin D2 reverses induced pulmonary hypertension in the newborn lamb. J Pediatr. 1982;100:458–463. doi: 10.1016/s0022-3476(82)80460-5. [DOI] [PubMed] [Google Scholar]
- 22.Soifer S.J., Clyman R.I., Hermann M.A. Effects of prostaglandin D2 on pulmonary arterial pressure and oxygenation in newborn infants with persistent pulmonary hypertension. J Pediatr. 1988;112:774–777. doi: 10.1016/s0022-3476(88)80701-7. [DOI] [PubMed] [Google Scholar]
- 23.Dobyns E.L., Wescott J.Y., Kennaugh J.M., et al. Eicosanoids decrease with successful extracorporeal membrane oxygenation therapy in neonatal pulmonary hypertension. Am J Respir Crit Care Med. 1994;149:873–880. doi: 10.1164/ajrccm.149.4.8143049. [DOI] [PubMed] [Google Scholar]
- 24.Robbins I.M., Barst R.J., Rubin L.J., et al. Increased levels of prostaglandin D2 suggest macrophage activation in patients with primary pulmonary hypertension. Chest. 2001;120:1639–1644. doi: 10.1378/chest.120.5.1639. [DOI] [PubMed] [Google Scholar]
- 25.Chen G., Zuo S., Tang J., et al. Inhibition of CRTH2-mediated Th2 activation attenuates pulmonary hypertension in mice. J Exp Med. 2018;215:2175–2195. doi: 10.1084/jem.20171767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia D., Bai P., Wan N., et al. Niacin Attenuates pulmonary hypertension through H-PGDS in macrophages. Circ Res. 2020;127:1323–1336. doi: 10.1161/CIRCRESAHA.120.316784. [DOI] [PubMed] [Google Scholar]
- 27.Cheng H., Huang H., Guo Z., et al. Role of prostaglandin E2 in tissue repair and regeneration. Theranostics. 2021;11:8836–8854. doi: 10.7150/thno.63396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norel X. Prostanoid receptors in the human vascular wall. Scientific World Journal. 2007;7:1359–1374. doi: 10.1100/tsw.2007.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozen G., Benyahia C., Mani S., et al. Bronchodilation induced by PGE2 is impaired in group III pulmonary hypertension. Br J Pharmacol. 2020;177:161–174. doi: 10.1111/bph.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H., Fang B., Du S., et al. Endothelial cell prostaglandin E2 receptor EP4 is essential for blood pressure homeostasis. JCI Insight. 2020;5(13) doi: 10.1172/jci.insight.138505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jose P., Niederhauser U., Piper P.J., et al. Degradation of prostaglandin F2alpha in the human pulmonary circulation. Thorax. 1976;31:713–719. doi: 10.1136/thx.31.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams J.W., Migita D.S., Yu M.K., et al. Prostaglandin F2 alpha stimulates hypertrophic growth of cultured neonatal rat ventricular myocytes. J Biol Chem. 1996;271:1179–1186. doi: 10.1074/jbc.271.2.1179. [DOI] [PubMed] [Google Scholar]
- 33.Vankova M., Snetkov V.A., Knock G.A., et al. Euhydric hypercapnia increases vasoreactivity of rat pulmonary arteries via HCO3− transport and depolarisation. Cardiovasc Res. 2005;65:505–512. doi: 10.1016/j.cardiores.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 34.Semenza G.L. Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J. 2017;36:252–259. doi: 10.15252/embj.201695204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman W.H., Frankis M.B., Halushka P.V. Increased myocardial release of prostacyclin in dogs with heart failure. J Cardiovasc Pharmacol. 1983;5:194–201. doi: 10.1097/00005344-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Ueda S., Fukunaga K., Takihara T., et al. Deficiency of CRTH2, a prostaglandin D2 receptor, aggravates bleomycin-induced pulmonary inflammation and fibrosis. Am J Respir Cell Mol Biol. 2019;60:289–298. doi: 10.1165/rcmb.2017-0397OC. [DOI] [PubMed] [Google Scholar]
- 37.Li K., Zhao J., Wang M., et al. The roles of various prostaglandins in fibrosis: a review. Biomolecules. 2021;11(6):789. doi: 10.3390/biom11060789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oga T., Matsuoka T., Yao C., et al. Prostaglandin F2alpha receptor signaling facilitates bleomycin-induced pulmonary fibrosis independently of transforming growth factor-beta. Nat Med. 2009;15:1426–1430. doi: 10.1038/nm.2066. [DOI] [PubMed] [Google Scholar]
- 39.Mulvaney E.P., Reid H.M., Bialesova L., et al. NTP42, a novel antagonist of the thromboxane receptor, attenuates experimentally induced pulmonary arterial hypertension. BMC Pulm Med. 2020;20:85. doi: 10.1186/s12890-020-1113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin C., Zheng X., Lin S., et al. Mechanotransduction regulates the interplays between alveolar epithelial and vascular endothelial cells in lung. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.818394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hecker M., Bara A.T., Bauersachs J., et al. Characterization of endothelium-derived hyperpolarizing factor as a cytochrome P450-derived arachidonic acid metabolite in mammals. J Physiol. 1994;481(Pt 2):407–414. doi: 10.1113/jphysiol.1994.sp020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards G., Dora K.A., Gardener M.J., et al. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 43.Zhao W., Zhang J., Lu Y., et al. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunt V.E., Minson C.T. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol. 2012;590:3523–3534. doi: 10.1113/jphysiol.2012.236398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alkayed N.J., Cao Z., Qian Z.Y., et al. Control of coronary vascular resistance by eicosanoids via a novel GPCR. Am J Physiol Cell Physiol. 2022;322:C1011–C1021. doi: 10.1152/ajpcell.00454.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandow S.L., Hill C.E. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 47.Si R., Zhang Q., Cabrera J.T.O., et al. Chronic hypoxia decreases endothelial connexin 40, attenuates endothelium-dependent hyperpolarization-mediated relaxation in small distal pulmonary arteries, and leads to pulmonary hypertension. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.018327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonkusare S.K., Bonev A.D., Ledoux J., et al. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markewitz B.A., Farrukh I.S., Chen Y.X., et al. Regulation of endothelin-1 synthesis in human pulmonary arterial smooth muscle cells: effects of transforming growth factor-beta and hypoxia. Cardiovasc Res. 2001;49:200–206. doi: 10.1016/s0008-6363(00)00221-2. [DOI] [PubMed] [Google Scholar]
- 50.Xu S.W., Chen Y.L., Denton C.P., et al. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15:2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barton M., Yanagisawa M. Endothelin: 30 years from discovery to therapy. Hypertension. 2019;74:1232–1265. doi: 10.1161/HYPERTENSIONAHA.119.12105. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi M., Matsushita Y., Iijima Y., et al. Purification and characterization of endothelin-converting enzyme from rat lung. J Biol Chem. 1993;268:21394–21398. [PubMed] [Google Scholar]
- 53.Bohm F., Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76:8–18. doi: 10.1016/j.cardiores.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Masuda Y., Miyazaki H., Kondoh M., et al. Two different forms of endothelin receptors in rat lung. FEBS Lett. 1989;257:208–210. doi: 10.1016/0014-5793(89)81535-2. [DOI] [PubMed] [Google Scholar]
- 55.Davie N., Haleen S.J., Upton P.D., et al. ETA and ETB receptors modulate the proliferation of human pulmonary artery smooth muscle cells. Am J Respir Crit Care Med. 2002;165:398–405. doi: 10.1164/ajrccm.165.3.2104059. [DOI] [PubMed] [Google Scholar]
- 56.Pollock D.M., Keith T.L., Highsmith R.F. Endothelin receptors and calcium signaling. FASEB J. 1995;9:1196–1204. doi: 10.1096/fasebj.9.12.7672512. [DOI] [PubMed] [Google Scholar]
- 57.Miyagawa K., Emoto N. Current state of endothelin receptor antagonism in hypertension and pulmonary hypertension. Ther Adv Cardiovasc Dis. 2014;8:202–216. doi: 10.1177/1753944714541511. [DOI] [PubMed] [Google Scholar]
- 58.Kostov K. The causal relationship between endothelin-1 and hypertension: focusing on endothelial dysfunction, arterial stiffness, vascular remodeling, and blood pressure regulation. Life (Basel) 2021;11:986. doi: 10.3390/life11090986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tabeling C., Gonzalez CR Calera, Lienau J., et al. Endothelin B receptor immunodynamics in pulmonary arterial hypertension. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.895501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Michel R.P., Langleben D., Dupuis J. The endothelin system in pulmonary hypertension. Can J Physiol Pharmacol. 2003;81:542–554. doi: 10.1139/y03-008. [DOI] [PubMed] [Google Scholar]
- 61.Chen S.J., Chen Y.F., Meng Q.C., et al. Endothelin-receptor antagonist bosentan prevents and reverses hypoxic pulmonary hypertension in rats. J Appl Physiol (1985) 1995;79:2122–2131. doi: 10.1152/jappl.1995.79.6.2122. [DOI] [PubMed] [Google Scholar]
- 62.Elton T.S., Oparil S., Taylor G.R., et al. Normobaric hypoxia stimulates endothelin-1 gene expression in the rat. Am J Physiol. 1992;263:R1260–R1264. doi: 10.1152/ajpregu.1992.263.6.R1260. [DOI] [PubMed] [Google Scholar]
- 63.Stelzner T.J., O’Brien R.F., Yanagisawa M., et al. Increased lung endothelin-1 production in rats with idiopathic pulmonary hypertension. Am J Physiol. 1992;262:L614–L620. doi: 10.1152/ajplung.1992.262.5.L614. [DOI] [PubMed] [Google Scholar]
- 64.Miyauchi T., Yorikane R., Sakai S., et al. Contribution of endogenous endothelin-1 to the progression of cardiopulmonary alterations in rats with monocrotaline-induced pulmonary hypertension. Circ Res. 1993;73:887–897. doi: 10.1161/01.res.73.5.887. [DOI] [PubMed] [Google Scholar]
- 65.Dupuis J., Cernacek P., Tardif J.C., et al. Reduced pulmonary clearance of endothelin-1 in pulmonary hypertension. Am Heart J. 1998;135:614–620. doi: 10.1016/s0002-8703(98)70276-5. [DOI] [PubMed] [Google Scholar]
- 66.Giaid A., Yanagisawa M., Langleben D., et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 67.Rubens C., Ewert R., Halank M., et al. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest. 2001;120:1562–1569. doi: 10.1378/chest.120.5.1562. [DOI] [PubMed] [Google Scholar]
- 68.Gao Y., Yokota R., Tang S., et al. Reversal of angiogenesis in vitro, induction of apoptosis, and inhibition of AKT phosphorylation in endothelial cells by thromboxane A(2) Circ Res. 2000;87:739–745. doi: 10.1161/01.res.87.9.739. [DOI] [PubMed] [Google Scholar]
- 69.Ashton A.W., Ware J.A. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circ Res. 2004;95:372–379. doi: 10.1161/01.RES.0000138300.41642.15. [DOI] [PubMed] [Google Scholar]
- 70.Shenker A., Goldsmith P., Unson C.G., et al. The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J Biol Chem. 1991;266:9309–9313. [PubMed] [Google Scholar]
- 71.Klages B., Brandt U., Simon M.I., et al. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J Cell Biol. 1999;144:745–754. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palombo C., Lubrano V., Sampietro T. Oxidative stress, F2-isoprostanes and endothelial dysfunction in hypercholesterolemia. Cardiovasc Res. 1999;44:474–476. doi: 10.1016/s0008-6363(99)00367-3. [DOI] [PubMed] [Google Scholar]
- 73.Delannoy E., Courtois A., Freund-Michel V., et al. Hypoxia-induced hyperreactivity of pulmonary arteries: role of cyclooxygenase-2, isoprostanes, and thromboxane receptors. Cardiovasc Res. 2010;85:582–592. doi: 10.1093/cvr/cvp292. [DOI] [PubMed] [Google Scholar]
- 74.Yi S.L., Kantores C., Belcastro R., et al. 8-Isoprostane-induced endothelin-1 production by infant rat pulmonary artery smooth muscle cells is mediated by Rho-kinase. Free Radic Biol Med. 2006;41:942–949. doi: 10.1016/j.freeradbiomed.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 75.Hart C.M., Karman R.J., Blackburn T.L., et al. Role of 8-epi PGF2alpha, 8-isoprostane, in H2O2-induced derangements of pulmonary artery endothelial cell barrier function. Prostaglandins Leukot Essent Fatty Acids. 1998;58:9–16. doi: 10.1016/s0952-3278(98)90124-7. [DOI] [PubMed] [Google Scholar]
- 76.Christman B.W., McPherson C.D., Newman J.H., et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327:70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- 77.Crane B.H., Maish T.L., Maddox Y.T., et al. Effect of prostaglandin I2 and analogs on platelet aggregation and smooth muscle contraction. J Pharmacol Exp Ther. 1978;206:132–138. [PubMed] [Google Scholar]
- 78.Smyth E.M., Grosser T., Wang M., et al. Prostanoids in health and disease. J Lipid Res. 2009;50(suppl):S423–S428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clapp L.H., Gurung R. The mechanistic basis of prostacyclin and its stable analogues in pulmonary arterial hypertension: role of membrane versus nuclear receptors. Prostaglandins Other Lipid Mediat. 2015;120:56–71. doi: 10.1016/j.prostaglandins.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 80.McLaughlin V.V., Gintner D.E., Panella M.M., et al. Reduction in pulmonary vascular resistance with long-term epoprostenol (prostacyclin) therapy in primary pulmonary hypertension. N Engl J Med. 1998;338:273–277. doi: 10.1056/NEJM199801293380501. [DOI] [PubMed] [Google Scholar]
- 81.Maron B.A., Leopold J.A. The role of the renin-angiotensin-aldosterone system in the pathobiology of pulmonary arterial hypertension (2013 Grover Conference series) Pulm Circ. 2014;4:200–210. doi: 10.1086/675984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang F., Chen A., Pan Y., et al. Research progress on pulmonary arterial hypertension and the role of the angiotensin converting enzyme 2-angiotensin-(1-7)-Mas axis in pulmonary arterial hypertension. Cardiovasc Drugs Ther. 2021;36:363–370. doi: 10.1007/s10557-020-07114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Forrester S.J., Booz G.W., Sigmund C.D., et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98:1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fried N.D., Morris T.M., Whitehead A., et al. Angiotensin II type 1 receptor mediates pulmonary hypertension and right ventricular remodeling induced by inhaled nicotine. Am J Physiol Heart Circ Physiol. 2021;320:H1526–H1534. doi: 10.1152/ajpheart.00883.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peng Y., Yang X., Li H., et al. Salt-contaminated water inducing pulmonary hypertension and kidney damage by increasing Ang II concentration in broilers. Environ Sci Pollut Res Int. 2022;29(1):1134–1143. doi: 10.1007/s11356-021-13358-y. [DOI] [PubMed] [Google Scholar]
- 86.Shao X., Dong X., Cai J., et al. Oxygen enrichment ameliorates cardiorespiratory alterations induced by chronic high-altitude hypoxia in rats. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.616145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang H., Chang C.Y., Lee H.J., et al. Magnolol ameliorates pneumonectomy and monocrotaline-induced pulmonary arterial hypertension in rats through inhibition of angiotensin II and endothelin-1 expression. Phytomedicine. 2018;51:205–213. doi: 10.1016/j.phymed.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 88.Shen H., Zhang J., Wang C., et al. MDM2-mediated ubiquitination of angiotensin-converting enzyme 2 contributes to the development of pulmonary arterial hypertension. Circulation. 2020;142:1190–1204. doi: 10.1161/CIRCULATIONAHA.120.048191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma R.K., Oliveira A.C., Yang T., et al. Gut pathology and its rescue by ACE2 (angiotensin-converting enzyme 2) in hypoxia-induced pulmonary hypertension. Hypertension. 2020;76:206–216. doi: 10.1161/HYPERTENSIONAHA.120.14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee S.L., Wang W.W., Lanzillo J.J., et al. Regulation of serotonin-induced DNA synthesis of bovine pulmonary artery smooth muscle cells. Am J Physiol. 1994;266:L53–L60. doi: 10.1152/ajplung.1994.266.1.L53. [DOI] [PubMed] [Google Scholar]
- 91.Welsh D.J., Harnett M., MacLean M., et al. Proliferation and signaling in fibroblasts: role of 5-hydroxytryptamine2A receptor and transporter. Am J Respir Crit Care Med. 2004;170:252–259. doi: 10.1164/rccm.200302-264OC. [DOI] [PubMed] [Google Scholar]
- 92.Hoyer D., Clarke D.E., Fozard J.R., et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 93.Launay J.M., Herve P., Callebert J., et al. Serotonin 5-HT2B receptors are required for bone-marrow contribution to pulmonary arterial hypertension. Blood. 2012;119:1772–1780. doi: 10.1182/blood-2011-06-358374. [DOI] [PubMed] [Google Scholar]
- 94.Launay J.M., Herve P., Peoc’h K., et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med. 2002;8:1129–1135. doi: 10.1038/nm764. [DOI] [PubMed] [Google Scholar]
- 95.Hood K.Y., Mair K.M., Harvey A.P., et al. Serotonin signaling through the 5-HT1B receptor and NADPH oxidase 1 in pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2017;37:1361–1370. doi: 10.1161/ATVBAHA.116.308929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Delaney C., Gien J., Roe G., et al. Serotonin contributes to high pulmonary vascular tone in a sheep model of persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol. 2013;304:L894–L901. doi: 10.1152/ajplung.00043.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li X., Zhu L.J., Lv J., Cao X. Purinoceptor: a novel target for hypertension. Purinergic Signal. Published online February 18, 2022 doi: 10.1007/s11302-022-09852-8. [DOI] [Google Scholar]
- 98.Cai Z., Tu L., Guignabert C., et al. Purinergic dysfunction in pulmonary arterial hypertension. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fullerton D.A., Jones S.D., Grover F.L., et al. Adenosine effectively controls pulmonary hypertension after cardiac operations. Ann Thorac Surg. 1996;61:1118–1123. doi: 10.1016/0003-4975(95)01149-8. [DOI] [PubMed] [Google Scholar]
- 100.Konduri G.G., Woodard L.L., Mukhopadhyay A., et al. Adenosine is a pulmonary vasodilator in newborn lambs. Am Rev Respir Dis. 1992;146:670–676. doi: 10.1164/ajrccm/146.3.670. [DOI] [PubMed] [Google Scholar]
- 101.Gui Y., Walsh M.P., Jankowski V., et al. Up4A stimulates endothelium-independent contraction of isolated rat pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2008;294:L733–L738. doi: 10.1152/ajplung.00403.2007. [DOI] [PubMed] [Google Scholar]
- 102.Horgan M.J., Pinheiro J.M., Malik A.B. Mechanism of endothelin-1-induced pulmonary vasoconstriction. Circ Res. 1991;69:157–164. doi: 10.1161/01.res.69.1.157. [DOI] [PubMed] [Google Scholar]
- 103.Ivey M.E., Osman N., Little P.J. Endothelin-1 signalling in vascular smooth muscle: Pathways controlling cellular functions associated with atherosclerosis. Atherosclerosis. 2008;199:237–247. doi: 10.1016/j.atherosclerosis.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 104.Iglarz M., Clozel M. Mechanisms of ET-1-induced endothelial dysfunction. J Cardiovasc Pharmacol. 2007;50:621–628. doi: 10.1097/FJC.0b013e31813c6cc3. [DOI] [PubMed] [Google Scholar]
- 105.Boulanger C., Luscher T.F. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sancho M., Klug N.R., Mughal A., et al. Adenosine signaling activates ATP-sensitive K+ channels in endothelial cells and pericytes in CNS capillaries. Sci Signal. 2022;15 doi: 10.1126/scisignal.abl5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Quinn K.V., Giblin J.P., Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res. 2004;94:1359–1366. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- 108.Galie N., Brundage B.H., Ghofrani H.A., et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119 doi: 10.1161/CIRCULATIONAHA.108.839274. 2894-U65. [DOI] [PubMed] [Google Scholar]
- 109.Nagaya N., Uematsu M., Oya H., et al. Short-term oval administration of L-arginine improves hemodynamics and exercise capacity in patients with precapillary pulmonary hypertension. Am J Respir Crit Care Med. 2001;163:887–891. doi: 10.1164/ajrccm.163.4.2007116. [DOI] [PubMed] [Google Scholar]
- 110.Barr F.E., Tirona R.G., Taylor M.B., et al. Pharmacokinetics and safety of intravenously administered citrulline in children undergoing congenital heart surgery: Potential therapy for postoperative pulmonary hypertension. J Thorac Cardiovasc Surg. 2007;134:319–326. doi: 10.1016/j.jtcvs.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 111.Granton J., Langleben D., Kutryk M.B., et al. Endothelial NO-synthase gene-enhanced progenitor cell therapy for pulmonary arterial hypertension: the PHACeT trial. Circ Res. 2015;117:645–654. doi: 10.1161/CIRCRESAHA.114.305951. [DOI] [PubMed] [Google Scholar]
- 112.Simon M.A., Vanderpool R.R., Nouraie M., et al. Acute hemodynamic effects of inhaled sodium nitrite in pulmonary hypertension associated with heart failure with preserved ejection fraction. JCI Insight. 2016;1 doi: 10.1172/jci.insight.89620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roy B., Mo E., Vernon J., et al. Probing the presence of the ligand-binding haem in cellular nitric oxide receptors. Br J Pharmacol. 2008;153:1495–1504. doi: 10.1038/sj.bjp.0707687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dasgupta A., Bowman L., D’Arsigny C.L., et al. Soluble guanylate cyclase: a new therapeutic target for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Clin Pharmacol Ther. 2015;97:88–102. doi: 10.1002/cpt.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schermuly R.T., Stasch J.P., Pullamsettl S.S., et al. Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur Respir J. 2008;32:881–891. doi: 10.1183/09031936.00114407. [DOI] [PubMed] [Google Scholar]
- 116.Ghofrani H.A., Galie N., Grimminger F., et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 117.Chester M., Seedorf G., Tourneux P., et al. Cinaciguat, a soluble guanylate cyclase activator, augments cGMP after oxidative stress and causes pulmonary vasodilation in neonatal pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2011;301:L755–L764. doi: 10.1152/ajplung.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lapp H., Mitrovic V., Franz N., et al. Cinaciguat (BAY 58-2667) improves cardiopulmonary hemodynamics in patients with acute decompensated heart failure. Circulation. 2009;119:2781–2788. doi: 10.1161/CIRCULATIONAHA.108.800292. [DOI] [PubMed] [Google Scholar]
- 119.Murray F., MacLean M.R., Pyne N.J. Increased expression of the cGMP-inhibited cAMP-specific (PDE3) and cGMP binding cGMP-specific (PDE5) phosphodiesterases in models of pulmonary hypertension. Br J Pharmacol. 2002;137:1187–1194. doi: 10.1038/sj.bjp.0704984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nagendran J., Archer S.L., Soliman D., et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 121.MacLean M.R., Johnston E.D., McCulloch K.M., et al. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J Pharmacol Exp Ther. 1997;283:619–624. [PubMed] [Google Scholar]
- 122.Hoeper M.M., Welte T. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2006;354:1091–1093. doi: 10.1056/NEJMc053442. [DOI] [PubMed] [Google Scholar]
- 123.Jing Z.C., Yu Z.X., Shen J.Y., et al. Vardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2011;183:1723–1729. doi: 10.1164/rccm.201101-0093OC. [DOI] [PubMed] [Google Scholar]
- 124.Corbin J.D., Francis S.H. Molecular biology and pharmacology of PDE-5-inhibitor therapy for erectile dysfunction. J Androl. 2003;24:S38–S41. doi: 10.1002/j.1939-4640.2003.tb02744.x. [DOI] [PubMed] [Google Scholar]
- 125.Hu L., Li L., Chang Q., et al. Discovery of novel pyrazolo[3,4-b] pyridine derivatives with dual activities of vascular remodeling inhibition and vasodilation for the treatment of pulmonary arterial hypertension. J Med Chem. 2020;63:11215–11234. doi: 10.1021/acs.jmedchem.0c01132. [DOI] [PubMed] [Google Scholar]
- 126.Hiremath J., Thanikachalam S., Parikh K., et al. Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trial. J Heart Lung Transplant. 2010;29:137–149. doi: 10.1016/j.healun.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 127.Waxman A., Restrepo-Jaramillo R., Thenappan T., et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. 2021;384:325–334. doi: 10.1056/NEJMoa2008470. [DOI] [PubMed] [Google Scholar]
- 128.White R.J., Jerjes-Sanchez C., Bohns GM Meyer, et al. Combination therapy with oral treprostinil for pulmonary arterial hypertension. A double-blind placebo-controlled clinical trial. Am J Respir Crit Care Med. 2020;201:707–717. doi: 10.1164/rccm.201908-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hirata T., Narumiya S. Prostanoids as regulators of innate and adaptive immunity. Adv Immunol. 2012;116:143–174. doi: 10.1016/B978-0-12-394300-2.00005-3. [DOI] [PubMed] [Google Scholar]
- 130.Asaki T., Kuwano K., Morrison K., et al. Selexipag: an oral and selective IP prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. J Med Chem. 2015;58:7128–7137. doi: 10.1021/acs.jmedchem.5b00698. [DOI] [PubMed] [Google Scholar]
- 131.Nagata T., Uehara Y., Hara K., et al. Thromboxane inhibition and monocrotaline-induced pulmonary hypertension in rats. Respirology. 1997;2:283–289. doi: 10.1111/j.1440-1843.1997.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 132.Horiguchi T., Enzan K., Mitsuhata H., et al. Heparin-protamine complexes cause pulmonary hypertension in goats. Anesthesiology. 1995;83:786–791. doi: 10.1097/00000542-199510000-00018. [DOI] [PubMed] [Google Scholar]
- 133.Kataoka M., Nagaya N., Satoh T., et al. A long-acting prostacyclin agonist with thromboxane inhibitory activity for pulmonary hypertension. Am J Respir Crit Care Med. 2005;172 doi: 10.1164/rccm.200501-102OC. 1575-1280. [DOI] [PubMed] [Google Scholar]
- 134.Prie S., Stewart D.J., Dupuis J. Endothelin, receptor blockade improves nitric oxide-mediated vasodilation in monocrotaline-induced pulmonary hypertension. Circulation. 1998;97:2169–2174. doi: 10.1161/01.cir.97.21.2169. [DOI] [PubMed] [Google Scholar]
- 135.Dupuis J., Prie S. The ETA-receptor antagonist LU 135252 prevents the progression of established pulmonary hypertension induced by monocrotaline in rats. J Cardiovasc Pharmacol Ther. 1999;4:33–39. doi: 10.1177/107424849900400106. [DOI] [PubMed] [Google Scholar]
- 136.Prie S., Leung T.K., Cernacek P., et al. The orally active ETA receptor antagonist (+)-(S)-2-(4,6-dimethoxy-pyrimidin-2-yloxy)-3-methoxy-3,3-diphenyl-propionic acid (LU 135252) prevents the development of pulmonary hypertension and endothelial metabolic dysfunction in monocrotaline-treated rats. J Pharmacol Exp Ther. 1997;282:1312–1318. [PubMed] [Google Scholar]
- 137.Abman S.H. Role of endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Annu Rev Med. 2009;60:13–23. doi: 10.1146/annurev.med.59.110106.212434. [DOI] [PubMed] [Google Scholar]
- 138.Dai Y., Chen X., Song X., et al. Immunotherapy of endothelin-1 receptor type A for pulmonary arterial hypertension. J Am Coll Cardiol. 2019;73:2567–2580. doi: 10.1016/j.jacc.2019.02.067. [DOI] [PubMed] [Google Scholar]
- 139.Walther D.J., Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 140.Eddahibi S., Guignabert C., Barlier AM-Mur, et al. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension—critical role for serotonin-induced smooth muscle hyperplasia. Circulation. 2006;113:1857–1864. doi: 10.1161/CIRCULATIONAHA.105.591321. [DOI] [PubMed] [Google Scholar]
- 141.Morecroft I., White K., Caruso P., et al. Gene therapy by targeted adenovirus-mediated knockdown of pulmonary endothelial Tph1 attenuates hypoxia-induced pulmonary hypertension. Mol Ther. 2012;20:1516–1528. doi: 10.1038/mt.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ciuclan L., Hussey M.J., Burton V., et al. Imatinib attenuates hypoxia-induced pulmonary arterial hypertension pathology via reduction in 5-hydroxytryptamine through inhibition of tryptophan hydroxylase 1 expression. Am J Respir Crit Care Med. 2013;187:78–89. doi: 10.1164/rccm.201206-1028OC. [DOI] [PubMed] [Google Scholar]
- 143.Aiello R.J., Bourassa P.A., Zhang Q., et al. Tryptophan hydroxylase 1 inhibition impacts pulmonary vascular remodeling in two rat models of pulmonary hypertension. J Pharmacol Exp Ther. 2017;360:267–279. doi: 10.1124/jpet.116.237933. [DOI] [PubMed] [Google Scholar]
- 144.Daniell H., Mangu V., Yakubov B., et al. Investigational new drug enabling angiotensin oral-delivery studies to attenuate pulmonary hypertension. Biomaterials. 2020;233 doi: 10.1016/j.biomaterials.2019.119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hemnes A.R., Rathinasabapathy A., Austin E.A., et al. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur Respir J. 2018;51 doi: 10.1183/13993003.02638-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Simon M.A., Hanrott K., Budd D.C., et al. An open-label, dose-escalation study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of single doses of GSK2586881 in participants with pulmonary arterial hypertension. Pulm Circ. 2022;12 doi: 10.1002/pul2.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang R., Su H., Ma X., et al. MiRNA let-7b promotes the development of hypoxic pulmonary hypertension by targeting ACE2. Am J Physiol Lung Cell Mol Physiol. 2019;316:L547–L557. doi: 10.1152/ajplung.00387.2018. [DOI] [PubMed] [Google Scholar]
- 148.Wang P., Deng J., Zhang Q., et al. Additional use of prostacyclin analogs in patients with pulmonary arterial hypertension: a meta-analysis. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.817119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Galie N., Barbera J.A., Frost A.E., et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373:834–844. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 150.Burks M., Stickel S., Galie N. Pulmonary arterial hypertension: combination therapy in practice. Am J Cardiovasc Drugs. 2018;18:249–257. doi: 10.1007/s40256-018-0272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chin K.M., Sitbon O., Doelberg M., et al. Three- versus two-drug therapy for patients with newly diagnosed pulmonary arterial hypertension. J Am Coll Cardiol. 2021;78:1393–1403. doi: 10.1016/j.jacc.2021.07.057. [DOI] [PubMed] [Google Scholar]