Abstract
Excess nutrient flux into the cellular energy system results in a scenario of cellular metabolic stress in diseases involving insulin resistance, such as type 2 diabetes, referred to as nutri-stress and results in cellular bioenergetic imbalance, which leads to insulin resistance and disease. Under nutri-stress, the heat shock response system is compromised due to metabolic abnormalities that disturb energy homeostasis. Heat shock proteins (HSPs) are the chief protectors of intracellular homeostasis during stress. Heat shock response (HSR) impairment contributes to several metabolic pathways that aggravate chronic hyperglycaemia and insulin resistance, highlighting a central role in disease pathogenesis. This article discusses the role of nutri-stress-related molecular events in causing insulin resistance and the nature of the roles played by heat shock proteins in some of the crucial checkpoints of the molecular networks involved in insulin resistance. Ample evidence suggests that the heat shock machinery regulates critical pathways in mitochondrial function and energy metabolism and that cellular energy status highly influences it. Weakening of HSPs, therefore, leads to loss of their vital cytoprotective functions, propagating nutri-stress in the system. Further research into the mechanistic roles of HSPs in metabolic homeostasis will help widen our understanding of lifestyle diseases, their onset, and complications. These inducible proteins may be crucial to attenuating lifestyle risk factors and disease management.
Keywords: Metabolic flexibility, Heat shock protein, Sirtuin, Oxidative stress, Mitochondrial dysfunction, Insulin resistance
Introduction
Hyperglycaemia has chronic harmful effects on diabetes. Glycaemic control, if not started at a very early stage of the disease, cannot wholly suppress complications. Evolutionarily, the human body has adapted to nutrient resource scarcity and high activity levels and has not entirely adjusted to the rapidly changing lifestyle, where activity has grown minimum and food is abundant. As our system is forced to deal with this nutrient surplus state, the response in the form of metabolic reprogramming manifests as the lifestyle diseases seen today, like diabetes (Hooper & Hooper 2009). This state of overnutrition induces stress in the system and culminates in dysfunction and disease. Excess nutrient-induced cellular stress, an effect of caloric abundance due to lesser expenditure or greater intake, is specifically called nutri-stress (Prentki et al. 2020). It has been linked to chronic low-grade inflammation (CLGI) or sterile stress-induced inflammation, a well-known feature of insulin resistance and a hallmark of metabolic syndrome and lifestyle diseases.
The literature suggests that the fulcrum in most diseases associated with lifestyle appears to be the dysregulation of stress response machinery. It propagates the effects of various stressors such as oxidative, nitrosative, carbonyl, and endoplasmic reticular stress, and genotoxic stressors unchecked, promoting nutri-stress. This review briefly discusses nutri-stress and its role in the onset of insulin resistance. This work aims to describe evidence regarding the implication of heat shock response at the beginning of insulin resistance and discuss the association between dysregulation of HSP and lifestyle factors contributing to insulin resistance. Therefore, our discussion is centred on highlighting the implication of HSP and its significance in cellular and molecular events altered by the effects of overnutrition and sedentary lifestyle that have a potentially causal role in the onset of insulin resistance. We have begun the narrative with discussions on the role of lifestyle factors in altering the energetic dynamics of the system, following with the impact upon mitochondria. The various mitochondrial events during and preceding insulin resistance are briefly discussed. Finally, the interlinking of lifestyle factor-associated cellular events with heat shock response is discussed in detail, focusing on insulin resistance.
Nutri-stress and low aerobic capacity—factors preceding insulin resistance
This section briefly discusses the onset of insulin resistance due to gluco- and lipo-toxicity. An appraisal of the events leading up to insulin resistance is required to set the perspective on the factors associated with and the significance of cellular energetics-related mitochondrial changes in insulin resistance.
Insulin resistance (IR) in any pathology is preceded by a state of impaired glucose tolerance, followed by a rise in insulin secretion, i.e. hyperinsulinaemia, to compensate for the impaired tolerance to glucose (Kahn et al. 2014). Hyperinsulinaemia is one of the earliest signs of metabolic syndrome leading up to type 2 diabetes mellitus (T2DM). Long-term exposure to high glucose in the presence of high insulin was found to impair insulin-stimulated glucose transport in human and rat adipocytes (Renström et al. 2007). Hyperinsulinaemia thus aggravates the intolerance of cells towards glucose. Christopher J. Nolan’s (2015, 2019) work suggested that the peripheral impairment of insulin action is a protective event occurring under chronic nutri-stress to mitigate nutri-stress-induced dysfunction of cells. Insulin resistance may be brought into effect to prevent the insulin-mediated entry of glucose or fatty acids into peripheral cells. The chronic nutri-stress, i.e. gluco- and lipo-toxicity, disrupts the fuel homeostasis in the cell, and the cells thus respond by preventing their further entry (Nolan et al. 2015; Nolan & Prentki 2019).
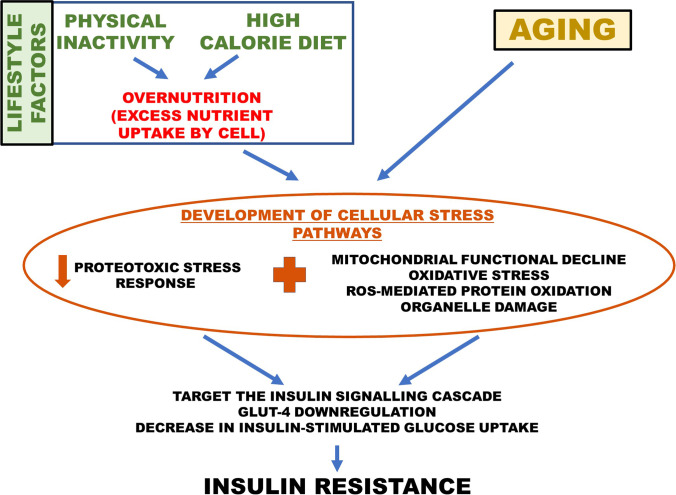
Altered energy homeostasis could have a substantial impact on the mitochondria, the powerhouse of the cell. This impact is known to result in the development of metabolic inflexibility. It is the loss of ability to switch between glucose and lipids as fuels for substrate oxidation, depending on the fuel availability, i.e. in fasting and fed state. Metabolic flexibility is a function critically dependent on mitochondrial functional stability, and its loss under chronic nutri-stress could be detrimental to the system. The phenomenon of metabolic inflexibility has been appreciated in the pre-diabetic stage of type 2 diabetes mellitus (Færch & Vaag 2011). Gluco- and lipo-toxicity at a chronic level, resulting in altered energetic dynamics in the cell, could be attributed to the development of metabolic inflexibility, which culminates in the failure of the system to cope with nutri-stress and results in insulin resistance (Fig. 1). Apart from nutrient toxicity resulting from overnutrition, sedentary behaviour is also a significant cause of metabolic inflexibility (Rynders et al. 2018). The Maastricht Study observed that sedentary individuals were metabolically unhealthy compared to their healthy peers, irrespective of obesity (de Rooij et al. 2016). It is attributed to low aerobic capacity, i.e. mitochondrial oxidative capacity, and reduced ATP production by the mitochondria (Distefano et al. 2018; Rovira-Llopis et al. 2017). Low aerobic capacity has been associated with an increased mortality rate in middle-aged men in a longitudinal study of 45-year follow-up (Ladenvall et al. 2016). It could not be fully restored with exercise in older adults and exhibited an attenuated increase compared to the increase seen in younger adults. With the increase in age, lack of physical activity at younger age seems to manifest as an impaired ability to regain significant aerobic capacity and could be linked to survival.
Fig. 1.
Outline of the onset of insulin resistance in ageing and lifestyle disease
Lowering aerobic capacity could be another manifestation of metabolic inflexibility resulting from lack of physical activity. Evidence suggests that overnutrition and sedentary lifestyle altogether increase nutrient flux into the cell, leading to low aerobic capacity and nutri-stress. The resulting loss of metabolic flexibility of the mitochondria could lead to its dysfunction and abnormal biogenesis. The decline in mitochondrial function is a vital phenomenon common to ageing, sedentary lifestyle, and T2DM (Kelley et al. 2002; Montaigne et al. 2014). However, lifestyle impacts mitochondrial aerobic capacity more than ageing (Rimbert et al. 2004). Concomitantly, mitochondrial function was comparable in young and old subjects lacking exercise. Post-exercise, there was an improvement in the major protein markers of mitochondrial biogenesis and activity, independent of age (Konopka et al. 2014). Thus, the decline of mitochondrial function observed in lifestyle-associated metabolic disorders could be strongly linked to the development of metabolic inflexibility due to nutrient excess.
The link between low aerobic capacity, the inability to restore it, and the possible relationship to ageing and survival in metabolically unhealthy people are crucial to understanding the factors involved in the onset of insulin resistance in individuals predisposed by lifestyle. Several studies have previously revealed the interplay between low aerobic capacity and the development of insulin resistance. Increasing aerobic capacity with physical activity could vastly improve the age-associated decline of insulin sensitivity (Bunprajun et al. 2013; Zając-Gawlak et al. 2021). Amati et al. (2009) compared insulin sensitivity between young and old subjects matched for their activity levels and observed no difference. However, they observed a significant decrease in insulin sensitivity in sedentary individuals compared to active individuals, irrespective of age. Insulin sensitivity declined with physical activity level and not with ageing (Amati et al. 2009). Physical exercise has also been shown to improve glycaemic control and reduce oxidative stress, limiting insulin resistance in T2DM (Konopka et al. 2015; Nojima et al. 2008).
It should be noted here that the protective effects of exercise on deterring metabolic inflexibility, mitochondrial dysfunction, and ultimately insulin resistance show that a potential metabolic regulator is involved in the molecular underpinnings of the cellular bioenergetic pathways. Evidence has been presented here about the impact of physical activity on ageing and insulin resistance. This suggests that the association between insulin resistance and ageing, as well as insulin resistance and nutri-stress, has an apparent commonality. As noted above, this has an implication for survival and longevity, which could be linked to lifestyle factors (Ladenvall et al. 2016). The significance of the interlinking factor and its involvement in metabolic disease will be detailed in the coming sections.
Nutri-stress leads to oxidative stress via mitochondrial dysfunction
The previous section discussed altered energy dynamics and subsequent metabolic inflexibility in mitochondrial dysfunction. Here, we briefly discuss the mechanistic aspects involved and the evidence revealing the role of nutrient excess in mitochondrial dysfunction and its significance in insulin resistance.
Oxidative stress (OS) is well implicated in insulin resistance and CLGI (Hurrle & Hsu 2017). In insulin-resistant and T2DM patients, perturbations of the genes responsible for protection against oxidative stress have been reported (Alibegovic et al. 2010; Cheng et al. 2011). Many mechanisms of high glucose intake inducing OS in the system have been elucidated. Oxidative stress-related genes are also involved in physical inactivity-induced insulin resistance (Alibegovic et al. 2010). ROS generation was found to be increased in healthy subjects after the ingestion of high-carbohydrate meal (Ehlers et al. 2014; Mohanty et al. 2000). Their healthy ADS ensures that the ROS increase is only transient. However, during nutrient excess, the ROS production is more and leads to prolonged activation of nuclear factor kappaB (NK-κB), amongst other molecules, and promotes its translocation to the nucleus (McKeegan et al. 2021; Nakajima & Kitamura 2013). This post-prandial increase in ROS indicates a possible feedback mechanism for preventing excess entry of glucose into cells. NF-κB is a known central effector molecule of ROS-mediated inflammatory pathways. It transcriptionally activates c-Jun N-terminal kinase (JNK) and inhibitor of kappaB kinase-β (IKK-β), the two central enzymes impairing the insulin signalling cascade. They lead to the suppression of GLUT-4 (glucose transporter-4) expression and prevent further glucose entry into the cell (Newsholme et al. 2019).
Studies done on ageing models of insulin resistance have also reported similar findings. Gupte et al. (2008) and Mylabathula et al. (2006) showed that in human muscular tissue, the increased expression and activation of three important stress kinases: JNK, IKKβ, and glycogen synthase kinase-3β (GSK-3β), with the increase in age (Gupte et al. 2008; Mylabathula et al. 2006). JNK and IKKβ are primarily involved in the suppression of insulin receptor substrate (IRS-1), thus leading to insulin resistance (Newsholme et al. 2019), and are mainly expressed under the influence of metabolic stress due to nutrient excess. GSK-3β is a known down-regulator of heat shock factor (HSF1), the transcriptional activator of the heat shock response (Wang et al. 2020). These molecules are highly expressed in metabolic diseases, mainly T2DM. Thus, age-associated IR could also be due to oxidative stress resulting from several pathways.
Impaired mitophagy and resulting dysregulation of mitochondrial fission–fusion dynamics is a major cause of intracellular oxidative stress (Sun et al. 2022). This is closely tied to cellular metabolic status, reflected by molecules such as AMPK and sirtuins (Rovira-Llopis et al. 2017). Although several mechanisms exist for the onset of oxidative stress, nutrient toxicity due to overnutrition is a significant mediator of oxidative stress, precipitated via mitochondrial dysfunction. An interesting observation was made by Houstis et al. (2006) when they triggered insulin resistance in human adipocytes. They observed the onset of oxidative stress long before any detectable level of insulin resistance developed (Houstis et al. 2006). This suggests that oxidative stress precedes the development of insulin resistance. Yu et al. (2006) observed that high glucose in the cell results in ROS generation by inducing changes in mitochondrial dynamics. Alterations in mitochondrial morphology and fragmentation preceded ROS generation under hyperglycaemic stress (Yu et al. 2006). Another report on weight-discordant twin pairs observed similar results in obesity. They stated that, in type 2 diabetes and other IR-related metabolic conditions, nutrient-induced mitochondrial morphological impairment and downregulation of oxidative metabolism-associated gene expression occurred at the sub-clinical stage and before the onset of other metabolic complications (Heinonen et al. 2015). Thus, high glucose in the cell sets the stage for impaired mitochondrial oxidative functions, resulting in a decrease in mitochondrial oxygen consumption, which increases free radical levels, ultimately triggering oxidative stress-associated molecular events (Heinonen et al. 2015). Due to nutrient excess, increased glycolytic and free fatty acid (FFA) flux occurs. The excess reducing equivalents generated require conversion to ATP, causing an overload of the mitochondrial oxidative pathways and electron transport complex. This event results in high mitochondrial membrane potential, with subsequent electron leaks across the membrane and, notably, ROS generation (Veeranki et al. 2016). Loss of mitochondrial respiratory complex activity leads to increased mitochondrial and cellular free radicals. The overproduction of ROS eventually overrides the cell’s antioxidant defence system (ADS), leading to OS (Capó et al. 2020; Muoio 2014). This further affects vital mitochondrial proteins, and many are known to be downregulated in T2DM (Diaz-Morales et al. 2016). It is also involved in the onset of organelle degradation, especially OS-mediated ER and mitochondrial apoptosis, via caspase-9 and 12, respectively. This has been reported to result in high glucose-mediated cardiomyocyte death in diabetic cardiomyopathy (Zhou et al. 2018a, b).
In concluding this section, it could be said that several molecular factors involved in nutri-stress-associated insulin resistance are also altered in ageing models of IR. Thus, overnutrition and a sedentary lifestyle result in nutri-stress, which could, in a manner of saying, accelerate the molecular mechanisms of ageing, where a decline in aerobic capacity is demonstrated. The main outcomes are loss of energy homeostasis and oxidative stress. Prolonged oxidative stress occurring under nutri-stress is important in developing insulin resistance. The cytoprotective ADS is deterred under nutri-stress, suggesting that there could be mechanisms required during stress that are potentially being downregulated in nutri-stress conditions, resulting in insulin resistance. Figure 2 illustrates the interaction of regulatory metabolic pathways in the onset of nutri-stress to insulin resistance.
Fig. 2.
Interactions of vital metabolic pathways that lead to insulin resistance in nutri-stress. Nutrient excess due to external lifestyle factors leads to metabolic inflexibility and dysregulation of the bioenergetic chemical signals, i.e. NAD/NADH ratio and AMP/ATP ratio. The onset of oxidative stress occurring parallel to excess oxidation in mitochondria, along with the decreased NAD and increased ATP, jointly involves in the dysregulation of the heat shock response. The blue-coloured portions represent the canonical HSP expression pathway; the grey-coloured represents the oxidative stress-associated pro-inflammatory NF-kB pathway and their interactions with the insulin signalling cascade (green portion) and HSP pathway. GSK-3beta is also included for its role in oxidative stress and HSP repression
Molecular chaperones in metabolic homeostasis, insulin resistance, and senescence
Heat shock proteins (HSPs), i.e. chaperone proteins, are engaged in a cell defence mechanism known as heat shock response (HSR) or proteotoxic stress response (PSR). They sequester proteins that are damaged or misfolded due to stress and refold them or trigger their elimination. This prevents the misfolded protein aggregates from triggering pro-inflammatory and auto-apoptotic pathways, thereby protecting them from cell damage and death, ultimately preserving cell lifespan. HSPs are physiologically involved in maintaining cellular homeostasis, even in the absence of stressors (Cornelius et al. 2013).
The proteostatic role of HSPs in preserving cellular homeostasis is vital for delayed senescence and longevity. In the skeletal muscle of ageing Vervet monkeys, high basal tissue levels of HSP-70 led to the preservation of tissue insulin sensitivity (Chichester et al. 2015). Retainment of insulin sensitivity could thus be a vital aspect of longevity and may be protected by HSPs, contributing to the ablation of senescence-associated diseases. Senescent cells are also potential drivers of chronic metabolic disorders (Kirkland & Tchkonia 2017). HSP72 downregulation triggers cell senescence via both p53-dependent and p53-independent pathways. Senescence is characterised by oxidative stress-induced damage. In this regard, HSP72 is involved in the modulation of focal adhesion kinase activity, c-Met, and Akt (Radons 2016). Thus, the delay of senescence or ageing, which potentially includes preservation of insulin sensitivity, is a principal physiological purpose of HSPs.
The cytoprotective roles of HSPs, under stress, include the regulation of apoptotic processes and mechanisms of autophagy. HSP-70 stabilises the lysosomal membrane and prevents lysosomal hydrolases from being released into the cytosol, where they can cause apoptosis-like programmed cell death. This is supported by the discovery of increased lysosomal cathepsin activity in neuronal cells with extra-lysosomal localisation (Zhu et al. 2014). They help to prevent ageing or premature ageing and may also help reduce the onset of metabolic dysfunction, mainly associated with insulin resistance.
In IR-associated metabolic conditions, especially in type 2 diabetes, the proteostatic stress response is impaired. HSP72 and several essential HSPs are downregulated. In T2DM, intracellular HSP72 expression is decreased and reported in humans as well as animal models (Bruce et al. 2003; Chung et al. 2008; Kavanagh et al. 2009; Rodrigues-Krause et al. 2012; Rogers et al. 2016). Type 2 diabetes patients are also known to have dysregulated autophagy mechanisms. This could be partly due to the lack of cytoprotective effect of HSR (Henriksen et al. 2019). Not many works have discussed the centrality of the role played by HSPs in insulin resistance and lifestyle-associated metabolic disorders. However, as we will see in later sections, dysregulation of HSR could be occurring in tandem with the early events implicated in the onset of insulin resistance. We discuss reports showing the close association of HSP expression with metabolic status and its instrumental role in preserving mitochondrial stability, thereby insulin sensitivity. A slew of articles has shown the benefits of exercise on improving insulin resistance and longevity in individuals with metabolic diseases. We propose that the heat shock machinery could be involved in these events, and the protection of HSPs by lifestyle modification could be the underlying protective factor in disease management. The significance given to HSR has to be, therefore, discussed greatly in terms of the onset and progression of insulin resistance.
Literature suggests that impairment of HSP machinery could be attributed to the effects of excess nutrient intake in metabolic disorders. The resulting loss of intracellular homeostasis, which we broadly refer to as nutri-stress, is the driving factor which sustains the vicious cyclical process of mitochondrial dysfunction and insulin resistance. HSP expression can be attributed to its trans-activator, HSF-1. HSF-1 activation is closely tied to regulators of metabolic flexibility, mainly sirtuins, AMPK, peroxisome proliferator-activated receptor (PPAR), and peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α). These molecules are central to the sustenance of metabolic flexibility, and their significance in the maintenance of HSP response will be detailed in oncoming sections. The association of these proteins with metabolic homeostasis implicates that proteostasis is governed or regulated by the cellular metabolic status.
Impairment of HSP expression is linked to altered cellular energetic status under nutri-stress
Sirtuins, being sensors of metabolic status, drive cytoprotection during excess nutrient stress by stimulating the PSR via HSF1. In response to stress, SIRT1 (sirtuin 1) promotes HSF1 expression and activity. It enhances the DNA-binding activity of HSF1 by maintaining it in the deacetylated state (Westerheide et al. 2009). This enables HSF1 to bind stably to the promoter sites of HSP genes and increase their expression. This was confirmed when SIRT1 knockdown could result in diminished HSR (Zheng et al. 2017). They are deacetylases involved in the metabolic reprogramming associated with senescence. Sirtuins are known to decline with age and in nutri-stress. Their downregulation strongly impacts HSF1 function, leading to HSP downregulation (de Kreutzenberg et al. 2010; Westerheide et al. 2009). In ageing and nutri-stress, the decline in the NAD+/NADH ratio in the cytosol occurs due to excess flux of nutrients into oxidative mitochondrial pathways (Fan et al. 2020). As NAD+ hydrolysis is essential for the deacetylase activity of sirtuins, a low NAD+/NADH ratio results in the downregulation of sirtuin function in nutri-stress. The same is observed in senescent cells, where restoration of the NAD levels, and thereby sirtuins, is associated with improved mitochondrial function, metabolic health, and delay in ageing (Okabe et al. 2019). Though SIRT1 modulates the expression of several transcription factors, such as FOXO and NF-κB (Raynes et al. 2013), its impact on HSF1 and thereby upon HSR potentially offsets the survival potential of the cell under stress (Purwana et al. 2017) (Fig. 2).
Physical activity and calorie restriction studies have helped in highlighting the functions of HSPs and sirtuins regarding their association with modulation of cellular function according to energy demands and activity-induced stress. In exercising individuals, the expression of HSP72 was reportedly improved when coupled with caloric restriction. It was associated with decreased oxidative stress parameters (Capó et al. 2020; Dimauro et al. 2016). A recent study on type 2 diabetic animal models demonstrated that HSP72 expression was higher in exercising animals than in sedentary ones and associated with improved glucose tolerance. Sedentary individuals had significantly lower expression of HSP72, coupled with poor glycaemic tolerance. Exercise could, therefore, mediate a healthy induction of the HSR, which could be implicated in the protection against nutri-stress-induced oxidative stress, thus preventing insulin resistance (Tsuzuki et al. 2017). The experiment by Rogers et al. (2016) demonstrates the significance of low physical activity and intrinsic aerobic capacity on HSP induction when exposed to stress. They compared rats selectively bred for low-capacity running (LCR) and high-capacity running (HCR). LCR rats had a deficient expression of HSP compared to HCR rats. When exposed to a high-fat diet (HFD), they exhibited metabolic inflexibility, improper lipid handling, and loss of insulin sensitivity. The low HSP expression in LCR rats occurred with a corresponding decrease in sirtuin 1 and HSF-1, indicating their significance in metabolic inflexibility. However, the HCR rats were well protected against metabolic disturbances and exhibited a higher HSP expression, with whole-body insulin sensitivity, even on exposure to HFD. Heat treatment in LCR rats resulted in a blunted induction of HSR (compared to HCR), which worsened after HFD exposure. Physical inactivity could limit the HSP induction and result in the decline of aerobic capacity and mitochondrial content, as seen in the LCR rats. When these animals are subjected to HFD, they are not protected against metabolic stress and develop metabolic inflexibility. Heat treatment induced an attenuated HSP72 expression, with associated improvement in insulin sensitivity and fat accumulation. This sufficiently demonstrated the significance of intrinsic physical inactivity as a primer of HSR decline, which predisposed the system to further high nutrient-induced anomalies. Interestingly, HSP72 was found crucial for insulin sensitivity. When HSP72 induction was silenced, insulin-stimulated glucose uptake did not occur in both HCR and LCR rats (Rogers et al. 2016).
Anckar and Sistonen (2011)observed that dietary restriction could delay the progression of age-related diseases, such as cancer, cardiovascular diseases, and diabetes. Studies on the effect of calorie restriction in humans reported the preservation of healthy mitochondrial function in subjects (Anckar & Sistonen 2011; Capó et al. 2020). Due to nutri-stress, sirtuin-associated stress response mechanisms are shunted. However, reversal of the age-associated decline in HSF-1 activity could be achieved in cells by calorie restriction and involves restoring the stress response mechanism via sirtuins. This suggests that HSPs might be involved in mediating the downstream functions of sirtuins with respect to delaying the onset of ageing-associated metabolic diseases. Thus, metabolic energetic balance (NAD/NADH ratio) has direct regulatory control over the activation of the proteotoxic stress response. The need for HSR regulation by a metabolic stress sensor, i.e. sirtuins, implies the significance of HSR in metabolic homeostasis.
Cellular energy status reflected by the cytosolic AMP/ATP ratio is also involved in HSP expression and functions. As a result of impaired autophagy, functional cells lose their homeostatic control and can potentially develop the senescence-associated secretory phenotype (SASP). SASP could be involved in the sustenance of chronic low-grade inflammation and contribute to the vicious cycle of inflammation and insulin resistance, as seen in diabetic complications (Kirkland & Tchkonia 2017; Rea et al. 2018). In this respect, the involvement of autophagy-associated protein, mammalian target of rapamycin (mTOR), is crucial. Under exposure to any cellular stress, mTOR is usually suppressed (Su and Dai 2017). Short-term stress activates mTOR, whereas chronic or long-term stress inhibits its expression (Selvarajah et al. 2015). Oxidative stress due to mitochondrial ROS could induce mTOR activation via the PI3K-Akt pathway (Heberle et al. 2019; Kim et al. 2018). In conditions of excess nutrient intake, mTOR is upregulated and is associated with metabolic diseases such as metabolic-associated fatty liver disease (NAFLD). Under nutri-stress, the loss of metabolic flexibility leads to excessive glycolysis and decreased OXPHOS and fatty acid beta-oxidation. This decreases the intracellular AMP to ATP ratio, increasing mTOR expression. This is further followed by a decrease in autophagy, accumulation of intracellular lipids, and decreased expression of AMPK (Singh et al. 2020; Wang et al. 2022). These events are associated with the activation of inflammatory transcriptional factors, coupled with decreased autophagy, resulting in SASP. This major event is attributed to cell and organ dysfunction, resulting in ageing and disease complications.
The expression of AMPK in acute energetic and chronic stress is directly tied to mTOR inhibition when the AMP to ATP ratio is high (Gwinn et al. 2008). But, under nutri-stress, the AMP to ATP ratio is lowered due to increased glycolysis, causing suppression of AMPK and de-inhibition of mTOR (Sancak et al. 2007). HSP72 overexpression increased the expression and activity of AMPK (AMP-activated protein kinase) and SIRT1 in HSP72 + / + skeletal muscle of experimental animals (Chowdhury et al. 2011). HSP72 expression is also enhanced by mTOR and Akt1. Akt1 strongly stimulated HSF1 expression compared to mTOR or other factors (Lu et al. 2022). In tandem with mTOR expression, HSP expression could be induced by the cell under stress to protect against SASP-associated molecular events that might occur due to the effects of mTOR. Apart from chaperone-mediated and mTOR-mediated autophagic mechanisms, HSPs are also involved in ATG-associated autophagy.
LC3BII-LC3B1 ratio is a marker of ATG5/ATG7-mediated autophagy. As discussed earlier, the autophagic mechanisms in insulin resistance conditions are impaired, and consequently, the LC3BII-LC3B1 ratio is reduced. The cleavage of LC3I to form LC3II is an essential step in ATG5/7-associated macroautophagy. HSP72 expression is associated with increased autophagy, as observed by studies reporting elevation of the LC3BII-LC3B1 ratio in experimental conditions of insulin resistance (Song 2014). The impact of heat shock protein 72 expression on mitochondrial function and insulin action). Although the mechanisms involved are yet to be understood clearly, the result of this work shows that HSP72 is involved in delaying senescence. This function of HSP72 may play a role in ageing and IR-associated metabolic diseases. An experimental model of atherosclerosis also demonstrated the pro-autophagic, anti-inflammatory, antioxidant, and anti-hyperglycaemic effects, i.e. the anti-senescent function of the HSF1-HSP72 expression. Atherosclerotic mice exhibited increased pro-inflammatory profile, and insulin resistance, with decreased expression of SIRT1, HSF1, and several HSPs. When heat stimulation of HSP expression was performed, amelioration of SASP was observed. Induced HSF1 and HSP72 expression were accompanied by the decline of the pro-inflammatory protein expression, insulin resistance, oxidative stress, and improvement in the disease severity indices. HSP expression is strongly associated with anti-senescent pathways, which are crucial to mitigating the chronic low-grade inflammation associated with SASP development in cells resistant to insulin (Bruxel et al. 2019). Restoration of HSP response is therefore essential to delay senescence, improve autophagy, and improve longevity, which are disturbed due to the lifestyle factors contributing to insulin resistance.
Significance of HSPs in mitochondrial integrity under nutri-stress
Several HSP overexpression experiments in diabetic and ageing animal models have shown the vital role of HSP72 in maintaining organelle integrity. Its decline in ageing and metabolic disorders could be an essential factor for mitochondrial and ER stress.
Almost all organisms consist of a stress-sensitive protective system to (1) prevent damage to cellular components under stress and retain metabolic homeostasis and (2) dispose of damaged components and organelles impacted by the stressor, to prevent activation of apoptotic mechanisms and oxidative stress. At basal levels or under exposure to stress, eukaryotic cells utilise the heat shock response system or autophagy to perform these critical cytoprotective functions (Dokladny et al. 2015; Klionsky & Codogno 2013). In metabolic diseases, the impairment of HSP-mediated autophagic mechanisms and cytoprotective functions could be involved in mitochondrial dysfunction. Impairment of mitophagy is a significant aspect of mitochondrial dysfunction and is seen in insulin resistance. It is a major contributor to oxidative stress (Onyango 2018; Z. Su et al. 2019). In this regard, HSP72 has been proven to restore mitochondrial morphology in the hepatocyte model of non-alcoholic fatty liver disease (NAFLD) (Archer et al. 2018). It also restores AMPK expression and activity. Decreased AMPK is associated with increased mitochondrial fission and free radical generation. This HSP72-mediated restoration of AMPK could help re-establish the AMP to ATP levels by helping in mitochondrial preservation as well as restoration of OXPHOS enzyme activity (Chowdhury et al. 2011; Zhou et al. 2018a, b).
In an animal model, the skeletal muscle with depleted HSP72 expression exhibited impaired mitophagy due to decreased HSP72-mitofuscin complex formation. This complex is essential for the degradation of depolarised mitochondria and involves Parkin-mitofuscin2 interaction. Due to the depletion of HSP72, the chaperoning of mitofuscin2 into the mitochondria does not occur, preventing the Parkin-mitofuscin2 complex-mediated mitophagy. As a result, depletion of HSP72 impairs natural mitophagy leading to dysmorphic mitochondria and reduction in mitochondrial aerobic capacity, which are associated with insulin insensitivity and oxidative stress (Drew et al. 2014). Subjects with T2DM or obesity also exhibit lesser levels of HSP60 than lean individuals and athletes, in addition to increased mitochondrial fragmentation and impaired mitonuclear protein balance. The HSP60 levels were also positively correlated with aerobic capacity and insulin sensitivity in biopsied human skeletal muscle tissue (Houzelle et al. 2021). Overall, experimental evidence from exercise studies strongly points to the stress machinery’s protective role in preserving insulin sensitivity and metabolic fuel switching. This is a vital physiological role that has not received much traction in terms of molecular pathogenesis. The benefits of heat therapy, calorie restriction, and exercise as management modalities in lifestyle diseases, in both young and elderly, are already known. Some of the works reporting on the crucial involvement of HSPs in these benefits have been discussed in the “Impairment of HSP expression is linked to altered cellular energetic status under nutri-stress” section.
In earlier sections, we discussed the decline in mitochondrial metabolic enzymes and their activity in insulin resistance. At least in part, this can be attributed to the chaperoning function of HSPs, lost in nutri-stress. The upregulation of SIRT1 and AMPK due to overexpression of HSP72 facilitates the expression of PGC-1α, which promotes mitochondrial biogenesis and substrate oxidation through PPARs (Chowdhury et al. 2011). This promotion of mitochondrial activity is implicated in preventing the accumulation of free fatty acid (FFA) by increasing oxidative metabolism (Boutant & Cantó 2013). An outcome of the accumulated FFAs and their intermediates, such as diacyl-glycerol and ceramide, is the activation of pro-inflammatory and pro-oxidant JNK. HSP expression mitigates this event through its inhibitory effect on JNK expression (MohammadTaghvaei et al. 2012), resulting in the reduction of insulin resistance. HSP72 overexpression also improves the oxidative capacity of skeletal muscle mitochondria. Two major enzymes of mitochondrial oxidation, namely, beta-hydroxy acyl-CoA dehydrogenase and citrate synthase, exhibited maximum activity when HSP72 was overexpressed, suggesting its importance in promoting the aerobic capacity of mitochondria (Chung et al. 2008). In myocardial mitochondria, heat-induced HSP70 expression is associated with enhanced activity of cytochrome C oxidase, a key regulator of mitochondrial respiratory capacity (Vogt et al. 2019).
HSPs are crucial to preserving mitochondria, including their ultrastructure, biogenesis, membranal import complexes, respiratory complex activity, and metabolic enzyme functions. The significance of HSPs in these roles has been well documented in metabolic diseases and other non-metabolic diseases, such as ischemia–reperfusion injury, Alzheimer’s disease, and cardiomyopathy. HSPs have been well documented to improve the energetic capacity of mitochondria. Their expression is significant in improving the structure and respiratory complex activities of cardiac mitochondria (Sammut & Harrison 2003). HSP70 is also significant to the maintenance of the mitochondrial import complex. Under stress, proteins prone to aggregation in the cytosol are migrated to the mitochondrial intermembrane space and matrix, and their timely degradation depends on cytosolic HSP70 expression. Ruan et al. propose that this mitochondria-mediated proteostasis mechanism could link stress-mediated protein aggregation and mitochondrial dysfunction. Lack of HSPs could cause inefficiency of the mitochondrial import complex, allowing excess protein aggregates to accumulate inside the mitochondria, resulting in mitochondrial stress and dysfunction (Ruan et al. 2017). HSP-mediated preservation of mitochondrial enzyme activities and respiratory capacity has been linked to Alzheimer’s disease. HSP expression was found to prevent the release of intra-neuronal beta-amyloid and cytochrome c from the dysfunctional mitochondria and prevent caspase activation. HSPs elicited the protection of cell viability and ATP generation. The latter was attributed to the preservation of respiratory complex IV by HSP 60, 70, and 90 under the stress of beta-amyloid accumulation (Veereshwarayya et al. 2006). HSPs thus protect mitochondrial function to prevent the activation of apoptotic pathways under stress and in ageing. Their major role is to preserve respiratory chain activity, maintain mitochondrial import complex, and redox balance. Loss of this crucial protective machinery leads to irreversible damage to the cells, resulting in chronic inflammation as in metabolic conditions or misfolded protein accumulation and apoptotic events.
Successful clearance of dysfunctional cellular organelles and cells themselves is essential to delay ageing and ageing-associated degenerative changes in homeostasis. By preserving the activity and stability of several metabolic enzymes, HSPs help protects the cell against nutri-stress and prevent mitochondrial dysfunction.
Crosstalk between HSF-1 and PGC-1α under stress potentially protect against mitochondrial dysfunction and insulin resistance
PGC-1α is integral in maintaining mitochondrial oxidative processes, its biogenesis, and redox balance. Under physiological conditions, it can cause direct transcriptional repression of HSF-1 (Minsky & Roeder 2015). However, when cells are exposed to acute stress such as heat or lipopolysaccharide (LPS)-induced stress, PGC-1α has been found to transcriptionally cooperate with HSF-1, promoting the expression of antioxidant HSP-70, thereby protecting against oxidative damage (Dang et al. 2019; Xu et al. 2016). In line with these findings, PGC-1α-specific binding sites were found on the heat shock elements (HSE) of the HSP promoter sequence (Charos et al. 2012). Coincidentally, heat shock elements (HSE) were also present on the promoter of PGC-1α, where HSF1 and PGC-1α could physically co-occupy and upregulate PGC-1α expression (Ma et al. 2015). Thus, efficient crosstalk exists between HSR and PGC-1α in maintaining mitochondrial health during stress under the influence of Sirtuin signalling. This suggests that HSP transcription is vital to the efficient biogenesis of healthy mitochondria.
PGC-1α is essential for metabolic flexibility, which is lost in ageing and metabolic diseases. It is proposed to occur due to Sirtuin and AMPK inactivation triggered by altered NAD/NADH and AMP/ATP ratios in reaction to nutrient excess (Chowdhury et al. 2011). Heat stress induced due to physical activity upregulates HSPs, followed by PGC-1α and AMPK expression, resulting in increased respiratory capacity in human and animal skeletal muscle tissues (Hafen et al. 2018). Post-exercise, HSP60-mediated PGC-1α expression has been reported (Barone et al. 2016). PGC-1α expression is vital to the metabolic switching from incomplete to complete oxidation, i.e. improved fuel processing ability. PGC-1α overexpression was found to cause disposal of accumulated intermediates of incomplete fatty acid oxidation, alleviating excess nutrient-induced disturbance in energetic flux. These effects of PGC-1α were also observed post-exercise and suggest the PGC-1α-dependent mechanism in mediating exercise-induced restoration of metabolic flexibility (Koves et al. 2005). The interplay between PGC-1α and HSPs could be fundamental to this beneficial effect and highlight the role of HSPs in assisting metabolic flexibility and warrant future research involving interactions between HSPs and PGC-1α. HSPs are important regulatory proteins required for the protection of essential cellular processes, and therefore, an intricate crosstalk network exists in every vital checkpoint of cellular metabolism. This explains their diminished expression in several pathways associated with metabolic diseases and the cytoprotective effects elicited by their restoration.
HSPs in insulin signalling
As a result of oxidative stress prevailing in T2DM subjects with chronic hyperglycaemia, several pro-inflammatory proteins are upregulated and activated. In peripheral blood mononuclear cells of obese humans, an increase in active pJNK and pIKK-β, serine-phosphorylated IRS-1, with an associated decrease in GLUT-4 was observed compared to non-obese controls. But induction of HSP72 with heat exposure resulted in decreased pJNK, pIKK-β, and Ser-pIRS-1 (serine-phosphorylated insulin receptor substrate 1) (Simar et al. 2012). However, basal HSP72 expression is decreased in insulin-resistant human skeletal muscle, with overexpression of pJNK. HSP72, and its associated co-chaperone CHIP, regulate the functional state of JNK by modulating the expression of DLK (dual leucine zipper-bearing kinase) and MKP (MAP kinase phosphatase-1), the two proposed activators of JNK. Physiologically, HSP72 downregulates DLK and MKP, preventing phosphorylation of JNK and thereby preventing active JNK formation. Additionally, HSPs inhibit JNK activity by directly binding to it (Chow et al. 2009). Accordingly, in obese mice, Hsp72 overexpression resulted in decreased JNK phosphorylation, causing an improvement in insulin sensitivity (Chung et al. 2008). Kitano et al. (2019) also reported the onset of endoplasmic reticulum stress, pJNK upregulation, and suppression of insulin signalling coupled with glucose intolerance, leading to the onset of insulin resistance in hepatic cells of HSP72-KO mice (Kitano et al. 2019). Thus, intracellular HSP decline favours phosphorylation and activation of JNK to pJNK, resulting in the downregulation of GLUT-4, causing insulin resistance. With progression in age, HSPs decline, while JNK and IKKβ are increased (Gupte et al. 2008; Westerheide et al. 2009). This suggests a parallel of events occurring in ageing and metabolic disease, where risk factors of metabolic diseases could target and impair the HSR to impair insulin signalling, thus causing disease preemptively. Induction of HSP activity by exercise or caloric restriction could serve to ameliorate the insulin resistance conferred by nutri-stress-induced JNK overactivation (Hao et al. 2021; Kim et al. 2015; Rogers et al. 2016) and help restore mitochondrial function, to promote metabolic flexibility, against excess nutrient-induced damage. Therefore, loss of HSP response is an important component of metabolic inflexibility, leading to the decline in mitochondrial function, as both are regulated and maintained actively by the HSR. The significance of HSP72 in protecting insulin sensitivity is evident also from several mild electrical stimulation (MES) studies. Obese subjects exposed to MES and heat therapy exhibited an increase in HSP72 and AMPK, which was well associated with improvements in insulin sensitivity and glycaemic tolerance (Kondo et al. 2016). The downregulation of the HSP72 in T2DM could therefore be strongly associated with the development of oxidative stress and insulin resistance. A summarised diagram depicting the interaction of HSP with the above-discussed processes involved in insulin resistance is given in Fig. 3.
Fig. 3.
Role of heat shock proteins in nutri-stress. Cellular energetic signals, mainly AMP/ATP ratio and NAD/NADH ratio, are involved in regulating heat shock protein expression. The interplay between SIRT1, AMPK, HSP, and PGC-1α is central to the regulation of cellular energy status. HSP participates via its chaperone function by stabilising mitochondrial structural and functional integrity. It also helps suppress the activity of the NF-kB-associated pro-inflammatory pathway molecules. It directly suppresses the activation of IKKβ and JNK. However, the oxidative stress pathways in chronic stress diminish the HSP function by enabling the activation of GSK3β, which has known inhibitory roles towards HSP72 and HSF-1
Heat shock proteins are essential for cellular energetic homeostasis, organelle integrity, and maintenance of essential protein functions. These roles have made HSPs a crucial target affected by lifestyle-associated nutri-stress. Furthermore, with respect to insulin resistance, HSPs play a significant role in regulating enzymes of the signalling cascade and repression of proteins associated with decreased response to insulin. This direct role in preserving insulin signalling shows that HSPs are crucial to managing glucose intolerance in tissues sensitive to insulin. This could be the basis for the beneficial role of HSP restoration in decreasing insulin resistance.
Conclusion
This review has focused on the pathways involving HSPs that could be attributed to insulin resistance occurring due to lifestyle risk factors, which result in the nutrient-excess condition called nutri-stress in the system. The weakening of HSR due to metabolite derangement in overnutrition leaves the system vulnerable to nutri-stress, where the cytoprotective functions of HSPs are unavailable. To summarise, HSPs inactivate stress kinases in normal conditions and prevent ROS-mediated damage to mitochondrial and endoplasmic proteins during oxidative metabolism via their chaperone function. They cooperate with metabolic sensors such as sirtuins and PGC-1α and help restore metabolic homeostasis under stress. The fact that calorie restriction and exercise alleviate nutri-stress-induced insulin resistance and metabolic inflexibility, and possess HSPs as their central effectors, goes to show that a healthy HSR is vital for healthy ageing and metabolism.
Acknowledgements
We sincerely thank JIPMER, Puducherry, for providing the Intramural Funding required to carry out the research associated with this manuscript.
Author contribution
Jayashree Kuppuswami: conceptualization and writing—original draft preparation.
GP Senthilkumar: supervision and writing—reviewing and editing
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jayashree Kuppuswami, Email: k.jayashree.msc@gmail.com.
Gandhipuram Periyasamy Senthilkumar, Email: biosenthilkumar@gmail.com.
References
- Alibegovic AC, Sonne MP, Højbjerre L, Bork-Jensen J, Jacobsen S, Nilsson E, et al. Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. Am J Physiol. Endocrinol Metab. 2010;299(5):E752–E763. doi: 10.1152/ajpendo.00590.2009. [DOI] [PubMed] [Google Scholar]
- Amati F, Dubé JJ, Coen PM, Stefanovic-Racic M, Toledo FGS, Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care. 2009;32(8):1547–1549. doi: 10.2337/dc09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-09520. [DOI] [PubMed] [Google Scholar]
- Archer AE, Rogers RS, Von Schulze AT, Wheatley JL, Morris EM, McCoin CS, et al. Heat shock protein 72 regulates hepatic lipid accumulation. Am J Physiol. Regul Integr Comp Physiol. 2018;315(4):R696–R707. doi: 10.1152/ajpregu.00073.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone R, Macaluso F, Sangiorgi C, Campanella C, Marino Gammazza A, Moresi V, et al. Skeletal muscle heat shock protein 60 increases after endurance training and induces peroxisome proliferator-activated receptor gamma coactivator 1 α1 expression. Sci Rep. 2016;6:19781. doi: 10.1038/srep19781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutant M, Cantó C. SIRT1 metabolic actions: integrating recent advances from mouse models. Mol Metab. 2013;3(1):5–18. doi: 10.1016/j.molmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52(9):2338–2345. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- Bruxel MA, Tavares A, Zavarize Neto LD, de Souza BV, Schroeder HT, Bock, , et al. Chronic whole-body heat treatment relieves atherosclerotic lesions, cardiovascular and metabolic abnormalities, and enhances survival time restoring the anti-inflammatory and anti-senescent heat shock response in mice. Biochimie. 2019;156:33–46. doi: 10.1016/j.biochi.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Bunprajun T, Henriksen TI, Scheele C, Pedersen BK, Green CJ. Lifelong physical activity prevents aging-associated insulin resistance in human skeletal muscle myotubes via increased glucose transporter expression. PLoS ONE. 2013;8(6):e66628. doi: 10.1371/journal.pone.0066628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capó X, Martorell M, Ferrer MD, Sureda A, Pons V, Domingo JC, et al. Calorie restriction improves physical performance and modulates the antioxidant and inflammatory responses to acute exercise. Nutrients. 2020;12(4):930. doi: 10.3390/nu12040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charos AE, Reed BD, Raha D, Szekely AM, Weissman SM, Snyder M. A highly integrated and complex PPARGC1A transcription factor binding network in HepG2 cells. Genome Res. 2012;22(9):1668–1679. doi: 10.1101/gr.127761.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Siow RC, Mann GE. Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: a role for mitochondria and the nuclear factor-E2-related factor 2-Kelch-like ECH-associated protein 1 defense pathway. Antioxid Redox Signal. 2011;14(3):469–487. doi: 10.1089/ars.2010.3283. [DOI] [PubMed] [Google Scholar]
- Chichester L, Wylie AT, Craft S, Kavanagh K. Muscle heat shock protein 70 predicts insulin resistance with aging. J Gerontol - Ser A Biol Sci Med Sci. 2015;70(2):155–162. doi: 10.1093/gerona/glu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow AM, Steel R, Anderson RL. Hsp72 chaperone function is dispensable for protection against stress-induced apoptosis. Cell Stress Chaperones. 2009;14(3):253–263. doi: 10.1007/s12192-008-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SK, Dobrowsky RT, Fernyhough P. Nutrient excess and altered mitochondrial proteome and function contribute to neurodegeneration in diabetes. Mitochondrion. 2011;11(6):845–854. doi: 10.1016/j.mito.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2008;105(5):1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius C, Perrotta R, Graziano A, Calabrese EJ, Calabrese V. Stress responses, vitagenes and hormesis as critical determinants in aging and longevity: mitochondria as a “chi”. Immunity & Ageing : I & A. 2013;10(1):15. doi: 10.1186/1742-4933-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang X, Du G, Hu W, Ma L, Wang P, Li Y. Peroxisome proliferator-activated receptor gamma coactivator-1α/HSF1 axis effectively alleviates lipopolysaccharide-induced acute lung injury via suppressing oxidative stress and inflammatory response. J Cell Biochem. 2019;120(1):544–551. doi: 10.1002/jcb.27409. [DOI] [PubMed] [Google Scholar]
- de Kreutzenberg SV, Ceolotto G, Papparella I, Bortoluzzi A, Semplicini A, Dalla Man C, et al. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: potential biochemical mechanisms. Diabetes. 2010;59(4):1006–1015. doi: 10.2337/db09-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij BH, van der Berg JD, van der Kallen CJ, Schram MT, Savelberg HH, Schaper NC, et al. Physical activity and sedentary behavior in metabolically healthy versus unhealthy obese and non-obese individuals - the Maastricht Study. PloS one. 2016;11(5):e0154358. doi: 10.1371/journal.pone.0154358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Morales N, Rovira-Llopis S, Bañuls C, Escribano-Lopez I, de Marañon AM, Lopez-Domenech S, et al. Are mitochondrial fusion and fission impaired in leukocytes of type 2 diabetic patients? Antioxid Redox Signal. 2016;25(2):108–115. doi: 10.1089/ars.2016.6707. [DOI] [PubMed] [Google Scholar]
- Dimauro I, Mercatelli N, Caporossi D. Exercise-induced ROS in heat shock proteins response. Free Radical Biol Med. 2016;98:46–55. doi: 10.1016/j.freeradbiomed.2016.03.028. [DOI] [PubMed] [Google Scholar]
- Distefano G, Standley RA, Zhang X, Carnero EA, Yi F, Cornnell HH, et al. Physical activity unveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. J Cachexia Sarcopenia Muscle. 2018;9(2):279–294. doi: 10.1002/jcsm.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokladny K, Myers OB, Moseley PL. Heat shock response and autophagy–cooperation and control. Autophagy. 2015;11(2):200–213. doi: 10.1080/15548627.2015.1009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew BG, Ribas V, Le JA, Henstridge DC, Phun J, Zhou Z, et al. HSP72 is a mitochondrial stress sensor critical for Parkin action, oxidative metabolism, and insulin sensitivity in skeletal muscle. Diabetes. 2014;63(5):1488–1505. doi: 10.2337/db13-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers K, Brand T, Bangert A, Hauner H, Laumen H. Postprandial activation of metabolic and inflammatory signalling pathways in human peripheral mononuclear cells. Br J Nutr. 2014;111(12):2167–2175. doi: 10.1017/S0007114514000208. [DOI] [PubMed] [Google Scholar]
- Færch K, Vaag A. Metabolic inflexibility is a common feature of impaired fasting glycaemia and impaired glucose tolerance. Acta Diabetol. 2011;48(4):349–353. doi: 10.1007/s00592-010-0245-x. [DOI] [PubMed] [Google Scholar]
- Fan L, Cacicedo JM, Ido Y. Impaired nicotinamide adenine dinucleotide (NAD+ ) metabolism in diabetes and diabetic tissues: implications for nicotinamide-related compound treatment. J Diabetes Investig. 2020;11(6):1403–1419. doi: 10.1111/jdi.13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Geiger PC. Age-related differences in skeletal muscle insulin signaling: the role of stress kinases and heat shock proteins. J Appl Physiol. 2008;105(3):839–848. doi: 10.1152/japplphysiol.00148.2008. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafen PS, Preece CN, Sorensen JR, Hancock CR, Hyldahl RD. Repeated exposure to heat stress induces mitochondrial adaptation in human skeletal muscle. J Appl Physiol (Bethesda, Md. : 1985) 2018;125(5):1447–1455. doi: 10.1152/japplphysiol.00383.2018. [DOI] [PubMed] [Google Scholar]
- Hao D, Li Y, Shi J, Jiang J. Baicalin alleviates chronic obstructive pulmonary disease through regulation of HSP72-mediated JNK pathway. Mol Med. 2021;27(1):53. doi: 10.1186/s10020-021-00309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberle AM, RazquinNavas P, Langelaar-Makkinje M, Kasack K, Sadik A, Faessler E, et al. The PI3K and MAPK/p38 pathways control stress granule assembly in a hierarchical manner. Life Sci Alliance. 2019;2(2):e201800257. doi: 10.26508/lsa.201800257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen S, Buzkova J, Muniandy M, Kaksonen R, Ollikainen M, Ismail K, et al. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes. 2015;64(9):3135–3145. doi: 10.2337/db14-1937. [DOI] [PubMed] [Google Scholar]
- Henriksen TI, Wigge LV, Nielsen J, Pedersen BK, Sandri M, Scheele C. Dysregulated autophagy in muscle precursor cells from humans with type 2 diabetes. Sci Rep. 2019;9(1):8169. doi: 10.1038/s41598-019-44535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper PL, Hooper PL. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones. 2009;14(2):113–115. doi: 10.1007/s12192-008-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Houzelle A, Jörgensen JA, Schaart G, Daemen S, van Polanen N, Fealy CE, et al. Human skeletal muscle mitochondrial dynamics in relation to oxidative capacity and insulin sensitivity. Diabetologia. 2021;64(2):424–436. doi: 10.1007/S00125-020-05335-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurrle S, Hsu WH. The etiology of oxidative stress in insulin resistance. Biomedical Journal. 2017;40(5):257–262. doi: 10.1016/j.bj.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet (london, England) 2014;383(9922):1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Zhang L, Wagner JD. Tissue-specific regulation and expression of heat shock proteins in type 2 diabetic monkeys. Cell Stress Chaperones. 2009;14(3):291–299. doi: 10.1007/s12192-008-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Kim JH, Choi TG, Park S, Yun HR, Nguyen N, Jo YH, et al. Mitochondrial ROS-derived PTEN oxidation activates PI3K pathway for mTOR-induced myogenic autophagy. Cell Death Differ. 2018;25(11):1921–1937. doi: 10.1038/s41418-018-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Lee YH, Choi DY, Yi HK (2015) Expression of heat shock proteins (HSPs) in aged skeletal muscles depends on the frequency and duration of exercise training. J Sports Sci Med 14(2):347–353. [PMC free article] [PubMed]
- Kirkland JL, Tchkonia T. Cellular Senescence: a Translational Perspective. Ebiomedicine. 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano S, Kondo T, Matsuyama R, Ono K, Goto R, Takaki Y, et al. Impact of hepatic HSP72 on insulin signaling. Am J Physiol. Endocrinol Metab. 2019;316(2):E305–E318. doi: 10.1152/AJPENDO.00215.2018. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Codogno P. The mechanism and physiological function of macroautophagy. J Innate Immun. 2013;5(5):427–433. doi: 10.1159/000351979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Goto R, Ono K, Kitano S, Sato SMA, M, , et al. Activation of heat shock response to treat obese subjects with type 2 diabetes: a prospective, frequency-escalating, randomized, open-label, triple-arm trial. Sci Rep. 2016;6:35690. doi: 10.1038/srep35690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka AR, Suer MK, Wolff CA, Harber MP. Markers of human skeletal muscle mitochondrial biogenesis and quality control: effects of age and aerobic exercise training. J Gerontol - Ser A Biol Sci Med Sci. 2014;69(4):371–378. doi: 10.1093/gerona/glt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka AR, Asante A, Lanza IR, Robinson MM, Johnson ML, Man CD, et al. Defects in mitochondrial efficiency and H2O2 emissions in obese women are restored to a lean phenotype with aerobic exercise training. Diabetes. 2015;64(6):2104–2115. doi: 10.2337/db14-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, et al. Peroxisome proliferator-activated receptor-γ co-activator 1α-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280(39):33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- Ladenvall P, Persson CU, Mandalenakis Z, Wilhelmsen L, Grimby G, Svärdsudd K, et al. Low aerobic capacity in middle-aged men associated with increased mortality rates during 45 years of follow-up. Eur J Prev Cardiol. 2016;23(14):1557–1564. doi: 10.1177/2047487316655466. [DOI] [PubMed] [Google Scholar]
- Lu WC, Omari R, Ray H, Wang J, Williams I, Jacobs C, et al. AKT1 mediates multiple phosphorylation events that functionally promote HSF1 activation. FEBS J. 2022;289(13):3876–3893. doi: 10.1111/febs.16375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Xu L, Alberobello AT, Gavrilova O, Bagattin A, Skarulis M, et al. Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1-PGC1α transcriptional axis. Cell Metab. 2015;22(4):695–708. doi: 10.1016/j.cmet.2015.08.005. [DOI] [PubMed] [Google Scholar]
- McKeegan K, Mason SA, Trewin AJ, Keske MA, Wadley GD, Della Gatta PA, et al. Reactive oxygen species in exercise and insulin resistance: working towards personalized antioxidant treatment. Redox Biol. 2021;44:102005. doi: 10.1016/j.redox.2021.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minsky N, Roeder RG. Direct link between metabolic regulation and the heat-shock response through the transcriptional regulator PGC-1α. Proc Natl Acad Sci USA. 2015;112(42):E5669–E5678. doi: 10.1073/pnas.1516219112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MohammadTaghvaei N, Taheripak G, Taghikhani M, Meshkani R. Palmitate-induced PTP1B expression is mediated by ceramide-JNK and nuclear factor κB (NF-κB) activation. Cell Signal. 2012;24(10):1964–1970. doi: 10.1016/j.cellsig.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85(8):2970–2973. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- Montaigne D, Marechal X, Coisne A, Debry N, Modine T, Fayad G, et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation. 2014;130(7):554–564. doi: 10.1161/CIRCULATIONAHA.113.008476. [DOI] [PubMed] [Google Scholar]
- Muoio DM. Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell. 2014;159(6):1253–1262. doi: 10.1016/j.cell.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylabathula DB, Rice KM, Wang Z, Uddemarri S, Kinnard RS, Blough ER. Age-associated changes in MAPK activation in fast- and slow-twitch skeletal muscle of the F344/NNiaHSD X Brown Norway/BiNia rat model. Exp Gerontol. 2006;41(2):205–214. doi: 10.1016/j.exger.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Kitamura M. Bidirectional regulation of NF-κB by reactive oxygen species: a role of unfolded protein response. Free Radical Biol Med. 2013;65:162–174. doi: 10.1016/j.freeradbiomed.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Keane KN, Carlessi R, Cruzat V. Oxidative stress pathways in pancreatic β-cells and insulin-sensitive cells and tissues: importance to cell metabolism, function, and dysfunction. Am J Phys Cell Physiol. 2019;317(3):C420–C433. doi: 10.1152/ajpcell.00141.2019. [DOI] [PubMed] [Google Scholar]
- Nojima H, Watanabe H, Yamane K, Kitahara Y, Sekikawa K, Yamamoto H, et al. Effect of aerobic exercise training on oxidative stress in patients with type 2 diabetes mellitus. Metab: Clin Exp. 2008;57(2):170–176. doi: 10.1016/j.metabol.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Nolan CJ, Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: time for a conceptual framework shift. Diab Vasc Dis Res. 2019;16(2):118–127. doi: 10.1177/1479164119827611. [DOI] [PubMed] [Google Scholar]
- Nolan CJ, Ruderman NB, Kahn SE, Pedersen O, Prentki M. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes. 2015;64(3):673–686. doi: 10.2337/db14-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe K, Yaku K, Tobe K, Nakagawa T. Implications of altered NAD metabolism in metabolic disorders. J Biomed Sci. 2019;26(1):34. doi: 10.1186/s12929-019-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango AN. Cellular stresses and stress responses in the pathogenesis of insulin resistance. Oxid Med Cell Longev. 2018;2018:4321714. doi: 10.1155/2018/4321714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M, Peyot ML, Masiello P, Murthy Madiraju SR. Nutrient-induced metabolic stress, adaptation, detoxification, and toxicity in the pancreatic β-cell. Diabetes. 2020;69(3):279–290. doi: 10.2337/dbi19-0014. [DOI] [PubMed] [Google Scholar]
- Purwana I, Liu JJ, Portha B, Buteau J. HSF1 acetylation decreases its transcriptional activity and enhances glucolipotoxicity-induced apoptosis in rat and human beta cells. Diabetologia. 2017;60(8):1432–1441. doi: 10.1007/s00125-017-4310-7. [DOI] [PubMed] [Google Scholar]
- Radons J. The human HSP70 family of chaperones: where do we stand? Cell Stress Chaperones. 2016;21(3):379–404. doi: 10.1007/S12192-016-0676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynes R, Brunquell J, Westerheide SD. Stress inducibility of SIRT1 and its role in cytoprotection and cancer. Genes Cancer. 2013;4(3–4):172–182. doi: 10.1177/1947601913484497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renström F, Burén J, Svensson M, Eriksson JW. Insulin resistance induced by high glucose and high insulin precedes insulin receptor substrate 1 protein depletion in human adipocytes. Metab: Clin Exp. 2007;56(2):190–198. doi: 10.1016/j.metabol.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Rimbert V, Boirie Y, Bedu M, Hocquette JF, Ritz P, Morio B. Muscle fat oxidative capacity is not impaired by age but by physical inactivity: association with insulin sensitivity. FASEB J: Off Publ Fed Am Socr Exp Biol. 2004;18(6):737–739. doi: 10.1096/fj.03-1104fje. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Krause J, Krause M, O'Hagan C, De Vito G, Boreham C, Murphy C, Newsholme P, Colleran G. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones. 2012;17(3):293–302. doi: 10.1007/s12192-011-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RS, Morris EM, Wheatley JL, Archer AE, McCoin CS, White KS, et al. Deficiency in the heat stress response could underlie susceptibility to metabolic disease. Diabetes. 2016;65(11):3341–3351. doi: 10.2337/db16-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM. Mitochondrial dynamics in type 2 diabetes: pathophysiological implications. Redox Biol. 2017;11:637–645. doi: 10.1016/j.redox.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan L, Zhou C, Jin E, Kucharavy A, Zhang Y, Wen Z, et al. Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature. 2017;543(7645):443–446. doi: 10.1038/NATURE21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynders CA, Blanc S, DeJong N, Bessesen DH, Bergouignan A. Sedentary behaviour is a key determinant of metabolic inflexibility. J Physiol. 2018;596(8):1319–1330. doi: 10.1113/JP273282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammut IA, Harrison JC. Cardiac mitochondrial complex activity is enhanced by heat shock proteins. Clin Exp Pharmacol Physiol. 2003;30(1–2):110–115. doi: 10.1046/J.1440-1681.2003.03799.X. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25(6):903–915. doi: 10.1016/J.MOLCEL.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Selvarajah J, Elia A, Carroll VA, Moumen A. DNA damage-induced S and G2/M cell cycle arrest requires mTORC2-dependent regulation of Chk1. Oncotarget. 2015;6(1):427–440. doi: 10.18632/ONCOTARGET.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simar D, Jacques A, Caillaud C. Heat shock proteins induction reduces stress kinases activation, potentially improving insulin signalling in monocytes from obese subjects. Cell Stress Chaperones. 2012;17(5):615–621. doi: 10.1007/s12192-012-0336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BK, Tripathi M, Sandireddy R, Tikno K, Zhou J, Yen PM. Decreased autophagy and fuel switching occur in a senescent hepatic cell model system. Aging. 2020;12(14):13958–13978. doi: 10.18632/aging.103740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X (2014) The impact of Heat Shock Protein 72 expression on mitochondrial function and insulin action. UCLA. ProQuest ID: Song_ucla_0031N_12898. Merritt ID: ark:/13030/m5qg1166. Retrieved from https://escholarship.org/uc/item/7tr0t9zw
- Su Z, Nie Y, Huang X, Zhu Y, Feng B, Tang L, et al. Mitophagy in hepatic insulin resistance: therapeutic potential and concerns. Front Pharmacol. 2019;10:1193. doi: 10.3389/fphar.2019.01193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su KH, & Dai C (2017) mTORC1 senses stresses: coupling stress to proteostasis. BioEssays: News Rev Mol, Cell Dev Biol 39(5):10.1002/bies.201600268. 10.1002/bies.201600268 [DOI] [PMC free article] [PubMed]
- Sun D, Wang J, Toan S, Muid D, Li R, Chang X, et al. Molecular mechanisms of coronary microvascular endothelial dysfunction in diabetes mellitus: focus on mitochondrial quality surveillance. Angiogenesis. 2022;25(3):307–329. doi: 10.1007/s10456-022-09835-8. [DOI] [PubMed] [Google Scholar]
- Tsuzuki T, Kobayashi H, Yoshihara T, Kakigi R, Ichinoseki-Sekine N, Naito H. Attenuation of exercise-induced heat shock protein 72 expression blunts improvements in whole-body insulin resistance in rats with type 2 diabetes. Cell Stress Chaperones. 2017;22(2):263–269. doi: 10.1007/s12192-017-0767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranki S, Givvimani S, Kundu S, Metreveli N, Pushpakumar S, Tyagi SC. Moderate intensity exercise prevents diabetic cardiomyopathy associated contractile dysfunction through restoration of mitochondrial function and connexin 43 levels in db/db mice. J Mol Cell Cardiol. 2016;92:163–173. doi: 10.1016/J.YJMCC.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veereshwarayya V, Kumar P, Rosen KM, Mestril R, Querfurth HW. Differential effects of mitochondrial heat shock protein 60 and related molecular chaperones to prevent intracellular beta-amyloid-induced inhibition of complex IV and limit apoptosis. J Biol Chem. 2006;281(40):29468–29478. doi: 10.1074/jbc.M602533200. [DOI] [PubMed] [Google Scholar]
- Vogt S, Irqsusi M, Naraghi H, Sattler A, Ruppert V, Weber P, et al. Mitochondrial active and relaxed state respiration after heat shock mRNA response in the heart. J Therm Biol. 2019;80:106–112. doi: 10.1016/J.JTHERBIO.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen Z, Li Y, Ma L, Zou Y, Wang X, et al. Low density lipoprotein receptor related protein 6 (LRP6) protects heart against oxidative stress by the crosstalk of HSF1 and GSK3β. Redox Biol. 2020;37:101699. doi: 10.1016/j.redox.2020.101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YL, Zhou X, Li DL, Ye JM. Role of the mTOR-autophagy-ER stress pathway in high fructose-induced metabolic-associated fatty liver disease. Acta Pharmacol Sin. 2022;43(1):10–14. doi: 10.1038/s41401-021-00629-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide SD, Anckar J, Stevens SM, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT. Science. 2009;323(5917):1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Ma X, Bagattin A, Mueller E. The transcriptional coactivator PGC1α protects against hyperthermic stress via cooperation with the heat shock factor HSF1. Cell Death Dis. 2016;7(2):e2102–e2102. doi: 10.1038/cddis.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA. 2006;103(8):2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zając-Gawlak I, Pelclová J, Groffik D, Přidalová M, Nawrat-Szołtysik A, Kroemeke A, et al. Does physical activity lower the risk for metabolic syndrome: a longitudinal study of physically active older women. BMC Geriatr. 2021;21(1):11. doi: 10.1186/s12877-020-01952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Xu F, Liang H, Cao H, Cai M, Xu W, Weng J. SIRT1/HSF1/HSP pathway is essential for exenatide-alleviated, lipid-induced hepatic endoplasmic reticulum stress. Hepatology. 2017;66(3):809–824. doi: 10.1002/hep.29238. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018;15:335–346. doi: 10.1016/J.REDOX.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Yue Y, Wang J, Ma Q, Chen Y. Melatonin therapy for diabetic cardiomyopathy: a mechanism involving Syk-mitochondrial complex I-SERCA pathway. Cell Signal. 2018;47:88–100. doi: 10.1016/J.CELLSIG.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Zhu H, Yoshimoto T, Yamashima T. Heat shock protein 70.1 (Hsp70.1) affects neuronal cell fate by regulating lysosomal acid sphingomyelinase. J Biol Chem. 2014;289(40):27432–27443. doi: 10.1074/JBC.M114.560334. [DOI] [PMC free article] [PubMed] [Google Scholar]