Abstract
Pili of Neisseria gonorrhoeae are phase-variable surface structures that mediate adherence to host target cells. Each pilus is composed of thousands of major pilus subunits, pilins, pilus-associated protein PilC, and possibly other components. Piliated and nonpiliated gonococcal clones may secrete a soluble smaller pilin (S-pilin) that is cleaved after amino acid 39 of the mature pilin protein. Here, purified S-pilin was found to migrate as a 61- to 64-kDa double band on nondenaturing gels, suggesting the formation of tetrameric S-pilin proteins with two isomeric forms. In situ studies of binding to formalin-fixed tissue sections demonstrated the binding of S-pilin to human tissue but not to tissue from mouse or rat organs, showing the presence of a human-specific receptor-binding domain within the pilin polypeptide. Pretreatment of the target tissues with proteinase K decreased gonococcal binding dramatically, whereas pretreatment with neuraminidase and meta-periodate, which cleave carbon-carbon linkages between vicinal hydroxyl groups in carbohydrates, did not affect gonococcal binding. In overlay assays, purified S-pilin bound to a band with a migration pattern and size similar to those of CD46, a cellular pilus receptor. Further, binding of N. gonorrhoeae to target cells and tissues could be blocked by both CD46 antibodies and purified S-pilin. These data argue that S-pilin interacts with a protein domain(s) of the CD46 receptor on human cells.
Neisseria gonorrhoeae is an organism adapted to the human host, having no other ecological niche. The initial step in N. gonorrhoeae infection involves bacterial attachment to the epithelial cell surface, a process facilitated by pili, fimbrial appendages that extend from the bacterial surface (30, 37). Each pilus is composed of thousands of major pilin subunits, i.e., PilE proteins, of 20 kDa. PilE is expressed as a precursor (propilin) with a seven-amino-acid leader sequence. This leader peptide is cleaved off during transport across the inner membrane. Gonococcal pili undergo both phase and antigenic variations, and variant expression of pili on the gonococcal surface is reflected in colony morphology. Structural variation of pili results predominantly from recombination events between silent (pilS) and expressed (pilE) pilin gene sequences, giving rise to a vast number of pilin structural variants. Many derivatives with a nonpiliated colony morphology express a soluble pilin, S-pilin, that is released into the culture supernatant at high concentrations (7). S-pilin is cleaved after amino acid 39 of the mature pilin. Changes in the pilin sequence produce a spectrum of S-pilin production and pilus expression levels that may influence epithelial cell adherence (17). Glycosylation of pilin favors the production of S-pilin but is not required for S-pilin production in N. gonorrhoeae (19). The role of S-pilin in N. gonorrhoeae pathogenesis and adherence has not been determined, although S-pilin secretion has been proposed to allow the release of toxic pilin monomers that cannot be efficiently assembled into pili (7, 11).
PilC is a 110-kDa pilus-associated protein that is involved in biogenesis and adherence functions of the pilus (10, 12, 23, 26, 27). Most strains carry two homologous but not identical pilC genes. The expression of each gene can be turned on and off by frameshift mutations in a poly(G) tract in the signal peptide coding region (10). Spontaneous nonpiliated, PilC− gonococcal derivatives of strain MS11 may still express one or two pili but have often acquired new sequences in the expression locus (11). Both nonpiliated PilC+ and PilC− gonococcal clones secrete S-pilin. PilC is present at the tip of the pilus fiber, and purified PilC inhibits gonococcal adherence to host cells (28, 29). However, the protein is also present in the membranes and is exposed on the bacterial cell surface (25). In addition to PilC, adherence of N. gonorrhoeae to host cells is modulated by sequence changes in PilE (12, 22, 26).
The gonococcal pilus interacts with CD46 (13), also called membrane cofactor protein (MCP), a cell surface glycoprotein involved in complement regulation. The protein is found on virtually all human cell types except erythrocytes. CD46 protects host tissue from complement activation by binding to C3b and C4b and serves as a cofactor for factor I-mediated degradation of C3b and C4b. The CD46 structure contains four complement control protein repeats (CCP-1 to CCP-4) of about 60 amino acids each, a serine-threonine-proline-rich domain, a 12-amino-acid area whose function is undefined, a transmembrane hydrophobic domain, a cytoplasmic anchor, and a cytoplasmic tail. CD46 is expressed in four major isoforms, BC1, BC2, C1, and C2, depending on alternative splicing and choice of cytoplasmic tail, Cyt-1 or Cyt-2. Adherence of N. gonorrhoeae strain MS11 is highest to cells expressing the BC isoforms (14). Further, the serine-threonine-proline-rich domain CCP-3 and the cytoplasmic tail Cyt-1 are crucial for the adherence of piliated N. gonorrhoeae to host cells.
The initial pilus-mediated adherence by CD46 is followed by a second step of tight attachment. Opacity (Opa) proteins facilitate this next step of bacterial adhesion and invasion of target epithelial cells. Several eucaryotic receptors for Opa have been identified. CD66, an immunoglobulin superfamily cell adhesion molecule belonging to the carcinoembryonic antigen family, is a eucaryotic receptor for the majority of Opa proteins (4, 6, 35, 36). Also, certain gonococcal Opa proteins interact with cell surface-associated heparan sulfate proteoglycan receptors (3, 34). Invasion of epithelial cells by piliated or Opa-expressing neisseriae involves cytoskeletal rearrangements (20, 21). It has been demonstrated that interaction of Opa52-expressing bacteria and CD66 on human neutrophils activates a signaling cascade via Src-like protein tyrosine kinases rac1 and PAK (8).
In this study, S-pilin from PilC− gonococcal clones was found to bind to human tissue sections but not to mouse or rat tissue. Secreted S-pilin migrated as a 61- to 64-kDa band on nondenaturing gels, suggesting the formation of a tetrameric unit with two isomeric forms. Taken together, the data argue that S-pilin interacts with the epithelial cell surface, most likely with the cellular pilus receptor CD46.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
N. gonorrhoeae MS11mk(P+) and P−n have been described previously (31). The MS11mk(P+) strain sample used in these studies is designated MS11mk(P+)-u and is referred to as MS11 in this paper. Variants MS11-8, MS11-6, MS11-5, and MS11-3 are spontaneous nonpiliated PilC− derivatives of MS11 (11). A spontaneous pilE mutant derivative of variant 8 was isolated on plates. This clone was lacked the 5′ end of pilE, as demonstrated by PCR amplification and Southern blotting. Transmission electron microscopy confirmed that most bacteria of variants MS11-8, MS11-6, MS11-5, and MS11-3 were nonpiliated. One or two pili were seen on ∼10% of the cells. Bacteria were grown on GCB plates with Kellogg's supplement (15) at 37°C in a 5% CO2 atmosphere and passaged every 18 to 20 h. Outer membranes of the different variants and mutants were prepared as previously described (10) in order to examine the presence of Opa proteins. Aliquots of the outer membrane preparations were heated at 100°C for 10 min or at 37°C for 10 min and subsequently separated by sodium dodecyl sulfate (SDS)–12% polyacrylamide gel electrophoresis (PAGE) and stained with Coomassie brilliant blue. All of the variants used in this study lacked detectable levels of Opa protein (data not shown).
S-pilin isolation.
Nonpiliated N. gonorrhoeae MS11-8 (PilC− pilin+), MS11-6 (PilC− pilin+), MS11-5 (PilC− pilin+), MS11-3 (PilC− pilin+), and P−n (PilC+ pilin−) were grown in Catlin's defined medium (2) for 18 to 44 h to an optical density at 550 nm of 1. The cultures were centrifuged for 1 h at 10,000 × g, whereafter the supernatant was recentrifuged under the same conditions. Two liters of culture supernatant was concentrated in Amicon cells to a final volume of 2 ml with YM10 diaflo ultrafiltration membranes, followed by ultracentrifugation in a Ty65 rotor for 2 h at 100,000 × g to remove high-molecular-weight material. All steps were performed on ice and in the presence of 1 mM phenylmethylsulfonyl fluoride if possible. Extracts were tested in immunoblots and on Coomassie blue-stained SDS-polyacrylamide gels for the presence of S-pilin and lack of mature pilin. The total protein extracts contained approximately 5 to 10% S-pilin. The presence of S-pilin was confirmed by determination of the amino-terminal protein sequence. For N-terminal sequence determination, S-pilin was subjected to SDS-PAGE, the pilin band was transferred to an Immobilon polyvinylidene difluoride filter by electroblotting, and the proteins were stained and sequenced as previously described (10). Concentrated supernatants were applied to mono-Q HR 10/10 (Pharmacia Inc.) and Superdex 75HR 10/30 columns. The final preparation contained less than 1% contaminating proteins.
DNA sequencing.
The pilE gene from gonococcal variants was PCR amplified by using the primers 5′-AAATTTAAGGCCTATTTGCC-3′ and 5′-TTTCCCCTTTCAATTAGG AGT-3′. Automated sequencing of purified PCR products was performed by using the Taq DyeDeoxy terminator kit for cycle sequencing (Applied Biosystems).
Cell lines and adherence assays.
ME180 cells (ATCC HTB33), an epithelial cell-like cell line derived from a human cervical carcinoma, was maintained in McCoy's 5A medium supplemented with 10% fetal calf serum. The Wong-Kilbourne derivative of Chang conjunctiva (ATCC CCL20.2), an epithelial cell line, was grown in medium 199 with Earle's salts supplemented with 10% fetal calf serum. For adherence assays, the cells were seeded into six-well plates at 105 cells/well and incubated in 5% CO2 at 37°C for 1 to 2 days. Nonconfluent monolayers were overlaid with 200 μl of S-pilin preparations (2 μg/ml) or bacteria (107 bacteria/ml), incubated for 1 h in 5% CO2 at 37°C, and washed six times for 5 min each time in phosphate-buffered saline (PBS, pH 7.4). Bound S-pilin was detected with PilE antiserum (diluted 1:50 in blocking buffer) for 15 min, washed in PBS, incubated with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit immunoglobulin A (IgA; Sigma, St. Louis, Mo.) diluted 1:80 in blocking buffer) for 15 min, and finally washed for 5 min in PBS. Binding was evaluated by visual inspection. Bound bacteria were counted on 100 cells in each experiment.
Immunoblots and antibodies.
For separation of eucaryotic cells by SDS-PAGE, confluent layers of cells were washed in medium, scraped off of the plastic bottle, washed twice in PBS (pH 7.4), and thoroughly resuspended in PBS. Whole-cell extracts were subjected to SDS–12% PAGE for detection of CD46. Proteins were transferred onto polyvinylidene difluoride membranes and overlaid with digoxigenin (DIG)-labeled S-pilin preparations (10 μg/ml) or unlabeled S-pilin preparations and with polyclonal antiserum directed against MCP (diluted 1:1,000) for 1 h at room temperature. Bound S-pilin or MCP antibodies were detected with alkaline phosphatase (AP)-conjugated monoclonal antibodies against DIG (diluted 1:1,000) (Boehringer Mannheim) or IgG (Bio-Rad), respectively. For separation of bacterial cells by SDS-PAGE, bacteria were scraped off of the plate, washed twice in PBS (pH 7.4), and thoroughly resuspended in PBS. Bacterial lysates (10 μg) or protein preparations were subjected to SDS–15% PAGE. The proteins were transferred from the gel to nitrocellulose sheets and identified with polyclonal pilus antiserum or stained with Coomassie brilliant blue. S-pilin was detected with pilin-specific antibodies and AP-conjugated monoclonal antibodies against DIG (diluted 1:1,000) (Boehringer Mannheim) or AP-conjugated anti-IgG (Bio-Rad). All samples were boiled at 100°C prior to electrophoresis. The pilus antiserum was raised in rabbits by using highly purified pilus preparations of N. gonorrhoeae MS11(P+) as previously described (10), and the pilus antiserum was generated to the gel-purified 20-kDa pilin band in pilus preparations of strain MS11 separated by SDS PAGE (10).
Tissues and tissue-binding assays.
The human tissues used have already been described (12). Rat stomach and intestine were from male 6- to 8-week-old Sprague-Dawley rats, and the mouse organs were from 6- to 8-week-old FVB/N mice (33). Tissues were formalin fixed and embedded in paraffin in according with a standard procedure (32). Sections of paraffin-embedded tissues were deparaffinized, rinsed in water and PBS (pH 7.4), and incubated for 15 to 30 min in blocking buffer (0.2% BSA in PBS, pH 7.4) as previously described (12). Tissue sections were overlaid with 200 μl of S-pilin preparations (2 μg/ml) or with 107 bacteria, incubated for 1 h in 5% CO2 at 37°C, and washed six times for 5 min each time in PBS, pH 7.4. The slides were then incubated with PilE antiserum (diluted 1:50 in blocking buffer) for 15 min, washed for 5 min in PBS, incubated with FITC-conjugated anti-rabbit IgA (Sigma) diluted 1:80 in blocking buffer) for 15 min and finally washed for 5 min in PBS.
Sodium periodate oxidation and proteinase K treatment.
Cleavage of vicinal hydroxyl groups on carbohydrates by mild periodate oxidation, followed by sodium borohydride reduction, was performed as previously described (5, 38). Deparaffinized tissue sections were incubated with 10 mM sodium meta-periodate in 50 mM sodium acetate buffer, pH 4.5, for 1 h at room temperature in the dark, followed by incubation for 30 min in sodium borohydride to reduce the aldehyde groups generated by periodate oxidation to alcohols. Control tissue slides were incubated in 50 mM sodium acetate buffer without sodium meta-periodate. In order to confirm that polypeptide antigenicity remained intact after periodate oxidation, sections were overlaid with polyclonal antiserum specific for MCP. Carbohydrate signals in corneal and cervical tissues were detected by using the concanavalin A lectin and in endometrial tissue by using the Dolichos biflorus agglutinin lectin. Deparaffinized tissue sections were incubated with 200 mU of proteinase K from Tritirachium album (Sigma) for 1 h at 37°C or with 200 mU of neuraminidase from Vibrio cholerae (Boehringer Mannheim) for 2 h at 37°C in a total volume of 200 ml. The tissue sections were then washed in PBS three times for 5 min each time, blocked for 30 min in blocking buffer, and overlaid with a bacterial suspension. Bacterial, carbohydrate, and protein signals were detected as described above. Inhibition was evaluated by visual inspection of the tissue.
RESULTS
S-pilin migrates as a tetramer on nondenaturing gels.
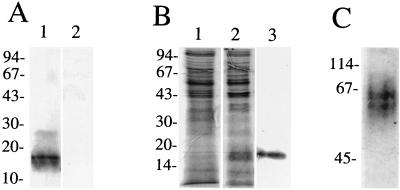
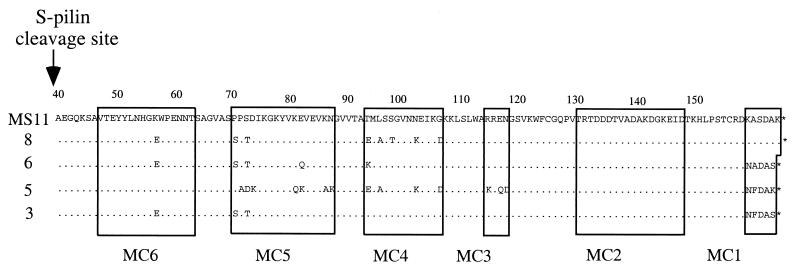
S-pilin was purified from MS11-8, a nonpiliated PilC− pilin+ derivative of strain MS11. Pilin produced from MS11-8 is cleaved predominantly into S-pilin, as determined by immunoblotting with PilE antiserum (Fig. 1A). Culture supernatants of MS11-8 were concentrated by ultrafiltration. Such crude extracts contained approximately 5 to 10% S-pilin. S-pilin was further purified on a mono-Q ion-exchange column. All fractions were analyzed by immunoblotting with PilE-specific antiserum. Fractions containing S-pilin (16 kDa) were pooled and purified on a Superdex 12 HR column. The purified preparation obtained contained >99% S-pilin (Fig. 1B). The presence of S-pilin was confirmed by determination of the amino-terminal protein sequence. N-terminal sequencing of the 16-kDa protein from variant 8 showed that the 16-kDa product is the result of cleavage between Leu39 and Ala40 of the mature pilin (data not shown). Purified S-pilin preparations ran as a 61- to 64-kDa doublet band on a nondenaturing polyacrylamide gel, suggesting that S-pilin from variant MS11-8 is released as a tetramer and that there may be two isomeric forms of this pilin complex (Fig. 1C). Similar purification procedures were performed with three other nonpiliated PilC− pilin+ derivatives of MS11, i.e., MS11-6, MS11-5, and MS11-3. The PilE sequences of these clones were determined and are shown in Fig. 2.
FIG. 1.
Purified S-pilin migrates as a tetrameric unit under nondenaturing conditions. (A) Immunoblot of gonococcal whole-cell lysates using PilE-specific antiserum. Lanes: 1, MS11-8, a PilC−, S-pilin producing derivative of MS11; 2, MS11-P−n. (B) Concentrated culture supernatants of MS11-P−n (lane 1) and MS11-8 (lane 2). Lane 3, purified S-pilin preparation of MS11-8. The proteins were separated by SDS–15% PAGE and stained with Coomassie brilliant blue. (C) S-pilin separated on a nondenaturing polyacrylamide gel and stained with Coomassie brilliant blue. S-pilin migrates as a tetrameric unit. Shown on the left are molecular size markers (kilodaltons). N-terminal sequencing of the 16-kDa protein showed that the 16-kDa product is S-pilin.
FIG. 2.
Deduced amino acid sequences of the pilE genes in nonpiliated, PilC− derivatives of N. gonorrhoeae MS11. The S-pilin cleavage site is indicated by an arrow. Amino acids identical to those of parental strain MS11 are marked by dots. MC1 to MC6 mark the six variable minicassettes.
S pilin binds specifically to human tissue.
In a previous study, we demonstrated that piliated, but not nonpiliated, gonococci adhere to fixed human tissue sections but not to mouse or rat tissue (12). Bacteria bound to both epithelial and subepithelial tissues, which is in agreement with the distribution of the human pilus receptor CD46 (13). Little is known about whether gonococcal S-pilin may adhere to receptors in mammalian tissue. To study this, tissue sections and cells were incubated with purified S-pilin. Binding of S-pilin was estimated by fluorescence activity after detection with pilin-specific antiserum, followed by FITC-conjugated anti-rabbit IgG.
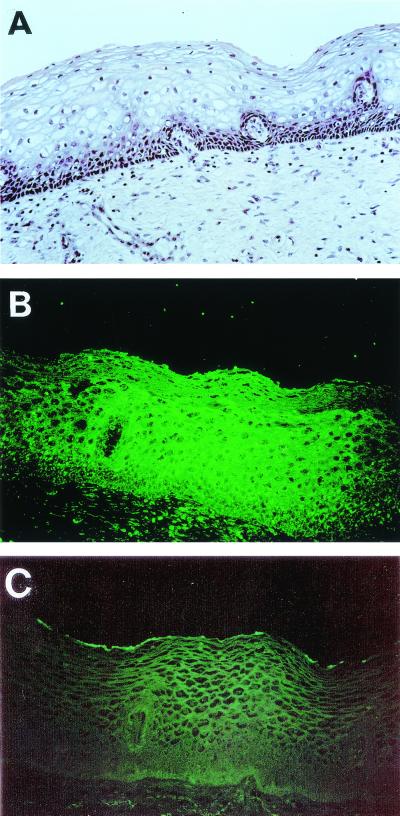
All four S-pilin variants, MS11-8, MS11-6, MS11-5, and MS11-3, bound to human cervix, endometrium, intestine, stomach, midbrain, and meninges (Table 1). Concentrated supernatants of P−n gonococci did not show detectable interaction with human tissues. As illustrated in Fig. 3, binding to the complete tissue section, including both epithelial and subepithelial tissues, occurred. S-pilin variants 5 and 6 differed from variants 3 and 8 by binding much less efficiently to human cornea and conjunctiva tissue (Table 1). Similarly, S-pilin variants 5 and 6 bound at low levels to Chang conjunctiva cells whereas all four S-pilin variants bound to the ME180 cervical cell line (Table 1). The finding that preincubation of the S-pilin with pilin antibodies prevented detectable binding of S-pilin to target tissues or cells supported the presence of a eucaryotic receptor for S-pilin (data not shown). Binding of S-pilin was specific for human tissues and cells, since purified S-pilin (2 μg/ml) showed only low levels of fluorescence when added to tissue sections of mouse or rat organs (Table 1). Binding of S-pilin to cells and tissues was strictly temperature dependent; the most efficient binding to human tissue occurred at 37°C, intermediate binding occurred at 25°C, whereas no binding of S-pilin was detected at +4°C (data not shown).
TABLE 1.
Measurement of S-pilin binding to tissue sections by determination of fluorescence activity
| Tissue or cells | Bindinga of S-pilinb from:
|
Binding of P−n supernatantc | |||
|---|---|---|---|---|---|
| MS11-8 | MS11-6 | MS11-5 | MS11-3 | ||
| Human tissues | |||||
| Cervix | +++ | +++ | +++ | +++ | − |
| Endometrium | |||||
| Small intestine | ++ | +++ | +++ | +++ | − |
| Large intestine | |||||
| Stomach | |||||
| Midbrain | |||||
| Meninges | |||||
| Cornea | +++ | (+) | (+) | +++ | − |
| Conjunctiva | |||||
| Human cell lines | |||||
| ME180 | +++ | +++ | +++ | +++ | − |
| Chang conjunctiva | ++ | (+) | (+) | +++ | − |
| Mouse tissues | |||||
| Uterus | − | − | − | − | − |
| Intestine | |||||
| Stomach | |||||
| Midbrain | |||||
| Cornea | |||||
| Rat tissues | |||||
| Uterus | − | − | − | − | − |
| Intestine | |||||
| Stomach | |||||
| Kidney | |||||
+++, high fluorescence; ++, medium fluorescence; (+), low fluorescence; −, very low fluorescence.
Purified S-pilin at 2 μg/ml was used.
Concentrated culture supernatant at 20 μg/ml was used.
FIG. 3.
In situ assay of binding of S-pilin to fixed sections of human cervical tissue. (A) Tissue section stained with hematoxylin-eosin. (B) Tissue section overlaid with purified S-pilin preparation of MS11-8 (1.5 μg/ml). (C) Tissue section overlaid with concentrated supernatant of the P−n variant (20 μg/ml). Bound S-pilin was detected with PilE antiserum and FITC-conjugated anti-rabbit IgG. The low fluorescence level still detected represents the background of the FITC-labeled secondary antibody.
S-pilin-binding sites in human tissue possess protein characteristics.
In an earlier study, we showed that adherence of piliated gonococci to human tissue was blocked by pretreatment of the tissue section with proteinase K (12). Adherence was unaffected by pretreatment with meta-periodate. Mild meta-periodate oxidation under acidic conditions destroys many (16), but not all (24), carbohydrate determinants by cleaving carbon-carbon linkages between vicinal hydroxyl groups without altering the structure of polypeptide chains.
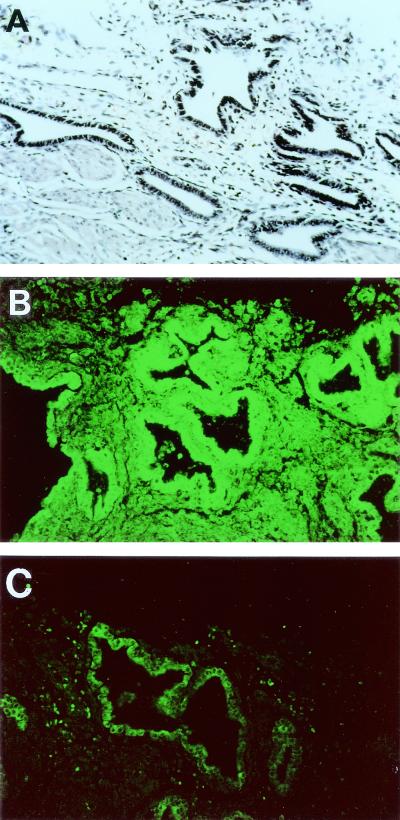
In order to study the nature of a possible eucaryotic receptor for S-pilin, tissue sections were pretreated with meta-periodate, neuraminidase, or proteinase K before incubation with bacteria. During these experiments, carbohydrate and protein signals were carefully monitored by using appropriate lectins and protein antiserum (Table 2). Pretreatment of tissue with proteinase K abolished S-pilin-associated fluorescence, whereas periodate oxidation, neuraminidase treatment, or both neuraminidase and periodate pretreatments of human tissue did not affect the binding of S-pilin (Table 2 and Fig. 4). Tissues incubated with P−n supernatants did not show any fluorescence. Taken together, these data argue that S-pilin may interact with a protein or glycoprotein on eucaryotic cells.
TABLE 2.
Chemical and enzymatic pretreatments of human endometrial tissue sections
| Pretreatment | Fluorescence level after addition of:
|
|||
|---|---|---|---|---|
| S-pilins 8, 6, 5, 3a | P− n supernatantb | Control lectinc | Control MCP antiserumd | |
| Proteinase K | − | − | +++ | − |
| PBS (control) | +++ | − | +++ | +++ |
| Periodate | +++ | − | − | +++ |
| Na acetate (control) | +++ | − | − | +++ |
| Neuraminidase | +++ | − | − | +++ |
| PBS (control) | +++ | − | +++ | +++ |
| Neuraminidase + periodate | +++ | − | − | +++ |
| PBS-Na acetate (control) | +++ | − | +++ | +++ |
Purified S-pilin at 2 μg/ml was added. +++; high fluorescence; −, low fluorescence.
Concentrated P− n supernatant at 20 μg/ml was added. −, low fluorescence.
The control for periodate treatment was the Dolichos biforus agglutinin lectin recognizing N-acetyl-α-d-galactosaminyl (α-d-GalNAc). The control for neuraminidase treatment was the lectin from Maakia amurensis recognizing glycoconjugate side chains containing sialic acid α2-3Gal. +++, strong lectin binding; −, no lectin binding detected.
MCP (CD46) antiserum, followed by FITC-conjugated antibody, was used to detect protein. +++, strong fluorescence; −, weak fluorescence.
FIG. 4.
Inhibition of S-pilin binding to human endometrial tissue. (A) Tissue stained with hematoxylin-eosin. (B) Tissue overlaid with purified S-pilin preparation of MS11-8 (1.5 μg/ml). (C) Preincubation of the tissue with proteinase K prior to addition of the MS11-8 S-pilin preparation. Bound S-pilin was detected with PilE antiserum and FITC-conjugated anti-rabbit IgG. The low fluorescence level still detected represents the background of the FITC-labeled secondary antibody.
S-pilin interacts with the pilus receptor CD46.
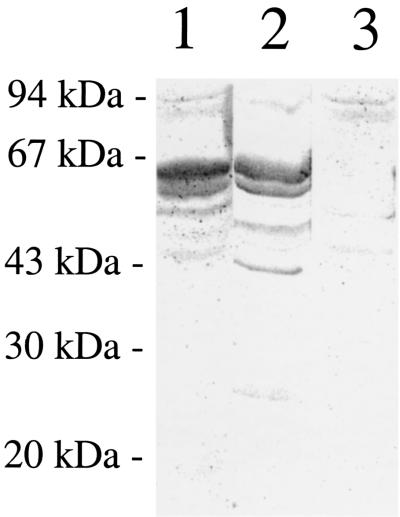
CD46 acts as a cellular pilus receptor (13, 14). The protein is expressed as four major isoforms that arise by alternative splicing, giving rise to two classes of glycoproteins with molecular masses of 51 to 58 kDa and 59 to 68 kDa. We have previously shown that purified gonococcal pili bind to a doublet band of 55 to 60 kDa in overlay assays of separated ME180 cells (13). To test whether the soluble form of pilin, S-pilin, binds in a similar manner, whole-cell extracts of ME180 were separated by SDS-PAGE and blotted onto nitrocellulose membranes. Membranes were overlaid with a DIG-labeled S-pilin-preparation of MS11-8. Bound S-pilin was detected with antibodies directed against DIG or pili. Similarly to pilus organelles, a double band of 55 to 60 kDa was labeled with S-pilin (Fig. 5). The DIG-specific antibody did not cross-react with ME180 components in the size range of 50 to 60 kDa (data not shown). An antiserum directed against CD46 labeled bands of the same size as S-pilin, i.e., 55 to 60 kDa. Further, there was no binding of S-pilin to separated Chinese hamster ovary (CHO) cells that lack CD46 expression. These data suggest that S-pilin contains a CD46-binding domain.
FIG. 5.
Purified gonococcal S-pilin binds to a protein of the same size as CD46. SDS-PAGE of whole-cell lysates blotted onto a nitrocellulose membrane. Lanes: 1, ME180 cells overlaid with DIG-labeled S-pilin MS11-8; 2, ME180 cells overlaid with CD46 antiserum and AP-conjugated IgG antibodies; 3, CHO cells overlaid with DIG-labeled S-pilin of MS11-8.
Purified S-pilin inhibits bacterial binding to epithelial cells.
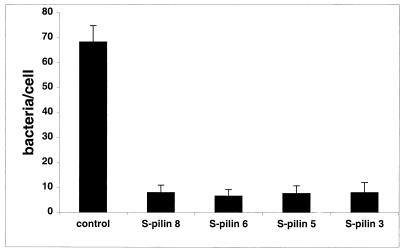
If S-pilin interacts with the CD46 cell surface receptor, purified S-pilin of MS11-8, MS11-6, MS11-5, and MS11-3 should inhibit the adherence of piliated N. gonorrhoeae to target cells. Figure 6 shows that preincubation of ME180 cells with of S-pilin at 2 μg/ml dramatically reduced bacterial binding. In addition, purified S-pilin blocked the binding of piliated N. gonorrhoeae to human tissues (data not shown). As a control in these assays, we used concentrated supernatants of a pilE derivative of MS11-8. In this clone, the 5′ end of pilE was deleted, as determined by PCR and Southern blotting. The control supernatant did not inhibit the binding of S-pilin to target tissue (Fig. 6). Our earlier data have demonstrated that polyclonal CD46 antiserum inhibits the adherence of piliated gonococci to fixed tissue sections and to ME180 cells (13). Incubation of tissue with CD46 antiserum prior to overlay with S-pilin resulted in much-reduced fluorescence (data not shown), supporting the interaction between CD46 and S-pilin.
FIG. 6.
S-pilin inhibits adherence of P+ gonococci to target cells. ME180 cells were preincubated with S-pilin (2 μg/ml) for 30 min prior to the addition of piliated N. gonorrhoeae MS11. Binding was allowed to occur for 1 h, and bound bacteria were detected with gonococcal antiserum and FITC-conjugated IgG. The control shows adherence of P+ MS11 to ME180 cells preincubated with supernatant of a pilE derivative of MS11-8 for 30 min. Bound bacteria were counted on 100 randomly selected cells. Shown are the average of four independent experiments and the standard deviation.
DISCUSSION
In this paper, we show that gonococcal pilin fragments (S-pilins) attach to human target tissue but not to mouse or rat tissue, indicating the presence of a receptor-binding domain within this pilin polypeptide. The findings that adherence of piliated N. gonorrhoeae to human tissue or viable cells was blocked by purified S-pilin and that binding of S-pilin was blocked by pilin antibodies supported the presence of an S-pilin receptor on human cells. Purified S-pilin bound to a wide variety of human tissues, and the receptor had the characteristics of a protein or a glycoprotein, as evidenced by chemical and enzymatic treatment of fixed tissue sections. Overlay assays with purified S-pilin demonstrated binding to a double band of 55 to 60 kDa similar in size to CD46. Data showing that CD46 antiserum inhibited the S-pilin interaction with human target cells and tissue support the idea that S-pilin plays an important role in bacterial interaction with eucaryotic host cell surfaces.
There are two major forms of pilins, S-pilin and L-pilin. L-pilin is an extra-long pilin that is neither secreted nor assembled into pili and that results from insertion of tandem pilin copies into the expressed copy. S-pilin is a truncated soluble pilin fragment that is spontaneously released into the medium. Posttranslational proteolytic cleavage of the full-size pilin occurs after amino acid 39 of the mature pilin, removing the hydrophobic amino-terminal region, and subsequent export and release of the truncated protein into the supernatant (7). Here we show that purified S-pilin migrates as a 61- to 64-kDa band on nondenaturing gels, indicating the formation of a tetrameric unit. Since a double band was present on nondenaturing protein gels, it is possible that two isomeric forms are secreted. The variation may represent incomplete processing at the amino terminus, partial phosphorylation, or the presence of similar but isoelectrically distinct proteins.
S-pilin isolated from PilC− clones attached to human cervical and endometrial tissue, but not to mouse tissue, indicating that PilC is not involved in this interaction. Further, S-pilin bound to the complete tissue section, and the distribution of N. gonorrhoeae receptors was not cell lineage specific. However, the possibility cannot be excluded that the S-pilin preparation contains trace amounts of a hitherto unidentified pilus adhesin. Such an adhesin may be associated with S-pilin and could be present in minor amounts not detectable by SDS-PAGE and staining. The fluorescence observed with specific antibodies would thus detect an S-pilin attached to an adhesin. No matter how this interaction is accomplished, it is obvious that bacteria release a protein(s) that interacts with the eucaryotic cell surface. Since preincubation of S-pilin with pilin antibodies prevented the binding of S-pilin to target cells, it is most likely that a receptor-binding site remains within the S-pilin polypeptide.
The finding that S-pilin may be secreted as a tetrameric unit supports the possibility that a receptor-binding pocket is built up from a combination of domains of different pilin subunits. It is tempting to speculate that S-pilin may have a function in sending signals into the eucaryotic cell. Since S-pilin interacts with human cells, it may transfer a signal through the plasma membrane. It is, however, also possible that S-pilins are required at an intracellular step or needed for bacteria to be released from epithelial cells into the subepithelial space. We have previously showed that piliated bacteria are released from HEC-1 B cells in a nonpiliated S-pilin-producing form (9). Since purified S-pilin inhibits the adherence of piliated N. gonorrhoeae MS11 to target cells, it is possible that secretion of S-pilin assists in the spread of the bacteria to other organs or from one individual to another.
In this work, S-pilin variants 5 and 6 did not bind to corneal tissue sections or Chang conjunctiva cells whereas S-pilin variants 3 and 8 bound well. These four S-pilin variants all carry slightly different pilE sequences, suggesting that sequence variation in S-pilin affects its interaction with human cells. Since S-pilin variants 3 and 6 differ by only three amino acids, it is clear that minor changes in pilE may dramatically influence the cell-binding capacity of the protein. These data are in agreement with what has been observed for the adherence of whole gonococci to epithelial cells. In an earlier study, we showed that sequence changes in the major pilus subunit of N. gonorrhoeae lead to variation in tropism to human tissue (12). Even earlier, Rudel et al. (26) demonstrated that PilE sequence changes affect the pilus-mediated adherence of N. gonorrhoeae to human epithelial cells. The same observation has been reported for N. meningitidis, namely, that antigenic variation of pilin regulates the adhesion of meningococci to human epithelial cells (22). Thus, the interaction of different S-pilin sequence variants with target cells follows a pattern similar to that of antigenically different pilus structures expressed in bacteria.
Cervical and endometrial tissues must express a different arsenal of S-pilin receptor epitopes relative to corneal tissue, because different binding degrees were obtained by using S-pilin variants with different sequences. However, it may be that the receptor is structurally changed or that the receptor concentration is lower in certain cell types. In a recent paper, we reported that the adherence of piliated N. gonorrhoeae depends upon which isoform is expressed in a specific cell type (14). The adherence of wild-type strain MS11P+ to cells expressing the BC isoform of CD46 is most robust. In Chang conjunctiva cells, the C1 isoform is dominant although expression of the BC1 isoform is also detected, whereas ME180 cells express only the BC1 and BC2 isoforms (14). In this work, we demonstrate that S-pilin also binds better to ME180 cells than to Chang cells. CD46 expression has been reported on both the apical and basolateral sides of epithelial cells (1, 18) Therefore, it is most likely that S-pilins have the ability to bind both apical and basolateral membranes. Nonpiliated, revertible S-pilin-producing bacteria still express a few pili and adhere at moderate levels. These bacterial variants may still deliver S-pilin to the mucosal surface. In addition, S-pilin may also have an important function inside the cells.
It has been shown that PilC is located at the tip of the pilus (28), where it theoretically has the potential to interact with CD46. Since S-pilin inhibits bacterial adherence to human target cells, the pilus tip structure is likely to interact with a receptor domain close to the S-pilin-binding site. However, it is possible that the intact pilus and soluble pilin interact with different domains of CD46, giving rise to two distinct cellular responses. The adherence of piliated neisseriae is modulated by PilE sequence variation. However, expression of PilC is required for the adherence of N. gonorrhoeae to target cells (12, 23, 27). We have data showing that PilC also interacts with CD46 (B. Albiger and A.-B. Jonsson, Abstr. 12th Int. Pathogenic Neisseria Conf., abstr. 156, 2000). This finding is in agreement with the involvement of PilC and PilE sequence variation in the attachment of piliated N. gonorrhoeae to host target cells.
The data in this work suggest that a eucaryotic receptor(s) for S-pilin of N. gonorrhoeae is distributed on a wide variety of human tissues and has the characteristics of a protein or a glycoprotein, as evidenced by chemical and enzymatic treatments of fixed tissue sections. Further, we demonstrate that S-pilin interacts with CD46, a cellular receptor for the pilus structure. A future characterization of the interaction between S-pilin and its cell surface receptor will certainly lead to a better understanding of the role and function of this secreted molecule in Neisseria pathogenesis. Also, long-term experiments to investigate the exact biological function of S-pilins and to tease out the epitopes on pilin that are responsible for binding will be of great importance.
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Medical Research Council (Dnr 10846), the Swedish Foundation for Strategic Research/Infection and Vaccinology Program, the Swedish Cancer Society, the Swedish Society of Medicine, the Magnus Bergvalls Stiftelse, the Åke Wibergs Stiftelse, and the Anders Otto Svärds Stiftelse; by Karolinska Institutet Research grants; and by the Clas Grochinsky Foundation. B.A was supported by grants from the Wenner-Gren Foundation.
REFERENCES
- 1.Blau D M, Compans R W. Entry and release of measles virus are polarized in epithelial cells. Virology. 1995;210:91–99. doi: 10.1006/viro.1995.1320. [DOI] [PubMed] [Google Scholar]
- 2.Catlin B W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973;128:178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- 3.Chen T, Belland R J, Wilson J, Swanson J. Adherence of pilus− Opa+ gonococci to epithelial cells in vitro involves heparan sulfate. J Exp Med. 1995;182:511–517. doi: 10.1084/jem.182.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen T, Gotschlich E C. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci USA. 1996;93:14851–14856. doi: 10.1073/pnas.93.25.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falk P, Roth K A, Borén T, Westblom T F, Gordon J I, Normark S. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage specific tropism in the human gastric epithelium. Proc Natl Acad Sci USA. 1993;90:2035–2039. doi: 10.1073/pnas.90.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray-Owen S D, Dehio C, Haude A, Grunert F, Meyer T F. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 1997;16:3435–3445. doi: 10.1093/emboj/16.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas R, Schwarz H, Meyer T F. Release of soluble pilus antigen coupled with gene conversion in Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1987;84:9079–9083. doi: 10.1073/pnas.84.24.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauck C R, Meyer T F, Lang F, Gulbins E. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J. 1998;17:443–454. doi: 10.1093/emboj/17.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilver D, Källström H, Normark S, Jonsson A-B. Transcellular passage of Neisseria gonorrhoeae involves reversible pilus phase variation. Infect Immun. 1998;166:469–473. doi: 10.1128/iai.66.2.469-473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonsson A-B, Nyberg G, Normark S. Phase variation of gonococcal pili by frame shift mutation in pilC, a novel gene for pilus assembly. EMBO J. 1991;10:35–43. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonsson A-B, Pfeifer J, Normark S. Neisseria gonorrhoeae PilC expression provides a selective mechanism for structural diversity of gonococcal pili. Proc Natl Acad Sci USA. 1992;89:3204–3208. doi: 10.1073/pnas.89.8.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonsson A-B, Ilver D, Falk P, Pepose J, Normark S. Sequence changes in the major pilus subunit of Neisseria gonorrhoeae lead to tropism variation to human tissue. Mol Microbiol. 1994;14:416–428. [Google Scholar]
- 13.Källström H, Liszewski K M, Atkinson J P, Jonsson A-B. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor of pathogenic Neisseria. Mol Microbiol. 1997;25:639–647. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- 14.Källström H, Albiger B, Liszewski K M, Atkinson J P, Jonsson A-B. Attachment of Neisseria gonorrhoeae to the cellular pilus receptor CD46: identification of domains important for bacterial adherence. Cell Microbiol. 2001;3:133–143. doi: 10.1046/j.1462-5822.2001.00095.x. [DOI] [PubMed] [Google Scholar]
- 15.Kellogg D S, Jr, Cohen I R, Norins L C, Schroeter A L, Reising G. Neisseria gonorrhoeae. II. Clonal variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968;96:596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knibbs R N, Goldstein I J, Ratcliffe R M, Shibyua N. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. J Biol Chem. 1991;266:83–88. [PubMed] [Google Scholar]
- 17.Long C D, Madraswala R N, Seifert H S. Comparisons between colony phase variation of Neisseria gonorrhoeae FA1090 and pilus, pilin, and S-pilin expression. Infect Immun. 1998;66:1918–1927. doi: 10.1128/iai.66.5.1918-1927.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maisner H, Zimmer M, Liszewski M K, Lublin D M, Atkinson J P, Herrner G. Membrane cofactor protein (CD46) is a basolateral protein that is not endocytosed. J Biol Chem. 1997;272:20793–20799. doi: 10.1074/jbc.272.33.20793. [DOI] [PubMed] [Google Scholar]
- 19.Marceau M, Nassif X. Role of glycosylation at Ser63 in production of soluble pilin in pathogenic Neisseria. J Bacteriol. 1999;181:656–661. doi: 10.1128/jb.181.2.656-661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mertz A J, So M. Attachment of piliated, Opa− and Opc− gonococci and meningococci to epithelial cells elicits cortical actin rearrangements and clustering of tyrosine-phosphorylated proteins. Infect Immun. 1997;65:4341–4349. doi: 10.1128/iai.65.10.4341-4349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mertz A J, Enns C A, So M. Type IV pili of pathogenic neisseriae elicit cortical plaque formation in epithelial cells. Mol Microbiol. 1999;32:1316–1332. doi: 10.1046/j.1365-2958.1999.01459.x. [DOI] [PubMed] [Google Scholar]
- 22.Nassif X, Lowy J, Stenberg P, O'Gaora P, Ganji A, So M. Antigenic variation of pilin regulates adhesion of Neisseria meningitidis to human epithelial cells. Mol Microbiol. 1993;8:719–725. doi: 10.1111/j.1365-2958.1993.tb01615.x. [DOI] [PubMed] [Google Scholar]
- 23.Nassif X, Beretti J-L, Lowy J, Stenberg P, O'Gaora P, Pfeifer P, Normark S, So M. Roles of pilin and PilC in adhesion of Neisseria meningitidis to human epithelial and endothelial cells. Proc Natl Acad Sci USA. 1994;91:3769–3773. doi: 10.1073/pnas.91.9.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ota H, Katsuyama T, Ishii K, Nakayama J, Shiozawa T, Tsukahara Y. A dual method for identifying mucins of different gastric epithelial mucous cells. Histochem J. 1991;23:22–28. doi: 10.1007/BF01886504. [DOI] [PubMed] [Google Scholar]
- 25.Rahman M, Källström H, Normark S, Jonsson A-B. PilC of pathogenic Neisseria is associated with the bacterial cell surface. Mol Microbiol. 1997;25:11–25. doi: 10.1046/j.1365-2958.1997.4601823.x. [DOI] [PubMed] [Google Scholar]
- 26.Rudel T, van Putten J P M, Gibbs C P, Haas R R, Meyer T F. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol Microbiol. 1992;6:3439–3450. doi: 10.1111/j.1365-2958.1992.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 27.Rudel T, Boxberger H J, Meyer T F. Pilus biogenesis and epithelial cell adherence of Neisseria gonorrhoeae pilC double knock-out mutants. Mol Microbiol. 1995;1:1057–1071. doi: 10.1111/j.1365-2958.1995.mmi_17061057.x. [DOI] [PubMed] [Google Scholar]
- 28.Rudel T, Scheuerpflug I, Meyer T F. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature. 1995;373:357–359. doi: 10.1038/373357a0. [DOI] [PubMed] [Google Scholar]
- 29.Scheuerpflug I, Rudel T, Ryll R, Pandit J, Meyer T F. Roles of PilC and PilE proteins in pilus-mediated adherence of Neisseria gonorrhoeae and Neisseria meningitidis to human erythrocytes and endothelial and epithelial cells. Infect Immun. 1999;67:834–843. doi: 10.1128/iai.67.2.834-843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973;137:571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swanson J, Bergstrom S, Robbins K, Barrera O, Corwin D, Koomey J M. Gene conversion involving the pilin structural gene correlates with pilus+ pilus− changes in Neisseria gonorrhoeae. Cell. 1986;47:267–276. doi: 10.1016/0092-8674(86)90449-6. [DOI] [PubMed] [Google Scholar]
- 32.Sweetser D A, Birtenmeier E H, Hoppe P C, McKeel D W, Gordon J I. Mechanisms underlying generation of gradients in gene expression within intestine: an analysis using transgenic mice containing fatty acid binding protein-human growth hormone fusion genes. Genes Dev. 1988;2:1318–1332. doi: 10.1101/gad.2.10.1318. [DOI] [PubMed] [Google Scholar]
- 33.Taketo M, Schroeder A C, Morbraaten L E, Gunning K B, Hanten G, Fox R R, Roderick R H, Stewart C L, Lilly F, Hansen C T, Overbecek P A. FVB/N: an inbred mouse strain preferable for transgenic analysis. Proc Natl Acad Sci USA. 1991;88:2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Putten J P, Paul S M. Binding of syndecan-like surface proteoglycan receptors for Neisseria gonorrhoeae entry into human mucosal cells. EMBO J. 1995;14:2144–2154. doi: 10.1002/j.1460-2075.1995.tb07208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virji M, Watt S M, Barker S, Makepeace K, Doyonnas R. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol Microbiol. 1996;22:929–939. doi: 10.1046/j.1365-2958.1996.01548.x. [DOI] [PubMed] [Google Scholar]
- 36.Virji M, Makepeace K, Ferguson J P, Watt S M. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol. 1996;22:941–950. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- 37.Watt P J, Ward M E. Adherence of Neisseria gonorrhoeae and other Neisseria species to mammalian cells. In: Beachey E H, editor. Bacterial adherence. Receptors and recognition, series B. Vol. 6. London, England: Chapman & Hall; 1980. pp. 253–288. [Google Scholar]
- 38.Woodward M P, Young W W, Jr, Bloodgood R A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985;78:143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]