Abstract
Dietary quality and patterns may influence SARS‐CoV‐2 infection and outcomes, but scientific data and evidence to support such a role are lacking. Therefore, this meta‐analysis aims to elucidate the effect of prepandemic diet quality on the risk of COVID‐19 infection and hospitalization. PubMed/MEDLINE, CENTRAL, Scopus, and EMBASE were systematically searched for articles published up to September 1, 2022. A systematic review and meta‐analysis were performed to calculate each outcome's risk ratio (RR) and 95% confidence interval (CI). Five studies including 4 023 663 individuals (3 149 784 high‐quality diet individuals and 873 881 controls) were included in the present meta‐analysis. The effectiveness of high‐quality dietary pattern against SARS‐CoV‐2 infection and hospitalization was 28% (95% CI 19%–36%) and 62% (95% CI 25%–80%); respectively. Subgroup analysis based on different levels of diet quality showed no difference between middle and high levels of diet quality in reducing the risk of COVID‐19 infection. Interestingly, subgroup analysis based on the different types of high‐quality diets and the risk of COVID‐19 infection revealed that the effectiveness of plant‐based diet against SARS‐CoV‐2 infection was 50% (95% CI 30%–65%); while the effectiveness of Mediterranean diet against SARS‐CoV‐2 infection was 22% (95% CI 12%–31%). Adherence to a high‐quality dietary pattern is associated with a lower risk of COVID‐19 infection and hospitalization. More studies are required to confirm these findings, and future studies should determine the biological mechanisms underlying the association between diet quality and risk of COVID‐19 infection.
Keywords: COVID‐19, diet quality, Mediterranean diet, meta‐analysis, plant‐based diet
1. INTRODUCTION
Many factors related to COVID‐19 severity have been reported, including a physically active lifestyle, age, history of chronic diseases, and cultural status. 1 , 2 It has been demonstrated that nutritional quality and status play a key role in innate and adaptive immunity, and are associated with the pathogenesis and progression of COVID‐19, as in other viral infections. 3
Several dietary patterns, which are mostly considered high quality diets, including plant‐based diet, 4 , 5 , 6 Mediterranean diet, 7 , 8 pre and probiotic diet, 9 and low‐fat diet 10 have been documented to reduce the risk and severity of COVID‐19. It is possible for poor diets to induce inflammation and immune system dysfunction, which can disrupt the body's immune system in dealing with respiratory viral infections. 11 Furthermore, a significant negative association has been reported between the severity of COVID‐19 symptoms and increased habitual intake frequency of specific food groups such as legumes and grains, 3 while individuals living in countries with a diet containing more milk, food products with potent anti‐ACE activity, and cabbage products have a lower severity and mortality rate of COVID‐19. 10 It has been documented that polyphenolic compounds in special plant‐based products significantly inhibit viral infection and prolong cellular survival after virus infection. 12 Altogether, these findings clearly indicate that dietary patterns could be associated with the risk and severity of COVID‐19 infection.
Given the potential positive effect of high‐quality diet patterns on the risk and severity of COVID‐19, this meta‐analysis aims to explore the infection and hospitalization rates of COVID‐19 in individuals with a history of high‐quality diet before the onset of the pandemic.
2. METHODS
The current systematic review and meta‐analysis were carried out following methodological guidelines from the Cochrane Handbook for Systematic Reviews, and the findings were reported following the Preferred Reporting Items for Systematic Review and Meta‐Analyses statement (Supporting Information: S1). 13 This systematic review followed a preplanned but unpublished protocol, owing to the importance of the topic and the necessary need for the timely dissemination of the present findings. Data is available on reasonable request from the corresponding author.
2.1. Search strategy
Three electronic databases, including PubMed/Medline, CENTRAL, Scopus, and EMBASE, were systematically searched by two researchers (M. A. and R. F.) up to September 2022. The search strategy and terms are listed in Supporting Information: S2. We searched all reference lists of included studies to find other eligible articles. Language restriction was not considered in our systematic search.
2.2. Eligibility criteria
The Eligibility criteria for the present systematic review and meta‐analysis followed PICOs question. 14 The Population; Intervention; Comparator; Outcome question was as follows: in general adult population (P), does plant‐based diet or Mediterranean dietary pattern (I) compared to the normal dietary pattern (C), affect COVID‐19 infection and hospitalization (O)?
We included studies that evaluated the effects of prepandemic dietary patterns on the risk of COVID‐19 infection and hospitalization. We included prospective or retrospective cohort studies and cross‐sectional studies on individuals who had high‐quality dietary patterns or habits before the onset of the pandemic. Studies were excluded if they reported improved dietary patterns or habits during the pandemic. Finally, abstracts with insufficient data and studies with no reported sample size were excluded from the present systematic review and meta‐analysis.
2.3. Data extraction and quality assessment
The following data were extracted from the eligible studies: study design, country, age and gender, type of diet, diet assessment method, diet assessment period before the pandemic onset, history of diabetes, cardiovascular and pulmonary diseases, and relative outcomes. The quality of included cohort and cross‐sectional studies was assessed using the Newcastle–Ottawa Scale (NOS). 15 Data extraction and quality assessment were independently performed by two reviewers (M. R. and R. F.), and discrepancies were solved by consensus with a third researcher (J. I. Sh) before the meta‐analysis.
2.4. Subgroup analysis
We performed four sets of subgroup analyses. First, we did a subgroup analysis based on different study types (cohorts vs. cross‐sectional studies). Second, we performed another subgroup analysis based on different levels of diet quality (middle vs. high) to determine the possible dose–response of improved dietary patterns on the risk of COVID‐19 infection. Third, we performed another subgroup analysis based on different dietary patterns (plant‐based vs. Mediterranean diet). Fourth, we performed another subgroup analysis based on different dietary assessment periods (1 vs. 4 and 10 year).
2.5. Statistical analyses
All meta‐analyses in the current study were conducted using Review manager (Version 5.4, The Nordic Cochrane Centre, Copenhagen, The Cochrane Collaboration, 2014), and a p value less than 0.05 was considered significant. Dichotomous outcomes were pooled and expressed as mean risk ratio (RR) with corresponding 95% confidence intervals (CI). 16 The pooled analysis results were classified based on study types into two categories, cohort, and cross‐sectional studies, and the pooled effect sizes were estimated using the random‐effect model. 17 Moreover, Cochran's Q statistics and I 2 were used to calculate heterogeneity. The potential for publication bias was assessed using funnel plots with Egger weighted regression test. Finally, to assess the robustness of summary estimates and to detect if any particular study accounted for a large proportion of heterogeneity, the overall pooled effect size of the respective outcomes was re‐estimated by the one study removed methods to perform sensitivity analysis. 1
3. RESULTS
3.1. Study identification and characteristics
We identified five studies involving 4 023 663 individuals (3 149 784 high‐quality diet individuals and 873 881 controls) addressing the effects of different high‐quality dietary patterns on COVID‐19 infection and hospitalization (Figure 1). Reports were published between 2021 and 2022 using the following experimental designs: three cohorts 6 , 7 , 8 and two cross‐sectional studies. 4 , 5 Except for two cross‐sectional studies, all cohorts reported the prevalence of comorbidity, including hypertension, diabetes mellitus, cardiovascular disease, and pulmonary disease among included individuals. The included studies used different types of diet screening and diagnostic tools, including self‐reported questionnaire, 4 , 5 Leeds Short Form Food Frequency Questionnaire, 6 and Mediterranean Diet Score. 7 , 8 Participants’ data range regarding dietary pattern were collected from 1 year, 4 , 5 , 6 4 years, 8 and to 10 years 7 before the pandemic. Association between dietary pattern with COVID‐19 outcomes was evaluated in the included studies using the following strategies: plant‐based diet, 4 , 5 , 6 and Mediterranean diet. 7 , 8 All the cohort and cross‐sectional studies were of mild to high quality, with NOS scores between 6 and 8 (Supporting Information: S3).
Figure 1.
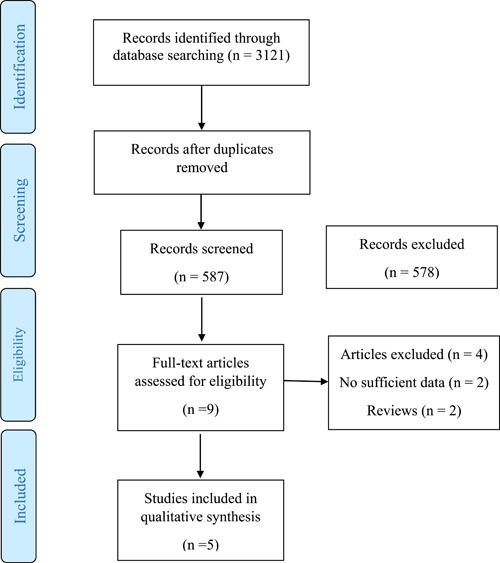
PRISMA flow diagram of study selection. PRISMA, Preferred Reporting Items for Systematic Review and Meta‐Analyses.
3.2. The effect of adherence to high‐quality dietary pattern on the risk of COVID‐19 infection
Five studies involving 4 907 637 individuals (3 149 784 high‐quality diet individuals and 1 757 853 controls) reported the risk of COVID‐19 infection in individuals with high‐quality dietary pattern. 4 , 5 , 6 , 7 , 8 , 18 Overall pooled analyses showed a 28% decrease of COVID‐19 infection was associated with high‐quality dietary patterns (RR = 0.72, 95% CI 0.64–0.81, p = 0.00001; Figure 2). Significant heterogeneity was observed among the included studies (I 2 = 85%, p = 0.00001). According to the study types, the pooled main effect analyses in cohorts and cross‐sectional studies were RR, 0.79 (95% CI: 0.74, 0.85; p = 0.00001), and RR, 0.37 (95% CI: 0.15, 0.93; p = 0.03), respectively. Subgroup analysis based on different levels of high‐quality diet showed no difference between middle and high levels of diet quality in reducing the risk of COVID‐19 infection (Figure 3). Interestingly, subgroup analysis based on the different types of high‐quality diets and the risk of COVID‐19 infection showed that the effectiveness of plant‐based diet against SARS‐CoV‐2 infection was 50% (95% CI 30%–65%, 3 129 796 high‐quality diet individuals and 1 740 267 controls, I 2 = 97%). While, the effectiveness of Mediterranean diet against SARS‐CoV‐2 infection was 22% (95% CI 12%–31%, 20 266 high‐quality diet individuals and 17 586 controls, I 2 = 66%; Figure 4). Further, subgroup analysis based on different dietary assessment periods showed no difference between 1, 4, and 10 years of assessment in reducing the risk of COVID‐19 infection (Figure 5).
Figure 2.
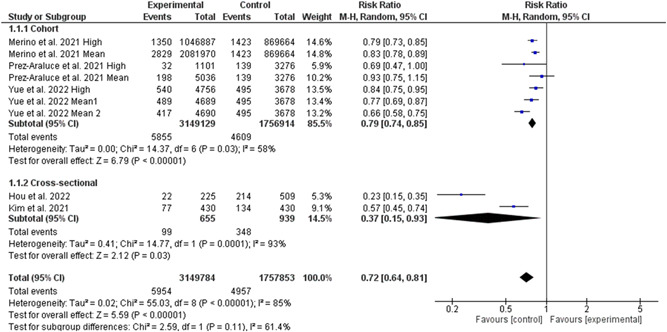
Meta‐analysis of the risk of COVID‐19 infection change means for adherence to high‐quality dietary pattern. CI, confidence interval; IV, inverse‐variance method; SD, standard deviation; SMD, standardized mean difference; MD, mean difference.
Figure 3.
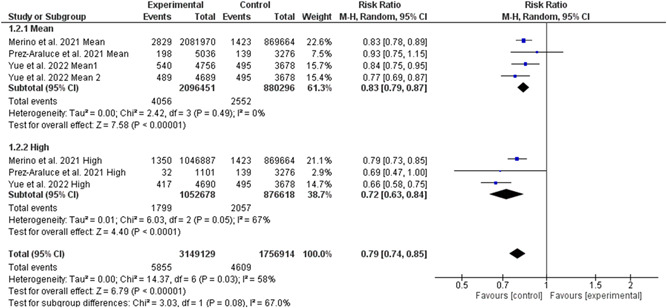
Subgroup analysis based on different levels of diet quality in reducing the risk of COVID‐19 infection. CI, confidence interval; IV, inverse‐variance method; SD, standard deviation; SMD, standardized mean difference; MD, mean difference.
Figure 4.
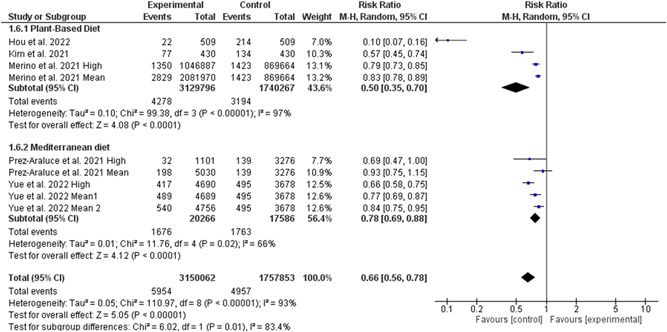
Subgroup analysis based on different types of diet in reducing the risk of COVID‐19 infection. CI, confidence interval; IV, inverse‐variance method; SD, standard deviation; SMD, standardized mean difference; MD, mean difference.
Figure 5.
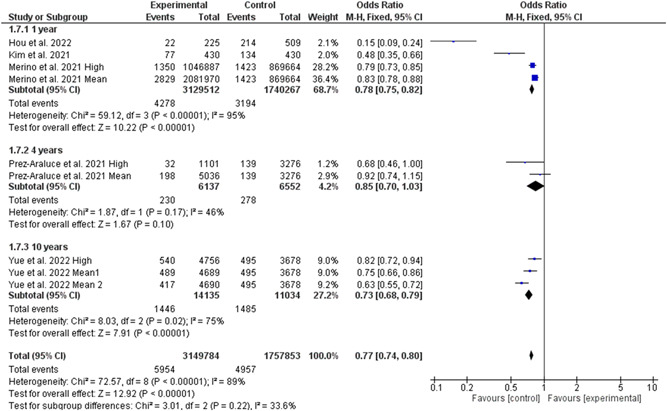
Subgroup analysis based on different dietary assessment periods in reducing the risk of COVID‐19 infection. CI, confidence interval; IV, inverse‐variance method; SD, standard deviation; SMD, standardized mean difference; MD, mean difference.
3.3. The effect of adherence to high‐quality dietary pattern on the risk of COVID‐19 hospitalization
The random‐effect model analyses including three studies involving 4 009 271 individuals (3 136 629 high‐quality diet individuals and 872 642 controls) showed the risk of COVID‐19 hospitalization was reduced by 62% in association with high‐quality dietary pattern (RR = 0.38, 95% CI 0.20–0.75, p = 0.005; Figure 6). The χ 2 statistic implicated significant heterogeneity among the included studies (I 2 = 95%, p = 0.00001). The pooled main effects were comparable for the different study designs: RR = 0.80, 95% CI: 0.67, 0.96; p = 0.02 (in cohorts), RR = 0.17, 95% CI: 0.11, 0.28; p = 0.00001 (in cross‐sectionals). The number of studies was too small to permit subgroup analysis based on the levels of diet quality and types.
Figure 6.
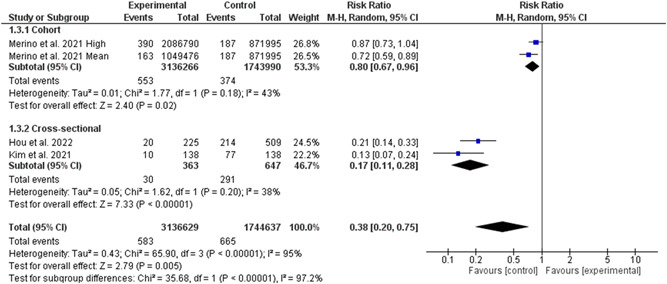
Meta‐analysis of the risk of COVID‐19 hospitalization change means for adherence to high‐quality dietary pattern. CI, confidence interval; IV, inverse‐variance method; SD, standard deviation; SMD, standardized mean difference; MD, mean difference.
3.4. The effect of adherence to high‐quality dietary pattern on the risk of critical COVID‐19
Only one study reported the risk of critical COVID‐19 in individuals with high‐quality dietary pattern. Hou et al., in a retrospective evaluation of 509 patients who had been diagnosed with COVID‐19, indicated that from 79 patients who were admitted to the intensive care unit, only one of them followed vegetarian diet. They found that COVID‐19 patients with adherence to nonvegetarian diet had significantly higher odds of developing critical COVID‐19 (adjusted odds ratio (OR) = 5.434, 95% CI: 1.624–18.826, p = 0.005).
3.5. Publication bias
Funnel plots suggested no noticeable bias in the studies of the present meta‐analysis. Further, Begg's correlation rank and Egger's regression did not show significant publication bias (Supporting Information: S3).
4. DISCUSSION
In the present systematic review and meta‐analysis, we performed a pooled analysis to estimate the effect of prepandemics' dietary pattern on the risk and severity of COVID‐19 infection. Based on the results of five eligible articles, the present meta‐analysis shows that previous high‐quality dietary patterns reduce the risk of COVID‐19 infection and hospitalization.
It has been well documented that healthy dietary patterns may play a role in the incidence or disease course of COVID‐19 by improving the immune response. 4 , 5 , 6 , 18 The standard nutritional guideline reduces nutritional risk by correcting energy intake, oxidative stress and inflammatory responses, and also helps to strengthen the immune system to fight against infectious diseases. 19 Higher fiber, vitamins A, C, and E, folate, and mineral (iron, potassium, magnesium) intake have been reported among those with highest versus lowest adherence to plant‐based diets. 20 Vitamin A plays an important role in regulating immune function and its deficiency leads to decreased immunity and thus increases the chance of contracting infectious diseases. 21 In addition, vitamin C is a potent antioxidant and protects against free radicals released from phagocytes in viral infections. 22 By accumulating in phagocytic cells such as neutrophils, vitamin C improves chemotaxis and phagocytosis, and ultimately removes bacteria and viruses and supports the immune system from viral infection. 23 Vitamin E also helps to strengthen the immune system and destroy bacteria and viruses by maintaining the integrity of the membrane of T‐cells. 24
Moreover, higher adherence to a Mediterranean‐style dietary pattern has been associated with a lower risk of respiratory infections and COVID‐19‐related deaths. 25 Mediterranean diet, as a rich source of iron, zinc, selenium, and omega‐3, as potent antioxidants, can reduce lipid peroxidation, increase the immune response, and prevent various infectious diseases. 26 Importantly, vitamin D can prevent respiratory infections, especially those of a viral nature, by maintaining strong connections between immune cells, killing viruses by releasing Cathelicidin and Alpha defensin, and reducing the production of inflammatory cytokines through innate immunity, thus reducing the risk of pneumonia‐induced cytokine storm. 27 A meta‐analysis study on 39 randomized clinical studies showed that vitamin D leads to an 11% reduction in the rate of COVID‐19 infection. 28 Taken together, these studies show that diets containing sufficient amounts of vitamins and micronutrients, such as vegetables, fruits, and dairy products, can reduce the risk of SARS‐CoV‐2 infection (Table 1).
Table 1.
General characteristics of included studies
| Study | Design | Country | Group (gender: %M) | Age (year) | Type of diet: diet assessment method | Diet assessment perioda | History of diabetes, % | History of CVD, % | History of Pulmonary disease, % | Outcome measure |
|---|---|---|---|---|---|---|---|---|---|---|
| Hou et al. (2022) 4 | Cross‐sectional | Switzerland | 509 (52.8) | 52.2 ± 16.6 | Plant‐based: self‐reported questionnaire (vegetarian, vegan, ovo‐lacto vegetarian, lacto‐vegetarian, ovo‐vegetarian) | 1 year | NR | NR | NR | Infection, hospitalization |
| Kim et al. (2021) 5 | Cross‐sectional | France, Germany, Italy, Spain, UK, USA | 2884 (27.5) | 48 ± 10 | Plant‐based: self‐reported questionnaire for 22 food items (fruits, vegetables, potatoes, legumes, nuts, refined grains, dark or whole grain breads, sweets and desserts, eggs, dairy, poultry, meat, fish and seafood, soups, croquettes, sugar‐sweetened beverages, fruit juices, vegetable oil, butter, alcohol, coffee, and tea) | 1 year | NR | NR | NR | Infection, hospitalization |
| Merino et al. (2021) 6 | Cohort | UK, USA | Low: 148143 (60.5) | 52 ± 10.5 | Plant‐based: leeds short form food frequency questionnaire for 18 food items (wholegrains, fruits, vegetables, nuts, legumes, vegetable oils, tea and coffee, fruit juice, refined grains, potatoes, sugar sweetened beverages, sweets and desserts, animal fats, dairy, egg, fish and seafood, meat, miscellaneous) | 1 year | 4.1 | 3.5 | 11.8 | Infection, hospitalization |
| Middle: 296286 (68.4) | 57 ± 10.5 | 3.4 | 3.6 | 10.5 | ||||||
| 2.6 | 3 | 9.6 | ||||||||
| High: 148142 (75.5) | 57 ± 10 | |||||||||
| Perez‐Araluce et al. (2021) 7 | Cohort | Spain | Low: 3276 (56.2) | 50.1 ± 10.8 | Mediterranean: Mediterranean diet score for 10 food items (cereals, fruits, nuts, vegetables, legumes, fish, meat, dairy products, alcohol, and ratio of monounsaturated to saturated fat) | 10 year | 2.4 | 4.3 | 6.9 | Infection |
| Middle: 5036 (55.5) | 53.4 ± 12.7 | 3.6 | 4.7 | 6.1 | ||||||
| High: 1101 (50.1) | 57.7 ± 13.8 | 4.3 | 5.2 | 4.5 | ||||||
| Yue et al. (2022) 8 | Cohort | USA | T: 4466 (2) | 66.2 ± 6.1 | Mediterranean: Mediterranean diet score for 9 food items (vegetables, fruits, nuts, whole grains, legumes, fish, meat, alcohol, and ratio of monounsaturated to saturated fat) | 4 year | 7.4 | 0.7 | NR | Infection |
| C: 4213 (6.5) | 66.8 ± 6 | 3.5 | 0.4 |
Abbreviations: C, control group; CVD, cardiovascular disease; not reported; T, treatment group.
This period indicates the time period before the date of COVID‐19 diagnosis.
Another interesting result of the present study is the different effectiveness between a plant‐based diet and a Mediterranean diet, where the plant‐based diet showed better results on the infection rate of COVID‐19. This finding may partly explain the higher infection rates in southern European countries with higher adherence to the Mediterranean diet than others. 29 , 30 Although this finding was extracted from just four eligible studies and due to the paucity of included studies, it must be interpreted with caution. Previous studies have reported that a plant‐based diet decreases the risk of respiratory infections, such as the common cold and pneumonia, and can shortens the duration of these illnesses. 31 , 32 It is worth mentioning that consuming five main food groups, with an emphasis on whole grains and consumption of gemma varieties, whole grains containing B vitamins and selenium can support health of the immune system. 33 Although to provide protein, consuming meat, eggs, fish, legumes, almonds, walnuts, and hazelnuts which contain omega‐3, is beneficial to strengthen the immune system responses. 33 , 34 Therefore, diets containing vegetable and marine oils and dairy products can strengthen the body's immune system against SARS‐CoV‐2 and reduce the infection rate.
Findings from the present systematic review and meta‐analysis must be interpreted in light of its limitations. First, the results of the present meta‐analysis were extracted from just five eligible studies, and due to the paucity of included studies, its results must be interpreted with caution. More high‐quality research is needed to determine the impacts of dietary patterns on the risk and severity of COVID‐19 infection. Second, different dietary patterns were enrolled in included studies, making it difficult to determine which dietary pattern is the best for reducing the risk and severity of COVID‐19 infection. Third, in five included studies in the present meta‐analysis, the data related to dietary patterns were assessed in a vast range of periods from 1 year to 10 years before the pandemic. Forth, all included studies enrolled data for COVID‐19 infection rates from the first wave only, and data from other variants are limited. Fifth, considering the present meta‐analysis's forest plots indicate an exaggerated effect size in cross‐sectional studies than in cohort studies. Finally, data for the association between severe to critical COVID‐19 patients had not been included in most studies which prevented meta‐analysis for intensive care unit admission and mortality rates.
5. CONCLUSIONS
In conclusion, the present systematic review and meta‐analysis provides evidence that adherence to high‐quality dietary pattern is associated with reduced risk of COVID‐19 infection and hospitalization rates. Additional high‐quality research is necessary to determine the real impacts of dietary patterns on the risk and severity of COVID‐19 during the global pandemic.
AUTHOR CONTRIBUTIONS
Masoud Rahmati and Jae Il Shin developed the idea and designed the study and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Masoud Rahmati and Rouholah Fatemi ran the search strategy; Masoud Rahmati and Rouholah Fatemi selected articles and extracted data; Masoud Rahmati evaluated the quality of the literature. Masoud Rahmati, Rouholah Fatemi, Ai Koyanagi, Dong K Yon, Seung Won Lee, Jae Il Shin, and Lee Smith wrote the manuscript. All listed authors reviewed and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Rahmati M, Fatemi R, Yon DK, et al. The effect of adherence to high‐quality dietary pattern on COVID‐19 outcomes: a systematic review and meta‐analysis. J Med Virol. 2022;95:e28298. 10.1002/jmv.28298
Contributor Information
Masoud Rahmati, Email: rahmati.mas@lu.ac.ir.
Jae Il Shin, Email: shinji@yuhs.ac.
DATA AVAILABILITY STATEMENT
All data relevant to the study are included in the article or uploaded as Supporting Information. The data are available by accessing the published studies listed in Table1.
REFERENCES
- 1. Rahmati M, Shamsi MM, Khoramipour K, et al. Baseline physical activity is associated with reduced mortality and disease outcomes in COVID‐19: a systematic review and meta‐analysis. Rev Med Virol. 2022;32:e2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Summers C, Do Vale ML, Haines L, et al. A web‐based survey assessing perceived changes in diet, physical activity and sleeping behaviours in adults with type 1 and type 2 diabetes during the COVID‐19 pandemic in the UK. BMJ Nutr Prev Health. 2022; e000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salazar‐Robles E, Kalantar‐Zadeh K, Badillo H, et al. Association between severity of COVID‐19 symptoms and habitual food intake in adult outpatients. BMJ Nutr Prev Health. 2021;4(2):469‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hou Y‐C, Su W‐L, Chao Y‐C. COVID‐19 illness severity in the elderly in relation to vegetarian and non‐vegetarian diets: a single‐center experience. Front Nutr. 2022;9:837458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim H, Rebholz CM, Hegde S, et al. Plant‐based diets, pescatarian diets and COVID‐19 severity: a population‐based case–control study in six countries. BMJ Nutr Prev Health. 2021;4(1):257‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Merino J, Joshi AD, Nguyen LH, et al. Diet quality and risk and severity of COVID‐19: a prospective cohort study. Gut. 2021;70(11):2096‐2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perez‐Araluce R, Martinez‐Gonzalez MA, Fernández‐Lázaro CI, Bes‐Rastrollo M, Gea A, Carlos S. Mediterranean diet and the risk of COVID‐19 in the ‘Seguimiento Universidad de Navarra’ cohort. Clin Nutr. 2021;S026‐1‐5614(21):0019‐0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yue Y, Ma W, Accorsi EK, et al. Long‐term diet and risk of SARS ‐CoV‐2 infection and Coronavirus Disease 2019 (COVID‐19) severity. Am J Clin Nutr. 2022;nqac2019 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olaimat AN, Aolymat I, Al‐Holy M, et al. The potential application of probiotics and prebiotics for the prevention and treatment of COVID‐19. NPJ Science of Food. 2020;4(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bousquet J, Anto JM, Iaccarino G, et al. Is diet partly responsible for differences in COVID‐19 death rates between and within countries? Clin Transl Allergy. 2020;10(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morais AHA, Aquino JS, da Silva‐Maia JK, Vale SHL, Maciel BLL, Passos TS. Nutritional status, diet and viral respiratory infections: perspectives for severe acute respiratory syndrome Coronavirus 2. Br J Nutr. 2021;125(8):851‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin S‐C, Ho CT, Chuo WH, Li S, Wang TT, Lin CC. Effective inhibition of MERS‐CoV infection by resveratrol. BMC Infect Dis. 2017;17(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg. 2010;8(5):336‐341. [DOI] [PubMed] [Google Scholar]
- 14. Eriksen MB, Frandsen TF. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc. 2018;106(4):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. [DOI] [PubMed] [Google Scholar]
- 16. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101‐129. [Google Scholar]
- 17. Lee SW. Methods for testing statistical differences between groups in medical research: statistical standard and guideline of life cycle committee. Life Cycle. 2022;2:e1. [Google Scholar]
- 18. Louca P, Murray B, Klaser K, et al. Modest effects of dietary supplements during the COVID‐19 pandemic: insights from 445 850 users of the COVID‐19 symptom study app. BMJ Nutr Prev Health. 2021;4(1):149‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao X, Li Y, Ge Y, et al. Evaluation of nutrition risk and its association with mortality risk in severely and critically ill COVID‐19 patients. J Parenter Enteral Nutr. 2021;45(1):32‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim H, Rebholz CM, Garcia‐Larsen V, Steffen LM, Coresh J, Caulfield LE. Operational differences in plant‐based diet indices affect the ability to detect associations with incident hypertension in middle‐aged US adults. J Nutr. 2020;150(4):842‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Speakman L, Michienzi S, Badowski M. Vitamins, supplements and COVID‐19: a review of currently available evidence. Drugs Context. 2021;10:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Calder P, Carr A, Gombart A, Eggersdorfer M. Optimal nutritional status for a well‐functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12(4):1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jovic TH, Ali SR, Ibrahim N, et al. Could vitamins help in the fight against COVID‐19? Nutrients. 2020;12(9):2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beigmohammadi MT, Bitarafan S, Hoseindokht A, et al. Impact of vitamins A, B, C, D, and E supplementation on improvement and mortality rate in ICU patients with coronavirus‐19: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greene MW, Roberts AP, Frugé AD. Negative association between Mediterranean diet adherence and COVID‐19 cases and related deaths in Spain and 23 OECD countries: an ecological study. Front Nutr. 2021;8:591964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taghdir M, et al. A review on some nutrition‐based interventions in Covid‐19. J Mil Med. 2020;22(2):169‐176. [Google Scholar]
- 27. Bui L, Zhu Z, Hawkins S, Cortez‐Resendiz A, Bellon A. Vitamin D regulation of the immune system and its implications for COVID‐19: a mini review. SAGE Open Med. 2021;9:205031212110140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Butler‐Laporte G, Nakanishi T, Mooser V, et al. Vitamin D and Covid‐19 susceptibility and severity: a Mendelian randomization study. PLoS Med. 2021;1:e1003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Konstantinoudis G, Cameletti M, Gómez‐Rubio V, et al. Regional excess mortality during the 2020 COVID‐19 pandemic in five European countries. Nat Commun. 2022;13(1):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta‐analysis of observational studies. Cancer Med. 2015;4(12):1933‐1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;2013(1):CD000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;8(8):CD005532. [DOI] [PubMed] [Google Scholar]
- 33. Detopoulou P, Demopoulos CA, Antonopoulou S. Micronutrients, phytochemicals and Mediterranean diet: a potential protective role against COVID‐19 through modulation of PAF actions and metabolism. Nutrients. 2021;13(2):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kahleova H, Barnard ND. Can a plant‐based diet help mitigate Covid‐19? Eur J Clin Nutr. 2022;76:911‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as Supporting Information. The data are available by accessing the published studies listed in Table1.


