Abstract
Variable-region-identical mouse immunoglobulin G1 (IgG1), IgG2b, and IgG2a monoclonal antibodies to the capsular polysaccharide of Cryptococcus neoformans prolong the lives of mice infected with this fungus, while IgG3 is either not protective or enhances infection. CD4+ T cells are required for IgG1-mediated protection, and CD8+ T cells are required for IgG3-mediated enhancement. Gamma interferon is required for both effects. These findings revealed that T cells and cytokines play a role in the modulation of cryptococcal infection by antibodies and suggested that it was important to more fully define the cytokine requirements of each of the antibody isotypes. We therefore investigated the efficacy of passively administered variable-region-identical IgG1, IgG2a, IgG2b, and IgG3 monoclonal antibodies against intravenous infection with C. neoformans in mice genetically deficient in interleukin-12 (IL-12), IL-6, IL-4, or IL-10, as well as in the parental C57BL/6J strain. The relative inherent susceptibilities of these mouse strains to C. neoformans were as follows: IL-12−/− > IL-6−/− > C57BL/6J ≈ IL-4−/− ≫ IL-10−/−. This is consistent with the notion that a Th1 response is necessary for natural immunity against cryptococcal infection. However, none of the IgG isotypes prolonged survival in IL-12−/−, IL-6−/−, or IL-4−/− mice, and all isotypes significantly enhanced infection in IL-10−/− mice. These results indicate that passive antibody-mediated protection against C. neoformans requires both Th1- and Th2-associated cytokines and reveal the complexity of the mechanisms through which antibodies modulate infection with this organism.
Cryptococcus neoformans is an encapsulated yeast that is a frequent cause of life-threatening meningoencephalitis in patients with impaired immunity. The prevalence of cryptococcal meningitis in patients with AIDS ranges from 8% in the United States to 30% in Africa (11, 12, 84). Current therapy is inadequate, as 10 to 20% of patients treated with antifungal drugs die from cryptococcal meningitis (10, 76). Furthermore, individuals who survive beyond the initial treatment period must be maintained on lifelong suppressive therapy to prevent relapse (62). Because of these therapeutic limitations, better treatments for C. neoformans infections are needed.
One new approach to improving therapy for cryptococcosis is the use of monoclonal antibodies (MAbs) to the glucuronoxylomannan (GXM) component of the C. neoformans capsular polysaccharide as adjuncts to antifungal drugs. Certain MAbs to GXM can protect mice against C. neoformans infection and enhance the efficacy of antifungal therapy (17, 18, 52–56). A murine immunoglobulin G1 (IgG1) MAb is currently undergoing phase I evaluation for the treatment of cryptococcal meningitis in patients with AIDS (7). Studies using MAbs to GXM have demonstrated that antibody-mediated protection in murine models of systemic cryptococcal infection is dependent on the antibody isotype. Comparisons of variable-region-identical antibodies of the IgG1, IgG2a, IgG2b, and IgG3 isotypes have consistently shown that all isotypes, except IgG3, prolong survival of mice infected with C. neoformans (61, 79, 82). This difference is not dependent on antigen clearance because all IgG isotypes accelerate clearance of GXM in infected animals in a similar manner (43). These observations indicate that functions mediated by the constant regions of these MAbs are crucial for determining their protective potential. While Fc receptors play a role in antibody-mediated protection (80), the exact mechanisms responsible for these phenomena are not understood.
It is our hope that a better understanding of the variables that mediate antibody efficacy will lead to the design of more-effective antibody-based therapeutics. Prior experiments on immunodeficient mice showed that CD4+ T cells and gamma interferon (IFN-γ) are necessary for protection by IgG1 and that CD8+ T cells and IFN-γ are required for enhancement of infection by IgG3 (81). These results revealed the importance of T cells and the Th1 cytokine IFN-γ in modulating the protective efficacy of the different isotypes. Before attempting to identify the detailed mechanisms responsible for the interaction of antibodies, T cells, cytokines, effector cells, and the organism, it was important to more fully define the types of cytokines that could affect this process. To do this, we investigated the capacity of passively administered IgG subclasses to protect mice deficient in either the Th1 cytokine interleukin-12 (IL-12), the proinflammatory cytokine IL-6, or the Th2 cytokines IL-4 and IL-10 against cryptococcal infection. We first studied the innate susceptibility of each of these genetically deficient mice to cryptococcal infection. The results demonstrated that C. neoformans infection was accelerated in IL-12−/− and IL-6−/− mice, while IL-4−/− mice were as susceptible as the background strain, C57BL/6J. In contrast, IL-10−/− mice were very resistant to infection. This confirmed that Th1 cytokines contributed to the natural resistance of mice to cryptococcal infection. We then examined the effect of each of the antibody isotypes and found that none of the isotypes protected IL-12−/−, IL-6−/−, or IL-4−/− mice against C. neoformans, while all isotypes greatly enhanced infection in IL-10−/− mice. These results revealed that antibody-mediated protection against C. neoformans is dependent on both Th1- and Th2-associated cytokines and further highlight the interdependence of cellular and antibody-mediated immunity.
MATERIALS AND METHODS
Mice.
The mice with targeted disruption of specific cytokine genes used in these experiments, including IL-12p40−/− (46), IL-6−/− (38), IL-4−/− (42), and IL-10−/− (41) mice, have been reported previously. Fully backcrossed breeding pairs were obtained from The Jackson Laboratory (Bar Harbor, Maine) and were bred and maintained in isolator cages in a pathogen-free barrier facility within the Animal Care Institute at Albert Einstein College of Medicine, where they were checked daily. All mice had been backcrossed onto C57BL/6J for at least 10 generations, and, therefore, C57BL/6J mice were used as controls. The genotype of the breeders was confirmed by PCR of tail DNA using primers described previously (38, 41, 42, 46). Mice were used at 6 to 10 weeks of age; control mice were age matched.
Antibodies.
The 3E5 IgG3 hybridoma was obtained previously by fusing NSO cells to spleen cells from a mouse immunized with GXM conjugated to tetanus toxoid (8). The IgG1, IgG2b, and IgG2a switch variants of MAb 3E5 were generated by in vitro isotype switching (68, 79, 82). The variable-region sequences of these MAbs are identical, and all bind GXM (unpublished data; 82). Ascites fluid was obtained by injecting 5 × 106 hybridoma cells suspended in Hanks' buffered saline into the peritoneal cavities of pristane-primed (Sigma, St. Louis, Mo.) SCID mice. The ascites fluid was collected in a sterile fashion and centrifuged at 1,000 × g to remove cells. Lipids and cell debris were removed with Cleanascite HC (LigoChem, Fairfield, N.J.), and the ascites fluid was sterilized by passage through a 0.2-μm-pore-size filter. Antibody concentration relative to isotype-matched standards was then determined by enzyme-linked immunosorbent assay (ELISA). Ascites fluid was stored at 4°C and checked for activity by ELISA prior to each survival experiment. Each batch of ascites fluid was tested for contaminating isotypes, which were present at <0.001%. For some experiments, antibodies were purified by protein A affinity chromatography (20).
Murine infection
C. neoformans serotype D strain 24067 was obtained from the American Type Culture Collection (Manassas, Va.) and stored in sucrose at −80°C. This strain was selected for study because it has been extensively analyzed (25) and was used in previous studies of antibody efficacy (23, 51, 52, 54–56, 61, 79–82). C. neoformans was grown at 37°C in Sabouraud's dextrose broth (Difco Laboratories, Detroit, Mich.) to log phase. Yeast cells were then washed three times with phosphate-buffered saline (PBS), and the inoculum was determined by counting in a hemocytometer. The C. neoformans inoculum was diluted and plated on Sabouraud's dextrose agar (Difco) to confirm CFU estimates. Organisms were suspended in PBS and injected into the lateral tail vein in a volume of 0.2 ml. For survival studies, the number of mice per group ranged from 8 to 11. Twenty-four hours prior to infection with C. neoformans, mice were given an intraperitoneal injection of SCID mouse ascites fluid containing 1 mg of a single 3E5 IgG isotype or, as a control, 1 ml of ascites fluid made from NSO cells, the nonproductive mouse myeloma fusion partner used to make MAb 3E5. For survival studies with cytokine-deficient mice, an additional control group of NSO cell ascites fluid-treated C57BL/6J mice was included to ensure consistency of organism inoculum and virulence between experiments. In separate experiments, we have shown that mice infected with C. neoformans and treated with PBS or ascites fluid from NSO cells have the same survival as untreated mice. In a few survival experiments, mice were given purified MAbs and PBS was used as the control to determine if factors in the ascites fluid other than the MAb were influencing survival. No significant differences in survival were noted between animals treated with NSO cell ascites fluid and animals treated with PBS or between animals treated with purified antibody and animals treated with the same antibody in SCID mouse ascites fluid. Serum was obtained 14 days after infection, and GXM concentration was measured by capture ELISA as previously described (9).
CFU preparation and pathological examination.
Mice were killed by cervical dislocation on day 10 or 17 after infection, and their organs were removed in a sterile manner. The right upper lobe of the lung, caudal half of the spleen, accessory lobe of the liver, and right hemisphere of the brain were fixed in 10% buffered formalin and embedded in paraffin. Five mice per group were examined. Sections (5 μm thick) stained with hematoxylin and eosin (H&E) or mucicarmine were reviewed by light microscopy (one to three sections per organ). CFU were determined by homogenizing the remaining brain, lung, liver, and spleen tissue, which was then diluted in PBS and plated on Sabouraud's dextrose agar.
In vitro phagocytosis.
Peritoneal macrophages were obtained by peritoneal lavage from mice 5 days after intraperitoneal stimulation with 1.5 ml of 4% thioglycolate. Thioglycolate-stimulated mice were killed, and their peritoneal cavities were washed with 10 ml of Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). Alveolar macrophages were obtained from bronchoalveolar lavage. After the mice were killed, a 20-guage Angiocath (Becton Dickinson, Franklin Lakes, N.J.) was inserted into the trachea and the lungs were irrigated with 10 ml of PBS. Peritoneal or alveolar cells were counted, suspended in DMEM supplemented with 10% FCS, and plated on 96-well tissue culture plates (Costar, Corning, N.Y.) at a density of 3 × 104 to 4 × 104 mononuclear cells per well. Nonadherent cells were washed away after 2 h of incubation at 37°C. Adherent cells were incubated overnight without IL-10 or with 2 ng of murine recombinant IL-10 (R&D Systems, Minneapolis, Minn.)/ml. Three micrograms of purified 3E5 IgG1, IgG2a, IgG2b, or IgG3 MAb was then added to each well 20 min before adding 5 × 105 heat-killed C. neoformans cells per well and incubating for 4 h at 37°C. Wells were then washed three times with cold PBS to remove nonphagocytosed organisms, fixed with cold methanol for 25 min, and stained with a 1:10 solution of Giemsa (Sigma) for 20 min. The stain was then replaced with PBS. Multiple fields from three wells per condition were examined by inverted light microscopy at ×400 magnification; phagocytosis was expressed as the phagocytic index (percentage of cells with two or more internalized organisms).
Statistical methods.
Data were analyzed with StatView statistical software (SAS Institute, Cary, N.C.). Serum GXM and CFU data were compared using the Mann-Whitney U test for nonparametric data. Survival data were subjected to Kaplan-Meier analysis, and statistical significance was determined by the log rank (Mantel-Cox) test. A P value of less than 0.05 was considered statistically significant.
RESULTS
IgG1, IgG2b, and IgG2a prolonged the lives of C57BL/6J mice with cryptococcal infection, while IgG3 did not.
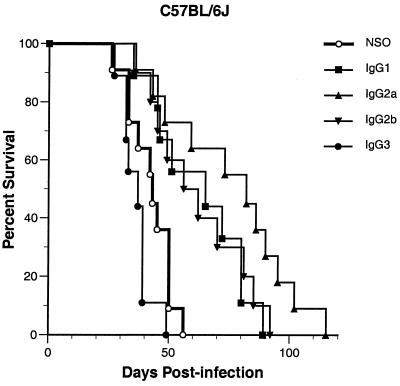
In previously reported experiments, we showed that the 3E5 IgG3 MAb to GXM does not prolong survival and that its in vitro switch variant 3E5 IgG1 protects C57BL/6J mice against intravenous cryptococcal challenge (81). To confirm these results and to further examine the efficacy of the other IgG isotypes in this mouse strain, which is the background strain for all the mice used in the experiments reported here, we gave C57BL/6J mice an intraperitoneal injection of SCID mouse ascites fluid containing 1 mg of either 3E5 IgG3 or one of its switch variants of the IgG1, IgG2b, or IgG2a isotype. SCID mouse ascites fluid made from NSO cells, the nonproductive hybridoma fusion partner used to generate MAb 3E5, was used as a control. After 24 h, animals were infected intravenously with 106 CFU of C. neoformans. The survival data in Fig. 1 demonstrate that the variable-region-identical 3E5 IgG1, IgG2b, and IgG2a MAbs significantly prolonged the lives of infected C57BL/6J mice (P < 0.006). IgG2a appeared to offer the most protection, but this tendency was not significant (P = 0.08 versus IgG1 and P = 0.09 versus IgG2b). IgG3, on the other hand, did not protect these mice and seemed to exacerbate infection somewhat, although this trend did not reach statistical significance (P = 0.09).
FIG. 1.
Survival of MAb-treated C57BL/6J mice infected with C. neoformans. Mice were given an intraperitoneal injection of SCID mouse ascites fluid containing either 1 mg of the anti-GXM 3E5 IgG3, one of its in vitro switch variants of the IgG1, IgG2b, or IgG2a isotype, or 1 ml of NSO cell SCID mouse ascites fluid as a control and then 24 h later were infected intravenously with 106 C. neoformans CFU. Administration of IgG1, IgG2b, or IgG2a was significantly protective compared to the control (P < 0.006). Animals treated with IgG3 fared worse than control mice, but this did not reach statistical significance (P = 0.09). Mean survival in days by treatment group: NSO cell ascites fluid, 41; IgG1, 63; IgG2a, 75; IgG2b, 62; IgG3, 36.
IL-12−/− and IL-6−/− mice were more susceptible than C57BL/6J to cryptococcal infection, while IL-10−/− mice were very resistant.
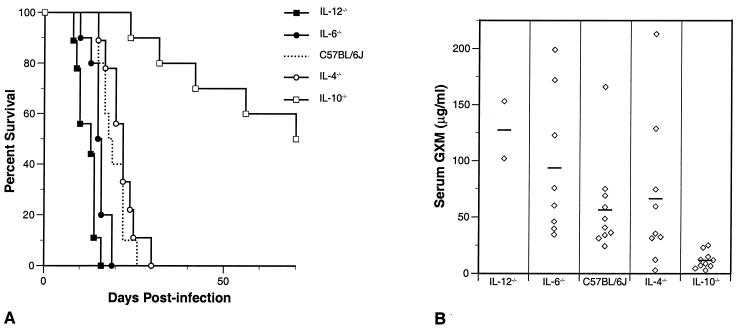
Next, we examined the course of C. neoformans infection in IL-4−/−, IL-6−/−, IL-10−/−, and IL-12−/− mice. Decken and colleagues have recently shown that IL-12−/− mice are highly susceptible to cryptococcal infection, while IL-4−/− mice are resistant to infection (15). Blackstock et al. have reported that IL-10−/− mice are resistant to infection (5). The susceptibility of IL-6−/− mice to C. neoformans has not been reported previously. To compare the inherent susceptibilities of the cytokine-deficient mice with that of the parental C57BL/6J mice and to anticipate studies with MAbs, animals were treated with NSO cell ascites fluid and infected with C. neoformans. Figure 2A shows the survival of IL-4−/−, IL-6−/−, IL-10−/−, and IL-12−/− mice in comparison to that of the parental C57BL/6J strain, all given 2.5 × 106 CFU of C. neoformans. As expected, IL-12−/− mice were highly susceptible to cryptococcal infection (P < 0.0001). IL-6−/− mice were also more susceptible to cryptococcal infection than the parental strain (P = 0.02). In contrast, IL-4−/− mice appeared slightly more resistant to infection than the parental strain, but the trend did not achieve statistical significance (P = 0.4). As reported previously (5), IL-10−/− mice were significantly more resistant to infection than the parental strain (P < 0.0001); 40% of the animals were still alive at 18 weeks, when the experiment was terminated. While CFU were not determined for these survivors, they all had significant serum GXM levels (mean = 87 μg/ml), indicating that they had not cleared the infection. Since the GXM levels all of the IL-10 −/− mice were low at 14 days (Fig. 2B), this suggests that there was a progressive increase in fungal burden that ultimately led to the death of the animals.
FIG. 2.
(A) Survival of untreated cytokine-deficient mice and the parental C57BL/6J mice infected intravenously with 2.5 × 106 C. neoformans CFU. IL-12−/− and IL-6−/− mice were more susceptible to infection than control mice (P < 0.0001 and P < 0.02, respectively), while IL-10−/− mice were very resistant to cryptococcal challenge (P < 0.0001). IL-4−/− mice resembled the parental C57BL/6J mice (P = 0.4). The experiment was terminated on day 140 postinfection, when there was still 40% survival in the IL-10−/− mice. Mean survival in days by mouse strain: IL-12−/−, 12; IL-6−/−, 15; C57BL/6J, 19; IL-4−/−, 21; IL-10−/−, 79. (B) Serum GXM levels measured on day 14 postinfection from mice in panel A. By this time, two mice in the IL-6−/− group and seven in the IL-12−/− group had died. Horizontal lines, mean serum concentrations. In comparison to C57BL/6J, only IL-10−/− mice had significantly different levels of circulating GXM (P < 0.003).
Serum GXM levels were determined on day 14 postinfection (Fig. 2B). By this time, two IL-6−/− mice and seven IL-12−/− mice had already died from the infection. In comparison to C57BL/6J mice, IL-10−/− mice had very low levels of circulating GXM (P = 0.003), while the more-susceptible IL-6−/− mice had a trend toward higher serum GXM levels, but this was not statistically significant (P = 0.3). Serum GXM levels in IL-4−/− mice were heterogeneous: four mice had high serum GXM (>50 μg/ml), and mice in this subgroup died earlier than the five mice with levels below 50 μg/ml (P = 0.02). In this experiment, serum GXM levels correlated with survival. However, in subsequent experiments using antibody treatment, serum GXM levels determined when the mice began to die did not correlate with outcome or any other measured parameter (data not shown).
IgG isotypes did not protect IL-12−/− mice against infection with C. neoformans
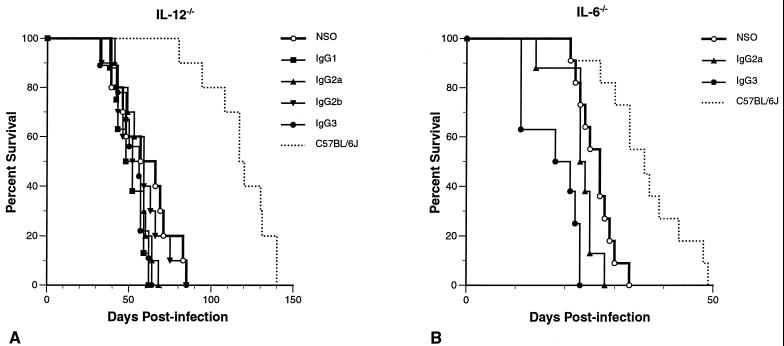
Given the susceptibility of IL-12−/− mice to C. neoformans, we decreased the initial inoculum 25-fold to 105 CFU in an attempt to detect smaller differences in antibody efficacy. This modification resulted in an increased mean survival time of control IL-12−/− mice from 12 to 60 days. Control NSO cell ascites fluid-treated C57BL/6J mice given the same inoculum survived substantially longer as well (mean survival = 116 days; Fig. 3A), again illustrating the increased susceptibility of IL-12−/− mice. None of the IgG isotypes were protective compared to control (P > 0.2).
FIG. 3.
Survival of MAb-treated IL-12−/− and IL-6−/− mice. (A) IL-12−/− mice were infected with 105 C. neoformans CFU. NSO cell ascites fluid-treated C57BL/6J given the same inoculum are also shown so that the effect of this lower inoculum on the wild-type mice can be seen. Passive-antibody administration was neither protective nor enhancing compared to the control (P > 0.2). Mean survival in days by treatment group: NSO cell ascites fluid, 60; IgG1, 50; IgG2a, 55; IgG2b, 56; IgG3, 52; C57BL/6J mice treated with NSO cell ascites fluid, 116. (B) Survival of MAb-treated IL-6−/− mice infected with 106 C. neoformans CFU. 3E5 IgG2a was not protective and 3E5 IgG3 enhanced infection in comparison to the control (P = 0.23 and 0.0009, respectively). Mean survival in days by treatment group: NSO cell ascites fluid, 26; IgG2a, 23; IgG3, 18; C57BL/6J mice treated with NSO cell ascites fluid, 36.
In IL-6−/− mice, IgG2a did not protect against, while IgG3 enhanced, infection with C. neoformans.
We studied the efficacy of our most and least protective 3E5 isotypes, IgG2a and IgG3, respectively, in IL-6−/− mice infected with 106 CFU of C. neoformans (Fig. 3B). Compared to control, IgG2a was not protective, while IgG3 enhanced infection (P = 0.23 and 0.0009, respectively). These results indicated that proinflammatory cytokine IL-6 was necessary for the protective efficacy of IgG2a but was not required for IgG3-mediated enhancement of cryptococcal infection.
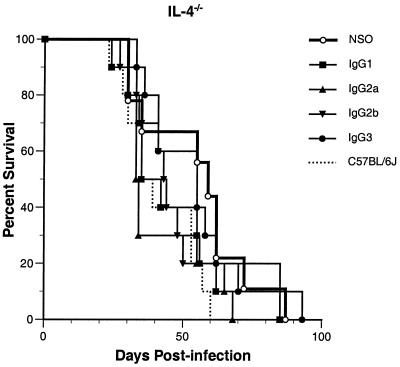
IgG isotypes did not protect IL-4−/− mice against infection with C. neoformans.
Mice that lack prototypic Th2 cytokine IL-4 have high circulating levels of IFN-γ (57). To our knowledge, there are no studies examining the importance of Th2 responses in passive antibody protection, but, given the seeming importance of IFN-γ in antibody protection against cryptococcal infection, we expected that these mice would be highly protected by IgG1, IgG2b, and IgG2a. To our surprise, none of the 3E5 IgG isotypes protected IL-4−/− mice infected with 106 CFU of C. neoformans (P > 0.2; Fig. 4). As we observed above (Fig. 2), IL-4−/− mice appeared slightly more resistant to infection than C57BL/6J mice, but at this lower inoculum the difference was statistically significant (P = 0.03). We repeated the experiment, this time using purified antibodies and PBS as the control with virtually identical results (data not shown), indicating that the effect was due to the MAbs and not to other factors in the ascites fluid.
FIG. 4.
Survival of MAb-treated IL-4−/− mice infected with 106 C. neoformans CFU. The antibody was neither protective nor enhancing compared to the control (P > 0.2). At this lower inoculum, control IL-4−/− mice were more resistant to infection than C57BL/6J mice (P = 0.03). Mean survival in days by treatment group: NSO cell ascites fluid, 55; IgG1, 46; IgG2a, 42; IgG2b, 45; IgG3, 54; C57BL/6J mice treated with NSO cell ascites fluid, 41.
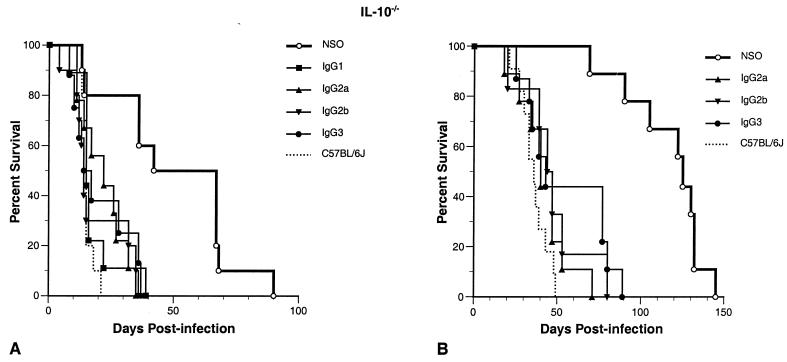
All IgG isotypes significantly reduced survival of IL-10−/− mice infected with C. neoformans.
Because IL-10−/− mice were so resistant to infection, we increased the inoculum to 5 × 106 organisms to examine antibody efficacy in these mice. C57BL/6J control mice given this inoculum were all dead by week 3 postinfection (Fig. 5A). As expected (5), the control IL-10−/− mice were much more resistant to infection, with a median survival of 7 weeks. Unexpectedly, we found that all isotypes enhanced infection in a highly significant fashion (P < 0.006). This experiment was repeated using a lower inoculum of 106 CFU, which resulted in longer survival times for all groups but otherwise produced very similar results (Fig. 5B). We again saw similar results when we performed this experiment with purified antibodies (data not shown). Since these findings were surprising and since we are concerned about anything that enhances infection, we carried out further experiments to try to understand the mechanism(s) underlying the enhancement of cryptococcal infection seen with antibody treatment in the IL-10−/− mice.
FIG. 5.
(A) Survival of MAb-treated IL-10−/− mice infected with 5 × 106 C. neoformans CFU. All antibodies greatly enhanced cryptococcal infection compared to the control (P > 0.006). Mean survival in days by treatment group: NSO cell ascites fluid, 50; IgG1, 17; IgG2a, 22; IgG2b, 19; IgG3, 20; C57BL/6J mice treated with NSO cell ascites fluid, 15. (B) Survival of IL-10−/− mice treated as above but given a lower inoculum of 106 C. neoformans CFU. Again, all antibodies greatly enhanced cryptococcal infection compared to the control (P > 0.002). Mean survival in days by treatment group: NSO cell ascites fluid, 114; IgG2a, 42; IgG2b, 47; IgG3, 55; C57BL/6J mice treated with NSO cell ascites fluid, 37.
Fungal burden in antibody-treated IL-10−/− mice was not different from that in control mice.
Organ CFU have been correlated with antibody protection against cryptococcal infection in many of our previous studies (23, 52, 54, 55, 79, 81, 82). To determine if fungal burden or differences in cryptococcal dissemination could explain why the antibody-treated IL-10−/− mice were dying earlier, antibody-treated IL-10−/− mice were infected with 106 CFU of C. neoformans and killed when the first mouse died (day 17 postinfection) and C. neoformans CFU from the brain, liver, spleen, and lung were tallied (Table 1). Interestingly, there were no differences in CFU that could explain why antibody-treated animals were dying earlier, though IgG1-treated animals had significantly higher organism burdens in the liver and spleen. Despite the lack of major differences in CFU, most antibody-treated mice looked sick (as determined by lack of preening, decreased activity, and weakness), while all of the IL-10−/− control mice appeared robust. To determine if there were differences in fungal burden at an earlier time, we examined organ CFU on day 10 postinfection (Table 1; data from animals in the experiment are shown in Fig. 5B). The control IL-10−/− mice had slightly lower CFU in all organs, and, in some cases, the decrease in CFU compared to CFU in antibody-treated mice was statistically significant. We conclude that antibody-mediated enhancement of cryptococcal infection in IL-10−/− mice was not explained solely by differences in fungal burden.
TABLE 1.
Organ CFU from IL-10−/− mice infected with C. neoformans
| Organ | Treatmenta | Mean CFU ± SE (104) on day:
|
|
|---|---|---|---|
| 10b | 17c | ||
| Brain | NSO | 38 ± 16 | 150 ± 32 |
| IgG1 | 160 ± 28 | ||
| IgG2a | 50 ± 17 | 150 ± 30 | |
| IgG2b | 160 ± 56 | 120 ± 19 | |
| IgG3 | 98 ± 24 | 150 ± 26 | |
| C57/NSO | 58 ± 24 | 170 ± 23 | |
| Liver | NSO | 82 ± 12 | 8.4 ± 3.0 |
| IgG1d | 19 ± 3.0 | ||
| IgG2a | 105 ± 44 | 7.8 ± 3.2 | |
| IgG2b | 410 ± 160 | 6.0 ± 1.2 | |
| IgG3e | 240 ± 42 | 7.2 ± 2.7 | |
| C57/NSOf | 110 ± 10 | 75 ± 11 | |
| Lung | NSOg | 25 ± 6.0 | 110 ± 28 |
| IgG1 | 130 ± 30 | ||
| IgG2a | 76 ± 25 | 150 ± 25 | |
| IgG2b | 110 ± 15 | 110 ± 36 | |
| IgG3 | 100 ± 30 | 93 ± 15 | |
| C57/NSO | 110 ± 26 | 91 ± 8.6 | |
| Spleen | NSO | 6.6 ± 1.0 | 3.4 ± 1.1 |
| IgG1h | 19 ± 4.6 | ||
| IgG2a | 9.2 ± 2.1 | 3.7 ± 1.0 | |
| IgG2b | 21 ± 11 | 2.4 ± 0.8 | |
| IgG3i | 84 ± 24 | 3.0 ± 0.5 | |
| C57/NSOj | 37 ± 15 | 12 ± 2.4 | |
Mice received ascites fluid containing either 1 mg of 3E5 IgG3, one of its switch variants of the IgG1, IgG2b or IgG2a isotype, or 1 ml of NSO cell ascites fluid as a control (NSO). C57BL/6J mice treated with NSO cell ascites fluid (C57/NSO) are also shown.
Mice killed on day 10 postinfection. There was no IgG1 treatment group for this experiment (n = 4).
Mice killed day 17 postinfection (n = 5).
P < 0.0005 versus liver CFU for all treatment groups on day 17.
P < 0.03 versus liver CFU for NSO and C57/NSO treatment groups on day 10.
P < 0.05 versus liver CFU for all treatment groups on day 17.
P < 0.04 versus lung CFU for IgG3 and C57/NSO treatment groups on day 10.
P < 0.02 versus spleen CFU for all treatment groups except C57/NSO on day 17.
P < 0.05 versus spleen CFU for NSO and IgG2a treatment groups on day 10.
P < 0.02 versus spleen CFU for all treatment groups on day 17.
Histopathology of antibody-treated IL-10−/− mice following cryptococcal infection.
We examined organ histopathology from the same animals that we evaluated for fungal burden. There was little difference between C57BL/6J and IL-10−/− mice, regardless of treatment. In the lung and the liver, infection was characterized by the presence of diffuse foci of granulomatous inflammation, in which macrophages, epithelioid cells, and multinucleated giant cells represented the predominant cell types (Fig. 6). The cytoplasm of many of these cells stained with mucicarmine, suggesting the presence of capsular polysaccharide. These inflammatory foci also contained various proportions of neutrophils, lymphocytes, and eosinophils. In the lung, yeast cells were also seen in alveolar spaces without inflammatory cells. In animals sacrificed on day 17, both control and MAb-treated IL-10−/− mice appeared to have more abundant inflammatory infiltrates near infectious foci than did the C57BL/6J mice and MAb-treated IL-10−/− mice had fewer free yeast cells than did control IL-10−/− or C57BL/6J mice. However, on day 10 postinfection, these differences in lung pathology were not observed.
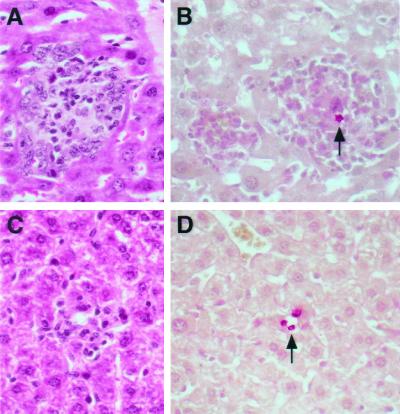
FIG. 6.
Liver pathology in IL-10−/− mice 17 days after infection with C. neoformans. (A) H&E staining of the livers of IL-10−/− mice in the control (NSO cell ascites fluid) group showed granulomatous cryptococcal lesions that contained macrophages with a mixture of other leukocytes. (B) Mucicarmine staining showed small numbers of organisms in these lesions (arrow) and demonstrated pink cytoplasmic staining of macrophages, which is consistent with phagocytosis of cryptococcal capsular polysaccharide. (C) In contrast, H&E staining of sections from C57BL/6J mice showed smaller, less-organized inflammatory responses to cryptococcal infection. (D) Mucicarmine staining demonstrated extracellular yeast in sinusoids (arrow) without associated inflammation. Magnification (all panels), ×400.
In the liver, many of the granulomatous lesions were located perivascularly, and yeast cells outside of inflammatory foci were present in hepatic sinusoids and in Kupffer cells. On day 10 postinfection, the hepatic granulomas of both the MAb-treated and control IL-10−/− mice were more cellular and had a more epithelioid appearance than those of C57BL/6J mice, such that one of us (A.C.) was able to correctly distinguish between the IL-10−/− controls and C57BL/6J mice in sections from four different mice of each group in a blinded experiment. However, this difference was not noted on day 17 postinfection. In both C57BL/6J and IL-10−/− mice, infection in the brain was characterized by the presence of diffuse foci of infection with numerous yeast cells and minimal inflammatory response. In some lesions, the brain tissue bordering the collection of yeast cells contained intracellular yeast cells and the cytoplasm of cells with yeast stained with mucicarmine. In the spleen, yeast cells were seen in all mice in the red pulp, predominantly in the venous sinuses. In sections from mice in which yeast cells appeared to be more numerous, intra- and extracellular yeast cells were also seen in the periarteriolar lymphoid sheaths, in marginal-zone macrophages, and in lymphoid nodules. In general, the pathology did not reveal a sustained increase in inflammation in the IL-10−/− mice.
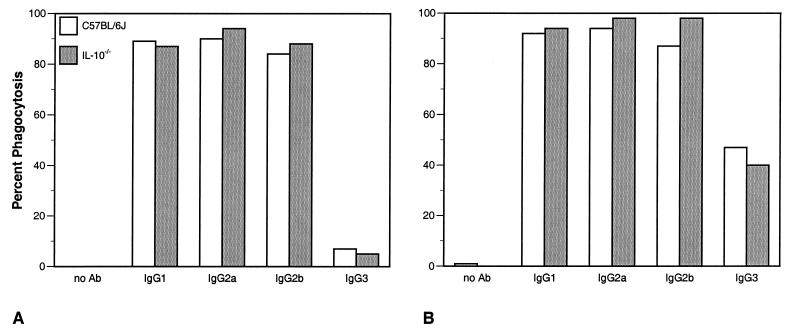
Levels of in vitro phagocytosis of C. neoformans by primary macrophages from IL-10−/− mice and C57BL/6J mice were similar.
We compared the phagocytic properties of macrophages from IL-10−/− and C57BL/6J mice both with and without IL-10 pretreatment (Fig. 7). In the absence of antibodies to GXM, there was little or no phagocytosis of C. neoformans by alveolar or peritoneal macrophages from either C57BL/6J or IL-10−/− mice, even after stimulation with IL-10. In macrophages from both C57BL/6J and IL-10−/− mice that were not treated with IL-10, phagocytosis was greatly increased after addition of IgG1, IgG2a, and IgG2b capsule-specific antibodies and there was a small but detectable increase in phagocytosis with IgG3 (Fig. 7A). However, there were no significant differences in phagocytosis by macrophages from C57BL/6J mice and IL-10−/− mice in the presence of antibodies. The addition of IL-10 in vitro increased phagocytosis in both C57BL/6J and IL-10−/− macrophages to equivalent degrees (Fig. 7B). Stimulation with IL-10 increased the phagocytosis of IgG3-treated organisms, but there were no discernible differences in the phagocytic properties of IL-10−/− macrophages that explained either the resistance of IL-10−/− mice to cryptococcal infection or the enhancement of infection by addition of IgG1, IgG2b, or IgG2a MAbs.
FIG. 7.
Phagocytic indices of peritoneal macrophages from C57BL/6J and IL-10−/− mice pretreated overnight without (A) or with (B) recombinant murine IL-10 (2 ng/ml) and then incubated with either IgG1, IgG2a, IgG2b, or IgG3 3E5 MAbs to GXM and heat-killed C. neoformans for 4 h. This experiment was repeated three times, once with alveolar macrophages, with similar results.
DISCUSSION
There is a consensus that a Th1-driven cell-mediated response is necessary for the control of C. neoformans infections (reviewed in reference 10). This is consistent with the suggestion that this organism is a facultative intracellular pathogen (22). In this regard, the effective tissue response is granulomatous inflammation, which is dependent on a T-cell immune response. Numerous studies have established that T-cell deficiencies correlate with significantly enhanced susceptibility to cryptococcal infection (27, 31, 32, 81). In contrast, a role for humoral immunity in protection against infection has been more difficult to establish, and, in fact, Th2 responses are ineffective in clearing cryptococcal infection from the lung and brain (30, 34, 45). However, several studies have shown that administration of antibodies directed against the cryptococcal capsular polysaccharide can modify the course of infection to the benefit of the host by prolonging survival, clearing serum antigen, and, in some cases, reducing fungal burden (17, 18, 23, 50–54, 81, 82). Antibody efficacy has been shown to depend on such characteristics as isotype and specificity (51, 61, 79, 82) and on host factors such as T-cell immune function, the presence of IFN-γ, and Fc receptor competence (80, 81).
The different efficacies of IgG3 and the other IgG isotypes, the unexplained requirement for CD8+ T cells in the IgG3-mediated enhancement of infection, and the need for IFN-γ in antibody-mediated modulation of infection suggested that cytokines might be playing a broader role than just facilitating the phagocytosis and killing of the organism by macrophages. To begin to define that broader role, we have now evaluated the contribution of cytokines IL-4, IL-6, IL-10, and IL-12 to host defense and to passive antibody efficacy with variable-region-identical MAbs representing the four murine IgG isotypes. We first examined the course of cryptococcal infection in mice deficient in IL-12, IL-6, IL-4, and IL-10 in comparison to that in C57BL/6J mice. One of the primary effects of IL-12 is to promote production of IFN-γ, which is an essential cytokine for defense against cryptococcosis (15, 29, 35, 36). IL-12−/− mice have impaired Th1 responses with decreased levels of IFN-γ (46) and are susceptible to diseases where IFN-γ plays an important protective role, such as infections with mycobacteria (37), Toxoplasma gondii (19), Candida albicans (48), and Leishmania major (47). Our observation that IL-12-deficient mice were more susceptible to infection with C. neoformans is consistent with these studies and confirms a recent report by Decken et al. showing increased susceptibility of both IL-12p35−/− and IL-12p40−/− mice to cryptococcal infection (15).
IL-6 is a pleiotropic cytokine with proinflammatory effects that also appears to be important in generating Th2 responses (28, 64). However, IL-6−/− mice are more susceptible than wild-type mice to infection by Listeria monocytogenes and vaccinia virus (14, 38). In both infections, Th1 responses confer resistance while Th2-associated cytokines are deleterious to the host (75). IL-6 administration reduces the severity of intracerebral C. neoformans infection (6), suggesting a role for this cytokine in host defense against cryptococcosis. Consistent with this view, we found IL-6-deficient mice to be more susceptible to cryptococcal infection.
IL-4−/− mice have deficient Th2 responses, increased serum IFN-γ, and decreased levels of IL-6 and IL-10 (40). Treatment with MAbs to IL-4 prolongs survival of mice infected with C. neoformans (34). Decken et al. have shown that (C57BL/6 × 129/Sv/Ev)F2 IL-4−/− mice are significantly resistant to infection with C. neoformans (15). However, we found that IL-4−/− mice, backcrossed to C57BL/6J for at least 10 generations, were equally or only slightly more resistant to infection with C. neoformans than the parental strain. Other studies with IL-4−/− mice have given results that conflict with or do not seem to fit the Th1/Th2 paradigm. For example, while Kopf et al. found IL-4−/− mice on a BALB/c background to be more resistant to Leishmania major infection (39) than wild-type mice, Noben-Trauth et al. found these same mice to be as susceptible as wild type mice (60). In addition, IL-4−/− mice are more susceptible to Toxoplasma gondii infection where IFN-γ is necessary for protection (65). Our differences with Decken et al. (15) may be attributable to mouse strain, cryptococcal strain and inoculum, or both. In addition, while IL-4 is considered a prototypic Th2 cytokine, the lack of resistance by IL-4−/− mice to infections that require Th1 responses for protection observed by us and others is consistent with recent studies showing that endogenous IL-4 is needed to effectively sustain a protective Th1 response in candidal infection (49) and that IL-4−/− mice have an impaired ability to produce IFN-γ in the later stages of Toxoplasma gondii infection (72).
IL-10-deficient mice were significantly more resistant than the parental strain to C. neoformans infection (5; this paper). IL-10 is secreted by T cells, macrophages, and B1 cells and is commonly classified as a Th2-associated cytokine. While most murine studies show that IL-10 inhibits Th1, but not Th2, responses, studies with human cells indicate that IL-10 can also inhibit production of Th2-associated cytokines (16, 63, 67, 78). In addition, the observations that in mice IL-10 downregulates IL-5 production (83) and that it inhibits CD86 expression (24) suggest a broader regulatory capacity for IL-10 in humans as well as mice, and the current view is that IL-10 is a negative regulator of inflammation that acts by inhibiting release of both Th1 and Th2 proinflammatory cytokines (2, 58, 70). The intrinsic resistance of IL-10−/− mice to cryptococcal infection is consistent with recent reports that these mice are also protected against other infections that require a Th1 response for protection, such as those with Candida albicans (77), Mycobacterium tuberculosis (59), and Listeria monocytogenes (13). However, other studies with IL-10−/− mice illustrate the difficulty in predicting results based on the Th1/Th2 paradigm because these mice die rapidly from infection by the intracellular parasites Toxoplasma gondii (26) and Trypanosoma cruzi (33) due to systemic overproduction of inflammatory mediators such as IFN-γ and tumor necrosis factor alpha (TNF-α). We conclude that, despite the inadequacies of the Th1/Th2 model in reconciling certain findings (1, 58), our observations concerning the course of C. neoformans infection in cytokine-deficient mice are consistent with the belief that a Th1-driven cell-mediated response is critical for host defense against C. neoformans.
To our knowledge, the effects of Th1 and Th2 cytokines on antibody efficacy have not been evaluated. Passive-antibody-protection experiments have consistently shown that IgG1, IgG2a, and IgG2b are protective against cryptococcal infection in several different mouse strains including BALB/c and C57BL/6J (53, 81). Our earlier experiments with different mouse strains seem to suggest that passive antibodies are protective under both Th1 and Th2 conditions. More recently, we provided evidence that antibody-mediated protection against C. neoformans is dependent on cell-mediated immune responses and requires IFN-γ (81). Consistent with this finding, we observed a lack of antibody-mediated protection in IL-12−/− mice. However, none of the IgG isotypes were protective in mice deficient in IL-4, IL-6, and IL-10, indicating that these Th2-associated cytokines are as important for antibody-mediated protection as prototypic Th1 cytokine IFN-γ.
Probably our most dramatic finding is the observation that antibody administration greatly enhanced C. neoformans infection in the otherwise resistant IL-10-deficient mice. Since IL-10 upregulates macrophage expression of FcγRI and increases phagocytic capacity of macrophages (73), we considered the possibility that yeast cells were not being avidly phagocytosed in these mice. However, in vitro phagocytosis studies showed no significant difference between IL-10−/− and C57BL/6J mouse macrophages and organisms were seen within macrophages and Kupffer cells in the antibody-treated IL-10−/− mice. Given the potent anti-inflammatory role of IL-10, it is possible that the decreased survival we observed in the antibody-treated IL-10−/− mice resulted from increased inflammation induced by immune complexes formed when the exogenous antibody to GXM was administered. Immune complexes induce the release of many proinflammatory cytokines such as IL-1β, IL-6, and TNF-α, as well as IL-10 and vasoactive substances such as platelet-activating factor (3, 4, 74). In the absence of the inhibition of inflammation that is usually caused by IL-10, these mediators may act unopposed, thereby causing intense inflammatory damage to the host. As described in Results, all IL-10−/− mice showed more-abundant inflammatory infiltrates near infectious foci than did C57BL/6J mice. We did not, however, see evidence on histopathology of further increased inflammation in antibody-treated IL-10−/− mice compared to that in control IL-10−/− mice. While there were three to seven times more organisms in the spleens and livers of the IgG1-treated IL-10−/− mice than in controls that did not receive the antibody, there were no significant differences in the CFU in the mice treated with the other isotypes. This suggests that the enhancement of infection in the antibody-treated mice was not due to a decrease in the ability of effector cells to kill the organism in vivo in the antibody-treated IL-10−/− mice.
While human immunodeficiency virus (HIV) infection alters cytokine balance, the profile is more complex than polarization to Th1 or Th2 (21). Increased levels of IL-10 are associated with progressive HIV infection (69, 71). Lortholary et al. have shown that AIDS patients with disseminated cryptococcosis have elevated levels of IL-10 and TNF-α (44). While it is simplistic to look at any one factor, these studies suggest that AIDS patients with cryptococcosis are likely to have IL-10 levels that would be deleterious in cryptococcal infection but that would not appear to negatively impact passive-antibody therapy. As such, our studies provide further support for the current phase I evaluation of the murine IgG1 MAb in conjunction with antifungal therapy for the treatment of cryptococcal meningitis in AIDS patients (7). Our results also suggest that it may be important to evaluate the underlying cytokine milieu of the host before embarking on antibody therapy for C. neoformans. However, with further insight into the interplay of various cytokines and passive-antibody treatment, it may be possible to shift the cytokine balance to maximize the efficacy of antibodies and favorably to improve therapeutic outcome.
The Th1/Th2 paradigm has proven useful as a framework to predict the host response to certain infections and to investigate fundamental immunologic pathways (66). However, the discrimination is an artificial one that does not apply universally (1). As our understanding of immunologic phenomena becomes increasingly sophisticated, this duality will likely become less useful as a construct for understanding the immune system. In fact, our results provide strong support for the current view that Th1-associated responses are necessary for the control of C. neoformans infection. However, our observations from passive-antibody studies in cytokine-deficient mice highlight a previously unsuspected dependence on the ability of the host to mount a Th2-associated response. The studies described here have alerted us to the fact that we do not understand the detailed mechanisms through which antibodies modulate cryptococcal infection in mice. It is remarkable that, although the humoral immune response has been intensely studied for over a century, we are just beginning to understand the elements involved in mediating antibody protection. Since a mouse MAb is currently being used in combination with antifungal agents to treat AIDS patients with chronic cryptococcal infection, we have used a mouse model in which the organism is administered intravenously to mimic the hematogenous spread that occurs in such patients. We have shown that the absence of both Th1 and Th2 cytokines affects the ability of antibodies of different isotypes to modify cryptococcal infection. One of the goals of treating with both antibodies and antifungals is to lower the fungal burden so that even an immunodeficient host might eradicate the organism. The administered antibodies also form antigen-antibody complexes with the organism and the shed capsular polysaccharide, which should activate both innate and adaptive immune responses and which may be protective or enhancing depending on the cytokine environment of the host. Our observations illustrate the need for additional studies to understand the variables that determine antibody efficacy. For example, antibody-treated IL-10-deficient mice should be examined for the levels of cytokines in their tissues and wild-type mice should be acutely depleted of IL-10. In addition, the cell-mediated and humoral immune responses of IL-10−/− mice subsequent to antibody administration should be studied. Such studies would make it less likely that unexpected defects in the development of the immune response or in the physiology of genetically defective mice are responsible for the results we have observed. In addition, detailed in vitro assays with T cells and macrophages from infected and antibody-treated mice would allow us to begin to understand the mechanisms involved. Such studies are all the more important because the results reported here may also be relevant to vaccine protocols, in particular those evaluating antibody responses using adjuvants that shift the response to Th1, as our findings suggest that the absence of IL-4 or IL-10 can neutralize antibody efficacy. Manipulation of cytokines in such systems will allow us to establish whether the protective efficacy of endogenous antibody responses is similarly affected by defects in the cytokine network.
ACKNOWLEDGMENTS
This research was supported by grants from the National Institutes of Health: AI 01434 (D.O.B), T32 GM 07288 (S.S.), AI 01341 (M.F.), AI 33774, AI 13342, HL 59842 (A.C.), AI 43937, and AI 42997 (M.D.S.). M.D.S. is also supported by the Harry Eagle Chair provided by the Women's Division of the Albert Einstein College of Medicine.
We thank Jieru Zhang and Jin Oh for technical support and Alvin Watford for assistance in animal breeding. We also thank Sherie Morrison, Betty Diamond, and Anne Davidson for helpful comments and critical review of the manuscript.
REFERENCES
- 1.Allen J E, Maizels R M. Th1-Th2: reliable paradigm or dangerous dogma? Immunol Today. 1997;18:387–392. doi: 10.1016/s0167-5699(97)01102-x. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J. Converging and diverging properties of human interleukin-4 and interleukin-10. Behring Inst Mitt. 1995;96:58–77. [PubMed] [Google Scholar]
- 3.Berger S, Ballo H, Stutte H J. Immune complex-induced interleukin-6, interleukin-10 and prostaglandin secretion by human monocytes: a network of pro- and anti-inflammatory cytokines dependent on the antigen:antibody ratio. Eur J Immunol. 1996;26:1297–1301. doi: 10.1002/eji.1830260618. [DOI] [PubMed] [Google Scholar]
- 4.Berger S, Chandra R, Ballo H, Hildenbrand R, Stutte H J. Immune complexes are potent inhibitors of interleukin-12 secretion by human monocytes. Eur J Immunol. 1997;27:2994–3000. doi: 10.1002/eji.1830271136. [DOI] [PubMed] [Google Scholar]
- 5.Blackstock R, Buchanan K L, Adesina A M, Murphy J W. Differential regulation of immune responses by highly and weakly virulent Cryptococcus neoformans isolates. Infect Immun. 1999;67:3601–3609. doi: 10.1128/iai.67.7.3601-3609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blasi E, Barluzzi R, Mazzolla R, Pitzurra L, Puliti M, Saleppico S, Bistoni F. Biomolecular events involved in anticryptococcal resistance in the brain. Infect Immun. 1995;63:1218–1222. doi: 10.1128/iai.63.4.1218-1222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman D L, Kozel T R, Lendvai N, Mukherjee J, Pirofski L A, Rivera J, Rosas A L, Scharff M D, Valadon P, Westin K, Zhong Z. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998;42:1437–1446. doi: 10.1128/aac.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadevall A, Mukherjee J, Devi S J, Schneerson R, Robbins J B, Scharff M D. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992;165:1086–1093. doi: 10.1093/infdis/165.6.1086. [DOI] [PubMed] [Google Scholar]
- 9.Casadevall A, Mukherjee J, Scharff M D. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J Immunol Methods. 1992;154:27–35. doi: 10.1016/0022-1759(92)90209-c. [DOI] [PubMed] [Google Scholar]
- 10.Casadevall A, Perfect J R. Cryptococcus neoformans. Washington, D.C.: ASM Press; 1998. [Google Scholar]
- 11.Clumeck N, Sonnet J, Taelman H, Mascart-Lemone F, De Bruyere M, Vandeperre P, Dasnoy J, Marcelis L, Lamy M, Jonas C, et al. Acquired immunodeficiency syndrome in African patients. N Engl J Med. 1984;310:492–497. doi: 10.1056/NEJM198402233100804. [DOI] [PubMed] [Google Scholar]
- 12.Currie B P, Casadevall A. Estimation of the prevalence of cryptococcal infection among patients infected with the human immunodeficiency virus in New York City. Clin Infect Dis. 1994;19:1029–1033. doi: 10.1093/clinids/19.6.1029. [DOI] [PubMed] [Google Scholar]
- 13.Dai W J, Kohler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 14.Dalrymple S A, Lucian L A, Slattery R, McNeil T, Aud D M, Fuchino S, Lee F, Murray R. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun. 1995;63:2262–2268. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decken K, Kohler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately M K, Alber G. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Prete G, De Carli M, Almerigogna F, Giudizi M G, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–360. [PubMed] [Google Scholar]
- 17.Dromer F, Charreire J, Contrepois A, Carbon C, Yeni P. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect Immun. 1987;55:749–752. doi: 10.1128/iai.55.3.749-752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dromer F, Perronne C, Barge J, Vilde J L, Yeni P. Role of IgG and complement component C5 in the initial course of experimental cryptococcosis. Clin Exp Immunol. 1989;78:412–417. [PMC free article] [PubMed] [Google Scholar]
- 19.Ely K H, Kasper L H, Khan I A. Augmentation of the CD8+ T cell response by IFN-gamma in IL-12-deficient mice during Toxoplasma gondii infection. J Immunol. 1999;162:5449–5454. [PubMed] [Google Scholar]
- 20.Ey P L, Prowse S J, Jenkin C R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-Sepharose. Immunochemistry. 1978;15:429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- 21.Fakoya A, Matear P M, Filley E, Rook G A, Stanford J, Gilson R J, Beecham N, Weller I V, Vyakarnam A. HIV infection alters the production of both type 1 and 2 cytokines but does not induce a polarized type 1 or 2 state. AIDS. 1997;11:1445–1452. doi: 10.1097/00002030-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun. 2000;68:4225–4237. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldmesser M, Mukherjee J, Casadevall A. Combination of 5-flucytosine and capsule-binding monoclonal antibody in the treatment of murine Cryptococcus neoformans infections and in vitro. J Antimicrob Chemother. 1996;37:617–622. doi: 10.1093/jac/37.3.617. [DOI] [PubMed] [Google Scholar]
- 24.Flores Villanueva P O, Reiser H, Stadecker M J. Regulation of T helper cell responses in experimental murine schistosomiasis by IL-10. Effect on expression of B7 and B7-2 costimulatory molecules by macrophages. J Immunol. 1994;153:5190–5199. [PubMed] [Google Scholar]
- 25.Franzot S P, Mukherjee J, Cherniak R, Chen L C, Hamdan J S, Casadevall A. Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect Immun. 1998;66:89–97. doi: 10.1128/iai.66.1.89-97.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 27.Hill J O, Aguirre K M. CD4+ T cell-dependent acquired state of immunity that protects the brain against Cryptococcus neoformans. J Immunol. 1994;152:2344–2350. [PubMed] [Google Scholar]
- 28.Hirano T. Interleukin 6 and its receptor: ten years later. Int Rev Immunol. 1998;16:249–284. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 29.Hoag K A, Lipscomb M F, Izzo A A, Street N E. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 30.Huffnagle G B, McNeil L K. Dissemination of C. neoformans to the central nervous system: role of chemokines, Th1 immunity and leukocyte recruitment. J Neurovirol. 1999;5:76–81. doi: 10.3109/13550289909029748. [DOI] [PubMed] [Google Scholar]
- 31.Huffnagle G B, Yates J L, Lipscomb M F. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med. 1991;173:793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huffnagle G B, Yates J L, Lipscomb M F. T-cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect Immun. 1991;59:1423–1433. doi: 10.1128/iai.59.4.1423-1433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunter C A, Ellis-Neyes L A, Slifer T, Kanaly S, Grunig G, Fort M, Rennick D, Araujo F G. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol. 1997;158:3311–3316. [PubMed] [Google Scholar]
- 34.Kawakami K, Hossain Qureshi M, Zhang T, Koguchi Y, Xie Q, Kurimoto M, Saito A. Interleukin-4 weakens host resistance to pulmonary and disseminated cryptococcal infection caused by combined treatment with interferon-gamma-inducing cytokines. Cell Immunol. 1999;197:55–61. doi: 10.1006/cimm.1999.1557. [DOI] [PubMed] [Google Scholar]
- 35.Kawakami K, Kohno S, Kadota J, Tohyama M, Teruya K, Kudeken N, Saito A, Hara K. T cell-dependent activation of macrophages and enhancement of their phagocytic activity in the lungs of mice inoculated with heat-killed Cryptococcus neoformans: involvement of IFN-gamma and its protective effect against cryptococcal infection. Microbiol Immunol. 1995;39:135–143. doi: 10.1111/j.1348-0421.1995.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 36.Kawakami K, Tohyama M, Xie Q, Saito A. IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clin Exp Immunol. 1996;104:208–214. doi: 10.1046/j.1365-2249.1996.14723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi K, Yamazaki J, Kasama T, Katsura T, Kasahara K, Wolf S F, Shimamura T. Interleukin (IL)-12 deficiency in susceptible mice infected with Mycobacterium avium and amelioration of established infection by IL-12 replacement therapy. J Infect Dis. 1996;174:564–573. doi: 10.1093/infdis/174.3.564. [DOI] [PubMed] [Google Scholar]
- 38.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 39.Kopf M, Brombacher F, Kohler G, Kienzle G, Widmann K H, Lefrang K, Humborg C, Ledermann B, Solbach W. IL-4-deficient Balb/c mice resist infection with Leishmania major. J Exp Med. 1996;184:1127–1136. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kopf M, Le Gros G, Coyle A J, Kosco-Vilbois M, Brombacher F. Immune responses of IL-4, IL-5, IL-6 deficient mice. Immunol Rev. 1995;148:45–69. doi: 10.1111/j.1600-065x.1995.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 41.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 42.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 43.Lendvai N, Casadevall A, Liang Z, Goldman D L, Mukherjee J, Zuckier L. Effect of immune mechanisms on the pharmacokinetics and organ distribution of cryptococcal polysaccharide. J Infect Dis. 1998;177:1647–1659. doi: 10.1086/515329. [DOI] [PubMed] [Google Scholar]
- 44.Lortholary O, Improvisi L, Rayhane N, Gray F, Fitting C, Cavaillon J M, Dromer F. Cytokine profiles of AIDS patients are similar to those of mice with disseminated Cryptococcus neoformans infection. Infect Immun. 1999;67:6314–6320. doi: 10.1128/iai.67.12.6314-6320.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lovchik J A, Wilder J A, Huffnagle G B, Riblet R, Lyons C R, Lipscomb M F. Ig heavy chain complex-linked genes influence the immune response in a murine cryptococcal infection. J Immunol. 1999;163:3907–3913. [PubMed] [Google Scholar]
- 46.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C Y, Ferrante J, Stewart C, Sarmiento U, Faherty D A, Gately M K. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 47.Mattner F, Magram J, Ferrante J, Launois P, Di Padova K, Behin R, Gately M K, Louis J A, Alber G. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- 48.Mencacci A, Cenci E, Del Sero G, Fe d'Ostiani C, Mosci P, Trinchieri G, Adorini L, Romani L. IL-10 is required for development of protective Th1 responses in IL-12-deficient mice upon Candida albicans infection. J Immunol. 1998;161:6228–6237. [PubMed] [Google Scholar]
- 49.Mencacci A, Del Sero G, Cenci E, d'Ostiani C F, Bacci A, Montagnoli C, Kopf M, Romani L. Endogenous interleukin 4 is required for development of protective CD4+ T helper type 1 cell responses to Candida albicans. J Exp Med. 1998;187:307–317. doi: 10.1084/jem.187.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukherjee J, Kozel T R, Casadevall A. Monoclonal antibodies reveal additional epitopes of serotype D Cryptococcus neoformans capsular glucuronoxylomannan that elicit protective antibodies. J Immunol. 1998;161:3557–3568. [PubMed] [Google Scholar]
- 51.Mukherjee J, Nussbaum G, Scharff M D, Casadevall A. Protective and nonprotective monoclonal antibodies to Cryptococcus neoformans originating from one B cell. J Exp Med. 1995;181:405–409. doi: 10.1084/jem.181.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukherjee J, Pirofski L A, Scharff M D, Casadevall A. Antibody-mediated protection in mice with lethal intracerebral Cryptococcus neoformans infection. Proc Natl Acad Sci USA. 1993;90:3636–3640. doi: 10.1073/pnas.90.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mukherjee J, Scharff M D, Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun. 1992;60:4534–4541. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukherjee J, Zuckier L S, Scharff M D, Casadevall A. Therapeutic efficacy of monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan alone and in combination with amphotericin B. Antimicrob Agents Chemother. 1994;38:580–587. doi: 10.1128/aac.38.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mukherjee S, Lee S, Mukherjee J, Scharff M D, Casadevall A. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun. 1994;62:1079–1088. doi: 10.1128/iai.62.3.1079-1088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukherjee S, Lee S C, Casadevall A. Antibodies to Cryptococcus neoformans glucuronoxylomannan enhance antifungal activity of murine macrophages. Infect Immun. 1995;63:573–579. doi: 10.1128/iai.63.2.573-579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller W, Rajewsky K, Kuhn R. Interleukin-4-deficient mice. Res Immunol. 1993;144:637–638. doi: 10.1016/s0923-2494(05)80018-5. [DOI] [PubMed] [Google Scholar]
- 58.Muraille E, Leo O. Revisiting the Th1/Th2 paradigm. Scand J Immunol. 1998;47:1–9. doi: 10.1111/j.1365-3083.1998-47-1.00383.x. [DOI] [PubMed] [Google Scholar]
- 59.Murray P J, Young R A. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67:3087–3095. doi: 10.1128/iai.67.6.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noben-Trauth N, Kropf P, Muller I. Susceptibility to Leishmania major infection in interleukin-4-deficient mice. Science. 1996;271:987–990. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- 61.Nussbaum G, Yuan R, Casadevall A, Scharff M D. Immunoglobulin G3 blocking antibodies to the fungal pathogen Cryptococcus neoformans. J Exp Med. 1996;183:1905–1909. doi: 10.1084/jem.183.4.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Powderly W G. Recent advances in the management of cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1996;22(Suppl. 2):S119–S123. doi: 10.1093/clinids/22.supplement_2.s119. [DOI] [PubMed] [Google Scholar]
- 63.Punnonen J, de Waal Malefyt R, van Vlasselaer P, Gauchat J F, de Vries J E. IL-10 and viral IL-10 prevent IL-4-induced IgE synthesis by inhibiting the accessory cell function of monocytes. J Immunol. 1993;151:1280–1289. [PubMed] [Google Scholar]
- 64.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell R A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roberts C W, Ferguson D J, Jebbari H, Satoskar A, Bluethmann H, Alexander J. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect Immun. 1996;64:897–904. doi: 10.1128/iai.64.3.897-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 67.Schandene L, Alonso-Vega C, Willems F, Gerard C, Delvaux A, Velu T, Devos R, de Boer M, Goldman M. B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J Immunol. 1994;152:4368–4374. [PubMed] [Google Scholar]
- 68.Spira G, Scharff M D. Identification of rare immunoglobulin switch variants using the ELISA spot assay. J Immunol Methods. 1992;148:121–129. doi: 10.1016/0022-1759(92)90165-p. [DOI] [PubMed] [Google Scholar]
- 69.Srikanth P, Castillo R C, Sridharan G, John T J, Zachariah A, Mathai D, Schwartz D H. Increase in plasma IL-10 levels and rapid loss of CD4+ T cells among HIV-infected individuals in south India. Int J STD AIDS. 2000;11:49–51. doi: 10.1258/0956462001914904. [DOI] [PubMed] [Google Scholar]
- 70.Stordeur P, Goldman M. Interleukin-10 as a regulatory cytokine induced by cellular stress: molecular aspects. Int Rev Immunol. 1998;16:501–522. doi: 10.3109/08830189809043006. [DOI] [PubMed] [Google Scholar]
- 71.Stylianou E, Aukrust P, Kvale D, Muller F, Froland S S. IL-10 in HIV infection: increasing serum IL-10 levels with disease progression—down-regulatory effect of potent anti-retroviral therapy. Clin Exp Immunol. 1999;116:115–120. doi: 10.1046/j.1365-2249.1999.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suzuki Y, Yang Q, Yang S, Nguyen N, Lim S, Liesenfeld O, Kojima T, Remington J S. IL-4 is protective against development of toxoplasmic encephalitis. J Immunol. 1996;157:2564–2569. [PubMed] [Google Scholar]
- 73.te Velde A A, de Waal Malefijt R, Huijbens R J, de Vries J E, Figdor C G. IL-10 stimulates monocyte Fc gamma R surface expression and cytotoxic activity. Distinct regulation of antibody-dependent cellular cytotoxicity by IFN-gamma, IL-4, and IL-10. J Immunol. 1992;149:4048–4052. [PubMed] [Google Scholar]
- 74.Tripp C S, Beckerman K P, Unanue E R. Immune complexes inhibit antimicrobial responses through interleukin-10 production. Effects in severe combined immunodeficient mice during Listeria infection. J Clin Investig. 1995;95:1628–1634. doi: 10.1172/JCI117837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van den Broek M, Bachmann M F, Kohler G, Barner M, Escher R, Zinkernagel R, Kopf M. IL-4 and IL-10 antagonize IL-12-mediated protection against acute vaccinia virus infection with a limited role of IFN-gamma and nitric oxide synthetase 2. J Immunol. 2000;164:371–378. doi: 10.4049/jimmunol.164.1.371. [DOI] [PubMed] [Google Scholar]
- 76.van der Horst C M, Saag M S, Cloud G A, Hamill R J, Graybill J R, Sobel J D, Johnson P C, Tuazon C U, Kerkering T, Moskovitz B L, Powderly W G, Dismukes W E. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. N Engl J Med. 1997;337:15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 77.Vazquez-Torres A, Jones-Carson J, Wagner R D, Warner T, Balish E. Early resistance of interleukin-10 knockout mice to acute systemic candidiasis. Infect Immun. 1999;67:670–674. doi: 10.1128/iai.67.2.670-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Warren H S, Kinnear B F, Phillips J H, Lanier L L. Production of IL-5 by human NK cells and regulation of IL-5 secretion by IL-4, IL-10, and IL-12. J Immunol. 1995;154:5144–5152. [PubMed] [Google Scholar]
- 79.Yuan R, Casadevall A, Spira G, Scharff M D. Isotype switching from IgG3 to IgG1 converts a nonprotective murine antibody to Cryptococcus neoformans into a protective antibody. J Immunol. 1995;154:1810–1816. [PubMed] [Google Scholar]
- 80.Yuan R, Clynes R, Oh J, Ravetch J V, Scharff M D. Antibody-mediated modulation of Cryptococcus neoformans infection is dependent on distinct Fc receptor functions and IgG subclasses. J Exp Med. 1998;187:641–648. doi: 10.1084/jem.187.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan R R, Casadevall A, Oh J, Scharff M D. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc Natl Acad Sci USA. 1997;94:2483–2488. doi: 10.1073/pnas.94.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan R R, Spira G, Oh J, Paizi M, Casadevall A, Scharff M D. Isotype switching increases efficacy of antibody protection against Cryptococcus neoformans infection in mice. Infect Immun. 1998;66:1057–1062. doi: 10.1128/iai.66.3.1057-1062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zuany-Amorim C, Creminon C, Nevers M C, Nahori M A, Vargaftig B B, Pretolani M. Modulation by IL-10 of antigen-induced IL-5 generation, and CD4+ T lymphocyte and eosinophil infiltration into the mouse peritoneal cavity. J Immunol. 1996;157:377–384. [PubMed] [Google Scholar]
- 84.Zuger A, Louie E, Holzman R S, Simberkoff M S, Rahal J J. Cryptococcal disease in patients with the acquired immunodeficiency syndrome. Diagnostic features and outcome of treatment. Ann Intern Med. 1986;104:234–240. doi: 10.7326/0003-4819-104-2-234. [DOI] [PubMed] [Google Scholar]