Abstract
Physical inactivity is common in people with chronic airways disease (pwCAD) and associated with worse clinical outcomes and impaired quality of life. We conducted a systematic review and meta-analysis to characterise and evaluate the effectiveness of interventions promoting step-based physical activity (PA) in pwCAD. We searched for studies that included a form of PA promotion and step-count outcome measure. A random-effects model was used to determine the overall effect size using post-intervention values. 38 studies (n=32 COPD; n=5 asthma; n=1 bronchiectasis; study population: n=3777) were included. Overall, implementing a form of PA promotion resulted in a significant increase in step-count: median (IQR) 705 (183–1210) when compared with usual standard care: −64 (−597–229), standardised mean difference (SMD) 0.24 (95% CI: 0.12–0.36), p<0.01. To explore the impact of specific interventions, studies were stratified into subgroups: PA promotion+wearable activity monitor-based interventions (n=17) (SMD 0.37, p<0.01); PA promotion+step-count as an outcome measure (n=9) (SMD 0.18, p=0.09); technology-based interventions (n=12) (SMD 0.16, p=0.01). Interventions promoting PA, particularly those that incorporate wearable activity monitors, result in a significant and clinically meaningful improvement in daily step-count in pwCAD.
Short abstract
Utilising wearable activity monitors in conjunction with established behaviour change techniques leads to the greatest improvement in step-based physical activity in people with chronic airways disease. https://bit.ly/3Ujs8y7
Introduction
Chronic lung disease affects over 550 million people worldwide and is a leading cause of morbidity and mortality [1]. Collectively, common obstructive airway diseases such as asthma and COPD contribute significantly to the overall prevalence of non-communicable disease [2] and are projected to remain a major burden on society for the foreseeable future [3]. Despite this outlook, prevention and intervention strategies exist to slow physiological deterioration, optimise prognosis and improve quality of life [4].
Exertional dyspnoea and activity limitation are often the earliest clinical indications of underlying respiratory disease due to airflow impairment and/or gas exchange abnormalities (and cardiovascular dysfunction and/or peripheral muscle wasting in those with comorbid illness) [5]. It is therefore common for people with chronic airways disease (pwCAD) to avoid physical activity (PA) or strenuous exercise in an attempt to minimise or control their respiratory symptoms [4, 6]. However, this approach is considered ineffective on the basis that physical inactivity leads to deconditioning, which ultimately contributes to increased symptom burden and lower functional capacity [7, 8]. Furthermore, physical inactivity (assessed via daily steps) is now recognised as an independent risk factor for both mortality and hospitalisation in people with COPD [9–11].
To counteract this “cycle of physical inactivity”, it is therefore recommended that pwCAD should be referred to pulmonary rehabilitation programmes that encompass exercise training, education and PA promotion, to encourage long-term adherence to health-enhancing behaviours [4]. Despite substantial evidence supporting the clinical value of pulmonary rehabilitation [12], access and resources remain limited [13, 14], and without effective maintenance strategies, the associated improvements in PA typically diminish within 1–2 years [15, 16].
Improvements in functional capacity following pulmonary rehabilitation also often fail to translate into increased daily PA [17, 18]. The reasons for this are complex and relate to physiological, psychological, social, cultural, environmental and economic factors which may affect behaviour in relation to PA [19]. Historically, PA promotion strategies have primarily centred on goal setting, action planning, support mechanisms, self-affirmation and motivational techniques [20]. However, novel behaviour change techniques to promote activity continue to emerge [21] and technological developments over the past decade (i.e., wearable activity monitors, in-built smartphone pedometers and mobile applications) have also shown promise in this setting [22]. Despite this, there is currently limited guidance concerning the optimal or most effective form of PA promotion to elicit long-term behaviour change and/or lifestyle modification in pwCAD [23].
The primary aim of this study was therefore to conduct a systematic review and meta-analysis to characterise and evaluate the effectiveness of interventions promoting step-based PA in pwCAD. A secondary objective was to identify unmet need, provide direction for research and inform the design of future interventions.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [24]. The review was registered prospectively with the PROSPERO database (registration number: CRD42019134918).
Study selection and eligibility criteria
PubMed, CINAHL, PsycINFO, Embase and EBSCO were used to search for published articles between January 2010 and July 2022. The search strategy comprised broad terms including: “asthma” OR “chronic obstructive pulmonary disease” OR “COPD” OR “emphysema” OR “chronic bronchitis” OR “bronchiectasis” OR “cystic fibrosis” OR “airways disease” OR “airway obstruction” OR “bronchoconstriction” OR “expiratory airflow limitation” AND “physical activity” OR “exercise” OR “step-count”. The results were combined and duplicate articles removed. Any additional relevant articles identified by the authors or sourced from the reference list of identified studies were also included.
Inclusion and exclusion criteria
Studies were required to meet the following PICOS criteria: 1) participants: adults >18 years of age with a prior diagnosis of airways disease; 2) intervention: a form of PA promotion (e.g. educational resources, face-to-face or remote support, feedback on PA, behavioural techniques); 3) comparator or control group (i.e., no PA promotion or usual standard care); 4) outcomes: PA objectively assessed via change in step-count (pre-to-post intervention); and 5) study design: randomised controlled trials and non-randomised controlled trials. Studies were excluded if they were published in a non-English language, reviews, expert opinion, editorials, qualitative or consensus position papers. Studies were also excluded if there was no control arm or incomplete pre-to-post intervention data (i.e., mean±sd) was not provided or could not be calculated. Two independent reviewers (C. Reilly and J. Sails) screened the titles and abstracts of all studies against the inclusion and exclusion criteria. Any disparity between the two reviewers was resolved by a third independent reviewer (O. Price).
Data extraction
C. Reilly and J. Sails independently performed study screening (titles and abstracts) and extracted data using a standardised data extraction template developed specifically for this review. Information concerning year of publication, title, study design, sample size, participant characteristics (specific type of airways disease, severity of condition and sub-type, sex and age), intervention (form of PA promotion employed, study duration and follow-up) and outcome measures (type of PA monitor, steps per day (pre-to-post intervention)) were extracted. If mean differences in step-count pre-to-post intervention were not reported, corresponding authors were approached to provide the data. Studies were excluded from the analysis if authors did not respond within 2 weeks or were unable to provide the requested data.
Quality assessment
C. Reilly and J. Sails evaluated eligible studies using the Downs and Blacks checklist which consists of a 27-item instrument including five domains: reporting, external validity, internal validity, confounding assessment and statistical power [25]. All studies were scored and assigned a quality grade: excellent (26–28); good (20–25); fair (15–19); and poor (<14). Any disparity between the two reviewers was resolved by a third independent reviewer (O. Price).
Data synthesis and analysis
A random-effects model was used to determine the overall effect size using post-intervention values (mean step-count) to calculate the standardised mean difference (SMD) between studies and 95% confidence intervals (CIs). p-values were calculated from the CIs. For studies that reported step-count data as medians, interquartile ranges and CIs, means and standard deviations were estimated using established referenced formulas [26, 27]. The post-intervention values were used to calculate the effect size rather than change scores as it was not possible to calculate the standard deviation of the mean change in step-count for each study. The comparison of final measurements is considered to produce the same estimate as a comparison of change from baseline when examining randomised controlled trials [27] but does mean that baseline step-count is not accounted for. Accordingly, subgroup analysis was undertaken to assess the impact of baseline step-count (<4000 or ≥4000 steps) [28–30]. A random-effects model was used, based on the assumption that study effect sizes are different and that the collected studies represent a random sample from a larger population of studies. Heterogeneity was measured using I2 statistics and Cochran's Q statistic. An I2 value of 25% was considered to demonstrate low heterogeneity, 25–50% moderate and >50% high [27]. For the Cochran's Q test, p<0.05 was used to define statistically significant heterogeneity. The effect size (SMD) was calculated using Hedges’ g formula:
The pooled weighted standard deviation (sd*pooled) was calculated using the following formula:
Hedges’ g was employed to account for small and variable sample sizes between intervention and control groups [27]. All statistical analyses were conducted using STATA version 15.1 (Stata Corporation, College Station, TX, USA).
Results
Study characteristics and quality assessment
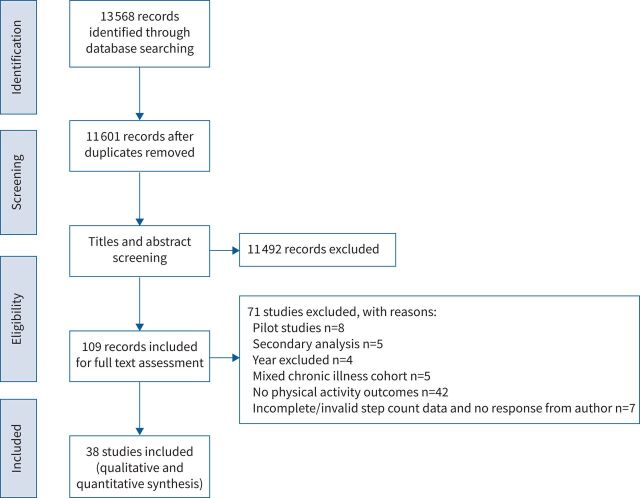
In total, 13 568 studies were identified. Of these, 38 studies (n=37 randomised controlled trials [28–64]; n=1 non-randomised controlled trial [65]) were considered eligible for inclusion in the systematic review and meta-analysis (figure 1). The included studies resulted in a combined study population of n=3777 (intervention: n=1995 and control: n=1782) (male: 65%). Of these, 32 studies included people with COPD (n=3498), five studies included people with asthma (n=216) and one study included people with bronchiectasis (n=63). Study variables and characteristics including the type of PA monitor employed are summarised for reference in table 1. Downs & Black Quality Assessment Scores ranged from 16 to 25, and studies were rated as fair (n=4) and good (n=34) (table 2).
FIGURE 1.
PRISMA flowchart representing search results.
TABLE 1.
Summary of key study variables and characteristics
| Intervention/studies | Population | FEV1 % pred | Sample size (n) | Study duration weeks | Physical activity monitor | |
| Intervention | Control | |||||
| Physical activity promotion+wearable activity monitor-based intervention | ||||||
| Altenburg et al. [28] | COPD | 60 | 65 | 55 | 12 | Yamax Digiwalker SW-200 |
| Armstrong et al. [31] | COPD | 50 | 24 | 24 | 8 | Actigraph Wgt3x |
| Bender et al. [32] | COPD | 54 | 50 | 50 | 12 | Omron |
| Bertici et al. [65] | COPD | 41 | 21 | 21 | 3 | Canyon |
| Cheng et al. [33] | COPD | 56 | 34 | 34 | 6 | activPAL |
| Coelho et al. [34] | Asthma | 81 | 15 | 15 | 12 | Actigraph Wgt3x |
| Cruz et al. [29] | COPD | 66 | 16 | 16 | 12 | Yamax Digiwalker SW-200 |
| Freitas et al. [35] | Asthma | 70 | 28 | 28 | 13 | Yamax Power Walker EX-510 |
| Freitas et al. [36] | Asthma | 66 | 26 | 26 | 8 | Actigraph GT9X |
| Geidl et al. [37] | COPD | 54 | 160 | 160 | 6 | Actigraph Wgt3x |
| Hiles et al. [38] | Asthma | 75 | 9 | 9 | 12 | Actigraph Wgt3x |
| Hornikx et al. [39] | COPD | 43 | 15 | 15 | 6 | Fitbit Ultra |
| Mendoza et al. [40] | COPD | 66 | 47 | 47 | 12 | Tanita PD724 |
| Nolan et al. [41] | COPD | 51 | 57 | 57 | 8 | Yamax Digiwalker CW700 |
| Nyenhuis et al. [42] | Asthma | 62 | 28 | 28 | 24 | Actigraph GT3XP |
| Varas et al. [30] | COPD | 49 | 16 | 16 | 8 | Omron HJ 320-e |
| Widyastuti et al. [43] | COPD | 66 | 18 | 18 | 6 | Omron HJ 321 |
| Physical activity promotion+step-count as an outcome measure | ||||||
| Effing et al. [44] | COPD | 50 | 68 | 68 | 26 | Yamax Digiwalker SW-200 |
| Evaristo et al. [45] | Asthma | 71 | 25 | 25 | 13 | PowerWalker SW610 |
| Holland et al. [46] | COPD | 50 | 33 | 33 | 8 | Sensewear Armband |
| José et al. [47] | Bronchiectasis | 53 | 28 | 28 | 9 | Actigraph Wgt3x |
| Ko et al. [48] | COPD | 48 | 57 | 57 | 8 | Actigraph Wgt3x |
| Lahham et al. [49] | COPD | 90 | 29 | 29 | 8 | Omron Walking Style Pro |
| Rausch et al. [50] | COPD | 81 | 18 | 18 | 17 | Sensewear Pro armband |
| Selzler et al. [51] | COPD | 56 | 85 | 85 | 8 | Fitbit Flex |
| Wootton et al. [52] | COPD | 43 | 39 | 39 | 10 | Sensewear Armband |
| Technology-based intervention | ||||||
| Arbillaga-Etxarri et al. [53] | COPD | 56 | 148 | 148 | 52 | Dynaport movemonitor |
| Benzo et al. [54] | COPD | 43 | 74 | 74 | 8 | Actigraph Wgt3x |
| Demeyer et al. [55] | COPD | 56 | 132 | 132 | 12 | Dynaport movemonitor/Actigraph |
| Moy et al. [56] | COPD | 68 | 68 | 18 | Omron HJ 720 ITC | |
| Moy et al. [57] | COPD | 84 | 84 | 52 | Omron HJ 720 ITC | |
| Park et al. [58] | COPD | 65 | 20 | 20 | 26 | Actigraph wGT-3X-BT |
| Robinson et al. [59] | COPD | 61 | 78 | 78 | 26 | Fitbit zip |
| Simmich et al. [60] | COPD | 6 | 6 | 3 | Fitbit Alta HR or Fitbit Charge HR 2 | |
| Spielmanns et al. [61] | COPD | 44 | 34 | 34 | 26 | POLAR A370 watch |
| Tabak et al. [62] | COPD | 52 | 16 | 16 | 4 | Yamax Digiwalker 200 |
| Vorrink et al. [63] | COPD | 56 | 67 | 67 | 12 | SenseWear PRO / MF-SW Mini armband |
| Wan et al. [64] | COPD | 63 | 52 | 52 | 12 | Omron HJ 720 ITC |
FEV1: forced expiratory volume in 1 s.
TABLE 2.
Downs and Black assessment checklist scores
| Intervention/studies | Reporting (out of 11) | External validity (out of 3) | Internal validity (out of 7) | Confounding bias (out of 6) | Power (out of 1) | Total score (out of 28) |
| Physical activity promotion+wearable activity monitor-based intervention | ||||||
| Altenburg et al. [28] | 10 | 2 | 5 | 2 | 1 | 20 |
| Armstrong et al. [31] | 11 | 2 | 5 | 2 | 1 | 21 |
| Bender et al. [32] | 8 | 2 | 5 | 2 | 0 | 17 |
| Bertici et al. [65] | 9 | 2 | 5 | 0 | 0 | 16 |
| Cheng et al. [33] | 10 | 2 | 7 | 4 | 1 | 24 |
| Coelho et al. [34] | 10 | 2 | 5 | 3 | 1 | 21 |
| Cruz et al. [29] | 11 | 2 | 6 | 3 | 1 | 23 |
| Freitas et al. [35] | 10 | 2 | 6 | 4 | 1 | 23 |
| Freitas et al. [36] | 10 | 2 | 7 | 3 | 1 | 23 |
| Geidl et al. [37] | 10 | 2 | 6 | 3 | 0 | 21 |
| Hiles et al. [38] | 11 | 2 | 5 | 4 | 0 | 22 |
| Hornikx et al. [39] | 11 | 2 | 5 | 3 | 1 | 22 |
| Mendoza et al. [40] | 10 | 2 | 7 | 4 | 1 | 24 |
| Nolan et al. [41] | 10 | 2 | 6 | 4 | 0 | 22 |
| Nyenhuis et al. [42] | 11 | 2 | 5 | 2 | 0 | 20 |
| Varas et al. [30] | 10 | 2 | 7 | 4 | 0 | 23 |
| Widyastuti et al. [43] | 10 | 2 | 5 | 2 | 1 | 20 |
| Physical activity promotion+step-count as an outcome measure | ||||||
| Effing et al. [44] | 11 | 2 | 5 | 3 | 1 | 22 |
| Evaristo et al. [45] | 11 | 2 | 6 | 4 | 1 | 24 |
| Holland et al. [46] | 11 | 2 | 7 | 4 | 1 | 25 |
| José et al. [47] | 11 | 2 | 5 | 3 | 1 | 22 |
| Ko et al. [48] | 10 | 2 | 6 | 4 | 1 | 23 |
| Lahham et al. [49] | 10 | 2 | 6 | 3 | 1 | 22 |
| Rausch et al. [50] | 11 | 2 | 6 | 3 | 0 | 22 |
| Selzler et al. [51] | 10 | 2 | 7 | 3 | 1 | 23 |
| Wootton et al. [52] | 10 | 2 | 7 | 4 | 1 | 24 |
| Technology-based intervention | ||||||
| Arbillaga-Etxarri et al. [53] | 11 | 2 | 6 | 3 | 1 | 23 |
| Benzo et al. [54] | 11 | 2 | 6 | 2 | 1 | 22 |
| Demeyer et al. [55] | 11 | 2 | 5 | 3 | 1 | 22 |
| Moy et al. [56] | 11 | 2 | 5 | 3 | 1 | 22 |
| Moy et al. [57] | 11 | 2 | 5 | 3 | 1 | 22 |
| Park et al. [58] | 11 | 2 | 5 | 3 | 1 | 22 |
| Robinson et al. [59] | 11 | 2 | 7 | 4 | 1 | 25 |
| Simmich et al. [60] | 10 | 2 | 5 | 2 | 0 | 19 |
| Spielmanns et al. [61] | 11 | 2 | 5 | 3 | 1 | 22 |
| Tabak et al. [62] | 10 | 2 | 5 | 2 | 0 | 19 |
| Vorrink et al. [63] | 11 | 2 | 6 | 2 | 1 | 22 |
| Wan et al. [64] | 10 | 2 | 6 | 2 | 1 | 21 |
PA promotion versus usual standard care (n=38)
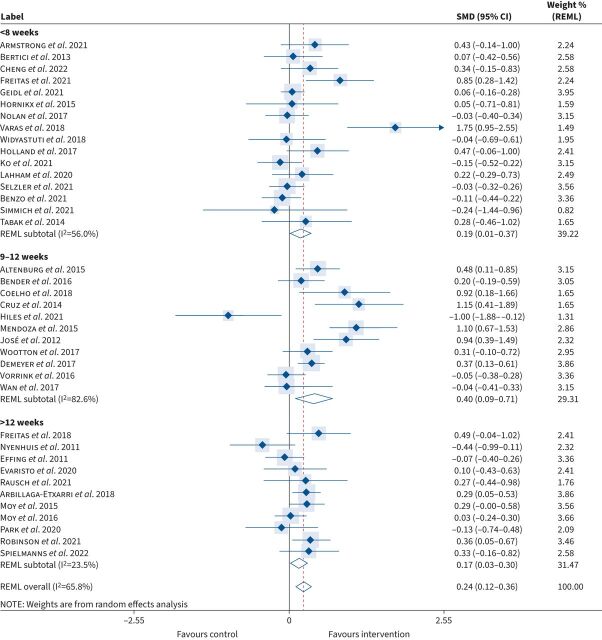
Five behaviour change techniques were employed across all 38 studies: 1) motivational interviewing, 2) real-time feedback on step-count, 3) diaries/logbooks, 4) face-to-face support and 5) remote support. The majority of interventions (95%) combined at least two techniques (table 3). Baseline daily step-count was not significantly different between intervention (5043±1653 steps) and control (5143±1542 steps) (p=0.359). However, PA promotion was associated with a larger effect size favouring intervention in those with a higher baseline step-count (≥4000 steps): SMD=0.28 (95% CI: 0.11–0.45) in comparison to those with lower baseline steps (<4000 steps): SMD=0.15 (95% CI: 0.02–0.29). The duration of the interventions was <8 weeks (n=8 studies), nine to 12 weeks (n=9 studies) and over 12 weeks (mean±sd: 27±14 weeks) (n=5 studies). The greatest improvement in step-count was observed for studies lasting between 9 and 12 weeks: median (IQR): 890 (360–1558); SMD 0.40 (95% CI: 0.09–0.71), p=0.01 (figure 2).
TABLE 3.
Breakdown of physical activity promotion strategies
| First author [ref.] | Motivational interviewing | Real-time feedback on step-count | Diaries and logbooks | Face-to-face support | Remote support | Total behaviour change strategies |
| Altenburg et al. [28] | ✓ | ✓ | ✓ | ✓ | × | 4 |
| Armstrong et al. [31] | ✓ | ✓ | ✓ | ✓ | × | 4 |
| Bender et al. [32] | ✓ | ✓ | × | ✓ | ✓ | 4 |
| Bertici et al. [65] | × | ✓ | ✓ | ✓ | ✓ | 4 |
| Cheng et al. [ 33 ] | ✓ | ✓ | ✓ | ✓ | ✓ | 5 |
| Coelho et al. [ 34 ] | × | ✓ | ✓ | ✓ | ✓ | 4 |
| Cruz et al. [ 29 ] | × | ✓ | ✓ | ✓ | ✓ | 4 |
| Freitas et al. [ 35 ] | × | ✓ | ✓ | ✓ | × | 3 |
| Freitas et al. [ 36 ] | ✓ | ✓ | ✓ | ✓ | × | 4 |
| Geidl et al. [ 37 ] | × | ✓ | ✓ | ✓ | × | 3 |
| Hiles et al. [ 38 ] | × | ✓ | ✓ | ✓ | × | 3 |
| Hornikx et al. [ 39 ] | × | ✓ | × | × | ✓ | 2 |
| Mendoza et al. [ 40 ] | × | ✓ | ✓ | ✓ | × | 3 |
| Nolan et al. [ 41 ] | × | ✓ | ✓ | ✓ | × | 3 |
| Nyenhuis et al. [ 42 ] | × | ✓ | ✓ | ✓ | ✓ | 4 |
| Varas et al. [ 30 ] | × | ✓ | ✓ | ✓ | ✓ | 4 |
| Widyastuti et al. [ 43 ] | × | ✓ | ✓ | ✓ | × | 3 |
| Effing et al. [ 44 ] | × | × | ✓ | ✓ | × | 2 |
| Evaristo et al. [ 45 ] | × | × | × | ✓ | × | 1 |
| Holland et al. [ 46 ] | ✓ | × | ✓ | ✓ | ✓ | 4 |
| José et al. [ 47 ] | × | × | ✓ | ✓ | ✓ | 3 |
| Ko et al. [ 48 ] | × | × | × | ✓ | ✓ | 2 |
| Lahham et al. [ 49 ] | ✓ | × | × | × | ✓ | 2 |
| Rausch et al. [ 50 ] | ✓ | × | × | ✓ | ✓ | 3 |
| Selzler et al. [ 51 ] | × | × | ✓ | ✓ | × | 2 |
| Wootton et al. [ 52 ] | × | × | × | ✓ | × | 1 |
| Arbillaga-Etxarri et al. [53] | ✓ | ✓ | ✓ | ✓ | ✓ | 5 |
| Benzo et al. [54] | ✓ | ✓ | ✓ | × | ✓ | 4 |
| Demeyer et al. [ 55 ] | ✓ | ✓ | ✓ | ✓ | ✓ | 5 |
| Moy et al. [ 56 ] | × | ✓ | × | × | ✓ | 2 |
| Moy et al. [ 57 ] | × | ✓ | × | × | ✓ | 2 |
| Park et al. [ 58 ] | × | ✓ | ✓ | ✓ | ✓ | 4 |
| Robinson et al. [ 59 ] | × | ✓ | ✓ | ✓ | ✓ | 4 |
| Simmich et al. [ 60 ] | × | ✓ | ✓ | × | ✓ | 3 |
| Spielmanns et al. [ 61 ] | × | ✓ | ✓ | × | ✓ | 3 |
| Tabak et al. [ 62 ] | × | ✓ | ✓ | × | ✓ | 3 |
| Vorrink et al. [ 63 ] | × | ✓ | ✓ | ✓ | ✓ | 4 |
| Wan et al. [ 64 ] | ✓ | ✓ | × | × | ✓ | 3 |
| Total | 12 | 29 | 28 | 29 | 25 |
FIGURE 2.
Standard mean difference (SMD) in daily step-count according to intervention duration (pre-to-post intervention). REML: restricted maximum likelihood.
Irrespective of the study duration, implementing any form of PA promotion resulted in a significant increase in step-count from baseline: median (IQR): 705 (183–1210) when compared with usual standard care: −64 (−597–229); SMD 0.24 (95% CI: 0.12–0.36), p<0.01 (small effect size) (figure 3). However, a high degree of heterogeneity was observed between studies (I2=66%), and thus to explore the effectiveness of specific interventions, studies were stratified into three distinct subgroups according to the primary methods of PA promotion (detailed below).
FIGURE 3.
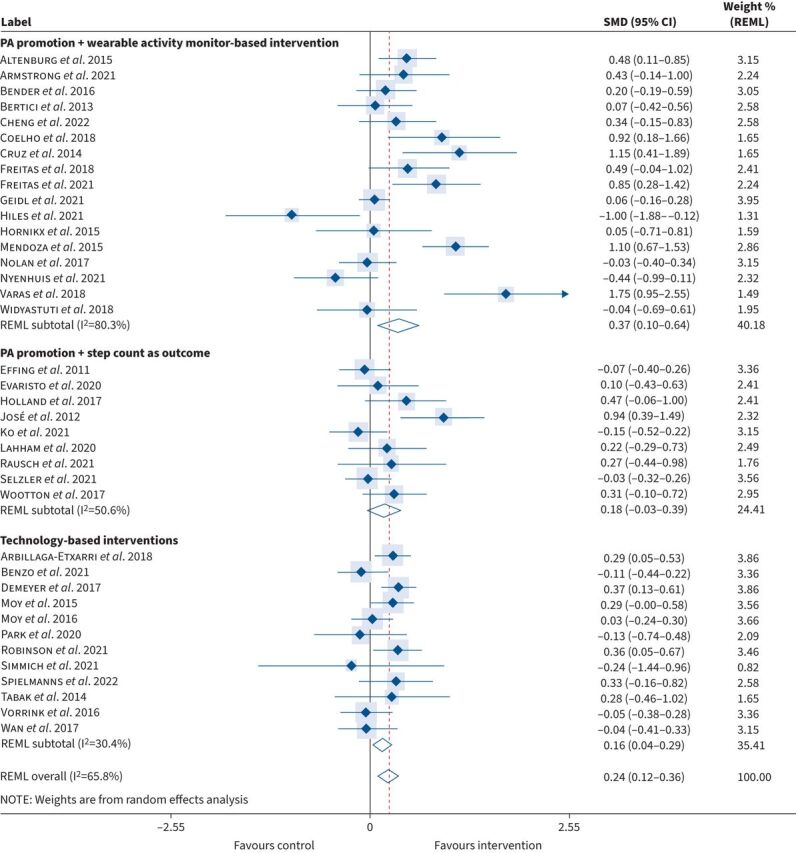
Standard mean difference (SMD) in daily step-count according to primary method of physical activity promotion (pre-to-post intervention). PA: physical activity; REML: restricted maximum likelihood.
PA promotion+wearable activity monitor-based interventions (n=17)
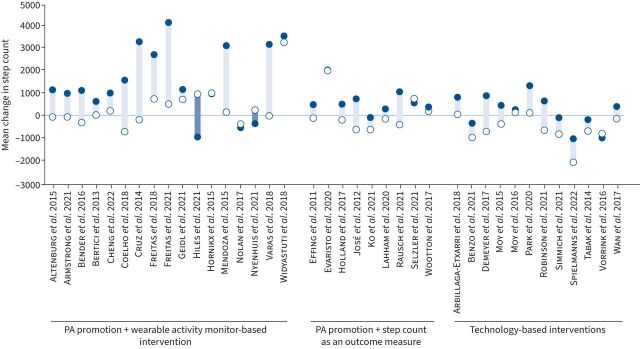
17 out of 38 studies (44.7%) (n=1304) included PA promotion with a wearable activity monitor-based intervention (i.e., pedometer or accelerometer incorporated as a tool to monitor and provide feedback on step-count throughout the intervention). This combination resulted in the greatest increase in step-count: median (IQR): 1153 (791–3199) when compared with usual standard care: 138 (−114–737); SMD 0.37 (95% CI: 0.10–0.64), p<0.01 (small effect size) (figures 3 and 4).
FIGURE 4.
Daily step-count stratified according to subgroups pre-to-post intervention (closed and open circles denote intervention and controls, respectively). PA: physical activity.
PA promotion+step-count as an outcome measure (n=9)
Nine out of 38 studies (23.7%) (n=797) utilised PA promotion+step-count as an outcome measure (i.e., pedometer or accelerometer only used to evaluate step-count pre-to-post intervention). This form of PA promotion also resulted in an increase in step-count (albeit to a lesser extent): median (IQR): 520 (332–902) compared to usual standard care: −106 (−497–490); SMD 0.18 (95% CI: −0.03–0.39), p=0.09 (small effect size) (figures 3 and 4).
Technology-based interventions (n=12)
12 out of 38 studies (31.6%) (n=1676) employed a technology-based intervention (i.e., using smartphone applications and/or website resources to provide information to promote PA). Importantly, all technology-based interventions also objectively monitored step-count throughout the study. This approach also led to a significant increase in step-count: median (IQR): 355 (−300–780) compared to usual standard care: −639 (−793–23); SMD 0.16 (95% CI: 0.04–0.29), p=0.01 (small effect size) (figures 3 and 4).
Discussion
Physical inactivity is common in pwCAD and associated with worse clinical outcomes and impaired quality of life [4, 6]. The development of effective strategies to promote PA to elicit long-term behaviour change and lifestyle modification therefore remains a priority. In this comprehensive systematic review and meta-analysis, we confirm that interventions promoting PA, particularly those that incorporate wearable activity monitors, led to a significant increase in step-based activity when compared to usual standard care. Importantly, the total increase in daily step-count met the current threshold or smallest effect associated with a clinically relevant or perceived beneficial outcome from data in people with COPD (600–1110 steps·day−1) [11].
The impact of interventions promoting PA, in the context of chronic airways disease, has been extensively evaluated over the past 5 years [66, 67]. However, improvements in PA have not been systematically demonstrated following any particular intervention [23]. In keeping with prior reports, this systematic review emphasises the diverse range of interventions employed in contemporary research. Indeed, a variety of behaviour change strategies, including motivational interviewing, real-time feedback on step-count, diaries and logbooks, and face-to-face and remote support, were included in the 38 studies, with all but one combining at least two techniques.
In the current systematic review, we applied a stringent inclusion and exclusion criteria (i.e., objective assessment of step-count pre-to-post intervention) in order to identify relevant studies. A lack of consistency and standardisation relating to the type of wearable activity monitors employed made it difficult to quantify the effect or relative benefit of specific PA interventions [18]. Accordingly, due to the considerable heterogeneity observed between studies, we stratified interventions by study duration (short-term: <8 weeks; medium-term: 9–12 weeks; long-term: >12 weeks) and the primary method of PA promotion (PA promotion+wearable activity monitor-based interventions; PA promotion+step-count as an outcome measure; technology-based interventions).
Longer-term pulmonary rehabilitation programmes (>12 weeks) have previously been reported to be more effective at increasing PA when compared to short-term interventions (<12 weeks) [17, 18]. In contrast to these findings, we found that (irrespective of the specific intervention) studies lasting between 9 and 12 weeks led to a greater improvement in daily step-count in comparison to shorter (<8 weeks) or longer-term (>12 weeks) interventions. This may relate to the challenges associated with maintaining participant interest over long-term periods and the burden associated with tracking and reporting progress (i.e., completing step-count diaries and logbooks) which may lead to patient dropout and/or lower rates of engagement [68]. Equally, it may be that short-term setbacks are recoverable at different time points, which moving forward, justifies considering how different techniques are combined over time to elicit effective and prolonged behavioural change.
Our findings support the concept that wearable activity monitors contribute to an increase in step-based activity. Indeed, a recent meta-analysis of randomised controlled trials in COPD concluded that incorporating pedometers either as a standalone intervention or in conjunction with pulmonary rehabilitation led to a significant improvement in daily steps, particularly in those with higher baseline step-count (≥4000 steps) [22]. Sub-dividing studies according to intervention (n=17) versus outcome (n=9) represents an important extension and unique aspect of the current analysis. Indeed, for the first time, our analysis indicates that the greatest improvement in daily steps occurs when wearable activity monitors are incorporated during the intervention (i.e., continuous monitoring with real-time feedback), as opposed to simply quantifying change pre-to-post intervention. These findings are consistent with a recent systematic review and meta-analysis that reported a strong association between the use of wearable activity trackers when combined with healthcare professional consultations and increased PA in people with cardiometabolic conditions [69]. From a behavioural perspective, it is plausible that utilising wearable devices to promote PA acts to support real-time self-regulatory mechanisms (e.g. goal setting and self-monitoring), i.e., established behaviour change techniques recognised to promote long-term health-enhancing behaviours [20, 70].
Our analysis also indicates that studies incorporating a technology-based intervention (n=12) had a comparable effect to those that used activity monitors as an outcome measure only (i.e., pre-to-post intervention). This was despite the fact that all technology-based studies objectively monitored and provided feedback on daily step-count throughout the intervention. While speculative, the disparity between traditional wearable (i.e., pedometer and accelerometer) and smartphone-based interventions may be due to the fact that some patients (i.e., particularly elderly individuals or those with severe disease) struggle to operate modern smartphone devices and/or access online resources. It is also plausible that some individuals may require a more personalised approach (i.e., face-to-face contact) to optimise and maintain PA. Irrespective of these potential limitations, advances in modern user-friendly remote technologies and continued global growth in smartphone users (with the functionality to quantify step-count accurately [71]) offer promise as a low cost and scalable solution to address physical inactivity in this setting moving forward.
A secondary aim of this systematic review was to identify unmet need and provide direction for future research. It was notable that the majority of studies focused on people with COPD, despite the significant global burden of other respiratory diseases such as asthma, bronchiectasis and cystic fibrosis, where exercise intolerance and activity limitation are central features [1, 2]. Despite the identification of sex-based differences across the spectrum of chronic airways disease (i.e., higher prevalence of asthma and bronchiectasis and rising incidence of COPD in females) [72], almost two-thirds of the study population were male. In the current era of personalised and precision medicine, a key focus for future research is therefore to quantify activity status using valid research grade objective assessment tools [73] and evaluate PA promotion strategies in more diverse and inclusive populations, with consideration for disease sub-type, severity, comorbid illness, age, sex and ethnicity. Ultimately, this approach will help to identify novel clinical end-points, establish the minimal important difference according to specific populations, and permit the implementation of targeted and effective PA promotion interventions.
Clinical implications and practical application
While many healthcare professionals acknowledge the importance of PA, factors such as time constraints during consultation, lack of knowledge and confidence may limit the advice provided [74]. Indeed, brief, non-individualised and generic recommendations featuring in many contemporary consultations may lack essential components to initiate change [75]. The best available evidence to date (albeit primarily in COPD) indicates that the greatest improvement in daily step-count occurs by combining established behaviour change techniques with wearable activity monitors during the intervention. In terms of practical application, healthcare professionals could therefore encourage the use of low-cost wearable activity monitors and/or in-built smartphone pedometers to record and track daily steps during medical consultation. The potential advantages of electronic information supplemented by encouragement from a clinician may reduce healthcare utilisation while ensuring PA promotion interventions adhere to best practice and current guidelines. This recommendation is particularly pertinent in view of the ongoing SARS-CoV-2 pandemic. Indeed, it is now recognised that low levels of PA are strongly associated with a higher risk of severe COVID-19 [76] and that a significant proportion of individuals experience long-term sequelae [77, 78] including impaired functional capacity and activity limitation [79].
Methodological considerations and future research
Several methodological limitations are worthy of consideration. First, an arbitrary classification was applied to sub-divide studies, and thus findings should be interpreted with a degree of caution. Second, our analysis emphasised differences in post-intervention step-count between control and intervention groups, yet the many features affecting PA may not be captured in a single measurement. For that reason, future PA promotion-based studies should therefore aim to adopt a more holistic approach to assessment, with consideration for other relevant aspects or refined markers of PA, such as sports participation/structured exercise, time spent in sedentary living, moderate-to-vigorous activity and/or activity-related energy expenditure [80]. Third, some studies failed to report whether PA promotion interventions were evaluated in isolation or embedded within a pulmonary rehabilitation programme, which limited our ability to provide a direct comparison. Fourth, PA is highly dependent on environmental conditions and seasonality, and we were unable to account for these factors in our analysis. Finally, few studies included long-term surveillance, which limits our ability to draw robust conclusions concerning sustained benefit.
Conclusion
In summary, our findings indicate that interventions promoting PA, particularly those that incorporate wearable activity monitors, result in a significant and clinically meaningful improvement in daily step-count in pwCAD. Further multicentre randomised controlled trials with longitudinal follow-up, in diverse and inclusive populations, according to airways disease sub-type and sex, remain an important avenue for future research.
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: C. Reilly: conception and design of the study, data acquisition: preparing and validation of search strategy, searching bibliographic databases, title and abstract screening, full-text screening, drafting the manuscript. J. Sails: data acquisition: preparing and validation of search strategy, searching bibliographic databases, title and abstract screening, full-text screening, data analysis, drafting of the manuscript A. Stavropoulos-Kalinoglou: contribution to conception and design of the study, participation in acquisition: preparing and validation of search strategy, data analysis, drafting the manuscript, critical revision and project supervision. R.J. Birch: data analysis, drafting the manuscript and critical revision. J. McKenna: drafting the manuscript and critical revision. I.J. Clifton: contribution to conception and design of the study, drafting the manuscript and critical revision. D. Peckham: drafting the manuscript and critical revision. K.M. Birch: drafting the manuscript and critical revision. O.J. Price: conception and design of the study, data acquisition: preparing and validation of search strategy, drafting the manuscript, critical revision and project supervision. All authors provided approval of the final version of this manuscript to be published.
Conflict of interest: I.J. Clifton reports personal fees from GlaxoSmithKline, outside the submitted work. The remaining authors have no conflicts to declare.
References
- 1.Soriano JB, Kendrick PJ, Paulson KR, et al. . Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 2020; 8: 585–596. doi: 10.1016/S2213-2600(20)30105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labaki WW, Han MK. Chronic respiratory diseases: a global view. Lancet Respir Med 2020; 8: 531–533. doi: 10.1016/S2213-2600(20)30157-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson GJ, Loddenkemper R, Lundbäck B, et al. . Respiratory health and disease in Europe: the new European Lung White Book. Eur Respir J 2013; 42: 559–563. doi: 10.1183/09031936.00105513 [DOI] [PubMed] [Google Scholar]
- 4.Spruit MA, Singh SJ, Garvey C, et al. . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188: e13–e64. doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 5.Vogiatzis I, Zakynthinos S. Factors limiting exercise tolerance in chronic lung diseases. Compr Physiol 2012; 2: 1779–1817. doi: 10.1002/cphy.c110015 [DOI] [PubMed] [Google Scholar]
- 6.Watz H, Pitta F, Rochester CL, et al. . An official European Respiratory Society statement on physical activity in COPD. Eur Respir J 2014; 44: 1521–1537. doi: 10.1183/09031936.00046814 [DOI] [PubMed] [Google Scholar]
- 7.Cordova-Rivera L, Gibson PG, Gardiner PA, et al. . Physical activity associates with disease characteristics of severe asthma, bronchiectasis and COPD. Respirology 2019; 24: 352–360. doi: 10.1111/resp.13428 [DOI] [PubMed] [Google Scholar]
- 8.Schneider LP, Furlanetto KC, Rodrigues A, et al. . Sedentary behaviour and physical inactivity in patients with chronic obstructive pulmonary disease: two sides of the same coin? COPD 2018; 15: 432–438. doi: 10.1080/15412555.2018.1548587 [DOI] [PubMed] [Google Scholar]
- 9.Gimeno-Santos E, Frei A, Steurer-Stey C, et al. . Determinants and outcomes of physical activity in patients with COPD: a systematic review. Thorax 2014; 69: 731–739. doi: 10.1136/thoraxjnl-2013-204763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaes AW, Garcia-Aymerich J, Marott JL, et al. . Changes in physical activity and all-cause mortality in COPD. Eur Respir J 2014; 44: 1199–1209. doi: 10.1183/09031936.00023214 [DOI] [PubMed] [Google Scholar]
- 11.Demeyer H, Burtin C, Hornikx M, et al. . The minimal important difference in physical activity in patients with COPD. PLoS ONE 2016; 11: e0154587. doi: 10.1371/journal.pone.0154587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy B, Casey D, Devane D, et al. . Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 2: CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogiatzis I, Rochester CL, Spruit MA, et al. . Increasing implementation and delivery of pulmonary rehabilitation: key messages from the new ATS/ERS policy statement. Eur Respir J 2016; 47: 1336–1341. doi: 10.1183/13993003.02151-2015 [DOI] [PubMed] [Google Scholar]
- 14.Rochester CL, Vogiatzis I, Powell P, et al. . Patients’ perspective on pulmonary rehabilitation: experiences of European and American individuals with chronic respiratory diseases. ERJ Open Res 2018; 4: 00085-2018. doi: 10.1183/23120541.00085-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egan C, Deering BM, Blake C, et al. . Short term and long term effects of pulmonary rehabilitation on physical activity in COPD. Respir Med 2012; 106: 1671–1679. doi: 10.1016/j.rmed.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 16.Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med 2000; 109: 207–212. doi: 10.1016/S0002-9343(00)00472-1 [DOI] [PubMed] [Google Scholar]
- 17.Mantoani LC, Rubio N, McKinstry B, et al. . Interventions to modify physical activity in patients with COPD: a systematic review. Eur Respir J 2016; 48: 69–81. doi: 10.1183/13993003.01744-2015 [DOI] [PubMed] [Google Scholar]
- 18.Langer D, Demeyer H. Interventions to modify physical activity in patients with COPD: where do we go from here? Eur Respir J 2016; 48: 14–17. doi: 10.1183/13993003.00762-2016 [DOI] [PubMed] [Google Scholar]
- 19.Bauman AE, Reis RS, Sallis JF, et al. . Correlates of physical activity: why are some people physically active and others not? Lancet 2012; 380: 258–271. doi: 10.1016/S0140-6736(12)60735-1 [DOI] [PubMed] [Google Scholar]
- 20.Greaves CJ, Sheppard KE, Abraham C, et al. . Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011; 11: 119. doi: 10.1186/1471-2458-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouyard T, Engelen B, Papanikitas A, et al. . Boosting healthier choices. BMJ 2022; 376: e064225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong M, Winnard A, Chynkiamis N, et al. . Use of pedometers as a tool to promote daily physical activity levels in patients with COPD: a systematic review and meta-analysis. Eur Respir Rev 2019; 28: 190039. doi: 10.1183/16000617.0039-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burge AT, Cox NS, Abramson MJ, et al. . Interventions for promoting physical activity in people with chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev 2020; 4: CD012626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page MJ, McKenzie JE, Bossuyt PM, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed) 2021; 74: 790–799. doi: 10.1016/j.recesp.2021.06.016 [DOI] [PubMed] [Google Scholar]
- 25.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998; 52: 377–384. doi: 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan X, Wang W, Liu J, et al. . Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. doi: 10.1186/1471-2288-14-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JT, Thomas J, Chandler J, et al. . Cochrane Handbook for Systematic Reviews of Interventions, version 6.3. February 2022. Available at: www.training.cochrane.org/handbook
- 28.Altenburg WA, ten Hacken NHT, Bossenbroek L, et al. . Short- and long-term effects of a physical activity counselling programme in COPD: a randomized controlled trial. Respir Med 2015; 109: 112–121. doi: 10.1016/j.rmed.2014.10.020 [DOI] [PubMed] [Google Scholar]
- 29.Cruz J, Brooks D, Marques A. Walk2Bactive: a randomised controlled trial of a physical activity-focused behavioural intervention beyond pulmonary rehabilitation in chronic obstructive pulmonary disease. Chron Respir Dis 2016; 13: 57–66. doi: 10.1177/1479972315619574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varas AB, Córdoba S, Rodríguez-Andonaegui I, et al. . Effectiveness of a community-based exercise training programme to increase physical activity level in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Physiother Res Int 2018; 23: e1740. doi: 10.1002/pri.1740 [DOI] [PubMed] [Google Scholar]
- 31.Armstrong M, Hume E, McNeillie L, et al. . Behavioural modification interventions alongside pulmonary rehabilitation improve COPD patients’ experiences of physical activity. Respir Med 2021; 180: 106353. doi: 10.1016/j.rmed.2021.106353 [DOI] [PubMed] [Google Scholar]
- 32.Bender BG, Depew A, Emmett A, et al. . A patient-centered walking program for COPD. Chronic Obstr Pulm Dis 2016; 3: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng SWM, Alison J, Stamatakis E, et al. . Six-week behaviour change intervention to reduce sedentary behaviour in people with chronic obstructive pulmonary disease: a randomised controlled trial. Thorax 2022; 77: 231–238. doi: 10.1136/thoraxjnl-2020-214885 [DOI] [PubMed] [Google Scholar]
- 34.Coelho CM, Reboredo MM, Valle FM, et al. . Effects of an unsupervised pedometer-based physical activity program on daily steps of adults with moderate to severe asthma: a randomized controlled trial. J Sports Sci 2018; 36: 1186–1193. doi: 10.1080/02640414.2017.1364402 [DOI] [PubMed] [Google Scholar]
- 35.Freitas PD, Silva AG, Ferreira PG DA, et al. Exercise improves physical activity and comorbidities in obese adults with asthma. Med Sci Sports Exerc 2018; 50: 1367–1376. doi: 10.1249/MSS.0000000000001574 [DOI] [PubMed] [Google Scholar]
- 36.Freitas PD, Passos NFP, Carvalho-Pinto RM, et al. . A behavior change intervention aimed at increasing physical activity improves clinical control in adults with asthma: a randomized controlled trial. Chest 2021; 159: 46–57. doi: 10.1016/j.chest.2020.08.2113 [DOI] [PubMed] [Google Scholar]
- 37.Geidl W, Carl J, Schuler M, et al. . Long-term benefits of adding a pedometer to pulmonary rehabilitation for COPD: the randomized controlled STAR trial. Int J Chron Obstruct Pulmon Dis 2021; 16: 1977–1988. doi: 10.2147/COPD.S304976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiles SA, Urroz PD, Gibson PG, et al. . A feasibility randomised controlled trial of Novel Activity Management in severe ASthma-Tailored Exercise (NAMASTE): yoga and mindfulness. BMC Pulm Med 2021; 21: 71. doi: 10.1186/s12890-021-01436-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hornikx M, Demeyer H, Camillo CA, et al. . The effects of a physical activity counseling program after an exacerbation in patients with Chronic Obstructive Pulmonary Disease: a randomized controlled pilot study. BMC Pulm Med 2015; 15: 136. doi: 10.1186/s12890-015-0126-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendoza L, Horta P, Espinoza J, et al. . Pedometers to enhance physical activity in COPD: a randomised controlled trial. Eur Respir J 2015; 45: 347–354. doi: 10.1183/09031936.00084514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nolan CM, Maddocks M, Canavan JL, et al. . Pedometer step count targets during pulmonary rehabilitation in chronic obstructive pulmonary disease. A randomized controlled trial. Am J Respir Crit Care Med 2017; 195: 1344–1352. doi: 10.1164/rccm.201607-1372OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nyenhuis SM, Shah N, Kim H, et al. . The feasibility of a lifestyle physical activity intervention for black women with asthma. J Allergy Clin Immunol Pract 2021; 9: 4312–21.e2. doi: 10.1016/j.jaip.2021.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Widyastuti K, Makhabah DN, Setijadi AR, et al. . Benefits and costs of home pedometer assisted physical activity in patients with COPD. A preliminary randomized controlled trial. Pulmonology 2018; 24: 211–218. doi: 10.1016/j.pulmoe.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 44.Effing T, Zielhuis G, Kerstjens H, et al. . Community based physiotherapeutic exercise in COPD self-management: a randomised controlled trial. Respir Med 2011; 105: 418–426. doi: 10.1016/j.rmed.2010.09.017 [DOI] [PubMed] [Google Scholar]
- 45.Evaristo KB, Mendes FAR, Saccomani MG, et al. . Effects of aerobic training versus breathing exercises on asthma control: a randomized trial. J Allergy Clin Immunol Pract 2020; 8: 2989–96.e4. doi: 10.1016/j.jaip.2020.06.042 [DOI] [PubMed] [Google Scholar]
- 46.Holland AE, Mahal A, Hill CJ, et al. . Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax 2017; 72: 57–65. doi: 10.1136/thoraxjnl-2016-208514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.José A, Holland AE, Selman JPR, et al. . Home-based pulmonary rehabilitation in people with bronchiectasis: a randomised controlled trial. ERJ Open Res 2021; 7: 00021-2021. doi: 10.1183/23120541.00021-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ko FW, Tam W, Siu EHS, et al. . Effect of short-course exercise training on the frequency of exacerbations and physical activity in patients with COPD: a randomized controlled trial. Respirology 2021; 26: 72–79. doi: 10.1111/resp.13872 [DOI] [PubMed] [Google Scholar]
- 49.Lahham A, McDonald CF, Moore R, et al. . The impact of home-based pulmonary rehabilitation on people with mild chronic obstructive pulmonary disease: a randomised controlled trial. Clin Respir J 2020; 14: 335–344. doi: 10.1111/crj.13138 [DOI] [PubMed] [Google Scholar]
- 50.Rausch Osthoff AK, Beyer S, Gisi D, et al. . Effect of counselling during pulmonary rehabilitation on self-determined motivation to be physically active for people with chronic obstructive pulmonary disease: a pragmatic RCT. BMC Pulm Med 2021; 21: 317. doi: 10.1186/s12890-021-01685-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selzler AM, Jourdain T, Wald J, et al. . Evaluation of an enhanced pulmonary rehabilitation program: a randomized controlled trial. Ann Am Thorac Soc 2021; 18: 1650–1660. doi: 10.1513/AnnalsATS.202009-1160OC [DOI] [PubMed] [Google Scholar]
- 52.Wootton SL, Hill K, Alison JA, et al. . Effects of ground-based walking training on daily physical activity in people with COPD: a randomised controlled trial. Respir Med 2017; 132: 139–145. doi: 10.1016/j.rmed.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 53.Arbillaga-Etxarri A, Gimeno-Santos E, Barberan-Garcia A, et al. . Long-term efficacy and effectiveness of a behavioural and community-based exercise intervention (Urban Training) to increase physical activity in patients with COPD: a randomised controlled trial. Eur Respir J 2018; 52: 1800063. doi: 10.1183/13993003.00063-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benzo RP, Ridgeway J, Hoult JP, et al. . Feasibility of a health coaching and home-based rehabilitation intervention with remote monitoring for COPD. Respir Care 2021; 66: 960–971. doi: 10.4187/respcare.08580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Demeyer H, Louvaris Z, Frei A, et al. . Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax 2017; 72: 415–423. doi: 10.1136/thoraxjnl-2016-209026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moy ML, Collins RJ, Martinez CH, et al. . An internet-mediated pedometer-based program improves health-related quality-of-life domains and daily step counts in COPD. Chest 2015; 148: 128–137. doi: 10.1378/chest.14-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moy ML, Martinez CH, Kadri R, et al. . Long-term effects of an Internet-mediated pedometer-based walking program for chronic obstructive pulmonary disease: randomized controlled trial. J Med Internet Res 2016; 18: e215. doi: 10.2196/jmir.5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park SK, Bang CH, Lee SH. Evaluating the effect of a smartphone app-based self-management program for people with COPD: a randomized controlled trial. Appl Nurs Res 2020; 52: 151231. doi: 10.1016/j.apnr.2020.151231 [DOI] [PubMed] [Google Scholar]
- 59.Robinson SA, Cooper JA, Goldstein RL, et al. . A randomised trial of a web-based physical activity self-management intervention in COPD. ERJ Open Res 2021; 7: 00158-2021. doi: 10.1183/23120541.00158-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simmich J, Mandrusiak A, Smith ST, et al. . A co-designed active video game for physical activity promotion in people with chronic obstructive pulmonary disease: pilot trial. JMIR Serious Games 2021; 9: e23069. doi: 10.2196/23069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spielmanns M, Gloeckl R, Jarosch I, et al. . Using a smartphone application maintains physical activity following pulmonary rehabilitation in patients with COPD: a randomised controlled trial. Thorax 2022; in press [ 10.1136/thoraxjnl-2021-218338]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tabak M, Brusse-Keizer M, van der Valk P, et al. . A telehealth program for self-management of COPD exacerbations and promotion of an active lifestyle: a pilot randomized controlled trial. Int J Chron Obstruct Pulmon Dis 2014; 9: 935–944. doi: 10.2147/COPD.S60179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vorrink SNW, Kort HSM, Troosters T, et al. . Efficacy of an mHealth intervention to stimulate physical activity in COPD patients after pulmonary rehabilitation. Eur Respir J 2016; 48: 1019–1029. doi: 10.1183/13993003.00083-2016 [DOI] [PubMed] [Google Scholar]
- 64.Wan ES, Kantorowski A, Homsy D, et al. . Promoting physical activity in COPD: insights from a randomized trial of a web-based intervention and pedometer use. Respir Med 2017; 130: 102–110. doi: 10.1016/j.rmed.2017.07.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertici N, Fira-Mlădinescu O, Oancea C, et al. . The usefulness of pedometry in patients with chronic obstructive pulmonary disease. Multidiscip Respir Med 2013; 8: 7. doi: 10.1186/2049-6958-8-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lahham A, McDonald CF, Holland AE. Exercise training alone or with the addition of activity counseling improves physical activity levels in COPD: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis 2016; 11: 3121–3136. doi: 10.2147/COPD.S121263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu S, Cai X, Wang X, et al. . Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis 2018; 12: 1753466618787386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laranjo L, Ding D, Heleno B, et al. . Do smartphone applications and activity trackers increase physical activity in adults? Systematic review, meta-analysis and metaregression. Br J Sports Med 2021; 55: 422–432. doi: 10.1136/bjsports-2020-102892 [DOI] [PubMed] [Google Scholar]
- 69.Hodkinson A, Kontopantelis E, Adeniji C, et al. . Interventions using wearable physical activity trackers among adults with cardiometabolic conditions: a systematic review and meta-analysis. JAMA Netw Open 2021; 4: e2116382. doi: 10.1001/jamanetworkopen.2021.16382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Conn VS, Hafdahl AR, Brown SA, et al. . Meta-analysis of patient education interventions to increase physical activity among chronically ill adults. Patient Educ Couns 2008; 70: 157–172. doi: 10.1016/j.pec.2007.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reilly C, Stavropoulos-Kalinoglou A, Clifton I, et al. . Smartphone pedometers in adults with asthma: a practical approach to physical activity assessment? A pilot validation study. J Asthma 2022; 59: 967–975. [DOI] [PubMed] [Google Scholar]
- 72.Somayaji R, Chalmers JD. Just breathe: a review of sex and gender in chronic lung disease. Eur Respir Rev 2022; 31: 210111. doi: 10.1183/16000617.0111-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Demeyer H, Mohan D, Burtin C, et al. . Objectively measured physical activity in patients with COPD: recommendations from an international task force on physical activity. Chronic Obstr Pulm Dis 2021; 8: 528–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lahham A, Burge AT, McDonald CF, et al. . How do healthcare professionals perceive physical activity prescription for community-dwelling people with COPD in Australia? A qualitative study. BMJ Open 2020; 10: e035524. doi: 10.1136/bmjopen-2019-035524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brannan M, Bernardotto M, Clarke N, et al. . Moving healthcare professionals: a whole system approach to embed physical activity in clinical practice. BMC Med Educ 2019; 19: 84. doi: 10.1186/s12909-019-1517-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sallis R, Young DR, Tartof SY, et al. . Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br J Sports Med 2021; 55: 1099–1105. doi: 10.1136/bjsports-2021-104080 [DOI] [PubMed] [Google Scholar]
- 77.Adeloye D, Elneima O, Daines L, et al. . The long-term sequelae of COVID-19: an international consensus on research priorities for patients with pre-existing and new-onset airways disease. Lancet Respir Med 2021; 9: 1467–1478. doi: 10.1016/S2213-2600(21)00286-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fabbri L, Moss S, Khan FA, et al. . Parenchymal lung abnormalities following hospitalisation for COVID-19 and viral pneumonitis: a systematic review and meta-analysis. Thorax 2022; in press [ 10.1136/thoraxjnl-2021-218275]. [DOI] [PubMed] [Google Scholar]
- 79.Thomas M, Price OJ, Hull JH. Pulmonary function and COVID-19. Curr Opin Physiol 2021; 21: 29–35. doi: 10.1016/j.cophys.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rist C, Karlsson N, Necander S, et al. . Physical activity end-points in trials of chronic respiratory diseases: summary of evidence. ERJ Open Res 2022; 8: 00541-2021. doi: 10.1183/23120541.00541-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]