Abstract
Interleukin-33 (IL-33) and thymic stromal lymphopoietin (TSLP) are alarmins that are released upon airway epithelial injury from insults such as viruses and cigarette smoke, and play critical roles in the activation of immune cell populations such as mast cells, eosinophils and group 2 innate lymphoid cells. Both cytokines were previously understood to primarily drive type 2 (T2) inflammation, but there is emerging evidence for a role for these alarmins to additionally mediate non-T2 inflammation, with recent clinical trial data in asthma and COPD cohorts with non-T2 inflammation providing support. Currently available treatments for both COPD and asthma provide symptomatic relief with disease control, improving lung function and reducing exacerbation rates; however, there still remains an unmet need for further improving lung function and reducing exacerbations, particularly for those not responsive to currently available treatments. The epithelial cytokines/alarmins are involved in exacerbations; biologics targeting TSLP and IL-33 have been shown to reduce exacerbations in moderate-to-severe asthma, either in a broad population or in specific subgroups, respectively. For COPD, while there is clinical evidence for IL-33 blockade impacting exacerbations in COPD, clinical data from anti-TSLP therapies is awaited. Clinical data to date support an acceptable safety profile for patients with airway diseases for both anti-IL-33 and anti-TSLP antibodies in development. We examine the roles of IL-33 and TSLP, their potential use as drug targets, and the evidence for target patient populations for COPD and asthma, together with ongoing and future trials focused on these targets.
Short abstract
Therapies targeting the alarmins IL-33 and TSLP form the next frontier for airway diseases. In addition to their role in adaptive immunity, emerging clinical data indicate these alarmins modulate innate immunity to address unmet needs in COPD and asthma. https://bit.ly/3tD8VM5
Introduction
Asthma and COPD are chronic inflammatory airway diseases characterised by obstructive airflow limitation. Both diseases have a significant burden on both patients and healthcare systems. While asthma affects 262 million people with 461 000 deaths globally [1], COPD carries an even greater burden of disease and is the third leading cause of death worldwide, responsible for ∼3.2 million deaths in 2019 [2]. Both diseases are heterogeneous in terms of their clinical presentation and underlying inflammatory mechanisms.
The most well-described biological phenotype is type 2 (T2)-high asthma driven by the T2 cytokines, interleukin (IL)-5, IL-4 and IL-13, and is associated with blood and sputum eosinophilia [3]. The identification of these critical pathways has led to the clinical success of biologic therapies antagonising these targets, benefiting a subset of asthma patients with an eosinophilic inflammatory phenotype. Less is known of the key pathologic drivers in non-T2 asthma and COPD. In patients with COPD, there is an increase in airway neutrophils, in addition to increased numbers of macrophages, T- and B-lymphocytes, with both innate and adaptive immune responses being involved in COPD pathogenesis, C-X-C motif chemokine ligand (CXCL) 1, C-X-C motif chemokine receptor 2 and T-helper (Th) 17. Some asthmatics may have disease driven by non-T2 pathways such as the activation of IL-1, IL-17 and IL-6 pathways, and characterised by neutrophilic inflammation [4–6]. However, this remains unproven without antibody trials in specific patient groups. Defining non-T2 inflammation via the absence of high eosinophils remains problematic since non-T2 biology may encompass heterogeneous endotypes. This review focuses on epithelial alarmins, another group of cytokines that may be biologically relevant to both T2 and non-T2 asthma, together with COPD.
In both diseases, airflow obstruction and acute exacerbation of symptoms remain hallmarks of disease severity, with acute exacerbations representing events with increased airway and systemic inflammation that are related to the exacerbation trigger. Common triggers for acute exacerbations include viral or bacterial infections, cigarette smoke, allergens and environmental factors such as air pollution; while allergens typically drive T2 inflammation and related exacerbations, several of these triggers can also drive non-T2 inflammation and exacerbations. Airway neutrophilia in COPD is related to exacerbation severity regardless of the exacerbation trigger, whereas higher sputum eosinophils are associated with viral exacerbations [7]. Acute exacerbations of asthma or COPD are significantly harmful events in the life of these patients because they contribute to symptomatic and functional decline, greater healthcare utilisation, and early mortality [8, 9].
Currently available standard-of-care treatment of combined inhaled β-adrenergic agonists and inhaled corticosteroids (ICS) mainly provide symptomatic relief with control of disease, and can often improve lung function and reduce exacerbation rates. However, the response is generally better in asthma compared to COPD and those with eosinophilic inflammation respond best to ICS [8, 10], although a subset of patients with asthma and COPD are insensitive to ICS. The introduction of ICS for COPD patients is usually made in those with a history of exacerbations. Biological treatments targeting specific inflammatory pathways, such as anti-immunoglobulin (Ig)E, anti-IL-5, anti-IL-5Rα and anti-IL-4Rα antibodies, are available for severe eosinophilic or severe allergic asthma as add-on maintenance therapy for patients exhibiting a T2 inflammatory endotype. These add-on biological therapies have reduced the rate of exacerbations, with variable effects on airflow obstruction, ranging from none to small improvements [11]. Patients with non-T2 asthma, as assessed by a low blood eosinophil count and low exhaled nitric oxide fraction, are not suitable candidates for these biologic therapies. Recently, tezepelumab (anti-thymic stromal lymphopoietin (TSLP)) has been approved by the Food and Drug Administration (FDA) for the treatment of severe asthma irrespective of the level of blood eosinophil count [12]. In contrast to asthma, there are currently no approved biologics for COPD, although recent clinical trials have indicated that IL-33 blockade may be a promising strategy for COPD [13–15], and treatment options remain largely limited to bronchodilators and/or ICS. While azithromycin and the phosphodiesterase-4 inhibitor, roflimulast, are available for reducing COPD exacerbations, these therapies have limited adoption due to side-effects. For azithromycin, side-effects are primarily gastrointestinal with potential for arrhythmias and development of antibiotic resistance; while with roflimulast, gastrointestinal side-effects with nausea predominate. Thus, a large unmet need remains in disease maintenance and acute exacerbation reduction for COPD as well as for asthma, with ongoing clinical trials evaluating new biologics targeting this unmet need.
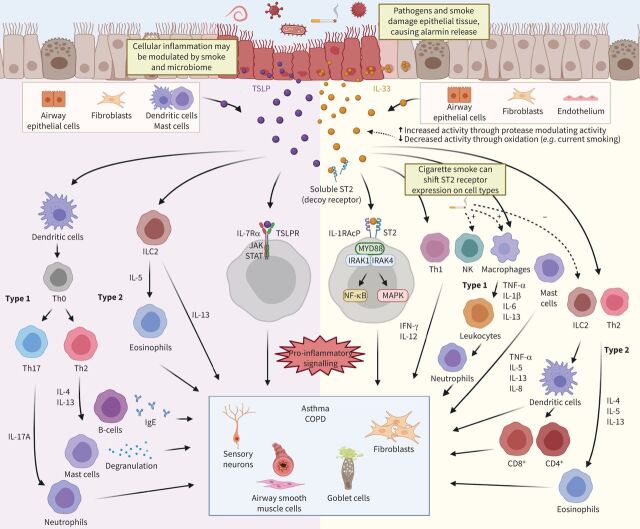
A new arena for biologic therapies focuses on the alarmins, IL-33 and TSLP, as important cytokines that can initiate and amplify innate and adaptive immune responses upon release from airway epithelial cells following external insults from pollutants, certain allergens, viral or microbial agents (figure 1) [16]. Both alarmins are found in multiple tissues and have been studied as potent activators of T2 immune responses [17, 18]. The classic T2 immune response is characterised by the induction of Th2 cells, which leads to the production of T2 cytokines such as IL-4, IL-5 and IL-13, and alarmins eliciting responses in multiple cell types, including mast cells, group 2 innate lymphoid cells (ILC2s), eosinophils, dendritic cells (DCs) and basophils. These cytokines also promote a skewing towards a B-cell IgE response, a classical trait of allergic inflammation. Blockade of another alarmin, IL-25, in mouse models of allergic disease, appears to attenuate allergic inflammation and airway hyperresponsiveness [19], suggesting a role for IL-25 in T2 inflammation. However, with several biological therapies already available to target T2 inflammation, development appears to be shifting to address the unmet need in terms of different biological pathways. Recent clinical evidence has highlighted the potential for IL-33 and TSLP in non-T2 inflammation, making these alarmins an attractive target for asthma and COPD.
FIGURE 1.
Interleukin (IL)-33 and thymic stromal lymphopoietin (TSLP) pathways in asthma and COPD. In response to insults to the airway epithelium, the alarmins IL-33 and TSLP are released from damaged epithelial cells, although other sources of the alarmins also exist. The alarmin IL-25 (not pictured here) is also released upon epithelial damage and has shown to induce type 2 (T2) inflammation and eosinophilia. IL-33 promotes inflammation through binding to its receptor, ST2 (IL1RL1), which is present on multiple cells including neutrophils, eosinophils, macrophages, basophils and mast cells, resulting in the production of both T2 and non-T2 cytokines. Binding of IL-33 to the ST2 receptor results in activation of downstream mitogen-activated protein kinase (MAPK) and NF-κB signalling. A decoy, soluble form of the ST2 receptor acts as a negative regulator of IL-33 activity. Full-length IL-33 can undergo cleavage from different proteases (e.g. neutrophil elastase), which can produce shorter isoforms that can either enhance or reduce IL-33 activity. Cigarette smoke has been shown to shift the type of immune response to IL-33, limiting the T2 while leading to an exaggerated T1 response. TSLP has a broad immune effect, activating multiple cell types and resulting in the production of cytokines typically associated with a T2 response, while also affecting mast cells, T-helper (Th) 1 cells, and other cells that result in the production of both T2 and non-T2 cytokines. TSLP signals through its receptor, TSLPR, to activate downstream Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathways with the absence of any decoy TSLPR. IL-33 and TSLP inflammatory pathways ultimately result in changes to the nonimmune components of the lung microenvironment, such as smooth muscle contraction/hyperreactivity (both), goblet cell mucus production (both), fibroblast activation (IL-33) and sensory neurons (TSLP), which contributes to the clinical symptoms and traits of asthma and COPD. Figure created using BioRender.com. IgE: immunoglobulin E; IRAK: IL-1 receptor-associated kinase; MYD88: myeloid differentiation primary response gene 88; NK: natural killer; TNF-α: tumour necrosis factor α.
Despite COPD usually being characterised by sputum neutrophilia, up to one third of COPD patients may have high sputum eosinophil levels [20]. Similarly, although asthma is considered to be driven in part by T2 inflammation, a significant proportion of patients with severe asthma have neutrophilic or pauci-granulocytic inflammation of the airways as measured by cell differentials of induced sputum [21]. Since IL-33 and TSLP contribute to both T2 and non-T2 pathways, they may represent an advance over targeting a single pathway (i.e. only T2 inflammation, such as with anti-IL-5 therapies) for asthma and COPD.
In this review, we examine the role of IL-33 and TSLP in asthma and COPD, and review their potential for multiple immune activation pathways in these diseases, and the clinical outcomes of novel therapeutics targeting these alarmins.
IL-33 pathway
IL-33 overview
IL-33, a member of the IL-1 family of cytokines first identified in 1999, plays a key role in both innate and adaptive immune responses (figure 1) [22]. It is expressed in endothelial, epithelial and fibroblast-like cells, and therefore is found in multiple organs, with well-recognised functions in cancer and allergic inflammation [17, 23, 24].
IL-33 is constitutively expressed in and mostly localised to barrier epithelial cells and endothelial cells [25]; other cell types have also been described, such as fibroblasts, myofibroblasts and airway smooth muscle cells [17, 26–29]. As IL-33 lacks a secretory signal motif and contains a chromatin binding domain, under normal and inflammatory conditions, it is a tightly sequestered protein in the cell nucleus bound to chromatin [30]. IL-33 is passively released as an “alarmin” upon tissue injury or necrosis, secondary to cigarette smoke, pollutants and viral or bacterial exposure [31–33]. Allergens and certain proteases such as calpain proteases can also activate IL-33 proteolytically, leading to increased release from airway epithelial cells [33]. IL-33 can be considered to be both having pro-inflammatory effects and can act as a nuclear transcription factor [17].
Although human full-length IL-33 is a 270-amino acid protein, shorter isoforms of IL-33 as a result of inflammatory and apoptotic protease processing can either enhance or reduce IL-33 activity [34]. Additionally, cleavage by proteases from different cell types, such as mast cells and neutrophils, could lead to differing IL-33 effects within microenvironments dependent on the cell types present or recruited to an inflammatory insult. In necrotic cells, IL-33 undergoes cleavage with caspase-1 and becomes the active form that is able to be bound by its receptor, ST2 (also known as IL-1RL1), with this cleaved IL-33 isoform being 10–30 times more potent in activating downstream signalling [28]. In apoptotic cells, IL-33 undergoes cleavage with caspase-3 and -7 to be inactivated upon cell death [28]. This is an important layer of regulation for homeostasis during cell turnover because apoptosis is a form of noninflammatory cell death. Constitutive signalling without additional protease processing has also been described, particularly with relation to changes in airway diseases [35, 36]; a spliced variant of IL-33 has been linked to increased T2 cytokine activity in airway epithelial cells from asthmatics [35], whereas another isoform, IL-33Δ34, is increased in airway cells from COPD patients [36].
IL-33 and ST2 genetics
IL-33 is encoded by the IL33 gene, whereas IL1RL1 encodes ST2 [37]. Loss-of-function mutations in IL33 have been associated with reduced risk of developing asthma and COPD, while gain-of-function mutations have been associated with increased risk of both diseases [13, 38, 39]. Many single nucleotide polymorphisms (SNPs) have been associated with increased susceptibility to disease in asthma [40–43]; however, not all IL33 SNPs have a detrimental effect on asthma. Loss of function mutations in IL33 appear protective against asthma while also causing a decrease in overall eosinophil counts [39]. A variant of IL33 with a deletion of exons 3 and 4 variant can be secreted and is associated with T2 inflammation in asthma [35]. Beyond the IL33 gene, genetic variants of IL1RL1 also play a role in regulating IL-33 signalling [44]. A protective variant of IL1RL1 in asthma has been shown to reduce overall IL-33 signalling activity via an increase in soluble ST2 (sST2) [45]. The genetics underlying the IL-33/ST2 axis could play a major role in determining disease progression. The loss-of-function rs146597587:C allele was associated with a 46% reduction in serum IL-33 protein levels, a reduction in blood eosinophil count and a reduction in the risk of development of asthma [13]. This same loss of function was associated with a 21% reduction in risk of developing COPD, supporting the role of this pathway in COPD pathobiology.
ST2 receptor
Binding of IL-33 occurs through the formation of a heterodimer complex between ST2, the functional receptor for IL-33, and IL-1 receptor accessory protein (IL1RAP), the shared receptor among the IL-1 family of cytokines [46, 47]. Ligand binding leads to the recruitment of (myeloid differentiation primary response gene 88, a Toll–IL-1R domain binding protein, and activation of IL-1R associated kinase, which in turn activates mitogen-activated protein kinases and NF-κB pathways, that requires tumour necrosis factor receptor associated factor 6 for activation. Given that some IL-1 family cytokines use IL1RAP, the specificity of the IL-33 signal lies in its binding to ST2.
ST2 is found on multiple cell types including airway endothelial cells, ILC2s, mast cells, myeloid cells, natural killer (NK) cells, Th cells (both T1 and T2), cytotoxic T-cells, NK T-cells, and basophils [27, 48–50]. The dynamics of IL-33 binding to ST2 are tightly regulated by the release of sST2, which serves as a decoy receptor [51–53]. sST2 sequesters free active IL-33, thereby preventing the binding of membrane ST2 and IL-33, and therefore acts as a negative regulator of downstream IL-33 signalling. Although the molecular mechanisms regulating the expression of sST2 are clear, it is highly induced following exposure to proinflammatory cytokines, including IL-33 itself [54, 55]. Furthermore, oxidation of IL-33 regulates the range and duration of its activity through formation of disulphide bridges that create a conformational change preventing binding between IL-33 and ST2, providing a further level of control of this interaction [56].
IL-33 and ST2 expression and actions in lung diseases
Epithelial expression of IL-33 is upregulated in both asthma and COPD, correlating with disease severity (figure 1) [26, 27, 57, 58]. In mouse models, cigarette smoke represents a key trigger for epithelial IL-33 release and cigarette smoke exposure primes the release of IL-33 upon epithelial damage from influenza virus infection [26, 27]. Simultaneously, cigarette smoke decreased ST2 expression on ILC2s while increasing ST2 expression on NK cells and macrophages, resulting in a limited Th2 response [27]. This synergistic effect leads to an exaggerated T1 inflammatory response to viral infections via IL-33-dependent amplification of tumour necrosis factor α (TNF-α), IL-12 and interferon-γ. Indeed, NK, NK T-cells, ILC1 and Th1 cells are excellent producers of interferon-γ when exposed to both IL-12 and IL-33, two cytokines that are commonly expressed in inflamed epithelial airways [59]. Differences in mouse and human IL-33 expression may limit translation of these findings; in mice, IL-33 is expressed by alveolar type II pneumocytes whereas, in humans, the predominant source is in bronchial epithelial cells [60].
IL-33 promotes both T1 and T2 inflammation in vivo. Acute house dust mite allergen exposure in mice increased eosinophils, IL-4, IL-5, IL-13, goblet cell metaplasia and IgE in the lungs, but long-term exposure led to a mixed eosinophilic and neutrophilic phenotype characterised by IL-1β and TNF-α with features of airway wall remodelling, and sustained IL-33 release which persisted even after cessation of allergen exposure [61]. Both neutrophil and eosinophil numbers were reduced following IL-33 neutralising antibody exposure, together with decreased ST2 expression. Thus, in this murine model, IL-33 played an important role in causing mixed granulocytic inflammation with airway remodelling. In mice, there is also evidence for a direct effect of IL-33 in promoting eosinophilic inflammation by supporting mature eosinophils through systemic IL-5 production and expanding the number of IL-5Rα-expressing precursor cells in the bone marrow [62].
Mast cells activated by IL-33 may contribute to the effects of IL-33. Studies using human cord blood-derived mast cells have shown that IL-33 can both enhance IgE-mediated responses, in addition to direct activation [63, 64]. Intriguingly, IL-33-activated mast cells released higher levels of IL-5 and IL-13 protein, but there was also increased production of the non-T2 mediators, TNF-α and IL-8 [65]. Furthermore, transcriptomic analysis of sputum cells from patients with severe asthma show that an IL-33-activating mast cell signature was enriched in patients with a mixed granulocytic and neutrophilic phenotype, whereas IgE-activated mast cell signatures were enriched in patients with a predominantly eosinophilic phenotype [65–69]. These data suggest that IL-33 activation of mast cells could be an important mechanism for determining a mixed phenotype of T1 and T2 inflammatory response.
TSLP pathway
TSLP overview
TSLP, a member of the IL-2 cytokine family, was initially identified ∼25 years ago as a cytokine necessary for B-cell development [70]. Similar to IL-33, TSLP can be produced by epithelial cells in multiple tissues such as lung, skin and the gastrointestinal tract, in addition to DCs, keratinocytes, stromal cells, basophils and mast cells (figure 1) [18, 71–73]. TSLP is also classified as an “alarmin”, and lung-derived parenchymal and immune cells have been shown to secrete TSLP upon exposure to respiratory viruses, air pollutants, allergens and stimuli such as IL-4, IL-13 and TNF-α [16].
In response to TSLP, DCs induce naive CD4+ T-cell proliferation and TH2 cell differentiation via upregulation of the OX40L on the DC. TSLP-activated DCs can prime CD4+ cells in an antigen-specific manner, resulting in Th2-differentiated cells displaying characteristics of T2 immune responses, with production of IL-4, IL-5, IL-13 and TNF-α. Additionally, TSLP-activated DCs can play a role in maintaining and further polarising chemoattractant receptor-homologous molecule expressed on Th2 (CRTH2+) Th2 memory cells [74]. In addition to playing a role in helper T-cell differentiation, TSLP plays a role in the development, function and recruitment of a subset of basophils to sites of T2 inflammation. TSLP can further augment T2 responses through enhanced cytokine production from NK T-cells, eosinophils and mast cells, with recent evidence implicating TSLP in the production of eosinophil extracellular traps in response to infection [75].
Beyond inducing CD4+ Th2 differentiation, TSLP also activates multiple cell types including DCs, ILC2, NK T-cells, CD8+ T-cells, B-cells, regulatory T-cells, eosinophils, neutrophils, monocytes, mast cells, macrophages, platelets and sensory neurons [72].
While TSLP has primarily been thought of as a Th2-inducing cytokine, there is evidence for a role as Th1 effector in the recruitment of cells through Th1 chemokines such as CXCL10 and CXCL11 in both patients with severe asthma and COPD [76]. Similar sources of production for TSLP and Th1- and Th2-attracting chemokines across both diseases suggest that similar regulatory mechanisms exist to regulate these responses regardless of their Th subsets. Additionally, TSLP modulates mast cell activation and induces both T2 and non-T2 cytokines and chemokines [77]. TSLP can synergise with neuropeptides such as substance P and pro-inflammatory cytokines such as IL-1β to activate mast cells and promote degranulation and cytokine release. TSLP also regulates mast cell development [78]. Furthermore, TSLP can activate sensory neurons in both T2 and non-T2 conditions, inducing itch in the skin [79, 80]. Thus, similar to IL-33, TSLP has been implicated as having broad inflammatory properties that are influenced by the inflammatory microenvironment.
TSLP has multiple variants with distinct functions [72]; the long isoform of TSLP (lfTSLP) plays a role in allergic inflammation, while the short isoform of TSLP (sfTSLP) is a potential splice variant [81]. Poly (I:C), an activator of the Toll-like receptor (TLR) 3 pathway and a viral mimic, induces the expression of lfTSLP by bronchial epithelial cells and contributes to Th2-mediated inflammation. TLRs such as TLR2, TLR3, TLR5 and TLR6 upregulate both sfTSLP and lfTSLP [81–84].
sfTSLP has been identified as primarily a homeostatic mediator in human keratinocytes, where it is constitutively expressed and was shown to have properties of an antimicrobial peptide [85]. Higher levels of lfTSLP than sfTSLP were induced via the TLR3 pathway. sfTSLP was also tested as an antimicrobial peptide across multiple species of bacteria and was effective in limiting bacterial growth when compared with lfTSLP. While these isoforms were initially believed to be TSLP alternative splice variants, they are actually regulated instead by differential promoter regions and by pro- and anti-inflammatory stimuli [86].
TSLP genetic variants
Similar to IL-33, TSLP genetic variants are also strong risk factors in asthma disease development. The gene encoding for TSLP leads to the production of both sfTSLP and lfTSLP with either two or four exons respectively [86]. Multiple genome-wide association studies have identified TSLP as a locus of interest in asthma [43, 87]. SNPs found in the TSLP-promoter locus are also associated with an increased susceptibility to asthma [88–91]. While protective variants against disease for TSLP have not been found in asthma or COPD, one such variant has been found in another allergic condition, atopic dermatitis [92]. Further work to identify TSLP SNPs is needed to determine their impact on lung disease.
TSLP receptor
Binding of TSLP is mediated through the TSLP receptor (TSLPR), a heterodimer of TSLPR and the IL-7Rα subunit [93, 94]. TSLPR can be found on multiple cell types in lung airways, including epithelial cells, endothelial cells, DCs, ILC2, NK T-cells, CD4+ T-cells, CD8+ T-cells, B-cells, regulatory T-cells, eosinophils, neutrophils, monocytes, mast cells, macrophages, platelets and sensory neurons [16, 72]. Elevated TSLPR expression in bronchial biopsies from severe COPD patients has been reported [95] and cigarette smoke increased TSLPR expression in human airway smooth muscle cells [96]. The requirement for both TSLPR and IL-7Rα dictates the specificity and regulation of TSLP activity in vivo. Unlike IL-33, TSLP does not appear to have a decoy receptor that regulates downstream signalling alongside its nuclear receptor [18].
TSLP expression in lung diseases
Elevated levels of TSLP messenger RNA (mRNA) and protein have been found in the bronchial mucosa of both COPD and asthma patients [97]; elevated TSLP levels have been correlated with increased asthma susceptibility and severity, but the role of TSLP in COPD is not as well understood [76, 98]. TSLP mRNA is higher in patients with severe asthma and elevated levels of Th2-attracting chemokines IL-4 and IL-13 are associated with higher TSLP concentrations in the lamina propria of asthmatic patients [99]. Beyond higher levels of TSLP protein expressed in the airway epithelium of asthmatic patients, respiratory viruses, bacteria, allergens and loss of airway epithelial integrity can also induce TSLP expression [82, 100–104].
TSLP plays a large role in the initiation of allergic T2 inflammation through DCs, both permitting a T2 inflammatory cascade and inducing Th2 cells (figure 1) [47]. As an activator of the response, TSLP upregulated major histocompatibility complex class II molecules and induced CD4+ Th2 cell differentiation into Th2 cells via upregulation of OX40L on DCs [105]. This induction further drives T2 responses as TSLP leads to an increase in Th2 cytokines in asthma by promoting proliferation and differentiation of naive CD4+ T-cells to produce IL-4 for a continued Th2 response [98, 106]. In an allergic asthma murine model, rhinovirus infection induced an increase in IL-13-producing ILC2 cells, with simultaneous increases in lung IL-13 and TSLP. In the same model, blocking TSLP effects via TSLPR knockout or TSLP-neutralising antibodies limited ILC2 response to infection [103]. These data suggest that TSLP impacts asthma exacerbations primarily via amplification of a T2 response, whereas data from murine models for asthma suggest a dual role for IL-33 in response to viruses, with attenuation of a T2 response in addition to modulating T1 responses and enhancing viral clearance and antiviral cytokines [107].
Serum TSLP levels appear much higher in patients with asthma than in patients with COPD [108, 109]. Together with greater expression of TSLP in asthma, a primary role for TSLP as a Th2-inducing cytokine implies that there may be a more limited role for TSLP in COPD than in asthma. TSLP release from airway smooth muscle cells in COPD patients in vitro and in vivo appears to be triggered by IL-1β and TNF-α, indicating that TSLP is an upstream pathway for airway inflammation [110]. In COPD, TSLP production in bronchial epithelial cells may be mediated by Th17, suggesting that anticholinergics may exert an anti-inflammatory effect in COPD via TSLP [111] and that the role of TSLP in COPD may be more limited to airway smooth muscles. Nonetheless, TSLP may play a role as a Th1 effector in the recruitment of cells through various Th1 chemokines such as CXCL10 and CXCL11 in both patients with severe asthma and COPD [40], and TSLP, together with TLR3, can promote differentiation of Th17 cells via DCs [111]. Further work is needed to understand a potential role for TSLP in non-T2 responses and exacerbations of COPD.
Insights from clinical trials
Within the last 1S0 years, trials with biological drugs targeting the IL-33/ST2 and anti-TSLP pathways in asthma and COPD have been and continue to be undertaken (table 1).
TABLE 1.
Completed and ongoing studies with drugs targeting airway diseases via anti-thymic stromal lymphopoietin (TSLP) and anti-interleukin (IL)-33/ST2 pathways
| Study/drug | Target population | Study design | Dose | Key inclusion criteria | Key results summary | Key results according to type 2 inflammation |
| Asthma | ||||||
| Anti-IL-33/ST2 | ||||||
| NCT02918019 [114] ZENYATTA study Astegolimab (anti-ST2) |
Severe asthma | n=502 (120 patients per arm) Primary end-point: annualised rate of asthma exacerbations at week 52 Stratified by screening blood eosinophil counts (<150, 150–<300, ≥300 cells·μL−1) |
1:1:1:1 70 mg every 4 weeks, 210 mg every 4 weeks, 490 mg every 4 weeks, placebo |
Age 18–75 years; ≥1 asthma exacerbation in prior 12 months; FEV1 40–80%; nonsmokers | 43% annualised asthma exacerbation rate reduction (p=0.0049) and FEV1 improvement of 0.128 L (nonsignificant) for 490 mg dose in comparison with placebo | Annualised rate of exacerbation reduction: 53.6% (p=0.0016) in patients with baseline blood eosinophils <300 cells·μL−1
versus 10.2% (p=0.7718) in patients with eosinophils ≥300 cells·μL−1 FEV1 improvement appeared to be higher in patients with baseline blood eosinophil counts <150 cells·μL−1 in the 490 mg group |
| NCT03387852 [112] Itepekimab (anti-IL-33) |
Moderate-to-severe asthma | n=296 (74 patients per arm) Primary end-point: loss of asthma control events from baseline to week 12 |
1:1:1:1 Itepekimab alone every 2 weeks, itepekimab+dupilumab every 2 weeks, dupilumab alone every 2 weeks, placebo |
Age 18–70; ≥1 asthma exacerbation in prior 12 months; FEV1 40–85%; nonsmokers | 22% of patients in the itepekimab group experienced an event indicating a loss of asthma control versus 27% in the combination group and 41% in the placebo group The corresponding odds ratio compared to placebo was 0.42 (95% CI 0.20–0.88; p=0.02) for itepekimab and 0.52 (95% CI 0.25–1.06; p=0.07) in the combination group |
In the itepekimab only group, for loss of asthma control events, patients with baseline blood eosinophils ≥300 cells·mm−3 had an odds ratio versus placebo of 0.39 (95% CI 0.14–1.05) compared to 0.46 (95% CI 0.15–1.41) in patients with baseline blood eosinophils <300 cells·mm−3 |
| NCT04570657 FRONTIER-3 study Tozorakimab (MEDI3506) (anti-IL-33) |
Moderate-to-severe uncontrolled asthma | n=228 (76 patients per arm) Primary end-point: change from baseline to week 16 in pre-BD FEV1 |
1:1:1 MEDI3506 dose 1, MEDI3506 dose 2, placebo |
Age 18–<65 years; ≥1 asthma exacerbation in prior 12 months, pre-BD FEV1 40–85%; nonsmokers | Study ongoing | |
| NCT03207243 Melrilimab (GSK3772847) (anti-ST2) |
Moderate-to-severe asthma | n=165 Primary end-point: percentage of participants with loss of asthma control over weeks 0–16 |
1:1 10 mg·kg−1 every 4 weeks i.v., placebo |
≥18 years of age, treatment with high dose ICS, ACQ-5 score 1.0–4.0, ≥1 asthma exacerbation within 12 months, nonsmokers | 67% of patients who received melrilimab intravenously every 4 weeks suffered loss of asthma control, compared to 81% of people on placebo Median rate ratio 0.82 (95% CI 0.66–0.99) |
|
| Anti-TSLP | ||||||
| NCT02054130 [109] PATHWAY study Tezepelumab (anti-TSLP) |
Severe asthma | n=550 (∼138 patients per arm) Primary end-point: annualised rate of asthma exacerbations at week 52 Randomisation stratified by blood eosinophil count of ≥250 or <250 cells·mL−1) |
1:1:1:1 70 mg every 4 weeks, 210 mg every 4 weeks, 280 mg every 2 weeks, placebo |
Age 18–75 years; ≥2 asthma exacerbations requiring glucocorticoids or ≥1 asthma exacerbation leading to hospitalisation in prior 12 months; FEV1 40–80%; nonsmokers or former smokers with smoking history ≤10 pack-years | 71% exacerbation rate reduction (p<0.001), FEV1 improvement of 0.13 L (p=0.009) for 210 mg dose in comparison with placebo | Annualised exacerbation reduction in the 210 mg every 4 weeks arm: 62% (p=0.021) in patients in high Th2 group versus 84% (p<0.001) in patients in low Th2 group# 65% (p=0.005) versus 79% (p<0.001) in patients with blood eosinophils <250 cells·mL−1 versus >250 cells·mL−1 |
| NCT03347279 [117] NAVIGATOR study Tezepelumab (anti-TSLP) |
Severe asthma | n=1061 (∼530 patients per arm) Primary end-point: annualised rate of asthma exacerbations at week 52 |
1:1 210 mg every 4 weeks, placebo |
Age 12–80 years; ≥2 asthma exacerbations in prior 12 months; FEV1 <80% (<90% for patients 12–17 years old) | 66% exacerbation rate reduction (p<0.001), FEV1 improvement of 0.13 L (p<0.001) for 210 mg dose in comparison with placebo | Annualised exacerbation rate reduction: 41% (p<0.001) in patients with blood eosinophils <300 cells·μL−1, versus 70% for patients with blood eosinophils ≥300 cells·μL−1 |
| NCT03688074 [116] CASCADE study Tezepelumab (anti-TSLP) |
Moderate-to-severe asthma | n=55 patients per arm Primary end-point: reduction in number of airway submucosal inflammatory cells (eosinophils, neutrophils, T-cells and mast cells) at 28 weeks Stratified by screening blood eosinophil counts (<150, 150–<300 cells·μL−1) |
1:1 210 mg every 4 weeks, placebo |
Age 18–75 years; FEV1 >50%, nonsmokers or former smokers with smoking history ≤10 pack-years | Airway submucosal eosinophils reduction in tezepelumab versus placebo group (ratio of geometric least-squares means 0.15 (nominal p<0.0010) No differences between treatment groups in the other cell types evaluated | Reduction in airway submucosal eosinophils was similar across all groups according to baseline blood eosinophils |
| NCT03406078 [123] SOURCE study Tezepelumab (anti-TSLP) |
Severe uncontrolled, OCS-dependent asthma | n=150 Primary end-point: percent reduction from baseline in the daily OCS dose while not losing asthma control at week 48 |
1:1 210 mg every 4 weeks, placebo |
Age 18–80 years; FEV1 >50%, nonsmokers or former smokers with smoking history ≤10 pack-years, medium- or high-dose ICS and LABA, OCS for at least 6 months, pre-BD FEV1 <80% predicted normal | The (cumulative) odds of achieving a category of greater percentage reduction in maintenance OCS dose at week 48 was numerically higher with tezepelumab than placebo (OR 1.28, 95% CI 0.69–2.35; p=0.43) | In patients with a baseline blood eosinophil count ≥150 and ≥300 cells·µL−1, the (cumulative) odds of achieving a category of greater percentage reduction in maintenance OCS dose at week 48 were 2.58 (95% CI 1.16–5.75) and 3.49 (95% CI 1.16–10.49) times higher with tezepelumab than placebo, respectively No effects of tezepelumab versus placebo on OCS dose reduction were observed in patients with low baseline blood eosinophil counts (<300 and <150 cells·µL−1) |
| NCT04410523 CSJ117 (inhaled anti-TSLP) |
Severe asthma | n=625 Primary end-point: change from baseline in pre-BD FEV1 |
0.5 mg, 1 mg, 2 mg, 4 mg, 8 mg and placebo | Age ≥18 and ≤75 years, treatment with medium/high dose ICS plus LABA with up to two additional controllers, pre-BD FEV1 of ≥40% and ≤85% of the predicted normal, ACQ-5 score of ≥1.5 | Data expected 2022 | |
| COPD | ||||||
| Anti-IL-33/ST2 | ||||||
| NCT03546907 [13] Itepekimab (anti-IL-33) |
Moderate-to-severe COPD | n=170 patients per arm Primary end-point: annualised rate reduction of moderate-to-severe COPD exacerbations during 24–52-week treatment period |
1:1 300 mg every 2 weeks, placebo |
Age 40–75 years, current and former smokers, chronic bronchitis, FEV1 30–80%; ≥2 moderate or ≥1 severe COPD exacerbations in prior 12 months | 19% exacerbation rate reduction (p=0.13) Pre-BD FEV1 improvement of 0.06 L (p=0.024) in comparison with placebo |
Annualised exacerbation rate reduction: 22% (p=0.28) in patients with eosinophils ≥250 cells·μL−1 versus 16% (p=0.32) in patients with eosinophils <250 cells·μL−1 42% (p=0.0061) in former smokers versus −9% (p=0.65) in current smokers |
| NCT03615040 [15] COPD ST2OP Astegolimab (anti-ST2) |
Moderate to very severe COPD | n=40 patients per arm Primary end-point: annualised rate reduction of moderate-to-severe COPD exacerbations during 48-week treatment period |
1:1 490 mg every 4 weeks, placebo | Age 40–75 years, current and former smokers, FEV1 30–80%; ≥2 moderate or severe exacerbations in prior 12 months | 22% annualised exacerbation rate reduction (p=0.195) Post-BD improvement in FEV1 of 40.0 mL (p=0.094) for astegolimab versus placebo group at 48 weeks Improvement in SQGRQ-c of −3.3 points (p=0.039) for astegolimab versus placebo group at 48 weeks |
Annualised exacerbation rate reduction: 37% reduction in patients with baseline blood eosinophils <300 per μL versus 37% increase in patients with blood eosinophils >300 cells·μL−1 (p=0.072) |
| NCT04701983 NCT04751487 AERIFY-1 and AERIFY-2 studies Itepekimab (anti-IL-33) |
Moderate-to-severe COPD | n=310 patients per arm Annualised rate reduction of moderate-to-severe COPD exacerbations in former smokers during 52-week treatment period |
1:1:1 300 mg every 2 weeks, every 4 weeks, placebo |
Age 40–85 years, former smokers¶, chronic bronchitis, ≥2 moderate or ≥1 severe COPD exacerbation in prior 12 months | Study ongoing | |
| NCT05037929 ALIENTO study Astegolimab (anti-ST2) |
Moderate to very severe COPD | n=310 patients per arm Annualised rate reduction of moderate-to-severe COPD exacerbations during 52-week treatment period |
1:1:1 476 mg every 2 weeks, 476 mg every 4 weeks, placebo | Age 40–90 years, current and former smokers, FEV1 20–80%; ≥2 moderate or severe exacerbations in 12-month period within prior 24 months | Study ongoing | |
| NCT04631016 FRONTIER-4 Tozorakimab (MEDI3506) (anti-IL-33) |
Moderate-to-severe COPD | n=114 (57 patients per arm) Primary end-point: change from baseline to week 12 in pre-BD FEV1 |
1:1 Tozorakimab, placebo |
Age 40–75; current or former smokers with COPD, chronic bronchitis, ≥1 moderate or severe COPD exacerbation in the previous 12 months, dual or triple therapy | Study ongoing | |
| NCT05166889 OBERON study Tozorakimab (MEDI3506) (anti-IL-33) |
Moderate to very severe COPD | n=1272 (424 patients per arm) Primary end-point: annualised rate of moderate-to-severe COPD exacerbations in participants who are former smokers+ |
1:1:1 Tozorakimab dose 1, tozorakimab dose 2, placebo |
Age ≥40, current and former smokers, FEV1 ≥20%, ≥2 moderate COPD exacerbations or ≥1 severe COPD exacerbation in the prior 12 months | Study ongoing | |
| NCT05158387 TITANIA study Tozorakimab (MEDI3506) (anti-IL-33) |
Moderate to very severe COPD | n=1272 (424 patients per arm) Primary end-point: annualised rate of moderate-to-severe COPD exacerbations in participants who are former smokers§ |
1:1:1 Tozorakimab dose 1, tozorakimab dose 2, placebo |
Age ≥40, current and former smokers, FEV1 ≥20%, ≥2 moderate COPD exacerbations or ≥1 severe COPD exacerbation in the prior 12 months | Study ongoing | |
| Anti-TSLP | ||||||
| NCT04039113 Tezepelumab (anti-TSLP) |
Moderate to very severe COPD | n=338 Primary end-point: moderate or severe COPD exacerbation rate ratio (tezepelumab versus placebo) |
1:1 Every 4 weeks or placebo |
Age 40–80 years, current and former smokers, FEV1 20–80%; ≥2 moderate or severe exacerbations in 12 months, CAT score ≥15, on triple therapy (ICS/LABA/LAMA) | Data expected 2023 | |
| NCT04882124 CSJ117 (inhaled anti-TSLP) |
COPD | n=300 Primary end-point: change from baseline in E-RS symptom score at 12 weeks |
1:1:1 4 mg, 8 mg and placebo inhaled once daily |
Age ≥40 years, former or current smokers with COPD on triple therapy (ICS/LABA/LAMA) | Data expected 2023 | |
ACQ: asthma control questionnaire; BD: bronchodilator; CAT: COPD Assessment Test; COPD: chronic obstructive pulmonary disease; E-RS: Evaluating Respiratory Symptoms–COPD; FEV1: forced expiratory volume in 1 s; ICS: inhaled corticosteroids; LABA: long-acting beta-agonists; LAMA: long-acting muscarinic antagonist; OCS: oral corticosteroids; SQGRQ-c: St George Respiratory Questionnaire-COPD; Th2: T-helper 2. #: Th2 status defined as: high=immunoglobulin E (IgE) >100 IU·mL−1 and eosinophil count ≥140 cells·μL−1; low IgE <100 IU·mL−1 or eosinophil count <140 cells·μL−1. ¶: AERIFY-2 contains an additional two arms (itepekimab every 2 weeks, placebo) with current smokers. +: Primary end-point will be assessed first in primary population (former smokers) and then assessed in the overall population. §: Primary end-point will be assessed first in primary population (former smokers) and then assessed in the overall population.
Anti-IL-33/anti-ST2
Anti-IL-33/ST2 therapies are under development by Regeneron/Sanofi, AstraZeneca, GSK and Genentech/Roche.
Asthma
Regeneron's anti-IL-33 antibody, REGN3500 or itepekimab, while failing to demonstrate superiority against dupilumab alone or in combination with dupilumab in patients with asthma, was efficacious in its own right; patients in the itepekimab arm had a 58% lower chance of asthma loss of control in comparison to placebo [112]. GSK discontinued development of its anti-ST2 molecule, melrilimab (GSK3772847), following phase 2a study where patients in the intervention arm had a 18% lower chance of asthma loss of control versus the placebo arm [113]. Genentech's anti-ST2 antibody, astegolimab, significantly reduced annualised asthma exacerbation rate by 43% compared with placebo in a phase 2b study (table 1) [114]. AstraZeneca's anti-IL-33 antibody, tozorakimab (MEDI3506), currently has a phase 2a study underway in asthma. In contrast to anti-TSLP therapy, the efficacy of anti-IL-33/ST2 antibodies therapy appears to be greater in, but not limited to, patients with lower baseline blood eosinophils in comparison to patients with high blood eosinophils. Astegolimab had the greatest reduction in asthma exacerbations in patients with blood eosinophils <300 cells·μL−1, with a 51.4% reduction in exacerbations compared to a 13.3% reduction in those with blood eosinophils >300 cells·μL−1 at the 490 mg dose [114].
COPD
The primary outcome data in phase 2 studies for anti-IL-33/ST2 has been negative; however, subgroup analyses from these trials have led to further pivotal studies being launched. Itepekimab, an anti-IL-33 antibody, is continuing to be developed for COPD with two phase 3 studies following phase 2a data showing a nonsignificant reduction of 19% compared with placebo in annualised exacerbation rate [13]. In this study, there was a greater nonsignificant exacerbation reduction in patients with high baseline blood eosinophil levels (22% for ≥250 cells·μL−1 versus 16% (p=0.32) for <250 cells·μL−1); a post hoc analysis also revealed a 40% exacerbation rate reduction in former smokers. A phase 2b study with astegolimab, an anti-ST2 antibody, in COPD is underway following a phase 2a study showing a nonsignificant reduction of 22% compared with placebo in annualised exacerbation rate [15]. In contrast to itepekimab, there was greater response in patients with low baseline eosinophil levels with astegolimab treatment (37% reduction for <300 cells·μL−1 versus 37% increase for >300 cells·μL−1 (p=0.072)). AstraZeneca's anti-IL-33 antibody, tozorakimab (MEDI3506), currently has phase 2a and phase 3 studies underway in COPD.
Clinical data suggest that anti-IL-33/ST2 therapies could benefit patients with both T2 and non-T2 asthma, the latter being an area where there remains high unmet need, as well as patients with COPD, where T1 immune responses are thought to play a primary role. This could be a result of the ST2 receptor playing a major role in establishing and regulating both T1 and T2 inflammation, but may also reflect the IL-33/ST2 blockade modulating the local inflammatory microenvironment according to the predominant type of inflammation present.
Targeting IL-33 directly should yield similar responses to targeting ST2; however, one potential effect of targeting IL-33 directly would be preventing the role of IL-33 as a chemoattractant during its release in tissue damage [115]. There could be other cell types (e.g. mast cells) beyond Th2 and/or ILC2 responsive cells that are recruited by IL-33 that could be key in determining the effects of anti-IL-33 therapy. The emergence of a potentially ST2-independent pathway and IL-33 signalling via RAGE will be an area of future research interest to understand whether this suggests a difference in clinical outcomes with targeting the IL-33 pathway with anti-IL-33 ligand versus anti-ST2 antibodies. Phase 2 studies of astegolimab and itepekimab showed opposite directionality in subgroup efficacy trends according to baseline blood eosinophils and smoking status; although data from larger pivotal studies will help identify differences, if any. Whether smoking status is an important selection factor for treatment with anti-IL-33/ST2 biologics will be informed by the current phase 2 and 3 studies with itepekimab, tozorikimab and astegolimab. Understanding potential clinical differences in targeting either IL-33 or ST2, due to the role of sST2 as a decoy receptor, is a question in both asthma and COPD; however, in the case of targeting the anti-IL-5 ligand versus anti-IL-5Rα receptor, clinical benefit and safety appear broadly similar, and this could prove to be true for the IL-33/ST2 pathway.
Anti-TSLP
Clinical data for airways diseases is currently only available for one anti-TSLP therapy developed by AstraZeneca, tezepelumab, in asthma. Additionally, Novartis is developing an inhaled anti-TSLP molecule, CSJ117, with phase 2 studies underway for asthma (NCT04410523) and COPD (NCT04882124).
Tezepelumab received FDA approval for the add-on maintenance treatment of severe asthma on the basis of annualised exacerbation rate reduction demonstrated across multiple studies (table 1) [109, 116, 117]. Tezepelumab is approved for treating asthma irrespective of blood eosinophil level, with phase 3 data in the PATHWAY and NAVIGATOR studies suggesting greater efficacy for exacerbation reduction in patients with high blood eosinophil count and/or fractional exhaled nitric oxide, taken to represent predominantly T2 inflammation. In the CASCADE study, additional to an effect on asthma exacerbations, tezepelumab resulted in a greater reduction in airway submucosal eosinophils compared to placebo, although there was no measurable effect on submucosal neutrophils, T-cells or mast cells [116]. However, anti-TSLP treatment improved mannitol-induced airway hyperresponsiveness, an effect that has not been associated with other eosinophil-targeted biologics, suggesting that TSLP antagonism may involve non-T2 mechanisms such as mast cell degranulation and airway smooth muscle hyperresponsiveness [118]. Tezepelumab also attenuated allergen-induced early and late asthmatic responses in patients with mild asthma [119]. Anti-epithelial cytokine therapy may have disease-modifying potential, as evidenced by a decrease in biomarkers of inflammation (IL5R and pregnancy-associated plasma protein A) and matrix remodelling (matrix metalloproteinase-10 and periostin) in the phase 2B PATHWAY study of anti-TSLP, which additionally showed efficacy in exacerbation reduction with non-T2 asthma [109]. However, whether these effects are sustained after discontinuation of biologic therapy, indicative of true disease modification, will require longitudinal studies. A phase 2a study for tezepelumab in COPD is underway (NCT04039113).
Benefits/risks of IL-33 or TSLP blockade
Efficacy and non-T2 potential of IL-33 or TSLP blockade
Corticosteroids, whether inhaled or oral, are a key element of maintenance therapy to help prevent and control chronic airway inflammation that can lead to worsening of symptoms and airway remodelling in asthma and COPD. There are reports that T2 airway inflammation in response to allergens can trigger steroid resistance or insensitivity through synthesis of TSLP and induction of the IL-33 pathway in conjunction with CD4+ or natural helper cells [120–122]. Furthermore, in bronchoalveolar lavage fluid from patients with asthma, the presence of elevated TSLP was associated with steroid-resistant ILC2 cells, with reversal of resistance following inhibition of TSLP-signalling pathways such as MAPK kinase (MEK) or signal transducer and activator of transcription 5. Together, both anti-TSLP and anti-IL-33/ST2 therapies may have an important role in reversing steroid insensitivity in underlying severe asthma. Notably, in the 150-patient SOURCE study (NCT03406078), tezepelumab had a positive effect on exacerbations, forced expiratory volume in 1 s (FEV1) and symptoms, but failed to significantly reduce oral corticosteroid (OCS) dose in patients with OCS-dependent asthma (although there were better cumulative odds for reduction in OCS use in the tezepelumab group versus placebo in the subgroup with blood eosinophils >150 cells·μL−1 at baseline) [123].
The role of eosinophilic inflammation in COPD has been the subject of intense research interest. While early studies of oral and inhaled corticosteroids have shown benefit in treating COPD patients in terms of reduction in exacerbation rates [124–126], the results of clinical trials targeting eosinophilic inflammation in COPD with mepolizumab (anti-IL-5) and benralizumab (anti-IL-5R) have not been as robust [127, 128]. Further studies will be needed to elucidate the role of eosinophils as a driver as contrast to a bystander role in COPD.
In mice, expression of lung-specific TSLP induced airway inflammation and airway hyperresponsiveness [129]. In the exploratory studies CASCADE and UPSTREAM, blocking TSLP reduced mannitol-associated airway hyperresponsiveness, suggesting that alarmins play a significant role in airway hyperresponsiveness [118, 130]. Human bronchial epithelial and airway smooth cells express IL-33 and, in asthmatics, this expression correlates with airway hyperresponsiveness through upregulation of mast cell-derived IL-13 [131]. Clinical studies of the effectiveness of blocking TSLP and the IL-33/ST2 axis in reducing airway hyperresponsiveness are needed.
Exacerbations remain the most common primary end-point for pivotal clinical trials of biologics in asthma and COPD [132]. However, other measures such as the health-related quality of life, functional capacity, disease control or modification and airway hyperresponsiveness may be complementary outcomes that can provide additional insight into the effectiveness of biological therapy [133, 134]. For trials of biologics, benefit in terms of FEV1 improvement has been small, especially in COPD, and the results for patient-reported outcomes have been mixed across studies, suggesting that the reduction in exacerbations does not necessarily confer a benefit in other outcomes. However, reducing exacerbations remains an area of high unmet need and add-on therapies that reduce exacerbations by even 20–25% in COPD can be clinically meaningful since few available therapies specifically target exacerbations, and are limited either by the intended patient population (e.g. severe COPD associated with chronic bronchitis for roflumilast) or potential side-effects (e.g. azithromycin).
The IL-6 transignalling pathway (a neutrophilic phenotype) has been previously described; IL-33 may also activate this IL-6 transignalling pathway and may be a non-T2 mechanism of anti-IL-33/ST2 [5].
Safety
Both anti-TSLP and anti-IL-33/ST2 therapies appear to have good tolerability and safety profiles for airway diseases thus far. There is experimental evidence that IL-33 plays a protective role in helminth infections due to its modulation of T2 immunity and viral infections via its effects on ILC2s [135] and T1 immunity [136], respectively. However, there has been no evidence for an increased risk of infections in published trials of tezepelumab in patients with moderate or severe asthma and in phase 2 studies of itepekimab and astegolimab in both asthma and COPD patients [13, 15, 117]. Continued monitoring in future trials is essential. The effects of these treatments on the airway microbiome of such patients should be studied.
Conclusions and future perspectives
Although our understanding of both IL-33 and TSLP has expanded beyond their role as specific inducers of T2 immune responses, many questions remain unanswered. The mechanism of action for both alarmins requires further evaluation to determine their role in treating pulmonary disease. Understanding the role of different immune cells during IL-33/ST2 and TSLP blockade, in response to different pathogenic and environmental factors (e.g. smoking), and effects of alarmin release on current treatments should serve as a focus area in identifying patient populations most likely to benefit from anti-IL-33 and TSLP therapy and may help determine optimal treatment regimens for symptom alleviation and improved quality of life.
Clinical interest in IL-33 and TSLP as targets that regulate both T1 and T2 immune responses in asthma and COPD has yielded multiple clinical investigations. In asthma, studies for both anti-TSLP and anti-IL-33/ST2 in asthma met their primary end-point. For COPD, data from ongoing phase 2 and 3 trials of anti-IL-33/ST2 and anti-TSLP antibodies are eagerly awaited since phase 2 studies of anti-IL-33/ST2 blockade, although encouraging, did not meet their primary end-point, and for anti-TSLP blockade, there is no clinical data available yet. Clinical data to date suggest that anti-alarmin therapies hold particular promise for patients with non-T2 inflammation for whom treatment options remain limited. Given their ability to modulate T1 and T2 responses, further investigation for the role of anti-alarmin therapies in patients who are refractory to treatment with existing biologics is warranted, as is investigation into the role of anti-alarmin therapies in the asthma–COPD overlap [137]. To date, anti-IL-33/ST2 and anti-TSLP therapies in development have an acceptable safety profile that support their future use as maintenance therapies, although it remains uncertain as to whether their use would impact on OCS use in patients with asthma or COPD. With the recent approval of tezepelumab in asthma, real-world evidence will become available as to the impact of anti-TSLP therapy on disease control. Currently, pivotal studies for both anti-IL-33/ST2 and anti-TSLP have focused on exacerbation reduction as the primary end-point, as well as (short-term) asthma control. However, future trials should additionally evaluate the role of these alarmins in long-term disease modification and asthma remission. Although both TSLP and IL-33/ST2 are both epithelial alarmins, they represent distinct biological processes. Investigating the interaction between these two pathways should also represent an important future research avenue.
Acknowledgements
Editing and writing assistance was provided by Andrew Occiano (Genentech, Inc.) and was funded by Genentech, Inc.
Provenance: Submitted article, peer reviewed.
Conflict of interest: A.A. Calderon has nothing to declare. C. Dimond, D.F. Choy, R. Pappu, M.A. Grimbaldeston and D. Mohan are employees of Genentech, Inc., a member of the Roche group, and are Roche stockholders. D.F. Choy and M.A. Grimbaldeston are co-inventors on patents that have been filed or are pending relating to the diagnosis and treatment of chronic respiratory diseases for Genentech, Inc. D. Mohan was previously an employee and shareholder of GSK. K.F. Chung received personal payments for service on an advisory board for Roche, Merck, Rickett-Beckinson and Shinogi, data safety monitoring board for Nocion, and for speaking engagements from Novartis and AstraZeneca; and received the MRC, EPSRC, and GSK grants for his institution.
Support statement: Funding was provided by Genentech. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.GBD Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1204–1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . The top 10 causes of death. www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death Date last accessed: 22 February 2022. Date last updated: 9 December 2020.
- 3.Choy DF, Arron JR. Beyond type 2 cytokines in asthma – new insights from old clinical trials. Expert Opin Ther Targets 2020; 24: 463–475. doi: 10.1080/14728222.2020.1744567 [DOI] [PubMed] [Google Scholar]
- 4.Kuo CS, Pavlidis S, Loza M, et al. T-helper cell type 2 (Th2) and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in U-BIOPRED. Eur Respir J 2017; 49: 1602135. doi: 10.1183/13993003.02135-2016 [DOI] [PubMed] [Google Scholar]
- 5.Jevnikar Z, Ostling J, Ax E, et al. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J Allergy Clin Immunol 2019; 143: 577–590. doi: 10.1016/j.jaci.2018.05.026 [DOI] [PubMed] [Google Scholar]
- 6.Ostling J, van Geest M, Schofield JPR, et al. IL-17-high asthma with features of a psoriasis immunophenotype. J Allergy Clin Immunol 2019; 144: 1198–1213. doi: 10.1016/j.jaci.2019.03.027 [DOI] [PubMed] [Google Scholar]
- 7.Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006; 173: 1114–1121. doi: 10.1164/rccm.200506-859OC [DOI] [PubMed] [Google Scholar]
- 8.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global strategy for the diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease. 2022. Available from: https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf
- 9.Bourdin A, Bjermer L, Brightling C, et al. ERS/EAACI statement on severe exacerbations in asthma in adults: facts, priorities and key research questions. Eur Respir J 2019; 54: 1900900. doi: 10.1183/13993003.00900-2019 [DOI] [PubMed] [Google Scholar]
- 10.Global Initiative for Asthma . Global strategy for asthma management and prevention. 2021. Available from: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf Date last accessed: 28 October 2022.
- 11.McGregor MC, Krings JG, Nair P, et al. Role of biologics in asthma. Am J Respir Crit Care Med 2019; 199: 433–445. doi: 10.1164/rccm.201810-1944CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amgen Inc. TEZSPIRE™ (tezepelumab-ekko) injection, for subcutaneous use. Thousand Oaks, CA: Amgen Inc; 2021. www.accessdata.fda.gov/drugsatfda_docs/label/2021/761224s000lbl.pdf [Google Scholar]
- 13.Rabe KF, Celli BR, Wechsler ME, et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med 2021; 9: 1288–1298. doi: 10.1016/S2213-2600(21)00167-3 [DOI] [PubMed] [Google Scholar]
- 14.Yousuf A, Ibrahim W, Greening NJ, et al. T2 biologics for chronic obstructive pulmonary disease. J Allergy Clin Immunol Pract 2019; 7: 1405–1416. doi: 10.1016/j.jaip.2019.01.036 [DOI] [PubMed] [Google Scholar]
- 15.Yousuf AJ, Mohammed S, Carr L, et al. Astegolimab, an anti-ST2, in chronic obstructive pulmonary disease (COPD-ST2OP): a phase 2a, placebo-controlled trial. Lancet Respir Med 2022; 10: 469–477. doi: 10.1016/S2213-2600(21)00556-7 [DOI] [PubMed] [Google Scholar]
- 16.Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest 2019; 129: 1441–1451. doi: 10.1172/JCI124606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol 2016; 16: 676–689. doi: 10.1038/nri.2016.95 [DOI] [PubMed] [Google Scholar]
- 18.Takai T. TSLP expression: cellular sources, triggers, and regulatory mechanisms. Allergol Int 2012; 61: 3–17. doi: 10.2332/allergolint.11-RAI-0395 [DOI] [PubMed] [Google Scholar]
- 19.Ballantyne SJ, Barlow JL, Jolin HE, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol 2007; 120: 1324–1331. doi: 10.1016/j.jaci.2007.07.051 [DOI] [PubMed] [Google Scholar]
- 20.Singh D, Kolsum U, Brightling CE, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J 2014; 44: 1697–1700. doi: 10.1183/09031936.00162414 [DOI] [PubMed] [Google Scholar]
- 21.Rossios C, Pavlidis S, Hoda U, et al. Sputum transcriptomics reveal upregulation of IL-1 receptor family members in patients with severe asthma. J Allergy Clin Immunol 2018; 141: 560–570. doi: 10.1016/j.jaci.2017.02.045 [DOI] [PubMed] [Google Scholar]
- 22.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005; 23: 479–490. doi: 10.1016/j.immuni.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 23.Afferni C, Buccione C, Andreone S, et al. The pleiotropic immunomodulatory functions of IL-33 and its implications in tumor immunity. Front Immunol 2018; 9: 2601. doi: 10.3389/fimmu.2018.02601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan BCL, Lam CWK, Tam LS, et al. IL33: roles in allergic inflammation and therapeutic perspectives. Front Immunol 2019; 10: 364. doi: 10.3389/fimmu.2019.00364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin'? PLoS ONE 2008; 3: e3331. doi: 10.1371/journal.pone.0003331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byers DE, Alexander-Brett J, Patel AC, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest 2013; 123: 3967–3982. doi: 10.1172/JCI65570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearley J, Silver JS, Sanden C, et al. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection. Immunity 2015; 42: 566–579. doi: 10.1016/j.immuni.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 28.Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol 2016; 17: 122–131. doi: 10.1038/ni.3370 [DOI] [PubMed] [Google Scholar]
- 29.Prefontaine D, Lajoie-Kadoch S, Foley S, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol 2009; 183: 5094–5103. doi: 10.4049/jimmunol.0802387 [DOI] [PubMed] [Google Scholar]
- 30.Travers J, Rochman M, Miracle CE, et al. Chromatin regulates IL-33 release and extracellular cytokine activity. Nat Commun 2018; 9: 3244. doi: 10.1038/s41467-018-05485-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cayrol C, Girard JP. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol Rev 2018; 281: 154–168. doi: 10.1111/imr.12619 [DOI] [PubMed] [Google Scholar]
- 32.Kouzaki H, Iijima K, Kobayashi T, et al. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol 2011; 186: 4375–4387. doi: 10.4049/jimmunol.1003020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott IC, Majithiya JB, Sanden C, et al. Interleukin-33 is activated by allergen- and necrosis-associated proteolytic activities to regulate its alarmin activity during epithelial damage. Sci Rep 2018; 8: 3363. doi: 10.1038/s41598-018-21589-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lefrancais E, Roga S, Gautier V, et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA 2012; 109: 1673–1678. doi: 10.1073/pnas.1115884109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon ED, Simpson LJ, Rios CL, et al. Alternative splicing of interleukin-33 and type 2 inflammation in asthma. Proc Natl Acad Sci USA 2016; 113: 8765–8770. doi: 10.1073/pnas.1601914113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz-Kiriakos E, Steinberg DF, Kluender CE, et al. Epithelial IL-33 appropriates exosome trafficking for secretion in chronic airway disease. JCI Insight 2021; 6: e136166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ketelaar ME, Portelli MA, Dijk FN, et al. Phenotypic and functional translation of IL33 genetics in asthma. J Allergy Clin Immunol 2021; 147: 144–157. doi: 10.1016/j.jaci.2020.04.051 [DOI] [PubMed] [Google Scholar]
- 38.Demenais F, Margaritte-Jeannin P, Barnes KC, et al. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat Genet 2018; 50: 42–53. doi: 10.1038/s41588-017-0014-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith D, Helgason H, Sulem P, et al. A rare IL33 loss-of-function mutation reduces blood eosinophil counts and protects from asthma. PLoS Genet 2017; 13: e1006659. doi: 10.1371/journal.pgen.1006659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonnelykke K, Sleiman P, Nielsen K, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet 2014; 46: 51–55. doi: 10.1038/ng.2830 [DOI] [PubMed] [Google Scholar]
- 41.Gudbjartsson DF, Bjornsdottir US, Halapi E, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet 2009; 41: 342–347. doi: 10.1038/ng.323 [DOI] [PubMed] [Google Scholar]
- 42.Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med 2010; 363: 1211–1221. doi: 10.1056/NEJMoa0906312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torgerson DG, Ampleford EJ, Chiu GY, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet 2011; 43: 887–892. doi: 10.1038/ng.888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Portelli MA, Dijk FN, Ketelaar ME, et al. Phenotypic and functional translation of IL1RL1 locus polymorphisms in lung tissue and asthmatic airway epithelium. JCI Insight 2020; 5: e132446. doi: 10.1172/jci.insight.132446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramirez-Carrozzi V, Dressen A, Lupardus P, et al. Functional analysis of protective IL1RL1 variants associated with asthma risk. J Allergy Clin Immunol 2015; 135: 1080–1083.e1083. doi: 10.1016/j.jaci.2014.10.028 [DOI] [PubMed] [Google Scholar]
- 46.Lingel A, Weiss TM, Niebuhr M, et al. Structure of IL-33 and its interaction with the ST2 and IL-1RAcP receptors–insight into heterotrimeric IL-1 signaling complexes. Structure 2009; 17: 1398–1410. doi: 10.1016/j.str.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol 2007; 120: 238–244. doi: 10.1016/j.jaci.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 48.Allakhverdi Z, Smith DE, Comeau MR, et al. Cutting edge: the ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol 2007; 179: 2051–2054. doi: 10.4049/jimmunol.179.4.2051 [DOI] [PubMed] [Google Scholar]
- 49.Dagher R, Copenhaver AM, Besnard V, et al. IL-33-ST2 axis regulates myeloid cell differentiation and activation enabling effective club cell regeneration. Nat Commun 2020; 11: 4786. doi: 10.1038/s41467-020-18466-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smithgall MD, Comeau MR, Yoon BR, et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol 2008; 20: 1019–1030. doi: 10.1093/intimm/dxn060 [DOI] [PubMed] [Google Scholar]
- 51.Hayakawa H, Hayakawa M, Kume A, et al. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem 2007; 282: 26369–26380. doi: 10.1074/jbc.M704916200 [DOI] [PubMed] [Google Scholar]
- 52.Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 2015; 42: 1005–1019. doi: 10.1016/j.immuni.2015.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oshikawa K, Yanagisawa K, Tominaga S, et al. Expression and function of the ST2 gene in a murine model of allergic airway inflammation. Clin Exp Allergy 2002; 32: 1520–1526. doi: 10.1046/j.1365-2745.2002.01494.x [DOI] [PubMed] [Google Scholar]
- 54.Kumar S, Tzimas MN, Griswold DE, et al. Expression of ST2, an interleukin-1 receptor homologue, is induced by proinflammatory stimuli. Biochem Biophys Res Commun 1997; 235: 474–478. doi: 10.1006/bbrc.1997.6810 [DOI] [PubMed] [Google Scholar]
- 55.Mildner M, Storka A, Lichtenauer M, et al. Primary sources and immunological prerequisites for sST2 secretion in humans. Cardiovasc Res 2010; 87: 769–777. doi: 10.1093/cvr/cvq104 [DOI] [PubMed] [Google Scholar]
- 56.Cohen ES, Scott IC, Majithiya JB, et al. Oxidation of the alarmin IL-33 regulates ST2-dependent inflammation. Nat Commun 2015; 6: 8327. doi: 10.1038/ncomms9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bianchetti L, Marini MA, Isgro M, et al. IL-33 promotes the migration and proliferation of circulating fibrocytes from patients with allergen-exacerbated asthma. Biochem Biophys Res Commun 2012; 426: 116–121. doi: 10.1016/j.bbrc.2012.08.047 [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Wang W, Lv Z, et al. Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol 2018; 200: 2253–2262. doi: 10.4049/jimmunol.1701455 [DOI] [PubMed] [Google Scholar]
- 59.Freeman BE, Raue HP, Hill AB, et al. Cytokine-mediated activation of NK cells during viral infection. J Virol 2015; 89: 7922–7931. doi: 10.1128/JVI.00199-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yasuda K, Muto T, Kawagoe T, et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci USA 2012; 109: 3451–3456. doi: 10.1073/pnas.1201042109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allinne J, Scott G, Lim WK, et al. IL-33 blockade affects mediators of persistence and exacerbation in a model of chronic airway inflammation. J Allergy Clin Immunol 2019; 144: 1624–1637.e1610. doi: 10.1016/j.jaci.2019.08.039 [DOI] [PubMed] [Google Scholar]
- 62.Johnston LK, Hsu CL, Krier-Burris RA, et al. IL-33 precedes IL-5 in regulating eosinophil commitment and is required for eosinophil homeostasis. J Immunol 2016; 197: 3445–3453. doi: 10.4049/jimmunol.1600611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ronnberg E, Ghaib A, Ceriol C, et al. Divergent effects of acute and prolonged interleukin 33 exposure on mast cell IgE-mediated functions. Front Immunol 2019; 10: 1361. doi: 10.3389/fimmu.2019.01361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silver MR, Margulis A, Wood N, et al. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflamm Res 2010; 59: 207–218. doi: 10.1007/s00011-009-0088-5 [DOI] [PubMed] [Google Scholar]
- 65.Nagarkar DR, Ramirez-Carrozzi V, Choy DF, et al. IL-13 mediates IL-33-dependent mast cell and type 2 innate lymphoid cell effects on bronchial epithelial cells. J Allergy Clin Immunol 2015; 136: 202–205. doi: 10.1016/j.jaci.2015.01.036 [DOI] [PubMed] [Google Scholar]
- 66.Chtanova T, Newton R, Liu SM, et al. Identification of T cell-restricted genes, and signatures for different T cell responses, using a comprehensive collection of microarray datasets. J Immunol 2005; 175: 7837–7847. doi: 10.4049/jimmunol.175.12.7837 [DOI] [PubMed] [Google Scholar]
- 67.Jayapal M, Tay HK, Reghunathan R, et al. Genome-wide gene expression profiling of human mast cells stimulated by IgE or FcεRI-aggregation reveals a complex network of genes involved in inflammatory responses. BMC Genomics 2006; 7: 210. doi: 10.1186/1471-2164-7-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suurmond J, Habets KLL, Tatum Z, et al. Repeated FcεRI triggering reveals modified mast cell function related to chronic allergic responses in tissue. J Allergy Clin Immunol 2016; 138: 869–880. doi: 10.1016/j.jaci.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 69.Tiotiu A, Badi Y, Kermani NZ, et al. Association of differential mast cell activation to granulocytic inflammation in severe asthma. Am J Respir Crit Care Med 2021; 205: 397–411. doi: 10.1164/rccm.202102-0355OC [DOI] [PubMed] [Google Scholar]
- 70.Friend SL, Hosier S, Nelson A, et al. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol 1994: 22: 321–328. [PubMed] [Google Scholar]
- 71.Sokol CL, Barton GM, Farr AG, et al. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol 2008; 9: 310–318. doi: 10.1038/ni1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Varricchi G, Pecoraro A, Marone G, et al. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front Immunol 2018; 9: 1595. doi: 10.3389/fimmu.2018.01595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, Zhou X, Zhou B. DC-derived TSLP promotes Th2 polarization in LPS-primed allergic airway inflammation. Eur J Immunol 2012; 42: 1735–1743. doi: 10.1002/eji.201142123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang YH, Ito T, Wang YH, et al. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity 2006; 24: 827–838. doi: 10.1016/j.immuni.2006.03.019 [DOI] [PubMed] [Google Scholar]
- 75.Morshed M, Yousefi S, Stockle C, et al. Thymic stromal lymphopoietin stimulates the formation of eosinophil extracellular traps. Allergy 2012; 67: 1127–1137. doi: 10.1111/j.1398-9995.2012.02868.x [DOI] [PubMed] [Google Scholar]
- 76.Ying S, O'Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol 2008: 181: 2790–2798. doi: 10.4049/jimmunol.181.4.2790 [DOI] [PubMed] [Google Scholar]
- 77.Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med 2007; 204: 253–258. doi: 10.1084/jem.20062211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han NR, Oh HA, Nam SY, et al. TSLP induces mast cell development and aggravates allergic reactions through the activation of MDM2 and STAT6. J Invest Dermatol 2014; 134: 2521–2530. doi: 10.1038/jid.2014.198 [DOI] [PubMed] [Google Scholar]
- 79.Lee WJ, Shim WS. Cutaneous neuroimmune interactions of TSLP and TRPV4 play pivotal roles in dry skin-induced pruritus. Front Immunol 2021; 12: 772941. doi: 10.3389/fimmu.2021.772941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilson SR, Thé L, Batia LM, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013; 155: 285–295. doi: 10.1016/j.cell.2013.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harada M, Hirota T, Jodo AI, et al. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol 2009; 40: 368–374. doi: 10.1165/rcmb.2008-0041OC [DOI] [PubMed] [Google Scholar]
- 82.Kato A, Favoreto S Jr, Avila PC, et al. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol 2007; 179: 1080–1087. doi: 10.4049/jimmunol.179.2.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Le TA, Takai T, Vu AT, et al. Flagellin induces the expression of thymic stromal lymphopoietin in human keratinocytes via toll-like receptor 5. Int Arch Allergy Immunol 2011; 155: 31–37. doi: 10.1159/000318679 [DOI] [PubMed] [Google Scholar]
- 84.Vu AT, Baba T, Chen X, et al. Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll-like receptor 2–Toll-like receptor 6 pathway. J Allergy Clin Immunol 2010; 126: 985–993, 993 e981–983. doi: 10.1016/j.jaci.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 85.Bjerkan L, Schreurs O, Engen SA, et al. The short form of TSLP is constitutively translated in human keratinocytes and has characteristics of an antimicrobial peptide. Mucosal Immunol 2015; 8: 49–56. doi: 10.1038/mi.2014.41 [DOI] [PubMed] [Google Scholar]
- 86.Fornasa G, Tsilingiri K, Caprioli F, et al. Dichotomy of short and long thymic stromal lymphopoietin isoforms in inflammatory disorders of the bowel and skin. J Allergy Clin Immunol 2015; 136: 413–422. doi: 10.1016/j.jaci.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hirota T, Takahashi A, Kubo M, et al. Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat Genet 2011; 43: 893–896. doi: 10.1038/ng.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harada M, Hirota T, Jodo AI, et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol 2011; 44: 787–793. doi: 10.1165/rcmb.2009-0418OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He JQ, Hallstrand TS, Knight D, et al. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. J Allergy Clin Immunol 2009; 124: 222–229. doi: 10.1016/j.jaci.2009.04.018 [DOI] [PubMed] [Google Scholar]
- 90.Hunninghake GM, Soto-Quiros ME, Avila L, et al. TSLP polymorphisms are associated with asthma in a sex-specific fashion. Allergy 2010; 65: 1566–1575. doi: 10.1111/j.1398-9995.2010.02415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Masuko H, Sakamoto T, Kaneko Y, et al. Lower FEV1 in non-COPD, nonasthmatic subjects: association with smoking, annual decline in FEV1, total IgE levels, and TSLP genotypes. Int J Chron Obstruct Pulmon Dis 2011; 6: 181–189. doi: 10.2147/COPD.S16383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao PS, Rafaels NM, Mu D, et al. Genetic variants in thymic stromal lymphopoietin are associated with atopic dermatitis and eczema herpeticum. J Allergy Clin Immunol 2010; 125: 1403–1407.e1404. doi: 10.1016/j.jaci.2010.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pandey A, Ozaki K, Baumann H, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol 2000; 1: 59–64. doi: 10.1038/76923 [DOI] [PubMed] [Google Scholar]
- 94.Park LS, Martin U, Garka K, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med 2000; 192: 659–670. doi: 10.1084/jem.192.5.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Di Stefano A, Caramori G, Barczyk A, et al. Innate immunity but not NLRP3 inflammasome activation correlates with severity of stable COPD. Thorax 2014; 69: 516–524. doi: 10.1136/thoraxjnl-2012-203062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smelter DF, Sathish V, Thompson MA, et al. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J Immunol 2010; 185: 3035–3040. doi: 10.4049/jimmunol.1000252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Redhu NS, Gounni AS. Function and mechanisms of TSLP/TSLPR complex in asthma and COPD. Clin Exp Allergy 2012; 42: 994–1005. doi: 10.1111/j.1365-2222.2011.03919.x [DOI] [PubMed] [Google Scholar]
- 98.Ying S, O'Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol 2005; 174: 8183–8190. doi: 10.4049/jimmunol.174.12.8183 [DOI] [PubMed] [Google Scholar]
- 99.Shikotra A, Choy DF, Ohri CM, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol 2012; 129: 104–111 e101–109. doi: 10.1016/j.jaci.2011.08.031 [DOI] [PubMed] [Google Scholar]
- 100.Calven J, Yudina Y, Hallgren O, et al. Viral stimuli trigger exaggerated thymic stromal lymphopoietin expression by chronic obstructive pulmonary disease epithelium: role of endosomal TLR3 and cytosolic RIG-I-like helicases. J Innate Immun 2012; 4: 86–99. doi: 10.1159/000329131 [DOI] [PubMed] [Google Scholar]
- 101.Han J, Dakhama A, Jia Y, et al. Responsiveness to respiratory syncytial virus in neonates is mediated through thymic stromal lymphopoietin and OX40 ligand. J Allergy Clin Immunol 2012; 130: 1175–1186 e1179. doi: 10.1016/j.jaci.2012.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee HC, Headley MB, Loo YM, et al. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol 2012; 130: 1187–1196 e1185. doi: 10.1016/j.jaci.2012.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stier MT, Bloodworth MH, Toki S, et al. Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J Allergy Clin Immunol 2016; 138: 814–824 e811. doi: 10.1016/j.jaci.2016.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dong H, Hu Y, Liu L, et al. Distinct roles of short and long thymic stromal lymphopoietin isoforms in house dust mite-induced asthmatic airway epithelial barrier disruption. Sci Rep 2016; 6: 39559. doi: 10.1038/srep39559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Omori M, Ziegler S. Induction of IL-4 expression in CD4+ T cells by thymic stromal lymphopoietin. J Immunol 2007; 178: 1396–1404. doi: 10.4049/jimmunol.178.3.1396 [DOI] [PubMed] [Google Scholar]
- 106.Rochman Y, Dienger-Stambaugh K, Richgels PK, et al. TSLP signaling in CD4+ T cells programs a pathogenic T helper 2 cell state. Sci Signal 2018; 11: eaam8858. doi: 10.1126/scisignal.aam8858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Werder RB, Zhang V, Lynch JP, et al. Chronic IL-33 expression predisposes to virus-induced asthma exacerbations by increasing type 2 inflammation and dampening antiviral immunity. J Allergy Clin Immunol 2018; 141: 1607–1619 e1609. doi: 10.1016/j.jaci.2017.07.051 [DOI] [PubMed] [Google Scholar]
- 108.Korosec P, Korn S, Skrgat S, et al. TSLP in patients with asthma, COPD and ACOS. Am J Respir Crit Care Med 2016; 193: A1008. doi: 10.1164/ajrccm-conference.2016.193.1_MeetingAbstracts.A1008 [DOI] [Google Scholar]
- 109.Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med 2017; 377: 936–946. doi: 10.1056/NEJMoa1704064 [DOI] [PubMed] [Google Scholar]
- 110.Zhang K, Shan L, Rahman MS, et al. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: role in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2007; 293: L375–L382. doi: 10.1152/ajplung.00045.2007 [DOI] [PubMed] [Google Scholar]
- 111.Anzalone G, Albano GD, Montalbano AM, et al. IL-17A-associated IKK-alpha signaling induced TSLP production in epithelial cells of COPD patients. Exp Mol Med 2018; 50: 1–12. doi: 10.1038/s12276-018-0158-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wechsler ME, Ruddy MK, Pavord ID, et al. Efficacy and safety of itepekimab in patients with moderate-to-severe asthma. N Engl J Med 2021; 385: 1656–1668. doi: 10.1056/NEJMoa2024257 [DOI] [PubMed] [Google Scholar]
- 113.ClinicalTrials.gov . Efficacy and safety study of GSK3772847 in subjects with moderately severe asthma. ClinicalTrials.gov identifier: NCT03207243. https://clinicaltrials.gov/ct2/show/results/NCT03207243 Date last updated: 1 March 2020. Date last accessed: 29 July 2022.
- 114.Kelsen SG, Agache IO, Soong W, et al. Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: a randomized clinical trial. J Allergy Clin Immunol 2021; 148: 790–798. doi: 10.1016/j.jaci.2021.03.044 [DOI] [PubMed] [Google Scholar]
- 115.Komai-Koma M, Xu D, Li Y, et al. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol 2007; 37: 2779–2786. doi: 10.1002/eji.200737547 [DOI] [PubMed] [Google Scholar]
- 116.Emson C, Diver S, Chachi L, et al. CASCADE: a phase 2, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the effect of tezepelumab on airway inflammation in patients with uncontrolled asthma. Respir Res 2020; 21: 265. doi: 10.1186/s12931-020-01513-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med 2021; 384: 1800–1809. doi: 10.1056/NEJMoa2034975 [DOI] [PubMed] [Google Scholar]
- 118.Diver S, Khalfaoui L, Emson C, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2021; 9: 1299–1312. doi: 10.1016/S2213-2600(21)00226-5 [DOI] [PubMed] [Google Scholar]
- 119.Gauvreau GM, O'Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med 2014; 370: 2102–2110. doi: 10.1056/NEJMoa1402895 [DOI] [PubMed] [Google Scholar]
- 120.Kabata H, Moro K, Fukunaga K, et al. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun 2013; 4: 2675. doi: 10.1038/ncomms3675 [DOI] [PubMed] [Google Scholar]
- 121.Liu S, Verma M, Michalec L, et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: the role of thymic stromal lymphopoietin. J Allergy Clin Immunol 2018; 141: 257–268 e256. doi: 10.1016/j.jaci.2017.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mato N, Hirahara K, Ichikawa T, et al. Memory-type ST2+CD4+ T cells participate in the steroid-resistant pathology of eosinophilic pneumonia. Sci Rep 2017; 7: 6805. doi: 10.1038/s41598-017-06962-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wechsler M, Gow AM, Brightling CE, et al. Oral corticosteroid-sparing effect of Tezepelumab in adults with severe asthma. Am J Respir Crit Care Med 2021; 203: A1197. [Google Scholar]
- 124.Albert RK, Martin TR, Lewis SW. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med 1980; 92: 753–758. doi: 10.7326/0003-4819-92-6-753 [DOI] [PubMed] [Google Scholar]
- 125.Burge PS, Calverley PM, Jones PW, et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000; 320: 1297–1303. doi: 10.1136/bmj.320.7245.1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med 1999; 340: 1941–1947. doi: 10.1056/NEJM199906243402502 [DOI] [PubMed] [Google Scholar]
- 127.Criner GJ, Celli BR, Brightling CE, et al. Benralizumab for the prevention of COPD exacerbations. N Engl J Med 2019; 381: 1023–1034. doi: 10.1056/NEJMoa1905248 [DOI] [PubMed] [Google Scholar]
- 128.Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med 2017; 377: 1613–1629. doi: 10.1056/NEJMoa1708208 [DOI] [PubMed] [Google Scholar]
- 129.Zhou B, Comeau MR, De Smedt T, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol 2005; 6: 1047–1053. doi: 10.1038/ni1247 [DOI] [PubMed] [Google Scholar]
- 130.Sverrild A, Hansen S, Hvidtfeldt M, et al. The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM). Eur Respir J 2022; 59: 2101296. doi: 10.1183/13993003.01296-2021 [DOI] [PubMed] [Google Scholar]
- 131.Kaur D, Gomez E, Doe C, et al. IL-33 drives airway hyper-responsiveness through IL-13-mediated mast cell: airway smooth muscle crosstalk. Allergy 2015; 70: 556–567. doi: 10.1111/all.12593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mathioudakis AG, Moberg M, Janner J, et al. Outcomes reported on the management of COPD exacerbations: a systematic survey of randomised controlled trials. ERJ Open Res 2019; 5: 00072–2019. doi: 10.1183/23120541.00072-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008; 31: 416–469. doi: 10.1183/09031936.00099306 [DOI] [PubMed] [Google Scholar]
- 134.Tejwani V, Chang HY, Tran AP, et al. The asthma evidence base: a call for core outcomes in interventional trials. J Asthma 2021; 58: 855–864. doi: 10.1080/02770903.2020.1744641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 2011; 12: 1045–1054. doi: 10.1038/ni.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bonilla WV, Frohlich A, Senn K, et al. The alarmin interleukin-33 drives protective antiviral CD8+ T cell responses. Science 2012; 335: 984–989. doi: 10.1126/science.1215418 [DOI] [PubMed] [Google Scholar]
- 137.Milne S, Mannino D, Sin DD. Asthma-COPD overlap and chronic airflow obstruction: definitions, management, and unanswered questions. J Allergy Clin Immunol Pract 2020; 8: 483–495. doi: 10.1016/j.jaip.2019.10.044 [DOI] [PubMed] [Google Scholar]