Abstract
The spontaneous regression or remission (SR) of cancer, often described as the partial or complete disappearance of a malignant tumor in the absence of all medical treatment and therapy, is a well-documented phenomenon. With efforts ongoing to establish cancer treatments that limit undesirable outcomes and adverse effects, these uncommon occurrences of SR carry significant implications for novel therapies and warrant further investigation. While several case studies have reported instances of SR in gastrointestinal (GI) malignancies, a comprehensive review of previous manifestations of SR in the GI tract remains lacking. The inclusion criteria for the rare phenomenon are also in need of an appropriate update that takes recent scientific advancements and emerging new medical technologies into account. Our analysis of 390 cases of SR in the GI tract focuses primarily on neoplasms of the hepatobiliary system and proposes an updated version of the older inclusion criteria for spontaneous regression.
Keywords: hepatobiliary system, neoplasms, oncology, gastroenterology, hepatology, gastrointestinal cancers, hepatocellular carcinoma, spontaneous remission, spontaneous regression
Introduction and background
In 2021 alone, 372,470 new cases of primary gastrointestinal (GI) cancer were reported worldwide, and approximately 124,348 deaths occurred as a result, comprising 19.6% and 20.4% of total new cancer cases and deaths, respectively [1]. Nonetheless, with the introduction of various novel diagnostic, therapeutic, and antineoplastic modalities, the mortality rates of GI cancers have declined significantly over the past several years [2]. These new modalities have led to a greater understanding of the pathogenesis of cancer and of achieving remission. Spontaneous regression (SR) is defined as the complete or partial disappearance of a primary and/or disseminated lesion of a histologically diagnosed metastatic disease in the absence of any medical treatment or therapy known to have antitumor effects. Spontaneous regression has been found to occur throughout the entire body, including the GI tract [3]. But it is not equivalent to a cure, as cancer may reappear or spread elsewhere in the body. The frequency of spontaneous regression varies based on the type of cancer, as it is most commonly reported in renal cell carcinoma, melanoma, and neuroblastoma [3-5]. Occurring at a rate of about one out of every 60,000 to 100,000 cases of all cancers, these extremely rare occurrences of SR have the potential to serve as an instructive in vivo model of biological tumor regulation and control [6].
A number of putative mechanisms have been proposed for the observed spontaneous disappearance of malignancies, including inflammation, apoptosis, ischemia, and immunological responses [7]. Other mechanisms proposed to cause SR include epigenetic modifications, hormonal responses, oncogenes, tumor suppressors, cytokines and growth factors, and psychological mechanisms. Unfortunately, several of these postulated mechanisms are based on association and speculation alone, with the exact mechanistic modalities surrounding the SR of GI cancer yet to be elucidated. Regardless, an immunological anti-tumoral response of a patient’s body to specific malignancies is among the most prevalently described mechanistic hypotheses for the observed spontaneous disappearance of neoplasms. Since the very inception of the term, spontaneous regression has historically been speculated to be a dynamic interplay of immunological anti-tumor responses. In 1956, Everson and Cole defined the criterion for SR as the partial or complete disappearance of a malignant tumor in the absence of all treatment or in the presence of therapy that is considered inadequate to exert a significant influence on neoplastic disease. At that time, they theorized that the phenomenon must be an opportunistic by-product of an activated immune response. Cases of SR linked to infections have significantly influenced the discovery of several different anticancer therapies that facilitate the targeting of cancer cells by the host’s immune system. For example, immune checkpoint inhibitors have revolutionized modern cancer treatment by targeting inhibitory receptors (e.g., PD-1, CTLA-4, LAG-3), ligands (e.g., PD-L1) expressed on T cells, antigen-presenting cells, and tumor cells, which result in an anti-tumor response by stimulating the host immune system.
Focusing chiefly on malignancies of primary GI origin, this observational review of the literature hopes to bring further attention to the phenomenon of SR while also identifying some potential mechanisms that have been purported to contribute to this largely unreported phenomenon. Secondarily, this comprehensive review aims to introduce a revised and up-to-date version of the older inclusion criteria for SR throughout the body. This updated criterion has been modified in a way that takes into account recent scientific advancements and emerging new medical technologies, with the intent that it will also be easy to follow for physicians and clinical researchers alike. Finally, this study seeks to broaden the scope of how SR is perceived by clinicians and members of the medical community by encouraging a holistic view of the exceptionally rare phenomenon as a dynamic interplay of various modalities.
Review
Materials and methods:
Search Strategy
A literature search across five databases (PubMed, Medline, Google Scholar, Semantic Scholar, and Jstage) was performed employing the following main keywords: gastrointestinal cancer, spontaneous regression, spontaneous remission, spontaneous necrosis, and abscopal effect. A full list of searched keywords is included in Appendix A. The clinical characteristics of each occurrence of SR within the GI pathway and the related long-term outcomes were then extracted. Articles were excluded if any systemic treatment was used or if any treatment directed at the lesion was utilized before the documented regression. All diseases were limited to the GI tract, spanning the oral cavity, esophagus, stomach, liver, bile duct, gallbladder, pancreas, mesenteries, peritoneum, small intestine, colon, and rectum. A manual search of each work’s citations was performed, utilizing additional published works listed in the supplementary materials or reference sections of each of the aforementioned studies. No restriction was applied to the date of publication, the form of publication, or the primary language of the publication.
Inclusion Criteria
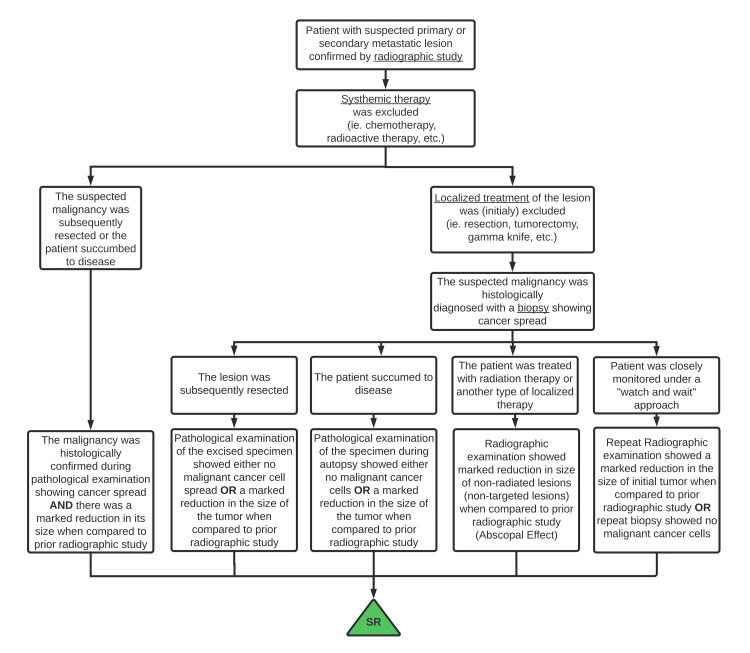
Only publications that described the true SR of a histologically confirmed GI cancer were included following the inclusion criteria depicted in Figure 1. These criteria are based on the original criteria proposed by Cole, modified to emphasize histological diagnosis, and adapted to fit multiple clinical scenarios (2). These criteria are summarized as follows: (1) Partial or complete disappearance of the primary tumor or secondary metastasis was radiographically or pathologically demonstrated in the absence of systemic therapy; (2) localized therapy to the lesion prior to the observed shrinkage was excluded; and (3) the malignant neoplasm was histologically proven at some point during this course. Patients with primary neoplasms histologically determined to have originated from outside the GI pathway but demonstrating SR were excluded, even if they demonstrated SR of a secondary metastasis within the GI tract. Patients demonstrating regression of an extra-digestive lesion, histologically determined to have arisen in the GI tract, were included regardless of whether the primary GI lesion had also regressed.
Figure 1. Clinician guidelines or criteria for reporting spontaneous regression.
From a clinician’s initial encounter with a patient with a suspected cancerous lesion to the demonstration of tumor shrinkage or disappearance, potential clinical manifestations of spontaneous regression are schematically investigated and shown. Original figure by the authors.
Data Extraction and Analysis
The following information was extracted and recorded from each article: patient age and sex, location and histological typing of the primary tumor, the site of regression, the period of regression or remission, and the etiological mechanism of regression proposed by the author. The demonstrated recurrence of cancer was also noted in some patients. Limited to each author’s interpretation and the duration of follow-up included in each study, the period of remission was defined as one of the following, whichever was found to be the longest: (1) the total period of time during which the tumor demonstrated a shrinkage in size, beginning with the date when the tumor’s size was found to be at its maximum to the date when the tumor’s size was found to be at its minimum, (2) The total period of time between the partial or complete disappearance of cancer and the most recent follow-up date in which the patient continued to show no signs of metastatic spread or recurrence of the malignancy; (3) the total period of time during which the tumor demonstrated a shrinkage in size prior to its resection, from the proposed date when the tumor’s size was found to be at its maximum size to the date of the resection. After the tumor was resected, the specimen was pathologically found to have shrunk or disappeared.
Results
Of the 390 cases of SR of GI malignancies reported meeting our criteria, a majority were noted in men (272 cases, 69.7%) compared to women (118 cases, 30.3%). The mean patient age was 63 years, with a majority of patients between 65 and 74 years of age (114 cases, 29.2%) or 55 and 64 years of age (95 cases, 24.4%). Overall, the literature search demonstrated a global incidence of SR, with cases spanning all six inhabited continents.
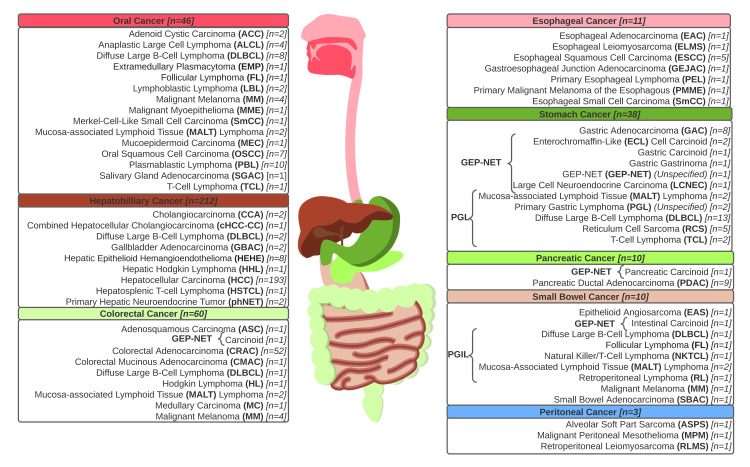
All reported cases detailing the SR of GI malignancies throughout the clinical literature are comprehensively reviewed in Appendix B, with pertinent findings summarized in Table 1. These reported cases of SR included various cases of carcinoma (289 cases, 74.1%), primary gastrointestinal and oral lymphomas (67 cases, 17.2%), and a few neuroendocrine tumors (12 cases, 3.1%), among other primary gastrointestinal cancers (22 cases, 5.6%). Hepatocellular carcinoma (HCC) represented almost half of all reported cases of SR in GI cancers (193 cases, 49.5%). Several rare forms of cancer, including extramedullary plasmacytoma (EMP), peritoneal alveolar soft-part sarcoma (ASPS), and gastric gastrinoma, were also observed to spontaneously regress. A complete list of reported histological manifestations of GI malignancies recorded to have undergone SR is in Figure 2.
Table 1. Baseline characteristics of spontaneous regression within the study sample.
| Patient characteristics* | Oral (n=46) | Esophageal (n=11) | Gastric (n=38) | Peritoneal (n=3) | Hepatobiliary (n=212) | Pancreatic (n=10) | Small bowel (n=10) | Colorectal (n=60) | Total (n=390) |
| Age (Mean (SD)) | 61.2 (17.4) | 59.0 (16.2) | 60.1 (19.0) | 47.7 (15.9) | 64.7 (13.5) | 50.4 (18.2) | 51 (16.2) | 62.1 (15.0) | 63.1 (14.7) |
| Age group (n (%)) | |||||||||
| <19 | 1 (2.2%) | 0 | 1 (2.6%) | 0 | 0 | 0 | 0 | 1 (1.7%) | 3 (0.8%) |
| 19-34 | 3 (6.5%) | 1 (9.1%) | 2 (5.3%) | 0 | 6 (2.8%) | 2 (20.0%) | 1 (10.0%) | 1 (1.7%) | 16 (4.1%) |
| 35-44 | 1 (2.2%) | 1 (9.1%) | 7 (18.4%) | 2 (66.7%) | 6 (2.8%) | 1 (10.0%) | 2 (20.0%) | 7 (11.7%) | 27 (6.9%) |
| 45-54 | 11 (23.9%) | 1 (9.1%) | 2 (5.3%) | 0 | 21 (9.9%) | 1 (10.0%) | 1 (10.0%) | 7 (11.7%) | 44 (11.3%) |
| 55-64 | 8 (17.4%) | 4 (36.4%) | 10 (26.3%) | 0 | 49 (23.1%) | 2 (20.0%) | 5 (50.0%) | 17 (28.3%) | 95 (24.4%) |
| 65-74 | 9 (19.6%) | 2 (18.2%) | 5 (13.2%) | 1 (33.3%) | 82 (38.7%) | 1 (10.0%) | 1 (10.0%) | 13 (21.7%) | 114 (29.2%) |
| 75-84 | 11 (23.9%) | 2 (18.2%) | 10 (26.3%) | 0 | 43 (20.3% | 1 (10.0%) | 0 | 12 (20.0%) | 79 (20.3%) |
| 85+ | 2 (4.3%) | 0 | 1 (2.6%) | 0 | 4 (1.9%) | 0 | 0 | 2 (3.3%) | 9 (2.3%) |
| Sex (n (%)) | |||||||||
| Male | 25 (54.3%) | 8 (72.7%) | 23 (60.5%) | 2 (66.7%) | 168 (79.2%) | 6 (60.0%) | 5 (50.0%) | 35 (58.3%) | 272 (69.7%) |
| Female | 21 (45.7%) | 3 (27.3%) | 15 (39.5%) | 1 (33.3%) | 44 (20.8%) | 4 (40.0%) | 5 (50.0%) | 25 (41.7%) | 118 (30.3%) |
| Site of Regression (n (%)) | |||||||||
| Primary tumor/Recurrence | 41 (89.1%) | 8 (72.7%) | 35 (92.1%) | 0 | 194 (91.5%) | 10 (100.0%) | 7 (70.0%) | 48 (80.0%) | 288 (73.8%) |
| Lung metastases | 2 (4.3%) | 3 (27.3%) | 0 | 1 (33.3%) | 28 (13.2%) | 0 | 1 (10.0%) | 2 (3.3%) | 37 (9.5%) |
| Liver metastases | 0 | 0 | 1 (2.6%) | 1 (33.3%) | 2 (0.9%) | 2 (20.0%) | 2 (20.0%) | 10 (16.7%) | 18 (4.6%) |
| Lymph metastases | 5 (10.9%) | 2 (18.2%) | 1 (2.6%) | 0 | 2 (0.9%) | 0 | 3 (30.0%) | 2 (3.3%) | 15 (3.8%) |
| Other metastases | 1 (2.2%) | 1 (9.1%) | 1 (2.6%) | 1 (33.3%) | 13 (6.1%) | 0 | 1 (10.0%) | 6 (10.0%) | 24 (6.2%) |
| Extent of regression (n (%)) | |||||||||
| Complete | 43 (93.5%) | 9 (81.8%) | 32 (84.2%) | 2 (66.7%) | 156 (73.6%) | 8 (80.0%) | 10 (100.0%) | 57 (95.0%) | 317 (81.3%) |
| Partial | 3 (6.5%) | 2 (18.2%) | 6 (15.8%) | 1 (33.3%) | 56 (26.4%) | 2 (20.0%) | 0 | 3 (5.0%) | 73 (18.7%) |
| Histological profile (n (%)) | |||||||||
| Carcinoma | 11 (23.9%) | 8 (72.7%) | 8 (21.1%) | 0 | 198 (93.4%) | 9 (90.0%) | 1 (10.0%) | 54 (90.0%) | 289 (74.1%) |
| Primary lymphoma | 28 (60.9%) | 1 (9.1%) | 24 (63.2%) | 0 | 4 (1.9%) | 0 | 6 (60.0%) | 4 (6.7%) | 67 (17.2%) |
| NET | 1 (2.2%) | 0 | 6 (15.8%) | 0 | 2 (0.9%) | 1 (10.0%) | 1 (10.0%) | 1 (1.7%) | 12 (3.1%) |
| Other | 6 (13.0%) | 2 (18.2%) | 0 | 3 (100.0%) | 8 (3.8%) | 0 | 2 (20.0%) | 1 (1.7%) | 22 (5.6%) |
| Period of regression (n (%)) | |||||||||
| <1 month | 4 (8.7%) | 0 | 3 (7.9%) | 0 | 6 (2.8%) | 0 | 0 | 0 | 13 (3.3%) |
| 1-1.5 months | 3 (6.5%) | 1 (9.1%) | 5 (13.2%) | 0 | 11 (5.2%) | 1 (10.0%) | 0 | 9 (15.0%) | 30 (7.7%) |
| 2-5 months | 2 (4.3%) | 2 (18.2%) | 6 (15.8%) | 1 (33.3%) | 27 (12.7%) | 0 | 3 (30.0%) | 13 (21.7%) | 54 (13.8%) |
| 6-11 months | 6 (13.0%) | 3 (27.3%) | 3 (7.9%) | 0 | 16 (7.5%) | 1 (10.0%) | 3 (30.0%) | 1 (1.7%) | 33 (8.5%) |
| 12-23 months | 6 (13.0%) | 2 (18.2%) | 5 (13.2%) | 0 | 41 (19.3%) | 2 (20.0%) | 0 | 9 (15.0%) | 65 (16.7%) |
| 24-35 months | 6 (13.0%) | 1 (9.1%) | 4 (10.5%) | 1 (33.3%) | 30 (14.2%) | 1 (10.0%) | 0 | 3 (5.0%) | 46 (11.8%) |
| 36-47 months | 5 (10.9%) | 0 | 2 (5.3%) | 0 | 14 (6.6%) | 1 (10.0%) | 1 (10.0%) | 5 (8.3%) | 28 (7.2%) |
| 48 months+ | 8 (17.4%) | 1 (9.1%) | 8 (21.1%) | 1 (33.3%) | 33 (15.6%) | 4 (40.0%) | 2 (20.0%) | 17 (28.3%) | 74 (19.0%) |
| Unspecified | 6 (13.0%) | 1 (9.1%) | 2 (5.3%) | 0 | 34 (16.0%) | 0 | 1 (10.0%) | 3 (5.0%) | 47 (12.1%) |
| Malignancy recurrence (n (%)) | |||||||||
| Reported | 5 (10.9%) | 0 | 1 (2.6%) | 0 | 14 (6.6%) | 1 (10.0%) | 2 (20.0%) | 1 (1.7%) | 24 (6.2%) |
| Not reported | 41 (89.1%) | 11 (100.0%) | 37 (97.4%) | 3 (100.0%) | 198 (93.4%) | 9 (90.0%) | 8 (80.0%) | 59 (98.3%) | 366 (93.8%) |
Figure 2. Histological manifestations of gastrointestinal malignancies are recorded to have undergone spontaneous regression throughout the clinical literature.
Biopsy-confirmed cancers of the gastrointestinal pathway, including various carcinomas, gastroenteropancreatic neuroendocrine tumors (GEP-NETs), and primary oral and gastrointestinal lymphomas, have been shown to demonstrate spontaneous regression. These cases have been observed throughout the entirety of the alimentary canal, spanning all of the organs of digestion. Cases of each distinct histological denomination were enumerated and systematically organized by the anatomical distribution of the primary lesion. Original figure by the authors.
The vast majority of cases of partial or complete regression occurred within the primary tumor (288 cases, 73.8%); nonetheless, multiple cases demonstrated regression of liver metastases (18 cases, 4.6%), lung metastases (37 cases, 9.5%), lymph metastases (15 cases, 3.8%), or other metastases (24 cases, 6.2%). The period of regression (as defined above) varied greatly in these cases, with some cases reporting just a few days of regression and others expressing several years of remission. Cancer recurrence was reported in 24 cases of SR, comprising 6.2% of total cases of SR in the GI pathway (noted with an asterisk "*" in Appendix B).
The authors proposed various putative mechanisms of SR, which are summarized in Table 2.
Table 2. Proposed mechanisms of spontaneous regression within the gastrointestinal pathway.
| Proposed mechanism | n (%) |
| Immunological | 202 (51.8%) |
| Abscopal effect | 10 (2.5%) |
| Endocrine factors | 4 (1.0%) |
| Restored immunogenicity | 18 (4.6%) |
| Eradication of oncogenic virus | 7 (1.8%) |
| Fever/Infection | 35 (9.0%) |
| Inflammatory response | 14 (3.6%) |
| Transfusions | 1 (0.3%) |
| Treatment of primary/Metastases | 7 (1.8%) |
| Other/Not specified | 106 (27.2%) |
| Ischemic | 146 (37.4%) |
| Anti-angiogenic factors | 3 (0.8%) |
| Vascular ischemia/ thrombosis | 26 (6.7%) |
| Tumor ablation/Biopsy/Angiography | 44 (11.3%) |
| Tumor hypoxia/ Hypoperfusion | 30 (7.7%) |
| Tumor microenvironment disruption | 3 (0.8%) |
| Unpredictable/Rapid growth | 14 (3.6%) |
| Other/Not specified | 26 (6.7%) |
| Idiopathic | 92 (23.6%) |
| Apoptotic tumor cell death | 6 (1.5%) |
| Dislodged | 10 (2.6%) |
| Drugs | 14 (3.6%) |
| Genetic | 5 (1.3%) |
| Herbal medicines | 20 (5.1%) |
| Metabolic/Nutritional | 7 (1.8%) |
| Psychoneurological | 5 (1.3%) |
| Withdrawal of carcinogenic agent | 16 (4.1%) |
| Other | 9 (2.3%) |
| Not specified | 68 (17.4%) |
The majority of the reviewed authors provided at least one conjectural mechanism (322 cases, 82.6%), with most citing immunological (202 cases, 51.8%), ischemic (146 cases, 37.4%), or idiopathic (92 cases, 23.6%) processes.
Discussion
This systematic review includes the presentation of 390 cases reported in 346 scientific papers, journals, case studies, and published books. These publications were generated over the past 95 years. Interestingly, 325 cases were published in the modern era, defined as cases published in the past 30 years. Following the review of these articles, multiple common factors were revealed, including a tendency for SR to occur in patients over the age of 55 (297 patients, 76.2%), patients of the male sex (272 patients, 69.7%), and patients with primary liver tumors (209 patients, 53.6%) or secondary liver metastases (18 patients, 4.6%). The clinical features and proposed mechanisms surrounding these cases of SR within the GI pathway, along with the location and duration of remission, are documented in Appendix B. Patients within this cohort of reported cases displayed varied periods of stability, ranging from just a few days of observed partial tumor regression to several years of cancer remission, up to 20 years cancer-free.
Mechanisms of Spontaneous Regression
Historically, SR has been speculated to occur in the setting of a prolonged febrile illness due to viral or bacterial infection; nonetheless, only a fraction of cases of SR (35 patients, or 5.1%) have been attributed to a hyperthermic state and infection [8]. In cases of SR occurring during times of acute febrile infections, immune cell infiltrations and signaling cascades are postulated to lead to tumor cell death and cancer tissue necrosis via the release of interleukins, tumor necrosis factors, and interferons (specifically IL-2, IL-6, and IL-8) [9-17]. Viral infections notably induce the production of interferons, which are capable of their own immunomodulatory effects involving macrophages, B-cells, and monocytes, alongside the induction of IL-2 receptors in some cancers [6-8]. Most recently, tumor regression has been reported after COVID-19 vaccination and infection with its wide-ranging pro-inflammatory effects on the host immune system [18-23].
Enhanced antitumoral immunogenicity is proposed to play a profound role in the involution of several GI cancers. In fact, examples displaying the correlation between SR and the elimination of immunodisruptive factors (e.g., medications, viral infection, checkpoint proteins) are perhaps the best evidence supporting the involvement of immunological mechanisms in the achieved SR of GI cancer [24,25]. Tumors have also occasionally been found to regress following systemic or localized treatment for some other disease process. For example, certain localized therapies have been observed to cause tumor regression of both the target lesion and any untreated tumors [26]. Described as the "abscopal effect," this phenomenon is purportedly mediated by a systemic anti-tumor response that follows after receiving radiation therapy for a metastatic lesion or an entirely separate neoplasm. Overall, more than half of the reported cases of SR within the GI tract have been attributed to immunological processes (202 cases, 51.8%), with authors also suggesting the involvement of endocrine factors (four cases, 1.0%) and inflammatory responses (14 cases, 3.6%).
Ischemic models of regression are also proposed to play a key role in the dynamic interplay of antitumoral mechanisms described in the SR of cancer. Tumor cells require an ample supply of blood, so limiting their blood supply and perfusion could intuitively starve the cells to death [27-29]. Consequently, systemic and tumoral hypoperfusion (30 cases, 7.7%), rapid and unpredictable growth (14 cases, 3.6%), anti-angiogenic factors (three cases, 0.8%), and vascular compromise (26 cases, 6.7%) are all theorized to lead to the SR of GI cancer [30-34]. For example, there are multiple cases of SR described as following profound systemic hypoperfusion associated with hemodialysis, surgical invasion, or GI hemorrhage [30-34]. Several reviewed cases of SR (44 patients, 11.3%) have been specifically attributed to diagnostic biopsy procedures alongside tumor ablation and angiographic techniques [3,7,35]. In addition to impairing the adequate delivery of essential nutrients and oxygenation to the remaining (AL3) malignant tissue, these procedures are known to set forth a landslide of tumor-derived antigens into circulation, thus acting as a therapeutic vaccine [4,36].
While endocrinologic mechanisms are largely considered to play a secondary role in the course of tumor regulation, notable hormonal changes are considered possible antecedents to SR [37]. In a case describing a presumed appendiceal neuroendocrine tumor (NET) during pregnancy, Sewpaul et al. observed rapid regression following the patient’s completion of her pregnancy, suggesting that the pregnancy did not worsen the course of the disease but instead may have contributed to tumor regression [38]. Additional influences on the endocrine system by psychological events, such as trauma and stress, suggest that a patient’s psychological status might also influence the course of tumor development. In a case study detailing the SR of one patient’s recurrent oral squamous cell carcinoma (OSCC), Oya et al. describe how the 73-year-old patient was unable to understand the state of his recurrent cancer following cerebral infarction and dementia and postulate how this "unconsciousness" functioned as a preferable psychological condition for tumoral regression [39].
Spontaneous Regression in Cancers of Specific Pathohistology
Hepatocellular carcinoma: While testicular germ cell tumors, neuroblastomas, and renal carcinomas are conventionally the most frequent types of histologically diagnosed tumors presenting this phenomenon, several recent studies report an increasing incidence of SR within the GI pathway, particularly in primary hepatic lesions [6,40]. Correspondingly, we found that HCC was by far the most frequently observed type of cancer within the GI pathway to have undergone SR, with 199 total cases reported in the literature from 1982 to 2021. The reviewed cases proposed several mechanisms surrounding the involution of HCC, primarily citing ischemic and immunological antitumoral models of regression.
To prevent a barrage of immune responses to innocuous materials while still enabling immunity to pathogens, the complex cellular, functional, and molecular modeling of the liver allows for a dynamic, multifaceted approach to immune surveillance that incorporates the tolerogenic organ’s inherently immunosuppressive microenvironment and its distinct hepatic regulatory pathways [41]. It is possible that any manipulation of this multipronged system, such as through the abatement of the tolerogenic characteristics of hepatic APCs or the enhancement of effector lymphocyte function, could potentially have the desired effect of increased anti-tumor activity and tumor regression [42].
Interestingly, several of the changes associated with the SR of the poorly prognosed tumor can also be observed following transarterial chemoembolization (TACE) treatment, thus suggesting that the SR of HCC should, to some degree, involve ischemic processes [43]. Regression of HCC has also been linked to rapid tumor infiltration, in which the notably hypervascular tumor grows more rapidly than its blood supply, leading to local or centralized ischemia, intratumoral bleeding, and hemorrhagic necrosis of the lesion [44]. These distinct immunologic and vascular attributes of the liver combine to form a tumoral environment wherein an intrahepatic malignancy is uniquely positioned to respond to immune and ischemic changes compared to tumors of other organs of the GI tract. Otherwise, abstinence from alcohol, persistent fever, withdrawal from androgens, blood transfusions, and the use of herbal medicines have also been described as leading to the SR of primary hepatic lesions.
Primary oral and gastrointestinal lymphoma: Cases detailing the SR of primary oral and GI lymphomas were observed to span the entirety of the alimentary canal, from several primary extranodal lymphomas of the oral cavity to four cases of rectal lymphoma that regressed spontaneously. Regarding the spontaneous regression of aggressive NHLs of the digestive tract, several cases have been reported demonstrating the spontaneous involution of lymphoma following improved immunological status, particularly in HIV-infected patients receiving antiretroviral therapy [27-32].
While SR is an exceptionally rare occurrence in aggressive lymphomas, such as DLBCL and ALCL, it can occur relatively frequently in low-grade lymphomas such as follicular lymphomas (FLs) and mucosa-associated lymphoid tissue (MALT) lymphomas. Generally, well- or moderately-differentiated forms of cancer are considered low immunogenic tumors due to their limited mutational load and concomitant limited neoantigen expression. In a retrospective analysis of 209 cases of NHL from 1965 until 1978, Gattiker et al. reported the occurrence of SR in 18 out of 140 (12.9%) cases of nodular type malignant lymphoma and 2 out of 69 (2.9%) cases of diffuse type malignant lymphoma [45]. The relationship between gastric mucosa-associated lymphoid tissue (MALT) lymphoma and H. pylori is very well established, and low-grade gastric MALT lymphomas are known to regress following the bacteria’s eradication [46]. This reversible reactivity of low-grade MALT lymphomas to H. pylori infection is a clearly documented phenomenon; hence, cases detailing the regression of low-grade MALT lymphomas involving H. pylori eradication through the use of eradication therapy were excluded from the scope of this careful review.
Pancreatic ductal adenocarcinoma: With only a few cases reported in the literature, pancreatic tumors are seldom known to undergo SR, leaving clinicians skeptical of this lethal tumor’s ability to truly demonstrate involution when left untreated. Despite numerous molecular and immunological approaches, pancreatic cancer is typically poorly responsive to existing chemotherapeutic and immunological antineoplastic agents. This lack of response to immunotherapies is largely due to cancer’s low mutational burden and tendency to favor an immunosuppressive microenvironment characterized by self-isolating dense desmoplastic tissue and an exceptionally low number of infiltrating T cells [47,48]. In a recently published article investigating the possibility of misdiagnosis leading to a presumptive finding of SR in pancreatic cancer, Herreros-Villaneuva et al. emphasized how different types of pancreatic carcinomas must be cautiously distinguished from otherwise benign tumors, insulinomas, and immunoglobulin G4 (IgG4)-associated autoimmune pancreatitis during the process of recording SR [49]. Regardless, four additional cases of pancreatic ductal adenocarcinoma (PDAC) have since been published, citing various multifactorial models of SR, including acute pancreatitis and bacterial or fungal infection in the vicinity of the pancreatic tumor, leading to improved immunogenic tumor presentation [48,50-52].
Colorectal cancer: Like pancreatic cancer, colorectal cancer has long been considered poorly immunogenic, largely based on indirect data from epidemiological studies on the lack of SR in colorectal cancer [53,54]. This lack of immunogenicity in this cancer can be attributed to the failure of tumor-infiltrating lymphocytes to demonstrate substantial lytic activity against cancer cells, as demonstrated in in vitro models [55,56]. While colorectal cancer constitutes more than 15% of all malignancies, it represents less than 2% of all tumors to demonstrate SR [57]. Still, several other rare forms of GI cancer, including Merkel cell-like small cell carcinoma of the parotid gland and multiple gastroenteropancreatic neuroendocrine tumors (GEP-NETs) of the stomach, bile duct, and pancreas, were observed to spontaneously regress.
Strengths and Limitations
While prior retrospective analyses have investigated the incidence of SR for specific cancers and its occurrence within the individual organs of digestion, an observational study of this scope, broadly examining all prior cases of SR throughout the entire GI pathway, has never been published to date. This first-of-its-kind study systematically and thoroughly extracts and organizes information from an array of 390 individual cases of SR within the GI pathway. Although the majority of reports were restricted to the English literature, cases in other languages, including Spanish, Chinese, German, and Japanese, are included in this broad review in order to better demonstrate the global incidence of the otherwise rare phenomenon. Putative mechanisms for SR, including immunological, ischemic, and idiopathic modalities, are also explored and discussed in a detailed manner with the hopes of aiding in an understanding of SR as a dynamic interplay of complex and interconnected antitumoral responses.
In general, SR remains a poorly understood and somewhat vaguely defined phenomenon. Our review has multiple limitations. Recognizing true SR as a host response to specific tumors may continue to be obscured by bias in how the regression is reported. In addition to the possible underreporting of cases of SR by certain physicians, there is also a significant amount of variability in how SR is defined. Distinguishing SR from abscopal effects and tumor regression instigated by eradication therapy remains highly subjective and may result in the misreporting of true spontaneous antitumoral host responses to specific cancers. Overall, the literature is quite heterogeneous, and not every case study reported the duration of follow-up or the duration of remission in a similar manner as would be expected in a retrospective review of this kind.
Conclusions
SR is an extremely rare occurrence. Nonetheless, certain recurrent patterns in cases of SR, as demonstrated in this review, deserve ample consideration. To better study SR in the future, there must be an emphasis on standardizing how SR is reported. A well-defined registry would also be helpful. Ultimately, this broadly encompassing yet focused assessment is meant to bring attention to the phenomenon of SR and perhaps aid in the investigative efforts in the burgeoning field of immunotherapies.
Acknowledgments
Authors' contributions: Conceived and designed the analysis: CDM, PL, CC, VR. Collected the data: CDM, PL, CC. Contributed data or analysis tools: CDM, PL, CC, AP. Performed the analysis: CDM, PL, AP, GA, AL. Wrote the manuscript: CDM, PL, AP, CC, GA, AL, VR. Approved final manuscript version: CDM, PL, AP, CC, GA, AL, VR.
Appendices
Appendix A
Table 3. Keywords included in search criteria.
Each word from cohort P was cross-searched with a word or phrase from cohorts 1 or 2a and 2b. Phrases from cohort 1 were searched individually after being paired with a word from cohort P. Phrases searched with words from Cohort 2a were matched with a single word from cohort 2b (if applicable), and this pairing was used across the five databases.
| Cohort P | Cohort 1 | Cohort 2a | Cohort 2b |
| Abscopal effect | Alveolar soft-part sarcoma | Anus (anal) | Adenocarcinoma |
| Spontaneous necrosis | Cholangiocarcinoma | Bile duct (biliary/hepatobiliary) | Anaplastic lymphoma |
| Spontaneous regression | Epithelioid hemangioendothelioma | Cecum (Ileocecal) | Cancer |
| Spontaneous remission | Extramedullary plasmacytoma | Colon (colorectal) | Carcinoid |
| Gastrinoma | Duodenum (duodenal) | Carcinoma | |
| Insulinoma | Esophagus (esophageal) | Diffuse large B-cell lymphoma | |
| Islet cell cancer | Gallbladder | Follicular lymphoma | |
| Malignant myoepithelioma | Gastroesophageal junction | Hodgkin lymphoma | |
| Malignant peritoneal mesothelioma | Gastrointestinal | Leiomyosarcoma | |
| Mucoepidermoid carcinoma | Ileum (ileal) | Lymphoblastic lymphoma | |
| Pseudomyxoma peritonei | Jejunum (jejunal) | Lymphoma | |
| Liver (hepatic/hepatocellular) | Malignant melanoma | ||
| Mesentery (mesenteric) | MALT lymphoma | ||
| Omentum (omental) | Natural killer lymphoma | ||
| Oral cavity (oral) | Neuroendocrine tumor | ||
| Pancreas (pancreatic) | Plasmablastic lymphoma | ||
| Peritoneum (peritoneal) | T-cell lymphoma | ||
| Rectum (rectal) | |||
| Salivary gland (adenoid) | |||
| Small bowel/intestine (enteric) | |||
| Tongue |
Appendix B
Table 4. Clinical features of cases demonstrating the spontaneous regression of gastrointestinal cancers.
| Ref | Age/ Sex | Location | Pathologic Histology | Site of regression | Period of Remission | Proposed mechanism | ||
| Oral cancer | 1 | Roxburgh (1935) | 60s/F | Tongue | OSCC | Primary tumor | 7 years | Partial Resection |
| 2 | Grillet et al (1984) | 26/M | Parotid gland | ACC | Lung metastases | 7 years | Diet | |
| 3 | Grillet et al (1984) | 53/M | Submandibular gland | ACC | Lung & nasolabial metastases | 3 years | Not reported | |
| 4 | Grem et al (1986) | 54/F | Vallecula | DLBCL | Primary tumor | 4 years | Immunological; viral/bacterial infection; Biopsy | |
| 5 | Poppema et al (1988) | 12/M | Oropharynx | LBL | Primary tumor & cervical lymph node | 3 years | Immunological (cytotoxic) | |
| 6 | Savarrio et al (1999) | 77/M | Soft palate | ALCL | Primary tumor | 1 year* | Immunological; Biopsy | |
| 7 | King et al (2001) | 52/M | Parotid gland | MM | Primary tumor & regional lymph nodes | 6 weeks | Immunological | |
| 8 | Notani et al (2002) | 77/M | Tongue | ALCL (TCL) | Primary & multiple oral recurrences | multiple | Not reported | |
| 9 | Koga et al (2003) | 78/F | Maxillary mingiva | DLBCL | Primary tumor | 3 years | Biopsy | |
| 10 | Yamamato et al (2003) | 80/F | Maxilla | DLBCL | Primary tumor | 1.5 years | Biopsy | |
| 11 | Yokoyama et al (2003) | 46/F | Hard palate | MALT Lymphoma | Primary tumor | 2.5 years | Biopsy | |
| 12 | Heibel H et al (2004) | 70/M | Mandible | DLBCL | Primary tumor | 1.5 years | Biopsy | |
| 13 | Lester et al (2004) | 50/M | Palate | PBL | Primary tumor | 4 months* | Restoration of immune function (HAART) | |
| 14 | Sakuma et al (2006) | 70/F | Palatine salivary gland | MALT Lymphoma | Primary tumor | 3 years | Biopsy; Localized infection | |
| 15 | Armstrong et al (2007) | 35/M | Maxilla | PBL | Primary tumor (partial) | 2 weeks | Restoration of immune function (antiretroviral) | |
| 16 | Kurita et al (2007) | 67/M | Tongue | OSCC | Cervical lymph node metastases | 10 months | Enhanced Apoptosis | |
| 17 | Oya andikemura K (2007) | 73/M | Tongue | OSCC | Primary tumor | 3.5 years | Psychoneurological ("unconsciousness to cancer" S/P cerebral infarct & dementia); Immunological | |
| 18 | Rujirojindakul et al (2007) | 26/M | Submandibular gland | LBL | Primary tumor | multiple* | Not reported | |
| 19 | Daly et al (2008) | 56/M | Maxillary gingiva | TCL | Primary tumor | 4 years | Biopsy | |
| 20 | Mulder et al (2009) | 78/M | Parotid gland | Merkel cell-like SmCC | Primary tumor | 5 months* | T-cell mediated response triggered by trauma; Apoptosis | |
| 21 | Brachet et al (2011) | 58/F | Hard palate | DLBCL | Primary tumor | 15 days* | Biopsy | |
| 22 | Corti et al (2011) | 55/F | Oral | PBL | Primary tumor | 10 months | Restoration of immune function (antiretroviral cART) | |
| 23 | Tamás et al (2011) | 66/F | Vallecula/tongue | DLBCL | Primary tumor | 7 years | Not reported | |
| 24 | Fitzpatrick et al (2012) | 88/F | Labial mucosa | ALCL (TCL) | Primary tumor | 2 weeks | Biopsy | |
| 25 | García-Noblejas et al (2013) | 78/F | Buccal mucosa | PBL | Buccal & cervical lymph node lesions | 2 years | Restoration of immune function (removal of methotrexate) | |
| 26 | Choi et al (2014) | 52/F | Buccal mucosa | OSCC | Cervical lymph node metastases | Not reported | Tumor Microenvironment modification | |
| 27 | Sousa et al (2014) | 62/M | Mouth floor | OSCC | Primary tumor | 3 months | Biopsy; Immunological | |
| 28 | Cuenca-Jimenez et al (2015) | 65/M | Parotid gland | OSCC | Primary tumor | Not reported | Not reported | |
| 29 | Igawa et al (2015) | 80/M | Maxillary gingiva | PBL | Primary tumor | 8 months | Genetic (immunosenescence) | |
| 30 | Kaibuchi et al (2015) | 87/M | Gingiva | DLBCL | Primary tumor | 2.5 years | Biopsy | |
| 31 | Gonzalez-Perez et al (2016) | 75/M | Mandibular gingiva | EMP | Primary tumor | 1.5 years | Immunological (cytokines. Growth factors); Biopsy | |
| 32 | Wagner et al (2016) | 33/F | Mandibular gingiva | PBL | Primary tumor | 1.5 months | Restoration of immune function (HAART) | |
| 33 | Daroit et al (2017) | 66/F | Maxilla | PBL | Primary tumor | 1 year | Restoration of immune function (HAART) | |
| 34 | Kitamura et al (2017) | 61/M | Maxillary gingiva | PBL | Primary tumor | 2 years | Restoration of immune function (antiretroviral) | |
| 35 | Rajan & Samant et al (2017) | 49/F | Hard palate | MM | Primary tumor | Not reported | Immunological | |
| 36 | Yao et al (2017) | 80/M | Maxillary gingiva | PBL | Primary tumor | Not reported | Traumatic factors (Biopsy) | |
| 37 | Miyagawa et al (2018) | 46/M | Upper lip | ALCL | Primary tumor | 1 year | Biopsy | |
| 38 | Gamarra et al (2018) | 50/F | Maxilla | MEC | Primary tumor (partial) | 10 years | Not reported | |
| 39 | Ono et al (2019) | 69/M | Mandibular gingiva | PBL | Primary tumor | 2 years | Not reported | |
| 40 | Curioni et al (2020) | 51/F | Submandibular gland | SGAC | Primary tumor | 7 years | Metabolic derangement (hypoglycemia); Immunological | |
| 41 | Oliveira et al (2020) | 52/F | Hard palate | MM | Primary tumor | 6 years | Inflammatory; Immunological | |
| 42 | Peeters et al (2020) | 59/M | Buccal/Masticator space | FL | Primary tumor | 6 months | Biopsy | |
| 43 | Aoki et al (2021) | 84/M | Maxillary gingiva | DLBCL | Primary tumor | 2 years | Biopsy | |
| 44 | Lau et al (2021) | 66/F | Oropharyngeal tonsil | OSCC | Primary tumor | 7 months | Biopsy (Anti-tumoral T-cell response); Hyperthermal state (COVID-19 vaccine) | |
| 45 | Ueno et al (2021) | 83/F | Mandibular gingiva | MM | Primary tumor (partial) | 26 days | Not reported | |
| 46 | Sousa et al (2022) | 61/F | Parotid gland | MME | Primary tumor | 9 months | Inflammatory response (COVID-19 vaccine) | |
| Esophageal cancer | 47 | Rees et al (1983) | 49/M | Lower esophagus | EAC | Lung metastases | 1 year | Abscopal effect |
| 48 | Oshwada et al (1990) | 78/M | Thoracic esophagus | ESCC | Primary tumor (partial) & pulmonary metastases | 7 months | Change in T-cell subsets; surgical trauma | |
| 49 | Vergeau et al (1991) | 36/M | Mid esophagus | ESCC | Primary tumor (partial) | 1 year | Leukocyte infiltration; Inflammation | |
| 50 | Hahm et al (1993) | 30/M | Thoracic esophagus | PEL | Primary tumor | Not reported | H2-blocker (Cimetidine) | |
| 51 | Saruki et al (1994) | 82/F | Thoracic esophagus | ESCC | Primary tumor | 2 years | Infection (Pneumonia) | |
| 52 | Takemura et al (1999) | 63/F | Thoracic esophagus | ELMS | Pleural & splenic metastases | 10 months | Removal of Primary | |
| 53 | Chang (2000) | 57/M | Lower esophagus | ESCC | Primary tumor | 9 years | Infection (Pneumonia); Inflammation | |
| 54 | Kubota et al (2003) | 73/M | Thoracic esophagus | SCEC | Primary tumor | 1 month | Infection (Hepatitis C) | |
| 55 | Hornby et al (2015) | 57/M | GEJ | MM | Primary tumor | 2 months | Immunological; Occult Primary | |
| 56 | Mitchell et al (2021) | 58/F | GEJ | GEJAC | Local & supraclavicular lymph metastases | 6 months | Not Reported | |
| 57 | Kahn et al (2021) | 66/M | Lower esophagus | ESCC | Primary tumor | 3 months | Immunological (T-cell response) | |
| Stomach cancer | 58 | Takeuchi et al (1971) | 39/M | Corpus-antrum | PGL (RCS) | Primary tumor (partial) | 2 months | Not reported |
| 59 | Nakano et al (1972) | 55/F | Corpus | PGL (RCS) | Primary tumor (partial) | 3 weeks | "malignant cycle" of early gastric cancer | |
| 60 | Rosenberg et al (1972) | 51/M | Lesser curvature | GAC | Liver metastases | 12 years | Fever | |
| 61 | Ohashi et al (1973) | 42/M | Corpus-antrum | PGL (RCS) | Primary tumor (partial) | 1 month | "malignant cycle" of early gastric cancer | |
| 62 | Tietjen et al (1974) | 60/F | Gastric antrum duodenum | PGL (RCS) | Primary tumor | 5 years | "malignant cycle" of early gastric cancer | |
| 63 | Yamazaki et al (1974) | 39/M | Pyloric antrum | PGL (RCS) | Primary tumor (partial) | 20 days | Not reported | |
| 64 | Kimura et al (1987) | 85/F | Residual stomach | GAC | Primary tumor | Not reported | Not reported | |
| 65 | Strauchen et al (1987) | 73/M | Pyloric antrum | PGL (DLBCL) | Primary tumor | 3 weeks | H2-receptor antagonist | |
| 66 | Strauchen et al (1987) | 84/M | Pyloric antrum | PGL (DLBCL) | Primary tumor | 1 month | H2-receptor antagonist | |
| 67 | Harvey et al (1988) | 78/F | Gastric body/fundus | GEP-NET (ECL-cell Carcinoid) | Multifocal gastric lesions (majority) | 10 years | Not reported | |
| 68 | Harvey et al (1988) | 55/M | Stomach | GEP-NET (ECL-cell Carcinoid) | Multifocal gastric lesions | 5 years | Not reported | |
| 69 | Sawant et al (1989) | 40/F | Unspecified stomach | GEP-NET (Carcinoid) | Primary tumor | 1 year | Biopsy | |
| 70 | Shigematsu et al (1989) | 40/F | Pyloric antrum | PGL (DLBCL) | Primary tumor (partial) | 2 months | Not reported | |
| 71 | Shigematsu et al (1989) | 73/M | Pyloric antrum | PGL (DLBCL) | Primary tumor | 2 months | Not reported | |
| 72 | Rebollo et al (1990) | 77/M | Gastric body/fundus | GAC | Primary tumor | 8 months | Infection (abdominal wall abscess) | |
| 73 | Yoshimine et al (1991) | 69/F | Gastric angulus | PGL | Primary tumor | 2.5 months | Dislodged (ulceration) | |
| 74 | Hayakawa et al (1992) | 62/F | Lesser curvature | PGL (DLBCL) | Primary tumor | 1 year | Necrosis & Detachment | |
| 75 | Takehara et al (1992) | 44/M | Gastric angulus/Antrum | PGL | Primary tumor (partial) | 1 month | H2-blocker | |
| 76 | Matsusaki et al (1996) | 64/F | Pyloric antrum | PGL (DLBCL) | Primary tumor | 1 month | Not reported | |
| 77 | Ogawa et al (1998) | 63/F | Gastric corpus | PGL (DLBCL) | Primary tumor | 13 months | H Pylori eradication | |
| 78 | Bariol et al (2001) | 24/M | Gastric antrum | PGL (TCL) | Primary tumor | 2 years | H Pylori eradication | |
| 79 | Salam et al (2001) | 73/F | Greater curvature | PGL (DLBCL) | Primary tumor | 2.5 years | H Pylori eradication | |
| 80 | Pentimone et al (2002) | 84/M | Residual stomach | PGL (MALT) | Recurrences | 15/5 years | Not reported | |
| 81 | Chung et al (2003) | 48/M | Lesser curvature | GAC | Primary tumor | 4 years | Ischemia (angiography) | |
| 82 | Watanabe et al (2003) | 22/M | Gastric body & Antrum | PGL (TCL) | Primary tumor | 1 month | Infection (Severe EBV viremia in CAEBV) | |
| 83 | Watari et al (2005) | 60/F | Gastric angulus/Antrum | PGL (DLBCL) | Primary tumor | 1 year | H2-blocker; H. pylori eradication | |
| 84 | Watari et al (2005) | 61/M | Gastric angulus | PGL (DLBCL) | Primary tumor | 6 months | H2-blocker; H. pylori eradication | |
| 85 | Ohno et al (2006) | 14/M | Lower gastric corpus | PGL (MALT) | Primary tumor | 10 years | Immunological (Cessation of exposure to H pylori antigen) | |
| 86 | Lee et al (2010) | 84/M | Cardia & Lower body | GAC | Primary tumor | 1 year | Not reported | |
| 87 | Ip et al (2011) | 77/M | Gastric cardia | GEP-NET (LCNEC) | Primary tumor | 3 months | Infection (cytomegalovirus); Cross-autoimmune reaction against neuronal cells | |
| 88 | Yang et al (2012) | 77/M | Gastric body | GAC | Primary tumor & recurrences | Multiple | Not reported | |
| 89 | Rojas-Hernandez et al (2014) | 57/M | Greater curvature | PGL (DLBCL) | Primary tumor | 2 years | Immunological (B-cell stimulation by HCV) | |
| 90 | Shibata et al (2016) | 75/M | Gastric antrum | GEP-NET | Peripancreatic lymph metastases | 6 months* | EUS-FNA; Bacterial infection | |
| 91 | Sugiyama et al (2018) | 62/F | Gastric body | PGL (DLBCL) | Primary tumor | 10 years | Not reported | |
| 92 | Bonilla et al (2019) | 78/F | Gastric antrum | GAC | Retroperitoneal adenopathies | 3 months | Abscopal effect | |
| 93 | Hatsuse et al (2019) | 82/F | Unspecified stomach | PGL (DLBCL) | Primary tumor | 2 years | Not reported | |
| 94 | Okamoto et al (2021) | 37/M | Gastric antrum | GEP-NET (Gastrinoma) | Primary tumor | 3 years | Biopsy; Resection of omental metastases | |
| 95 | Zafar et al (2021) | 74/M | Lesser curvature | GAC | Primary tumor | 6 years | Not reported | |
| Primary peritoneal cancer | 96 | Schwartz et al (1991) | 39/M | Peritoneum, omentum | MPM | Local regression | 8 years | Fever; Rheumatoid factor |
| 97 | BaniHani et al (2009) | 38/M | Mesentery | ASPS | Abdominal mass, heart & lung metastases | 5 months | Immunological; Herbal medicine | |
| 98 | Jagodic et al (2018) | 66/F | Retroperitoneal space | RLMS | Liver metastases | 2 years | Not reported (possible delayed response to ChT) | |
| Hepatobiliary Cancer | 99 | Gottfried et al (1982) | 65/M | Diffuse hepatic | HCC | Primary tumor | 4 years | Abstinence from alcohol; A-P shunt; Portal vein thrombosis |
| 100 | Lam et al (1982) | 50/M | Unspecified liver | HCC | Primary tumor & lung metastases | 13 years | Chinese herbal medicine; Bronchopneumonia | |
| 101 | McCaughan et al (1985) | 28/M | Right lobe | HCC | Primary tumor | 6.5 years | Androgen withdrawal | |
| 102 | McCaughan et al (1985) | 40/M | Right lobe | HCC | Primary tumor | 9 years | Androgen withdrawal | |
| 103 | Sato et al (1985) | 78/M | Right lobe | HCC | Primary tumor & bone metastases | 5 years | Ischemia (GI Bleeding) | |
| 104 | Takayasu et al (1986) | 38/M | Unspecified liver | HCC | Primary tumor (partial) | 2 months | Subintintimal injury (angiography) | |
| 105 | Takayasu et al (1986) | 58/F | Unspecified liver | HCC | Primary tumor | 2.5 years | Subintintimal injury (angiography) | |
| 106 | Andreola et al (1987) | 75/M | S6/7 | HCC | Primary tumor | 18 days | Venous thrombosis | |
| 107 | Saez-Royeula (1989) | 66/M | Unspecified liver | HCC | Primary tumor | 2.5 years | Not reported | |
| 108 | Suzuki et al (1989) | 65/M | Posterior right lobe | HCC | Primary tumor | 6 years | Rapid growth | |
| 109 | Ayres et al (1990) | 63/F | Diffuse hepatic | HCC | Primary tumor (partial) | 1 year | Not reported | |
| 110 | Gaffey & Joyce (1990) | 63/M | Right lobe | HCC | Primary tumor (partial) | 1.5 years | Ischemia (GI Bleeding); Macrobiotic diet | |
| 111 | Tocci, G et al (1990) | 79/M | Hepatic hilum | HCC | Primary tumor | 3 months | Ischemia (GI Bleeding) | |
| 112 | Mochizuki et al (1991) | 61/M | Unspecified liver | HCC | Primary tumor (partial) | 1.5 years | Abscopal Effect | |
| 113 | Yamamoto et al (1991) | 58/M | Unspecified liver | HCC | Primary tumor | Not reported | Ischemia (hemorrhage) | |
| 114 | Yamamoto et al (1991) | 68/F | Unspecified liver | HCC | Primary tumor | Not reported | Not reported | |
| 115 | Chien et al (1992) | 65/M | Right lobe | HCC | Primary tumor | 2.5 years | Herbal Remedies | |
| 116 | Imaoka et al (1994) | 65/M | Left lateral lobe | HCC | Primary tumor | Not reported | Arterial thrombosis | |
| 117 | McDermott & Khettry (1994) | 23/F | Left lobe | Clear cell HCC | Primary tumor (partial) | 5 years | Not reported | |
| 118 | Grossmann et al (1995) | 52/M | Diffuse hepatic | HCC | Primary tumor (partial) | 1 year | Abstinence from alcohol | |
| 119 | Herrera et al (1996) | 76/M | Unspecified liver | HCC | Primary tumor | 1 year | Not reported | |
| 120 | Ozeki et al (1996) | 69/F | S3 | HCC | Primary tumor | 1 year | Herbal Remedies | |
| 121 | Markovic et al (1996) | 62/M | S5/6 | HCC | Primary tumor | 8 years | Fever; Ischemia (hemorrhage S/P biopsy); Biological effects by cytokines | |
| 122 | Yoshimitsu et al (1996) | 34/M | Intrahepatic (left lobe) | CCA | Primary tumor | 4 months* | Fibrous component | |
| 123 | Iwasaki et al (1997) | 72/F | Posterior/lateral | HCC | Primary tumor (partial) | 1.5 years | Tumor's rapid growth | |
| 124 | Van Halteren et al (1997) | 72/F | Right lobe | HCC | Primary tumor | 2 years | Ischemia & infarction due to Cirrhosis | |
| 125 | Kaczynski et al (1998) | 73/M | Central part/Hilum | HCC | Primary tumor | 3 years | Not reported | |
| 126 | Ohba et al (1998) | 76/M | S5 | HCC | Primary tumor (partial) | 2 years | Abscopal Effect | |
| 127 | Magalotli et al (1998) | 66/M | Unspecified liver | HCC | Primary tumor | 4 years* | Not reported | |
| 128 | Megalotli et al (1998) | 75/F | Unspecified liver | HCC | Primary tumor (partial) | 3 years* | Not reported | |
| 129 | Sanz et al (1998) | 66/M | Right lobe | HCC | Primary tumor | 1 year | Immunological | |
| 130 | Stoelben et al (1998) | 56/M | S6 | HCC | Primary tumor | 2 years | Immunological (Resection of tumor); Infection (abscess) | |
| 131 | Stoelben et al (1998) | 74/M | S6 | HCC | Primary tumor | 3.5 years | Immunological (resection of tumor); Infection (abscess) | |
| 132 | Takeuchi et al (1998) | 53/M | S8 | HCC | Primary tumor | Not reported | Ischemia (thrombus) | |
| 133 | Itoh et al (1999) | 58/M | S5 | HCC | Primary tumor | 13 days | Tumor Hypoxia (Thick capsule) | |
| 134 | Toyoda et al (1999) | 82/M | Right lobe | HCC | Primary regression (Primary & Lung metastases) | 1.5 years | Transition from necrosis to fibrosis | |
| 135 | Izuishi et al (2000) | 50/M | S2/3 | HCC | Primary tumor | 5 years | Ischemia; Immunological; Angiography | |
| 136 | Jang et al (2000) | 54/F | Right lobe | HCC | Primary tumor | 4 years | Not reported | |
| 137 | Lee et al (2000) | 44/M | S4/8 | HCC | Partial regression (Primary) | 1 year* | Infection; Abstinence from alcohol | |
| 138 | Lee et al (2000) | 63/M | Right lobe | HCC | Partial regression (Primary) | 3 years | Infection; Arterial thrombosis/intimal injury (angiography) | |
| 139 | Takeda et al (2000) | 68/M | S4/5/6/7/8 | HCC | Primary tumor | 1 year | Herbal Remedies | |
| 140 | Terasaki et al (2000) | 72/F | S5 | HCC | Primary tumor, peritoneal & splenic metastases | 2 years | Apoptosis | |
| 141 | Uenishi et al (2000) | 65/M | Right lobe | HCC | Primary tumor (partial) | 1 year | Abstinence from alcohol; A-P shunt; Portal vein thrombosis | |
| 142 | Ikeda et al (2001) | 75/M | S7 | HCC | Primary tumor (partial) | 6 years | Not reported | |
| 143 | Jung et al (2001) | 58/M | Right lobe | HCC | Primary tumor (partial) & lung metastases | 1.5 years* | Herbal Remedies; cessation of smoking; | |
| 144 | Kawai et al (2001) | 58/M | S6 | HCC | Primary tumor | 1 month | Ischemia; Immunological; Angiography | |
| 145 | Matsuo et al (2001) | 72/M | S5 | HCC | Primary tumor (partial) | 1 year | Immunological; Hypoxia; Inflammatory cell infiltration | |
| 146 | Nakai et al (2001) | 76/M | Residual liver | HCC | Primary tumor | 2 years | Immunological (NK cell response) | |
| 147 | Sakurai et al (2001) | 65/M | Gallbladder fundus | GBAC | Primary tumor | Not reported | Ischemia; Inflammation; Pancreaticobiliary maljunction | |
| 148 | Serrano et al (2001) | 71/M | Left lobe | HCC | Primary tumor | 3 years | Growth factors; Ischemia (hepatic artery) | |
| 149 | Abiru et al (2002) | 70/M | Unspecified liver | HCC | Primary tumor, lung & bone metastases | 2 years | Immunological (IL-18) | |
| 150 | Abiru et al (2002) | 65/F | Unspecified liver | HCC | Primary tumor, lung & lymph metastases | 4 months | Immunological (IL-18) | |
| 151 | Abiru et al (2002) | 65/M | Unspecified liver | HCC | Primary tumor, lung & bone metastases | 1 year | Immunological (IL-18) | |
| 152 | Lee et al (2002) | 70/M | S2/3 | HCC | Primary tumor | 24 days | Occlusion of feeding artery | |
| 153 | Misawa et al (2002) | 62/M | Anterior segment | HCC | Primary tumor | 1 year | Biological effects by A-P shunt | |
| 154 | Morimoto et al (2002) | 73/M | S2/3 | HCC | Primary tumor | 1 year | Arterial thrombosis | |
| 155 | Zimmermann et al (2002) | 56/M | S6 | Medullary-like HCC | Primary tumor | 2 years | Immunological (Cytotoxic pathway); Apoptosis | |
| 156 | Iiai et al (2003) | 69/M | S6/7 | HCC | Primary tumor | 4 years | Portal vein thrombosis; cessation of smoking | |
| 157 | Jozuka et al (2003) | 52/M | Hepatic surface | HCC | Primary tumor | 2.5 years | Psychoneurological; Antidepressants; Immunological | |
| 158 | Li et al (2003) | 53/M | S6 | HCC | Primary tumor | Not reported | Biological effects by cytokines | |
| 159 | Ohta et al (2003) | 74/M | S2/3 | HCC | Primary tumor | 1 year | Immunological; hypoxia (arterial sclerosis) | |
| 160 | Blondon et al (2004) | 64/M | Diffuse hepatic | HCC | Local regression | 9 months | Infection (peritonitis); Ischemia (Intraperitoneal bleed) | |
| 161 | Blondon et al (2004) | 70/F | Diffuse hepatic | HCC | Local regression | 3 years | Infection (peritonitis); Ischemia (Intraperitoneal bleed); tamoxifen | |
| 162 | Cheng et al (2004) | 74/M | Medial left lobe | HCC | Primary tumor | 6 years | Herbal remedies | |
| 163 | Erturk et al (2004) | 69/M | Left lobe | HCC | Primary tumor | 3 years | Blood transfusion | |
| 164 | Feo et al (2004) | 71/F | S3/5 | HCC | Primary tumor (partial) | 1.5 years | Ischemia | |
| 165 | Kato et al (2004) | 72/M | Right lobe | HCC | Primary tumor (partial) | 2 years | Not reported | |
| 166 | Kato et al (2004) | 77/M | Right lobe | HCC | Primary tumor & lung metastases | 1 year | Abstinence from smoking | |
| 167 | Lin et al (2004) | 42/M | S8 | HCC | Primary tumor (partial) | 2 years | Herbal remedies | |
| 168 | Nakajima et al (2004) | 80/M | S4/6 | HCC | Partial regression (Primary) | 6 months | Ischemia; Intratumoral bleeding/hemorrhagic necrosis | |
| 169 | Jeon et al (2005) | 72/M | Right lobe | Clear Cell HCC | Primary tumor (partial) & chest wall metastases | 9 months | Metabolic derangement (hypoglycemia & HLD) | |
| 170 | Moon et al (2005) | 72/M | S6 | HCC | Primary tumor | 2 years | Alcohol cessation | |
| 171 | Nam et al (2005) | 65/M | Right lobe | HCC | Liver & bone metastases (partial) | 1 year | Abscopal Effect | |
| 172 | Nouso et al (2005) | 85/M | S5/6/7/8 | HCC | Primary tumor (partial) | 2 years | Ischemia; Vitamin K administration | |
| 173 | Ohtani et al (2005) | 69/M | S4 | HCC | Primary tumor (partial) | 3 years | Tumor Hypoxia (Thick capsule) | |
| 174 | Randolph et al (2005) | 56/M | Left lateral lobe | HCC | Primary tumor | 1.5 years | Alcohol cessation, ischemia (obstruction of portal vein thrombosis); Infection (Pneumonia) | |
| 175 | Rizell et al (2005) | 58/M | Central liver | HCC | Primary tumor (partial) | 1.5 years | Sirolimus (Immunosuppressive) | |
| 176 | Yano et al (2005) | 71/F | S8 | HCC | Primary tumor (partial) | 2 years | Hypoxia (artery rupture) | |
| 177 | Otrock et al (2006) | 75/F | Diffuse hepatic | HEHE | Primary tumor | 3.5 years | Not reported | |
| 178 | Kojima et al (2006) | 79/M | S8 | HCC | Lung metastases | 6 months | Steroids, Hormones, or Herbal Remedies | |
| 179 | Kondo et al (2006) | 67/M | S4 | HCC | Primary tumor (partial) | 2 months | Immunological | |
| 180 | Kondo et al (2006) | 67/M | S5/3 | HCC | Primary tumor & lung metastases | 4 years | CAM's; Immunological | |
| 181 | Kondo et al (2006) | 70/M | Right lobe | HCC | Primary tumor (partial) | 5 years | Bleeding (esophageal varicies); Immunological | |
| 182 | Kondo et al (2006) | 75/M | S7 | HCC | Lung metastases (partial) | 2 years | Immunological | |
| 183 | Shibuya et al (2006) | 71/M | S5 | HCC | Primary tumor | 2 months | Ischemia; Immunological; Angiography | |
| 184 | Heianna et al (2007) | 70/F | Unspecified liver | HCC | Lung metastases | 5 years | Immunological (Host cytokines); Systemic inflammatory (TACE of primary) | |
| 185 | Matsunaga et al (2007) | 71/F | Left lateral lobe | Sarcomatoid HCC | Peritoneal metastases | 4 months* | Ischemia (rapid growth) | |
| 186 | Meza-Junco et al (2007) | 56/F | S5 | HCC | Primary tumor | 2 years | Hypoxia (Thick capsule) | |
| 187 | Peddu et al (2007) | 57/M | S4 | HCC | Primary tumor | 2 months | Auto-infarction | |
| 188 | Vardhana et al (2007) | ?/M | S2/3/4 | HCC | Primary tumor | 8 months | Immunological | |
| 189 | Arakawa et al (2008) | 78/F | S2/3 | HCC | Primary tumor | 2.5 years | Immunological; Portal vein thrombosis | |
| 190 | Hori et al (2008) | 71/M | Gallbladder | GBAC | Primary tumor | Not reported | Increased intraluminal pressure (PBM); Pancreatic enzymes | |
| 191 | Sibartie et al (2008) | 76/M | S5 | HCC | Primary tumor (partial) | 2 years | Ischemia (disturbance of blood flow) | |
| 192 | Del Poggio et al (2009) | 77/F | S6 | HCC | Primary tumor (partial) | 1.5 years | Immunological (tumor antigens) | |
| 193 | Hsu et al (2009) | 66/M | S7/8 | HCC | Primary tumor (partial) | 1.5 years | Hypoxia; Immunological; Silymarin; Portal vein thrombosis | |
| 194 | Kanzaki et al (2009) | 52/M | S8 | HCC | Primary tumor | 8 months | Tumor Hypoxia (thick capsule) | |
| 195 | Nishijima et al (2009) | 86/F | S7 | HCC | Primary tumor (partial) | 4 months | Tumor infarction | |
| 196 | Oquiñena et al (2009) | 54/M | S6 | HCC | Primary tumor | 2 years | Vascular ischemia; Immunological | |
| 197 | Oquiñena et al (2009) | 61/M | S1 | HCC | Primary tumor | 1.5 years | Vascular ischemia; Immunological | |
| 198 | Oquiñena et al (2009) | 60/M | Right lobe | HCC | Primary tumor | 3 years | Vascular ischemia; Immunological | |
| 199 | Park et al (2009) | 57/M | S5/6/7/8 | HCC | Primary tumor (partial) | 5 years | Infiltrating lymphocytes | |
| 200 | Harada et al (2010) | 70/M | S7 | HCC | Primary tumor | 2 years | Ischemia; Herbal remedies | |
| 201 | Hong et al (2010) | 67/M | Resection margin | HCC | Primary tumor & lung metastases | 1 year | TACE of Primary | |
| 202 | Kai et al (2010) | 58/F | S6/7 | HCC | Primary tumor | 1 month | intimal injury (angiography) | |
| 203 | Kai et al (2010) | 49/M | S6 | HCC | Primary tumor | 3 weeks | intimal injury (angiography) | |
| 204 | Satou et al (2010) | 83/M | Right lobe | HCC | Primary tumor | Not reported | NSAIDS (Ketoprofen) | |
| 205 | Storey et al (2010) | 52/M | S5/6 | HCC | Primary tumor | 3 years | Cessation of alcohol | |
| 206 | Alqutub et al (2011) | 65/M | Right lobe | HCC | Primary tumor | 2 years | Ischemia (Rapid growth; Intratumoral hemorrhage) | |
| 207 | Arora et al (2011) | 54/M | Right lobe | HCC | Primary tumor | 2 years | Immunological; Necrosis | |
| 208 | Fukushima et al (2011) | 69/M | Right lobe | HCC | Lung metastases | 7 years | Immunological (TACE of Primary) | |
| 209 | Maejima et al (2011) | 68/M | S3/5 | HCC | Primary tumor | 3 months | Ischemia; Immunological | |
| 210 | Okano et al (2011) | 68/M | Right lobe | HCC | Tumor recurrence | 2 years | PVT; Ischemia (rapid growth) | |
| 211 | Okuma et al (2011) | 63/M | Right lobe | HCC | Lung metastases | 3 years | Abscopal Effect | |
| 212 | Bastawrous et al (2012) | 63/M | Right lobe | HCC | Primary tumor (partial) | Not reported | Ischemia | |
| 213 | Harimoto et al (2012) | 73/M | S6/7 | HCC | Primary tumor & lung metastases | 1 year | Ischemia (hypotension during dialysis) | |
| 214 | Komatzu et al (2012) | 65/M | Right lobe | HCC | Primary tumor & recurrences | 6 months (x3) | Not reported | |
| 215 | Nakayama et al (2012) | 92/F | Right lobe | HCC | Primary tumor | Not reported | Ischemia (rapid growth); Immunological | |
| 216 | Takeura et al (2012) | 69/F | Unspecified liver | HCC | Primary tumor & bone metastases | 10 months | Inflammatory (trauma) | |
| 217 | Takeura et al (2012) | 84/F | Unspecified liver | HCC | Primary tumor & peritoneal carcinomatosis | 1.5 years | Inflammatory (trauma) | |
| 218 | Yamamoto et al (2012) | 60/M | S4-8 | HCC | Primary tumor | 3 weeks | Immunological; Diabetes Control | |
| 219 | Yokoyama et al (2012) | 80/M | S4 | HCC | Primary tumor | 1 month | Immune; Ischemia (thrombus) | |
| 220 | Katayama et al (2013) | 74/M | S5/6 | HCC | Primary tumor | 1 month | Tumor Hypoxia (thick capsule) | |
| 221 | Okano et al (2013) | 77/M | S4/6/7/8 | HCC | Primary tumor (partial) | 1 year | Ischemia (Disruption of feeding artery); Abstinence from alcohol | |
| 222 | Sasaki et al (2013) | 79/M | S2 | HCC | Primary tumor | 2 months | Not reported | |
| 223 | Tomishige et al (2013) | 76/F | S6 | HCC | Primary tumor | Not reported | Not reported | |
| 224 | Ueda et al (2013) | 63/F | S7 | HCC | Primary tumor | Not reported | Ischemia (Hypotension during dialysis) | |
| 225 | Bhardwaj et al (2014) | 74/M | Left lobe | HCC | Primary tumor | Not reported | Not reported | |
| 226 | Chiesara et al (2014) | 65/M | S6 | HCC | Primary tumor (partial) | 2 years | Herbal remedies; Ischemia; Inflammatory Processes | |
| 227 | Inoue et al (2014) | 57/M | S5 | HCC | Primary tumor | Not reported | Drugs (Inhibition of angiogenesis by rifampicin & minocycline) | |
| 228 | Lim et al (2014) | 64/M | Right lobe | HCC | Primary tumor | 6 months | Immunological; Herbal medicine | |
| 229 | Miyake et al (2014) | 79/M | S6/8 | HCC | Primary tumor | 1 month | Tumor Hypoxia (thick capsule) | |
| 230 | Saito et al (2014) | 74/M | S8 | HCC | Primary tumor (partial) | 2 months | Immunological; Cessation of drinking & smoking | |
| 231 | Tomino et al (2014) | 77/M | S1 | HCC | Primary tumor | 1 month | Hypoxia; Fever; Biopsy | |
| 232 | Tsai et al (2014) | 74/M | Left lobe | HCC | Primary tumor | 4 years | Not reported | |
| 233 | Zhao et al (2014) | 22/F | Diffuse hepatic | HEHE | Primary tumor (partial) | 3 years | Not reported | |
| 234 | Parks et al (2015) | 69/M | S8 | HCC | Recurrent Hepatic Lesions | 6 months* | Immunological (Vitiligo autoimmunity) | |
| 235 | Parks et al (2015) | 63/M | S7 | cHCC-CC | Retroperitoneal lymph metastases | 2 months | Immunological | |
| 236 | Parks et al (2015) | 67/M | Left lobe | HCC | Primary tumor | 5 months | Immunological | |
| 237 | Kim et al (2015) | 57/M | S6 | HCC | Primary tumor | Not reported | Immunological; Ischemia | |
| 238 | Kohda et al (2015) | 80/M | S1 | HCC | Primary tumor | Not reported | Ischemia (rapid growth) | |
| 239 | Kuwano et al (2015) | 84/M | S4 | HCC | Primary tumor | Not reported | Ischemia | |
| 240 | Matsuoka et al (2015) | 67/M | S6 | HCC | Primary tumor | 3 years | Hypoxia; Hepatic arterial & portal vein thromboses | |
| 241 | Okano et al (2015) | 73/M | S8 | HCC | Primary tumor | 6 months | Ischemia; Angiography | |
| 242 | Sugamoto et al (2015) | 77/F | S3 | HCC | Primary tumor | 9 months | Immunological (weight loss) | |
| 243 | Takeda et al (2015) | 68/M | S4 | HCC | Primary tumor (partial) | 1 year | Hypoxia; Vessel thrombosis | |
| 244 | Tazawa et al (2015) | 77/M | S7 | HCC | Primary tumor | 3 months | Ischemia (postoperative hypotension) | |
| 245 | Verla-Tebit et al (2015) | 53/M | Right lobe | HCC | Primary tumor & lung metastases (partial) | 1.5 years | Anti-hepaciviral medication for Hepatitis C (sorafenib & ribavirin) | |
| 246 | Wang et al (2015) | 50/M | S7/8 | HCC | Primary tumor | Not reported | Immunological | |
| 247 | Yang et al (2015) | 59/M | S6 | HCC | Primary tumor | 6 months | Seroconversion of HBV | |
| 248 | Yoo et al (2015) | 62/M | S5 | HCC | Primary tumor (partial) | 2 years | Immunological; Hypoxia/Ischemia | |
| 249 | Gunasekaran et al (2016) | 49/M | Left lobe | HCC | Pulmonary metastases | 5 months | Consumption of Guaynabo fruit extract | |
| 250 | Heron et al (2016) | 61/F | S7 | HCC | Primary tumor | 1 year | Withdrawal of azathioprine in Crohn's Disease; Biopsy | |
| 251 | Jianxin et al (2016) | 64/M | S6 | HCC | Tumor recurrence and omental metastases | 2.5 years | Immunological; Herbal medicine | |
| 252 | Kumar et al (2016) | 40/M | S2/3/5 | HCC | Primary tumor | 7 years | Cessation of immunosuppressive therapy | |
| 253 | Kumar et al (2016) | 74/M | Right | HCC | Primary tumor (partial) | 8 months | Cessation of immunosuppressive therapy | |
| 254 | Luo et al (2016) | 61/F | S4 | HCC | Primary tumor | 2.5 years | Cirrhosis related hypoxia | |
| 255 | Mahmood et al (2016) | 59/M | S4/8 | HCC | Primary tumor | 4 months | Anti-hepaciviral medication for Hepatitis C (sorafenib & ribavirin) | |
| 256 | Ooka et al (2016) | 63/M | S7/8 | HCC | Primary tumor | 6 months | Ischemia (PVT) | |
| 257 | Pectasides et al (2016) | 53/M | S4 | HCC | Primary (partial) & lung metastases (partial) | 2 months | Portal vein thrombosis; Immunological reaction | |
| 258 | Sawatsubashi et al (2016) | 59/M | S5-8 | HCC | Primary tumor | 1 month | Tumor Hypoxia (thick capsule) | |
| 259 | Sugiura et al (2016) | 90/F | S6 | HCC | Primary tumor | Not reported | Not reported | |
| 260 | Alam et al (2017) | 65/M | S5/6 | HCC | Primary tumor (partial) | 3 months | Immunological | |
| 261 | Iwatani et al (2017) | 59/M | S8 | HCC | Primary tumor | Not reported | Ischemia (Duodenal Ulcer) | |
| 262 | Murata et al (2017) | 67/M | S1/8 | HCC | Primary tumor | 2 months | Ischemia | |
| 263 | Noij et al (2017) | 74/M | Diffuse hepatic | HCC | Primary (partial) & lung metastases | 6 months | Not reported | |
| 264 | Oyama et al (2017) | 79/M | Diffuse hepatic | HCC | Primary tumor | Not reported | Hypoxia | |
| 265 | Oyama et al (2017) | 78/F | Left lobe | HCC | Primary tumor | Not reported | Inflammatory; Immunological; Infection (Bacterial) | |
| 266 | Sakamaki et al (2017) | 78/M | S8 | HCC | Primary tumor & lymph metastases | 3 months | Immunological; hemodialysis | |
| 267 | Sano et al (2017) | 30/F | Common bile duct | NET | Primary tumor | Not Reported | Biopsy; Central necrosis | |
| 268 | Yamaguchi et al (2017) | 63/M | Right lobe | HCC | Primary tumor | Not reported | Not reported | |
| 269 | Yamaguchi et al (2017) | 67/M | S5 | HCC | Primary tumor | Not reported | Not reported | |
| 270 | Yamaguchi et al (2017) | 84/F | S7 | HCC | Primary tumor | Not reported | Not reported | |
| 271 | Yamaguchi et al (2017) | 60/M | S8/S1 | HCC | Primary tumor | Not reported | Not reported | |
| 272 | El-Badrawy et al (2018) | 45/F | Porta hepatis | DLBCL | Primary tumor | 18 days | Biopsy (Aspiration); Regional immune reaction | |
| 273 | Goto et al (2018) | 64/M | S6/7 | HCC | Primary tumor | 1 month | Portal vein thrombosis; Immunological | |
| 274 | Koya et al (2018) | 83/M | S2/3/4 | HCC | Primary tumor | 1 year | PVT | |
| 275 | Lee et al (2018) | 67/M | Diffuse hepatic | HCC | Primary tumor | 1 year | Infection (diabetic foot); Ischemia (obstruction of portal vein thrombosis) | |
| 276 | Taniai et al (2018) | 74/M | S7 | HCC | Primary tumor | 2 years | Not Reported | |
| 277 | Taniguchi et al (2018) | 70/M | S3 | HCC | Primary tumor | Not reported | Ischemia (dialysis); Drugs (Elythrocin Steroids) | |
| 278 | Alhatem et al (2019) | 60/M | Right lobe | DLBCL | Primary tumor | 4 years | Immunological (HIV, Hep C); Genetic | |
| 279 | Chohan et al (2019) | 79/F | S6 | HCC | Primary (partial) & lung metastases | 1.5 years* | Ischemia; Immunological | |
| 280 | Fujikawa et al (2019) | 78/M | S2/7 | HCC | Primary tumor | Not reported | Anemia (fracture) | |
| 281 | Hirota et al (2019) | 67/M | S7 | HCC | Primary tumor | Not reported | Ischemia; Cessation of alcohol and smoking | |
| 282 | Kim et al (2019) | 70/M | Bile duct | CCA | Liver Metastasis | 3 months | Abscopal effect; Post-radiotherapy antitumoral immunity | |
| 283 | Kawaguchi et al (2019) | 68/M | S3 | HCC | Primary tumor | 2.5 months | Antiangiogenesis (SGLT2i) | |
| 284 | Lee et al (2019) | 78/F | S5-8 | HCC | Primary tumor | 1 month | Immunological; Ischemia (rapid tumor growth or disruption of feeding artery) | |
| 285 | Yoshida et al (2019) | 71/F | Right lobe | Clear cell HCC | Primary tumor | 1 year | Not reported | |
| 286 | Arjunan et al (2020) | 53/M | Liver | HCC | Pulmonary, Omental, retroperitoneal metastases | 5 years | Immunological | |
| 287 | Arjunan et al (2020) | 48/M | Left Lobe | HCC | Pulmonary metastases | 13 years | Immunological | |
| 288 | Arjunan et al (2020) | 62/M | Liver | HCC | Systemic metastases | 11 years | Immunological | |
| 289 | Arjunan et al (2020) | 73/M | Liver | HCC | Primary tumor | 6 years | Immunological | |
| 290 | Costa-Santos et al (2020) | 68/M | S4 | HCC | Hepatic lesions | 5 years | Megestrol; Herbal remedies | |
| 291 | Hokkoku et al (2020) | 77/M | S6 | HCC | Primary tumor | Not reported | Not reported | |
| 292 | Muroya et al (2020) | 78/M | Right lobe | HCC | Lung metastases | 1 year | Hypoxia; Immunological; Dialysis | |
| 293 | Nakamoto et al (2020) | 74/M | Right lobe | HSTCL | Hepatic, splenic, & osseous lesions | 1.5 months | Biopsy | |
| 294 | Ohmatsu et al (2020) | 77/M | Right lobe | HCC | Lung metastases | 1 month | Abscopal Effect | |
| 295 | Onishi et al (2020) | 28/M | Left lobe | HEHE | Primary lesion (partial) | 6 years* | Unpredictable growth (new lesions) | |
| 296 | Onishi et al (2020) | 44/M | Unspecified liver | HEHE | Primary lesion (partial) | 4 years* | Unpredictable growth (new lesions) | |
| 297 | Onishi et al (2020) | 47/M | Unspecified liver | HEHE | Primary lesion (partial) | 12 years | Calcification | |
| 298 | Onishi et al (2020) | 51/F | S6 | HEHE | Primary lesion (partial) | 11.5 years* | Unpredictable growth (new lesions) | |
| 299 | Onishi et al (2020) | 61/F | Unspecified liver | HEHE | Primary lesion (partial) | 5.5 years* | Unpredictable growth (new lesions) | |
| 300 | Onishi et al (2020) | 63/M | Unspecified liver | HEHE | Primary lesion (partial) | 6 years* | Unpredictable growth (new lesions) | |
| 301 | Raufi et al (2020) | 63/M | Porta hepatis | PHNEC | Pulmonary metastases | 2 months | Immunological | |
| 302 | Sakamoto et al (2020) | 62/M | S3 | HCC | Primary tumor | Not reported | Ischemia | |
| 303 | Sakamoto et al (2020) | 75/F | S4 | HCC | Primary tumor | Not reported | Tumor hypoxia (bleeding from rectal varicose veins) | |
| 304 | Sonbare et al (2020) | 74/M | S8 | HCC | Primary tumor | 1 year | Immunological; Ischemia | |
| 305 | Franses et al (2021) | 64/M | S4 | HCC | Primary tumor | 2 months | Immunological | |
| 306 | Kakuta et al (2021) | 71/M | Not reported | HCC | Lung metastases | 3 months* | TACE of Primary | |
| 307 | Kimura et al (2021) | 84/F | S8 | HCC | Primary tumor (partial) | Not reported | Immunological | |
| 308 | Liu et al (2021) | 67/M | Diffuse hepatic | HCC | Pulmonary metastases | 5 months | Immunological; Chinese herbal remedies | |
| 309 | Obu et al (2021) | 83/M | S2 | HCC | Primary tumor | 1 year | Ischemia (rapid growth); Capsule formation; PVT | |
| 310 | Tanaka et al (2021) | 71/F | Diffuse hepatic | HHL | Hepatic lesions | 1 year | Cessation of immunosuppressive therapy | |
| Pancreatic cancer | 311 | Shapiro (1967) | ?/F | Unspecified pancreas | PDAC | Primary tumor | 7.5 years | Not reported |
| 312 | Lokich et al (1973) | 42/M | Pancreatic head | PDAC | Primary tumor | 2 years* | Not reported | |
| 313 | Eidemiller et al (1971) | ?/M | Pancreatic head | PDAC | Primary tumor | 6 years | Not reported | |
| 314 | Tchertkoff et al (1974) | 21/M | Pancreatic head | PDAC | Primary tumor | 12 years | Bacterial Infection | |
| 315 | Cann et al (2003) | 50/M | Pancreatic body | PDAC | Primary tumor | 6 months | Acute febrile response; alternative therapies; Chinese herbs; High-dose Vitamin C | |
| 316 | Chin et al (2017) | 77/M | Pancreatic head | PDAC | Primary tumor & liver metastases | 1 year | Leukocyte activation; Fever; Allergenic & hormonal influences | |
| 317 | Sreevathsa et al (2018) | 32/M | Pancreatic body | pNET (Carcinoid) | Primary tumor | 19 years | Apoptosis (cytokines); VEGF blockade | |
| 318 | Lemus et al (2019) | 56/F | Pancreatic body/tail | PDAC | Primary tumor & liver metastases | 3 years | Immunogenic; angiogenic effects on the tumor microenvironment. | |
| 319 | Ibrahimi et al (2019) | 59/F | Residual pancreas | PDAC | Primary tumor (partial) | 1 year | Acute pancreatitis; Bacterial/fungal infection (abscess) | |
| 320 | Kawaguchi et al (2021) | 66/F | Pancreatic tail | PDAC | Primary tumor (partial) | 1 month | Not reported | |
| Small bowel cancer | 321 | Sroujieh et al (1988) | 55/M | Occult (Ileal lesion) | MM | Intestinal lesion (occult primary) | 7 years | Not Reported |
| 322 | Nagashima et al (1996) | 58/M | Duodenum | MALT Lymphoma | Primary tumor | 1 year | Eradication of H. Pylori | |
| 323 | Rayson et al (1996) | 45/F | Ileum | GEP-NET (Carcinoid) | Liver metastases | 5 months* | Valvular surgery for carcinoid heart disease | |
| 324 | Horiuhi et al (2003) | 74/F | Diffuse enteric | NKTCL | Upper Abdominal tumors (non-radiated) | 1 year | Abscopal Effect | |
| 325 | Makino et al (2010) | 38/M | Terminal Ileum | MALT Lymphoma | Primary tumor & ileocecal lymphadenopathy | 1 year | Resolution of infection; Inflammatory | |
| 326 | Hayashi et al (2013) | 64/F | Duodenum | FL | Primary tumor | 5.5 years | Eradication of H. Pylori | |
| 327 | Tanaka et al (2014) | 61/F | Duodenum | SBAC | Primary tumor & liver metastases | 4 months | Methotrexate | |
| 328 | Sasaki et al (2016) | 60/M | Ileum | RL | Primary tumor | Not reported* | Radiography Radiation | |
| 329 | Hori et al (2017) | 20/F | Small intestine | EAS | Lung & mediastinal lymph metastases | 2 months | Immunological; Biopsy (transbronchial); Inflammatory | |
| 330 | Tanaka et al (2019) | 35/M | Small intestine | DLBCL | Primary tumor & lymph metastases | 3 years | Immunological (PD-L1/PD-1 axis) | |
| Colorectal cancer | 331 | Most (1927) | 57/M | Rectum | CRAC | Local recurrence | 9 years | Sepsis |
| 332 | Henry (1944) | 60/M | Rectum | CRAC | Primary tumor & liver metastases | 11 years | Not reported | |
| 333 | Fergeson (1954) | 45/M | Descending colon | CRAC | Primary tumor with local extension | 10 years | Severe sepsis (abscess) | |
| 334 | Dunphy (1956) | 46/M | Rectum | CRAC | Primary tumor & liver metastases | 8 years | Severe debilitation; Fecal diversion | |
| 335 | Ellison (1956) | 59/M | Rectum | CRAC | Peritoneal carcinomatosis | 3 years | Persistent high fever (Pneumonia) | |
| 336 | Fallis (1959) | 42/M | Transverse colon | CRAC | Primary tumor with local extension | 18 years | Severe sepsis (abscess); Fecal diversion; Religious rituals | |
| 337 | Brown (1961) | 54/F | Sigmoid colon | CRAC | Primary tumor & liver metastases | 3 years | Not reported | |
| 338 | Brunschwig et al (1963) | 68/F | Rectum | CRAC | Local recurrence | 14 years | Not reported | |
| 339 | Mayo et al (1963) | 63/F | Descending colon | CRAC | Liver metastases | 16 years | Not reported | |
| 340 | Fullerton & Hill (1963) | 58/F | Transverse colon | Anaplastic CRAC | Primary tumor | 16 years | Not reported | |
| 341 | Rankin et al (1965) | 44/M | Rectum | CRAC | Liver metastases | 9 years | Not reported | |
| 342 | Margolis & West (1967) | 71/M | Rectum | CRAC | Peritoneal carcinomatosis | 1 year | Fecal diversion | |
| 343 | Synder et al (1968) | 62/F | Sigmoid colon | CRAC | Primary tumor with local extension | 15 years | Persistent high fever (wound infection); Immunologic; Genetic | |
| 344 | Synder et al (1968) | 60/M | Cecum | CRAC | Peritoneal carcinomatosis | 9 years | Severe Sepsis (abscess w/ fecal fistula); Immunologic; Genetic | |
| 345 | Weinstock (1977) | 40/F | Sigmoid colon | CRAC | Primary tumor with local extension & liver metastases | 20 years | Psychological | |
| 346 | Meares (1979) | 64/M | Rectum | CRAC | Primary tumor | 1 year | Intensive meditation | |
| 347 | Glasser et al (1979) | 36/M | Ascending colon | CRAC | Primary tumor & peritoneal carcinomatosis | 28 years | Genetic factors | |
| 348 | Beechy et al (1986) | 23/F | Ascending colon | CRAC | Primary tumor with local extension, peritoneal, & liver metastases | 4 years | Not reported | |
| 349 | Tominaga et al (1999) | 44/F | Transverse colon | CRAC | Primary tumor | 3 years | Dislodged | |
| 350 | Wadsworth et al (1999) | 67/M | Rectum | CRAC | Pulmonary metastases | 3 years | Infection (Pneumonia) | |
| 351 | Okamura et al (2000) | 54/M | Rectum | MALT Lymphoma | Primary tumor | Not reported | Not reported | |
| 352 | Kamesui et al (2000) | 66/F | Ascending colon | CRAC | Primary tumor | 1 year | Dislodged | |
| 353 | Takenaka et al (2000) | 76/F | Rectum | MALT Lymphoma | Primary tumor | 1.5 years | Not reported | |
| 354 | Ikuta et al (2002) | 60/M | Rectum | ASC | Liver metastases | 2 years | Interruption of blood supply; growth factors | |
| 355 | Abdelrazeq et al (2005) | 51/M | Rectum | CRAC | Local recurrence & peritoneal carcinomatosis | 16 years | Immunologic; Metabolic; Endocrine; Diversion of carcinogen | |
| 356 | Itano et al (2006) | 63/M | Rectum | MM | Primary tumor | 2 years | Dislodged | |
| 357 | Tomiki et al (2007) | 80/F | Rectum | CRAC | Primary tumor | 5 years | Dislodged | |
| 358 | Kochi et al (2008) | 80/M | Transverse colon | CRAC | Primary tumor | 3 years | Dislodged | |
| 359 | Bir et al (2009) | 86/F | Ascending colon | CRAC | Peritoneal lymph node metastases | 2 years | Immunologic | |
| 360 | Sakamoto et al (2009) | 80/M | Rectum | CRAC | Primary tumor | 3 months | Immunological | |
| 361 | Shimizu et al (2010) | 80/M | Transverse colon | CRAC | Primary tumor | 5 years | Physical stimulation (peristaltic movement; dislodged) | |
| 362 | Sakuma et al (2011) | 64/M | Sigmoid colon | CRAC | Primary tumor | 3 months* | Not reported | |
| 363 | Nakashima et al (2012) | 76/F | Ileocecal junction | CRAC | Primary tumor | 2 months | Dislodged | |
| 364 | Flynn et al (2013) | 36/M | Descending colon/rectum | DLBCL | Primary tumor | 6 months | Cessation of immunosuppressive therapy (infliximab & azathioprine) | |
| 365 | Flynn et al (2013) | 52/M | Sigmoid colon | HL | Primary tumor | 1 year | Cessation of immunosuppressive therapy (infliximab & azathioprine) | |
| 366 | Nakamura et al (2013) | 60/M | Rectum | CRAC | Primary tumor | 1.5 years | Biopsy; Immunological | |
| 367 | Sekiguchi et al (2013) | 76/F | Cecum | CRAC | Primary tumor | 1.5 months | Hyperimmunity & perioperative stress (lung surgery) | |
| 368 | Kihara et al (2014) | 64/M | Transverse colon | CRAC | Primary tumor | 1.5 months | Immunological | |
| 369 | Mitchell et al (2014) | 75/F | Cecum | MC | Regional lymph metastases | 1 year | Immunological | |
| 370 | Sewpaul et al (2014) | 35/F | Appendix (presumed) | GEP-NET (Carcinoid) | Primary tumor | 1 year | Pregnancy | |
| 371 | Kyoichi et al (2015) | 65/M | Transverse colon | CRAC | Primary tumor | 1.5 months | Drugs (Metformin); Immunological | |
| 372 | Serizawa et al (2015) | 75/M | Transverse colon | CRAC | Primary tumor | 2.5 months | Immunological; Apoptosis | |
| 373 | Ito et al (2016) | 73/M | Ascending colon | CRAC | Primary tumor | 3.5 months | Biopsy; mechanical stimulation (intestinal peristalsis) | |
| 374 | Nemésio et al (2016) | 51/F | Splenic angle | CRAC | Liver metastases | Not reported | Removal of Primary; Tumor necrosis | |
| 375 | Chida et al (2017) | 80/M | Transverse colon | CRAC | Primary tumor | 1 month | Immunological (CD4 +T-cells) | |
| 376 | Matsuki et al (2016) | 72/F | Ascending colon | CRAC | Liver metastases | 2 months | Infection (Biliary S/P pancreaticoduodenectomy) | |
| 377 | Chuang et al (2018) | 74/F | Occult | CRAC | Lung metastases | 2 months | Abscopal effect | |
| 378 | Yoshida et al (2018) | 73/M | Transverse colon | CRAC | Primary tumor | 2.5 months | Not reported | |
| 379 | Fukutomi et al (2019) | 87/F | Transverse colon | CRAC | Primary tumor | 1 month | Immunological (CD8+ T-cells) | |
| 380 | Karakuchi et al (2019) | 70/M | Transverse colon | CRAC | Primary tumor | 2 months | Immunological; Biopsy | |
| 381 | Kawakita et al (2019) | 62/M | Ascending colon | CRAC | Primary tumor | 1 month | Immunological (CD4 +T-cells) | |
| 382 | Tanaka et al (2019) | 63/F | Sigmoid colon | CRAC | Primary tumor | 2.5 months | Injection for non-lifting sign; Ischemia (vasoconstriction S/P epinephrine) | |
| 383 | Utsumi et al (2020) | 78/M | Ascending colon | CRAC | Primary tumor | 1 month | Immunological (dMMR) | |
| 384 | Utsumi et al (2020) | 66/M | Ascending colon | CRAC | Primary tumor | 1.5 months | Immunological (dMMR) | |
| 385 | Utsumi et al (2020) | 73/M | Ascending colon | CRAC | Primary tumor | 1.5 years | Immunological (dMMR) | |
| 386 | Mehawej et al (2020) | 18/F | Occult | CRAC | Primary tumor | Unknown | Not reported | |
| 387 | Nishiura et al (2020) | 67/F | Transverse colon | CRAC | Primary tumor | 3 months | Immunological | |
| 388 | Yokota et al (2020) | 76/F | Transverse colon | CRAC | Primary tumor | 2 months | Immunological | |
| 389 | Yokota et al (2020) | 64/F | Cecum | CRAC | Primary tumor | 3 months | Immunological | |
| 390 | Yokota et al (2020) | 64/M | Transverse colon | CRAC | Primary tumor | 1 month | Immunological |
| 1 | Roxburgh, D.: Spontaneous Regression of Cancer. Brit. M. J., 1: 39, 1935 |
| 2 | Grillet B, Demedts M, Roelens J, Goddeeris P, Fossion E. Spontaneous regression of lung metastases of adenoid cystic carcinoma. Chest. 1984 Feb;85(2):289-91. doi: 10.1378/chest.85.2.289. PMID: 6319089 |
| 3 | Grillet B, Demedts M, Roelens J, Goddeeris P, Fossion E. Spontaneous regression of lung metastases of adenoid cystic carcinoma. Chest. 1984 Feb;85(2):289-91. doi: 10.1378/chest.85.2.289. PMID: 6319089. |
| 4 | Grem, J. L., Hafez, G. R., Brandenburg, J. H. et a/. (1986) Spontaneous remission in diffuse large cell lymphoma. Cancer 57: 2042-2044. |
| 5 | Poppema S., Postma L., Brinker M., Jong B. Spontaneous regression of a small non-cleaved-cell malignant lymphoma (non-Burkitt’s lymphoblastic lymphoma): morphologic, immunohistochemical and immunoglobulin gene analysis. Cancer. 1988;62:791–794. |
| 6 | Savarrio L, Gibson J, Dunlop DJ, O'Rourke N, Fitzsimons EJ. Spontaneous regression of an anaplastic large cell lymphoma in the oral cavity: first reported case and review of the literature. Oral Oncol. 1999 Nov;35(6):609-13. doi: 10.1016/s1368-8375(99)00034-2. PMID: 10705098. |
| 7 | King M, Spooner D, Rowlands DC. Spontaneous regression of metastatic malignant melanoma of the parotid gland and neck lymph nodes: a case report and a review of the literature. Clin Oncol (R Coll Radiol). 2001;13(6):466-9. doi: 10.1053/clon.2001.9315. PMID: 11824888. |
| 8 | Notani, Kenichi & Shindoh, Masanobu & Takami, Tsuyoshi & Yamazaki, Yutaka & Kohgo, Takao & Fukuda, Hiroshi. (2002). Anaplastic Large Cell Lymphoma (ALCL) in the Oral Mucosa with Repeating Recurrence and Spontaneous Regression of Ulceration: Report of a case. Oral Medicine & Pathology. 7. 79-82. |
| 9 | Koga M, Kusukawa J, Hayabuchi N. Spontaneous regression of extranodal malignant lymphoma occurred in the gingiva. Oral Oncol. 2003 Apr;39(3):323-4. doi: 10.1016/s1368-8375(02)00122-7. PMID: 12618208. |
| 10 | Yamamoto, K., Ohgi, K., Yasumoto, J., Imai, Y., Kawakami, M., & Kirita, T. (2003). A case of spontaneous regression of malignant lymphoma of the upper gingiva. In Japanese) J Jpn Soc Oral Tumors, 15, 7-12. |
| 11 | Yokoyama T, Miyazawa K, Otawa M, Kawakubo K, Kuriyama Y, Serizawa H, Mukai K, Ohyashiki K. [Spontaneous regression of MALT lymphoma of the hard palate accompanied by Sjögren syndrome]. Rinsho Ketsueki. 2003 Jul;44(7):468-70. Japanese. PMID: 12931566. |
| 12 | Heibel H, Knödgen R, Bredenfeld H, Wickenhauser C, Scheer M, Zöller JE. Complete spontaneous remission of an aggressive non-Hodgkin's lymphoma with primary manifestation in the oral cavity. Leuk Lymphoma. 2004 Jan;45(1):171-4. doi: 10.1080/1042819031000139747. PMID: 15061215. |
| 13 | Lester R, Li C, Phillips P, Shenkier TN, Gascoyne RD, Galbraith PF, Vickars LM, Leitch HA. Improved outcome of human immunodeficiency virus-associated plasmablastic lymphoma of the oral cavity in the era of highly active antiretroviral therapy: a report of two cases. Leuk Lymphoma. 2004 Sep;45(9):1881-5. |
| 14 | Sakuma H, Okabe M, Yokoi M, Eimoto T, Inagaki H. Spontaneous regression of intraoral mucosa-associated lymphoid tissue lymphoma: molecular study of a case. Pathol Int. 2006 Jun;56(6):331-5. doi: 10.1111/j.1440-1827.2006.01967.x. PMID: 16704497. |
| 15 | Armstrong R, Bradrick J, Liu YC. Spontaneous regression of an HIV-associated plasmablastic lymphoma in the oral cavity: a case report. J Oral Maxillofac Surg. 2007 Jul;65(7):1361-4. doi: 10.1016/j.joms.2005.12.039. PMID: 17577503. |
| 16 | Kurita M, Hirano K, Ebihara S, et al. Spontaneous regression of cervical lymph node metastasis in a patient with mesopharyngeal squamous cell carcinoma of the tongue: possible association between apoptosis and tumor regression. Int J Clin Oncol. 2007; 12(6): 448- 454. |
| 17 | Oya, Ikemura K. Spontaneous regression of recurrent squamous cell carcinoma of the tongue. Int J Clin Oncol. 2004 Aug;9(4):339-42. doi: 10.1007/s10147-004-0404-6. |
| 18 | Rujirojindakul P, Kayasut K, Rohitoprakarn M, Lekhakula A. A unique case of transient spontaneous regression complicated with tumor lysis syndrome of T-cell lymphoblastic lymphoma in HIV-infected patient without antiretroviral therapy. J Med Assoc Thai. 2007 Sep;90(9):1930-3. PMID: 17957940. |
| 19 | Daly RM, Healy CM, Toner ME, Flint SR. Spontaneous regression of non-Hodgkin's lymphoma in the oral cavity after incisional biopsy. Br J Oral Maxillofac Surg. 2008 Apr;46(3):223-225. doi: 10.1016/j.bjoms.2007.03.010. Epub 2007 May 2. PMID: 17478018. |
| 20 | Mulder, D. C., Rosenberg, A. J. W. P., Storm-Bogaard, P. W., & Koole, R. (2010). Spontaneous regression of advanced Merkel-cell-like small cell carcinoma of the parotid gland. British Journal of Oral and Maxillofacial Surgery, 48(3), 199-200. |
| 21 | Brachet P, de Leval L, Chantrain G, Loeb I, Saussez S. Régression spontanée d'un lymphome B à grandes cellules du palais [Hard palate B cell lymphoma with spontaneous regression]. Rev Stomatol Chir Maxillofac. 2011 Jun;112(3):180-2. French. doi: 10.1016/j.stomax.2011.01.011. Epub 2011 Apr 8. Erratum in: Rev Stomatol Chir Maxillofac. 2012 Jun;113(3):199. Deleval, L [corrected to de Leval, L]. PMID: 21481900. |
| 22 | Corti M, Villafañe MF, Bistmans A, Campitelli A, Narbaitz M, Baré P. Oral cavity and extra-oral plasmablastic lymphomas in AIDS patients: report of five cases and review of the literature. Int J STD AIDS. 2011 Dec;22(12):759-63. |
| 23 | Tamás L, Sári E, Répássy G, Szabó P, Bagdi E, Krenács L, Demeter J. Spontaneous remission in localized diffuse large B-cell lymphoma. Pathol Oncol Res. 2011 Sep;17(3):779-84. doi: 10.1007/s12253-011-9379-6. Epub 2011 Jun 10. PMID: 21660524. |
| 24 | Fitzpatrick, S. G., Bowers, L. M., Al-Quran, S. Z., Fox, E. G., Cohen, D. M., & Bhattacharyya, I. ALK negative anaplastic large cell lymphoma of the oral cavity showing spontaneous regression. |
| 25 | García-Noblejas, A., Velasco, A., Cannata-Ortiz, J., & Arranz, R. (2012). Spontaneous regression of immunodeficiency associated plasmablastic lymphoma related to methotrexate after decrease of dosage. Medicina Clinica, 140(12), 569-570. |
| 26 | Choi, N., Cho, J. K., Baek, C. H., Ko, Y. H., & Jeong, H. S. (2014). Spontaneous regression of metastatic cancer cells in the lymph node: a case report. BMC research notes, 7(1), 1-6. |
| 27 | de Andrade Sousa A, Lopes Rena R, Souza Silva G, Marcos Arantes Soares J, Porcaro-Salles JM, Nunes L, Alves Mesquita R, Jham BC. Spontaneous remission of a squamous cell carcinoma of the floor of the mouth. J Craniomaxillofac Surg. 2014 Oct;42(7):1536-9. |
| 28 | Cuenca-Jimenez, T., Blanco-Guzman, M., & Holwill, S. (2015). Case report: extensive spontaneous regression of metastatic parotid keratinizing squamous cell carcinoma. British Journal of Oral and Maxillofacial Surgery, 53(10), e48-e49. |
| 29 | Igawa T, Sato Y, Kawai H, Kondo E, Takeuchi M, Miyata-Takata T, Takata K, Yoshino T. Spontaneous regression of plasmablastic lymphoma in an elderly human immunodeficiency virus (HIV)-negative patient. Diagn Pathol. 2015 Oct 6;10:183. doi: 10.1186/s13000-015-0421-y. PMID: 26445485; PMCID: PMC4596369. |
| 30 | Kaibuchi, N., Okamoto, T., Kataoka, T., Kumasaka, A., & Ando, T. (2015). A case of spontaneous regression of lymphoma in the mandibular gingiva after biopsy. Oral and Maxillofacial Surgery Cases, 1, 33-37. |
| 31 | Gonzalez-Perez, Luis Miguel & Borrero-Martín, Juan Jose. (2015). An elderly man with a gingival mass that spontaneously regressed. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology. 121. 348-352. |
| 32 | Wagner VP, Ortiz L, da Silva HP, Meurer L, da Cunha Filho JJ, Martins MA, Martins MD. Impact of highly active antiretroviral therapy in the development and remission of oral plasmablastic lymphoma. Indian J Dent Res. 2016 Sep-Oct;27(5):559-562. |
| 33 | Daroit, Natália & Silva, Viviane & Maraschin, Bruna & Visioli, Fernanda & Aleixo, Pedro & Oliveira, Márcia & Rados, Pantelis. (2017). Spontaneous Regression of an Oral Manifestation of Plasmablastic Lymphoma: Literature Review and Commentary about the Phenomena. British Journal of Medicine and Medical Research. 21. 1-7. |
| 34 | Kitamura, N., Mizobuchi, T., Ohno, S., Yoshizawa, Y., & Yamamoto, T. (2017). A case of AIDS-related plasmablastic lymphoma of the upper gingiva. Journal of Japanese Society of Oral Oncology, 29(4), 233–239. |
| 35 | Rajan, R., & Samant, S. (2008). Spontaneous regression of mucosal melanoma. Otol Head Neck Surg, 139(2 Suppl 1), 128. |
| 36 | Yao, M., Yoshioka, N., Hasegawa, K., Shimo, T., & Sasaki, A. (2017). A case of spontaneous regression of plasmablastic lymphoma in the upper gingiva. International Journal of Oral and Maxillofacial Surgery, 46, 315. |
| 37 | Miyagawa, F., Ogawa, K., & Asada, H. (2017). A case of CD4+/CD8+ double-positive primary cutaneous anaplastic large cell lymphoma of the lip involving spontaneous regression after biopsy. European Journal of Dermatology, 27(1), 68-69. |
| 38 | M F Vargas Gamarra, O Natsuki, M Flores, M Armengot Carceller, Spontaneous partial regression of low-grade mucoepidermoid carcinoma of the maxilla, Oxford Medical Case Reports, Volume 2018, Issue 7, July 2018, omy026 |
| 39 | Ono, K., Okui, T., Ibaragi, S., Kawai, H., Obata, K., Fujita, M., & Sasaki, A. (2019). A case of intraoral plasmablastic lymphoma spontaneously regressed after biopsy in HIV-negative patient. Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology. |
| 40 | Curioni OA, Andrade Filho P, Prates AJ, Rapoport A, Dedivitis RA. Spontaneous regression of adenocarcinoma of submandibular gland. Braz J Otorhinolaryngol. 2021 Jul-Aug;87(4):486-488. doi: 10.1016/j.bjorl.2020.10.014. Epub 2020 Nov 22. PMID: 33303413. |
| 41 | de Oliveira, A. A., Lee, I., & Teixeira, G. V. (2020). Spontaneous regression of oral mucosal malignant melanoma. Archives of Head and Neck Surgery, 49, 0-0. |
| 42 | Peeters M, Geusens J, Van der Cruyssen F, Michaux L, de Leval L, Tousseyn T, Vandenberghe P, Politis C. Case Report: Spontaneous Remission of an Infraorbital Follicular B-Cell Lymphoma: Case Report and Review of the Literature. Pathol Oncol Res. 2021 Apr 8;27:642433. doi: 10.3389/pore.2021.642433. PMID: 34257608; PMCID: PMC8262163. |
| 43 | Aoki Y, Hasegawa S, Miyabe S, Nagao T. Spontaneous regression of malignant lymphoma of the maxillary gingiva following biopsy. Int J Oral Maxillofac Surg. 2021 Sep 21:S0901-5027(21)00326-X. doi: 10.1016/j.ijom.2021.09.006. Epub ahead of print. PMID: 34561111. |
| 44 | Lau, K., Lee, C., Tustin, H., & Stafford, F. (2021). Spontaneous regression of advanced stage oropharyngeal squamous cell carcinoma. The Journal of Laryngology & Otology, 1-8. doi:10.1017/S0022215121002899 |
| 45 | Ueno, S., Yamada, K., Kamitani, M., & Shima, M. (2021). A Case of Oral Malignant Melanoma with Spontaneous Partial Regression. Japanese Journal of Oral Diagnosis / Oral Medicine, 34(3), 208–213. |
| 46 | Sousa LG, McGrail DJ, Li K, Marques-Piubelli ML, Gonzalez C, Dai H, Ferri-Borgogno S, Godoy M, Burks J, Lin SY, Bell D, Ferrarotto R. Spontaneous tumor regression following COVID-19 vaccination. J Immunother Cancer. 2022 Mar;10(3):e004371. doi: 10.1136/jitc-2021-004371. PMID: 35241495. |
| 47 | Rees, G.J.; Ross, C.M. Abscopal regression following radiotherapy for adenocarcinoma. Br J Radiol 1983, 56, 63-66, doi:10.1259/0007-1285-56-661-63. |
| 48 | Ohwada S, ~iyamoto Y, Fujii T, Oyama T, Joshita T, Izuo~. Spontaneous regression of esophageal carci- noma with pulmonary metastases: Case report. Jpn J Clin Onco11990; 20: 193-8. |
| 49 | Vergeau B, ~olinie C, Grandpierre G, Vindrios J. Spontaneous partial elimination of a carcinoma of the esophagus. Gastrointest Endosc 1991; 37: 591. |
| 50 | HAHM, K. B., SHIM, Y. J., YIM, D. S., KIM, W. H., MOON, Y. M., KANG, J. K., ... & PARK, C. I. (1993). Spontaneous Regression of Primary Malignant Lymphoma of the Esophagus. Korean Journal of Gastrointestinal Endoscopy, 335-339. |
| 51 | Saruki, K. et al. A Case of Spontaneous Regression of the Esophageal Cancer during Endoscopic Follow in 2 Years. Prog. Dig. Endosc. 45, 114–116 (1994). |
| 52 | Takemura, Osugi, Tokuhara, Kinoshita & Higashino. Case of spontaneous regression of metastatic lesions of leiomyosarcoma of the esophagus. Dis. Esophagus 12, 317–320 (1999). |
| 53 | Chang WYM (2000) Complete spontaneous regression of cancer: four case reports, review of literature, and discussion of possible mechanisms involved. Hawaii Med J 59:379–387 |
| 54 | Kubota, M. et al. Spontaneous regression in small cell esophageal carcinoma. Jpn. J. Thorac. Cardiovasc. Surg. 51, 660–664 (2003). |
| 55 | Hornby, S. T., Newman, P. A., Hassan, U. & Robb, W. B. Spontaneous Regression of Malignant Melanoma of the Gastro-Oesophageal Junction. Am. J. Cancer Case Rep. 3, 154–160 (2015). |
| 56 | Mitchell R, Kaur A, Munoh Kenne F, Khan A, Zafar W. Spontaneous Regression of Metastatic Lesions of Adenocarcinoma of the Gastro-Esophageal Junction. Cureus. 2021 Oct 14;13(10):e18784. doi: 10.7759/cureus.18784. PMID: 34796071; PMCID: PMC8590531. |
| 57 | Khan, H., Casey, P., Hayes, S., Tokala, A., & Sultan, J. (2021). Spontaneous regression of oesophageal squamous cell carcinoma. BMJ Case Reports CP, 14(6), e241344. |
| 58 | Takeuchi T, Itoh M et al. A case of early gastric reticulum cell sarcoma : follow-up study with x-ray and gastroendoscope during the early stage. I to Cho (Stomach and Intestine) 1971;6:211-9. (in Japanese) |
| 59 | Nakano H, Nakazawa S, Itoh Z et al. A case of reticulum cell sarcoma of the stomach. I to Cho (Stomach and Intestine) 1972 ; 7 : 375-82. (in Japanese) |
| 60 | Rosenberg, S. A., Fox, E. & Churchill, W. H. Spontaneous regression of hepatic metastases from gastric carcinoma. Cancer 29, 472–474 (1972). |
| 61 | Ohashi Y, Kumakura K, Sugiyama N et al. Early reticulum cell sarcoma of the stomach: report of a case showing remarkable changes within a short time. I to Cho (Stomach and Intestine) 1973;8:195- 203. (in Japanese) |
| 62 | Tietjen GW, McAllister FF. Spontaneous regression of gastric reticulum cell sarcoma. N Y State J Med. 1974 Apr;74(4):680-3. PMID: 4596541. |
| 63 | Yamazaki S, Nakaizumi H, Shirasaki S. A case of reticulum cell sarcoma of the stomach undergoing changes within a short time. I to Cho (Stomach and Intestine) 1974 ; 9 : 51-5. (in Japanese) |
| 64 | Kimura W, Ohtsubo K, Miura H, Kino K. Spontaneous regression of a carcinoma of the residual stomach with latent carcinoma of the pancreas: autopsy report. Jpn J Clin Oncol. 1987 Jun;17(2):187-92. PMID: 3613142. |
| 65 | Strauchen JA, Moran C, Goldsmith M, Greenberg M. Spontaneous regression of gastric lymphoma. Cancer. 1987 Oct 15;60(8):1872-5. doi: 10.1002/1097-0142(19871015)60:8<1872::aid-cncr2820600833>3.0.co;2-6. PMID: 3652015. |
| 66 | Strauchen JA, Moran C, Goldsmith M, Greenberg M. Spontaneous regression of gastric lymphoma. Cancer. 1987 Oct 15;60(8):1872-5. doi: 10.1002/1097-0142(19871015)60:8<1872::aid-cncr2820600833>3.0.co;2-6. PMID: 3652015. |
| 67 | Harvey, R. (1988). Spontaneous resolution of multifocal gastric enterochromaffin-like cell carcinoid tumours. The Lancet, 331(8589), 821. |
| 68 | Harvey, R. (1988). Spontaneous resolution of multifocal gastric enterochromaffin-like cell carcinoid tumours. The Lancet, 331(8589), 821. |
| 69 | Sawant PD, Nanivadekar SA, Shroff CP, Srinivas A, Dewoolkar VV. Spontaneous regression of large gastric carcinoid. Indian J Gastroenterol. 1989 Oct;8(4):289-90. PMID: 2689331. |
| 70 | Shigematsu A, Iida M, Lien GS, Imamura T, Okada M, Fuchigami T, Fujishima M, Itoh H, Iwashita A. Spontaneous regression of primary malignant lymphoma of the stomach in two nontreated Japanese. J Clin Gastroenterol. 1989 Oct;11(5):511-7. doi: 10.1097/00004836-198910000-00006. PMID: 2794430. |
| 71 | Shigematsu A, Iida M, Lien GS, Imamura T, Okada M, Fuchigami T, Fujishima M, Itoh H, Iwashita A. Spontaneous regression of primary malignant lymphoma of the stomach in two nontreated Japanese. J Clin Gastroenterol. 1989 Oct;11(5):511-7. doi: 10.1097/00004836-198910000-00006. PMID: 2794430. |
| 72 | Rebollo J, Llorente I, Yoldi A. Regresión tumoral espontánea en un paciente con cáncer gástrico metastásico. Communicación de un caso adicional [Spontaneous tumor regression in a patient with metastatic gastric cancer. Communication of an additional case]. Rev Med Univ Navarra. 1990 Jul-Sep;34(3):141-2. Spanish. PMID: 2151656. |
| 73 | Yoshimine T, Hamada T, Kubota H et aE. Primary malignant lymphoma of the stomach with spontane- ous regression, report of a case. I to Cho (Stomach and Intestine) 1991;26 :814-8. |
| 74 | HAYAKAWA, K., FUKUCHI, S., YOSHIDA, Y., HASHIMOTO, M., HOSHIHARA, Y., MIYAKOSHI, S., ... & UNAKAMI, M. (1992). A Case of Spontaneous Regression of Primary Malignant Lymphoma of the Stomach. Digestive Endoscopy, 4(3), 267-273. |
| 75 | Takehara Y, Haruma K, Tsuda T, Sumii K, Kajiyama G. Apparent spontaneous regression of primary malignant lymphoma of the stomach. Endoscopy. 1992 Oct;24(8):735-6. doi: 10.1055/s-2007-1010573. PMID: 1425471. |
| 76 | MATSUSAKI, K., KAWANO, T., MIURA, O., TODA, T., MINAMISONO, Y., NAGASAKI, S., & YAO, T. (1996). Primary malignant lymphoma of the stomach with a complete and spontaneous regression in a short period-a case report. The journal of the Japanese Practical Surgeon Society, 57(9), 2198-2202. |
| 77 | Ogawa D, Uemura N, Sasaki N, et al. Spontaneous regression of malignant lymphoma of the stomach. Journal of Medicine. 1998 ;29(5-6):381-393. PMID: 10503173. |
| 78 | Bariol C, Field A, Vickers CA, et al (2001) Regression of gastric T cell lymphoma with eradication of Helicobacter pylori. Gut 48:269–271 |
| 79 | Salam, I., Durai, D., Murphy, J. K., & Sundaram, B. (2001). Regression of primary high-grade gastric B-cell lymphoma following Helicobacter pylori eradication. European journal of gastroenterology & hepatology, 13(11), 1375-1378. |
| 80 | Pentimone F, Moncini C, Pastine F, Gerini A, Lucchesi Q. Spontaneous regression and recurrence of primary low-grade B-cell gastric lymphoma on the gastric stump 15 and 20 years after gastroresection. Panminerva Med. 2002 Sep;44(3):271-4. PMID: 12094145. |
| 81 | Chung, H. W., Son, D. K., Ji, J. S., Chung, D. Y., Chung, J. S., Kim, J. I., ... & Sun, H. S. (2003). A case of spontaneous regression of advanced gastric cancer. Korean Journal of Gastrointestinal Endoscopy, 216-219. |
| 82 | Watanabe N, Okazaki K, Yazumi S, Ohana M, Uchida K, Chiba T. Spontaneous regression of Epstein-Barr virus-associated T-cell lymphoma of the stomach. Gastrointest Endosc. 2003 Mar;57(3):414-7. doi: 10.1067/mge.2003.132. PMID: 12612533. |
| 83 | Watari, J., Saitoh, Y., Fujiya, M. et al. Spontaneous remission of primary diffuse large B-cell gastric lymphoma. J Gastroenterol 40, 414–420 (2005). |
| 84 | Watari, J., Saitoh, Y., Fujiya, M. et al. Spontaneous remission of primary diffuse large B-cell gastric lymphoma. J Gastroenterol 40, 414–420 (2005). |
| 85 | Ohno Y, Kosaka T, Muraoka I, et al. Remission of primary low-grade gastric lymphomas of the mucosa-associated lymphoid tissue type in immunocompromised pediatric patients. World J Gastroenterol 2006;12:2625-2628. |
| 86 | Lee HS, Cheung DY, Kim JI, Cho SH, Park SH, Han JY, Kim JK. A case of spontaneous regression of advanced gastric cancer. J Korean Med Sci. 2010 Oct;25(10):1518-21. doi: 10.3346/jkms.2010.25.10.1518. Epub 2010 Sep 20. PMID: 20890436; PMCID: PMC2946665. |
| 87 | Ip, Y.-T., Pong, W.-M., Kao, S.-S. & Chan, J. K. C. Spontaneous Complete Regression of Gastric Large-Cell Neuroendocrine Carcinoma: Mediated by Cytomegalovirus-Induced Cross-Autoimmunity? Int. J. Surg. Pathol. 19, 355–358 (2011). |
| 88 | A Patient with Distinct Evidences of Spontaneous Regression and Recurrence of Gastric Cancer for 13 Years. (2012). Ann Geriatr Med Res, 16(3), 149–152. doi:10.4235/jkgs.2012.16.3.149 |
| 89 | Rojas-Hernandez, C. M., Coleman, J. F., Czuchlewski, D. R. & Fekrazad, M. H. Spontaneous regression of high grade primary gastric Lymphoma in an untreated viral hepatitis infection. Leuk. Lymphoma 55, 2643–2645 (2014). |
| 90 | Shibata, M., Matsubayashi, H., Todaka, A., Kurai, H., Tsutsumi, N., Sasaki, K., & Ono, H. (2016). The Disappearance of Lymph Node Metastasis from Neuroendocrine Carcinoma after Endoscopic Ultrasound-guided Fine Needle Aspiration. Internal Medicine, 55(19), 2805–2809. |
| 91 | Sugiyama, T., Arita, K., Shinno, E., & Nakajima, T. (2018). Spontaneous remission of diffuse large B cell lymphoma in the stomach and the continuation of remission for 10 years. Case reports in gastroenterology, 12(3), 699-703. |
| 92 | Bonilla, C.E.; Esguerra, J.; Mendoza Díaz, S.; Álvarez, A.; Morales, R.L. Abscopal Effect after Palliative Radiotherapy in a Patient with a Gastric Adenocarcinoma Disseminated to Retroperitoneal Space: Case Report from a Latin American Reference Center and Review of the Literature. Cureus 2019, 11, e6235. |
| 93 | Hatsuse M, Ide D, Matsui S, Fuchida SI, Murakami S, Shimazaki C. [Spontaneous regression of primary gastric EBV-positive diffuse large B-cell lymphoma]. Rinsho Ketsueki. 2019;60(11):1573-1576. Japanese. |
| 94 | Okamoto T, Yoshimoto T, Ohike N, Fujikawa A, Kanie T, Fukuda K. Spontaneous regression of gastric gastrinoma after resection of metastases to the lesser omentum: A case report and review of literature. World J Gastroenterol. 2021 Jan 7;27(1):129-142. doi: 10.3748/wjg.v27.i1.129. PMID: 33505155; PMCID: PMC7789063. |
| 95 | Zafar M, Paracha AW, Ashraf M, Muhammad T, Whitehead M, Toqeer M. Delayed Spontaneous Regression of Metastatic Gastric Cancer: A Case Report of a Rare Finding. Cureus. 2021 Dec 7;13(12):e20224. doi: 10.7759/cureus.20224. PMID: 34900506; PMCID: PMC8649674. |
| 96 | Schwartz, E., Maayan, C., Mouallem, M., Engelberg, S., & Friedman, E. (1991). Malignant peritoneal mesothelioma: Long‐term spontaneous clinical remission. Medical and pediatric oncology, 19(4), 325-328. |
| 97 | BaniHani MN, Al Manasra AR. Spontaneous regression in alveolar soft part sarcoma: case report and literature review. World J Surg Oncol. 2009 Jun 10;7:53. doi: 10.1186/1477-7819-7-53. PMID: 19515237; PMCID: PMC2703639. |
| 98 | Jagodic, M. (2010, July). 3. P. 35 “SPONTANEOUS” REGRESSION OF ADVANCED RETROPERITONEAL LEIOMYOSARCOMA AFTER THE END OF SALVAGE CHEMOTHERAPY. In Orthopaedic Proceedings (Vol. 92, No. SUPP_III, pp. 447-447). The British Editorial Society of Bone & Joint Surgery. |
| 99 | Gottfried EB, Steller R, Paronetto F, Lieber CS. Spontaneous regression of hepatocellular carcinoma. Gastroenterology 1982; 82:770– 4. |
| 100 | Lam KC, Ho JC, Yeung RT. Spontaneous regression of hepatocellular carcinoma: a case study. Cancer. 1982 Jul 15;50(2):332-6. doi: 10.1002/1097-0142(19820715)50:2<332::aid-cncr2820500228>3.0.co;2-o. PMID: 6282440. |
| 101 | McCaughan GW, Bilous MJ, Gallagher ND (1985) Long-term survival with tumor regression in androgen-induced liver tumors. Cancer 56:2622–2626 |
| 102 | McCaughan GW, Bilous MJ, Gallagher ND (1985) Long-term survival with tumor regression in androgen-induced liver tumors. Cancer 56:2622–2626 |
| 103 | Sato Y, Fujiwara K, Nakagawa S, Kanishima S, Ohta Y, Oka Y, Hayashi S, Oka H. A case of spontaneous regression of hepatocellular carcinoma with bone metastasis. Cancer. 1985 Aug 1;56(3):667-71. doi: 10.1002/1097-0142(19850801)56:3<667::aid-cncr2820560339>3.0.co;2-s. PMID: 2408740. |
| 104 | Takayasu K, Muramatsu Y, Shima Y, Moriyama N, Yamada T, Yoshida T, Makuuchi M, Kishi K. Necrosis of hepatocellular carcinoma as a result of subintimal injury incurred by hepatic angiography: report of two cases. Am J Gastroenterol. 1986 Oct;81(10):979-83. PMID: 3020974. |
| 105 | Takayasu K, Muramatsu Y, Shima Y, Moriyama N, Yamada T, Yoshida T, Makuuchi M, Kishi K. Necrosis of hepatocellular carcinoma as a result of subintimal injury incurred by hepatic angiography: report of two cases. Am J Gastroenterol. 1986 Oct;81(10):979-83. PMID: 3020974. |
| 106 | Andreola S, Audisio RA, Mazzaferro V, Doci R, Milella M: Spontaneous massive necrosis of a hepatocellular carcinoma. Tumori 1987;73:203–207. |
| 107 | Royuela, S.F., Prolonged course of hepatocellular carcinoma A spontaneous regression. Gastroenterlogia Y. Hepatologia, 1989. 12(10). |
| 108 | Suzuki, M., et al., Spontaneous Regression of Hepatocellular Carcinoma--a case report, Suzuki M, Okazaki, Hepatogastroenterology, 1989. pdf. New York, 1989. 36: p. 160-163. |
| 109 | Ayres, R.C., et al., Spontaneous regression of hepatocellular carcinoma. Gut, 1990. 31(6): p. 722-4. |
| 110 | Gaffet, M.J. and J.P. Joyce, . Cancer, 1990. |
| 111 | Tocci, G., et al., Spontaneous remission of hepatocellular carcinoma after massive gastrointestinal haemorrhage Coffee consumption as trigger for insulin-dependent diabetes mellitus in childhood. 1988. 300. |
| 112 | Mochizuki, T. and Y. Takehara, Regression of hepatocellular carcinoma. AJR, 1991. |
| 113 | Masakazu Yamamoto, Ken Takasaki, Takaho Watayō, et al. Spontaneous Necrosis Due to a Hepatocellular Carcinoma. Cancer clinical 1991; 37: 491-496 |
| 114 | Masakazu Yamamoto, Ken Takasaki, Takaho Watayō, et al. Spontaneous Necrosis Due to a Hepatocellular Carcinoma. Cancer clinical 1991; 37: 491-496 |
| 115 | Chien, R.-N., T.J. Chen, and Y.-F. Liaw, Spontaneous Regression of Hepatocellular Carcinoma,Chien RN, Chen TJ, Liaw YF, American Journal Gastroenterology, 1992.pdf. 1992, The American Journal of Gastroenterology. |
| 116 | Imaoka, S. and Y. Sasaki, Necrosis of hepatocellular Carcinoma Caused by Spontaneously Arising Arterial Thrombus 1994. Hepato-Gastroenterology, 1994. 41: p. 359-362. |
| 117 | McDermott, W.V. and U. Khettry, Clear cell carcinoma of the liver with spontaneous regression of metastases. J Surg Oncol, 1994. 57(3): p. 206-209. |
| 118 | Grossmann M, Hoermann R, Weiss M, Jauch KW, Oertel H, Staebler A, Mann K, Engelhardt D. Spon- taneous regression of hepatocellular carcinoma. Am J Gastroenterol 1995; 90: 1500-1503 |
| 119 | Herrera A, Erdozain JC, Molina E, Conde P, Palomo V. Spontaneous regression of hepatocellular carcinoma. Am J Gastroenterol 1996; 91:1288 – 1289. |
| 120 | Ozeki Y, Matsubara N, Tateyama K, Kokubo M, Shimoji H, Katayama M. Spontaneous complete necrosis of hepatocellular carcinoma. Am J Gastroenterol. 1996;91:391–2. |
| 121 | Markovic S, Ferlan-Marolt V, Hlebanja Z. Spontaneous regression of hepatocellular carcinoma. Am J Gastroenterol. 1996;91:392–3. |
| 122 | Yoshimitsu, K., Honda, H., Kaneko, K., Fukuya, T., Irie, H., Aibe, H., ... & Masuda, K. (1996). Temporary spontaneous regression of intrahepatic cholangiocarcinoma. Computerized medical imaging and graphics, 20(2), 115-118. |
| 123 | Iwasaki, M., et al., Spontaneous regression of hepatocellular carcinoma: a case report. Japanese journal of clinical oncology, 1997. 27(4): p. 278-281. |
| 124 | Van Halteren, H.K., J.M.J.I. Salemans, and H. Peters. Journal of Hepatology, 1997. 27: p. 211-215. |
| 125 | Kaczynski, J., et al., Spontaneous regression of hepatocellular carcinoma Case report. 1998: p. 147-150. |
| 126 | Ohba, K., et al., Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut, 1998. 43(4): p. 575-577. |
| 127 | Magalotli, D., C. Gueli, and M. Zoli, Transient spontaneous regression of hepatocellular carcinoma. 1998. p. 2369-2371. |
| 128 | Magalotli, D., C. Gueli, and M. Zoli, Transient spontaneous regression of hepatocellular carcinoma. 1998. p. 2369-2371. |
| 129 | Gómez Sanz R, Moreno Gonzalez E, Colina Ruiz- Delgado F, Garcia-Muñoz H, Ochando Cerdan F, Gonzalez-Pinto I. Spontaneous regression of a re- current hepatocellular carcinoma. Dig Dis Sci 1998; 43: 323-328 |
| 130 | Stoelben E, Koch M, Hanke S, Lossnitzer A, Gaert- ner HJ, Schentke KU, Bunk A, Saeger HD. Spontaneous regression of hepatocellular carcinoma confirmed by surgical specimen: report of two cases and review of the literature. Langenbecks Arch Surg 1998; 383: 447-452 |
| 131 | Stoelben E, Koch M, Hanke S, Lossnitzer A, Gaert- ner HJ, Schentke KU, Bunk A, Saeger HD. Spontaneous regression of hepatocellular carcinoma confirmed by surgical specimen: report of two cases and review of the literature. Langenbecks Arch Surg 1998; 383: 447-452 |
| 132 | Takeuchi, K., Nishino, H., Ohira, M., & Sowa, M. (1998). A case of hepatocellular carcinoma with massive spontaneous necrosis presented unusual image. Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association), 59(6), 1614–1618. |
| 133 | Itoh, T., Kanamaru, T., Morita, Y., Yamamoto, M., Kuroda, Y., Hayashi, Y., & Itoh, H. (1999). A Case Report of Spontaneous Complete Necrosis of Hepatocellular Carcinoma. The Japanese Journal of Gastroenterological Surgery, 32(1), 32–35. |
| 134 | Toyoda H, Sugimura S, Fukuda K, Mabuchi T. Hepa- tocellular carcinoma with spontaneous regression of multiple lung metastases. Pathol Int 1999; 49: 893-897 |
| 135 | Izuishi K, Ryu M, Hasebe T, Kinoshita T, Konishi M, Inoue K. Spontaneous total necrosis of hepatocellular carcinoma. Hepatogastroenterology. 2000;47:1122–4. |
| 136 | Jang TJ, Lee JI, Kim DH, Kim JR, Lee HK. Spontane- ous regression of hepatocellular carcinoma--a case report. Korean J Intern Med 2000; 15: 147-150 |
| 137 | Lee HS, Lee JS, Woo GW, Yoon JH, Kim CY. Recur- rent hepatocellular carcinoma after spontaneous regression. J Gastroenterol 2000; 35: 552-556 |
| 138 | Lee HS, Lee JS, Woo GW, Yoon JH, Kim CY. Recur- rent hepatocellular carcinoma after spontaneous regression. J Gastroenterol 2000; 35: 552-556 |
| 139 | Takeda Y, Togashi H, Shinzawa H, Miyano S, Ishii R, Karasawa T, Takeda Y, Saito T, Saito K, Haga H, Matsuo T, Aoki M, Mitsuhashi H, Watanabe H, Takahashi T. Spontaneous regression of hepatocellular carcinoma and review of literature. J Gastroen- terol Hepatol 2000; 15: 1079-1086 |
| 140 | Terasaki T, Hanazaki K, Shiohara E, Matsunaga Y, Koide N, Amano J. Complete disappearance of recurrent hepatocellular carcinoma with peritoneal dissemination and splenic metastasis: a unique clinical course after surgery. J Gastroenterol Hepatol 2000; 15: 327-330 |
| 141 | Uenishi T, Hirohashi K, Tanaka H, Ikebe T, Kinoshi- ta H. Spontaneous regression of a large hepatocellular carcinoma with portal vein tumor thrombi: report of a case. Surg Today 2000; 30: 82-85 |
| 142 | Ikeda M, Okada S, Ueno H, et al. Spontaneous regression of hepatocellular carcinoma with multiple lung metastases: a case report. Jpn J Clin Oncol 2001; 31 (9): 454―458 |
| 143 | Jung, D. Y., Kim, Y. B., Lee, Y. H., Lee, Y. K., Kim, J. R., Bae, Y. O., & Cho, I. S. (2001). A Case of Spontaneous Regression of Metastatic Hepatocellular Carcinoma. The Korean Journal of Gastroenterology, 38(6), 436-439. |
| 144 | Kawai, M., Hara, H., Dohi, T., Morita, S., Tanigawa, N., Takeshita, A., … Shibayama, Y. (2001). Spontaneous complete coagulative necrosis of hepatocellular carcinoma. Kanzo, 42(9), 471–476. |
| 145 | Matsuo R, Ogata H, Tsuji H, Kitazono T, Shimada M, Taguchi K, et al. Spontaneous regression of hepatocellular carcinoma. Hepatogastroenterology. 2001 Nov–Dec;48(42):1740–2. |
| 146 | Nakai T, Shimomura T, Hirokawa F. Spontaneous 33 regression of recurrent hepatocellular carcinoma after TAE: possible mechanisms of immune mediation. Int J Clin Oncol 2001; 6: 149-152 |
| 147 | Sakurai Y, Shoji M, Matsubara T, Suganuma M, Hasegawa S, Imazu H, Ochiai M, Funabiki T, Urano M, Mizoguchi Y, et al. Spontaneous necrosis of gallbladder carcinoma in patient with pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg. 2001;8:95–100. |
| 148 | Garrido Serrano A, Guerrero Igea FJ, Lepe Jiménez JA, Palomo Gil S. [Spontaneous regression of hepatocellular carcinoma in a cirrhotic patient]. Gastro- enterol Hepatol 2001; 24: 503-505 |
| 149 | Abiru S, Kato Y, Hamasaki K, Nakao K, Nakata K, Eguchi K. Spontaneous regression of hepatocellular carcinoma associated with elevated levels of inter- leukin 18. Am J Gastroenterol 2002; 97: 774-775 |
| 150 | Abiru S, Kato Y, Hamasaki K, Nakao K, Nakata K, Eguchi K. Spontaneous regression of hepatocellular carcinoma associated with elevated levels of inter- leukin 18. Am J Gastroenterol 2002; 97: 774-775 |
| 151 | Abiru S, Kato Y, Hamasaki K, Nakao K, Nakata K, Eguchi K. Spontaneous regression of hepatocellular carcinoma associated with elevated levels of inter- leukin 18. Am J Gastroenterol 2002; 97: 774-775 |
| 152 | Lee SC, Chung HW, Chung JB, Park YN, Ahn SH, Park SW, Chun CY, Moon YM, Kang JK, Park IS. Total necrosis of hepatocellular carcinoma due to spontaneous occlusion of feeding artery. Yonsei Med J 2002; 43: 123-127 |
| 153 | Misawa K, Hata Y, Manabe K, Matsuoka S, Saito M, Takada J, Sano F. Spontaneous regression of hepatocellular carcinoma. J Gastroenterol 1999; 34: 410-414 |
| 154 | Morimoto Y, Tanaka Y, Itoh T, Yamamoto S, Mizuno H, Fushimi H. Spontaneous necrosis of hepatocellular carcinoma. Dig Surg. 2002;19:413–8. |
| 155 | Zimmermann, A., Kappeler, A., Friess, H., & Büchler, M. W. (2002). Hepatocellular carcinoma with an unusual medullary-like histology and signs of regression (“medullary-like hepatocellular carcinoma”). Digestive and Liver Disease, 34(10), 748-753. |
| 156 | Iiai T, Sato Y, Nabatame N, Yamamoto S, Makino S, Hatakeyama K. Spontaneous complete regression of hepatocellular carcinoma with portal vein tumor thrombus. Hepatogastroenterology. 2003 Sep–Oct;50(53):1628–30. |
| 157 | Jozuka H, Jozuka E, Suzuki M, Takeuchi S, Takatsu Y . Psycho-neuro-immunological treatment of hepatocellular carcinoma with major depression--a single case report. Curr Med Res Opin 2003; 19: 59-63 |
| 158 | Li AJ, Wu MC, Cong WM, Shen F, Yi B. Spontaneous complete necrosis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2003 Feb;2(1):152–4. |
| 159 | Ohta H, Sakamoto Y, Ojima H, Yamada Y, Hibi T, Takahashi Y, et al. Spontaneous regression of hepatocellular carcinoma with complete necrosis. Abdom Imaging. 2005 Nov–Dec;30(6):734–7. |
| 160 | Blondon H, Fritsch L, Cherqui D. Two cases of spontaneous regression of multicentric hepatocellular carcinoma after intraperitoneal rupture: possible role of immune mechanisms. Eur J Gastroenterol Hepatol 2004; 16 (12): 1355―1359 |
| 161 | Blondon H, Fritsch L, Cherqui D. Two cases of spontaneous regression of multicentric hepatocellular carcinoma after intraperitoneal rupture: possible role of immune mechanisms. Eur J Gastroenterol Hepatol 2004; 16 (12): 1355―1360 |
| 162 | Cheng HM, Tsai MC. Regression of hepatocellular carcinoma spontaneous or herbal medicine related? Am J Chin Med 2004; 32 (4): 579―585 |
| 163 | Erturk, S., Yuceyar, S., Yigitbasi, R., Onur, E., Saner, H., & Uygun, N. (2004). Spontaneous regression of hepatocellular carcinoma. A case report. CHIRURGIA-TORINO-, 17(3), 95-98. |
| 164 | Feo CF, Marrosu A, Scanu AM, et al. Spontaneous regression of hepatocellular carcinoma: report of a case. Eur J Gastroenterol Hepatol 2004; 16 (9): 933― 936 |
| 165 | Kato H, Nakamura M, Muramatsu M, Orito R, Ueda R, Mizokami M. Spontaneous regression of hepatocellular carcinoma: two case reports and a literature review. Hepatol Res 2004; 29:180-190 |
| 166 | Kato H, Nakamura M, Muramatsu M, Orito R, Ueda R, Mizokami M. Spontaneous regression of hepatocellular carcinoma: two case reports and a literature review. Hepatol Res 2004; 29:180-190 |
| 167 | Lin TJ, Liao LY, Lin CL, et al. Spontaneous regression of hepatocellular carcinoma: a case report and literature review. Hepatogastroenterology 2004; 51 (56): 579―582 |
| 168 | Nakajima T, Moriguchi M, Watanabe T, et al. Recurrence of hepatocellular carcinoma with rapid growth after spontaneous regression. World J Gastroenterol 2004 Nov 15; 10 (22): 3385―3387 |
| 169 | Jeon SW, Lee MK, Lee YD, et al. Clear cell hepatocellular carcinoma with spontaneous regression of primary and metastatic lesions. Korean J Intern Med 2005; 20 (3): 268―273 |
| 170 | Moon TG, Joon Hyeok Lee, Moon Seok Choi, Kwang Cheol Koh, Jae J. Kim, Seung Woon Paik, Byung Cheol Yoo, Jong Chul Rhee. A Case of Complete Remission after Multiple Sessions of Local Treatment in Metastatic Hepatocellular Carcinoma. (2006). J Liver Cancer, 6(1), 70–76. |
| 171 | Nam SW, Han JY, Kim JI, et al. Spontaneous regression of a large hepatocellular carcinoma with skull metastasis. J Gastroenterol Hepatol 2005 ; 20 ( 3 ) : 488―492 |
| 172 | Nouso K, Uematsu S, Shiraga K, et al. Regression of hepatocellular carcinoma during vitamin K ad-ministration. World J Gastroenterol 2005; 11 ( 42 ) : 6722―6724 |
| 173 | Ohtani H, Yamazaki O, Matsuyama M, Horii K, Shimizu S, Oka H, et al. Spontaneous regression of hepatocellular carcinoma. Surg Today. 2005;35(12):1081–6. |
| 174 | Randolph AC, Tharalson EM, Gilani N. Spontaneous regression of hepatocellular carcinoma is possible and might have implications for future therapies. Eur J Gastroenterol Hepatol 2008; 20 (8): 804― 809 |
| 175 | Rizell, M., et al., Impressive regression of primary liver cancer after treatment with sirolimus. Acta Oncologica, 2005. 44(5): p. 496-496. |
| 176 | Yano Y, Yamashita F, Kuwaki K, et al. Partial spontaneous regression of hepatocellular carcinoma: a case with high concentrations of serum lens culinaris agglutinin-reactive alpha-fetoprotein. Ku- rume Med J 2005; 52 (3): 97―103 |
| 177 | Otrock, Z. K., Al-Kutoubi, A., Kattar, M. M., Zaatari, G., & Soweid, A. (2006). Spontaneous complete regression of hepatic epithelioid haemangioendothelioma. The lancet oncology, 7(5), 439-441. |
| 178 | Kojima H, Tanigawa N, Kariya S, Komemushi A, Shomura Y, Sawada S, Arai E, Yokota Y. A case of spontaneous regression of hepatocellular carcinoma with multiple lung metastases. Radiat Med. 2006 Feb;24(2):139-42. doi: 10.1007/BF02493281. PMID: 16715676. |
| 179 | Kondo S, Okusaka T, Ueno H, et al. Spontaneous regression of hepatocellular carcinoma. Int J Clin Oncol 2006; 11 (5): 407―411 |
| 180 | Kondo S, Okusaka T, Ueno H, et al. Spontaneous regression of hepatocellular carcinoma. Int J Clin Oncol 2006; 11 (5): 407―411 |
| 181 | Kondo S, Okusaka T, Ueno H, et al. Spontaneous regression of hepatocellular carcinoma. Int J Clin Oncol 2006; 11 (5): 407―411 |
| 182 | Kondo S, Okusaka T, Ueno H, et al. Spontaneous regression of hepatocellular carcinoma. Int J Clin Oncol 2006; 11 (5): 407―411 |
| 183 | Shibuya, K., Bando, T., Onishi, Y., Nagata, T., Yamagishi, F., & Tsukada, K. (2006). A case of spontaneous complete necrosis of hepatocellular carcinoma. Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association), 67(9), 2152–2156. |
| 184 | Heianna J, Miyauchi T, Suzuki T, Ishida H, Hashi- moto M, Watarai J. Spontaneous regression of multiple lung metastases following regression of hepatocellular carcinoma after transcatheter arterial embolization. A case report. Hepatogastroenterology 2007; 54: 1560-1562 |
| 185 | Matsunaga, K., Uesaka, K., Maeda, A., Kanemoto, H., & Furukawa, H. Spontaneous Regression of Sarcomatous Hepatocellular Carcinoma-Report of a Case. |
| 186 | Meza-Junco J, Monta!o-Loza AJ, Martinez-Benítez B, et al. Spontaneous partial regression of hepatocellular carcinoma in a cirrhotic patient. Ann Hepatol 2007; 6 (1): 66―69 |
| 187 | Peddu P, Huang D, Kane PA, et al. Vanishing liver tumours. Clin Radiol 2008; 63 (3): 329―339 |
| 188 | Vardhana HG, Panda M. Spontaneous regression of hepatocellular carcinoma: potential promise for the future. South Med J 2007; 100 (2): 223―224 |
| 189 | Arakawa Y, Mori H, Ikegami T, Hanaoka J, Kanamoto M, Kanemura H, et al. Hepatocellular carcinoma with spontaneous regression: report of the rare case. Hepatogastroenterology. 2008 Sep–Oct;55(86–87):1770–2. |
| 190 | Hori T, Wagata T, Takemoto K, Shigeta T, Takuwa H, Hata K, Uemoto S, Yokoo N. Spontaneous necrosis of solid gallbladder adenocarcinoma accompanied with pancreaticobiliary maljunction. World J Gastroenterol. 2008 Oct 14;14(38):5933-7. |
| 191 | Sibartie V, Moriarty J, Crowe J. Spontaneous regression of hepatocellular carcinoma. Am J Gastroenterol. 2008 Apr;103(4):1050-1. doi: 10.1111/j.1572-0241.2007.01772_14.x. PMID: 18397439. |
| 192 | Del Poggio P, Mattiello M, Gilardoni L, et al. The mysterious case of spontaneous disappearance of hepatocellular carcinoma. Dig Liver Dis 2009; 41 (7): e21―25 |
| 193 | Hsu CY, Sun PL, Chang HC, Perng DS, Chen YS. Spontaneous regression of advanced hepatocellular carcinoma: a case report. Cases J 2009; 2: 6251 |
| 194 | Kanzaki, A., Hibino, S., Nishi, T., Kawagoe, T., Ito, A., & Sakakibara, S. (2009). A case of spontaneous complete necrosis of hepatocellular carcinoma. Kanzo, 50(5), 244–249. |
| 195 | Nishijima N, Marusawa H, Kita R, et al. Education and Imaging. Hepatobiliary and pancreatic: spontaneous regression of hepatocellular cancer demonstrated by contrast-enhanced ultrasonography. J Gastroenterol Hepatol 2009; 24 (6): 1153 |
| 196 | Oquiñena S, Iñarrairaegui M, Vila JJ, et al. Spontaneous regression of hepatocellular carcinoma: three case reports and a categorized review of the literature. Dig Dis Sci 2009; 54 (5): 1147―1153 |
| 197 | Oquiñena S, Iñarrairaegui M, Vila JJ, et al. Spontaneous regression of hepatocellular carcinoma: three case reports and a categorized review of the literature. Dig Dis Sci 2009; 54 (5): 1147―1153 |
| 198 | Oquiñena S, Iñarrairaegui M, Vila JJ, et al. Spontaneous regression of hepatocellular carcinoma: three case reports and a categorized review of the literature. Dig Dis Sci 2009; 54 (5): 1147―1153 |
| 199 | Park HS, Jang KY, Kim YK, et al. Hepatocellular carcinoma with massive lymphoid infiltration: a regressing phenomenon? Pathol Res Pract 2009; 205 (9): 648―652 |
| 200 | Harada, T., Sakaguchi, T., Inaba, K., Nakamura, T., Kurachi, K., Fukazawa, A., … Konno, H. (2010). Spontaneous regression of hepatocellular carcinomas. Nippon Shokakibyo Gakkai Zasshi, 107(3), 432–441. |
| 201 | Hong JH, Seo DD, Jeon TJ, Oh TH, Shin WC, Choi WC, Cho HS. [A case of spontaneous regression of hepatocellular carcinoma with multiple lung metastases]. Korean J Gastroenterol. 2010 Feb;55(2):133-8. Korean. doi: 10.4166/kjg.2010.55.2.133. PMID: 20168060. |
| 202 | Kai, K., Miyoshi, A., Ario, K., Kitahara, K., Mizuta, T., Kawazoe, S., … Miyazaki, K. (2010). The two cases of massive spontaneous necrosis of hepatocellular carcinoma after angiography. Kanzo, 51(6), 312–318. |
| 203 | Kai, K., Miyoshi, A., Ario, K., Kitahara, K., Mizuta, T., Kawazoe, S., … Miyazaki, K. (2010). The two cases of massive spontaneous necrosis of hepatocellular carcinoma after angiography. Kanzo, 51(6), 312–318. |
| 204 | 佐藤政弘. プロスタグランジン合成阻害剤ケトプロフェンにより一過性の腫瘍退縮現象を生じたと考えられる肝細胞癌の1例. 秋田県医師会雑誌 2010; 60: 96 |
| 205 | Storey RE, Huerta AL, Khan A, Laber DA. Spontaneous complete regression of hepatocellular carcinoma. Med Oncol. 2011 Dec;28(4):948–50. |
| 206 | Alqtub A, Peck D, Marotta P. Spontaneous regression of large hepatocellular carcinoma: case report. Ger Med Sci 2011; 9: Doc07 |
| 207 | Arora, N., & Madhusudhana, S. (2011). Spontaneous regression of hepatocellular cancer: case report and review of literature. Gastrointestinal Cancer Research: GCR, 4(4), 141. |
| 208 | Fukushima, H., Shibayama, T., Hishiki, S., Yamamoto, C., & Okada, Y. (2011). A case of hepatocellular carcinoma (HCC) with multiple lung metastases, with disappearance of both the primary HCC and lung metastases after transcatheter arterial chemoembolization (TACE) for the primary HCC and no recurrence for more than 7 years after the TACE. |
| 209 | Maejima R, Nakayama H, Horii T, Tosa M, Dairaku N, Shiina M, Dairaku N, Oriuchi T, Ikeda T, Ueno T, Kusano M, Ikeya S, Hiwatashi N. [A case of spontaneous complete necrosis of hepatocellular carcinoma sized 10cm in diameter demonstrated by the resected specimen]. Nihon Shokakibyo Gakkai Zasshi. 2011 Nov;108(11):1902-9. |
| 210 | Okano A, Takakuwa H, Nakamura T, et al. Spontaneous regression of diffuse intrahepatic recurrence with portal vein tumor thrombus after resection of hepatocellular carcinoma. Clin J Gastroenterol 2011; 4: 43―48 |
| 211 | Okuma, K., Yamashita, H., Niibe, Y., Hayakawa, K., & Nakagawa, K. (2011). Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: a case report. Journal of medical case reports, 5(1), 1-4. |
| 212 | Bastawrous, S., M.J. Kogut, and P. Bhargava, Spontaneous regression of hepatocellular carcinoma in a cirrhotic patient: Possible vascular hypothesis. Singapore Medical Journal, 2012. 53(10): p. 2-5. |
| 213 | Harimoto, N., et al., Spontaneous regression of multiple pulmonary recurrences of hepatocellular carcinoma after hepatectomy: Report of a case. Surgery Today, 2012. 42(5): p. 475-478. |
| 214 | Komatsu H, Imamura S, Shimizu T, et al. Clinical Journal of Gastroenterology 2012; 5: 35―41 |
| 215 | Nakayama S. Spontaneous regression of hepatocellular carcinoma. Indian J Gastroeterol 2012 ; 31 :267―270 |
| 216 | Takeura C, Tokoro T, Tanahashi Y, Yoshida J, Kagawa T, Kato Y, et al. Two cases of spontaneous regression of hepatocellular carcinoma with extrahepatic metastasis. 2012. Poster session at the 2012 American HepatoPancreatoBiliary Association Annual Meeting, Miami, FL. |
| 217 | Takeura C, Tokoro T, Tanahashi Y, Yoshida J, Kagawa T, Kato Y, et al. Two cases of spontaneous regression of hepatocellular carcinoma with extrahepatic metastasis. 2012. Poster session at the 2012 American HepatoPancreatoBiliary Association Annual Meeting, Miami, FL. |
| 218 | Yamamoto, S., Tokuhara, T., Nishikawa, M., Nishizawa, S., Nishioka, T., Nozawa, A., … Kubo, S. (2012). Spontaneous regression of hepatocellular carcinoma after improving diabetes mellitus: possibly responsible for immune system. Kanzo, 53(3), 164–174. |
| 219 | Yokoyama T, Yoshida H, Hirakata A, Makino H, Maruyama H, Suzuki S, et al. Spontaneous complete necrosis of advanced hepatocellular carcinoma. J Nippon Med Sch. 2012;79(3):213–7. |
| 220 | Katayama, Y., Morinaga, S., Numata, M., Godai, T., Masuda, M., & Akaike, M. (2013). A Case of Resection for Complete Spontaneous Necrosis of Hepatocellular Carcinoma. Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association), 74(10), 2863–2868. |
| 221 | Okano A, Ohana M, Kusumi F, et al. Spontaneous regression of hepatocellular carcinoma due to dis- ruption of the feeding artery. Case Rep Oncology 2013; 6: 180―185 |
| 222 | Sasaki, T., Fukumori, D., Yamamoto, K., Yamamoto, F., Igimi, H., & Yamashita, Y. (2013). Management considerations for purported spontaneous regression of hepatocellular carcinoma: a case report. Case Reports in Gastroenterology, 7(1), 147-152. |
| 223 | Tomishige, H., Morise, Z., Mizoguchi, Y., Kawabe, N., Nagata, H., Ohshima, H., ... & Isetani, M. (2013). A case of solitary necrotic nodule treated with laparoscopic hepatectomy: spontaneous regression of hepatocellular carcinoma?. Case Reports in Hepatology, 2013. |
| 224 | 植田 茂, 調 憲, 山下洋市, 他. 1度自然消退するも4年後に再燃した肝細胞癌の1切除例. 臨床と研究 2013; 90: 131-134 |
| 225 | Bhardwaj, N., Li, M., Price, T., & Maddern, G. J. (2014). Spontaneous regression of a biopsy confirmed hepatocellular carcinoma. Case Reports, 2014, bcr2014204897. |
| 226 | F. Chiesara, A. Spagnolo, M. Koch, and A. Moretti, “A case of hepatocellular carcinoma: spontaneous regression?,” Digestive and Liver Disease, vol. 46, no. 7, pp. 659-660, 2014. |
| 227 | Masafumi Inoue, Takuya Kimura, Yasuo Matsuda, et al. A case of spontaneous necrosis of hepatocellular carcinoma: possible induction by medication. Rinsho Geka 2014; 69: 377-381 |
| 228 | Lim, D. H., Park, K. W., & Lee, S. I. (2014). Spontaneous complete regression of multiple metastases of hepatocellular carcinoma: A case report. Oncology letters, 7(4), 1225-1228. |
| 229 | Miyake, M., Sugita, M., Mogaki, M., Fukushima, T., Masui, H., Nagahori, K., & Tsuura, Y. (2014). A Case of Spontaneous Complete Necrosis of Hepatocellular Carcinoma. Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association), 75(7), 1972–1978. |
| 230 | Saito R, Amano H, Abe T, Fujikuni N, Nakahara M, Yonehara S, Teramen K, Noriyuki T. Complete spontaneous necrosis of hepatocellular carcinoma confirmed on resection: A case report. Int J Surg Case Rep. 2016;22:70-4. |
| 231 | Tomino T, Yamashita Y, Iguchi T, et al. Spontane- ous massive necrosis of hepatocellular carcinoma with narrowing and occlusion of the arteries and portal veins. Case Rep Gastroenterol 2014; 8: 148― 155 |
| 232 | Tsai SC, Kao JL, Shiao CC. Spontaneous regression of a hepatoma with ring calcification. Acta Clin Belg. 2014;69:130–131. |
| 233 | Zhao, X.Y., Rakhda, M.I., Habib, S., Bihi, A., Muhammad, A., Wang, T.L., & Jia, J. (2014). Hepatic epithelioid hemangioendothelioma: A comparison of Western and Chinese methods with respect to diagnosis, treatment and outcome. Oncology Letters, 7, 977-983. |
| 234 | Parks AL, McWhirter RM, Evason K, Kelley RK. Cases of spontaneous tumor regression in hepatobiliary cancers: implications for immunotherapy? J Gastrointest Cancer. 2015;46:161–165. |
| 235 | Parks AL, McWhirter RM, Evason K, Kelley RK. Cases of spontaneous tumor regression in hepatobiliary cancers: implications for immunotherapy? J Gastrointest Cancer. 2015;46:161–165. |
| 236 | Parks AL, McWhirter RM, Evason K, Kelley RK. Cases of spontaneous tumor regression in hepatobiliary cancers: implications for immunotherapy? J Gastrointest Cancer. 2015;46:161–165. |
| 237 | Kim SB, Kang W, Shin SH, Lee HS, Lee SH, Choi GH, et al. Spontaneous neoplastic remission of hepatocellular carcinoma. Korean J Gastroenterol. 2015 May;65(5):312–5. |
| 238 | 幸田昌樹, 杉浦 愛, 森 弘樹. 経過中に一時自然退縮を認めた肝細胞癌の1例. 浜松医療センター学術誌 2015; 9: 130-133 |
| 239 | 桑野哲史, 宮ケ原典, 多喜研太郎, 他. 自然退縮が疑われた肝腫瘤の1例. 診断と治療 2015; 103: 697-701 |
| 240 | Matsuoka S, Tamura A, Moriyama M, Fujikawa H, Mimatsu K, Oida T, Sugitani M. Pathological evidence of the cause of spontaneous regression in a case of resected hepatocellular carcinoma. Intern Med. 2015;54:25–30. |
| 241 | Okano A, Ohana M. Spontaneous regression of hepatocellular carcinoma: its imaging course leading to complete disappearance. Case Rep Oncol 2015; 8: 94―100 |
| 242 | 菅本祐司, 太田拓実, 木村正幸, 他. 肥満患者の減量中に自然退縮した肝細胞癌の1例. 千葉医学 2015; 91: 59-63 |
| 243 | Takeda Y, Wakui N, Asai Y, Dan N, Yamauchi Y, Ueki N, et al. Spontaneous complete necrosis of hepatocellular carcinoma: a case report and review of the literature. Oncol Lett. 2015 Apr;9(4):1520–6. |
| 244 | Tazawa, H., Fukuda, S., Fujisaki, S., Sakimoto, H., Eto, T., Moriya, T., … Nishida, T. (2015). Suggesting of spontaneous complete necrosis of hepatocellular carcinoma: Report of a case. Kanzo, 56(12), 645–654. |
| 245 | Verla-Tebit E, Rahma OE. Regression of hepatocellular carcinoma after treatment of hepatitis C: a case report. J Gastrointest Oncol. 2015 Jun;6(3):E52-4. |
| 246 | Wang Z, Ke ZF, Lu XF, Luo CJ, Liu YD, Lin ZW, Wang LT. The clue of a possible etiology about spontaneous regression of hepatocellular carcinoma: a perspective on pathology. Onco Targets Ther 2015; 8: 395-400 |
| 247 | Yang SZ, Zhang W, Yuan WS, Dong JH. Recurrence of Hepatocellular Carcinoma With Epithelial-Mesenchymal Transition After Spontaneous Regression: A Case Report. Medicine (Baltimore) 2015;94:e1062. |
| 248 | Yoo YJ, Kim JH. [Spontaneous Complete Remission of Hepatocellular Carcinoma]. Korean J Gastroenterol. 2015 Dec;66(6):359-62. Korean. doi: 10.4166/kjg.2015.66.6.359. PMID: 27175457. |
| 249 | Gunasekaran SS, Emmadi R, Landers LA, Gaba RC. Regression of Hepatocellular Carcinoma Lung Metastases after Guyabano Fruit Extract Consumption. J Diet Suppl. 2016;13(3):237-44. doi: 10.3109/19390211.2015.1008613. Epub 2015 Feb 9. PMID: 25664807. |
| 250 | Heron V, Fortinsky KJ, Spiegle G, Hilzenrat N, Szilagyi A. Resected hepatocellular carcinoma in a patient with Crohn’s disease on azathioprine. Case Rep Gastroenterol 2016;10:50-6. |
| 251 | Jianxin C, Qingxia X, Junhui W, Qinhong Z. A Case of Recurrent Hepatocellular Carcinoma Acquiring Complete Remission of Target Lesion With Treatment With Traditional Chinese Medicine. Integr Cancer Ther. 2016 Epub ahead of print. |
| 252 | Kumar A, Le DT. Hepatocellular Carcinoma Regression After Cessation of Immunosuppressive Therapy. J Clin Oncol. 2016;34:e90–e92. |
| 253 | Kumar A, Le DT. Hepatocellular Carcinoma Regression After Cessation of Immunosuppressive Therapy. J Clin Oncol. 2016;34:e90–e92. |
| 254 | Luo, S. C., Wu, C. C., Jheng, S. B., & Huang, Z. Y. (2016). Spontaneous remission of hepatocellular carcinoma without any treatment. Journal of Cancer Research and Practice, 3(4), 128-131. |
| 255 | Mahmood, Shafaq & Gul, Aleesha & Saif, Tayyaba. (2016). Regression of hepatocellular carcinoma after treatment with Sofosbuvir - A case report. JPMA. The Journal of the Pakistan Medical Association. 66. 1507-1509. |
| 256 | Ooka, Y., Chiba, T., Inoue, M., Wakamatsu, T., Saito, T., Sekimoto, T., … Yokosuka, O. (2016). A case of hepatocellular carcinoma with spontaneous regression of a tumor thrombus invading the main portal trunk. Kanzo, 57(4), 178–185. |
| 257 | Pectasides E, Miksad R, Pyatibrat S, Srivastava A, Bullock A. Spontaneous regression of hepatocellular carcinoma with multiple lung metastases: A case report and review of the literature. Dig Dis Sci 2016;61:2749-54. |
| 258 | Sawatsubashi, Y., Abe, Y., Nishihara, K., Takesue, S., Nakashima, Y., & Nakano, T. (2016). A Spontaneous Complete Regression of Hepatocellular Carcinoma. The Japanese Journal of Gastroenterological Surgery, 49(6), 488–495. |
| 259 | 杉浦 玄.高齢女性に発症し自然退縮した肝細胞が んの 1 例.内科 2016;117:329―331 |
| 260 | Alam MA, Das D. Spontaneous Regression of Hepatocellular Carcinoma-a Case Report. J Gastrointest Cancer. 2017;48:194–197. |
| 261 | Iwatani, Y., Kuroda, D., Abe, T., Kohama, T., Urade, T., Murata, K., … Oka, S. (2017). A case of complete spontaneous necrosis with residual intrahepatic metastasis of hepatocellular carcinoma. Kanzo, 58(3), 170–175. |
| 262 | Murata, R., Kamiyama, T., Kanno, H., Yokoo, H., Orimo, T., Wakayama, K., … Taketomi, A. (2017). Spontaneous Complete Regression of a Hepatocellular Carcinoma with Hepatic Vein Tumor Thrombosis. The Japanese Journal of Gastroenterological Surgery, 50(7), 535–543. |
| 263 | Noij DP, van Der Linden PW.Spontaneous regression of hepatocellular carcinoma in a Caucasian male patient: A case report and review of the literature. Mol Clin Oncol 2017;6:225-8. |
| 264 | 大山 潤,山下 航,川田秀一,他.肝細胞癌の自 然退縮と考えられた 2 例.臨床放射線 2017;62: 469―473 |
| 265 | 大山 潤,山下 航,川田秀一,他.肝細胞癌の自 然退縮と考えられた 2 例.臨床放射線 2017;62: 469―474 |
| 266 | Sakamaki A, Kamimura K, Abe S, et al. Spontaneous regression of hepatocellular carcinoma: A mini-review. World J Gastroenterol 2017; 23 (21): 3797― 3804 |
| 267 | Sano I, Kuwatani M, Sugiura R, Kato S, Kawakubo K, Ueno T, Nakanishi Y, Mitsuhashi T, Hirata H, Haba S, Hirano S, Sakamoto N. Hepatobiliary and Pancreatic: A rare case of a well-differentiated neuroendocrine tumor in the bile duct with spontaneous regression diagnosed by EUS-FNA. |
| 268 | 山口晃典,藪崎哲史,加藤扶美,他.肝細胞癌の自 然退縮と考えられた 4 症例.臨床放射線 2017;62: 781―789 |
| 269 | 山口晃典,藪崎哲史,加藤扶美,他.肝細胞癌の自 然退縮と考えられた 4 症例.臨床放射線 2017;62: 781―789 |
| 270 | 山口晃典,藪崎哲史,加藤扶美,他.肝細胞癌の自 然退縮と考えられた 4 症例.臨床放射線 2017;62: 781―789 |
| 271 | 山口晃典,藪崎哲史,加藤扶美,他.肝細胞癌の自 然退縮と考えられた 4 症例.臨床放射線 2017;62: 781―789 |
| 272 | El-Badrawy A, Abd El-Wahab R, Emarah Z, Eisa N. Rapid Spontaneous Regression of Diffuse Large B-Cell Lymphoma after Fine Needle Aspiration Cytology: A Case Report. Clin Oncol. 2018; 3: 1455. |
| 273 | Goto Y, Uchino Y, Sasaki S, et al. Complete spontaneous necrosis of hepatocellular carcinoma accompanied by portal vein tumor thrombosis: A case report. Int J Surg Case Rep 2018; 44: 220―225 |
| 274 | Koya Y, Suzuki T, Tai M, Ichii O, Matsuhashi N, Ejiri Y, et al. Spontaneous regression of hepatocellular carcinoma with portal vein tumor thrombus. Case Rep Gastroenterol 2018;12:411-9. |
| 275 | Lee J, Lee N, Yoon K, et al. Cystic degeneration of hepatocellular carcinoma mimicking mucinous cystic neoplasm. Korean J Gastroenterol 2019; 73: 303―307 |
| 276 | Taniai T, Shirai Y, Shiba H, Sakamoto T, Furukawa K, Yanaga K. Spontaneous pathological complete regression of hepatocellular carcinoma. Case Rep Gastroenterol 2018;12:653-9. |
| 277 | Taniguchi, M., Sakuma, Y., Koizumi, M., Sasanuma, H., Lefor, A. K., & Sata, N. (2018). Spontaneous Complete Necrosis of Hepatocellular Carcinoma—A Case Report—. Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association), 79(9), 1922–1927. |
| 278 | Alhatem, A., Badeti, S., Liu, C., Liu, D., & Cai, D. (2019). Spontaneous Regression of Hepatic Diffuse Large B-cell Lymphoma in HIV and HCV Positive Patient: A Novel Case Study. Int J Cancer Clin Res, 6, 127. |
| 279 | M.B.Y. Chohan, N. Taylor, C. Coffin, K.W. Burak, O.F. Bathe, Spontaneous regression of hepatocellular carcinoma and review of reports in the published English literature, Case Rep. Med. 2019 (2019), 9756758. |
| 280 | Koichi Fujikawa, Ken Fujishiro, Harufumi Makino, et al. Spontaneous regression of multiple hepatocellular carcinoma and peritoneal disseminations. Rinsho Geka 2019; 74: 763-767 |
| 281 | Masashi Hirota, Hiroki Murakami, Koichi Omoto, et al. A case of hepatocellular carcinoma with spontaneous regression caused by temperance and non-smoking. Surgery 2019; 81: 284-287 |
| 282 | Kim, J.O.; Kim, C.A. Abscopal Resolution of a Hepatic Metastasis in a Patient with Metastatic Cholangiocarcinoma Following Radical Stereotactic Body Radiotherapy to a Synchronous Early Stage Non-small Cell Lung Cancer. Cureus 2019, 11, e4082. |
| 283 | Kawaguchi T, Nakano D, Okamura S, et al. Spontaneous regression of hepatocellular carcinoma with reduction in angiogenesis-related cytokines after treatment with sodium-glucose cotransporter 2 inhibitor in a cirrhotic patient with diabetes mellitus. Hepatology Research 2019; 49: 479―486 |
| 284 | Lee J, Lee N, Yoon K, et al. Cystic degeneration of hepatocellular carcinoma mimicking mucinous cystic neoplasm. Korean J Gastroenterol 2019; 73: 303―307 |
| 285 | Yoshida, T., Yoshida, S., Yamagishi, T., Yamane, H., Matsumoto, T., Kobayashi, A., … Tominaga, M. (2019). Clear Cell Type of Hepatocellular Carcinoma with a Slow Progression Accompanied by Natural Necrosis. The Japanese Journal of Gastroenterological Surgery, 52(9), 513–520. |
| 286 | Arjunan, V., Hansen, A., Deutzmann, A., Sze, D. Y., & Dhanasekaran, R. (2021). Spontaneous Regression of Hepatocellular Carcinoma: When the Immune System Stands Up to Cancer. Hepatology (Baltimore, Md.), 73(4), 1611-1614. |
| 287 | Arjunan, V., Hansen, A., Deutzmann, A., Sze, D. Y., & Dhanasekaran, R. (2021). Spontaneous Regression of Hepatocellular Carcinoma: When the Immune System Stands Up to Cancer. Hepatology (Baltimore, Md.), 73(4), 1611-1614. |
| 288 | Arjunan, V., Hansen, A., Deutzmann, A., Sze, D. Y., & Dhanasekaran, R. (2021). Spontaneous Regression of Hepatocellular Carcinoma: When the Immune System Stands Up to Cancer. Hepatology (Baltimore, Md.), 73(4), 1611-1614. |
| 289 | Arjunan, V., Hansen, A., Deutzmann, A., Sze, D. Y., & Dhanasekaran, R. (2021). Spontaneous Regression of Hepatocellular Carcinoma: When the Immune System Stands Up to Cancer. Hepatology (Baltimore, Md.), 73(4), 1611-1614. |
| 290 | Costa-Santos, M. P., Gonçalves, A., Ferreira, A. O., & Nunes, J. (2020). Spontaneous regression of hepatocellular carcinoma: myth or reality? BMJ Case Reports CP, 13(2), e233509. |
| 291 | Hokkoku D, Morimoto O, Takiuchi D, Wada R, Ikeshima R, Wada N, Munakata K, Akamaru Y, Ota H, Shibata K, Ohashi H. [A Case Report of Hepatocellular Carcinoma with Complete Spontaneous Regression]. Gan To Kagaku Ryoho. 2020 Dec;47(13):2385-2387. Japanese. |
| 292 | Muroya, D., Sato, T., Sakai, H., Hisaka, T., Akagi, Y., & Okuda, K. (2021). Spontaneous regression of lung metastases in hepatocellular carcinoma: A case report. International Journal of Surgery Case Reports, 78, 378-381. |
| 293 | Nakamoto R, Okuyama C, Oka S. Complete Spontaneous Regression of Hepatosplenic T-Cell Lymphoma After Surgical Biopsy. Clin Nucl Med. 2020 Feb;45(2):e88-e91. |
| 294 | Ohmatsu, K.; Hashimoto, Y.; Kawanishi, M.; Ishii, Y.; Kono, S.; Kuribayashi, S.; Ariizumi, S.; Karasawa, K. Abscopal complete regression of hepatocellular carcinoma with multiple pleural metastases. Int. Cancer Conf. J. 2021, 10, 54–58. |
| 295 | Onishi, Y., Kusumoto, M., Motoi, N., Hiraoka, N., Sugawara, S., Itou, C., & Sone, M. (2021). Natural history of epithelioid hemangioendothelioma of the liver: CT findings of 15 cases. Academic Radiology, 28(6), 778-782. |
| 296 | Onishi, Y., Kusumoto, M., Motoi, N., Hiraoka, N., Sugawara, S., Itou, C., & Sone, M. (2021). Natural history of epithelioid hemangioendothelioma of the liver: CT findings of 15 cases. Academic Radiology, 28(6), 778-782. |
| 297 | Onishi, Y., Kusumoto, M., Motoi, N., Hiraoka, N., Sugawara, S., Itou, C., & Sone, M. (2021). Natural history of epithelioid hemangioendothelioma of the liver: CT findings of 15 cases. Academic Radiology, 28(6), 778-782. |
| 298 | Onishi, Y., Kusumoto, M., Motoi, N., Hiraoka, N., Sugawara, S., Itou, C., & Sone, M. (2021). Natural history of epithelioid hemangioendothelioma of the liver: CT findings of 15 cases. Academic Radiology, 28(6), 778-782. |
| 299 | Onishi, Y., Kusumoto, M., Motoi, N., Hiraoka, N., Sugawara, S., Itou, C., & Sone, M. (2021). Natural history of epithelioid hemangioendothelioma of the liver: CT findings of 15 cases. Academic Radiology, 28(6), 778-782. |
| 300 | Onishi, Y., Kusumoto, M., Motoi, N., Hiraoka, N., Sugawara, S., Itou, C., & Sone, M. (2021). Natural history of epithelioid hemangioendothelioma of the liver: CT findings of 15 cases. Academic Radiology, 28(6), 778-782. |
| 301 | Raufi, A. G., May, M., Greendyk, R. A., Iuga, A., Ahmed, F., Mansukhani, M., & Manji, G. A. (2020). Spontaneous Regression and Complete Response to Immune Checkpoint Blockade in a Case of High-Grade Neuroendocrine Carcinoma. JCO Precision Oncology, (4), 1006–1011. |
| 302 | 坂本愛子,高木慎太郎,福原崇之,他.自然壊死肝 細胞癌の 2 例.広島医学 2020;73:573―576 |
| 303 | 坂本愛子,高木慎太郎,福原崇之,他.自然壊死肝 細胞癌の 2 例.広島医学 2020;73:573―577 |
| 304 | Sonbare D, J, Bandi R, Sharma V, Cacciarelli T, Shaikh O, S: Spontaneous Regression of Advanced Hepatocellular Carcinoma. Case Rep Gastroenterol 2020;14:491-496. |
| 305 | Joseph W. Franses, Irun Bhan, Amaya Pankaj, David T. Ting, Vikram Deshpande, and Kenneth Tanabe: Spontaneous Immune-Mediated Regression of Hepatocellular Carcinoma With High Tumor Mutational Burden. JCO Precision Oncology 2021:5, 1040-1043 |
| 306 | Kakuta, N., Yasumoto, T., Yoshida, Y., Tsuda, M., Miyazaki, A., Miyamoto, S., ... & Naito, M. (2021). Spontaneous regression of lung metastases after transarterial chemoembolization for hepatocellular carcinoma. Radiology Case Reports, 16(6), 1530-1534. |
| 307 | Kimura T, Goi T, Yokoi S, Ohnishi K, Togawa T, Iida A, Ishida M, Sato Y. Possible spontaneous regression of hepatocellular carcinoma with unique histopathological features confirmed by surgical resection: a case report. Surg Case Rep. 2021 Jul 13;7(1):162. doi: 10.1186/s40792-021-01246-z. PMID: 34255193; PMCID: PMC8276907. |
| 308 | Liu Z, Zou JW, Wang WL, Li Z. Spontaneous regression of recurrent hepatocellular carcinoma with multiple lung metastases. J Cancer Res Ther. 2020;16(7):1710-1713. |
| 309 | Obu, M., Ishii, K., Oki, Y., Fujiya, M., Yonemoto, T., Takahashi, T., … Azemoto, R. (2022). A case of spontaneous regression of a rapidly-grown hepatocellular carcinoma. Kanzo, 63(2), 51–61. |
| 310 | Tanaka, Y., Asai, S., Hashimoto, A., & Shinzato, I. (2020). Hepatic Hodgkin Lymphoma Presenting as Solitary Hepatic Mass Following Other Iatrogenic Immunodeficiency-Associated Lymphoproliferative Disorder in a Patient With Rheumatoid Arthritis. Journal Of Medical Cases, 12(2), 79-83. |
| 311 | Shapiro SL. Spontaneous regression of cancer. Eye Ear Nose Throat Mon. 1967 Oct;46(10):1306-10. PMID: 6076351. |
| 312 | Lokich JJ, Brooks JR. Disappearance of disseminated pancreatic carcinoma with combined chemotherapy. Ann Surg. 1973;177:13–14. |
| 313 | Eidemiller LR, Fletcher WS, Dennis DL, et al. . Spontaneous remission of proven cancer. Northwest Med 1971;70:539–43. |
| 314 | Tchertkoff V, Hauser AD. Carcinoma of head of pancreas with spontaneous regression. N Y State J Med 1974;74:1814–7. |
| 315 | Gunn HD, Cann SAH, Gunn HD, et al. . Spontaneous regression of pancreatic cancer. 2016. |
| 316 | Chin KM, Chan CY, Lee SY. Spontaneous regression of pancreatic cancer: a case report and literature review. Int J Surg Case Rep. 2018;42: 55–59. |
| 317 | Sreevathsa MR, Koirala N (2018) Three decades of survival in Pancreatic Neuroendocrine Tumor with Unresectable Liver Metastases. Ann Pancreat Disord Treatm 2(1): 002-006. |
| 318 | Saade Lemus P, Anderson K, Smith M, Bullock A. Spontaneous regression of pancreatic cancer with liver metastases. BMJ Case Rep. 2019 May 31;12(5):e229619. |
| 319 | Ibrahimi S, Mukherjee S, Alhyari L, Rubin E, Aljumaily R. Spontaneous Regression of Metastatic Pancreatic Cancer: A Role for Recurrent Inflammation. Pancreas. 2019 Jan;48(1):e4-e6. |
| 320 | Kawaguchi S, Ohtsu T, Enokida K, Terada S, Endo S, Shirane N, Kanemoto H, Taku K, Muramatsu A. [A case of poorly differentiated pancreatic adenocarcinoma with spontaneous regression wherein retrospective rim enhancement findings were important for diagnosis]. Nihon Shokakibyo Gakkai Zasshi. 2021;118(4):358-365. Japanese. doi: 10.11405/nisshoshi.118.358. PMID: 33840717. |
| 321 | Sroujieh AS. Spontaneous regression of intestinal malignant melanoma from an occult primary site. Cancer. 1988 Sep 15;62(6):1247-50. |
| 322 | Nagashima R, Takeda H, Maeda K, et al. Regression of duodenal mucosa-associated lymphoid tissue lymphoma after eradication of Helicobacter pylori. Gastroenterology 111: 1674-1678, 1996. |
| 323 | Rayson D, Pitot HC, Kvols LK. Regression of metastatic carcinoid tumor after valvular surgery for carcinoid heart disease. Cancer. 1997 Feb 1;79(3):605-11. PMID: 9028374. |
| 324 | Horiuchi, T., Nomura, J., Okuda, M., & Ichinohasama, R. (2003). Abscopal effect of small intestinal NK/T-cell lymphoma. Rinsho Ketsueki, 44(9), 940–945. |
| 325 | Makino Y, Suzuki H, Nishizawa T, Kameyama K, Hisamatsu T, Imaeda H, Mukai M, Hibi T. Ileal Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma with a Large-Cell Component That Regressed Spontaneously. Gut Liver. 2010 Mar;4(1):117-21. |
| 326 | Hayashi, H., Onishi, Y., Mitsuoka, H., Ogura, T., Maeda, M., Nishigami, T., & Harada, M. (2013). Regression of Follicular Lymphoma of the Duodenum Following Eradication of H. pylori Infection. Internal Medicine, 52(23), 2611–2614. |
| 327 | Tanaka, K., Ohtsuka, M., Shimizu, H., Yoshidome, H., Kato, A., Furukawa, K., Yoshitomi, H., Kishimoto, T., Nakatani, Y., & Miyazaki, M. (2014). Spontaneous Regression of Duodenal Cancer with a Metastatic Liver Cancer. The Japanese Journal of Gastroenterological Surgery, 47, 11-17. |
| 328 | Sasaki J, Kurihara H, Nakano Y, Kotani K, Tame E, Sasaki A. Apparent spontaneous regression of malignant neoplasms after radiography: Report of four cases. Int J Surg Case Rep. 2016;25:40-3. doi: 10.1016/j.ijscr.2016.05.049. Epub 2016 May 31. |
| 329 | Hori S, Tachihara M, Tamura D, Kobayashi K, Nakata K, Kamiryo H, Sakai Y, Itoh T, Hirose T, Nishimura Y. Spontaneous Regression of Epithelioid Angiosarcoma in a Young Woman. Intern Med. 2017 Dec 15;56(24):3333-3339. doi: 10.2169/internalmedicine.6754-15. Epub 2017 Oct 11. |
| 330 | Tanaka Y, Ishihara M, Miyoshi H, Hashimoto A, Shinzato I, Ohshima K. Spontaneous regression of diffuse large B-cell lymphoma in the small intestine with multiple lymphadenopathy. J Clin Exp Hematol. 2019;59(1):17-21. |
| 331 | Everson TC, Cole WH (1966) Spontaneous regression of cancer. WB Saunders, Philadelphia, PA |
| 332 | Boyd W (1966) The spontaneous regression of cancer. Charles C. Thomas, Springfield, IL |
| 333 | Fergeson JO, Black BM (1954) Disappearance, probably spontaneous of locally inoperable carcinoma of the descending colon: report of case. Mayo Clin Proc 29:407–410 |
| 334 | Everson TC, Cole WH (1966) Spontaneous regression of cancer. WB Saunders, Philadelphia, PA |
| 335 | Everson TC, Cole WH (1966) Spontaneous regression of cancer. WB Saunders, Philadelphia, PA |
| 336 | Jerry LM, Challis EB. Rakel RE. Oncology, Textbook of Family Practice, 19843rd edn (pg. 1061-81) |
| 337 | Brown CH. Regression of metastatic lesions: report of two cases. Am Pract (Clin Pediatr) 1961;12:655–6. |
| 338 | Brunschwig A (1963) Spontaneous regression of cancer. Surgery 53:423–431 |
| 339 | Mayo CW (1963) Tumor clinic conference. Cancer Bull 15:78–79 |
| 340 | Fullerton JM, Hill RD. Spontaneous regression of cancer. Br Med J 1963;21:1589–90. |
| 341 | Rankin GB, Brown CH, Crile G Jr (1965) Spontaneous regression of hepatic metastases from a carcinoma of the colon: 10-year follow up of a patient with familial polyposis. Ann Surg 162:156–159 |
| 342 | Margolis J, West D (1967) Spontaneous regression of malignant disease: report of three cases. J Am Geriatr Soc 15:251–253 |
| 343 | Synder W, Clark R, Rubini J. Long-term survival of mother and son with widespread metastatic adenocarcinoma of colon. Cancer 1968;21:129–33. |
| 344 | Synder W, Clark R, Rubini J. Long-term survival of mother and son with widespread metastatic adenocarcinoma of colon. Cancer 1968;21:129–33. |
| 345 | Weinstock C. Notes on “spontaneous” regression of cancer. J Am Soc Psycosom Dent Med 1977;24:106–10. |
| 346 | Meares A. Regression of cancer of the rectum after intensive meditation. Med J Aust 1979;2:539–40. |
| 347 | Glasser M, Rosenberg MZ, Gaito R. Widespread adenocarcinoma of the colon with survival of 28 years. JAMA 1979;241:2542–3. |
| 348 | Beechey RT, Edwards BE, Kellands CH. Adenocarcinoma of the colon: an unusual case. Med J Aust 1986;144:211–3. |
| 349 | Tominaga, K., Arai, J., Imamura, Y., Ohta, A., Sato, K., Tada, T., … Nagao, J. (1999). A case of colonic early carcinoma suspected autoamputation. Progress of Digestive Endoscopy(1972), 55(2), 96–97. |
| 350 | Wadsworth SJ, Davies CW, Gray W, Gleeson FV. Spontaneous regression of pulmonary metastases demonstrated by CT. Br J Radiol 1999 Mar;72(855):304e7. |
| 351 | Okamura S, Katsuhiko H, Satoh T. Regression of rectal mucosa-associated lymphoid tissue lymphoma unrelated to Helicobacter pylori. Ann Intern Med. 2000 Feb 1;132(3):247. |
| 352 | Kamesui T, Munemoto Y, Fujisawa K, Kasahara Y, Mitsui T, Asada Y, Iida Y, Miura S, Fujisawa M, Tsukioka N. A case of spontaneous dislodging of early colon cancer. Gastroenterol Endosc. 2000;42:1218–22. |
| 353 | Takenaka R, Tomoda J, Sakata T, et al. Mucosa-associated lymphoid tissue lymphoma of the rectum that regressed spontaneously. J Gastroenterol Hepatol 2000;15:331-335. |
| 354 | Ikuta, S. I., Miki, C., Ookura, E., Tonouchi, H., & Kusunoki, M. (2002). Spontaneous regression of a metastatic liver tumor: report of a case. Surgery today, 32(9), 844-848. |
| 355 | Abdelrazeq AS, Lund JN, Leveson SH (2005) Spontaneous regression of peritoneal carcinomatosis from a rectal cancer. Eur J Gastroenterol Hepatol 17:1421–1423 |
| 356 | Satoshi Itano, Norihiko Terada, Sadayuki Horiki et al .: A case of anorectal malignant melanoma falling off spontaneously and surviving over two years. Surgical treatment 2006; 94: 967-969 |
| 357 | Tomiki Y, Gonda H, Seki E, et al : Spontaneous decapitation of a small colorectal cancer : follow-up of the spontaneous course. Endoscopy 2007 ; 39 : 290-291 |
| 358 | Kochi, M., Yamake, H., Kaiga, T., Ookubo, R., Fujii, M., & Takayama, T. (2008). Spontaneous regression of advanced colon cancer—case report—. Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association), 69(7), 1717–1720. |
| 359 | Bir, A. S., Fora, A. A., Levea, C., & Fakih, M. G. (2009). Spontaneous regression of colorectal cancer metastatic to retroperitoneal lymph nodes. Anticancer research, 29(2), 465-468. |
| 360 | Sakamoto S, Fu K, Kobayashi O, Matsuyama S, Miyazaki A, Ogura K, et al. Spontaneous complete regression of a rectal cancer. Endoscopy. 2009;41: 910–2. |
| 361 | Shimizu H, Kochi M, Kaiga T, Mihara Y, Fujii M, Takayama T. A case of spontaneous regression of advanced colon cancer. Anticancer Res. 2010;30: 2351–4. |
| 362 | Sakuma, A., Yoshimatsu, K., Yokomizo, H., Osawa, G., Shimazaki, A., Matsumoto, A., … Ogawa, K. (2011). Radical Excision of Suspected Recurrent Sigmoid Colon Cancer after Spontaneous Regression. Nippon Daicho Komonbyo Gakkai Zasshi, 64(2), 83–87. |
| 363 | Nakashima M, Hori K, Kimura Y, Hayashi K, Yokomizo H, et al. A case of spontaneous regression of colon cancer. The journal of the Japan Surgical Association. 2012;73(6):1482–5. |
| 364 | Ann D. Flynn, MD, Jose M. Azar, MD, Michael V. Chiorean, MD, Spontaneous Regression of Colonic Lymphoma Following Infliximab and Azathioprine Withdrawal in Patients With Crohn’s Disease, Inflammatory Bowel Diseases, Volume 19, Issue 5, 1 April 2013, Pages E69–E70, |
| 365 | Ann D. Flynn, MD, Jose M. Azar, MD, Michael V. Chiorean, MD, Spontaneous Regression of Colonic Lymphoma Following Infliximab and Azathioprine Withdrawal in Patients With Crohn’s Disease, Inflammatory Bowel Diseases, Volume 19, Issue 5, 1 April 2013, Pages E69–E70, |
| 366 | Nakamura F, Sakamoto T, Nakajima T, Saito Y, Taniguchi H, Matsuda T. A case of rectal tumor in which the shape altered with regression in short period. BMC Gastroenterol. 2013;13:146. |
| 367 | Sekiguchi M, Mtsumoto N. Spontaneously disappearing colon cancer. Japan Gastroenterological Endoscopy Society. 2013;25:84–93. |
| 368 | Kihara K, Fujita S, Ohshiro T, Yamamoto S, et al. Spontaneous regression of colon cancer. Jpn J Clin Oncol. 2015;45(1):111–4. |
| 369 | Mitchell, A., & Bendavid, Y. (2014). Medullary colon cancer presenting with total necrosis of all regional lymph node metastases: morphologic description of a presumed immune-mediated event. Diagnostic Pathology, 9(1), 1-5. |
| 370 | Sewpaul A, Bargiela D, James A, Johnson SJ, French JJ. Spontaneous Regression of a Carcinoid Tumor following Pregnancy. Case Rep Endocrinol. 2014;2014:481823. |
| 371 | Kihara, K., Fujita, S., Ohshiro, T., Yamamoto, S., & Sekine, S. (2015). Spontaneous regression of colon cancer. Japanese Journal of Clinical Oncology, 45(1), 111-114. |
| 372 | Serizawa M, Kawarabayashi N. A case of spontaneous regression of transverse colon cancer. Gastroenterol Endosc. 2015;57(7):1490–5. |
| 373 | Ito, S., Kobayashi, O., Ohta, K., Kojima, T., Hashimoto, S., Miyoshi, Y., … Watanabe, S. (2016). Spontaneous regression of ascending colon cancer : Case report. Progress of Digestive Endoscopy, 88(1), 152–153. |
| 374 | Nemésio, R. A., Martins, R., Cipriano, M. A., Tralhão, J. G., & e Sousa, F. C. (2016). Spontaneous regression of colorectal liver metastases. Archives in Cancer Research, 4(1), 0-0. |
| 375 | Chida K, Nakanishi K, Shomura H, et al. Spontaneous regression of transverse colon cancer: a case report. Surgical Case Reports. 2017;3:65. |
| 376 | Matsuki, R., Sugiyama, M., Yoshiike, S., Shibahara, J., Kogure, M., Yokoyama, M., ... & Mori, T. (2018). Spontaneous regression of colorectal liver metastasis. Clinical Journal of Gastroenterology, 11(4), 263-267. |
| 377 | Chuang, C.-H.; Hsu, J.-F.; Shen, Y.-T.; Yang, C.-J. Regression of a metastatic lung mass after receiving whole brain irradiation: Can the abscopal effect cross the blood-brain barrier? Asia Pac. J. Clin. Oncol. 2018, 14, e548–e550. |
| 378 | Yoshida K, Sawaya M, Mikami T, et al. A case of transverse colon cancer that underwent spontaneous regression after biopsy. Gastroenterol Endosc. 2018;60:2303–9. |
| 379 | Fukutomi, T., Sato, A., Chiba, H., & Itakura, Y. (2019). A Case of Spontaneous Regression of Advanced Transverse Colon Cancer. Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association), 80(8), 1501–1507. |
| 380 | Karakuchi N, Shimomura M, Toyota K, Hinoi T, Yamamoto H, Sadamoto S, Mandai K, Egi H, Ohdan H, Takahashi T. Spontaneous regression of transverse colon cancer with high-frequency microsatellite instability: a case report and literature review. World J Surg Oncol. 2019 Jan 15;17(1):19. |
| 381 | Kawakita I, Takeda K, Tanaka Y, et al. Spontaneous regression of ascending colon cancer. Jpn J Gastroenterol Surg. 2019;52:106–11. |
| 382 | Tanaka, F., Hiraki, M., Yamada, K., Tominaga, N., Ikeda, O., Kido, S., … Kitahara, K. (2019). A Case of Spontaneous Regression of Sigmoid Colon Cancer after Local Injection for Non-lifting Sign Evaluation. Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association), 80(2), 368–372. |
| 383 | Utsumi T, Miyamoto S, Shimizu T, et al. Spontaneous regression of mismatch repair-deficient colorectal cancers: a case series. Dig Endosc 2020. Epub 2020/05/18. |
| 384 | Utsumi T, Miyamoto S, Shimizu T, et al. Spontaneous regression of mismatch repair-deficient colorectal cancers: a case series. Dig Endosc 2020. Epub 2020/05/18. |
| 385 | Utsumi T, Miyamoto S, Shimizu T, et al. Spontaneous regression of mismatch repair-deficient colorectal cancers: a case series. Dig Endosc 2020. Epub 2020/05/18. |
| 386 | Mehawej, J., El Helou, N., Pejovic, T., & Mhawech-Fauceglia, P. (2020). Metastatic colorectal carcinoma to one ovary with vanishing primary: A case report in an 18-year-old patient. Clin Obstet Gynecol, 6, 1-3. |
| 387 | Nishiura, B., Kumamoto, K., Akamoto, S., Asano, E., Ando, Y., Suto, H., ... & Suzuki, Y. (2020). Spontaneous regression of advanced transverse colon cancer with remaining lymph node metastasis. Surgical Case Reports, 6(1), 1-6. |
| 388 | Yokota, T., Saito, Y., Takamaru, H., Sekine, S., Nakajima, T., Yamada, M., ... & Matsuda, T. (2021). Spontaneous Regression of Mismatch Repair-Deficient Colon Cancer: A Case Series. Clinical Gastroenterology and Hepatology, 19(8), 1720-1722. |
| 389 | Yokota, T., Saito, Y., Takamaru, H., Sekine, S., Nakajima, T., Yamada, M., ... & Matsuda, T. (2021). Spontaneous Regression of Mismatch Repair-Deficient Colon Cancer: A Case Series. Clinical Gastroenterology and Hepatology, 19(8), 1720-1722. |
| 390 | Yokota, T., Saito, Y., Takamaru, H., Sekine, S., Nakajima, T., Yamada, M., ... & Matsuda, T. (2021). Spontaneous Regression of Mismatch Repair-Deficient Colon Cancer: A Case Series. Clinical Gastroenterology and Hepatology, 19(8), 1720-1722. |
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Cancer Stat Facts: Cancer of Any Site. 2022. https://seer.cancer.gov/statfacts/html/all.html https://seer.cancer.gov/statfacts/html/all.html
- 2.Gastrointestinal cancer: recent developments in medical oncology. Chong G, Cunningham D. https://doi.org/10.1016/j.ejso.2005.02.026. Eur J Surg Oncol. 2005;31:453–460. doi: 10.1016/j.ejso.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Spontaneous regression of cancer: preliminary report. Cole WH, Everson TC. Ann Surg. 1956;144:366–383. doi: 10.1097/00000658-195609000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efforts to explain spontaneous regression of cancer. Cole WH. J Surg Oncol. 1981;17:201–209. doi: 10.1002/jso.2930170302. [DOI] [PubMed] [Google Scholar]
- 5.The spontaneous regression of cancer. A review of cases from 1900 to 1987. Challis GB, Stam HJ. Acta Oncol. 1990;29:545–550. doi: 10.3109/02841869009090048. [DOI] [PubMed] [Google Scholar]
- 6.Spontaneous regression of malignant tumors: importance of the immune system and other factors (Review) Ricci SB, Cerchiari U. Oncol Lett. 2010;1:941–945. doi: 10.3892/ol.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The spontaneous remission of cancer: current insights and therapeutic significance. Radha G, Lopus M. Transl Oncol. 2021;14:101166. doi: 10.1016/j.tranon.2021.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacteria and cancer: cause, coincidence or cure? A review. Mager DL. J Transl Med. 2006;4:14. doi: 10.1186/1479-5876-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spontaneous regression of tumour and the role of microbial infection--possibilities for cancer treatment. Kucerova P, Cervinkova M. Anticancer Drugs. 2016;27:269–277. doi: 10.1097/CAD.0000000000000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dr William Coley and tumour regression: a place in history or in the future. Hoption Cann SA, van Netten JP, van Netten C. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1742910/ Postgrad Med J. 2003;79:672–680. [PMC free article] [PubMed] [Google Scholar]
- 11.Anti-cancer peptides from bacteria. Karpinski TM, and A. K. Szkaradkiewicz. Bangladesh J Pharmacol. 2013;8:8. [Google Scholar]
- 12.Tumour necrosis factor as an anticancer agent. Balkwill FR, Naylor MS, Malik S. Eur J Cancer. 1990;26:641–644. doi: 10.1016/0277-5379(90)90097-d. [DOI] [PubMed] [Google Scholar]
- 13.Interleukin 6: a fibroblast-derived growth inhibitor of human melanoma cells from early but not advanced stages of tumor progression. Lu C, Vickers MF, Kerbel RS. Proc Natl Acad Sci U S A. 1992;89:9215–9219. doi: 10.1073/pnas.89.19.9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regulation of interleukin-8 expression in human melanoma cells by the organ environment. Gutman M, Singh RK, Xie K, Bucana CD, Fidler IJ. https://pubmed.ncbi.nlm.nih.gov/7758001/ Cancer Res. 1995;55:2470–2475. [PubMed] [Google Scholar]
- 15.Interleukin-2 antitumor and effector cell responses. Hawkins MJ. https://pubmed.ncbi.nlm.nih.gov/8284693/ Semin Oncol. 1993;20:52–59. [PubMed] [Google Scholar]
- 16.Interferons in the treatment of human cancer. Kirkwood JM, Ernstoff MS. J Clin Oncol. 1984;2:336–352. doi: 10.1200/JCO.1984.2.4.336. [DOI] [PubMed] [Google Scholar]
- 17.Jerry LM, Challis EB. Textbook of family practice. pp. Philadelphia: WB Saunders; 1983. Oncology; pp. 1061–1081. [Google Scholar]
- 18.Spontaneous tumor regression following COVID-19 vaccination. Sousa LG, McGrail DJ, Li K, et al. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2021-004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Can the host immune response against SARS-CoV2 also cause an anticancer effect? Kahraman S, Akinci MB, Sendur MA, Yalcin B. Med Oncol. 2021;38:90. doi: 10.1007/s12032-021-01533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Complete remission of follicular lymphoma after SARS-CoV-2 infection: from the "flare phenomenon" to the "abscopal effect". Sollini M, Gelardi F, Carlo-Stella C, Chiti A. Eur J Nucl Med Mol Imaging. 2021;48:2652–2654. doi: 10.1007/s00259-021-05275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SARS-CoV-2-induced remission of Hodgkin lymphoma. Challenor S, Tucker D. Br J Haematol. 2021;192:415. doi: 10.1111/bjh.17116. [DOI] [PubMed] [Google Scholar]
- 22.Spontaneous regression of metastatic renal cell carcinoma after SARS-CoV-2 infection: a report of two cases. Buchler T, Fiser L, Benesova J, Jirickova H, Votrubova J. Curr Oncol. 2021;28:3403–3407. doi: 10.3390/curroncol28050294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unexpected tumor reduction in metastatic colorectal cancer patients during SARS-Cov-2 infection. Ottaiano A, Scala S, D'Alterio C, et al. Ther Adv Med Oncol. 2021;13:17588359211011455. doi: 10.1177/17588359211011455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spontaneous regression of cancer. Cole WH. CA Cancer J Clin. 1974;24:274–279. doi: 10.3322/canjclin.24.5.274. [DOI] [PubMed] [Google Scholar]
- 25.Biologic control of cancer. The James Ewing lecture. Baker HW. Arch Surg. 1986;121:1237–1241. doi: 10.1001/archsurg.121.11.1237. [DOI] [PubMed] [Google Scholar]
- 26.A patient-level data meta-analysis of the abscopal effect. Hatten SJ Jr, Lehrer EJ, Liao J, et al. Adv Radiat Oncol. 2022;7:100909. doi: 10.1016/j.adro.2022.100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spontaneous regression of primary renal cell carcinoma following image-guided percutaneous biopsy. Dickerson EC, Davenport MS, Liu PS. Clin Imaging. 2015;39:520–524. doi: 10.1016/j.clinimag.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Spontaneous regression of cancer: possible mechanisms. Papac RJ. https://pubmed.ncbi.nlm.nih.gov/9891219/ In Vivo. 1998;12:571–578. [PubMed] [Google Scholar]
- 29.Spontaneous regression of massive infiltrative hepatocellular carcinoma with change in hepatic contour: a case report with literature review. Lee EH, Oh MJ. J Liver Cancer. 2018;18:55–62. [Google Scholar]
- 30.Spontaneous regression of hepatocellular carcinoma. Kondo S, Okusaka T, Ueno H, Ikeda M, Morizane C. Int J Clin Oncol. 2006;11:407–411. doi: 10.1007/s10147-006-0591-4. [DOI] [PubMed] [Google Scholar]
- 31.Spontaneous remission of hepatocellular carcinoma after massive gastrointestinal haemorrhage. Tocci G, Conte A, Guarascio P, Visco G. BMJ. 1990;300:641–642. doi: 10.1136/bmj.300.6725.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.A case of spontaneous regression of hepatocellular carcinoma with bone metastasis. Sato Y, Fujiwara K, Nakagawa S, et al. Cancer. 1985;56:667–671. doi: 10.1002/1097-0142(19850801)56:3<667::aid-cncr2820560339>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 33.Spontaneous regression of multiple pulmonary recurrences of hepatocellular carcinoma after hepatectomy: report of a case. Harimoto N, Shirabe K, Kajiyama K, Gion T, Takenaka M, Nagaie T, Maehara Y. Surg Today. 2012;42:475–478. doi: 10.1007/s00595-011-0030-7. [DOI] [PubMed] [Google Scholar]
- 34.Spontaneous regression of hepatic adenoma in a patient with glycogen storage disease type I after hemodialysis: ultrasonographic and CT findings. Iijima H, Moriwaki Y, Yamamoto T, Takahashi S, Nishigami T, Hada T. Intern Med. 2001;40:891–895. doi: 10.2169/internalmedicine.40.891. [DOI] [PubMed] [Google Scholar]
- 35.A case of spontaneous regression of lymphoma in the mandibular gingiva after biopsy. Kaibuchi N, Okamoto T, Kataoka T, Kumasaka A, Ando T. https://www.sciencedirect.com/science/article/pii/S2214541915000176 Oral Maxillofac Surg Cases. 2015;1:33–37. [Google Scholar]
- 36.Cancer vaccines. Butterfield LH. BMJ. 2015;350:0. doi: 10.1136/bmj.h988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirshberg C. 1993. Spontaneous Remission. The Spectrum of Self-Repair. [Google Scholar]
- 38.Spontaneous regression of a carcinoid tumor following pregnancy. Sewpaul A, Bargiela D, James A, Johnson SJ, French JJ. Case Rep Endocrinol. 2014;2014:481823. doi: 10.1155/2014/481823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spontaneous regression of recurrent squamous cell carcinoma of the tongue. Oya R, Ikemura K. Int J Clin Oncol. 2004;9:339–342. doi: 10.1007/s10147-004-0404-6. [DOI] [PubMed] [Google Scholar]
- 40.Studies on cases of spontaneous regression of cancer in Japan in 2011, and of hepatic carcinoma, lung cancer and pulmonary metastases in the world between 2006 and 2011 [Article in Japanese] Iwanaga T. http://www.pieronline.jp/content/article/0385-0684/40110/1475. Gan To Kagaku Ryoho. 2013;40:1475–1487. [PubMed] [Google Scholar]
- 41.Induction of immunological tolerance by porcine liver allografts. Calne RY, Sells RA, Pena JR, et al. Nature. 1969;223:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 42.Immunotherapeutic approaches to hepatocellular carcinoma treatment. Miamen AG, Dong H, Roberts LR. Liver Cancer. 2012;1:226–237. doi: 10.1159/000343837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spontaneous regression of hepatocellular carcinoma is most often associated with tumour hypoxia or a systemic inflammatory response. Huz JI, Melis M, Sarpel U. HPB (Oxford) 2012;14:500–505. doi: 10.1111/j.1477-2574.2012.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spontaneous regression of hepatocellular carcinoma-a case report. Alam MA, Das D. J Gastrointest Cancer. 2017;48:194–197. doi: 10.1007/s12029-016-9812-x. [DOI] [PubMed] [Google Scholar]
- 45.Spontaneous regression in non-Hodgkin's lymphoma. Gattiker HH, Wiltshaw E, Galton DA. Cancer. 1980;45:2627–2632. doi: 10.1002/1097-0142(19800515)45:10<2627::aid-cncr2820451023>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 46.Helicobacter and gastric MALT lymphoma. Stolte M, Bayerdörffer E, Morgner A, Alpen B, Wündisch T, Thiede C, Neubauer A. Gut. 2002;50 Suppl 3:0–24. doi: 10.1136/gut.50.suppl_3.iii19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The pancreas cancer microenvironment. Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spontaneous regression of pancreatic cancer with liver metastases. Saade Lemus P, Anderson K, Smith M, Bullock A. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2019-229619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spontaneous regression of pancreatic cancer: real or a misdiagnosis? Herreros-Villanueva M, Hijona E, Cosme A, Bujanda L. World J Gastroenterol. 2012;18:2902–2908. doi: 10.3748/wjg.v18.i23.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spontaneous regression of pancreatic cancer: a case report and literature review. Chin KM, Chan CY, Lee SY. Int J Surg Case Rep. 2018;42:55–59. doi: 10.1016/j.ijscr.2017.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spontaneous regression of metastatic pancreatic cancer: a role for recurrent inflammation. Ibrahimi S, Mukherjee S, Alhyari L, Rubin E, Aljumaily R. Pancreas. 2019;48:0–6. doi: 10.1097/MPA.0000000000001193. [DOI] [PubMed] [Google Scholar]
- 52.A case of poorly differentiated pancreatic adenocarcinoma with spontaneous regression wherein retrospective rim enhancement findings were important for diagnosis [Article in Japanese] Kawaguchi S, Ohtsu T, Enokida K, et al. Nihon Shokakibyo Gakkai Zasshi. 2021;118:358–365. doi: 10.11405/nisshoshi.118.358. [DOI] [PubMed] [Google Scholar]
- 53.Immunology and immunotherapy of colorectal cancer. Dalerba P, Maccalli C, Casati C, Castelli C, Parmiani G. Crit Rev Oncol Hematol. 2003;46:33–57. doi: 10.1016/s1040-8428(02)00159-2. [DOI] [PubMed] [Google Scholar]
- 54.Spontaneous regression of colon carcinoma. Serpick AA. https://pubmed.ncbi.nlm.nih.gov/1025474/ Natl Cancer Inst Monogr. 1976;44:21. [PubMed] [Google Scholar]
- 55.Patterns of human tumor-infiltrating lymphocytes in 120 human cancers. Balch CM, Riley LB, Bae YJ, Salmeron MA, Platsoucas CD, von Eschenbach A, Itoh K. Arch Surg. 1990;125:200–205. doi: 10.1001/archsurg.1990.01410140078012. [DOI] [PubMed] [Google Scholar]
- 56.Karnofsky Memorial Lecture. The immunotherapy and gene therapy of cancer. Rosenberg SA. J Clin Oncol. 1992;10:180–199. doi: 10.1200/JCO.1992.10.2.180. [DOI] [PubMed] [Google Scholar]
- 57.Spontaneous regression of colorectal cancer: a review of cases from 1900 to 2005. Abdelrazeq AS. Int J Colorectal Dis. 2007;22:727–736. doi: 10.1007/s00384-006-0245-z. [DOI] [PubMed] [Google Scholar]