Abstract
In this study, we examined the link between plasma Alzheimer’s disease (AD) biomarkers and physical functioning outcomes within a community-dwelling, multiethnic cohort. Data from 1 328 cognitively unimpaired participants (n = 659 Mexican American and n = 669 non-Hispanic White) from the ongoing Health & Aging Brain Study—Health Disparities (HABS-HD) cohort were examined. Plasma AD biomarkers (amyloid beta [Aβ]40, Aβ42, total tau [t-tau], and neurofilament light chain [NfL]) were assayed using the ultra-sensitive Simoa platform. Physical functioning measures were the Timed Up and Go (TUG) and the Short Physical Performance Battery (SPPB). Cross-sectional linear regression analyses revealed that plasma Aβ 40 (p < .001), Aβ 42 (p = .003), and NfL (p < .001) were each significantly associated with TUG time in seconds. Plasma Aβ 40 (p < .001), Aβ 42 (p < .001), t-tau (p = .002), and NfL (p < .001) were each significantly associated with SPPB Total Score. Additional analyses demonstrate that the link between plasma AD biomarkers and physical functioning outcomes were strongest among Mexican Americans. Plasma AD biomarkers are receiving a great deal of attention in the literature and are now available clinically including use in clinical trials. The examination of AD biomarkers and physical functioning may allow for the development of risk profiles, which could stratify a person’s risk for neurodegenerative diseases, such as AD, based on plasma AD biomarkers, physical functioning, ethnicity, or a combination of these measures prior to the onset of cognitive impairment.
Keywords: Biomarkers, Brain aging, Health disparities, Mexican American, Physical functioning
Alzheimer’s disease (AD) is the most common neurodegenerative dementia (1,2) and is considered to disproportionately affect underserved populations. Work conducted examining biomarkers of AD have supported that biomarkers can be effective in discriminating neurodegenerative diseases (AD, Parkinson’s disease [PD], and dementia with Lewy bodies) and can also be used to aid in the decision-making process for when to refer to specialty clinics (3–7). Even among cognitively unimpaired populations, AD biomarkers including those of neurodegeneration, tau-related pathology, and neuroinflammation have been found to correlate with amyloid beta (Aβ) pathology (8), suggesting that such pathological changes can and do occur early in the preclinical stage. Cullen et al. described how AD biomarkers such as Aβ42/40, phosphorylated tau (p-tau) 217, and neurofilament light chain (NfL) could be combined with demographic data to predict change in cognitive function, which could lead to the development of screening algorithms for AD clinical trials (9).
Despite the fact that U.S. Hispanics/Latinos are expected to experience the greatest increase in AD and AD-related dementias (ADRDs) by 2060 (10), this group remains severely underrepresented in AD research (2,11,12). In our own work, we have previously demonstrated that AD biomarkers (13–16), and AD risk factors (13,17–19), are different among Mexican Americans (65% of the U.S. Hispanic/Latino population) when compared with non-Hispanic Whites. In fact, findings have shown that Mexican Americans present with earlier age of onset for cognitive loss (13,18,19) and neurodegeneration (15) yet tend to exhibit lower rates of amyloid positivity (13) and APOE4 genotype presence (18,19). To date, limited research has been conducted on the link between AD biomarkers and physical functioning performance specifically among community-based Mexican Americans, who also exhibit higher rates of medical comorbidities (13,17–19), such as diabetes, that may impact physical functioning outcomes during aging.
Similar to AD biomarkers, physical functioning, including measures of mobility and balance such as the Timed Up and Go (TUG) and the Short Physical Performance Battery (SPPB), have been increasingly explored due to changes observed among those with AD (20). A cross-sectional study showed that people living with dementia, including those in the mild/moderate stages, had reduced physical functioning compared with cognitively unimpaired individuals (21). Because of this work, physical functioning measures have received increased attention as potential metrics for changes among individuals with cognitive impairment including those with AD and even in the earlier disease stages such as mild cognitive impairment (MCI).
Of the work conducted examining the TUG test (22), research has supported that this measure can map onto cognitive declines among older adults with findings that a decline of 3 points on the Mini-Mental State Examination (MMSE) over multiple years corresponds with reduced TUG walking performance (23). Ansai et al. identified in regression models that after adjusting for age and sex, fluency measures were associated with performance on TUG subtests such as turn-to-walk and turn-to-sit among cognitively unimpaired individuals while among individuals with AD, visuospatial abilities were found to be associated with performance on the same TUG subtests (24). Such work conducted examining the TUG in combination with a semantic fluency task (Naming Animals) found a negative correlation between this dual task and cerebral spinal fluid (CSF) measures of AD including total tau (t-tau) and p-tau (25). Of note, no significant correlations were found in the study for CSF measures of Aβ (25).
The SPPB is another measure of physical functioning that has been increasingly used in older adult populations and is a well-validated assessment (26). In a cohort of adults age 90+, findings support that lower scores on the SPPB were associated with greater WMH, hippocampal atrophy, and reduced cognitive performance on composite measures; however, the latter was found to be mediated by hippocampal volume (27). Another study found that SPPB in a cross-sectional study was associated with increases in plasma NfL, a marker of general neuronal injury and neurodegeneration. Similar to prior work, no significant associations were found with Aβ (28).
The literature establishes that biomarkers are effective in distinguishing between neurodegenerative diseases (3–7), and studies have demonstrated associations between physical functioning and biomarkers of neurodegeneration (24,25,28). Gaps in the literature include a lack of examination of the relationship between physical function and AD biomarkers in diverse populations including among cognitively unimpaired individuals. Clinically, treatment goals have increasingly focused on intervening prior to symptom onset (clinical/functional declines). Subtle deficits in physical functioning may coincide with changes in biomarkers of AD, which creates an ideal opportunity for intervention. The impact of such interventions could be more powerful because they occur before decline. Therefore, this study sought to address gaps in the literature and examine the link between physical functioning and plasma AD biomarkers (Aβ 40, Aβ 42, t-tau, and NfL) among cognitively unimpaired, community-dwelling Mexican Americans and non-Hispanic Whites of the HABS-HD study.
Materials and Methods
Participants and Assessment
The Health & Aging Brain Study—Health Disparities (HABS-HD; formally the Health & Aging Brain among Latino Elders, [HABLE] study) study is an ongoing, longitudinal, community-based project examining health disparities in MCI and AD among Mexican Americans and non-Hispanic Whites with recent expansion to enroll African Americans. HABS-HD methods have been published (13) and are briefly outlined below. Participants were recruited using a community-based participatory research approach and the study replaces for attrition in order to maintain the target enrollment size (13). The study is currently working toward incorporating recruitment data with study enrollment information to further understand study enrollment and attrition. The data included in this study encompass baseline examinations from Mexican American and non-Hispanic White participants because the recruitment of the African American participants is ongoing. All aspects of the study protocol can be conducted in Spanish or English. The HABS-HD study is conducted under IRB approved protocols and each participant (or his/her legal representative) signs written informed consent. All HABS-HD data are available to the scientific community through the UNTHSC Institute for Translational Research (ITR) website (29).
Clinical Assessment
The HABS-HD protocol includes an interview, neuropsychological testing, clinical and biorepository blood draw, MRI and PET scans, and a functional exam. Generally, there are no more than 3 months between the study components; however, some exceptions were made during COVID. An informant interview is conducted for completion of the Clinical Dementia Rating (CDR) Scale (30) by clinicians with expertise in dementia to evaluate for functional declines and is reviewed during consensus review.
Functional Exam
The TUG is a clinical measure of mobility requiring an individual to rise from an armchair, walk straight ahead, pass a line marked on the floor, turn around and walk back and sit down again, and it is measured in seconds. The test is a timed measure that has been shown to be well correlated with gait speed including among elderly and cognitively impaired populations and can be used as a fall risk screener (22,31). The SPPB is a combined functional exam that includes a balance test, a 4-m walk test for walking speed, and a chair stand test (32,33). The SPPB scoring ranges from 0 to 12 with each component allowing for a maximum score of 4 with the lower score indicating a poorer performance on the functional measure. The TUG and SPPB are different conceptualizations of physical functioning as the TUG is a clinical measure with a single score contrasting with the SPPB, which is a combination exam consisting of subscale functioning measures and a total score.
Blood Biomarkers
Fasting blood samples were collected, processed, and stored per previously published international guidelines (34). Assay preparation was completed using custom automated StarPlus system from Hamilton Robotics (Reno, NA). Plasma markers of amyloid (Aβ 40, Aβ 40), tau (t-tau), and neurodegeneration (NfL) were assayed using the ultra-sensitive Simoa (single molecule array technology) on the HD-X platform (Quanterix.com, Billerica, MA) (13,14,16). Coefficients of variation for all assays were of ≤5%.
Diagnostic Classification
Cognitive diagnoses were assigned algorithmically (decision tree). The CDR and neuropsychological testing were considered when assigning a cognitive diagnosis. The cognitive diagnoses were verified at consensus review as follows: Cognitively unimpaired: no cognitive complaints, CDR sum of boxes score of 0 (35,36), and cognitive tests scores broadly within normal limits (ie, performance greater than that defined as meeting diagnostic criteria for MCI [ie, ≤1.5 SD below the normative range]); Mild cognitive impairment (MCI): cognitive complaint (self or other), CDR sum of boxes score between 0.5 and 2.0 (35,36), and at least one cognitive test score falling ≤1.5 SD below normative ranges; Dementia: CDR sum of boxes score ≥ 2.5 (35,36) and at least 2 cognitive test scores 2 SD below normative ranges. In this study, only data from participants assigned as cognitively unimpaired were included. Medical research diagnoses were assigned based on a participant’s past medical history and objective measures. These were assigned by a licensed clinician at consensus review.
Statistical Analyses
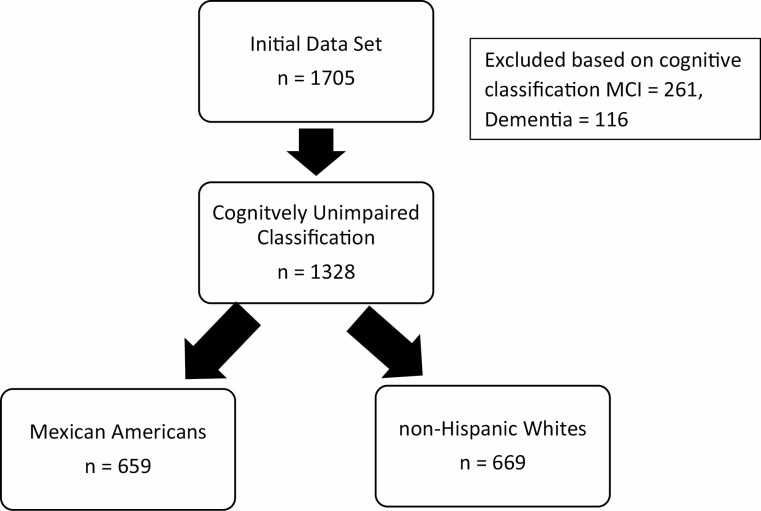
Statistical analyses were conducted in SPSS 28 (IBM). Figure 1 shows the participants of the cohort included in the analysis. Normality tests were performed, and the data met the assumptions of the statistical tests used in the subsequent analyses. Chi-square and ANOVAs were utilized to compare groups on demographic variables. Cross-sectional linear regression models were created with TUG and SPPB entered as the outcome variables and plasma AD biomarkers as the predictor variables with age, gender, education, and ethnicity entered as covariates. Coefficients were examined in addition to β weights to aid in the interpretation of the models. A moderator analysis was also performed between ethnicity, plasma AD biomarkers, and physical functioning measures. Linear regressions models were further run split by ethnicity. In a follow-up analysis, linear regression models were conducted with the addition of medical comorbidities entered as covariates in order to examine the potential impact on the relationship between AD biomarkers and physical functioning. Statistical significance was set at p < .05.
Figure 1.
STROBE diagram.
Results
The demographic characteristics of the cohort are provided in Table 1. Data from 1 328 cognitively unimpaired participants (659 Mexican American and 669 non-Hispanic White) were included in the analyses. Mexican American participants were found to be significantly younger, achieved fewer years of formal education, and were more likely to be female (all p < .001). The non-Hispanic White participants performed significantly better on the TUG (p < .001) and SPPB (total score, p < .001). Mexican Americans participants had significantly higher Aβ 40 (p < .001), lower Aβ 42 (p < .001), lower t-tau (p < .001), and lower NfL levels (p < .001).
Table 1.
Descriptive Characterization of the HABS-HD Sample Included in the Analyses
| Total Cohort N = 1 328 |
Mexican American N = 669 |
Non-Hispanic White N = 659 |
Statistic | |
|---|---|---|---|---|
| Age | 66.10 (8.51) | 68.92 (8.33) | 63.23(7.70) | F = 166.95, p < .001 |
| Education | 12.68 (4.7) | 15.59 (2.58) | 9.71 (4.54) | F = 847.59, p < .001 |
| Gender (% male) | 36% | 30% | 41% | χ 2 = 18.52, p < .001 |
| MMSE | 28.07 (2.30) | 29.19 (1.01) | 26.94 (2.66) | F = 419.06, p < .001 |
| Tug (s) | 9.66 (2.30) | 9.39 (2.03) | 9.94 (2.51) | F = 18.29, p < .001 |
| SPPB total | 10.88 (1.82) | 11.04 (1.68) | 10.72 (2.51) | F = 9.71, p < .001 |
| Aβ 40 pg/mL | 250.81 (65.93) | 264.98 (64.36) | 236.42 (64.42) | F = 62.63, p < .001 |
| Aβ 42 pg/mL | 11.99 (3.25) | 12.22 (3.12) | 11.77 (3.36) | F = 6.03, p = .014 |
| Tau pg/mL | 2.44 (.928) | 2.33 (.933) | 6.39 (.911) | F = 17.86, p < .001 |
| NfL pg/mL | 18.18 (11.37) | 19.87 (11.00) | 16.46 (11.48) | F = 29.23, p < .001 |
| Hypertension (% yes) | 61.8% | 65.4% | 58.3% | χ 2 = 7.10, p < .008 |
| Hyperlipidemia (% yes) | 64.6% | 66.6% | 63.1% | χ 2 = 1.38, p < .001 |
| Diabetes mellitus (% yes) | 23.3% | 35.0% | 11.8% | χ 2 = 18.52, p < .001 |
Notes: Aβ40 = plasma beta amyloid 40; Aβ42 = plasma beta amyloid 42; MMSE = Mini Mental State Examination; NfL = plasma neurofilament light; SPPB = Short Physical Performance Battery; tau = plasma total tau. All p values < .005.
Associations Between Functional Status and Biomarkers
To examine whether the AD biomarkers were related to functional status when controlling for age, gender, education, and ethnicity, linear regressions were performed (see Table 2). In the total cohort, plasma AD biomarkers were significantly related to the TUG and SPPB. Most plasma AD biomarkers were found to be significantly positively related to TUG scores (reflecting poorer physical functioning performance): Aβ 40 (β = .128, 95% CI: 0.003, 0.006, p < .001), Aβ 42 (β = .080, 95% CI: 0.019, 0.094, p = .003) and NfL (β = .11, 95% CI: 0.011, 0.034, p < .001) with a trend toward significance for t-tau (β = 0.077, 95% CI: 0.067, 0.005, p = .050). The plasma AD biomarkers were also found to be significantly negatively associated with the SPPB total scores (reflecting poorer physical functioning): Aβ 40 (β = −0.097, 95% CI: −0.004, −0.001, p < .001), Aβ 42 (β = −0.098, 95% CI: −0.084, −0.025, p < .001), t-tau (β = −0.086, 95% CI: −0.272, −0.065, p = .002), and NfL (β = −0.132, 95% CI: −0.030, −0.012, p < .001). Structure coefficients were examined in addition to β weights. In models with the TUG as the dependent variable, all of the independent variables were significantly correlated with the observed effects of the model but ranged in variance explained by the model with education explaining (41%–44%) and age (36%–%39%) followed by biomarkers (11%–22%), ethnicity (10%–11%), and gender contributing a scant (0.1%–6%). In models with the SPPB as the dependent variable, all the independent variables were significantly correlated with the observed effects of the model but ranged in variance explained by the models with age (44%–45%) and education (30%–33%) explaining the most then biomarkers (14%–29%), ethnicity (6%–7%), and gender (3%–4%).
Table 2.
Association Between Physical Function and Plasma Alzheimer’s Disease Biomarkers
| Aβ 40 | Aβ 42 | Total Tau | NfL | |
|---|---|---|---|---|
| TUG (TIME) | (0.128, <0.001) [0.003, 0.006] |
(0.080, 0.003) [0.019, 0.094] |
(0.077, 0.050) [0.058, 0.322] |
(0.11, <0.001) [0.011, 0.034] |
| SPPB Total | (−0.097, <0.001) [−0.004, −0.001] | (−0.098, <0.001) [−0.084, −0.025] | (−0.086, 0.002) [−0.272, −0.065] | (−0.132, <0.001) [−0.030, −0.012] |
Notes: Aβ40 = plasma beta amyloid 40; Aβ42 = plasma beta amyloid 42; MMSE = Mini Mental State Examination; NfL = plasma neurofilament light; SPPB = Short Physical Performance Battery; total tau = plasma total tau. All models are adjusted for age, sex, ethnicity, education. (β, p-value) [95% confidence interval]. All p values < .05 are bolded.
Supplementary regression models were also run with the addition of medical comorbidities (hypertension, diabetes mellitus, and hyperlipidemia) entered as covariates (see Table 3). In the total cohort, poorer TUG performance was again found to be significantly associated with elevated Aβ 40 (β = 0.004, 95% CI: 0.002, 0.006, p < .001), Aβ 42 (β = 0.041, 95% CI: 0.003, 0.079, p = .036), NfL (β = −0.018, 95% CI: 0.006, 0.029, p = .002), and t-tau (β = 0.144, 95% CI: 0.012, 0.276, p = .033). Similar to models without the covariate of medical comorbidities, in the total sample, poorer SPPB performance was also associated with higher Aβ 40 (β = −0.002, 95% CI: −0.004, −0.001, p = .005), Aβ 42 (β = −0.045, 95% CI: −0.075, −0.015, p = .004), t-tau (β = −0.139, 95% CI: −0.243, −0.035, p = .009) and NfL levels (β = −0.019, 95% CI: −0.028, −0.010, p < .001).
Table 3.
Association Between Physical Function and Plasma Alzheimer’s Disease Biomarkers Covarying for Medical Comorbidity
| Aβ 40 | Aβ 42 | Total Tau | NfL | |
|---|---|---|---|---|
| TUG | (0.004, <0.001) [0.002, 0.006] |
(0.041, 0.036) [0.003, 0.079] |
(0.144, 0.033) [0.012, 0.276] |
(0.018, 0.002) [0.006, 0.029] |
| SPPB | (−0.002, 0.005) [−0.004, −0.001] |
(−0.045, 0.004) [−0.075, −0.015] |
(−0.139, 0.009) [−0.243, 0.035] |
(−0.019, <0.001) [−0.028, −0.010] |
Notes: Aβ40 = plasma beta amyloid 40; Aβ42 = plasma beta amyloid 42; MMSE = Mini Mental State Examination; NfL = plasma neurofilament light; SPPB = Short Physical Performance Battery; total tau = plasma total tau. (β, p-value) [95% confidence interval]. Bold indicates a significant moderating effect between ethnicity and the biomarker. All p values <.05 are bolded.
Associations Between Functional Status and Biomarkers in Mexican American and non-Hispanic Whites
Next, a moderator analysis was performed, which revealed a moderating effect of ethnicity for NfL and TUG (β = 0.029, 95% CI: 0.007, 0.052, p = .010) as well as for NfL and SPPB (β = −0.023, 95% CI: −0.040, −0.005, p = .011). Because a moderating effect was found for select plasma AD biomarkers, additional models were rerun split by ethnicity (see Table 4).
Table 4.
Link Between Plasma Alzheimer’s Disease Biomarkers and Physical Function by Ethnicity
| Aβ 40 | Aβ 42 | Total Tau | NfL | |
|---|---|---|---|---|
| NHW | ||||
| TUG | (0.003, <0.001) [0.001, 0.006] |
(0.039, 0.116) [−0.010, 0.088] |
(0.182, 0.028) [0.020, 0.345] |
(0.006, 0.466) [−0.009, 0.021] |
| SPPB | (−0.002, 0.055) [−0.004, 0.000] |
(−0.028, 0.054) [−0.078, 0.001] |
(−0.134, 0.044) [−0.265, −0.003] |
(−0.007, 0.232) [−0.020, 0.005] |
| MA | ||||
| TUG | (0.006, <0.001) [0.003, 0.009] |
(0.068, 0.020) [0.011, 0.126] |
(0.180, 0.095) [−0.032, 0.391] |
(0.037, <0.001) [0.020, 0.054] |
| SPPB | (−0.003, 0.004) [−0.006, −0.001] |
(−0.065, 0.005) [−0.110, −0.020] |
(−0.191, 0.023) [−0.355, −0.026] |
(−0.033, <0.001) [−0.046, −0.020] |
Notes: Aβ 40 = plasma beta amyloid 40; Aβ 42 = plasma beta amyloid 42; MA = Mexican American; NfL = plasma neurofilament light chain; NHW = non-Hispanic White; total tau = plasma total tau. All models are adjusted for age, gender, and education: (β, p-value) [95% confidence interval]. All p values < .05 are bolded.
Mexican Americans
Poorer TUG performance (reflected in higher TUG scores) was found to be associated with higher levels of plasma Aβ 40 (β = 0.006, 95% CI: 0.003, 0.009, p < .001), Aβ 42 (β = .068, 95% CI: 0.011 0, 0.126, p = .020), and NfL (β = .037, 95% CI: 0.020, 0.054, p < .001). Also, poorer SPPB performance (reflected in higher SPPB scores) was also found to be associated with higher levels of Aβ 40 (β = −0.003, 95% CI: −0.006, −0.001, p = .004), Aβ 42 (β = −0.065, 95% CI: −0.110, −0.020, p = .005), t-tau (β = −0.191, 95% CI: −0.355, −0.026, p = .023), and NfL (β = −0.033, 95% CI: −0.046, −0.020, p < .001).
Non-Hispanic Whites
Among non-Hispanic Whites, higher plasma Aβ 40 (β = 0.003, 95% CI: 0.001, 0.006, p < .001) and t-tau (β = 0.182, 95% CI: 0.020, 0.345, p = .028) were associated with poorer TUG performance. T-tau (β = −0.134, 95% CI: −0.265, −0.003, p = .044) was the only plasma AD biomarker associated with a worse performance on the SPPB for this ethnic group.
Discussion
The present work reflects, to our knowledge, the first large-scale study of the link between plasma AD biomarkers and physical functioning outcomes among a community-dwelling, multiethnic cohort. Our results suggest that plasma AD biomarkers are significantly related to physical functioning outcomes across ethnic groups; however, the link appears to be more consistent and stronger among Mexican Americans. This line of work is important as it focuses on the AD biomarkers comprised in the National Institute on Aging-Alzheimer’s Association (NIA-AA) research framework. This framework consists of A = amyloid, T = tau, and N = neurodegeneration (37), with the focus on understanding how such biomarkers relate to AD disease progression and development including during the preclinical stage. The aim of the framework is to spur research into the study of the relationship between pathological processes and cognitive symptoms (37).
In our prior work focused on the AT(N) framework, we have demonstrated that AD biomarkers are different among Mexican Americans as compared to non-Hispanic Whites (13,15,38). In fact, in a recent study from the HABS-HD cohort (14), we demonstrated that blood-based biomarker profiles were highly accurate in detecting MCI and AD among Mexican Americans and non-Hispanic Whites; however, the relative importance of the biomarkers varied across both diagnostic and ethnic groups. In that work, the plasma AD-specific biomarkers (measured in this study) were only ranked in the top half of the variable importance plots among non-Hispanic Whites and were not weighted of importance among Mexican Americans (14).
The current findings support that ethnicity can contribute to differences in functional outcomes. This could be due to increased rates of medical comorbidities observed among certain ethnic groups, which, in and of themselves, have also previously been shown to be related to plasma AD biomarkers (39). Prior work from our own group has shown a link between biomarkers of metabolic functioning (including insulin and glucagon) and brain amyloid specifically among Mexican Americans (40). Supplemental analyses conducted as part of this study found that for the total sample, the significant relationship between AD biomarkers and poorer physical functioning (both on TUG and on SPPB) remained after controlling for medical comorbidities (hypertension, diabetes mellitus, and dyslipidemia).
Prior work has demonstrated a link between plasma AD biomarkers and functional outcomes. Ding et al. (41) found that plasma Aβ 42 was negatively associated with postural instability gait difficulty type of PD. He et al. looked at plasma Aβ 42/Aβ 40 ratio, NfL, and Progranulin (PGRN) among participants of the Multidomain Alzheimer’s Prevention Trial (MAPT) in relation to SPPB (28). This work found that cross-sectionally, higher plasma NfL were found to be associated with lower SPPB scores; however, longitudinally, PGRN levels were associated with decreased grip strength (28). In an independent study of 452 participants of the MAPT trial. He et al. (42) found no significant cross-sectional links between Aβ 42/Aβ 40 ratio positivity, NfL positivity, and cognitive or physical functioning; however, longitudinally elevations in these markers was associated with declines in both cognitive and physical measures. Our findings reflect both increased statistical power due to larger sample size as well as the capacity to examine the differential links across ethnic groups. Therefore, the current findings add significantly to the existing literature. Given that functional and cognitive outcomes are typically measured in clinical trials targeting AD (prevention and treatment), additional work needs to be conducted to determine the relationship between plasma biomarkers and physical function and then how each could be used separately or in combination to aid in interpreting clinical trial outcomes.
The current findings provide additional evidence that biomarkers of AD vary across racial and ethnic groups and requires additional investigation. Despite the fact that Hispanic and African Americans in the United States have a discorporate burden of AD/ADRD (1,10), and reflect the 2nd largest non-White portions of the population (43), these groups remain severely underrepresented in AD science as do other racial/ethnic groups across the globe (2). The singular recruitment of non-Hispanic White participants has led to a myopic understanding of these AD biomarkers that may not directly apply to diverse populations. In fact, we have previously demonstrated that underlying cerebral amyloid burden is lower among clinically diagnosed MCI and AD Mexican Americans as compared to non-Hispanic Whites (13) and our unpublished findings demonstrate the same among African Americans. In the A4 study, cerebral amyloid positivity was also lower among African Americans as compared to non-Hispanic Whites (44). These findings, within the context of other work, suggest that our conceptual framework of AT(N)-pathology may not be directly applicable to diverse populations. These fundamental questions must be addressed to have population-informed clinical trials designed to treat and/or prevent AD across all communities.
Although the sample size, community-based nature, and ethnic diversity are significant strengths of the study, there are weaknesses to the current work. First and foremost, these are cross-sectional analyses. However, the HABS-HD study is longitudinal and, therefore, the link between plasma AD biomarkers and physical functioning over time will be examined in the near future. An additional limitation is inclusion of only those cognitively unimpaired in the sample. Although the aim was to examine the relationship of the AD biomarkers and physical functioning measures among those prior to experiencing cognitive decline, additional follow-up work is planned to expand the application to those with cognitive impairment (MCI and dementia). Another limitation of the study was the exclusion of physical activity from the analyses. Given the known link between physical activity and physical function it will be important for future work to include this variable when examining the link with AD biomarkers. Future work will also need to seek to examine possible interaction effects of vascular and socioeconomic factors. Additionally, this work only included Mexican Americans and non-Hispanic Whites. However, the HABS-HD study is currently enrolling 1,000 African Americans and, therefore, follow-up work will be conducted examining all 3 groups simultaneously, which reflects 75% of the U.S. population, both cross-sectionally and longitudinally. Finally, recent work suggests that additional plasma AD biomarkers (p-tau 217, glial fibrillary acidic protein, etc.) (45,46) may be of significant importance and, therefore, these markers are being assayed in the HABS-HD study and will be included in future examinations. Overall, the current findings add significantly to the extant literature and highlight the need for additional work in this area across diverse communities.
Acknowledgments
HABS-HD Study Team: MPIs: Sid E. O’Bryant, Kristine Yaffe, Arthur Toga, Robert Rissman, and Leigh Johnson, and the HABS-HD Investigators: Meredith Braskie, Kevin King, Matthew Borzage, James R. Hall, Melissa Petersen, Raymond Palmer, Robert Barber, Yonggang Shi, Fan Zhang, Rajesh Nandy, Roderick McColl, David Mason, Bradley Christian, Nicole Philips, and Stephanie Large.
Contributor Information
Sid E O’Bryant, Institute for Translational Research, University of North Texas Health Science Center, Fort Worth, Texas, USA; Department of Family Medicine, University of North Texas Health Science Center, Fort Worth, Texas, USA.
Melissa Petersen, Institute for Translational Research, University of North Texas Health Science Center, Fort Worth, Texas, USA; Department of Family Medicine, University of North Texas Health Science Center, Fort Worth, Texas, USA.
James R Hall, Institute for Translational Research, University of North Texas Health Science Center, Fort Worth, Texas, USA; Department of Family Medicine, University of North Texas Health Science Center, Fort Worth, Texas, USA.
Stephanie Large, Institute for Translational Research, University of North Texas Health Science Center, Fort Worth, Texas, USA; Department of Pharmacology and Neuroscience, University of North Texas Health Science Center, Fort Worth, Texas, USA.
Leigh A Johnson, Institute for Translational Research, University of North Texas Health Science Center, Fort Worth, Texas, USA; Department of Pharmacology and Neuroscience, University of North Texas Health Science Center, Fort Worth, Texas, USA.
HABS-HD Study Team:
Sid E O’Bryant, Kristine Yaffe, Arthur Toga, Robert Rissman, Leigh Johnson, Meredith Braskie, Kevin King, Matthew Borzage, James R Hall, Melissa Petersen, Raymond Palmer, Robert Barber, Yonggang Shi, Fan Zhang, Rajesh Nandy, Roderick McColl, David Mason, Bradley Christian, Nicole Philips, and Stephanie Large
Funding
This work was supported by the National Institute on Aging of the National Institutes of Health under Award Numbers R01AG054073 and R01AG058533. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The research team also thanks the local Fort Worth community and the participants of the HABS-HD study.
Conflict of Interest
S.E.O. has multiple patents on precision medicine for neurodegenerative diseases and is the founding scientist of Cx Precision Medicine. The other authors declare no conflict of interest.
Author Contributions
S.E.O. = conceptualization and design of study; acquisition, analysis, and interpretation of data; drafting and revising manuscript; final approval of version to be published; agreement to be accountable for the accuracy and integrity of the work. L.A.J. = conceptualization and design of study; acquisition, analysis, and interpretation of data; drafting and revising manuscript; final approval of version to be published; agreement to be accountable for the accuracy and integrity of the work. J.R.H. = design of study; acquisition and interpretation of data; drafting and revising manuscript; final approval of version to be published; agreement to be accountable for the accuracy and integrity of the work. M.P. = design of study; acquisition and interpretation of data; drafting and revising manuscript; final approval of version to be published; agreement to be accountable for the accuracy and integrity of the work. S.L. = design of study; acquisition and interpretation of data; drafting and revising manuscript; final approval of version to be published; agreement to be accountable for the accuracy and integrity of the work.
Ethics Approval and Consent to Participate
This study protocol was reviewed and approved by the UNTHSC IRB protocols UNTHSC 2016-128 and 2020-125. Each participant (or his/her legal representative) signed written informed consent to participate in the study.
Data Availability
The data is available to the scientific community through the UNTHSC Institute for Translational Research (ITR) website.
References
- 1. Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dementia. 2020;16:391–460. doi: 10.1002/alz.12068 [DOI] [Google Scholar]
- 2. Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: update and areas of immediate need. Alzheimers Dement. 2019;15:292–312. doi: 10.1016/j.jalz.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O’Bryant SE, Zhang F, Petersen M, Johnson L, Hall J, Rissman RA. A precision medicine approach to treating Alzheimer’s disease using Rosiglitazone therapy: a biomarker analysis of the REFLECT trials. J Alzheimers Dis. 2021;81(2):557–568. doi: 10.3233/JAD-201610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Bryant SE, Edwards M, Zhang F, et al. Potential two-step proteomic signature for Parkinson’s disease: pilot analysis in the Harvard Biomarkers Study. Alzheimers Dement (Amst). 2019;11:374–382. doi: 10.1016/j.dadm.2019.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Bryant SE, Ferman TJ, Zhang F, et al. A proteomic signature for dementia with Lewy bodies. Alzheimers Dement (Amst). 2019;11:270–276. doi: 10.1016/j.dadm.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Bryant SE, Xiao G, Zhang F, et al. Validation of a serum screen for Alzheimer’s disease across assay platforms, species, and tissues. J Alzheimers Dis. 2014;42(4):1325–1335. doi: 10.3233/JAD-141041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rauchmann BS, Schneider-Axmann T, Perneczky R; Alzheimer’s Disease Neuroimaging Initiative (ADNI). Associations of longitudinal plasma p-tau181 and NfL with tau-PET, Aβ-PET and cognition. J Neurol Neurosurg Psychiatry. 2021;92(12):1289–1295. doi: 10.1136/jnnp-2020-325537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma LZ, Hu H, Wang ZT, et al. P-tau and neurodegeneration mediate the effect of β-amyloid on cognition in non-demented elders. Alzheimers Res Ther. 2021;13(1):200. doi: 10.1186/s13195-021-00943-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cullen NC, Leuzy A, Janelidze S, et al. Plasma biomarkers of Alzheimer’s disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nat Commun. 2021;12(1):3555. doi: 10.1038/s41467-021-23746-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15:17–24. doi: 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. NACC. NACC Researcher Home Page, NACC, Alzheimer’s Disease Research, FTLD, NIA/NIH, Database, Neuropathology. n.d. https://www.alz.washington.edu/ (accessed December 7, 2020).
- 13. O’Bryant SE, Johnson LA, Barber R, et al. ; Ya for the HS Team. The Health and Aging Brain among Latino Elders (HABLE) study methods and participant characteristics. Alzheimers Dement. 2021;13(1):e12202. doi: 10.1002/dad2.12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Bryant SE, Zhang F, Petersen M, et al. for the HABLE Study Team. A blood screening tool for Alzheimer’s disease among community-dwelling Mexican Americans and non-Hispanic Whites: a method for increasing representation of diverse populations in research and trails. Alzheimers Dement. 2021;18(1):1–11. doi: 10.1002/alz.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Bryant SE, Zhang F, Petersen M, et al. ; HABLE Study Team. Neurodegenration from the AT(N) framework is different among Mexican Americans compared to non-Hispanic Whites: a Health & Aging Brain among Latino Elders (HABLE) Study. Alzheimers Dement. 2022;14(1):e12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Bryant S, Petersen M, Hall J, et al. ; HABLE Study Team. Characterizing plasma NfL in a community-dwelling multi-ethnic cohort: results from the HABLE study. Alzheimers Dement. 2021;18(2):240–250. doi: 10.1002/alz.12404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Bryant SE, Humphreys JD, Schiffer RB, Sutker PB. Presentation of Mexican Americans to a memory disorder clinic. J Psychopathol Behav Assess. 2007;29:137–140. doi: 10.1007/s10862-006-9042-9 [DOI] [Google Scholar]
- 18. O’Bryant SE, Johnson L, Reisch J, et al. Risk factors for mild cognitive impairment among Mexican Americans. Alzheimers Dement. 2013;9:622–631.e1. doi: 10.1016/j.jalz.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Bryant SE, Johnson L, Balldin V, et al. Characterization of Mexican Americans with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2013;33:373–379. doi: 10.3233/JAD-2012-121420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sverdrup K, Selbæk G, Bergh S, et al. Physical performance across the cognitive spectrum and between dementia subtypes in a population-based sample of older adults: the HUNT study. Arch Gerontol Geriatr. 2021;95:104400. doi: 10.1016/j.archger.2021.104400 [DOI] [PubMed] [Google Scholar]
- 21. Taraldsen K, Helbostad JL, Follestad T, Bergh S, Selbæk G, Saltvedt I. Gait, physical function, and physical activity in three groups of home-dwelling older adults with different severity of cognitive impairment—a cross-sectional study. BMC Geriatr. 2021;21(1):670. doi: 10.1186/s12877-021-02598-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ries JD, Echternach JL, Nof L, Blodgett MG. Test–retest reliability and minimal detectable change scores for the timed “Up & Go” test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys Ther. 2009;89:569–579. doi: 10.2522/PTJ.20080258 [DOI] [PubMed] [Google Scholar]
- 23. Greene BR, Kenny RA. Assessment of cognitive decline through quantitative analysis of the timed up and go test. IEEE Trans Biomed Eng. 2012;59:988–995. doi: 10.1109/TBME.2011.2181844 [DOI] [PubMed] [Google Scholar]
- 24. Ansai JH, de Andrade LP, Nakagawa TH, et al. Cognitive correlates of timed up and go subtasks in older people with preserved cognition, mild cognitive impairment, and Alzheimer’s disease. Am J Phys Med Rehabil. 2017;96:700–705. doi: 10.1097/PHM.0000000000000722 [DOI] [PubMed] [Google Scholar]
- 25. Åhman HB, Giedraitis V, Cedervall Y, et al. Dual–task performance and neurodegeneration: correlations between timed up-and-go dual-task test outcomes and Alzheimer’s disease cerebrospinal fluid biomarkers. J Alzheimer’s Dis. 2019;71:S75–S83. doi: 10.3233/JAD-181265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kameniar K, Mackintosh S, Van Kessel G, Kumar S. The psychometric properties of the Short Physical Performance Battery to assess physical performance in older adults: a systematic review. J Geriatr Phys Ther. 2022. Online ahead of print. doi: 10.1519/JPT.0000000000000337 [DOI] [PubMed] [Google Scholar]
- 27. Legdeur N, Badissi M, Yaqub M, et al. What determines cognitive functioning in the oldest-old? The EMIF-AD 90+ Study. J Gerontol B Psychol Sci Soc Sci. 2021;76:1499–1511. doi: 10.1093/GERONB/GBAA152 [DOI] [PubMed] [Google Scholar]
- 28. He L, de Souto Barreto P, Giudici KV, et al. Cross-sectional and longitudinal associations between plasma neurodegenerative biomarkers and physical performance among community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2021;76:1874–1881. doi: 10.1093/GERONA/GLAA284 [DOI] [PubMed] [Google Scholar]
- 29. Institute for Translational Research. n.d. https://apps.unthsc.edu/itr/ (accessed December 19, 2020).
- 30. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 31. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/J.1532-5415.1991.TB01616.X [DOI] [PubMed] [Google Scholar]
- 32. Treacy D, Hassett L. The Short Physical Performance Battery. J. Physiother. 2018;64:61. doi: 10.1016/J.JPHYS.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 33. Ack J, Uralnik MG, Uigi L, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 2009;332:556–562. doi: 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O’Bryant SE, Gupta V, Henriksen K, et al. Guidelines for the standardization of preanalytic variables for blood-based biomarker studies in Alzheimer’s disease research. Alzheimers Dement. 2015;11(5):549–560. doi: 10.1016/j.jalz.2014.08.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O’Bryant SE, Lacritz LH, Hall J, et al. Validation of the new interpretive guidelines for the clinical dementia rating scale sum of boxes score in the National Alzheimer’s Coordinating Center database. Arch Neurol. 2010;67:746–749. doi: 10.1001/archneurol.2010.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O’Bryant SE, Waring SC, Cullum CM, et al. Staging dementia using Clinical Dementia Rating Scale sum of boxes scores. Arch Neurol. 2008;65:1091–1095. doi: 10.1001/archneur.65.8.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O’Bryant S, Petersen M, Hall J, et al. Characterizing plasma NfL in a community-dwelling multi-ethnic cohort: Results from the HABLE study. Alzheimers Dement. 2021;18(2):240–250. doi: 10.1002/alz.12404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Syrjanen JA, Campbell MR, Algeciras-Schimnich A, et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. 2021;18(6):1128–1140. doi: 10.1002/alz.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O’Bryant SE, Petersen M, Hall J, Johnson L; HABS-HD Study Team. Metabolic factors are related to brain amyloid among Mexican Americans: a HABS-HD study. J Alzheimers Dis. 2022;86(4):1745–1750. doi: 10.3233/JAD-215620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ding J, Zhang J, Wang X, et al. Relationship between the plasma levels of neurodegenerative proteins and motor subtypes of Parkinson’s disease. J Neural Transm. 2017;124:353–360. doi: 10.1007/s00702-016-1650-2 [DOI] [PubMed] [Google Scholar]
- 42. He L, de Souto Barreto P, Aggarwal G, et al. Plasma Aβ and neurofilament light chain are associated with cognitive and physical function decline in non-dementia older adults. Alzheimers Res Ther. 2020;12:128. doi: 10.1186/s13195-020-00697-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. U.S. Census Bureau. U.S. Census Bureau QuickFacts: United States. Quick Facts 2020:2019–21. https://www.census.gov/quickfacts/fact/table/US/PST045219 (accessed December 7, 2020).
- 44. Deters KD, Napolioni V, Sperling RA, et al. Amyloid PET imaging in self-identified non-Hispanic Black participants of the anti-amyloid in asymptomatic Alzheimer’s disease (A4) Study. Neurology. 2021;96:e1491–e1500. doi: 10.1212/WNL.0000000000011599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Janelidze S, Berron D, Smith R, et al. Associations of plasma phospho-Tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA Neurol. 2021;78:149–156. doi: 10.1001/jamaneurol.2020.4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mielke MM, Hagen CE, Xu J, et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 2018;14:989–997. doi: 10.1016/j.jalz.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available to the scientific community through the UNTHSC Institute for Translational Research (ITR) website.