Abstract
Murine immunization with Trypanosoma cruzi KMP11-HSP70 fused genes but not the KMP11 gene alone elicited both an immunoglobulin G2a long-lasting humoral immune response against KMP11 protein and activation of CD8+ cytotoxic T lymphocytes specific for two KMP11 peptides containing A2 motifs. Moreover, protection against the parasite challenge was observed after immunization with the chimeric gene.
In the 1990s, vaccine development received a new impetus from the discovery that antigen-encoding DNA plasmids were able to induce cellular and humoral immune responses against pathogenic viruses, bacteria, and parasites (8, 27). Although different experimental studies performed primarily with mice have shown that the immunity generated by DNA vaccines can confer protection against pathogen challenges (20, 33), it has also become clear that the efficacy of the vaccine decreases when the same regimen is applied to higher organisms such as primates. In attempts to enhance the immune responses generated by DNA vaccines, the coinjection of plasmids encoding the foreign antigen fused to genes encoding immunostimulatory molecules has been assayed (5, 9). Moreover, different studies have demonstrated that immunization of animals with haptens coupled to or antigens fused to heat shock proteins (HSPs) in the absence of an adjuvant elicits hapten- or antigen-specific immune responses (2, 16, 22, 23).
Trypanosoma cruzi is an intracellular protozoan parasite that infects humans and causes Chagas' disease, one of the major public health problems in many countries of Central and South America (25). Conventional chemotherapy has low efficacy (7), so viable parasites and chronic local inflammations may be detected during the whole life of the patient (31), making necessary the search for new alternatives to prevent or ameliorate the disease. Vaccines probably constitute the most appropriate approach. The kinetoplastid-specific KMP11 protein was first described for Leishmania donovani associated with the lypophosphoglycan molecule. It has been reported to be a potent inducer of immune cellular responses, and it is thought to have a role in protective immunity (12, 30). It has been demonstrated recently that the T. cruzi KMP11 protein is located mainly in the parasite's flagellar pocket and that it is associated with the cytoskeleton (28), structures critical for the mobility of the parasite and for its attachment to the host cell. In the present study, we addressed the questions of whether T. cruzi HSP70 within a DNA vaccine context would have any immunomodulatory effect on the KMP11 antigen to which it is fused and whether this chimeric molecule confers protection against lethal infection by T. cruzi.
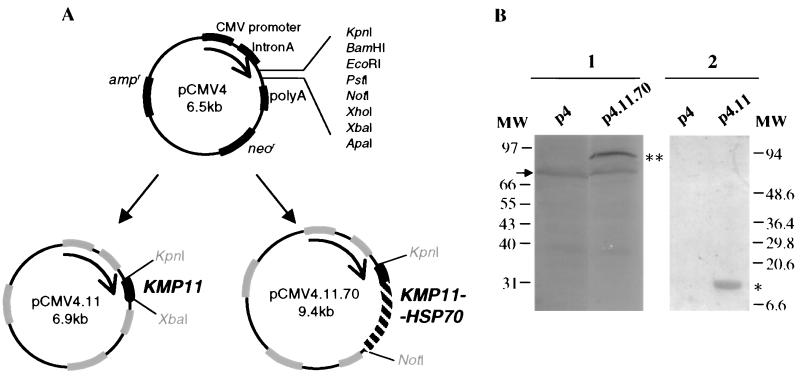
To generate the DNA vaccine vectors shown in Fig. 1A, KMP11 and HSP70 genes were obtained from the TcKMP11n clone (28) and the pQE-70 clone (14), respectively. All the transformants were identified by restriction analysis, and their identities were further confirmed by automatic sequencing. Plasmid DNAs were purified using an Endofree Plasmid Gigakit (Qiagen). The recombinant plasmids (Fig. 1A) express the KMP11 protein and the KMP11-HSP70 fusion protein, as demonstrated by Western blotting of transfected COS-7 cells using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The immunoblots, through the use of polyclonal anti-KMP11 (28) and anti-HSP70 (15) antibodies, showed (Fig. 1B) two bands of approximately 11 and 83 kDa in the p4.11 and p4.11.70 lanes, respectively. The slightly stained bands of approximately 70 kDa present in the panel incubated with the anti-HSP70 antibody should correspond to the 70-kDa HSP of COS-7 cells.
FIG. 1.
(A) Construction of the DNA vaccines. T. cruzi KMP11 and KMP11-HSP70 genes were cloned separately between the cytomegalovirus promoter sequence and the bovine growth hormone polyadenylation sequence in the pCMV4 expression vector, whose characteristics are summarized in this figure, generating pCMV4.11 and pCMV4.11.70 clones. To construct the vector pCMV4.11.70 containing the fused genes, the KMP11 coding sequence with the stop codon deleted was cloned upstream and in frame with the HSP70 gene previously cloned in the pCMV4 vector. (B) Expression of KMP11 and KMP11-HSP70 proteins in COS-7 cells. Protein expression was checked in vitro by plasmid transient transfection with lipofectin (Gibco) into COS-7 cells, followed by Western blotting of the cell extracts (29). Antisera produced in rabbits and directed against the GMPG repeated motif located at the C termini of the T. cruzi HSP70 protein (15) and the KMP11 protein (24) were used (panels 1 and 2, respectively). Lanes p4, cells transfected with the control vector; lane p4.11.70, cells transfected with the vector bearing the coding sequence for the KMP11-HSP70 fusion protein; lane p4.11, cells transfected with the DNA plasmid containing the gene coding for the KMP11 protein. Double and single asterisks indicate the locations of the KMP11-HSP70 fusion protein and the KMP11 protein, respectively. MW, molecular weights of standard proteins in thousands.
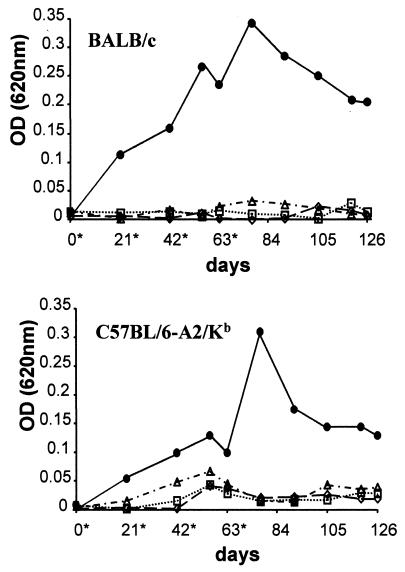
We investigated whether mice of different haplotypes (BALB/c-H2Kd and C57BL/6-H2Kb obtained from IFFA-CREDO (CRIFFA, Lyon, France) would elicit an anti-KMP11 humoral response after inoculation with the vector containing the KMP11-encoding gene alone as well as that containing the KMP11-encoding gene fused to the HSP70 gene. Female mice (6 to 8 weeks old) of both strains and C57BL/6-A2.1/Kb transgenic mice (32) received intramuscularly DNA vaccines four times at 3-week intervals. As a control, we immunized mice with the empty vector or with saline solution. The anti-KMP11-specific antibody levels were determined by enzyme-linked immunosorbent assay (ELISA) using purified KMP11 recombinant protein as an antigen, obtained as previously described (29). The antibody response (immunoglobulin G [IgG]) induced by the DNA constructs is shown in Fig. 2. Only the sera from the animals vaccinated with the construct expressing the KMP11-HSP70 fusion protein presented high antibody titers, and these titers were slightly higher in the BALB/c strain than in the C57BL/6 strain. In both mouse strains, enhancement of the humoral response occurred in a dose-dependent manner, with a maximum level achieved 2 weeks after the fourth immunization. Moreover, the antibody response was long-lived, since positive anti-KMP11 reactivity could be detected 9 weeks after the last immunization. For both mouse strains, analysis of the IgG subclasses in the pooled sera revealed that immunization with the construct containing the KMP11-HSP70 fused genes induced a clear IgG2a antibody bias (Fig. 3), which is indicative of a predominant Th1 response.
FIG. 2.
Detection of anti-KMP11 IgG antibody levels in the sera of mice immunized with DNA plasmids or saline solution. BALB/c (top panel) and C57BL/6-A2.1/Kb (bottom panel) mice were immunized intramuscularly four times with saline solution (◊) or 100 μg of each the DNA vectors pCMV4 (□), pCMV4.11 (▵), and pCMV4.11.70 (●). Production of IgG antibodies to KMP11 was evaluated by ELISA (29) on days 0, 21, 42, 56, 63, 77, 91, 105, 119, and 126 using 1 μg of recombinant KMP11 protein/well. Data are optical density (OD) values of pooled sera from six mice per group. These and all subsequent data show representative results of at least three independent experiments. Asterisks indicate immunization days.
FIG. 3.
IgG isotype level generated against KMP11 protein in mice immunized with pCMV4.11.70. The antibody level was determined by ELISA with sera from BALB/c (top panel) and C57BL/6-A2.1/Kb (bottom panel) mice, intramuscularly immunized with pCMV4.11.70 DNA, using 1 μg of recombinant KMP11 protein/well. IgG1 (○) and IgG2a (●) antibodies produced against KMP11 were evaluated in serum samples obtained on days 0, 21, 42, 56, 63, 77, 91, 105, 119, and 126. Data are optical density (OD) values of pooled sera from 6 mice. Asterisks indicate immunization days.
In an attempt to analyze the ability of HSP70 to induce a KMP11-specific cytotoxic response, we studied the presence of cytotoxic T lymphocytes (CTLs) in C57BL/6-A2.1/Kb transgenic mice immunized with DNA plasmids. Cells were cultured in complete medium consisting of Dulbecco's modified Eagle medium (Gibco BRL) supplemented with 10% fetal calf serum (Life Technologies), 2 mM l-glutamine (Gibco BRL), 50 μM 2-mercaptoethanol (Sigma), 100 IU of penicillin (Sigma)/ml, and 100 μg of streptomycin (Sigma)/ml. Ten units of recombinant murine interleukin-10 (Boehringer Mannheim)/ml was added for the cytotoxicity assays. KMP11-specific peptides (Fig. 4A) containing theoretically estimated A2.1 binding motifs (17) were tested using spleen cells from the immunized mice. Two weeks after the last immunization, pooled splenocytes from two mice per group were stimulated in vitro with EL4-A2.1/Kb cells loaded separately with peptide K1, K2, K3, or K4. An EL4-A2.1/Kb cell line, expressing the product of the HLA-A2.1/Kb chimeric gene (32), was grown in the presence of 400 μg of G418 sulfate (Sigma)/ml. After 6 days of stimulation, a classical chromium assay was carried out. The results are shown in Fig. 4B. CTL activity with specificity towards the EL4-A2.1/Kb target cells loaded with the K1 and K4 peptides (60 and 55%, respectively) was observed only in the mice immunized with the plasmid containing the KMP11-HSP70 fused genes. Analysis of the surface phenotype of the generated CTL lines, observed after two in vitro restimulations, showed it to be composed of CD8+ cells (results not shown). This A2.1-restricted cytotoxic response is very relevant, since the HLA-A2 allele is the most common HLA type in sera from people living in areas where Chagas' disease is endemic (13).
FIG. 4.
KMP11 peptide-specific CTL response. (A) KMP11 deduced amino acid sequence and composition of synthesized peptides containing theoretically estimated A2 union motifs (17). The asterisk indicates the protein stop codon. The positions of the designed A2 peptides K1, K2, K3, and K4 are marked. (B) KMP11 peptide-specific CTLs elicited after immunization with the DNA vector carrying the KMP11-HSP70 fusion. Spleens from C57BL/6-A2.1/Kb mice immunized with saline solution (□) or the pCMV4 (∗), pCMV4.11 (▵), and pCMV4.11.70 (⧫) vectors were removed 2 weeks after the last immunization. Splenocytes were used as effector cells after being incubated for 6 days with EL4-A2.1/Kb cells treated with 50 μg of mitomycin/ml and loaded separately with each of the four KMP11 A2 peptides. CTL activity was measured against EL4-A2.1/Kb cells pulsed with or without each one of the respective peptides by a standard 51Cr release assay (19). Each panel corresponds to results with one A2 peptide. The level of lysis of EL4-A2.1/Kb cells in the absence of peptide was <5% for all groups (data not shown). Specific lysis was calculated using the following formula: percent specific lysis = (experimental release [cpm] − spontaneous release [cpm]/(total release [cpm] − spontaneous release [cpm] × 100), where cpm is counts per minute. Spontaneous release represents the counts obtained when the target cells were incubated in culture medium without effectors, and total release was obtained after treatment of target cells with 2.5% Triton X-100. Experiments with more than 20% spontaneous lysis were discarded. Data are representative of results with three mice per group.
In order to determine whether immunization of BALB/c mice with DNA plasmids provides some degree of protection against T. cruzi infection, we carried out challenges with 103 blood trypomastigote forms 9 weeks after the fourth immunization. It has been reported that BALB/c mice are susceptible to T. cruzi experimental infection (10, 18). The results (Fig. 5A) show that challenged BALB/c mice immunized with the KMP11-HSP70 fused genes or the KMP11 gene have a lower degree of parasitemia than that detected in control mice inoculated with an empty plasmid or with saline solution. Remarkably, we observed that only the mice immunized with the plasmid containing the fused genes survived lethal infection by T. cruzi (three out of six mice) (Fig. 5B) and presented IgG2a antibodies against KMP11 protein after challenge (Fig. 6). Recent studies have shown that CTLs against parasite antigens and/or an immune response mediated by CD8+ T cells is required to generate a protective immunity in the initial phase of the disease in order to control T. cruzi infection (26). Moreover, a humoral immune response, associated mainly with the presence of antibodies of the IgG2a isotype, seems to be essential to maintain the long-term survival of infected animals (3).
FIG. 5.
Parasitemia and survival percentages for immunized mice after challenge with T. cruzi. Mice were immunized with saline solution (□) or pCMV4 (▵), pCMV4.11 (○), and pCMV4.11.70 (⧫). Six mice per group were challenged with 103 T. cruzi blood trypomastigotes (Y Brazil strain) 9 weeks after the fourth immunization (A). The levels of parasites in the bloodstream were determined individually for three mice per group using a Neubauer chamber. Values are means ± standard deviations (SD) of the means of results for three mice. (B) Survival percentages of immunized mice challenged 2 weeks after the last immunization were regularly recorded.
FIG. 6.
Anti-KMP11 IgG2a antibodies in immunized mice after challenge with T. cruzi. Nine weeks after the last immunization, six BALB/c mice from each group were challenged with 103 T. cruzi blood trypomastigotes (Y Brazil strain). On days 10, 14, 17, and 22 after challenge, anti-KMP11 IgG2a levels in all the mice were individually tested by ELISA using 1 μg of recombinant KMP11 protein per well. The bars represent the means of optical density (OD) values ± SD of the results for six mice immunized with saline solution (□), pCMV4 (▤), or pCMV4.11 (▨). ■ and  represent the means of optical density (OD) values ± SD of the sera of three mice immunized with pCMV4.11.70 that survived or died, respectively, after the T. cruzi challenge.
represent the means of optical density (OD) values ± SD of the sera of three mice immunized with pCMV4.11.70 that survived or died, respectively, after the T. cruzi challenge.
Although there are many data supporting the adjuvant-like effects of HSP70 molecules, little is known about the action mechanism of genetic vaccines. A question still unresolved is whether the immune response induced after intramuscular immunization of DNAs is promoted by direct priming (using products expressed by transfected antigen-presenting cells) or by a cross-presentation process. Our findings suggest that the KMP11-HSP70 fusion protein may be expressed and released by monocytes and cross-presented by untransfected antigen-presenting cells. Recently, specific HSP receptors in macrophages and dendritic cells (DCs) have been described (1). Thus, DCs capture the KMP11-HSP70 fusion antigen, which acts to induce maturation and Th1 cytokine production, and consequently DCs are ready to prime CTL activity. Moreover, it has recently been reported that immunization with OVA protein fused to M. tuberculosis HSP70 protein elicited an OVA-specific CTL response independent of CD4 T cells (11). That this is the mechanism proposed to overcome the participation of CD4+ T cells in the induction of CD8+ CTLs implies that fused HSPs have the capacity to stimulate DCs, upregulating the levels of major histocompatibility complex classes I and II and costimulatory (B7.2) molecules (4). Preliminary studies (data not shown) support this hypothesis, as we have detected in vitro that KMP11-HSP70 protein is able to promote maturation of murine DCs. In addition, studying the capability of HSP70 to improve the translocation of proteins to different subcellular compartments (6) and to induce the breaking of intracellular proteins (21) would also facilitate an understanding of why HSP70 fused to an antigen leads to a major compatibility complex class I processing pathway and elicits CD8+ CTLs against the antigen. In conclusion, the results shown indicate that immunization with DNA vectors containing the HSP70 gene fused to sequences coding for appropriate antigens such as, for example, KMP11 could be used for the rational design of efficacious vaccines against T. cruzi infection.
Acknowledgments
We thank the following for providing us with some of the materials used in this study: R. Tascon for the pCMV4 vector, P. Romero for the EL4 and EL4-A2.1/Kb cells, C. Terhorst for the 2.4G2 hybridoma, and L. Sherman for the C57BL/6-A2.1/Kb mouse strain. We also thank M. E. Patarroyo and F. Guzman for synthesis of the peptides.
This work was supported by the Fondo de Investigaciones Sanitarias (grant FIS98/0914) and Plan Nacional I+D-FEDER (DGESIC) (grant 1FD1997-0630-C02-01), Spain. M. C. Thomas was supported by FIS-ISCII postdoctoral fellowship 97/4207, and L. Planelles was supported by an MEC predoctoral fellowship.
REFERENCES
- 1.Binder R J, Han D K, Srivastava P K. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 2.Blachere N E, Li Z, Chandawarkar R Y, Suto R, Jaikaria N S, Basu S, Udono H, Srivastava P K. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodskyn C I, Silva A M, Takehara H A, Mota I. IgG subclasses responsible for immune clearance in mice infected with Trypanosoma cruzi. Immunol Cell Biol. 1989;67:343–348. doi: 10.1038/icb.1989.50. [DOI] [PubMed] [Google Scholar]
- 4.Burleigh B A, Andrews N W. The mechanisms of Trypanosoma cruzi invasion of mammalian cells. Annu Rev Microbiol. 1995;49:175–200. doi: 10.1146/annurev.mi.49.100195.001135. [DOI] [PubMed] [Google Scholar]
- 5.Chow Y H, Chiang B L, Lee Y L, Chi W K, Lin W C, Chen Y T, Tao M H. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998;160:1320–1329. [PubMed] [Google Scholar]
- 6.Cyr D M, Neupert W. Roles for hsp70 in protein translocation across membranes of organelles. In: Feige U, Morimoto R I, Yahara I, Polla B S, editors. Stress-inducible cellular responses. Basel, Switzerland: Birkhaüser Verlag; 1996. pp. 25–40. [DOI] [PubMed] [Google Scholar]
- 7.de Andrade A L, Zicker F, de Oliveira R M, Almeida Silva S, Luquetti A, Travassos L R, Almeida I C, de Andrade S S, de Andrade J G, Martelli C M. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996;348:1407–1413. doi: 10.1016/s0140-6736(96)04128-1. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 9.Flo J, Tisminetzky S, Baralle F. Modulation of the immune response to DNA vaccine by co-delivery of costimulatory molecules. Immunology. 2000;100:259–267. doi: 10.1046/j.1365-2567.2000.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoft D F, Lynch R G, Kirchhoff L V. Kinetic analysis of antigen-specific immune response in resistant and susceptible mice during infection with Trypanosoma cruzi. J Immunol. 1993;151:7038–7047. [PubMed] [Google Scholar]
- 11.Huang Q, Richmond J F, Suzue K, Eisen H N, Young R A. In vivo cytotoxic T lymphocyte elicitation by mycobacterial heat shock protein 70 fusion proteins maps to a discrete domain and is CD4(+) T cell independent. J Exp Med. 2000;191:403–408. doi: 10.1084/jem.191.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen A T, Gasim S, Ismail A, Gaafar A, Kurtzhals J A, Kemp M, El Hassan A M, Kharazmi A, Theander T G. Humoral and cellular immune responses to synthetic peptides of the Leishmania donovani kinetoplastid membrane protein-11. Scand J Immunol. 1998;48:103–109. doi: 10.1046/j.1365-3083.1998.00370.x. [DOI] [PubMed] [Google Scholar]
- 13.Krausa P, Brywka M, Savage D, Hui K M, Bunce M, Ngai J L, Teo D L, Ong Y W, Barouch D, Allsop C E, et al. Genetic polymorphism within HLA-A∗02: significant allelic variation revealed in different populations. Tissue Antigens. 1995;45:223–231. doi: 10.1111/j.1399-0039.1995.tb02444.x. [DOI] [PubMed] [Google Scholar]
- 14.Marañon C, Planelles L, Alonso C, López M C. HSP70 from Trypanosoma cruzi is endowed with specific cell proliferation potential leading to apoptosis. Int Immunol. 2000;12:1685–1693. doi: 10.1093/intimm/12.12.1685. [DOI] [PubMed] [Google Scholar]
- 15.Martin F, Requena J M, Martin J, Alonso C, López M C. Cytoplasmic-nuclear translocation of the Hsp70 protein during environmental stress in Trypanosoma cruzi. Biochem Biophys Res Commun. 1993;196:1155–1162. doi: 10.1006/bbrc.1993.2372. [DOI] [PubMed] [Google Scholar]
- 16.Perraut R, Lussow A R, Gavoille S, Garraud O, Matile H, Tougne C, van Embden J, van der Zee R, Lambert P H, Gysin J, Del Giudice G. Successful primate immunization with peptides conjugated to purified protein derivative or mycobacterial heat shock proteins in the absence of adjuvants. Clin Exp Immunol. 1993;93:382–386. doi: 10.1111/j.1365-2249.1993.tb08189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rammensee H G, Friede T, Stevanoviic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 18.Rowland E C, Lozykowski M G, McCormick T S. Differential cardiac histopathology in inbred mouse strains chronically infected with Trypanosoma cruzi. J Parasitol. 1992;78:1059–1066. [PubMed] [Google Scholar]
- 19.Schoenberger S P, van der Voort E I, Krietemeijer G M, Offringa R, Melief C J, Toes R E. Cross-priming of CTL responses in vivo does not require antigenic peptides in the endoplasmic reticulum of immunizing cells. J Immunol. 1998;161:3808–3812. [PubMed] [Google Scholar]
- 20.Sedegah M, Hedstrom R, Hobart P, Hoffman S L. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherman M Y, Goldberg A L. Involvement of molecular chaperones in intracellular protein breakdown. In: Feige U, Morimoto R I, Yahara I, Polla B S, editors. Stress-inducible cellular responses. Basel, Switzerland: Birkhaüser Verlag; 1996. pp. 57–58. [DOI] [PubMed] [Google Scholar]
- 22.Suzue K, Young R A. Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J Immunol. 1996;156:873–879. [PubMed] [Google Scholar]
- 23.Suzue K, Zhou X, Eisen H N, Young R A. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94:13146–13151. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura Y, Peng P, Liu K, Daou M, Srivastava P K. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 25.Tanowitz H B, Kirchhoff L V, Simon D, Morris S A, Weiss L M, Wittner M. Chagas' disease. Clin Microbiol Rev. 1992;5:400–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarleton R L. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J Immunol. 1990;144:717–724. [PubMed] [Google Scholar]
- 27.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 28.Thomas M C, Garcia-Perez J L, Alonso C, Lopez M C. Molecular characterization of KMP11 from Trypanosoma cruzi: a cytoskeleton-associated protein regulated at the translational level. DNA Cell Biol. 2000;19:47–57. doi: 10.1089/104454900314708. [DOI] [PubMed] [Google Scholar]
- 29.Thomas M C, Longobardo M V, Carmelo E, Marañón C, Planelles L, Patarroyo M E, Alonso C, López M C. Mapping of the antigenic determinants of the Trypanosoma cruzi kinetoplastid membrane protein-11. Identification of a linear epitope specifically recognized by human Chagasic sera. Clin Exp Immunol. 2001;123:465–471. doi: 10.1046/j.1365-2249.2001.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tolson D L, Jardim A, Schnur L F, Stebeck C, Tuckey C, Beecroft R P, Teh H-S, Olafson R W, Pearson T W. The kinetoplastid membrane protein 11 of Leishmania donovani and African trypanosomes is a potent stimulator of T-lymphocyte proliferation. Infect Immun. 1994;62:4893–4899. doi: 10.1128/iai.62.11.4893-4899.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viotti R, Vigliano C, Armenti H, Segura E. Treatment of chronic Chagas' disease with benznidazole: clinical and serologic evolution of patients with long-term follow-up. Am Heart J. 1994;127:151–162. doi: 10.1016/0002-8703(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 32.Vitiello A, Marchesini D, Furze J, Sherman L A, Chesnut R W. Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in transgenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J Exp Med. 1991;173:1007–1015. doi: 10.1084/jem.173.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wizel B, Garg N, Tarleton R L. Vaccination with trypomastigote surface antigen 1-encoding plasmid DNA confers protection against lethal Trypanosoma cruzi infection. Infect Immun. 1998;66:5073–5081. doi: 10.1128/iai.66.11.5073-5081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]