Abstract
Over the times, carbapenems have been the choice of drug for treating multidrug-resistant (MDR) and extended spectrum beta-lactamase (ESBL)-producing organisms. The current study aimed at determining the occurrence of metallo beta-lactamase (MBL) and AmpC beta-lactamase (ABL) in gram negative bacteria isolated from clinical samples. A cross-sectional study was conducted amongst the patients visiting Manmohan Memorial Medical College and Teaching Hospital (MMTH), Kathmandu, Nepal from August 2017 to January 2018. A total of 4351 samples including urine, pus, wound swab, endotracheal tip, catheter tip, and blood were collected from the patients and processed by standard conventional microbiological methods. Antibiotic susceptibility testing (AST) of the isolates was performed by Kirby-Bauer disk diffusion method. Double disc synergy test was performed on carbapenem resistant organisms to detect production of MBL and inhibitor-based test was used for the detection of ABL production. Of the 4351 samples, 421 bacterial isolates belonging to 16 different genera were recovered, of which 303 (71.97%) were Gram negative bacilli (GNB). E. coli (189/303) and S. aureus (80/118) were the most prevalent among gram negatives and gram positives, respectively. Bacterial incidence was found significantly associated with gender, specimen type, and the department where the patients were enrolled. Colistin-sulfate and polymycin-B were the most effective drug against GNB, whereas imipenem against gram positives. Prevalence of MDR and methicillin-resistant S. aureus (MRSA) was 35.15% and 60%, respectively. The prevalence of MBL and ABL-producing isolate was 11(3.6%) and 13(4.3%), respectively. Pseudomonas aeruginosa (5/11) and E. coli (9/13) were the major MBL and ABL producers, respectively. MBL and ABL production was found to be significantly associated with the age of the patient and the specimen type. A regular antibiotic surveillance activity with screening for MBL and ABL-producing bacterial isolates in the hospital settings to curb the incidence and transmission of such difficult-to-treat pathogens.
Keywords: AmpC, β-lactams, MBL, multidrug-resistant
Introduction
β-Lactam antibiotics are frequently used in the treatment for infections caused by gram negative as well as gram positive bacteria. These antibiotics are named so because of the presence of the 4-membered β-lactam ring which is active chemical component of the drug and includes the 6-membered ring structured penicillins, monobactams, and carbapenems; and the 7-membered ring structured cephalosporins and cephamycins.1 β-lactam antibiotics can hinder the growth of organisms by binding covalently with penicillin binding proteins (PBPs) present in the cytoplasm. The increase in antibiotic resistance is an intimidating major concern in Gram positive cocci, non-Enterobacteriaceae bacilli and Enterobacteriaceae family in clinical as well as non-clinical settings.2-6 Among Gram negative bacteria, production of beta-lactamase remains the important contributing factor to beta lactams resistance as enzyme β-lactamase hydrolyses the β-lactam ring present in the antibiotics.7 Classification of β-lactamases enzymes has usually been based on either the functional characteristics of the enzymes or their primary structure.8 The simplest mode of classification is by protein sequence, based on conserved and distinguishing amino acid motifs from where the β-lactamases are divided into 4 molecular classes, A, B, C, and D.9 Classes A, C, and D include enzymes that hydrolyze their substrates by forming an acyl enzyme through an active site serine, whereas class B β-lactamases are metallo-enzymes that utilize at least 1 active-site zinc ion to help Β-lactam hydrolysis.8 Initially, class C β-lactamases (also known as Amp-C type enzymes) were described as chromosomally encoded enzymes found in several Gram-negative bacilli including Pseudomonas aeruginosa, Acinetobacter baumannii, and some members of the family Enterobacteriaceae (eg, Citrobacter freundii, Enterobacter cloacae, Serratia marcescens, and Morganella morganii), but several studies have reported plasmid encoded transferable class C enzymes.10,11 These plasmid-encoded AmpC-type β-lactamases are usually produced constitutively, and their prevalence among Enterobacteriaceae is increasing.10 Penicillins and many cephalosporins (including extended spectrum cephalosporins) are susceptible to Class C β-lactamases, are not inhibited by clavulanic acid, sulbactam, or tazobactam, but are inhibited by cloxacillin and avibactam.12 Class B β-lactamases are zinc-dependent enzymes, so known as Metallo β-Lactamases (MBL) and largely related with their constant and efficient carbapenemase activity, and their spectrum often extends to most other β-lactams.13 MBL are not susceptible to therapeutic serine β-lactamases inhibitors (such as clavulanate and sulfones). Moreover, their genes are carried on highly mobile elements, allowing easy dissemination.1 MBLs are found as chromosomally-encoded resident enzymes in some environmental species of low pathogenic potential (Stenotrophomonas maltophilia, Aeromonas hydrophila), but since the mid-1990s several plasmid-encoded MBLs have emerged as acquired carbapenemase in gram-negative non-fermenters and Enterobacteriales. In recent years, MBL genes have spread from P. aeruginosa to members of the Enterobacteriaceae. The VIM, NDM, and IMP-type enzymes are currently the most prevalent and widespread acquired MBLs encountered among clinical isolates.12 Globally, ABL and MBL producers have emerged with varying rate in different places.14,15 Despite a higher prevalence rate of AmpC β-lactamase producing Enterobacteriales in neighboring countries, it is very little known in Nepal.16 Looking at the current trends of ABL and MBL production in bacterial isolates, it is very important to detect the production of such enzymes, at least phenotypically, if not possible by molecular methods in the country like ours’. Unfortunately, it is not a regular practice to investigate the incidence of such isolates in Nepal. In light of this, the current study was undertaken to investigate the prevalence of ABL and MBL-producing bacterial isolates in the clinical specimens and get an insight into some pertinent factors that affect their production.
Methodology
Study design and setup
A hospital based cross-sectional study was conducted at the Department of Microbiology, Manmohan Memorial Medical College and Teaching Hospital (MMTH), a 300-bedded tertiary care hospital, in Swaymbhu, Kathmandu, Nepal. Study hospital is a referral center boasting state-of-the-art facilities withmedical, surgical, gynecological, pediatric, geriatric, and other specialties. The duration of the study was 6 months (August 2017 to January 2018). All the patients of both sexes and any age group visiting both out-patient department (OPD) and in-patient department (IPD) were recruited in the study.
Sample size determination
The general formula Sample size (N) = (Zα)2 × p × q/e2 was used to calculate the sample size. As the highest prevalence for ABL and MBL as revealed by the literature search was 27.5% and 50.2%, respectively,17 an average prevalence of 39% was considered. Taking “P” value as .39 and allowable error of .05 (absolute error), sample size was calculated as follows:
Bacterial isolates
Altogether, 421 clinical strains were isolated from 4351 different clinical specimens such as urine, blood, pus/wound swab, sputum, body fluids etc. received in the clinical bacteriology laboratory, from the patients suspected with infections. The isolation of the bacteria and their identification were done according to the standard protocol suggested by American Society for Microbiology [ASM].18
Antimicrobial susceptibility testing
Kirby-Bauer disk diffusion method was used to find out the resistant pattern of the isolates on Mueller Hinton Agar (HiMedia Laboratories India). Standard zone size interpretative chart recommended by Clinical and Laboratory Standards Institute (CLSI) was used to determine the antimicrobial pattern of the isolates.19 Antibiotics used in this study were Amikacin (30 µg) Ampicillin (10 µg), Ceftazidime (30 µg), Colistin-Sulfate (10 µg), Cotrimoxazole (25 µg), Cefotaxime (30 µg), Cefoxitin (30 µg), Gentamicin (10 µg), Imipenem (10 µg), Levofloxacin (5 µg), Meropenem (10 µg), Nitrofurantoin (300 µg), Ofloxacin (5 µg), Polymyxin B (300 units), Piperacillin-Tazobactam (100/10 µg), Azithromycin (30 µg), and Oxacillin (1 µg).
Screening of MDR, MBL, and ABL producers
The isolates resistant to at least 1 antibiotic of 3 different classes of commonly used antibiotics were regarded as multidrug resistant (MDR).20 Isolates showing resistance to imipenem (10 µg) and ceftazidime (30 µg) were further subjected to the detection of possible MBL production. Similarly, isolates with zone diameter less than 18 mm against cefoxitin (30 µg) were suspected as AmpC positive and selected for the confirmation of AmpC production.
Phenotypic confirmation of MBL and ABL
To identify MBL producer, two 10 µg imipenem disks were placed on the surface of agar plate, and 750 µl of 0.5 M EDTA solution of pH 8 was added on one of them. The inhibition zones of imipenem and imipenem-EDTA disks were compared after 16 to 18 hours of incubation in aerobic condition at 37°C. All of the MBL positive isolates were separated from MBL negative isolates by the criterion of a ⩾7 mm increase in inhibition zone with the disks to which EDTA was added.21
Currently there are no standardized phenotypic methods for the screening and detection of AmpC enzymes. In the current study, the ABL production was confirmed by inhibitor based method by using phenyl boronic acid (PBA) and AmpC disk test with EDTA as described by Coudon et al22 and Black et al,23 respectively. In inhibitor based method, phenyl boronic acid solution was prepared by dissolving 120 mg of phenyl boronic acid in 3 mL of DMSO (dimethyl sulfonic acid), to which 3 mL of sterile distilled water was added. A 20 µl stock solution of phenyl boronic acid solution was dispensed on cefoxitin (30 µg) and cefoxitin (30 µg) disk with phenyl boronic acid (400 µg) was placed on MHA plate inoculated with the test isolate. The inoculated plates were then incubated overnight at 37°C for 24 hours. Organisms showing an increase of ⩾5 mm of zone diameter around the cefoxitin disk with PBA as compared to that of cefoxitin disk alone was considered as ABL producer.22
In AmpC disk test, AmpC disks were prepared by adding 20 μL of a 1:1 mixture of saline and Tris-EDTA to sterile filter paper disks and then dried and refrigerated. Immediately prior to use, AmpC disks were rehydrated with 20 mL of saline and several colonies of each test organism were applied to a disk. A 30 μg cefoxitin disk was placed at the inoculated surface of the Mueller-Hinton agar containing E. coli ATCC 25922. The inoculated AmpC disk was then placed nearly touching the antibiotic disk with the inoculated disk face coming in contact with the agar surface. The plate was then inverted and incubated overnight at 35°C in ambient air. An indentation or a flattening of the zone of inhibition, indicating enzymatic inactivation of cefoxitin was considered as positive.23 During identification and antibiotic susceptibility testing of organisms, control strain of E. coli ATCC 25922 was tested primarily.
Data analysis and interpretation
Information on patients’ demographic information such as age, sex, specimens, and bacterial isolates were entered into Statistical Package for Social Sciences [SPSS™] version 20.0 [IBM, Armonk, NY, USA] and results were interpreted by descriptive statistics. Chi-squared test was used to draw association between relevant variables and a value of P ⩽ .05 was considered significant.
Results
Patient demographics
During the study period, a total of 421 bacteria were isolated from 4351 different clinical specimens such as urine (n = 2640), pus/wound swab (n = 187), and sputum (n = 592). The male to female ratio in the study population was 1:1.5. Patients from inpatient department (IPD) accounted for larger fraction of samples as compared to the outpatient department (OPD). Samples were collected largely from the age group 25 to 44 (35.53%). Average year of the patients enrolled in this study was found to be 40.03 years.
Distribution of isolates
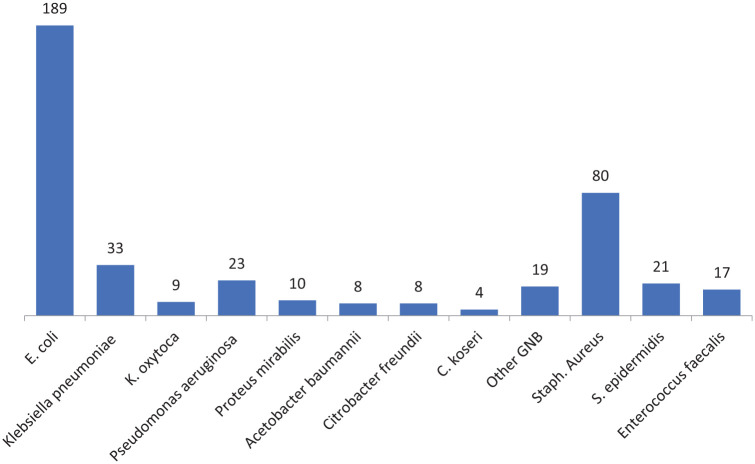
Out of 421 bacteria recovered, 303 isolates were Gram negative, whereas only 118 were Gram positive. Among 303 Gram negative isolates, 267 (88.1%) belonged to Enterobacteriaceae family, while other 36 (11.9%) were non-Enterobacteriaceae. E. coli was isolated in 189 (44.9%) culture positive specimens, whereas S. aureus was the second most frequently isolated organism (19%) after E. coli. Figure 1 represents the prevalence of isolated bacteria from the total sample during the study.
Figure 1.
Distribution of bacterial isolates (number) recovered from clinical samples.
Bacterial incidence and associated factors
Bacterial incidence in female 275 (10.43%) was higher as compared to male counterparts 146 (8.51%) and the relation was statistically significant (P < .05). Growth rate was higher in age group 45 to 54 (11.715%) in comparison to other age groups. However, bacterial occurrence according to the age group of the patient was not statistically significant (P > .05). Prevalence of bacterial infection in patients from IPD (10.96%) was more than the patients from OPD (8.35%) (P < .05). Incidence of bacteria in pus sample (49.73%) was higher than any other specimen types (P < .05) (Table 1).
Table 1.
Distribution of bacterial isolates with respect to different variables.
| Attributes | Parameters | Sample size | Growth rate (%) | P-value |
|---|---|---|---|---|
| Gender | Male | 1715 | 146 (8.51) | .02* |
| Female | 2636 | 275 (10.43) | ||
| Age | <15 | 366 | 40 (10.93) | .12 |
| 15-24 | 817 | 82 (10.04) | ||
| 25-44 | 1546 | 125 (8.09) | ||
| 45-54 | 487 | 57 (11.715) | ||
| 55-65 | 431 | 41 (9.51) | ||
| >65 | 704 | 76 (10.79) | ||
| Specimens | Urine | 2640 | 265 (10.04) | .004* |
| Pus | 187 | 93 (49.73) | ||
| Sputum | 592 | 30 (5.07) | ||
| Blood | 639 | 8 (1.255) | ||
| Others | 293 | 25 (8.53) | ||
| Departments | OPD | 2143 | 179 (8.35) | .003* |
| IPD | 2208 | 242 (10.96) |
Significant at 5% level of significance.
Antimicrobial resistance pattern of the isolates
On performing antimicrobial susceptibility test, we found that most of the Enterobacteriaceae isolates were resistant to third generation cephalosporins (37.5% and 60.9% to ceftazidime and cefotaxime, respectively). A small fraction of the isolates were also resistant to carbapenems (23.2% to imipenem and 36.4% to meropenem). These cephalosporins and carbapenems were significantly effective against non-Enterobacteriaceae as compared to Enterobacteriaceae isolates. Two most effective drugs against both groups were colistin-sulfate and polymixin-B (0.8%). Only 110 (36.3%) isolates were found to be MDR among gram negative isolates. Of those 110 MDR isolates, E. coli accounted for 54.5%, P. aeruginosa accounted for 10.9% and Klebsiella spp. accounted for 10%. All of the MBL and ABL producers were MDR. One of the ABL producing Klebsiella pneumoniae was resistant toward all antibiotics including Polymyxin and Colistin. In Gram positive isolates, imipenem was completely effective against all the isolates while 91.53%-Gram positive isolates were resistant to ampicillin. Of the 80 S. aureus isolates, 60% (48) were MRSA (Table 2).
Table 2.
Antibiotic susceptibility pattern of the isolated organisms.
| Antibiotics | Resistant % of isolates | ||
|---|---|---|---|
| Enterobacteriaceae | Non-Enterobacteriaceae | Gram positive | |
| Amikacin (30 µg) | 15.20 | 11.68 | 22.03 |
| Ampicillin (10 µg) | 80.08 | – | 91.53 |
| Ceftazidime (30 µg) | 37.53 | 22.28 | – |
| Colistin-sulfate (10 µg) | 0.78 | 0.00 | – |
| Cotrimoxazole (25 µg) | 35.63 | 37.50 | 44.92 |
| Cefotaxime (30 µg) | 60.90 | 26.50 | 35.59 |
| Gentamicin (10 µg) | 15.30 | 15.90 | 32.20 |
| Imipenem (10 µg) | 23.23 | 19.30 | 0.00 |
| Levofloxacin (5 µg) | 19.28 | – | – |
| Meropenem (10 µg) | 36.40 | 19.15 | – |
| Nitrofurantoin (300 µg) | 24.48 | – | – |
| Ofloxacin (5 µg) | 47.38 | 20.25 | – |
| Polymyxin B (300 units) | 0.78 | 0.00 | – |
| Piperacillin/Tazobactam (100/10 mcg) | 27.10 | 16.98 | – |
| Azithromycin (30 µg) | – | 22.28 | 43.22 |
| Chloramphenicol (30 µg) | – | 19.15 | 61.02 |
| Oxacillin (1 µg) | – | – | 58.47 |
Frequency of MBL and ABL isolates
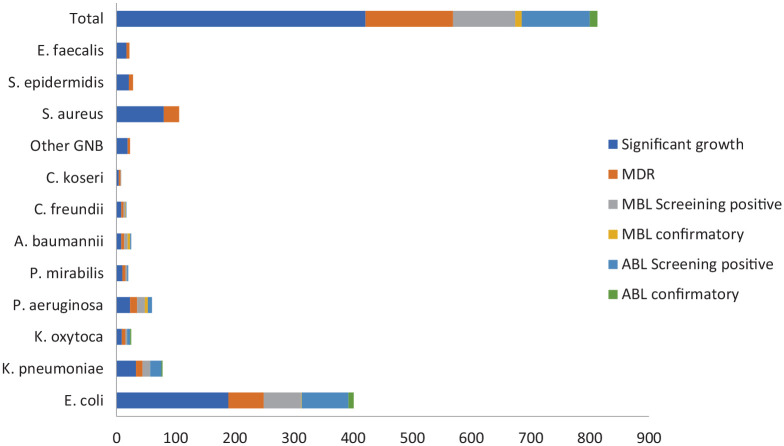
Among 303 Gram negative isolates, on performing phenotypic tests, 11 (3.6%) were MBL producers, whereas 13 (4.3%) were ABL producers. Of the 11 MBL producers, P. aeruginosa comprised of 45.5% isolates, whereas A. baumannii complex comprised of 36.4%. Among 13 ABL producers, 9 (69.2%) were E. coli and remaining 4 (30.8%) were of Klebsiella spp. Figure 2 deciphers the detailed information of ABL and MBL producers.
Figure 2.
Distribution of isolates according to MDR and production of MBL and ABL.
Distribution of MDR, MBL, and ABL producing organisms
Samples from male patients were found to be harboring more MDR bacteria as well as MBL and ABL producing organisms (37.22%, 6%, and 5%, respectively), as compared to females (34.15%, 2.46%, and 3.94%, respectively), but none of the associations was statistically significant (P > .05). Prevalence of MDR (46.51%) as well as MBL (9.09%) producing bacteria was significantly higher in the age group >6, however the association was statistically significant (P < .05) with only MDR bacteria. Meanwhile, the ABL production rate was higher in those bacteria from the age group 55 to 65 (16.12%) which was statistically significant (P < .05). Lower rate of MDR (31.23%) as well as MBL producing organism was found in bacteria isolated from urine as compared to other specimens and the finding was statistically significant in both the cases (P < .05). Higher fraction (12.5%) of bacteria isolated from the sputum were ABL producers compared to other samples though the result was statistically insignificant (P > .05). Higher incidence of MDR (38.91% vs 30.21%), MBL (4.51% vs 2.38%) and ABL (5.65% vs 2.38%) was observed in IPD as compared to OPD, but statistical association was found only with MDR bacteria (P < .05) (Table 3).
Table 3.
Distribution of MDR, MBL, and ABL producer according to different parameters.
| Attributes | Parameters | Sample size | MDR rate | P-value | Sample size | MBL rate | P-value | ABL rate | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Gender | Male | 137 | 51 (37.22) | .536 | 100 | 6 (6) | .121 | 5 (5) | .668 |
| Female | 284 | 97 (34.15) | 203 | 5 (2.46) | 8 (3.94) | ||||
| Age | <15 | 34 | 11 (32.35) | .005* | 24 | 0 | .12 | 1 (4.17) | .012* |
| 15-24 | 71 | 18 (25.35) | 48 | 1 (2.08) | 1 (2.08) | ||||
| 25-44 | 135 | 36 (26.67) | 92 | 2 (2.18) | 2 (2.17) | ||||
| 45-54 | 55 | 25 (45.45) | 42 | 2 (4.76)) | 0 | ||||
| 55-65 | 40 | 18 (45) | 31 | 0 | 5 (16.12) | ||||
| >65 | 86 | 40 (46.51) | 66 | 6 (9.09) | 4 (9) | ||||
| Specimens | Urine | 317 | 99 (31.23) | .005* | 223 | 6 (2.69) | .037* | 7 (3.13) | .132 |
| Pus | 44 | 16 (36.36) | 32 | 4 (12.5) | 4 (12.5) | ||||
| Sputum | 36 | 20 (55.56) | 29 | 1 (3.45) | 1 (3.45) | ||||
| Others | 24 | 13 (54.17) | 19 | 0 | 1 (5.26) | ||||
| Departments | OPD | 182 | 55 (30.21) | .064 | 126 | 3 (2.38) | .326 | 3 (2.38 | .166 |
| IPD | 239 | 93 (38.91) | 177 | 8 (4.51) | 10 (5.65) |
Significant at 5% level of significance.
Discussion
The present study was carried out in order to determine the prevalence of ABL and MBL production in the clinical isolates of bacteria. Infections caused by gram negative bacteria as well as gram positive cocci are considered one of the major burdens in low and middle income countries like Nepal which get further complicated due to the acquisition of various resistant genes by bacteria.24,25
In the current study, urine constituted the highest fraction of all samples collected (60.68%). Similar observation has been reported in various studies carried out in Nepal, where urine occupies the larger fraction of all the clinical samples.24-28 Contrary to this, a study done in Qatar has found respiratory specimens harboring higher fraction of the isolates.29 Higher fraction of urine may be attributed to greater cases of urinary tract infection (UTI), a common problem, among patients attending the hospitals.30 E. coli was the most prevalent isolate followed by S. aureus in our study. Among the gram negatives, K. pneumoniae occupied the second position after E. coli in terms of the incidence. This result is in alignment with some other studies done in Nepal earlier such as at Shaheed Gangalal National Heart Center,25 Universal College of Medical Sciences,26 and Padma Nursing Hospital.31 Prevalence of other bacterial isolates such as P. aeruginosa and Acinetobacter baumannii complex. observed in our study is similar to the studies previously mentioned.25,26,30 Meanwhile, a study done by Sid Ahmed et al29 found Klebsiella spp as predominant organism. One probable reason why E. coli was the most predominant organism in our study may be due to the fact that a higher faction of the samples processed comprised of urine of UTI-suspected patients, and several studies suggest E. coli as the most common culprit in such patients.31 This study recorded a higher bacterial growth rate in females (10.43%) as compared to male counterparts (8.51%). Similar result was revealed in a study done in Meerut, India32; whereas a study done in Lumbini Medical College has found a larger bacterial growth among females.33 Higher bacterial growth rate observed in females can be attributed to higher susceptibility of the females to UTIs compared to males33 and higher number of urine of UTI-suspected patients being processed in the study. In addition, non-specific immunity in females decreases with aging promoting bacterial infection mainly in urinary tract and external wounds.34 Incidence of bacterial infection was almost similar in all age groups with fairly higher rate being in the age group 45 to 54 (11.71%) and lower in the age group 25 to 44 (8.09%). The result suggests that age does not play major role on influencing bacterial growth. However, slightly higher growth rate noted in all age group after 25 to 44, can be imperative as immunity level of a person decreases with aging.35 Higher bacterial incidence observed in pus/wound swab sample (49.73%) as compared to other specimens in our study is similar to some studies carried out in different regions all of which have revealed higher bacterial load in pus/wound swab.26,28 Wounds are generally considered being contaminated with surrounding skin flora due to various anthropogenic activity, plus the fact that when wound when loses its outer defense mechanism, that is, skin, it is more easier for bacteria to establish infection36 promoting bacterial growth in pus/wound. Patients enrolled at hospital in IPD accounted for larger fraction of bacterial growth in comparison to patients visiting OPD. Similar result was observed in a study done at International Friendship hospital.28 In contrast, another similar study from Om Hospital and Research Center37 has reported larger fraction of growth from OPD. Variation in sample size of the patients from OPD and IPD may have influenced the variation in the rate of bacterial growth.37 On performing AST, both colistin-sulfate and polymyxin-B were more effective as only 0.78% Enterobacteriaceae and none of non-Enterobacteriaceae isolates were resistant to them. A study done few years ago in Bhairahawa, Nepal reported that both these drugs were completely effective against all non-Enterobacteriaceae bacteria isolated from clinical samples,26 whereas another study done in Kathamandu, Nepal reported colistin being 100% effective toward all Klebsieslla spp.38 Non-surprisingly, carbapenems such as meropenem (36.4%) and imipenem (23.2%) were resisted at fair level by Enterobacteriaceae. Similar studies carried out in different places have reported even higher resistance against carbapenems with resistant rate 48.86% and 51.13% against meropenem and imipenem, respectively.38 Conversely, most of the study done so far have reported slightly lower resistant rates such as one done by Bora et al39 (19.95% against carbapenems), Sah et al25 (12.8% against imipenem, 9.17% against meropenem), Kayastha et al28 (9.81% against imipenem) as compared to this study. The pattern of resistivity shows that rate of bacterial resistance toward carbapenem is increasing day by day. Ampicillin was found to be the least effective drug against both gram negative (80.08%) and gram positive isolates (91.53%). Our findings are consistent with the previous studies carried out at Shahid Gangalal Hospital (100%),25 International Friendship Hospital (77.77%),28 and Universal College of Medical Science (93.8%).26 Against gram positive isolates, amikacin (22.03%), and gentamicin (32.2%) were more effective drugs besides imipenem which was hundred per cent effective. This result is fairly similar to the work carried out in Kathmandu, which has reported gentamicin being resisted by 24% of the isolates.40 One of the plausible reasons for an increase in bacterial resistance against array of drugs could be due to the production of several β-lactamase enzymes.28 Also, overdependence on carbapenems might lead to unwanted increase of metallo-beta lactamase producing isolates, thus increasing the number of bacteria resistant to carbapenems.41 Besides, the current study has also found several bacteria resistant to drugs such as fluoroquinolones, and tetracyclines at a significant rate. Presence of R-plasmid in bacteria can be a plausible reason that bacteria are resistant toward those drugs.42 This study found that 60% of S. aureus were MRSA. The finding is in line with some other studies done in various places like Iran (46.3%)43 and Bharatpur, Nepal (60.34%),5 but another study has reported a lower rate of MRSA among clinical isolates in Pokhara, Nepal (15.4%).44 MRSA poses a significant threat for patients visiting hospital as it is one of the leading causes of hospital acquired infections and hence rapid and accurate identification of MRSA and effective antibiotic treatment is crucial.45,46 We observed a high prevalence of MDR isolates (35.15%) in the present study. This finding is commensurate with the finding of some other studies like the one carried out by Thakur et al27 (38.75%), Ansari et al41 (46.9%), Sah et al25 (51.3%), and Kayastha et al28 (62.1%). MDR is a global concern which has red-signaled the concerned authorities to take stern measures to curb its dissemination. MDR isolates are abruptly increasing in hospital settings, with its burden varying from region to region, and lower middle income countries (LMIC) are in difficult situation due to multitude of factors embedded in the characteristics of the health system, policy, and the practice.47 The rate of MBL and ABL production was found to be 3.6% and 4.3%, respectively, among GNB isolates in this study. This rate is lower than some previous studies such as one done in Bharatpur Hospital (11.4% and 2.86%), College of Medical Sciences (19.95% MBL), and Om Hospital and Research Center (3.95% MBL), Kathmandu Model Hospital (46.3% ABL, 11.2% MBL)48 in Nepal. Some earlier studies have reported a very high prevalence of MBL producers such as a report from Allama Iqbal Medical College, Lahore (86.61%)15 and Annapurna Neurological Institute and Allied Sciences, Kathmandu (77.6%).41 P. aeruginosa was the chief MBL producing organism (45.5%) followed by Acinetobacter baumannii complex (36.4%) in the current study. This result is similar to the one carried out by Ansari where P. aeruginosa and A. baumannii were chief MBL producers.41 Various studies have reported E. coli and Klebsiella spp. as potent MBL producers,4,38,39 but in this study only one isolate of E. coli and none of Klebsiella were found to be MBL producers. All the 13 ABL producing isolates belonged to Enterobacteriaceae [E. coli 9 (69.2%) and Klebsiella spp 4 (30.8%)] in this study. A previous study in Nepal has also reported similar kind of result where ABL producer among various GNB isolates were from Enterobacteriaceae only.4 At present, there are many reports worldwide indicating increasing incidence of MDR isolates including MBL and ABL producers. It is high time the concerned authorities be serious in order to save the future where antibiotics will no longer be effective to treat bacterial infections.3-5,15,43 It has been reported that carbapenemase producers are usually associated with many other non-beta-lactam resistance determinants, which give rise to MDR and PDR isolates.49 We also observed that all the MBL and ABL producing isolates were MDR. Similar result has been reported earlier by Ansari and his friends who showed that almost all the MBL isolates were non-MDR.41 The class B metallo-β lactamases (MBLs) showing resistance to carbapenems are significant problems in many other parts of the world as many genera of Gram negative bacilli including P. aeruginosa, Acinetobacter spp., and Enterobacteriaceae have been found to possess this enzyme.4 Interestingly, the prevalence of MDR, MBL, and ABL producing bacterial isolates was observed higher in males (37.22%, 6%, and 5%) as compared to females (34.15%, 2.46%, and 3.94%) in this study. Few studies done earlier have also reported a higher rate of MDR27 and MBL15 producing organism among men. The rate of MDR, MBL, and ABL was almost similar in all the age groups although it was slightly higher in age group <65. With age, immunity level of the person decreases35 and requires higher and larger dose of antibiotics to be used to treat infection and that might have resulted drug resistance.50 Similarly, incidence of MBL and ABL was noted higher in GNB isolated from pus (12.5% each) in our study. Bora et al39 in the past reported similar result where larger fraction of bacteria isolated from pus and wound swab were MBL and ABL producers. In contrast, a study from National Reference Laboratory revealed urine as the most prevalent ABL and MBL-harboring specimen.51 Patients enrolled in IPD were seen harboring more MDR (38.91%), MBL (4.51%), and ABL (5.65%) bacteria in comparison to the patients visiting OPD. A similar study done in Bharahava, Bharatpur, and Kathmandu, Nepal separately indicated that patients staying at IPD are more frequently encountered with MDR,39 MBL,26 and ABL16 producing organisms. Further studies should encompass molecular aspects of other beta-lactamases besides MBL/ABL and should also look for investigating other mechanisms of resistance to beta-lactams besides enzyme production.
Conclusion
The current cross-sectional study executed at a medical college and teaching hospital based on the Nepalese capital city showed the incidence of MDR, MBL, and ABL-producers among the clinical isolates. MBL and ABL production by the isolates were found to be significantly associated with the age of the patient and the specimen type. We recommend that the regular antibiotic surveillance activity should incorporate screening tests for MBL and ABL-producing bacterial isolates in the hospital settings in order to abate their dissemination.
Acknowledgments
We would like to express our sincere thanks to all the staffs and faculties of Central Department of Microbiology, Tribhuvan University, Kirtipur and Manmohan Memorial Teaching Hospital, Kathmandu, Nepal, for their support and guidance to complete this study.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by departmental fund of Central Department of Microbiology, Tribhuvan University, Kirtipur, Kathmandu.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All the authors made substantial contribution to the study. MP collected samples, investigated and recorded the laboratory findings. BPR, MRB, KRR and PG conceived and devised the methodology of the study. SA, RSR and KRR wrote the manuscript. SA, BD and KRR reviewed several versions of the manuscript. All authors read and approved the final manuscript.
Availability of Data and Materials: All data pertaining to this study are within the manuscript
Ethical Approval and Consent to Participate: Institutional Review Committee of Manmohan Memorial Institute of Health Science Institutional Review Committee approved this research (Ref No. 388/2074/2075, dated 2074/8/28). Written consent was obtained from the patients. Strict adherence to the ethical guidelines was taken and we declare that this research is free from selection bias.
References
- 1. Behera B, Mathur P, Das A, Kapil A, Sharma V. An evaluation of four different phenotypic techniques for detection of metallo-beta-lactamase producing Pseudomonas aeruginosa. Indian J Med Microbiol. 2008;26:233-237. [DOI] [PubMed] [Google Scholar]
- 2. Adhikari S, Regmi RS, Pandey S, Paudel P, Neupane N. Bacterial etiology of bronchoalveolar lavage fluid in tertiary care patients and antibiogram of the isolates. J Inst Sci Technol. 2021;26:99-106. [Google Scholar]
- 3. Regmi RS, Khadka S, Sapkota S, et al. Bacterial etiology of sputum from tuberculosis suspected patients and antibiogram of the isolates. BMC Res Notes. 2020;13:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adhikari S, Khadka S, Rana JC, et al. Prevalence of β -lactamase producing carbapenem-resistant enterobacteriaceae among the patients attending Bharatpur hospital. Biosci Discov. 2019;10:64-71. [Google Scholar]
- 5. Regmi RS, Khadka S, Sapkota S, et al. Phenotypic detection of inducible clindamycin resistance among clinical isolates of staphylococcus aureus in Bharatpur hospital. J Coll Med Sci. 2020;16:178-183. [Google Scholar]
- 6. Sapkota S, Khadka S, Adhikari S, Parajuli A, Kandel H, Regmi RS. Microbial diversity and antibiotic susceptibility pattern of bacteria associated with motorcycle helmets. Int J Microbiol. 2020;2020:8877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pitout JD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159-166. [DOI] [PubMed] [Google Scholar]
- 8. Bush K, Jacoby GA. Updated functional classification of β-lactamases. Antimicrob Agents Chemother. 2010;54:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Philippon A, Arlet G, Jacoby GA. Plasmid-determined AmpC-type beta-lactamases. Antimicrob Agents Chemother. 2002;46:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacoby GA. AmpC β-lactamases. Clin Microbiol Rev. 2009;22:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smet A, Martel A, Persoons D, et al. Diversity of extended-spectrum β-lactamases and class C β-lactamases among Cloacal Escherichia coli Isolates in Belgian Broiler farms. Antimicrob Agents Chemother. 2008;52:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016;4:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tooke CL, Hinchliffe P, Bragginton EC, et al. β-Lactamases and β-lactamase inhibitors in the 21st century. J Mol Biol. 2019;431:3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deshmukh DG, Damle AS, Bajaj JK, Bhakre JB, Patwardhan NS. Metallo-β-lactamase-producing clinical isolates from patients of a tertiary care hospital. J Lab Physicians. 2011;3:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ain NU, Iftikhar A, Bukhari SS, et al. High frequency and molecular epidemiology of metallo-β-lactamase-producing gram-negative bacilli in a tertiary care hospital in Lahore, Pakistan. Antimicrob Resist Infect Control. 2018;7:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baral P, Neupane S, Shrestha B, Ghimire KR, Marasini BP, Lekhak B. Clinical and microbiological observational study on AmpC β-lactamase-producing Enterobacteriaceae in a hospital of Nepal. Braz J Infect Dis. 2013;17:256-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parajuli NP, Acharya SP, Mishra SK, Parajuli K, Rijal BP, Pokhrel BM. High burden of antimicrobial resistance among gram negative bacteria causing healthcare associated infections in a critical care unit of Nepal. Antimicrob Resist Infect Control. 2017;6:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leber AL. Clinical Microbiology Procedures Handbook. 4th ed. ASM Press; 2016. [Google Scholar]
- 19. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 24th Informational Supplement (M100-S28). CLSI; 2016.
- 20. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-281. [DOI] [PubMed] [Google Scholar]
- 21. Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046-5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coudron PE, Moland ES, Thomson KS. Occurrence and detection of AmpC Beta-Lactamases among Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates at a veterans medical center. J Clin Microbiol. 2000;38:1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Black JA, Moland ES, Thomson KS. AmpC disk test for detection of plasmid-mediated AmpC β-lactamases in enterobacteriaceae lacking chromosomal AmpC β-lactamases. J Clin Microbiol. 2005;43:3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dhungana K, Awal BK, Dhungel B, et al. Detection of Klebsiella pneumoniae Carbapenemase (KPC) and Metallo-Beta Lactamase (MBL) producing gram negative bacteria isolated from different clinical samples in a transplant center, Kathmandu, Nepal. Acta Sci Microbiol. 2019;2:60-69. [Google Scholar]
- 25. Sah RSP, Dhungel B, Yadav BK, et al. Detection of TEM and CTX-M Genes in Escherichia coli Isolated from clinical specimens at tertiary care heart Hospital, Kathmandu, Nepal. Diseases. 2021;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raut S, Rijal KR, Khatiwada S, et al. Trend and characteristics of Acinetobacter baumannii infections in patients attending Universal College of Medical Sciences, Bhairahawa, Western Nepal: a longitudinal study of 2018. Infect Drug Resist. 2020;13:1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thakur P, Ghimire P, Rijal K, Singh G. Antimicrobial resistance pattern of Esherichia coli isolated from urine samples in patients visiting tertiary health care centre in Eastern Nepal. Sunsari Tech Coll J. 2012;1:22-26. [Google Scholar]
- 28. Kayastha K, Dhungel B, Karki S, et al. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella species in pediatric patients visiting International Friendship Children’s Hospital, Kathmandu, Nepal. Infect Dis (Auckl). 2020;13:117863372090979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sid Ahmed MA, Bansal D, Acharya A, et al. Antimicrobial susceptibility and molecular epidemiology of extended-spectrum beta-lactamase-producing Enterobacteriaceae from intensive care units at Hamad Medical Corporation, Qatar. Antimicrob Resist Infect Control. 2016;5:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pandit R, Awal B, Shrestha SS, Joshi G, Rijal BP, Parajuli NP. Extended-spectrum β-lactamase (ESBL) genotypes among multidrug-resistant uropathogenic Escherichia coli clinical isolates from a teaching hospital of Nepal. Interdiscip Perspect Infect Dis. 2020;2020:6525826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tamang K, Shrestha P, Koirala A, Khadka J, Gautam N, Rijal KR. Prevalence of bacterial uropathogens among diabetic patients attending Padma Nursing Hospital of Western Nepal. Himal J Sci Technol. 2017;1:15-19. [Google Scholar]
- 32. Prakash D, Saxena RS. Distribution and antimicrobial susceptibility pattern of bacterial pathogens causing urinary tract infection in urban community of Meerut City, India. ISRN Microbiol. 2013;2013:749629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maharjan N, Mahawal BS. Bacteriological profile of wound infection and antibiotic susceptibility pattern of various isolates in a tertiary care center. J Lumbini Med Coll. 2020;8:218-224. [Google Scholar]
- 34. Bouman A, Schipper M, Heineman MJ, Faas MM. Gender difference in the non-specific and specific immune response in humans. Am J Reprod Immunol. 2004;52:19-26. [DOI] [PubMed] [Google Scholar]
- 35. Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nepal K, Pant ND, Neupane B, et al. Extended spectrum beta-lactamase and metallo beta-lactamase production among Escherichia coli and Klebsiella pneumoniae isolated from different clinical samples in a tertiary care hospital in Kathmandu, Nepal. Ann Clin Microbiol Antimicrob. 2017;16:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pyakurel S, Ansari M, Kattel S, et al. Prevalence of carbapenemase-producing Klebsiella pneumoniae at a tertiary care hospital in Kathmandu, Nepal. Trop Med Health. 2021;49:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bora A, Sanjana R, Jha BK, Narayan Mahaseth S, Pokharel K. Incidence of metallo-beta-lactamase producing clinical isolates of Escherichia coli and Klebsiella pneumoniae in central Nepal. BMC Res Notes. 2014;7:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rai S, Yadav UN, Dutt N, et al. Bacteriological profile and antimicrobial susceptibility patterns of bacteria isolated from pus/wound swab samples from children attending a tertiary care hospital in Kathmandu, Nepal. Int J Microbiol. 2017;2017:2529085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ansari M, Aryal SC, Rai G, et al. Prevalence of multidrug-resistance and blaVIM and blaIMP genes among gram-negative clinical isolates in tertiary care hospital, Kathmandu, Nepal. Iran J Microbiol. 2021;13:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bergogne-Bérézin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dibah S, Arzanlou M, Jannati E, Shapouri R. Prevalence and antimicrobial resistance pattern of methicillin resistant Staphylococcus aureus (MRSA) strains isolated from clinical specimens in Ardabil, Iran. Iran J Microbiol. 2014;6:163. [PMC free article] [PubMed] [Google Scholar]
- 44. Subedi S, Brahmadathan KN. Antimicrobial susceptibility patterns of clinical isolates of Staphylococcus aureus in Nepal. Clin Microbiol Infect. 2005;11:235-237. [DOI] [PubMed] [Google Scholar]
- 45. Johnson AP. Methicillin-resistant Staphylococcus aureus: the European landscape. J Antimicrob Chemother. 2011;66 Suppl 4:iv43-iv48. [DOI] [PubMed] [Google Scholar]
- 46. Erdem H, Dizbay M, Karabey S, et al. Withdrawal of Staphylococcus aureus from intensive care units in Turkey. Am J Infect Control. 2013;41:1053-1058. [DOI] [PubMed] [Google Scholar]
- 47. Pokharel S, Raut S, Adhikari B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob Heal. 2019;4:e002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shrestha UT, Shrestha S, Adhikari N, et al. Plasmid profiling and occurrence of β-lactamase enzymes in multidrug-resistant uropathogenic Escherichia coli in Kathmandu, Nepal. Infect Drug Resist. 2020;13:1905-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nordmann P, Poirel L, Dortet L. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2012;18:1503-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. Pharm Ther. 2015;40:277. [PMC free article] [PubMed] [Google Scholar]
- 51. Kuinkel S, Acharya J, Dhungel B, et al. Biofilm formation and phenotypic detection of ESBL, MBL, KPC and AmpC enzymes and their coexistence in Klebsiella spp. isolated at the National Reference Laboratory, Kathmandu, Nepal. Microbiol Res. 2021;12:683-697. [Google Scholar]