Abstract
The rapid development of mRNA vaccines has contributed to the management of the current coronavirus disease 2019 (COVID-19) pandemic, suggesting that this technology may be used to manage future outbreaks of infectious diseases. Because the antigens targeted by mRNA vaccines can be easily altered by simply changing the sequence present in the coding region of mRNA structures, it is more appropriate to develop vaccines, especially during rapidly developing outbreaks of infectious diseases. In addition to allowing rapid development, mRNA vaccines have great potential in inducing successful antigen-specific immunity by expressing target antigens in cells and simultaneously triggering immune responses. Indeed, the two COVID-19 mRNA vaccines approved by the U.S. Food and Drug Administration have shown significant efficacy in preventing infections. The ability of mRNAs to produce target proteins that are defective in specific diseases has enabled the development of options to treat intractable diseases. Clinical applications of mRNA vaccines/therapeutics require strategies to safely deliver the RNA molecules into targeted cells. The present review summarizes current knowledge about mRNA vaccines/therapeutics, their clinical applications, and their delivery strategies.
Keywords: mRNA therapeutics, mRNA vaccine, RNA delivery, RNA therapeutics
INTRODUCTION
RNA-based drugs have recently received significant attention mainly because of the great success of developing mRNA vaccines to cope with the coronavirus disease 2019 (COVID-19) pandemic (Jackson et al., 2020; Kwon et al., 2018; Wang et al., 2020). mRNA is a molecule that, when delivered into cells, can be translated to produce target proteins (Lee et al., 2018; Sahin et al., 2014). mRNA can be applied to develop therapeutic agents to cure diseases for which treatment options require the expression of specific types of therapeutic proteins. Compared with DNA, another type of nucleic acid capable of producing encoded proteins in cells, mRNA has higher translation efficiency and can induce transient protein expression. Several hurdles had to be overcome, however, prior to the therapeutic application of mRNA. First, mRNA structures are easily degraded in serum. Thus, strategies were required to protect and deliver mRNA into targeted cells. For this purpose, various types of biomaterials, including lipid nanoparticles (LNP), liposomes, cationic polymers, and inorganic nanoparticles, have been developed as mRNA delivery materials (Paunovska et al., 2022). Second, mRNA itself can induce innate immune reactions by being recognized by various pattern recognition receptors (PRRs) upon delivery into cells (Schlee and Hartmann, 2016). The immune responses induced by mRNAs may have negative effects on disease treatment, thus limiting the therapeutic applications of mRNA.
Meanwhile, for the development of effective vaccines, the induction of immune responses and the expression of target antigens in our body is necessary. Since the mRNA can induce translation and immune activation simultaneously, it can satisfy the requirements for successful vaccines. From this point of view, mRNA has been regarded as a novel vaccine platform with great potential. Most clinical applications of mRNA-based drugs to date have been for the development of vaccines, either for cancer immunotherapy or against infectious diseases (Sahin et al., 2014). In addition to its use in vaccine development, mRNA has recently shown promising results in the treatment of various diseases. These applications include protein replacement therapy, in which mRNA is used to produce clinically available therapeutic proteins, and novel therapeutic strategies such as ex vivo/in vivo cell engineering and gene editing (Berraondo et al., 2019; Magadum et al., 2019; Warren and Lin, 2019).
This concise review will discuss mRNA-based therapeutics/vaccines and their clinical applications. mRNA can be utilized in clinical situations that require the expression of specific proteins. Also, mRNA can be developed as a vaccine by inducing antigen expression and immune responses. Delivery strategies are required for the therapeutic applications of mRNA-based therapeutics/vaccines. The present review will describe mRNA-based drugs and their delivery strategies.
mRNA VACCINES
mRNA vaccines are considered a game changer in vaccine development. Although the process of vaccine development has usually required more than 10 years, vaccines against SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), the virus causing COVID-19, took less than 1 year for complete development, with the first vaccines approved by the U.S. Food and Drug Administration (FDA) being mRNA vaccines. The rapid development of mRNA vaccines has enabled their use in the management of urgent pandemic and epidemic situations. The processes for the development of mRNA vaccines were mainly based on platform technology that included the sequence optimization of all aspects of the structures of in vitro transcribed (IVT) mRNA, their delivery strategies, and the manufacturing process. Successful establishment of the mRNA platform technology can enable the manufacture of mRNA vaccines for various antigens within 1 month, simply by altering the ORF (open reading frame) sequence of the DNA templates utilized in the IVT reactions. This simple development process enabled the rapid development of COVID-19 mRNA vaccines. Based on the tremendous potential, various applications of mRNA vaccines for preventing infectious diseases are currently in clinical trials (Table 1).
Table 1.
mRNA vaccines currently in clinical trials
| Name | Developer | Type | Clinical phase | Target | Route of administration |
|---|---|---|---|---|---|
| mRNA-1644 | Moderna | eOD-GT8 60mer mRNA | Phase 1 | HIV-1-infection | IM |
| MRT5407 | Sanofi Pasteur | Quadrivalent influenza mRNA | Phase 1/2 | Influenza | IM |
| MRT5500 | Sanofi Pasteur and Translate Bio | 2P/GSAS S mRNA | Phase 2 | COVID-19 | IM |
| DS-5670a | Daiichi Sankyo Co., Ltd. | Coronavirus-modified uridine RNA vaccine | Phase 2/3 | COVID-19 | IM |
| HDT-301 | SENAI CIMATEC | Self-replicating mRNA | Phase 2/3 | COVID-19 | IM |
| ARCT-154 | Arcturus Therapeutics, Inc. | Self-amplifying mRNA vaccine | Phase3 | COVID-19 | IM |
| EG-COVID | EyeGene Inc. | Lyophilized, liposome-based vaccine | Phase 1/2 | COVID-19 | IM |
| CV2CoV | CureVac AG | Non-chemically modified mRNA | Phase 1 | COVID-19 | IM |
| mRNA-1010 | Moderna TX, Inc. | mRNA encoding hemagglutinin (HA) glycoproteins of the four influenza strains recommended by the World Health Organization | Phase 3 | Seasonal influenza | IM |
| mRNA-1345 | Moderna TX, Inc. | mRNA encoding prefusion F glycoprotein | Phase 3 | Respiratory syncytial virus (RSV) | IM |
| GSK4382276A | GlaxoSmithKline/CureVac | mRNA-based monovalent vaccine | Phase 1 | Influenza | IM |
| PF-07852352 | Pfizer | Self- amplifying ribonucleic acid (saRNA) vaccine | Phase 1 | Influenza | IM |
| BNT113 | BioNTech SE | mRNA encoding human papillomavirus type 16 (HPV-16) oncoproteins E6 and E7 | Phase 1/2 | Carcinoma, squamous cell head and neck; Neoplasms, including cervical, penile and malignant neoplasms | IV |
| mRNA-1215 | Moderna TX, Inc. | mRNA encoding secreted prefusion stabilized F component covalently linked to G monomer (pre-F/G) of Malaysian strain NiV | Phase 1 | Nipah virus infection | IM |
| CVSQIV | GlaxoSmithKline/CureVac | Seasonal quadrivalent influenza mRNA vaccine (unmodified) | Phase 1 | Influenza | IM |
IM, intramuscular injection; IV, intravenous injection.
Two key technologies were responsible for the success of mRNA vaccines: the use of LNP as mRNA delivery materials, and the chemical modification of mRNA to avoid unwanted immune responses. At the start of COVID-19 vaccine development, three major companies attempted to develop mRNA vaccines: CureVac, BioNTech, and Moderna. The COVID-19 mRNA vaccines developed by BioNTech and Moderna were approved by the FDA, whereas the vaccine developed by CureVac did not show sufficient efficacy in phase 3 clinical trials. The main difference between the vaccines developed by BioNTech and Moderna and the CureVac vaccine was that the first two companies utilized chemically modified mRNAs, whereas CureVac used unmodified mRNA. In 2005, Karikó and Weissman reported that the immunogenicity of mRNA could be reduced by introducing chemically modified uridine into mRNA structures instead of unmodified uridine (Karikó et al., 2005; 2008). Furthermore, mRNAs chemically modified with N1-methylpseudouridine were found to encode higher amounts of protein than unmodified mRNAs (Karikó et al., 2012). Whereas the BioNTech and Moderna COVID-19 vaccines used mRNA containing the N1-methylpseudouridine modification, the CureVac vaccine attempted to leverage the immunogenic response to unmodified mRNA. The failure of the CureVac COVID-19 vaccine in clinical trials suggested that chemical modification is a prerequisite for practical applications of mRNA. However, additional studies are required to determine whether unmodified mRNA is unsuitable for vaccines or therapeutics.
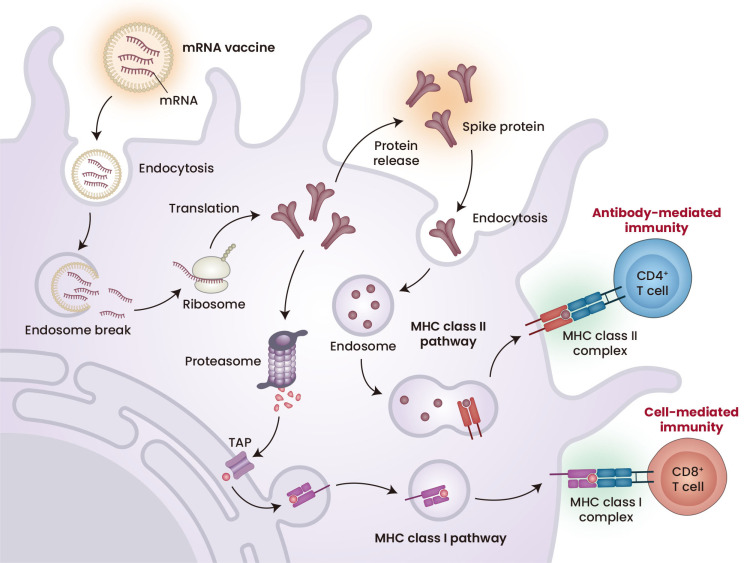
The mechanism of action of the mRNA vaccines differs from the mechanisms of conventional types of vaccines, such as inactivated and live-attenuated vaccines (Pardi et al., 2018; Yoon et al., 2022). The latter vaccines contain inactivated or weakened forms of whole disease-causing pathogens, including all types of antigens and epitopes. After vaccination with these vaccines, host immune cells recognize the administered pathogens primarily as exogenous antigens and trigger immune responses, most of which are mediated by the major histocompatibility complex (MHC) class II system. By contrast, mRNA vaccines are designed to encode specific antigens rather than whole pathogens. To induce antigen-specific immune responses, mRNA vaccines must be translated into antigens after being delivered into cells. The translated antigens can be secreted and taken up by cells as exogenous antigens, eliciting MHC class II-mediated immunity. In addition, when the mRNA vaccines are delivered to antigen presenting cells, the translated antigens are regarded by the host as endogenous antigens, which primarily activate MHC class I immune responses (Fig. 1). Because the MHC class I system is associated with CD8+ T-cell-mediated cellular immunity, the mRNA vaccines tend to induce greater cellular immunity than inactivated or live-attenuated vaccines. The induction of tumor antigen-specific cellular immunity, especially antigen-specific cytotoxic T-cell immunity, by cancer vaccines determines their therapeutic efficacy in cancer immunotherapy. Because mRNA vaccines can effectively induce cellular immunity, they are potent types of vaccine in the treatment of cancer.
Fig. 1. Mechanism of action of mRNA vaccines.
mRNA vaccines to prevent COVID-19 were developed by encapsulating chemically modified mRNAs in lipid nanoparticles. After endocytosis, mRNA can be delivered into the cytoplasm, resulting in the induction of antigen expression. The antigens translated by mRNA vaccines can induce both cell-mediated and antibody-mediated immunity. MHC, major histocompatibility complex; TAP, The transporter associated with antigen processing. Created with BioRender.com.
In addition, by being recognized by PRRs, mRNA vaccines can act as adjuvants that stimulate innate immune responses. Because mRNA vaccines can simultaneously express encoded antigens and stimulate the immune system, they can be regarded as vaccines with self-adjuvating properties. Although mRNA vaccines have immunostimulatory effects, however, they can also suppress the efficiency of translation, reducing antigen expression and antigen-specific immunity. Currently available mRNA vaccines utilize a modified form of mRNA that does not mediate immune responses. These modified mRNA vaccines require agents that can stimulate the immune system to successfully induce immunity. The immunostimulatory effects of vaccines containing modified mRNA are thought to be caused by the LNP utilized to deliver mRNA. In this regard, there have been extensive efforts to develop a system that can successfully control the immunostimulatory effects of mRNA while simultaneously delivering the mRNA into target tissues. Additional details of RNA delivery materials are described below.
mRNA THERAPEUTICS
mRNA therapeutics utilize RNAs that can be translated into specific proteins in targeted cells. Expression of specific proteins in cells can have various therapeutic effects, including the regulation of pathogenic processes. mRNA therapeutics can replace defective proteins, with this protein replacement therapy being used to treat diseases associated with protein deficiency. Although most approaches to protein replacement therapy are still in the preclinical stage, mRNA therapeutics may have application in various diseases, including cancers, neurological disorders, cardiac diseases, and metabolic diseases (Berraondo et al., 2019; Magadum et al., 2019; Uchida et al., 2013; Vallazza et al., 2015). In addition, gene editing, such as the clustered regularly interspaced short palindromic repeat (CRISPR)-associated protein (CRISPR/Cas) nuclease system, has been applied to mRNA therapeutics (Cheng et al., 2020). The CRISPR/Cas system, which has shown great potential for gene editing in clinical studies, requires expression of Cas protein and intracellular delivery of guide RNA. mRNA therapeutics can also mediate the cellular expression of Cas protein required for CRISPR/Cas-mediated gene editing. Other applications of mRNA therapeutics include cell engineering, such as chimeric antigen receptor (CAR) T-cell therapy and stem cell engineering (Billingsley et al., 2020; Warren and Lin, 2019). CAR T-cell therapy utilizes T-cells expressing CAR, which requires the intracellular ex vivo delivery of mRNAs. In stem cell engineering, it is critical to modulate transcription factor expression. Because mRNA therapeutics can induce transient expression of various transcription factors without the risk of mutagenesis, mRNA therapeutics can be utilized to develop various therapeutic agents, including cell engineering as tools to express specific proteins. Several mRNA therapeutic agents are currently undergoing clinical trials (Table 2).
Table 2.
mRNA therapeutics currently in clinical trials
| Name | Developer | Type | Clinical phase | Target | Route of administration |
|---|---|---|---|---|---|
| SAFE-T-HBV | Lion TCR Pte. Ltd. | mRNA HBV/TCR T-cells | Phase 1 | Hepatocellular carcinoma | IV infusion |
| Amaretto | UTC Therapeutics Inc. | mRNA-engineered anti-mesothelin (MESO) chimeric antigen receptor T-Cell (CAR T-cells) therapy | Phase 1 | Refractory malignant solid neoplasm | IV infusion |
| mRNA-3927 | Moderna TX, Inc. | mRNA encoding two proteins that form the deficient enzyme (PCCA [PA type I]/PCCB [PA type II]) | Phase 1/2 | Propionic acidemia | IV infusion |
| mRNA-3705 | Moderna TX, Inc. | mRNA encoding MUT enzyme (methylmalonic CoA mutase) | Phase 1/2 | Methylmalonic acidemia | IV |
| ARCT-810 | Arcturus Therapeutics, Inc. | mRNA encoding ornithine transcarbamylase (OTC) | Phase 2 | Ornithine transcarbamylase deficiency | IV infusion |
| mRNA-3745 | Moderna TX, Inc. | mRNA encoding glucose 6-phosphatase (G6Pase) enzyme | Glycogen storage disease | IV infusion |
IV, intravenous injection.
RNA DELIVERY SYSTEM
mRNA-based drugs require strategies to protect the mRNA from degradation and deliver it to desired target tissues. In contrast to DNA therapeutics, which require delivery to the nucleus, mRNA-based drugs can induce therapeutic effects once delivered to the cytoplasm. Thus, the cellular delivery process after the cellular uptake is simpler for mRNA- than DNA-based drugs. Among the various mRNA delivery strategies developed to date are nanomaterials, including polymeric, lipid, and inorganic nanoparticles (Byun et al., 2022; Hou et al., 2021). Physical interactions between mRNA structures and lipid, polymer, or inorganic materials allow mRNA-based drugs to be formulated within these nanomaterials. The LNP system has been considered the most potent platform for delivering RNA therapeutics including mRNA therapeutics/vaccines. Indeed, the LNP system was utilized to develop the first FDA-approved siRNA therapeutics, Onpattro. In general, LNP systems are composed of four lipid components: structural lipids, ionizable lipids, poly(ethylene glycol) (PEG)-lipids, and cholesterol. Protonation of ionizable lipids under acidic conditions results in the formation of cationic lipids, which interact with anionic RNA structures by charge interactions. RNA structures surrounded by ionizable lipids subsequently recruit other lipid components by hydrophobic interactions, resulting in the assembly of LNPs encapsulating the RNA structures. Most systemically administered LNP systems can be successfully delivered, especially into liver tissue, by absorbing apolipoprotein E (ApoE) protein (Sebastiani et al., 2021). LNP systems can be engineered to target specific tissue by screening the chemical structures of ionizable lipids, adding specific targeting ligands, or changing the composition of the LNP system. The serum proteins that bind to the surface of LNPs depend on the physicochemical characteristics of these LNPs. Furthermore, the pattern of serum proteins absorbed on LNP surfaces has been found to determine the protein corona affecting the tissue targetability of these LNPs. In addition to LNPs, several cationic lipid materials were found to mediate the cellular delivery of RNA structures by forming nano-sized complexes called lipoplexes. The cationic lipid materials can interact with anionic RNA structures through electrostatic interaction. For example, lipofectamine, a commercially available transfecting agent, is a cationic lipid material that forms lipoplexes with RNA structures. Cationic polymers can also form condensed complexes with RNA structures called polyplexes. Lipoplexes and polyplexes, which are based on electrostatic interactions between the delivery materials and RNA structures, can be easily dissociated in the systemic circulation. Many attempts have therefore been made to develop stable forms of lipoplexes and polyplexes for the systemic delivery of RNA therapeutic agents (Zelphati et al., 1998).
The development of mRNA therapeutics/vaccines requires delivery strategies based on nanomaterials such as LNP because the mRNA do not have activities in the absence of these nanomaterials. siRNA therapeutics differ, however, as their RNA structures can be chemically synthesized by oligo synthesizers, allowing the introduction of chemical modifications into these siRNA structures. In the absence of RNA delivery strategies, some of these chemical modifications provide protection to siRNA structures against degradation by the systemic circulation. RNA structures can also be delivered into desired tissues and cells by conjugating various targeting ligands to the RNA structures through chemical modifications. This conjugation-based RNA delivery system eliminates extra RNA-delivering materials, reducing potential toxicity and other limitations associated with RNA-delivering materials. Following the first FDA approval of a siRNA therapeutics, the LNP system has not been extensively utilized in developing siRNA therapeutics. Rather, the recent trend has been to develop chemical conjugation-based RNA delivery systems, which introduce chemical modifications that protect RNA structures and induce their cellular uptake. Specifically, various chemical modifications conferring RNase resistance have been introduced into synthesized siRNA structures. In addition, N-acetylgalactosamine (GalNAc) has been chemically conjugated to siRNA structures as a targeting ligand inducing cellular uptake, especially in liver tissues. Most clinically available siRNA therapeutic agents to date are based on GalNAc-conjugated siRNA structures without nanomaterial-based RNA delivery materials. Chemical conjugated-based RNA delivery systems may also be used to develop mRNA therapeutics/vaccines, significantly reducing the potential toxicity of mRNA-based drugs. However, for this purpose, it is highly required to develop chemical modification techniques to make the mRNA structures resistant to enzymatic degradation.
CONCLUSION
This review has described the mechanism of action of mRNA therapeutics/vaccines (Fig. 2). The high versatility of RNA therapeutics suggests that they have great potential in modulating various cellular mechanisms. By utilizing mRNA therapeutics, almost any protein can be produced in cells, simply by changing the sequences of the mRNA structures without changing the manufacturing processes. Therefore, mRNA therapeutics can be used to treat diseases associated with the suppression of specific protein(s). In addition, the simple process to generate mRNA therapeutics has contributed to the rapid development of prophylactic vaccines to manage patients with COVID-19. Because of their rapid generation, mRNA platforms are considered the most potent vaccine platforms to treat epidemic and pandemic conditions.
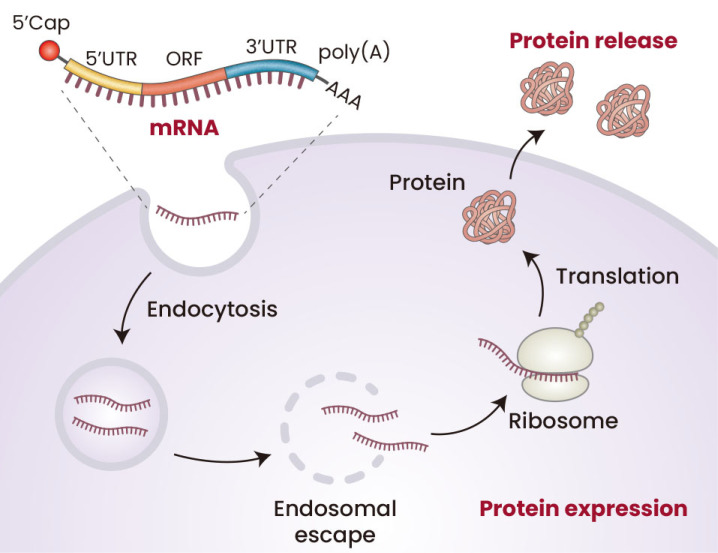
Fig. 2. mRNA therapeutics/vaccines for inducing protein expression.
Conventional types of mRNA therapeutics includes 5′cap, 5′UTR, open reading frame (ORF), 3′UTR, and poly(A), which induce cap-dependent translation following transfection into delivered cells, resulting in protein expression. During the development of mRNA therapeutics and vaccines, the ORF region was designed to express therapeutic proteins and antigens, respectively. Created with BioRender.com.
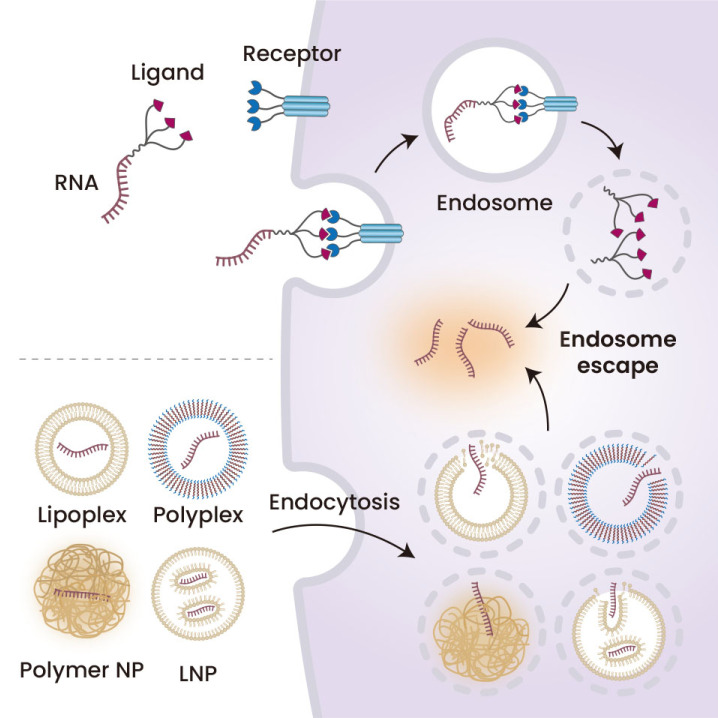
Optimizing delivery strategies is required in the development of RNA therapeutics (Fig. 3). We also summarized some RNA delivery strategies. These delivery strategies should (1) protect RNA molecules from degradation in the systemic circulation and (2) deliver the RNA therapeutics to desired tissue and cells. Various nanomaterials, including LNP, polymeric nanoparticles, lipoplexes, and polyplex, as well as conjugation-based delivery strategies, have been developed to deliver RNA therapeutics.
Fig. 3. Strategies for RNA delivery.
Encapsulation of RNA therapeutic agents by various nanomaterials, including lipoplexes, polyplexes, polymer nanoparticles (polymer NP), and lipid nanoparticles (LNP), protecting the RNAs and delivering them to desired cells. By chemical conjugation with various targeting ligands, these RNAs, especially short RNAs such as siRNAs and miRNAs, can be delivered into cells. Created with BioRender.com.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Science & ICT (Basic Science Research Program (2020R1C1C1007820) and the Bio & Medical Technology Development Program (2022M3E5F1017743), and by a grant (22203MFDS402, 22203MFDS405) from the Ministry of Food and Drug Safety in 2022.
Footnotes
AUTHOR CONTRIBUTIONS
S.S. and K.L. wrote the manuscript.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Berraondo P., Martini P.G.V., Avila M.A., Fontanellas A. Messenger RNA therapy for rare genetic metabolic diseases. Gut. 2019;68:1323–1330. doi: 10.1136/gutjnl-2019-318269. [DOI] [PubMed] [Google Scholar]
- Billingsley M.M., Singh N., Ravikumar P., Zhang R., June C.H., Mitchell M.J. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20:1578–1589. doi: 10.1021/acs.nanolett.9b04246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun M.J., Lim J., Kim S.N., Park D.H., Kim T.H., Park W., Park C.G. Advances in nanoparticles for effective delivery of RNA therapeutics. Biochip J. 2022;16:128–145. doi: 10.1007/s13206-022-00052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q., Wei T., Farbiak L., Johnson L.T., Dilliard S.A., Siegwart D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020;15:313–320. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson N.A.C., Kester K.E., Casimiro D., Gurunathan S., DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 2020;5:11. doi: 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Karikó K., Muramatsu H., Keller J.M., Weissman D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol. Ther. 2012;20:948–953. doi: 10.1038/mt.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikó K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H., Kim M., Seo Y., Moon Y.S., Lee H.J., Lee K., Lee H. Emergence of synthetic mRNA: in vitro synthesis of mRNA and its applications in regenerative medicine. Biomaterials. 2018;156:172–193. doi: 10.1016/j.biomaterials.2017.11.034. [DOI] [PubMed] [Google Scholar]
- Lee K., Kim M., Seo Y., Lee H. Development of mRNA vaccines and their prophylactic and therapeutic applications. Nano Res. 2018;11:5173–5192. doi: 10.1007/s12274-018-2095-8. [DOI] [Google Scholar]
- Magadum A., Kaur K., Zangi L. mRNA-based protein replacement therapy for the heart. Mol. Ther. 2019;27:785–793. doi: 10.1016/j.ymthe.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunovska K., Loughrey D., Dahlman J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022;23:265–280. doi: 10.1038/s41576-021-00439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U., Karikó K., Tureci O. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- Schlee M., Hartmann G. Discriminating self from non-self in nucleic acid sensing. Nat. Rev. Immunol. 2016;16:566–580. doi: 10.1038/nri.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiani F., Yanez Arteta M., Lerche M., Porcar L., Lang C., Bragg R.A., Elmore C.S., Krishnamurthy V.R., Russell R.A., Darwish T., et al. Apolipoprotein E binding drives structural and compositional rearrangement of mRNA-containing lipid nanoparticles. ACS Nano. 2021;15:6709–6722. doi: 10.1021/acsnano.0c10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Itaka K., Uchida H., Hayakawa K., Ogata T., Ishii T., Fukushima S., Osada K., Kataoka K. In vivo messenger RNA introduction into the central nervous system using polyplex nanomicelle. PLoS One. 2013;8:e56220. doi: 10.1371/journal.pone.0056220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallazza B., Petri S., Poleganov M.A., Eberle F., Kuhn A.N., Sahin U. Recombinant messenger RNA technology and its application in cancer immunotherapy, transcript replacement therapies, pluripotent stem cell induction, and beyond. Wiley Interdiscip. Rev. RNA. 2015;6:471–499. doi: 10.1002/wrna.1288. [DOI] [PubMed] [Google Scholar]
- Wang F., Zuroske T., Watts J.K. RNA therapeutics on the rise. Nat. Rev. Drug Discov. 2020;19:441–442. doi: 10.1038/d41573-020-00078-0. [DOI] [PubMed] [Google Scholar]
- Warren L., Lin C. mRNA-based genetic reprogramming. Mol. Ther. 2019;27:729–734. doi: 10.1016/j.ymthe.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon B.K., Oh T.G., Bu S., Seo K.J., Kwon S.H., Lee J.Y., Kim Y., Kim J.W., Ahn H.S., Fang S. The peripheral immune landscape in a patient with myocarditis after the administration of BNT162b2 mRNA vaccine. Mol. Cells. 2022;45:738–748. doi: 10.14348/molcells.2022.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelphati O., Nguyen C., Ferrari M., Felgner J., Tsai Y., Felgner P.L. Stable and monodisperse lipoplex formulations for gene delivery. Gene Ther. 1998;5:1272–1282. doi: 10.1038/sj.gt.3300707. [DOI] [PubMed] [Google Scholar]