Abstract
Background: Epilepsy is a devastating neurological disorder that affects nearly 70 million people worldwide. Epilepsy causes uncontrollable, unprovoked and unpredictable seizures that reduce the quality of life of those afflicted, with 1-9 epileptic patient deaths per 1000 patients occurring annually due to sudden unexpected death in epilepsy (SUDEP). Predicting the onset of seizures and managing them may help patients from harming themselves and may improve their well-being. For a long time, electroencephalography (EEG) devices have been the mainstay for seizure detection and monitoring. This systematic review aimed to elucidate and critically evaluate the latest advancements in medical devices, besides EEG, that have been proposed for the management and prediction of epileptic seizures. A literature search was performed on three databases, PubMed, Scopus and EMBASE.
Methods: Following title/abstract screening by two independent reviewers, 27 articles were selected for critical analysis in this review.
Results: These articles revealed ambulatory, non-invasive and wearable medical devices, such as the in-ear EEG devices; the accelerometer-based devices and the subcutaneous implanted EEG devices might be more acceptable than traditional EEG systems. In addition, extracerebral signal-based devices may be more efficient than EEG-based systems, especially when combined with an intervention trigger. Although further studies may still be required to improve and validate these proposed systems before commercialization, these findings may give hope to epileptic patients, particularly those with refractory epilepsy, to predict and manage their seizures.
Conclusion: The use of medical devices for epilepsy may improve patients' independence and quality of life and possibly prevent sudden unexpected death in epilepsy (SUDEP).
Keywords: Electroencephalography, electrophysiology device, seizure prediction, wearable device, extracerebral signals, SUDEP
1. INTRODUCTION
Epilepsy is defined by the International League Against Epilepsy as at least two unprovoked seizures occurring more than 24 hours apart, diagnosis of an epileptic syndrome, or one unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures [1]. Reports suggest that about 80% of people diagnosed with epilepsy live in low-to-middle income countries, with the majority of them failing to receive any proper or effective treatment, contributing to a reduced quality of life and an increased economic burden [2]. Epilepsy may account for a significant proportion of the global disease burden, where the estimated proportion of active or uncontrollable epilepsy within the general population at any given time is between 4 to 10 per 1000 people [2]. Epilepsy may be associated with a range of other disorders (comorbidities), such as depression, anxiety, stroke, arthritis and dementia [3], which may further reduce patient’s quality of life.
One of the most devastating possible outcomes of uncontrollable or improperly treated epilepsy is a sudden unexpected death in epilepsy (SUDEP), seen in 1-9 deaths per 1000 patients annually [4]. Thus, it is crucial that epilepsy is effectively treated and managed to improve the quality of life of patients and their caregivers. Accurately predicting and diagnosing epilepsy and its symptoms may aid in the management of epilepsy as well. This is because, with an effective prediction of seizure onset/occurrence, proper medication and personalized treatment/management strategies may be sought after by patients.
Currently, electroencephalography (EEG) remains the mainstay of epilepsy diagnosis and management; however, with recent advancements in the field of technology, less bulky and easily accessible devices, such as the utilization of wristwatches, have been introduced as a possible aiding technology for the effective prediction of seizures and their management [5, 6]. This advancement in technology allows patients to monitor their seizure activity on a daily basis without the hassle of seeking an EEG-equipped hospital. Certainly, effective prediction of seizures and accurate diagnosis of the type of epilepsy may help clinicians better prescribe treatment strategies.
Anti-seizure drugs (ASD), formerly known as anti-epileptic drugs (AED), are often prescribed as the first treatment strategy against epilepsy. However, nearly 30% of epileptic patients may not respond or negatively respond to ASD, thus being diagnosed with drug-resistant/ refractory epilepsy [7]. Drug-resistant epilepsy or refractory epilepsy is defined as failure of adequate trials of two tolerated, appropriately chosen and used antiepileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom [8]. Factors that may influence a patient's response to ASDs include the suitability/compatibility of the ASD against the type of epilepsy, age, and medical/genetic history. When ASD monotherapy or combination therapy fails, neurostimulator implantation through vagal nerve stimulation and deep brain stimulation treatments may be suggested for drug resistant epileptic patients [9]. These treatments may be invasive and expensive but have proven to increase the efficiency of managing epilepsy, especially when in combination with ASD [10]. Nevertheless, both ASD and surgical treatments are accompanied by various side effects [11, 12], impacting the quality of life of patients, thereby negating the benefits of managing the seizure condition.
Recently, the utilization of advanced technology, such as artificial intelligence, has been proposed to overcome these hurdles with ASD by using algorithms in the detection and prediction of epileptic seizures and the selection of compatible ASD for epileptic patients [13]. This will possibly reduce the possibility of side effects and increase the effectiveness of ASD without going through invasive surgical procedures. Similarly, other technology-based medical devices have also been proposed in the past couple of years to help epileptic patients. A systematic review by van Andel and colleagues, published in 2016, elucidated the various ambulatory seizure detection devices that many researchers have proposed over the years, with the most reaching good sensitivity and accuracy but having high false alarm rate and low predictive ability [14].
However, similar strengths and weaknesses of epilepsy prediction, management and treatment using various medical devices have not been discussed previously. Thus, this systematic review aims to critically evaluate the current medical technologies and devices presented in the literature, besides conventional EEG and other commercially available devices that may be used to manage, predict, and treat epilepsy. This review also aims to elucidate the potential of these medical devices to be commercially used as a prediction, management and treatment tool for epileptic patients in the future, thereby improving their quality of life and decreasing the burden on the future economy.
2. METHODOLOGY
2.1. Literature Search Method
A systematic literature search was performed to identify and extract all currently available literature related to medical devices or technology utilized in the prediction, treatment and/or management of epilepsy. Since a recent systematic review has been conducted on seizure detection devices [14], this paper focused on utilizing medical devices for the prediction, management, and treatment of epilepsy, and not just seizures. Thus, the search term (“epilepsy”) was searched in combination with the following search strings: (“medical device” OR “medical technology” OR “treatment device” OR “treatment technology”), (“management” AND (“technology” OR “device”)), (“predict” AND (“technology” OR “device”)) in three databases, PubMed, EMBASE, and Scopus. The Boolean operator AND was used to link the search term with the respective search strings on all databases. All searches were performed based on the title, abstract and keyword in all databases. Articles were first screened through their titles and abstracts before proceeding with the full-text screening of relevant articles.
2.2. Study Selection and Eligibility Criteria
The inclusion criteria applied in the study selection were: 1) Original research articles that utilized medical devices/technology in the prediction, treatment and management of epilepsy, and 2) English articles with full-text availability. The exclusion criteria that were applied in the study selection were: 1) non-original research articles labeled as editorials, conference papers, book chapters, reviews, systematic reviews, and case reports, 2) duplicated articles and 3) articles that do not align with the aim of the study. The study selection was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [15]. Two reviewers independently assessed and mutually reached a consensus on the selection and quality analysis of relevant articles included in this review.
2.3. Data Extraction and Risk of Bias Assessment
Data extracted from the selected studies included study information (study design and sample size), sample characteristics (type of epilepsy, age range and gender), type of medical device/technology utilized, significant results (device accuracy, sensitivity, specificity) and limitations (Tables 1-3). The articles that were selected for critical appraisal in this systematic review were assessed using various quality appraisal tools. The prospective and retrospective population studies were evaluated using the Effective Public Health Practice Project (EPHPP) (Table S1) [16], while the Cochrane risk-of-bias tool for randomized trials was used to appraise randomized clinical/controlled trials (RCTs) studies (Table S2) [17]. The Systematic Review Centre for Laboratory Animal Experimentation Risk of Bias (SYRCLE RoB) tool was used to appraise preclinical animal-based studies (Table S3) [18]. Articles that were algorithm-based were not quality assessed due to the unavailability of proper assessment tools for the aforementioned type of studies.
Table 1.
Utilization of medical devices/technology in clinical management and prediction of epilepsy.
| System Type | System Function |
Type of Study
(Sample Size) |
Medical
Technology |
Participants
(Age, Gender) |
Significant Findings | Refs. |
|---|---|---|---|---|---|---|
| Electro-encephalography (EEG)-based Systems | Monitoring System | Retrospective cohort [35] |
Robot-assisted stereo-electro-encephalography (SEEG) | Pharmaco-resistant epileptic children (3-17 years; 14F, 21M) | → SEEG electrodes were implanted using frameless robotic guidance. → More effective and safer at identifying Epileptic Zones in children with pharmaco-resistant partial epilepsy. → Shorter surgery time compared to the Talairach frame-based method. → Allow more electrode trajectory possibilities, enabling better localization of epileptic networks. Limitation: Invasive in nature with possible complications arising, including death. |
[19] |
| Retrospective Cohort [42] |
Video EEG-surface EMG combination (EMG - deltoid and biceps only) |
Patients admitted to the Epilepsy Monitoring Unit (11-62 years; 17F, 25M) | → Quantitative parameters, such as seizure phase duration, clonic bursts, silent periods and evolution dynamics, computed from surface EMGs in the deltoid and brachial bicep muscles were studied during convulsive seizures. → Identified seizures of increased risk with an accuracy of 85%, sensitivity up to 97%, and specificity of 90%. → Ictal quantitative surface EMG parameters correlated with postictal generalized EEG suppression (PGES) in real-time. → Non-invasive method. → Algorithms developed may be implemented into the wearable detection device. |
[20] | ||
| Prospective Longitudinal [24] | Behind-the-Ear EEG for Wearable Seizure Detection System | Patients with refractory focal epilepsy (19-64 years; 6F, 6M) |
→ Four electrodes were glued to the skin behind the ears, and the potential difference between both ears was measured, acting as an EEG channel. → Had 94.5% sensitivity, very similar to traditional scalp EEG. → Had a lower false detection rate of 0.52/hour compared to scalp EEG. → No visible artifacts (eye-blinking). Limitation: Unsuitable for frontal lobe epilepsy due to distance of EEG channels. |
[21] | ||
| Prospective Cohort [9] |
Long-term Subcutaneous EEG | Epileptic patients (27-64 years; 7F, 2 M) | → The implant consists of three leads which were surgically implanted subcutaneously through an incision behind the ear to ensure close proximity of the leads to the temporal lobe. → External logging device was attached to patient’s clothing by a strong magnet. → Feasible and well-tolerated, even after 3 months with home monitoring. → Moderate compliance (73%) and inter-individual variability (45-91%). → Minimal impact on daily activities. → Prevents under-/over-reporting as seen with seizure diaries. Limitation: Minimally invasive and may cause adverse effects, such as post-implantation soreness; low sample size and only TLE patients; the possibility of error due to artifacts; and the lack of validation data. |
[22] | ||
| Prospective Observational [1] | In-ear EEG system | Volunteer (NA; NA) |
→ The sensors were in contact with the mastoid of each ear as positions for reference and ground electrodes, respectively. → All-in-one portable system that is suitable to be used in combination with a smartphone and tablet. → Suitable for EEG recordings and real-time processing in everyday situations. |
[23] | ||
| - | - | - | - | - | → Low-cost, small-sized, and individualized device, increasing acceptability and tolerability by patients. Limitations: Spatial limitation, primarily in measuring temporal lobe activity; limited number of signal channels may constraint the system; currently available minicomputer limits the possibility of real-time processing; a single volunteer may not be sufficient to test the validity, reliability and safety of the system. |
- |
| - | - | Prospective Longitudinal [32] | An iEEG based prediction system for focal seizure evaluation | Epileptic patients (21-59 years; 15F,17M) | → Time and frequency of the implanted intracranial EEG readings were used to study the progression of EEG readings. → Successfully predicted the progression of focal seizures into bilateral tonic-clonic seizures in more than 60% of the cases. → Allows presurgical evaluation of seizure activity which improves safety. Limitation: Sample size and low number of seizures may deter the determination of effectivity, reliability and validity of the system; invasive implantation. |
[24] |
| - - |
- Prediction System |
Retrospective [20] | A patient-independent algorithm-based detection device of childhood absence epilepsy seizures | Childhood absence epileptic patients (7.5 ±1.8 years; 13F, 7M) |
→ Algorithm utilized support vector machines to classify seizures and maximum thresholds for the absolute amplitude of EEG signals were applied to recognize artefacts. → 97.2% of absence seizures were successfully detected. → No false positives detected. Limitation: Absence seizures were undetected during dosing; reliability and validation may require further verification; detect paroxysms longer than 2 seconds only; subtle manifestations may be missed. |
[25] |
| Retrospective [21] | Patient-specific EEG prediction system + LS-SVM classifier | Epileptic patients (10-50 years; 13F, 8 M) | → The proposed system analyzes EEG signals from different brain locations and predicts seizures onset in an automated method. → The proposed system successfully predicted seizures with a prediction accuracy of 95.4% and a false positive rate of 0.03/h. → The prediction system outperforms six unspecified existing prediction systems. Limitation: Validity is still unclear for prospective data. |
[26] | ||
| Prediction System - |
Prospective Cohort [10] |
Seizure prediction system utilizing deep learning classifier + TrueNorth processor chip | Epileptic patients (NA;NA) | → iEEG recordings were fed into the deep learning system in the training phase. The prediction system classified EEG signals into either pre-ictal or interictal phase, and self-optimized with parameters updated monthly with incoming data. → Proposed prediction system achieved mean sensitivity of 69% and mean time in warning of seizures at 27%, significantly surpassing an equivalent random predictor. → Proposed prediction system may be easily wearable, real-time and always on recording, and patient-specific seizure warning system. → Low power consumption with reliable long-term performance, a similar concept to hearing aid device. |
[27] | |
| Prospective Cohort [15] |
Seizure advisory system + iEEG | Epileptic patients with partial onset seizures (20-62 years; 6F, 9M) |
→ Patient-specific algorithms were configured for each patient prior to surgery. Automated and manual audio recordings of seizure diaries were correlated with the iEEG activity. → Implantation well-tolerated and feasible for drug-resistant patients. Limitations: Invasive and high incidents of adverse events were reported after 4 months; success rate was very low (only 2 patients); high variability in seizure warning times; patients were required to make significant lifestyle changes. |
[28] | ||
| Prospective Cohort [15] |
Seizure advisory system + implantable 16-channel subdural neural monitoring | Epileptic patients (20-61 years; 6F, 9M) | → A system with a vast high-quality database of EEG recordings, a structured algorithm, an implantable 16-channel subdural neural monitoring, and a seizure advisory system, was designed and built. → It was able to predict the high likelihood of seizure in subjects. → Long mean seizure advance warning time, allowing sufficient time for patients to take an intramuscular or oral antiepileptic drug to prevent the occurrence of the predicted seizure. → High efficacy. Limitation: Invasive method; a larger sample required to establish validity and reliability. |
[29] | ||
| - Extracerebral Signal-Based Systems |
Alarm-System | Retrospective [5] | Cloud Based Seizure Prediction | 4 healthy patients, 1 epileptic patient (NA; NA) | → An automatic, efficient and scalable mobile-based system to monitor and predict epileptic seizures in real-time and long-term, using wearable EEG sensors and cloud computing with GPS tracking as a hospital alert system. →The cloud-based system consists of a GPS alert system and processes the EEG readings, whereas the smartphone was bluetooth linked to a scalp EEG headset. → Accuracy up to 94.6%, sensitivity up to 93.8%, and specificity up to 92.3% in seizure classification. Limitation: Small sample size, not accurately representing the population. |
[30] |
| Monitoring System | Prospective Longitudinal [30] | Wristwatch accelerometer biosensor + online seizure database | Epileptic patients (19-66 years; 10F, 20 M) | → Patients were monitored continuously with video, audio, ECG and EEG sensors where the watch detected rhythmic and repetitive limb movements. → The watch successfully and automatically captured 12 out of 13 Generalized Tonic-Clonic Seizures with high accuracy. → Better feasibility than seizure diaries, especially for illiterate patients. Limitation: Low accuracy with 48% false positives was recorded; low sensitivity at 24.2%; artificial environment may not represent the general population. |
[31] | |
| Extracerebral Signal-Based Systems - |
Monitoring System - |
Prospective [7] |
Wearable system + Smartphone app with pre-calibrated MSPC model | Focal epileptic Japanese patients (9-54 years; 4F, 3M) |
→ Disposable ECG electrodes were attached to smartphone bluetooth-connected telemeter, which measured the interval time between R-waves on the ECG. → Small in size and follows smartphone battery (chargeable), and being easy for patients to use. → A sensitivity of 85.7% along with sufficient accuracy and reliability when compared to EEG video-monitoring and ECG data. Limitation: Motion artifacts of daily activities need optimization to avoid false positives; limited conditions may limit representation to the general population; Japanese population with focal epilepsy and during awake hours. |
[32] |
| Randomized controlled clinical trials [31] | Cardiac-based seizure detection algorithm (CBSDA) | Epileptic VNS candidates (19-66 years, 19F, 12 M) |
→ Continuous video-EEG and ECG monitoring was utilised, while heart electrical activity was monitored using VNS device monitors and lead electrode. → CBSDA has a high sensitivity for seizure prediction of more than 80%. → CSBDA has an acceptable specificity for triggering VNS, where almost 30% of the participants had more than 50% reduction in seizures. → Improved quality of life, reduced anxiety/depression, and improved social and cognition. Limitation: Invasive with 29% of developing dysphonia as an adverse effect. |
[33] | ||
| Prospective Longitudinal [75] | Accelerometry-sensor based wrist-wearable system to detect tonic-clonic seizures (TCSs) | Epilepsy surgery candidates (18-77 years; 47F, 28M) |
→ Two types of sensors were used for data collection. → High TCSs detection sensitivity (90-100%), comparable with a commercial wrist device. → Low false positive rate achieved with a single modality used. → Patients had no movement restrictions during video-EEG recording. Limitation: Missing data in the study, with a high rate of non-adherence to wrist-worn sensors. |
[34] | ||
| - | Prediction System | Prospective Cohort [70] |
Wearable devices based on extracerebral signals | Epileptic patients (20 to 69 years; majority identified as female) | → Commercially available wearable sensors measuring physiological signals, like accelerometry, electrodermal activity, photoplethysmography, and EEG, were utilized. → Patients showed a significant preference for wrist-worn devices. → Current wearable devices may provide high-quality data for routine use. → Signal quality metrics between the wearable devices provided good discrimination in the data quality as well as against noise artifacts. Limitation: Data quality, consistency, validity and management may need more improvement. |
[35] |
| Prediction System Alarm System |
Prospective Cohort [18] |
Self-aware wearable system | Epileptic patients (NA; NA) | → Prediction based on cardiorespiratory function, and achieves a sensitivity of 88.66% and a specificity of 85.65%. → Machine learning techniques that significantly improve detection quality, better than any other existing real-time wearable seizure detection system. → Energy-efficient where the battery lifetime outperforms the state-of-the-art techniques. → Patients were able to perform daily activities without constraints. Limitation: May require a larger sample size for validation of results. |
[36] | |
| Prospective Longitudinal [2] | Ethernet body-worn motion sensors-based alarm system | Epileptic patients (NA; NA) | → The motion sensors were attached to the subject's torso and wrist, which sent messages to nurses when motion abnormalities were detected. → Accurately detected seizures accounted for 90% with a low average number of false alarms per night. → This method is readily accepted by epileptic young adults; it is a non-invasive system. → This new system supports biomedical applications in hospitals or nursing homes, and increases accessibility and compliance. Limitation: Validation, sensitivity and specificity rate still underway; low sample size. |
[37] |
Note: NA: not available; F: female; M: male; VNS: vagal nerve stimulation; EEG: Electro-encephalography; GPS: global positioning system; LS-SVM: least squares support vector machines; MSPC: multivariate statistical process control.
Table 3.
Utilization of algorithm-based medical devices/technology in management and prediction of epilepsy.
| Main Method of Algorithm | System Function | Significant Findings | Refs. |
|---|---|---|---|
| Customizable multi-domain features in the seizure detection algorithm | Monitoring System | → High detection accuracy with low dimension, which reduces computational complexities. → Customisable and optimisable for wearable device. Limitation: Only 5 datasets were used to test the algorithm, which may lower the reliability and validity of results. |
[42] |
| Adaptive seizure prediction based on EEG synchronization | Prediction System | → 84% sensitivity and 63% specificity to seizures are achieved. → Adaptive learning capabilities allowing improvements in performance over time. → Fast processing time allowing embedment into mobile devices. Limitation: Some false positives could be due to eye movement artifacts. |
[43] |
| EEG rhythm decomposition using Jacobi polynomial transforms (JPTs) and linear discrimination analysis (LDA) |
Prediction System | → Processing chain for seizure detection yields a 96.25–100% accuracy → Able to discriminate between seizure-free, healthy and seizure conditions. Limitation: Computational time for processing chain is long. |
[44] |
| 1D convolution neural network | Prediction System | → Able to learn a lot of features. → Better precision and accuracy compared to existing standard models. → 98.33% accuracy, may be useful for the development of automated systems. Limitation: Training accuracy and validation were found to be good after 20 epochs. |
[45] |
Note: EEG: Electro-encephalography; 1D: 1 dimension.
3. RESULTS
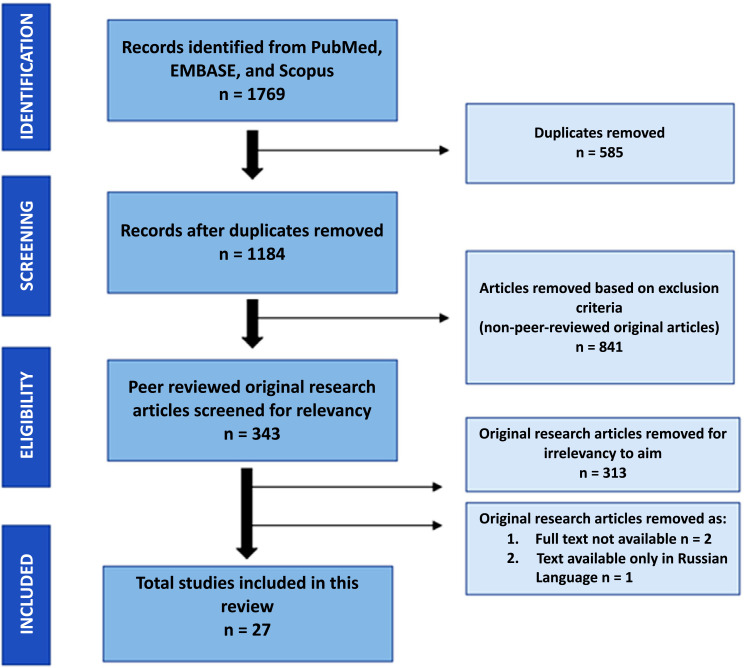
The initial literature search retrieved a total of 1769 articles, i.e., 271 articles from PubMed, 415 articles from EMBASE, and 1083 from Scopus databases. Among these, 585 duplicated articles were removed. Another 841 articles were also excluded based on the inclusion and exclusion selection criteria as per the PRISMA guidelines (Fig. 1). Finally, the abstracts of the remaining 343 articles were screened for relevance towards the aim of this systematic review, where 313 articles were found to be irrelevant as they mainly consisted of surveys/opinions on the medical/technology devices used for management of epilepsy without any utilization of description. Unfortunately, three articles were further removed from the selected articles as they were neither available as a full text nor were in English language [45-47]. Thus, a final total of 27 articles were selected for critical appraisal and were systematically evaluated and included in this review.
Fig. (1).
Study selection based on PRISMA flowchart.
These 27 articles were related to medical devices utilized mostly in the prediction and monitoring of epilepsy; none were found for the use in epilepsy treatment solely, but one article discussed the combination of prediction and treatment. These articles can be categorized broadly as follows: 19 clinical studies, four preclinical animal studies, and four algorithm-based research studies. Clinical and preclinical studies were further categorized into system type (EEG-based or extracerebral-signal based) and system function (monitoring, prediction or alarm system). A summary of these articles, including their significant findings, is provided in Table 1 (clinical studies), Table 2 (preclinical animal studies), and Table 3 (algorithm-based studies).
Table 2.
Utilization of medical devices/technology in management and prediction of epilepsy based on preclinical studies.
| System Type | System Function | Medical Technology |
Subjects
(Age, Gender) |
Significant Findings | Refs. |
|---|---|---|---|---|---|
| EEG-based System | Monitoring System | Implantable Continuous iEEG device | 8 dogs with spontaneous seizures (6 mixed hounds and 2 beagles) (8 to 43 months; 50%F, 50%M) |
→ Electrode arrays were placed in the subdural space. → The device proves valuable for guiding patient-administered antiepileptic therapy. → Two-way communications with other devices and a dictionary of stimulation paradigms, machine neuro-evolution. → Well-tolerated device with low-false positives. → Better than inpatient video EEG monitoring in terms of diagnosis and management of seizures. Limitation: Invasive and requires further validation in detecting human seizures. |
[38] |
| Prediction System | Support Vector Machine (SVM) + 16-channel ambulatory iEEG device | 8 dogs with naturally occurring epilepsy (NA; NA) |
→ The electrodes were anchored to the frontal bone, while the classification of seizures was done using SVM. → Prediction performance was significantly better than a time-matched Poisson random predictor. → Subject-specific tuning of the device is possible. Limitation: Generic placement of electrodes may hinder the prediction of a clear and consistent seizure onset zone; invasive and requires battery charging which may conflict the results and even cause data loss. |
[39] | |
| Alarm System |
Seizure Advisory System (SAS) | 5 dogs with naturally occurring idiopathic epilepsy (NA; NA) | → SAS devices were implanted. → May lead to seizure prediction that alerts clinicians and caregivers to administer the appropriate medication in a timely manner, which will prevent the seizure. → SAS provides information to clinicians regarding onset, duration and frequency of seizures. Limitation: Some focal seizures could not be detected due to spatial coverage limitation with strip electrodes in dogs. |
[40] | |
| Non-EEG based system | Prediction System | Implantable device with detection algorithm design space | 5 Kainate-treated Long Evans rats (NA; 5F) |
→ Microelectrodes were implanted ventrally to the cortex in the dentate gyrus, equivalent to the human temporal lobe. → Low power device and cost-effective. → The combined algorithm of an implantable monitoring device and implanted stimulator exhibited a 33% increase in seizure detection efficacy compared to other algorithm computations. Limitation: Invasive and requires further validation with a larger sample size. |
[41] |
Note: NA: not available; F: female; M: male; EEG: Electro-encephalography; iEEG: intracranial electro-encephalography.
4. DISCUSSION
This systematic review aimed to elucidate the beneficial potential and limitations of upcoming medical devices or technology utilized for the prediction and management of epilepsy that have been presented in the current literature. In this review, commercially available medical devices, such as the EEG, protective headgears, seizure-only alarms/ detection, and medication reminders were not included. However, this review highlighted that most of the upcoming devices and systems in the literature might be an improvement/advancement to these already established medical devices to increase efficiency, accuracy, sensitivity, and specificity towards epilepsy prediction and management in both children and in the adult population, since epilepsy affects all ages. Previous studies have shown that the age of the epileptic patient may play a role in EEG recommendation for seizure prediction and management [6], therefore similar factors should be taken into account for other medical devices as well.
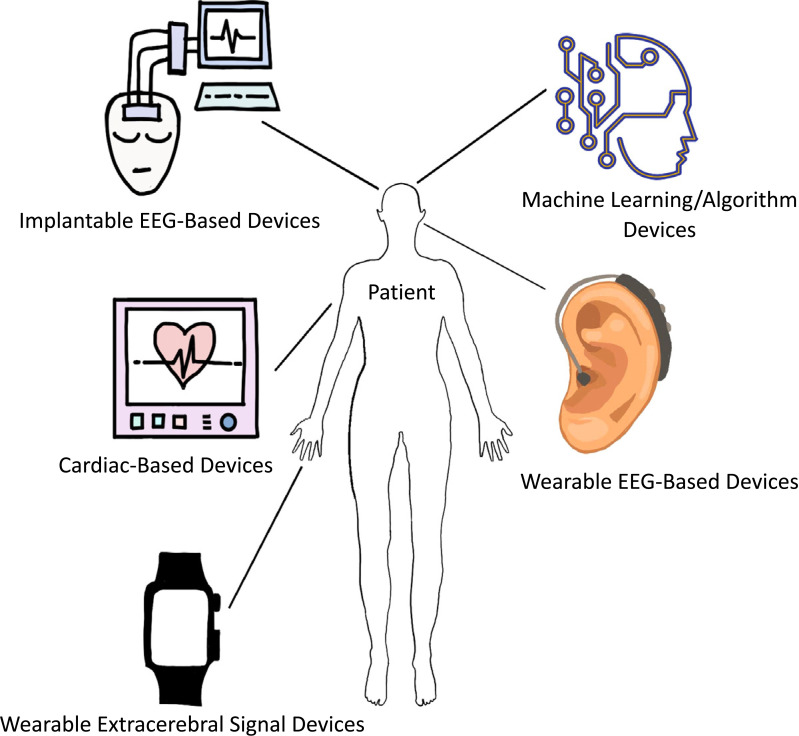
In total, there were 19 clinical studies, four preclinical studies and four algorithm studies that investigated new medical devices for epilepsy prediction and management (monitoring). A majority of the medical devices were based on the former EEG and seizure advisory systems, whilst some utilized newer deep learning or machine learning technology and even smartphones, which mainly focused on the external signatures of epilepsy, such as cardiorespiratory output and body motion (Fig. 2). Among the clinical studies, 5 studies utilized invasive, mainly implantation methods. Additionally, the majority of the animal studies also involved invasive methods. Invasive procedures may lead to medical complications and may pose a financial burden, as evident with even commonly used invasive devices in the hospitals [46]. Thus, invasive medical devices may require further improvements in being cost-effective with minimal side effects prior to recommendation to patients.
Fig. (2).
Medical devices/technology utilized for epilepsy prediction and management.
A majority of the clinical papers used adult epileptic patients as subjects, with only two papers [19, 25] focusing on epileptic children. This aligned with the prevalence and incidence rate of epilepsy within the global population, where adults outweighed children in the prevalence of active epilepsy and SUDEP incidence [47, 48]. However, the suitability of these medical devices in children should be investigated in future studies, particularly taking into account their safety and the precision of the device in the pediatric population. While the age of the participants in the clinical studies may represent the population, the sample sizes of these studies may not be accurately representative, as the sample size ranged from 2 to 75 subjects only. The small sample size utilized by some of the clinical studies may reduce the validity of the findings in these studies, and thus were also labelled as of poor quality with a possible risk of bias based on the EPHPP and Cochrane quality analysis tools (Supplementary File 1 (481KB, pdf) and 2 (481KB, pdf) , respectively). Sample size may influence the validity and reliability of research outcomes, regardless of the results obtained, but depending on the aim of the study, even a small sample size may be valid and reliable [49]. Unfortunately, since the clinical studies presented in Table1 mostly reported the accuracy, specificity and sensitivity of medical devices, a sample size of 1 to 20 participants may not be conclusive, especially due to the variability in seizure presentation between patients. Interestingly, Yamakawa and colleagues believe that their sample size of 8 Japanese focal epileptic patients was appropriate for their study findings to be reliable, as their proposed model was already established in their previous study with 14 patients and that this model utilized 14 seizure data from 7 patients, which created statistical significance [32]. However, the recruitment of a small population (heritage and type of epilepsy) with only age and sex variability may still hold the reliability and validity of the results in question.
As for the preclinical studies, three papers used 5 to 8 dogs as subjects in their studies (38-40) and only 1 paper [41] used 5 rats as subjects. The sample size of five rats may render the quality of this paper weak with a high risk of bias, as analyzed via the SYRCLE RoB tool (Supplementary File 3 (481KB, pdf) ), and may question the validity of the study’s findings. As dogs are able to develop spontaneous naturally occurring seizures, which mimic human genetic epilepsies and have been commonly used in EEG-based seizure detection algorithms [50], 5 to 8 dogs per study may be adequate for determining the initial validation from the modified EEG-based seizure detection medical devices.
Nevertheless, the concept and findings of the medical devices or technology proposed by the selected studies in this review should also be considered and critically reviewed before acknowledging their potential for commercialization.
4.1. Electroencephalogram for Epilepsy Prediction and Management
Electroencephalogram (EEG) was first used in humans in the 1920s [51], but it was not until the 1970s did clinicians started utilizing EEG for seizure predictions [52]. Today, it is one of the most commonly used devices for epilepsy prediction and management. This is mainly because the EEG remains to be easily available in many countries, even in low-to-middle income countries [53].
Although the EEG has achieved reliable sensitivity and specificity in detecting electrical activity of the brain despite the various forms of seizures in epilepsy, it is invasive and often has an intolerance to motion artifacts, thereby providing false-positive readings [32]. Moreover, hospitals use EEG to diagnose seizures as a presurgical screening procedure and to monitor patients’ seizure progression, thereafter prescribing them with anti-seizure medications [54], with no possibility of predicting the seizure occurrence in the patient after discharge and not knowing if the prescription made actively reduces seizures occurrence, other than relying on patients’ seizure diaries. The preictal period remains as the most difficult period to be detected as it is not clinically annotated and has no presence of recurrent pattern [55]. However, there are some arguments regarding whether the preictal and interictal spikes/epochs captured by EEG and their non-real-time detection [32, 56] may not provide sufficient information for seizure prediction and management as well as subsequent epilepsy treatment. Thus, with many limitations in the common EEG prediction system, improved medical devices, particularly those with ambulatory and portable options, may be proposed to further help epileptic patients in their seizure prediction and subsequent management.
4.2. Medical Devices Based on Electroencephalogram (EEG) System
Due to familiarity with the EEG system, innovation or improvement upon the existing scalp-EEG technology have been proposed in the development of a new medical device for epilepsy. Ambulatory EEG systems, non-invasive EEG system, and EEG systems with improved algorithms, are some of the innovations that have been proposed. In this systematic review, 12 clinical studies and 3 animal studies had proposed an improved EEG-based prediction and monitoring system for epilepsy (Tables 1-2).
For example, Weisdorf and colleagues described the use of ambulatory subcutaneous EEG, instead of the traditional hospital scalp EEGs, which may allow for easy, hassle-free home-monitoring of epilepsy up to 3 months [22]. The device consisted of three leads which were surgically implanted subcutaneously through an incision behind the ear to ensure close proximity of the leads to the temporal lobe. An external logging device was attached to the patient’s clothing by a strong magnet. Most patients concluded that the device was user friendly and had no issues with its daily operations [22]. This may allow patients to monitor their seizures during their daily activities and sleep, with the time of seizure occurrence specified, thus allowing more efficient seizure management and possibly for treatment administration as well. Even though the patients have reported good tolerability and compliance with the subcutaneous EEG device, some have reported soreness at the site of implantation of the device [22]. Furthermore, the accuracy and reliability of this device were compared to the manual reporting of seizures by patients/caregivers using their seizure diaries, instead of a more reliable comparison with hospital-based EEG systems.
There were also some other limitations discussed regarding the device proposed by Weisdorf, such as the detection of artifacts which may give false positives and the distance between implanted rods and seizure foci, which may miss the detection range of some seizures [22]. The position of the electrodes in relation to the seizure foci plays an important role in the detection accuracy and specificity of seizures [22, 57, 58]. This spatial limitation of seizure detection was also witnessed in the other selected studies, even those involving children and sleep [23, 25, 39, 40], which may not be ideal, as seizure detection during sleep may play a crucial role in preventing SUDEP. This is because SUDEP may be associated with sleep [59], thus identifying true seizure spikes during sleep may be important for SUDEP intervention.
Similarly, the false positive or artifact limitation was also reported in the other non-EEG-based devices studies selected in this review [31, 32], whereby eye movements created noise artifacts that were difficult to discriminate between seizures. Artifact-related false positives have been a common problem in traditional EEG recordings as well. The rhythmic patterns generated from a faulty electrode or ocular and muscle movements during EEG may mimic epileptiform activity, thereby misinterpreting the epileptic seizures [60, 61]. Interestingly, this limitation was only observed by Weisdorf among the EEG-based systems.
Since there have been many studies that have applied various methods or algorithms, including the utilization of principal component analysis (PCA), support vector machine classifiers or energy interval histograms to overcome or discriminate the artifact detection [61-63], these methods/algorithms may be applied in the development of future medical devices. Table 1 presents some clinical studies (EEG-based and non-EEG based system) applying these artifact elimination methods/algorithms which have been successful in reducing their false positive rates while still maintaining their seizure sensitivity rate [21, 25, 26, 34, 35, 37]. In one study, the prediction device with the appropriate classifiers encouraged the development of a patient-specific system, whereby the extraction, classification and regularization features of the classifier allowed the distinction of preictal/ictal and interictal EEG signals, thereby increasing the sensitivity and specificity of the device [26]. Kiral-Kornek, however, showed low sensitivity in seizure prediction when iEEG data was coupled with the newer but less optimized deep learning classifier [27]. In this study, iEEG recordings were fed into the deep learning system in the training phase. The prediction system classified EEG signals to preictal or interictal phase, which then self-optimized with parameters updated each month with incoming data. Despite their results, they still argued that with further studies, the deep learning classifiers together with the neuromorphic chip, may provide the foundation for real-time, always on, low power, and patient-specific wearable medical device with reliable long-term performance, making it the ideal seizure prediction and monitoring device for epileptic patients.
Canine studies [38, 39] and a rodent study [41] also utilized algorithms to improve the accuracy, sensitivity and specificity of seizure prediction medical devices. Davis’s paper even argued that their seizure detection algorithm, which was developed using human EEG where electrode arrays were placed in the subdural space, performed equally well in predicting canine ictal patterns with precision [38]. The support vector machine algorithm used in the intracranial EEG in Brinkmann’s study showed a subject-specific seizure prediction capability, which may require further validation [39]. In this study, electrodes were anchored to the frontal bone, and classification of seizures was done using SVM. This later invention may resolve the patient variability issues in seizure prediction and management. Moreover, if the detection algorithm design space and co-optimization method proposed by Raghunathan are to be implemented in future human seizure prediction devices [41], then the effectiveness of algorithm may be determined for each type of seizures displayed by the patients, thereby increasing the specificity of many EEG-based and non-EEG-based prediction devices. Similarly, another study proposed a customizable multi-domain feature pool that enhanced the seizure detection and prediction accuracy of wearable EEG-based medical devices in real-time and at low power consumption [42], making it ideal to be used in ambulatory seizure prediction devices. This, together with the 1D Convolution Neural Network model proposed by Ranga and colleagues and the single-step processing chain proposed in the study by Djoufack Nkengfack’s, may highly improve the currently available EEG-based seizure prediction and management devices worldwide, in terms of precision, accuracy, specificity and sensitivity. The 1D Convolution model will enable automated analysis of EEG-signals [45], while the processing chain involving the Jacobi polynomial transforms and linear discriminant analysis may lead to the development of treatment triggering prediction devices [44], thus saving time and increasing the efficiency of ambulatory EEG-based devices in preventing seizures in patients.
Among the clinical studies, the non-invasive EEG-based devices were found to be more tolerable by patients, as the invasive devices have reported incidences of adverse effects [19, 22, 29, 64]. In a 2015 survey, a majority of the patients voiced their preference for easily removable devices rather than those requiring implantation [65]. The behind-ear EEG system [21], in-ear EEG system [23], as well as the video-EEG and surface EMG combination system [20], may be the most tolerable and efficient EEG-based medical devices proposed, with a high rate of sensitivity, accuracy and specificity towards seizure detection and prediction. However, thus far, these devices, such as the in-ear EEG device, seem to be more optimized for the prediction of temporal lobe seizures [23], compared to other types of seizures.
Another promising medical device may be the one proposed by Sareen and colleagues, which utilizes wearable EEG sensors coupled with the cloud computing technology, enabling a mobile-based seizure prediction and management system equipped with a GPS alarm system for fast intervention action [30]. The EEG synchronization method proposed by Ibrahim and Sohaib, where the baseline, normal and pre-seizure EEG data were synchronized to predict upcoming seizures in an adaptive manner [43], may allow mobile EEG-based systems to be implemented for patients.
Similar to Sareen’s proposed device, an alarm system-based EEG device was also suggested in a preclinical study, where veterinarians were alerted immediately to administer anti-seizure drugs as a result of the seizure advisory EEG system [40]. This suggests that an alarm system in seizure prediction devices may improve the quick management of these seizures. However, thus far, these EEG-based systems have only been proven feasible against a single type of epilepsy, which may be due to the small sample sizes of these studies. Only one study showed the successful prediction of epilepsy progression from focal seizures to the secondary generalization of bilateral tonic-clonic seizures, but the reliability of the result may be questionable due to the small sample size and low seizure frequencies [24]. Therefore, more studies, preferably with larger sample sizes, should be conducted to investigate the feasibility of these proposed EEG-based medical devices for a more diverse collection of epilepsies.
4.3. Medical Devices Based on Extracerebral Signals System
Instead of detecting cerebral signals like the EEG-based system, the extracerebral signals of epilepsy, such as body/muscular motion, cardiorespiratory outputs and electrodermal activity, may also be used to develop non-EEG-based medical devices for the prediction and monitoring of epilepsy. In this systematic review, 7 clinical studies utilized the extracerebral signals in their proposed medical device systems. None of the preclinical animal studies selected in this review utilized these signals in their devices.
Predicting seizures based on extracerebral signals may provide various benefits; they make it robust and easy to record the seizures, are simpler to process and analyze, involve the usage of already commercialized and inexpensive tools, may be used in small and portable devices, and may be better tolerated among patients [66]. In fact, a couple of studies included in this review have shown the potential of wrist-worn devices, similar to the smartwatch, for epilepsy prediction and management, having high accuracy for seizure detection but still lacking validity for sensitivity and specificity parameters [31, 35]. Wrist-worn devices would be ideal since they would allow patients to be independent and regain their quality of life with reduced hinderance in their daily activities. A review in 2018 suggested that wrist-worn devices still have much room for improvements, particularly in the battery life, resistance to environmental factors and setting standards [67]. Privacy may also be a concern for some patients, especially when their data may be transferred to an online system to allow real-time prediction and monitoring of seizures [31]. Wrist-worn devices were equipped with accelerometers and principal component analysis, which accurately detected and recorded movement acceleration, as seen in smartwatches for fitness [68]. Accelerometer equipped devices have shown to be able to predict seizures with a low false-positive rate that may be comparable to video EEG [34] and may be important for managing nocturnal seizures [37], suggesting the potential of these devices to be worn by epileptic patients in the prevention of SUDEP. Although the device suggested by Bonnet and colleagues was equipped with an emergency alarm system that warns caregivers when the patient is about to have a seizure, allowing timely medication administration [37], the 2015 survey suggested that 50% of patients found the alarm system very tiresome [65], creating a phenomenon known as ‘alarm fatigue’ [14].
On the other hand, Nasseri and colleagues managed to show that wrist-worn devices may detect more than just accelerated movements, where detection by photoplethysmography (blood volume changes) and electrodermal activity (electrical discharges in the skin) were also capable to predict and manage epilepsy to the level of an EEG system [35]. However, the paper did conclude that improvements may be needed in these wrist-worn devices before recommendation to epileptic patients.
Another extracerebral signal that has been proposed to be utilized in medical devices for epilepsy prediction and management involves the cardiorespiratory outcomes of epilepsy. Cardiorespiratory comorbidities have been found to be prevalent in epileptic patients and have even been suggested as a risk contributor towards SUDEP [69]. In this systematic review, three clinical studies have used cardiac-based detection system for seizure prediction and management, with one looking into the respiratory signal potential as well. Tachycardia, a common outcome displayed by epileptic patients, may manifest prior to EEG-electrographic signals [70]. Likewise, respiratory rate may also change drastically during ictal/postictal seizures, which may be distinguishable from the respiratory rate of exercise [66, 71]. Therefore, these suggest that cardiac and respiratory signals may be more important than EEG signals for seizure prediction and management.
Forooghifar’s paper proposed a real-time wearable medical device, which utilizes the cardiac and respiratory responses of patients [36]. Their paper concluded the specificity and sensitivity of above 80% for the proposed medical device in predicting epileptic seizures. Unfortunately, their study included only a sample size of 18 epileptic patients, thus compromising the validity of the results. Similarly, while Yamakawa’s proposed device also showed an above 80% sensitivity in seizure prediction via heart rate variability monitoring through a smartphone application [32], their sample size was also small and not representative of the general population.
This review found the medical device proposed by Boon and colleagues to be most promising. They presented a seizure prediction and management device which utilized the cardiac-based seizure detection algorithm (CBSDA) as well as automatically intervened the seizures through vagus nerve stimulation (VNS) [33]. VNS is one of the common interventions for epilepsy, the others being surgical resection, ablative implantation of probes and anti-convulsive drugs [12]. Boon’s device managed to reduce the seizure activity by 50% in nearly 30% of their sample size. Their results showed that the CBSDA exhibited high sensitivity and specificity towards seizure prediction that allowed accurate triggering of the VNS [33]. When a patient’s heart rate increases above the set threshold for at least one second, the VNS will be automatically triggered. Although, for now, this intervention may only be used for epileptic patients with ictal tachycardia, one study suggested the use of ECG patch that will be able to detect atrial fibrillation as well, but the automatic algorithm for atrial fibrillation detection may still be underway [72]. Although Boon’s paper claim that further studies may be required, especially in correcting the adverse effect (dysphonia) seen in some patients, if successful, this medical device may be recommended for nocturnal seizure monitoring and for the prevention of nocturnal seizure-related physical injuries and SUDEP.
Nevertheless, there may still be much improvements to be made in these non-EEG-based devices, particularly in terms of sensitivity and specificity towards seizures-related changes in extracerebral activity and not the changes resulting from daily activities. Multivariant-based or multi-extracerebral signal detection system may enhance the sensitivity and specificity of the automated device, whereby different combinations of signals may be programmed to determine the type and extent of the seizure activity.
CONCLUSION
This systematic review found that EEG-based seizure prediction and management (monitoring) devices remain the most investigated form of medical devices for epileptic seizures compared to devices that utilize extracerebral signals. However, this review believes that extracerebral signals, especially cardiac signals such as tachycardia, which may present themselves before cerebral signals, may be more appropriate and efficient at predicting seizures in a timelier manner. In addition, wearable ambulatory medical devices, such as in-ear EEG and wristwatch sensors, are more preferred among patients due to their lower interference with daily activities as well as their safety in terms of side effects, compared to invasive implantable devices. These may also be advantageous and effective for detecting nocturnal epileptic seizures, which therefore may reduce the occurrence of SUDEP in epileptic patients, particularly when combined with an automated alarm system or intervention trigger, VNS or ASDs. However, given the limitations of the current studies, there is still a need for further studies for the validation of these proposed devices in terms of reliability, safety, accuracy, sensitivity and specificity in detecting and predicting patient-specific seizures. With the rapid development in artificial intelligence, the potential for improvements in these medical devices may be endless.
ACKNOWLEDGEMENTS
Declared none.
AUTHORS’ CONTRIBUTIONS
JO and SW performed the literature search and selection, quality analysis and wrote the manuscript; AA guided, reviewed and edited the manuscript; JLW reviewed the manuscript, while MFS conceptualized the idea and reviewed the manuscript. All authors read and approved the final manuscript.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines have been followed.
FUNDING
This project is funded by Monash University Malaysia NEED (NEtwork for Equity through Digital Health) Grant Scheme 2020 (MED/NEED/11-2020/002).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material and PRISMA checklist are available on the publisher’s website along with the published article.
REFERENCES
- 1.Fisher R.S., Acevedo C., Arzimanoglou A., Bogacz A., Cross J.H., Elger C.E., Engel J., Jr, Forsgren L., French J.A., Glynn M., Hesdorffer D.C., Lee B.I., Mathern G.W., Moshé S.L., Perucca E., Scheffer I.E., Tomson T., Watanabe M., Wiebe S. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Epilepsy: World Health Organization (WHO); 2019 [cited 2021 28/5/2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/epilepsy
- 3.Keezer M.R., Sisodiya S.M., Sander J.W. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15(1):106–115. doi: 10.1016/S1474-4422(15)00225-2. [DOI] [PubMed] [Google Scholar]
- 4.Stewart M. An explanation for sudden death in epilepsy (SUDEP). J. Physiol. Sci. 2018;68(4):307–320. doi: 10.1007/s12576-018-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson P. Wearable Device Clears a First 'Milestone' in Seizure Detection: Medscape; 2020. Available from: https://www. medscape.com/viewarticle/942344
- 6.Smith S.J.M. EEG in the diagnosis, classification, and management of patients with epilepsy. J. Neurol. Neurosurg. Psychiatry. 2005;76(Suppl. 2):ii2–ii7. doi: 10.1136/jnnp.2005.069245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahab A. Difficulties in treatment and management of epilepsy and challenges in new drug development. Pharmaceuticals (Basel) 2010;3(7):2090–2110. doi: 10.3390/ph3072090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwan P., Arzimanoglou A., Berg A.T., Brodie M.J., Allen Hauser W., Mathern G., Moshé S.L., Perucca E., Wiebe S., French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 9.Fisher R.S. Therapeutic devices for epilepsy. Ann. Neurol. 2012;71(2):157–168. doi: 10.1002/ana.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwan P., Brodie M.J. Combination therapy in epilepsy: when and what to use. Drugs. 2006;66(14):1817–1829. doi: 10.2165/00003495-200666140-00004. [DOI] [PubMed] [Google Scholar]
- 11.Mutanana N., Tsvere M., Chiweshe M.K. General side effects and challenges associated with anti-epilepsy medication: A review of related literature. Afr. J. Prim. Health Care Fam. Med. 2020;12(1):e1–e5. doi: 10.4102/phcfm.v12i1.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jette N., Reid A.Y., Wiebe S. Surgical management of epilepsy. CMAJ. 2014;186(13):997–1004. doi: 10.1503/cmaj.121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An S., Kang C., Lee H.W. Artificial intelligence and computational approaches for epilepsy. J. Epilepsy Res. 2020;10(1):8–17. doi: 10.14581/jer.20003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Andel J., Thijs R.D., de Weerd A., Arends J., Leijten F. Non-EEG based ambulatory seizure detection designed for home use: What is available and how will it influence epilepsy care? Epilepsy Behav., 2016, 57(Pt A), 82-89. [DOI] [PubMed]
- 15.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EPHPP EPHPP. Effective Public Healthcare Panacea Project EPHPP. 1999.
- 17.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., Emberson J.R., Hernán M.A., Hopewell S., Hróbjartsson A., Junqueira D.R., Jüni P., Kirkham J.J., Lasserson T., Li T., McAleenan A., Reeves B.C., Shepperd S., Shrier I., Stewart L.A., Tilling K., White I.R., Whiting P.F., Higgins J.P.T. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 18.Hooijmans C.R., Rovers M.M., de Vries R.B.M., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14(1):43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abel T.J., Varela Osorio R., Amorim-Leite R., Mathieu F., Kahane P., Minotti L., Hoffmann D., Chabardes S. Frameless robot-assisted stereoelectroencephalography in children: technical aspects and comparison with Talairach frame technique. J. Neurosurg. Pediatr. 2018;22(1):37–46. doi: 10.3171/2018.1.PEDS17435. [DOI] [PubMed] [Google Scholar]
- 20.Arbune A.A., Conradsen I., Cardenas D.P., Whitmire L.E., Voyles S.R., Wolf P., Lhatoo S., Ryvlin P., Beniczky S. Ictal quantitative surface electromyography correlates with postictal EEG suppression. Neurology. 2020;94(24):e2567–e2576. doi: 10.1212/WNL.0000000000009492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu Y., Cleeren E., Dan J., Claes K., Van Paesschen W., Van Huffel S., Hunyadi B. Comparison between scalp EEG and behind-the-ear EEG for development of a wearable seizure detection system for patients with focal epilepsy. Sensors (Basel) 2017;18(1):29. doi: 10.3390/s18010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisdorf S., Duun-Henriksen J., Kjeldsen M.J., Poulsen F.R., Gangstad S.W., Kjaer T.W. Ultra-long-term subcutaneous home monitoring of epilepsy-490 days of EEG from nine patients. Epilepsia. 2019;60(11):2204–2214. doi: 10.1111/epi.16360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sintotskiy G., Hinrichs H. In-ear-EEG - a portable platform for home monitoring. J. Med. Eng. Technol. 2020;44(1):26–37. doi: 10.1080/03091902.2020.1713238. [DOI] [PubMed] [Google Scholar]
- 24.Karthick P.A., Tanaka H., Khoo H.M., Gotman J. Prediction of secondary generalization from a focal onset seizure in intracerebral EEG. Clin. Neurophysiol. 2018;129(5):1030–1040. doi: 10.1016/j.clinph.2018.02.122. [DOI] [PubMed] [Google Scholar]
- 25.Duun-Henriksen J., Madsen R.E., Remvig L.S., Thomsen C.E., Sorensen H.B.D., Kjaer T.W. Automatic detection of childhood absence epilepsy seizures: toward a monitoring device. Pediatr. Neurol. 2012;46(5):287–292. doi: 10.1016/j.pediatrneurol.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Parvez M.Z., Paul M. Seizure prediction using undulated global and local features. IEEE Trans. Biomed. Eng. 2017;64(1):208–217. doi: 10.1109/TBME.2016.2553131. [DOI] [PubMed] [Google Scholar]
- 27.Kiral-Kornek I., Roy S., Nurse E., Mashford B., Karoly P., Carroll T., Payne D., Saha S., Baldassano S., O’Brien T., Grayden D., Cook M., Freestone D., Harrer S. Epileptic seizure prediction using big data and deep learning: Toward a mobile system. EBioMedicine. 2018;27:103–111. doi: 10.1016/j.ebiom.2017.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cook M.J., O’Brien T.J., Berkovic S.F., Murphy M., Morokoff A., Fabinyi G., D’Souza W., Yerra R., Archer J., Litewka L., Hosking S., Lightfoot P., Ruedebusch V., Sheffield W.D., Snyder D., Leyde K., Himes D. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. Lancet Neurol. 2013;12(6):563–571. doi: 10.1016/S1474-4422(13)70075-9. [DOI] [PubMed] [Google Scholar]
- 29.DiLorenzo D.J., Leyde K.W., Kaplan D. Neural state monitoring in the treatment of epilepsy: Seizure prediction-conceptualization to first-in-man study. Brain Sci. 2019;9(7):E156. doi: 10.3390/brainsci9070156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sareen S., Sood S.K., Gupta S.K. An automatic prediction of epileptic seizures using cloud computing and wireless sensor networks. J. Med. Syst. 2016;40(11):226. doi: 10.1007/s10916-016-0579-1. [DOI] [PubMed] [Google Scholar]
- 31.Velez M., Fisher R.S., Bartlett V., Le S. Tracking generalized tonic-clonic seizures with a wrist accelerometer linked to an online database. Seizure. 2016;39:13–18. doi: 10.1016/j.seizure.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Yamakawa T., Miyajima M., Fujiwara K., Kano M., Suzuki Y., Watanabe Y., Watanabe S., Hoshida T., Inaji M., Maehara T. Wearable epileptic seizure prediction system with machine-learning-based anomaly detection of heart rate variability. Sensors (Basel) 2020;20(14):3987. doi: 10.3390/s20143987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boon P., Vonck K., van Rijckevorsel K., El Tahry R., Elger C.E., Mullatti N., Schulze-Bonhage A., Wagner L., Diehl B., Hamer H., Reuber M., Kostov H., Legros B., Noachtar S., Weber Y.G., Coenen V.A., Rooijakkers H., Schijns O.E., Selway R., Van Roost D., Eggleston K.S., Van Grunderbeek W., Jayewardene A.K., McGuire R.M. A prospective, multicenter study of cardiac-based seizure detection to activate vagus nerve stimulation. Seizure. 2015;32:52–61. doi: 10.1016/j.seizure.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Johansson D., Ohlsson F., Krýsl D., Rydenhag B., Czarnecki M., Gustafsson N., Wipenmyr J., McKelvey T., Malmgren K. Tonic-clonic seizure detection using accelerometry-based wearable sensors: A prospective, video-EEG controlled study. Seizure. 2019;65:48–54. doi: 10.1016/j.seizure.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 35.Nasseri M., Nurse E., Glasstetter M., Böttcher S., Gregg N.M., Laks Nandakumar A., Joseph B., Pal Attia T., Viana P.F., Bruno E., Biondi A., Cook M., Worrell G.A., Schulze-Bonhage A., Dümpelmann M., Freestone D.R., Richardson M.P., Brinkmann B.H. Signal quality and patient experience with wearable devices for epilepsy management. Epilepsia. 2020;61(Suppl. 1):S25–S35. doi: 10.1111/epi.16527. [DOI] [PubMed] [Google Scholar]
- 36.Forooghifar F., Aminifar A., Cammoun L., Wisniewski I., Ciumas C., Ryvlin P. A self-aware epilepsy monitoring system for real-time epileptic seizure detection. Mob. Netw. Appl. 2019 doi: 10.1007/s11036-019-01322-7. [DOI] [Google Scholar]
- 37.Bonnet S., Jallon P., Bourgerette A., Antonakios M., Guillemaud R., Caritu Y. An Ethernet motion-sensor based alarm system for epilepsy monitoring. IRBM. 2011;32(2):155–157. doi: 10.1016/j.irbm.2011.01.021. [DOI] [Google Scholar]
- 38.Davis K.A., Sturges B.K., Vite C.H., Ruedebusch V., Worrell G., Gardner A.B., Leyde K., Sheffield W.D., Litt B. A novel implanted device to wirelessly record and analyze continuous intracranial canine EEG. Epilepsy Res. 2011;96(1-2):116–122. doi: 10.1016/j.eplepsyres.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brinkmann B.H., Patterson E.E., Vite C., Vasoli V.M., Crepeau D., Stead M., Howbert J.J., Cherkassky V., Wagenaar J.B., Litt B., Worrell G.A. Forecasting seizures using intracranial EEG measures and SVM in naturally occurring canine epilepsy. PLoS One. 2015;10(8):e0133900. doi: 10.1371/journal.pone.0133900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coles L.D., Patterson E.E., Sheffield W.D., Mavoori J., Higgins J., Michael B., Leyde K., Cloyd J.C., Litt B., Vite C., Worrell G.A. Feasibility study of a caregiver seizure alert system in canine epilepsy. Epilepsy Res. 2013;106(3):456–460. doi: 10.1016/j.eplepsyres.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raghunathan S., Gupta S.K., Markandeya H.S., Roy K., Irazoqui P.P. A hardware-algorithm co-design approach to optimize seizure detection algorithms for implantable applications. J. Neurosci. Methods. 2010;193(1):106–117. doi: 10.1016/j.jneumeth.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Jiang Z., Zhao W. Optimal selection of customized features for implementing seizure detection in wearable electroencephalography sensor. IEEE Sens. J. 2020;20(21):12941–12949. doi: 10.1109/JSEN.2020.3003733. [DOI] [Google Scholar]
- 43.Ibrahim S., Majzoub S. Adaptive epileptic seizure prediction based on EEG synchronization. J. Biomimetics. Biomater. Biomed. Eng. 2017;33:52–58. doi: 10.4028/www.scientific.net/JBBBE.33.52. [DOI] [Google Scholar]
- 44.Djoufack Nkengfack L.C., Tchiotsop D., Atangana R., Louis-Door V., Wolf D. EEG signals analysis for epileptic seizures detection using polynomial transforms, linear discriminant analysis and support vector machines. Biomed. Signal Process. Control. 2020;62:102141. doi: 10.1016/j.bspc.2020.102141. [DOI] [Google Scholar]
- 45.Ranga V., Gupta S., Meena J., Agrawal P. Automated human mind reading using EEG signals for seizure detection. J. Med. Eng. Technol. 2020;44(5):237–246. doi: 10.1080/03091902.2020.1791988. [DOI] [PubMed] [Google Scholar]
- 46.Bennett E.E., VanBuren J., Holubkov R., Bratton S.L. Presence of Invasive Devices and Risks of Healthcare-Associated Infections and Sepsis. J. Pediatr. Intensive Care. 2018;7(4):188–195. doi: 10.1055/s-0038-1656535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zack M.M., Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 2017;66(31):821–825. doi: 10.15585/mmwr.mm6631a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitney R., Donner E.J. Risk factors for sudden unexpected death in epilepsy (SUDEP) and their mitigation. Curr. Treat. Options Neurol. 2019;21(2):7. doi: 10.1007/s11940-019-0547-4. [DOI] [PubMed] [Google Scholar]
- 49.Faber J., Fonseca L.M. How sample size influences research outcomes. Dental Press J. Orthod. 2014;19(4):27–29. doi: 10.1590/2176-9451.19.4.027-029.ebo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grone B.P., Baraban S.C. Animal models in epilepsy research: legacies and new directions. Nat. Neurosci. 2015;18(3):339–343. doi: 10.1038/nn.3934. [DOI] [PubMed] [Google Scholar]
- 51.Berger H. Über das Elektrenkephalogramm des Menschen. Arch. Psychiatr. Nervenkr. 1929;87(1):527–570. doi: 10.1007/BF01797193. [DOI] [Google Scholar]
- 52.Viglione S.S., Walsh G.O. Proceedings: Epileptic seizure prediction. Electroencephalogr. Clin. Neurophysiol. 1975;39(4):435–436. [PubMed] [Google Scholar]
- 53.McLane H.C., Berkowitz A.L., Patenaude B.N., McKenzie E.D., Wolper E., Wahlster S., Fink G., Mateen F.J. Availability, accessibility, and affordability of neurodiagnostic tests in 37 countries. Neurology. 2015;85(18):1614–1622. doi: 10.1212/WNL.0000000000002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iasemidis L.D. Epileptic seizure prediction and control. IEEE Trans. Biomed. Eng. 2003;50(5):549–558. doi: 10.1109/TBME.2003.810705. [DOI] [PubMed] [Google Scholar]
- 55.Pinto M.F., Leal A., Lopes F., Dourado A., Martins P., Teixeira C.A. A personalized and evolutionary algorithm for interpretable EEG epilepsy seizure prediction. Sci. Rep. 2021;11(1):3415. doi: 10.1038/s41598-021-82828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagaraj V., Lee S.T., Krook-Magnuson E., Soltesz I., Benquet P., Irazoqui P.P., Netoff T.I. Future of seizure prediction and intervention: closing the loop. J. Clin. Neurophysiol. 2015;32(3):194–206. doi: 10.1097/WNP.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lieb J.P., Walsh G.O., Babb T.L., Walter R.D., Crandall P.H. A comparison of EEG seizure patterns recorded with surface and depth electrodes in patients with temporal lobe epilepsy. Epilepsia. 1976;17(2):137–160. doi: 10.1111/j.1528-1157.1976.tb03392.x. [DOI] [PubMed] [Google Scholar]
- 58.Tao J.X., Baldwin M., Ray A., Hawes-Ebersole S., Ebersole J.S. The impact of cerebral source area and synchrony on recording scalp electroencephalography ictal patterns. Epilepsia. 2007;48(11):2167–2176. doi: 10.1111/j.1528-1167.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 59.Ali A., Wu S., Issa N.P., Rose S., Towle V.L., Warnke P., Tao J.X. Association of sleep with sudden unexpected death in epilepsy. Epilepsy Behav. 2017;76:1–6. doi: 10.1016/j.yebeh.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 60.Skupch A.M., Dollfuß P., Fürbaß F., Hartmann M., Perko H., Pataraia E. EEG artifact detection using spatial distribution of rhythmicity. APCBEE Procedia. 2013;7:16–20. doi: 10.1016/j.apcbee.2013.08.005. [DOI] [Google Scholar]
- 61.Jiang X., Bian G-B., Tian Z. Removal of artifacts from EEG signals: A review. Sensors (Basel) 2019;19(5):987. doi: 10.3390/s19050987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Regan S., Faul S., Marnane W. Automatic detection of EEG artefacts arising from head movements using EEG and gyroscope signals. Med. Eng. Phys. 2013;35(7):867–874. doi: 10.1016/j.medengphy.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 63.Park H.J., Jeong D.U., Park K.S. Automated detection and elimination of periodic ECG artifacts in EEG using the energy interval histogram method. IEEE Trans. Biomed. Eng. 2002;49(12 Pt 2):1526–1533. doi: 10.1109/TBME.2002.805482. [DOI] [PubMed] [Google Scholar]
- 64.Lazarou I., Nikolopoulos S., Petrantonakis P.C., Kompatsiaris I., Tsolaki M. EEG-Based Brain-Computer Interfaces for Communication and Rehabilitation of People with Motor Impairment: A Novel Approach of the 21 st Century. Front. Hum. Neurosci. 2018;12:14. doi: 10.3389/fnhum.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoppe C., Feldmann M., Blachut B., Surges R., Elger C.E., Helmstaedter C. Novel techniques for automated seizure registration: Patients’ wants and needs. Epilepsy Behav., 2015, 52(Pt A), 1- 7. [DOI] [PubMed]
- 66.Osorio I., Schachter S. Extracerebral detection of seizures: a new era in epileptology? Epilepsy Behav. 2011;22(Suppl. 1):S82–S87. doi: 10.1016/j.yebeh.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 67.Al-Eidan R.M., Al-Khalifa H., Al-Salman A.M. A review of wrist-worn wearable: Sensors, models, and challenges. J. Sens. 2018;2018:5853917. doi: 10.1155/2018/5853917. [DOI] [Google Scholar]
- 68.Balli S., Sağbaş Ensar A., Peker M. Human activity recognition from smart watch sensor data using a hybrid of principal component analysis and random forest algorithm. Meas. Control. 2019;52(1-2):37–45. doi: 10.1177/0020294018813692. [DOI] [Google Scholar]
- 69.Akyüz E., Üner A.K., Köklü B., Arulsamy A., Shaikh M.F. Cardiorespiratory findings in epilepsy: A recent review on outcomes and pathophysiology. J. Neurosci. Res. 2021;99(9):2059–2073. doi: 10.1002/jnr.24861. [DOI] [PubMed] [Google Scholar]
- 70.Leutmezer F., Schernthaner C., Lurger S., Pötzelberger K., Baumgartner C. Electrocardiographic changes at the onset of epileptic seizures. Epilepsia. 2003;44(3):348–354. doi: 10.1046/j.1528-1157.2003.34702.x. [DOI] [PubMed] [Google Scholar]
- 71.Seyal M., Bateman L.M., Albertson T.E., Lin T-C., Li C-S. Respiratory changes with seizures in localization-related epilepsy: analysis of periictal hypercapnia and airflow patterns. Epilepsia. 2010;51(8):1359–1364. doi: 10.1111/j.1528-1167.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 72.Shao M., Zhou Z., Bin G., Bai Y., Wu S. A wearable electrocardiogram telemonitoring system for atrial fibrillation detection. Sensors (Basel) 2020;20(3):606. doi: 10.3390/s20030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material and PRISMA checklist are available on the publisher’s website along with the published article.